
Project Gutenberg's Identification of the Larger Fungi, by Roy Watling This eBook is for the use of anyone anywhere at no cost and with almost no restrictions whatsoever. You may copy it, give it away or re-use it under the terms of the Project Gutenberg License included with this eBook or online at www.gutenberg.org/license Title: Identification of the Larger Fungi Author: Roy Watling Editor: Antony Kenney Release Date: August 24, 2019 [EBook #60159] Language: English Character set encoding: ISO-8859-1 *** START OF THIS PROJECT GUTENBERG EBOOK IDENTIFICATION OF THE LARGER FUNGI *** Produced by MFR, Eric Lehtonen, Harry Lamé and the Online Distributed Proofreading Team at http://www.pgdp.net. This file was produced from images generously made available by The British Mycological Society and special thanks and appreciation are extended to the Author and Editor of the book for granting permission to release it to the public domain.
Please see the Transcriber’s Notes at the end of this text.

DEDICATION
To my parents who encouraged my interests in mushrooms and toadstools and my wife who, later, was sympathetic to my studies and assisted in the production of the manuscript.
Hulton Group Keys
IDENTIFICATION OF THE LARGER
FUNGI
by
ROY WATLING, B.Sc., Ph.D., M.I.Biol.
Principal Scientific Officer,
Royal Botanic Garden, Edinburgh
Editor of series: Antony R. Kenney, M.A., B.Sc.
©
1973
R. Watling
A. R. Kenney
ISBN 0 7175 0595 2
First published 1973 by Hulton Educational Publications Ltd.,
Raans Road, Amersham, Bucks.
Reproduced and printed by photolithography and bound in
Great Britain at The Pitman Press, Bath
[5]
This is one of a series of books intended to introduce field-biology to students, particularly the sixth form and early university student. The present work is ecologically biased in order to emphasise a rather neglected aspect of the higher fungi.
Few books on fungi have ever been designed for students. This book is aimed primarily at this level, but if the interested amateur is assisted and encouraged by this same text my hopes will have been doubly achieved. Many amateurs interested in higher fungi wish only to name their collections, or know approximately what they are before sampling them as an addition to their diet. An understanding of our commoner species at an early age will allow the ‘budding’ mycologist to tackle the much needed study of the more critical forms. Mycology is still at a descriptive stage, but it is hoped this will soon be changed and fungi of all kinds will be studied as part and parcel of courses in ecology.
It is of course quite impossible to cover all the species in such a small volume as this present one, but it is hoped that the examples which have been carefully chosen are sufficiently common throughout the country for any student to collect them in a single season. The examples, except for very few, in fact appear in the list of higher fungi found about the Kindrogan Field Centre, Perthshire, Scotland, compiled from the collections made by students attending my field course there.
The present work is arranged in three parts: the agarics are dealt with first, the non-agarics next, both with particular reference to their major habitat preferences, and lastly a catalogue of those more specialised habitats which are frequently encountered. All parts are supported at the end by lists in tabular form of those species expected to be found in any one habitat. Keys to the major groups, families and genera, are included to widen the scope of the book and place the examples chosen and illustrated in the text in their position in classification.
In the description the synonymy has been very severely pruned and only covers the commonly seen names; they are included as part of the general information under each species. In order for the student[6] to expand unfamiliar names a list of references is added at the end of the work. The common names of the fungi, whenever possible, have been adopted from a list produced by Dr Large, the author of The Advance of the Fungi, an exciting tale of fungal parasites. The authorities for the names of the fungi described have been reduced to accord with the minimum requirements set out by the Code of Botanical Nomenclature. After each description a list of references to coloured plates is given and while some of these illustrations are not of the highest quality they are adequate, and, more important, they are widely available. Any technical terms appearing in the description are explained in the glossary, although they have been kept to a minimum; the difficulty of expressing colours has been overcome by consistently referring to one colour chart only, (a chart designed originally for the use of mycologists and available from Her Majesty’s Stationery Office).
I have not indicated the edibility of a particular species unless there is no doubt as to the edibility of it, related species and those species with which it might be easily confused. Many fungi are notoriously difficult to identify and when one has approximately 3,000 species of larger fungi in the country the task is even more difficult. It would be folly therefore to indicate edibility for all the fungi described in a book such as this; the golden rule which should be adopted is not to eat any of the fungi one collects in the woods and fields. A fault of most popular treatments is that they are biased towards the human diet and selection of species is done on this basis; in the present work selection of examples within the 270 pages has been difficult and two factors have been particularly considered to ensure that (i) representatives of all the major groups of fungi and genera have been covered and (ii) a coverage has been attempted of all the common ecological niches.
I am fully aware that the taste of a fungus may be distinctive to that species or to a group of closely related species, but it is only a spot character and the tasting of one’s finds is neither necessary nor advisable; indeed it is not used in this book. The odour, however, has been indicated whenever distinctive.
[7]
| Page | |||||
|---|---|---|---|---|---|
| Preface | 5 | ||||
| Introduction | 9 | ||||
| Where to look | 9 | ||||
| Collecting | 10 | ||||
| Examination | 11 | ||||
| Microscopic examination | 12 | ||||
| Key to major groups of Larger Fungi | 21 | ||||
| A. | Agarics and their relatives | 22 | |||
| Key to major genera | 22 | ||||
| (i) | Agarics of woodlands and copses | 27 | |||
| (a) | Mycorrhizal formers | 27 | |||
| (b) | Parasites | 59 | |||
| (c) | Saprophytes—Wood-inhabiting or lignicolous agarics | 64 | |||
| (d) | Saprophytes—Terrestrial agarics | 78 | |||
| (ii) | Agarics of pastures and meadows | 95 | |||
| (a) | Agarics of rough & hill-pastures | 95 | |||
| (b) | Agarics of chalk-grassland & rich uplands | 108 | |||
| (c) | Agarics of meadows and valley-bottom grasslands | 114 | |||
| (d) | Fairy-ring formers | 118 | |||
| (e) | Agarics of urban areas—lawn and parkland agarics | 122 | |||
| (f) | Agarics of wasteland and hedgerows | 126 | |||
| B. | Bracket fungi and their relatives | 135 | |||
| Key to major genera | 135 | ||||
| (i) | Pored and toothed fungi | 140 | |||
| (a) | Colonisers of tree trunks, stumps and branches | 140 | |||
| (b) | Destroyers of timber in buildings | 154 | |||
| (c) | Colonisers of cones | 158 | |||
| (d) | Terrestrial forms | 160 | |||
| (ii) | Cantharelles and related fungi | 162 | |||
| (iii) | Fairy-club fungi | 166 | |||
| (iv) | Resupinate fungi | 176 | |||
| C. | The Jelly fungi—Key to major groups with examples | 179 | |||
| D. | The Stomach fungi; puff-balls and their relatives—Key to major groups with examples | 186 | |||
| E. | Cup fungi and allies[8] | 198 | |||
| F. | Specialised Habitats | 207 | |||
| (i) | Fungi of dung and straw heaps | 207 | |||
| (ii) | Fungi of bonfire sites | 216 | |||
| (iii) | Fungi of bogs and marshes | 222 | |||
| (a) | Sphagnum bogs | 222 | |||
| (b) | Alder-carrs | 226 | |||
| (iv) | Fungi of beds of herbaceous plants | 227 | |||
| (v) | Fungi of moss-cushions | 230 | |||
| (vi) | Heath and mountain fungi | 231 | |||
| (a) | Moorland fungi | 231 | |||
| (b) | Mountain fungi & Basidiolichens | 236 | |||
| (vii) | Sand-dune fungi | 238 | |||
| (viii) | Subterranean fungi | 243 | |||
| (ix) | Fungal parasites | 246 | |||
| G. | Appendix | 249 | |||
| (i) | Species lists of specialised habitats | 249 | |||
| (ii) | Glossary | 260 | |||
| (iii) | Simple experiments with Fairy-rings | 264 | |||
| (iv) | Development of the Agaric fruit-body | 266 | |||
| (v) | References | 269 | |||
| H. | Index | 271 | |||
Cover transparency supplied by John Markham, F. R. P. S., F. Z. S.
[9]
The term larger fungus refers to any fungus whose study does not necessarily require more than a low-powered lens to see most of the important morphological features. Using such a term cuts across the existing scientific classification, for it includes the more obvious fungi bearing their spores on specialised reproductive cells called basidia, fig. 5, and a few of those whose spores are produced inside specialised reproductive cells called asci. The term is useful, however, even though it embraces a whole host of unrelated groups of fungi; it includes the polypores, fairy-clubs, hedgehog-fungi, puff-balls and elf-cups, as well as the more familiar mushrooms and toadstools—or puddockstools as they are often called in Scotland. Specimens of all these groups will find their way some time into the collecting baskets of the naturalist when he is out fungus-picking, along with probably a few jelly-fungi and less frequently one or two species of the rather more distantly related group, the morels. The biggest proportion of the finds, however, on any one collecting day in the autumn, when the larger fungi are in their greatest numbers, will be of the mushrooms and toadstools; these are, collectively, more correctly called the agarics.
The early botanists and pioneer mycologists of the nineteenth century recognised the fact that the fungi both large and small are ecologically connected to the herbaceous plants and trees among which they grow, but many mycologists since have tended to neglect these early observations. Although the importance of the fungi in the economy of the woodland, copse, field and marsh is well-known, mycologists and ecologists alike have been rather slow to appreciate that the fungi can be just as good indicators of soil conditions, if not better, than many other plants. Perhaps it is rash to attempt such a treatment as you find here because we know so little of the reasons why a particular fungus prefers one habitat to another. However, it is envisaged and hoped that, if a framework is provided, accurate field-notes can gradually be accumulated and many of the secrets yet to be uncovered explained.
Fungi can be found in most situations which are damp at some time of the year. Searching for fungi can begin as soon as the spring days become warm, although even in the colder periods of winter several[10] finds can be made. In summer it gets very dry and this necessitates collecting in damper areas, such as marshes, alder-carrs, swamps and moorland bogs. After a heavy storm in summer, on the edges of paths and roadsides, woodland banks, in clearings in woods and in gardens, fungi can be collected within a few days of the rain, but collecting normally reaches a climax in August-September, the precise date depending on the locality and the individual character of the particular year.
All woodlands are worth visiting, particularly well-established woods with a mixture of trees. Pure pine-woods do not seem to be as good as pine-woods with scattered birch; plantations are often disappointing except after heavy rain or late in the season, even well into November in mild years. Pure birch and beech, the latter particularly when on chalky soils, are excellent areas to visit. Oak is possibly not as good but areas with willow and alder have many unique species. The edge of woods, sides of paths or clearings are usually more productive areas to search in than is the depth of the wood, and a small plot of trees can be much more rewarding than a large expanse of woodland. After some time one is able to judge the sort of place which will yield fungi. Rotten and burnt wood are very suitable substrates for they retain the moisture necessary for growth of fungi even in dry conditions, so allowing fructification to take place.
Grasslands including hill-pastures, established sand-dunes, etc., are often excellent, but of course they are much more dependent on the weather to produce favourable conditions for fungal development than woodland areas where the changes in the humidity and temperature are less extreme; prolonged mist or mild showery weather favour the fruiting of the grassland fungi. Dung in both woods and fields is an excellent although ephemeral substrate; many species of fungi characterise dung whilst others will grow in manured fields, on straw-heaps or where man has distributed the habitat.
The collecting of larger fungi should not be considered a haphazard pursuit; careless collecting often results in many frustrating hours being spent on the identification of inadequate material, which is also not suitable after for preservation as reference material. A few good specimens are infinitely better than several poor ones; one is always tempted to collect too much and then collections are inevitably discarded. Always try to select specimens showing all the possible stages of development[11] from the smallest buttons to the expanded caps. Sometimes such a range is not possible and one must be satisfied with either a couple or only one fruit-body.
Carefully dig up or cut from the substrate the entire fungus and handle it as little as possible. A strong pen-knife or fern-trowel is admirable for the job. The associated plants should be noted, especially trees, and if one is unable to identify the plants or woody debris retain a leaf or a piece of wood for later identification. One should note in a field-notebook any features which strike one as of interest, such as smell, colour, changes on bruising, presence of a hairy or viscid surface.
For transporting home the specimens should be placed in tubes, tins or waxed paper which are themselves kept in a basket. The smallest specimen can go in the first, the intermediate-sized forms in the tins or waxed paper and the larger ones laid in the basket or placed in large paper bags; plastic bags are not suitable except for very woody fungi. Thus an assortment of tins, tubes and various sizes of pieces of waxed paper are essential before setting out on a collecting trip. The specimens should be placed in the waxed paper such that they can be wrapped once or twice and the ends twisted as if wrapping a sweet.
Once home always aim at examining the specimens methodically.
The first necessity is to determine whether the fungus, which has been collected, has its spores borne inside a specialised reproductive cell (ascus) i.e. Ascomycete, or on a reproductive cell (basidium) i.e. Basidiomycete. By taking a small piece of the spore-bearing tissue, mounting in water, gently tapping it and examining under a low power of the microscope this can be easily ascertained. The tapping out is best done with the clean eraser of a rubber-topped pencil. There are several different shaped asci and basidia; the latter structures are more important in our study because the Ascomycetes are in the main composed of microscopic members.
The following procedure is necessary for the examination of your find:—
Select a mature cap of an agaric from each collection, cut off the stem and set the cap gills down on white paper, or if the specimen is small or is a woody or toothed fungus, or consists of a club or flattened irregular plate, place the spore-bearing surface (hymenium) face down on a microscope glass slide. The smaller specimens must be placed in tins with a drop of water on the cap to prevent drying out. Even with[12] the larger specimens it is desirable to place a glass slide somewhere under the cap between the gills and the paper, and if possible to enclose the species carefully in waxed paper or in a tin. Whilst you are waiting for the spore-print to form, notes must be made on the more easily observable features; one is not required at this stage to examine the microscopic characters.
All the characters which may change on drying must be noted immediately, and these include colour, stickiness, shape, smell and texture. A sketch, preferably in colour, however rough, can give much more information than many score words.
Cut one fruit-body, longitudinally down with a razor or scalpel or a sharp knife if the fruit-body is woody, and sketch the cut surfaces, fig. 1A-B. These sketches and the rest of the collection notes should be made such that identification and future comparisons can be achieved. Thus always note the characters in the same order for each description. A table of the important characters is provided here, but this is meant as a guide not as a questionnaire. The attachment of the gills, pores or teeth to the fruit-bodies when once the fungus is in section should be always noted (see p. 20).
The spore-print when complete should be allowed to dry under normal conditions and then the spore-mass scraped together into a small pile. A microscope cover-slip should be placed on the top of the pile and lightly pressed down. The colour of the spore-print (or deposit) can then be compared with a standard colour chart and the spores making up the print examined in water under a microscope.
When one is more experienced with fungi it will be found necessary to carry out many microscopic observations, but when commencing the study it is necessary only to have an ordinary microscope; a calibrated eyepiece-micrometer is an advantage as is an oil-immersion lens. An examination of the spores is always necessary; the examination of features such as the sterile cells on the gill and stem, etc., varies with the fungus under observation. Spores should if at all possible be taken from a spore-print and mounted on a microscope slide, either in water or in a dilute aqueous solution of household ammonia. Although for mycologists it is often necessary to measure spores to within a 1⁄2 micron (µm) this book has been so arranged that one only really has to distinguish between a spore which is small (up to 5 µm), medium (5-10 µm), long (10-15 µm), or large if globose and very long (if over 15 µm); this is not strictly accurate, but serves the purpose for an introductory text. It is important to describe the character of the spore, i.e. ornamentations, whether a hole (germ-pore) is present at one end and/or a beak (apiculus) at the other (fig. 5). With white or pale coloured spores it is useful to stain either the spore or the surrounding liquid with a dye—10% cotton blue solution is admirable, or a solution of 1·5 g iodine in 100 ml of an aqueous mixture containing 5 g of potassium iodine and 100 g of chloral hydrate. Both these dyes must be accurately made up if the study of the fungi is to be taken at all seriously; because some of the chemicals used above are not normally required by students, a chemist must make up the reagents for you. Often the spores turn entirely or partially blue-black or pale blue or purplish red in the iodine solution—a useful character.
[13]
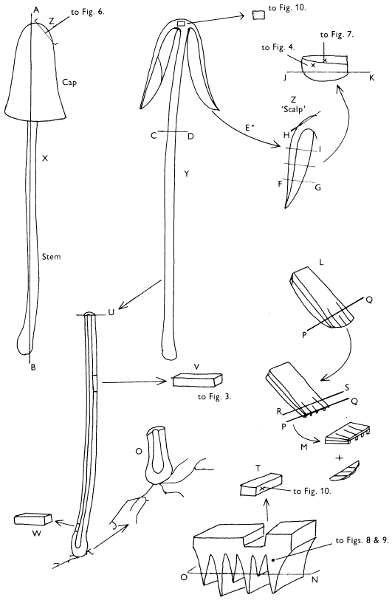
Fig. 1. Dissection of a toadstool as recommended by the author. For explanation see text.
[14]
Material in good condition is always required and one of the first things the student needs to do is train himself to collect sufficient material in good condition. The steps by which all the structures of the fungus used in the text can be observed are outlined below:—
Fig. 1 shows the cuts required to furnish suitable sections in order to observe the various structures and patterns of tissue which are important.
1. Carefully place the longitudinal section (AB) of the fruit-body which has been sketched gill-face down under a low power or dissecting microscope. Hairs or gluten on the cap, if present, will be made visible by focusing up and down (figs. 2 and 3A) and/or those on the stem (fig. 3B). When any part of the cut fruit-body is not being examined retain it in a chamber containing damp paper or moist moss; this will assist the cells to retain their turgidity, for they frequently collapse on drying and are difficult to observe except after performing often lengthy and special techniques.
If only one fruit-body is available, then cut along CD and mount in a tin box on a slide in order to obtain a spore-print (otherwise see paragraph 6).
2. Cut off a complete gill (E) and quickly mount on a dry slide. Under the low power of a microscope, the cystidia on the gill-margin will be visible (fig. 4); it will be seen whether the spores are arranged in a particular pattern (fig. 5) and whether the basidia are 2-spored or 4-spored. In white-spored toadstools it is difficult sometimes to determine whether the basidia are 2- or 4-spored so one must confirm the observations by other techniques.
[15]
[16]
A section of the gill accompanied by a small piece of cap-tissue, as in E, will confirm the presence or absence of noticeable cystidia (or hairs) on the cap. Now mount the section bounded by FG and HI in a drop of water containing either a drop of washing-up liquid and/or glycerine; the soapy liquid helps to expel any water which may tend to cling to the gill-margin amongst the cystidia and the glycerine stops the mount from drying out whilst further sections for comparison are cut and examined. It is at this time that the structure of the outermost layer of the cap can be examined, e.g. whether it is made up of a turf-like structure; the presence or absence of cystidia on the cap can be also confirmed (fig. 7A-C). It is frequently necessary to tap the mount in order to spread the tissue slightly and expose the elements; this can be done very efficiently by light pressure from the end of a pencil to which an eraser is attached. Cut off along line JK to eliminate marginal cystidia from confusing the picture and mount both pieces separately.
3. Cut out a wedge of tissue from the fruit-body (L) so as to have several gills attached to some cap-tissue; until one is familiar with the variability of facial and marginal cystidia, carefully cut along the line PQ (note: the cut is made one-third of the distance from the cap margin, thus eliminating the possibility of large numbers of marginal cystidia being examined in error for facial cystidia). Now make a second cut along the line of RS so that finally a small block of tissue remains (M).
Mount on a dry slide with the plane through PQ face down on the slide and observe under a low magnification, to assess whether cystidia on the gill-face are present or absent, and if present their general shape and whether numerous or infrequent (fig. 8).
Mount in water/washing-up mixture as outlined above and tap gently with the rubber attached to the end of a pencil; evenly distributed pressure should be given. If the gills appear to be too close then rotate the rubber a little whilst pressing in order to spread the tissue.
4. Using a low power of a microscope and looking down into the plane RS of the unmodified block M or a similar block, one obtains by this simple technique a very accurate idea as to the structure of the trama of the gill (fig. 9). The organisation of this tissue is very important in classification, some groups of toadstools having what has been described as regular trama (fig. 9C), others irregular (fig. 9D), inverse (fig. 9B) or divergent (fig. 9A). This same tissue may be thick or sparse to wanting, coloured or not. Such sections are often better than attempts at very thin sections unless very specialised techniques are used. There are few satisfactory thicknesses between the two extremes; the thick sections you can do and the very thin requiring expert techniques.
5. Take out a small block of tissue T as indicated in the figure (fig. 1). Mount immediately and repeat as in 3. This will allow the outer layer of the cap to be more clearly seen (fig. 7A-C) and also the structure of the flesh (fig. 10). The latter may be composed of a mixture of filaments and ‘packets’ or ‘nests’ of rounded cells (i.e. heteromerous), or of filaments, only some of which may be inflated (i.e. homoiomerous); but when individual cells are swollen they never form distinct groups. By very similar techniques it is possible to show that the more woody fungi can have flesh composed of one of four types of cells (Corner, 1932): these types depend on whether distinctly thickened cells (plate 47) are present with the actively growing hyphae or not (pp. 140-150), whether hyphae are present which bind groups of hyphae together, etc. (plate 46).
6. Remove stem along line CD and cut out small blocks of tissue as indicated (U, V and W). Mount immediately and examine as in paragraph 3, for cystidia, etc. (see fig. 3).
Whilst all these sections are being cut and processed a second fruit-body, if available, should be set to drop spores; this is done by cutting off the cap from the stem and placing it either entirely or in part, and with gill-edges down, on a slide in a tin.
7. Z is a ‘scalp’ of a cap; a thin sliver from the cap is placed on a slide in a drop of water (modified with washing-up liquid, etc. as above). After placing a cover-slip over the tissue it is tapped gently; this will show if the cap is composed of globose to elliptic elements or if it is composed of strictly filamentous units (figs. 6A & B). Care must be taken not to reverse the section when transferring it to the mountant, either by turning the scalpel or by allowing the surface tension of the liquid to pull the section upside down. The construction of any veil fragments will also be seen in this mount, and if a loose covering of veil is present this should be removed before observation so that it does not obscure the fundamental structures.
8. Examine the stipe of the fruit-body used above under a low power or with a dissecting microscope in order to ascertain whether there are any remains of veil and/or vegetative mycelium. If found, mount immediately in the solution containing iodine mentioned above and examine.
[19]
Of course it is difficult to carry out the above system the first time and be successful in seeing everything, indeed in being able to cut all the sections 1-8. Practice makes perfect, so why not practise with a 1⁄4 lb of mushrooms from the grocer before the autumn season starts. In this way you will have overcome the difficulties without having to experiment with your collections.
[20]
| CHARACTERISTICS FOR THE IDENTIFICATION OF HIGHER FUNGI WITH CAPS | |||||
| Locality | G. Ref. | Date | |||
| Habitat notes | soil type | pH | |||
| vegetational community | |||||
| solitary; in troops or rings | |||||
| Draw or preferably paint exterior and vertical section of fruit-body | |||||
| MACROSCOPIC CHARACTERS | |||||
| CAP | |||||
| General characters: | |||||
| diameter | shape | consistency | |||
| colour: | when immature | when mature | |||
| when wet | when dry | ||||
| Surface | |||||
| dry, moist, greasy, viscid, glutinous, peeling easily or not, smooth, matt, polished, irregularly roughened, downy, velvety, scaly, shaggy |
|||||
| Margin | |||||
| regular, wavy | incurved or not | ||||
| smooth, rough, furrowed | striate or not | ||||
| Veil, if present | |||||
| colour | abundance or scarcity | ||||
| distribution at margin, whether appendiculate or dentate | |||||
| consistency, whether filamentous, membranous | |||||
| GILLS, or pores or teeth etc. | |||||
| remote, free, adnate, adnexed, emarginate, subdecurrent, decurrent | |||||
| crowded or distant | distinctly formed or not | ||||
| shape | interveined or not | ||||
| easily separable from the cap-tissue or not | |||||
| consistency (whether brittle, pliable, fleshy or waxy) | |||||
| thickness | width | ||||
| colour: | when immature | at maturity | |||
| number of different lengths or number of layers | |||||
| obvious features of gill-edge, tube-edge, e.g. colour, consistency | |||||
| STEM | |||||
| central, eccentric or lacking | shape | ||||
| dimensions: length | thickness | ||||
| hollow or not | |||||
| colour: | when immature | when mature | |||
| consistency (whether fleshy, stringy, cartilaginous, leathery or woody) | |||||
| surface characters (whether fibrillose, dry, viscid, scaly or smooth) | |||||
| characters of stem-base | |||||
| Veil, if present | characters | ||||
| Volva, if present | characters | ||||
| Ring, if present | |||||
| whether single or double | whether membranous or filamentous | ||||
| whether persistent, fugacious or mobile | whether thick or thin | ||||
| whether apical, median or basal | |||||
| FLESH | |||||
| colour in cap: | when wet | when dry | |||
| colour in stem: | when wet | when dry | |||
| colour changes if any when exposed to air | |||||
| presence or absence of milk-like or coloured fluid | |||||
| (note: colour when exuded on fruit-body immediately and after some time and when dabbed on to a clean cloth or paper handkerchief and exposed to the air). | |||||
| SMELL | before and after cutting | —relate to a common every day odour | |||
| MICROSCOPIC CHARACTERS | |||||
| BASIDIOSPORES | |||||
| colour in mass | colour under microscope. | ||||
| shape | size | type of ornamentation, if any | |||
| size and shape of germ-pore, if present | |||||
| iodine reaction of spore-mass:—blue-black to dark violet (amyloid); red-purple | |||||
| (dextrinoid); yellow-brown or brown (non-amyloid) | |||||
| BASIDIA | number of sterigmata | ||||
| CAP-FLESH | type of constituent cells | ||||
| GILL-TISSUE | type and arrangement of cells between adjacent hymenial faces | ||||
| CAP-SURFACE | type of cells composing the outermost layer—whether filaments or rounded cells | ||||
| STERILE CELLS—CYSTIDIA | |||||
| presence or absence of sterile cells:— | |||||
| on gill-edge | on gill-margin | ||||
| on cap | on stem | ||||
| shape, estimation of size, thick or thin-walled, hyaline or not | |||||
| types of ornamentation, etc. | |||||
[21]
[22]
[27]
Cap: width 45-150 mm. Stem: length 70-200 mm; width 20-30 mm.
Description: Plate 1.
Cap: convex and becoming only slightly expanded at maturity, pale brown, tan or buff, soft, surface dry, but in wet weather becoming quite tacky, smooth or streaky-wrinkled and cap-margin not overhanging the tubes.
Stem: white, buff or greyish, roughened by scurfy scales which are minute, pale and arranged in irregular lines at the stem-apex, and enlarged and dark brown to blackish towards the base.
Tubes: depressed about the stem, white becoming yellowish brown at maturity, with small, white pores which become buff at maturity and bruise distinctly yellow-brown or pale pinkish brown when touched.
Flesh: watery, very soft in the cap lacking distinctive smell and either not changing on exposure to the air or only faintly becoming pinkish or pale peach-colour.
Spore-print: brown with flush of pinkish brown when freshly prepared.
Spores: very long, spindle-shaped, smooth, pale honey-coloured under the microscope and more than 14 µm in length (14-20 µm long × 5-6 µm broad).
Marginal cystidia: numerous and flask-shaped. Facial cystidia: sparse, similar to marginal cystidia.
Habitat & Distribution: Found in copses and woods containing birch trees, or even accompanying solitary birches.
General Information: This fungus is recognised by the pale brown cap, the white, unchanging or hardly changing flesh and the cap-margin not overhanging the tubes. There are several closely related fungi which also grow with birch trees but they need some experience in order to distinguish them. This fungus was formerly placed in the[28] genus Boletus, indeed it will be found in many books under this name. Species of Leccinum are edible and considered delicacies in continental Europe. The majority can be separated from the other fleshy fungi with pores beneath the cap, i.e. boletes, by the black to brown scaly stem and rather long, elongate spores. The scales on the stem give rise to the common name ‘Rough stalks’ which is applied to this whole group of fungi.
Illustrations: F 39C; Hvass 253; LH 122; NB 1556; WD 891.
Cap: width 30-100 mm. Stem: width 15-20 mm; length 50-70 mm.
Description: Plate 2.
Cap: convex or umbonate at first, later expanding and then becoming plano-convex, golden-yellow or rich orange-brown, very slimy because of the presence of a pale yellow sticky fluid.
Stem: apex reddish and dotted or ornamented with a fine network, cream-coloured about the centre because of the presence of a ring which soon collapses, ultimately appearing only as a pale yellow zone; below the ring the stem is yellowish or rusty brown, particularly when roughly handled.
Tubes: adnate to decurrent, deep yellow but becoming flushed wine-coloured on exposure to the air, with angular and small sulphur-yellow pores which become pale pinkish brown to lilaceous or pale wine-coloured when handled.
Flesh: with no distinctive smell, pale yellow immediately flushing lilaceous when exposed to the air, but finally becoming dingy red-brown, sometimes blue or green in the stem-base.
Spore-print: brown with distinct yellowish tint when freshly prepared.
Spores: long, ellipsoid, smooth and pale honey when under the microscope, less than 12 µm in length (8-11 µm long × 3-4 µm broad).
Marginal cystidia: in bundles and encrusted with amorphous brown, oily material. Facial cystidia: similar in shape and morphology to marginal cystidia.
Habitat & Distribution: Found on the ground accompanying larch trees either singly or more often in rings or troops.
General Information: This fungus is easily recognised by the poorly developed ring, overall golden-yellow colour and pale yellow viscidness on the cap which comes off on to the fingers when the fruit-body is handled. There are several closely related fungi which also grow with coniferous trees, e.g. Suillus luteus Fries, ‘Slippery jack’, but many need experience in order to identify them. All these fungi were formerly placed in the genus Boletus, because of the fleshy fruit-body with pores beneath the cap. The larch-bolete receives its common name from the close relationship of the fungus with the larch. On drying S. luteus and S. grevillei may strongly resemble one another but the former can be distinguished when fresh by the chocolate brown, sepia, or purplish brown cap and the large whitish, lilac-tinted ring.
[29]
[30]
[31]
Species of Suillus are edible and rank highly in continental cook-books, although they have disagreeably gelatinous-slimy caps, a character, in fact, which helps to separate them from other fleshy pore-fungi.
Illustrations: F 41a; Hvass 257; ML 187; NB 1044; WD 842.
Cap: width 70-130 mm. Stem: width 34-37 mm; length 110-125 mm. (36-40 mm at base).
Description: Plate 3.
Cap: hemispherical, minutely velvety, but soon becoming smooth and distinctly viscid in wet weather, red-brown flushed with date-brown and darkening even more with age and in moist weather to become bay-brown.
Stem: similarly coloured to the cap but paler particularly at the apex, smooth or with faint, longitudinal furrows which are often powdered with minute, dark brown dots.
Tubes: adnate or depressed about the stem, lemon-yellow but immediately blue-green when exposed to the air and with angular, rather large similarly coloured, pores which equally rapidly turn blue-green when touched.
Flesh: strongly smelling earthy, pale yellow but becoming pinkish in centre of the cap, and blue in the stem and above the tubes when exposed to the air, but finally becoming dirty yellow throughout.
Spore-print: brown with a distinct olivaceous flush.
Spores: long, spindle-shaped, smooth, honey-coloured under the microscope and greater than 12 µm in length (13-15 µm long × 5 µm broad).
[32]
Marginal cystidia: numerous, flask-shaped and slightly yellowish.
Facial cystidia: scattered and infrequent and similar to marginal cystidia in shape.
Habitat & Distribution: Found in woods, especially accompanying pine trees, but often found fruiting on the site of former coniferous trees, even years after the trunks or the stumps have been removed.
General Information: This fungus is recognised by the rounded, red-brown cap, coupled with the pale yellow flesh and greenish yellow tubes, both of which become greenish blue when exposed to the air. There are several species in the genus Boletus which stain blue at the slightest touch or when the flesh is exposed to the air, e.g. B. erythropus (Fries) Secretan, a common bolete with a dark olivaceous cap, orange pores and red-dotted stem.
The flesh of some species of Boletus, e.g. B. edulis Fries, however, remains unchanged or at most becomes flushed slightly pinkish. Although many people say they recognise B. edulis, the ‘Penny-bun’ bolete—a name derived from the colour of the cap, there is some doubt as to whether the true B. edulis is common in Britain as we are led to believe. B. edulis and its relatives are highly recommended as edible (see p. 35). B. badius is also edible, but it is ill-advised to eat any bolete which turns blue when cut open.
Illustrations: B. badius—F 38c; Hvass 248 (not very good); LH 191; NB 1095; WD 851. B. edulis: F 42a; Hvass 246; LH 191; NB 1433.
There are nearly seventy boletes recorded for the British Isles and evidence of others which have as yet not been fully documented. As a group they are characterised by being fleshy, possessing a central stem and producing their spores within the tubes, and not on gills as in the common mushroom. It is the first character by which the boletes differ so markedly from the other pored fungi, such as the ‘Scaly Polypore’ (see p. 140).
The boletes have long been classified in the genus Boletus, but instead of referring all the pored, fleshy fungi to a single large genus several genera are now recognised. The separation of these genera is based on differences in colour of the spore-print and differences in the anatomy of the tubes, cap and stem, etc., e.g. members of the genus Suillus have colourless or pale coloured dots on the stem exuding a resin-like liquid in wet weather, which is clear and glistening in some species but turbid and whitish in others, gradually darkening and hardening so that the stem is ultimately covered in dark brown or reddish smears or spots; members of the genus Leccinum on the other hand never exude liquid and have coarse or fine roughenings on the stem which are usually dark, but may commence white and ultimately darken depending on the species; many species of Boletus possess a very distinct raised network all over the stem, whilst others have it present only in part, or have minute, often brightly coloured, dots replacing it.
[33]
[34]
Within this single, yet not particularly large, group of fungi, several biological phenomena are demonstrable. There is good evidence that the majority of British boletes are mycorrhizal; several species are known to be associated only with one species of tree or group of closely related tree-species. Thus Suillus grevillei and S. aeruginascens (Secretan) Singer grow in association with larch trees; S. luteus and Boletus badius in contrast grow in association with pine trees; Leccinum scabrum with birch trees; L. aurantiacum (Fries) S. F. Gray, with poplar trees and L. quercinum (Pilát) Green & Watling, with oak trees.
Boletus edulis can be separated into several distinct subspecies which are associated with different trees; the two commonest subspecies are those associated with birch and with beech trees. It is well known that although present in this country during the warmer periods of the Ice-Age, larch neither survived the intense cold of the last advance of the ice nor migrated back into Britain after the ice had melted. Thus all larches which we see in Britain have been planted by man. There is little doubt that mycelia of many fungi were introduced along with these plants very probably including the mycelium of the larch-bolete. A similar pattern can be seen with other introduced trees, although not to such a marked degree, e.g. spruce trees. The beech tree, however, is native to the south of England, unlike the larch returning to this country after the ice had melted; it has been planted extensively outside its former range in northern areas of the British Isles taking with it its associated fungi. There is some evidence that some stocks of beech and fungi have been introduced from continental Europe in comparatively recent times.
A parallel, yet inexplicable association is found between the bolete Suillus bovinus (Fries) O. Kuntze and its close relative Gomphidius[35] roseus (Fries) Karsten where the mycelium of two fungi are found intertwined forming a close association! Parasitism although rare is also found amongst the boletes, and an uncommon parasitism at that—a fungus on a fungus; for example in Britain although infrequent Boletus parasiticus Fries grows attached and ultimately replaces the spore-tissue of the common earth-ball (Scleroderma, see p. 192).
Those fungi which grow on dead and decaying substrates are called saprophytes and although the greater number of higher fungi would be included in this class of organisms the character is infrequent amongst the boletes. One British example of this type of fungus is the rare Boletus sphaerocephalus Barla which grows on woody debris.
Chemists have long been interested in boletes, for as noted above the flesh of some species when exposed to the atmosphere turns vivid colours, a feature often incorporated into the Latin name, e.g. Boletus purpureus Persoon, from the purple colours produced whenever the fruit-body is handled. The reaction appears to be an oxidation where in the presence of an enzyme and oxygen a pigmented substance or substances are produced. What the significance of these colour-changes is in nature is as yet unknown; however, what is interesting is that many of the chemicals involved are unique and have only recently been analysed completely; they are related to the quinones.
There is little doubt that it is this rapid and intense blueing of the flesh of many boletes that has lead to a belief that they are poisonous. It is uncertain whether there are any truly toxic species of Boletus but several have unpleasant smells and tastes which make them very unattractive. Boletus edulis is the important ingredient, however, which gives the distinctive taste to so-called dried mushroom soup. Thousands of fruit-bodies are collected annually in the forests of Europe to be later dried and processed for incorporation into soup. Boletes appear to form an important part of the diet of several rodents and deer and in Scandinavia in the diet of reindeer.
Probably one of the most obscure of our British boletes is Strobilomyces floccopus (Fries) Karsten, the ‘Old Man of the Woods’. It has a black, white and grey woolly, scaly cap and stem, and the flesh distinctly reddens when exposed to the air. The spores are almost spherical, purple-black in colour and covered in a coarse network when seen under the microscope. All these characters readily separate Strobilomyces from all other European boletes; however, in Australasia, members of this and related genera form a very important part of the flora.
[36]
Cap: width 30-150 mm. Stem: width 10-18 mm; length 60-120 mm.
Description:
Cap: convex with a pronounced often sharp umbo, wine-coloured, flushed with bronze-colour at centre and yellow or ochre at margin, viscid but soon drying and then becoming paler and quite shiny.
Stem: yellowish orange, apricot-coloured or peach-coloured, streaked with dull wine-colour, spindle-shaped or narrowed gradually to the apex from a more or less pointed base.
Gills: arcuate-decurrent, distant, at first greyish sepia then dingy purplish with paler margin, but finally entirely dark purplish brown.
Flesh: lacking distinctive smell and reddish yellow or pale tan in the cap, rich apricot- or peach-colour towards the stem-base.
Spore-print: purplish black.
Spores: very long, spindle-shaped, smooth, olivaceous purple and greater than 20 µm in length (20-23 × 6-7 µm).
Marginal cystidia: cylindrical to lance-shaped and up to 100 × 15 µm.
Facial cystidia: similar to marginal cystidia.
Habitat & Distribution: Found in pine woods, usually solitary or in small groups. Fairly common throughout the British Isles and characteristic of Scots Pine woods.
General Information: This fungus can be distinguished by the purplish or wine-coloured cap and the gills being pigmented from youth. There is only one other British species of this genus, i.e. C. corallinus Miller & Watling.
Chroogomphus is separated from Gomphidius by the flesh having an intense blue-black reaction when placed in solutions containing iodine, and the gills being coloured from their youth. In many books Chroogomphus is placed in synonymy with the genus Gomphidius. However, Gomphidius glutinosus (Fries) Fries, G. roseus (Fries) Karsten and G. maculatus Fries all have whitish gills when immature which gradually darken, and their flesh simply turns orange-brown in solutions of iodine. G. glutinosus is uniformly grey in colour and is most frequently found under spruce and other introduced conifers: G. roseus has a pale-pinkish coloured cap and white stem, and grows with pine; G. maculatus grows under larch and is flushed lilaceous at first but becomes strongly spotted with brown when handled.
Illustrations: Hvass 192; LH 213; WD 833.
[37]
[38]
Cap: width 50-120 mm. Stem: width 8-15 mm; height 30-75 mm.
Description:
Cap: at first convex with a strongly inrolled, downy margin, but then expanded and later frequently depressed towards the centre, clay-coloured, ochre or yellow-rust, slightly velvety but becoming smooth or sticky particularly in wet weather and readily bruising red-brown when fresh.
Stem: central or slightly eccentric, thickened upwards, fibrillose-silky, similarly coloured to the cap but typically streaked with red-brown particularly with age.
Gills: ochre or yellow-brown then rust and finally darker brown, decurrent, crowded, often branched and united about the apex of the stem; easily peeled from the flesh with the fingers and rapidly becoming red-brown on handling.
Flesh: thick, soft and with slightly astringent smell and yellowish to brownish but becoming red-brown after exposure to the air.
Spore-print: rust-brown.
Spores: medium-sized, ellipsoid, smooth, deep yellow-brown and rarely greater than 10 µm in length (8-10 × 5-6 µm).
Marginal cystidia: numerous lance-shaped or spindle-shaped.
Facial cystidia: scattered and similar in shape to marginal cystidia.
Habitat & Distribution: Found on heaths and in mixed woods, particularly where birch has or is now growing, or even accompanying solitary birch trees.
General Information: This fungus is easily recognisable by the strongly inrolled, woolly margin of the cap and yellow-brown gills which are easily separable from the cap-flesh. P. rubicundulus P. D. Orton is similar but grows under alder and has yellow gills unchanging when handled and dark scales on the cap. P. atrotomentosus (Fries) Fries and P. panuoides (Fries) Fries both grow on coniferous wood and have smaller spores; the former is recognised by the dark brown to almost black shaggy stem and the latter by the shell-shaped cap devoid almost completely of a stem.
Illustrations: F 41c; Hvass 189; LH 185; NB 1158; WD 702.
[39]
[40]
Cap: width 60-125 mm. Stem: width 15-25 mm; length up to 180 mm.
Description:
Cap: bell-shaped or bluntly conical only slightly expanding with maturity, smooth or wrinkled at centre but often furrowed at the margin, slimy, brown with a distinct olive flush when in fresh condition and becoming ochraceous brown and shiny when dry.
Stem: usually swollen to some degree about the middle, slimy particularly towards the base, whitish throughout when young except for a faint amethyst or violaceous flush in the lower part; as the slime dries the stem becomes shiny and the outer surface breaks up into fibrillose scales or scaly, irregular ring-zones.
Flesh: lacking distinct smell, white with ochraceous flush in the cap, white in the stem, thick and soft in the cap but fibrous in the stem.
Gills: adnate, broad, rather thick, frequently veined and distant, ochraceous brown and finally deep rust-brown.
Spore-print: rust-colour.
Spores: long, slightly almond-shaped in side view, finely warted throughout and not less than 12 µm in length (13-14 × 7-8 µm).
Marginal cystidia: ellipsoid or club-shaped, hardly different from the surrounding undeveloped basidia.
Facial cystidia: absent.
Habitat & Distribution: Found on the ground in copses and woods especially those containing beech.
General Information: Recognised by the conical, grooved cap and the slimy spindle-shaped stem with a distinct violaceous flush; this fungus is often misnamed C. elatior Fries but this is a much less common fungus. There are several closely related fungi, but these grow with other tree-species and need much more experience to distinguish one from the other. C. pinicola P. D. Orton is one such species growing in the litter under Pinus sylvestris, Scots Pine; this species is fairly common in the remnant pine woods of Northern Scotland. The large size, sticky or glutinous cap and stem indicate that this fungus belongs to Cortinarius, subgenus Myxacium.
Illustrations: Hvass 145; LH 162; NB 119; WD 601.
[41]
[42]
The genus Cortinarius is the largest genus of agarics in the British Isles, indeed in Europe and North America—perhaps in the world. It includes some of our most beautiful agarics, yet it is one of the least satisfying to the mycologist because of the difficulties experienced in identifying collections—partly because many species are so seldom seen.
Cortinarius contains just under two hundred and fifty recognisable British species, although recent research has shown that many more are yet to be described from this country as new to science. Except for some very characteristic species the individual members within the genus Cortinarius are often very difficult to separate one from the other; however, Cortinarius is one of our least difficult genera to recognise in the field owing to the presence when mature of rust-coloured gills and a cobwebby veil which extends from the margin of the cap to the stem. This structure is termed a cortina (Fig. 14) and in young specimens covers the gills with delicate filaments. As the cap expands the cottony or cobwebby filaments are stretched and either disappear entirely or may collapse to form a ring-like zone of filaments on the stem. In some species a second completely enveloping veil is also found, and this veil is viscid in one distinct group of which C. pseudosalor already described is a member. The gills in the genus are variable in colour when young although constant for a single species; they may be lilaceous purple, orange, brown, red, yellow-ochraceous or tan, but ultimately in all members at maturity they become rust-colour. The spores under the microscope are richly coloured, yellow to red-brown and are frequently strongly warted; in mass they are rust-brown and this character coupled with the presence of the cobweb-like veil characterises the genus.
Within the genus Cortinarius there is a wide range of characters varying from species with distinctly sticky caps and stems, some with sticky caps and dry stems to those with both dry caps and stems. A few species are very large and fleshy whilst others are quite slender and many of the latter rapidly change colour on drying out and are then said to be hygrophanous. However, although there is such a large spectrum of characters in a single genus the species all have in common the cortina and rust-coloured gills, the latter often appearing as if powdered with rusty dust.
[43]
Utilising the characters mentioned above this very large genus can be split into the following six sections, called by the mycologist subgenera:
In several continental books some or all of these divisions are recognised as distinct genera in their own right. The subgenus Telamonia which occurs in many texts was formerly thought to differ from Hydrocybe in the presence of a universal veil; the universal veil is a second veil which completely envelopes the fruit-body when it is young and is in addition to the cortina. However, the modern treatment would seem to suggest that the presence of the universal veil is not of the utmost importance and so the two subgenera are incorporated into one. The name Hydrocybe reflects the character of changing colour as it dries out because of the loss of water. Within each subgenus the species are distinguished by the colour of the young gills and of the cap, the veil colour and texture, and microscopic characters of the spores, particularly their size.
The majority of species of Cortinarius are mycorrhizal and like the boletes possess very specific relationships with tree species. Thus some are typical of coniferous woodland and others typical of deciduous woodland in general, whilst others typify woods of a particular tree, e.g. beech, oak, birch, pine, larch. Some species are characteristic of woods on limestone or chalky soils (calcareous) whilst others are characteristic of woods on sandy, heathy acidic soils. For example, Cortinarius armillatus (Fries) Fries which is found in damp woods and possesses one or more cinnabar-red or scarlet zones on the stem and[44] red fibrils at the stem-base appears to be connected with birch. Several species are associated with native trees whilst others have undoubtedly been introduced from abroad. They are very important in the economy of the woodland ecosystem.
One of the most beautiful and easily distinguished of our British species is Cortinarius violaceus (Fries) Fries which has uniformly deep violet-coloured stem and cap and coloured cystidia on the gill-margin, a character unusual in Cortinarius.
No species are known to be truly poisonous and many species are known to be edible, but many are too small to be of any value. Some of the larger species are regarded as good to eat, but frequently are too scarce. Thus the necessity for experience to recognise the different species, coupled with their often unpleasant tastes make them an unimportant group of agarics for eating.
[45]
Cap: width 50-100 mm. Stem: width 20-35 mm; length 50-100 mm.
Description: Plate 7.
Cap: yellow-ochre or dull yellow becoming paler with age, or flushed faintly greyish green, convex but soon expanding and becoming flat or depressed in the centre, smooth, or granular when young and slightly tacky in wet weather, faintly striate at the margin.
Stem: white at first then flushed slightly greyish, smooth or wrinkled, firm at first but quickly becoming soft and fragile.
Flesh: brittle, firm at first then soft, white, yellow under cap-centre.
Gills: white at first then flushed pale cream-colour, brittle, adnexed to free, rather distant.
Spore-print: faintly cream when freshly prepared.
Spores: medium-sized, hyaline, broadly ellipsoid or subglobose to almost globose, coarsely ornamented with prominent warts which stain blue-black when mounted in solutions containing iodine and which are faintly interconnected by low ridges, about 8 × 7 µm in size (9-10 × 7-8 µm).
Marginal cystidia: prominent, lance- to spindle-shaped and often filled with oily material.
Facial cystidia: similar in shape to marginal cystidia and projecting some distance from the gill-face.
Habitat & Distribution: Commonly found in mixed woods from summer until late autumn.
General Information: Easily recognised by the ochre-yellow cap, very pale cream-coloured spore-print and greying stem. Two other yellow-capped species of Russula are commonly found. R. claroflava Grove with yellow spore-print and blackening fruit-body which grows with birches in boggy places, and R. lutea (Fries) S. F. Gray which is much smaller, having a cap up to 50 mm and very deep egg-yellow gills and spore-print; it grows in deciduous woods.
Illustrations: F 22a; Hvass 226; LH 119; NB 1371; WD 491.
A large genus with nearly one hundred distinct species in the British Isles and several others yet unrecognised or undocumented. This genus is composed generally of large toadstools often beautifully coloured, indeed the majority have brightly coloured caps in reds, purples,[46] yellows or greens depending on the species although a few are predominantly white bruising reddish brown or grey to some degree.
Such large and distinctive fungi one would think would be the easiest members of our flora to identify, unfortunately they are not. They form a group quite isolated in their relations, the only close relatives being members of the genus Lactarius, to be dealt with later (see p. 50). The flesh of members of both Lactarius and Russula contains groups of rounded cells, a feature unique amongst agarics and explains why in Russula the fruit-bodies, cap and gills and sometimes the stem are brittle and easily break if crushed between the fingers. The fruit-body does not exude a milky liquid when the flesh is broken.
The spore-print varies, depending on the species involved, from white to deep ochre and individual spores are covered in a coarse ornamentation which is composed of isolated warts or warts interconnected by raised lines, or mixtures of both. The ornamentation stains deep blue-black when the spores are mounted in solutions containing iodine and the pattern which is produced appears in many cases to be of a specific character.
The majority of the species, if not all north-temperate species are mycorrhizal and the familiar host-tree fungus relationship can be recognised:—
R. claroflava Grove, with birch in boggy places, R. emetica (Fries) S. F. Gray with pine in wet places, R. betularum Hora with birch in grassy copses and R. sardonia Fries with pines. Brief notes are here included giving the basic characters of eight common species, but it must be appreciated the identification of many species within this genus is difficult.
Cap: width 50-100 mm. Stem: width 14-25 mm; length 60-80 mm.
Cap: deep reddish purple but becoming spotted with either cream-colour or white blotches.
Stem: white but becoming flushed greyish or stained brownish with age.
Gills: white then very pale yellow.
Flesh: white in cap and stem.
Spore-print: white.
On the ground in mixed woods and copses, particularly those containing oak.
[47]
[48]
Cap: width 50-150 mm. Stem: width 10-30 mm; length 50-100 mm.
Cap: lilac, bluish to purple often with green tints.
Stem: pure white.
Gills: pure white.
Flesh: white.
Spore-print: white.
Common in deciduous woods, especially beech-woods.
Cap: width 50-100 mm. Stem: width 8-15 mm; length 25-70 mm.
Cap: bright scarlet fading with age to become spotted pinkish, slightly viscid when moist.
Stem: spongy, fragile.
Flesh: white.
Gills: pure white.
Spore-print: pure white.
In pine woods usually in boggy areas.
Cap: width 40-75 mm. Stem: width 10-20 mm; length 30-75 mm.
Cap: tacky when fresh, straw-coloured or pale tawny brown.
Stem: similarly coloured to the cap.
Gills and flesh: pale straw-colour and smelling of House Geraniums (i.e. Pelargoniums).
Spore-print: cream-coloured.
Common under beech.
Cap: width 70-170 mm. Stem: width 15-30 mm; length 50-90 mm.
Cap: slimy, dingy yellow to tawny, margin strongly furrowed and ornamented with raised bumps.
Stem: whitish then flushed or spotted with rust-brown.
Gills: straw-coloured, often spotted brown with age and beaded with watery droplets when growing under moist conditions.
Flesh: white to cream, brittle and with foetid-oily smell.
Spore-print: pale cream-colour.
Common in deciduous woods.
[49]
Cap: width 30-75 mm. Stem: width 7-15 mm; length 35-70 mm.
Cap: scarlet red but developing creamy areas with age, dry.
Stem and gills: white but with a distinct although faint greenish grey flush, the former fairly firm.
Flesh: white.
Spore-print: pure white.
Commonly accompanying beech, even individual trees in gardens.
Cap: width 75-200 mm. Stem: width 15-35 mm; length 25-75 mm.
Cap: cream-coloured then flushed sooty brown, finally black as if scorched by proximity to bonfire.
Stem: white then dark brown.
Gills: pale ochre reddening when bruised, thick and very distant.
Flesh: white slowly dull red on cutting then brown and finally changing soot-colour after some time.
Spore-print: white.
Common in deciduous woods.
Cap: width 50-140 mm. Stem: width 15-30 mm; length 40-60 mm.
Cap: deep blood-red or brownish red.
Stem: white with a flush of red towards the base.
Gills: cream then ochraceous.
Flesh: white staining brownish and smelling strongly of fish- or crab-paste, and staining dark green when a crystal of green iron sulphate is rubbed into it.
Spore-print: deep cream-colour.
Common in mixed woods; a very variable fungus with many colour-forms, but easily recognised by the green reaction with ferrous sulphate.
[50]
Cap: width 60-200 mm. Stem: width 10-25 mm; length 40-75 mm.
Description:
Cap: firm, convex usually with a central depression at maturity, dark olive-brown or dark greyish olive with a yellow-tawny, woolly margin when young which soon disappears, and the whole cap becomes sticky with age and turns deep purple when a drop of household ammonia is placed on it.
Stem: short, stout, similarly coloured to the cap except for the distinctly ochraceous apex, slimy and pitted.
Gills: crowded, cream-coloured to pale straw-coloured, but soon spotted with dirty brown, particularly when bruised.
Flesh: white or greyish ochre exuding a milk-like liquid which lacks a distinct smell and is white and unchanging when exposed to the air.
Spore-print: pale pinkish buff.
Spores: subglobose or ellipsoid and covered in a network of strongly developed, raised lines interconnected by finer ones, both of which stain blue-black in solutions containing iodine, generally 8 × 6 µm in size (7-8 × 6-7 µm).
Marginal cystidia: lance- or spindle-shaped and filled with oily contents.
Facial cystidia: similar to marginal cystidia.
Habitat & Distribution: Common in woods and copses, or on heaths especially in boggy places but always where birch is growing.
General Information: Easily recognised by the dull colours and purple reaction with alkali; there is no British species with which L. turpis can be mistaken. The purple reaction is similar to that found in the familiar school laboratory reagent litmus, for the compound found in L. turpis turns purple in alkali and reddens in acidic solutions. First discovered by Harley in 1893 this reaction marked the beginning of a whole series of chemical studies on the agarics which has led to the discovery of many unique compounds.
Illustrations: Hvass 214 (but too green); LH 213; NB 1133; WD 381.
There is little doubt that the genus Russula and the genus Lactarius are closely related; in fact they stand aside from the other agarics in the very important character mentioned on page 46. In Europe the easiest distinction between the two genera is that members of the genus Lactarius exude a milk-like juice which may be white or variously coloured depending on the species involved (e.g. purple in L. uvidus (Fries) Fries, yellow in L. chrysorheus Fries). The cap, stem and frequently the gills are brittle and when broken liberate the milk-like liquid; when the fruit-body is dry, however, the presence of this liquid may be difficult to demonstrate. The spores have a blue-black ornamentation under the microscope when mounted in iodine, and although when in mass the colours are not as varied as those found in the genus Russula there is every likelihood that they will play an important role in the classification of the group in the future. The colour of the spore-print has been rather neglected, although the genus includes some rather unusual fungi.
[51]
[52]
The odours of many species are very distinct and vary from the smell of coconut and spice to those of various flowers; an odour commonly met with is termed ‘oily rancid resembling butter which has become mouldy’; in early books it was described as being the smell of bed-bugs!
The majority of the species are undoubtedly mycorrhizal: thus L. torminosus is found with birch, L. deliciosus and L. rufus with conifers and L. quietus with oak. Brief notes are given on additional species:—
Cap: width 20-50 mm. Stem: length 20-50 mm; width 4-6 mm.
Cap and stem: red-brown.
Gills: reddish brown.
Flesh: reddish buff with an aromatic odour resembling spices which becomes very strong when dried and exudes a pale thin milk-like liquid.
Common in conifer woods and plantations.
Cap: width 50-120 mm. Stem: length 20-60 mm; width 15-25 mm.
Cap: viscid, dirty greyish ochre with flush of tawny but soon becoming greenish with age.
Stem: dirty buff or greyish ochre, spotted with green particularly with age or on handling.
Gills: orange-yellow bruising deep orange but becoming green with time.
[53]
Flesh: pinkish to apricot-coloured but becoming green with age and exuding a rich orange-red fluid which gradually becomes greyish green.
Frequent in conifer woods and plantations.
Cap: width 20-50 mm. Stem: length 30-50 mm; width 5-8 mm.
Cap: usually with a central ‘bump’, greyish lilac, dull and minutely scaly or velvety.
Stem: white to pale yellowish.
Gills: pale yellowish to flesh-coloured then flushed lilaceous.
Flesh: pale yellowish or flushed lilaceous, smelling strongly of desiccated coconut and exuding a white unchanging milk-like liquid.
In woods and on heaths, particularly where birch is growing.
Cap: width 30-80 mm. Stem: length 40-80 mm; width 10-15 mm.
Cap and stem: milky cocoa-coloured, zoned with reddish brown.
Gills: pale ochraceous then flushed red-brown.
Flesh: similar to gills, smelling strongly of rancid oil, and exuding a white, thin milk-like liquid which becomes very, very faintly yellow on exposure to the air.
Common wherever oak is growing.
Cap: width 50-90 mm. Stem: length 50-90 mm; width 10-15 mm.
Cap: dark red-brown with a distinct, usually sharp ‘bump’ in centre.
Stem: pale red-brown throughout or whitish at base.
Gills: pale reddish brown and exuding a white, unchanging milk-like fluid.
In pine woods and less frequently with birches on acid heaths.
Cap: width 40-150 mm. Stem: length 60-100 mm; width 15-30 mm.
Cap: pale strawberry-pink or pale salmon colour, distinctly zoned, slimy when wet at centre and strongly shaggy fibrillose at margin.
Stem and gills: pale strawberry colour.
Flesh: tinged salmon-pink and exuding a white unchangeable milk-like liquid.
Frequent where birches grow.
[54]
Cap: width 100-175 mm. Stem: width 30-40 mm; length 150-275 mm.
Description:
Cap: bright scarlet to orange-red with scattered whitish or yellowish fragments of veil particularly towards the centre and hanging down from the margin, viscid when moist, striate at margin with age.
Stem: white, striate above the soft easily torn, although prominent, ring which is white above and yellow below; stem-base swollen and ornamented with patches of yellowish or white veil-fragments which form concentric rings or ridges of tissue.
Gills: white, free, crowded, fairly thick, minutely toothed at their edge.
Flesh: soft, lacking distinctive smell, or at times slightly earthy and white, yellowish below cap-centre.
Spore-print: white.
Spores: long, hyaline under the microscope, ellipsoid, smooth about 10 × 7 µm in size (10-13 × 7-8 µm).
Marginal cystidia: composed of chains of swollen, hyaline cells.
Facial cystidia: absent.
Habitat & Distribution: Found in birch-woods, less frequently collected in the vicinity of conifers; wide-spread and fairly common, but it is erratic in its appearance giving the impression of being absent from a locality until one season it suddenly fruits in profusion.
General Information: An easily recognised fungus because of its striking colour. It is also very familiar and well-known because it appears so often on Christmas cards, and features commonly in illustrations in children’s story-books. The fungus contains a poison which formerly was used to kill flies—hence the common name of ‘Fly agaric’ and the scientific name from the latin name for the house-fly. The red skin of the cap, where the major amount of the poison resides, was cut up with a little milk and sugar or honey; flies attracted to this sweet concoction inadvertently ate the poison and later perished. This fungus has a very well documented and long history and appears in the legends of many countries. It is featured in Greek mythology, Slavic and Scandinavian folk-lore and indeed appears in the pre-history of Indian tribes of N.E. Asia. It has even been connected with the formation of certain sects within the early Christian church.
Illustrations: F. frontispiece; Hvass 1; LH 117; NB 1131; WD 21.
[55]
[56]
The genus Amanita contains many important mycorrhizal fungi including the ‘Blusher’, A. rubescens (Fries) S. F. Gray, the ‘Tawny grisette’, A. fulva Secretan, and the ‘False death-cap’, A. citrina S. F. Gray. The first grows on heaths and in woods with a variety of trees; A. fulva frequently grows with birch and A. citrina with several leafy trees although its var. alba (Gillet) E. J. Gilbert appears to be confined to beech woods. However, there is some evidence that many members of the genus in drier more southern countries than Britain, are non-mycorrhizal. In fact the genus as a whole may be southern-temperate in its distribution. In the British Isles the number of species of Amanita recorded decreases as one goes north, or the frequency of single species except for a few widespread forms falls off northwards. In a few cases a more familiar southern species is replaced in similar habitats by another species, e.g. A. phalloides (Fries) Secretan is replaced by A. virosa Secretan the ‘Destroying angel’ in Scotland, and A. citrina frequently in the north by A. porphyria (Fries) Secretan. Species of Amanita are usually large conspicuous fungi and the genus contains some of our best known agarics. One, A. muscaria (Fries) Hooker has already been mentioned, but the genus also includes the ‘Death-cap’ A. phalloides and ‘Caesar’s mushroom’ A. caesarea (Fries) Schweinitz, a fungus not found in this country but considered to be superior in edibility to all other fungi; thus edible and deadly poisonous species are found closely related and this simply emphasises how important it is not to eat the agarics one finds in the woods and fields except when accompanied by a ‘real’ expert. Deaths or near fatalities in Europe and North America are recorded annually due to the eating of fungi belonging to this genus.
The poisonous qualities of the fungi in this genus—only a very small amount of poison is often sufficient to produce fatal results—has led to a close connection between these fungi and black magic and the supernatural. This connection is even more emphasised when it is learnt that some have an intoxicating effect. Hence the long history mentioned earlier.
Members of the genus Amanita are characterised by their anatomy and certain macroscopic features; the former is illustrated under A. muscaria, i.e. the divergent gill-trama. The main macroscopic character of note is the presence of a volva at the base of the stem[57] and it is the details of this volva which helps to distinguish different species. A. phalloides has a distinct, loose, membranous sheath, in A. citrina the volva is reduced to a narrow rim around the bulbous stem and in A. rubescens and A. muscaria the volva is simply a series of concentric zones of woolly scales. All the four species noted above possess a ring, but A. fulva the ‘Tawny grisette’ and A. vaginata (Fries) Vittadini the ‘Grisette’ only possess a volva; this has lead to the use of the generic name Amanitopsis in many books, now no longer considered necessary.
The veil in Amanita is probably the most highly developed amongst our common agarics and from Appendix iv it can be seen how the scaly cap and stem originate and how the volva differs from the ring. The volva and cap-scales constitute what has been called the universal veil and the ring which stretches from the cap margin to the stem has been termed the partial veil.
The spores of species of Amanita are large and their shape and chemical reactions help to distinguish the different species within the genus. One of the most interesting features, however, is that the spore-mass, although usually described as white, in many species is not white but flushed greenish grey, etc. The slight subtleties in colour of the spore-print assist in classification.
The following notes may be instructive in conjunction with the information above (for common names see above).
Cap: width 55-80 mm. Stem: width 18-22 mm; length 70-80 mm.
A lemon-yellow or whitish capped agaric with bulbous stem-base, white patches of volva on cap and white stem with flesh strongly smelling of new potatoes.
Spores: almost globose and measuring 9-10 × 7-8 µm.
Cap: width 75-140 mm. Stem: width 20-28 mm; length 85-120 mm.
A greyish or brownish capped agaric with clavate stem-base, grey patches of volva on the cap and white concentrically scaly stem with flesh unchanged on exposure to the air.
Spores: broadly ellipsoid and measuring 9-10 × 8-9 µm.
[58]
Cap: width 70-120 mm. Stem: width 12-25 mm; length 65-100 mm.
A reddish fawn or pinkish buff capped agaric with swollen stem-base, pinkish or flesh-coloured patches of volva on cap and reddish concentrically scaly stem with flesh becoming reddish when exposed to the air.
Spores: ellipsoid and measuring 9-10 × 5-6 µm.
Cap: width 48-95 mm. Stem: width 12-20 mm; length 65-100 mm.
An olive-brown or smoky brown capped agaric with only slightly swollen stem-base, white patches of volva on the cap and white concentrically scaly stem with unchanging flesh.
Spores: ellipsoid and measuring 8-12 × 7 µm.
Cap: width 70-85 mm. Stem: width 12-20 mm; length 85-120 mm.
A greenish or yellow-olive capped agaric with stem sheathed in membranous volva, white patches of volva on cap and smooth, white stem with white flesh.
Spores: broadly ellipsoid and measuring 10-12 × 7 µm.
Cap: width 40-60 mm. Stem: width 10-15 mm; length 100-150 mm.
A thin, tawny-brown agaric with stem sheathed in membranous volva and pale tawny, slightly scaly stem.
Spores: globose and 10-12 µm in diameter.
Differs from A. fulva in the cap being metallic grey or silvery in colour.
[59]
Cap: width 50-150 mm. Stem: width 10-12 mm; length 75-150 mm.
Description: Plate 10.
Cap: at first convex then more or less flattened or slightly depressed, very variable in colour, yellowish, olive, buff, sand-coloured or some shade of brown, at first covered in small, brownish or ochraceous scales which give the young cap a velvety aspect, but gradually the scales disappear with age except at the cap-centre; margin striate and usually paler than centre of the cap.
Stem: equal or swollen at base, often several grouped together, white at apex above a whitish, rather thick, ring which is flushed with olive-yellow or red-brown at its margin; stem-base fibrillose, whitish but finally red-brown at maturity.
Gills: adnate or slightly decurrent, whitish then flushed flesh colour and developing brownish spots with age or in cold, wet weather.
Flesh: with rather strong and unpleasant smell, white or flushed pinkish in the cap, brown and stringy in the stem.
Spore-print: very pale cream colour.
Spores: medium-sized, hyaline, ellipsoid, less than 10 µm in length (8-9 × 5-6 µm).
Marginal cystidia: variable, hyaline, cylindric and not well-differentiated.
Facial cystidia: absent.
Habitat & Distribution: This fungus grows in troops or is found joined at the base to form clusters. It is always attached to old trees, trunks, stumps and buried wood, either directly or by its vegetative stage which darkens and aggregates to form strands resembling boot-laces which are called rhizomorphs.
General Information: This rather variable, and therefore often perplexing, fungus causes a destructive rot of trees and can travel long distances through the soil with the use of its rhizomorphs. It commonly grows on several species of broad-leaved trees, but can also colonise conifer trees. It also attacks garden shrubs, such as privet-hedges, and is particularly destructive to Rhododendrons causing a wilt of the whole shrub and subsequent death; it has also been recorded as attacking potatoes. The actively growing mycelium which can often be found growing under the bark of infected trees, exhibits a luminosity[60] if freshly exposed and placed in a darkened room. The rhizomorphs of A. mellea are highly specialised structures composed of mycelial threads some of which have become rather more differentiated than is normally found in the vegetative stage of other agarics.
Illustrations: F 27a; Hvass 26; LH 93; NB 1411; WD 43.
Cap: width 50-120 mm. Stem: width 17-25 mm; length 95-125 mm.
Description: Plate 11.
Cap: convex, but expanding and becoming flattened with a slight central umbo, ochre-yellow to yellowish rust-colour and covered with dark brown recurved scales which are particularly dense at the centre.
Stem: variable in length and thickness depending on how it is attached to the substrate, whether in a deep crack or wound, or in a depression, and how many specimens are in the cluster; its colour is similar to that of the cap, exhibits a small, dark brown fibrillose, torn ring or ring-zone and is ornamented with recurved red-brown scales below that ring.
Gills: broadly adnate with a decurrent tooth and crowded, yellowish at first then rust-coloured.
Flesh: with strong, pleasant but pungent smell, yellowish brown, soft in the cap, fibrous in the stem.
Spore-print: rich rust-brown.
Spores: medium-sized, pale brown under the microscope, smooth, ellipsoid, and 6-8 × 4 µm in size.
Marginal cystidia: spindle-shaped, hyaline, numerous.
Facial cystidia: flask-shaped with a small apical appendage and becoming rich yellow when immersed in solutions containing ammonia.
Habitat & Distribution: Common in clusters in woods, gardens or parks, on wood or at the base of the trunks of broad-leaved trees in summer and autumn.
[61]
[62]
General Information: Although rather a common easily recognisable and aesthetically pleasing fungus growing in its characteristic clusters at the base of trees, it is a weak parasite entering the living tissue after invading decayed areas of the tree. This is the reason why when branches are broken off trees by wind, snow or storms, they should be carefully trimmed to remove ragged edges and the wound treated with a protective tar to stop the entry of rain, cold and fungus spores. Other more destructive fungi may enter a tree through such wounds; P. squarrosa frequently attacks mountain ash or rowan.
It is recognised by the dry scaly cap and stem which helps to distinguish it from the sticky capped P. aurivella (Fries) Kummer with similar habitat preferences but wider spores (6-9 × 4-5 µm). P. adiposa (Fries) Kummer is found on beech trees and it, too, has a viscid cap, but the spores are 5-6 × 3-4 µm in dimensions.
Illustrations: Hvass 134; LH 149; WD 542.
[63]
[64]
Cap: width 20-50 mm. Stem: width 6-13 mm; length 40-100 mm.
Description:
Cap: sulphur-yellow, flushed with sand-colour or red-brown at centre then ochraceous yellow throughout, convex at first with margin incurved and clothed with fibrillose remnants of a yellow-olive veil, but then becoming flattened and losing evidence of that veil.
Stem: equal or flexuous, usually with several joined at base, similarly coloured to the cap, fibrillose streaky or with some fibrils from the veil stretching from the cap to the stem in young specimens.
Gills: sinuate and crowded, at first sulphur-yellow then olive-green, but finally with a flush of purple-brown.
Flesh: with rather strong and unpleasant smell, yellow throughout.
Spore-print: purple-brown.
Spores: medium-sized, ellipsoid or ovoid, smooth, purple-brown and less than 10 µm in length (6-8 × 4 µm).
Marginal cystidia: flasked-shaped, short, cylindric and hyaline.
Facial cystidia: more swollen than marginal cystidia and with silvery contents which yellow in solutions containing ammonia.
Habitat & Distribution: The sulphur-tuft grows in dense clusters on and around old stumps of broad-leaved trees, and can be found throughout the year; it also grows on conifers, but less frequently.
General Information: It may be recognised by the greenish tint of the immature gills and of the young cap. H. capnoides (Fries) Kummer grows on the wood of coniferous trees and has a much more ochraceous brown cap and stem than the sulphur-tuft and slightly larger spores—7-8 × 4-5 µm. H. sublateritium (Fries) Quélet grows on hardwoods but is bigger than H. fasciculare and has a brick-coloured cap and very sturdy stem (spores 6-7 × 3-4 µm).
Illustrations: F 37b; Hvass 176; LH 147; NB 1415; WD 762.
[65]
[66]
Cap: width 20-80 mm. Stem: width 5-10 mm; length 35-60 mm.
Description:
Cap: bright sand-colour or slightly red-brown at centre, convex at first then flattened with age, smooth, slimy because of the presence of a sticky elastic skin, rather rubbery to the touch.
Stem: cylindrical or slightly swollen towards the base, dark brown and densely hairy or velvety, tough and rubbery to handle.
Gills: adnexed, very unequal and somewhat distant, pale yellow, gradually becoming buff as the spores mature.
Flesh: with rather pleasant smell, yellowish, watery and soft.
Spore-print: white.
Spores: medium-sized, hyaline, ellipsoid and about 8 × 3-4 µm in Size (7-9 × 3-4 µm).
Marginal cystidia: hyaline, elongate, broadly flask-shaped.
Facial cystidia: similar to marginal cystidia.
Habitat & Distribution: Found in clusters on old stumps, fallen trunks and on the wounded parts of standing trees.
General Information: This fungus can be recognised by the clustered habit, the viscid, bright tawny cap and the dark velvety stem. This is one of the few agarics which occurs regularly late in the season, even appearing in the winter, although it can be seen growing in its familiar groups at almost any time of the year. This fungus holds a rather isolated position in classification and was once placed in the genus Collybia. It may be found in several books under this last genus.
Illustrations: F 18b; Hvass 80; LH 109; NB 1413; WD 214.
[67]
[68]
Cap: width 25-50 mm. Stem: width 3-6 mm; length 50-125 mm.
Description:
Cap: conical or bell-shaped then expanding but retaining a central umbo, never completely flattened, smooth, greyish, pale sepia or dirty white and striate with darker lines from the margin to the centre.
Stem: similarly coloured to the cap, smooth, shiny, tough and usually noticeably downy at base.
Gills: at first white flushed distinctly pale pink with age, uncinate, rather distant and sometimes with interconnecting veins.
Flesh: white with little or no distinctive smell.
Spore-print: white.
Spores: medium-sized, hyaline, broadly ellipsoid, smooth, about 10 × 7 µm in size (9-12 × 6-8 µm) and staining bluish grey when mounted in solutions containing iodine.
Marginal cystidia: club-shaped but the apex ornamented with blunt hairs of varying lengths.
Facial cystidia: absent.
Habitat & Distribution: Commonly found, in all but the coldest months, in woods, parks or gardens, often in dense clusters on stumps and fallen trunks of broad-leaved trees.
General Information: This is one of our commonest members, and one of the largest in the genus Mycena; many species in this genus are quite small yet are nevertheless very important components of the woodland flora decomposing leaves, twigs, etc., and contributing in this way to the recirculating of organic matter.
The name Mycena is derived from the same Greek word as that which refers to the country around the ancient city of Mycenae in the plain of Argos, and from whence Agamemnon came and gathered his forces to invade Troy to reclaim Helen his wife. It has been suggested that this similarity in name came about through the necessity for an army stationed in Argos, early in the history of Ancient Greece, to rely on the mushrooms found on the plains about to save the soldiers from starvation.
Illustrations: F 17a; Hvass 119; LH 109; NB 1338; WD 263.
[69]
[70]
Cap: width 40-100 mm. Stem: width 10-15 mm; length 75-125 mm.
Description:
Cap: conical, rapidly expanding and then becoming plano-convex or flattened with only a slight but persistent umbo, dark brown, umber or vandyke brown, viscid when wet and often with radiating fibrils.
Stem: white, streaked to varying degrees with dark brown fibrils, cylindrical or slightly swollen towards the base, where it is attached to the substrate.
Gills: remote, very crowded, thin, at first white then distinctly salmon-pink.
Flesh: with pleasant smell, white and soft.
Spore-print: dull salmon-pink.
Spores: medium-sized, very faintly buff under the microscope, broadly ellipsoid and 7-8 × 5-6 µm in size.
Marginal cystidia: flask-shaped, the majority with three or four hooks at the distinctively thick-walled apex.
Facial cystidia: similar to marginal cystidia but sometimes intermixed with those lacking hooks.
Habitat & Distribution: This fungus grows singly or in groups on old stumps and fallen trunks throughout the year except for the most wintry months; it is commonest in autumn.
General Information: This fungus may also grow on old sawdust heaps, a habitat which is often very worth while examining in detail by the interested amateur during wet seasons. In summer sawdust heaps dry out but after a good soaking, which, of course, can be applied artificially by frequent watering with a hose or watering-can, many interesting fungi develop. On sawdust heaps containing conifer debris a larger species with black or dark brown edge to the gills is found—P. atromarginatus Kühner.
The peculiar pointed cystidia found on the gill-edge and on the gill-face of P. cervinus were thought by some early mycologists to stop mites and insect larvae from crawling up between the gills and damaging the developing spores. There is no evidence that this actually takes place in nature; the real purpose of these obscure structures is unknown and has been little studied.
Illustrations: Hvass 127; LH 121; NB 1351; WD 502.
[71]
[72]
Cap: width 20-50 mm. Stem: width 4-7 mm; length 20-50 mm.
Description:
Cap: convex then becoming flattened at maturity, dry, slightly scaly, golden tawny, or rusty yellow and when young with the remnants of a rapidly disappearing yellow cortina hanging from the margin.
Stem: yellow above and red-brown or orange-tawny below and darkening on bruising; veil forming a delicate fibrillose zone in the upper part of the stem which is soon lost on excessive handling.
Gills: adnate to slightly decurrent, thin and crowded, at first golden yellow, but soon spotted rust colour.
Flesh: yellow and lacking distinctive smell.
Spore-print: rich orange-tawny.
Spores: medium-sized, ellipsoid, finely roughened and deep yellow brown under the microscope, less than 10 µm in length (7-8 × 5-4 µm).
Marginal cystidia: hyaline, flask-shaped with long often slightly irregular neck.
Facial cystidia: similar to the marginal cystidia, but often broader.
Habitat & Distribution: This fungus is found on sticks or twigs or chips of coniferous wood, particularly in plantations.
General Information: Although it has only comparatively recently been recognised in Britain it is very wide-spread. It has been confused with, indeed described under, the name of the less-common fungus Gymnopilus sapineus (Fries) Maire which also grows in conifer woods; it is easily distinguished, however, by its spotted gills. Both the fungi above can be found in books under the old name Flammula, from the bright colour of the caps of many of its constituent members, but Flammula has been used for a genus of flowering plants also and this has precedence.
Illustrations: F 29a; Hvass 152 not very good; LH 175 not very good; NB 1096.
[73]
[74]
One of the common features of lignicolous fungi is the fact that they lack a distinct stem or if one is present it is attached to one side of the cap, i.e. lateral. However, in the past the correlation of the habitat with lack of stem has induced mycologists to define a single family to include all these forms. After studying the anatomy and microscopic characters this grouping has been found to be entirely artificial and simply reflects how the morphology is tied up intimately with the ecology of a species.
In this one family members of the genera Panus, Panellus, Lentinus, Lentinellus, Crepidotus, Pleurotellus, and Pleurotus have all been grouped together, but some of the genera are more related to the polypores referred to later (p. 135); many of those with brown spores are better placed with Cortinarius and some of those with white or cream-coloured spores are better placed close to Mycena and Tricholoma. This leaves as a residue the genus Pleurotus, a genus which although rather heterogeneous contains one familiar member, i.e. the common Oyster mushroom, Pleurotus ostreatus.
Grows up to 150 mm across.
Cap: flattened, shell-shaped, smooth or slightly cracked, deep bluish grey, gradually becoming brownish with age and finally dark buff.
Stem: absent or very short, passing gradually into one side of the cap.
Gills: white flushing dirty yellow with age, rather distant and deeply decurrent.
Flesh: white, soft and with very pleasant smell.
Spore-print: pale lilac.
Spores: long, hyaline, oblong under the microscope and 10-11 × 4 µm in size.
Marginal and facial cystidia: absent.
Habitat & Distribution: Common, clustered in tiers on stumps, trunks, posts, etc.
General Information: This fungus is not infrequent on old telephone-poles and forms white sheets of mycelium immediately under the bark of fallen trees. Although frequent in autumn it may be found throughout the year and is easily recognised by its size and bracket-like, shell-shaped caps. It surprisingly has a pale lilac spore-print and not as might be expected a white spore-print. In the var. columbinus Quélet the young caps are a beautiful peacock-blue; this variety frequently grows on poplars.
Illustrations: F 1252; Hvass 109; LH 107; NB 1252; WD 311.
[75]
Plate 17. Wood-inhabiting, fleshy but leathery fungi: Spores whitish or brownish and borne on gills—‘Pleurotaceae’
[76]
is a tough, funnel-shaped, yellowish cinnamon fungus with oblong-ellipsoid, small, hyaline spores measuring 5-6 × 3 µm and changing yellowish not bluish grey in iodine solutions.
forms tiers of pale cinnamon-brown, more or less kidney-shaped, scurfy caps on old wood and has egg-shaped, hyaline, small spores measuring 4 × 2-3 µm which become bluish grey in iodine solutions.
forms irregular lobed and twisted, flattened or funnel-shaped dirty brownish caps with a fragrant smell, toothed gill-edges and almost spherical, small, hyaline spores measuring 5 × 4 µm which become bluish grey in iodine solutions.
Lentinellus apparently has very close affinities to Auriscalpium, ‘the Ear pick fungus’, (p. 158) both in the structure of the spores and the anatomy of the fruit-body.
forms very tough fruit-bodies with convex or flattened, pale yellowish caps and stems ornamented with dark tawny or brown scales. The stem is often eccentric and buried in cracks or soft rotten wood on which it grows; the spores are non-amyloid. It grows on pine stumps but also on decaying or unprotected railway sleepers and wooden paving blocks, joists, etc., made of conifer wood. When the fungus fruits in a darkened environment, such as a cellar, the mushroom-like fruit-bodies are not produced but are replaced by slender branched structures similar to the ‘Stag’s horn’ or ‘Candle-snuff fungus’ (p. 206), or to certain of the Fairy Club fungi (p. 172). Similar growths have been recorded for Polyporus squamosus which grows on hard wood timber and is described in detail later (p. 140).
[77]
Cap: up to 45 mm across and in tiers, sessile, shell-shaped or kidney-shaped, smooth, rubbery and brownish ochre in colour.
Gills: pale buff then cinnamon-brown and finally flushed snuff-brown, thin and crowded.
Flesh: watery, gelatinous beneath the skin of the cap and whitish buff.
Spore-print: warm brown.
Spores: ellipsoid, smooth, medium-sized, pale buff under the microscope and 8-9 × 5-5·5 µm in size.
Easily recognised by the soft elastic cap which can be stretched without breaking, the brown gills and pale buff spores. (See Plate 49, p. 153.)
Illustrations: LH 177; NB 1453; WD 691.
The artificiality of classifying all those agarics with both a spoon-shaped or bracket-shaped fruit-body, and a reduced (or lacking) stem is further exemplified by the presence of similar genera in other groups of fungi. For instance Claudopus is typified by pink, angular spores (Plate 28) and Clitopilus is characterised by longitudinally ridged spores, i.e. they are not angular in all optical sections but only when seen end on (see p. 101). An example of the former is C. parasiticus (Quélet) Ricken which grows on dead remains of woody fungi, and of the latter C. passackerianus (Pilát) Singer which may invade mushroom beds. Both species are quite small though the last fungus is similarly coloured to the more familiar Clitopilus prunulus (Fries) Kummer, ‘The Miller’, so common in woods and fields.
Thus in the British Isles agarics with eccentric stems may be found, in the white, brown and pink-spored groups—and in the tropics and subtropics the picture is completed by the existence of the genus Melanotus in the black-spored agarics. M. bambusinus Pat. grows on bamboos and M. musae (Berk. & Curt.) Singer grows on dead leaves and debris of bananas; the latter is also a probable agent in the decay of fibres in the tropics.
[78]
Cap: width 40-110 mm. Stem: width 50-80 mm; length 50-90 mm.
Description:
Cap: dark brown, umber or vandyke when moist, hygrophanous and becoming very much paler on drying almost tan, convex then flattened sometimes umbonate, smooth or wrinkled.
Stem: white or whitish covered in brownish fibrils which increase in number with age or after handling; solid, rather elastic and slightly swollen towards the base.
Gills: white, broad, crowded and as if cut out from behind before joining the stem.
Flesh: with pleasant smell, soft, white, becoming brownish with age, particularly in the stem.
Spore-print: very pale ivory-colour.
Spores: medium-sized, ellipsoid, hyaline under the microscope and roughened by distinct dots which become blue-black when mounted in solutions containing iodine, 8 × 4-5 µm.
Marginal cystidia: spear- or sword-shaped, roughened with crystals at the top and appearing as if barbed like fish-spines.
Facial cystidia: numerous and similar to marginal cystidia.
Habitat & Distribution: Common in autumn in woods; also found in pastures.
General Information: A very common fungus which is rather confusing to the beginner because of its variation in colour, brought about by the change in colour with change in content of water. However, this fungus can be easily recognised by the unusually ornamented cystidia found on the gill-faces and gill-margins. This character and the fact that the spores possess amyloid ornamentation define in part the genus Melanoleuca. In many books this common fungus is found under the genus Tricholoma; however, members of this latter genus have neither amyloid ornamented spores nor barbed cystidia.
Illustrations: LH 103; WD 131.
[79]
[80]
Cap: width 20-60 mm. Stem: width 8-13 mm; length 35-75 mm.
Description:
Cap: yellowish ochre flushed slightly pinkish buff or cinnamon but later pale tan on ageing or drying, funnel shaped.
Stem: colour like cap or slightly darker, flexible but firm and solid.
Gills: white or faintly flushed buff, decurrent and crowded.
Flesh: with pleasant slightly floral smell, white, soft and fairly thin.
Spore-print: white.
Spores: medium-sized, hyaline, tear-drop shaped, smooth, 6-7 × 3-4 µm and not blueing when mounted in solutions containing iodine.
Marginal cystidia: little different from young basidia in dimension and shape, although some may have a short apical prolongation.
Facial cystidia: absent.
Habitat & Distribution: Woods, copses, heaths and hill-pastures from summer to autumn.
General Information: An easily recognisable fungus because of its graceful stature, thin, funnel-shaped pinkish buff cap and tear-drop-shaped spores. Several Clitocybe species grow in woodlands, many of them appearing later in the season when colourful agarics are rarer.
The genus Clitocybe is characterised by the fleshy cap with incurved margin when young, fibrous, fleshy stem and decurrent gills. C. clavipes (Fries) Kummer has a smoky brown, top-shaped cap, fragile stem which also has a distinct swelling at its base, and strong rather unpleasant smell. C. nebularis (Fries) Kummer is similar, but is pale cloudy grey, has a less fragile stem and a fairly pleasant smell. This species if often covered in a bloom which develops further as the fruit-body deteriorates. The agaric Volvariella surrecta (Knapp) Singer is a rare parasite of C. nebularis (see p. 247) and it has been suggested that this bloom may in fact belong to this species. However, I have on several occasions tried to encourage the bloom to reproduce by keeping hoary looking fruit-bodies of C. nebularis in a damp-chamber, but as yet I have never been successful.
Nevertheless, it is an exercise which would be of great interest to continue and a source of great excitement if the small pink-spored agaric were produced. C. fragrans (Fries) Kummer is a small, sweetly aromatic-smelling species found in frondose woods, and C. langei Hora, is a mealy-smelling species of conifer plantations.
Illustrations: F 16a; Hvass 55; LH 95; WD 162.
[81]
[82]
Cap: width 40-80 mm. Stem: width 8-12 mm; length 38-85 mm.
Description:
Cap: pale yellow buff or pale tan with a distinct reddish buff or cinnamon-brown tint, darkening only slightly with age; smooth, at first tacky to the fingers, but then dry and shiny at centre, convex and hardly expanding.
Stem: cylindrical or slightly swollen towards the base, whitish and with a flush of pinkish buff at apex, and covered all over in small, white scales.
Gills: sinuate, crowded, pale clay-colour or buff, but finally dull dark yellow ochre except for the distinct white margins which are beaded in wet weather with droplets of liquid.
Flesh: whitish with a very strong smell of radishes.
Spore-print: dark clay-colour.
Spores: long, slightly almond-shaped, pale brown under the microscope, distinctly warted and about 11 × 6 µm in size (10-12 × 6-7 µm).
Marginal cystidia: cylindrical to skittle-shaped with slightly to distinctly swollen apex.
Facial cystidia: absent.
Habitat & Distribution: Common in autumn on the ground by pathsides and in woodland clearings.
General Information: Recognisable by the uniform cinnamon or pinkish buff cap, white woolly scales on the stem and distinctive, strong smell of radish. There is some evidence that this species may on occasions be mycorrhizal; further field studies are required.
There are several closely related fungi which are difficult for the amateur to differentiate from H. crustuliniforme; there is no doubt that there are several species present in the British Isles which do not appear in the Check List of British Agarics & Boleti; in fact, it would appear that there are several yet to be described as new to science. Although individual species are fairly difficult to delimit, the genus Hebeloma itself is easily recognised, most members being medium sized with brown sinuate gills, whitish, yellowish, or pinkish, i.e. pale, caps and white-powdered stems. The word ‘crustulin’ which appears in the Latin name of H. crustuliniforme is itself from the Latin and means small cake, referring to the cap-shape, which remains fairly constant throughout the fungus’ growth. The common name is derived from this also.
[83]
[84]
Cap: width 10-25 mm. Stem: width 3-6 mm; length 30-50 mm.
Description:
Cap: conical with incurved margin then bell-shaped and retaining a distinct umbo even when mature, silvery white then ivory and finally pale tan particularly centrally and silky fibrillose throughout.
Stem: slender, cylindrical but for a small swelling at the base, silky and shining with a few fibrils from a former cortina which may be brownish due to spores adhering to it at maturity.
Gills: adnexed to free, crowded, pale ochraceous becoming clay-coloured.
Flesh: white with smell of newly dug potatoes, strong when fresh.
Spore-print: clay-colour.
Spores: medium sized, ellipsoid or slightly French-bean-shaped, smooth, yellow-brown under the microscope and 9-11 × 4-5 µm in size.
Marginal and facial cystidia: flask- to spindle-shaped with distinctly thickened walls and frequently ornamented with crystals apically.
Habitat & Distribution: Common in troops in woodland clearings, by pathsides or on the edges of ditches bordering woods.
General Information: This fungus is easily recognised by the very pale uniform colour, the colour of the spore-print, silky umbonate cap and small size. The cortina connects the cap-margin and the stem and consists of a cobwebby structure which collapses at maturity.
A violet coloured variety, var. lilacina Gillet is frequently found, in fact, even accompanying var. geophylla; it differs only in the lilac-colour of the cap and stem. I. geophylla is a member of the very large genus Inocybe, further members of which will be dealt with later (see p. 238).
The genus is well defined with dull-yellow spore-print, well differentiated sterile cells on the gill-edge (and often on the gill-face) and the cobweb-like veil, or cortina, stretching from the cap-margin to the stem and easily observed in young specimens. The genus is split into three distinct groups: those with smooth spores, those with nodulose spores and those with subglobose spores ornamented with long projections. I. geophylla is included in the first group. The group which includes the nodulose-spored members has been elevated to the rank of genus by some authors, i.e. Astrosporina—a name referring to the spore-shape eg., I. asterospora.
Illustrations: F 13a (too blue); LH 155; NB 1395; WD 654.
[85]
[86]
Cap: width 12-28 mm. Stem: width 4-8 mm; length 15-60 mm.
Description:
Cap: hygrophanous, reddish brown or brick-colour becoming ochraceous on drying, but can be rapidly returned to the original colour by placing on the top a drop of water which is rapidly absorbed; fragile, convex at first then flattened or depressed about centre, smooth or surface scaley, striate at margin when moist.
Stem: similarly coloured to the cap, fibrous, cylindrical, tough and usually with white woolly base.
Gills: adnate with or without a decurrent tooth, thick, distant and pinkish or pale reddish-brown, powdered with white when mature.
Flesh: red-brown, soft in the cap and fibrous in the stem.
Spore-print: pure white.
Spores: medium sized, hyaline under the microscope and spherical, 7-8 µm in diameter and beautifully spiny.
Marginal and facial cystidia: absent.
Habitat & Distribution: Common in troops in woodland, copses, on heaths; in fact it may be found in nearly all possible habitats.
General Information: This is a very common agaric which in the future will probably be split into several distinct species; unfortunately it is as variable as it is common, hence the common name ‘deceiver’; it is often mistaken at first glance for many other species quite unrelated. I have seen even the most experienced mycologist pick up rather unfamiliar specimens of Laccaria laccata in mistake for a species of Lactarius or a species of Collybia, etc. I would hate to say more because I have been ‘deceived’ myself on more than one occasion. L. laccata appears to be a composite species, but because of the difficulty in defining some of the characters the splitting of the species has not as yet been satisfactorily solved. The smell, however, may well give a clue for some specimens smell very strongly of radish whilst others are odourless.
, differs in having ellipsoid spores; it is larger in stature and is common in wet places.
, differs in the deep violet or amethyst-colour of the fruit-body and commonly grows in shaded woods.
, which is less frequent, has lilaceous gills and violaceous mycelium at the base of the stem.
Illustrations: Hvass 66; NB 1331; WD 202.
[87]
[88]
Cap: width 10-17 mm. Stem: width 2-4 mm; length 50-80 mm.
Description:
Cap: bell-shaped or conical expanding only slightly with age and so remaining umbonate, reddish-brown, striate to the margin from the darker apex and blotched age with red-brown spots.
Stem: pale reddish brown, very slender, fragile, woolly at the base and exuding a red-brown juice when broken.
Gills: adnate, fairly distant, whitish to flesh-colour with a dark red-brown edge and not noticeably becoming blotched with red-brown.
Flesh: with no distinctive smell, reddish-brown and very thin.
Spore-print: white.
Spores: medium sized, hyaline, ellipsoid to pip-shaped, smooth about 10 µm long (9-10 × 4-5 µm) and becoming bluish grey when mounted in solutions containing iodine.
Marginal cystidia: awl-shaped, pointed at the apex, swollen below and filled with dark red-brown contents.
Facial cystidia: absent.
Habitat & Distribution: Solitary or in small groups on poorly kept lawns, in woods and copses; it is particularly frequent in the beds of needles found in pine woods.
General Information: This fungus is easily recognised by the slender habit, reddish juice exuded when broken and habitat preferences. Mycena haematopus (Fries) Kummer is larger and grows in tufts on wood, but also has a red-brown juice which, however, spots the gills. Another very common species of Mycena is M. galopus (Fries) Kummer which has a greyish or brownish cap and exudes a milk-like juice. The related M. leucogala (Cooke) Saccardo is almost black (see p. 216). These agarics exuding juice when broken have a flesh composed of filaments, a very different flesh-structure to species of Lactarius (see p. 50) and although their spores are amyloid they do not turn blue-black in iodine because of the presence of amyloid crests and warts. There are few additional species of agaric which exude a milk-like liquid, but the majority of these are tropical or subtropical. The second names or epithets for the four species mentioned above all refer to the ‘latex’—sanguinolenta—bleeding, haematopus blood-foot; galopus, milk-foot and leucogala, white milk. For notes on Mycena one is referred to p. 68 describing M. galericulata (Fries) S. F. Gray.
Illustrations: WD 284.
[89]
[90]
Cap: width 80-130 mm. Stem: width 5-20 mm; length 50-158 mm.
Description:
Cap: white but soon becoming spotted with reddish-brown, finally cream-colour with red-brown blotches, convex then becoming flattened, fleshy, firm and tough.
Stem: white becoming streaked red-brown, thickest in the middle, longitudinally furrowed or striate and often narrowed downwards into a long irregular root embedded in the deep litter.
Gills: very crowded, cream-coloured, becoming spotted red-brown with age.
Flesh: with pleasant smell, white and fibrous in the stem.
Spore-print: pinkish cream-colour.
Spores: small, almost spherical, hyaline under the microscope, about 5 µm in diameter (4-5 × 5 µm) and not blueing when placed in solutions containing iodine.
Marginal and facial cystidia: absent.
Habitat & Distribution: Common in troops in woods, particularly beech but also found in pine woods and on heaths.
General Information: Easily recognised by the crowded, narrow, cream coloured gills and the cap being entirely white when young, but which rapidly becomes spotted red-brown as it develops. ‘Maculatus’ means spotted and refers to the red-brown blotches which develop irregularly on the cap, stem and gills as the fruit-body matures.
The genus Collybia is characterised by the fruit-body being tough, the cap-margin incurved at first and the spore-print white or whitish. The common fungus C. maculata has always been assumed to have a white spore-print but if a cap is placed on a piece of white paper gills-down and left for twelve hours there is a surprise in store for the careful observer.
Illustrations: F 15a; Hvass 77; LH 101; NB 1034; WD 212.
[91]
Plate 24. Fleshy fungi with tough stem: Spores white to cream and borne on gills
[92]
A whole series of very small fungi are found in woodland communities which appear to be closely related one to another because their caps are usually tough, although membranous, dry rapidly yet do not decay, and, moreover, revive on remoistening. Their gills are also rather tough and their spores always white in mass. They are placed in the genus Marasmius. Collybia or Marasmius peronatus (Fries) Fries the ‘wood woolly foot’ is one of our larger more familiar agarics related to this group, but whereas it grows on all kinds of leafy detritus, even wood, these small fungi appear to be very specific to the substrate on which they grow.
grows both on heather and on pine-needles (see p. 231).
Cap: whitish or pinkish buff.
Stem: black and hair-like.
Spores: pip-shaped and 7-9 × 3-4 µm.
grows on box leaves.
grows on ivy leaves.
grows on grass stems.
Cap: red-brown.
Stem: dark brown.
Spores: pip-shaped, 8-12 × 4-6 µm.
grows on holly leaves.
grows on pine needles (now placed in the genus Micromphale).
grows on bracken stems.
Cap: reddish brown or greyish and wrinkled.
Spores: egg-shaped, 8-9 × 6-7 µm.
Except for their rather special requirements as to substrate preference, these species have in common small size, rather tough horny stems and cap composed of erect ornamented cells.
Several agarics which grow on cones have also been placed in Marasmius. They are frequent in spring and early summer the fruit-bodies being attached by a very long rooting stem and cord of fluffy hyphae to buried cones in conifers. The biology of these fungi is still unknown, but the cones to which they are attached are always closed yet buried often several inches beneath the surface of the soil. It is yet to be found whether the spores of the agaric infect the cones after they drop or whether the cones fall because they have become infected. How do the cones become so deeply buried? Are squirrels or rodents involved? All the species which grow on cones have brown or tawny caps and yellowish brown stems.
[93]
Plate 25. Fleshy fungi with wiry to tough stem: Spores white and borne on gills, fruit-body frequently reviving when moistened
[94]
has cystidia with rounded heads and grows on pine-cones.
has pointed cystidia and grows on pine-cones.
has lance-shaped cystidia and grows on spruce cones.
is tough and pale-coloured and is similar in general characters to species of Strobilurus, but has amyloid spores and fruits on pine-cones in the autumn.
When discussing the specialised plant-substrates, such as cones, one must mention the small brown-spored, pale buff coloured agaric Tubaria dispersa (Persoon) Singer, or Tubaria autochthona (Berkeley & Broome) Saccardo, which grows on the ground under hawthorns, often in troops in summer and autumn, attached to old hardened hawthorn berries.
[95]
Cap: width 20-80 mm. Stem: width 5-12 mm; length 30-70 mm.
Description:
Cap: convex then expanding to become plano-convex with a broad low umbo, tan, pale russet or even yellowish buff throughout or slightly darker at the centre.
Stem: gradually thickened upwards, similarly coloured to the cap or paler if the cap is dark russet.
Gills: pale buff, deeply decurrent and often connected up at their bases by veins.
Flesh: buff or pale tan, thick and soft in the cap, slightly fibrous in the stem.
Spore-print: white.
Spores: medium-sized, ellipsoid to egg-shaped, hyaline under the microscope, 7-8 × 5 µm in size and not becoming bluish grey in solutions containing iodine.
Marginal and facial cystidia: absent.
Habitat & Distribution: Common in pastures or on heaths from early summer to late autumn.
General Information: A fungus easily recognised by the uniform buff-colour of the stem, cap and gills. As one might expect from the common name it is edible; it is held in high regard by many mushroom-pickers.
Although ‘pratensis’ specifically means fields, reflecting the habitat of the fungus, this and related species can also be found on heaths and pastures often intermixed and forming a most interesting flora. The following are perhaps the most commonly seen:
H. lacma (Fries) Orton & Watling and H. cinerea (Fries) Orton & Watling are similar in stature, but metallic grey in colour except for the persistently yellow stem-base in H. lacma.
H. subradiata (Secretan) Orton & Watling is flesh-coloured or brownish and H. virginea (Fries) Orton & Watling is white.
H. nivea (Fries) Orton & Watling and H. russocoriacea (Berkeley & Miller) Orton & Watling are much smaller, the former white and odourless and the latter off-white with a very strong smell of incense.
Illustrations: F 12b; Hvass 95; LH 77; NB 332; WD 333.
[96]
[97]
Cap: width 12-25 mm. Stem: width 3-8 mm; length 30-60 mm.
Description:
Cap: very slimy with colourless sticky fluid, deep bluish green when fresh, but becoming more and more ochraceous-orange with age or completely fading out to a yellow ochre, bell-shaped at first then expanded except for central umbo.
Stem: like the cap very slimy, apple-green or bluish green throughout but becoming ochraceous like the cap except at the apex which is persistently green.
Gills: adnate yellow or apricot-coloured, greenish towards their base, broad, distant and rather tough.
Flesh: whitish, tinged green in the cap and yellow or apricot-colour in the stem.
Spore-print: white.
Spores: medium-sized, hyaline, ellipsoid, not blue-grey in solutions containing iodine and 8-9 × 4-5 µm in size.
Marginal and facial cystidia: absent.
Habitat & Distribution: Common in grassland and hill-pastures, but it also occurs in copses and woodlands.
General Information: This fungus is easily recognised by the distinctive colours, but it is rather deceptive for the cap and the stem soon become faded; however, the green colouration persists at the apex of the stem and it is by this that in the faded state the fungus can still be identified. H. laeta (Fries) Kummer fades to similar colours but the cap is flesh-colour at first or sordid brown and the gills are flesh-coloured or greyish; it prefers upland pastures and heathland: its spores are smaller, being 5-7 × 4 µm.
Illustrations: F 12a; Hvass 92; LH 79; NB 336; WD 345.
Hygrophori are some of our most colourful groups of agarics, many are brightly coloured with caps in reds, greens, yellows, oranges, etc., the colour often accentuated by the usually slimy aspect. Traditionally the genus Hygrophorus has been split into three groups as follows:—
[98]
Limacium with slimy cap, adnate to decurrent gills and slimy or tacky stem which may also often be ornamented with dots, especially towards the top.
Camarophyllus with dry cap, smooth and fibrous stem and decurrent gills.
Hygrocybe with thin, fragile, sticky or moist cap, smooth fibrillose stem and gills varying from free to decurrent.
The last two sections have been joined together into the single genus Hygrocybe and all the members seem to be saprophytic or intimately associated with grassland communities. The first section Limacium now makes up the genus Hygrophorus and its members are thought to be mycorrhizal with trees, e.g. H. hypothejus (Fries) Fries with pine, the ‘Herald of the winter’ because it occurs at the end of the fungus season and H. chrysaspis Métrod, a whitish, sickly-smelling fungus under beech. Results from examining the anatomy of the gills appears to confirm these divisions. All the Hygrophori have a homogeneous flesh, white spores, central, fleshy stem and thick, waxy gills; microscopically this group of fungi can be recognised by the very long basidia.
The following are common examples of the genus Hygrocybe:—
Fayod has a rose-pink, conical cap which expands to become upturned at the edge with age.
has a bright scarlet cap which becomes yellow-ochre on drying and a yellow base to a scarlet stem.
has an orange to red stem and sharply conical cap which turns blackish with age and whose gills when cut exude a clear watery liquid.
has a slimy, golden yellow cap and similarly coloured stem.
is similar, but has a lemon-yellow cap and stem.
is a large and robust species, similar in colour to H. coccinea but with a white base to the stem.
has a smoky grey, very slimy cap and stem.
is as dull coloured as H. unguinosa, but is not slimy, and in addition strongly smells of cleaning fluid or bleaching-powder. It is one of three dull coloured, strong bleaching-powder-smelling species found in Britain. H. ovina is another, but is darker than H. nitrata and becomes red when bruised or cut.
[99]
Plate 27. Fleshy brightly coloured fungi: Spores white and borne on thick, waxy gills
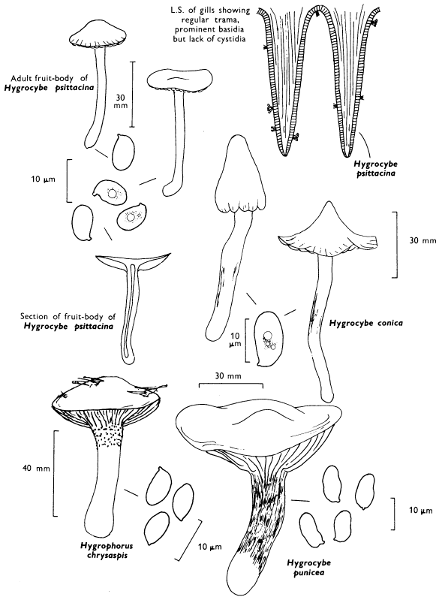
[100]
has a sooty brown fibrillose-streaky cap and stem. The gills are distant and grey, and the fruit-body may reach up to 100 mm across. It is probably the biggest of our native species of Hygrocybe.
For completion examples of Hygrophorus include:
has a slimy orange-yellow cap, yellow gills and yellow, slimy, smooth stem. It is found under larch trees.
has ivory white cap, stem and gills which soon become flushed with rust-brown and finally the whole fruit-body becomes red-brown. The stem is slimy and white dotted at the apex. It grows in beech woods.
has a slimy cream-coloured cap flushed with pale peach colour. The gills and stem are cream and the latter slimy and dotted at the top. It is found in pine woods.
has an olive-brown slimy cap, yellow stem and gills; the stem is slimy and smooth. It is found in pine woods and under pines on heaths.
has an ash-grey cap brownish towards its centre, viscid white stem with dark grey dots at the apex and white gills. H. agathosmus (Secretan) Fries is similar, but smells strongly of bitter almonds. Both species are found in plantations.
Species of the genus Hygrophorus are infrequently encountered in Britain, although twenty species are recorded for the British Isles. They are ecologically distinct from members of the genus Hygrocybe in preferring woodland communities to grassland areas; they are probably mycorrhizal. The anatomy of the fruit-body is also rather different to that found in Hygrocybe; the gill-trama is bilateral as in Leccinum (p. 27), Suillus (p. 28), Boletus (p. 31), Chroogomphus (p. 36), Paxillus (p. 38) and Amanita (p. 54). Members of the genus Hygrocybe have regular to irregular gill-tramas. In fact, although both genera are united into a single family, the Hygrophoraceae is based on one character common to both, i.e. the long basidium; there is every indication that the genus Hygrocybe has greater affinity to Omphalina in the Tricholomataceae (p. 232).
Surprisingly enough in North America many of our familiar grassland species including H. pratensis are to be found in deep shaded woodland!
[101]
The name of the family refers to the pink gills and it unites all those fungi with a salmon-pinkish buff spore-print and whose spores are angular in all optical sections. There are a few agarics, e.g. Clitopilus prunulus (Fries) Kummer with ridged spores which appear angular in end-on view, but which are ellipsoid in both side and face views and so are considered less related.
The family Rhodophyllaceae by some authorities contains one genus Rhodophyllus, more correctly called Entoloma; in the British Isles five constituent genera are recognised, but they will have to be more critically defined to make a more meaningful classification. At the moment, many of the species are poorly documented and it would appear that anatomical studies will assist in the future in the recognition of species-groups.
If one selects the eight most distinctive shaped spore-types exhibited in members of this family, then when their spores are examined side-on a feature is available for correlation with the traditional field characters, such as cap scaliness and gill-attachment. The most distinctive spore-shape is Type G, found in Nolanea staurospora Bresadola, which is probably the most common and widespread species of the family. It grows in woodlands, grassland and on lawns and will be dealt with later (p. 122). The other spore types are illustrated and range from irregularly rhomboid to elongate angular.
The majority of the members of this group grow in grassland, hill-pastures and meadows and distinct communities containing members of this family and of the Hygrophoraceae can be recognised. It is not proposed to deal in detail with any individual members because they can be so easily confused with each other by the specialist let alone by the amateur.
However, the genera as at present accepted are as follows:—
in its original sense contains agarics with fleshy caps, fibrous stems and sinuate or adnexed gills, e.g. Ent. clypeatum (Fries) Kummer with grey to yellow-brown cap, found growing with members of the apple and rose-family in the summer and early autumn. This genus corresponds to Calocybe in the white-spored agarics (p. 110).
[102]
contains those agarics with rather thin caps whose margin is incurved, cartilaginous stems and adnate to adnexed, rarely decurrent, gills and whose cap flesh is indistinct from that of the stem, e.g. Lept. serrulata (Fries) Kummer with dark blue to violet-blue cap and dark blue edge to the gills. This genus approaches the tough-shanks (Collybia) in the white-spored genera (p. 90).
is characterised by agarics with delicate caps, whose flesh is distinct from that of the stem and whose edge is straight and pressed against the fragile stem when young, and the adnexed or adnate, rarely decurrent, gills, e.g. N. staurospora (see p. 122). N. cetrata (Fries) Kummer with yellow-brown to tan-coloured cap is found from spring to autumn in conifer woodland, especially plantations. The genus corresponds to Mycena in the white-spored agaric genera (p. 68).
is a small genus containing agarics with thin, membranous caps and distinctly decurrent gills, e.g. E. sericeonitida P. D. Orton with convex, then umbilicate, silky greyish brown cap. This genus corresponds to Omphalina in the white-spored agarics (p. 232).
has three British representatives, all of which have a very small stem which may even be absent, e.g. C. depluens (Fries) Gillet grows on soil and C. parasiticus (Quélet) Ricken grows on old decaying fruit-bodies of woody fungi. This genus corresponds to Pleurotellus in the white-spored genera and to Crepidotus in the brown-spored genera (p. 77).
[103]
Plate 28. Fleshy fungi: Spores pinkish and angular and borne on gills - Rhodophyllaceae
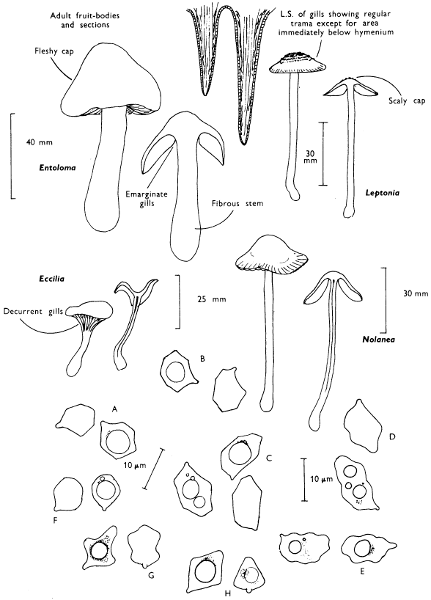
[104]
Cap: width 15-35 mm. Stem: width 4-8 mm; length 15-30 mm.
Description:
Cap: pale ochraceous yellow to sand-colour, convex then expanded, with central umbo and often radially wrinkled-reticulate, covered completely in powdery granules when fresh but these gradually disappear with age or on excessive handling.
Stem: slender, white above a narrow, easily lost ring which is composed of floccose, ochraceous yellow granules which also clothe the lower part of the stem.
Gills: adnate, cream-coloured and crowded.
Flesh: yellowish with a strong smell of new-mown hay.
Spore-print: white.
Spores: small to medium sized, hyaline under the microscope, smooth, ellipsoid, 5-7 × 3-4 µm and becoming blue-grey when mounted in solutions containing iodine.
Marginal and facial cystidia: absent.
Habitat & Distribution: Frequently found amongst grass on heaths, in hill-pastures and in woodlands from summer to autumn.
General Information: This fungus is recognised by the gill-attachment and the powdery-scurfy cap formed by the breaking up of an enveloping veil composed of thick-walled, rounded cells, similar to those on the surface of the stem.
This fungus was formerly placed in the genus Lepiota because of the ring but the veil in Cystoderma amianthinum is formed in quite a different way to the ring in the true parasol mushrooms. The gills are also adnate and not free as in the true species of Lepiota (see p. 112). C. carcharias (Secretan) Fayod is found under similar conditions, but is white or flesh-coloured. C. cinnabarinum (Secretan) Fayod is also found in short grass and moss, but has a cinnabar-red, floccose cap and C. granulosum (Fries) Fayod is yellowish brown with non-amyloid spores and adnexed gills.
Many authorities prefer to connect this small group of closely related species more to members of the Tricholomataceae (i.e. the family which contains the Wood Blewits (p. 131), Mycena (p. 68, etc.) than to the parasol mushrooms—Lepiota (p. 112).
Illustrations: Hvass 23; LH 129; NB 1037; WD 84.
[105]
[106]
Cap: width 25-70 mm. Stem: width 4-7 mm; length 25-50 mm.
Description:
Cap: bright orange-yellow or apricot, fleshy, soft, depressed at centre and with wavy, incurved, slightly downy margin.
Stem: yellow at apex, rich red-brown or orange about the middle and sometimes dark brown at the very base.
Gills: decurrent, deep orange, thin, crowded, repeatedly forked and easily separable from the cap-tissue.
Flesh: yellowish, pale in the cap, darker in the stem.
Spore-print: white.
Spores: medium sized, hyaline under the microscope, ellipsoid or pip-shaped, smooth, 7-8 × 4 µm and red-brown when mounted in solutions of iodine.
Marginal and facial cystidia: absent.
Habitat & Distribution: Common in woodlands, particularly with pines, and on heaths or in rough hill-pastures.
General Information: This fungus is recognisable by the orange or yellow cap and stem and the decurrent gills. It was formerly placed in Cantharellus because of the colours, white spores and the decurrent gills, but it really differs in many other respects. It is true, however, that it is frequently confused with the true Chanterelle (Cantharellus cibarius Fries, p. 162) by those who do not inspect their specimens carefully. The gills are thin, plate-like as in other agarics and not fold-like as in Cantharellus (see p. 162). The Chanterelle is edible and sought after as a delicacy, but there are varying reports as to the edibility of Hygrophoropsis. Certainly it is not of the best quality and there is evidence for it causing upsets: therefore it is best to take the name ‘False Chanterelle’ at face value and treat this fungus as truely false; ‘aurantiaca’ means orange-coloured and refers to the colour of the fungus.
A pale form is frequently collected, particularly in hill-pastures, and is probably worthy of specific recognition. The cap is ochraceous yellow to cream and the stem distinctly dark in the lower half.
There is some confusion as to the true position in classification of this fungus. The anatomical details of the fruit-body parallel those of Paxillus involutus (Fries) Fries (see p. 38) although the spore-print is white. There is little doubt that future research will answer this problem.
Illustrations: Hvass 183; LH 185; NB 1031; WD 163.
[107]
[108]
Cap: width 40-100 mm. Stem: width 12-20 mm; length 40-80 mm.
Description:
Cap: rounded then expanding to become plano-convex, fleshy with the margin incurved at first, initially pure white, but soon becoming cream-colour and at maturity streaked brownish particularly at the centre.
Stem: white with a simple, very thin, white ring which becomes brownish on rubbing and is easily lost with age or by handling.
Gills: free, pink but finally umber-brown at maturity.
Flesh: white, flushed reddish when cut especially in the stem.
Spore-print: cigar-brown, with hint of purple.
Spores: medium sized, ellipsoid or egg-shaped, smooth, small, 7-8 × 4-5 µm and dark brown under the microscope.
Marginal and facial cystidia: absent. Basidia 4-spored.
Habitat & Distribution: The field-mushroom grows amongst grass in pastures, etc., and also on old lawns where it may form fairy-rings.
General Information: This is the common wild, edible mushroom for which many people have in the past unwisely substituted many quite unrelated species. Deaths have often been caused by lack of careful observation when selecting wild fungi for the table; this only emphasises why white mushrooms found in fields should not be casually eaten.
Secretan the Horse-mushroom is also edible, but is much bigger (up to 180 mm), creamy white and bruises slightly yellowish on handling; it also has larger spores (7-10 × 5 µm), club-shaped cells on the gill-edge, gills commencing white and not pink, and the presence of a complex ring.
Genevier the ‘Yellow-staining mushroom’ has even smaller spores than the field mushroom, i.e. 5-6 × 4 µm and a rather strong, unpleasant smell; if eaten many people subsequently suffer from stomach-pains and this shows that even amongst those fungi which the scientist would call true mushrooms, i.e. those fungi in the genus Agaricus, there are some poisonous members. Thus it is always necessary to have wide experience before one collects fungi for eating and until this is achieved all specimens should be discarded.
Illustrations: Field mushroom—Hvass 163; LH 133; NB 316; WD 712. Horse mushroom—Hvass 160; LH 135; WD 721. Yellow-staining mushroom—Hvass 159; WD 713.
[109]
[110]
Cap: width 70-100 mm. Stem: width 15-25 mm; length 50-70 mm.
Description:
Cap: creamy white, ivory or light buff, slightly darker at the centre with age, fleshy, rounded and with wavy margin, finally expanding to become plane-convex; the margin is incurved and slightly downy at first.
Stem: firm, rather thick, white at the top, creamy or buff below and slightly downy when fresh.
Gills: sinuate to adnexed with a slight decurrent tooth, white to pale buff.
Flesh: with a very strong smell of meal, white and thick.
Spore-print: white.
Spores: small, ellipsoid, smooth, hyaline under the microscope, 5-6 × 3-4 µm and not becoming blue-grey with solutions containing iodine.
Marginal and facial cystidia: absent.
Habitat & Distribution: Found amongst grass in base rich pastures, often in fairly large rings from April to June and on golf-courses particularly those near the sea.
General Information: The common name refers to the early appearance of this agaric; St George’s Day is April 23rd, and this mushroom is found about this time in favourable years, its fruiting often extending into early June, particularly if the fruiting is retarded by a cold and wet spring. It is easily recognised by the pale colour of the cap, strong mealy smell, but particularly by its appearance in spring. In each new year it is probably the first of the larger agarics to appear. This species will be found in most books under the genus Tricholoma, but differs from typical members of this group in the anatomy and chemistry of the gill-tissues.
The Latin name ‘gambosum’ is derived from ‘gamba’ meaning a hoof and this reflects the shape of the fleshy cap as it pushes up through the grass. Another much older name is Tricholoma georgii (Fries) Quélet which was used by Clusius and is derived from the legend of St George.
Illustrations: Hvass 28; LH 83; WD 92.
[111]
[112]
Cap: width 70-200 mm. Stem: width 12-20 mm; length 100-250 mm.
Description:
Cap: dull brown or greyish brown, oval or rounded at first, but later becoming bell-shaped, finally expanding but for the central umbo and the surface breaking up into shaggy scales.
Stem: straight, tapering upwards from a slightly bulbous base, felty at first but then the surface breaking up into small patches which finally resemble the pattern of a snake-skin; there is also a large, thick, white ring which is brown below and becomes loose on the stem.
Gills: remote, white, crowded and fairly broad.
Flesh: white, thin, soft.
Spore-print: white.
Spores: very long, ellipsoid with a germ-pore, hyaline under the microscope about 16 × 10 µm (14-17 × 9-12 µm), and becoming reddish brown in solutions containing iodine.
Marginal cystidia: variable, elongate balloon-shaped and hyaline.
Facial cystidia: absent.
Habitat & Distribution: Found from summer until mid-autumn, on the outskirts of copses, in fields, at edges of woodland or in woodland clearings; it is sometimes found in very large rings.
General Information: When this fungus first appears through the soil it resembles a drum-stick with the margin of the unexpanded cap tightly hugging the stem. It is an easily recognised fungus because of its straight and graceful stature with large cap and tall stem. It is one of our best edible fungi and cannot be confused with any other agaric. L. rhacodes (Vittadini) Quélet is not as elegant and has much smaller spores.
Illustrations: F 26a; Hvass 15; LH 125; NB 311; WD 51.
[113]
[114]
Cap: width 8-14 mm; height up to 18 mm. Stem: width 4-6 mm; length 50-70 mm.
Description:
Cap: sharply conical, in fact often with a very distinct apical point, never or very rarely becoming expanded, often fluted and puckered at the incurved margin, smooth, viscid, pale buff or clay colour, but soon flushed with greyish green at maturity and becoming free of the fibrils of veil which ornament the margin when young.
Stem: slender, tough and smooth, similarly coloured to the cap and sometimes blueing at the base when picked.
Gills: adnate to adnexed, crowded, purplish black except for white edge.
Flesh: white or pallid.
Spore-print: purple-brown.
Spores: long, ellipsoid, slightly lemon-shaped, smooth and with a distinct germ-pore at one end and 12-14 × 7 µm in size.
Marginal cystidia: bottle-shaped with an elongate tapering neck, with thin walls which at most become pale honey in solutions containing ammonia, unlike the cystidia of Hypholoma (p. 64).
Habitat & Distribution: Commonly growing amongst grass in fields near farm-yards, on heaths and by roadsides; often it occurs in small troops.
General Information: Psilocybe semilanceata is recognised by the uniquely shaped cap; ‘semilanceata’ means half spear-shaped, from the papilla at the top of the cap, giving it a pointed aspect. However, the common name is more descriptive and comes from the fact that these caps resemble the helmets worn by French soldiers in the early part of the century.
This fungus was once very isolated amongst British agarics, but now it has been united with a group of small purplish brown-spored fungi formerly placed in the genus Deconica. What is of more interest is the fact that unlike many British agarics the cap often does not expand fully in order to release the spores. In this way it allows mycologists to hypothesise on how certain of the enclosed, stalked Gastromycetes evolved in some of the desert regions of the world.
Illustrations: LH 149; NB 3311; WD 787.
[115]
[116]
Cap: width 10-20 mm. Stem: width 3-6 mm; length 70-100 mm.
Description:
Cap: very hygrophanous, sand colour, orange-yellow or ochraceous brown tinted cinnamon when fresh but drying uniformly yellow-ochre, thin, fragile, striate when moist, but soon non-striate as water is lost from the cap.
Stem: tall, slender and similarly coloured to the cap, straight, fragile, minutely striate from the top to bottom with what appears to be minute powdery granules.
Gills: adnate then becoming free, crowded, ochraceous and finally cinnamon-rust in colour.
Flesh: russet when moist but rapidly becoming yellowish as the fruit-body dries.
Spore-print: rust-brown.
Spores: long, ellipsoid, with thick, bright yellow-brown walls and distinct germ-pores at their ends when seen under the microscope, and over 10 µm in length (11-12 × 6 µm.)
Marginal cystidia: pinheaded or skittle-shaped.
Facial cystidia: absent.
Habitat & Distribution: This fungus grows in ones and twos, more rarely in troops amongst grass.
General Information: This is one member of a whole complex group of ochraceous, brown, tawny or cinnamon-brown capped agarics which superficially appear to be the same, but on closer examination the expert can split them into several distinct species. The use of microscopic characters is essential and outside the scope of this book or the ordinary mushroom-picker’s manual. However, the closely related C. lactea (J. Lange) Métrod can be more easily distinguished for it has a white or cream-coloured cap and stem. It also has larger broadly ellipsoid spores, measuring 12-14 × 6-9 µm, but the same shaped cells on the gill-edge.
Illustrations: LH 153; NB 354; WD 682.
[117]
[118]
Many agarics grow in circles, but not all of them produce zones in the vegetation. It is the distinct zonation caused by the ‘fairy-ring champignon’ Marasmius oreades (Fries) Fries and related fungi which have given rise to the name of Fairy-ring and which resulted in the foundation of many folk tales.
A fairy-ring can be divided into four distinct zones, a central zone of fairly normally developed vegetation on the outside of which is a green, actively growing zone of grass; outside this is a zone composed of brown or dead vegetation. The outermost zone again appears to be far more lush than the normal grass in the vicinity and it is in this last zone that the fruit-bodies of the fungus causing the pattern appear.
A generalised explanation of the zoning appears to be as follows:—
In the outermost zone the actively growing mycelium decomposes soil constituents and liberates nitrogenous material which is in turn taken up by the plant roots nearby and utilised for their growth. In the penultimate zone the grass is dead, probably not caused by a direct parasitic attack but by the mycelial threads filling the air-spaces in the soil and so inhibiting water flow. This destruction of the delicate balance of water and air found in any soil induces drying out and gradual death of the plants whose roots permeate the soil. Behind the dead-zone is vegetation which shows increased vigour apparently due to plant-nutrients being released by the decaying mycelium and plant-material, whose death has been caused by the presence of the fungus. The innermost zone is not so stimulated.
With nothing more than graph and tracing paper, a tape-measure, note-book and pencil, pieces of cane about four inches long, and coloured dye or indian ink, it is exciting to assess the annual radial growth of fairy-rings and to correlate these with environmental conditions. This can be carried out on a school lawn or on a home lawn; the method and further experiments are given in Appendix iii.
[119]
[120]
Cap: width 25-60 mm. Stem: width 5-9 mm; length 30-80 mm.
Description:
Cap: pinkish tan with slight flush of brown at centre, hygrophanous and drying out buff-coloured or clay-coloured, convex at first then expanding to become plane, but for an obtuse umbo which is retained at the centre.
Stem: pale buff, tough, flexible and smooth.
Gills: adnexed, pale cream colour or pinkish buff and fairly distant.
Flesh: whitish or pinkish tan, smelling of cherry laurel (cyanic).
Spore-print: white.
Spores: medium sized, hyaline, pip-shaped, smooth, not staining bluish grey when mounted in solutions containing iodine and about 10 × 6 µm in size (9-11 × 5-6 µm).
Marginal and facial cystidia: absent.
Habitat & Distribution: This agaric is very common from May to October on lawns and grass-verges.
General Information: M. oreades forms well developed fairy-rings, and is easily recognised by its tough nature, pale colours and ability to revive after having been dried. This ability to revive in moist weather even after the fruit-body has been dried by the sun or wind is a character which was used to distinguish members of the genus Marasmius. However, this is a very subjective character and since microscopic techniques were introduced and used widely in the study of agarics the genus has been delimited rather more critically. Marasmius is close to Collybia (p. 90), in fact many species appear in one book in one genus and in another book in the second genus; M. oreades itself is not a typical member of the genus. Marasmius seems to be a much more important genus in the tropical and subtropical regions of the world; we have already mentioned how some of the small species of Marasmius in Europe grow only on leaves of a particular plant (see p. 92). M. androsaceus (Fries) Fries (see p. 231) is the horse-hair fungus.
Illustrations: F 19a; Hvass 81; LH 115; NB 351; WD 2410 (not very good).
[121]
Plate 37. Fleshy fungi reviving when moistened even after drying: Spores white and borne on gills
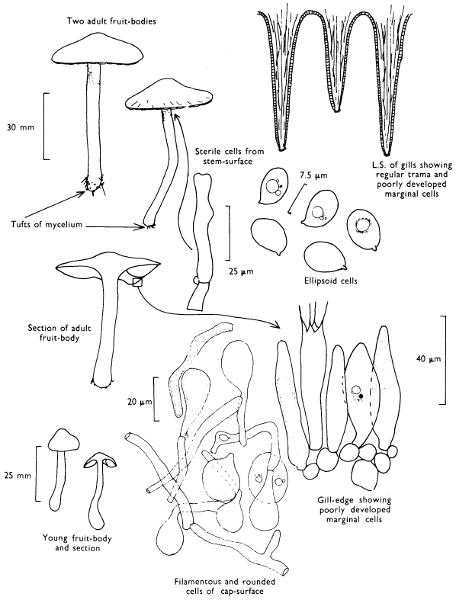
[122]
Cap: width 20-40 mm. Stem: width 3-5 mm; length 45-70 mm.
Description:
Cap: bell-shaped at first then expanded, hygrophanous, date-brown, striate when moist but pale fawn or tan and non-striate when dry, and usually becoming quite silky-shiny.
Stem: slender, fragile, greyish brown, silky fibrillose-striate and shiny.
Gills: almost free, crowded and pale greyish brown when young, but finally flesh coloured.
Flesh: brownish and smelling very strongly of meal when cut or broken between the fingers.
Spore-print: salmon-pink with flush of cinnamon.
Spores: medium sized, fawn under the microscope, star-shaped with 4-6 prominent angles, 9-10 × 7-9 µm, smooth and with no germ-pore.
Marginal and facial cystidia: absent.
Cap: width 25-40 mm. Stem: width 5-9 mm; length 25-50 mm.
Description:
Cap: convex then flattened or with slight umbo, umber-brown with a greyish cast which becomes accentuated as the cap dries out and finally becoming silky-shiny; the margin is incurved and striate at first but on expanding it becomes non-striate with time.
Stem: short, fibrillose, greyish brown, shining and white at the base, very fragile and often snaps just above the soil-level when collected.
Gills: crowded, adnate and pale greyish brown then pinkish brown.
Flesh: with a strong smell of new meal, brownish becoming paler as it dries out.
Spore-print: salmon-pink.
Spores: medium sized, smooth, pale fawn under the microscope, angular almost cubic and 10-13 × 8-9 µm in size.
Marginal and facial cystidia: absent.
General Information: Nolanea staurospora is very common amongst grass, in many habitats such as on heaths, and in woodlands and copses, but it is particularly common in pastures and on lawns. It is difficult to separate from close relatives on field-characters, except for the strong mealy smell; however, it is recognised immediately by the spore-shape, in fact stauro—means a cross and spora—spore!
[123]
[124]
Because of the flattened cap and gill-shape N. sericea (Mérat) P. D. Orton was first placed in Entoloma, but for a long time it was one of the smallest members of that genus. The European species of Nolanea have recently been critically analysed, and now that closely related species to the silky Nolanea have been found, it appears better placed in Nolanea although it is still found under Entoloma in many books. The Latin word ‘sericeum’ means silky and refers to the silky cap and stem of this fungus which is a very noticeable feature when the fungus is collected in the dry state. The common name which has been given to this fungus also refers to the silky nature of the fruit-body.
Illustrations: N. staurospora—LH 181; ND 312; WD 522. N. sericea—LH 181; WD 525.
Cap: width 12-28 mm. Stem: width 3-6 mm; length 40-60 mm.
Description:
Cap: semiglobate to convex and hardly expanding even with age, smooth, expallent, dull cinnamon-brown or dark tan-colour, becoming clay-colour or pale cinnamon-colour from centre outwards on drying and so sometimes appearing as if it is zoned.
Stem: slender, fragile, smooth and pale cinnamon-brown, except at apex where it is dotted with white; it is usually more brownish below.
Gills: adnate, crowded, pale brown and mottled, but becoming more uniformly umber-brown except for whitish margin.
Flesh: whitish or pale cinnamon colour.
Spore-print: purple-brown.
Spores: long, lemon-shaped under the microscope, dull brown, warted all over but for the distinct germ-pore; 12-15 × 7-8 µm in size.
Marginal cystidia: variable spindle-shaped with flexuous neck and subcapitate apex, about 5-6 µm wide.
Facial cystidia: absent.
Habitat & Distribution: Common amongst short grass on lawns, in pastures, on grass-verges, etc., from May until October.
[125]
[126]
General Information: P. foenisecii is recognised under the microscope by the ornamented spores; this character was used to separate this fungus in the new genus Panaeolina. However, although the spore-print is not exactly black the stature, mottled gills and anatomy conform closely with Panaeolus sphinctrinus (Fries) Quélet and P. rickenii Hora (see p. 210 and below respectively). The same fungus has been placed in Psilocybe (see p. 114), but it has little in common with members of that genus. The word ‘foenisecii’ means hay-harvest, reflecting the habitat of growing in fields. This fungus is variable in colour depending on its state of turgidity; it can be easily confused with other species of Panaeolus when moist and with certain species of Conocybe when dry. P. rickenii is an equally common agaric growing on similar or slightly less base-rich soil-types. It has a distinctly bell-shaped reddish brown cap with a pale incurved margin which in wet weather is, like the entire stem, beaded with droplets of liquid. This gives the fungus a glistening appearance when seen fresh and as it dries these droplets are lost and the cap becomes slightly zoned. The stem is pale reddish-brown with a strong frosted appearance because of the minute hairs which cover it. I have no doubt that the classification of these fungi will be assisted by careful analysis of the shapes of the hairs found in the different species.
Illustrations: Panaeolina foenisecii—LH 145; WD 784. Panaeolus rickenii—LH 145.
Cap: width 30-60 mm; height 80-200 mm. Stem: width 10-20 mm; length 80-250 mm.
Description: Plate 40.
Cap: at first cylindrical or oval then bell-shaped, fleshy, fragile, white and covered with woolly, whitish, shaggy scales which have brown tips; the centre of the cap is smooth and yellow to ochraceous whilst the margin becomes striate and lilaceous and finally black as the tissue liquefies (autodigests) and the margin rolls up to expose new areas of spore-bearing tissue.
Stem: tall, white, smooth and tapered towards the apex, with a white ring which can easily move up and down the stem with handling, and which soon disappears with age.
[127]
Plate 40. Fleshy fungi becoming reduced to an inky mass: Spores black and borne on gills
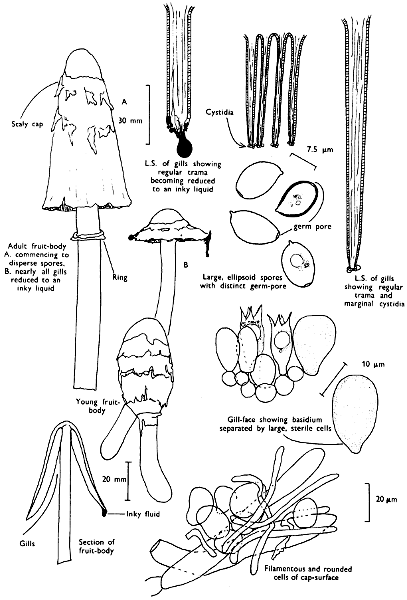
[128]
Gills: free at first, white then pink and finally black, becoming gradually dissolved into a black fluid from the base of the cap upwards.
Flesh: white, thin, except immediately in the central area of the cap.
Spore-print: blackish-purple.
Spores: long, elongate-ellipsoid, large and about 13 × 5-8 µm in size, (12-15 × 7-9 µm).
Marginal cystidia: elongate club-shaped to balloon-shaped, hyaline and thin-walled.
Facial cystidia: absent.
Habitat & Distribution: Grows in clusters on rich ground, in gardens, on sides of newly prepared roads and central reservations of motor-ways, on path-sides, in cultivated fields and on rubbish dumps; it grows from spring to autumn and sometimes occurs in huge troops.
General Information: Easily recognised by its size, the shape of the cap with its scaly surface and from its resemblance to a ‘judge’s wig’; it is frequently called the ‘lawyer’s wig’ and whereas some common names are not very descriptive and one has to use a lot of imagination to conjure up what the common name implies, in this case it is not so. It is also known as the ‘shaggy cap’ or ‘shaggy ink-cap’. Ink or inky cap is, however, a common name for many species of the genus Coprinus (see p. 211-4).
The unrelated Lyophyllum decastes (Fries) Singer and L. connatum (Fries) Singer are also common fungi growing on roadsides, on soil and compost-heaps. They too break through embankments, soil, paths, etc., producing large craters and mounds of debris.
Illustrations: Coprinus comatus—F 34b; Hvass 172; LH 137; NB 355; WD 822. Lyophyllum decastes—LH 81; WD 142.
Cap: width 45-90 mm. Stem: width 8-14 mm; length 50-125 mm.
Description: Plate 41.
Cap: convex then expanded with obtuse central umbo, dull clay-brown or date-brown and at first covered with flattened, woolly fibrils which are gradually lost with age; the margin is incurved and fringed with remnants of the veil.
Stem: fragile, pale dingy-coloured or clay-coloured at apex, dull brown below the ring-zone which consists of white fibrils; later in development these fibrils catch the spores and the stem becomes black and fibrillose-scaly, particularly below the ring-zone.
[129]
[130]
Gills: sinuate, crowded and very dark brown or almost black with distinct white margin which is covered in tiny beads of liquid in moist weather.
Flesh: pale buff.
Spore-print: almost black.
Spores: long, dark brown, lemon-shaped and warted with distinct and prominent germ-pore and 10-12 × 6-7 µm in size.
Marginal cystidia: club-shaped or with a distinctly rounded head.
Facial cystidia: absent.
Habitat & Distribution: Common on the ground near newly built houses, on roadsides, tips and paths in woods, either solitary or in groups; it is also found in pastures.
General Information: The fibrillose scaly cap and stem and the almost black gills which frequently have liquid droplets at their edge separate this species from all other agarics and microscopically it can be easily recognised by the warted spores. ‘Velutina’ means velvety and refers to the texture of the cap-surface, of the young fruit-body. The genus name Lacrymaria refers to this peculiar, but certainly not unique, phenomenon, of exuding liquid from cells on the gill-edge. This has been compared with weeping and ‘lacrymans’ means weeping; the common name reflects this also—weeping widow (cf. p. 154).
This fungus has had a chequered history, for it is also known in some books as Hypholoma lacrymabunda (again meaning weeping) or H. velutina; the anatomy of the fungus, however, is quite different to Hypholoma (e.g. H. fasciculare p. 64). More recently it has found a place in Psathyrella, but it seems unsatisfactorily placed there because of the warty spores, black spore-print and fibrillose cap-surface; it warrants a separate genus, i.e., Lacrymaria. L. pyrotricha (Fries) Konrad & Maublanc is the only other British species of this genus but it has a bright orange cap colour; it is rare.
Illustrations: Hvass 180; LH 141; WD 863.
[131]
Cap: width 70-100 mm. Stem: width 10-15 mm; length 70-100 mm.
Description: Plate 42.
Cap: rounded then flattened or slightly depressed in the centre, smooth, bluish lilac, or violaceous when young but gradually with age becoming reddish-brown, with or without a flush of wine colour.
Stem: similarly coloured to the cap, equal, fleshy, elastic, fibrillose and streaky.
Gills: adnate with or without a decurrent tooth, crowded, lilac and easily separable from the cap-tissue by the fingers.
Flesh: bluish violaceous, but drying out dirty buff in the base of the stem.
Spore-print: flesh-coloured.
Spores: medium-sized, ellipsoid appearing smooth but very minutely roughened under the microscope, although it is very difficult to see except with a good instrument (6-8 × 4-5 µm in size).
Marginal and facial cystidia: absent.
Habitat & Distribution: Widespread in troops or small groups in copses and under hedgerows and not uncommon in flower-beds in gardens in late autumn and early winter especially on compost heaps and in rhubarb patches which have been mulched with piles of moribund leaves.
General Information: This fungus was originally placed in Tricholoma, but due to differences in anatomy and the distinctly coloured and ornamented spores it has been placed along with ‘common blewits’ T. personatum (Fries) Kummer (or better L. saeva (Fries) P. D. Orton), in the genus Lepista. This genus which is also called Rhodopaxillus, again referring to the pinkish spore-print, is not found in many of the easily obtainable books. One should look for the fungus under Tricholoma, from which it can be separated easily by the beautiful colour.
Both the ‘wood blewits’ and ‘common blewits’ have been regularly sold in markets in England within the last fifty years. They are edible and considered of high quality. In their fresh state they are easily recognised, but as they age they become browned and so resemble many other less desirable fungi.
Illustrations: F 17d; Hvass 49; LH 91; NB 1252; WD 123 (a bit too pastel).
[132]
[133]
Cap: width 40-100 mm. Stem: width 15-25 mm; length 50-75 mm.
Description: Plate 43.
Cap: rounded gradually expanding to become plane, whitish with numerous brown radiating fibrils and with the margin irregular because of fragments from the ring which are left there after expansion of the cap.
Stem: short, cylindrical, smooth, bruising reddish-brown when handled and with a narrow ring which soon collapses and disappears.
Gills: free, pink at first then purple-brown, narrow and crowded.
Flesh: solid, thick, firm and slowly flushing brownish on cutting.
Spore-print: purple-brown.
Spores: medium-sized, broadly ellipsoid, purple-brown under the microscope, less than 10 µm long, (6-8 × 5-6 µm).
Marginal cystidia: club-shaped, 10-12 µm at apex.
Facial cystidia: absent.
Basidia: 2-spored.
Habitat & Distribution: Frequent on manure heaps, straw heaps, on road scrapings and around garden plants.
General Information: This fungus is recognised by the dark fibrils on the cap, the 2-spored basidia easily seen with the low power of a microscope, and the pink gills when young. Much confusion has existed over this fungus and its nearest relatives. It is similar to the ‘Cultivated mushroom’, A. hortensis (Cooke) Pilát, which is offered for sale in shops. However, it differs in several minor details and it may be that A. bisporus is the fungus from which the cultivated mushroom developed, very probably unconsciously by man, but the history of the cultivated mushroom is very obscure. The cultivated mushroom when bought in British shops is white but in the United States two varieties are sold, one with the brownish fibrils predominating and a snow-white one where the fibrils do not darken; the former is frequently found in Europe. The white form is sometimes found in gardens where spent-mushroom spawn is used as mulching around fruit-trees but it has a rounder cap than A. bisporus. The cultivated mushroom accounts for an annual income of £14 million in the British Isles.
Illustrations: A. hortensis—LH 133 (as the forma albida); NB 317; WD 711. A. bisporus—Hvass 161; LH 133.
[134]
[135]
A group of fungi which includes the bracket fungi, hedgehog fungi, fairy-clubs and their relatives; in the majority of species the margin continues to grow through the favourable part of the season and so often envelopes leaves, grass, etc.
[140]
Cap: 100-300 mm. Stem: width 25-50 mm; length 25-75 mm.
Description:
Cap: fan-shaped or semicircular, spreading horizontally with age, ochre-yellow or straw-coloured with dark brown, flattened scales in concentric zones which are much more dense at the centre.
Stem: short, stout, white at apex and netted with pale creamy buff about middle, but dark brown or black towards the base and attached to the side of the cap.
Tubes: whitish to yellowish and decurrent with large, angular, irregularly fringed, whitish or cream-coloured pores.
Flesh: with strong, not very pleasant smell, cream-coloured or white.
Spore-print: white.
Spores: long, oblong or elongate ellipsoid, hyaline under the microscope (10-15 × 4-5 µm) and not blueing in solutions containing iodine.
Habitat & Distribution: An easily recognisable fungus growing on stumps and old living trees, especially of sycamore and elm where it often forms tiers of caps from late spring until autumn; however, they decompose rapidly and almost completely disappear by the next year when new fruit-bodies may appear in the same place, a phenomenon which may take place for several consecutive seasons.
General Information: The genus Polyporus is in most text-books, a big and unwieldy genus joining together all fleshy, annual fungi possessing tubes; even the boleti (see p. 32) have been included! Many of these species are now considered less closely related one to another than previously thought. Boleti differ from polypores, however, in their less tough and distinctly putrescent fruit-body, and in the fact that the margin of the cap extends but does not continue to grow during the life-cycle; the margin of the polypore fruit-body is active and may burst into growth again when favourable weather conditions occur. The ‘Scaly polypore’ has a flesh which consists of two types of hyphae: (i) hyphae of unlimited growth with abundant protoplasmic contents which stain easily and which collapse on drying; and (ii) thick-walled, strengthening hyphae which bind the thin walled hyphae together. Laetiporus sulphureus (Fries) Murrill ‘Sulphur polypore’ has a single type of hyphae in the tubes, i.e. thin walled generative, and only a few binding hyphae in the flesh. It has an orange cap with a rather thick, sulphur or chrome-yellow margin, sulphur-yellow tubes and pores and yellow, then pale buff, flesh. The spore-print is white and the spores hyaline, pip-shaped and medium sized, (5-7 × 4-5 µm).
[141]
[142]
Illustrations: P. squamosus—F 43b; Hvass 267; LH 75; NB 1291; WD 941. L. sulphureus—Hvass 268; LH 73; NB 1293; WD 942.
Cap: 75-200 mm, kidney-shaped or hoof-shaped, smooth, covered by a thin, separable and greyish silvery or pale brownish skin; cap-margin thick, incurved and projects beyond the tubes.
Stem: rudimentary, simply a small hump below which the fungus develops.
Tubes, pores and spore-print: white.
Spores: sausage-shaped, and thin-walled hyaline under the microscope and very narrow, (5-6 × 1-2 µm). It grows on birch throughout the country where it causes a sap wood-rot which finally converts the inner timber to a red-brown friable mass. The flesh, which contains thickened binding hyphae, is used for mounting insects and for sharpening knives, hence the common name ‘Razor-strop fungus’.
Illustrations: Hvass 269; LH 67; NB 1174; WD 933.
Cap: 100-250 mm, kidney-shaped, yellow-brown to rust-brown, but finally almost black, at first covered with shaggy hairs, but these tend to mat together with age.
Stem: absent.
Tubes and flesh: rust-colour; pores at first yellow, but finally red-brown.
Spore-print: yellow-brown.
Spores: medium sized (8-9 × 7-8 µm) and globose under the microscope. It grows on various broad leaved trees, especially ash where it causes a spongy, white heart-wood rot. The flesh contains hyphae with thick, brown walls.
Illustrations: LH 63; WD 961.
[143]
[144]
Cap: 100-300 mm, bracket-shaped or tub-shaped, dark brown with a knobbly, velvety, roughened and grooved surface; margin at first golden yellow.
Stem: absent or short, thick and brown.
Tubes and pores: greenish yellow.
Flesh: deep rust-brown.
Spore-print: greenish yellow.
Spores: medium sized, greenish under the microscope, ellipsoid and about 8 × 4 µm in size, (7-8 × 3-4 µm). This fungus is found on conifers or near conifer stumps where it is attached to the roots; it causes a brown cubical heart-wood rot; the flesh of the fruit-body is composed of only one type of hyphae.
Illustrations: LH 67; NB 1113; WD 951.
Cap: 75-100 mm, or even up to 200 mm wide, grouped and forming a tuft of caps up to 750 mm across. The individual caps are fan-shaped, pliable, rather thin and yellow-brown to snuff-brown with their margins wavy and cream colour or yellowish.
Stem: replaced by a united mass of caps.
Tubes, pores and flesh: white and very soft, but becoming black on bruising.
Spores: small, pip-shaped, hyaline under the microscope and 5-6 × 4-5 µm. This fungus is a common sight forming masses at the base of broad-leaved trees; it is common on beech. It is a soft, fibrous polypore as a result of the lack in the flesh of thick-walled specialised hyphae.
Illustrations: Hvass 277; LH 73; NB 1294; WD 931.
The spores of all the annual polypores described above do not blue when placed in solutions containing iodine.
[145]
Cap: 25-60 mm. Stem: absent.
Description: Plate 46.
Cap: semi-circular, flattened, thin, tough and flexible when fresh with the surface velvety and marked with smoother, paler concentric zones giving a pattern of yellow-brown, grey or darker greenish grey zones; the margin is thin and is the palest of the zones and may be wavy or lobed.
Tubes: white with small, round and rough-edged to angular white or cream-coloured pores which become yellowish with age.
Flesh: white, tough and continuous with the tube tissue and so not allowing one to detect any difference between the tissues.
Spore-print: white.
Spores: medium sized, oblong and hyaline under the microscope, and 6-8 × 2-3 µm; not blueing in solutions containing iodine.
Habitat & Distribution: Very common on stumps, trunks and fallen branches of various trees, especially beech; it is to be found throughout the year.
General Information: It is often associated with nodulose masses of fungal tissue which are covered in small poroid areas and are very confusing when found by the beginner; they are simply growth-forms of Coriolus versicolor; such forms are frequently found on old house-timbers exposed to the weather, particularly window frames where it forms a distinct rot. Its flesh consists of thin-walled hyphae and binding hyphae as in Polyporus squamosus as well as an additional thick-walled type called skeletal hyphae. It would appear that several polypores are capable of producing the amorphous growths mentioned above, some of which contain hyphal fragments called conidia.
The bands of colour on the cap of the ‘many zoned polypore’ are retained after drying and from a group of fruit-bodies the most attractively zoned can be selected, mounted on small pieces of wood or cardboard and fitted at the back with a pin. Such preparations make very attractive brooches and have been used even by modern designers to contrast with their fashion creations.
There are many pale tubed polypores growing on wood. Daedalea quercina Fries ‘Mazegill’, grows on oak and has irregular maze-like pores; Lenzites betulina (Fries) Fries, grows on birch, has tough plates which resemble the gills of an agaric. Datronia mollis (Fries)[146] Donk forms thick spreading resupinate patches on beech, sometimes with irregular dark brown caps formed by the upturned margin. Several species of Tyromyces occur in Britain and are characterised by their white pores and tubes and the white or pale-coloured caps. Bjerkandera adusta (Fries) Karsten has a grey pore-surface and is also frequently found on beech.
Illustrations: F 44a; LH 69; NB 1173; WD 512.
Cap: 100-350 mm. Stem: absent.
Description: Plate 47.
Cap: bracket-shaped, rather flat at margin but humpy and irregular about the middle, frequently concentrically zoned, smooth and only slightly shiny; its margin is whitish or pale greyish.
Tubes: red-brown or cinnamon-brown, obscurely layered and with small, white pores flushed with pale cinnamon-brown, but deep red-brown when rubbed or with age.
Flesh: with a fragrant smell, deep red brown and felty-fibrous.
Spore-print: dark cinnamon-brown.
Spores: long, oval with truncate apex, smooth, but reticulate on the inner surface of the inner wall giving the spores a patterned appearance when seen under the microscope; 10-11 × 6-7 µm in size.
Habitat & Distribution: This fungus is common on various trees, especially beech and can be found throughout the year.
General Information: This common Ganoderma is perennial and distinguished from other polypore groups by the complex spores. G. applanatum (Fries) Karsten is closely related, but differs in the thinner fruit-body with a thin margin, and the pale cinnamon-brown flesh; the flesh of both species contains thick-walled binding and strengthening hyphae as well as the generative hyphae.
So sensitive are the pores to bruising that if a drawing or writing is executed on the lower surface with a pin, needle or similar sharp instrument and the fungus dried, the red-brown lines produced are retained and the pattern preserved. Several fungus paintings prepared in this way were made in the early part of the century, many beautiful ones having originated in the eastern part of North America.
[147]
Plate 46. Woody fungi: Spores white and borne within tubes or on thickened plates
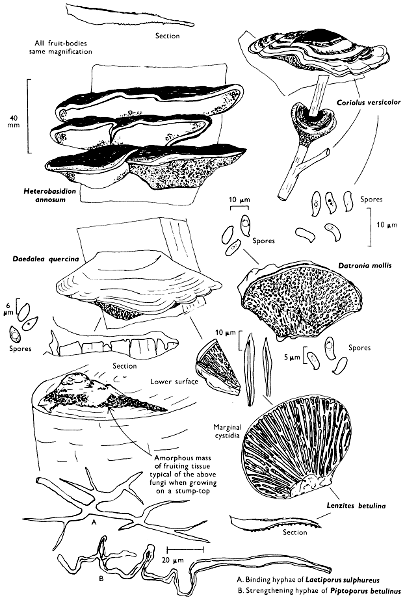
[148]
Fomes fomentarius whose important characters are described below has frequently been confused with Ganoderma europaeum. It is common growing on birch in Scotland, but is less frequent south of Perth, and then grows probably more frequently on beech which is similar to the pattern found on the continent of Europe. However, it has grown in former periods in England on birch, for it was found commonly amongst birch timbers in an excavation of an early Mesolithic lake side village near Scarborough, Yorkshire.
Illustrations: NB 1253; WD 1602.
Cap: 90-300 mm, hoof-shaped, thick, broadly attached to the substrate, zoned with yellow-brown and shades of grey; its margin is blunt and fawn or pale brownish.
Tubes: layered, cinnamon-brown with pale cinnamon pores with a whitish bloom.
Flesh: cinnamon-brown or buff and woolly.
Spore-print: white.
Spores: elongate, ellipsoid, very long, hyaline under the microscope, 15-18 × 5-6 µm, and not ornamented. The flesh contains both thick- and thin-walled hyphae. It grows on birch and less frequently on beech. The flesh has been used in dentistry, in manufacturing fancy articles, such as mats, and was the basis of the tinder used in flint-boxes.
Illustrations: LH 65; NB 1171; WD 1001.
, grows on willows and causes their heart-rot. It is a rust-brown, woody fungus with a hard crust and brown tubes and flesh. The spore-print is white and composed of small, spherical, hyaline spores, 5-6 µm in diameter. The flesh contains thin- and thick-walled hyphae.
Illustrations: LH 63; WD 993.
[149]
[150]
, grows on various sorts of broad-leaved trees, particularly poplars and often becomes covered in mosses and algae. It has a pale buff or cream-coloured cap, white flesh, pores, tubes and spores.
Illustrations: LH 67.
, grows on conifers often several feet above the ground. It has a woody, deeply cracked upper surface, dark red-brown flesh, tubes and pores. Its spores are small, broadly ellipsoid and brown.
Variable, sometimes possessing a cap, sometimes resupinate except for the upturned margin, flattened or shell-shaped, red-brown to blackish at the centre but pale at the margin, which when seen from below is always white. The tubes are in layers and like the pores, flesh and spore-print are white. The spores are broadly ellipsoid, small, smooth, hyaline and 4-5 × 4 µm. The flesh is fairly tough as it contains both generative hyphae and skeletal hyphae. It is frequent on the roots and lower parts of stems of many trees and shrubs causing a rapid heart-rot of conifers and extensive damage to young trees.
Illustrations: LH 67; NB 1111; WD 981.
The spores of all the perennial polypores described above do not blue when placed in solutions containing iodine.
[151]
[152]
Cap: 10-25 mm. Stem: width 2-4 mm; length 2-4 mm.
Description:
Cap: greyish fawn becoming whitish when dry, fan or kidney-shaped, often lobed and covered in close-set hairs and with incurved margin.
Stem: absent or the cap simply narrows into a stem-like bump.
Gills: replaced by a series of grey-brown plates which when dry appear as if to split longitudinally and their edges roll back.
Flesh: brownish but drying whitish.
Spore-print: white.
Spores: medium sized, oblong, hyaline under the microscope, not blueing in solutions containing iodine and 6-7 × 2-5 µm in size.
Facial and marginal cystidia: absent.
Habitat & Distribution: Grows on fallen branches, trunks, dead wood, etc.
General Information: Easily recognised by the ‘gills’ radiating from a point and becoming ‘split’ when dry. Specimens of Schizophyllum sealed by A. H. R. Buller in a tube in 1911 have been shown on remoistening to unroll their gills and shed variable spores, after 521⁄2 years—probably a world record! The split-gill is a rather unique British fungus which appears to be much more closely related to the polypores than to the agarics—although it has for a long time been associated with the Oyster mushroom (p. 74). In fact, the splitting gills are two adjacent shallow dishes with spores produced on their inner surfaces. The cups separate on drying and therefore only superficially resemble gills splitting down the centre.
Another fungus which can also be associated with the idea of cups is Fistulina hepatica Fries ‘the Beef-steak fungus’. This fungus is a polypore in the widest sense. It may grow up to 250 mm wide and is reddish-brown or liver-coloured with reddish tubes and pale flesh-coloured pores; the tubes although free are aggregated together and can be easily separated individually with the fingers. This fungus is edible although very strong in taste, it produces a serious decay of oaks.
Illustrations: S. commune—LH 105; NB 1256; WD 693. F. hepatica—F 43a (lower figure); Hvass 278; LH 75; NB 1292; WD 1014.
[153]
[154]
Description:
Fruit-body: usually widely spreading, but sometimes forming a distinct bracket with the upper surface silvery or smokey grey, flushed with lilac or rose or yellowish.
Stem: absent and replaced by a series of dirty white or greyish mycelial threads or strands which can be traced up to 100 mm over the substrate.
Flesh: thin, dirty yellowish and composed of only one type of hypha.
Spores: borne in shallow pores which are part of a complicated network of rust-brown folds and ridges.
Spore-print: rust-brown.
Spores: medium sized, golden yellow, thick-walled and broadly ellipsoid, and 8-10 × 5-6 µm in size.
Cystidia: absent.
Habitat & Distribution: On worked wood in buildings and less commonly in timber-yards. It can be found throughout the year.
General Information: This fungus forms fan-like structures and strands of mycelium which pass along beams and joists and through plaster. Where there is a bad case of dry-rot, the room or building will have an unpleasant musty smell and when actually growing the fungus exudes droplets of water on the mycelium and fruit-body, i.e. weeping, hence the name ‘lacrymans’—weepy. It is a very important and destructive agent causing damage to floors and skirting boards, to joists and beams. It is a frequent pest of old houses and therefore of many of our cities. This fungus does not appear to have been found in the wild in Europe, but there is a record from the Himalayas. There are, however, very closely related species found on soil or wood-detritus. The Dry-rot fungus darkens the wood and produces a rot which makes the wood crack into small cubes or rectangular blocks.
This fungus was formerly placed in Merulius, but this genus should be retained for hyaline-spored fungi, e.g. M. tremellosus Fries, a species which grows even in winter on stumps of various trees in our woods.
Illustrations: LH 53; WD 1033.
[155]
Plate 50. Dry-rot fungi—leathery and tough spores borne in shallow irregular pores
[156]
Description:
Fruit-body: variable in size, resupinate, composed of one type of hypha only and with a sterile whitish cream or yellow margin.
Spore-bearing tissue: an irregularly wrinkled or humpy, yellowish surface which then becomes olive-green or bronze-colour.
Spore-print: olivaceous brown.
Spores: olive-brown under the microscope, smooth, ellipsoid, thick-walled and 12-14 × 8-9 µm in size.
Cystidia: absent.
Habitat & Distribution: This fungus causes wet-rot in houses, but may also be found on stumps and fallen trunks in woodland.
General Information: The fungus causes a discolouration of worked timber and induces longitudinal cracking with only a few lateral hair-like cracks unlike timber attacked by the dry-rot fungus (see p. 154).
Illustrations: WD 1035.
Description:
Fruit-body: a resupinate layer of pores with cream-coloured or white sterile radiating margin.
Spore-bearing tissue: distributed within a series of small often shallow, white or ivory tubes.
Spore-print: white.
Spores: smooth, hyaline under the microscope, oblong 5-7 × 3-4 µm.
Cystidia: absent.
Habitat & Distribution: The dry-rot of houses, particularly in roof-systems.
General Information: Fibuloporia vaillantii is recognised by the white, resupinate pore-surface and fairly tough nature due to the presence of strengthening hyphae. Just as the genus Polyporus was found to be composed of several quite different elements (see pp. 140-44) and has since been split up into a number of different genera, the genus Poria has also been fragmented; one of the constituent genera is Fibuloporia. Amyloporia xantha (Fries) Bondarsev & Singer differs in having amyloid tissue and cystidia encrusted with crystals. The flesh contains both simple hyphae and thickened structural hyphae. It is yet another member of the large old unwieldy genus Poria and causes decay of worked wood, particularly the timbers of benching and staging in greenhouses. A. xantha has a sulphur-yellow pore-surface and is rather cheesy when handled.
[157]
Plate 51. Wet and Dry-rot fungi—leathery and tough and spores borne within shallow pores or on an uneven surface
[158]
Cap: 8-12 mm. Stem: width 4-6 mm; length 40-75 mm.
Description:
Cap: kidney-shaped or semicircular, thin, date- or umber-brown, hairy, but paler towards the margin.
Stem: erect, slender, hairy, particularly at the base, and attached at the side of the cap (excentric).
Gills: replaced by flesh-coloured, then greyish brown spines.
Flesh: brown.
Spore-print: white.
Spores: small, hyaline, minutely spiny, spherical, 4-5 µm in diameter, and becoming blue-grey in solutions containing iodine.
Cystidia: flask-shaped with oily contents.
Habitat & Distribution: This fungus is always found on fallen pine-cones and occurs from early summer to autumn.
General Information: The ear-pick fungus is easily recognised by the slender, elegant habit, excentrically placed cap, substrate preference and dark colours. It cannot be confused with any other fungus. Recently it has been shown that the ‘agaric’ Lentinellus cochleatus (Fries) Karsten (p. 76) is more closely related to Auriscalpium than this fungus is to other spine-bearing forms and Lentinellus is to the other agarics. Both fungi possess thick-walled cells in the flesh and oil-containing hyphae; they are placed in the family Auriscalpiaceae.
Another laterally stemmed Hedgehog fungus differs in possessing distinctly gelatinised teeth and preference for conifer wood and not cones. Examination of the basidia of this fungus shows that it is more closely related to the jelly-fungi, Exidia and Tremella (p. 184) than to Hedgehog fungi such as Auriscalpium or Hyndum repandum Fries (p. 160). This false nature is reflected in the name of the genus to which it belongs, Pseudohydnum, and the very gelatinous texture in the specific name ‘gelatinosum’: the fungus is Pseudohydnum gelatinosum, or as it used to be called Tremellodon gelatinosum.
Illustrations: Auriscalpium vulgare—WD 1036. Pseudohydnum gelatinosum—WD 1059.
[159]
Plate 52. Tough or leathery fungi: Spores white and borne on spines—Ear pick fungus
[160]
Cap: 50-75 mm width. Stem: width 10-17 mm; length 45-65 mm.
Description:
Cap: rather thick, fleshy, pinkish buff or tan, paler at its incurved and often lobed margin.
Stem: short, stout and powdered with white roughenings and often attached to the cap to one side of the centre.
Gills: replaced by awl-shaped, pinkish buff spines which are unequal in length and run down the top of the stem.
Flesh: white, firm and with a pleasant smell.
Spore-print: whitish.
Spores: medium sized, hyaline under the microscope, smooth, broadly ellipsoid, 7 × 6-7 µm, and not becoming bluish grey in solutions containing iodine.
Cystidia: absent.
Habitat & Distribution: The ‘wood-hedgehog’ grows on the ground in mixed woods and is easily recognised by its colour and fleshy texture.
General Information: The closely related, smaller, red-brown species H. rufescens Persoon grows with conifers. Hydnum was formerly a genus which contained several entities, now not considered closely related. Thus the following genera have been delimited in addition to those related to Hydnum repandum and H. rufescens, and Auriscalpium described on p. 158.
Sarcodon: Fruit-body fleshy: spores brown and ornamented with irregular bumps, e.g. S. imbricatum (Fries) Karsten.
Phellodon: Fruit-body tough and fibrous: spores white and ornamented with small spines, e.g. P. niger (Fries) Karsten.
Hydnellum: Fruit-body tough and fibrous: spores brown and ornamented with irregular bumps and bosses, e.g. H. scrobiculatum (Secretan) Karsten.
Bankera: Fruit-body fleshy: spores white and ornamented with small spines, e.g. B. fuliginoalbum (Fries) Pouzar.
Illustrations: Hvass 280; LH 61; NB 1533; WD 534; Z 61.
[161]
[162]
Cap: 30-100 mm. Stem: width 15-25 mm; length 30-70 mm.
Description:
Cap: convex then flattened, irregularly wavy, more or less top-shaped, depressed and smooth or slightly roughened at centre, egg-yellow or lemon-chrome with flush of orange and with the margin incurved at first.
Stem: short, stout, tapered downwards, fleshy and similarly coloured to the cap.
Gills: replaced by irregularly branched yellow folds which may form a network near the margin and at the apex of the stem over which the folds run down irregularly (decurrent).
Flesh: with pleasant, fruity smell, yellow but paler on drying.
Spore-print: pale cream-colour.
Spores: medium sized, ellipsoid, thin-walled, smooth, 8-10 × 5-6 µm in size and not becoming bluish grey in solutions containing iodine.
Marginal and facial cystidia: absent.
Basidia: 2-8 spored.
Habitat & Distribution: Very common in troops in deciduous woods especially those with beech and oak.
General Information: Easily recognised by its folds and absence of true gills beneath the cap and the overall yellow colour. This fungus is the edible chanterelle of the continental market, where it is considered of very high quality; it can be purchased in this country in tins. C. friesii Quélet is of a bright apricot colour with lilaceous or rose-coloured flesh. The ‘false chanterelle’ Hygrophoropsis aurantiaca (Fries) Maire already discussed (see p. 106) has true gills and is reddish orange in colour.
Illustrations: Hvass 182; LH 59; NB 1232; WD 831.
[163]
Plate 54. Fleshy but firm fungi: Spores pale-coloured and borne on irregular folds (False gills)
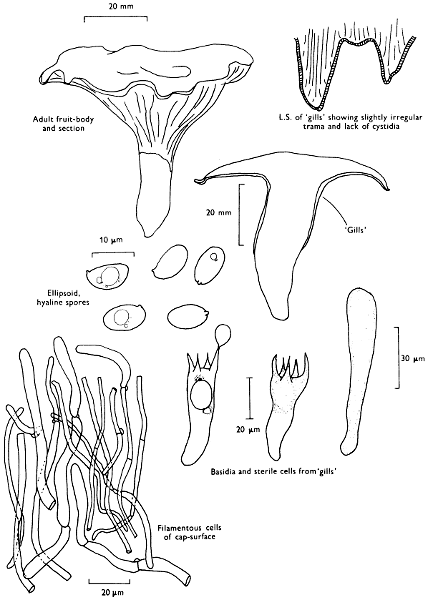
[164]
Cap: 22-80 mm. Stem: width 15-25 mm; length 25-80 mm.
Description:
Cap: funnel-shaped, membranous to leathery, but limp, dark brown or almost black in wet weather, but on drying becoming dull brown or sepia, slightly scaly and with irregularly wavy margin.
Stem: short, blackish and hollow.
Gills: absent, replaced by a smooth to irregularly wrinkled, ash-grey surface.
Flesh: sepia but drying out greyish ochre.
Spore-print: cream-colour.
Spores: medium sized, hyaline under the microscope, ellipsoid, smooth, 10-11 × 6-7 µm in size and not blueing in solutions containing iodine.
Marginal and facial cystidia: absent.
Basidia: usually 2-spored.
Habitat & Distribution: Often in very large troops in woods, especially under beech.
General Information: This fungus is recognised by the peculiar shape and dull colours which conceal it so well amongst the dead leaves and woodland debris; in the shade of the tree-canopy it is easily overlooked. Craterellus sinuosus (Fries) Fries is a much smaller species with dirty ochraceous fertile surface and brownish grey cap and stem.
‘Cornucopioides’ means like (oides) a horn of plenty, a familiar object in mediaeval paintings as part of heathen festivities full and overflowing either with fruit or wine, or both!
Illustrations: Hvass 186; LH 59; NB 1231; WD 834.
[165]
Plate 55. Fleshy but leathery fungi: Spores pale-coloured and borne on irregular wrinkles
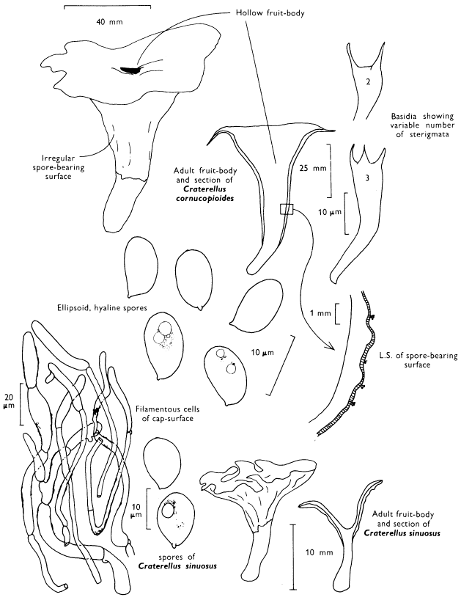
[166]
Cap: absent. Fruit-body: length 50-100 mm; width 7-13 mm.
Description:
Fruit-body: club-shaped, simple with blunt apex or irregular blunt branches, white or dirty cream colour, often thickened upwards and marked with longitudinal wrinkles or grooves and the whole surface of the club bearing spores.
Stem: absent or extremely short.
Flesh: white.
Spore-print: white.
Spore: medium sized, broadly ellipsoid to subglobose, hyaline under the microscope and not turning bluish grey in iodine solutions, 9-10 × 7-8 µm in size.
Cystidia: absent.
Basidia: 2-spored.
Habitat & Distribution: Frequent on the ground in woods, especially in the shade of beech trees or in conifer plantations.
General Information: Two very closely related species are to be found in similar localities and are equally as common; they are C. cristata (Fries) Schroeter with strongly branched white fruit-body, each branch ending in pinkish or lavender-white, divided, sharply pointed branchlets and C. cinerea (Fries) Schroeter with irregular greyish or dark grey branches with a flush of violaceous.
These three species are very closely related; in fact so many intermediates between the extreme morphological forms are known that some authorities have considered them simply forms of a single species. All these species lack cystidia.
rugosa—wrinkled, referring to the spore-bearing surface.
cristata—crested, referring to the branchlets.
cinerea—ash-grey, referring to the colour.
All these species are often found blackened by the growth of the microscopic fungus, Helminthosphaeria clavariae (Tulasne) Fuckel.
Illustrations: C. rugosa—LH 55; WD 1045. C. cristata—LH 55; NB 1535; WD 1042. C. cinerea—WD 1041.
[167]
Plate 56. Fleshy but firm fungi: Spores pale-coloured and borne on club-shaped fruit-bodies
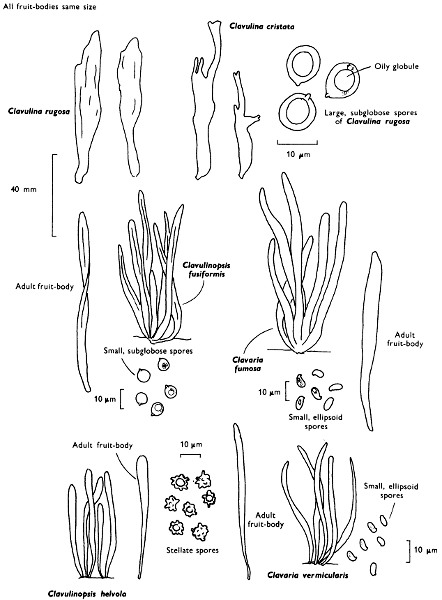
[168]
Cap: absent. Fruit-body: width 6-10 mm; length 50-85 mm.
Description: Plate 56.
Simple or very rarely branched, but not forked below the soil-level, densely tufted, spindle-shaped, pure white with sharp, often slightly brownish, tips, when old it is wavy, often twisted, compressed and fragile.
Stem: absent.
Flesh: whitish.
Spore-print: white.
Spores: small, pip-shaped, smooth, hyaline under the microscope, 4-5 × 3 µm in size, and not becoming bluish grey in iodine solutions.
Cystidia: absent.
Habitat & Distribution: Common in autumn amongst grass in fields, less frequent in woods.
General Information: Clavulinopsis fusiformis (Fries) Corner, ‘Golden spindles’ is similar to C. vermicularis, but forms dense tufts of canary-yellow, very fragile clubs joined in 2’s or 3’s below the soil level; the spores are also slightly different, being almost globose, hyaline under the microscope and 5-7 µm in diameter.
Clavaria fumosa Fries is similar to C. vermicularis and forms tufts of very fragile mouse-grey clubs with brownish tips; it produces elongate ellipsoid spores measuring 6-8 × 3-4 µm which are hyaline under the microscope. C. vermicularis and C. fumosa differ from Clavulinopsis in hyphal construction, but the differences are rather difficult to demonstrate to the beginner. Clavulinopsis helvola favours similar habits to C. fusiformis and although yellow in colour differs in the more orange-yellow colouration, but more particularly in the spores being rounded, 5-6 µm in diameter with large angular spines.
The earth-tongues, i.e. members of the family Geoglossaceae which are also found in pastures belong to an unrelated group of fungi, the Ascomycetes. If the clubs are crushed and examined under the microscope rows of sacs (asci) containing long thread-like ascospores are found—no basidia are to be seen.
Illustrations: Clav. fusiformis—WD 1049. C. vermicularis—WD 10410. C. fumosa—Hvass 303; WD 10411. Clav. helvola—Hvass 300; WD 1051.
[169]
[170]
Cap: absent. Fruit-body: complex; width 20-30 mm; length 20-40 mm.
Description: Plate 57.
Fruit-body: shape depending on the length of grass in which it grows but always branching strongly from its base, composed of a dense compact tuft of egg-yellow or orange-tawny branches which are either irregular or of equal length and so they form a flattened top to the fruit-body complex, the branchlets are slender, forked 2- or 3-times, with their apices narrowed or curved.
Stem: very downy at the base.
Flesh: pale yellow.
Spore-print: white.
Spores: medium sized, hyaline under the microscope, smooth, spherical and 5-7 µm in diameter, not becoming bluish grey in iodine solutions.
Cystidia: absent.
Habitat & Distribution: Common amongst grass in fields or on grassy path sides in woodland.
General Information: Clavulinopsis corniculata is recognised by the branched habit and the smooth spores; Ramaria ochraceo-virens is of similar form, but has an overall duller colour and turns green on bruising, grows in pinewoods and has finely roughened brownish spores. Calocera viscosa also has an erect, bright golden or orange-yellow fruit-body which becomes more orange on drying. It is repeatedly branched and usually has a long, tough-rooting base. However, the spore-print is dirty yellowish and the fruit-body, which grows on coniferous wood, is viscid and elastic, a character reflected in the name ‘viscosa’. Microscopically the basidium of Calocera is shaped like a tuning-fork and is not clavate as in Clavulinopsis corniculata. It appears to be more related to the jelly-fungi (see p. 180).
Illustrations: Clavulinopsis corniculata—LH 55; NB 6; WD 1043. Calocera viscosa—Hvass 304; LH 225; NB 1493; WD 1078.
[171]
Cap: absent. Fruit-body up to 20 mm high.
Description:
Fruit-body: upper fertile portion club-shaped and not more than half the length, white, surmounting a reddish brown, thread-like, often wavy or twisted stem which is attached at its base to an ellipsoid bead-like structure, called a sclerotium.
Spore-print: white.
Spores: oblong, smooth, hyaline under the microscope, 6-7 × 2 µm in size and not becoming bluish grey in iodine solutions.
Cystidia: absent.
Habitat & Distribution: Not uncommon on dead leaves and twigs or dead herbaceous stems.
Cap: absent. Fruit-body: up to 10 mm high.
Description:
Club-shaped or oblong, rose-pink hardly differentiated from the similarly coloured stem, and arising at most from a small pad of filaments.
Spore-print: white.
Spores: broadly ellipsoid to pip-shaped, smooth, hyaline under the microscope, about 10 × 6 µm (8-11 × 5-7 µm) in size and not becoming bluish grey in iodine solutions.
Cystidia: absent.
Habitat & Distribution: Not uncommon on dead herbaceous stems and leaves, especially those in damp places.
Illustrations: T. erythropus WD 10510. P. micans WD 1057.
[172]
Early mycologists believed that the club-shaped nature of the fruit-body was important in the classification of these fungi. Thus the Earth Tongues (Geoglossum, see Plate 57), the Stag’s horn fungi and relatives (Xylosphaera see p. 204), both Ascomycete groups, the Dacrymycetales (a group of jelly-fungi, see p. 180) and the true fairy-clubs were all classified together. It was the ‘Father of Mycology’, the Swede, Elias Fries, who in 1821, as in many other groups of fungi, made an attempt to make some sense of the chaos. By very careful observations, and what is so amazing without using a microscope, he was able to separate the tough stemmed and gelatinous stemmed groups from the more slender or coral-like ones. Fries was a very keen observer and noticed features which many modern authorities miss in the field because they rely too heavily on the microscope. Fries’ system was used almost unchanged until the second half of this century; its beauty was its simplicity in that it joined together in one group all those fungi with simple basidia and the spore-bearing tissue distributed all around a simple club or around the branches of a complex fruit-body resembling a coral. However, by a careful examination of the microscopic structures, such as the spores and hyphae and the development of the fruit-body, it has been found necessary to separate these fungi still further. The ecology of the club-fungi has assisted in an understanding of these proposed divisions.
The larger many branched clavarias, more correctly placed in the genus Ramaria, are to be found on bare soil in woodlands and plantations; R. ochraceo-virens is common in conifer plantations and can be recognised by the long ornamented spores, which characterise this group of fungi, and the fact that the sandy-coloured fruit-body becomes dark olive-green on bruising (see p. 170).
Clavariadelphus pistillaris is the largest of our simple club-fungi; it may grow up to 200 mm high and 50 mm wide. This fungus has a wrinkled outer surface and sometimes the apex of the club becomes flattened and lacks basidia; this suggests a possible relationship, perhaps evolutionary, to the primitive chanterelles (see p. 162)—also woodland fungi. Clavulina, a complex group of dull or whitish, branched fruit-bodies, has been described earlier and the genus is characterised by the large spores and 2-spored basidia; they are woodland fungi also.
The grassland species are often simple in structure belonging in the[173] main to the genus Clavulinopsis (see p. 170) and the now much reduced genus Clavaria (see p. 168). Although really complex, some of these species of Clavulinopsis are branched only below the soil level and thus appear as single clubs amongst the grass. Perhaps the single club has evolved especially to grow amongst blades of grass. C. corniculata, however, is well branched and the head is tight and compact and often flattened close to the ground. The same fungus in woodland is more open and because of this it was thought to be a different species to the grassland form. It is the simple club which dominates the form of those species which grow on herbaceous debris and grass-stems; indeed several species of Typhula cause diseases of grass particularly those of lawns where they have suffered damage because of cold or long periods under the snow. Some of these small fungi produce a small hard mass of fungal tissue about the size of a lupin seed (called a sclerotium). This is a resting body from which the club-shaped almost filament-like fruit-body later develops.
[174]
Cap: absent. Fruit-body: width 20-40 mm; height 30-50 mm.
Description:
Fruit-body: erect, fan-shaped or effused with upturned margin, tough but thin and fibrous, chocolate-brown or cocoa-coloured, scaly from radiating fibrils and with fringed, pale buff or wine-coloured margin.
Gills: absent and replaced by a wrinkled or irregularly granular, dark lilaceous grey or cocoa-coloured surface.
Flesh: brown and thin.
Spore-print: purplish brown.
Spores: medium sized, dark brown under the microscope, warted-angular and 8-9 × 6-7 µm in size.
Cystidia: absent but basidia often filled with brown contents.
Basidia: 2-4 spored.
Habitat & Distribution: Found on the ground in woods, especially pine woods; also on heathland growing up vegetation and incorporating it into the fruit-body’s shape.
General Information: There is some evidence to suggest that this fungus can form mycorrhiza with pine trees under certain conditions.
Although it may be easily passed over because it is perfectly camouflaged it is quite easy to recognise when collected. T. palmata (Bulliard) Patouillard, is a bigger, less frequently seen species more coral-like in shape; it also grows in pine woods. When the fruit-body of T. terrestris spreads over the soil or plant debris it resembles other members of the family to which it belongs, i.e. Thelephoraceae; species of Tomentella. They also have warty angular spores, purplish brown colours, and wrinkled or puckered spore-bearing surfaces. Tomentella spp., however, are resupinate or encrusting and so do not form caps, even at the margin of the fruit-body. Tomentella is one of the many genera which were classed collectively as resupinate fungi because they lack a cap and form crusts. This group ‘the resupinates’ consists of a whole series of quite unrelated fungi.
Illustrations: LH 53; NB 478.
[175]
[176]
When mycologists talk generally about ‘resupinates’ they are referring to a whole group of Basidiomycetes whose spore-bearing layer is exposed, the cap highly reduced or completely lacking, and the fungus adhering to the surface of the substrate which may be soil, wood, grasses, etc., at the point which would have been the cap of an agaric. Probably members of the group are the most commonly seen yet it is one of the most commonly ignored groups of fungi—by naturalists and mycologists alike; they form ‘white wash’ on old sticks, dark coloured discolourations on trunks, etc. It is an entirely artificial group of many quite unrelated elements united on the common factor of having either a reduced or primitive fruit-body consisting only of a sheet of tissue. However, these same fungi have a uniting factor in that they frequent the same ecological sites, e.g. on muddy soil in bogs, under overhangs of banks and stream sides, undersides of logs, trunks, branches and twigs, hidden in cracks of old stumps or spreading over carpets of conifer needles or dead leaves and sedges.
By studying the anatomy of the fruit-body and the characters of the spores certain relationships can be found which relate many of these fungi to several other groups of fungi we have dealt with in earlier chapters.
It is only possible to mention here the group as a whole for all the species really require very careful examination, often necessitating several hours of microscope work. They should be left by the beginner until more experience is obtained and advice of an expert easily available.
Although the group mainly contains saprophytes, a few are parasitic. ‘Silver-leaf disease’ of almonds and fruit trees is caused by Stereum (chondrostereum) purpureum (Persoon) Fries; it has a purple fruiting surface, and greyish upper surface when ever this is formed at the margin.
There are several species of Stereum in Britain, three species of which when handled in the fresh state stain red: S. sanguinolentum (A. & S.) Fries, a pale coloured species on conifer wood, S. rugosum (Pers.) Fries a similar coloured species on beech, birch and especially hazel, and S. gausapatum Fries an ochraceous yellow species on oak, often forming a pocket rot of the timber. However, the commonest member of the genus is an orange-tawny coloured species with a greyish buff, hairy cap, S. hirsutum (Willd) Fries. It grows on many trees of broad-leaved wood and can be found wherever twigs, branches, trunks or stumps have been lying out in the rain; it does not bleed.
[177]
[178]
Those species of resupinate fungi which resemble members of this genus, i.e. those with a distinct tough, although poorly developed, cap, are called stereoid.
‘Red thread disease’ of grass which often causes unsightly red patches on lawns and school and corporation playing-fields is caused by Corticium fuciforme (Berkeley) Wakefield. Fungi belonging to this genus produce fruit-bodies which ‘scramble’ over the substrate; for example, if one searches old elder trees throughout the year one will certainly find a ‘white wash’ fungus of this type, Hyphodontia sambuci (Pers.) J. Eriksson. Fungi with this type of fruit-body are called corticoid.
The two major types are illustrated along with some of the bizarre microscopic structures one finds in the resupinates; such structures are useful in classification and identification, and their beauty and intricacy make up for the surprisingly simple fruit-body shape and texture.
[179]
The jelly fungi or Hymenomycetous heterobasidiae is a complex group of fungi and not only includes our common jelly fungi but many microscopic forms some of which are parasitic. The group is divided into three main divisions depending on the position of the cross-walls within the basidium, or whether the basidium is in the shape of a tuning-fork. They are probably not closely related one to another.
Fruit-body: 3-6 mm.
Description:
Fruit-body: cushion or brain-like, often irregular, lacking any evidence of stem, yellow or orange, gelatinous, covered entirely by spore-bearing tissue.
Spore-print: yellowish.
Spores: long, cylindrical or oblong, and slightly curved and 12-15 × 5-6 µm in size; they characteristically have 2 to 4 cross-walls dividing the interior of the spore (see below).
Cystidia: absent.
Habitat & Distribution: Common on all sorts of old wood, particularly on fence-posts, wooden railway-sleepers and other worked timber outside, e.g. sides of summer-houses and garden sheds. It is also found on twigs and branches in woods and copses.
General Information: This fungus is found throughout the year, but it is much more obvious under damp conditions when it is strongly gelatinised and very soft; when dry it almost disappears. The tissue bearing the basidia (perfect state) is yellow, when orange there is a predominance of asexually produced spores called arthrospores (conidia).
D. deliquescens is only another name for the same fungus. There are several species of Dacrymyces with which D. stillatus can be confused, but can only be separated with certainty by using a microscope. The Coral-spot fungus, frequently found in gardens, produces gelatinous, pink protuberances on wood especially that of sycamore, and may easily be mistaken for species of Dacrymyces. It consists entirely of asexually produced spores (conidia) of the Ascomycete Nectria cinnabarina. The perfect state appears late in the year as grouped, small, blood-red flask-shaped fruit-bodies containing envelopes of spores. It is quite unrelated to Dacrymyces.
[181]
Calocera viscosa (Fries) Fries described earlier (p. 170) is closely related to Dacrymyces. The much smaller, and probably equally as common, Calocera cornea (Fries) Fries is simple, club-shaped and yellow, but darkens to become orange on drying. It grows up to 10 mm high and occurs on all sorts of wood; it is especially common on wet beech trunks. It approaches Dacrymyces more than the much larger C. viscosa.
Illustrations: D. deliquescens—LH 225; NB 1497; WD 10710. C. cornea—WD 1079.
[182]
Fruit-body: width 20-75 mm.
Description:
Fruit-body: cup or ear-shaped, red-brown or deep wine-colour, gelatinous with its upper surface, velvety and clothed in greyish or olivaceous hairs.
Spore-bearing layer: reddish or purplish brown, smooth or veined and translucent.
Spore-print: white.
Spores: very long, hyaline under the microscope, oblong, curved and narrowed towards their base, 16-18 × 6-8 µm in size.
Cystidia: absent.
Habitat & Distribution: On dead branches of all kinds and particularly common throughout the year on elder.
General Information: Easily recognised by the wine-coloured, cup-shaped or ear-shaped fruit-body; it is often called Auricularia judae in many books. Its Latin name is reflected in the common name:—auricula ear and judae, of a jew. This fungus is supposed to be a reappearance, as a warning to us all, of Judas, who on betrayal of Christ hung himself from an elder tree.
, ‘Tripe-fungus’, is bracket-shaped with a hairy upper surface and reddish purple or deep purple lower surface which when fresh has a greyish bloom due to the formation of the spores.
There are several fungi in the group Auriculariales in Britain, but many of them are inconspicuous and are identified with difficulty except by the expert. Sebacina incrustans (Fries) Tulasne is a common more obvious example of the resupinate forms. It grows as a cream or ivory-coloured, soft fruit-body encrusting twigs, leaves, grass and soil.
Illustrations: LH 225; NB 1491; WD 1071.
[183]
[184]
Fruit-body: width 15-50 mm.
Description:
Fruit-body: sessile or shortly stalked, blackish, variable in shape, rounded, flattened, disc-shaped or convolute, gelatinous with its under surface tomentose and free from the substrate.
Fruiting surface: uppermost, wavy and folded, and with numerous wart-like projections.
Spore-print: white.
Spores: long, hyaline, cylindrical, sausage-shaped and 12-15 × 5 µm in size.
Cystidia: absent.
Habitat & Distribution: Frequent in crowded groups on stumps, logs and fallen branches of broad-leaved trees, especially those of ash; common throughout the year.
General Information: Tremella foliacea (S. F. Gray) Persoon and Tremella mesenterica Hooker are similar but more convoluted with leaf-like lobes. The former is cinnamon brown and occurs on conifer wood and its spores are 7-9 × 5-7 µm, whilst the latter is bright golden yellow or orange-yellow and occurs on broad-leaved trees. T. mesenterica has spores 7-8 × 5-6 µm, often accompanied or replaced by small, asexually produced spores.
Glandulosa—means full of glands and refers to the glands of the upper surface of the Witch’s butter.
The convoluted fruit-body of the Tremella spp. is reflected in the word foliacea—leafy, and mesenterica—middle intestine. The last species is also often called the ‘Yellow brain-fungus’.
Illustrations: Exidia glandulosa—LH 225; NB 1494; WD 1073. Tremella mesenterica—LH 225; NB 1495; WD 1076.
[185]
[186]
The Gasteromycetes are a complex mixture of higher fungi united in virtue of their spores being enclosed in a fruit-body and not forcibly ejected from the basidium; the group includes the puff-balls and their relatives.
The Gasteromycetes is an unnatural group of predominantly saprophytic higher fungi many of which are extremely grotesque and strange in their morphology. Instead of the spores being formed asymmetrically on the basidium as is found in the agarics, the spores of members of this group are usually more or less symmetrically attached to their sterigmata or may even be seated directly (sessile) on the basidium. The whole group, even if unnatural, can, however, be regarded under one heading as a biological unit. Until something better is suggested and[187] supported by evidence the existence of this group is very convenient.
Usually the basidia project into cavities within the fruit-body in which the spores themselves are released as the fruit-body gradually matures—hence the name Gastero-mycetes: ‘stomach-fungi’. In a few more advanced forms, the puff-balls of temperate countries, for instance, the spores escape from these cavities through a pore or pores in the outer wall of the fruit-body, and in the stinkhorns the spores are exposed as a sticky mass because the smell of the material in which they are held is attractive to flies. In forms which have subterranean (or hypogeous p. 243) fruit-bodies there is no special opening and here the spores are dispersed by insects and small mammals. In the bird’s nest fungi the spores are enclosed in separate packets within a saucer or cup-like open structure.
Recently it has been shown by examination of the microscopic structure of the fruit-bodies and spores that certain genera of the Gasteromycetes are more closely related to the agarics than many of them are between themselves.
It is believed that some of the Gasteromycetes may have evolved from more familiar fungi by adaptation to arid or semi-arid conditions. Although this is not true for all the Gasteromycetes within this one group of fungi, a whole series of methods of overcoming the disadvantages connected with non-violent disposal of spores has evolved. These methods include both changes in structure and ecology; only a few have evolved a mycorrhizal relationship with higher plants.
[188]
Fruit-body: width 20-50 mm; height 40-75 mm.
Description:
Fruit-body: more or less pear-shaped, pale brownish often with a slight hump on the top, scurfy on the outside with tiny pointed granules which soon fall off or become rubbed off by abrasion, particularly after careless handling.
Stem: consisting of rather small cells and connected at the base by long, white, branched cords of mycelium which permeate the substrate.
Spore-mass: white at first then greenish yellow and finally olive-brown and formed around a sterile column.
Spores: small, olive, minutely warted but appearing smooth under the student microscope; 4 µm in diameter and intermixed with long, olive coloured, branched hyphal threads 4-5 µm broad and of irregular wall thickness.
Habitat & Distribution: This puff-ball grows in huge clusters on stumps and logs, or can be traced to buried pieces of wood; it occurs from summer to late autumn.
General Information: There are several species of Lycoperdon in this country, some quite small and several rather infrequent. L. pyriforme is the only one which grows on wood; ‘pyriforme’ means pear-shaped and is derived from the shape of the fruit-body.
is also a common puff-ball; it is pestle-shaped or top-shaped, whitish or tan with minutely roughened, globose spores measuring 4 µm in diameter. The fruit-body is covered in a mixture of large and small, fragile spines which leave a network when rubbed off. It grows in woods and on heaths.
is similar to L. perlatum, but the spines are umber or vandyke-brown; it also grows both in woods and upland pastures, particularly the latter.
Illustrations: L. pyriforme—Hvass 316; LH 219; NB 1553; WD 1093. L. perlatum—Hvass 315; LH 217; NB 1552; WD 1102.
[189]
[190]
Fruit-body: diameter 300-450 mm (-1,050 mm).
Description:
Fruit-body: round or slightly flattened on the top, smooth or cracked into small scales, white but becoming flushed yellowish with age and finally olive-brown when old, frequently the outer layer dries and breaks away to expose the powdery spore-mass within.
Stem: absent or only present as a small cone of tissue.
Spore-mass: whitish, cream-coloured and finally olive-brown.
Spores: small, brownish, minutely warted and spherical, 4-5 µm in diameter and intermixed with thick-walled, branched, brown hyphae, 3-5 µm broad.
Habitat & Distribution: On the ground in copses, at the edges of woods, under hedges or on refuse tips, and sometimes in gardens. It may appear in the same place year after year, and has been recorded growing beneath the rafters in houses.
General Information: When young it is white inside or cream-coloured before the spores have developed and can then be cut into slices and cooked. I have seen it on sale in markets in N. America and it is collected for food by many in Europe. Its pumpkin-shape with a circumference of anything up to 1,050 mm makes this fungus easily recognisable. The number of spores produced by a fruit-body measuring 400 × 280 mm has been calculated by A. H. R. Buller as 7,000,000,000,000 spores!
has a goblet-like shape and a distinct, sterile base composed of large cells with a prominent membrane separating them from the spore-mass; the spores are 4-5 µm diameter, smooth and spherical.
is pestle-shaped with a well developed stem. The spore-mass is composed of warted, globose spores, 4-5 µm in diameter.
is very similar in shape to the Giant puff-ball, but is very much smaller; it lacks a stalk, being attached to the substrate only by mycelial cords. It commences white, but then darkens to become purplish brown at maturity when it also breaks from its moorings and rolls about in the wind.
The last three species are found on heaths, in pastures or on the ground in woods.
Illustrations: C. gigantea—Hvass 312; LH 217; NB 371; WD 1097. B. nigrescens—Hvass 311; LH 219; NB 373.
[191]
[192]
The earth-stars, i.e. species of Geastrum, are closely related to the puff-balls, but differ in having two very distinct and separate enclosing walls, the outer one splitting at maturity to expose a ‘puff-ball’ within; an example of the genus is G. triplex Jungh, found in parks or under beech trees or G. rufescens Pers. (illustrated) in mixed woodland. The outer skin splits in different ways in different species: in some it splits like a star—hence the common name of Earth-star, in some the spore-mass is raised as if on stilts. There are several species of Geastrum recorded for Britain, but they are decidedly uncommon.
The Earth-balls are, however, far from uncommon and may be met with from early summer until late autumn in any wood particularly those on sandy soils. They are unrelated to the earth-stars.
Fruit-body: diameter 25-75 mm.
Description:
Fruit-body: rounded or flattened on top, sometimes lobed, very firm, yellow or clay colour, scaly, thick, white within or pinkish, if cut when immature, and then purplish black as the spores mature.
Stem: absent or reduced to a small group of mycelial cords.
Spore-mass: purplish black.
Spores: medium to large, dark brown, 8-13 µm in diameter and covered with a delicate network.
Habitat & Distribution: On the ground in woods or on heaths.
General Information: This fungus is found in many books under the name of S. aurantium. S. verrucosum Persoon is closely related, but has a stem-like rooting base and an umber brown spore-mass. The spores are also slightly different; they are 10-14 µm in diameter and ornamented with spines and ridges.
The earth-balls appear to have characters in common with the false truffles, indeed sometimes they grow partially buried in the sandy soil of woods. Like the false truffles they have been used to adulterate pâté as a cheap substitute for true truffles (see p. 244). It is not wise, however, to eat earth-balls as there are cases of poisoning known. Although truffle-like, they should be avoided except under the guidance of an expert, as with agarics.
Illustrations: Geastrum triplex—Hvass 307; LH 221; NB 1551. Scleroderma citrinum—Hvass 320; LH 223; NB 1555; WD 1113.
[193]
[194]
Fruit-body: Egg: 30-60 mm in diameter—then Cap: 25-40 mm and Stem: width 18-25 mm; length 100-150 mm.
Description:
Fruit-body: commencing as a white, silky egg-like structure full of jelly in which is embedded a conical cap attached only at its apex to a cylindrical white, spongy, hollow stem.
Cap: covered in a slimy mass of dark olive-coloured spores at maturity.
Stem: cylindrical, rapidly elongating, white, spongy and hollow.
Spore-mass: dark olive-green, smelling strongly, foetid.
Spores: small, pale olive, oblong and 3-5 × 2 µm in size.
Habitat & Distribution: Common from summer to autumn on the ground in woods and in gardens.
General Information: Easily recognised by its shape and evil smell which can be detected at some distance. The unburst eggs are called ‘witches eggs’. Under favourable conditions the egg bursts and the stem elongates carrying the cap and spore-mass with it. The spore-mass is attractive to flies and they feed upon it; spores stick to their feet and so are transported from one place to another.
The very similar P. hadriani Persoon is frequently found in sand-dunes; it differs in having a lilaceous colour to the egg. An interesting variety of the common stinkhorn is uncommonly found and differs in having a skirt-like frill beneath the cap. The jelly in the egg is a water-store and is used by the fungus to expand rapidly.
, the ‘Dogs stinkhorn’, is found around old stumps or on piles of leaves. It has the spore-mass covering an orange-red pear-shaped cap which is itself fused to the stem.
The stinkhorns and their allies appear to be commoner in warmer countries where they take on many bizarre shapes. Other than the three species noted above stinkhorns are rarely found in this country, but when they are it would appear they have been introduced with foreign imports such as timber, ornamental plants, vegetables etc.
Eggs of phalloids brought into the laboratory can be surrounded by wet tissues or blotting paper and then allowed to develop further in a dish or box. Provided the skin covering the spores is not broken or injured the fungus will not smell and therefore before it becomes unpleasant, the whole mechanism of expansion can be studied.
Illustrations: Hvass 323; LH 215; NB 1531; WD 1081.
[195]
[196]
Fruit-body: diameter 8-12 mm.
Description:
Fruit-body: ochraceous brown or sand-colour, downy and then smooth, truncate, cup-shaped with the cup at first closed by a yellowish membrane which finally splits to expose a group of pale brown or dingy whitish, circular, lens-shaped ‘eggs’ (peridioles), scattered on a shiny pale ochraceous interior.
Spores: medium-sized, in packets within ‘eggs’, ellipsoid, hyaline, smooth and 8-10 × 4-6 µm in size.
Habitat & Distribution: Common in crowded groups on dead twigs, fern stems, straw and wheat stubble.
General Information: Cyathus differs from Crucibulum in the more complex fruit-body which consists of three layers, and the peridioles forming on distinct stalks. Two species are frequently seen: Cyathus striatus Persoon has a grey, fluted inner surface to the cup and strongly hairy red-brown outer surface; the spores measure 16-22 × 9-10 µm. Cyathus olla Persoon has a smooth, shiny, grey surface and minutely silky, yellowish grey outer surface. C. striatus is found on twigs, and about dead stumps; C. olla is more frequent in gardens on herbaceous debris and dead pieces of perennial flowers—or even in plant pots.
is more distantly related and grows on decaying leaves, bracken fronds, partially buried twigs and dung. It is an intriguing fungus because it possesses a remarkable spore-dispersal mechanism. The inner layer of the fruit-body when ripe suddenly turns inside out catapulting the inner spore-mass to distances of anything up to 4,200 mm, that is a distance of 1,000 times the size of the fruit-body. The fruit-body is externally whitish or pale yellow, but this layer splits into lobes like a star exposing the bright orange inner surface and pale spore-mass.
Illustrations: Crucibulum laeve—LH 223; WD 1117. Cyathus striatus—LH 223; WD 1119. Sphaerobolus stellatus—LH 223; WD 1115.
[197]
[198]
The Ascomycetes differ from all the other fungi so far dealt with in that the spores develop enclosed in a microscopic envelope or sac—called the ascus. Usually eight spores are produced in each ascus and they are often dispersed violently into the air. Elf-cups and morels are typical Ascomycetes, but the group also includes innumerable minute forms of the microscopic fungi, small discs, minute flask-like structures, some of which are parasitic on leaves and stems of higher plants. In number the large species of Ascomycetes are few when compared with the others and therefore can only be given but a mention in the present account. When collected the Ascomycetes can be distinguished from the Basidiomycetes by simply examining a slice of the spore-producing tissue where the tell-tale asci will be seen (see p. 21). If the fruit-body is placed in a tin when collected and opened in a warm room all the mature asci explode at once producing a cloud of spores visible in the air immediately over the fruit-body.
Fruit-body: diameter 25-50 mm.
Description:
Fruit-body: cup-shaped then undulating and becoming flattened, irregular, sometimes split and lacking a stem.
Inner surface: bright orange.
Outer surface: whitish and minutely downy.
Flesh: thin and white.
Spores: very long, ellipsoid, ornamented with a coarse network which projects at each end, and 17-24 × 9-11 µm in size; eight contained in an elongate, cylindric ascus.
Habitat & Distribution: Common on bare soil in woods and open spaces, on road verges, between stone sets and on lawns.
[199]
[200]
Fruit-body: diameter 20-50 mm.
Description: Plate 67.
Fruit-body: cup-shaped with white, crenulate margin, becoming expanded and undulating, and lacking a stem.
Inner surface: light chestnut brown.
Outer surface: whitish or pale fawn and finely scurfy.
Flesh: whitish or fawn, and appearing as if layered.
Spores: very long, ellipsoid, smooth and 15-16 × 9-10 µm in size; eight contained in an elongate, cylindrical ascus.
Habitat & Distribution: On bare soil in woods, farm-yards, hedgerows, etc.
General Information: There are many different species of Peziza classified on the shape and ornamentation of the spores and colour of the fruit-body—see pp. 216 and 220. P. badia is darker, although similar in other ways; it is found on pathsides in woods and has roughened spores.
Cap: width 30-40 mm; length 35-60 mm. Stem: width 15-25 mm; length 50-80 mm.
Description:
Fruit-body: consisting of a head with a honeycomb-like arrangement of narrow ridges surrounding angular and often slightly elongated, shallow pits, on a cylindric or swollen stem.
Cap: brownish grey then reddish brown or ochraceous brown.
Stem: cylindrical or slightly enlarged at the base, brittle, hollow, minutely scurfy and/or furrowed.
Flesh: ochraceous.
Spore-print: cream.
Spores: very long, broadly ellipsoid, pale honey, smooth but for some small granules at each end, and 16-23 × 11-14 µm in size; eight contained in an elongate cylindrical ascus.
Habitat & Distribution: Infrequent in gardens, on river-banks, sites of bonfires, etc., in spring.
Illustrations: F7c; LH 41; NB 413.
[201]
[202]
Cap: width 30-40 mm; length 35-45 mm. Stem: width 15-25 mm; length 50-80 mm.
Description: Plate 68.
Fruit-body: consisting of a subglobose, more or less lobed, wrinkled and convoluted head on a short stem.
Cap: yellow-brown to reddish brown and becoming hollow or chambered.
Stem: flesh-coloured or creamy grey and powdery.
Flesh: yellow-buff, darker in the cap.
Spores: very long, ellipsoid, usually containing two or more yellowish oil drops and 18-22 × 9-12 µm in size; eight contained in an elongate cylindrical ascus.
Habitat & Distribution: This fungus is found in the spring, under conifers, but also on railway embankments, river banks, etc. This fungus is also known as the ‘Lorel’ or ‘Elephant’s ears’.
General Information: Mitromorpha semilibera (Fries) Léville differs from species of Morchella in that the head is for its greater length free from the stalk. It is frequently found in the spring in gardens, tennis courts, etc.
Illustrations: G. esculenta—F 6d; Hvass 327; LH 39.
Cap: width 18-28 mm. Stem: width 8-12 mm; length 40-65 mm.
Description:
Fruit-body: consisting of a saddle-shaped cap on a short stem.
Cap: convoluted towards the centre, two lobed, wavy at the margin, white or cream-coloured.
Stem: cylindric and hollow, white or cream-coloured and unevenly and deeply longitudinally furrowed.
Flesh: thin and pale.
Spores: very long, broadly ellipsoid, with a large central oil drop and 18-20 × 10-13 µm in size; eight contained in an elongate cylindrical ascus.
Habitat & Distribution: Frequent in damp woods with deciduous trees, from early summer until autumn.
[203]
General Information: Plate 68.
is similar in stature but differs in being ash-grey or dark grey.
is better placed in the genus Helvella. It differs in having a slender, smooth, cylindric stem and irregularly 2-3 lobed, yellow or tan-coloured cap.
is sometimes placed in Helvella. It differs in having a grey cup-shaped cap on a long, slender stem. The spore-bearing tissue in the last species is the inner surface of the cup whilst in Helvella and Leptopodia it is on the outer surface of the saddle-like cap.
, is a similar fungus growing in spring to early autumn on old leaves and detritus in swamps. It is widespread and has a bright orange head on a white stem—as the common name might suggest. It grows to a height of about 20 mm.
Illustrations: H. crispa—F 6e; Hvass 331; LH 41. H. lacunosa—F 6b; Hvass 330; LH 39; NB 1534. L. elastica—Hvass 332; LH 39. C. macropus—F 6a; LH 39.
Fruit-body: width 20-60 mm, or several coalescing.
Description: Plate 69.
Fruit-body: chestnut-brown to rust colour with a distinct white or cream margin, fleshy, smooth, concave and thrown up into irregular humps.
Stem: lacking, but undersurface pale, ochraceous, and bearing numerous cylindrical branched, whitish root-like structures, 1-2 mm thick.
Flesh: reddish brown, tough and fibrous.
Spores: very, very long, spindle-shaped with two or more internal droplets, with hyaline extensions at each end, and 22-40 × 8-11 µm in size; eight contained in an elongate cylindrical ascus.
Habitat & Distribution: Infrequent in pine woods but common at the sites of bonfires in pine woodlands.
[204]
Fruit-body: diameter 20-40 mm × 20-60 mm.
Description:
Fruit-body: date-brown at first finally black or dark brownish black, tough, minutely pimply over entire surface although at first covered in a powdery dust of asexual spores (conidia).
Stem: lacking.
Flesh: pale grey or buff, concentrically zoned with darker purplish black layers below which are small, black dots.
Spores: very long, black, ellipsoid with one flattened side and 12-17 × 6-9 µm in size; eight contained in an elongate cylindrical ascus.
Habitat & Distribution: Common on old deciduous wood, particularly of ash and beech.
General Information: These two fungi are unrelated; the first is related to the disc-fungi, like species of Peziza, whilst Daldinia is related to the Dead man’s finger fungus. Rhizina undulata has been shown to be able to attack roots of pine or larch trees and cause death. Daldinia is a pure saprophyte rotting down wood into more simple compounds later to be incorporated into the soil-system. The common name ‘Cramp-balls’ refers to the old belief that if one of the fruit-bodies is carried in the pocket it saves the possessor from cramp and rheumatism. The other common name for the same fungus is ‘King Alfred’s cakes’. The black colour of the fruit-body is like that of charred cakes—resembling the cakes in the legend which King Alfred allowed to burn.
Illustrations: R. undulata—LH 37; NB 1116. D. concentrica—F 7b; LH 47; NB 1477.
Fruit-body: width 10-20 mm; length 30-60 mm.
Description:
Fruit-body: more or less club-shaped, irregularly or evenly lobed at apex, at first light brown due to development of asexually produced spores (conidia) but finally almost black.
Stem: black and short.
Flesh: white, fibrous and tough.
[205]
[206]
Crust: black, thin, pimply with the protruding tips of the perithecia, and sometimes irregularly furrowed.
Spores: very long, fusiform with one flattened side, black and 20-32 × 5-9 µm in size; eight contained in an elongate cylindrical ascus.
Habitat & Distribution: Common either solitary or in clusters on dead stumps or on buried wood, especially that of beech. This fungus may be found throughout the year.
Fruit-body: width 4-8 mm; length 25-60 mm.
Description: Plate 69.
Fruit-body: slender, subcylindrical to strap-shaped and usually forked repeatedly near the tip, white at first due to production of conidia and then black or dark brown and covered in pimples.
Stem: black and hairy.
Spores: very long, bean-shaped, black and 11-14 × 5-6 µm in size; eight in an elongate ascus.
General Information: Another name for X. hypoxylon is ‘Candle-snuff fungus’. Other club-shaped ascomycetes include members of the genus Geoglossum (already mentioned p. 172) and members of the genus Cordyceps. Plate 69.
Cordyceps militaris (St Amans) Link, the ‘Scarlet caterpillar fungus’, produces orange-red or orange, minutely roughened fruit-bodies up to 50 mm high, which grow on larvae and pupae of moths buried in the soil. It is not infrequent late in the autumn in pasture land.
C. ophioglossoides (Fries) Link produces long (up to 100 mm high) yellow stemmed, dark and rough headed fruit-bodies growing on the subterranean fungus Elaphomyces—see p. 244.
C. capitata (Fries) Link also grows on fungi beneath the soil surface but has a rounded head. Leotia lubrica Persoon the ‘Gum-drop fungus’ is similarly coloured but grows on soil under trees and is gelatinous. It grows in autumn and is quite common and in fact more related to the Discomycetes than to Cordyceps.
Illustrations: X. polymorpha—F 7d; LH 47; NB 1476. X. hypoxylon—F 7e; LH 47; NB 1475. C. militaris—LH 48.
[207]
Cap: width 20-40 mm. Stem: width 2-5 mm; length 30-60 mm.
Description:
Cap: chrome-yellow or lemon-yellow when young, paling with age at margin to become cinnamon-buff, bell-shaped but rapidly expanding to become plane or slightly umbonate, smooth, viscid but soon drying; margin striate then radially grooved, often split and the whole cap soon collapsing.
Stem: slender, whitish, cream colour to pale yellow, at apex covered in small, white floccose scales but downy at the base, fragile and soon collapsing.
Gills: adnexed or free, cinnamon-buff, thin and crowded.
Flesh: yellowish, very thin and lacking distinct smell.
Spore print: rust-brown.
Spores: long, yellow-brown under the microscope, ellipsoid with a very distinct germ-pore about 13 × 8 µm in size (11-15 × 6-9 µm).
Facial cystidia: rare, balloon-shaped.
Marginal cystidia: swollen, flask-shaped with a variable, elongate neck.
General Information: This fungus is common on horse droppings or other manures, but it may also be found amongst grass in pastures and in sand-dunes, and gardens on piles of rotting grass stems or straw. It is easily recognised by the colour and rapid expansion of the cap and the sudden collapse of the whole fruit-body. ‘Vitellinus’ means yolk of an egg and refers to the persistently bright yellow cap-centre, so obvious even when the fruit-body collapses. This collapsing is not one of autodigestion as described for members of the genus Coprinus. It is variable both in size and habitat, and I even have records of the fungus growing within herbaceous stems.
Illustrations: LH 153; WD 806.
[208]
Cap: width 10-35 mm. Stem: width 4-7 mm; length 25-50 mm.
Description:
Cap: hemispherical or slightly umbonate, sometimes flattened and hardly expanding even with age, very viscid, smooth, pale yellow-ochre or yellowish tan.
Stem: slender, straight, white then yellowish, smooth, viscid, but then dry and shiny below an imperfectly formed, thin ring.
Gills: adnate, almost triangular in shape, crowded, dark brown to purplish black, but with ochraceous areas at maturity.
Flesh: pale ochre.
Spore-print: purplish brown.
Spores: very long, dark brown under the microscope, smooth, ellipsoid with large germ-pore and about 18 × 10 µm in size (17-20 × 9-10 µm).
Facial cystidia: spindle-shaped, thin-walled and filled with amorphous contents which become yellow in solutions containing ammonia.
Marginal cystidia: spindle-shaped or flask-shaped, numerous, thin-walled and typically yellowing as above.
General Information: ‘Semiglobata’ means hemispherical and refers to the shape of the cap of S. semiglobata; it is a very variable fungus in both size of the cap and the prominence of the ring. The Dung-roundhead grows only on dung which is acidic in its soil status, whilst Panaeolus semiovatus (Fries) Lundell next described (p. 210) grows on slightly to distinctly base-rich dung. This may explain why in Britain the Dung-roundhead is the commoner of the two species. However, P. semiovatus was formerly placed in the genus Stropharia because of its blackish spores and distinct ring. The spores of Stropharia in the mass are violaceous black whilst those of P. semiovatus are brownish black. Under the microscope they are also differently coloured and have different chemical compositions as their reaction with dilute solutions of ammonia shows; the spores of the first species turn purplish olive in ammonia and those of the second species become very dark brown.
Illustrations: F 33b; Hvass 171; LH 153; NB 315; WD 753.
[209]
[210]
Cap: width 20-70 mm. Stem: width 5-10 mm; length 80-160 mm.
Description:
Cap: oval or bell-shaped, not expanding, dingy whitish or pale clay colour, smooth, slimy when moist, but soon drying and then becoming shiny, often wrinkled and cracked, and ornamented with fragments of veil at the margin.
Stem: dull, straight, rather rigid, tapering upwards, white, and striate at apex above a whitish erect and membranous, often collapsing, ring; yellowish below the ring and whitish and cottony at the slightly swollen base.
Gills: adnate, greyish then black, mottled and crowded.
Flesh: whitish or pale ochre.
Spore-print: black.
Spores: very long, very dark brown under the microscope with large obvious germ-pore and 18 × 10 µm (16-20 × 9-11 µm) in size.
Facial cystidia: flask-shaped and with amorphous contents.
Marginal cystidia: numerous, flask-shaped.
Cap: width 15-35 mm. Stem: width 3-6 mm; length 60-95 mm.
Description:
Cap: bell-shaped, hardly expanding, expallent, dark grey to olivaceous black, much paler when dry and zoned when half dry; margin ornamented with a white fringe of veil fragments.
Stem: long, slender, straight, rather rigid but fragile, grey and completely powdered with white.
Gills: adnate, crowded and grey then blackened, mottled throughout except at the white fringed edge.
Flesh: reddish brown.
Spore-print: black.
Spores: long, very dark brown under the microscope, broadly lemon-shaped with large germ-pore, smooth and 14-15 × 9-10 µm in size (14-19 × 8-10 × 10-12 µm).
Facial cystidia: absent.
Marginal cystidia: numerous, cylindrical, flexuous and hyaline.
General Information: P. sphinctrinus is recognised by the overall grey colouration and very distinct white fringe to the cap-margin.
P. campanulatus (Fries) Quélet which is said to be common is in[211] fact infrequent and many records really refer to P. sphinctrinus. The word semiovatus means half ovate and refers to the shape of the cap in P. semiovatus. Sphinctrinus means banded, referring to the zoned cap of the fungus when it is partially dry.
Illustrations: P. semiovatus—LH 145; WD 773. P. sphinctrinus—NB 415; WD 781.
Cap: width 20-40 mm. Stem: width 4-8 mm; length 50-100 mm.
Description:
Cap: oval then rapidly expanding, covered at first in a mass of dense, white or greyish woolly scales which break up into patches and finally leave the cap shiny, brownish grey at centre and striate and dark grey at the margin.
Stem: white, covered particularly towards the base with white, woolly scales, long, fragile, tapering upwards and at the base often elongated into a ‘tap root’ buried in the dung.
Gills: free, white but then rapidly dissolving into a black liquid.
Flesh: thin and whitish.
Spore-print: violaceous black.
Spores: medium sized, ellipsoid, smooth with a distinct germ-pore and 10-12 × 5-6 µm in size.
Facial cystidia: absent.
Marginal cystidia: inflated and large.
General Information: It is found on manure heaps, on straw dung and on silage heaps: very common throughout the year.
C. macrocephalus (Berkeley) Berkeley is very closely related to C. cinereus, but differs in having much larger spores over 12-15 × 7-9 µm, a long cap and a stem which lacks a rooting base.
Coprinus radiatus (Fries) S. F. Gray is smaller in stature and also differs in spore-size (11-14 × 6-7 µm). C. pseudoradiatus Kühner & Josserand is minute and has even smaller spores (7-9 × 4-5 µm). The dung-heap ink-cap has long been used by scientists in genetic studies, usually under the name of C. lagopus (Fries) Fries. However, this latter species, although similar, grows only on woodland detritus; it has narrower spores. The dung-heap ink-cap may be referred to in other books as C. fimetarius Fries or C. macrorhizus (Fries) Rea and whilst cinereus means grey referring to the colour, fimetarius means dung—from the habitat, and macrorhizus refers to the long rooting base found in some specimens.
Illustrations: LH 137; NB 4110; WD 814.
[212]
The genus Coprinus is easily recognised from all other agarics by the structure and development of the fruit-body. In the field, most species of the genus can be recognised by the gradual conversion of the gills, and often the cap tissue into a black liquid resembling ink—hence the name inky-caps. The conversion of the gills to an inky mass is called autodigestion and the process is complete within a few hours; this mechanism enables spores to be dispersed immediately they have ripened. Unlike other agarics the spores are not shot off into the spaces between the gills, but directly into the air. The gills are parallel-sided in Coprinus and not wedge-shaped as in more normal agarics, and in order to achieve spore dispersal the gills must disintegrate; Coprini are very specialised.
Coprinus is a large genus with over seventy members in the British Isles, many of which are strictly dung-loving. It is impossible to give more than one example in full here, for although many of the large species can be recognised on sight the smaller ones require the aid of a microscope. The interested student must therefore refer to more advanced texts, but in order to demonstrate the diversity of the Coprini and how they are classified the following key to the sections of Coprinus will be found useful.
[213]
[214]
Dung fungi are highly satisfactory for demonstrating the diversity and morphology of a group of related organisms within a single ecological system, as representatives of most of the major groups of fungi usually grow on dung after a period of incubation. Dung will always produce characteristic fungi whatever time of year it is collected.
Dung is best incubated in a light place, for example on a window sill, in a warm room on layers of blotting paper or other absorbent material. For rabbit-pellets and samples of similar size petri-dishes are ideal, but for cow, horse and similar types of dung large covered dishes such as casseroles or sandwich containers are very good. Samples should not be kept in airtight containers for long periods of time as under such conditions animal life present rapidly breaks down the dung and induces anaerobic conditions. Instead larvae and earthworms should be excluded from the sample as they decompose the dung and inhibit fungal growth but their activity can be reduced, if causing a problem, by spraying the sample lightly with a proprietary fly-kill aerosol.
By keeping the dung under constant observation during incubation a whole succession of fungi can be seen. Thus the first fungi to appear are the moulds which although numerous need a microscope for their identification. The moulds are followed by a series of Ascomycetes (Sporormia & Sordaria with flask-shaped fruit-bodies and Iodophanus, Coprobia and Cheilymenia with disc-shaped fruit-bodies), which are best sought with the use of a powerful hand-lens or a stereoscopic binocular microscope when their full beauty will be revealed. However, because they need the aid of instruments even to see them they cannot be considered larger fungi. The fruit-bodies of the Basidiomycetes are readily seen with the naked eye, but a hand-lens is still very useful for observing features of the cap and stem, particularly the veil characters. The Basidiomycetes usually conclude the succession of fungi found on dung and soon after this state the dung is colonised by mosses and higher plants and later it is fully incorporated into the soil.
[215]
[216]
Dung is a very useful substrate for studying succession. However, equally interesting results can be obtained from observing the fungi which appear on a stump, colonise a newly laid lawn, or indeed those growing on refuse such as a cast-out rug; microscopic and larger fungi are all to be found.
If the dung cannot be incubated immediately it should be dried quickly, for most dung-fungi will survive such treatment and grow when the sample is remoistened. The blotting-paper on which the dung is placed should be kept moist throughout the incubation period.
One large discomycete (up to 80 mm across) occurring on manure-heaps must, however, be mentioned, this is Peziza vesiculosa St Amans (see p. 200); the inner surface of this cup-fungus becomes detached from the flesh at maturity and forms blisters.
Cap: width 20-50 mm. Stem: width 4-8 mm; length 25-60 mm.
Description: Plate 73.
Cap: convex then flattened and slightly umbonate, smooth, very sticky at first, but becoming shiny when dry, orange-yellow to sand-colour; the margin is first incurved and ornamented with filaments from the veil, but these are soon lost.
Stem: dirty yellow, darker towards the base, cylindric or narrowed downwards and covered in small fibrillose scales.
Gills: clay-coloured then dull brown, adnate and crowded.
Flesh: yellowish.
Spore-print: dull rust-brown.
Spores: medium-sized, ellipsoid, smooth, dull brown under the microscope and 7-8 × 3-4 µm in size.
Facial cystidia: spindle-shaped with obtuse apex.
Marginal cystidia: similar to facial cystidia but usually smaller.
General Information: This fungus which occurs from spring to autumn is recognised by the habitat, colour of the fruit-body and the spore-size.[217] It is known in many books as Flammula carbonaria (Fries) Kummer, but the genus Flammula is no longer used for it refers to a flowering plant in the buttercup family.
P. highlandensis is the same fungus as that referred to as Pholiota carbonaria by European Mycologists, but this name cannot be used for it refers to an entirely different N. American species. ‘Highlandensis’, in fact, refers to the locality where the present fungus was first found in the United States of America. The true P. carbonaria A. H. Smith has only been found once in Europe and this only recently in the south of England. It differs in the reddish orange scales on the stem; indeed it is a much brighter fungus than the common charcoal Pholiota.
Cap: width 1-4 mm. Stem: width 1 mm; length 2-5 mm.
Description: Plate 73.
Cap: blackish when wet, drying sooty brown, slightly depressed in the centre, smooth, and viscid.
Stem: sooty brown, tough and smooth.
Flesh: sooty brown.
Gills: whitish then grey, adnate and not very crowded.
Spores: medium sized, subglobose, 4-6 × 4-5 µm in diameter and minutely roughened.
Spore-print: white, not blueing in solutions of iodine.
General Information: T. atrata also grows on burnt soil and is very closely related, but differs in its spores being broadly ellipsoid and smooth. Mycena leucogala also grows on burnt soil (see p. 88).
Illustrations: T. anthracophila—LH 83. T. atrata—WD 4b.
[218]
Cap: width 10-30 mm. Stem: width 1-3 mm; length 30-40 mm.
Description:
Cap: conical or bell-shaped then expanding and slightly umbonate, whitish because of a coating of dense fibrils, but soon becoming brownish as these are lost.
Stem: short, stout, white and densely floccose.
Gills: slightly adnate, pale brownish grey with pink tinge, then dark-brown.
Spore-print: purplish brown.
Spores: medium sized, oval, ellipsoid with an obvious germ-pore, purplish brown under the microscope and 8-9 × 4-5 µm in size.
Marginal & facial cystidia: flask-shaped, hyaline with either a short or long neck.
The brown-spored Hebeloma anthracophila Maire is similar.
Cap: width 4-25 mm. Stem: 1-3 mm; length 15-30 mm.
Description:
Cap: dark red-brown at first, then orange-brown, especially at the margin and appearing as if frosted all over, conical at first but rapidly expanding at the margin and becoming grey-brown, strongly striate and deliquescent, leaving finally only a central red-brown umbo.
Stem: white and minutely hairy.
Gills: free, dirty whitish then black.
Spore-print: black-brown.
Spores: medium sized, dark brown under the microscope, lobed like the hat of a bishop and 8-11 × 6-8 × 5-7 µm in size.
Marginal cystidia: bottle-shaped, very variable.
Facial cystidia: similar to marginal cystidia.
General Information: It must be noted that this fungus has spores which require three quite different measurements to describe the dimension. Another species of Coprinus found on burnt soil is C. lagopides Karsten which resembles C. cinereus (Fries) S. F. Gray (p. 211); it is typified, however, by the rounded spores.
[219]
[220]
Several common fungi found at the sites of bonfires have their closest relatives amongst various groups of microscopic fungi more than amongst the large forms already discussed. Keeping a close watch at the site of a former bonfire day by day, week by week and month by month is very rewarding and shows a further example, like the dung habitat, of a tightly knit community of various groups of fungi.
Peziza repanda Persoon has been discussed in detail above (p. 200); its close relatives P. petersii Berkeley & Curtis (brown with grey tints and with spores finely warted and measuring 10-12 × 5-6 µm), P. praetervisa Bresadola (violet or mauve and with spores finely warted and measuring 11-13 × 6-8 µm), P. violacea Persoon (dark violet with smooth spores measuring 13-15 × 7-9 µm) and P. echinospora Karsten (dark chocolate brown with spores densely warted and 14-18 × 7-10 µm in size) all grow on the sites of old bonfires or around charred root stumps. Rhizina undulata also found by charred stumps has been described on p. 203. These are large to medium sized disc-fungi, but there are many much smaller species which cannot be dealt with here, such as species of Anthracobia and Trichophaea. Pyrenomycetes are also found on charred wood and soil. Probably the commonest species of fungus met with is a pale reddish orange to rose-pink disc-fungus seated on a white mycelial mat; this is Pyronema omphalodes (St Amans) Fuckel. Morchella esculenta St Amans and M. elata Fries (see p. 200) appear to grow on the sites of garden bonfires or where cinders have been spread on the soil surface. The stimulus for fruiting appears to be due to the release of mineral nutrients during the process of burning. Competition from other fungi appears to be reduced so rapid colonisation by the bonfire fungi (carbonicoles) after the period of sterilisation ensures their development. Many similar fungi were found about bomb- and shell-craters on the continent during the two World Wars.
One microscope fungus, however, must be mentioned when considering bonfires and that is Neurospora sitophila Shear & Dodge so much used in genetical studies. It can be found as the conidial state on burnt soil and is called ‘Baker’s mould’ because it is frequently found growing on refuse in the hot moist conditions of bakers’ kitchens.
[221]
[222]
Cap: width 12-20 mm. Stem: width 3-5 mm; length 50-80 mm.
Description: Plate 75.
Cap: bell-shaped but rapidly expanding to become plane, honey-yellow with a greyish green tint, slightly striate at the margin and also with a few remnants of a fibrillose veil when very young, but these are soon lost.
Stem: slender, smooth, whitish at the apex and yellow-brown or honey-yellow below.
Gills: adnate and distant, pale ochraceous honey-yellow then lilaceous grey and finally sepia.
Flesh: yellowish in the cap, red-brown in the stem and lacking a distinct smell.
Spore-print: purplish brown.
Spores: long, ellipsoid, fairly thick-walled, olivaceous brown under the microscope and with a small germ-pore, smooth and 10-12 × 6-7 µm in size.
Marginal cystidia: flask-shaped and hyaline.
Facial cystidia: flask-shaped with contents which turn yellowish in solutions containing ammonia.
General Information: This fungus which appears from early summer to late autumn is recognised by the almost uniform ochraceous colour with hint of olive and its habit of growing in troops. The word elongatum means elongated and refers to the shape of the stem which pushes up through the Sphagnum and in order to disperse its spores it must elongate so that it just pushes up above the bog-surface. H. polytrichi is closely related to H. elongatum but has a paler cap and stem and it grows in moss, particularly Polytrichum in woodlands; the spores of H. polytrichi are paler, slightly narrower and slightly thinner, but they have a much more distinct germ-pore.
Both the above species have been formerly placed in Psilocybe, but they are more correctly classified in Hypholoma along with the sulphur-tuft fungus (see p. 64) because of the cortina-like veil and specialised facial cystidia.
Illustrations: WD 785.
[223]
Cap: width 12-30 mm. Stem: width 3-5 mm; length 50-75 mm.
Description: Plate 75.
Cap: bell-shaped then plane-convex, but finally depressed at centre, watery buff to greyish with flush of ochre or smoky grey, striate to centre when moist, but drying out non-striate and uniformly ochraceous buff.
Stem: thin, rather long, smooth, similarly coloured to the cap or paler, fragile and whitish woolly at the base.
Gills: dirty whitish, adnate with a tooth and not very crowded.
Flesh: thin, watery buff, drying out ochraceous and with a strong smell of new meal.
Spore-print: white.
Spores: medium sized, hyaline under the microscope, oval, not turning blue-grey in solutions of iodine, and 6-7 × 4-5 µm in size.
Marginal and facial cystidia: absent.
General Information: This fungus which grows from late spring to autumn is usually associated with a greying and finally a killing of the Sphagnum, noticeable from a distance even in the absence of the fruiting-bodies as paler patches in the rich green bog. Another agaric found only in Sphagnum bogs is Omphalina sphagnicola (Berkeley) Moser with decurrent gills and long, elongate, hyaline spores.
At the margin of Sphagnum bogs, the fungus Mycena bulbosa can be found attached to the base of tufts of rushes.
Potting up a sward of Sphagnum and retaining it in a warm greenhouse during winter favours the bog agarics to fruit when other larger fungi are not available.
Cap: width 3-6 mm. Stem: width 1 mm; length 10-15 mm.
Description:
Cap: dirty white, greyish and very gelatinous.
Stem: very thin, hyaline with a very distinct hairy, basal disc.
Gills: crowded, adnexed, very short and whitish.
Spore-print: white, but because it is so small it is often difficult to see.
Spores: medium sized, hyaline under the microscope, ellipsoid, not blueing in solutions of iodine, and 8-10 × 4 µm in size.
Marginal cystidia: clavate or ventricose, hyaline and smooth.
Facial cystidia: absent.
Illustrations: T. palustris LH 83.
[224]
Cap: width 10-20 mm. Stem: width 3-5 mm; length 50-90 mm.
Description:
Cap: conico-convex expanding slightly but retaining the central umbo, striate to half-way, sand-colour to red-brown, hygrophanous, minutely floccose because of remnants of veil distributed over its surface, but soon becoming smooth.
Stem: long, buried amongst the Sphagnum, red-brown and flecked with white fibrils, except at the finely hairy apex, the fibrils typically form a distinct but easily lost ring.
Gills: almost horizontal, adnate to subdecurrent, pale at first and then rust-brown.
Spore-print: rust-brown.
Spores: medium-sized, ovate to slightly lemon-shaped, minutely warty, honey-brown under the microscope and about 10 × 6 µm in size, (9-11 × 6-7 µm).
Facial cystidia: absent.
Marginal cystidia: hyaline, almost cylindrical or bottle-shaped with an inflated base.
General Information: This species grows from spring to early autumn in Sphagnum bogs; several other species of Galerina are also found in the same localities:—
(i) G. sphagnorum (Fries) Kühner has a convex cap, fibrillose silky and ochraceous brown stem, but it lacks the ring-zone so typical of G. paludosa. The smell is like that of meal when crushed and the gills are emarginate.
(ii) G. tibiicystis (Atkinson) Kühner has a rapidly expanding cap which becomes plano-convex or depressed at maturity; it also lacks a ring-zone, but the stem in this species is finely hairy because of the presence of numerous pin-shaped cells which can be seen only with the aid of a lens. The gills are broadly adnate.
Illustrations: G. paludosa—LH 175.
[225]
[226]
Cap: width 12-30 mm. Stem: width 1-3 mm; length 25-45 mm.
Description: Plate 76.
Cap: pale yellowish ochre, but becoming darker ochraceous with age, scurfy, convex but then flattened, or with its edge upturned; the margin is slightly striate when moist.
Stem: slender, pale to dirty yellowish ochre but darker brown at base, slightly fibrillose, particularly at first because of filaments from a veil, but these are soon lost.
Gills: pale tan to brownish ochre with a paler, floccose margin, adnate and crowded.
Flesh: yellowish ochre but lacking a distinct smell.
Spore-print: clay-colour.
Spores: medium sized, almond-shaped, pale brown under the microscope, warted and 10-11 × 5-6 µm in size.
Marginal cystidia: swollen below, but drawn out into a hair-like apex.
Facial cystidia: absent.
General Information: Although this is a common species growing in damp places under alder it is difficult except with an expert eye to separate it from several closely related species which are also found in similar places. At present it is not known whether these fungi are favoured by the water-logged base-rich, reducing soils found nowhere else except under alder, or if they have a special relationship with the tree. There is ample evidence that soil conditions in alder woods are rather different from those found in other woodlands, but whatever the reason Naucoria escharoides is only found under alder—in fact this species has been placed in the genus Alnicola because of this character—cola meaning inhabitant and Alnus the tree of that name. Willow-carrs have not been as extensively studied as alder-carrs but there is evidence that a store of mycological information is still to be obtained from these places. Several species of Naucoria have been described from only willow-carrs, while others are to be found under both alder and willows; about eight species are known to grow under alder. The word escharoides means scab-like and refers to the cap which when freshly collected is minutely scaly and appears scabby.
Illustrations: LH 163; WD 671.
[227]
Beds of herbaceous plants provide protection for many small agarics and collecting can be conducted in these situations from spring to early winter. The buffered environments under the herbs is humid and relatively still, and this allows the development of the small often delicate fruit-bodies of certain species to continue unimpeded. Nettle-beds or mixtures of nettle and dog’s mercury have very rich floras under the shelter of their leaves and stems, either on the bare soil or plant debris.
Cap: width 4-7 mm. Stem: width 1 mm; length 10-15 mm.
Description: Plate 76.
Cap: white then greyish, globose at first and then expanding to become plane with upturned margin covered, at first, with scales from a veil which at the centre are white-tipped with ochre.
Stem: white and slightly downy.
Spore-print: brownish black.
Spores: elliptic-ovoid, only slightly compressed with distinct germ-pore, dark brown under the microscope and 6-8 × 5 µm in size.
Marginal cystidia: ellipsoid to pyriform and hyaline.
Facial cystidia: elongate cylindric larger than marginal cystidia.
Cap: 5-15 mm. Stem: width 1 mm; length 20-50 mm.
Description: Plate 76.
Cap: grey to sepia or greyish brown entirely scaly-hairy, at first, but then fibrillose.
Stem: silvery grey to grey-sepia and silky fibrillose.
Gills: greyish pink.
Spore-print: greyish pink.
Spores: very long, wavy angular in outline, very pale honey under the microscope and 14-20 × 7-9 µm.
Marginal cystidia: club-shaped or balloon-shaped and hyaline.
Facial cystidia: absent.
So very different to other species of Leptonia is it that it should be classified in Dr. Pilát’s genus Pouzaromyces.
[228]
Cap: width 5-10 mm. Stem: width 1 mm; length 10-40 mm.
Description:
Cap: pale to deep ochraceous or buff, minutely tomentose.
Stem: flexuous, whitish or very pale ochraceous.
Gills: pale buff then ochraceous.
Spore-print: ochraceous.
Spores: medium sized, ellipsoid or slightly almond-shaped with small germ-pore and 6-8 × 3-4 µm in size.
Cap: 4-15 mm. Stem: width 1 mm; length 10-25 mm.
Description:
Cap: ochraceous to date-brown, darker at the centre and granular scaly throughout.
Stem: similarly coloured to the cap and similarly roughened, except for the slightly smoother paler apex.
Spores: ellipsoid to almond-shaped, very pale brown under the microscope and 8-10 × 4-5 µm in size.
Marginal cystidia: cylindric-wavy, hyaline.
Facial cystidia: absent.
Depending on the herbaceous constituents the fungus-flora will vary. Certain species are found on all sorts of herbaceous debris, but others are much more specific to their substrate preferences. Beds of Butterbur, Coltsfoot or Impatiens are also good hunting places, as are beds of sedges in fenland. In many of these localities agarics with reduced fruit-bodies looking like disc-fungi are frequently seen. We have already discussed the specific requirements of certain species of Marasmius (see p. 92).
[229]
[230]
Many small species grow amongst moss cushions on tree trunks, tucked in crevices in walls or on the tops of old buildings. However, there is one genus of agarics, i.e. Galerina which is probably more typical than any other of such situations. There are many members of this genus whose small caps are found in the autumn pushing up through the moss plants. Plate 78.
Cap: width 4-6 mm. Stem: width 1 mm; length 20-40 mm.
Description:
Cap: hemispherical or bell-shaped, hygrophanous, orange-yellow, sand-colour, smooth and striate almost to the cap-centre.
Stem: smooth and similarly coloured to the cap.
Gills: yellow-tawny then rust-coloured, adnate emarginate, rather broad and somewhat distant.
Flesh: thin, yellow-tawny and with a smell of new meal.
Spore-print: rust-colour.
Spores: medium-sized, almond-shaped, golden yellow under the microscope, slightly roughened and 10-11 × 6-7 µm in size.
Marginal cystidia: flask-shaped or cylindrical with slight swelling at the apex.
Facial cystidia: absent.
Cap: width 6-15 mm. Stem: width 1 mm; length 30-60 mm.
Description:
Cap: similarly coloured to G. hypnorum, but with a few white silky fibrils.
Stem: coloured as the cap, but with white silky fibrils when young.
Gills and flesh: as in G. hypnorum, but it has no smell.
Spore-print: rust-colour.
Spores: medium-sized, ellipsoid, pale golden yellow under the microscope, smooth and 9-11 × 5-6 µm in size.
Marginal cystidia: club-shaped, cylindrical and with distinct rounded heads.
Facial cystidia: absent.
[231]
General Information: G. mniophila (Lasch) Kühner is similar to or slightly larger than C. mycenopsis, but differs in its dull honey-coloured cap and stem, and distinctly roughened spores. G. calyptrata P. D. Orton is small and has been long confused with G. hypnorum; it, however, is of a much brighter orange-colour, with distinct white fibrils on the cap and has spores which have a distinct envelope, sometimes separating as a loose covering. G. vittaeformis (Fries) Moser is a red-brown fungus with 2-spored basidia, facial cystidia, minutely hairy stem, and very rough spores; it grows in moss in pastures as well as on moss-cushions.
Cap: width 5-15 mm. Stem: width 1 mm; length 30-60 mm.
Description: Plate 77.
Cap: whitish to pale smoke-brown with a distinct wine-coloured tinge, membranous, flattened, or umbilicate and radially wrinkled.
Stem: thread-like, black or very dark brown, horny and usually springing from a black horse-hair-like mycelium.
Gills: whitish or dirty flesh-colour, adnate and crowded.
Flesh: white in the pileus and black in the stem.
Spore-print: white.
Spores: medium-sized, pip-shaped, not blueing in solutions containing iodine and measuring 7-9 × 3-4 µm in size.
Marginal cystidia: oval or ellipsoid, covered on the upper half with small pimple-like projections.
Facial cystidia: absent.
General Information: This fungus is common in troops from late summer until winter on dead and dying heather. It is also found in woods on leaves and twigs, particularly in plantations on conifer needles. It is easily recognised by the dark horse-hair-like stem which becomes bent and twisted on drying and the small, pinkish flesh-coloured cap. The word androsaceus means, and refers to, the stem which resembles the tough and wiry fronds of some of the red algae, such as Ahnfeldtia which is found around our sea-shores.
Illustrations: LH 115; NB 471; WD 244.
[232]
Cap: width 5-20 mm. Stem: width 2 mm; length 10-20 mm.
Description:
Cap: variable in colour, straw-colour, cream-colour, bistre or grey, convex then flat or slightly depressed, radially grooved to the centre when moist; the margin is scalloped.
Stem: slender, similarly coloured to the cap, except for a brownish wine-coloured zone at the very apex, thickened upwards and smooth with a white and woolly base.
Gills: adnate to decurrent, white then cream-colour or yellowish, triangular in shape, very distant and often connected by veins.
Flesh: pale cream-colour.
Spore-print: white.
Spores: medium sized, hyaline under the microscope, broadly ellipsoid, or pip-shaped, not becoming bluish grey in solutions of iodine, 8-10 × 5 µm in size.
Marginal and facial cystidia: absent.
General Information: This fungus is common and often in large troops on peaty ground in woods as well as in moorland and mountain regions. In mountains O. ericetorum must be carefully distinguished from some of the truly mountain species of Omphalina dealt with on p. 236. O. wynniae (Berkeley & Broome) P. D. Orton is similar but pale lemon-yellow and is found on stumps of conifers. The word ericetorum refers to the habit of growing on heaths—Erica is the Latin name for heath. In many books this same fungus is called O. umbellifera which reflects the shape of the cap—umbrella shaped.
Illustrations: Hvass 116; LH 99; NB 857; WD 299.
Cap: width 25-75 mm. Stem: width 2-6 mm; length 25-55 mm.
Description:
Cap: finely or minutely velvety at centre, fibrillose or white silky as if frosted towards the margin, sepia or bistre, or mouse-grey, dull-coloured but with a hint of violaceous brown.
Stem: equal or slightly thickened at the apex, sometimes club-shaped, thickened at the base, greyish brown and pale cream-colour at the base.
Flesh: dark sepia in the cap, whitish in the stem and smelling strongly of meal.
[233]
[234]
Gills: white or whitish at first then dirty pinkish brown, adnate and emarginate.
Spore-print: dull salmon-pink.
Spores: medium to long, angular, ellipsoid-oblong, slightly cinnamon-colour under the microscope and 9-12 × 7-8 µm in size.
Marginal cystidia: conspicuous, spindle or bottle-shaped and with subcapitate apex.
Facial cystidia: absent.
Cap: width 15-30 mm. Stem: width 4-7 mm; length 50-100 mm.
Description:
Cap: fleshy, convex, later becoming flattened but remaining slightly umbonate at the centre, viscid at first, smooth and shining when dry, bright reddish to sand-colour or brown.
Stem: slender, yellow above, brown below, smooth and tough.
Gills: adnate or adnexed, purplish black with a whitish margin and fairly crowded.
Flesh: yellowish or red-brown in the stem.
Spore-print: purple-brown.
Spores: long, dark purple-brown, broadly ellipsoid and 12-15 × 7-9 µm in size.
Marginal cystidia: cylindrical or flask-shaped.
Facial cystidia: flask-shaped and filled with contents which become yellowish in solutions containing ammonia.
Fruit-body: height 20-60 mm.
Description:
Fruit-body: club-shaped, blunt or rounded at the apex, cylindrical or compressed and often grooved, yellow ochraceous or buff.
Stem: distinct but short and yellowish.
Flesh: yellowish.
Spore-print: white.
Spores: medium-sized, hyaline under the microscope, smooth and 10-11 × 5-6 µm in size.
All these three species are typical of bare peaty soil, or moss covered peat amongst or around Heather or Ling (Calluna vulgaris) bushes.
[235]
[236]
Omphalina ericetorum (Fries) M. Lange has already been described (p. 232): it grows on acidic soils and ascends into mountain areas where it frequently grows on algal scum which accumulates around Sphagnum plants.
Under these conditions the algal cells enter the base of the fungus and grow in the cavity of the stem and amongst those hyphae which constitute the base. This association, however, appears to be much closer in the two lichens Coriscium viride (Acharius) Vain and Botrydina vulgaris Meneghini which have long been classified as species of lichen of unknown affinity because no perfect state was known. Coriscium viride consists of blue-green overlapping plates or scales with narrow rounded often paler margins and which dry out greenish brownish grey. Botrydina vulgaris, in contrast, consists of dark green, gelatinous blobs drying out greenish brown.
Coriscium is now considered to be an association of an algae and a Basidiomycete, the latter being the agaric, Omphalina hudsoniana (Jennings) Bigelow, which resembles O. ericetorum but for the pinkish coloured stem. Botrydina may be a complex of several separate associations of an algae with different species of Omphalina. In the high mountains the association is with O. luteovitellina (Pilát & Nannfeldt) M. Lange a small uniformly bright yellow agaric, whilst in Sphagnum bogs it is with O. sphagnicola (Berkeley) Moser. Myxomphalia maura (Fries) Hora, a fungus typical of burnt ground, is also reported to take up this association in lowland woods and O. velutina (Quélet) Quélet appears to be capable of forming a loose relationship with algal cells also. This is a most interesting association and research work is still at an early stage. In the tropics and subtropical regions of the world, similar associations are found on rotten and decomposing trunks and stumps. In these examples the Basidiomycetes are frequently fairy-clubs, particularly species of Multiclavula (‘many small clubs’). A few species of this genus may be found also in North temperate woodlands. Botrydina also grows in Europe with Stereum fasciatum (Schw.) Fries and Athelia viride (Bres.) Parm. (see p. 176), and Odontia bicolor (Fries) Quélet is rarely collected without green algal cells buried in the thallus. Perhaps associations like this are much commoner than at first supposed.[237] Probably the most remarkable of this group of poorly known organisms is Cora pavonia (Sw.) Fries which produces masses of interlocking fans; it is tropical and found in Brazil.
There are several groups of mountain fungi, some mycorrhizal formers, some which prefer peaty soil and some which are associated with algae forming a loose relationship—the Basidiolichens. When the mountain top is covered with such dwarf willows as Salix herbacea or S. reticulata the leaves are cast each year, woody tissue develops above and below the ground; in fact all the processes taking place in our familiar woodlands are also taking place in these communities, the only difference being that the trees are dwarf. Indeed it looks quite odd to see normal sized agarics growing amongst the woody stalks of dwarf trees, the leaves of which are often one-tenth the size of the fruit-bodies, but this is what happens.
The mycorrhizal formers in these conditions include species of Russula (e.g. Russula alpina Möller & Schaeffer, R. xerampelina var. pascua Favre (see p. 45)), Lactarius (e.g. Lactarius lacunarum Hora see p. 50), Cortinarius (e.g. C. anomalus (Fries) Fries see p. 42) and Amanita (e.g. Amanita nivalis Greville see p. 56). Subterranean fungi are also found, e.g. Elaphomyces see p. 244, and, just as woodlands, valley bottoms have a saprophytic ground flora of toadstools so do the high mountain ‘woods’, and many familiar fungi of the lowerland areas are to be found there also, e.g. Mycena epipterygia (Fries) S. F. Gray, Mycena olivaceo-marginata (Massee) Massee (see p. 88.)
The barer tops of the mountains, where large areas of moss are only to be found, support species of Hygrocybe, e.g. H. lilacina (Laestadius) Moser and H. subviolacea (Peck) P. D. Orton & Watling (see p. 97).
In the moist atmosphere on the hills in western Scotland, woodland-like floras containing familiar flowering plants are found on the mountain sides often much higher than in central Scotland. It is in such communities that typical woodland fungi are also to be found, e.g. Nolanea cetrata (Fries) Kummer (see p. 101).
[238]
Cap: width 27-75 mm. Stem: width 4-10 mm; length 35-80 mm.
Description:
Cap: convex then expanded, usually broadly umbonate, pale or dirty ochraceous paler at the margin, reddish brown at the centre, smooth, radially fibrillose towards the margin and sometimes showing the remains of a pale greyish buff veil.
Stem: equal with marginate or rounded bulb at the base, white or whitish, then becoming discoloured pinkish or brownish, powdered with white, at first, but finally silky.
Gills: free or narrowly adnate, subcrowded, whitish then clay-buff, finally snuff-brown with whitish edge.
Flesh: white or whitish, tinted ochraceous or dirty pinkish and with strong smell of rancid oil.
Spore-print: snuff-brown.
Spores: medium to long, ellipsoid-oblong, indistinctly nodulose or wavy-angular and 9-12 × 6-7 µm in size.
Facial cystidia: swollen, spindle-shaped with short, broad neck, thick-walled and crested with crystals.
Marginal cystidia: spindle-shaped and crested with crystals.
General Information: This fungus is often buried to half-way in the sand of slacks near dwarf willows (Salix spp.). Three other species of Inocybe grow in dune-slacks I. halophila Heim, I. serotina Peck and I. devoniensis P. D. Orton, but all differ in their spores being smooth and elongate-cylindric. Astrosporina, a name referring to the shape of the spore, has been considered a genus of agarics in its own right and to this group I. dunensis would belong. However, as the members show the same range of characters as those species with the smooth spores it seems unnecessary to split Inocybe into two. The cystidia in many species are unusual, being crested with a bundle of crystals which have been reported as being calcium oxalate, although even the simplest school-laboratory tests have been rarely applied to them (see p. 84).
[239]
[240]
Cap: width 20-40 mm. Stem: width 4-8 mm; length 40-80 mm.
Description: Plate 79.
Cap: semiglobate to convex, pale dingy clay-colour or dark tan to dirty brownish, non-striate, rather fleshy and usually sand covered.
Stem: deeply rooting in sand and club-shaped towards the base, similarly coloured to the cap except for the whitish apex.
Gills: adnate, subfuscous or dark dirt-brown.
Flesh: dirty buff and with no distinct smell.
Spore-print: pale snuff-brown with purplish flush.
Spores: long, ovoid, yellowish-grey brown under the microscope with a distinct germ-pore and 10-12 × 7 µm in size.
Marginal cystidia: balloon-shaped, obtuse or somewhat bottle-shaped and hyaline.
Facial cystidia: sparse, similar to the marginal cystidia, voluminous.
General Information: This is a very distinct fungus found amongst stems of Marram grass in sand-dune systems. At first sight it appears as if it is growing in the bare sand, but by careful excavation it usually is found attached to pieces of Marram grass, indeed the hyphae enter the roots of the grass, but apparently do not kill them.
This fungus was first described in the genus Psilocybe (see p. 114) because of its brownish purple spore-print, but the cap-surface is composed of rounded cells and so is related to all the other species of Psathyrella.
Psathyrella flexispora Wallace & P. D. Orton grows in similar habitats amongst Ammophila and other seashore grasses. It is easily recognised by the chocolate, umber or date-brown cap and the peculiar shaped spores, which look as if they have been slightly twisted during their development.
, resembling a little mushroom (i.e. Agaricus) is also found in sand-dune systems and, just as species of Psathyrella, it possesses purplish black spores. However, the cap is ochraceous yellow with a whitish margin formed of veil fragments. The stem is white becoming yellow with age and possesses a narrow, white striate ring. The spores are ellipsoid and measure 8-9 × 4-5 µm and it has filamentous cells in the cap. Unlike P. ammophila it is not confined to sand-dune systems but it is also to be found in pastures and on heaths.
[241]
[242]
Cap: width 10-30 mm. Stem: width 2-4 mm; length 40-100 mm.
Description: Plate 80.
Cap: conical then conico-expanded, date-brown, dull sand-colour or dark liver-colour, drying buff or ochraceous, expallent, not or indistinctly striate when moist.
Stem: whitish or pale ochraceous then darker ochraceous or dirty brownish from the base up, lower part whitish and buried in the sand.
Flesh: thin and pale ochraceous.
Gills: adnate, whitish but soon pale honey and finally rusty honey.
Spore-print: rust-brown.
Spores: long, ellipsoid or slightly amygdaliform, golden brown under the microscope with large germ-pore and 12-14 × 7-8 µm in size.
Facial cystidia: absent.
Marginal cystidia: capitate.
General Information: C. dunensis differs from C. tenera in its dull colours (see p. 116) and habitat preferences. Conocybe dunensis, Stropharia coronilla, the two species of Psathyrella are all dull-coloured. However, in the sand-dunes colourful agarics are also found. The most common is Hygrocybe conicoides (P. D. Orton) Orton & Watling; Laccaria maritima (Theodowicz) Moser is indeed an unusual but rewarding find. ‘Lac’ as in Laccaria is a red-brown resinous substrate produced by the lac-insect and resembles the cap colour of many species of the genus, including L. maritima, L. laccata and L. proxima (see p. 86). All these fungi were formerly placed in Clitocybe, but they differ in the warted or spiny spores which at maturity give the rather thick gills the appearance of being heavily talced. L. maritima can be distinguished from all other species of Laccaria by the elongated spores which are minutely spiny and not strongly warted as in L. laccata. Hygrocybe conicoides (P. D. Orton) Orton & Watling has a conical to conico-convex, acutely umbonate cap with wavy-lobed margin; it is scarlet or cherry-red, discolouring blackish with age or on bruising. The gills are at first chrome-yellow then become flushed red and the stem is yellow or greenish lemon becoming streaky blackish after handling. The spores are 10-13 × 4-5 µm in size and slightly French bean-shaped. It can be readily distinguished from close relatives, e.g. H. conica (Fries) Kummer by the gills soon turning reddish, the reddish cap and the narrow spores.
[243]
The adaptive habit of growing completely submerged beneath the surface of the ground has developed in all the major groups of fungi. Thus the simplest form related to the common bread-mould have taken up the character just as certain relatives of the disc-fungi (discomycetes) and of the flask-fungi (pyrenomycetes). In the higher fungi in several foreign countries even agarics, polypores and stinkhorns have become hypogeous, but in this country we have a very depauparate flora composed of some twenty-eight species of false (Basidiomycete) truffle. The following key may assist in identifying the different groups of hypogeous fungi for some of these species are of commercial value and includes the French or Perigord truffle, Tuber melanospermum Vittadini which is used as a constituent of Pâté de Foie Gras, and many of the fungi used as poor quality substitutes. There is a long folk-history surrounding truffles and they have been utilised in the production of aphrodisiacs for centuries. Seeking them out was a difficulty and has been overcome in different countries in different ways. Thus in continental Europe, pigs have been used to sniff them out but on finding them the pigs cannot eat the truffles because of a ring placed through their nose. In Dorset a particular breed of dog was developed to do the same job—the Dorset hounds.
A simple key would read as follows:—
[244]
Description:
Fruit-body: globular to tubiform and up to 60 mm broad, partly covered in mycelial cords, dirty white, later reddish-tawny gradually reddish and finally olive-brown, it soon becomes tawny on bruising when fresh and young.
Spores: medium sized, narrowly ellipsoid, smooth at first, hyaline then pale olive under the microscope and measuring 8-11 × 4 µm.
Habitat & Distribution: This fungus is not uncommon on the edges of paths, in pine woods just pushing up through the soil surface.
Description:
Fruit-body: globose to ovoid, 20-40 mm broad, pale ochraceous, covered in small pyramidal warts, and when it is cut it shows three layers, an outer thin yellowish zone, an inner thicker compact white zone and within this a purplish black area full of spores separated into chambers by bands of sterile white tissue; the first two zones make up the ‘rind’.
Spores: spherical, blackish brown, warty, 24-32 µm in diameter; eight contained in globose asci.
Habitat & Distribution: This fungus is not uncommon in the surface layers of pine woods at the junction of needle debris and mineral soil. E. muricatus Fries is similar, but differs in the marbled flecked interior.
Description:
Fruit-body: subglobose except for basal flattening, up to 80 mm broad, covered in 5-6-sided pyramidal scales, dark brown to violaceous, white then greyish brown within, separated by a network of veins radiating from the basal cavity.
Spores: very large, ellipsoid, light or yellowish brown and ornamented with a prominent network, borne in two’s and sixes in subglobose asci and variable in size, 20-40 × 15-30 µm.
[245]
[246]
Habitat & Distribution: This fungus is to be found buried in the surface layers of soil in beech woods. T. rufum is smaller and smoother and the spores are not crested but simply minutely spiny.
Illustrations: R. luteolus—Hvass 322; LH 215. El. granulatus—Hvass 325; LH 49. T. aestivum—LH 43. Melanogaster variegatus—LH 215 (see p. 243). Hymenogaster tener—LH 215 (see p. 243).
Description: Plate 81.
Cap: bell-shaped then becoming expanded, silky dirty white, but gradually grey with a flush of lilac with age.
Stem: slender, white and smooth except for the base.
Gills: pallid but soon becoming brownish, adnate or adnate with tooth, thick and distant alternately long and short and contorted or united with age.
Flesh: dark brown.
Spore-print: buff.
Spores: small, hyaline under the microscope, ovoid, 5-6 × 3-4 µm but usually replaced completely or in part by ovoid, smooth, thick-walled and pale brownish asexually produced spores (chlamydospores) measuring about 15 × 10 µm in size.
Habitat & Distribution: This fungus grows in clusters on old decaying specimens of various species of Russula and Lactarius (Russulaceae)—see p. 45.
General Information: N. asterophora Fries is closely related and also grows on decaying specimens of various species of Russula, particularly R. nigricans (Fries) Fries. It differs, however, in the cap being fawn-coloured and very mealy when touched; it is recognised by the poorly formed often developmentally hindered gills on which chlamydospores are formed. Unlike the smooth asexual spores in N. parasitica this species has chlamydospores with conical, blunt humps—i.e. star-shaped; asterophora in fact means ‘I bear stars’. These fungi have been associated by some mycologists with the common chanterelle (Cantharellus cibarius Fries, see p. 162) in virtue of them possessing reduced fold-like gills. However, the fold-like gills are secondary in nature, correlated with the active production[247] of chlamydospores and the suppression of the formation of basidiospores. The gills are not therefore of a primitive type. The genus Nyctalis is related to fungi such as Tephrocybe palustris (Peck) Donk (see p. 223).
There are several rather uncommon ‘agaric-parasites’ of agarics or other higher fungi, e.g. Volvariella surrecta (Knapp) Singer, but their formal description must be left to other more advanced texts. However, the intriguing bolete, Boletus parasiticus Fries, which grows on Scleroderma (earth-balls) in this country has been mentioned and figured previously (p. 35 & Plate 64). It is of interest to note that a close relative of B. parasiticus in Japan lives on another group of Gasteromycetes.
Illustrations: N. parasitica—F 11a; LH 81; WD 257. N. asterophora—LH 81; WD 258.
Many beginners are confused on finding specimens which, although appearing agaric-like, are covered in long hairs or irregularly shaped bumps. Indeed many of these abnormalities are true agarics attacked by microscopic fungi, and I know of one textbook on mushrooms and toadstools which includes such an abnormality amongst the discussion on the normal fruit-bodies. Thus Sporadinia grandis Link, which is a primitive fungus, attacks many fungi reducing them to a grey velvety mass of fungal filaments. Specimens of several species of Mycena (p. 88) are common in autumn, covered in whiskers with small nobbles on the top. These whiskers are produced by the parasitic Spinellus megalocarpus (Corda) Karsten, another primitive fungus—a phycomycete.
In some wet seasons the orange and green coloured Lactarius deliciosus (Fries) S. F. Gray is to be found contorted and covered in small pinkish to lilac pimples of the ascomycete Byssonectria lactaria (Fries) Petch, and other species of Lactarius are attacked by Byssonectria viridis (Berkeley & Broome) Petch which converts the fruit-bodies into a hardened mass of green tissue. In North America, species of Lactarius are frequently attacked by Hypomyces lactifluorum (Schweintz) Tulasne and the whole fungus is reduced to a contorted acidic-smelling mass of fungal tissue with vivid orange pimples or warts on the outer surface. These parasitic fruit-bodies are eaten as a delicacy in their own right[248] whereas the same consumer will be less enthusiastic about eating the same agaric before it is so deformed.
Boletes particularly B. subtomentosus Fries, B. chrysenteron St Amans and B. edulis Fries are frequently converted into yellow powdery masses due to the production of asexual spores of the fungus Sepedonium chrysospermum Fries; the sexual stage occurs on the remains after they have collapsed into the soil surface—this stage is called Apiocrea chrysosperma (Tulasne) Sydow. Several closely related fungi in the genus Hypomyces also attack agarics.
The yellow pustules found on the spore-bearing surface of the birch polypore Piptoporus (p. 142) is Hypocrea pulvinata Fuckel; it is only one of several lower fungi which grow on bracket fungi. The genus Cordyceps has been mentioned previously (p. 206) and in the discussion it was indicated that certain hypogeous fungi are attacked by members of this genus.
White gelatinous pustules found amongst the fruit-bodies of Stereum sanguinolentum (p. 176) have a hard white centre. On examination these ‘nuclei’ are aborted structures of the stereum covered in the jelly-fungus Tremella encephala Persoon. This fungus is apparently parasitic; it is closely related to Tremella foliacea and T. mesenterica described on page 184.
[249]
Although some fungi prefer one type of woodland more than another many fungi are less specialised and may be found in all kinds of woods. Indeed many fungi which we usually associate with a woodland fungus flora can also be commonly seen in pastures and gardens, e.g. Laccaria laccata (Fries) Cooke, Hygrophoropsis aurantiaca (Fries) Maire.
It is useful to consider the fungi of different woodland types separately, but this in some cases is very difficult because some species are not exclusive; indeed some species may grow in completely contrasting habitats, e.g. Amanita muscaria (Fries) Hooker in both birch and conifer woods, or on contrasting substrates, e.g. Fomes fomentarius (Fries) Kickx on birch in Scotland and beech on the continent of Europe. The picture becomes even more complex because frequently woods, in fact, often include several tree species growing in close proximity and it is then difficult to draw connections between a fungus and the tree with which it is truly growing—we know little or nothing except for mycorrhizal fungi, why certain fungi prefer certain habitats.
A parallel example is that phenomenon seen in certain polypores which only attack twigs or branches and not stumps or trunks, whilst others grow exclusively on stumps. We know little of the reasons for these demarcations, even when they occur within the same host. Mycology, therefore, offers to the beginner and the professional many opportunities in physiology and ecology.
In grassland areas it is difficult to know where to draw the line between one plant-community and another when listing species, for although ecologically distinct both would come under the name grassland. In the field, however, this is often very obvious and there is little doubt that fungi can give just as accurate an indication as to the soil-type, as many mosses or vascular plants. In sand-dune systems, the mobile dunes offer a different ecological niche to that of the fixed dunes which in many ways resemble grasslands. Thus although the lists below are split into easily manageable units, some flexibility must still be allowed. It is meant only as a guide—and will differ in some cases from one place to another, even within the British Isles.
[250]
[251]
characterised by species of Suillus, Chroogomphus, Gomphidius, several Lactarius and Russula spp.
[257]
[260]
Specialised colours are placed in capitals
Adnate (of the gills or tubes), broadly attached to the stem at least for one quarter of their length. See p. 267.
Adnexed (of the gills or tubes), narrowly attached to the stem by less than one quarter of their length. See p. 267.
Amygdaliform (of the spore), almond-shaped.
Amyloid (of the spore-walls, spore-ornamentation or hyphal walls), greyish or bluish or blackish violet in solutions containing iodine.
Apiculus (of the spore), the short peg-like structure at the basal end of the spore by which it is attached to the basidium. See Fig. 5, p. 15.
Arcuate-decurrent (of the gills or tubes), curved and extending down the stem. See p. 267.
Ascus, a clavate to cylindrical or subglobose cell in which the (asco-) spores are borne, usually in eights.
Basidium, a clavate or subcylindrical cell on which the (basidio-) spores are borne, externally on stalks. See Fig. 5, p. 15.
Cap (of the fruit-body), that structure which bears the spore-bearing layers beneath it (= pileus).
Caespitose (of the fruit-body), aggregated into tufts.
CINNAMON-BROWN, the colour of cinnamon powder obtainable from the grocer.
Clavate (of the stem, or cystidia), club-shaped.
Convex (of the cap), curving outwards. See Plate 9, p. 55.
Cortex (of the cap or stem), outer layers of the tissue.
Cortina, a cobweb-like veil at first connecting the margin of the cap and stem, but at maturity often only present as remnants on the stem and/or cap-margin. See p. 267.
Cystidium, a differentiated terminal cell usually on the surface and edges of the cap, gill and stem: facial cystidia occurring on the gill-face: marginal cystidia occurring on the gill-margin. See Fig. 4, p. 15.
DATE-BROWN, the colour of packed dates.
Decurrent (of the gills and tubes), with a part attached to and descending down the stem. See p. 267.
[261]
Deliquescent (of the gills, cap or entire fruit-body), changing into a liquid at maturity.
Depauperate poorly developed.
Depressed (of the cap), having the central portion sunken, and (of the tubes) sunken about the apex of the stem. See Plate 1, p. 29.
Dentate see toothed.
Distant (of the gills), greater than their own thickness apart.
Divergent (of the gill-trama in transverse longitudinal section), with the hyphae curving downwards and outwards on both sides of a central zone as if combed. See Fig. 9A, p. 17.
Ellipsoid (of the spores), elliptic in outline in all planes.
Emarginate (of the gills), notched near the stem. See Sinuate, p. 263.
Excentric (of the cap), laterally placed on the stem.
Expallent (of the cap), becoming paler when drying.
Expanded (of the cap), opened out when mature. See Plate 10, p. 61.
Fibrillose (of the cap and stem-surfaces), almost smooth but for distinct parallel longitudinal filaments (fibrils).
Fleshy (of the fruit-body), of a rather soft consistency: readily decaying.
Floccose, with loose, cottony surface; diminutive—flocculose.
Free (of the gills and tubes), not attached to the stem. See p. 267.
Frondose trees, broad-leaved trees.
Fruit-body, the whole agaric (toadstool or mushroom, polypore, etc.), as usually understood.
Germ-pore, a differentiated apical, usually thin-walled portion of the spore. See Fig. 5, p. 15.
Gill, the structure on which the reproductive tissue is borne in agarics, resembling plates.
Globose (of the spore), round in outline in all planes.
Glutinous (of the cap or stem), provided with a sticky jelly-like coating.
Heteromerous (of the cap and stem-flesh), with discrete nests of rounded cells in a background of filamentous cells: characterises members of the Russulaceae. See Fig. 10B, p. 17.
Homoiomerous (of the cap and stem-flesh), not sharply differentiated into two types of cells, although some may be swollen: characterises agarics other than members of the Russulaceae. See Fig. 10A, p. 17.
Hygrophanous (of the cap), translucent when wet, opaque and often paler on drying.
Hymenium, the superficial layer of cells in which basidia occur. See Fig. 9A-D, p. 17.
[262]
Hyaline, appearing as if clear glass.
Hypogeous, growing under ground.
Hypha, a fungus filament composed of a chain of several cells; plural—hyphae; adjective—hyphal.
Inverse, (of the gill-trama in transverse longitudinal section), with the hyphae curving upwards and outwards on both sides of a central zone. See Fig. 9B, p. 17.
Irregular (of the gill-trama in transverse longitudinal section), lacking any clear pattern as to hyphal arrangement. See Fig. 9D, p. 17.
Mealy, covered in powdery granules, resembling meal.
Mycelium, a mass of fungus-filaments (hyphae).
Mycorrhiza, a symbiotic association of a fungus and the roots of a higher plant.
Non-amyloid (of the spore-wall, spore-ornamentation and hyphal walls), remaining uncoloured or becoming yellowish in solutions containing iodine.
OCHRACEOUS, bright clay-colour: colour of ochre (yellow-brown).
OLIVACEOUS BROWN, a dull clay-brown with an additional but distinct hint of dirty green.
Plano-convex (of the cap), regularly rounded although almost flat. See Plate 13, p. 67—adult fruit-body.
Pruinose (of the cap and stem-surfaces), finely powdered.
Pubescent (of the cap and stem-surfaces), with short, soft hairs.
Putrescent (of the fruit-body), soft and very easily decaying.
Pyriform (of the spore), pear-shaped.
Regular (of the gill-trama in transverse longitudinal section), with hyphae showing no distinct curvature and practically parallel to the gill-surfaces. See Fig. 9C, p. 17.
Remote (of the gills or tubes), separate from the stem by a zone of cap-flesh. See p. 267.
Resupinate (of the fruit-body), spore-bearing tissue facing outward and attached to support by what would have been the cap had the fungus been a normal agaric.
Ring, a girdling veil on the stem. See p. 267.
Rugulose (of a surface), covered in small wrinkles.
RUST-BROWN, the colour of rusty iron.
Saprophyte (of an organism), using dead material for active growth.
Scurfy (of the cap and stem surfaces), with small irregular loosely attached scales.
[263]
Sessile (of the fruit-bodies), lacking a stem.
Septate (of the structural units of the fruit-body), with cross-walls; septum—cross-wall.
Sinuate (of the gills), having a concave indentation of that part of the edge nearest the stem. See Plate 32, p. 111.
SNUFF-BROWN, a dull dark clay-brown said to resemble the colour of snuff.
Spore-print (or deposit), the mass of spores obtained by allowing the fruit-body to discharge its spores at maturity.
Stem (of the fruit-body), that structure which supports the cap (= stipe).
Sterile, a tissue or structure not involved in the reproductive process, or failing to take part.
Sterigma, the point-like structure at the apex of the basidium actually bearing the spores.
Striate (of a surface), having minute furrows or lines.
Subdecurrent (of the gills or the tubes), having the gill-attachment extending slightly down the stem. See p. 267.
TAWNY, sand-coloured.
Tomentose (of the cap and stem surfaces), densely matted and woolly.
Toothed (of the gills or cap-margin), as if with teeth (= dentate).
Trama (of the gills), the tissue between the layers bearing basidia (hymenia).
Umbilicate (of the cap), having a central, small depression. See p. 267.
Umbonate (of the cap), provided with a broad, flattened, raised centre (the umbo).
Uncinate (of the gills), emarginate, but with a long descending decurrent tooth because the cap does not expand. See Plate 14, p. 69.
Veil, a general term for the tissues which protect the whole or part of the developing fruit-body.
Viscid (of the cap or stem), very slippery to the touch.
Volva, a persistent cup-like structure at the base of the stem. See p. 267.
Waxy (of the gills), lustrous because they are thick and watery.
[264]
Text-figures and line-drawings of the greater number of the fungi mentioned in the text have been included in the book. It is impossible to supply colour pictures of a high quality in a book such as this without raising the price of the publication astronomically. The plates in six easily obtainable popular books have been used to represent whenever possible the fungus described in the text, as accurate colour illustrations are very useful in identification. The titles of these books have been abbreviated for clarity.
F—Findlay, W. P. K. (1967), Wayside and Woodland Fungi, London.
Hvass—Hvass, E. & H. (1961), Mushrooms and Toadstools in Colour, London.
LH—Lange, M. & Hora, F. B. (1963), Collins Guide to Mushrooms and Toadstools, London.
NB—Nicholson, B. E. & Brightman, F. H. (1966), Oxford Book of Flowerless Plants, Oxford.
WD—Wakefield, E. & Dennis, R. W. G. (1950), Common British Fungi, London.
Z—Zeitlmayr, L. (1968), Wild Mushrooms, London.
Object: To assess the annual radial growth of fairy-rings and to correlate this with any obvious environmental change.
Materials: Graph and tracing papers, tape-measures, note-book, pencil and rule, small pieces of cane about four inches long and coloured dye (e.g. Eosin solution, Janus Green).
Method: Select a fairy-ring on the school cricket pitch or hockey pitch, school lawn, local golf course or park at a time when the fruit-bodies are first visible. Carefully mark the centre of the ring by driving into the soil a piece of cane until the top is only just visible. Plot this point on graph paper and relate it to any prominent feature nearby, e.g. post, tree or hedge.
Carry out weekly observations throughout the fruiting season[265] plotting the individual fruit-bodies on tracing paper, which is trimmed so as to make a replica of the original graph-sheet. A small dab of coloured dye placed on a fruit-body will assist one in recognising fruit-bodies from previous observations. During the fruiting season observe and plot the zones of differently coloured vegetation—devise some method of describing (and measuring) these colours perhaps by comparison with a colour-chart, printed or hand prepared. Continue observations on the ring at monthly or fortnightly intervals after the disappearance of the fruit-bodies, and record subsequent changes in the vegetation for twelve months.
This project can be continued for several years and for different species of fungus. Weather conditions may be noted simultaneously with the growth observations, or obtained from a reliable source of similar information close by. In this way not only is the increase in ring size measured but the results can be considered in the light of climatic data; fungal growth appears to be dependent on favourable weather conditions.
Further experiments:
[266]
In the soil or substrate the hyphae of agarics frequently grow in close contact with each other, indeed the intertwining of such hyphae to form small knots is common in many fungi. In these intertwining hyphae, those close together divide and branch, later branching again to form a heap of tissue. The fruit-body develops from, or within, this knot and at its earliest stage is usually covered by loosely branched and irregularly arranged hyphae. To the unaided eye the primordium, for this is what such a structure or early beginning is called, appears to be enveloped in a mass of pale hyphal strands, often giving the fruit-body a woolly appearance when seated on the soil, wood, herbaceous debris, etc. If more than one primordium develops in close proximity, usually all but one abort early in development, or they remain checked in formation at this stage until those close by have matured. Some species which grow on wood are caespitose, that is clustered together, and in these cases all or many more of the primordia develop fully and simultaneously.
Often it is possible to search and find these primordia in the fields and woods, and if they are examined under the low-power of a microscope it is possible to study how the fruit-body subsequently develops from its small beginnings and the part played by the ring and volva in the development determined. Thus the origin of the veil can be located, its development followed as well as its disintegration. When the fungus is grown in pure culture on sterile dung, or soil, or wood, or simply on artificial media prepared in the laboratory the full sequence of events can be more easily followed. This is how the professional mycologist conducts his observations. By very careful studies it has been found in recent years that the development of the fruit-body, the origin of the gills, etc. can assist in the classification of the higher fungi. Thus some species have no protective tissue around the developing gills (gymnocarpic) whilst others have one or even two, simple or complex, tissues around the developing gills or pores (hemiangiocarpic). It is these tissues which give rise to the ring, volva, cortina, etc. This most exciting part of the study of the higher fungi is illustrated in the accompanying figures (Figs. 12 & 13) along with the various types of gill-attachment mentioned in the text (Fig. 11 A-H). If the agaric has two tissues surrounding it as the cap expands and matures, first the outer tissue or skin breaks leaving pieces on the stem and/or cap and then the second skin breaks as the cap expands still further. The last skin leaves remnants on the stem and sometimes bits and pieces at the cap margin. Only now can the agaric shed its spores from the fully exposed gills.
[269]
Some references have already been given on p. 264. Findlay, Hvass & Hvass, Lange & Hora, Nicholson & Brightman, Wakefield and Dennis and Zeitlmayr.
In addition to these the following texts are suggested:
Henderson, D. M., Orton, P. D. & Watling, R. (1969). British Fungus Flora: Agarics and Boleti: Introduction, H.M.S.O., Edinburgh.
Hennig, E. (1958-60). Handbuch für Pilzfreunde, Jena (in German).
Haas, H. (1969). The Young Specialist looks at Fungi, London.
Pilát, A. & Usak, O. (1951). Mushrooms, London.
Pilát, A. & Usak, O. (1961). Mushrooms and other fungi, London.
Ramsbottom, J. (1951). Handbook of Larger fungi, London.
Ramsbottom, J. (1953). Mushrooms and Toadstools, New Naturalist, London.
Romagnesi, H. (1963). Petit Atlas des Champignons, Bordas (in French).
Smith, A. H. (1963). Mushroom Hunters’ Field-guide, Michigan.
Wakefield, E. M. (1954). Observer’s book of Common fungi, London.
Watling, R. (1970). British Fungus Flora: Agarics & Boleti, Part I, H.M.S.O., Edinburgh.
Talbot, P. M. B. (1971). Principles of Fungal Taxonomy, London.
Webster, J. (1970). Introduction to Fungi, Cambridge.
Bulletin Trimestriel de la Société Mycologique de France, Paris. (Official organ of the French Mycological Society.)
Coolia, Leiden. (Official organ of the Dutch Mycological Society.)
Mycologia, New York. (Official organ of the American Mycological Society.)
Schweizerische Zeitschrift für Pilzkunde. (Official organ of the Swiss Mycological Society.)
[270]
Transactions of the British Mycological Society, (Official organ of the British Society: Hon. Sec. Dr B. E. Wheeler, Imperial College of Science and Technology Field Station, Silwood Park, Sunninghill, Ascot, Berks, also publishes a Bulletin intended for the amateur.)
Dennis, R. W. G., Orton, P. D. & Hora, F. B. (1960). New Check List of British Agarics and Boleti, suppl. Trans. British Mycological Soc.
Moser, M. (1967). in Gams Kleine Kryptogamenflora, Band IIb Stuttgart (in German).
Kühner, R. & Romagnesi, H. (1953). Flore Analytique des Champignons Supérieurs de France, Paris (in French).
Rea, C. (1922). British Basidiomycetae, Cambridge.
Revue de Mycologie (journal) Paris (in French).
[271]
Numbers in bold italics refer to pages with illustrations.

Inconsistent formatting, spelling and hyphenation have been retained, except as listed below.
Links to larger images are provided (available in browser html, but not necessarily in other formats; see www.gutenberg.org).
The differences between the Table of Contents and the body text have not been standardised, except as mentioned below.
The name Léville may be a variant or misspelling of (Joseph-Henri) Léveillé.
Page 10, where man has distributed the habitat: possibly an error for ... disturbed the habitat.
Page 49, changing soot-colour: possibly an error for changing to soot-colour.
Page 68, to reclaim Helen his wife: Helen was his brother’s wife.
Page 88, blotched age: there may be one or more words missing.
Page 104, Many authorities prefer ...: a closing parenthesis is missing.
Page 187, non-violent disposal of spores: possibly an error for dispersal.
Changes made:
Illustrations have been moved out of text paragraphs and some lists and tables.
Some obvious minor misprints and typographical and punctuation errors have been corrected silently.
Page 22: reference to key 24 changed to key 25
Page 27: width 70-200 mm; length 20-30 mm changed to length 70-200 mm; width 20-30 mm
Page 45: Stem: changed to Stem:
Page 52: mm inserted after 20-50
Page 95: H. subradiat changed to H. subradiata;
Page 98: H. calytraeformis changed to H. calyptraeformis
Page 158: Hyndum changed to Hydnum
Page 174: 8-9 · 6-7 µm changed to 8-9 × 6-7 µm
Page 202: Marchella changed to Morchella; Léveille changed to Léville
Page 207: (i) added in section heading Fungi of dung and straw heaps
Page 212: patoullardii changed to patouillardii
Page 222: (a) added in section heading Sphagnum bogs
Page 231: (a) added in section heading Moorland fungi
Page 236: (b) added in section heading Mountain fungi and the so-called Basidiolichens
Page 256: G. glutinosum changed to G. glutinosus; Marchella changed to Morchella
Page 257: Hebeoloma anthracophilum changed to Hebeloma anthracophilum; Tephrocybe arthracophila changed to Tephrocybe anthracophila; Myxomphalina changed to Myxomphalia
Page 262: closing parenthesis added after fruit-body
Page 269, Bulletin Trimestriel ... formatted as other journals
Page 270: Flore Analytique des Champignons Superiéurs de France changed to Flore Analytique des Champignons Supérieurs de France
Index: some entries moved to proper alphabetical order
Page 271: Baespora changed to Baeospora; Bjerkandera adjusta changed to Bjerkandera adusta; pantharina changed to pantherina
Page 273: serioceocybe changed to sericeocybe
Page 274: tibiicytis changed to tibiicystis
Page 275: chrysorhaeus changed to chrysorheus
Page 276: Myxomphalina changed to Myxomphalia
Page 277: vesciculosa changed to vesiculosa
Page 280: Paté de Foi Gras changed to Pâté de Foie Gras
End of Project Gutenberg's Identification of the Larger Fungi, by Roy Watling
*** END OF THIS PROJECT GUTENBERG EBOOK IDENTIFICATION OF THE LARGER FUNGI ***
***** This file should be named 60159-h.htm or 60159-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/6/0/1/5/60159/
Produced by MFR, Eric Lehtonen, Harry Lamé and the Online
Distributed Proofreading Team at http://www.pgdp.net. This
file was produced from images generously made available
by The British Mycological Society and special thanks and
appreciation are extended to the Author and Editor of the
book for granting permission to release it to the public
domain.
Updated editions will replace the previous one--the old editions
will be renamed.
Creating the works from public domain print editions means that no
one owns a United States copyright in these works, so the Foundation
(and you!) can copy and distribute it in the United States without
permission and without paying copyright royalties. Special rules,
set forth in the General Terms of Use part of this license, apply to
copying and distributing Project Gutenberg-tm electronic works to
protect the PROJECT GUTENBERG-tm concept and trademark. Project
Gutenberg is a registered trademark, and may not be used if you
charge for the eBooks, unless you receive specific permission. If you
do not charge anything for copies of this eBook, complying with the
rules is very easy. You may use this eBook for nearly any purpose
such as creation of derivative works, reports, performances and
research. They may be modified and printed and given away--you may do
practically ANYTHING with public domain eBooks. Redistribution is
subject to the trademark license, especially commercial
redistribution.
*** START: FULL LICENSE ***
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full Project
Gutenberg-tm License (available with this file or online at
http://gutenberg.org/license).
Section 1. General Terms of Use and Redistributing Project Gutenberg-tm
electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or destroy
all copies of Project Gutenberg-tm electronic works in your possession.
If you paid a fee for obtaining a copy of or access to a Project
Gutenberg-tm electronic work and you do not agree to be bound by the
terms of this agreement, you may obtain a refund from the person or
entity to whom you paid the fee as set forth in paragraph 1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this agreement
and help preserve free future access to Project Gutenberg-tm electronic
works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the Foundation"
or PGLAF), owns a compilation copyright in the collection of Project
Gutenberg-tm electronic works. Nearly all the individual works in the
collection are in the public domain in the United States. If an
individual work is in the public domain in the United States and you are
located in the United States, we do not claim a right to prevent you from
copying, distributing, performing, displaying or creating derivative
works based on the work as long as all references to Project Gutenberg
are removed. Of course, we hope that you will support the Project
Gutenberg-tm mission of promoting free access to electronic works by
freely sharing Project Gutenberg-tm works in compliance with the terms of
this agreement for keeping the Project Gutenberg-tm name associated with
the work. You can easily comply with the terms of this agreement by
keeping this work in the same format with its attached full Project
Gutenberg-tm License when you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are in
a constant state of change. If you are outside the United States, check
the laws of your country in addition to the terms of this agreement
before downloading, copying, displaying, performing, distributing or
creating derivative works based on this work or any other Project
Gutenberg-tm work. The Foundation makes no representations concerning
the copyright status of any work in any country outside the United
States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other immediate
access to, the full Project Gutenberg-tm License must appear prominently
whenever any copy of a Project Gutenberg-tm work (any work on which the
phrase "Project Gutenberg" appears, or with which the phrase "Project
Gutenberg" is associated) is accessed, displayed, performed, viewed,
copied or distributed:
This eBook is for the use of anyone anywhere at no cost and with
almost no restrictions whatsoever. You may copy it, give it away or
re-use it under the terms of the Project Gutenberg License included
with this eBook or online at www.gutenberg.org/license
1.E.2. If an individual Project Gutenberg-tm electronic work is derived
from the public domain (does not contain a notice indicating that it is
posted with permission of the copyright holder), the work can be copied
and distributed to anyone in the United States without paying any fees
or charges. If you are redistributing or providing access to a work
with the phrase "Project Gutenberg" associated with or appearing on the
work, you must comply either with the requirements of paragraphs 1.E.1
through 1.E.7 or obtain permission for the use of the work and the
Project Gutenberg-tm trademark as set forth in paragraphs 1.E.8 or
1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any additional
terms imposed by the copyright holder. Additional terms will be linked
to the Project Gutenberg-tm License for all works posted with the
permission of the copyright holder found at the beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including any
word processing or hypertext form. However, if you provide access to or
distribute copies of a Project Gutenberg-tm work in a format other than
"Plain Vanilla ASCII" or other format used in the official version
posted on the official Project Gutenberg-tm web site (www.gutenberg.org),
you must, at no additional cost, fee or expense to the user, provide a
copy, a means of exporting a copy, or a means of obtaining a copy upon
request, of the work in its original "Plain Vanilla ASCII" or other
form. Any alternate format must include the full Project Gutenberg-tm
License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works provided
that
- You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is
owed to the owner of the Project Gutenberg-tm trademark, but he
has agreed to donate royalties under this paragraph to the
Project Gutenberg Literary Archive Foundation. Royalty payments
must be paid within 60 days following each date on which you
prepare (or are legally required to prepare) your periodic tax
returns. Royalty payments should be clearly marked as such and
sent to the Project Gutenberg Literary Archive Foundation at the
address specified in Section 4, "Information about donations to
the Project Gutenberg Literary Archive Foundation."
- You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or
destroy all copies of the works possessed in a physical medium
and discontinue all use of and all access to other copies of
Project Gutenberg-tm works.
- You provide, in accordance with paragraph 1.F.3, a full refund of any
money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days
of receipt of the work.
- You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project Gutenberg-tm
electronic work or group of works on different terms than are set
forth in this agreement, you must obtain permission in writing from
both the Project Gutenberg Literary Archive Foundation and Michael
Hart, the owner of the Project Gutenberg-tm trademark. Contact the
Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
public domain works in creating the Project Gutenberg-tm
collection. Despite these efforts, Project Gutenberg-tm electronic
works, and the medium on which they may be stored, may contain
"Defects," such as, but not limited to, incomplete, inaccurate or
corrupt data, transcription errors, a copyright or other intellectual
property infringement, a defective or damaged disk or other medium, a
computer virus, or computer codes that damage or cannot be read by
your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH 1.F.3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium with
your written explanation. The person or entity that provided you with
the defective work may elect to provide a replacement copy in lieu of a
refund. If you received the work electronically, the person or entity
providing it to you may choose to give you a second opportunity to
receive the work electronically in lieu of a refund. If the second copy
is also defective, you may demand a refund in writing without further
opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS' WITH NO OTHER
WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO
WARRANTIES OF MERCHANTABILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of damages.
If any disclaimer or limitation set forth in this agreement violates the
law of the state applicable to this agreement, the agreement shall be
interpreted to make the maximum disclaimer or limitation permitted by
the applicable state law. The invalidity or unenforceability of any
provision of this agreement shall not void the remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in accordance
with this agreement, and any volunteers associated with the production,
promotion and distribution of Project Gutenberg-tm electronic works,
harmless from all liability, costs and expenses, including legal fees,
that arise directly or indirectly from any of the following which you do
or cause to occur: (a) distribution of this or any Project Gutenberg-tm
work, (b) alteration, modification, or additions or deletions to any
Project Gutenberg-tm work, and (c) any Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of computers
including obsolete, old, middle-aged and new computers. It exists
because of the efforts of hundreds of volunteers and donations from
people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need, are critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future generations.
To learn more about the Project Gutenberg Literary Archive Foundation
and how your efforts and donations can help, see Sections 3 and 4
and the Foundation web page at http://www.pglaf.org.
Section 3. Information about the Project Gutenberg Literary Archive
Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Its 501(c)(3) letter is posted at
http://pglaf.org/fundraising. Contributions to the Project Gutenberg
Literary Archive Foundation are tax deductible to the full extent
permitted by U.S. federal laws and your state's laws.
The Foundation's principal office is located at 4557 Melan Dr. S.
Fairbanks, AK, 99712., but its volunteers and employees are scattered
throughout numerous locations. Its business office is located at
809 North 1500 West, Salt Lake City, UT 84116, (801) 596-1887, email
[email protected]. Email contact links and up to date contact
information can be found at the Foundation's web site and official
page at http://pglaf.org
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
[email protected]
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To
SEND DONATIONS or determine the status of compliance for any
particular state visit http://pglaf.org
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including checks, online payments and credit card donations.
To donate, please visit: http://pglaf.org/donate
Section 5. General Information About Project Gutenberg-tm electronic
works.
Professor Michael S. Hart is the originator of the Project Gutenberg-tm
concept of a library of electronic works that could be freely shared
with anyone. For thirty years, he produced and distributed Project
Gutenberg-tm eBooks with only a loose network of volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as Public Domain in the U.S.
unless a copyright notice is included. Thus, we do not necessarily
keep eBooks in compliance with any particular paper edition.
Most people start at our Web site which has the main PG search facility:
http://www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.