
Left wing of Bubo virginianus, from below (reduced one third).
r, radius; u, ulna; c, cuneiform; s, scapho-lunar; os p, os prominens; epa, tendon of extensor patagii longus.
Transcriber’s Note:
New original cover art included with this eBook is granted to the public domain.
| NUMBER I. | |
| Page. | |
|---|---|
| On an apparently New Heron from Florida. By Robert Ridgway. | 1 |
| List of Birds observed at Houston, Harris Co., Texas, and Vicinity, and in the Counties Montgomery, Galveston, and Ford Bend. By H. Nehrling. | 6 |
| On the Sesamoid at the front of the Carpus in Birds. By F. Amory Jeffries. | 13 |
| Notes on Some of the Birds observed near Wheatland, Knox Co., Indiana, in the Spring of 1881. By Robert Ridgway. | 15 |
| Notes on the Habits and Changes of Plumage of the Acadian Owl (Nyctale acadica), with some additional Records of its Breeding in Massachusetts. By William Brewster. | 23 |
| Description of a New Race of Peucæa ruficeps from Texas. By Nathan Clifford Brown. | 26 |
| On Kennicott’s Owl and some of its Allies, with a Description of a proposed New Race. By William Brewster. | 27 |
| A Reconnoissance in Southwestern Texas. By Nathan Clifford Brown. | 33 |
| RECENT LITERATURE. | |
| Memorial Volume of Garrod’s Scientific Papers, 43; Shufeldt’s Osteology of the North American Tetraonidæ, 44; Illustrations of Ohio Nests and Eggs, 45; Shufeldt’s “The Claw on the Index Digit of the Cathartidæ,” 46; Papers on Minnesota Birds, 47; Freke on the Birds of Amelia County, Virginia, 48; Langdon’s Field Notes on Louisiana Birds, 48; Krider’s Field Notes, 49; Langdon’s Zoölogical Miscellany, 50; Hoffman on the Birds of Nevada, 51. | |
| GENERAL NOTES. | |
| ivThe Tufted Titmouse on Staten Island, N. Y., 52; Nesting of the White-bellied Wren (Thryothorus bewicki leucogaster), 52; An Erroneous Record of the Orange-crowned Warbler (Helminthophaga celata) in New Hampshire, 53; On the Generic Name Helminthophaga, 53; Dendræca palmarum again in Massachusetts, 54; Ampelis cedrorum as a Sap-sucker, 54; Capture of Plectrophanes lapponicus in Chester, S. C., 54; Occurrence of Coturniculus lecontei in Chester County, South Carolina, 54; The Sharp-tailed Finch in Kansas, 55; Note on Mitrephanes, a New Generic Name, 55; Nesting of Empidonax minimus and Helmintherus vermivorus in Pennsylvania and New Jersey, 55; Cuckoos laying in the Nests of other Birds, 56; Melanerpes erythrocephalus about Boston, 57; The Barn Owl in Maine; a Retraction, 58; The Snowy Owl at Fort Walla Walla, W. T., 58; Capture of the Golden Eagle in Crawford County, Pennsylvania, 58; The Swallow-tailed Kite in Dakota, 59; A Remarkable Specimen of the Pinnated Grouse (Cupidonia cupido), 59; Wilson’s Plover (Ægialites wilsonius) in New England, 59; Capture of Baird’s Sandpiper on Long Island, N. Y., 60; An Addition to the Maine Fauna, 60; Capture of Larus leucopterus near Boston, 60; The Great Black-backed Gull (Larus marinus) from a new Locality, 60; The Snake-bird in Kansas, 61; Capture of the Sea Dove 150 Miles from the Sea, 61; Additions to the Catalogue of North American Birds, 61; Notes on Some Birds of the Belt Mountains, Montana Territory, 61; Remarks on Some Western Vermont Birds, 63. | |
| Erratum | 64 |
| NUMBER II. | |
| On a Collection of Birds lately made by Mr. F. Stephens in Arizona. By William Brewster. | 65 |
| Notes on the Os Prominens. By Frederic A Lucas. | 86 |
| A List of Birds from the Lower Mississippi Valley, observed during the Summer of 1881, with brief Notes. By O. P. Hay. | 89 |
| Impressions of some Southern Birds. By William Brewster. | 94 |
| Notes on some of the Rarer Birds of Southern New Brunswick. By Montague Chamberlain. | 104 |
| Notes on the Summer Birds of the Upper St. John. By Charles F. Batchelder. | 106 |
| RECENT LITERATURE. | |
| Dr. Coues’ New Check List and Dictionary, 111; Nests and Eggs of Ohio Birds, 112; Professor Macoun’s Report of Exploration, 113; Knowlton’s Revised List of the Birds of Brandon, Vermont, 113; Krukenberg on the Coloring Matter of Feathers, 114; Minor Ornithological Papers, 115. | |
| GENERAL NOTES. | |
| vDescription of a Nest of the Water Ouzel, 118; The Short-billed Marsh Wren in New Hampshire, 118; Early Arrival of the Yellow Rump in Southern Maine, 119; Late Stay (probable Wintering) of Dendrœca pinus in Massachusetts, 119; The Hooded Warbler in Western New York, 119; Breeding of the Pine Grosbeak (Pinicola enucleator) in Lower Canada, 120; Coturniculus lecontei, C. henslowi, and Cistothorus stellaris in Florida, 121; Ammodramus caudacutus—a somewhat Inland Record on the Atlantic Coast, 122; The White-throated Sparrow in Winter near Worcester, Mass., 122; Peucæa ruficeps eremœca, 122; The Canada Jay at Portland, Maine, 122; The White-throated Swift breeding on Belt River, Montana, 122; Capture of the Golden Eagle (Aquila chrysaëtus canadensis) near Columbus, Ohio, 123; The Little Blue Heron in Maine, 123; Baird’s Sandpiper on Long Island, N. Y.—a Correction, 123; Pelidna subarquata on the Maine Coast, 124; The King Rail in New England, 124; Purple Gallinule (Ionornis martinica) in Rhode Island, 124; Note on the Habits of the Young of Gallinula galeata and Podilymbus podiceps, 124; Rhynchops nigra—an Early Record for the Massachusetts Coast, 125; Notes on the Habits of the Kittiwake Gull, 125; Sterna forsteri breeding off the Eastern Shore of Virginia, 126; Note on the Foot of Accipiter fuscus, 126; Supplementary Notes on two Texas Birds, 127; Addenda to the Preliminary list of Birds ascertained to occur in the Adirondack Region, Northeastern New York, 128. | |
| Errata | 128 |
| NUMBER III. | |
| The Colors of Feathers. (Plate I.) By J. Amory Jeffries | 129 |
| On a Collection of Birds lately made by Mr. F. Stephens in Arizona. By William Brewster | 135 |
| Notes on the Summer Birds of the Upper St. John. By Charles F. Batchelder | 147 |
| A Sketch of the Home of Hylocichla aliciæ bicknelli, Ridgway, with some Critical Remarks on the Allies of this New Race. By Eugene P. Bicknell | 152 |
| Short Notes on the Birds of Bayou Sara, Louisiana. By Charles Wickliffe Beckham | 159 |
| List of Birds observed at Houston, Harris Co., Texas, and in the Counties Montgomery, Galveston, and Ford Bend. By H. Nehrling | 166 |
| RECENT LITERATURE. | |
| Bailey’s Index to Forest and Stream, 175; Chamberlain’s Catalogue of the Birds of New Brunswick, 176; Krukenberg on the Coloring Matter of Feathers, Second Part, 177; Stejneger’s Nomenclatural Innovations, 178; Ingersoll’s Birds’-Nesting, 179. | |
| GENERAL NOTES. | |
| Note on Mimus polyglottus, 180; The Nest of the House Wren, 180; Remarkable plumage of the Orchard Oriole, 181; The Nest and Eggs of Perisoreus canadensis, 181; Notes on the Plumage of Nephæcetes niger borealis, 182; Plumage of the Young of Eclectus polychlorus, 183; An Owl’s Egg laid in Confinement, 183; Buteo brachyurus—a Correction, 184; The Turkey Buzzard in New Hampshire, 184; Rapacious Birds in Confinement, 184; Note on Mareca americana, 185; Destruction of Birds by the Cold Wave of May 21st and 22d, 185; More Definite Statistics needed in regard to the Abundance of Birds, 186; Remarks on Five Maine Birds, 189; Maine Notes, 190; Stray Notes from Lookout Mountain, Tenn., 191. | |
| Errata | 192 |
| vi | |
| NUMBER IV. | |
| On a Collection of Birds lately made by Mr. F. Stephens in Arizona. By William Brewster | 193 |
| Notes upon the Osteology of Cinclus mexicanus. By R. W. Shufeldt | 213 |
| List of Birds observed at Houston, Harris Co., Texas, and in the Counties Montgomery, Galveston, and Ford Bend. By H. Nehrling | 222 |
| Notes on some Birds collected by Capt. Charles Bendire at Fort Walla Walla, Washington Territory. By William Brewster | 225 |
| List of Birds ascertained to occur within Ten Miles from Point de Monts, Province of Quebec, Canada, based chiefly upon the Notes of Napoleon A. Comeau. By C. Hart Merriam | 233 |
| RECENT LITERATURE. | |
| The Coues Check List and Ornithological Dictionary, 242; Gentry’s Nests and Eggs of Birds of the United States, 246. | |
| GENERAL NOTES. | |
| Dendræca palmarum at Sing Sing, N. Y., 249; Nest and Eggs of Setophaga picta—a Correction, 249; The Summer Tanager (Pyranga æstiva) in New Brunswick, 249; The Evening Grosbeak in New York, 250; The Black-throated Bunting in Florida, 250; Distribution of the Fish Crow (Corvus ossifragus), 250; The Swallow-tailed Kite (Elanoïdes forficatus) taken in Southern Michigan, 250: Garzetta candidissima at Nantucket, Massachusetts, 251; The Snow Goose (Chen hyperboreus) at Sing Sing, N. Y., 251: Note on the Long-tailed Duck, 251; Lomvia arra brünnichi and L. troile in New England, 251; Rare Warblers in Massachusetts, 252; The Unusual “Wave” of Birds during the Spring Migration of 1882, 252; Birds new or rare in the District of Columbia, 253; Notes on some Birds and Eggs from the Magdalen Islands, Gulf of St. Lawrence, 253; Second Addendum to the Preliminary List of Birds ascertained to occur in the Adirondack region, Northeastern New York, 256; List of Additions to the Catalogue of North American Birds, 257. | |
| Index | 259 |
The following facts in relation to an apparently hitherto unnoticed large Heron found in Southwestern Florida, I am kindly permitted to lay before the readers of the Nuttall Bulletin, by Mr. Charles W. Ward, of Pontiac, Michigan, who spent several weeks at the breeding grounds of the bird in question, and was thus enabled to make many very interesting observations on its habits, etc. Mr. Ward’s memoranda are especially interesting in connection with the question of Ardea occidentalis Aud. and A. würdemanni Baird, but unfortunately the matter, in the light of the evidence which he adduces, becomes involved in greater obscurity than before.
Under date of September 3 (1881), Mr. Ward writes as follows:—
“My observations of the Herons during the past season do not correspond with those of Mr. N. B. Moore, as recorded on page 232 of your article[1], in regard to their feeding habits. I found them generally living in communities, roosting, nesting, and feeding together, like Pigeons, and often observed flocks of the Little White, Reddish, and other Egrets, feeding together 2like Teal Ducks. Two specimens of A. occidentalis were seen feeding quietly within twenty feet of one of the Herons procured by me [A. wardi, nobis]. They were feeding on a mud bar at low tide. I was once concealed in the low brush near a small pool watching three Louisiana Egrets chasing minnows, when two of them making for the same minnow squared off for a knock-down, while the third coolly appropriated the prize, leaving the combatants situated like complainant and defendant at the close of a law suit. In all my observations of the Herons I have seen nothing to lead to a conclusion that one of these birds held any particular antipathy against its own species while feeding. In the many squabbles between Herons on their feeding grounds the encounters occurred quite as often between different species as members of the same species. It may be that during the breeding season they are more friendly than at other times. In order that you may understand my opportunities for observing these birds, I enclose a rough map of Mound Key and surroundings, my camping place from January 20 till April 10. As you will see by the figures marked ... it was in the midst of their feeding grounds, these places being mud- and sand-bars, bare at low tide. Regarding the Reddish Egret, among many thousands of them I saw only one in the pure white plumage, and no white young; but one of my dark specimens has white feathers on the head and in the tail, while one of the secondary quills has the outer web chiefly white. My companion of last winter’s Florida trip reports that he saw no Reddish Egrets with white except on the secondaries.
“Regarding the large Herons [i.e., A. wardi], I am much inclined to think them a geographical variety ... the specimens being very uniform in color.... I examined some thirty nests at least, fifteen of which contained young, all being dark colored, with one exception. These birds are common in Southwestern Florida, and their nests are frequently found along the coast. From all the information at my command, connected with my own observations, I am almost convinced that the bird in question is separate and distinct from A. occidentalis and A. würdemanni, and the fact that Audubon found the former in immense numbers among the mangrove islands of Eastern Florida is strong evidence that he happened in the vicinity of one of their rookeries. As you will observe by examining the diagram 3of my camping place and noting the rookeries of large Herons ... these birds were quite common in that vicinity, while I saw only a few specimens of A. occidentalis. The white bird found in the nest with the blue might have come there from an adjoining empty nest, some 30 or 40 feet distant, as it could easily have done, being nearly full-grown. This surmise is strengthened by the circumstance that I saw a large white Heron on the island marked ‘*,’ and my companion killed a similar, if not the same, specimen on the large island marked ‘2,’ which he threw away, supposing it to be a common White Egret [Herodias egretta]. These I now believe to have been A. occidentalis; the other [H. egretta] was then laying its eggs, while the description of A. occidentalis corresponds to my recollection of the bird he killed. At the time, I was not familiar with the description of A. occidentalis.
“In the Little Blue Heron [Florida cærulea] and Reddish Egret (Dichromanassa rufa), where dichromatism appears to be an established fact, each species presents different phases and mixtures of both colors, especially the Little Blue, which shows almost every variety of curious markings of blue and white; while in the Reddish Egret, one specimen shows white on the head, tail, and wings, and others reported by Mr. Adams show white on the wings.
“As before said, I believe the bird to be a geographical variation of A. herodias, residing permanently and breeding in South Florida. I think that further search and observation will develop more evidence concerning A. occidentalis and A. würdemanni, which may result in confirming your theory of their being one and the same species. You will pardon my opposing your opinion, but my convictions are so strong that only the finding of white birds with blue young and more cases of blue parents with white young, or adults showing mixtures of both phases, would overcome them.”
Assuming that the large white birds observed by Mr. Ward were really a white phase of the dark-colored birds obtained by him, and which were so numerous in the locality, it certainly appears strange that so few of the former were seen. The case of the Reddish Egret, which he cites, affords, however, an exact parallel, and it is now considered established beyond question that “Peale’s Egret” (Ardea pealei Bonap.—a pure white bird) 4is merely a white phase of this species. As to the comparative rarity of these large white birds, in the locality where observed by Mr. Ward, militating against any theory of their specific identity with the dark-colored birds, it should be remembered that in the case of nearly every dichromatic species of bird this condition is more or less variable with locality. A pertinent example may be cited in the case of Demiegretta sacra, a Heron of wide distribution in the Far East. This species inhabits a considerable number of islands in the Polynesian group, and it has been noticed and recorded by naturalists who have visited that region, that on some islands all or nearly all the birds of this species are dark colored, on others all or nearly all are white, while on others still there may be a more equal proportion of the two phases. It may be remarked that the two phases in this species are even more distinct in coloration than in the case of Dichromanassa rufa, the colored phase being darker than in the latter species. Upon the whole, even admitting the possibility of the white young bird seen by Mr. Ward having of its own volition taken up its abode in a nest containing dark-colored young, I am strongly inclined to believe that it belonged to the same species with the latter, the question of its parentage (i.e., whether its parents were white or dark-colored birds) being a comparatively unimportant consideration, as affecting the main question. But in adopting the view of their specific identity a problem arises which in the light of our present knowledge appears unsolvable, and which may be briefly stated thus:—
The large “blue” Herons obtained by Mr. Ward are, in every respect as regards size and proportions, identical with Ardea occidentalis Aud. and A. würdemanni Baird; in coloration they agree exactly with the latter, except only in the pattern of the head and tint of the neck, which are precisely as in A. herodias. The bird in question is apparently “dichromatic,” having a white phase; hence, assuming that A. occidentalis and A. würdemanni are dichromatic phases of one species, it necessarily follows that white individuals of the bird in question would be absolutely indistinguishable from white examples of A. occidentalis! Still, in view of the fact that the colored phase differs from A. würdemanni in its most essential feature of coloration, i.e., the pattern of the head markings, it seems impossible to unite them, unless it can be shown that the type of A. würdemanni does not represent 5the perfect colored phase of that species.[2] There are hence several hypotheses which might be plausibly argued upon theoretical grounds, and which may be stated as follows: (1) That A. occidentalis, A. würdemanni, A. wardi, and A. herodias all belong to a single species, which reaches its extremes of variation in the first- and last-named; (2) That these names include three distinct races or species: A. herodias, which is never white; A. occidentalis, which is dichromatic (having separate white and colored phases), and A. wardi, also dichromatic, its white phase indistinguishable from that of A. occidentalis, and its colored phase distinguishable from that of the same species (A. würdemanni) by the different pattern and color of the head and neck alone; and (3) that there are two species, A. occidentalis and A. herodias, which in Florida hybridize on an extensive scale, producing the intermediate specimens which have been distinguished as A. würdemanni and A. wardi.
Of these hypotheses I have, after careful consideration of them all, concluded to adopt the second as being most consistent with known facts, and accordingly propose for the bird in question the name
With the following characters:—
Ch.—Colored phase exactly like A. würdemanni (= dark phase of A. occidentalis?), but with the head colored as in A. herodias. Differing from herodias in much larger size (culmen 6.50–7.00 inches, tarsus, 8.50–9.00 inches), lighter general coloration, and (in dried skin) light brown instead of black legs. Dichromatic; the white phase being indistinguishable from that of A. occidentalis (?).
Adult ♂ (No. 82,329, U. S. Nat. Mus., Oyster Bay, Florida, March, 1881; Chas. W. Ward): Head white, with the sides of the crown and entire occiput (including the lengthened plumes) deep black;[3] neck lavender-gray (much lighter than in the type of würdemanni), the fore-neck 6white thickly streaked with black for the lower two-thirds; jugular plumes chiefly white, their lengthened tapering portion entirely so. Upper surface uniform bluish plumbeous, the lengthened scapular plumes hoary whitish or pale silvery gray. Upper breast uniform black; abdomen and lower breast white, rather indistinctly streaked with dark gray; anal region mixed black and white, in longitudinal dashes (the black rather predominating); crissum immaculate pure white. Tibiæ uniform light cinnamon; edge of the wing (especially near the bend) deeper cinnamon, but this much mixed with white toward the bases of the quills; lining of the wing, axillars, sides, and flanks, uniform plumbeous. Bill, apparently, entirely olivaceous-yellow; naked portion of tibiæ very pale brown (evidently yellowish or flesh-colored in life); tarsi light brown (olivaceous in life?), darker in front; toes light brown. Wing, 20.50: culmen, 6.75; depth of bill through nostril, 1.10; tarsus, 8.75; middle toe, 5.10; naked portion of tibiæ, 5.50.
Mr. W. H. Collins, of Detroit, who kindly presented the specimen described above to the National Museum, has sent me measurements of two other specimens, one in his own possession, the other mounted for Mr. Ward. As may be seen below they agree closely in dimensions with the type, their measurements being, respectively, wing 20.00–20.50; culmen 6.50–7.00; depth of bill through nostril, 1.25; tarsus, 8.75–9.00; middle toe, 5.25–5.45; naked portion of tibia, 5.75–6.00.
1. Turdus migratorius, L. Robin.—Very common in the woods from November to April. Very shy and retiring during their stay; only a few have been observed in the larger gardens of Houston. Feeds abundantly on the berries of the holly (Ilex opaca) and the myrtle-holly (Oreophila myrtifolia). About the 15th of April all have departed for the North.
2. Turdus mustelinus, Gmel. Wood Thrush.—Arrives from the North early in October when the aromatic berries of the Magnolia grandiflora are ripe, on which they eagerly feed. On account of this food the flesh is very delicate and large numbers are killed by pot hunters, who call them “Grassets.” In the winter months they appear not to be common and inhabit swampy thickets and bottom woods.
73. Turdus fuscescens, Steph. Wilson’s Thrush.—Only a few observed during the fall migration.
4. Turdus swainsoni, Cab. Olive-backed Thrush.—Not rare during the migrations.
5. Mimus polyglottus, Boie. Mockingbird.—A very abundant resident. Only a few remain to winter, in protected localities; the majority migrate further south. They arrive from their winter quarters early in March and are by the end of that month again common. Nest-building commences usually in the middle of April. Many are killed by farmers and gardeners on account of their fondness for ripe figs and grapes. Besides insects, they feed eagerly on the berries of the poke (Phytolacca decandra), the elder (Sambucus canadensis), and the Mexican mulberry (Callicarpa americana). In winter the berries of the myrtle-holly (Oreophila myrtifolia) and those of the mistletoe (Phoradendron flavescens) are their principal food.
6. Mimus carolinensis, Gray. Catbird.—I first observed a single specimen of this bird April 25, 1879. It was then my opinion that this bird must be a very rare migrant, as I did not meet with another that year. It was this year (1881), May 5, when I wandered through the thick underbrush in the woods on Spring Creek that I heard the peculiar cry of the Catbird, and a few minutes after I discovered the nest, which was built in a young oak sapling, about ten feet above the ground. They are not the familiar and confident birds of the Northern States, but extremely shy and retiring in their habits. They kept a good distance from me when I took the nest.
7. Harporhynchus rufus, Cab. Brown Thrush.—Common during the winter months in the thick underbrush of the woods near Spring Creek, in the northern part of Harris County. Very silent and extremely shy.
8. Sialia sialis, Hald. Bluebird.—A very abundant winter sojourner and a common summer resident; but not so abundant as in the Northern States, and not so familiar. Commences to breed as early as February 15. I found a nest March 6, which contained newly hatched young. A nest discovered April 29 contained four pure white eggs.
9. Regulus calendula, Licht. Ruby-crowned Kinglet, and
10. Regulus satrapa, Licht. American Golden-crested Kinglet.—Both are common during the winter months, when, in company with Titmice, they inhabit the pine woods near Houston. Are to be observed during the whole winter in the mountain cedars (Juniperus occidentalis texanus), which are common in the gardens of the city.
11. Polioptila cærulea, Sclat. Blue-gray Gnatcatcher.—Common in the heavy wooded bottom lands on the Brazos, Spring Creek, and San Jacinto, and especially abundant on Buffalo Bayou when the magnificent Magnolia grandiflora is in bloom. Almost with the agility and grace of a Hummingbird, it flies around the showy flowers in pursuit of insects. Nest-building commences early in May. This beautiful little domicile is built very high, in small branches of elms, swamp oaks (Quercus palustris) and other densely leaved forest trees.
812. Lophophanes bicolor, Bon. Tufted Titmouse.—A very common bird and resident throughout the year, even in the city gardens, where it is exceedingly tame and confiding. Breeds as early as the beginning of March. Nests in deserted Woodpeckers’ holes, in old stumps, in cedar-posts, in hollow branches, etc.
13. Parus carolinensis, Aud. Southern Chickadee.—Very common and familiar. Resident throughout the year. April 15 I discovered a nest of this diminutive bird in an old fence-post; it contained six nearly fledged young. The cavity was filled up about nine inches with soft mosses, cow’s hair, and the fur of smaller animals. Usually the nest is built in the hollow of a branch.
14. Thryothorus ludovicianus, Bon. Carolina Wren.—Very common in all low wooded localities with dense underbrush. Thickets of smilax, blackberry bushes, snowball (Viburnum molle and V. dentatum), Rhamnus carolinianus, Bumelia lanuginosa, intermixed with a few larger trees (oaks or elms), which are commonly overgrown by the mustang-grape and the grotesque forms of the supple jack (Berchemia volubilis), are its favorite resorts. In a few instances I have known a pair to build their nest in a bird-box near a dwelling.
15. Thryothorus bewicki, Bonap. Long-tailed House Wren.—Abundant in all suitable localities and very familiar, breeding in bird-boxes, stables, corn-cribs, and even in houses over doors, etc. One pair built their nest in the pocket of an old coat, hanging out doors.
16. Troglodytes aëdon, Vieill. House Wren.—Only a winter visitant, occurring in considerable numbers in secluded localities.
17. Cistothorus palustris, Baird. Long-billed Marsh Wren.—Rare during the migrations.
18. Cistothorus stellaris, Cab. Short-billed Marsh Wren.—Observed so late as May 2 in the marshy prairie districts in the northern part of Harris County, and in September in the sugar-cane fields on the Brazos in Ford Bend County. Probably breeds.
19. Anthus ludovicianus, Licht. American Pipit; Titlark.—Very common during winter, from the middle of November to the second week in April. Comes fearlessly in the streets of the city and in the door-yards.
20. Neocorys spraguei, Sclat. Missouri Skylark.—Observed small flocks early in November on the prairies near Houston. They were often associated with Passerculus savanna, and in habits resembled very closely the Titlark. All disappeared soon.
21. Mniotilta varia, Vieill. Black-and-white Creeper.—Not uncommon during the migrations. Noted first March 22. At the 15th of April the majority depart for the north, only few remaining to breed.
22. Parula americana, Bon. Blue Yellow-backed Warbler.—This beautiful little Warbler is rather common during the migrations in all wooded portions, especially in the river bottoms, where almost every tree is covered with the long gray Spanish moss (Tillandsia usneoides). Some remain to breed, as I have seen the parents feeding the young in July and August.
923. Protonotaria citrea, Bd. Prothonotary Warbler.—A not uncommon summer resident in marshy localities on Spring Creek and in Ford Bend County in the Brazos bottom, where so-called lakes are abundant. It breeds in hollows of trees, deserted Woodpeckers’ holes, and in stumps standing in the water. I usually met with this bird in localities where the Little Blue Heron (Florida cærulea) and the Snowy Heron (Garzetta candidissima) were common. I can add nothing to the unsurpassable life history of this bird given by Mr. William Brewster in this Bulletin, Vol. III, pp. 153–162.
24. Helmintherus vermivorus, Bon. Worm-eating Swamp Warbler.—A few seen April 6, 1881, in a flowering plum tree in a city-garden.
25. Helminthophaga chrysoptera, Cab. Golden-winged Warbler.—Common during the migrations, in October and April.
26. Helminthophaga peregrina, Cab. Tennessee Warbler.—Not uncommon during migrations.
27. Helminthophaga celata, Bd. Orange-crowned Warbler.—Seen only during migrations and very rare.
28. Dendrœca æstiva, Bd. Summer Yellow Bird.—Very abundant during migrations. Not a very common summer sojourner, but quite regularly distributed.
29. Dendrœca coronata, Gray. Yellow-rumped Warbler.—The most common of all the Warblers from November to April. Winters abundantly in this region and numbers visit the gardens, even those in the interior of the city.
30. Dendrœca maculosa, Bd. Black-and-yellow Warbler, and
31. Dendrœca blackburniæ, Bd. Blackburnian Warbler, are both, so far as I observed, exceedingly rare during migrations.
32. Dendrœca pennsylvanica, Bd. Chestnut-sided Warbler.—Somewhat common in the latter part of April and early in May.
33. Dendrœca castanea, Bd. Bay-breasted Warbler.—This elegant Warbler is one of the most common of its family during the spring migration. I observed small flocks of from eight to ten so late as May 5.
34. Dendrœca striata, Bd. Black-poll Warbler.—Transient; arrives from winter quarters late in April, when the host of Warblers pass northward. Tolerably common.
35. Dendrœca virens, Bd. Black-throated Green Warbler.—Abundant during migrations. Moves in flocks of from four to ten.
36. Dendrœca dominica albilora, Ridg. Yellow-throated Warbler.—A very rare summer resident and very difficult to observe in the high moss-grown forest trees of the river bottoms. The song resembles that of Dendrœca æstiva, but is louder and more varied. I think it is almost impossible to discover a nest of this bird in the high trees, so densely covered with Tillandsia.
37. Dendrœca pinus, Bd. Pine Warbler.—Winters in small companies in the woods in the northern part of Harris County, near Spring Creek.
10I did not find so many Warblers as I expected, although I kept a diligent lookout. I did not observe D. palmarum, D. canadensis, D. discolor, or D. cærulea.
38. Siurus auricapillus, Sw. Golden-crowned Thrush.—Transient and not common.
39. Siurus nævius, Coues. Water Thrush.—Not uncommon in suitable localities during migrations.
40. Oporornis formosa, Bd. Kentucky Warbler.—A common summer resident; exceeding in numbers even the Maryland Yellow-throat, with which it occupies the same localities. Common in wet fields with patches of low bushes, and in the dense undergrowth near water. Visits frequently the country gardens. Very abundant on Spring Creek, in the northern part of Harris County, and in Montgomery County. Arrives about April 21. Commences nest-building early in May. Nest very difficult to find.
41. Geothlypis trichas, Cab. Maryland Yellow-throat.—Arrives about April 15, from its winter quarters. A common summer sojourner. Like the preceding species, most common in grassy localities with thickets interspersed. On a farm near Houston is a wet piece of land containing about two acres, where I found three pairs breeding. Through this runs a ditch and the whole ground is covered with high broom-grass (Andropogon macrurus) with briar patches, thickets of water oak. Viburnum dentatum, black haw (V. pruneifolium), etc. The field is surrounded by an almost impenetrable hedge of Cherokee roses (Rosa lævigata). Here the Yellow-throats occur with Kentucky Warblers, White-eyed Vireos, Yellow-throated Vireos, Painted Finches, and Blue Grosbeaks, all living in harmony. Two broods are raised yearly in this latitude. In almost every nest of this bird, and also of the Kentucky Warbler, eggs of the Cow Bird are to be found.
42. Geothlypis philadelphia, Bd. Mourning Warbler.—Transient and rather rare.
43. Icteria virens, Bd. Yellow-breasted Chat.—A common summer resident, arriving from its winter quarters about April 15. Many winter in sheltered places. Its most favorable resorts are brier-patches in fields, thickets on the edge of woods, myrtle-holly thickets overgrown with tangled Smilax laurifolia, and similar localities. Nest in the interior of thickets near the ground; it has some resemblance to the Catbird’s, and is built of nearly the same material.
44. Myiodioctes mitratus, Aud. Hooded Warbler.—This beautiful species is common during migrations. Arrives from the South in the last part of April, when the host of Warblers migrate northward. I never observed the bird during the summer months and do not think that any remain to breed.
45. Myiodioctes canadensis, Aud. Canadian Flycatching Warbler.—Not very common during the spring migration.
46. Myiodioctes pusillus, Bon. Black-capped Warbler.—I consider this the most common species of the genus during migrations.
1147. Setophaga ruticilla, Sw. American Redstart.—Moves northward late in April and early in May, when the throng of Warblers migrate to their summer quarters in high northern latitudes.
48. Vireosylvia olivacea, Bon. Red-eyed Vireo.—A common summer resident in all the deciduous woods.
49. Vireosylvia gilva, Cass. Warbling Vireo.—Evidently a rare species, even during the migrations.
50. Lanivireo flavifrons, Bd. Yellow-throated Vireo.—Abundant and breeding. The first nest, beautifully constructed, I discovered April 28 in a high blackberry-bush about four feet above the ground, near Houston. It contained four fresh eggs and one of the Dwarf Cowbird (Molothrus ater obscurus). Nest and eggs in my collection. Many more nests were discovered during the months of May and June, and many contained one and two eggs of the Cowbird.
51. Lanivireo solitarius, Bd. Solitary Vireo.—Rare during migrations.
52. Vireo noveboracensis, Bon. White-eyed Vireo. A common summer resident in localities where Viburnum dentatum, V. molle, V. pruneifolium, Rhamnus carolinensis, Cornus florida, laurel-oaks (Quercus imbricaria), and elms are growing, especially on the borders of woods, in open thickets, peach gardens, etc.
53. Vireo belli, Aud. Bell’s Vireo.—A common summer sojourner. A not quite finished nest was discovered April 15 on a horizontal branch of a Viburnum dentatum on the edge of a thicket, about five feet above the ground. It contained three fresh eggs. The nests of this Vireo are more purse-shaped and deeper than any other Vireo nests I am acquainted with.
54. Lanius ludovicianus excubitorides, Coues. White-rumped Shrike.—A generally dispersed summer resident, but not abundant. Prefers to build in the hedges of the osage orange.
55. Ampelis cedrorum, Vieill. Cedar Bird.—Abundant migrant. Observed flocks of from thirty to fifty as late as May 6. None remain to breed.
56. Progne subis, Bd. Purple Martin.—Abundant summer resident. Arrives March 1 from the South. Breeds in large numbers under the wooden awnings of sidewalks, even in the business part of Houston and Galveston. Abundant also in the country where bird-boxes are put out for its convenience. Two broods are commonly raised in this latitude.
57. Petrochelidon lunifrons, Lawr. Cliff Swallow.—Seen in great numbers during September, but does not breed in this region.
58. Hirundo erythrogastra, Bodd. Barn Swallow.—Large numbers seen in the latter part of August, but not found breeding.
59. Tachycineta bicolor, Cab. White-bellied Swallow.—Common during migrations. A few observed in summer on the borders of woods.
1260. Cotyle riparia, Boie. Bank Swallow.—A few pairs remain to breed in such localities as the banks of Buffalo Bayou and Galveston Bay.
61. Stelgidopteryx serripennis, Bd. Rough-winged Swallow.—A very abundant summer resident. Often nests under the roofs of sidewalks and on old buildings in Houston, but is more a companion to the preceding on the high banks on Buffalo Bayou and Galveston Bay.
62. Pyranga rubra, Vieill. Scarlet Tanager.—A moderately common bird during the migrations. Arrives from the South about April 15 and passes without lingering to its more northern breeding range.
63. Pyranga æstiva, Vieill. Summer Redbird.—A common summer resident, particularly in oak woods. It is an elegant species, as are all the members of this family, but is more retired in its habits and quicker and more restless in its motions than the preceding. The song is more varied, louder, and wilder. The nest is usually built on the horizontal branch of an oak, from seven to twenty feet above the ground. It is a very open-worked inartificial structure, and the eggs cannot with certainty be distinguished from those of the Scarlet Tanager.
64. Astragalinus tristis, Cab. Goldfinch.—A very abundant winter sojourner. Feeds almost entirely on the seeds of the sycamore or button-wood (Platanus occidentalis).
65. Chrysomitris pinus, Bon. Pine Finch.—A somewhat rare winter sojourner.
66. Passerculus savanna, Bon. Savanna Sparrow.—Common resident throughout the year. Breeds on the low grassy prairies, but the nest is difficult to find.
67. Poœcetes gramineus, Bd. Grass Finch.—Only to be found during migrations. None remain, so far as I know, to winter or to breed.
68. Coturniculus passerinus, Bon. Yellow-winged Bunting.—Seen occasionally during the winter months.
69. Ammodromus caudacutus, Sw. Sharp-tailed Finch.—Observed near the coast of the Gulf of Mexico and Galveston Bay. Doubtless breeds.
70. Chondestes grammicus, Bon. Lark Finch.—This interesting, lively bird is the most common of its family in all suitable localities, that is, on the prairies, near woods. Departs for the South late in September and early in October; arrives from his winter quarters again in April. Breeds in May, June, and July, and two or even three broods are raised yearly. Nests in gardens on mulberry-trees, in the corners of rail-fences, in cotton fields on the ground, but most commonly on a low horizontal branch of an oak densely covered with Tillandsia, on the borders of woods, where they are exceedingly difficult to discover. After breeding-time the birds assemble in large flocks.
71. Zonotrichia albicollis, Bon. White-throated Sparrow.—Rare and occurs only in winter.
72. Zonotrichia leucophrys, Sw. White-crowned Sparrow.—Abundant in winter.
73. Zonotrichia gambelli intermedia, Ridg. Gambel’s Finch.—Not uncommon in winter.
1374. Spizella socialis, Bon. Chipping Bird.—Abundant in October and November, and again in March.
75. Spizella pallida, Bon. Clay-colored Bunting.—Abundant in winter near thickets and in fields with brier-patches.
76. Spizella pusilla, Bon. Field Sparrow.—Not uncommon during winter.
77. Junco hiemalis, Sclat. Common Snowbird.—Abundant winter visitor.
78. Melospiza fasciata, Scott. Song Sparrow.—Common during the winter months.
79. Melospiza lincolni, Bd. Lincoln’s Sparrow.—Common in winter in the thick undergrowth on the borders of woods.
80. Peucæa cassini, Bd. Cassin’s Finch.—A common summer resident on the open grassy prairies. It runs like a mouse through the grass, and is very shy and difficult to observe. A nest I never discovered.
81. Pipilo erythrophthalmus, Vieill. Ground Robin.—A rare summer resident. A few pairs breed in the woods on Spring Creek.
82. Calamospiza bicolor, Bon. Lark Bunting.—Abundant in winter on the prairies.
83. Euspiza americana, Bon. Black-throated Bunting.—A common summer resident. Breeds abundantly in all the prairie districts.
84. Cardinalis virginianus, Bon. Cardinal Grosbeak.—This well-known bird is the most abundant of the family and resident throughout the year.
85. Guiraca cærulea, Sw. Blue Grosbeak.—Regularly distributed summer resident, but nowhere abundant. Nests discovered always in brier-patches in fields, on roadsides, and on the border of woods.
86. Cyanospiza ciris, Bd. Painted Finch.—Inhabits with the preceding similar localities. Very common from April to October. Nest usually in blackberry-bushes, but always well hidden and not easy to find. These birds are very shy and exceedingly quick in all their motions.
87. Cyanospiza cyanea, Bd. Indigo Bird.—Observed only during the migrations. None I think remain to breed.
In the Bulletin for October, 1881, is a paper by Dr. Shufeldt entitled “On the Ossicle of the Antibrachium as found in some of the North American Falconidæ,” in which the author describes 14the sesamoid ossicle at the distal end of the radius in the Marsh Hawk (Circus hudsonius) as a new bone. Dr. Shufeldt says: “It does not seem possible that a bone the size of one which I am now about to describe could have been entirely overlooked by ornithologists, yet after a careful perusal of such parts of the works of the most prominent writers, as refer to the skeletology of the upper extremity I fail to discover the barest mention as to the existence of any such an one.” Now this bone was figured, as it occurs in Aquila fucsa, by Milne-Edwards in his famous work on the Fossil Birds of France, the publication of which began in 1866, so that the bone as it occurs in the Falconidæ can scarcely be considered unknown to anatomists. The “os prominens” as it occurs in the Falconidæ is a modification of the sesamoid ossicle which very often occurs in the tendon of the tensor petagii longus where it passes over the carpus;[4] its function here being that of a simple sesamoid over the carpus. In many of the Falconidæ[5] this sesamoid becomes bound to the distal end of the radius, and lengthened out at right angles to the long axis of that bone, as figured by Dr. Shufeldt. By this means the function of the ossicle becomes very much altered. It no longer slides over the carpus, but serves, since the tendon of the extensor petagii longus includes only its free end, to keep that tendon off the carpus, thus avoiding friction at the joint. Again, since the ossicle attains considerable length,—6 centimeters (millimeters?) according to Dr. Shufeldt in Circus,—it materially alters the action of the extensor petagii longus so that it tends much more to extend the hand and draw the thumb away from the index. In this way the extensor petagii longus seems to antagonize the slip of the flexor longus digitorum sublimis, and since its tendon is elastic, owing to the amount of yellow fibrous tissue in it, the action must be to a considerable degree automatic.
My views of the functions of this ossicle are, it will be seen,
very different from those of Dr. Shufeldt, who considers it to
protect the carpus and greatly increase the area of the wing.
This bone, standing up as it does on the anterior edge of the
15wing, would seem to be particularly liable to injury, sufficient,
we should think, to offset the amount it may protect the compact
carpals below. The extra area covered by the wing on account
of the ossicle is easily measured. It is simply the area of a triangle,
which has for its base the difference in altitude between the
process of the metacarpus and the sesamoid ossicle, 3 millimeters
say, and for its altitude the distance between the carpus and
the origin of the extensor petagii longus, say 2.5 decimetres.
Absolute measurements cannot be given since no Hawks are to be
got in Boston at present. So the entire increase of area would be
3.75 square centimetres, and this increase is at the base of the wing,
where it would least increase the resistance of the wing. This difference
becomes quite small in the ratio √2 a
∛weight where a,
the area of one wing, represents hundreds of square centimeters.
Yet the ratio is that of the supporting power of the wing to the
weight of the body, other things being equal. In the above calculation
it is assumed that Dr. Shufeldt meant millimeters not
centimeters,[6] when giving the dimensions of the “os prominens.”
To sum up, the bone serves: (1) To keep the friction of the extensor petagii longus muscle off the carpus. (2) To increase the power of that muscle to abduct the thumb. (3) To slightly increase the supporting power of the wing. (4) To protect the carpus (?).
Here it may not be improper to state that during the winter of 1880–81, the writer showed a specimen of the carpus of Accipiter fuscus, and explained his views as here stated of the function of the “os prominens,” at a meeting of the Nuttall Ornithological Club.
Monteur’s Pond, situated about ten miles east of Vincennes and two miles west of the village of Wheatland, on the O. & M. R. R., is of considerable extent, being about nine miles long by 16a mile in average width. It is rather a swamp, however, than a pond, probably less than half its area being open water, the remainder filled with trees, chiefly willows (Salix nigra) averaging 50–60 feet high, mixed in places with a larger growth, chiefly ashes (Fraxinus americana, F. sambucifolia and F. pubescens), red maple, and swamp cottonwood (Populus heterophylla), the latter chiefly around the margin of the pond, where grow also swamp, white, and water oaks, sweet-gums, and an occasional catalpa (C. speciosa). The surrounding country, where not cleared, consists chiefly of original forest of various oaks and hickories, “poplar” (Liriodendron), beech, elm, and other trees in great variety, coniferous species being wholly absent.
The pond is never very deep, probably nowhere or at anytime exceeding four feet, and in seasons of drouth becomes absolutely dry, then forming an excellent pasturage for the stock of the neighboring farmers. Even when filled with water, the latter is, in the season of vegetable growth, entirely hidden by a luxuriant growth of aquatic plants, rendering the passage of a boat, of any description, impossible, while numerous muskrat holes and the intricate submerged stems render wading difficult and fatiguing in the extreme. For these reasons the pond was but slightly explored, while it was wholly neglected after the use of a boat became out of the question. I am therefore quite ignorant as to what species may have been breeding in the recesses of the pond, my investigations having been wholly confined to the surrounding fields and woodland, the northern portion of the pond and its immediate vicinity having been the scene of my ornithological investigations from April 15 to May 27.
Notwithstanding the very unusual lateness of the season I found on my arrival (April 15) that many of the migratory birds had preceded me, but subsequent arrivals were carefully noted up to May 6, and are presented herewith.
April 15. Prairie Warbler (Dendrœca discolor).
April 17. Yellow-throated Warbler (Dendrœca dominica albilora), Yellow-throated Vireo (Lanivireo flavifrons), Least Flycatcher (Empidonax minimus).
April 18. Prothonotary Warbler (Protonotaria citrea), Canada Flycatching Warbler (Myiodioctes canadensis), Blue Yellow-backed Warbler (Parula americana), Scarlet Tanager (Pyranga rubra), Summer Redbird (P. æstiva), Lark Finch (Chondestes grammica), Summer Yellowbird 17(Dendrœca æstiva), Maryland Yellow-throat (Geothlypis trichas), White-eyed Vireo (V. noveboracensis), Wood Thrush (Hylocichla mustelina), Black-throated Green Warbler (Dendrœca virens), Indigo Bird (Passerina cyanea).
April 19. Great-crested Flycatcher (Myiarchus crinitus), Kingbird (Tyrannus carolinensis), Catbird (Galeoscoptes carolinensis), Pine-creeping Warbler (Dendrœca pinus).
April 20. Golden-crowned Thrush (Siurus auricapillus), Kentucky Warbler (Oporornis formosa).
April 21. Red-eye Vireo (Vireosylvia olivacea), Tawny Thrush (Hylocichla fuscescens).
April 22. Yellow-breasted Chat (Icteria virens).
April 23. Blue-winged Yellow Warbler (Helminthophaga pinus).
April 24. Warbling Vireo (Vireosylvia gilva), Ruby-throated Humming Bird (Trochilus colubris), Baltimore Oriole (Icterus galbula), Chestnut-sided Warbler (Dendrœca pennsylvanica), Worm-eating Warbler (Helminthotherus vermivorus), Nighthawk (Chordeiles popetue).
April 25. Rose-breasted Grosbeak (Zamelodia ludoviciana[7]), Blue Warbler (Dendrœca cærulea[7]), Hooded Warbler (Myiodioctes mitratus), Yellow-billed Cuckoo (Coccyzus americanus).
April 26. Black-throated Bunting (Spiza americana), Yellow-winged Sparrow (Coturniculus passerinus), Wood Pewee (Contopus virens), Oak-woods Sparrow (Peucæa æstivalis illinoensis).
April 30. Bay-breasted Warbler (Dendrœca costanea), Long-billed Marsh Wren (Telmatodytes palustris).
May 2. Black-throated Blue Warbler (Dendrœca cærulescens), Black-and-yellow Warbler (D. maculosa), Chestnut-sided Warbler (D. pennsylvanica), Red-poll Warbler (D. palmarum).
May 3. Blackburnian Warbler (D. blackburniæ).
May 6. Nashville Warbler (Helminthophaga ruficapilla), Cape May Warbler (Perissoglossa tigrina), Mourning Warbler (Geothlypis philadelphia).
May 7. Tennessee Warbler (Helminthophaga peregrina).
Among the migratory species which had already arrived by the 15th were the Large-billed Water Thrush (Siurus motacilla), numbers of which were heard singing in the swamp, the Black-and-white Creeper (Mniotilta varia borealis), Blue-gray Gnatcatcher (Polioptila cærulea), and a few others.
The nesting season began much later than usual, as the following list, of the earliest date on which the eggs of any species were obtained, will show.[8]
18April 27. Yellow-crowned Night Heron (Nyctherodius violaceus).
April 30. Hairy Woodpecker (Picus villosus), two sets; Grass Finch (Poœcetes gramineus).
May 2. Field Sparrow (Spizella pusilla), Chewink (Pipilo erythrophthalmus).
May 9. Redbird (Cardinalis virginianus).
May 18. Red-eyed Vireo (Vireosylvia olivacea).
May 19. Prothonotary Warbler (Protonotaria citrea), Wood Thrush (Hylocichla mustelina).
May 20. Acadian Flycatcher (Empidonax acadicus).
May 22. Yellow-breasted Chat (Icteria virens), Maryland Yellow-throat (Geothlypis trichas), Indigo Bird (Passerina cyanea), Black-billed Cuckoo (Coccyzus erythrophthalmus).
May 24. Green Heron (Butorides virescens).
Although situated about 20 miles north and the same distance east of Mt. Carmel, the bird-fauna was entirely the same, allowing for differences in the character of the country, the environs of Wheatland being much less varied, and therefore not such as to attract so great a variety of species. Nearly all the characteristic summer birds found further south were abundant near Wheatland, however, even Peucæa illinoensis occurring there. Among the more numerous species were the Cerulean, Blue-winged Yellow, Kentucky and Prothonotary Warblers, all of which were quite as numerous as near Mt. Carmel. At the time of my arrival, the most abundant bird was probably the Cardinal Grosbeak, it being no unusual sight to see several males at one time along the railroad track, picking up grain dropped from passing cars, while the swamp and surrounding woods were filled with their sweet but monotonous whistlings. Later in the season, however, other species became rather more numerous, it being difficult to decide between the Redstart and Red-eyed Vireo, as to first rank in point of numbers. Other species almost as well represented as those mentioned, were the Red-headed Woodpecker, Tufted Titmouse, Blue Jay, and Red-winged Blackbird, and, for a brief season, the Rose-breasted Grosbeak and Cedarbird. Hawks were very plentiful, especially the Red-shouldered and Red-tailed, and on one occasion eight of the former (all adults) were observed soaring about, near together, uttering their clamorous cries. Barred Owls were exceedingly numerous among the trees growing in the swamp, and at night afforded much amusement by their “family squabbles.” Ducks 19and Geese which had been very plenty on the pond during the winter, had gone northward prior to the middle of April, except a few Mallards, Shovellers, and Blue-winged Teal, which remained until about the end of the month, as did also multitudes of Coots (Fulica americana).
The following list of course includes only a small proportion of the total number of species observed.
Gray-cheeked Thrush (Hylocichla aliciæ).—The exact date of arrival of this species was not noted, but was somewhere near the 20th of April. During the last week of April and the first three weeks of May it was very common, perhaps more so than any other of the small Thrushes. Specimens were shot May 23, and others were observed as late as the 28th of that month, the date of my departure.
Tawny Thrush (Hylocichla fuscescens).—Arrived April 21 and remained until toward the last of May. Less common than H. aliciæ but frequenting the same localities and having nearly identical manners.
Bewick’s Wren (Thryomanes bewicki).—Rather common, found only about the out-buildings of farms and in the village.
House Wren (Troglodytes aëdon).—Less common than Bewick’s Wren, and noticed only about brush-heaps and along old fences.
Prothonotary Warbler (Protonotaria citrea).—Very abundant among the “elbow-brushes” (Cephalanthus occidentalis) and willows in the pond, nesting in hollows of the latter.
Blue-winged Yellow Warbler (Helminthophaga pinus).—Very abundant among the undergrowth in thick woods, chiefly in the bottoms.
Golden-winged Warbler (Helminthophaga chrysoptera).—Not uncommon for a few days during the early part of May.
Tennessee Warbler (Helminthophaga peregrina). As usual, very numerous for several days, arriving May 7.
Nashville Warbler (Helminthophaga ruficapilla).—Rather rare during the middle portion of May, arriving about the 6th.
Cape May Warbler (Perissoglossa tigrina). Probably not uncommon, four specimens being obtained, all shot from the top branches of tall trees, and not recognized until after being shot.
Black-and-yellow Warbler (Dendrœca maculosa).—Much the most abundant of the migratory species.
Bay-breasted Warbler (Dendrœca castanea).—Rather common for a few days.
Blue Warbler (Dendrœca cærulea).—Very abundant summer resident, first noticed about the 25th of April. Diligent search failed to discover a single nest, though pairs evidently having nests were met with on every hand through the woods.
Yellow-throated Warbler (Dendrœca dominica albilora).—Unaccountably rare, only two having been obtained, and one or two others 20heard. I am at a loss to account for the scarcity of this species, unless it be the rarity of sycamore (Platanus) trees in the locality under consideration.
Since there is evidently a general misapprehension of the characters distinguishing this race from true D. dominica, it may be as well to state here that the latter is larger, with a constantly and very decidedly longer bill, while the yellow over the lores is never absent. Var. albilora frequently has the yellow over the lores almost as distinct as in typical dominica, but the bill is always much smaller, and somewhat differently shaped.
Pine-creeping Warbler (Dendrœca pinus).—Rather rare.
Prairie Warbler (Dendrœca discolor).—Heard singing among the bushes in an old field on the day of my arrival, and frequently afterward.
Connecticut Warbler (Oporornis agilis).—Not uncommon about the middle of May, but very shy. Frequented the borders of the swamp, and escaped into the thick button-bushes when surprised.
Kentucky Warbler (Oporornis formosa).—One of the most abundant of the summer residents.
Mourning Warbler (Geothlypis philadelphia).—Became suddenly very common May 6. Frequented chiefly brush-piles and old fences. Most of the specimens observed were males in fine plumage.
Black-capped Yellow Warbler (Myiodioctes pusillus).—Rare during migration.
Canada Flycatching Warbler (Myiodioctes canadensis).—One of the most numerous of the migratory species; first noted April 18, but not common until a week later.
Hooded Warbler (Myiodioctes mitratus).—Rather common in deep woods, but much less so than in the vicinity of the Cypress swamp, further south.
Solitary Vireo (Lanivireo solitarius).—Rare.
Cedarbird (Ampelis cedrorum).—Exceedingly numerous among the willows in the swamp, where feeding upon the larvæ of Diabrotica 12–maculata infesting these trees.
Summer Redbird (Pyranga æstiva).—Rather common, but owing to the comparative absence of high, dry woods, much less so than near Mt. Carmel. A female, killed at the same shot with her mate, resembled the male except in the tint of the red, which was of a brick-red rather than vermilion, the male also being in the parti-colored plumage of the immature bird, the red occupying, in both male and female, one-half or more of the plumage. The ovaries of the female were well developed.
Grass Finch (Poœcetes gramineus).—Common in the meadows, a nest with four eggs being taken April 30.
Lark Finch (Chondestes grammica).—Rather common, chiefly in fields near roadsides.
White-crowned Sparrow (Zonotrichia leucophrys).—Became common about the middle of May.
White-throated Sparrow (Zonotrichia albicollis).—Very abundant up to the middle of May, and a female was started among some bushes 21near the edge of the swamp about the 27th or 28th of the month, her actions and notes strongly suggesting a nest in the vicinity, but I was unable to discover one.
Field Sparrow (Spizella pusilla).—A very common bird. Remarkable variations were noticed in the song of this species, several individuals repeating the usual song three times without stopping. Another had such peculiar notes that it was followed and shot for a strange bird.
Oak-woods Sparrow (Peucæa æstivalis illinoensis).—Rare, and observed only on one occasion, on the 26th of April. The locality was a “woods pasture,” about one-half cleared of trees, with occasional old logs and brush-piles on the open portion, and plenty of dead standing trees, the ground high and rolling. Immediately upon sighting the locality I thought of this bird, and at almost the same instant heard one sing. This one was shot, as he sat upon a brush-pile. Two or three others were heard at a distance, but I failed to discover them.
Lincoln’s Sparrow (Melospiza lincolni).—Very abundant about brush-piles in swampy clearings.
Cardinal Grosbeak (Cardinalis virginianus). By far the most numerous of the resident Fringillidæ, and one of the most abundant of all birds. It was a very common thing to hear several males singing at the same time, and I once saw three males and two females near together on the railroad track, picking up grain scattered from the cars.
Rose-breasted Grosbeak (Zamelodia ludoviciana).—Exceedingly common during the greater part of the month of May. The first were seen April 25. They were most numerous among the willows in the swamp, engaged in feeding upon a small green beetle (Diabrotica 12–maculata) which infested the trees. They were also common in the sugar-maple groves, and were in full song during their stay.
Blue Grosbeak (Guiraca cærulea).—A single specimen seen but not obtained (date forgotten).
Bronzed Grackle (Quiscalus purpureus æneus).—Very numerous, breeding among the willows in the swamp. The “love note” of this bird is decidedly more metallic and more musical than that of Q. purpureus.
Red-headed Woodpecker (Melanerpes erythrocephalus).—Much the most numerous of the Woodpeckers.
Barred Owl (Strix nebulosa).—Exceedingly numerous, the swamp resounding at night with their hootings.
Cooper’s Hawk (Accipiter cooperi).—Common, breeding.
Red-shouldered Hawk (Buteo lineatus).—Much the most numerous of the Hawks. On one occasion eight adults were observed circling together overhead, all uttering their clamorous cries.
Mourning Dove (Zenaidura carolinensis).—Abundant. All the specimens shot had the ends of the toes frozen off, showing that they had remained during the past severe winter.
Wild Turkey (Meleagris gallopavo americana).—Common. Scarcely a day but what one or more were seen, and on one occasion a flock of 22fourteen was met with. When surprised they fly into the swamp, where, alighting on the trees, they are secure from pursuit. The inhabitants pay no attention whatever to the game laws, and it is owing entirely to the safe retreat afforded by the swamp that the Turkeys have not been more nearly exterminated.
Virginia Quail (Ortyx virginiana).—Almost exterminated by the severe winter of 1880–81.
Green Heron (Butorides virescens).—Abundant. A small colony had their nests in a second-growth thicket, some distance from the swamp. The nests (seven in number) were placed in saplings at 12–15 feet from the ground, and, with two exceptions, contained five eggs each.
Yellow-crowned Night Heron (Nyctherodius violaceus).—Abundant, a colony of perhaps a hundred pairs having their nests among the tall ash and sweet-gum trees in a creek bottom, near the edge of the pond. The nests were mostly at a considerable height, and few of them readily accessible. They had just begun to lay, and were frightened away from the locality during a “wet spell” by squirrel hunters. A female was shot from her nest April 27, and a perfect egg cut from her oviduct. Several fine specimens of the bird were secured, and it was noticed that the delicate, almost luminous, yellowish buff of the forehead very soon faded.
American Woodcock (Philohela minor).—Common, breeding.
Solitary Sandpiper (Rhyacophilus solitarius).—Common, and undoubtedly breeding, about small ponds in the woods.
Sora Rail (Porzana carolina).—Common among the sedges in the swamp.
Florida Gallinule (Gallinula galeata).—Probably common in the swamp. A fine specimen with its neck broken was picked up on the railroad track near the depot in Vincennes, having been killed by flying against the telegraph wires.
American Coot (Fulica americana).—Exceedingly numerous in the swamp during latter half of April and early part of May, but toward the last of the latter month the greater part had disappeared.
Mallard (Anas boscas).—Very numerous at the time of our arrival and for a week or two afterward. A few pairs are said to breed in the swamp.
Shoveller Duck (Spatula clypeata).—Much the most numerous of the Ducks at the time of my arrival (April 15).
Blue-winged Teal (Querquedula discors).—Abundant, even up to the latter part of May, and undoubtedly breeding.
Summer Duck (Aix sponsa).—Common and breeding in the swamp.
Hooded Merganser (Lophodytes cucullatus).—More common than A. sponsa, breeding, like that species, in hollow trees in the swamp.
Thick-billed Grebe (Podilymbus podiceps).—Very common in the swamp, where it was breeding.
At the time of my arrival the Ducks had mostly departed for the North, while the Geese had entirely disappeared. Both had passed the winter in 23the swamp, in immense numbers. A thorough exploration of the swamp would no doubt have added largely to the list of Water Birds, but I could not afford the time and labor necessary to accomplish even a partial exploration after the birds had begun breeding.
In the Bulletin for July, 1881, I gave an account of the breeding of the Acadian Owl at Tyngsboro’, Massachusetts, with a description of a set of eggs taken there by Mr. Perham on April 5. Early in June of the same season Mr. Perham sent me a brood of four young Saw-whets which he had taken from the nest about the 15th of the preceding month. They were all in the plumage of N. “albifrons,” and showed little individual variation, save in respect to size, the two females being slightly larger than their brothers. In their fresh, silky feathering they were beautiful little creatures, the warm sepia-brown of the upper parts harmonizing well with the rich fulvous beneath, and their white foreheads showing in strong contrast with both. Nor were their manners less engaging than their plumage, for, unlike most Owls, they were perfectly gentle from the first, never attempting to bite or scratch those who handled them. With each other they were really affectionate, often going through a caressing performance with their bills, and showing a mutual forbearance at meal-times which was very pleasing. They eat all kinds of meat with avidity, but seemed especially fond of mice. The latter were invariably skinned and the flesh torn in shreds and devoured, the skins being swallowed afterwards as dessert. I often saw them eject those peculiar pellets of bones, fur, and other indigestable fragments which all Owls and many Hawks are in the habit of depositing 24about their haunts. The operation was a peculiar one. The Owl would gape several times, then the head would be violently shaken sideways, and finally the pellet, coated with mucous, would shoot forth, frequently falling several inches in front of the spot where the bird was sitting. After it was all over the little fellow assumed an expression of relief and contentment which was very comical.
Although not less grave and solemn than other Owls, their movements were much more animated and restless. They were continually flying or hopping from place to place, even in the day-time, and they had a frequent habit of oscillating the head, at the same time lengthening and shortening the neck. This was apparently done for the purpose of fixing the exact position of some distant object, as afterwards the bird usually flew to the top of some door or book-case towards which its eyes had evidently been directed. Their only cry at this time was a shrill bat-like squeaking, which was frequently given by all four at once. Altogether they were unusually interesting pets and when the time came for preparing three of them as specimens, I found it very hard to break up the affectionate and attractive little family.
I believe it is now generally admitted by ornithologists, that the so called “N. albifrons” is simply the young of N. acadica. Indeed, Mr. Ridgway satisfactorily settled this point when he cited[9] the testimony of Dr. J. W. Velie of Chicago who kept a live “albifrons” “until it moulted and became a fine specimen of Nyctale acadica.” But as no one seems to have published a detailed account of the transition it may be worth while to briefly record some observations made on the survivor of the brood just mentioned.
This bird was placed in a large cage where it had abundant room to fly about, and was kept well supplied with food. Through June and July there was absolutely no change in its plumage, but on August 1 I noticed a few medially spotted feathers pushing their way through the uniformly brown ones of the fore part of the crown. Through the next two weeks they gradually increased and developed until the full-face aspect of the head was that of an adult Saw-whet. At this stage there was no 25indication of any second plumage on the other parts, but about August 15 a few streaked feathers appeared along the central line of the breast and abdomen, while a little later the moult began over the back and wings and quickly became general. Through the last two weeks of the month the new plumage gained daily, and by Sept. 1 the final stage was perfected and the bird had become a remarkably beautiful Saw-whet Owl. From this it appears that the “albifrons” condition is simply the first plumage, which in the Saw-whet is apparently better defined (as contrasted with the earlier downy stage and later autumnal plumage), as well as longer worn, than in most other Owls.
The specimen just mentioned is still (at the date of this writing, Dec. 1) alive and well. It has become rather wilder and less gentle than formerly, and lately has acquired a habit of swelling its plumage and snapping the bill when closely approached. Shortly after the moult it began a new cry, which is now frequently heard at night and occasionally also in the day-time. This utterance consists of a series of five or six low, chuckling but nevertheless whistled calls, which remind one of that peculiar, drawling soliloquy sometimes indulged in by a dejected hen on a rainy day. I cannot reconcile these notes with descriptions of the saw-filing ones which are supposed to have given the species its name, but they perhaps represent the unfinished performance of a young bird. The bat-like squeaking was discontinued before the bird began to whistle, and has never since been heard.
At the time of writing the article already referred to I received the impression that the nest then mentioned was the only one that Mr. Perham had found. But I have since learned that, including the two taken the present season, he has actually examined no less than seven during the past ten years, all of which occurred in or near the township of Tyngsboro’. Most of these nests were, however, broken up by red squirrels before the full complement of eggs was laid. The nesting places were usually of the artificial sort which I have already described, but occasionally use was made of a deserted Flicker’s hole. Mr. Perham frequently hears the notes of Saw-whets during the month of March, and believes that many pairs breed about Tyngsboro’ every season. The region is a heavily wooded one and apparently offers exceptional attractions to all kinds of Raptorial birds.
Peucæa ruficeps eremœca.[10] General aspect dull gray. Dorsal region grayish-ash, the feathers brownish centrally and with their shafts almost black. Top of head rufous, much admixed with grayish. A black frontlet, divided at the culmen by a white line, as in ruficeps and var. boucardi. Breast and sides clear gray. Abdomen whitish. Crissum and flanks tinged with fulvous. A black maxillary stripe. Length of fresh specimen, 6.25; extent, 8.62; wing and tail about 2.75. Sexes alike.
The above description characterizes a bird very unlike Peucæa ruficeps both in size and in coloration. It is much larger and entirely lacks the peculiar rufous tint of the upper parts seen in P. ruficeps. Var. boucardi, which is simply a larger race of ruficeps, the present form therefore resembles only in size and in the distribution of its markings. Indeed it is so unlike both described races that, but for thorough investigations by Mr. Robert Ridgway which fail to justify such a procedure, I should urge the claims of the new form to specific rank. Mr. Ridgway has with great kindness made a careful comparison of several of my specimens with all accessible material bearing upon the matter, and writes me that he finds the former insufficiently differentiated from ruficeps, through boucardi, to stand as a species. An interesting fact, incidentally brought to light by Mr. Ridgway, is that of the few Mexican examples upon which Dr. Sclater based his Zonotrichia boucardi, those from Orizaba are apparently referable to the race I have named eremœca. The National Museum possesses one of the three original Orizaba skins.
The specimens above described were taken, during the months of Dec., 1879 and Jan., Feb., and March, 1880, at Boerne, Kendall Co., Texas. Some account of their habits may be found on another page of the present number of the Bulletin.
Since the date of its first description in 1867, Kennicott’s Owl (Scops asio kennicotti) has remained a very rare bird, and ornithologists have gained but little additional knowledge regarding either its distribution or variations of color. The prominent characters of Elliot’s type were its large size and tawny or umber-brown plumage, and as the few specimens subsequently recognized have closely resembled it, this peculiar coloring has come to be regarded as constant and diagnostic. But not long since Capt. Bendire sent me a Screech Owl from Fort Walla Walla, Washington Territory, which, although equaling kennicotti in size and resembling it in some other respects, was colored more nearly like S. asio in its gray dress. Being unable to reconcile the peculiarities of this bird with any of the standard descriptions, I set to work, at Capt. Bendire’s request, to bring together a sufficiently large number of specimens to determine its identity or relationship. In this I have at length succeeded, thanks to the kind assistance of Professor Baird and Mr. Ridgway of the National Museum, Mr. Allen of the Cambridge Museum of Comparative Zoölogy, Capt. Charles Bendire, U. S. A., Mr. H. W. Henshaw, Mr. Purdie and several other friends, all of whom have been most generous in placing their material at my disposal.
The series now before me comprises about fifty specimens, and includes representatives of all the known North American forms of Scops except S. flammeolus. Among the number are two typical kennicotti, a fine suite of asio, illustrating its numerous variations of plumage, and no less than nine examples referable to the large gray form already mentioned as coming from Fort Walla Walla. A comparison of the latter with asio and kennicotti shows that while a few of the grayer specimens bear a strong superficial resemblance to asio in its corresponding condition, the evidence of the series as a whole points to a stronger affinity 28with kennicotti. In regard to size, they are fully up to the standard of the latter, the difference from asio in this respect being so decided that the smallest male of the series is considerably larger than any female which I have from the East. Moreover, the purely gray style is represented by only a small proportion of the number, the majority being more or less tinged with tawny-rufous, in this as well as some other respects indicating evident approaches to the supposed typical characteristics of kennicotti. In short, the intermediate character of several of these specimens is so unmistakable that, although the transition is not completely shown, they furnish ample evidence that the gray form actually does intergrade with brown kennicotti.
The bearing of this testimony is not doubtful. Geographical considerations preclude our regarding the two birds as allied races, for one of the most typical examples of kennicotti comes from Idaho (No. 59,068 Coll. Nat. Mus., Dr. Whitehead), while I have a specimen referable to the gray condition from the coast of Oregon (Portland, Capt. Bendire), thus showing that they cannot be assigned different habitats. Clearly, then, the only alternative remaining is the assumption that kennicotti, like asio, is dichromatic, the purely gray birds from Fort Walla Walla representing the extreme of one phase, as the tawny brown type probably does that of the other. And considered in connection with its bearing on similarly variable allied forms, the hypothesis of dichromatism certainly offers a very easy and natural way out of the difficulty. Nor is there anything inconsistent in the fact that one or the other style apparently predominates in many sections of their mutual range, and in some is perhaps the exclusive representative, for a similar state of affairs is well known to obtain with other dichromatic members of this genus.[11]
Assuming the preceding conclusions to be granted, the gray condition of kennicotti may be characterized as follows:—
Scops asio kennicotti. Gray phase; adult (♀, no. 6456 author’s collection, Fort Walla Walla, W. T., October 22, 1881, Capt. Bendire). Ground-color above brownish-ash, darkest on the head, palest on the wings, with confused, often nearly obsolete transverse mottling and shaft-stripes of dull black, broadest and most numerous on the crown. Outer webs of scapulars and alula-coverts cream-color, the former tipped and narrowly 29margined with black. Secondaries and inner webs of primaries crossed by from six to seven bars of pale reddish-brown. Outer webs of primaries with broad, quadrate spots of brownish-white. Tail regularly but faintly barred with light reddish-brown. Feathers of the sides of head and neck thickly but minutely mottled with dusky upon a lighter ground. Lores nearly pure white. A somewhat broken facial-circle of black or chestnut spots and blotches. Beneath ashy-white, lightest on the abdomen, with numerous fine, regular, transverse bars of black and coarse shaft-stripes of the same color; the only immaculate space being that along the middle of the abdomen. Lining of wings and concealed silky plumage of sides under the wings, pale ochraceous. Tarsi, dull chestnut. Wing, 7.10; culmen, .61; tarsus, 1.77; tail, 4.10; middle toe, .75; ear-tufts, 1.45.
The above description is of a specimen representing the extreme grayish phase so far as shown by the series before me. Six others from the same locality vary a good deal in color and markings, some of them being very dark with coarse shaft-stripes, both above and below, while one or two have the dorsal surface nearly like that of asio in its corresponding condition. In all, however, the plumage of the under parts is somewhat different from that of asio, the transverse bars being usually much finer and more regular and the ground-color ashy instead of clear white. These differences seem to be most strongly marked in the purely gray specimens which otherwise afford the nearest approaches to asio.
Among the darker birds are three which may be considered as about intermediate between the extreme brown and gray phases. The first, from Mr. Henshaw’s collection (Fort Walla Walla, Nov. 7, 1880, Capt. Bendire) has the dorsal plumage dark brown with an umber cast, while the tibiæ, lining of wings, outer webs of scapulars, and numerous pairs of rounded spots forming a band or collar across the nape, are tawny-ochraceous of nearly as deep a shade as in typical brown birds. The dark shaft-stripes in this specimen are broader and blacker than in any of the others and the usual ashy cast beneath is replaced by an ochraceous one. The remaining two birds are similarly characterized but to a less marked degree. All three combine the gray and brown coloring of the respective extreme phases, precisely as do many of the eastern specimens before me, the gray and red conditions of S. asio.
The Portland specimen already mentioned, although in some respects an intermediate, is on the whole nearer the gray than 30the brown condition. Its general coloring is essentially similar to that of Mr. Henshaw’s bird, but the ground shade above is darker and the scapular spots are confined to the edges of two or three of the outer feathers, while the ochraceous wash beneath occurs only on the sides, lining of the wings, and tibiæ, the ground-color of the under parts being otherwise clear ashy-white.
An unusually large female from Hellgate, Montana (No. 18,299, Nat. Mus.), which Mr. Ridgway very naturally treated as asio in the “Birds of North America” (Vol. III, p. 50), agrees closely with Capt. Bendire’s specimens and with them must now be referred to kennicotti.
In the light of the present evidence it becomes necessary to rearrange the typical characters of this Owl. I accordingly offer the following diagnosis:—
Scops asio kennicotti. Wing, 6.40 to 7.60. Dichromatic, assuming either a gray or a tawny brown condition. Gray phase similar to that of asio, but with the plumage beneath thickly barred and streaked along the median line. Brown phase characterized by a general dusky-umber or tawny-ochraceous coloring unlike that of any other North American form.[12]
The following table includes the most essential measurements of all the specimens of kennicotti which I have examined, together with some taken at second hand, of Elliot’s type of the race.
| Gray and Intermediate. | |||||||
|---|---|---|---|---|---|---|---|
| Wing. | Tail. | ||||||
| 6457, | W. B. | ♂ | ad. | Ft. Walla Walla, W.T. | Nov. 20, 1881. | 7.50 | 4.07 |
| 6458, | W. B. | ♂ | ad. | „ „ | Apr. 25, 1881. | 7.07 | 4.05 |
| 82,330, | Nat. Mus. | ♂ | ad. | „ „ | Dec. 22, 1880. | 7.06 | 4.25 |
| 6459, | W. B. | ♂ | juv. | John Day River. Ore. | Aug. 6, 1881. | 6.92 | 3.65 |
| 30,624, | C. Mus. | ♂ | ad. | Ft. Walla Walla. W.T. | Feb. 12, 1881. | 7.00 | 4.22 |
| H. W. H. | ♀ | ad. | „ „ | Nov. 7, 1880. | 7.05 | wanting | |
| 6456, | W. B. | ♀ | ad. | „ „ | Oct. 22, 1881. | 7.10 | 4.10 |
| 18,299, | Nat. Mus. | ♂ | ad. | Hellgate, Mon. | 7.60 | 4.10 | |
| 6466, | W. B. | ad. | Portland, Oregon. | 6.40 | 3.82 | ||
| Brown. | |||||||
| 4.530, | Nat. Mus. | Washington Ter. | 6.80 | 4.07 | |||
| 59,068, | Nat. Mus. | Idaho. | 6.67 | 3.65 | |||
| 59,847, | Nat. Mus. | ♂ | ad. | Sitka, Alaska. | March, 1866. | 7.40 | 4.00 |
31During the course of the preceding investigation I had occasion to compare a large number of Eastern specimens of Scops asio with some California examples from Nicasio and Alameda County. Somewhat to my surprise, I detected several apparently constant differences which, taken in connection with the pretty definitely settled fact that the California bird is not, like asio, subject to dichromatism, seem to me to warrant the varietal separation of the two. I accordingly propose a new race as follows:—
Ch. Sp. Similis S. asioni, sed auribus brevioribus; colore subtus magis cinerario, transversis lineis tenuioribus, pallidioribus, ac in medio haud interruptis. Nulla rubra conditione cognita.
Adult ♀ (No. 1,546, author’s collection, Nicasio, California, April 24, 1877, C. A. Allen). Above essentially similar to asio in its gray dress. Beneath ashy-white, every where thickly barred and streaked with black; the transverse bars being fine, numerous and regular, the shaft-stripes coarse and generally distributed from the throat to the crissum, both markings occurring as thickly on the median line of the breast and abdomen as along their sides. Wing, 6.20; tail, 3.30; tarsus, 1.50; culmen, .60; ear-tufts, 1.15.
Another adult from the same locality (♀, May 18, 1878, Coll. H. A. Purdie), measures: wing, 6.22; tail, 3.18; ear-tufts, 1.05: while seven unsexed specimens from Alameda county furnish the following extremes: wing, 6.01–6.52; tail, 3.22–3.72, ear-tufts, 1.05–1.25.
The above detailed characters, so far as my series goes, are sufficient to distinguish the California specimens from any gray examples of asio taken in the Eastern States. The chief difference is in the ground-color and markings of the plumage beneath. In asio the central line of the breast and abdomen is nearly always immaculate, while there is frequently a broad, entirely unspotted gular space: in bendirei these parts are as thickly barred and streaked as are the sides, while the ashy tinge of the entire lower surface and the much finer character of the transverse pencilling gives the plumage a clouded appearance which, although difficult of description, is very characteristic. The ear-tufts, also, are usually shorter than those of S. asio.
32Among the nine examples before me there is remarkably little individual variation, much less in fact than with any equal number of asio which I have ever examined. The Alameda County specimens as a rule are rather more finely and faintly barred than the Nicasio ones and the ground-color beneath is of a slightly different shade, inclining more to clayey than ashy-white. In one bird the under surface is decidedly dull clay-color, which is so generally and evenly distributed that there is positively no approach to clear white even on the throat, lores, forehead or abdomen. But the essential characters already given are so well maintained on the whole that the description of the one chosen as the type will apply nearly as well to them all. This uniformity is doubtless largely owing to the absence in this race of any tendency to dichromatism, for much of the variation among the dichromatic ones can be traced to the combination in varying degrees of the colors of both phases, purely colored birds of either style being, at least in some sections, of comparatively rare occurrence. It is of course to be expected that larger suites of specimens will furnish occasional aberrant ones some of which may approach asio; but, so far as the present material is concerned, the tendency of variation is rather towards kennicotti and “tricopsis.” Indeed, as will be seen by comparing my diagnoses, the general coloring and markings of bendirei are so nearly like those of kennicotti in its extreme gray phase, that were it not for their wide difference in size it might be difficult to separate some of the specimens. That bendirei grades into the larger bird at the point where their respective habitats meet is shown by a specimen (No. 16,027, Nat. Mus.) from Fort Crook, Northern California, which is almost exactly intermediate in size, although more nearly like kennicotti in color and markings. As to our bird of the Southwest border, I believe that Mr. Ridgway is still undecided whether it really represents the tricopsis of Wagler or not, but he writes me that however this may turn out, he is now convinced that it intergrades with the form found over California at large and must hence be reduced to a variety of Scops asio. After a careful comparison of specimens I can unhesitatingly endorse this opinion, my Arizona examples of “tricopsis” differing from some of the more faintly barred bendirei only in the purer ash and sharper streaking of their dorsal plumage.
Save in cases where this fresh material has thrown new light 33on old data, I have deemed it unnecessary to go over any of the ground trodden by Mr. Ridgway in his elaborate and invaluable monograph of the genus Scops,[14] but the bearing of some of the present testimony has proved so far reaching that I venture, in concluding, to suggest the following rearrangement of the North American Screech Owls belonging to the S. asio group.
Scops asio. Habitat, United States north of the Gulf States and east of the Rocky Mountains.
Scops asio floridanus. Habitat, Florida and Southern Georgia.
Scops asio maccalli. Habitat, Highlands of Guatemala, Eastern Mexico, and Valley of the Lower Rio Grande in Texas.
Scops asio kennicotti. Habitat, Northwest Coast from Sitka to Oregon and eastward across Washington Territory into Idaho and Montana.
Scops asio bendirei. Habitat, Coast region of California.
Scops asio tricopsis? Habitat, Western Mexico and the extreme southwestern border of the United States.
Scops asio maxwellæ. Habitat, Mountains of Colorado.
The village of Boerne in Southwestern Texas, with its environing country, was the field of my ornithological labors between December 21, 1879 and April 4, 1880. Boerne is situated about thirty miles northwest of San Antonio, and less than that distance 34westerly from New Braunfels, where Messrs. Werner and Ricksecker made their collection, a few years ago.[16] It lies in a country of hills and “flats,” scantily watered and largely unproductive, beyond which timber and general vegetation rapidly disappear, as the westward-bound traveller nears the desolation of the Great Plains. Live-oak grows in scattering groves, the postoak in more compact clusters, and cedar occurs in small “brakes” of some density. There are also, along the creek to which the village owes its existence, two or three small oases of deciduous trees admixed with vines, no one of them, perhaps, an acre in extent. The mesquite, which is so common on the prairies to the south and east, is not seen, but is replaced by a small variety of live-oak growing in the form of chaparral. Throughout my stay in it, the country had a very inhospitable and dreary aspect, on account of the almost total lack of grass of any kind; and by its absence the number of the local birds is of course materially diminished.
In presenting a list of the birds observed in this locality, I wish to call especial attention to the curious admixture of geographical races found here. Among the species which are subject to climatic variation, several are represented by two distinct varieties and with them confused and indeterminable intermediate forms. In others but one constant form is found. And in a third class the bird occurs in a varying, transitional phase of plumage which, however, occasionally becomes typical of some described race.
1. Hylocichla unalascæ (Gm.) Ridg. Dwarf Thrush.—Uncommon resident. Not heard to sing. Several of my specimens very closely approach the variety auduboni. I saw nothing of the eastern pallasi, which I have received from Mr. Geo. H. Ragsdale, of Gainesville.
2. Merula migratoria propinqua, Ridg. Western Robin.—Irregularly abundant.
3. Mimus polyglottus (Linn.) Boie. Mockingbird.—Rare resident.
4. Sialia sialis (Linn.) Haldem. Bluebird.—Comparatively common during the winter. All of my specimens were in most beautiful plumage. Not one male in a dozen showed the slightest brownish edging to the feathers of the back. I was particularly struck with this in view of the fact that almost every individual in a large series collected in Alabama, in the winter of 1878, exhibited more or less of this brownish edging.
355. Sialia arctica, Swains. Rocky Mountain Bluebird.—Abundant winter visitor. Generally in dull plumage.
6. Polioptila cærulea (Linn.) Scl. Blue-gray Gnatcatcher.—Apparently a common summer resident. Arrived March 8.
7. Regulus calendula (Linn.) Licht. Ruby-crowned Kinglet.—Abundant up to the last week in March.
8. Regulus satrapa, Licht. Golden-crested Kinglet.—Not common. Last seen about March 22.
9. Lophophanes atrocristatus, Cassin. Black-crested Tit.—Very abundant resident.
10. Parus carolinensis, Aud. Carolina Chickadee.—Uncommon during my stay. Usually seen in pairs.
11. Certhia familiaris rufa (Bartr.) Ridg. Brown Creeper.—Rare. Only two individuals observed: one Jan. 16, the other Jan. 29.
12. Salpinctes obsoletus (Say) Cab. Rock Wren.—I obtained a single female on March 4, in a cañon of the Cibalo Creek. It was very shy and was secured with difficulty.
13. Catherpes mexicanus conspersus, Ridg. White-throated Wren.—About three pairs were resident in the cañon above referred to. They lurked almost constantly in the interstices of the rocks, and had it not been for their delightful song would many times have entirely escaped observation.
14. Thryothorus ludovicianus (Gm.) Bp. Carolina Wren.—Uncommon resident.
15. Thryomanes bewicki leucogaster, Baird. Texan Bewick’s Wren.—Very common resident. Sang throughout the winter.
16. Anthus ludovicianus (Gm.) Licht. Titlark.—Abundant winter visitor. Became uncommon towards the last of March.
17. Neocorys spraguei (Aud.) Scl. Missouri Skylark.—Mr. Sennett having detected this species at Galveston,[17] it was, of course, to have been expected in the present locality. Since, however, I observed no examples until the 16th of March, it is to be inferred that the bird’s winter habitat lies much farther to the south than has been supposed. I met with specimens up to within a few days of my departure, but never in abundance and, I believe, all upon one “flat” containing about twenty acres.
While according to Dr. Coues[18] the manners and habits of this bird and the Titlark agree so closely during the breeding season, they were quite unlike at the time of my own observations. At Boerne the flight of the Skylark was peculiarly characteristic, being made slowly, at a height of but a few inches from the ground and with the regular, undulating movement of the Goldfinch. When several birds were associated together—as was usually the case—they were invariably much scattered about upon the ground, and in flight never closed ranks sufficiently to form anything 36like a flock. The Titlarks, on the contrary, as I have also found them at the North, were birds of erratic and more rapid flight, frequently ascending to a considerable height and always preserving the semblance of a flock, however straggling their order.
18. Mniotilta varia (Linn.) Vieill. Black-and-white Creeper.—Rather common after March 13.
19. Helminthophaga ruficapilla (Wils.) Bd. Nashville Warbler.—Two specimens,—March 30 and April 1.
20. Helminthophaga celata (Say) Bd. Orange-crowned Warbler.—Arrived the first week in March and thereafter was the most abundant of the Warblers. One of my specimens is a partial albino, the first, I believe, that has been detected in this peculiar phase of plumage.
21. Parula americana (Linn.) Bp. Blue Yellow-back.—Rare migrant. Arrived March 20 in full song.
22. Dendrœca coronata (Linn.) Gray. Yellow-rump.—An abundant winter visitor, seen throughout my stay.
23. Dendrœca blackburnæ (Gm.) Bd. Blackburnian Warbler.—A single male taken March 31.
24. Dendrœca dominica albilora, Bd. White-browed Yellow-throat.—Uncommon migrant, first seen on March 19. The song of this variety is very different from that of its eastern analogue, and is a close reproduction of the Field Sparrow’s familiar chant, without his decrescendo termination.
25. Dendrœca chrysoparia, Scl. and Salv. Golden-cheeked Warbler.—Previous to the capture of my Boerne specimens, there were only about seven[19] skins of this elegant Warbler in existence. It was a rare bird at Boerne, and my own series was not brought up to a total of seven without special exertion. The first individual made his appearance on March 12. Within forty-eight hours from that time, under the influence of a biting norther, the mercury sank to 29° and hovered about that figure for several days. So that in his semi-tropical habitat this little bird is sometimes called upon to endure pretty severe weather. The remaining examples were taken at intervals up to March 24, after which I saw none. I found them usually in cedar brakes; never more than a few rods distant from them. They were sometimes very shy, at other times easily approached, but almost always pursued their various avocations rather silently. I did not hear the song at all, until by this I was attracted to the last specimen that I procured. The notes were an exact counterpart of the song of Dendrœca discolor, as I heard it in Alabama, and, indeed, for the utterances of that bird I mistook them.
By the few examples of this species hitherto existing in cabinets, the plumage of the adult male has been represented with much green on the 37back. Four of my five males conform to this pattern of coloration, but the fifth is in a much more beautiful dress, undoubtedly showing the male bird in full perfection. In this specimen the back is deep black, glossy and continuous. Upon close examination, faint and irregular traces of greenish are perceptible, but in much too slight a degree to materially affect the groundwork. This high state of plumage greatly enhances the bird’s beauty and renders its wearer one of the handsomest of the Sylvicolidæ.
26. Dendrœca virens (Gm.) Bd. Black-throated Green Warbler.—An uncommon migrant, first seen on March 13. Found in hardwood growth and never in company with the preceding species. On March 25 I heard a male singing the plaintive song so familiar in northern woods.
27. Siurus motacilla (Vieill.) Coues. Large-billed Water Thrush.—A single male taken, March 25, in one of the “oases” of the creek.
28. Lanivireo flavifrons (Vieill.) Bd. Yellow-throated Vireo.—A pair taken on March 25.
29. Vireo atricapillus, Woodh. Black-capped Vireo.—One specimen, March 27. Could I have remained a few days later, other specimens would have undoubtedly been detected.
30. Vireo noveboracensis (Gm.) Bp. White-eyed Vireo.—Common summer resident, first seen on March 13.
31. Lanius ludovicianus excubitorides (Sw.) Coues. White-rumped Shrike.—Of irregular and uncommon occurrence. I obtained one specimen of ludovicianus proper.
32. Ampelis cedrorum (Vieill.) Bd. Cedar Bird.—Very irregular in its occurrence, and never common.
33. Progne subis (Linn.) Bd. Purple Martin.—Common summer resident. Arrived from the south, Feb. 17.
34. Petrochelidon lunifrons (Say) Lawr. Eave Swallow.—Common summer resident. Arrived about March 20. The cañon, to which I have several times alluded, contained many nests of this bird.
35. Hirundo erythrogastra, Bodd. Barn Swallow.—Common summer resident. A single individual seen on March 4, but no others noticed until the 10th of the month when there was a general arrival.
36. Stelgidopteryx serripennis (Aud.) Bd. Rough-winged Swallow.—But two observed,—March 3 and 4.
37. Pyranga æstiva (Linn.) Vieill. Summer Redbird.—One specimen taken in April.
38. Astragalinus tristis (Linn.) Cab. Goldfinch.—Rather common winter visitant.
39. Centrophanes ornatus (Towns.) Cab. Chestnut-collared Longspur.—This and the following species apparently do not winter here. I first met with them in the second week of February. They were often associated together, sometimes with the addition of a few Horned Larks. The present species, though not common, was the more numerous and lingered later, being taken up to March 2.
3840. Rhynchophanes maccowni (Lawr.) Bd. McCown’s Longspur.—Uncommon migrant, taken between Feb. 11 and 21.
41. Passerculus sandwichensis alaudinus (Bp.) Ridg. Western Savanna Sparrow.—Common, throughout my stay, in cultivated fields.
42. Poœcetes gramineus confinis, Bd. Western Grass Finch.—Abundant in cultivated fields and less common elsewhere, throughout my stay. Several specimens were taken in plumage intermediate between this and the eastern form; and one which can hardly be referred to anything but gramineus proper.
43. Coturniculus passerinus (Wils.) Bp. Yellow-winged Sparrow.—A single specimen, Feb. 14.
44. Chondestes grammica (Say) Bp. Lark Finch.—Rare during the winter. A general arrival on March 11, after which it was common.
45. Zonotrichia querula (Nutt.) Gamb. Harris’s Sparrow.—Excepting two specimens taken by Mr. Dresser near San Antonio, this species has no Texas record. I found it very abundant during the winter, and in smaller numbers up to within a few days of my departure.
46. Zonotrichia leucophrys (Forst.) Sw. White-crowned Sparrow.—Uncommon winter visitor.
47. Zonotrichia gambeli intermedia, Ridg. Ridgway’s Sparrow.—More common than the preceding, tarrying into March, if not later.
48. Spizella domestica arizonæ (Coues) Ridg. Western Chipping Sparrow.—Rare during the winter. More numerous after Feb. 13. This form is new to the State.
49. Spizella breweri, Cass. Brewer’s Sparrow.—One specimen, March 5, amongst sterile hills. Doubtless is not rare in suitable localities, of which there are none in the immediate vicinity of the village.
50. Spizella pusilla (Wils.) Bp. Field Sparrow.—Common during my stay.
51. Junco hyemalis (Linn.) Scl. Black Snowbird.—Common during my stay.
52. Junco oregonus (Towns.) Scl. Oregon Snowbird.—Uncommon. In addition to the specimens typical of the two Juncos here given, I acquired a series of very puzzling examples intermediate between the two. Such connecting links between the accepted species are perhaps best accounted for under Mr. Ridgway’s theory[20] of hybridization, until it can be decisively shown that they are an effect of climatic causes.
53. Peucæa ruficeps eremœca,[21] Brown. Rock Sparrow.—This beautiful Sparrow was uncommon though apparently resident at Boerne. I found it altogether in rocky localities, usually in close proximity to the creek, but occasionally upon barren hills, a mile or more from water. It has the same shy, skulking habits which are familiar in other species of the genus, rarely taking wing, on the approach of an intruder, so long as 39rock, bush or weed affords a hiding place. The male’s song, which I first heard on Feb. 25, is a pretty warble, not strongly accentuated, and quite unsparrowlike,—equalling neither in sweetness nor in quality of music, the beautiful chant of P. æstivalis. Before becoming thoroughly familiar with it, I more than once attributed it to some unknown Warbler. The call-note is extremely fine and sharp, suggesting the eep of Ampelis cedrorum.
54. Melospiza fasciata (Gm.) Scott. Song Sparrow.—Rare throughout my stay. Specimens are not typical of this form, but are not referable to any of the western varieties.[22]
55. Melospiza lincolni (Aud.) Bd. Lincoln’s Finch.—Arrived March 4; common thereafter.
56. Passerella iliaca (Merrem) Sw. Fox Sparrow.—Two or three individuals met with. This species was detected in the valley of the Brazos by Mr. L. Kumlien,[23] but is not included in the papers of other Texas collectors.
57. Pipilo maculatus megalonyx (Bd.) Coues. Spurred Towhee.—To this form I refer a large series of Pipilos, which is by far the most remarkable of the many curious series from this locality. The relation of some specimens to restricted maculatus and the variety arcticus is indicated in the extract from Mr. Ridgway’s letter, under M. fasciata. Other examples are links in the chain of evidence that is gradually accumulating against the specific distinctness of Pipilo erythrophthalmus. Indeed, I am not sure that they may not be considered as establishing the intergradation between that form and the maculatus group. The extreme approach to the eastern bird is seen in a single specimen, in which the white spotting, partially concealed, appears upon the outer scapulars alone, and there only in very slight measure.
58. Cardinalis virginianus (Briss.) Bp. Cardinal.—Abundant resident. In a series of fifty specimens, two or three are typical, the remainder exhibiting to a greater or less degree the characters of both virginianus as restricted and var. igneus. In one specimen the black band across the culmen is hardly perceptible, but in none does the red of the forehead reach completely to the bill.
59. Calamospiza bicolor (Towns.) Bp. Lark Bunting.—One specimen, in a scattering grove of post oaks, March 24.
4060. Molothrus ater (Bodd.) Gray. Cowbird.—A few females shot out of flocks of the following variety, in March.
60 b. Molothrus ater obscurus (Gm.) Coues. Dwarf Cowbird.—On Jan. 20 I shot the first females that I had observed, after which they soon became common. No males were detected until Feb. 25, but from that time both sexes were found in abundance.
61. Agelæus phœniceus (Linn.) Vieill. Red-winged Blackbird.—Abundantly represented, during the winter, but by females only, so far as my observations went. The males are said by the villagers to occur rarely.
62. Sturnella neglecta, Aud. Western Field Lark.—Abundant during my stay.
63. Icterus spurius (Linn.) Bp. Orchard Oriole.—One individual seen in April.
64. Scolecophagus cyanocephalus (Wagl.) Cab. Brewer’s Blackbird.—Found throughout my stay; in great abundance up to the middle of March.
65. Corvus corax carnivorus (Bartr.) Ridg. Raven.—Uncommon. Usually solitary, but on Jan. 28, I noticed a flock of a dozen.
66. Corvus frugivorus, Bartr. Crow.—Rare.
67. Eremophila alpestris chrysolæma (Wagl.) Coues. Mexican Horned Lark.—Abundant up to the first week of March, after which none were seen until March 27. From this time occasional individuals only were observed.
68. Milvulus forficatus (Gm.) Sw. Scissor-tail.—Arrived March 24, and became at once common.
69. Myiarchus crinitus (Linn.) Cab. Great-crested Flycatcher.—One specimen taken March 30.
70. Sayornis fuscus (Gm.) Bd. Pewee.—Found rather uncommonly throughout my stay.
71. Caprimulgus vociferus, Wils. Whip-poor-will.—One specimen taken April 2.
72. Picus scalaris, Wagl. Texas Woodpecker.—An abundant resident.
73. Sphyrapicus varius (Linn.) Bd. Yellow-bellied Woodpecker.—Rare and irregular.
74. Centurus carolinus (Linn.) Bp. Red-bellied Woodpecker.—The rarest species of this family: but three seen.
75. Centurus aurifrons, Wagl. Golden-fronted Woodpecker.—Uncommon resident. Unlike Mr. Sennett,[24] I found it always very shy.
76. Colaptes auratus hybridus (Bd.) Ridg. Hybrid Flicker.—Uncommon and of irregular occurrence. This form does not appear to have been met with in Texas limits before. The present locality is at all events exceptionally southern.
4176 b. Colaptes auratus mexicanus (Sw.) Ridg. Red-shafted Flicker.—One specimen, taken Jan. 2. Others doubtless occurred amongst the shy Flickers which escaped my gun.
77. Ceryle alcyon (Linn.) Boie. Belted Kingfisher.—A pair seen on Feb. 18, one of which was shot by a friend on Feb. 21.
78. Geococcyx calfornianus (Less.) Bd. Chaparral Cock.—Though said by the inhabitants to be usually numerous, I found it rare during my stay.
79. Tinnunculus sparverius (Linn.) Vieill. Sparrow Hawk.—Common winter visitant.
80. Accipiter fuscus (Gm.) Bp. Sharp-shinned Hawk.—Common winter visitant.
81. Cathartes aura (Linn.) Illig. Turkey Buzzard.—Common resident.
82. Catharista atrata (Wils.) Less. Black Vulture.—Common resident.
83. Zenaidura carolinensis (Linn.) Bp. Carolina Dove.—In great numbers throughout my stay.
84. Meleagris gallopavo, Linn. Mexican Turkey.—The Boerne Hotel occasionally favored its guests with Wild Turkey obtained of ranchmen from the surrounding country, but I did not meet with the bird myself.
85. Ortyx virginiana texana (Lawr.) Coues. Texas Quail.—Uncommon resident. Nearly all of my specimens lack the outer one or two joints of all the toes,—a result, perhaps, of excessive cold.
86. Ardea herodias, Linn. Great Blue Heron.—Occasionally observed.
87. Charadrius dominicus, Müll. Golden Plover.—Uncommon after March 9, which was the date of its arrival.
88. Oxyechus vociferus (Linn.) Reich. Killdeer.—Abundant resident.
89. Podasocys montanus (Towns.) Coues. Mountain Plover.—Occurs uncommonly in the migrations. A flock of about twenty individuals encountered on Jan. 2; two specimens taken on March 15; and a flock of a dozen or more seen on March 17. They were very tame, but, from some peculiar constitutional trait, difficult to kill. This Plover was not procured in southern Texas by Mr. Sennett nor by Dr. Merrill. It was, however, met with by Mr. Dresser,[25] and two specimens obtained in the State by other collectors are catalogued in the ninth volume of Pacific Railroad Reports.
90. Gallinago media wilsoni (Temm.) Ridg. Wilson’s Snipe.—In the course of the winter I met with perhaps a dozen individuals, at one particular spot in the bed of the creek, where a little grass afforded partial cover. Specimens which I shot are exactly similar to eastern examples in plumage, but when freshly killed all agreed in having pale, 42flesh-colored legs and feet—those of the female being tinged with greenish-yellow. So far as my own experience goes, this is a peculiarity never seen in eastern Snipe, in which the legs and feet are olivaceous.
91. Actodromas maculata (Vieill.) Coues. Grass-bird.—One specimen, March 21.
92. Actodromas bairdi, Coues. Baird’s Sandpiper.—One specimen, March 16. A Sandpiper seen on Feb. 18, and two small flocks seen in March were also probably of this species.
93. Totanus melanoleucus (Gm.) Vieill. Great Yellow-legs.—One seen, Jan. 1; three others observed in the last week of March.
94. Rhyacophilus solitarius (Wils.) Cass. Solitary Sandpiper.—One specimen, March 25.
95. Bartramia longicauda (Bechst.) Bp. Upland Plover.—First seen on March 22, and but few noted subsequently.
96. Numenius longirostris, Wils. Sickle-billed Curlew.—Two observed, Dec. 21.
97. Numenius borealis (Forst.) Lath. Esquimaux Curlew.—Rather common migrant, first seen on March 9.
98. Grus canadensis (Linn.) Temm. Sandhill Crane.—Solitary individuals occasionally noted.
99. Anas obscura, Gm. Black Duck.—Small flocks rather unfrequently found in the creek.
100. Chaulelasmus streperus (Linn.) Gray Gadwall.—I did not detect this species until March 25, after which I found it uncommonly.
101. Nettion carolinensis (Gm.) Bd. Green-winged Teal.—Rare. First seen Feb. 6.
102. Fulix collaris (Donov.) Bd. Ring-billed Black-head.—One of three shot, Feb. 27.
103. Mergus merganser americanus (Cass.) Ridg. Goosander.—Small flocks observed in January.
104. Plotus anhinga, Linn. Snake-bird.—A female shot by a friend, on March 24.
To the foregoing list of species actually taken or identified beyond question, are to be added six others which I was unable to fix decisively. These are a Hawk, believed to have been Ictinia subcærulea, seen in pursuit of a Buzzard, on March 4; a red-tailed Buteo of which I saw a pair, Feb. 26; a shy, black Buteo, almost undoubtedly Buteo abbreviatus, frequently observed about the village; an Owl, apparently Strix nebulosa, several times scared up in an unusually dense grove of deciduous trees; a Hummer, noted a few times towards the close of my stay; and a beautiful Larus which hovered over the stage as it forded the creek, on my return journey to San Antonio.
Memorial Volume of Garrod’s Scientific Papers.[26] Garrod’s work is apparently not so well known in this country as it must eventually become, forming as it does a permanent way-mark in the progress of the science, and contributing indispensable material for the solving of the most vexed problem in ornithology—we mean a sound, rational classification of birds, based on morphological data according to the theory of genetic relationship, and as such one which any considerable number of ornithologists can agree to adopt and stand by. As is well understood, those of us who have no classification of our own to advance, fall back upon some convention as make-shift, practically waiving the points at issue. As far as taxonomy is concerned, the present attitude of ornithology is thoroughly iconoclastic; but, while we agree that much of what has been set up must be upset, few claim to know what ought to replace the broken images, and fewer still agree on that point. There is nevertheless a large amount of material at hand, the soundness and utility of which no one questions; and of late years Garrod has been both indefatigable and successful in setting bricks and mortar. Of the anatomical papers in the present volume, some 73 in number, more than half relate to birds, describing conditions of the osseous, muscular, respiratory, vascular, digestive and nervous systems which appear to promise most of value in taxonomy, and discussing in candid and scientific spirit, from a vantage-ground of long experience, the bearing of the anatomical points upon classification. Of the accuracy and high rate of reliability of these papers there can be no question; they are sufficiently lucid to shine with their own light, and there is a certain “finish” about them which is truly admirable. This is seen when the author is drawing the comparisons which his extensive knowledge enables him to adduce, and summing his conclusions. These are always clean-cut and luminous, so that we know exactly where to find Garrod, whether we like him and agree with him or not. It is scarcely possible that he has been exempt from the all but inevitable tendency of the mind’s eye to magnify the particular subjects there focussed for the time, and so get them more or less out of perspective of the whole range of vision; but he seems to have known and guarded against this most scrupulously, unless, perhaps the “ambiens” muscle proved too much for him. On the whole, we do not think that even the warm praise of the editor, his personal friend and admirer, is too much to say, and we quote with pleasure:
44“Of his zoölogical papers indeed, the ornithological ones must probably, on account of their more novel character, and as affording entirely new data for the solution of the various problems connected with the classification of Birds, which he revolutionized, be considered of the greater importance. No future worker in that group can neglect the facts or ideas concerning it that we owe to Garrod, and they alone suffice to put his name in the very first rank of those who have ever studied these creatures, and to stamp his work on Birds as truly ‘Epochmachende.’ „
Garrod’s numerous papers, covering the period of 1871–79, are scattered through various periodicals; and it is a subject for congratulation that they have been collected in one convenient volume, under careful editorship. At a meeting of the Zoölogical Club to consider the wish of friends to possess some permanent memorial of Garrod, it was decided, with wisdom and good taste which none can impugn, “that the most appropriate and desirable one would be the publication, in a collected form, of all the papers published by Garrod in various scientific journals and periodicals, with a portrait and memoir of the author.” This decision has been ably carried into effect by Mr. Forbes, whose own contributions to the same subject already prove him to be one on whom the mantle may fittingly descend. We wish there were more work of this kind, even if not of the same highest quality, done by our own countrymen; but at present no one of them seems especially interested excepting Dr. Shufeldt, whose studies thus far possess much value and give still more promise. Noticing only two or three American names on the list of subscribers, we venture to hint that the work may be procured by others in the usual way.
We cannot of course go into any examination of these papers in an editorial notice like the present, or even adduce the leading results of the author. It must suffice to say that among them is an entirely new classification of birds, primarily based upon the ambiens. Among the more important papers we may mention those on the carotid arteries; on certain muscles of the leg (Garrod’s pièce de resistance); on the anatomy of Pigeons, of Parrots, and of Passerine Birds; and on the trachea in Gallinæ. All these are of general import, bearing on broad questions of taxonomy, as distinguished from minor papers, however valuable, in which special points are examined. The editor has done well to preserve the original pagination of the text and numeration of the illustrations for facility of citation, and the plates are said to be faithfully reproduced.—E.C.
Shufeldt’s Osteology of the North American Tetraonidæ.[27]—This osteological memoir is, so far as we know, the most complete of any on American birds of one group. In general the descriptions, with the aid of the numerous plates, can be easily understood. In treating of the skull Dr. Shufeldt adopts the old theory that it is nothing but the modified end of the back bone, and gives a diagramatic figure of the skull of Centrocercus much like that given by Owen of the Ostrich. This view will 45of course be rejected by all who do not consider the membrane and cartilage bones of the skull to be from the same source. The use of “hyoid arch” when speaking of all the tongue bones is, we think, liable to lead many young students astray; we would suggest “hyobranchial arches,” or “hyoid arches.”
A point of considerable interest is a small ossicle which occurs at the inner side of the II metacarpal—III metacarpal of Dr. Shufeldt’s homologies of the hand—near its base. This bone is compared to the pisiform bone of the Mammalia by the author. Besides this, two proximal and two distal carpal bones are found, just as in the chick. Thus the chick and the young Centrocercus have the same structure of the hand except the presence of a IV metacarpus in the first and a “pisiform” in the second. We notice that the “index” is described as being composed of only one phalanx; this we believe to be an oversight of the author; at all events most of the European Gallinæ have two phalanges, the last one bearing a claw. On reference to fig. 57 it will be seen that the distal end of the first phalanx in Centrocercus is very large and looks as if there should be another joint. As regards the tarsus, Dr. Shufeldt has been able to demonstrate the existence of a fibulare, tibiale, and intermedium, which ultimately become anchylosed with the tibia. Dr. Shufeldt also states that as a whole the different parts of this skeleton in Centrocercus are slow to anchylose, thus rendering the bird an extremely favorable one for the study of the separate elements of the skeleton.
The description of the osteology of Lanius ludovicianus excubitorides,[28] by the same author, is short, concise, and may be summed up in the statement that the skeleton of this bird is strictly Passerine.—J. Amory Jeffries.
Illustrations of Ohio Nests and Eggs.[29]—We are glad to record the progress of this great work, of which we have had former occasions to speak so highly. The ninth fascicle is the last which has reached us, carrying the number of plates to twenty-seven, each with its sheet or so of letter-press. The high standard of the work is on the whole maintained, although, to our eye at least, the plates lack somewhat of the peculiar attractiveness that the earlier ones had for us. It may, however, be only the charm of novelty that we miss; and there is certainly no falling off in the conscientious endeavor to unite fidelity to nature with artistic excellence in depicting these beautiful objects. Should the project be carried to completion, the work will certainly become a standard of reference. It deserves to be better known and more widely circulated than it appears thus far to have become, and we trust that time will serve to make its merit fully appreciated.
46The following are the plates of the two parts before us. (In No. 8) Pl. 22, Cardinalis virginianus (the eggs shown in their remarkable extremes of size and coloring); Pl. 23, fig. 1, Vireo gilvus and fig. 2, V. olivaceus; Pl. 24, Zenaidura carolinensis; (in No. 9) Pl. 25, fig. 1, Trochilus colubris, fig. 2, Polioptila cærulea (and one is interested to see that these nests are of identical orders of architecture and ornamentation, however different in materials); Pl. 26, Spizella socialis; Pl. 27, Butorides virescens.
The text continues as heretofore to consider the subjects under the formal heads of—Locality—Position—Materials—Eggs—Differential Points—Remarks; the latter head usually covering the most matter. We are glad to see that the authors now fill, as a rule, their sheets of letter-press—there is certainly enough to be said on the subject for that! The pagination of the letter-press reaches p. 104 with the end of No. 9.
It is never untimely to suggest that when works published in this manner come to be bound, especially if the parts are made up in any other order than sequence of publication, the original cover-titles should be preserved; there being no intrinsic evidence, either in the text or on the plates, of dates of publication or of contents of Parts; and it may not be too early to suggest to the authors that explicit indication of these points should be given with the permanent title, contents, etc., of the finished work.—E. C.
Shufeldt’s “The Claw on the Index Digit of the Cathartidæ.”[30]—We regret being obliged to make unfavorable criticisms, but this paper contains such important errors, both in regard to the structure of birds and the literature of the subject, that some rectification seems necessary. Dr. Shufeldt describes the claw at the end of the first finger of Catharista atrata as a new discovery, considering that claws outside the Ostrich groups have not hitherto been described, and also states that it is a point of distinction between the Old and New World Vultures. Unfortunately Nitzsch[31] long ago described the claw on the first finger of birds in the following words: “Die Analogie, welche die Flügel der Vögel mit den Vorderfüssen der Säugthiere und Reptilien haben, zeigt sich auch in den Spuren von Nägel- oder Klauenbildung, welche an den Finger jener Glieder oftmals gefunden werden. Dieser Bildung macht es zugleich wahrscheinlich, dass die Urform der Flügel in der Fussform, oder doch in einer, dieser sehr ähnlichen, bestand; denn die Nägel gehören den Füssen an, sie haben im Kreise der Flügelfunkzion keine Bedeutung, und sind da wohl nur durch zweckloses Nachahmen und Ueberbleiben der Fussform.” Farther on he describes the skeleton of the hand as follows: “Die Hand der Vögel hat drei Finger, 1) den Daumen, welcher (ohne das Nagelglied) aus einem Stücke, 2) den grossen Finger, der (ohne das Nagelglied) aus 47zwei Stücken oder Gliedern, und 3) den kleinen Finger, der stets nur aus einen Stücke besteht.” Since Nitzsch’s memoir was written his observations have been extended, and mentioned by many anatomists, as Meckel, in his Anatomy, by Blainville, by Selenka in Bronn’s “Thiereichs,” by myself in this Bulletin for 1881, by Professor Morse in the “Anniversary Memoirs” of the Boston Society of Natural History. Accordingly the claw on the first finger is anything but an unknown object. It is constantly demonstrating its existence to practical ornithologists by pricking their fingers while measuring bird’s wings. That the claw is absent in the Old World Vultures is also an error if we may trust the high authority of Nitzsch, who wrote as follows: “Unter den Raubvögeln einiges Geier, Adler, Falken; aber nicht die Eulen—Am Vultur percnopterus ist sie ziemlich stark, ungefähr einen halb Zoll long zugespitzt und bräunlich vom Horne.” In fact, a claw on the first finger is of very common occurrence, and is found, according to the authorities given above, in the Accipitres, Herodiones, Palamedeæ, Anseres, Gallinæ, Fulicariæ, Alectorides, Limicolæ, Gaviæ, Pygopodes, Crypturi, and Struthiones.
Here it may not be out of place to add that a claw has also been found on the end of the second finger, by myself and Professor Morse, in certain of the Winter Birds, and perhaps in some embryo Hawks: and that as a rule the claws are much more conspicuous in young than in adult birds.—J. Amory Jeffries.
Papers on Minnesota Birds.[32]—Although the report containing these papers was not generally circulated in 1881, a copy reached us in December of that year. Dr. Hatch contributes a list of 281 species briefly annotated—usually with only a line or two to each species respecting the manner and character of its appearance in the State. In explanation of its cursory style the author states that, as we regret to learn, the original copy was destroyed by fire, “and it has been impossible to give its re-writing the measure of carefulness which the first manuscript received.” The most interesting entry is that of Querquedula cyanoptera, which thus appears far from its recognized range.
Mr. Roberts’ article treats much more fully of 52 species known to occur in the State in winter, divided into the categories of “permanent residents” (23), “winter visitants” (14), “half hardy” species (9), and “accidental” ones (6), the information given conveying a good idea of the bird-fauna at that season of the year. Doubtless owing to circumstances for which neither author is responsible, each paper bristles with typographical errors, few of which are corrected in the accompanying erratum slip. We understand that a full list will accompany the volumes as finally published.—E.C.
48Freke on the Birds of Amelia County, Virginia.[33]—Our knowledge of the birds of Eastern Virginia is so largely inferential that Mr. Freke has done good service in publishing the results of six years’ observations in Amelia County, at a point “about thirty miles south of Richmond.” His list, which is freely annotated, includes 112 species. The Barn Swallow is catalogued as a spring and fall migrant; the Tree Sparrow (Spizella montana), as a rather uncommon winter visitor; the Field Sparrow, as resident but most common in winter; the Chipping Sparrow as arriving from the south late in March and as leaving during November; the Song Sparrow as wintering but not breeding; the Blue Grosbeak as not uncommon during the latter part of April and early in May, but, rather unaccountably, as not being found in summer; the Ruffed Grouse as plentiful in the mountains but not common in the low country, although a few regularly nest there in thick pine woods.
The author has evidently fallen into some confusion regarding the spotted-breasted Thrushes of the genus Turdus. Thus T. “pallasi” is characterized as a “resident species, apparently not migrating even in the most partial manner.” In view of our very definite knowledge of the Hermit’s distribution, such a statement by itself would be open to the gravest suspicion, but when we add that Mr. Freke does not mention the Wilson’s, Olive-backed, or Wood Thrushes as occurring at any season, it is quite plain that the Hermit (verus) did duty as the winter bird, the Olive-backed or Wilson’s Thrush filled the gap during the migrations, and the Wood Thrush was the species that “builds its clay-lined nest in the fork of some cedar or dogwood bush, at the height of eight or ten feet from the ground, and there lays its blue eggs.” The statement that Dendræca coronata “is one of the commonest warblers in the district, and spends [a] great part of the year there,” is not so easily explained: but despite the still more explicit assurance that “they come about the end of April, or the beginning of May, and remain until very late in the autumn,” we cannot help thinking that some mistake was made in the identification of the individuals seen in summer.
Save in the last-named instances, however, there is no reason to doubt that the author’s commendable practise of “verifying my observations, as far as possible, by securing specimens and preserving skins” was conscientiously carried out, and his paper will be read with interest, not only as an exponent of the ornithology of a previously unworked section, but also as embodying a foreigner’s pleasantly told impressions of many of our familiar birds.—W. B.
Langdon’s Field Notes on Louisiana Birds.[34]—These notes comprise “a record of ornithological observations and collections made by 49the writer during the month ending April 17th, 1881, at ‘Cinclaire’ plantation, situated in the parish of West Baton Rouge, Louisiana, on the right bank of the Mississippi, one hundred and twenty-seven miles by river above New Orleans.”
The locality is described as “flat and uninteresting.... The cultivated grounds are mainly comprised in a strip ranging from one to three miles in width, along the rivers and principal bayous, the remainder of the state being chiefly occupied by extensive forests and swamp lands.”
The author considers the list “of quite as much interest for what it does not include, as for what it does,” and comments on the apparent absence of the Catbird, Long-billed Marsh Wren, Black-and-white Creeper, Yellow-rumped, White-browed, Black-throated Green, Yellow Red-poll, and Kentucky Warblers, Large-billed Water Thrush, Redstart, Song Sparrow, and Common Pewee; to which he might with equal propriety have added the Prothonotary and Blue-winged Yellow Warblers and the Acadian Flycatcher. But we cannot believe with him that the non-occurrence, on the present occasion, of most of these species has any special significance, either as affecting their general distribution in, or usual migration through, the region of which the paper treats. The country about “Cinclaire” may have been unsuited to the habits of some of them, while the early date of Dr. Langdon’s departure, taken in connection with the exceptional lateness of the season, will sufficiently explain his failure to detect a number of the migratory ones which have been found near the mouth of the Mississippi by Mr. Henshaw, and which are well known to extend over the Mississippi valley at large only a few hundred miles further to the northward.
Dr. Langdon’s thoroughness and energy as a field collector are, however, so well known through the medium of his valuable papers on Ohio birds, that we may rest assured that his work at “Cinclaire” was well done, and the paper will be welcomed as an acceptable contribution to our knowledge of a region which has been nearly a terra incognita to ornithologists since the days of Audubon.—W. B.
Krider’s Field Notes.[35]—In an unpretending little pamphlet of some eighty odd pages Mr. Krider has “endeavored to describe and give the history of only those species of birds of the United States” which he has “collected and mounted,” and whose nests have come under his personal observation. Had this plan been carried out with only ordinary forethought and intelligence it could scarcely have failed to result in a valuable contribution to our knowledge of North American birds, for Mr. Krider’s long experience as a field collector must have afforded unusual opportunities for original investigation and observation. But a casual glance through 50the pages of his work is enough to show that these opportunities have been sadly neglected. Important records are given without dates and often with only a vague or inferential assignment of locality, while improbable statements and palpable errors are of frequent occurrence. In short, it is only too evident that Mr. Krider’s “Notes” are the offspring of a fading memory rather than the carefully kept data of a systematic worker. Moreover, the author writes from a standpoint at least twenty-five years behind the times, and consequently ignores all the various developments affecting classification and the relationship of allied species and races. From all this chaff it is of course possible to separate some sound grain, but most of the really important records were published long ago by Turnbull, Cassin, and other writers. Of the literary execution of the present work we can say nothing favorable. It is to be regretted that the author could not have recognized his unfitness in this respect, and, as on a former occasion, have secured the services of a competent editor.—W. B.
Langdon’s Zoölogical Miscellany.[36]—In the last issue of its well-known “Journal,” the Cincinnati Society of Natural History publishes the first of a series of articles entitled “Zoölogical Miscellany,” the aim and scope of which are thus tersely defined by the editor, Dr. F. W. Langdon:—
“Under the above caption it is proposed to bring together from time to time such facts as may be deemed worthy of record, respecting the structure, the life history, or the geographical distribution of the various species of animals constituting the Ohio Valley Fauna.”
The part before us includes sections on mammalogy, ornithology, herpetology, ichthyology, conchology, and entomology. In general terms, it may be said that all of these are well sustained, but in the present connection we have to do only with the one relating to birds. This contains a number of interesting notes, a large proportion of which are from the editor’s pen, although a few are signed by Mr. E. R. Quick, Mr. A. W. Butler, Dr. Howard E. Jones, and other more or less well-known names. Most of these notes relate chiefly to the local presence or distribution of certain birds within the Ohio Valley, but one or two possess a wider interest. Among the latter we notice an announcement by Dr. Langdon of the detection of the Oak-woods Sparrow (Peucæa æstivalis illinoensis, Ridgway) near Bardstown, Nelson County, Kentucky, “about one hundred miles southwest of Cincinnati.” The specimen was taken April 28, 1877, by Mr. C. W. Beckham, who referred it to Dr. Langdon for identification.
In addition to his numerous notes, the editor contributes a short but useful paper on the “Introduction of European Birds.” From this it appears that “during the years 1872, ’73 and ’74, about nine thousand dollars were expended in the purchase and importation of European birds, their average cost to import being about four dollars and fifty cents a pair. 51According to this estimate some four thousand individuals were introduced.” This great outlay was borne by the “Acclimation Society of Cincinnati” and we believe that most of the birds were turned out in the neighborhood of that city; but, according to Dr. Langdon, the experiment has practically proved a failure.
If the present instalment of “Zoölogical Miscellany” may be taken as a fair criterion of future issues, its favorable reception by naturalists is a matter of no uncertainty, and under Dr. Langdon’s able editing we look to see its popularity widely extended, even though its field be restricted to the Ohio Valley.—W. B.
Hoffman on the Birds of Nevada.[37]—In the present paper Dr. Hoffman has done good service to ornithology by tabulating the two hundred and fifty species and varieties of birds which he considers are entitled to a place in the avi-fauna of Nevada. The list is based partly upon the writer’s personal experience in the field during the season of 1871, but mainly upon the previously published reports of Mr. Ridgway, Mr. Henshaw and Dr. Yarrow, and Dr. J. G. Cooper. It hence partakes largely of the nature of a compilation, although the author’s original notes are by no means few or uninteresting.
The paper begins with a pertinent chapter entitled “Remarks on the distribution of vegetation in Nevada as affecting that of the avi-fauna” and closes with a bibliographical list of the chief publications relating to the region considered, and an excellent map of the state.
The list proper is freely annotated and the numerous and often extended quotations are always apt and interesting. The work, generally, has been so well done that we find few points open to adverse criticism. There is however an evident tendency on the author’s part to swell the number of species and varieties by the enrollment of many which have been taken or observed near the borders of the state but not as yet actually within its limits. We are aware that Dr. Hoffman has some high authority for adopting this course but we are none the less inclined to deprecate it, believing that it is time enough to catalogue a species when it has actually been found within the limits treated. In the present case, however, it must be admitted that there are good grounds for supposing that most of these extra-limitals will eventually turn up in Nevada.
Dr. Hoffman’s paper ranks easily among the higher class of publications to which it belongs and should find a place in the hands of every working ornithologist.—W. B.
The Tufted Titmouse on Staten Island, N. Y.—I shot a specimen of this species (Lophophanes bicolor) on the 24th of August, 1881, in a thick wood, a few miles south of Port Richmond, a small town on the north shore of Staten Island, N. Y.—Daniel E. Moran, Brooklyn, N.Y.
Nesting of the White-bellied Wren (Thryothorus bewicki leucogaster).—This Wren is abundant in Northern Arizona, where I saw it and heard it singing most constantly, during the month of June, while traveling from Fort Whipple to view the Grand Cañon of the Colorado. The birds were particularly numerous in the vicinity of cañons and arroyos, and in the patches of red cedar and piñon pine that stretch away from mountain sides to the valley ground of the Colorado Plateau. At a water-hole about midway on my journey, it so happened that my tent was pitched beneath a cedar where, as I was soon satisfied by their vehement scolding, a pair of the Wrens were protesting against such intrusion upon their privacy. In a little while, however, finding themselves unmolested they quieted down, resumed their song at intervals, and were soon after busily engaged in bringing insects to their family. Having explored a deserted Woodpecker’s hole, only to find it empty, I at length saw one of the birds disappear in the hollow end of a blasted horizontal bough about eight feet from the ground. The entrance was too narrow to admit my arm, but by breaking away some of the rotten wood I at length got a glimpse of the nest, and could just put a finger over the edge of it far enough to feel the little birds. I should have despoiled the household had there been eggs; but as it was I refrained, and for a day or two was much interested in watching the happy, devoted pair, bubbling over with joyous music as they assiduously cared for their little family, now coming and going undisturbed by the group of men who shared the luxury of this fragrant cedar shade. This was June 7; returning a week afterward, the pretty spot was a “banquet hall deserted”; so that I did not hesitate to break into the bough and remove the nest. It contained two dead young ones, upon which a troop of ugly carrion-beetles were rioting and feasting. The nest was quite unlike what a House Wren’s would have been under the same circumstances, having none of the trash with which these queer birds would have surrounded it; it rested upon the horizontal floor of the cavity, upon a bed of wood-mould and cedar-berries, about a foot from the ragged entrance of the hollow. It was a neat structure, about 4 inches across outside, by half as much in internal diameter, cupped to a depth of an inch and a half. Outside was a wall of small cedar twigs interlaced, and next came a layer of finely frayed inner bark strips from the same tree; but the bulk of the nest consisted of matted 53rabbit-fur stuck full of feathers, among which those of the Carolina Dove were conspicuous. These latter birds are extremely abundant all over Arizona and in the dry season they are often at such straits for water as to congregate in immense flocks at the water-holes, few and far between, which alone render it possible to traverse some parts of the unblest Territory. On the morning of which I write, reveille was sounded by the clapping and whistling of a thousand eager wings, now venturing near, then frightened from the coveted water where men and animals were crowding. In other times, the Dove brought tidings of dry land; in Arizona now, where everything goes by contraries, river-sites are many, but the sight of a Dove is a surer sign of water.—Elliott Coues, Washington, D. C.
An Erroneous Record of the Orange-crowned Warbler (Helminthophaga celata) in New Hampshire.—In Vol. III, pp. 96, 97 of this Bulletin, Mr. John Murdoch recorded the capture of an Orange-crowned Warbler at the Isles of Shoals, New Hampshire, by the Messrs. Bangs of Boston. I have lately had an opportunity of examining this specimen and find it to be a Tennessee Warbler (Helminthophaga peregrina), in the ordinary autumnal plumage. It is but just to the Messrs. Bangs to state that they are not to be held responsible for this blunder, the bird having been submitted by them to an ornithologist of some standing, one in whose determination they placed perfect confidence. Nor can Mr. Murdoch (who I believe took all his facts at second hand) be blamed for accepting the same supposed good authority.—William Brewster, Cambridge, Mass.
On the Generic Name Helminthophaga.—The change of a generic name, especially one long established, is in any case unfortunate, and in the present instance seems particularly so; yet the plain rules of zoölogical nomenclature leave no alternative. The generic name Helminthophaga, proposed in 1850 by Cabanis for a well-known group of American Warblers, was used in a sub-generic sense about forty-seven years previously, by Bechstein, who, in 1803 (Taschenbuch, p. 548), included under this name the Nightingale and Redbreast of Europe (Luscinia philomela and Erithacus rubecula); in consequence of which (no other name having, apparently, been proposed for the group in question) it becomes necessary to rename the genus so long called Helminthophaga. In proposing a new name, which I am very reluctant to do, I have selected the term Helminthophila, on account of its similarity to the one so long in use. It is proper to state here that my attention was called to this point by Dr. L. Stejneger, the eminent Norwegian ornithologist.
Leaving out H. lawrencei and H. leucobronchialis, which Mr. Brewster has pretty clearly proven to be hybrids of H. pinus and H. chrysoptera, the known species of this genus are as follows:—
1. Helminthophila bachmani (Aud.).
2. Helminthophila chrysoptera (Linn.).
3. Helminthophila pinus (Linn.).
544. Helminthophila ruficapilla (Wils.).
5. Helminthophila virginiæ (Baird).
6. Helminthophila celata (Say).
7. Helminthophila peregrina (Wils.).
8. Helminthophila luciæ (Cooper).—Robert Ridgway, Washington, D. C.
Dendrœca palmarum again in Massachusetts.—The first capture of Dendrœca palmarum in Massachusetts was that of a single bird taken by Mr. Arthur Smith at Brookline, about the middle of October, 1878. (See note by Mr. Ruthven Deane, Bull. Nutt. Club, Vol. IV, page 60.) I have the pleasure of announcing the capture of two additional specimens. The first was taken at Cambridge, September 13, 1880, and was shot on an apple tree while in company with several other Warblers. The second was shot at Belmont, September 7, 1881, from the top of a yellow pine. The marked difference in the intensity of the yellow of the breast and under tail-coverts first attracted my attention to this bird. Never having met with D. palmarum hypochrysea in the autumn, I thought both birds to be of this variety until quite recently, when my friend Mr. William Brewster identified them for me and found them to be genuine D. palmarum.—Henry M. Spelman, Cambridge, Mass.
Ampelis cedrorum as a Sap-sucker.—The Cedar, or Cherry-Bird seems never to be very abundant in this section of the State; but early in the spring, when the birds first arrived from the south, I saw quite a large number of them, and observed what was to me a new habit. They resorted to the maple trees for the purpose of gathering the sap flowing from wounds made by the ice in the bark of the smaller branches. The birds would grasp a branch or twig with their claws, and partially swing themselves under it and drink the sap where it hung in drops. For a week or more these birds were so plentiful and so intent upon their sap-gathering that one was almost certain to find a flock wherever there was a group of maples. I took considerable pains to ascertain if this habit was shared by any other bird, but did not observe a single instance. In the Eastern States I have often seen squirrels drinking sap from the branches in this way, but never before saw it done by a bird.—F. E. L. Beal, Ames, Iowa.
Capture of Plectrophanes lapponicus in Chester, South Carolina.—Mr. Leverett M. Loomis writes me that on January 1, 1881, he shot a single individual of this species from a small flock of Shore Larks, which were feeding upon offal in a barnyard. There appears to be no previous record of the occurrence of this species in South Carolina.—J. A. Allen, Cambridge, Mass.
Occurrence of Coturniculus lecontei in Chester County, South Carolina.—Near the town of Chester, S. C., on the dividing ridge between the Broad and Catawba Rivers, there is an “old field” of some 55two hundred acres that has been lying out, until recently, for a number of years. Here and there are patches of newly-sown grain, but the greater portion is now in broom-sedge and weedy stubble and corn land. Near the middle there is a small “wet-weather branch,” which empties into a large creek a mile distant. November 11, 1881, in this locality, in the weedy stubble, my first specimen of Le Conte’s Bunting was secured. Nov. 16, a second was taken in the broom-sedge near the same spot. Nov. 17, a third was shot, and several others were seen. Dec. 3, three more were captured; two in the broom-sedge, and the remaining one in the swamp grass bordering the “branch.” Dec. 10, my last visit to the field, six additional specimens were taken, and as many more were seen. I am not aware that the species has hitherto been reported as occurring so far east as South Carolina—Leverett M. Loomis, Chester, S. C.
The Sharp-tailed Finch in Kansas.—Col. N. S. Goss, of Neosho Falls, Kansas, wrote me under date of Oct. 17, 1881, that he had killed what he thought was a male Nelson’s Sharp-tailed Finch. Two days later he shot another, which he kindly sent me. The bird proved to be, as Mr. Goss supposed, Ammodramus caudacutus nelsoni. The birds were killed “at the edge of a slough, on the low bottom lands of the Neosho River, about two miles from Neosho Falls.” This discovery is of special interest as indicating that the Sharp-tailed Finch, formerly supposed to be strictly maritime in its distribution, may be found locally over a wide range in the interior.—J. A. Allen, Cambridge, Mass.
Note on Mitrephanes, a new Generic Name.—The name Mitrephorus of Sclater, P. Z. S., 1859, p. 44, is preoccupied in Coleoptera by Mitrephorus, Schönh., 1837, emended Mitrophorus, Burm., 1844. It may therefore be changed to Mitrephanes; type Mitrephanes phæocercus (Scl.); including Mitrephanes aurantiiventris (Lawr.), if not also Mitrephanes fulvifrons (Grd.), and its var. pallescens (Coues).—Elliott Coues, Washington, D. C.
Nesting of Empidonax minimus AND Helmintherus vermivorus in Pennsylvania and New Jersey.—Although instances of the breeding of the Least Flycatcher within the limits of Pennsylvania and New Jersey have been affirmed by Turnbull and one or two other authorities, a precise record cannot perhaps be found that will prove it to breed as far south as Philadelphia. Having found a nest and clutch of eggs belonging to this species, June 1, 1881, and satisfactorily identified the parent birds by shooting them, it is thought that this notice may prove of interest as perhaps removing doubts as to the accuracy of Turnbull’s statement. E. minimus escaped the notice of the writer till the spring of 1880, when two pairs were noticed in June in the suburbs of Philadelphia, but any nests which may have existed escaped my observation. The present year (1881) I first noticed them in Delaware County, Pa., two pairs taking up their abode in an orchard surrounding the house. Here the above mentioned 56nest was found, placed on a drooping branch of an apple tree fifteen feet above the ground. The species was seen and heard singing about six miles west of Camden, New Jersey, in June, and again in July at the same place; is it not just therefore to suppose this pair had a nest near the spot?
Worm-eating Warblers were noticed in full song in the vicinity of Marple, Delaware County. Pa., as early as the last week in April, and whilst on a collecting trip in May I procured three males and a female in southern Chester County, and on dissecting the latter I was surprised to find in her oviduct a partly shelled egg. On the 16th of June, 1881, a ramble in the woods resulted in finding a brood of young of this species scarcely able to fly; one of them is now in my collection and another just missed the same claim to immortality. The old birds were exceedingly solicitous but so wary that three shots failed to procure either of them.
Near Camden, New Jersey, I procured a female Worm-eating Warbler in the latter part of July, 1880; its actions and the time of year caused me to infer it had young near by.—Samuel N. Rhoads, Haddonfield, N. J.
Cuckoos Laying in the Nests of other Birds.—As far as my knowledge extends, there are only four instances known, in which the eggs of Coccygus americanus have been found in other bird’s nests, namely, the two given by Nuttall, in nests of Catbird and one by Langdon in Robin’s, and that mentioned by Ridgway in Coccygus erythrophthalmus. I was not a little astonished to find last Saturday, June 4. 1881, an egg of the Yellow-billed Cuckoo in a Catbird’s nest, and near by another one in the nest of a Black-billed Cuckoo. The Catbird’s nest contained only one egg of its rightful owner; another Catbird’s egg was found broken on the ground. The Cuckoo’s egg was fresh, but the Catbird’s egg was incubated. The nest of the Black-billed Cuckoo contained besides the parasitic egg, which was fresh, two eggs, both incubated, but one much more than the other, the embryo being fully developed. The parent bird (Coccygus erythrophthalmus) was sitting, but left when the tree was ascended and stationed itself on a near tree to watch our movements.
The circumstances attending the discovery of these two eggs make me think that such cases of parasitic Cuckoo’s eggs might not be so very exceptional and still evade the watchful eye of the collecting oölogist or of the observing ornithologist. I went out to look for nests of Empidonax acadicus. I took my nephew, a lad of fifteen, with me to assist in taking down nests from trees. In passing a thicket by the wayside, he looked in and immediately called out, “a big nest, blue eggs.” Judging from the surroundings, I replied without taking the trouble to look at the thing, “a Catbird’s nest; let it alone.” We passed on and after a little while a Catbird crossed our way. He saw the bird and I told him that this was the Catbird whose nest he had just found. He wondered that a bird of this size lays such large eggs. Inquiring how large the egg was, he showed the size with thumb and index. I smiled and said it was not exactly 57that big, but he insisted, and I concluded to walk back and look at the eggs, when the discovery was made. Who cares to look into each of the dozen of Catbird’s nests we find in the course of a season? We are satisfied to know that this is the nest of the Robin, the Wood thrush, the Catbird; but we do not think of taking the trouble to look every time at their eggs or young.
Still more likely to elude discovery would the strange egg be in the other Cookoo’s nest. In this neighborhood at least are the Cuckoo’s nests generally amidst such a terribly entangled mass of wild vine that we do not care to go up for mere pleasure. I do not know how regular egg-collectors go to work; other ornithologists may operate differently. My case may be no measure. I give it only to draw attention to the matter, and I have made up my mind to despise no more Catbirds’s nests in future.—O. Widman, St. Louis, Mo.
[Mr. Widman has overlooked a note which appeared in an early number of this Bulletin (Vol. II, p. 110), where three instances of the laying of our Cuckoos in other bird’s nests are given. Years ago when I used to take many Cuckoo’s nests each season in the apple orchards about Cambridge it was no uncommon thing to find an egg of the Black-billed species in a clutch of the Yellow-bills, and on more than one occasion, but less often, the situation would be reversed. An instance of the latter kind came under my notice in 1878, when at Belmont, Mass., I found a nest of the Black-billed Cuckoo which contained, besides two eggs of the rightful proprietor, a single one of the Yellow-bill. Speaking from memory, and without consulting my notes on the subject, I should say that at least ten per cent of the Cuckoo’s nests that I have found contained eggs of both species. But in no case have I ever seen the eggs of either kind in the nests of other birds.—William Brewster.]
Melanerpes erythrocephalus about Boston.—Massachusetts, at least the extreme eastern part, has shared in the flight of Red-headed Woodpeckers that has been reported as visiting Southern Connecticut last fall.[38] During the latter part of September, through October and into November, the oak groves in the suburbs of Boston were tenanted by numbers of these truly handsome birds. I should judge that about one third were in full plumage, and their conspicuous dress attracting attention many were shot. Twelve years ago the individual occurrence of this species among us was thought worthy of record. Of late years, during the months above named, it has become a more frequent though irregular visitor, but never in such numbers as have recently shown themselves. In spring or summer it is rarely seen, yet an instance of its nesting in Brookline is given me by Mr. H. K. Job, who early in June, 1878, found five eggs in the hole of an apple tree. According to Dr. C. Hart Merriam, this Woodpecker is a common resident of Lewis County, N. Y.[39] May not our visitors have come from that direction?—H. A. Purdie, Newton, Mass.
58The Barn Owl in Maine: A Retraction.—In the Bulletin for January, 1877, p. 28, I added the Barn Owl (Aluco flammeus americanus) to the catalogue of Maine birds, basing the record upon a specimen, which I had examined, in the possession of a taxidermist then of Portland. I very much regret to say that I now believe the account given me of this bird’s capture within our state limits to have been false. Several other statements in relation to ornithology have since been made me by the same man, of a character so improbable and with such contradictory details that they can only be regarded as wilfully and utterly untrue. Their author has recently left the city under circumstances which dispel any doubts which may previously have existed as to the reliability of his word. I cannot longer be responsible for a statement emanating from such a source, and wish to formally withdraw the name of the Barn Owl from the list of birds known to occur in Maine.—Nathan Clifford Brown, Portland, Maine.
The Snowy Owl at Fort Walla Walla, W. T.—On November 10, 1881, one of my men shot here a female of this species (Nyctea scandiaca), which I have made into a fine skin. I reported the capture of one on December 1, 1880 (see this Bulletin, Vol. VI, p. 128), and these two are the only records known to me for the Pacific coast. The occurrence of this species here seems to be much rarer than in the Eastern States.—Charles Bendire, Fort Walla Walla, W. T.
Capture of the Golden Eagle in Crawford County, Pennsylvania.—A Golden Eagle (Aquila chrysaëtus canadensis) was shot in Rookdale Township this (Crawford) County on December 10, under the following circumstances. A farmer, by the name of Hull, early one morning saw the bird fly from a carcass in his field to the woods some distance off. He conceived the idea that it would return to the carrion and at once made a blind of the rails of a fence near by. The following morning he repaired to the blind long before daylight with gun in hand, and, although he was well concealed and waited patiently until nearly noon, no bird put in an appearance. Nothing daunted, however, he repeated the watching on the second morning, and about eight o’clock was rewarded by the return of the bird, which he shot. The eagle was purchased by Mr. Roe Reisinger of our city and is now mounted. It is the first recorded specimen, I believe, of this species taken in this county. The sex I could not ascertain, as the entire contents of the bird’s body were drawn by Mr. Hull before bringing it to town, but from the following dimensions I should judge it to be a young female: Extent, 83 inches; wing, 24.50 inches; tail, 15 inches. Tail about two-thirds white. The black terminal zone was about four inches deep on outer quills and about one and one-half inches deep on the centre ones. The general color of the bird is brown, with wings almost deep black. The hood extends well down on the nape and is of a light tawny brown, approaching the golden hue probably as much as any of them do. The tarsus is well covered with feathers to the toes. On the whole it is a very clean and perfect specimen.—George B. Sennett, Meadville, Pa.
59The Swallow-tailed Kite in Dakota.—On November 14, 1881, when a short distance west of Jamestown, Dakota Territory, I saw several Swallow-tailed Kites (Elanoïdes forficatus) flying around apparently in search of food. The day was clear and the Kites were much separated; one even was seen alone skimming along an alkali lake, showing every indication of searching for food. On November 17, farther to the west, about midway between Jamestown and Bismark, near the line of the Northern Pacific Railroad, I saw some fifty more of these beautiful birds, but this time in a flock, and each movement being common with them all it was a glorious sight. The weather had changed from that of the 14th, and was now cloudy with a brisk wind from the northwest, accompanied at times by a slight shower of rain, but this change they seemed to enjoy. So easily did they ride the storm, so beautiful were their evolutions, so much at home did they appear in mid-air, that when they had passed out of sight I was pained, for in this northern latitude such a sight is of very rare occurrence.—D. H. Talbot, Sioux City, Ia.
A Remarkable Specimen of the Pinnated Grouse (Cupidonia cupido).—While overhauling some Grouse in the Boston markets a few years since I came across a specimen which exhibits the following peculiarities of plumage:
Adult ♂ (No. 2691, author’s collection, Boston Markets, February 27, 1873—said to have come from Iowa). Ground-color above warm, brownish-cinnamon. Shorter neck-tufts or pinnate coverts, bright reddish-brown. Breast, reddish-chestnut, becoming almost clear chestnut anteriorly. A band or collar of broad, stiff feathers extends continuously around the neck in front and across the lower portion of the jugulum about in a line with the neck-tufts. These feathers although less stiff than the longest ones in the neck-tufts, are nevertheless quite as much so as the shorter ones. They make a conspicuous ruff which is mainly black mixed with a good deal of reddish-chestnut. The latter color on the shorter and overlapping feathers occurs in the form of narrow central stripes, which in some cases are nearly orange in tint; on the longer ones as a more or less broad, lateral margining.
I offer the above description solely for the purpose of calling attention to this remarkable specimen for I am entirely at a loss to account for its peculiarities. Several who have seen it have suggested that it may be a hybrid between the Prairie Hen and the Ruffed Grouse, but this hypothesis seems hardly a probable one, inasmuch as none of the combined characters which would be expected in such an offspring are here presented. The ruff does indeed remotely suggest that of Bonasa, but otherwise the bird shows all the well-marked structural characters of Cupidonia. To simply say that it is abnormal will hardly satisfy the numerous investigators of this pushing age of inquiry.—William Brewster, Cambridge, Mass.
Wilson’s Plover (Ægialites wilsonius) in New England.—Mr. W. A. Stearns sends me a letter from Mr. Arthur S. Fiske, dated Gurnet, Conn., Aug. 22, 1877. “This morning I shot a bird of this species on 60the beach at the south of the hotel. It was alone, though there were several flocks of other Plovers near at hand. In note and actions it closely resembled the Piping Plover, but was larger and lighter colored. Capt. Hall called it the ‘Pale Ring-neck,’ and said he had seen it at the Gurnet before.” The description given by Mr. Fiske (length 7.75 inches; bill fully 1 inch, black, etc.) leaves no doubt that the bird was Wilson’s Plover.—Elliott Coues, Washington, D. C.
Capture of Baird’s Sandpiper on Long Island.—On September 22, 1880, I shot a specimen of Tringa bairdi on Montauk, Long Island. The bird was in a flock of “Peeps” (Ereunetes pusillus), feeding on the beach of Great Pond, a brackish lake often in communication with the Sound. It so closely resembled the “Peeps” that I only noticed it on account of its larger size. The skin I preserved, though badly cut by the shot.—Daniel E. Moran, Brooklyn, N. Y.
[This is apparently the first known occurrence of this species on the Atlantic Coast south of New England.—Edd.]
An Addition to the Maine Fauna.—On October 8, 1881, I received from Mr. Alpheus G. Rogers, of Portland, an immature specimen of Rallus elegans, the King Rail, which he shot on Scarborough Marsh, on the morning of that day. This species is new to the State of Maine, and has occurred in New England only about half a dozen times.
Its previous New England record is as follows: (1) Stratford, Conn., breeding, Linsley, Am. Jour. Sci. and Arts, Vol. XLIV, No. 2, p. 267. (2) Portland. Conn., one specimen: (3) Saybrook, Conn., one specimen, Merriam, Rev. Birds Conn., p. 115. (4) Nahant, Mass., one specimen, Purdie, this Bulletin, Vol. II. p. 22. (5) Sudbury Meadows, Mass., one specimen, Purdie, this Bulletin, Vol. III, p. 146.—Nathan Clifford Brown, Portland, Maine.
Capture of Larus leucopterus near Boston.—In November last Mr. Charles I. Goodale showed me an immature specimen of Larus leucopterus in the flesh, which he stated was shot near Boston. The bird is now in my collection.—Charles B. Cory, Boston, Mass.
The Great Black-backed Gull (Larus marinus) from a new Locality.—Mr. Howard Saunders, in his excellent synopsis of the Larinæ (P. Z. S., 1878, pp. 155–212), p. 180, in defining the known range of this species, says that there is “no record from the American side of the Pacific,” but that he had “examined undoubted specimens from Japan,” this being considered “a very great extension of its previously known range.” During the present year the National Museum has received specimens of this species, in alcohol, from Herald Island, in the Arctic Ocean, northwest of Behring’s Straits, and from Port Clarence on the American side of the Straits, the former collected by Captain C. M. Hooper, of the U. S. Revenue Cutter “Corwin,” the latter by Dr. T. H. Bean, of the National Museum.—Robert Ridgway, Washington, D. C.
61The Snake-bird in Kansas.—Prof. F. H. Snow, of the University of Kansas, writes as follows: “I have the pleasure of informing you of the capture of a specimen of the Snake-bird, Plotus anhinga, in the Solomon Valley in Western Kansas. It was taken in August of this year by C. W. Smith, Esq., of Stockton, and the skin is now in my possession.”—Elliott Coues, Washington, D. C.
Capture of the Sea Dove 150 Miles from the Sea:—On November 8th, 1881, a Sea Dove (Alle nigricans), was shot in the Hudson River, at Lansingburg, by Alfred Benjamin of that village. The bird was mounted by William Gibson of the same place, and is in his collection.—Austin F. Park, Troy, N. Y.
Additions To the Catalogue of North American Birds.—The following list includes all the species that have been added to the North American fauna since the publication of the “Nomenclature of North American Birds.” The numbers given these additional species indicate their position in the list; and I would suggest that any author publishing a species new to our fauna do the same, so that collectors and others may know its number.
440.* Buteo fuliginosus Scl. Little Black Hawk.
440.** Buteo brachyurus Vieill. Short-tailed Hawk; White-fronted Hawk.
708.* Puffinus borealis Cory. Northern Shearwater.
717.* Œstrelata gularis (Peale) Brewster. Peale’s Petrel.—Robert Ridgway, Washington, D. C.
Notes on Some Birds of the Belt Mountains, Montana Territory.—The following observations were made in the southern range of the Belt Mountains, latitude about 46° 30′, some miles to the west and south of the head-waters of the Musselshell, from which the land, intersected by frequent smaller streams, gradually rises to the foot of the low mountains, which are mostly forest-clad and of some 6,000 feet elevation. The streams have little or no timber save in the mountains or among the foot-hills where scattering firs appear; but willows grow in dense thickets along the bank, striving apparently by numbers to make up for any lack in size.
The notes extend from June 22 to July 3, 1880, three days excepted, when the writer was absent. All the birds were found within an area of a square mile, perhaps less, but the locality was unusually favorable, including several patches of burnt timber, a large open tract stretching up the mountain side to almost the summit, and two streams flowing in rather open cañons with clumps of willows on either bank.
Several interesting birds which were sought for unsuccessfully at this time I have since found in the Belt Range, viz. Cinclus mexicanus, Cyanocitta stelleri (macrolopha?) and Tetrao canadensis franklini. Skins of most of the species mentioned were preserved.
621. Turdus migratorius propinquus.—Common. A bird nesting June 25.
2. Turdus fuscescens.—Found only in the cañons. Common.
3. Sialia arctica.—Nesting in deserted Woodpecker’s holes.
4. Regulus calendula.—Everywhere among the firs.
5. Parus montanus.—Common. It never whistles more than two successive notes, at least I have never heard it.
6. Sitta carolinensis aculeata.—One pair found breeding in the knot-hole of a large fir. Young hatched on or shortly before the 25 June.
7. Neocorys spraguei.—A pair breeding on a high, grass-covered knoll just outside the timber. The male was often observed flying high overhead, constantly shifting his position, but keeping at about the same elevation while uttering his song—a rather monotonous carol, unless one is sufficiently near to hear the wonderful resonance of the blended notes.
8. Dendrœca auduboni.—Common.
9. Pyranga ludoviciana.—Rather common. A female observed nest-building June 26, the male meantime singing in a neighboring treetop. July 3 the nest was apparently completed but without eggs. It was built in a fir some thirty feet from the ground and about midway on a small horizontal limb where several twigs projected out on either side.
10. Cotyle riparia.—Swallows apparently of this species were seen flying high overhead. Their homes were found lower down on the streams.
11. Vireo gilvus swainsoni.—A common bird in the cañons.
12. Carpodacus purpureus.—Two individuals observed.
13. Chrysomitris pinus.—A flock of these restless little creatures appeared almost daily, uttering their querulous notes.
14. Poœcetes gramineus confinis.—Common on the grassy slopes.
15. Melospiza fasciata fallax.—Occasional among the willows of the streams.
16. Junco oregonus.—Apparently this form was not uncommon.
17. Spizella socialis.—Abundant in the patches of dead timber.
18. Cyanospiza amœna.—Not uncommon but confined to the willows etc. along the streams.
19. Sturnella magna neglecta.—Breeding on the grassy hillsides.
20. Picicorvus columbianus.—Occasional. Much commoner lower down among the scattered firs of the coulées.
21. Perisoreus canadensis capitalis.—A single bird shot July 2. It was almost full-grown, but in the “fluffy” plumage peculiar to young birds.
22. Contopus borealis.—One bird seen.
23. Contopus virens richardsoni.—Common.
24. Chordiles virginianus henryi.—In dead timber, common.
25. Picus villosus.—Young of perhaps a week old were found on the 25th of June.
26. Picoides arcticus.—Rather common.
27. Picoides americanus dorsalis.—Two or three specimens noted.
6328. Melanerpes erythrocephalus.—One bird observed.
29. Colaptes mexicanus.—Common. The young of this species doubtless hatching on June 28, as an old bird was seen carrying out and dropping, a hundred or two yards from the nest, the fragment of an egg shell at that time.
30. Buteo borealis.—Hawks apparently of this species occasionally observed.
31. Bonasa umbellus umbelloides.—Not common. Is mostly found in the cottonwood timber of the valleys.
32. Tetrao obscurus richardsoni.—Not as common here as in some other localities of the Belt Mountains. They prefer rough and rocky ledges with only a moderate growth of fir to denser forests. Occasionally one finds them outside of the mountains, but only among the scattered clumps of fir growing on the high bluffs of some of the streams. Their “tooting” is a low, muffled sort of cooing, uttered without vigor, or any visible effort on the bird’s part, which may be squatting on some rock at the time.
33. Tringoides macularius.—Found on the streams.—R. S. Williams, Benton, W. T.
Remarks on Some Western Vermont Birds.—The Red-headed Woodpecker (Melanerpes erythrocephalus, Sw.), is a strangely erratic species. Mr. C. S. Paine has taken but a single specimen in the eastern part of the State, and five years ago it was a very rare species about here (Brandon). Now they are nearly as abundant as the common Golden-wings. At Orwell, only ten miles to the west, they outnumber the Golden-wings, and appear to be on the increase. Dr. C. H. Merriam mentions (Bull. Nutt. Ornith. Club, Vol. III, No. 3, p. 124) their remaining in Northern New York during some of the severest winters known. I have never observed them in this vicinity later than the 2d of October, except in one instance (January 7, 1879), when I took a single specimen. At Rutland, sixteen miles south of Brandon, Mr. Jenness Richardson informs me that they are a resident species, being as abundant in winter as in summer. They were particularly abundant about here during August and September, 1879, being attracted, no doubt, by the great abundance of black cherries (Prunus serotina), which they appear to relish greatly. I have frequently observed this species to employ the same nest for several successive seasons.
The Pileated Woodpecker (Hylotomus pileatus, Bd.), is by no means as rare as might be expected in so thickly populated a section. Not a year passes but that from one to five specimens are taken. I have notes of at least fifteen specimens, taken during the last four or five years, all of which occurred from the month of September to May, inclusive; the last record being the capture of two young females, September 28, 1881. Of the remaining Picidæ, Sphyrapicus varius is a rather rare summer visitant; Picoides arcticus, a very rare winter visitant: while Picus pubescens and P. villosus are resident species, the former being by far the most abundant.
64During the winter of 1880–81, no less than seven specimens of the little Acadian Owl (Nyctale acadica) were taken, all within a few days’ time. Two specimens of the Snowy Owl (Nyctea scandiaca) were also taken at the same time. During the fall of 1879, a fine specimen of the American Raven (Corvus corax carnivorus) remained in this immediate vicinity for nearly a month, but successfully eluded capture. A single specimen of the Canada Jay (Perisoreus canadensis) was taken in December, 1874.
Although the recorded instances of the breeding of the Loggerhead Shrike (Lanius ludovicianus) in New England are rather numerous, the following notes may not be entirely devoid of interest. One rainy day last season (June 5, 1880) as I was seated on the porch of a neighbor’s house, my attention was attracted by a Shrike flying past several times. I watched the bird and saw it fly to the top of an old apple tree. The tree was not more than two rods from the house, and was densely overrun with a large grape vine. I climbed the tree, and, about twenty feet from the ground, found the nest, and, much to my disappointment, found no eggs, but four nearly fledged young. The old birds were very tame, and flew about within a few feet of my head.
This season I visited the locality May 16, and was fortunate enough to find a nest and four fresh eggs. The nest was in an apple tree, perhaps three rods from the nest of last year; was composed of coarse sticks and weeds, very deeply hollowed, and lined with wool and twine. I took both parent birds with the nest, thus rendering the identification positive.
A few days after this (May 23, 1881) some boys told me they had found a “Cat Bird’s” nest in an apple tree about a mile from the vicinity of the other nests. They had climbed the tree, and said “the old bird flew at them, and snapped her bill hard!” I knew this to be a Shrike, and, when I visited the place, had the pleasure of securing another nest, containing six eggs, with the female parent. The nest was much like the other, but was perhaps deeper, and lined entirely with feathers.
The Great Northern Shrike (Lanius borealis) is a rather rare species, being most frequently observed in spring.
The Scarlet Tanagers (Pyranga rubra) first made their appearance about here in the summer of 1875, when a single pair nested. Since then they have gradually increased until probably twenty pairs nested the past season. Strange as it may seem, I have never taken the common Titlark (Anthus ludovicianus) during the spring migrations, although they are usually abundant in the fall.—F. H. Knowlton, Brandon, Vt.
Erratum.—In Vol. VI, p. 199, lines 9 and 10. for “centimeters” read millimeters.
Early in 1881 I wrote to Mr. Stephens asking him to get me some Arizona birds during the following spring and summer. He replied that he was on the point of starting by wagon for California, but that being provided with a camping outfit, and feeling under no necessity of hurrying by the way, he was willing to give his whole attention, for several months at least, to collecting in my interest. It was accordingly arranged that the journey should take in as great a variety of country as possible, and, that the most productive points should be thoroughly worked. The energy, intelligence, and conscientiousness with which this plan was carried out are sufficiently attested by the material results upon which the present paper is based.
The route traversed was substantially as follows: Leaving Galeyville on March 3, Mr. Stephens drove southward to Cave Creek, where a few days’ collecting yielded a limited number of birds. At the end of this time he retraced his steps to Galeyville, and continuing northward, passed Camp Bowie, and crossed to the western side of the Chiricahua Mountains. Here a halt was made at Morse’s Mill, after a journey of seventy miles by wagon-road from Cave Creek, although the distance is less than twelve 66miles in an air line. This place is described in the notes as being at the head of a cañon, in a sort of basin, elevated about seven thousand feet above the sea, and encircled by mountains which rise from two to three thousand feet higher.
From some further remarks on the general character of the range, I quote the following: “The Chiricahua Mountains are situated in the southeast corner of Arizona, some of the foot-hills even reaching the line of New Mexico and the Mexican state of Sonora. Several small streams run east and west from their summits, those of the former division emptying into the San Simon Valley; of the latter into the Sulphur Spring and San Bernardino Valleys. The first two water-sheds are comprised in the Rio Gila system, while the San Bernardino Valley stretches southward, and water from it flows into the Pacific near Guaymas.”
“These valleys are usually grassy plains, but there are scattering bushes, mostly mesquite, in some of them. The scrub oaks begin with the foot-hills; they are evergreen, the leaves being insensibly replaced with new ones in May. A little higher the juniper (called ‘cedar’ by the people here) comes in. Still higher, on the north side of the hills, there is a little piñon and scrub pine, while the summits are heavily timbered with red and black pines. In the gulches some fir grows, and on the hillsides, mostly near the summits and facing the north, occasional patches of aspen.”
At Morse’s Mill three weeks were very profitably spent, and on April 1 a start was made for Tucson, the next objective point. The route led through Sulphur Spring Valley, Tombstone, and Cienega Station, and at all these places, as well as at some intermediate points, a longer or shorter stay was made for the purpose of collecting. These delays consumed so much time that Tucson was not reached until April 18.
The country lying about this town and the neighboring station, Camp Lowell, proved so rich in desirable birds that it engaged Mr. Stephens’ attention for nearly the whole of the two succeeding months, during which, however, a brief visit was paid to the Santa Rita Mountains, where some important observations were made.
The season practically ended with June, for the wagon-journey, begun on the 29th of that month, across the arid plains and scorching deserts of middle and western Arizona, was attended 67with such privations, and often positive suffering, that little attention could be paid to birds. Mr. Stephens arrived at Yuma on July 15, and by August 1 reached his final destination, Riverside, California.
The entire trip yielded about six hundred and fifty skins besides a fairly large number of nests and eggs. Under the terms of our agreement I had all the birds, a representative series of the nests and eggs, and the field notes relating to both. This collection, embracing the results of four months’ uninterrupted work in a region as yet only imperfectly known, seems to me too complete in itself to be merely skimmed of its cream. Accordingly in preparing the following paper I have included every species which is represented among the specimens or mentioned in the collector’s notes. It should be understood, however, that the latter were not kept with reference to this plan, and it is not unlikely that certain common birds, which are known to occur in Arizona, were inadvertently omitted. For similar reasons, the number of specimens obtained can seldom be taken as an exponent of the relative abundance of the species to which they belong, as a decided preference was given to the rarer kinds. Three species new to the “North American” fauna have already been announced (this Bulletin, Vol. VI, p. 252.).
A few technical points require explanation. The catalogue numbers are usually those of the collector’s field-book, but in certain cases—as of specimens taken as types, or with birds obtained by Mr. Stephens before starting on the present trip—I have used my own numbers, either alone or in connection with the original ones. This double system need cause no confusion, however, for the field-numbers never reach 700, while those of my general catalogue are always above 5,000. Of the measurements, the length and stretch were taken in the field, the others from the dry skins. The biographical matter is of course based on Mr. Stephens’ notes, which are sometimes paraphrased, sometimes literally quoted, as convenience dictates. The frequent quotations of Mr. Henshaw’s experience or opinions are always, unless otherwise stated, from his Report in Volume V of “Explorations and Surveys West of the One Hundredth Meridian.”
1. Turdus unalascæ Gmel. Dwarf Thrush.—The only Hermit Thrush in the present collection is unmistakably referable 68to var. unalascæ. In fact it gives nearly the same measurements as the smallest extreme in the large series examined by Mr. Henshaw.[40] Mr. Stephens marks it as the first which he has seen in Arizona where, however, it was found sparingly by Mr. Henshaw in October, 1873.
283, ♀ ad., Tucson, April 25. Length, 6.40; extent, 10.10; wing. 3.26; tail, 2.61; culmen, .52. “Bill dark brown, yellowish at base of lower mandible; legs pale brownish; iris brown.”
2. Turdus ustulatus Nutt. Russet-backed Thrush.—Under this heading I include with some hesitation, a Thrush killed May 17, in the Santa Rita Mountains. The specimen unfortunately was one of three or four which were accidentally destroyed while in the collector’s possession, but Mr. Stephens is positive that it was referable to the above variety. As he is perfectly familiar with ustulatus, having previously met with it in California, there can, I think, be little doubt of the correctness of his determination. This record, if accepted, will make the first for Arizona.
397, ♀ ad., Santa Rita Mountains, May 17. Length, 6.90; extent, 10.70; “Iris dark brown; bill black, brownish at base of lower mandible; legs very pale brown.”
3. Turdus migratorius propinquus Ridgw. Western Robin.—Robins were met with only in or near the Chiricahua Mountains, where perhaps a dozen individuals were seen. The one mentioned below is typical of the slightly differentiated, but still apparently constant western race.
75, ♂ ad., Morse’s Mill, March 20. Length, 10; extent, 16.40; wing, 5.38; tail, 4.36. “Iris dark brown.”
4. Oreoscoptes montanus (Towns.) Baird. Mountain Mockingbird. There is no mention of this species among the notes made during the late trip.
6313 (author’s coll.), ♀ ad., San Pedro River, Dec. 25, 1880. Length, 8.90; extent, 12.40.
5. Mimus polyglottus (Linn.) Boie. Mockingbird.—“Generally distributed and common, but not as abundant as in Southern California” (Camp Lowell). “Common in the valleys; they are found but a short distance up the foot-hills of the mountain ranges” (near Tombstone).
181, ♀ ad., near Tombstone, April 8. Length, 9.80; extent, 13.10; wing, 4.30; tail, 5.03.
69550, ♂ ad., Camp Lowell, June 20. Length, 10.20; extent, 14.10; wing, 4.40; tail, 5.20. “Iris golden brown; bill and legs black.”
6. Harporhynchus bendirei Coues. Bendire’s Thrasher.—Mr. Stephens’ notes contain few references to this species, and judging from the limited number of specimens which he obtained, it must be less abundant in Arizona than either H. crissalis or H. curvirostris palmeri, a status which is in strict accordance with Mr. Henshaw’s experience. About half of the skins collected during the past season are labeled either Camp Lowell or Tucson, while the remainder were taken at various points directly north or south of the latter place, and not over twenty-five miles distant in either direction. Outside the limits of this desert region the bird was not anywhere met with, although it was common at Phœnix in February, 1880.
A nest taken June 16 near Tucson, and identified by the capture of one of the parent birds, was placed in a “cat-claw mesquite” at a height of about five feet from the ground. It is a deeply-hollowed, smoothly-lined structure, composed of fine grasses and soft, hemp-like vegetable fibres, which are protected externally, in a manner common to the nests of nearly all Thrashers, by a bristling array of interlaced twigs and thorny sticks. The interior cup measures two inches in depth by three in width. The two eggs which it contained, like those described by Dr. Coues, are readily separable from eggs of H. palmeri by their grayish-white instead of dull green ground-color. They are faintly marked with reddish-brown and lavender, the spots being confined chiefly to the larger ends, where many of them assume the character of blotches or dashes of color. These eggs measure respectively 1.02 × .79 and .96 × .79. The greatest number of eggs found in any of the several nests examined by Mr. Stephens was three, but two seemed to be the usual complement.
Of the birds before me four are in first plumage, a stage which, if I am not mistaken, has never been previously examined. The first of these (No. 426, twenty-five miles south of Tucson, May 22) was unable to fly, and was taken from the nest. It differs from the adult in the following particulars: The upper parts, with nearly the same ground-color, have a tinge of reddish-brown which, on the rump, wing-coverts, and tips and outer webs of the primaries and secondaries, shades into brownish-chestnut. The sprouting rectrices are also tipped with the same color. The under parts generally are warm fulvous, which becomes nearly pure cinnamon on the sides and crissum, and along the median line pales to 70fulvous-white. The breast and abdomen are everywhere thickly but finely spotted with dull black, these markings becoming finer and fainter where they border on the anal region. The remaining three (Nos. 538, ♀; 539, —; and 540, ♂: twenty-five miles north of Tucson, June 16) have the wings fully developed, and were all out of the nests when shot. They are apparently of about the same respective ages, but nevertheless exhibit a good deal of individual variation. No. 538 has the breast and sides finely spotted with dark brown, but a central space extending forward along the abdomen nearly to the breast is entirely unmarked. No. 535 has large, rounded, but indistinct blotches of light brown, thickly and evenly distributed over the entire under parts, excepting the throat, anal region and crissum. No. 539 has a cluster of faint, sagittate spots on the centre of the breast, but otherwise is entirely immaculate beneath. All three are essentially similar above, and differ from No. 426 in having the crown, nape, back, wing-coverts and outer webs of the secondaries pale reddish-brown, which, on the rump, is only tinged with chestnut. The primaries are dark brown edged with hoary; the rectrices, dull black with a terminal band of pale reddish-chestnut crossing both webs of all the feathers, but most broadly those of the outer pairs.
The adults making up the rest of this series vary a good deal with the season at which they were taken. A specimen killed in February is clear grayish-brown above, with the breast and abdomen thickly spotted; and one or two others shot early in May are nearly as deeply colored and distinctly marked. But most of the breeding birds are either entirely immaculate beneath, or with only a few faint specks scattered here and there upon the abdomen. Several of the latter are nearly as pale as my specimens of H. lecontei, and equally devoid of any special markings. This condition apparently is due mainly to the wearing off of the tips of the feathers, although the continued action of the sun’s rays doubtless lends its aid, and still further bleaches the plumage.
453, ♂ ad., Camp Lowell, May 30. Length, 10.30; extent, 13.30.
4987, (author’s coll.) ♂ ad., Tucson, Feb. 28, 1880. Wing, 4.25; tail, 4.84; culmen (chord), .99.
423, ♂ ad., twenty-five miles south of Tucson, May 21. Length, 10.40; extent, 14.20; wing, 4.30; tail, 4.92; culmen, 1.06.
425, ♂ ad., same locality, May 22. Length, 10.30; extent, 13.10; wing, 4.01; tail, 4.96; culmen, 1.05.
455, ♂ ad., Camp Lowell, May 30. Length, 10.18; extent, 13.30; wing, 4.20; tail, 4.96; culmen, 1.05.
537, ♂ ad., twenty-five miles north of Tucson, June 16. Length, 10.10; extent, 12.70; wing, 4.14; tail, 4.78; culmen, 1.01.
583, ♂ ad., Camp Lowell, June 24. Length, 10.50; extent, 13; wing, 3.99; tail, 4.95; culmen, 1.05.
454, ♀ ad., Camp Lowell, May 30. Length, 10.10; extent, 12.70; wing, 3.95; tail, 4.43; culmen, 1.
529, ♀ ad., twenty-five miles north of Tucson, June 16. Length, 10.20; extent, 12.10; wing, 3.63; tail, 4.50; culmen, 1.01. “Iris yellow; legs dull bluish.”
71557, ♀ ad., Camp Lowell, June 21. Length, 10; extent, 13.20; wing, 4.10; tail, 4.60; culmen, .95.
426, ♀ juv. first plumage, twenty-five miles south of Tucson, May 22. Length, 6.10; extent, 9.40; “Iris light gray; bill dark brown, lighter below; legs pale bluish.” Taken from the nest; wings and tail only partly developed.
538, ♀ juv. first plumage, twenty-five miles north of Tucson, June 16. Length, 10.10; extent, 12.50; wing, 3.77; tail, 4.59; culmen, .96.
539, — juv. first plumage, same locality and date. Length, 9.80; extent, 12.70; wing, 3.92; tail, 4.67; culmen, .92.
540, ♂ juv. first plumage, same locality and date. Length, 10; extent, 12.80; wing, 3.90; tail, 4.55; culmen, .95.
7. Harporhynchus curvirostris palmeri Ridgw. Palmer’s Thrasher.—During the present trip this Thrasher was met with at various points in the desert region about Tucson and Camp Lowell, where it was one of the most abundant and characteristic summer birds. Its favorite haunts were barren wastes covered with cactuses and stunted mesquites; but, like many other desert species, it occasionally visited the more fertile valleys to drink at the springs and water-holes. At these latter places specimens were obtained without much difficulty, but on all other occasions they were exceedingly shy and wary. In February, 1880, Mr. Stephens found Palmer’s Thrasher at Phœnix, and he also took winter specimens along the San Pedro River.[41]
Numerous nests were taken. The one before me was placed in a cholla at a height of about seven feet. It is composed outwardly of large twigs, and is lined with bleached grasses. Although by no means a rude structure, it suffers by comparison with the nest of H. bendirei, its construction being simpler, and all the materials much coarser. The three eggs which it contained were only slightly incubated on June 14. They measure respectively 1.05×.82, 1.09×.82, and 1.08×.83. They are pale greenish-blue, finely and very evenly spotted with brown and lavender. The number of eggs making up this set was not exceeded in any of the others examined by Mr. Stephens.
The series of skins embraces no less than twenty-two examples, and very fully illustrates all the variations of age and season. Among the number are several in the hitherto undescribed first plumage. The
72youngest of these (No. 480, ♂?, Camp Lowell, June 2), although well feathered, has the wings and tail undeveloped, and was taken from the nest. Its entire upper plumage is rusty brown with a chestnut tinge which deepens on the rump and outer webs of the secondaries to decided chestnut brown. The general coloring of the under parts is pale fulvous with a strong tinge of rusty chestnut across the breast, along the sides, and over the anal region and crissum. The breast is obsoletely spotted, but the plumage elsewhere, both above and below, is entirely immaculate. An older bird (No. 577, Camp Lowell, June 23) with the wings and tail fully grown out, differs in having the back (excepting a narrow anterior space bordering on the nape), with the exposed webs and coverts of the wings, and a broad tipping on the tail feathers, bright rusty;—while in a third of about the same age (No. 614, ♂, Camp Lowell, June 28), the rusty color, although paler, is uniformly distributed over the entire upper surface save upon the wings and tail feathers, which are only edged and tipped with that color. This last example is so faintly marked beneath that the plumage at first sight appears immaculate; but a closer inspection reveals a few spots here and there among the central feathers of the breast. A fourth (No. 487, Camp Lowell, June 3), although apparently no older, has the breast and sides spotted more sharply than in any of the adults, while the rusty tinge above is chiefly confined to the rump, posterior half of the back, and the outer webs of the wing feathers.
Several of these young birds are so nearly similar to specimens of H. bendirei in corresponding stages that they can be separated only with great difficulty. The stouter bill and entirely black lower mandible of palmeri may, however, always be depended upon as distinguishing characters; and, moreover, the pectoral spotting of bendirei is usually (but not invariably) finer and sharper, and the rusty tinge above paler and less extended.
The adults present a good deal of variation, most of which is apparently seasonal. Winter specimens have the lower abdomen, with the anal region and crissum, rich rusty-fulvous, while the markings beneath are similar in character to those of true curvirostris, and the spots equally distinct, numerous and widely distributed. With the advance of the season, and the consequent wear and tear of the plumage, the spots gradually fade or disappear. Indeed some of the June specimens are absolutely immaculate beneath, although most of them, like Mr. Ridgway’s types, have a few faint markings on the abdomen. In this condition the general coloring is also paler and grayer, and the fulvous of the crissum and neighboring parts often entirely wanting.
But although the evidence of this series tends to demolish several of the characters upon which palmeri has been based, enough remain to separate it from its ally the true curvirostris of Mexico and the Rio Grande Valley in Texas. The best of these, perhaps, is to be found in the different marking of the tail feathers. In curvirostris the three outer pairs are broadly tipped with pure white which, on the inner web, extends twice as deep, basally, as on the outer one, and has its boundaries everywhere 73sharply defined; in palmeri the outer rectrices are, at the most, barely tipped with pale brown, which either extends squarely across both webs, or fades insensibly into the darker color of the feather. The bill of palmeri, also, is usually longer and more curved than that of curvirostris.
8. Harporhynchus lecontei Bonap. Leconte’s Thrasher.—The great rarity of Leconte’s Thrasher, even in the heart of the desolate regions where alone it has so far been found, is still further attested by Mr. Stephens’ experience during the past season, for although he searched for it carefully in all suitable places between Camp Lowell and Riverside (California), he met with only two individuals. These occurred about fifteen miles west of Maricopa, Arizona, in a locality which the accompanying notes describe as follows: “Near the middle of ‘Forty-five-mile Desert,’ between Maricopa Wells and Gila Bend. No chollas or other cactuses in the immediate neighborhood, but some giant cactuses about a mile away in the hills; a few mesquites and much scattering low brush in the vicinity; nearest water twenty miles away.”
Dr. Cooper is said to have found the species “rather common” in the desert between Fort Mohave and the San Bernardino Mountains, California, but Mr. Stephens has thrice traversed this route without seeing a single specimen. In a recent number[42] of the American Naturalist, however, Mr. E. Holterhoff, Jr., speaks of seeing the bird “on the Colorado desert, at a station called Flowing Wells,” and gives an interesting description of a nest and set of eggs taken there. “The nest was placed in a palo verde tree, and was a very bulky affair, measuring externally nine inches in depth and six in width; the hollow of the nest was fully three inches in depth. It was so awkwardly situated that much of the base of the nest had evidently been filled in to firmly support the structure. The two eggs were somewhat smaller than those of H. redivivus, lighter in color, and marked all over with finer reddish spots, thicker at the larger end.”
I am inclined to consider the Maricopa specimens above referred to as adults, although this is not so clear in the case of the male, portions of whose plumage suggest that of a young bird. Both are in worn, ragged condition, but there is no indication of any moult, save upon the wings and tail, where many of the feathers have been replaced by new ones which are conspicuous among the others by their fresher coloring.
74On a former occasion[43] I urged the specific distinctness of this Thrasher from H. redivivus, and to this conviction I still hold, although a comparison of additional specimens of both species inclines me to believe with Dr. Coues that Leconte’s Thrasher is, on the whole, more nearly related to redivivus than to any other United States form.
616, ♂ ad., near Maricopa Wells, July 5. Length, 10.80; extent, 12.30; wing, 3.85; tarsus, 1.27; tail, 5.35; culmen (chord), 1.30; bill from nostrils, .91; width below posterior angle of nostrils, .23.
617, ♀ ad., same locality and date. Length, 10.60; extent, 12; wing, 3.78; tarsus, 1.32; tail, 4.91; bill (chord of culmen), 1.32; bill from nostril, .94; width below posterior angle of nostril, .24. “Iris reddish-brown; bill black; legs nearly black. Stomach contained a small species of katydid and some ants.”
9. Harporhynchus crissalis Henry. Crissal Thrasher.—Not uncommon near Tombstone, Tucson and Camp Lowell.
Dr. Coues, comparing this species with Le Conte’s, Palmer’s, and Bendire’s Thrashers, concludes:[44] “and we are led to infer that when the ‘topography’ of the other three species is fully determined, it will be found no less extensive. For there is nothing peculiar in the economy or requirements of any one of the four in comparison with the rest.” This view, however, is hardly supported by the testimony of observers who have had the best opportunities of studying these birds. The Crissal Thrasher, according to Captain Bendire,[45] “appears to prefer damp localities near water-courses, and confines itself principally to spots where the wild currant is abundant.” Mr. Henshaw says: “According to my experience, it is not a bird of the plains, but inhabits by preference the rough sides of rocky cañons or the hillsides covered with broken débris, interspersed with straggling bushes.” Mr. Stephens’ evidence is not less explicit. He found the Crissal Thrasher in copses in valleys, and along streams. It was especially fond of well-shaded undergrowth, and spent much of its time on the ground, searching for food under the bushes. It never occurred among cactuses, and the only place where he saw it actually associating with Bendire’s and Palmer’s Thrashers, was at Camp Lowell, where the latter species, with other desert birds, came to drink at a water-hole and thus occasionally mingled with the Crissal Thrashers which inhabited the neighboring thickets. The contrast which these traits afford 75when compared with the ones characterizing the other three species named by Dr. Coues, is sufficiently apparent.[46]
A nest received from Mr. Stephens is precisely similar to those found by Captain Bendire. The three eggs which it contained measure respectively, 1.14×.76, 1.14×.75, and 1.08×.77. Like all the specimens which have been previously reported they are entirely unspotted, and both in size and color closely resemble eggs of the common Robin.
Juv., first plumage (♀, No. 546, Camp Lowell, June 20). Above dull reddish-brown. Rump and a broad tipping on the tail, brownish-chestnut. Under parts nearly uniform, brownish-fulvous. Crissum chestnut, of nearly the same shade as in the adult. Maxillary stripes dusky brown. No trace of spots or other dark markings either above or beneath.
Five other young birds in the series are essentially similar and call for no special comment. I cannot find any description of the first plumage of either H. redivivus or H. lecontei, but with the exception of these, H. crissalis is the only North American species in the sub-family Miminæ whose young are entirely unmarked beneath. It is interesting to note that with respect to the color of the upper parts, especially that of the rump, they resemble the young of both H. bendirei and H. palmeri.
The individual variation presented by the adults before me is chiefly confined to the relative length and curvature of the bill, the general coloring of all being nearly uniform, although the breeding birds are slightly paler than those taken early in the season.
166, ♂ ad., near Tombstone, April 5. Length, 12.10; extent, 12.30; “Iris light brown. Stomach contained insects and a small lizard.”
251, ♂ ad., Tucson, April 21. Length, 12.60; extent, 12.60; wing, 4.11; tail, 6.25; chord of culmen, 1.56. “Iris light gray,—almost white.”
278, ♂ ad., Tucson, April 25. Length, 12.10; extent, 12.50; wing, 3.84; tail, 6.20; culmen, 1.47.
309, ♂ ad., Tucson, April 30. Length, 11.70; extent, 12.70; wing, 4.05; tail, 5.85; culmen, 1.53.
434, ♂ ad., Tucson, May 25. Length, 11.20; extent, 12.30; wing, 4.02; tail, 5.52; culmen, 1.43.
503, ♂ ad., Tucson, June 8. Length, 11.40; extent, 12.10; wing, 3.85; tail, 5.85; culmen, 1.46.
578, ♂ ad., Camp Lowell, June 23. Length, 11.60; extent, 12.60; wing, 4.05; tail, 5.75; culmen, 1.45.
437, ♂ juv., first plumage, Tucson, May 26. Length, 11.30; extent, 12.40; wing, 3.92; tail, 5.50; culmen, 1.18.
76595, ♂ juv., first plumage, Camp Lowell, June 25. Length, 11.60; extent, 12.50; wing, 3.84; tail, 6.18; culmen, 1.35.
596, ♂ juv., first plumage, Camp Lowell, June 25. Length, 11.80; extent, 12.60; wing, 3.86; tail, 6.12; culmen, 1.40.
436, ♀ ad., Tucson, May 25. Length, 11.80; extent, 12.40; wing, 3.90; tail, 5.90; culmen, 1.55. Parent of No. 435.
435, ♀ juv., first plumage, same locality and date. Length, 11.30; extent, 12.20; wing, 4.02; tail, 5.55; culmen, 1.20.
546, ♀ juv., first plumage, Camp Lowell, June 20. Length, 11.60; extent, 12.40; wing, 4.95; tail, 6.02; culmen, 1.38.
555, ♀ juv., first plumage, Camp Lowell, June 21. Length, 11.30; extent, 12.20; wing, 3.73; tail, 5.65; culmen, 1.42.
10. Cinclus mexicanus Swains. American Water Ouzel.—The following notes relate to the only specimen met with:
“My attention was called to the song of some bird which came from the mountain brook running past camp. There was a steep, rocky wall on the further side, and the notes echoing from it, and mingling with the purling of the water, sounded exquisitely sweet. On looking for the author, I noticed some ripples rolling out from behind the willows that fringed the nearer shore, and soon discovered an Ouzel dabbling in the shallow water. My shot wounded the bird, but did not disable its wings, for it repeatedly dived, using them as propelling agents when beneath the surface. The sun shining on the air-bubbles that clung to its plumage made it look like a ball of silver flying through the water. On the surface it paddled along very much in the manner of a Phalarope.”
79, ♂ ad., Morse’s Mill, Chiricahua Mountains, March 20. Length, 7.90; extent, 12.10; wing, 3.85; tail, 2.50. “Iris hazel. The flesh was dark and tough with a fishy smell. The inside of the skin looked like that of a small Wader. Stomach contained insects.”
11. Sialia mexicana Swains. Western Bluebird.—A single pair, taken in the Chiricahua Mountains in March, are accompanied by the note, “abundant in all kinds of timber.”
12. Sialia arctica Swains. Arctic Bluebird.—This species is noted as “rare in the low valleys” among the Chiricahua Mountains. A small flock was also seen near Galeyville on “grassy plains,” where “they flew from one weed-stalk to another.” They were “restless and rather shy.” The single specimen obtained was shot on this latter occasion.
13. Myiadestes townsendi (Aud.) Caban. Townsend’s Solitaire.—Three specimens were obtained in the Chiricahua 77Mountains, where they occurred sparingly among piñons. “They are rather tame, and have a habit of sitting perfectly still for several minutes at a time. Flight slow. Food insects.” A fourth, taken May 13, in the Santa Rita Mountains, completes the series.
14. Phaïnopepla nitens (Swain.) Scl. Black-crested Flycatcher.—The life history of this singular bird has been so fully given by Dr. Coues in “Birds of the Colorado Valley,” that there is little chance of adding anything new. Most of the specimens obtained by Mr. Stephens are from Camp Lowell and Tucson, but he did not find it abundant at either of these points. He speaks of it as having “a sweet but not loud song,” and remarks on its known fondness for mistletoe berries. “Iris red.”
15. Polioptila cærulea (Linn.) Scl. Blue-gray Gnatcatcher.—Eight specimens, representing the following localities: Chiricahua Mountains (two ♂, two ♀, April 1–6); Tombstone (♂, April 5); Cienega Station (♂, April 16); Tucson (♂, April 20); Santa Rita Mountains (♂, May 20).
16. Polioptila plumbea Baird. Black-capped Gnatcatcher.—This Gnatcatcher was observed at Tucson, Camp Lowell, and near Yuma, specimens being taken in all these localities. A female shot at the first-named point on April 23 had evidently finished laying, but a nest found June 27 near Camp Lowell contained a perfectly fresh egg, while another taken at Yuma, July 15, had a single egg of its owner and one of the Dwarf Cowbird. These dates indicate that the species breeds at least twice during the season.
The Yuma nest, although a delicate structure, will not compare with that of P. cærulea. It entirely lacks the exterior coating of lichens so effectively employed by the commoner bird, and in its general appearance closely resembles the Redstart’s well-known domicile, being similarly felted of soft bark strips and hemp-like vegetable fibres. It is lined with down from plants, a few feathers, and the hair of some small quadruped. Externally it measures 2.25 in width by 1.55 in depth; internally 1.45 by 1. The egg is pale greenish-blue, coarsely and very evenly spotted with reddish-brown. Its measurements are .53×.42. This nest was placed in a bunch of mistletoe, at a height of about eight feet from the ground. It is accompanied by the male parent, who revealed its position by repeatedly entering the mistletoe. 78and showing other signs of anxiety respecting its contents. The position of the Camp Lowell nest is not mentioned.
Juv., first plumage, ♀ (No. 619, Yuma, July 15). Crown pale cinereous; rest of upper parts faded brown. The wings are uniform with the back, but all the primaries and secondaries have a broad white edging on their outer webs. The tail is dull black, with white areas on the outer rectrices corresponding in extent and purity with those of the adult. Beneath, pale ashy-white.
A study of the large series of Gnatcatchers collected during the past season confirms the views which I lately advanced (this Bulletin, Vol. VI, p. 101) regarding the affinity of P. plumbea and P. “melanura,” and also affords additional evidence of the assumed specific distinctness of P. californica. The Yuma examples of P. plumbea are quite as typical as those taken at Tucson and Camp Lowell, while seven specimens of californica, collected at Riverside after Mr. Stephens’ return to that place, still further attest the constancy of most of the characters which I assigned to the latter bird. That relating to the brown edging of the secondaries will, however, have to be abandoned, for plumbea proves to be similarly characterized when in worn breeding dress; the supposed shorter tail of californica also is now shown to be an inconstant feature. All of the three young males taken at Riverside have black lateral crown-stripes like those of immature plumbea.
267, ♂ ad., Tucson, April 23. Length, 4.60; extent, 5.80; wing, 1.85; tail, 2.15; bill (from nostril) .25; tarsus, .67. “Iris dark brown;” lores ashy mixed with black; eyelids white.
500, ♂ ad., Tucson, June 7. Length, 4.60; extent, 5.80; wing, 1.81; tail, 2.12; bill (from nostril), .25; tarsus, .65. Lores ashy mixed with black; upper eyelid white.
564, ♂ ad., Camp Lowell, June 22. Length, 4.55; extent, 5.80; wing, 1.84; tail, 2.19; bill (from nostril), .25; tarsus, .70. Lores black; both eyelids white.
567, ♂ ad., Camp Lowell, June 22. Length, 4.40; extent, 5.60; wing, 1.84; tail, 2.16; bill (from nostril), .26; tarsus, .70. Lores and superciliary line white mixed with black.
581, ♂ ad., Camp Lowell, June 24. Length, 4.40; extent, 5.80; wing, 1.98; tail, 2.20; bill (from nostril), .28; tarsus, .70. Lores ashy.
618, ♂ ad., Yuma, July 15. Length, 4.40; extent, 5.80; wing, 1.90; tail, 2.15; bill (from nostril), .26; tarsus, .68. Lores, with broad superciliary lines meeting across the forehead, white.
621, ♂ juv., first plumage, Yuma, July 16. Length, 4.40; extent, 5.60; wing, 1.76; tail, 2.13; bill (from nostril), .26; tarsus, .72. Sides of head ashy-white; ill-defined, black, lateral crown-stripes partially concealed.
272, ♀ ad., Tucson, April 23. Length, 4.50; extent, 5.50; wing, 1.78; tail, 2.21; bill (from nostril), .27; tarsus, .68. “Had just finished laying.”
458, ♀ ad., Camp Lowell, May 31. Length, 4.50; extent, 5.50; wing, 1.86; tail, 2.13; bill (from nostril), .26; tarsus, .68.
79601, ♀ ad., Camp Lowell, June 27. Length, 4.60; extent, 5.50; wing, 1.74; tail, 2.18; bill (from nostril), .27; tarsus, .70. “Taken with the nest and one fresh egg.”
619, ♀ juv., first plumage, Yuma, July 15. Length, 4.40; extent, 5.60; wing, 1.86; tail, 2.12; bill (from nostril), .26; tarsus, .70.
566,—juv., first plumage, Camp Lowell, June 22. Length, 4.40; extent, 5.60; wing, 1.85; tail, 2.22; bill (from nostril), .27; tarsus, .68.
For comparison I add measurements of the seven specimens of P. californica above mentioned.
656, ♂ juv., fall plumage, Riverside, Sept. 16. Length, 4.55; extent, 5.70; wing, 1.67; tail, 2.20; bill (from nostril), .29; tarsus, .75.
658, ♂ juv., fall plumage, same locality and date. Length, 4.70; extent, 5.80; wing, 1.89; tail, 2.21; bill (from nostril) .26; tarsus, .75.
688, ♂ juv., fall plumage, Riverside, Sept. 23. Length, 4.50; extent, 5.90; wing, 1.73; tail, 2.11; bill (from nostril), .30; tarsus, .75.
657, ♀ juv., fall plumage, Riverside, Sept. 16. Length, 4.60; extent, 5.80; wing, 1.85; tail, 2.14; bill (from nostril), .30; tarsus, .72.
686, ♀ juv., fall plumage, Riverside, Sept. 23. Length, 4.45; extent, 5.90; wing, 1.92; tail, 2.17; bill (from nostril), .30; tarsus, .75.
687, ♀ juv., fall plumage, same locality and date. Length, 4.50; extent, 5.80; wing, 1.85; tail, 2.20; bill (from nostril), .28; tarsus, .70.
655, ♀ juv., fall plumage, Riverside, Sept. 16. Length, 4.45; extent, 5.75; wing, 1.86; tail, 2.15; bill (from nostril), .28; tarsus, .75.
17. Regulus calendula (Linn.) Licht. Ruby-crowned Kinglet.—“Common among the Chiricahua Mountains, especially in deciduous timber. I think a few summer and breed.” The following specimens are identical with eastern ones:
28, ♂ ad., Cave Creek, Chiricahua Mountains, March 8. Length, 4.60; extent, 6.50; wing, 2.32.
122, ♂ ad., Morse’s Mill, March 28. Length, 4.20; extent, 6.90; wing, 2.38.
18. Lophophanes inornatus (Gamb.) Cass. Plain Titmouse.—Mentioned in Mr. Stephens’ notes as rare on the foot-hills of the Chiricahua Mountains, but no specimens are included in his collection.
19. Lophophanes wollweberi Bonap. Wollweber’s Titmouse.—This species was abundant in the Chiricahua Mountains, where a fine series was collected. They were usually seen in flocks of six or eight, and often associated with other small birds. They were rarely met with excepting in the groves of “scrub oaks,” but their food appeared to be wholly insects. A single pair taken in the Santa Rita Mountains in May are unaccompanied by any special remarks.
20. Parus meridionalis Scl. Mexican Chickadee.—In a late number of the Bulletin (Vol. VI, p. 252) I briefly 80announced this important addition to the North American fauna. The series obtained by Mr. Stephens comprises nine specimens, all of which were taken near Morse’s Mill. They occurred upon the sides or summits of the surrounding mountains, at elevations varying from seven to ten thousand feet, and were usually found in pairs, although they not unfrequently associated with other birds, among which are mentioned Psaltriparus plumbeus, Lophophanes wollweberi, Sitta pygmæa, and Peucedramus olivaceus. They were for the most part silent, but occasionally uttered a “chee-wee-wee,” as well as notes resembling those of P. montanus.
Previous writers have compared this species with P. atricapillus, but to me it seems nearer related to P. montanus. With the latter it agrees in certain peculiarities of size and proportions, while the general coloring and markings of the two are so similar that almost the only appreciable points of difference are presented by the white forehead and head-stripes of montanus. These characters are, of course, enough to instantly separate the birds, but their importance is somewhat weakened by the fact that one of my specimens of meridionalis (No. 124) possesses a head-stripe which, though ill-defined and considerably shorter, is nevertheless similar in appearance and position to that of montanus. While it would be rash to argue any varietal affinity on the strength of this single specimen, the outcropping of such a well-marked characteristic certainly shows a close relationship between the two species, unless indeed No. 124 be regarded as a hybrid.
65, ♂ ad., Morse’s Mill, March 18. Length, 5.20; extent, 8.50; wing, 2.74; tail, 2.60. “Iris dark brown. Stomach contained insects.”
82, ♀ ad., Morse’s Mill, March 21. Length, 5.10; extent, 8.10; wing, 2.73; tail, 2.62.
83, ♂ ad., same locality and date. Length, 5.10; extent, 8.50; wing, 2.90; tail, 2.69.
99, ♀ ad., Morse’s Mill, March 24. Length, 4.70; extent, 7.90; wing, 2.63; tail, 2.42.
100, ♂ ad., same locality and date. Length, 5.10; extent, 8.60; wing, 2.76; tail, 2.65.
104, ♂ ad., Morse’s Mill, March 25. Length, 5.10; extent, 8.30; wing, 2.75; tail, 2.40.
105, ♂ ad., same locality and date. Length, 5.10; extent, 8.20; wing, 2.66; tail, 2.56.
124, ♂ ad., Morse’s Mill, March 29. Length, 5.10; extent, 8.70; wing, 2.85; tail, 2.68.
125, ♂ ad., same locality and date. Length, 5; extent, 8.20.
21. Psaltriparus plumbeus Baird. Lead-colored Tit.—Of the eight specimens of this species which are included in the collection, seven were taken in the Chiricahua Mountains, the 81remaining one being from the Santa Rita Mountains. Mr. Stephens does not appear to have found it elsewhere, and in his notes characterizes it as rather uncommon. It was oftenest seen among the oaks of the foot-hills, where it associated with Wollweber’s Titmouse, the Ruby-crowned Kinglet, and several other small birds.
22. Auriparus flaviceps (Sundev.) Baird. Yellow-headed Tit.—Mr. Henshaw while in Arizona met with but few specimens of this curious little species. He attributed their apparent rarity to the lateness of the season at which his observations were made, and doubtless this explanation is the true one; for during the past spring Mr. Stephens found them in abundance both at Cienega Station and Tucson. Nevertheless it is probable that some individuals pass the winter in Arizona, for one of my specimens is dated November 29, and another was killed early in March. A nest taken at Tucson contained three fresh eggs on April 20.
23. Sitta carolinensis aculeata (Cass.) Allen. Slender-billed Nuthatch.—This Nuthatch was common in the pine forests of the Chiricahua Mountains, but the notes do not mention its occurrence elsewhere.
24. Sitta pygmæa Vig. Pygmy Nuthatch.—Equally common with the preceding species in the same locality.
25. Certhia familiaris mexicana (Gloger) Ridgw. Mexican Creeper.—Various writers have attributed the Mexican Creeper to our fauna, either on purely inferential grounds, or from a misconception, which at one time prevailed, regarding the relationship of the form found in California; for up to the present time no undoubted specimens of mexicana have been taken within our boundaries. It accordingly gives me much pleasure to announce the actual occurrence in Arizona of this well-characterized race, of which the specimen mentioned below is perfectly typical. It is the only Creeper which Mr. Stephens met with during the past season, but in the previous year two others, which I have not examined, but which he considers identical with this, were taken in the same locality. All the Arizona specimens obtained by Mr. Henshaw were referred to our eastern form.
66, ♀ ad., Morse’s Mill, Chiricahua Mountains, March 18. Length, 4.80; extent, 7.10; wing, 2.45; tail, 2.25; culmen, .50. “Iris dark brown.”
8226. Campylorhynchus brunneicapillus (Lafr.) Gray. Cactus Wren.—I notice little of special interest among the notes accompanying the eight skins which Mr. Stephens collected. He found the bird abundant in all suitable localities, and took several nests and sets of eggs. The unsophisticated young were easily shot, but the adults, even when breeding, were shy and hard to secure.
27. Salpinctes obsoletus (Say) Caban. Rock Wren.—Mr. Stephens makes no mention of finding this species in Arizona during the past season, but he sends me a single specimen taken December 25, 1880, on the San Pedro River.
28. Thryomanes bewicki leucogaster Baird. White-bellied Wren.—The collection includes five specimens of this form, which was apparently met with only in the Chiricahua Mountains and about Tucson. In the former locality it was common along the banks of streams where, however, it kept so closely hidden among the weeds and brush that it was oftener heard than seen. The examples before me are typical.
29. Troglodytes aëdon Vieill. House Wren.—The only House Wren taken is absolutely indistinguishable from many of my Massachusetts specimens, and I accordingly refer it here. Furthermore, I fail to find the characters supposed to distinguish var. parkmani, in any of the several California specimens included in my series. If the latter form really possesses any constant differential characters, I believe they have yet to be defined.
169, ♀, near Tombstone, April 6. Length, 4.80; extent, 6.40; wing, 2.10. “Iris dark brown. Shot among low brush. Not common.”
30. Anthus ludovicianus (Gm.) Licht. American Titlark.
271, ♀ ad., Tucson, April 23. Length, 6.50; extent, 10.60. “Bill brown, paler at base below; legs brown.” Several seen in marshes along the stream.
31. Helminthophila luciæ (Coop.) Ridgw. Lucy’s Warbler.—Although this diminutive Helminthophila has been known to ornithologists for nearly twenty years, few specimens have found their way into the cabinets of private collectors, and up to the present time the species has remained a very rare one. On this account the acquisition of a good series of skins was among the main objects of Mr. Stephens’ trip, and the success which rewarded his labors is very gratifying.
83The first specimen was shot April 15 at Cienega Station, where, during the succeeding three days, six more were obtained. They frequented large willows along the banks of a stream and, like Kinglets, spent much of their time searching for food at the extremity of the branches. Although active and restless, they were not at all shy. The only note heard here was a sharp “tseep.” On April 18 Mr. Stephens reached Tucson, where almost the first birds met with were Lucy’s Warblers. During the early part of his stay they were more abundant among the mesquites than any other species, and their “tseeping” could be heard on every side. They were continually in motion, flying from tree to tree, and occasionally visiting some low brush in the vicinity. By the 28th their numbers became perceptibly diminished, but many remained to breed in the surrounding country. The presence of the species at Camp Lowell is attested by a single young specimen, barely large enough to fly, which was taken there on June 1st, but which is unaccompanied by any special remarks. An adult male from the Santa Rita Mountains, however, comes to me with the following comments, under date of May 19:—“This is the only one of the species which I have seen here. It was near the banks of a stream below the mouth of a cañon, where there were a few mesquites interspersed among the oaks. I watched it for some time. It lingered among the mesquites, seeming to prefer them to the oaks, in which, however, it occasionally alighted for a moment.”
In addition to the above, Mr. Stephens’ notes supply some very important information regarding the previously doubtful nesting habits of this species. A female taken April 25, proved on dissection to be about to lay, but no eggs were actually taken until May 8, when a full set of five was found near Tucson. After that date many nests containing either eggs or young were examined. Their sites were variable; the characteristic place, like that of the specimen discovered by Captain Bendire, was behind the loosened bark of a large tree, but use was frequently made of old Woodpeckers’ nests, knot-holes, and in short all sorts of crevices. A brood of nearly fledged young (one of which is before me) was actually taken from the deserted domicile of a Yellow-headed Titmouse, which had been appropriated by the new tenant without any apparent repairs or alterations. Among Helminthophilæ this Wren-like mode of nidification is, I believe, peculiar to this species.
84I have the Tucson nest just alluded to. It is composed outwardly of twigs and weed-stalks; inwardly of hemp-fibres; while there is a scanty lining of horse-hairs and feathers. Like most hole nests it is rather flat, and the rim is thin in places where the walls of the cavity encroached on the space within. The eggs are white, handsomely wreathed about the larger ends with reddish-brown and umber spots, a few of which are also scattered over their general surfaces. They measure respectively .58×.46; .58×.46; .62×.46; .60×.47. The notes accompanying this set are as follows:—“Nest about six feet above the ground in a crevice nearly covered by bark. The bottom of the hole contained an old nest; over this were droppings of wood-rats, and the whole filled the cavity nearly to its top. The tree (a mesquite) stood within twenty feet of a frequented road. Female sitting. Eggs fresh; one had been broken and crowded in behind the nest by the parent bird.” None of the other sets found by Mr. Stephens contained more than three eggs and the present clutch is probably an exceptionally large one.
Juv., first plumage (♀ No. 471, Camp Lowell, June 1).—Wing-coverts and inner secondaries broadly tipped and edged with pale brownish-fulvous. Primaries and rectrices edged and tipped with hoary white. Rump and upper tail-coverts yellowish-chestnut. No chestnut on the crown. Otherwise colored like the adult.
Among a number of adults before me the range of individual variation is very limited, and is chiefly confined to the females. While it is true that some of the latter are indistinguishable from the brightest males, the majority have the rump and crown-patches considerably duller, the chestnut being either diluted in shade, or mixed with the color of the back. In No. 206 the crown-patch is concealed, the chestnut being restricted to the basal portion of the feathers.
225, ♂ ad., Tucson, April 18. Length, 4.40; extent, 6.70.
229, ♂ ad., Tucson, April 19. Length, 4.40; extent, 6.80.
231, ♂ ad., Tucson, April 19. Length, 4.40; extent, 7; wing, 2.35; tail, 1.93.
232. ♂ ad., Tucson, April 19. Length, 4.30; extent, 6.80; wing, 2.35; tail, 1.95.
253, ♂ ad., Tucson, April 21. Length, 4.40; extent, 6.70; wing, 2.21; tail, 1.87.
254, ♂ ad., Tucson, April 21. Length, 4.30; extent, 6.70; wing, 2.21; tail, 1.95.
255, ♂ ad., Tucson, April 21. Length, 4.50; extent, 7.10; wing, 2.23; tail, 1.93.
280, ♂ ad., Tucson, April 25. Length, 4.40; extent, 7; wing, 2.25; tail, 1.95.
85299, ♂ ad., Tucson, April 28. Length, 4.40; extent, 6.70.
326, ♂ ad., Tucson, May 4. Length, 4.30; extent, 7; wing, 2.20; tail, 1.93.
340, ♂ ad., Tucson, May 7. Length, 4.40; extent, 7; wing, 2.21; tail, 1.93.
410, ♂ ad., Santa Rita Mountains, May 19. Length, 4.10; extent, 6.90; wing, 2.22; tail, 1.82.
516, ♂ ad., Tucson, June 10. Length, 4.30; extent, 7; wing, 2.12; tail, 1.85.
524, ♂ juv., first plumage, Tucson, June 11. “Taken from nest, which also contained a young Molothrus ater obscurus.”
197, ♀ ad., Cienega Station, April 15. Length, 4.10; extent, 6.40; wing, 2.12; tail, 1.78. “Iris dark brown; bill black above, bluish beneath; legs black.”
206, ♀ ad., Cienega Station, April 16. Length, 4.40; extent, 6.50; wing, 2.17; tail, 1.80.
208, ♀ ad., Cienega Station, April 16. Length, 4.20; extent, 6.60; wing, 2.09; tail, 1.82.
217, ♀ ad., Cienega Station, April 17. Length, 4.30; extent, 6.70; wing, 2.21; tail, 1.84.
218, ♀ ad., Cienega Station, April 17. Length, 4.10; extent, 6.60; wing, 2.10; tail, 1.85.
228, ♀ ad., Tucson, April 19. Length, 4.30; extent, 6.70; wing, 2.10; tail, 1.85.
230, ♀ ad., Tucson, April 19. Length, 4.30; extent, 6.70; wing, 2.07; tail, 1.84.
256, ♀ ad., Tucson, April 21. Length, 4.20; extent, 6.60.
260, ♀ ad., Tucson. April 22. Length, 4.30; extent, 6.60; wing, 2.08; tail, 1.85.
261, ♀ ad., Tucson, April 22. Length, 4.30; extent, 6.70; wing, 2.25; tail, 1.92.
279, ♀ ad., Tucson, April 25. Length, 4.30; extent, 6.70; wing, 2.10; tail, 1.82. “About to lay.”
433, ♀ ad., Tucson, May 25. Length, 4.50; extent, 6.50. “With nest and three eggs; set completed.”
449, ♀ ad., Tucson, May 29. Length, 4.40; extent, 6.90; wing, 2.11; tail, 1.77. “With nest and three eggs; set completed.”
439, ♀ juv., first plumage, Tucson, May 26. Nearly feathered, but unable to fly. “Taken from a deserted nest of Auriparus flaviceps.”
471, ♀ juv., first plumage, Camp Lowell, June 1. Length, 4.20; extent, 6.60; wing, 2.10; tail, 1.71. Fully feathered.
32. Helminthophila celata lutescens Ridgw. Western Orange-crowned Warbler.—A few were seen late in April near Tucson.
Although not perfectly typical of lutescens, both of the Orange-crowned Warblers obtained by Mr. Stephens are clearly referable to that race. They are not quite as yellow beneath as Nicasio (California) specimens, but they come within a shade of it, and are brighter by many shades than any of the same sex among my eastern examples; while in the vividness of the 86olive-green on the upper parts, they fully equal any of the California females. The supposed difference in the tail markings of these races does not hold in the series before me, for a male from Nicasio has the edging on the inner webs of the rectrices quite as broad and pure as that of any of the Florida ones. The loss of this character, however, would be of little consequence, as the two forms could be readily separated by the wide difference in their general coloring. Mr. Henshaw considers his Arizona specimens true celata, and lutescens is now for the first time announced from that Territory.
290, ♀ ad., Tucson, April 26. Length, 5; extent, 7.30; wing, 2.45; tail, 2.10. “Iris dark brown; bill black, lighter at base below; legs dark brown. Not common.”
291, ♀ ad., same locality and date. Length, 4.70; extent, 7.10; wing, 2.37; tail, 2.09. Same remarks.
My attention was first directed to this bone by Dr. Shufeldt’s article in this Bulletin for October, 1881, and subsequently by Mr. Jeffries’ paper in the number for January, 1882. With the view of ascertaining in what birds the os prominens is present, and what is its use, I have since examined quite an extensive series of birds. Lack of time has prevented as extended an examination as could be wished for; and as regards discovering any special use for this sesamoid, it must be confessed that the results of the investigation are not wholly satisfactory, being rather negative than positive in their character. But such as they are, they are submitted, in the hope that they may prove of service to some better skilled physiologist.
Through a lack of good material Dr. Shufeldt failed to discover the existence of the os prominens in any of the Owls, but it would seem to be specially characteristic of the Bubonidæ, since it is present in one particular shape, and with a constant mode of articulation, in the following species of that family: Ketupa ceylonensis, K. javanensis, Bubo ignavus, B. bengalensis, 87B. virginianus, Scops brasilianus, S. asio, Nyctea scandiaca, Ninox albigulare, Asio otus, Syrnium nebulosum, and S. uralense. It is not present in Strix flammea or S. perlata, and should it prove to be present in other genera of the Bubonidæ than those noted above, it may serve as an additional, though trivial, point of distinction between the families Bubonidæ and Strigidæ.

Left wing of Bubo virginianus, from below (reduced one third).
r, radius; u, ulna; c, cuneiform; s, scapho-lunar; os p, os prominens; epa, tendon of extensor patagii longus.
The accompanying cut, drawn from a fresh specimen of B. virginianus, explains the form and position of the os prominens.
It will be noticed that it is situated on the anterior surface of the distal end of the radius, and runs almost parallel with that bone, instead of standing erect as in the Falconidæ. The radial portion of the tensor patagii longus terminates in the os prominens, and is not continued to the first metacarpal.
Apart from the Owls above noted, this bone has been found in Otogyps calvus, Heterospizias meridionalis, Buteo melanoleucus, B. pennsylvanicus, B. lineatus, Circus gouldi, Asturina pucherani, and Haliæetus albicilla.

A. Os prominens of Otogyps calvus, full size.
B. Os prominens of Bubo virginianus, seen from above to show articulation with radius, full size.
88It is absent in Polyborus tharus, Milvago chimango, and the following peculiar forms which were examined to see if they would throw any light upon the subject: Nyctibius, Strigops, Nestor, Megapodius, Ocydromus, and Atagen. Neither was any trace of it to be found in two specimens of Pandion haliæetus from N. Africa and the Duke of York group. Dr. Shufeldt’s theory that the os prominens is for the purpose of extending the wing area struck me, as it did Mr. Jeffries, as being untenable, from the fact that the increase of surface thus obtained was too slight to be of any value.[48]
The first proposition of Mr. Jeffries’ summary is that the bone serves to keep the friction of the extensor patagii longus from the carpus. Were this the case it ought surely to be present in the Albatross and Gull, birds which in a fresh breeze are continually flexing and extending their wings according to the direction of their flight and the varying force of the wind. But in both these birds the os prominens is absent,[49] and moreover, as we see in the Owls, it may be so situated as not to prevent the friction of the ulnar portion of the tendon. Second, that it serves only to a limited extent to increase the power of the extensor patagii longus to abduct the thumb, is shown by the fact that in the majority of cases that tendon is inserted in the first metacarpal. The exceptions to this, so far observed by me, are in Otogyps calvus and Haliæetus albicilla, where there is a strong tendon running from the os prominens to the first phalanx of the thumb. The third proposition has already been considered, and the fourth (that it protects the carpus) must be rejected, both for the reason given by Mr. Jeffries, and because as we see it in Owls it frequently does not lie over the carpus at all. Only in Otogyps calvus does the os prominens seem to exist as a simple sesamoid, and in that bird it is imbedded in the tendon of the extensor patagii longus, and glides over the scapho-lunar. Were I to venture a suggestion it would be that 89by its serving as a point of attachment for the tensor patagii longus, that tendon is freed from all duties save that of “puckering up” the anterior margin of the wing; but, as stated before, that theory is by no means entirely satisfactory to me.
During the summer of 1881 the writer and two companions spent a little more than a month in the South, especially in the State of Mississippi, travelling and studying its zoölogy. Our primary object was to collect fresh water fishes; and to this we devoted the greater part of our time and efforts. Incidentally, however, we collected and made observations on other animals. Hence this list of birds and the few notes concerning them. I did not intend to publish this list until I had opportunity to make additions to it; but the recent publication by Dr. F. W. Langdon of his field notes on birds observed by him, early in the spring, at a point a little farther south, has made it seem proper that I should contribute my little toward making known the ornithology of this region.
Our observations and collections were made of course under difficulties, and no attempt was made to secure nests and eggs, or, in any special manner, notes on the breeding habits of birds. Still, on account of the season when our trip was made, this list may be of some value as indicating that the birds observed are summer residents. The number of species recorded is not large, but I include only birds that I am reasonably sure were seen. In nearly all cases the birds were shot, and identified by means of descriptions. Others were seen, but as they were not identified with certainty, they are not included in the list.
The birds noted as found at Memphis, Tenn., were really seen in Arkansas just across the river from Memphis. Most of our other notes were obtained at Vicksburg and Jackson, Miss.
90The nomenclature adopted is that of Mr. Robert Ridgway, issued by the U. S. National Museum, 1881.
1. Hylocichla mustelina (Gmel.) Baird. Wood Thrush.—This species was seen and specimens were shot at Memphis and at Vicksburg. Its song was frequently heard; and it would appear to be quite common.
2. Mimus polyglottus (Linn.) Boie. Mockingbird.—Very abundant at all points visited. At the time we were at Vicksburg, July 1, the young had not yet left the nest, as negro boys were offering them captured in their nests for sale. In the “History of N. A. Birds” Dr. Brewer has stated that the Mockingbird in the South nests early in April, and that the young birds appear a month later. If this is the case these birds must remain in the nest six weeks or two months. I was informed that a law in Mississippi prohibits the keeping of these birds in confinement.
3. Galeoscoptes carolinensis (Linn.) Caban. Catbird.—This bird was quite common at Memphis. I did not note it at any point farther south.
4. Harporhynchus rufus (Linn.) Caban. Brown Thrush.—A single specimen seen at Jackson.
5. Sialia sialis (Linn.) Haldem. Bluebird.—Seen in considerable numbers at Memphis, Vicksburg, and Jackson.
6. Lophophanes bicolor (Linn.) Bonap. Tufted Titmouse.—Specimens of this species were obtained at Memphis and at Jackson. It may be worth noting here that it occurs as far north as Indianapolis, and I have seen it here during the present winter.
7. Parus carolinensis Aud. Carolina Chickadee.—Seen only at Memphis.
8. Thryothorus ludovicianus (Gm.) Bonap. Carolina Wren.—We observed this active bird at Memphis and at Jackson, at both of which places it appeared to be very abundant.
9. Mniotilta varia (Linn.) Vieill. Black-and-white Creeper.—Observed at Memphis and Jackson. It will probably be found to breed at both these points.
10. Protonotaria citrea (Bodd.) Baird. Prothonotary Warbler.—Specimens of this species were shot at Memphis, and others were seen at Jackson.
11. Parula americana (Linn.) Bp. Blue Yellow-backed Warbler.—This was found to be one of the most common of the smaller birds at Memphis, Vicksburg, and Jackson. We were constantly shooting them while hunting for other species. In the “History of N. A. Birds” it is said to be nowhere abundant; but a day’s hunt in the Mississippi lowlands would, I think, convince any ornithologist that this is an error. I have no doubt whatever that it breeds all through the South, although we found no nests. Audubon was probably correct in saying that it breeds in Louisiana, however much he may have erred in regard to the structure of the nest.
9112. Oporornis formosa (Wils.) Baird. Kentucky Warbler.—This sprightly little bird was observed, and specimens were handled, at both Vicksburg and Jackson.
13. Geothlypis trichas (Linn.) Caban. Maryland Yellow-throat.—A specimen was shot at Memphis; others were seen.
14. Myiodioctes mitratus (Gmel.) Aud. Hooded Warbler.—Specimens, male and female, of this bird were obtained at Jackson. It appeared to be moderately common.
15. Setophaga ruticilla (Linn.) Swains. American Redstart.—During our stay at Hopefind, Ark., opposite Memphis, a number of specimens of the Redstart were seen. Afterwards, while at Jackson, about July 10, a male and a female were killed. Their presence so far south at this season, and in such numbers, would indicate that they breed here. Up to this time I am not aware that it is known to breed south of the Potomac River and Illinois. The finding of the nest and eggs in Mississippi may be expected.
16. Vireosylvia olivacea (Linn.) Bonap. Red-eyed Vireo.—Very abundant at all the stations visited. Its clear, musical notes could be heard everywhere in the deep forests. A specimen was shot at Vicksburg, which had apparently just become fledged. Memphis, Vicksburg, Jackson.
17. Vireo noveboracensis (Gmel.) Bonap. White-eyed Vireo.—Specimens of this Vireo were obtained at Memphis and at Jackson.
18. Lanius ludovicianus Linn. Loggerhead Shrike.—A specimen of Shrike was seen at Jackson; but, as it was not shot, I am unable to say whether it belongs to this variety or to excubitorides.
19. Progne subis (Linn.) Baird. Purple Martin.—Common about Vicksburg.
20. Hirundo erythrogastra Bodd. Barn Swallow.—This species was observed to be quite common about Jackson together with the next.
21. Tachycineta bicolor (Vieill.) Caban. White-bellied Swallow.—Seen flying about the outskirts of Jackson.
22. Cotile riparia (Linn.) Boie. Bank Swallow.—Seen at various points along the Mississippi River near Memphis.
23. Pyranga æstiva (Linn.) Vieill. Summer Redbird.—A male of this species was shot at Memphis, another at Vicksburg, and a male and a female at Jackson. It is apparently a very common bird.
24. Spizella pusilla (Wils.) Bonap. Field Sparrow.—A single specimen of this species was shot at Jackson. Its occurrence there at that season was hardly to be expected. This individual may have been left behind in its winter quarters by its migrating comrades; or it may be that the species will be found to breed even as far south as Jackson.
25. Cardinalis virginianus (Briss.) Bonap. Cardinal Grosbeak.—One of the most conspicuous birds at every point visited.
26. Passerina cyanea (Linn.) Gray. Indigo Bunting.—The Indigo Bird was observed at Memphis, and again at Jackson.
9227. Passerina ciris (Linn.) Gray. Painted Bunting.—This beautiful bird was seen at the crossing of the Vicksburg and Meridian R. R. over the big Black River, and again at Jackson. Females were shot at both places, but the males eluded capture. They seem to be quite common.
28. Spiza americana (Gm.) Bonap. Black-throated Bunting.—Seen in the lowlands along the river in Louisiana opposite Vicksburg.
29. Agelæus phœniceus (Linn.) Vieill. Red-wing Blackbird.—Very abundant in the swamps in the vicinity of Vicksburg.
30. Sturnella magna (Linn.) Swains. Meadow Lark.—Not many were seen. One specimen at Vicksburg, and another along the railway while en route to Jackson.
31. Icterus spurius (Linn.) Bonap. Orchard Oriole.—Many of these were observed, and some shot, in Louisiana opposite Vicksburg.
32. Icterus galbula (Linn.) Coues. Baltimore Oriole.—Quite common at Memphis and at Vicksburg.
33. Quiscalus purpureus (Bartr.) Licht. Purple Grackle.—Common at Memphis and at Vicksburg.
34. Corvus frugivorus Bartr. Common Crow.—Seen at Memphis, Vicksburg, and at several intermediate points along the river.
35. Cyanocitta cristata (Linn.) Strickl. Blue Jay.—A common bird at Memphis and Vicksburg.
36. Tyrannus carolinensis (Linn.) Temm. Kingbird.—A very common bird at Memphis and Vicksburg.
37. Myiarchus crinitus (Linn.) Caban. Great Crested Flycatcher.—Seen at all points visited. Apparently more common than at the North.
38. Contopus virens (Linn.) Caban. Wood Pewee.—This bird was found to be quite common at Memphis and at Jackson.
39. Empidonax acadicus (Gmel.) Baird. Acadian Flycatcher.—A specimen was shot at Jackson.
40. Trochilus colubris Linn. Ruby-throated Hummingbird.—A single specimen was shot at Vicksburg.
41. Chætura pelasgica (Linn.) Baird. Chimney Swift.—Seen flying about at Jackson.
42. Chordeiles popetue (Vieill.) Baird. Night Hawk.—Observed at Jackson.
43. Campephilus principalis (Linn.) Gray. Ivory-billed Woodpecker.—No specimens of this species were seen, but their existence in the denser and less frequented forests in the neighborhood of Vicksburg and at other points, was confirmed by hunters and trappers. It is possible that the bird referred to here is the Logcock (Hylotomus pileatus), but as special mention was made by my informant, a professional hunter, of the white bill, I think the Ivory-billed Woodpecker must have been seen. Doubtless the other bird also occurs.
44. Picus pubescens Linn. Downy Woodpecker.—A single individual of this species was obtained at Vicksburg.
9345. Melanerpes erythrocephalus (Linn.) Sw. Red-headed Woodpecker.—This Woodpecker is apparently not so common as at the North, but it was observed at Memphis, Vicksburg, and Jackson.
46. Colaptes auratus (Linn.) Sw. Yellow-shafted Flicker.—A not uncommon bird about Vicksburg.
47. Ceryle alcyon (Linn.) Boie. Belted Kingfisher.—Quite common. Seen at Memphis and Vicksburg and intermediate points along the river.
48. Coccyzus americanus (Linn.) Bonap. Yellow-billed Cuckoo.—Apparently common. A specimen was secured at Vicksburg.
49. Conurus carolinensis (Linn.) Kuhl. Carolina Parakeet.—None were seen by ourselves. Inquiry concerning this rapidly disappearing species was made of various persons, and especially of hunters. It is still occasionally seen; but, for the most part, it maintains itself in the dense cane-brakes and forests, away from contact with man. I heard of its having been seen recently along the Mississippi River, about half-way down the state of Mississippi; also that it had been seen in southeastern Arkansas. A gentleman in Jackson stated that he had, within a year or two, seen a flock of Parakeets pass over that city. These items, together with the information obtained by Dr. F. W. Langdon, communicated in his recent paper, would indicate that this bird has not yet disappeared from the Mississippi Valley.
50. Scops asio (Linn.) Bonap. Little Screech Owl.—A single individual of this species, in the shabbiest of plumage, was shot along the Big Black River between Vicksburg and Jackson.
51. Buteo lineatus (Gm.) Jard. Red-shouldered Hawk.—A specimen of this hawk was shot and brought to me by a hunter at Jackson.
52. Cathartes aura (Linn.) Illig. Turkey Buzzard.—A common bird everywhere. Seen in great numbers at Jackson in company with the next.
53. Catharista atrata (Wils.) Less. Carrion Crow.—Not observed at any place but Jackson, although doubtless common everywhere. Readily distinguished from the Turkey Buzzard by its smaller size and its manner of flight.
54. Zenaidura carolinensis (Linn.) Bonap. Mourning Dove.—Common everywhere. Memphis, Vicksburg, Jackson.
55. Meleagris gallopavo americana (Bartr.) Coues. Wild Turkey.—None were seen, but hunters stated that they were quite abundant, even in the immediate vicinity of the city of Jackson. In the spring of 1880 I saw a fine gobbler that had been shot by a party of hunters in the pine woods of Kemper County, near the eastern border of the State.
56. Ortyx virginiana (Linn.) Bonap. Bob White.—The call notes of these birds were frequently heard as we passed down the river. At Vicksburg they appeared to be abundant in the bottom lands. We were extremely sorry that we could procure none of their skins.
57. Ardea herodias Linn. Great Blue Heron.—Several of these birds were seen flying about in the swamps near Vicksburg.
9458. Herodias alba egretta (Gmel.) Ridgw. American Egret.—A number of this snow-white species were observed in the swamps across the “lake” from Vicksburg. One was shot, and was found to have the long dorsal train of plumes.
59. Oxyechus vociferus (Linn.) Reich. Killdeer.—Observed only at Vicksburg. Will probably be found to breed here.
60. Philohela minor (Gmel.) Gray. American Woodcock.—One specimen was shot at Vicksburg.
61. Sterna antillarum (Less.) Coues. Least Tern.—This beautiful little Tern was very abundant on a sandy point across the “lake,” or old bend of the river, opposite Vicksburg. We were told that these birds lay their eggs on the bare sand, and that these eggs hatch in an extraordinarily short time.
Looking back on my first winter in the South I can recall no pleasanter experience than that of a stay of some four weeks at St. Mary’s, a town situated on the very border line of Southern Georgia. This place was then scarcely known to Northerners, although the crowded Florida steamers, on their way across Cumberland Sound, passed within sight of it and occasionally even touched at its wharf for some chance freight or a supply of fuel. But the village still retained a primitive quiet and simplicity that was all the more restful from its contrast with the bustling world outside. Now there are rumors of a railroad and daily trains from Savannah, with all the accompanying desecrations. It is a pity that the march of modern improvements cannot spare a few such peaceful spots, but the “levelling process” seems universal and inevitable.
A Northerner passing his first spring in the South will miss the marked distinction between the seasons upon which he has been accustomed to rely. The vegetation does indeed take a partial rest during the winter months, but it is checked rather than suppressed, and the reign of summer begins without that interval of preparation which we call spring. Most of the trees 95are evergreen, but some of them, curiously enough, assume bright autumn tints and cast their leaves in April. This at least is true of the live-oaks and magnolias: during my stay at St. Mary’s one of the latter, a remarkably fine tree which I often passed in my daily walks, was at one time nearly denuded, while the ground beneath was strewn with scarlet and orange-tinted leaves.
By the middle of April the fields and forests wore that mature appearance which we associate with August and early September. At noonday cicadas shrilled in the sultry woods, and crickets chirped all night long in the shrubbery about the house. Yet few birds had begun to nest, and many of the northern ones still lingered. I saw Yellow-rumped Warblers, Blue Yellow-backed Warblers and Cedar Birds nearly to the end of April, and a White-throated Sparrow as late as May 2. Many of the Blue Yellow-backed Warblers remained to breed, or rather were breeding, for long before this (on April 9) I had found a nearly finished nest. The local birds, however, did not mingle with the strangers, the former being found in pairs, and only where the trees were hung with Spanish moss; while the latter occurred in all kinds of timber, and in flocks made up largely of Redstarts, Kinglets, Black-poll Warblers and other northern species. The same was true of the Catbirds, Brown Thrushes, Pine Warblers, Towhees and several others. It was especially marked in the case of the Towhees, for the resident individuals belonged to a different and readily recognizable race.
One needed but to pass the boundaries of St. Mary’s to be fairly in the country, for the village had not then overflowed its limits, and the few outlying plantations were scarcely less wild and unkempt than the woods which surrounded them. One of my favorite haunts was the “Bay-gall” (I could never learn the origin of this name), a tract of swampy forest less than a quarter of a mile distant from the house at which we were staying. This place was sure to be alive with birds, and I rarely entered it without making some pleasing discovery. My first visit was on April 6, the day after our arrival. As I approached the woods a Red-bellied Woodpecker started from a solitary tree within a few feet of my head, and alighting at the base of one near by scrambled hurriedly up, dislodging the scales of loose bark in his ascent. He was immediately joined by his mate and 96the two began a game of hide-and-seek around the trunk and among the branches, uttering a rolling wor’r’r’roo very like that of a Flicker.
Forcing my way through the brambly outskirts, I entered the swamp and paused a moment to look around. Grand old wateroaks and sweet-gums thickly hung with Spanish moss cast a dense shade over the ground beneath, and the few sunbeams that struggled through flickered in the gloom like dying torches. There was little undergrowth, and the eye could penetrate far in every direction. In the branches above Blue Yellow-backed Warblers were singing incessantly, and occasionally the note of a Great-crested Flycatcher echoed sharply among the trees. There were other sounds; the rolling tapping of Woodpeckers, the shrill cry of the Blue Jay; and, from the clearing outside, pleasantly softened by distance, the songs of Mockingbirds and Cardinal Grosbeaks.
Passing deeper into the forest I came to an opening where the morning sun lay warm on a thicket of bushes that surrounded a shallow pool. Here I found an interesting little company of tired migrants resting after the fatigues of their last night’s journey and preparing for that still before them. There were six or eight Hooded Warblers, all males in full spring livery, a number of Worm-eating Warblers, a female Prothonotary Warbler, and several Ruby-crowned Kinglets and Redstarts. All were busily engaged in catching insects, but occasionally one of them would pause to sing a few notes in a listless undertone. The Prothonotary was the first that I had ever met with, and it was the only one that I saw at St. Mary’s. The Hooded and Worm-eating Warblers were common for a week or more afterwards, when all departed for some more northern breeding-ground.
During subsequent visits to the “Bay-gall” I met many interesting birds, several of which were new to me. Occasionally I would startle a Chuck-will’s-widow from its noonday slumbers on some mossy knoll, and if a chance shot through the leaves succeeded in stopping its erratic, bat-like flight, there was the pleasure of smoothing its soft plumage and admiring the rich brown coloring before consigning the bird to the paper wrapper that formed its temporary tomb. I believe I never shot one without indulging myself in this way. There is much to be learned, too, from the examination of a freshly-killed bird. For instance, 97I had never known the wonderful beauty of this Goat-sucker’s eye until I held the bird in my hand, and the size of its mouth would hardly be suspected from the examination of a dried skin.
On April 17 the Acadian Flycatchers arrived. I was first made aware of their presence by their emphatic queep’ éep which so closely resembled that of Traill’s Flycatcher that I immediately suspected the identity of the singers, although it was some time before I could get a sight at one. They had another note also which was much like the whistling of wings. I afterwards satisfied myself that this sound was a vocal one.
I never left the “Bay-gall” without reluctance in the days when I was perhaps the only invader of its secret recesses; and now, in recalling it, the feeling is scarcely less strong. But the country about St. Mary’s held other attractions which must not be neglected. The open space surrounding the town was bordered on the north by a pine forest that stretched an indefinite number of miles into the interior, and my walks often tended in this direction. Following some grass-grown road that wandered aimlessly among the trees, I often paused to watch the gambols of the Brown-headed Nuthatches which fairly swarmed in these woods. They are exceedingly social little birds, and it was no uncommon thing, even in the middle of their breeding season, to see five or six rollicking together. In their motions they closely resemble Sitta canadensis, and they have the same habit of exploring the ends of the pine branches and hanging head downward, like Titmice, among the tufts of pine needles. But they are decidedly more active, and their notes are shriller, more varied and altogether unlike those of either the Red or White-bellied species. Whick-whick-whee’e’e’ whick-whicker-whicker is the usual utterance, but when several come together their shrill excited piping altogether baffles description. These little companies were by no means wholly composed of Nuthatches, but usually included a more or less numerous escort of Pine Warblers, Bluebirds, Titmice and Woodpeckers. As the motley troop rambled through the woods, its members were continually chasing one another from tree to tree, chirping, calling and singing as their various moods dictated. I noticed that the Bluebirds usually led the van, while the Woodpeckers invariably brought up the rear.
Unlike the Red-bellied, Downy, Hairy and Golden-winged species, which inhabited all sorts of timber, the Red-cockaded 98Woodpecker was exclusively a bird of the pines. It was not common about St. Mary’s and I had difficulty in getting as many specimens as I wanted. Its notes to my ear almost exactly resembled those of Sitta pusilla. On the 1st of May I started a female from her nesting-hole, which was about thirty feet above the ground in a large and apparently perfectly sound pine. I was unable to climb the tree but the bird acted as if her eggs had already been laid.
The pine lands of the South have an open park-like character that is a continual surprise to one accustomed only to New England forests. The trees rarely stand in close proximity to one another, and they are often so widely scattered that the general effect is that of an opening rather than a forest. Unless a hummock interrupts the view, the eye may sometimes roam for half a mile in every direction over a perfectly level plain, interspersed with occasional trees whose tufted heads throw waving shadows upon the bright green beds of saw-palmetto that cover most of the ground beneath. Were it not for the half-wild cattle that range at will through the country, the palmetto would probably usurp every inch of ground; but these creatures keep it within reasonable limits, and many spaces of closely cropped grass and stunted blueberries intervene. About such places I used to find the Bachman’s Finch, a retiring little bird which might easily be overlooked by one unacquainted with its habit of skulking among the herbage and lying concealed until nearly trodden on. But no one with the slightest ear for bird music can long remain in ignorance of its presence after the breeding season has set in, for the male possesses vocal powers of a very rare order. His song is a prolonged, leisurely chant composed of several distinct bars or sets of notes, with brief pauses between, as if the bird stopped to take breath. The final notes of each bar have sometimes a rising, sometimes a falling, inflection, and the tone is varied in the most subtle manner. Now it has a full bell-like ring that seems to fill the air around; next it is soft and low and inexpressibly tender; now it is clear again, but so modulated that the sound seems to come from a great distance. The whole performance is very simple and I hardly know the secret of its charm. To be fully appreciated it should be heard in the soft twilight of an April evening, when the still woods are filled with dusky shadows. At such times it has moved me more deeply than I care to confess.
99The male always sings from an elevated perch, usually a dead twig close to the trunk of a southern pine. He sits perfectly motionless and is unaccountably hard to see. I have often stood directly beneath one for several minutes, vainly straining my eyes in the direction from whence the sound came, and perhaps finally discovered him within ten feet of my head in plain view. The ventriloquous character of many of his notes increases this difficulty. If disturbed in the midst of his song, he pitches to the ground beneath and at once seeks shelter in the grass.
Another characteristic inhabitant of these grassy openings was the Meadow Lark. It was much tamer than our northern bird, and its notes had a wild, ringing inflection that harmonized well with the surroundings.
In the thicker groves I often heard the voice of the Summer Tanager (Pyranga æstiva). His song is rich, flowing, and not unlike that of the Rose-breasted Grosbeak, although some of its notes recall those of the Robin. The call-note used by both sexes is a peculiar chuck’l-chuckl’ut. The bright colors of the male make him a conspicuous object among the branches of the southern pine which, at least in Georgia, is his favorite tree.
The Yellow-throated Warbler also was sure to be met with in these walks. His song to my ear has a far-a-way sound, even when the bird is near at hand. It is simple and monotonous, but nevertheless sweet and plaintive. This bird has all the habits of the Pine Warbler, with which it often associates.
A totally different phase of bird life was presented when, as was often the case, I visited the plantations. The fields themselves rarely offered anything more attractive than Yellow-winged Sparrows, Grass Finches and, late in April, migratory troops of Bobolinks that settled among the last year’s weeds for a moment before resuming their northward journey with rollicking snatches of song. But the fence corners and similar neglected places around the outskirts of the cultivated lands were filled with bushes over which trailed Cherokee roses, trumpet-vines and other luxuriant creepers. In these places I was sure to find Mockingbirds, Cardinals, Catbirds, Brown Thrushes, White-eyed Vireos and the brilliant little Painted Buntings.
Next to the always self-assertive Mockingbird the White-eyed Vireo was perhaps the most conspicuous inhabitant of such thickets. Not that he was often seen, but at almost any time of 100the day one might hear his emphatic, jerky little strain, coming from half a dozen points at once. I noticed that the note varied considerably from that which we hear in New England, and, moreover, scarcely two of the southern birds sang exactly alike. Some individuals even seemed to have a talent for mimicry. One that I remember imitated the note of the Loggerhead Shrike so closely that I was completely deceived. The nest of this bird is a wonderfully delicate and beautiful structure. One that I got at St. Mary’s contained its complement of four eggs on April 26. I discovered it twelve days previously when the birds were busily employed on the framework. The male took an equal part in this task and it was amusing to see him try to sing with his bill full of moss or bark.
The Painted Buntings or Nonpareils, as they are universally called by the townspeople, arrived April 23 and through the remainder of the month were abundant. I used to find them in flocks about the openings where they spent much of their time on the ground. They were timid rather than shy, flying to the thickets upon the slightest alarm, but when once conscious of being pursued, it was difficult to get a shot at one. The brilliant plumage of the adult male makes him a conspicuous object either on the ground or in green foliage, but it is no easy matter to see one among the flowers of the trumpet-vine where they often seek refuge, apparently fully conscious of the protection afforded by the clusters of scarlet blossoms. The young males during the first year are colored precisely like the females. They sing, and for aught I know, breed, while in this condition. The song is a low, pleasing warble very un-Finch-like in character. I should compare it to that of the Canada Flycatcher, but the notes are less emphatic, though equally disconnected. The bird almost invariably sings in the depths of some thicket, and its voice ceases at the slightest noise. Both sexes have a sharp chirp of alarm which closely resembles that of the Indigo Finch. Most of the Nonpareils left St. Mary’s by May 1, but a few pairs remained up to the time of my departure, when they were apparently preparing to breed. Another familiar inhabitant of these thickets was the Towhee Bunting. Two distinct races of this bird were to be met with during the same walk, but never, so far as my observation went, actually in company. The Red-eyed or northern form, erythrophthalmus proper, apparently occurred only as a winter 101visitor, while var. alleni represented the resident or local race. The latter was chiefly a bird of the oak scrub, although it was also to be found in open pine woods where it haunted the beds of saw-palmetto. Its note differed widely from that of erythrophthalmus; the “chewink” was shorter and harsher, and in addition to this cry, both sexes occasionally uttered a sharp, clear whistle that sounded like a sportsman’s call to his dog. I am not sure that I heard the song, or at least identified it. These Towhees were hard to obtain, for they were shy and retiring, rarely venturing far from their secure retreats. The irides of all the specimens that I examined were brownish-yellow or dull, opaque amber; never white, as is said to be the case with examples from Southern Florida.
It would be difficult to find a plantation in the South that did not have one or more pairs of Mockingbirds. About St. Mary’s they were especially abundant, and nowhere more so than in the gardens of the village. Here they were half-domesticated, building their nests in the shrubbery that surrounded the houses, and hopping about, like Robins, upon the grass-plats and gravelled walks. An orange tree directly in front of the windows of my room was appropriated by a remarkably fine singer. There is a noticeable difference in the performances of most males, but the voice of this bird possessed a compass and perfection of tone that I have never heard equalled. His repertoire included the notes of nearly all the birds of the surrounding region besides many of the characteristic village sounds, and most of the imitations were simply perfect. Moreover he was continually adding to his accomplishments. An interesting instance of this occurred one afternoon, when several of us were sitting on the veranda. A Greater Yellow-leg passing over the town was attracted by my answering whistle, and circled several times above the house reiterating his mellow call. The Mockingbird up to this time had been singing almost uninterruptedly, but at the sound of these strange notes he relapsed into silence and retreated into the thickest foliage of his favorite tree; after a while we heard him trying them in an undertone. The first note came pretty readily, but the falling inflection of the succeeding three troubled him. Whenever I ventured to prompt he would listen attentively, and at the next attempt show an evident improvement. Finally he abandoned the task, as we thought in 102despair, and at sunset that evening for the first and only time during my stay his voice was missing in the general chorus. But at daylight the next morning the garden rang with a perfect imitation of the Yellow-leg’s whistle. He had mastered it during the night, and ever afterwards it was his favorite part. The discomfiture of the rival males in the neighborhood was as amusing as it was unmistakable. Each in turn tried it, but not one of them succeeded.
Another frequenter of the village shrubbery was the Orchard Oriole. His flute-like voice, which bears some resemblance to that of the Fox Sparrow, could be heard almost any time after April 10. Our garden offered especial attractions to these Orioles, for the hedge of wild olive trees that bordered it on two sides was overrun with Cherokee roses and trumpet-vines among which they found a congenial shelter. They were fond, too, of sipping the honey from the trumpet-flowers, and it was no uncommon thing to see half a dozen collected about a single cluster. In this occupation they were almost invariably joined by numerous Hummingbirds;—and such a group, with its setting of green leaves and scarlet and white blossoms, formed the prettiest picture imaginable.
To our garden also came the Blue Jays; bold, familiar birds very different in bearing from the outcast that boys and would-be sportsmen pursue so relentlessly in the northern woods. Everywhere at the South this Jay is as much an inhabitant of the cultivated grounds as of the forests, and if not actually encouraged, it is universally tolerated. In Jacksonville I have heard them screaming among the live-oaks that shade the busiest streets, and at St. Mary’s they were scarcely less tame and confiding than the Mockingbirds.
The average Georgian is indifferent to the shooting of most of the birds that inhabit his plantation; but it is little short of a crime in his eyes to take the life of either a Turkey Buzzard or a Mockingbird. The killing of one of the former is considered an offence against the State, which protects them on account of their services as scavengers. But the Mockingbirds are treasured as personal property, and any interference with them is sure to be promptly resented. The natural result of this sentiment is that both species are universally abundant and familiar. The Buzzards, especially, are ubiquitous. At 103all hours of the day, in every kind of weather, they float over the cities, villages, plantations, pine woods, hummocks, cypress swamps, salt marshes and even the beaches of the Sea-islands. Go where you will, it is almost impossible to look upward without seeing the picturesque forms drifting about in the sky. Some are soaring almost beyond the reach of human vision. Others at a lower elevation cross and recross each other in interminable mazy lines; while still others glide across the landscape passing just above the tops of the trees. Both species occurred at St. Mary’s, but the Black Vulture was much the less common. It associated freely with the Turkey Buzzards, among which it could be recognized at almost any distance by its different color, shape and manner of flying. The tail is so short as to be altogether out of proportion with the body and wings, while its square tip gives it the appearance of having been cut off. This bird’s flight is heavy, awkward and generally straight forward, although it occasionally soars. The wings are flapped every few seconds in a hurried, nervous manner that seems to betoken a lack of power or confidence. The flight of the Turkey Buzzard, on the contrary, is a picture of repose in motion. The bird rarely moves its wings, save to alter their inclination, and its dark form drifts through miles of space without the slightest perceptible effort. The impression of entire freedom from exertion which its movements convey, is curiously in accord with the general enervating influence of southern life and its surroundings. Its impassive flight may perhaps be regarded as the most characteristic feature of a southern landscape, as it certainly is one of the most attractive. But the observer who would keep this impression untarnished will be wise to refrain from looking too closely into the useful side of the bird’s character.
The Buzzard’s flight will not bear comparison however with that of the Swallow-tailed Kite. The latter is equally easy and graceful of wing, and, in addition, its movements are characterized by a certain dash and energy of purpose that one looks for in vain in the calm, emotionless flight of the Vulture. I hardly know a more attractive sight than that presented by one of these Kites playing about an opening in the woods. For a moment it floats motionless, as if suspended by an invisible wire; the next, it glides close over the ground crossing and recrossing every yard 104of space. The long, thin wings, firmly set, cleave the air like knife-blades and the forked tail, spread to its fullest, is inclined to one side or the other as the bird changes its swift course. When it turns, the snowy head and breast are contrasted against the green background and its steel blue back glances in the sunlight. Finally rising to a level with the tree-tops it is gone as it came, like a beautiful vision.
But my space is exhausted, although many interesting birds remain to be mentioned. Perhaps at some future time I may take up the threads where this sketch leaves them.
1. Sialia sialis. Bluebird.—About the middle of March, 1877, Mr. Harold Gilbert saw one at Mount Pleasant, a suburb of St. John. Some time early in June, 1879, Mr. J. W. Banks saw one at Milledgeville, with food in its mouth, apparently for its young. On April 26, 1881, Mr. Henry Gilbert shot one at Rothesay, nine miles north of St. John.
2. Dendrœca pennsylvanica. Chestnut-sided Warbler.
3. Dendrœca castanea. Bay-breasted Warbler.
4. Dendrœca blackburnæ. Blackburn’s Warbler.—These three species are but rarely found here. In my note-book is a record of one of each taken during the summer of 1881, and I can learn of none others having been seen or heard.
5. Vireo noveboracensis. White-eyed Vireo.—Mr. Harold Gilbert shot one specimen of this bird at South Bay, a few miles northwest from St. John, on May 24, 1877, and this is the only known instance of its occurrence in this vicinity.
6. Pyranga rubra. Scarlet Tanager.—I saw an adult male of this species sitting on a fence in the suburbs of St. John on June 20, 1879, and have examined two specimens taken near Hampton during the summer of 1880.
7. Ammodromus caudacutus. Sharp-tailed Finch.—On June 21, 1881, five individuals of this species were taken by Mr. H. A. Purdie, Mr. Fred. W. Daniel and myself, on a marsh near Hampton. This marsh is watered by the Kenebecasis, a tributary of the St. John, and lies some twenty-five miles up the former river. The junction of the two rivers 105takes place about five miles from the mouth of the St. John. The marsh is some twenty miles, air line, from the nearest point on the Bay of Fundy shore, and at the time we visited it, the water running past it did not taste in the least brackish.[50]
8. Pipilo erythrophthalmus. Towhee.—A specimen, now in the collection of the Natural History Society of St. John, was shot at Irishtown on May 8, 1881, by Mr. J. Belyea.
9. Zamelodia ludoviciana. Rose-breasted Grosbeak.—I have examined the skin of one of this species taken near Hampton in June, 1879.
10. Passerina cyanea. Indigo Bunting.—There is a skin in the collection of James McGivern, Esq., said to have been taken about six miles north of St. John in June, 1880. I can learn of no other occurrence of this bird near here, though I have frequently seen specimens taken on the western, or Bay of Fundy shore of Nova Scotia.
11. Zenaidura carolinensis. Mourning Dove.—This bird has been but rarely met with here; one taken at Hampton in June, 1880, one at Rothesay on September 30, 1881, and one at Milkish on October 17, 1881, are the only specimens I have heard of.
12. Ardetta exilis. Least Bittern.—Between the spring of 1877 and the fall of 1880 there were five individuals of this species taken on the Bay of Fundy shore, about ten miles to the eastward of St. John.
13. Micropalama himantopus. Stilt Sandpiper.—The only known occurrence of this bird in this vicinity is of three seen by Mr. F. W. Daniel on the sand flats back of St. John on September 8, 1881. He secured one of them, which is now in the museum of the Natural History Society.
14. Recurvirostra americana. Avocet.—Mr. William Ellis of St. Martins, a village on the shore of the Bay of Fundy, says he has shot one or more of these birds each year for the last five years, usually meeting two together. A specimen taken by him in 1880 is in the museum of the Natural History Society.
15. Himantopus mexicanus. Black-necked Stilt.—I procured one of this species in September, 1880, from Mr. John Ellis of Mace’s Bay, an arm of the Bay of Fundy, lying some thirty miles to the westward of St. John, and was told by Mr. Ellis that several had been taken there during former years.
16. Ionornis martinica. Purple Gallinule.—Since obtaining the male, announced by Mr. Wm. Brewster in this Bulletin for July, 1881, I have had the good fortune to get possession of a female which was shot near Gagetown, a village on the St. John River, about forty miles from its mouth. The bird was taken in the early part of September, 1880.
17. Chen hyperboreus. Snow Goose.—One of these birds was taken at Gagetown in December, 1880, and sent by me to Mr. E. O. Damon of Northampton, Mass.
18. Anas boscas. Mallard.—A pair in the museum of the Natural History Society were shot near Hampton by the late Col. Otty some fifteen years ago. The only late occurrences of this species are of one mounted 106by J. H. Carnell, taxidermist, and a flock of some six or eight seen by Mr. Henry Gilbert on the Kenebecasis River in August, 1880, from which he obtained a male and female.
19. Æthyia vallisneria. Canvas-back.—Carnell has mounted one of this species taken within the Province, and E. C. Sutton, Esq., of Sutton, who is familiar with their appearance, saw a flock on the St. John River, about four miles from the city, several times during the fall of 1879.
20. Pelecanus erythrorhynchus. American White Pelican.—One of these birds in the collection of the Natural History Society was shot on the shore of the Gulf of St. Lawrence near Pt. du Chene by Mr. Robert Bustin, and I have very good authority for announcing the occurrence of another at Cape Spencer, some five miles east of St. John, during the first week in April, 1881.
During the spring of 1879 Messrs. W. A. Jeffries and J. Amory Jeffries spent some time at Grand Falls, New Brunswick, collecting and studying the birds of that neighborhood; and at about the same time Mr. J. Dwight, Jr., and myself were similarly engaged at Fort Fairfield, Aroostook Co., Maine.
Owing to the limited time of our stay the number of species observed was not large, but as it included almost all the commoner summer residents,—the species really characteristic of the fauna of the region—it has been thought worth while to lay the results of our observations before the public, especially as the fauna is in some respects peculiar. One might be led to expect, from the latitude of the region, that the fauna would be thoroughly Canadian in its character. Our experience shows, however, that it has a strong tinge of the Alleghanian.
Grand Falls is situated on the right bank of the St. John River at about N. Lat. 47° 03′, and W. Long. 67° 50′. The river below the falls runs through a narrow valley, almost all of which is under cultivation. On the higher land above the falls and about the town are farms devoted chiefly to hay, potatoes and buckwheat. The country is hilly, and is scantily watered, the few 107rapid streams and brooks draining directly into the river. There are no lakes or ponds, except a few insignificant puddles, although there are occasional cedar swamps and “barrens.” The tributary streams below the falls have cut narrow*, steeply walled ravines in their passage to the river. These were cold and damp, and apparently without birds.
In some places forests of hard woods exist, tall maples, elms and birches that have no doubt stood there for ages. There is but little underbrush in these woods, and they have a rather park-like aspect. The second growth and the woods on the low lands along the river consist of firs, spruces and hemlocks of all sizes, and often have an almost impenetrable underbrush. Where fires have spread large tracts are stripped of their woods, and are covered with fallen trunks overgrown with vines, with here and there tall dead “stubs” still standing.
Mr. W. A. Jeffries’ observations extended from May 21 to June 19. He was joined by his brother on the 9th of June. During the ten days following this latter date the weather was cold—there was a frost June 15, and rain fell every day except the 9th and the 15th.
Fort Fairfield is twenty miles south of Grand Falls. It is situated on the Aroostook River, about five miles in an air line west of its junction with the St. John. It is in a rolling country containing but few ponds and swamps, and watered merely by small brooks which empty into the Aroostook River. The river itself is broad, with a rapid current, and flows between banks which though not very high, are yet never swampy. Much of the original forest has been removed, especially in the neighborhood of the town and along the river, where the stretches of wooded land are interspersed with clearings, pastures and cultivated fields, large crops of buckwheat and potatoes being raised on the fertile soil. The woods are mostly evergreen—the several species of Abies and the arbor vitae—intermingled, of course, with a few yellow birches and an occasional maple, but few tracts being wholly covered by deciduous trees.
Our collecting was done mostly within two or three miles of the town. Our notes were made between June 14 and July 1. On our arrival we found the trees by no means in full leaf, and were told that the season was very backward, and had been very wet. Heavy frosts occurred on the 15th and 19th of the month.
108The nights were generally cold, the days warm—even hot during the latter part of our stay.
Fort Fairfield is 415 feet above the sea, and has a mean annual temperature of 38.11° F.
Through the kindness of Mr. H. A. Purdie I have been enabled to supplement our observations by extracts from some manuscript notes on the birds occurring at Houlton, Maine, made by Mr. Robert R. McLeod. These notes were written in 1877, and are based on his experience during a residence of four years at Houlton.
This town is in the southeastern part of Aroostook County, on the Meduxnekeag River about twelve miles from its junction with the St. John. It is forty-five miles south of Fort Fairfield, its Latitude being 46° 8′. I quote the following from Mr. McLeod’s notes: “The country round about is well watered with lakes and streams. Much of the land is under cultivation, but where it is not, the old forests are standing in great tracts of many miles in extent. The first snow falls about the 10th of November, and it generally remains on the fields till the middle of April, and in the woods until the last of May.”
At each locality several species, that would doubtless have been much more abundant had local conditions been favorable, were absent or represented by but few individuals.
All statements are given on the authority of both the observers at the locality to which they refer, except in some few cases, in which the initials of the observer are appended. All references to Houlton are, unless otherwise stated, on the authority of Mr. McLeod.
1. Turdus migratorius Linn. Robin.—Rather common at Fort Fairfield. At Grand Falls it was abundant everywhere.
2. Turdus fuscescens Steph. Wilson’s Thrush.—Rare at Grand Falls. On June 16 a nest with four fresh eggs was found on top of a stump. Not met with at Fort Fairfield. Mr. McLeod says that it appears at Houlton by May 15, and by the 10th of June becomes common. Breeds.
3. Turdus pallasi Caban. Hermit Thrush.—Common. One nest taken May 30 at Grand Falls was about three feet from the ground in a small fir tree.
4. Turdus ustulatus swainsoni (Caban.) Coues. Olive-backed Thrush.—Common at Grand Falls, especially in the hard woods and more open fir woods. At Fort Fairfield it appeared to be rather common, though seldom seen. Common and breeding at Houlton.
1095. Mimus carolinensis (Linn.) Gr.. Catbird.—At Houlton “very rare. A pair has bred in this vicinity each year since I have been here” (R. R. McL.). Not met with at Fort Fairfield or Grand Falls.
6. Sialia sialis (Linn.) Hald. Bluebird.—At Grand Falls they were frequently seen, as many as seven or eight in the course of a day. Apparently not common at Fort Fairfield. At Houlton “very rare,” one pair breeding.
7. Parus atricapillus Linn. Black-capped Chickadee.—At Grand Falls it was not uncommon. Some days four or five pairs would be seen, on others none at all. At Fort Fairfield it was not very common, though seen occasionally. At Houlton “very common.”
8. Parus hudsonicus Forst. Hudsonian Chickadee.—About half a dozen were seen at Grand Falls, mostly in hardwood brush or small woods. It was not seen at Fort Fairfield.
9. Sitta carolinensis Gmel. White-bellied Nuthatch.—Common in the hard woods at Grand Falls. Breeding.
10. Sitta canadensis Linn. Red-bellied Nuthatch.—One shot at Fort Fairfield. Both species are said to be common at Houlton.
11. Certhia familiaris Linn. Brown Creeper.—Seen occasionally at Fort Fairfield. Breeds. Rare at Grand Falls. “Common” at Houlton.
12. Troglodytes aëdon Vieill. House Wren.—At Grand Falls one pair was noticed which had a nest in the frame work of a barn.
13. Anorthura troglodytes hyemalis (Vieill.) Coues. Winter Wren.—This species is common at Houlton,[51] and no doubt occurs throughout this region; it was seen and heard at Grand Falls, but not observed at Fort Fairfield.
14. Mniotilta varia (Linn.) Vieill. Black-and-white Creeper.—We saw several at Fort Fairfield. Not seen at Grand Falls.
15. Helminthophaga ruficapilla (Wils.) Bd. Nashville Warbler.—Apparently not very common at Fort Fairfield. It was not observed at Grand Falls.
16. Dendrœca æstiva (Gmel.) Bd. Yellow Warbler.—Rather common at Fort Fairfield. Not met with at Grand Falls.
17. Dendrœca cærulescens (Linn.) Bd. Black-throated Blue Warbler.—Rather common at Fort Fairfield. At Grand Falls it was common in hard woods where the underbrush was thick.
18. Dendrœca coronata (Linn.) Gray. Yellow-rumped Warbler.—It was common at Fort Fairfield. At Grand Falls it was rare during May. All had left before the 9th of June.
19. Dendrœca maculosa (Gm.) Bd. Black-and-yellow Warbler.—Common.
20. Dendrœca pennsylvanica (Linn.) Bd. Chestnut-sided Warbler.—Common.
21. Dendrœca blackburnæ (Gm.) Bd. Blackburnian Warbler.—This bird was seldom seen while we were at Fort Fairfield, and was not met with at Grand Falls.
22. Dendrœca virens (Gm.) Bd. Black-throated Green Warbler.—Rather common at Fort Fairfield. Not met with at Grand Falls.
11023. Dendrœca tigrina (Gm.) Bd. Cape May Warbler.—I shot a male at Fort Fairfield, June 23, in a thick second growth of spruces on the edge of a path.
24. Siurus auricapillus (Linn.) Swains. Golden-crowned Thrush.—Rather common at Fort Fairfield. At Grand Falls it was seen only in the hard woods, where it was not common.
25. Siurus nævius (Bodd.) Coues. Water Thrush.—Breeding at Fort Fairfield, but not very common. It was not met with at Grand Falls.
26. Geothlypis philadelphia (Wils.) Bd. Mourning Warbler.—Common in suitable places. It was almost sure to be found in “burnt lots,” where the fallen trunks lay, half hidden by a luxuriant growth of tall weeds, or thickly overrun with vines. Under the shelter thus afforded they undoubtedly nested, safely screened from the most searching eyes.
27. Geothlypis trichas (Linn.) Caban. Maryland Yellow-throat.—Common.
28. Myiodioctes pusillus (Wils.) Bp. Wilson’s Black-cap.—At Fort Fairfield this bird was common. We usually found them in thickets of willow bushes, often in rather wet places. The birds were apt to go in companies of three or four or more. June 23 Mr. Dwight caught a young bird, just able to fly two or three yards at a time. The nest was no doubt close at hand, but the ground among the willow bushes was covered so deeply with brush that a diligent search for the nest showed nothing—except that it was not built in the bushes. The youngster showed in the most marked way the energy of disposition and restless activity that characterize the adults. The species was not common at Grand Falls.
29. Myiodioctes canadensis (Linn.) Aud. Canada Flycatching Warbler.—Rare at Grand Falls, but common at Fort Fairfield.
30. Setophaga ruticilla (Linn.) Swains. Redstart.—This species was exceedingly abundant at Grand Falls wherever there were hard woods. It was a common bird at Fort Fairfield.
31. Hirundo erythrogastra Bodd. Barn Swallow.—Common.
32. Tachycineta bicolor (Vieill.) Caban. White-bellied Swallow.—At Grand Falls it was common in suitable localities. None were seen about the town. It was abundant at Fort Fairfield.
33. Petrochelidon lunifrons (Say) Lawr. Eave Swallow.—Common at Grand Falls. Abundant at Fort Fairfield.
34. Cotile riparia (Linn.) Boie. Bank Swallow.—Common.
35. Progne subis (Linn.) Bd. Purple Martin.—Common, breeding in martin-houses at Fort Fairfield. This bird seems to be generally distributed throughout eastern Maine and the adjoining parts of New Brunswick, where there are settlements. While on our way to Fort Fairfield we noticed it at a number of places between Bangor and Woodstock, N. B., as well as at various points along the St. John River between Fredericton, N. B., and Fort Fairfield. It is also common at Houlton.
36. Ampelis cedrorum (Vieill.) Bd. Cedarbird.—It was not uncommon at Grand Falls. At Fort Fairfield we found it common.
11137. Vireo olivaceus (Linn.) Vieill. Red-eyed Vireo.—Common.
38. Vireo philadelphicus Cass. Philadelphia Vireo.—Taken only at Grand Falls in May, singing in the hard woods.
39. Vireo solitarius Vieill. Solitary Vireo.—This species was apparently not very common at Fort Fairfield. It was not seen at Grand Falls. Mr. McLeod gives it in his notes as “quite common” at Houlton.
40. Pyranga rubra (Linn.) Vieill. Scarlet Tanager.—Not rare in the hard woods at Grand Falls. The people there call them “war-birds.” We did not see them at Fort Fairfield, though we have reason to think that they occur. At Houlton Mr. McLeod says they are “rare. They arrive May 29. I have not found the nest, but have a young one taken here. They remain all summer.”
Dr. Coues’ New Check List and Dictionary.[52]—Judging from advance sheets lately received, this new treatise by Dr. Coues will occupy a previously unclaimed place among ornithological works; for, as its title indicates, it is much more than a catalogue of North American birds. Its novel feature is a dictionary of etymology, orthography and orthoëpy of scientific names, to which is devoted the lower portion of each page of the running list. In this department the generic, specific and varietal names—duplicated from the text above with the addition of the diacritical marks for quantities, accents and division of syllables—are exhaustively treated; their derivation and meaning being explained, their construction scrutinized, their spelling revised, and their applicability in each particular case carefully considered. The erudition and scholarly research involved in this undertaking must be apparent to the most casual reader. The practical value of the work is equally plain, and perhaps it is not too much to say that it calls for a fuller measure of gratitude on the part of ornithologists than anything which its versatile author has hitherto produced.
A detailed consideration of the Check List proper must necessarily be deferred until the appearance of the complete work; pending this, we may simply say that the plan followed by Dr. Coues is essentially to make a second edition of his original list, with all the required additions and corrections to date, and such revision of nomenclature as seemed desirable 112and practicable. Ten species are subtracted, and one hundred and twenty added, while names are changed for various reasons in probably more than a hundred cases. A simple system of reference numbers forms a concordance of the present and original edition, as well as with Baird’s list of 1858 and Ridgway’s of 1880. The total number of species and varieties enumerated is eight hundred and eighty-eight.
It should be mentioned that the introductory portion of the work includes an analysis of the present list as compared with that of 1874, and an important chapter entitled “Remarks on the use of names.” The latter is devoted to a general consideration of the technique of Greek and Latin scientific names and the principles governing their derivation, spelling and pronunciation.
The book ends with a catalogue complete to date of the author’s ornithological publications. We understand that the edition will be offered to the public before the close of the present month. May it meet with the cordial reception which it so richly merits.—W. B.
Nests and Eggs of Ohio Birds.—It is always a pleasure to record the progress of this notably meritorious work—a pleasure which we trust will be ours until the completion of the design which the authors have thus far carried out so successfully. As we have before remarked, there has been nothing since Audubon in the way of pictorial illustration of American Ornithology to compare with the present work—nothing to claim the union of an equal degree of artistic skill and scientific accuracy. We have no knowledge of the financial aspects of the case; but, as such a work is necessarily expensive, we can only trust that it continues to receive the support it so richly deserves. It is, we believe, sold only by subscription. The last number which has reached us is a double one, being parts 10 and 11, dating Oct. 1881 and Jan. 1882, containing Plates XXVIII-XXXIII, and pages 107–118. Plate 18 is perhaps the first in which the authors have introduced a bird—being the head of the Purple Martin protruding from the orifice of the C gourds so frequently put up in the South for its accommodation. This figure shows that Mrs. Jones can draw and paint a bird as well as its nest and eggs—and we should not be surprised if other birds appeared with their nests in future numbers. The temptation thus to enlarge upon the original plan of the work must be at times almost irresistible. Plate 29 is Euspiza americana, the simple nest of which gives less scope for the artist’s skill than the elaborately finished surroundings of the Song Sparrow’s nest of Plate 30. The extremes of size and coloring of the eggs of Melospiza are well portrayed, as are those of the Thrasher, the rough exterior of whose nest fairly bristles on Plate 31. One of the most artistic pictures of the whole series is the lowly nest of Helminthophaga pinus (Plate 32), with its characteristic surroundings at the foot of a slight bush clump. It is interesting to note in this case the curious “protective mimicry” by which the nest resembles a bunch of dead leaves and dried bark strips blown and caught among the roots of a bramble. One would have sharp eyes who would 113at first glance see it was something else. The last plate (33) represents the nest of the Summer Tanager, furnishing a good illustration of a “saddled” nest—by which we mean one placed directly upon a large horizontal bough, only confined by a few slight upright twigs. The text consists, as usually heretofore, of a folio to each plate, and continues to be prepared by Dr. Howard E. Jones. We find it to be a perfectly reliable account of the objects represented. The authors evidently have spared no pains or expense in maintaining the high standard of excellence they set for themselves at the beginning.—E. C.
Prof. Macoun’s Report of Exploration.[53]—We hear so seldom from our friends of the Dominion, as far as ornithology is concerned, that the present contribution would be welcome as an index of their activity, even were it of less importance than we find it to be. It is difficult to cite the brochure correctly, as it has no title-page and bears no date or place of publication, and may be an “extra” of a portion of some more extensive government publication. However this may be, the pamphlet which reaches us through Professor Macoun’s kind attentions is the report of the Surveyor General to the Minister of the Interior, consisting chiefly (pp. 8–40) of Professor Macoun’s own report of his explorations during the summer of 1880 of that portion of the Souris River Valley lying within British Territory and of the adjoining region to the west and north—that is to say, north of our territories of Dakota and Montana. The region is one seldom examined even incidentally in the interests of ornithology, and the present paper possesses decided value, as the observer appeared to have paid special attention to the distribution of birds in the wide area traversed. After a résumé of the leading ornithological features of the region is presented an annotated list of the species secured, 109 in number. This list may be profitably examined in connection with the article on the birds observed along the parallel of 49° by the Northern Boundary Commission in 1873 and 1874. We feel at liberty to call attention to some manuscript alterations made by the author in our copy. For Coturniculus passerinus read Zonotrichia albicollis; for Myiarchus crinitus, read Tyrannus verticalis; for Archibuteo lagopus, read A. ferrugineus, the range of which is thus carried beyond any point hitherto given; for Tringa canutus read T. bairdi; for Podilymbus podiceps, read Podiceps californicus. We could wish the report were better printed; but poor presswork is the usual fate of public documents, English or American.—E. C.
Knowlton’s Revised List of the Birds of Brandon, Vermont.[54]—This is a briefly annotated list of 149 species occurring in the immediate 114vicinity of Brandon. The author says: “A few more species doubtless occur, especially among the Waders and Swimmers, but as they have never been actually noted, they have been rigidly excluded.” An examination of the List shows that, with perhaps one or two exceptions, he has succeeded in adhering to this principle, the result being a very reliable list as far as it goes. The further application of this rule doubtless accounts for the fact that many of the species are not stated to breed that yet no doubt do so.
The chief interest of the List lies in its bearing upon the extent of the Alleghanian fauna in the Champlain valley. The breeding of such species as Dendrœca striata and Zonotrichia leucophrys, the occurrence of Perisoreus canadensis and Picoides arcticus, and the absence of Ortyx virginiana and one or two other species, are almost the only exceptions to an otherwise strictly Alleghanian fauna.
A number of species, especially among the migrants, would seem, from what the writer says, to be by no means numerous at this locality, and no doubt his statements are strictly in accordance with his experience. We have reason to believe, however, that a more thorough search might reveal greater numbers of some of these species.
It is to be regretted that Mr. Knowlton’s List could not have appeared elsewhere than in the columns of a newspaper, both for the sake of giving it a more permanent form, and of avoiding the typographical errors inevitable under such circumstances. It may be worth while here to mention that by a slip of the pen Mr. Knowlton has recorded Wilson’s Plover (Ochthodromus wilsonius) instead of Wilson’s Snipe.—C. F. B.
Krukenberg on the Coloring Matter of Feathers.[55]—This paper, the first of a series, seems to be the product of more careful work than previous publications on the subject. The author first states positively that the color may change after growth, the feather becoming lighter or darker as the case may be, but postpones deciding whether the change is the result of external or internal causes. Judging from the effects of stimulants upon Canaries with fully grown feathers, I have no doubt that internal changes play an important part. At least, almost white Canaries will become very yellow, gray sometimes appearing, if properly fed.
Turacin, a red or purple-violet pigment, found in the feathers of the Musophagidæ is first considered. Attention was first called to this pigment by Verreaux, who found that the purple-violet in the wing feathers of Corythaix albicristatus was destroyed by wetting, but returned on drying. Later it was observed that the water in which these birds bathed became colored dark red. Facts worthy of consideration by all systematic ornithologists. Turacin is soluble in weak alkalies, insoluble in acids, and slightly soluble in water, especially if warm. It may be precipitated as an amorphous red powder by the action of acids. In solution the 115spectrum of Turacin is marked by two absorption bands, between D and E, much resembling those of oxyhemoglobin. Carbon dioxide and oxygen, however, have no effect on the color or the spectrum. As to its chemical composition the author differs from his predecessors in that he denies the presence of nitrogen, though copper and iron are both present in considerable quantities. By the action of concentrated sulphuric acid two products are formed, named α Turaceïn and β Turaceïn by the author.
Zoönerythrin, another red pigment of much wider distribution, is found in red feathers, as those of the Flamingo and the Cardinal Grosbeak. It is soluble in alcohol, ether, bisulphide of carbon, and the like, from which it can be precipitated by evaporation. The solution of this pigment is often favored by first digesting the feather in a trypsin or pepsin solution. Unlike Turacin, Zoönerythrin has no absorption bands, but all is absorbed beyond E.
Zoöfulvin, a yellow pigment of much the same solubility as the preceding, occurs in the yellow feathers of the European Oriole, the Canary, and the like. The spectrum has two bands between F and G which vary in position according to the solvent used.
As yet Dr. Krukenberg has been unable to extract any green, blue, or purple pigment from feathers, so that he agrees with Bogdanon that blue feathers have no pigment as proved by transmitted light. Of this any one can at once convince himself by holding the feather of a Bluebird immersed in water between himself and a window.—J. Amory Jeffries.
Minor Ornithological Papers.—161. The Ruddy Duck (Erismatura rubida). By Spencer Trotter, Chicago Field, Vol. XIII, p. 23.—Brief general account, including reference to their occasional great abundance in Chesapeake Bay.
162. Bibliographical Manuals of American Naturalists. Chapter II. Dr. Elliott Coues, U. S. A. By William Hosea Ballou. Ibid., XIII, pp. 92, 103, 123, 189, 205, 221.—Rather more than 400 titles of papers and works, relating mainly to ornithology.
163. Nomenclature of the North American Grouse. By Spencer Trotter. Ibid., XIII. pp. 314, 315.—Common and scientific names of North American Grouse, with their principal synonymy and habitats.
163. The California Quails in Missouri. By H. Clay Ewing. Ibid. XIII, p. 413.—Six or seven pairs, turned out near the junction of the Missouri and Osage Rivers in March 1879, raised broods the following season near where they were liberated.
164. Bibliographical Manual of American Naturalists Chapter III. The Literature of Prof. Edward D. Cope. By Wm. Hosea Ballou. Ibid. XIV, pp. 19, 20.—Contains a few ornithological titles.
165. Can the Pinnated Grouse be successfully propagated? By H. W. Merrill. Forest and Stream, XVI, Feb. 10, 1881, p. 28.—Believes they can be “successfully propagated” with proper “regard to cover, food and range.”
166. Pine Grosbeak (Pinicola enucleator, L., V.) and Robin (Turdus migratorius, L.) in Winter [in Nova Scotia]. By. J. Matthews Jones. 116Ibid., XVI. March 13, 1881, p. 86.—The former “quite common”; small flocks of the latter frequent the spruce woods every winter, in Point Pleasant Park, Halifax peninsula.
167. The “Crane’s Back.” By J. C. Merrill. Ibid., XVI, March 10, 1881, p. 105.—A Cree Indian account of the napite-shu-utle, a bird said to migrate by taking passage on the backs of Cranes. The bird is believed to be a Grebe.
168. A Hawk new to the United States. By Robert Ridgway. Ibid., XVI, Apr. 14, 1881, 206.—From Oyster Bay, Fla., provisionally referred to Buteo fuliginosus. (See this Bull., VI, Oct. 1881, p. 207.)
169. The Pine Grosbeak. By Chas. E. Ingalls. Ibid., XVI. Apr. 14, 1881, pp. 206, 207.—Observations on its habits in winter in Massachusetts.
170. Our unique Spoon-billed Sandpiper, Eurinorhynchus pygmæus (Linn.). By Tarleton H. Bean. Ibid., XVI, Apr. 21. 1881, p. 225.—Brief general history of the species, with record of its capture at Plover Bay, Eastern Siberia, and Point Barrow, Alaska.
171. Domesticated Quail. By Henry Benbrook. Ibid., XVI, May 5, 1881, p. 266.—Ortyx virginianus successfully reared in captivity to the third generation. Believes that under favorable circumstances they could be bred “as easily as Turkeys.”
172. Great Carolina Wren. By William Dutcher. Ibid., XVI, July 14, 1881, p. 473.—Record of its capture at Greenville, N. J., within four miles of New York City.
173. The Rail we shoot. [By George B. Grinnell.] Ibid.. XVII, Sept. 22, 1881, pp. 146, 147.—Classification, diagnoses and habitats of the Rallidæ of the United States.
174. Range and Rotary Movements of Limicolæ. By W. Hapgood. Ibid., XVII, Oct. 20, 1881, pp. 225–228.—An important and suggestive paper on the migrations and range of American Limicolæ. The greater part of the species of this group are noticed at length. The paper relates especially to the winter haunts of these birds, and the conclusion is pretty fairly sustained that many of them pass beyond the tropics to winter in the Southern Hemisphere.
175. Migration of Shore Birds. By M. H. Simons. Ibid., XVII, Nov. 10, 1881, p. 288.—Apropos of Mr. Hapgood’s paper (see No. 174). the writer calls attention to the fact that many kinds of Shore Birds winter in Florida and the other Gulf States. “Didymus.” under the same caption, has some pertinent suggestions in reference to Mr. Hapgood’s paper.
176. The Herring Gull and the Ring-bill on Georgian Bay. By Rev. J. A. Langille. Ibid., XVII, Nov. 17, 1881, p. 307.—On the habits, etc., of these species at their breeding haunts in Georgian Bay.
177. Beechnuts and Woodpeckers. By C. Hart Merriam, M.D. Ibid., XVII, Dec. 1, 1881, p. 347.—A reply to several pseudonymous articles in previous numbers of this journal (Forest and Stream) in reference to the Red-headed Woodpecker’s habit of eating beechnuts. Other notes on the same subject, by various contributors, follow in this and succeeding numbers.
117178. The Enemies of Game Birds. By Adolphe B. Covert [and others]. Ibid., XVII, Dec. 8, 1881, p. 366, Dec. 22, p. 407, and Dec. 29, p. 428.—Various enemies are mentioned, among whom the Red Squirrel is prominent.
179. Habits of Woodpeckers. By W. Beeke [and others]. Ibid., XVII, Dec. 15, 1881, p. 387.—In reference to their laying up stores of beechnuts for winter use, particularly refers to the Red-headed Woodpecker.
180. Inquiries about the Snow Grouse [lege Goose]. By William Dutcher. Ibid., XVII, Dec. 22, 1881, p. 407.—In reference to the distribution of Anser hyperboreus on the Atlantic coast, and to the change of plumage in the Blue Goose (A. cærulescens) in captivity.
181. The Sparrow Curse in Australia. Ibid., XVII, Dec. 22, 1881, pp. 407, 408.—Abstract of a “progress report” of a government commission appointed to investigate “alleged injuries caused to fruit growers, gardeners, farmers and others by [the imported] Sparrows.” The analysis of the testimony taken is suggestive reading in its bearing upon the “Sparrow Pest” of our own country.
182. The Snow Goose and Blue Goose. By C. S. Wescott. Ibid., XVII, Jan. 5, 1882, p. 447.—Respecting their specific diversity, and on the occurrence of the Snow Goose in Delaware Bay. This is followed by a communication (under the same caption) from Arthur Edward Brown, who states that seven Blue Geese have lived seven years in the Philadelphia Zoölogical Garden without showing any material change of color.
183. Der Schwalbenweih (Nauclerus forficatus). Von H. Nehrling. Ornithologisches Centralblatt, VI. No. 2, 15 Jan. 1881, pp. 9, 10.—Account of its habits, etc., as observed in Texas.
184. Der Gelbkopfstärling oder Gelbkopftrupial (Xanthocephalus icterocephalus Baird). Von H. Nehrling. Ibid., VI, No. 11. 1 Juni, 1881, pp. 81–84, No. 13, 1 Juli, 1881, pp. 97, 98.—General history.
185. Die Wandertaube [Ectopistes migratorius]. Von Chas L. Mann. Ibid., VI, No. 21, 1 Nov. 1881, pp. 164–166. (Aus: Jahresber. des Naturhist. Vereins in Wisconsin 1880–81.)—On the great numbers destroyed by pigeon hunters for the market. Contains interesting statistics of the slaughter and the manner in which it is prosecuted.
186. Zwei amerikanische Prairiefinken. Von H. Nehrling. Monatsschrift des Deutschen Vereins zum Schuke der Vogelwelt, VI Jahrg., No. 3, März, 1881, pp. 58–64.—General account of the “Lerchenfink (Chondestes grammica Bp.)” and the “Savannenfink (Passerculus savanna Bp.).”
187. Ornithologische Beobachtungen aus Texas. II. Von H. Nehrling. Ibid., VI, No. 5, Mai, 1881, pp. 111–121. (See this Bulletin, VI, p. 109.)
188. Nordamerikanische Vögel im Freileben geschildert. Von H. Nehrling. Die gefiederte Welt. Zeitschrift für Vogelliebhaber, -Zuchter und -Händler, X Jahrg., 1881.—Under this title Dr. Nehrling contributes a series of well-written popular articles on various North American birds. In the present volume are the following: (1) Das Rubingoldhähnchen (Regulus calendula Lichtst.), l. c. pp. 14–16, 24–26. (2) Der blauköpfige 118oder Brewer’s Stärling, Scolecophagus Breweri, Nehrl. (S. cyanocephalus Cab....) pp. 44–46, 57, 58. (3) Der Kentuckysänger oder Buschsänger (Sylvia-Opornis [sic.]—formosa Wils. ...), pp. 100–102. (4) Die Einfiedlerdrossel (Turdus Pallasi Cab. ...), pp. 173, 174. (5) Der Gold- oder Kukukspecht (Colaptes auratus Swns. ...), pp. 228–230, 240, 241, 251–253, 265, 266. (6) Der Scherentyrann, Scheren- oder Gabelschwanz (Milvulus forficatus, Swains. ...), pp. 325, 326, 333–335. (7) Der blaugraue Fliegenfänger oder Mückenfänger (Polioptila cærulea Scl.), pp. 368–370, 380, 381, 393. (8) Der Satrap oder das Gelbkrongoldhähnchen (Regulus satrapa, Lichsts. ...), pp. 435, 436. (9) Die Bergdrossel (Oreoscoptes montanus Brd. ...), pp. 528–530.
189. Rocky Mountains-Hüttensänger oder Steinschmätzer (... Sialia arctica Swns.) Eine Vogelstudie aus den Felsingebergen. Von Fr. Trefz. Ibid., p. 81.
Description of a Nest of the Water Ouzel.—The nest of the Water Ouzel (Cinclus mexicanus) is perhaps not so well known as to make the following description of one wholly uninteresting. The nest when found was in good condition, and had evidently been used the past season. It was built under a slightly overhanging wall of limestone, on a ledge projecting seven or eight inches from the wall, and about four feet above low-water mark, the deepest part of a swift mountain stream flowing directly beneath. The material of construction was a bright green moss, forming a rather conspicuous object for some distance along the opposite bank. The nest has a nearly spherical interior seven inches in diameter. The entrance is triangular, one side of the triangle forming the top and being three and one-half inches across and three inches above the lower angle. The most exposed side of the nest varies from three to four inches in thickness, the top and remainder being only an inch and a half through. At time of finding, the interior of the nest was perfectly clean, but outside, just below the opening, the rock was discolored for some distance by excrement of the birds. Side by side with this nest was an older one partially destroyed, and I fancied I could see traces of still another on the same ledge not far off. The birds had evidently lived in the locality for some time.—R. S. Williams, Gold Run, Montana.
The Short-billed Marsh Wren in New Hampshire.—On the 24th of August, 1881, while investigating the recesses of a fresh water marsh at Rye Beach, N. H., I found a colony of Short-billed Marsh Wrens (Cistothorus stellaris) in a small meadow about a mile from the sea. One bird was shot, and five or six others seen and heard.
119Mr. Wm. Brewster in 1872 found this bird in the same vicinity, but in a locality about five miles farther inland.
These two records extend the northern range of the Short-billed Marsh Wren, and give it a place among the birds of New Hampshire.—Henry M. Spelman, Cambridge, Mass.
Early Arrival of the Yellow-rump in Southern Maine.—This morning—March 21, 1882—I found a solitary Yellow-rumped Warbler (Dendræca coronata) flitting about in a straggling growth of spruces, on Cape Elizabeth. His arrival is unprecedentedly early for this vicinity. The Yellow-rumps usually reach Portland in the last week of April, sometimes not until after May 1, and up to to-day I have never seen one before April 21, which was the date of their appearance in 1879. My little friend of this morning was probably only an accidental and temporary visitor. Snow still lies from two to three feet deep in the woods, and much blustering, wintry weather must be expected, before the earliest Warblers come to us in earnest.—Nathan Clifford Brown, Portland, Maine.
Late Stay (probable Wintering) of Dendrœca pinus in Massachusetts.—A few individuals of the Pine-creeping Warbler remained so late with us the last season, that their courage deserves a record. I found four of them on December 5, 1881, in company with Chickadees, in a rocky run thickly set with maples and alders. There were no pines, but a small bunch of them not far away. I shot one, according to rule, to make sure of the species. Being desirous of ascertaining if they proposed to spend the winter in that cheerful company, on January 1, 1882, I sent a young friend, who is well posted and a good observer, to the locality, and he reported seeing two of the Warblers so near at hand, perhaps twenty feet, as to make the identification positive. I intended to look for them again in February, but was unable to do so.—F. C. Browne, Framingham, Mass.
The Hooded Warbler in Western New York.—From various points in the dense forest, on the balmy days of May, comes the common and familiar song of the Hooded Warbler,—che-reek, che-reek, che-reek, chi-dì-eê, the first three notes with a loud bell-like ring, and the rest in very much accelerated time, and with the falling inflection. Arriving early in May, this is one of our common summer residents throughout the dense upland forests, occupying the lower story of the woodland home, while the Cœrulean Warbler occupies the upper. Here let me say that in addition to its alarm note, a sharp whistling or metallic chip which is very clearly characterized, the Hooded Warbler has two distinct songs, as different as if coming from different species. Never shall I forget how I was once puzzled by this trick. I was strolling in a thick forest, near the corner of a slashing, in an evening twilight in June, when I was surprised by a strange whistling melody.—whee-reeh, whee-ree-eeh—with 120a marked emphasis on the second syllable, and a still more marked one on the last. Part of the time this utterance was somewhat varied, a few notes being sometimes added, and again a few being dropped. My curiosity was greatly excited, for I had supposed myself familiar with the voices of all the birds in the neighborhood; but it became too dark to identify the bird. For nearly a week I went to that spot every day, always hearing the song, but never being able to get a clear sight of the bird. It seemed exceedingly shy. In vain did I crawl on hands and knees among the undergrowth to get near to it; for just as I would seem about to gain a good view of it the song would cease at the point under observation and come from one more distant. Just as I was about to give the matter up one evening, down came the singer, stage by stage through the thick foliage, and alighting within a few feet of me and in clear sight, gave the full effect of his whistling song. I have since heard the same song a number of times and in different places from the Hooded Warbler. So I conclude that in the case of this species there are, occasionally at least, two distinct and altogether different songs.
The Hooded Warbler is one of those which make their home on or near the ground. Here it keeps itself for the most part well concealed among the foliage of the thick undergrowth, having a rather slow and dignified movement for a bird of its kind.
It builds its nest from a foot to eighteen inches from the ground, generally in the upright or somewhat leaning fork of a little bush. I once found it on a beech limb, lying on the ground, but still retaining the dry leaves. It is somewhat bulky, but quite neat, the lower part being of dry or skeleton leaves, the upper part, especially the high and well-defined rim, of long fibrous bark, as that of the grape vine, ash, basswood, or elm, laid almost as nicely as coiled cords, the whole structure being bound together by a webby material, and lined with fine grasses, bark-fibres, and horse-hair. In location, material, and structure, it is quite unique, and, like most other birds’ nests, is a much more certain means of identification than the eggs themselves. These, two to four in number, varying from .63×.52 to .75×.50, are clear white, delicately specked and spotted, sometimes even blotched, with reddish, brown, and lilac. In form and coloration the eggs are very variable. They may be found fresh from the last week in May till the middle of June. A second set may be found in July. The male aids in incubation.
Confined to the eastern part of the United States, and barely entering the southern part of New England, Western New York, and Central New York where it is quite common, must be about the northern limit of this species.—J. H. Langille, Knowlesville, Orleans Co., N. Y.
Breeding of the Pine Grosbeak (Pinicola enucleator) in Lower Canada.—Last summer I had the rare good fortune to accompany, as his guest, the Hon. Judge H. E. Taschereau (Chief Justice Supreme Court of Canada) on his annual salmon fishing excursion to the Godbout River, which empties into the St. Lawrence from the north, about six miles from the Pointe des Monts where the river widens into the Gulf.
121One rainy afternoon about the middle of July, while the Judge was catching salmon at the famous “Upper Pool” on the Godbout, Mr. Nap. A. Comeau and I climbed a high and densely wooded hill that rises from the western border of the pool, and when near the summit saw a Pine Grosbeak, in the slate and golden plumage, hopping about amongst the branches of a large Balsam (Abies balsamea). I was within twenty feet from the bird, but having only a rifle was unable to secure it. Mr. Comeau, who lives at the mouth of the Godbout, told me that this species was by no means rare here, and that he regarded it as a resident. He has since written me that he shot several after I left, and that “the bird is quite common here both summer and winter.” Although he has never taken its nest, he says “I have no doubt they breed here, and I have often seen them in the early part of the fall while out trapping. They seem to be fond of keeping near streams and lakes.”
Dr. Coues found the Pine Bullfinch breeding on the Labrador Coast, and I have no doubt that it breeds all along the north shore of the Gulf of St. Lawrence, and perhaps extends even as far west as the Saguenay, along the north shore of the St. Lawrence River. It is asserted, on high authority, that it breeds in some parts of Northern New England.—C. Hart Merriam, M.D., Locust Grove, N. Y.
Coturniculus lecontei, C. henslowi, and Cistothorus stellaris in Florida.—Mr. C. J. Maynard has kindly placed at my disposal the following notes made during his recent trip to Florida. In November, 1881, he spent three weeks collecting at Rosewood, a small settlement on the northern edge of the Gulf Hummock, about eighteen miles northeast of Cedar Keys. Around the outskirts of this town were a number of old fields, grown up to rank grass and tall weeds, but nevertheless perfectly dry. Here he found Leconte’s Buntings, Henslow’s Buntings, Yellow-winged Sparrows, and Short-billed Marsh Wrens, associating together in comparative numbers ranking in the order in which their names are mentioned. The first C. lecontei was shot November 4. Shortly afterwards they became so abundant that as many as twenty were sometimes seen in a day, but notwithstanding their numbers, it was by no means easy to obtain specimens. The chief difficulty arose from their excessive tameness, for they could rarely be forced to take wing, while in the long grass it was impossible to see them at a greater distance than a few yards. Indeed so very fearless were they that on several occasions Mr. Maynard nearly caught them in his insect net. All four species were apparently established for the winter.
The detection of Leconte’s Bunting at Coosada, Alabama, by Mr. Brown,[56] and more recently in Chester County, South Carolina, by Mr. Loomis,[57] has prepared us to expect it almost anywhere in the Southern States, but I believe that this is its first Florida record. The occurrence 122of Henslow’s Bunting is also of importance, as confirming Audubon’s more or less discredited statement that it wintered numerously in Florida; while that of the Short-billed Marsh Wren is interesting from the exceptional character of the locality and the distinguished society in which the little bird was found.—William Brewster, Cambridge, Mass.
Ammodramus caudacutus.—A somewhat inland Record on the Atlantic Coast.—On June 21, 1881, in company with my friends Messrs. Chamberlain and Daniel, of St. John. N. B., I found a few pairs of Sharp-tailed Finches in the tall grassy marshes bordering the Kenebecasis River at Hampton, which is about twenty miles to the north of the above named city and the Bay of Fundy, and about at the head of tide water. The birds were singing, and undoubtedly breeding, but a severe hunt for their nests was unsuccessful. Although a closely allied variety (nelsoni) is known to occur in certain western States, I think our maritime form has not before been observed away from the immediate coast on the Atlantic seaboard. It might however be looked for up our rivers and creeks as far as or a little above the flow of tide water. See this Bulletin. II pp. 27, 28; III, pp. 48, 98; V, p. 52.—H. A. Purdie, Newton, Mass.
The White-throated Sparrow in Winter near Worcester, Mass.—I saw White-throated Sparrows (Zonotrichia albicollis) at different dates during December, 1879. I also saw some on January 1, 1880. I, myself, had not observed it before, though possibly it may not be uncommon.—J. A. Farley, Worcester, Mass.
Peucæa ruficeps eremœca.—In Gillespie County, Texas, which adjoins Kendall Co. on the north, where Mr. Nathan C. Brown’s specimens were taken, I collected on April 24, 1878, a pair of Sparrows which Mr. J. A. Allen identified as Peucæa ruficeps. From the fact that Mr. Brown collected no typical ruficeps it is more than likely that my specimens were var. eremæca.
My specimens were sent to the late Greene Smith, Esq., Peterboro, New York, and are Nos. 961 and 962 in his Museum.—G. H. Ragsdale, Gainesville, Texas.
The Canada Jay at Portland, Maine.—A specimen of the Canada Jay (Perisoreus canadensis) was killed in Scarborough on October 15, 1880, by Mr. Luther Redlon, of Portland, and delivered into my hands a few hours after its capture. The specimen is worth noting from its being the first that I have ever known to occur in the vicinity of Portland, although its kind is said by Professor Verrill (Proc. Ess. Inst., Vol. III, p. 151) to winter commonly at Norway, Maine, only forty miles farther north.—Nathan Clifford Brown, Portland, Maine.
The White-throated Swift Breeding on Belt River, Montana.—About the middle of last July, while hunting on Belt River, I happened to approach the edge of the high limestone cliffs which rise above the 123stream for several miles after leaving the mountains. Watching the Violet-green and Crescent Swallows, which were abundant, for some time, I was about to leave, when I noticed a Swift evidently flying directly towards me. It passed only a few yards overhead, displaying at the same time the extensive white throat-patch of Cypselus saxatilis. Further search revealed some half a dozen altogether. A small opening in the rock which a bird of this species was seen to enter and reappear from several times, I approached, near enough to hear a vigorous twittering at each visit of the parent bird, from which I presume the young were well advanced. This is the only species of Swift I have yet seen in the Territory.—R. S. Williams, Gold Run, M. T.
Capture of the Golden Eagle (Aquila chrysaëtus canadensis) near Columbus, O.—December 13, 1881, I received a male specimen of the Golden Eagle, killed five miles west of the city.
This bird, according to information which I have gathered from various sources, had caused the farmers in the neighborhood in which it was killed a great amount of annoyance. A reward was offered, and published in our city papers, for the capture of a “Bald Eagle” (as they called it), which had killed several young calves. By further inquiry I ascertained that the bird was seen eating at two of the calves, but was not seen in the act of killing them.—Oliver Davie, Columbus, O.
The Little Blue Heron in Maine.—During the summer of 1881 a small white Heron took up his abode in a dense swamp bordering the eastern side of Scarborough Marsh. He foraged regularly about the neighboring ponds and rivers, and before autumn had been seen and unsuccessfully shot at by many covetous gunners. In September, however, he fell captive to the wiles of Mr. Winslow Pilsbury, and now reposes in the cabinet of Mr. Chas. H. Chandler, of Cambridge, Mass. Upon writing Mr. Chandler, to ascertain the species represented by his specimen, I learned that Mr. Henry A. Purdie[58] had seen the bird and pronounced it the Little Blue Heron (Florida cærulea). No previous instance of its occurrence in Maine is on record.—Nathan Clifford Brown, Portland, Maine.
Baird’s Sandpiper on Long Island, N. Y.—A Correction.—In the Bulletin for January, 1882, p. 60, it is stated that the record of a specimen of this species from Long Island is apparently its first from any point south of New England. A note to the editors from Dr. E. A. Mearns calls attention to a previous record of the species for Long Island in an article by Newbold T. Lawrence, entitled “Notes on Several Rare Birds Taken on Long Island, N. Y.,” published in “Forest and Stream,” Vol. X, No. 13, p. 235, May 2, 1878, as follows:—
124“Tringa bairdii, Baird’s Sandpiper.—Four specimens taken at Rockaway. The first two in September, 1872, shot on a small piece of meadow, out of a flock of Tringa minutilla. The third was taken August 26, 1873, while snipe shooting on a low strip of sand that separates the ocean and bay. My attention was first called to it by hearing a peculiar long-drawn whistle, and soon after I perceived a small snipe flying very high. The next moment it darted down and settled among my decoys, where I secured it. The fourth was taken in the same locality as the first two, September 20, 1874. Three of the above specimens were males.”—Edd.
Pelidna subarquata on the Maine Coast.—I have to thank Mr. C. H. Chandler of Cambridge, for allowing me to view a mounted specimen of the Curlew Sandpiper, which he shot on the beach at Pine Point, Scarborough, Cumberland Co., on September 15, 1881. The plumage is immature—probably a bird of the year. It was in company with Peeps, but its larger size and lighter coloration were noticed, hence this visit to American shores is registered. The species is new to the Maine fauna, at least this is the first instance of actual capture within the limits of that State.[59]—H. A. Purdie, Newton, Mass.
The King Rail in New England.—It seems that in making up the New England record of the King Rail (Rallus elegans)[60] I overlooked a note on this species, published in “Forest and Stream” of March 11, 1880. In this note Mr. Jno. H. Sage announces the capture of a female specimen at Portland, Conn., September 19, 1879.—Nathan Clifford Brown, Portland, Maine.
Purple Gallinule (Ionornis martinica) in Rhode Island.—Mr. Newton Dexter states that some years ago Mr. P. W. Aldrich showed him a fine Purple Gallinule just received in the flesh from Westerly, R. I. Mr. Dexter bought, and now has the bird. He is not able to give the exact year, but thinks it was in 1857.—Fred. T. Jencks, Providence, R. I.
Note on the Habits of the Young of Gallinula galeata and Podilymbus podiceps.—Mr. N. R. Wood, who collected quite a number of young Grebes and Gallinules this summer at Montezuma Marsh, near Clyde, N. Y., tells me that the little Gallinules use the thumb to aid them in moving about. The thumb in the young of this bird is quite long and sharp, and the nestlings, when unable to walk, hook it into any yielding substance, and drag themselves along. The young Grebes are more vigorous than the Gallinules, and progress by little hops.—Frederic A. Lucas, Rochester, N. Y.
125Rhynchops nigra.—An early Record for the Massachusetts Coast.—Champlain,[61] while cruising along the sandy shores of Cape Cod on a voyage of exploration in July, 1605, makes mention of the Black Skimmer, as his narration, p. 87, shows.
“We saw also a sea-bird with a black beak, the upper part slightly aquiline, four inches long and in the form of a lancet; namely, the lower part representing the handle and the upper the blade, which is thin, sharp on both sides, and shorter by a third than the other; which circumstance is a matter of astonishment to many persons, who cannot comprehend how it is possible for this bird to eat with such a beak. It is of the size of a pigeon, the wings being very long in proportion to the body, the tail short, as also the legs, which are red; the feet being small and flat. The plumage on the upper part is gray-brown, and on the under part pure white. They go always in flocks along the seashore, like the pigeons with us.”
That this species was found on our shores early in this century is proved by the older natives of the Cape telling me, since the bird’s recent occurrence, that “them cutwater or shearwater birds used to be with us summer times.” Also Mr. Brewster informs me that Nantucket fishermen assert that Skimmers bred on Muskegat Island fifty years ago.—H. A. Purdie, Newton, Mass.
Notes on the Habits of the Kittiwake Gull.—Some fishermen whom I lately employed to get a few Kittiwake Gulls on the winter fishing grounds off Swampscott, Massachusetts, gave me the following interesting account of the habits of this species, and the way in which my specimens were procured.
A number of small schooners sail from Swampscott every winter morning, and reach the fishing banks, which are some twelve miles off shore, about daybreak. The men then take to their dories, and buckets of bait—generally cod-livers or other refuse—are thrown out to attract the fish to the spot. Of this custom the Kittiwakes—or “Pinny Owls,” as these men invariably call them—are well aware, and swarms of them quickly collect around the boats to pick up the morsels before they sink. They are very tame, and if one of the flock is shot the others hover over it as Terns will do on similar occasions. The usual way of taking them, however, is with hook and line, the bait being allowed to float off on the surface, when it is quickly seized by one of the greedy horde. In this manner great numbers are annually taken by the fishermen, who either skin and stew them or use the flesh for bait. I was assured that a “Pinny Owl” stew is by no means an unpalatable dish.
After the morning fishing is at an end the vessels start for their anchorage in Swampscott harbor, and the fish are dressed on the way. This gives the Gulls another chance which is not neglected, for the entire flock 126follows closely in their wake. When the catch has been a large one, and the work of cleaning the fish is continued at the anchorage, they remain about the spot for hours picking up this offal directly under the sides of the vessels. Here again the poor birds are often mercilessly slaughtered by city gunners who shoot them for sport or practice, leaving the dead and wounded to float out to sea with the ebbing tide. The fishermen admit that their numbers have greatly diminished of late years, but they are said to be still very abundant through the winter months.—William Brewster, Cambridge, Mass.
Sterna forsteri breeding off the Eastern Shore of Virginia.—An impression seems to prevail among ornithologists that Forster’s Tern breeds only in the interior of North America. At least I cannot learn that Dr. Coues’ comparatively recent ruling[62] to that effect has been publicly corrected, or that it is generally known that the bird nests on the Atlantic Coast.[63] On this account it may be worth while to state that during a visit to Cobb’s Island, Va., in July, 1880, I found Forster’s Terns breeding in moderate numbers on all the neighboring islands. They nested apart from the other Terns, but often in company with Laughing Gulls, on the salt marshes or on marshy islets, where their eggs were almost invariably laid on tide-rows of drift-weed that fringed the muddy shores. The largest colony seen in any one place comprised perhaps twenty-five pairs, but it was more usual to find from six to a dozen mingled with a countless number of Gulls. I was late for the eggs, but secured a few far advanced in incubation, besides several downy young and many adult birds in full nuptial dress.—William Brewster, Cambridge, Mass.
Note on the Foot of Accipiter fuscus.—On the plantar surfaces of each foot of the Sharp-shinned Hawk two papillae may be noticed, which differ from the others, more properly described as pads, in their greater length and more symmetrical form. These pads are placed at the second phalangeal joint of the third toe, and at the third phalangeal joint of the fourth toe, that is, at the bases of the penultimate phalanges of the third and fourth toes. These papillae are shown to be modified pads, the same as those at the other two joints, by the less developed papillae of Circus, Astur, and others. This transition can readily be traced in the sketches of the feet given in the systematic works on Hawks, though the special prominence of the papillae in the Sharp-shinned Hawk does not seem to be particularly noted. On removing the skin, however, a marked difference at once comes in view. While all the pads are nearly obliterated, the papillae still remain as solid cones of connective tissue (?), having much the same shape and sizes as the entire papillae. These cones 127or cores are internally connected with the superficial fascia of the toes and seem to straddle the flexor tendons running below.
On noting the structural difference, the cause or function of these papillae at once becomes a point of interest. Why have these two pads been modified into long papillae (.12 inch in a dried specimen), and provided with a solid core? Now the foot of Accipiter is so constructed that the first toe opposes the second toe, and their claws move in nearly parallel arcs. This is not the case with the third and fourth toes, which are longer and not opposable to one another. Thus the claws can be opposed to nothing except the middle portions of the toes to which they belong. But when the claw is thus flexed a small space well adapted for grasping twigs and feathers is formed by the papillae, the penultimate phalanx and the claw, the point projecting beyond resembling the feet of certain crustacea and lice. Hence the function of the papillae would seem to be to aid the third and fourth claws in grasping small objects, and it is an interesting point to notice that the foot of Accipiter fuscus is thus drawn in North American Birds, by Baird, Brewer and Ridgway.
How far the same considerations hold in other species I cannot say, but as mentioned above, allied forms seem to possess the character to a less degree.—J. Amory Jeffries, Boston, Mass.
Supplementary Notes on two Texas Birds.—In a recent paper[64] on a collection of birds made in southwestern Texas, I referred a series of Hylocichla unalascæ to the restricted form, with the remark that several specimens closely approached var. auduboni. Upon reading the article, an esteemed correspondent wrote me that one of these aberrant examples, which had passed into his hands, appeared to him to be true auduboni. In this opinion, after a reëxamination of the specimen, I concur. The bird in question has a wing of 3.82 inches, which, though decidedly under the average of auduboni, is more than should be allowed unalascæ proper.[65] Here, then, is another species, besides those previously cited, which is represented by two distinct varieties in the tract of country explored.
The single specimen of Coturniculus passerinus taken in the same locality represents the western variety perpallidus, under which, by an oversight, it was not included.—Nathan Clifford Brown, Portland, Me.
128Addenda to the Preliminary List of Birds ascertained to occur in the Adirondack Region, Northeastern New York.[66]—
178. Dendrœca striata (Forst.) Baird. Black-poll Warbler.—In the collection of the late A. Jenings Dayan (of Lyons Falls, N. Y.) is a female of this species that he killed in the town of Lyonsdale in Lewis Co., May 23, 1877.
179. Dendrœca pinus (Wilson) Baird. Pine-creeping Warbler.—Mr. Dayan took a full-plumaged male D. pinus at Lyonsdale, Lewis Co., May 8, 1877. I have never observed the species within the limits of the Adirondack Region, and it must be regarded as a rare bird here.
180. Asio accipitrinus (Pallas) Newton. Short-eared Owl.—I have seen two specimens of the Short-eared Owl that were taken within the limits of the Adirondack Region, in Lewis County. They were both killed east of the Black River Valley—one in the town of Greig, and the other in Lyonsdale.
181. Nyctiardea grisea nævia (Bodd.) Allen. Night Heron.—I have seen a Night Heron that was shot at Crown Point (in Essex Co.) on Lake Champlain. There were two of them together, and both were killed.
182. Calidris arenaria (Linn.) Illig. Sanderling.—On the 5th of October, 1881 Mr. O. B. Lockhart killed, from a flock, four Sanderlings at Lake George, in Warren Co. (Dr. A. K. Fisher.)
183. Chen hyperboreus (Pallas) Boie. Snow Goose.—Dr. A. K. Fisher writes me that he saw a flock of one hundred and fifty or two hundred Snow Geese on Lake George (in Warren County) Nov. 19, 1881. In company with Mr. O. B. Lockhart he rowed out to within a hundred yards of them, when they were frightened by another boat and took flight, showing plainly the black tips of their primaries as they left.
184. Phalacrocorax dilophus (Sw. and Rich.) Nuttall. Double-crested Cormorant.—Mr. F. H. Knowlton, from Brandon, Vermont, writes me: “I shot, on September 24, 1879, at St. Regis’ Lake [Franklin County], two miles from Paul Smith’s, a young female example of Graculus dilophus. The bird was not wild and was easily shot from the shore.”
185. Dytes auritus (Linn.) Ridgway. Horned Grebe.—On Little Tuppers Lake (Hamilton Co.), Oct. 22, 1881. Dr. A. K. Fisher and I saw about eight Horned Grebes and I killed one of them. While crossing Raquette Lake, the same day, Dr. Fisher shot another. At Big Moose Lake (in Hamilton and Herkimer Counties) we saw this species every day from Oct. 26 to Nov. 8, 1881. Nov. 5 I shot one out of a flock of nine. They were all in the plain fall dress, so that the size alone enabled us to distinguish young from old. In all the iris was of a bright orange red. They are excellent divers and can remain under water an astonishingly long period.—C. Hart Merriam, M.D., Locust Grove, N. Y.
In Vol. VII, page 26, line 6, for “An indistinct, dusky” read “A black.” Same page, foot note, for “οὐκέω” read “οἰκέω.”

Bull. Nutt. Ornith. Club., Vol. VII., No. 3. Plate 7.
Jeffries & Blake, del. The Heliotype Printing Co. 211 Tremont St. Boston
Feathers have been studied from the earliest days of the microscope, indeed long before the modern microscope came into existence. Malpighei, Hooke and Leeuwenhoek all wrote on the subject, and not a little of our knowledge dates from their time. Since then authors have constantly written on feathers and their colors, until the papers on the subject may be counted by hundreds. Accordingly little that is new can be expected from this short article, nor even a history of the literature of the subject. My only object is to give an idea, so far as is known, how the colors of feathers are produced, the literature of the subject being out of the track of most American ornithologists.
Color may be the result of any one or more of the following causes: a pigment, interference and diffraction of light in their various phases, fluorescence, and phosphorescence. Of these causes only three have been called upon to explain the colors of feathers, the last two apparently playing no part. The fluorescence noted by Dr. Krukenberg in solutions of certain feather-pigments probably plays no part, or at most an insignificant one, in the colors of feathers. Pigments act by absorbing all rays of light but those which enter into their color, that is turn them into heat.
130Interference acts in several different ways, all of which are based on the same principle, and so films may be taken as an example. If a beam of light, xy (figure 1), is allowed to fall on any thin plate, or film, part of the rays will be reflected in the direction yz, the angles byx and ayz being equal. The rest of the rays will pass through the film to the other surface, being slightly refracted in their course. Here part will be reflected, and being again refracted at the first surface, will emerge in a line wz′ nearly coincident with yz, the balance passing out into the air. Now the waves composing the white light of two beams yz and wz′ will run together and partially obliterate each other, after the manner of ripples on water. Accordingly certain waves will be obliterated, and since white light is due to the blending of waves of the different colors, the light reflected from the film will be that of the colors not interfered with, the waves thus obliterated depending upon their length and the thickness of the film traversed. So as we look at the film from different points the conditions vary, and with them the resultant color.
Interference may also produce colored light by means of fine particles diffused through another substance, as milk in water, the particles in the air, and the like. Colored light produced in this way is known as opalescent, the transmitted light tending to the red end of the spectrum, and the reflected to the other portions. This result can be obtained by mixing black and white grains, an experiment which all have tried as school boys, by soaking chalk in ink, the result being a bluish color.
Diffraction acts apparently by bending the light rays different amounts, and thus spreading out the spectrum. Explanations of the various phenomena of this sort are difficult, and need not be entered into here.
Feathers are classed, according to their appearance, into ordinary, metallic and iridescent, the peculiarities of which are well known and so need not delay us.
The ordinary feathers are colored by simple pigments, by contrast of light and darkness and mechanically, as in the case of the Bluebird (Sialia sialis). Pigments of various colors are known to occur in feathers, and have received special names, as turacin, zoönerythrin, zoöfulvin, zoöxanthin, zoöchlorin, zoömelanin. These evenly distributed, as turacin, zoönerythrin, and zoöfulvin, or in patches, as zoömelanin, impart their respective colors to the 131feather parts in which they exist.[67] The color of the mass of the feather may, however, owing to various colors in the small feather parts, be different from that of any part.
Of these pigments none seem to be peculiar except turacin. This pigment is altered by wetting the feathers, and comes from the feathers into the water in which the birds bathe, a fact of considerable interest, since the birds maintain their normal color, thus necessitating a new supply of pigment.
White feathers are the result of the light being reflected as a whole from the finely divided feather parts. Some grays are the result of small black nodes in the barbules, which nodes are of considerable size, and do not disperse the light, being distributed along the barbules. Other grays are the result of a small quantity of black pigment.
Yellow feathers colored with zoöfulvin receive their hue from this pigment, which is pretty evenly distributed through the texture like a dye.
Red feathers, as those of the Flamingo, Cardinal Bird, and the like, are so colored by a red pigment similar to the yellow one. Brown feathers are colored by a brown pigment in the feathers, which is for the most part collected in patches within the cells of the feather.
Violet pigments are said by some to exist, while others have never been able to extract them, so the causes of this color still remain in doubt.
Green feathers owe their color to various causes. In some it is due to a green pigment, as Turacoverdin or zoöchlorin, in others it is said to be due to a mixture of yellow and blue dots. The olive-greens are sometimes produced by a yellow pigment overlying a dark brown or black.
132All the above pigments seem to be blended and used in gaudily colored birds much after the manner of paints by artists. So that a great variety of colors may be produced from a few pigments by the skilful hand of nature.
Metallic feathers, properly speaking, are those which partake of the characters shown by the red crests of the Woodpeckers. The metallic appearance is limited to the barbs, the barbules not showing this peculiarity, and being quickly shed. If a feather from the crest of a Woodpecker, say Picus pubescens, be examined, it will at once be noticed that the red barbs have few if any barbules, and that the barbs themselves are enlarged. Such barbules as are present, are not red but black, and only serve to diminish the effects of the red parts. They would seem accordingly to be properly classed among useless hereditary organs. That the red color is due to a pigment is proved by dissolving it out and by its persistence when examined by transmitted light. But what causes the brilliancy which has led to their being called metallic? This is due to the extreme smoothness of the barbs, the horn-cells of which they are composed being fused together and solid. Thus the unabsorbed rays of the beam of light which strikes them are reflected as a whole, instead of being sent in every direction by the walls of the cells as in most cases. The metallic feathers differ from ordinary feathers in the same way that window or glass paintings differ from ordinary pictures. They simply give off much more light, and thus produce more marked effects on our eyes.
The colors of metallic feathers seem to be limited to the red end of the spectrum, the colors varying from yellow or orange to red; blue, green or purple feathers constructed on this principle do not seem to abound.
So far we have only had to deal with pigments, and all has been plain sailing, but the various accidental colors shown by feathers are far more difficult of explanation. Not only are the parts extremely small, but the entire subject of accidental colors as regards organic structures has been in large part dealt with from a theoretical point of view. The question has not been how is the feather part made, but what kinds of structures will produce such color effects. Accordingly divers opinions have been expressed on the subject, the most probable of these we shall now endeavor to sketch out.
133Blue colors seem to be accidental, that is, the result of other causes than pigments. Not only have all efforts to extract the pigments failed, but blue feathers appear gray when examined by transmitted light. Again, no blue can be found in transverse sections of blue feather parts. This method of studying the colors of feathers is worthy of more extended use than it has yet had. By this means all physical effects of the outer coat are avoided, and the exact position of the pigments can be seen. Sections are quickly prepared by fastening the feather on to a piece of pith with collodion, and mounting sections pith and all. The pith keeps the sections on end, a result otherwise difficult to obtain.
Gray-blues, such as those seen in Dendrœca cœrulescens, are due to opalescence. The feather is full of fine granules of black or darkish pigment, which in a manner already described produces a blue color.
Brilliant blues, as those shown by Sialia sialis, Cyanospiza cyanea, Cœreba lucida, and the like, do not seem to be susceptible of a like explanation. The color is too intense and pure to be produced in such a small space by opalescence. So most authors have simply ascribed it to some other form of interference, as a thin outer plate, which would seem on examination to be the true cause. Figure 2, drawn from a section of a Bluebird’s barb enlarged about one thousand diameters, will give an idea of the structure found in such cases. The central cells are full of some dark pigment, probably zoömelanin, while the surface is bounded by a transparent layer of horn varying from ¹⁄₃₀₀₀₀ to ¹⁄₁₀₀₀₀ of an inch in thickness. Thus we have a contrivance not ill adapted to the production of interference colors, the black pigment absorbing all rays which are not reflected by the horn coat on the outside. Yet there are decided difficulties in this view. Thin as it is, the outer horn coat is thick compared to the length of light waves, and again the blue color is constant. However, in spite of these objections, the color must be ascribed to the action of the outer coat of cells. The structure of other bright blue feathers is much the same, though differences in minutiae exist. Thus the outer layer of cells, the external walls of which form the outer coat of the barb, are devoid of pigment in the Blue Jay. (Fig. 3.)
134Here it is of interest to note that the barbs of the brown female Indigo bird differ but slightly from the bright blue barbs of the male. In the female the pigment is more diffuse, and the outer horny coat is thicker and less dense and lustrous.
The above feathers with their smooth outer coat are connected with true iridescent feathers by an intermediate group. I refer to the highly-colored blue and green feathers of such birds as Chlorophanes atrocristatus (Fig. 2) and Cœreba lucida. In these the ends of the barbs are enlarged and the barbules reduced to a minimum, after the manner of the Woodpeckers; unlike them, however, the surface is rough, each cell being rounded out. When examined under a microscope such barbs appear as if covered with a mosaic of gems. Sections show, whatever may be the shape of the barb, that the walls of the iridescent parts are extremely thin, so thin that exact measurements cannot be made with the instruments at my disposal. The thickness got when reduced to fractions of an inch, is approximately ¹⁄₁₀₀₀₀₀₀ of an inch, a film sufficiently thin for all purposes of interference. Many of these feathers when magnified show that the color is not uniform, but that all the colors contribute their quota to the final color. The figure of a section of a barb of Chlorophanes atrocristatus will give some idea of such a feather. In this case the final color seems to be the result of mixing the light reflected from the dark end with that from the yellow triangular part.
We now naturally come to the true iridescent feathers, of which the Peacock may be taken as an example. The iridescent barbules are made up of flat, wonderfully thin cells, arranged end to end, as shown in figure 5. When examined with transmitted light, they are seen to be films full of a brownish pigment more or less evenly dispersed through the mass. When cut in sections and looked at on edge they resemble, even under quite high powers, the edge of a piece of paper. Here we have the most admirable contrivance for the production of iridescent light, the plates being fully thin enough, and all white light which may get through the walls being taken up by the brown pigment within. All the parts of the eye are constructed on the same plan, and only provided with brownish pigments, hence the color must be due to variations in the thickness. Here it is well to notice that the colors are quite constant.
135The brilliant colors of these feathers have often been ascribed to irregularities of surface, the traces of the cell cavities being mistaken for pits on the surface. That this is an error is at once shown by examining a section.
Before leaving the subject I cannot refrain from calling attention to the wonderful diversity of means employed, as well as their complexity in the production of feather colors. Among the Parrots we have the most skilful painting combined with accidental colors. Yet all ornithologists base specific differences on slight variations of color, and this in spite of the fact that birds may change their color according as they are wet or dry, owing to the nature of their food, or to slight differences in the quantity of pigment.
In this they are no doubt often right, but when we come to varieties based on the very faintest distinctions of color and form, we may well pause till more is known of avian physiology.
Fig. 1. Diagramatic representation of the effect of a film on light.
Fig. 2. Transverse section of a barb of Chlorophanes atrocristatus; Hartnack 3–9 im. the light part yellow, the dark part dark brown.
Fig. 3. Transverse section of a barb of Cyanocitta cristata. Hart. 3–9 im.
Fig. 4. Same of Cyanospiza cyanea ♂.
Fig. 5. Two sections of a barbule of a Peacock.
Fig. 6. Section of barb of Sialia sialis much magnified.
33. Peucedramus olivaceus (Giraud) Coues. Olive-headed Warbler.—The Olive-headed Warbler, one of Giraud’s famous “sixteen” Texas species, has found an unquestioned place in our fauna only on the strength of three Arizona specimens, taken by Mr. Henshaw at Mount Graham, in September, 1361874. Accordingly, the acquisition of the fine series catalogued below can scarcely fail to be a matter of much interest. As will appear from the accompanying data, Mr. Stephens met with the bird in only a single locality in the Chiricahua Mountains where it was apparently not uncommon in March: but he writes of a previous specimen (an adult male) taken among the Santa Catarina Mountains, in February, 1880, a date which seems to imply that the species winters in the latter range. His observations throw no light on its still unknown breeding haunts.
The specimens obtained during the past season were found in pine woods on the mountain sides at an elevation of from ten to twelve thousand feet. Although individuals often occurred not far from one another, two were rarely seen in actual companionship. The only exception to this is noted under date of March 24, when a small flock was met with on a steep slope near the summit of one of the mountains. In their actions these Warblers reminded Mr. Stephens of Dendrœca occidentalis. They spent much of their time at the extremities of the pine branches where they searched among the bunches of needles for insects, with which their stomachs were usually well filled. Occasionally one was seen to pursue a falling insect to the ground, where it would alight for a moment before returning to the tree above. The only song heard consisted of “a few low notes” which were rarely uttered, but a peculiar “cheerp” was repeated at frequent intervals.
The examples before me illustrate a fact which I do not find mentioned by previous writers, viz., that during the first year the males wear a plumage similar to that of the females. I have three in this condition; two of them, although in unworn dress, are absolutely undistinguishable from adults of the opposite sex; the third (No. 77), however, has the throat appreciably tinged with the brownish-saffron of the adult male. The females show some variation in respect to the dusky patch on the side of the head. In most of them it is confined to the auriculars, and even there is much mixed with yellow; but No. 46 has a continuous, dull-black stripe extending from the bill through the eye, and spreading over the auriculars in a broad, well-marked patch. Nos. 94 and 101 differ from the others in having the crown so slightly washed with olive-green that the whole upper surface is nearly uniform, a condition which I take to be the immature one of this sex. The adult males show but little individual variation. Both sexes and all ages have the basal half of the lower mandible light brown.
44. ♂ ad., Morse’s Mill, Chiricahua Mountains, March 14, Length, 5.10; extent, 9; wing, 3.12; tail, 2.35; culmen, .56; tarsus, .72.
13745, ♂ ad., same locality and date. Length, 5.40; extent, 9.20; wing, 3.16; tail, 2.55; culmen, .55; tarsus, .69. Iris dark brown.
72, ♂ ad., Morse’s Mill, March 19. Length, 5.40; extent, 8.90.
91, ♂ ad., Morse’s Mill, March 24. Length, 5.40; extent, 9; wing, 3.08; tail, 2.50; culmen, .55; tarsus, .75.
92, ♂ ad., same locality and date. Length, 5.20; extent, 8.90.
102, ♂ ad., Morse’s Mill, March 25. Length, 5.30; extent, 8.80; wing, 3.10; tail, 2.44; culmen, .56; tarsus, .75.
77, ♂ im., Morse’s Mill, March 20. Length, 5.20; extent, 8.90; wing, 3.03; tail, 2.37; culmen, .55; tarsus, .77. In plumage of the ♀.
90, ♂ im., Morse’s Mill, March 24. Length, 5.10; extent, 8.50; wing, 2.85; tail, 2.30; culmen, .56; tarsus, .71. Same remarks.
103, ♂ im., Morse’s Mill, March 25. Length, 5.10; extent, 8.50; wing, 2.90; tail, 2.33; culmen, .57; tarsus, .67. Same remarks.
46, ♀ ad., Morse’s Mill, March 14. Length, 5.20: extent, 8.50; wing, 2.93; tail, 2.35; culmen, .56; tarsus, .73.
47, ♀ ad., same locality and date. Length, 5; extent, 8.30; wing, 2.87; tail, 2.18; culmen, .58; tarsus, .73.
81, ♂ ad., Morse’s Mill, March 21. Length, 5; extent, 8.50; wing, 2.76; tail, 2.35; culmen, defective; tarsus, .72.
93, ♀ ad., Morse’s Mill, March 24. Length, 5.20; extent, 8.80.
94, ♀ ad., same locality and date. Length, 5; extent, 8.20: wing, 2.84; tail, 2.18; culmen, defective; tarsus, .71.
101, ♀ ad., Morse’s Mill, March 25. Length, 5.10; extent, 8.50: wing, 2.87; tail, 2.22; culmen, .58; tarsus, .75.
34. Dendrœca æstiva (Gmel.) Baird. Yellow Warbler.
210, ♂ ad., Cienega Station, April 16. Length, 5; extent, 7.50; wing, 2.75; tail, 2.20; tarsus, .74. “Iris dark brown; bill dark horn color above, lighter below; legs pale brown. Common in the migrations.”
35. Dendrœca coronata (Linn.) Gray. Yellow-rumped Warbler.—Chiricahua Mountains; a single specimen, taken March 26.
From its general dispersion over North America, the Yellow-rumped Warbler was of course to be expected in Arizona, at least as a visitor, but I cannot learn that it has been previously detected within the limits of that Territory. Mr. Stephens, however, sends me an adult female which must be referred to coronata, although it is in some respects peculiar, if not intermediate between that species and auduboni. The wing-bands are as distinctly separated as in coronata (with females and immature males of both species this character is not always well-defined), and the throat, generally, is equally white, but on its left side, adjoining the maxillary line, there is a small patch of the faintest possible yellow. The light superciliary stripes, which should be at least indicated in female coronata, are also entirely wanting.
114, ♀ ad., Chiricahua Mountains, March 26. Length, 5.50; extent, 8.70; wing, 2.98; tail, 2.52. “Iris brown.”
13836. Dendrœca auduboni (Towns.) Baird. Audubon’s Warbler.
343, ♂ ad., Tucson, May 7. Length, 5.80; extent, 9.52; wing, 3.05; tail, 2.75. “Iris dark brown; bill and legs black.”
37. Dendrœca nigrescens (Towns.) Baird. Black-throated Gray Warbler.—On April 1, Mr. Stephens secured five males of this species among the Chiricahua Mountains. The only additional specimens in the collection are two females taken late in the season (No. 203, ♀ ad., Cienega Station, April 15. No. 357, Santa Rita Mountains, May 12.).
38. Dendrœca townsendi (Nutt.) Baird. Townsend’s Warbler.
2.98, ♀ ad., Tucson, April 28. Length, 5.10; extent, 7.70; wing, 2.45. “Iris dark brown; bill and legs black; soles of the feet yellow. Among mesquites.”
373, ♂ ad., Santa Rita Mountains, May 13. Length, 5.30; extent, 8.10; wing, 2.64.
374, ♀ ad., same locality and date. Length, 4.90; extent, 7.40; wing, 2.44. “Iris dark brown; soles of feet yellowish. Water oaks of foot-hills; very fat.”
Even the most adult males of this species seem to have the throat-patch slightly sprinkled with yellow. At least I have yet to see one with the black absolutely pure and unmixed.
39. Siurus nævius (Bodd.) Coues Northern Water Thrush.—A single specimen taken May 4, at Tucson. It was among willows on the borders of a stream.
This example differs from New England ones in being darker above and less yellowish beneath. In these respects, as well as some minor ones, it resembles a rather peculiar style from West Virginia to which I once called attention.[68] Mr. Ridgway kindly furnishes the following opinion regarding its relationship with S. notabilis. “The Siurus from Tucson is very different in proportions from the type of notabilis, with which I have compared it, but it may be a small individual of that form. The wing is about the same length, but the bill and tail are very much shorter, and the tarsi more slender. The color above is grayer, the streaks beneath much narrower, and the spots on the throat much smaller.” Notabilis, based as it is on a single specimen, and instituted in a species which varies to an unusual degree in size, color and markings, seems to me, however, to be, at best, a very doubtful race.
329, ♂ ad., Tucson, May 4. Length, 6.20; extent, 9.50; wing, 3.10; tail, 2.32; tarsus, .85; culmen, .64. “Iris brown; bill black above, brown below; legs light brown. Very fat. Stomach contained insects.”
13940. Geothlypis macgillivrayi (Aud.) Baird. Macgillivray’s Warbler.—Two specimens collected at Tucson (♀ April 20, ♂ June 8). “I have not found it common in either Arizona or New Mexico.”
41. Geothlypis trichas (Linn.) Caban. Maryland Yellow-throat.—Mr. Stephens found this species “abundant along streams,” an experience at variance with that recorded by Mr. Henshaw, who met with it but twice while in Arizona.
The only specimen taken agrees closely with some examples from the Truckee River, Nevada, and differs from my eastern representatives, in having the upper parts yellowish-olive instead of olive-green; the crown-band much broader and creamy white in color; the wings and tail longer; the yellow beneath richer, and extending more over the abdomen. Mr. Ridgway has already called attention[69] to some of these differences which, as he now writes me, would be enough to warrant the varietal separation of the western bird, were it not that specimens from both sections of the country occasionally vary in such a manner as to invalidate any characters that could at present be proposed. With the acquisition of better series, however, it is probable that the representatives of two regions, as yet undefined, will be found to present sufficiently constant characteristics to deserve distinctive names.
219, ♂ ad., Cienega Station, April 17. Length, 5.40; extent, 6.90; wing, 2.16; tail, 2.40; culmen, .55. “Iris brown; bill black, bluish beneath; legs pale brown.”
42. Icteria virens longicauda (Lawr.) Coues. Long-tailed Chat.—This bird was observed only in the vicinity of Tucson. The first specimen was taken April 30, and it soon afterwards became abundant.
310, ♂ ad., Tucson, April 30. Length, 7.50; extent, 9.40; wing, 3.12; tail, 3.52. “Bill and legs black.”
318, ♂ ad., Tucson, May 3. Length, 7.70; extent, 9.60; wing, 3.05; tail, 3.61.
335, ♂ ad., Tucson, May 5. Length, 7.30; extent, 9.70; wing, 3.12; tail, 3.45.
521, ♂ ad., Tucson, June 11. Length, 7.10; extent, 9.40; wing, 3.15; tail, 3.36.
43. Myiodioctes pusillus pileolatus (Pall.) Ridgw. Pileolated Warbler.
Although Mr. Henshaw referred all his Arizona Black-capped Flycatchers to pusillus, mine are absolutely typical of pileolatus; in fact they are brighter than some specimens from Nicasio (California), the yellow below being richer, and the upper surface more yellowish, while the bill is equally
140narrow and several shades lighter in color. Compared with eastern examples they of course present an even greater contrast. Dr. Coues was undoubtedly right in saying (Birds of the Colorado Valley, p. 327) that pileolatus “is not confined to the Pacific coast region”; but I cannot agree with him in thinking it an inconstant form. On the contrary, I find its characters, as proposed by Mr. Ridgway, so well maintained that any one of my western birds can be separated at a glance when placed in a series of twenty-one specimens from the Atlantic States.
221, ♂ ad., Cienega Station, April 17. Length, 4.70; extent, 6.80; wing, 2.17; tail, 2.23; width of bill below nostrils, .12. “Iris brown; bill dark above, pale brown below. Common here in willows and underbrush along streams.”
257, ♂ ad., Tucson, April 21. Length, 4.90; extent, 7; wing 2.27; tail, 2.30; width of bill below nostrils, .12.
44. Setophaga picta Swains. Painted Redstart.—During the past season this beautiful species was met with only among the Chiricahua and Santa Rita Mountains, but in 1876 Mr. Stephens found it in New Mexico, a Territory from which I believe it has not previously been reported. In the Chiricahua Mountains it was not uncommon after March 21, and many specimens were taken near Morse’s Mill, at an elevation of fully seven thousand feet. They occurred most numerously among pines, in a cañon where they had been previously observed in April, 1880. This experience, it will be observed, differs somewhat from that recorded by Mr. Henshaw, who says: “It appears not to inhabit the high mountains nor the extreme lowlands, but to occupy an intermediate position, and to find the rocky hills covered with a sparse growth of oak most congenial to its habits.”
In the Santa Rita Mountains, where it was rather common in May, Mr. Stephens had the good fortune to find its previously unknown nest and eggs. The nest, which is now before me, is large, flat and shallow. It is composed of bark, coarse fibres from weed-stalks, and fine, bleached grasses, the latter, with a few hairs, forming a simple lining. The cup measures 2.10 inches in width by 1 inch in depth; while the external diameter of the whole structure is rather more than 5 inches, and its depth about 1.50. The eggs, which were three in number, measure respectively .64×.51; .64×.50; and .66×.49. They are clear, dead white, delicately spotted with light reddish-brown, the markings being sparsely distributed over the general surface of the egg, and handsomely wreathed about its larger end. Neither nest 141nor egg resembles that of S. ruticilla. But a greater surprise is the character of the nesting-site, which was “under a projecting stone, in a bank near a small stream.” This position is so unexpected that, from an unproved collector, I should hesitate to accept the accompanying evidence of identification, which is a simple statement that the parent was sitting, and was distinctly seen. But knowing as well as I do Mr. Stephens’ unusual accuracy and conscientiousness in such matters I cannot doubt the correctness of his determination, especially as the Painted Redstart is a bird of such striking colors and markings that it could not possibly be mistaken by one who is so familiar with its appearance in life.[70] After all the case is not more peculiar than that presented among Helminthophilæ by Lucy’s warbler which, as has just been shown, departs from the normal nesting habits of the genus and builds in holes, behind loose bark and in all sorts of unexpected places. The nest above described was taken May 18, when the eggs were sufficiently advanced in incubation to show that the clutch was complete.
Mr. Henshaw comparing the sexes, says: “The adult plumage of the sexes differs little, though the coloration in the female is quite perceptibly duller throughout. The black is less lustrous; the wings are blackish brown instead of pure black; the white on the wing confined to the coverts, and only just visible on the edges of the secondaries.” These differences, however, are not always maintained for one of the two adult females before me is quite as bright as the average male, while the black is not less lustrous, and the white edging on the secondaries is even broader. The other is more like those examined by Mr. Henshaw, but seems to be peculiar in having the sides, with a broad collar across the nape, fine stone-gray.
14245. Vireo gilvus (Vieill.) Bonap. Warbling Vireo.—Found among all the well-timbered mountains visited, but nowhere as a common bird.
Of the several characters which are said to distinguish var. swainsoni from gilvus proper, I can appreciate only the slightly different shape of the bill. The relative length of the wing-quills is an absolutely inconstant characteristic with birds from any of the localities represented in my series, while I do not find that western specimens—at least California and Arizona ones—are either paler or grayer than many we get in the Atlantic States. Indeed, nearly the darkest one in my whole suite comes from Arizona. In view of these facts I cannot regard swainsoni as worthy of varietal recognition.
46. Vireo solitarius cassini (Xantus) Ridgw. Cassin’s Vireo.—Common among the foot-hills of the mountains.
Mr. Henshaw has so satisfactorily defined[71] the characters which respectively distinguish the Cassin’s and Plumbeous Vireos from solitarius proper, as well as from each other, that there is no room for any further remarks on what, previous to his examination, was a very tangled problem. The specimens mentioned below are all unmistakably referable to cassini, although one or two of them present slight approaches to plumbeus. It is a singular fact that Mr. Stephens did not meet with any typical examples of the latter race.
209, ♂ ad., Cienega Station, April 16. Length, 5.40; extent, 8.70. “Iris brown; bill dark horn-color above, lighter below; legs dark bluish.”
214, ♀ ad., same locality and date. Length, 5.60; extent, 9.10; wing, 3; tail, 2.44.
236, ♀ ad., Tucson, April 19. Length, 5.60; extent, 8.70; wing, 2.89; tail, 2.41.
316, ♀ ad., Tucson, May 2. Length, 5.30; extent, 8.50; wing. 2.71; tail, 2.26.
346, ♀ ad., Tucson, May 7. Length, 5.30; extent, 9; wing, 2.76; tail, 2.23. “Very fat. Would not have laid for a long time.”
354, ♂ ad., Santa Rita Mountains, May 11. Length, 5.10; extent, 8.80; wing, 2.82; tail, 2.27. “Iris brown; bill nearly black, bluish at base below; legs lead-color.”
47. Vireo huttoni stephensi var. nov. Stephens’ Vireo.
Ch. Sp.—♂ ♀ Similis V. huttoni sed rostro robustiori, alis longioribus. Supra griseo-cinereus, infra fusco-albidus. Uropygio et marginibus caudæ sordide virenti-olivaceis. Alis albo bifasciatis; remigibus albo-marginatis. Loris et orbe circum-oculari (macula fusco-brunnea in palpebra superiori excepta), cinereo-albis.
Adult ♂ (No. 5,728, author’s collection—collector’s No. 41—Chiricahua Mountains, Arizona, March 14, 1881. F. Stephens). Bill stout; wings from .30 to .40 inches longer than tail. Above grayish-ash; the crown, 143vertex and sides of head and neck nearly pure; the back faintly tinged with olive; the rump and an edging on the tail feathers, dull olive-green. Wings with two nearly confluent bands on the coverts, and the outer edges of the inner secondaries, broadly white; outer quills edged more narrowly with the same color. Beneath brownish or smoky-white, with a mere wash of yellowish on the sides and crissum. Upper eyelid dusky brown; remainder of orbital region, with the lores, ashy-white in decided contrast with the nearly clear cinereous of the head generally. Lining of wings white.
Dimensions. Length, 5.20; extent, 8.50; wing, 2.90; tail, 2.25; culmen, .50.
Habitat. Arizona and New Mexico.
Four additional specimens offer no variations affecting any of the characters above detailed.
In its generally dull, grayish coloration, with little trace of olive or yellow shades, this Vireo is curiously like V. pusillus, but the under parts are obscured with brownish, while the differences in size and proportions are too evident to require detailed comparison. From the smaller, much brighter-colored V. huttoni, which is unmistakably its nearest United States relative, it may be distinguished by the following diagnoses.
V. huttoni.—Wing, 2.28 to 2.37. Olive-green above and olivaceous-yellowish beneath. No clear white anywhere.
V. huttoni stephensi.—Wing, 2.55 to 2.90. Grayish-ash above with no decided olive-green excepting on the rump and tail. Beneath brownish-white, untinged with yellowish excepting on the sides and crissum. Wing-bands pure white and nearly confluent.
It will be observed that the above differences are closely parallel to those which separate Vireo belli and V. pusillus, while they are in no respect less important. Indeed were I disposed to emphasize certain peculiarities presented in the wing-formula of my type, it would not be difficult to make out an equally good case of specific distinctness, but unfortunately, the relative length of the wing-quills (including the spurious primaries) proves to be quite as variable in V. huttoni and its Arizona race, stephensi, as I find it to be in V. pusillus and V. belli, and, I might add, in all closely allied species which I have so far studied. In short, I am convinced that this feature, if ever of any diagnostic value, is so with only a small proportion of the birds to which it has been so freely and confidently applied.
In naming this Vireo after its discoverer, Mr. F. Stephens, I have paid but a deserved compliment to that gentleman’s zeal and energy as a field ornithologist. He notes the bird as “not uncommon in scrub oaks” among both the Chiricahua and Santa Rita Maintains. He also writes me that he has taken specimens in New Mexico, where, near Fort Bayard, a nest with four eggs was obtained in 1876. In both Territories it seems to be confined to the mountain ranges, where it undoubtedly breeds in all suitable localities.
14441, ♂ ad., Morse’s Mill, Chiricahua Mountains, March 14. Length, 5.20; extent, 8.50; wing, 2.90; tail, 2.25; tarsus, .73; culmen, .50; depth of bill at nostrils, .15. “Iris brown.”
50, ♂ ad., Morse’s Mill, March 16. Length, 4.90; extent, 8; wing, 2.55; tail, 2.20; tarsus, .73; depth of bill at nostrils, .15.
118, ♂ ad., Morse’s Mill, March 28. Length, 5; extent, 7.90; wing, 2.68; tail, 2.30; tarsus, .70; culmen, .50; depth of bill at nostrils, .15.
140, ♂ ad., Chiricahua Mountains, March 31. Length, 5.10; extent, 8.40; wing, 2.65; tail, 2.25; tarsus, .73; culmen, .49; depth of bill at nostrils, .15.
353, ♂ ad., Santa Rita Mountains, May 11. Length, 5; extent, 8.10; wing, 2.74; tail, 2.25; tarsus, .70; culmen, .48; depth of bill at nostrils, .15.
Seven California specimens of V. huttoni measure as follows:—
1443, ♂, Nicasio. Wing, 2.35; tail, 2.20; tarsus, .75; culmen, .50; depth of bill, .11.
1445, ♂, Nicasio. Wing, 2.31; tail, 2.15; tarsus, .76; culmen, .51; depth of bill, .11.
1444, ♀, Nicasio. Wing, 2.35; tail, 2.25; tarsus, .76: culmen, .49; depth of bill, .10.
1446, ♀, Nicasio. Wing, 2.32; tail, 2.28; tarsus, .74; culmen, .50; depth of bill, .14.
6800, ♂, Berkeley Co. Wing, 2.37; tail, 2.30; tarsus, .75; culmen, .46; depth of bill, .11.
6801, ♀, Berkeley Co. Wing, 2.28; tail, 2.15; tarsus, .75; culmen, .51; depth of bill, .11.
6339, ♀, Riverside. Wing, 2.34; tail, 2.14; tarsus, .75; culmen, .52; depth of bill, .14.
48. Vireo pusillus Coues. Least Vireo.—An abundant summer species frequenting willows along streams and, near Tucson, thickets of mesquites. “It is active, restless and very noisy.”
Numerous nests were taken. The only one sent me is a shallower, but nevertheless rather more elaborate structure, than that of V. belli to which, however, it bears a strong resemblance. It is mainly composed of fibrous shreds, apparently obtained from the stalks of some herbaceous plant. The lining is of delicate, bleached grasses, which are very neatly arranged. The eggs are white with a cluster of small black spots about the larger ends. The clutch comprised three, a number which was not exceeded in any of the other nests. The notes relating to this set are as follows: “Tucson, June 11. Nest pensile between the forks of a small mesquite branch, about five feet from the ground, in a 145thicket of weeds and brush. Incubation commenced. Female shot. This species seems to abandon a nest if it is found before any eggs are laid.”
205, ♂ ad., Cienega Station, April 15. Length, 5; extent, 7.10; wing, 2.21; tail, 2.25. “Iris dark brown; bill dark above, light below; legs dark.”
235, ♀ ad., Tucson, April 19. Length, 5.10; extent, 7.30; wing, 2.23; tail, 2.25.
262, ♂ ad., Tucson, April 22. Length, 6; extent, 7.10; wing, 2.28; tail, 2.34.
275, ♂ ad., Tucson, April 25. Length, 5; extent, 7; wing, 2.21; tail, 2.25.
276, ♀ ad., same locality and date. Length, 4.90; extent, 6.90; wing, 2.18; tail, 2.25.
282, ♂ ad., same locality and date. Length, 5; extent. 7.10; wing, 2.30; tail, 2.30.
461, ♀ ad., Camp Lowell, May 31. Length, 5; extent, 6.90; wing, 2.21; tail, 2.25. “Laying.”
499, ♀ ad., Tucson, June 7. Length, 5; extent, 6.90. Skin lost.
589, ♀ ad., Camp Lowell, June 24. Length, 4.80; extent, 6.80; wing, 2.21; tail, 2.25.
49. Vireo vicinior Coues. Gray Vireo.—The only individuals met with were a male and female—apparently a mated pair—which were taken at Tucson, on April 26. “They were in low brush and were very shy.”
286, ♀ ad., Tucson, April 26. Length, 5.60; extent, 8.20; wing, 2.63; tail, 2.67; tarsus, .80.
287, ♂ ad., same locality and date. Length, 5.60; extent, 8.30; wing, 2.58; tail, 2.70; tarsus, .80. “Iris dark brown; bill plumbeous, darkest above; legs light plumbeous.”
50. Lanius ludovicianus excubitorides (Sw.) Coues. White-rumped Shrike.—“Common and generally distributed.”
It is unfortunate that so much prominence has been given to the white rump of excubitorides as a distinguishing character, for I have yet to see a good series of Shrikes from any Western locality, excepting, possibly, Arizona, which did not afford a considerable percentage of dark-rumped birds; and conversely, it is by no means difficult to find light-rumped specimens in the East. The same instability also affects most of the other characters which have been assigned to excubitorides, as is sufficiently shown by the various conflicting rulings of the authorities regarding the precise definition and limits of distribution of this troublesome race. The only differential points which seem to me to hold good with any number of specimens, are the lighter, purer ash of the upper parts as compared with those of ludovicianus, and the smaller and very much weaker bill. 146But if these alone are to be depended upon, it becomes necessary to limit the distribution of ludovicianus proper to the Gulf States, Georgia and the Carolinas, if not strictly to Florida, and to refer all representatives from the United States at large, east of California, to excubitorides: and this course, I believe, will ultimately have to be adopted. The proper position of the dark California form which is so curiously like ludovicianus remains to be satisfactorily determined.
51. Ampelis cedrorum (Vieill.) Baird. Cedar Waxwing.—Met with but once, at Galeyville, where on January 12, 1881, several were shot from a small flock. Mr. Henshaw took a single specimen near Camp Apache, in September, 1873.
52. Progne subis (Linn.) Baird. Purple Martin.—“Common.”
438, ♂ ad., Tucson, May 26. Length, 7.6; extent, 15.7; wing, 5.45. “Iris dark brown; bill black; legs blackish.”
53. Petrochelidon lunifrons (Say) Lawr. Cliff Swallow.—At Yuma. “They were breeding abundantly along a bluff above the town.”
54. Tachycineta bicolor (Vieill.) Caban. White-bellied Swallow.—“Common in the migrations.”
195, ♂ ad., Cienega Station, April 15. “Iris dark brown; bill black; legs brown.”
55. Tachycineta thalassina (Swains.) Caban. Violet-green Swallow. “Common.”
212, ♀ ad., Cienega Station, April 16. “Iris dark brown; bill and legs black.”
56. Stelgidopteryx serripennis (Aud.) Baird. Rough-winged Swallow.—Common. Breeds.
211, ♀ ad., Cienega Station, April 16. “Iris and legs dark brown.”
57. Pyranga ludoviciana (Wils.) Bp. Louisiana Tanager.—Santa Rita Mountains. “They frequent oaks, and are not very common.”
408, ♂ ad., Santa Rita Mountains, May 18. Length, 7.30; extent, 7.60; wing, 3.80; tail, 3.17. “Iris dark brown; bill blackish horn-color above, greenish-yellow below.”
58. Pyranga hepatica Swains. Liver-colored Tanager.—This Tanager was not uncommon in the Santa Rita Mountains, where the first specimen was taken on May 12. “They range from the foot-hills, through the oaks to the lower pines on the mountains.”
359, ♀ ad., Santa Rita Mountains, May 12. Length, 7.80; extent, 12.10; wing, 3.75. “Bill black above, bluish horn-color below; legs lead-color; iris brown.”
147377, ♂ ad., Santa Rita Mountains, May 14. Length, 8.20; extent, 12.70; wing, 4.20.
380, ♀ ad., Santa Rita Mountains, May 14. Length, 8.10; extent, 12.40; wing, 4.07. “This bird would have laid in about ten days.”
386, ♂ ad., Santa Rita Mountains, May 15. Length, 8.20; extent, 12.80; wing, 4.10.
59. Pyranga æstiva cooperi Ridgw. Cooper’s Tanager.—Mr. Stephens found this bird rather common at a point about five miles south of Tucson, where it frequented the cottonwoods along a small river. He also informs me that in May, 1875, he took several specimens on the Rio Grande River, between Albuquerque and Mesilla, and some others on the Gila, in New Mexico, during May and June.
227, ♂ ad., Tucson, April 19. Length, 8.10; extent, 12.40; wing, 3.83; tail, 3.50. “Iris brown; bill pale horn-color; legs pale brown. Skin very tender. The first seen this season.”
268, ♂ ad., Tucson, April 23. Length, 8.20; extent, 12.40.
297, ♂ ad., Tucson, April 27. Length, 7.90; extent, 12.20; wing, 4; tail, 3.60.
515, ♂ ad., Tucson, June 10. Length, 8.10; extent, 12.20; wing, 3.85; tail, 3.60.
522, ♂ im. Tucson, June 11. Length, 8; extent, 12.20; wing, 3.78; tail, 3.46. In mixed yellow and red plumage.
526, ♂ ad., same locality and date. Length, 8.10; extent, 12.50; wing, 3.89; tail, 3.45.
579, ♂ ad., Camp Lowell, June 23. Length, 8; extent, 11.60; wing, 3.99; tail, 3.58.
339, ♀ ad., Tucson, May 7. Length, 7.90; extent, 12.20; wing, 3.75; tail, 3.39.
41. Carpodacus purpureus (Gm.) Bd. Purple Finch.—Common.
42. Astraga1inus tristis (Linn.) Cab. Goldfinch.—Common. Though somewhat beyond the limits of my subject, I quote the following from Mr. McLeod’s notes: “This winter [1876–77] they have been abundant, although the season is very severe. I have seen them at this time of year but once before.” The Goldfinch has been supposed not to winter north of Massachusetts.
14843. Chrysomitris pinus (Wils.) Bp. Pine Finch.—Seen in May at Grand Falls. Mr. H. A. Purdie tells me that he observed it at Houlton in June, 1878.
44. Passerculus sandwichensis savanna (Wils.) Ridgw. Savanna Sparrow.—Common in the pastures at Grand Falls. At Fort Fairfield it was common. It was found in grassy fields, especially along the roadsides.
45. Poœcetes gramineus (Gm.) Bd. Grass Finch.—Common at Fort Fairfield. Some seen in the open fields at Grand Falls.
46. Melospiza fasciata (Gm.) Scott. Song Sparrow.—Abundant at Grand Falls. It was common at Fort Fairfield.
47. Melospiza palustris (Wils.) Bd. Swamp Sparrow.—“Not common” at Houlton. Not found at Fort Fairfield or Grand Falls.
48. Junco hyemalis (Linn.) Scl. Black Snowbird; “Bluebird.”—Very common at Fort Fairfield. At Grand Falls it was very abundant everywhere.
49. Spizella socialis (Wils.) Bp. Chipping Sparrow.—This bird was quite abundant at Grand Falls. The nests found were not the loose structures they are in Massachusetts, but were well lined with hair. It was rather common at Fort Fairfield.
50. Zonotrichia albicollis (Gm.) Bp. White-throated Sparrow.—Very abundant at Grand Falls wherever there was dead wood on the ground. At Fort Fairfield also it was very abundant; this bird and Junco hyemalis were the commonest species. The nests were apt to be in a clearing near the edge of woods, and frequently were in damp places. They were often under a fallen branch, or at the foot of a sapling, and were but slightly concealed.
The White-crowned Sparrow is probably only a migrant through this section. With regard to its abundance, however, I quote the following from Mr. McLeod’s notes: “These Sparrows make their first appearance from May 10th to 18th. Some seasons they are very abundant, scores of them at a time feeding in my garden. By June 1 they have disappeared. In the autumn I have seen but one flock of them.”
51. Zamelodia ludoviciana (Linn.) Coues. Rose-breasted Grosbeak.—Common in low hard woods at Grand Falls. Rather common at Fort Fairfield, apparently more so than in eastern Massachusetts. Rather common at Houlton.
52. Dolichonyx oryzivorus (Linn.) Swains. Bobolink.—Apparently not rare at Fort Fairfield. Found in grassy fields and meadows near the river. Not observed at Grand Falls. At Houlton “arrives by the 25th of May, common by June 15.” July 2, on our return from Fort Fairfield, Mr. Dwight and I saw them at several places along the St. John River above Fredericton.
53. Agelæus phœniceus (Linn.) Vieill. Red-winged Blackbird.—“Quite common at Eel River, ten miles from Houlton” (R. R. McL.). It does not occur at Fort Fairfield or Grand Falls.
14954. Quiscalus purpureus æneus Ridgw. Crow Blackbird.—Common at Fort Fairfield, in the town, along the river, and about a small pond back in the woods. At Grand Falls it was not uncommon about the town. “Very common” at Houlton.
55. Corvus corax Linn. Raven.—Rare at Grand Falls. Not met with at Fort Fairfield. “Very rare” at Houlton.
56. Corvus americanus Aud. Crow.—Common.
57. Cyanocitta cristata (Linn.) Strickl. Blue Jay.—Common at Grand Falls. At Fort Fairfield it was rather common, but shy and seldom seen.
58. Perisoreus canadensis (Linn.) Bp. Canada Jay.—At Houlton: “very common. These birds do not often appear in the thickly settled part of the town, but are very abundant around the lumber camps in this vicinity.” This no doubt explains the fact that the species was not seen by any of us at Grand Falls and Fort Fairfield.[72]
59. Tyrannus carolinensis (Linn.) Bd. Kingbird.—Rather common at Fort Fairfield. At Grand Falls several were seen, but it was not common.
60. Myiarchus crinitus (Linn.) Caban. Great Crested Flycatcher.—In June, 1878, Messrs. H. A. Purdie and Ruthven Deane observed a pair nest-building at a point in New Brunswick about six miles east of Houlton.
61. Sayornis fuscus (Gm.) Bd. Pewee.—One was observed at Fort Fairfield, June 28. “Very rare” at Houlton.
62. Contopus borealis (Swains.) Bd. Olive-sided Flycatcher.—Common in the woods at Grand Falls. This species was rather common at Fort Fairfield. We usually saw them perched on the tops of tall dead trees in clearings. They were rather shy.
63. Contopus virens (Linn.) Caban. Wood Pewee.—At Fort Fairfield it appeared to be not uncommon. It was not met with, however, at Grand Falls.
64. Empidonax flaviventris Bd. Yellow-bellied Flycatcher.—At Fort Fairfield this species was rather common in wet evergreen woods, especially in those that had small streams flowing through them. It was not observed at Grand Falls. Messrs. Purdie and Deane found it rather common at Houlton in June, 1878.[73]
65. Empidonax trailli (Aud.) Bd. Traill’s Flycatcher.—Not common at Grand Falls. They were to be found mostly where there were scattered dead trees. We did not find it at Fort Fairfield. Mr. H. A. Purdie informs me that it was not uncommon at Houlton in June, 1878.
66. Empidonax minimus Bd. Least Flycatcher.—Very abundant in hard woods at Grand Falls. At Fort Fairfield it was rather common.
67. Caprimulgus vociferus Wils. Whip-poor-will.—Mr. McLeod 150notes that there are a few at Houlton during the summer. The species was neither seen nor heard at Fort Fairfield and Grand Falls.
68. Chordeiles popetue (Vieill.) Bd. Nighthawk.—Very abundant at Grand Falls. At Fort Fairfield it was common; they frequented burnt lands.
69. Chætura pelasgica (Linn.) Bd. Chimney Swift.—At Fort Fairfield they were common, breeding both in chimneys and in hollow trees. Common in the burnt country at Grand Falls. Not many were breeding in chimneys, the people disliking to have them there.
70. Trochilus colubris Linn. Ruby-throated Hummingbird.—Common at Grand Falls. At Fort Fairfield it was apparently rather common—we saw several.
71. Ceryle alcyon (Linn.) Boie. Belted Kingfisher.—Rather common at Fort Fairfield. At Grand Falls it was to be seen wherever there was good fishing ground.
72. Picus villosus Linn. Hairy Woodpecker.—Common.
73. Picus pubescens Linn. Downy Woodpecker.—At Fort Fairfield this species was much less common than P. villosus. It was not uncommon at Grand Falls.
74. Picoides arcticus (Swains.) Gray. Black-backed Three-toed Woodpecker.—Common at Grand Falls in burnt cedar swamps. At Fort Fairfield we shot two, all we saw.
75. Sphyropicus varius (Linn.) Bd. Yellow-bellied Woodpecker.—Common—the commonest Woodpecker—at Fort Fairfield. They were generally found about recent clearings, or in the more open mixed woods. At Grand Falls they were common in hard woods.
76. Hylotomus pileatus (Linn.) Bd. Pileated Woodpecker.—At Grand Falls half a dozen pairs were seen. Probably there is too little of the heavy forest left in the immediate neighborhood of Fort Fairfield to suit their tastes, as we did not meet with them. “Common” at Houlton.
77. Colaptes auratus (Linn.) Sw. Golden-winged Woodpecker.—Rather common at Fort Fairfield. Not common at Grand Falls.
78. Coccyzus erythrophthalmus (Wils.) Bd. Black-billed Cuckoo.—Mr. McLeod records this bird in his notes, but without comments. It was not seen at Fort Fairfield or Grand Falls.
79. Strix nebulosa Forst. Barred Owl.—“Very common” at Houlton. We were shown a mounted specimen by Mr. Frank P. Orcutt at Fort Fairfield. He considered it the commonest Owl.
80. Nyctale acadica (Gm.) Bd. Saw-whet Owl.—This bird is not uncommon at Houlton. Mr. Frank P. Orcutt told us that it was tolerably common at Fort Fairfield.
81. Bubo virginianus (Gm.) Bd. Great Horned Owl.—“Very common” at Houlton. Mr. Orcutt said it was rather common at Fort Fairfield.
82. Circus hudsonius (Linn.) Vieill. Marsh Hawk.—Rare at Houlton. One seen at Fort Fairfield.
83. Accipiter cooperi Bp. Cooper’s Hawk.—Not common at Grand Falls. Not observed at Fort Fairfield or Houlton.
15184. Accipiter fuscus (Gm.) Bp. Sharp-shinned Hawk.—“Not common” at Houlton.
85. Falco sparverius Linn. Sparrow Hawk.—Commonest Hawk at Grand Falls. Not met with at Houlton or Fort Fairfield, though Mr. Orcutt considers it common at the latter place.
86. Buteo borealis (Gm.) Vieill. Red-tailed Hawk.—Not common at Grand Falls. Not observed at Fort Fairfield. “Common” at Houlton.
87. Buteo pennsylvanicus (Wils.) Bp. Broad-winged Hawk.—Not common at Grand Falls. It was found breeding at Houlton, but not met with at Fort Fairfield.
88. Haliæetus leucocephalus (Linn.) Savig. Bald Eagle.—“Not common” at Houlton.
89. Ectopistes migratorius (Linn.) Sw. Wild Pigeon.—Breeding at Grand Falls, but not common.
90. Canace canadensis (Linn.) Bp. Spruce Partridge.—At Houlton “mostly found in the deep fir thickets, or in the swamps of firs and cedars.” Not met with at Fort Fairfield and Grand Falls, though of course it occurs there.
91. Bonasa umbellus (Linn.) Steph. Ruffed Grouse.—Rather common at Fort Fairfield. At Grand Falls only a few were seen—in the hard woods.
92. Ardea herodias Linn. Great Blue Heron.—“Common” at Houlton.
93. Nyctiardea grisea nævia (Bodd.) Allen. Night Heron.—“Not common” at Houlton.
94. Botaurus lentiginosus (Montag.) Steph.—Bittern.—“Common” at Houlton. One seen at Grand Falls.
95. Philohela minor (Gm.) Gray. Woodcock.—One seen on Little River Flats near Grand Falls. At Fort Fairfield we saw a specimen in the collection of Mr. Frank P. Orcutt, who considered it rare in that neighborhood. “A few breed in the vicinity” of Houlton.
96. Rhyacophilus solitarius (Wils.) Cass. Solitary Sandpiper.—At Grand Falls some were seen along the river June 9 (J. A. J.).
97. Tringoides macularius (Linn.) Gray. Spotted Sandpiper.—At Fort Fairfield it was very numerous along the Aroostook River, and was also noticed in one or two other places. It was abundant along the rivers at Grand Falls. At Houlton too it was very common.
98. Porzana carolina (Linn.) Bd. Carolina Rail.—One seen at Fort Fairfield, June 20, in a wet meadow partly grown up with alder bushes (J. D.).
99. Anas obscura Gm. Black Duck.—“Very common, breeding” at Houlton.
100. Aix sponsa (Linn.) Boie. Wood Duck.—“Quite common” at Houlton.
101. Clangula glaucium americana (Bp.) Ridgw. Golden-eye.—A few seen at Grand Falls.
152102. Mergus merganser americanus (Cass.) Ridgw. Sheldrake.—Not uncommon at Grand Falls.
103. Mergus serrator Linn. Red-breasted Merganser.—“Very common, breeding,” at Houlton.
104. Larus argentatus smithsonianus Coues. Herring Gull.—At Houlton it is common on the neighboring lakes, where it breeds.
105. Podilymbus podiceps (Linn.) Lawr. Pied-billed Grebe.—Rare, breeds, Houlton.
That there remained unrecognized at this late day a bird regularly inhabiting one of the must populous portions of our country; or, indeed, that a species of eminently boreal habitat during its breeding season, and not known to occur at all at such time within the limits of the United States, should have a representative race regularly breeding in our midst, are facts for which we were little prepared. Mr. Ridgway’s recent paper[74] announcing these facts being necessarily of a technical nature, and confined to a formal description of the new Thrush, it has been thought well on the present occasion to allude more particularly to the character of the locality inhabited by the bird, and to some of its associates there, in connection with other sequential considerations. As the general physical character of the Catskill Mountains and the faunal features of the region will be treated by the writer elsewhere, it will be unnecessary to extend the range of the present relation from the summit of Slide Mountain in Ulster Co.,[75] where the new race was discovered.
On June 15, 1881, nearing the summit of this mountain, the forests of a more northern latitude were forcibly suggested. A shower had fallen during the ascent, and the sun was still obscured, 153while a sharp wind from the northwest piercing the wet woods and sighing among the balsams, blasted and weather beaten, heightened an impression of remoteness and desolation. The evergreens, constituting the principal arboreal growth, extended off on all sides, clothing the rocky and moss-grown slopes, and presenting the striking contrast of a young and fragrant second growth clustering about the branchless and spiny trunks of their sires tottering in decay; or, with tangled and matted branches outlined here and there, as we approached the summit, against a gray and cheerless sky. Owing to the comparatively short life of these trees, that high portion of the mountain where their tribe had pitched was brought into grim contrast with its surroundings. Old age and death, continually present invading their ranks, had everywhere left their traces; flourishing clusters had been stricken in their fellowship, groups and gatherings had been divided and scattered, and like a contagion the destroyer had spread among their hosts. But the younger generations are continually forming their associations, and with green and fragrant grouping filling in deserted chambers and screening the devastation that has gone before, although only to furnish material for its continuance in the future. All this, with an occasional undergrowth of greater or less luxuriance, gave a diversified and somewhat open character to the surroundings, entirely dissimilar to that of the environing forest; conditions, which, in conjunction with humidity and elevation, have brought this mountain top into some relation with the swampland of a more northern region.
Reaching a more elevated portion of the ridge where the ground was more level and the surface less rocky, that north-woods tree, the Paper Birch (Betula papyracea) occasionally appeared, and more abundantly the Mountain Ash. Almost the only remnant of the dense mountain forests below was the Yellow Birch (Betula lutea) which, joining the undergrowth, persisted with small and stunted stature to the summit. On all sides were to be seen the white blossoms of Viburnum lantanoides which, though also found in the valley woodlands, had there long since flowered and was now bearing green fruit. Another characteristic shrub was Amelanchier canadensis oligocarpa; lower down had been found the var. botryapium, but here, the northern form was well marked, seeming almost specifically distinct. In the deep, damp moss, covering and filling in the rocks beneath the balsam growth, 154and relieving the ruggedness of the slopes, northern plants were growing in greater or less profusion. The Dwarf Cornel (Cornus canadensis) grew in such close luxuriance in congenial spots, that its snowy bracts imparted an almost uniform whiteness to whole beds. With, or near it, blossomed the Wood Sorrel (Oxalis acetosella) with delicately violet-veined petals, and the appropriately-named Gold-thread (Coptis trifolia) of evanescent bloom but shining evergreen leaves, and the little Star Flower (Trientalis americana) were often also associates. Excepting the pale yellow bells of Clintonia borealis, and the purplish tinge, or veining, of the blossoms of several other species, all the plants noticed in bloom at this time upon the mountain bore flowers of some shade of white. The more open ground about our course along the ridge supported a luxuriant and graceful growth of that lovely fern Aspidium spinulosum, and with it, in openings about the summit, grew abundantly the Mountain Golden-rod (Solidago thyrsoidea) which, although yet many weeks from bloom, heralded a royal emblem to light the mountain’s brow ere the white locks of winter should again possess it.
At the elevation where these plants first appeared the trees nowhere attained more than a medium stature, those which seemed best to have surmounted the difficulties of their situation, the Balsam and the Paper Birch, never rising to a height of more than, perhaps, twenty-five feet. This growth completely encompassed the range of vision, but an occasional scantiness in the foliage permitted glimpses of surrounding mountains rolling off like huge green billows into the blue distance.
From these evergreens came the leisurely call of the Canada Nuthatch (Sitta canadensis), and on closer approach the low, plaintive notes of the little Yellow-bellied Flycatcher (Empidonax flaviventris). The brief warble of the Black-and-Yellow Warbler (Dendrœca maculosa) told of the presence of its unseen author in the surrounding trees, while among the undergrowth the less frequent, but louder and more sustained song of the Mourning Ground-warbler (Geothlypis philadelphia) showed that this species, which had been left at the foot of the mountain, had here reappeared. At intervals, faintly mingling with these songs, from some hidden fastness below, came the fantasia of the Winter Wren, a melody that seemed to pass from the spirit of unclaimed nature, voicing some mystery of the mountains. 155The clamor of a party of Blue Jays occasionally arose and died away in the forest, but here, in this mountain solitude, their screams seemed more subdued than in less primitive regions, and lacked that suggestion of consciousness which individuals constantly within human hearing, seem to acquire. Busily roaming Chickadees (Parus atricapillus) at times came about our path, and the Snowbird (Junco hyemalis) was present with its simple song. Olive-backed Thrushes (Hylocichla ustulata swainsoni) too, were constantly to be heard, and finally, guided by its near song, one was followed up and secured. A moment later another Thrush darted across the path, and disappearing through a young balsam growth, immediately began to sing a few rods off. The song was different from that of the bird which had just been shot, so much so, in fact, as to be remarked even by my guide. It seemed to be more uniform in character, with less variation and definition of the notes; as I wrote in my note-book at the time—more suggestive of the song of H. fuscescens. A conspicuous point of difference was that it was more subdued in tone, in fact of a somewhat ventriloquous nature. On examining the bird, in hand, although I had thought myself familiar with all our eastern Hylocichlæ, I must confess to having been puzzled. It was obviously neither the Olive-backed nor the Hermit Thrush, the only species of our own smaller Thrushes which from the distribution of their group (as then understood) could possibly be expected to occur. I at once noted its general resemblance to the Gray-cheeked Thrush, but it seemed impossible that this Hudsonian bird could be found so far south at this season; and though a second specimen pointed more strongly toward it, it was not until I had reached home and made actual comparisons, that I could feel satisfied that its true relationship was with that species. I had long noticed certain somewhat constant differences between examples of aliciæ occurring at New York on their migrations, and incited by these specimens went carefully over my series of seventeen examples and found them separable into two forms, characterized by slight differences in coloration and a notable difference in size. The examples from the Catskills were more closely allied to the smaller of the two forms, and these, with, subsequently, my entire series, were submitted to Mr. Ridgway, the result being the recognition of a new bird, belonging to our eastern fauna.
156But to return to the mountain. It would hardly be justifiable to make a positive statement about a difficult song that had been but once identified, but I feel positive that the Thrushes which were last heard that evening about our camp on the extreme summit of the mountain were of the new form. Night was rapidly falling, and the valleys were in darkness, when one sang several times near the camp, and for some time afterwards a single call-note was occasionally heard, and the varying distance of the sound showed that the birds were still active. Excepting these sounds, the last bird notes heard were those of the Yellow-bellied Flycatcher.
The sharp northwest wind continued late, and the night became clear and cold. Shortly after midnight the bright moon showed the temperature, by a thermometer which I had hung beside the camp, to be 35°, and at sunrise it stood at 32°. Before daylight I was standing on a boulder of conglomerate on the dim mountain’s brow listening for the awakening of the birds. The first songs heard were those of the Hermit Thrush, Snowbird, and Yellow-bellied Flycatcher, which began almost simultaneously, followed a little later by those of the Olive-backed Thrush and the Mourning Warbler, but H. bicknelli was not heard, or at least not near enough to be distinguished among the other species.
The increasing light upon the mountain seemed to attract the birds from below, whither, perhaps, they had retired for the night, and soon many different notes were to be heard about the camp; not, however, in that boisterous chorus with which the day is often announced about our homes, in which the notes of many individuals of many species are blended in such confused medley that separate voices are almost indistinguishable, but simply the association of a few vocalists, the very isolation of whose position endowed their voices with an additional interest and charm.
After those already mentioned the Black-poll Warbler (Dendrœca striata) began its unpretending notes, which always to me suggest a short dotted line, and this song, with that of the Black-and-Yellow Warbler, occasionally alternated about us in agreeable contrast. Now and then a Canada Nuthatch, on its morning tour, tarried to inspect some dead trunk or thinly clothed tree, upon the projecting apex of which, or that of some companion, 157a solitary Purple Finch occasionally alighted, and with a few wild fugitive notes was gone, to other mountain tops or the forests of the descending slopes.
But to revert to the Thrushes. The two specimens of the new form which were obtained were both males, and were unquestionably breeding,[76] though no nest known to belong to their species was found.
It remains to briefly consider some facts furnished by the birds’ occurrence as narrated. These facts bear directly on the long contested question of the relationship which H. aliciæ and H. swainsoni bear to one another, and it can scarcely be denied that the present evidence on this point is conclusive. Not only have we representatives of both birds preserving their respective identities at the same locality, under identical conditions of environment, but examples of each taken under these circumstances, display, except in size, even a greater dissimilitude than those which occur together on their migrations. There is but one tenable interpretation of these facts: the birds—Hylocichla aliciæ and H. ustulata swainsoni—are wholly and entirely distinct. Any theory of dichromatism which might be advanced, aside from its extreme unlikelihood, would be shown inadequate by the relative differences in proportions of parts which the two birds exhibit. These differences, as well as those of color are illustrated by the Catskill birds. A specimen of H. swainsoni taken at the top of Slide Mountain was in every way typical of its species, and conspicuously unlike the examples of bicknelli taken at the same time. Aside from differences in the proportions of parts, the two birds were strikingly different in color, the decided grayish olive tinge of the superior surface of swainsoni contrasting strongly with the much darker brownish cast of its congener. One example of the latter instead of showing indications of a buffy tinge about the sides of the head and on the breast, which under the circumstances we should expect to be the case, were it in any way specifically related to swainsoni, has absolutely no indications whatever of this shade about the sides of the head, and actually less on the breast than any specimens 158of true aliciæ that I have seen, and this little most evident low down where the corresponding shade in swainsoni begins to pale. It seems probable that this newly recognized race of aliciæ is responsible for much of the ambiguity which the discussion of both species by different writers has occasioned. Indeed, it seems to occupy the same position relative to aliciæ proper which, by some, swainsoni was supposed to hold, viz., the more southern-born individuals of the species, but that it represents a link specifically connecting the two, the facts already presented refute. As it occurs with true aliciæ on the autumn migration most specimens of the new form are paler and more brownish in color above, and their general size is nearly that of swainsoni,[77] and these differences may be regarded by some as approaches towards the latter species. In both species there is a wide individual variation, but the closest approach of each towards the other never exceeds that limit within which each may vary without its specific distinctness being compromised. I have yet to see a specimen of either which would admit of the slightest question as to its identity. I speak thus of adult birds. In such closely related species the young must almost necessarily approximate, and to these we must appeal for light on the things that have been—on the question of origin—whether one has been derived from the other, or both species from a common ancestor. Such obscure insight into this point as I have been permitted seems to indicate that the latter alternative will be found to be the more correct, but, for the present, from lack of the necessary data this important subject is proscribed.
It is unnecessary here to repeat the diagnosis of the new form of Hylocichla aliciæ given by Mr. Ridgway in the paper before cited. As this writer states, the race breeds “probably in other mountainous districts of the northeastern United States” than the single locality where it was discovered, and it seems very singular that up to the present time we have no knowledge of its occurrence in the summer season elsewhere, even in regions where the two congeneric species with which it was here associating—H. 159nanus[78] and H. swainsoni—are well known to be common summer residents. The occurrence of a representative of H. aliciæ in the United States at all during its breeding season is a matter of surprise, especially when we recollect the boreal distribution of the typical form during that period, and read[79] that so far towards the north as the Yukon and the Great Slave Lake it occurs “only as a bird of passage to and from more northern breeding grounds.” Additional information respecting the distribution of the new race will be awaited with great interest.
As the avian fauna of the lower Mississippi Valley is now receiving some attention,[80] it seems well that I should contribute my mite of information to the general fund.
Bayou Sara and the adjoining town of St. Francisville, in the parish of West Feliciana, are situated on the east bank of the Mississippi River, 170 miles above New Orleans by that stream and about 80 miles in an air line northwest of it. It is 30 or 40 miles north of Baton Rouge, near which place Dr. Langdon made his observations in April, 1881. The following notes were made principally on and near “Wyoming,” two miles from the river, the plantation of Ex-Gov. R. C. Wickliffe, a place possessing peculiarly agreeable ornithological associations on account of its former owner, Gen. Dawson, having entertained Audubon as his guest for several months. It will be remembered that the type specimen of Buteo harlani was captured here.
160The topography is much more interesting, and is quite different from that farther south and that immediately opposite on the west side of the river. A level plateau, 100 feet above the levee, begins about a quarter of a mile from the river and extends back into the State of Mississippi. This plateau is deeply cut by numerous creeks and ravines, the banks of which are generally densely wooded, with water oak, sweet-gum, cedar, prickly ash, magnolias, etc. All of the level ground on top is in a state of cultivation; cotton being the principal crop. A few miles farther up the high ground does not extend so near the river, the intervening space being occupied by several small lakes and swamps—a great resort for water birds of all kinds. On account of the high water I did not have an opportunity of visiting this interesting field.
My observations extended only over a period of five days from April 15th to 19th, 1882, inclusive, but a great deal of ground was canvassed in that time; nearly the whole of each day being spent in the field. A good many birds were shot, but few were preserved, as taxidermy was necessarily subordinated to field-work. Dr. Langdon in his interesting paper particularly remarks the absence of the Catbird, Black-and-White Creeper, White-browed Yellow-throat, Kentucky Warbler, Large-billed Water Thrush, and the Redstart, but I found all of these at “Wyoming,” together with many others not noted by either him or Mr. Hay, the Catbird and Kentucky Warbler being particularly abundant.
The writer was greatly assisted in his work by Mr. Robert Wederstraudt of “Wyoming,” a young man whose unusually close and accurate observations of birds and bird life rendered his help peculiarly valuable. Many of the following notes are credited to him entirely. I have followed the nomenclature of the Smithsonian list of 1881.
1. Hylocichla mustelina (Gm.) Bd. Wood Thrush.—Common in woodland, and several seen in the yard near the house.
2. Merula migratoria (L.) Sw. and Rich. American Robin.—Not observed. They appear here in large numbers early in February to feed on the fruit of the “wild peach,” and hundreds are shot for the table. They leave early in March.
3. Mimus polyglottus (L.) Boie. Mockingbird.—Very abundant, both in the town about gardens and yards, and in the country. Frequenting open ground exclusively. Four sets of eggs were taken; two perfectly fresh, and two about half incubated. Mr. Wederstraudt called my attention to a curious foraging habit of this bird. We noticed one hopping 161along the ground in an open grassy place, pausing at every three or four hops to extend and close its wings. It repeated this several times until a grasshopper was flushed, when the bird immediately “reached” for it, and having captured it, made off to a neighboring bush to eat it. Mr. Wederstraudt says that he has observed this interesting performance many times.
4. Galeoscoptes carolinensis (L.) Cab. Catbird.—Abundant in the shrubbery in the creek bottoms. None were seen near the dwellings.
5. Harporhynchus rufus (L.) Cab. Brown Thrasher.—Abundant in same places as the last. Three clutches of three eggs each were taken, in one of which incubation was very far advanced, and on the 19th a nest was found containing two young nearly able to fly.
6. Sialia sialis (L.) Haldem. Bluebird.—Observed several pairs in town and in the country. Not as common as in Kentucky.
7. Polioptila cærulea (L.) Scl. Blue-gray Gnatcatcher.—A common, and, on account of its active and noisy habits, conspicuous bird.
8. Lophophanes bicolor (L.) Bp. Tufted Titmouse.—Not very common. Frequenting principally the tops of trees.
9. Parus carolinensis Aud. Carolina Chickadee.—But few observed. A pair bred in a hole in a cedar post within twenty yards of the house last year.
10. Thryothorus ludovicianus (Gm.) Bp. Carolina Wren.—Very abundant everywhere. A clutch of three eggs was taken on the 19th from a nest in a small recess formed by the junction of several timbers, under the piazza, which was frequented at all times of the day. The nest was empty on the 16th, one egg was deposited on the 17th, one on the 18th, and one on the 19th. I saw neither of the old birds about the place at all, and it was only by capturing the female on the nest at night, that the eggs were positively identified. A pair have bred about this piazza for many years, I am informed.
11. Mniotilta varia (L.) V. Black-and-white Creeper.—A male, the only one seen at all, was captured in a dense wood on the 17th.
12. Parula americana (L.) Bp. Blue-yellow-backed Warbler.—Very abundant. A persistent but weak vocalist.
13. Dendrœca æstiva (Gm.) Bd. Summer Yellowbird.—Common in open places.
14. Dendrœca blackburniæ (Gm.) Bd. Blackburnian Warbler.—Common in large trees about open ground.
15. Dendrœca dominica albilora Bd. White-browed-yellow-throated Warbler.—A male, the only one seen, was shot out of a magnolia tree on the 10th. In all of my Kentucky specimens of this bird the anterior portion of the superciliary line has a trace of yellow. In this one no yellow is perceptible.
16. Dendrœca pinus (Wils.) Bd. Pine-creeping Warbler.—Apparently not uncommon. Preferring open ground. In song.
17. Siurus auricapillus (L.) Sw. Golden-crowned Thrush.—One specimen captured in a thicket on the 15th.
18. Siurus motacilla (V.) Coues. Large-billed Water Thrush.—Heard 162one singing in a densely wooded ravine on the 17th. Mr. Wederstraudt has often seen them in pairs along the smaller water-courses.
19. Oporornis formosa (Wils.) Bd. Kentucky Warbler.—One of the most abundant inhabitants of the dense growth along the ravines. Two or three were often heard singing at the same time.
20. Geothlypis trichas (L.) Cab. Maryland Yellow-throat.—Abundant in the usual places.
21. Icteria virens (L.) Bd. Yellow-breasted Chat.—Very abundant. In full song.
22. Myiodioctes mitratus (Gm.) Aud. Hooded Warbler.—Found in same places, and almost as abundant as the Kentucky Warbler. An inhabitant of the undergrowth principally. In song; its note being uttered at intervals of 15 or 20 seconds as it hops from branch to branch in pursuit of insects.
23. Setophaga ruticilla (L.) Sw. Redstart.—A single specimen, a male, captured in a swamp. It was in company with a female.
24. Vireosylvia olivacea (L.) Bp. Red-eyed Vireo.—Very abundant everywhere.
25. Vireosylvia gilva (V.) Cass. Warbling Vireo.—Heard one singing in a shade tree in Bayou Sara on the 15th.
26. Vireo noveboracensis (Gm.) Bp. White-Eyed Vireo.—Very abundant and voluble everywhere.
27. Lanius ludovicianus L. Loggerhead Shrike.—Not observed. Mr. Wederstraudt says that they are not uncommon here in the fall. He once saw one kill and devour a small bird in a thorn tree.
28. Ampelis cedrorum (V.) Bd. Cedar Waxwing.—Observed several small flocks. Said to be very abundant here in winter when numbers are shot for the table. Known here as the “ortolan”—the fourth bird, I believe, embraced under that comprehensive name.
29. Progne subis (L.) Bd. Purple Martin.—Common about Bayou Sara and St. Francisville.
30. Stelgidopteryx serripennis (Aud.) Bd. Rough-winged Swallow.—Very abundant. Beginning to breed. Several holes examined but no eggs found. One was shot out of a dead tree.
31. Pyranga æstiva (L.) V. Summer Redbird.—Abundant about dwellings and open ground. In song.
32. Passerculus sandwichensis savanna (Wils.) Ridgw. Savanna Sparrow.—Common in old wet fields. One individual captured, a female, had a very large tumor on the bill and several smaller ones on the toes.
33. Zonotrichia albicollis (Gm.) Bp. White-throated Sparrow.—Abundant in parties of six or eight in the undergrowth about open places in the low lands.
34. Peucæa æstivalis illinoensis Ridgw. Oak-woods Sparrow.—Two specimens of this interesting form were taken; both males. One was shot from the top of a small bush near the edge of an old corn field; the other from the top of an isolated pine on the edge of a cotton field. 163Both were singing when shot. No others were observed. This, I believe, is the most southeasterly “record” of the form.
35. Melospiza palustris (Wils.) Bd. Swamp Sparrow.—Not uncommon in the usual places.
36. Pipilo erythrophthalmus (L.) V. Chewink; Towhee.— Abundant. Locally known as the “Joree.”
37. Cardinalis virginianus (Briss.) Bp. Cardinal Grosbeak.—Very abundant. Took a set of three fresh eggs on the 17th. Nest as usual.
38. Passerina cyanea (L.) Gray. Indigo Bunting.—Rather common about open places, but very shy. Not in song.
39. Passerina ciris (L.) Gray. Painted Bunting; Nonpareil.—First seen on the 16th. A male in full song captured on the 19th—the only two seen. Mr. Wederstraudt, who has trapped them, using a captive male as a decoy, says that the same individual is always to be found within a few hundred feet of the place where first observed. I saw several males in confinement in New Orleans, and observed that the red of the underparts was heavily blotched and obscured by yellow, and attributed it to immaturity, but was informed that it was due to the confinement. They are called “Pops” here, the derivation of which name I could not make out.
40. Agelæus phœniceus (L.) V. Red-and-buff-shouldered Blackbird.—Abundant in swampy places.
41. Sturnella magna (L.) Sw. Meadow Lark.—Common in old fields. Their note seemed to me to be different from that of the Kentucky bird.
42. Icterus spurius (L.) Bp. Orchard Oriole.—Common about open ground.
43. Icterus galbula (L.) Coues. Baltimore Oriole.—Observed several singing in shade trees in Bayou Sara and St. Francisville.
44. Quiscalus purpureus (Bartr.) Licht. Purple Grackle.—A common Grackle about the river and bayou at Bayou Sara is referred to this form, as the one found forty or fifty miles down the river is according to Dr. Langdon the Purple, and not the Bronzed Grackle.
45. Corvus frugivorus Bartr. Common Crow.—Common.
46. Cyanocitta cristata (L.) Strickl. Blue Jay.—Common.
47. Tyrannus carolinensis (L.) Temm. Kingbird; Bee Martin.—Common.
48. Myiarchus crinitus (L.) Cab. Great-crested Flycatcher.—A common and conspicuous inhabitant of yards and orchards.
49. Contopus virens (L.) Cab. Wood Pewee.—Common in dense timber.
50. Empidonax acadicus (Gm.) Bd. Acadian Flycatcher.—Common in same places as last.
51. Trochilus colubris L. Ruby-throated Hummingbird.—Very abundant about cultivated ground.
52. Chætura pelasgica (L.) Boie. Chimney Swift.—Common.
53. Antrostomus carolinensis (Gm.) Gould. Chuck-will’s-widow.—Heard but one, on the night of the 19th, near the house, but I am told that they are quite common.
16454. Chordeiles popetue (V.) Bd. Night Hawk.—Saw one about dusk on the evening of the 19th, high in air, giving the peculiar call common to the males during the breeding season.
55. Picus pubescens (L.) Downy Woodpecker.—Only two individuals were observed during my visit.
56. Hylotomus pileatus (L.) Bd. Pileated Woodpecker.—Not observed, but it is said to be common in heavy timber along the borders of the swamp.
57. Centurus carolinensis (L.) Bp. Red-bellied Woodpecker.—Rather common. At the time of my departure a pair had begun digging a hole for their nest in a large china tree within thirty yards of the house.
58. Melanerpes erythrocephalus (L.) Sw. Red-headed Woodpecker—A familiar and common bird here; preferring open to densely wooded country.
59. Colaptes auratus (L.) Sw. Yellow-shafted Flicker.—Not observed. Mr. Wederstraudt and others pronounce it an abundant bird here.
60. Ceryle alcyon (L.) Boie. Belted Kingfisher.—Common in open places along Alexander’s Creek and its branches. A clutch of six fresh eggs was taken from a hole in a perpendicular bank on the 16th. The orifice was about thirty-five feet from the bottom, and three and a half from the top of the bank. The hole extended horizontally into the bank for a distance of six feet. The old birds circled around a few times after we began digging for the eggs, and then flew off, apparently unconcerned at our operations.
61. Coccyzus americanus (L.) Bp. Yellow-billed Cuckoo.—One individual observed on the 19th in a large live-oak near the house. In song.
62. Conurus carolinensis (L.) Kuhl. Carolina Parakeet.—Not seen by me. Gov. Wickliffe says that twenty years ago it was quite common here at times in large flocks, and Mr. Wederstraudt has several times observed it within the last few years, but never more than two or three together at a time. About eighteen months ago he saw one in an orchard near “Wyoming.”
63. Scops asio (L.) Bp. Little Screech Owl.—Found here, according to Mr. Wederstraudt.
64. Bubo virginianus (Gm.) Bp. Great horned Owl.—Given as a common inhabitant by Mr. Wederstraudt.
65. Pandion haliaëtus carolinensis (Gm.) Ridgw. American Osprey; Fish Hawk.—Often seen here, according to the natives.
66. Haliaëtus leucocephalus (L.) Savig. Bald Eagle.—Said to occasionally occur here.
67. Cathartes aura (L.) Illig. Turkey Buzzard.—Common.
68. Catharista atrata (Wils.) Less. Black Vulture; Carrion Crow.—Very abundant. I flushed thirty or forty, one day, from the carcass of a dead horse.
16569. Zenaidura carolinensis (L.) Bp. Mourning Dove.—Abundant about open places. I took a clutch of two fresh eggs on the 19th from a nest on a horizontal limb of a water oak, eight feet from the ground.
70. Ortyx virginianus (L.) Bp. Bob white; American Quail.—Abundant in pairs about cultivated ground. They are not much hunted here as the shooting is very difficult, for when flushed they immediately make for the thickets.
71. Ardea herodias (L.) Great Blue Heron.—One was seen on the 19th flying towards the swamp
72. Herodias alba egretta (Gm.) Ridgw. American Egret.—A flock of eight was observed on the 19th flying towards the swamp.
73. Butorides virescens (L.) Bp. Green Heron.—A common bird about the creeks and ponds.
74. Oxyechus vociferus (L.) Reich. Killdeer.—Saw a party of eight on the creek. They were very tame.
75. Philohela minor (Gm.) Gray. American Woodcock.—Not observed. Said to be common here in the fall, when they are hunted in the cotton fields at night with torches.
76. Gallinago media wilsoni (Temm.) Ridgw. Wilson’s Snipe.—Not observed, but said to be common here in early spring.
77. Rhyacophilus solitarius (Wils.) Cass. Solitary Sandpiper.—Two individuals were several times noted about a pond of stagnant water.
78. Tringoides macularius (L.) Gray. Spotted Sandpiper.—Several times observed along the creeks.
79. Rallus elegans Aud. Red-breasted Rail.—One several times seen in a small pond thickly overgrown with small trees, water-lillies etc.
80. Rallus virginianus L. Virginia Rail.—One seen in same place as the last. Both eluded capture.
81. Fulica americana Gm. American Coot.—Not observed, but said to be common here in fall and early spring. Known here by the Creole name of “Poulette d’Eau.”
82. Anas boschas L. Mallard.—Not observed, but it is said to be common here during the migrations.
83. Querquedula discors (L.) Steph. Blue-winged Teal.—Two were shot out of a flock of eight on the 16th on Alexander’s Creek.
84. Aix sponsa (L.) Boie. Wood duck; Summer Duck.—Not observed, but common in the swamp, I am informed.
85. Pelecanus fuscus (L.) Brown Pelican.—Said to breed in the lakes above Bayou Sara.
86. Podilymbus podiceps (L.) Lawr. Thick-billed Grebe.—Not observed, but well known here.
87. Molothrus ater Gray. Cowbird.—Very abundant throughout the year. They come in large flocks into the streets of the city in the winter months to search for food; they also associate at that season with Scolecophagus cyanocephalus Cab. I have never seen anywhere else such numbers of these birds as here, and in the breeding season most of the nests of our small birds contain eggs of this parasite.
87a. Molothrus ater obscurus Coues. Dwarf Cowbird.—A common bird during the breeding season. It is smaller than its near relative, and quicker in its motions. Moves usually in flocks of from two to ten. I first observed the bird when it was just leaving the nest of Lanivireo flavifrons Bd., and found its egg in it, besides four of the Vireo’s. The egg is smaller and not so thickly sprinkled as that of the common Cowbird.
88. Xanthocephalus icterocephalus Bd. Yellow-headed Blackbird.—Very common in marshy localities from the latter part of October to March and April. I think some remain to breed, as I observed small flocks during May in the low prairie districts overgrown with reeds and other water plants. The best opportunity I ever had to study the breeding habits of this beautiful but very locally distributed Blackbird was in the Calumet Marshes near Kensington, about eighteen miles south of Chicago, where I discovered in a single day about fifty nests among the reeds. During the winter months they associate with Molothrus ater, Agelæus phœniceus, and Scolecophagus cyanocephalus; many migrate further south, and in cold winters only a few remain near Houston.
89. Agelæus phœniceus Vieill. Red-winged Blackbird.—Common in swamps, but not so abundant as I have found these birds to be in Wisconsin and Illinois. May 6, 1881, I discovered a nest in a somewhat strange position, in a blackberry-bush (Rubus villosus) on the edge of a thicket; there was no swamp within a mile. This was in the northern part of Harris County. Only a few remain to winter, the greater part migrating farther south.
90. Sturnella magna Swains. Meadow Lark.—Common summer sojourner, and very abundant during winter; many thousands are killed in the latter season by pot hunters. During summer the Meadow Lark is strictly a prairie bird, always to be looked for on the open grassy savannas; 167I never found the bird breeding in a cotton field or corn field. In winter, however, they change their habits, and in large flocks visit the sugar-cane, cotton, and corn fields.
91. Icterus spurius Bp. Orchard Oriole.—Common during migrations.
91a. Icterus spurius affinis Coues. Southern Orchard Oriole.—Very common summer sojourner; breeds in all suitable localities, especially in peach gardens. The bird is decidedly smaller than the northern variety; it is also more active and quicker in its motions. The song is much more varied, and louder, quicker and more beautiful, reminding one of the song of the Baltimore Oriole. The nest is smaller, but it is built of the same materials—green grasses, lined with cotton. May 8, 1881, I discovered a very curious but not quite finished nest near Spring Creek, only a few yards from a dwelling. For several days I had observed a pair of these birds carrying fresh green grasses to a laurel oak (Quercus imbricaria), that was densely covered with large hanging bunches of Spanish moss (Tillandsia usneoides); they disappeared every time into a bunch of moss, yet I could see no nest. At last, on taking down the bunch of moss, I was surprised to find a beautiful structure in my hands. The grasses and moss were all woven firmly together; the entrance was on the side.
92. Scolecophagus ferrugineus Swains. Rusty Blackbird.—Very rare. A few seen in March, 1881, among flocks of the following species.
93. Scolecophagus cyanocephalus Cab. Brewer’s Blackbird.—During winter the most common of the family Icteridæ. They are very abundant in Houston from the early part of November to April, when they disappear for the north; by the end of that month only a few remain to breed in suitable localities. I found several nests May 5, 1881, in thick, small oaks near the Rose Hill Post Office in the northern part of Harris County. They were built in the tops of young post oaks (Quercus obtusiloba), about twelve to fifteen feet from the ground, and contained from two to five eggs each. The nests were composed exteriorly of strong slender plant stems and coarse grasses, and were lined with fine grasses. These birds are very unsuspicious and bold during winter, running about in even the most crowded city streets, and also frequenting door-yards. On cold days they are easily caught. I had a pair over a year in a cage; they soon became reconciled to confinement, and were lively, interesting pets.
94. Quiscalus purpureus æneus Ridgw. Bronzed Grackle.—The most abundant of all the Blackbirds during the breeding season, arriving from their more southern winter quarters early in March. None remain, so far as my observations go, during winter. They breed abundantly in the larger gardens of Houston, especially in the mountain cedars (Juniperus occidentalis texana), and the live and water oaks (Quercus virens et Q. aquatica). In the thick young oak grove near Rose Hill Post Office I found a large colony of about two hundred pairs breeding and in their company also the Boat-tailed Grackles (Quiscalus major) and Brewer’s Blackbirds (Scolecophagus cyanocephalus), but each species had its own 168limited nesting range. Every nest was built in the top of a slender oak and all the nests examined were neat, strong, and large structures; they were constructed of plant stems, slender grasses, fragments of corn-husks, intermingled with sheep’s wool, and lined with finer grasses. In some nests a layer of mud was also to be found.
95. Quiscalus major Vieill. Boat-tailed Grackle.—Quite regularly distributed over the coast region of Texas. I found the birds breeding in the colonies of the Little Blue Heron (Florida cærulea) and the Snowy Heron (Garzetta candidissima), on the button bushes (Cephalanthus occidentalis) standing in the water. May 6, 1881, I observed a colony of about twenty pairs near Rose Hill Post Office. They were all busily engaged in building their nests in the tops of young oaks. Only a few nests were finished, and only one contained eggs, four in number. Nest composed of weed-stalks, grasses and sheep’s wool, lined with finer grasses; cavity very shallow if compared with nests of Quiscalus purpureus æneus and Scolecophagus cyanocephalus. The male has a few very fine songlike notes, different from those of every other Blackbird.
96. Corvus frugivorus Bartr. Common Crow.—In winter numbers are to be observed on Galveston Bay, near bayous, and on the sugar cane fields near the Brazos. In spring they scatter over the country, breeding in all suitable localities, but they are then nowhere common in the coast region.
97. Cyanocitta cristata Strickl. Blue Jay.—A very common resident; breeds abundantly in all woody localities; also often in gardens on mountain cedars and sometimes on the beautiful Japan medlars (Eriobotrya japonica). Very bold and tame when well treated, coming then into door-yards and even into houses.
98. Milvulus forficatus Sw. Scissor-tailed Flycatcher; “Texan Bird of Paradise”; “Fork-tail.”—Very common summer sojourner; breeds frequently in the “bosquets” on the prairies, on the borders of woods, on isolated trees in the fields, and even in gardens. As the nest in this part of Texas is in most cases placed in trees, densely covered with Tillandsia, it is almost impossible to discover it. These beautiful birds are not at all retiring in their habits; in many instances they are so tame as to breed in close proximity to dwellings. They arrive from their winter quarters late in March, sometimes in the first days of April. Very often two broods are raised yearly. I found fresh eggs as late as July 4. The nests in the coast region are built partly of grasses but especially of gray Spanish moss. In September, after the breeding season, they gather in large flocks, visiting the cotton fields, where multitudes of cotton worms (Aletia argillacea) and their moths abound, on which they, with many other small birds, eagerly feed; early in October they depart for the South.
99. Tyrannus carolinensis Temm. Kingbird.—Common summer resident. Arrives from the South late in March or early in April, when the beautiful native yellow jessamine (Gelsemium sempervirens) fills the air with its pleasant odor. Nests commonly in the honey locust (Gleditschia 169triacanthos) and also in the common locust (Robinia pseudacacia). In many cases two broods are raised yearly.
100. Myiarchus crinitus Cab. Great-crested Flycatcher.—Common summer sojourner, even in Houston, where it sometimes breeds in bird-boxes, but more commonly in knot-holes of the cedar and sycamore (Platanus occidentalis) and in old Woodpeckers’ holes. Their loud whistling cries are almost always to be heard from early April till the latter part of August; departs for winter quarters early in October. It is not a shy bird, but knows well how to escape danger. They are, with Kingbirds and other species, very busy during the time the Magnolia grandiflora is in bloom, about which millions of various insects abound.
101. Sayornis fuscus Bd. Phoebe Bird; Pewee.—Common in winter, from December to March, especially in the gardens of Houston. The common notes I heard were quite different from their familiar pewee, sounding like tsip, tsip, tsip, zewee. None remain to breed.
102. Sayornis sayus Brd. Say’s Pewee.—This Pewee I have observed only in April, on the borders of thickets and in the shrubbery near woods, and always singly.
103. Contopus virens Cab. Wood Pewee.—Common summer sojourner in open woods, particularly in the “post oak,” where its loud characteristic notes can be heard throughout the summer. Although this bird is common, I did not succeed in finding a nest. Arrives early in April; departs early in October.
104. Empidonax acadicus Bd. Acadian Flycatcher.—Common summer resident, and the only one of this attractive genus that breeds in this part of Texas. They are common in all the woods, particularly where a beautiful light green lichen (Usnea barbata) hangs from the trees. In all the deciduous woods of Harris County, and also in the mixed bottom woods near Spring Creek, they are common, but I was not so fortunate as to discover the nest, although I always kept a diligent lookout for it.
105. Empidonax trailli Bd. Traill’s Flycatcher.—Common during migrations, but none, I think, remain to breed.
106. Empidonax minimus Bd. Least Flycatcher.—Common during migrations in April and October.
107. Trochilus colubris Linn. Ruby-throated Hummingbird.—Very common summer sojourner. I observed them from early April to the middle of October. Very abundant when the Wistaria chinensis, Lonicera japonica, Gardenia florida, Pittosporum tobira, Cydonia japonica, etc., are in flower.
I have several times seen another species of Hummingbird, but I did not succeed in securing it.
108. Chætura pelasgica Bd. Chimney Swift.—On August 20, 1880, I saw numbers on the borders of woods near Spring Creek. During May, June and July I have seen only a few pairs.
109. Antrostomus carolinensis Gld. Chuck-will’s-widow.—Common during the breeding season in dry woods, with much undergrowth. Arrives late in April from its winter quarters; the time of departure I do 170not know. They remain silent during day-time, and commence their peculiar cries soon after dusk of evening. The eggs are laid on the bare ground in dry places, and are commonly well hidden by thick shrubbery. In the dry woods near Spring Creek they are common, but not in the wet wooded tracts near Houston.
110. Chordiles popetue Bd. Nighthawk.—Seen in very large numbers. I saw thousands during a cloudy, rainy day in the early part of May, near the borders of woods. They all soon disappeared.
111. Chordiles acutipennis texensis Ridgw. Texan Nighthawk.—A regular but somewhat rare summer sojourner. Differs from the preceding in many respects. They are more retiring in their habits; they also sail very low over ponds and pools of water, where myriads of insects, especially mosquitoes, abound. Four to six are often seen together, flying quite near each other. I never heard them utter a sound, and do not know where they breed, but I think they have their nests on the shrubby borders of woods, where they are most commonly to be observed when flying. They are readily distinguishable from their near relatives, our familiar northern Nighthawk, by their peculiar, low, and quiet sailing, and also by their smaller size.
112. Campephilus principalis Gray. Ivory-billed Woodpecker.—Very rare; I have found it only in the large and dense pine forests in the northern part of Harris County and in Montgomery County far from human habitations. Very shy and not easy to approach.
113. Picus villosus Linn. Hairy Woodpecker.—Frequently seen during winter, but only a few times during the breeding season.
114. Picus scalaris Wagl. Texas Woodpecker.—This beautiful little Woodpecker is quite numerous in all wooded districts; it comes often during winter into the gardens of Houston, and is then very unsuspicious. I can give no particulars about its nesting habits, as I have never found a nest.
115. Picus pubescens Linn. Downy Woodpecker.—Common; breeds in all wooded districts, but is by no means so abundant as I have found it to be in Wisconsin.
116. Picus borealis Vieill. Red-cockaded Woodpecker.—The Picus querulus of Wilson is resident in all the large pine woods; it is very shy, restless, and noisy. The male is very wary during the breeding season, and every pair has its own limited breeding range. I discovered a nest in an old high pine stump, but it was out of reach. These birds are not rare in heavily wooded districts. I never have seen one in the deciduous woods.
117. Sphyropicus varius Bd. Yellow-bellied Woodpecker.—Winter resident from November to March, and then not uncommon. Always seen singly.
118. Hylotomus pileatus Bd. Pileated Woodpecker.—Common resident in all the wooded tracts, in the “post oak” as well as in the bottom and pine forests. A very noisy species; its drumming is almost as loud as that of the Ivory-billed Woodpecker. It is not a shy and retiring 171species, but, on the contrary, is very often seen near farm houses. Especially abundant where during the previous winter or spring many trees have been cut down; these they search for worms, and very soon have all the bark hammered away from them. They often excavate a hole for their nest in a post oak, in a sycamore, and also in elms, often at a considerable height. The cavity is from 10 to 20 inches deep and so large that a man can easily put his hand into it. The eggs, from four to six, are of a brilliant white color. Only one brood is raised, and the young follow their parents till late in the fall.
119. Centurus carolinus Bonap. Red-bellied Woodpecker.—Another very common Woodpecker. Its loud, harsh croaks, sounding like crirrk, are almost continually to be heard in the woods. Prefers deciduous woods. It is resident throughout the year, and is not shy or of retiring habits, but often visits the larger gardens. In the winter months I have often observed them on the ground searching for insects, but it can not walk as easily as Colaptes auratus. Breeds usually on the borders of woods, and raises only one brood yearly.
120. Melanerpes erythrocephalus Sw. Red-headed Woodpecker.—The most abundant of its family in and near Houston; breeds commonly in the city in sycamores, water and swamp oaks, and in magnolias along Buffalo Bayou. Very confiding and tame; hammers often on houses and stables, on church towers, telegraph posts, etc. Two broods are raised each season. I have seen, late in August, young just from the nest. Once I discovered the nest in a sycamore in a street, about fifteen feet from the ground, the tree standing only a few yards from a house. Resident throughout the year. Many are killed by negro boys with so-called “nigger-shooters,” and not only this species, but also numberless other birds are thus destroyed by them.
121. Colaptes auratus Sw. Golden-winged Woodpecker; “Flicker.”—Rare during the breeding season, abundant in the winter months. Frequently seen in pairs and small companies of from four to ten, and even more. Spends its time during this season mostly on the ground, where it searches for food. The first companies arrive late in October, and they steadily increase in numbers till December, when they are exceedingly abundant. They begin to migrate northward late in February.
122. Ceryle alcyon Boie. Belted Kingfisher.—Seen only occasionally. In the western part of Texas, where the rivers and creeks have clear water, the bird is evidently more common.
123. Coccyzus americanus Bonap. Yellow-billed Cuckoo.—Common summer sojourner; breeds abundantly in the thickets on the edges of woods, and is in this part of our country a very unsuspicious bird, as it is not only often seen in gardens, but sometimes breeds in them, in pomegranate bushes, in Banksia and Cherokee-rose thickets, etc. The first nests I found late in April, the last, July 5. This, a typical nest for this region, was placed in a young sweet-gum tree (Liquidambar styraciflua), about ten feet above the ground, and was almost hidden among Smilax laurifolia, with which the tree was overgrown. It was built of 172sticks, fragments of leaves, Spanish moss and a few grass-stems lined with the leaves of the loblolly pine (Pinus tæda). It contained two eggs, one somewhat advanced in incubation, the other quite fresh. I think two broods are, in many cases, reared each season.
124. Coccyzus erythrophthalmus Bd. Black-billed Cuckoo.—Common during the middle of April, but I do not think that any remain to breed.
125. Strix flammea americana Coues. American Barn Owl.—More or less common in all suitable localities. Breeds usually in hollow stumps, but last year (May 6, 1881) I discovered a nest in the side of a high bank of a “gully” near Spring Creek. The nest was about two feet from the entrance and nearly horizontal; a few feathers were the only lining. Eggs, three in number, dirty white.
126. Brachyotus palustris Gld. Short-eared Owl.—Not uncommon late in autumn and during winter near thickets and marshes, where many little birds associate, on which it feeds almost entirely. Very shy, and not easily secured.
127. Syrnium nebulosum Gray. Barred Owl; “Hoot Owl”; “Bottom Owl.”—Very common, especially in all the bottom woods and in the thick woods bordering Buffalo Bayou. Their curious notes are heard every night from the dusk of evening till dawn, and also in dark cloudy and rainy days. These notes are easily imitated, and often three or four of the birds may be thus attracted. During night time they come fearlessly near farm houses, and, with their loud, laughing, unearthly sounds, make a terrible noise. I have often heard four or five at one time near a house. Their flight is easy and quick. In Texas where the hens, turkeys, etc., roost on trees, this Owl is very destructive. They do not kill old poultry, but like half-grown chickens, and soon depopulate a whole poultry-yard. The nest is usually built high up in trees, mostly in pin oaks (Quercus palustris) and elms, sometimes also in pines, of strong twigs and sticks, without a lining. They also use old Crow’s and Hawk’s nests, which they repair a little.
128. Scops asio maccalli Ridgw. Texan Screech Owl.—This little Owl seems to be quite common. If they are disturbed, they hide in the hollow of a tree or stump. All their movements are exceedingly quick and elegant, and the flight gliding and noiseless. I have never had an opportunity to examine a nest.
129. Bubo virginianus Bonap. Great Horned Owl.—Common; resident; breeds. Nests abundantly in all the large woods; especially common in dense bottom woods. Their loud cries are to be heard not only during the nights, but also in the day-time, when the weather is cloudy and rainy. They are very destructive to the poultry; they, like the Barred Owls, come near the farm houses and commence their ludicrous cries about nine o’clock in the evening; they utter their cries only during the breeding season; later they are almost silent. The flight is very quick and easy. The nest is placed from thirty to seventy feet from the ground in the top of a large forest tree; it is composed of sticks and 173twigs, and is sometimes lined with a bunch of Spanish moss, but this may be accidental.
130. Speotyto cunicularia hypogæa Ridgw. Burrowing Owl.—This little Owl is every year increasing in numbers. Breeds in the higher prairies, and also in waste fields, in holes. They also breed in the burrows of the salamander, a species of Geomys, probably Geomys pinetis. I have not seen their eggs.
131. Falco mexicanus polyagrus Coues. Prairie Falcon.—This noble bird is resident on the borders of woods near prairies, but it is by no means a common bird. Its flight is graceful, but always low; its food is said to consist especially of Prairie Chickens and domestic fowl.
132. Æsalon columbarius Kaup. Pigeon Hawk.—Common in fall and winter, as soon as the large flocks of Blackbirds and different Finches appear from the north, among which it makes great havoc. None remain to breed. They disappear quite early, usually in the first days of March.
133. Tinnunculus sparverius Vieill. Sparrow Hawk.—Common in fall and winter, but never observed during the breeding season. This bird also does great harm among our small birds.
134. Polyborus cheriway Cab. Caracara Eagle; Mexican Eagle; “Mexican Buzzard.”—Regularly distributed, but in this part of Texas is not so common as farther in the interior. It is a very showy bird, and the flight is extremely elegant and quick. Although it is very shy and not easily to be approached, it often builds its nest in trees not far from farm houses. The farmers say they are as harmless as Turkey Buzzards. The nest is usually from twenty-five to thirty feet above the ground and is built of sticks, sometimes lined with bits of cotton and Spanish moss; the cavity is shallow. Often the birds, commonly single individuals, are to be observed with Vultures feeding together on carrion.
135. Elanoïdes forficatus Ridgw. Swallow-tailed Kite; Fork-tailed Kite.—Abundant summer sojourner from the first part of March to October. A beautiful bird, and one of the most characteristic species of this locality. Especially abundant in the bottom woods near prairies or fields. Nest very high in slender trees in the river and creek bottoms; it is built of sticks and Spanish moss. I never had an opportunity to collect eggs of this bird as the nests, in almost every case, were out of reach. In August and September the birds are often seen in cotton fields, where they feed on cotton worms and other insects. They are particularly fond of small snakes, such as Leptophis, Rhinostoma coccinea, lizards (Anolius carolinensis and Ameiva sex-lineata). I never have seen them take a bird or a small quadruped.
136. Elanus glaucus Coues. White-tailed Kite.—This rare and beautiful bird I have seen several times sailing over cotton fields. Its flight is easy and graceful, but not rapid; sometimes it stops a few moments and then descends with great velocity to the ground to capture a lizard or a snake. It is not shy, and is easily recognized by its white tail.
137. Ictinia subcærulea Coues. Mississippi Kite.—Not a common summer resident, and very shy and retiring in its habits. It is generally 174found in the same localities with Elanoïdes forficatus. Its sailings are extremely beautiful and sometimes the bird is so high in the air as to be almost invisible. Like the Swallow-tailed Kite, it is often seen about cotton fields, where it feeds on the cotton worms and on small snakes and lizards. I have a few times seen the nest high up in the top of gigantic pines, pin oaks and sycamores, entirely out of my reach.
138. Circus hudsonius Vieill. Marsh Hawk.—Common resident in the marshy prairies in the northern part of Harris County; also common near the sugar-cane fields on the Brazos. It is very destructive to all the smaller prairie birds, but it also feeds on snakes, frogs and lizards. I never found a nest.
139. Accipiter cooperi Bonap. Cooper’s Hawk.—This very common and impudent robber is the most destructive of the Raptores to the barnyard fowls; in a short time all the young chickens, turkeys, and ducks are killed by it. It is so bold as to seize the poultry before the farmer’s eyes, and in only few cases can the bird be punished, as it is very difficult to shoot. The flight is easy, very quick, and usually low. Nests found in April had already half-grown young. They were similar to Crows’ nests, built of twigs in the tops of middle sized trees, and lined with bunches of Tillandsia.
140. Accipiter fuscus Bonap. Sharp-shinned Hawk.—Common in winter.
141. Buteo pennsylvanicus Bonap. Broad-winged Hawk.—Not uncommon during the winter months, and a few remain to breed, nesting in the high trees near the rivers and creeks.
142. Buteo swainsoni Bonap. Swainson’s Hawk.—Not uncommon during the breeding season; often seen on the prairies near woods. Many are killed, as they commit great havoc among the poultry. The nest is built in the tallest trees, in an almost inaccessible position.
143. Haliaëtus leucocephalus Savig. Bald Eagle; White-headed Eagle.—This is not a common bird, but is known to breed in certain parts of this region. They build their nests in the tallest trees of the river bottoms. Two young, taken out of a nest in the spring of 1880, became very tame pets.
144. Cathartes aura Illig. Turkey Buzzard.—Very abundant, and resident throughout the year. Nests on the ground.
145. Catharista atrata Less. Black Vulture; Carrion Crow.—Common but not abundant; about one-twentieth as common as the Turkey Buzzard. Breeds on the ground in the grassy prairies.
146. Ectopistes migratoria Sw. Passenger Pigeon.—Occasionally common during the migrations. In September and October, 1881, I saw immense numbers in the post oak woods, where they were feeding on acorns.
147. Zenaidura carolinensis Bonap. Mourning Dove.—Very abundant, and resident throughout the year. In very cold winters many migrate farther south. They raise, at least in this part of the country, three broods yearly. On the prairies the nest is not unfrequently placed upon the ground.
175148. Chamæpelia passerina Sw. Ground Dove.—A rare summer sojourner. Have never seen more than two together.
149. Meleagris gallopavo (americana Coues?). Wild Turkey.—I can not state with certainty whether the Wild Turkey under consideration is the Meleagris gallopavo americana or M. gallopavo, but I think it is the first-named variety. I have found the bird abundant in all the heavily wooded districts, especially common in the thick woods with much underbrush near Spring Creek. Eggs are often put under a tame hen, but the young are not easily domesticated; as soon as they are grown they become very wild, and many go off again to their favorite woods. Early in May I have seen the mother bird with about a dozen young ones, but they were so extremely wild that they suddenly disappeared among the almost impenetrable thickets of blackberries (Rubus villosus) and Smilax (Smilax laurifolia and S. lanceolata). When the pecans are ripe, they assemble in flocks of from ten to twenty and even thirty, and feed particularly on these nuts. Later in the season they feed on several kinds of acorns, and in winter when food becomes scarce, they eat the berries of the myrtle-holly (Oreophila myrtifolia) and other berries.
150. Cupidonia cupido Bd. Prairie Hen.—Common resident on all the flat grassy prairies. Is becoming scarcer every year.
151. Ortyx virginiana Bonap. American Quail; “Bob white.”—Very abundant resident. Two broods are raised yearly. They are exceedingly tame and confiding, breeding sometimes in close proximity to the habitations of men. In winter from fifty to one hundred are usually seen in cotton and sugar-cane fields.
Bailey’s Index to Forest and Stream.[81]—The newspaper thus indexed as to the bird-matter contained in its first twelve volumes has always given much space to ornithological articles, which have become of late years more valuable from a scientific standpoint than newspaper pieces generally are, being authenticated by the signatures of the writers instead of some silly pen-name, and being on the whole scarcely below or not below the grade of the bird notes that one finds in periodicals of professed technical character. No one who has had any experience in hunting for what he wants through the scantily indexed pages of a weekly issue can 176fail to appreciate the good office Mr. Bailey has rendered us all; and every one upon whom the bibliographical blight has descended knows what an immense amount of industry that curse entails. The author has our hearty sympathy in the latter, and our best thanks for the former. His work is more than a mere alphabetical list of names, followed by reference figures; for it includes, as the title says, a summary of each article indexed—often giving just the points wanted, thus rendering it unnecessary to look up the reference. The Index also includes authors’ names, and among these the authorship of many pseudonyms and initial-signatures are for the first time properly exposed. The summation of the bird-matters seems to be quite complete, and is certainly extensive, in the cases of some common game birds occupying several pages. We presume the work is not free from faults and errors of all sorts, because nothing of the kind can be; but we have found it more reliable than its mechanical execution would lead one to expect. Considering how great a favor Mr. Bailey has conferred upon the publishers, and how much good his Index will do the paper, by “setting it up” in the estimation of working ornithologists higher even than it was before, his work might have been better dressed.—E. C.
Chamberlain’s Catalogue of the Birds of New Brunswick.[82]—As many of our readers are doubtless aware, Mr. Montague Chamberlain has been engaged, for some time past, in investigating the bird fauna of New Brunswick, and an interesting result of his labors is now before us in the form of a catalogue of the birds of that Province. This paper, which forms by far the most important one in the publication of which it is a part, comprises some forty-three pages which are divided into two sections; “Section A” being restricted to “species which occur in St. John and King’s Counties”; while “Section B” embraces “species which have not been observed in Saint John or King’s Counties but which occur in other parts of the Province.”
The former division treats of a region to which the author has evidently paid special attention, and the text, being mainly based on his personal observations or investigations, includes many interesting and several important notes and records. From these we gather that the rather marked Alleghanian tinge which is known to pervade the bird-fauna of the entire coast region of Maine, as far as Eastport and Calais, extends still further eastward. Thus the Catbird, White-eyed Vireo, Towhee Bunting, Cowbird, Meadow Lark, Baltimore Oriole, Carolina Dove, Least Bittern, Florida Gallinule, and a few others scarcely less characteristic of the more southern fauna, have been found within the area treated by the present paper, but all are marked as rare, and the greater number as merely accidental visitors. Many of the more important records have already been published elsewhere.
177The annotations in this section are often full and always interesting. The author writes clearly and simply and his style is characterized by a modest frankness that is very attractive. We fear, however, that some of his views respecting the distribution of races are hardly orthodox. Thus he thinks that “two races of Loon spend the summer in New Brunswick, and breed here. They have plumage of similar colors and markings, but one is smaller than the other, being some six inches less in length. The larger bird is common on the lakes and rivers in all sections of the Province, seldom seeking the salt water until the rivers freeze over, while the smaller is rarely found away from the sea shore”; and again that a light form of the Ruffed Grouse “resembling the descriptions given of umbelloides” occurs with typical umbellus and that it is “not improbable that both the Brown and Gray varieties are represented here, with numerous hybrids”; a condition of affairs which, if true, is certainly deplorable.
“Section B” is almost wholly compiled, the authorities mainly drawn on being Boardman, Herrick, and Dr. A. Leith Adams. Several of the records left by the latter writer are, in the light of our present knowledge, of very doubtful value.
Mr. Chamberlain’s work, so far as it has gone, has evidently been done carefully and well, a fact which makes it the more to be regretted that the publication of his report could not have been longer delayed, for in many respects it lacks the completeness that is desirable in a paper of its kind. Any adequate exploration of a region so extensive as that embraced within the limits of New Brunswick cannot be accomplished in one or two seasons only. It is rather the task of a lifetime. But we must bear in mind that the present “Catalogue” is offered simply as a “starting point,” to be “supplemented by additions and revisions as opportunity for further investigation occurs”; and considered from this standpoint it is in every way a highly creditable production. That its author is qualified to carry out an undertaking which he has so satisfactorily begun can be a matter admitting of no doubt, and we shall look for many interesting developments in the field which he has chosen.—W. B.
Krukenberg on the Coloring Matter of Feathers. Second Part.[83]—Turacoverdin, a green pigment which occurs in the green feathers of the Musophagidæ is first considered. This pigment is soluble in alkalies, such as soda and the like, but is insoluble in acids, chloroform, ether and the alcohols. Concentrated sulphuric acid added to the pigment in solution turns it violet red. Turacoverdin in solution emits a weak red fluorescent light, and when examined by the spectroscope shows an absorption band near D. It contains a considerable quantity of iron, but little copper or manganese, and probably, like Turacin, lacks sulphur and nitrogen. A point of considerable interest is its identity with a green 178pigment procured by Church by boiling a solution of Turacin for a long time.
Zoörubin, a red-brown pigment occurring in Cicinnurus regius is next described. In solubility it much resembles the preceding, but has no absorption band, though all of the spectrum beyond D is absorbed. When treated with a very small quantity of copper-sulphate, Zoörubin instantly becomes cherry-red, a characteristic reaction. This pigment occurs in the brown female paradise bird though not in other brown birds, as Strix flammea and Alcedo ispida. As regards the colors of Eclectus polychlorus, where green, blue, red, yellow and brown may all be found, the author has brought out some very interesting points. The blue and green are mechanical, or rather the blue is mechanical and the green is the result of a yellow pigment overlying a brown one. The true pigments of the feathers are brown, yellow, and red. If the feathers be blackened on their under surfaces with lampblack or sepia, they become blue. If the yellow feathers are treated in a similar way, they become green. The yellow pigment is Zoöfulvin, the red probably Zoönerythrin.
Lastly the author describes the yellow pigment, Coriosulfurin, found in the tarsus of the birds of prey. This substance is unlike any known to occur in feathers. It has three absorption bands between F and G.—J. A. J.
Stejneger’s Nomenclatural Innovations.[84]—Proposing to use “the oldest available name in every case,” the author shows that many of our current names must give way if the “inflexible law of priority” is to be observed. For ourselves, we believe that the surest way out of the nomenclatural difficulties that beset us is to be found in some such simple rule as this, and that to upset every name that can be upset according to any recognized principle is really the shortest road to that fixity of nomenclature for which we now all sigh like furnaces. Still such a paper as this makes us wish, as so many others have done, that some counteractive “statute of limitation” could come into operation, by which a bird resting in undisturbed enjoyment of its name for, say, a century or half a century, should not be liable to eviction under the common law of priority. Human welfare and happiness on the whole is the final cause of all law, and in the case of titles to real estate it is we believe statutory that undisturbed possession for a certain period shall exempt property-holders from litigation on account of any adverse claim, however otherwise sound, which is not presented within a certain number of years. This seems to be necessary for the security of any title and to proceed upon the theory that if owners don’t take the trouble to make good their title in due time they ought to forfeit it. The logic of a bird’s right to its name and a possessor’s right to any other property is the same in theory, and might properly be carried into effect. Fifty years of unchallenged usage might do, and a 179hundred certainly would suffice, to eliminate the factor of “contemporaneous courtesy,” and the shades of a few departed greatnesses might not be offended by being invited to yield a point now and then for the benefit of the many whom natural selection has not yet eliminated from the struggle for existence.
Stejneger’s points seem to be well taken in the main; and though we have not yet had opportunity of verifying them, we presume the restitutions and substitutions he proposes are available if not indeed necessary under the priority statute. But has he in all cases taken up names which rest upon diagnosis? Does indication of a type-species make a generic name valid? Some other objections might also be raised. We pass no judgment, pendente lite, but simply note the following propositions advanced:—Phænicurus Forst., 1817, for Ruticilla Naum., 1822.—Cinclus merula Schäff., 1789, for C. aquaticus Bechst.—Regulus cristatus V., 1807, for R. satrapa Licht., 1823.—Chelidon Forst., 1817, for Hirundo L. et auct. (rustica, etc.).—Hirundo L., 1758, for Chelidon Boie, 1822.—Clivicola sive Riparia Forst., 1817, for Cotile Boie, 1822.—Calcarius Bechst., 1803, for the birds now commonly called Centrophanes, and Plectrophenax, g. n., for “Plectrophanes” nivalis.—Otocoris Bp., 1839, for Eremophila, preocc. in botany, and by Eremophilus in ichthyology.—Archibuteo norvegicus Gunnerus, 1767, for A. lagopus Gm. (but there is A. lagopus Brünn, 1764).—Morinella M. & W., 1810, for Strepsilas Ill., 1811.—Vanellus capella Schäff., 1789, for V. cristatus M. & W., 1803.—Ægialitis alexandrinus, L., 1758, for Æ. cantianus Lath., 1790.—Gallinago cælestis Freuzel, 1801, for G. media Leach, 1816.—Totanus nebularius Gunnerus, 1767, for the Greenshank.—Pavoncella Leach, 1816, for Machetes Cuv., 1817.—Tadorna dameatica Hasselq., 1762, for T. cornuta Gm., 1788.—Harelda hyemalis L., 1758, for H. glacialis L., 1766.—Eniconetta Gray, 1840, for Polysticta Eyt., 1836, preocc. by Polysticte Smith, 1835, and for “Stellaria”! Bp., 1838, preocc. in botany.—Gavia Boie, 1822, for Pagophila Kaup, 1829, and the species G. alba (Gunn., 1767, for P. eburnea) Phipps, 1774.—(Larus hyperboreus Gunnerus, 1767, for L. glaucus Brünn, 1764.)—Hydrochelidon nigra (L., 1758, p. 137) for H. lariformis (Ibid., p. 153).—The short and long-tailed Jägers to be respectively Stercorarius parasiticus (L., 1758, p. 136), and S. longicaudatus (V., 1819).—Urinator Cuv., 1799, for Colymbus auct., nec Briss., 1760; U. immer (Brünn, 1764, p. 38) instead of U. torquatus (id., ibid., p. 41) and U. lumme Brünn, 1764, for C. septentrionalis L., 1766.—E. C.
Ingersoll’s Birds’-Nesting.[85]—This little book is intended for a guide to the beginner, and as such it will no doubt be of service. The book may be summarized as a readable account of the various modes of collecting birds’ eggs and nests. There are, however, a few points which we regard with suspicion, as the contrivances for descending cliffs; such things in 180careless hands would become instruments of self-destruction. A long account of the various paraphernalia for blowing and marking eggs is given. To the novice such things may be amusing, but are sure sources of disaster. A keen eye, accuracy of hand and a mind to govern, not patent scissors and forceps, are the requisites for blowing eggs.
The list of unknown nests, which does not claim to be free from faults of omission, contains faults of admission, though these are not numerous. Finally, we would heartily indorse all advice for absolute identification of eggs and the avoidance of gummed labels.—J. A. J.
Note on Mimus polyglottus.—In the summer of 1879 I found on the Platte River, about a mile west of Fort Fetterman, Wyoming, in Lat. 42° 23′ 35″ N. and Long. 105° 21′ 4″ W., a pair of Mockingbirds (Mimus polyglottus) breeding; the nest was placed in a low cottonwood, very near the river bank. In the following year these birds, undoubtedly the same pair, returned and reared a brood in identically the same place. This time I secured the male bird; and the specimen is now in my private collection.
In the “Birds of the Colorado Valley” Dr. Coues tells us, when referring to the limits of Mimus, that “the northermost records generally quoted fix the limit in Massachusetts; but Dr. Brewer speaks of a single individual seen near Calais, Me., by Mr. George A. Boardman. Another record from an extreme point, given by Dr. P. R. Hoy, is above quoted; the extension of the bird to Wisconsin, as there indicated, has been commonly overlooked. Other States in which the bird is known to have occurred are New York, Ohio, Indiana, Illinois, Missouri, Iowa, and Kansas. The parallel of 40° N. has been named as its usual or normal limit.”
In view of these facts, and what I have learned from other ornithologists, it seems to me that this case is entitled to record, as another interesting instance, extending the limits of this bird.—R. W. Shufeldt. Washington, D. C.
The Nest of the House Wren.—Some writer speaks of the well known habit of the House Wren of filling up any cavity within which it builds its nest with sticks and rubbish, as a “survival” of an old habit for which there would seem to be no present use. I think I have seen this statement in some of the writings of Dr. Elliott Coues, though I cannot refer to the book or page. Possibly it may have been stated by some one else. But it is a generally recognized fact that if a box holds half a peck the little birds will fill it up full! It seems to me, however, that while this 181may be really a “survival,” it is still a most useful habit. When a hole or space is so filled the nest proper is generally built on the side of the mass of rubbish opposite to the entrance and as far as possible from it. Manifestly there is a clear purpose in this—viz: that of protection from any enemy seeking an entrance. I have observed many nests, in large cigar boxes, and in the majority find this state of things to exist. The interior space will be filled with sticks, leaving a little passage way over the top, through which the bird can reach the nest on the back side of the rubbish. It seems to me that this is clearly a defensive habit, necessary at this time. When they build a nest in the skull of a horse or ox, it will be found that they follow the same rule, and that it will be very difficult to get at the nests.
But their practices are sometimes varied. If a box is not too large, and the hole is only large enough to admit of the passage of the birds, they will often carry in only just enough material to build the nest, leaving the space all open above. I have often known them to pursue this course in building in a cigar box where a small hole had been made at the middle of one of the sides. But if the box is a large one with a large hole cut through the end near the top, as it is suspended on a tree or the side of a building, then they will carry in “fully a peck of rubbish,” and build the soft nest down on the side opposite the entrance.—Charles Aldrich, Webster City, Iowa.
Remarkable Plumage of the Orchard Oriole.—There is in the collection here a very curiously marked specimen of the Orchard Oriole (Icterus spurius) from Columbia, Pa. It is evidently a male bird in the transition stage of plumage from young to that of the adult. Young males of this species usually exhibit “confused characters of both sexes,” but in this case the male plumage is confined to the right side of the bird, and the female plumage to the left side, the two colorations uniting on median lines above and below. So distinctly is this peculiarity marked, that a bilateral section of the bird would divide the phases about equally. The left side, however, shows very slight traces of black and chestnut, yet not so distinct as to lessen the general yellowish-olive appearance of the female. There is more of the white on the coverts of the left wing than usual.—Charles H. Townsend, Acad. Nat. Science, Philadelphia, Pa.
The Nest and Eggs of Perisoreus Canadensis.—The nest upon which the following description is based was found by Mr. P. S. Glasier on April 7th, 1881, twenty-three miles from Grand Falls, New Brunswick. It was built in a small fir tree with few branches, about ten feet from the ground. The tree was in “mixed land” beside a brook, on the south side of a hill and near a lumber camp. From the men in the camp it was learned that the bird built the nest about the middle of March, and had been sitting for ten days. The parent bird was found on the nest, shot, and forwarded to me, so that there can be no doubt of identity.
182The nest is rather a large structure, between nine and ten inches in diameter and five inches deep. The cavity is slightly oval, measuring three and six-tenths by three and two-tenths, and is two inches deep.
The bottom is formed of large pieces of rotten wood, which must have been torn from some neighboring stump, while the sides are supported by a scraggy structure of long twigs. The walls are formed of strips of bark and the subjacent rotten wood, apparently of cedars, cocoons, the remains of wasp nests, lichens and the like. All this material is closely packed together, but not woven, so that were it not for the outer coat of twigs the whole would quickly fall apart. On one side, snarled up among the twigs, is a long piece of white twine, which shows that the neighboring camp was called upon to pay its tribute. The lining is quite thick, and offers a decided contrast to the walls. Rootlets of various kinds form the greater part, though grass and the remains of wasp nests form the floor. A few feathers are scattered throughout the structure and about as many more are to be found inside. By far the greater part of these are from the Jays themselves, and they might be regarded as of accidental occurrence were it not for a few from some species of Grouse. As a whole the nest is a substantial structure, admirably adapted to keep the eggs and nestlings warm.
The eggs were three in number, and are of about the same size and form as those of the Blue Jay. Their ground-color is a light green of much the same color as the Field Sparrow’s egg. Two of the eggs are thickly covered with fine spots of lavender and light brown, the spots being most abundant at the large end. The third has less lavender and more brown, while the spots are of considerable size and evenly distributed.—J. Amory Jeffries, Boston, Mass.
Notes on the Plumage of Nephœcetes niger borealis.—An examination of ten birds of this species, taken at Howardsville, Colorado, in 1880 and 1881, leads me to believe that four years are necessary for them to acquire their complete plumage. A young male of the year, taken Sept. 17, was marked as follows. General color dull black, every feather tipped with white, scarcely appreciable on upper back and throat, broader on upper tail-coverts and rump. Crissum almost pure white. In birds of the second year the general plumage has a brownish cast; feathers of back tipped with brown, the head whitish, belly feathers yet broadly tipped with white. The third year the color is black, with a very faint edging of white on under tail-coverts. In the fourth year pure black, forehead hoary, neck with a brownish wash. Feathers bordering the black loral crescent whitish.
Tail in young of first year, rounded; in second year, slightly rounded; in third year slightly emarginate, feathers becoming more acute. In adult, forked, outer feathers three-eighths of an inch longer than inner.
I do not know when they come—some time late in June—but they remain until long after the Violet-green Swallows leave. They always hunt in flocks, range far above 13,000 feet and breed up to at least 11,000 feet. Those I have shot have had their crops filled with Ephemeridæ, and it 183is only when a cloud of insects is discovered low down that the birds come within gunshot range. Often one will sweep down almost to the earth and, swinging on in the same ellipse, soar far up entirely out of sight.
Measurements from dried skins of eight specimens give an average length of six and seven-sixteenths inches, with extremes of seven and one-half inches—an adult male, and five and seven-eighths inches—a young female; and an average wing of six and five-sevenths inches, with extremes of six and seven-eighths and six and three-eighths inches.—Frank M. Drew, Bunker Hill, Ill.
Plumage of the Young of Eclectus Polychlorus.—Dr. A. B. Meyer in the P. Z. S. for 1877, p. 801, says in an article on Eclectus polychlorus: “Formerly I discussed the question whether the young bird in both sexes is plain green or not; but I now believe that it is red in both sexes, i.e. bears the dress which the female keeps during its whole life.” This conclusion would seem to be incorrect, since among a series of these birds in the possession of Prof. H. A. Ward, there is one bird so young as not to be fully fledged, but which is nevertheless of the same bright green color as the adult males. This substantiates the statement of the Rev. George Brown that the young birds have the same colored plumage as the adults.—F. A. Lucas, Rochester, N. Y.
[This is a large Parrot found in the Malacca and Papuan Islands. The occurrence of “young red-and-blue birds” has already been recorded (see Ibis for 1878).—J. Amory Jeffries.]
An Owl’s Egg Laid in Confinement.—The history of my Acadian Owl, given in a late number of this Bulletin,[86] has an interesting sequel. On February 4, 1882, the bird (then but nine months old) astonished its friends—and perhaps itself as well—by laying an egg in the bottom of its cage. This, when first brought to me, was of normal size and shape, but soft and leathery to the touch, like the egg of a turtle. One side was fractured; and soon afterward the shell around the edges of the hole began to curl inward until, in a short time, the whole egg became shrivelled and distorted. Finally, in the course of a day or two, the shell crumbled and scaled off in small fragments leaving only the half-dried yelk and albumen.
Of course more eggs were looked for, and in anticipation, the floor of the cage was lined with saw-dust and a hollow stump even supplied to serve as a nesting-place. But despite these attentions the bird obstinately refused to gratify our hopes. For several days after the removal of her egg she was restless and irritable, continually flying from perch to perch, and fiercely attacking any one who ventured to approach her. Indeed, it was two or three weeks before she recovered her wonted gentleness.
184I cannot now recall an instance of the breeding of Owls in confinement, but the present occurrence would apparently indicate that it might be accomplished with Saw-whets, which, as captives, seem to be more animated and cheerful than most of the members of their sedate family.—William Brewster, Cambridge, Mass.
Buteo brachyurus—A Correction.—An inaccuracy, comparatively so unimportant that I have hitherto neglected to call attention to it, will be found in the paper “On a Tropical American Hawk to be added to the North American Fauna” (this Bulletin, Vol. VI, p. 207). The Hawk in question was shot Feb. 22., 1881—not Feb. 1, as stated in the article referred to. I was at Palatka at the time, and saw the bird in the flesh the day it was shot. It was secured on the outskirts of the town, early in the morning, by a young taxidermist, Mr. Wm. Dickinson, since deceased. We could not determine the species, and he would not part with the specimen, a very fine one, but “set it up” for himself. A short time afterwards he presented it to Mr. G. A. Boardman.—J. Dwight, Jr., New York City.
The Turkey Buzzard in New Hampshire.—A specimen of Cathartes aura was shot this spring near Hampton Falls, N. H., by Mr. Frank Percell. The bird was killed April 6th or 7th. and received by Mr. C. I. Goodale on the 8th. When I examined it on the 10th it was still quite fresh.—Charles B. Cory, Boston. Mass.
Rapacious Birds in Confinement.—In the winter of 1874 I spent several months with a friend who had a number of rapacious birds in confinement. There were a couple of Barred Owls, a Great Horned Owl, and a Rough-legged Hawk, living together upon excellent terms in one apartment; in another, half a dozen Mottled Owls; and in another a superb Bald Eagle. Most of these birds became quite tame after a short period of captivity, tolerating our presence in their quarters, taking food from our hands, and even submitting to caresses. One little Scops developed especial docility. My friend, who was a taxidermist, used to place it upon a perch at his side and copy strigine attitudes from nature. The accommodating bird would sit content for half an hour at a time, and never objected to any sort of gentle handling. One of its brethren. however, was vicious and untameable. He nipped our fingers whenever occasion offered, snapped and spat if even approached, and finally sealed his own doom by decapitating his gentle associate.
We did not succeed in cultivating a spirit of great tractability in the Eagle. Aside from the amusement he occasionally afforded in tackling living quarry, generally some superfluous cat, he was a rather uninteresting captive. One morning we omitted his breakfast, but in the course of the forenoon introduced a kitten into his apartment. He eyed her sharply for a few moments, then persistently ignored her and in the evening she was removed unscathed. Upon this we instituted upon the royal bird a brief 185course of starvation, and then submitted the unfortunate kitten again. This time her reception was very different. At sight of her he manifested great excitement, and in a very few minutes left his perch with a jump and a flop, and seized the poor beast in his talons. He struck her very nicely, pinning fore paws and head together with one foot, the hind paws together with the other, thus preventing the slightest resistance. My remorse at this stage of the proceedings was somewhat alleviated by the fact that the kitten did not even quiver, having apparently been instantly killed by the force of the blow. However, the Eagle at once put an end to what little life may have been left by breaking her spine with his beak. He thereupon tore a hole in her abdomen, and cast the intestines daintily aside. The contents of the stomach were examined and, with the exception of a single tid-bit which appeared to be a piece of bread, rejected. The rest of the body was then rapidly devoured. On the following morning a full-grown tom-cat was turned loose in the cage. The Eagle attacked him several times but was valiantly repelled, and up to the end of the third day, when he made his escape, Thomas remained master of the situation. Dissatisfied with this experiment, my friend subsequently introduced the cat in a half-stunned condition, and after getting well scratched the Eagle succeeded in overcoming him.—Nathan Clifford Brown, Portland, Maine.
Note on Mareca Americana.—I shot at Wayland, Mass., October 1, 1881, a young male Widgeon (Mareca americana). It was flying in company with a flock of twelve others, apparently of the same species.—A. Thorndike, Brookline, Mass.
Destruction of Birds by the Cold Wave of May 21st and 22nd.—It seems worthy of note that, judging from indications in this vicinity, the destruction of bird life by the recent cold wave must have been very considerable.
On the morning of May 21st, a specimen of Helminthophila peregrina was picked up so nearly chilled to death that it died shortly afterwards. The same was also true of a specimen of Dendræca pennsylvanica On the morning of May 22nd, three other specimens of the following species were picked up here which had apparently died of cold: Dendrœca maculosa, Myiodioctes pusillus, and Empidonax minimus.
These facts suggest that the abundance of bird life may, to a considerable extent, be influenced by sudden extreme changes of temperature, as well as by heavy gales.—F. H. King, River Falls, Wis., May 24, 1882.
A “Tidal Wave” of Birds in Washington.—In the twenty-five years during which I have paid more or less attention to birds hereabouts I have never seen anything like the “wave” that rolled up in the second and third weeks of May of this year. The highest spring “season” is usually the month from April 20 to May 20, at which latter date the tide has usually ebbed equably from its greatest height at the middle of May. This year the birds seemed to be held back by the cold and wet, and such 186an accumulation has seldom if ever been seen before. The streets and parks were full of the birds, and the daily papers all had their say upon the unwonted apparition. In the Smithsonian Grounds, for example, I saw one day a flock of a hundred or more Orchard Orioles, mixed with Baltimores. There were flocks of Scarlet Tanagers, Rose-breasted Grosbeaks, etc., and any quantity of Thrushes, Vireos, Flycatchers and Warblers—among the latter the rare beauty Dendrœca tigrina. Of the latter Dr. Prentiss took several—the only ones we have known to be captured here for many years. The cause of this gathering of the clans was doubtless the cold wave Mr. King speaks of in the preceding paragraph.—Elliott Coues, Washington, D. C.
More Definite Statistics Needed in Regard To the Abundance of Birds.—It is deeply to be regretted, it seems to me, that we have so little specific information in regard to the abundance of birds in the various portions of the United States from which lists of species have been published.
Such terms as “common”, “not common,” “abundant,” “rare,” “rather rare,” etc., may have such different values in the minds of different observers, as to render them of but little value for any but the most general considerations. They are absolutely valueless in the discussion of such economic questions as, Can birds ever become abundant in thickly settled districts? and, What birds, if left to themselves, are likely to become most abundant in thickly settled sections?
The table given below indicates the character and kind of information which is much needed in the discussion of many important ornithological questions.
The first four columns are compiled from notes made in Jefferson County, Wisconsin, between July 31 and August 7, 1877; those in the last four columns are from notes taken in the vicinity of Ithaca, N. Y., in 1878.
In each column, opposite the name of the species, is given the number of individuals which were observed in travelling the distance indicated near the foot of each column. The item, “birds seen or heard but not named.” includes those individuals which were known to exist in the territory passed over, but which for various reasons could not be identified with certainty.
The salient features of the two localities, briefly stated, are these:—
In the vicinity of Ithaca, there is a long, deep, and narrow valley, having somewhat rolling, glen-cut sides: in it lies Cayuga Lake, deep and weedless, stretching, like a broad river, to the northward. Its east and west banks are abrupt and rocky and cut, at intervals, by deep wooded glens. A small grass swamp, bearing a few trees, at the south end of the lake and running up into the city, is about the only low land in the vicinity. Formerly a mixed deciduous and evergreen forest covered the hills. Now, mere remnants stand near together upon small closely packed farms on both sides of the valley. The houses are numerous, the orchards large, and there are few fields not having some trees standing in them.
187In the portion of Jefferson County where the notes were taken, the country is nearly level, with gentle undulations, and is traversed by Bark and Rock Rivers. The streams make a sharp line between prairies and openings on one side and heavy hard and soft-wood timber on the other. Marshes trend along the streams, and shallow reedy ponds are common. Compared with the vicinity of Ithaca, the farms are larger, the houses less numerous, the orchards smaller, the woods and groves larger, and but few trees stand in the fields.
Route 1 led from a point about half a mile north of Bark River out across cultivated fields. Routes 2 and 3 each led east from Rock River, north of Jefferson, alternately through pieces of heavy timber and across dry cultivated fields. Route 4 led from the Crayfish west upon the prairie southwest of Aztelan, traversing dry treeless fields and leading through two small groves. Route 5 led from the University buildings west across the valley, leading through a pasture, through the north end of the city, through the swamp, and up the railroad, bordered on one side by cultivated fields, and by tangled thickets on the other. Route 6 led directly east from the campus to Varna, and then southwest along the railroad. On this trip only cultivated fields were crossed and one small piece of woods traversed. Route 7 led up the valley from Ithaca along the east side, and then across to Enfield Falls. On this tramp we passed in turn along the railroad, bordered with small scattering thickets on both sides, across the inlet through low fields, and then past cultivated fields and small pieces of woods. Route 8 lay ten miles east of Ithaca, and led from McLean off to the southeast of Dryden, and then through Dryden to Freeville. A branch of Fall Creek was crossed twice, and, with the exception of a small marsh near Freeville, only cultivated fields and small pieces of wood were passed.
| NAME. | ROUTES. | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Turdus migratorius | 11 | 3 | 20 | 13 | 31 | 44 | ||
| Turdus fuscescens | 2 | 2 | 4 | |||||
| Mimus carolinensis | 2 | 8 | 3 | 2 | 12 | 25 | 7 | |
| Sialia sialis | 1 | 2 | 2 | 8 | 5 | 17 | ||
| Parus atricapillus | 9 | |||||||
| Sitta carolinensis | 3 | 7 | 2 | 1 | 2 | 3 | ||
| Troglodytes aëdon | 1 | 5 | ||||||
| Eremophila alpestris | 3 | |||||||
| Cistothorus stellaris | 1 | |||||||
| Dendrœca æstiva | 1 | 2 | 5 | 5 | ||||
| Geothlypis trichas | 1 | 3 | ||||||
| Setophaga ruticilla | 2 | 15 | 5 | 2 | ||||
| Pyranga rubra | 1 | 3 | ||||||
| Hirundo horreorum | 5 | 5 | 12 | 7 | 20 | |||
| Tachycineta bicolor | 2 | |||||||
| Petrochelidon lunifrons | 2 | 12 | 10 | 55 | ||||
| Cotyle riparia | 13 | |||||||
| Progne purpurea | 2 | 1 | ||||||
| Ampelis cedrorum | 8 | 4 | 7 | 12 | 4 | |||
| Vireo olivaceus | 1 | 13 | 13 | 1 | ||||
| Vireo gilvus | 1 | 3 | ||||||
| 188Vireo flavifrons | 10 | 4 | ||||||
| Lanius excubitorides | 1 | |||||||
| Chrysomitris tristis | 9 | 27 | 5 | 4 | 6 | 28 | 32 | 44 |
| Poœcetes gramineus | 5 | 10 | 16 | 19 | 28 | |||
| Melospiza melodia | 6 | 5 | 8 | 17 | 7 | 33 | 23 | 73 |
| Melospiza palustris | 1 | |||||||
| Spizella socialis | 3 | 1 | 1 | 7 | 33 | 17 | 36 | |
| Spizella pusilla (shot) | 2 | |||||||
| Cyanospiza cyanea | 5 | 2 | 3 | 3 | ||||
| Pipilo erythrophthalmus | 3 | 3 | ||||||
| Dolichonyx oryzivorus | 18 | 3 | 5 | 22 | 52 | |||
| Molothrus pecoris | 2 | 10 | ||||||
| Agelæus phœniceus | 1 | 12 | 10 | 12 | ||||
| Sturnella magna | 1 | 2 | 2 | 8 | 5 | 11 | ||
| Icterus baltimore | 7 | 11 | 5 | 3 | ||||
| Quiscalus purpureus | 2 | |||||||
| Corvus americanus | 1 | 2 | 3 | 10 | 8 | 28 | ||
| Cyanurus cristatus | 1 | |||||||
| Tyrannus carolinensis | 8 | 10 | 4 | 4 | 8 | |||
| Sayornis fuscus | 2 | 4 | 2 | 22 | 11 | |||
| Contopus virens | 3 | 20 | 15 | 4 | 2 | 4 | ||
| Empidonax minimus | 1 | |||||||
| Chætura pelasgica | 4 | 3 | 13 | 12 | ||||
| Trochilus colubris | 1 | 5 | 1 | 1 | 1 | 1 | ||
| Ceryle alcyon | 2 | 7 | ||||||
| Coccyzus erythrophthalmus | 2 | 1 | ||||||
| Picus villosus | 2 | 1 | ||||||
| Picus pubescens | 1 | |||||||
| Sphyrapicus varius | 1 | 2 | 4 | |||||
| Melanerpes erythrocephalus | 4 | 2 | 2 | 3 | ||||
| Colaptes auratus | 7 | 6 | 2 | 2 | 1 | |||
| Circus hudsonicus | 5 | |||||||
| Falco sparverius | 1 | |||||||
| Hawk | 2 | 2 | ||||||
| Zenaidura carolinensis | 5 | 4 | 1 | |||||
| Bonasa umbellus | 10 | |||||||
| Ægialites vociferus | 17 | 2 | ||||||
| Tringoides macularius | 1 | 11 | 4 | 3 | ||||
| Actiturus bartramius | 2 | |||||||
| Ardea herodias | 2 | |||||||
| Ardea virescens | 2 | 3 | ||||||
| Rallus virginianus | 1 | 1 | 1 | |||||
| Podilymbus podiceps | 1 | |||||||
| Birds seen or heard, but not named | 20 | 36 | 18 | 15 | 20 | 69 | 100 | 101 |
| Total number of birds observed | 137 | 141 | 112 | 95 | 127 | 282 | 405 | 626 |
| Number of miles traveled | 4 | 5 | 3 | 3 | 2¼ | 5 | 7 | 11 |
| Average number of birds per mile | 34 | 28 | 37 | 32 | 56 | 56 | 58 | 57 |
| Total number of species | 35 | 27 | 18 | 17 | 23 | 22 | 31 | 32 |
Total average number of birds per mile in Jefferson County is about thirty-three.
Total average number per mile in the vicinity of Ithaca is about fifty-seven.
189The notes from which these tables are prepared were obtained by walking continuously over the routes named, without retracing steps in any case. When a bird was observed a record was made in the form of a dot placed against the name of the bird. The dots were placed for convenience in groups of five each separated by straight lines.
It seems a little remarkable that the four averages of the two localities should so nearly coincide. The fact that they do coincide so closely suggests that, unless we have here an unusual recurrence of figures, the averages represent a tolerably definite factor of the bird population of the two localities at the time the observations were made. The statistics do not indicate the actual bird-population in the two localities; but they do show, it seems to me, the relative abundance in the two sections, and, to a large extent, the relative abundance of the various species in each locality.
It is to be observed that the notes from the vicinity of Ithaca were taken in June before many of the young birds were upon the wing, while those from the other locality were made after the breeding season. The two localities should not be compared, therefore, without taking this fact into account. For instance, all the Bobolinks observed on trip 8 were, with two exceptions, males. Hence the figures probably show only about one-half the number of birds of this species that existed in the territory at the time of the visit.
In July, 1878, about the middle of the month, I went over route 5 and 6 a second time to see what effect upon the average the addition of the young birds would have. The whole number of birds observed was a little more than double that observed in June.
Perhaps some one will suggest a better method of obtaining the facts recorded in this connection.—F. H. King, River Falls, Wis., May 24, 1882.
Remarks on Five Maine Birds.—It appears that no formal announcement of the occurrence of the Gray-cheeked Thrush (Hylocichla aliciæ) in the State of Maine has ever been made, though the course the bird is known to pursue in its migrations renders such an announcement of slight importance. It may be stated, however, for the benefit of compilers, that this Thrush is a regular, not very common, spring and fall migrant in southern Maine, reaching Portland in spring about the middle of May, and in autumn about September 20.
Apropos of Dr. Coues’ recent prediction[87] that the Titlark (Anthus ludovicianus) will yet be ascertained to breed occasionally along the Maine coast, is there anything but inferential evidence to indicate that it occurs there at all in spring or summer? Being known to pass through Massachusetts in spring and to occur on the island of Grand Manan[88] at that 190season, it is fair to suppose that the Titlark also touches at favorable points in Maine while en route to its breeding grounds. Nevertheless neither my own observations nor the records of other observers substantiate this hypothesis.
The once prized Ipswich Sparrow (Passerculus princeps) must now take its place among the common autumnal migrants of southern Maine, though restricted, so far as I am aware, to the sea-coast. In spring, however, it is uncommon if not rare. Since the capture of the first Maine specimen,[89] March 20, 1875, I have seen but two other spring specimens. These I found upon Old Orchard Beach, March 28, 1882, and one of them is now in my collection. In their autumnal migration the birds reach Cumberland County about Oct. 13, remaining at least until Nov. 6, later than which I have never looked for them. Upon almost any day between these dates the collector may find a dozen or more individuals along the sandy shore between Scarborough Beach and the Saco River.
In the Proceedings of the Portland Society of Natural History for April, 1882, I spoke of the Ring-necked Duck (Fulix collaris) as having but once been taken in the vicinity of the city within my experience. On the very morning upon which my paper left the press, I found in one of the city markets two adult males which were killed in the Presumpscot River, March 31, 1882. On April 12 I found another male in the market; the next day I purchased a pair from a sportsman in Deering; and on April 17 detected another male in the market. That the bird’s occurrence in such numbers is very unusual there can be no doubt. In fact, so far as I have been able to learn, our most experienced hunters of wild fowl either knew the species only by tradition, before this year, or else were wholly unacquainted with it.
Mr. Brewster has more than once advanced good evidence to the effect that the Short-tailed Tern (Hydrochelidon lariformis) should be considered a regular and not uncommon visitor to suitable localities on the New England coast.[90] Specific records for Maine are, notwithstanding, few as yet.[91] Two recent specimens should go on the list. One of these was killed in Scarborough, the other at Wells Beach, York County, in the autumn of 1881.—Nathan Clifford Brown, Portland, Maine.
Maine Notes.—Oporornis agilis (Wils.) Baird. Connecticut Warbler.—Mr. Nathan Clifford Brown, in a paper read before the Portland Society of Natural History April 3, 1882, gives this bird for the first time a place in the Maine fauna. He met with it Aug. 30, 1878, on Cape Elizabeth. I would record a specimen which I took in August, 1879, at Ebeme Lake. This makes the second record for this State.
Hylocichla unalascæ pallasi (Caban.) Ridgw. Hermit Thrush.—These birds breed commonly with us every year (Bangor). Their eggs 191are usually taken early in June, but I find among my notes the record of a set taken August 5, 1873, at Dedham, Maine, the eggs being but slightly incubated. This would seem to be presumptive evidence for the belief that these birds raise two broods in a season.
Lomvia arra brünnichi (Scl.) Ridgw. Brünnich’s Guillemot; and Lomvia troile (Linn.) Brandt. Common Guillemot.—These birds are found on our coast in the winter season, Brünnich’s Guillemot being quite numerous, while the Common Guillemot is more rare. Some idea of their comparative numbers may perhaps be obtained from the fact that during the past two years I have procured some thirty specimens from different points on our coast (from Grand Manan to South Bristol) and out of this number only one was a representative of the Common Guillemot (L. troile.) The experience of Mr. N. A. Eddy of this city is exactly similar, and out of about an equal number of specimens he has obtained but a single example of troile. Other collectors in this vicinity who have received numbers of Guillemots have not obtained a specimen of Lomvia troile.
Actodromas fuscicollis (Vieill.) Ridgw. Bonaparte’s Sandpiper.—This bird is not given as a resident of our State in Hamlin’s, Verrill’s or Maynard’s lists, but is still a not uncommon autumnal migrant along our coast. They are seldom met with in the interior, and the only records of their capture away from the coast, so far as I can learn, are here given. Nathan C. Brown furnishes me the first record from his notes as follows: “Oct. 16, 1876. During the past two weeks our party has taken only three specimens of this bird at Lake Umbagog. One was shot about Oct. 2, the two others upon Oct. 14.” On October 23, 1881, I came upon a flock of four at a small pool near this city (Bangor), and obtained three of them. Mr. N. A. Eddy afterwards took one at the same place.—Harry Merrill. Bangor, Maine.
Stray Notes From Lookout Mountain, Tenn.—The following notes were taken on Lookout Mountain, Tenn., from March 17 to April 4, 1882. The “Mountain,” so-called, is a ridge, some twenty miles or more in length, extending nearly due north and south. Its altitude ranges from 2200 to 2450 feet above the sea, and from 1500 to 1750 feet above the Tennessee River, which touches the base at its most northern point: its width, at the top, is from half a mile to two miles. About two miles of its northern end is in Tennessee, the rest being in Georgia. My collecting was done mostly on the Tennessee portion, but occasionally I went into Georgia, my longest trip into that State being five miles. The country is, for the most part, heavily wooded, although towards the northern end a great deal of the timber was destroyed during the late war and the new growth is still quite small. There are numerous streams in the ravines, along the banks of which laurels, blackberries, etc., grow luxuriantly. On the east side of the ridge there are, for half a mile, huge boulders, and the trees, principally pines, on and around them, were, I found, a favorite resort 192for the smaller birds. The whole number of species noted during my stay was fifty, but I give only such notes as may, perhaps, be of general interest.
1. Sitta canadensis Linn. Red-bellied Nuthatch.—Met with but once, on March 29, in a partial clearing.
2. Dendrœoa virens (Gmel.) Baird. Black-throated Green Warbler.—First seen March 19. Taken March 20. After this date it was not at all uncommon, and could be heard singing at almost any hour of the day.
3. Peucæa æstivalis illinoensis Ridgw. Oak-wood Sparrow.—First noted April 3. Two males procured April 4, both in song. These were both well-marked examples of illinoensis, one, indeed, carrying the differentiation to an extreme degree. In this specimen the back was of a reddish-brown color, entirely without streaks, and exactly resembled extreme specimens from Illinois. The other had the back distinctly streaked with black, and closely resembled a specimen from Alabama, taken by Mr. N. C. Brown. I found these birds both in groves of small pines and in open fields where there were plenty of brush-piles. They seemed to be quite common, as I heard several singing, at the same time, in different parts of the field. I was enabled to compare my specimens with those of the Smithsonian Institution through the kindness of Mr. R. Ridgway, and for this and many other favors I wish to tender him my grateful thanks.
4. Corvus corax carnivorus (Bartr.) Ridgw. American Raven.—Quite common. Said to breed on the cliffs. I have seen as many as eight or ten chasing each other through the air at one time.
5. Catharista atrata (Wils.) Less. Carrion Crow.—Quite common. Breeds. They seem to keep in flocks more than Cathartis aura.
6. Bonasa umbella (Linn.) Steph. Ruffed Grouse.—Once seen and once heard “drumming.” The local sportsmen report them as being quite scarce.—W. H. Fox, Washington, D. C.
Vol. VII, page 119, line 8, for “struggling” read “straggling”; page 122, line 9 from bottom, for “Rellon” read “Redlon”; page 123, line 28, for “Before” read “Upon.”
60. Carpodacus frontalis (Say) Gray. House Finch.
571, ♀ ad., Camp Lowell, June 22.
61. Loxia curvirostra mexicana (Strickl.) Baird. Mexican Crossbill.—Chiricahua Mountains; most numerous on the eastern side. Young just able to fly were taken March 7.
All of the following specimens are referable to true mexicana.
16, ♂ ad., Chiricahua Mountains, March 6. Length, 7.10; extent, 11.90; wing, 4; tail, 2.73; culmen, .87.
17, ♀ ad., same locality and date. Length, 7.10; extent, 11.80; wing, 3.88; tail, 2.75; culmen, .85. “Iris dark brown. The jaw muscles were extraordinarily developed on the side toward which the lower mandible crossed.”
24, ♀ ad., Chiricahua Mountains, March 7. Length, 6.80; extent, 11.40; wing, 3.70; tail, 2.52; culmen, .81.
25, ♀ juv., first plumage, same locality and date. This bird had been out of the nest but a few days and the tips of the mandibles had not begun to cross.
116, ♂ juv., first plumage, Chiricahua Mountains, March 26. Length, 6.90; extent, 12; wing, 4; tail, 2.75: culmen. .65. Wings and tail fully grown; mandibles decidedly crossed.
19462. Chrysomitris psaltria (Say) Bp. Arkansas Goldfinch.—“Common in only a few localities. I have not found much difference among the examples that occur here and have taken few that answered the description of var. arizonæ. California specimens are almost identical with those from New Mexico.”
130, ♂ ad., Chiricahua Mountains, March 30. Length, 4.50; extent, 7.80; wing, 2.65; tail, 1.90. “Iris brown.”
63. Chrysomitris pinus (Wils.) Bp. Pine Finch.—Common among the Chiricahua Mountains.
20, ♂ ad., Chiricahua Mountains, March 7. Length, 5; extent, 8.90; wing, 2.91; tail, 2.20.
128, ♂ ad., Chiricahua Mountains, March 29. Length, 4.90; extent, 8.60; wing, 2.96; tail, 2.14; “Iris dark brown.”
64. Poœcetes gramineus confinis Baird. Western Grass Finch.—“Common on prairies.”
The utility of recognizing this race of the Grass Finch seems to me questionable, although the western bird certainly possesses slight differential characters; these, however, are so largely comparative that they are difficult of adequate description, and any one attempting to determine examples by the books without the aid of large series of specimens, will be likely to abandon the task in despair.
158, ♀ ad., Sulphur Spring Valley, April 4. Length, 6.20; extent, 10.20; wing, 3.20; tail, 2.90.
164, ♂ ad., near Tombstone, April 5. Length, 6.40; extent, 10.80; wing, 3.35; tail, 3.04.
65. Spizella socialis arizonæ Coues. Western Chipping Sparrow.—Noted only at Cave Creek. “A large flock; they keep much among trees.”
11, ♂ ad., Cave Creek, March 5. Length, 5.50; extent, 8.90. “Iris dark brown; bill dark flesh-color; legs pale brownish.”
66. Spizella breweri Cass. Brewer’s Sparrow.—Four specimens, all taken April 5, near Tombstone. Eight were killed by one shot into a flock which had gathered about a water-hole, but they were in such ragged plumage, owing to the progress of the spring moult, that half of them had to be thrown away.
67. Junco oregonus (Towns.) Scl. Oregon Snowbird.—A single specimen obtained March 5, on Cave Creek.
68. Junco cinereus caniceps[92] (Woodh.) Coues. Gray-headed Snowbird.
19510, ♂ ad., Cave Creek, March 5. Length, 6.20; extent, 9.20. Iris dark brown; bill and legs flesh-color.
15, ♀ ad., same locality and date. Length, 6.30; extent, 9.
141, ♀ ad., Chiricahua Mountains, March 31. Length, 6.10; extent, 9.30. Iris dark brown.
69. Junco cinereus dorsalis (Henry) Henshaw. Red-backed Snowbird.
108, ♀ ad., Chiricahua Mountains, March 26. Length, 6.50; extent, 9.50; wing, 3.05; tail, 3.18. “Not as plenty here as J. cinereus.”
70. Junco cinereus (Swains.) Caban. Mexican Snowbird.—Nine specimens, all taken during March, in the Chiricahua Mountains.
71. Amphispiza bilineata (Cass.) Coues. Black-throated Sparrow.—Mr. Stephens found this Sparrow on barren plains sparsely covered with low bushes; he considers it a permanent resident in Arizona.
Juv., first plumage ♂ (No. 613, Camp Lowell, June 28). Crown, lores, orbital region and sides of head generally, dull brownish-ash; a white superciliary line as in the adult; back faded brown with shaft-stripes of a darker shade on most of the feathers; wing-coverts and outer webs of inner secondaries, reddish-buff; beneath dull white with the breast and sides of the abdomen thickly but finely streaked with dull black.
In addition to the bird just mentioned the collection includes five adults from the following localities: San Pedro River (♂, Dec. 25); Sulphur Spring Valley (♂, April 4); Tucson (♀, May 3); Santa Rita Mountains (♀, May 20); Camp Lowell (♂, May 30).
72. Peucæa cassini (Woodh.) Baird. Cassin’s Sparrow.—Although special efforts were made to obtain specimens of this species, only one was secured during the trip. “The song of the male is peculiar; about midway it drops several notes and is finished on one key. Several others seen. They were all very wild.”
159, ♀ ad., Sulphur Spring Valley, April 4. Length, 6.30; extent, 7.80; wing, 2.50; tail, 2.82. “Iris brown.”
73. Peucæa carpalis Coues. Rufous-winged Sparrow.—Found sparingly about Tucson and Camp Lowell. It inhabited the mesquite thickets, keeping closely hidden in the bunches of “sacaton” grass, from which, when flushed, it flew into the branches above.
233, ♀ ad., Tucson, April 19. Length, 5.70; extent, 7.90; wing, 2.42; tail, 2.82.
234, ♂ ad., same locality and date. Length, 5.90; extent, 8; wing, 2.57; tail, 3. “Iris brown; bill dark brown above, paler below; legs pale brown.”
196432, ♀ ad., Tucson, May 25. Length, 5.80; extent, 7.80; wing, 2.46; tail, 2.75. With nest and three eggs.
442, ♂ ad., Tucson, May 27. Length, 5.80; extent, 8; wing, 2.58; tail, 3.
582, ♂ ad., Camp Lowell, June 24. Length, 5.90; extent, 8.20; wing, 2.55; tail, 2.91.
74. Peucæa ruficeps boucardi (Scl.) Ridg. Boucard’s Sparrow.—These Sparrows were met with at Cave Creek, near Morse’s Mill, and in the Santa Rita Mountains. Among some notes taken at the first-named place I find the following: “I saw five of these Sparrows to-day [March 4] but two of them escaped me. They were in scrub oaks on rocky hillsides, and were apparently mated. They acted somewhat like Wrens, hiding among the rocks and flushing from the grass at a point some distance beyond where I would mark them down. Two went into the low branches of the oaks, from which I easily shot them. I have not found the species before in Arizona, but I took several near Fort Bayard, New Mexico, in 1876.” A specimen taken near the end of March was shot “on a ridge among thick brush,” while two others, obtained in the Santa Rita Mountains in May, occurred at a high elevation on similar ground.
1, ♂ ad., Cave Creek, March 4. Length, 6.60; extent, 8.30; wing, 2.80; tail, 3.29.
2, ♀ ad., same locality and date. Length, 6.40; extent, 7.90; wing, 2.60; tail, 3.02.
3, ♂ ad., same locality and date. Length, 6.50; extent, 8.20. “Iris brown; legs pale flesh-color; bill dark bluish slate-color.”
138, ♂ ad., Chiricahua Mountains, March 31. Length, 6.50; extent, 8.30; wing, 2.56; tail, 3.15.
387, ♀ ad., Santa Rita Mountains, May 16. Length, 6.10; extent, 8.10; wing, 2.58; tail, 2.95. “Iris brown; bill blackish above, light bluish below; legs pale flesh-color.”
413, ♀ ad., Santa Rita Mountains, May 20. Length, 6.30; extent, 7.80; wing, 2.50; tail, 3.
The specimens enumerated above represent true boucardi and are readily separable from Texas examples by the characters which my friend Mr. Brown has lately pointed out[93] in his diagnosis of the new race, eremœca.
75. Melospiza fasciata fallax Baird. Western Song Sparrow.—Rather common about Tucson where they haunted willow thickets and tall marsh grass near water.
258, ♀ ad., Tucson, April 21. Length, 6.30; extent, 8.20; wing, 2.60; tail, 2.98; culmen, .58. “Iris dark brown; bill dark above, light below; legs light brown. With nest and three eggs.”
197270, ♀ ad., Tucson, April 23. Length, 6.10; extent, 7.90; wing, 2.42; tail, 2.86; culmen, .54.
319, ♂ ad., Tucson, May 3. Length, 6.30; extent, 8.40; wing, 2.60; tail, 2.99; culmen, .55.
338, ♀ ad., Tucson, May 6. Length, 6.10; extent, 7.80; wing, 2.52; tail, 2.97; culmen, .53. “With nest and three eggs: set completed.”
510, ♂ ad., Tucson, June 8. Length, 6.50; extent, 8.40; wing, 2.74; tail, 3.16; culmen, .52.
76. Melospiza lincolni (Aud.) Baird. Lincoln’s Finch.—“Common along streams” in March. Two specimens (Cave Creek, March 5).
77. Passerella townsendi schistacea (Baird) Coues. Slate-colored Sparrow.—None were met with during 1881, but I have a specimen taken by Mr. Stephens near Tucson, in February, 1880.
78. Pipilo maculatus megalonyx (Baird) Coues. Spurred Towhee.—Two males, Chiricahua Mountains, March 26 and 28. “Common in brush, usually along streams. They have a variety of calls, some of which resemble those of the Catbird. The song, uttered while the bird is sitting on a tree, sounds like jack-jacksonii.”
The North American Towhees of the maculatus group are at present involved in much confusion. The trouble seems to be that each locality furnishes a race of its own which either possesses certain slight individual characteristics, or combines, in varying degrees, the characters of two or more recognized forms. The case, however, is not peculiar; for to a greater or less extent the same state of things obtains among the Song Sparrows, Shore Larks, and several other species, in which the forces of evolution are still actively working.
79. Pipilo chlorurus (Towns.) Baird. Green-tailed Towhee.—Several specimens taken late in April. “Not common; usually found in low brush.”
80. Pipilo fuscus mesoleucus (Baird) Ridgw. Cañon Towhee.—“Common in rocky localities on plains, and in valleys.” A nest containing three eggs was taken June 15 at a point about twenty-five miles north of Tucson. The eggs are grayish-white with numerous, short, zigzag lines of black about the larger end and occasional spots or dashes of brown and dull lavender scattered over the general surface of the shell. They measure respectively .91 × .69, .94 × .69, and .92 × .69. The nest, which was placed about four feet above the ground in a “cat-claw” mesquite, is firmly and rather compactly built of fibrous shreds 198from the stalks of herbaceous plants, with a few twigs and whole stems supporting the outside, and a scanty lining of horse-hair. Its external diameter is about five inches; its depth two. The cavity is two inches wide and one and a half deep. Both nest and eggs differ somewhat from California examples of crissalis in my collection, the eggs being smaller and whiter, the nest softer and more compact.
177, ♂ ad., Tombstone, April 7. Length, 8.80; extent, 11.60. “Iris light brown.”
186, ♀ ad., Tombstone, April 9. Length, 8.10; extent, 10.90; wing 3.50; tail, 4.15.
416, ♂ ad., Santa Rita Mountains, May 20. Length, 8.50; extent, 11.50; wing, 3.73; tail, 4.45.
81. Pipilo aberti Baird. Abert’s Towhee.—“I have found this species common along the Colorado and Gila Rivers, and I took several on the San Pedro in December, 1880. They appear to be restricted to the vicinity of streams and usually to thick brush, although they frequent trees more than most of the members of this genus. I have seen them hunting insects in the bark of large trees in a manner similar to that of Wrens. They are rather shy. The usual note is a sharp chirp. The song is difficult to describe; it is rapid and near the middle rises to a higher key, quickly falling again and ending on the initial note. The nest is rather bulky; it is sometimes built in bushes near the ground, and again in trees. I found one in a bunch of mistletoe at a height of at least thirty feet.”
A nest found May 28, at Tucson, was built on the top of a mesquite stump, where it was kept in place by the surrounding sprouts. It contained three fresh eggs which measure respectively .91 × .72, .92 × .72, and .90 × .71. They are elliptical in shape, and in the character and distribution of their markings they resemble the above described eggs of P. mesoleucus from which, however, they differ in having a faint but decided bluish cast. The nest is large and loosely built. It is composed mainly of broad strips or ribbons of bark with which are mingled small, pliant twigs and the green stems and leaves of the mesquite(?). The whole structure is homogeneous and, strictly speaking, it has no lining, but the materials surrounding the cavity are rather softer than the rest, while they are arranged with some regard to smoothness. The external diameter of this nest is about seven inches; its depth three. The cavity is three inches wide and two deep.
199Juv., first plumage (No. 520, Tucson, June 10). Above uniform light brown; wing-coverts, outer edges of the inner secondaries and a narrow tipping on the tail, brownish-ochraceous; beneath brownish-fulvous with an ochraceous tinge on the throat, abdomen, and crissum, and a broad band of coarse but obscure black spots extending across the breast; head markings as in the adult, but duller.
Eight specimens were collected. “Iris light brown; bill brownish horn-color above, bluish beneath; legs brown.”
82. Cardinalis virginianus igneus (Baird) Coues. Saint Lucas Cardinal.—Found only at Tucson, where it occurred sparingly in low brush, usually near streams.
269, ♂ ad., Tucson, April 23. Length, 9.40; extent, 12.40; wing, 4.12; tail, 4.92; longest feathers of crest, 1.35. “Iris dark brown; legs brown.”
83. Pyrrhuloxia sinuata Bonap. Texan Cardinal.—In the latter part of April three of these Cardinals were taken near Tucson, and several others were seen in the same place during March, 1880. They were found among mesquites, along brush fences and in the shrubbery of an arroya. “Iris dark brown; bill yellowish horn-color; legs pale brown. Food seeds, green buds and insects.”
84. Zamelodia melanocephala (Swains.) Coues. Black-headed Grosbeak.—Common at high elevations among the mountains.
367, ♂ ad., Santa Rita Mountains, May 13. Length, 8.10; extent, 12.90; wing, 4.17; tail, 3.75. “Iris dark brown; legs light plumbeous.”
391, ♀ ad., Santa Rita Mountains, May 16. Length, 8.40; extent, 12.80; wing, 4.28; tail, 3.70.
In addition to being considerably larger than any of my more northern specimens, these examples are peculiar in having the interscapular feathers so broadly edged with brownish-orange (brownish-yellow in the ♀) that the back appears to be about equally streaked with light and dark color.
85. Guiraca cœrulea (Linn.) Swains. Blue Grosbeak.—Only a few were seen during the present trip, but Mr. Stephens found them common on the Gila River in 1876. “They are late migrants.”
445, ♂ ad., Tucson, May 28. Length, 7.20; extent, 11.10; wing, 3.60; tail, 3.27. “Iris dark brown; bill black above, bluish below; legs black.”
86. Passerina amœna (Say) Gray. Lazuli Bunting.—Two specimens, obtained April 25, at Tucson, are noted as “the first ones seen.” One of them, a male, has the blue almost completely obscured by rufous, which forms a broad tipping on all the feathers of the upper parts. The throat, however, remains nearly pure blue.
20087. Calamospiza bicolor (Towns.) Bonap. Lark Bunting.—Several large flocks were seen April 13, in the neighborhood of Tombstone. Most of the males were in parti-colored dress, not above one per cent having put on the black breeding-plumage. The stomachs of all which were killed contained “buds and seeds.”
88. Molothrus ater obscurus (Gmel.) Coues. Dwarf Cowbird.
277, ♂ ad., Tucson, April 25. Length, 7.30; extent, 12.40; wing, 4.02; tail, 3.20. “Iris dark brown.”
417, ♂ ad., Santa Rita Mountains, May 20. Length, 7.10; extent, 12.10; wing, 4.01; tail, 3.17.
89. Agelæus phœniceus (Linn.) Vieill. Red-winged Blackbird.
511, ♀ ad., Tucson, June 8. Length, 8.10; extent, 13.20; wing, 4.22; tail, 3.40.
90. Icterus parisiorum Bonap. Scott’s Oriole.—Although this Oriole was oftenest seen among the foot-hills it occasionally occurred on the most barren plains, where it seemed content with the scanty shelter afforded by the cactus thickets. In the hill country it frequented the oak belt, and was seldom observed at a high elevation. During the breeding season it was seen near Tucson, as well as among the Santa Rita Mountains, but no nests were found in either locality.
Juv., first plumage (♀. No. 528, Tucson, June 14). Generally like the adult, but with all the wing feathers edged and tipped with white, the wing-bands yellowish, the tail tipped with yellow, the breast obscured with brownish, and the yellow of the under parts paler and greener.
Only a small proportion of the males collected by Mr. Stephens have the adult plumage perfected. A female (No. 189, Tombstone, April 10) has a black throat-patch extending from the chin to the breast, and small, sagittate black spots on the crown.
“Iris dark brown; bill black, bluish at base below; legs dark bluish. Food, insects.”
91. Icterus cucullatus Swains. Hooded Oriole.—An uncommon species, found only in the valleys, where it seemed to prefer cottonwoods to other trees.
The specimens taken are all adults, with the exception of a male which, although evidently a bird of the previous year, differs from the females only in having a black throat-patch and several concealed black spots on the interscapulars. One of the females is also peculiar in having many half-concealed black spots on the throat and jugulum. Some of the richest-colored males have the interscapular feathers tipped with yellow.
20192. Icterus bullocki (Swains.) Bonap. Bullock’s Oriole.—Only two of these Orioles were taken during 1881: but in the previous summer Mr. Stephens found them not uncommon in the foot-hills of the Chiricahua Mountains.
93. Corvus corax carnivorus (Bartr.) Ridgw. American Raven.—Incidentally mentioned as common about Tucson.
94. Corvus cryptoleucus Couch. White-necked Raven.—A small proportion of the Ravens seen about Tucson were recognized as belonging to this species. Their notes differed widely from those of the common Raven, and “at times sounded somewhat like the quacking of a Duck.”
324, ♀ ad., Tucson, May 4. Length, 19.90; extent, 40.70; wing, 14.06; tail, 8.94. “Iris dark brown.”
95. Cyanocitta stelleri macrolopha (Baird) Ridgw. Long-crested Jay.—Five specimens, Chiricahua Mountains, March 24 to 26. “These Jays are common in the pines well up the mountain sides, but they are wary and difficult of approach. When pursued they fly from one tree to the lower branches of the next and jumping from limb to limb, take flight again as soon as they reach the top. If one can follow fast enough to get within range before the bird reaches the top of the tree he may obtain a shot, but it is necessary to keep behind some object while accomplishing this. They are noisy and have a variety of calls, some of which are disagreeably harsh. I think they are shyer here than in other localities where I have met with them.” One of Mr. Stephens’ specimens (No. 106) has the crest strongly tinged with blue, thus approaching var. diademata of Mexico.
96. Aphelocoma woodhousii (Baird) Ridgw. Woodhouse’s Jay.—One specimen, Galeyville. January 29, 1881.
97. Aphelocoma sordida arizonæ Ridgw. Arizona Jay.—Mr. Stephens met with this Jay in the Chiricahua and Santa Rita Mountains, and judging from the number of specimens obtained it must be rather abundant in both ranges. “They go in flocks of from five to twenty, and are generally seen in the foot-hills. They are restless, and in most localities shy, but around mills, where they congregate to feed on the grain in horse droppings, they become used to the presence of human beings and are more easily approached. Their food is chiefly broken acorns.”
A nest found May 16, in the Santa Rita Mountains, is a bulky structure composed chiefly of yellowish rootlets with some coarse 202dead twigs protecting its exterior and a scanty lining of fine grasses. The female was sitting on four eggs. which were on the point of hatching. The only specimen saved measures 1.13 × .82. It is pale greenish-blue, absolutely without markings, and closely resembles a Robin’s egg. “The others were similar, as were three eggs of a set taken in 1876, and two of one found in 1880.”
Of the fifteen specimens collected only four have the bill wholly black. With all the others there is more or less flesh-color, which, although usually confined to the base and tip of the lower mandible, sometimes spreads over nearly the whole of the bill below as well as encroaching on the maxilla at the tomia, and occasionally even occupies a narrow central space along the ridge of the culmen above the nostrils. Mr. Henshaw has remarked on this feature, which he considers peculiar to young birds. If this view be correct it must require several years for the bill to become unicolor.
98. Eremophila alpestris chrysolæma (Wagl.) Coues. Mexican Shore Lark.—The only Shore Lark in the collection, a young bird in first plumage, taken on the plains at the base of the Santa Rita Mountains, has been referred by Mr. Ridgway to the above race.
99. Tyrannus verticalis Say. Arkansas Flycatcher.—Although this species was much less numerous than the following, especially after the spring migrants had gone, a few pairs were found breeding about Camp Lowell, where a nest containing three slightly incubated eggs was taken on June 20. The collection includes skins from Tucson and Camp Lowell.
100. Tyrannus vociferans Swains. Cassin’s Flycatcher.—“Abundant in summer. Neither verticalis nor vociferans winters in Arizona.” Specimens were collected at Tombstone, Tucson, and among the Santa Rita Mountains.
The peculiar attenuation of the primaries in this species has been freely commented on by authors, but no one seems to have noticed that this character, at least as applied in diagnoses, is to be found in only the male of T. vociferans. Nevertheless this is true of the somewhat large series of specimens before me, among which there is a decided and very constant sexual difference in the shape of the outer four primary feathers. All the adult males have them abruptly and deeply notched on the inner webs about half an inch from the tip, the emargination extending more than half-way to the shaft and reducing the width of the feather, terminally, to about .12 of an inch. In the females these feathers show no well-defined notching, the tips being simply tapered, usually with a slightly concave outline, although the outline is sometimes actually rounded. A young male from Riverside, 203California (No. 6380, Sept. 19, 1881), taken during its first autumnal moult, has the old primaries (1–2) almost without attenuation, their tips being only slightly tapered, while the new ones (3–5) are as deeply notched as in any of the adults. Hence it is probable that males in first plumage will be found to have the primaries shaped like those of the female.
The sexes of T. verticalis differ in a similar manner but less markedly, for the first primary of the female, although broader than that of the male, usually has the same falcate shape. I have one or two females, however, which, by the wing characters alone, can with difficulty be distinguished from females of vociferans.
101. Myiarchus mexicanus cooperi[94] (Kaup) Baird. Cooper’s Flycatcher.—This large Myiarchus which, as I lately announced,[95] Mr. Stephens has the credit of first finding within our boundaries, was ascertained to be a common summer resident about Camp Lowell. Of its occurrence in New Mexico, also, I now have positive evidence, a previously undetermined specimen, taken by Mr. Stephens near the Gila River, June 12, 1876, proving on comparison to be identical with the Arizona ones.
The collector’s notes relating to the habits of this Flycatcher are disappointingly brief. It frequented low mesquites and was tame and rather noisy, having a variety of loud calls, some of which resembled those of M. cinerescens, while others were “almost Thrasher-like.” Its food seemed to consist largely of beetles. On June 27 a nest was found at Camp Lowell. “Both parents were distinctly seen and positively identified. The nest was in an old Woodpecker’s hole in a giant cactus about eighteen feet from the ground. It was lined with soft, downy weed-seeds, and contained two young just hatched and an addled egg.” The egg, unfortunately, is so badly broken that accurate measurements are impossible, but an approximation would be 1.04 × .74. In ground-color and markings it closely resembles eggs of M. crinitus, the shell being a dull clayey-buff over which are numerous longitudinal lines and dashes of purplish-brown or lavender. 204These markings are pretty evenly distributed, but are coarsest at the larger end of the egg.
462, ♂ ad., Camp Lowell, May 31. Length, 9.90; extent, 14.10; wing, 4.40; tail, 4.40; culmen, 1.15. “Iris brown; bill and legs black.”
468, ♂ ad., Camp Lowell, June 1. Length, 10; extent, 14.30; wing, 4.35; tail, 4.44; culmen, 1.10.
472, ♂ ad., Camp Lowell, June 2. Length, 9.90; extent, 14.10; wing, 4.40; tail, 4.37; culmen, 1.27.
473, ♂ ad., same locality and date. Length, 10; extent, 14.20; wing, 4.40; tail 4.60; culmen, 1.25.
491, ♂ ad., Camp Lowell, June 4. Length, 9.60; extent, 14.20; wing, 4.40; tail, 4.40; culmen, 1.13.
492, ♂ ad., same locality and date. Length, 9.80; extent, 14.30; wing, 4.38; tail, 4.49; culmen, 1.15.
558, ♂ ad., Camp Lowell, June 21. Length, 9.80; extent, 14.30; wing, 4.37; tail, 4.47; culmen, 1.16.
592, ♂ ad., Camp Lowell, June 25. Length, 9.80; extent, 13.80; wing, 4.23; tail, 4.35; culmen, 1.16.
463, ♀ ad., Camp Lowell, May 31. Length, 9.60; extent, 13.70; wing, 4.12; tail, 4.34; culmen, 1.10.
464, ♀ ad., same locality and date. Length, 9.50; extent, 13.60; wing, 4.16; tail, 4.32; culmen, 1.11.
493, ♀ ad., Camp Lowell, June 4. Length, 9.60; extent, 13.70; wing, 4.16; tail, 4.16; culmen, 1.10.
559, ♀ ad., Camp Lowell, June 21. Length, 9.40; extent, 13.40; wing, 4.04; tail, 4.10; culmen, 1.10.
591, ♀ ad., Camp Lowell, June 25. Length, 9.40; extent, 13.60; wing, 4.15; tail, 4.10; culmen, 1.12.
102. Myiarchus cinerescens Lawr. Ash-throated Flycatcher.—Specimens were obtained at Tombstone, Tucson, and Camp Lowell. In the latter locality the bird was common through June and was presumably breeding, although no nests were actually found. At all the points in Arizona where they were observed these Flycatchers frequented the timber in valleys and along streams, none being seen among the denser forests of the mountains.
103. Myiarchus lawrencii (Giraud) Baird. Lawrence’s Flycatcher.—This pretty Myiarchus, scarcely larger than our common Phoebe, was met with only among the Santa Rita Mountains, where it was apparently not uncommon, although its distribution seemed to be very local, most of Mr. Stephens’ specimens being taken in a single cañon. They haunted the banks of streams, perching on dead limbs and taking frequent flights after insects. The only note heard was a short, mournful 205“peeúr.” No nests were found, but a female shot May 17 was laying.
In my preliminary announcement[96] of the occurrence of this species in Arizona I inadvertently gave the number of specimens as eight, whereas nine were really obtained. These show little variation in color or markings, but the females are slightly smaller than the males. The characters which separate M. lawrencii from its respective allies, M. tristis of Jamaica and M. nigricapillus of Central America, are well maintained in this series.
360, ♂ ad., Santa Rita Mountains, May 12. Length, 7.20; extent, 10.50; wing, 3.25; culmen, .76; tail, 3.38. “Iris dark brown; bill and legs black.”
361, ♂ ad., same locality and date. Length, 7.20; extent, 10.30; wing, 3.25; culmen, .80; tail, 3.43.
364, ♂ ad., same locality and date. Length, 7.30; extent, 10.30; wing, 3.20; culmen, .80; tail, 3.35.
400, ♂ ad., same locality, May 17. Length, 7.10; extent, 10.20; wing, 3.20; culmen, .77; tail, 3.36.
412, ♂ ad., same locality, May 19. Length, 7.30; extent, 10.50; wing, 3.26; culmen, .82; tail, 3.32.
388, ♀ ad., same locality, May 16. Length, 7.10; extent, 10; wing, 3.20; culmen, .81; tail, 3.20.
401, ♀ ad., same locality, May 17. Length, 7; extent, 10; wing, 3.05; culmen, .74; tail, 3.05.
402, ♀ ad., same locality and date. Length, 7.10; extent, 10.
403, ♀ ad., same locality and date. Length, 7; extent, 9.80; wing, 3.10; culmen, .85; tail, 3.16. “Laying.”
104. Sayiornis sayi (Bonap.) Baird. Say’s Pewee.—“Common on prairies; usually found singly, perching on weed-stalks. They do not frequent timber. Iris dark brown; bill and legs black.” Several specimens collected.
105. Sayiornis nigricans (Swains.) Bonap. Black Pewee.—Found more or less abundantly along streams, but rarely at a great elevation in the mountains. “The nest is similar to that of S. fusca, and is built under bridges or sometimes in deserted dwellings. Iris dark brown; bill and legs black.” Several specimens taken.
106. Contopus borealis (Swains.) Baird. Olive-sided Flycatcher.—Two specimens were obtained in May in the Santa Rita Mountains, where it was “not very common.”
107. Contopus pertinax Caban. Coues’s Flycatcher.
392, ♀ ad., Santa Rita Mountains, May 16. Length, 7.70; extent, 12.50; wing, 4.12; tail, 3.30; culmen, .78. “Iris dark brown; bill black above, yellow below with dusky tip; legs black.”
206108. Contopus virens richardsoni (Swains.) Coues. Western Wood Pewee.
371, ♂ ad., Santa Rita Mountains, May 13. Length, 6.40; extent, 10.70. “Iris dark brown; bill black above, dusky below.”
109. Empidonax flaviventris difficilis Baird. Western Yellow-bellied Flycatcher.
Both of the following specimens are more decidedly ochraceous than are my California examples, the latter, like many Pacific Coast birds, showing a closer approach to the eastern form. Difficilis, however, seems to be a pretty strongly characterized race, if not, as Mr. Ridgway has lately ranked it, a distinct species.
484, ♂ ad., Camp Lowell, June 3. Length, 5.50; extent, 8.10; wing, 2.60; tail, 2.46.
517, ♀ ad., Tucson, June 10. Length, 5.50; extent, 8.10; wing, 2.46; tail, 2.52.
110. Empidonax pusillus (Swains.) Baird. Little Flycatcher.—A common bird about Tucson, where it inhabited willow thickets near water. Numerous nests were taken: the one sent me is a loosely woven structure composed chiefly of dry grasses, with a neat lining of horse-hair. It agrees closely with northern New England nests of E. trailli, and like them differs widely from the compact, Yellow-Warbler-like nests which trailli builds in the region about Columbus, Ohio, and at St. Louis, Missouri.[97]
The series of skins is a full one, and the specimens uniformly sustain the characters ascribed to pusillus, a race which seems to me quite as constant as many which have been regarded with less suspicion and disfavor.
111. Empidonax hammondi (Xantus) Baird. Hammond’s Flycatcher.
172, ♀ ad., near Tombstone, April 12. Length, 5.40; extent, 8.90.
237, ♂ ad., Tucson, April 19. Length, 5.40; extent, 8.70.
363, ♀ ad., Santa Rita Mountains, May 12. Length. 5.30; extent, 8.30.
No. 237 has the outer web of the external rectrices as white as in average specimens of E. obscurus. I have Colorado examples also which cannot be separated from obscurus by this character alone.
112. Empidonax obscurus (Swains.) Baird. Wright’s Flycatcher.—This species was noted only in the vicinity of Tombstone, where a few were found early in April among scattered clumps of trees.
The four specimens collected have the lower mandible pale orange. 207passing into dusky at the tip, and in this respect differ from some more northern ones in which the part is flesh-color.
113. Empidonax fulvifrons pallescens Coues. Buff-breasted Flycatcher.—A single specimen from the Santa Rita Mountains is accompanied by the following remarks: “Rare here; more numerous in the Chiricahua Mountains last season [1880]; and rather common near Fort Bayard, New Mexico, in 1876. One of its notes is a chirp similar to a Warbler’s.”
395, ♂ ad., Santa Rita Mountains, May 17. Length, 5.10; extent, 7.90. “Iris dark brown; bill black, yellow below; legs black.”
114. Pyrocephalus rubineus mexicanus (Scl.) Coues. Vermilion Flycatcher.—This beautiful species was found at Cienega Station in April; near Tucson and among the Santa Rita Mountains during May; and about Camp Lowell in early June. In all these localities it was abundant among undergrowth, usually near water. “Their motions resemble those of other Flycatchers, excepting that they have a habit of poising over one spot for several seconds at a time, maintaining their position by a rapid fluttering of the wings very nearly in the manner of a Sparrow Hawk.”
A nest taken April 25, at Tucson, was placed in the horizontal fork of a stout mesquite branch to which it was attached in such a manner that its upper surface was flush with that of the embracing supports. This nest is composed outwardly of small twigs, and is lined with horse and cow hair and a few feathers. It entirely lacks the exterior coating of lichens spoken of by Dr. Merrill,[98] but in other respects it agrees well with his description of the Fort Brown (Texas) specimen. The three eggs which it contained are creamy white with rounded blotches of brown and pale lilac wreathed about their larger ends. They measure respectively .72 × .53, .71 × .53, .70 × .52. Mr. Stephens found other nests similar in construction and position to the present one. He considers three eggs the full complement.
Juv., first plumage, ♂ (No. 6153 (Coll.’s No. 466) Camp Lowell, June 1). Above similar to the adult female, but with the rump golden brown; the wing-coverts and outer webs of the secondaries, brownish-fulvous; and the feathers of the occiput, nape and interscapular region tipped with brownish-white; beneath white with a tinge of lemon-yellow on the 208sides and crissum; the breast and sides of the abdomen thickly marked with rounded spots of clear brown.
The series of adults is a very full one and includes several interesting styles of plumage. Some of the males have the brown of the back mixed with ashy, which has a tendency to form a collar on the nape, and gives the interscapular region a patched appearance. In others the red of the under parts as well as that of the crown is replaced by orange; while one specimen has a large patch of lemon-yellow on the right side of the breast, which shows in striking contrast with the otherwise clear carmine of the lower surface. These variations present a curious analogy to certain similar ones which occur in the Scarlet Tanager and Summer Redbird.
115. Ornithium imberbe ridgwayi, var. nov. Ridgway’s Beardless Flycatcher.
Ch. Sp. ♂ Similis O. imberbi, sed rostro robustiore; colore obscuriore ac magis cinerario.
Adult ♂ (No. 6000, author’s collection—collector’s No. 313. Tucson, May 1, 1881. F. Stephens). Above ashy brown; crown nearly pure brown in decided contrast with the back; rump pale brown with a faint olive tinge; wings and tail brown, edged with ashy-white; greater and middle wing-coverts tipped with fulvous, forming two wing-bars; edge of wing and under wing-coverts pale lemon-yellow; lores and sides of head posteriorly, ashy; a narrow frontal line continued backward above and nearly around the eye, ashy-white; under parts ash, shading posteriorly to ashy-white on the belly, and with the faintest possible lemon tinge on the jugulum and crissum; bill stout; upper mandible much curved, brown; under mandible slightly curved, brown at tip, brownish-orange at base; commissure reddish-orange.
Dimensions. Length, 4.60; extent, 7.20; wing, 2.23; tail, 1.96; culmen, .42; tarsus, .56; depth of bill at nostrils, .15.
Adult ♀ (No. 6133, author’s collection—collector’s No. 446. Tucson, May 28, 1881. F. Stephens). Smaller than the male, slightly more yellowish below and with a faint tinge of olive on the back.
Dimensions. Length, 4.50; extent, 6.70; wing, 2.04; tail, 1.78; culmen, .40; tarsus, .52; depth of bill at nostrils, .14.
Juv., first plumage, ♂ (No. 6138, author’s collection—collector’s No. 451. Tucson, May 29, 1881. F. Stephens). Crown plumbeous; back olive-brown; wing and tail-coverts, outer edges of secondaries, and a broad tipping on all the rectrices, dull brownish-chestnut; beneath delicate ashy-buff, shading to yellowish-white on the belly and crissum; bill orange, dusky at tip of upper mandible.
Habitat, Arizona.
The chief points of difference between the above race and imberbe proper may be briefly expressed as follows:
O. imberbe.—Depth of bill at nostrils, .11 to .13. Above olivaceous-ash; entire under parts strongly tinged with lemon-yellow.
209O. imberbe ridgwayi.—Depth of bill at nostrils, .14 to .15. Above ashy brown; beneath ash or ashy-white with scarcely any yellowish.
In the present connection I have examined seven specimens of O. imberbe. Five of these, from the collection of the National Museum, represent the following localities: Texas (Rio Grande Valley), Mexico (Mazatlan and Tehuantepec) and Yucatan (Merida). The remaining two, in my own cabinet, were taken at Lomita Ranch, Texas, in March, 1880. The result of a careful comparison of this material is that the Texas examples prove to be identical with those from Mexico and Central America, while the Arizona birds differ very constantly from all the others in respect to the points mentioned above. The entire series is, of course, a small one, but its evidence seems sufficient to warrant the varietal separation of the Arizona form.
The detection of this Flycatcher in Arizona is perhaps the most interesting discovery resulting from Mr. Stephens’ late trip. O. imberbe has only recently been added to our fauna by Mr. Sennett, and the locality of his single specimen—Lomita, Texas—was so far beyond the previously known range of the species that its occurrence seemed hardly likely to prove more than a mere accident. In 1880, however, Mr. M. A. Frazar secured additional specimens at Lomita, and now an allied, but apparently distinct race, turns up in Arizona.
Mr. Stephens found the curious little bird only at Tucson, where his first specimen was taken April 28. Afterwards others were shot in the same locality, but they were by no means common. The males had a habit of perching on the tops of the tallest trees in the vicinity of their haunts, and at sunrise occasionally uttered a singular song which Mr. Stephens transcribes as “yoop-yoop-yoopeédeedledeè, the first half given very deliberately, the remainder rapidly.” A commoner cry, used by both sexes in calling to one another, was a shrill “piér pièr pièr pièr, beginning in a high key and falling a note each time.” They were very shy, and specimens were obtained only at the expense of much trouble and perseverance. Their loud calls were frequently heard, but when the spot was approached the bird either relapsed into silence or took a long flight to resume its calling in another direction. In their motions they resembled other small Flycatchers, but their tail was less frequently jerked.
On May 28 Mr. Stephens met with a young bird which had but just left the nest. It was accompanied by the female parent, who showed much solicitude and frequently uttered her shrill cries, to which the offspring responded in nearly similar tones. Both 210individuals were secured, but neither the nest nor the remainder of the brood—if indeed there were any more—could be found. On the following day this episode was repeated, a second female being found in attendance on another young bird of nearly the same age as that obtained on the previous occasion.
308, ♂ ad., Tucson, April 29. Length, 4.80; extent, 7.20; wing, 2.28; tail, 2.04; culmen, .40; tarsus, .55. “Iris dark brown; bill black, basal half of lower mandible reddish-brown; legs black. Contents of stomach worms and insects.”
313, ♂ ad., Tucson, May 1. Length, 4.60; extent, 7.20; wing, 2.23; tail, 1.96; culmen, .42; tarsus, .56.
446, ♀ ad., Tucson, May 28. Length, 4.50; extent, 6.70; wing, 2.04; tail, 1.78; culmen, .40; tarsus, .52. Parent of the next.
447, ♂ juv., first plumage, same locality and date.
450, ♀ ad., Tucson, May 29. Length, 4.30; extent, 6.80. Parent of the following.
451, ♂ juv., first plumage, same locality and date.
116. Trochilus alexandri Bourc. & Muls. Black-chinned Hummingbird.—The first specimen met with was a female which, with a nest and two eggs, was taken at Tucson on April 23. The species was also found breeding among the Santa Rita Mountains, as well as near Camp Lowell. At all these points it was decidedly the most abundant of the Hummingbirds.
Six of the seven examples collected are females, and Mr. Stephens remarks on the apparent absence of the males during the breeding season.
The nest just mentioned, and another obtained April 28 in the same locality, are now in my possession. Both were built in willows, one being saddled on a small branch, while the other rested lightly in the fork of a slender twig. Their construction is homogeneous, the only material used being a creamy-white down, probably from willow catkins. One nest, however, has a few delicate, faded leaves attached to its exterior. The eggs are indistinguishable from those of T. colubris. The first set was fresh, the second slightly incubated.
117. Calypte costæ (Bourc.) Gould. Costa’s Hummingbird.
289, ♀ ad., Tucson, April 26. Length, 3.70; extent, 4.60. “Iris dark brown; bill and legs black.”
294, ♂ im., Tucson, April 27. Length, 3.55; extent, 4.52. This specimen lacks the ruffs of the adult male, but has a patch of violet feathers on the centre of the throat.
211118. Selasphorus platycercus (Swains.) Bonap. Broad-tailed Hummingbird.
366, ♂ ad., Santa Rita Mountains, May 13. Length, 4; extent, 5.50. “Iris dark brown; bill black; feet black, their soles lighter.”
385, ♂ ad., Santa Rita Mountains, May 15. Length, 4.70; extent, 5.90.
119. Iache latirostris (Swains.) Elliot. Broad-billed Hummingbird.—From the known fact of its occurrence among the Chiricahua Mountains, as ascertained by Mr. Henshaw in 1874, it was of course to be expected that this Hummer would eventually be found, under similar conditions, in other parts of Arizona, a probability which Mr. Stephens has confirmed by the capture of five specimens in the Santa Rita Mountains. In addition to these, several others were seen at various times during his short stay in that range, and I infer from his notes that the birds were not uncommon there. They were always found near water, and usually along the streams which flowed through cañons, high among the mountains. They seemed to prefer sycamores to other trees, and invariably perched on dead twigs where they could command an open view. “Their notes were flat and differed from those of other Hummers.”
356, ♂ ad., Santa Rita Mountains, May 12. Length, 4.10; extent, 5.05; wing, 2.11; bill, .91. “Iris dark brown; point of bill below, with terminal third above, black; rest of upper mandible reddish-brown; of lower, purplish-red; feet black.”
365, ♀ ad., Santa Rita Mountains, May 13. Length, 3.95; extent, 5.05; wing, 1.98; bill, .92. “Bill above, and its tip below, black; remainder of lower mandible reddish. Not near laying.”
382, ♂ ad., Santa Rita Mountains, May 14. Length, 4; extent, 5.02.
405, ♂ ad., Santa Rita Mountains, May 18. Length, 3.88; extent, 4.98; wing, 1.99; bill, .88.
411, ♀ ad., Santa Rita Mountains, May 19. Wing, 2.03; bill, .90.
120. Cypselus saxatilis Woodh. White-throated Swift.—In some notes made at Cave Creek, under date of March 4, Mr. Stephens incidentally refers to this Swift as follows: “We camped here last night chiefly for the purpose of investigating some caves said to contain large quantities of bird-droppings. I went to one of the largest of these to-day and found the floor covered with tons of bat droppings as well as a little from birds. There were also a few feathers (primaries and rectrices) of Cypselus saxatilis and some of Falco sparverius.”
121. Antrostomus vociferus arizonæ Brewster. Stephens’ Whip-poor-will.—During 1881 this Whip-poor-will was 212again met with in Arizona among the Santa Rita Mountains, where, however, it was less numerous than it had been in the Chiricahua range in 1880. The only specimen obtained was an adult male which was shot, by moonlight, in oaks near a stream.
Through Mr. Stephens’ kindness I am now enabled to present descriptions of the female and egg alluded to in a letter quoted in connection with the original description[99] of the race.
Adult ♀ (6309, author’s collection, Chiricahua Mountains, Arizona, July 4, 1880. F. Stephens). General coloring similar to that of the male, but lighter, the ground tints more ochraceous; the white of the tail replaced by reddish-fulvous which forms a narrow tipping on the outer three pairs of rectrices; the tawny gular crescent continued around the sides of the neck, the ends meeting behind and forming an uninterrupted collar.
Dimensions. Length, 9.60; extent, 18.80; wing, 6.27; tail, 5.03; culmen, .80; tarsus, .70; longest rictal bristle, 1.40.
This specimen differs even more widely from the female, than does my type from the male of A. vociferus. The ochraceous of the lores, superciliary-stripe, and neck-collar, spreads over the entire plumage both above and beneath, giving it a tawny tinge which overlies and obscures the usual dark markings. On the shoulders, breast, lores and throat this color deepens to a fine reddish-chestnut, and elsewhere it replaces the ashy, dirty white and other light tints of the eastern birds. In its general coloring the plumage strikingly resembles that of the brown phase of Scops asio kennicotti. The ochraceous neck-collar is also present in the male from the Santa Rita Mountains, but it is less distinctly defined, being somewhat obscured, especially on the nape, by dusky mottling. In all other respects this example agrees closely with my type.
The egg is white with a dull gloss. At first sight it appears to be immaculate, but a closer inspection reveals a few faint blotches of the palest possible purple, so faint indeed that they might pass for superficial stains were it not for the fact that they underlie the external polish. The absence of well-defined markings may probably be explained by the assumption that the bird had laid one or more clutches earlier in the season, thus exhausting her supply of coloring pigment. The specimen measures 1.17 × .87.
355, ♂ ad., Santa Rita Mountains, May 11. Length, 9.90; extent, 18.70; wing, 6.50; tail, 5.15; culmen, .76; tarsus, .70; longest rictal bristle, 1.73.
It has never been my good fortune to enjoy the opportunity of studying the habits and manners of our American Dipper in its native haunts, but this seems to have been due more to my ill-luck, than to any neglect on my part to seize upon every chance to visit the localities where this bird, one that I have so often longed to see alive, certainly should have occurred; I refer to the rocky, mountain streams that course down the gorges of the Big Horn Mountains and the Laramie Hills. Many a time I have scrambled alone up through the rocky cañon that marked the bed of one of these noisy, bounding torrents with the vain hope of finding Cinclus, but, like many a naturalist before me, I was obliged to leave the country where these birds undoubtedly occur without ever having seen one of them. So that of my own personal experience I have nothing to add, so far as its life history is concerned, to the many beautiful descriptions of this bird given in our standard ornithologies, familiar to all lovers of the science, and to those read in its literature.
Of skins of Cinclus I have examined many a score, as has every one who from time to time has gone through large collections, but the very nearest, the most intimate acquaintance that I can boast of ever having made with this little bird, was with a pair and three young that had been stowed away by themselves in alcohol for several years in the large collection at the Smithsonian Institution. Of this material I was kindly allowed to avail myself, or of so much of it at least as was necessary to develop the facts that I now have the pleasure of presenting to my reader in this paper.
I did very little with the viscera, and this part of its anatomy has been laid aside for some future study, my attention having been directed more particularly to the skeleton, and to the extremely interesting points that it presented for consideration. These I shall endeavor to describe, as minutely and elaborately as the limits of this article will permit, at the same time suppressing 214as many of the technicalities in terms, as is compatible with exactness, and in accord with the tastes of those who have not devoted themselves especially to anatomical reading and work.
In studying the skeletons of birds, or of anything else for that matter, the student must keep the fact ever present in his mind, that the great value of such studies and the descriptions that may follow them, rests almost entirely upon the comparisons that he makes; the more carefully and minutely he compares the form he may have under consideration with nearly related forms, the greater will be the value of his results; to this end tend all the studies of biologists of the present day.
With respect to the skull of Cinclus, our space will not permit us to enter upon the engaging part of the subject as to the mode of formation of this part of the skeleton in the adult from the many segments found in the cranium of the chick, it being enough for us to say that the usual bones ossify, unite, and leave the ordinary ones free, as the pterygoids, the ossa quadrata, and the lower jaw. The superior mandible is drawn out into a sharp point, and the bony nostril on either side occupies considerable space, being long and elliptical in outline; as in all nearly related genera these apertures are not separated by a bony partition or septum, but below we detect a delicate vomer in the median plane.
The eye-cavities or orbits are well shut off from the nasal chambers beyond them by broad bony walls composed of the usual elements, and here each is of a quadrate figure, as seen in so many genera of birds. The upper and outer angles of these osseous partitions are rounded. The almost complete separation existing between the two cavities just referred to by no means exists between the orbits themselves, for here we find an extremely deficient septum, and a large aperture leading into the brain-case at the usual site of the exit of the nasal nerves, the openings for the optic nerves being circular and entire.
On the inferior aspect of the skull we find maxillo-palatines, of a more or less spongy composition, existing between maxillaries and the delicate palatines, which latter are slightly bent downwards from the horizontal plane. The pterygoids are very slender, and articulate in the usual manner with the quadrates and the palatines.
215The external form of the brain-case is more or less globular, the supra-occipital prominence being well developed behind. Above in the median line a shallow furrow is carried forward as far as the fronto-maxillary suture.
There is but little of interest to note in the lower mandible, to illustrate the points we have in view.
From this slight sketch of this part of the skeleton we are prepared to look a little into how Cinclus compares with other forms of near kin. The writer, to illustrate his remarks, offers the student the four accompanying cuts of the superior aspects of the skulls of birds chosen with the view of showing the comparable points.
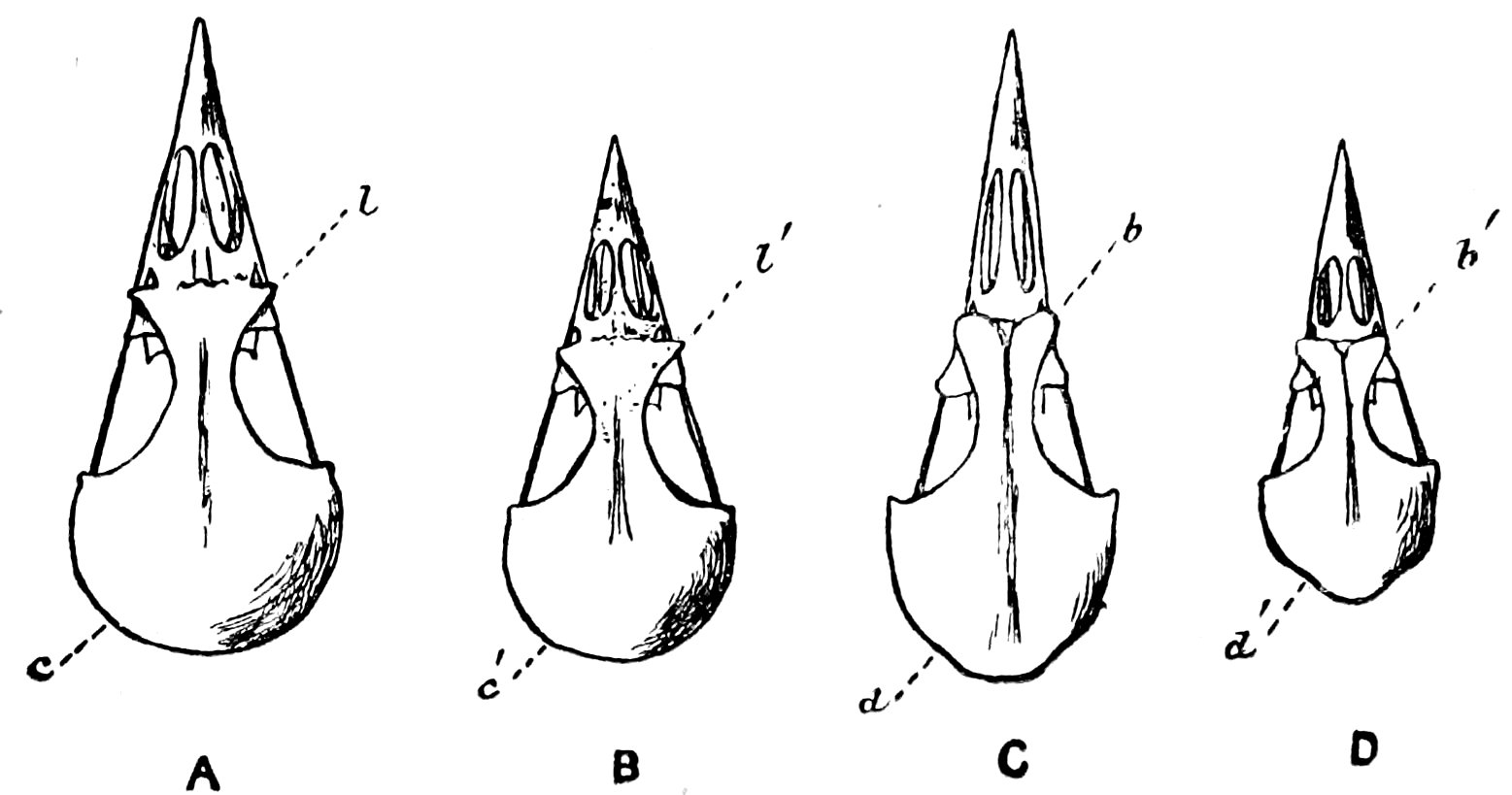
A is of Oreoscoptes montanus, B of Sialia mexicana, C of Cinclus itself, and D of Siurus nævius.
In the figures, the angle formed at l, l′, b, and b′ is due to the lachrymal bone on that side; viewed from above in such forms as Sialia, Turdus grayi, Oreoscoptes, Hylocichla unalascæ, and no doubt Merula and Mimus, less so in Harporhynchus, this projection is markedly angular; while in Siurus, the Wrens, and rather less so in Anthus, it is rounded, as shown in Siurus and also in Cinclus itself.
Of the forms we have examined, Siurus appears to be closer to the Dipper in this respect than any other genus, the Wrens (Salpinctes) next, and Anthus next. This also applies to the manner in which the median furrow at the summit of the cranium approaches the fronto-maxillary suture, also shown in C 216and D in the cuts, this feature in the opposed forms mentioned above occupying a position between the superior orbital margins.
There is still another very marked distinction among the birds we have thus far compared, and that is in the general external form of the brain-case proper. A and B show the form assumed by the genera we mentioned above in connection with them; smooth, large, and globular, all indicating the possession of a brain of no mean size as compared with the owner. In Cinclus, Siurus, and the Troglodytinæ the prominence of the supra-occipital eminence causes depressions to exist at d and d′ that are not present in A and B at c and c′.
With regard to this last characteristic the outline assumed by Siurus seems to claim the nearer place, over the other forms mentioned.
So much for the skull, and the writer must reluctantly and with as good grace as possible allow the student to observe other interesting points of difference for himself, though he would be only too glad to assist him in this part of the skeleton.
There are fourteen cervical vertebræ in Cinclus, the last two bearing each a pair of free ribs, the ultimate pair possessing uncinate processes; this arrangement holds good in Siurus and Salpinctes, but we remember that in Eremophila[100] we found only thirteen cervical vertebræ; the number of ribs varied however. Cinclus also possesses, in common with the form mentioned, four dorsal ribs; these are connected with the sternum by sternal ribs, the first sacral vertebra possessing an additional pair, but its sternal ribs only articulate along the hind border, on either side of the true sternal and last pair. This condition obtains, we know, in very many birds.
If we do not include the pygostyle or last coccygeal vertebræ, we observe that Cinclus has seven caudal vertebræ, Siurus and Salpinctes each only five, Oreoscoptes having six, so that the number of these segments may vary more or less among the genera we have quoted above.
The general pattern of the pelvis of the Dipper, the Wrens, the Thrushes, and Sialia is pretty much the same for all, that is it would be very hard to point out decided differences among them upon casual examinations; of course they are proportionate 217in size to that of their respective owners, and we might, in extensive series of each, by exceedingly careful measurements, detect relative differences. These remarks cannot be applied to the genus Harporhynchus, as the pelvis there has a very striking form, best expressed by saying that it is more angular than the others cited, the processes are more pronounced and sharper. In Cinclus, as in other forms noted, the bone is broad across, with the distal extremities of the pubic bones and ischia flaring well outwards; the ilio-neural canals open; the sacral vertebræ very broad, with numerous foramina or openings existing among them.
What we have just said in regard to the pelvis applies with equal force to the shoulder girdle and sternum; indeed, this latter bone is singularly alike among the various genera that I have referred to; the shape it assumes is that described by Professor Owen in his Anatomy of Vertebrates, as the “Cantorial sternum,” it being the pattern allotted to the vast majority of the class Aves. In front we find the manubrium bifurcated, and supported upon a stout and produced base, directed upwards and outwards. The body behind is 1–notched, the lateral xiphoidal processes thus formed having dilated ends. The keel is deep, convex below, sharp and concave in front, forming an acute cardinal angle at the point of meeting. The costal processes are very lofty, broad and directed forwards, having the facets for the sternal ribs placed along their posterior borders, which meet on either side the xiphoidal borders at a very obtuse angle. The “merry-thought” of Cinclus is delicately formed, having expanded upper extremities and a median plate below.
Our subject has, in addition to the usual number of bones in the pectoral limb, quite a sizable sesamoid, to be found at the back of the elbow; this bonelet is likewise found in Oreoscoptes and may be a common character of other birds we have mentioned. The arm seems to be completely non-pneumatic, indeed I have failed to find the apertures for the entrance of air in any of the bones composing it. Several months ago my attention was directed to a note, I think in the Proceedings of the Zoölogical Society of London, in which some English observer says the same of the European Dipper. This non-pneumatic condition of the long bones, not only of the upper but also of the lower extremities, seems to hold good among all the other forms and genera we have thus far referred to in this article.
218The proximal extremity of the humerus is very much expanded, and rather abruptly bent in the direction of the bird’s body, the member being considered in a position of rest. The “crest” we know curls over the usual site of the pneumatic fossa, which depression is divided by a bony partition from a lesser cavity above. This characteristic is also more or less strongly marked in the Rock Wren, Siurus, and others, and is feebly shown in Harporhynchus.
The articular cavity of the shoulder joint is increased in the Dipper by a good sized os humero-scapalare, a sesamoid that we are aware is to be found among other orders.
We will present the reader here with a table showing the relative lengths, etc., of some of the bones we have thus far examined, in order that a study of their comparative development may be made. (The measurements are given in centimeters.)
| Sternum | ||||||
|---|---|---|---|---|---|---|
| Species. | Length from bifurcation of manubrium to end of body. | Depth of keel. | Humerus. | Radius and ulna. | Hand. | Long axis of skull. |
| Cinclus mexicanus. | 2.7 | 0.8 | 2.2 | 2.6 | 2.6 | 4.4 |
| Siurus nævius. | 1.9 | 0.6 | 1.7 | 2.1 | 1.7 | 3.1 |
| Salpinctes obsoletus. | 1.6 | 0.5 | 1.7 | 2.0 | 1.7 | 3.6 |
| Oreoscoptes montanus. | 2.3 | 0.7 | 2.2 | 2.7 | 2.4 | 4.2 |
| Sialia mexicana. | 2.3 | 0.8 | 2.0 | 2.8 | 2.3 | 3.5 |
| Anthus ludovicianus. | 2.1 | 0.7 | 1.8 | 2.5 | 2.1 | 2.9 |
| Merula migratoria. | 3.4 | 1.1 | 2.9 | 3.4 | ||
| Hesperocichla nævia. | 3.0 | 1.6 | 2.5 | 3.1 | 3.1 | 4.6 |
A great many points of extreme interest and of the highest importance reward the ornithotomist’s study of the pelvic limb of Cinclus; some of these the writer has already remarked upon in papers now in press, but he offers them here again, confident of the fact that they will be of interest to ornithologists generally, 219particularly to those whose aim it is to pursue the study more than “skin deep.”
In the adult Dipper the pelvic limb, as far as its skeleton is concerned, is made up of the most usual number of bones; the thigh having the femur, the leg the tibia and fibula, a patella, the tarsus the bone tarso-metatarsus, and finally a foot arranged upon the plan of four toes, with first, second, third, and fourth digit composed of 2, 3, 4, and 5 joints respectively.
I have already said that these bones are non-pneumatic, they are also of lengths proportionate to the size of the bird, the claws being curved about as much as they are in a typical Thrush. Anatomists have described certain general points for examination on these long bones composing the leg; many of these are present, but we shall only call the student’s attention to a few of them, so as to make clear what we have to point out hereafter. Nothing of striking variance marks the femur, as distinguishing it from the common form of the bone among birds of this class. The same might be said of the tibia, but we must note the two large flaring processes at the anterior and upper end of this, the larger bone of the leg; in this bone, too, the condyles are well developed below. The tarso-metatarsus, or the bone of the tarsus, we observe in the old bird, has rather a slender shaft, presenting for examination at its upper end the usual dilatation, crowned by a smooth, undulating surface to articulate with the tibia; behind this, at the same end, we find a tuberous process that has given comparative anatomists no little trouble to name; but we will speak of this further on. The lower end of the tarso-metatarsus has the little lateral facet for the diminutive first tarsal bone, and the three trochleæ for the other toes.
Let us now, after this brief survey of the bones in the adult take up the young of this species. We find first that the femur has grown in the usual manner, its lower end bearing the two large condyles has been formed by one epiphysis which included both of these articulate surfaces. Nothing of particular interest is to be observed in the development of the fibula or the small “splint bone” of the leg. The superior end of the tibia has been formed by the epiphysis including the two large processes that I spoke of above. These plates are called the procnemial and the ectocnemial processes, the inner and outer one respectively. They are turned slightly outwards, springing abruptly 220from the shaft in the adult, very much as I figured them in Lanius.
Such of my readers as have read my account of the development of Centrocercus in the Osteology of the Tetraonidæ, will remember what we had to say in regard to the lower end of the tibia and its growth, and also all that Professor Morse has done for us in that direction. The specimen we have of the young of Cinclus does not admit of the demonstration of the intermedium; the fibulare and the tibiale seem to ossify separately, however. We must admit, then, that in this instance we are no nearer solving the problem of the homologies of the avian tarsal segments than we were before, but a little light at least is thrown on the subject when we come to examine the next bone, the tarso-metatarsus.
In nearly all birds this bone has at the back part of its upper end a tuberous process, amalgamated with the shaft in the adult, that assumes various forms in different members of the class. This bony process has long been regarded with suspicion, as to whether it was one of the ankle or rather tarsal bones or not. Let us hear what a few of the authorities have to say in this matter. Professor Owen tells us in Vol. II of his Anatomy of the Vertebrates, when speaking in general terms of this process, that: “One or more longitudinal ridges at the back of the upper end of the metatarsal are called ‘calcaneal’; they intercept or bound tendinal grooves which, in some instances, are bridged over by bone and converted into canals; the ridges may be expanded and flattened.” This would lead one to think that the Professor might regard this process as the homologue of the os calcis, a tarsal bone.
Professor Huxley, in his Anatomy of Vertebrated Animals, page 254, tells us, in speaking of this process, that: “Again in most birds, the posterior face of the proximal end of the middle metatarsal, and the adjacent surface of the tarsal bone, grow out a process, which is commonly, but improperly, termed “calcaneal.” The inferior surface of this hypo-tarsus is sometimes simply flattened, sometimes traversed by grooves or canals, for the flexor tendons of the digits.”
Mivart says, when referring to birds: “Thus no projection corresponding with the tuberosity of the os calcis exists in this compound bone.” (Elementary Anatomy, p. 206.)
221Coues, in his Osteology of Colymbus torquatus, leaves no doubt in our mind how he regards this projection of the tarso-metatarsus; this author says:—“The process of bone representing the os calcis, rises at the superior end of the bone, on its posterior aspect, as a very conspicuous crest.”
Professor Morse, in his Tarsus and Carpus of Birds (Ann. Lyc. Nat. Hist., N. Y., Vol. X, 1872), speaks of the centrale, but not in connection with this process.
In the chick of Centrocercus I found that the centrale did not include this process, consequently in my Osteology of the Tetraonidæ (Bull. U. S. Geol. Surv., Vol. VI) I declared that this process had nothing whatever to do with the os calcis, and in the osteology of Lanius, termed it the tendinous process, from the fact that the flexor tendons in so many birds either pass over or through it. Now our young of Cinclus mexicanus, just before it leaves the nest, has its metatarsal bones still ununited, and crowned by a separate segment that has apparently ossified from one single centre, a segment that not only includes the centrale, but the entire process of which we have been speaking. So between Cinclus and Centrocercus we must still look for other forms to throw light upon this problem. The subject is an extremely engaging one for the ornithologist to look into and investigate.
The shaft of the tarso-metatarsus of this bird develops after the usual rule set forth in works upon the subject, and the same may be said of the phalanges.
The writer only hopes that his sketch, necessarily brief, and far from being exhaustive, will have at least the tendency to induce other ornithologists to record their observations upon this subject whenever the opportunity offers.
Our studies, as far as we have carried them, seem to point pretty conclusively to the fact that our American Dipper is quite closely related to the genus Siurus, and not far removed from some of the Wrens.
152. Ægialites vociferus Bonap. Killdeer Plover.[101]—Common resident throughout the year, but most abundant during the spring and fall migrations.
153. Ægialites semipalmatus Bonap. Semipalmated or Ring Plover.—Rare and only observed during migrations.
154. Ægialites wilsonius Ord. Wilson’s Plover.—Common during the breeding season, but I did not succeed in finding a nest.
155. Strepsilas interpres Illig. Turnstone.—Seen on Galveston Bay and on the Gulf Coast.
156. Recurvirostra americana Gmel. Avocet.—Winters, but not noticed in summer.
157. Gallinago wilsoni Bonap. Wilson’s Snipe.—Common during migrations; arriving from the north usually in the middle of October, sometimes earlier, sometimes later. I think none remain here to breed, and all go farther south to winter. The time of arrival from their winter quarters is unknown to me.
158. Tringa maculata Vieill. Jack Snipe; Grass Snipe.—Common in September and again in April. None remain to winter or to breed.
159. Tringa minutilla Vieill. Least Sandpiper.—Not uncommon in winter.
160. Actiturus bartramius Bonap. Bartramian Sandpiper; Upland Plover.—Abundant on the prairies during March and April and again in October. None remain to breed or to winter.
161. Limosa fœda Ord. Marbled Godwit.—Rare; seen only in March and October.
162. Totanus semipalmatus Temm. Willet; Tattler.—This well-known bird is also common in this region in all suitable localities. Resident throughout the year; breeds.
163. Numenius longirostris Wils. Long-billed Curlew.—Common during migrations; occasionally seen during the breeding season.
164. Tantalus loculator Linn. Wood Ibis.—This bird is common in all marshy localities near the Gulf Coast. I have seen it frequently in the marshes and ponds near Spring Creek and the Brazos, in company with Herons and other water fowl.
165. Platalea ajaja Linn. Roseate Spoon-bill.—Common in the breeding season. Never seen in companies, but always singly, associated 223with Herons, Ducks, etc. Particularly common on the prairie ponds in the northern part of Harris County, Texas.
166. Ardea herodias Linn. Great Blue Heron.—Quite regularly distributed, but nowhere common; breeds on trees near ponds in the woods.
167. Herodias egretta Gray. White Heron; Great White Egret.—Abundant summer resident; breeds. This beautiful bird is to be observed in numbers in all the prairie ponds. They breed in communities on bushes in swamps. The nests are bulky, built of sticks; the nesting cavity is very flat; eggs three or four in number. The birds begin to breed in the latter part of April.
168. Garzetta candidissima Bonap. Snowy Heron; Little White Heron.—Exceedingly abundant during a large part of the year. I have seen these birds by thousands in the marshes near the Brazos River and on the Gulf Coast. Large colonies breed in the marshes near Spring Creek, where they build their nests on bushes, or, more frequently, in the lower horizontal branches of forest trees, bordering ponds and marshes. None remain to winter.
169. Florida cœrulea Bd. Little Blue Heron.—This beautiful bird is exceedingly abundant in all suitable localities. Many are resident throughout the year, but most migrate further south in winter. They nest in large colonies in swamps and marshes overgrown thickly with bushes. I have always found the nest in the top of button-bushes (Cephalanthus occidentalis). Eggs three or four, in one case five, in number. I have seen hundreds of nests in one pond. They are built entirely of sticks without any lining. In the second week of May many eggs were already hatched.
170. Butorides virescens Bonap. Green Heron.—Common summer resident; breeds; never observed in flocks, but always in pairs or singly.
171. Hydranassa tricolor ludoviciana Ridgw. Louisiana Heron.—One specimen, shot May, 1880, on Spring Creek. Seems to be not very common. Breeds in the swampy woods.
172. Nyctiardea grisea nævia Allen. Black-crowned Night Heron.—Not common and very shy. Breeds in the swamps where other Herons have their nests.
173. Botaurus lentiginosus Steph. American Bittern.—Occurs during migrations; none observed in the breeding season or in winter.
174. Ardetta exilis Gray. Least Bittern.—Common during migrations; rare in summer; breeds in the marshes of tule reeds and water shrubs, such as Cephalanthus occidentalis and Pinckneya pubescens, in company with Herons and other water fowl.
175. Grus americana Temm. Whooping Crane.—From November to the end of March these beautiful birds are exceedingly abundant on all the low prairies in the vicinity of Houston. Very shy.
176. Grus canadensis Temm. Sandhill Crane.—Even more abundant than the preceding. Observed flocks of many hundreds on the low prairies in the western and northern parts of Harris County. Very shy.
177. Porzana carolina Bd. Carolina Rail; Sora.—Seen in summer, breeds. but I have not discovered the nest.
224178. Porzana noveboracensis Cass. Little Yellow Rail.—Very rare during migrations.
179. Porzana jamaicensis Cass. Little Black Rail.—One taken April 29, 1879.
180. Gallinula galeata Bonap. Florida Gallinule.—Common during the breeding season in all marshes where reeds and bushes grow, but especially so where the magnificent Nymphæa odorata (Water-Lily) opens its fragrant flowers, and where Nuphar advena (Yellow Pond Lily) and another beautiful aquatic, Nelumbium luteum (Water Chinquepin), are found; over the broad leaves of which plants the little Florida Gallinule runs with exceeding quickness, searching for water insects and other food.
181. Fulica americana Gmel. American Coot; Mud Hen.—Decidedly more numerous than the preceding. Especially common in the large prairie swamps.
182. Cygnus buccinator Rich. Trumpeter Swan.—Every winter there are large numbers on Galveston Bay and on the Gulf of Mexico near the coast.
183. Cygnus americanus Sharp. American or Whistling Swan.—Sometimes these birds winter abundantly on Galveston Bay.
184. Anser hyperboreus Pall. Snow Goose; White Brant.—Exceedingly abundant on Galveston Bay, also on the rivers and bayous near the Gulf Coast in winter.
185. Anser albifrons gambeli Coues. American White-fronted Goose.—This is the first Goose to arrive from the north in autumn, but they all migrate farther south.
186. Bernicla canadensis Boie. Canada Goose.—Exceedingly abundant during winter. Large flocks are to be observed on the wet prairies in company with Cranes.
187. Anas boscas Linn. Mallard.—Very common during migrations and in winter.
188. Anas obscura Gmel. Black Duck; Dusky Duck.—Common during the breeding season. A pair of these Ducks are seen in almost every pond among Herons, Roseate Spoonbills, Anhingas, Gallinules, and Blackbirds (Agelæus phœniceus).
189. Dafila acuta Bonap. Pintail Duck.—Common during migrations.
190. Chaulelasmus streperus Gray. Gadwall.—Exceedingly abundant during winter.
191. Mareca americana Steph. American Widgeon.—Common during migrations.
192. Querquedula carolinensis Steph. Green-winged Teal.—Very common in autumn and spring, rather rare in winter.
193. Querquedula discors Steph. Blue-winged Teal.—Very common during migrations but all pass further south.
194. Querquedula cyanoptera Cass. Cinnamon Teal.—Not common during migrations; none remain to winter.
195. Spatula clypeata Boie. Shoveller; Spoon-bill Duck.—Abundant in winter.
225196. Aix sponsa Boie. Wood Duck; Summer Duck.—Common during migrations; some remain to breed.
197. Fulix marila Bd. Scaup Duck.—Common in winter on Galveston Bay.
198. Fulix affinis Bd. Little Black-head.—Very common in winter.
199. Aythya vallisneria Boie. Canvas-back.—Abundant in winter on Galveston Bay and on all marshy districts near the Gulf Coast.
200. Bucephala albeola Bd. Butter-ball; Buffle-head.—Abundant in winter near the coast.
201. Erismatura rubida Bonap. Ruddy Duck.—Very common during migrations; none remain to winter, but many breed.
202. Pelecanus erythrorhynchus Gmel. American White Pelican.—Common during winter, especially near the coast.
203. Pelecanus fuscus Linn. Brown Pelican.—Common during the breeding season on all the rivers, creeks, and bayous near the coast.
204. Plotus anhinga Linn. American Anhinga; Snake-Bird; “Water Turkey.”—Breeds in all marshy localities and is very common.
205. Larus atricilla Linn. Laughing Gull.—Abundant near the Gulf Coast; breeds on the small sand islands in Galveston Bay.
206. Sterna anglica Montag. Gull-billed Tern.—Breeds abundantly on the islands of Galveston Bay.
207. Sterna regia Gambel. Royal Tern.—Breeds in considerable numbers on the islands of Galveston Bay.
208. Sterna cantiaca acuflavida Ridgw. Cabot’s Tern, and—
209. Sterna forsteri Nutt. Forster’s Tern.—These and a few other Terns breed in abundance on the islands near the coast, especially on the sand-bars of Galveston Bay, where they lay their eggs on the bare sand. It was impossible for me to distinguish the eggs, as the birds all leave the nests as soon as they are approached.
The following paper is based on a collection of about two hundred and fifty birds obtained in the immediate vicinity of Fort Walla Walla during the autumn and winter of 1881–82, and submitted to me for determination by Capt. Bendire, who has kindly consented to my publishing any notes respecting them, that seem of sufficient interest.
226As an exponent of the workings of geographical variation in species easily modified by their surroundings, this material is especially instructive. The region represented apparently constitutes a sort of neutral ground between the Pacific and Middle Provinces and naturally its fauna is a mixed one. Setting aside species not subject to geographical modification, and migrants from the north which have only an indirect bearing on the general question, we find the collection divisible into three classes: (1) Forms identical with or most nearly like Pacific coast types; (2) Forms about intermediate between representatives inhabiting the Pacific and Middle Provinces; (3) Forms to a certain extent intermediate between Pacific and Middle Province representatives, but differing from both in certain original characteristics. The locality seems to be nearly lacking in typical representatives of the Middle Province; and its fauna, on the whole, must be regarded as closely related to that of the coast region.
The third class, although least numerous, includes many of the most interesting birds. The majority of these are resident forms, a fact which sufficiently explains many of their peculiarities, for it is well known that sedentary species are, of all others, the most subject to local variation.
But while the philosophic bearing of this material is not doubtful, there are certain systematic difficulties in the way of its satisfactory presentation. I refer to the naming of these intermediate forms. The practice has been to use the name of the race to which the bird seems most nearly related, and this I have been forced to adopt in default of a better way. But the method obviously fails to meet the requirements of such cases, while to a certain extent it is unscientific and inaccurate. The evil, however, is not likely to be remedied, for it is difficult to conceive of a system of nomenclature that would adequately designate the numberless intermediate and local types.
In the present connection I would gratefully mention the assistance received from my friend, Mr. Ridgway, who, during my study of the collection, has given me every facility for examining the matchless series in the National Museum, and to whom I am further indebted for many valuable suggestions. My obligations to Capt. Bendire are greater than I can adequately express, for, in addition to other kind attentions, he has generously 227presented me with many valuable specimens included among those about to be discussed.
6. Parus atricapillus occidentalis (Baird) Coues. Oregon Chickadee.—A series of six specimens furnishes satisfactory proof—which I believe has been heretofore wanting—that P. occidentalis is simply a dark, geographical race of P. atricapillus. One example is absolutely typical of occidentalis, while the others grade evenly into a form that is essentially undistinguishable from atricapillus. Indeed the lightest colored specimen is so nearly like some Massachusetts birds taken at the same season that I have been unable, after a most careful comparison, to detect the slightest difference in either color or markings: the wing of the Walla Walla skin, however, is slightly shorter. There are no apparent approaches in this series to P. septentrionalis.
16. Spizella monticola ochracea var. nov. Western Tree Sparrow.—Ch. Subsp. ♂ ♀ Similis S. monticolæ, sed colore suprà dilutiore; strigis dorsalibus rarioribus, angustioribus et magis acutè in tergo pallidiore depictis; lateribus gulâque magis ochraceis; vertice, in auctumnalibus quidem avibus, sæpissimè magis cinereo.
♂ (Fort Walla Walla, Washington Territory, Nov. 8, 1881. Capt. Bendire.) Back and rump pale sandy-brown or brownish-ochraceous, the back with sharply defined black streaks which, excepting on the scapulars, have no chestnut bordering; crown invaded centrally, from the nape, by a broad space of pale ash which tinges most of the feathers to their bases and confines the usual chestnut to a small area on the forehead and two narrow, lateral stripes; lores and sides of head pale fulvous; entire under parts washed with warm ochraceous, deepest on the sides and abdomen, palest on the throat where it only partially conceals the ashy beneath. Otherwise similar to S. monticola.
Dimensions. Wing, 2.94; tail, 2.73; culmen, .43.
Habitat. Western North America, east to Dakota, north to Arctic Ocean: Alaska?
The specimen above described differs widely from its nearest approaches among my eastern examples. The ground-color of the back is decidedly paler, bringing out the dark streaks in sharper contrast, which is heightened by the absence of their usual chestnut edging; the ash of the throat and sides of the head is much fainter, and in many places replaced by brownish-fulvous; the under parts, especially the sides and abdomen, are more strongly ochraceous; and the broad, ashy crown-patch gives the head a very different appearance.
Upon testing these characters by comparison with the extensive material in the National Museum, I find the different ground-color and markings of the back to be constant in western birds, while the ochraceous tint of the throat and sides of the head, although most conspicuous in fall and winter specimens, is also a good distinction; the ashy hood is apparently confined to autumnal birds, and with these is variable in extent, as well as sometimes wanting; but as it never occurs in eastern examples it is not wholly lacking in diagnostic value.
229A comparison of measurements taken from a large number of specimens of both races shows little average difference in size, although the western birds usually have smaller and narrower bills.
18. Melospiza fasciata guttata (Nutt.) Ridgw. Rusty Song Sparrow.—The thirteen Song Sparrows sent me from Fort Walla Walla represent a form very nearly intermediate between fallax and guttata. Most of these specimens are decidedly browner above and more heavily streaked beneath than true fallax; but on the other hand none of them are as dark as typical guttata, although several closely approach that form. One of the lighter examples is even grayer than a Utah skin, and, taken by itself would necessarily be referable to fallax. But the series as a whole may perhaps best be referred to guttata.
25. Cyanocitta stelleri annectens (Baird) Ridgw. Black-headed Jay.—An interesting series of Jays collected by Capt. Bendire includes five typical representatives of annectens, two nearly typical stelleri and four birds about intermediate between these forms. The differential characteristics of the three styles may be briefly given as follows: The first-named has a well-defined and conspicuous patch of white over the eye; the second entirely lacks this marking; the third has it merely indicated by a narrow gray line. In all, the crest is glossy black; the rest of the head, with the breast anteriorly, plumbeous-black; the back plumbeous-brown; and the throat streaked with bluish-white. All have the head above more or less streaked with blue, but the shade and extent of this marking bear no apparent relation to the presence or absence of the white patch over the eyes. Thus examples of each style have the forehead and crown, to a point half an inch behind the eye, thickly marked with blue or bluish-white, while with all there is a more or less complete gradation from this pattern to one in which a few pale streaks are confined to the forehead. Similarly, the greater wing-coverts are distinctly barred with black, faintly crossed with fine dark lines, or entirely immaculate, without regard to the character of the features already mentioned.
The above evidence clearly goes to show that annectens grades directly into stelleri; but it does not necessarily preclude the recognition of the former as a well-defined geographical race, for the locality under consideration abounds in similarly intermediate forms.
33. Asio accipitrinus (Pall.) Newton. Short-eared Owl.—A female, taken Oct 7, has the ground-color of the plumage, both above and beneath, rich, almost rusty, ochraceous; the markings, also, are unusually dark and broad. Three males represent the other extreme, their coloring, especially beneath, being remarkably pale and almost free from any ochraceous tinge.
36. Bubo virginianus saturatus[111] Ridgw. Dusky Horned Owl.—During the autumn of 1881 Great Horned Owls were unusually abundant 230about Fort Walla Walla, and Capt. Bendire secured no less than fourteen specimens, of which twelve are now before me. In a general way these are referable as follows: eight to saturatus, two to subarcticus, and two to a form apparently about intermediate between these races. Five of the representatives of saturatus are typical, while the remaining three grade into the intermediate form which, in turn, approaches one of the light specimens referred to subarcticus. The latter example is not typical, but its companion differs from an Arizona skin only in having slightly darker dorsal markings and a little stronger rufous cast about the face and across the breast, the color and markings elsewhere being essentially the same.
The occurrence of these three forms together is not remarkable, for two of them may reasonably be regarded as migrants from distant and probably widely separated regions. The third possibly represents a resident type, but on this point I have no direct evidence.
38. Falco columbarius suckleyi? Ridgw. Black Merlin.—A beautiful adult male Pigeon Hawk, taken at Fort Walla Walla Oct. 18, 1881, presents such a puzzling combination of characters that, after carefully comparing it with all the material available, I am still at a loss for a definite opinion regarding its precise identity or relationship. It most closely resembles highly colored, autumnal adults of F. columbarius, but the under parts, excepting the throat and a small central space on the abdomen, are rich rusty-ochraceous—almost orange-chestnut on the breast and tibiæ, while the usual cinereous above is intensified on the back to a nearly pure plumbeous; the markings of the under parts, also, are unusually coarse and numerous. In these respects it agrees with a bird in the National Museum from Santa Clara, California, but it differs from this specimen, as well as from every other adult that I have seen, in having the outer webs of all the primaries, excepting the first two, conspicuously marked with rounded spots of pale ochraceous.
With F. richardsoni it cannot be consistently associated, for the adult, as well as the young of that species, always has six distinct light bars on the tail, while the example under consideration possesses but five. Moreover, the adult male of richardsoni is very much lighter colored than the adult of columbarius, whereas the present bird is decidedly darker. The adult of suckleyi is unknown, but we should expect to find it, like the young, with sparse, inconspicuous spotting on the lining of the wings. In the Walla Walla bird these markings are as numerous and well-defined as in columbarius.
Taking all these considerations into account, and bearing in mind the unstable character of so many of the types furnished by this locality, it seems most reasonable to assume that Capt. Bendire’s specimen represents the adult plumage of a form which, although referable to suckleyi, is more or less intermediate between that race and true columbarius. But additional material must be forthcoming before the question can be definitely settled.
39. Falco richardsoni Ridgw. Richardson’s Merlin.—Of this well-marked species the collection contains two immature females, dated 231respectively Oct. 13 and Oct. 21, 1881. Neither of these calls for any special comment, but I take the present opportunity to characterize the adult plumage of the male, which apparently has not been previously described.[112]
Falco richardsoni, adult ♂ (author’s collection, Colorado Springs, Colorado, C. E. Aiken). Above pale ashy-blue, most of the feathers of the back, as well as the inner secondaries and many of the scapulars, with fine, black shaft-lines; crown tinged with ochraceous (probably wanting in the highest conditions of plumage), the black shaft-lines here very numerous, each feather being conspicuously marked; forehead and sides of head light ochraceous, the former with narrow black streaks, the latter with broader brownish ones; a well-defined nuchal collar of rusty-ochraceous with darker mottling; secondaries and primary coverts concolor with the back, but with light bars on their inner webs; primaries plumbeous-brown, margined with bluish-white and marked conspicuously on both webs with the same color, the markings on the inner webs being pure white and extending in transverse bars from the shaft to the edge of the feather, those of the outer webs ashy-white and in the form of conspicuous, rounded or quadrate spots; tail crossed by five dark and six light bars, the last of the latter terminal and pure white, the others more or less bordered by pale ashy-blue; all of the dark bars clear black excepting the basal two, which, on the central rectrices, are nearly uniform with the back, but decidedly darker than the light ones with which they alternate; throat pure white and immaculate; remainder of under parts pale ochraceous, deepest on the tibiæ and crissum, where it is decidedly tinged with rusty; feathers of the breast, abdomen, flanks and sides with median stripes of clear reddish-brown, these stripes broadest on the flanks (where they are sometimes actually transverse), narrowest across the anterior part of the breast, and everywhere with fine but inconspicuous dark shaft-lines; crissum entirely unmarked; under tail-coverts and tibiæ with conspicuous shaft-lines of dark brown; edges of wings pale ochraceous; under wing-coverts white, barred with reddish-brown; all the markings of the primaries showing distinctly on their under surfaces. Dimensions. Wing, 8.21; tail, 5.18; culmen (from cere), .50.
Were further proof wanting to establish this Falcon’s specific distinctness from F. columbarius, the difference in the adult plumage of the two would settle the question. The adult male of F. richardsoni has the mantle almost as light as that of a Herring Gull, while the conspicuous ashy-white spots on the outer webs of the primaries and the six light tail bands constitute equally well-marked characters. The specimen above described is essentially similar to five examples in the National Museum.
42. Astur atricapillus (Wils.) Bonap. American Goshawk.—The present collection includes four Goshawks, one an adult male, the remaining 232three young, or at least immature, birds in brown plumage. The adult is absolutely identical with Massachusetts specimens, and must be considered typical atricapillus. Two of the young agree well with Mr. Ridgway’s description of young striatulus,[113] but the third does not have the markings either darker or more extensive than do several of my New England examples, and the dorsal feathers have an even broader light (ochraceous) edging; the under parts, also, are strongly ochraceous, while the stripes on the flanks are neither cordate nor transverse. The latter characters, however, are probably worthless for they occur in a Tyngsboro (Mass.) bird.
Without going further into details I may sum up my conclusions as follows: (1) That two of Capt. Bendire’s specimens (the adult and the young bird just mentioned) are undistinguishable from typical atricapillus; (2) That the other two examples (both young or immature) differ from eastern birds in having broader, more linear black markings beneath and a narrower light edging on the feathers above, and are probably referable to a form more or less distinct from atricapillus; (3) That true atricapillus ranges westward at least to Fort Walla Walla, Washington Territory; (4) That striatulus, as at present defined, is a doubtfully tenable variety.
I am not at liberty to pursue the subject further, for I understand that Mr. Nelson is about to propose a new Pacific coast race which occurs, at least as a migrant, in the Western United States, and upon the young of which Mr. Ridgway apparently based his description of young striatulus.[114]
49. Bonasa umbella sabinii (Dougl.) Coues. Oregon Ruffed Grouse.—The series of Ruffed Grouse embraces twelve specimens, all from the immediate vicinity of Fort Walla Walla. These birds apparently represent a dark, or more properly speaking, non-rufescent phase of sabinii, corresponding to the gray phase of umbella, and bearing the same relation to typical sabinii that the Walla Walla Scops does to what has been considered typical S. kennicotti. This peculiar plumage may be characterized as follows:
Gray phase; adult ♂. Above with the ground-color clear, dark ash, nearly uniform and unmixed with reddish even on the wings and tail; throat and breast tinged with reddish-yellow; remainder of under parts white, occasionally with a trace of ochraceous; markings as in typical sabinii.
The above description is taken from a bird which probably represents the extreme gray condition, all the others having more or less reddish-brown on the upper parts, especially on the back and wings, although the tail is usually clear ashy. Two specimens, however, show a decided approach 233to what may now be called the red phase of sabinii, in having the breast, with the entire dorsal surface, including that of the tail, strongly tinged with orange-chestnut which is scarcely duller than in examples from the coast region. Some of the grayer birds present a general resemblance to umbelloides, but the ground tint of their plumage is always deeper, the dorsal markings richer and blacker, and the under parts very much more thickly barred. It is probable that this style of coloration will prove to be more or less characteristic of all the Ruffed Grouse inhabiting the region between the Coast Range and the Rocky Mountains.
50. Pediœcetes phasianellus columbianus (Ord) Coues. Common Sharp-tailed Grouse.—Three specimens, taken at Fort Walla Walla, differ considerably from eastern birds. The entire upper parts are darker and duller, the usual rusty-ochraceous ground-color being replaced by plain wood brown; the dorsal markings, also, are finer, while those of the under parts are blacker and more generally distributed, the only immaculate area being the centre of the abdomen. These differences do not seem to indicate any approach to true P. phasianellus, which is an altogether differently colored bird. They probably have only a local significance, but the region in question is so poorly represented by the material to which I have had access, that I have not been able to form a definite opinion on this point.
Point de Monts is the southward termination of a high rocky promontory that separates the river from the Gulf of St. Lawrence, on the north shore. It is in latitude 49° 19′ north. The country is well wooded, the forests consisting chiefly of spruce (both white and black) and balsam. Scattered about are a few birches, poplars, cedars, and tamaracks; and on a sandy terrace near the Godbout River is a quantity of the northern scrub pine (Pinus banksiana) that here attains a height of thirty and sometimes forty feet. The region is so far north that not only are the oaks and hickories absent, but even the hardy beech and maple do not grow here.
I visited this section of the coast in July, 1881, and again in July, 1882; and with the observations made at these times I have 234incorporated the notes kindly placed at my disposal by Mr. Napoleon A. Comeau, guardian of Godbout.
The nomenclature followed is that of the second edition of Dr. Coues’s Check List of North American Birds.
1. Turdus migratorius. Robin.—A common summer resident. Arrives about the first of May, and remains till late in November. Seen Dec. 22, 1879.
2. Turdus unalascæ nanus. Hermit Thrush.—Tolerably common; breeds.
3. Turdus ustulatus swainsoni. Olive-backed Thrush.—Not uncommon; breeds.
4. Sialia sialis. Bluebird.—Extremely rare. During a residence of many years at Godbout Mr. Comeau has seen but one pair of these birds; they nested in a stump near his house in July, 1880.
5. Regulus calendula. Ruby-crowned Kinglet.—A male was shot June 4, 1882.
6. Parus atricapillus. Black-capped Chickadee.—A common resident.
7. Parus hudsonicus. Hudsonian Chickadee.—A common resident, like the last.
8. Sitta canadensis. Red-bellied Nuthatch.—Tolerably common in winter, but not observed in summer.
9. Eremophila alpestris. Horned Lark.—First seen April 21, 1882, after which they were common for about three weeks and then disappeared. I found a young one, dead, at Godbout in July, 1881.
10. Anthus ludovicianus. Titlark.—Tolerably common summer resident, and doubtless breeds. I have seen flocks of them in July feeding on the beach at low water. First seen May 7, 1882.
11. Helminthophila peregrina. Tennessee Warbler.—A tolerably common summer resident. First shot June 6, 1882.
12. Dendrœca æstiva. Summer Warbler.—Not very common. First seen June 6, 1882.
13. Dendrœca virens. Black-throated Green Warbler.—A tolerably common summer resident.
14. Dendrœca coronata. Yellow-rumped Warbler.—A rather common summer resident. First seen May 29, 1882.
15. Dendrœca blackburnæ. Blackburn’s Warbler.—Rather rare. Shot June 9, 1882.
16. Dendrœca striata. Black-poll Warbler.—Rare. Mr. Comeau shot a male, June 7, 1882.
17. Dendrœca maculosa. Black-and-Yellow Warbler.—The commonest Warbler, breeding abundantly. Earliest seen May 29, 1882.
18. Siurus nævius. Water Thrush.—Rather rare. Shot June 6, 1882. Others seen.
19. Geothlypis trichas. Maryland Yellow-throat.—Not common. Saw two in the clearing about Mr. Allan Gilmour’s camp on the Godbout.
23520. Myiodioctes pusillus. Black-capped Yellow Warbler.—Rather rare. Shot June 9, 1882. Others seen.
21. Myiodioctes canadensis. Canadian Flycatching Warbler.—A tolerably common summer resident.
22. Setophaga ruticilla. Redstart.—Tolerably common. First seen June 9, 1882.
23. Hirundo erythrogastra horreorum. Barn Swallow.—Rare, and not known to breed. Mr. Comeau shot one May 29, 1882.
24. Iridoprocne bicolor. White-bellied Swallow.—Common; breeds plentifully. First seen May 12, 1882.
25. Petrochelidon lunifrons. Cliff Swallow.—A small colony nested in the deserted Hudson’s Bay Trading Post at Godbout this year.
26. Ampelis cedrorum. Cedarbird.—A tolerably common summer resident.
27. Lanius borealis. Great Northern Shrike.—Occurs, but is not known to breed.
28. Pinicola enucleator. Pine Grosbeak.—A tolerably common resident. In autumn it feeds extensively upon the berries of the mountain ash. I have already published a note on the breeding of this species at Godbout.[115]
29. Carpodacus purpureus. Purple Finch.—Not very common. First seen April 26, 1882.
30. Loxia leucoptera. White-winged Crossbill.—Tolerably common, but somewhat irregular in appearance. I found this species to be very abundant here in July, 1881, while in July, 1882, I did not see any.
31. Ælgiothus linaria. Red-poll.—Very abundant in winter, large flocks being seen nearly every day. They all seem to move in one direction, following the shore westward.
32. Chrysomitris pinus. Pine Linnet.—Generally common, but somewhat irregular.
33. Astragalinus tristis. American Goldfinch.—Rather rare. I saw a small flock in July, 1882.
34. Plectrophanes nivalis. Snow Bunting.—Very common in flocks in winter. Seen as late as the middle of May.
35. Centrophanes lapponicus. Lapland Longspur.—Large flocks of this species appear on this part of the coast during the latter part of April, remaining till about the middle of May. They are then very abundant, occurring both alone and in flocks with the preceding.
36. Passerculus sandvicensis savana. Savanna Sparrow.—Tolerably common, breeding on the thinly grassed sand-fields about the mouth of the Godbout. Mr. Comeau shot one as early as April 21, 1882.
37. Melospiza fasciata. Song Sparrow.—A rather common summer resident in suitable places, arriving early in May. Particularly numerous in the clearing about Allan Gilmour’s camp on the Godbout.
38. Junco hiemalis. Black Snowbird.—Very common. First seen May 16, 1882.
23639. Zonotrichia albicollis. White-throated Sparrow.—The commonest Sparrow, breeding everywhere. First seen May 14, 1882. This bird is the “Nightingale” of the Canadians.
40. Zonotrichia leucophrys. White-crowned Sparrow.—Breeds, but is not common.
41. Agelæus phœniceus. Red-shouldered Blackbird.—Very rare. The only one ever seen here was a female, and was shot by Mr. Comeau May 22, 1882.
42. Xanthocephalus icterocephalus. Yellow-headed Blackbird.—An accidental straggler from the west. Mr. Comeau shot a male of this species in his door yard, at Godbout, early in September, 1878.[116]
43. Quiscalus purpureus. Crow Blackbird.—Rare. Sometimes seen in flocks in spring.
44. Corvus corax. Raven.—A common resident. May 12, 1882, Mr. Comeau found one of their nests on the face of a cliff about half-way between Godbout and Point de Monts. It contained four full-fledged young that must have been at least three or four weeks old.
45. Corvus frugivorus. Crow.—A common summer resident, sometimes wintering. I have observed that the Crows here find much of their food along the beach at low water.
46. Cyanocitta cristata. Blue Jay.—Resident but not very common.
47. Perisoreus canadensis. Canada Jay.—A tolerably common resident.
48. Tyrannus carolinensis. Kingbird.—Not rare. Earliest seen June 9, 1882.
49. Empidonax flaviventris. Yellow-bellied Flycatcher.—I have seen a specimen that Mr. Comeau shot June 15, 1882.
50. Chordeiles popetue. Nighthawk.—A common summer resident. First seen June 5, 1883. I saw Night-hawks flying about overhead nearly every day while at Godbout, both in July, 1881, and July, 1882.
51. Chætura pelasgica. Chimney Swift.—Generally tolerably common, but not seen this year.
52. Ceryle alcyon. Belted Kingfisher.—A rather common summer resident, arriving about the first of May. About June 13, 1882, Mr. Comeau found three Kingfisher’s nests in a bank, and each contained seven fresh eggs.
53. Hylotomus pileatus. Pileated Woodpecker.—Very rare. Mr. Comeau has shot but one here.
54. Picus villosus. Hairy Woodpecker.—A tolerably common resident, being particularly fond of the burnt-over scrub-pine barren near Godbout.
55. Picus pubescens. Downy Woodpecker.—A tolerably common resident, like the last.
56. Picoides arcticus. Black Three-toed Woodpecker.—Resident; not rare.
23757. Colaptes auratus. Golden-winged Woodpecker.—A tolerably common summer resident. First seen May 14, 1882.
58. Bubo virginianus. Great Horned Owl.—A rather common resident.
59. Asio wilsonianus. Long-eared Owl.—Rare. Mr. Comeau shot three in May, 1877 or 1878.
60. Asio accipitrinus. Short-eared Owl.—A rather rare summer resident. Earliest seen May 9, 1882.
61. Strix nebulosa. Barred Owl.—A tolerably common resident.
62. Nyctea scandiaca. Snowy Owl.—Very irregular in appearance; sometimes very abundant in winter, and sometimes not seen for several years. Mr. Comeau shot one May 17, 1882, and Mr. Gregoire Labrie killed one May 31, 1880. These are the latest dates at which they have been seen in this section.
63. Surnia funerea. Hawk Owl.—Common in winter, generally appearing in November and not remaining later than February.
64. Nyctala tengmalmi richardsoni. Richardson’s Owl.—A common winter resident, and very tame. This Owl has a low liquid note that resembles the sound produced by water slowly dropping from a height; hence the Montagne Indians call it pillip-pile-tshish, which means “water-dripping bird.” These Indians have a legend that this was at one time the largest Owl in the world, and that it had a very loud voice. It one day perched itself near a large waterfall and tried not only to imitate the sound of the fall but also to drown the roaring of the torrent in its own voice. At this the Great Spirit was offended and transformed it into a pygmy, causing its voice to resemble slowly dripping water instead of the mighty roar of a cataract.
65. Nyctala acadica. Saw-whet Owl.—Not very common. In winter Mr. Comeau once saw one of these little Owls fly out from within the carcass of a great northern hare that had been caught in a snare. The Owl had eaten away the abdomen and was at work within the thoracic cavity when frightened away.
66. Circus cyaneus hudsonius. Marsh Harrier.—A tolerably common summer resident. Three individuals were seen as early as May 5, 1882.
67. Astur atricapillus. Goshawk.—Not rare.
68. Falco sacer obsoletus. Labrador Gyrfalcon.—Mr. Comeau has killed several of these rare Falcons in the vicinity of Godbout.
69. Falco columbarius. Pigeon Hawk.—Not rare, and doubtless breeds.
70. Falco sparverius. Sparrow Hawk.—Rare. One shot May 5, 1882.
71. Archibuteo lagopus sancti-johannis. Rough-legged Buzzard.—Breeds, and is rather common. The southward migration commences about the last of September and continues into November. During this period large numbers of these Hawks are constantly passing over this part of the coast on the way to their winter quarters.
23872. Pandion haliaëtus. Fish Hawk.—A few pairs of Fish Hawks breed in this vicinity every year. They were first seen May 2, 1882. They depart in November.
73. Aquila chrysaëtus. Golden Eagle.—Breeds, and is not particularly rare. Mr. Comeau has shot three, and knows of half a dozen that were caught in steel-traps.
74. Haliaëtus leucocephalus. White-headed Eagle.—Tolerably common; breeds. They arrive in March, and remain till December or January. Mr. Comeau found a nest, early in June, that contained three young about the size of Crows.
75. Ectopistes migratorius. Wild Pigeon.—A rather rare and very irregular visitor.
76. Zenaidura carolinensis. Carolina Dove.—Of this southern species Mr. Comeau has killed two at Godbout: the first, a male, he shot October 10, 1881, and the second, a female, June 6, 1882.
77. Canace canadensis. Spruce Grouse.—A resident species, but rather rare.
78. Bonasa umbella. Ruffed Grouse.—A resident, like the last, but not common. This appears to be the northern limit of the Grouse on the east coast, and I was unable to find any evidence of its presence lower down along the north shore of the Gulf.
79. Lagopus albus. Willow Ptarmigan.—Very abundant during the early part of some winters, but during other years it does not occur at all. They generally arrive about the first of December, and a few remain till the first of May. They are always most abundant in December, and Mr. Comeau once killed six hundred before Christmas! He has shot as many as eighty-two in a single morning.
80. Squatarola helvetica. Black-bellied Plover.—Rather rare and irregular in occurrence. Mr. Comeau has shot it in May and September.
81. Charadrius dominicus. Golden Plover.—Tolerably common in September, and sometimes seen in spring.
82. Ægialites vociferus. Killdeer Plover.—Mr. Comeau says that this species breeds and is not rare.
83. Ægialites semipalmatus. Ring-neck.—Occurs in spring.
84. Strepsilas interpres. Turnstone.—Tolerably common in September.
85. Steganopus wilsoni. Wilson’s Phalarope.—Mr. Comeau tells me that this Phalarope occurs during the fall migration, but is not common.
86. Phalaropus fulicarius. Red Phalarope.—Not rare in September.
87. Gallinago wilsoni. Snipe.—A rather rare migrant.—Earliest killed May 9, 1882.
88. Macrorhamphus griseus. Red-breasted Snipe.—Occurs during the fall migration.
89. Ereunetes pusillus. Semipalmated Sandpiper.—Tolerably common. First seen during the latter part of May, and common in August and September.
23990. Actodromas minutilla. Least Sandpiper.—Rather common in spring and fall.
91. Actodromas maculata. Pectoral Sandpiper.—Occurs in fall, but is not common.
92. Actodromas bonapartii. White-rumped Sandpiper.—Mr. Comeau shot one May 31, 1882.
93. Calidris arenaria. Sanderling.—Occurs in the fall migration.
94. Totanus melanoleucus. Greater Tattler.—Common spring and fall. Earliest shot May 9, 1882. Passes south in September.
95. Totanus flavipes. Yellow-shanks.—Common during the migrations. Occurs with the preceding.
96. Rhyacophilus solitarius. Solitary Tattler.—Tolerably common, breeding about the fresh water lakes and streams.
97. Tringoides macularius. Spotted Sandpiper.—A tolerably common summer resident.
98. Numenius borealis. Eskimo Curlew.—Common in August and September.
99. Numenius hudsonius. Hudsonian Curlew.—Rather rare. Mr. Comeau has shot it in August.
100. Ardea herodias. Great Blue Heron.—Rather rare, and generally seen in September.
101. Ardea egretta. Great White Egret.—Accidental straggler from the south. One seen June 9, 1882, on an island in Godbout River.
102. Botaurus mugitans. American Bittern.—Rare. Mr. Comeau has shot several here, and tells me that they are common at Manacougan, thirty miles west of Godbout.
103. Cygnus SP.—? A swan was shot at Point de Monts by an Indian in 1870.
104. Chen hyperboreus. Snow Goose.—Rare. Mr. Comeau has shot it in October.
105. Bernicla brenta. Brant Goose.—Breeds, and is by no means rare. Arrives in April, remaining into November and sometimes December.
106. Bernicla canadensis. Canada Goose.—A common migrant, arriving during the latter part of March and departing in November. They breed at Natashquan, Western Labrador.
107. Anas obscura. Black Duck.—A tolerably common summer resident, breeding about the fresh water lakes.
108. Dafila acuta. Pintail.—The only one Mr. Comeau ever saw here he shot June 7, 1882.
109. Querquedula carolinensis. Green-winged Teal.—Rare here, but they breed at Manacougan.
110. Querquedula discors. Blue-winged Teal.—Rare, but oftener seen than the preceding. Has been shot early in May.
111. Fuligula affinis. Scaup Duck.—Tolerably common in October.
112. Fuligula collaris. Ring-neck Duck.—Mr. Comeau has killed two in spring.
240113. Clangula glaucium. Golden-eye.—A resident species, and tolerably common. Breeds on fresh water only. Remains throughout the winter.
114. Clangula islandica. Barrow’s Golden-eye.—A common resident, breeding, like the foregoing, on fresh water, and remaining on the Gulf all winter.
115. Clangula albeola. Butter-ball.—Rare. Has been shot in October.
116. Harelda glacialis. Old Wife.—Resident. Very abundant in winter, the largest flocks being seen in December, January, and February. Mr. Comeau took one in full summer plumage as early as April 23, 1882. Tolerably common in summer, and supposed to breed.
117. Histrionicus minutus. Harlequin Duck.—Rare, and only seen during the latter part of April and early in May. This year Mr. Comeau saw two April 16, and shot one May 8, out of a flock of four.
118. Somateria mollissima. Eider Duck.—A permanent resident, but rather rare.
119. Somateria spectabilis. King Eider.—Rare. Has been known to breed.
120. Œdemia americana. Black Scoter.—Common from early in April till some time in November. They do not remain through the winter.
121. Œdemia fusca. Velvet Scoter.—A common resident. The largest flocks are seen in April and November, and the species is common all the year round.
122. Œdemia perspicillata. Surf Duck.—Very common from April to November, but does not winter. The males greatly preponderate over the females in this species, and Mr. Comeau tells me that the proportion is always about seven males to one female.
123. Mergus merganser. Sheldrake.—Tolerably common, breeding about the fresh water.
124. Mergus serrator. Red-breasted Merganser.—Very common, frequenting both fresh and salt water.
125. Sula bassana. Gannet.—Occasional. I have found it breeding in numbers at the west end of Anticosti, but do not think it nests farther up in the Gulf.
126. Phalacrocorax carbo. Common Cormorant.—Rare. but Mr. Comeau has shot several here.
127. Phalacrocorax dilophus. Double-crested Cormorant.—Mr. Comeau shot a female May 19, 1882.
128. Stercorarius pomatorhinus. Pomatorhine Jaeger.—Rare.
129. Stercorarius parasiticus. Parasitic Jaeger.—Rather rare. Mr. Comeau shot six in one day about the middle of May, 1874.
130. Larus glaucus. Glaucous Gull; Ice Gull.—Rather rare. Usually seen in February, March, and April. I have a handsome male which was shot by Mr. Comeau April 29, 1882.
131. Larus leucopterus. White-winged Gull.—Not common. Commonly appears and disappears with the last. Mr. Comeau has shot it as late as May 1.
241132. Larus marinus. Great Black-backed Gull.—Breeds, and is tolerably common. It is absent only in January and February. July 17, 1882, I found one of their nests on Great Baule, one of the Seven Islands. It consisted of a little coarse grass placed in a slight depression in the rock, and was lined with a sort of pad, about four inches in diameter, of beautiful soft down, on which reposed a single egg. The egg had been incubated, but failed to hatch.
133. Larus argentatus smithsonianus. Herring Gull.—Very abundant, breeding plentifully on suitable rocks. Arrives about the middle or latter part of April, remaining into November.
134. Rissa tridactyla. Kittiwake.—Breeds abundantly. Arrives late in April or early in May, remaining into December. This and the preceding are the commonest Gulls along this part of the coast, and are constantly seen, both singly and in immense flocks. They follow the receding tide and cover the sand flats that are exposed at low water, feeding upon the molluscs and other marine animals that abound in such situations. I have seen more than a thousand at one time.
135. Pagophila eburnea. Ivory Gull.—Very rare. Mr. Comeau shot a male in April, 1877, at Point de Monts. The specimen was presented to the Museum at Bersimis Mission, where it is now preserved.
136. Chroïcocephalus philadelphia. Bonaparte’s Gull.—A tolerably common summer resident, arriving late in May.
137. Sterna macrura. Arctic Tern.—Very abundant at certain places, where it breeds. Mr. Comeau once killed sixteen at one shot, flying. It arrives early in June.
138. Cymochorea leucorrhoa. Leach’s Petrel.—Common in summer.
139. Colymbus torquatus. Loon.—Common. Breeds about the fresh water lakes of the interior. I saw many, and heard others, in the Gulf, near Point de Monts, in July. Earliest seen April 12, 1882.
140, Colymbus septentrionalis. Red-throated Diver.—Common, breeding with the last, but not arriving so early, usually coming in May.
141. Podicipes griseigena holbœlli. Red-necked Grebe.—Rare; one shot in September.
142. Podilymbus podicipes. Dab-chick; Hell Diver.—Not rare; killed both spring and fall.
143. Fratercula arctica. Puffin; Sea Parrot.—Not common as far up as Point de Monts, but very abundant on the Mingan Islands, where they breed by thousands.
144. Alle nigricans. Dovekie.—Very abundant in flocks during some winters, arriving early in December and remaining till some time in February. During other winters it is rare or does not occur at all.
145. Uria grylle. Black Guillemot; Sea Pigeon.—A common resident, breeding not only here, but even on the islands off the mouth of the Saguenay, an hundred and fifty miles farther up the St. Lawrence.
146. Lomvia troile. Foolish Guillemot; Murre.—Like the Dovekie, the Murre is sometimes very abundant here in winter, while during 242other winters it does not occur at all. It is not wary, and does not even know enough to keep out of the way of dogs along the shore. It is well named the “Foolish” Guillemot, for both its habits and appearance deserve this appellation. In fact it looks like a perfect idiot, swimming over on one side as if one leg were broken, and staring vacantly at its enemies without attempting to escape. Its tout ensemble is stupid and gawky.
During the winter of 1875 they were so exceedingly abundant that Mr. Comeau shot about a thousand for their feathers, and his dog caught over fifty. They were all in very poor flesh, some being little more than animated skeletons, and a great many died and were washed ashore.
147. Utamania torda. Razor-billed Auk.—Not common here, but breeds on the Mingan Islands.
The Coues Check List and Ornithological Dictionary.[117]—The April number of the Bulletin contained (p. 111) a brief preliminary notice of this work, prepared from advance proof sheets. It was not published until June, and therefore too late for the appearance in our July number of a satisfactory review. As stated in the title the work is a second edition of the “Check List” which originally appeared in 1873 and was reissued in 1874 in connection with “Field Ornithology,” as a reflex of the classification and nomenclature of the “Key to North American Birds” (1872), though containing a few additional species. The original List gave 778 names; the present one gives 888, subtracting 10[118] and adding 120.
“In revising the List,” says the author, “for the main purpose of determining the ornithological status of every North American bird, the most scrupulous attention has been paid to the matter of nomenclature,—not only as a part of scientific classification, determining the technical relations of genera, species, and varieties to each other, but also as involved in writing and speaking the names of birds correctly. The more closely the matter was scrutinized, the more evidences of inconsistency, 243negligence, or ignorance were discovered in our habitual use of names. It was therefore determined to submit the current catalogue of North American birds to a rigid examination, with reference to the spelling, pronunciation, and derivation of every name—in short, to revise the list from a philological as well as an ornithological standpoint.”
“The purpose of the present ‘Check List’ is thus distinctly seen to be two-fold: First, to present a complete list of the birds now known to inhabit North America, north of Mexico, and including Greenland, to classify them systematically, and to name them conformably with current rules of nomenclature; these being ornithological matters of science. Secondly, to take each word occurring in such technical usage, explain its derivation, significance, and application, spell it correctly, and indicate its pronunciation with the usual diacritical marks; these being purely philological matters, affecting not the scientific status of any bird, but the classical questions involved in its name” (pp. 3, 4).
The analysis of the two editions shows that of the 120 additions to the old list the large majority are bona fide species, and actual acquisitions to the North American list, being birds discovered since 1873 in Texas, Arizona, and Alaska, together with several long known to inhabit Greenland, which had never been formally included in the “North American” list at the time Dr. Coues’s first Check List was issued, though the Greenland Fauna, even then, was generally claimed and conceded to be North American. Beside these, the increment is represented by species or varieties named as new to science since 1873, by a few restored to the list, and by two (Passer montanus and Coturnix dactylisonans) imported and now naturalized species.
The author states that the list includes the names of some twenty or thirty sub-species which “my conservatism would not have allowed me to describe as valid, and the validity of which I can scarcely endorse,” but which are retained because “I preferred, in preparing a ‘Check List’ for general purposes, rather to present the full number of names in current usage, and let them stand for what they may be worth, than to exercise any right of private judgment, or make any critical investigation of the merits of disputed cases.” In view of this declaration, however, we fail to understand why such names as Carpodacus purpureus californicus, Chondestes grammicus strigatus, Picus villosus leucomelas, Bubo virginianus subarcticus, Bubo virginianus saturatus, and Oreortyx picta plumifera should have been denied a place. Nor can we approve the exclusion of certain Audubonian species “not since identified,” as well as some of Giraud’s, which there is no good reason to doubt were actually taken in Texas. “A few Cape St. Lucas birds have been so long in the ‘North American’ list that it is not thought worth while to displace them”; but does not this consideration apply with equal force to many of the Mexican species which are excluded? Our present southern boundary is a political, not a natural one, but this is all the more reason why it should be rigidly adhered to if followed at all. As Dr. Coues remarks, however, it would be far more satisfactory, from a scientific standpoint, to ignore the present 244arbitrary line and include the whole “Nearctic Region,” thus taking in the table lands of Mexico nearly to the Isthmus of Tehuantepec.
To the analyses and comparisons succeed “Remarks on the use of Names,” ten pages being devoted to the principles which have guided the author in his philological researches so far as the etymology, orthography, and orthoepy are concerned. This portion of the work has something more than an indirect value, for it forms a condensed, readily available grammar of the subject to which it pertains. The assistance here rendered by his literary associate, Mrs. S. Olivia Weston-Aiken, is fittingly acknowledged in the Introduction.
In the body of the Check List the names are printed in bold type, both English and Latin, and are numbered 1 to 888. Sub-generic names are entirely discarded, as is the sign of “var.” between specific and subspecific terms. The nomenclature of sub-species is therefore trinomial, without the slightest disguise. The technical name is followed by the name of the original describer of the bird, and by that of the authority for the particular combination adopted. The “concordance of previous lists,” mentioned in the title, is effected by referring by number to Baird’s List of 1858, Coues’s Check List of 1874, and Ridgway’s Catalogue of 1880, in the case of every species.
On each page the names are duplicated in smaller type, divided into syllables marked for quantity and accent, and their pronunciation therefore shown, according to the system of orthoepy advocated. The most important point secured, however, is the etymology or derivation of the scientific words. “On the whole,” say the authors of this part of the work, “it has not been our intention to go beyond a good fair definition of these Greek and Latin words, considering that all practical purposes are thus subserved.” The etymologies are really, however, traced far back in many cases. “Nothing of the sort has been done before, to the same extent at any rate, and it is confidently expected that the information here given will prove useful to many who, however familiar they may be with the appearance of the names on paper, have comparatively little notion of the derivation, signification, and application of the words, and who unwittingly speak them as they usually hear them pronounced, that is to say, with glaring impropriety. No one who adds a degree of classical proficiency to his scientific acquirements, be the latter never so extensive, can fail to handle the tools of thought with an ease and precision so greatly enhanced, that the merit of ornithological exactitude may be adorned with the charm of scholarly elegance” (p. 4).
The Check List proper is concluded with “a list of words defined,” alphabetically arranged, and therefore serving as an index to the work.
The volume finishes with a chronological list of Dr. Coues’s writings on ornithology.
Aside from modifications which affect the ornithological or scientific status of the “Check List,” the changes in nomenclature are numerous and radical. Under our accepted, but in certain ways pernicious, system of ornithological nomenclature most of these were probably necessary; 245but we have little sympathy with the recent upheaval in this respect, nor do we believe that the names at present advocated will prove more stable than those which have preceded them. Stejneger has lately shown[119] that neither Coues nor Ridgway reached the foundations; and doubtless some one of an equally enquiring mind and with an imagination still better adapted to interpreting ancient descriptions of uncertain application, will yet come forward and work fresh havoc. The trouble with this kind of investigation is that sufficient regard is rarely paid to the rule that a description must be clearly defined, and that “definition properly implies a distinct exposition of essential characters.” We have not forgotten Mr. Allen’s eloquent protest against the adoption of certain Bartramian names, and there can be no doubt that his objections will apply equally well to the descriptions of many other early authors. Moreover, while we distinctly disclaim any personal application of such a thought, we cannot help believing that if the practice of giving the authority for the arrangement of names were discontinued, there would be less of this meddling with nomenclature. At all events the evil is a terrible one, and it must be stopped, even if the whole code has to be thrown overboard and a new one instituted. So extreme a course, however, is probably unnecessary, for some simple statute of limitation can doubtless be devised which will answer all the required ends. Dr. Coues’s recent suggestion,[120] that fifty years of unchallenged usage shall fix a name forever, is an excellent one, but the time of probation might, with advantage, be reduced to twenty-five years. Such a provision, with one requiring all proposed changes to be referred to a tribunal composed of not less than three prominent ornithologists, who might meet for the purpose at intervals of say once in four years, would effectually prevent any further tampering with a system which should be sacred, but which has become a mere football.
With respect to genera we are sorry to notice that Dr. Coues has abandoned certain old-time principles and adopted many of the sub-divisions which he rejected in the edition of 1873. Chief among these are Actodromas, Arquatella, Pelidna, and Ancylochilus, in Tringa; Symphemia and Rhyacophilus in Totanus; Herodias, Garzetta, Hydranassa, Dichromanassa, Florida, and Butorides in Ardea, and Chroïcocephalus in Larus. Turdus, however, is retained for all the Thrushes of the sub-family Turdinæ, and Vireo, in its euphonious simplicity, stands for all the Vireos. While we would not be understood as condemning all the above changes, we consider the majority of them arbitrary, and hence uncalled for. The ever increasing tendency to institute new genera on differences of structure which in other classes of Vertebrates would be considered no more than well-marked specific characters, is one of the banes of modern ornithology. Our systematists seem to have lost sight of the uses for which genera were primarily intended. Of this 246school, however, Dr. Coues is perhaps among the more conservative members.
Having fulfilled our duty of critic by finding all possible fault with the “Coues Check List” we turn to the much pleasanter task of mentioning some of its many good qualities. Of its several departments the introductory chapters may be characterised as terse, practical, and to the point; the Check List proper as carefully and in the main wisely framed; the “dictionary” as an exhaustive treatise of high scholarly excellence and of unquestionable utility. Concerning the whole work we can say nothing stronger than that it is in every way worthy of its brilliant and distinguished author, who has evidently made it one of his most mature and carefully studied efforts. Its favorable reception can be a matter of no uncertainty, for it fills a field of usefulness peculiarly its own, and one which need in no way conflict with that so ably covered by Mr. Ridgway’s recent “Nomenclature.”[121]—W. B.
Gentry’s Nests and Eggs of Birds of the United States.[122]—It is now several months since the appearance of the twenty-fifth part, the final number of this work, which was published by subscription. The text is written by Mr. Gentry himself, while the plates were executed by Mr. Edwin Sheppard, “subject to the suggestions and dictations of the author.” The title is misleading, for instead of treating of all the species found in the United States, it deals with but fifty—less than one-fifth the number known to occur within this area.
The typography and press work are good, but the plates fall far short of deserving the same praise. In the early numbers the nests and eggs were generally figured alone, but the author soon acceded to the popular demand and furnished colored representations of the birds on all plates commencing with the seventh part; with the final number appeared four extra plates, on which were shown the birds that were omitted in the first six parts.
247In a general way it may be said of most of the plates that the perspective is very bad—if not absent altogether; that a large number of the nests look as if temporarily balanced, like so many saucers, upon the branches on which they rest, and from which they seem ready to tumble on the slightest jar; and that nearly all have the appearance of cheap chromo-lithographs, while none attain to the degree of excellence essential to first-class workmanship. In order to give the subscribers as much paint as possible for the money, the artist has endeavored to supply backgrounds to many of the plates. Some of these seem intended to represent distant mountains, but the greater number consist of dense, and sometimes shapeless masses of solid green. At other times we are treated to glimpses of the sky and ocean that rival, in depth and intensity of color, the rich ultramarine-blue of the head of the Nonpareil.
Turning now to the letter-press let us examine its claim to rank among the contributions to ornithological literature. A few brief quotations will suffice to show both the scope of the work and the author’s estimate of its value. In the preface he says: “Especial pains have been taken with the text. The aim of the author has been to present a short, plain, and detailed account of the habits of each species described....
“Throughout the work, considerable prominence has been given to those interesting and curious phases of bird life which are present during the breeding period, and which have been the principal study of the author for many years. Extraneous matter has been sedulously omitted, and nothing permitted to appear about which there could be serious doubts of accuracy.
“With these few preliminary remarks, we send this beautiful book out into the world, trusting that it may meet with a cordial reception everywhere.”
That the work does not contain anything approaching a complete “detailed account of the habits” of a single species is evident from the most cursory examination of the biographies. On the other hand, we are given an amount of detail and exact data, concerning some of the most inaccessible points connected with the breeding habits of birds, that excite, first, admiration (for the author’s extraordinary acuteness of observation); next, astonishment (at the possibility of attaining a knowledge of certain peculiarities mentioned); and finally, incredulity (regarding the reliability of the author’s statements).
To be more explicit: Not only does Mr. Gentry tell us the exact number of days consumed in building the nest, in depositing the eggs, in incubation, the period the young remain in the nest, and the length of time they are afterwards fed by the parents; but he goes further and states how much time is devoted to courting, gives the period of mating and the duration of the honeymoon, and tells us how many days are spent in the selection of a suitable and satisfactory site for the nest, not omitting, in some cases, to mention which sex governs in making the choice. A few citations, in the author’s own words, will suffice, to demonstrate his unparalleled perspicacity in these matters.
248Speaking of the Wood Pewee he says: “The assumption of matrimonial relations, however, is not a matter that is entered into without more or less consideration.... The ceremony of mating being over—which business is ordinarily of short continuance, seldom lasting for a greater period than two days—the newly-wedded pair now set out to discover a suitable place for the building of a home. This is a matter of considerable moment, often requiring the performance of long and extended tours of observation and exploration. These reconnoissances generally last for a week,.... The site being mutually agreed upon, the happy pair proceed with all possible dispatch and diligence to construct a domicile: the male to collect and bring in the necessary materials; the female to fix them in their proper places.... Having finished their home, only a day or so intervenes when oviposition becomes the controlling instinct. The female now proceeds to deposit her complement of four eggs, which she does on consecutive days, at the rate of a single egg daily. This is followed, on the day succeeding the last deposit, by the trying duty of incubation. Upon the female devolves this arduous and irksome labor.”
Of the nesting of the Catbird he tells us that “ordinarily a week or ten days are spent in making a choice of locality.”
With the Orchard Oriole “Mating does not occur,” he says, till “more than two weeks after the advent of the sexes.... The sexes having come together in a wise and business-like way, with little or none of the bluster that is customary on such occasions, a conference ensues, which results in a temporary separation for mutual good; one bird going in one direction and the other in an entirely opposite course. The selection of a suitable spot for a home is the vera causa of this divergence.... In five or six days from the time of the assumption of matrimonial relations the nest is started, and through the united efforts of both birds for the period of a week is brought to completion.”
Of the Hummingbird he writes, “The sexes, tired as it were, of the riotous and luxurious lives they have been leading, come together by mutual agreement, and enter into matrimonial relations. This being accomplished, they separate for a brief period, and each proceeds to scour the country for miles around in quest of a suitable tree in which to locate. When one is selected by either bird the other is summoned to the spot to talk over, in true bird language, the merits thereof. Should the parties differ as to the advantageousness of the site, no quarrelling or bickering is indulged in, but, in the most friendly manner, they separate and renew the search until one is found which gives satisfaction.”
In his biography of the Chewink occurs the following: “The females wholly entranced, yield to the persuasions of their would-be lords, and conjugal relations are entered into.... But the happy couple are not yet ready to begin nest-building. They must needs celebrate the occasion of their marriage. Accordingly they set out on a wedding trip, so to speak, visiting adjoining lots and thickets, and enjoying the delights and scenes around them. This continues for four or five days, when the lovers, thoroughly surfeited, return and quietly settle down to prosy life.”
249Such statements as the foregoing cast a shadow of suspicion upon remarks that otherwise might be regarded as authentic, and attach to the work the stigma of untrustworthiness.
The account of the nocturnal habits of the Virginia Rail, although the wording is changed, savors strongly of the latter part of the 537th page of Coues’s “Birds of the Northwest.”
Enough has been said to show that instead of becoming an authority, worthy of place amongst the standard works on North American ornithology, Mr. Gentry’s book on nests and eggs must inevitably find its level alongside such unreliable and worthless productions as Jasper’s “Birds of North America” and similar trash. In other words, instead of a work of scientific value, we have a popular picture-book, well-adapted for the amusement of children.—C. H. M.
Dendrœca palmarum at Sing Sing, New York.—On April 29, 1882, while collecting at this place, I killed a specimen of the true D. palmarum. The bird is unusually yellow beneath, but Mr. Robert Ridgway, who kindly compared it, says: “We have several specimens from Wisconsin and Illinois which will match it.” It was busily engaged, when captured, in catching winged insects in a low swampy thicket.—A. K. Fisher, M. D., Sing Sing, N. Y.
Nest and Eggs of Setophaga picta—a Correction.—Mr. W. E. Bryant has kindly called my attention to the fact that he described two nests and sets of eggs of the Painted Redstart in Vol. VI of this Bulletin (pp. 176, 177). The clutch found by Mr. Stephens and mentioned by me in the last number of the Bulletin (Vol. VII, July 1882, pp. 140, 141) is, therefore, the third, instead of the first authentic one known. I take this opportunity for correcting the mistake, and at the same time tender my apology to Mr. Bryant for the inadvertent oversight of his note.—William Brewster, Cambridge, Mass.
The Summer Tanager (Pyranga æstiva) in New Brunswick.—While staying at Grand Manan, N. B., in June, last year, I saw in the possession of Mr. J. F. C. Moses a Summer Tanager which had been taken there a few weeks before. It was shot at North Head, Grand Manan, about the 12th on 14th of May, 1881, by a boy who brought it in the flesh to Mr. Moses, by whom it was mounted. The bird—which was undoubtedly a male, though dissection had been neglected—was in full plumage, 250and showed no signs of previous captivity. Indeed in that thinly settled region the capture of an escaped cage bird would be an unlikely event. The specimen is now in the collection of Mr. George A. Boardman.
This adds another case to the list of southern birds that have occasionally found their way to the neighborhood of the Bay of Fundy. The causes of their coming still remain hidden, and more light is needed before the facts can be satisfactorily explained.—Charles F. Batchelder, Cambridge, Mass.
The Evening Grosbeak in New York.—Mr. Charles F. Earle writes me from Syracuse, N. Y., July 11th, as follows: “On the 8th of the present month I saw a male Evening Grosbeak (Hesperophona vespertina) near Marcellus Station, Onondaga County, N. Y. Being engaged in fly-fishing at the time, I was unable to secure the bird; but there is no question of the identification, as I had a good view of it at reasonably close quarters.”—Elliott Coues, Washington, D.C.
The Black-throated Bunting in Florida.—Neither Professor Allen in his “Winter Birds of East Florida,” nor Mr. Maynard in his work on the birds of Eastern North America, includes the Black-throated Bunting (Spiza americana) as an inhabitant of Florida; hence the following note of its capture there may be worth recording. While walking along the fence row of an old field near Fernandina on April 22d, 1881, looking for Shrikes and Ground Doves, I heard the familiar note of this well-dressed Bunting in a small tree near the fence. He was immediately secured, but although I afterwards searched diligently for others, none were found.—C. W. Beckham, Bardstown, Ky.
Distribution of the Fish Crow (Corvus ossifragus.)—During a recent trip to Charlottesville, Albemarle Co., Virginia, I was much surprised to find the Fish Crow exceedingly common—quite as numerous, in fact, as the Common Crow (C. frugivorus). The locality in question is entirely surrounded by mountains—Monticello and Ragged Mountains to the east and south, the Blue Ridge only about twelve miles to the westward—and is distant at least sixty miles from the nearest tide water.—Robert Ridgway, Washington, D. C.
The Swallow-tailed Kite (Elanoïdes forficatus) taken in Southern Michigan.—Two fine specimens, male and female, of the Swallow-tailed Kite, were taken near this place, June 19, 1882, by Mr. Charles Chittenden. When first discovered by him they were foraging about his dove house, and causing a great commotion among the inmates.
The female was shot and instantly killed, while her mate, who was only slightly wounded, was secured alive. The latter is now in the possession of Dr. N. Paquette of Petersburg. They were properly identified by comparison with a nicely mounted specimen in my collection, which came 251from Georgia. As far as I am aware this is the first recorded capture of this species within the State. Dr. Morris Gibbs in his List of the Birds of Michigan, 1879, admits it on the authority of Hon. D. D. Hughes of Grand Rapids, but cites no recorded example having been taken.—Jerome Trombley, Petersburg, Munroe County, Michigan.
Garzetta candidissima at Nantucket, Massachusetts.—Visiting the above-named island, Aug. 12, 1882, I saw in the shop of Mr. H. S. Sweet, a mounted specimen of the Little White or Snowy Egret, which he said was shot near the southwest shore, at Hummock pond, last March, by one of the men of the Life-saving Station. A straggler to New England, the species has occurred far less frequently than its larger relative the White Heron (Herodias egretta), and this capture in early spring is remarkable.—H. A. Purdie, Newton, Mass.
The Snow Goose (Chen Hyperboreus) at Sing Sing, New York.—On the morning of April 9th, 1882, a large flock of two or three hundred Snow Geese visited this place. They alighted several times at the mouth of the Croton, where it empties into the Hudson, but being disturbed by the gunners, who were anxious for a shot at them, they at last flew farther up the river. I examined them by the aid of a powerful field-glass, at a distance of a few hundred yards, and being on elevated ground I could look down upon the flock and easily distinguish the black wing-tips of the adults as they flew. A few days previous I saw a single individual flying, who seemed to be taking the lay of the country. I was informed that the flock again passed down the river on the night of the 10th.—A. K. Fisher, M. D., Sing Sing, N. Y.
Note on the Long-tailed Duck.—On February 5, 1881, one of my friends procured a male specimen of the Long-tailed Duck (Harelda glacialis), at Latrobe, Westmoreland Co., Pennsylvania. The bird was shot on the only unfrozen spot noticed on the creek at the time—it was during the coldest “snap” of the season—and was in a very emaciated condition. The occurrence of this species so far inland (west of and near the mountains) is noteworthy. It was altogether unknown to the gunners thereabouts, and was brought to me for identification.—Chas. H. Townsend, Acad. Nat. Sciences, Philadelphia.
Lomvia Arra Brünnichi and L. Troile in New England.—Mr. Merrill’s note on these birds in the July number of this Bulletin (p. 191) was a timely correction of a long established error, for the common Murre found in winter off the New England coast is, as he has stated, Lomvia arra brünnichi, and not L. troile. At different times during the past ten years I have examined specimens from various points along the shores of Maine, New Hampshire, and Massachusetts, and all of the numerous birds that have come under my notice have proved to be Brünnich’s 252Guillemots. Indeed the example of L. troile mentioned by Mr. Merrill is the only New England one of which I have any knowledge. Dr. Coues says that the young of L. troile in their first winter plumage “are colored precisely like the adults, but may be always distinguished by their much shorter and slenderer bills which are in great part light colored (yellowish).”[123] If the latter peculiarity be constant it will afford a ready mark of distinction between young of the two species, for the bill in young brünnichi, so far as I have seen, is invariably black.—William Brewster, Cambridge, Mass.
Rare Warblers in Massachusetts.—In the wonderful flight or bird wave, especially of the Mniotiltidae, that took place with us May 21 and 22 last, and for some species continued during a few succeeding days, three Mourning Warblers, all males, were shot near Fresh pond, Cambridge. These, in the flesh, were kindly shown me by Mr. C. J. Maynard.
At Framingham,[124] on the above-named dates, Mr. Browne and myself identified twenty species of Warblers—among them specimens of the Cape May, Tennessee, and Bay-breasted; of the last two several were obtained in Eastern Massachusetts. Among New England Warblers, collectors here consider Geothlypis philadelphia to be the rarest, and Dendrœca tigrina next in scarcity. Helminthophila peregrina and Dendrœca castanea follow, though in the fall migrations this latter species occurs in moderate numbers with more or less regularity.—H. A. Purdie, Newton, Mass.
The Unusual “Wave” of Birds during the Spring Migration Of 1882.—A note by Dr. Coues in the July Bulletin[125] describes the remarkable “tidal wave” of our smaller birds that occurred at Washington, D.C., during the spring migration this year, and it may be worth while to throw a little light upon its further course.
As Dr. Coues says, the vast number of birds was doubtless due to the cold and rainy weather that prevailed, checking the progress of the migration beyond the latitude of Washington. When the weather changed, the gradually accumulated throng was let loose, and rushed in a great wave towards the northern breeding grounds. In the vicinity of New York, as I learn from my friend Mr. J. Dwight, Jr., after prolonged cold and wet weather a change came on the morning of May 20, and with the pleasant weather the rush of birds began. Almost all the Warblers and Thrushes were in great numbers, and continued very abundant at least throughout the following day. In the latitude of Boston birds had been unusually scarce for some days. The change to clear and warmer weather took place about noon of the 2lst, and before the rain ceased the rush of birds had begun. All day long the smaller birds came in 253unheard of numbers, stopping awhile to feed, and then hurrying on. The next morning the host was even greater, and the trees fairly swarmed with Warblers. Before noon of that day most of the birds had passed on, but for a day or two afterward the number of loiterers was sufficient to be noticeable, compared with ordinary migrations, though they seemed but a few stragglers after the army that had swept over the country during the previous days. Almost all the species of Warblers that occur in the spring migration through New England were observed. Among the rarer ones were Helminthophila peregrina, Dendrœca tigrina, D. castanea, and Geothlypis philadelphia. A White-crowned Sparrow was also shot in Cambridge.
Dr. Coues suggests that the cold wave spoken of by Mr. King[126] was the cause of this accumulation of birds. Such could hardly have been the case, as that occurred on the 21st and 22d, whereas by that time the accumulated hosts had reached Massachusetts.
It would be interesting to hear further of the course and magnitude of this “bird wave” as observed at other points.—Charles F. Batchelder, Cambridge, Mass.
Birds new to or rare in the District of Columbia.
1. Bewick’s Wren (Thryomanes bewicki). An adult ♂, taken at Arlington, Virginia (immediately opposite Washington), April 10, 1882, by W. Palmer, is in the collection of the U. S. National Museum (No. 86,218).
2. Yellow-throated Warbler (Dendrœca dominica). The National Museum also possesses a fine young ♂ of this species, taken at Arlington by Mr. Palmer, September 7th, 1881 (No. 84,858).
3. Loggerhead Shrike (Lanius ludovicianus). Several specimens of this irregularly distributed, and everywhere more or less local, species, have within the last few years been taken in the vicinity of Washington, and are now in the collection of the National Museum. Most if not all of them were obtained in winter.
4. Sharp-tailed Finch (Ammodromus caudacutus). In the mounted collection of the National Museum there is a fine adult of this species labeled, “Washington City, September, 1862; C. Drexler.” (Nat. Mus. Catal. No. 25,905.)—Robert Ridgway, Washington, D. C.
Notes on Some Birds and Eggs From the Magdalen Islands, Gulf of St. Lawrence.—The following notes, made by Mr. M. A. Frazar during a collecting trip to the Magdalen Islands in June and July, 1882, seem of sufficient importance to merit publication, although many of them are not absolutely new. Some of the points which they cover, however, have been previously involved in more or less obscurity, while the others will be none the worse for fresh data. The specimens described, and most of those mentioned, are now in the writer’s collection, and the descriptions are on his authority.
1. Dendrœca striata. Black-poll Warbler.—A set of three fresh eggs, identified by the capture of the female parent, was taken June 23. 254The nest was built in a low, thick spruce which stood on the edge of a swamp, near a brook. It was placed on a horizontal branch at a height of about three feet, and was well concealed by the clusters of densely-imbricated needles above. Externally it measures 5 inches wide by 2.50 inches deep; internally 1.80 by 1.50 inches. The walls in places are 1.50 inches in thickness. The main body of the structure is composed of Usnea moss, weed-stalks, and dry grasses, closely matted and protected outwardly by coarser stalks and a few dead spruce twigs. The lining is of slender, black moss-stems (which curiously resemble horse-hair), cows’-hair, and a few feathers. The whole affair is remarkably solid and bulky for a Warbler’s nest.
The eggs are white, with brown specks scattered over the general surface of the shell and numerous spots and blotches of reddish-brown and lavender about the larger end. They measure respectively .75 × .56, .76 × .56, and .75 × .57.
2. Pinicola enucleator. Pine Grosbeak.—The Pine Grosbeak was apparently rare among the Magdalens for Mr. Frazar met with only five individuals, four of which were secured. The first pair, taken June 18, on Amherst Island, evidently had a nest among some low spruces, for both birds showed unmistakable signs of anxiety when the spot was approached, and the female proved, on examination, to be incubating. The female of the second pair, shot June 29, on Grindstone Island, had laid all her eggs but one, which, although in the oviduct and of full size, was unfortunately without a shell. Mr. Frazar searched long and carefully for both nests but without success.
Our knowledge respecting the breeding of this Grosbeak, as found in America, is so very imperfect that the above data are both interesting and valuable. The inference is that the eggs are laid late in the season, a fact which the analogy furnished by kindred species would scarcely have suggested.
3. Loxia leucoptera. White-winged Crossbill.—Mr. Frazar met with these Crossbills on all the islands of the Magdalen group, where they were among the most abundant of the land birds. At the time of his arrival (June 6) they had already collected in large flocks which were composed chiefly of young birds and females, a company of fifty or more often containing only one or two males in red plumage. The latter were also found singly, and from the fact that such individuals were often in full song Mr. Frazar inferred that they might still be in attendance on sitting mates, or unfledged young. The average development of the numerous young birds collected would indicate, however, that the regular breeding season was somewhat earlier, although none of them could have been hatched much before the middle of May. Assuming, then, that the past season was not an exceptionally late one, the proper time to look for fresh eggs in this locality would be not far from May 1.
As I can find no detailed description of the first plumage of this species I append the following:—
Juv., first plumage (♀, Magdalen Islands, June 14, 1882. M. A. Frazar). Entire plumage of head and body thickly streaked with dull black 255on an ochraceous ground; greater and middle wing-coverts, with the tertials, broadly tipped with fulvous-white; primaries and rectrices black, edged with pale fulvous.
A male (June 26) somewhat older, but still in first plumage, differs from the specimen just described in having the dark streaks broader and blacker, the wing-bands nearly pure white, and the under parts less strongly ochraceous.
4. Ægiothus linaria. Common Red-poll.—In his list of the birds of the Magdalen Islands,[127] Mr. Cory included this species “with great hesitation,” a single specimen, so badly mangled that it could not be positively identified, being the only one which came under his notice. Mr. Frazar, however, found it abundant on both Amherst and Grindstone Islands where many large flocks were seen feeding among the spruces. Owing to lack of time and the pressure of other duties he secured only two specimens, but as these are both in first plumage the breeding of the species there may be considered assured. The following description is taken from the younger of the two examples just mentioned.
Juv., first plumage (♂, Magdalen Islands, June 29, 1882. M. A. Frazar). Entire plumage of the head and body, excepting the throat, cheeks, and abdomen, thickly and coarsely streaked with dull black on a pale ochraceous or brownish-white ground; tips of the greater and middle wing-coverts with the outer edges of the tertials, ochraceous-white; throat black; cheeks brownish-ochraceous; center of the abdomen brownish-white and immaculate; no red on the vertex.
5. Falco columbarius. Pigeon Hawk.—A set of four eggs from Amherst Island was taken under the following circumstances: Mr. Frazar was passing a spruce-clad knoll surrounded by a boggy swamp, when he noticed a pair of Pigeon Hawks circling above the trees. Approaching, he quickly discovered their nest, built in a dense spruce at the intersection of a horizontal branch with the main stem and at a height of about ten feet. As he climbed the tree the Hawks, now thoroughly alarmed for the safety of their charge, dashed wildly about his head, frequently passing within a few feet and uttering shrill screams of anger or dismay. After taking the eggs he made a close examination of the nest, which was found to be very bulky—in fact “as large as a Crow’s,” and composed chiefly of bark with some coarse sticks surrounding the exterior, and a neat, soft lining of finer bark and horse-hair. From its general appearance he felt convinced that it was constructed by the Hawks themselves. This was June 9; returning five days later he found both birds flying about the knoll and their actions indicated that they had built another nest somewhere near, but it could not be found. As he was then on the point of leaving the island he shot the male, a fine adult specimen which accompanies the eggs.
The latter, now before me, are almost perfectly elliptical in shape, and measure respectively 1.57 × 1.27, 1.55 × 1.23, 1.59 × 1.24, and 1.56 × 1.25. The ground-color, in three of them, is apparently pinkish-buff, but this is 256almost wholly overlaid by numerous, nearly confluent blotches of dilute chocolate and purplish-brown which, with a few black spots and dashes, are uniformly spread over the entire surface of the shell. The fourth specimen has some immaculate spaces of creamy-buff about the smaller end, although the markings elsewhere are even denser than in the other three. The general coloring of these eggs is extremely rich and handsome and, excepting in size, they bear a close resemblance to the notoriously beautiful egg of the Duck Hawk.—William Brewster, Cambridge, Mass.
Second Addendum to the Preliminary List of Birds ascertained to occur in the Adirondack region, Northeastern New York.[128]
186. Telmatodytes palustris. Long-billed Marsh Wren.—Dr. A. K. Fisher writes me that he took a nest and three eggs of this species at Lake George, in Warren Co., August 2, 1882.
187. Passer domesticus. House Sparrow.—Common in the villages along the outskirts of the wilderness, on both sides of the Adirondacks.
188. Squatarola helvetica. Black-bellied Plover.—Occurs along Lake Champlain during the migration.
189. Charadrius dominicus. Golden Plover.—Very common about Lake Champlain during October in some seasons.
190. Ægialites semipalmatus. Semipalmated Plover; Ring-Neck.—Abundant along Lake Champlain during the fall migration, arriving about the middle of September.
191. Tringa canutus. Knot; Robin Snipe.—Occurs during the migrations.
192. Actodromas minutilla. Least Sandpiper.—Very abundant about Lakes George and Champlain during the fall migration.
193. Pelidna alpina americana. Red-backed Sandpiper; American Dunlin.—Occurs during the migrations.
194. Limosa fœda. Marbled Godwit.—Sometimes tolerably common about Lake Champlain in October.
195. Bartramia longicauda. Field Plover.—Breeds in dry fields bordering the Adirondacks, on both sides of the mountains.
196. Numenius longirostris. Long-billed Curlew.—A specimen was shot near Plattsburg, on Lake Champlain, several years ago.
197. Rallus virginianus. Virginian Rail.—Tolerably common about the borders of the wilderness.
198. Chaulelasmus streperus. Gadwall.—Rare. Mr. Henry Prentiss shot one on Lake Champlain in April, 1882.
199. Dafila acuta. Pintail.—Rather rare. Occurs both in spring and fall.
200. Mareca americana. Baldpate.—Rare along Lake Champlain.
201. Fuligula marila. Scaup Duck.—Occurs during the migrations, but is not common.
257202. Fuligula affinis. Little Black-head.—Tolerably regular fall migrant. Taken on Lake Champlain.
203. Fuligula vallisneria. Canvas-back.—Rare fall migrant.
204. Fuligula americana. Redhead.—Rare. Has been killed on Lake Champlain in November.
205. Larus glaucus. Glaucous Gull; Ice Gull.—I have seen a specimen of this boreal species that was killed while feeding on carrion, in the town of Bangor in Franklin Co., about two years ago.—C. Hart Merriam, M.D., Locust Grove, N. Y.
List of Additions to the Catalogue of North American Birds.—In this Bulletin for January, 1882 (page 61), there was published a list of species of birds which had been added to the fauna of North America since the publication of the last “Smithsonian” catalogue, or Nomenclature of North American Birds. I now give a list of subsequent additions for the benefit of those who, for various reasons, are not able to “keep the run” of all the new discoveries; and a supplement with each number of the Bulletin is contemplated, in order that all interested may keep posted in the matter.
The number prefixed indicates the position of each species in the catalogue in question.
2a. Hylocichla fuscescens salicicola Ridgw. Willow Thrush.—Proc. U. S. Nat. Mus., Vol. IV, 1882, p. 374. (Rocky Mountain district of U. S.)
3a. Hylocichla aliciæ bicknelli Ridgw. Bicknell’s Thrush.—Proc. U. S. Nat. Mus., Vol. IV, 1882, p. 377. (Breeding on the Catskill Mts., New York.)
35a. Chamæa fasciata henshawi Ridgw. Pallid Ground Tit.—Proc. U. S. Nat. Mus., Vol. V, 1882, p. 13. (Interior of California.)
38a. Lophophanes inornatus griseus Ridgw. Gray Titmouse.—Proc. U. S. Nat. Mus., Vol. V, 1882, p. 344. (Middle Province of U. S.)
55b. Certhia familiaris montana Ridgw. Rocky Mountain Creeper.—Proc. U. S. Nat. Mus., Vol. V, 1882, p. 114. (Middle Province of North America.)
55c. Certhia familiaris occidentalis Ridgw. California Creeper.—Proc. U. S. Nat. Mus. Vol. V, 1882, p. 115. (Pacific coast of U. S.)
59b. Catherpes mexicanus punctulatus Ridgw. Punctulated Wren.—Proc. U. S. Nat. Mus., Vol. V. 1882, p. 343. (California.)
69.* Motacilla ocularis Swinh. Swinhoe’s Wagtail.—Cf. Proc. U. S. Nat. Mus., Vol. IV, 1882, p. 414. (La Paz, Lower California; straggler from eastern Asia.)
93.* Dendrœca vieilloti bryanti Ridgw. Chestnut-headed Yellow Warbler.—Dendrœca vieilloti var. bryanti Ridgw., in Hist. N. Am. B., I, 1874, p. 218. Cf. Proc. U. S. Nat. Mus., Vol. IV, 1882, p. 414. (Common at La Paz, Lower California.)
122.* Geothlypis beldingi Ridgw. Belding’s Yellow-throat.—Proc. U. S. Nat. Mus., Vol. V, 1882, p. 344. (San José del Cabo, Lower California.)
258144a. Vireo huttoni stephensi Brewst. Stephens’s Vireo.—Bull. Nutt. Orn. Club, Vol. VII, July, 1882, p. 142. (Arizona and New Mexico.)
230b. Peucæa ruficeps eremœca Brown. Rock Sparrow.—Brown, Bull. Nutt. Orn. Club, Vol. VII, Jan. 1882, pp. 26, 38. (Kendall Co., Texas.)
297c. Perisoreus canadensis nigricapillus Ridgw. Labrador Jay.—Proc. U. S. Nat. Mus., Vol. V, 1882, p. 15. (Labrador.)
311a. Myiarchus mexicanus cooperi (Baird). Cooper’s Flycatcher.—Myiarchus cooperi Brewst. Bull. Nutt. Orn. Club, Vol. VI, Oct. 1881, p. 252. (Camp Lowell, Arizona.)
354a. Caprimulgus vociferus arizonæ (Brewst.). Stephens’s Whip-poor-will.—Antrostomus vociferus arizonæ Brewst. Bull. Nutt. Orn. Club, Vol. VI, April, 1881, p. 69. (Arizona.)
402e. Scops asio bendirei Brewst. California Mottled Owl.—Bull. Nutt. Orn. Club, Vol. VII, Jan. 1882, p. 31. (California.)
452.* Gyparchus papa (Linn.). King Vulture.—Sarcorhamphus papa Coues, Bull. Nutt. Orn. Club, Vol. VI, Oct. 1881, p. 248. (Rio Verde, Arizona.)
475a. Lagopus mutus reinhardti (Brehm.). Greenland Ptarmigan.—Cf. Turner, Proc. U. S. Nat. Mus., Vol. V, 1882, p. 229. (Greenland and west side of Cumberland Gulf.)
475b. Lagopus mutus atkhensis Turner. Atkhan Ptarmigan.—Proc. U. S. Nat. Mus., Vol. V, 1882. p. 230. (Atkha Island, Aleutian chain.)
486.* Ardea wardi Ridgw. Ward’s Heron.—Bull. Nutt. Orn. Club, Vol. VII, Jan. 1882, p. 5. (Oyster Bay, West Florida.)
569.* Rallus beldingi Ridgw. Belding’s Rail.—Proc. U. S. Nat. Mus., Vol. V, 1882, p. 345. (Espiritu Santo Island, Gulf of California.)
701.* Diomedea melanophrys Temm. Spectacled Albatross.—Cf. Bean, Proc. U. S. Nat. Mus., Vol. V, 1882, p. 170. (Off coast of California, in lat. 40° 30′ N., long. 142° 23′ W.)—Robert Ridgway, Washington, D. C.
| Vol. | VI, | page | 199, | line | 10, | for “centimeters” read “millimeters.” |
| „ | VII, | „ | 9, | „ | 12, | for “Blue-winged Yellow” read “Golden-winged.” |
| „ | „ | „ | 26, | „ | 6, | for “An indistinct, dusky” read “A black.” |
| „ | „ | „ | 26, | foot | note, | for “οὐκέω” read “οἰκέω.” |
| „ | „ | „ | 47, | „ | „ | line 3, for “Water” read “Winter.” |
| „ | „ | „ | 119, | line | 8, | for “struggling” read “straggling.” |
| „ | „ | „ | 122, | „ | 9, | from bottom, for “Rellon” read “Redlon.” |
| „ | „ | „ | 123, | „ | 28, | for “Before” read “Upon.” |
| „ | „ | „ | 164, | „ | 11, | for “chince” read “china.” |
| „ | „ | „ | 165, | „ | 31, | for “‘Poulet Dean’” read “‘Poulette d’Eau.’” |
| „ | „ | „ | 178, | „ | 3, | for “Cincinnurus” read “Cicinnurus.” |
1. Cf. Bull. U. S. Geol. Geog. Survey Terr. Vol. IV, No. 1, pp. 231, 232.
2. After many careful examinations of the type specimen, I am led to the conclusion that it does represent the perfect colored phase, since no combination, or division, of the markings of A. herodias and A. occidentalis—or, in other words, no partial development of the head-pattern of the former—would give the peculiar markings which distinguish A. würdemanni.
3. The pattern of coloration of the head exactly as in A. herodias, and not at all like A. würdemanni.
4. This bone is described in Mivart’s “Lessons in Elementary Anatomy,” p. 320, fig. 289; and by Alix in his “Essai sur l’Appareil locomoteur des Oiseaux,” p. 403. Being out of town fuller references cannot be given.
5. In his “Essai sur l’Appareil locomoteur des Oisseaux,” Alix figures (pl. II. fig. 12) the carpus of a Kestrel with a simple sesamoid.
7. These all common on the date when first observed.
8. The difference between the season just passed in the arrival and time of nesting of the birds, may be illustrated by the fact that in the spring of 1880, Setophaga ruticilla was noted near Wheatland April 1, while in the spring of 1878, eggs of Protonotaria citrea were obtained near Mt. Carmel April 27.
9. Baird, Brewer and Ridgway’s Birds N. Am., Vol. III, p. 45.
10. Eremœca = ἔρημος + οἰκέω.
11. Mr. Ridgway has found that fully ninety-five per cent of the Screech Owls of the Wabash Valley, in southern Illinois, are red.
12. The small quadrate spots on the primaries and the indistinct tail bands, characters which have been held as diagnostic, are both shown by my series to be inconstant and of no varietal significance.
13. As my material is not at present sufficiently comprehensive to enable me to define the limits of distribution of this race I leave the compilation of its synonymy to those who may have better opportunities in this respect.
14. “Review of the American Species of the genus Scops.” Proc. U. S. Nat. Mus., Vol. I, pp. 85–117.
15. This arrangement leaves a large portion of the Middle Province without any characteristic representative, maxwellæ being an Alpine form apparently confined to the Rocky Mountains, while kennicotti and “tricopsis” respectively invade only its northern and southern borders. Our knowledge of the subject is not as yet sufficiently comprehensive to enable me to fill this gap, but all the available evidence goes to show that asio, at least as above defined, is not found to the westward of the Rocky Mountain range.
16. See Brewster, this Bull., Vol. IV, pp. 75–80 and 91–103.
17. See Orn. Lower Rio Grande, Bull. U.S. Geol. Surv., IV, No. 1, 1878, p. 10.
18. Birds Dak. and Mont., Bull. U.S. Geol. Surv., IV, No. 3, 1878, p. 561.
19. Four specimens were known before Mr. Werner explored Comal Co., in 1878. In his article on Werner’s Birds (this Bull., Vol. IV, p. 77), Mr. Brewster does not state just how many were taken.
20. Hist. N. A. Birds, Vol. I, 1874, p. 579.
21. See anteà, p. 26.
22. Mr. Ridgway acquiesces in the identification made of my inconstant examples of this species and Pipilo maculatus, in a letter from which I here make an extract: “The Pipilos appear to be neither true arcticus nor true megalonyx, and are almost as near (one of them at least) to maculatus of Mexico. They are, however, less like arcticus than either.... You will notice that one of the specimens has a very considerable admixture of grayish on the upper parts. Now, were this color more olivaceous, the specimen in question would be exactly like maculatus. The Song Sparrows are about equally like M. fasciata and M. fallax, but in colors appear to me to be nearer the former, as fallax has the markings less sharply contrasted. The specimens are, however, more like fallax in the grayness of the plumage. Upon the whole, I would say that they are nearer fasciata than fallax.”
23. See Field and Forest, Feb. 1877, p. 131.
24. See Orn. Lower Rio Grande, Bull. U. S. Geol. Surv., IV, No. 1, 1878, p. 39.
25. See Ibis, 1866, p. 33.
26. In Memoriam. The Collected Scientific Papers of the late Alfred Henry Garrod, M. D., F. R. S., etc. Edited, with a biographical memoir of the author, by W.A. Forbes, B. A., etc. London: R. H. Porter: 6 Tenterden Street. 1881. 1 vol. 8vo. pp. xxvi, 538, pll. 33, frontisp. (portrait), and many cuts in text.
27. Osteology of the North American Tetraonidæ. By Dr. R. W. Shufeldt, U.S.A. Bull. U.S. Geol. and Geog. Surv. Territories, Vol. VI, No. 2, pp. 309–350, pll. V-XIII.
28. Osteology of Lanius ludovicianus excubitorides. By Dr. R. W. Shufeldt, U.S.A. Bull. U.S. Geol. and Geog. Surv. Territories, Vol. VI, No. 2, pp. 351–359, pl. XIV.
29. Illustrations of the Nests and Eggs of the Birds of Ohio. Part VIII, April, 1881 Part IX, July, 1881. Pll. xxii-xxvii. fol.
30. American Naturalist, Nov., 1881, pp. 906–908.
31. Osteografische Beiträge zur Naturgeschichte der Vögel. Ueber das Nagelglieder der Flügelfinger, besonders der Daumen. Leipzig, 1811, S. 89.
32. A List of the Birds of Minnesota. By Dr. P. L. Hatch. Ninth Ann. Rep. Geol. and Nat. Hist Surv. Minn., for 1880, 1881, pp. 361–372.
The Water Birds of Minnesota. By Thomas S. Roberts. Op. cit., pp. 373–383.
33. On birds observed in Amelia County, Virginia. By Percy E. Freke. Scientific Proceedings of the Royal Dublin Society, Vol. III, Part III. [Read Feb. 21st, 1881.]
34. Field Notes on Louisiana Birds. By Dr. F. W. Langdon. Jour. Cincinnati Soc. Nat. Hist., July, 1881, pp. 145–155.
35. Forty Years’ Notes of a Field Ornithologist, by John Krider, Member of the Philadelphia Academy of Natural Sciences and author of Krider’s Sporting Anecdotes, Philadelphia. Giving a description of all birds killed and prepared by him. Philadelphia, 1879, 8vo. pp. i-xi, 1–84.
36. Zoölogical Miscellany, edited by Dr. F. W. Langdon. Jour. Cincinnati Soc. Nat. Hist., Vol. IV, Dec., 1881, pp. 336–346.
37. Annotated List of the Birds of Nevada. By W. J. Hoffman, M. D. Bull. U. S. Geol. and Geog. Survey of the Territories, Vol. VI, No. 2, Sept. 19, 1881, pp. 203–256, and map.
38. Ornithologist and Oölogist, Vol. VI, pp. 78, 79.
39. This Bulletin, Vol. III, p. 123.
40. See this Bulletin, Vol. IV, p. 137.
41. Its distribution in Arizona is apparently limited to a comparatively small area which, according to Mr. Stephens’ experience, is bounded on the east by the valley of the San Pedro; on the west by a point “a few miles east of the Hassayampa, on the desert between it and Salt River.”
42. Vol. XV, No. 3, March, 1881.
43. This Bulletin, Vol. VI, p. 67.
44. Birds of the Colorado Valley, p. 74.
45. Birds of the Colorado Valley, p. 75.
46. In a recent letter Mr. Stephens adds:—“From my own observations I should characterize the respective haunts of the Arizona Thrashers as follows: H. lecontei is exclusively a bird of the deserts. H. bendirei is a desert bird approaching the valleys. H. palmeri occurs along the edge of deserts, occasionally appearing in valleys. H. crissalis haunts valleys and broad cañons, seldom venturing into the deserts.”
47. The name “os prominens,” proposed by Dr. Shufeldt, has been adopted by me because it seems eminently proper that so large a sesamoid, frequently equalling the patella in size, should receive a distinctive appellation.
48. The English Sparrow, which is but an indifferent flyer, can be deprived of one-half of the secondaries and one-fourth of the primaries of both wings, in the long axis of the pinion, without apparently impairing its flight. See Pettigrew.
49. I find that this statement must be modified in regard to Gulls, if not retracted altogether, for since this paper was written I have found the os prominens in Larus glaucus and L. dominicanus. It is present as a small, elongated, trihedral prism, imbedded in the tendon of the extensor patagii longus, and playing over the flattened surface of the scapho-lunar.
50. [See p. 122 of this issue.—Edd.]
51. For an account of its breeding at Houlton see this Bulletin, Vol. IV, pp. 37–39.
52. The Coues Check List of North American Birds, revised to date and entirely rewritten under direction of the author, with a Dictionary of the Etymology, Orthography and Orthoëpy of the scientific names, the Concordance of previous lists, and a Catalogue of his Ornithological Publications. Boston: Estes and Lauriat. 1882. 1 vol. roy. 8vo. pp. 165.
53. Extract from a Report of Exploration by Professor John Macoun, M. A., F.L.S. Report of Department of Interior (n. d., n. p. Ottawa, 1881? 8vo, pp. 48.)
54. A Revised List of the Birds of Brandon, Vt. and vicinity. By F. H. Knowlton. The Brandon Union (newspaper), February 10, 1882. See also, by the same author:—A Partial List of the Birds of Brandon, Vt. The Brandon Union, December 13, 1878.
Remarks on some Western Vermont Birds. Bull. Nutt. Ornith. Club, Vol. VII, January, 1882, pp. 63, 64.
55. Dr. C. Fr. W. Krukenberg. Die Farbstoffe der Federn, in Dessen Vergleichendphysiologische Studien. I Reihe, V Abth., 1881, s. 72–92. Plate III.
56. See this Bulletin, Vol. IV, p. 8.
57. See this Bulletin, Vol. VII, pp. 54–55.
58. It should be stated that Mr. Purdie, with characteristic courtesy, declines to publish this note as, after discovering his prior knowledge of the specimen, I requested him to do.
59. See Brewer, Proc. Bost. Soc. Nat. Hist. XVII, 1875, p. 446.
60. This Bulletin, Vol. VII, p. 40.
61. Voyages of Samuel de Champlain, translated from the French by Charles Pomeroy Otis, Ph.D., with historical illustrations, and a Memoir, by Rev. Edmund F. Slafter, A. M. Vol. II, 1604–1610, Boston, published by Prince Society, 1878.
62. Birds of the Northwest, 1874, pp. 679, 680.
63. Mr. Sennett and Dr. Merrill found it breeding on the Lower Rio Grande in Texas. (Sennett, B. Rio Grande, 1878, pp. 65, 66; Merrill, Ornith. Southern Texas, 1878, p. 172.)
64. This Bulletin, Vol. VII, p. 33.
65. For an excellent review of the races of H. unalascæ, by Mr. H. W. Henshaw, see this Bulletin, Vol. IV, p. 134. Several errors, perhaps typographical, are apparent in the tables of measurements given in this paper. For example, the bill of var. pallasi is said to average .53 inch, whereas the largest specimen of that form is afterwards credited with a bill of only .51. Again, var. nanus (i.e., unalascæ) does not appear from the table of extreme measurements to have been found with a smaller bill than .49, though it had previously been said to average .48. The difference in length of bill exhibited by the three races of this species is almost microscopic. A much more tangible character, not mentioned by Mr. Henshaw, lies in the disproportionate slenderness of the bill of the western varieties. In a rather large (wing 3.67) example of unalascæ before me, the bill measured across the base of the culmen is but .20 wide, while in a specimen of var. pallasi of the same site it is .25 wide.
66. Bull. Nutt. Ornith. Club, Vol. VI, pp. 225–235.
67. Descriptions of the various pigments may be found in:
Krukenberg, Dr. C. Fr. W.; Vergl.-phys. Studien, 1 R, v. Abth. 1881, SS. 72–99, u. 2 R, 1 Abth., 1882, SS. 151–171.
Bogdanow, A., Note sur le pigment des touracos. Compt. rend., T. LIV, 1862, pp. 660–663. Études sur les causes de la coloration des Oiseaux. Compt. rend T. XLVI, 1858, pp. 780, 781
Church, H. H., Researches on Turacine, an animal pigment containing copper. Chemical News, vol. XIX, 1869, No. 496.
Blasius, W., A. D. Sitzungsb des Vereins f. Naturwiss, zur Braunschweig. Braunschweigische Anzeigen, 1877, Nr. 29.
68. Annals N. Y. Lyceum Nat. Hist., Vol. XI, p. 136.
69. Hist. N. A. Birds, Vol. I, 1874, pp. 297–298.
70. A letter just received from Mr. Stephens contains the following very satisfactory confirmation of the above evidence. “The identification of your nest of S. picta is positive. I saw the parent plainly, and could easily have shot her. Indeed I should never have found the nest had not my attention been called to it by the birds flying from it as I brushed past almost within touching distance. When first found, the nest contained three eggs. I thought it best to leave them until next day to see if more might not be laid. * * * When I returned, however, the bird was not at home and as it was a long, rough walk to camp, I took the nest, their being no occasion to visit the spot again. * * * The locality was a wide part of a cañon between the two Santa Rita peaks, perhaps two miles from the top of the high ridge connecting them. Up this cañon passed an old Mexican road to the pine timber above. It had not been used for many years. In its course it cut through an occasional projecting bank, and in one of these places was the nest. It was under a small boulder in the side of a nearly perpendicular bank, which was but two or three feet high. The vicinity was heavily timbered with oak and sycamore. I regard the position as exceptional: still, it may be the rule.”
71. U. S. Geol. Surveys W. 100 Merid., 1879, pp. 291–293.
72. For an account of the nesting of this species at Grand Falls, see this Bulletin, Vol. VII, p. —.
73. For descriptions of the nesting of this species at Houlton and Fort Fairfield see this Bulletin, Vol. III, pp. 166–168, and Vol. IV, pp. 241, 242.
74. “Descriptions of two new Thrushes from the United States.” Proceedings U. S. National Museum, Vol. 374, pp. 374–9.
75. The highest peak of the Catskills,—4,205 feet altitude.
76. Both birds were carefully examined and the evidence on this point was positive and unequivocal. A Thrush’s nest containing spotted eggs discovered near the top of Slide Mountain may have been either that of this form or of swainsoni, but as positive identification was prevented, further allusion to it is, for the present, withheld.
77. Though averaging of greater length, in proportions this bird averages smaller than swainsoni, and some specimens are much smaller than any I have seen of the latter species. The wide difference from true aliciæ here implied may be illustrated by the following extreme measurements given by the birds of my series:—
| aliciæ, | length, | 8.00; | extent, | 13.12; | wing, | 4.35; | tail, | 3.40. |
| bicknelli, | „ | 6.55; | „ | 10.56; | „ | 3.40; | „ | 2.60. |
78. See “The Coues Check List of North American Birds,” p. 24.
79. Birds of North America, p. 12.
80. Field Notes on Louisiana Birds. By Dr. F. W. Langdon. Journal of the Cincinnati Society of Natural History, July, 1881, pp. 145–155. A List of Birds from the Lower Mississippi Valley, Observed During the Summer of 1881, with Brief Notes. By O. P. Hay. Bull. Nutt. Ornith. Club, Vol. VII, pp. 89–94.
81. “Forest and Stream” Bird Notes. An index and summary of all the ornithological matter contained in “Forest and Stream,” Vols. I-XII. Compiled by H. B. Bailey. New York: F. & S. Pub. Co., 39 Park Row. 1881. 8vo., paper, pp. iv, 195.
82. A Catalogue of the Birds of New Brunswick, with brief notes relating to their migrations, breeding, relative abundance, etc. By Montague Chamberlain. Bulletin of the Natural History Society of New Brunswick. No. 1, pp. 23–68. Published by the Society. Saint John, N. B., 1882.
83. Dr. C. Fr. Krukenberg. Die Farbstoffe der Federn. 2 Mittheilung, in Dessen Verg.—phys. Stud., 2 R., I. Abth., 1882, SS 151–171.
84. On some generic and specific appellations of North American Birds. By Leonhard Stejneger. Proc. U. S. Nat. Mus., June, 1882, pp. 28–43.
85. Ingersoll, Ernest. Birds’-Nesting: A Handbook of Instruction in Gathering and Preserving the Nests and Eggs of Birds for the Purposes of Study. Salem, 1882.
86. Vol. VII, pp. 23–25.
87. N. E. Bird Life, p. 104, foot note.
88. See Herrick, Birds of Grand Manan, p. 6.
89. See Rod and Gun, Vol. VI, p. 65.
90. See especially this Bulletin, Vol. VI, pp. 124–25.
91. See this Bulletin, Vol. IV, p. 108, and Vol. V, p. 63.
92. In citing this and the next form as races of cinereus, I follow Mr. Henshaw, with whose views respecting the affinity of the three birds I fully agree.
94. The question of the relationship which M. cooperi, M. erythrocercus, M. mexicanus and M. crinitus bear to one another, and that of the respective names which should be used for each, has been recently discussed at some length. (See Bull. U. S. Geolog. Surv., Vol. IV, pp. 32–33; ibid., Vol. V, No. 3, pp. 402–404; Proc. U. S. Nat. Mus., Vol. 1, p. 139; and ibid., Vol. 3, pp. 13–15.) While I cannot claim to have personally investigated the points at issue, I am at present inclined to follow Mr. Ridgway’s ruling, at least so far as M. cooperi is concerned.
95. This Bulletin, Vol. VI, p. 252.
96. This Bulletin, Vol. VI, p. 252.
97. See this Bulletin, Vol. I. pp. 14–17 and 75–76, and Vol. V, pp. 20–25.
98. Proceedings U. S. Nat. Mus., Vol. I, p. 142.
99. This Bulletin, Vol. VI, pp. 69–72.
100. See Bull. U. S. Geol. Surv., Vol. V, Art. 5.
101. Of Grallatores, Lamellirostres, etc., I can give only a very incomplete list, as I have never had favorable opportunity to observe these birds.
102. Typical; the occurrence of both forms seems at first thought anomalous, but migratorius may be a migrant from Alaska, where it is the representative bird.
103. Var. nov. See page 228 of this number.
104. Nearly typical, but showing slight approaches to var. oregonus.
105. Typical, and not approaching var. caurinus of the coast region.
106. Typical.
107. One specimen, with a complete red nuchal band.
108. See my late paper on this Owl (this Bulletin, Vol. VII, pp. 27–33). Six examples in the present collection offer no new points affecting the position there taken.
109. Slightly aberrant; see remarks under B. saturatus (p. 230).
110. See remarks under A. atricapillus (pp. 231, 232).
111. As Mr. Ridgway has lately pointed out, Cassin’s pacificus was clearly based on specimens of subarcticus, a very distinct race first recognized by Hoy in 1852. Hence the name pacificus must give place to saturatus, proposed by Mr. Ridgway for “a northern littoral form, of very dark colors.”
112. The supposed adult, described by Mr. Ridgway in the “History of North American Birds” (Vol. III, p. 148), proves to be an immature bird in its second year. The real adult, however, was figured in the second edition of this work.
113. “Darker (brownish-black) markings prevailing in extent over the lighter (nearly clear white) ones. Stripes beneath broad, brownish-black; those on the flanks cordate and transverse.”
114. The type of the adult striatulus has turned out to be merely a light-colored, faintly marked example of atricapillus.
115. See this Bulletin, Vol. VII, pp. 120, 121.
116. See this Bulletin, Vol. VI, p. 246.
117. The Coues Check List of North American Birds. Second Edition, Revised to Date, and entirely Rewritten, under Direction of the Author, with a Dictionary of the Etymology, Orthography, and Orthoëpy of the Scientific Names, the Concordance of previous Lists, and a Catalogue of his Ornithological Publications. [Monogram.] Boston. Estes and Lauriat. 1882. 1 vol. imp. 8vo. pp. 165.
118. The 10 species retired are: Ægiothus fuscescens; Centronyx ochrocephalus; Sphyropicus williamsoni; Lampornis mango; Agyrtria linnæi; Momotus cœruleiceps; Ibis thalassina; Ardea wuerdemanni; Sterna “longipennis” (S. pikii Lawr.); Podiceps cristatus. The list of added species (too long to print here) is given on pp. 6–8 and 10 of the Check List.
119. Proc. U. S. Nat. Mus., June, 1882, pp. 28–43.
120. This Bulletin, Vol. VII, pp. 178, 179.
121. While it is unfortunate that there should be two check lists of North American birds, Dr. Coues’s right to publish his views in this form was undeniably established when his first list was issued and accepted. Moreover, we see no reason why others should be debarred from the same privilege, and we fancy that a third list, representing a different and more conservative school of thought, especially in the matter of nomenclature, would have a large following. As regards a choice of names, in the comparatively few cases where the present authorities differ we should weigh well before accepting either. Many persons, doubtless, have neither the time nor the inclination to do this, and such, necessarily, must be guided by individual preferences in favor of one or the other author. In all cases of publication, however, a simple statement of the authority followed will be sufficient to prevent any confusion or misunderstanding.
122. Illustrations of Nests and Eggs of Birds of the United States, with Text, by Thos. G. Gentry. Philadelphia: J. A. Wagenseller, Publisher, No. 23 North Sixth Street. Copyright by J. A. Wagenseller, 1881. [4to, parts 1–25, pp. 1–300. 54 col. chromo-lithographs, and chromo-portrait frontispiece of the author. Price, $25.00. 1880–82.]
123. “Monograph of the Alcidæ,” Proc. Phila. Acad., Vol. XX, 1868, p. 77.
124. See F. C. Browne, Forest and Stream, Vol. XVIII, June 15, 1882, p. 386.
125. Vol. VII, p. 185.
126. This Bulletin, Vol. VII, p. 185.
127. “A Naturalist in the Magdalen Islands,” p. 42.
128. See this Bulletin, Vol. VI, p. 225, and Vol. VII, p. 128.