
The cover image was created by the transcriber and is placed in the public domain.
The Project Gutenberg EBook of Useful Knowledge: Minerals. Volume 1 (of 3)., by
William Bingley
This eBook is for the use of anyone anywhere in the United States and most
other parts of the world at no cost and with almost no restrictions
whatsoever. You may copy it, give it away or re-use it under the terms of
the Project Gutenberg License included with this eBook or online at
www.gutenberg.org. If you are not located in the United States, you'll have
to check the laws of the country where you are located before using this ebook.
Title: Useful Knowledge: Minerals. Volume 1 (of 3).
or A familiar account of the various productions of nature
Author: William Bingley
Release Date: September 14, 2018 [EBook #57898]
Language: English
Character set encoding: UTF-8
*** START OF THIS PROJECT GUTENBERG EBOOK USEFUL KNOWLEDGE: MINERALS, VOL 1 ***
Produced by Chris Curnow, Barry Abrahamsen, and the Online
Distributed Proofreading Team at http://www.pgdp.net (This
file was produced from images generously made available
by The Internet Archive)

The cover image was created by the transcriber and is placed in the public domain.

Comparative Height of Mountains, Cities and Lakes
British Islands Continent of Europe Islands not British Asia America
(Click on image to see a larger version.)
J. Shury sculp.

The mode in which instruction has hitherto been conveyed, on the peculiar subjects of the present work, has chiefly been by small books, in question and answer, denominated catechisms. But such, however respectable in themselves, or however advantageous for children, are wholly insufficient for persons who are in search of extended knowledge, and desirous of furnishing their minds with useful information.
On these subjects there has not hitherto been published any work in which they are collectively to be found; nor could a knowledge of them be obtained but by the consultation of many and expensive writings. That they are generally important to be known will not probably be denied.
It has consequently been the object of the author to compress all the interesting information that could be obtained respecting them, within as narrow a compass, and at the same time to render this information as entertaining, and as devoid of technical words and phrases, as possible.
The scheme of the work will, it is hoped, be found sufficiently simple. The passage in smaller characters at the head of each article, is in general ivso arranged as to reply to the questions, “What is?” “What are?” or “How do you know?” For instance: “What is flint?” (See Vol. I. p. 53.) The answer will be found thus: “Flint is a peculiarly hard and compact kind of stone, generally of smoke-grey colour, passing into greyish white, reddish, or brown. It is nearly thrice as heavy as water, and, when broken, will split in every direction, into pieces which have a smooth surface.” The author is aware that, in many instances, the definitions are defective: but this has, in general, arisen from a necessity of rendering them short, and at the same time of using such terms as would be likely to convey information to the minds of persons who have had no previous knowledge of the systems of natural history.
After the definition, a further illustration sometimes follows; and in the large characters will be found a brief detail of the history and uses of the object described. The articles are numbered, for the greater convenience both of reference and explanation, but particularly the latter. Thus, under the explanation of Carbon, it is stated that “in combination with oxygen (21) it forms carbonic acid (26), and that it is the chief component part of pit-coal (217), petroleum (213), and other bituminous substances.” By a reference to the vnumbers inserted, each of the words, against which they stand, will be explained: whilst at least three of them would otherwise have been incomprehensible by the generality of unscientific readers.
It must be remarked that the reader will not here find an account of every production of nature, which is employed for the use of man, nor even all the uses of such objects as are described. The most important of the productions, and the principal of the uses, are all that he trusts can reasonably be required in a work of the present extent. On this ground it is that a great number of animals, which are in request only for food, have been wholly omitted.
The figures that are inserted have been drawn upon as small and economical a scale as was compatible with a sufficiently accurate representation of the objects to which they relate. If the reader be desirous of reference to further illustration, he will derive much satisfaction from the invaluable figures of Mr. Sowerby in his British and Exotic Mineralogy, and English Botany, and Woodville’s Medical Botany; as well as from those in Dr. Shaw’s General Zoology, and Bewick’s Histories of Quadrupeds and British Birds. There are also many figures of useful animals in the author’s own work, entitled “Memoirs of British Quadrupeds.”
Since this work was first printed, the author has made in it considerable improvements. The first volume, particularly, contains many additional articles, and more than half of it has been re-written. The plates also have been re-engraved. For the plate of the mountains a new drawing has been made, that the scale might be extended, and many particulars might be introduced which before were omitted. For the plates of vegetables every drawing has been corrected; and, in place of such figures as were most defective, new ones have been inserted.
| Fig. | MINERAL DEPOSITS. |
| 1. | Horizontal beds or strata. |
| a. Veins or dykes. | |
| 2. | Bending strata. |
| 3. | Minerals in detached masses. |
| 4. | Disjoined strata. |
| b. A fault. |
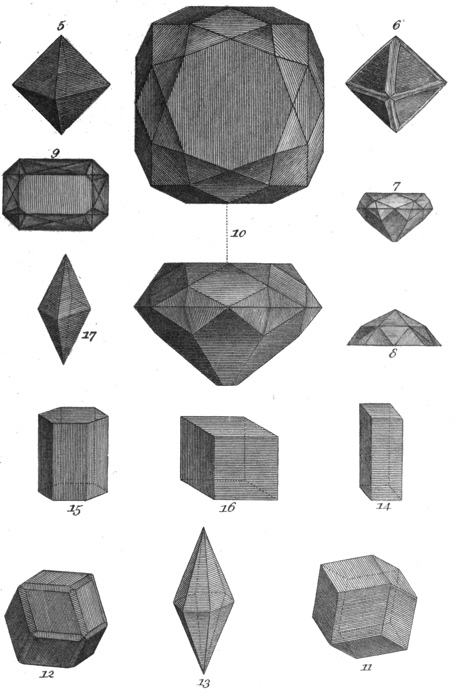
| 5. | Octohedron. |
| 6. | Rough diamond. |
| 7. | Profile of a brilliant-cut stone. |
| 8. | Profile of a rose-cut stone. |
| 9. | Plane of a table-cut stone. |
| 10. | Plane and profile of the Pitt diamond. |
| 11. | Dodecahedron. |
| 12. | Rough garnet. |
| 13. | Six-sided pyramids, joined base to base. |
| 14 | Regular four-sided prism. |
| 15. | Six-sided prism. |
| 16. | Cube. |
| 17. | Four-sided pyramid having a rhomb for its base. |
| Fig. | |
| 1. | Granite. |
| 2. | Gneiss. |
| 3. | Mica-slate. |
| 4. | Clay-slate. |
| a. Lime-stone. | |
| b. Quartz. | |
| 5. | Primitive lime-stone. |
| 6. | Grey-wacka. |
| 7. | Transition lime-stone. |
| 8. | Old red sand-stone. |
| 9. | Alternating strata of lime-stone and sand-stone. |
| 10. | Alluvial strata of clay, gravel, &c., &c. |
The BINDER is desired to insert all the Plates, except the Frontispieces, immediately after the Explanations in the respective Volumes.

Pl. 1. Vol. I.
J. Shury. sculp.
Sections of Strata &c.
Pl. 2. Vol. I.
CRYSTALS &c.

Pl. 3. Vol. I.
Section of Rocks. J Shury sculp.
1. Minerals are natural bodies destitute of organization and life: and Mineralogy is that branch of natural science which treats of the properties and relations of such bodies.
2. If we penetrate beneath the surface of the earth, we observe there a very remarkable arrangement. Instead of a generally uniform appearance, as we see on the surface, we pass through divers substances, as clay, gravel, sand, and numerous others, deposited in beds or strata of various thickness, from a few inches to a great many feet (Pl. I. Fig. 1). These lie, for the most part, nearly horizontal: but in some instances, particularly in mountainous countries, they take different degrees of inclination; and, in places where the country consists of gently sloping hills and vales, the beds have a waving or bending form (Pl. 1, Fig. 3). The strata of which the earth is composed, as deep as the curiosity or the necessities 2of mankind have induced them to explore, satisfactorily demonstrate the wisdom which has been displayed in the arrangement of materials requisite for the use of men and animals.
The first layer is frequently a rich, black mould, formed almost wholly of animal and vegetable remains. This yields sustenance to the vegetable productions; and thereby becomes the actual, though not the immediate, support of the whole animal creation.—Beneath this is often found a thick bed of clay, that furnishes to man a substance of which to make bricks, tiles, various kinds of pottery, and innumerable other articles for the comfort of social life.—Next are deposited vast beds of gravel, that are of use in numerous points of view.—Underneath this are the infinitely varying strata of sandstone, limestone, &c. which not only serve for the construction of buildings, and for other important purposes, but also frequently surround mines which contain the valuable metals.—Beneath a slaty stratum are usually discovered those immense beds of coal so requisite for the comfort, and, in some situations, even for the existence of man.
These strata, it is true, are not always found together, nor are they always discovered in the same order; but the statement will suffice to show the general nature of their arrangement.
3. Minerals are sometimes observed in detached masses of various size, and situated at various depths in the earth (Pl. I, Fig. 1).
4. They are also found in a kind of natural clefts which cross the regular mineral beds or strata in different directions (Pl. I, Fig. 1, a, Pl. I, Fig. 4, b). When these 3contain metallic ores, they are styled veins; but when they contain only stony or earthy matters, the miners call them dykes. They vary much both in magnitude and length. Six thousand feet are considered an unusual length for veins, though, in some instances, veins have been traced upwards of four miles. Few veins extend more than 1200 feet below the surface of the mountains in which they are situated. They are usually much inclined; but they sometimes descend in a direction parallel with the beds of rock in which they occur.
5. At the places where dykes or veins pass through the earth, they occasionally disjoint the strata in a very singular manner (Pl. I, Fig. 4). Some of the coal strata, for instance, are thrown down or raised on one side of a dyke upwards of a hundred yards; and the miner, after penetrating through this dyke, instead of finding the same coal again, meets, on the opposite side, with beds of stone or clay. Hence he is frequently at a loss how to proceed in searching for the coal of which he is in pursuit; and hence it is that to such dykes the peculiar name of faults has sometimes been given.
6. In England the metallic ores are generally found in veins, that form a considerable angle with the regular strata. This in Cornwall is uniformly the case. And it is remarkable, concerning the veins of tin and copper of that county, that they run in a direction nearly east and west; whilst the dykes, or veins of other substances, run for the most part north and south.
7. The thickness of veins, and the quantity and quality of the ores they contain, differ in every mine. Some are only a few inches wide, whilst others extend 4to the width of several feet. The vein at Dalcooth mine, in Cornwall, varies from two or three to forty feet and upwards; and, in some parts, it contracts so as to be little more than six inches across.
8. In Cornwall the first traces of tin and copper are usually found at the surface of the ground, and thence to the depth of 80 or 100 feet beneath; and it is said that no miner has ever yet seen the bottom of a vein, although several have been wrought to the depth of more than 1000 feet. The veins of these metals have, in some instances, been worked to the length of three or four miles.
9. It is frequently observed that metallic veins are separated, from the substances they intersect, by a thin wall, or lining, of minerals different from these substances, and also by a layer of clay on each side of the vein. It is also remarked that the same substance which forms the outer coat of the vein is often intermixed with the ore, or forms layers alternately with it. This has usually the denomination of matrix or gangue.
10. There are few mines of any considerable depth that would not be flooded with water from internal springs were not means adopted for drawing off this fluid. The steam engines that are employed for this purpose in some of the Cornish mines are so powerful as to discharge incessantly, both by night and day, a quantity of water, equal to at least 1000 gallons, or near twenty hogsheads, every minute.
11. To a superficial observer, perhaps nothing would appear more easy than to describe a mineral. This, 5however, is by no means the case. The same general appearance sometimes prevails in substances that are very different from each other; and the same stone, in its different states, is often extremely varied in its appearance. To these difficulties it must be added, that the combinations of mineral substances are multiplied to a great extent. A little application, however, particularly if the student be possessed of a collection of arranged and named specimens, which he will have no difficulty in procuring at a reasonable price, will enable him to overcome all the obstacles that otherwise might impede his progress in beginning to acquire a knowledge of this interesting science.[1]
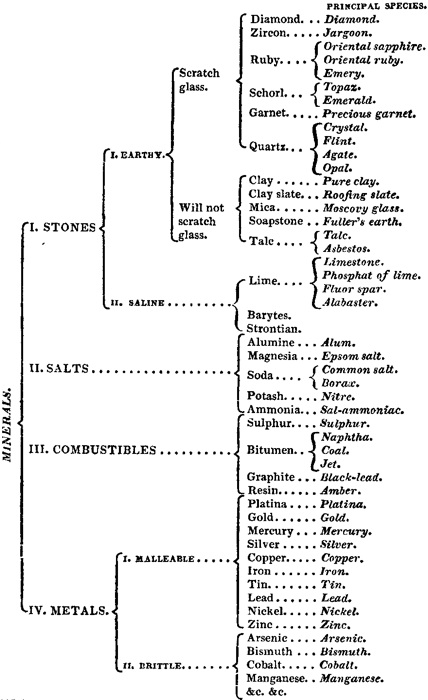
12. The most simple and natural division of minerals is into four classes, of, 1. Stones; 2. Salts; 3. Combustibles; and, 4. Metals; and the following table, which has chiefly been arranged from the system of Werner, the well-known German mineralogist, will exhibit a tolerably correct outline of the classification of these substances. To reduce the whole within the compass of a single page, many of the families, however, have necessarily been omitted.
1. Such collections are supplied by Mr. Mawe, No. 149, Strand, London. His terms, for collections containing from 100 to 200 specimens, are 5 guineas; from 200 to 300 specimens, 10 guineas; and from 300 to 400 specimens, 15 guineas. For collections containing from 350 to 400 specimens, more select, and comprising a better suite of precious stones, he charges from 20 to 30 guineas; and for larger collections, from 50 to 100 guineas. At the particular request of the author, Mr. Mawe has arranged a few collections of minerals, and numbered them in such manner as to correspond with, and illustrate the present volume.
713.To complete a general view of the different productions of the mineral kingdom, it is requisite to subjoin a tabular arrangement of the various kinds of rocks.

8
14. For the purpose of ascertaining the names and characters of minerals, attention must be paid to their form, surface, lustre, fracture, or the appearance of their internal surface when broken; structure, transparence, streak, or the mark left when scratched by any hard body; stain, or trace left when rubbed upon paper; cohesion, whether solid, friable, or fluid: hardness, or the resistance which they oppose when scratched; tenacity, or the resistance which they oppose to the stroke of a hammer; flexibility, or their property of bending without breaking; feel, or the sensation communicated by their surfaces when handled; smell, taste, adherence to the tongue, sound, specific gravity, or weight in comparison with that of water; colour and electricity.
15. To ascertain the chemical properties of minerals, one of the most important instruments is the blow-pipe. This is a tube which terminates in a cavity as fine as a small wire, and through which the air is forced, and made to play upon the flame of a candle. The flame is thus concentrated, and directed against small particles of the mineral to be examined, which is placed upon a bit of charcoal in a spoon of platina or silver. The air is forced into the blow-pipe by the mouth of the person using it, or by bellows attached to it for that purpose. Under this operation we have an opportunity of trying the action of other bodies upon minerals at a very high temperature; and the properties which these experiments bring into view enable us, in many cases, to ascertain, not only the nature, but even the component parts, of minerals.
16. As a necessary introduction to the study of minerals, it is requisite to describe, in a brief manner, such 9simple substances as form their constituent parts. Few of these, it is true, are to be found in a separate, uncombined state; yet that they do exist, and that they are to be obtained from the minerals with which they are united, we have the proof of every day’s experience.
17. There are some kinds of unconfinable fluids, the existence even of which is manifested only by their contact with other bodies, or becoming separated from them. They are of a nature too subtile to be collected or confined in our vessels for the purpose of examination, and the investigation of their properties has consequently been attended with peculiar difficulty. Those at present known are caloric, light, electricity, and magnetism; but of these the first only is immediately connected with the subjects of our present investigation.
18. Caloric.—Every one is acquainted with the different sensations of heat and cold. That matter which produces on our bodies the sensation of heat has the name of Caloric; heat being only an effect, of which caloric is the cause. This is extended in a greater or less degree through the whole extent of space, and penetrates into the interior of even the most solid bodies: in so doing it expands the particles of which they are composed, augments their bulk, and diminishes their solidity. The sun is the principal fountain from which the earth is supplied with this fluid; and it passes thence to us at the rate of 12,000,000 of miles per minute. The defect of caloric in any substance occasions the sensation called cold.
Were the world deprived of caloric, every species of organized being would, from that moment, cease to 10exist. It is the cause of all fluidity: to it every production of the earth has been most essentially indebted, even for its form and structure; and in no respect do the power and goodness of the Almighty appear more conspicuous than in the creation, dispersion, and continuance, of this most subtile and astonishing fluid.
19. All the various substances with which we are acquainted must be considered either as solid or fluid. Every substance is defined to be a solid in which the parts are so united or connected that it requires an external force to separate them. A fluid, on the contrary, is a body the parts of which are so loosely connected that they not only yield easily to any force impressed upon them, but also move freely amongst each other; and every fluid is a combination of caloric with some other substance.
20. Fluids are of two kinds: one of these, called liquids, have, when at rest, a smooth and distinct surface, and are distinguishable both by the sight and touch; the other, denominated gas, or gaseous fluids, have the appearance of air, and are not perceptible either to the sight or touch, except under certain circumstances. The latter are principally oxygen (21), azote or nitrogen, and hydrogen (45). We shall at present have occasion to speak only of the first.
21. Oxygen, like caloric, is a fluid never found in an uncombined state. It forms one of the component parts of the air that we breathe, and of the water we drink; but it approaches nearest to a state of purity in combination with caloric (18), when it has the name of oxygen gas. It was formerly called vital air, because 11no breathing animal can live for a moment in any air or gas which has not in it a mixture of oxygen; every kind of combustible burns with great splendour in it, and without it ceases to burn. It unites with a great number of substances, and changes both their appearance and properties in a very remarkable manner. Of the metals it entirely destroys the metallic lustre, and gives them an earthy form and texture. Substances in this state have the name of oxides.
Lead, for instance, combined with oxygen becomes the well-known red and heavy substance used by painters, under the name of minium or red lead (239). This, if deprived of its oxygen, loses its red colour, and returns to its former metallic state. Some of the metals are oxidized by merely being exposed to moisture. Thus the rust which is so readily contracted by iron is an oxide of that metal, produced by its attracting oxygen from the air or from water.
22. It is one of the most remarkable properties of oxygen to impart to most of those bodies called acids their peculiar character of acidity. Oxygen does not itself possess the properties of an acid, nor is it an essential ingredient in all acids, though it is the acidifying principle in the greater number of them.
23. Acid is a word originally synonymous with sour. It has, however, been gradually extended in its signification, and now comprehends all substances possessed of the properties of exciting upon the tongue the sensation called sour; of changing the blue colours of vegetables to red; of uniting with water in almost any proportion; of combining with alkalies (42), metallic oxides, 12and earths, and of forming with them certain compounds called salts.
24. Sulphuric Acid, or Spirit of Vitriol, as it is commonly called, is a liquid of a somewhat oily consistence, transparent and colourless as water, formed by a combination of oxygen (21) with sulphur (46). Like other acids, it never occurs in nature in a pure state, for it can no sooner be formed than it unites with earths (31), alkalies (42), or metals, and forms, with them, several well-known salts, which have the name of sulphats. Thus alabaster (192) and Epsom salts (199) are respectively formed by an union of sulphuric acid with lime and magnesia, and are denominated by chemists sulphat of lime and sulphat of magnesia. In like manner, blue vitriol (209) is sulphat of copper; green vitriol (208), sulphat of iron; and white vitriol (210), sulphat of zinc.
25. Phosphoric Acid is produced by a combination of oxygen (21) with phosphorus (47); and, when obtained in a state of purity, is not a fluid, but a white and flaky substance. This acid, when combined with mineral productions, forms those salts which have the name of phosphats. It is very soluble in water; and, in dissolving, makes a hissing noise, similar to that produced by plunging hot iron into water.
26. Carbonic Acid is a compound of oxygen (21) and carbon, or pure charcoal (48): and in a state of gas (20) it forms a constituent part of the atmospheric air. It is also emitted in great abundance from wine, beer, and other liquors, in a state of fermentation, and is sometimes found in the lowest parts of mines, where it is known to the miners by the name of choke damp, from the circumstance of its immediately extinguishing 13flame, and suffocating all animals that are immersed in it. This gas, which was formerly called by chemists fixed air, is about twice the weight of common air. In combination with lime it forms chalk, marble, and limestone; and it constitutes part of several other mineral substances, which are thence denominated carbonats.
27. Fluoric Acid is a gas of very singular nature, which is held in combination with lime, in the Derbyshire or fluor spar (194); and may be separated from it by pouring sulphuric acid, or spirit of vitriol (24), upon powdered spar, in a leaden vessel called a retort, and applying to it a gentle heat. The salts formed by fluoric acid have the name of fluats.
28. The Boracic is a peculiar kind of acid, which, in combination with soda (200), forms the substance that we import from the East Indies under the name of borax (204). When extracted from borax this acid does not assume the form of a fluid, but appears in thin six-sided scales or flakes, of white colour, which adhere slightly together, and feel somewhat greasy in handling. To the taste it is at first sour, then bitterish; and at last it leaves an agreeable sweetness on the palate.
29. Muriatic Acid is a gas formed by the combination of oxygen (21) with some base that is not yet known. It is an invisible and elastic fluid, which, in mechanical properties, resembles common air, and has a pungent and very peculiar smell. This gas unites with alkalies (42), earths (31), and the oxides (21) of metals; and with them forms the compounds called muriats, of which common salt, or muriat of soda (202), is one of the principal. The liquid muriatic acid, or muriatic acid gas combined with water, is frequently denominated spirit of salt (202).
1430. Nitric Acid is a compound of oxygen and azote, or nitrogen, in the proportion of twenty-five parts, by weight, of the latter to seventy-five of the former. It is one of the constituent parts of nitre, or saltpetre (206); and, in a pure state, is transparent and colourless, like water. By the action of light, however, it soon becomes yellow; and, if exposed to the air, it emits yellow fumes, which even tinge the air of the same colour. To the taste it is extremely acid. It dyes the skin a yellow colour, which is very difficult to be removed; and it is so corrosive as to destroy almost every substance into which it penetrates. If poured upon oils, it sets them on fire. With various bases it forms compounds called nitrats. This acid, which hitherto has never otherwise been obtained than mixed with water, is chiefly known in commerce by the name of aqua fortis (206).
31. The solid contents of the globe are composed of several elementary substances, amongst which have been enumerated no fewer than nine different kinds of earth:
These, when freed from foreign admixture, are, for the most part, of white colour, not soluble in water, not combustible, and do not exceed four times the weight of water.
32. The whole of these earths have, till lately, been considered simple and uncombined substances; but, by the discoveries of Sir Humphrey Davy, it has been ascertained that four of them have a metallic basis, and are in fact metallic oxides, or compounds consisting of 15a metal united with oxygen (21). These, which have the same affinity with their respective bases as rust has to iron, are silex, lime, barytes, and alumine. Until, however, some further light be thrown upon their nature and constitution, they must continue to hold their former situation of simple earths.
33. Silex, or Siliceous Earth, is the basis of all substances known by the name of quartz and silex (76). In a state of nature it has never been found pure; but, in combination with other substances, it abounds in almost every country of the globe. Common flint (90) contains ninety-seven parts in a hundred of silex: it consequently has given its name to this earth, silex being the Latin word for flint. When purified it is a white powder, the particles of which are harsh to the touch, as if they consisted of very minute grains of sand. It is not quite three times as heavy as water, and has neither taste nor smell. Water will not dissolve it, nor any kind of acid, except fluoric. Sir H. Davy has discovered it to have a metallic basis, to which he has given the name of silicium.
34. Alumine is a kind of earth, so called from its forming the basis of alum (197). It is soft, compact, and tenacious; about twice the weight of water, and, when breathed upon, has a smell which is peculiar to all clayey productions. In the fire it shrinks, and becomes so hard as even to yield sparks when struck against steel. It readily absorbs water, and is dissolved by most acids. Some writers state that pure alumine has been discovered in a native state near Halle, in Germany. It is found in a crystallized form, and nearly in a state of purity, in the Oriental ruby and sapphire. The name of argil, or clay, has sometimes 16been applied to it; but, in mineralogy, this name has usually been given to a mixture of alum, quartz, and other substances. Sir H. Davy has obtained from alumine a metallic basis, called aluminum.
35. Zircon, when freed from those substances with which it is combined, is a white and somewhat rough powder, insipid to the taste, insoluble in water, and about four times as heavy as that fluid. It is found in the two kinds of precious stones called jargoon and hyacinth, and has not hitherto been applied to any useful purpose.
36. Glucine is a kind of earth of peculiar nature, which is found in the emerald and beryl, and, when purified, forms a soft and white powder, without smell, and of sweetish taste. To the last of these qualities it is indebted for its name, which is derived from a Greek word signifying sweet. It is somewhat unctuous to the touch, and about three times as heavy as water. The uses of this earth, whatever they may be, are not known.
37. Yttria is an earth which, among other particulars, differs from glucine by its weight, as it is nearly five times heavier than water. In a natural state it occurs as the basis of a black Swedish mineral, called gadolinite. When cleansed, by chemical process, from all its impurities, it is a fine, white, and inodorous powder.
38. Barytes is a white, porous, and very heavy earth, which can only be obtained pure by chemical process. It is easily reduced to powder, and is soluble in all kinds of acids. To the taste it is harsh and caustic; and, if taken into the stomach, proves an extremely virulent poison. In some respects it agrees with the alkalies (42), particularly in its property of changing blue vegetable colours to green, and in corroding, like 17them, though with less energy, all kinds of animal substances. From these circumstances it has sometimes been denominated an alkaline earth. Saturated with sulphuric (24) and carbonic acid (26), it constitutes the minerals denominated sulphat and carbonat of barytes (196). It has been discovered to have a metallic base, which is called barium.
39. Strontian is an earth which, like barytes, is not found otherwise than in combination with sulphuric and carbonic acids. It occurs in various parts of the world, and, when purified, forms a porous mass of greyish white colour, acrid taste, and somewhat alkaline nature. This earth converts vegetable blue colours to green, but does not act so strongly on animal bodies as barytes, nor is it poisonous, like that substance.
40. Lime, the basis of all those substances which are denominated calcareous, is only to be obtained in a state of purity by artificial process. Combined with carbonic acid (26) it forms limestone (140), chalk, and marble; all of which are capable of being converted into lime by burning. Lime may also be obtained from oyster and other sea shells. When pure, it is of white colour, and moderately hard substance, though it is easily reducible to powder. Its taste is burning and acrid; and, like the alkalies, it changes vegetable blue colours to green. It has likewise the property of corroding and destroying animal substances. Lime, when pure, absorbs water rapidly, becomes hot, and falls into powder. Even if exposed to the open air it gradually attracts moisture, and assumes a powdery form; soon after which it becomes saturated with carbonic acid (26) from the atmosphere, and is thereby again converted into carbonat of lime (140). It occurs abundantly in almost every 18country, but always in combination with some acid, carbonic (26), sulphuric (24), boracic (28), fluoric (27), or phosphoric (25). This substance has a metallic basis, which has been denominated calcium.
41. Magnesia is a light and perfectly white kind of earth, of soft powdery appearance, without taste or smell, and somewhat more than twice as heavy as water. It is not found in this pure state in nature, but may be prepared from Epsom salt, which consists of magnesia in union with sulphuric acid (24). The slightly acrid taste that is perceptible in the magnesia used in medicine arises from a portion of lime which it contains. This substance does not dissolve in water, but is soluble in every kind of acid. It has the property of changing delicate blue colours to green.
42. Alkalies are substances which enter into the composition of several kinds of minerals, and are known by their property of changing the colour of blue vegetable juices to green, and by a peculiarly acrid, caustic, and nauseous taste, which it is impossible to describe, but which, after it has been once experienced, will easily be recollected. Alkalies corrode and dissolve animal substances, and unite with oil and fat in such manner as to form the well known compound called soap. They readily dissolve in water; and, when mixed with acids, form what have been denominated neutral salts.
43. The alkalies at present known are three in number; potash (205), soda (200), and ammonia (207). Of these the two former, although till lately they have been considered simple substances, have been shown by Sir H. Davy to have metallic bases.
44. By this term we are to understand all those mineral substances, capable of combustion, which have not been discovered to consist of more than a single component part. They are four in number; hydrogen, sulphur, phosphorus, and carbon.
45. Hydrogen, as its name imports, is a principal constituent part of water; for, singular as it may appear, that well-known fluid is formed by a combination of two species of air or gas, called hydrogen and oxygen (21), and in the proportion of about fifteen parts of the former and eighty-five parts of the latter. This gas had formerly the denomination of inflammable air, and has long been known in mines under the name of fire-damp. It is about twelve times lighter than atmospheric air. When pure it soon destroys such animals, and extinguishes all such flaming substances, as are immersed in it. Mixed with atmospheric air, it explodes with great violence on the application of any ignited body.
46. Sulphur is a simple combustible substance, of yellow colour, which is found pure, or native, in several parts of the world, and is sufficiently familiar to us under the name of brimstone (211). It strongly attracts oxygen (21), and is thereby converted into sulphuric acid (24). It frequently occurs in combination with mineral substances, such as arsenic, antimony, copper, and other metallic ores.
47. Phosphorus is a combustible substance which, when pure, somewhat resembles bees’-wax both in colour and consistence; and, when exposed to the air under the usual temperature of our atmosphere, is luminous in the dark, and has a smell somewhat resembling 20that of garlic. It is so combustible that, when melted, it should be kept under water, as it cannot be exposed to the air during this process without great risk of catching fire. This substance is not known in a native state; and the whole of what is used in philosophy and commerce is obtained by different artificial processes. In union with oxygen (21) it becomes converted into an acid, called phosphoric acid (25), and, under this form, in conjunction with lime, it constitutes the bones of men and animals. The greater part of the phosphorus of the shops is obtained from bones.
48. Carbon is a name given to the pure inflammable part of charcoal. It is abundantly diffused throughout nature, for it enters into the composition of several minerals, and of all vegetable and animal bodies. The purest form under which carbon is known to exist is in the diamond (50). It may, however, be obtained sufficiently pure, for all common purposes, by burning a piece of wood, covered with sand, in a vessel called a crucible. In combination with oxygen (21) it forms carbonic acid (26). Carbon is a chief component part of pit-coal (217), petroleum (213), and other bituminous substances.
Gems, or precious stones, as they are frequently called, are, for the most part, transparent, and have a vitreous or glassy appearance. Their different colours are occasioned by metallic oxides (21) of various kinds, with which they are impregnated. Some writers have classed them by their colours, but this is a very uncertain mode, as different gems have not unfrequently the same colour; and, in many cases, the same gems are of different colours. The usual distinction of gems into Oriental and Occidental is also liable to error, as the best gems, from whatever part of the world they are brought, are always called Oriental. The most estimable of all the kinds are the diamond (50), ruby (54), emerald (67), and sapphire (53); and stones a grain in weight, and equal in quality, are valued in the following proportions, at 8l. per carat for diamonds, 4l. for rubies, and 3l. for each of the others. The amethyst (79), topaz (61), and aqua-marine (61), are considered of nearly equal value with each other; and the garnet (70) is the cheapest of precious stones.
22The ancients engraved upon several kinds of gems; but they appear to have been ignorant of the art of cutting the diamond, the ruby, and the sapphire, which were too hard for them to operate upon. The emerald and the noble opal (102) were too highly esteemed as precious stones to have often found their way into the hands of engravers. It has been asserted that the ancients did not use the topaz for engraving; but there is extant a beautiful intaglio, representing an Indian Bacchus, which is said to be a topaz. The garnet was often engraved upon: and there are many master-pieces of the art in calcedony (91) and carnelian (93.) Onyx and sardonyx (92) were employed for that species of engraving in relief called cameos; and, in many instances, it is pleasing to observe with what dexterity the ancient artists availed themselves of the different colours in the alternate zones to express the different parts and shades of their figures.
Most of the gems may be imitated by artificial preparations of glass, coloured by different metallic substances; and it is not easy, by mere inspection, to distinguish the better kinds of factitious stones from real gems. They are, however, discoverable by a deficiency of lustre, and being so soft as, even in the most perfect kinds, to yield to the point of a steel instrument.
The cutting and polishing of gems is the work of the lapidary, and is in general thus performed:—The shape most proper to be given to any particular gem being determined on, the stone is cemented to the end of a stick, and the different facets are formed by a mill contrived for the purpose. This mill is a plate of copper, or an alloy of lead and tin, to which an horizontal motion is given by very simple machinery, and the surface of which is charged either with diamond powder and oil, or with fine emery and water. A thick peg of wood called a gauge, pierced with small holes in all directions, is set upright on the lapidary’s bench, close to the mill, and the process of shaping the facets thus takes place. The stone is placed on the surface of the 23mill, the opposite end of the stick to which it is cemented being inserted in one of the holes of the gauge. In this position it is kept steady by the workman, with his right hand, whilst, with the other, he puts the mill in motion. The skill of the lapidary depends on regulating the velocity of the mill, and pressing with more or less force on the stick, with an almost imperceptible tendency to one or other direction in different stages of the work, examining each facet at very short intervals, in order to give as great precision as possible to its size and form. This part of the business being completed, the cutting mill is taken out, and replaced by one of brass, on which the polishing is performed by means of fine emery (58), tripoli, and rotten stone (119), exactly in the same manner as is practised in the first stage of the process for setting the facets.
50. The DIAMOND, or ADAMANT of the ancients, is the most valuable of gems, and the hardest of all known bodies; when pure, it is perfectly transparent.
In a rough state, diamonds have usually either the form of rounded pebbles, with a shining surface, or they are crystallized in the shape of octohedrons, or double four-sided pyramids. (Pl. II, Fig. 5, 6.) Though for the most part colourless, they are sometimes yellow, green, blue, blackish, or rose-coloured.
The best diamonds are brought from the East Indies. The principal mines are those of Raolconda and Coulour, in the province of Golconda; and that of Soumelpour, or Goual, in Bengal. At Raolconda they are found in the deep crevices of rocks. Persons, by means of long iron rods, with hooks at the end, draw out from these crevices the loose contents, and afterwards wash them in tubs, for the purpose of discovering the diamonds.
The first discovery of diamonds at Coulour was about two centuries ago, by a countryman, who, on digging his ground to sow millet, accidentally found one of these stones of large size. From that period the whole 24adjacent plain began to be searched to the depth of from ten to fourteen feet; and the work was, at one time, so extensively pursued, that nearly 6,000 persons were employed in it. At Soumelpour the diamonds are found amongst the sand and gravel of the river.
Diamonds are likewise found in the island of Borneo, and in several parts of South America. The mode by which they are obtained from one of the rivers of Brazil has been described by Mr. Mawe. The current is turned, and part of the bed of the river being laid dry, the mud is taken up and washed, by negroes, in places prepared for the purpose, through which a portion only of the stream is allowed to flow. As soon as all the earthy particles have been washed away, the gravel-like matter that remains is raked together, the stones are thrown out, and what diamonds happen to be present are found amongst the refuse that is left.
To ascertain whether a stone, that has been found, be really a diamond, the workmen have a mode of placing it upon a hard substance, and striking it with a hammer. If it either resists the blow or separate into leaves, it must be a diamond; but, in the latter case, the discovery is sometimes made at an immense expense, as, by thus diminishing the size, its value must also, of course, be greatly diminished.
Diamonds are generally exported from Madras in a rough state; and in small parcels neatly sewed in muslin, and sealed by the merchants who send them. These, we are informed by Mr. Milburne in his valuable work on oriental commerce, are, for the most part, sold in Europe by the invoice, as it is called; that is, without being opened: and he says that they are always found to contain the value for which they were sold in India.
Of all transparent substances, none for brilliancy can be compared with the diamond. Its hardness is such, that no steel instrument whatever can make any impression upon it. Notwithstanding this, at a temperature not so high as that which is required for the 25melting of silver, it gradually dissipates and burns. Diamonds have been shown to consist principally of carbon or charcoal in a pure and crystallized state.
The ancients, ignorant of the art of cutting diamonds, were contented to set them in a native state; and for this purpose they preferred such stones as had naturally a crystallized form. The four large diamonds which ornament the clasp of the Imperial mantle of Charlemagne, and which are still preserved in Paris, are uncut stones of this description. The extreme hardness of the diamond baffled all attempts to polish it in such manner as to exhibit its peculiar beauty, until the year 1456, when a young man of Bruges, whose name was Berquin, endeavoured to polish two diamonds by rubbing them against each other. Having succeeded in this, he next constructed a wheel, on which, by means of diamond powder, he was enabled to cut and polish these gems in a manner beyond his greatest expectation. Since this period the art of polishing them has been greatly improved both by the Dutch and British jewellers.
In the choosing and valuing of diamonds in a rough state, attention is paid to their colour, their being free from extraneous matter, and their shape. Those that are most perfect are crystalline, and resemble a drop of clear spring water, in the middle of which is to be perceived a strong light, that plays with great spirit on moving them about. When they have a yellowish or greenish tinge they are considered to be bad. Many diamonds have a kind of confused structure, which lapidaries compare to knots formed in wood. These are rejected, from the impossibility of polishing them properly.
Mr. Mawe remarks that diamonds, when rubbed together, have a peculiarly and scarcely to be described grating sound, which is one of their most remarkable characteristics. By this alone rough diamonds may be accurately and expeditiously distinguished from every other gem.
26It is usual to cut diamonds into three principal forms, called brilliant (Pl. II, Fig. 7), rose (Fig. 8), and table diamonds (Fig. 9). Brilliants are, for the most part, cut from such of the stones as have naturally a crystallized shape, and rose diamonds from the flat varieties. The former are so called from their great lustre, in consequence of the facets on both sides being cut. These are always set upon a black ground, whilst rose diamonds, which are much thinner, are set upon a white foil speckled with black, for the purpose of adding to their lustre. Rose-cut diamonds are of course much less estimable than brilliants; so much so indeed, that of late many of them, brought from Holland, have been re-cut into brilliants, notwithstanding the additional expense, and the loss of size necessarily attendant on this operation. The table diamond is the least beautiful of any. This mode of cutting is only adopted for such stones, or rather fragments, as, with a considerable breadth, have only a very trifling depth. The diamond-cutters of England are considered to be the best in Europe, but their number is so small as to occasion many stones to be sent to Holland to be cut.
The value of diamonds is ascertained by their weight in carats; and this value increases, in a very high ratio, according to their magnitude. For instance, a diamond weighing one carat will be worth about 10l. whilst another of five carats will be worth 150l. and of ten carats 800l.[2] This rule, however, can only be taken for diamonds of twenty carats and under. The larger ones, in consequence of the scarcity of purchasers, are generally disposed of at prices greatly inferior to their estimated worth. The value of some diamonds that are peculiarly perfect exceeds the above 27ratio; whilst, for a stone that is cloudy, foul, or of bad colour, even three quarters of the estimated value will perhaps be deducted.
2. A Carat is equal to four jeweller’s grains, seven grains of which are equal to six grains troy. To ascertain the value of wrought diamonds the weight must be doubled, about half being supposed to be lost in the working. This sum must be multiplied into itself, and the product by two. Thus to find the value of a diamond of twenty carats 20 × 2 = 40 × 40 = 1600 × 2 = 3,200l.
No diamonds are so valuable as those that are perfectly transparent, and of snow-white colour. The green and yellow varieties are, however, much esteemed: the blue kinds were formerly more valued than at present; and the least valuable are those that have a grey or brownish tint. Black diamonds are much prized by collectors.
The principal use of the diamond is in jewellery. It is also used by lapidaries, for slitting hard stones, and for cutting and engraving upon other gems; by clock-makers in the finer kinds of clock-work; in the glass-trade for squaring large pieces or plates of glass, and among glaziers for cutting their glass.
The largest diamond ever known (if it be such, and not a white topaz, as some people have imagined) is in the possession of the Queen of Portugal, and weighs 1,680 carats, or more than eleven ounces. It was found in Brazil, and sent to Lisbon in the year 1746. It is still uncut, and has been valued at 5,644,800l.
The Rajah of Mattan, in the island of Borneo, possesses a large diamond, shaped like an egg, with an indented hollow near the smaller end. It was found in that island about eighty years ago, is said to be of the finest water, and to weigh 367 carats, or more than two ounces and a quarter. Several years ago the Governor of Batavia, desirous of purchasing this gem, sent a Mr. Stuvart to the Rajah, authorizing him to offer for it 150,000 dollars, two large brigs of war, with their guns and ammunition, together with a certain number of great guns, and a quantity of powder and shot. The Rajah, however, refused to deprive his family of so valuable an hereditary possession; for the Malays not only attach to it the miraculous power of curing all diseases by means of water in which it is dipped, but also believe that the fortune of the family is sustained by its continuing in their possession.
28Tavernier, the French Traveller, saw in the possession of the Great Mogul a diamond which weighed near 280 carats. In form and size it resembled half a hen’s egg. This diamond had been obtained from the mine of Coulour, about the year 1550; and was valued at more than 700,000l. sterling.
The sceptre of the Emperor of Russia is adorned with an oriental diamond about the size of a pigeon’s egg, which weighs 195 carats. This diamond is said to have once been placed as the eye of an idol in Seringham, in the Carnatic. A grenadier, who had deserted from the French service in India, contrived to become one of the priests of the idol, in the hope of being able to steal this eye. He at length effected his purpose, and escaped with the diamond to Madras, where he sold it to the captain of a ship for a sum equal to 2,500l. of British money. It was afterwards transferred to a Jew for 18,000l. Coming into the hands of a Greek merchant, he offered it for sale at Amsterdam, in 1766; and the Russian Prince Orloff bought it for the Empress Catharine for about 90,000l. sterling, and an annuity of 4,000l. during the life of the person who sold it.
The Pitt, or Regent diamond (Pl. II, Fig. 10), which lately was set in the handle of the sword of state of Buonaparte, and is now possessed by the king of France, is a brilliant of the most beautiful kind, and weighs 136¾ carats. It was brought from India by Thomas Pitt, Esq. Governor of Fort George. Mr. Pitt has himself stated, respecting it, that, in December, 1701, whilst resident in Madras, several valuable stones, in a rough state, were brought to him for sale by an eminent diamond merchant. One of these, the diamond here spoken of, was so large that the merchant asked for it the sum of 85,000l. After much bargaining, Mr. Pitt purchased it for 20,400l. He afterwards sold it for 135,000l. to the Regent Duke of Orleans; and by him it was placed among the crown jewels of France.
The Pigot diamond weighs forty-seven and a half carats. This, which is an extremely fine stone, was 29disposed of by lottery, in 1800, for 22,000l.; and is now in the possession of Messrs. Rundell and Bridge, jewellers in London.
A large star, cross, and chain, worn on grand gala days by the Prince of Brazil, as Sovereign of the different Portuguese orders of knighthood, are each ornamented with a great number of magnificent diamonds, set in gold. The centre diamond of the star is alone valued at 800,000l.
When the diamond is rubbed it will attract bits of straw, feathers, hairs, and other small objects; and if exposed to the rays of the sun, and immediately taken into a dark place, some diamonds will appear luminous.
51. JARGOON is a gem usually of smoky yellow or brownish colour, and sometimes limpid: if placed upon any object, it exhibits of it a very distinct double image.
The primitive form of its crystal is an octohedron (Pl. II, Fig. 5), but it is frequently crystallized in right-angled prisms, terminated by four-sided pyramids.
In hardness this stone does not much exceed that of the emerald. The greyish white and yellowish white varieties of jargoon are valuable chiefly on account of their resemblance to the diamond. The darker-coloured varieties can be deprived of their colour by heat; and, in this state, though in lustre they are infinitely inferior to them, they are sometimes substituted for diamonds. Jargoons are now seldom used except for the jewelling of watches and time-pieces. About a century ago, they were much used in mourning ornaments, for which the dark tone of their colour, and their almost adamantine lustre, were supposed to be peculiarly appropriate.
The jargoon is principally brought from the island of Ceylon; but it is occasionally found in France, and Spain, and in granite rocks near Cuffel, in Dumfrieshire, Scotland.
3052. The Hyacinth, or Jacinth, is a dark orange-red variety of jargoon. It is also chiefly imported from Ceylon, where it is generally found in the sand of rivers, in irregularly round pieces, but seldom of large size without flaws.
This stone is indebted for its name to a supposed resemblance in colour to that flower, which, according to the Pagan mythology, Apollo raised from the blood of his favourite youth, Hyacinthus.
When bright, and free from flaws, the hyacinth is a superb ring stone; but it is not of usual occurrence in modern jewellery.
53. The ORIENTAL SAPPHIRE is a gem of blue colour, the shades of which vary from a full and deep tint to a nearly colourless appearance, and sometimes it is party-coloured.
It is found crystallized in six-sided pyramids much lengthened and joined base to base (Pl. II, Fig. 13); and also in rounded or pebble-shaped fragments. It has a foliated texture, is extremely hard, and about four times as heavy as water.
We are chiefly indebted for the sapphire to the East Indies and the Island of Ceylon, where it is found amongst the sand of the rivers. When brought into Europe, it is cut by means of diamond powder, and polished with emery. It is now usually set with a foil of its own colour; but it was formerly the practice, instead of foil, to place under this stone the blue part of a peacock’s feather.
In hardness the sapphire ranks next to the ruby (54); and in value it is about equal to the emerald (67). A good sapphire of ten carats’ weight is worth about fifty guineas. In the Museum of Natural History at Paris there is a sapphire which weighs upwards of sixty-six carats: it was placed there from the wardrobe of the crown.
We are informed by M. Hauy that sapphires are found in Bohemia and France, particularly in one part 31of the Ville du Puy, among the sand of a rivulet near Expailly. In the summer-time, when the rivulet is nearly dry, they are collected by persons, each of whom is furnished with a small tray and a linen bag. Where-ever there are small depressions in which the water has been stationary, these persons enter them, and fill their trays with the sand. This they wash in water in such manner that the lighter particles are carried away; whilst the heavier ones of gravel, sapphire, and other articles, remain at the bottom.
Some sapphires exhibit a kind of opalescence, or whitish floating light in their interior. Sapphires lose all their colour in the fire; and, after having been subjected to heat, they are so hard and transparent as sometimes to be sold for diamonds.
54. ORIENTAL RUBY is a precious stone of intense and bright red colour, occasionally varied with blue, and sometimes party-coloured.
In the general form of its crystals it much resembles the sapphire (53).
The ruby is imported into this country from the East Indies, though seldom in a rough state, as the stones are almost always first cut by the Indians for the purpose of ascertaining their value. They are said to be found in the sand of certain streams near the town of Sirian, the capital of Pegu; and with sapphires in the sand of rivers in Ceylon. But they are so seldom seen of large size, that a ruby above thirty-one carats’ weight, of perfect colour, and without flaws, is even more estimable than a diamond of equal weight. The ruby is usually set with a foil; but, if peculiarly fine, it is sometimes set without bottom, that the stone may be seen through.
Tavernier, the Eastern traveller, states that, in the throne of the Great Mogul, he saw 108 rubies, which, on an average, weighed from 100 to 200 carats each. Among the jewels of the King of Candy, that were sold by auction in London, on the 13th of June, 1820, 32was a ruby which measured two inches in length, and one inch in breadth. It was, however, interesting only as a specimen for a cabinet, for it had, in various directions, a great number of small hair-like tubes running through it.
The hardness of this stone is such that the ancients do not appear to have possessed the art of cutting it; and, in the improvements which of late have been made by Mr. Earnshaw in the construction of time-keepers, no stones have been found sufficiently hard for jewelling the holes, except the ruby and the diamond.
There are several modes of counterfeiting rubies; and some persons have succeeded so well in imitating these stones, that even the most able lapidaries, till they try the hardness, may be deceived.
55. The Oriental Amethyst is an extremely rare gem, usually of purple colour, apparently formed by an union of the colouring matter of the sapphire and the ruby. This stone, if heated, loses its colour, and becomes transparent. After this process its brilliancy is such that it is scarcely distinguishable from the diamond; and, in jeweller’s work, it is occasionally substituted for that gem. The common amethyst (79), or that which is chiefly seen, is nothing more than a violet-coloured rock crystal (78).
56. The Oriental Topaz and Emerald are each varieties of the oriental ruby, the former straw-coloured and the latter green. This kind of emerald is imported from Pegu, and some other parts of the East Indies, and is an extremely rare gem.
57. The SPINEL and BALAIS RUBY are two kinds of precious stones, which differ from each other principally in colour, the former being of a carmine, and the latter a cochineal red.
They vary from the oriental ruby (54) in being less hard; in the primitive form of their crystals being regular octohedrons (Pl. II, Fig. 5), and in their not being much more than 3 times heavier than water.
33Although these two kinds of rubies are inferior, both in lustre and colour, to the oriental ruby; yet, when they exceed a certain size, they are much esteemed. A spinel that weighs more than four carats is valued at half as much as a diamond of the same weight, and is not unfrequently imposed upon ignorant purchasers for the oriental species. It is easily wrought, takes a high polish, and is certainly a beautiful gem. Being too expensive for necklaces, it is usually set in rings and brooches, surrounded by brilliants.
The spinel ruby is found amongst sand, in one of the rivers of Ceylon, which flows from the high mountains, towards the middle of the island. It is also found in Brazil; and in Hungary, Bohemia, and Silesia.
The Balais ruby is so named from Balacchan, the Indian appellation of Pegu, from which country it is chiefly imported.
58. EMERY is a very hard opaque mineral, of blackish or bluish grey colour, which is chiefly found in shapeless masses, and mixed with other minerals. It is about four times as heavy as water.
The best emery is brought from the Levant, and chiefly from Naxos, and other islands of the Grecian Archipelago, where it occurs abundantly, in large, loose masses, at the foot of primitive mountains. It is also found in some parts of Spain; and is obtained from a few of the iron mines in our own country.
In hardness it is nearly equal to adamantine spar; and this property has rendered it an object of great request in various arts. It is employed by lapidaries in the cutting and polishing of precious stones; by opticians, in smoothing the surface of the finer kinds of glass, preparatory to their being polished; by cutlers, and other manufacturers of iron and steel instruments; by masons, in the polishing of marble: and, in their respective businesses, by locksmiths, glaziers, and numerous other artisans.
For all these purposes it is pulverized in large iron 34mortars, or in steel mills; and is afterwards separated, according to the several degrees of fineness that are required, by washing it in water, and suffering the grosser particles to deposit themselves. By this operation the finer particles, which remain suspended in the water, and which are obtained by decanting the water off, and suffering it to stand for a considerable time, are separated. The particles first deposited are again ground, and again agitated in the water, to separate the finest. By these successive operations the emery is reduced to a powder so fine that, when rubbed between the fingers, it communicates no sensation whatever of grittiness. In general those particles only of the emery which remain suspended in the water, after it has stood about half an hour, are used to polish metals.
59. ADAMANTINE SPAR, or IMPERFECT CORUNDUM, is a very hard and nearly opaque stone, which varies much in colour, but is chiefly grey, with a greenish, brown, or bluish tint.
It is usually found in the form of six-sided prisms, but it sometimes occurs in shapeless masses, has a foliated texture, and is about four times as heavy as water.
The name of adamantine spar was given, by the British lapidaries, to this substance from its hardness being nearly equal to that of the diamond. It was originally discovered among the granite rocks of China; but it has since been found, and in greater purity, in Bengal and Ceylon.
In a powdered state this substance has long been used by the artists of India and China for the cutting and polishing of precious stones, and even of the diamond; but, though it will in some degree operate upon that gem, it is not sufficiently hard to bring out the peculiar beauty of it in a degree at all comparable to that which is effected by the European lapidaries with diamond powder. The Chinese also use adamantine spar for polishing steel, and in the composition of the finer kinds of porcelain or earthenware. For the cutting 35of seals and precious stones European workmen consider it preferable to emery; but, for minute engraving, it is much inferior to diamond powder.
60. CHRYSOBERYL is a gem of yellowish or brownish green colour, harder than quartz (76), and sometimes transparent; but often only semi-transparent, in which case it exhibits a bluish light, floating in the interior of the stone.
It is usually found in rounded pieces, but is sometimes crystallized in compressed six-sided prisms, and in double six-sided pyramids.
So little is this gem in request in Europe, that it is seldom to be found in the possession of jewellers; but in Brazil it is considered inferior only to the diamond. It is usually procured from South America; yet it occurs in Saxony; and, with the ruby and sapphire, amongst sand in the rivers of Ceylon.
Such is the hardness of the chrysoberyl, that, when properly polished, which is a difficult operation, it is capable of receiving a lustre nearly equal to that of the diamond. We are informed that, a few years ago, a considerable number of these gems were imported into this country from Brazil, but that the greater part of them were entirely spoiled by inferior workmen, and that the rest were so ill-cut that they remained unnoticed, and without value. The smaller stones are said to appear to most advantage in circular ear-drops; and the larger specimens form necklaces and ring stones of great beauty.
The variety which exhibits an opalescent appearance, or presents a bluish light, undulating as it were in the interior of the stone, and changing its situation according to the position of the observer, is chiefly valuable as an article of curiosity: the transparent kind is always preferred by the jeweller.
61. The TOPAZ is a gem usually of a wine-yellow colour, but sometimes orange, pink, blue, and even colourless, like rock 36crystal; of a lamellar or foliated structure, harder than quartz, but not so hard as ruby.
It varies considerably in its crystallization; is 3½ times heavier than water; and, when placed upon any object, shows a double image of it.
The name of topaz is derived from an island in the Red Sea, where the ancients found a stone, but very different from ours, which they denominated topaz. The best topazes are of a deep colour, and are imported from Brazil; the most brilliant ones are supposed to be those of Saxony; but the latter are generally of very pale colour. This species of gem is found in many parts of Europe, but defective in transparency, and sometimes even opaque. It occurs in large crystals, and rolled masses, in an alluvial soil (269), in the upper parts of Aberdeenshire, Scotland; and in veins, along with tin-stone, at St. Anne’s, in Cornwall. Topazes, more than a pound in weight, have been found in Scotland.
Mr. Mawe speaks of a topaz mine at Capon, near Villa Rica, in Brazil. In two breaks or slips of the rocks, he says, there were little soft places where the negroes found the topazes by scraping in them with pieces of iron. He himself observed at least a cart-load of inferior topazes, any number of which he might have taken away; but all that he saw were defective and full of flaws.
These stones vary much in size; some, particularly those of Siberia, being extremely small, and others being upwards of an inch in thickness. In the Collection of Natural History at Paris there is a Brazilian topaz which weighs four ounces and a quarter. These stones are not sufficiently scarce to be, in general, much valued by the jeweller or lapidary. The deep yellow variety is preferred to the pale sort, although the latter is often superior to it both in size and hardness.
Figures have sometimes been engraved on the topaz; and these, when well executed, are of great value. In the National Museum at Paris there is a superb Indian Bacchus engraven on a topaz. The cabinet of the Emperor 37of Russia contains several fine topazes of this description.
Some of the coarse kinds of topaz are broken down, pounded, and used instead of emery for the cutting of hard minerals; and powdered topaz was formerly kept in apothecaries’ shops, and sold as an antidote against madness.
It is a somewhat singular circumstance, that, if the Saxon topaz be gradually exposed to a strong heat in a crucible, it will become white and, on the contrary, that Brazilian topazes by the same process become red or pink. By exposure to a still stronger heat, the Brazilian topaz changes its colour to a violet-blue.
Jewellers usually divide topazes into the following kinds:
62. Brazilian and Saxon, already mentioned.
63. Bohemian.—These are found chiefly in the tin mines of Bohemia, are of small size, deficient in transparency, have only grey or muddy white colours, and are of little value.
64. Blue Topaz.—This is a large Brazilian gem, which varies in size from one or two carats to two or three ounces. A fine blue topaz, without flaw, and which weighed an ounce and a quarter, was sold for 200 guineas. It is sometimes difficult to distinguish a blue topaz from an aqua marine (68).
65. Pink Topaz.—Some beautiful rose-coloured varieties of topaz have been brought from Asia Minor, and others are found in South America; but the pink topazes in the jewellers’ shops are chiefly stones of the yellow Brazilian kind, which have had their colour changed by heat.
66. The White, or Nova Mina Topaz, is a perfectly colourless and transparent variety. It generally occurs of small size, and is in considerable estimation in Brazil for ear-rings, or for being set round yellow topazes. 38Small stones of this description have recently been found at St. Michael’s Mount, in Cornwall.
There is imported from Brazil a yellow kind of crystal (83), which is so similar, in its appearance, to the yellow topaz as sometimes to be imposed upon purchasers for that stone.
67. The EMERALD is a well-known gem, of pure green colour, and somewhat harder than quartz.
Its natural form is a short six-sided prism; but it is sometimes found massive, and rounded like a pebble.
By the ancients the emerald was a gem much in request, and particularly for engraving upon. They denominated it smaragdus, and are said to have procured it from Ethiopia and Egypt; but, besides the true emerald, Pliny, under this title, includes green jasper (96), malachite (231), fluor spar (194), and some other green minerals. The pillars of emerald in the temple of Hercules at Tyre, mentioned by Herodotus, and the large emeralds described by Pliny as having been cut into columns and statues, cannot be referred to the true emerald.
The deepest coloured and most valuable emeralds that we are acquainted with are brought from Peru. They are found in clefts and veins of granite, and other primitive rocks; sometimes grouped with the crystals of quartz (76), felspar (110), and mica (123); and, not unfrequently, loose in the sand of rivers. The most ancient emerald mine is that of Manta, in Peru, but it has been some time exhausted; and most of the emeralds that are now brought to Europe are obtained from a mine situated in the valley of Tunca, between the mountains of New Grenada and Popayan.
The emerald is one of the softest of the precious stones; and is almost exclusively indebted for its value to its charming colour. The brilliant purple of the ruby, the golden yellow of the topaz, the celestial blue of the sapphire, are all pleasing tints; but the green of the emerald is so lovely, that the eye, after glancing 39over all the others, finds delight in resting upon this. In value it is rated next to the ruby; and, when of good colour, is set without foil and upon a black ground, like a brilliant diamond. Emeralds of inferior lustre are generally set upon a green gold foil. These gems appear to greatest advantage when table cut (Pl. II, Fig. 9), and surrounded by brilliants, the lustre of which forms an agreeable contrast with the quiet hue of the emerald. They are sometimes formed into pear-shaped ear-drops; but the most valuable stones are generally set in rings. A favourite mode of setting emeralds among the opulent inhabitants of South America is to make them up into clusters of artificial flowers on gold stems.
The largest emerald that has been mentioned is one said to have been possessed by the inhabitants of the valley of Manta, in Peru, at the time when the Spaniards first arrived there. It is recorded to have been as big as an ostrich’s egg, and to have been worshipped by the Peruvians, under the name of the Goddess, or Mother of Emeralds. They brought smaller ones as offerings to it, which the priests distinguished by the appellation of daughters. Many fine emeralds are stated to have formerly been bequeathed to different monasteries on the Continent; but most of them are said to have been sold by the monks, and to have had their place supplied by coloured glass imitations. These stones are seldom seen of large size, and at the same time entirely free from flaws.
The emerald, if heated to a certain degree, assumes a blue colour; but it recovers its proper tint when cold. When the heat is carried much beyond this, it melts into an opaque coloured mass.
The precious stone called oriental emerald (56) is a green and very scarce variety of the oriental ruby.
68. The BERYL, or AQUA MARINE, is a light or mountain green variety of the emerald, sometimes straw-coloured, bluish, yellow, or even white.
These stones are of such frequent occurrence, even 40in large pieces perfectly clear and free from flaws, they are in general so soft, and have so little the brilliancy of other gems, that they are usually considered of inferior value. The most beautiful kinds are brought from Dauria, on the frontiers of China, from Siberia, and from Brazil. They are also found in Saxony and the South of France, and are very common at Baltimore, in North America. Specimens of aqua marine have been obtained from the upper parts of Aberdeenshire, Scotland, where they sometimes occur in alluvial soil, along with rock crystal and topaz. These stones have also been found, embedded in granite, near Lough Bray, and Cronebane, in the County of Wicklow, Ireland; and also in mountain rock, in some parts of Devonshire.
They are cut by means of emery (58), and polished with tripoli (119). The darkest green specimens are set upon a somewhat steel-coloured foil; and the pale ones are either placed, like the diamond, on a black ground, or upon a silvery foil. The aqua marine is usually made into necklaces; but it is likewise employed for brooches, and not unfrequently for steel stones and intaglios. The larger ones are in much esteem among the Turks for the handles of stilettos.
69. The TOURMALINE is a stone belonging to the same family as the emerald, and generally of a smoky blackish colour: sometimes, however, it is green, red, blue, or brown; and, when not very thick, it is transparent.
It is occasionally found in shapeless masses, but more frequently crystallized in three, six, or nine-sided prisms, variously truncated or terminated; and its weight is somewhat more than three times that of water.
This stone was first made known in Europe, about the beginning of the last century, by the Dutch merchants, who brought it from the island of Ceylon, where it is principally found. When strongly heated it becomes electric; one of the summits of the crystal negatively, and the other positively. An early writer, by whom it is mentioned, says, that “it has the property 41not only of attracting ashes from the warm or burning coals, but that it also repels them again, which is very amusing: for as soon as a small quantity of ashes leaps upon it, and appears as if endeavouring to writhe themselves by force into the stone, they in a little time spring from it again, as if about to make a new attempt. It was on this account that the Dutch called it the ashes drawer.”
Since the above period, tourmaline has been found in Brazil; and in Norway, Germany, France, and several other parts of Europe. It generally occurs embedded in different kinds of mountain rock; and, in these, is rather confined to single beds or strata, than disseminated through the whole mass of the mountain. A piece of tourmaline, of cylindrical form, and brownish grey colour, was some time ago discovered in the neighbourhood of Kitt-hill, near Callington, Cornwall. Black tourmaline, both in large and small crystals, is found in granite rock, in the vicinity of the Logan, or Rocking-stones, near Treryn, in the same county.
When laid on a table, the tourmaline appears a dark and opaque stone; but, when held against the light, it has generally a pale brownish hue. It is sometimes cut, polished, and worn as a gem; but, on account of the muddiness of its colours, it is not in general much esteemed. Those persons who wear tourmalines set in rings consider them more as objects of curiosity than of elegance: they show them as small electrical instruments, which, after being heated a little while by the fire, will attract and repel light bodies.
In the superb collection of minerals of the British Museum, there is a magnificent specimen of red tourmaline, or rubellite, which has been valued at 1000l. sterling. It was presented by the King of Ava to the late Colonel Symes, when on an embassy to that country, and was afterwards deposited by the latter in Mr. Greville’s collection; with that collection it became the property of the British Museum.
70. The PRECIOUS, or NOBLE GARNET, is a gem of crimson colour, which, when crystallized, has the form of a twelve-sided solid (Pl II, Fig. 11, 12). It is sufficiently hard to scratch quartz, and is about four times as heavy as water.
This stone is found abundantly in many mountains (particularly of primitive rock), in different parts of the world. But garnets of the hardest and best quality are brought from Bohemia, where there are regular mines of them; and a great number of persons are there employed in collecting, cutting, and boring them. The boring is performed by an instrument having a diamond at its extremity, which is rapidly turned by a bow. The work is so expeditiously performed, that an expert artist can bore 150 garnets, or he can cut and polish thirty, in a day. In Suabia there are two towns in which upwards of 140 persons are employed in these operations.
In general garnets are stones of inferior value. When compared with the ruby, those even of finest quality have a very sombre appearance. The kinds most esteemed are such as have a clear and intense red colour, or a rich violet or purplish tinge. The best garnets are cut in the manner of other precious stones, and are usually set upon a foil of the same colour. To heighten the colour and transparency of certain garnets, jewellers either form them into what are called doublets, by attaching to the lower part of the stone a thin plate of silver, or they hollow them underneath.
Crystals of garnet sometimes occur three or four inches in diameter. These are cut into small vases; which, if of good colour, and free from defects, are highly valued. Many fine engravings have been executed on garnet. One of the most beautiful that is known is a figure of the dog Sirius, in the possession of Lord Duncannon.
The coarser kinds of garnet are used as emery for 43the polishing of other minerals; and are thus prepared. They are made red-hot, then quenched in water, reduced to powder in an iron mortar, and lastly diffused through water, poured into other vessels, and allowed to settle, in order to obtain an uniform powder. This powder is known to artists by the name of red emery.
It has been conjectured that our garnet was the same kind of stone which, on account of its colour, the ancients denominated carbuncle.
71. Common Garnet.—A very inferior variety of garnet, of brown or greenish brown colour, is found in our own country, and particularly amongst rocks near Huntley, in Aberdeenshire, Scotland. These garnets, however, are, in general, so soft as to be of little value to the lapidary; and consequently are seldom cut or polished for ornamental purposes. But being easily fused, and abounding in iron, they are occasionally employed as a flux in the smelting of rich iron ores: and as an addition to poor ones.
72. Syrian Garnets are distinguished by their violet or purplish tinge. Some writers state that they have their name from the word Soranus, which signifies a red stone; and others from Sirian, a town in Pegu, where they are said to be found in great beauty.
73. Pyrop Garnets are of a dark blood-red colour, which, when the stones are held between the eye and the light, falls strongly into yellow: they are chiefly brought from Bohemia: are employed in almost every kind of jewellery, and generally set with a gold foil. At Waldkirch, in Suabia, there are twenty-four mills for the cutting and polishing of pyrop garnets: and 140 masters are occupied in manufacturing these stones.
74. Vesuvian is a liver-brown kind of garnet, that was originally found among rocks ejected from Mount Vesuvius; and in the vicinity of which mountain it still occurs in considerable abundance. At Naples it 44is cut into stones for rings and other ornaments. Vesuvian has of late years been found in other parts of Europe; and even at Kilranelagh, and Donegal, in Ireland.
75. Cinnamon Stone is a kind of garnet of hyacinth-red colour, which is found in angular and roundish pieces among the sand of rivers in the island of Ceylon. It is cut as a precious stone; and, when of good colour, and free from flaws, is of considerable value.
76. COMMON QUARTZ is a hard and foliated substance, usually of white or grey colour, and more or less transparent.
It is generally found in shapeless masses, which are nearly thrice as heavy as water, and the fracture of which is glassy. When crystallized, it most commonly has the form of a six-sided prism, terminated by a pyramid of six sides.
This kind of stone forms a constituent part of many mountains, and is very common in our own, as well as in most other countries. It is sufficiently hard to scratch iron and steel; and it has the property, after having been several times successively made red-hot, and dipped into water, of communicating to that fluid a certain degree of acidity.
Quartz is employed, in place of sand, for making the finer kinds of glass; and also in the manufacture of porcelain. For the latter purpose great quantities are collected from the mountains of Wales, ground into powder, and in that state shipped to Liverpool, and other parts. After having been burnt and reduced to powder, it is sometimes mixed with clay, and formed into bricks for the construction of glass furnaces: these are capable of resisting the intense heat which is requisite in the fusion of glass.
77. Burrstone is a vesicular and corroded variety of quartz, which forms a most excellent and valuable 45kind of millstone. It is chiefly found in France; but is so much esteemed by the English millers, that the Society of Arts, in London, for many successive years, offered a considerable reward for its discovery in Great Britain. At length a vein of burrstone was discovered in the Moel y Golfa hills, North Wales, by a Mr. Evans, who, in consequence received a premium from the Society. About the same time another vein was opened near Conway; and the same Society, in 1800, gave a premium of 100l. to the widow and orphan children of the discoverer. Both these quarries were sufficiently convenient for water carriage; yet the demand for the Cambrian burr did not answer the expectation, and millstones of French production were still preferred to them.
The mode of splitting these stones, as it is practised in some parts of France, is singular, and affords a proof of the extraordinary power of capillary attraction. The blocks are first cut into the form of cylinders, sometimes many feet in height. To split these horizontally into millstones, circular indentations are made round them, at proper distances, according to the thickness that is to be given to the stones; wedges of willow, that have been dried in an oven, are then driven into the indentations with a mallet. When these have been sunk to a proper depth, they are moistened with water; and, after a few hours, the several stones that have been marked out are found to be perfectly separated.
78. ROCK CRYSTAL is an extremely beautiful kind of quartz, sometimes perfectly transparent, and sometimes shaded with grey, yellow, green, brown, or red. It occurs in the form of crystals with six sides, each terminated by a six-sided prism.
The name of this substance was considered by the ancients to signify ice, or water crystallized; and they imagined that crystal was produced from a congelation of water.
46Its uses are numerous. It is cut into vases, lustres, and snuff-boxes; and many kinds of toys of extremely beautiful appearance are made of it. When pure and perfectly transparent, it is much in request by opticians, who make of it those glasses for spectacles which are called pebbles, and who use it for various kinds of optical instruments. The best crystal is imported from Brazil and Madagascar, in blocks, not unfrequently from fifty to a hundred pounds in weight.
This stone is wrought into the different shapes that are required, by sawing, splitting, and grinding. The sawing is effected by an extended copper wire fixed to a bow: the wire is coated with a mixture of oil and emery, and is drawn backward and forward until the operation is performed. But, as this process is a tedious one, particularly when the mass is large, a more expeditious, although less certain, method is sometimes adopted. The crystal is heated red hot, and a wet cord is drawn across, in the direction that the workman intends to split it. By the rapid cooling thus effected, in the direction of the cord, the stone easily splits by a single blow of the hammer, and generally in the direction required. The grinding is performed by means of emery: and the polishing effected by tin ashes and tripoli.
The ancients held vases that were made of this stone in great estimation, particularly when they were of large size. Of two cups which the tyrant Nero broke into pieces in a fit of despair, when informed of the revolt that caused his destruction, one was estimated to be worth more than 600l. of our money. The most valuable kind of crystal that was known to the ancients was obtained from the island of Cyprus; but it was often faulty in particular parts, having flaws, cracks, and blemishes. When the crystal was used for the engraving of intaglios and cameos, the artist could sometimes conceal these defects amongst the strokes of his work; but, when it was to be formed into cups or vases, this could not be done, and for the latter purpose the purest pieces only could be employed.
47In the counties of Cornwall and Derby, in the neighbourhood of Bristol, and amongst the mountains of North Wales, small crystals of this kind are frequently found: these are respectively called Cornish, Buxton, Bristol, and Snowdon diamonds. We are informed that the crevices of some parts of Mont Blanc and the Alps contain rock crystal in such abundance as to be perfectly bristled with it.
Some crystals contain in their substance drops of water, or other kind of fluid; and these, as curiosities, are usually sold at a rate considerably higher than others. There are in the British Museum specimens of crystal which enclose many kinds of foreign substances, such as ironstone, needle antimony, and asbestos (136).
Various means have been devised for communicating colours to rock crystal. If it be heated and plunged into a solution of indigo, or copper, it acquires a blue colour; or if into a decoction of cochineal, a red colour. A clove-brown colour may be given by exposing it to the vapour of burning wood. Artists sometimes communicate beautiful colours to rock crystals, by forming them into what are called doublets. Two modes of doing this are adopted. In one, a stone that is brilliant-cut at the top is hollowed underneath, filled with the colour that the stone is intended to exhibit, and then closed at the bottom by a plate of glass. If this kind of doublet be dexterously executed, the deception is not easily discovered; for the whole mass will appear of an uniform tint. The second kind of doublet is formed by cementing a coloured plate of glass on the base of a rose or brilliant-cut crystal: by this the whole stone acquires the colour of the plate.
There are found in nature, many coloured kinds of crystal. These are often confounded with precious stones; and, as such, are made into female ornaments of different kinds. The following are the principal of them.
4879. Common Amethyst.—This is a violet-coloured crystal, which acquires considerable brilliancy in polishing, and is sometimes of sufficient size to be formed into columns more than a foot in height, and several inches in diameter. When the colour is good, and uniformly diffused, amethysts are cut into necklaces, bracelets, ear-rings, and seals; and, when less pure, they are manufactured into snuff-boxes. They are valued in proportion to the depth of their colour, and to their perfect transparency. The most favourite form in which they are made up is in necklaces; and as it is not easy to find a number of perfect stones with precisely the same tint of colour, necklaces of this description are very valuable. The finest that is known was in the possession of her late Majesty. When the colour is not uniformly diffused, jewellers sometimes expose amethysts, for a little while, in a mixture of sand and iron-filings, to a moderate heat; and, by this process, their appearance is rendered more uniform.
The amethyst being almost the only coloured stone that can be worn with mourning, it derives, from this circumstance, a considerable addition of value.
This species of gem was well known to the ancient Greeks and Romans, and was held by them in great esteem. Its name is derived from the Greek language, and implies a power of preventing intoxication; which (originating no doubt in the resemblance of its colour to that of wine, and the absurd doctrine of sympathies) it was believed by the ancients to possess. They ascribed to it many other virtues, equally surprising and equally absurd; particularly that the wearing of it would expel melancholy, procure the confidence and friendship of princes, render people happy, and even dispel storms of wind and hail. The ancients frequently engraved upon amethyst; and their favourite subject was the representation of Bacchus and his followers.
The most valuable amethysts are imported into 49Europe from India and Ceylon. These, although they are with truth denominated oriental, must be carefully distinguished from the true oriental amethyst (55), which is a much more valuable gem. The amethysts next in esteem are found in Brazil, and are procured in the mining districts of that country. Siberia, and various countries in Europe, especially Germany and Spain, also furnish very beautiful amethysts; and inferior stones of this description are even found in the mountainous districts of some parts both of Scotland and Ireland.
80. False Ruby is a crystal of red colour, and found in Bohemia, Silesia, and Barbary.
81. False, or Water Sapphire is a blue crystal, which does not differ much in appearance from the true sapphire, but is considerably less hard. This kind is found in Bohemia, Silesia, and some parts of Switzerland, but it is not so valuable as the last.
82. False Emerald is a green variety of crystal, the scarcest and most valuable of all the coloured kinds. It is chiefly found in Saxony and Dauphiny.
83. Yellow, or Topazine Crystal is a stone of wine-yellow colour. It is found in Brazil and Bohemia, but has no other alliance with the true topaz than its colour.
84. Cairn Gorum Crystals are obtained in various parts of Scotland, but particularly from a mountain of that name in the county of Aberdeen. They are usually of smoky yellow or brown colour, and are, at this time, so much in request for ornamental articles of dress, that several lapidaries have been induced to settle in Aberdeen, who are constantly employed in cutting them for seals, rings, necklaces, brooches, and other trinkets. When these crystals are of deep and good colour, they are nearly as estimable as topazes; and, if clear and large, they are sold at a high rate. The 50price of inferior seal-stones varies from ten shillings to three or four pounds each; but those of superior beauty will produce from five to ten guineas. Such specimens as have a pure and full yellow colour are often sold for topazes. When they are muddy, the lapidaries have the art of entirely dissipating the colour, and giving them a transparent lustre. This is done by means of heat, which will dissipate the colour of every species of crystal.
85. AVANTURINE is a quartz, generally of reddish colour, sprinkled with yellowish shining points of mica (123), which are dispersed through its whole substance.
A French artist, some years ago, having by accident, or “par aventure,” suffered a quantity of brass filings to fall into a vessel of melted glass, afterwards found that it was admirably calculated for vases and different kinds of ornamental work. Hence he denominated it avanturine, a name which mineralogists have since applied to those natural objects of which this production of art was an apparent imitation.
Avanturine is found in some of the countries bordering upon the White Sea, in Spain, and some parts of France. In the late Leverian Museum there was a piece which weighed near five pounds, and was unique both for beauty and magnitude. It had been discovered in 1788, amongst the ruins of the triumphal arch of Julius Cæsar in the valley of Suse, in Piedmont; and was purchased of the person who found it for 200 guineas. Avanturine is cut into various ornamental articles, which are sometimes sold at a very high price.
Imitations of it are very common, and are formed by the simple operation of throwing brass or copper filings into coloured glass in a state of fusion.
86. CATS-EYE is a stone of brownish grey colour, tinged with green, yellow, white, or red; semi-transparent, and reflecting from its interior a splendid white line or speck, which 51varies according to the direction in which the stone is held to the light.
It is found in pieces that are rounded, massive, or blunt-edged.
These stones are considered by some writers as varieties of quartz (76), and by others as a kind of opal (102). They are sometimes found in Hanover, but are chiefly brought from the island of Ceylon. It is usual to cut them before they are exported, and generally in a convex and oblong form, without facets, and in such manner as to bring the streak which intersects them into the centre. Among the king of Candy’s jewels, which were sold by auction in London, in June 1820, was a cat’s-eye of extraordinary magnitude and beauty. It was two inches in diameter, of dark colour, and nearly hemispherical. This stone was set in gold, with small rubies round it, and was sold for more than 400l.
Cat’s-eyes are chiefly used for setting in rings. Their size seldom exceeds that of a hazel nut; but there was one in the cabinet of the Dukes of Tuscany, which was nearly an inch in diameter. Those that are the most highly esteemed are of an olive-green, or red colour.
87. WOODSTONE is a very hard mineral substance, supposed to have been wood petrified with a siliceous mineral called hornstone.
It is of various colours; and has not only the external appearance, but the internal organization of wood.
This extraordinary mineral is found embedded in sandy loam, in alluvial soil (269), and occurs in various parts both of Europe and Asia. It has been found in ferruginous sand, near Woburn, in Bedfordshire, and near Nutfield, in Surrey. Immense pieces of it are discovered in some places in the original shape of the trees; trunks, branches, and roots. In the year 1752 the whole under part of the trunk of a tree, with its branches and roots, was found, in a state of woodstone, 52near Chemnitz, in Saxony; and, in the Electoral Cabinet at Dresden, there is part of the trunk of a tree, from the same place, which measures five feet in length and as many in thickness.
Woodstone is in considerable request by lapidaries. It takes a good polish, and is made into beads for necklaces, and other female ornaments. In the East Indies it is generally called Petrified Tamarind Tree.
88. COMMON SAND is a granulated kind of quartz; or consists of rounded grains of small size, which have a vitreous or glassy surface.
It is usually of white or yellowish colour; but is sometimes blue, violet, or black.
In the torrid regions of Africa and Asia there are immense tracts of desert covered only with sand, so dry and light as to be moveable before the wind, and to be formed into vast hills and boundless plains. These are incessantly changing their place, and frequently overwhelm and destroy the travellers whose necessities require them to enter these dreary realms.
Sand has numerous uses. When mixed in due proportion with lime, it forms that hard and valuable cement called mortar. Melted with soda (200) and potash (205) it is formed into glass; white sand being used for the finer kinds, and coarse and more impure sand for bottle glass. A very pure kind of sand which is found in Alum Bay, on the west side of the Isle of Wight, and on some parts of the coasts of Norfolk, is in great request by glass-makers. Sand is also employed in the manufacture of earthenware; and its utility in various branches of domestic economy, but particularly for the scouring and cleaning of kitchen utensils, is well known. In agriculture sand is used by way of manure, to all soils of clayey lands: as it renders the soil more loose and open than it would otherwise be. The best sand for this purpose is that which is washed by rains from roads or hills, or that which is taken from the beds of rivers.
53There is a kind of sand which is naturally mixed with clay, and has the name of Founder’s Sand, from its being chiefly employed in the formation of moulds to cast metals in. At Neuilly, in France, there is a bed of perfectly transparent and crystalline sand. Each grain, when examined with a magnifying glass, is seen to consist of a perfect six-sided prism, terminated by two six-sided pyramids.
The uses of the different kinds of Sandstone will be enumerated in the account of the rocks (267, 268).
89. LYDIAN STONE is a kind of flinty-slate, of greyish or velvet-black colour, not quite so hard as flint, opaque, and about twice and a half as heavy as water.
It is usually massive, and, internally, has a glimmering appearance.
This mineral occurs in beds in primitive clay-slate (257); and is found in Bohemia and Saxony, and also in the Pentland hills near Edinburgh. It was first noticed in Lydia, whence it derived its name.
It is sometimes used as a touchstone to ascertain the purity of gold and silver. This was its use among the ancients. The metal to be examined is drawn along the stone so as to leave a mark, and its purity is judged by the colour of the metallic streak. A good touchstone should be harder than the metals, or metallic compounds to be examined; if softer, the powder of the stone mixes with the trace of the metal and obscures it. A certain degree of roughness on the surface of the best stone is also requisite, that the metal to be tried may leave a trace or streak sufficiently distinct. It must not, however, be too rough, otherwise the particles of the metal will be hid amongst its inequalities, and no distinct trace will be formed. The touchstone should also be of black colour, as this tint shows the colour of the streak better than any other.
90. FLINT is a peculiarly hard and compact kind of stone, generally of smoke-grey colour, passing into greyish white, 54reddish, or brown. It is nearly thrice as heavy as water, and when broken will split, in every direction, into pieces which have a smooth surface.
It is very common in several parts of England, generally among chalk, arranged in a kind of strata or beds, and in pieces that are for the most part either rounded or tubercular.
The property which flint possesses of yielding sparks, when struck against steel, has rendered it an article of indispensable utility in the system of modern warfare. To this substance the sportsman also is indebted for a means of obtaining his game. The art of cutting, or rather of breaking, this stone into gun-flints is of modern date, and was for a long time kept secret. The most absurd and contradictory accounts have been given of it by various writers; and it is only of late that the true mode has been rendered public. It consists in striking the stone repeatedly with a kind of mallet, and bringing off at each stroke a splinter which is sharp at one end and thick at the other. These splinters are afterwards shaped, by placing them upon a sharp iron instrument, and then giving them repeatedly small blows with a mallet. During the whole operation the workman holds the stone in his hand, or merely supports it on his knee: and the operation is so simple, that a good workman has no difficulty in making 1500 flints in a day. The manufacture of gun-flints is chiefly confined to England, and two or three departments in France. In Prussia an attempt was once made to substitute a kind of earthenware or porcelain for flint; and such was, for some time, used by the Prussian soldiers. All the kinds of flint are not equally adapted for guns: the best are the yellowish grey; the dark smoke and ash-grey varieties are also used, but they are neither so easy to be split, nor do they afford such thin fragments as the other; and, owing to their greater hardness, they wear the lock sooner.
Flint is employed in the manufacture of porcelain and glass. For this purpose it is heated red hot, and, in 55that state, is thrown into cold water. It is then of a white colour, and capable, without difficulty, of being reduced to powder, either in a mortar or by a mill. After this powder has been passed through fine sieves, some aqua fortis is poured upon it, to dissolve any particles of iron which it may have acquired in the grinding. The powder is then several times washed in hot water, and afterwards dried for use. The glass that is manufactured from this substance is perfectly transparent and faultless.
Glass is made by mixing sand, or prepared flint, with a certain proportion of soda (200) or potash (205); and exposing these substances, in a furnace, to a violent heat. When they are in a perfectly fluid state, part of the melted matter is taken out at the end of a long hollow tube. This is done by dipping the tube into it, and turning it about until a sufficient quantity is taken up; the workman, at each turn, rolling it gently upon a piece of iron, to unite it more intimately. He then blows through the tube till the melted mass, at the extremity, swells like a bubble; after which he rolls it again on a smooth surface to polish it, and repeats the blowing until the glass is brought as nearly to the size and form of the vessel required, as he thinks necessary.
If he be forming a common bottle, the melted matter at the end of the tube is put into a mould of the exact size and shape of the body of a bottle; and the neck is formed by drawing out the ductile glass at the upper extremity.
If he be making a vessel with a large or wide orifice, the glass, in its melted state, is opened and widened with an iron tool; after which, being again heated, it is whirled about with a circular motion, and, by the centrifugal force thus produced, is extended to the size required. Should a handle, foot, or any thing of similar kind be required, that is made separately, and stuck on in its melted state.
Window glass is made in a similar manner, except that the mass at the end of a tube is formed into a 56cylindrical shape. This being cut longitudinally by scissars or shears, is gradually bent back until it becomes a flat plate.
Large plate glass for looking-glasses is made by suffering the mass, in a state of complete fusion, to flow upon a casting table, with iron ledges. These confine the melted matter, and, as it cools, a metallic roller is passed over it, to reduce it to an uniform thickness.
Glass utensils, unless very small and thin, require to be gradually cooled in an oven. This operation is called annealing, and is necessary in order to prevent them from cracking by change of temperature, wiping, or slight accidental scratches.
It appears that the manufacture of glass was known very early; but glass perfectly transparent was esteemed of extremely high value. It is stated that the Emperor Nero purchased two glass cups with handles for a sum which was equivalent to 50,000l. of our money. The windows of some of the houses of the ancient city of Pompeii, which was buried by an eruption of Mount Vesuvius, in the year 79, were glazed, but the glass was thick, and not transparent.
By many persons flint is used as a test for ascertaining the purity of silver coins. This is done by rubbing them upon the flint; and if the mark which they leave be not perfectly white, they are rejected as counterfeit.
91. CALCEDONY is a species of quartz, generally of whitish, bluish, or smoke-grey colour; and, when broken, it appears internally dull, and somewhat splintery.
It is generally found in a massive state, is harder than flint, generally semi-transparent, and 2½ times heavier than water.
The name of this stone is derived from Chalcedon, in Upper Asia, whence it appears to have been originally obtained, and where it is still found in considerable abundance. Several superb specimens of calcedony have been found in Britain, and particularly in 57some of the tin and copper mines of Cornwall. It occurs in several parts of Scotland; and in many of the countries of the Continent. In the Leverian Museum there was a specimen of calcedony, which weighed more than 200 pounds. Its whole surface appeared such that, at first sight, one might imagine it to have formerly been in a liquid state: it had much the appearance that thick oil has while boiling.
Few stones are susceptible of a higher or more beautiful polish than calcedony. Hence the different varieties of it are cut into ring and seal stones, necklaces, ear-pendants, small vases, cups, and snuff-boxes.
92. ONYX is a kind of calcedony, generally marked alternately with stripes of white and black, or white and brown.
Its name is derived from the Greek language, and has been given on account of its resemblance in colour to the whitish band at the base of the human nail. The distinction which appears to be made betwixt onyx and sardonyx, arises from the colours of the former being arranged either concentrically, or in a somewhat confused manner, and those of the latter in regular stripes or bands.
Both these kinds are highly esteemed by lapidaries, for the formation of vases, snuff boxes, and trinkets of various kinds. Of sardonyx the ancients made those beautiful cameos, many of which still ornament our cabinets. The ingenuity they have shown, in the accommodation of the natural veins and marks of the stone to the figures engraven upon them, is such as to excite, in many instances, the greatest admiration.
It is said that we are entirely ignorant of the country whence the ancient artists obtained the large specimens of sardonyx which are now found in some cabinets.
Onyx is imported from the East Indies, Siberia, Germany, and Portugal.
93. CARNELIAN is another kind of calcedony usually of a red or flesh colour, though sometimes white, orange, or yellow.
58On several of the British shores carnelians are found with other pebbles: but the most beautiful and valuable kinds are imported from the East Indies. These are sometimes so large as to measure nearly three inches in diameter. The kinds principally in request are those of pure white, and bright red colour; and jewellers have the art of changing the colour of the yellow varieties to red, by heat.
No stone is so much in request for seals as carnelian. It is likewise cut into beads for necklaces, and stones for ear-rings; into crosses, bracelets, and other trinkets, which, in India, form a considerable branch of traffic. The amount of the sale value of different kinds of carnelian goods vended by the East India Company in 1807, was 11,187l.: but, in other years, it has not usually been so much as half that sum.
Formerly carnelians were exported from Japan to Holland; and thence were carried to Oberstein, in France, to be exchanged for the agates of that country, which were exported to China.
The carnelian was much esteemed by the ancients; and many fine engraved carnelians are preserved in different collections.
94. CHRYSOPRASE, an extremely hard kind of stone, of clear and delicate apple-green colour, is considered to be a kind of calcedony.
This beautiful mineral has hitherto been found only in the vicinity of Kosemitz, and in a few other parts of Lower Silesia. It is susceptible of a high polish, and is much prized by jewellers when its colour is deep and pure. Its colour, however, is so fugitive, that, if kept in a warm and dry situation, it loses the greatest part of it; and if exposed to moisture it becomes much altered. Lapidaries assert, that great care ought to be taken in the polishing of it;—pretending that if, from want of sufficient moisture, or by the too rapid motion of the wheel, it be over-heated, it will become whitish or turbid.
59Chrysoprase is generally cut into a convex form, or what jewellers call en cabochon; and is set with green taffeta beneath it, as foil. It is used for ring stones, brooches, and other ornaments; and is found to harmonize well with diamonds and pearls. The larger and more impure masses are cut into snuff-boxes, seal stones, and similar articles. Some of the finest specimens of chrysoprase that are known, are to be seen in the cathedral church of Prague, where a small closet is inlaid with them.
Imitations of chrysoprase are sometimes imposed upon the public; but these are easily known by persons who are acquainted with the nature of precious stones.
95. BLOODSTONE, or HELIOTROPE, is an opaque stone of the quartz family, generally of dark green colour, with a somewhat bluish cast, and marked with blood-red spots or stripes.
It usually occurs in masses of irregular form; and, when cut thin, is sometimes translucent at the edges.
The most valuable kinds of bloodstone are imported from the East. They are not so opaque as those which are found in Germany, and are marked with more vivid spots. As bloodstone is capable of a high polish, and is even better calculated for engraving upon than carnelian (93), it is in great request for seal stones, for the tops and bottoms of snuff-boxes, and other articles on which costly gold mountings are frequently bestowed. Its dark colour and opaque appearance prevent its being much used for beads. Great quantities of it are consumed in China as ornaments to the girdle clasps of the superior ranks of people. Absurd as it may appear, many persons entertain a notion that this stone worn in the dress will prevent bleeding at the nose. Good bloodstone and carnelian are considered to be about the same value.
There are many cameos and intaglios, both by ancients and moderns, executed in bloodstone. In the National Library at Paris, there is a fine engraved stone of this kind, representing the head of Christ whilst undergoing 60the punishment of scourging, and so cut that the red spots are made to represent drops of blood.
The ancients procured bloodstones chiefly from Ethiopia; but, at present, the most highly esteemed varieties are brought from Bucharia, Great Tartary, and Siberia. A kind of mineral nearly resembling this is found in Rum, one of the western isles of Scotland.
The spots in bloodstone are particles of red jasper.
96. JASPER is a species of quartz, and one of the hardest stones with which we are acquainted. It varies much in colour, being red, green, yellow, blue, olive, violet, black, and often variegated, spotted, or veined with several other colours. It is usually opaque, but is capable of receiving a beautiful polish.
This stone is found in large and shapeless masses, and constitutes an ingredient in mountains of various parts of the world.
Such is the hardness of jasper, that the savages of Canada avail themselves of it for the fabrication of the heads of javelins, and sometimes also of arrows. It is used by artists for the formation of vases, snuff-boxes, seals, and trinkets of various kinds; and formerly cups and saucers were sometimes made of it. Many beautiful antique engravings have been made upon jasper.
In the province of Andalusia, in Spain, there are four fine quarries of jasper. One of these is celebrated for a blood-red stone, streaked with white, exceedingly hard and very handsome, of which the beautiful columns of the tabernacle in the Escurial are made. This quarry is in the territory of Cogullus, in the archbishopric of Seville, and was purchased by the Crown in 1581; but was afterwards so far neglected that even the place where it lay was not remembered. It was, however, again discovered about the end of the reign of Charles the Third, after a very expensive search made by order of the government.
Jasper occurs in the Pentland hills, near Edinburgh, and in several other parts of Scotland; in the Shetland Islands, and Hebrides. It has been observed in most of 61the countries of the Continent; and is found, in great abundance, in Siberia.
97. Red Jasper is an opaque red stone which is found embedded in red clay-ironstone in Baden; and is cut and polished for various ornamental purposes. There are extant many fine antique engravings on red jasper.
98. Egyptian Pebble is a kind of jasper, that is found in globular or rounded pieces, and is distinguishable when cut or broken, by its numerous colours, arranged in concentric stripes or layers. It is chiefly brought from Egypt; and, as it is capable of receiving a fine polish, and when polished is very beautiful, it is manufactured into several kinds of ornamental articles. From the great abundance in which it is supplied, it is, however, much less valuable than carnelian (93). The colours of the Egyptian pebble frequently assume very singular forms. There was one in the Leverian Museum which exhibited, in the centre, the resemblance of a pantaloon, or a man wearing a fool’s cap.
99. Striped, or Ribbon Jasper, is marked with alternate stripes of different colours; and is found in Siberia, Saxony, and even in the Pentland hills, near Edinburgh. It receives an excellent polish, and is frequently cut into the tops and bottoms of snuff-boxes. The red and green layers of jasper, being well defined and regular, this kind is used for several purposes of ornament, particularly for cameos.
100. AGATE, or AGATE JASPER, as some mineralogists denominate it, is a semi-transparent stone of the quartz family, which is capable of receiving a high and very beautiful polish.
These stones are always found in a shapeless or massive form, and nearly of all colours, except bright red and green.
The name of agate is derived from the river Achates, in Sicily, in the vicinity of which these stones were obtained by the ancients in great abundance. They are now found in several parts of Scotland; in Iceland, Saxony, and Hungary; and they are occasionally brought into Europe from China and the East Indies.
62Agates are used in several kinds of ornamental work, and particularly for necklaces and seals. They are occasionally made into cups, the handles of knives and forks, hilts of swords and hangers, and the tops and bottoms of snuff-boxes. The less ornamental kinds are manufactured into small mortars, which are employed by enamellers and others, for pounding such substances as are too hard to be reduced in any other way. They are also made into instruments for grinding colours, and into polishers for the glazing of linen. In the Electoral Cabinet at Dresden, and the Ducal Cabinet in Brunswick, there are several elegant vases formed of agate.
The most beautiful agates which our island produces are known by the name of Scots Pebbles. These are found in various parts of Scotland, but principally on the sea-shore, in the neighbourhood of Dunbar. Agate pebbles are found on several of the English shores, as those of Suffolk, Dorset, Scotland, Wales, and Ireland; and sometimes even in gravel pits. Many of them will bear cutting and polishing as well as the best agates of foreign countries.
Agates are occasionally seen to be figured in very singular manner; but this, in some instances at least, is suspected to be the work of art. One is mentioned in the church of St. Mark, at Venice, which had the representation of a king’s head surmounted by a diadem. On another, was represented a man in the attitude of running. But the most remarkable of all seems to have been one which contained a representation of the nine Muses, with Apollo in the midst of them!
It must be remarked that agate is not, as some mineralogists imagine, a simple mineral, but that it is composed of various species of the quartz family, intimately blended together. It consists chiefly of calcedony (91), with flint, hornstone, carnelian (93), jasper (96), cacholong (105), amethyst (79), and quartz (76). Of these minerals sometimes only two, and sometimes three or more, occur in the same agate. Its varieties, consequently, are extremely numerous.
63101. Mochoa Stone is a kind of agate, which has on its surface the resemblance of moss; and this so nearly approaching a natural appearance, that some persons have actually supposed it to be occasioned by a condensation of moss into stone. Its name is derived from mocks, the German word for moss.
These stones are used for several ornamental purposes; and are not unfrequently imitated, by spreading a solution of copper in nitric acid or aqua fortis (30) over the surface of a plain agate, and then setting a small iron nail on its head in the middle. The acid unites with the iron, and deposits the copper in beautiful ramifications from the centre. The nail must then be removed, and the surface carefully washed by dipping the stone into warm water. Afterwards, on the application of a moderate heat, the copper becomes black. As, however, the deposition is merely superficial, it requires to be covered with glass, to preserve it from injury.
102. OPALS are a semi-transparent kind of stones, which have a milky cast, and, when held betwixt the eye and the light, exhibit a changeable appearance of colour.
They are always found in a shapeless or massive state, are brittle, and considerably less hard than most other precious stones.
The only opal mines in the world are those of Hungary. About four centuries ago, opals were obtained, in such abundance, from these mines, that upwards of three hundred persons were employed in them. They still produce opals, some of which are so valuable as to pass, in commerce, under the appellation of oriental opals, whilst others are so poor as to be of no value whatever to the jeweller. Opals are also found in other parts of Europe; and in the island of Sumatra and several parts of the East Indies.
Few precious stones are more beautiful than opals. Their elegant play of colours, brilliant blue, green, red, and yellow, variously modified, has procured for 64them a distinguished rank among gems. Notwithstanding this, they are but ill suited to the purposes of jewellery, on account of their softness, their great frangibility, and their sometimes splitting on a sudden change of temperature. They are usually set without bottoms; but sometimes with a black bottom, and sometimes with a foil of red, blue, or gold colour. Their value is such that a fine oriental opal is considered worth about twice as much as an oriental sapphire of the same size. By the Turks they are so peculiarly esteemed, that a fine opal of moderate size has sometimes been sold at the price of a diamond. The esteem in which they were held among the ancient Romans was such, that Nonius, the Roman senator, is stated to have preferred banishment to parting with a favourite opal which Mark Antony was anxious to possess.
In the abbey of St. Denys, near Paris, there was formerly a curious ancient opal which was green on the outside, and, when viewed against the light, exhibited a fine ruby colour: and in the Imperial Cabinet at Vienna, there are two pieces of opal, from the mines in Hungary, one of which is about five inches long, and 2½ inches broad; and the other the size and shape of a hen’s egg. Both these stones exhibit a very rich and splendid play of colours.
In the purchasing of opals great caution is requisite, as fine glass pastes have not unfrequently been substituted for them, and sold at enormous prices.
103. Hydrophanous Opal, or Oculus Mundi, is a kind of opal, the distinguishing characteristic of which is, that it gradually becomes transparent, and exhibits a beautiful play of colour after being immersed in water. It is either of a whitish brown, yellowish green, milky grey, or yellow colour, and opaque; and, when touched by the tongue, adheres to it.
The name of oculus mundi has been given to these stones from an internal luminous spot, which changes 65its position according to the direction in which they are held to the light. The countries in which they are chiefly found are Hungary and Iceland.
They are sometimes set in rings; and the prices at which they were formerly valued were, in the highest degree, unaccountable and absurd. At present their value is considerably lower, though they are still in great request as objects of curiosity. The phenomenon of their becoming transparent in water is supposed to be occasioned by that fluid soaking through their whole substance, in the same manner as the transparency of paper is occasioned by immersing it in oil. An hydrophanous opal weighing 27½ grains was kept four minutes in water, and, on being taken out, weighed 32½ grains, having received in this short period an augmentation of five grains, or more than one sixth part of its whole weight. When taken from the water, these stones as they dry become again opaque.
To preserve them in beauty and perfection, care should be taken not to immerse them in any but pure water, and to take them out as soon as they have acquired their full transparency. If these precautions be neglected, the pores will soon become filled with earthy particles: the stones will cease to exhibit their peculiar property, and will ever afterwards continue opaque.
104. Common Opal is a semi-transparent kind of opal, which does not exhibit any changeable refraction of colour. It is found in Germany, France, Italy, and other countries of the Continent, and is employed for brooches and other ornaments. A green-coloured Saxon variety is sometimes cut into ring-stones.
105. Mother-of-Pearl Opal, or Cacholong, is a milk-white, yellowish, or greyish-white kind of opal, which occurs in Iceland, Greenland, Spain, and the island of Elba. It is sometimes cut into a concave form, for brooches, and other female ornaments. Italian artists also use it for mosaic work.
66106. Wood Opal appears to be wood that, by some extraordinary operation of nature, has been converted into opal. Some specimens exhibit, very beautifully, the ligneous texture. This kind of opal is chiefly cut into plates for the tops and bottoms of snuff-boxes. It is found in alluvial land in some parts of Germany and Hungary. Several years ago the trunk of a tree, penetrated with opal, and so heavy that eight oxen were requisite to draw it, was found in Hungary.
107. OBSIDIAN is a kind of glass, generally of blackish colour, formed in volcanoes, from which it issues in thick streams.
This substance has been used for various purposes. It is possible to cut and polish it; but its brittleness and frangibility are so great, that, without much care, it will fly into pieces during the working. The reflectors of telescopes are sometimes formed of it. In Mexico and Peru obsidian is cut into mirrors; and the inhabitants of those countries used formerly to manufacture it into knives and other cutting instruments. Hernandez says that he saw more than a hundred of these knives made in an hour. Cortez, in a letter to the Emperor Charles the Fifth, relates that he saw razors that had been formed of obsidian. The natives of Easter and Ascension Islands use this substance for cutting instruments; and also for pointing their lances and spears, and, in place of flint, for striking fire with. According to the account that has been given by Pliny, the ancients sometimes formed obsidian into mirrors, and ornamental articles of different kinds. The Danish lapidaries, who obtain considerable quantities of it from Iceland, cut it into snuff-boxes, ring-stones, and ear-pendants.
Obsidian is found near Mount Hecla, and in other parts of Iceland. Sir George Mackenzie, during his journey through that island, observed an immense mass of this substance, which appeared to him to have been 67part of a stream that had flowed from a volcano. It is also found in Sicily, and several other islands of the Mediterranean; and in nearly all parts of the world where there are volcanoes.
108. PUMICE is an extremely light and porous mineral, of somewhat fibrous texture, and of white, grey, reddish, brown, or black colour.
From the texture of this mineral, which is chiefly brought from the neighbourhood of volcanoes, some persons have imagined it to be asbestos decomposed by the action of fire. Its lightness is such that, placed in water, it will float.
To mechanics and other artists pumice stone is a very useful mineral. It is employed for cleansing and smoothing the surface of wood, leather, metal, stones, glass, and other substances; and it is used by parchment-makers, curriers, and hat-makers. Hence it forms a considerable article of trade: and is exported from the Lipari Islands, in great quantities, to the different countries of Europe. Sailors in the Mediterranean rub their beards off with pumice, instead of shaving. On account of its porosity, it is used in Teneriffe as a filtering stone. It forms a pernicious ingredient in some kinds of tooth powder; and in Italy is ground and used instead of sand, in the making of mortar. Pumice occurs in Ireland, along with obsidian (107); and it abounds in several islands of the Grecian Archipelago.
109. LAPIS LAZULI, AZURE STONE, or LAZULITE, is a mineral of azure-blue colour in various shades, and generally accompanied with white or clouded spots, and also with pyrites (236), which have the appearance of golden veins or spots. Its texture is earthy, and fracture uneven. It is opaque, or nearly so, and, in some parts, is sufficiently hard to strike fire with steel. We are not informed that lapis lazuli is otherwise found than in shapeless masses or lumps.
68About fifty years ago this stone was an article much in fashion for various ornamental parts of dress. Being capable of very high polish, it was cut into beads, stones for rings, bracelets, and necklaces. It was also cut into ornamental vases, small statues, and the tops and bottoms of snuff-boxes; but of late it has been almost wholly out of use for these purposes. Before the French Revolution it was imported, to considerable extent, into that country from the Persian Gulf for the inlaying of richly-decorated altars; and its value was appreciated according to the proportion of its yellow spots or veins: these, by many persons, were erroneously considered to be of gold.
The most important purpose to which lapis lazuli is now applied is in the manufacturing of the beautiful and brilliant blue colour so much esteemed by painters, called ultramarine. For the making of this, such pieces are selected as contain the greatest proportion of blue substance, and consequently the least yellow or white. These are burned or calcined, reduced to a fine powder, made into a paste with wax, linseed oil, and resinous matters of different kinds, and afterwards separated by washing. The powder that is left in this operation, which requires much time and great attention to perform, is ultramarine.
There are few colours so little susceptible of change from the effects of time as ultramarine: the consequence of this has been that, as several of the ancient painters introduced it for the representation of blue drapery, their pictures, in many instances, are now devoid of harmony, as this colour alone has stood, whilst all the others have changed.
Lapis lazuli is principally brought from Persia, Natolia, and China; but it is also found in Siberia and Tartary. In Europe it has been discovered only in Germany, and among the ruins of Rome.
A coarse imitation of it is sometimes made by throwing copper filings into blue enamel whilst in a melted state.
110. COMMON FELSPAR is a hard kind of stone which varies much in colour, being flesh-red, bluish grey, yellowish white, milk-white, or brownish yellow.
It is found in a massive state, disseminated or crystallized in four, six, and ten-sided prisms; will strike fire with steel, and is sometimes opaque and coloured, sometimes transparent and whitish.
The name of felspar is derived from the German language, and signifies spar of the fields. It is a very common substance, and constitutes a principal part of many of the highest mountains of the world. When exposed to weather, it gradually acquires an earthy appearance, and at length passes into porcelain clay (118).
Felspar is of great use in the manufacture of the finer kinds of earthenware. Of the two substances which chiefly compose the porcelain of China, one called petunzé is a whitish laminar kind of felspar. This mineral is used in the celebrated porcelain that is manufactured at Sevres, near Paris, for the purpose of giving to it a white and transparent appearance. Previously to being used, it is pulverized, made into a paste, and suffered to dry. It is sometimes applied to the surface of ornamental vases in the form of enamel.
111. Amazon Stone is a green variety of felspar, which is found in small rolled pieces on the bank of the river of Amazons, in South America, whence it has its name. It is susceptible of a beautiful polish, and is often cut into ring-stones, brooches, and the tops of snuff-boxes. Lapidaries consider it to be most estimable when accompanied by mica, which gives it a kind of speckled perlaceous appearance.
112. LABRADOR FELSPAR is a very beautiful stone, of smoky grey colour, intermingled with veins and shades of blue, green, and golden yellow, exhibiting a brilliant play of 70colours, according to the position with respect to the light in which it is held.
The original discovery of this singular mineral was by the Moravian missionaries, on the island of St. Paul, near the coast of Labrador; but it has since been found in various parts of Norway and Siberia. Persons who have passed in boats along the rivers of Labrador, have described the extremely brilliant and beautiful appearance which the rocks of this substance frequently exhibit in shallow places, at the bottom of the water. The visitors of the late Leverian Museum will, no doubt, recollect a remarkably fine mass of Labrador felspar, the surface of which was polished, and exhibited some of the most splendid and beautiful colours that can be imagined. It was considered to have been the most capital specimen that was ever brought to England.
This mineral, on account of its hardness, its brilliancy, and its capability of receiving a high polish, is in considerable estimation among lapidaries for different kinds of ornamental work, particularly for the tops and bottoms of snuff-boxes, for brooches, and necklaces.
113. MOONSTONE, or ADULARIA, is the purest kind of felspar that is known; and is considered to have the same relation to common felspar that rock crystal has to common quartz. Its colour is white, sometimes with a shade of yellow, red, or green.
The translucent varieties of this stone, when viewed in a certain direction, sometimes exhibit a pearly and silvery play of colour. These are valued by jewellers, who cut them into a semi-globular form, and sell them under the name of moonstone. Those specimens are considered most estimable which, when cut in a very low oval, present the silvery spot in the centre of the stone. They are generally used for rings and brooches; and when set round with diamonds, their pearly lustre exhibits a striking and agreeable contrast with the brilliancy of that gem.
71Adularia is said to have been first discovered by an Italian mineralogist, near Mount St. Gothard, in Switzerland. He named it Adularia felspar, in the belief that the mountain on which he had found it was named Adula. This, however, was not the case; for Mount Adula is at some distance from St. Gothard, in the Grisons. This mineral has since been found in the granite of the island of Arran, in France, and Germany. The finest specimens are brought from Ceylon.
114. Clay is a mixture of alumine (33) and silex (38), and is too well known to require much description.
It is opaque, has an earthy texture, is about twice as heavy as water, when moistened is very ductile, adheres slightly to the tongue; and with its peculiar smell (called clayey) every one is acquainted.
115. COMMON CLAY, or POTTER’s CLAY, which is found in nearly every country of the world, is sometimes white, has a blue or yellowish tinge, or is brown or reddish.
It is the peculiar quality of this substance to become so hard by heat that it will even strike fire with steel. The ductility of clay, and its property of thus hardening in the fire, have rendered it an article of indispensable utility to mankind in all civilized countries. It is formed into eating vessels of almost every description; plates, dishes, cups, basins, bowls, and pans for keeping provisions in. For these almost any kind of clay may be advantageously used; but it is necessary to mix it with sand, for the purpose of rendering the vessels 72that are made of it more firm and strong. Those that are applied to culinary, and other uses in which it is requisite for them not to be penetrable by water, are covered with a glazing. This glazing, for coarse ware, is sometimes made with lead, and sometimes by throwing a certain portion of salt into the furnace. In the formation of the better kinds of earthenware, the clay is made into a paste with water, moulded into the requisite shape upon an horizontal wheel, the inside being formed by one hand of the potter, and the outside by the other, as the wheel turns round. When the pieces have been baked, they are dipped into a glazing mixture, consisting of white lead, ground flints, and water, and are exposed a second time to the fire. The different colours of earthenware are obtained by means of various kinds of metallic oxides (21).
The coarser kinds of clay are manufactured into bricks for the building of houses, and tiles for the covering and paving of them. These are formed in moulds of the requisite shape, afterwards dried for some time in the sun, and finally piled in kilns, and there baked to a proper degree of hardness. The earth for bricks ought to be sufficiently fine, free from pebbles, and not too sandy, which would render them heavy and brittle; nor ought it to be entirely free from sand, as this would make them crack in drying.
Clay is a substance of inestimable value for forming the bottoms of ponds, and the bottoms and sides of canals and reservoirs, to prevent the water from draining away. It also composes, in a great measure, those tenacious earths called arable soils. What is peculiarly denominated clay land is known by its holding water, and not soon drying when wetted. Such land requires much labour from the husbandman, before it can be sufficiently pulverized, or brought to a fit state for being productive of corn or grass.
116. Pipe Clay is a fine and yellowish white variety of common clay. It is very plastic, adheres strongly to 73the tongue; and, in a strong heat, is hardened, and rendered perfectly white.
It is of this clay that tobacco pipes are made, by the simple process of casting them in moulds, forming a hole through the stems by means of a wire, generally dipping the small end into some glazing material, and then baking them. Pipe clay is also formed into oblong pieces, dried, and employed for cleaning white woollen cloths, and for various purposes of domestic utility. It is likewise the basis of the yellow, of what is called Queen’s ware pottery. This is glazed in a manner somewhat different from that of common pottery. The glazing mixture consists of a certain proportion of carbonat of lead (239), ground flint, and flint glass, worked with water to the thickness of cream. The ware, before it is glazed, is baked, and thus acquires the property of strongly imbibing moisture. It is then dipped into the above composition; exposed a second time to the fire, by which the glaze it has imbibed is melted. A thin glossy coat is thus formed upon its surface, which is more or less yellow, according to the greater or less proportion of lead that has been used.
117. LOAM is a yellowish or brownish kind of clay; sometimes containing a considerable proportion of sand. It occurs in immense beds, and is found in almost every part of the world.
This substance, when mixed with straw or hair, to prevent it from cracking, is extensively used for the building of what are called mud cottages or houses. These are generally reared on a foundation of stone, or brickwork, to secure them from injury by the moisture of the earth. It is said to be the most advantageous practice to form the loam into bricks, and to dry these in the shade, and afterwards in the sun. The use of such bricks is of great antiquity. We are informed that the ancient city of Damascus, and even the walls of Babylon, were constructed of bricks made of loam.
74118. PORCELAIN CLAY is generally of white or reddish white colour, sometimes inclining to yellowish or grey. When dry, it absorbs moisture rapidly; and it becomes very tenacious when kneaded.
It is known from the other clays by the fineness of its particles, its soiling the fingers much when handled, and its fine but meagre feel.
The usual distinction betwixt earthen ware and porcelain is, that the former is opaque, and the latter semi-transparent. In the manufacture of porcelain the clay is sometimes used alone, and sometimes intermixed with other earths, or with felspar (110). The earliest manufacture of porcelain is supposed to have been that in China and Japan. The quantity produced in China must formerly have been extremely great; as not only a considerable portion of the eastern parts of the world, but almost the whole of Europe, was supplied with it. In a single province it is said that nearly a million of persons were at one time employed in this manufacture.
The manufactory at Sevres, in France, has long been celebrated both for the excellence and elegance of its porcelain. There are well-known manufactories of porcelain at Meissen in Saxony, at Berlin, and in Austria; but none of these are at present superior to our own, in Worcestershire and Staffordshire.
Porcelain clay occurs chiefly in countries which abound with granite (251) and gneiss (255). It is found in small quantity in Cornwall, and other granite districts of England, as well as in those of Scotland and Ireland. But the most valuable kinds of this clay are found in China and Japan.
The mineral is not used in the state in which it is found in the earth; but is previously washed several times to free it from impurities. After the process of washing, only about fifteen parts of pure clay remain: this is the kaolin of the Chinese. To form the composition of the porcelain, this clay is mixed, in certain proportions, with quartz (76), flint, gypsum (192), 75steatite (124), or other substances; and the mixture is sifted several times through hair sieves. It is afterwards moistened with rain water, and, in the form of a paste, is put into covered casks. Here a fermentation soon takes place, which changes its smell, colour, and consistence. Its colour passes from white into dark grey; and the matter becomes both tougher and more soft than before. The peculiar mode of preparing this mixture, and the art of rightly managing it, are secrets in most porcelain manufactories.
The next operation consists in giving to the paste thus formed the requisite shape of the vessels. This is done first by kneading it with the hands; and then by taking up certain portions of it, and turning it on a lathe, in the manner of common pottery (115), but with more care.
The third operation is the baking or firing. This is done in furnaces of a particular construction, and generally lasts from thirty-six to forty-eight hours. The state of the baking is shown by proof pieces, as they are called, which are placed in convenient situations, and can be drawn out, from time to time, for examination. The porcelain in this state, is named biscuit porcelain; and figures, and such other porcelain articles as are neither to be painted nor exposed to water are in the state of biscuit.
A fourth operation is covering the surface of the biscuit with a varnish or enamel. This is composed of pure white quartz (76), white porcelain, and calcined crystals of gypsum (192); and sometimes principally of felspar (110). These substances are carefully ground, then diffused through water, and formed into a paste. When used, the paste is diluted in water, so as to give it considerable fluidity; and the pieces of biscuit porcelain are separately plunged into it, in such manner as to cover their whole surface. These are then exposed to a heat sufficient to melt the enamel or covering: and in this state they constitute white porcelain.
If the porcelain is to be painted, it must again be 76exposed to heat in the furnace. The colours used for the painting of it are all derived from metals; and many of them, though dull when applied, acquire considerable lustre by the action of the fire. The colours are always mixed with some kind of flux, such as a mixture of glass (204), borax (208), and nitre, melted together, and afterwards ground.
Gum or oil of lavender is used for mixing up the colours. When the painting is finished, the pieces are exposed to a heat sufficient to melt the flux, and thus fix the colour.
119. TRIPOLI is a kind of clay of yellowish grey, brown, or white colour, sometimes striped or spotted, and of an earthy texture.
It feels harsh and dry to the touch; is soft, scarcely adheres to the tongue, and will not take a polish from the nail.
This substance obtained its name from having formerly been imported into Europe from Tripoli, on the north coast of Africa. It is, however, now found in several parts of Germany; and a granulated kind has been discovered in England.
Tripoli is used for the polishing of metals and stones. For this purpose, it is mixed with sulphur, in the proportion of two parts of tripoli to one of sulphur. These are well rubbed together on a marble slab, and are applied to the stone or metal with a piece of leather.
When tripoli is combined with red ironstone, it is used for the polishing of optical glasses. It is sometimes made into moulds, in which small metallic or glass figures and, medallions are cast; and a kind of tripoli is found near Burgos, in Spain, which is used as an ingredient in the manufacture of porcelain.
In Derbyshire,, and several parts of Staffordshire, is dug a kind of tripoli which has the name of rotten stone. This is considered to be a produce of limestone shale, which has undergone a decomposition by exposure to the air and moisture. It is used for most of the same purposes as tripoli.
120. CLAY SLATE, or ROOFING SLATE, is a kind of stone of foliated texture, and greyish, black, brown, green, or bluish colour.
It breaks into splinters, does not adhere to the tongue, yields generally a clear sound when struck, and is nearly thrice as heavy as water.
Vast and extensive beds of slate occur in different parts of the world; and this mineral sometimes constitutes even a principal portion of mountains. In our own country there are many important quarries of it, particularly in Westmoreland, Yorkshire, Wales, and Derbyshire.
The uses of slate are numerous and important; but its principal use is for the roofing of houses. For this purpose it is split into thin plates or laminæ. These are fastened to the rafters by pegs driven through them; and are made to lap over each other at the edges, in such manner as to exclude the rain and other moisture. The kinds which are preferred for this purpose are such as have the smoothest surface, and split into the thinnest plates. It is requisite that slates should be damp when they are split, otherwise this cannot be done without difficulty. Hence it is generally customary to split the masses as soon as possible after they have been separated from the rock.
Slate should not be porous. If it be so, rain and snow water will pass through it, and destroy the wood-work of the house on which it is placed. Porous slate is also liable to have moss and lichens grow upon and cover it. These plants retain moisture long, and keep the surface, and even the interior of the slate, moist; so that, during the winter season, by the freezing of the moisture, the slate is apt to split and fall into pieces. To ascertain whether the slate be of requisite compactness, it should be completely dried, then weighed, and afterwards soaked for some time in water. When taken 78out it is to be wiped with a cloth, and again weighed. If it have not acquired any considerable increase of weight, it is a proof of its being sufficiently compact. If, on the contrary, it have absorbed much of the water, and have become considerably heavier by the immersion, it is shown to be of a porous texture. Slates that are brittle are bad. If they emit a tolerably clear sound, when struck with a hammer, it is considered a proof that they are not too brittle: if, on the contrary, the sound be dull, they are soft and shattery. A good slate ought also to resist the action of a considerable degree of heat.
The slates that are principally used in London are brought from North Wales, from quarries that are worked near Bangor. There are also extensive slate quarries near Kendal, in Westmoreland; and the Kendal slates, which are of a bluish green colour, are more highly esteemed than those from Wales. They are not of large size, but they possess great durability, and give a peculiarly neat appearance to the roofs on which they are placed. The slate quarries near Easdale, in Scotland, are so extensive as to furnish annually more than 5,000,000 in number, and to give employment to upwards of 300 men.
French slates were much used in London about seventy years ago; but they have been found too small, thin, and light, to resist the winds and storms of this changeable climate.
Dark-coloured, compact, and solid slates are manufactured into writing slates, or table slates, as they are sometimes called. In the preparation of these, the slate, after it is split of proper thickness, is smoothed with an iron instrument. It is then ground with sandstone, and slightly polished with tripoli (119), and, lastly, rubbed with charcoal powder. It is cut into the requisite shape, set in a wooden frame, and is then ready for use.
For writing on these slates, pencils are used which are also made of slate. These, which are called slate pencils, 79are made of a particular kind of slate, that, on splitting, falls into long splintery fragments. It is necessary that the pencils should be considerably softer than the slate to be written upon, so that they may leave a whitish streak on its surface, without scratching it. Such is the shivery nature of the slate of which they are made, that, if it be exposed for some time to the action of the sun or frost, it is rendered useless. Hence, workmen are careful to cover it up and sprinkle it with water, as soon as it is taken from the quarry, and to preserve it in damp cellars. The pieces are afterwards split by a particular instrument, and then wrought into the requisite shape.
In some of the quarries in Derbyshire and Wales the slate is so thick as to admit of being split into large and tabular pieces. These are used for gravestones, and for slabs for dairies and cellars. Paving stones and mile-stones are also formed of them; and vessels for the salting of meat, and setting of milk in dairies. For the latter use slate is peculiarly well adapted, on account of its resistance of greasy or oily substances. But this property renders it unfit for any purpose for which it is requisite to be painted; as, the oil not entering the stone, the paint soon peels off, and leaves the stone as black as it was at first. Cut into narrow strips, slate has also been applied, in the neighbourhood of Bangor, North Wales, for the formation of fences.
When sufficiently solid for the purpose, slate is cut into inkstands, and turned into vases, and fancy articles of various kinds. And a singular circumstance has been remarked, that, if a window or door be suddenly opened, in an apartment where the workmen are turning these, they will sometimes fly in pieces; though, after the work is finished, they may be exposed to the usual changes of temperature without injury.
Pounded slate is advantageously used for cleaning iron and other works in metal. When well ground, and mixed with a certain proportion of loam, slate is made into moulds for the casting of metals in; and, when 80burned and coarsely ground, is used instead of sand in the making of a solid and impermeable mortar or cement, for the parts of buildings that are covered with water.
121. BLACK CHALK, or DRAWING SLATE, is an earthy substance, of slaty texture; generally of a greyish, sometimes a bluish black colour.
It is soft and smooth to the touch, and, in handling, stains the fingers.
To crayon painters, and other artists, black chalk is a very useful article. Considerable quantities of it are imported from France, Spain, and Italy. The best is brought from Italy. This is more free from gritty particles, more firm and compact in its texture, and in its touch much smoother than the chalk of any other country. It contains somewhat more than one-tenth part of its weight of charcoal. When prepared for use, it is cut into square pieces, which are sometimes enclosed in wooden cases, like black lead pencils. These pencils are said to become dry, hard, and unfit for use, by long keeping. To preserve them in greatest perfection, they should be kept in a moist place. Some artists prefer pencils that are made of the chalk finely ground, mixed with a certain proportion of gum water, and cast in moulds. Care should be taken not to put too much gum, as the pencils will not, in such case, leave any mark on the paper.
Drawing slate is sometimes used as a black colour for painting. For this purpose it is pounded or ground, and then mixed with oil or size, according to the kind of work for which it is required. When black chalk is strongly heated, it loses its colour, and assumes that of a reddish grey.
122. HONE, or WHET SLATE, is a well-known kind of stone, of somewhat slaty texture, and generally of dull white, or greenish grey colour. Its surface is smooth, and feels unctuous to the touch.
81These stones, when properly cut and smoothed, are of indispensable utility to carpenters, cutlers, and others, for sharpening their cutting instruments upon. Those of the finest grain are used for lancets, penknives, and razors. For this purpose their surface, when used, is covered with a small quantity of oil; by which, after a while, they are rendered considerably harder than they were at first. They ought to be kept in damp and cool places; for, if much exposed to the sun, they become too hard and dry for many purposes to which they are applied.
There is a vulgar and erroneous notion that hones are holly wood, which by lying in petrifying water, have been thereby converted into stone. The greater number of them have a fine and a coarse side. From the circumstance of their having been originally brought into this country from Turkey and the Levant, they are sometimes called Turkey stones. They are now found in Saxony and Bohemia, in North Wales, and near Drogheda, in Ireland.
The powder of whet slate is sometimes used, instead of emery, for the cutting and polishing of metals.
123. COMMON MICA, GLIMMER, or MUSCOVY GLASS, is a mineral substance of foliated texture, which is capable of being divided into extremely thin leaves that have a sensible elasticity, and are transparent.
The colour of mica is greenish, sometimes nearly black, reddish, brown, yellow, or silvery white, with, occasionally, a metallic lustre on the surface. Mica is so soft as easily to be scratched; and, when divided across the plates, seems rather to tear than break.
This is one of the most abundant mineral substances that is known. It not only occurs in a massive and crystallized state, but it enters into the composition of many rocks; is found filling up their fissures, or crystallized 82in the cavities of the veins which traverse them. In some countries, as in Siberia, it is an article of commerce, and is obtained from mines like other minerals. From these it is extracted by hammers and chisels. It is then washed, to free it from the impurities which adhere to it; split into thin leaves or pieces; and assorted into different kinds, according to their goodness, purity, and size. We are informed by the Abbé Haüy, that plates of mica a yard or more in width have been obtained from the mines in some parts of Russia.
Thin plates of mica are adopted, in many parts of Siberia and Muscovy, to supply the place of glass for windows. In the shipping of Russia it is considered preferable to glass, as the concussion produced by the firing of the guns does not shatter it. It is employed instead of window glass in Peru and New Spain; and also in Pennsylvania. Mica may be advantageously substituted for horn in lanterns, as it is not only more transparent, but is not susceptible of injury from the flame of the candle. It has, however, the inconvenience of soon becoming dirty; and of having its transparency destroyed by long exposure to the air. Mica is used for enclosing objects that are intended to be viewed by microscopes.
So plentiful is this substance in Bengal, that, for the value of five shillings, as much of it may be purchased as will yield a dozen panes, each measuring about twelve inches in length and nine in breadth, and so clear as to allow of ordinary objects being seen through them at the distance of twenty or thirty yards.
Mica, when powdered, is sold by stationers on the Continent, in place of sand, for absorbing ink in writing, but it does not dry sufficiently quick to be of much use in this respect. In Russia it is employed in different kinds of inlaid work. It is sometimes powdered, and intermixed with the glaze in particular kinds of earthen ware. The heat which melts the glaze has no effect on 83the mica: hence it appears, dispersed throughout the glaze, like plates or scales of silver or gold. Some artists use it in making artificial avanturines (85).
It must be observed that the best mica is of a pure pearl colour; and, when split into leaves, presents a smooth surface.
124. STEATITE, or SOAPSTONE, is a soft and unctuous substance, which has much the appearance of soap; and is generally of a white or grey colour, intermixed with greenish or yellowish shades.
It is somewhat more than twice as heavy as water; and is distinguished from indurated talc (135) by not splitting, like that substance, into slaty fragments.
In the counties of Devon and Cornwall, and the islands in the vicinity of the Lizard Point, this mineral is found in considerable abundance. It possesses many of the same properties as fullers’ earth, and is, like that substance, employed in the scouring of woollen cloths. When mixed with water it may be formed into a paste; and, in this state, it is easily worked, like clay, for the manufacture of earthen ware. In the porcelain manufactory at Worcester considerable quantities of steatite are employed. According to Dr. Shaw, the Arabs use it in their baths, instead of soap, to soften the skin.
As it becomes hard in the fire, and does not alter its shape, this substance has been successfully adopted for imitations of engraved gems. The subjects are engraved upon it with great ease in its natural state; it is then exposed to a strong heat; afterwards polished, and then coloured by means of certain metallic solutions.
We are informed by travellers, that some of the savage tribes eat steatite, either alone, or mixed with their food, to deceive hunger. The inhabitants of New Caledonia eat considerable quantities of it. Humboldt, the South American traveller, assures us that the Otomacks, a savage race of people, who live on the banks 84of the Orinoco, are almost wholly supported, during three months of the year, by eating species of steatite, or potter’s clay, which they first slightly bake, and then moisten with water. M. Golberry says that the negroes near the mouth of the Senegal mix their rice with a white kind of steatite, and eat it without inconvenience.
In some parts of Spain a variety of steatite is found, which is used by artists under the name of Spanish chalk. When slightly burned, this mineral is sometimes used as the basis of rouge.
125. Figure Stone is a kind of steatite, which has, internally, a glimmering and resinous lustre, and a slaty or splintery fracture.
From its softness, and yet solidity of texture, this mineral can easily be fashioned into various shapes, even with a knife. Hence in China, where it frequently occurs, it is cut into grotesque figures of various kinds, which the French call magots de la Chine, into cups, vases, pagodas, snuff-boxes, and other articles.
126. MEERSCHAUM, or SEA-FROTH, is a singular kind of mineral, of yellowish or greyish white colour, sometimes so light as to float in water: when fresh dug it has nearly the consistence of wax.
If exposed to a strong heat, it becomes so hard as to yield sparks with steel.
The principal use to which meerschaum is applied is in the formation of the bowls or heads of tobacco-pipes used by the Turks, and the quantity consumed for this purpose is very great. It is found in a fissure of grey, calcareous earth, about six feet wide, near Konie, in Natolia, where upwards of six hundred men are employed in the digging and preparation of it; and the sale of it supports a monastery of dervises established at that place. The workmen assert that it grows again in the fissure, and puffs itself up like froth. It is prepared for use by being first agitated with water in great 85reservoirs, then allowed to remain at rest for some time. The mixture soon passes into a kind of fermentation, and a disagreeable odour, resembling that of rotten eggs, is exhaled. As soon as this smell ceases, the mass is further diluted with water, which, after a while, is poured off. Fresh water is repeatedly added, until the mass is sufficiently washed and purified. The meerschaum, in this state, is dried to a certain degree. It is then pressed into a brass mould, and, some days afterwards, is hollowed out so as to form the head of the pipe. It is subsequently dried in the shade, and lastly is baked. In this state the pipe heads are brought to Constantinople, where they are subjected to further processes. They are first bailed in milk, and next in linseed oil and wax; and, when perfectly cool, are polished with rushes and leather. The boiling in oil and wax renders them capable of receiving a higher polish than could otherwise be given. When thus impregnated, they also acquire, by use, various shades of red and brown, which are thought to add considerably to their beauty. In Turkey, and even in Germany, meerschaum pipes that have been much used are more valued than those newly made, and this solely on account of the colouring they possess. Indeed there are people in those countries whose only employment consists in smoking tobacco pipes, until they acquire the favourite tints of colour. By long use, the heads become black: but if boiled in milk and soap, they are soon rendered white again.
It is asserted that the Turks spread meerschaum on bread, and eat it as a medicine; and that they cover with it the heads and eyes of dead bodies, previously to interment. As it lathers with water like soap, it is used by the Turkish women for washing their hair; and, as it absorbs oily matters, it is occasionally used, as fuller’s earth is with us, for the cleansing and scouring of cloth.
We are informed by Pliny, that a kind of bricks were made by the ancients, so light that, when dried, they 86would float in water. He describes them to have been formed of a spongy kind of earth, and to have had some resemblance to pumice stone, which he says might perhaps be applied to the same purposes as these bricks, if it could be obtained and wrought in sufficient quantity. Bricks of similar description have lately been made of a mineral substance found near Sienna, in Italy, and which is supposed to be meerschaum.
A kind of meerschaum has lately been discovered, in veins, in the serpentine (132) of Cornwall.
127. BOLE is an earthy mineral, of yellowish or reddish brown colour, soft, and somewhat unctuous to the touch, and generally found in a massive state.
It exhibits internally a glimmering lustre; and, when put into water, immediately absorbs it, and breaks down into small pieces with a crackling noise. This mineral is farther distinguished by its fracture being conchoidal, or appearing somewhat like the impression of a shell; and by its adhering strongly to the tongue.
Although bole is at present little used except as a basis of tooth powder, and a coarse kind of paint, it was formerly considered an important article in medicine, and used as an astringent. We are informed that tobacco pipes are sometimes made of this mineral; and that it is employed as an ingredient in the glaze of some kinds of earthen ware.
It is chiefly imported from the Levant; though it has also been found in considerable beds in Silesia and Saxony.
128. LEMNIAN EARTH is a kind of bole of yellowish grey, or yellowish white colour, sometimes marbled with rust-like spots.
It is distinguished from bole by being dry and not unctuous to the touch, dull internally, adhering slightly to the tongue, and its fracture being earthy.
With the ancients this mineral was considered an almost invaluable medicine. They procured it chiefly 87from Armenia, and the island of Lemnos, in the Grecian Archipelago. The Lemnian bole was held so sacred that it was dug in the presence of the priests of Venus, and, after having been mixed by them with goat’s blood, was moulded into cakes, which were impressed with the figure of a goat, to authenticate them. This done, it was administered as a consecrated remedy; and, even so lately as the sixteenth century, the vein of bole in Lemnos was annually opened on the sixth of August, and, after certain prayers by the priests, so much of the earth was taken out as was thought sufficient for the consumption of the ensuing year. The entrance was then closed, and the severest punishments were denounced against any one who should open it without permission. A portion of the earth was sent to Constantinople, where it was made into small cakes, and sealed by the ministers of the Emperor; the remainder was prepared in the island, and was impressed with the seal of the Governor. Not many years ago, it was customary with certain empirics on the Continent, to sell this substance in sealed packets, as a nostrum of great value, and particularly as possessing astringent properties of very extraordinary nature.
129. FULLER’s EARTH is a well-known mineral, generally of greenish colour, more or less mixed with brown, grey, or yellow: of soft and almost friable texture, and somewhat unctuous to the touch.
When put into water it immediately absorbs it, and breaks down into a fine pulp.
This earth is valuable for its property of taking grease out of woollen and other cloths, which, on a large scale, is effected by the operation called fulling, whence its name has been derived. This operation, which is performed by a kind of water mill, called a fulling mill, is particularly necessary with respect to new cloths, to extract from them the grease and oil that have been used in their preparation.
Fuller’s earth was formerly considered an article of 88such importance in England that its exportation was prohibited under severe penalties. It was then employed for most of those purposes for which soap has since been so extensively applied. In the dressing of cloth it is now so indispensable, that foreigners, although they can procure the wool, are never able, without fuller’s earth, to reach the perfection of the English cloths: and, in this country, incalculable quantities of it are consumed. As an article of domestic utility, it might be much more frequently used than it is, as a substitute for soap, in the cleaning and scouring of wooden floors and wainscots.
There are extensive beds of fuller’s earth in several of the counties of England. London is principally supplied from those of Kent, Sussex, and Surrey. At Wavedon, near Woburn, in Bedfordshire, a peculiarly fine kind is dug up from pits at the depth of ten or twelve feet below the surface of the ground; and no country in the world is known to produce fuller’s earth of quality so excellent as that obtained in England.
130. JADE, or NEPHRITE, is a very hard and tough species of stone, of greenish or olive colour, somewhat unctuous to the touch, and looking as if it had imbibed oil.
It is found massive, in blunt-edged or rounded pieces.
Nothing has so much tended to make this stone known, as a superstitious notion that a piece of it suspended to the neck will dissolve stones in the kidneys. Hence has been attained its appellation of nephrite, or divine stone; and hence have originated all those numerous amulets in the form of oval plates, hearts, fishes, birds, &c. pierced with holes for ribbons to pass through, which are seen in collections of the curious. Some of the Indian nations make talismans of jade.
From the roughness and tenacity of this stone, in addition to its hardness, it is very difficult to be cut and polished; and even the best polish which it is 89capable of taking as so imperfect, that a person ignorant of its nature might consider it to be merely smoothed and rubbed with oil. The ancient artists executed in it many beautiful and delicate figures; and it is impossible but to admire the industry and perseverance by which they produced even chains, and other hollow kinds of work, in jade.
The Turks cut it into handles for sabres and daggers, and into several kinds of vessels, to which they attach great value.
Jade occurs in granite (251) and gneiss (255) in Switzerland; but the most beautiful specimens of this mineral are brought from Persia, Egypt, and Siberia.
131. Axestone is a kind of jade, but differs from it in having a slaty texture; and in being less transparent and less tough. This stone is found in China, New Zealand, and on the banks of the river of Amazons, in America. And it is said that several of the tribes of American Indians form of it the axes which they use in place of iron. To explain how these people have been enabled to work a substance so rebellious as this is even to the file, and to other instruments of steel (of which they know not the use), it has been presumed that, when the stone is first taken from the earth, it is considerably less hard than when, by drying, its humidity is evaporated: that in this state they work it, and subsequently harden it, in some peculiar manner, by exposure to heat.
132. SERPENTINE is a stone which, when polished, has a near resemblance to marble, is of dark green colour, or reddish; variously streaked, and spotted with lighter green, red, brown, and yellow.
It is found in beds, and in a massive state; is translucent at the edges; and, when pounded, the powder feels soapy to the touch.
There are few stones likely to prove more valuable in ornamental architecture, both for beauty and durability, than this. It admits of an excellent polish, which is not easily injured by the effects of air or water. It 90is also too hard to suffer the same inconveniences of being scratched or broken as marble; and its colours are stated to be indestructible. And such is the size of many of the blocks of serpentine, that columns of almost any dimensions may be wrought out of them.
Of the serpentine obtained from the Island of Anglesea, and lately known by the name of Mona marble, a great proportion was sent to London by Messrs. Bullock and Co. who, until the death of Mr. Bullock, had a large warehouse and polishing rooms for it in Oxford-street. The prevailing colours of this stone are red and green. The quarries were worked by them to considerable extent. They manufactured it into chimney pieces, slabs, columns, and other articles; and its great beauty, and its excellence, in many respects, over the generality of marbles, will recommend it strongly to the public notice.
The chief places in which serpentine has hitherto been found are near Bareuth, and Zöblitz, in Saxony; in some districts of Cornwall; about six miles west of the Paris copper mine, in the island of Anglesea; at Portsoy, in Bamffshire, and other parts of Scotland; and at Cloghan Lee, in the county of Donegal, Ireland.
At Zöblitz there are some extensive manufactories, in which serpentine is made into vessels and ornaments of various shapes, that are carried for sale over nearly all parts of Germany. Several hundred persons are there employed in the working of this stone.
The name of serpentine is derived from some of the varieties appearing coloured and spotted like a serpent’s skin. This stone, when found intermixed with primitive limestone, or crystalline white marble, differs in no respect from the celebrated verde antique marble (149).
133. POTSTONE, or LAPIS OLLARIS, is a greenish grey stone, unctuous to the touchy and so soft when first taken from the quarry as to yield to the pressure of the nail, yet not easily broken.
It is found in a massive state.
91In consequence of the softness and tenacity of this stone, it can be turned upon a lathe, and otherwise cut and wrought with great ease. Hence, in Egypt, Lombardy, Norway, and other countries where it is found, it is formed into various kinds of culinary vessels and lamps, which harden in drying, and are capable of withstanding the strongest action of fire. Vessels of this description were known to the ancients; and are particularly mentioned by Pliny, the Roman naturalist, who speaks of some that were highly wrought being very valuable.
Potstone is used in some countries for the lining of stoves, furnaces, and ovens; and it is so durable as to have, in some instances, stood unimpaired for several hundred years.
On the banks of the Lake Como, there were some extensive quarries of potstone, which had been worked from the beginning of the Christian era. These quarries, however, fell in, on the 25th of August, 1618, and destroyed the neighbouring town of Pleurs; which had previously obtained by means of them an annual revenue of about sixty thousand ducats.
134. COMMON, or VENETIAN TALC, is an earthy stone, capable of being divided into plates or leaves, which are soft and unctuous to the touch, somewhat transparent, and usually of greenish silvery white colour.
It leaves a white trace when rubbed upon any object.
Mica and talc have a near resemblance to each other; but the plates of the former, when bent, are elastic, while those of the latter are not.
Venetian talc is very abundant in the Tyrol and the Valteline. In a state of powder it renders the skin soft and shining; a property which appears to have suggested the idea of employing it as the basis of the cosmetic named rouge. This is prepared by rubbing together, in a warm mortar, certain proportions of carmine, or extract of the flowers of carthamus tinctorius, 92with finely powdered talc, and a certain portion of oil of benzoin.
The Romans prepared a beautiful blue or purple colour, by combining pounded talc with the colouring fluid of some particular kinds of testaceous animals, that are found among the submarine rocks of the coasts of the Mediterranean. According to Tavernier, the French traveller, the Persians whiten the walls of their houses and gardens with lime, and then powder them with a silvery white kind of talc; which, he says, gives to them a very beautiful appearance. Talc is now used by the Chinese, and was formerly used by the Europeans, in medicine,
135. INDURATED TALC, or FRENCH CHALK, is a heavy mineral, of close texture, and generally of greenish grey colour; unctuous to the touch, and having a somewhat slaty fracture.
It is found in a massive state; and leaves a white trace when rubbed upon any object.
This is a well known substance, which is in great request by carpenters, tailors, hat-makers, and others, as the lines that are drawn with it are not so easily effaced as those that are made with chalk, and particularly as they remain unaltered even under water. If lines be traced with it on glass, they remain invisible, or at least are scarcely perceptible by the naked eye, till breathed upon. This, it has been conjectured, in part depends on the comparative softness of the substance with which the impression is made; the condensation of the breath taking place more readily on the glass than on the talc that covers it, and the impression of the talc becoming more apparent by the contrast.
Indurated talc, when reduced to powder, is frequently employed for the purpose of removing stains, occasioned by grease, from silk and cloth. This it does effectually, and, in general, without injuring even the most delicate colour. Like potstone, it is sometimes manufactured into culinary vessels.
93This mineral is found in several parts of the continent of Europe; and in Cornwall, Scotland, and the Shetland Islands.
136. ASBESTOS is a greenish or silvery white mineral, of fibrous texture, which is found in many mountainous countries of the Continent, in the island of Anglesea, and in Scotland. It occurs in shapeless masses, and varies much both in weight and hardness.
The name of asbestos is derived from the Greek language, and signifies that which is inconsumable. This mineral, and particularly a silky variety of it, in long slender filaments, called amianthus, was well known to the ancients. They made it into an incombustible kind of cloth, in which they burned the bodies of their dead, and, by which means, they were enabled to collect and preserve the ashes without mixture. In the manufacture of this article they were not able to weave the asbestos alone; but, in the loom, were obliged to join with it linen or woollen threads, which were afterwards burned away.
Incombustible cloth was purchased by the Romans at an enormous expense. Sir J. E. Smith, when at Rome, saw a winding sheet of amianthus in the Museum of the Vatican. It was coarsely spun, but as soft and pliant as silk. The person who attended him set fire to one corner of it; and the same part burned repeatedly with great rapidity and brightness, without being at all injured. This interesting relic was discovered, in the year 1702, in a funeral urn, and contained burned bones, together with a quantity of ashes. It was nine Roman palms long, and about seven in width, and had been deposited in the library of the Vatican by order of Pope Clement the Eleventh.
Cloth made of amianthus, when greased, or otherwise contaminated with dirt, may be cleansed by throwing it into a bright fire. In this process the stains are burned out, and the cloth is restored to a dazzling white colour. Pliny, the Roman naturalist, informs us that he had himself seen table-cloths, towels, and napkins 94of amianthus taken from the table of a great feast, thrown into the fire, and burned before the company: and by this operation, he says, they became better cleansed than if they had been washed.
The inhabitants of some parts of Siberia manufacture gloves, caps, and purses of amianthus; and in the Pyrenees it is wrought into girdles, ribbons, and other articles. The finest girdles are made by weaving the most beautiful and silky filaments with silver wire. These are much prized by the women, not only on account of their beauty, but from certain mysterious properties they are supposed to possess.
The shorter fibres of amianthus have sometimes been manufactured into paper, but this is too hard for use. It has, indeed, been proposed to preserve valuable documents from fire, by writing them on paper made of amianthus. Such a plan might deserve consideration, if we possessed fire-proof ink; but until this be obtained, the fire-proof paper will be of little use.
When several of the long fibres of this mineral are placed together, they may be formed into wicks for lamps; and it has been asserted that such wicks are incombustible. Kircher, the German philosopher, had a wick made of amianthus which burned for two years without injury, and was at last destroyed by accident. It is said that the inhabitants of Greenland make use of amianthus for the wicks of their lamps.
This substance, although it will long continue unaltered in considerable heat, yet if the heat be much increased it ceases to withstand it, and is melted into a dense kind of scoria. In the island of Corsica asbestos is advantageously employed in the manufacture of pottery. Being reduced into fine filaments, it is kneaded with clay; and vessels made of this mixture are said to be lighter, less brittle, and more capable of sustaining sudden alterations of heat and cold than common earthenware.
137. CHRYSOLITE, or PERIDOT, is a soft gem, usually of yellowish green colour, though sometimes it is grass-green, or bluish green, but with a tinge of brown.
It is generally found in fragments and rounded pieces, and rarely crystallized. In the latter case its regular form is an eight, ten, or twelve-sided prism.
Though scarcely harder than glass, and consequently inferior to most other gems in lustre, these stones are not unfrequently used in jewellery, particularly for necklaces and ornaments for the hair; and, when well matched in colour, and properly polished, their effect is very good. They are, however, too soft for ring stones; for, by wearing, they soon become dull on the surface. But it is said that their lustre may, in some degree, be restored by immersing them in olive oil.
To give the greatest brilliancy to this stone, we are informed by Mr. Mawe, that a copper wheel is used, on which a little sulphuric acid, or spirit of vitriol (24), is dropped; and that, during the process, an highly suffocating odour is given out. But he is of opinion that the most advantageous way of working it would be that in which glass is cut.
Chrysolite is imported from the Levant, and is said to be found in Upper Egypt, and on the shores of the Red Sea.
138. BASALT is a greyish black and coarse grained stone, which is usually found either in globular distinct pieces or in groups of large columns, each of which has from three to eight sides, and is divided horizontally into numerous stones, that very exactly lie upon, or fit into each other.
The most remarkable assemblages of basaltic columns that are known are those called the Giants’ Causeway, on the coast of Antrim, in Ireland, and the Cave of Fingal, in the island of Staffa, one of the Hebrides, or Western Islands of Scotland.
96The former, which is believed by the common people to have been an artificial production, the vast labour of giants who formerly inhabited the country, consists of an irregular group of many thousand jointed pillars. Most of these are of considerable height; are in general five-sided, fifteen or sixteen inches in diameter, and each perfectly distinct from top to bottom, though so closely and compactly arranged that it is scarcely possible to introduce any thing betwixt them. This assemblage of columns extends into the sea to a distance unknown, and along a tract of the sea coast of nearly six miles.
The Cave of Fingal is accessible only by sea, and is formed by ranges of massive basaltic columns, fifty feet and upwards in height. The stone of which these columns are formed very much resembles that of the Giants’ Causeway.
In several parts of the world large masses of basalt are discovered, composing entire insulated mountains, of somewhat conical form. They are considered by some writers as volcanic productions, but the proofs of this are by no means satisfactory.
Amongst the uses to which basalt has been applied, two of the most important are as materials of an excellent and durable kind for building and paving. When burned and pulverized, these stones impart to mortar with which they are mixed the property of hardening under water. They easily melt, without any addition, into an opaque and black glass; and from them, under a certain modification, bottles of olive-green colour, and of extreme lightness, but great strength and solidity, have been formed. Some of the kinds have been advantageously employed as millstones. Basalt is occasionally used by artists for touch or teststones, to ascertain the purity of gold and silver; and goldbeaters and bookbinders, on the Continent, usually make their anvils or beating blocks of it.
Basalt, though harder, more brittle, and less pleasing in its colours than marble, was in considerable esteem 97among the sculptors of antiquity, on account of its great durability. Many fine works were consequently executed by them in this stone. Pliny, who has described several, states that the columns of it were sometimes so large as to admit of several figures being wrought out of them. The Emperor Vespasian had an entire statue, accompanied by the figures of sixteen children, cut out of a single column of basalt; this statue he placed in the Temple of Peace, and dedicated it to the Nile. The famous statue of Minerva, at Thebes, is described by travellers to have been formed of basalt. Antiques of basalt are always in a much better state of preservation than those of marble. Even such as are dug out of the earth still retain their original polish; and the finest touches of the chisel upon them are still unimpaired.
139. Lime, after it has been freed from extraneous matters by burning, is a mineral of whitish colour, and pungent, acrid, and caustic taste. It has the property of changing vegetable blue colours to green, and of corroding and destroying animal substances.
This mineral is found in nearly every country of the globe: but, in a native state, has not hitherto been discovered except in combination with some acid.[3] The 98process of purifying lime, or depriving it of the acid with which it is combined, is by burning. This is done in a large kind of furnace, called a kiln, where the limestone and fuel are heaped in alternate layers. After it has gone through this process it is called quick-lime, and has the above-mentioned appearance and qualities.
3. With carbonic acid (26) it forms common limestone, marble, chalk, and some other substances; with sulphuric acid (24) it constitutes alabaster, or gypsum; and with fluoric acid (27) it becomes that beautiful production, the Derbyshire spar.—All these, having lime for their bases, are denominated CALCAREOUS SUBSTANCES.
The uses of lime are numerous and important. The principal of these is in the formation of mortar, or cement for buildings. For this purpose it is first slaked, by having water poured upon it: a violent heat is thereby excited, and the lime falls into powder: it is then formed into paste by working it with water and sand. This, when dry, becomes extremely solid, hard, and durable. Various examples might be mentioned of buildings nearly two thousand years old, where the lime is, at this day, as hard as the stones which it cements together. Lime is also used for agricultural purposes: when spread upon land it is supposed to hasten the dissolution and putrefaction of all kinds of animal and vegetable substances, and to impart to it a power of retaining the moisture which is necessary for the vigorous growth of corn or grass. It is employed in the refining of sugar, in the manufacture of soap, in the melting of iron, and by tanners, in a state of solution, for dissolving the gelatinous parts of skins, and removing the hair from them. The manufacturers of glue mix it with that article, for the purpose of adding to its strength, and preventing its becoming flexible by the absorption of moisture. This mineral, if well dried, pounded, and mingled with gunpowder, in the proportion of one pound to two, is of great utility in the rending of stones and rocks: the mixture, it is said, will cause an explosion equal in force to three pounds’ weight of gunpowder. Lime, if swallowed or inhaled, is a virulent poison. Hence persons employed in lime-works are subject to very distressing complaints; and hence, if bread be adulterated with lime, it is extremely injurious. Notwithstanding this pernicious quality, lime is of considerable use in medicine. It is chiefly given in a 99state of solution, and in the proportion of half a pound of quick-lime to twelve pints of boiling distilled water. This preparation is called lime water.
The superb basin of Lampi, one of the principal reservoirs which furnishes the canal of Languedoc with water, was, some years ago, found to leak at the junction of the stones. The engineer who had the direction of the works caused lime to be slacked in the water. This, passing through the apertures betwixt the stones, formed a crust, or very white covering, over its whole surface, of so hard and durable a nature, that it now constitutes one solid and undivided substance, which the water cannot penetrate.
CARBONAT OF LIME.
140. COMMON LIME is a variety of carbonat of lime, or of lime in combination with carbonic acid (26), which is harder and heavier than chalk, usually of a greyish colour, and is always found in a massive state.
Vast mountains of limestone occur in several countries of the globe; but no where is lime more abundant than in some parts of England and Wales. It forms, in particular, nearly the whole mountainous districts of Derbyshire and Shropshire, and encloses, in its substance, numerous veins of lead ore, calamine, and other important mineral productions.
Its uses have been already described (139).
141. CHALK is a white or yellowish kind of limestone, too well known to need any description.
It is found abundantly in many of the southern counties of England, and is usually procured from large open places, called chalk-pits, by digging. In some parts of Kent, however, the workmen save themselves, in this respect, much trouble. They undermine the sides of hills to a certain depth, then dig a trench at the top as far distant from the edge as the mining extends at the bottom. This trench they fill with water, which soaks through during the night, and the whole 100mass is thereby loosened, and falls down before morning.
The harder and more compact kinds of chalk are cut into blocks, and used as building stones. When burned and formed into lime, chalk becomes an excellent mortar: nearly all the houses in London are cemented with chalk mortar. It is also used as lime in agriculture. As it readily imbibes water, it is used by starch-makers, chemists, and others, to dry precipitates upon. With isinglass or the white of eggs it forms a valuable lute or cement. By artists it is in request for the construction of moulds to cast metals in; and by carpenters and others, as a material to mark with. Chalk is one of the most useful absorbents that are employed in medicine: it likewise gives name to an officinal mixture, to a powder, and a potion.
When pounded and cleared from gritty particles, it has the name of whiting. In this state it is used for the cleaning and polishing of metallic and glass utensils; for whitening the ceilings of rooms, and numerous other purposes. Spanish white is the same substance cleansed with peculiar care; and the Vienna white, which is used by artists, is perfectly purified chalk.
142. MARBLE is a compact and close-grained kind of limestone; so hard as to admit of being polished. It is this quality which principally distinguishes it from other calcareous substances.
Although nearly all the numerous kinds of marble may be burned, and thus converted into quick-lime, their use in ornamental architecture, &c. is so important as, in general, to prevent their application to the inferior purpose of mortar. Marble has been known from a very early period. The Book of Esther, in the Old Testament, describes the palace of Ahasuerus to have had “pillars of marble,” and the pavement of “red, and blue, and white, and black marble.”
It would be impossible, in an elementary work like the present, to describe, or even to enumerate, all the different kinds of marble which were known to the ancients, 101or are known to the moderns. But it is, perhaps, requisite that an account should be given of some of the most important of them.
GREEK MARBLES.—143. Pentelic Marble is of beautiful white colour, and nearly resembles the Parian marble (145) of the Italians; but it is in coarser granulations. Sometimes it is splintery. It was obtained from quarries on Mount Pentelicus, near Athens, and was generally preferred, by the Grecian artists, to Parian marble. The Pantheon was built entirely of Pentelic marble; and many of the Athenian statues, and works carried on near Athens during the administration of Pericles, were executed in it. Dr. Clarke, however, has observed that while the works wrought of Parian marble remain perfect to the present time, those of Pentelic marble have been decomposed by the atmosphere, and sometimes exhibit a surface as rude and earthy as common lime-stone. There are numerous examples of Pentelic marble in those works of Phidias which form the Elgin collection in the British Museum.
144. Greek White Marble.—The Marmo Greco, of Italian artists, is of snow-white colour, in fine granulations; and somewhat harder, and consequently capable of higher polish, than most other white marbles. It is found near the river Coralus, in Phrygia.
ITALIAN MARBLES.—145. Parian Marble is of snow-white colour, inclining to yellowish white. It is obtained from quarries in the island of Paros, is finely granular, and, when polished, has somewhat of a waxy appearance. Parian marble hardens by exposure to the air, and is one of the most permanent kinds that is known. Varro and Pliny each state that it was named lychnites, by the ancients, from a Greek word signifying a lamp, because it was generally hewn in quarries by the light of lamps. The finest Grecian sculpture that has been preserved to the present time is of Parian marble. The principal statues of it now extant are the Medicean 102Venus, the Diana Venatrix, and Venus leaving the Bath. It is also Parian marble on which the celebrated tables at Oxford are inscribed.
146. Carrara Marble, the purest of all the kinds with which we are acquainted, is to this day obtained from quarries near the town of Carrara. It is of brilliant white colour, has a granular texture; and, when broken, sparkles like sugar. This marble, which is almost the only one in use by modern sculptors, was also quarried and wrought by the ancients.
It is susceptible of a high polish, and is applicable to every species of sculpture, except when, as is too often the case, dark veins intrude, and spoil the beauty of the work. In the centre of the blocks a beautiful kind of rock crystals, called Carrara diamonds, are sometimes found.
During the late war with France, the exportation of statuary marble from the countries under the dominion of Buonaparte was prohibited; and, at one time, it became so scarce in England as to be sold at the rate of more than seven guineas per cubic foot. The block of marble for the statue of his late Majesty in the great Council Chamber at Guildhall, London, was stated by the public prints to have cost twelve hundred guineas.
147. Luni Marble is a snow-white, compact, and finely granular variety, which was obtained by the ancients from quarries on the coast of Tuscany. It was preferred by the Grecian sculptors, both to the Parian and Pentelic marbles; and it is usually supposed that the Belvidere Apollo, as well as the Antinous of the Capitol, was wrought out of this marble. There is now found at Luni a white marble, variegated with red spots and dots.
148. Green antique Marble, or Verde antique of the Italians, is a mixture of white marble and green serpentine (132). This is believed to have been obtained from some part of Italy, but the quarries are not now known.
103149. Sienna Marble is of close texture, and yellowish colour, disposed in large irregular spots, surrounded with veins of bluish red, passing sometimes into purple. It is not uncommon in the vicinity of Sienna, and is in great request, throughout Europe, for chimney-pieces and ornamental furniture.
150. Brocatello Marble is somewhat like the last; but is also irregularly marked with various shades of red, and, in some parts, with white.
151. Mandelato Marble is of light red colour, with yellowish white spots. It is found at Lugezzana, in the Veronese. Another variety, bearing the same name, occurs at Preosa.
152. Verde di Prato Marble is a green marble, marked with darker green spots, which is found near the town of Prato in Tuscany.
153. Lago Maggiore Marble is a beautiful kind, white, with black spots and dots. It has been employed for decorating the interior of many churches in the Milanese.
154. Bretonico Marble.—This beautiful marble, which is found near the village of Bretonico, in the Veronese, is varied with yellow, grey, and rose colour.
FRENCH MARBLES.—155. Many valuable kinds of marble are obtained from different parts of the French territory.
156. Campan Marble.—Three kinds of marble are known by this name, all of them procured from immense quarries at Campan, near Bagnere, in the Pyrenees. The first, called Green Campan, is of pale sea-green colour, and exhibits, on its surface, lines of much deeper green, forming a kind of net-work. The second, called Isabel Campan, is of delicate rose colour, with undulating green veins. The third variety, the Red Campan, is of deep red colour, with veins of still deeper red. The green variegations in this stone are formed by a 104talcy mineral, intermixed with the lime-stone.—The Campan marble is well adapted for slabs, tables, chimney-pieces, and other ornamental purposes in the interior of buildings; but, if exposed to the weather, the talcose substances perish, and leave hollow spaces which render its surface rough and uneven.
157. Griotte Marble is of a deep brown colour, with blood-red oval spots, formed by shells. Its name has been obtained from its brownish colour, being similar to that of the cherries that are called by the French griotte. This marble has, of late, been much used in the decoration of public monuments, and in splendid furniture, in France. Some of the ornaments of the Triumphal Arch of the Carousel are made of it. The department of Herault is the part of France from which it is obtained. It sometimes contains large white veins, which destroy the harmony of the other tints.
158. Marquese Marble.—This, which is obtained from quarries, near the village of Marquese, between Calais and Boulogne, is marked with different shades and variegations of white and brown. Of this marble Buonaparte commenced a magnificent column on the heights near the sea, at Boulogne, to commemorate his victories; but, since his dethronement, the erection of this structure has been discontinued.
159. Sarencolin Marble is distinguished by exhibiting large zones, and angular spots of yellow or blood-red colour. It is found at Sarencolin, in the High Pyrenees.
160. St. Beaume, or Languedoc Marble, is of light red colour, marked with white and grey zones, formed by madrepores. The eight columns which adorn the Triumphal Arch, in the Carousel at Paris, are of this marble. It is obtained from quarries at St. Beaume, in the department of Aude.
161. Breccia Marble of the Pyrenees.—One kind of this marble contains black, grey, and red, middle-sized spots in a brownish red ground. It admits of 105a good polish. Another kind has an orange-yellow-coloured ground, containing small fragments of snow-white colour. Both these are found in the Pyrenees.
SPANISH MARBLES.—162. Few countries are more productive of marble than Spain; and in few countries are the public monuments and buildings more profusely decorated with marble. The vault of the theatre of Toledo is supported by 350 marble columns; and an ancient mosque at Cordova is ornamented with 1200 columns, most of which are of Spanish marble. The palace and church of the Escurial, and many of the churches in Madrid, are decorated with marbles of the most beautiful description.
163. White Spanish Marble.—Near Cordova; at Filabres, three leagues from Almeria, in Grenada; and in some other parts of Spain, white marble is obtained, which is susceptible of a good polish, and is well adapted to the general purposes of sculpture.
164. Seville Marble is a beautiful red variety, with shining red and white spots and veins. In the vicinity of Tortosa is found a kind of marble which has a violet ground, spotted with bright yellow; and near Grenada a marble of green colour, which somewhat resembles the celebrated verde antique (149).
165. Spanish Breccia.—There are several beautiful varieties of breccia in Spain. At Riela, in Arragon, there is one, composed of angular portions or fragments of black marble, embedded in a reddish yellow base. The breccia marble of Old Castile is of bright red colour, dotted with yellow and black, and encloses fragments of pale yellow, brick-red, deep brown, and blackish grey colour.
GERMAN MARBLES.—166. Germany abounds in marbles, and affords many kinds which are remarkable both for beauty and singularity. Of these the kind best known is
106167. Lumachelli Marble.—This exhibits beautiful iridescent colours, which are sometimes prismatic internally, but more commonly of various shades of red or orange; whence it has also obtained the name of fire marble. Few kinds of marble are more generally admired than this. It has a dark ground, and is marked throughout with the appearance of small whitish shells, which, in certain parts, refract the most beautiful and brilliant colours. This marble is cut into the tops and bottoms of snuff-boxes, and several other ornamental articles. It is found in veins at Bleyberg, in Carinthia.
168. Many beautiful kinds of marble are obtained from the island of Sicily, particularly one called Sicilian jasper, which is red, with stripes like ribbons, white, red, and sometimes green. Switzerland abounds in marbles; Portugal, Sweden, and Norway afford few. In the Russian empire many have been noticed, particularly among the Uralian mountains. The late Empress Catharine caused an immense palace to be built for her favourite Orloff, which is entirely coated, both inside and outside, with marble. She built the church of Isaac with marbles of different kinds, on a vast space, near the statue of Peter the Great, in Petersburgh. We are at present very imperfectly acquainted with the marbles of Asia. Dr. Shaw mentions a red marble obtained from Mount Sinai; and Mr. Morier, in his journey through Persia, speaks of a beautiful translucent kind which he calls marble of Tabriz, and the colours of which are light green, with veins sometimes of red, sometimes of blue. He says it is cut into large slabs, some of which he describes to have measured nine feet in length, and five feet in breadth.—No account has hitherto been published of the marbles of Africa.—In the United States of America many kinds of marble have been discovered, some of which have been wrought, and polished; but very imperfect descriptions have yet been given of them.
169. Few countries produce a greater variety of excellent 107marbles than the British Islands. Although these marbles are seldom noticed much beyond the limits of the districts in which they occur, many of them are admirably adapted for ornamental purposes; particularly for slabs and chimney-pieces. It is much to be regretted that we should send to foreign countries for stones which, in many instances at least, could certainly be as well supplied from our own. The following is an enumeration of a few of the most important kinds.
ENGLISH MARBLES.—170. Petworth Marble, when cut into slabs, is equal, both in beauty and quality, to many of the marbles imported from the Continent. The Earl of Egremont has, at Petworth, several chimney-pieces formed of it. Much of this marble was used in the cathedral church of Canterbury. The pillars, monuments, vaults, pavement, and other parts of that venerable structure, have been formed of it. The archbishop’s chair is an entire piece of Petworth marble. This marble is found in greatest perfection upon an estate of the Earl of Egremont, at Kirdford. It lies at the distance of from ten to twenty feet under the surface of the ground, and in flakes or strata nine or ten inches in thickness. Petworth marble is also an excellent stone for walls; and, for paving, it cannot be excelled. When burned, it also constitutes a valuable manure, superior, as some farmers imagine, even to chalk.
171. Purbeck Marble is obtained from the island of Purbeck, in Dorsetshire. It is of dark colour, and contains numerous small round shells, which, when it is cut and polished, mark it with roundish variegations of brown, dark green, and grey. This marble was formerly more used than it is at present. Several of the small columns, and many of the monuments, in the churches of Dorsetshire, and the adjacent counties, are formed of it. But it is not so durable as many other kinds. Wherever it is long exposed to the weather, the surface cracks, splits off, and becomes defaced.
108172. Babbicombe Marble is one of the most beautiful kinds that is found in any country. It varies in colour, from light brown to deep red; and large slabs of it have been obtained that are elegantly and diversely marked, some in streaks, others in spots, and others in different coloured shades.
This kind is quarried at Babbicombe, in Torbay, Devonshire, and is extensively manufactured into chimney-pieces in the West of England. An attempt was lately made to introduce it in London; but, from its not being the production of a foreign country, this has failed of success.
173. Derbyshire Marble.—There are, in Derbyshire, several kinds of marble, most of which contain an abundance of fossil shells, and other remains of marine animals. At Wetton, near Ashbourne, a beautiful kind is obtained, of greyish black colour, which contains a vast number of whitish and very minute shells. This has the name of bird’s eye marble. Near Monyash a beautiful variety is found, of a cheerful colour, inclining to brown red, and full of large marine figures in all directions; these, when the marble is cut, appear white, and afford a pleasing contrast.
174. Kendal Marble.—Some varieties of black, grey, and brown marble, are wrought near Kendal, in Westmoreland. These somewhat resemble the Derbyshire marbles; and, like them, are manufactured into chimney-pieces, and ornamental slabs for houses. Several of the slabs are found to contain corallines, and the remains of other marine animals, which vary their appearance in a very pleasing manner.
The Mona Marble is a species of serpentine intermixed with white limestone: it has been already described (132).
SCOTTISH MARBLES.—Scotland affords many valuable and beautiful varieties of marble.
175. Tirie Marble.—Few of the British kinds of 109marble have been more admired than that obtained from Tirie, one of the Western Islands of Scotland. It is of a reddish, sometimes a delicate rose-coloured tint, and sometimes white; and is always intermixed with other minerals which add to its beauty. The most common of these is of black colour, and called hornblende; the others are pale green sahlite, blackish brown mica (123), and green chlorite. In some varieties the hornblende is more abundant than the marble.
176. Assynt Marble.—At Assynt, in Sutherland, a white marble has been discovered, which is perfectly solid and pure, and entirely free from blemishes or stains. Blocks or slabs of it may be cut of almost any size that can be required. This marble acquires a smooth surface, but remains of a dead hue; whence, of course, its uses as an ornamental marble are much circumscribed.
177. Isle of Sky Marble.—There is found in the Isle of Sky a marble of pure white colour, which appears capable of yielding large and valuable blocks. Its fracture is granular and splintery, and its texture fine. It is harder, heavier, and more compact than the marble of Carrara (146); and is apparently well fitted for all the purposes of sculpture. But it has the defect of being very unequally hard. While some parts of the stone are nearly as easy to work as that of Carrara, other parts are so hard as to add a charge of near fifty per cent. to the cost of the working.
178. Sutherland Marble.—Some beautiful specimens of marble of dark brown colour, veined with whitish, light red, or light brown, have lately been brought from the county of Sutherland. These appear of close texture, are susceptible of a beautiful polish, and are capable of being wrought into extremely beautiful slabs for chimney-pieces and other ornamental purposes.
179. Glen Tilt Marble is of white or grey colour, 110and veined or spotted with yellow or green; some specimens are nearly white. The granulations are peculiarly large; and, in its aspect and composition, the Glen Tilt has great general resemblance to the Pentelic marble (143). This marble has of late attracted the notice of the Duke of Athol, through the suggestion of Dr. Macculloch; and chimney-pieces of it have since been made. It is obtained from a valley of the same name in the county of Perth.
180. Blairgowrie Marble.—A few miles from Blairgowrie, in Perthshire, there is an excellent granulated broad-bedded marble, of sugar-loaf texture, and as white as the finest statuary marble. It may be easily raised in blocks and in slabs of great size, perfectly free from blemishes. This marble is supposed to be well adapted for ornamental architecture, but its large sparry texture renders it unfit for the sculptor.
181. Glenavon Marble is of white colour, with large granular concretions, somewhat like spangles, and as large as the scales of fishes. This is a valuable kind; but its situation in the forest of Glenavon, on the property of the Duke of Gordon, is remote and difficult of access.
182. Ballichulish Marble.—On the north side of the ferry of Ballichulish, in Lochaber, there is a rock of marble, of beautiful ash-grey colour, and of a fine, regular, and uniform grain, which is capable of being wrought into blocks or slabs of any size, and is susceptible of a fine polish. This marble is finely sprinkled throughout with grains and specks of pyrites (236), and with grains and specks of a beautiful lead ore, which to the eye appears to be rich in silver. If used for ornamental purposes, it would be a bright and beautiful metallic marble.
183. Blairmachyldach Marble.—In the bed of a river, at the farm of Blairmachyldach, about three miles south of Fort William, is a singular marble, consisting of a black ground, flowered with white. It is of 111fine close grain, but not very hard. The flowering in it is light, and beautiful, like fine needle-work, or rather resembling the frosty fret-work upon glass windows, in a winter morning.
The cutting and polishing of marble appear to have been performed by the ancients nearly in the same manner as it is with us. In polishing, the first substance employed is a sharp, coarse-grained sand. Afterwards a finer sand is used, then emery (58) in different degrees of fineness. These are followed by a red powder called tripoli (119): and the last polish is given with putty.
184. BLACK MARBLE is a species of limestone, of uniform black colour, and easily distinguishable, by an excessively disagreeable smell, which is emitted on rubbing two pieces of it together, or striking it with a hammer.
Few minerals are susceptible of a more beautiful polish than this. It is consequently much used for chimney-pieces, small columns, vases, and other ornamental work. There are two quarries of black marble near Bakewell, in Derbyshire: and it is manufactured to a considerable extent by Messrs. Brown and Co. at Derby, who have fixed up in their ware-rooms a large slab of it as a looking-glass.
By the ancients it was much prized. Marcus Scaurus is said to have ornamented his palace with columns of black marble, each thirty-eight feet high; and many of the monuments of ancient Persepolis were executed in it. M. D’Avejan, Bishop of Alais, used a kind of black marble for paving the apartments of his palace; but the friction and heat rendered it so fetid that his successors were compelled to substitute another species of stone in its place.—The pavements, however, of many churches, and of the porticos of several galleries, on the Continent, are of black marble.
185. CALCAREOUS ALABASTER is a species of limestone of somewhat whitish or yellowish colour, translucent, and internally splendent or shining.
It is nearly a pure carbonat of lime; and occurs in masses, 112hanging, like immense icicles, from the roofs of lime-stone caverns, and also coating the sides of such caverns.
The formation of this substance is deserving of notice. The water which oozes through the crevices of limestone rocks, becomes strongly impregnated with minute particles of lime. This water, when it has reached the roof or side of a cavern, is generally suspended, for a considerable time, before a drop of sufficient size to fall by its own weight is formed. In the interval which thus elapses, some of the particles of lime are separated from the water, owing to the escape of the carbonic acid (26), and adhere to the roof. In this manner successive particles are separated, and are attached to each other, until what is called a stalactite, having somewhat the appearance of an icicle, is formed. These stalactites are sometimes solid, having a lamellar structure; sometimes of a fibrous texture, radiating from the centre to the circumference, as may be observed when they are broken; and sometimes hollow. If the water collects and drops too rapidly to allow time for the formation of a stalactite, it falls upon the floor, and there forms an irregular lump of alabaster, which has the name of stalagmite. In some caverns, the separation of the calcareous matter takes place both at the roof and on the floor; and, in course of time, the substance upon each increasing, they meet, and form pillars, sometimes of great magnitude.
Caverns of this kind occur in almost every country. Those of Derbyshire are well known; but the most celebrated stalactitic cave in the world is that of Antiparos, in the Grecian Archipelago.
The kind of limestone formed in the above manner is what the ancients generally denominated alabaster. It was employed by them for the same purposes as marble, was cut into tables, columns, vases, and sometimes even into statues. They also used it in the manufacture of vases or boxes for containing unguents. It is supposed to have been a vessel formed of this stone that is mentioned in the Gospel of St. Matthew, 113where it is said there came unto our Saviour “a woman having an alabaster box of precious ointment.” In the National Museum at Paris there is a colossal figure of an Egyptian deity, which is cut in a kind of alabaster brought from the mountains between the Nile and the Red Sea.
186. TUFA, or INCRUSTING CARBONAT OF LIME, is a calcareous substance deposited by such water as is impregnated with lime.
It clothes, with a stony coat, the smaller branches of trees, leaves, moss, plants, and other substances; and thus preserves them from decay, by protecting them from the action of the atmosphere.
Most of the substances termed by the common people petrifactions belong to this kind of lime. They are, however, merely covered with, and by no means converted into stone.
The dropping well at Knaresborough, in Yorkshire, is particularly celebrated for them. An overhanging rock, several yards in depth, has been gradually formed of the calcareous matter which the water holds in solution; and, from this rock, it incessantly drops into the basin below. The persons who have the care of the place constantly keep these petrified articles for sale. Even old wigs and hair brooms are subjected to the powers of the water, to furnish subjects for attraction to the visitors. There are other springs of this description in Oxfordshire and Somersetshire, and particularly at Matlock, in Derbyshire. We are informed that at Dalton, on the south side of Mendip, the workmen not unfrequently discover large pieces of oak enveloped in blocks of stone which are four or five tons in weight.
Blocks of tufa are, in some countries, cut and used for building stones; and this substance, when burned, becomes an excellent lime. Pieces of it are sometimes hollowed, and used as filtering stones.
In the British Museum there is a human skull completely incrusted with stone, which was found in the river Tiber.
114The warm baths of Hungary are often so thickly coated at the sides and bottom with tufa, that, during certain intervals, it actually fills up the tubes and canals through which they are supplied. The fur in teakettles is a somewhat similar deposit from water in boiling.
187. PORTLAND STONE, BATH STONE, KETTON STONE, are different kinds of limestone; and, of a texture so hard and compact as to be used in building.
They have their names from the places where they are respectively found, in Portland Island, near Bath, and at Ketton, in the county of Rutland.
Of Ketton stone several of the colleges in Cambridge are built. Its grain has a singular resemblance to the petrified roe of a fish, whence also it is sometimes called roestone. The bridges, St. Paul’s Cathedral, the Monument, and nearly all the buildings of late date in London, are constructed of Portland stone.
Some of these kinds of stone, when first dug out of the quarry, are so soft that they are readily worked into any form which use or ornament may require. This is owing to the moisture with which they are naturally impregnated; but when they once become hardened, by exposure to the sun and air, they are extremely firm and solid. On the contrary, other kinds of limestone that are used for buildings imbibe and retain the moisture of the atmosphere, in consequence of which they burst or are crumbled by frost.
We are informed that Portland stone was first used in London in the reign of James the First, that monarch, by the advice of his architects, having employed it in the construction of the banquetting house at Whitehall. After the great fire in London, it was brought into general use by Sir Christopher Wren.
188. MARL is a combination of clay, silex (76), and lime: and is denominated calcareous, argillaceous, or siliceous, as the lime, clay, or silex, is most abundant.
The calcareous part of marl is frequently composed 115of shells, whence it frequently has the name of shell marl; and where these are predominant, it affords an excellent manure for sandy, dry, gravelly, or light lands. Marl likewise produces very beneficial effects on mossy and clayey soils; and these effects, where it has been properly applied, have been observable for twelve or fourteen years. Some kinds of marl that contain but a small portion of lime have been successfully used in the manufacture of earthenware.
This mineral is usually found at the depth of from five to nine feet beneath the surface of the ground, and deposited between beds of clay and sand. It is dug out with spades; and, in the digging of it, in Ireland, the workmen not unfrequently meet with the horns of deer and other curious fossils.
The usual mode by which persons, generally unacquainted with minerals, distinguish this from other clayey substances, is, to break a small piece of dry marl into a glass of vinegar. If it be marl it will immediately dissolve with considerable effervescence; and the briskness of the effervescence will be in proportion to the quantity of lime which it contains.
189. FLORENCE MARBLE is a kind of indurated or hardened marl, and is remarkable for presenting, when polished, the appearance of ruined edifices or rocks.
This kind of marble is never used in architecture. Little slabs of it are cut for Mosaic work, and to be framed like pictures; and the latter, when of considerable dimensions, are sometimes purchased at a high price. If held at a distance from the eye, an inexperienced observer might mistake a slab of Florence marble for a drawing in bistre. Here, observes a French writer, we remark a shattered Gothic castle, there the mouldering fragments of a cathedral; in one part ruined walls, and in another shattered bastions and towers. But, when we approach the picture, the illusion vanishes, and those imaginary figures which, at a distance, appeared to be so correctly drawn, become 116changed into irregular spots, lines, and shades, which present nothing distinct to the view.
190. Cottam Marble, which, when cut and polished, also exhibits the appearance of a landscape, is a kind of compact marl. It has its name from being found at Cottam, near Bristol.
191. LIAS, or CALP is a kind of limestone of bluish black, or greyish blue colour, and composed chiefly of lime, silex (76), clay, and oxide of iron (21).
This stone, when burned, forms a cement which has the property of setting very strongly under water. It has also, of late years, been employed in a manner which merits particular notice, for the multiplying of copies of drawings and penmanship. A drawing is made on prepared paper with a peculiar kind of ink. A slab of lias, about an inch thick, is then heated; the drawing is placed upon it, and both are passed through a rolling press. The paper is afterwards wetted, and washed from the stone; but the ink, being of a gummy or glutinous quality, becomes in part absorbed by the stone, and remains. The stone is then ready for the printer. Previously to taking off each impression, the stone is wetted with a sponge; fresh ink (which is said somewhat to resemble printers’ ink, and is put on with a ball similar to that used by letter-press printers) is then applied. This is prevented, by the water, from adhering to any part except to the ink that had been absorbed, by the stone, from the paper on which the drawing was originally made. Paper is then placed on the stone, both are passed through a rolling press as before, and a perfect impression of the drawing is made upon the paper.
This art has been practised in Germany with great success; and with the difference only of the original drawing being made upon the stone instead of paper. Many beautiful specimens of drawings, taken from slabs of lias, may be seen in this country. It is said that copies of military drawings and orders were, to a very 117large amount, multiplied by this means at the headquarters of the armies lately employed on the Continent.
An artificial composition is sometimes used instead of lias.
Considerable quarries of this stone are wrought in Germany. It is also found at Leixlip, near Dublin; in beds at Aberthaw, in Glamorganshire; in Dorsetshire, and near Bath.
SULPHAT OF LIME.
192. ALABASTER, or GYPSUM, is a kind of sulphat of lime, or of lime in combination with sulphuric acid (24), which has a shivery and glittering texture; and is of white colour tinged with grey or red, and sometimes striped, veined, or spotted. When crystallized, the primitive form of its crystals is a regular four-sided prism (Pl. II, Fig. 14.)
Being considerably softer than marble, this mineral is not capable of receiving a good polish. From this circumstance it is, however, the more easily worked. It is manufactured into chimney-pieces, columns, busts, ornamental vases, and lamps; the latter of which transmit a soft and pleasing light. Such is sometimes the transparency of alabaster, that it has been employed for windows; and, at Florence, there is now a church which receives its light through the medium of this substance.
The ancients, though acquainted with the art of making glass, had not attained the knowledge of reducing it into thin transparent plates; and frequently employed alabaster for windows. Of this stone the Temple of Fortune, which was built by order of the Emperor Nero, was erected. It had no windows whatever, and received only a soft kind of light through its walls; appearing rather as if the light issued from the interior, than that it was admitted from without.
The hot springs of St. Philip, which supply the baths of Tuscany, are so strongly impregnated with alabaster, that artists take advantage of this to obtain impressions of bas-reliefs, by merely exposing their moulds to a 118current of the water until they become filled with the earthy deposit. These impressions, when taken out, are found to be as hard as marble, and are very beautiful. There are, in the British Museum, some casts of medals formed from the water of these springs.
When alabaster is heated, it falls into a soft white powder, which, on being mixed with water, absorbs it so rapidly, that if it be formed into a paste, it dries and becomes hard in a few minutes. In this state it is called plaster of Paris; and is employed for the making of statues, casts, and other ornamental work, which, though of a beautiful white colour, are very brittle. When mixed with coloured gummy or glutinous substances, it yields plasters of different hues, and has the name of stucco; and, in this state, is used for lining the walls and ceilings of rooms. This plaster is much in request in the northern counties of England, for the floors of dairies, store-rooms, granaries, and other apartments; and, when properly formed, it constitutes a very smooth and durable flooring.
The fine white varieties of gypsum are used as an ingredient in the composition of earthenware and porcelain; and the glaze, or enamel, with which porcelain is covered, has the purest gypsum for one of its ingredients. Of late years this mineral has been advantageously employed as a manure for fertilizing the soil.
Gypsum is found in Cheshire and Derbyshire, as well as in several parts of the Continent. That which is imported into this country from Italy and Spain is considered the best.
193. Fibrous Gypsum.—There is a variety of gypsum which has a somewhat fibrous appearance, and which, when cut in a convex form, and polished, reflects a light not much unlike that of the cats-eye (86). Hence it is sometimes sold to ignorant persons for that stone. It has also been imposed upon purchasers for the gem called moonstone (113). Fibrous gypsum is cut into ear-pendants, crosses, beads for necklaces, and other female ornaments; but its softness is such as to 119allow of its being easily injured both by dirt and friction.
FLUAT OF LIME.
194. FLUOR SPAR, or DERBYSHIRE SPAR, is a mineral formed by the combination of lime with fluoric acid (27).
It sometimes occurs in a massive, and sometimes in a crystallized state; the primitive form of its crystals being a regular octohedron (Pl. II, Fig. 5). Its colour is usually bluish, green, yellow, whitish, or a mixture of some of these.
When heated, this substance cracks, and shines brightly in the dark. But if kept hot for some time, it ceases to be luminous, and this property cannot be restored to it. If also two pieces be rubbed strongly together, they become luminous in the dark.
From this spar are made several kinds of ornamental vases of considerable size, columns, and toys, which, from being extremely varied in their colours and appearance, and admitting of a high polish, are very beautiful. When a piece of fluor spar is to be wrought into a vase, or any similar article, it is first carved with a mallet and chisel into a somewhat spherical form. It is then fixed to a turner’s lathe, and, with great care, is formed into the shape that is required. When this is complete, it has to be polished, which is done first with gritstone and pumice (108), and lastly with emery (58) and putty. The lathes formerly in use were worked by the foot; but those now adopted are worked by machinery, the advantage of the more steady motion of which has been that ornaments of much more delicate structure can now be formed than before. The manufacture of articles from fluor spar gives employment to a great number of industrious families in Derbyshire. This mineral occurs in several parts of that county, where it has the name of Blue John, and where it is obtained from caverns at a considerable depth beneath the surface of the earth. It is also found in various countries both of the European and American continents.
120The acid produced from fluor spar is called fluoric acid (27), and has the peculiar property of corroding glass and flint, and consequently cannot be kept in glass bottles. Artists, by means of fluoric acid, are enabled to etch on glass, in the same manner as, with aqua fortis (nitric acid), they do on copper. The process is sufficiently simple. The glass is first a little heated, for the purpose of covering it thinly over with wax; then, with a needle or other fine point the drawing is to be made, by cutting through the wax to the surface of the glass. The edges are next to have a little wall of wax raised upon them. This done, the glass must be placed in an horizontal position, and sifted over with fluor finely pounded; and lastly, a mixture of one part of spirit of vitriol or sulphuric acid (24) with two or three parts of water is to be poured gently upon it. The acid will be prevented from running off by the wax; and, in the course of a little while, if these be cleared away, the glass will be found corroded in all the lines along which the needle passed.
The mode of obtaining fluoric acid for chemical purposes is, by pouring sulphuric acid upon powdered spar in a leaden retort, and applying to it a gentle heat. This acid should be used with great caution; for, when applied to the skin, it instantly disorganizes it, and produces very painful sores.
195. These minerals are sometimes called ponderous earths, and have their name from a Greek word signifying heavy. They comprehend all the combinations of barytes with acids.
When purified, they form a greyish white, porous substance, which is easily reducible to powder; has no perceptible smell, but has a harsh and more burning taste than lime, and changes the blues of vegetable colours to green.
Although barytes is one of the most useful chemical tests that we are acquainted with, it is not much employed in the arts, because, when purified, it is found too expensive. It is capable of being made into a very 121tenacious cement; and painters use a preparation that is made from it as a white colour which will not change. This is sold in the shops under the name of “Hume’s permanent white.” Barytes taken into the stomach proves a virulent poison; yet a preparation of it is used in medicine, and particularly for the removal of scrophulous complaints. When finely pounded and mixed with oatmeal, carbonat of barytes has been found an efficacious poison for rats.
196. SULPHAT of BARYTES is a mineral formed by the combination of sulphuric acid (24) with barytes.
It sometimes occurs in a state of powder, frequently in shapeless masses, and often crystallized: the primitive form of its crystals being a four-sided prism. It is not soluble in any other than sulphuric acid.
With us sulphat of barytes is of no use in the arts. The Chinese, however, employ it as an ingredient in the composition of porcelain; and it is said to form a good manure for clover fields.
The Bologna Phosphorus, or Bononian Stone, a very remarkable kind of barytes, has its name from being found near Bologna in Italy. This substance, when detached, is usually observed in roundish, flat, kidney-shaped pieces, from about the size of a walnut to that of an orange, which have a shining and somewhat fibrous texture within.
When the outer coat of this stone is washed away by heavy rains, it has sometimes the appearance of burnished silver. An Italian shoemaker, in the year 1630, deceived by this appearance, carried home several pieces, hoping, by means of fire, to extract silver from them. But at the same time that he was disappointed in this expectation, he was surprised by a very unlooked-for phenomenon. All the pieces which he had thus attempted to melt, when they were afterwards exposed to the light, became themselves luminous. It is the singular property of the Bologna phosphorus, after it has undergone calcination in a particular manner, to 122become capable of imbibing so much light on exposure, for a little while, to the light of the sun, or even to the flame of a candle, that it will afterwards shine in the dark for an interval of from eight to fifteen minutes, like a glowing coal, but without any sensible heat. The light which it emits is sufficient to read by, provided the letters be placed near it. If well prepared, the stone will retain this extraordinary property for five or six years.
The preparation of it is thus conducted. Pieces of sulphat of barytes are made red hot, for a few minutes, in a covered crucible placed in the middle of a fire, and then left to cool. When cool, they are pounded in a stone mortar, and sifted. The powder thus formed is made into a paste with a little gum arabic, and divided into long cakes, or cylinders, each about a quarter of an inch thick. These pieces are dried in a moderate heat, and then, by degrees, are exposed to a more violent heat, among charcoal, in a wind furnace. As soon as the coals of the furnace are half consumed, it must be filled a second time, and the phosphorus must be left undisturbed. When the coals are quite consumed the ashes must be carefully blown off with a pair of bellows, and the phosphorus will be found at the bottom of the grate.
197. ALUM is a substance of yellowish or greyish white colour, usually opaque, but sometimes transparent. When purified, it consists of slender, irregular, hair-shaped fibres, and has a sweetish, astringent taste.
The alum of commerce is an artificial production from the different kinds of stones which contain it. That called Roman alum, from its being procured from the neighbourhood of Rome, is usually considered preferable to the other sorts; but good alum of our own manufacture is equal to it in quality. The Levant, or Roche alum, is said to have had its name from the village of Rocca, the present Edessa, in Syria.
There is a famous alum mine at Tolfa, near Civita Vecchia, in Italy. The alum is obtained from this mine nearly in a pure state; and it is so extremely hard, that it can only be wrought by means of pickaxes, and gunpowder. At Solfatara, near Naples, and in other volcanic countries, an abundance of alum is found, in a state of efflorescence, from the lava.
The alum of our own country is manufactured from a kind of slaty stone which is found near Whitby, in Yorkshire. This manufactory was first established about the conclusion of the sixteenth century, by Sir Thomas Chaloner, who is supposed to have obtained his knowledge of the process, from the alum works which had then lately been introduced into Germany and Spain. The rock of alum slate, near Whitby, is supposed to be nearly twelve miles in extent: and affords an abundant supply of alum. The workmen tear open the 124rock; after which the different fragments are loosened, in the form of slaty leaves or plates, that are of a dark grey colour. To obtain the alum, a bed of fagots is formed from ten to twelve feet in depth. By the side of this a scaffold is erected, which enables the workmen to form a pile of mineral about fifty feet long, and forty feet high. While this pile is forming, the fagots are lighted. By the gradual operation of the heat, a calcination takes place, in consequence of which the alum is afterwards rendered capable of being more easily separated than it otherwise would be from the stone in which it was contained, and from other extraneous matters that are combined with it. After this, the mineral is washed in shallow vessels, so arranged that the water may be poured from one into the other. By this process the alum becomes suspended in the water, while all the earthy particles subside to the bottom. The next operation is to evaporate the water saturated with alum. This is done by boiling it in large leaden caldrons, fixed, on cast iron bars, over a furnace. As soon as the contents of the caldrons are brought to a proper state, they are drawn off into casks, where the alum concretes into a mass. The hoops are then taken off, and the alum is broken and left to dry; after which it is packed in casks for sale.
Alum is an article of indispensable importance to dyers, not only on account of its cleansing and opening the pores of the substances to be dyed, and thus rendering them fit to receive the colouring particles, but also from its more essential property of fixing the colours in such manner that they cannot afterwards be washed out. By tanners it is in great request for giving firmness to the skins after they have been rendered flaccid in the lime-pits. It is employed in the manufacture of paper, and by engravers, and other artists. In the making of candles, alum is added to the tallow, to render it glossy, and to give it greater firmness and consistence; and, mixed with cream, it aids the separation 125of butter. It has a tendency to retard ignition. Paper soaked in alum water does not easily take fire, and is thereby better fitted for the preservation of gunpowder. Such paper is likewise used in the whitening of silver, and the silvering of brass. It has been recommended that ladies’ muslin dresses should be dipped in a solution of this substance, for the purpose of rendering them less liable to catch fire. A solution of alum also retards the putrefaction of animal substances, and affords useful, as well as economical, means of preserving natural productions that are imported from foreign countries. Alum is frequently mixed with paste, to prevent its losing its tenacity by the absorption of moisture. It is asserted that bakers occasionally use it as an ingredient in bread, and that its presence may be discovered by thrusting a heated knife into a loaf before it is cold: if free from alum, scarcely any alteration will be visible on the blade, but if the contrary, the surface, when cool, will appear slightly covered with an incrustation of alum. A very important purpose to which alum may be applied is in the purifying and sweetening of water that has become fetid and unfit for use; from five to ten grains of burned alum, and double or treble that quantity of pounded charcoal, will correct the fetor of a gallon of water. Printers’ cushions, and the blocks used for the printing of calicos, are rubbed with burned alum to remove any greasiness, which otherwise would prevent the ink or colour from sticking. This substance is also occasionally employed by surgeons to stop the bleeding of small vessels, to corrode fungous or proud flesh, and for other purposes in medicine.
This is a family of minerals which comprehends all the combinations of magnesia with acids.
When freed from extraneous matters, magnesia is a powdery substance of limpid white colour.
126199. EPSOM SALTS, or SULPHAT OF MAGNESIA, consist of magnesia in conjunction with sulphuric acid (24).
It is said that Epsom salts have been found in the Alps, and in Switzerland, under a powdery form, and sometimes even in masses, or a state of incrustation on stones and rocks. They are, however, chiefly found dissolved in mineral waters, and particularly in those at Epsom in Surrey, and Sedlitz in Bohemia. Their taste is bitter and unpleasant. So little are they affected by exposure to the air, that the Abbé Haüy kept some by him for more than twelve years without any sensible alteration.
These salts are much used in medicine, and are sometimes manufactured from the waters of Epsom (290) and Sedlitz (289), but more frequently, and in much greater abundance, from sea-water.
The magnesia of the shops is prepared by dissolving Epsom salts in water, and adding to the solution half their weight of potash (205). The substance that sinks to the bottom is magnesia; and this, washed with a sufficient quantity of water and dried, has the appearance of a light, soft, and white powder, of insipid taste.
Magnesia is used in medicine, both in a simple state and when calcined or burned. It is also employed in some chemical processes; and is in considerable request in the manufacture of enamel and porcelain. If putrid water be agitated with a small quantity of magnesia, it will lose a considerable portion of its bad taste and smell.
Soda, like potash (205), is an extremely caustic alkali (42). It has a greyish white colour, and agrees exactly with potash (205) in taste, smell, and corrosive quality, but it is not so heavy.
In a mineral state soda has hitherto been found only in combination with some acid.
Common salt (202) is a compound of soda with muriatic acid (29).
127The soda of commerce is obtained from sea-water; and from the ashes of different kinds of plants that grow on the sea-shores, but particularly from that called salsola soda, which is found in great abundance on the coasts of the southern parts of Europe; and from which it has its name. It is sometimes called barilla, from the salsola soda being so denominated in Spain.
This alkali is of essential use in the arts. When melted with flint or sand, it forms glass, and answers much better for this purpose than potash. In conjunction with oil and lime, it is employed in the manufacture of soap; and it is used as a substitute for soap in the cleaning and bleaching of linen, flannels, and worsted goods. If a weak solution of soda be poured into foul bottles, or casks in which wine has long been kept, it will cleanse them. It may also be successfully used for the cleansing of vessels in which milk has become acid. Saddles, bridles, or boot-tops, may be effectually cleaned by means of this liquor, and restored nearly to their original colour and appearance.
The art of soap-boiling may easily be illustrated by the following experiment. Take a piece of quick-lime, slake it gradually by sprinkling on it a sufficient quantity of water. When it is completely slaked, add to it about twenty times its weight of water. To this mixture add two parts, by weight, of common subcarbonat of soda, previously dissolved in a sufficient quantity of water. Boil the whole for about half an hour, strain it through a cloth, and boil it till so much of the water is evaporated that a phial that will contain an ounce of water will hold one ounce, seven pennyweights and a half, of this ley. Then mix in an earthenware pipkin or basin, one part of the ley, with two parts of olive oil. Place the mixture in a gentle heat, capable only of making the liquor simmer, and allow it to simmer, stirring the liquor continually, with a wooden stick, till, by letting a few drops of it fall on a plate, the soap will be found to coagulate, and the water become speedily separated from it. After which, pour out the contents 128into a cup, and suffer it to cool.—Soap may also be prepared without heat. If one part of the ley be mixed with two parts of olive oil, in a glass or stone ware vessel, and the mixture be stirred, from time to time, with a wooden spoon or spatula, it will become thick, and white; in seven or eight days afterwards the combination will be completed, and a white and firm soap will be obtained.
White soap is formed of ingredients similar to those that have just been mentioned. Yellow soap is made with tallow, resin, and soda. Soap may be formed by boiling shreds of woollen cloth with ley till the whole has acquired a certain consistence. This kind of soap has been made, and applied with success, in several manufactories in France.—The combination of oil and other ingredients with potash (205), instead of soda, affords what is called soft soap.
201. NATRON, or CARBONAT of SODA, is a salt which consists of soda (200) in combination with carbonic acid (26). It is massive, of greyish colour, soluble in water, and has a disagreeable alkaline taste.
This salt is found in Egypt, on the surface of the earth, and particularly near the margins of certain lakes called natron lakes. In the summer season the water of these lakes is evaporated by the heat of the sun, leaving a bed of natron generally about two feet in thickness. This is broken with wedges and hammers; and packed up for sale in the European markets. The waters of some of the lakes contain both common salt and natron; and these, on evaporation, crystallize in successive beds. Natron is found in considerable quantity under the form of an efflorescence, on the surface of the earth, in the plains of Debreczin in Hungary. It is likewise found in small quantity in the ashes of most vegetables, but particularly in those of salsoda and salicornia.
The ancient Egyptians are said to have made great use of natron for the preservation of dead bodies, by macerating 129them in it for several months previously to their being embalmed. Large quantities of this salt are sometimes imported into England, by the East India Company’s ships, from China, and other parts of the East. It is employed in the manufacture of soap, and for the washing of linen. Glass-makers mix it with sand for the formation of glass. On the continent it is administered as a medicine in complaints of the bowels and liver. The ancients sometimes employed a mixture of natron for soaking their seed corn, under an impression, that, when afterwards committed to the earth, it would thereby be rendered more fertile.
202. COMMON SALT, or MURIAT of SODA, though found in some countries in a solid and massive state, is for the most part an artificial preparation from sea-water, and from the water of salt lakes and brine springs. It consists of soda (200) in combination with muriatic acid (29).
Few productions, either natural or artificial, are in so much request as common salt. It is used by the inhabitants of nearly all countries, for correcting the insipidity of food. When applied in small quantities, it accelerates the putrid fermentation; and, in this case, is considered to aid digestion, by promoting the decomposition of the aliments. In larger quantity it has a contrary effect, and tends to preserve organic substances from corruption. Salt is used for glazing the surface of coarse earthenware; and is employed in several processes of dyeing.
When this substance is dug out of the earth it has the appellation of rock salt: and immense masses of it are found in different countries of the world. The most considerable, as well as the most celebrated salt mines, with which we are acquainted, are those about five miles from Cracow, in Poland; and it is supposed that they contain more salt than would be sufficient to supply the wants of the whole world for several thousand years. On descending to the bottom of these mines, a stranger is astonished to find a kind of subterraneous 130republic, consisting of many families, who have their own peculiar laws and polity. Here are likewise public roads, and carriages, horses being employed to draw the salt to the mouths of the mine, where it is taken up by engines. The horses, when once they are down, never more see the light of day; and many of the people seem buried alive in this immense abyss. Some are born there, and never stir out; others, however, have occasional opportunities of breathing the fresh air in the fields, and enjoying the light of the sun. The subterraneous passages or galleries are very spacious; and, in many of them, chapels are hewn out of the salt. In these are set up crucifixes, and the images of saints, before which lights are kept continually burning. In some parts of the mine huge columns of salt are left standing to support the rock. Its windings are so numerous and intricate, that workmen have frequently lost their way: the lights they carried have been burned out, and they have perished before they could be found. The salt is taken from these mines in blocks so large as, sometimes, to measure nine feet in length, four feet in width, and two or three feet in thickness. In the year 1780, the greatest depth to which the workmen had penetrated was about 320 yards, and the mass of salt was considered to be in some places more than 240 yards thick, and to extend at least three leagues.
Near the town of Cardona, about fifty miles northwest of Barcelona, in Spain, there is a mountain of salt, without cleft or crevice, 500 feet high, and nearly three miles in circumference. In the province of Lahore, in Hindostan, travellers have described a mountain of the same mineral, not inferior to this in magnitude; and the elevated regions of Peru afford rock salt at the height of 7000 feet above the level of the sea.
At Northwich and Nantwich, in the county of Chester, there are salt mines of great depth and extent. These are frequently visited by travellers, and are found amply to repay the trouble and inconvenience of descending into them. There are two principal beds of 131this substance; the upper one is about forty-two yards below the surface, and twenty-six yards thick. This was originally discovered about a century and a half ago, in searching for coal. The lower bed has already been examined to the depth of forty yards, without coming to the bottom; and it is about the centre of this bed that the purest salt has been discovered. The average depth of the cavity, formed by the workmen along the vein of salt in the different mines, is supposed to be about sixteen feet. In some of the mines, where pillars six or eight yards square are left to support the roof, the appearance of the cavity is singularly beautiful: and the effect is greatly increased when the mine is illuminated by numerous candles fixed to the side of the rock. The scene so formed would almost seem to realize the notion of the magic palaces of Eastern poets. Some of the mines are worked in aisles or streets. The methods employed in working out the salt offer nothing worthy of notice. Larger masses are separated from the body of the rock, by blasting with gunpowder; and are afterwards broken down with pickaxes, hammers, and other instruments. The present number of mines in the vicinity of Northwich is eleven or twelve, from which there are raised, on an average, 50,000 or 60,000 tons of salt per annum. The greater part of this quantity is exported to Ireland and the Baltic; and the remainder is employed in Cheshire, and the adjacent counties.
Salt is also made from brine springs in Cheshire, Cumberland, Staffordshire, and Worcestershire; but the kind most commonly used in England is that which is made from sea water, and has the name of sea salt. The mode of manufacturing it is very simple. The water is first pumped into shallow reservoirs of earth, called salt pans, or salterns. In these it remains exposed to the sun until a certain proportion of the water is evaporated, so as to leave it about seven times stronger than in its original state. It is then conducted by another pump into flat iron pans, eight or nine feet square, and as many inches deep. These, being placed over a 132hot fire, the liquor or brine is boiled until nearly all the remaining particles of water have passed off by evaporation, and nothing is left in the pans but salt. This is thrown together into proper vessels, for a few days, to drain, after which it is fit for use.
In some countries the whole evaporation is performed by the heat of the sun; and, in extreme northern climates, where the sun would not have sufficient power for the operation, a very different process is adopted. The water is suffered to freeze in the salterns, and that portion of it which continues uncongealed is so strongly saturated that it requires only a moderate heat to evaporate the remainder of the water, and to crystallize the salt.
Bay salt is that which is produced from the evaporation of sea-water by the heat of the sun only.
The inhabitants of Cardona, in Spain, make of the rock salt in their neighbourhood various transparent articles, which they vend at a cheap rate. These, which consist of small altars, figures of saints, crosses, chandeliers, salt-cellars, &c. are as clear as crystal, and, to appearance, as lasting. They are chiefly purchased by strangers as curiosities, and are distributed over various parts of Spain and the south of France.
The decomposition of salt furnishes the muriatic acid (29), or spirit of salt of commerce. This liquid, which is much used in the arts, and is in great request by chemists, is prepared, for common purposes, by mixing one part of common salt with seven or eight parts of clay, and distilling the mixture; or by distilling common salt and spirit of vitriol or sulphuric acid (24), and receiving the product into a vessel containing water.
It has been discovered that muriatic acid, in a state of gas, is an excellent means of correcting putrid exhalations. In the year 1773, the cathedral church of Dijon was so much infected by the corruption of bodies which had been interred within its walls, that it was entirely deserted. The professor of Chemistry at Dijon having been applied to for assistance, placed, on a few 133burning coals, in the middle of the church, a glass vessel containing six pounds of common salt. Upon this he poured two pounds of sulphuric acid (24), precipitately withdrew, and shut all the doors. The gas soon filled the whole cathedral. After twelve hours the doors were thrown open, and a current of air was made to pass through to remove the gas, which had entirely destroyed every putrid odour.
The following has been recommended as an eligible mode of fumigating rooms for the prevention of infectious disorders. Take six drachms of powdered nitre (206), and six drachms of sulphuric acid (spirit of vitriol); and mix them in a tea-cup, by adding to the nitre one drachm at a time of the oil. During the preparation the cup must be placed on a piece of heated iron, and the mixture stirred with a tobacco pipe or piece of glass. As soon as the fumes arise, the cup must be moved about to different parts of the room or house that are to be fumigated.
203. GLAUBER SALT, or SULPHAT of SODA, is a salt which consists of soda (200) in combination with sulphuric acid (24). It occurs in an efflorescent or powdery state, on the borders of salt lakes; or, more commonly, in a state of solution, in certain mineral waters.
This salt, which was originally discovered by a German chemist whose name was Glauber, has a nauseously bitter and saline taste. It is found, in an efflorescent state, on meadow ground at Eger, in Bohemia; and on the walls of old galleries in mines, at Grenoble, in France. It is also abundant in the ashes of some kinds of vegetables, especially of sea weeds. The waters of the Mediterranean yield a great proportion of it; and the Glauber salt used for commercial purposes is chiefly prepared from sea-water, or by decomposing common salt, in order to procure muriatic acid (29). It may also be obtained by saturating soda with sulphuric acid (24).
The use of this salt in medicine is well known; and, in some countries, it is employed as a substitute for soda 134(200), in the manufacture of white glass. It ought to be kept in well-corked bottles, as otherwise the crystals soon fall into powder.
The following is a pleasing experiment, which shows a singular and almost instantaneous crystallization of Glauber’s salt. Dissolve this salt by adding portions of it gradually to water kept boiling until the water will dissolve no more. Pour the solution, whilst boiling, into common medicine phials previously warmed, and immediately cork them. Set the phials in a quiet place without shaking them. The solution, when cool, will remain perfectly fluid till the cork is taken out; but the moment this is done, and the air is admitted, it will begin to crystallize on its upper surface, in fine satin-like crystals, which will shoot downward, like a dense white cloud. In this act so much heat becomes evolved as to make the phial feel sensibly warm to the hand. When the crystallization is complete, the whole mass generally becomes so solid, that, on inverting the bottle, not a drop of it will fall out. If the crystallization should not immediately ensue on opening the phial, this may instantly be effected by dropping into it a minute crystal of the same salt. The experiment may be exhibited any number of times afterwards, by merely placing the phial in boiling water, till the salt it contains be again completely liquefied; and letting it stand, as before, to cool.
204. BORAX is a salt composed of boracic acid (28) and soda (200), and is imported chiefly from the East Indies, in the form of a brownish grey, impure, shapeless salt, of sweetish taste; or in detached prismatic crystals, each about an inch in length.
Although borax has long been known as an article of traffic, there is scarcely any production with the origin of which we have been, till lately, less acquainted. It is found in a native, though impure state, in a mountain lake, situated about fifteen days’ journey from the capital of Thibet in the East Indies. This lake is so encompassed with hills as to have no stream either falling into 135it or flowing from it. The water is salt to the taste, and contains both borax and common salt; and the edges and shallow parts are covered with a stratum of this substance, which is dug up in considerable masses for exportation. It has here the name of tinkal, and is usually brought into Europe enveloped in a kind of fatty substance. The mode of refining it was for a long time kept, by the Dutch and Venetians, amongst those secrets which a want of sufficient research alone prevented from being generally known. When refined, it is called borax.
The uses of borax are numerous. It is employed as a flux for metals, being found to produce a more perfectly limpid fusion than any other substance. For the same reason it is made an ingredient in the finest kinds of glass, and particularly in some of the coloured glass pastes which are manufactured in imitation of gems. But its chief use is to jewellers and goldsmiths, to facilitate the soldering of gold and silver. Borax is also used in medicine.
Potash is an alkaline substance (42), of white colour, and of smell somewhat resembling that which is perceived during the slaking of quick-lime (137). It is extremely corrosive, and remarkably acrid to the taste.
In a mineral state it is found only in combination with nitric acid (30).
Potash principally exists under the form of a salt, in vegetable substances; and is obtained by burning them, afterwards repeatedly washing the ashes with water, and then filtering and evaporating these to dryness. The appellation of potash was given to this salt from its having formerly been prepared in large iron pots.
The uses to which it is applied are numerous. In chemistry it is employed for a variety of purposes; and also in many arts and manufactures, in scouring, washing, bleaching, dyeing, glass-making, and several others. Its corrosive property is such that it is often used by 136surgeons under the name of potential cautery, to open abscesses, and to destroy useless or hurtful excrescences.
Potash, after it has been made red hot, is rendered whiter and more pure. In this state it has the name of pearl ash.
206. NITRE, or SALTPETRE, is a salt which consists of potash in combination with nitric acid (30).
Its colour is whitish or limpid; and it does not liquefy by the action of the air. It is usually observed in the form of fine capillary crystals, though it is sometimes found in a massive state. When pure, it crystallizes into six-sided prisms (Pl. II, Fig. 15) which have a rectangular base. It is denominated by chemists nitrat of potash.
Nitre is found incrusted on the surface of the earth, in some parts of India, Africa, and Spain, and, in such abundance, as to admit of being swept off at certain seasons of the year, twice or three times a week. In our own country it not unfrequently occurs in a state of white efflorescence, on old plaster walls that are sheltered from rain. Nitre is also produced in stables and cart-houses, from the mixture of animal and vegetable substances in a state of putrescence.
Many kinds of plants, which grow in soils favourable to the production of it, contain nitre: this is particularly the case with pellitory, borage, and the large sunflower.
Immense quantities of nitre are annually required for the purposes of war. From its constituting one of the most important substances in the composition of gunpowder, it has been found necessary to adopt artificial modes of procuring it. In several districts of the East Indies there are places called saltpetre grounds. From these large quantities of the earth are dug, and put into cavities through which water is passed. This brings away with it the salt which the earth contains, and which is afterwards separated from the water by boiling. The East India Company, for more than a century past, has been under engagements to import into this country, and supply the board of ordnance, for his Majesty’s service, 137with 500 tons of nitre annually, at given rates and prices in times of peace and war.
In France this article is obtained in what are called nitrières, or nitre beds. These consist of the refuse of animal and vegetable substances, which undergo putrefaction, mixed with calcareous and other earths; and the nitre is obtained from them by water, as above-mentioned.—The principal requisites for the formation of nitre are said to be lime, animal and vegetable matters, heat, and an open, but not too free communication with dry atmospheric air.
The discovery of gunpowder has completely changed the modern art of war. The earliest notice that has occurred respecting the use of this article in Europe is, that it was employed in the wars of Germany, somewhat before the year 1373. It is said, however, to have been known in China long anterior to that period. Its component parts are nitre, charcoal, and sulphur, in the proportion of seventy-six, fifteen, and nine parts, in every hundred. These ingredients are first reduced to a fine powder separately, and then mixed with water, so as to form a thick paste. After this has dried a little, it is placed upon a kind of sieve full of small holes, through which it is forced. By this process it is divided into grains, the size of which depend of course upon the size of the holes through which it has been squeezed. It afterwards undergoes some other operations before it is ready for use.
Nitre is frequently administered in medicine; and it is used very extensively in different arts. A mixture of equal parts of nitre and tartar, burned together in a crucible, forms what is called white flux, which is used for melting and reducing different kinds of metallic substances. And a mixture of one part of nitre and two parts of tartar burned in the same manner forms what is called black flux. Nitre possesses antiseptic qualities in a considerable degree, whence it is much used, in conjunction with common salt and bay salt, for the preserving of animal food from putrefaction.
138Aqua-fortis, or nitric acid (30) as it is denominated by chemists, is prepared from this mineral. The mode of obtaining it in large manufactories is by distilling a mixture of nitre and clay in glass or stone retorts, each capable of containing seventy or eighty pounds’ weight of this mixture. But the acid thus procured being weak and impure, chemists, for nicer purposes, generally prepare it by distilling, in a glass apparatus, a proportion of three parts of nitre and one of sulphuric acid (24). The uses of aqua-fortis are various and important. All kinds of metals, except gold and platina, are capable of being dissolved in it. Hence, among other uses, it is employed by dyers, for dissolving tin, and forming with madder a scarlet colour; and, by hatters, for dissolving mercury (228) for some processes in the preparation of hats. Jewellers use it for several purposes.
207. SAL-AMMONIAC, or MURIAT OF AMMONIA, is a salt compounded of ammonia and muriatic acid (22). It is occasionally found in a state of powder, sometimes in a massive form, and sometimes in very irregularly shaped crystals, the primitive form of which is an octohedron (Pl II, Fig. 5). It is, however, more frequently an artificial production from the soot of burned animal matter.
The name of sal-ammoniac was acquired by this substance from its having been found by the ancients in great abundance amongst sand near the temple of Jupiter Ammon, in Africa. It is at present found in Persia; and, accompanying sulphur, amongst volcanic matter near Mount Vesuvius.
This salt was formerly imported from Egypt in the form of conical loaves, or of round cakes, which were convex on one side and concave on the other; but it is now made in Europe, by burning at the same time soot, bones, oil, and salt. The deposit formed by the vapour consists of sal-ammoniac, in conjunction with other substances, which are separated from it by a subsequent process. When good, it is white, transparent, and dry 139within; and externally of yellowish grey, or blackish colour.
Sal-ammoniac is applied to many useful purposes. Occasionally it is used in medicine. A considerable portion of it is consumed by dyers, to give brightness to some of their colours. It is also employed in the assay of metals, to discover the presence of iron; and having the property of rendering lead brittle, is sometimes used in the manufacture of shot. By coppersmiths and tinners it is used for cleansing the surface of the metals which they are about to cover with tin. In certain manufactories sal-ammoniac is mixed with tobacco, to give that article, or the snuff that is made from it, additional stimulant properties. Sal-ammoniac dissolved in nitric acid (30) forms the fluid named aqua-regia, which is employed in the solution of gold.
208. GREEN VITRIOL, IRON VITRIOL, or COPPERAS, is a mineral salt formed on a decomposition of pyrites (236) by the moisture of the atmosphere. It is also called SULPHAT OF IRON.
Its colour is bright green, and its taste very astringent; a solution of it in water dropped on oak bark instantly produces a black spot.
Although copperas is occasionally found in grottoes, caverns, the galleries of mines, and other places; yet, being much in request by dyers, tanners, and the manufacturers of ink, it is artificially prepared from pyrites. This mineral being moistened and exposed to the air, a crust is formed upon it, which is afterwards dissolved in water; and from this the crystals of vitriol are obtained by evaporation.
140The principal use of vitriol is in dyeing woollen articles, hats, and other manufactures, black. It is the basis of ink, and is used in the manufacture of Prussian blue. If it be reduced to powder by the action of fire in a crucible, and mixed with powder of galls, it forms a dry portable ink. Sulphuric acid (24) may be obtained from this kind of vitriol by distillation. The residue, after the process is completed, is used as a red paint; and when washed, is employed for the polishing of steel.
209. BLUE VITRIOL, or SULPHAT OF COPPER, is a blue salt formed by a combination of copper with sulphuric acid (24).
This substance, though sometimes found in a state of concretion, or in the form of powder disseminated over the surface of stones that have been in contact with water impregnated with it, is more frequently an artificial preparation obtained from evaporating the water which runs through copper mines. In the mines of Neussol, in Hungary, at the depth of 380 feet beneath the surface of the ground, are several vats, placed at different distances, for the purpose of collecting the water impregnated with copper, and which flows into them through a kind of gallery above. From this water the vitriol is afterwards separated by evaporation. A process somewhat similar is pursued in our own country.
In the principal blue vitriol manufactories established in France, the operation is thus carried on. Pieces of copper are first dipped into water, and their surface, while wet, is covered with a stratum of powdered sulphur. The copper thus prepared is put into an oven, and heated to redness. After some time, it is taken out, and, while hot, is plunged into a vessel filled with water. These operations are repeated several times, till the whole of the copper is dissolved, and the water becomes loaded with vitriol. Thus saturated, the water is placed 141over a fire till all the fluid particles are dissipated, and the vitriol alone is left.
Blue vitriol is used by artists and manufacturers in various ways. It is employed in dyeing: and enters into the composition of black colours, to which it gives depth and solidity. Blue feathers are stained by plunging them into a hot solution of it. The beautiful grass-green colour of the shops, called mineral green, is made from blue vitriol; and fowling-pieces and tea-urns are browned by washing them with a preparation of it.
210. WHITE VITRIOL, or SULPHAT OF ZINC, is a whitish, yellowish, or greenish white salt, formed by a combination of zinc (241) with sulphuric acid (24).
Although the white vitriol that is used in commerce is chiefly an artificial preparation, this salt sometimes occurs in a natural state, in mineral repositories that contain blende (241); and it appears to be formed by a decomposition of that ore. It is found at Holywell, in Flintshire, and in some parts of Cornwall.
When white vitriol is artificially prepared, the blende is roasted, and thrown, while red hot, into a vessel filled with water; in which it is allowed to remain about eighteen hours. This process is repeated several times; and, after the solution has become clear, it is removed into leaden vessels, and the water is evaporated by means of heat. On cooling, it crystallizes. After this the crystals are melted in a copper vessel, and the surface of the solution is skimmed with a hair sieve. It is then poured into a wooden vessel, and stirred till it becomes cool, and acquires a sufficient degree of consistence, when it is formed into loaves for sale. In this state it has the appearance and colour of refined sugar. White vitriol is chiefly manufactured in Germany.
It is used in medicine; and is employed in great quantities by varnishers, to make their oil varnishes dry more readily than they otherwise would. A fine white colour, called zinc-white, which is more durable 142than white lead, is prepared from it. Dyers use a considerable quantity of white vitriol to render deeper the colours produced by madder, cochineal, and other substances.
A pleasing experiment is made by mixing in a phial a small quantity of solution of white vitriol with a little liquid ammonia. Though each of the fluids is transparent when separate, yet the zinc will now be immediately precipitated in a white mass; and, what is peculiarly deserving of remark, if then shaken, it will almost as instantly be re-dissolved.
211. COMMON SULPHUR, or BRIMSTONE, is a yellow, dry, and brittle substance, which, in burning, yields a suffocating fume: the smell of this, under the denomination of sulphureous, is well known.
Sulphur is found in a pure or native state in nearly all volcanic countries: it is about twice as heavy as water; and is sometimes crystallized in the form of octohedrons, whose bases are rhombs. It exists abundantly in a state of combination with several metallic substances, and is also formed in putrid animal remains.
A great proportion of the sulphur which is used in commerce is obtained by the process of roasting copper, and other ores, previously to their being smelted. It passes off in the form of vapour, and, on being received into chambers constructed for the purpose, is there deposited in a powdery state. The substance thus formed is the flour of sulphur of the shops. It is afterwards melted in large pans, and cast in wooden tubes, to make the hard, or roll brimstone. Nearly all the sulphur used in France comes from the Solfatara of Italy. This volcanic country every where exhibits indications of the agency of subterraneous fires. Nearly the whole ground is bare and white; and, in every part, is warmer than the atmosphere during the greatest heat of summer. A sulphureous vapour is constantly emitted from the earth, and sulphur is condensed in various parts, and in great abundance. This is collected, packed in casks, and exported to Marseilles, where it undergoes certain preparations that are necessary towards purifying and rendering it fit for sale.
A considerable quantity of sulphur is employed in the 144composition of gunpowder (206). Its readiness of taking fire is the reason of its being employed in the making of matches. Sulphur gives a blue colour to artificial fire-works. Its vapour is used for the whitening of silk and wool, and also for the bleaching of straw used for making ladies’ hats.
Modellers employ sulphur to make moulds for various kinds of casts; and artists are enabled, by means of it, to take sharp and beautiful impressions of medals and engraved stones. The mode of doing this is very simple. The sulphur is put into an earthen vessel called a crucible, and placed on a hot fire. It soon melts; and if kept some time over the fire, becomes thick and dark-coloured. When poured into water in this state, it is as soft as wax. It may now be easily worked between the fingers into any given form: and, if pressed upon a seal or engraved stone, will be found to retain a perfect impression of it. It is this property of sulphur of which Mr. Tassie, of Leicester-fields, London, has availed himself, to furnish extremely elegant impressions of many antique gems.
Sulphur was much used by the ancients in medicine; and it is now occasionally administered both as an external and internal remedy. The compounds formed from it are employed to considerable extent in various processes of dyeing and calico printing. Many of the mineral waters, those, for instance, of Harrowgate (299) and Moffat (300), are indebted to sulphur for their most valuable qualities.
This substance has the property of becoming electric by rubbing. On exposure to a gentle heat, it melts; but if the heat be increased, it is entirely consumed, and passes off in vapour. When ignited, and the combustion is slow, it burns with a suffocating and acid fume, and blue flame; but when the combustion is quick it burns with a white and vivid flame. If exposed to a sudden, though gentle heat, by holding it, for instance, in a hand when that is warm, it will sometimes break in pieces with a crackling noise.
145It is a remarkable circumstance, that, if a bar of iron be heated to perfect whiteness, and then touched with a roll of sulphur, the two bodies combine, and drop down together, in a fluid state, forming what is called sulphuret of iron, a compound of the same nature as iron pyrites (236). A piece of iron rolled out very thin may be apparently melted in the hand, by putting it, when heated to whiteness, upon a thick piece of solid sulphur. It is, however, necessary, that this experiment be performed with great care; and under a chimney, or in a place where there is a current of air, to carry off the suffocating vapour.
Useful as sulphur is, in various ways, its most important application is supposed to be for the production of sulphuric acid, or spirit of vitriol (24). One mode in which this acid is obtained for the purposes of commerce, is by burning a mixture of sulphur and nitre (206) in large chambers lined with lead. In this process the nitre supplies a considerable portion of oxygen (21) to the sulphur, and the air of the atmosphere furnishes the rest. Thus a substance which, in a natural state, is one of the mildest that we are acquainted with, is by this operation converted into a corrosive and dangerous, though useful fluid. Its taste is strongly acid: and, when applied to animal or vegetable substances, it soon corrodes, and destroys their texture.
The properties of sulphuric acid have rendered it extremely valuable for numerous purposes, both in the arts and in the laboratory. It has been long employed by chemists, as one of their most useful and frequent agents.
The fluid that is put into the bottles for procuring instantaneous light is no other than sulphuric acid; and it is poured among filaments of asbestos (which it will not corrode), for the same purpose as ink is sometimes poured upon cotton. The matches are slips of wood dipped in a mixture of equal weights of sugar or charcoal powder, and what the chemists call hyperoxy-muriat of potash. These are to be rubbed together in a mortar, but with great care, as by strong friction the 146mixture is apt to explode. To obtain a light, nothing farther is requisite than to dip a match, thus formed, into a bottle containing the acid.
212. NAPHTHA, or ROCK OIL, is a yellow or brownish bituminous fluid, of strong penetrating odour, somewhat greasy to the touch, and so light as to float even on spirit of wine.
By exposure to the air, the consistence of naphtha is increased, and it passes into petroleum (213).
There are copious springs of naphtha at Baku, on the shore of the Caspian Sea; and also in some parts of Italy, particularly at Monte-Chiaro, near Piacenza. At Pitchford, in Shropshire, extensive strata or beds of sandstone are saturated with this mineral fluid, which is obtained from the stone by distillation, and is sold, as a remedy against sprains and rheumatism, under the name of Betton’s British oil.
By the Persians and Russians naphtha is used internally as a cordial. On the shores of the Caspian it is burned in lamps, instead of oil; and, in some parts of Italy it is employed in the lighting of churches and streets. When mixed with certain vegetable oils, it forms an excellent varnish.
It is the property of naphtha to take fire on the approach of a light, and to burn with great readiness and a white flame, leaving scarcely any residuum. The town of Broseley, in Shropshire, was formerly celebrated for a burning spring, which was first discovered in the month of June, 1711. Its original issuing from the ground was announced by a terrible noise in the night, which awakened several persons who lived near the spot. Some of these, on going out to ascertain the cause of the alarm, perceived, about two hundred yards from the river Severn, an extraordinary shaking of the earth, and a little bubbling of water through the grass. On digging round the spot, the water sprang up to a great height, and a candle which one of them held in his hand, set it on fire. This circumstance excited great curiosity; and 147many persons, from different parts of the adjacent country, came to visit what was called the “burning well.” To prevent this spring from being destroyed, an iron cistern was placed upon it, with a small hole in the cover, through which the water might be viewed. When a lighted candle was put into this hole, the water immediately took fire, darting and flashing in a violent manner, much in the same way as spirits do in a lamp, but with greater agitation. It would sometimes burn for forty-eight hours successively, and without any sensible diminution: and a tea-kettle, full of water, by being placed upon the hole, has been made to boil in nine minutes. In 1747, this spring had been lost for many years; but another was shortly afterwards discovered, the issuing of which was announced by a rumbling noise under ground, similar to that which had been formerly heard. This, however, also disappeared in the year 1756, by the sinking of a coal-pit in the neighbourhood.
213. PETROLEUM, or MINERAL OIL, is a fluid bitumen, of somewhat greater consistency than naphtha: of black, brown, or sometimes dingy green colour.
By exposure to the air it assumes the consistence of tar, and is then called MINERAL TAR (214).
This substance exudes spontaneously from the earth, or from clefts of rocks, and is found in nearly all countries, particularly in the East Indies, Italy, France, Spain, Germany, and England. In the neighbourhood of Rangoon, in Pegu, there are several hundred wells of petroleum. These are of square form, of considerable depth, and each lined with cassia wood staves. The oil is drawn from them pure, and in a liquid state, and is conveyed thence in small jars. The whole annual produce of this district is estimated at more than 400,000 hogsheads.
At Colebrook Dale, in Shropshire, there is a spring of petroleum. This was discovered at the depth of about thirty yards beneath the surface of the earth, in digging an archway for the conveying of coals from a 148very deep pit. The petroleum was at first found to ooze from between the crannies of the rock, but it soon afterwards poured forth in a considerable stream. The utility of this fluid having been made known, large iron pipes were formed from the spring into pits sunk for the purpose of receiving it. From these pits it is conveyed into immense caldrons, where it is boiled until it attains the consistency of pitch. Since the first discovery of this substance, three different springs of it have broken out. One of these is near the celebrated iron bridge; and the fluid that issues from it is almost pellucid, but, at the same time, is thicker than treacle.
Petroleum easily takes fire, and, in burning, yields a strong, sharp, and somewhat unpleasant odour; and a thick and disagreeable smoke. In cold weather it congeals in the open air.
In Pegu, and other parts of the East, petroleum is used in place of oil for lamps. Boiled with a species of resin, it is employed for painting the timber of houses, and covering the bottoms of boats and other vessels. In the latter respect it is considered to be particularly efficacious, by protecting the timber from the attacks of marine worms. It is also used by the inhabitants of eastern countries as a lotion in cutaneous eruptions, and as an embrocation in bruises and rheumatic affections. The ancient Egyptians used it in the embalming of dead bodies. In some countries lumps of earth are soaked with petroleum, and are employed as fuel.
214. MINERAL TAR, or BARBADOES TAR, is a fluid kind of bitumen, somewhat thicker than petroleum, and nearly of the consistence of common tar. It is viscid, of a black, brownish black, or reddish colour.
In burning its smell is disagreeable, but less pungent than that of most other kinds of bitumen. Its weight is somewhat greater than that of water.
In the West Indies, where this substance is principally found, it is applied to many of the purposes for which the preceding species is used; but its principal 149repute has been obtained from its being thought useful in disorders of the breast and lungs, though this application of it is considered very improper. It is likewise used as an external remedy in paralytic disorders.
215. ELASTIC BITUMEN, or MINERAL CAOUT-CHOUC, has a strong resemblance to Indian rubber. In some instances it is elastic, and so soft as to adhere to the fingers, and in others brittle, and so hard as nearly to resemble asphalt (216).
Its colour is yellowish, reddish brown, or blackish. One kind of this mineral, when fresh cut, nearly resembles fine cork, both in texture and colour.
This extraordinary substance, which will expunge the marks of black lead in the same manner as Indian rubber, was first discovered, about the year 1786, in cavities of the lead mine of Odin, near Castleton, in Derbyshire, and it has not hitherto been found elsewhere. Elastic bitumen appears to be a peculiar modification of petroleum, in its passage to asphalt: and probably owes its elasticity to its cellular texture, and to the moisture with which it is combined.
216. ASPHALT, or SOLID BITUMEN, is a brittle substance, of black or brownish black colour, and of consistence somewhat harder than pitch.
It has nearly the same weight as water, is smooth to the touch, does not stain the fingers, and has little or no smell unless it be rubbed or heated. When heated, it melts, swells and inflames; and, if pure, burns without leaving any ashes.
The ancients were well acquainted with this substance, which is nothing more than mineral tar (214) in an indurated or hardened state. It is found on the surface of volcanic productions, and floats, in solid pieces, and in considerable abundance, on the Asphaltic Lake, in Syria, which has thence received its name. This lake is also called the Dead Sea, from a notion that the odour arising from the asphalt destroys even birds which fly over it: Maundrell, however, states that this is not true, as he saw several birds fly about and over it, without experiencing the slightest injury.
150Asphalt is also found near ancient Babylon; and there is reason to suppose that the mortar so celebrated amongst the ancients, and with which the walls of Babylon and of the Temple of Solomon were cemented, was nothing more than a preparation of asphalt. We are informed by Herodotus that a composition of heated bitumen, mixed with the tops of reeds, was used by the ancients as a cement. This account is confirmed by modern travellers, who assert that the remains of buildings have been discovered in which bitumen was formerly thus employed. It is presumed to be the same substance which, in our translation of the Old Testament, is called pitch, and which was used by Noah, as an exterior and interior coating of the ark; by the mother of Moses as a coating for the little vessel in which he was exposed; and on various other occasions.
As an article of modern utility, it is to be remarked that the Arabians dissolve asphalt in oil, and, with the mixture, smear their horse harness, to preserve it from the effects of weather, and the attacks of insects. In a state of solution it is applied, in several eastern countries, as a covering for timber and the bottoms of ships. It is occasionally used in the cleansing and healing of ulcers, and other sores. In France it is manufactured into a substance which is in considerable request for greasing the wheels of carriages. It is used by the makers of watch-dials, who mix it with lamp black, and oil of turpentine; but its chief use is as an ingredient in certain varnishes, and particularly in the varnish used by copper-plate engravers. It is frequently adulterated by a mixture with common pitch; but this is easily discovered by the smell.
Besides the countries and places already mentioned, asphalt is found in several parts of America, in the island of Trinidad, in the province of Neufchatel, and many parts of the Continent of Europe.
217. The component parts of coals are principally carbon or charcoal (48), and bitumen (216).
151Some kinds of coal are laminar, and others compact. They in general burn freely, with a bituminous odour, and leave a considerable residuum.
This invaluable mineral is found in beds, or strata, frequently betwixt clay slate (257) and sandstone (267), and seldom betwixt those of limestone (140). It chiefly occurs in the northern hemisphere, particularly in countries which lie nearly in the same latitudes with Great Britain; in Siberia, Germany, Sweden, France, Canada, and Newfoundland; and in some of the northern parts of China. It is stated to be abundant in New Holland; but we have no distinct account of coal in the continent of Africa. No fewer than seventy different kinds of coals are brought to the London market, the value and prices of which greatly differ. Of these the coals called Wall’s-end, from the name of the pit, near Newcastle, whence they are obtained, usually bear the highest price.
218. COMMON COAL, or PIT COAL, is of black colour, and has generally a slaty structure and foliated texture.
When handled it stains the fingers; and when burnt it cakes more or less during combustion. Its component parts are usually charcoal (48) and bitumen (216), with a small portion of clay, and sometimes with pyrites, or sulphat of iron (236). What is called slaty coal contains a greater portion of clay than other kinds.
Some foreign writers have ascribed the great wealth possessed by this country to the coals which are here produced in such abundance, and which facilitate, in a very essential degree, nearly all its manufactures, and consequently are a means of promoting its commerce to an extent which is possessed by few other countries. All our great manufacturing towns, Birmingham, Sheffield, Leeds, Glasgow, &c. are situated either in the midst of coal districts, or in places to which coals are conveyed, with little expense, by canal carriage.
Coals are principally obtained from the neighbourhood of Newcastle-upon-Tyne, Sunderland, and Whitehaven. 152The particular places whence they are obtained have the name of collieries, and the mines from which they are dug are called pits. The deepest of these are in Northumberland, and are worked at more than 900 feet below the surface of the earth. At Newcastle there is a coal-pit near 800 feet in depth, and which, at that depth, is wrought five miles horizontally, quite across, and beneath the bed of the river Tyne, and under the adjacent part of the county of Durham. At Whitehaven the mines are of great depth, and are extended even under the sea, to places where there is above them sufficient depth of water for ships of great burthen, and in which the miners are able sometimes to hear the roaring of the water. On the contrary, in some parts of Durham the coal lies so near the surface of the earth that the wheels of carriages lay it open, and in such quantity as to be sufficient for the use of the neighbourhood.
The beds of coal are of various thicknesses, from a few inches to several feet; and in some places, it is found advantageous to work them at a very great depth, although their thickness does not exceed four or five feet. The thickest bed of English coal, of any extent, is that of the main coal in Staffordshire, which measures about thirty feet. In many places there are several beds above, and parallel to, each other, separated by strata of slate, sandstone, and other minerals. Coal is never found in chalk, and very rarely in limestone.
At Whitehaven, the principal entrance to the coalmine, both for men and horses, is by an opening at the bottom of a hill, through a long passage hewn in a rock. This, by a steep descent, leads to the lowest bed of coal. The greatest part of the descent is through spacious galleries, which intersect other galleries; all the coal having been cut away, except large pillars, which, in deep parts of the mine, are three yards high, and about twelve yards square at the base, such great strength being there required to support the ponderous roof. There are three distinct and parallel strata 153of coal, which lie at a considerable distance above each other, and which have a communication by pits that are sunk between them. These strata are not always regularly continued in the same plane. The miners occasionally meet with veins of hard rock, which interrupt their further progress, and, at such places, the earth, on one side of the vein, appears to have sunk down, while that on the opposite side has its ancient situation. These breaks the miners call dykes (4). When they come to one of them, their first care is to discover whether the coal, in the part adjoining, be higher or lower than that in which they have been working; or, to use their own terms, whether it be cast down or cast up. For this purpose they examine attentively the mineral strata on the opposite side, to see how far they correspond with those which they have already passed through. If the coal be cast down, they sink a pit to it: but if it be cast up, the discovery of it is often attended with great labour and expense.
In general the entrance to coal mines is by perpendicular shafts, and the coals and workmen are drawn up by machinery. As the mines frequently extend to great distances, horizontally, beneath the surface of the earth, peculiar care is necessary to keep them continually ventilated with currents of fresh air, for the purpose, not only of affording to the workmen a constant supply of that vital fluid, but also to expel from the mines certain noxious exhalations which are sometimes produced in them.
One of these, denominated fire damp, is occasioned by the generation of hydrogen gas, or inflammable air (45). This gas, when mixed with the common air of the atmosphere, explodes, with great violence, on the approach of a lighted candle, or any other flame; and has, at different times, occasioned the loss of many valuable lives. It is a singular circumstance, that although it is immediately set on fire by a flame, yet it cannot be kindled by red hot iron, nor by sparks produced from the collision of flint and steel. Hence a machine 154was, some years ago, adopted in the mines near Whitehaven and Workington, in which a wheel formed of steel, and in shape somewhat like that of a razor-grinder, was turned round with very rapid motion against a series of flints, and in such manner as to yield to the miners sufficient light to carry on their work in places where the flame of a candle would occasion the most dreadful explosions. Sir Humphrey Davy has lately invented, for the use of mines where this gas is prevalent, what is called a safety lamp. This is a lamp enclosed in a wire cylinder, the interstices of which are so extremely small as, whilst it gives light, will not explode the gas.
Another injurious exhalation in coal mines arises from the formation of carbonic acid gas, or fixed air (26), and is called choke damp. It is the property of inflammable air to rise to the upper parts; but this, on account of its weight, occupies principally the lower parts of mines, and occasions death by suffocation, though it is by no means so fatal as the former. In some mines a prevention of injury arising from each of these gases is attained, by ascertaining the particular crevices in the coal from which they issue, confining them at those places within a narrow space, and, if possible, conveying them out of the mines, through long pipes, into the open air.
There is yet another danger attending coal mines which requires to be provided against, and this is inundation. Many mines have been destroyed by the flooding of water, which springs up within them. The modes by which this was formerly extracted were extremely laborious, and, in numerous instances, entirely inefficacious. By means, however, of the fire or steam engines now in use, the quantity of water raised from mines is perfectly astonishing. Four engines in one of the collieries at Whitehaven discharge more than twenty hogsheads per minute, or upwards of 30,000 hogsheads in every twenty-four hours.
The coal trade, which at present affords so important 155a nursery for our seamen, and, in numerous other respects, yields advantages of the most beneficial description to this country, was entirely unknown a few centuries ago. Coals were not generally adopted as fuel until the beginning of the reign of Charles I. They were, however, noticed in documents anterior to the reign of Henry III., for, that monarch, in the year 1234, renewed a charter, granted by his father, to the inhabitants of Newcastle, by which they were permitted to dig coal upon payment of 100l. per annum. Coals had been introduced into London before 1306; for in that year, the use of them as fuel was prohibited, from the supposed tendency of their smoke to corrupt the air. About the beginning of the sixteenth century, the best coals were sold in London at the rate of 4s. 1d. per chaldron, and at Newcastle for no more than 2s. 6d. During the ensuing century, however, they were received into such general use, that, in 1648, on a scarcity of coal in London, many of the poor are said to have died from want of fuel. The whole quantity of coals imported into London has been estimated, on an average of four years, ending in March, 1815, to amount to 1,170,000 chaldrons per annum.
Some writers have imagined coal to be the remains of antediluvian timber, which floated upon the waters of the deluge until several strata of mineral substances had been formed: others conceive it to have been antediluvian peat bog. It is called pit coal, from the circumstance only of its being obtained from mines or pits; and, in London, for no better reason than its having been conveyed thither by sea, it has the name of sea coal.
Its uses as fuel are too extensively known to need here any observations. By the distillation of coal an inflammable gas is produced, which has of late been introduced for the lighting of manufactories, and lighting several of the streets and shops of the metropolis. This gas is conveyed by pipes, from the reservoir in which it is collected, to great distances; and the light which 156it yields is peculiarly brilliant and beautiful. It was at the foundery belonging to Messrs. Boulton and Watts, at Birmingham, that the first public display of gas lights was made, in the year 1802, on the occasion of the rejoicings for peace. In 1805 the cotton mills of Messrs. Phillips and Lee, at Manchester, were lighted with gas, to the exclusion of lamps, candles, and every other source of artificial light. In the beginning of 1816 it was estimated that, at the three gas-light stations, in Peter-street, Westminster, Worship-street, and Norton Falgate, London, twenty-five chaldrons of coals were used daily; and that these were sufficient to supply with gas 125,000 large lamps. At the works in Dorset-street, Fleet-street, the daily consumption of coal was about three chaldrons, which afforded gas for 1,500 lamps.
The production of the gas light is easily effected in miniature, by putting common coal, pounded small, into the bowl of a tobacco-pipe, and closely covering this with clay made into a stiff lute with water. When the clay is dry, the bowl of the pipe must be put into the fire, and there heated gradually. In a few minutes a stream of gas will issue from the end of the pipe. This may be set on fire with a piece of paper, and will burn with a bright flame. When the gas is no longer disengaged, there will be found in the bowl of the pipe the remains of the coals, in the form of coke.
It is estimated that one chaldron of good coals will afford from 17,000, to 20,000 cubical feet of gas; and that one of the large burners in the shops of London, consumes about four cubical feet per hour.
Soot is produced from the smoke of burned coal, and is used as a manure for cold, moist, and clayey meadows and pastures: and pounded coal has been applied to the same purpose in some parts of the Continent. By a process called charring, coal is divested of its humid, acid, and bituminous particles, and is converted into a kind of cinder called coke. This is employed in 157cases where intense heat is requisite, as for the smelting of iron ore; and likewise where acid and bituminous particles of coal would be detrimental, as in the drying of malt.
What is usually termed culm is the refuse or dusty coal, produced in working the common coals. It contains much earthy matter, will not kindle in an ordinary fire-place, but produces considerable heat and flame in a furnace, where a strong current of air is introduced. In England it is exempted from the high duty imposed on other coals, and is sold at a very low price. It is used for burning lime, making salt, and in steam engines.
219. CANNEL COAL is of black colour, with little lustre, is not laminar, but breaks in any direction, like pitch, and does not stain the fingers.
This highly inflammable kind of coal is found abundantly in the neighbourhood of Wigan, in Lancashire, where there is an entire stratum of it about four feet in thickness. It is also found near Whitehaven, in some of the pits at Newcastle, and in some parts of Scotland. Doubts have been entertained respecting the name of this coal; but when it is recollected that in Lancashire, whence it is chiefly brought, the word candle is usually pronounced with the omission of the letter d, and that, in many instances, the coal is used by the poor as a substitute for candles, these will be immediately removed. In Scotland it has the name of parrot coal.
No kind of coal takes fire so readily, nor burns with so cheerful and brilliant a flame as this: and its not soiling the fingers, like pit coal, renders the use of it peculiarly pleasant; but it does not cake, and soon burns away. When first kindled, it crackles and splinters very much; and, on this account, would be dangerous, were it not easily prevented from so doing by being previously immersed for a little while in water. Cannel coal has much the appearance of jet. It admits of being turned in a lathe, and takes a good polish; and snuff-boxes and trinkets made of it have in many instances 158been sold as jet (222). Of all the kinds of coal that are used for gas-lights, none are said to be so useful as this.
220. STONE COAL, KILKENNY COAL, WELSH COAL, or GLANCE COAL, is of a dark iron-black colour, with a metallic lustre and foliated texture; and consists almost entirely of charcoal.
Unlike most other kinds of coal, this occurs both in stratified masses, and in lumps, nested in clay. It is found in several countries of the Continent, in Wales, Scotland, and near Kilkenny in Ireland.
When laid on burning coals, it becomes red hot, emits a blue lambent flame in the same manner as charcoal; and is, at length, slowly consumed, leaving behind a portion of red ashes. No smoke nor soot is produced from this coal; but, on the contrary, it whitens the places where the fume is condensed; and the effluvia which it gives out are extremely suffocating.
This coal is chiefly used in the drying of malt.
221. BOVEY COAL, BROWN COAL, or BITUMINOUS WOOD, is of brown colour, and in shape exactly resembles the stems and branches of trees, but is usually compressed. It is soft, somewhat flexible, and so light as nearly to float when thrown into water.
The greatest abundance of this coal occurs at Bovey, near Exeter, from which place it derives its name. The lowest stratum is worked at the depth of seventy-five feet beneath the surface of the earth. It is also found in Scotland, Ireland, and Germany.
As fuel, the Bovey coal is used only by the poorest classes of the community, as, notwithstanding its burning with a clear flame, it emits a sweetish but extremely disagreeable sulphureous gas, which is injurious to the health of the inhabitants. It is principally used for the burning of lime, and for the first baking of earthen ware.
222. JET, or PITCH COAL, is a solid, black, and opaque mineral, harder than coal, and found in detached 159masses from an inch to seven or eight feet in length, having a fine or regular structure, and a grain resembling that of wood.
It has sometimes been confounded with cannel coal (219), but it is easily distinguished by its superior hardness: Jet cannot without difficulty be scratched with a knife, whilst cannel coal may be marked by the simple pressure of the nail.
The name of jet has been derived from Gages, a river of Lycia, whence the ancients are said to have obtained this substance. It is frequently cast ashore on the eastern coasts of England, together with pieces of amber and curious pebbles, particularly near Lowestoft in Suffolk, and in some parts of Yorkshire, where many persons employ their leisure in searching for it, and forming it into various kinds of trinkets. Jet is found in several countries of the Continent.
It is stated that in the district of Aude, in France, there are more than 1,000 persons constantly employed in the fabrication of jet into rosaries, buttons, ear-rings, necklaces, bracelets, snuff-boxes, and trinkets of different kinds. Near fifty tons weight of it are annually used for this purpose; and articles to the value of 18,000 livres are said to be sold in Spain alone. In Prussia the amber diggers call it black amber, because it is found accompanying that substance; and because, like amber, it is faintly electric, or attracts feathers and other light objects when rubbed. They manufacture it into various ornamental articles, and sell these to ignorant persons, as black amber, at a great price.
In different parts of the globe the trunks of trees, which have been long buried, have passed into the state of jet; and, in almost all these trees may be traced the distinctive characters of the species to which they belong. They are more or less brittle, more or less unctuous, according to the species, the degree of alteration, and the nature of the soil. All of them have a smooth and glassy fracture, but all are not adapted for the tool of the workman. When, for instance, the texture of the tree presents only a mass of dry fibres, 160the jet obtained is dry and brittle; and cannot be used in the forming of trinkets. But, if the texture be unctuous the fibre acquires a considerable degree of softness, is susceptible of being properly wrought, and receives a perfect and beautiful polish.
A fictitious kind of jet is made of glass; and several varieties of mineral pitch, and cannel coal, are imposed upon ignorant purchasers for jet.
When jet is once set on fire it burns with a green flame, and continues to burn for a considerable time, exhaling a strong bituminous smell. If the heat be rendered greater, it melts.
223. BLACK LEAD, or PLUMBAGO, is an inflammable mineral, which consists of carbon, or charcoal (48), combined with iron, in the proportion of about nine parts of the former to one of the latter.
It is of dark iron-grey colour, with a strong metallic lustre, and so soft that it is easily scratched with a knife. To the touch it is soft and greasy; and, when handled, it stains the fingers. In weight it is about twice as heavy as water.
The name of black lead has very improperly been given to this substance from its appearance only, as it has no alliance whatever with lead. It is usually found in kidney-shaped lumps of various size, and occurs in several countries of Europe, but no where of such excellent quality as in Borrowdale, Cumberland, where it has the name of wadd. The vein of black lead lies between strata of slate, and is from eight to nine feet thick. This mine is not opened more than once every three or four years, the quantity thus obtained being found fully sufficient for the demand. The only other mine of black lead in Britain is in Ayrshire, Scotland.
Artists in water-colours, if deprived of this mineral, would find great difficulty in making their sketches; as the marks that are erroneously made with it are more easily expunged than those of almost any other substance. Hundreds of thousands of pencils are every 161year formed of black lead. For this purpose the mineral is sawed into slender square pieces. These are fixed into grooves, of the same shape, cut in cedar, or some other soft wood; another piece of wood is then glued upon this, and the whole is worked into a circular form. The finer kinds of black lead are prepared for use by being boiled in oil before they are cut. The coarser kinds, and the refuse of the sawings, are melted with sulphur, and then cast into coarser pencils for carpenters. These may, in general, be easily distinguished by their sulphureous smell. The pencils that are manufactured in England are more esteemed on the Continent than any others.
The powder produced in the sawing of pencils is employed for numerous purposes. It is used for giving a bright gloss to cast-iron grates and stoves, and defending them from rust, and from the action of fire. It may also be advantageously applied to the inner surface of wooden screws, to packing presses, the axles of various sorts of machines, to slides, and other wood work, which are subject to friction. In this respect it is far superior either to grease or soap. The makers of razor-strops occasionally employ black lead in the composition which they spread upon leather for the sharpening of razors; and, on the Continent, it is sometimes used for blackening the hair. A coarser kind of black lead is used for making the vessels that are used by chemists, called crucibles.
224. AMBER is a substance usually of golden yellow colour, semi-transparent, and of shining and somewhat resinous lustre. It is occasionally seen of yellowish white colour, and nearly opaque.
The origin of amber is unknown. From the ants and other insects which it frequently contains, there can be no doubt that it has once been in a fluid state: and some writers have thought that it is a resinous juice, gradually modified by the action of sulphuric acid 162(24); but this is entirely conjecture. The ancients called it electron, and attributed its formation to the sisters of Phaëton, who, lamenting the death of their brother, were converted into poplar trees; these, it was said, instead of tears, yielded every year this substance; which, issuing from them in a fluid state, ran into the river, and there became hardened.
Amber is usually found in rounded and detached pieces, on the south coast of the Baltic, on the eastern shores of England, and in small quantity, on those of Sicily and the Adriatic; and a substance greatly resembling it is occasionally found in gravel pits near London. The only mines of amber at present known are in Prussia. These are worked in the usual way, by shafts and galleries, to the depth of about 100 feet. The amber is imbedded in a stratum of fossil wood, and occurs in rounded pieces, from a few grains to three and even five pounds in weight. The largest piece of amber ever known to be discovered in a detached state was found near the surface of the ground, in Lithuania, about twelve miles from the Baltic Sea. It weighed more than eighteen pounds, and was deposited in the cabinet of the King of Prussia at Berlin. Very lately a mass of amber, weighing thirteen pounds, was also found in Prussia. For this piece 5000 dollars are said to have been offered; but the Armenian merchants assert that it might have been sold in Constantinople for more than 30,000 dollars.
Anterior to the discovery or general dispersion of precious stones from India, amber was considered of great value as a jewel, and was employed in all kinds of ornamental dresses. The ancient Romans were so partial to this substance that Pliny, reprobating the great demand for it, says, the Roman females would give larger sums for a puppet or figure in amber, resembling a man or woman, however small its size, than they would for the finest man or the most valiant soldier. Under the Emperor Nero, persons were sent from Rome, for the purpose of collecting and purchasing 163amber; and so much of it was at length obtained, that it was used for ornamenting the nets and cordage employed in the theatres for preventing the wild animals from approaching the populace there assembled. It was likewise used to ornament the armour, the biers, and funeral apparatus of such persons as were killed.
Amber is now chiefly in request by Greek and Armenian merchants, but it is uncertain where they dispose of it. Some persons conjecture that it is purchased by pilgrims previously to their journey to Mecca; and that, on their arrival in that place, they burn it in honour of Mahomet.
The kind most in esteem is of a bright golden yellow colour. This is occasionally manufactured into snuff-boxes, small vases, necklaces, bracelets, cane-heads, and other ornamental articles, many of which are purchased by the Turks, Russians, and Poles; but the general demand for them has of late very much decreased. Some years ago the German artists paid great attention to this substance; and many experiments were made for the purpose of discovering means of removing its defects, and improving its beauty. It is said that they possessed the art of liquefying it to such a degree, that it could be run into moulds without injuring its beauty; and that specimens of this liquefied amber are preserved in the Electoral Cabinet at Dresden. There are still considerable manufactories of amber at Stolpen, Konigsberg, Dantzic, and Lubeck.
Amber, when wrought into ornaments, is first split on a leaden plate, and then turned on a particular kind of whetstone. The polishing of it is performed with chalk and water, or chalk and oil; and the work is finished by rubbing the whole with clean flannel. Without great attention it becomes very hot, and either flies into pieces, or takes fire during the operation.
After having been roasted or melted, amber is readily soluble in oil, and, in this state, constitutes the basis of several kinds of varnish. It was formerly much used in medicine, but, in this respect, it is now almost wholly 164neglected. Some persons, however, have still an absurd notion that a collar or necklace of amber, tied round an infant’s neck, will enable it to cut its teeth in safety. Oil of amber combined with liquid ammonia constitutes a white soapy liquor called eau-de-luce.
It has already been mentioned that insects are occasionally found in amber. These are generally in a very perfect state, and consist of flies, small moths, &c. Grains of sand, pieces of iron pyrites, and the leaves of plants, are also sometimes found in it. Insects, sand, and other substances, are likewise remarked in a species of gum, called gum animè, which, in colour, appearance, and qualities, so nearly resembles amber, that it is almost impossible to distinguish the two substances from each other. Large productions, which were formerly supposed to have been made of amber, such as a column ten feet high in the Florentine Museum, are now usually considered to have been formed of this gum; and many of the large beads of what are sold as amber necklaces are made of it.
If a piece of amber be fixed on the point of a knife and lighted, it will burn entirely away, emitting at the same time a white smoke, and a somewhat agreeable though sickly odour. When rubbed it has the property of attracting light bodies; hence one of the ancient Greek philosophers attributed to it a certain kind of life. From the name of electron, which was given to it by them, in consequence of this property, we derive our word electricity.
225. METALS, in a perfect state, are easily distinguished from other minerals, by a peculiar brilliancy which pervades their whole substance, and which has the name of metallic lustre; by their complete opacity, and their great weight in proportion to that of other mineral substances.
When taken from the earth they are found in one or other of the four following states: 1. In a native or metallic state, 2. Combined with sulphur, 3. In a state of oxide (21) 4. Combined with acid.
Metals, when found in a state of combination with other substances, have the name of ores. They are in general deposited in veins (4), of various thickness, and at various depths in the earth. The mode of obtaining them is to penetrate from the surface of the earth to the vein, and there to follow it, in whatever direction it may lie. The hollow places thus formed are called mines, and the men employed in them are denominated miners. When the veins are at a great depth, or extend to any considerable distance beneath the surface of the earth, it is necessary, at intervals, to make openings, or shafts, to the surface, for the admission and circulation of the air; and also to draw off the water which collects at the bottom, by drains, pumps, or steam-engines, as the situation or circumstances require.
After the metallic ores are drawn from the mine, they, in general, go through several processes before they are in a state fit for use. Some of them are first washed in running water, to clear them from earthy 166particles. They are then piled with combustible substances, and burnt or roasted, for the purpose of ridding them of the sulphur or arsenic with which they may happen to be combined, and which rises from them in a state of fume or smoke. Thus, having been freed from impurities, they undergo the operation of melting, in furnaces constructed according to the nature of the respective metals, or the uses to which they are to be subsequently applied.
The knowledge of metals is a subject of great importance to mankind. Their use in trade is so frequent, and in the arts so various and so interesting, that few objects can be more worthy of attention than these.
OR, SUCH AS ARE CAPABLE OF BEING FLATTENED OR ELONGATED BY THE HAMMER, WITHOUT TEARING OR BREAKING.
226. PLATINA, the most ponderous of all the metals with which we are acquainted, is, when purified, about twenty times heavier than water. It is also one of the hardest and most difficult to be melted, is of white colour, but darker and not so bright as silver, and is found only in small blunted and angular grains or scales in the sands of some of the rivers in South America.
If platina could be obtained in sufficient quantity, it would perhaps be the most valuable of all metals. The important uses to which it is applicable may easily be imagined when we state that it is nearly as hard as iron, and that the most intense fire and most powerful acids have scarcely any effect upon it. Platina is not fusible by the heat of a forge, but requires either the concentrated rays of the sun in a burning mirror, the 167galvanic electricity, or a flame produced by the agency of oxygen gas.
It is admirably adapted for the uses of the philosophical chemist: although vessels made of it must always be found expensive, from its being necessary to solder them with gold; and although it has the disadvantage of being subject to corrosion by the application or use of caustic alkalies. Vessels made of it are not liable to be broken, and are as indestructible as those made of gold. When properly refined, its colour is somewhat betwixt that of silver and iron. Not being liable to tarnish like silver, platina is manufactured into several kinds of trinkets.
Its ductility is so great that it may be rolled into plates, or drawn into wire; and platina wire, for strength and tenacity, is considered much preferable to that either of gold or silver of equal thickness. Platina is also made into mirrors for reflecting telescopes, into mathematical instruments, pendulums, and clock-work; particularly where it is requisite that the construction of these should be more than usually correct, as platina is not only free from liability to rust, but is likewise subject to very little dilatation by heat. It is sometimes beaten into leaves and applied to porcelain, in the same manner as leaf gold; and its oxide (21) is used in enamel painting, and might be used, with great advantage, in the painting and ornamenting of porcelain. The platina employed for all these purposes is repeatedly melted with arsenic, as without the aid of this it could only be obtained in very small masses, owing to the intense heat that is required for its fusion.
This extraordinary metal was unknown in Europe until about the year 1735, when it was first brought from South America by Don Antonio Ulloa.
227. GOLD is a metal distinguished by its yellow colour; by its being next in weight to platina, softer than silver, but considerably more hard than tin; and being more easily melted than copper.
It is found in various states, massive, in grains, small 168scales, and capillary, or in small branches. It cannot be dissolved in any acid except that called aqua regia (207), and is more than nineteen times heavier than water.
The countries of hot climates are those chiefly in which gold is discovered. It abounds in the sands of many African rivers, and is very common in several districts both of South America and India. The gold mines of Lima and Peru have had great celebrity; but, since the late commotions in the Spanish colonies, the working of them has been much neglected. It is from Brazil that the greatest part of the gold which is seen in commerce is brought. The annual produce of the various gold mines in America has been estimated at nearly 9,500,000l. sterling.
The principal gold mines in Europe are those of Hungary, and next to them those of Saltzburg. Spain is probably very rich in gold. Considerable mines were worked there in former times, particularly in the province of Asturia; but, after the discovery of America, these were given up or lost. Gold has been found in Sweden and Norway, and also in several parts of Ireland, but particularly in the county of Wicklow.—Among the sands of a mountain stream in that county, and among the sand of the valley on each side, lumps of gold are occasionally found. Pieces have been discovered which weighed twenty-two ounces, but they are generally much smaller, from two or three ounces to a few grains. It is said that lumps of gold, of large size, have been used as weights in some of the common shops, and that others have been placed to keep open the doors of cottages and houses in some parts of Ireland, the owners not knowing what they were. Gold is also occasionally found in Cornwall, and some other counties of England. Wherever it occurs it is commonly observed in a state of alloy with copper or silver, and in the form of grains, plates, or small crystals.
Gold was formerly obtained in Scotland. It is asserted that, at the marriage of James V. there were covered dishes filled with coins made of Scottish gold, 169and that a portion of these was presented to each of the guests by way of dessert. Very extensive operations for the discovery of gold were carried on during the reign of Queen Elizabeth, at Leadhills, in Lanarkshire, under the direction of an Englishman whose name was Bulmer. The trenches, the heaps of soil that were turned up, and other marks of these operations, are yet visible near the road between Leadhills and Elvanfoot. It is said that 300 men were then employed; and that, in the course of a few years, a quantity of gold was collected, equal in value to 100,000l. sterling. Not many years ago similar operations were commenced under the superintendence of a celebrated manager of the Scottish lead mines. The gold was found immediately under the vegetable soil; and the method of obtaining it was to direct a small stream of water, so as to carry the soil along with it, to basins or hollow places, where the water might deposit the matters carried down by the force of its current. The matter thus deposited was repeatedly washed, till the whole of the earthy substances were carried off. The gold, being heaviest, sunk to the bottom, and remained behind. The soil still furnishes gold; but the produce would by no means be equal to the expense of collecting it. Searching for gold, therefore, is now regarded only as an amusement, and not as a source of profit. Grains of this metal are sometimes found, after great floods, among the sand of brooks in different parts of Scotland.
The mode of extracting gold from its ore is by reducing it into a fine powder, and mixing this powder with quicksilver (228). The latter having the quality of uniting with itself every particle of the precious metal, but being incapable of union with the other substances, extracts it even from the largest portions of earth. The quicksilver, which has absorbed the gold, is then separated by means of heat; it flies off in vapour, and leaves the other metal in the vessel used for the operation.
170Gold has been known, and in request, from the very earliest ages of the world. By the assent of civilized nations, it has become the representative of wealth under the form of money; and it is now an universal circulating medium for the purchase of all kinds of commodities. It has been chosen to occupy this important place on account of its scarcity, its weight, and other valuable properties.
As gold is not liable to tarnish or rust, it is frequently employed for ornaments of dress. But, beyond its use in the coinage, its most important uses are for goldsmith’s work, in jewellery, and for gilding. In each of these its standard or purity is different. That denominated coinage, or sterling gold, consists of an alloy of about twenty-two parts of gold with two parts of copper; whilst gold of the new standard, of which gold plate, watch-cases, and many other articles are made, consists of only eighteen parts of gold, and six parts of copper. Each of these is stamped at Goldsmiths’ Hall; the former with a lion, a leopard’s head (the mark of the goldsmith’s company), a letter denoting the year, the king’s head, and the manufacturer’s initials; the latter is stamped with the king’s head, letter for the year, a crown, the number 18 to designate its quality, and the manufacturers initials. The coinage gold of Portugal and America is of the same standard as our own; that of France is somewhat inferior; and Spanish gold is inferior to the French. The Dutch ducats and some of the Moorish coins are of gold unalloyed. Trinket gold, which is unstamped, is in general much less pure than any of the above; and the pale gold which is used by jewellers is an alloy of gold with silver.
The ductility and tenacity of this metal, particularly when alloyed with copper, are extremely remarkable, and are fully proved by the great extent to which a very small quantity of it may be beaten into leaves, or drawn into wire. Leaves of gold may be beaten so thin, that a single grain may be made into fifty-six 171leaves, each an inch square. These leaves are only 1/282000 of an inch thick; and the gold leaf which is used to cover silver wire is but the twelfth part of that thickness. An ounce of gold upon silver wire is capable of being extended more than 1,300 miles in length: and sixteen ounces of gold, which, in the form of a cube, would not measure more than an inch and a quarter on each side, will completely gild a silver wire in length sufficient to compass the whole earth like a hoop.
Gold is beaten into leaves upon a smooth block of marble, fitted into the middle of a wooden frame about two feet square, in such manner that the surfaces of the marble and of the frame are exactly level. On three of the sides there is a high ledge; and the front, which is open, has a flap of leather attached to it, which the man who beats the gold uses as an apron for preserving the fragments that fall off. In this process there are three kinds of animal membranes used, some of which are laid between the leaves to prevent their uniting together, and others over them to defend them from being injured by the hammer. The exterior cover is of parchment. For interlaying with the gold, the smoothest and closest vellum that can be procured is first used; and, when the gold becomes thinner, this is exchanged for much finer skin, made of the entrails of oxen, prepared for this express purpose, and hence called gold beater’s skin. After the leaf has been beaten to a sufficient degree of thinness, it is taken up by a cane instrument, and thrown flat upon a leathern cushion, where it is cut to a proper size with a square frame of cane, or wood edged with cane. These pieces are then fitted into books of twenty-five leaves each, the paper of which has been well smoothed, and rubbed with red bole (127), to prevent them from sticking. The leaves are about three inches square, and the gold of each book weighs somewhat more than four grains and a half.
172It was anciently the custom to beat gold into thin plates, and to gild the walls of apartments, the surfaces of dishes, drinking utensils, and other articles, by covering them with such. But this was not only an expensive, but it must have been a most clumsy mode of ornament. The present modes of gilding are very different. When wood is to be gilded, the surface is first smeared with an adhesive kind of oil, or with a kind of glue called size; and the gold leaf, above mentioned, is then spread upon it by a tuft of cotton or other soft substance.
The gilding of iron or copper is performed by cleaning and polishing its surface, and then heating it till it has a blue colour. When this has been done, a layer of gold leaf is put on, slightly burnished down, and exposed to a gentle fire. It is usual, in common work, to place three such layers, or four at the most, each consisting of a single leaf. The heating is repeated at each layer, and last of all the work is burnished. For gilding in or moulu, as it is denominated by the French, an amalgam consisting of ten parts of mercury and one part of gold is used. This is spread upon the metal, and is afterwards exposed to the action of a fire sufficiently strong to evaporate the mercury and leave the gold behind. The gilding in or moulu is much more solid and permanent than that by the former method.
When gilding is pale and dirty, it may be revived by means of what is called gilding wax, a composition of yellow wax, bole (127), verdigris (230), and alum.
A very beautiful gilding upon metals, and particularly upon silver, is effected by soaking clean linen rags in a solution of gold made by aqua regia (207). The rags are dried and burnt; and the ashes are carefully preserved. These ashes are used by taking a sound cork, moistening it with a little water, dipping it into the ashes, and then rubbing strongly a portion of them on the surface of the silver, which should be perfectly clean and bright. By this simple and economical process, it will be covered 173with an extremely thin coating of gold, the colour and brilliancy of which may be heightened by burnishing. The ornaments upon snuff-boxes, fans, and various kinds of trinkets, are merely thin plates of silver, gilded in this manner.
The edges of tea-cups, and other similar articles, may be gilded, though not in a very durable manner, by applying a thin coat of amber varnish (224), and then placing leaf-gold upon it. When the varnish is dry, the gold is to be burnished.
Gold, in a state of solution, is sometimes used for staining marble, ivory, ornamental feathers, and other articles, a purple-red colour, which cannot be effaced. By chemical processes an oxide (21) is obtained from this metal, which is employed for giving those beautiful shades of lilac, rose colour, red and purple, which we observe in glass and porcelain.
A gold powder for painting may be made by uniting one part of gold with eight parts of mercury (228), and afterwards evaporating the latter by heat.
The article denominated gold wire is generally silver wire gilded, very little wire being made entirely of gold. Its uses are chiefly for embroidery and filagree work. Gold thread consists of flatted silver gilt wire, laid over a thread of yellow silk, by twisting it in a machine with iron bobbins. It is of this, and not of gold, that the article called gold lace is made. The Chinese, instead of flatted wire, use slips of gilt paper, which they interweave in their stuffs, and twist upon silk threads.
228. MERCURY, in its native state, is called quicksilver, and is found in small globules of shining, silvery appearance, scattered through different kinds of stones, clay, and ores. It is nearly fourteen times heavier than water.
The principal ore of mercury, and that from which the metal is chiefly obtained, is cinnabar. This is of red colour, and consists of mercury mineralized with sulphur. It is sometimes found in a massive state, sometimes in grains, and sometimes crystallized; and chiefly among rocks of the coal formation.
174The most productive mines of cinnabar are in the palatinate of Germany, at Idria in Carniola, and at Almaden in Spain. Those of Idria are supposed to be more valuable than any of the others. Their first discovery, which was somewhat more than three hundred years ago, was made in a very extraordinary manner. This part of the country was then much inhabited by coopers; and one of the men, on retiring from work in the evening, placed a new tub under a dropping spring, to try if it would hold water; and, when he came in the morning, he found it so heavy that he could scarcely move it. Examining into the cause of this extraordinary circumstance, the man observed that it was owing to a shining and ponderous fluid which was at the bottom. The affair was noised abroad, and a society of persons was formed to search further, and discover the mine from which this quicksilver had flowed. Such was their success that the reigning Duke of Austria paid them a compensation for the discovery, and took the mine into his own possession. The greatest perpendicular depth of this mine is now more than 830 feet. It is descended by buckets, or by ladders placed obliquely in a zigzag direction. In some parts of the mine the pure metal flows in small streams, so that in six hours a man has been known to collect more than thirty-six pounds weight of it. In other parts it is found in a multitude of little drops, either in ores or in clay. The whole produce of the mine is said to exceed a hundred tons weight of mercury per annum.
It has been asserted that, several years ago, in digging out clay for the foundation of a house opposite to the King’s Arms inn, in the street called Hyde-hill, in Berwick-upon-Tweed, a quantity of native mercury was discovered. The clay, when dug out, lay for some time in the place to which it was conveyed; and the mercury was observed to exude from the small fissures or cracks that were formed as it dried. It is said that, several years afterwards, in making some alteration in the yard of the same house, the workmen penetrated into the 175same bed of clay; and that it then appeared to be impregnated with native mercury, which ran out in small globules.
Mercury is sometimes imported into Europe from Peru, and from the East Indies.
The mode of extracting it from cinnabar is said to be by mixing this ore either with pounded chalk, or with half its weight of iron filings, and distilling it in a stoneware retort. By this process the sulphur combines with the iron, and the mercury, in a state of purity, passes into the receiver.
When pure or native mercury occurs in mixture with other substances, these are stamped or ground into a coarse powder. Water is poured upon them; they are briskly stirred until the water becomes thick and turbid, and then are left to settle. This operation is repeated till the water runs off perfectly clear. The substance at the bottom, which is principally mercury, is then put into large iron retorts and the metal is obtained, free from all extraneous matters, by distillation.
It is the singular property of this metal, which has no other alliance whatever with silver than its appearance, to be capable of division, by the least effort, into an indefinite number of particles, each of which assumes a spherical form; and to be always in a fluid state in the common temperature of our atmosphere. Even during the most intense frost, it still retains its fluidity. By the effect, however, of extreme cold artificially produced, mercury becomes a solid metal, and in this state may be beaten with a hammer and extended without breaking; but care must be taken that it does not touch the fingers, as it would blister them and cause unpleasant sores, in the same manner as any burning substance.
Mercury has been known from the remotest ages; and it was employed by the ancients in gilding, and in the operations of separating gold and silver from their 176ores, in the same manner as at present. Being the heaviest of all fluids of which we have any knowledge, and not congealing in the temperature of our climate, it has been preferred, before all others, for barometers, as a measure of the weight of the atmosphere. And, as heat dilates mercury similarly to other fluids, it is likewise made into thermometers. Mercury is sometimes used in medicine in its pure metallic state.
The combinations of mercury with other metals are termed amalgams. That of mercury and gold is formed so readily, that if gold be dipped into mercury, its surface immediately becomes as white as silver. An amalgam of mercury and gold is employed for the gilding, and of mercury and silver for the silvering of metals.
Mercury and tin combined together form the substance that is used for the silvering of looking-glasses. The process is as follows: A quantity of tin-foil, equal in size to the glass, is evenly placed on a flat stone or table; and mercury, in which some tin has been dissolved, is poured upon it, and spread with a feather, or bunch of cloth, until its union has covered every part. A plate of glass is then cautiously slided upon it, from one end to the other, in such manner that part of the redundant mercury is driven off, or swept away before its edge. The remainder is now united to the tin. The glass is then loaded with weights all over, so as to press out still more of the mercury. By inclining the table, this remaining mercury becomes discharged; and, in a few hours, the rest of the tin-foil and mercury adhere so firmly to the glass, that the weight may be removed without any danger of its falling. About two ounces of mercury are requisite for covering, in this manner, three square feet of glass.
By means of mercury a fulminating powder is made, which, when struck with a hammer on an anvil or flat iron, such as is used by laundresses, explodes with a stunning and disagreeable report, and with such force 177as to indent both the anvil and the hammer. Four or five grains are as much of this powder as ought to be used for such experiments. Its force is much greater than that of gunpowder, but does not extend so far. Hence it is a substance which might be rendered of great use in the blasting of rocks.
Corrosive sublimate is an extremely poisonous preparation from mercury. Among other uses, it is employed by dyers as a mordant to fix their colours. From certain proportions of corrosive sublimate rubbed together, until they are perfectly incorporated, is formed calomel; a salt which, of late years, has been extensively and most usefully employed in medicine.
A valuable red colour or pigment called vermilion, or artificial cinnabar, which was as well known to the ancients as it is to the moderns, is usually formed of three parts of mercury and one of sulphur, melted together, heated to redness, and then sublimated out of contact of the air. The manufacture of vermilion was long kept a secret by the Dutch; and it is stated that, before the late war, nearly 50,000 pounds weight of it were annually made, in three furnaces, by four workmen, near Amsterdam. Native cinnabar is sometimes used for the same purpose; but the artificial kind is preferred on account of the purity and brightness of its colour.
229. SILVER is a white, brilliant, sonorous, and ductile metal, somewhat more than ten times heavier than water.
It is found in different states. Of these the principal is denominated native silver, from its being nearly in a state of purity. Native silver sometimes occurs in small lumps, sometimes in a crystallized form, and sometimes in leaves, threads, or wire. In many instances the latter are so connected with each other as to resemble the branches of trees, in which case the ore is called dendritic. There are also several ores of silver, in which this metal is combined with lead, antimony, arsenic, sulphur, and other substances.
The silver that is produced from the mines of Potosi, in South America, is of the dendritic kind; and is considered 178by the Spaniards as the purest that is known. A range of mountains near Potosi, about twenty miles in circumference, is said to be perforated by more than 300 shafts, or openings of mines, and to produce, in the whole, from 30,000 to 40,000 dollars’ worth of ore per week. The annual produce of the silver mines in America has been estimated at near 2,400,000l. sterling.
Silver is also found in several parts of Europe; and, some years ago, there were mines of this metal, worked to a great extent, at Konigsberg in Norway. These were discovered in 1623, and they were found so profitable, that in 1751 forty-one shafts and twelve veins were wrought there; and 3,500 officers, artificers, and labourers, were employed. The perpendicular depth of the principal shaft was more than 750 feet. Specimens of native silver are not uncommon from some of the copper-mines of Cornwall; and, many years ago, a vein of silver ore was, for a short time, wrought with considerable advantage in the parish of Alva, Stirlingshire, Scotland. It is said that from 40,000l. to 50,000l. worth of silver was obtained from it before the repository was exhausted. We are informed that a mass of capillary native silver was found, in veins traversing the blue-coloured limestone of Isla, one of the Western Islands of Scotland. Great quantities of silver are extracted from lead. There was lately melted in one refining house in London 50,000l. worth of this metal, from lead of the Beralston mines in Devonshire.
Different methods are employed, in different countries, to extract silver from its ore. In Mexico and Peru the mineral is pounded, roasted, washed, and then mixed with mercury in vessels filled with water; a mill being employed for the more perfectly agitating and mingling them. By this process the silver combines with the mercury. The alloy thus obtained, after undergoing some further processes, is submitted to the action of heat, by which the mercury passes off in a state of vapour, leaving the silver behind. The silver is then melted and cast into bars or ingots. In 179other countries, after the earthy matters are cleared from the silver ore by pounding and washing, the remainder is melted with lead: which, by a subsequent process, is separated, and leaves the silver alone and pure.
This metal ranks next in value to gold. Like gold, it is coined into money, and is manufactured into various kinds of utensils, such as goblets, vases, spoons, and dishes, which have the general appellation of silver plate. For all these purposes it is alloyed with copper, which does not affect its whiteness, and is not easily detected, unless it be in too great proportion: the intention of this is to render it harder than it would otherwise be, and thereby the better to adapt it to receive fine and sharp impressions on being cast. Our standard silver is composed of somewhat more than 12¼ parts of pure metal and one part of copper; and the metal of this standard is used, both for silver plate, and in the coinage. The mark or stamp which is given to it at Goldsmiths’ Hall is similar to that which has been explained for sterling gold.
After platina (226) and gold (227), silver is considered the most unchangeable of all metals. The air does not easily act upon its surface in such manner as to injure it; but, when long exposed to the atmosphere, especially in frequented or smoky places, it acquires a covering or rust of dark brown colour, which, on examination, is found to be what chemists denominate sulphuret of silver. The fumes of sulphur and other inflammable substances blacken silver. Various powders have been contrived with a view to restore to plate its original lustre; but these should be used with caution, as some of them are very injurious.
Silver is nearly as ductile as gold. It may be beaten into leaves so thin that a single grain in weight will cover a space of more than fifty-one inches; and it may be drawn into wire much finer than a human hair, indeed so fine that a single grain of silver has, in this 180form, been extended nearly to the length of 400 feet. It is this wire gilded that has the name of gold wire; and what is denominated gold lace (227) is but flatted silver thread gilt, twisted round silk, and woven.
The plating of copper with silver is a very useful operation, and is thus performed. Plates of silver are bound with iron wire, upon small ingots of copper. The quantity generally allowed is one ounce of silver to twelve ounces of copper. The surface of the plate of silver is made not quite so large as that of the copper; and upon the edges of the copper, which are not covered by the silver, a little borax (204) is put. By exposing the whole to a strong heat, the borax melts; and, in melting, contributes to fuse that part of the silver to which it is contiguous, and to attach it, in that state, to the copper. The ingot, with its silver plate, is then rolled between steel rollers moved by machinery, till it is of proper thickness. It is afterwards cut into such sizes and to such shapes as may be required for use. An ounce of silver is thus often rolled out into a surface of three square feet, having its thickness, upon the copper, not more than the three-thousandth part of an inch. Hence we ought not to be surprised at the silver being soon worn from the sharp edges of plated goods. To prevent this, it is customary, with the best articles, to have all the edges, and the parts liable to be worn, formed, to a considerable thickness, of silver.
What is called French plate is made by heating copper, or more frequently, brass, to a certain degree, then applying leaf-silver to the surface, and strongly rubbing it with a burnisher. The durability of this plating depends of course on the number of leaves which are applied on a given surface. For ornaments that are not much used ten leaves may be sufficient; but a hundred will not last long, if the metal be exposed to frequent handling or washing.
Besides the above, there are various modes of silvering metal articles, or, as it is called, washing them with 181silver. All these are performed by different chemical preparations of this metal.
The article denominated shell-silver, used by painters, is prepared, by carefully grinding silver-leaf, with a little honey or gum water upon a slab, or in a mortar, and separating the honey or gum by means of water. When this is washed away, the silver may be put on paper, or kept in shells, for use. When it is to be used, it must again be diluted with gum water.
The application of silver-leaf for the silvering of paper or wood is similar to that of gold-leaf (227).
Silver, dissolved in aqua fortis (nitric acid, 30), yields crystals, which, afterwards melted in crucibles, form that grey mass usually called lunar caustic, and by chemists nitrat of silver. This preparation is of considerable use in surgical cases, being employed to keep down fungous or proud flesh, in wounds and ulcers, and also for the consuming of warts, small wens, and other excrescences upon the skin. It is likewise, though a most violent medicine, sometimes given internally, but in very small doses, to persons subject to epileptic fits. The liquid in which the silver is dissolved becomes excessively caustic. It gives to the skin, the hair, and almost all animal substances, an indelible black colour. Hence it is often used as a specific for dyeing the human hair. No person, however, would employ it for this purpose, who was acquainted with its injurious qualities, not only to the hair itself, but also to the skin, if permitted to come in contact with it.
The article called indelible, or permanent marking ink, for marking linen, and other wearing apparel, is formed by dissolving, in a glass mortar, two drachms of nitrat of silver, in six drachms of pure water, and then adding to them two drachms, by measure, of thick gum water. This is the ink for writing on the linen.—In another vessel dissolve half an ounce of salt of tartar, or of the subcarbonat of soda, of commerce, in four ounces of water; and add to the solution half an ounce, by measure, of thick gum water. This forms the preparatory 182liquor. With this the linen is to be thoroughly wetted at the part intended to be marked. The linen is to be dried, and then to be written upon by a clean pen dipped in the marking ink. The letters will at first be pale, but by exposure to light and heat, they will soon become black; and be so permanently fixed, that no washing nor bleaching can efface them.
The attention of the curious has of late been turned to a very extraordinary compound called fulminating silver, which explodes without heat, and with even the slightest degree of friction. Of this compound little fulminating balls have been made. These are globules of thin glass, each somewhat larger than a pea, and containing a grain or two of fulminating silver. After the silver is put in, it is secured by a piece of soft paper, pasted over the ball, so as completely to cover it. These balls explode by merely crushing them under the heel of the shoe. What are called fulminating bombs are similar balls, but of the size of hazel nuts. No one should attempt to explode these by crushing them with the shoe, as their explosive effect is so violent as sometimes to prove injurious.
Fulminating silver requires the utmost care. It should never be put into phials, nor should it be in any way handled so as to produce much friction. It is the most dangerous preparation that is known. The mere touch of a hard substance will sometimes explode it; and its very preparation is so hazardous that this ought never to be attempted without a mask upon the face with strong glass eyes.
The following are three pleasing experiments with preparations of silver:
1. Mix or amalgamate together four parts of silver leaf with two parts of mercury (228) and dissolve this in diluted aqua fortis. To the solution add as much water as will be equal to thirty times the weight of the metals employed. Pour a portion of the above mixture into a phial, and place at the bottom a small piece of silver. After it has stood awhile, little filaments of silver 183will be seen to shoot up from it somewhat in the form of a shrub. This apparent vegetation is popularly called the tree of Diana.
2. A production nearly similar may be obtained by adding a little quicksilver to a solution of nitrat of silver in water.
3. Drop upon a clean plate of copper a small quantity of solution of lunar caustic, or nitrat of silver. In a short time a metallic vegetation will be perceptible, branching out in pleasing forms, and in various directions.
230. COPPER is a red or orange-coloured metal, about nine times heavier than water. It is the most sonorous of all metals, and, except iron, the most elastic.
It is found under a great variety of forms, sometimes in masses of pure metal, but, more frequently, in combination with other substances, particularly sulphur.
There are valuable copper mines in every quarter of the world; and the use of copper is probably of greater antiquity than that of any other metal. It is mentioned in the Old Testament; and, at a very early period, domestic utensils and instruments of war were made of bronze, or a compound of copper and tin. Even during the Trojan war, as we learn from Homer, the combatants had no other armour than what was made of bronze. The Greek and Roman sculptors are said to have executed fine works of art in porphyry, granite, and other hard minerals, by means of copper instruments; whence historians have been induced to believe that the ancients possessed the secret of rendering this metal as hard as steel: some of them even imagined that they had the means of converting it into steel.
Copper is very abundant in several parts of Great Britain, particularly in the island of Anglesea. The copper mines of Anglesea are situated on the top of a mountain, and form an enormous cavity more than five hundred yards long, a hundred yards broad, and a hundred yards deep. The ore is got from the mine by pickaxes, and blasting with gunpowder. It is then 184broken with hammers into small pieces, an operation which is chiefly performed by women and children. After this, it is piled into kilns of great length, and each about six feet high; from the upper parts of which flues are attached that communicate with what are called sulphur chambers. The kilns are closely covered; and fires are lighted in different parts, that the ore may undergo the process of roasting. The whole mass gradually kindles, and the sulphur, which is combined with the ore, is expelled in fumes, by the heat, and is conveyed, through the flues, to the sulphur chamber. This process occupies from three to ten months, according to the size of the kilns; and, during that period, the sulphur chamber is cleared four or five times. When the operation is complete, or the ore is freed from the sulphur, it is taken to places denominated slacking pits. It is subsequently conveyed to the smelting houses, where, by intense heat, the pure metal is drawn off in a fluid state.
As the water, which passes through several parts of the Paris mine, is strongly impregnated with sulphat of copper (209), or copper held in solution by sulphuric acid (24), the proprietors turn the course of this water through certain large and shallow pits, which they have formed for the purpose, and in each of which they place a quantity of iron. A decomposition here takes place: the iron is corroded, and, at length, entirely dissolved, and the copper, in the form of a brown mud, falls to the bottom. One ton weight of iron, thus immersed, will produce nearly two tons of copper mud, each of which, when melted, will yield sixteen hundred weight of metal. This mode of obtaining copper is said to have been an accidental discovery from one of the workmen, several years ago, having left a shovel in the water, which, when afterwards taken out, appeared changed into copper.
The magnitude of the above mentioned copper works may readily be conceived, when it is stated that the beds of ore are, in some places, more than sixty feet in depth: 185that the proprietors employ more than 1000 workmen; and that they ship, from the adjacent port of Amlwch, upwards of 20,000 tons of copper, annually.
There is at Ecton, in Staffordshire, a copper mine which is now worked at the depth of 1416 feet below the surface of the ground. This is the deepest mine in England.
The uses of copper are numerous and important. When rolled into sheets, betwixt large iron cylinders, it is employed for the covering of houses, sheathing the bottoms of ships, and other purposes. As a covering for houses, copper is lighter than slate, but whether it be more durable has not been yet ascertained. The coppering of ships tends to facilitate their progress through the water, by presenting a smoother surface than that of wood, and not permitting shell animals to fasten to it as they do to wood. It likewise preserves the bottoms of the ships from being punctured by marine worms; and consequently secures to them a longer duration than they would otherwise have. Plates, or flat pieces of copper, are used by artists for engraving pictures upon, either by cutting them with a sharp steel instrument, or corroding them with aqua fortis (206), in lines drawn by a needle through a thin coat of wax spread upon their surface.
Copper is manufactured into various kinds of cooking utensils. Great care, however, ought to be taken that acid liquors, or even water intended for drinking, or to be mixed with food, be not suffered to stand long in such vessels, otherwise they will dissolve so much of the metal as to give them disagreeable and even poisonous qualities. Yet, it is remarkable that, while acid liquors are kept boiling, they do not seem to dissolve any of the metal. Hence it is that confectioners, by skilful management, prepare the most acid syrups in copper vessels, without their receiving any unpleasant taste or injurious quality from the metal. All vessels formed of this metal which are employed in cookery, 186ought to have their inner surface covered with a coat of tin (238).
As copper does not, like iron, strike fire by collision, it has on this, as well as on some other accounts, been substituted for iron in the machinery which is employed in gunpowder mills. It is also made into water pipes, and sometimes into sash frames. Under the hammer it is capable of being beaten into thin leaves like gold. Copper wire is much employed by bell-hangers and other artisans. The filings of this metal are used for giving a green colour to some kinds of artificial fire-works.
Several preparations of copper are employed in medicine, some of them internally, and others externally; but most of the former are violently emetic.
Verdigris is a rust or oxide (21) of copper, usually prepared from that metal by corroding it with vinegar. There is a large manufactory of verdigris at Montpelier in France. The workmen place alternate strata of copper plates and husks of grapes, the latter of which speedily become acid and corrode the metal. The verdigris, thus formed, is scraped off as it collects on the surface; it is afterwards dried, and put in bags or casks for sale. A manufactory of verdigris has lately been established at Deptford, near London.
A solution of this substance in distilled vinegar affords permanent crystals, which are improperly called distilled verdigris, and are made into a green paint. Verdigris is principally consumed by dyers in combination with logwood, for striking a black colour. It is a virulent poison.
Oxide of copper is employed for giving a beautiful green colour to porcelain. It also imparts the same colour to glass, and hence is frequently employed for the formation of artificial emeralds.
Of all metals that are known, copper is the most susceptible 187of alloy. The most frequent and useful of these alloys are made with copper and zinc, in different proportions.
Brass is an alloy composed of three parts of copper, and about a fourth part of zinc (241). It is a beautiful, useful, and well-known yellow metal. Not being so apt to tarnish and rust as copper, and being, in other respects, better adapted for the purpose than that metal, it is much used for clock-work, and for mathematical and astronomical instruments. It is more ductile than either copper or iron, and hence is peculiarly fitted to be made into wire, for the strings of musical instruments, and other purposes. Sieves are woven with brass wire, after the manner of cambric weaving, and of such extreme fineness that similar ones could not possibly be made with copper wire. Brass wire, flatted and gilded, is sometimes made into lace. The finest brass is manufactured at Geneva. It unites great beauty of colour to a high degree of ductility; and is used chiefly for escapement wheels, and other nicer parts of watch-making. For work in which there is no friction it is necessary to cover brass with a kind of varnish or laquer, to improve its colour, and prevent it from being tarnished by exposure to the atmosphere.
Prince’s Metal, or Pinchbeck, is an alloy containing three parts of zinc (241), and four of copper. This metal has nearly the same colour as gold, and was formerly much in use for the manufacture of ornamental articles of different kinds.
Dutch Gold is formed by the cementation of copper-plates with calamine (241), hammered out into leaves. This article is chiefly manufactured in Holland and Germany, and has about five times the thickness of gold leaf.
Bronze, and the metal of which cannons are made, consist of from six to twelve parts of tin (238) combined with 100 parts of copper. This alloy is brittle, heavier than copper, and of a yellow colour. Before the method 188of working iron was brought to perfection, it was used by the ancients for the manufacture of sharp-pointed instruments; and it is supposed to have been the æs or brass of the Romans.
Bell Metal, or the metal of which bells are formed, is usually composed of three parts of copper and one of tin. Its colour is greyish white; and it is very hard, sonorous, and elastic.
Bronze and bell metal are not, however, always made of copper and tin only. They frequently have other admixtures, consisting of lead, zinc, or arsenic. Bell-makers sometimes abuse the vulgar credulity by pretending that they add a certain quantity of silver to the alloy, for the purpose of rendering the bells more melodious: but they are better acquainted with their business than to employ so valuable a metal in the operation.
White Copper is an alloy composed of equal parts of copper and arsenic (242). The metal produced by this mixture is of a whitish colour, but with a coppery tinge. It is freed from the latter by being melted several times; and, by this process, is at last rendered as white as silver. White copper is very brittle; but, if the arsenic be evaporated by heat, it resumes its ductility, and still preserves its white colour. When the operation is well performed, it is easy, at the first glance, to mistake white copper for silver; but the difference may immediately be ascertained from the properties inherent to the two metals.
White copper is employed in the manufacture of many kinds of trinkets: and of a great number of domestic utensils; such as tea-pots, coffee-pots, and candle-sticks.
231. MALACHITE is a solid green copper ore, the surface of which has frequently a bubbled appearance, and the interior is marked with numerous irregular zones, and layers of different shades of green. It is somewhat more than three times as heavy as water, and is so soft as to be easily scratched by a knife.
189In its appearance, malachite somewhat resembles green jasper; but it is by no means so hard. It is, however, capable of being cut and polished as a gem, and is manufactured into various kinds of trinkets, which of late years have been much in request for necklaces, brooches, and bracelets. It is also cut into slabs, and mounted into snuff-boxes. Such is the size of which it is sometimes found, that M. Patrin saw, at Petersburgh, a plate of malachite thirty-two inches long and seventeen inches broad, which was valued at 20,000 livres; but the finest specimens in Europe are some slabs that are adapted as the tops of tables, sideboards, &c. at Trianon, in the Park of Versailles: the largest of these are nearly four feet in length and two feet wide. They may indeed have been formed by various pieces joined together; but, if so, the joints are so completely concealed as not to be discoverable even by the closest examination. Malachite is sometimes employed for the engraving of cameos, but is seldom cut in intaglio. Smaller pieces of this substance, that are used for trinkets, are about the same value as carnelian. Independently of its use, in the above respects, and also as an ore of copper, malachite, when pure, is ground into powder, and employed as a green pigment.
The Vosges Mountains in Lorraine, and certain copper mines of Saxony, are celebrated for producing very fine specimens of malachite. This beautiful mineral is also found in our own country, in the copper mines of Cornwall and Wales.
232. TURQUOISE. The beautiful light blue substances that are called turquoises have usually been considered as the bones or teeth of animals, impregnated with blue oxide (21) of copper; but they are sometimes found in nodules which are certainly not of an osseous nature.
Turquoises are frequently set in rings, necklaces, brooches, and other female ornaments. In Persia they are very common; and, amongst the Turks, are held in such estimation that persons of rank almost constantly wear them in some part of their dress, as ring-stones, 190and to adorn the handles of stilettoes. They are imported into England from Russia, stuck with pitch upon the ends of straws; because if mixed together in parcels, the purchaser would not easily be able, in turning them over, to observe their colour, and ascertain their value.
In the turquoise there is nothing that can recommend it to notice except the agreeable softness of its colour, which is particularly distinguishable by candle-light; this alone has rendered it so fashionable as an ornament in female dress, for rings, ear-drops, and brooches, that the demand for it is at present greater than the supply. Imitations of turquoise are easily made in paste, and not unfrequently imposed upon the ignorant purchaser; but in these, though the colour is correctly given, there is a glassy lustre much higher than that of the real stone.
Of late years a spurious kind of turquoise has also found its way into Europe, which is much softer than the genuine kind; has more of a green than a blue cast, and is by no means capable of so good a polish.
233. IRON is a well-known metal, of livid greyish colour, hard and elastic, and capable of receiving a high polish. Its weight is nearly eight times as great as that of water.
It is seldom found in a truly native state, but occurs, abundantly, in almost every country of the world, in a state of oxide (21), and mineralized with sulphuric (24), carbonic (26), and other acids.
Iron is found in plants, in several kinds of coloured stones, and even in the blood of animals.
Of all the metals there are none which, in the whole, are so useful, or are so copiously and variously dispersed as iron. Its uses were ascertained at a very early period of the world. Moses speaks of furnaces for iron, and of the ores from which it was extracted, and tells us that swords, knives, axes, and instruments for cutting stones, were, in his time, all made of this metal.
The most considerable iron mines at present existing 191are those in Great Britain and France. After iron ore is dug out of the earth, it is crushed or broken into small pieces, by machinery. It is next washed, to detach the grosser particles of earth which adhere to it. This operation ended, it is roasted in kilns, formed for the purpose, by which the sulphur, and some other substances that are capable of being separated by heat, are detached. It is then thrown into a furnace, mixed with a certain portion of limestone and charcoal, to be melted. Near the bottom of the furnace there is a tap-hole, through which the liquid metal is discharged into furrows made in a bed of sand. The larger masses, or those which flow into the main furrow, are called sows; the smaller ones are denominated pigs of iron; and the general name of the metal in this state is cast iron.
With us iron is employed in three states, of cast iron, wrought iron, and steel.
Cast iron is distinguishable, by its properties of being, in general, so hard as to resist both the hammer and the file; being extremely brittle, and for the most part, of a dark grey or blackish colour.
A great number of useful and important articles are formed of cast iron, such as grates, chimney backs, pots, boilers, pipes, and cannon shot. These are made by casting ladles full of the liquid metal into moulds that are shaped, for the purpose, in sifted sand.
Wrought iron. The process of converting cast iron into wrought or malleable iron, is called blooming. The cast iron is thrown into the furnace, and kept melted by the flame of combustibles which is made to play upon its surface. Here it is suffered to continue for about two hours, a workman constantly stirring it, until, notwithstanding the continuance of the heat, it gradually acquires consistency, and congeals. It is then taken out, while hot, and violently beaten with a large hammer worked by machinery. In this state it is formed into bars for sale.
The value of iron is beyond all estimate, and infinitely 192greater than even that of gold. By means of this metal the earth has been cultivated and subdued. Without it houses, cities, and ships, could not have been built; and few arts could have been practised. It forms also the machinery by which the most useful and important mechanical powers are generated and applied.
Steel is usually made by a process called cementation. This consists in keeping bars of iron in contact with powdered charcoal, during a state of ignition, for several hours, in earthen troughs, or crucibles, the mouths of which are stopped up with clay. Steel, if heated to redness, and suffered to cool slowly, becomes soft; but if plunged, whilst hot, into cold water, it acquires extreme hardness. It may be rendered so hard as even to scratch glass; and at the same time, it becomes more brittle and elastic than it was before. Although thus hardened, it may have its softness and ductility restored, by being again heated, and suffered to cool slowly. A piece of polished steel, in heating, assumes first a straw-yellow colour, then a lighter yellow, next becomes purple, then violet, then red, next deep blue, and at last of all bright blue. At this period it becomes red hot, the colours disappear, and metallic scales are formed upon, and encrust its surface. All these different shades of colour indicate the different tempers that the steel acquires by the increase of heat, from that which renders it proper for files, to that which fits it for the manufacture of watch springs. Mr. Stoddart has availed himself of this property to give to surgical, and other cutting instruments, those degrees of temper which their various uses require.
The kind of steel which has been most celebrated in this country is that imported from Syria under the name of Damascus steel. Germany is also noted for its steel. The best steel manufactured in Britain is known by the name of cast steel; and the making of it, although it was long kept a profound secret, is now discovered to be a simple process. It consists merely in fusing it with carbonat of lime (140), or in what is called cementation, 193with charcoal powder, in a peculiar kind of furnace. The iron produced in Sweden is considered superior to that of any other country in Europe for the manufacture of steel.
All kinds of edge tools, where excellence is required, are made of steel; and a steel instrument may be immediately known from an iron one, by letting fall upon it a drop of nitric acid or aqua fortis (206), somewhat diluted with water. If it be steel, this will occasion a black spot; but if it be iron, it will not have this effect. Steel is attracted by the magnet, and is capable of receiving a permanent magnetic property, which has led to the discovery of the mariner’s compass. Had iron been productive of no other advantages to mankind than this, it would on this account alone have been entitled to their greatest attention.
Iron, when exposed to the moisture of the atmosphere, becomes gradually covered with a brown, or yellowish substance, known by the name of rust, which, if suffered to continue without interruption, will corrode the entire substance of the iron. The rust or oxide of iron (21) is a substance in considerable request by calico printers for a dye. Iron-moulds are spots on linen occasioned by its exposure to iron in damp situations; these are removeable only by the application of an acid.
There are various modes of preserving iron and steel from rust. The following is recommended by an eminent French chemist as one of the best. Mix copal varnish, made greasy with oil, with about four-fifths of the best spirit of turpentine. Apply this by means of a sponge, over the whole surface, and allow it to dry. This varnish may be successfully used for all the metals; and particularly for the preservation of such philosophical instruments as, by being brought into contact with water, are liable to lose their splendour, and become tarnished.
234. METEORIC STONES are a species of iron ore, which have at different times been known to fall from the atmosphere.
194They have been seen only in shapeless masses, of from a few ounces to several hundred pounds in weight. Their texture is granular. They are covered externally with a thin blackish crust, and are, internally, of an ashy grey colour, mixed with shining minute particles.
There is sufficient evidence to show that solid masses of stone have been observed to fall from the air at a period considerably anterior to the Christian era. Notwithstanding this, so very extraordinary was the phenomenon, that, until the year 1802, it was generally regarded by philosophers as a vulgar error. Mr. Howard, in that year, submitted to the Royal Society a paper which contained an accurate examination of the testimonies connected with events of this kind; and described a minute analysis of several of the substances which had been said to have fallen in different parts of the globe. The result of his examination was that all these stony bodies differ completely from every other known stone; that they all resemble each other, and are all composed of the same ingredients.
The greatest number of the stones which have fallen from the air have been preceded by the appearance of luminous bodies or meteors. These meteors have burst with an explosion, and then the shower of stones has fallen to the earth. Sometimes the stones have continued luminous until they sunk into the earth, but most commonly their luminousness disappeared at the time of the explosion. Their motion through the air is surprisingly rapid, in a direction nearly horizontal; but they seem to approach the earth before they explode. In their flight they have frequently been heard to yield a loud whizzing sound. They are hot when they first reach the earth; and exhibit, on their surface, visible marks of fusion.
A general tradition has prevailed in almost all ages, and amongst all people, of the fall of solid bodies from the atmosphere, under various denominations, but, with us, more particularly, under that of thunderbolts. In barbarous and uncivilized countries, these have usually 195been ascribed to the miraculous judgment of the deity; and they may be considered as the true origin of the worship of stones. The image of Diana, mentioned in the Acts of the Apostles, as believed by the Ephesians to have fallen down from Jupiter, and the Palladium or sacred statue of Minerva, which also is said to have fallen from Heaven, and to have been preserved in Troy, as a treasure, on the safety of which that of the city depended, had each, no doubt, this origin. The Psalmist evidently alludes to the falling of meteoric stones, when, speaking of the Almighty, he says, “He made darkness his secret place; his pavilion round about him with dark water, and thick clouds to cover him. At the brightness of his presence his clouds removed; hailstones and coals of fire. The Lord also thundered out of Heaven, and the Highest gave his thunder; hailstones and coals of fire.”
Among numerous other instances of these stones, it is recorded that, on the seventh of November, 1492, betwixt eleven and twelve o’clock at noon, a dreadful clap of thunder was heard at Ensisheim, a considerable town in Alsace, and that a huge stone was seen to fall on a field lately sown with wheat. On several of the neighbours going to the place, the hole it had formed was found to be about three feet in depth, and the stone when dug out, weighed two hundred and sixty pounds. It was preserved in the cathedral of Ensisheim until the beginning of the French Revolution, when it was conveyed to the public library at Colmar. There are in the British Museum two small pieces of this stone, and fragments of several other meteoric stones which have fallen in different parts of the world.
Two stones fell near Verona in Italy, in the year 1672, one of which weighed three hundred, and the other two hundred pounds.
Mr. Sowerby, the publisher of English Botany, and of several other highly estimable works, possessed a meteoric stone which fell near Wold Newton in Yorkshire, in the afternoon of the thirteenth of December, 1795, 196and weighed fifty-six pounds. Whilst this stone was in motion through the air, several persons perceived a body passing along the clouds, although they were unable to ascertain what it was. It passed over several different villages, and was also accurately and distinctly heard. The day was foggy; and, though there was some thunder and lightning at a distance, it was not until the stone fell that an explosion took place which alarmed all the adjacent country; and created, distinctly, a sensation that something very extraordinary had happened. A shepherd belonging to Captain Topham was within a hundred and fifty yards of the place where it fell; George Sawden, a carpenter, within sixty yards; and John Shepley, one of Captain Topham’s farming servants, was so near that he was forcibly struck by some of the mud and earth that were raised by the stone dashing into the ground. In its fall the stone excavated a place nineteen inches in depth (seven inches of which were in a solid rock of chalk), and somewhat more than three feet in diameter, fixing itself so firmly that some labour was required to dig it out.
Another stone of considerable size fell in Scotland on the fifth of April, 1704. A misty commotion was observed in the atmosphere, and, nearly at the time of the stone falling, a report was heard as loud as if three or four cannon had been fired at a little distance. The report was succeeded by a violent rushing or whizzing noise; and, almost immediately afterwards, the stone fell into a drain, in the presence of two men and two boys, splashing the water to a distance of twenty feet around. The stone, when dug out, was found to have sunk about eighteen inches into the earth.
On the fifth of November, 1814, about half past four o’clock in the afternoon, a dreadful peal of thunder was heard in the Doab in Persia, and was immediately succeeded by a shower of large stones, many of them from twenty-six to thirty pounds weight each. Several inhabitants of the adjacent country were present at the time; and not fewer than nineteen of the stones were collected.
197Professor Pallas, many years ago, discovered lying on the surface of a hill in Siberia, a mass of native iron, which weighed 1680 pounds. It was considered by the natives as a holy relic, and was believed by them to have fallen from heaven. M. de Bougainville, the French circumnavigator, discovered, on the banks of the river La Plata, in South America, an enormous mass of native iron, which he calculated to have weighed about 100,000 pounds. And a mass of native iron, appearing in every respect to have been of meteoric origin, was, some years ago, discovered in the district of St. Jago del Estro, in South America. It was in the middle of a great plain, and had no rock nor mountain near it, and was calculated to have weighed about thirty tons.
The origin of meteoric stones is involved in great obscurity. Some writers have imagined that they might be projected from distant volcanoes; others, that they may have been detached from rocks, and had their substance considerably changed by a concurrence of natural causes; others, that they may have been generated in the air by a combination of mineral substances; and others, that they may have been projected from the moon. The latter was the opinion of La Place the astronomer, who says that a mass, if thrown by a volcano from the moon, with a velocity of about a mile and half per second, it will thence be projected beyond the sphere of the moon’s attraction, and into the confines of that of the earth; the consequence of which will be, that the mass must presently fall to the earth, and become a part of it.
235. LOADSTONE, or MAGNETIC IRONSTONE, is a compact blackish kind of iron ore, which is possessed of the power of attracting iron, as well as every substance which contains ferruginous particles. It is betwixt four and five times as heavy as water.
This mineral is found in masses of different form and size in most of the iron mines of Europe and America, and, when submitted to the furnace, it yields a considerable 198proportion of metal. It makes excellent bar iron, but very indifferent cast iron. In Sweden, and particularly at Roslager, magnetic iron stone is found quite pure, and the iron that is wrought from it is imported in considerable quantities into Great Britain, for the purpose of being manufactured into steel.
The appellation of load, or leading stone, has been given to this kind of iron from its magnetic virtues; for it is not only endowed with the property of attracting iron, but also of pointing itself, and even enabling a needle touched with it to point, towards the poles of the world. We are, however, entirely ignorant what is the cause of this very extraordinary property.
Artificial magnets, constructed of steel, not only possess all the essential virtues of the genuine loadstone, but even in a much higher degree. The natural magnet is consequently now little esteemed except as an object of curiosity.
236. PYRITES, or MARCASITE, is a mineral substance, formed by a combination of iron with sulphur.
It is usually of a bronze, yellow, or brownish colour, very various in form, being massive, globular, club-shaped, oval, or crystallized; and so hard as to strike fire with flint.
Few minerals are more common than this, as it occurs, in some state or other, in almost every rock and vein. It is often found among coals; and, when heated, decrepitates with a loud unpleasant noise and sulphureous smell. To the decomposition of this mineral it is that the hot temperature of almost all the mineral waters may be ascribed.
The name of pyrites, which in the Greek language signifies firestone, has been obtained by this mineral from its property of striking sparks from steel. It was formerly used for fire-arms, as we now use flints. In commerce it is known by the name of marcasite. Some years ago it was much used, particularly in France, for the making of buttons and buckles; and was cut and polished, by lapidaries, for trinkets, particularly for the rims and hands of watches, and various kinds of female 199ornaments. If skilfully cut in the form of small rose diamonds, although an opaque substance, it has somewhat the appearance of a diamond. In the tombs of the Peruvian princes, with whom a considerable portion of their valuables was always interred, there have been found polished plates of marcasite, which appear to have served them as mirrors.
This mineral is never worked as an ore of iron; and it is principally valued on account of the sulphur which can be obtained from it by means of heat; and the green vitriol, or copperas (208), which it affords by exposure to the air.
Ignorant persons frequently mistake iron pyrites for gold; but it is easily distinguished from that precious metal by its brittleness. It breaks when hammered, whereas gold is malleable, or may be extended by hammering: it also strikes fire with steel, which gold will not.
237. RED OCHRE, REDDLE, or RED CHALK, is an iron ore of blood-red colour, which is sometimes found in powder, and sometimes in a hardened state. It has an earthy texture, and stains the fingers when handled.
The principal use of red chalk is for drawing: the coarser kinds are employed by carpenters and other mechanics, and the finer kinds by painters. For the latter purpose it should be free from grit, and not too hard. In order to free it from imperfections, and render it better for use, it is sometimes pounded, washed, mixed with gum, and cast into moulds of convenient shape and size.
Under the name of reddle, this substance is much used for the marking of sheep; and (when mixed with oil) for the painting of pales, gates, and the wood-work of out-buildings.
Another kind of iron ore, or rather a compound of the ores of iron and manganese, is called umber. This mineral, which is of a brown colour, is found in beds in the island of Cyprus, and is used as a kind of paint both in a raw state and burnt.
200238. TIN is a white metal, somewhat like silver in appearance, but is considerably lighter, and makes a squeaking or crackling noise when bent. It is very soft and ductile, and has but little elasticity.
This metal is always found either in a state of oxide (21), or in combination with sulphur and copper; and is about seven times as heavy as water.
The principal tin mines which are known to us are those of Cornwall, Devonshire, Germany; the island of Banca, and peninsula of Malacca, in India; and Chili and Mexico in America. Of these the most celebrated are the mines of Cornwall, which are known to have been worked before the commencement of the Christian era. Diodorus Siculus, who wrote forty years before the birth of Christ, gives an account of these mines, and says that their produce was conveyed to Gaul, and thence to different parts of Italy. This species of metal was used in the time of Moses, and is mentioned in the writings of Homer.
Tin is found in veins, or beds, but chiefly in veins, running through granite and other rocks. In some of the valleys and low grounds of Cornwall, the tin ore is found in rounded grains and masses. In these situations, small grains of gold are sometimes found with it. To separate the tin from earthy and other matters with which it is intermixed, streams of water are passed over them; and these deposits have the name of stream-works.
When the tin ore has been dug from the earth, or has been collected at these stream-works, it is thrown into heaps, and broken to pieces. After this it is washed, and subsequently roasted in an intense heat, for the purpose of dissipating some of the substances with which it is combined. It is lastly melted in a furnace, and thereby reduced to a metallic state. The metal is then poured into quadrangular moulds of stone, each containing about 320 pounds weight. These have the denomination of block-tin, and are stamped by officers of the Duke of Cornwall, with the impression of a 201lion, the arms of that duchy. This is rendered a necessary operation before the tin can be offered for sale; and on stamping, it pays a duty of four shillings per hundred weight to the Prince of Wales, as Duke of Cornwall, who thence derives a very considerable income.
The article usually called tin, or tin-plate, and, in Scotland, white iron, of which saucepans, boilers, drinking vessels, and other utensils of domestic economy are made, consists only of thin iron plate coated with tin. It is thus formed. The iron plates are immersed in water rendered slightly acid by spirit of salt (muriatic acid, 202) or spirit of vitriol (sulphuric acid, 211): after which, to clean them completely, they are scoured quite bright. These plates are then each dipped into a vessel filled with melted tin, the surface of which is covered with suet, pitch, or resin, to prevent the formation of dross upon it. The tin not only covers the surface of the iron, but completely penetrates it, giving to its whole substance a white colour.
In a manner similar to this, stirrups, buckles, bridle-bits, and other articles, are tinned.
Iron is usually tinned before, but copper always after it has been formed into utensils. The object to be attained by the tinning of copper is to prevent the vessels made of that metal from being corroded, and to preserve the food prepared in them from being mixed with any particles of that poisonous substance called verdigris, which is formed by such corrosion. In the tinning of copper vessels, their interior surface is first scraped very clean with an iron instrument, and then rubbed over with sal-ammoniac (207), for the purpose of more completely cleansing them, and also of preventing the formation of verdigris from the copper during the operation. The vessel is then heated, and a little pitch is thrown into it. While quite hot, a piece of tin is applied to the copper, and this, instantly uniting with it, soon clothes the whole surface with tin.
This metal, when amalgamated with mercury, is used for the silvering of looking-glasses (228). When tin is 202melted in an open vessel, its surface is soon found to be covered with a grey powder, which is an oxide (21) of the metal, and is generally called dross. If the heat be continued, the colour of this powder becomes yellow. In this state it is known by the name of tin-putty, and is employed in polishing glass, steel, and other hard substances. When the heat is very violent, the metal takes fire, and is converted into a fine white oxide, which is used to render glass opaque, for the forming of enamel. Oxide of tin is also an important article to dyers. It is employed by them, in large quantities, to give brightness to such colours as are used in forming scarlets and other reds: and to precipitate the colouring matter of other dyes.
Tin is an essential ingredient in bell-metal, bronze, pewter, and various other compounds. It may be combined with lead, in any proportion, by fusion; and this alloy is harder, and possesses much more tenacity than tin. The hardest alloy is a composition of three parts of tin and one of lead. The presence of the tin destroys, in a great measure, the noxious qualities of the lead. It is sometimes customary to tin copper vessels with this mixture, and it has been ascertained that such vessels are in no respects injurious.
There are three kinds of pewter in common use. These are called plate, trifle, and ley pewter. The first, which is made into plates and dishes, is formed of tin, with a small proportion of lead and antimony (245). The second, or trifle pewter, which is made in somewhat different proportions, is used for the quart and pint pots of the publicans: and the ley pewter, which is formed of three parts of tin and one of lead, is manufactured into wine and spirit measures.
Tin may be beaten into leaves or plates that are much thinner than paper. But, when it is thus worked, several leaves must be joined together. They then support each other, and yield to the hammer without tearing. These leaves are used for the silvering of glass globes, and the plating of other metals. Those that are 203used for the silvering of looking-glasses are much thicker. The article called tin-foil is an alloy, consisting generally of two parts of tin and one of lead; and capable of being beaten to less than the thousandth part of an inch in thickness.
239. LEAD is a heavy metal, of pale and livid grey colour when broken, not sonorous when pure, very flexible, and so soft that it may be marked with the nail. It stains paper or the fingers of a bluish colour, and is about eleven times heavier than water.
The most common state in which lead is found is in combination with sulphur and a small portion of silver. This ore is known by the name of galena, and is frequently in the form of blackish cubical crystals. Lead is also found in union with arsenic (242) and many acids.
Great Britain possesses the most important lead-mines in the world; and those that are best known are in the counties of Flint and Derby. The latter are supposed to have been worked even in the time of the Romans.
Lead mines are entered sometimes by perpendicular shafts, and sometimes (when in the sides of hills) by levels. In some of the Derbyshire mines, where the depth of the veins will admit of it, the men work, at different heights, of from four to six feet above each other, along what are called stoops; the uppermost men being two or three yards before those next in succession, and thus forming a kind of steps. The implements used are picks, hammers, and strong iron wedges; and the rocks are also frequently loosened by means of gunpowder.
When the ore is brought out of the mine, it is sorted and washed, to free it from dirt and rubbish. After this it is spread on a board; the best pieces are picked out and separated; and those containing ore mixed with spar (194) or other substances, are placed separate, to be broken, and again picked. After the ore, by pickings and washing, has been sufficiently cleansed from extraneous matters, it is roasted in a kind of kiln to free 204it from the sulphur that is combined with it. The next process is to mix it with a certain quantity of coke, charcoal, or peat, and submit it to the smelting furnace. In this furnace there are tap-holes, which, when the lead is melted, are opened, and the metal, in a fluid state, runs into a large iron pan. The dross which floats on its surface is now skimmed off; and the metal is taken out by ladles, and poured into cast iron moulds, with round ends. The lead thus formed, is ready for use, and has the name of pig lead. According to their size, the pieces that are thus cast have the appellation of pigs, and half-pigs.
Lead is mentioned in the Sacred Writings; and is described by Homer as in common use at the period of the Trojan war. The ancients seem to have considered it as nearly allied to tin. The Romans employed it to sheathe the bottoms of their ships, fastening it to the planks and timbers by nails made of bronze.
When first melted, lead is bright, but it soon tarnishes by exposure to the air. It melts at a temperature very low in comparison with most other metals; and when a strong heat is applied, it boils and evaporates.
Lead is much employed in the useful arts. When rolled between iron cylinders to a requisite state of thinness and uniformity, it is used for the covering of houses and churches, notwithstanding the danger, in case of fire, to persons within, who are exposed to a shower of burning metal. It is cast into pipes, cisterns, and reservoirs for water, as well as into large boilers for chemical purposes. But all culinary or domestic vessels made of lead, particularly if intended for the keeping of acid liquors, should carefully be avoided, as the surface of the lead is thereby corroded, and the liquid contained in them is rendered poisonous. Hence arises that dreadful complaint, too well known where cyder is kept in leaden cisterns, called the Devonshire colic; hence also the injury which sometimes follows from the use of lead in the glazing of coarse earthenware.
205Great quantities of lead are consumed for the making of shot. For this purpose the metal is alloyed with arsenic (242), to render it more brittle; and to render the grains more round and perfect than they otherwise would be. Shot is formed by dropping the melted alloy into water, through an iron or copper frame, perforated with round holes, according to the size required. For the smallest shot the elevation is about ten feet above the water; and for the largest about a hundred and fifty feet.
An alloy of lead and tin, in the proportion of two parts of lead and one of tin, forms the solder which is used by plumbers. The types that are used by printers for very large characters are sometimes composed of an alloy of lead and copper. Lead is also used, with tin, in the manufacture of pewter.
The different oxides (21) of lead are easily soluble in oil, and consequently are of great use to painters. Of these the following are the most important:
White Lead, or Ceruse.—This is made by suspending thin plates of lead over heated vinegar, in such manner that the vapour which rises from the acid may circulate about the plates. By this process the plates become at length entirely corroded, and converted into a heavy white powder. The manufacture of white lead is a most unhealthy trade, and is confined to a few persons, who have large conveniences for the purpose. This substance, when mixed with oil, is used as a paint for wood-work both of the outsides and insides of buildings. The fumes that are emitted from white paint are extremely noxious. Persons who breathe them are frequently seized with pains, and experience symptoms not much unlike those that precede palsy; and the danger which attends the inhabiting of apartments recently painted is well known. The odour of vinegar will correct the pernicious effect of these exhalations, by acting 206as a solvent, and combining with, and precipitating them. We are informed that white lead, dangerous as it is, was in great request among the Roman ladies as a cosmetic. It is sometimes used as an external application for ulcers and other kinds of sores.
Massicot is a mineral substance of yellow colour, used for painting, and prepared from the dross or pellicle that is formed by the melting of lead.
Red Lead, or Minium, is a mineral substance of red colour, used for painting, and made, by a tedious and troublesome process, from massicot. For this purpose the massicot is ground to a fine powder, put into a furnace, and constantly stirred, whilst the flame of the burning coals plays against its surface for about forty-eight hours, when it is converted into a red powder, which is the article under consideration. It is subsequently passed through very fine iron sieves. The use of red lead as a pigment is well known; but as it is liable to turn black, vermilion is generally preferred to it. It is sometimes employed in medicine as an external application for abating inflammations, for cleansing and healing ulcers, and the like; and is used in the manufacture of glass.
Litharge is another kind of oxide of lead. This is prepared by exposing calcined lead to a brisk fire for a certain length of time. The substance, on cooling, concretes into a flaky matter. Litharge is used by potters for the glazing of earthenware, but vessels that are glazed with it are thereby rendered unwholesome. It is also employed, in the composition of the finer kinds of glass, for the purpose, not only of giving them greater transparency, but also of rendering them capable of sustaining sudden changes from heat to cold, and of giving to them a susceptibility of being cut without breaking. It, however, adds considerably to the weight of the glass.
Litharge Plaster, or Diachylon Plaster, as it is more frequently called, is prepared by boiling two pints of olive oil with one pint of litharge, adding water, and 207constantly stirring the mixture till they are duly incorporated. This plaster is applied in excoriations of the skin, slight wounds, and other sores.
Sugar of Lead is a preparation either from the metal itself, or from white lead and distilled vinegar. It is usually observed in the form of small slender crystals, which have a glossy appearance like satin. This substance is employed, in considerable quantity, by dyers and calico printers; and is the basis of a liquid frequently used in medicine, called Goulard, or Goulard’s Extract. Although in itself a most virulent poison, it is often used by unprincipled dealers for correcting the rancidity of oil of almonds and olive oil; and a similar pernicious fraud is practised by dissolving a portion of it in wines which are becoming acid, in order to correct their acidity. These frauds, however, are easily detected by preparations or tests, which are sold by chemists for that purpose. Perhaps the best and simplest test is Harrowgate water: a little of this poured into the suspected compound will discover the presence of lead by giving to the fluid a dark brown or blackish tinge.
The following is a pleasing experiment. Dissolve an ounce of sugar of lead in about a quart of water; filter the solution through a piece of blotting paper, and put it into a glass decanter, suspending in it a piece of zinc by a brass wire. A decomposition will take place; the lead will be set at liberty, and will attach itself to the zinc, forming there a sort of metallic tree.
It has been stated that silver is usually a component part of lead ore. To disengage this, where the quantity is sufficient to repay the expense, the lead, after it has been smelted, is subjected to the action of what is called a refining furnace. A continued blast of fresh air is thrown upon its surface by means of large bellows, while the lead is kept in a state as intensely hot as possible. This by degrees converts the lead into a yellow scaly oxide or dross. The oxide, thus formed, is driven off from the melted metal as it rises, and the silver is 208left alone at the bottom, in a metallic state. After the operation is complete, the oxide is fused with charcoal, and again reduced to metallic lead.
We must not omit to mention that, in some of the mines of Derbyshire, there is a singular variety of lead ore called slickenside. This is a kind of galena, which presents, to the eye, a smooth and bright surface, appearing as if it were plated. Sometimes it forms the sides of cavities; and it has the extraordinary property, when merely pierced with the miner’s tool, of rending with great violence, and exploding with a crackling noise. Some miners, fearless of danger, venture to scratch it with their tools; and, on coming again to the spot, they often find that, during their absence, the slickenside has exploded, and fallen off in considerable quantity. Sometimes, however, they suffer for their imprudence. Mr. Mawe, in his account of the Mineralogy of Derbyshire, says, that he has seen a man come out of a mine cut violently, as if he had been stabbed about the neck and in other parts of the body, in consequence of the explosion of slickenside which he had pierced. The cause of this extraordinary phenomenon has not been explained.
240. NICKEL, when pure, is a fine white metal, somewhat resembling silver in appearance, but it is attracted by the magnet, and has itself the property of attracting iron.
It is ductile and malleable, difficult of fusion, and about nine times heavier than water. This metal is always mixed with arsenic (242) and iron.
Nickel is found in Cornwall, and in some other counties of England; in Germany, Sweden, France, Spain, and several parts of Asia. The Chinese employ it in making white copper; and, in conjunction with copper and zinc, they manufacture it into various kinds of children’s toys. Nickel gives a certain degree of whiteness to iron. It is used, with advantage, by some of the Birmingham manufacturers, in combination with that metal, and by others in combination with brass. If 209it were possible to discover an easy method of working nickel, there can be little doubt but it would be found a very valuable metal for surgical instruments, for compass needles, and other articles, as it is not, like iron, liable to rust. When nickel is freely suspended, it points to the north and south, in precisely the same manner as the common magnetic needle.
Oxide of nickel is used for giving colours to enamels and porcelain. In different mixtures it produces brown, red, and grass-green tints.
241. ZINC, or SPELTER, as it is sometimes called, is a bluish white metal formed in thin plates adhering together. It has a very perceptible taste, is about seven times heavier than water, rather harder than silver; and possesses but a small degree of malleability and ductility, except under certain circumstances.
This metal is never found in a pure state; and the principal ores from which it is procured are known by the names of Calamine and Blende. Of these the former is an oxide (21) of zinc combined with carbonic acid (26), and the latter is a combination of zinc with sulphuric acid (24).
The ores of zinc are very abundant in many countries. We are informed that nearly the whole of Flintshire in North Wales abounds with calamine; and that, so entirely ignorant were the inhabitants of its use, as, till after the middle of the eighteenth century, to have even mended their roads with it. These roads, however, have since been turned up in many places, and the materials have been converted to more valuable purposes. Derbyshire affords a great quantity of the ores of zinc, particularly calamine. This is found at various depths, generally in beds of yellow, or reddish brown clay, and usually near some vein of lead ore.
The mode of extracting zinc from its ore is by distillation. The process adopted, in some parts of Saxony, is equally simple and ingenious. An inclined stone is placed near the anterior part of a furnace, in which the ore of lead containing zinc is fused. A great part of the zinc condenses upon this stone, and flows, in 210drops or globules, into a quantity of charcoal placed at the bottom to receive it. These globules are afterwards again melted, to run the metal into a mass.
When exposed to the air, the surface of zinc is soon tarnished, but it scarcely undergoes any other change. It has a certain degree of ductility. When heated a little above 218° of Fahrenheit, it is malleable; and, when annealed, may be passed through rollers, and formed into thin sheets or leaves. Although, previously to being thus heated, it is brittle; on now cooling, it continues soft, flexible, and ductile. The inconvenience arising from the brittleness of the zinc being removed, this metal is applicable to many useful purposes. It may even be drawn into wire, but the tenacity of this is not great: a piece of zinc wire, one tenth of an inch in diameter, will sustain only a weight of twenty-six pounds without breaking. It has been proposed to substitute zinc in the place of tin for the lining of copper vessels; but it has not hitherto been ascertained whether this can be done with effect, and without injury. Prizes have of late been offered, to a considerable extent, in France, for the ascertainment of this fact. In China, zinc is employed as a current coin of the country; and for this purpose it is used in the utmost purity. The Chinese also, as well as the artists of our own country, employ it to a great extent in various alloys. It is used in the manufacture of brass, pinchbeck or prince’s metal, and bronze, all of which consist of this metal in combination with different proportions of copper (230). Tutenag is a well known white metal, made principally of zinc, and used for forming candlesticks and other articles. When tutenag is well manufactured, it is of good colour, and not more disposed to tarnish than silver. Zinc is one of the metals employed to form the galvanic or voltaic apparatus; and its filings are mixed with gunpowder, to produce those brilliant stars and spangles which are seen in the best kinds of artificial fire-works. Preparations of zinc are occasionally used in medicine. If a thin 211plate of zinc be applied to the upper surface of the tongue, and a shilling to the lower surface, and both metals, after a little while, be brought into contact, a very peculiar taste will, at that instant, be perceived. The same sensation will be perceived, though in a weaker degree, if the silver be placed at the top and the zinc at the bottom.
If a silver probe be introduced high up one of the nostrils, and be brought into contact with a piece of zinc placed on the tongue, a sensation not unlike that of a strong flash of light will be produced in the corresponding eye. A similar perception will result, both at the moment of contact and that of separation, if one of the metals be applied as high as possible between the gums and upper lip, and the other in a similar situation with the under lip, or even under the tongue.
A white oxide (21) prepared from zinc, was, some years ago, proposed as a substitute for white lead in house painting. This oxide is not dangerous in its application; and does not become yellow when mixed with oil. But these advantages are counterbalanced by some defects, which have hitherto caused it to be rejected. It is lighter than white lead: does not cover the surface so equally, nor so well; and is of considerably higher price.
Calamine.—The principal use of calamine is in the manufacture of brass (230); and the mines of Derbyshire, and of Limbourg in the Netherlands, supply with this mineral nearly all the brass works in Europe. After the calamine is dug out of ground, it is reduced to pieces not in general larger than a nut. It is then roasted for five or six hours, in what is called a reverberating furnace. The large pieces are separated, and the small ones are passed through a sieve. It is washed; and, when dry, is ground in a mill. In this state it is sold. The principal demand for it is at Birmingham, for the different brass founderies in that town.
The use of calamine in the composition of brass was 212known at a very early period. It is mentioned by Aristotle, who also makes a distinction between the compound resulting from the mixture of copper and calamine or brass, and that resulting from the mixture of copper and tin or bronze.
OR SUCH AS ARE NOT CAPABLE OF BEING FLATTENED OR ELONGATED BY THE HAMMER WITHOUT TEARING OR BREAKING.
242. ARSENIC, in a metallic state, has a bluish white colour, and considerable brilliancy; it is remarkably brittle, is the softest of all known metals, and is somewhat more than eight times heavier than water.
It is found nearly pure, and in considerable abundance, in different parts of Germany; usually occurring in masses of various shapes, and in combination with a small portion of iron, gold, or silver.
The arsenic sold in the shops, and too well known for its poisonous qualities, is an oxide (21) of this metal artificially prepared.
In some mines on the Continent arsenic is very abundant, and is found extremely injurious to the workmen. Being very volatile, its fumes affect and destroy the lungs, and occasion death in a short time to many of them. One of its ores, arsenical pyrites, is found abundantly in Cornwall and Devonshire, accompanying ores of copper and tin; and, in combination with other metals, it occurs, in a greater or less proportion, in almost all mines.
Arsenic is occasionally used in the arts. It is employed in various metallic combinations where a white colour is required, and, particularly, for the whitening or bleaching of copper, which is thereby also rendered 213capable of taking a fine polish; hence its use in many of the compositions for the mirrors of reflecting telescopes, and for other optical instruments. The manufacturers of glass frequently employ the oxides of arsenic in the fabrication of that article. Arsenic is used in the processes of dyeing and calico printing; and for the imparting of different artificial shades and colours to furs. It is also used in the manufacture of small shot, from its rendering the lead more brittle, and better capable of being formed into grains, than it would be without such admixture.
The arsenic of commerce is prepared to a great extent in Bohemia and Saxony, by roasting cobalt ores for the manufacture of zaffre (247). White arsenic is made, by mixing the common oxide with potash, and submitting it to a certain degree of heat, in vessels adapted to the purpose; the arsenic, rising in fumes, is separated, leaving the sulphur behind, united to the potash. This process is called sublimation.
Of all substances with which we are acquainted this is perhaps the most deadly. If only a few grains of it be taken into the stomach, it proves fatal; and it has frequently proved the more injurious from its deceitful appearance, in which it somewhat resembles salt or white sugar. Carelessly left in places open to the access of children, arsenic has not unfrequently been mistaken by them for sugar, and has been attended with the most dreadful consequences. If thrown on heated coals, however, it is immediately known, by the smell of garlic, and the white fumes which it gives out. The best remedy for this poison is said to be a few scruples of liver of sulphur (sulphuret of potash), dissolved in half a pint or a pint of water, and administered a little at a time, as the patient can bear it.
Notwithstanding its deleterious qualities, arsenic is occasionally used in medicine, though in extremely small doses; and it has, in particular, been found efficacious in many cases of intermittent fever.
214It is employed as a poison for rats and mice; and, diluted with water, it attracts and poisons flies, whence it is sometimes called by the French, poudre a mouches. There cannot, however, be too great caution used either in the preparation, or in the application, of this fatal poison.
243. YELLOW ORPIMENT is a mineral substance of lemon colour, which consists of arsenic in combination with sulphur; and in the proportion of about fifty-seven parts of the former and forty-three of the latter.
It is about thrice as heavy as water; and is found both in a massive and crystallized state; but the crystals are so confused that their figures cannot easily be determined.
The orpiment of commerce is an artificial production, and is chiefly imported from different parts of the Levant. The Turks, and other Orientals, use it in the depilatories which serve to render bald the top of the head. A very beautiful, but fugitive pigment, called King’s yellow, is prepared from this mineral; and other preparations of orpiment are occasionally used by painters, and also by dyers and calico printers. The whole of these, however, are extremely poisonous.
Orpiment is found in a natural state, along with copper and other ores, in Natolia, Servia, Hungary, Turkey, and some other countries.
244. REALGAR, or RED ORPIMENT, is a mineral substance of red or orange colour, which consists of arsenic in combination with sulphur; and in the proportion of seventy-five parts of the former, and twenty-five of the latter.
It is somewhat more than three times as heavy as water; and occurs sometimes in a crystallized, and sometimes in a massive or disseminated state.
This production, which, by ignorant persons, is not unfrequently mistaken for red lead, is in considerable request by painters, dyers, and calico printers. In China it is manufactured into small pagodas and other ornaments. And the Chinese form it into medical cups, and use lemon juice which has stood for some hours in 215them, as a cathartic. Realgar is poisonous, but by no means so much so as arsenic (242).
It is found in Sicily, Hungary, and various parts of Germany: and is very common in several districts of China.
245. ANTIMONY is a compact metallic substance of brilliant and slightly bluish white colour, destitute of ductility, and about seven times heavier than water.
Its texture is laminated, the plates crossing each other in almost every different direction. It is as hard as silver, and so brittle that it may easily be reduced to powder, in a mortar.
In the state of the Connecticut, North America, it is said that antimony, in a pure metallic form, is found in such abundance that, in some places, large masses of it may be seen lying on the surface of the ground. The principal supply of antimony in Europe is from an ore which is found in Hungary and Norway, called sulphuret of antimony. The process of bringing it into a state for use is very simple. The mineral is put into pots, each of which has a hole in the bottom, and which is placed on another pot bedded in the earth. The upper pots, which are filled with the mineral, are heated. As soon as the antimony is fused it flows into the lower pots, while the substances with which it was combined remain in the upper ones. The antimony fixes, and forms cakes of the shape of the pots which receive it. In this state the metal presents, in its fracture, a surface thick-set, with long needle-shaped crystals, which, lying by the side of each other, compose, as it were, the whole of the mass. It is afterwards re-melted and cast into cakes for sale.
This metal, in a pure state, or alloyed only with a very small portion of silver and iron, is found in veins of mountains in some parts of France and Sweden, occurring in massive and kidney-shaped lumps of white colour.
The only mine of antimony in Britain, which is of any importance, is at Glendinning in Dumfries-shire. It was discovered in 1760, in searching for lead ore, but 216was not regularly worked till 1763. In the first five years about a hundred tons’ weight of antimony were obtained from it. This at 84l. per ton, produced the sum of 8400l. The undertaking was afterwards relinquished, but, as the price of antimony is now at least thrice what it then was, it is supposed that this work, if resumed, might prove an advantageous speculation. The vein of ore is only from eight inches to a foot and a half in thickness.
Antimony was known to the ancients. The earliest account we have of it is in the Sacred Writings. The passage in the Second Book of Kings,[4] which states that, on the approach of Jehu to the city of Jezreel, “Jezebel painted her face,” implies, in the original, that she stained her eyes and eyebrows with antimony, for the purpose of making them look black and large, a custom which, at that period, was prevalent in several of the Eastern countries. Antimony was likewise considered by the ancients a remedy against inflammations of the eyes.
This metal is the basis of many of the officinal preparations which are now in use; and it was the basis of many others which were formerly used, but are now discontinued. No mineral substance has so much attracted the attention, or so much divided the opinion of physicians, as antimony. One party extolled it as an infallible specific for almost every disease; whilst another described it as a virulent poison, which ought to be expunged from the list of medicines. It was on this metal that the alchemists of the middle ages principally founded their hope of discovering the philosopher’s stone; and, by a kind of good fortune, of which we can cite but few examples, it has happened that, in pursuing a chimera, they hit upon a succession of important realities. To the unremitted perseverance with which they tormented this metal, if we may so express it, the art of healing has been most essentially indebted.
4. Ch. ix. v. 30. See also Ezek. Ch. xxiii. v. 40.
217The first rational account of the properties of antimony was given, about the end of the seventeenth century, by a French chemist, whose name was Lemeri. Its great importance in medicine will be seen by an enumeration of some of the most valuable preparations of it which are still in use.
Antimonial Wine is prepared from antimony, in conjunction with white Lisbon wine. It is employed as an emetic; but, if mixed with milk, this quality is said to be completely destroyed, and it becomes narcotic.
Emetic Tartar, which is much more employed in this country than all the other antimonial preparations put together, is formed from antimony mixed with its own weight of tartar, and a certain proportion of water, and afterwards boiled, filtered, and suffered to crystallize.
Butter of Antimony is obtained from a combination of antimony with corrosive sublimate. It is denominated by chemists muriat of antimony, and is usually a thick fatty mass of greyish white colour.
Glass of Antimony is a vitreous substance of reddish brown colour, which is occasionally used in medicine, but more frequently in colouring the imitations of yellow diamond, Oriental, Brazil, and Saxon topaz, hyacinth, emerald, and beryl.
James’s Powder, or Antimonial Powder, is a well-known medicine, composed of phosphat of lime and antimony.
An alloy consisting of sixteen parts of lead and one part of antimony constitutes the metal of which printers’ types are formed. This alloy does not differ from lead except in being considerably harder and more tenacious. The plates on which music is engraved are formed of a mixture of tin and antimony; and the oxides of antimony are used for the colouring of glass.
246. BISMUTH is a reddish white semi-metal, harder than silver, and composed of broad brilliant plates adhering together.
218It is nearly ten times heavier than water, and is so brittle as readily to break under the hammer. None of the semi-metals are so easy to be fused as this; it melts even in the flame of a wax candle, and long before it becomes red hot, and has the singular property of expanding as it cools.
The ores of bismuth chiefly occur in Sweden, Norway, Germany, France, and England. This metal appears to have been known to the ancients. It was confounded by them with tin; and, even in our own manufactories, it is known to the workmen by the name of tin-glass.
It is not of much use in the arts; but its fusibility renders the working of it very simple and easy. It is employed in the composition of some of the soft kinds of solder; and is also used for giving hardness to tin and other metals. Amalgamated with mercury it renders that metal less fluid; and the addition of it to mercury and tin is found useful in the foliating or silvering of looking-glasses. Some manufacturers use it in the composition of pewter; but it is said that this ought not to be done, particularly for the formation of vessels intended to contain food, as bismuth partakes of the noxious properties of lead, and sometimes contains even arsenic. It is also occasionally employed in the fabrication of printers’ types.
A very singular metal is formed by melting together eight parts of bismuth, five of lead, and three of tin. Tea-spoons formed of this metal surprise all who are unacquainted with their nature: they have somewhat the appearance of common spoons, but they melt as soon as they are put into boiling water.
Bismuth reduced to powder, mixed with the white of eggs and applied to wood, gives it, when gradually dried and rubbed with a polisher, the appearance of being silvered. If this metal be dissolved in aquafortis (30), and water be poured into the solution, a white powder precipitates, which is an oxide of bismuth, and which, after being well washed, is used as a pigment, under the name of pearl-white. From its beautiful appearance, 219this powder is sometimes employed by ladies for painting their skin; a practice which cannot be too much condemned, both on account of the danger with which it is attended, and from its soon injuring both the texture and natural colour of the skin. It has the further disadvantage of turning black when touched by the fumes of fetid and other substances; and ladies, who have used this cosmetic, and have afterwards bathed in the Harrowgate waters, have come from the bath a perfectly tawny colour. It was probably the oxide of bismuth which the Roman ladies used for whitening their skin; for Martial, in speaking of a lady, who made too free an use of cosmetics, describes her as afraid even of the sun. The oxide of bismuth is used in the composition of most of the pomades employed in France for painting the face.
A preparation of bismuth has lately been employed in medicine, as a remedy against spasmodic affections of the stomach.
The following is a pleasing experiment, illustrative of metallic crystallization. Melt a ladleful of bismuth, and allow it to cool slowly and quietly till a thin crust is formed on the surface: then, with a pointed iron, make two small opposite apertures through the crust: and, through one of these, quickly pour out the fluid portion, as carefully and with as little motion of the mass as possible. The air having entered by the other aperture, there will appear, on removing the upper crust by means of a chisel, when the vessel has become cold, a cup-shaped concavity, studded with very brilliant crystals, and more or less regular according to the quantity of metal employed, the tranquillity and slowness with which it has cooled, and the dexterity with which the fluid portion of the mass was poured off before it became solid. The same effect may be produced by melting bismuth in a crucible which has a hole in the bottom, lightly closed by an iron rod or stopper; this is to be drawn out when the mass begins to congeal. 220By so doing, the upper portion, which is fluid, is made to run off, and a cake studded with crystals will be left.
247. COBALT is a semi-metal of grey colour with a shade of red, brittle, somewhat harder than silver, nearly eight times as heavy as water, is attracted by the magnet, and is itself capable of being rendered permanently magnetical.
The ores of cobalt are not numerous, and are, for the most part, combinations of this substance with other metals, or of its oxides (24) with arsenic, or with sulphuric acid (21).
The name of this metal implies an evil being, (Kobold, German, goblin) and is said to have been given on account of the vapour of arsenic, which issues from it, tormenting the miners, and making them believe that they are afflicted by wicked spirits. Hence it was once customary in Germany to introduce into the church service a prayer that God would preserve miners and their works from cobalts and spirits.
Cobalt is found in several parts of Europe, but most plentifully in the southern borders of France, and in Saxony; and the cobalt ores of Hesse, although they were formerly used for no other purpose than the mending of roads, are said now to yield a clear profit of nearly 15,000l. a year. Some parts of our own country yield this substance in considerable abundance, particularly the Mendip Hills in Somersetshire, and a mine near Penzance in Cornwall.
After the ore is taken from the earth, it is broken into pieces about the size of a hen’s egg, and the stony parts are picked out. The sorted mineral is then pounded in mills, and sifted through brass-wire sieves. The lighter particles are next carried off by water. After undergoing some other preparations, to rid it of the impurities and foreign matters with which it is connected, it appears in the form of a dark grey oxide. The working of the cobalt ores in Germany is considered so injurious, on account of the arsenic with which they are combined, that much of the labour is 221performed by criminals who are condemned to it for the commission of crimes which, by the laws of the country, have deserved the punishment of death.
As a metal, cobalt was unknown till the year 1733, when it was discovered by a celebrated Swedish chemist whose name was Brant. In its metallic state it is not employed in the useful arts; but in a state of oxide it is found extremely valuable in the colouring of porcelain, in painting, enamelling, and for other purposes. Cobalt and ultramarine form the most permanent blue colours with which we are acquainted. The old painters generally used them for the representation of the sky and of blue drapery, and this is the reason why these parts in some old pictures have been found so much more durable than any others.
Zaffre is an oxide of cobalt mixed with about three times its own weight of calcined and pounded flint. It has been chiefly imported into this country from Saxony and Bohemia, but it is now also manufactured from cobalt dug from mines in the Mendip Hills and in Cornwall. In Staffordshire there are several persons who carry on a considerable trade in preparing this colour for the earthenware manufacturers of that county.
This substance is extremely valuable for the colouring of porcelain and glass; as it resists without change, the effects of the most intense heat. Hence also it is advantageously used for giving various shades of blue to enamels, and to glass manufactured in imitation of lapis lazuli, turquoise, sapphire, and various precious stones. So intense is the colour imparted by it that a single grain of zaffre will give a full blue tint to 240 grains of glass.
Smalt is a kind of glass, of dark blue colour, formed by melting zaffre with three parts of sand and one of potash; when this substance is ground to a coarse powder, it has the name of strewing-smalt, and is much used by sign painters, as an ornamental filling up of the vacant space betwixt the letters of signs. In Germany it is frequently employed instead of sand for the purpose 222of drying ink after writing. The same substance reduced to a perfectly fine or impalpable powder, is the article which is sold under the name of powder-blue, and which is not only used by laundresses and others in the getting up of linen, but also as the basis of several kinds of paint; and by the manufacturers of writing and printing papers, to give a blue tinge to those articles.
A solution of the oxide of cobalt in spirit of salt (muriatic acid, 29) and afterwards diluted till nearly the whole of its colour disappears, forms one of the most beautiful sympathetic inks with which we are acquainted. If a landscape be drawn with Indian ink, and, afterwards, the foliage be washed over with this solution, it will have no peculiar appearance; but, on holding the paper near the fire, the part representing the vegetation will gradually assume a green tint, which will subside on removing the paper into a cool situation.
248. MANGANESE, in the state that we usually see it, is a black oxide of a metal which is of a silvery grey colour, of leafy or foliated texture, and somewhat more than six times as heavy as water.
Mines of manganese have long been worked in several parts of Great Britain, but particularly in the counties of Devon and Somerset. Near Exeter and in the Mendip Hills this mineral is found in great abundance.
It is employed for various useful purposes. In the manufacture of the finer kinds of glass it is used in a double capacity, both as a colouring material and as a destroyer of colour. As a colouring ingredient, the imitators of several precious stones are indebted to it for the red and purple tints which they give to the oriental ruby, the balais ruby, and the amethyst.
The violet colour given to porcelain is obtained from manganese. This substance is also used for the glazing of black earthen ware, as a paint, and an ingredient in 223printers’ ink. As a discharger of colour it is applied in small quantities, and, by the oxygen which it gives out, it is said completely to destroy any tinge left in the glass, by the presence of iron, and some other colouring matters. This property has obtained for it the appellation of the soap of glass.
It is from manganese that all the oxygen gas (21) used by chemists is obtained. By the application of a red heat this is yielded in such abundance that an ounce of the oxide of this metal will yield about two quarts of gas. The consumption of manganese has, of late years, become very considerable by the discovery of the oxygenated muriatic acid, which is now extensively used in the bleaching of linen and cotton; that liquor being made by the distillation of the oxide of manganese with spirit of salt (muriatic acid, 29).
249. There exist considerable masses of minerals in a state of combination, or aggregation with each other. These constitute the rocks and soil of which the globe of the earth is composed; and the study of them is called GEOLOGY. The opinions of learned men relative to their structure, and original formation, have produced various systems denominated theories of the earth; but, when we consider that the greatest depth beneath the surface to which the art and industry of man have been able to penetrate, does not exceed 1/35000 part of the earth’s diameter, we must confess that this is very insufficient to allow of any correct opinion being thereby formed concerning the structure of the whole.
Modern geologists, for the more convenient arrangement of the compound minerals, have divided them into four classes, which they denominate primitive rocks, secondary rocks, alluvial depositions, and volcanic rocks.
250. These are so called from their being considered by geologists, to belong to the first formed parts of the globe.
Rocks of this description are of a nature extremely hard. They contain no vestiges whatever of animal or organic remains; and the substances of which they are composed are crystallized. They rise through other rocks at various elevations, in every quarter of the globe; and never either alternate with, or rest upon rocks that enclose organic remains, though they are themselves frequently covered by such.
The following are the principal kinds.
225251. GRANITE or moonstone is a compound rock composed of felspar (110), quartz (76), and mica (123), each in crystalline grains of various size, and promiscuously arranged; sometimes one and sometimes the other of these ingredients predominates, but generally the felspar.
This is one of the most common and most widely extended rocks that are known; and is considered as the foundation on which the secondary rocks are deposited. In Cornwall it is very abundant, and veins both of copper and tin are found in it. Granite forms the summits of the highest mountains in Scotland, of the highest of the Grampian Hills, the Alps, and the Pyrenees; and indeed the loftiest parts of most of the countries of the world. The Logan or rocking stones, in Cornwall, are immense blocks of granite.
The uses of this stone are numerous and important. Millstones, steps, troughs for stamping mills, and innumerable other articles, are made of it. The streets of London are chiefly paved with granite, and its hardness and durability render it peculiarly eligible for this use. Weather has little effect upon it. Consequently, when applied to architectural purposes it is found infinitely preferable to Portland stone, of which nearly all the public buildings of modern date in London have been constructed, and many of which are fast going to decay. This circumstance induced the proprietors of the Waterloo Bridge to adopt granite in the construction of that edifice. Mr. Smeaton also chose it for the outer walls of the Eddystone Lighthouse.
252. Scottish Granite.—Scotland is remarkable for many kinds of granite, some of which are susceptible of an excellent polish. The greatest part of the mountain of Ben Nevis, near Fort William, is composed of a reddish granite, one of the best and most beautiful that is known. This mountain is nearly a mile in perpendicular height, and is said to contain granite enough for all the kingdoms of the earth, although they should be as partial to this stone as the ancient Egyptians 226were. Columns and obelisks of any size and height might be cut from it: for the rock is one uniform mass, without appearance of strata, division, or fissure of any kind. A convincing proof has been given of the strength and hardness of this granite, in a fragment of several tons’ weight, which fell from nearly the top of a precipice five hundred yards in height, upon a hard and solid rock below, and yet continued entire.
253. Granite of Ingria.—A beautiful red granite is found in some parts of Russia, remarkable on account of the felspar (110) that it contains, appearing in round or oval pieces, from half an inch to two inches in diameter. This granite, when polished, exhibits shining spots of round or oval shape, which give to it somewhat the appearance of being studded with precious stones.
The royal summer garden at Petersburg is decorated with a superb colonnade of Ingrian granite. The columns are sixty in number, and each of a single piece twenty feet high, and three feet in diameter. Many of the public buildings in Petersburg are of this granite. An immense block of it thirty-two feet long, twenty-one feet broad, and seventeen feet high, forms the pedestal of the celebrated equestrian statue of Peter the Great, in that city.
254. Graphic Granite.—A singular kind of granite has been discovered in the island of Corsica, and lately near Portsoy in the north of Scotland. The ground of this granite is a whitish or reddish yellow felspar, in which are embedded crystals of quartz each from an inch to an inch and half long, and several lines in diameter. The name of graphic granite was given to it in consequence of an imaginary resemblance which the sections of these crystals have to Hebrew, or Arabic, and sometimes to musical characters.
255. GNEISS is a primitive rock, consisting, like granite, of felspar (110), quartz (76), and mica (123), but differing from that rock in its structure, being slaty.
227Mountains of gneiss are not so steep as those of granite, and their summits are usually rounded. Ben Lomond and others in Scotland, and mount Rosa in Italy, are almost wholly of gneiss, as well as the middle part of the Pyrenees. It is not an uncommon rock, but in Britain is of less frequent occurrence than granite.
Many valuable metallic ores are found in veins of gneiss. This rock also sometimes contains crystals of garnet (70), and tourmaline (69).
256. MICA SLATE, or MICACEOUS SCHISTUS, is a primitive rock of slaty structure, consisting principally of quartz (76) and mica (123).
Like gneiss, it is rich in ores. It often contains beds of magnetic ironstone (235), galena (239), copper, blende (241), cinnabar (228), and sometimes even gold. It frequently has garnets, and sometimes tourmalines (69), interspersed in different parts of it.
Mica slate occurs in many parts of Scotland; the mountain of Schehallien, and the rocky adjacent country, are in a great degree composed of it.
257. CLAY SLATE is a primitive rock generally of dull blue colour, more or less compact, always slaty, and always stratified.
Under the appellation of clay slate are included roofing slate (120), whet slate (122), drawing slate (121), and some other kinds already described.
Few rocks abound more in veins and beds of valuable metals than slate. In different countries it contains ores of tin, lead, cobalt (247), silver, and copper; and gold, and mercury (228) sometimes occur in it. The celebrated quicksilver mines of Idria (228), and the immense mass of copper at Parys mountain in the island of Anglesea (230), are in clay slate. Crystals of pyrites (236), and sometimes garnets (70), and thin layers of quartz (76), and felspar (110), are all occasionally found embedded in it.
228This is a widely-extended rock; it sometimes forms whole mountains, and even chains of mountains; but these usually have a gentle acclivity. The summit of the celebrated mountain called Skiddaw in Cumberland is of clay slate.
258. PRIMITIVE LIMESTONE is a simple mountain rock of crystalline or granular structure; and generally of white, yellowish, greenish, or reddish colour.
To this species of rock belong many of the rich and beautiful kinds of marble already described (143, &c.). Carrara, or statuary marble (146), is a familiar instance of it. Whole mountains in Stiria, Carinthia, Carniola, and the Pyrenees, and three mountains in Switzerland, 10,000 feet in height, are of primitive limestone. The mountain of Filabres in Spain, is said to consist of one block of white granular marble, 2,000 feet high, and three miles in circuit; without intermixture of other earths or stones, and almost without a fissure.
Various mineral ores, in beds and veins, as lead, zinc (241), and iron, are occasionally found in this kind of rock.
259. PRIMITIVE TRAP is a mountain rock composed of a black mineral called hornblende, mixed, in some varieties, with felspar (110), and, in others, with mica (123).
The word trap is of German origin, signifying a stair; and rocks of this formation are called trap rocks, because their strata, when exposed, usually jut out, one beneath the other, somewhat like a stair. Under this term is comprehended a series of rocks, distinguished chiefly by the hornblende, which they all contain.
Rocks belonging to this formation are numerous. They occur in Scotland; and abundantly in Derbyshire and some other parts of England. In many countries they constitute considerable hills. They abound in ores.
260. SERPENTINE is a primitive rock, usually consisting of quartz (76), magnesia (198), alumine (197), with a portion of oxide (21) of iron.
229This rock and its various uses, have been already described (132). It generally occurs in shapeless masses and beds; and seldom in distinct strata. It is found in Cornwall, the island of Anglesea, and several parts of Scotland; but it rarely forms mountains.
Ores of lead, silver, and copper, are sometimes found in serpentine.
261. PORPHYRY is a primitive rock, consisting of quartz (76) or felspar (110), or both, embedded in a solid and compact cement or ground.
The ground or basis of porphyry varies in the different kinds. In some it is claystone, in others pitchstone, hornstone, or compact felspar.
When not covered by other formations, porphyry sometimes forms single rocks; but it never constitutes elevated mountains. It occurs in beds of considerable magnitude, but never appears in distinct and well-defined strata.
There are many beautiful and splendid works in porphyry. Obelisks, statues, and columns, wrought in it, have had great celebrity. It is susceptible of a polish as high as that of marble, but is so hard, that the expense of working it has caused it to be much neglected by the moderns. This hardness, however, renders it very durable, and also constitutes it a material of great utility for mortars, slabs for grinding colours upon, and for several other purposes.
Porphyry was much esteemed by the ancient Egyptians; and Pliny informs us that the procurator-general in Egypt, under Claudius Cæsar, brought thence, for that Emperor, certain statues of porphyry, which he conceived to be very valuable: this act, however, was not much approved, and the example was not followed by any other Roman.
The principal quarries of porphyry are in Egypt; but this stone is also found in Italy, Germany, and various parts of the European continent. It may be traced from Norway to the borders of the Black Sea, 230and it has been discovered in some of the western and northern parts of Great Britain.
262. SIENITE is a rock composed chiefly of felspar (110) and hornblende. Its colours are usually reddish and black.
Some varieties of it contain quartz (76) and mica (123), with but little hornblende. In these the colours are various.
Although this is a less abundant rock than any of those that have yet been mentioned, it occurs, in great abundance, at Mount Mado, in the island of Jersey. There are extensive quarries of it in that mountain, not only for the use of the island, but for exportation to England, and other distant countries. The cliffs, for a long space, and an elevation of a hundred feet or more, consist entirely of sienite, in large masses, which are apparently uninterrupted by a single fissure. Shafts for columns of considerable length have been taken from these quarries; and, were the demand sufficient to call for new openings, it is imagined that columns of twenty feet and upwards in length might be raised. The felspar is of a flesh colour, and the stone is capable of a beautiful polish.
A somewhat similar kind of sienite is found at Grande Roque, in the island of Guernsey, in large masses, which are quarried for building stones. Sienite also occurs in some parts of Scotland and Derbyshire; in Saxony, Hungary, the island of Cyprus, and Egypt. Its name has been derived from that of the city of Syene, in Upper Egypt, where it is found in great abundance.
Sienite was much used by the ancients in ornamental architecture. What was called the red granite of Egypt (for this rock has usually been considered a granite) furnished numerous magnificent obelisks and columns, of a single piece, which have been much admired in Rome and other places. The ancient artists sometimes cut this kind of stone into statues, vases, monumental and other works. The celebrated column in Egypt, upwards of ninety feet high, and known by the name of Pompey’s Pillar, is formed of sienite.
231In veins of this rock are found, in different countries, many kinds of metallic ores: among others, silver, iron, tin, copper, and lead.
263. QUARTZ ROCK is a simple mountain rock, usually of granular texture, and whitish colour.
It sometimes contains mica, in which case it has a slaty form.
In certain mountains of Scotland, and the Scottish islands, quartz rock is very abundant. On the Continent it appears in Saxony, Bohemia, Silesia, and several other countries. We are informed that a mountain, 350 feet high, and near 5000 feet broad and long, one of the Altaisch chain, in Siberia, consists entirely of a milk-white quartz.
The uses of quartz have been already described (76, &c.) This kind of rock does not contain metallic ores of any description.
264. Secondary Rocks are composed of, or at least contain within them, the mineralized remains of organic substances. These must necessarily have been formed at a period subsequent to the formation of those organized bodies the remains of which they enclose; and they have apparently been formed by the deposition of water. Hence it is that, to distinguish them from rocks of the preceding class, they have received the appellation of secondary. They always rest upon or cover primitive mountains, and sometimes lean upon their sides or invest them.
Werner, the celebrated German mineralogist, makes two divisions of secondary rocks. The first of these he denominates transition rocks, and states that they are less perfectly crystallized than the primitive rocks; and that they enclose the remains of marine animals, no species of which are at this time known to exist: the other division he terms floetz, or flat rocks, because they 232are generally disposed in horizontal or flat strata. Some of the latter contain the fossil remains of marine animals and shells, approaching in character and appearance to the kinds which are now found in the ocean; and others contain shells precisely similar to those now known to exist. These rocks usually occur at the foot of primitive mountains, or in deep valleys.
265. TRANSITION LIMESTONE is distinguished by containing marine petrifactions of corals, and other zoophytes which are supposed no longer to exist. It often contains veins of calcareous spar, and exhibits a variety of colours, which give to it a marbled appearance.
This species of limestone occurs in immense beds, and forms a great portion of the mountainous parts of Derbyshire and Scotland; but it does not rise so high, on the sides of mountains, as primitive rocks (250).
It often contains veins of valuable metallic ores. When cut and polished, many of the varieties of transition limestone are beautiful marbles; some of them have been already described.
266. GREY WACKA is a transition rock, composed of pieces of quartz (76), flinty slate, felspar (110), and clay slate (120), cemented together by a basis of clay slate.
It has various appearances, the pieces being sometimes as large as a hen’s egg, and sometimes so small that they can scarcely be perceived by the naked eye.
When the rocks of grey wacka are not covered by those of any other formation, they form round-backed hills, usually insulated towards the tops, and intersected by deep valleys. They are widely distributed: and are often extremely rich in ores, both in beds and veins. Almost all the mines of copper, lead, and zinc, in the Hartz, are in grey wacka; and, in Transylvania, this species of rock is traversed by numerous small veins of gold.
267. OLD RED SANDSTONE, or MILLSTONE GRIT, is a floetz or flat rock, composed of large grains of sand or quartz (76), coloured by oxide (21) of iron, and usually cemented together by a kind of clay.
In several parts of Derbyshire this kind of rock forms the uppermost stratum; and in some places, is known to be 120 yards thick.
What are known by the name of peak millstones are formed of millstone grit. They are chiefly obtained from quarries near Nether Padley, in Hathersede, Derbyshire; a very inaccessible part of the country, but where the stone is of better quality than it can elsewhere be procured. These millstones are made of different dimensions, from two feet in diameter, and eight inches thick, to five feet and half in diameter, and seventeen inches thick.
Some of the beds of millstone grit, which have spherical stains in them, of light red colour, are said to be infusible; and are consequently a valuable stone for lining the hearths of iron and other furnaces, where an intense heat is required. These are called firestones, and Roches quarry, near Upper Town, in Ashover, Derbyshire, is famous for them.
The upper beds of this kind of rock are often thin, and capable of division, so as to make excellent paving stones, or flags. There is a particular bed of it at Stanton, in the Peak of Derbyshire, so porous that it is made into filtering stones for the cleansing of turbid water.
268. THIRD SANDSTONE, GRITSTONE, or FREESTONE, is another kind of floetz or flat rock, formed of very small agglutinated particles of sand. It is opaque, usually of whitish colour, and found in large masses, of various degrees of hardness.
The name of freestone has been given to this kind of rock, from its capability of being broken or hewn, with nearly equal facility, in any direction. Hence, as well 234as from its great durability, it is peculiarly esteemed for buildings. It is also formed into cisterns and troughs of various kinds; into pillars for supporting corn ricks; into rolling stones; and into grinding stones for cutlers, edge-tool makers, and workers in polished steel. Paviors’ flags, or the stones used for the paving of footpaths, yards, kitchens, and out-houses, are generally flat pieces of freestone.
Scythestones, or stones for the sharpening of scythes upon, are made of freestone. Considerable numbers of these are wrought in Derbyshire; and the dexterity that is displayed in cleaving and forming them is somewhat remarkable. The workmen use sharp-pointed picks, several very small wedges, and a hammer. A proper block of stone being selected, two or three of these small wedges are set in a row, by gentle blows of the hammer. These blows are successively repeated till the stone splits. The wedges are then set in a straight line into the face of the piece split off, and the stone is cleft again in that direction. In this manner the sub-divisions are continued, until a piece remains of size to make two scythestones, each an inch and a half square, and about twelve inches long. This the workman holds in his left hand, nearly upright; with the point of his pick he traces a deep nick down the middle of first one side and then the other; and then by a slight blow of his pick he separates it, into two, so dexterously, that not more than three or four in a hundred are broken in the cleaving. Such stones as are intended for round rubbers, are first reduced into an octagonal shape by the point of the pick, and then handed over to women and boys, who grind or rub them in a notch formed in a hard stone, until they are of the requisite shape. The square ones are finished by being ground on a flat stone.
Other rocks, belonging to what is called the floetz, or flat formation, have been already mentioned, under the heads of lime-stone (140), gypsum (192), rock salt (202), chalk (141), and coal (217).
269. These are described to comprehend all such substances as have been formed from previously existing rocks, of which the materials have been worn down, by long exposure to the agency of water and air, and afterwards deposited in nearly horizontal beds on the surface of the land. Alluvial deposits have been formed, and are still forming in every quarter of the globe. They occur both in mountainous regions and in flat countries, filling up the valleys or hollows in the one; and often forming vast and extended plains in the other.
They consist of peat, sand, gravel, loam, clay, and other substances.
IV. VOLCANIC ROCKS.
270. Volcanic rocks are composed of such mineral substances as have been ejected from volcanoes, or have been formed by the agency of subterraneous fires, and have undergone certain changes in such fires.
They are of two kinds; the one called pseudo volcanic, such as burnt clay, porcelain jasper, and earth-slag, which have been altered in consequence of the burning of beds of coal in their neighbourhood; and the other, called true volcanic minerals, such as stones, ashes, and lava, which have been thrown out of real volcanoes.
271. It will somewhat tend to illustrate the history of the mineral kingdom, to state, in conclusion, under a tabular form, the relative heights of the principal mountains, or masses of rocks, which occur in the different countries of the world; previously remarking, that the most lofty and magnificent of these, respecting which any account sufficiently authentic has hitherto been obtained, are the mountains of Nepaul and Thibet, in Asia, one of the former being 27,667, and the highest of the latter measuring at least 23,262 feet, or from 4½ miles to 5¼ miles in perpendicular height above the level 236of the sea. Previously to the knowledge that has lately been attained respecting the Asiatic mountains, those of the Andes, on the continent of South America, had been considered by far the highest in the world. One of them, Chimborazo, is 20,900 feet in height. Of the European mountains, the highest is Mont Blanc, in Switzerland, which measures 15,680 feet, or about 2¾ miles. The loftiest summit within the British islands is Ben Nevis, in Inverness-shire, Scotland, which does not exceed 4,380 feet, or somewhat more than three quarters of a mile; and the great pyramid of Egypt, the loftiest work of human art and industry with which we are acquainted, and which will serve as a point in the scale, measures only 477 feet.
272. It has been remarked that the greatest altitude at which bananas and other palm-trees grow in America is about 3280 feet above the level of the sea (Frontispiece, Fig. 48): that in the torrid zone, the superior limits of oaks is about 10,500 feet (49), of pines 12,000 feet (50), and of lichen plants 18,225 feet (51). The American travellers, Messrs. Humboldt and Bonpland, on the twenty-third of June, 1802, ascended the mountain of Chimborazo to the height of 19,400 feet (52). The highest flight that has been remarked of the South American vulture, called the condor, was 21,000 feet (53). M. Lussac, on the 16th of September, 1804, ascended in a balloon from Paris, to the height of 22,900 feet. In Switzerland, the limit of perpetual snow is above the altitude of 9000 feet (54).
| Frontispiece. | |||
|---|---|---|---|
| Ft. above | |||
| Fig. | the sea | ||
British Islands.
| Scotland | Ben Nevis, Inverness-shire | 1 | 4380 |
| Ben Lawers, Perthshire | 2 | 4051 | |
| England | Skiddaw, Cumberland | 3 | 3530 |
| Cross Fell, Cumberland | 4 | 3390 | |
| Helvellyn, Cumberland | 5 | 3324 | |
| Wharnside, Yorkshire | 6 | 2480 | |
| Ingleborough, Yorkshire | 7 | 2380 | |
| Wales | Snowdon, Caernarvonshire | 8 | 3568 |
| Cader Idris, Merionethshire | 9 | 3550 | |
| Ireland | Macgillicuddy’s Reeks, Kerry | 10 | 3404 |
| Sleibh-Dorin, Londonderry | 11 | 3150 |
Continent of Europe.
| France | Mont d’Or, Auvergne | 12 | 6707 |
| Puy de Sausi, Auvergne | 13 | 6700 | |
| Pyrenees | Mont Perdu, the highest of the Pyrenees | 14 | 11,283 |
| Le Pic Blanc, Spain | 15 | 10,205 | |
| Alps | Loucira | 16 | 14,451 |
| Loupilon | 17 | 14,144 | |
| Switzerland | Mont Blanc, highest mountain in Europe | 18 | 15,680 |
| Mont Rosa | 19 | 15,555 | |
| Mont St. Gothard | 20 | 10,014 | |
| Italy | Mont Cimone | 21 | 6401 |
| Vesuvius | 22 | 3900 | |
| Germany | Ortler-Spitze, Tyrol | 23 | 15,430 |
| Ostelle, Saltzburg | 24 | 12,800 | |
| Carpathian Mountains, highest summit | 25 | 8640 | |
| Lomnitz Peak | 26 | 8640 | |
| Sweden | Areskutan, Jempland | 27 | 6180 |
Islands.
| Teneriffe | Peak of Teneriffe | 28 | 12,236 |
| Sicily | Ætna | 29 | 10,963 |
| Jamaica | Blue Mountains | 30 | 7431 |
| Iceland | Snæfiel | 31 | 6860 |
| Hecla | 32 | 4900 |
Asia.
| India | Dhawalgeri in Nepaul | 46 | 27,667 |
| Mountains of Thibet | 47 | 23,262 | |
| Turkey | Mount Lebanon, estimated at | 33 | 9520 |
| in Asia | Mount Ararat, estimated at | 34 | 9500 |
| Mount Ida | 35 | 4960 |
America.
| Andes, South | Chimborazo, Quito | 36 | 20,900 |
| America. | Cotopaxi, Quito | 37 | 18,880 |
| Alps | Lake of Lausanne | 38 | 8640 |
| Lake of Lanzon, on the mountain of Olan | 39 | 6797 | |
| Switzerland | Lake of Lucerne | 40 | 1408 |
| South America | City of Riobamba, Quito | 41 | 18,800 |
| City of Quito | 42 | 9356 | |
| North America | City of Mexico | 43 | 7424 |
| Austria | Town of Eisenerz | 44 | 2056 |
| Egypt | The great Pyramid | 45 | 477 |
273. WATER, generally speaking, is a transparent and nearly incompressible fluid, the component parts of which are two kinds of gas, called hydrogen (45) and oxygen (21).
It is liquid in the common temperature of our atmosphere, assumes a solid state under the denomination of ice, in a cold temperature (32° of Fahrenheit’s thermometer); and, by heat at 212°, is converted into an elastic vapour of almost incredible force, called steam. At any temperature betwixt these two points, it returns, unaltered, to its liquid state. The weight of water is about 816 times greater than that of atmospheric air.
Water abounds in, and may be considered as, a kind of general cement to all solid bodies. It performs the most important functions both in the animal and vegetable kingdoms, and even enters largely into their composition.
A chief part of the nutrition of vegetables is the water which they absorb from the earth through the pores of their roots. The great quantity so absorbed may readily be imagined, when it is stated that the driest and most compact kinds of wood, such as even heart of oak, when converted into charcoal, lose, during the process, full three-fourths of their weight; and that the fluid which escapes is nearly pure water. This fluid is found in the driest of solid bodies, whatever be their description. A piece of hartshorn kept for forty years, and thereby become as hard and dry as metal (so that if struck against a flint it would give sparks of fire), upon being distilled, was found to yield an eighth part of its weight of water.
239Every being with life, in a great degree, lives by it; and whatever grows, through it receives its growth; and wherever it enters, it promotes and sustains life, preserving the whole of created nature in their proper classes of existence. And whether we consider it as productive of health to animals and vegetables, as requisite to the existence and beauty of the earth, or as one of the great powers by which the Almighty works in the support of the world, we cannot but admire and adore the wisdom by which it has been ordained.
In the various kinds of water, even of that which is commonly used in drinking, and for the preparation of food, there is great difference both of taste and appearance. This difference is chiefly occasioned by the foreign matters which they hold in solution or suspension. In some cases the quantity of these is so minute as to have but little influence on the taste; but in others they alter its properties altogether, and render the water noxious, or medicinal, or unfit for the preparation of food.
The chemical analysis of water, for the purpose of ascertaining the different substances which it holds in solution, is one of the most difficult and complicated operations that is known in this branch of science; and one that exercises, in a peculiar degree, both the skill and industry of the operator. The difficulty arises not only from the diversity of the bodies which occur, but from the very minute quantities of some of them.
These bodies are discovered by an addition to the water of certain substances, the consequence of which is some change in its appearance, and this change indicates the presence or absence of the bodies suspected.
The substances thus employed are very numerous, and have the name of tests. The methods of ascertaining the exact proportion of each of these ingredients are much too complicated to require a place in the present work.
240Water cannot be obtained in any state of perfect purity except by the artificial process of distillation.
274. RAIN WATER is considered to be next in purity to distilled water, from its having undergone a natural distillation. Its foreign contents vary according to the state of the air through which it falls. Hence, for instance, when it passes through the atmosphere of a smoky town, it becomes impure: and when collected in towns, it frequently acquires a small quantity of sulphat of lime (192), and calcareous matter from the mortar and plaster of the houses.
This water is always very soft; and is, consequently, well calculated for the dissolving of soap, in washing and other processes. It is also peculiarly adapted to the solution of alimentary or colouring matter, in the preparation of food and dyeing, and is accordingly used to great extent for these purposes. By the addition of a small quantity of a solution of barytes (195), it may be rendered sufficiently pure for all chemical uses.
If rain water be long kept, especially in hot climates, it acquires a disagreeable smell, and becomes putrid, and full of animalculæ.
275. ICE and SNOW WATER are equal to rain water in purity; and the air having been expelled from them during the process of freezing, they are consequently devoid of air when first melted.
Ice and snow, in their natural state, are highly important to mankind. It is a general law of nature that all bodies become more dense and heavy by exposure to cold; but the freezing of water is an exception to this law, and for a purpose of extreme benefit to mankind. By this ordination it is that ice always rises to 241the surface of the water, and thus preserves from the effects of the surrounding cold, a vast body of heat in the fluid beneath; and is itself ready to receive its own accustomed quantity upon the first change of the atmosphere. The expansion of water, in freezing, is owing to its assuming a crystallized form; and this expansion is often so great that glass bottles, filled with water, are burst by it.
During the intense cold of winter, snow, which is of a soft and spongy texture, is considered of great utility in preventing the immediate access of the atmospheric air to the ground; it has doubtless been designed by Providence as a garment to protect the incipient vegetation, at that inclement season, from injury.
The inhabitants of all the extreme northern parts of the world use thawed snow for their constant beverage during winter; and the vast masses of ice which float in the polar seas afford an abundant supply of fresh water to the navigators of those dreary regions. Snow water has, however, long lain under the imputation of occasioning those extraordinary swellings in the neck, which deform the inhabitants of some of the alpine valleys of Switzerland; but this opinion is not supported by any well-authenticated facts. Indeed it is rendered quite improbable by the frequency of this disease in the island of Sumatra, where ice and snow are never seen: and by its being quite unknown in Chili and Thibet, though the rivers of these countries are chiefly supplied by the melting of the snows.
276. SPRING WATER is nothing more than rain water, which having gradually filtered through the earth, collects at the bottom of declivities, and there makes its way to the surface.
It is obvious that spring water must be nearly as various in its contents as the substances through which it flows.
Ordinary springs pass insensibly into mineral springs, according as their foreign contents become more abundant; but it has not unfrequently happened that waters have acquired great medical reputation from their 242purity only. Although by far the greater number of springs are cold, some are hot, or at least are of a temperature which, at all times, exceeds that of summer heat: and this warmth is so little influenced by the state of the atmosphere, that it is nearly the same both in summer and winter.
The water of almost every spring is of such nature that it will not dissolve, but curdles, soap; and cannot be used for dressing several kinds of food. Water of this description is denominated hard, a property owing to the great proportion of earthy salts which it holds in solution, and which, at the same time, are not in such abundance as to impair its taste. The most common of these salts is selenite, or sulphat of lime (192), and chalk, or carbonat of lime (140); when it contains only the latter, the water is easily rendered soft by boiling, which expels the excess of carbonic acid (26), and thus causes the chalk to be precipitated. Hence originates the earthy crust or fur in such tea kettles as have had hard water several times boiled in them.
The water of deep wells is for the most part much harder than that of springs which overflow their channels.
277. RIVER WATER is a mixture of spring and rain water, which, from much agitation, and by long exposure to the air, in the course of its channel, becomes, in general, tolerably soft and free from earthy salts.
For washing, and other purposes of domestic economy, river water, from its softness and purity, is not only preferable to spring water, but also serves for many uses to which the latter cannot be at all applied. As a beverage, however, it is in general vapid and unpleasant.
The waters of some rivers, particularly where the beds, over which they flow, are sandy or stony, are remarkably pure. This is the case with several of those in Switzerland, Wales, Scotland, and the northern counties of England. The river Seine has great repute in France on this account: it has been found, on accurate examination, even more pure than Bristol water.
243That of the river Thames, impregnated as it would appear to be with putrid remains, is soft and good, when taken up at low water; and, after rest and filtration, is found to contain but a small portion of any thing either noxious or unpleasant. It is preferred, by mariners, to most other water for sea store; but it soon becomes putrid, and undergoes a remarkable spontaneous change. When, after having been kept a month or two, a cask is opened, a quantity of inflammable air escapes, and the water is black and nauseous. If, in this state, it be racked off into large earthen vessels (oil jars it is said are commonly used for the purpose), and exposed to the air, it gradually deposits a portion of black slimy mud, and becomes perfectly clear, sweet, and fit for use.
278. STAGNANT WATER contains greater impurities than any other. In ponds and marshes particularly, it is filled with the remains of animal and vegetable matters, which are there undergoing a gradual decomposition.
The water of lakes is not, in general, so much contaminated as this; but from the same cause, it frequently has a slimy appearance, a brownish colour, and an unpleasant taste.
From the putrefying contents of stagnant water, nutriment is afforded to various living plants and insects which there supply the place of those that perish. Its taste is vapid, unpleasant, and wholly destitute of that agreeable freshness which is found in spring water. It is, however, generally soft, and, by filtration, it may be freed from many of its impurities.
The air which issues from marshes and stagnant pools is extremely noxious, and is the cause of agues and other distressing complaints, to such persons as reside in the neighbourhood of them or are much exposed to them; and the injurious effects of such air have also been considered to extend to the internal use of these waters.
279. SEA WATER is a very heterogeneous compound, not only containing a considerable portion of saline substances, but holding also suspended in it an infinite number of minute animal and vegetable particles, to the gradual putrefaction of which its peculiarly nauseous and bitter taste, at the surface, is in some measure to be attributed.
The average quantity of salt in sea water is estimated to amount to about one-thirtieth part of its weight. It likewise contains a certain portion of muriat of magnesia, sulphat of magnesia or Epsom salts (199), and a small quantity of sulphat of lime (192). Sea water, taken from a great depth, has not the bitterness which the water of the surface has: it is only saline.
No natural waters, if we except certain brine springs and salt lakes, are so saline as those of the ocean; and the latter differ, in this respect, in different parts of the world. At the tropic, the sea is in general more salt than it is at the poles, a wise ordination to preserve it, in those climates, from the great tendency to putrefaction: and, at a considerable depth, it is always found more salt than at the surface. The water of the Baltic is much less salt than that of the Atlantic; and it is a remarkable circumstance, that its saline contents are increased by a west wind, but still more so by a gale from the north west.
Some philosophers have endeavoured, but to little purpose, to account, from second causes, for the saltiness of the ocean. Dr. Halley persuaded himself that it might have been gradually acquired, in very minute portions, by a deposit of salt washed down from the land by rivers, and that, as it could not be carried off by evaporation, instead of being diminished, it must be constantly increasing. But this idea of salting the sea 245with fresh water, is, to say the least of it, somewhat absurd, more particularly as it presumes that the sea was originally unimpregnated with salt. Had this been the case, the putrefaction of the immense mass of animal and vegetable substances which it gradually contained, would, in a short time, have proved fatal to the whole inhabitants of the earth.
The temperature of the sea, although it must necessarily vary in the different seasons, is much more uniform than that of any inland water exposed to the atmosphere. This is, in a great measure, attributable to its vast body of water, and the perpetual agitation to which it is exposed.
Sea water, when congealed by frost, is found to reject all, or nearly all, its saline particles; and consequently, when thawed, its ice yields water so fresh that it may be drunk without unpleasantness. The freezing of sea water is not unfrequently practised in the northern parts of the world, with a view to lessen the trouble and expense of extracting salt from it, for domestic and other uses (202). Salt water may likewise be rendered fresh and palatable by distillation, a mode which is now very generally practised at sea.
The sea shore has of late become so much frequented by invalids, for the purpose of bathing, that there is scarcely a fishing village, on the whole extent of our coast, but which is provided with some accommodation for bathers. As a cold bath, sea water is employed, with advantage, in all those cases of debility for which cold bathing has, in general, been recommended. It is also used as an external application in tumours and some other complaints; and, taken internally, as a remedy in various disorders.
It is to sea water that we are chiefly indebted for the salt which we use at table, and for all the purposes of domestic economy (202). From this water is also obtained those salts used in medicine, called Glauber’s (203) and Epsom salts (199).
280. MALVERN WATER is a simple cold water, perfectly bright and pellucid: it has an agreeable, and somewhat pungent flavour; but, in other respects, it does not differ in taste from pure and good soft water.
It contains carbonic acid (26), and a very small portion of earth, either lime or magnesia; but, the carbonic acid perhaps excepted, the foreign bodies are less in quantity than those even of our common spring water.
The spring from which this water principally issues is denominated the Holy Well: and is situated high up the hill, about midway between the villages of Great and Little Malvern, in Worcestershire.
Both as an external and internal application, the waters of Malvern have been considered beneficial in many obstinate complaints. It is a singular circumstance respecting them that, notwithstanding their apparent purity, if they be exposed to the air in an open vessel, they will soon acquire a fetid and unpleasant smell.
Malvern is principally frequented during the summer season.[5]
5. Adjoining to Great Malvern, and a little higher up the hill, there is a very light and pleasant chalybeate water.
281. BRISTOL HOT-WELL WATER is pure, warm, and slightly acidulated, clear, sparkling, and agreeable to the palate, but without any very decided taste. It is also destitute of smell. When poured into a glass it sends forth numerous air-bubbles. The heat of this spring is very moderate, the average 247being about 74° of Fahrenheit’s thermometer; and this heat does not sensibly vary during summer or winter.
The foreign contents of the hot-well water are muriat of magnesia, common salt, Glauber’s salt, sulphat of lime (192), and chalk: but these are in extremely small quantity. It also contains at the rate of about thirty cubic inches of carbonic acid gas, or fixed air (26), in every gallon.
This water springs from the bottom of the southern extremity of St. Vincent’s rock, a lofty cliff of limestone situated on the north bank of the river Avon, and about a mile below the city of Bristol. And, although it is considerably higher than the river, it is so far affected by the spring tides as to become, thereby, in some degree turbid. The discharge of water amounts to about forty gallons in a minute.
There is another spring at Clifton, on the summit of the same hill, from the bottom of which the waters of the hot-well issues. This is called the Sion spring, and is one or two degrees colder, but, in other respects, it very nearly resembles the water of the hot-well.
Its discovery was somewhat remarkable. A Mr. Morgan, an attorney of Bristol, having erected a house near the spot, sunk a well for the supply of his family with water. The workmen had proceeded to the depth of nearly 240 feet without success, when they were suddenly alarmed by the gushing forth of such an abundance of water that they were compelled to retreat with precipitation. The proprietor was so far disappointed of his hopes as to find that this was a spring of warm instead of cold water. But the circumstance induced him to erect an engine to raise the water for medicinal purposes; and, since that period, a pump room and bathing houses have been prepared for the accommodation of visitors.
The water of each of these springs, besides being used medicinally in pulmonary consumptions and other complaints, is employed very extensively at table, and for all domestic purposes. It is remarkable for softness and purity; and, from its quality of continuing untainted 248for a great length of time, even in hot climates, is a valuable water for long voyages, and is accordingly exported in considerable quantities to distant parts.
The season of general resort to Clifton and the hot-wells is from about the middle of April to the end of October.
282. MATLOCK WATER is a simple warm water, which, in its sensible properties, exclusive of its temperature, which is only about 66° of Fahrenheit, is scarcely different from good spring water. It is beautifully clear, and exhales no steam, except in very cold weather.
The medicinal virtues of this water have chiefly been ascribed to its temperature. Its supply is very copious, and from several different sources. Though recommended in some internal complaints, it is principally employed as a bath; and, in this respect, it forms a medium betwixt the waters of Bath or Buxton and those of the generality of cold baths.
Matlock, which is a beautifully romantic village, situated in a hilly part of Derbyshire, and at the distance of 143 miles north of London, was first brought into public notice about the year 1698, shortly after which period the first bath was erected. It is chiefly frequented from the month of May to that of October; or, if the weather continue fine, till near the beginning of November.
283. BUXTON WATER is a simple warm water, which contains so little foreign matter, as scarcely to be distinguishable from common spring water heated to the same temperature. It has neither smell nor taste; and, though it sparkles a little in the glass, when first drawn, this is not apparently more than what is observable in the water of many common springs.
Its temperature, in the bath called the Gentleman’s bath, is invariably 82°.
Buxton has been celebrated, for its warm springs, nearly two centuries and a half. As early as the year 1572 a treatise on their virtues was published: this states them to have been at that time much resorted to 249by persons from all the adjacent counties. The water is employed both externally and internally, and to great extent. Its principal value, as a bath, arises from its very copious supply, its purity, and its high temperature. The sensation which is felt from bathing in it is considered to be such as would be experienced from any bath heated to the highest temperature which is compatible with giving some sensation of cold when the body is first plunged into it. This water is also used as an internal medicine; and is frequently used by the inhabitants as their common beverage, and for such domestic purposes as its hardness will admit.
There are several springs and several distinct baths; but the original and most ancient of them is called St. Ann’s Well, and is enclosed in an elegant stone building. These waters are frequented by persons afflicted with the rheumatism, gout, diseases of the alimentary organs, and kidneys, and various other complaints: and the chief influx of company is during the summer and autumnal months.
The situation of Buxton is in a narrow and funnel-shaped valley, surrounded by wild, bleak, and dreary mountains, in the midst of the county of Derby, and about 160 miles north of London.
284. BATH WATER is a hot carbonated chalybeate. When first drawn, it appears clear and colourless, nor does it afford any signs of briskness or effervescence. The temperature of the water drawn from the King’s Bath, which is that usually employed for drinking, is 116° of Fahrenheit, and that of the Cross Bath is 112°. No odour whatever is perceptible from a glass of fresh water; but from a large body of it the nose is affected by a slight degree of pungency. When the water is hot from the pump, it fills the mouth with a strong chalybeate impression without any pungency, and accompanied with scarcely any kind of saline taste; and, what is remarkable, as soon as the water cools, the chalybeate taste is entirely lost, and nothing but an extremely slight saline sensation remains upon the palate.
The foreign contents of Bath water are sulphat of lime (192), 250chalk, Glauber’s salt (203), and common salt; together with a very small portion of oxide of iron (21), yet sufficient to give iron mould stains to the linen of the bathers. The water curdles soap, and is so hard as to be unfit for many domestic purposes.
The city of Bath has been celebrated for its hot springs even from the time of the Romans. These are of higher temperature than any within the British dominions; and indeed are the only natural waters which we possess that are at all hot to the touch, the other thermal waters being of heat below the animal temperature.
There are three principal sources of these waters, called the King’s Bath, the Cross Bath, and the Hot Bath; and they differ slightly in their properties. The springs arise within a short distance of each other, at the lower part of the city; and yield so copious a supply that all the large reservoirs used for bathing are filled every evening with water fresh from their respective fountains.
The application of the water externally is either general or local. The latter consists in pumping it for a considerable time on the part affected. This is called dry pumping, because in it only one part of the body is wetted, whilst the rest is kept dry; and in many cases, it is found an excellent remedy.
The diseases for which these waters are resorted to are very numerous, and are amongst the most important and difficult to be cured that come under medical treatment.
285. AIX-LA-CHAPELLE or AKEN WATER, is an alkaline sulphureous water, much hotter than that of any of the springs in England, varying in temperature in the different baths from 112° to 143°. It has a saline, bitterish, and somewhat alkaline taste; and its smell precisely resembles, but is greatly more powerful than, that of Harrowgate water (299).
It contains a small quantity of chalk, common salt, and carbonat of soda (201), the latter of which renders it soapy to the touch. But the most striking feature in this water is 251the unusual quantity of sulphur which it contains; and which is so extremely volatile on the application of heat, that none of it is left in the residuum after evaporation. In this water there is also a considerable portion of carbonic acid.
The city of Aix-la-Chapelle is in the circle of Westphalia, betwixt the rivers Meuse and Rhine, about seventy miles east of Brussels, and in a rich and fertile country. Its waters have been in great medical repute, and have attracted a numerous concourse of visitants for many centuries past. Their reputation was so well established, even in the time of Charlemagne, that he frequently resided at Aix: and he is said to have been so much delighted in the use of the waters as to have sometimes even held his levee at the baths.
In this city, and in the small territory that belongs to it, there are several sources of hot water. Of these the principal spring is enclosed in a stone cistern, which is vaulted and almost conical at the top, and the parts of which are connected with the utmost care, to prevent the vapour from escaping. From this spring the water flows, in a copious stream, into several spacious and elegant baths, in the different parts of the city, distinguished by the names of the Emperor’s Bath, the Nobles’ Bath, the Poor’s Bath, and other appellations. In most of these there is every necessary apparatus for bathing by immersion, for vapour bathing, and for pumping on any particular parts of the body.
The water rises, with great quickness, from the springs, and sends forth bubbles of air, which burst with a slight noise when they reach the surface. It is at first perfectly colourless and pellucid, and emits a large portion of steam, and with it a strong sulphureous smell, which is perceptible at a great distance.
Its temperature is so high, that, in the large baths, it requires to stand from fifteen to eighteen hours before it is sufficiently cooled for tepid bathing; and it is one of the few natural springs which are hot enough to be employed as a vapour bath without the addition of artificial heat. On standing to cool, it gradually loses 252its clearness, acquires a milky hue, and deposits an earthy sediment, which is entirely calcareous. At the same time it loses its offensive smell, and, when cold, has scarcely any odour.
Wherever a large quantity of this water passes hot from the spring through a confined place, the upper covering becomes encrusted with sulphur. This is particularly the case with respect to the dome of the vault that encloses the great source which supplies the Emperor’s bath, and which is opened, from time to time, for the purpose of having the sulphur brushed off.
From the waters of Aix-la-Chapelle, though only internally used, the body acquires a sulphureous smell; and even silver worn in the pocket becomes tarnished.
286. BORSET WATER is of two kinds. One of these resembles the water of Aix in every respect, except as to the impregnation of sulphur, which is much weaker: its temperature is 132°. The other contains no sulphur: it is, however, equally alkaline, and the heat is as high as 152°, which much exceeds the hottest of the waters of Aix.
Both these waters are used by fullers and cloth-workers, on account of the convenience they afford, without expense, of a sufficiency of hot and somewhat alkaline fluid which is well adapted for the cleansing of woollen cloth.
In the latter of the above-mentioned springs a large portion of earth is suspended. This, as the water cools, is deposited, and forms hard incrustations of considerable thickness round every substance with which it comes in contact. It is not, however, on this account found less useful for the scouring of cloth, boiling of vegetables for the table, or any of those domestic purposes for which soft water is required.
In this spring there is a considerable portion of carbonic acid gas, or fixed air (26), which is continually escaping from the fresh water, and is in sufficient quantity to corrode, in a short time, the leaden covering that is used for the vapour baths, and any iron within its reach.
253After having supplied several baths, the stream flows into a large fish pond, where it is still of blood heat. In this pond we are informed that carp and tench multiply very fast, and grow to an enormous size; but that their flesh is soft and without flavour, until they have been removed, for about six months, into a pond of cold water, where they become perfectly firm and good for the table.
In their medicinal application these waters are chiefly employed externally, and their great heat allows of every convenience for vapour, hot, warm, and tepid bathing. The village of Borset, or Bordscheit, in which they are found, is situated about a quarter of a mile south of Aix-la-Chapelle (285).
287. THE VICHY WATERS are hot, saline, and chalybeate. They vary in some degree in the different springs, have a salt and somewhat bitter taste, and a considerable pungency of smell. They are alkaline, and about the temperature of 120°.
There are, at Vichy, a small town on the banks of the river Allier, about 180 miles south-east of Paris, no fewer than six different springs of hot water, which vary somewhat in their temperature, and in the proportion of their foreign contents. The valley in which this town is situated is highly fertile and beautiful, and abounds in vineyards and fruit-trees.
It is remarkable that sheep, cows, and other animals, crowd to drink this water with great eagerness, and even to lick the stones and sides of the channel through which it flows. Their partiality for it is so great that, at certain times, they are known to swim across the river Allier, in considerable numbers together, without even tasting of that water, and to proceed, without interruption onward, until they reach this their favourite beverage.
254288. CARLSBAD WATER is hot, saline, and chalybeate, having an unpleasant alkaline and bitter taste, though scarcely any smell. Its constant temperature is 165°. It contains chalk, Glauber’s salt (203), common salt, and carbonat of soda (201), together with a small portion of iron; and carbonic acid gas, or fixed air (26), in considerable quantity.
The town of Carlsbad, situated on the river Eger, in Bohemia, and its springs (which have the name of Caroline baths), received their appellation from the Emperor Charles the Fourth, who is said to have himself discovered the latter, in the year 1370, whilst hunting; and, since that period, few waters have more engaged the attention of chemists and physicians than these. Carlsbad is now much frequented during the summer months, and has good accommodations as a watering place. Its water is remarkable for a rapid and copious deposition of calcareous earth, which takes place always on cooling, and forms a very hard and beautiful crust on the inner surface or tube of any channel through which it flows; and forms petrifactions round moss, pieces of straw, or other extraneous substances which are put into the stream, even for so short a time as twenty-four hours. All the iron which the fresh water contains is also precipitated by cooling, and rather sooner than the calcareous earth. A very fine laminated calcareous stone in variegated colours is thus formed in large masses around the channel of the stream, which, when polished, is almost equal in beauty to jasper.
Of the hot springs of this neighbourhood the principal is called the Sprudel. It boils up, with great violence, and discharges about 352 cubic feet of water hourly, through a curious natural vault or incrustation which it has gradually formed. This water supplies the greater number of the baths. The other springs are, in general, of much lower temperature: they do not exceed from 114° to 125°, and they differ somewhat from each other in their chemical properties. They all contain 255a large portion of carbonic acid gas, or fixed air, and this is given out in such quantity by the water, that it fills several caverns, in the rocks adjoining to the springs, rendering them fatal to all animals which incautiously enter them.
The waters of Carlsbad are used for the removal of a great variety of disorders, but particularly such as are connected with indigestion. They are likewise used in obstructions of the bowels, and diseases of the kidneys. About five pints, divided into fourteen portions, are, on an average, drunk by each individual every day.
The Sprudel spring is better than that of any mineral waters which are employed medicinally. It requires to be considerably cooled before it can either be used as a bath, or drunk. Its heat is such that it is occasionally employed, in place of water artificially heated, for several domestic purposes, such as the scalding of fowls and hogs, the feathers and hair of which it immediately loosens.
Several hundred pounds weight of Glauber’s salt are annually prepared from this water.
289. SEDLITZ WATER is very salty and bitter. It contains a small portion of chalk, some sulphat of lime (192), carbonat of magnesia, muriat of magnesia, and a very great proportion of Epsom salt (199), to which its bitter taste and medicinal virtues are principally attributed.
The spring for which the village of Sedlitz, in Bohemia, has long been celebrated, was, for many years, wholly neglected by the inhabitants, on account of the bitter and nauseous taste of its water, which rendered it unfit for nearly all domestic purposes. Its virtues, as a medicine, were first brought into notice about the year 1721, by Hoffman, the celebrated Prussian physician.
This water is considered a valuable remedy in cases of indigestion, for removing scorbutic humours, and in several other complaints.
256290. EPSOM WATER is saline, and partakes, in some degree, of the nature and qualities of Sedlitz water, but it is by no means so powerful. It is transparent and colourless; and, when first taken into the mouth, has scarcely any taste, but it leaves a decidedly bitter and saltish taste on the palate.
This water contains sulphat of magnesia, or Epsom salt (199), selenite, and a small portion of chalk.
Although the Epsom waters, on account of their deficiency of strength, are now scarcely ever employed in medicine, yet they were among the first saline cathartic springs which were brought into use in this country. The salt to which they owe their property, and which is known throughout Europe by the name of Epsom salt, was, for many years, prepared almost exclusively from them and from Sedlitz water. But the quantity which they supplied was found so very inadequate to the increasing demands for this salt in medicine, that Epsom Salt has, for some time past, been manufactured from sea water.
Epsom water, if closely corked, may be kept for several months without injury: but, otherwise, it soon becomes putrid. The spring from which it issues is situated about half a mile from the town of Epsom in Surrey, sixteen miles south of London.
There are, in the neighbourhood of London, many springs of similar quality to this of Epsom: of these the principal are at Acton, Kilburne, Bagnigge Wells, and formerly the Dog and Duck in St. George’s Fields; but they are, in general, so weak as to render very large quantities of the water necessary to produce any sufficient medical effect.
291. SELTZER WATER is an highly carbonated alkaline water. When fresh, or well preserved, it is perfectly clear, and sparkles much when poured into a glass. It is somewhat pungent, slightly saline, and a little alkaline to the taste.
It contains chalk, carbonat of magnesia, carbonat of soda (201), and common salt; and more carbonic acid gas, or fixed
257air (26), than any water hitherto known. It is hard, and curdles with soap.
The spring which supplies this water is situated in Nieder Seltzer, a village in a fine woody country, within the bishopric of Treves; and there are few mineral springs which have acquired so much celebrity for medical virtues as this. The diseases, for the removal of which it has been successfully applied, are too numerous to be here particularized.
To the taste it is very agreeable, and when drunk in moderate quantity, it exhilarates the spirits, increases the appetite, and produces no particular determination to the bowels. It is to the strong impregnation with carbonic acid, and the small proportion of soda which it contains, that its most important benefits are owing.
If it be closely corked and sealed, Seltzer water may be kept without injury, or even alteration, for a very considerable time; but, if exposed to the air, it soon becomes fetid. It is used as a common drink at table in many parts of Germany and Holland, and is even brought into England in stone bottles, each containing about three pints. A large proportion of Seltzer water, either genuine or artificial, is consumed in this country.
292. Are such as contain a portion of iron. This is easily detected by the property which it has of striking a black colour with tincture of nutgalls.
293. TUNBRIDGE WATER is a carbonated chalybeate, the small portion of iron which it contains being held in solution by carbonic acid (26). It is, however, neither brisk nor acidulous. To the taste it is simply chalybeate; and that only in a slight degree.
Its foreign contents are oxide of iron (21), a small portion of common salt, muriat of magnesia, and sulphat of lime (192), carbonic acid gas or fixed air (26), and other gases, but these only in small quantity.
258Tunbridge Wells is a populous village, situated in a sandy but romantic valley in the county of Kent, about five miles from the town of Tunbridge, and thirty-six miles south of London. There are, at this place, many chalybeate springs, all of which nearly resemble each other in their chemical properties. Two of them, however, are chiefly used, each of which yields about a gallon of water in a minute.
When first taken from the stone basin into which it flows, the water is perfectly clear and bright, and exhales no particular smell. It does not sparkle in the glass, but a few bubbles slowly separate, and adhere to the sides of the vessel. When it has stood for some hours exposed to the air, it becomes turbid, and otherwise undergoes a very material change. As it does not properly curdle soap, it may be denominated a soft water.
The original discovery of this water, as to its medical properties, is usually considered to have been in the reign of James the First. The season for drinking it commences as early as March or April, and continues till November.
294. SPA WATER, the celebrity of which has given a general appellation to most other mineral springs, is a highly carbonated chalybeate water, which contains a great proportion of carbonic acid (26). It has an agreeable acidulous taste, mixed with a strong impression of chalybeate, which remains on the palate for a considerable time after it has been drunk.
It contains oxide of iron (21), chalk, carbonat of magnesia, carbonat of soda (201), and common salt, together with about forty-five parts in a hundred of carbonic acid gas or fixed air (26); and is sufficiently soft to mix both with milk and soap without curdling.
Spa is a small but celebrated town in the Netherlands. It is situated on the little river Weze, about twenty miles south-east of Liege, and seven miles south-west of Linsburg; and is surrounded by rude and uncultivated 259mountains, many of which are covered with wood, and others with heath or morasses. In its neighbourhood there are no fewer than sixteen mineral springs, five of which are more celebrated than the others. The most copious and most frequented of the whole is the Pouhon spring, in the market-place of Spa. This is a large, slow, and deep spring, the descent to which is by several steps. In cold dry weather the water, when first taken up, appears colourless and perfectly transparent: it scarcely sparkles, but it soon covers the inside of the glass with small air-bubbles, which it also emits very copiously when shaken. During moist weather the surface of the well appears somewhat turbid: and, on the approach of rain, a whistling or humming noise is heard, which is called by the country people the music of the spring.
If this water be bottled, and then set in a warm place, it will generally force out the cork, with a loud explosive noise. In preserving it for exportation, it is consequently necessary to wire the corks firmly down. In this state, if well cemented, it may be kept perfectly good for more than two years.
It is somewhat remarkable, respecting this water, that if it be taken in a full draught, particularly in hot weather, or upon an empty stomach, it produces a swimming in the head, and a degree of intoxication, which frequently continues for half an hour or upwards, and is very similar to that which arises from the drinking of spirituous liquor, but it does not leave the same debility.
295. PYRMONT WATER is a highly carbonated chalybeate. When recently taken from the spring, it is clear and pellucid, and sends forth a copious stream of bubbles for a considerable time. In this respect it far exceeds any of the mineral waters with which we are acquainted. Its taste is pleasant, being strongly acidulated, and having a pungency not unlike that of brisk Champagne wine; but it is at the same time strongly chalybeate, and a little bitterish.
260It chiefly contains oxide of iron (21), chalk, carbonat of magnesia, Epsom salt (199), sulphat of lime (193), and common salt, and a great proportion of carbonic acid gas, or fixed air. It is very hard.
Pyrmont is a town of Westphalia, and about thirty-eight miles south-west of Hanover. It is the capital of a county, has a strong fort, and is well known on account of its mineral springs.
The water which issues from these springs constantly emits so large a quantity of gas as to have a sensible pungency of smell to those who stand around, and even to make the water-servers giddy. It forms an atmosphere over the surface of the well which proves fatal to ducks and small birds that attempt to swim across. The gas contained in the water is estimated to be nearly equal in bulk to the water. It is owing to this, that Pyrmont water, if bottled and well corked, and afterwards removed into a warm place, will frequently burst the bottles.
When drawn fresh from the spring and drunk copiously, it produces a temporary kind of intoxication. It also enlivens the spirits and increases the appetite. This water is sent in bottles, by the Weser, to Bremen, whence it is exported to various parts of the world.
296. CHELTENHAM WATER is a saline, carbonated, chalybeate, which has a slight sulphureous smell, and a brackish, somewhat bitter, and chalybeate taste, but no briskness nor pungency.
Its foreign contents are Glauber’s salts (203), muriat and carbonat of magnesia, common salt, and oxide of iron, together with a portion of carbonic acid gas, and some other kinds of gas.
The original discovery of the mineral spring at Cheltenham was about the year 1716. The water of this spring issues slowly, and in a scanty stream of not more than 35 pints in an hour, from a bed of sand intermixed with blue clay. The well is sunk to the depth of six feet, and is excluded from communication 261with the external air. This spring is denominated the Old Spa.
In the year 1788, on digging a well for a private house, another spring was accidentally discovered, which is of nearly the same nature as this, and produces a much more abundant supply of water. It is about a hundred yards distant from it, is upwards of forty feet deep, and is drawn by a pump.
When Cheltenham water is fresh drawn, it appears tolerably clear, though not perfectly transparent. After standing some time, it becomes more turbid, and air-bubbles, in small quantity, rise from it. It contains more salt than perhaps any other waters, except those of the sea and some brine springs; and by far the greatest part of the salts are of a purgative kind. It is also a very strong chalybeate, and has a slight impregnation of sulphur.
This water cannot long be kept, nor can it be transported to any distance without being materially altered. In order, however, to reduce its valuable parts to a more convenient form, for carriage and keeping, the salts are extracted from it on the spot, by evaporation, and crystallizing the residuum. These salts are much used, in addition to the fresh water, for the purpose of increasing its operation on the bowels.
Cheltenham is a small town in the county of Gloucester. It is about ninety-five miles north-west of London, situated in a sandy vale, surrounded with hills of moderate height, and in the midst of a fertile and well cultivated country.
297. BRIGHTON CHALYBEATE WATER is a vitriolated chalybeate, which, when fresh, has a peculiar and faint smell not uncommon in ferruginous waters, and a strong though not unpleasant chalybeate taste.
It contains sulphat of iron or vitriol (208), sulphat of lime (182), common salt, muriat of magnesia, siliceous earth, and a certain portion of carbonic acid gas (26).
262Brighton is a well-known market-town, situated on the coast of Sussex, and about fifty-four miles south of London. The chalybeate spring is at Wick, on the declivity of a small eminence nearly a mile west of the town, and a quarter of a mile from the sea. A small but neat building has been erected immediately over the spot from which the water issues, and where it is received, a few feet under ground, into a basin of Portland stone. This reservoir contains only a few gallons of water, but it fills again almost as soon as it is emptied.
The water is so hard as instantly to curdle soap. It is considered useful in cases of debility, indigestion, and such diseases for which chalybeate and tonic remedies are required. The sea-bathing at Brighton is, in many cases, an additional and important advantage to those persons who use the chalybeate water.
298. HEPATIC, or SULPHUREOUS WATERS, are so strongly impregnated with sulphur, united either to hydrogen (45), or to an alkali, or both, as thereby to acquire a very sensible smell and taste. They have the property of blacking silver and lead; and are immediately known by the smell, which is very fetid, and like that which arises from the scouring of a foul gun-barrel, or, as some persons suppose, like the smell of rotten eggs.
The taste of these waters is peculiar, and rather sweetish. They constitute a drink which, at first, is very unpalatable, but which, by habit, is soon reconciled to the drinker. None of them will bear carriage to any distance.
299. HARROWGATE WATER is a cold sulphureous water, which has a very strong and fetid smell, like that of a damp rusty gun-barrel. To the taste it is bitter, nauseous, and strongly saline.
Its foreign contents are common salt, muriat of lime, muriat of magnesia, chalk, carbonat of magnesia, Epsom salt (199), carbonic acid gas, or fixed air (26), azotic gas, and sulphureted hydrogen gas.
263There are, at Harrowgate, four distinct sulphureous springs, which appear to have their rise in a large bog, at a small distance from the wells. The water of all these springs is similar in its properties and its distinguishing characters, but as one of them is more strongly impregnated with sulphur than the others, this alone is used for drinking, whilst the other three are employed to supply the baths.
When the water of the former of these springs is first taken up, it is perfectly clear and transparent; and sends forth a few air-bubbles. Notwithstanding both its nauseous smell and taste, such is the power of habit in reconciling it to the palate, that, after a little while, nearly all persons who drink this water do it without disgust.
When exposed to the air it loses its transparency, and assumes a somewhat greenish colour: the sulphureous odour abates; and, at last, the sulphur is deposited on the bottom and sides of the vessel in which it is kept.
Such is the nature of Harrowgate water that a secret correspondence has often been carried on by means of it. A letter written with solution of sugar of lead is illegible; but if dipped into this water the writing will not merely become visible, but, in a short time, will appear almost black. Hydrogen has the property of reviving the metallic oxides: hence also it is that ladies who have used metallic cosmetics have become of a dark tawny colour by bathing in these waters.
Harrowgate has long been celebrated for its sulphureous waters. It has also two very valuable chalybeate springs, called the Old Spa, and the Tewit Well, the water of which was formerly used internally, whilst the other water was confined to external use. But, at present, the latter is employed to very great extent as an internal medicine.
The two villages of High and Low Harrowgate are situated in a pleasant open country, in the centre of 264the county of York, near the town of Knaresborough, and about 212 miles north of London.
300. MOFFAT WATER is a cold sulphureous water, the smell of which is precisely similar to that of Harrowgate water, and the taste simply saline, and without any bitterness.
Its foreign contents are common salt, together with carbonic acid gas, azotic gas, and sulphureted hydrogen gas. It is consequently very simple in its composition.
Moffat is a village situated, at the head of a valley, on the banks of the river Annan, and about fifty-six miles south-west of Edinburgh. It is surrounded by hills, some of which are very lofty. This village has obtained so much celebrity, on account of its waters, as to be considered the Harrowgate of North Britain. These issue from a rock which is at a little distance below a bog, whence, probably, they derive their sulphureous ingredients. The principal spring is contained within a stone building, and affords a sufficient quantity of water to supply every demand. It is drawn by a pump.
When the water is first taken from the well it appears somewhat milky and bluish. It sparkles a little; but, on being exposed to the air, it becomes turbid, and throws up a thin film, which, on examination, will be found pure sulphur. This change takes place even in close vessels, so that it cannot be sent to any distance with advantage.
The common people so much esteem this water that many of them drink at the rate of from six to ten quarts of it in a morning, and one instance has been stated of a person drinking thirty-two quarts of it in eight hours.
End of the Project Gutenberg EBook of Useful Knowledge: Minerals. Volume 1
(of 3)., by William Bingley
*** END OF THIS PROJECT GUTENBERG EBOOK USEFUL KNOWLEDGE: MINERALS, VOL 1 ***
***** This file should be named 57898-h.htm or 57898-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/5/7/8/9/57898/
Produced by Chris Curnow, Barry Abrahamsen, and the Online
Distributed Proofreading Team at http://www.pgdp.net (This
file was produced from images generously made available
by The Internet Archive)
Updated editions will replace the previous one--the old editions will
be renamed.
Creating the works from print editions not protected by U.S. copyright
law means that no one owns a United States copyright in these works,
so the Foundation (and you!) can copy and distribute it in the United
States without permission and without paying copyright
royalties. Special rules, set forth in the General Terms of Use part
of this license, apply to copying and distributing Project
Gutenberg-tm electronic works to protect the PROJECT GUTENBERG-tm
concept and trademark. Project Gutenberg is a registered trademark,
and may not be used if you charge for the eBooks, unless you receive
specific permission. If you do not charge anything for copies of this
eBook, complying with the rules is very easy. You may use this eBook
for nearly any purpose such as creation of derivative works, reports,
performances and research. They may be modified and printed and given
away--you may do practically ANYTHING in the United States with eBooks
not protected by U.S. copyright law. Redistribution is subject to the
trademark license, especially commercial redistribution.
START: FULL LICENSE
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full
Project Gutenberg-tm License available with this file or online at
www.gutenberg.org/license.
Section 1. General Terms of Use and Redistributing Project
Gutenberg-tm electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or
destroy all copies of Project Gutenberg-tm electronic works in your
possession. If you paid a fee for obtaining a copy of or access to a
Project Gutenberg-tm electronic work and you do not agree to be bound
by the terms of this agreement, you may obtain a refund from the
person or entity to whom you paid the fee as set forth in paragraph
1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this
agreement and help preserve free future access to Project Gutenberg-tm
electronic works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the
Foundation" or PGLAF), owns a compilation copyright in the collection
of Project Gutenberg-tm electronic works. Nearly all the individual
works in the collection are in the public domain in the United
States. If an individual work is unprotected by copyright law in the
United States and you are located in the United States, we do not
claim a right to prevent you from copying, distributing, performing,
displaying or creating derivative works based on the work as long as
all references to Project Gutenberg are removed. Of course, we hope
that you will support the Project Gutenberg-tm mission of promoting
free access to electronic works by freely sharing Project Gutenberg-tm
works in compliance with the terms of this agreement for keeping the
Project Gutenberg-tm name associated with the work. You can easily
comply with the terms of this agreement by keeping this work in the
same format with its attached full Project Gutenberg-tm License when
you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are
in a constant state of change. If you are outside the United States,
check the laws of your country in addition to the terms of this
agreement before downloading, copying, displaying, performing,
distributing or creating derivative works based on this work or any
other Project Gutenberg-tm work. The Foundation makes no
representations concerning the copyright status of any work in any
country outside the United States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other
immediate access to, the full Project Gutenberg-tm License must appear
prominently whenever any copy of a Project Gutenberg-tm work (any work
on which the phrase "Project Gutenberg" appears, or with which the
phrase "Project Gutenberg" is associated) is accessed, displayed,
performed, viewed, copied or distributed:
This eBook is for the use of anyone anywhere in the United States and
most other parts of the world at no cost and with almost no
restrictions whatsoever. You may copy it, give it away or re-use it
under the terms of the Project Gutenberg License included with this
eBook or online at www.gutenberg.org. If you are not located in the
United States, you'll have to check the laws of the country where you
are located before using this ebook.
1.E.2. If an individual Project Gutenberg-tm electronic work is
derived from texts not protected by U.S. copyright law (does not
contain a notice indicating that it is posted with permission of the
copyright holder), the work can be copied and distributed to anyone in
the United States without paying any fees or charges. If you are
redistributing or providing access to a work with the phrase "Project
Gutenberg" associated with or appearing on the work, you must comply
either with the requirements of paragraphs 1.E.1 through 1.E.7 or
obtain permission for the use of the work and the Project Gutenberg-tm
trademark as set forth in paragraphs 1.E.8 or 1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any
additional terms imposed by the copyright holder. Additional terms
will be linked to the Project Gutenberg-tm License for all works
posted with the permission of the copyright holder found at the
beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including
any word processing or hypertext form. However, if you provide access
to or distribute copies of a Project Gutenberg-tm work in a format
other than "Plain Vanilla ASCII" or other format used in the official
version posted on the official Project Gutenberg-tm web site
(www.gutenberg.org), you must, at no additional cost, fee or expense
to the user, provide a copy, a means of exporting a copy, or a means
of obtaining a copy upon request, of the work in its original "Plain
Vanilla ASCII" or other form. Any alternate format must include the
full Project Gutenberg-tm License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works
provided that
* You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is owed
to the owner of the Project Gutenberg-tm trademark, but he has
agreed to donate royalties under this paragraph to the Project
Gutenberg Literary Archive Foundation. Royalty payments must be paid
within 60 days following each date on which you prepare (or are
legally required to prepare) your periodic tax returns. Royalty
payments should be clearly marked as such and sent to the Project
Gutenberg Literary Archive Foundation at the address specified in
Section 4, "Information about donations to the Project Gutenberg
Literary Archive Foundation."
* You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or destroy all
copies of the works possessed in a physical medium and discontinue
all use of and all access to other copies of Project Gutenberg-tm
works.
* You provide, in accordance with paragraph 1.F.3, a full refund of
any money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days of
receipt of the work.
* You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project
Gutenberg-tm electronic work or group of works on different terms than
are set forth in this agreement, you must obtain permission in writing
from both the Project Gutenberg Literary Archive Foundation and The
Project Gutenberg Trademark LLC, the owner of the Project Gutenberg-tm
trademark. Contact the Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
works not protected by U.S. copyright law in creating the Project
Gutenberg-tm collection. Despite these efforts, Project Gutenberg-tm
electronic works, and the medium on which they may be stored, may
contain "Defects," such as, but not limited to, incomplete, inaccurate
or corrupt data, transcription errors, a copyright or other
intellectual property infringement, a defective or damaged disk or
other medium, a computer virus, or computer codes that damage or
cannot be read by your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH 1.F.3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium
with your written explanation. The person or entity that provided you
with the defective work may elect to provide a replacement copy in
lieu of a refund. If you received the work electronically, the person
or entity providing it to you may choose to give you a second
opportunity to receive the work electronically in lieu of a refund. If
the second copy is also defective, you may demand a refund in writing
without further opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS', WITH NO
OTHER WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT
LIMITED TO WARRANTIES OF MERCHANTABILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of
damages. If any disclaimer or limitation set forth in this agreement
violates the law of the state applicable to this agreement, the
agreement shall be interpreted to make the maximum disclaimer or
limitation permitted by the applicable state law. The invalidity or
unenforceability of any provision of this agreement shall not void the
remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in
accordance with this agreement, and any volunteers associated with the
production, promotion and distribution of Project Gutenberg-tm
electronic works, harmless from all liability, costs and expenses,
including legal fees, that arise directly or indirectly from any of
the following which you do or cause to occur: (a) distribution of this
or any Project Gutenberg-tm work, (b) alteration, modification, or
additions or deletions to any Project Gutenberg-tm work, and (c) any
Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of
computers including obsolete, old, middle-aged and new computers. It
exists because of the efforts of hundreds of volunteers and donations
from people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need are critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future
generations. To learn more about the Project Gutenberg Literary
Archive Foundation and how your efforts and donations can help, see
Sections 3 and 4 and the Foundation information page at
www.gutenberg.org
Section 3. Information about the Project Gutenberg Literary Archive Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Contributions to the Project Gutenberg Literary
Archive Foundation are tax deductible to the full extent permitted by
U.S. federal laws and your state's laws.
The Foundation's principal office is in Fairbanks, Alaska, with the
mailing address: PO Box 750175, Fairbanks, AK 99775, but its
volunteers and employees are scattered throughout numerous
locations. Its business office is located at 809 North 1500 West, Salt
Lake City, UT 84116, (801) 596-1887. Email contact links and up to
date contact information can be found at the Foundation's web site and
official page at www.gutenberg.org/contact
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
[email protected]
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To SEND
DONATIONS or determine the status of compliance for any particular
state visit www.gutenberg.org/donate
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including checks, online payments and credit card donations. To
donate, please visit: www.gutenberg.org/donate
Section 5. General Information About Project Gutenberg-tm electronic works.
Professor Michael S. Hart was the originator of the Project
Gutenberg-tm concept of a library of electronic works that could be
freely shared with anyone. For forty years, he produced and
distributed Project Gutenberg-tm eBooks with only a loose network of
volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as not protected by copyright in
the U.S. unless a copyright notice is included. Thus, we do not
necessarily keep eBooks in compliance with any particular paper
edition.
Most people start at our Web site which has the main PG search
facility: www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.