Obvious typographical errors have been silently corrected. Variations in hyphenation other spelling and punctuation remains unchanged. In particular the words height and hight are used about equally. As hight is a legitimate spelling, it has not been changed.
Some of the larger tables have been re-organised to improve clarity and avoid excessive width.
The cover was prepared by the transcriber and is placed in the public domain.
WITH SOME NOTES ON LEAD MINING
EDITED BY
WALTER RENTON INGALLS
NEW YORK AND LONDON
THE ENGINEERING AND MINING JOURNAL
1906
Copyright, 1906,
By The Engineering and Mining Journal.
ALSO ENTERED AT
Stationers’ Hall, London, England.
ALL RIGHTS RESERVED.
This book is a reprint of various articles pertaining especially to the smelting and refining of lead, together with a few articles relating to the mining of lead ore, which have appeared in the Engineering and Mining Journal, chiefly during the last three years; in a few cases articles from earlier issues have been inserted, in view of their special importance in rounding out certain of the subjects treated. For the same reason, several articles from the Transactions of the American Institute of Mining Engineers have been incorporated, permission to republish them in this way having been courteously granted by the Secretary of the Institute. Certain of the other articles comprised in this book are abstracts of papers originally presented before engineering societies, or published in other technical periodicals, subsequently republished in the Engineering and Mining Journal, as to which proper acknowledgment has been made in all cases.
The articles comprised in this book relate to a variety of subjects, which are of importance in the practical metallurgy of lead, and especially in connection with the desulphurization of galena, which is now accomplished by a new class of processes known as “Lime Roasting” processes. The successful introduction of these processes into the metallurgy of lead has been one of the most important features in the history of the latter during the last twenty-five years. Their development is so recent that they are not elsewhere treated in technical literature, outside of the pages of the periodicals and the transactions of engineering societies. The theory and practice of these processes are not yet by any means well understood, and a year or two hence we shall doubtless possess much more knowledge concerning them than we have now. Prompt information respecting such new developments is, however, more desirable than delay with a view to saying the last word on the subject, which never can be said by any of us, even if we should wait to the end of the lifetime. For this reason it has appeared useful to collect and republish in convenient form the articles of this character which have appeared during the last few years.
W. R. Ingalls.
August 1, 1906.
| PART I | |
| Notes on Lead Mining | |
| PAGE | |
| Sources of Lead Production in the United States (Walter Renton Ingalls) | 3 |
| Notes on the Source of the Southeast Missouri Lead (H. A. Wheeler) | 10 |
| Mining in Southeastern Missouri (Walter Renton Ingalls) | 16 |
| Lead Mining in Southeastern Missouri (R. D. O. Johnson) | 18 |
| The Lead Ores of Southwestern Missouri (C. V. Petraeus and W. Geo. Waring) | 24 |
| PART II | |
| Roast-Reaction Smelting | |
| SCOTCH HEARTHS AND REVERBERATORY FURNACES | |
| Lead Smelting in the Scotch Hearth (Kenneth W. M. Middleton) | 31 |
| The Federal Smelting Works, near Alton, Ill. (O. Pufahl) | 38 |
| Lead Smelting at Tarnowitz (Editorial) | 41 |
| Lead Smelting in Reverberatory Furnaces at Desloge, Mo. (Walter Renton Ingalls) | 42 |
| PART III | |
| Sintering and Briquetting | |
| The Desulphurization of Slimes by Heap Roasting at Broken Hill (E. J. Horwood) | 51 |
| The Preparation of Fine Material for Smelting (T. J. Greenway) | 59 |
| The Briquetting of Minerals (Robert Schorr) | 63 |
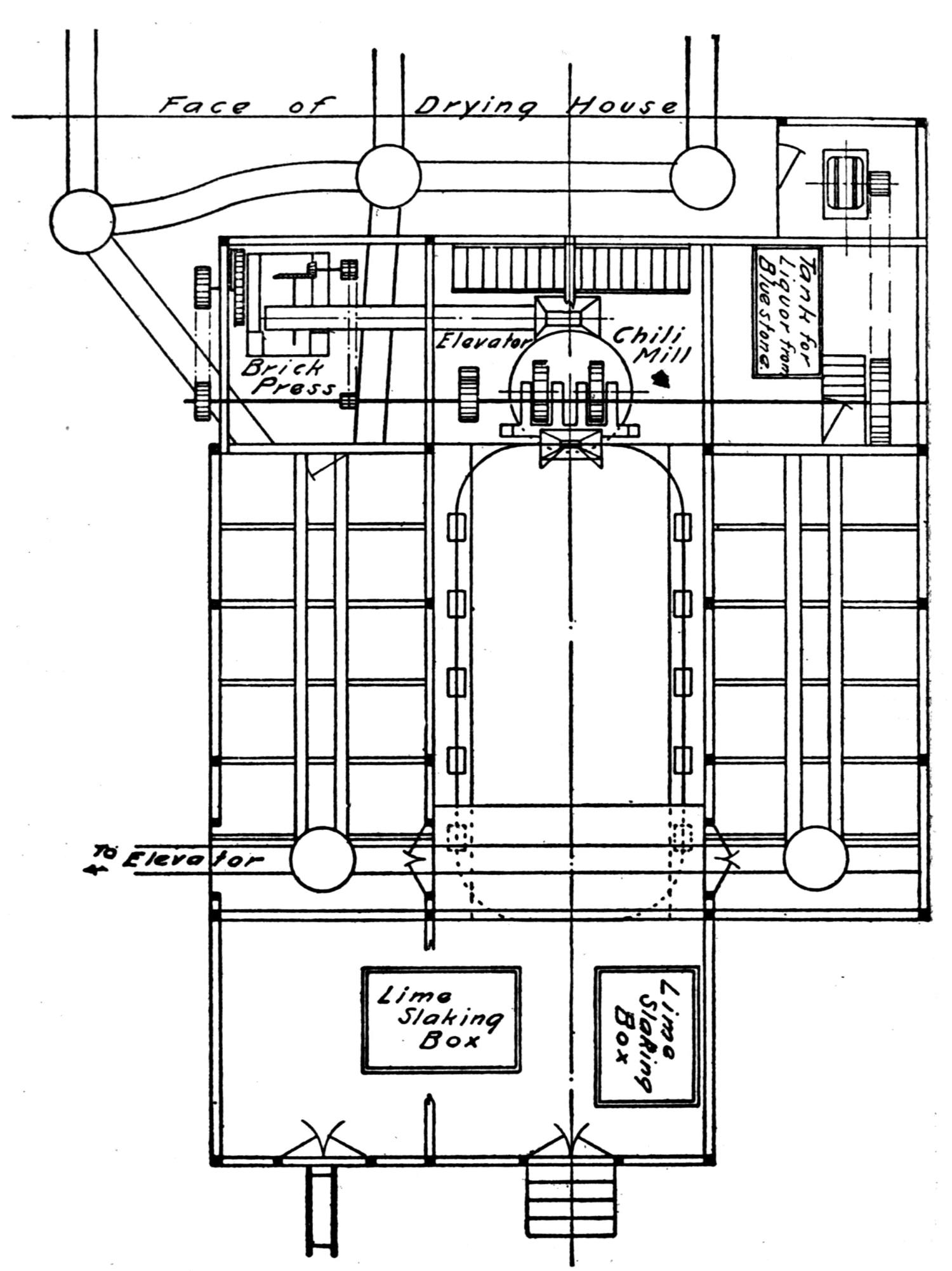
| A Bricking Plant for Flue Dust and Fine Ores (Jas. C. Bennett) | 66 |
| PART IV | |
| Smelting in the Blast Furnace | |
| Modern Silver-Lead Smelting (Arthur S. Dwight) | 73 |
| Mechanical Feeding of Silver-Lead Blast Furnaces (Arthur S. Dwight) | 81 |
| Cost of Smelting and Refining (Malvern W. Iles) | 96 |
| Smelting Zinc Retort Residues (E. M. Johnson) | 104 |
| Zinc Oxide in Slags (W. Maynard Hutchings) | 108 |
| PART V | |
| Lime-Roasting of Galena | |
| The Huntington-Heberlein Process | 113 |
| Lime-Roasting of Galena (Editorial) | 114 |
| The New Methods of Desulphurizing Galena (W. Borchers) | 116 |
| Lime-Roasting of Galena (W. Maynard Hutchings) | 126 |
| Theoretical Aspects of Lead-Ore Roasting (C. Guillemain) | 133 |
| Metallurgical Behavior of Lead Sulphide and Calcium Sulphate (F. O. Doeltz) | 139 |
| The Huntington-Heberlein Process (Donald Clark) | 144 |
| The Huntington-Heberlein Process at Friedrichshütte (A. Biernbaum) | 148 |
| The Huntington-Heberlein Process from the Hygienic Standpoint (A. Biernbaum) | 160 |
| The Huntington-Heberlein Process (Thomas Huntington and Ferdinand Heberlein) | 167 |
| Making Sulphuric Acid at Broken Hill (Editorial) | 174 |
| The Carmichael-Bradford Process (Donald Clark) | 175 |
| The Carmichael-Bradford Process (Walter Renton Ingalls) | 177 |
| The Savelsberg Process (Walter Renton Ingalls) | 186 |
| Lime-Roasting of Galena (Walter Renton Ingalls) | 193 |
| PART VI | |
| Other Methods of Smelting | |
| The Bormettes Method of Lead and Copper Smelting (Alfredo Lotti) | 215 |
| The Germot Process (Walter Renton Ingalls) | 224 |
| PART VII | |
| Dust and Fume Recovery | |
| FLUES, CHAMBERS AND BAG-HOUSES | |
| Dust Chamber Design (Max J. Welch) | 229 |
| Concrete in Metallurgical Construction (Henry W. Edwards) | 234 |
| Concrete Flues (Edwin H. Messiter) | 240 |
| Concrete Flues (Francis T. Havard) | 242 |
| Bag-houses for Saving Fume (Walter Renton Ingalls) | 244 |
| PART VIII | |
| Blowers and Blowing Engines | |
| Rotary Blowers vs. Blowing Engines for Lead Smelting (Editorial) | 251 |
| Rotary Blowers vs. Blowing Engines (J. Parke Channing) | 254 |
| Blowers and Blowing Engines for Lead and Copper Smelting | |
| (Hiram W. Hixon) | 256 |
| Blowing Engines and Rotary Blowers (S. E. Bretherton) | 258 |
| PART IX | |
| Lead Refining | |
| The Refining of Lead Bullion (F. L. Piddington) | 263 |
| The Electrolytic Refining of Base Lead Bullion (Titus Ulke) | 270 |
| Electrolytic Lead Refining (Anson G. Betts) | 274 |
| PART X | |
| Smelting Works and Refineries | |
| The New Smelter at El Paso, Texas (Editorial) | 285 |
| New Plant of the American Smelting and Refining Company at Murray, Utah (Walter Renton Ingalls) | 287 |
| The Murray Smelter, Utah (O. Pufahl) | 291 |
| The Pueblo Lead Smelters (O. Pufahl) | 294 |
| The Perth Amboy Plant of the American Smelting and Refining Company (O. Pufahl) | 296 |
| The National Plant of the American Smelting and Refining Company (O. Pufahl) | 299 |
| The East Helena Plant of the American Smelting and Refining Company (O. Pufahl) | 302 |
| The Globe Plant of the American Smelting and Refining Company (O. Pufahl) | 304 |
| Lead Smelting in Spain (Hjalmar Eriksson) | 306 |
| Lead Smelting at Monteponi, Sardinia (Erminio Ferraris) | 311 |
(November 28, 1903)
Statistics of lead production are of value in two directions: (1) in showing the relative proportion of the kinds of lead produced; and (2) in showing the sources from which produced. Lead is marketed in three principal forms: (a) desilverized; (b) soft; (c) antimonial, or hard. The terms to distinguish between classes “a” and “b” are inexact, because, of course, desilverized lead is soft lead. Desilverized lead itself is classified as “corroding,” which is the highest grade, and ordinary “desilverized.” Soft lead, referring to the Missouri product, may be either “ordinary” or “chemical hard.” The latter is such lead as contains a small percentage of copper and antimony as impurities, which, without making it really hard, increase its resistance against the action of acids, and therefore render it especially suitable for the production of sheet to be used in sulphuric-acid chamber construction and like purposes. The production of chemical hard lead is a fortuitous matter, depending on the presence of the valuable impurities in the virgin ores. If present, these impurities go into the lead, and cannot be completely removed by the simple process of refining which is practised. Nobody knows just what proportions of copper and antimony are required to impart the desired property, and consequently no specifications are made. Some chemical engineers call for a particular brand, but this is really only a whim, since the same brand will not be uniformly the same; practically one brand is as good as another. Corroding lead is the very pure metal, which is suitable for white lead manufacture. It may be made either from desilverized or from the ordinary Missouri product; or the latter, if especially pure, may be classed as corroding without further refining. Antimonial lead is really an alloy of lead with about 15 to 30 per cent. antimony, which is produced as a by-product by the desilverizers of 4 base bullion. The antimony content is variable, it being possible for the smelter to run the percentage up to 60. Formerly it was the general custom to make antimonial lead with a content of 10 to 12 per cent. Sb; later, with 18 to 20 per cent.; while now 25 to 30 per cent. Sb is best suited to the market.
The relative values of the various grades of lead fluctuate considerably, according to the market place, and the demand and supply. The schedules of the American Smelting and Refining Company make a regular differential of 10c. per 100 lb. between corroding lead and desilverized lead in all markets. In the St. Louis market, desilverized lead used to command a premium of 5c. to 10c. per 100 lb. over ordinary Missouri; but now they sell on approximately equal terms. Chemical hard lead sells sometimes at a higher price, sometimes at a lower price, than ordinary Missouri lead, according to the demand and supply. There is no regular differential. This is also the case with antimonial lead.[1]
The total production of lead from ores mined in the United States in 1901 was 279,922 short tons, of which 211,368 tons were desilverized, 57,898 soft (meaning lead from Missouri and adjacent States) and 10,656 antimonial. These are the statistics of “The Mineral Industry.” The United States Geological Survey reported substantially the same quantities. In 1902 the production was 199,615 tons of desilverized, 70,424 tons of soft, and 10,485 tons of antimonial, a total of 280,524 tons. There is an annual production of 4000 to 5000 tons of white lead direct from ore at Joplin, Mo., which increases the total lead production of the United States by, say, 3500 tons per annum. The production of lead reported as “soft” does not represent the full output of Missouri and adjacent States, because a good deal of their ore, itself non-argentiferous, except to the extent of about 1 oz. per ton in certain districts, is smelted with silver-bearing ores, going thus into an argentiferous lead; while in one case, at least, the almost non-argentiferous lead, obtained by smelting the ore unmixed, is desilverized for the sake of the extra refining.
Lead-bearing ores are of widespread occurrence in the United States. Throughout the Rocky Mountains there are numerous districts in which the ore carries more or less lead in connection 5 with gold and silver. For this reason, the lead mining industry is not commonly thought of as having such a concentrated character as copper mining and zinc mining. It is the fact, however, that upward of 70 per cent. of the lead produced in the United States is derived from five districts, and in the three leading districts from a comparatively small number of mines. The statistics of these for 1901 to 1904 are as follows:[2]
| Production, Tons | Per cent. | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| District | 1901 | 1902 | 1903 | 1904 | 1901 | 1902 | 1903 | 1904 | Ref. |
| Cœur d’Alene | 68,953 | 74,739 | 89,880 | 98,240 | 24.3 | 26.3 | 32.5 | 32.5 | a |
| Southeast Mo. | 46,657 | 56,550 | 59,660 | 59,104 | 16.4 | 19.9 | 21.2 | 19.6 | b |
| Leadville, Colo. | 28,180 | 19,725 | 18,177 | 23,590 | 10.0 | 6.9 | 6.6 | 7.8 | c |
| Park City, Utah | 28,310 | 36,300 | 36,534 | 30,192 | 10.0 | 12.8 | 13.2 | 10.0 | d |
| Joplin, Mo.-Kan. | 24,500 | 22,130 | 20,000 | 23,600 | 8.6 | 7.8 | 7.2 | 7.8 | i>e |
| Total | 196,600 | 209,444 | 224,251 | 234,726 | 69.3 | 73.7 | 81.0 | 77.7 | |
a. The production in 1901 and 1902 is computed from direct returns from the mines, with an allowance of 6 per cent. for loss of lead in smelting. The production in 1903 and 1904 is estimated at 95 per cent. of the total lead product of Idaho.
b. This figure includes only the output of the mines at Bonne Terre, Flat River, Doe Run, Mine la Motte and Fredericktown. It is computed from the report of the State Lead and Zinc Mine Inspector as to ore produced, the ore (concentrates) of the mines at Bonne Terre, Flat River and Doe Run being reckoned as yielding 60 per cent. lead.
c. Report of State Commissioner of Mines.
d. Report of Director of the Mint on “Production of Gold and Silver in the United States,” with allowance of 6 per cent. for loss of lead in smelting.
e. From statistics reported by “The Mineral Industry,” reckoning the ore (concentrates) as yielding 70 per cent. lead.
Outside of these five districts, the most of the lead produced in the United States is derived from other camps in Idaho, Colorado, Missouri and Utah, the combined output of all other States being insignificant. It is interesting to examine the conditions under which lead is produced in the five principal districts.
Leadville, Colo.—The mines of Leadville, which once were the largest lead producers of the United States, became comparatively unimportant after the exhaustion of the deposits of carbonate ore, but have attained a new importance since the successful 6 introduction of means for separating the mixed sulphide ore, which occurs there in very large bodies. The lead production of Leadville in 1897 was 11,850 tons; 17,973 tons in 1898; 24,299 tons in 1899; 31,300 tons in 1900; 28,180 tons in 1901, and 19,725 tons in 1902. The Leadville mixed sulphide ore assays about 8 per cent. Pb, 25 per cent. Zn and 10 oz. silver per ton. It is separated into a zinc product assaying about 38 per cent. Zn and 6 per cent. Pb, and a galena product assaying about 45 per cent. Pb, 10 or 12 per cent. Zn, and 10 oz. silver per ton.
Cœur d’Alene.—The mines of this district are opened on fissure veins of great extent. The ore is of low grade and requires concentration. As mined, it contains about 10 per cent. lead and a variable proportion of silver. It is marketed as mineral, averaging about 50 per cent. Pb and 30 oz. silver per ton. The production of lead ore in this district is carried on under the disadvantages of remoteness from the principal markets for pig lead, high-priced labor, and comparatively expensive supplies. It enjoys the advantages of large orebodies of comparatively high grade in lead, and an important silver content, and in many cases cheap water power, and the ability to work the mines through adit levels. The cost of mining and milling a ton of crude ore is $2.50 to $3.50. The mills are situated, generally, at some distance from the mines, the ore being transported by railway at a cost of 8 to 20c. per ton. The dressing is done in large mills at a cost of 40 to 50c. per ton. About 75 per cent. of the lead of the ore is recovered. The concentrates are sold at 90 per cent. of their lead contents and 95 per cent. of their silver contents, less a smelting charge of $8 per ton, and a freight rate of $8 per ton on ore of less than $50 value per ton, $10 on ore worth $50 to $65, and $12 on ore worth more than $65; the ore values being computed f. o. b. mines. The settling price of lead is the arbitrary one made by the American Smelting and Refining Company. With lead (in ore) at 3.5c. and silver at 50c., the value, f. o. b. mines, of a ton of ore containing 50 per cent. Pb and 30 oz. silver is approximately as follows:
| 1000 × 0.90 = 900 lb. lead, at 3.5c. | $31.50 |
| 30 × 0.95 = 28.5 oz. silver, at 50c. | 14.25 |
| Gross value, f. o. b. mines | $45.75 |
| Less freight, $10, and smelting charge, $8 | 18.00 |
| Net value, f. o. b. mines | $27.75 |
Assuming an average of 6 tons of crude ore to 1 ton of concentrate, the value per ton of crude ore would be about $4.62½, and the net profit per ton about $1.62½, which figures are increased 23.75c. for each 5c. rise in the value of silver above 50c. per ounce.
The production of the Cœur d’Alene since 1895, as reported by the mines, has been as follows:
| Year | Lead, Tons | Silver, oz. | Ratio [3] |
|---|---|---|---|
| 1896 | 37,250 | 2,500,000 | 67.1 |
| 1897 | 57,777 | 3,579,424 | 61.9 |
| 1898 | 56,339 | 3,399,524 | 60.3 |
| 1899 | 50,006 | 2,736,872 | 54.7 |
| 1900 | 81,535 | 4,755,877 | 58.3 |
| 1901 | 68,953 | 3,349,533 | 48.5 |
| 1902 | 74,739 | 4,489,549 | 60.0 |
| 1903 | [4]100,355 | 5,751,613 | 57.3 |
| 1904 | [4]108,954 | 6,247,795 | 57.4 |
The number of producers in the Cœur d’Alene district is comparatively small, and many of them have recently consolidated, under the name of the Federal Mining and Smelting Company. Outside of that concern are the Bunker Hill & Sullivan, the Morning and the Hercules mines, control of which has lately been secured by the American Smelting and Refining Company.
Southeastern Missouri.—The most of the lead produced in this region comes from what is called the disseminated district, comprising the mines of Bonne Terre, Flat River, Doe Run, Mine la Motte and Fredericktown, of which those of Bonne Terre and Flat River are the most important. The ore of this region is a magnesian limestone impregnated with galena. The deposits lie nearly flat and are very large. They yield about 5 per cent. of mineral, which assays about 65 per cent. lead. The low grade of the ore is the only disadvantage which this district has, but this is so much more than offset by the numerous advantages, that mining is conducted very profitably, and it is an open question whether lead can be produced more cheaply here or in the Cœur d’Alene. The mines of southeastern Missouri are only 60 to 100 miles 8 distant from St. Louis, and are in close proximity to the coalfields of southern Illinois, which afford cheap fuel. The ore lies at depths of only 100 to 500 ft. below the surface. The ground stands admirably, without any timbering. Labor and supplies are comparatively cheap. Mining and milling can be done for $1.05 to $1.25 per ton of crude ore, when conducted on the large scale that is common in this district. Most of the mining companies are equipped to smelt their own ore, the smelters being either at the mines or near St. Louis. The freight rate on concentrates to St. Louis is $1.40 per ton; on pig lead it is $2.10 per ton. The total cost of producing pig lead, delivered at St. Louis, is about 2.25c. per pound, not allowing for interest on the investment, amortization, etc.
The production of the mines in the disseminated district in 1901 was equivalent to 46,657 tons of pig lead; in 1902 it was 56,550 tons. The milling capacity of the district is about 6000 tons per day, which corresponds to a capacity for the production of about 57,000 tons of pig lead per annum. The St. Joseph Lead Company is building a new 1000 ton mill, and the St. Louis Smelting and Refining Company (National Lead Company) is further increasing its output, which improvements will increase the daily milling capacity by about 1400 tons, and will enable the district to put out upward of 66,000 tons of pig lead. In this district, as in the Cœur d’Alene, the industry is closely concentrated, there being only nine producers, all told.
Park City, Utah.—Nearly all the lead produced by this camp comes from the Silver King, Daly West, Ontario, Quincy, Anchor and Daly mines, which have large veins of low-grade ore carrying argentiferous galena and blende, a galena product being obtained by dressing, and zinkiferous tailings, which are accumulated for further treatment as zinc ore, when market conditions justify.[5]
Joplin District.—The lead production of southwestern Missouri and southeastern Kansas, in what is known as the Joplin district, is derived entirely as a by-product in dressing the zinc ore of that district. It is obtained as a product assaying about 77 per cent. Pb, and is the highest grade of lead ore produced, in large quantity, anywhere in the United States. It is smelted partly for the production of pig lead, and partly for the direct 9 manufacture of white lead. The lead ore production of the district was 31,294 tons in 1895, 26,927 tons in 1896, 29,578 tons in 1897, 26,457 tons in 1898, 24,100 tons in 1899, 28,500 tons in 1900, 35,000 tons in 1901, and 31,615 tons in 1902. The production of lead ore in this district varies more or less, according to the production of zinc ore, and is not likely to increase materially over the figure attained in 1901.
(March 31, 1904)
The source of the lead that is being mined in large quantities in southeastern Missouri has been a mooted question. Nor is the origin of the lead a purely theoretical question, as it has an important bearing on the possible extension of the orebodies into the underlying sandstone.
The disseminated lead ores of Missouri occur in a shaly, magnesian limestone of Cambrian age in St. François, Madison and Washington counties, from 60 to 130 miles south of St. Louis. The limestone is known as the Bonne Terre, or lower half of “the third magnesian limestone” of the Missouri Geological Survey, and rests on a sandstone, known as “the third sandstone,” that is the base of the sedimentary formations in the area. Under this sandstone occur the crystalline porphyries and granites of Algonkian and Archean age, which outcrop as knobs and islands of limited extent amid the unaltered Cambrian and Lower Silurian sediments.
The lead occurs as irregular granules of galena scattered through the limestone in essentially horizontal bodies that vary from 5 to 100 ft. in thickness, from 25 to 500 ft. in width, and have exceeded 9000 ft. in length. There is no vein structure, no crushing or brecciation of the inclosing rock, yet these orebodies have well defined axes or courses, and remarkable reliability and persistency. It is true that the limestone is usually darker, more porous, and more apt to have thin seams of very dark (organic) shales where it is ore-bearing than in the surrounding barren ground. The orebodies, however, fade out gradually, with no sharp line between the pay-rock and the non-paying, and the lead is rarely, if ever, entirely absent in any extent of the limestone of the region. While the main course of the orebodies seems to be intimately connected with the axes of the 11 gentle anticlinal folds, numerous cross-runs of ore that are associated with slight faults are almost as important as the main shoots, and have been followed for 5000 ft. in length. These cross-runs are sometimes richer than the main runs, at least near the intersections, but they are narrower, and partake more of the type of vertical shoots, as distinguished from the horizontal sheet-form.
Most of the orebodies occur at, or close to, the base of the limestone, and frequently in the transition rock between the underlying sandstone and the limestone, though some notable and important bodies have been found from 100 to 200 ft. above the sandstone. This makes the working depth from the surface vary from 150 to 250 ft., for the upper orebodies, to 300 to 500 ft. deep to the main or basal orebodies, according as erosion has removed the ore-bearing limestone. The thickness of the latter ranges from 400 to 500 ft.
Associated with the galena are less amounts of pyrite, which especially fringes the orebodies, and very small quantities of chalcopyrite, zinc blende, and siegenite (the double sulphide of nickel and cobalt). Calcite also occurs, especially where recent leaching has opened vugs, caves, or channels in the limestone, when secondary enrichment frequently incrusts these openings with crystals of calcite and galena. No barite ever occurs with the disseminated ore, though it is the principal gangue mineral in the upper or Potosi member of the third magnesian limestone, and is never absent in the small ore occurrences in the still higher second magnesian limestone.
While the average tenor of the ore is low, the yield being from 3 to 4 per cent. in pig lead, they are so persistent and easy to mine that the district today is producing about 70,000 tons of pig lead annually, and at a very satisfactory profit. As the output was about 2500 tons lead in 1873, approximately 8500 tons in 1883, and about 20,000 tons in 1893, it shows that this district is young, for the principal growth has been within the last five years.
Of the numerous but much smaller occurrences of lead elsewhere in Missouri and the Mississippi valley, none resembles this district in character, a fact which is unique. For while the Mechernich lead deposits, in Germany, are disseminated, and of even lower grade than in Missouri, they occur in a sandstone, 12 and (like all the lead deposits outside of the Mississippi valley) they are argentiferous, at least to an extent sufficient to make the extraction of the silver profitable; and on the non-argentiferous character of the disseminated deposits hangs my story.
Of the numerous hypotheses advanced to account for the origin of these deposits, there are only two that seem worthy of consideration: (a) the lateral secretion theory, and (b) deposition from solutions of deep-seated origin. Other theories evolved in the pioneer period of economic geology are interesting, chiefly by reason of the difficulties under which the early strugglers after geological knowledge blazed the pathway for modern research and observation.
The lateral secretion theory, as now modernized into the secondary enrichment hypothesis, has much merit when applied to the southeastern and central Missouri lead deposits. For the limestones throughout Missouri—and they are the outcropping formation over more than half of the State—are rarely, if ever, devoid of at least slight amounts of lead and zinc, although they range in age from the Carboniferous down to the Cambrian.
The sub-Carboniferous formation is almost entirely made up of limestones, which aggregate 1200 to 1500 ft. in thickness. They frequently contain enough lead (and less often zinc) to arouse the hopes of the farmer, and more or less prospecting has been carried on from Hannibal to St. Louis, or 125 miles along the Mississippi front, and west to the central part of the State, but with most discouraging results.
In the rock quarries of St. Louis, immediately under the lower coal measures, fine specimens of millerite of world-wide reputation occur as filiform linings of vugs in this sub-Carboniferous limestone. These vugs occur in a solid, unaltered rock which gives no clue to the existence of the vug or cavity until it is accidentally broken. The vugs are lined with crystals of pink dolomite, calcite and millerite, with occasionally barite, selenite, galena and blende. They occur in a well-defined horizon about 5 ft. thick, and the vugs in the limestone above and below this millerite bed contain only calcite, or less frequently dolomite. Yet this sub-Carboniferous formation in southwestern Missouri, about Joplin, carries the innumerable pockets and sheets of lead and zinc that have made that district the most important zinc producer in the world. While faulting and limited folding occur 13 in eastern and central Missouri to fully as great an extent as in St. François county or the Joplin district, thus far no mineral concentration into workable orebodies has been found in this formation, except in the Joplin area.
The next important series of limestones that make up most of the central portion of Missouri are of Silurian age, and in them lead and zinc are liberally scattered over large areas. In the residual surface clays left by dissolution of the limestone, the farmers frequently make low wages by gophering after the liberated lead, and the aggregate of these numerous though insignificant gopher-holes makes quite a respectable total. But they are only worked when there is nothing else to do on the farm, as with rare exceptions they do not yield living wages, and the financial results of mining the rock are even less satisfactory. Yet a few small orebodies have been found that were undoubtedly formed by local leaching and re-precipitation of this diffused lead and zinc. Such orebodies occur in openings or caves, with well crystallized forms of galena and blende, and invariably associated with crystallized “tiff” or barite. I am not aware of any of these pockets or secondary enrichments having produced as much as 2000 tons of lead or zinc, and very few have produced as much as 500 tons, although one of these pockets was recently exploited with heroic quantities of printer’s ink as the largest lead mine in the world. Yet there are large areas in which it is almost impossible to put down a drill-hole without finding “shines” or trifling amounts of lead or zinc. That these central Missouri lead deposits are due to lateral secretion there seems little doubt, and it is possible that larger pockets may yet be found where more favorable conditions occur.
When the lateral secretion theory is applied to the disseminated deposits of southeastern Missouri, we are confronted by enormous bodies of ore, absence of barite, non-crystallized condition of the galena except in local, small, evidently secondary deposits, and well-defined courses for the main and cross-runs of ore. The Bonne Terre orebody, which has been worked longest and most energetically, has attained a length of nearly 9000 ft., with a production of about 350,000 tons or $30,000,000 of lead, and is far from being exhausted. Orebodies recently opened are quite as promising. The country rock is not as broken nor as open as in central Missouri, and is therefore much less favorable 14 for the lateral circulation of mineral waters, yet the orebodies vastly exceed those of the central region.
Further, the Bonne Terre formation is heavily intercalated with thick sheets of shale that would hinder overlying waters from reaching the base of the ore-horizon, where most of the ore occurs, so that the leachable area would be confined to a very limited vertical range, or to but little greater thickness than the 100 ft. or so in which most of the orebodies occur. While I have always felt that such large bodies, showing relatively rapid precipitation of the lead, could not be satisfactorily explained except as having a deep-seated origin, the fact that the disseminated ore is practically non-argentiferous, or at least carries only one to three ounces per ton, has been a formidable obstacle. For the lead in the small fissure-veins that occasionally occur in the adjacent granite has always been reported as argentiferous. Thus the Einstein silver mine, near Fredericktown, worked a fissure-vein from 1 to 6 ft. wide in the granite. It had a typical complex vein-filling and structure, and carried galena that assayed from 40 to 200 oz. per ton. While the quantity of ore obtained did not justify the expensive plant erected to operate it, the galena was rich in silver, whereas in the disseminated ores at the Mine la Motte mine, ten miles distant, only the customary 1.5 oz. per ton occurs. Occasionally fine-grained specimens of galena that I have found in the disseminated belt would unquestionably be rated as argentiferous by a Western miner, but the assay showed that the structure in this case was due to other causes, as only about two ounces were found. An apparent exception was reported at the Peach Orchard diggings, in Washington county, in the higher or Potosi member of the third magnesian limestone, where Arthur Thacher found sulphide and carbonate ore carrying 8 to 10 oz. of silver per ton; and a short-lived hamlet, known as Silver City, sprang up to work them. I found, however, that these deposits are associated with little vertical fissure-veins or seams that unquestionably come up from the underlying porphyry.
Recently I examined the Jackson Revel mine, which has been considered a silver mine for the last fifty years. It lies about seven miles south of Fredericktown, and is a fissure-vein in Algonkian felsite, where it protrudes, as a low hill, through the disseminated limestone formation. A shaft has just been sunk 15 about 150 ft. at less than 1000 ft. from the feather edge of the limestone. The vein is narrow, only one to twelve inches wide, with slicken-sided walls, runs about N. 20 deg. E., and dips 80 to 86 deg. eastward. White quartz forms the principal part of the filling; the vein contains more or less galena and zinc blende. Assays of the clean galena made by Prof. W. B. Potter show only 2.5 oz. silver per ton, or no more than is frequently found in the disseminated lead ores. As the lead in this fissure vein may be regarded as of undoubted deep origin, and it is practically non-argentiferous, this would seem to remove the last objection to the theory of the deep-seated source of the lead in the disseminated deposits of southeast Missouri.
(February 18, 1904)
The St. Joseph Lead Company, in the operation of its mines at Bonne Terre, does not permit the cages employed for hoisting purposes to be used for access to the mine. Men going to and from their work must climb the ladders. This rule does not obtain in the other mines of the district. The St. Joseph Lead Company employs electric haulage for the transport of ore underground at Bonne Terre. In the other mines of the district, mules are generally used. The flow of water in the mines of the district is extremely variable; some have very little; others have a good deal. The Central mine is one of the wettest in the entire district, making about 2000 gal. of water per minute. Coal in southeastern Missouri costs $2 to $2.25 per ton delivered at the mines, and the cost of raising 2000 gal. of water per minute from a depth of something like 350 ft. is a very considerable item in the cost of mining and milling, which, in the aggregate, is expected to come to not much over $1.25 per ton.
The ore shoots in the district are unusually large. Their precise trend has not been identified. Some consider the predominance of trend to be northeast; others, northwest. They go both ways, and appear to make the greatest depositions of ore at their intersections. However, the network of shoots, if that be the actual occurrence, is laid out on a very grand scale. Vertically there is also a difference. Some shafts penetrate only one stratum of ore; others, two or three. The orebody may be only a few feet in thickness; it may be 100 ft. or more. The occurrence of several overlying orebodies obviously indicates the mineralization of different strata of limestone, while in the very thick orebodies the whole zone has apparently been mineralized.
The grade of the ore is extremely variable. It may be only 1 or 2 per cent. mineral, or it may be 15 per cent. or more. How 17ever, the average yield for the district, in large mines which mill 500 to 1200 tons of ore per day, is probably about 5 per cent. of mineral, assaying 65 per cent. Pb, which would correspond to a yield of 3.25 per cent. metallic lead in the form of concentrate. The actual recovery in the dressing works is probably about 75 per cent., which would indicate a tenure of about 4.33 per cent. lead in the crude ore.
(September 16, 1905)
The lead deposits of southeastern Missouri carry galena disseminated in certain strata of magnesian limestone. Their greater dimensions are generally horizontal, but with outlines extremely irregular. The large orebodies consist usually of a series of smaller bodies disposed parallel to one another. These smaller members may coalesce, but are generally separated from one another by a varying thickness of lean ore or barren rock. The vertical and lateral dimensions of an orebody may be determined with a fair degree of accuracy by diamond drilling, and a map may be constructed from the information so obtained. Such a map (on which are plotted the surface contours) makes it possible to determine closely the proper location of the shaft, or shafts, considering also the surface and underground drainage and tramming.
The first shafts in the district were sunk at Bonne Terre, where the deposits lie comparatively near the surface. The early practice at this point was to sink a number of small one-compartment shafts. As the deposits were followed deeper, this gave way to the practice of putting down two-compartment shafts equipped much more completely than were the shallower shafts.
At Flat River (where the deposits lie at much greater depths, some being over 500 ft.) the shafts are 7 × 14 ft., 6½ × 18 ft., and 7 × 20 ft. These larger dimensions give room not only for two cage-ways and a ladder-way, but also for a roomy pipe-compartment. The large quantities of water to be pumped in this part of the district make the care of the pipes in the shafts a matter of first importance. At Bonne Terre only such a quantity of water was encountered as could be handled by bailing or be taken out with the rock; there the only pipe necessary was a small air-pipe down one corner of the shaft. When the quantity 19 of water encountered is so great that the continued working of the mine depends upon its uninterrupted removal, the care of the pipes becomes a matter of great importance. A shaft which yields from 4000 to 5000 gal. of water per minute is equipped with two 12 in. column pipes and two 4 in. steam pipes covered and sheathed. Moreover, the pipe compartment will probably contain an 8 in. air-pipe, besides speaking-tubes, pipes for carrying electric wires, and pipes for conducting water from upper levels to the sump. To care for these properly there are required a separate compartment and plenty of room.
Shafts are sunk by using temporary head frames and iron buckets of from 8 to 14 cu. ft. capacity. Where the influx of water was small, 104 ft. have been sunk in 30 days, with three 8 hour shifts, two drills, and two men to each drill; 2¾ in. drills are used almost exclusively; 3¼ in. drills have been used in sinking, but without apparent increase in speed.
The influence of the quantity of water encountered upon the speed of sinking (and the consequent cost per foot) is so great that figures are of little value. Conditions are not at all uniform.
At some point (usually before 200 ft. is reached) a horizontal opening will be encountered. This opening invariably yields water, the amount following closely the surface precipitation. It is the practice to establish at this point a pumping station. The shaft is “ringed” and the water is directed into a sump on the side, from which it is pumped out. This sump receives also the discharge of the sinking pumps.
The shafts sunk in solid limestone require no timbering other than that necessary to support the guides, pipes, and ladder platforms. These timbers are 8 × 8 in. and 6 x 8 in., spaced 7 or 8 ft. apart.
Shafts are sunk to a depth of 10 ft. below the point determined upon as the lower cage landing. From the end at the bottom a narrow drift is driven horizontally to a distance of 15 ft.; at that point it is widened out to 10 ft. and driven 20 ft. further. The whole area (10 × 20 ft.) is then raised to a point 28 or 30 ft. above the bottom of the drift from the shaft. The lower part of this chamber constitutes the sump. Starting from this chamber (on one side and at a point 10 ft. above the cage landing, or 20 ft. above the bottom of the sump), the “pump-house” is cut out. This pump-house is cut 40 ft. long and is as wide as the 20 sump is long, namely, 20 ft. A narrow drift is driven to connect the top of the pump-house with the shaft. Through this drift the various pipes enter the pump-house from the shaft.
The pumps are thus placed at an elevation of 10 ft. above the bottom of the mine. Flooding of mines, due to failure of pumps or to striking underground bodies of water, taught the necessity of placing the pumps at such an elevation that they would be the last to be covered, thus giving time for getting or keeping them in operation. The pumps are placed on the solid rock, the air pumps and condensers at a lower level on timbers over the sump.
With this arrangement, the bottom of the shaft serves as an antechamber for the sump, in which is collected the washing from the mine and the dripping from the shaft. The sump proper rarely needs cleaning.
The pumps are generally of high-grade, compound-and triple-expansion, pot-valved, outside-packed plunger pattern. Plants with electrical power distribution have recently installed direct-connected compound centrifugal pumps with entire success.
Pumps of the Cornish pattern have never been used much in this region. One such pump has been installed, but the example has not been followed even by the company putting it in.
The irregular disposition of the ore renders any systematic plan of drifting or mining (as in coal or vein mining) impossible. On each side of the shaft and in a direction at right angles to its greater horizontal dimension, drifts 12 to 14 ft. in width are driven to a distance of 60 or 70 ft. In these broad drifts are located the tracks and also the “crossovers” for running the cars on and off the cage.
When a deposit is first opened up, it is usually worked on two, and sometimes three, levels. These eventually cut into one another, when the ore is hoisted from the lower level alone.
The determination of the depth of the lower level is a matter of compromise. Much good ore may be known to exist below; when it comes to mining, it will have to be taken out at greater expense; but the level is aimed to cut through the lower portions of the main body. It is generally safe to predict that the ore lying below the upper levels will eventually be mined from a lower level without the expense of local underground hoisting and pumping.
The ore has simply to be followed; no one can say in advance 21 how it is going to turn out. The irregularity of the deposits renders any general plan of mining of little or no value. Some managers endeavor to outline the deposits by working on the outskirts, leaving the interior as “ore reserves.” Such reserves have been found to be no reserves at all, though the quality of the rock may be fairly well determined by underground diamond drilling. Many of the deposits are too narrow to permit the employment of any system of outlining and at the same time keeping up the ore supply.
The individual bodies constituting the general orebody are rarely, if ever, completely separated by barren rock; some “stringers” or “leaders” of ore connect them. The careful superintendent keeps a record on the monthly mine map of all such occurrences, or otherwise, or of blank walls of barren rock that mark the edge of the deposit. This precaution finds abundant reward when the drills commence to “cut poor,” and when a search for ore is necessary.
The method of mining is to rise to the top of the ore and to carry forward a 6 ft. breast. If the ore is thick enough, this is followed by the underhand stope. Drill holes in the breast are usually 7 or 8 ft. in depth; stope holes, 10 to 14 feet.
Both the roof and the floor are drilled with 8 or 10 ft. holes placed 8 or 10 ft. apart. These serve to prospect the rock in the immediate neighborhood; in the roof they serve the further very important purpose of draining out water that might otherwise accumulate between the strata and that might force them to fall. The condition or safety of the roof is determined by striking with a hammer. If the sound is hollow or “drummy,” the roof is unsafe. If water is allowed to accumulate between the loose strata, obviously it is not possible to determine the condition of the roof.
It is the duty of two men on each shift to keep the mine in a safe condition by taking down all loose and dangerous masses of rock. These men are known as “miners.” It sometimes happens that a considerable area of the roof gets into such a dangerous condition that it is either too risky or too expensive to put in order, in which case the space underneath is fenced off. As a general thing, the mines are safe and are kept so. There are but few accidents of a serious nature due to falling rock.
The roof is supported entirely by pillars; no timbering what 22ever is used. The pillars are parts of the orebody or rock that is left. They are of all varieties of size and shape. They are usually circular in cross-section, 10 to 15 ft. in diameter and spaced 20 to 35 ft. apart, depending upon the character of the roof. Pillars generally flare at the top to give as much support to the roof as possible. The hight of the pillars corresponds, of course, to the thickness of the orebody.
All drilling is done by 2¾ in. percussion drills. In the early days, when diamonds were worth $6 per carat, underground diamond drills were used. Diamond drills are used now occasionally for putting in long horizontal holes for shooting down “drummy” roof. Air pressure varies from 60 to 80 lb. Pressures of 100 lb. and more have been used, but the repairs on the drills became so great that the advantages of the higher pressure were neutralized.
Each drill is operated by two men, designated as “drillers,” or “front hand” and “back hand.” The average amount of drilling per shift of 10 hours is in the neighborhood of 40 ft., though at one mine an average of 55 ft. was maintained.
In some of the mines the “drillers” and “back hands” do the loading and firing; in others that is done by “firers,” who do the blasting between shifts. When the drillers do the firing, there is employed a “powder monkey,” who makes up the “niphters,” or sticks of powder, in which are inserted and fastened the caps and fuse; 35 per cent. powder is used for general mining.
Battery firing is employed only in shaft sinking. In the mining work this is found to be much more expensive; the heavy concussions loosen the stratum of the roof and make it dangerous.
Large quantities of oil are used for lubrication and illumination. “Zero” black oil and oils of that grade are used on the drills. Miners’ oil is generally used for illumination, though some of the mines use a low grade of felsite wax.
Two oil cans (each holding about 1½ pints) are given to each pair of drillers, one can for black oil and one for miners’ oil. These cans, properly filled, are given out to the men, as they go on shift, at the “oil-house,” located near the shaft underground. This “oil-house” is in charge of the “oil boy,” whose duty it is to keep the cans clean, to fill them and to give them out at the beginning of the shift. The cans are returned to the oil-house at the end of the shift.
Kerosene is used in the hat-lamps in wet places.
The “oil-houses” are provided with three tanks. In some instances these tanks are charged through pipes coming down the shaft from the surface oil-house. These tanks are provided with oil-pumps and graduated gage-glasses.
Shovelers or loaders operate in gangs of 8 to 12, and are supervised by a “straw boss,” who is provided with a gallon can for illuminating oil. The cars are 20 cu. ft. (1 ton) capacity. Under ordinary conditions one shoveler will load 20 of these cars in a shift of 10 hours. They use “half-spring,” long-handled, round-pointed shovels.
Cars are of the solid-box pattern, and are dumped in cradles. Loose and “Anaconda” manganese-steel wheels are the most common. Gage of track, 24 to 30 in., 16 lb. rails on main lines and 12 lb. on the side and temporary tracks. Cars are drawn by mules. One mine has installed compressed-air locomotives and is operating them with success.
Shafts are generally equipped with geared hoists, both steam and electrically driven. Later hoists are all of the first-motion pattern.
Generally the cars are hoisted to the top and dumped with cradles. One shaft, however, is provided with a 5-ton skip, charged at the bottom from a bin, into which the underground cars are dumped. Upon arriving at the top the skip dumps automatically. This design exhibits a number of advantages over the older method and will probably find favor with other mine operators.
(October 21, 1905)
The lead ore of southwestern Missouri, and the adjoining area in the vicinity of Galena, Kan., is obtained as a by-product of zinc mining, the galena being separated from the blende in the jigging process. Formerly the galena (together with “dry-bone,” including cerussite and anglesite) was the principal ore mined from surface deposits in clay, the blende being the subsidiary product. In the deeper workings blende was found largely to predominate; this is shown by the shipments of the district in 1904, which amounted to 267,297 tons of zinc concentrate and 34,533 tons of lead concentrate.
The lead occurs in segregated cubes, from less than one millimeter up to one foot in diameter. The cleavage is perfect, so that each piece of ore when struck with a hammer breaks up into smaller perfect cubes. In this respect the ore differs from the galena encountered in the Rocky Mountain regions, where torsional or shearing strains seem in most instances to have destroyed the perfect cleavage of the minerals subsequent to their original deposition. Cases of schistose and twisted structure occur in lead deposits of the Joplin district but rarely, and they are always quite local.
The separation of the galena from the blende and marcasite (“mundic”) in the ordinary process of jigging is very complete; the percentage of zinc and iron in the lead concentrate is insignificant. As an illustration of this, the assays of 100 recent consecutive shipments of lead ore from the district, taken at random, are cited as follows:
Fourteen shipment samples, ranging from 70 to 84.4 per cent. lead, were tested for zinc and iron. These averaged 2.24 per cent. Fe and 1.78 per cent. Zn, the highest zinc content being 4.5 per cent. No bismuth or arsenic, and only very minute traces of antimony, have ever been found in these ores. They contain only about 0.0005 per cent. of silver (one-seventh of an ounce per ton) and scarcely more than that of copper (occurring as chalcopyrite).
The pig lead produced from these ores is therefore very pure, soft and uniform in quality, so that the term “soft Missouri lead” has become a synonym for excellence in the manufacture of lead alloys and products, such as litharge, red and white lead, and orange mineral. Its freedom from bismuth, which is generally present in Colorado lead, makes it particularly suitable for white lead; also for glass-maker’s litharge and red lead. These oxides, for use in making crystal glass, must be made by double refining so as to remove even the small quantities of silver and copper that are present. The resulting product, made from soft Missouri lead, is far superior to any refined lead produced anywhere in this country or in Europe, even excelling the famous Tarnowitz lead. It gives a luster and clarity to the glass that no other lead will produce. Lead from southeastern Missouri, Kentucky, Illinois, Iowa, and Wisconsin yields identical results, but the refining is more difficult, not only because the lead contains a little more silver and copper, but also because it contains more antimony.
The valuation of the lead concentrate produced in the Joplin district is based upon a wet assay, usually the molybdate or ferrocyanide method. The price paid is determined variously. One buyer pays a fixed price for average ore, making no deductions; as, for example, at present rates, $32.25 per 1000 lb. whether the ore assays 75 or 84 per cent. Pb, pig lead being worth $4.75 at St. Louis.[6] Another pays $32.25 for 80 per cent. ore, or over, deducting 50c. per unit for ores assaying under 80 per cent. Another pays for 90 per cent. of the lead content of the ore as shown by the assay, at the St. Louis price of pig lead, less a smelting charge of, say, $6 to $8 per ton of ore.
The history of the development of lead ore buying in the Joplin district is rather curious. In the early days of the district the ore was smelted wholly on Scotch hearths, which, with the 26 purest ores, would yield 70 per cent. metallic lead. No account was taken of the lead in the rich slag, chemical determinations being something unknown in the district at that time; it being supposed generally that pure galena contained 700 lb. lead to the 1000 lb. of ore, the value of 700 lb. lead, less $4.50 per 1000 lb. of ore for freight and smelting costs, was returned to the miner. The buyers graded the ore, according to their judgment, by its appearance, as to its purity and also as to its behavior in smelting; an ore, for example, from near the surface, imbedded in the clay and coated more or less with sulphate, yielded its metal more freely than the purer galenas from deeper workings.
This was the origin of the present method of buying—a system that would hardly be tolerated except for the fact that the lead is, as previously stated, considered a by-product of zinc mining.
Originally all the lead ore from the Missouri-Kansas district was smelted in the same region, either in the air furnace (reverberatory sweating-furnace) or in the water-back Scotch hearth. Competition gradually developed in the market. Lead refiners found the pure sulphide of special value in the production of oxidized products. Some of the ore found its way to St. Louis, and even as far away as Colorado, where it was used to collect silver. Since the formation of the American Smelting and Refining Company and the greatly increased output of the immense deposits of lead ore in Idaho, no Missouri lead ore has gone to Colorado.
Up to 1901, one concern had more or less the control of the southwestern Missouri ores. At the present time, lead ore is bought for smelters in Joplin, Carterville, and Granby, Mo., Galena, Kan., and Collinsville, Ill., and complaint is heard that present prices are really too high for the comfort of the smelters. Yet the old principle of paying for lead ores upon the supposed yield of 70 per cent., irrespective of the real lead content, is still largely in vogue.
Any one interested in the matter will find it quite instructive to calculate the returning charges, or gross profits, in smelting these ores, on the basis of 70 per cent. recovery, at $32.25 per 1000 lb. of ore, less 50c. per ton haulage, with lead at $4.77 per 100 lb. at St. Louis. No deduction, it should be remarked, is ever made for moisture in lead ores in this district. It is of 27 interest to observe that Dr. Isaac A. Hourwich estimates (in the U. S. Census Special Report on Mines and Quarries recently issued) the average lead contents of the soft lead ores of Missouri in 1902 at 68.2 per cent., taking as a basis the returns from five leading mining and smelting companies of Missouri, which reported a product of 70,491 tons of lead from 103,428 tons of their own and purchased ore. The average prices for lead ore in 1902 were reported as follows, per 1000 lb.: Illinois, $19.53; Iowa, $24.48; Kansas, $23.51; Missouri, $22.17; Wisconsin, $23.29; Rocky Mountain and Atlantic Coast States, $10.90. In 1903, according to Ingalls (“The Mineral Industry,” Vol. XII), the ore from the Joplin district commanded an average price of $53 per 2000 lb., while the average in the southeastern district was $46.81.
SCOTCH HEARTHS AND REVERBERATORY FURNACES
(July 6, 1905)
In view of the fact that the Scotch hearth in its improved form is now coming to the front again to some extent in lead smelting, it may prove interesting to give a brief account of its present use in the north of England.
Admitting that, where preliminary roasting is necessary, the best results can be obtained with the water-jacketed blast furnace (this being more especially the case where labor is an expensive item), we have still as an alternative the method of smelting raw in the Scotch hearth. At one works, which I recently visited, all the ore was smelted raw; at another, all the ore received a preliminary roast, and it is instructive to compare the results obtained in the two cases. The following data refer to a fairly “free-smelting” galena assaying nearly 80 per cent. of lead.
When smelting raw ore in the hearth, fully 7½ long tons can be treated in 24 hours, the amount of lead produced direct from the furnace in the first fire being 8400 to 9000 lb.; this is equivalent to 56 to 60 per cent. of lead, the remaining 24 to 20 per cent. going into the fume and the slag.
When smelting ore which has received a preliminary roast of two hours, 12,000 lb. of lead is produced direct from the hearth, this being equivalent to 65 per cent. of the ore. When the ore is roasted, the output of the hearth is practically the same for all ores of equal richness; but when smelting raw, if the galena is finely divided, the output may fall much below that given herewith; while, on the other hand, under the most favorable conditions it may rise to 12,000 lb. in 24 hours, or even more.
I had an opportunity of seeing a parcel of galena carrying 84 per cent. of lead (but broken down very fine) smelted raw. The ore was kept damp and the blast fairly low; but, in spite of that, a quantity of the ore was blown into the flue, and only 5100 lb. of lead was produced from the hearth in 24 hours.
Galena carrying only 65 per cent. of lead does not give nearly as satisfactory results when smelted raw in the hearth; barely six tons of ore can be smelted in 24 hours, and only 4500 to 5400 lb. of lead can be produced directly. This is equivalent to, say, 43 per cent. of the ore in the first fire; the remaining 22 per cent. goes into the slag or to the flue as fume. Moreover, the 65 per cent. ore requires 1500 lb. of coal in 24 hours, while the 80 per cent. galena uses only 1000 lb.
Turning now for a moment to the costs of smelting raw and of smelting after a preliminary roast, we find that (in the case of the two works we have been considering) the results are all in favor of smelting raw, so far as a galena carrying nearly 80 per cent. is concerned.
The cost of smelting, per ton of lead produced, is given herewith:
ORE SMELTED RAW
| Smelters’ wages | $2.04 |
| Smelters’ coal (425 lb.) | 0.38 |
| Total | $2.42 |
A very small quantity of lime is also used in this case for some ores, but its cost would never amount to more than 4c. per ton of lead produced.
ORE RECEIVING A PRELIMINARY ROAST
| Roasters’ wages | $0.61 |
| Roasters’ coal (425 lb.) | 0.65 |
| Smelters’ wages | 1.08 |
| Smelters’ coal (75 lb.) | 0.11 |
| Peat and lime | 0.08 |
| Total | $2.53 |
It should be noted also that the smelters at the works where the ore was not roasted receive higher pay. In the eight-hour shift they produce about 1½ tons of lead; and as there are two of them to a furnace, they make $3.06 between them, or $1.53 each. The two men smelting roasted ore produce about two tons in an eight-hour shift, and therefore each receives $1.08 per shift.
Coming now to fume-smelting in the hearth, we can again compare the results obtained in smelting raw and after roasting. It is well to bear in mind, also, that, while only 6½ per cent. of the lead goes in the fume when smelting roasted ores in the hearth, a 33 considerably larger proportion is thus lost when smelting raw ores. When fume is smelted raw, it is best dealt with when containing about 40 per cent. of moisture. One man attends to the hearth (instead of two as when smelting ore), and in 24 hours 3000 lb. of lead is produced, the amount of coal used being 2100 lb. No lime is required.
When smelting roasted fume, two men attend to the hearth and the output is 6000 lb. in 24 hours, the amount of coal used being 1800 lb. In this latter case fluorspar happens to be available (practically free of cost), and a little of it is used with advantage in fume-smelting, as well as a small quantity of lime.
The cost of fume-smelting per ton of lead produced is given herewith:
FUME SMELTED RAW
| Smelters’ wages | $2.88 |
| Smelters’ coal (1400 lb.) | 2.13 |
| Total | $5.01 |
FUME RECEIVING A PRELIMINARY ROAST
| Roasters’ wages | $2.08 |
| Roasters’ coal (1450 lb.) | 2.18 |
| Smelters’ wages | 2.04 |
| Smelters’ coal (600 lb.) | 0.92 |
| Peat and lime | 0.08 |
| Total | $7.30 |
In this case, as in that of ore, the smelter of the raw fume gets better pay; he has $1.44 per eight-hour shift, while the smelter of the roasted ore has only $1.02 per eight-hour shift.
Fume takes four hours to roast, as compared to the two hours taken by ore.
From these facts regarding Scotch-hearth smelting, it would seem that with galena carrying, say, over 70 per cent. lead (but more especially with ore up to 80 per cent. in lead, and, moreover, fairly free from impurities detrimental to “free” smelting), very satisfactory results can be obtained by smelting raw. Against this, however, it must be said that at the works where the ore is roasted attempts at smelting raw have been made several times without sufficient success to justify the adoption of this method, although the ores smelted average 75 per cent. lead and seem quite suitable for the purpose.
Probably this may be accounted for by the fact that the method of running the furnace when raw ore is being smelted is rather different from that adopted when dealing with roasted ore. Moreover, at the works under notice the furnaces are not of the most modern construction; and, as the old custom of dropping a peat in front of the blast every time the fire is made up still survives, it is necessary to shut off the blast while this is being done, and the fire is then apt to get rather slack.
The gray slag produced in the hearth is smelted in a small blast furnace, a little poor fume, and sometimes a small quantity of fluorspar, being added to facilitate the process. Some figures regarding slag-smelting may be of interest. The slag-smelters produce 9000 lb. of lead in 24 hours. The cost of slag-smelting per ton of lead produced is as follows:
| Smelters’ wages | $1.60 |
| Coke (1500 lb.) | 3.42 |
| Peat | 0.06 |
| Total | $5.08 |
Recent analyses of Weardale (Durham county) lead smelted in the Scotch hearth, and slag-lead smelted in the blast furnace, are given herewith:
| Fume-Lead from Hearth | Silver-Lead from Hearth | Slag-Lead from Blast Furnace | |
|---|---|---|---|
| Lead | 99.957 | 99.957 | 99.013 |
| Silver | 0.0035 | 0.0200 | 0.0142 |
| (1 oz. 2 dwt. 21 gr. per Long Ton) |
(6 oz. 10 dwt. 16 gr. per Long Ton) |
(4 oz. 12 dwt. 18 gr. per Long Ton) |
|
| Tin | nil | nil | nil |
| Antimony | nil | nil | 0.874 |
| Copper | nil | nil | 0.024 |
| Iron | 0.019 | 0.019 | 0.023 |
| Zinc | nil | nil | nil |
| 99.9795 | 99.9960 | 99.9482 |
The ordinary form of the Scotch hearth is probably too well known to need much description. The dimensions which have been found most suitable are as follows: Front to back, 21 in.; width, 27 in.; depth of hearth, 8 to 12 in. Formerly the distance from front to back was 24 in., but this was found too much for the blast and for the men.
The cast-iron hearth which holds the molten lead is set in 35 brickwork; if 8 in. deep and capable of holding about ¾ ton of lead, it is quite large enough. The workstone or inclined plate in front of the hearth is cast in one piece with it, and has a raised holder on either side at the lower edge, and a gutter to convey the overflowing lead to the melting-pot. The latter is best made with a partition and an opening at the bottom through which clean lead can run, so that it can be ladled into molds without the necessity for skimming the dross off the surface. It is well also to have a small fireplace below the melting-pot.
On each side of the hearth, and resting on it, is a heavy cast-iron block, 9 in. thick, 15 in. high, 27 to 28 in. long. To save metal, these are now cast hollow and air is caused to pass through them. On the back of the hearth stands another cast-iron block known as the “pipestone,” through which the blast comes into the furnace. In the older forms of pipestone the blast comes in through a simple round or oval pipe, a common size being 3 or 4 in. wide by 2½ in. high, and the pipestone is not water-cooled. With this construction the hearth will not run satisfactorily unless the pipestone is set with the greatest care, so as to have the tuyere exactly in the center, and as there is no water-cooling the metal quickly burns away when fume is being smelted. Moreover, the blast is apt to be stopped by slag adhering to the end of the pipe. As already mentioned, a peat is dropped in front of the blast every time the fire is made up, with the object of keeping a clear passage open for the blast. This old custom has, however, several serious disadvantages; first, it prevents the blast being kept on continuously; and, second, it makes it necessary to have the hearth open at the top so that the smelter-man can go in by the side of it. In this case the ore is fed from the side by the smelter-man, who works under the large hood placed above the furnace to carry away the fume. Even when he is engaged in shoveling back the fire from the front and is not underneath the hood, it is impossible to prevent some fume from blowing out; and there is much more liability to lead-poisoning than when the hearth is closed at the top by the chimney and the smelter-men work from the front. The best arrangement is to have the hearth entirely closed in by the chimney, except for the opening at the front, and to have a small auxiliary flue above the workstone leading direct to the open air to catch any fume that may blow out past the shutter in front of the hearth.
In an improved form of pipestone, a pipe connected to the blast-main fits into the semicircular opening at the back and is driven tight against a ridge in the flat side of the opening. Going through the pipestone, the arch becomes gradually flatter, and the blast emerges into the hearth, about 2 in. above the level of the molten lead, through an oblong slit 12 in. long by 1 in. wide, with a ledge projecting 1½ in. immediately above it. The back and front are similar, so that when one side gets damaged the pipestone can be turned back to front.
Water is conveyed in a 2½ in. iron pipe to the pipestone, and after passing through it is led away from the other end to a water-box, which stands beside the hearth and into which the red-hot lumps of slag are thrown to safeguard the smelters from the noxious fumes.
On the top of the pipestone rests an upper backstone, also of cast iron; it extends somewhat higher than the blocks at the sides. All this metal above the level of the lead is necessary because the partially fused lumps which stick to it have to be knocked off with a long bar, so that if fire-bricks were used in place of cast iron they would soon be broken up and destroyed.
With a covered-in hearth, when the ore is charged from the front, the following is the method adopted in smelting raw ore: The charge floats on the molten lead in the hearth, and at short intervals the two smelters running the furnace ease it up with long bars, which they insert underneath in the lead. Any pieces of slag adhering to the sides and pipestone are broken off. After easing up the fire, the lumps of partially reduced ore, mixed with cinders and slag, are shoveled on to the back of the fire; the slag is drawn out upon the workstone (any pieces of ore adhering to it being broken off and returned to the hearth), and it is then quenched in a water-box placed alongside the workstone. One or two shovelfuls of coal, broken fairly small and generally kept damp, are thrown on the fire, together with the necessary amount of ore, which is also kept damp if in a fine state of division. It is part of the duty of the two smelters to ladle out the lead from the melting-pot into the molds. In smelting ore a fairly strong, steady blast is required, and it is made to blow right through so as to keep the front of the fire bright. A little lime is thrown on the front of the fire when the slag gets too greasy.
When smelting raw fume one man attends to the furnace. It 37 does not have to be made up nearly as frequently, the work being easier for one man than smelting ore is for two. The unreduced clinkers and slag are dealt with exactly as in smelting ore; and coal is also, in this case, thrown on the back of the fire, but the blast does not blow right through to the front. On the contrary, the front of the fire is kept tamped up with fume, which should be of the coherency of a thick mud. The blast is not so strong as that necessary for ore. The idea is partially to bake the fume before submitting it to the hottest part of the furnace, or to the part where the blast is most strongly felt. It is only when smelting fume that it is necessary to keep the pipestone water-cooled.
To start a furnace takes from two to three hours. The hearth is left full of lead, and this has to be melted before the hearth is in normal working order. Drawing the fire takes about three-quarters of an hour; the clinkers are taken off and kept for starting the next run, and the sides and back of the hearth are cleaned down.
(June 2, 1906)
The works of the Federal Lead Company, near Alton, Ill., were erected in 1902. They have a connection with the Chicago, Peoria & St. Louis Railway, by which they receive all their raw materials, and by which all the lead produced is shipped.
The ore smelted is galena, with dolomitic gangue, and a small quantity of pyrites (containing a little copper, nickel, and cobalt) from southeastern Missouri, and consists chiefly of fine concentrates, containing 60 to 70 per cent. lead. In addition thereto a small proportion of lump ore is also smelted.
A striking feature at these works is the excellent facility for handling the materials. The bins for the ore, coke and coal are made of concrete and steel and are filled from cars running on tracks laid above them. For transporting the materials about the works a narrow-gage railway with electric locomotives is used.
The ores are smelted by the Scotch-hearth process. There are 20 hearths arranged in a row in a building constructed wholly of steel and stone. The sump (4 × 2 × 1 ft.) of each furnace contains about one ton of lead. The furnaces are operated with low-pressure blast from a main which passes along the whole row. The blast enters the furnace from a wind chest at the back through eight 1 in. iron pipes, 2 in. above the bath of lead. The two sides and the rear wall are cooled by a cast-iron water jacket of 1 in. internal width.
Two men work, in eight-hour shifts, at each of the furnaces, receiving 4.75 and 4.25c. respectively for every 100 lb. of lead produced. The ore is weighed out and heaped up in front of the furnaces; on the track near by the coke is wheeled up in a flat iron car with two compartments. The furnacemen are chiefly 39 negroes. At the side of each furnace is a small stock of coal, which is used chiefly for maintaining a small fire under the lead kettle. Only small quantities of coal are added from time to time during the smelting operation.
Over each furnace is placed an iron hood, through which the fumes and gases escape. They pass first through a collecting pipe, extending through the whole works, to a 1500 ft. dust flue, measuring 10 × 10 ft., in internal cross-section. Near the middle of this is placed a fan of 100,000 cu. ft. capacity per minute, which forces the fumes and gases into the bag-house, where they are filtered through 1500 sacks of loosely woven cotton cloth, each 25 ft. long and 18 in. in diameter, and thence pass up a 150 ft. stack.
The dust recovered in the collecting flue is burnt, together with the fume caught by the bags, the coal which it contains furnishing the combustible. It burns smolderingly and frits together somewhat. The product (chiefly lead sulphate) is then smelted in a shaft furnace, together with the gray slag from the hearth furnaces. The total extraction of lead is about 98 per cent., i.e., the combined process of Scotch-hearth and blast-furnace smelting yields 98 per cent. of the lead contained in the crude ore.
The direct yield of lead from the Scotch hearths is about 70 per cent. They also produce gray slag, containing much lead, which amounts to about 25 per cent. of the weight of the ore. About equal proportions of lead pass into the slag and into the flue dust. When working to the full capacity, with rich ore (80 per cent. lead and more) the 20 furnaces can produce about 200 tons of lead in 24 hours. The coke consumption in the hearth furnaces amounts to only 8 per cent. of the ore. The lead from these furnaces is refined for 30 minutes to one hour by steam in a cast-iron kettle of 35 tons capacity, and is cast into bars either alone or mixed with lead from the shaft furnace. The “Federal Brand” carries nearly 99.9 per cent. lead, 0.05 to 0.1 per cent. copper, and traces of nickel and cobalt.
The working up of the between products from the hearth-furnaces is carried out as follows: Slag, burnt flue dust and roasted matte from a previous run, together with a liberal proportion of iron slag (from the iron works at Alton), are smelted in a 12-tuyere blast furnace for work-lead and matte. The furnace is provided 40 with a lead well at the back. The matte and slag are tapped off together at the front and flow through a number of slag pots for separation. The shells which remain adhering to the walls of the pots on pouring out the slag are returned to the furnace. All the waste slag (containing about 0.5 per cent. lead) is dumped down a ravine belonging to the territory of the smeltery.
The lead from the shaft furnace is liquated in a small reverberatory furnace, of which the hearth consists of two inclined perforated iron plates. The residue is returned to the shaft furnace, while the liquated lead flows directly to the refining kettle, which is filled in the course of four hours. Here it is steamed for about one hour and is then cast into bars through a Steitz siphon, after skimming off the oxide. The matte is crushed and roasted in a reverberatory furnace (60 ft. long).
The power plant comprises three Stirling boilers and two 250 h. p. compound engines, of which one is for reserve; also one steam-driven dynamo, coupled direct to the engine, furnishing the current for the entire plant, for the electric locomotives, etc.
The coke is obtained from Pennsylvania and costs about $4 a ton, while the coal comes from near-by collieries and costs $1 per ton.
In the well-equipped laboratory the lead in the ores and slags is determined daily by Alexander’s (molybdate) method, while the silver content of the lead (a little over 1 oz. per ton) is estimated only once a month in an average sample. When the plant is in full operation it gives employment to 150 men. Cases of lead-poisoning are said to occur but rarely, and then only in a mild form.
The account of the introduction of the Huntington-Heberlein process at Tarnowitz, Prussia, published elsewhere in this issue, is of peculiar interest inasmuch as it tells of the complete displacement by the new process of one of the old processes of lead smelting which had become classic in the art. The roast-reaction process of lead smelting, especially as carried out in reverberatory furnaces, has been for a long time decadent, even in Europe. Tarnowitz was one of the places where it survived most vigorously.
Outside of Europe, this process never found any generally extensive application. It was tried in the Joplin district, and elsewhere in Missouri, with Flintshire furnaces in the seventies. Later it was employed with modified Flintshire and Tarnowitz furnaces at Desloge, in the Flat River district of Missouri, where the plant is still in operation, but on a reduced scale.
The roast-reaction process of smelting, as practised at Tarnowitz, was characterized by a comparatively large charge, slow roasting and low temperature, differing in these respects from the Carinthian and Welsh processes. It was not aimed to extract the maximum proportion of lead in the reverberatory furnace itself, the residue therefrom, which inevitably is high in lead, being subsequently smelted in the blast furnace. Ores too low in lead to be suitable for the reverberatory smelting were sintered in ordinary furnaces and smelted in the blast furnace together with the residue from the other process. In both of these processes the loss of lead was comparatively high. One of the most obvious advantages of the Huntington-Heberlein process is its ability to reduce the loss of lead. The result in that respect at Tarnowitz is clearly stated by Mr. Biernbaum, whose paper will surely attract a good deal of attention.[8]
(December 16, 1905)
The roast-reaction method of lead smelting in reverberatory furnaces never found any general employment in the United States, although in connection with the rude air-furnaces it was early introduced in Missouri. The more elaborate Flintshire furnaces were tried at Granby, in the Joplin district, but they were displaced there by Scotch hearths. The most extensive installation of furnaces of the Flintshire type was made at Desloge, in the Flat River district of southeastern Missouri. This continued in full operation until 1903, when the major portion of the plant was closed, it being found more economical to ship the ore elsewhere for smelting. However, two furnaces have been kept in use to work up surplus ore. As a matter of historic interest, it is worth while to record the technical results at Desloge, which have not previously been described in metallurgical literature.
The Desloge plant, which was situated close to the dressing works connected with the mine, and was designed for the smelting of its concentrate, comprised five furnaces. The furnaces were of various constructions. The oldest of them was of the Flintshire type, and had a hearth 10 ft. wide and 14 ft. long. The other furnaces were a combination of the Flintshire and Tarnowitz types. They were built originally like the newer furnaces at Tarnowitz, Upper Silesia, with a rather large rectangular hearth and a lead sump placed at one side of the hearth near the throat end; but good results were not obtained from that construction, wherefore the furnaces were rearranged with the sump at one side, but in the middle of the furnace, as in the Flintshire form. The rectangular shape of the Tarnowitz hearth was, however, retained. Furnaces thus modified had hearths 11 ft. wide and 16 ft. long, except one which had a hearth 13 ft. wide.
The same quantity of ore was put through each of these fur 43naces, the increase in hearth area being practically of no useful effect, because of inability to attain the requisite temperature in all parts of the larger hearths with the method of heating employed. The men objected especially to a furnace with hearth 13 ft. wide, which it was found difficult to keep in proper condition, and also difficult to handle efficiently. Even the width of 11 ft. was considered too great, and preference was expressed for a 10 ft. width. In this connection, it may be noted that the old furnaces at Tarnowitz were 11 ft. 9 in. long and 10 ft. 10 in. wide, while the new furnaces were 16 ft. long and 8 ft. 10 in. wide (Hofman, “Metallurgy of Lead,” fifth edition, p. 112). All of these dimensions were exceeded at Desloge.
The Flintshire furnaces at Desloge had three working doors per side; the others had four, but only three per side were used, the doors nearest the throat end being kept closed because of insufficient temperature in that part of the furnace. The furnace with hearth 11 × 14 ft. had a grate area of 6.5 × 3 ft. = 19.5 sq. ft.; the 11 × 16 furnaces had grates 8 × 3 = 24 ft. sq. The ratios of grate to hearth area were therefore approximately 1:8 and 1:7.3, respectively. (Compare with ratio of 1:10 at Tarnowitz, and 1:6⅔ at Stiperstones.) The ash pits were open from behind in the customary English fashion. The grate bars were cast iron, 36 in. long. The bars were 1 in. thick at the top, with ⅝ in. spaces between them. The open spaces were 32 in. long, including the rib in the middle. The bars were 4 in. deep at the middle and 2 in. at the ends. The distance from the surface of the grate bars to the fire-door varied in the different furnaces. Some of those with hearths 11 × 16 ft. and grates 8 × 3 ft. had the bars 6 in. below the fire-door; in others the bars were almost on a level with the fire-door.
The furnaces were run with a comparatively thin bed of coal on the grate, and combustion was very imperfect, the percentage of unburned carbon in the ash being commonly high. This was unavoidable with the method of firing employed and the inferior character of the coal (southern Illinois). The excessive consumption of coal was due largely, however, to the practice of raking out the entire bed of coal at the beginning of the operation of “firing down” (beginning the reaction period), when a fresh fire was built with cordwood and large lumps of coal.
Each furnace had two flues at the throat, 16 × 18 in. in size, 44 each flue being provided with a separate damper. Each furnace had an iron chimney approximately 55 ft. high, of which 13 ft. was a brick pedestal (64 × 64 in.) and the remaining 42 ft. sheet steel, guyed. The chimneys were 42 in. in diameter. The distance from the outside end of the furnace to the chimney was approximately 6 ft., and there was consequently but little opportunity for flue dust to collect in the flue. About once a month, however, the chimney was opened at the base and about two wheelbarrows (say 600 lb.) of flue dust, assaying about 50 per cent. lead, was recovered per furnace.
The furnace house was a frame building 45 ft. wide, with boarded sides and a corrugated-iron pitch roof, supported by steel trusses. The furnaces were set in this house, side by side, their longitudinal axes being at right angles to the longitudinal axis of the building. The distance from the outside of the fire-box end of the furnace to the side of the building was 10 ft. The coal was unloaded from a railway track alongside of the building and was wheeled to the furnace in barrows. Some of the furnaces were placed 18 ft. apart; others 22 ft. apart. The men much preferred the greater distance, which made their work easier, an important consideration in this method of smelting.
The hight from the floor to the working door of the furnace was approximately 36 in. The working doors were formed with cast-iron frames, making openings 7 × 11 in. on the inside and 15 × 28 in. on the outside. On the side of the furnace opposite the middle working door was placed a cast-iron hemispherical pot, set partially below the floor-line. This pot was 16 in. deep and 24 in. in diameter; the metal was ¼ in. thick. The distance from the top of the pot to the line of the working door was 31 in.; from the top of the pot to the bottom of the tap-door was 7 in. The tap-door was 4 in. wide and 9 in. high, opening through a cast-iron plate 1½ in. thick. Below the tap-door and on a line with the upper rim of the pot was a tap-hole 3½ in. in diameter. The frames of the working doors had lugs in front, against which the buckstaves bore, to hold the frames in position. All other parts of the sides of the furnace, including the fire-box, were cased with ⅝ in. cast-iron plates, which were obviously too light, being badly cracked.
The cost of a furnace when built in 1893 was approximately $1400, not including the chimney; but with the increased cost of 45 material the present expense would probably be about $2000. Notwithstanding the light construction of the furnaces, repairs were never a large item. Once a month a furnace was idle about 24 hours while the throat was being cleaned out, and every two months some repairing, such as relining the fire-boxes, etc., was required. If repairs had to be made on the inside of the furnace, two days would be lost while it was cooling sufficiently for the men to enter. In refiring a furnace, from 8 to 12 hours was required to raise it to the proper temperature. Out of the 365 days of the year, a furnace would lose from 20 to 25 days, for cleaning the throat and making repairs to the fire-box, arch, etc.
When a furnace was run with two shifts the schedule of operation was as follows:
| Drop charge | 4 a.m. |
| Begin work | 7 a.m. |
| Begin firing down | 11 a.m. |
| Begin first tapping | 1 p.m. |
| Rake out slag | 2.30 p.m. |
| Begin second tapping | 3 p.m. |
| Drop charge | 4 p.m. |
| Begin working | 5.30 p.m. |
| Begin firing down | 11 p.m. |
| Begin first tapping | 1 a.m. |
| Rake out slag | 2.30 a.m. |
| Begin second tapping | 3 p.m. |
With three shifts on a furnace, the schedule was as follows:
| Drop charge | 7 a.m. |
| Begin firing down | 12 a.m. |
| Begin tapping | 1 p.m. |
| Rake out slag | 2 p.m. |
| Begin tapping | 2.30 p.m. |
| Drop charge | 3 p.m. |
| Begin firing down | 8 p.m. |
| Begin tapping | 9 p.m. |
| Rake out slag | 10 p.m. |
| Begin tapping | 10.30 p.m. |
| Drop charge | 11.00 p.m. |
| Begin firing down | 4 a.m. |
| Begin tapping | 5 a.m. |
| Rake out slag | 6 a.m. |
| Begin tapping | 6.30 a.m. |
The hearths were composed of about 8 in. of gray slag beaten 46 down solidly on a basin of brick, which rested on a filling of clay, rammed solid. The hearth was patched if necessary after the drawing of each charge.
The system of smelting was analogous to that which was practiced in Wales rather than to the Silesian, the charges being worked off quickly, and with the aim of making a high extraction of lead directly and a gray slag of comparatively low content in lead. The average furnace charge was 3500 lb. At the beginning of the reaction period about 85 to 100 lb. of crushed fluorspar was thrown into the furnace and mixed well with the charge. The furnace doors were then closed tightly and the temperature raised, the grate having previously been cleaned. At the first tapping about 1200 lb. of lead would be obtained. A small quantity of chips and bark was thrown into the lead in the kettle, which was then poled for a few minutes, skimmed, and ladled into molds, the pigs weighing 80 lb. The skimmings and dross were put back into the furnace. The pig lead was sold as “ordinary soft Missouri.” The gray slag was raked out of the furnace, at the end of the operation, into a barrow, by which it was wheeled to a pile outside of the building. Shipments of the slag were made to other smelters from time to time, 95 per cent. of its lead content being paid for when its assay was over 40 per cent., and 90 per cent. when lower.
Each furnace was manned by one smelter ($1.75) and one helper ($1.55) per shift, when two shifts per 24 hours were run. They had to get their own coal, ore and flux, and wheel away their gray slag and ashes. In winter, when three shifts were run, the men were paid only $1.65 and $1.50 respectively. There was a foreman on the day shift, but none at night. The total coal consumption was ordinarily about 0.8 to 0.9 per ton of ore. Run-of-mine coal was used, which cost about $2 per ton delivered. The coal was of inferior quality, and it was wastefully burned, as previously referred to, wherefore the consumption was high in comparison with the average at Tarnowitz, where it used to be about 0.5 per ton of ore.
The chief features of the practice at Desloge are compared with those at Tarnowitz, Silesia and Holywell (Flintshire), and Stiperstones (Shropshire), Wales, in the following table, the data for Silesia and Wales being taken from Hofman’s “Metallurgy of Lead,” fifth edition, pp. 112, 113.
| Detail | Holywell | Stiper-stones | Tarnowitz | Tarnowitz | Desloge |
|---|---|---|---|---|---|
| Hearth length, ft. | 12.00 | 9.75 | 11.75 | 16.00 | 16.00 |
| Hearth width, ft. | 9.50 | 9.50 | 10.83 | 8.83 | 11.00 |
| Grate length, ft. | 4.50 | 4.50 | 8.00 | 8.00 | 8.00 |
| Grate width, ft. | 2.50 | 2.50 | 1.67 | 1.67 | 3.00 |
| Grate area: hearth area | 1:8 | 1:6⅔ | 1:10 | 1:10 | 1:7-1/3 |
| Charges per 24 hr., | 3 | 3 | 2 | 2 | 3 |
| Ore smelted per 24 hr., lb. | 7,050 | 7,050 | 8,800 | 16,500 | 10,500 |
| Assay of ore, % Pb | 75-80 | 77.5 | 70-74 | 70-74 | 70 |
| Gray slag, % of charge | 12 | 15 | 30 | 27 | |
| Gray slag, % Pb | 55 | 38.8 | 56 | 38 | |
| Men per 24 hr. | 6 | 4 | 4 | 6 | 6 |
| Coal used per ton ore | 0.57-0.76 | 0.56 | 0.46 | 0.50 | 0.90 |
The regular furnace charge at Desloge was 3500 lb. The working of three charges per 24 hours gave a daily capacity of 10,500 lb. per furnace. These figures refer to the wet weight of the concentrate, which was smelted just as delivered from the mill. Its size was 9 mm. and finer. Assuming its average moisture content to be 5 per cent., the daily capacity per furnace was about 10,000 lb. (5 tons) of dry ore.
The metallurgical result is indicated by the figures for two months of operation in 1900. The quantity of ore smelted was 1012 tons, equivalent to approximately 962 tons dry weight. The pig lead produced was 523.3 tons, or 54.4 per cent. of the weight of the ore. The gray slag produced was 262.25 tons, or about 27 per cent. of the weight of the ore. The assay of the ore was approximately 70 per cent. lead, giving a content of 673.4 tons in the ore smelted. The gray slag assayed approximately 38 per cent. lead, giving a content of 99.66 tons. Assuming that 90 per cent. of the lead in the gray slag be recoverable in the subsequent smelting in the blast furnace, or 89.7 tons, the total extraction of lead in the process was 523.3 + 89.7 ÷ 673.4 = 91 per cent. The metallurgical efficiency of the process was, therefore, reasonably high, especially in view of the absence of dust chambers.
The cost of smelting with five furnaces in operation, each treating three charges per day, was approximately as follows:
On the basis of 6.25 tons of wet ore, this would be $4.65 per ton. The actual cost in seven consecutive months of 1900 was as follows: Labor, $1.98 per ton; coal, $1.86; flux and supplies, $0.51; blacksmithing and repairs, $0.39; miscellaneous, $0,017; total, $4.757. If the cost of smelting the gray slag be reckoned at $8 per ton, and the proportion of gray slag be reckoned at 0.25 ton per ton of galena concentrate, the total cost of treatment of the latter comes to about $6.75 per ton of wet charge, or about $7 per ton of dry charge. This cost could be materially reduced in a larger and more perfectly designed plant.
The practice at Desloge did not compare unfavorably, either in respect to metal extracted or in smelting cost, with the roast-reduction method of smelting or the Scotch hearth method, as carried out in the plants of similar capacity and approximately the same date of construction, smelting the same class of ore, but the larger and more recent plants in the vicinity of St. Louis could offer sufficiently better terms to make it advisable to close down the Desloge plant and ship the ore to them. One of the drawbacks of the reverberatory method of smelting was the necessity of shipping away the gray slag, the quantity of that product made in a small plant being insufficient to warrant the operation of an independent shaft furnace.
(August 22, 1903)
It is well known that, owing to the intimate mixture of the constituents of the Broken Hill sulphide ores, a great deal of crushing and grinding is required to detach the particles of galena from the zinc blende and the gangue; and it will be understood, therefore, that a considerable amount of the material is converted into a slime which consists of minute but well-defined particles of all the constituents of the ore, the relative proportions of which depend on the dual characteristics of hardness and abundance of the various constituents. An analysis of the slime shows the contents to be as follows;
| Galena (PbS) | 24.00 |
| Blende (ZnS) | 29.00 |
| Pyrite (FeS2) | 3.38 |
| Ferric oxide (Fe2O3) | 4.17 |
| Ferrous oxide (FeO) contained in garnets | 1.03 |
| Oxide of manganese (MnO) contained in rhodonite and garnets | 6.66 |
| Alumina (Al2O3) contained in kaolin and garnets | 5.40 |
| Lime (CaO) contained in garnets, etc. | 3.40 |
| Silica (SiO2) | 22.98 |
| Silver (Ag) | .06 |
| 100.48 |
Galena, being the softest of these, is found in the slimes to a larger extent than in the crude ore; it is also, for the same reason, in the finest state of subdivision, as is well illustrated by the fact that the last slime to settle in water is invariably much the richest in lead, while the percentages of the harder constituents, zinc blende and gangue, show a corresponding reduction in 52 quantity, by reason of their being generally in larger sized particles and consequently settling earlier.
The fairly complete liberation of each of the constituent minerals of the ore that takes place in sliming tends, of course, to help the production of a high-grade concentrate by the use of tables and vanners, and undoubtedly a fair recovery of lead is quite possible, even with existing machines, in the treatment of fine slimes; but, owing to the great reduction in the capacity of the machines, which takes place when it is attempted to carry the vanning of the finer slimes too far, and the consequently greatly increased area of the machines that would be necessary, the operation, sooner or later, becomes unprofitable.
The extent to which the vanner treatment of slimes should be carried is, of course, less in the case of those mines owning smelters than with those which have to depend on the sale of concentrates as their sole source of profit. In the case of the Proprietary Company, all slime produced in crushing is passed over the machines after classification. A high recovery of lead in the form of concentrates is, of course, neither expected nor obtained, for reasons already explained; but the finest lead-bearing slimes are allowed to unite with the tailings, which are collected from groups of machines, and are then run into pointed boxes, where, with the aid of hydraulic classification, the fine rich slimes are washed out and carried to settling bins and tanks, where the water is stilled and allowed to deposit its slime, and pass over a wide overflow as clear water. The slime thus recovered amounts to over 1200 tons weekly, or about 11 per cent., by weight, of the ore, and assays about 20 per cent. lead, 17 per cent. zinc, and 18 oz. silver, and represents, in lead value, about 11 per cent. of the original lead contents of the crude ore and rather more than that percentage in silver contents. These slimes are thus a by-product of the mills, and their production is unavoidable; but as they are not chargeable with the cost of milling, they are an asset of considerable value, more especially so since it has been demonstrated that they can be desulphurized sufficiently for smelting purposes by a simple operation, and, at the same time, converted into such a physical condition as renders the material well suited for smelting, owing to its ability to resist pressure in the furnaces.
The Broken Hill Proprietary Company has many thousands 53 of tons of these slimes which the smelters have hitherto been unable to cope with, owing to the roasters being fully occupied with the more valuable concentrates. Moreover, the desulphurization of slimes in Ropp mechanical roasters is objectionable for various reasons, namely, owing to the large amount of dust created with such fine material, resulting injuriously to the men employed; also on account of the reduction in the capacity of the roasters, and consequent increase in working cost, owing to the lightness of the slime, especially when hot, as compared with concentrates, and the necessity for limiting the thickness of material on the bed of the roasters to a certain small maximum. Further, the desulphurization of the slimes is no more complete with the mechanical roasters than in the case of heap roasting, and the combined cost of roasting and briquetting being quite three shillings (or 75c.) per ton in excess of the cost of heap roasting, the latter possesses many advantages. These heaps are being dealt with, preparatory to roasting, by picking down the material in lumps of about 5 in. in thickness, while the fine dry smalls, unavoidably produced, are worked up in a pug mill with water, and dealt with in the same way as the wet slime produced from current work.
The slime, as produced by the mills, is run from bins into railway trucks in a semi-fluid condition, and shortly after being tipped alongside one of the various sidings on the mine is in a fit condition to be cut with shovels into rough bricks, which dry with fair rapidity, and when required for roasting are easily reloaded into railway trucks. As each man can cut about 20 tons of bricks per day, the cost is small. Various other methods of lumping the slime were tried, including trucking the semi-fluid material on movable trams, alongside which were set laths, about 9 in. apart, which enabled long slabs to be formed 9 in. wide and 5 in. thick, which were, after drying, picked up in suitable lumps and loaded in platform trucks, thence on railway trucks. Owing to the inferior roasting that takes place with bricks having flat sides, which are liable to come into close contact in roasting, and to the rather high labor cost, this method was discontinued. Another method was to allow the slime to dry partially after being emptied from railway trucks, and to break it into lumps by means of picks; but this method entailed the making of an increased amount of smalls, besides taking up more siding room, 54 owing to the extra time required for drying, as compared with the method now in use. Ordinary bricking machines could, of course, be used, but when the cost of handling the slime before and after bricking is counted, the cost would be greater than with the simple method now in use; the material being in too fluid a condition for making into bricks until some time elapses for drying, a double handling would be necessitated before sending it to the bricking machine. If, however, the slime could be allowed time to dry sufficiently in the trucks, bricking by machinery would probably be preferable. Rather more than 10 per cent. of smalls is made in handling the lumps in and out of the railway trucks, and this is, as already noted, worked up with water in a pug mill at the sintering works, and used partly for covering the heaps with slime to exclude an excessive amount of air. The balance is thrown out and cut into bricks, as already described.
At the heaps the lumps are at present being thrown from one man to another to reach their destination in the heap, but the sidings have been laid out in duplicate with a view to enabling traveling cranes to be used on the line next the heap, the lumps to be loaded primarily into wooden skips fitting the trucks. It is probable, however, that the lumps will require to be handled out of the skips into their place in the heap, as the brittle nature of the material may be found to render automatic tipping impracticable. A considerable saving in labor would nevertheless accompany the use of cranes, which would likewise be advantageous in loading the sintered material.
In order to reduce the inconvenience arising from fumes, length is very desirable in siding accommodation, so that heap building may be carried on at a sufficient distance from the burning kilns. It is for the same reason preferable to build in a large tonnage at one time, lighting the heaps altogether. As the heaps burn about two weeks only, long intervals intervene, during which the fumes are absent.
In the experimental stages of slime roasting, fuel, chiefly wood, was used in quantities up to 5 per cent., and was placed on the ground at the bottom of the heap, where also a number of flues, loosely built bricks, were placed for the circulation of air. The amount of fuel used has, however, been gradually reduced, until the present practice of placing no fuel whatever in the bottom was arrived at; but instead less than 1 per cent. of wood 55 is now burned in small enlargements of the flues, under the outer portion of the pile, and placed about 12 ft. apart at the centers. This is found to be sufficient to start the roasting operation within 24 hours of lighting, after which no further fuel is necessary.
As regards the dimensions of the heaps, the width found most suitable is 22 ft. at the base, the sides sloping up rather flatter than one to one, with a flat section on top reaching about 7 ft. in hight. As there is always about 6 in. of the outer crust imperfectly roasted, it is advisable to make the length as great as possible, thus minimizing the surface exposed. The company is building heaps up to 2000 ft. long.
During roasting care is required to regulate the air supply, the object being to avoid too fierce a roast, which tends to sinter and partially fuse the material on the outer portions of the lumps, while inside there is raw slime. By extending the roast over a longer period this is avoided, and a more complete desulphurization is effected. Experiments conducted by Mr. Bradford, the chief assayer, demonstrated that, at a temperature of 400 deg. C., the sulphide slime is converted into basic sulphate, while at a temperature of 800 deg. C. the material becomes sintered owing to the decomposition of the basic sulphate and the formation of fusible silicate of lead.
In practice, the sulphur contents of the material, which originally are about 14 per cent., become reduced to from 6.5 to 8.5 per cent., half in the form of basic sulphate and half as sulphides; much of the material sinters and becomes matted together in a fairly solid mass. The heaps are built without chimneys of any kind; a strip about 5 ft. wide along the crest of the pile is left uncovered by plastered slime, and this, together with the open way in which the lumps are built in, allows a natural draft to be set up, which can be regulated by partly closing the open ends of the flues at the base of the pile. Masonry kilns were used in the earlier stages with good results, which, however, were not so much better than those obtained by the heap method as to justify the expense of building, taking into consideration, too, the extra cost of handling the roasted material in the necessarily more confined space.
Much interest has been taken in the chemical reactions which take place in the operation of desulphurization of these slimes, it being contended, on the one hand, that the unexpectedly rapid 56 roast which takes place may be due to the sulphide being in a very fine state of subdivision, and more or less porous, thus allowing the air ready access to the sulphur, producing sulphurous acid gas (SO2). On the other hand, others, of whom Mr. Carmichael is the chief exponent, claim that several reactions take place during the operation, connected with the rhodonite and lime compounds present in the slimes, which he describes as follows:
“The temperature of the kilns having reached a dull red heat, the rhodonite (silicate of manganese) is converted into manganous oxide and silica; at a rather higher temperature the calcium compounds are also split up, with formation of calcium sulphide, the sulphur being provided by the slimes. The air permeating the mass oxidizes the manganese oxide and calcium sulphide into manganese tetroxide and calcium sulphate respectively, as shown as follows;
and, as such, are carriers of a form of concentrated oxygen to the sulphide slimes, with a corresponding reduction to manganous oxide and calcium sulphide, as shown by the following equation, in the case of lead:
The oxidation of the manganous oxide and calcium sulphide is repeated, and these alternate reactions recur until the desulphurization ceases, or the kiln cools down to a temperature below which oxidation cannot occur. These reactions, being heat-producing, provide part of the heat necessary for desulphurization, which is brought about by certain concurrent reactions between metallic sulphates and sulphide.
“The first that probably occurs is that in which two equivalents of the metallic sulphide react on one of the metallic sulphate with reduction to the metal, metallic sulphide, and sulphurous acid, as shown by the following equation in the case of lead:
“The metal so formed, in the presence of air, is oxidized, and in this state reacts on a further portion of the metallic sulphide produced, with an increased formation of metal and evolution of sulphurous acid, according to the following equation, in the case of lead:
“The metal so produced in this reaction is wholly reoxidized by the oxygen of the air current, and being free to react on still further portions of the metallic sulphide, repeats the reaction, and becomes an important factor in the desulphurizing of the undecomposed portion of the material. As the desulphurization proceeds, and the sulphate of metal accumulates, reactions are set up between the metallic sulphide and different multiple proportions of the metallic sulphate, with the formation of metal, metallic oxide, and evolution of sulphurous acid, as follows:
“With two equivalents of metallic sulphate to one equivalent of metallic sulphide, in the case of lead, according to the following equation:
“With three equivalents of metallic sulphate to one of metallic sulphide, in the case of lead, according to the following equation:
The volatility of sulphide of lead—especially in the presence of an inert gas such as sulphurous acid—being greater than that of the sulphate, oxide, or the metal itself, it might be thought that the conditions are conducive to a serious loss of lead. This, however, is reduced to a minimum, owing to the easily volatilized sulphide being trapped, as non-volatile sulphate, by small portions of sulphuric anhydride (SO3), which is formed by a catalytic reaction set up between the hot ore, sulphurous acid, and the air passing through the mass. Owing to the non-volatility of the silver compounds in the slimes, the loss of this metal has been found to be inappreciable. The zinc contents of the slime are reduced appreciably, thus rendering the material more suitable for smelting. After desulphurization ceases, a few days are allowed for cooling off. On the breaking up of the mass for 58 despatch to the smelters, as much of the lower portion of the walls is left intact as possible, so that it can be utilized for the next roast, thus avoiding the re-building of the whole of the walls.[10]
(January 12, 1905)
In the course of smelting, at the works of the company known as the Broken Hill Proprietary Block 14, material which consisted chiefly of silver-lead concentrate and slime, resulting from the concentration of the Broken Hill complex sulphide ore, I had to contend with all the troubles which attend the treatment of large quantities of finely divided material in blast furnaces. With the view of avoiding these troubles, I experimented with various briquetting processes; and, after a number of more or less unsatisfactory experiences, I adopted a procedure similar to that followed in manufacturing ordinary bricks by what is known as the semi-dry brick-pressing process. This method of briquetting not only converts the finely divided material cheaply and effectively into hard semi-fused lumps, which are especially suitable for the heavy furnace burdens required by modern smelting practice, but also eliminates sulphur, arsenic, etc., to a great extent; therefore, it is capable of wide application in dealing with concentrate, slime, and other finely divided material containing lead, copper and the precious metals.
This briquetting process comprises the following series of operations:
1. Mixing the finely divided material with water and newly slaked lime.
2. Pressing the mixture into blocks of the size and shape of ordinary bricks.
3. Stacking the briquettes in suitably covered kilns.
4. Burning the briquettes, so as to harden them, without melting, at the same time eliminating sulphur, arsenic, etc.
1. The material is dumped into a mixing plant, together with such proportions of screened slaked lime (usually from three to five per cent.) and water as shall produce a powdery mixture 60 which will, on being squeezed in the hand, cohere into dry lumps. In preparing the mixture, it is well to mix sandy material with suitable proportions of fine, such as slime, in order that the finer material may act as a binding agent.
The mixer used by me consists of an iron trough, about 8 ft. long, traversed by a pair of revolving shafts, carrying a series of knives arranged screw-fashion; and so placed that the knives on one shaft travel through the spaces between the knives on the other shaft. The various materials are dumped into one end of the mixing trough, from barrows or trucks, and are delivered continuously at the other end of the trough, into an elevator which conveys the mixture to the brick-pressing plant.
2. The plant employed was the semi-dry brick-press. This machine receives the mixture from the elevators, and delivers it in the form of briquettes, which can at once be stacked in the kilns. It was found that such material as concentrate and slime has comparatively little mobility in the dies during the pressing operation; this necessitates the use of a device which provides for the accurate filling of the dies. It was also found that the materials treated by smelters vary in compressibility, and this renders necessary the adoption of a brick-pressing plant having plungers which are forced into the dies by means of adjustable springs, brick-presses having plungers actuated by rigid mechanism being extremely liable to jam and break.
3. Briquettes made from such material as concentrate and slime vary in fusibility; they are also combustible, and while being burned they produce large quantities of smoke containing sulphurous acid and other objectionable fumes. It is therefore necessary that such briquettes be burned in kilns provided with arrangements for accurately controlling the burning operations, and for conveniently disposing of the smoke. Suitable kilns, which will contain from 30 to 50 tons of briquettes per setting, are employed for this purpose. Regenerative kilns of the Hoffman type might be used for dealing with some classes of material, but, for general purposes, the kilns as designed here will be found more convenient.
The briquettes are stacked according to the character of the material and the object to be obtained. The various methods of stacking, and the reasons for adopting them, can be readily learned by studying ordinary brick-burning operations in any 61 large brick-yard. After the stacking is complete the kiln-fronts are built up with burnt briquettes produced in conducting previous operations, and all the joints are well luted.
4. In burning briquettes made from pyrite or other self-burning material, it is simply necessary to maintain a fire in the kiln fireplaces for a period of from 10 to 20 hours. When it is judged that this firing has been continued long enough, the fire-bars are drawn and the fronts are luted with burnt briquettes in the same manner as the kiln-fronts. Holes about two inches square are then made in these lutings, through which the air required for the further burning of the briquettes is allowed to enter the kilns under proper control. After the fireplaces are thus closed the progress of the burning, which continues for periods of from three to six days, is watched through small inspection holes made in the kiln-fronts; and when it is seen that the burning is complete the fronts are partially torn away, in order to accelerate the cooling of the burnt briquettes, which are broken down and conveyed to the smelters as soon as they can be conveniently handled.
When briquettes made from pyrite concentrate, or of other free-burning material, are thus treated, they are not only sintered but they are also more or less effectively roasted, and it may be taken for granted that any ore which can be effectively roasted in the lump form in kilns or stalls will form briquettes that will both sinter and roast well; indeed, one may say more than this, for briquettes which will sinter and roast well can be made from many classes of ore that cannot be effectively treated by ordinary kiln-and stall-roasting operations; and, moreover, good-burning briquettes may be made from mixtures of free-burning and poor-burning material. Briquettes containing large proportions of pyrite or other free-burning material will, unless the air-supply is properly controlled, often heat up to such an extent as to fuse into solid masses, much in the same manner as matte of pyritic ore will melt when it is unskilfully handled in roasting. In dealing with material which will not burn freely, such as roasted concentrate, the briquetting is conducted with the intention of sintering the material; and in this case the firing of the kilns is continued for periods of from three to four days, the procedure being similar in every way to that followed in burning ordinary bricks.
When conducting my earlier briquetting operations I made the briquettes by simply pugging the finely divided material, following a practice similar to that adopted in producing “slop-made” bricks by hand. This method of making the briquettes was attended with a number of obvious disadvantages, and was abandoned as soon as the semi-dry brick-pressing plant became available. The extent to which this process, or modifications of it, may be applied is shown by the fact that, following upon information given by me, the Broken Hill Proprietary Company adopted a similar method of sintering and roasting slime, consisting of about 20 per cent. galena, 20 per cent. blende, and 60 per cent. silicious gangue. The procedure followed in this case consisted of simply pugging the slime, and running the pug upon a floor to dry; afterward cutting the dried material into lumps by means of suitable cutting tools, and then piling the lumps over firing foundations, following a practice similar to that pursued in conducting ordinary heap-roasting. This company is now treating from 500 to 1000 tons of slime weekly in this manner. It is, however, certain that better results would attend the treatment of this material by making this slime into briquettes and burning them in kilns.
The cost of briquetting and burning material in the manner first described, with labor at 25c. per hour, and wood or coal at $4 per ton, amounts to from $1 to $1.50 per ton of material.
(November 22, 1902)
The value of briquetting in connection with metallurgical processes and the manufacture of artificial stone is well understood and appreciated. In smelting plants there is always more or less flue dust, fine ores, and sometimes fine concentrates to be treated, but the charging of such fine material directly into a furnace would cause trouble and irregularities, and would lessen its capacity also. As mineral briquetting cannot be effected without considerable wear upon the machinery and without quite appreciable expense in binder, labor, and handling, many smelters try to avoid it.
The financial question, however, is not as serious as it may at first appear, and taking the large output of modern briquetting machines in consideration, the cost for repairs amounts only to a few cents per ton of briquetted material. The total cost depends in the first place on the cost of labor, power and the binder, and in most American smelters it varies between $0.65 and $1.25 per ton of briquettes.
Ordinary brick presses, with clay as a binder, were used in Europe as well as in this country, but they are too slow and expensive for large propositions and the presence of clay is usually undesirable.
The English Yeadon (fuel) press has also been used for some years at the Carlton Iron Company’s Works at Ferryhill in England, and at the Ore and Fuel Company’s plant at Coatbridge in the same country; also by some Continental firms. Dupuis & Sons, Paris, furnished a few presses which are mostly used for manganese and iron ores and pyrites. In some localities coke dust is added. The making of clay briquettes or mud-cakes is the crudest form of briquetting; but while heat has to be expended to evaporate the 40 to 50 per cent. of moisture in them, and while considerable flue dust is made, this method is better than feeding fine ore or flue dust directly into the furnace.
The only other method of avoiding briquetting is by fusing ore fines in slagging reverberatory furnaces and by adding flue dust in the slagging pit, thus incorporating it with the slagging ore. This is practised sometimes in silver-lead smelters, but in connection with copper or iron smelters it is not practicable.
In briquetting minerals a thorough mixing and kneading is of the first importance. If this is done properly a comparatively low pressure will suffice to create a good and solid briquette, which after six to eight hours of air-drying, or after a speedier elimination of the surplus of moisture in hot-air chambers, will be ready for the furnace charge. A good briquette should permit transportation without excessive breakage or dust a few hours after being made, and it should retain its shape in the furnace until completely fused, so as to create as little flue dust as possible. The briquette should be dense, otherwise it will crumble under the influence of bad weather.
The two presses on the American machinery market are the type built by the Chisholm, Boyd & White Company, of Chicago, and the briquetting machine manufactured by the H. S. Mould Company, of Pittsburg. Both are extensively used, and in many metallurgical plants it will pay well to adopt them.
From 4 to 6 per cent. of milk of lime is generally used as binder, and this has a desirable fluxing influence also. A complete outfit comprises, besides the press, a mixer for slacking the lime, and a feed-pump which discharges the liquid in proportion into the main mixer wherein the ore fines, flue dust, or concentrates are shoveled.
The Chisholm, Boyd & White Company’s press makes 80 briquettes per minute, which, with a new disk, are of 4 in. diameter and 2½ in. hight, thus giving about 872 cu. ft. of briquette volume per 10 hours, or 50 to 80 tons, depending on the weight of the material. With the wear of the disk the hight of the briquettes is reduced and consequently the capacity of the machine also. The disk weighs about 1600 lb., and as most large smelters have their own foundries it can be replaced with little expense. About 30 effective horse-power is usually provided for driving the apparatus. The machine is too well known to metallurgists and engineers to require further comment or description.
The H. S. Mould Company has also succeeded in making its machine a thorough practical success. This machine is a plunger 65-type press. The largest press built employs six plungers, and at 25 revolutions it makes 150 briquettes of 3 in. diameter and 3 in. hight, or 1080 cu. ft. per 10 hours. Its rated capacity is 100 tons per 10 hours.
In using a plunger-type press the material should not contain more than 7 per cent. mechanical moisture. If wet concentrates have to be briquetted it is necessary to add dry ore fines or flue dust to arrive at a proper consistency. The briquettes are very solid and only air-drying for a few hours is necessary.
The cylindrical shape of briquettes is very good, as it insures a proper air circulation in the furnace and consequently a rapid oxidation and fusion.
The wear of the Mould Company’s press is mostly confined to the chilled iron bushings and to the pistons. Auxiliary machinery consists of the slacker, the feeder and the main mixer. The press is of a very substantial design, and it is claimed that the cost of repairs does not amount to more than 3c. per ton of briquettes.
Wear and tear is unavoidable in a crude operation like briquetting; to treat flue dust, ore fines, and fine concentrates successfully, it is almost absolutely necessary to resort to it.
Edison used a number of intermittent-acting presses at his magnetic iron-separation works in New Jersey, but this plant shut down some time ago.
(September 15, 1904)
The plant, which is here described, for bricking fine ores and flue dust, was designed and the plans produced in the engineering department of the Selby smelter. The machinery contained in the plant consists of a Boyd four-mold brick press, a 7 ft. wet pan or Chile mill, a 50 h.p. induction motor, and a conveyor-elevator, together with the necessary pulleys and shafting.
The press, Chile mill, and motor need no special mention, as they all are from standard patterns and bought, without alterations, from the respective builders. The Chile mill was purchased from the builders of the brick press. The conveyor-elevator was built on the premises and consists of a 14 in. eight-ply rubber belt, with buckets of sheet steel placed at intervals of 6 in., running over flanged pulleys. The buckets, or more properly speaking the flights, are made from No. 12 steel plate, flanged to produce the back and ends, with the ends secured to the flanged bottom by one rivet in each. The plant has been in operation for sixteen months and there have been few or no repairs to the elevator, except to renew the belt, which is attacked by the acid contained in the charges. This first belt was in continuous use for nine months. As originally designed, the capacity was 100 tons per day of 12 hours, but this was found to require a speed so high that the workmen were unable to handle the output of the press. The speed was, consequently, reduced about 25 per cent., which brings the output down to about 75 tons per day. This output, as expressed in weight, naturally varies somewhat owing to the variation in the weight of the material handled.
It is probable that the capacity could be increased to about 90 tons by enlarging the bricks, which could be done, but would require a considerable amount of alteration in the machine, as it is designed to produce a standard sized building brick. By this method of increase, however, the work of handling would 67 not be materially increased, because the number of bricks would be the same as with the present output of 75 tons; there would be about 16 per cent. more to handle, by weight. Working on the basis of 100 tons capacity, the bins were designed to afford storage room for about three days’ run, or a little over 300 tons. The bins are made entirely of steel, in order that the hot material may be dumped into them directly from the roasting furnaces, thus saving one handling. In order that there may be room for several kinds of material, the bins are divided into seven compartments, three on one side and four on the other. The lower part is of ⅜ in. steel plate, and the upper, about one-half the hight, of 5/16 in. plate.
It may be well to call attention to the method of handling the material, preparatory to its delivery to the brick press. The bins are constructed, as will be seen by the drawing, with their floor set 2.5 ft. above the working floor, which enables the workmen to reach the material with a minimum effort. The floor of the bins project 2.5 ft. in front of the face, thus forming a platform on which the shoveling may be done without the necessity of bending over. In this projecting platform are cut rectangular holes 12 × 18 in., which are placed midway between the openings in the front of the bins and furnished with screens to stop any stray bolts or other coarse material that might injure the press. This position of the holes through the platform was adopted so that, in the event of the material running out beyond the opening in the face, it would not fall directly upon the floor. Two buckets are provided, with a capacity of 7 cu. ft. each, which is the size of a single charge of the Chile mill. These buckets have a hopper-shaped bottom fixed with a swinging gate which is operated by the foot; thus the bucket can be run over the pan of the Chile mill and the charge dumped directly into it. The buckets run on an overhead iron track (1 in. by 3 in.) hung 7 ft. in the clear, above the floor.
The method of making up the charge is as follows: The bucket is run under the hole in the platform nearest to the compartment containing the material of which the charge is partly composed, and a predetermined number of shovelfuls is drawn out and put into the bucket, which is then pushed on to the next compartment from which material is wanted, where the operation is repeated. After charging into the bucket the requisite amount of ore or 68 flue dust, the bucket is run to the back of the building, where the necessary amount of lime (slaked) is added. By putting the lime in last, it is so surrounded by the dust or ore that it has not the opportunity to stick to the sides of the bucket in discharging, as it otherwise would.

The number of men required to operate the entire plant, exclusive of those employed in bringing the material to the bins and emptying the cars into them, is 12, placed as follows; One preparing the lime for use, one removing the charge from the mill and supplying the elevator-conveyor, which is accomplished by means of a specially shaped, long-handled shovel; one keeping the supply spout of the press clear (an attempt was made to do this mechanically, but was found to be unsuccessful, owing to the extremely sticky nature of the material, and so was discarded in favor of manual labor); one to control the press in case of mishap and to keep the dies clean; one oiler; three receiving the bricks from the press and taking the brick-loaded cars from the press to the drying-house, and two placing the bricks on the shelves.
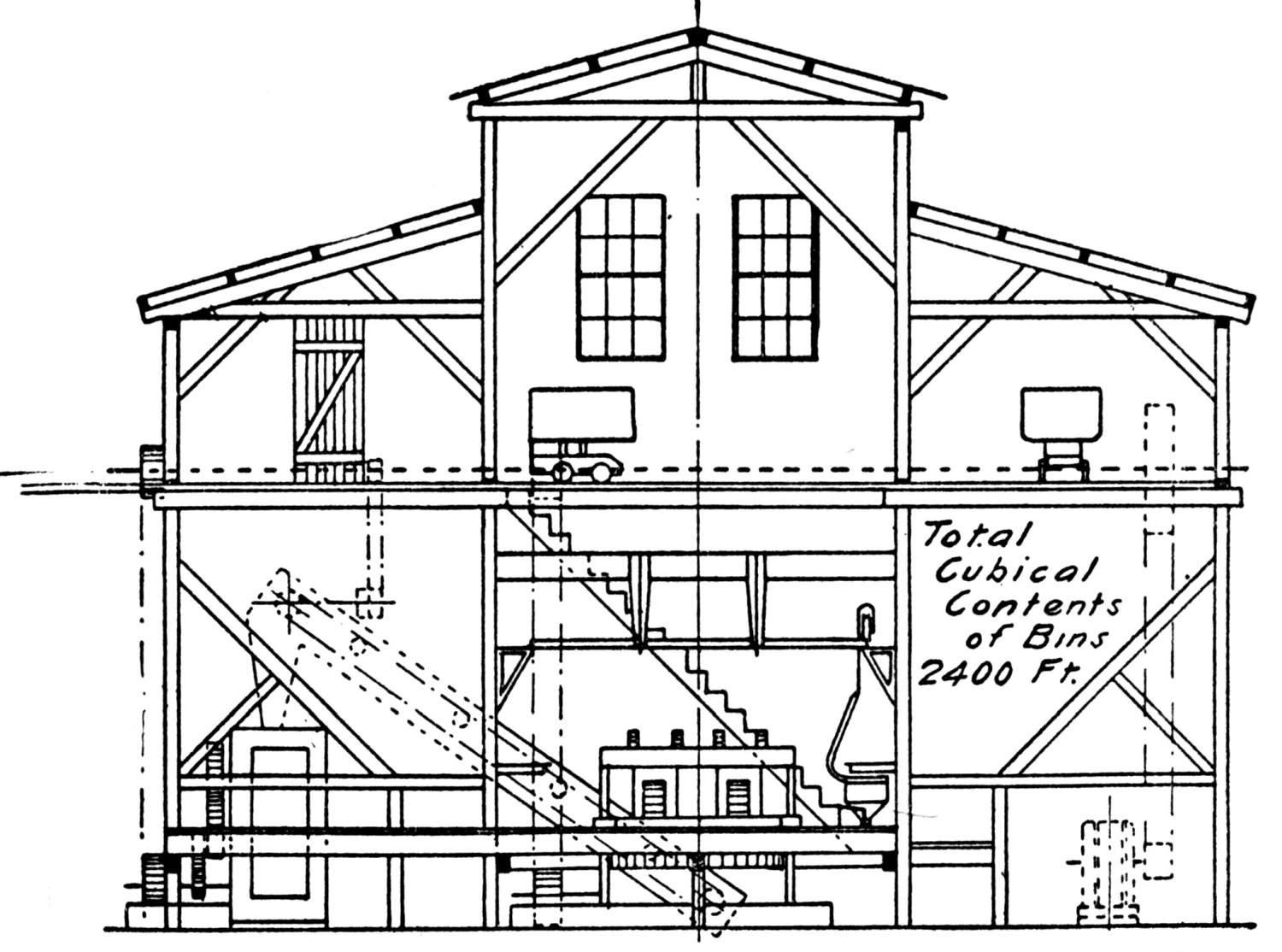
The drying-house scarcely requires description; it is but a roofed shed, without sides, fitted with stalls into which the bricks are set on portable shelves, as close as working conditions will permit. The means of drying, at the present time, is by the natural circulation of air, but a mechanical system is in contem 70plation, by which the air will be drawn into the building from the outside and forced to find its way out through the bricks. The drying-house is adjacent to the pressing plant, in fact forms the back of it, so that there is a minimum distance to haul the product. The time required for drying the bricks sufficiently for them to withstand the necessary handling is, depending on the weather, from two to eight days, the usual time being about three days.
(January 10, 1903)
The rectangular silver-lead blast furnace developed in the Rocky Mountains has an area of 42 × 120 to 48 × 160 in. at the tuyeres; 54 × 132 to 84 × 200 in. at the top; and hight from tuyere level to top of charge of 15 to 21 ft. Such a furnace smelts 80 to 200 tons of charge (ore and flux, but not slag and coke) per 24 hours. The slag that has to be resmelted amounts to 20 to 60 per cent. of the charge. Coke consumption is 12 to 16 per cent. of the charge. The blast pressure ranges from 1.5 to 4 lb. per square inch, averaging close to 2 lb. Gases of hand-charged furnaces are taken off through an opening below the charge-floor, the furnace being fed through a slot (about 20 in. wide, extending nearly the whole length of the furnace) in the iron floor-plates; or through a hood (brick or sheet iron) above the charge-floor level, with a down-take to the flues, charge-doors being provided on each side of the hood, extending preferably the whole length of the furnace and usually having a sill a few inches high which compels the feeder to lift his shovel.
When a silver-lead blast furnace is operating satisfactorily, the following conditions should obtain; (1) A large proportion of the lead in the charge should appear as direct bullion-product at the lead-well. (2) The slag should be fluid and clean. (3) The matte should be low in lead. (4) The furnace should be cool and quiet on top, making a minimum quantity of lead-fume and flue-dust, and the charges should descend uniformly over the whole area of the shaft. (5) The furnace speed should be good. (6) The furnace should be free from serious accretions and crusts; that is to say, the tuyeres should be reasonably bright and open, 74 and the level of the lead in the lead-well should respond promptly to variations of pressure, caused by the blast and by the hight of the column of molten slag and matte inside the furnace—an indication that ample connection exists between the smelting column and the crucible. Good reduction (using that term to express the degree in which the furnace is manifesting its reducing action) is obtained when the first three of the above conditions are satisfied.
For any given furnace there are five prime factors, the resultant of which determines the reduction, namely: (a) Chemical composition of the furnace charges; (b) proportion and character of fuel; (c) air-volume and pressure, to which might perhaps also be added temperature of blast; for, although hot blast has not yet been successfully applied in lead-smelting practice, I believe it is only a question of time when it will be; (d) dimensions and proportions of smelting furnace; (e) mechanical character and arrangement of the smelting column.
All but one of the above factors can be intelligently gaged. The mechanical factor, however, can be expressed only in generalities and indefinite terms. A wise selection of ores and proper preliminary preparation, crushing the coarse and briquetting the fine, will do much to regulate it, but all this care may be largely nullified by careless feeding. The importance and possibilities of the mechanical factor are generally overlooked and its symptoms are wrongly diagnosed. For instance, the importance of slag-types has undoubtedly been considerably exaggerated at the expense of the mechanical factor. Slags seldom come down exactly as figured. We must know our ores and apply certain empirical corrections to the iron, sulphur, etc., based on previous experience with the ores; but these empirical corrections may represent also an unformulated expression of the influence of the mechanical factor on the reduction—a function, therefore, of the ruling physical complexion of the ores, and the peculiarities of the feeding habitually maintained in the works concerned. With a given ore-charge large reciprocal variations may be produced in the composition of slag and matte by merely changing the mechanical conditions of the smelting column, and since the efficient utilization of both fuel and blast must be controlled in the same way, the mechanical factor may be considered, perhaps, the dominating agent of reduction. Inasmuch as there is no 75 way of gaging it, however, the only recourse is to seek a correct adjustment and maintain it as a positive constant, after which slag, fuel and blast may be with much greater certainty adjusted toward efficiency of furnace work and metal-saving.
Behavior of Iron.—The output of lead is so dependent upon the reactions of the iron in the charge that the chief attention may well be fixed upon that metal as the key to the situation. The success of the process depends largely upon reducing just the right amount of iron to throw the lead out of the matte, the remainder of the iron being reduced only to ferrous oxide and entering the slag. Too much iron reduced will form a sow in the hearth. Iron is reduced from its oxides principally by contact with solid incandescent carbon, and by the action of hot carbon monoxide. Reduction by solid carbon is the more wasteful, but there is in lead smelting an even more serious objection to permitting the reduction to be accomplished by that means, which leads to comparatively hot top and more or less volatilization of lead. Reduction by carbon monoxide is the ideal condition for the lead furnace. It means keeping the zone of incandescence low in the charge column, leaving plenty of room above for the gases to yield up their heat to, and exercise their reducing power on, the descending charge, so that by the time they escape they will be well-nigh spent. Their volume and temperature will be diminished, and the low velocity of their exit will tend to minimize the loss of lead in fume and flue dust.
The idea that high temperatures in lead blast furnaces should be avoided is based on a misconception. Temperatures must exist which are sufficiently high to volatilize all the lead in the charge, if other conditions permit. A high temperature before the tuyeres means fast smelting; and fast smelting, under proper conditions, means a shortening of the time during which the lead is subject to scorifying and volatilizing influences. A rapidly descending charge, constantly replenished with cold ore from above, absorbs effectively the heat of the gases and acts as a most efficient dust and fume collector. In considering long flues, bag-houses, etc., it should be kept in mind that the most effective dust collector ought to be the furnace itself.
In the practice of twelve years ago and earlier, particularly when using mixed coke and charcoal, reduction by carbon was probably the rule; and the percentage of fuel required was very 76 high. There is good reason to think we have still much room for improvement along this line in our average practice of today.
Volume of Blast.—It is customary to supply a battery of furnaces from a large blast main, connected with a number of blowers. Inasmuch as the air will take preferably the line of least resistance, if the internal resistance of any one furnace be increased the volume of air it will take will be diminished and the others will be favored unduly. Only by keeping all the furnaces on approximately the same charge, with the same hight of smelting column, can anything like uniformity of operation and close regulation be secured. The rational plan would seem to be to have a separate blower, of variable speed, directly connected to each furnace, but this plan, which has had a number of trials, has usually been abandoned in favor of the common blast main. Trials by myself, extending over considerable periods, have been so uniformly favorable, however, that I am forced to ascribe the failure of others to some outside reason.
The peculiar atmosphere required in the lead blast furnace depends upon the correct proportion of two counteractive elements, carbon and oxygen. If given too much air the furnace will show signs of deficient reduction, commonly interpreted as calling for more fuel, which will be sheer waste since its object is to burn up surplus air. There will be an additional waste through the extra coal burned under the steam boilers. The true remedy would be to cut down the quantity of air. Burning up excessive coke is as hard work as smelting ore. Too much fuel invariably slows up a furnace; it also drives the fire upward and gives predominance to reduction by solid carbon. The maintenance of a minimum fuel percentage, with a correctly adjusted volume of air, will tend to promote the conditions under which iron will be reduced by the gases, rather than by solid carbon.
Pressure of Blast.—Pressure necessarily involves resistance; and the blast-pressure, as registered by a simple mercury-gage on the bustle-pipe, may be increased in two ways: (1) By increasing the volume of air forced through the interstices in the charge. This is the wrong way; but, unfortunately, it is only too common in our practice, and therefore deserves to be mentioned, if only to be condemned. (2) By leaving the volume of air unchanged, but increasing the friction offered by the interstitial channels, either 77 by making them smaller in aggregate cross-section (which means a finer charge), or by making them longer (which means a higher smelting column). A correctly graduated internal resistance is, therefore, the only true basis for a high blast furnace, which, when so produced, will bring about rapid smelting, a low zone of incandescence, and a very vigorous action upon the ores by the gases in their retarded ascent through the charge column. These conditions promote the reduction of iron by CO. The adjustment of internal resistance, which is thus clearly the main factor, can be accomplished only by the correct feeding of the furnace.
Feeding the Charge.—It is self-evident that, the more thorough the preliminary preparation of the charge before it reaches the zone of fusion, the more rapidly can the actual smelting proceed. A piece of raw ore that finds itself prematurely at the tuyeres, without having been subjected to the usual preparatory processes of drying, heating, reduction, etc., must remain there until it is gradually dissolved or carried away mechanically in the slag. Any such occurrence must greatly retard the process. It would seem, by the same reasoning, that an intimate mixture of the ingredients of the charge should expedite the smelting, and I advocate the intimate mixture of the charge ingredients in all cases.
The theory of feeding is simple, but not so the practice. If the charge column were composed of pieces of uniform size, the ascending gases would find the channel of least resistance close to the furnace walls and would take it preferably to the center of the shaft. The more restricted channel would necessitate a higher velocity, so that not only would the center of the charge be deprived of the action of the gases, but also the portion traversed would be overheated; many particles of ore would be sintered to the walls or carried off as flue dust; slag would form prematurely; fuel would be wasted; in short, all the irregularities and losses which accompany over-fire would be experienced. In practice the charge is never uniform, but is a mixture of coarse and fine. By lodging the finer material close to the walls and placing the coarser in the center, an adjustment may be made which will cause the gases to ascend uniformly through the smelting column. A furnace top smoking quietly and uniformly over its whole area is the visible sign of a properly fed furnace.
Effect of Large Charges.—It has frequently been remarked 78 that, within certain limits, large charges give more favorable results than small ones; and numerous attempts have been made to account for this fact. My observations lead me to offer the following as a rational explanation—at least in cases where ore and fuel are charged in alternate layers. Large ore-charges mean correspondingly large fuel-charges. The gases can pass readily through the coke; and hence each fuel-zone tends to equalize the gas currents by giving them another opportunity to distribute themselves over the whole furnace area, while each layer of ore subsequently encountered will blanket the gases, and compel them to force a passage under pressure, which is the manner most favorable to effective chemical action.
In mechanically fed furnaces the charges of ore and fuel are usually dropped in simultaneously from a car and the separate layers thus obliterated, and the distributing zones which are such a safeguard against the consequences of bad feeding are lacking, hence more care must be exercised to secure proper placing of the coarse and fine material. This may throw some light on the failure of most of the early attempts at mechanical feeding.
Mechanical Character of Charge.—Very fine charges blanket the gases excessively and cause them to break through at a few points, forming blow-holes, which seriously disturb the operation, cause loss of raw ore in the slag, and are accompanied by all the evils of over-fire. A charge containing a few massive pieces, the rest being fine, is a still more unfavorable combination. A very coarse charge permits too ready an exit to the gases, and in the end tends likewise to over-fire and poor reduction. The remedy is to briquette the fine ore (though preferably not all of it), and crush the coarse to such degree as to approach an ideal result, which may be roughly described as a mixture in which about one-third is composed of pieces of 5 to 2 in. in diameter, one-third pieces of 2 to 0.5 in., and the remaining third from 0.5 in. down. The coke is better for being somewhat broken up before charging, and a reasonable amount of coke fines, such as usually accompanies a good quality of coke, is not in the least detrimental. The common practice of handling the coke by forks and throwing away the fines is to be condemned as an unwarranted waste of good fuel. The slag on the charge should be broken to pieces at most 6 in. in diameter. The common 79 practice of throwing in whole butts of slag-shells is bad. There is no economy in using the slag hot; cold charges, not hot, are what we want. A reasonable amount of moisture in the charge is beneficial, providing it be in such form as to be readily dried out. It is often advantageous to wet the ore mixtures while bedding them, or to sprinkle the charges before feeding. The driving off of this water must consume fuel, but not so much as if the smelting zone crept up. Large doses of water applied directly to the furnace are unpardonable under any circumstances, however, though they are sometimes indulged in as a drastic measure to subdue excessive over-fire when other and surer means are not recognized. One of the chief merits of moderate sprinkling before charging is that it gives in many cases a more favorable mechanical character, approximating a lumpy condition in too fine a charge, and assisting to pack a too coarse one.
Different Behavior of Coarse and Fine Ore.—In taking up a shovelful of ore, the fine will be observed to predominate in the bottom and center, and the coarse on the top and sides. When thrown from the shovel, the coarse will outstrip the fine and fall beyond it. In making a conical pile the coarse ore will roll to the base, leaving the fine near the apex. This difference in the action of the mobile coarse ore and the sluggish fines is the key to the practical side of feeding, both manual and mechanical. It is not sufficient to tell the feeder to throw the coarse in the middle and the fine against the sides; if it be easier to do it some other way such instructions will count for little. The desired result can be best secured by making the right way easier than the wrong way.
It is generally conceded that the open-top furnaces, fed by hand through a slot in the floor-plates, do not give as satisfactory results as the hooded furnaces with long feed-doors on both sides. In the open-top furnace it is comparatively difficult to throw to the sides; the narrower the slot the greater the difficulty. The major part of the charge will drop near the center, making that place higher than the sides. The fine ore will tend to stay where it falls, while the coarse will tend to roll to the sides, thus leading to an arrangement of the charge just the reverse of what it ought to be. In the hooded furnace most of the material will naturally fall near the doors, causing the sides to be higher than the center toward which the coarse will roll, while the force of 80 the throw as the ore is shoveled in will also have a tendency to concentrate the coarse material in the center.
Once a proper balance of conditions has been found, absolute regularity of routine is the secret of good results. An experienced and intelligent feeder owes his merit to his conscientious regularity of work. He may have to vary his program somewhat when he encounters a furnace that is suffering from the results of bad feeding by a predecessor; but his guiding principle is first to restore regularity, and then maintain it. A poor feeder can bring about, in a single shift, disorders that will require many days to correct, if indeed they are corrected at all during the campaign. The personal element is productive of more harm than good.
Mechanical Feeding.—If it be admitted that the work of a feeder is the better the more it approximates the regularity of that of a machine, it ought to be desirable to eliminate the personal factor entirely and design a machine for the purpose, which would be a comparatively simple matter if it be known just what we want to accomplish. No valid ground now exists for prejudice against mechanical feeding in lead smelting. It is in successful operation in a number of large works, and is being installed in others. Our furnaces have outgrown the shovel; we have passed the limit of efficiency of the old methods of handling material for them. We must come to mechanical feeding in spite of ourselves. But whatever may be the motive leading to its introduction, its chief justification will be discovered, after it has been successfully installed and correctly adjusted, in the consequent great improvement of general operating results, metal saving, etc. It will remove one of the most uncertain factors with which the metallurgist has to deal, thereby bringing into clearer view for study and regulation the other factors (fuel and blast proportion, slag composition, etc.) in a way that has hardly been possible under the irregularities consequent upon hand feeding.
(January 17, 1903)
Historical.—A silver-lead furnace fed by means of cup and cone was in operation in 1888 at the works of the St. Louis Smelting and Refining Company at St. Louis, Mo., but it is probable that previous attempts had been made, since Hahn refers (“Mineral Resources of the United States,” 1883) in a general way to experiments with this device, which were unsuccessful because the heat crept up in the furnace and gave over-fire. At the time of my visit to the St. Louis works (in 1888) the furnaces were showing signs of over-fire, but this may not have been their characteristic condition. A. F. Schneider, who built the St. Louis furnaces, afterward erected, at the Guggenheim works at Perth Amboy, N. J. , round furnaces with cup and cone feeders, but although good results are said to have been obtained, the running of refinery products is no criterion of what they would do on general ore smelting.
Cup and Cone Feeders.—The cup and cone is an entirely rational device for feeding a round furnace, but is quite unsuitable for feeding a rectangular one. Furnaces of the latter type were installed for copper smelting at Aguas Calientes, Mex., with two sets of circular cup and cone feeders, but disastrous results followed the application of this device to lead furnaces. The reason is clear when it is considered that a circular distribution cannot possibly conform to the requirements of a rectangular furnace. A more rational device was designed for the works at Perth Amboy, N. J.
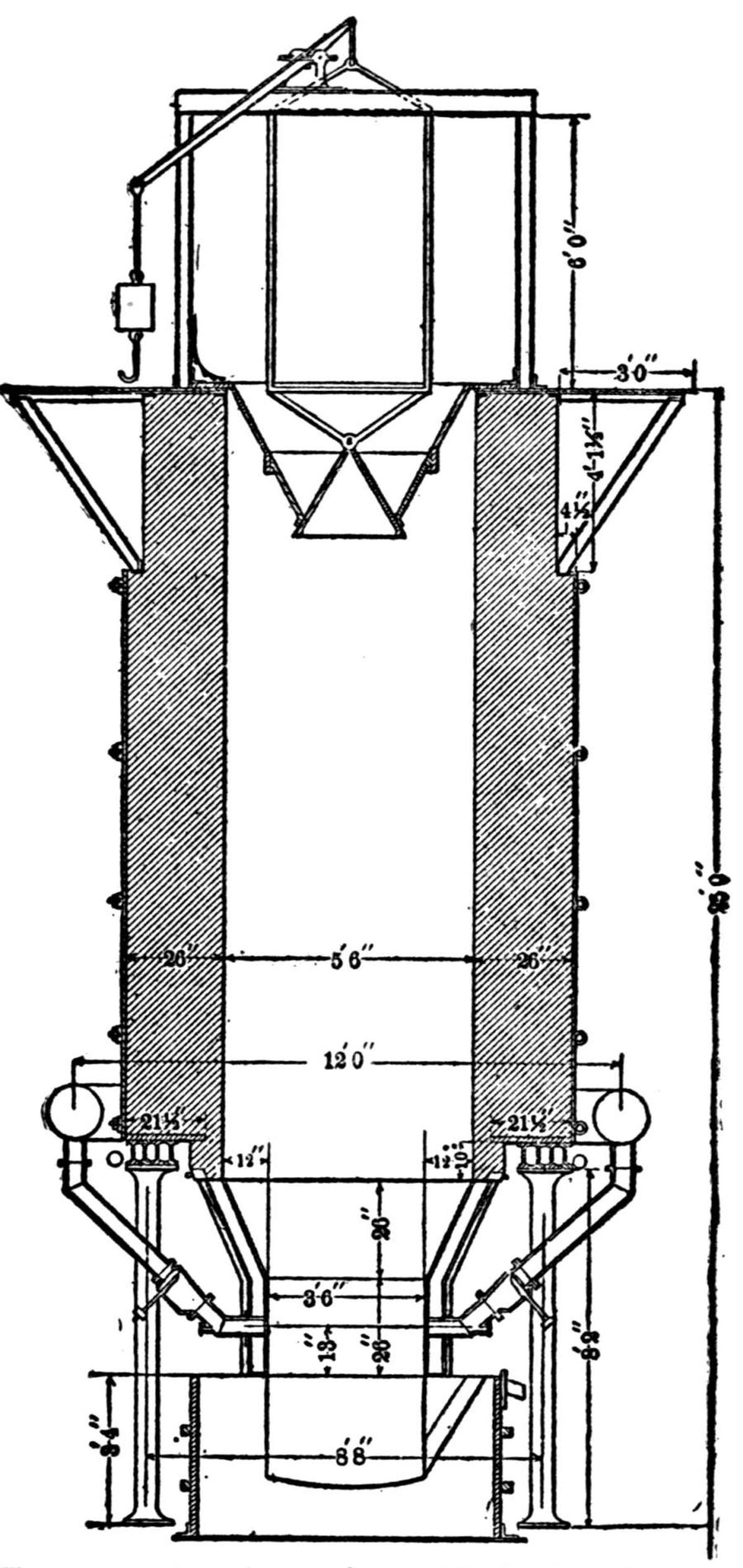
Pfort Curtain.—About ten years ago some of the American smelters adopted the Pfort curtain, which, as adapted to their 82 requirements, consisted of a thimble of sheet iron hung from the iron deck plates so as to leave about 15 in. of space between it and the furnace walls, this space being connected with the down-take of the furnace. The thimble was kept full of ore up to the charge-floor. This device was popular for a time, chiefly because it prevented the furnace from smoking and diminished the labor of feeding, but it was found to give bad results in the furnaces, it being impossible to observe how the charge sunk (except by 83 dropping it below the thimble), while the curtain had to be removed in order to bar down accretions, and, most important, it caused irregular furnace work and high metal losses, because it effected a distribution of the coarse and fine material which was the reverse of correct, the evil being emphasized by the taking off of the gases close to the furnace walls.
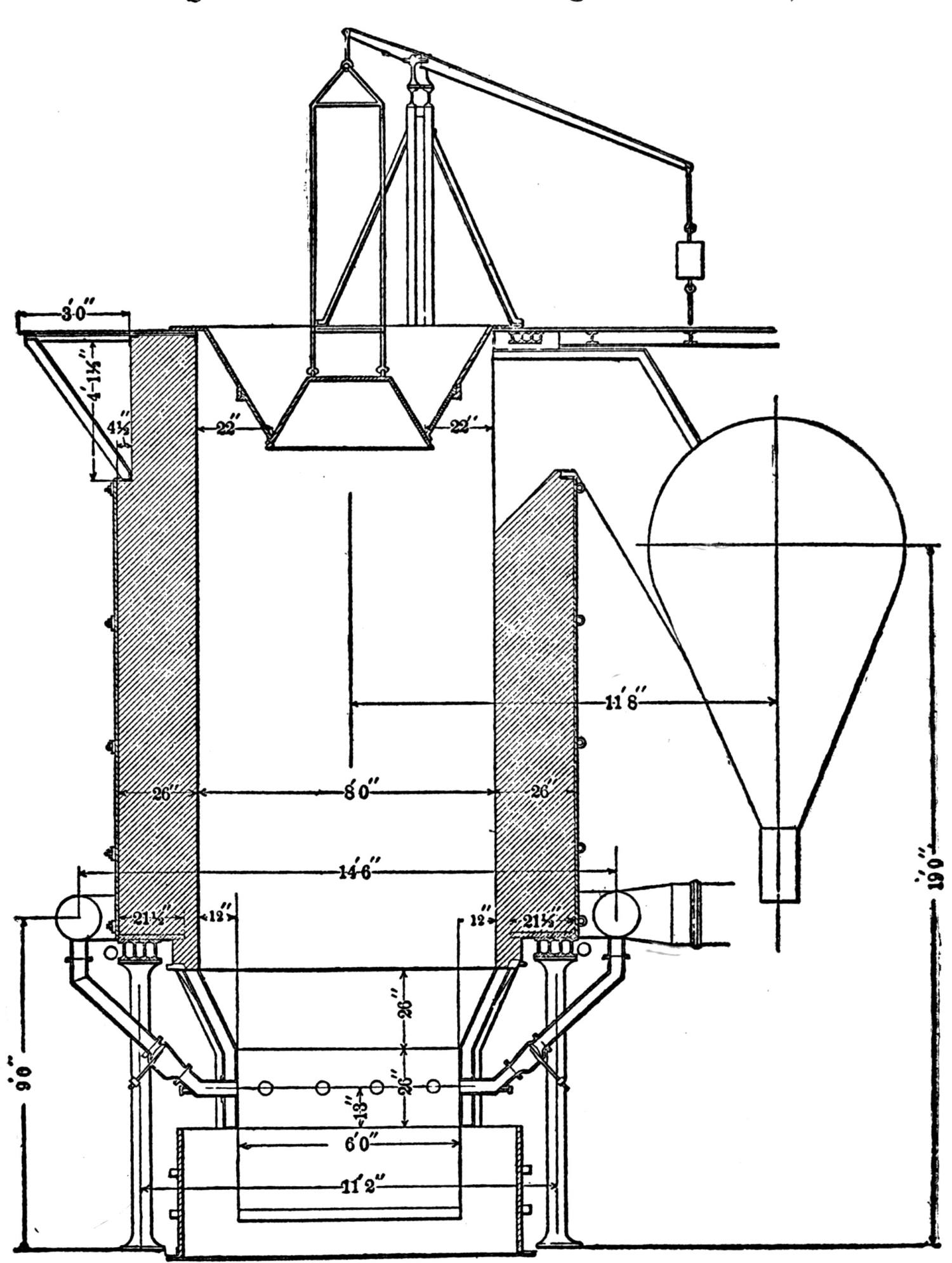
Terhune Gratings.—R. H. Terhune designed a device (United States patent No. 585,297, June 29, 1897), which comprised two grizzlies, one on each side of the furnace, sloping downward from the edge of the charge-floor toward the center line of the furnace. The bars tapered toward the center of the furnace, the open spaces tapering correspondingly toward the sides, so that as the charge was dumped on them a classification of coarse and fine would be effected. This device is correct in conception.
Pueblo System.—In the remodeling of the plant of the Pueblo Smelting and Refining Company in 1895, under the direction of W. W. Allen, mechanical feeding was introduced, and the system was the first one to be applied successfully on a large scale. The furnaces of this plant are 60 × 120 in. at the tuyeres, with six tuyeres, 4 in. in diameter on each side, the nozzles (water cooled) projecting 6 in. inside the jackets. The hight of the smelting column above the tuyeres is 20 ft. The gases are taken off below the charge-floor, and the furnace tops are closed by hinged and counter-weighted doors of heavy sheet iron, opened by the attendant, just previous to dumping the charge-car. In the side walls of the shaft are iron door-frames, ordinarily bricked up, but giving access to the shaft for repairs or barring out without interfering with the movement of the charge-car. Extending across the shaft, about 18 in. above the normal stock line, are three A-shaped cast-iron deflectors, dividing the area of the shaft into four equal rectangles.
The general arrangement of the plant is shown in Fig. 4. From the charge-car pit there extends an inclined trestle, on an angle of 17 deg. to the charge-floor level, in line with the battery of furnaces. The gage of the track is approximately equal to the length of the furnaces at the top. The charge-car, actuated by a steel tail-rope, moves sideways on this track from the charging-pit to any furnace in the battery. The hoisting drums are located at the crest of the incline, inside of the furnace building. At the far end of the latter there is a tightener sheave, with a weight to keep proper tension on the tail-rope. The charge-car has a capacity of 5 tons. It has an A-shape bottom, and is so arranged that one attendant can quickly trip the bolt and discharge the car.
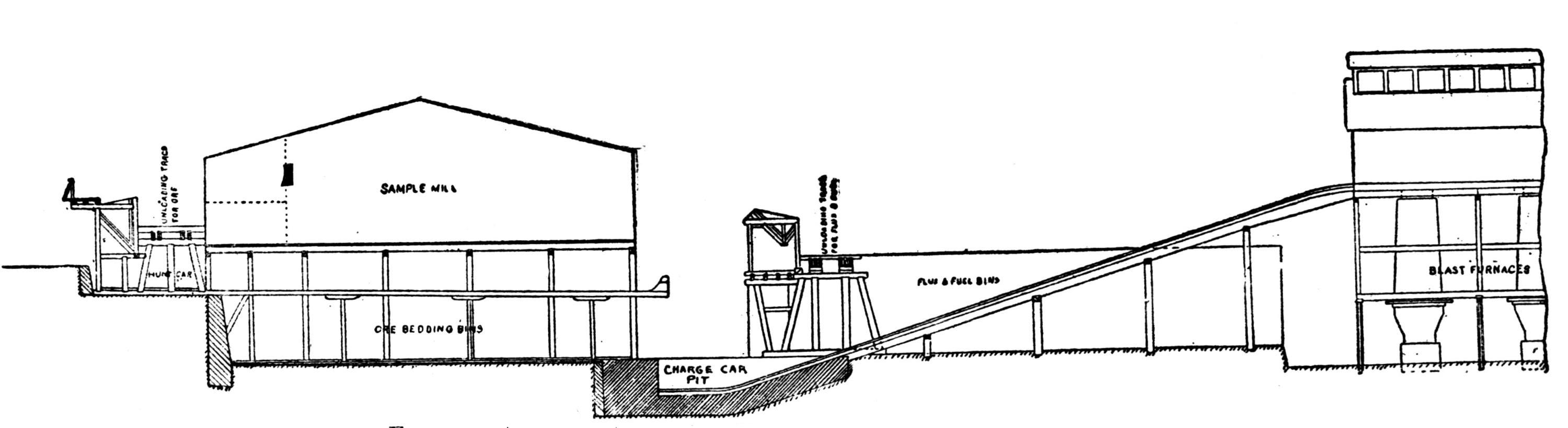
While the car is making its trip the charge-wheelers are filling
their buggies, working in pairs, each man weighing up a half 85
86charge
of a particular ingredient. They then separate, each
taking his proper place in the line of wheelers on either side.
When the car has returned, the wheelers successively discharge
their buggies into opposite ends of the car. The coke is added
last, to avoid crushing. The system is not strictly economical
of labor, since the wheelers, who must always be ready for their
car, have to wait for its return, which necessitates more wheelers
than would otherwise be required. Figs. 5, 6 and 7 show the car.
A vertical section through the car filled by dumping from the two ends will show an arrangement of coarse and fine, which is far from regular. Analyzing its structure, we shall find a conical pile near each end, with a valley between them, in which coarse ore will predominate. The deflectors in the furnace, previously referred to, serve to scatter the fines as the charge is dropped in. Without them the feeding of the furnace would be a failure; with them it is successful, though not so completely as might be, the furnaces having a tendency to run with hot tops. With the battery of seven furnaces, each smelting an average of 100 tons of ore per day, the saving, as compared with hand-feeding, was $63 per day, or 9c. per ton of ore, this including cost of steam, but not wear and tear on the machinery. This is distinctly a maximum figure; with fewer furnaces the fixed charges of the mechanical feed would soon increase the cost per ton to such a figure that the two systems would be about equal in economy.
East Helena System.—This was introduced at the East Helena plant of the United Smelting and Refining Company by H. W. Hixon. The plant comprised four lead furnaces, each 48 × 136 in., with a 21 ft. smelting column. They were all open-top furnaces, fed through a slot over the center, the gases being taken off below the floor. They were capable of smelting about 180 tons of charge (ore and flux) per 24 hours, using a blast of 30 to 48 oz., furnished by two Allis duplex, horizontal, piston blowers, air-cylinders 36 in. diam., 42 in. stroke, belted from electric motors. The Hixon feed was designed to meet existing conditions, without irrevocably cutting off convenient return to hand feeding in case of an emergency. As shown in Fig. 9 there is a track-way at right angles to the line of furnaces. The car hoisted up the incline is landed on a transfer carriage, on which, after detaching the cable, it can be moved over the tops of the furnaces by means of a tail-rope system. The gage of the charge-car is 4 ft. 9 in.; of the transfer carriage, 11 ft. 8 in. A switch at the lower end of the incline permits two charge-cars to be employed, one being filled while the other is making the trip. In sending down the empty car a hand winch is necessary to start it from the transfer carriage. Figs. 10 and 11 show the charge-car; Fig. 12 the transfer carriage.
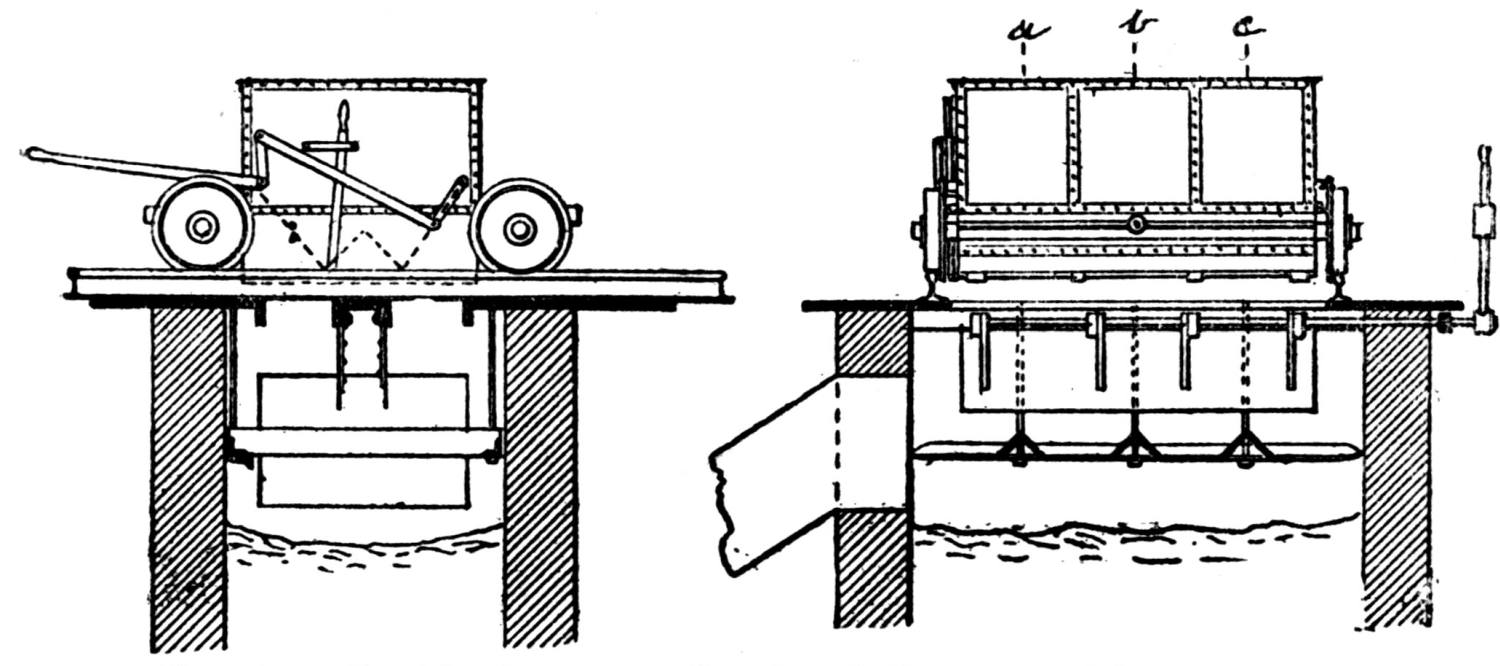
The charge-car is 10 × 4 × 3.5 ft., and has capacity for 6 tons of ore, flux, slag and fuel, the total of ore and flux being usually 8800 lb. Its bottom is flat, consisting of two doors, hinged along the sides and kept closed by means of chains wound about a longitudinal windlass on top of the car. The charging pits are decked with iron plates, leaving a slot along the center of 89 each car exactly like the slot in the furnace top. The loaded ore-buggies are taken from the wheelers by two men, who carefully distribute the contents of each buggy along the whole length of the charge-car by dragging it along the slot while in the act of dumping. Each buggy contains but one ingredient; they follow one another in a prescribed order, so as to secure thin layers in the charge-car. The coke is divided into three or more layers.
The first few trials of this device were not satisfactory. The furnaces quickly showed over-fire, and decreased lead output, which would not yield to any remedy except a return to hand 90 feeding. The total charge being dropped in the center of the furnace, a central core of fines was produced, the lumps tending to roll toward the walls. This wrong tendency was emphasized by the presence of the chains supporting the bottom of the charge-car. On unwinding them to dump the car, the doors were prevented from dropping by the wedging of the chains in the charge, which in turn arched itself more or less against the sides of the car; hence the doors opened but slowly, and often had to be assisted by an attendant with a bar. In consequence of this slow opening, considerable fine ore sifted out first and formed a ridge in the center of the furnace, from the slopes of which the coarser part of the charge, the last to fall, naturally rolled toward the sides. This fact, determined during a visit of the writer in April, 1899, proved to be the key to the situation. The attendant operating the tail-rope mechanism was instructed to move the transfer carriage rapidly backward and forward over the slot while the first one-third or one-half of the charge was dropping, and during the rest of the discharge to let the car stand directly over the slot and permit the coarser material to fall in the center of the furnace. Two piles of comparatively fine material were thus left on the charge-floor, one on each side of the slot. These were subsequently fed in by hand, with instructions to throw the material well to the sides of the furnace.
The furnaces were running very hot on top when this modified procedure was begun. In a few hours the over-fire had disappeared; the lead output was increasing; and the furnaces were running normally. This was done about May 1, 1899, and from 91 that time until about February 20, 1900, the Hixon feed, as modified above, was continuously in operation. In October, 1898, with three furnaces in operation and hand feeding, the labor cost per furnace was $42.06 per day; in October, 1899, with the same number of furnaces and mechanical feeding, it was $41 per day, the saving being only 0.6c. per ton of charge.
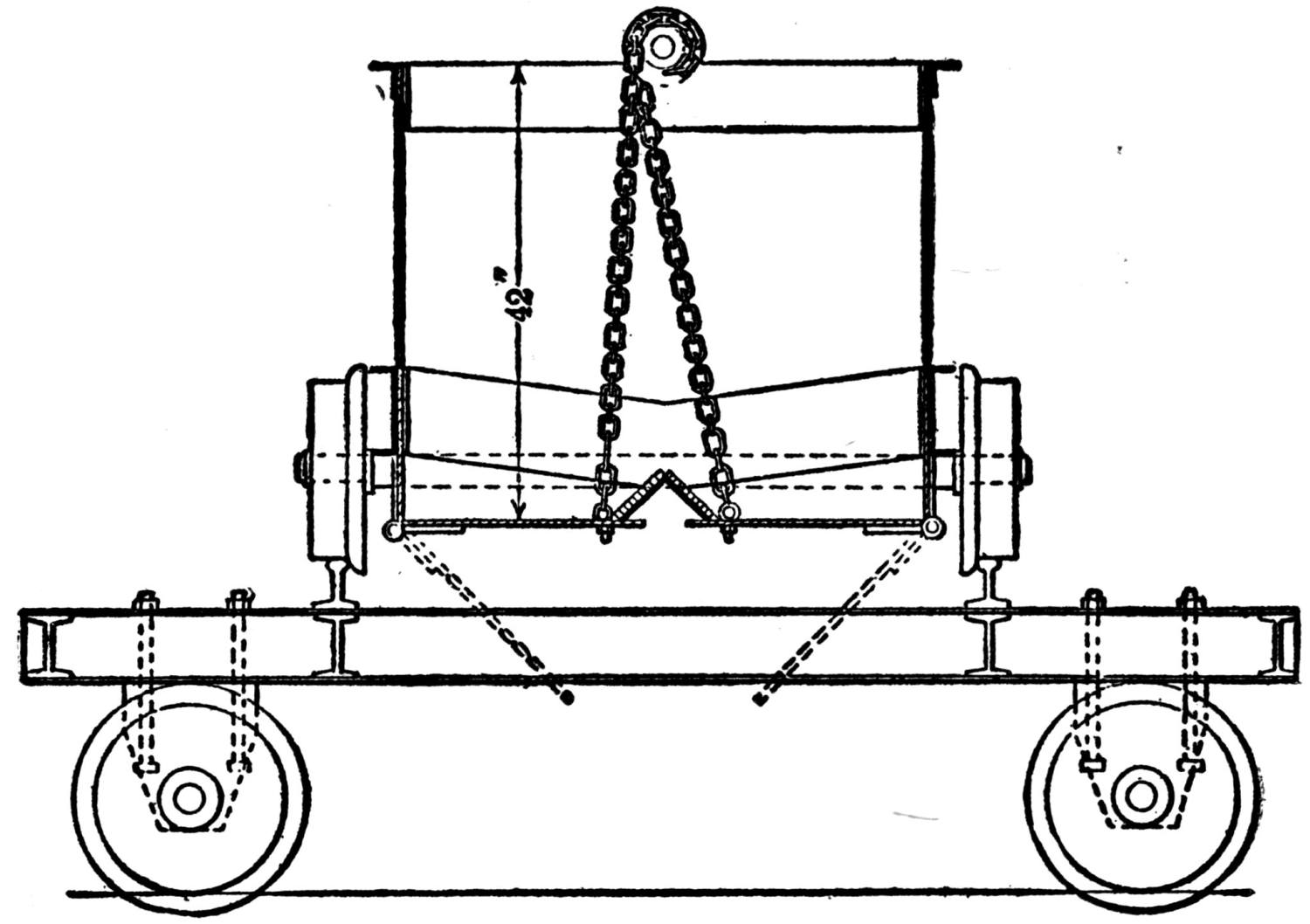
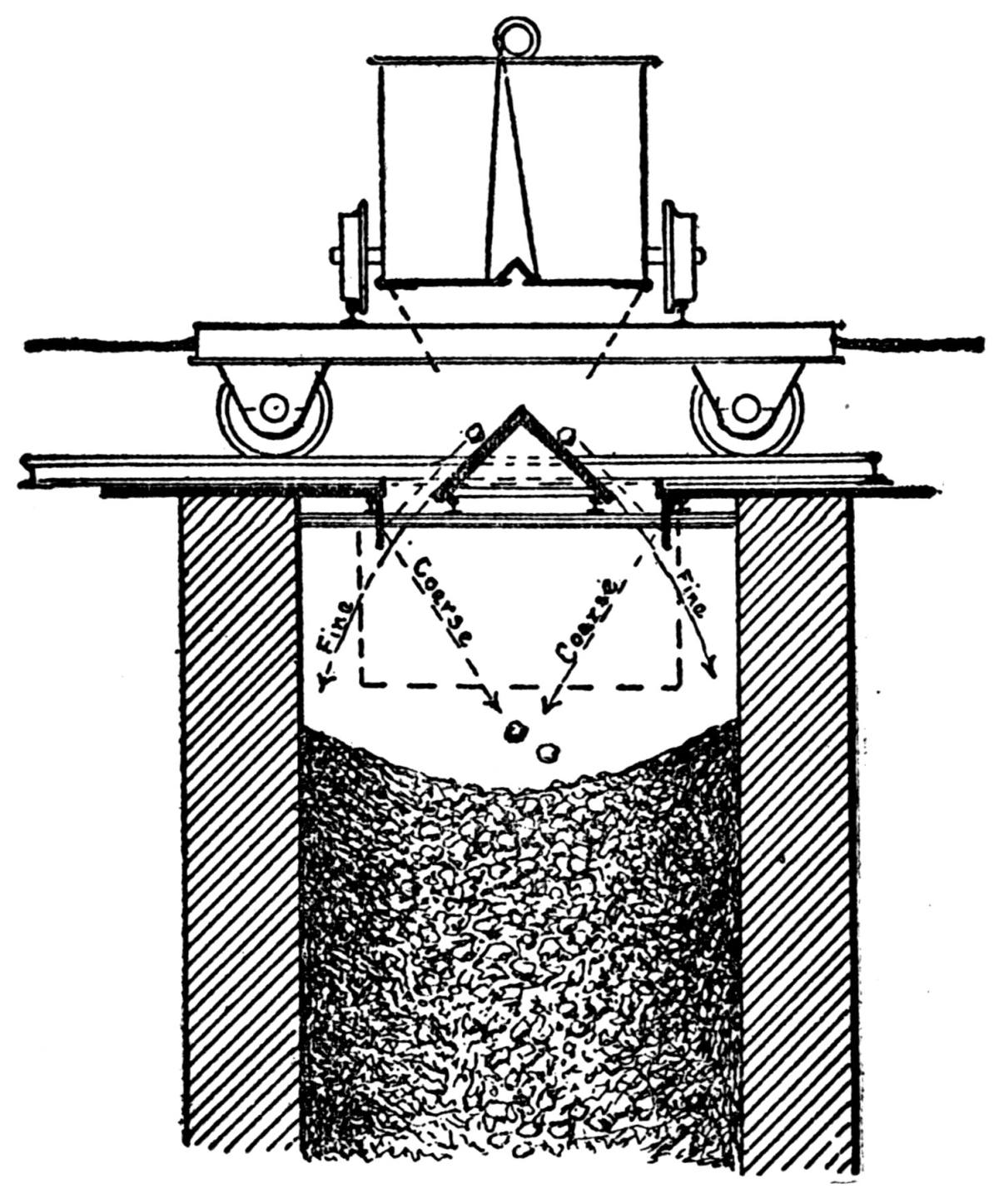
Dwight Spreader and Curtain.—In January, 1900, the writer again had occasion to visit the East Helena plant, to investigate why a certain cheap local coke could not be used successfully instead of expensive Eastern coke. Strange as it may seem, the peculiar behavior of the cokes was traced to improper feeding of the furnaces. Further study of the mechanical feeding system, then in operation for nine months, showed that it was far from perfect, and it appeared desirable to design a spreader which would properly distribute the material discharged from the Hixon car and dispense with hand feeding entirely. An experimental construction was arranged, as shown in Fig. 13. The flanged cast-iron plates around the feeding slot were pushed back and a roof-shaped spreader, with slopes of 45 deg., was set in the gap, leaving openings about 8 in. wide on each side. The plan pro 92vided for two iron curtains to be hung, one on each side of the spreader, and so adjusted that the fine ore sliding down the spreader would clear the edge of the curtain and shoot toward the sides of the furnace, while the coarse ore would strike the curtain and rebound toward the center of the furnace. The classification effected in this manner was capable of adjustment by raising or lowering the curtain. This arrangement was found to work surprisingly well. The first furnace equipped with it immediately showed improvement. It averaged better in speed, with lower blast, lower lead in slag and matte, and better bullion output than the other furnaces operating under the old system. The success of the spreader and curtain being established, the furnaces were provided with permanent constructions, the only modifications being that the ridge of the spreader was lowered to correspond with the level of the floor and the curtains were 93 omitted, the feeding being apparently satisfactory without their aid. In their absence, the lowering of the spreader was a proper step, as it distributed the material fully as well, and caused less abrasion of the walls. The final form is shown approximately in Fig. 14. It has given complete satisfaction at East Helena since February, 1900, and has been adopted as the basis for the mechanical feeding device in the new plant of the American Smelting and Refining Company at Salt Lake, Utah.
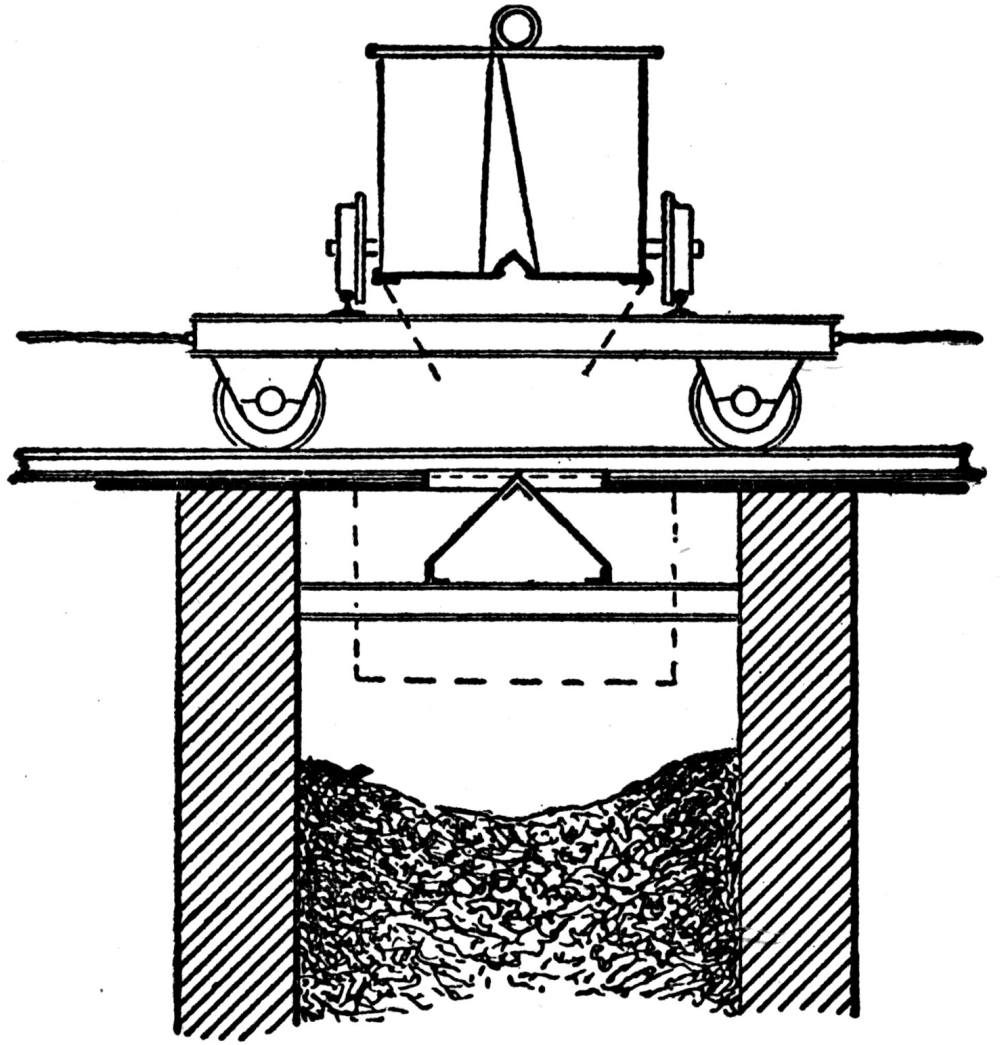
Comparison of Systems.—In mechanical design the Pueblo system is better than the East Helena, being simpler in construction and operation. No time is lost in attaching and changing cables, operating transfer carriage, etc. In both systems the track runs directly over the tops of the furnaces, and this is an inconvenience when furnace repairs are under way. The Pueblo car is the simpler, and makes the round trip in about half the time of a car at East Helena, so the two cars of the latter do not make much difference in this respect. The system of filling the charge-car at Pueblo is also the quicker. It may be estimated 94 roughly that per ton of capacity it takes 2.5 to 3 times as long to fill the East Helena car; and this means longer waiting on the part of the wheelers, and consequently greater cost of moving the material, representing probably 7 or 8c., in favor of Pueblo, per ton of charge handled. However, both systems are wasteful of labor. As to furnace results, it is believed that the better distribution of the charge in the East Helena system leads to greatly increased regularity of furnace running, less tendency to over-fire, some economy in fuel, less accretions on the furnace walls and larger metal savings. If the half of these conclusions are true, the difference of 7 or 8c. per ton in favor of the Pueblo system, which can be traced almost entirely to the cost of filling the charge-car, sinks into insignificance in comparison with the important advantages of having the furnaces uniformly and correctly fed.
True Function of the Charge-Car.—The radically essential feature of a mechanical feeding device is that part which automatically distributes the material in the furnace, whatever approximate means may have been used to effect the delivery.
Taking a hasty review of the numerous feeding devices that have been tried in lead-smelting practice, we cannot but remark the fact that those which depended upon dumping the charge into the furnace from small buggies or barrows failed generally to secure a proper classification and distribution of coarse and fine, and, consequently, were abandoned as unsuccessful, while the adoption of the idea of the charge-car for transporting the material to the furnace in large units seems to have been coincident with a successful outcome. It is natural enough, therefore, that the car should be regarded by many as the vital feature. This view of the question is not, however, in accordance with the true perspective of the facts, and merely limits the field of application in an entirely unnecessary way. It must be apparent that the essential function of the charge-car is cheap and convenient transportation. The distribution of the charge is an entirely different matter, in which, however, the charge-car may be made to assist, as in the Pueblo system; or entirely distinct and special means may be employed for the distribution, as in the East Helena system.
To follow the argument to its conclusion, let us imagine for the moment that the East Helena plant were arranged on the 95 terrace system, with the furnace tops on a level with the floor of the ore-bins. Certain precautions being observed, the spreader would give as good results with small units of charge delivered by buggies as it now does with the large units delivered by the charge-car, and the expense of delivery to the furnaces would be practically no more than it now is to the charge-car pit. The furnace top would, of course, have to be arranged so that the buggies, in discharging, could be drawn along the slot, so as to give the necessary longitudinal distribution parallel to the furnace walls, just as is now done in filling the charge-car. The ends of the spreader, if built like a hipped roof, would secure proper feeding of the front and back.
Thus, by eliminating the charge-car, and with it the necessity for powerful hoisting machinery, with its expensive repairs and operating costs, we may greatly simplify the problem of mechanical feeding, and open the way for the adoption of successful automatic feeding in many existing plants where it is now considered impracticable.
(August 18, 1900)
In the technical literature of lead smelting there is a lamentable lack of data on the subject of costs. The majority of writers consider that they have fulfilled their duties if they discuss in full detail the chemical and engineering sides of the subject, leaving the industrial consideration of cost to be wrought out by experience. When an engineer or metallurgist collects data on the costs involved in the various smelting operations, he generally hesitates to give this special information to the public, as he regards it as private, or reserves it as stock in trade to be held for his own use.
The following tables of cost have been compiled from actual results of smelting and refining at the Globe works, Denver, Colo., and are offered in the hope that they will prove a valuable addition to the literature of lead smelting. These results are offered tentatively, and, while true for the periods stated, they require considerable adjustment to meet the smelting conditions of the present time.
COST OF HAND-ROASTING PER TON (2000 LB.) OF ORE
| 1887 | $3.975 | │ | 1893 | — |
| 1888 | 4.280 | │ | 1894 | 3.429 |
| 1889 | 4.120 | │ | 1895 | 2.806 |
| 1890 | 3.531 | │ | 1896 | 2.840 |
| 1891 | 3.530 | │ | 1897 | 2.740 |
| 1892 | — | │ | 1898 | 2.620 |
At first the roasting was done mainly by hand roasters; later two Brown-O’Harra mechanical furnaces were used, and the cost was reduced, but not to the extent usually conceded to this type of furnace, as the large amount of repairs and the consequent loss of time diminished the apparent gain due to greater output. The figures quoted above may be considered somewhat higher than the average, as the roasters were charged in proportion with expenses of general management, office, etc.
In viewing the yearly reduction of costs one must take into consideration many changes in the furnace construction and working, as well as the items of labor, fuel, etc. From 1887 to 1899 the principal changes in the construction of the hand-roasting furnaces consisted in an increase of width, 2 ft., which allowed an addition of 200 lb. to each ore charge, and corresponded to a total increase per furnace of 1200 lb. in 24 hours. In the working of the charge an important change was made in the condition of the product. Formerly the material was fused in the fusion-box and drawn from the furnace in a fused or slagged condition; and while this gave an excellent material for the subsequent treatment in the shaft furnace in that there was very little dusting of the charge, and a considerable increase in the output of the furnace, the disadvantages of large losses of lead and silver greatly over-balanced the advantages, and called for an entire abandonment of the fusion-box. As a result of experience it was found that the best condition of product is a semi-fused or sintered state, in which the particles of roasted ore have been compressed by pounding the material, which has been drawn into the slag pots, with a heavy iron disk. The amount of “fines” under these conditions is quite small and depends upon the percentage of lead in the ore, the degree of heat employed, and the extent of the compression.
The total cost was partly reduced from the lessened labor cost following the financial disturbance of 1893, and partly from the reduction in the fuel cost, the former expensive lump coal being replaced by the slack coals from southern Colorado.
The comparison of the cost of labor by the two methods shows a gain of 54c. a ton in favor of the mechanical furnaces. However, I consider that this gain is a costly one, and is more than offset by the large amount of high-grade fuel required, and the expense of repairs not shown in the following table. Indeed, I believe that at the end of five or ten years the average cost of roasting per ton by the hand roasters will be even smaller than by these mechanical roasters.
To illustrate the details of roasting cost and to furnish a comparison of the hand roasters and mechanical furnaces, the following table has been prepared:
DETAILS OF AVERAGE MONTHLY COST FOR 1898 OF HAND ROASTERS AND MECHANICAL FURNACES
| Month | Hand Roasters | Brown-O’Harra Mechanical Furnaces | ||||||
|---|---|---|---|---|---|---|---|---|
| Total Tons Roasted Per Day | Tons Roasted Per Day | Labor | Coal | General Expense | Labor | Coal | General Expense | |
| January | 5,691 | 184 | $1.47 | $0.53 | $0.80 | $0.92 | $0.80 | $1.32 |
| February | 5,677 | 203 | 1.44 | 0.44 | 0.99 | 0.72 | 0.58 | 1.01 |
| March | 5,821 | 188 | 1.51 | 0.53 | 0.64 | 0.76 | 0.64 | 0.62 |
| April | 5,472 | 182 | 1.47 | 0.47 | 0.71 | 0.80 | 0.69 | 0.87 |
| May | 5,444 | 176 | 1.55 | 0.51 | 0.84 | 0.80 | 0.69 | 0.81 |
| June | 4,859 | 162 | 1.58 | 0.48 | 0.71 | 0.90 | 0.68 | 1.17 |
| July | 5,691 | 184 | 1.59 | 0.48 | 0.75 | 0.72 | 0.56 | 0.64 |
| August | 5,910 | 191 | 1.55 | 0.46 | 0.83 | 0.72 | 0.55 | 0.75 |
| September | 5,677 | 189 | 1.55 | 0.45 | 0.74 | 0.73 | 0.55 | 0.67 |
| October | 6,254 | 202 | 1.48 | 0.49 | 0.72 | 0.65 | 0.50 | 0.60 |
| November | 6,291 | 213 | 1.42 | 0.47 | 0.80 | 0.66 | 0.53 | 0.70 |
| December | 5,874 | 198 | 1.45 | 0.48 | 0.78 | 0.79 | 0.63 | 0.81 |
| Average | $1.50 | $0.48 | $0.77 | $0.76 | $0.62 | $0.83 | ||
| Total | 2.75 | 2.21 | ||||||
Cost of Smelting.—The lead-ore mixtures of the United States, in addition to lead, contain gold, silver and generally copper, and are treated to save these metals. The total cost of smelting is made up of a large number of items. The questions of locality and transportation, fuel, fluxes and labor are the principal factors, to which must be added the handling of the material to and from the furnace; the furnace itself, its size, shape, and method of smelting, the volume and pressure of blast, etc. The following table of costs, from 1887 to 1898, shows in a general way the great advance that has been made in the development of smelting, and the consequent reduction in cost per ton of ore treated:
AVERAGE COST OF SMELTING, PER TON
| 1887 | $4.644 | 1891 | 4.170 | 1895 | 2.786 |
| 1888 | 4.530 | 1892 | 4.906 | 1896 | 2.750 |
| 1889 | 4.480 | 1893 | 3.375 | 1897 | 2.520 |
| 1890 | 4.374 | 1894 | 3.029 | 1898 | 2.260 |
In connection with this table of smelting cost should be considered the changes developed during the interval 1887-1889, outlined as follows:
CONDITIONS OF SMELTING IN 1886 AND 1899 CONTRASTED TO SHOW THE PROGRESS OF DEVELOPMENT
| Area of Furnace at Tuyeres, In. | Height of Charge from Tuyeres, Ft. | Blast Pressure, Lb. per Sq. In. | Fore Hearth Capacity, Cu. Ft. | Slag Settled | Fuel | Slag Removed, Lb. per Trip | Matte Removed, Lb. per Trip | |
|---|---|---|---|---|---|---|---|---|
| 1886 | 30 × 100 | 11 | 1 | 6 | In pots | Charcoal | By hand 280 | By hand 200 |
| 1899 | 42 × 140 | 16 | 3 to 4 | 128 | In furnaces | Coke | By locomotive 3000-6000 | By horse 2000-3000 |
I believe that there is room for further improvement in the substitution of mechanical transportation within the works for hand labor, and that the fuel cost can be materially reduced by replacing the coke, which at present contains 16 to 22 per cent. of ash, by a fuel of purer and better quality.
Cost of Refining by the Parkes Process.—In general it may be stated that the average cost of refining base bullion is from $3 to $5 a ton. This amount is based on the cost of labor, spelter, coal, coke, supplies, repairs and general expenses. When the additional items of interest, expressage, brokerage and treatment of by-products are considered, which go to make up the total refining cost, the amount may be stated approximately as $10 per ton of bullion treated.
Variations in the cost occur from time to time, and are due to several causes, principally the irregularity of the bullion supply and its consequent effect on the work of the plant. When the amount of bullion available for treatment is small, the plant cannot be run to its maximum capacity, and the cost per ton will naturally be increased. To illustrate this variation, the average cost per ton of base bullion refined during nine months in 1893 was:
January, $4.864; February, $5.789; March, $5.024; April, $3.915; May, $5.094; June, $4.168; July, $4.231; August, $4.216; September, $5.299.
The yearly variation shows but little change, as the average cost per ton was for 1893, $4.75; for 1894, $3.99; for 1895, $4.21; for 1896, $3.90. In considering the total cost of refining, the additional factors of interest, expressage, parting, brokerage, and reworking of by-products must be considered. As the doré silver is treated at the works or elsewhere, so will the total cost be less 100 or greater. The following table gives the cost in detail, when the parting is done at the same works:
AVERAGE MONTHLY COST OF REFINING PER TON OF BULLION TREATED
| Items | 1895Jan. to July | 1895July to Dec. | 1896Jan. to July | Average |
|---|---|---|---|---|
| Labor | $2.351 | $1.718 | $1.836 | $1.968 |
| Spelter | 0.757 | 0.840 | 0.987 | 0.861 |
| Coal | 0.585 | 0.442 | 0.461 | 0.496 |
| Coke | 0.634 | 0.418 | 0.511 | 0.521 |
| Supplies, repairs and general expenses |
0.343 | 0.273 | 0.252 | 0.289 |
| Interest | 1.808 | 1.075 | 1.070 | 1.317 |
| Expressage | 1.360 | 1.015 | 0.882 | 1.085 |
| Parting and brokerage | 2.483 | 2.084 | 1.796 | 2.121 |
| Reworking by-products | 1.567 | 1.286 | 1.625 | 1.492 |
| Totals | $11.888 | $9.151 | $9.420 | $10.151 |
| Tons bullion refined 5,511.58 | 9,249.07 | 10,103.43 | 8,287.99 |
An analysis of the different items of cost is important, and a brief summary is given below.
Labor and Attendance.—The cost for this item varies but little from year to year, and its reduction depends, for the most part, on a larger yield per man rather than on a reduction of wages. If a man at the same or slightly increased cost can give a larger output, so will the labor cost per ton be diminished. This result is accomplished by enlarging the furnace capacity and by using appliances which will handle the bullion and its products in an easier and quicker manner. The small size of the furnaces, settlers and retorts used at modern refineries is open to criticism; I believe that great improvement can be made in this direction.
Spelter.—The cost of this item varies with the market conditions, and will probably be changed but little in the future, as the amount necessary per ton of bullion seems to be fixed.
Coal.—The amount required per ton of bullion is fairly constant, and while lessened cost for fuel may be attained by the substitution of oil or gaseous fuel, the fuel cost in comparison with the aggregate cost is very small, and leaves little opportunity for improvement in this line.
Supplies.—This item includes brooms, shovels, wheelbarrows, etc., and the amount is small and fairly constant from year to year.
Repairs.—This item is quite small in works properly con 101structed; and in this connection I wish to call particular attention to the floor covering, which should be made of cast-iron plates from 1.5 to 2 in. thick, and placed on a 2 to 3 in. layer of sand spread over the well-tamped and leveled ground. The constant patching of brick floors is not only an annoyance, but is costly from the additional labor required. Furthermore, a brick floor does not permit a close saving of the metallic scrap material.
It will be found economical in the long run to protect all exposed brickwork of furnaces or kettles with sheet iron.
In the construction of the refinery building I should advise brick walls except at the end or side, where there is the greatest likelihood of future extension; here corrugated iron may be used. The roof should not be made of corrugated iron, as condensed or leakage water is liable to collect and drop on those places where water should be scrupulously avoided. The presence of water in a mold at the time of casting, even though small in amount, will cause explosions and will scatter the molten lead, endangering the workmen.
The item of repair for the ordinary corrugated iron roof may be diminished by constructing it of 1 in. boards with intervening spaces of half an inch, the whole overlaid with tarred felt, and covered with sheets of iron at least No. 27 B. W. G., painted with graphite paint and joined together with parallel rows of ribbed crimped iron.
General Expenses.—This item is generally constant, and calls for no special comment.
Interest.—This important item is, as a rule, considerable, as the stock of bullion and other gold-and silver-bearing material is quite large. For this reason special attention should be given to prevent the accumulation of stock or by-products. The occasional necessity of additional capital to run the business should preferably be met by an increase of working capital, rather than by a direct loan.
Expressage.—This item, as a rule, is large, and should be taken into consideration in the original plans for the location of the refining works.
Parting.—The item of parting and brokerage is the largest of the refinery costs, and for obvious reasons a modern smelting plant should have a parting plant under its own control.
The Working of the By-Products.—This constitutes a large item 102 of cost, and considerable attention should be devoted to the improvement of present methods, which seem faulty, slow and expensive.
Summary.—The items of smaller cost with their respective amounts per ton of base bullion treated are: Spelter, $0.85; coal, $0.50; coke, $0.50; supplies, repairs and general expenses, $0.35; total, $2.10. It is doubtful whether much improvement can be made in the reduction of these costs.
The items of larger cost are: Labor, $2; interest, $1.32; expressage, $1.10; parting and brokerage, $2; reworking by-products, $1.50; total, $7.92. The general manager usually attends to the items of interest, expressage and brokerage, leaving the questions of labor and working of by-products to the metallurgist.
The cost quoted for smelting practice, as employed at Denver, will differ necessarily from those at other localities, where the cost of labor, freight rates on spelter, fuel, etc., are changed. Refining can doubtless be done at a lower cost at points along the Mississippi River, and even more so at cities on the Atlantic seaboard, as Newark or Perth Amboy, N. J.
The consolidation of many of the more important smelting plants of the United States under one management will doubtless alter the figures of cost given above, particularly as the interest cost there stated is at the high rate of 10 per cent., a condition of affairs now changed to 5 per cent. Other factors have lessened the cost of refining; the bullion produced at the present time is softer, or contains a smaller amount of impurities, and admits of easier working with shorter time and less labor. By proper management larger tonnages are turned out per man, and the Howard stirrer and Howard press have simplified and cheapened the working of the zinc skimmings. To illustrate the comparatively recent conditions of cost I have compiled the following table for each month of the year 1898:
COST OF REFINING DURING 1898, INCLUDING LABOR, SPELTER, COAL, COKE, SUPPLIES, REPAIRS AND GENERAL EXPENSES.
| January | $3.59 | May | 3.38 | September | 3.35 |
| February | 3.28 | June | 3.56 | October | 3.45 |
| March | 3.26 | July | 3.65 | November | 3.20 |
| April | 3.59 | August | 3.54 | December | 3.56 |
| Average cost during the year, $3.45. | |||||
It is understood, of course, that these figures do not include cost of interest, expressage, parting, brokerage and reworking of by-products.
[Although this article refers to conditions in 1898, since which time there have been improvements in practice, the latter have not been of radical character and the figures given are fairly representative of present conditions.—Editor.]
(March 22, 1906)
The following notes were taken from work done at the Cherokee Lanyon Smelter Company, Gas, Kansas, in 1903. It was practically an experiment. The furnace was only 36 × 90 in. at the crucible, with a 10 in. side bosh and a 6 in. end bosh. There were five tuyeres on each side with a 3 in. opening. The side jackets measured 4.5 ft. × 18 in. The distance from top of crucible to center of tuyeres was 11.5 in.
The blast was furnished by one No. 4½ Connellsville blower. The furnace originally was only 11 ft. from the center of tuyeres to the feed-floor, and had only been saving about 60 per cent. of the lead. This loss of lead, however, was not entirely due to the low furnace. As no provision had been made to separate the slag and matte, upon assuming charge I raised the feed-floor 3 ft., thereby changing the distance from the tuyere to top of furnace from 11 ft. to 14 ft. Matte settlers were also installed. These two changes raised the percentage of lead saved to 92, as shown by monthly statements. The furnace being small, and a high percentage of zinc oxide on the charge, the campaigns were naturally short. The longest run was about six weeks. This was made on some residue that had been screened from the coarse coal, and coke, and had weathered for several months. This particular residue also carried about 10 per cent. lead. The more recent residue that had not been screened and weathered, and was low in lead, did not work so well. Although these residues consisted of a large proportion of coal and coke, it seemed impossible to reduce the percentage of good lump coke on the charge lower than 12.5 or 13 per cent. At the same time the reducing power of the residue was strong, and with the normal amount of coke caused some trouble in the crucible.
When residue containing semi-anthracite coal was smelted, the saving in lead dropped, and the fire went to the top of the furnace, burning with a blue flame, thereby necessitating the reduction of this class of material. This residue had been screened through a five-mesh screen, and wet down in layers, becoming so hard that it had to be blasted. The low saving of lead with this class of material was a surprise, as it has been claimed that the substitution of part of the fuel by anthracite coal did not affect the metallurgical operations of the furnace.
The slag was quite liquid and flowed very well at all times. However, there was a marked variation in the amount at different tappings. This, I am satisfied, was not due to irregular work on the furnace, but may be accounted for in the following manner. The residue (not screened or weathered to any extent), consisting approximately of one-half coal and coke, was very bulky, and while there was about 35 per cent. of it on the charge by weight, there was over 50 per cent. of it by bulk, not including slag and coke. In feeding, therefore, it was a difficult matter to mix the whole of it with the charge. Several different ways of feeding the furnace were tried. The one giving the most satisfactory results was to feed nearly all of the residue along the center of the furnace, in connection with the lime-rock, coarse ore and coarse iron ore, and the fine and easy smelting ores along the sides. The slag was spread uniformly over the whole furnace, while the sides were favored with the coke. The charge would drop several inches at a time, going down a little faster in the center than on the sides.
It is possible that a small proportion of the residue in connection with the easy smelting, leady, neutral ore, iron ore and lime-rock formed the type of slag marked No. 1.
| SiO2 | FeO | MnO | CaO | ZnO | Pb | Ag | |
|---|---|---|---|---|---|---|---|
| 1 | 33.7 | 34.1 | 1.0 | 16.5 | 7.5 | 0.9 | 0.7 |
| 2 | 31.0 | 36.1 | 1.2 | 16.0 | 9.6 | 1.3 | — |
This being tapped with a good flow of slag, the charge would drop, bringing a proportionately large amount of residue in the fusion zone which formed the type of slag marked No. 2. There was also a marked variation in the slag-shells from different pots. 106 The above cited irregularities of course exist to a certain extent in any blast furnace.
AVERAGE ANALYSIS OF MATERIALS SMELTED
| Name | SiO2 | FeO | CaO | MgO | ZnO | Al2O3 | Fe2O3 | S | Pb | Cu | Ag | Au |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mo. iron ore | 10.0 | 65.0 | ; | ; | ; | ; | ; | ; | ; | ; | ; | ; |
| Lime rock | 1.5 | ; | 52.0 | ; | ; | ; | ; | ; | ; | ; | ; | ; |
| Mo. galena | 1.5 | 2.4 | ; | ; | 9.5 | ; | ; | 11.0 | 74.0 | ; | ; | ; |
| Av. of beds | 50.8 | 16.2 | ; | ; | 4.6 | ; | ; | 3.3 | 9.1 | ; | ; | ; |
| Residue[14] | 10.5 | 38.5 | ; | ; | 18.0 | ; | ; | 4.8 | 2.2 | 1.0 | 10.0 | 0.03 |
| Roasted matte[15] | 9.0 | 48.0 | 3.0 | ; | 10.0 | ; | ; | 4.0 | 9.9 | 3.0 | 21.0 | 0.06 |
| Barrings | 18.8 | 24.4 | 5.0 | ; | 14.5 | ; | ; | 6.0 | 25.4 | ; | 13.0 | 0.07 |
| Coke ash | 27.0 | ; | 14.9 | 4.5 | ; | 19.7 | 31.6 | ; | ; | ; | ; | ; |
| H2O | V.M. | F.C. | Ash | S | ; | ; | ; | ; | ; | ; | ; | |
| Coke[16] | 1.2 | 2.3 | 85.7 | 11.1 | 0.9 | ; | ; | ; | ; | ; | ; | ; |
ANALYSIS OF BULLION, SLAG AND MATTE PRODUCED
| Bullion | Slag | Matte | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ag | Au | SiO2 | FeO | MnO | CaO | ZnO | Pb | Ag | Ag | Au | Pb | Cu | |
| Feb. | 90.0 | 1.15 | 31.2 | 35.9 | 1.0 | 14.5 | 10.3 | 0.88 | 0.98 | 19.0 | 0.04 | 8.7 | 1.5 |
| March | 93.1 | 1.63 | 31.3 | 37.2 | 1.0 | 13.9 | 11.1 | 0.71 | 1.30 | 21.0 | 0.06 | 8.0 | 2.5 |
| April | 104.3 | 1.59 | 29.8 | 37.7 | 2.7 | 13.9 | 11.4 | 0.52 | 1.40 | 23.0 | 0.07 | 7.0 | 3.5 |
| May | 90.0 | 1.24 | 30.0 | 37.3 | 2.2 | 14.1 | 9.3 | 0.86 | 1.10 | 25.4 | 0.07 | 5.1 | 4.0 |
| July | 78.7 | 1.00 | 32.2 | 37.4 | 1.0 | 13.9 | 9.8 | 0.50 | 1.15 | 21.3 | 0.03 | 8.9 | 4.0 |
| Aug. | 90.8 | 1.21 | 31.2 | 37.1 | 1.7 | 13.7 | 9.6 | 1.10 | 1.60 | 23.1 | 0.08 | 9.8 | 3.0 |
| Sept. | 65.3 | 2.58 | 32.0 | 39.7 | 0.8 | 14.1 | 8.1 | 0.80 | 1.30 | 18.6 | 0.06 | 7.6 | 2.3 |
| Average | 87.5 | 1.49 | 31.1 | 37.5 | 1.5 | 14.1 | 10.0 | 0.77 | 1.26 | 21.6 | 0.06 | 7.8 | 3.0 |
MONTHLY RECORD OF FURNACE OPERATIONS
| Blast Ounces | Tons per F. D. | Per Cent. Pb. on Charge | Per Cent. Coke on Charge | Per Cent. Slag on Charge | Per Cent. S on Charge | Matte Produced | Saving | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Ag | Au | Pb | ||||||||
| Feb. | 21 | 42.5 | 9.0 | 12.0 | 30.0 | 3.7 | 8.0} | 84.4 | 83.0 | 90.3 |
| March | 21 | 44.8 | 9.7 | 13.5 | 37.0 | 4.0 | 9.0} | |||
| April | 21 | 43.7 | 9.0 | 13.5 | 35.0 | 4.3 | 10.0 | 97.9 | 70.5 | 96.6 |
| May | 21 | 49.4 | 10.0 | 13.5 | 30.0 | 3.5 | 6.5 | 95.6 | 109.5 | 88.8 |
| July | 17 | 41.0 | 9.8 | 12.5 | 34.0 | 3.8 | 6.0 | 97.9 | 90.0 | 92.9 |
| August | 18 | 47.0 | 9.3 | 13.0 | 32.0 | 3.7 | 6.3 | 86.2 | 107.5 | 87.6 |
| Sept.[17] | 15 | 51.0 | 7.3 | 13.0 | 30.0 | 2.8 | 4.6 | 92.9 | 94.0 | 95.6 |
| Average | 45.6 | 9.1 | 13.0 | 32.6 | 3.7 | 7.2 | 90.8 | 92.4 | 92.0 | |
I believe that, in smelting residues high in zinc oxide, better metallurgical results would be obtained by using a dry silicious ore in connection with a high-grade galena ore, provided the residue be low in sulphur. This was confirmed to a certain degree in actual practice, as the furnace worked very well upon increasing the percentage of Cripple Creek ore on the charge. This would also seem to indicate that alumina had no bad effect on a zinky slag.
(December 24, 1903)
From time to time, in various articles and letters on metallurgical subjects in the Engineering and Mining Journal, the question of the removal of zinc oxide in slags is referred to, and the question is raised as to the form in which it is contained in the slags.
I gather that opinion is divided as to whether zinc oxide enters into the slags as a combined silicate, or whether it is simply carried into them in a state of mechanical mixture.
For many years I have taken great interest in the composition of slags, and have studied them microscopically and chemically. The conclusion to which I have been led as regards zinc oxide is, that in a not too basic slag it is originally mainly, if not wholly, taken up as silicate along with the other bases. On one occasion, one of my furnaces for several days produced a slag in which beautiful crystals of willemite were very abundant, both free in cavities and also imbedded throughout the mass of solid slag, as shown in thin sections under the microscope. In the same slag was a large amount of magnetite, all of which contained a considerable proportion of zinc oxide combined with it. Magnetite crystals, separated out from the slag and treated with strong acid, yielded shells of material retaining the form of the original mineral, rich in zinc oxide; an inter-crystallized zinc-iron spinel, in fact. I have seen and separated zinc-iron spinels very rich in zinc oxide from other slags. They have been seen in the slags at Freiberg; and of course everybody knows the very interesting paper by Stelzner and Schulze, in which they described the beautiful formations of spinels and willemite in the walls of the retorts of zinc works.
I think there is thus good ground for concluding that zinc oxide is slagged off as combined silicate, and that free oxide does not exist in slags; though zinc oxide does occur in them after solidification, combined with other oxides, in forms ranging 109 from a zinkiferous magnetite to a more or less impure zinc-iron, or zinc-iron-alumina spinel, these minerals having crystallized out in the earlier stages of cooling.
The microscope showed that the crystals of willemite, mentioned above, were the first things to crystallize out from the molten slag. The main constituent was well-crystallized iron-olivine-fayalite.
(July 6, 1905)
It is a fact, not generally known, that the American Smelting and Refining Company is now preparing to introduce the Huntington-Heberlein process in all its plants, this action being the outcome of extensive experimentation with the process. It is contemplated to employ the process not only for the desulphurization of all classes of lead ore, but also of mattes. This is a tardy recognition of the value of a process which has been before the metallurgical profession for nine years, the British patent having been issued under date of April 16, 1896, and has already attained important use in several foreign countries; but it will be the grandest application in point of magnitude.
The Huntington-Heberlein is the first of a new series of processes which effect the desulphurization of galena on an entirely new principle and at great advantage over the old method of roasting. They act at a comparatively low temperature, so that the loss of lead and silver is reduced to insignificant proportion; they eliminate the sulphur to a greater degree; and they deliver the ore in the form of a cinder, which greatly increases the smelting speed of the blast furnace. They constitute one of the most important advances in the metallurgy of lead. The roasting process has been the one in which least progress has been made, and it has remained a costly and wasteful step in the treatment of sulphide ores. In reducing upward of 2,500,000 tons of ore per annum, the American Smelting and Refining Company is obliged to roast upward of 1,000,000 tons of ore and matte.
The Huntington-Heberlein process was invented and first applied at Pertusola, Italy. It has since been introduced in Germany, Spain, Great Britain, Mexico, British Columbia, Tasmania, and Australia, in the last at the Port Pirie works of the Broken Hill Proprietary Company. Efforts were made to introduce it in the United States at least five years ago, without success and with little encouragement. The only share in this metallurgical improvement that this country can claim is that Thomas Huntington, one of the inventors, is an American citizen, Ferdinand Heberlein, the other, being a German.
(September 22, 1905)
The article of Professor Borchers (see p. 116) is, we believe, the first critical discussion of the reactions involved in the new methods of desulphurizing galena, as exemplified in the processes of Huntington and Heberlein, Savelsberg, and Carmichael and Bradford, although the subject has been touched upon by Donald Clark, writing in the Engineering and Mining Journal. It is perfectly obvious from a study of the metallurgy of these processes that they introduce an entirely new principle in the oxidation of galena, as Professor Borchers points out. Inasmuch as there are already three of these processes and are likely to be more, it will be necessary to have a type-name for this new branch of lead metallurgy. We venture to suggest that it may be referred to as the “lime-roasting of galena,” inasmuch as lime is evidently a requisite in the process; or, at all events, it is the agent which will be commonly employed.
When the Huntington-Heberlein process was first described, it did not even appear a simplification of the ordinary roasting process, but rather a complication of it. The process attracted comparatively little attention, and was indeed regarded somewhat with suspicion. This was largely due to the policy of the company which acquired the patent rights in refusing to publish the technical information concerning it that the metallurgical profession expected and needed. The history of this exploitation is another example of the disadvantage of secrecy in such matters. The Huntington-Heberlein process has only become thoroughly established as a new and valuable departure in metallurgy, a departure which is indeed revolutionary, nine years after the date of the original patent. In proprietary processes time is a particularly valuable element, inasmuch as the life of a patent is limited.
From the outset the explanation of Huntington and Heberlein as to the reactions involved in their process was unsatisfactory. Professor Borchers points out clearly that their conception of 115 the formation of calcium peroxide was erroneous, and indicates strongly the probability that the active agent is calcium plumbate. It is very much to be regretted that he did not go further with his experiments on this subject, and it is to be hoped that they will be taken up by the professors of metallurgy in other metallurgical schools. The formation of calcium plumbate in the process was clearly forecasted, however, by Carmichael and Bradford in their first patent specification; indeed, they considered that the sintered product consisted largely of calcium plumbate.
Even yet, we have only a vague idea of the reactions that occur in these processes. There is undoubtedly a formation of calcium sulphate, as pointed out by Borchers and Savelsberg; but that compound is eventually decomposed, since it is one of the advantages of the lime-roasting that the sintered product is comparatively low in sulphur. Is it true, however, that the calcium eventually becomes silicate? If so, under what conditions is calcium silicate formed? The temperature maintained throughout the process is low, considerably lower than that required for the formation of any calcium silicate by fusion.
Moreover, it is not only galena which is decomposed by the new method, but also blende, pyrite and copper sulphides. The process is employed very successfully in the treatment of Broken Hill ore that is rather high in zinc sulphide, and it is also to be employed for the desulphurization of mattes. What are the reactions that affect the desulphurization of the sulphides other than lead?
There is a wide field for experimental metallurgy in connection with these new processes. The important practical development is that they do actually effect a great economy in the reduction of lead sulphide ores.
(September 2, 1905)
An important revolution in the methods of smelting lead ore, which had to a large extent remained for centuries unchanged in their essentials, was wrought by the invention of Huntington and Heberlein in 1896. More especially is this true of the roast-reduction method of treating galena, which consists of oxidizing roasting in a reverberatory furnace and subsequent smelting of the roasted product in a shaft furnace.
The first stage of the roast-reduction process, as carried out according to the old method, viz., the oxidizing roast of the galena, serves to convert the lead sulphide into lead oxide:
PbS + 3O = PbO + SO2.
Owing to the basic character of the lead oxide, the production of a considerable quantity of lead sulphate was of course unavoidable:
PbO + SO2 + O = PbSO4.
As this lead sulphate is converted back into sulphide in the blast-furnace operation, and so adds to the formation of matte, it has always been the aim (in working up ores containing little or no copper to be concentrated in the matte) to eliminate the sulphate as completely as possible, by bringing the charge, especially toward the end of the roasting operation, into a zone of the furnace wherein the temperature is sufficiently high to effect decomposition of the sulphate by silica:
PbSO4 + SiO2 = PbSiO3 + SO3.
But in the usual mode of carrying out the roast in reverberatory 117 furnaces, the roasting itself on the one hand, and the decomposition of the sulphates on the other, were effected only incompletely and with widely varying results.
Little attention has been paid in connection with the roast-reduction process to the reaction between sulphates and undecomposed sulphides, which plays so important a part in the roast-reaction method of lead smelting. As is well known, lead sulphate reacts with lead sulphide in varying quantities, forming either metallic lead or lead oxide, or a mixture of both. A small quantity of lead sulphate reacting with lead sulphide yields under certain conditions only lead:
PbSO4 + PbS = Pb2 + 2SO2.
Within certain temperature limits this reaction even proceeds with liberation of heat. In order to encourage it, it is necessary to create favorable conditions for the formation of considerable quantities of sulphate right at the beginning of the operation. This was first achieved by Huntington and Heberlein, but not in the simplest nor in the most efficient manner. And, indeed, the inventors were not by any means on the right track as to the character of their process, so far as the chemical reactions involved are concerned.
At first sight the Huntington-Heberlein process does not even appear as a simplification, but rather as a complication, of the roasting operation. For in place of the roast carried out in one apparatus and continuously, there are two roasts which have to be carried out separately and in two different forms of apparatus; nevertheless, the ultimate results were so favorable that the whole process is presumably acknowledged, without reservation, by all smelters as one of the most important advances in lead smelting.
It is useful to examine in the light of the German patent specification (No. 95,601 of Feb. 28, 1897) what were the ideas of its originators regarding the operation of this process and the reactions leading to such remarkable results. They stated:
“We have made the observation that when powdered lead sulphide (PbS), mixed with the powdered oxide of an alkaline earth metal, e.g., calcium oxide, is exposed to the action of air at bright red heat (about 700 deg. C.), and is then allowed to 118 cool without interrupting the supply of air, an oxidizing decomposition takes place when dark-red heat (about 500 deg. C.) is reached, sulphurous acid being expelled, and a considerable amount of heat evolved; if sufficient air is then continuously passed through the charge, dense vapors of sulphurous acid escape, and the mixture gradually sinters together to a mass, in which the lead of the ore is present in the form of lead oxide, provided the air blast is continued long enough; there is no need to supply heat in this process—the heat liberated in the reaction is quite sufficient to keep it up.”
The inventors explained the process as follows:
“At a bright-red heat the calcium oxide (CaO) takes up oxygen from the air supplied, forming calcium peroxide (CaO2), which latter afterward, in consequence of cooling down to dark-red heat, again decomposes into monoxide and oxygen; this nascent oxygen oxidizes a part of the lead sulphide to lead sulphate, which then reacts with a further quantity of lead sulphide, with evolution of sulphur dioxide and formation of lead oxide.”
Assuming the formation of calcium peroxide (CaO2), the process leading to the desulphurization would therefore be represented as follows:
| 1. at 700° C. | CaO + O = CaO2 |
| 2. at 500° C. | 4CaO2 + PbS = 4CaO + PbSO4 |
| 3. at the melting point | PbS + PbSO4 = 2PbO + 2SO2 (?) |
Reactions 1 and 2 combined, assuming the presence of sufficient oxygen, give:
PbS + 4CaO + 4O = PbSO4 + 4CaO.
Now the invention consists in applying the observation described above to the working up of galena, and other ores containing lead sulphide, for metallic lead; and the essential novelty of the process therefore consists in passing air through the mass cooled to a dark-red heat (500 deg. C.).
This feature sharply distinguishes it from other known processes. It is true that in previous processes (compare the Tarnowitz reverberatory-furnace process, the roasting process used at Munsterbusch near Stolberg, and others) the lead ore was mixed with limestone or dolomite (which are converted into oxides in 119 the early stage of the roast) and the heat was alternately raised and lowered; but in all cases only a surface action of the air was produced, the air supply being provided simply by the furnace draft. Passing air through the mass cooled down, as indicated above, leads to the important economic advantages of reducing the fuel consumption, the losses of lead, the manual labor (raking) and the dimensions of the roasting apparatus.
In order to carry out the process of this invention, the powdered ore is intimately mixed with a quantity of alkaline earth oxide, e.g., calcium oxide, corresponding to its sulphur content; if the ore already contains alkaline earth, the quantity to be added is reduced in accordance. The mixture is heated to bright-red heat (700 deg. C.) in the reverberatory furnace, in a strongly oxidizing atmosphere, is then allowed to cool down to dark-red heat (500 deg. C.), also in strongly oxidizing atmosphere, is transferred to a vessel called the “converter,” and atmospheric air is passed through at a slight pressure (the inventors have found a blast corresponding to 35 to 40 cm. head of water suitable).[19] The heat liberated is quite sufficient to keep the charge at the reaction temperature, but, if desired, hot blast may also be used. The mixture sinters together, and (while sulphurous acid gas escapes) it is gradually converted into a mass consisting of lead oxide, gangue and calcium sulphate, from which the lead is extracted in the metallic form, by any of the known methods, in the shaft furnace. The operation is concluded as soon as the mass, by continued sintering, has become impermeable to the blast. If the operation is properly conducted, the gas escaping contains only small quantities of volatile lead compounds, but on the other hand up to 8 per cent. by volume of sulphur dioxide. This latter can be collected and further worked up.
“In place of the oxide of an alkaline earth, ferrous oxide (FeO) or manganous oxide (MnO) may also be used.”
According to the reports on the practice of this process which have been published,[20] conical converters of about 1700 mm. (5 ft. 6 in.) upper diameter and 1500 mm. (5 ft.) depth are used in Australian works. At a new plant at Port Pirie (Broken Hill Proprietary Company) converters 2400 mm. (7 ft. 10 in.) in 120 diameter and 1800 mm. (5 ft. 11 in.) deep have been installed. These latter will hold a charge of about eight tons. In the lower part of these converters, at a distance of about 600 mm. (2 ft.) from the bottom, there is placed an annular perforated plate, and upon this a short perforated tube, closed above by a plate having only a limited number of holes.
No details have been published with regard to the European installations. The general information which the Metallurgische Gesellschaft[21] placed at my disposal upon request some years ago, for use in my lecture courses, was restricted to data regarding the consumption of fuel and labor in roasting and smelting the ores, which was figured at about one-third or one-half of the consumption in the former processes, to the demonstration of the large output of the comparatively small converters, and to the reduced size of the roasting plant as the result. But the European establishments which introduced this process were bound by the owners of the patents, notwithstanding the protection afforded by the patents, to give no information whatever regarding the process to outsiders, and not to allow any inspection of the works.
On the other hand, a great deal appeared in technical literature which was calculated to excite curiosity. Moreover, as professor of metallurgy, it was my duty to instruct my pupils concerning this process among others, and it was therefore very gratifying to me that one of the students in my laboratory took a special interest in the treatment of lead ore. I gave him opportunity to install a small converter, in order to carry out the process on a small scale, and in spite of the slender dimensions of the apparatus the very first experiments gave a complete success.
However, I could not harmonize the explanation of the process given by the inventors with the knowledge which I had acquired in my many years’ practical experience in the manufacture of peroxides. It is clear from the patent specification that in the roasting operation at 700 deg. C. a compound must be formed which functions as an excellent oxygen carrier, for on cooling to 500 deg. C. the further oxidation then proceeds to the end not only without any external application of heat, but even with vigorous evolution of heat. No more striking instance than this could be desired by the theorists who have of recent years again become 121 so enthusiastic over the idea of catalysis. Huntington and Heberlein regarded calcium peroxide as the oxygen carrier, but that is a compound which cannot exist at all under the conditions which obtain in their process. The peroxides of the alkaline earths are so very sensitive that in preparing them the small quantities of carbon dioxide and water must be extracted carefully from the air, and yet in the process, in an atmosphere pregnant with carbon dioxide, water, sulphurous acid, etc., calcium peroxide, the most sensitive of the whole group, is supposed to form! This could not be.
The only compounds known as oxygen carriers, and capable of existing under the conditions of the process, are calcium plumbate and plumbite. I have emphasized this point from the first in my lectures on metallurgy, when dealing with the Huntington-Heberlein process, and, in point of fact, this assumption has since been proved to be correct by the work of L. Huppertz, one of my students.
During my practical activity (1879-1891) I had prepared barium peroxide and lead peroxide in large quantities on a manufacturing scale, the last-mentioned through the intermediate formation of plumbites and plumbates:
2NaOH + PbO + O = Na2PbO3 + H2O
or:
4NaOH + PbO + O = Na4PbO4 + 2H2O.
An experiment made in this connection showed that calcium plumbate is formed just as readily from slaked lime and litharge as the sodium plumbates above. Litharge is an intermediate product, produced in large quantities in lead works, and must in any case be brought back into the process. If, then, the litharge is roasted at a low temperature with slaked lime, the roasting of the galena could perhaps be entirely avoided by introducing that ore together with calcium plumbate into the converter, after the latter had once been heated up. Mr. Huppertz undertook the further development of this process, but I have no information on the later experimental results, as he placed himself in communication with neighboring lead works for the purpose of continuing his investigation, and has not since then given me any precise data. I will therefore confine myself to the statement 122 that the fundamental idea for the experiments, which Mr. Huppertz undertook at my suggestion, was the following:
To dispense with the roasting of the galena, which is necessary according to Huntington and Heberlein; in other words, to convert the galena by direct blast, with the addition of calcium plumbate, the latter being produced from the litharge which is an unavoidable intermediate product in the metallurgy of lead and silver. (Borchers, “Elektrometallurgie,” 3d edition, 1902-1903, p. 467.)
This alone would, of course, have meant a considerable simplification of the roast, but the problem of the roasting of galena has been solved in a better way by A. Savelsberg, of Ramsbeck, Westphalia, who has determined the conditions for directly converting the galena with the addition of limestone and water and without previous roasting. He has communicated the following information regarding these conditions:
In order that, in blowing the air through the mixture of ore and limestone, an alteration of the mixture may not take place owing to the lighter particles of the limestone being carried away, it is necessary (quite at variance with the processes in use hitherto, in which for the sake of economy stress is laid on the precaution of charging the ore as dry as possible into the apparatus) to add a considerable quantity of water to the charge before introducing it into the converter. The water serves this purpose perfectly, also preventing any change in the mixture of ore and limestone, which invariably occurs if the ore is used dry. The water, moreover, exerts a very beneficial action in the process, inasmuch as it aids materially in the formation and temporary retention of sulphuric acid, which latter then, by its oxidizing action, greatly enhances the reaction and consequently the desulphurization of the ore. Furthermore, the water tends to moderate the temperature in the charge by absorbing heat in its volatilization.
In carrying out the process the converter must not be filled entirely all at once, but first only in part, additional layers being charged in gradually in the course of the operation. In this way a uniform progress of the reaction in the mass is secured.
The following mode of procedure is advantageously adopted: A small quantity of glowing fuel (coal, coke, etc.) is introduced into the converter, which is provided at the bottom with a grate (perforated sheet iron), the grate being first covered with a thin layer of crushed limestone in order to protect it from the action 123 of the red-hot coals and ore. Upon this red-hot fuel a uniform layer of the wetted mixture of crude ore and limestone is placed. When the surface of the first layer has acquired a uniform red heat, a fresh layer is charged on, and this is continued, layer by layer, until the converter is quite full. While the layers are still being put on, the blast is passed in at quite a low pressure, and only when the converter is entirely filled is the whole force of the blast, at a rather greater pressure, turned on. There then sets in a kind of slag formation, which, however, is preceded by a very vigorous desulphurization. After the termination of the process, which can be recognized by the fact that vapors cease to be evolved, and that the surface of the ore becomes hard, the converter is tipped over, and the desulphurized mass drops out as a solid cone of slag, which is then suitably broken up for the subsequent smelting in the shaft furnace.
Savelsberg explains the reaction of this process as follows:
“1. The particles of limestone act mechanically, gliding in between the particles of lead ore and separating them from one another. In this way a premature sintering is prevented, and the whole mass is rendered loose and porous.
“2. The limestone moderates the reaction temperature produced in the combustion of the sulphur, so that the fusion of the galena, the formation of dust and the separation of metallic lead are avoided, or at least kept within the limits permissible. The lowering of the temperature of reaction is due partly to the decomposition of the limestone into caustic lime and carbon dioxide, in which heat is absorbed, and partly to the consumption of the quantity of heat which is necessary in the further progress of the operation for the formation of a slag from the gangue of the ore and the lead oxide produced.
“3. The limestone gives rise to chemical reactions. By its decomposition it produces lime, which, at the moment of its formation, is converted into calcium sulphate at the expense of the sulphur in the ore. The calcium sulphate at the time of slag formation is converted into silicate by the silica present, sulphuric acid being evolved. The limestone therefore assists directly and forcibly in the desulphurization of the ore, causing the formation of sulphuric acid at the expense of the sulphur in the ore, the sulphuric acid then acting as a strong oxidizing agent toward the sulphur in the ore.”
The most conclusive proof for the correctness of the opinion which I expressed above, that it is very important to create at the beginning of the operation the conditions for the formation of as much sulphate as possible, has been furnished by Carmichael and Bradford. They recommend that gypsum be added to the charge in place of limestone. At one of the works of the Broken Hill Proprietary Company (where their process has been carried on successfully, and where lead ores very rich in zinc had to be worked up) the dehydrated gypsum was mixed with an equal quantity of concentrate and three times the quantity of slime from the lead ore-dressing plant, as in the table given herewith:
| Contents | Of the Slime | Of the Concentrate | Of the Calcium Sulphate | Sulphate Whole Charge |
|---|---|---|---|---|
| Galena | 24 | 70 | — | 29 |
| Zinc blende | 30 | 15 | — | 21 |
| Pyrites | 3 | — | — | 2 |
| Ferric oxide | 4 | — | — | 2.5 |
| Ferrous oxide | 1 | — | — | 1 |
| Manganous oxide | 6.5 | — | — | 5 |
| Alumina | 5.5 | — | — | 3 |
| Lime | 3.5 | — | 4.1 | 10 |
| Silica | 23 | — | — | 14 |
| Sulphur trioxide | — | — | 59 | 12 |
The charge is mixed, with addition of water, in a suitable pug-mill. The mass is then, while still wet, broken up into pieces 50 mm. (2 in.) in diameter, which are then allowed to dry on a floor in contact with air; in doing so they set hard, owing to the rehydration of the gypsum.
As in the case of the Savelsberg process, the converters are heated with a small quantity of coal, are filled with the material prepared in the manner above described, and the charge is blown, regulating the blast in such manner that, after the moisture present has been dissipated, a gas of about 10 per cent. SO2 content is produced, which is worked up for sulphuric acid in a system of lead chambers.
The reactions are in this case the same as in the Savelsberg process, for here also calcium sulphate is formed transitorily, 125 which, like other sulphates, reacts partly with sulphides, partly with silica.
Where gypsum is available and cheap, the Carmichael-Bradford process must be given preference; in all other cases unquestionably the Savelsberg process is superior, owing to its great simplicity.
(October 21, 1905)
Much interest attaches to the paper by Professor Borchers, recently presented in the ,Engineering and Mining Journal (Sept. 2, 1905) on “New Methods of Desulphurizing Galena,” together with an editorial on “Lime-Roasting of Galena”; it is a curious coincidence that the same issue contained also an article on the “Newer Treatment of Broken Hill Sulphides,” in which is shown the importance of the new methods as a contribution to actual practice.
For some years it had been a source of surprise to me that a new process, so interesting and so successful as the Huntington-Heberlein treatment of sulphide ores, should have received scarcely any notice or discussion. This lack, however, now appears to be remedied. The suggestion that the subject should be discussed in the Journal is good, as is also that of the designation “Lime-Roasting” for a type-name. Such observations and experiments on the subject as I have had occasion to record have, for many years, figured in my note-books under that heading.
Whatever may be the final results of the later processes, now before the metallurgical world or still to come, there can be no doubt whatever that full and exclusive credit must be given to Huntington and Heberlein, not only for first drawing attention to the use of lime, but also for working out and introducing practically the process. It has been a success from the first; and so far as part of it is concerned, it seems to be an absolute and fundamental necessity which later inventors can neither better nor set aside. The other processes, since patented, however good they may be, are simply grafts on this parent stem.
It is, however, quite certain that Huntington and Heberlein, in the theoretical explanation of the process, failed to understand the most important reactions. Their attributing the effect to 127 the formation and action of calcium peroxide affords a sad case of a priori assumption devoid of any shred of evidence. As Professor Borchers points out, calcium peroxide, so difficult to produce and so unstable when formed, is an absolute and absurd impossibility under the conditions in question. Probably many rubbed their eyes with astonishment on reading that part of the patent on its first appearance, and hastened to look up the chemical authorities to refresh their minds, lest something as to the nature of calcium peroxide might have escaped them.
Fortunately the patent law is such that there was no danger of a really good and sound invention being invalidated by a wrong theoretical explanation by its originators. But, nevertheless, it was a misfortune that the inventors did not understand their own process. Had they known, they could have added a few more words to their patent-claims and rendered the Carmichael patent an impossibility.
Professor Borchers appears to consider that the active agent in the new process is calcium plumbate. That this compound may play a part at some stage of the process may be true; this long ago suggested itself to some others. We may yet expect to hear that the experiments undertaken by Professor Borchers himself, and by others at his instigation (in which calcium plumbate is separately prepared and then brought into action with lead sulphide), have given good results. But it does not appear so far that there is any real proof that calcium plumbate is formed in the Huntington-Heberlein or other similar processes; and it is difficult to see at what stage or how it would be produced under the conditions in question. This is a point which research may clear up, but it should not be taken for granted at this stage. Indeed, it seems to me that the results obtained may be fairly well explained without calling calcium plumbate into play at all.
Of course the action of lime in contact with lead sulphide excited interest many years before the new process came into existence. My own attention to it dates back more than a dozen years before that time (I was in charge of works where I found the old “Flintshire process” still in use).
Percy pointed out, in his work on lead smelting, that on the addition of slaked lime to the charge, at certain stages, to “stiffen it up,” the mixture could be seen to “glow” for a time. When I myself saw this phenomenon, I commenced to make some 128 observations and experiments. Also (as others probably had done), I had observed that charges of lead with calcareous gangue are roasted more rapidly and better than others, and to an extent which could not be wholly explained by simple physical action of the lime present.
Simple experiments made in assay-scorifiers in a muffle, on lime roasting, are very striking, and I think quite explain a good part of what takes place up to a certain stage in the processes now under consideration. I tried them a number of years ago, on many sorts of ore, and again more recently, when studying the working of the new patents. For illustration, I will take one class of ore (Broken Hill concentrate), using a sample assaying; Pb, 58 per cent.; Fe, 3.6 per cent.; S, 14.6 per cent.; SiO2, 3 per cent. The ore contained some pyrite. If two scorifiers are charged, one with the finely powdered ore alone, and one with the ore intimately mixed with, say, 10 per cent. of pure lime, and placed side by side just within a muffle at low redness, the limed charge will soon be seen to “glow.” Before the simple ore charge shows any sign of action, the limed charge rapidly ignites all over, like so much tinder, and heats up considerably above the surrounding temperature, at the same time increasing noticeably in bulk. This lasts for some time, during which hardly any SO2 passes off. After the violent glowing is over, the charge continues to calcine quietly, giving off SO2, but is still far more active than its neighbor. If, finally, the fully roasted charge is taken out, cooled and rubbed down, it proves to contain no free lime at all, but large quantities of calcium sulphate can be dissolved out by boiling in distilled water. For instance, in one example where weighed quantities were taken of lime and the ore mentioned, the final roasted material was shown to contain nearly 23 per cent. of CaSO4; the quantity actually extracted by water was 20.2 per cent. Further tests show that the insoluble portion still contains calcium sulphate intimately combined with lead sulphate, but not extractable by water.
There is no doubt that when lead sulphide (or other sulphide) is heated with lime, with free access of air, the lime is rapidly and completely converted into sulphate. The strong base, lime, apparently plays the part of “catalyzer” in the most vigorous manner, the first SO2 evolved being instantly oxidized and combined with the lime to sulphate, with so strong an evolution of 129 heat that the operation spreads rapidly and still goes on energetically, even if the scorifier is taken out of the muffle. Also, the “catalytic” action starts the oxidation of the sulphides at a far lower temperature than is required when they are roasted alone.
If, in place of lime, we take an equivalent weight of pure calcium carbonate and intimately mix it with ore, we obtain just the same action, only it takes a little longer to start it. Once started, it is almost as vigorous and rapid, and with the same results. It does not seem correct to assume (as is usually done) that the carbonate has first to be decomposed by heat, the lime then coming into action. The reaction commences in so short a time and while the charge is still so cool, that no appreciable driving off of CO2 by heat only can have taken place. The main liberation of the CO2 occurs during the vigorous exothermic oxidation of the mixture, and is coincident with the conversion of the CaO into CaSO4.
If, in place of lime or its carbonate, we use a corresponding quantity of pure calcium sulphate and mix it with the ore, we see very energetic roasting in this case also, with copious evolution of sulphur dioxide, only it is much more energetic and rapid and occurs at a lower temperature than in the case of a companion charge of ore alone.
It is very easily demonstrated that the CaSO4 in contact with the still unoxidized ore (whether it has been introduced ready made or has been formed from lime or limestone added) greatly assists the further roasting, in acting as a “carrier” and enabling calcination to take place more rapidly and easily and at a lower temperature than would otherwise be the case.
The result of these experiments (whether we mix the ore with CaO, CaCO3, or CaSO4) is that we arrive with great ease and rapidity at a nearly dead-sweet roast. The lime is converted into sulphate, and the lead partly to sulphate and partly to oxide. Two examples out of several, both from the above ore, gave results as follows:
No. 1—Roasted with 20 per cent. CaCO3 (= 11.2 per cent. CaO); sulphide sulphur, 0.02 per cent.; sulphate sulphur, 9.30 per cent.; total sulphur, 9.32 per cent.
No. 8—Roasted with 27.2 per cent. CaSO4 (= 11 per cent. CaO); sulphide sulphur, 0.05 per cent.; sulphate sulphur, 11.28 per cent.; total sulphur, 11.33 per cent.
If these calcined products are now intimately mixed with additional silica (in about the proportions used in the Huntington-Heberlein process) and strongly heated, fritting is brought about and the sulphur content is reduced by the decomposition of the sulphates by the silica. Thus, the resultant material of experiment No. 1, above, when treated in this manner with strong heat for three hours, was sintered to a mass which was quite hard and stony when cold, and which contained 6.75 per cent. of total sulphur. Longer heating drives out more sulphur, but a very long time is required; in furnaces, and on a large scale, it is with great difficulty and cost that a product can be obtained comparable with that which is rapidly and cheaply turned out from the “converters” of the new process.
To return to the Huntington-Heberlein process, working, for example, on an ore more or less like the one given above, we may assume that, during the comparatively short preliminary roast, the lime is all rapidly converted into CaSO4 and that some PbSO4 is also formed (but not much, as the mixture to be transferred from the furnace to the converter requires not less than 6 to 8 per cent. of sulphur to be still present as sulphide, in order that the following operation may work at its best). As the blast permeates the mass, oxidation is energetic; no doubt that CaSO4 here plays a very important part as a carrier of oxygen, in the same manner as we can see it act on a scorifier or on the hearth of a furnace.
What the later reactions are does not seem so clear. They are quite different from those on the scorifier or on the open hearth of a furnace, and result in the rapid formation (in successive layers of the mixture, from the bottom upward) of large amounts of lead oxide, fluxing the silica and other constituents to a more or less slaggy mass, which decomposes the sulphates and takes up the CaO into a complex and easily fused silicate. It is true that, as a whole, the contents of a well-worked converter are never very hot, but locally (in the regions where the progressive reaction and decomposition from below upward is going on) the temperature reached is considerable. This formation of lead oxide is so pronounced at times that one may see in the final product considerable quantities of pure uncombined litharge.
When the work is successful, the mass discharged from the converters is a basic silicate of PbO, CaO, and oxides of other 131 metals present, and nearly all the sulphates have disappeared. A large piece of yellow product (which was taken from a well-worked converter) contained only 1.1 per cent. of total sulphur.
It may be that calcium plumbate is formed and plays a part in these reactions; but its presence would be difficult to prove, and its formation and existence during these stages would not be easy to explain. Neither does it seem necessary, as the whole thing appears to be capable of explanation without it.
While the mixture in the converter is still dry and loose, energetic oxidation of the sulphides goes on, with the intervention of the CaSO4 as a carrier. As soon as the heat rises sufficiently, fluxing commences in a given layer and sulphates are decomposed. The liberated sulphuric anhydride, at the locally high temperature and under the existing conditions, will act with the greatest possible vigor on the sulphides in the adjacent layers; these layers will then in their turn flux and act on those above them, till the whole charge is worked out. The column of ore is of considerable hight, requiring a blast of 1½ lb., or perhaps more, in the larger converters now used. This pressure of the oxidizing blast (and of the far more powerfully oxidizing sulphuric anhydride, continuously being liberated within the mass of ore, locally very hot) constitutes a totally different set of conditions from those obtained on the hearth of a furnace with the ore in thin layers, where it is neither so hot nor under any pressure. It is to these conditions, in which we have the continued intense action of red-hot sulphuric anhydride under a considerable pressure (together with the earlier action of the CaSO4), that the remarkable efficiency of the process seems to me to be due.
In the Carmichael process, the preliminary roast is done away with, CaSO4 being added directly instead of having to be formed during the operation from CaO and the oxidized sulphur of the ore. The charge in the converter has to be started by heat supplied to it, and the work then goes forward on the same lines as in the Huntington-Heberlein process, so that we may assume that the reactions are the same and come under the same explanation.
Carmichael was quick to see what was really an important part and a correct explanation of the original process. He was not misled by wrong theory about any mythical calcium peroxide, 132 and so he obtained his patent for the use of CaSO4 and the dispensing of the roast in a furnace.
This process would always be limited in its application by the comparative rarity of cheap supplies of gypsum, but it appears to be a great success at Broken Hill; there it is not only of importance in working the leady ores, but also for making sulphuric acid for the new treatment of mixed sulphides by the Delprat and Potter methods. For this purpose, the use of CaSO4 will have the additional advantage that the mixture to be worked in the converter will contain not only the sulphur of the ore, but also that of the added gypsum; on decomposition, it will yield stronger gases for the lead chambers of the acid plant.
Finally comes the Savelsberg patent, which is the simplest of all; not only (like the Carmichael process) avoiding the preliminary roast with its extra plant, but also not requiring the use of ready-made CaSO4, as it uses raw ore and limestone directly in the converter. I have no knowledge as to actual results of this process; and, so far as I am aware, nothing on the subject has been published. But Professor Borchers evidently has some information about it, and regards it as the most successful of the methods of carrying out the new ideas. On the face of it, there seems no reason why it should not attain all the results desired, as the chemical and physical actions of the CaO, and of the CaSO4 formed from it, should come into play in the same manner and in the same order as in the original process; as it is carried out in the identical converter used by Huntington and Heberlein, the final reactions (as suggested above) will take place under the same conditions as to continuous decomposition under considerable heat and pressure, which I regard as the most vital part of the whole matter.
It is well to emphasize again the fact that the idea, and the means of obtaining these vital conditions, owe their origination to Huntington and Heberlein.
(March 10, 1906)
It is well known that the process of roasting lead ores in reverberatory furnaces proceeds in various ways according to the composition of the ore in question. Thus in roasting a sulphide lead ore rich in silica, one of the reactions is:
PbS + 3O = PbO + SO2.
But this reaction is incomplete, for the gases which pass on in the furnace are rich in SO2 and in SO3. And so it is found that whatever lead oxide is formed passes over almost immediately into lead sulphate, according to the reaction:
PbO + SO2 + O = PbSO4.
This reaction is the chief one which takes place. Whether the silicious gangue serves as a catalyzer for the sulphur dioxide, or whether it serves merely to keep the galena open to the action of the gases, the end result of the roast is usually the formation of lead sulphate according to the above reaction.
In the case of an ore rich in galena, a slow roast is essential, for it is desired to have the following reaction take place during the latter part of the roast:
PbS + 3PbSO4 = 4PbO + 4SO2.
Now, if the heating were too rapid, not enough lead sulphate would be found to react with the unaltered galena. The quick roasting of a rich ore would result in the early sintering of the charge, and sintering prevents the further formation of lead sulphate. Whether this sintering (which takes place so easily and which is so harmful in the latter part of the process) is due 134 to the low melting point of the lead sulphide, whether the heat evolved by the reaction
PbS + 3O = PbO + SO2
is sufficient to melt the lead sulphide, or whether other thermochemical effects (notably the preliminary sulphatizing of the lead sulphide) come into play, must for the present be undecided. Suffice it to say that the sintering of the charge works against a good roast.
In the Tarnowitz process a definite amount of lead sulphide is converted into lead sulphate by a preliminary roast. The sulphate then reacts with the unaltered lead sulphide, and metallic lead is set free, thus:
PbS + PbSO4 = 2Pb + 2SO2.
But when a very little of the sulphide has been transformed into sulphate, and when there is so little of the latter present that only a small amount of lead sulphide can be reduced to metallic lead, the mass of ore begins to sinter and grow pasty. Very little lead could be formed were it not for the addition of crushed lime to the charge just before the sintering begins. This lime breaks up the charge and cools it, prevents any sintering, and allows the continued formation of lead sulphate.
It scarcely can be held that the lime has any chemical effect in forming lead sulphate, or in forming a hypothetical compound of lead and calcium. Even if such theories were tenable from a physico-chemical point of view, they would be lessened in importance by the fact that other substances, such as purple ore or puddle cinder, act just as well as the lime.
There are now to be mentioned several new processes of lead-ore roasting whose operations fall so far outside the common ideas on the subject that their investigation is full of interest. For a long time the attempt had been made to produce lead directly by blowing air through lead sulphide in a manner analogous to the production of bessemer steel or the converting of copper matte. In the case of the lead sulphide, the oxidation of the sulphur was to furnish the heat necessary to carry on the process.
After many attempts along this line, Antonin Germot has 135 perfected a method wherein, by blowing air through molten galena, metallic lead is obtained.[23] About 60 per cent. of a previously melted charge of galena is sublimed as lead sulphide, and the rest remains behind as metallic lead. The disadvantages of the process are the difficulties of collecting all of the sublimate and of working it up. Moreover, it is impossible as yet to secure two products of which one is silver-free and the other silver-bearing. The silver values are in both the metallic lead and in the sublimed lead sulphide.
While the process just described answers for pure galena, it fails with ores which contain about 10 per cent. of gangue. In the case of such ores, they form a non-homogeneous mass when melted, and the blast penetrates the charge with difficulty. If the pressure is increased the air forces itself out through tubes and canals which it makes for itself, and the charge freezes around these passages.
Messrs. Huntington and Heberlein have gone a little farther. Although they are unable to obtain metallic lead directly, they prepare the ore satisfactorily for smelting in the blast furnace, after their roasting is completed. The inventors found that if lead sulphide is mixed with crushed lime, heated with access of air, and then charged into a converter and blown, the sulphur is completely removed in the form of sulphur dioxide. The charge, being divided by the lime, remains open uniformly to the passage of air, and sinters only when the sulphur is eliminated.
The inventors announce, as the theory of their process, that at 700 deg. C. the lime forms a dioxide of calcium (CaO2) which at 500 deg. C. breaks down into lime (CaO) and nascent oxygen. This nascent oxygen oxidizes the lead sulphide to lead sulphate according to the reaction:
PbS + 4O = PbSO4.
Furthermore it is claimed that the heat evolved by this last reaction is large enough to start and keep in operation a second reaction, namely
PbS + PbSO4 = 2PbO + 2SO2.
The theory, as just mentioned, cannot be accepted, and some of the reasons leading to its rejection will be given.
It is well established that the simple heating of lime with access of air will not result in further oxidation of the calcium. The dioxide of calcium cannot be formed even by heating lime to incandescence in an atmosphere of oxygen, nor by fusing lime with potassium chlorate. Moreover, calcium stands very near barium in the periodic system. And as the dioxide of barium is formed at a low temperature and breaks up on continued heating, it seems absurd to suppose that the dioxide of calcium would act in exactly the opposite manner. Moreover, a consideration of the thermo-chemical effects will disclose more inconsistencies in the ideas of the inventors. The breaking up of CaO2 into CaO and O is accompanied by the evolution of 12 cal. The reaction of the oxygen (thus supposed to be liberated) upon the lead sulphide is strongly exothermic, giving up 195.4 cal. So much heat is produced by these two reactions that, if the ideas of the inventors were true, the further breaking up of the calcium dioxide would stop, as the whole charge would be above 500 deg. C. It appears, then, that the explanations suggested by Messrs. Huntington and Heberlein are untrue.
In the usual roasting process, as carried out in reverberatory furnaces, it is well established that the gangue, and whatever other substances are added to the ore, prevent mechanical locking up of charge particles, since they stop sintering. It is not at all improbable that in the new roasting process the chief, if not the only, part played by the lime is the same as that played by the gangue in reverberatory-furnace roasting. A few observations leading to this belief will be given.
It is known that other substances will answer just as well as lime in this new roasting process. Such substances are manganese and iron oxides. Not only these two substances, but in fact any substance which answers the purpose of diminishing the local strong evolution of heat, due to the reaction:
PbS + 3O = PbO + SO2,
serves just as well as the lime. This fact is proved by exhaustive experiments in which mixtures of lead sulphide on the one hand, and quartz, crushed lead slags, iron slags, crushed iron ores, crushed copper slags, etc., on the other hand, were used for blowing. All these substances are such that any chemical action, 137analogous to the splitting up of CaO2, or the formation of plumbates as suggested by Dr. Borchers, cannot be imagined. The time is not yet ripe, without more experiments on the subject, to assert conclusively that there is no acceleration of the process due to the formation of plumbates through the agency of lime. But the facts thus far secured point out that such reactions are, at least, not of much importance.
Theoretical considerations point out that it ought to be possible to avoid the injurious local increase of temperature during the progress of this new roasting process, without having to add any substance whatever. To explain: The first reaction taking place in the roasting is
PbS + 3O = PbO + SO2 + 99.8 cal.
Now the heat thus liberated may be successfully dispersed if there is, in simultaneous progress, the endothermic reaction:
PbS + 3PbSO4 = 4PbO + 4SO2 - 187 cal.
Hence if there could be obtained a mixture of lead sulphide and of lead sulphate in the proportions demanded by the above reaction, then such a mixture ought to be blown successfully to lead oxide without the addition of any other substance. Such a process has, in fact, been carried out. The original galena is heated until the required amount of lead sulphate has been formed. Then the mixture of lead sulphide and of lead sulphate is transferred to a converter and blown successfully without the addition of any other substance.
The adaptability of an ore to the process just mentioned depends on the cost of the preliminary roast and the thoroughness with which it must be done. As is known, when lead sulphide is heated with access of air, it is very easy to form sintered incrustations of lead sulphate. If these incrustations are not broken up, or if their formation is not prevented by diligent rabbling, the further access of air to the mass is prevented and the oxidation of the charge stops. If ores with such incrustations are placed in the converter without being crushed, they remain unaltered by the blowing. If the incrustations are too numerous the converting becomes a failure.
It has been found that the adoption of mechanical roasting furnaces prevents this. Such furnaces appear to stop the frequent failures of the blowing which are due to the lack of care 138 on the part of the workmen during the preliminary roasting. Moreover, in such mechanical furnaces a more intimate mixture of the sulphide with the sulphate is obtained, and the degree of the sulphatizing roast is more easily controlled.
As a summary of the facts connected with this new blowing process, it may be stated that the best method of working can be determined upon and adopted if one has in mind the fact that the amount of substance (lime) to be added is dependent on: 1, the amount of sulphur present; 2, the forms of oxidation of this sulphur; 3, the amount of gangue in the ore; 4, the specific heats of the gangue and of the substance added; 5, the degree of the preparatory roasting and heating.
For example, with concentrates which run high in sulphur, there is required either a large amount of additional material, or a long preliminary roast. The specific heat of the added material must be high, and the heat evolved by the oxidation of the sulphur in the preliminary roast must be dispersed. Oftentimes it is necessary to cool the charge partially with water before blowing. On the other hand, if the ore runs low in sulphur, the preliminary roast must be short, and the temperature necessary for starting the blowing reactions must be secured by heating the charge out of contact with air. Not only must no flux be added, but oftentimes some other sulphides must be supplied in order that the blowing may be carried out at all.
The opportunity for the acquisition of more knowledge on this subject is very great. It lies in the direction of seeing whether or not the strong local evolution of heat cannot be reduced by blowing with gases poor in oxygen rather than with air. Mixtures of filtered flue gases and of air can be made in almost any proportion, and such mixtures would have a marked effect upon the possibility of regulating the progress of the oxidation of the various ores and ore-mixtures which are met with in practice.
(January 27, 1906)
In his British patent,[25] for desulphurizing sulphide ores, A. D. Carmichael states that a mixture of lead sulphide and calcium sulphate reacts “at dull red heat, say about 400 deg. C.,” forming lead sulphate and calcium sulphide, according to the equation:
PbS + CaSO4 = PbSO4 + CaS.
Judging from thermo-chemical data, this reaction does not seem probable. According to Roberts-Austen,[26] the heats of formation (in kilogram-calories) of the different compounds in this equation are as follows: PbS = 17.8; CaSO4 = 318.4; PbSO4 = 216.2; CaS = 92. Hence we have the algebraic sum:
-17.8 - 318.4 + 216.2 + 92 = -28.0 cal.
As the law of maximum work does not hold, experiment only can decide whether this decomposition takes place or not. The following experiments were made:
Experiment 1.—Coarsely crystalline and specially pure galena was ground to powder. Some gypsum was powdered, and then calcined. The powdered galena and calcined gypsum were mixed in molecular proportions (PbS + CaSO4), and heated for 1½ hours to 400 deg. C., in a stream of carbon dioxide in a platinum resistance furnace. The temperature was measured with a Le Chatelier pyrometer. The material was allowed to cool in a current of carbon dioxide.
The mixture showed no signs of reaction. Under the magnifying glass the bright cube-faces of galena could be clearly dis 140tinguished. If any reaction had taken place, in accordance with the equation given above, no bright faces of galena would have remained.
Experiment 2.—A similar mixture was slowly heated, also in the electric furnace, to 850 deg. C., in a stream of carbon dioxide, and was kept at this temperature for one hour.
It was observed that some galena sublimed without decomposition, being redeposited at the colder end of the porcelain boat (7 cm. long), in the form of small shining crystals. The residue was a mixture of dark particles of galena and white particles of gypsum, in which no evidence of any reaction was visible under the microscope. That galena sublimes markedly below its melting point has already been noted by Lodin.[27]
Experiment 3.—In order to determine whether the inverse reaction takes place, for which the heat of reaction is + 28.0 cal., the following equations are given:
PbSO4 + CaS = PbS + CaSO4;
- 216.2 - 92 + 17.8 + 318.4 = 28.
A mixture of lead sulphate and calcium sulphide was heated in a porcelain crucible in a benzine-bunsen flame (Barthel burner). The materials were supplied expressly “for scientific investigation” by the firm, C. A. F. Kahlbaum.
The white mixture turned dark and presently assumed the color which would correspond to its conversion into lead sulphide and calcium sulphate. This experiment is easy to perform.
Experiment 4.—The same materials, lead sulphate and calcium sulphide, were mixed in molecular ratio (PbSO4 + CaS), and were heated for 30 minutes to 400 deg. C., on a porcelain boat in the electric furnace, in a current of carbon dioxide. The mixture was allowed to cool in a stream of carbon dioxide, and was withdrawn from the furnace the next day (the experiment having been made in the evening).
The mixture showed a dark coloration, similar to that of the last experiment; but a few white particles were still recognizable. The material in the boat smelled of hydrogen sulphide.
Experiment 5.—A mixture of pure galena and calcined 141gypsum, in molecular ratio (PbS + CaSO4), was placed on a covered scorifier and introduced into the hot muffle of a petroleum furnace, at 700 to 800 deg. C. The temperature was then raised to 1100 deg. C.
From 5 g. of the mixture a dark-gray porous cake weighing 3.7g. was thus obtained. There was some undecomposed gypsum present, recognizable under the magnifying glass. No metallic lead had separated out. When hot hydrochloric acid was poured over the mixture, it evolved hydrogen sulphide. The fracture of the cake showed isolated shining spots. The supposition that it was melted or sublimed galena was confirmed by the aspect of the cake when cut with a knife; the surface showed the typical appearance of the cut surface of melted galena. On cutting, the cake was found to be brittle, with a tendency to crumble. On boiling with acetic acid, a little lead went into solution. Wetting with water did not change the color of the crushed cake.
Experiment 6.—In his experiments for determining the melting point of galena, Lodin[28] found that, in addition to its sublimation at a comparatively low temperature, the galena also undergoes oxidation if carbon dioxide is used as the “neutral” atmosphere. Lodin was therefore compelled to use a stream of nitrogen in his determination of the melting point of galena. Now the temperature of experiment 2 (850 deg. C.), described heretofore, is not as high as the melting point of galena (which lies between 930 and 940 deg. C.); therefore experiment 2 was repeated in a stream of nitrogen, so as to insure a really neutral atmosphere. A mixture of galena and calcined gypsum in molecular ratio (PbS + CaSO4) was heated to 850 deg. C., was kept at this temperature for one hour, and allowed to cool, the entire operation being carried out in a stream of nitrogen.
Again, galena had sublimed away from the hotter end of the porcelain boat (6.5 cm. long), and had been partially deposited in the form of small crystals of lead sulphide at the colder end. The material in the boat consisted of a mixture of particles having the dark color of galena, and others with the white color of gypsum, the original crystals of gypsum and the bright surfaces of the lead sulphide being distinctly recognizable under the magnifying glass. The loss in weight was 1.9 per cent.
Experiment 7.—For the same reason as in 2, experiment 5 was also repeated, using a current of nitrogen. A mixture of 142 galena and calcined gypsum, in molecular ratio (PbS + CaSO4) was heated in a porcelain boat to 1030 deg. C., in a platinum-resistance furnace, and allowed to cool, being surrounded by a stream of nitrogen during the whole period.
Some sublimation of lead sulphide again took place. The mixture was seen to consist of white particles of gypsum, and others dark, like galena. The loss in weight was 3.5 per cent. The mixture had sintered together slightly; with hot hydrochloric acid, it evolved hydrogen sulphide. On boiling with acetic acid, a little lead (only a trace) went into solution. There was, therefore, practically no lead oxide present; no metallic lead had separated out.
Experiment 8.—In experiment 3, lead sulphate and calcium sulphide were mixed roughly and by hand (i.e., not weighed out in molecular ratio); in this experiment such a mixture of lead sulphate and calcium sulphide in molecular ratio (PbSO4 + CaS) was heated in a porcelain crucible in a benzine-bunsen flame. It presently turned dark, and a dark gray product was obtained, as in the former experiment.
Experiment 9.—In a mixture of lead sulphate and sodium sulphide in molecular ratio (PbSO4 + Na2S), the constituents react directly on rubbing together in a porcelain mortar. The mass turns dark gray, with formation of lead sulphide and sodium sulphate.
If a similar mixture is heated, it also turns dark gray. On lixiviation with water, a solution is obtained which gives a dense white precipitate with barium chloride.
Experiment 10.—If lead sulphate and calcium sulphide are rubbed together in a mortar, the mass turns a grayish-black.
Conclusion.—From these experiments I infer that the reaction
PbS + CaSO4 = PbSO4 + CaS
does not take place, but, on the contrary, that when lead sulphate and calcium sulphide are brought together, the tendency is to form lead sulphide and calcium sulphate.
Nevertheless, on heating a mixture of galena and gypsum in contact with air, lead sulphate will be formed along with lead oxide; not, however, owing to any double decomposition of the galena with the gypsum, but rather to the formation of lead 143 sulphate from lead oxide and sulphuric acid produced by catalysis, thus:
PbO + SO2 + O = PbSO4.
This is the well-known process which always takes place in roasting galena, the explanation of which was familiar to Carl Friedrich Plattner. That the presence of gypsum has any chemical influence on this process seems to be out of the question according to the above experiments.
(October 20, 1904)
The process was patented in 1897, and is based on the fact that galena can be desulphurized by mixing it with lime and blowing a current of air through the mixture. If the temperature is dull red at the start, no additional source of heat is necessary, because the reaction causes a great rise in temperature. The chemistry of the process cannot be said at present to have been worked out in detail.
The reactions given by the patentees are not satisfactory, since calcium dioxide is formed only at low temperatures and is readily decomposed on gently warming it; lead oxide, however, combines with oxygen under suitable conditions at a temperature not exceeding 450 deg. C. and forms a higher oxide, and it is probable that this unites with the lime to form calcium plumbate. The reaction between sulphides and lime when intimately mixed and heated may be put down as
CaO + PbS = CaS + PbO.
In contact with the air the calcium sulphide oxidizes to sulphite, then to sulphate, then reacts with lead oxide, giving calcium plumbate and sulphur dioxide,
CaSO4 + PbO = CaPbO3 + SO2.
Further, calcium sulphate will also react with galena, giving calcium sulphide and lead sulphate; the calcium sulphide is oxidized, by air blown through, to calcium sulphate again, the ultimate reaction being
CaSO4 + PbS + O = CaPbO3 + SO2.
In all cases the action is oxidizing and desulphurizing. It was 145 found that oxides of iron and manganese will, to a certain extent, serve the same purpose as lime, and on application to complex ores, especially those containing much blende, that these may be desulphurized as well as galena. In the case of zinc sulphide the decomposition is probably due to the interaction of sulphide and sulphate.
ZnS + 3ZnSO4 = 4ZnO + 4SO2.
The process has now been adopted by the Broken Hill Proprietary Company at its works at Port Pirie, the Tasmanian Smelting Company, Zeehan, the Fremantle Smelting Works, West Australia, and the Sulphide Corporation’s works at Cockle Creek, New South Wales.
The operations carried on at the Tasmania Smelting Works comprise mixing pulverized limestone, galena and slag-making materials and introducing the mixture either into hand-rabbled reverberatories or mechanical furnaces with rotating hearths. After a roast, during which the materials have become well mixed and most of the limestone converted into sulphate and about half of the sulphur expelled, the granular product is run while still hot into the Huntington-Heberlein converters. These consist of inverted sheet-iron cones, hung on trunnions, the diameter being 5 ft. 6 in. and the depth 5 ft. A perforated plate or colander is placed as a diaphragm across the apex of the cone, the small conical space below serving as a wind-box into which compressed air is forced. A hood above the converter serves to carry away waste gases. As soon as the vessel is filled, air under a pressure of 17 oz. is forced through the mass, which rapidly warms up, giving off sulphur dioxide abundantly. The temperature rises and the mixture fuses, and in from two to four hours the action is complete. The sulphur is reduced from 10 to 1 per cent., and the whole mass is fritted and fused together. The converter is emptied by inverting it, when the sintered mass falls out and is broken up and sent to the smelters. There are 12 converters, of the size indicated, for the two mechanical furnaces, of 15 ft. diameter. Larger converters of the same type were erected to deal with the product from the hand-rabbled roasters.
At Cockle Creek, New South Wales, the galena concentrate is reduced to 1.5 mm., more than 60 per cent. of the material 146 being finer; the limestone is crushed down to from 10 to 16 mesh; silica is also added, if it does not exist in the ore, so that, excluding the lead, the rest of the bases will be in such proportion as to form a slag running about 20 per cent. silica. The mixture may contain from 25 to 50 per cent. lead, and from 6 to 9 per cent. lime; if too much lime is added the final product is powdery, instead of being in a fused condition. This is given a preliminary roast in a Godfrey furnace.
The Godfrey furnace is characterized by a rotating, circular hearth and a low dome-shaped roof. Ore is fed through a hopper at the center and deflected outward by blades attached to a fixed radial arm. At each revolution the ore is turned over and moved outward, the mount of deflection of the blades, which are adjustable, and rate of rotation of the hearth, determining the output.
The hot semi-roasted ore is discharged through a slot at the circumference of the roaster. This may contain from 12 to 6.5 per cent. of sulphur, but from 6.5 to 8 per cent. is held to be the most suitable quantity for the subsequent operations. Thorough mixing is of the utmost importance, for if this is not done the mass will “volcano” in the converter; that is, channels will form in the mass through which the gases will escape, leaving lumps of untouched material alongside. The action can be started if a little red-hot ore is run into the converter and cold ore placed above it; the whole mass will become heated up, and the products will fuse, and sinter into a homogeneous mass showing none of the original ingredients. At Cockle Creek the time taken is stated to be five hours; a small air-pressure is turned on at first, and ultimately it is increased to 20 oz.
Operations at Port Pirie are conducted on a much larger scale. A mixture of pulverized galena, powdery limestone, ironstone and sand is fed into Ropp furnaces, of which there are five, by means of a fluted roll placed at the base of a hopper. Each roaster deals with 100 tons of the mixture in 24 hours. About 50 per cent. of the sulphur is eliminated from the ore by the Ropps (the galena in this case being admixed with a large amount of blende, there being only 55 per cent. of lead and 10 per cent. of zinc in the concentrate produced at the Proprietary mine). The hot ore from the roasters is trucked to the converters, there being 17 of these ranged in line. The converters here are large 147 segmental cast-iron pots hung on trunnions; each is about 8 ft. diameter and 6 ft. deep, and holds an 8-ton charge. At about two feet from the bottom an annular perforated plate fits horizontally; a shallow frustrum of a cone, also perforated, rests on this; while a plate with a few perforations closes the top of the frustrum. The whole serves as a wind-box. A conical hood with flanged edges rests on the flanged edges of the converter, giving a close joint. This hood is provided with doors which allow the charge to be barred if necessary. A pipe about 1 ft. 9 in. diameter, fitted with a telescopic sliding arrangement, allows for the raising or lowering of the hood by block and tackle, and thus enables the converter to be tilted up and its products emptied. The cast-iron pots stand very well; they crack sometimes, but they can be patched up with an iron strap and rivets. Only two pots have been lost in 18 months.
Air enters at a pressure of about 24 oz. and the time taken for conversion is about four hours. The sulphur contents are reduced to about three per cent. It is found that the top of the charge is not so well converted as the interior. There is practically no loss of lead or silver due to volatilization and very little due to escape of zinc. It has also been found that practically all the limestone fed into the Ropp is converted into calcium sulphate; also that a considerable portion of lead becomes sulphate, and it is considered that lead sulphate is as necessary for the process as galena.
The value of the process may be judged from the fact that better work is now done with 8 blast furnaces than was done with 13 before the process was adopted. In addition to the sintered product from the Huntington-Heberlein pots, sintered slime, obtained by heap roasting, and flux consisting of limestone and ironstone, are fed into the furnaces, which take 2000 long tons per day of ore, fluxes and fuel. The slags now being produced average: SiO2, 25 to 26 per cent.; FeO, 1 to 3 per cent.; MnO, 5 to 5.5; CaO, 15.5 to 17; ZnO, 13; Al2O3, 6.5; S, 3 to 4; Pb, by wet assay, 1.2 to 1.5 per cent.; and Ag, 0.7 oz. per ton. Although this comparatively large quantity of sulphur remains, yet no matte is formed.
(September 2, 1905)
Nothing, for some time past, has caused such a stir in the metallurgical treatment of lead ores, and produced such radical changes at many lead smelting works, as the introduction of the Huntington-Heberlein process. This process (which it may be remarked, incidentally, has given rise to the invention of several similar processes) represents an important advance in lead smelting, and, now that it has been in use for some time at the Friedrichshütte, near Tarnowitz, in Upper Silesia, and has there undergone further improvement in several respects, a comparison of this process with the earlier roasting process is of interest.
At the above-mentioned works, up to 1900 the lead ore was treated exclusively (1) by smelting in reverberatory furnaces (Tarnowitzeröfen), and (2) by roasting in reverberatory-sintering furnaces roasted material in the shaft furnace. The factor which determined whether the treatment was to be effected in the reverberatory-smelting or in the roasting-sintering furnace was the percentage of lead and zinc in the ores; those comparatively rich in lead and poor in zinc being worked up in the former, with partial production of pig-lead; while those poorer in lead and richer in zinc were treated in the latter. About two-fifths of the lead ores annually worked up were charged into the reverberatory-smelting furnaces, and three-fifths into the sintering furnaces.
In 1900 there were available 10 reverberatory-smelting and nine sintering furnaces. These were worked exclusively by hand.
The sintered product of the roasting furnaces, and the gray slag from the reverberatory-smelting furnaces, were transferred to the shaft furnaces for further treatment, and were therein 149 smelted together with the requisite fluxes. Eight such furnaces (8 m. high, and 1.4 m., 1.6 m., and 1.8 m. respectively in diameter at the tuyeres), partly with three and partly with five or eight tuyeres, were at that time in use.
Now that the Huntington-Heberlein process has been completely installed, the reverberatory-smelting furnaces have been shut down entirely, and the sintering furnaces also for the most part; all kinds of lead ore, with a single exception, are worked up by the Huntington-Heberlein process, irrespective of the contents of lead and zinc. An exceedingly small proportion of the ore treated, viz., the low-grade concentrate (Herdschlieche) containing 25 to 35 per cent. Pb, is still roasted in the old sintering furnace, together with various between-products (such as dust, fume, scaffoldings, and matte); these are scorified by the aid of the high percentage of silica in the material.
For roasting lead ores at the present time there are six round mechanical roasters of 6 m. diameter, one of 8 m. diameter, and two ordinary, stationary Huntington-Heberlein furnaces. The latter (which represent the primitive Huntington-Heberlein furnaces, requiring manual labor) have recently been shut down, and will probably never be used again. In the mechanical Huntington-Heberlein furnace, roasting of lead ore is carried only to such a point that a small portion of the lead sulphide is converted into sulphate. The desulphurization of the ore is completed in the so-called converter (made of iron, pear-shaped or hemispherical in form) in which the charge, up to this stage loosely mixed, is blown to a solid mass.
Owing to the ready fusibility of this product (which still contains, as a rule, up to 1.5 per cent. sulphur as sulphide), it is possible to use shaft furnaces of rather large dimensions; therefore a round shaft furnace (2.4 m. diameter at the tuyeres, 7 m. high, and furnished with 15 tuyeres) was built. In this furnace nearly the whole of the roasted ore from the Huntington-Heberlein converters is now smelted, some of the smaller shaft furnaces being used occasionally. The introduction of the new process has caused no noteworthy change in the subsequent treatment of the work-lead.
In the following study I shall discuss the treatment of a given annual quantity of ore (50,000 tons), which is the actual figure at the Friedrichshütte at the present time.
1. Roasting Furnaces.—A reverberatory-smelting furnace used to treat 5 tons of ore in 24 hours; a roasting-sintering furnace, 8 tons. Assuming the ratios previously stated, the annual treatment by the former process would be 20,000 tons, and by the latter 30,000 tons. On the basis of 300 working days per year, and no prolonged stoppages for furnace repairs (though considering the high temperatures of these furnaces this record would hardly be expected), there would be required:
20,000 ÷ (5 × 300) = 13.3 (or 13 to 14 reverberatory furnaces).
30,000 ÷ (8 × 300) = 12.5 (or 12 to 13 sintering furnaces).
The capacity of a stationary Huntington-Heberlein furnace is 18 tons; hence in order to treat the same quantity of ores there would be required:
50,000 ÷ (18 × 300) = 9.3 (or 9 to 10 Huntington-Heberlein furnaces).
With the revolving-hearth roasters (of 6 m. diameter) working a total charge of at least 27 tons of ore, there would be required:
50,000 ÷ (27 × 300) = 6.1 (or 6 to 7 roasters).
Still better results are obtained with the 8 m. round roaster, which has been in operation for some time; in this, 55 tons of ore can be roasted daily. Three such furnaces would therefore suffice for working up the whole of the ore charged per annum.
Now, making due provision for reserve furnaces, to work up 50,000 tons of ore would require:
| Reverberatory (15) and sintering furnaces (15) | 30 |
| Stationary Huntington-Heberlein furnaces | 12 |
| 6 m. revolving-hearth furnaces | 8 |
| 8 m. revolving-hearth furnaces | 4 |
Similar relations hold good regarding the number of workmen attending the furnaces, there being required, daily, six men for the reverberatory furnace; eight men for the sintering furnace; ten men for the stationary; and six men for the mechanical Huntington-Heberlein furnace; or, for 14 reverberatory furnaces, daily, 84 men; for sintering furnaces, daily, 104 men; total, 188 men. While for 10 stationary Huntington-Heberlein furnaces, 100 men are required; and for 7 mechanical Huntington-Heberlein furnaces, daily, 42 men. It is expected that only 14 men (working 151 in two shifts) will be required to run the new installation with 8 m. round roasters.
It is true that the exclusion of human labor here has been carried to an extreme. The roasters and converters will be charged exclusively by mechanical means; thus every contact of the workmen with the lead-containing material is avoided until the treatment of the roasted material in the converters is completed.
From the data given above, the capacity of each individual workman is readily determined, as follows: With the reverberatory-smelting furnace, each man daily works up 0.83 tons; with the sintering furnace, 1 ton; with the stationary Huntington-Heberlein furnace, 1.8 tons; with the 6 m. revolving-hearth furnace, 4.5 tons; and with the 8 m. revolving-hearth furnace, 11.8 tons.
A significant change has also taken place in coal consumption. Thus, when working with the reverberatory and sintering furnaces in order to attain the requisite temperature of 1000 deg. C., there was required not only a comparatively high-grade coal, but also a large quantity of it. A reverberatory furnace consumed about 503 kg., a sintering furnace about 287 kg., of coal per ton of ore. For roasting the ore in the stationary and also in the mechanical Huntington-Heberlein furnaces, a lower temperature (at most 700 deg. C.) is sufficient, as the roasting proper of the ore is effected in the converters, and the sulphur furnishes the actual fuel. For this reason, the consumption of coal is much lower. The comparative figures per ton of ore are as follows: In the reverberatory furnace, 50.3 per cent.; in the sintering furnace, 28.7 per cent.; in the stationary Huntington-Heberlein furnace, 10.3 per cent.; and in the Huntington-Heberlein revolving-hearth furnace, 7.3 per cent.
But there is another technical advantage of the Huntington-Heberlein process which should be mentioned. It is well known that the volatilization of lead at high temperatures is an exceedingly troublesome factor in the running of a lead-smelting plant; the recovery of the valuable fume is difficult, and requires condensing apparatus, to say nothing of the unhealthful character of the volatile lead compounds. This volatilization is of course particularly marked at the high temperatures employed when working with reverberatory-smelting furnaces; the same is true, in a somewhat less degree, of the sintering furnaces. In conse 152quence of the markedly lower temperature to which the charge is heated in the Huntington-Heberlein furnace, and also of the peculiar mode of completing the roast in blast-converters, the production of fume is so reduced that the difference between the values recovered in the old and the new processes is very striking. Whereas, in 1900, in working up 12,922 tons of ore in the reverberatory-smelting furnace, and 14,497 tons in the sintering furnace (27,419 tons in all), there was recovered 2470 tons (or 9 per cent.) as fume from the condensers and smoke flues, the quantity of fume recovered, in 1903, fell to 879 tons (or 1.8 per cent.), out of the 48,208 tons of ore roasted, and this notwithstanding the fact that in the meantime fume-condensing appliances had been considerably expanded and improved, whereby the collection was much more efficient.
Lastly, the zinc content of the ores no longer exerts the same unfavorable influence as in the old process (wherein it was advisable to subject ore containing much blende to a final washing before proceeding to the actual metallurgical treatment). In the new process, the ores are simply roasted without regard to their zinc content. In this connection it has been found that a considerable proportion of the zinc passes off with the fume, and that the roasted material usually contains a quantity of zinc so small that it no longer causes any trouble in the shaft furnace. It may also be mentioned here that the ore-dressing plants recently installed in the mines of Upper Silesia have resulted in a more perfect separation of the blende.
Shaft Furnaces.—The finished product from the Huntington-Heberlein blast-converters is of a porous character, and already contains a part of the flux materials (such as limestone, silica and iron) which are required for the shaft-furnace charge. It is just these two characteristics of the roasted product (its porous nature, on the one hand, leading to its more perfect reduction by the furnace gases; and, on the other hand, the admixture of fluxes in the molten condition, resulting in a more complete utilization of the temperature), which, together with its higher lead and lower zinc content, determine its ready fusibility. If we further consider that it is possible in the new process to make the total charge of the shaft furnace richer in lead than formerly (two-thirds of the total charge as against one-third), and that a higher blast pressure can be used without danger, it follows immediately 153 that the capacity of a shaft furnace is much greater by the new process than by the old method of working. The daily production of the shaft furnaces on the old and the new process is as shown in the table given herewith:
| Type of Shaft Furnace | Character of Charge | Charge per Day, Tons | Work-lead Produced per Day, Tons |
|---|---|---|---|
| Low-pressure Blast | |||
| 3 tuyeres | Gray slag from reverberatory furnaces and sintered concentrate | 36 | 6 to 7 |
| 8 tuyeres | ” | 36 to 38 | 6 to 8 |
| 3 tuyeres | Roasted product of Huntington-Heberlein process | 36 | 11 to 12 |
| High-pressure Blast | |||
| 8 tuyeres | ” | 65 to 72 | 24 to 26 |
| 15 tuyeres | ” | 270 | 90 to 100 |
It should be noted that the figure given for the furnace with 15 tuyeres represents the average for 1904; this average is lowered by the circumstance that during this period there was frequently a deficiency of roasted material, and the furnace had to work with low-pressure blast. A truer impression can be gained from the month of March, 1905, for instance, during which time this furnace worked under normal conditions; the results are as follows:
The average for March, 1905, was: Ore charged, 8,269.715 tons; coke, 652.441 tons; total, 8,922.156 tons. Or, in 24 hours: Ore charged, 266.765 tons; coke, 21.046 tons; total, 287.811 tons. The production of work-lead was 3,133.245 tons, or 101.069 tons per day.
The maximum production of roasted ore was 210 tons, on June 30, 1905, when the total charge was: Ore, 327.38 tons; coke, 25.2 tons; total, 352.58 tons. The quantity of work-lead produced on that day was 120.695 tons, while the largest quantity 154 previously produced in one day was 124.86 tons. It should also be mentioned that the lead tenor of the slag is almost invariably below 1 per cent.; it usually lies between 0.3 and 0.5 per cent.
As in the case of the roasting furnaces, the productive capacity of the shaft furnace also comes out clearly if we figure the number of furnaces required, on the basis of an annual consumption of 50,000 tons of ore. If we consider 1 ton of the roasted material as equivalent to 1 ton of ore (which is about right in the case of the Huntington-Heberlein material, but is rather a high estimate in the case of the product of the sintering furnace), then, in the old process (where one-third of the charge was lead-bearing material), 12 tons could be smelted daily. There would therefore be needed at least:
50,000 ÷ (12 × 300) = 14 three-tuyere shaft furnaces.
Since, as already mentioned, the lead-bearing part of the charge constitutes two-thirds of the whole in the Huntington-Heberlein process, the number of shaft furnaces of different types, as compared with the foregoing, would figure out:
3-tuyere shaft furnace, with product of sintering furnace, 50,000 ÷ (12 ×
300) = 14 furnaces;
3-tuyere shaft furnace, with product of Huntington-Heberlein furnace,
50,000 ÷ (24 × 300) = 7 furnaces;
8-tuyere shaft furnace, with product of Huntington-Heberlein furnace,
50,000 ÷ (48 × 300) = 3.4 (say 4) furnaces;
15-tuyere shaft furnace, with product of Huntington-Heberlein furnace,
50,000 ÷ (180 × 300) = 1 furnace.
Running regularly and without interruption, the large shaft furnace is therefore fully capable of coping with the Huntington-Heberlein roasted material at the present rate of production.
As regards the number of workmen and the product turned out per man, no such marked difference is produced by the introduction of the Huntington-Heberlein process in the case of the shaft furnace as there was noted for the roasting operation. This is chiefly due to the fact that the work which requires the more power (such as charging of the furnaces, conveying away the slag and pouring the lead) can be executed only in part by mechanical means. Nevertheless, it will be seen from the table given herewith that, on the one hand, the number of men required 155 for the charge worked up is smaller; and, on the other, the product turned out per man has risen somewhat.
| Type of Shaft Furnace | Character of Charge | Charge per Day, Tons | Number of Furnacemen | Charge per Man, Tons | Daily Outputof Work-lead, Tons | Output per Man, Tons |
|---|---|---|---|---|---|---|
| 3 tuyere | Sintered concentrate and gray slag from reverberatory furnace. | 36 | 6 | 6.0 | 6 | 1.0 |
| 3 tuyere | Huntington-Heberlein product. | 36 | 6 | 6.0 | 12 | 2.0 |
| 8 tuyere | Huntington-Heberlein product. | 72 | 12 | 6.0 | 26 | 2.1 |
| 15 tuyere | Huntington-Heberlein product. | 270 | 34 | 7.9 | 90 | 2.6 |
A slight difference only is produced by the new process in the consumption of coke; the economy is a little over 1 per cent., the coke consumed being reduced from 9.39 per cent. to 8.17 per cent. of the total charge. But with the high price of coke, even this small difference represents a considerable lowering of the cost of production.
With the great increase in the blast pressure, it would be supposed that the losses in fume would be much greater than with the former method of working. But this is not the case; on the contrary, all experience so far shows that there is much less fume developed. In 1904, for instance, the shaft-furnace fume recovered in the condensing system amounted to only 1.06 per cent. of the roasted material, or 0.64 per cent. of the total charge, as against 2.03 and 1.0 per cent., respectively, in former years. The observations made on the quantity of flue dust carried away with the gases escaping into the air through the stack showed that it is almost nil.
Now, from the loss in fume being slight, from the tenor of lead in the slag being low, and, on the one hand, from the quantity of lead-matte produced being much less than before, while on the other the losses in roasting the ore are greatly reduced—from all these considerations, it is clear that the total yield must have been much improved. As a matter of fact, the yield of lead and silver has been increased by at least 6 to 8 per cent.
Economic Results.—As regards the economical value of the new process, for obvious reasons no data can be furnished of the exact expenditure, i.e., the actual total cost of roasting and 156 smelting the ore. But this at least is placed beyond doubt by what has been developed above, namely, that considerable saving must be effected in the roasting, and especially in the smelting, as compared with the former mode of working. If we take into account only the economy which is gained in wages through the increase in the material which one workman can handle, and that resulting from the reduced consumption of coal and coke, these alone will show sufficiently that an important diminution of working cost has taken place. The objection which might be raised, that the saving effected by reducing manual labor may be neutralized by the expense of mechanical power (actuating the roasters, furnishing the compressed blast, etc.), cannot be regarded as justified, as the cost of mechanical work is comparatively low. Thus, for instance, the large 8 m. furnace and the small, round furnaces require 15 h.p. if worked by electricity. According to an exact calculation, the cost (to the producer) of the h.p. hour, inclusive of machinery, figures out to 3.6 pfennigs (0.9c.); hence the daily expense for running the revolving-hearth furnaces amounts to: 15 × 3.6 pfg. × 24 = 12.96 marks ($3.42). As the seven furnaces together work up: (6 × 27) + 55 = 217 tons of ore, the cost per ton of ore is about 0.06 mark (1.5c.).
The requisite blast is produced by means of single-compression Encke blowers, of which one is quite sufficient when running at full load, and then consumes 34 h.p. The daily expenses are accordingly: 34 × 3.6 pfg. × 24 = 29.28 marks ($7.32); or per ton of ore, 29.28 ÷ 217 = 0.14 mark (3.5c.). Therefore the total expense for the mechanical work in roasting the ore amounts to 0.06 + 0.14 = 0.20 mark (5c.).
However, the cost of roasting is much more affected by the expense for keeping the furnaces in repair; another important factor is the acquisition and maintenance of the tools. Both in the case of the sintering and also the reverberatory-smelting furnace, the cost of keeping in repair was high; the consumption of iron was especially large, owing to the rapid wear of the tools. This was not surprising, considering that a notably higher temperature prevailed in the reverberatory and sintering furnaces than in the new roasters, in which the temperature strictly ought not to rise above 700 deg. C. But in the old type of furnace the high temperature and the constant working with the iron tools caused their rapid wear, thus creating a large item for iron and 157 steel and smith work. In the new process (and more especially in the revolving-hearth roasters) this disadvantage does not arise. In this case there is practically no work on the furnace, and the wear and tear of iron is small. Also, the cost of keeping the furnaces in repair when working regularly is small as compared with the old process. In the year 1900, for instance, the cost of maintenance and tools for the reverberatory and sintering furnaces came to 20,701.93 marks ($5,175.48) for treating 27,419.75 tons of ore. Per ton of ore, this represents 0.75 mark (19c.). In the year 1903, on the other hand, only 9,074.17 marks ($2,268.54) were expended, although 48,208 tons of ore were worked up in the three stationary and six mechanical Huntington-Heberlein furnaces. The cost of maintenance was, therefore, in this case 0.18 mark (4.5c.) per ton of ore.
In the cost of smelting in the shaft furnace, only a slight difference in favor of the Huntington-Heberlein process is found if the estimate is based on the total charge; but a marked difference is shown if it is referred to the lead-bearing portion of the charge, or to the work-lead produced. Thus the cost of maintenance and total cost of smelting, figured for one ton of ore, without taking into account general expenses, have been tabulated as follows:
| Reduction in Expenses per Ton of | |||
|---|---|---|---|
| Total Charge | Lead Ore | Work-Lead | |
| (a) Cost of maintenance | 0.01M(0.25c) | 0.38M(9.5c) | 0.67M(16.75c) |
| (b) Total cost of smelting | 0.20M(5c) | 6.46M($1.615) | 11.48M($2.87) |
The marked reduction in the expenses, as referred to the lead-ore and the work-lead produced, is determined (as was pointed out above) by the greater lead content of the charge, and by the larger yield of lead consequent thereon. The advantage of longer smelting campaigns (which ultimately were mostly prolonged to one year) also makes itself felt; it would be still more marked, if the shaft furnace (which was still in working condition after it was blown out) had been run on for some time longer.
Finally, if we examine the question of the space taken up by 158 the plant (which, owing to the scarcity of suitably located building sites, would have been important at the Friedrichshütte at the time when the quantity of ore treated was suddenly doubled), here again we shall recognize the great advantage which this establishment has gained from the Huntington-Heberlein process.
As was calculated above, there would have been required 15 reverberatory and 15 sintering furnaces to cope with the quantity of ore treated. As a reverberatory requires, in round numbers, 120 sq. m. (1290 sq. ft.), and a sintering furnace 200 sq. m. (2153 sq. ft.); and as fully 100 sq. m. (1080 sq. ft.) must be allowed for each furnace for a dumping ground, therefore the 15 reverberatory furnaces would have required an area of 15 × 120 + 15 × 100 = 3300 sq. m.; the 15 sintering furnaces would have required 15 × 200 + 15 × 100 = 4500 sq. m.; in all 3300 + 4500 = 7800 sq. m. (83,960 sq. ft.). The 12 stationary Huntington-Heberlein furnaces (built together two and two) would take up a space of 6 × 200 + 12 × 100 = 2400 sq. m. (25,830 sq. ft.). Similarly, 8 small furnaces would require 8 × 100 + 8 × 100 = 1600 sq. m. (17,222 sq. ft.); while for the new installation of four 8-meter revolving-hearth furnaces and 10 large converters, only 1320 sq. m. (14,120 sq. ft.) have been allowed.
For shaft furnaces with three or eight tuyeres, which were run with low-pressure blast for the material roasted on the old plan, the total area built upon was 18 × 16.5 = 297 sq. m.; while a further area of 18 × 14 = 250 sq. m. was hitherto provided, and was found sufficient for dumping slag when working regularly. Therefore, the installation of shaft furnaces formerly in existence, after requisite enlargement to 14 furnaces, would have demanded a space of 7 × 297 + 7 × 250 = 3829 sq. m. (42,215 sq. ft.). If four of the small shaft furnaces had been reconstructed for eight tuyeres, and run with Huntington-Heberlein roasted material, using high-pressure blast, the area occupied would have been reduced to 2 × 297 + 2 × 250 sq. m. = 1094 sq. m. (11,776 sq. ft.).
Still more favorable are the conditions of area required in the case of the large shaft furnace. This furnace stands in a building covering an area of 350 sq. m. (3767 sq. ft.), which is more than sufficient room. The slag-yard (situated in front of this building, and amply large enough for 36 hours’ run) has an 159 area of 250 sq. m. (2691 sq. ft.); thus the space occupied by the large shaft furnace, including a yard of 170 sq. m. (1830 sq. ft.), is in all 780 sq. m. (8396 sq. ft.).
After completion of the new roasting plant and the large shaft furnace in connection with it, there would be occupied 1320 + 780 = 2100 sq. m. (2260 sq. ft.); and if the system of reverberatory and sintering furnaces had been continued (with the requisite additions thereto and to the old shaft-furnace system), there would have been required 11,629 sq. m. (125,214 sq. ft.). In the estimate above given no regard has been paid to any of the auxiliary installations (dust chambers, etc.), which, just as in the case of the old process, would have had to be provided on a large scale.
It is of course self-evident that both the principal and the auxiliary installations in the old process would not only have involved a high first cost, but would also, on account of their extensive dimensions, have caused considerably greater annual expense for maintenance.
(October 14, 1905)
With regard to the hygienic improvements which the Huntington-Heberlein process offers, we must first deal with the questions: What were the sources of danger in the old process, and in what way are these now diminished or eliminated? The only danger which enters into consideration is lead-poisoning, other influences detrimental to health being the same in one process as the other.
With the reverberatory-smelting and roasting-sintering furnaces, the chief danger of lead-poisoning lies in the metallic vapor evolved during the withdrawal of the roasted charge from the furnace. It is true that appliances may be provided, by which these vapors are drawn off or led back into the furnace during this operation; but, even working with utmost care, it is impossible to insure the complete elimination of lead fumes, especially in wheeling away the pots filled with the red-hot sintered product. Moreover, the work at the reverberatory-smelting and roasting-sintering furnaces involves great physical exertion, wherefore the respiratory organs of the workmen are stimulated to full activity, while the exposure to the intense heat causes the men to perspire freely. Hence, as has been established medically, the absorption of the poisonous metallic compounds (which are partially soluble in the perspiration) into the system is favored both by inhalation of the lead vapor and by its penetration into the pores of the skin, opened by the perspiration.
A further danger of lead-poisoning was occasioned by the frequently recurring work of clearing out the dust flues. The smoke from the reverberatory-smelting furnace especially contained oxidized lead compounds, which on absorption into the 161 human body might readily be dissolved by the acids of the stomach, and thus endanger the health of the workmen.
In the Huntington-Heberlein furnaces, on the other hand, although the charge is raked forward and turned over by hand, it is not withdrawn, as in the old furnaces, by an opening situated next to the fire, but is emptied at a point opposite into the converters which are placed in front of the furnace. Moreover, the converters are filled with the charge at a much lower temperature. Inasmuch as this charge has already cooled down considerably, there can be practically no volatilization of lead. The small quantity of gas which may nevertheless be evolved is drawn off by fans through hoods placed above the converters.
A further improvement, from the hygienic point of view, is in the use of the mechanical furnaces, from which the converters can be filled automatically (almost without manual labor, and with absolute exclusion of smoke). The converters are then placed on their stands and blown. This work also is carried out under hoods, as gas-tight as possible, furnished with a few closable working apertures. During the blowing of the material, the work of the attendant consists solely in keeping up the charge by adding more cold material and filling any holes that may be formed. It does not entail nearly as much physical strain as the handling of the heavy iron tools and the continued exposure of the workmen to the hottest part of the furnace, which the former roasting process involved.
Some experiments carried out with larger converters (of 4 and 10 ton capacity) have indicated the direction in which the advantages mentioned above may probably be developed to such a point that the danger of lead-poisoning need hardly enter into consideration. Both the charging of the revolving-hearth furnaces and the filling of the converters are to be effected mechanically. Furthermore, in the case of the large converters the filling up of holes becomes unnecessary, and no manual work of any kind is required during the whole time of blowing. The converters can be so perfectly enclosed in hoods that the escape of gases into the working-rooms becomes impossible, and lead-poisoning of the men can occur only under quite unusual circumstances.
The beneficial influence on the health of the workmen attending on the roasting furnaces, occasioned by the introduction of 162 the Huntington-Heberlein process, can be seen from the statistics of sickness from lead-poisoning for the years 1902 to 1904, as given herewith:
| Lead-poisoning | Cases Contracted | |||||||
|---|---|---|---|---|---|---|---|---|
| No. of Cases | Days of Sickness | |||||||
| Method of Working | Year | No. of Men | Total | Per 100 Persons | Total | Per 100 Persons | At Rever. and Sint. Fur. | At H. H. Fur. |
| Old | 1902 1903 |
93 86 |
15 12 |
16.1 13.9 |
246 222 |
264.5 258.1 |
11 7 |
4 5 |
| H.-H. | 1904 | 87 | 8 | 9.2 | 242 | 278.2 | 6 | 2 |
This shows a gratifying decrease in the number of cases, namely, from 16.1 to 9.2 per cent.; this decrease would have been still greater if Huntington-Heberlein furnaces had been in use exclusively. However, most of the time two or three sintering furnaces were fired for working up by-products, 16 to 18 men being engaged on that work. The Huntington-Heberlein furnaces alone (at which, in the year 1904, 69 men in all were occupied) show only 2.9 per cent. of cases. That the number of days of illness was not reduced is due to the fact that the cases among the gang of men working at the sintering furnaces were mostly of long standing and took some time to cure.
The noxious effects upon the health of the workmen in running the shaft furnaces are due to the fumes from the products made in this operation, such as work-lead, matte and slag, which flow out of the furnace at a temperature far above their melting points. Even with the old method of running the shaft furnaces the endeavor has always been to provide as efficiently as possible against the danger caused by this volatilization, and, wherever feasible, to install safety appliances to prevent the escape of lead vapors into the work-rooms; but these measures could not be made as thorough as in the case of the Huntington-Heberlein process.
The principal work in running the shaft furnaces, aside from the charging, consists in tapping the slag and pouring out the work-lead. Other unpleasant jobs are the barring down (which 163 in the old process had to be done frequently) and the cleaning out of the furnace after blowing out.
In the old process the slag formed in the furnace flows out continuously through the tap-hole into iron pots placed in front of the spout. A number of such pots are so arranged on a revolving table that as soon as one is filled the next empty can be brought up to the duct; thus the slag first poured in has time to cease fuming and to solidify before it is removed. The vapors arising from the slag as it flows out are conveyed away through hoods. At the same time with the slag, lead matte also issues from the furnace. Now the greater the quantity of lead matte, the more smoke is also produced; and, with the comparatively high proportion of lead matte resulting from the old process, the quantity of smoke was so great that the ventilation appliances were no longer sufficient to cope with it, thus allowing vapors to escape into the work-room.
The work-lead collects at the back of the furnace in a well, from which it is from time to time ladled into molds placed near by. If the lead is allowed to cool sufficiently in the well, it does not fume much in the ladling out. But when the furnace runs very hot (which sometimes happens), the lead also is hotter and is more inclined to volatilize. In this event the danger of lead-poisoning is very great, for the workman has to stand near the lead sump.
A still greater danger attends the work of barring down and cleaning out the furnace. The barring down serves the purpose of loosening the charge in the zone of fusion; at the same time it removes any crusts formed on the sides of the furnace, or obstructions stopping up the tuyeres. With the old furnaces, and their strong tendency to crust, this work had to be undertaken almost every day, the men being compelled to work for rather a long time and often very laboriously with the heavy iron tools in the immediate neighborhood of the glowing charge, the front of the furnace being torn open for this purpose. In this operation they were exposed without protection to the metallic vapors issuing from the furnace, inasmuch as the ventilating appliances had to be partially removed during this time, in order to render it at all possible to do the work.
In a similar manner, but only at the time of shutting down a shaft furnace, the cleaning out (that is to say, the withdrawing 164 of no longer fused but still red-hot portions of the charge left in the furnace) is carried out. In this process, however, the glowing material brought out could be quenched with cold water to such a point that the evolution of metallic vapors could be largely avoided.
Lastly, the mode of charging of the shaft furnace is also to be regarded as a cause of poisoning, inasmuch as it is impossible to avoid entirely the raising of dust in the repeated act of dumping and turning over the materials for smelting, in preparing the mix, and in subsequently charging the furnace.
By the introduction of the Huntington-Heberlein process, all these disadvantages, both in the roasting operation and in running the shaft furnaces, are in part removed altogether, in part reduced to such a degree that the danger of injury is brought to a minimum.
In furnaces in which the product of the Huntington-Heberlein roast is smelted, the slag is tapped only periodically at considerable intervals; and, as there is less lead matte produced than formerly, the quantity of smoke is never so great that the ventilating fan cannot easily take care of it. There is therefore little chance of any smoke escaping into the working-room.
As the production of work-lead, especially in the case of the large shaft furnace, is very considerable, so that the lead continually flows out in a big stream into the well, the hand ladling has to be abandoned. Therefore the lead is conducted to a large reservoir standing near the sump, and is there allowed to cool below its volatilizing temperature. As soon as this tank is full, the lead is tapped off and (by the aid of a swinging gutter) is cast into molds ready for this purpose. Both the sump and the reservoir-tank are placed under a fume-hood. The swinging gutter is covered with sheet-iron lids while tapping, so that any lead volatilized is conveyed by the gutter itself to a hood attached to the reservoir; thus the escape of metallic vapors into the working space is avoided, as far as possible.
This method of pouring does not entail the same bodily exertion as the ladling of the lead; moreover, as it requires but little time, it gives the workmen frequent opportunity to rest.
But one of the chief advantages of the Huntington-Heberlein process lies in the entire omission of the barring down. If the running of the shaft furnace is conducted with any degree of care, disorders in the working of the furnace do not occur, and one 165 can rely on a perfectly regular course of the smelting process day after day. No formation of any crusts interfering with the operation of the furnace has been recorded during any of the campaigns, which have, in each case, lasted nearly a year.
As regards the cleaning out of the furnace, this cannot be avoided on blowing out the Huntington-Heberlein shaft furnace; but at most it occurs only once a year, and can be done with less danger to the workmen, owing to the better equipment.
Further, the charge is thrown straight into the furnace (in the case of the large shaft furnace); thus the repeated turning over of the smelting material, as formerly practised, becomes unnecessary, and the deleterious influence of the unavoidable formation of dust is much diminished.
The accompanying statistics of sickness due to lead-poisoning in connection with the operation of the shaft furnace (referring to the same period of time as those given above for the roasting furnaces) confirm the above statements.
| Year | No. of Men | Lead-Poisoning—Shaft Furnaces | |||
|---|---|---|---|---|---|
| Cases | Days of Illness | ||||
| Total | Per 100 Persons | Total | Per 100 Persons | ||
| 1902 | 250 | 58 | 23.2 | 956 | 382.4 |
| 1903 | 267 | 59 | 22.1 | 1044 | 391.0 |
| 1904 | 232 | 24 | 10.3 | 530 | 228.4 |
If it were possible to make the necessary distinctions in the case of the large shaft furnace, the diminution in sickness from lead-poisoning would be still more apparent; for, among the furnace attendants proper, there has been no illness; all cases of poisoning have occurred among the men who prepare the charge, who break up the roasted material, and others who are occupied with subsidiary work. Some of these are exposed to illness through their own fault, owing to want of cleanliness, or to neglect of every precautionary measure against lead-poisoning.
Thus far we have dealt only with the advantages and improvements of the Huntington-Heberlein process; we will now, in conclusion, consider also its disadvantages.
The chief drawback of the new process lies in the difficulty of breaking up the blocks of the roasted product from the con 166verters, a labor which, apart from the great expense involved, is also unhealthy for the workmen engaged thereon. Seemingly this evil is still further increased by working with larger charges in the 10 ton converters, as projected; but in this case it is proposed to place the converters in an elevated position, and to cause the blocks to be shattered by their fall from a certain hight, so that further breaking up will require but little work. Trials made in this direction have already yielded satisfactory results, and seem to promise that the disadvantage will in time become less important.
Another unpleasant feature is the presence (in the waste gases from the converters) of a higher percentage of sulphur dioxide, the suppression of which, if it is feasible at all, might be fraught with trouble and expense.
That the roaster gases from the reverberatory-smelting and sintering furnaces did not show such a high percentage of sulphur dioxide must be ascribed chiefly to the circumstance that the roasting was much slower, and that the gases were largely diluted with air already at the point where they are formed, as the work must always be done with the working-doors open. In the Huntington-Heberlein process, on the other hand, the aim is to prevent, as far as possible, the access of air from outside while blowing the charge. The more perfectly this is effected, and the greater the quantity of ore to be blown in the converters, the higher will also be the percentage of sulphur dioxide in the waste gases. This circumstance has not only furnished the inducement, but it has rendered it possible to approach the plan of utilizing the sulphur dioxide for the manufacture of sulphuric acid. If this should be done successfully (which, according to the experiments carried out, there is reasonable ground to expect), the present disadvantage might be turned into an advantage. This has the more significance because an essential constituent of the lead ore—the sulphur—will then no longer, as hitherto, have to be regarded as wholly lost.[31]
(May 26, 1906)
This process for roasting lead sulphide ores has now fairly established itself in all parts of the world, and is recognized by metallurgical engineers as a successful new departure in the method of desulphurization. It offers the great advantage over previous methods of being a more scientific application of the roasting reactions (of the old well-used formulæ PbS + 3O = PbO + SO2 and PbS + PbSO4 + 2O = 2PbO + 2SO2) and admits of larger quantities being handled at a time, so that the use of fuel and labor are in proportion to the results achieved, and also there is less waste all around in so far as the factors necessary for the operation—fuel, labor and air—can be more economically used. The workman’s time and strength are not employed in laboriously shifting the ore from one part of the furnace to another with a maximum amount of exertion and a minimum amount of oxidation. The fuel consumed acts more directly upon the ore during the first part of the process in the furnace and its place is taken by the sulphur itself during the final and blowing stage, so that during the whole series of operations more concentrated gases are produced and consequently the large excess of heated air of the old processes is avoided to such an extent that the gases can be used for the production of sulphuric acid.
With a modern well-constructed plant practically all the evils of the old hand-roasting furnaces are avoided, and besides the notable economy achieved by the H.-H. process itself, the health and well-being of the workmen employed are greatly advanced, so that where hygienic statistics are kept it is proved that lead-poisoning has greatly diminished. It is only natural, therefore, that the H.-H. process should have been a success from the start, popular alike with managers and workmen once the difficulties inseparable from the introduction of any new process were overcome.
Simple as the process now appears, however, it is the result of many years of study and experiment, not devoid of disappointments and at times appearing to present a problem incapable of solution. The first trials were made in the smelting works at Pertusola, Italy, as far back as 1889, where considerable sums were devoted every year to this experimental work and lead ore roasting was almost continuously on the list of new work from 1875 on.
It may be interesting to mention that at this time the Montevecchio ores (containing about 70 per cent. lead and about 15 per cent. sulphur, together with a certain amount of zinc and iron) were considered highly refractory to roast, and the only ores approved of by the management of the works at this date were the Monteponi and San Giovanni first-class ores (containing about 80 per cent. lead), and the second-class carbonates (with at least 60 per cent. lead and 5 per cent. sulphur). It must be noted that a modified Flintshire reverberatory process was in use in the works, which could deal satisfactorily only with this class of ore, so that, as these easy ores diminished in quantity every year and their place was taken by the “refractory” Montevecchio type, the roasting problem was always well to the front at the Pertusola works.
It may be asserted that almost every known method of desulphurization was examined and experimented upon on a large scale. Gas firing was exclusively used on certain classes of ores for several years with considerable success, and revolving furnaces of the Brückner type—gas fired—were also tried. Although varying degrees of success were obtained, no really great progress was made in actual desulphurization; methods were cheapened and larger quantities handled at a time, but the final product—whether sintered or in a pulverulent state—seldom averaged much under 5 per cent. sulphur, while the days of the old “gray slags” (1 per cent. to 2 per cent. sulphur) from the reverberatories totally disappeared, together with the class of ores which produced them.
During the long period of these experiments in desulphurization various facts were established:
(1) That sulphide of lead—especially in a pulverulent state—could not be desulphurized in the same way as other sulphides, such as sulphides of iron, copper, zinc, etc., because if roasted in 169 a mechanical furnace the temperature had to be kept low enough to avoid premature sintering, which would choke the stirrers and cause trouble by the ore clogging on the sides and bottom of the furnace. If, however, the ore was roasted in a “dry state” at low temperature, a great deal of sulphur remained in the product as sulphate of lead, which was as bad for the subsequent blast-furnace work as the sulphide of lead itself. When air was pressed through molten galena—in the same way as through molten copper matte—a very heavy volatilization of lead took place, while portions of it were reduced to metal or were contained as sulphide in the molten matte, so that a good product was not obtained.
(2) That no complete dead roast of lead ores could be obtained unless the final product was thoroughly smelted and agglomerated.
(3) That a well roasted lead ore could be obtained by oxidizing the PbS with compressed air, after the ore had been suitably prepared.
(4) That metal losses were mainly due to the excessive heat produced in the oxidation of PbS to PbO, and other sulphides present in the ore.
It was by making use of these facts that the H.-H. roasting process was finally evolved, and by carefully applying its principles it is possible to desulphurize completely the ore to a practically dead roast of under 1 per cent. sulphur; in practice, however, such perfection is unnecessary and a well agglomerated product with from 2 to 2.5 per cent. sulphur is all that is required. During some trials in Australia, where a great degree of perfection was aimed at, a block of over 2000 tons of agglomerated, roasted ore was produced containing 1 per cent. sulphur (as sulphide); as the ores contained an average of about 10 per cent. Zn, this was a very fine result from a desulphurization point of view, but it was not found that this 1 per cent. product gave any better results in the subsequent smelting in the blast furnace than later on a less carefully prepared material containing 2.5 per cent. sulphur.
In the early stages of experiment the great difficulty was to obtain agglomeration without first fusing the sulphides in the ore, and turning out a half-roasted product full of leady matte. Simple as the thing now is, it seemed at times impossible to avoid this defect, and it was only by a careful study of the effects of an 170addition of lime, Fe2O3 or Mn2O3, and their properties that the right path was struck. Before the introduction of the H.-H. process lime was only used in the reverberatory process (Flintshire and Tarnowitz) to stiffen the charge, but as Percy tells us that after its addition the charge was glowing, it must have had a chemical as well as a mechanical effect. In recognition of this fact fine caustic lime or crushed limestone was mixed with the ore before charging it into the furnace and exposing it to an oxidizing heat.
It was thought probable that a dioxide of lime might be temporarily formed, which in contact with PbS would be decomposed immediately after its formation, or that the CaO served as Contactsubstanz in the same way as spongy platinum, metallic silver, or oxide of iron. As CaSO4 and not CaSO3 is always found in the roasted ore, this may prove that CaO is really a contact substance for oxygen (see W. M. Hutchings, Engineering and Mining Journal, Oct. 21, 1905, Vol. LXXX, p. 726). The fact that the process works equally well with Fe2O3 instead of CaO speaks against the theory of plumbate of lime. Whatever theory may be correct, the fact remains that CaO assists the roasting process and that by its use the premature agglomeration of the sulphide ore is avoided. A further advantage of lime is that it keeps the charge more porous and thus facilitates the passage of the air.
The shape and size of the blowing apparatus best adapted for the purpose in view occupied many months; starting from very shallow pans or rectangular boxes several feet square with a few inches of material over a perforated plate, it gradually resolved itself into the cone-shaped receptacle—holding about a ton of ore—as first introduced together with the process. In later years and in treating larger quantities a more hemispherical form has been adopted, containing up to 15 tons of ore.
It is probable about eight years were employed in actually working out the process before it was introduced on any large scale at Pertusola, but by the end of 1898 the greater part of the Pertusola ores were treated by the process. Its first introduction to any other works was in 1900, when it was started outside its home for the first time at Braubach (Germany). Since then its application has gradually extended, proceeding from Europe to Australia and Mexico and finally to America and Canada, where recognition of its merits was more tardy than elsewhere. It is now practically in general use all over the world and is recognized as a sound addition to metallurgical progress. It is 171 doubtless only a step in the right direction and with its general use a better knowledge of its principles will prevail, so that its future development in one direction or another, as compared with present results, may show some further progress.
The present working of the H.-H. process still follows practically the original lines laid down, and by preliminary roasting in a furnace with lime, oxide of iron, or manganese (if not already contained in the ore), prepares the ore for blowing in the converter. Mechanical furnaces have been introduced to the entire exclusion of the old hand-roasters, and the size of the converters has been gradually increased from the original one-ton apparatus successively to 5, 7 and 10 ton converters; at present some for 15 tons are being built in Germany and will doubtless lead to a further economy.
The mechanical furnace at present most in use is a single-hearth revolving furnace with fixed rabbles, the latest being built with a diameter of 26½ ft. and a relatively high arch to ensure a clear flame and rapid oxidation of the ore. The capacity of these furnaces varies, of course, with the nature of the ores to be treated, but with ordinary lead ores (European and Australian practice) of from 50 per cent. to 60 per cent. lead and 14 per cent, to 18 per cent. sulphur, the average capacity may be taken at about 50 to 60 tons of crude ore per day of 24 hours. The consumption of coal with a well-constructed furnace is very low and is always under 8 per cent.—6 per cent. being perhaps the average. These furnaces require very little attention, being automatic in their charging and discharging arrangements.
The ore on leaving the furnace is charged into the converters by various mechanical means (Jacob’s ladders, conveyors, etc.). The converter charge usually consists of some hot ore direct from the furnace, on top of which ore is placed which has been cooled down by storage in bins or by the addition of water. The converter is generally filled in two charges of five tons each, and the blowing time should not be more than 4 to 6 hours. The product obtained should be porous and well agglomerated, but easily broken up, tough melted material being due to an excess of silica and too much lead sulphide. Attention, therefore, to these two points (good preliminary roasting and correction of the charge by lime) obviates this trouble. This roasted ore should not contain more than about 1.5 to 2 per cent. sulphur, and in a 172 modern blast furnace gives surprisingly good results, the matte-fall being in most cases reduced to nothing, and the capacity of the furnace is largely increased, while the slags are poorer.
If the converter charge has been properly prepared, the blowing operation proceeds with the greatest smoothness and requires very little attention on the part of the workmen, the heat and oxidation rise gradually from the bottom and volatilization losses remain low, so that it is possible, if desired, to produce hot concentrated sulphurous gases suitable for the manufacture of sulphuric acid.
Besides the actual economy obtained in roasting ores by the process, a great feature of its success has been the remarkable improvement in smelting and reducing the roasted ore as compared with previous experience. This is due to the nature of the roasted material, which, besides being much poorer in sulphur than was formerly the case, is thoroughly porous and well agglomerated and contains—if the original mixture is properly made—all the necessary slagging materials itself, so that it practically becomes a case of smelting slags instead of ore, and to an expert the difference is evident.
Experience has shown that on an average the improvement in the capacity of the blast furnace may be taken at about 50 to 100 per cent., so that in works using the H.-H. process—after its complete introduction—about half the blast furnaces formerly necessary for the same tonnage were blown out. The matte-fall has become a thing of the past, so that, except in those cases where some matte is required to collect the copper contained in the ores, lead matte has disappeared and the quantity of flue dust as well as the lead and silver losses have been greatly reduced.
Referring to the latest history of the H.-H. process, and the theory of direct blowing, it may be remarked—putting aside all legal questions—that the idea, metallurgically speaking, is attractive, as it would seem that by eliminating one-half of the process and blowing the ores direct without the expense of a preliminary roast a considerable economy should be effected. Upon examination, however, this supposed economy and simplicity is not at all of such great importance, and in many cases, without doubt, would be retrogressive in lead ore smelting rather than progressive. When costs of roasting in a furnace are reduced 173 to such a low figure as can be obtained by using 50 ton furnaces and 10 to 15 ton converters, there is very little margin for improvement in this direction. From the published accounts of the Tarnowitz smelting works (the Engineering and Mining Journal, Sept. 23, 1905, Vol. LXXX, p. 535) the cost of mechanical preliminary roasting cannot exceed 25c. per ton, so that even assuming direct blowing were as cheap as blowing a properly prepared material, the total economy would only be the above figure, viz., 25c.; but this is far from being the case.
Direct blowing of a crude ore is considerably more expensive than dealing with the H.-H. product, because of necessity the blowing operation must be carried out slowly and with great care so as to avoid heavy metal losses, and whereas a pre-roasted ore can be easily blown in four hours and one man can attend to two or three 10 ton converters, the direct blowing operation takes from 12 to 18 hours and requires the continual attention of one man. In the first case the cost of labor would be: One man at say $3 for 50 tons (at least), i.e., 6c. per ton, and in the second case one man at $3 for 10 tons (at the best), i.e., 30c., a difference in favor of pre-roasting of 24c., so that any possible economy would disappear. Furthermore, as the danger of blowing upon crude sulphides for 12 or 18 hours is greater as regards metal losses than a quick operation of four hours, it is very likely that instead of an economy there would be an increase in cost, owing to a greater volatilization of metals.
These remarks refer to ordinary lead ores with say 50 per cent. lead and about 14 per cent. sulphur. With ores, however, such as are generally treated in the United States the advantages of pre-roasting are still more evident. These ores contain about 10 to 15 per cent. lead, 30 to 40 per cent. sulphur, 20 to 30 per cent. iron, 10 per cent. zinc, 5 per cent. silica, and lose the greater part of the pyritic sulphur in the preliminary roasting, leaving the iron in the form of oxide, which in the subsequent blowing operation acts in the same way as lime. For this reason the addition of extra fluxes, such as limestone, gypsum, etc., to the original ore is not necessary and only a useless expense.
In certain exceptional cases and with ores poor in sulphur, direct blowing might be applicable, but for the general run of lead ores no economy can be expected by doing away with the preliminary roast.
(August 11, 1904)
The Broken Hill Proprietary Company has entered upon the manufacture of sulphuric acid on a commercial scale. The acid is practically a by-product, being made from the gases emanating from the desulphurization of the ores, concentrates, etc., by the Carmichael-Bradford process. The acid can be made at a minimum of cost, and thus materially enhances the value of the process recently introduced for the separation of zinc blende from the tailings by flotation. The following particulars are taken from a recently published description of the process: The ores, concentrates, slimes, etc., as the case may be, are mixed with gypsum, the quantity of the latter varying from 15 to 25 per cent. The mixture is then granulated to the size of marbles and dumped into a converter. The bottom of the charge is heated from 400 to 500 deg. C. It is then subjected to an induced current of air, and the auxiliary heat is turned off. The desulphurization proceeds very rapidly with the evolution of heat and the gases containing sulphurous anhydride. The desulphurization is very thorough, and no losses occur through volatilization. The sulphur thus rendered available for acid making is rather more than is contained in the ore, the sulphur in the agglomerated product being somewhat less than that accounted for by the sulphur contained in the added gypsum. Thus from one ton of 14 per cent. sulphide ore it is possible to make about 12 cwt. of chamber acid, fully equaling 7 cwt. of strong acid.
The plant at present in use, which comprises a lead chamber of 40,000 cu. ft., can turn out 35 tons of chamber acid per week. This plant is being duplicated, and it has also been decided to erect a large plant at Port Pirie for use in the manufacture of superphosphates. It is claimed that the production of sulphuric acid from ores containing only 14 per cent. of sulphur establishes a new record.
(November 3, 1904)
Subsequent to the introduction of the Huntington-Heberlein process in Australia, Messrs. Carmichael and Bradford, two employees of the Broken Hill Proprietary Company, patented a process which bears their name. Instead of starting with lime, or limestone and galena, as in the Huntington-Heberlein process, they discovered that if sulphate of lime is mixed with galena and the temperature raised, on blowing a current of air through the mixture the temperature rises and the mass is desulphurized. The process would thus appear to be a corollary of the original one, and the reactions in the converter are identical. Owing to the success of the acid processes in separating zinc sulphide from the tailing at Broken Hill, it became necessary to manufacture sulphuric acid locally in large quantity. The Carmichael-Bradford process has been started for the purpose of generating the sulphur dioxide necessary, and is of much interest as showing how gases rich enough in SO2 may be produced from a mixture containing only from 13 to 16 per cent. sulphur.
Gypsum is obtained in a friable state within about five miles from Broken Hill. This is dehydrated, the CaSO, 2H2O being converted into CaSO4 on heating to about 200 deg. C. The powdered residue is mixed with slime produced in the milling operations and concentrate in the proportion of slime 3 parts, concentrate 1 part, and lime sulphate 1 part. The proportions may vary to some extent, but the sulphur contents run from 13 to 16 or 17 per cent. The average composition of the ingredients is as given in the table on the next page.
These materials are moistened with water and well mixed by passing them through a pug-mill. The small amount of water used serves to set the product, the lime sulphate partly becoming plaster of paris, 2CaSO, H2O. While still moist the mixture is broken into pieces not exceeding two inches in diameter and 176 spread out on a drying floor, where excess of moisture is evaporated by the conjoint action of sun and wind.
| Slime | Concentrate | Calcium Sulphate | Average | |
|---|---|---|---|---|
| Galena | 24 | 70 | — | 29 |
| Blende | 30 | 15 | — | 21 |
| Pyrite | 3 | — | — | 2 |
| Ferric oxide | 4 | — | — | 2.5 |
| Ferrous oxide | 1 | — | — | 1 |
| Manganous oxide | 6.5 | — | — | 5 |
| Alumina | 5.5 | — | — | 3 |
| Lime | 3.5 | — | 41 | 10 |
| Silica | 23 | — | — | 14 |
| Sulphur trioxide | — | — | 59 | 12 |
The pots used are small conical cast-iron ones, hung on trunnions, and of the same pattern as used in the Huntington-Heberlein process. Three of these are set in line, and two are at work while the third is being filled. These pots have the same form of conical cover leading to a telescopic tube, and all are connected to the same horizontal pipe leading to the niter pots. Dampers are provided in each case. A small amount of coal or fuel is fed into the pots and ignited by a gentle blast; as soon as a temperature of about 400 to 500 deg. C. is attained the dried mixture is fed in, until the pot is full; the cover is closed down and the mass warms up. Water is first driven off, but after a short time concentrated fumes of sulphur dioxide are evolved. The amount of this gas may be as much as 14 per cent., but it is usually kept at about 10 per cent., so as to have enough oxygen for the conversion of the dioxide to the trioxide. The gases are led over a couple of niter pots and thence to the usual type of lead chamber having a capacity of 40,000 cu. ft. Chamber acid alone is made, since this requires to be diluted for what is known as the saltcake process.
The plant has now been in operation for some time and, it is claimed, with highly successful results. The product tipped out of. the converter is similar to that obtained in the Huntington-Heberlein process, and is at once fit for the smelters, the amount of sulphur left in it being always less than that originally introduced with the gypsum; analysis of the desulphurized material shows usually from 3 to 4 per cent. sulphur.
(October 28, 1905)
As described in United States patent No. 705,904, issued July 29, 1902, lead sulphide ore is mixed with 10 to 35 per cent. of calcium sulphate, the percentage varying according to the grade of the ore. The mixture is charged into a converter and gradually heated externally until the lower portion of the charge, say one-third to one-fourth, is raised to a dull-red heat; or the reactions may be started by throwing into the empty converter a shovelful of glowing coal and turning on a blast of air sufficient to keep the coal burning and then feeding the charge on top of the coal. This heating effects a reaction whereby the lead sulphide of the ore is oxidized to sulphate and the calcium sulphate is reduced to sulphide. The heated mixture being continuously subjected to the blast of air, the calcium sulphide is re-oxidized to sulphate and is thus regenerated for further use. This reaction is exothermic, and sufficient heat is developed to complete the desulphurization of the charge of ore by the concurrent reactions between the lead sulphate (produced by the calcium sulphate) and portions of undecomposed ore, sulphurous anhydride being thus evolved. The various reactions, which are complicated in their nature, continue until the temperature of the charge reaches a maximum, by which time the charge has shrunk considerably in volume and has a tendency to become pasty. This becomes more marked as the production of lead oxide increases, and as the desired point of desulphurization is attained the mixture fuses; at this stage the calcium sulphide which is produced from the sulphate cannot readily oxidize, owing to the difficulty of coming into actual contact with the air in the pasty mass, but, being subjected to the strong oxidizing effect of the metallic oxide, it is converted into calcium plumbate, while sulphurous anhydride is set free. The mass then cools, as the exothermic reactions cease, and can be readily removed to a blast furnace for smelting.
The reactions above described are as outlined in the original American patent specification. Irrespective of their accuracy, the Carmichael-Bradford process is obviously quite similar to the Huntington-Heberlein, and doubtless owes its origin to the latter. The difference between them is that in the Huntington-Heberlein process the ore is first partially roasted with addition of lime, and is then converted in a special vessel. In the Carmichael-Bradford process the ore is mixed with gypsum and is then converted directly. The greatest claim for originality in the Carmichael-Bradford process may be considered to lie in it as a method of desulphurizing gypsum, inasmuch as not only is the sulphur of the ore expelled, but also a part of the sulphur of the gypsum; and the sulphur is driven off as a gas of sufficiently high tenor of sulphur dioxide to enable sulphuric acid to be made from it economically. Up to the present time the Carmichael-Bradford process has been put into practical use only at Broken Hill, N. S. W.
The Broken Hill Proprietary Company first conducted a series of tests in a converter capable of treating a charge of 20 cwt. These tests were made at the smelting works at Port Pirie. Exhaustive experiments made on various classes of ores satisfactorily proved the general efficacy of the process. The following ores were tried in these preliminary experiments, viz.:
First-grade concentrate containing: Pb, 60 per cent.; Zn, 10 per cent.; S, 16 per cent.; Ag, 30 oz.
Second-grade concentrate containing: Pb, 45 per cent.; Zn, 12.5 per cent.; S, 14.5 per cent.; Ag, 22 oz.
Slime containing: Pb, 21 per cent.; Zn, 17 per cent.; S, 13 per cent.; Ag, 18 oz.
Lead-copper matte containing: Fe, 42 per cent.; Pb, 17 per cent.; Zn, 13.3 per cent.; Cu, 2.4 per cent.; S, 23 per cent.; Ag, 25 oz.
Other mattes, of varying composition up to 45 per cent. Pb and 100 oz. Ag, were also tried.
The results from these preliminary tests were so gratifying that a further set of tests was made on lead-zinc slime, with a view of ascertaining whether any volatilization losses occurred during the desulphurization. This particular material was chosen because of its accumulation in large proportions at the mine, and the unsatisfactory result of the heap roasting which has 179 recently been practised. The heap roasting, although affording a product containing only 7 per cent. S, which is delivered in lump form and therefore quite suitable for smelting, resulted in a high loss of metal by volatilization (17 per cent. Pb, 5 per cent. Ag).
The result of nine charges of the slime treated by the Carmichael-Bradford process was as follows:
| Cwt. | Assays | Contents | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pb% | Ag oz. | Zn% | S% | Pb cwt. |
Ag. oz. |
Zn cwt. |
S cwt. |
||
| Raw slime | 128.1 | 21.3 | 18.0 | 16.8 | 13.1 | 27.28 | 115.3 | 26.2 | 16.78 |
| Raw gypsum | 54.9 | 9.88 | |||||||
| Total | 183.0 | 27.28 | 115.3 | 25.2 | 26.66 | ||||
| Sintered material | 109.88 | 20.7 | 17.2 | — | 4.80 | 22.74 | 94.5 | — | 5.27 |
| Middling | 14.47 | 17.7 | 15.7 | — | 6.20 | 2.56 | 11.3 | — | 0.89 |
| Fines | 11.12 | 19.0 | 14.8 | — | 7.50 | 2.11 | 8.2 | — | 0.83 |
| Total | 135.47 | — | — | — | 5.17 | 27.41 | 113.0 | — | 6.99 |
These results indicated practically no volatilization of lead and silver during the treatment, the lead showing a slight increase, viz., 0.47 per cent., and the silver 1.13 per cent. loss. A desulphurization of 70.4 per cent. was effected. A higher desulphurization could have been effected had this been desired. In the above tabulated results, the term “middling” is applied to the loose fritted lumps lying on the top of the charge: these are suitable for smelting, the fines being the only portion which has to be returned.
In order to test the practicability of making sulphuric acid, a plant consisting of three large converters of capacity of five tons each, together with a lead chamber 100 ft. by 20 ft. by 20 ft., was then erected at Broken Hill, together with a dehydrating furnace, pug-mill, and granulator. These converters are shown in the accompanying engravings.
A trial run was made with 108 tons of concentrate of the following composition: 54 per cent. lead; 1.9 per cent. iron; 0.9 per cent. manganese; 9.4 per cent. zinc; 14.6 per cent. sulphur; 19.2 per cent. insoluble residue, and 24 oz. silver per ton.
The converter charge consisted of 100 parts of the concentrate and 25 parts of raw gypsum, crushed to pass a 1 in. hole and 180 retained by a 0.25 in. hole, the material finer than 0.25 in. (which amounted to 5 per cent. of the total) being returned to the pug-mill. After desulphurization in the converter, the product assayed as follows: 48.9 per cent. lead; 1.80 per cent. iron; 0.80 per cent. manganese; 7.87 per cent. zinc; 3.90 per cent. sulphur; 1.02 per cent. alumina; 5.80 per cent. lime; 21.75 per cent. insoluble residue; 8.16 per cent. undetermined (oxygen as oxides, sulphates, etc.); total, 100 per cent. Its silver content was 22 oz. per ton. The desulphurized ore weighed 10 per cent. more than the raw concentrate. During this run 34 tons of acid were made.
A trial was then made on 75 tons of slime of the following composition: 18.0 per cent. lead; 16.6 per cent. zinc; 6.0 per cent. iron; 2.5 per cent. manganese; 3.2 per cent. alumina; 2.1 per cent. lime; 38.5 per cent. insoluble residue; total, 100 per cent. Its silver content was 19.2 oz. per ton.
The converter charge in this case consisted of 100 parts of raw slime and 30 parts of gypsum. The converted material assayed as follows: 16.1 per cent. lead; 14.0 per cent. zinc; 3.6 per cent. sulphur; 5.42 per cent. iron; 2.25 per cent. manganese; 4.10 per cent. alumina; 8.60 per cent. lime; 39.80 per cent. insoluble residue; 6.13 per cent. undetermined (oxygen, etc.); total, 100 per cent.; and silver, 17.5 oz. per ton. The increase in weight of desulphurized ore over that of the raw ore was 11 per cent. During this run 22 tons of acid were manufactured.
The analysis of the gypsum used in each of the above tests (at Broken Hill) was as follows: 76.1 per cent. CaSO4, 2H2O; 0.5 per cent. Fe2O3; 4.5 per cent. Al2O3; 18.9 per cent. insoluble residue.
The plant was then put into continuous operation on a mixture of three parts slime and one of concentrate, desulphurizing down to 4 per cent. S, and supplying 20 tons of acid per week, and additions were made to the plant as soon as possible. The acid made at Broken Hill has been used in connection with the Delprat process for the concentration of the zinc tailing. At Port Pirie, works are being erected with capacity for desulphurization of about 35,000 tons per annum, with an acid output of 10,000 tons. This acid is to be utilized for the acidulation of phosphate rock.
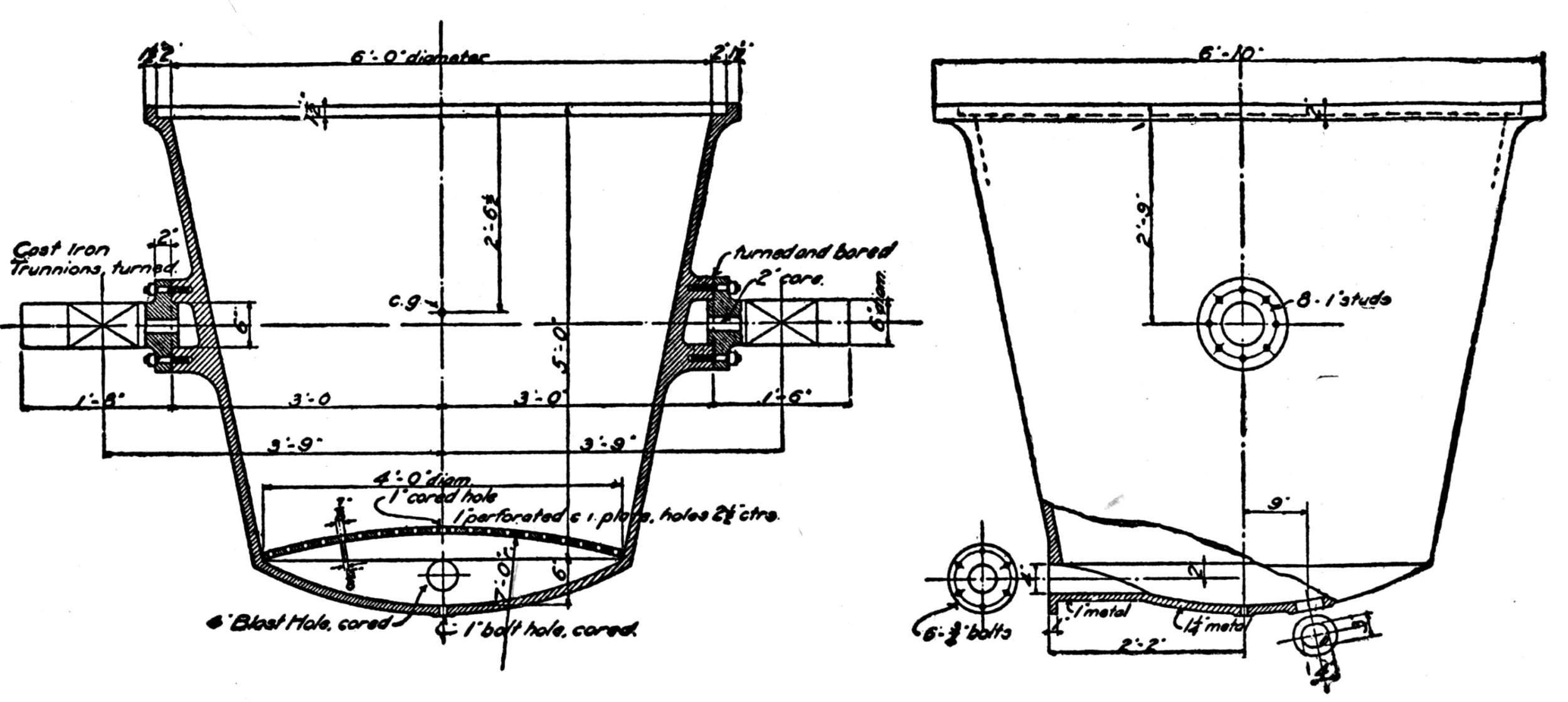
The cost of desulphurization of a ton of galena concentrate by the Carmichael-Bradford process, based on labor at $1.80 per 8 hours, gypsum at $2.40 per 2240 lb., and coal at $8.40 per 2240 lb., is estimated as follows:
| 0.25 ton of gypsum | $0.60 |
| Dehydrating and granulating gypsum | .48 |
| Drying mixture of ore and gypsum | .12 |
| Converting | .24 |
| Spalling sintered material | .12 |
| 0.01 ton coal | .08 |
| Total | $1.64 |
The lime in the sintered product is credited at 12c., making the net cost $1.52 per ton (2240 lb.) of ore.
The plant required for the Carmichael-Bradford process can be described with sufficient clearness without drawings, except the converters. The ore (concentrate, slime, etc.) to be desulphurized is delivered at the top of the mill by cars, conveyors, or other convenient means, and dumped into a bin. Two screw feeders placed inside the bin supply the mill with ore, uniformly and as fast as it is required. These feeders deliver the ore into a chute, which directs it into a vertical dry mixer.
A small bin, on the same level as the ore-bin, receives the crude gypsum from cars. Thence it is fed automatically to a disintegrator, which pulverizes it finely and delivers it into a storage bin underneath. This disintegrator revolves at about 1700 r.p.m. and requires 10 h.p. The body of the machine is cast iron, fitted with renewable wearing plates (made of hard iron) in the grinding chamber. The revolving parts consist of a malleable iron disc in which are fixed steel beaters, faced on the grinding surface with highly tempered steel. The bin that receives the floured gypsum contains a screw conveyor similar to those in the ore-bin, and dumps the material into push conveyors passing into the dehydrating furnace. They carry the crushed gypsum along at a speed of about 1 ft. per minute, and allow about 20 ft. to dehydrate the gypsum. This speed can, of course, be regulated to suit requirements.
The dehydrated gypsum runs down a chute into an elevator boot, and is elevated into a bin which is on the same level as the ore-bin. This bin also contains a screw conveyor, like that in the ore-bin. The speed of delivery is regulated to deliver the right proportion of dehydrated gypsum to the mixer.
The mixer is of the vertical pattern and receives the sulphide ore and dehydrated gypsum from the screw feeders. In it are set two flat revolving cones running at different speeds, thus ensuring a thorough mixture of the gypsum and ore. The mixed material drops from the cones upon two baffle plates, and is wetted just before entering the pug-mill. The pug-mill is a wrought-iron cylinder of ¼ in. plate about 2 ft. 6 in. diameter and 6 or 8 ft. long, and has the mixer fitted to the head. It contains a 3 ft. wrought-iron spiral with propelling blades, which forces the plastic mixture through ¾ in. holes in the cover. The material comes out in long cylindrical pieces, but is broken up and formed into marble-shaped pieces on dropping into a revolving trommel.
The trommel is about 5 ft. long, 2 ft. in diameter at the small end and about 4 ft. at the large end. It revolves about a wrought-iron spindle (2½ in. diameter) carrying two cast-iron hubs to which are fitted arms for carrying the conical plate ⅛ in. thick. About 18 in. of the small end of the cone is fitted with wire gauze, so as to prevent the material as it comes out of the pug-mill from sticking to it. The trommel is driven by bevel gearing at 20 to 25 r.p.m. The granulated material formed in the trommel is delivered upon a drying conveyor.
The conveyor consists of hinged wrought-iron plates flanged at the side to keep the material from running off. It is driven from the head by gearing, at a speed of 1 ft. per minute, passing through a furnace 10 ft. long to dry and set the granules of ore and gypsum. This speed can, of course, be regulated to suit requirements. The granulated material, after leaving the furnace, is delivered to a single-chain elevator, traveling at a speed of about 150 ft. per minute. It drops the material into a grasshopper conveyor, driven by an eccentric, which distributes the material over the length of a storage bin. From this bin the material is directed into the converters by means of the chutes, which have their bottom ends hinged so as to allow for the raising of the hood when charging the converters.
The converters are shown in the accompanying engravings,
but they may be of slightly different form from what is shown
therein, i.e., they may be more spherical than conical. They
will have a capacity of about four tons, being 6 ft. in diameter
at the top, 4 ft. in diameter at the false bottom, and about 5 ft. 184
185
deep. They are swung on cast-iron trunnions bolted to the
body and turned by means of a hand-wheel and worm (not shown).
They are carried on strong cast-iron standards fitted with bearings
for trunnions, and all necessary brackets for tilting gear. The
hood has a telescopic funnel which allows it to be raised or lowered,
weights being used to balance it. At the apex of the cone a
damper is provided to regulate the draft. A 4 in. hole in the
pot allows the air from the blast-pipe, 18 in. in diameter, to
enter under the false perforated bottom, the connection between
the two being made by a flexible pipe and coupling. Two Baker
blowers supply the blast for the converters. The material, after
being sintered, is tipped on the floor in front of the converters
and is there broken up to any suitable size, and thence dispatched
to the smelters.
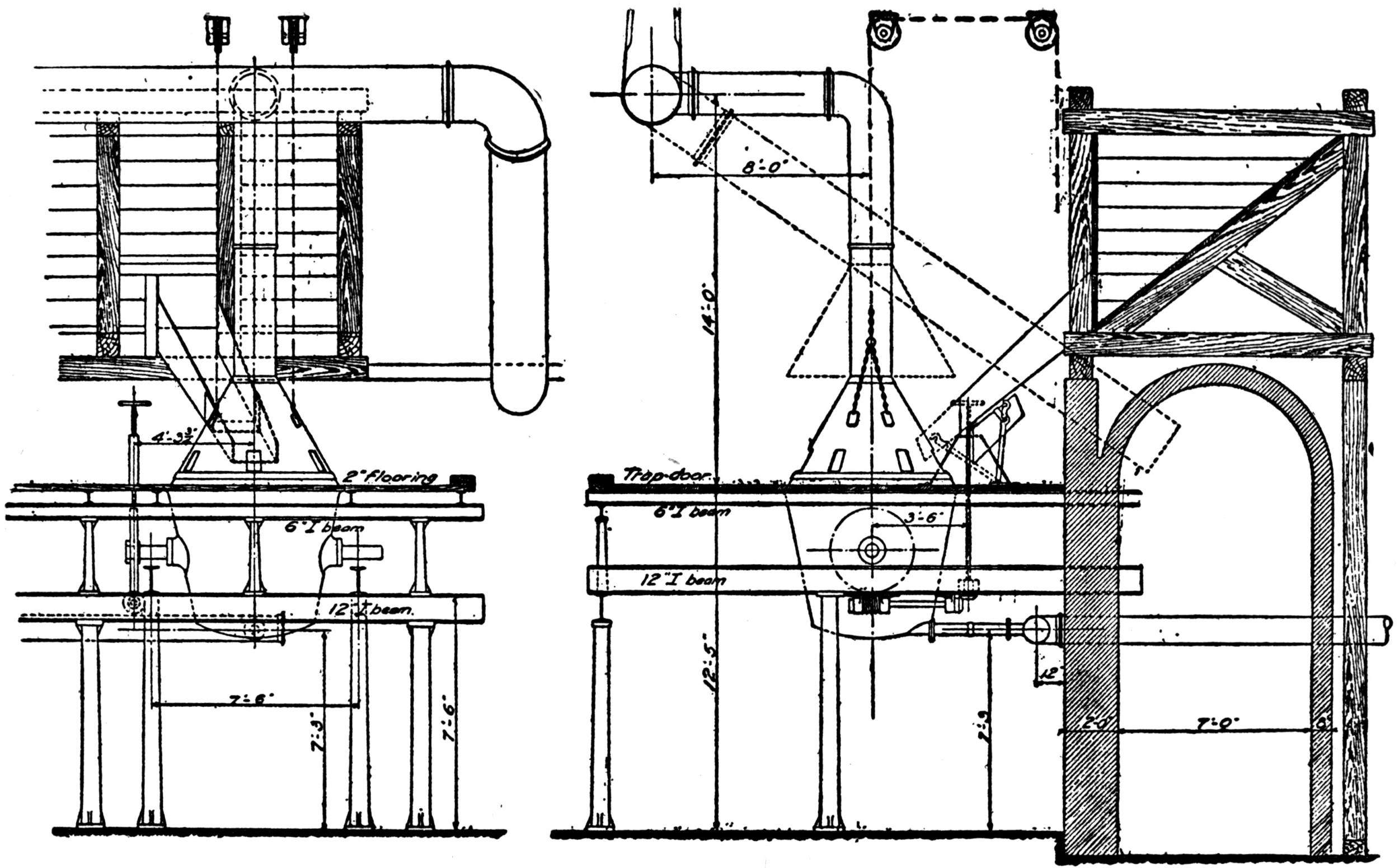
The necessary power for a plant with a capacity of 150 tons of ore per day will be supplied by a 50 h.p. engine.
(December 9, 1905)
There are in use at the present time three processes for the desulphurization of galena by the new method, which has been referred to as the “lime-roasting of galena.” The Huntington-Heberlein and the Carmichael-Bradford processes have been previously described. The third process of this type, which in some respects is more remarkable than either of the others, is the invention of Adolf Savelsberg, director of the smeltery at Ramsbeck, Westphalia, Germany, which is owned by the Akt. Gesell. f. Bergbau, Blei. u. Zinkhüttenbetrieb zu Stolberg u. in Westphalen. The process is in use at the Ramsbeck and Stolberg lead smelteries of that company. It is described in American patent No. 755,598, issued March 22, 1904 (application filed Dec. 18, 1903). The process is well outlined in the words of the inventor in the specification of that patent:
“The desulphurizing of certain ores has been effected by blowing air through the ore in a chamber, with the object of doing away with the imperfect and costly process of roasting in ordinary furnaces; but hitherto it has not been possible satisfactorily to desulphurize lead ores in this manner, as, if air be blown through raw lead ores in accordance with either of the processes used for treating copper ores, for example, the temperature rises so rapidly that the unroasted lead ore melts and the air can no longer act properly upon it, because by reason of this melting the surface of the ores is considerably decreased, the greater number of points or extent of surface which the raw ore originally presented to the action of the oxygen of the air blown through being lost, and, moreover, the further blowing of air through the molten mass of ore produces metallic lead and a plumbiferous slag (in which the lead oxide combines with the gangue) and also a large amount of light dust, consisting mainly of sublimated lead sulphide. Huntington and Heberlein have proposed to 187 overcome these objections by adopting a middle course, consisting in roasting the ores with the addition of limestone for overcoming the ready fusibility of the ores, and then subjecting them to the action of the current of air in the chamber; but this process is not satisfactory, because it still requires the costly previous operation in a roasting furnace.
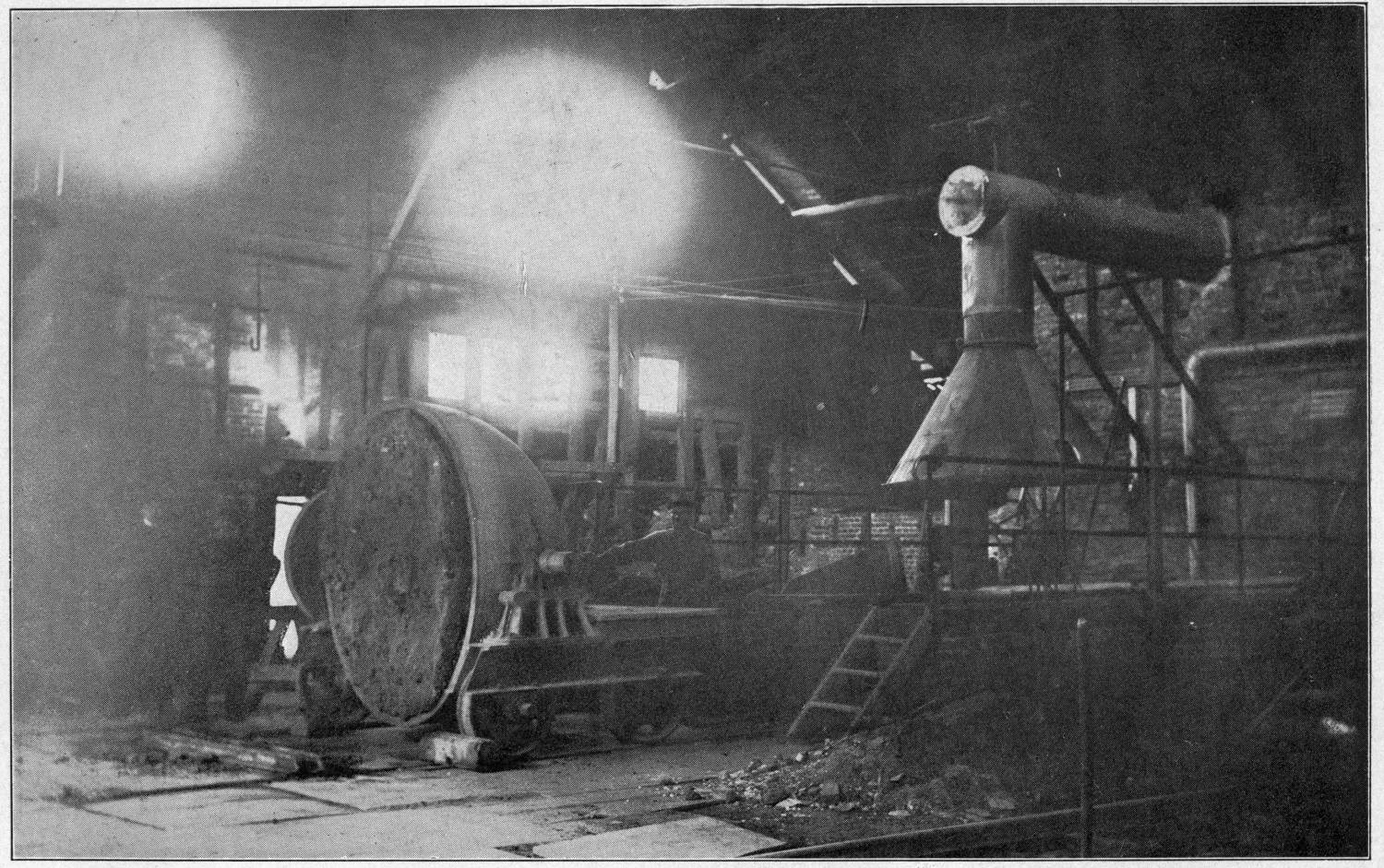
“My invention is based on the observation which I have made that if the lead ores to be desulphurized contain a sufficient quantity of limestone it is possible, by observing certain precautions, to dispense entirely with the previous roasting in a roasting furnace, and to desulphurize the ores in one operation by blowing air through them. I have found that the addition of limestone renders the roasting of the lead ore unnecessary, because the limestone produces the following effects:
“The particles of limestone act mechanically by separating the particles of lead ore from each other in such a way that premature agglomeration is prevented and the whole mass is loosened and rendered accessible to air; and, moreover, the limestone moderates the high reaction temperature resulting from the burning of the sulphur, so that the liquefaction of the galena, the sublimation of lead sulphide, and the separation of metallic lead are avoided. The moderation of the temperature of reaction is caused by the decomposition of the limestone into caustic lime and carbon dioxide, whereby a large amount of heat becomes latent. Further, the decomposition of the limestone causes chemical reactions, lime being formed, which at the moment of its formation is partly converted into sulphate of lime at the expense of the sulphur contained in the ore, and this sulphate of lime, when the scorification takes place, is transformed into calcium silicate by the silicic acid, the sulphuric acid produced thereby escaping. The limestone also largely contributes to the desulphurization of the ore, as it causes the production of sulphuric acid at the expense of the sulphur of the ore, which sulphuric acid is a powerful oxidizing agent. If, therefore, a mixture of raw lead ore and limestone (which mixture must, of course, contain a sufficient amount of silicic acid for forming silicates) be introduced into a chamber and a current of air be blown through the mixture, and at the same time the part of the mixture which is near the blast inlet be ignited, the combustion of the sulphur will give rise to very energetic reactions, and sulphurous 188 acid, sulphuric acid, lead oxide, sulphates and silicates are produced. The sulphurous acid and the carbon dioxide escape, while the sulphuric acid and sulphates act in their turn as oxidizing agents on the undecomposed galena. Part of the sulphates is decomposed by the silicic acid, thereby liberating sulphuric acid, which, as already stated, acts as an oxidizing agent. The remaining lead oxide combines finally with the gangue of the ore and the non-volatile constituents of the flux (the limestone) to form the required slag. These several reactions commence at the blast inlet at the bottom of the chamber, and extend gradually toward the upper portion of the charge of ore and limestone. Liquefaction of the ores does not take place, for although a slag is formed it is at once solidified by the blowing in of the air, the passages formed thereby in the hardening slag allowing of the continued passage therethrough of the air. The final product is a silicate consisting of lead oxide, lime, silicic acid, and other constituents of the ore, which now contains but little or no sulphur and constitutes a coherent solid mass, which, when broken into pieces, forms a material suitable to be smelted.
“The quantity of limestone required for the treatment of the lead ores varies according to the constitution of the ores. It should, however, amount generally to from 15 to 20 per cent. As lead ores do not contain the necessary amount of limestone as a natural constituent, a considerable amount of limestone must be added to them, and this addition may be made either during the dressing of the ores or subsequently.
“For the satisfactory working of the process, the following precautions are to be observed: In order that the blowing in of the air may not cause particles of limestone to escape in the form of dust before the reaction begins, it is necessary to add to the charge before it is subjected to the action in the chamber a considerable amount of water—say 5 per cent. or more. This water prevents the escape of dust, and it also contributes considerably to the formation of sulphuric acid, which, by its oxidizing action, promotes the reaction, and, consequently, also the desulphurization. It is advisable, in conducting the operation, not to fill the chamber with the charge at once, but first only partly to fill it and add to the charge gradually while the chamber is at work, as by this means the reaction will take place more smoothly in the mass.

“It is advantageous to proceed as follows: The bottom part of a chamber of any suitable form is provided with a grate, on which is laid and ignited a mixture of fuel (coal, coke, or the like) and pieces of limestone. By mixing the fuel with pieces of limestone the heating power of the fuel is reduced and the grate is protected, while at the same time premature melting of the lower part of the charge is prevented; or the grate may be first covered with a layer of limestone and the fuel be laid thereon, and then another layer of limestone be placed on the fuel. On the material thus placed in the chamber, a uniform charge of lead ore and limestone—say about 12 in. high—is placed, this having been moistened as previously explained. Under the influence of the air-blast and the heat, the reactions hereinbefore described take place. When the upper surface of the first layer becomes red-hot, a further charge is laid thereon, and further charges are gradually introduced as the surface of the preceding charge becomes red-hot, until the chamber is full. So long as charges are still introduced a blast of air of but low pressure is blown through; but when the chamber is filled a larger quantity of air at a higher pressure is blown through. The scorification process then takes place, a very powerful desulphurization having preceded it. During the scorification the desulphurization is completed.
“When the process is completed, the chamber is tilted and the desulphurized mass falls out and is broken into small pieces for smelting.”
The drawing on page 190, Fig. 17, shows a side view of the apparatus used in connection with the process, which will be readily understood without special description. The dotted lines show the pot in its emptying position. The series of operations is clearly illustrated in Figs. 18-20, which are reproduced from photographs.
This process has now been in practical use at Ramsbeck for three years, where it is employed for the desulphurization of galena of high grade in lead, with which are mixed quartzose silver ore (or sand if no such ore be available), and calcareous and ferruginous fluxes. A typical charge is 100 parts of lead ore, 10 parts of quartzose silver ore, 10 parts of spathic iron ore, and 19 parts of limestone. A thorough mixture of the components is essential; after the mixture has been effected, the charge is 190 thoroughly wetted with about 5 per cent. of water, which is conceived to play a threefold function in the desulphurizing operation, namely: (1) preservation of the homogeneity of the mixture during the blowing; (2) reduction of temperature during the process; and (3) formation of sulphuric acid in the process, which promotes the desulphurization of the ore.
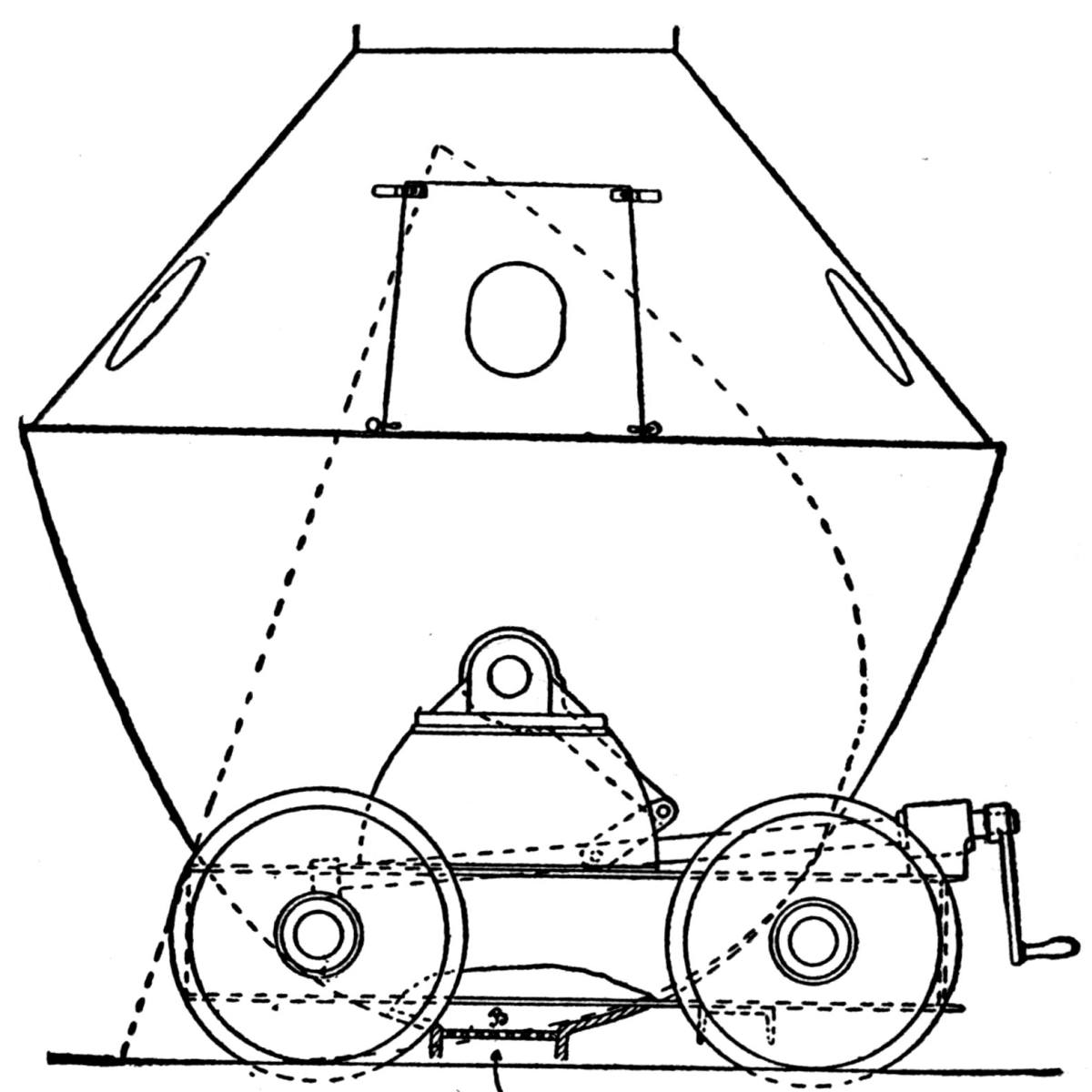
The moistened charge is conveyed to the converters, into which it is fed in thin layers. The converters are hemispherical cast-iron pots, supported by trunnions on a truck, as shown in the accompanying engravings. Except for this method of support, which renders the pot movable, the arrangement is quite similar to that which is employed in the Huntington-Heberlein process. The pots which are now in use at Ramsbeck have capacity for about 8000 kg. of charge, but it is the intention of the management to increase the capacity to 10,000 or 12,000 kg. Previously, pots of only 5000 kg. capacity were employed. Such a pot weighed 1300 kg., exclusive of the truck. The air-blast was about 7 cu. m. (247.2 cu. ft.) per min., beginning at a pressure 191 of 10 to 20 cm. of water (2¾ to 4½ oz.) and rising to 50 to 60 cm. (11½ to 13½ oz.) when the pot was completely filled with charge. The desulphurization of a charge is completed in 18 hours. A pot is attended by one man per shift of 12 hours; this is only the attention of the pot proper, the labor of conveying material to it and breaking up the desulphurized product being extra. One man per shift should be able to attend to two pots, which is the practice in the Huntington-Heberlein plants.
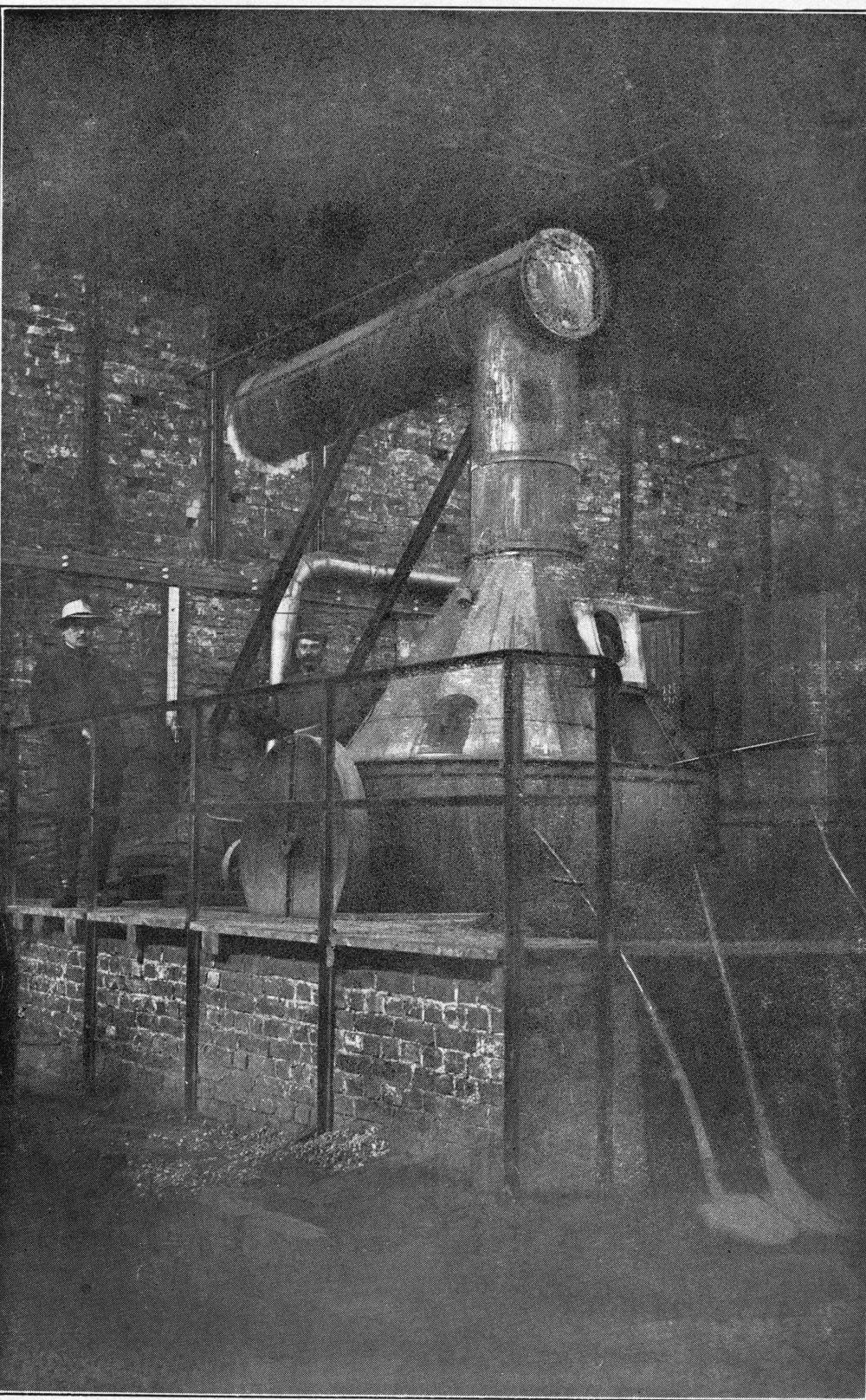
When the operation in the pot is completed, the latter is turned on its trunnions, until the charge slides out by gravity, which it does as a solid cake. This is caused to fall upon a vertical bar, which breaks it into large pieces. By wedging and sledging these are reduced to lumps of suitable size for the blast furnace. When the operation has been properly conducted the charge is reduced to about 2 or 3 per cent. sulphur. It is expected that the use of larger converters will show even more favorable results in this particular.
As in the Huntington-Heberlein and Carmichael-Bradford processes, one of the greatest advantages of the Savelsberg process is the ability to effect a technically high degree of desulphurization with only a slight loss of lead and silver, which is of course due to the perfect control of the temperature in the process. The precise loss of lead has not yet been determined, but in the desulphurization of galena containing 60 to 78 per cent. lead, the loss of lead is probably not more than 1 per cent. There appears to be no loss of silver.
The process is applicable to a wide variety of lead-sulphide ores. The ore treated at Ramsbeck contains 60 to 78 per cent. lead and about 15 per cent. of sulphur, but ore from Broken Hill, New South Wales, containing 10 per cent. of zinc has also been treated. A zinc content up to 7 or 8 per cent. in the ore is no drawback, but ores carrying a higher percentage of zinc require a larger addition of silica and about 5 per cent. of iron ore in order to increase the fusibility of the charge. The charge ordinarily treated at Ramsbeck is made to contain about 11 per cent. of silica. The presence of pyrites in the ore is favorable to the desulphurization. Dolomite plays the same part in the process that limestone does, but is of course less desirable, in view of the subsequent smelting in the blast furnace. The ore is best crushed to about 3 mm. size, but good results have been obtained with 192 ore coarser in size than that. However, the proper size is somewhat dependent upon the character of the ore. The blast pressure required in the converter is also, of course, somewhat dependent upon the porosity of the charge. Fine slimes are worked up by mixture with coarser ore.
In making up the charge, the proportion of limestone is not varied much, but the proportions of silica and iron must be carefully modified to suit the ore. Certain kinds of ore have a tendency to remain pulverulent, or to retain balls of unsintered, powdered material. In such cases it is necessary to provide more fusible material in the charge, which is done by varying the proportions of silica and iron. The charge must, moreover, be prepared in such a manner that overheating, and consequently the troublesome fusion of raw galena, will be avoided.
The essential difference between the Huntington-Heberlein and Savelsberg processes is the use in the former of a partially desulphurized ore, containing lime and sulphate of lime; and the use in the latter of raw ore and carbonate of lime. It is claimed that the latter, which loses its carbon dioxide in the converter, necessarily plays a different chemical part from that of quicklime or gypsum. Irrespective of the reactions, however, the Savelsberg process has the great economic advantage of dispensing with the preliminary roasting of the Huntington-Heberlein process, wherefore it is cheaper both in first cost of plant and in operation.
During the last two years, and especially during the last six months, a number of important articles upon the new methods for the desulphurization of galena have been published in the technical periodicals, particularly in the Engineering and Mining Journal and in Metallurgie. I proposed for these methods the type-name of “lime-roasting of galena,” as a convenient metallurgical classification,[33] and this term has found some acceptance. The articles referred to have shown the great practical importance of these new processes, and the general recognition of their metallurgical and commercial value, which has already been accorded to them. It is my present purpose to review broadly the changes developed by them in the metallurgy of lead, in which connection it is necessary to refer briefly to the previous state of the art.
The elimination of the sulphur content of galena has been always the most troublesome part of the smelting process, being both costly in the operation and wasteful of silver and lead. Previous to the introduction of the Huntington-Heberlein process at Pertusola, Italy, it was effected by a variety of methods. In the treatment of non-argentiferous galena concentrate, the smelting was done by the roast-reduction method (roasting in reverberatory furnace and smelting in blast furnace); the roast-reaction method, applied in reverberatory furnaces; and the roast-reaction method, applied in Scotch hearths.[34] Precipitation smelting, simple, had practically gone out of use, although its reactions enter into the modern blast-furnace practice, as do also those of the roast-reaction method.
In the treatment of argentiferous lead ores, a combination of the roast-reduction, roast-reaction and precipitation methods had been developed. Ores low in lead were still roasted, chiefly in hand-worked reverberatories (the mechanical furnaces not having proved well adapted to lead-bearing ores), while the high loss of lead and silver in sinter-or slag-roasting of rich galenas had caused those processes to be abandoned, and such ores were charged raw into the blast furnace, the part of their sulphur which escaped oxidation therein reappearing in the form of matte. In the roast-reduction smelting of galena alone, however, there was no way of avoiding the roasting of the whole, or at least a very large percentage of the ore, and in this roasting the ore had necessarily to be slagged or sintered in order to eliminate the sulphur to a satisfactory extent. This is exemplified in the treatment of the galena concentrate of southeastern Missouri at the present time.
Until the two new Scotch-hearth plants at Alton and Collinsville, Ill., were put in operation, the three processes of smelting the southeastern Missouri galena were about on an equal footing. Their results per ton of ore containing 65 per cent. lead were approximately as follows[35]:
| Method | Cost | Extraction |
|---|---|---|
| Reverberatory | $6.50-7.00 | 90-92% |
| Scotch hearth | 5.75-6.50 | 87-88% |
| Roast-reduction | 6.00-7.00 | 90-92% |
The new works employ the Scotch-hearth process, with bag-houses for the recovery of the fume, which previously was the weak point of this method of smelting.[36] This improvement led to a large increase in the recovery of lead, so that the entire extraction is now approximately 98 per cent. of the content of the ore, while on the other hand the cost of smelting per ton of 195 ore has been reduced through the increased size of these plants and the introduction of improved means for handling ore and material. The practice of these works represents the highest efficiency yet obtained in this country in the smelting of high-grade galena concentrate, and probably it cannot be equaled even by the Huntington-Heberlein and similar processes. The Scotch-hearth and bag-house process is therefore the one of the older methods of smelting which will survive.
In the other methods of smelting, a large proportion of the cost is involved in the roasting of the ore, which amounts in hand-worked reverberatory furnaces to $2 to $2.50 per ton. Also, the larger proportion of the loss of metal is suffered in the roasting of the ore, this loss amounting to from 6 to 8 per cent. of the metal content of such ore as is roasted. The loss of lead in the combined process of treatment depends upon the details of the process. The chief advantage of lime-roasting in the treatment of this class of ore is in the higher extraction of metal which it affords. This should rise to 98 per cent. That figure has been, indeed, surpassed in operations on a large scale, extending over a considerable period.
In the treatment of the argentiferous ores of the West different conditions enter into the consideration. In the working of those ores, the present practice is to roast only those which are low in lead, and charge raw into the blast furnace the rich galenas. The cost of roasting is about $2 to $2.50 per ton; the cost of smelting is about $2.50 per ton. On the average about 0.4 ton of ore has to be roasted for every ton that is smelted. The cost of roasting and smelting is therefore about $3.50 per ton. In good practice the recovery of silver is about 98 per cent. and of lead about 95 per cent., reckoned on basis of fire assays.
In treatment of these ores, the lime-roasting process offers several advantages. It may be performed at less than the cost of ordinary roasting.[37] The loss of silver and lead during the roasting is reduced to insignificant proportion. The sulphide fines which must be charged raw into the blast furnace are eliminated, inasmuch as they can be efficiently desulphurized in the lime-roasting pots without significant loss; all the ore to be smelted in the blast furnace can be, therefore, delivered to it in lump form, whereby the speed of the blast furnace is increased 196 and the wind pressure required is decreased. Finally, the percentage of sulphur in the charge is reduced, producing a lower matte-fall, or no matte-fall whatever, with consequent saving in expense of retreatment. In the case of a new plant, the first cost of construction and the ground-space occupied are materially reduced. Before discussing more fully the extent and nature of these savings, it is advisable to point out the differences among the three processes of lime-roasting that have already come into practical use.
In the Huntington-Heberlein process, the ore is mixed with suitable proportions of limestone and silica (or quartzose ore) and is then partially roasted, say to reduction of the sulphur to one half. The roasting is done at a comparatively low temperature, and the loss of metals is consequently small. The roasted ore is dampened and allowed to cool. It is then charged into a hemispherical cast-iron pot, with a movable hood which covers the top and conveys off the gases. There is a perforated grate in the bottom of the pot, on which the ore rests, and air is introduced through a pipe entering the bottom of the pot, under the grate. A small quantity of red-hot calcines from the roasting furnaces is thrown on the grate to start the reaction; a layer of cold, semi-roasted ore is put upon it, the air blast is turned on and reaction begins, which manifests itself by the copious evolution of sulphur fumes. These consist chiefly of sulphur dioxide, but they contain more or less trioxide, which is evident from the solution of copperas that trickles from the hoods and iron smoke-pipes, wherein the moisture condenses. As the reaction progresses, and the heat creeps up, more ore is introduced, layer by layer, until the pot is full. Care is taken by the operator to compel the air to pass evenly and gently through the charge, wherefore he is watchful to close blow-holes which develop in it. At the end of the operation, which may last from four to eighteen hours, the ore becomes red-hot at the top. The hood is then pushed up, and the pot is turned on its trunnions, by means of a hand-operated wheel and worm-gear, until the charge slides out, which it does as a solid, semi-fused cake. The pot is then turned back into position. Its design is such that the air-pipe makes automatic connection, a flanged pipe cast with the pot settling upon a similarly flanged pipe communicating with the main, a suitable gasket serving to make a tight joint. The pots are set 197 at an elevation of about 12 ft. above the ground, so that when the charge slides out the drop will break it up to some extent, and it is moreover caused to fall on a wedge, or similar contrivance, to assist the breakage. After cooling it is further broken up to furnace size by wedging and sledging; the lumps are forked out, and the fines screened and returned to a subsequent charge for completion of their desulphurization.
The Savelsberg process differs from the Huntington-Heberlein in respect to the preliminary roasting, which in the Savelsberg process is omitted, the raw ore, mixed with limestone and silica, being charged directly into the converter. The Savelsberg converter is supported on a truck, instead of being fixed in position, but otherwise its design and management are quite similar to those of the Huntington-Heberlein converter. In neither case are there any patents on the converters. The patents are on the processes. In view of the litigation that has already been commenced between their respective owners, it is interesting to examine the claims.
The Huntington-Heberlein patent (U. S. 600,347, issued March 8, 1898, applied for Dec. 9, 1896) has the following claims:
1. The herein-described method of oxidizing sulphide ores of lead preparatory to reduction to metal, which consists in mixing with the ore to be treated an oxide of an alkaline-earth metal, such as calcium oxide, subjecting the mixture to heat in the presence of air, then reducing the temperature and finally passing air through the mass to complete the oxidation of the lead, substantially as and for the purpose set forth.
2. The herein-described method of oxidizing sulphide ores of lead preparatory to reduction to metal, which consists in mixing calcium oxide or other oxide of an alkaline-earth metal with the ore to be treated, subjecting the mixture in the presence of air to a bright-red heat (about 700 deg. C.), then cooling down the mixture to a dull-red heat (about 500 deg. C.), and finally forcing air through the mass until the lead ore, reduced to an oxide, fuses, substantially as set forth.
3. The herein-described method of oxidizing lead sulphide in the preparation of the same for reduction to metal, which consists in subjecting the sulphide to a high temperature in the presence of an oxide of an alkaline-earth metal, such as calcium oxide, and oxygen, and then lowering the temperature substantially as set forth.
Adolf Savelsberg, in U. S. patent 755,598 (issued March 22, 1904, applied for Dec. 18, 1903) claims:
1. The herein-described process of desulphurizing lead ores, which consists in mixing raw ore with limestone and then subjecting the mixture to the simultaneous application of heat and a current of air in sufficient proportions to substantially complete the desulphurization in one operation, substantially as described.
2. The herein-described process of desulphurizing lead ores, which process consists in first mixing the ores with limestone, then moistening the mixture, then filling it without previous roasting into a chamber, then heating it and treating it by a current of air, as and for the purpose described.
3. The herein-described process of desulphurizing lead ores, which consists in mixing raw ores with limestone, then filling the mixture into a chamber, then subjecting the mixture to the simultaneous application of heat and a current of air in sufficient proportions to substantially complete the desulphurization in one operation, the mixture being introduced into the chamber in partial charges introduced successively at intervals during the process, substantially as described.
4. The herein-described process of desulphurizing lead ores, then moistening the mixture, then filling it without previous roasting into a chamber, then heating it and treating it by a current of air, the mixture being introduced into the chamber in partial charges introduced successively at intervals during the process, as and for the purpose described.
5. The herein-described process of desulphurizing lead ores, which process consists in first mixing the ores with sufficient limestone to keep the temperature of the mixture below the melting-point of the ore, then filling the mixture into a chamber, then heating said mixture and treating it with a current of air, as and for the purpose described.
6. The herein-described process of desulphurizing lead ores, which process consists in first mixing the ores with sufficient limestone to mechanically separate the particles of galena sufficiently to prevent fusion, and to keep the temperature below the melting-point of the ore by the liberation of carbon dioxide, then filling the mixture into a chamber, then heating said mixture and treating it with a current of air, as and for the purpose described.
The Carmichael-Bradford process differs from the Savelsberg by the treatment of the raw ore mixed with gypsum instead of limestone, and differs from the Huntington-Heberlein both in respect to the use of gypsum and the omission of the preliminary roasting. The Carmichael-Bradford process has not been threatened with litigation, so far as I am aware. The claims of its original patent read as follows[38]:
1. The process of treating mixed sulphide ores, which consists in mixing with said ores a sulphur compound of a metal of the alkaline earths, starting the reaction by heating the same, thereby oxidizing the sulphide and reducing the sulphur compound of the alkali metal, passing a current of air to oxidize the reduced sulphide compound of the metal of the alkalies preparatory to acting upon a new charge of sulphide ores, substantially as and for the purpose set forth.
2. The process of treating mixed sulphide ores, which consists in mixing calcium sulphate with said ores, starting the reaction by means of heat, thereby oxidizing the sulphide ores, liberating sulphurous-acid gas and converting the calcium sulphate into calcium sulphide and oxidizing the calcium sulphide to sulphate preparatory to treating a fresh charge of sulphide ores, substantially as and for the purpose set forth.
The process described by W. S. Bayston, of Melbourne (Australian patent No. 2862), appears to be identical with that of Savelsberg.
Irrespective of the validity of the Savelsberg and Carmichael-Bradford patents, and without attempting to minimize the ingenuity of their inventors and the importance of their discoveries, it must be conceded that the merit for the invention and introduction of lime-roasting of galena belongs to Thomas Huntington and Ferdinand Heberlein. The former is an American, and this is the only claim that the United States can make to a share in this great improvement in the metallurgy of lead. It is to be regretted, moreover, that of all the important lead-smelting countries in the world, America has been the most backward in adopting it.
The details of the three processes and the general results accomplished by them have been rather fully described in a series of articles recently published in the Engineering and Mining 200 Journal. There has been, however, comparatively little discussion as to costs; and unfortunately the data available for analysis are extremely scanty, due to the secrecy with which the Huntington-Heberlein process, the most extensively exploited of the three, has been veiled. Nevertheless, I may attempt an approximate estimation of the various details, taking the Huntington-Heberlein process as the basis.
The ore, limestone and silica are crushed to pass a four-mesh screen. This is about the size to which it would be necessary to crush as preliminary to roasting in the ordinary way, wherefore the only difference in cost is the charge for crushing the limestone and silica, which in the aggregate may amount to one-sixth of the weight of the raw sulphide and may consequently add 2 to 2.5c. to the cost of treating a ton of ore. The mixing of ore and fluxes may be costly or cheap, according to the way of doing it. If done in a rational way it ought not to cost more than 10c. per ton of ore, and may come to less. The delivery of the ore from the mixing-house to the roasting furnaces ought to be done entirely by mechanical means, at insignificant cost.
The Heberlein roasting furnace, which is used in connection with the H.-H. process, is simply an improvement on the old Brunton calciner—a circular furnace, with revolving hearth. The construction of this furnace, according to American designs, is excellent. The hearth is 26 ft. in diameter; it is revolved at slow speed and requires about 1.5 h.p. A flange at the periphery of the hearth dips into sand in an annular trough, thus shutting off air from the combustion chamber, except through the ports designed for its admittance. The mechanical construction of the furnace is workmanlike, and the mechanism under the hearth is easy of access and comfortably attended to.
A 26 ft. furnace roasts about 80,000 lb. of charge per 24 hours. In dealing with an ore containing 20 to 22 per cent. of sulphur, the latter is reduced to about 10 to 11 per cent., the consumption of coal being about 22.5 per cent. of the weight of the charge. The hearth efficiency is about 150 lb. per sq. ft., which in comparison with ordinary roasting is high. The coal consumption, however, is not correspondingly low. Two furnaces can be managed by one man per 8 hour shift. On the basis of 80 tons of charge ore per 24 hours, the cost of roasting should be approximately as follows:
| Labor—3 men at $2.50 | $ 7.50 | |
| Coal—18 tons at $2 | 36.00 | |
| Power | 3.35 | |
| Repairs | 3.35 | |
| Total | $50.20 | = 63c. per ton. |
In the above estimate repairs have been reckoned at the same figure as is experienced with Brückner cylinders, and the cost of power has been allowed for with fair liberality. The estimated cost of 63c. per ton is comparable with the $1.10 to $1.45 per ton, which is the result of roasting in Brückner cylinders in Colorado, reducing the ore to 4.5-6 per cent. sulphur.
The Heberlein furnace is built up to considerable elevation above the ground level, externally somewhat resembling the Pearce turret furnace. This serves two purposes: (1) it affords ample room under the hearth for attention to the driving mechanism; and (2) it enables the ore to be discharged by gravity into suitable hoppers, without the construction of subterranean gangways. The ore discharges continuously from the furnace, at dull-red heat, into a brick bin, wherein it is cooled by a water-spray. Periodically a little ore is diverted into a side bin, in which it is kept hot for starting a subsequent charge in the converter.
The cooled ore is conveyed from the receiving bins at the roasting furnaces to hopper-bins above the converters. If the tramming be done by hand the cost, with labor at 25c. per hour, may be approximately 12.5c. per ton of ore, but this should be capable of considerable reduction by mechanical conveyance.
The converters are hemispherical pots of cast iron, 9 ft. in diameter at the top, and about 4 ft. in depth. They are provided with a circular, cast-iron grate, which is ¾ in. thick and 6 ft. in diameter and is set and secured horizontally in the pot. This grate is perforated with holes ¾ in. in diameter, 2 in. apart, center to center, and is similar to the Wetherill grate employed in zinc oxide manufacture. The pot itself is about 2½ in. thick at the bottom, thinning to about 1½ in. at the rim. It is supported on trunnions and is geared for convenient turning by hand. The blast pipe which enters the pot at the bottom is 6 in. in diameter.
Two roasting furnaces and six converters are rated nominally as a 90 ton plant. This rating is, however, considerably in excess of the actual capacity, at least on certain ores. The time required 202 for desulphurization in the converter apparently depends a good deal upon the character of the ore. The six converters may be arranged in a single row, or in two rows of three in each. They are set so that the rim of the pot, when upright, is about 12 ft. above the ground level. A platform gives access to the pots. One man per shift can attend to two pots. His work consists in charging them, which is done by gravity, spreading out the charge evenly in the pot, closing any blow-holes which may develop, and at the end of the operation raising the hood (which covers the pot during the operation) and dumping the pot. The work is easy. The conditions under which it is done are comfortable, both as to temperature and atmosphere. Reports have shown a great reduction in liability to lead-poisoning in the works where the H.-H. process has been introduced.
A new charge is started by kindling a small wood or coal fire on the grate, then throwing in a few shovelfuls of hot calcines, and finally dropping in the regular charge of damp ore (plus the fluxes previously referred to). The charge is introduced in stages, successive layers being dropped in and spread out as the heat rises. At the beginning the blast is very low—about 2 oz. It is increased as the hight of the ore in the pot rises, finally attaining about 16 oz. The operation goes on quietly, the smoke rising from the surface evenly and gently, precisely as in a well-running blast furnace. While the charge is still black on top, the hand can be held with perfect comfort, inside of the hood, immediately over the ore. This explains, of course, why the volatilization of silver and lead is insignificant. There is, moreover, little or no loss of ore as dust, because the ore is introduced damp, and the passage of the air through it is at low velocity. In the interior of the charge, however, there is high temperature (evidently much higher than has been stated in some descriptions), as will be shown further on. The conditions in this respect appear to be analogous to those of the blast furnace, which, though smelting at a temperature of say 1200 deg. C. at the level of the tuyeres, suffers only a slight loss of silver and lead by volatilization.
At the end of the operation in the H.-H. pot, the charge is dull red at the top, with blow-holes, around which the ore is bright red. Imperfectly worked charges show masses of well-fused ore surrounded by masses of only partially altered ore, 203 a condition which may be ascribed to the irregular penetration of air through the charge, affording good evidence of the important part which air plays in the process. A properly worked charge is tipped out of the pot as a solid cake, which in falling to the ground breaks into a few large pieces. As they break, it appears that the interior of the charge is bright red all through, and there is a little molten slag which runs out of cavities, presumably spots where the chemical action has been most intense. When cold, the thoroughly desulphurized material has the appearance of slag-roasted galena. Prills of metallic lead are visible in it, indicating reaction between lead sulphide and lead sulphate.
The columns of the structure supporting the pots should be of steel, since fragments of the red-hot ore dumped on the ground are likely to fall against them. To hasten the cooling of the ore, water is sometimes played on it from a hose. This is bad, since some is likely to splash into the still inverted pot, leading to cracks. The cracked pots at certain works appear to be due chiefly to this cause, in the absence of which the pots ought to last a long time, inasmuch as the conditions to which they are subjected during the blowing process are not at all severe. When the ore is sufficiently cold it is further broken up, first by driving in wedges, and finally by sledging down to pieces of orange size, or what is suitable for the blast furnace. These are forked out, leaving the fine ore, which comes largely from the top of the charge and is therefore only partially desulphurized. The fines are, therefore, re-treated with a subsequent charge. The quantity is not excessive; it may amount to 7 or 8 per cent. of the charge.
The breaking up of the desulphurized ore is one of the problems of the process, the necessity being the reduction of several large pieces of fused, or semi-fused, material weighing two or three tons each. When done by hand only, as is usually (perhaps always) the practice, the operation is rather expensive. It would appear, however, to be not a difficult matter to devise some mechanical aids for this process—perhaps to make it entirely mechanical. When done by hand, a 6-pot plant requires 6 men per shift sledging and forking. With 8-hour shifts, this is 18 men for the breaking of about 60 tons of material, which is about 3⅓ tons per man per 8 hours. With labor at 25c. per hour, the cost of breaking the fused material comes to 60c. per ton. It may be remarked, for comparison, that in 204 breaking ore as it ordinarily comes, coarse and fine together, a good workman would normally be expected to break 5 to 5.5 tons in a shift of 8 hours.
The ordinary charge for the standard converter is about 8 tons (16,000 lb.) of an ore weighing 166 lb. per cu. ft. With a heavier ore, like a high-grade galena, the charge would weigh proportionately more. The time of working off a charge is decidedly variable. Accounts of the operation of the process in Australia tell of charge-workings in 3 to 5 hours, but this does not correspond with the results reported elsewhere, which specify times of 12 to 18 hours. Assuming an average of 16 hours, which was the record of one plant, six converters would have capacity for about 72 tons of charge per 24 hours, or about 58 tons of ore, the ratio of ore to flux being 4:1. The loss in weight of the charge corresponds substantially to the replacement of sulphur by oxygen, and the expulsion of carbon dioxide. The finished charge contains on the average from 3 to 5 per cent. sulphur. This is about the same as the result achieved in good practice in roasting lead-bearing ores in hand-worked reverberatory furnaces, but curiously the H.-H. product, in some cases at least, does not yield any matte, to speak of, in the blast furnace; the product delivered to the latter being evidently in such condition that the remaining sulphur is almost completely burned off in the blast furnace. This is an important saving effected by the process. In calculating the value of an ore, sulphur is commonly debited at the rate of 25c. per unit, which represents approximately the cost of handling and reworking the matte resulting from it. The practically complete elimination of matte-fall rendered possible by the H.-H. process may not be, however, an unmixed blessing. There may be, for example, a small formation of lead sulphide which causes trouble in the crucible and lead-well, and results in furnace difficulties and the presentation of a vexatious between-product.
It may now be attempted to summarize the cost of the converting process. Assuming the case of an ore assaying lead, 50 per cent.; iron, 15; sulphur, 22; silica, 8, and alumina, etc., 5, let it be supposed that it is to be fluxed with pure limestone and pure quartz, with the aim to make a slag containing silica, 30; ferrous oxide, 40; and lime, 20 per cent. A ton of ore will make, in round numbers, 1000 lb. of slag, and will require 344 lb. of lime 205stone and 130 lb. of quartz, or we may say roughly one ton of flux must be added to four tons of ore, wherefore the ore will constitute 80 per cent. of the charge. In reducing the charge to 3 per cent. sulphur it will lose ultimately through expulsion of sulphur and carbon dioxide (of the limestone) about 20 per cent. in weight, wherefore the quantity of material to be smelted in the blast furnace will be practically equivalent to the raw sulphide ore in the charge for the roasting furnaces; but in the roasting furnace the charge is likely to gain weight, because of the formation of sulphates. Taking the charge, which I have assumed above, and reckoning that as it comes from the roasting furnace it will contain 10 per cent. sulphur, all in the form of sulphate, either of lead or of lime, and that the iron be entirely converted to ferric oxide, in spite of the expulsion of the carbon dioxide of the limestone and the combustion of a portion of the sulphur of the ore as sulphur dioxide, the charge will gain in weight in the ratio of 1:1.19. This, however, is too high, inasmuch as a portion of the sulphur will remain as sulphide while a portion of the iron may be as ferrous oxide. The actual gain in weight will consequently be probably not more than one-tenth. The following theoretical calculation will illustrate the changes:
| Raw Charge | Semi-Roasted Charge | Finished Charge | |
|---|---|---|---|
| Ore | 1000 lb. Pb | 1154 lb. PbO | 1154 lb. PbO |
| 300 lb. Fe | 428 lb. Fe2O3 | 428 lb. Fe2O3(?) | |
| 160 lb. SiO2 | 160 lb. SiO2 | 160 lb. SiO2 | |
| 100 lb. Al2O3, etc. | 100 lb. Al2O3, etc. | 100 lb. Al2O3, etc. | |
| 440 lb. S | 300 lb. S | 68 lb. S | |
| Flux | 130 lb. SiO2 | 130 lb. SiO2 | 130 lb. SiO2 |
| 344 lb. CaCO3 | 193 lb. CaO | 193 lb. CaO | |
| 450 lb. O | |||
| —— | ——— | ——— | |
| 2474 lb. | 2915 lb. | 2233 lb. | |
| 10% S. | 3% S. |
It may be assumed that for every ton of charge (containing about 80 per cent. of ore) there will be 1.1 ton of material to go to the converter, and that the product of the latter will be 0.9 of the weight of the original charge of raw material.
Each converter requires 400 cu. ft. of air per minute. The blast pressure is variable, as different pots are always at different stages of the process, but assuming the maximum of 16 oz. pressure, with a blast main of sufficient diameter (at least 15 in.) and the blower reasonably near the battery of pots, the total requirement is 21 h.p. The cost of converting will be approximately as follows:
| Labor, 3 foremen at $3.20 | $ 9.60 | |
| Labor, 9 men at $2.50 | 22.50 | |
| Power, 21 h.p. at 30c | 6.30 | |
| Supplies, repairs and renewals | 5.00 | |
| Total | $43.40 | = 60c. per ton of charge. |
The cost of converting is, of course, reduced directly as the time is reduced. The above estimate is based on unfavorable conditions as to time required for working a charge.
The total cost of treatment, from the initial stage to the delivery of the desulphurized ore to the blast furnaces, will be, per 2000 lb. of charge, approximately as follows:
| Crushing 1.0 ton at 10c | $0.10 |
| Mixing 1.0 ton at 10c | .10 |
| Roasting 1.0 ton at 63c | .63 |
| Delivering 1.1 ton to converters at 12c | .13 |
| Converting 1.1 ton at 60c | .66 |
| Breaking 0.9 ton at 60c | .54 |
| Total | $2.16 |
The cost per ton of ore will be 2.16 ÷ 0.80 = $2.70. Making allowance for the crushing of the ore, which is not ordinarily included in the cost of roasting, and possibly some overestimates, it appears that the cost of desulphurization by this method, under the conditions assumed in this paper, is rather higher than in good practice with ordinary hand-worked furnaces, but it is evident that the cost can be reduced to approximately the same figure by introduction of improvements, as for example in breaking the desulphurized ore, and by shortening the time of converting, which is possible in the case of favorable ores. The chief advantage must be, however, in the further stage of the smelting. As to this, there is the evidence that the Broken Hill Proprietary Company was able to smelt the same quantity of ore in seven 207 furnaces, after the introduction of the Huntington-Heberlein process, that formerly required thirteen. A similar experience is reported at Friedrichshütte, Silesia.
This increase in the capacity of the blast furnace is due to three things: (1) In delivering to the furnace a charge containing a reduced percentage of fine ore, the speed of the furnace is increased, i.e., more tons of ore can be smelted per square foot of hearth area. (2) There is less roasted matte to go into the charge. (3) Under some conditions the percentage of lead in the charge can be increased, reducing the quantity of gangue that must be fluxed.
It is difficult to generalize the economy that is effected in the blast-furnace process, since this must necessarily vary within wide limits because of the difference in conditions. An increase of 60 to 100 per cent. in blast-furnace capacity does not imply a corresponding reduction in the cost of smelting. The fuel consumption per ton of ore remains the same. There is a saving in the power requirements, because the smelting can be done with a lower blast pressure; also, a saving in the cost of reworking matte. There will, moreover, be a saving in other labor, in so far as portions thereof are not already performed at the minimum cost per ton. The net result under American conditions of silver-lead smelting can only be determined closely by extensive operations. That there will be an important saving, however, there is no doubt.
The cost of smelting a ton of charge at Denver and Pueblo, exclusive of roasting and general expense, is about $2.50, of which about $0.84 is for coke and $1.66 for labor, power and supplies. General expense amounts to about $0.16 additional. If it should prove possible to smelt in a given plant 50 per cent. more ore than at present without increase in the total expense, except for coke, the saving per ton of charge would be 70c. That is not to be expected, but the half of it would be a satisfactory improvement. With respect to sulphur in the charge, the cost is commonly reckoned at 25c. per unit. As compared with a charge containing 2 per cent. of sulphur there would be a saving rising toward 50c. per ton as the maximum. It is reasonable to reckon, therefore, a possible saving of 75c. per ton of charge in silver-lead smelting, no saving in the cost of roasting, and an increase of about 3 per cent. in the extraction of lead, and per 208haps 1 per cent. in the extraction of silver, as the net results of the application of the Huntington-Heberlein process in American silver-lead smelting.
On a charge averaging 12 per cent. lead and 33 oz. silver per ton, an increase of 3 per cent. in the extraction of lead and 1 per cent. in the extraction of silver would correspond to 25c. and 35c. respectively, reckoning lead at 3.5c. per lb., and silver at 60c. per oz. In this, however, it is assumed that all lead-bearing ores will be desulphurized by this process, which practically will hardly be the case. A good deal of pyrites, containing only a little lead, will doubtless continue to be roasted in Brückner cylinders, and other mechanical furnaces, which are better adapted to the purpose than are the lime-roasting pots. Moreover, a certain proportion of high-grade lead ore, which is now smelted raw, will be desulphurized outside of the furnace, at additional expense. It is comparatively simple to estimate the probable benefit of the Huntington-Heberlein process in the case of smelting works which treat principally a single class of ore, but in such works as those in Colorado and Utah, which treat a wide variety of ores, we must anticipate a combination process, and await results of experience to determine just how it will work out. It should be remarked, moreover, that my estimates do not take into account the royalty on the process, which is an actual debit, whether it be paid on a tonnage basis or be computed in the form of a lump sum for the license to its use.
However, in view of the immense tonnage of ore smelted annually for the extraction of silver and lead, it is evident that the invention of lime-roasting by Huntington and Heberlein was an improvement of the first order in the metallurgy of lead.
In the case of non-argentiferous galena, containing 65 per cent. of lead (as in southeastern Missouri), comparison may be made with the slag-roasting and blast-furnace smelting of the ore. Here, no saving in cost of roasting may be reckoned and no gain in the speed of the blast furnaces is to be anticipated. The only savings will be in the increase in the extraction of lead from 92 to 98 per cent., and the elimination of matte-roasting, which latter may be reckoned as amounting to 50c. per ton of ore. The extent of the advantage over the older method is so clearly apparent that it need not be computed any further. In comparison with the Scotch-hearth bag-house method of smelting, 209 however, the advantage, if any, is not so certain. That method already saves 98 per cent. of the lead, and on the whole is probably as cheap in operation as the Huntington-Heberlein could be under the same conditions. The Huntington-Heberlein method has replaced the old roast-reaction method at Tarnowitz, Silesia, but the American Scotch-hearth method as practised near St. Louis is likely to survive.
A more serious competitor will be, however, the Savelsberg process, which appears to do all that the Huntington-Heberlein process does, without the preliminary roasting. Indeed, if the latter be omitted (together with its estimated expense of 63c. per ton of charge, or 79c. per ton of ore), all that has been said in this paper as to the Huntington-Heberlein process may be construed as applying to the Savelsberg. The charge is prepared in the same way, the method of operating the converters is the same, and the results of the reactions in the converters are the same. The litigation which is pending between the two interests, Messrs. Huntington and Heberlein claiming that Savelsberg infringes their patents, will be, however, a deterrent to the extension of the Savelsberg process until that matter be settled.
The Carmichael-Bradford process may be dismissed with a few words. It is similar to the Savelsberg, except that gypsum is used instead of limestone. It is somewhat more expensive because the gypsum has to be ground and calcined. The process works efficiently at Broken Hill, but it can hardly be of general application, because gypsum is likely to be too expensive, except in a few favored localities. The ability to utilize the converter gases for the manufacture of sulphuric acid will cut no great figure, save in exceptional cases, as at Broken Hill, and anyway the gases of the other processes can be utilized for the same purpose, which is in fact being done in connection with the Huntington-Heberlein process in Silesia.
The cost of desulphurizing a ton of galena concentrate by the Carmichael-Bradford process is estimated by the company controlling the patents as follows, labor being reckoned at $1.80 per eight hours, gypsum at $2.40 per 2240 lb., and coal at $8.40 per 2240 lb.:
| 0.25 ton of gypsum | $0.60 |
| Dehydrating and granulating gypsum | .48 |
| Drying mixture of ore and gypsum | .12 210 |
| Converting | 0.24 |
| Spalling sintered material | .12 |
| 0.01 ton coal | .08 |
| Total | $1.64 |
The value of the lime in the sintered product is credited at 12c., making the net cost $1.52 per 2240 lb. of ore.
The cost allowed for converting may be explained by the more rapid action that appears to be attained with the ores of Broken Hill than with some ores that are treated in North America, but the low figure estimated for spalling the sintered material appears to be highly doubtful.
The theory of the lime-roasting processes is not yet well established. It is recognized that the explanation offered by Huntington and Heberlein in their original patent specification is erroneous. There is no good evidence in their process, or any other, of the formation of the higher oxide of lime, which they suggest.
At the present time there are two views. In one, formulated most explicitly by Professor Borchers, there is formed in this process a plumbate of calcium, which is an active oxidizing agent. A formation of this substance was also described by Carmichael in his original patent, but he considered it to be the final product, not the active oxidizing agent.
In the other view, the lime, or limestone, serves merely as a diluent of the charge, enabling the air to obtain access to the particles of galena, without liquefaction of the latter. The oxidation of the lead sulphide is therefore effected chiefly by the air, and the process is analogous to what takes place in the bessemer converter or in the Germot process of smelting, or perhaps more closely to what might happen in an ordinary roasting furnace, provided with a porous hearth, through which the air supply would be introduced. Roasting furnaces of that design have been proposed, and in fact such a construction is now being tested for blende roasting in Kansas.
Up to the present time, the evidence is surely too incomplete to enable a definite conclusion to be reached. Some facts may, however, be stated.
There is clearly reaction to a certain extent between lead sulphide and lead sulphate, as in the reverberatory smelting 211 furnace, because prills of metallic lead are to be observed in the lime-roasted charge.
There is a formation of sulphuric acid in the lime-roasting, upon the oxidizing effect of which Savelsberg lays considerable stress, since its action is to be observed on the iron work in which it condenses.
Calcium sulphate, which is present in all of the processes, being specifically added in the Carmichael-Bradford, evidently plays an important chemical part, because not only is the sulphur trioxide expelled from the artificial gypsum, but also it is to a certain extent expelled from the natural gypsum, which is added in the Carmichael-Bradford process; in other words, more sulphur is given off by the charge than is contained by the metallic sulphides alone.
Further evidence that lime does indeed play a chemical part in the reaction is presented by the phenomena of lime-roasting in clay dishes in the assay muffle, wherein the air is certainly not blown through the charge, which is simply exposed to superficial oxidation as in ordinary roasting.
The desulphurized charge dropped from the pot is certainly at much below the temperature of fusion, even in the interior, but we have no evidence of the precise temperature condition during the process itself.
Pyrite and even zinc blende in the ore are completely oxidized. This, at least, indicates intense atmospheric action.
The papers by Borchers,[39] Doeltz,[40] Guillemain,[41] and Hutchings[42] may profitably be studied in connection with the reactions involved in lime-roasting. The conclusion will be, however, that their precise nature has not yet been determined. In view of the great interest that has been awakened by this new departure in the metallurgy of lead, it is to be expected that much experimental work will be devoted to it, which will throw light upon its principles, and possibly develop it from a mere process of desulphurization into one which will yield a final product in a single operation.
(September 30, 1905)
It is well known that, in order to obtain a proper fusion in lead and copper ore-smelting, it is not only advantageous, but often indispensable, that a suitable proportion of slag be added to the charge. In the treatment of copper matte in the converter, the total quantity of slag must be resmelted, inasmuch as it always retains a notable quantity of the metal; while in the smelting of lead ore in the blast furnace, the addition of slag is mainly intended to facilitate the operation, avoiding the use of strong air pressure and thus diminishing the loss of lead. The proportion of slag required sometimes amounts to 30 to 35 per cent. of the weight of the ore.
Inasmuch as the slag is usually added in lump form, cold, its original heat (about 400 calories per kilogram) is completely lost and an intimate mixture with the charge cannot be obtained. For this reason, I have studied the agglomeration of lead and copper ores with fused slag, employing a variable proportion according to the nature of the ore treated. In the majority of cases, and with some slight modifications in each particular case, by incorporating the dry or slightly moistened mineral with the predetermined quantity of liquid slag, and by rapidly stirring the mixture so as to secure a proper subdivision of the slag and the mineral, there is produced a spongy material, largely composed of small pieces, together with a simultaneous evolution of dense fumes of sulphur, sulphur dioxide, and sulphur trioxide. By submitting this spongy material to an air blast, the sulphur of the mineral is burned, the temperature rising in the interior of the mass to a clear red heat. Copious fumes of sulphur dioxide and trioxide are given off, and at times a yellowish vapor of sulphur, which condenses in drops, especially if the ore is pyritous.
At the end of from one to three hours, according to the quantity of sulphur contained in the material under treatment and the amount of the air pressure, the desulphurization of the ore, so far as it has come in contact with the air, is completed, and the mass, now thoroughly agglomerated, forms a spongy but compact block. It is then only necessary to break it up and smelt it with the requisite quantity of flux and coke. The physical condition of the material is conducive to a rapid and economical smelting, while the mixture of the sulphide, sulphate and oxide leads to a favorable reaction in the furnace.
In employing this method, it sometimes happens that ores rich in sulphur produce during the smelting a little more matte than when the ordinary system of roasting is employed. In such instances, in order to avoid or to diminish the cost of re-treatment of the matte, it is best to agglomerate a portion thereof with the crude mineral and the slag. This has the advantage of oxidizing the matte, which acts as a ferruginous flux in the smelting.
The system described above leads to considerable economy, especially in roasting, as the heat of the scoria, together with that given off in the combustion of the sulphur, is almost always sufficient for the agglomeration and desulphurization of the mineral; while, moreover, it reduces the cost of smelting in the blast furnace. Although the primary desulphurization is only partial (about 50 per cent.), it continues in the blast furnace, since the mineral, agglomerated with the slag, assumes a spongy form and thereby presents an increased surface to the action of the air. The sulphur also acts as a fuel and does not produce an excessive quantity of matte.
The system will prove especially useful in the treatment of argentiferous lead ore, since, by avoiding the calcination in a reverberatory furnace, loss of silver is diminished. It appears, however, that, contrary to the reactions which occur in the Huntington-Heberlein process, a calcareous or basic gangue is not favorable to this process, if the proportion be too great.
The following comparison has been made in the case of an ore containing 62 to 65 per cent. of lead, 16 to 17 per cent. sulphur, 10 to 11 per cent. zinc, 0.4 per cent. copper, and 0.222 per cent. silver, in which connection it is to be remarked that, in general, the less zinc there is in the ore the better are the results.
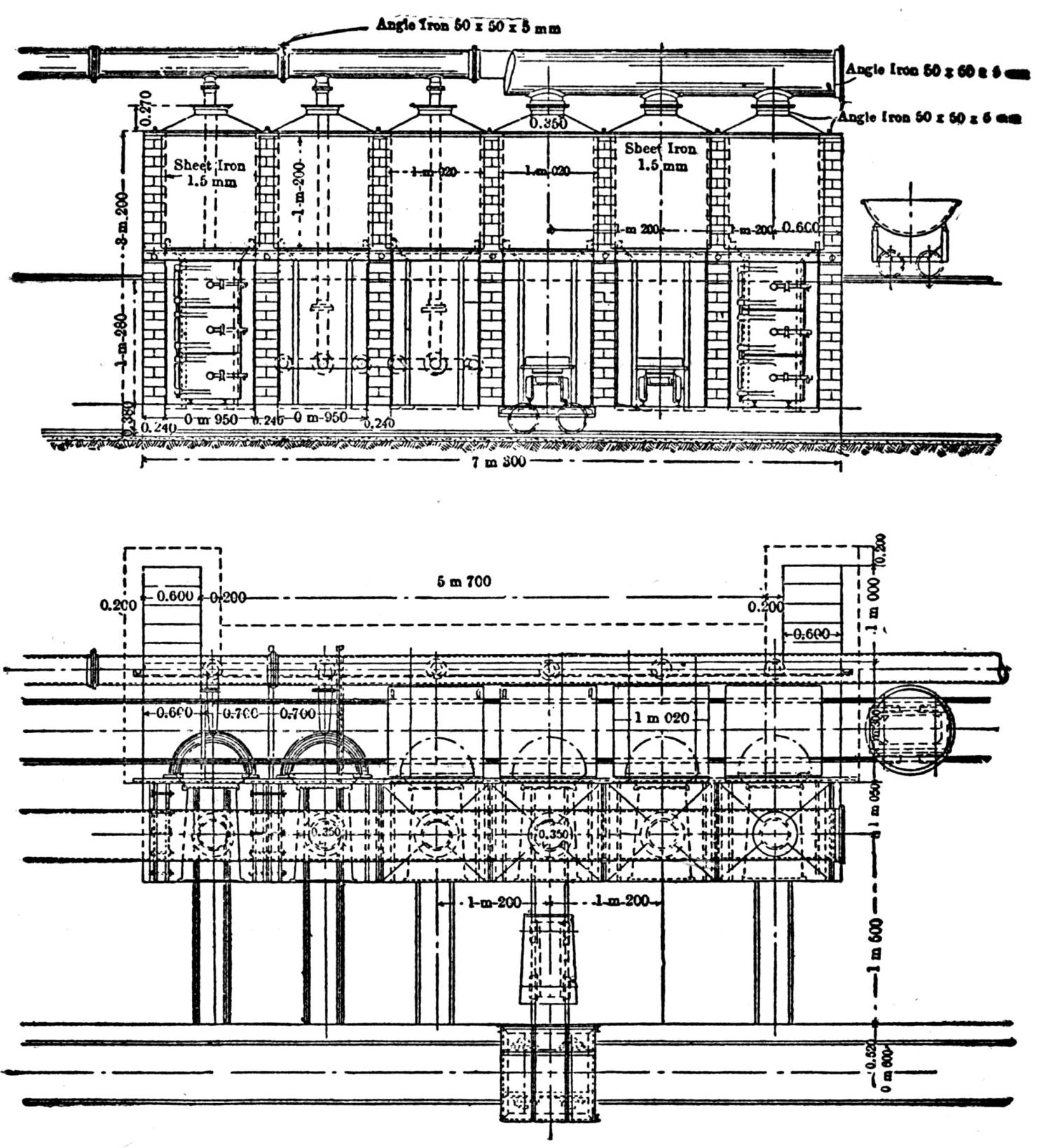
Ordinary Method.—Roast-reduction. Cost per 1000 kg. of crude ore:
| 1. Roasting in reverberatory furnace: | ||
| Labor | $0.70 | |
| Fuel | 1.50 | |
| Repairs and supplies | .05 | |
| $2.25 | ||
| 2. Smelting in water-jacket: | ||
| Labor | $1.01 | |
| Fuel | 2.20 | |
| Repairs and supplies | .03 | |
| Fluxes | .50 | |
| 3.74 | ||
| Total | $5.99 |
Bormettes Method.—Agglomeration with slag, pneumatic desulphurization and smelting in water-jacket:
| 1. Agglomeration and desulphurization: | ||
| Labor | $0.42 | |
| Repairs and supplies | 0.05 | |
| $0.47 | ||
| 2. Smelting in water-jacket: | ||
| Labor | $0.90 | |
| Fuel | 1.91 | |
| Repairs and supplies | .03 | |
| Fluxes | .42 | |
| 3.26 | ||
| Total | $3.73 |
This shows a difference in favor of the new method of $2.26 per ton of ore, without taking into account the savings realized by a much more speedy handling of the operation, which would further reduce the cost to approximately $2.50 per ton.
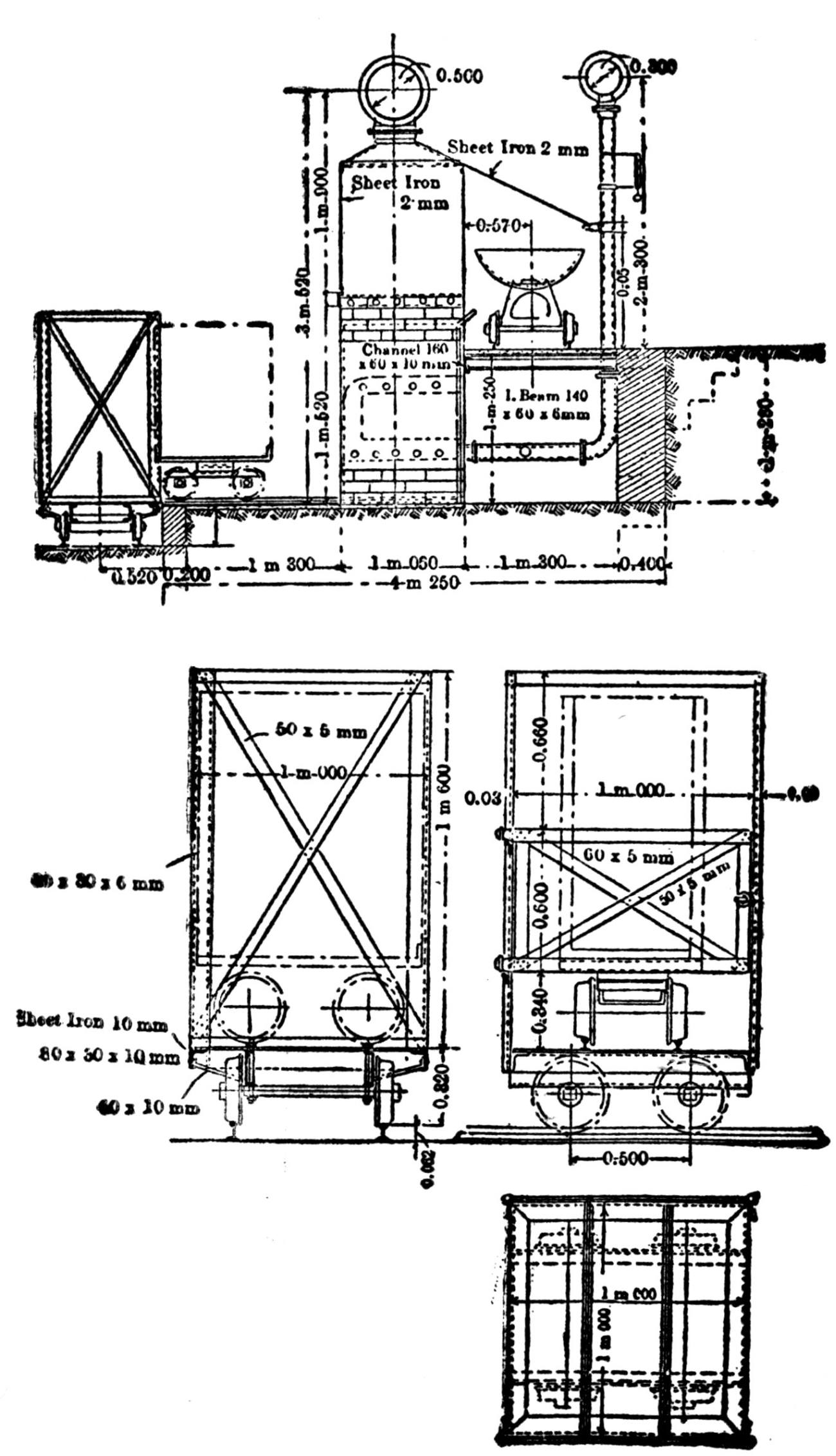
In the above figures, no account has been taken of general
expenses, which per ton of ore are reduced because of the greater
rapidity of the process, enabling a larger quantity of ore to be
smelted in a given time. Making allowance for this, the saving
will amount to an average of $2.40 per 1000 kg., a figure which
will naturally vary according to the prices for fuel, labor, and
the quantity of matte which it may be necessary to re-treat. 219
220
If the quantity of matte does not exceed 10 per cent. of the
weight of the ore, it can be desulphurized by admixture with the
ore, without use of other fuel. If, however, the proportion of
matte rises to 20 parts per 100 parts of ore (a maximum which
ought not to be reached in good working), it is necessary to
roast a portion of it. Under unfavorable conditions, consequently,
the saving effected by this process may be reduced to $2 @ $2.20
per 1000 kg., and even to as little as $1.40 @ $1.60. The above
reckonings are, however, without taking any account of the
higher extraction of lead and silver, which is one of the great
advantages of the Bormettes process.
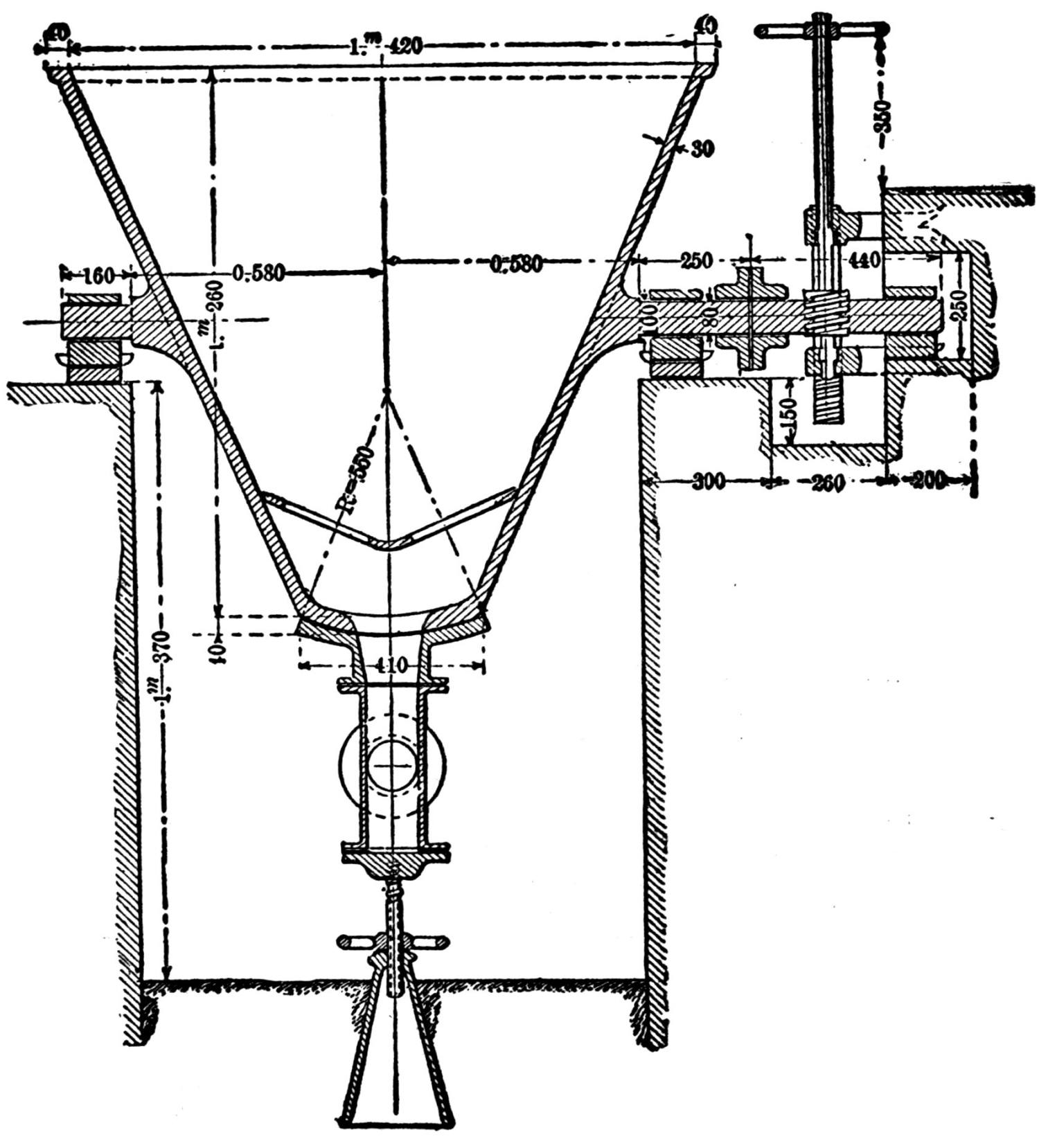
The technical results obtained in the smelting of an ore of the above mentioned composition are as follows:
| Ordinary Method |
Bormettes Method |
|
|---|---|---|
| Coke, per cent. of the charge | 14 | 12 |
| Blast pressure, water gage | 12 to 20 cm. | 12 to 14 cm. |
| Tons of charge smelted per 24 hr | 20 | 25 |
| Tons of ore smelted per 24 hr | 8 | 10 |
| Lead assay of slag | 0.80 to 0.90% | 0.20 to 0.40% |
| Matte-fall, per cent. of ore charged | 5 to 10 | 10 to 15 |
| Lead extraction | 90% | 92% |
| Silver extraction | 95% | 98% |
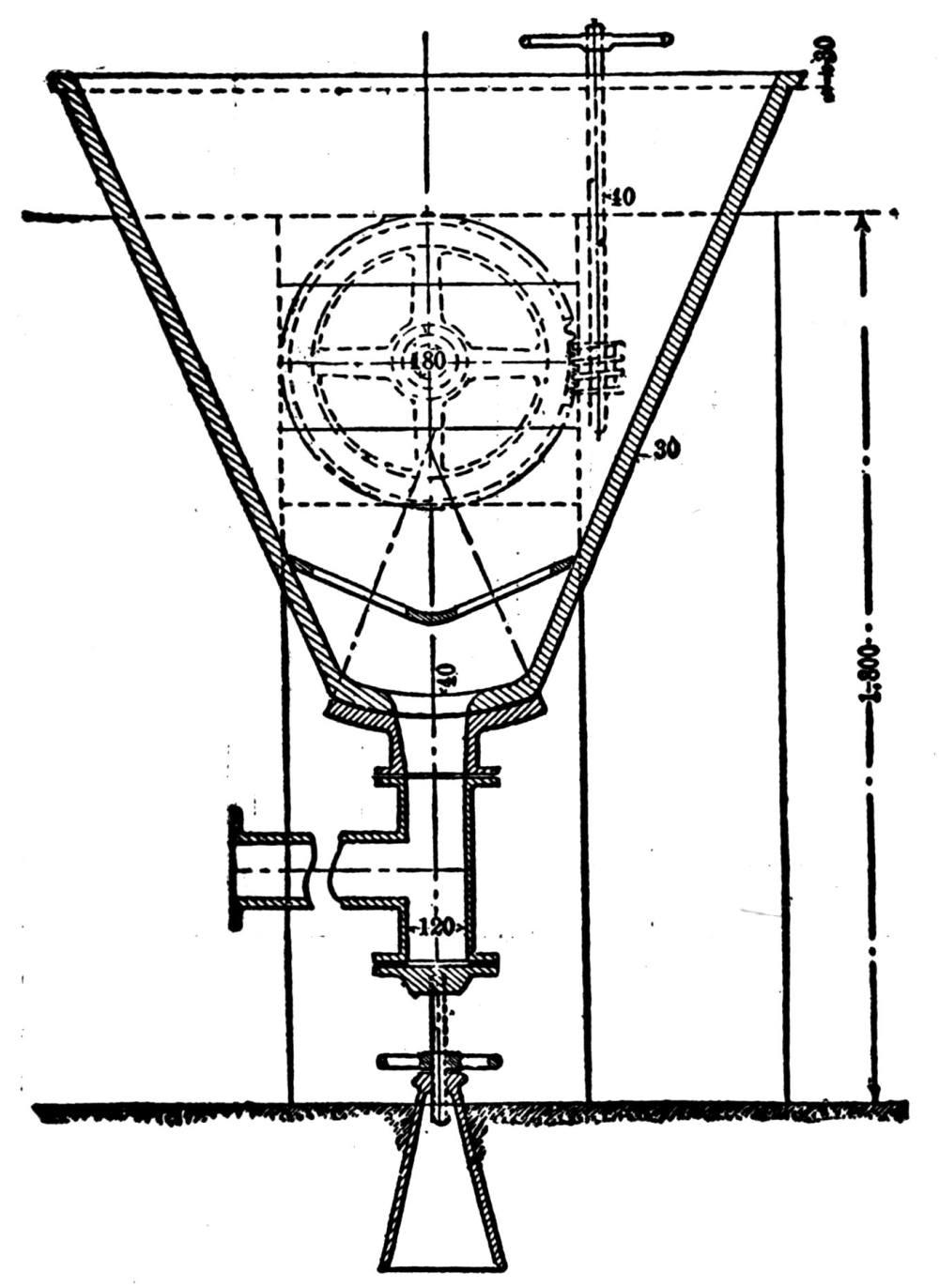
The higher extractions of lead and silver are explained by the fact that the loss of metals in roasting is reduced, while, moreover, the slags from the blast furnace are poorer than in the ordinary process of smelting. The economy in coke results from the greater quantity of sulphur which is utilized as fuel, and from the increased fusibility of the charge for the blast furnace.
The new system of desulphurization enables the charge to be smelted with a less quantity of fresh flux, by the employment in its place of a greater proportion of foul slag. The reduction in the necessary amount of flux is due not only to the increased fusibility of the agglomerated charge, but principally to the fact that in this system the formation of silicates of lead (which are produced abundantly in ordinary slag-roasting) is almost nil. It is therefore unnecessary to employ basic fluxes in order to reduce scorified lead.
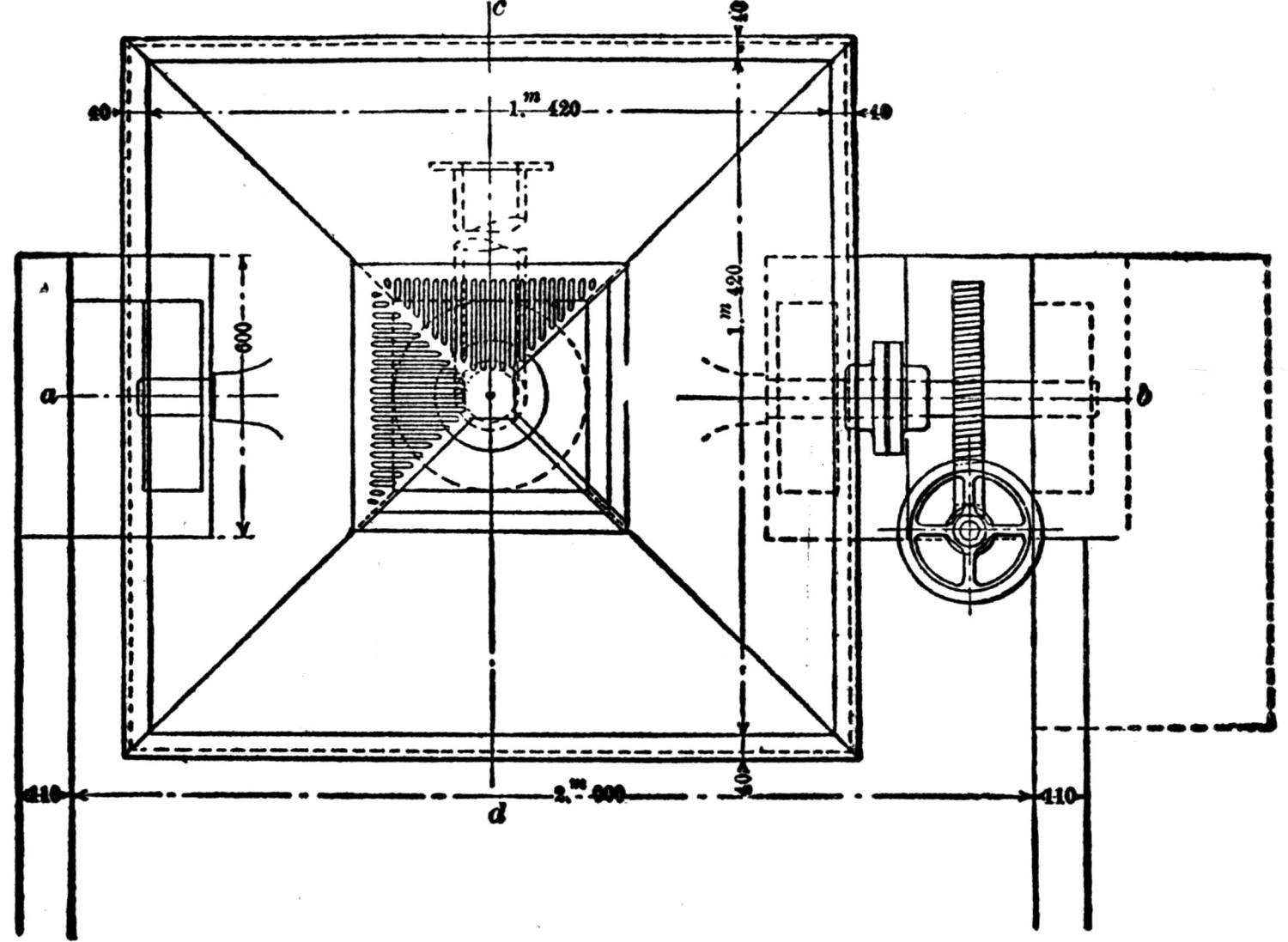
The losses of metal in the desulphurization are less than in the ordinary method, because the crude mineral remains only a short time (from one to three hours) in the apparatus for desulphurization and agglomeration, and the temperature of the process is lower. The blast-furnace slags are poorer, because there is no formation of silicate of lead during the agglomeration.
The Bormettes method, in so far as the treatment of lead ore is concerned, may be considered a combination process of roast-reaction, of roast-reduction, and of precipitation-smelting. It is 223 not, however, restricted to the treatment of lead ore. It may also be applied to the smelting of pyritous copper-bearing ores. In an experiment with cupriferous pyrites, containing 20 to 25 per cent. sulphur, it succeeded in agglomerating and smelting them without use of any fuel for calcination, effecting a perfect smelting, analogous to pyrite smelting, with the production of a matte of sufficient degree of concentration.
The first cost of plant installation is very much reduced by the Bormettes method, inasmuch as the ordinary roasting furnaces are almost entirely dispensed with, apparatus being substituted for them which cost only one-third or one-fourth as much as ordinary furnaces. The process presents the advantage, moreover, of being put into immediate operation, without any expenditure of excess fuel.
The apparatus required in the process is illustrated in Figs. 21-25. The apparatus for desulphurization and agglomeration consists of a cast-iron box, composed of four vertical walls, of which two incline slightly toward the front. These inclined walls carry the air-boxes. The other two walls are formed, the one in front by the doors which give access to the interior, and the other in the rear by a straight plate. The whole arrangement is surmounted by a hood. The four pieces when assembled form a box without bottom. Several of these boxes are combined as a battery. The pots in which the agglomeration and desulphurization are effected are moved into these boxes on suitable cars, in the manner shown in the first engraving. A later and more improved form is shown, however, in Figs. 23-25.
This process, which is the invention of A. Lotti and has been patented in all the principal countries, is in successful use at the works of the Société Anonyme des Mines de Bormettes, at Bormettes, La Londe (Var), France. Negotiations are now in progress with respect to its introduction elsewhere in Europe.
(November 1, 1902)
According to F. Laur, in the Echo des Mines (these notes are abstracted from Oest. Zeit., L., xl, 55, October 4, 1902), A. Germot, of Clichy, France, made experiments some years ago upon the production of white lead directly from galena. These led Catelin to attempt the recovery of metallic lead in a similar way. If air be blown in proper quantity into a fused mass of lead sulphide the following reaction takes place:
2PbS + 2O = SO2 + Pb + PbS.
Thus one-half of the lead is reduced, and it is found collects all the silver of the ore; the other half is sublimed as lead sulphide, which is free from silver. The reaction is exothermic to the extent that the burning of one-half the sulphur of a charge should theoretically develop sufficient heat to volatilize half of the charge and smelt the other half. This is almost done in practice with very rich galena, but not so with poorer ore. The temperature of the furnace must be maintained at about 1100 deg. C. throughout the whole operation, and there are the usual losses of heat by radiation, absorption by the nitrogen of the air, etc. Deficiencies in heat are supplied by burning some of the ore to white lead, which is mixed with the black fume (PbS) and by the well-known reactions reduced to metal with evolution of sulphur dioxide. The final result is therefore the production of (1) pig lead enriched in silver; (2) pig lead free from silver; (3) a leady slag; and (4) sulphur dioxide. In the case of ores containing less than 75 per cent. Pb the gangue forms first a little skin and then a thick hard crust which soon interferes with the operation, especially if the ore be zinkiferous. This difficulty is over 225come by increasing the temperature or by fluxing the ore so as to produce a fusible slag. A leady slag is always easily produced; this is the only by-product of the process. The theoretical reaction requires 600 cu. m. of air, assuming a delivery of 50 per cent. from the blower, and at one atmosphere pressure involves the expenditure of 18 h.p. per 1000 kg. of galena per hour.
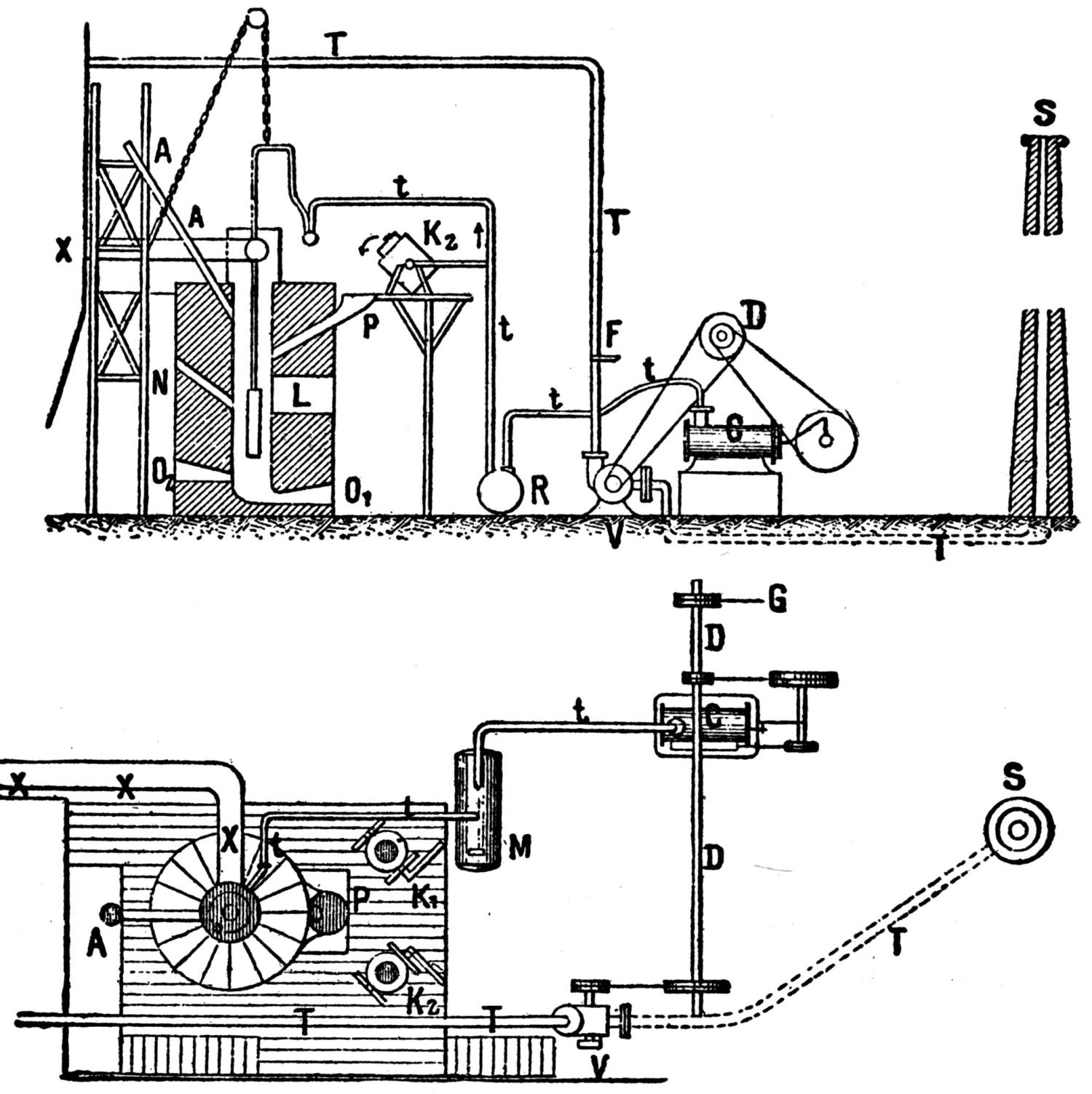
The arrangement of the plant at Clichy is shown diagrammatically in Fig. 26. There is a round shaft furnace, 0.54 meter in diameter and 4.5 meters high. Power is supplied to the blower C through the pulley G and the shaft DD. The compressed air is accumulated in the reservoir R, whence it is conducted by the pipe to the tuyere which is suspended inside of the furnace 226by means of a chain, whereby it can be raised or lowered. O1 and O2 are tap-holes. L is a door and N an observation tube. A is the charge tube. X is the pipe which conveys the gas and fume to the condensation chambers. T is the pipe through which the waste gases are drawn. V is the exhauster and S is the chimney. K1 and K2 are tilting crucible furnaces for melting lead and galena.
After the furnace has been properly heated, 100 kg. of lead melted in K1 are poured in through the cast-iron pipe P, and after that about 200 kg. of pure, thoroughly melted galena from K2. Ore containing 70 to 80 per cent. Pb must be used for this purpose. The blast of air is then introduced into the molten galena, and from 1000 to 3000 kg. of ore is gradually charged in through the tube A. During this operation black fume (PbS) collects in the condensation chamber. All outlets are closed against the external air. If the air blast is properly adjusted, nothing but black fume is produced; if it begins to become light colored, charging is discontinued and the blast of air is shut off. Lead is then tapped through O2, which is about 0.2 meter above the hearth, so there is always a bath of lead in the bottom of the furnace; but it is advisable now and then to tap off some through O1, so as gradually to heat up the bottom of the furnace. Hearth accretions are also removed through O1. The lead is tapped off through O2 until matte appears. The tap hole is then closed, the tuyere is lowered and the blast is turned into the lead in order to oxidize it and completely desulphurize the sulphur combinations, which is quickly done. The oxide of lead is scorified as a very fusible slag, which is tapped off through O2, and more ore is then charged in upon the lead bath and the cycle of operations is begun again.
(September 1, 1904)
Only a few years ago smelting companies began to recognize the advantage of large chambers for collecting flue dust and condensing fumes. The object is threefold: First, profit; second, to prevent law suits with surrounding agricultural interests; third, cleanliness about the plant. It is my object at present to discuss the materials used in construction and general types of cross-section.
Most of the old types of chambers are built after one general pattern, namely, brick or stone side walls and arch roof, with iron buckstays and tie rods. The above type is now nearly out of use, because it is short-lived, expensive, and dangerous to repair, while the steel and masonry are not used to good advantage in strength of cross-section.
With the introduction of concrete and expanded metal began a new era of dust-chamber construction. It was found that a skeleton of steel with cement plaster is very strong, light and cheap. The first flue of the type shown in Fig. 29 was built after the design of E. H. Messiter, at the Arkansas Valley smelter in Colorado. This flue was in commission several years, conveying sulphurous gases from the reverberatory roaster plant. The same company decided, in 1900, to enlarge and entirely rebuild its dust-chamber system, and three types of cross-section were adopted to meet the various conditions. All three types were of cement and steel construction.
The first type, shown in Fig. 27, is placed directly behind the blast furnaces. The cross-section is 273 sq. ft. area, being designed for a 10-furnace lead smelter. The back part is formed upon the slope of the hillside and paved with 2.5 in. of brick. The front part is of ribbed cast-iron plates. Ninety per cent. of the flue dust is collected in this chamber and is removed, through sliding doors, into tram cars. There is a little knack in designing 230 a door to retain flue dust. It is simply to make the bottom sill of the door frame horizontal for a space of about 1 in. outside of the door slide.
The front part of the chamber, Fig. 27, is of expanded metal and cement. The top is of 20 in. I-beams, spanning a distance of 24 ft. with 15 in. cross-beams and 3 in. of concrete floor resting upon the bottom flanges of the beams. This heavy construction forms the foundation for the charging floor, bins, scales, etc.
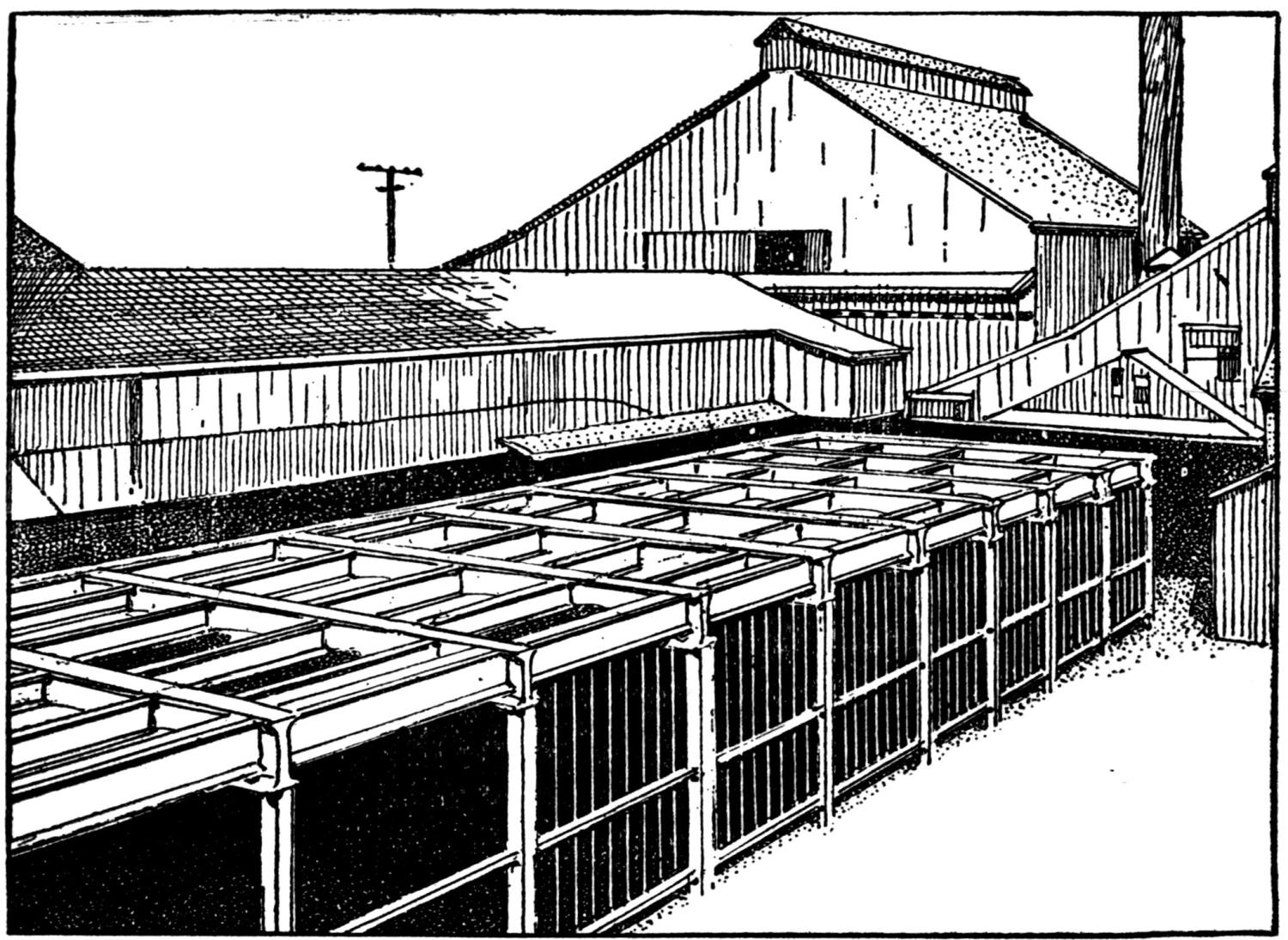
While dwelling upon this type of construction I wish to mention a most important point, that of the proper factor of safety. Flue dust, collected near the blast furnace, weighs from 80 to 100 lb. per cubic foot, and the steel supports should be designed for 16,000 lb. extreme fiber stress, when the chamber is three-quarters full of dust. If the dust is allowed to accumulate beyond this point, the steel, being well designed, should not be overstrained. Discussions as to strains in bins have been aired by the engineering profession, but the present question is “Where is a dust chamber a bin?” Experience shows that bin construction should be adopted behind, or in close proximity to, the blast furnaces.
Fig. 28 shows the second type of hopper-bottom flue adopted. It is of very light construction, of 274 sq. ft. area in the clear. The beginning of this flue being 473 ft. from the blast furnaces removes all possibility of any material floor-load, as the dust is light in weight and does not collect in large quantities. The hopper-bottom floor is formed of 4 in. concrete slabs, in panels, placed between 4 in. I-beams. Cast-iron door frames, with openings 12 × 16 in., are placed on 5 ft. centers. The concrete floor is tamped in place around the frames. The side walls and roof are built of 1 in. angles, expanded metal, and plastered to 2.5 in. thickness. At every 10 ft. distance, pilaster ribs built of 2 in. angles, latticed and plastered, form the wind-bracing and arch roof support.
Fig. 29 shows the beehive construction. This chamber is of 253 sq. ft. cross-sectional area. It is built of 2 in. channels, placed 16 in. centers, tied with 1 × 0.125 in. steel strips. The object of the strips is to support the 2 in. channels during erection. No. 27 gage expanded metal lath was wired to the inside of the channels and the whole plastered to a thickness of 3 in. The inside coat was plastered first with portland cement and sand, 232 one to three, with about 5 per cent. lime. The filling between ribs is one to four, and the outside coat one to three.
The above types of dust chamber have been in use over three years at Leadville. Cement and concrete, in conjunction with steel, have been used in Utah, Montana and Arizona, in various types of cross-section. The results show clearly where not to use cement; namely, where condensing sulphur fumes come in contact with the walls, or where moisture collects, forming sulphuric acid. The reason is that portland cement and lime mortar contain calcium hydrate, which takes up sulphur from the fumes, forming calcium sulphate. In condensing chambers, this calcium sulphate takes up water, forming gypsum, which expands and peels off.
In materials of construction it is rather difficult to get something that will stand the action of sulphur fumes perfectly. The lime mortar joints in the old types of brick flues are soon eaten away. The arches become weak and fall down. I noted a sheet steel condensing system, where in one year the No. 12 steel was nearly eaten through. With a view of profiting by past experience, let us consider the acid-proof materials of construction, namely, brick, adobe mortar, fire-clay, and acid-proof paint. Also, let us consider at what place in a dust-chamber system 233 are we to take the proper precaution in the use of these materials.
At smelting plants, both copper and lead, it is found that near the blast furnaces the gases remain hot and dry, so that concrete, brick or stone, or steel, can safely be used. Lead-blast furnace gases will not injure such construction at a distance of 6 or 8 ft. away from the furnaces. For copper furnaces, roasters or pyritic smelting, concrete or lime mortar construction should be limited to within 200 or 300 ft. of the furnaces.
Another type of settling chamber is 20 ft. square in the clear, with concrete floor between beams and steel hopper bottom. This chamber is built within 150 ft. distance from the blast furnaces, and is one of the types used at the Shannon Copper Company’s plant at Clifton, Arizona. After passing the 200 ft. mark, there is no need of expensive hopper design. The amount of flue dust settled beyond this point is so small that it is a better investment to provide only small side doors through which the dust can be removed. The ideal arrangement is to have a system of condensing chambers, so separated by dampers that either set can be thrown out for a short time for cleaning purposes, and the whole system can be thrown in for best efficiency.
As to cross-section for condensing chambers, I consider that the following will come near to meeting the requirements. One, four, and six, concrete foundation; tile drainage; 9 in. brick walls, laid in adobe mortar, pointed on the outside with lime mortar; occasional strips of expanded metal flooring laid in joints; the necessary pilasters to take care of the size of cross-section adopted; the top covered with unpainted corrugated iron, over which is tamped a concrete roof, nearly flat; concrete to contain corrugated bars in accordance with light floor construction; and lastly, the corrugated iron to have two coats of graphite paint on under side.
The above type of roof is used under slightly different conditions over the immense dust chamber of the new Copper Queen smelter at Douglas, Arizona. The paint is an important consideration. Steel work imbedded in concrete should never be painted, but all steel exposed to fumes should be covered by graphite paint. Tests made by the United States Graphite Company show that for stack work the paint, when exposed to acid gases, under as high a temperature as 700 deg. F., will wear well.
The construction of concrete flues of the section shown in Fig. 31 gives better results than that shown in Fig. 30, being less liable to collapse. It costs somewhat more to build owing to the greater complication of the crib, which, in both cases, consists of an interior core only. For work 4 in. in thickness and under, I recommend the use of rock or slag crushed to pass through a 1.5 in. ring. Although concrete is not very refractory, it will easily withstand the heat of the gases from a set of ordinary lead-or copper-smelting blast furnaces, or from a battery of calcining or roasting furnaces. I have never noticed that it is attacked in any way by sulphur dioxide or other furnace gas.
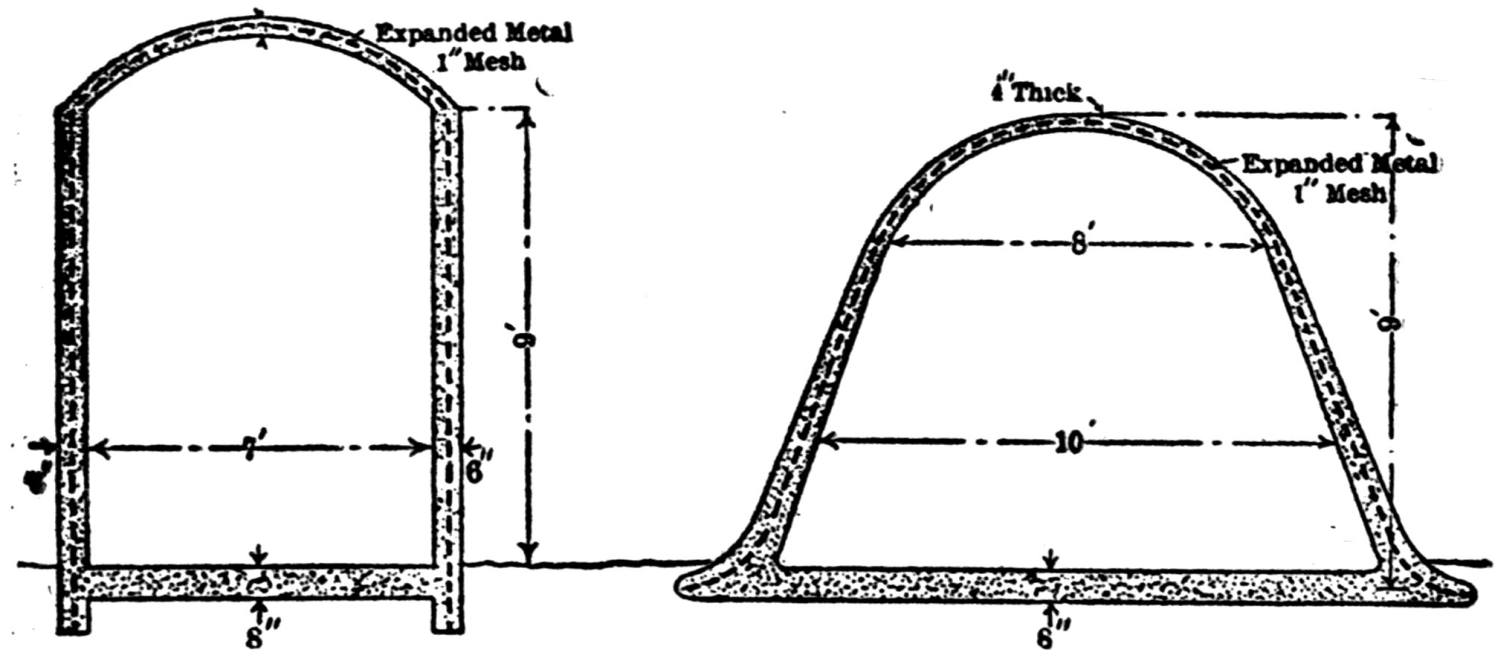
Shapes the most complicated to suit all tastes in dust chambers can be constructed of concrete. The least suitable design, so far as the construction itself is concerned, is a long, wide, straight-walled, empty chamber, which is apt to collapse, either inwards or outwards, and, although the outward movement can be 235 prevented by a system of light buckstays and tie-rods, the tendency to collapse inwards is not so simply controlled in the absence of transverse baffle walls. The tendency, so far as the collection of mechanical flue dust is concerned, appears to be towards a large empty chamber, without baffles, etc., in which the velocity of the air currents is reduced to a minimum, and the dust allowed to settle. In the absence of transverse baffle walls to counteract the collapsing tendency, it seems best to design the chamber with a number of stout concrete columns at suitable intervals along the side and end walls—the walls themselves being made only a few inches thick with woven-wire screen or “expanded metal” buried within them. The wire skeleton should also be embedded into the columns in order to prevent the separation of wall and the columns. This method of constructing is one that I have followed with very satisfactory results as far as the construction itself is concerned.
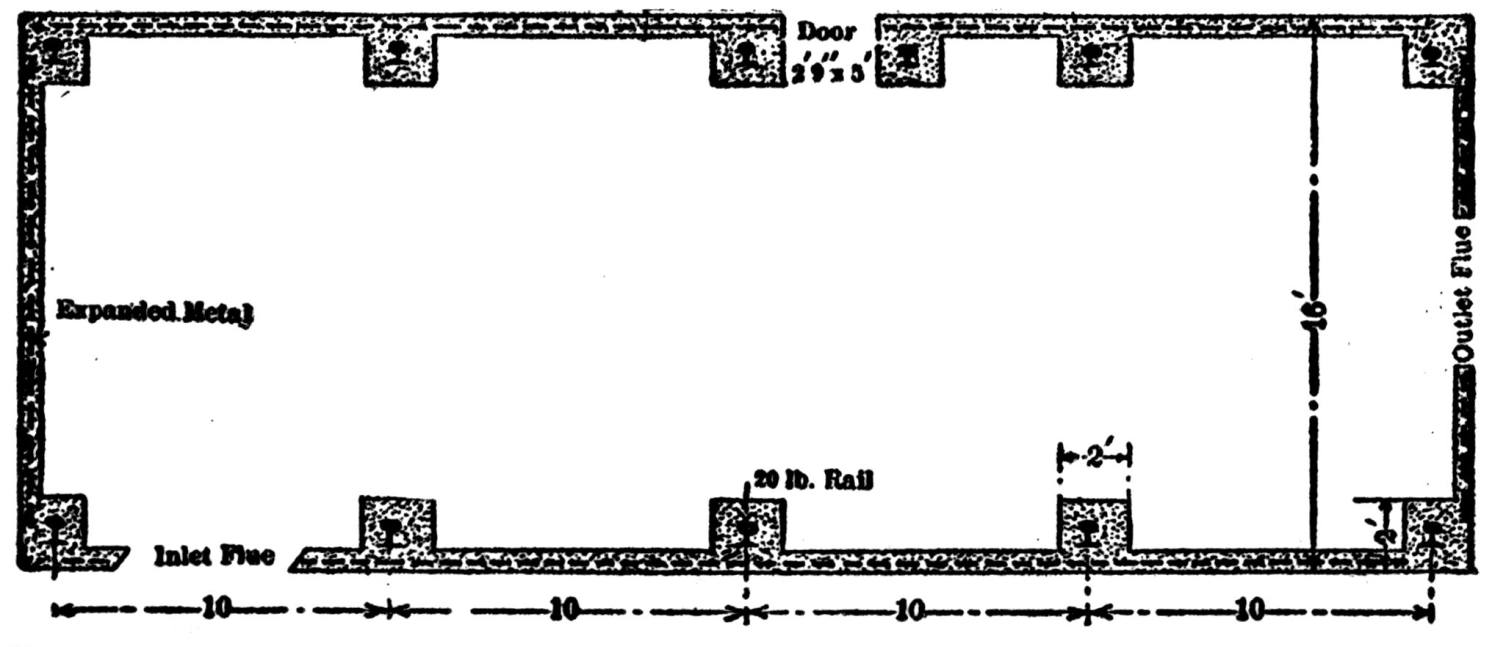
Figs. 32 and 33 show a chamber designed and erected at the Don Guillermo Smelting Works at Palomares, Province of Murcia, Spain. Figs. 34 and 35 show a design for the smelter at Murray Mine, Sudbury, Ontario, in which the columns are hollow, thus economizing concrete material. For work of this kind the columns are built first and the wire netting stretched from column to column and partly buried within them. The crib is then built on each side of the netting, a gang of men working from both sides, and is built up a yard or so at a time as the work progresses. Doors of good size should be provided for entrance into the 236 chamber, and as they will seldom be opened there is no need for expensive fastenings or hinges.
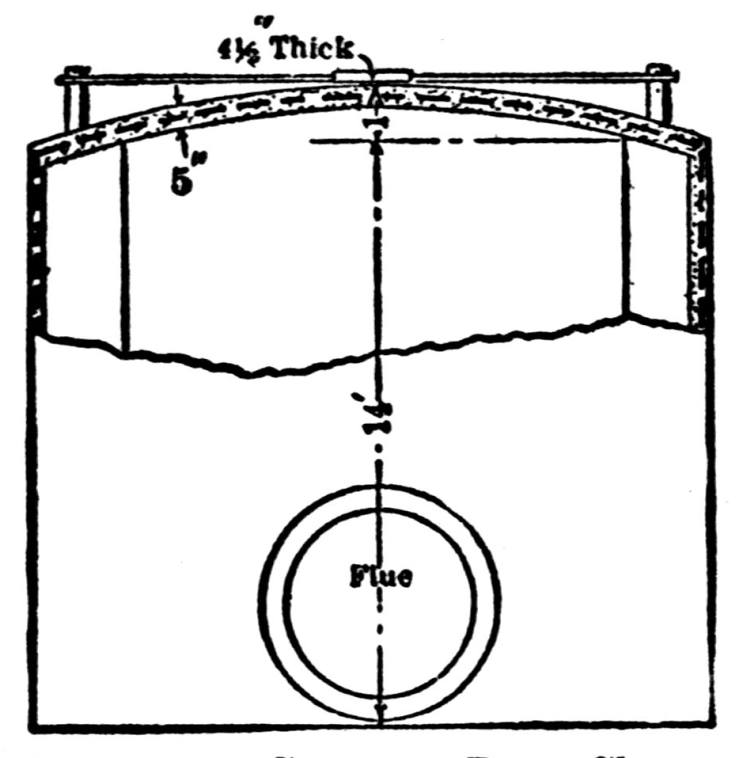
Foundations for Dynamos and other Electrical Machinery.—Dry concrete is a poor conductor of electricity, but when wet it becomes a fairly good conductor. Therefore, if it be necessary to insulate the electrical apparatus, the concrete should be covered with a layer of asphalt.
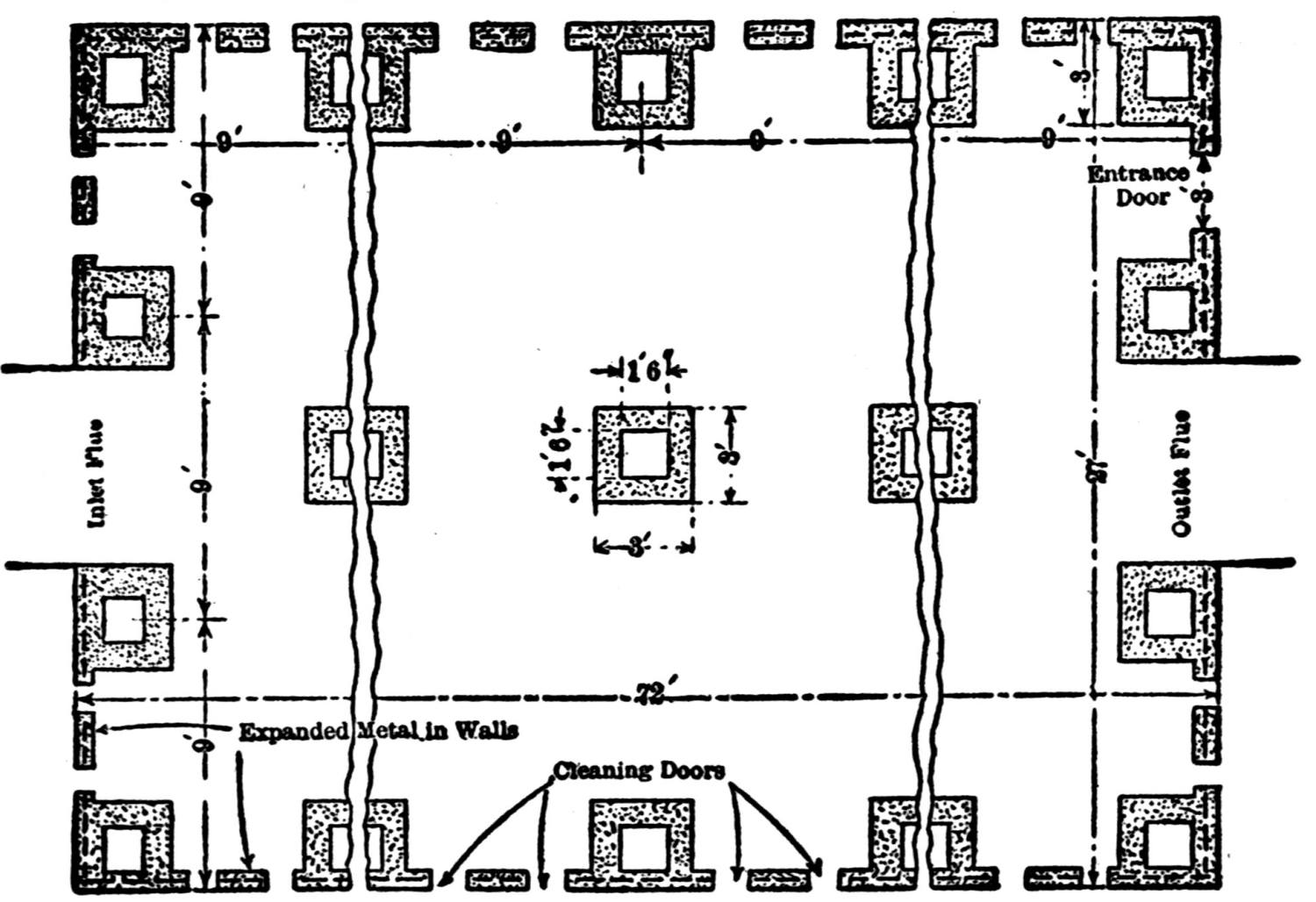
Chimney Bases.—Fig. 36 shows the base for the 90 ft. brick-stack at Don Guillermo. The resemblance to masonry is given by nailing strips of wood on the inside of the crib.
Retaining-Walls.—Figs. 37, 38, and 39 show three different styles of retaining-walls, according to location. These walls are shown in section only, and show the placing of the iron reenforcements. Retaining-walls are best built in panels (each panel being a day’s work), for the reason that horizontal joints in the concrete are thereby avoided. The alternate panels should be built first and the intermediate spaces filled in afterward. Should there be water behind the wall it is best to insert a few small pipes through the wall, in order to carry it off; this precaution is particularly important in places where the natural surface of the ground meets the wall, as shown in Figs. 37 and 38. If a wooden building is to be erected on the retaining-wall, it is best to bury a few 0.75 in. bolts vertically in the top of the wall, by which a wooden coping may be secured (see Figs. 37, 38, and 39), which forms a good commencement for the carpenter work.
Minimum thickness for a retaining-wall, having a liberal quantity of iron embedded therein, is 20 in. at the bottom and 10 in. at the top, with the taper preferably on the inner face. In the absence of interior strengthening irons the thickness of 238 the wall at the bottom should never be less than one-fourth the total hight, and at the top one-seventh of the hight; unless very liberal iron bracing be used, the dimensions can hardly be reduced to less than one-seventh and one-tenth respectively. Unbraced retaining-walls are more stable with the batter on the outer face. Dry clay is the most treacherous material that can be had behind a retaining-wall, especially if it be beaten in, for the reason that it is so prone to absorb moisture and swell, causing an enormous side thrust against the wall. When this material is to be retained it is best to build the wall superabundantly strong—a precaution which applies even to a dry climate, 239 because the bursting of a water-pipe may cause the damage. In order to avoid horizontal joints it is best, wherever practicable, to build the crib-work in its entirety before starting the concrete. In a retaining-wall 3 ft. thick by 16 ft. high this is not practicable. The supporting posts and struts can, however, be completed and the boards laid in as the wall grows, in order not to interrupt the regular progress of the tamping. A good finish may be produced on the exposed face of the wall by a few strokes of the shovel up and down with its back against the crib.
In conclusion I wish to state that this paper is not written for the instruction of the civil engineer, or for those who have special experience in this line; but rather for the mining engineer or metallurgist whose training is not very deep in this direction, and who is so often thrown upon his own resources in the wilderness, and who might be glad of a few practical suggestions from one who has been in a like predicament.
(September, 1904)
Under the heading “Flues,” Mr. Edwards refers to the Beehive construction, a cross-section of which is shown in Fig. 31 of his paper. A flue similar to this was designed by me about six years ago,[47] and in which the walls, though much thinner than those described by Mr. Edwards, gave entire satisfaction. These walls, from 2.25 in. thick throughout in the smaller flues to 3.25 in. in the larger, were built by plastering the cement mortar on expanded-metal lath, without the use of any forms or cribs whatever, at a cost of labor generally less than $1 per sq. yd. of wall. Of course, where plasterers cannot be obtained on reasonable terms, the cement can be molded between wooden forms, though it is difficult to see how it can be done with an interior core only, as stated by Mr. Edwards.
In regard to the effect of sulphur dioxide and furnace gases on the cement, I have found that in certain cases this is a matter which must be given very careful attention. Where there is sufficient heat to prevent the existence of condensed moisture inside of the flue, there is apparently no action whatever on the cement, but if the concrete is wet, it is rapidly rotted by these gases. At points near the furnaces there is generally sufficient heat not only to prevent internal condensation of the aqueous vapor always present in the gases, but also to evaporate water from rain or snow falling on the outside of the flue. Further along a point is reached where rain-water will percolate through minute cracks caused by expansion and contraction, and reach the interior even though internal condensation does not occur there in dry weather. From this point to the end of the flue the 241 roof must be coated on the outside with asphalt paint or other impervious material. In very long flues a point may be reached where moisture will condense on the inside of the walls in cold weather. From this point to the end of the flue it is essential to protect the interior with an acid-resisting paint, of which two or more coats will be necessary. For the first coat a material containing little or no linseed oil is best, as I am informed that the lime in the cement attacks the oil. For this purpose I have used ebonite varnish, and for the succeeding coats durable metal-coating. The first coat will require about 1 gal. of material for each 100 sq. ft. of surface.
In one of the earliest long flues built of cement in this country, a small part near the chimney was damaged as a result of failure to apply the protective coating, the necessity for it not having been recognized at the time of its construction. It may be said, in passing, that other long brick flues built prior to that time were just as badly attacked at points remote from the furnaces. In order to reduce the amount of flue subject to condensation, the plastered flues have been built with double lath having an intervening air-space in the middle of the wall.
In building thin walls of cement, such as flue walls, it is particularly important to prevent them from drying before the cement has combined with all the water it needs. For this reason the work should be sprinkled freely until the cement is fully set. Much work of this class has been ruined through ignorance by fires built near the walls in cold weather, which caused the mortar to shell off in a short time.
The great saving in cost of construction, which the concrete-steel flue makes possible, will doubtless cause it to supersede other types to even a greater extent than it has already done. If properly designed this type of construction reduces the cost of flues by about one-half. Moreover, the concrete-steel flue is a tight flue as compared with one built of brick. There is a serious leakage through the walls of the brick flues which is not easily observed in flues under suction as most flues are, but when a brick flue is under pressure from a fan the leakage is surprisingly apparent. In flues operating by chimney-draft the entrance of cold air must cause a considerable loss in the efficiency of the chimney, a disadvantage which would largely be obviated by the use of the concrete-steel flue.
In discussion of Mr. Edwards’s interesting and valuable paper, I beg to submit the following notes concerning the advantages and disadvantages of the concrete flues and stacks at the plant of the Anhaltische Blei-und Silber-werke. The flues and smaller stacks at the works were constructed of concrete consisting generally of one part of cement to seven parts of sand and jig-tailings but, in the case of the under-mentioned metal concrete slabs, of one part of cement to four parts of sand and tailings. The cost of constructing the concrete flue approximated 5 marks per sq. m. of area (equivalent to $0.11 per sq. ft.).
Effect of Heat.—A temperature above 100 deg. C. caused the concrete to crack destructively. Neutral furnace gases at 120 deg. C., passing through an independent concrete flue and stack, caused so much damage by the formation of cracks that, after two years of use, the stack, constructed of pipes 4 in. thick, required thorough repairing and auxiliary ties for every foot of hight.
Effect of Flue Gases and Moisture.—The sides of the main flue, made of blocks of 6 in. hollow wall-sections, 100 cm. by 50 cm. in area, were covered with 2 in. or 1 in. slabs of metal concrete. In cases where the flue was protected on the outside by a wooden or tiled roof, and inside by an acid-proof paint, consisting of water-glass and asbestos, the concrete has not been appreciably affected. In another case, where the protective cover, both inside and outside, was of asphalt only, the concrete was badly corroded and cracked at the end of three years. In a third case, in which the concrete was unprotected from both atmospheric influence on the outside, and furnace gases on the inside, the flue was quite destroyed at the end of three years. 243 That portion of the protected concrete flue, near the main stack, which came in contact only with dry, cold gases was not affected at all.
Gases alone, such as sulphur dioxide, sulphur trioxide, and others, do not affect concrete; neither is the usual quantity of moisture in furnace gases sufficient to damage concrete; but should moisture penetrate from the outside of the flue, and, meeting gaseous SO2 or SO3, form hydrous acids, then the concrete will be corroded.
Effect of the Atmosphere Alone.—For outside construction work, foundations and other structures not exposed to heat, moist acid gases and chemicals, the concrete has maintained its reputation for cheapness and durability.
Effect of Crystallization of Contained Salts.—In chemical works, floors constructed of concrete are sometimes unsatisfactory, for the reason that soluble salts, noticeably zinc sulphate, will penetrate into the floor and, by crystallizing in narrow confines, cause the concrete to crack and the floor to rise in places.
(July 15, 1905)
One of the most efficient methods of saving fume and very fine dust in metallurgical practice is by filtration through cloth. This idea is by no means a new one, having been proposed by Dr. Percy, in his treatise on lead, page 449, but he makes no mention of any attempt to apply it. Its first practical application was found in the manufacture of zinc oxide direct from ores, initially tried by Richard and Samuel T. Jones in 1850, and in 1851 modified by Samuel Wetherill into the process which continues in use at the present time in about the same form as originally. In 1878 a similar process for the manufacture of white lead direct from galena was introduced at Joplin, Mo., by G. T. Lewis and Eyre O. Bartlett, the latter of whom had previously been engaged in the manufacture of zinc oxide in the East, from which he obtained his idea of the similar manufacture of white lead. The difference in the character of the ore and other conditions, however, made it necessary to introduce numerous modifications before the process became successful. The eventual success of the process led to its application for filtration of the fume from the blast furnaces at the works of the Globe Smelting and Refining Company, at Denver, Colo., and later on for the filtration of the fume from the Scotch hearths employed for the smelting of galena in the vicinity of St. Louis.
In connection with the smelting of high-grade galena in
Scotch hearths, the bag-house is now a standard accessory. It
has received also considerable application in connection with
silver-lead blast-furnace smelting and in the desilverizing refineries.
Its field of usefulness is limited only by the character of
the gas to be filtered, it being a prerequisite that the gas contain
no constituent that will quickly destroy the fabric of which the
bags are made. Bags are also employed successfully for the
collection of dust in cyanide mills, and other works in which 245
246
fine crushing is practised, for example, in the magnetic separating
works of the New Jersey Zinc Company, Franklin, N. J. , where the
outlets of the Edison driers, through which the ore is passed, communicate
with bag-filtering machines, in which the bags are caused
to revolve for the purpose of mechanical discharge. The filtration
of such dust is more troublesome than the filtration of furnace
fume, because the condensation of moisture causes the bags to
become soggy.
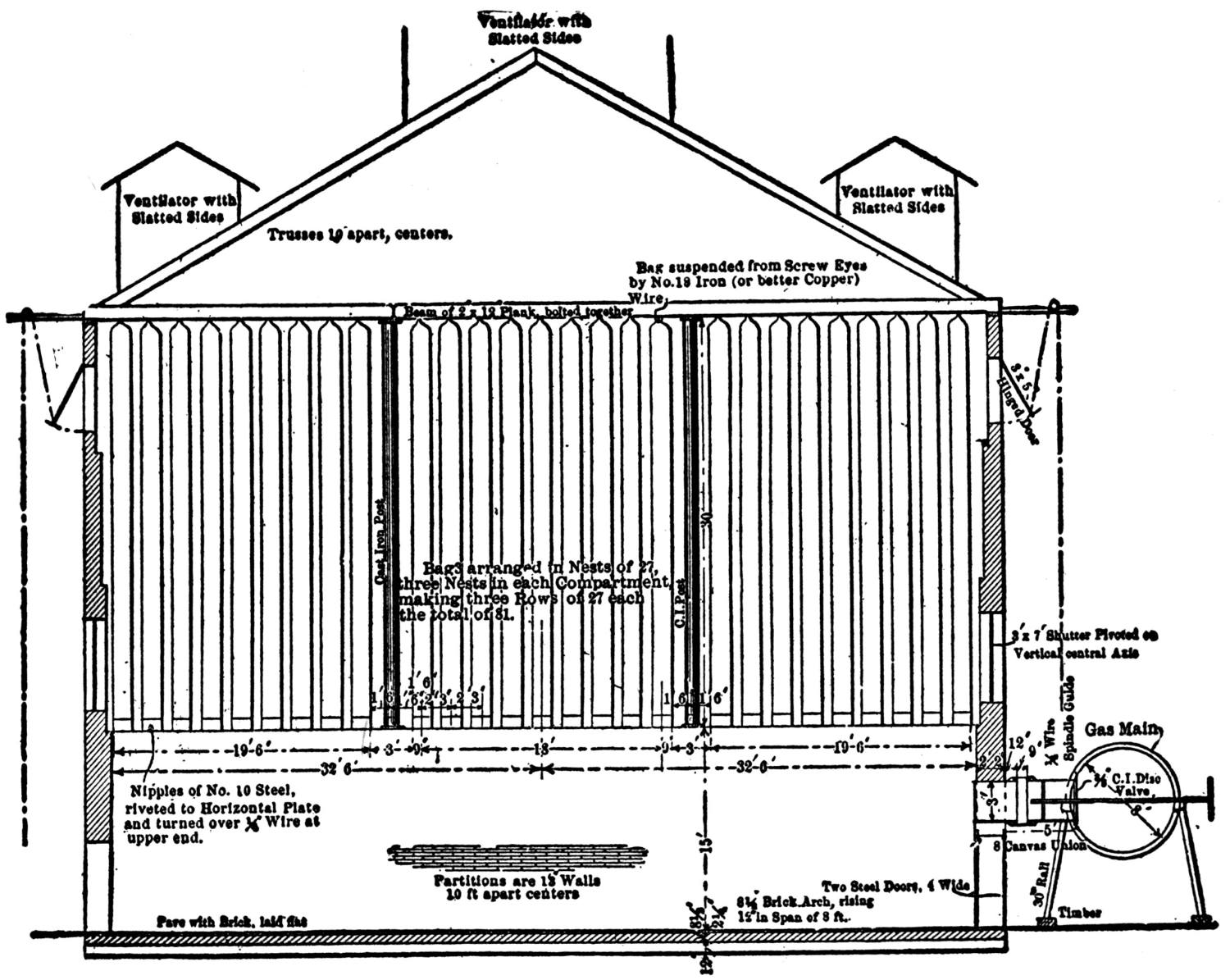
The standard bag-house employed in connection with furnace work is a large room, in which the bags hang vertically, being suspended from the top. The bags are simply tubes of cotton or woolen (flannel) cloth, from 18 to 20 in. in diameter, and 20 to 35 ft. in length, most commonly about 30 ft. In the manufacture of zinc oxide, the fume-laden gas is conducted into the house through sheet-iron pipes, with suitably arranged branches, from nipples on which the bags are suspended, the lower end of the bag being simply tied up until it is necessary to discharge the filtered fume by shaking. In the bag-houses employed in the metallurgy of lead, the fume is introduced at the bottom into brick chambers, which are covered with sheet-iron plates, provided with the necessary nipples; or else into hopper-bottom, sheet-iron flues, with the necessary nipples on top. In either case the bags are tied to the nipples, and are tied up tight at the top, where they are suspended. When the fume is dislodged by shaking the bags, it falls into the chamber or hopper at the bottom, whence it is periodically removed.
The cost of attending a bag-house, collecting the fume, etc., varies from about 10c. per ton of ore smelted in a large plant like the Globe, to about 25c. per ton in a Scotch-hearth plant treating 25 tons of ore per 24 hours.
No definite rules for the proportioning of filtering area to the quantity of ore treated have been formulated. The correct proportion must necessarily vary according to the volume of gaseous products developed in the smelting of a ton of ore, the percentage of dust and fume contained, and the frequency with which the bags are shaken. It would appear, however, that in blast furnaces and Scotch-hearth smelting a ratio of 1000 sq. ft. per ton of ore would be sufficient under ordinary conditions. The bag-house originally constructed at the Globe works had about 250 sq. ft. of filtering area per ton of charge smelted, but 247 this was subsequently increased, and Dr. Iles, in his treatise on lead-smelting, recommends an equipment which would correspond to about 750 sq. ft. per ton of charge. At the Omaha works, where the Brown-De Camp system was used, there was 80,000 sq. ft. of cloth for 10 furnaces 42 × 120 in., according to Hofman’s “Metallurgy of Lead,” which would give about 1000 sq. ft. per ton of charge smelted, assuming an average of eight furnaces to be in blast. A bag-house in a Scotch-hearth smeltery, at St. Louis, had approximately 900 sq. ft. per ton of ore smelted. At the Lone Elm works, at Joplin, the ratio was about 3500 sq. ft. per ton of ore smelted, when the works were run at their maximum capacity. In the manufacture of zinc oxide the bag area used to be from 150 to 200 sq. ft. per square foot of grate on which the ore is burned, but at Palmerton, Pa. (the most modern plant), the ratio is only 100:1. This corresponds to about 1400 sq. ft. of bag area per 2000 lb. of charge worked on the grate. In the manufacture of zinc-lead white at Cañon City, Colo., the ratio between bag area and grate area is 150:1.
Assuming the gas to be free, or nearly free, from sulphurous fumes, the bags are made of unbleached muslin, varying in weight from 0.4 to 0.7 oz. avoirdupois per square foot. The cloth should have 42 to 48 threads per linear inch in the warp and the same number in the woof. A kind of cloth commonly used in good practice weighs 0.6 oz. per square foot and has 46 threads per linear inch in both the warp and the woof.
The bags should be 18 to 20 in. in diameter. Therefore the cloth should be of such width as to make that diameter with only one seam, allowing for the lap. Cloth 62 in. in width is most convenient. It costs 4 to 5c. per yard. The seam is made by lapping the edges about 1 in., or by turning over the edges and then lapping, in the latter case the stitches passing through four thicknesses of the cloth. It should be sewed with No. 50 linen thread, making two rows of double lock-stitches.
The thimbles to which the bags are fastened should be of No. 10 sheet steel, the rim being formed by turning over a ring of 0.25 in. wire. The bags are tied on with 2 in. strips of muslin. The nipples are conveniently spaced 27 in. apart, center to center, on the main pipe.
The gas is best introduced at a temperature of 250 deg. F. 248 Too high a temperature is liable to cause them to ignite. They are safe at 300 deg. F., but the temperature should not be allowed to exceed that point.
The gas is cooled by passage through iron pipes of suitable radiating surface, but the temperature should be controlled by a dial thermometer close to the bag-house, which should be observed at least hourly, and there should be an inlet into the pipe from the outside, so that, in event of rise of temperature above 300 deg., sufficient cold air may be admitted to reduce it within the safety limit.
In the case of gas containing much sulphur dioxide, and especially any appreciable quantity of the trioxide, the bags should be of unwashed wool. Such gas will soon destroy cotton, but wool with the natural grease of the sheep still in it is not much affected. The gas from Scotch hearths and lead-blast furnaces can be successfully filtered, but the gas from roasting furnaces contains too much sulphur trioxide to be filtered at all, bags of any kind being rapidly destroyed.
(April 27, 1901)
A note in the communication from S. E. Bretherton on “Pyritic Smelting and Hot Blast,” published in the Engineering and Mining Journal of April 13, 1901, refers to a subject of great interest to lead smelters. Mr. Bretherton remarked that he had been recently informed by August Raht that by actual experiment the loss with the ordinary rotary blowers was 100 per cent. under 10 lb. pressure; that is, it was possible to shut all the gates so that there was no outlet for the blast to escape from the blower and the pressure was only 10 lb., or in other words the blower would deliver no air against 10 lb. pressure. For that reason Mr. Raht expressed himself as being in favor of blowing engines for lead blast furnaces. This is of special interest, inasmuch as it comes from one who is recognized as standing in the first rank of lead-smelting engineers. Mr. Raht is not alone in holding the opinion he does.
The rotary blower did good service in the old days when the air was blown into the lead blast furnace at comparatively moderate pressure. At the present time, when the blast pressure employed is commonly 40 oz. at least, and sometimes as high as 48 oz., the deficiencies of the rotary blower have become more apparent. Notwithstanding the excellent workmanship which is put into them by their manufacturers, the extensive surfaces of contact are inherent to the type, and leakage of air backward is inevitable and important at the pressures now prevailing. The impellers of a rotary blower should not touch each other nor the cylinders in which they revolve, but they are made with as little clearance as possible, the surfaces being coated with grease, which fills the clearance space and forms a packing. This will not, however, entirely prevent leakage, which will naturally increase with the pressure. Even the manufacturers of rotary blowers admit the defects of the type, and concede that for pres 252sures of 5 lb. and upward the cylinder blowing engine is the more economical. Metallurgists are coming generally to the opinion, however, that blowing engines are probably more economical for pressures of 4 lb. or thereabouts, and some go even further. With the blowing engines the air-joints of piston and cylinder are those of actual contact, and the metallurgist may count on his cubic feet of air, whatever be the pressure. Blowing engines were actually introduced several years ago by M. W. Iles at what is now the Globe plant of the American Smelting and Refining Company, and we believe their performance has been found satisfactory.
The fancied drawback to the use of blowing engines is their greater first cost, but H. A. Vezin, a mechanical engineer whose opinions carry great weight, pointed out five years ago in the Transactions of the American Institute of Mining Engineers (Vol. XXVI) that per cubic foot of air delivered the blowing engine was probably no more costly than the rotary blower, but on the contrary cheaper, stating that the first cost of a cylinder blower is only 20 to 25 per cent. more than that of a rotary blower of the same nominal capacity and the engine to drive it. The capacity of a rotary blower is commonly given as the displacement of the impellers per revolution, without allowance for slip or leakage backward. Mr. Vezin expressed the opinion that for the same actual capacity at 2 lb. pressure, that is, the delivery in cubic feet against 2 lb. pressure, the cylinder blower would cost no more than, if as much as, the rotary blower.
In this connection it is worth while making a note of the increasing tendency of lead smelters to provide much more powerful blowers than were formerly considered necessary, due, no doubt, in large measure to the recognition of the greater loss of air by leakage backward at the pressure now worked against. It is considered, for example, that a 42 × 140 in. furnace to be driven under 40 oz. pressure should be provided with a No. 10 blower, which size displaces 300 cu. ft. of air per revolution and is designed to be run at about 100 r.p.m.; its nominal capacity is, therefore, 30,000 cu. ft. of air per minute; although its actual delivery against 40 oz. pressure is much less, as pointed out by Mr. Raht and Mr. Bretherton. The Connersville Blower Company, of Connersville, Ind., lately supplied the Aguas Calientes plant (now of the American Smelting and Refining Company) 253 with a rotary blower of the above capacity, and duplicates of it have been installed at other smelting works. The force required to drive such a huge blower is enormous, being something like 400 h.p., which makes it advisable to provide each blower with a directly connected compound condensing engine.
In view of the favor with which cylindrical blowing engines for driving lead blast furnaces are held by many of the leading lead-smelting engineers, and the likelihood that they will come more and more into use, it will be interesting to observe whether the lead smelters will take another step in the tracks of the iron smelters and adopt the circular form of blast furnace that is employed for the reduction of iron ore. The limit of size for rectangular furnaces appears to have been reached in those of 42 × 145 in., or approximately those dimensions. A furnace of 66 × 160 in., which was built several years ago at the Globe plant at Denver, proved a failure. H. V. Croll at that time advocated the building of a circular furnace instead of the rectangular furnace of those excessive dimensions and considered that the experience with the latter demonstrated their impracticability. In the Engineering and Mining Journal of May 28, 1898, he stated that there was no good reason, however, why a furnace of 300 to 500 tons daily capacity could not be run successfully, but considered that the round furnace was the only form permissible. We are unaware whether Mr. Croll was the first to advocate the use of large circular furnaces for lead smelting, but at all events there are other experienced metallurgists who now agree with him, and the time is, perhaps, not far distant when they may be adopted.
(June 8, 1901)
In the issues of the Engineering and Mining Journal for April 13th and 27th reference was made to the relative efficiency of piston-blowing engines and rotary blowers of the impeller type, and in these articles August Raht was quoted as saying that, with an ordinary rotary blower working against 10 lb. pressure, the loss was 100 per cent. I have waited some time with the idea that some of the blower people would call attention to the concealed fallacy in the statement quoted, but so far have failed to notice any reference to the matter. I feel quite sure that Mr. Bretherton failed to quote Mr. Raht in full. The one factor missing in this statement is the speed at which the blower was run when the loss was 100 per cent.
The accepted method of testing the volumetric efficiency of rotary blowers is that of “closed discharge.” The discharge opening of the blower is closed, a pressure gage is connected with the closed delivery pipe, and the blower is gradually speeded up until the gage registers the required pressure. The number of revolutions which the blower makes while holding that pressure, multiplied by the cubic feet per revolution, will give the total slip of that particular blower at that particular pressure. Experience has shown that, within the practical limits of speed at which a blower is run, the slip is a function of the pressure and has nothing to do with the speed. If, therefore, it were found that the particular blower referred to by Mr. Raht were obliged to be revolved at the rate of 30 r.p.m. in order to maintain a constant pressure of 10 lb. with a closed discharge, and if the blower were afterward put in practical service, delivering air, and were run at a speed of 150 r.p.m., it would then follow that its delivery of air would amount to: 150-30 = 120. Its volumetric efficiency would be 120 ÷ 150 = 80 per cent. The above 255 figures must not be relied upon, as I give them simply by way of illustration.
About a year ago I had the pleasure of examining the tabulated results of some extensive experiments in this direction, made by one of the blower companies. I believe they carried their experiments up to 10 lb. pressure, and I regret that I have not the figures before me, so that I could give something definite. I do, however, remember that in the experimental blower, when running at about 150 r.p.m., the volumetric efficiency at 2 lb. pressure was about 85 per cent., and that at 3 lb. pressure the volumetric efficiency was about 81 per cent.
It is unnecessary in this connection to call attention to the horse-power efficiency of rotary blowers. This is a matter entirely by itself, and there is considerable difference of opinion among engineers as to the relative horse-power efficiency of rotary blowers and piston blowers. All agree that there is a certain pressure at which the efficiency of the blower becomes less than the efficiency of the blowing engine. This I have heard placed all the way from 2 lb. up to 6 lb.
At the smelting plant of the Tennessee Copper Company we have lately installed blast-furnace piston-blowing engines; the steam cylinders are of the Corliss type and are 13 and 24 in. by 42 in.; the blowing cylinders are two in number, each 57 × 42 in.; the air valves are all Corliss in type. These blowing engines are designed to operate at a maximum air pressure of 2½ lb. per square inch.
At the Santa Fe Gold and Copper Mining Company’s smelter we have recently installed a No. 8 blower directly coupled to a 14 × 32 in. Corliss engine. This blower has been in use about five months and is giving very good results against the comparatively low pressure of 12 oz., or ¾ lb.
During the coming summer it is my intention to make careful volumetric and horse-power tests on these two types of machines under similar conditions of air pressure, and to publish the results; but in the meantime I wish to correct the error that a rotary blower of the impeller type is not a practicable machine at pressure over 5 lb.
(July 20, 1901)
In the Engineering and Mining Journal for July 6th I note the discussion over the relative merits of blowers and blowing engines for lead and copper smelting, and wish to state that, irrespective of the work to be done, the blast pressure will depend entirely on the charge burden in any kind of blast-furnace work, and that the charge burden governs the reducing action of the furnace altogether. Along these lines the iron industry has raised the charge burden up to 100 ft. to secure the full benefit of the reducing action of the carbon monoxide on the ore.
In direct opposition to this we have what is known as pyritic smelting, wherein the charge burden governs the grade of the matte produced to such an extent that if a charge run with 4 to 6 ft. of burden above the tuyeres, producing 40 per cent. matte, is changed to a charge burden of 10 or 12 ft., the grade of the matte will decrease from 40 per cent. to probably less than 20 per cent. I can state this as a fact from recent experience in operating a blast furnace on heap-roasted ores under the conditions named, with the result as above stated.
I was exceedingly skeptical about pyritic smelting as advocated by some of your correspondents, and still continue to be so; but on making inquiries from some of my co-workers in this line, Mr. Sticht of Tasmania, and Mr. Nutting of Bingham, Utah, I have arrived at the following conclusion, to which some may take exception: That pyritic smelting without fuel, or with less than 5 per cent., with hot blast, is practically impossible; that smelting raw ore with a low charge burden to avoid the reducing action of the carbon monoxide, thereby securing oxidation of the iron and sulphur, is possible and practicable, under favorable conditions; and that a large portion of the sulphur is burned off, and the iron, without reducing action, goes into the 257 slag in combination with silica. These results can be obtained with cold blast.
A blowing engine would certainly be much out of place for operating copper-matting furnaces run with the evident intention of oxidizing sulphur and iron and securing as high a grade of matte as possible, for the reason that to do this it is necessary to run a low charge burden, and with a low charge burden a high pressure of blast cannot be maintained. With a 4 to 6 ft. charge burden the blast pressure at Victoria Mines at present is 3 oz., produced by a No. 6 Green blower run at 120 r.p.m.; and a blowing engine, delivering the same amount of air, would certainly not give more pressure. Under the conditions which we have, a fan would be more effective than a pressure blower, and a blowing engine entirely out of the question as far as economy is concerned.
I installed blowing engines at the East Helena for lead smelting where the charge burden was 21 ft. and the blast pressure at times went up as high as 48 oz. Under these conditions the blowing engines gave satisfaction, but I am of the opinion that the same amount of blast could have been obtained under that pressure with less horse-power by the best type of rotary blower. I do not believe that the field of the blowing engine properly exists below 5 lb., and if this pressure cannot be obtained by charge-burden conditions, their installation is a mistake.
I wish to add the very evident fact that varying the grade of the matte by feeding the furnace at different hights varies the slag composition as to its silica and iron contents and makes the feeder the real metallurgist. The reducing action in the furnace is effected almost entirely by the gases, and when these are allowed to go to waste, reduction ceases.
(August 24, 1901)
I have just read in the Engineering and Mining Journal of July 20th an interesting letter written by Hiram W. Hixon in regard to blowing engines versus the rotary blowers, and also the use of cold blast for pyritic smelting.
The controversy, which I unintentionally started in my letter in the Engineering and Mining Journal of April 13th last, about the advantages of using either blowers or blowing engines for blast furnaces, does not particularly interest me, with the exception that I have about decided, in my own mind, to use blowing engines where there is much back pressure, and the ordinary up-to-date blower for pyritic or matte smelting where much back pressure should not exist. I fully appreciate the fact that so-called pyritic smelting can be done to a limited extent, even with cold blast. Theoretically, enough oxygen can be sent into the blast furnace, contained in the cold blast, to oxidize both the fuel and the sulphur in an ordinary sulphide charge, but I have not yet learned where a high concentration is being made with unroasted ore and cold blast. I experimented on these lines at different times for three years, during 1896, 1897, and 1898, making a fair concentration with refractory ores, most of which had been roasted. I was myself interested in the profits and as anxious as any one for economy. We tried, for fuel in the blast furnace, coke alone, coke and lignite coal, lignite coal alone, lignite coal and dry wood, coal and green wood, and then coke and green wood, all under different hights of ore burden in the furnace.
A description of these experiments would, no doubt, be tiresome to your readers, but I wish to state that the furnace was frozen up several times on account of using too little fuel, when the cold blast would gradually drive nearly all the heat to the 259 top of the furnace, the crucible and between the tuyeres becoming so badly crusted that the furnace had to be cleaned out and blown in again, unless I was called in time to save it by changing the charge and increasing the fuel. We were making high-grade matte under contract, high concentration and small matte fall, which would, no doubt, aggravate matters.
After the introduction of hot blast, heated up to between 200 and 300 deg. F., we made the same grade of matte from the same character of ore, with the exception that we then smelted without roasting, and reduced the percentage of fuel consumption, increased the capacity of the furnace, and almost entirely obviated the trouble of cold crucibles and hot tops. I write the above facts, as they speak for themselves.
I nearly agree with Mr. Hixon, and do not think it practical to smelt with much less than 5 per cent. coke continuously; but there is a great saving between the amount of coke used with a moderately heated blast and cold blast. Regardless of either hot or cold blast, however, the fuel consumption depends very much on the character of the ore to be smelted, the amount of matte-fall and grade of matte made. It is not always advisable or necessary to use hot blast for a matting furnace; that is, where the supply of sulphur is limited. It may then be necessary to use as much fuel in the blast furnace to prevent the sulphur from oxidizing as will be sufficient to furnish the heat for smelting. Such conditions existed at Silver City, N. M. , at times, after our surplus supply of iron and zinc sulphide concentrates was used. I understand that they are now short of sulphur there, on account of getting a surplus amount of oxidized copper ore, and are only utilizing what little heat the slag gives them, without the addition of any fuel on top of the forehearth.
Before closing this, which I intended to have been brief, I wish to call your attention to a little experience we had with alumina in the matting furnace at Silverton, Col., where I was acting as consulting metallurgist. The ore we had to smelt contained, on an average, about 20 per cent. Al2O3, 30 per cent. SiO2, with 18 per cent. Fe in the form of an iron pyrite, and no other iron was available except some iron sulphide concentrates containing a small percentage of zinc and lead.
The question naturally arose, could we oxidize and force sufficient of the iron into the slag, and where should we class 260 the alumina, as a base or an acid? My experience in lead smelting led me to believe that Al2O3 could only be classed as an acid in the ordinary lead furnace, and that it would be useless to class it otherwise in a shallow matting furnace; and E. W. Walter, the superintendent and metallurgist in charge, agreed with me.
We then decided to make a bisilicate slag, classing the alumina as silica, and we obtained fairly satisfactory results. The slag made was very clean, but treacherous, which was attributed to two reasons: First, that it required more heat to keep the alumina slag liquid enough to flow than it does a nearly straight silica slag; and, second, that we were running so close to the formula of a bisilicate and aluminate slag (about 31½ per cent. SiO2, 27 per cent. Fe, 20 per cent. CaO, and 18 per cent. Al2O3, or 49½ per cent. acid) that a few charges thrown into the furnace containing more silica or alumina than usual would thicken the slag so that it would then require some extra coke and flux to save the furnace. At times the combined SiO2 and Al2O3 did reach 55 and 56 per cent. in the slag, which did not freeze up the furnace, but caused us trouble.
(October 3, 1903)
In presenting this account of the Parkes process of desilverizing and refining lead bullion no originality is claimed, but I hope that a description of the process as carried out at the works of the Smelting Company of Australia may be of service.
Introductory.—The Parkes process may be conveniently summarized as follows:
1. Softening of the base bullion to remove copper, antimony, etc.
2. Removal of precious metals from the softened bullion by means of zinc.
3. Refining the desilverized lead.
4. Liquation of gold and silver crusts obtained from operation No. 2.
5. Retorting the liquated alloy to drive off zinc.
6. Concentrating and refining bullion from No. 5.
Softening.—This is done in reverberatory furnaces. In large works two furnaces are used, copper, antimony, and arsenic being removed in the first and antimony in the second. The size of the furnaces is naturally governed by the quantity to be treated. In these works (refining about 200 tons weekly) a double set of 15-ton furnaces were at work. The sides and ends of these furnaces are protected by a jacket with a 2-in. water space, the jacket extending some 3 in. above the charge level and 6 in. to 9 in. below it. The furnace is built into a wrought-iron pan, and if the brickwork is well laid into the pan there need be no fear of lead breaking through below the jacket.
The bars of bullion (containing, as a rule, 2 to 3 per cent. of impurities) are placed in the furnace carefully, to avoid injuring the hearth, and melted down slowly. The copper dross separates 264 out and floats on top of the charge, which is stirred frequently to expose fresh surfaces. If the furnace is overheated some dross is melted into the lead again and will not separate out until the charge is cooled back. However carefully the work is done some copper remains with the lead, and its effects are to be seen in the later stages. The dross is skimmed into a slag pot with a hole bored in it some 4 in. from the bottom; any lead drained from the pot is returned to the charge. The copper dross is either sent back to the blast furnace direct or may be first liquated. By the latter method some 30 per cent. of the lead contents of the dross is recovered in the refinery.
Base bullion made at a customer’s smelter will often vary greatly in composition, and it is, therefore, difficult to give any hard and fast figures as to percentage of metals in the dross. As a rule our dross showed 65 to 70 per cent. lead, copper 2 to 9 per cent. (average 4 per cent.), gold and silver values varying with the grade of the original bullion, though it was difficult to detect any definite relation between bullion and dross. It was, however, noticed that gold and silver values increased with the percentage of copper.
Immediately the copper dross is skimmed off the heat is raised considerably, and very soon a tin (and arsenic, if present) skimming appears. It is quite “dry” and may be removed in an hour or so. It is a very small skimming, and the tin, not being worth saving, is put with the copper dross.
The temperature is now raised again and antimony soon shows in black, boiling, oily drops, gathering in time into a sheet covering the surface of the lead. When the skimming is about ½-inch thick, slaked lime, ashes, or fine coal is thrown on and stirred in. The dross soon thickens up and may be skimmed off easily. This operation is repeated until all antimony is eliminated. Constant stirring of the charge is necessary. The addition of litharge greatly facilitates the removal of antimony; either steam or air may be blown on the surface of the metal to hasten oxidation, though they have anything but a beneficial effect on the furnace lining. From time to time samples of the dross are taken in a small ladle, and after setting hard the sample is broken in two. A black vitreous appearance indicates plenty of antimony yet in the charge. Later samples will look less black, until finally a few yellowish streaks are seen, being the first 265 appearance of litharge. When all antimony is out the fracture of a sample should be quite yellow and the grain of the litharge long, a short grain indicating impurities still present, in which case another skimming is necessary. The analysis of a representative sample of antimony dross was as follows:
| PbO = | 78.11 per cent. |
| Sb2O4 = | 8.75 ” |
| As2O3 = | 2.18 ” |
| CuO = | 0.36 ” |
| CaO = | 1.10 ” |
| Fe2O3 = | 0.42 ” |
| Al2O3 = | 0.87 ” |
| Insol. = | 4.10 ” |
Antimony dross is usually kept separate and worked up from time to time, yielding hard antimonial lead, used for type metal, Britannia metal, etc.
Desilverization.—The softening being completed the charge is tapped and run to a kettle or pan of cast iron or steel, holding, when conveniently full, some 12 or 13 tons. The lead falling into the kettle forms a considerable amount of dross, which is skimmed off and returned to the softening furnace. By cooling down the charge until it nearly “freezes” an additional copper skimming is obtained, which also is returned to the softener. The kettle is now heated up to the melting point of zinc, and the zinc charge, determined by the gold and silver contents of the kettle, is added and melted. The charge is stirred, either by hand or steam, for about an hour, after which the kettle is allowed to cool down for some three hours and the first zinc crust taken off. When the charge is skimmed clean a sample of the bullion is taken for assay, and while this is being done the kettle is heated again for the second zinc charge, which is worked in the same way as the first; sometimes a third addition of zinc is necessary. The resulting crusts are kept separate, the second and third being added to the next charge as “returns,” allowing 3 lb. of zinc in returns as equal to 1 lb. of fresh zinc. An alternative method is to take out gold and silver in separate crusts, in which case the quantity of zinc first added is calculated on the gold contents of the kettle only. The method of working is the same, though subsequent treatment may differ in that the gold crusts are cupeled direct.
As to the quantity of zinc required:
1. Extracting the gold with as little silver as possible, the following figures were obtained:
| Total Gold in Kettle, oz. |
Amount taken out by 1 lb. zinc, oz. |
|---|---|
| 300 | 1.00 |
| 200 | 1.00 |
| 150 | 0.79 |
| 100 | 0.59 |
| 60 | 0.45 |
2. Silver zinking gave the following general results with 11-ton charges:
| Total Silver in Kettle, oz. |
Amount taken out by 1 lb. zic, oz. |
|---|---|
| 1,200 | 4.1 |
| 930 | 3.8 |
| 755 | 3.5 |
| 616 | 3.4 |
| 460 | 2.6 |
3. Extracting gold and silver together:
| Total Contents of Kettle | 1 Lb. Zinc Takes Out | ||
|---|---|---|---|
| Au. Oz. | Ag. Oz. | Au. Oz. | Ag. Oz. |
| 494 | 3,110 | 0.59 | 3.60 |
| 443 | 1,883 | 0.64 | 2.80 |
| 330 | 2,417 | 0.45 | 3.34 |
| 204 | 1,638 | 0.36 | 2.86 |
| 143 | 1,330 | 0.28 | 2.65 |
| 123 | 1,320 | 0.23 | 2.54 |
It will be noticed that in each case the richer the bullion the greater the extractive power of zinc. Experiments made on charges of rich bullion showed that the large amount of zinc called for by the table in use was unnecessary, and 250 lb. was fixed on as the first addition of zinc. On this basis an average of 237 charges gave results as follows:
| Total Contents | Zinc Used Lbs. | 1 Lb. Zinc Takes Out | ||
|---|---|---|---|---|
| Au. Oz. | Ag. Oz. | Au. Oz. | Ag. Oz. | |
| 520 | 1,186 | 507.5 | 1.27 | 2.91 |
The zinc used was that necessary to clean the kettle, added as follows: 1st, 250 lb.; 2d (average), 127 lb.; 3d (average), 57 lb. In 112 cases no third addition was required. From these figures it appears that in the earlier work the zinc was by no means saturated.
Refining the Lead.—Gold and silver being removed, the lead is siphoned off into the refining kettle and the fire made up. In about four hours the lead will be red hot, and when hot enough to burn zinc, dry steam, delivered by a ¾ in. pipe reaching nearly to the bottom of the kettle, is turned on. The charge is stirred from time to time and wood is fed on the top to assist dezinking and prevent the formation of too much litharge. In three to four hours the lead will be soft and practically free from zinc. When test strips show the lead to be quite soft and clean, the kettle is cooled down and the scum of lead and zinc oxides skimmed off. In an hour or so the lead will be cool enough for molding; the bar should have a yellow luster on the face when set; if the lead is too cold it will be white, if too hot a deep blue. The refining kettles are subjected to severe strain during the steaming process, and hence their life is uncertain—an average would be about 60 charges; the zinking kettles, on the other hand, last very much longer. Good steel kettles (if they can be obtained) are preferable to cast iron.
Treatment of Zinc Crusts.—Having disposed of the lead, let us return now to the zinc crusts. These are first liquated in a small reverberatory furnace, the hearth of which is formed of a cast-iron plate (the edges of the long sides being turned up some 4 in.) laid on brasque filling, with a fall from bridge to flue of ¾ in. per foot and also sloping from sides to center. The operation is conducted at a low temperature and the charge is turned over at intervals, the liquated lead running out into a small separately fired kettle. This lead rarely contains more than a few ounces of silver per ton; it is baled into bars, and returned to the zinking kettles or worked up in a separate charge. In two to three hours the crust is as “dry” as it is advisable to make it, and the liquated alloy is raked out over a slanting perforated plate to break it up and goes to the retort bin.
Retorting the Alloy.—This is carried on in Faber du Faur tilting furnaces—simply a cast-iron box swinging on trunnions and lined with firebrick. Battersea retorts (class 409) holding 560 lb. each are used; their average life is about 30 charges. The retorts are charged hot, a small shovel of coal being added with the alloy. The condenser is now put in place and luted on; it is made of ⅛ in. iron bent to form a cylinder 12 in. in diameter, open at one end; it is lined with a mixture of lime, clay and 268 cement. It has three holes, one on the upper side close to the furnace and through which a rod can be passed into the retort, a vent-hole on the upper side away from the furnace, and a tap-hole on the bottom for condensed zinc. In an hour or so the flame from the vent-hole should be green, showing that distillation has begun. When condensation ceases (shown by the flame) the condenser is removed and the bullion skimmed and poured into bars for the cupel. The products of retorting are bullion, zinc, zinc powder and dross. Bullion goes to the cupel, zinc is used again in the desilverizing kettles, powder is sieved to take out scraps of zinc and returned to the blast furnace, or it may be, and sometimes is, used as a precipitating agent in cyanide works; dross is either sweated down in a cupel with lead and litharge, together with outside material such as zinc gold slimes from cyanide works, jeweler’s sweep, mint sweep, etc., or in the softening furnace after the antimony has been taken off. In either case the resulting slag goes back to the blast furnace. The total weight of alloy treated is approximately 7 per cent. of the original base bullion. The zinc recovered is about 60 per cent. of that used in desilverizing. The most important source of temporary loss is the retort dross (consisting of lead-zinc-copper alloy with carbon, silica and other impurities), and it is here that the necessity of removing copper in the softening process is seen, since any copper comes out with the zinc crusts and goes on to the retorts, where it enters the dross, carrying gold and silver with it. If much copper is present the dross may contain more gold and silver than the retort bullion itself. In this connection I remember an occasion on which some retort dross yielded gold and silver to the extent of over 800 and 3000 oz. per ton respectively.
Cupellation.—Retort bullion is first concentrated (together with bullion resulting from dross treatment) to 50 to 60 per cent. gold and silver in a water-jacketed cupel. The side lining is protected by an inch water-pipe imbedded in the lining at the litharge level or by a water-jacket, the inner face of which is of copper; the cupel has also a water-jacketed breast so that the front is not cut down. The cupel lining may be composed of limestone, cement, fire-clay and magnesite in various proportions, but a simple lining of sand and cement was found quite satisfactory. When the bullion is concentrated up to 50 to 60 per cent. gold and silver, it is baled out and transferred to the finishing 269 cupel, where it is run up to about 0.995 fine; it is then ready either for the melting-pot or parting plant. The refining test, by the way, is not water-cooled.
Re-melting is done in 200-oz. plumbago crucibles and presents no special features. In the case of doré bullion low in gold, “sprouting” of the silver is guarded against by placing a piece of wood or charcoal on the surface of the metal before pouring, and any slag is kept back. The quantity of slag formed is, of course, very small, so that the bars do not require much cleaning.
The parting plant was not in operation in my time, and I am therefore unable to go into details. The process arranged for was briefly as follows: Solution of the doré bullion in H2SO4; crystallization of silver as monosulphate by dilution and cooling; decomposition of silver sulphate by ferrous sulphate solution giving metallic silver and ferric sulphate, which is reduced to the ferrous salt by contact with scrap iron. The gold and silver are washed thoroughly with hot water and cast into bars.
In conclusion, some variations in practice may be noted. The use of two furnaces in the softening process has already been mentioned; by this means the drossing and softening are more perfect and subsequent operations thereby facilitated; further, the furnaces, being kept at a more equable temperature, are less subject to wear and tear. Zinc crusts are sometimes skimmed direct into an alloy press in which the excess of lead is squeezed out while still molten; liquation is then unnecessary. Refining of the lead may be effected by a simple scorification in a reverberatory, the soft lead being run into a kettle from which it is molded into market bars.
(October 11, 1902)
Important changes in lead-refining practice are bound to follow, in my opinion, the late demonstration on a large scale of the low working cost and high efficiency of Betts’ electrolytic process of refining lead bullion. It was my good fortune recently to see this highly interesting process in operation at Trail, British Columbia, through the kindness of the inventor, A. G. Betts, and Messrs. Labarthe and Aldridge, of the Trail works.
A plant of about 10 tons daily capacity, which probably cost about $25,000, although it could be duplicated for perhaps $15,000 at the present time, was installed near the Trail smelting works. It has been in operation for about ten months, I am informed, with signal success, and the erection of a larger plant, of approximately 30 tons capacity and provided with improved handling facilities, is now completed.
The depositing-room contains 20 tanks, built of wood, lined with tar, and approximately of the size of copper-refining tanks. Underneath the tank-room floor is a basement permitting inspection of the tank bottoms for possible leakage and removal of the solution and slime. A suction pump is employed in lifting the electrolyte from the receiving tank and circulating the solution. In nearly every respect the arrangement of the plant and its equipment is strikingly like that of a modern copper refinery.
The great success of the process is primarily based upon Betts’ discovery of the easy solubility of lead in an acid solution of lead fluosilicate, which possesses both stability under electrolysis and high conductivity, and from which exceptionally pure lead may be deposited with impure anodes at a very low cost. With such a solution, there is no polarization from formation of lead peroxide on the anode, no evaporation of constituents except water, and no danger in its handling. It is cheaply 271 obtained by diluting hydrofluoric acid of 35 per cent. HF, which is quoted in New York at 3c. per pound, with an equal volume of water and saturating it with pulverized quartz according to the equation:
SiO2 + 6HF = HSiF6 + 2H2O.
According to Mr. Betts, an acid of 20 to 22 per cent. will come to about $1 per cu. ft., or to $1.25 when the solution has been standardized with 6 lb. of lead. One per cent. of lead will neutralize 0.7 per cent. H2SiF6. The electrolyte employed at the time of my inspection of the works contained, I believe, 8 per cent. lead and 11 per cent. excess of fluosilicic acid.
The anodes consist of the lead bullion to be refined, cast into plates about 2 in. thick and approximately of the same size as ordinary two-lugged copper anodes. Before being placed in position in the tanks, they are straightened by hammering over a mold and their lugs squared. No anode sacks are employed as in the old Keith process.
The cathode sheets which receive the regular lead deposits are thin lead plates obtained by electrodeposition upon and stripping from special cathodes of sheet steel. The latter are prepared for use by cleaning, flashing with copper, lightly lead-plating in the tanks, and greasing with a benzine solution of paraffin, dried on, from which the deposited lead is easily stripped.
The anodes and cathodes are separated by a space of 1½ to 2 in. in the tank and are electrically connected in multiple, the tanks being in series circuit. The fall in potential between tanks is only about 0.2 of a volt, which remarkably low voltage is due to the high conducting power of the electrolyte and to some extent to the system of contacts used. These contacts are small wells of mercury in the bus-bars, large enough to accommodate copper pins soldered to the iron cathodes or clamped to the anodes. Only a small amount of mercury is required.
Current strengths of from 10 to 25 amperes per sq. ft. have been used, but at Trail 14 amperes have given the most satisfactory results as regards economy of working and the physical and chemical properties of the refined metal produced.
A current of 1 ampere deposits 3.88 grams of lead per hour, or transports 3¼ times as much lead, in this case, as copper with an ordinary copper-refining solution. A little over 1000 kg., or 272 2240 lb., requires about 260,000 ampere hours. At 10 amperes per sq. ft. the cathode (or anode) area should be about 1080 sq. ft. per ton of daily output. Taking a layer of electrolyte 1.5 in. thick, 135 cu. ft. will be found to be the amount between the electrodes, and 175 cu. ft. may be taken as the total quantity of solution necessary, according to Mr. Betts’ estimate. The inventor states that he has worked continuously and successfully with a drop of potential of only 0.175 volt per tank, and that therefore 0.25 volt should be an ample allowance in regular refining. Quoting Mr. Betts; “260,000 ampere hours at 0.25 volt works out to 87 electrical h.p. hours of 100 h.p. hours at the engine shaft, in round numbers. Estimating that 1 h.p. hour requires the burning of 1.5 lb. of coal, and allowing say 60 lb. for casting the anodes and refined lead, each ton of lead refined requires the burning of 210 lb. of fuel.” With coal at $6 per ton the total amount of fuel consumed, therefore, should not cost over 60c., which is far below the cost of fire-refining base lead bullion, as we know.
In the Betts electrolytic process, practically all the impurities in the base bullion remain as a more or less adherent coating on the anode, and only the zinc, iron, cobalt and nickel present go into solution. The anode residue consists practically of all the copper, antimony, bismuth, arsenic, silver and gold contained in the bullion, and very nearly 10 per cent. of its weight in lead. Having the analysis of any bullion, it is easy to calculate with these data the composition of the anode residue and the rate of pollution of the electrolyte. Allowing 175 cu. ft. of electrolyte per ton of daily output, it will be found that in the course of a year these impurities will have accumulated to the extent of a very few per cent. Estimating that the electrolyte will have to be purified once a year, the amount to be purified daily is less than 1 cu. ft. for each ton of output. The amount of lead not immediately recovered in pure form is about 0.3 per cent., most of which is finally recovered. As compared with the ordinary fire-refined lead, the electrolytically refined lead is much purer and contains only mere traces of bismuth, when bismuthy base bullion is treated. Furthermore, the present loss of silver in fire refining, amounting, it is claimed, to about 1½ per cent. of the silver present, and covered by the ordinary loss in assay, is to a large extent avoided, as the silver in the electrolytic process is 273 concentrated in the anode residue with a very small loss, and the loss of silver in refining the slimes is much less than in treating the zinc crusts and refining the silver residue after distillation. The silver slimes obtained at Trail, averaging about 8000 oz. of gold and silver per ton, are now treated at the Seattle Smelting and Refining Works. There the slimes are boiled with concentrated sulphuric acid and steam, allowing free access of air, which removes the greater part of the copper. The washed residue is then dried in pans over steam coils, and melted down in a magnesia brick-lined reverberatory, provided with blast tuyeres, and refined. In this reverberatory furnace the remainder of the copper left in the slimes after boiling is removed by the addition of niter as a flux, and the antimony with soda. The doré bars finally obtained are parted in the usual way with sulphuric acid, making silver 0.999 fine and gold bars at least 0.992 fine.
Mr. Betts treated 2000 grams of bullion, analyzing 98.76 per cent. Pb, 0.50 Ag, 0.31 Cu, and 0.43 Sb with a current of 25 amp. per square foot in an experimental way, and obtained products of the following composition:
Refined Lead: 99.9971 per cent. Pb, 0.0003 Ag, 0.0007 Cu, and 0.0019 Sb.
Anode Residue: 9.0 per cent. Pb, 36.4 Ag, 25.1 Cu, and 2.95 Sb.
Four hundred and fifty pounds of bullion from the Compania Metalurgica Mexicana, analyzing 0.75 per cent. Cu, 1.22 Bi, 0.94 As, 0.68 Sb, and assaying 358.9 oz. Ag and 1.71 oz. Au per ton, were refined with a current of 10 amp. per square foot, and gave a refined lead of the following analysis: 0.00027 per cent. Cu, 0.0037 Bi, 0.0025 As, 0 Sb, 0.0010 Ag, 0.0022 Fe, 0.0018 Zn and Pb (by difference) 99.9861 per cent.
Although the present method for recovering the precious metals and by-products from the anode residue leaves much room for improvement, the use of the Betts process may be recommended to our lead refiners, because it is a more economical and efficient method than the fire-refining process now in common use. I will state my belief, in conclusion, that the present development of electrolytic lead refining signalizes as great an advance over zinc desilverization and the fire methods of refining lead as electrolytic copper refining does over the old Welsh method of refining that metal.
A solution of lead fluosilicate, containing an excess of fluosilicic acid, has been found to work very satisfactorily as an electrolyte for refining lead. It conducts the current well, is easily handled and stored, non-volatile and stable under electrolysis, may be made to contain a considerable amount of dissolved lead, and is easily prepared from inexpensive materials. It possesses, however, in common with other lead electrolytes, the defect of yielding a deposit of lead lacking in solidity, which grows in crystalline branches toward the anodes, causing short circuits. But if a reducing action (practically accomplished by the addition of gelatine or glue) be given to the solution, a perfectly solid and dense deposit is obtained, having very nearly the same structure as electrolytically deposited copper, and a specific gravity of about 11.36, which is that of cast lead.
Lead fluosilicate may be crystallized in very soluble brilliant crystals, resembling those of lead nitrate and containing four molecules of water of crystallization, with the formula PbSiF6,4H2O. This salt dissolves at 15 deg. C. in 28 per cent. of its weight of water, making a syrupy solution of 2.38 sp. gr. Heated to 60 deg. C., it melts in its water of crystallization. A neutral solution of lead fluosilicate is partially decomposed on heating, with the formation of a basic insoluble salt and free fluosilicic acid, which keeps the rest of the salt in solution. This decomposition ends when the solution contains perhaps 2 per cent. of free acid; and the solution may then be evaporated without further decomposition. The solutions desired for refining are not liable to this decomposition, since they contain much more than 2 per cent. of free acid. The electrical conductivity depends mainly on the acidity of the solution.
My first experiments were carried out without the addition 275 of gelatine to the fluosilicate solution. The lead deposit consisted of more or less separate crystals that grew toward the anode, and, finally, caused short circuits. The cathodes, which were sheet-iron plates, lead-plated and paraffined, had to be removed periodically from the tanks and passed through rolls, to pack down the lead. When gelatine has been added in small quantities, the density of the lead is greater than can be produced by rolling the crystalline deposit, unless great pressure is used.
The Canadian Smelting Works, Trail, B. C. , have installed a refinery, making use of this process. There are 28 refining-tanks, each 86 in. long, 30 in. wide and 42 in. deep, and each receiving 22 anodes of lead bullion with an area of 26 by 33 in. exposed to the electrolyte on each side, and 23 cathodes of sheet lead, about 1/16 in. thick, prepared by deposition on lead-plated and paraffined iron cathodes. The cathodes are suspended from 0.5 by 1 in. copper bars, resting crosswise on the sides of the tanks. The experiment has been thoroughly tried of using iron sheets to receive a deposit thicker than 1/16 in.; that is, suitable for direct melting without the necessity of increasing its weight by further deposition as an independent cathode; but the iron sheets are expensive, and are slowly pitted by the action of the acid solution; and the lead deposits thus obtained are much less smooth and pure than those on lead sheets.
The smoothness and the purity of the deposited lead are proportional. Most of the impurity seems to be introduced mechanically through the attachment of floating particles of slime to irregularities on the cathodes. The effect of roughness is cumulative; it is often observed that particles of slime attract an undue amount of current, resulting in the lumps seen in the cathodes. Samples taken at the same time showed from 1 to 2.5 oz. silver per ton in rough pieces from the iron cathodes, 0.25 oz. as an average for the lead-sheet cathodes, and only 0.04 oz. in samples selected for their smoothness. The variation in the amount of silver (which is determined frequently) in the samples of refined lead is attributed not to the greater or less turbidity of the electrolyte at different times, but to the employment of new men in the refinery, who require some experience before they remove cathodes without detaching some slime from the neighboring anodes.
Each tank is capable of yielding, with a current of 4000 276 amperes, 750 lb. of refined lead per day. The voltage required to pass this current was higher than expected, as explained below; and for this reason, and also because the losses of solution were very heavy until proper apparatus was put in to wash thoroughly the large volume of slime produced (resulting in a weakened electrolyte), the current used has probably averaged about 3000 amperes. The short circuits were also troublesome, though this difficulty has been greatly reduced by frequent inspection and careful placing of the electrodes. At one time, the solution in use had the following composition in grams per 100 c.c.: Pb, 6.07; Sb, 0.0192; Fe, 0.2490; SiF6, 6.93, and As, a trace. The current passing was 2800 amperes, with an average of about 0.44 volts per tank, including bus-bars and contacts. It is not known what was the loss of efficiency on that date, due to short circuits; and it is, therefore, impossible to say what resistance this electrolyte constituted.
Hydrofluoric acid of 35 per cent., used as a starting material for the preparation of the electrolyte, is run by gravity through a series of tanks for conversion into lead fluosilicate. In the top tank is a layer of quartz 2 ft. thick, in passing through which the hydrofluoric acid dissolves silica, forming fluosilicic acid. White lead (lead carbonate) in the required quantity is added in the next tank, where it dissolves readily and completely with effervescence. All sulphuric acid and any hydrofluoric acid that may not have reacted with silica settle out in combination with lead as lead sulphate and lead fluoride. Lead fluosilicate is one of the most soluble of salts; so there is never any danger of its crystallizing out at any degree of concentration possible under this method. The lead solution is then filtered and run by gravity into the refining-tanks.
The solution originally used at Trail contained about 6 per cent. Pb and 15 per cent. SiF6.
The electrical resistance in the tanks was found to be greater than had been calculated for the same solution, plus an allowance for loss of voltage in the contacts and conductors. This is partly, at least, due to the resistance to free motion of the electrolyte, in the neighborhood of the anode, offered by a layer of slime which may be anything up to ½ in. thick. During electrolysis, the SiF6 ions travel toward the anodes, and there combine with lead. The lead and hydrogen travel in the opposite 277 direction and out of the slime; but there are comparatively few lead ions present, so that the solution in the neighborhood of the anodes must increase in concentration and tend to become neutral. This greater concentration causes an e.m.f. of polarization to act against the e.m.f. of the dynamo. This amounted to about 0.02 volt for each tank. The greater effect comes from the greater resistance of the neutral solution with which the slime is saturated. There is, consequently, an advantage in working with rather thin anodes, when the bullion is impure enough to leave slime sticking to the plates. A compensating advantage is found in the increased ease of removing the slime with the anodes, and wiping it off the scrap in special tanks, instead of emptying the tanks and cleaning out, as is done in copper refineries.
It is very necessary to have adequate apparatus for washing solution out of the slime. The filter first used consisted of a supported filtering cloth with suction underneath. It was very difficult to get this to do satisfactory work by reason of the large amount of fluosilicate to be washed out with only a limited amount of water. At the present time the slime is first stirred up with the ordinary electrolyte several times, and allowed to settle, before starting to wash with water at all. The Trail plant produces daily 8 or 10 cu. ft. of anode residue, of which over 90 per cent. by volume is solution. The evaporation from the total tank surface of something like 400 sq. ft. is only about 15 cu. ft. daily; so that only a limited amount of wash-water is to be used—namely, enough to replace the evaporated water, plus the volume of the slime taken out.
The tanks are made of 2 in. cedar, bolted together and thoroughly painted with rubber paint. Any leakage is caught underneath on sloping boards. Solution is circulated from one tank to another by gravity, and is pumped from the lowest to the highest by means of a wooden pump. The 22 anodes in each tank together weigh about 3 tons, and dissolve in from 8 to 10 days, two sets of cathodes usually being used with each set of anodes. While 300 lb. cathodes can be made, the short-circuiting gets so troublesome with the spacing used that the loss of capacity is more disadvantageous than the extra work of putting in and taking out more plates. The lead sheets used for cathodes are made by depositing about 1/16 in. metal on paraffined steel sheets 278 in four of the tanks, which are different from the others only in being a little deeper.
The anodes may contain any or all of the elements, gold, silver, copper, tin, antimony, arsenic, bismuth, cadmium, zinc, iron, nickel, cobalt and sulphur. It would be expected that gold, silver, copper, antimony, arsenic and bismuth, being more electronegative than lead, would remain in the slime in the metallic state, with, perhaps, tin, while iron, zinc, nickel and cobalt would dissolve. It appears that tin stands in the same relation to lead that nickel does to iron, that is, they have about the same electromotive forces of solution, with the consequence that they can behave as one metal and dissolve and deposit together. Iron, contrary to expectation, dissolves only slightly, while the slime will carry about 1 per cent. of it. It appears from this that the iron exists in the lead in the form of matte. Arsenic, antimony, bismuth and copper have electromotive forces of solution more than 0.3 volt below that of lead. As there is no chance that any particle of one of these impurities will have an electric potential of 0.3 volt above that of the lead with which it is in metallic contact, there is no chance that they will be dissolved by the action of the current. The same is even more certainly true of silver and gold. The behavior of bismuth is interesting and satisfactory. It is as completely removed by this process of refining as antimony is. No other process of refining lead will remove this objectionable impurity so completely. Tin has been found in the refined lead to the extent of 0.02 to 0.03 per cent. This we had no difficulty in removing from the lead by poling before casting. There is always a certain amount of dross formed in melting down the cathodes; and the lead oxide of this reacts with the tin in the lead at a comparatively low temperature.
The extra amount of dross formed in poling is small, and amounts to less than 1 per cent. of the lead. The dross carries more antimony and arsenic than the lead, as well as all the tin. The total amount of dross formed is about 4 per cent. Table I shows its composition.
The electrolyte takes up no impurities, except, possibly, a small part of the iron and zinc. Estimating that the anodes contain 0.01 per cent. of zinc and soluble iron, and that there are 150 cu. ft. of the solution in the refinery for every ton of 279 lead turned out daily, in one year the 150 cu. ft. will have taken up 93 lb. of iron and zinc, or about 1 per cent. These impurities can accumulate to a much greater extent than this before their presence will become objectionable. It is possible to purify the electrolyte in several ways. For example, the lead can be removed by precipitation with sulphuric acid, and the fluosilicic acid precipitated with salt as sodium fluosilicate. By distillation with sulphuric acid the fluosilicic acid could be recovered, this process, theoretically, requiring but one-third as much sulphuric acid as the decomposition of fluorspar, in which the fluorine was originally contained.
The only danger of lead-poisoning to which the workmen are exposed occurs in melting the lead and casting it. In this respect the electrolytic process presents a distinct sanitary advance.
For the treatment of slime, the only method in general use consists in suspending the slime in a solution capable of dissolving the impurities and supplying, by a jet of steam and air forced into the solution, the air necessary for its reaction with, and solution of, such an inactive metal as copper. After the impurities have been mostly dissolved, the slime is filtered off, dried and melted, under such fluxes as soda, to a doré bullion.
The amount of power required is calculated thus: Five amperes in 24 hours make 1 lb. of lead per tank. One ton of lead equals 10,000 ampere-days, and at 0.35 volts per tank, 3500 watt-days, or 4.7 electric h.p. days. Allowing 10 per cent. loss of efficiency in the tanks (we always get less lead than the current which is passing would indicate), and of 8 per cent. loss in the generator, increases this to about 5.6 h.p. days, and a further allowance for the electric lights and other applications gives from 7 to 8 h.p. days as about the amount per ton of lead. At $30 per year, this item of cost is something like 65c. per ton of lead. So this is an electro-chemical process not especially favored by water-power.
The cost of labor is not greater than in the zinc-desilverization process. A comparison between this process and the Parkes process, on the assumption that the costs for labor, interest and general expenses are about equal, shows that about $1 worth of zinc and a considerable amount of coal and coke have been done away with, at the expense of power, equal to about 175 h.p. 280 hours, of the average value of perhaps 65c., and a small amount of coal for melting the lead in the electrolytic method.
More important, however, is the greater saving of the metal values by reason of increased yields of gold, silver, lead, antimony and bismuth, and the freedom of the refined lead from bismuth.
Tables II, III, and IV show the composition of bullion, slimes and refined lead.
Tables V, VI, VII, and VIII give the results obtained experimentally in the laboratory on lots of a few pounds up to a few hundred pounds. The results in Tables VI and VII were given me by the companies for which the experiments were made.
TABLE I.—ANALYSES OF DROSS
For analyses of the lead from which this dross was taken, see Table II
| No. | No. in Table II. |
Cu. Per Cent. |
As. Per Cent. |
Sb. Per Cent. |
Fe. Per Cent. |
Zn. |
|---|---|---|---|---|---|---|
| 1 | 2 | 0.0005 | 0.0003 | 0.0016 | 0.0016 | none |
| 2 | 3 | 0.0010 | 0.0008 | 0.0107 | 0.0011 | " |
TABLE II.—ANALYSES OF BULLION
| No. | Fe. Per Cent. |
Cu. Per Cent. |
Sb. Per Cent. |
Sn. Per Cent. |
As. Per Cent. |
Ag. Per Cent. |
Au. Per Cent. |
Pb. Per Cent. |
Ag. Per Cent. |
Au. Per Cent. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.0075 | 0.1700 | 0.5400 | 0.0118 | 0.1460 | 1.0962 | 0.0085 | 98.0200 | 319.7 | 2.49 |
| 2 | 0.0115 | 0.1500 | 0.6100 | 0.0158 | 0.0960 | 1.2014 | 0.0086 | 97.9068 | 350.4 | 2.52 |
| 3 | 0.0070 | 0.1600 | 0.4000 | 0.0474 | 0.1330 | 1.0738 | 0.0123 | 98.1665 | 313.2 | 3.6 |
| 4 | 0.0165 | 0.1400 | 0.7000 | 0.0236 | 0.3120 | 0.8914 | 0.0151 | 97.9014 | 260.0 | 4.42 |
| 5 | 0.0120 | 0.1400 | 0.8700 | 0.0432 | 0.2260 | 0.6082 | 0.0124 | 98.0882 | 177.4 | 3.63 |
| 6 | 0.0055 | 0.1300 | 0.7300 | 0.0316 | 0.1030 | 0.6600 | 0.0106 | 98.2693 | 192.5 | 3.10 |
| 7 | 0.0380 | 0.3600 | 0.4030 | — | tr. | 0.7230 | 0.0180 | 98.4580 | 210.9 | 5.25 |
TABLE III.—ANALYSES OF SLIMES
| Fe. Per Cent. |
Cu. Per Cent. |
Sb. Per Cent. |
Sn. Per Cent. |
As. Per Cent. |
Pb. Per Cent. |
Zn. Per Cent. |
Bi. Per Cent. |
|---|---|---|---|---|---|---|---|
| 1.27 | 8.83 | 27.10 | 12.42 | 28.15 | 17.05 | none | none |
| 1.12 | 22.36 | 21.16 | 5.40 | 23.05 | 10.62 | " | " |
TABLE IV.—ANALYSES OF REFINED LEAD
| No. | Cu. Per Cent. |
As. Per Cent. |
Sb. Per Cent. |
Fe. Per Cent. |
Zn. Per Cent. |
Sn. Per Cent. |
Ag. Oz. p. T. |
Ni,Co,Cd. Per Cent. |
Bi. Per Cent. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.0006 | 0.0008 | 0.0005 | — | — | — | — | — | — |
| 2 | 0.0003 | 0.0002 | 0.0010 | 0.0010 | none | — | — | — | — |
| 3 | 0.0009 | 0.0001 | 0.0009 | 0.0008 | " | — | 0.24 | — | — |
| 4 | 0.0016 | — | 0.0017 | 0.0014 | — | — | 0.47 | none | — |
| 5 | 0.0003 | — | 0.0060 | 0.0003 | — | — | 0.22 | — | — |
| 6 | 0.0020 | — | 0.0010 | 0.0046 | — | — | 0.22 | none | — |
| 7 | 0.0004 | none | 0.0066 | 0.0013 | none | 0.0035 | 0.14 | — | — |
| 8 | 0.0004 | — | 0.0038 | 0.0004 | " | 0.0035 | 0.25 | — | — |
| 9 | 0.0005 | — | 0.0052 | 0.0004 | " | 0.0039 | 0.28 | — | — |
| 10 | 0.0003 | none | 0.0060 | 0.0003 | " | 0.0049 | 0.43 | — | — |
| 11 | 0.0003 | " | 0.0042 | 0.0013 | " | 0.0059 | 0.32 | — | — |
| 12 | 0.0005 | " | 0.0055 | 0.0009 | " | 0.0049 | 0.22 | — | — |
| 13 | 0.0005 | " | 0.0055 | 0.0007 | " | 0.0091 | 0.11 | — | — |
| 14 | 0.0004 | " | 0.0063 | 0.0005 | " | 0.0012 | 0.14 | — | — |
| 15 | 0.0003 | " | 0.0072 | 0.0003 | " | 0.0024 | 0.24 | — | — |
| 16 | 0.0006 | " | 0.0062 | 0.0012 | " | 0.0083 | 0.22 | — | — |
| 17 | 0.0006 | " | 0.0072 | 0.0011 | — | 0.0080 | 0.23 | — | — |
| 18 | 0.0006 | " | 0.0057 | 0.0010 | — | 0.0053 | 0.34 | — | — |
| 19 | 0.0005 | " | 0.0066 | 0.0016 | — | 0.0140 | 0.38 | — | — |
| 19 | 0.0005 | " | 0.0044 | 0.0011 | — | 0.0108 | 0.35 | — | — |
| 20 | 0.0004 | " | 0.0047 | 0.0015 | — | 0.0072 | 0.22 | — | — |
| 20 | 0.0004 | " | 0.0034 | 0.0016 | — | trace | 0.23 | — | — |
| 21 | 0.0022 | " | 0.0010 | 0.0046 | none | 0.0081 | 0.38 | none | none |
TABLE V.—ANALYSES OF BULLION AND REFINED LEAD
| Ag. Per Cent. |
Cu. Per Cent. |
Sb. Per Cent. |
Pb. Per Cent. |
|
|---|---|---|---|---|
| Bullion | 0.50 | 0.31 | 0.43 | 98.76 |
| Refined lead | 0.0003 | 0.0007 | 0.0019 | 99.9971 |
TABLE VI.—ANALYSES OF BULLION AND REFINED LEAD
| Cu. Per Ct. |
Bi. Per Ct. |
As. Per Ct. |
Sb. Per Ct. |
Ag. Per Ct. |
Ag. Per Ct. |
Au. Oz. p.T. |
Fe. Per Ct. |
Zn. Per Ct. |
|
|---|---|---|---|---|---|---|---|---|---|
| Bullion | 0.75 | 1.22 | 0.936 | 0.6832 | 358.89 | — | 1.71 | — | — |
| Refined lead | 0.0027 | 0.0037 | 0.0025 | 0.0000 | — | 0.0010 | none | 0.0022 | 0.0018 |
TABLE VII.—ANALYSES OF BULLION, REFINED LEAD AND SLIMES
| Pb. Per Cent. |
Cu. Per Cent. |
As. Per Cent. |
Sb. Per Cent. |
Ag. Oz.p.T. |
Ag. Per Cent. |
Fe,Zn,Ni,Co. Per Cent. |
Bi. Per Cent. |
|
|---|---|---|---|---|---|---|---|---|
| Bullion | 96.73 | 0.096 | 0.85 | 1.42 | about 275[51] | — | — | — |
| Refined lead | — | 0.0013 | 0.00506 | 0.0028 | — | 0.00068 | 0.0027 | trace |
| Slimes (dry sample) |
9.05 | 1.9 | 9.14 | 29.51 | 9366.9 | — | 0.49 | trace |
TABLE VIII.—ANALYSES OF BULLION, REFINED LEAD AND SLIMES
| Pb. Per Cent. |
Cu. Per Cent. |
Bi. Per Cent. |
Ag. Per Cent. |
Sb. Per Cent. |
As. Per Cent. |
|
|---|---|---|---|---|---|---|
| Bullion | 87.14 | 1.40 | 0.14 | 0.64 | 4.0 | 7.4 |
| Lead | — | 0.0010 | 0.0022 | — | 0.0017 | trace |
| Slimes | 10.3 | 9.3 | 0.52 | 4.7 | 25.32 | 44.58 |
(April 19, 1902)
In July, 1901, the El Paso, Texas, plant of the Consolidated Kansas City Smelting and Refining Company[52] was almost completely destroyed by fire. The power plant, blast-furnace building and blast furnaces were entirely destroyed, and portions of the other buildings were badly damaged. The flames were hardly extinguished before steps were taken to construct a new, modern and enlarged plant on the ruins of the old one, and on April 15, 1902, nine months after the destruction of the former plant, the new furnaces were blown in. In rebuilding it was decided to locate the new power-house at some distance from the other buildings. The furnaces have all been enlarged, each of the new lead furnaces (of which there are seven) having about 200 tons daily capacity. These and the three large copper furnaces have been located in a new position in order to secure a larger building territory. The entire plant is modern and up to date in every particular. One of the interesting features is the substitution of crude oil as fuel in the boiler and roasting departments. It is intended to use Beaumont petroleum for the generation of power and the roasting of the ores instead of wood, coal or coke, and it is expected that a considerable economy will be effected by this means.
Power Plant.—The power plant is complete in all respects. It is a duplicate plant in every sense of the word, so that it will never be necessary to shut the works down on account of the failure of any one piece of machinery. There are seven boilers, having a total of 1250 h.p. The four blowers are unusually large, having a capacity of 30,000 cu. ft. of free air per minute. They are direct-connected to three tandem compound condensing Corliss engines. No belts are used in this plant, except for driving a small blower of 10,000 cu. ft. capacity, which will act as a regulator. A large central electric plant has been installed in the power-house, consisting of two direct-connected, direct-current generators, mounted on the shafts of two cross-compound condensing Nordberg-Corliss engines. The current from these 286 generators is transmitted through the plant, operating sampling works, briquetting machinery, pumps, hoists, motors, cars, etc., displacing all the small steam engines and steam pumps used in the old plant. The power plant is provided with two systems for condensing; one being a large Wheeler surface condenser, the other a Worthington central-elevated jet condenser, the idea being to use the surface condenser during a short period of the year when the water is so bad that it cannot be used in the boilers. During the remainder of the year the jet condenser is in service and the surface condenser can be cleaned. The condensed steam from the surface condenser, with the necessary additional water, goes back directly to the boilers when the surface condenser is in use. The power-house is absolutely fireproof throughout, being of steel and brick with iron and cement floors. It is provided with a traveling crane, and no expense has been spared to make this, as all other parts of the plant, complete in every respect. The main conductors from the generators pass out through a tunnel into a brick and steel lightning-arrester house, from which point the various distributing lines go to different parts of the plant.
Blast Furnaces.—There are seven large lead furnaces, each having a capacity of 200 to 250 tons of charge per day, and three large copper furnaces, each having a capacity of 250 to 300 tons per day. All of the furnaces are enclosed in one steel fireproof building, the lead furnaces being at one end and the copper furnaces at the other. Each of the furnaces has its independent flue system and stack. An entirely new system of feeding these furnaces has been devised, consisting of a 6 ton charge car operated by means of a street railroad motor and controller with third-rail system. The charge cars collect their charge at the ore beds, lime-rock and coke storage, and are run on to 15 ton hydraulic elevators. They are then elevated 38 ft. to the top of the furnaces, traveling over them to the charging doors, through which the loads are dumped directly into the furnaces. This system permits of two men handling about 1000 tons per day. The same system and cars are used for charging the copper furnaces, except that, as these furnaces are much lower than the lead furnaces, the charge is dropped into a large hopper, from which it is fed to the copper furnaces by a man on the copper furnace feed-floor level.
(June 28, 1902)
Murray is a few miles south of Salt Lake City, with which it is connected by a trolley line. The new works are situated within a few hundred yards of the terminus of the latter and in close juxtaposition to the old Germania plant, which is the only one of the Salt Lake lead-smelting works in operation at present. The new plant is of special interest inasmuch as it is the latest construction for silver-lead smelting in the United States, and may be considered as embodying the best experience in that industry, the designers having had access to the results attained at almost all of the previous installations. It will be perceived, however, that there has been no radical departure in the methods, and the novelties are rather in details than in the general scheme.
The new works are built on level ground; there has been no attempt to seek or utilize a sloping or a terraced surface, save immediately in front of the blast furnaces, where there is a drop of several feet from the furnace-house floor to the slag-yard level, affording room for the large matte settling-boxes to stand under the slag spouts. A lower terrace beyond the slag yard furnishes convenient dumping ground. Otherwise the elevations required in the works are secured by mechanical lifts, the ore, fluxes and coal being brought in almost entirely by means of inclines and trestles.
The plant consists essentially of two parts, the roasting department and the smelting department. The former comprises a crushing mill and two furnace-houses, one equipped with Brückner furnaces and the other with hand-raked reverberatories. The reverberatories are of the standard design, but are noteworthy for the excellence of their construction. Similar praise may be, indeed, extended to almost all the other parts of the works, in which obviously no expense has been spared on 288 false grounds of economy. The roasting furnaces stand in a long steel house; they are set at right angles to the longer axis of the building, in the usual manner. At their feed end they communicate with a large dust-settling flue, which leads to the main chimney of the works. The ore is brought in on a tramway over the furnaces and is charged into the furnaces through hoppers. The furnaces have roasting hearths only. The fire-boxes are arranged with step-grates and closed ash-pits, being fed through hoppers at the end of the furnace. The coal is dumped close at hand from the railway cars, which are switched in on a trestle parallel with the side of the building, which side is not closed in. This, together with a large opening in the roof for the whole length of the building, affords good light and ventilation. The floor of the house is concrete. The roasted ore is dropped into cars, which run on a sunken tramway passing under the furnaces. At the end of this tramway there is an incline up which the cars are drawn and afterward dumped into brick bins. From the latter it is spouted into standard-gage railway cars, by which it is taken to the smelting department. The roasted ore from the Brückner furnaces is handled in a similar manner. The delivery of the coal and ore to the Brückners and the general installation of the latter are analogous to the methods employed in connection with the reverberatories.
The central feature of the smelting department is the blast-furnace house, which comprises eight furnaces, each 48 by 160 in. at the tuyeres. In their general design they are similar to those at the Arkansas Valley works at Leadville. There are 10 tuyeres per side, a tuyere passing through the middle of each jacket, the latter being of cast iron and 16 in. in width; their length is 6 ft., which is rather extraordinary. The furnaces are very high and are arranged for mechanical charging, a rectangular brick down-take leading to the dust chamber, which extends behind the furnace-house. The furnace-house is erected entirely of steel, the upper floor being iron plates laid on steel I-beams, while the upper terrace of the lower floor is also laid with iron plates. As previously remarked, the lower floor drops down a step in front of the furnaces, but there is an extension on each side of every furnace, which affords the necessary access to the tap-hole. The hight of the latter above the lower terrace leaves room for the large matte settling-boxes, and the matte 289 tapped from the latter runs into pots on the ground level, dispensing with the inconvenient pits that are to be seen at some of the older works. The construction of the blast furnaces, which were built by the Denver Engineering Works Company, is admirable in all respects. The eight furnaces stand in a row, about 30 ft. apart, center to center. The main air and water pipes are strung along behind the furnaces. The slag from the matte-settling boxes overflows into single-bowl Nesmith pots, which are to be handled by means of small locomotives. The foul slag is returned by means of a continuous pan-conveyor to a brick-lined, cylindrical steel tank behind the furnace-house, whence it is drawn off through chutes, as required for recharging.
The charges are made up on the ground level, immediately behind the furnace-house. The ore and flux are brought in on trestles, whence the ore is unloaded into beds and the flux into elevated bins. These are all in the open, there being only two small sheds where the charges are made up and dumped into the cars which go to the furnaces. There are two inclines to the latter. At the top of the inclines the cars are landed on a transferring carriage by which they can be moved to any furnace of the series.
The dust-flue extending behind the furnace-house is arranged to discharge into cars on a tramway in the cut below the ground level. This flue, which is of brick, connects with the main flues leading to the chimney. The main flues are built of concrete, laid on a steel frame in the usual manner, and are very large. For a certain distance they are installed in triplicate; then they make a turn approximately at right angles and two flues continue to the chimney. At the proper points there are large dampers of steel plate, pivoted vertically, for the purpose of cutting out such section of flue as it may be desired to clean. Each flue has openings, ordinarily closed by steel doors, which give access to the interior. The flues are simple tunnels, without drift-walls or any other interruption than the arched passages which extend transversely through them at certain places. The chimney is of brick, circular in section, 20 ft. in diameter and 225 ft. high. This is the only chimney of the works save those of the boiler-house.
The boiler-house is equipped with eight internally fired corrugated fire-box boilers. They are arranged in two rows, face to 290 face. Between the rows there is an overhead coal bin, from which the coal is drawn directly to the hoppers of the American stokers, with which the boilers are provided. Adjoining the boiler-house is the engine-house; the latter is a brick building, very commodious, light and airy. It contains two cross-compound, horizontal Allis-Chalmers (Dickson) blowing engines for the blast furnaces, and two direct-connected electrical generating sets for the development of the power required in various parts of the works. A traveling crane, built by the Whiting Foundry Equipment Company, spans the engine-house. In close proximity to the engine-house there is a well-equipped machine shop. Other important buildings are the sampling mill and the flue-dust briquetting mill.
A noteworthy feature of the new plant is the concrete paving, laid on a bed of broken slag, which is used liberally about the ore-yard and in other places where tramming is to be done. The roasting-furnace houses are floored with the same material, which not only gives an admirably smooth surface, but also is durable. The whole plant is well laid out with service tramways and standard-gage spur tracks; the intention has been, obviously, to save manual labor as much as possible.
(May 26, 1906)
This plant has been in operation since June, 1902. It gives employment to 800 men. The monthly production consists of about 4000 tons of work-lead and 700 tons of lead-copper matte (12 per cent. lead, 45 per cent. copper). The work-lead is sent to the refinery at Omaha; the matte to Pueblo, Colo. Most of the ores come from Utah; but in addition some richer lead ores are obtained from Idaho, and some gold-bearing ores from Nevada.
For sampling the Vezin apparatus is used, cutting out one-fifth in each of three passes, crushing intervening, the sample from the third machine being 1/625 of the original ore; after further comminution of sample in a coffee-mill grinder, it is cut down further by hand, using a riffle. The final sample is bucked down to pass an 80-mesh sieve, but gold ores are put through a 120-mesh.
The steps in the smelting process are as follows: Roasting the poorer ores in reverberatory furnaces and in Brückner cylinders. Smelting raw and roasted ores, mixed, in water-jacketed blast furnaces, for work-lead and lead-copper matte, the latter containing 15 per cent. lead and 10 to 12 per cent. copper. Roasting the ground matte, containing 22 per cent. of sulphur, down to ¾ per cent. in reverberatory furnaces. Smelting the roasted matte together with acid flux in the blast furnace for a matte with 45 per cent. copper and 12 per cent. lead.
Only the pyritic ores are roasted in Brückner furnaces, the lead ores and matte being roasted in reverberatory furnaces. Each of the 20 Brückner furnaces, which constitute one battery, roasts 8 to 12 tons of ore in 24 hours down to 5½ to 6 per cent. sulphur, with a coal consumption of two tons. The charge weighs 24 tons. The furnaces make one turn in 40 minutes. To increase 292 the draft and the output, steam at 40 lb. pressure is blown in through a pipe; this has, however, resulted in increasing the quantity of flue dust to 10 to 15 per cent. of the ore charged. Ten furnaces are attended by one workman with one assistant, working in eight-hour shifts. For firing and withdrawing the charge five men are required.
The gases from the Brückners and reverberatory furnaces pass into a dust-flue 14 × 14 ft. in section and 600 ft. long, built of brickwork, with concrete vault; in the stack (225 ft. high, 20 ft. diameter) they unite with the shaft-furnace gases, the temperature of which is only 60 deg.
There are 12 reverberatory furnaces with hearths 60 ft. long and 16 ft. broad. They roast 14 tons of ore (or 13 tons of matte) in 24 hours down to 3½ to 4 per cent. sulphur, consuming 32 to 34 per cent. of coal figured on the weight of the charge. There are 12 working doors on each side. The small coal (from Rock Springs, Wyoming), which is burnt on flat grates, contains 5 per cent. ash and 3 to 5 per cent. moisture. The roasted product is dumped through an opening in the hearth, ordinarily kept closed with an iron plate, into cars which are raised by electricity on a self-acting inclined plane. Their content is then tipped over into a chute and cooled by sprinkling with water. From here the roasted matte is conveyed to the blast furnace in 30-ton cars. The roasted ore is tipped into the ore-bins.
There are eight blast furnaces, 48 × 160 in. at the tuyeres, of which there are 10 on each of the long sides. The hight from the tuyeres to the gas outlet is 20 ft., thence to the throat 6 ft.; the distance of the tuyeres from the floor is 4 ft. The base is water-cooled. The water-jackets of the furnace are 6 ft. high. The tuyeres (4 in.) are provided with the Eilers automatic arrangement for preventing the furnace gases entering the blast pipes. The blast pressure is 34 oz. The furnaces are furnished with the Arents lead wells; the crucible holds about 30 tons of lead. The slag and the matte run into a brick-lined forehearth (8 × 3 ft., 4 ft. deep), from which the slag flows into pots holding 30 cu. ft., while the matte is tapped off into flat round pans mounted on wheels.
The charge is conveyed to the feed-floor by electricity. The furnace charge is 8000 lb. and 12 per cent. coke, with 30 per cent, (figured on the weight of the charge) of “shells” (slag). 293 Occasionally as much as 230 tons of the (moist) charge, exclusive of coke and slag, has been handled by one furnace in 24 hours. During one month (September, 1904) 40,000 tons of charge were worked up, corresponding to a daily average of 166 tons per furnace.
The lead in the charge runs from 13 to 14 per cent. on an average. The limestone, which is added as flux, is quarried not far from the works. The coke used is in part a very ordinary quality from Utah; in part a better quality from the East, with 9 to 10 per cent. ash. The matte amounts to 10 per cent. The slag contains 0.6 to 0.7 per cent. lead and 0.1 to 0.15 per cent. copper. The slag has approximately the following composition: 36 per cent. silica, 23 per cent. iron (corresponding to 29.57 per cent. FeO), 23 per cent. lime, 3.8 per cent. zinc and 4 per cent. alumina.
The work-lead is transferred while liquid from the furnaces to kettles of 30 tons capacity, in which it is skimmed, and thence cast in molds through a Steitz siphon. First, however, a 5.5 lb. sample is taken out by means of a special ladle, and is cast into a plate. From this samples of 0.5 a.t. are punched out at four points for the assay of the precious metals.
For the purpose of precipitating the flue dust, the blast-furnace gases are passed into brickwork chambers in which a plentiful deposition of the heavier particles takes place. From here the gases go through an L pipe of sheet iron, 18 ft. in diameter, to the Monier flues, which have a cross-section of 256 sq. ft. and a total length of 2000 ft. A small part of the flues is also built of brick. The gases unite with the hot roaster gases just before entering the 225 ft. chimney. In the portion of the blast-furnace dust first precipitated the silver runs 22 oz. per ton, while that recovered nearer the stack contains only 8 oz. The flue dust is briquetted with a small proportion of lime, and, after drying, is returned to the blast furnaces.
(May 12, 1906)
At the Pueblo plant, ores containing over 10 per cent. lead are not roasted, but are added raw to the charge. For such material as requires roasting there are in use five Brückner furnaces. The charge is 24 tons for 48 to 60 hours; the furnaces make one revolution per minute and roast the ore down to 6 per cent. sulphur. There are also two O’Harra furnaces, each roasting 25 tons daily, and 10 reverberatory furnaces 75 ft. in length, each roasting 15 tons of ore daily down to 4 per cent. sulphur.
The charge for smelting is prepared from roasted ore, together with Idaho lead ore, Cripple Creek gold ore, briquetted flue dust, slag and limestone. There are seven water-jacketed furnaces, which smelt, each, 150 tons of charge per day. The furnaces have 18 tuyeres, blast pressure 34 oz., cross-section at the tuyeres 48 × 148 in. They are charged mechanically by a car of 4 tons’ capacity.
The output of lead is 11 to 15 tons per furnace. The matte, which is produced in small quantity, contains 8 to 12 per cent. lead and the same percentage of copper. It is crushed by rolls, roasted in reverberatory furnaces, and smelted with ores rich in silica. The matte resulting at this stage, running 45 to 50 per cent. in copper, is shipped to be further worked up for blister copper.
The work-lead is purified by remelting in iron kettles, the cupriferous dross being pressed dry in a Howard press, and sent to the blast furnaces. The work-lead is sent to the refineries at Omaha, Neb., or Perth Amboy, N. J.
To collect the flue dust the waste gases are passed through long brick flues. The chimneys are 150 to 200 ft. high, and 15 ft. in diameter. They stand 75 ft. above the ground level of the 295 blast furnaces. The comparatively small proportion of flue dust produced (0.9 per cent. of the charge) is briquetted, together with fine ore and 5 per cent. of a thick paste of lime. For this purpose a White press is used, which makes six briquets at a time, and handles 10 tons per hour.
According to a tabulation of the results of five months’ running, the proportion of flue dust at several works of the American Smelting and Refining Company is as follows:
| Globe Plant, Denver | 0.5% | of the charge. |
| Pueblo Plant, Pueblo | 0.9% | ” |
| Eilers’ Plant, Pueblo | 0.5% | ” |
| East Helena Plant, Helena | 0.3% | ” |
| Arkansas Valley Plant, Leadville | 0.2% | ” |
| Murray Plant, Murray, Utah | 1.2% | ” |
The fuel used is of very moderate quality. The coke (from beehive ovens) carries up to 17 per cent. ash, the coal 10 to 18 per cent. The monthly production is 2300 tons of work-lead and 150 tons of copper matte (45 to 50 per cent. copper).
At the Eilers plant all sulphide ores, except the rich Idaho ore, are roasted down to 5 to 7 per cent. S in 15 reverberatory furnaces, 60 to 70 ft. in length, each furnace roasting 15 tons per 24 hours, in six charges.
The flue dust is briquetted together with fine Cripple Creek ore, pyrites cinder from Argentine, Kan., Creede ores rich in silica and 10 per cent. lime. The residue from the zinc smeltery (U. S. Zinc Company), which is brought to this plant (600 tons a month containing nearly 10 per cent. lead), is taken direct to the blast furnaces. Of the latter there are six, each with 18 tuyeres, which handle per 24 hours 160 to 180 tons of charge, containing on an average 10 per cent. of lead in the ore, with 10 per cent. of coke, figured on the charge. The average monthly production of a furnace is about 360 tons of work-lead, which is purified at the Pueblo plant. The furnaces are charged by hand. Of the slag, 30 per cent., as shells, etc., is returned to the charge. The monthly production of work-lead is 2000 tons, carrying 150 oz. of silver and 2 to 6 oz. of gold per ton.
The matte amounts to about 8.3 per cent., and contains 12 per cent. copper. It is concentrated up to 45 per cent. Cu, which is shipped (150 tons a month) for smelting to blister copper.
(January 27, 1906)
These works were erected in 1895 by the Guggenheim Smelting Company. They are situated on Raritan Bay, opposite the southern point of Staten Island, in a position offering excellent facilities for transportation by land and by water. The materials worked up are base lead bullion and crude copper, containing silver and gold, chiefly drawn from the company’s smelteries in the United States and Mexico. Silver ore is received from South America. The ores and base metals from Mexico and South America are brought to Perth Amboy by the company’s steamships (American Smelters Steamship Company).
Ore Smelting.—The silver ore from South America (containing antimony and much silver, together with galena, iron and copper pyrites) is crushed by rolls and is roasted down from 26 per cent. to 3 per cent. S in 11 reverberatory furnaces, 70 ft. long and 15 ft. wide (inside dimensions). It is then mixed with rich galena from Idaho, pyrites cinder, litharge, copper skimmings, and residues from the desilverizing process, together with limestone, and is smelted for work-lead and lead-copper matte in three water-jacketed furnaces, using 12 per cent. coke, figured on the ore in the charge. Of these furnaces one has 12 tuyeres; it measures 42 × 96 in. in cross-section at the tuyeres, and 6 ft. 3 in. by 8 ft. at the charging level. The hight of charge is 16 ft. The other two furnaces have 16 tuyeres each, their cross-section at the tuyeres being 44 in. by 128 in., at the charging level 6 ft. 6 in. by 12 ft., and hight of charge 16 ft. The furnaces are operated at a blast pressure of 35 oz. per square inch. The temperature of the gases at the throat is 140 deg. F. (60 deg. C.) measured with a Columbia recording thermometer, which works 297 very well. These furnaces reduce, respectively, 100 to 120 and 130 to 140 tons of charge per 24 hours; they are also used for concentrating roasted matte.
Copper Refining.—The crude copper is melted in two furnaces of 125 tons aggregate daily capacity, and is molded into anodes by Walker casting machines. Twenty-six anodes are lifted out of the cooling vessel at a time, and are taken to the electrolytic plant.
The electrolytic plant comprises two systems, each of 408 vats. The current is furnished by two dynamos, each giving 4700 amperes at 105 volts. The cathodes remain in the bath for 14 days. The weight of the residual anodes is 15 per cent.
The anode mud is swilled down into reservoirs in the cellar as at Chrome (De Lamar Copper Refining Company), is cleaned, dried and refined in a similar manner.
For melting the cathodes there are two reverberatory furnaces of capacity for 75 tons per 24 hours. The wire-bars and ingots are cast with a Walker machine. About 3200 tons of refined copper are produced per month.
Copper Sulphate Manufacture.—The lyes withdrawn from the electrolytic process are worked up into copper sulphate, shot copper being added. This latter is prepared in a reverberatory furnace from matte obtained as a by-product in working up the lead. About 200 tons of copper sulphate are thus produced per month; the process used is the same as at the Oker works. Lower Harz, Germany. The crystals are rinsed, dried and packed in strong wooden barrels.
Lead Refining.—The working up of the Mexican raw lead is carried out under the supervision of the customs officers. The lead, which is imported duty free, must be exported again. From each bar a sample is cut from above and below by means of a punch entering half way into the bar. For refining the lead there are four reverberatory furnaces of 60 tons capacity, with hearths 17 ft. 9 in. by 12 ft. 6 in., a mean depth of 14 in., and a grate area of 2 ft. 6 in. by 6 ft.; in addition to these there is a furnace of 80 tons capacity with a hearth 19 ft. 7½ in. by 9 ft. 6 in., a mean depth of 18 in., and grate area of 3 ft. by 6 ft.
For desilverizing the softened lead there are five kettles, each of 60 tons capacity, 10 ft. 3 in. diameter and 39 in. depth. The zinc is stirred in with a Howard mechanical stirrer and the zinc 298 scum is pressed dry in a Howard press, which gives a very dry scum. The latter is then, while still warm, readily hammered into pieces for the retorts.
The desilverized lead is refined in five reverberatory furnaces, of which four take a charge of 50 tons each, and one of 65 tons. The production of desilverized lead is 5000 to 5500 tons a month.
The distillation of the zinc crusts is carried out in 18 oil-fired Faber du Faur tilting furnaces. Each retort receives a charge of 1200 lb. of broken-up crust and a little charcoal. The distillation lasts 6 to 7 hours. Fifty gallons of petroleum residues are consumed per charge. The oil is blown into the furnace with a compressed air atomizer. After withdrawing the condenser, which runs on a traveling support, the argentiferous lead is poured directly from the tilted retort into an English cupel furnace. Seven such furnaces (magnesia-lined, with movable test) are in use, of which each works up 4.5 to 5 tons of retort metal in 24 hours. The furnaces are water-jacketed. The blast is introduced by the aid of a jet of steam. Three tons of coal are used per 24 hours.
Gold and Silver Parting.—The doré bars are cast into anodes for electrolytic parting by the Moebius process. The plant consists of 144 cells in 24 divisions. The mean composition of the electrolytic bath is said to be as follows: 10 per cent. free nitric acid, 17 grams silver, and 35 to 40 grams copper per liter. The current is furnished by a 62 k.w. dynamo. One cell consumes 260 amp. at 1.75 volts. One k.w. gives a yield of 1600 oz. fine silver per 24 hours. The daily production of silver is almost 100,000 oz., and is exceeded at no other works. About $3,000,000 worth of metal is always on hand in the different departments.
(April 14, 1906)
This plant, at South Chicago, Ill., refines base lead bullion. It comprises four reverberatory furnaces, of which one takes a charge of 100 tons, one 80 tons, and the other two 30 tons each; one of the small furnaces is being torn down, and a 120 ton furnace is to be built in its place. The furnaces are fired with coal from Southern Illinois, which contains 11 per cent. of ash.
In softening the bullion, the time for each charge is 10 hours. The first portion tapped consists of dross rich in copper, which is followed by antimonial skimmings and litharge.
The copper dross is melted up in a small reverberatory furnace, together with galena from Wisconsin (containing 80 per cent. lead), for work-lead and lead-copper matte, the latter containing about 35 per cent. of copper; this matte is enriched to 55 per cent. copper by the addition of roasted matte, and is finally worked up for crude copper (95 per cent.) in a reverberatory furnace. All the copper so produced is used in the parting process for precipitating the silver. The antimonial skimmings are smelted in a reverberatory furnace, together with coke cinder, for lead and a slag rich in antimony, which is reduced to hard lead (27 per cent. antimony, 0.5 per cent. copper, 0.5 per cent. arsenic) in a small blast furnace, 14 ft. high, which has 8 tuyeres.
The softened lead is tapped off into cast-iron desilverizing pots, which usually outlive 200 charges; in isolated cases as many as 300. For desilverizing, zinc from Pueblo, Colo., is added in two instalments, being mixed in by means of a Howard stirrer. After the first addition there remains in the lead 7 oz. of silver per ton; after the second only 0.2 oz. The first scum is pressed 300 in a Howard press and distilled; the second is ladled off and is added to the next charge. The Howard stirrer is driven by a small steam engine suspended over the kettle; the Howard press by compressed air.
For distilling zinc scum, 12 Faber du Faur tilting retorts, heated with petroleum residue, are used. The argentiferous lead (with 9.6 per cent. silver) is transferred from the retort to a pan lined with refractory brick, which is wheeled to the cupelling hearth and raised by means of compressed-air cylinders, so as to empty its molten contents through a short gutter upon the cupelling hearth. The cupelling hearths are of the water-cooled English type, and are heated by coal with under-grate blast. The cast-iron test rings, with reinforcing ribs, are made in two pieces, slightly arched and water-cooled; they are rectangular, with rounded corners, and are mounted on wheels. The material of the hearth is marl.
Argentiferous lead is added as the operation proceeds, and finally the doré bullion is poured from the tilted test into thick bars (1100 oz.) for parting.
The desilverized lead is refined in charges of 28 tons (4 to 5 hours) and 80 to 90 tons (8 to 10 hours), introducing steam through four to eight half-inch iron pipes. The first skimmings contain a considerable proportion of antimony and are therefore added to the charge when reducing the antimonial slags in the blast furnace. The litharge is worked up in a reverberatory furnace for lead of second quality. The refined lead is tapped off into a kettle, from which it is cast into bars through a siphon.
The parting of the doré bullion is carried out in tanks of gray cast iron, in which the solution is effected with sulphuric acid of 60 deg. B. The acid of 40 deg. B. condensed from the vapors is brought up to strength in leaden pans. In a second larger tank, which is slightly warmed, a little gold deposits from the acid solution of sulphates. The solution is then transferred (by the aid of compressed air) to the large precipitating tank, and diluted with water. It is here heated with steam, and the silver is rapidly precipitated by copper plates (125 plates 18 × 8 × 1 in.) suspended in the solution from iron hooks covered with hard lead. After the precipitation, the vitriol lye is siphoned off, the silver is washed in a vat provided with a false bottom, is removed with a wooden shovel, and is pressed into cakes 10 × 10 × 6 in.
The refining is finished on a cupelling hearth fired with petroleum residue, adding saltpeter, and removing the slag by means of powdered brick. After drawing the last portion of slag the silver (0.999 fine) is kept fused under a layer of wood-charcoal for 20 minutes, and is then cast into iron molds, previously blackened with a petroleum flame. The bars weigh about 1100 oz.
The gold is boiled with several fresh portions of acid, is washed and dried, and finally melted up with a little soda in a graphite crucible. It is 0.995 fine.
The lye from the silver precipitation, after clearing, is evaporated down to 40 deg. B. in leaden pans by means of steam coils, and is transferred to crystallizing vats. The first product is dissolved in water, the solution is brought up to 40 deg. B. strength, and is allowed to crystallize. The purer crystals so obtained are crushed, and are washed and dried in centrifugal apparatus; they are then sifted and packed in wooden casks in two grades according to the size of grain. The very fine material goes back into the vats. From the first strongly acid mother liquor, acid of 60 deg. B. is prepared by concentrating in leaden pans, and this is used for the parting operation.
(April 28, 1906)
The monthly production of these works is about 1500 tons of base bullion (containing 150 oz. Ag and 4 to 6 oz. Au per ton), and 200 tons of 45 per cent. copper matte. The base bullion is shipped to South Chicago, the matte to Pueblo.
The ore-roasting is done in two batteries of eight reverberatory furnaces and 16 Brückner furnaces, the resulting product containing on an average 20 per cent. lead and 3 per cent. sulphur. The charge for the blast furnaces consists of roasted ore, rich galena, argentiferous red hematite, briquetted flue dust, slag (shells) from the furnace itself, lead skimmings, scrap iron and limestone.
Four tons of the charge are dumped over a roller into a low car, which is then drawn up an inclined plane to the charging gallery by an electric motor and is then dumped into the furnace.
The two rectangular blast furnaces (Eilers’ type) have eight tuyeres on each of their longer sides and cast-iron water-jackets of 6 ft. hight. The blast is delivered at a pressure of 40 oz. The lead is drawn off through a siphon tap into a cooling kettle. The furnace has a large forehearth for separating the matte and the slag. The slag is received by a two-pot Nesmith truck, having an aggregate capacity of 14 cu. ft. These trucks are hauled to the dump by an electric locomotive. The shells are returned to the furnace with the charge.
The matte (with about 6 per cent. Cu and the same percentage of lead) is tapped off into iron molds and after cooling is crushed to 0.25-in. size, to be roasted in the reverberatory furnaces and smelted up together with roasted ore for a 15 per cent. matte. The latter is crushed, roasted and separately smelted together 303 with silicious ore for 45 per cent. matte, which is then sent to Pueblo to be worked up into blister copper. The small quantity of speiss which is formed is broken up and returned to the blast furnaces with the charge. The slag contains 0.5 to 0.8 per cent. lead and 0.5 oz. silver per ton.
(May 5, 1905)
This plant produces 1800 tons of base bullion per month and 200 tons of lead-copper matte containing 45 to 52 per cent. of copper. The ores smelted are mostly from Colorado, but include also galena from the Cœur d’Alene and other supplies. The limestone is quarried 14 miles from Denver; coke and coal are brought from Trinidad, Colo.
All sulphides, except the slimes, concentrates and the rich Idaho ores, are roasted. For roasting there are:
(1) Fifteen reverberatory furnaces, five of which measure 60 × 14 ft., and the other ten 80 × 16 ft. externally. In 24 hours these roast six charges of 4400 lb. (average) of moist ore (2.15 tons of dry ore) from 28 to 30 per cent. down to 3 to 4 per cent. sulphur. Each furnace is attended by three men working in 12-hour shifts; the stoker earns $2.75; the roasters, $2.30.
(2) Two Brown-O’Harra furnaces, 90 ft. long, with two hearths, and a small sintering furnace attached. They have three grates on each long side, and each roasts 26 tons of ore in 24 hours down to ¾ per cent. sulphur.
(3) Twelve Brückner furnaces, each taking 24 tons’ charge, with under-grate blast, the air being fed into the cylinders by a steam jet. According to the zinc content of the ores the roasting operation lasts 70 to 90 hours, the furnace making one revolution per hour. The roasted product from the Brückner furnaces is pressed into briquets, together with fine ore, flue dust and lime.
The smelting is carried out in seven blast furnaces, with 16 tuyeres, blast at 2-lb. pressure, hight of furnace 18 ft. 6 in., section at the tuyeres 42 × 144 in. The charge is 120 to 150 tons exclusive of slag and coke. The slag and the matte are 305 tapped off together into double-bowl Nesmith cars, which are hauled, by an electric locomotive, to a reverberatory furnace (hearth 20 × 12 ft.) in which they are kept liquid, for several hours, in charges of 14 to 15 tons, in order to effect complete separation. A little work-lead is obtained in this operation, while the matte is tapped off into cast-iron pans of one ton capacity, and the slag, 0.5 to 0.6 per cent. lead, 0.6 to 0.7 oz. silver, is removed in 5-ton pots to the dump.
The matte is broken up, crushed to 0.25 in. size, roasted in the reverberatory furnaces, smelted for a 45 to 52 per cent. copper matte, which is shipped to be further worked up into blister copper. The crude matte contains 10 to 12 per cent. copper, 12 to 15 per cent. lead, 40 oz. silver and 0.05 oz. gold.
From the siphon taps of the blast furnaces the work-lead is transferred to a cast-iron kettle of 33 tons’ capacity. Here the copper dross is removed, the metal is mixed by introducing steam for 10 minutes, sampled, and the lead is cast into bars through siphons. It contains about 2 per cent. antimony, 200 oz. silver and 8 oz. gold. This product is refined at Omaha.
The blast-furnace gases pass through a flue 1200 ft. long, and enter the bag-house, in which they are filtered through 4000 cotton bags 30 ft. long and 18 in. in diameter. These bags are shaken every 6 hours. The material which falls to the floor is burnt where it lies, sintered and returned to the blast furnaces.
In the engine house there are four Connersville blowers, two of which are No. 8 and two of No. 7 size. Each blast furnace requires 45,000 cu. ft. of air a minute.
The works give employment to 450 men, whose wages (for 10-to 12-hour shifts) are $2 to $3.
(November 14, 1903)
A few notes, gathered during a couple of years while I was employed at one of the large lead works in the southeastern part of Spain, are of interest, not as showing good work, but for comparing the results obtained in modern practice with those obtained by what is probably the most primitive kind of smelting to be found today. The plant about to be described may serve as a general type for that country. As far as I know, the exceptions are a large plant at Mazarron, fully up to date and equipped with the most modern improvements in every line; a smaller plant at Almeria, also in good shape, and the reverberatory smelting of the carbonates at Linares. It should be kept in mind, however, that the conditions are peculiar, iron and machinery being very expensive and manual labor very cheap.
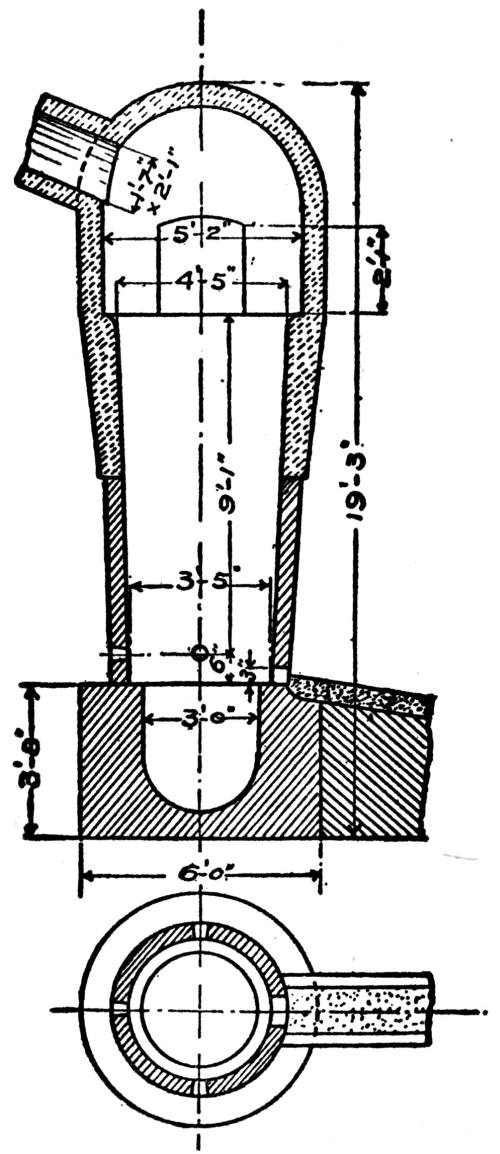
About 4 ft. above the tuyeres the furnace is built of uncalcined brick made of a black graphitic clay found in the mines near by; the upper part is of common red brick. The entire cost of one furnace does not reach $100. The flue leads to a main gallery 3.5 by 7 ft., which goes down to the ground, and extends several times around a hill, the chimney being placed on the top of the hill, considerably above the furnace level. The gallery is about 10,000 ft. long, and is laid down in the earth, with the arched roof just emerging. It is all built of rough stone, the inside being plastered with gypsum. The furnace has three tuyeres of 3 in. diameter. The blast pressure is generally 4 to 6 in. of water. Neither feeding floor nor elevators are used, only a couple of scaffolds, the charge being lifted up gradually by hand in small convenient buckets made of sea-grass. When charging the furnace, coke is piled up in the center, and the mixture of ore, fluxes and slag is charged around the walls. The slag and matte are left to run out together on an inclined sand-bed. The matte, flowing more quickly, goes further and leaves the slag behind, 307 but the separation thus obtained is, of course, very unsatisfactory. The charge mixture is weighed and made for each furnace every morning. When it is all put through, the furnace is run down very low, without any protecting cover on the top; several iron bars are driven through the furnace at the slag-tap level, for holding up the charge; the lead is all tapped out; a big hole is made in the crucible for the purpose of cleaning it out; all accretions are loosened with a bar; the hole is closed with mud of the graphitic clay; bars are removed, when the crucible is filled with 308 coke from the center and the charging is continued. In this way a furnace can be kept running for any length of time, but at a great loss of heat, and with a great increase of flue dust.
The current practice, in many parts of Spain, is to run the same number of ore-smelting and of matte-smelting furnaces. All the slag and the raw matte, produced by the ore-smelting furnaces, is re-smelted in the matte furnaces, together with some dry silver ores. No lead at all is produced in the matte furnaces, only a matte containing up to 150 oz. silver per ton and 25 to 35 per cent. of the lead charged on them. This rich matte is calcined in kilns, and smelted together with the ore charge.
The ores we smelted were galena ranging from 5 to 83 per cent. lead and about 250 oz. silver per ton of lead; dry silver ores containing up to 120 oz. silver per ton, and enough of the Linares carbonates for keeping the silver below 120 oz. per ton in the lead. The gangue of the galena was mainly iron carbonate. Most of that ore was hand picked and of nut size. Machine concentrates with more than 30 per cent. lead or containing much pyrite were calcined; everything else was smelted raw. The flux exclusively used, before I came, was carbonate of iron, which, by the way, was considered a “cure-for-all.” The slag analyses showed:
The specific gravity of the slag was about 5, or practically the same as that of the matte. The output of metallic lead was about 70 per cent.; of silver, 84 per cent. The working hight of the furnaces—tuyere level to top of charge—was at that time only 7 ft., and I was told that it had been still lower before.
To the working hight of the furnaces was added 2 ft., simply by putting up the charging doors that much. A very good limestone was found just outside the fence around the plant. Enough limestone was substituted for the iron carbonate, to keep the lime up to 12 per cent. in the slag, reducing the FeO to below 35 per cent. and the specific gravity to below four.
The result of these alterations was an increase in the output 309 of metallic lead, from 76 to 85 per cent.; of silver from 84 to 90 per cent.; a comparatively good separation of slag and matte, and a slag running about 0.5 to 0.75 per cent. Pb and 1.5 oz. Ag per ton.
Owing to the great extent of the gallery, and the consequent good condensation of the flue dust, the total loss of lead and silver was much smaller than would be expected; in no case being found above 4 per cent.
The composition of the charge was 55 per cent. ore and roasted matte, 13 per cent. fluxes, and 32 per cent. slag. Coke used was 11 per cent. on charge, or 20 per cent. on ore smelted. Each furnace put through 10 to 15 tons of charge, or 7 tons of ore, in 24 hours. Eight men and two boys were required for each furnace, including slag handling and making up of the charge. The cost of smelting was 17 pesetas per ton of ore, which at the usual premium (£1 = 34 pesetas = $4.85) equals $2.43. This cost is divided as follows:
| Coke | $1.47 |
| Fluxes | 0.04 |
| Labor | 0.65 |
| Coal for power | 0.10 |
| General expenses | 0.17 |
| Total | $2.43 |
This $2.43 per ton includes all expenses of whatever kind. The iron carbonate flux contained lead and silver, which was not paid for. The fluxes are credited for the actual value of this lead and silver. Without making this discount, the cost of flux would amount to 26c. per ton, making the entire smelting cost come to $2.65. As an explanation of the low cost of labor, it may be noted that the wages were, for the furnace-man, 2.25 pesetas, or 32c. a day; for the helpers, 1.75 pesetas, or 25c. a day.
The basis for purchasing the galena ore may here be given, reduced to American money; lead and silver are paid for according to the latest quotations for refined metals given by the Revista Minera, published at Cartagena. (The quotations are the actual value in Cartagena of the London quotations.)
The following discounts are made: 5 per cent. for both silver 310 and lead; $6.40 per ton on ore containing 7 per cent. Pb and below; this rises gradually to a discount of $7.75 per ton of ore containing 30 per cent. Pb and above.
The transportation is paid by the purchaser and amounts to about $1.20 per ton of ore.
The dry silver ores were cheaper than this and the lead carbonates much more expensive.
(October 28, 1905)
In dressing mixed lead and zinc carbonate ores by the old method of gradual crushing with rolls, middling products were obtained, which could be further separated only with much loss. Inasmuch as the losses in the metallurgical treatment of such mixed ore were reckoned to be less than in ore dressing, these between-products at Monteponi were saved for a number of years, until there should be enough raw material to warrant the erection of a small lead and zinc smeltery.
In 1894 the lead smeltery in Monteponi was put in operation; in 1899 the zinc smeltery was started. At about the same time the reserves of lead ore were exhausted, and the lead plant then began to treat all the Monteponi ores and a part of those from neighboring mines.
As will be seen from the plan (Fig. 42), the smelting works cluster in terraces around the mine shaft, covering an area of about 3000 sq. m. (0.75 acre); the ore stocks and the pottery of the zinc works are located in separate buildings.
During the first years of working, the slag had purposely been kept very rich in zinc, in the hope of utilizing it later for the production of zinc oxide. It had an average zinc content of 16.80 per cent., or 21 per cent. of zinc oxide, with about 32 per cent. SiO2, 25 per cent. FeO, and 14 per cent. lime. According to the recent experiments, this slag can very well be used for oxide manufacture, in connection with calamine rich in iron. The slag made at the present time has only 15 per cent. ZnO; 25 per cent. SiO2; 16 per cent. CaO; 3 per cent. MgO; 33 per cent. FeO; 2.5 per cent. Al2O3, and 2 per cent. BaO, and small quantities of alkalies, sulphur and lead (1 to 1.5 per cent).
The following classes of ore are produced at Monteponi:
1. Lead carbonates, with a little zinc oxide; these ores are 312 screened down to 10 mm. The portion held back by the screen is sent straight to the shaft furnaces; the portion passing through is either roasted together with lead sulphides, or is sintered by itself, according to circumstances.
2. Dry lead ores, mostly quartz, with 10 to 15 per cent. lead, which are mixed for smelting with the lead carbonates.
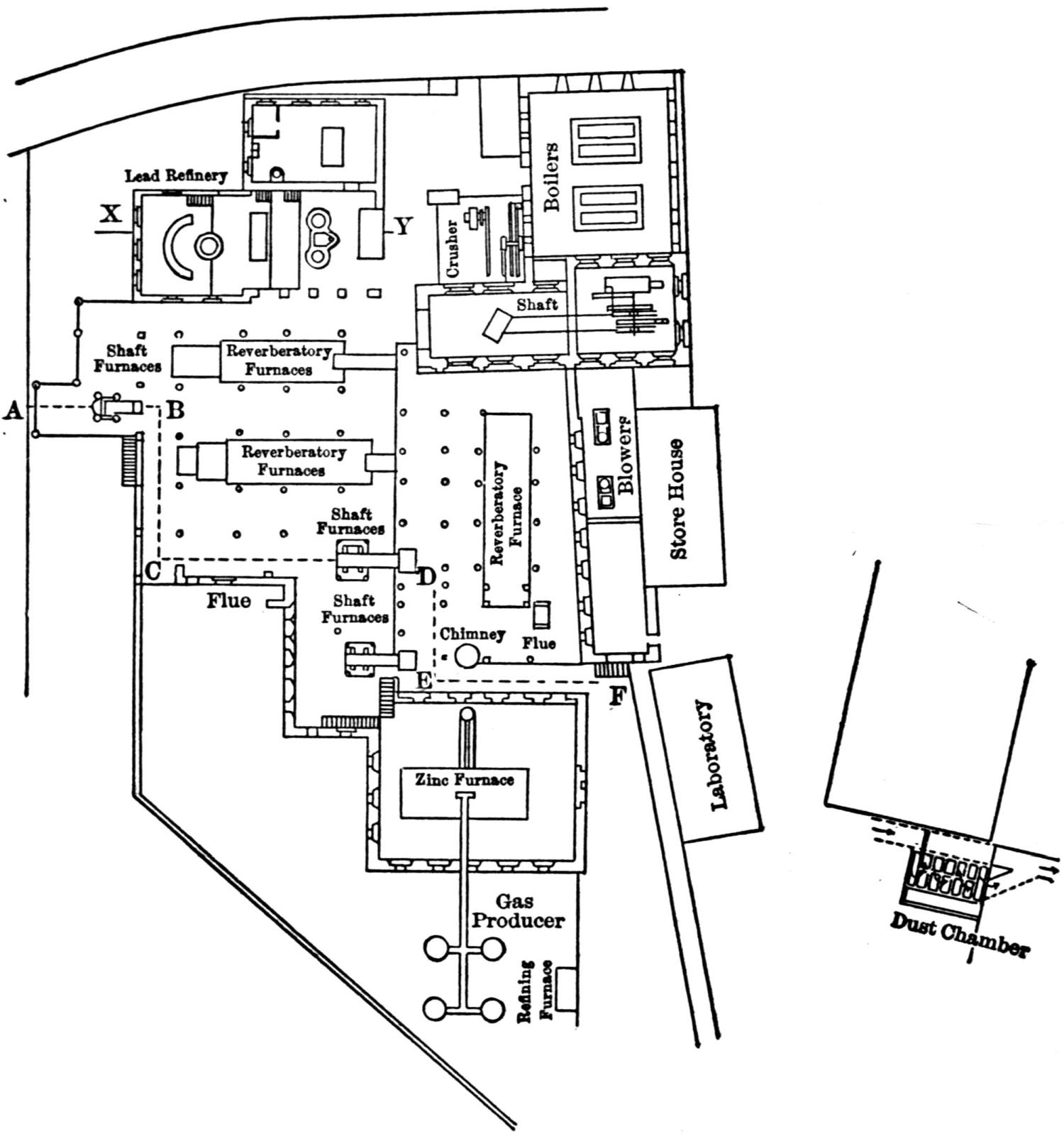
3. Lead sulphides, which are crushed fine and roasted dead. Quartz sand is added in the roasting, in order to decompose the lead sulphate and produce a readily fusible silicate; as quartz flux, fine sand from the dunes on the coast is used. This is a product of decomposition of trachyte, and contains 88 per cent. of silica, together with alkalies and alumina. The roast is effected 313 in two hand-raked reverberatory furnaces, 18 m. long, which turn out 12,000 kg. of roasted ore in 24 hours, consuming 1800 kg. of English cannel coal, or 2400 kg. of Sardinian lignite. There is also a third reverberatory furnace, provided with a fusion chamber, which is used for roasting matte and for liquating various secondary products.
The charge for the shaft furnace, as a rule, consists of 50 per cent. ore (crude and roasted), 20 per cent. fluxes and 30 per cent. slag of suitable origin. The fluxes used are limestone from the mine, containing 98 per cent. CaCO3, and limonite from the calamine deposits. This iron ore contains 48 per cent. Fe, not more than 4 per cent. Zn, a little lead and traces of copper and silver.
A shaft furnace will work up a charge of 60 tons, equal to 30 tons of ore, in 24 hours, with a coke consumption of 12 per cent. of the weight of the charge and a blast pressure of 50 mm. of mercury. There are three furnaces, of which two are used alternately for smelting lead ores, while one smaller furnace serves for smelting down products, such as hard lead, copper matte and copper bottoms.
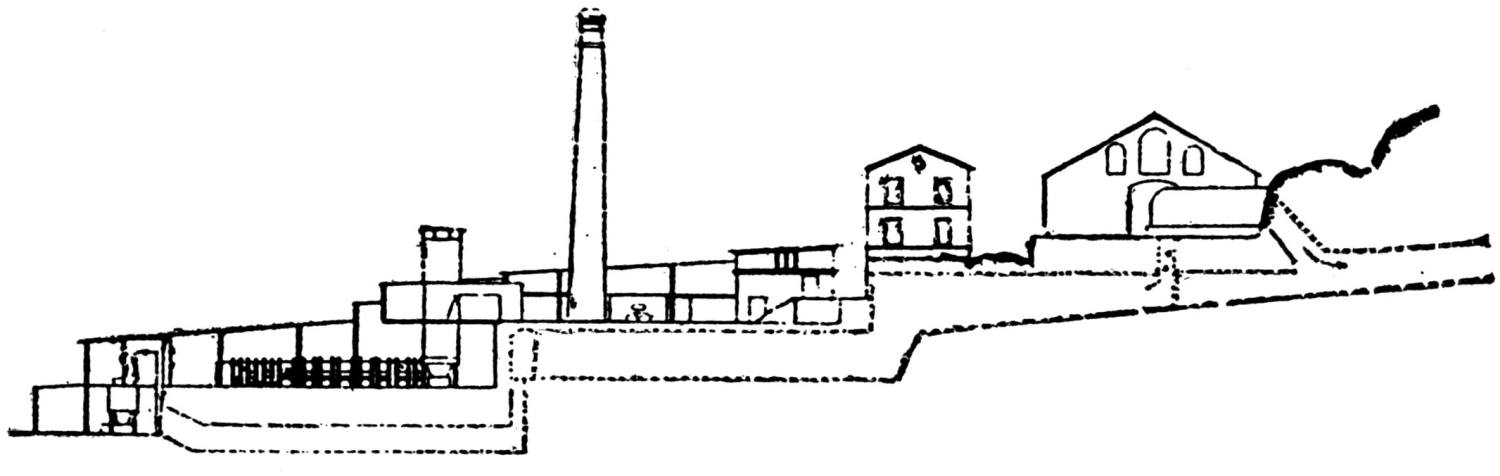
Figs. 43 to 46 show one of the furnaces. It will be seen at once that its construction is similar to that of the standard American furnaces. Pilz furnaces were tried in the first few years, but were finally abandoned, as they could not be kept running for any satisfactory length of time with slags rich in zinc. Diluting the slag, on the other hand, would have led to an increased coke consumption, and would have rendered the slag itself worthless. The furnace, however, differs in several respects from its American prototype; the following are some of the chief characteristics peculiar to it:
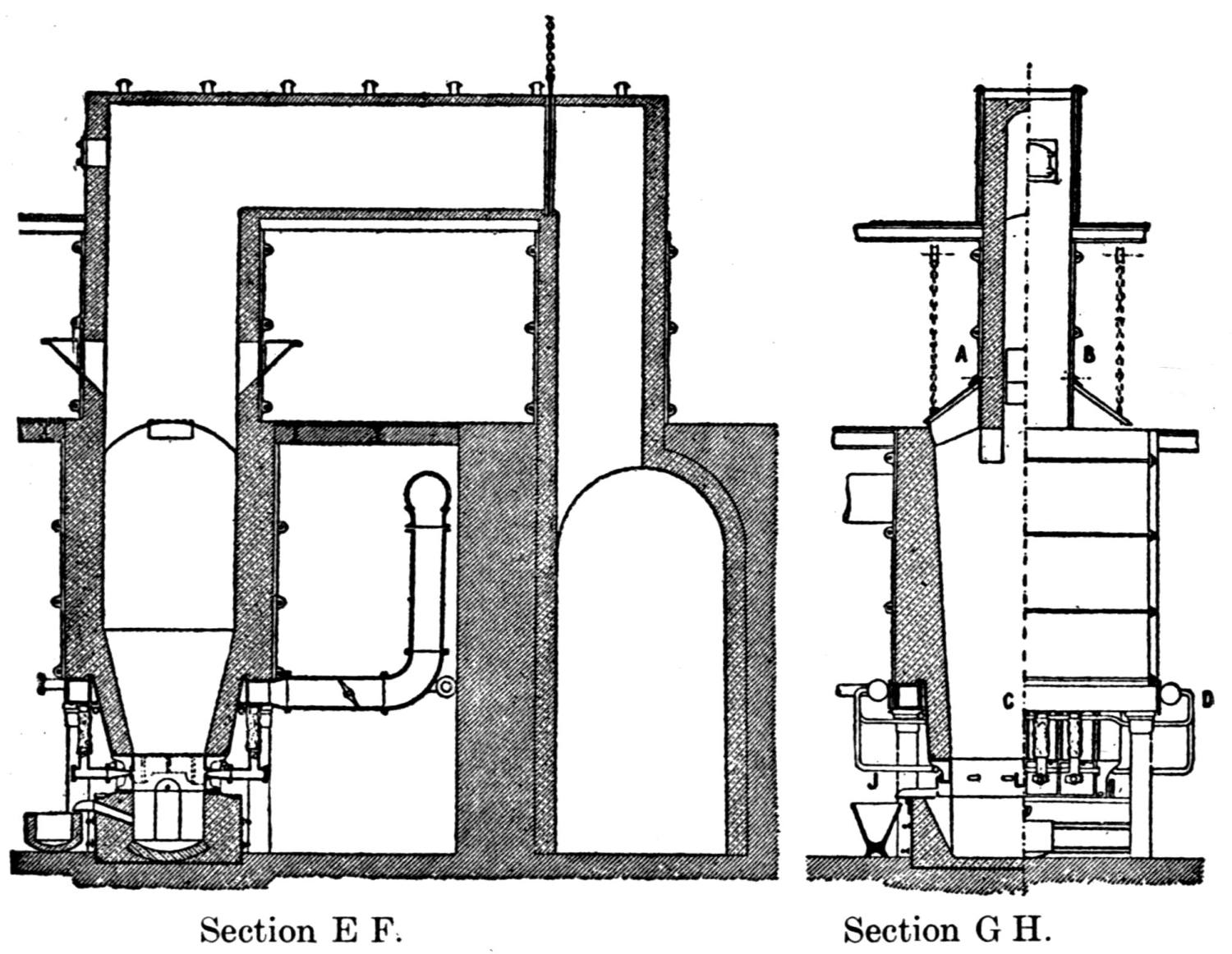
The chimney above the feed-floor covers one-third of the furnace shaft, and is turned down in the form of a siphon, to connect with the flue-dust chamber. The lateral faces, which are left open, serve as charging apertures; the central one of these, provided with a counterbalanced sheet-iron door, is used for charging from cars. The square openings at the ends, which are covered with cast-iron plates, are used for barring down the furnace shaft and may also be used for charging. By this arrangement, together with the two hoppers placed laterally on the chimney, it is possible to distribute the charge in any desired manner over the whole cross-section of the furnace. This arrangement greatly facilitates the removal of any accretions in the furnace shaft, as the centrally placed chimney catches all the smoke, while the charge-holes render the furnace accessible on all sides. In case of large accretions being formed, the whole furnace can be emptied, cleaned and restarted in 24 to 36 hours.
The smelting cone is enclosed by cast-steel plates 50 cm. high, instead of having a water-jacket. These are cooled as desired by turning a jet of water on them. The plates are con 315nected to the furnace shaft by a bosh wall 25 cm. thick, which is surrounded with a boiler-plate jacket. These jacket plates also are cooled from the outside by sprays of water. With this arrangement the consumption of water is less than with water-jackets, as a part of the water is vaporized, and the danger of leakage of the jackets is avoided. The cast-steel plates are made in two patterns; there are two similar side-plates, each with four slits for the tuyeres, and two end-plates, provided with a circular breast of 30 cm. aperture, for tapping the slag. The breast is cooled by water flowing down, and is closed in front by a plate of sheet iron, in which is the tap-hole for running off the slag. When cleaning out, this sheet-iron plate is removed and the breast is opened, thus providing easy access to the hearth. The four cast-steel plates are anchored together with bolts at their outer ribs, and rest on two long, gutter-shaped pieces of sheet iron, which carry off all the water which flows down, and keep it away from the brickwork of the hearth.
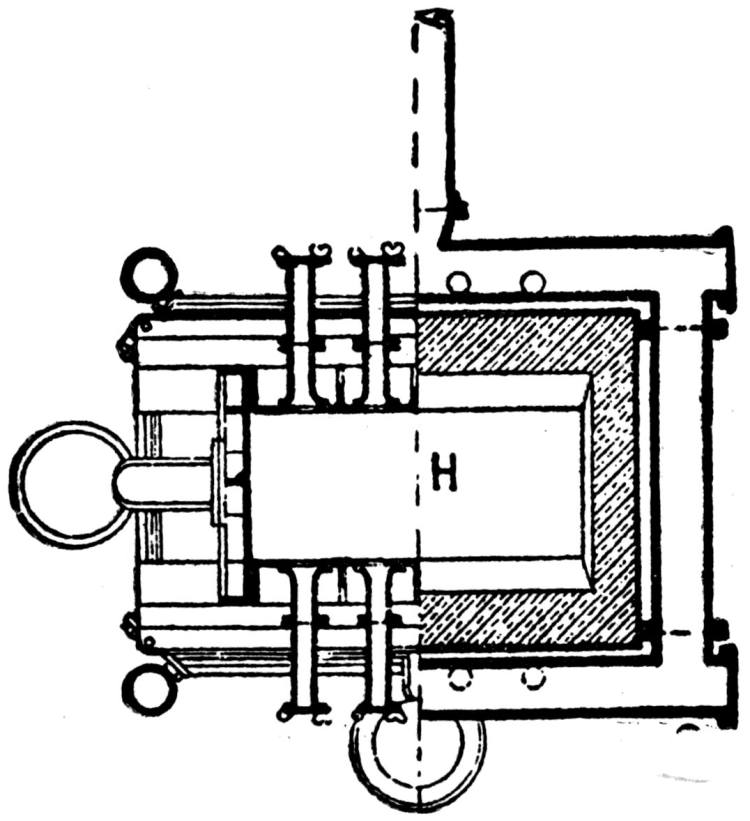
The hearth, cased with boiler plate and rails, has at the side a cast-iron pipe of 10 cm. diameter for drawing off the lead to the outside kettle; this pipe has a slight downward inclination, to prevent the slag flowing out; every 20 minutes lead is tapped, and the end of the pipe is then plugged up with clay.
The furnace shaft is supported upon a hollow mantel, which serves at the same time as blast-pipe. The blast-pipe has eight lateral tees, which are connected by canvas hose with the eight tuyeres. The mouth of the tuyeres has the form of a horizontal slit, whereby the air is distributed more evenly over the entire zone of fusion.
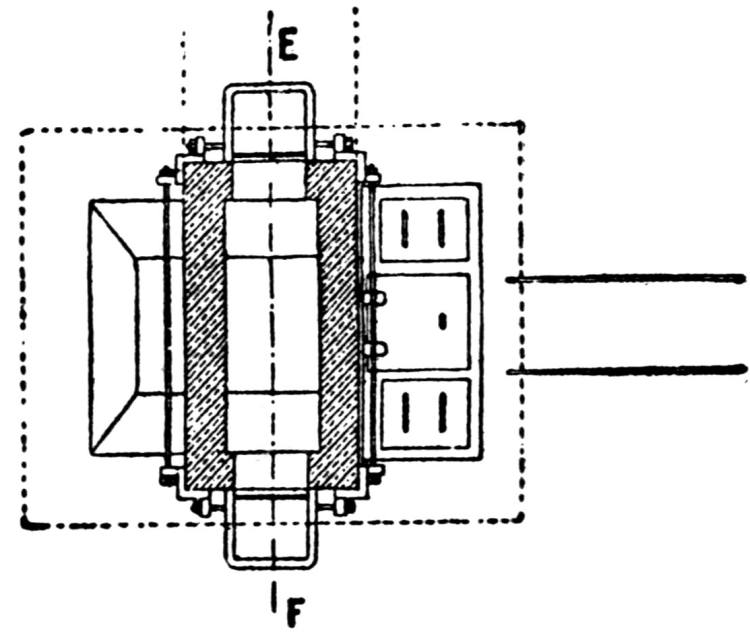
The precipitation of flue dust is effected in a brick condensing chamber, placed near the beginning of the main flue. The main flue terminates on the hill (see Fig. 43) in a chimney, the top of which is 160 m. above the ground level of the works, affording excellent draft. The condensing chamber (Figs. 49 to 51) consists of a vaulted room, 3.40 m. wide and 6.60 m. long, which is divided into twelve compartments by one longitudinal and five baffle walls. The gases change direction seven times, and pass over the longitudinal wall six times, being struck six times by fine sprays of water. The six atomizers for this purpose consume 1.5 liter of water per minute, of which four-fifths is vaporized, while one-fifth flows off to the lower water basin. By this means 10 to 15 per cent. of the total flue dust is precipitated in the condensing chamber itself, and is removed from time to time as mud through the lower openings, which are water-sealed. The remainder of the volatilized water precipitates the flue dust almost completely on the way to the stack, so that only a short column of steam is visible at the mouth of the stack. The flue to the stack passes for the most part underground through abandoned adits and galleries, thus providing a variety of changes in cross-section and in direction, and assisting materially the action of the condensing chamber.
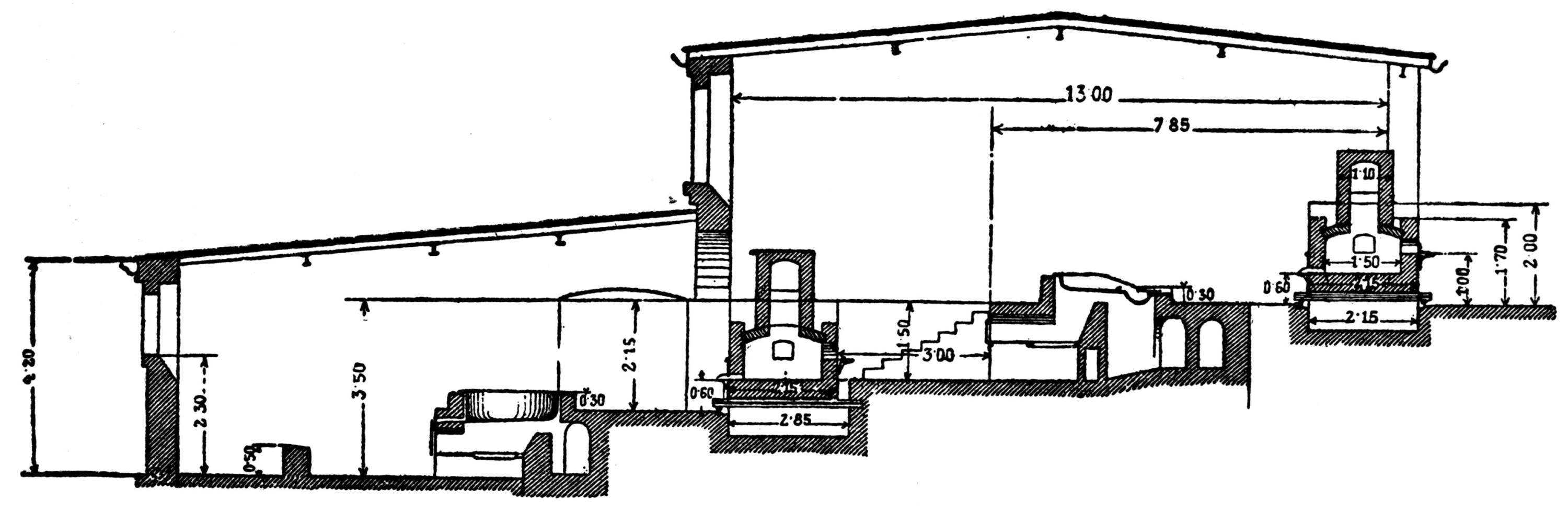
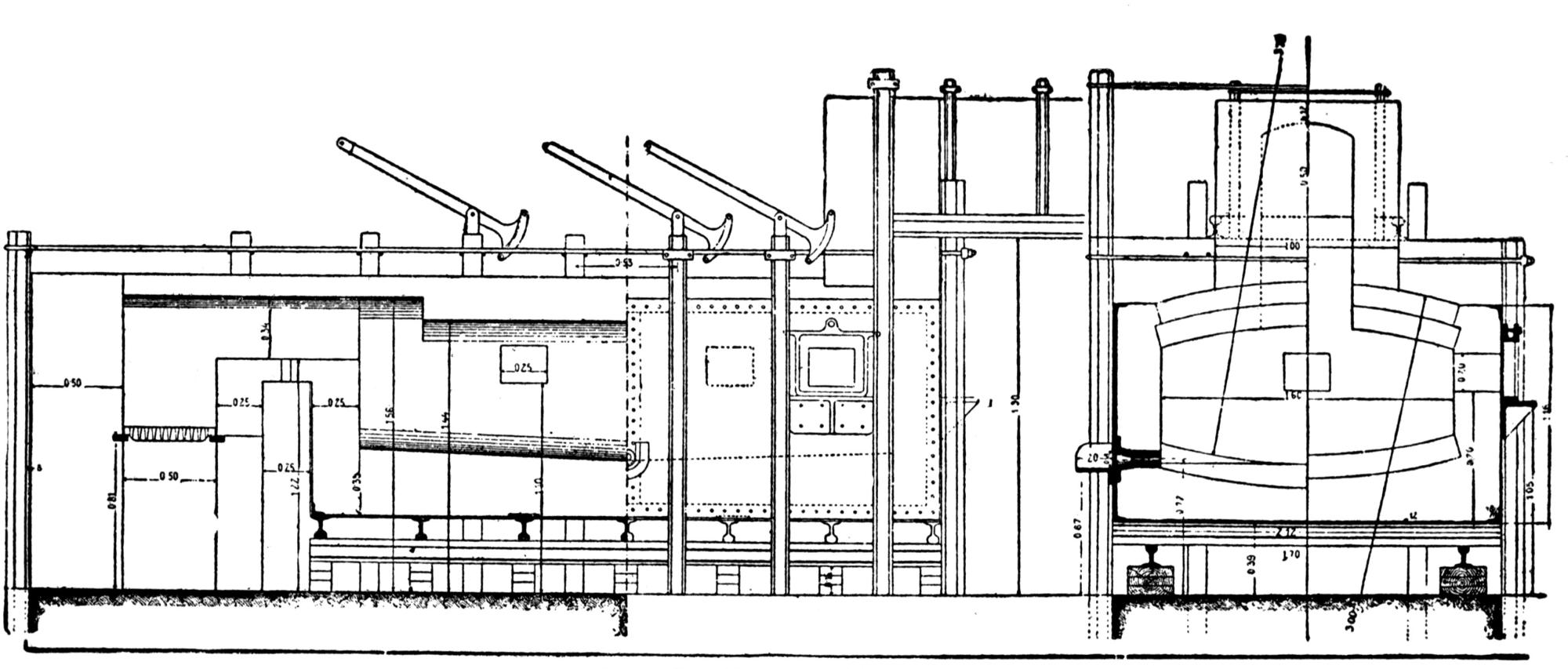
As the charge of the shaft furnaces is poor in sulphur, no real matte is produced, but only work lead and lead ashes (Bleischaum), which contains 90 per cent. of lead, 1.6 per cent. sulphur, 0.4 per cent. zinc, 0.85 per cent. Cu, 0.99 per cent. Fe, and 0.22 per cent. Sb. By liquation and a reducing smelt in a reverberatory furnace, most of the lead is obtained, along with a lead-copper matte, which is smelted for copper matte and antimonial lead in the blast furnace.
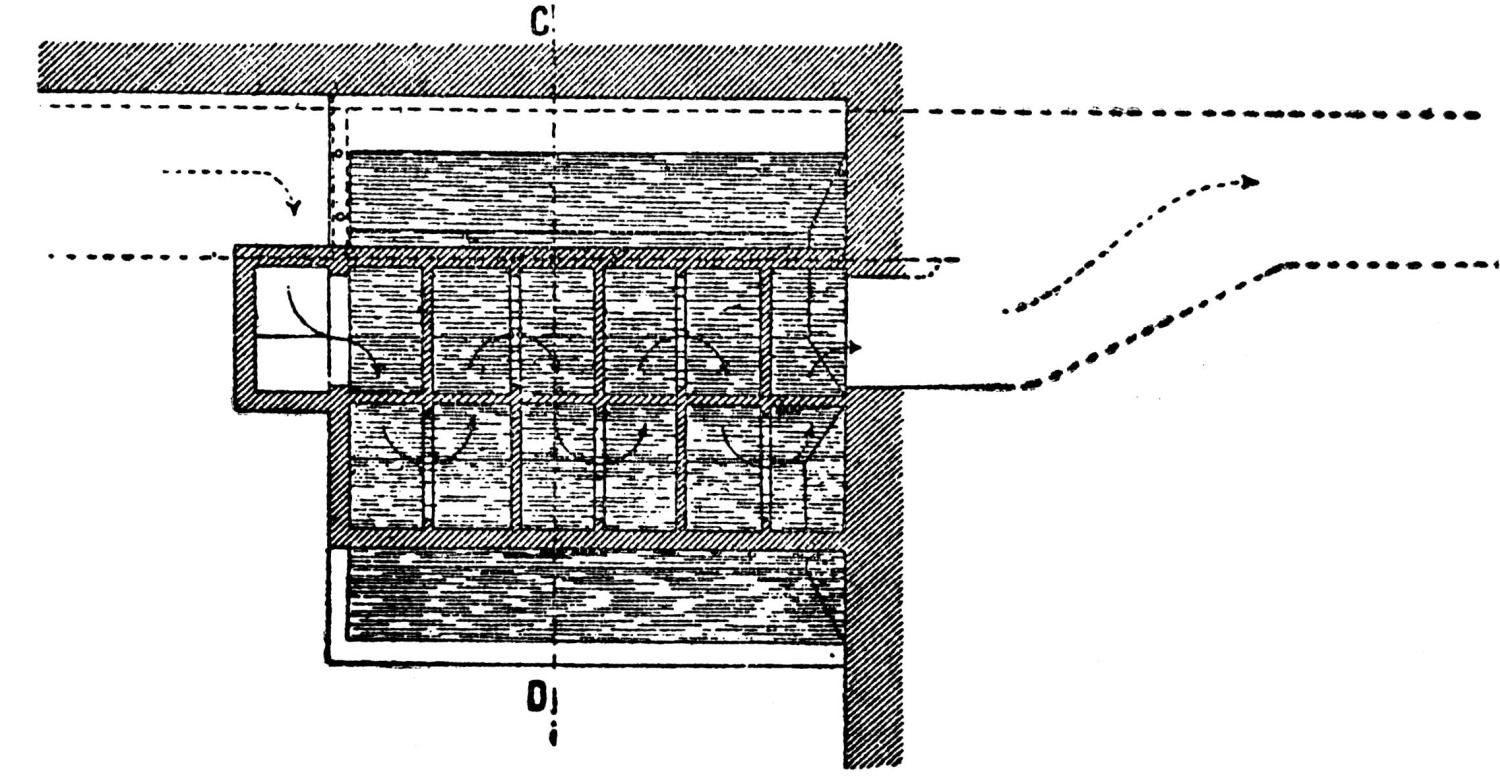
The copper matte, containing 18 per cent. Cu, 25 per cent. Fe, 30 per cent. Pb and 18.4 per cent. S, is roasted dead in a reverberatory furnace, is sintered, and melted to copper-bottoms in a small shaft furnace. These copper-bottoms, which contain 60 per cent. copper and 25 per cent. lead, are subjected to liquation, and finally refined to blister copper.
The zinc-desilvering plant, Fig. 47, consists of a reverberatory softening furnace, two desilvering kettles of 14 tons capacity, a pan for liquating the zinc crust, and a small kettle for receiving the lead from the liquation process.
This pan has the advantage over the ordinary liquating kettle, that the lead which drips off is immediately removed, before it can dissolve the alloy; the silver content of the liquated lead is scarcely 0.05 per cent., while the dry alloy contains 5 to 8 per cent.
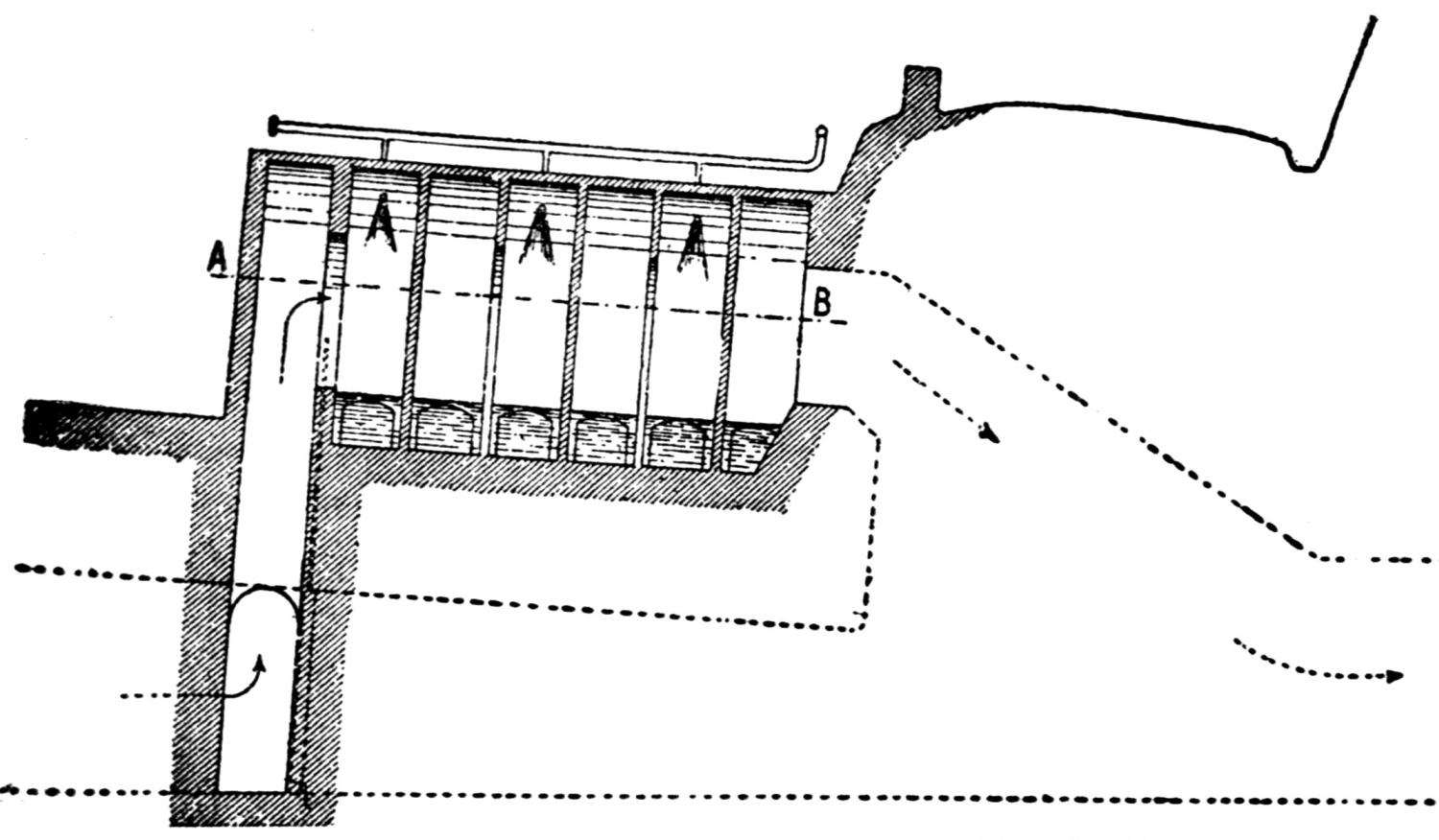
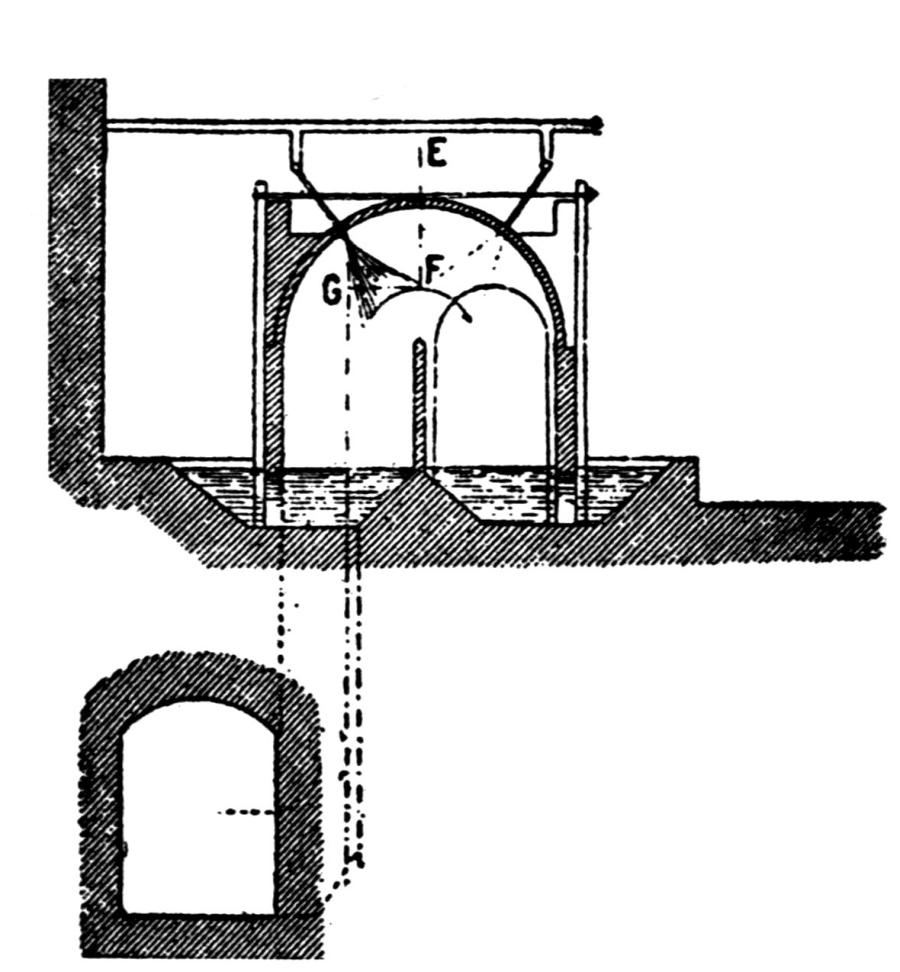
The removal of the zinc is effected in a second reverberatory furnace. Formerly the steam-method was used, but the rapid wear of the kettles, and the excessive formation of oxides called for a change in the process. The zinc-silver alloy is distilled in a crucible of 200 kg. capacity, and is cupeled in an English cupel furnace. The details of the reverberatory furnace are shown in Fig. 48.
The composition of the final products is shown by the following analyses; Lead: Zn, 0.0021 per cent.; Fe, 0.0047 per cent.; Cu, 0.0005 per cent.; Sb, 0.0030 per cent.; Bi, 0.0007 per cent.; Ag, 0.0010 per cent.; Pb, 99.998 per cent.; Silver, Ag, 99.720 per cent.; Cu, 0.121 per cent.; Fe, 0.005 per cent.; Pb, 0.018 per cent.; Au, 0.003 per cent.
[1] During 1905, antimonial lead commanded a premium of about 1c. per lb. above desilverized, owing to the high price for antimony.
[2] The figures for 1903 and 1904 have been added in the revision of this article for this book. The production of lead in the United States in 1903 was 276,694 tons; in 1904, it was 302,204 tons.
[3] Ounces of silver to the ton of lead.
[4] These figures are doubtful; they are probably too high. (See table on p. 5).
[5] The production of zinc ore in this district has now been commenced.
[6] The manuscript of this article was dated Oct. 5, 1905.
[7] Translated from Zeit. f. Berg.-Hütten-und Salinenwesen, LIII (1905, p. 450).
[8] This paper is published in pp. 148-166 of this book.
[9] Abstract from Transactions of the Australasian Institute of Mining Engineers, Vol. IX, Part 1.
[10] In the course of subsequent discussion Mr. Horwood stated that the losses in roasting were 12½ per cent. in lead and probably about 5 per cent. in silver. As compared to roasting in Ropp furnaces the loss in lead was 5 to 6 per cent. greater, but the difference of loss in silver was, he thought, not appreciable. Mr. Hibbard said that the Central mine had obtained satisfactory results with masonry kilns.—Editor.
[11] Abstract of portion of a paper presented at the Mexican meeting of the American Institute of Mining Engineers, under the title “The Mechanical Feeding of Silver-Lead Blast Furnaces.” Transactions, Vol. XXXII, pp. 353-395.
[12] Abstract of a paper (“The Mechanical Feeding of Silver-Lead Blast Furnaces”) presented at the Mexican meeting of the American Institute of Mining Engineers and published in the Transactions, Vol. XXXII. For the first portion of this paper see the preceding article.
[13] Abstract of a paper in Western Chemist and Metallurgist, I, VII, Aug., 1905.
[14] Much better work is being done at present, smelting the Western zinc ores, and the residue contains about one-third of the above figure, or 7.5 per cent. of zinc oxide. The high per cent. of ZnO left in residue was mainly due to poor roasting.
[15] There was also considerable coke used of an inferior grade, made from Kansas coal.
[16] Part of the ZnO in roasted matte came from being roasted in the same furnace the zinc ore had been roasted in.
[17] There was less residue on the charges during this month, which accounts for the larger tonnage with a lower blast.
[18] Translation of a paper read before the Naturwissenschaftlicher Verein at Aachen, and published in Metallurgie, 1905, II, i, 1-6.
[19] 35 to 40 cm. = 13.78 to 15.75 in. = 8 to 9.12 oz. per sq. in.
[20] Engineering and Mining Journal, 1904, LXXVIII, p. 630; article by Donald Clark; reprinted in this work, p. 144.
[21] Owner of the patents.—Editor.
[22] Abstract of a paper in Metallurgie, II, 18, Sept. 22, 1905, p. 433.
[23] This method is described further on in this book.
[24] Translated from Metallurgie, Vol. II, No. 19.
[25] British patent, No. 17,580, Jan. 30, 1902, “Improved process for desulphurizing sulphide ores.”
[26] W. C. Roberts-Austen, “An Introduction to the Study of Metallurgy,” London, 1902.
[27] A. Lodin, Comptes rendus, 1895, CXX, 1164-1167; Berg. u. Hüttenm. Ztg., 1903, p. 63.
[28] Comptes rendus, loc. cit.
[29] Translated from the Zeitschrift für das Berg.-Hütten-und Salinenwesen im. preuss. Staate, 1905, LIII, ii, pp. 219-230.
[30] Translated from the Zeitschrift für das Berg.-Hütten-und Salinenwesen im. preuss. Staate, 1905, LIII, ii, pp. 219-230.
[31] The manufacture of sulphuric acid from these gases has now been undertaken in Silesia on a working scale.—Editor.
[32] A paper presented before the American Institute of Mining Engineers, July, 1906.
[33] Engineering and Mining Journal, Sept. 2, 1905.
[34] This term is inexact, because the hearths employed in the United States are not strictly “Scotch hearths,” but they are commonly known as such, wherefore my use of the term.
[35] Percentages of lead in Missouri practice are based on the wet assay; among the silver-lead smelters of the West the fire assay is still generally employed.
[36] This improvement did not originate at either Alton or Collinsville. It had previously been in use at the works of the Missouri Smelting Company at Cheltenham, St. Louis, but the idea originated from the practice of the Picher Lead Company, of Joplin, Mo.
[37] This refers especially to the Savelsberg process.
[38] A. D. Carmichael, U. S. patent No. 705,904, July 29, 1902.
[39] Metallurgie, 1905, II, i, 1-6; Engineering and Mining Journal, Sept. 2, 1905.
[40] Metallurgie, 1905, II, 19; Engineering and Mining Journal, Jan. 27, 1906.
[41] Metallurgie, 1905, Sept. 22, 1905; Engineering and Mining Journal, March 10, 1906.
[42] Engineering and Mining Journal, Oct. 21, 1905.
[43] Translated by W. R. Ingalls.
[44] As originally published the title of this article was “Lead-Smelting without Fuel.” In this connection reference may well be made to Hannay’s experiments and theories, Transactions Institution of Mining and Metallurgy, II, 188, and Huntington’s discussion, ibid., p. 217.
[45] Excerpt from a paper, “Concrete in Mining and Metallurgical Engineering,” Transactions American Institute of Mining Engineers, XXXV (1905), p. 60.
[46] A Discussion of the Paper by Henry W. Edwards, on “Concrete in Mining and Metallurgical Engineering,” Transactions of the American Institute of Mining Engineers, XXXV.
[47] Engineering News, Nov 30, 1899, and U. S. Patent No. 665,250, Jan. 1 1901.
[48] A discussion of the paper of Henry W. Edwards, on “Concrete in Mining and Metallurgical Engineering,” Transactions of the American Institute of Mining Engineers, XXXV.
[49] Abstract from the Journal of the Chemical, Metallurgical and Mining Society of South Africa, May, 1903.
[50] Abstract of a paper in Transactions American Institute of Mining Engineers, XXXIV (1904), p. 175.
[51] Silver not given. This was the case, also, with the gold in the bullion. The slimes contained 0.131 per cent. of gold, or 39.1 oz. per ton.
[52] A constituent company of the American Smelting and Refining Company.
[53] Translated from Zeit. f. Berg.-Hütten.-und Salinenwesen im preuss. Staate, 1905, LIII, p. 433.
[54] Abstract from a paper in Zeit. f. Berg.-Hütten-und Salinenwesen im preuss. Staate, 1905, LIII, p. 439.
[55] Translated from Zeit. f. Berg.-Hütten.-und Salinenwesen im preuss. Staate, 1905, LIII, 490.
[56] Abstract from a paper in Zeit. f. Berg.-Hütten-und Salinenwesen im preuss. Staate, 1905, p. 400.
[57] Abstract from a paper in Zeit. f. Berg.-Hütten.-und Salinenwesen im preuss. Staate, 1905, p. 400.
[58] Abstract from an article in Zeit. f. Berg.-Hütten.-und Salinenwesen im preuss. Staate, 1905, LIII, p. 444.
[59] Translated from Oest. Zeit. f. Berg.-und Hüttenwesen, 1905, p. 455.