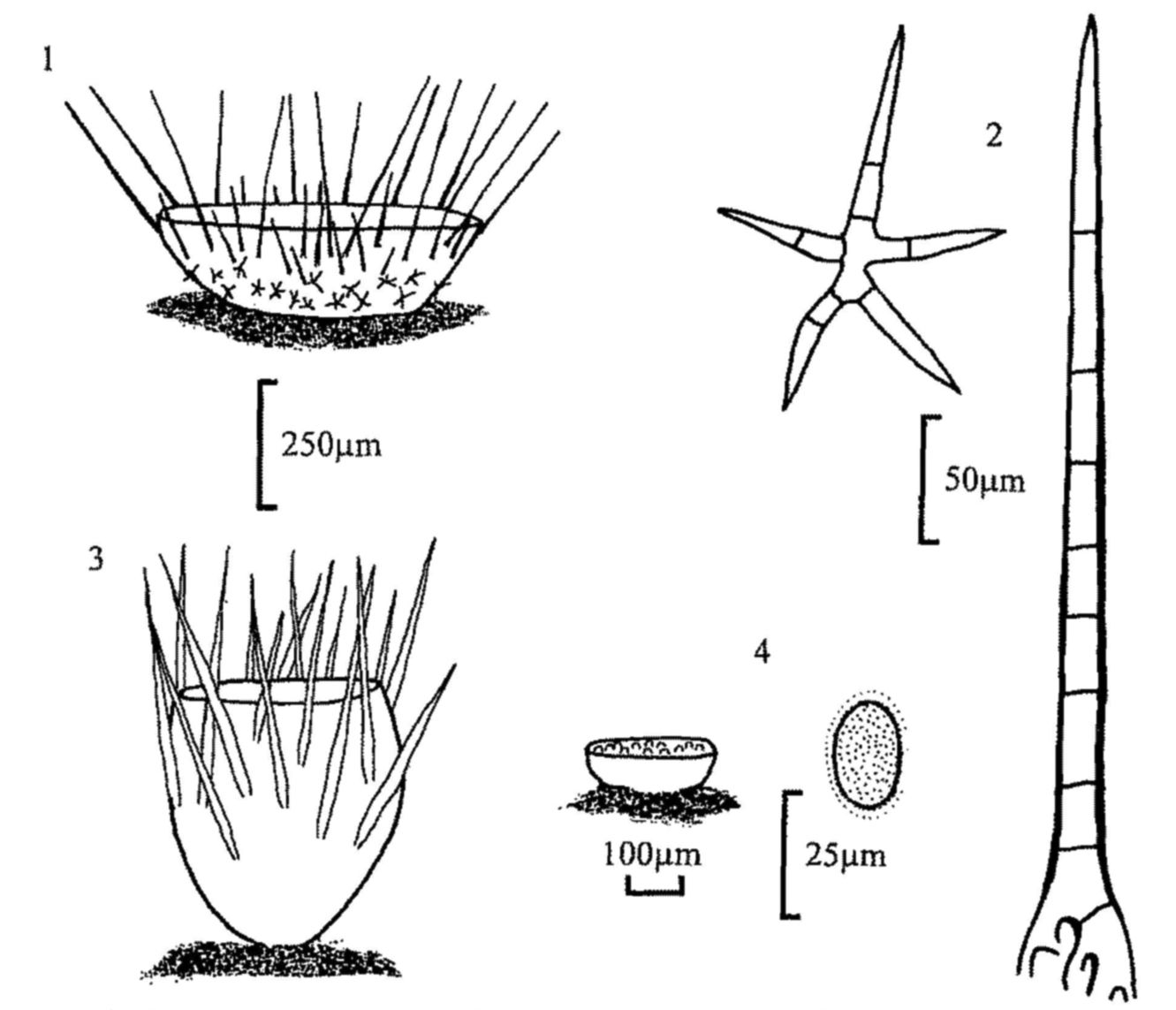
Fig. 1. Cheilymenia stercorea, apothecium.
Fig. 2. C. stercorea, stellate and rooted hairs.
Fig. 3. Lasiobolus ciliatus, apothecium.
Fig. 4. Iodophanus carneus, apothecium and spore.
Project Gutenberg's Keys to Fungi on Dung, by Mike Richardson and Roy Watling
This eBook is for the use of anyone anywhere in the United States and most
other parts of the world at no cost and with almost no restrictions
whatsoever. You may copy it, give it away or re-use it under the terms of
the Project Gutenberg License included with this eBook or online at
www.gutenberg.org. If you are not located in the United States, you'll have
to check the laws of the country where you are located before using this ebook.
Title: Keys to Fungi on Dung
Author: Mike Richardson
Roy Watling
Release Date: June 9, 2018 [EBook #57291]
Language: English
Character set encoding: ISO-8859-1
*** START OF THIS PROJECT GUTENBERG EBOOK KEYS TO FUNGI ON DUNG ***
Produced by Keith Edkins, Mary Glenn Krause, Eric Lehtonen
and the Online Distributed Proofreading Team at
http://www.pgdp.net
| Transcriber's note: | Corrigendum applied at the wish of the principle author: in Key 3 the pointers to couplets 56 and 66 were the wrong way round and have been corrected in this edition. |
KEYS TO FUNGI
ON DUNG
by
M. J. RICHARDSON
165 Braid Road,
EDINBURGH EH10 6JE
and
ROY WATLING
Royal Botanic Garden,
EDINBURGH EH3 5LR
Published by the British Mycological Society
PO Box 30, Stourbridge
West Midlands DY9 9PZ
© British Mycological Society 1997
Printed in Scotland by BPC-AUP Aberdeen Ltd
ISBN 0 9527704 2 3
The first edition of these keys was published in the Bulletin of the British Mycological Society 2, 18-43 (1968) and 3, 86-88, 121-124 (1969) in an attempt to bring together in one place information for the identification of coprophilous fungi which would be useful to teachers and others interested in these fungi. They were issued as a separate publication in 1972, and with corrections in 1974. They were reprinted in 1982 with additions. This latest edition is an update of all the earlier ones, with current nomenclature and recent references, and the inclusion of some additional species.
M.J.R.
R.W.
December 1996
Coprophilous fungi are highly satisfactory for demonstrating the diversity and morphology of a group of related organisms within an ecological system. Representative genera of most major groups of fungi can usually be guaranteed to appear on dung after a period of incubation. There is no shortage of dung in our fields and woods, and this material will always produce characteristic fungi at whatever time of year it is collected.
Dung is best incubated in a light place, for example on a table in a warm room, on layers of moist filter paper or other absorbent material. For rabbit pellets, and samples of similar size, Petri dishes are ideal; for horse 'apples', and larger types of dung, large covered dishes such as glass casseroles, plastic sandwich boxes or yoghurt pots are needed. The top third cut from a plastic lemonade or mineral water bottle fits neatly in a Petri dish, and replacing the screw cap with a cotton wool plug allows aeration and gives adequate height for developing basidiomycetes. Samples should not be kept in airtight containers for any length of time after collection, as in such conditions insects and nematodes tend to break down the dung, and anaerobic conditions which do not favour the fungi rapidly develop. If they cannot be set to incubate soon after collection they can be gently air dried, as most dung fungi will remain alive after such treatment and grow out when the sample is eventually moistened. The absorbent material should be kept moist. Although free water will not allow the best development of ascomycetes, the succession of basidiomycetes appears to vary with the wetness of the dung. Earthworms and insect larvae should be excluded from the samples as far as possible, for they break up the dung too much; activity of the latter can be reduced by spraying lightly with a household insecticide. If space is limited and cultures are kept nearby, it is very important to prevent mite infestation. Containers can be isolated by placing on glass plates lightly smeared with Vaseline, to which an acaricide (e.g. methyl benzoate) can be added.
Fungi are best sought with a stereoscopic binocular microscope, when their full beauty will be seen, but a hand lens or simple magnifier, although less convenient, is sufficient for all but the smallest forms. The larger ascomycetes and most of the basidiomycetes are readily seen with the unaided eye, but the binocular microscope is still very useful for observing the gross features of the veil of the basidiomycetes. Perithecia, apothecia and similar structures can be removed with fine needles or forceps quite cleanly for mounting, initially in water, on slides. Subsequent irrigation with iodine solution will allow any reaction of ascus wall, tip or pore to be observed, and mounting in diluted Indian ink can enhance the visibility of appendages, caudae and sheaths which occur on some spores. Spore discharge in the ascomycetes often occurs from mature asci when material is mounted in water, so mature spores can immediately be seen. Many of the coprophilous toadstools (agarics), because of their small size and/or rapidly deliquescent nature, often do not give spore prints in the normal way, but mature spores can usually be found on the stipe or in natural spore prints formed on the absorbent material on which the dung is supported. For accurate identification the ability to measure the size of spores and other structures will be necessary. Basic microscopical technique and mycological knowledge is assumed. Common species are well described and illustrated in popular books, and references are given to specialist works to allow descriptions of less common species to be found. It will be necessary to refer to these for critical taxa. Although this edition contains about one half more species than the 1982 edition, there are still many species to be described and new records and observations to be made, especially in the Ascomycotina.
Four keys are presented. Keys 1 and 2 (MJR) are to the coprophilous ascomycetes, a very diverse group which, although not covering all the possible types of reproductive structure found in the class, contains many of the important types. The information for the identification of these fungi is dispersed throughout the literature, and many new species are still being discovered and described. Some appear to be world-wide in their distribution, others more restricted, with a prevalence of reports from either arctic, temperate or tropical regions. These keys are not exhaustive, since there are far too many species to make it practical to include them all. They do, however, include most genera, and the commoner or well known species of temperate regions. Specific (and even generic) limits in some cases (e.g. Coprotus / Ascophanus / Ryparobius / Thelebolus) are still the subject of debate and the choice of names to use in the key for a few taxa has been a compromise. Key 2 includes the original 'plectomycete' key (RW), which contains fungi which may not be strictly coprophilous in the normal sense, but fungi which occur on hair, horn, bone and cadavers, and may thus be found on carnivore dung or pellets of owls and other birds of prey.
Key 3 (RW, p. 52) is to the basidiomycetes of dung and associated debris. The part of the key dealing with the agarics attempts to be as complete as possible. Since the toadstools have always been thought of as the best known of the coprophilous fungi, attention to their taxonomy has often been careless. In this key the opportunity has been taken to adopt a rather narrow species concept, and to provide in certain places indications of where distinct taxa, even autonomous species, may be found after further laboratory work. Many of these types have been cultured and appear to differ vegetatively in ways which support observations of gross morphology. Coprophilous agarics are popular material for genetic studies and additional information on veil structure, spore number etc. of individual species is given, even when these are not 'key characters'.
Key 4 (MJR, p. 63) is to the Zygomycota (phycomycetes) which are characteristic of dung and amongst the first to appear when freshly dropped dung is incubated. They soon disappear, however, but their fruiting can be prolonged by plating small portions of dung on a nutrient medium (e.g. potato carrot or potato dextrose agar) to which has been added a small amount of antibiotic to reduce bacterial growth. This method is especially suitable for the parasitic and predacious fungi. A cultural approach is essential for the identification of many of these fungi and the above media, and oatmeal agar, are suitable for culture as well as isolation. For this reason the study of this group of fungi is less easy than that of the ascomycetes and basidiomycetes but, because the asexual stages are characteristic, we have attempted to key out the commoner genera which might be found, with notes on common species. The asexual spores are sporangiospores formed in sporangia; some sporangia produce a single spore within a closely fitting sporangium, and have in the past been erroneously described as conidia. A great range of sporangial structure occurs within the orders concerned. The classical structure is the massive (up to 250µm diam.) multispored sporangium with an internal columella which remains after the spores have been dispersed (e.g. Mucor); those of Mortierella are similar, but smaller and without a columella. Other sporangia are much reduced and may be only 10-20µm diam., and contain only a small number of spores (Thamnidium) or one spore (Chaetocladium); these small globose structures are termed sporangioles. Spores may also form in chains; the chains are in terminal groups and are formed by the differentiation of the contents of cylindrical sporangia which are considered to be part-sporangia (merosporangia). When the sporangial wall has disappeared the spore chains may remain discrete and intact, or they may collapse into a wet droplet of spores (Syncephalastrum, some Piptocephalis). Members of the Kickxellaceae (e.g. Coemansia, Kickxella) have single spored merosporangia produced in serried ranks on boat-shaped or swollen structures (sporoclades). The sexual spores (zygospores) are rarely seen without culturing; oatmeal agar is one which favours their production. The key includes one member of the Entomophthorales, which also produces single-spored sporangia. Other members of this order may be found parasitising the various animals which live in dung; many other predacious fungi may also be seen, e.g. parasites of amoebae (Acaulopage). The key is of necessity far from complete, and omits members of the Dimargaritales, which have been found frequently on dung of small mammals in America.
Mitosporic fungi ('Fungi Imperfecti') and myxomycetes have been excluded, since they would expand the range of these keys beyond what was initially intended, although numerous species of both groups occur on dung when incubated in a damp chamber. For mitosporic fungi see Seifert, Kendrick & Murase (1983) and Ellis & Ellis (1988); for myxomycetes see Eliasson & Lundqvist (1979). As practical keys, rather than a taxonomic treatment, taxonomic authorities have not been cited. For ascomycetes, Cannon, Hawksworth & Sherwood-Pike (1985) have been followed, unless there is a more recent treatment of a group. For the basidiomycetes the 'New Checklist of British Agarics and Boleti' (Dennis, Orton & Hora, 1960, Supplement to the Transactions of the British Mycological Society 43) has been followed, and The British Fungus Flora (Orton & Watling, 1979 and Watling, 1982).
ASCOMYCETE REFERENCES
Ahmed, S. I. & Cain, R. F. (1972). Revision of the genera Sporormia and Sporormiella. Canadian Journal of Botany 50, 419-477. (Keys and descriptions of 66 spp.).
Apinis, A. E. (1964). Revision of the British Gymnoascaceae. Mycological Paper 96.
Arx, J. A. von (1971). On Arachniotus and related genera of the Gymnoascaceae. Persoonia 6, 371-380.
Arx, J. A. von (1975). Revision of Microascus with the description of a new species. Persoonia 8, 191-197.
Arx, J. A. von (1975). On Thielavia and some similar genera of Ascomycetes. Studies in Mycology 8.
Arx, J. A. von (1982). A key to the species of Gelasinospora. Persoonia 11, 443-449.
Arx, J. A. von (1986). The ascomycete genus Gymnoascus. Persoonia 13, 173-183.
Arx, J. A. von (1987). A re-evaluation of the Eurotiales. Persoonia 13, 273-300. (Keys to families and genera).
Arx, J. A. von, Dreyfuss, M. & Müller, E. (1984). A re-evaluation of Chaetomium and the Chaetomiaceae. Persoonia 12, 169-179. (Key to species).
Arx, J. A. von, Figueras, M. J. & Guarro, J. (1988). Sordariaceous Ascomycetes without Ascospore Ejaculation. Beihefte zur Nova Hedwigia 94, 1-104.
Arx, J. A. von, & Gams, W. (1967). Über Pleurage verruculosa und die zugehörige Cladorrhinum-Konidienform. Nova Hedwigia 13, 198-208.
Arx, J. A. von, Guarro, J. & van der Aa, H. A. (1987). Asordaria, a new genus of the Sordariaceae, and a new species of Melanocarpus. Persoonia 13, 263-272.
Barrasa, J. M. & Checa, J. (1990). Dothideales del Parque Natural de Monfragüe Cáceres. I. Boletín Sociedad Micológica de Madrid 15, 91-102.
Barrasa, J. M., Lundqvist, N. & Moreno, G. (1986). Notes on the genus Sordaria in Spain. Persoonia 13, 83-88.
Bell, A. & Mahoney, D. P. (1995). Coprophilous fungi in New Zealand. I. Podospora species with swollen agglutinated perithecial hairs. Mycologia 87, 375-396. (Key and descriptions of 8 spp.).
Bezerra, J. L. & Kimbrough, J. W. (1975). The genus Lasiobolus (Pezizales: Ascomycetes). Canadian Journal of Botany 53, 1206-1229. (Key and descriptions of 11 spp.).
Booth, C. (1961). Studies of pyrenomycetes: VI. Thielavia with notes on some allied genera. Mycological Paper 83.
Breton, A. & Faurel, L. (1968). Etudes des affinités du genre Mycorhynchus Sacc. et description de plusieurs especes nouvelles. Revue de Mycologie 32, 229-258.
Brummelen, J. van (1962). Studies on Discomycetes—II. On four species of Fimaria. Persoonia 2, 321-330.
Brummelen, J. van (1962). A World Monograph of the Genera Ascobolus and Saccobolus. Persoonia, Supplement Volume 1. (Key and descriptions of 66 spp., and a critical taxonomic treatment).
Brummelen, J. van (1980). Two species of Ascobolus new to Britain. Persoonia 11, 87-92.
Brummelen, J. van (1981). The genus Ascodesmis (Pezizales, Ascomycetes). Persoonia 11, 333-358.
Brummelen, J. van (1984). Notes on cup-fungi—2. Lasiobolus. Persoonia 12, 328-334.
Brummelen, J. van (1986). Notes on cup-fungi—3. On three species of Cheilymenia. Persoonia 13, 89-96.
Brummelen, J. van (1990). Notes on cup-fungi—4. On two rare species of Ascobolus. Persoonia 14, 203-207.
Cailleux, R. (1971). Recherches sur la mycoflore coprophile centrafricaine. Les genres Sordaria, Gelasinospora, Bombardia (Biologie, Morphologie, Systématique). Bulletin trimestriel de la Société Mycologique de France 87, 461-626 + 27 plates.
Cain, R. F. (1934). Studies of Coprophilous Sphaeriales in Ontario. University of Toronto Studies, Biological Series, No. 38. (Reprinted 1968 in Bibliotheca Mycologica, Band 9, by Cramer, Lehre).
Cain, R. F. (1961). Studies of coprophilous Ascomycetes. VII. Preussia. Canadian Journal of Botany 39, 1633-1666.
Cain, R. F. (1962). Studies of coprophilous Ascomycetes. VIII. New species of Podospora. Canadian Journal of Botany 40, 447-490.
Cain, R. F. & Kimbrough, J. W. (1969). Coprobolus, a new genus of the tribe Thelebolae (Pezizaceae). Canadian Journal of Botany 47, 1911-1914.
Cain, R. F. & Mirza, J. H. (1972). Three new species of Arnium. Canadian Journal of Botany 50, 333-336.
Cannon, P. F. & Hawksworth, D. L. (1982). A re-evaluation of Melanospora Corda and similar Pyrenomycetes, with a revision of the British species. Botanical Journal of the Linnean Society 84, 115-160.
Cannon, P. F., Hawksworth, D. L. & Sherwood-Pike, M. A. (1985). The British Ascomycotina. An Annotated Checklist. Commonwealth Agricultural Bureaux, Slough, U. K.
Cano, J. & Guarro, J. (1990). The genus Aphanoascus. Mycological Research 94, 355-377. (Key to species).
Currah, R. S. (1988). An annotated key to the genera of the Onygenales. Systema Ascomycetum 7, 1-12.
Dennis, R. W. G. (1978). British Ascomycetes. J. Cramer, Lehre. (or earlier edition, 1968 and 1960 (as British Cup Fungi and their allies), The Ray Society, London). (All groups).
Dissing, H. (1987). Three 4-spored Saccobolus species from north east Greenland. In Arctic and Alpine Mycology II (ed. G. A. Laursen, J. F. Ammirati & S. A. Redhead), pp. 79-86.
Dissing, H. (1989). Four new coprophilous species of Ascobolus and Saccobolus from Greenland (Pezizales). Opera Botanica 100, 43-50.
Dissing, H. (1992). Notes on the coprophilous pyrenomycete Sporormia fimetaria. Persoonia 14, 389-394.
Dissing, H. & Paulsen, M. D. (1976). Trichophaeopsis tetraspora, a New Coprophilous Discomycete from Denmark. Botanisk Tidsskrift 70, 147-151.
Elliott, M. E. (1967). Rutstroemia cuniculi, a coprophilous species of the Sclerotiniaceae. Canadian Journal of Botany 45, 521-524.
Guarro, J. & Arx, J. A. von (1987). The Ascomycete genus Sordaria. Persoonia 13, 301-313. (Key to 14 species and checklist).
Hawksworth, D. L. & Webster, J. (1977). Studies on Mycorhynchus in Britain. Transactions of the British Mycological Society 68, 329-340. (Key to 12 spp. and descriptions of some).
Jain, K. & Cain, R. F. (1973). Mycoarctium, a new genus in the Thelebolaceae. Canadian Journal of Botany 51, 305-307.
Jeng. R. S., Luck-Allen, E. R. & Cain, R. F. (1977). New species and new records of Delitschia from Venezuela. Canadian Journal of Botany 55, 383-392.
Khan. R. S. & Cain, R. F. (1972). Five new species of Podospora from East Africa. Canadian Journal of Botany 50, 1649-1661.
Kimbrough, J. W. (1969). North American species of Thecotheus (Pezizeae, Pezizaceae). Mycologia 61, 99-114. (Key and description of 5 spp.).
Kimbrough, J. W. & Korf. R. P. (1967). A synopsis of the genera and species of the tribe Thelebolae (Pseudoascobolaceae). American Journal of Botany 54, 9-23.
Kimbrough, J. W. & Luck-Allen, E. R. (1974). Lasiothelebolus, a new genus of the Thelebolaceae (Pezizales). Mycologia 66, 588-592.
Kimbrough, J. W., Luck-Allen, E. R. & Cain, R. F. (1969). Iodophanus, the Pezizeae segregate of Ascophanus (Pezizales). American Journal of Botany 56, 1187-1202. (Key and description of 10 spp.).
Kimbrough, J. W., Luck-Allen, E. R. & Cain, R. F. (1972). North American species of Coprotus (Thelebolaceae: Pezizales). Canadian Journal of Botany 50, 957-972. (Key and description of 18 spp.).
Krug, J. C. (1973). An enlarged concept of Trichobolus (Thelebolaceae, Pezizales) based on a new eight-spored species. Canadian Journal of Botany 51, 1497-1501. (With key to 4 spp.).
Krug, J. C. (1995). The genus Fimetariella. Canadian Journal of Botany 73, 1905-1916. (With key to 8 spp.).
Krug, J. C. & Cain, R. F. (1972). Additions to the genus Arnium. Canadian Journal of Botany 50, 367-373. (Key to 25 spp.).
Krug, J. C. & Cain, R. F. (1974). A preliminary treatment of the genus Podosordaria. Canadian Journal of Botany 52, 589-605. (Key and descriptions of 10 spp.).
Krug, J. C. & Cain, R. F. (1974). New species of Hypocopra (Xylariaceae). Canadian Journal of Botany 52, 809-843. (Descriptions and synoptic key to 30 spp.).
Krug, J. C. & Scott, J. A. (1994). The genus Bombardioidea. Canadian Journal of Botany 72, 1302-1310. (Description and key to 4 spp.).
Larsen, K. (1970). The Genus Saccobolus in Denmark. Botanisk Tidsskrift 65, 371-389.
Larsen, K. (1971). Danish Endocoprophilous Fungi and Their Sequence of Occurrence. Botanisk Tidsskrift 66, 1-32.
Lohmeyer, T. R. & Benkert, D. (1988). Poronia erici—eine neue Art der Xylariales (Ascomycetes). Zeitschrift fur Mykologie 54, 93-102.
Luck-Allen, E. R. & Cain, R. F. (1975). Additions to the genus Delitschia. Canadian Journal of Botany 53, 1827-1887. (Key to 46 spp. and descriptions/illustrations of most).
Lundqvist, N. (1967). On spore ornamentation in the Sordariaceae, exemplified by the new cleistocarpous genus Copromyces. Arkiv för Botanik, Series 2. 6(7), 327-337.
Lundqvist, N. (1969). Zygopleurage and Zygospermella (Sordariaceae s. lat., Pyrenomycetes). Botaniska Notiser 122, 353-374.
Lundqvist, N. (1970). New Podosporae (Sordariaceae s. lat., Pyrenomycetes). Svensk Botanisk Tidskrift 64, 409-420.
Lundqvist, N. (1972). Nordic Sordariaceae s. lat. Symbolae Botanicae Upsalienses XX. 1. 1-314. (Keys and descriptions of ca 100 spp., and critical taxonomic discussion).
Lundqvist, N. (1980). On the genus Pyxidiophora sensu lato (Pyrenomycetes). Botaniska Notiser 133, 121-144.
Lundqvist, N. (1980). Wawelia effusa Lundqvist, spec. nov. (Xylariaceae). Persoonia 14, 417-423.
Malloch, D. & Cain, R. F. (1970). The genus Arachnomyces. Canadian Journal of Botany 48, 839-845.
Malloch, D. & Cain, R. F. (1970). Five new genera in the new family of Pseudeurotiaceae. Canadian Journal of Botany 48, 1815-1825.
Malloch, D. & Cain, R. F. (1971). New genera of the Onygenaceae. Canadian Journal of Botany 49, 839-846.
Malloch, D. & Cain, R. F. (1971). Four new genera of cleistothecial Ascomycetes with hyaline ascospores. Canadian Journal of Botany 49, 847-854.
Malloch, D. & Cain, R. F. (1971). New cleistothecial Sordariaceae and a new family, Coniochaetaceae. Canadian Journal of Botany 49, 869-880.
Malloch, D. & Cain, R. F. (1972). New species and combinations of cleistothecial Ascomycetes. Canadian Journal of Botany 50, 61-72.
Minter, D. W. & Webster, J. (1983). Wawelia octospora sp. nov., a xerophilous and coprophilous member of the Xylariaceae. Transactions of the British Mycological Society 80, 370-373.
Mirza, J. H. & Cain, R. F. (1969). Revision of the genus Podospora. Canadian Journal of Botany 47, 1999-2048.
Moravec, J. (1990). A taxonomic revision of the genus Cheilymenia—3. A new generic and infrageneric classification of Cheilymenia in a new emendation. Mycotaxon 38, 459-484. (Synopsis of genus, including Coprobia).
Moravec, J. (1993). A taxonomic revision of the genus Cheilymenia—5. The section Cheilymenia. Czech Mycology 47, 7-37.
Moreau, C. (1953) Les Genres Sordaria et Pleurage. Encyclopédie mycologique 25, 1-330. (Sordaria and Pleurage (=Podospora/Schizothecium), and Coniochaeta, Hypocopra, Sporormiella, Trichodelitschia, and other pyrenomycetes for comparison).
Munk, A. (1957). Danish Pyrenomycetes. Dansk Botanisk Arkiv 17(1), 1-491.
Orr, G. F. & Kuehn, H. H. (1971). Notes on Gymnoascaceae. I. A review of eight species. Mycologia 63, 191-203.
Orr, G. F., Kuehn, H. H. & Plunkett, O. A. (1963). A new genus of the Gymnoascaceae with swollen peridial septa. Canadian Journal of Botany 41, 1439-1456. (Key to Auxarthron (Gymnoascus) species).
Orr, G. F., Kuehn, H. H. & Plunkett, O. A. (1971). The genus Myxotrichum Kunze. Canadian Journal of Botany 41, 1457-1480. (Key to species).
Paulsen, M. D. & Dissing, H. (1979). The genus Ascobolus in Denmark, Botanisk Tidsskrift 74, 67-78.
Rehm, H. (1887-1895). Ascomyceten: Hysteriaceen und Discomyceten. Vol. 1, Abt. 3 of Rabenhorst's Kryptogamen-Flora. (Discomycetes).
Renny, J. (1874). New species of the genus Ascobolus. Journal of Botany 12, 353-357 and 4 plates. (Description and illustration of 6 Ascozonus spp.).
Richardson, M. J. (1972). Coprophilous ascomycetes on different dung types. Transactions of the British Mycological Society 58, 37-48.
Samson, R. A. (1972). Notes on Pseudogymnoascus, Gymnoascus and related genera. Acta botanica neerlandica 21, 517-527.
Seth, H. K. (1970). The genus Lophotrichus Benjamin. Nova Hedwigia 19, 591-599.
Valldosera, M. & Guarro, J. (1987). Estudios sobre hongos copróphilos aislados en España. VI. Ascomycetes. Boletín Sociedad Micológica de Madrid 12, 51-56.
Valldosera, M. & Guarro, J. (1988). Some coprophilous ascomycetes from Chile. Transactions of the British Mycological Society 90, 601-605.
Valldosera, M. & Guarro, J. (1989). Estudios sobre hongos copróphilos aislados en España. XI. Ascomycetes. Boletín Sociedad Micológica de Madrid 14, 75-80.
Valldosera, M. & Guarro, J. (1989). Estudios sobre hongos copróphilos aislados en España. XV. El género Preussia (Sporormiella). Boletín Sociedad Micológica de Madrid 14, 81-94.
Valldosera, M. & Guarro, J. (1992). Estudios sobre hongos copróphilos en España. XVII. Ascomycotina. Boletín Sociedad Micológica de Madrid 17, 19-37.
Valldosera, M. & Guarro, J. (1992). Estudios sobre hongos copróphilos aislados en España. XVIII. Bibliographic catalogue of Ascomycotina. Boletín Sociedad Micológica de Madrid 17, 39-55.
Valldosera, M., Guarro, J. & Figueras, M. J. (1991). Two interesting coprophilous fungi from Spain. Mycological Research 95, 243-246.
Winter, G. (1884-1887). Ascomyceten: Gymnoasceen und Pyrenomyceten. Vol. 1, Abt. 2 of Rabenhorst's Kryptogamen-Flora. (Pyrenomycetes).
Yao, Y-J. (1996). Notes on British species of Lasiobolus. Mycological Research 100, 737-739.
Yao, Y-J. & Spooner, B. M. (1996). Notes on British species of Cheilymenia. Mycological Research 100, 361-367.
BASIDIOMYCETE REFERENCES
Moser, M. (1978), in Gams, H. (ed.). Kleine Kryptogamenflora von Mitteleuropa. Fischer Verlag.
Moser, M. (1983). Keys to Agarics and Boleti (English translation by S. Plant). Roger Phillips, London.
Orton, P. D. & Watling, R. (1979). British Fungus Flora: Coprinus. Her Majesty's Stationery Office, Edinburgh.
Phillips, R. (1981). Mushrooms and other fungi of Great Britain and Europe. Pan Books, London.
Watling, R. (1982). British Fungus Flora: Bolbitiaceae. Her Majesty's Stationery Office, Edinburgh.
PHYCOMYCETE REFERENCES
Benjamin, R. K. (1959). The merosporangiferous Mucorales. Aliso 4, 321-433.
Benjamin, R. K. (1961). Addenda to the merosporangiferous Mucorales. Aliso 5, 11-19.
Benjamin, R. K. (1963). Addenda to the merosporangiferous Mucorales. Aliso 5, 273-288.
Benjamin, R. K. (1965). Addenda to the merosporangiferous Mucorales. Aliso 6, 1-10. (The 4 papers above are an excellent account of Syncephalis, Piptocephalis, Coemansia and other unusual allied phycomycetes, republished (1967) as Bibliotheca Mycologica 5 by J. Cramer, Lehre).
Gams, W. & Moreau, R. (1959). Le genre Mortierella. Annales scientifiques de l'Université de Besançon, Series 2 3, 95-105.
Hesseltine, C. W. (1955). Genera of Mucorales with a note on their synonymy. Mycologia 47, 344-363. (With good key; many other papers by Hesseltine, with others, in Mycologia, American Journal of Botany, American Midland Naturalist and Lloydia).
Ingold, C. T. & Zoberi, M. H. (1963). The asexual apparatus of Mucorales in relation to spore liberation. Transactions of the British Mycological Society 46, 115-134.
Naumov, N. A. (1939). Clés des Mucorinées. Encyclopédie mycologique 9, 1-137.
Zycha, H., Siepmann, R. & Linneman, G. (1969). Mucorales. J. Cramer, Lehre. (A revision of Zycha, 1935).
GENERAL REFERENCES
Bell, A. (1983). Dung Fungi: an illustrated guide to coprophilous fungi in New Zealand. Victoria University Press, Wellington.
Bon, M. (1987). The Mushrooms and Toadstools of Britain and North-western Europe. Hodder & Stoughton, London.
Cacialli, G., Caroti, V. & Doveri, F. (1995). Funghi fimicoli e rari o interssanti del litorale Toscano. Schede di Micologia vol. 1. Fondazione Centro Studi Micologici Dell' A. M. B., Vicenza, Italy.
Domsch, K. H., Gams, W. & Anderson, T. H. (1980). Compendium of soil fungi. Academic Press, New York.
Ellis, M. B. & Ellis, J. P. (1988). Microfungi on Miscellaneous Substrates. Croom Helm, London & Sydney.
Gilman, J. C. (1957). A Manual of Soil Fungi. Iowa State College Press.
Eliasson, U. & Lundqvist, N. (1979). Fimicolous Myxomycetes. Botaniska Notiser 132, 551-568. (A list of 34 spp., with some descriptions and illustrations).
Hawksworth, D. L., Kirk, P. M., Sutton, B. C. & Pegler, D. N. (1995). Ainsworth & Bisby's Dictionary of the Fungi. 8th edn. CAB International, Wallingford.
Holden, M. (ed) (1982). Guide to the literature for the identification of British fungi, 4th Edition. Bulletin of the British Mycological Society 16, 36-55; 92-112.
Massee, G., & Salmon, E. S. (1901). Researches on coprophilous fungi. Annals of Botany, London 15, 313-357.
Seifert, K. A., Kendrick, W. B. & Murase, G. (1983). A key to hyphomycetes on dung. University of Waterloo Biology Series No. 27.
Webster, J. (1970). Coprophilous Fungi. Transactions of the British Mycological Society 54, 161-180.
| 1 | Ascoma either globose to flask shaped, usually with an easily observable pore or neck (perithecium or pseudothecium, figs 16, 18, 19, 22, 27, 30, 32, 34-37), or discoid (apothecium, figs 1, 3, 4, 7, 11-14). Spores usually 8 in each ascus (less frequently 4, 16, 32, 64, 128 etc.). Asci ellipsoid to cylindrical, borne in a distinct hymenium, thus appearing in fascicles or distinct groups when the fruit body is squashed. | 2 |
| - | Ascoma globose to subglobose, lacking a definite pore or neck (cleistothecium or gymnothecium, figs 38, 39, 46). Asci globose to subglobose, 8-spored, not in a distinct hymenium, appearing quite free when the fruit body is squashed. | |
| Key 2, 148 (p. 45) | ||
| 2(1) | Ascoma a perithecium or pseudothecium, usually dark in some part, not opening to a disc but remaining globose or flask shaped. Asci unitunicate, not operculate but often with an apical pore, which may stain blue in iodine, or bitunicate. | |
| Key 2, 1 (p. 24) | ||
| - | Ascoma an apothecium, white or lightly coloured, soft fleshed, opening out to a disc or cushion shape when mature. Asci unitunicate. | 3 |
| 3(2) | Asci opening by an operculum (fig. 8), a bilabiate vertical split down to a subapical ring of thickening (fig. 15), or apparently just bursting. | 4 |
| - | Asci inoperculate, with an apical pore. | 96 |
| 4(3) | Spores 8 (occasionally 4) in an ascus, colourless, purple or brown. | 5 |
| - | Spores more than 8 in an ascus, colourless. | 77 |
| 5(4) | Spores remaining colourless. | 6 |
| - | Spores purple or brown at maturity. | 39 |
| 6(5) | Apothecia with obvious hairs. | 7 |
| - | Apothecia without obvious hairs (microscopic hairs up to 50µm long may be present). | 14 |
| 7(6) | Hairs brown. Apothecia orange, red orange or yellow orange | |
| (Cheilymenia, fig. 1) 8 | ||
| - | Hairs colourless. Apothecia colourless or pinkish. | |
| (Lasiobolus, fig. 3) 12 | ||
| 8(7) | Apothecia with stellate hairs. Spores 14-20 × 8-11µm. | |
| Cheilymenia stercorea (figs 1, 2) | ||
| - | Apothecia without stellate hairs. | 9 |
| 9(8) | Spores 14.5-18 × 8-9.5µm. Asci 10-13µm diam. Apothecia 2mm diam. or more. | |
| Cheilymenia coprinaria | ||
| - | Spores larger, 17 × 10µm or more. | 10 |
| 10(9) | Apothecia reddish orange, up to 1mm diam., marginal hairs rooting, wall 2-4µm thick. Spores 21-26 × 10-13.8µm. | |
| Cheilymenia fimicola | ||
| - | Apothecia pale orange yellow, marginal hairs superficial, wall up to 2µm thick. | 11 |
| 11(10) | Asci up to 22µm diam. Spores 17-27 × 10-14.5µm. | |
| Cheilymenia pulcherrima | ||
| - | Asci wider, 25µm diam. or more. Spores 23-26.5 × 13-16.5µm. | |
| Cheilymenia raripila | ||

Fig. 1. Cheilymenia stercorea, apothecium.
Fig. 2. C. stercorea, stellate and rooted hairs.
Fig. 3. Lasiobolus ciliatus, apothecium.
Fig. 4. Iodophanus carneus, apothecium and spore.
| 12(7) | Hairs 600µm or longer. Spores 19-23 × 7-10µm. | |||
| Lasiobolus macrotrichus | ||||
| - | Hairs shorter, up to 600µm. | 13 | ||
| 13(12) | Asci clavate, 20µm diam. or wider. Spores 19-22 × 10.5-13.5µm. | |||
| Lasiobolus cuniculi | ||||
| - | Asci cylindrical, up to 20µm diam. Spores 18-22.5 × 9.5-11.5µm. | |||
| Lasiobolus ciliatus (fig. 3) | ||||
| 14(6) | Asci blue in iodine solution. | 15 | ||
| - | Asci not blue in iodine. | 24 | ||
| 15(14) | Spores large, 30-42 × 15-18µm, warted, ellipsoid with acute apices. | |||
| Thecotheus cinereus | ||||
| - | Spores smaller, smooth or only finely ornamented | 16 | ||
| 16(15) |
|
|||
| - | Apothecia pale, up to 4mm diam. Asci protruding from hymenium when ripe. | 17 | ||
| 17(16) | Apothecia white to pink, up to 2mm diam. Spores finely verruculose, 18-25 × 8-14µm. | |||
| Iodophanus carneus (fig. 4) | ||||
| - | Apothecia pale, variously coloured when fresh, but drying darker. Spores smooth. | |||
| (Thecotheus) 18 | ||||
| 18(17) | Spores apiculate at each end, smooth. | 19 | ||
| - |
|
|||
| 19(18) | Spores with a collar at the base of the apiculus. | 20 | ||
| - | Spores without a collar at the base of the apiculus, 16-21 × 8-12µm. | |||
| Thecotheus apiculatus | ||||
| 20(19) | Apothecia white. Spores 20-22 × 10-12µm, apiculus 4-6µm diam. | |||
| Thecotheus perplexans | ||||
| - | Apothecia yellowish. Spores 12-15 × 7.5-9µm, apiculus 2.5-3.5µm diam. | |||
| Thecotheus africanus | ||||
| 21(16) | Spores smooth, without guttules. | 22 | ||
| - | Spores verruculose or spinulose, 15-18 × 8-9µm, with 1 guttule. Paraphyses with clavate apices, with brown contents. Apothecia asymmetrical, extended on one side. | |||
| Peziza pleurota | ||||
| 22(21) | Spores 19-24 × 10.5-14µm. Apothecia yellowish brown, up to 10cm diam. | |||
| Peziza vesiculosa | ||||
| - | Spores up to 10µm wide. | 23 | ||
| 23(22) | Apothecia ca 1cm diam., umber with a paler margin. Spores 15-22 × 9-10µm. | |||
| Peziza bovina | ||||
| - | Apothecia up to 2cm diam., pale brown. Spores 13-16 × 7-9µm. | |||
| Peziza fimeti | ||||
| 24(14) | Apothecia robust, up to 4mm diam., orange or with brownish or purple tints. | 25 | ||
| - | Apothecia smaller, rarely more than 1mm, pale, yellowish green, orange, grey or chestnut. | 32 | ||
| 25(24) | Apothecia orange or red. | 26 | ||
| - |
|
|||
| 26(25) | Apothecia crowded, 1-3mm diam., orange, with a granular surface. Asci up to 190 × 15µm. Spores 15-18.5 × 7-9.5µm. Paraphyses strongly clavate to apex up to 14µm diam, filled with orange granules. | |||
| Coprobia granulata | ||||
| - | Apothecia discrete, 1-2mm diam., orange or red. Asci 240 × 10-12µm. Spores 12-15 × 7-8µm. Paraphyses yellow, only slightly swollen from 2µm to 3-4µm at apex. | |||
| Ascophanus bresadolae | ||||
| 27(25) |
|
|||
| - | Spores larger. | 28 | ||
| 28(27) |
|
|||
| - | Spores shorter. | 29 | ||
| 29(28) |
|
|||
| - | Spores 13-17 × 7-11µm. | 30 | ||
| 30(29) | Disc punctate with asci. Paraphysis tips swollen up to 3-5µm. Spores 14.5-16 × 9.5-11µm. | |||
| Fimaria leporum | ||||
| - | Disc not punctate with asci. Paraphysis tips not or only slightly swollen. | 31 | ||
| 31(30) | Apothecia pale yellowish. Spores 13-15.5 × 7.5-8.5µm. | |||
| Fimaria theioleuca | ||||
| - | Apothecia chestnut/purplish brown. Spores 14-17 × 7-8.5µm. | |||
| Fimaria cervaria | ||||
| 32(24) | Spores less than 10µm long. | 33 |
| - | Spores mostly longer than 10µm. | 36 |
| 33(32) | Paraphyses markedly capitate to 5-6µm, with yellowish green contents. Apothecia dull at first, yellowish at maturity. Spores 7-10 × 2-4.5µm. | |
| Thelebolus microsporus (fig. 5) | ||
| - | Paraphyses only slightly inflated above, without coloured contents. Apothecia whitish or grey. | 34 |
| 34(33) | Spores 5-7 × 3-4µm. Asci 38-42 × 6-7µm. Apothecia smoky grey, 0.3-0.4mm diam. | |
| Ascophanus cinerellus | ||
| - | Spores larger. Apothecia pale, white or yellowish. | 35 |
| 35(34) | Apothecia up to 1.2mm diam. Asci short stalked, 40-55 × 8-12µm. Spores 7.5-9 × 4.5-5.5µm. | |
| Coprotus glaucellus | ||
| - | Apothecia 0.2-0.5mm diam. Asci attenuate below, 65-85 × 10-15µm. Spores 8-10 × 5-6.5µm. | |
| Coprotus lacteus | ||
| 36(32) | Apothecia chestnut brown up to 1mm diam. Asci 160 × 13µm. Spores 13-16 x 8-11µm. Paraphyses forked, with swollen tips. | |
| Ascophanus misturae | ||
| - | Apothecia lighter coloured. Asci less than 150µm long. | 37 |
| 37(36) | Spores 14-18 × 9-11µm. Apothecia pale yellow/orange, up to 1.5mm diam. Asci cylindrical, 110-150 × 12-15µm. Paraphyses yellowish, slightly inflated to 4-5µm at apices. | |
| Coprotus ochraceus | ||
| - | Spores less than 15µm long. Apothecia up to 0.6mm diam. Asci less than 100µm long. | 38 |
| 38(37) | Apothecia bright yellow. Asci cylindrical clavate, attenuate below, 65-90 × 10-15µm. Spores 12-14 × 6-8.5µm. Paraphyses branched, apices inflated to 4-5µm, with yellow contents. | |||
| Coprotus aurorus | ||||
| - | Apothecia white/pale yellow, with darker margin. Asci broadly clavate, stalked below 40-55 × 15-30µm. Spores 9-15 × 6.5-9.5µm. Paraphyses inflated above to 5-8µm, hyaline. | |||
| Coprotus granuliformis | ||||
| 39(5) | Spores spherical or broadly ellipsoid, brown, ornamented with warts, anastomosing ridges or a reticulum. Asci clavate. Apothecium without excipulum. | |||
| (Ascodesmis, fig. 6) 40 | ||||
| - | Spores ellipsoid or spherical, hyaline at first, then purple, becoming brown at maturity; epispore smooth, finely verruculose, warted or cracked. Asci cylindrical. Excipulum present. | 45 | ||
| 40(39) |
|
|||
| - | Spores up to 16µm. | 41 | ||
| 41(40) | Spores ± spherical, L/B ratio mostly up to 1.2. | 42 | ||
| - | Spores ± broadly ellipsoidal, L/B ratio mostly 1.2 or more. | 43 | ||
| 42(41) | Spores ornamented with round warts, 8.5-11 × 8.3-10µm. | |||
| Ascodesmis nana | ||||
| - | Spores ornamented with a network of ridges, 10.5-14 × 9-12µm. | |||
| Ascodesmis sphaerospora | ||||
| 43(41) | Spores with a prominent reticulum of ridges (fig. 6), 11-15.5 × 8-13.5µm. Apothecia 150-300µm diam. | |||
| Ascodesmis microscopica (fig. 6) | ||||
| - | Spore ornament not a reticulum. | 44 | ||
| 44(43) | Spores with 1 simple or branched ridge and isolated or occasionally connected warts, 11-14.5 × 7-11.5µm. Apothecia up to 500µm diam. | |||
| Ascodesmis porcina | ||||
| - | Spores with isolated warts, some joined to form short ridges, but not a reticulum, often capitate, 9.5-12.5 × 7.5-10µm. Apothecia 50-150µm diam. | |||
| Ascodesmis nigricans | ||||
| 45(39) |
|
|||
| - | Spores firmly joined together, both in the ascus and after ejection (fig. 10). | |||
| (Saccabolus) 66 | ||||
| 46(45) | Spores spherical. | 47 | ||
| - | Spores ellipsoid. | 48 | ||
| 47(46) | Spores 10.5-13.5µm, epispore with numerous but isolated warts. | |
| Ascobolus brassicae (figs 8, 9) | ||
| - | Spores 11.5-13.5(15)µm, epispore with subparallel occasionally anastomosing lines. | |
| Ascobolus crosslandii | ||
| 48(46) | Spores very large, mostly 50-70 × 25-35µm, almost oblong with rounded ends, typically with few cracks in the epispore. | |
| Ascobolus immersus (figs 7, 9) | ||
| - | Spores smaller, with epispore smooth, warted or with cracks. | 49 |
| 49(48) | Epispore strongly and irregularly wrinkled with a vesiculose layer of pigment, 11.6-16 × 6.5-9.3µm. Paraphyses capitate up to 18µm. Apothecia up to 0.6mm diam. | |
| Ascobolus rhytidiosporus | ||
| - | Epispore not strongly wrinkled/vesiculose. | 50 |
| 50(49) | Epispore basically smooth or warted, perhaps with a few irregular cracks. | 51 |
| - | Epispore with a clear pattern of cracks or lines. | 56 |
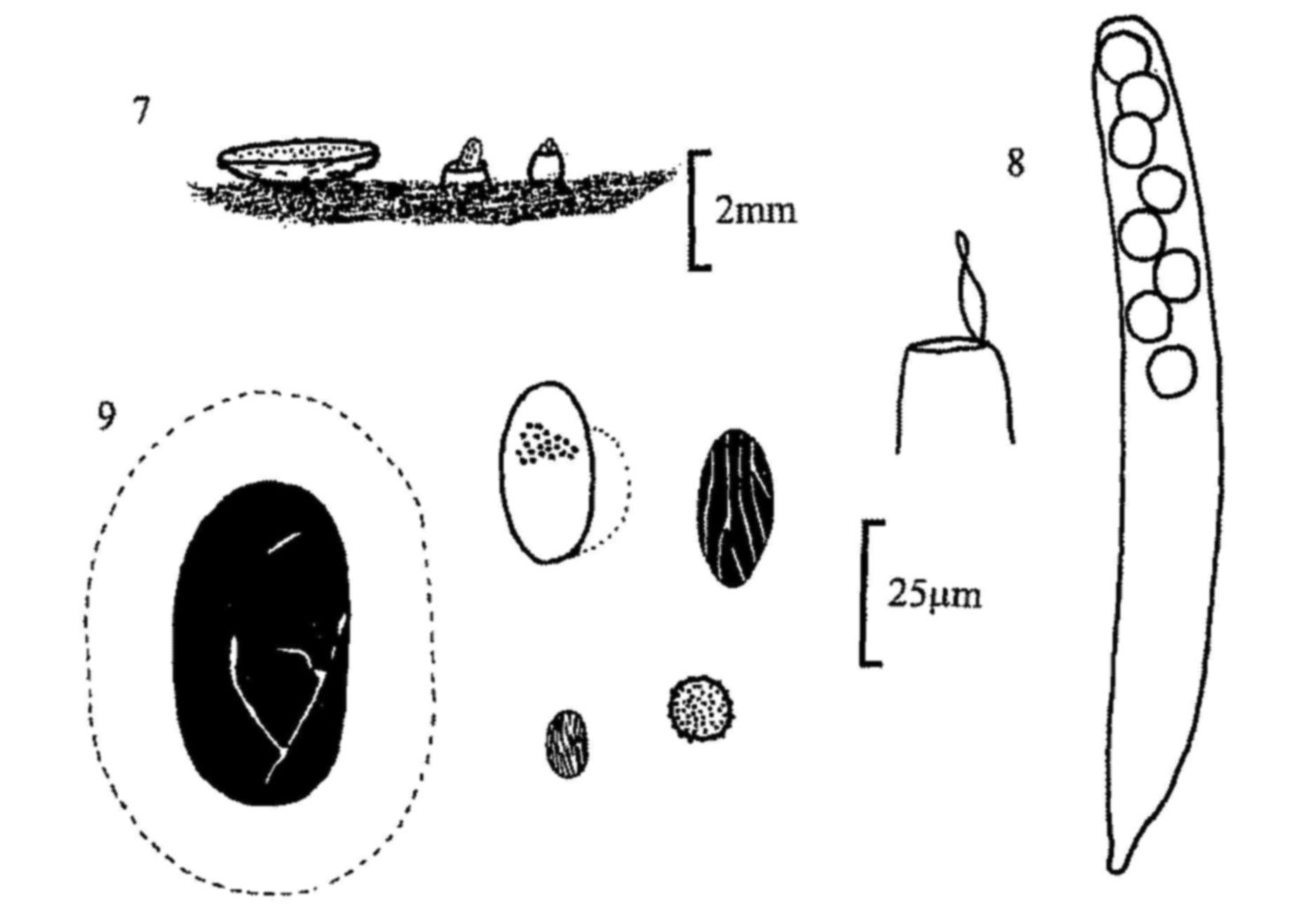
Fig. 7. Apothecia of, from left, Ascobolus furfuraceus, A.
immersus and A. albidus.
Fig. 8. A. brassicae, ascus with spores and detail of operculum.
Fig. 9. Ascospores of, clockwise from left, A. immersus, A. stictoideus,
A. albidus, A. brassicae and A. crenulatus.
| 51(50) | Spores up to 25µm long. | 52 |
| - | Spores longer, 25µm or more. | 54 |
| 52(51) | Epispore smooth, finely granular or punctate. Gelatinous material unilateral, not surrounding spore. | 53 |
| - | Epispore warted, spores 18.5-21(22.5) × (9)10-11.5µm, surrounded by gelatinous sheath. | |
| Ascobolus hawaiiensis | ||
| 53(52) | Spores 18-24 × 10-13µm. Hymenial mucus greenish yellow. Excipulum not brown. | |
| Ascobolus mancus | ||
| - | Spores 20-25 × 11-13µm. Hymenial mucus sulphur yellow. Excipulum with rich brown intercellular pigment. | |
| Ascobolus boudieri | ||
| 54(51) | Epispore smooth or finely granular, spores 23-29(32) × 12-17µm. | |
| Ascobolus elegans | ||
| - | Epispore warted. | 55 |
| 55(54) | Spores with a regular pattern of warts and intact epispore, 26-32 × 15-17.5µm. | |
| Ascobolus stictoideus (fig. 9) | ||
| - | Spores with irregular patches of thicker pigment, especially at the poles, 28-35 × 16-18µm. | |
| Ascobolus degluptus | ||
| 56(50) | Spores mostly 18 × 10µm or larger. | 57 |
| - | Spores mostly smaller than 20 × 10µm. | 61 |
| 57(56) | Apothecia small, mostly up to 1mm diam., colourless. Spores 20-35 × 11-14µm, epispore cracks distant, irregular, often anastomosing. | |
| Ascobolus albidus (figs 7, 9) | ||
| - | Apothecia larger, usually 1mm diam. or more, disc yellowish, greenish, purplish or brownish. | 58 |
| 58(57) | Apothecia crowded, purplish or purplish brown with intercellular pigment. Spores 18-28 × 10-12µm, with longitudinal anastomosing cracks. | |
| Ascobolus roseopurpurascens | ||
| - | Apothecia yellowish or greenish. | 59 |
| 59(58) | Spores 17-22 × 9.5-12µm with a few widely spaced and irregularly oriented cracks. | |
| Ascobolus michaudii | ||
| - | Spores with closely spaced, ± longitudinal, cracks, with varying degrees of anastomosis. | 60 |
| 60(59) | Apothecia furfuraceous, sessile. Ascus wall blue in iodine. Spores 19-28 × 10-14µm. | |
| Ascobolus furfuraceus (fig. 7) | ||
| - | Apothecia smooth, substipitate. Ascus wall only faintly blue in iodine. Spores 19-22 × 9.5-13µm. | |
| Ascobolus perplexans | ||
| 61(56) | Apothecia large, stipitate, 5-10mm diam. Spores 16-19.5 × 8.5-10µm, with subparallel, longitudinal, only rarely anastomosing lines. | |
| Ascobolus lignatilis | ||
| - | Apothecia up to 2mm diam. | 62 |
| 62(61) | Apothecia white. | 63 |
| - | Apothecia yellow, green or brownish. | 64 |
| 63(62) | Spores 13-17 × 7.5-8.5µm, with a coarse reticulum of fine cracks when mature. Only recorded on grouse, capercaillie etc. (Tetraonidae) dung. | |
| Ascobolus carletonii | ||
| - | Spores 16-20 × 8-10µm, with a pattern of longitudinal anastomosing cracks. Only recorded on deer dung. | |
| Ascobolus sacchariferus | ||
| 64(62) | Spores 14.5-16 × 8-9µm, epispore lines not densely crowded. | |
| Ascobolus cervinus | ||
| - | Spores smaller, epispore with densely crowded, rarely anastomosing cracks. | 65 |
| 65(64) | Apothecia greenish yellow, furfuraceous, with crenulate margin. Spores 9.5-15 × 6-8µm. | |
| Ascobolus crenulatus (fig. 9) | ||
| - | Apothecia brownish yellow to brown, smooth, with undifferentiated margin. Spores 12.5-14.5 × 7-8.5µm. | |
| Ascobolus minutus | ||
| 66(45) | Asci 4-spored. Spore clusters 42-58 × 14-20µm. Spores 16.5-23 × 9.5-12µm, smooth to finely punctate, but with a thick cap or girdle of reticulated or warted pigment. | |
| Saccobolus quadrisporus | ||
| - | Asci 8-spored. | 67 |
| 67(66) | Spore clusters ± globular, 17-26(39) × 15-20µm. | 68 |
| - | Spore clusters elongated, 2-3 times as long as wide. | 69 |
| 68(67) | Spore clusters compact, subglobose, with only the exposed surface of spores pigmented, ornamented with small and coarse warts. | |
| Saccobolus dilutellus | ||
| - | Spores loosely united in cluster, ornamented with small isolated warts covering most of their surface. | |
| Saccobolus globuliferellus | ||
| 69(67) | Apothecia yellow. Spores in 4 rows of 2 longitudinally arranged spores (fig. 10). | 70 | ||
| - | Apothecia hyaline or violaceous (some mature darker). Spores in 2 rows of 3 and 1 row of 2 (fig. 10). | 73 | ||
| 70(69) | Spore clusters 40µm or longer. | 71 | ||
| - | Spore clusters up to 40µm long. | 72 | ||
| 71(70) | Spore clusters 50-71 × 16-25µm. Spores 22-29 × 8.5-14.5µm, smooth or rarely finely punctate, with distant irregular cracks. | |||
| Saccobolus glaber (fig. 10) | ||||
| - | Spore clusters 43-51 × 14-17µm. Spores 16-22 × 7.5-9µm, with fine isolated warts. | |||
| Saccobolus citrinus | ||||
| 72(70) | Spores 14-17.5(19.5) × 7.5-8.5(10)µm, easily separated at maturity. Spore clusters becoming shorter and more rounded with maturity. Apothecia up to 300µm diam., inconspicuous due to their solitary nature and the predominantly brownish colour due to the mature spores. | |||
| Saccobolus truncatus (fig. 10) | ||||
| - |
|
|||
| 73(69) | Apothecia white, covered with tapering squamules composed of septate hyphae. Spore clusters 38-43 × 15-17µm. Spores 16-17.5 × 7-8.5µm, smooth or finely punctate. | |||
| Saccobolus caesariatus | ||||
| - | Apothecia not white, without tapering scales. | 74 | ||
| 74(73) | Spore clusters mostly over 40µm long. | 75 | ||
| - | Spore clusters mostly under 40µm long. | 76 | ||
| 75(74) | Spore clusters 38-62 × 14-19µm. Spores 13-21.5 × 6.5-9.5µm, smooth, finely warted or with reticulate cracks. Apothecia 0.2-2mm diam. | |||
| Saccobolus versicolor (fig. 10) | ||||
| - | Spore clusters 42-60 × 18-24µm. Spores very coarsely warted, 17.5-23 × 8.5-10µm (inc. warts). | |||
| Saccobolus beckii | ||||
| 76(74) | Spore clusters compact, 26-43 × 13-19µm. Spores 13.5-18 × 7.5-9.5µm, epispore with fine or coarse warts. Apothecia 0.3-0.8mm diam. | |
| Saccobolus obscurus | ||
| - | Spore clusters elongated, 28-37 × 10-13µm. Spores 10-14.5 × 5-7.5µm, epispore smooth or very finely granular. Apothecia 0.1-0.3mm diam. | |
| Saccobolus depauperatus | ||
| 77(4) | Asci operculate or bursting, without a subapical ring. Spores ellipsoid. | 78 |
| - | Apothecia white, often minutely hairy at the margin. Ascus dehiscing by a vertical slit; the slit is prevented from running right down the ascus by a subapical ring of thickening. Spores ellipsoid-fusiform. | |
| (Ascozonus, figs 14, 15) 90 | ||
| 78(77) | Asci 16-spored. Spores ellipsoid, 11-16 × 7-10µm. | |
| Coprotus sexdecemsporus | ||
| - | Asci more than 16-spored. | 79 |
| 79(78) | Asci 32-spored. | 80 |
| - | Asci more than 32-spored. | 84 |
| 80(79) | Asci very large, nearly 0.5mm long, spores 30-35 × 13-17µm (32-40 × 20-24µm in Kimbrough, 1969). Apothecia pale coloured. | |
| Thecotheus pelletieri | ||
| - | Asci and spores smaller. | 81 |
| 81(80) | Spores 10µm or longer. | 83 |
| - | Spores up to 10µm long. | 82 |
| 82(81) | Spores ellipsoid, with minute scattered warts visible under oil-immersion, 7-9 × 4-4.5µm. Apothecia densely crowded, 90-120µm diam., with 8-13 asci. Asci 32-55 × 16-18µm with (24-)32 spores. Paraphyses 1.5-2µm, clavate to 4-4.5µm. | |
| Thelebolus caninus | ||
| - | Spores subacute at apices, ca 6 × 4µm (described as 'minute'; this value is suggested by Boudier's comparison with R. dubius, for which measurements are given). Apothecia densely crowded, tawny yellowish-brown. | |
| Ryparobius brunneus | ||
| 83(81) | Spores 10-12.5 × 5-7.5µm. Asci clavate, 75-100 × 20-30µm. Paraphyses enlarged to 6µm at apex. | |
| Coprotus albidus | ||
| - | Spores 13.5-17.5 × 7-8µm. Asci 10-15 per apothecium, 120-175 × 50-75µm. Paraphyses filiform. | |
| Coprotus rhyparobioides | ||
| 84(79) | Asci with up to 64 spores. | 85 |
| - | Asci with many more than 64 spores—impractical to count. | 86 |
| 85(84) | Asci 64-spored, broad clavate with short stalk, 80-130 × 30-60µm. Spores 8-12 × 4-7µm. | |
| Coprotus niveus | ||
| - | Asci broadly clavate with up to 64 spores, 60-100 × 20-30µm. Spores 7-10 × 4.5-5.5µm. Apothecia superficial, on the surface of the substrate, yellowish brown, gregarious, united into a crust. | |
| Thelebolus crustaceus | ||
| 86(84) | Apothecia superficial, 400-600µm diam., with prominent, acuminate, superficial, 1-2-septate hairs, 80-190µm long, often roughened towards their apex, with one 1000+-spored ascus, 110-240 × 15-27µm. Spores very variable, 6.5-16 × 3.7-8.8µm (mostly 7.5-13 × 4.5-7µm). | |
| Lasiobolus monascus | ||
| - | Apothecia minute, rarely above 350µm diam., globose and immersed in substrate when young. Asci broad globose, with 100-200 spores. Usually only 1-3 asci in each apothecium, which dehisce by bursting at the apex. | 87 |
| (Other Ryparobius spp. will key out here [e.g. R. dubius, R. myriosporus, R. pachyascus and R. polysporus]. They all have scattered to gregarious, immersed to semi-immersed apothecia 100-200µm diam., with relatively few asci, each with 100-250 ellipsoid to subacuminate ca 5-7 × 3-4µm spores. There are insufficient modern observations to allow their identification and separation with confidence). | ||
| 87(86) | Apothecia with a few, but obvious, setae. Spores 9 × 7µm or larger. | 88 |
| - | Apothecia without setae. Spores ellipsoid, 6-9 × 3.5-4µm. | 89 |
| 88(87) | Spores ellipsoid, 9-11 × 7-9µm. Setae up to 600µm long. | |
| Trichobolus zukalii | ||
| - | Spores subglobose, 11-12 × 10-11µm. Setae up to 300µm long. | |
| Trichobolus sphaerosporus (fig. 11) | ||
| 89(87) | Apothecia and asci large, 170-250µm diam. | |
| Thelebolus stercoreus (fig. 12) | ||
| - | Apothecia and asci small, rarely above 80-90µm diam. | |
| Thelebolus nanus (fig. 13) | ||
| 90(77) | Asci 16(-24)-spored. Spores not closely aggregated into an imbricated mass, 13-14 × 6µm (8-9 × 4µm)[1]. Apothecial hairs rough, subulate. | |
| Ascozonus parvisporus | ||
| - | Asci with 32 or more spores. | 91 |
| 91(90) | Asci 32-spored. Spores 16.5-18 × 4.5-5µm (11-12 × 3-3.5µm)[1]. Apothecia with a single row of sharp, pointed, roughened hairs. | |
| Ascozonus crouanii | ||
| - | Asci more than 32-spored. | 92 |
| 92(91) | Asci 48-spored. Spores spindle-shaped, 12-14.5 × 2.5-4µm. | |
| Ascozonus leveillei | ||
| - | Asci more than 48-spored. | 93 |
| 93(92) | Asci 64-spored. | 94 |
| - | Asci more than 64-spored. | 95 |
| 94(93) | Apothecia with a short base of globose cells, with minutely roughened marginal hairs up to 30 × 8µm. Spores elliptic-fusoid, 12-14 × 3-5µm. | |
| Ascozonus woolhopensis (figs 14, 15) | ||
| - | Apothecia sessile, with aseptate smooth hairs. Spores 21 × 7.5µm (13-14 × 4.5-5µm)[1]. | |
| Ascozonus cunicularis | ||
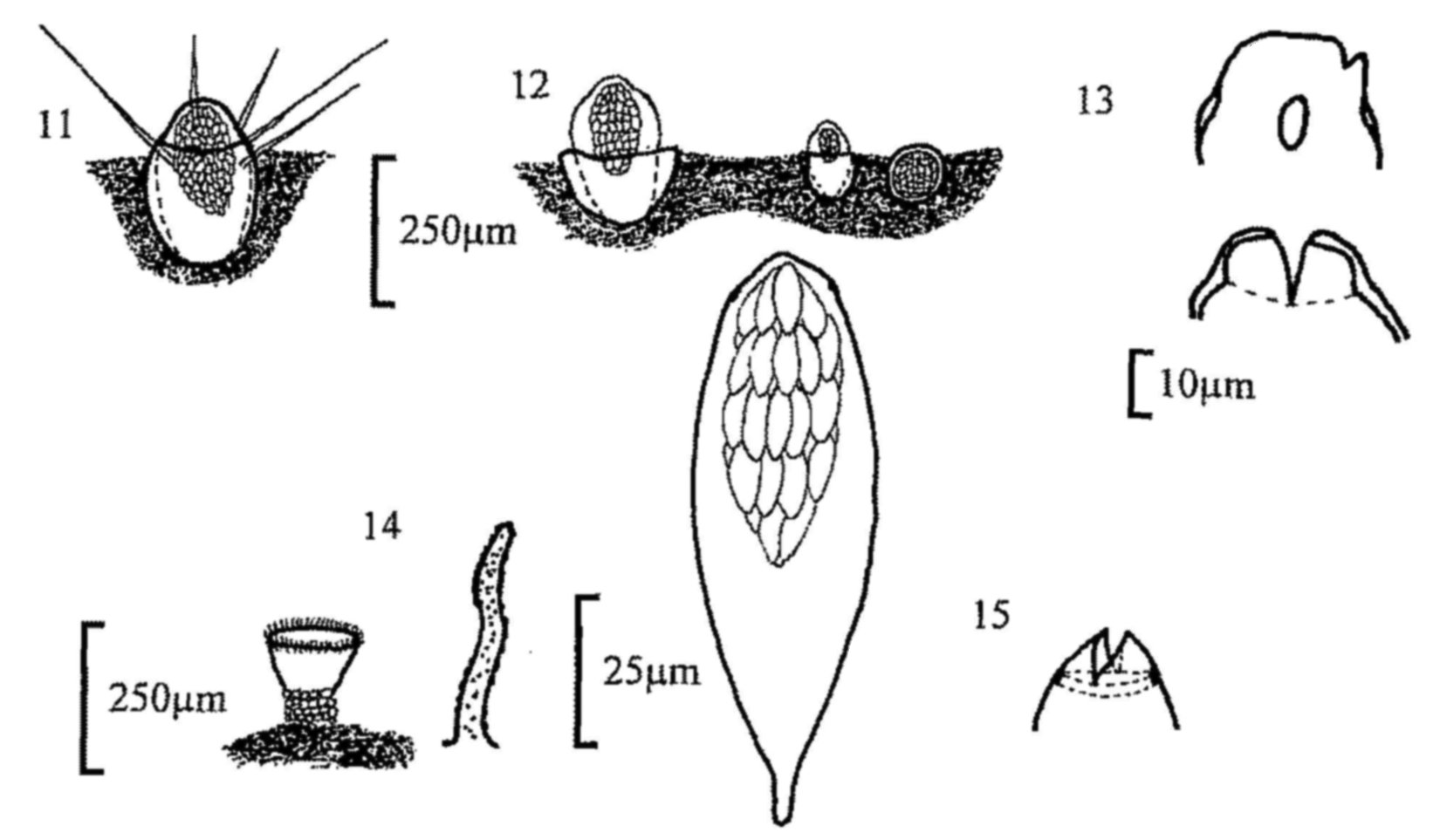
Fig. 11. Trichobolus sphaerosporus, apothecium.
Fig. 12. Thelebolus stercoreus, apothecium.
Fig. 13. T. nanus, mature and immature apothecia, and detail of ascus
dehiscence.
Fig. 14. Ascozonus woolhopensis, apothecium and apothecial hair.
Fig. 15. A. woolhopensis, ascus with spores and detail of dehiscence.
| 95(93) | Apothecia with a short base of globose cells, with short, irregular hairs. Asci 64-96-spored Spores elliptic-fusoid, 14-14.5 × 5-5.5µm (10-15 × 3.5-4µm)[1]. | |
| Ascozonus leveillanus | ||
| - | Apothecia sessile, dotted with hairs in connate groups of 2-3. Asci with 128 or more spores. Spores 10 × 5µm (7 × 3.5µm)[1]. | |
| Ascozonus subhirtus | ||
| 96(3) | Apothecia stalked. | 97 |
| - | Apothecia not stalked. | 98 |
| 97(96) | Apothecia up to 2mm diam., with a short cylindrical stalk, light brown. Asci 150 × 10µm. Spores hyaline, with 2 oil drops, occasionally 1-septate, 13-15 × 4.5µm. | |
| Lanzia cuniculi | ||
| - | Apothecia up to 3mm diam., pale olivaceous to grey, with a long, slender, reddish-brown stalk arising from a sclerotium in the dung. Asci 30-40 × 4-5µm. Spores ellipsoid, grey-brown, 4-4.5 × 2µm. | |
| Martininia panamaensis | ||
| 98(96) | Spores 7-11(14) × 1.75-2.75µm. ellipsoid, ellipsoid-fusiform or slightly clavate. Apothecia yellowish brown when fresh, drying darker, up to 1mm diam. Asci 42-60 × 7.5-9µm, pore weakly blue in iodine. | |
| Pezizella albula | ||
| - | Spores and asci smaller. | 99 |
| 99(98) | Spores linear, 3-5 × 1µm. Asci 30 × 5µm, cylindrical with a short stipe. Paraphyses not clavate but fused to form an epithecium. Apothecia pale pellucid, 0.5-1mm diam. | |
| Orbilia leporina | ||
| - | Spores longer, subulate, curved. | 100 |
| 100(99) | Spores 7-8.5 × 1.2-1.8µm. Asci 36-40 × 3-5µm, gradually tapering to a short base. Paraphyses enlarged to 3µm at apex, covered with brown granules. Apothecia light brown, 0.4-1.mm diam. | |
| Orbilia fimicola | ||
| - | Spores 8-10.55 × 0.9-1µm. Asci 30-45 × 3µm, cylindrical-clavate with narrow tapering base and truncate apex. Paraphyses 2µm diam., the tips with a crust-like secretion fusing together to form a shiny epithecium. Apothecia white to yellowish, 180-700µm diam. | |
| Orbilia fimicoloides | ||
| 1 (key 1,2) |
Perithecia occurring singly or in groups, but directly on the dung or buried in it (figs 16, 28, 19, 22, 27, 30, 32, 34-36). | 2 | ||
| - | Perithecia occurring in or on a mass of fungal tissue (stroma) growing in or on the dung (figs 32, 37). | 135 | ||
| 2(1) | Spores black, brown or dark olive-greenish. | 3 | ||
| - | Spores hyaline or pale coloured, at least under the microscope (may be coppery red en masse). | 117 | ||
| 3(2) | Spores smooth, without an ornamentation of hyaline pits. | 4 | ||
| - | Spores 1-celled, ornamented with hyaline pits. | |||
| (Gelasinospora) 114 | ||||
| 4(3) | Perithecia dark, olive, brown or black. | 5 | ||
| - | Perithecia reddish brown, orange or golden, globose, with a neck. Spores black, limoniform. | 116 | ||
| 5(4) | Perithecia globose, surmounted by a dense tuft of greyish green hairs, which may be branched or simple, straight or curly. Spores olivaceous, limoniform. Asci clavate, soon disappearing. (A large genus not characteristic of dung, but occurring occasionally). | |||
| Chaetomium (fig. 16) | ||||
| - | Perithecia more pyriform, or if globose then with a distinct neck, may be setose but not densely hairy, with clavate or cylindrical asci. | 6 | ||
| 6(5) | Each spore composed of 4 or more cells in a row (figs 17, 21). Asci bitunicate (figs 20, 23). | 7 | ||
| - | Spores 1- or 2-celled. Asci bitunicate or unitunicate. | 29 | ||
| 7(6) | Spores 16-32-celled, united firmly together in a bundle both in the ascus and after discharge. Germ slits usually absent. | |||
| (Sporormia) 8 | ||||
| - | Spores each with 4 or more cells, each spore free and surrounded by its own gelatinous sheath. Germ slits usually present. | |||
| (Sporormiella) 11 | ||||
| 8(7) | Spores 16-20-celled. | 9 | ||
| - |
|
|||
| 9(8) |
|
|||
| - | Spores smaller. | 10 | ||
| 10(9) | Spores 16-celled, 37-45 × 3µm. Asci 50-60 × 10-12µm. | |||
| Sporormia sp. (fig. 17) | ||||
| [recorded as S. fimetaria by Richardson (1972); see also Bell (1983) and Dissing (1992)] | ||||
| - | Spores 16-20-celled, 50-57 × 3.5-4.5µm. Asci 70-80 × 12-16µm. | |||
| Sporormia fimetaria | ||||
| (These two taxa may represent the extremes of S. fimetaria). | ||||
| 11(7) | Spores 4-celled. | 12 | ||
| - | Spores more than 4-celled. | 22 | ||
| 12(11) | Spores more than 65-70µm long. | 13 | ||
| - | Spores less than 65-70µm long. | 15 | ||
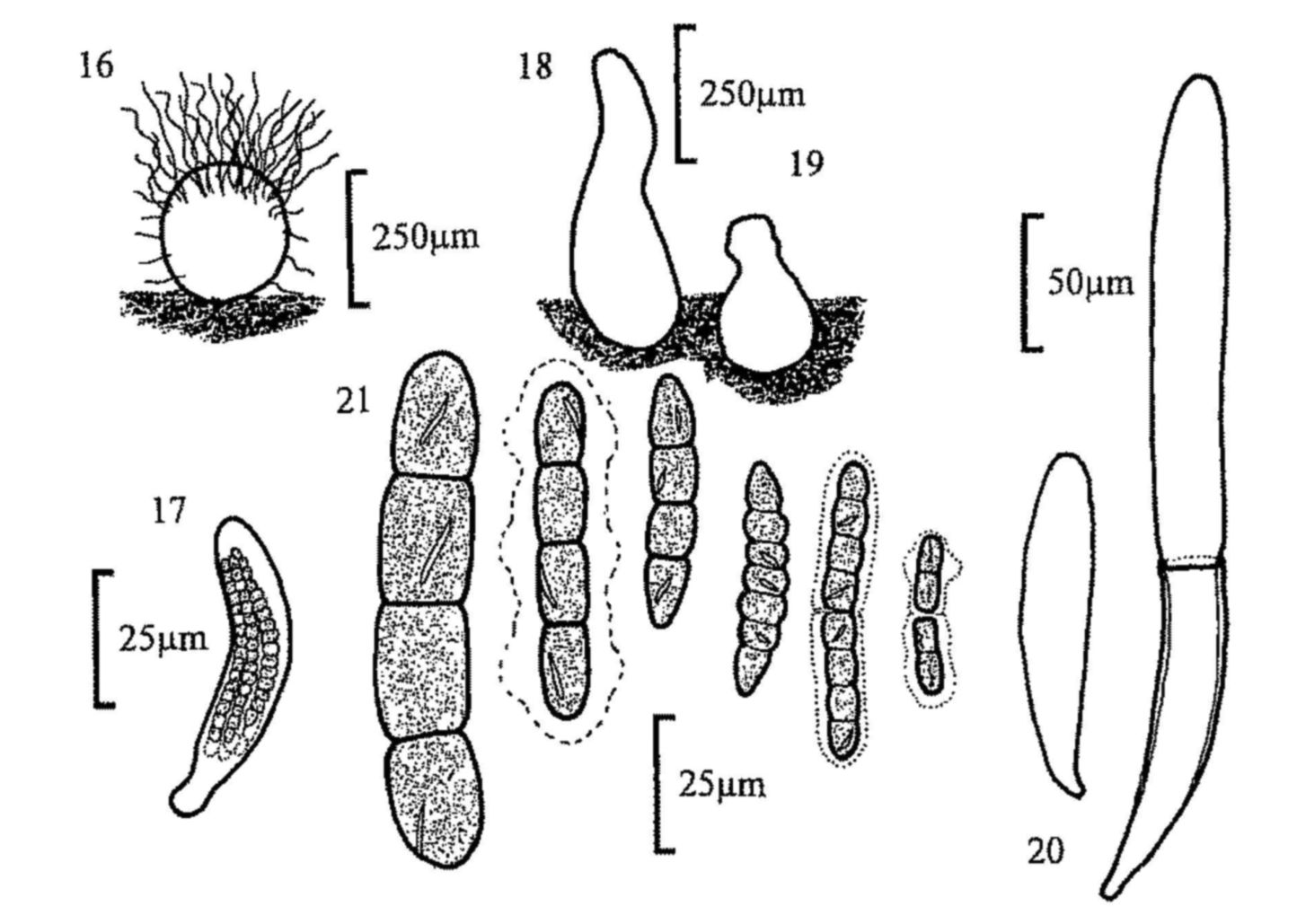
Fig. 16. Chaetomium sp., perithecium and spore.
Fig. 17. Sporormia sp., ascus and spores.
Fig. 18. Sporormiella ovina, pseudothecium.
Fig. 19. S. intermedia, pseudothecium.
Fig. 20. S. intermedia, immature bitunicate ascus and mature ascus with outer
layer ruptured.
Fig. 21. Ascospores of, from left, S. ovina, S. intermedia (with
gelatinous sheath characteristic of the genus), S. lageniformis, S. vexans,
S. bipartis and S. minima.
| 13(12) |
|
|||
| - | Spores longer than 90µm. | 14 | ||
| 14(13) | Spores 90-118 × 15-20µm. Asci tapering gradually from the broadest part near the apex to a 'stipe'. | |||
| Sporormiella ovina (figs 18, 21) | ||||
| - | Spores 91-114 × (14)18-21µm. Asci cylindrical, abruptly contracted below to a short 'stipe'. | |||
| Sporormiella borealis | ||||
| 15(12) | Spores mostly less than 35µm long. | 16 | ||
| - | Spores mostly between 35-60µm long. | 19 | ||
| 16(15) | Spores less than 25µm long. | 17 | ||
| - | Spores 25-35(38)µm long. | 18 | ||
| 17(16) | Spores (15)17-24(26) × 5-7µm, end cells broadly conical. Ascospores uniseriate. Asci 120-135µm long. Pseudothecia 250-300µm diam. | |||
| Sporormiella pulchella | ||||
| - | Spores 16-22 × 4.5-5.5µm, end cells subovate. Ascospores biseriate. Asci 95-125µm long. Pseudothecia 300-350µm diam. | |||
| Sporormiella nigropurpurea | ||||
| 18(16) | Spores 30-38.5 × 5.5-6.5µm. Asci clavate, tapering gradually below to a 'stipe'. | |||
| Sporormiella leporina | ||||
| - | Spores 27-36(38) × 4-6(8)µm, tending to break in two at the middle septum. Asci cylindrical, abruptly contracted below. | |||
| Sporormiella minima (fig. 21) | ||||
| 19(15) | Spores with end cells rounded. Asci cylindrical, abruptly contracted below. | 20 | ||
| - | Spores with end cells tapered and slightly conical. Asci clavate, tapering gradually to a long stalk. | 21 | ||
| 20(19) | Spores 45-65 × 8-11.5µm. | |||
| Sporormiella intermedia (figs 19-21) | ||||
| - |
|
|||
| 21(19) | Spores 45-60 × 11.5-14µm, germ slits parallel with long axis. | |||
| Sporormiella grandispora | ||||
| - | Spores 35-45(48) × 7-9(10)µm. | |||
| Sporormiella lageniformis (fig. 21) | ||||
| 22(11) |
|
|||
| - | Spores more than 5-celled. | 23 | ||
| 23(22) | Spores 7- or 8-celled. | 24 | ||
| - |
|
|||
| 24(23) | Spores 7-celled. | 25 | ||
| - | Spores 8-celled. | 26 | ||
| 25(24) | Spores 40-55 × 7-9µm, readily disarticulating, the end cells longer than wide, the rest shorter than wide. | |||
| Sporormiella vexans (fig. 21) | ||||
| - | Spores 70-80 × 16-18µm, end cells rounded. | |||
| Sporormiella heptamera | ||||
| 26(24) | Spores mostly longer than 45µm. | 27 | ||
| - | Spores less than 50µm long, not disarticulating at the central septum. | 28 | ||
| 27(26) | Spores 45-60 × 5-7.5µm, disarticulating at the central septum, all cells the same width. | |||
| Sporormiella bipartis (fig. 21) | ||||
| - | Spores 50-59 × 10-12µm, not disarticulating, 3rd cell down wider than the others. | |||
| Sporormiella corynespora | ||||
| 28(26) | Spores (33)37-40(49) × 7-9µm, cylindrical. Asci abruptly contracted below. | |||
| Sporormiella pascua | ||||
| - | Spores 40-48 × 7-8µm, fusiform cylindrical. Asci gradually tapered below. | |||
| Sporormiella octomera | ||||
| 29(6) | Spores obviously 2-celled at maturity. | 30 | ||
| - | Spores 1-celled, or appearing 1-celled at maturity. (Those of Podospora, Schizothecium etc. are 2-celled in early stages of their development, but only one cell matures to become pigmented; the other remains hyaline, often collapses, and may be difficult to see). | 47 | ||
| 30(29) | Spores 23-28 × 13-17µm, upper cell dark, 15-19µm, with close, blunt spines giving the impression of a pitted spore surface, with apical germ pore, the lower cell hyaline, 6-8.5µm, smoky-brown. Asci unitunicate, 4-spored. Perithecia 400µm diam. | |||
| Apiosordaria verruculosa (fig. 24) | ||||
| - | Both cells of spore similar in shape, size and colour. | 31 | ||
| 31(30) | Asci unitunicate. Spores with a 'gelatinous' appendage at each end. Perithecial neck with setae. | 32 | ||
| - | Asci bitunicate. Spores without gelatinous appendages, although a sheath may be present. | 33 | ||
| 32(31) | Spores 38-48 × 11-14µm, appendages longitudinally fibrillate. | |||
| Zygospermella striata | ||||
| - | Spores 46-68 × 11-17µm, appendages hollow, not fibrillate. | |||
| Zygospermella insignis (fig. 25) | ||||
| 33(31) | Spores with each end truncated by a germ pore. Pseudothecia with dark bristles at neck. | |||
| (Trichodelitschia) 34 | ||||
| - | Spores with rounded ends and germ slits along the sides. Pseudothecial neck smooth or hairy, but without setae. | |||
| (Delitschia, fig. 26) 36 | ||||
| 34(33) |
|
|||
| - | Spores smaller. | 35 | ||
| 35(34) | Spores 20-27.5 × 8-11µm. | |||
| Trichodelitschia bisporula (figs 22, 23) | ||||
| - |
|
|||
| 36(33) | Asci ca 256-spored. Spores 14-15 × 6-8µm. | |||
| Delitschia myriaspora | ||||
| - | Asci 8-spored. | 37 | ||
| 37(36) | Spores less than 20µm long. | 38 | ||
| - | Spores more than 20µm long. | 41 | ||
| 38(37) |
|
|||
| - | Spores 10-20µm long. | 39 | ||
| 39(38) |
|
|||
| - | Spores longer. | 40 | ||
| 40(39) | Spores 14-18 × 6-10µm, uniseriate. Asci 70-90 × 7-16µm. | |||
| Delitschia niesslii | ||||
| - | Spores (16)18-20(22.5) × 6-7.5µm, biseriate. Asci 80-145 × 20-25µm. | |||
| Delitschia consociata (fig. 26) | ||||
| 41(37) | Spores mostly wider than 20µm. | 42 | ||
| - | Spores mostly less than 20µm wide. | 43 | ||
| 42(41) |
|
|||
| - | Spores 50-70 × 25-33µm. | |||
| Delitschia winteri (fig. 26) | ||||
| 43(41) | Spores 20-25 × 4.5-6µm, the cells slightly tapered and almost completely separated. Pseudothecia hairless, globose, ca 200µm diam. | |
| Delitschia leptospora (fig. 26) | ||
| - | Spores longer and wider. | 44 |
| 44(43) | Spores transversely septate. | 45 |
| - | Spores obliquely septate, deeply constricted at the septum, 35-50 × 15-18µm. | |
| Delitschia didyma | ||
| 45(44) | Pseudothecia hairy. Spores 37-50 × 17-20µm, not deeply constricted at the septum. | |
| Delitschia chaetomioides | ||
| - | Pseudothecia smooth. | 46 |
| 46(45) | Spores biseriate, 45-55 × 13-16µm, one cell usually larger than the other, deeply constricted at the septum and readily separating. | |
| Delitschia canina | ||
| - | Spores uniseriate, 40-55 × 16-21µm, both cells equal. | |
| Delitschia patagonica | ||
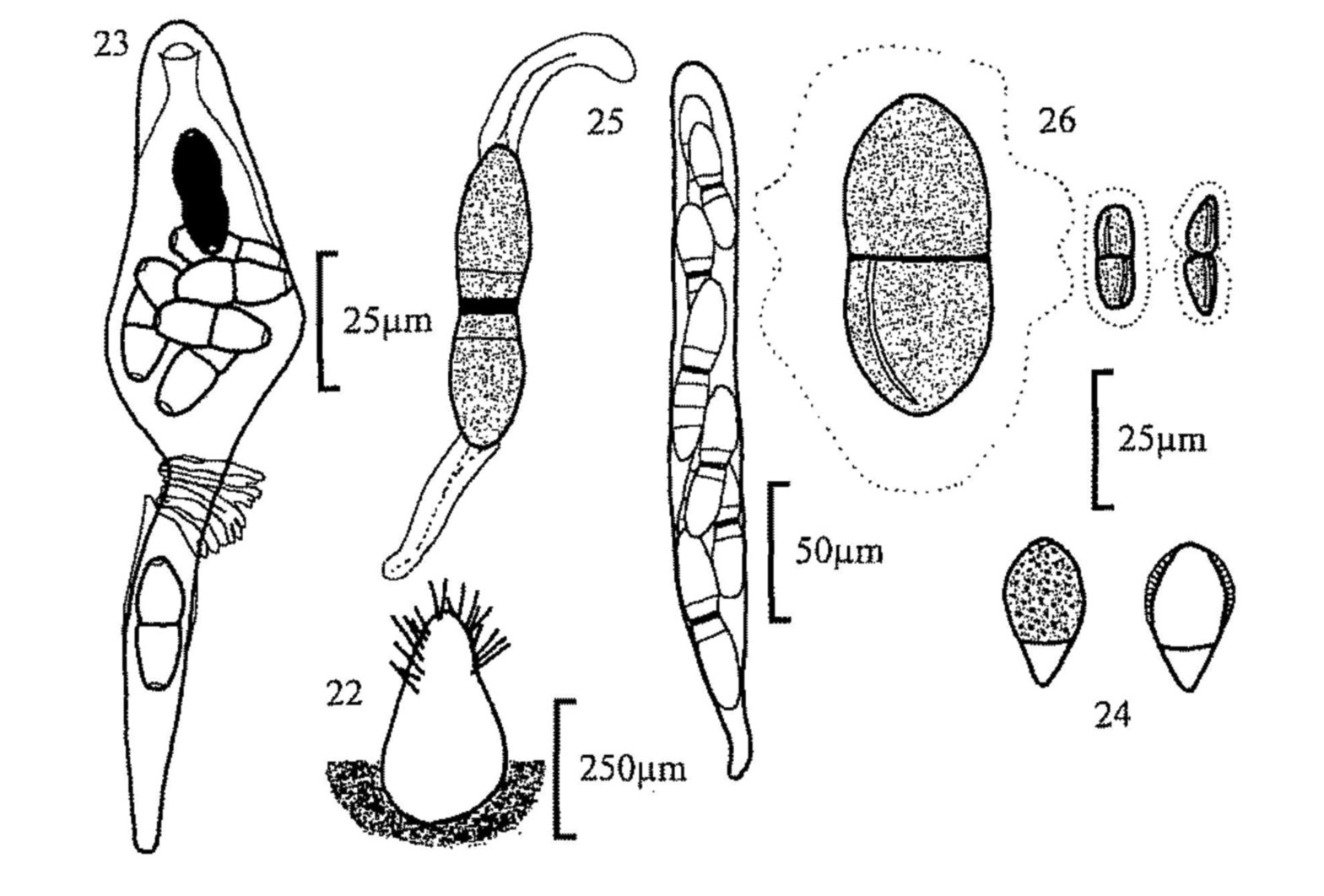
Fig. 22. Trichodelitschia bisporula, pseudothecium.
Fig. 23. T. bisporula, expanded ascus broken through the outer wall, with
spores.
Fig. 24. Apiosordaria verruculosa, ascospores.
Fig. 25. Zygospermella insignis, ascus and ascospore.
Fig. 26. Ascospores of, from left, Delitschia winteri, D. consociata and
D. leptospora.
| 47(29) | Spores with colourless 'gelatinous' secondary appendages (caudae, fig. 28) at one or both ends (not always easy to see; mounting in Indian ink is useful, and essential for some). A hyaline (empty) cell, the primary appendage (fig. 28), may also be present. | 48 |
| - | Spores without caudae, although a colourless gelatinous sheath may be present. Primary appendages present or absent. | 88 |
| 48(47) | Perithecia often hairy or tomentose when young. Immature spores long, wavy cylindrical, with a row of globules, and more likely to be seen than mature spores (fig. 29). Secondary appendages thin, simple, up to 60 × 3µm. Mature spores with a dark cell 14-25 × 7-13µm and pedicel (primary appendage) 25-50 × 3-6µm. | |
| (Cercophora) 49 | ||
| - | Perithecia often with scales or setae at the neck or tomentose. Caudae, simple or compound. Immature spores clavate or ellipsoid, not long, wavy cylindrical. Mature spores readily observed. | 51 |
| 49(48) | Immature spores 45-70 × 4-6µm. | 50 |
| - | Immature spores smaller, 38-52 × 3-3.5µm. Mature spores with upper (dark) cell 14-18 × 7-9µm; hyaline pedicel 27-36 × 3-3.5µm. | |
| Cercophora silvatica | ||
| 50(49) | Perithecia with white or grey tomentum. Young spores 45-65 × 4.5-6µm. Mature spores with upper cell 17-25 × 8.5-13µm and pedicel 30-50µm long. | |
| Cercophora coprophila (fig. 29) | ||
| - | Perithecia with flexuose brown hairs and, at the neck, tufts of agglutinated, swollen, obtuse hairs. Young Spores 52-68 × 4-5µm. Mature spores with upper cell 15-25 × 9-11µm and pedicel 35-45µm long. | |
| Cercophora mirabilis | ||
| 51(48) | Primary appendage absent. | |
| (Arnium, fig. 28) 52 | ||
| - | Primary appendage present. | 60 |
| 52(51) | Asci (64-)128-spored. Spores 18-26 × (10)12-15µm. Perithecial neck sometimes with rigid, brown, septate hairs up to 330µm. | |
| Arnium leporinum | ||
| - | Asci 4- or 8-spored. | 53 |
| 53(52) | Asci 4-spored. | 54 |
| - | Asci 8-spored. | 55 |
| 54(53) | Spores ellipsoid, sometimes inequilaterally flattened, 44-54 × 22-30µm, with 1 apical germ pore, caudae not swelling in water. Perithecium usually with lateral tufts of agglutinated hairs up to 550µm long. | |
| Arnium arizonense | ||
| - | Spores evenly ellipsoid-fusiform, 31-55 × 18-25µm, with germ pore at each end, caudae covering germ pores, 35-60 × 7-11µm, but rupturing and swelling to up to 130 × 50µm, and becoming diffuse and irregular. Perithecial neck covered with rigid hairs up to 190 × 2.5µm. | |
| Arnium hirtum | ||
| 55(53) | Perithecial neck distinctly setose with rigid hairs. | 56 |
| - | Perithecial neck without setae. | 57 |
| 56(55) | Spores evenly ellipsoid-fusiform, 31-55 × 18-25µm, with germ pore at each end, caudae covering germ pores, 35-60 × 7-11µm, but rupturing and swelling up to 130 × 50µm, and becoming diffuse and irregular. Perithecial neck covered with rigid hairs up to 190 × 2.5µm. | |
| Arnium hirtum | ||
| - | Spores slightly inequilateral, 35-43 × 17-23µm, caudae 50-75 × 5-8µm, not covering germ pores. Perithecial neck with brown hairs up to 250µm long. | |
| Arnium cervinum | ||
| 57(55) | Perithecia covered with a dense tomentum of septate flexuous hairs. Spores mostly longer than 45µm. Only occasionally fimicolous. | 58 |
| - | Perithecia without a tomentum. Spores up to 45µm. | 59 |
| 58(57) | Spores (40)45-54 × 25-35µm, uniseriate. Tomentum pale or grayish. | |
| Arnium olerum | ||
| - | Spores 47-70 x 20-30µm, biseriate above. Tomentum olivaceous brown. | |
| Arnium tomentosum | ||
| 59(57) | Spores somewhat inequilateral, rounded below, pointed above, 31-40 × 18-24µm, caudae 50-120 × 6-10µm, with 1 apical germ pore not covered by cauda. | |
| Arnium caballinum | ||
| - | Spores equilateral, 36-44 × 20-23µm, caudae 50-80 × 6-8µm, covering germ pores. | |
| Arnium mendax | ||
| 60(51) | Perithecia with scales at the neck, composed of inflated and agglutinated cells (fig. 27, S. conicum). | |
| (Schizothecium) 61 | ||
| - | Perithecia setose or hairy at the neck, but not with inflated cells, or neck black but almost hairless. | |
| (Podospora) 70 | ||
| 61(60) | Asci 4-spored. | 62 | ||
| - | Asci 8-spored. | 63 | ||
| 62(61) | Spores 11-14.5 × 6.5-9µm. | |||
| Schizothecium nanum (fig. 28) | ||||
| - |
|
|||
| 63(61) | Spores more than 30µm long. | 64 | ||
| - | Spores less than 30µm long. | 65 | ||
| 64(63) | Perithecia crowned with a fascicle of long agglutinated hairs at the neck, up to 335µm long. Spores 31-40 × 15-25µm, biseriate. | |||
| Schizothecium aloides | ||||
| - | Perithecia with shorter, less remarkable tufts. Spores 30-45 × 19-24µm, ± uniseriate. | |||
| Schizothecium glutinans | ||||
| 65(63) | Perithecial neck with rigid setae, as well as agglutinated hairs (which may be greatly reduced). Asci 140-210 × 19-25µm, broadest at the markedly rounded apex. Spores 18-23 × 11-14µm. | |||
| Schizothecium pilosum | ||||
| - | Perithecial neck without rigid setae. Asci broadest in the middle. | 66 | ||
| 66(65) | Spores mostly over 23µm long. | 67 | ||
| - | Spores up to 23µm long. | 69 | ||
| 67(66) | Spores 22-25(27) × 11-13µm. Scales at neck distinct. | |||
| Schizothecium hispidulum | ||||
| - | Spores wider, 12-19µm | 68 | ||
| 68(67) | Perithecia 0.5-1mm high, scales at neck usually well developed. Spores (23)26-30 × 12-17µm. | |||
| Schizothecium conicum (fig. 27) | ||||
| - | Perithecia 1-2mm diam., subpyriform, neck velvety with indistinct scales. Spores 24-28 × 15-19µm. | |||
| Schizothecium squamulosum | ||||
| 69(66) | Spores 17-23 × 8.5-13.5µm, primary appendage slender cylindrical, 6-8 × 2µm. Perithecia 0.25-0.7mm high, sometimes with poorly developed scales. | |||
| Schizothecium vesticola (fig. 28) | ||||
| - | Spores 11-14 × 6-8µm, primary appendage short, 2µm long, almost triangular. Perithecia 0.3-0.45mm high, with short agglutinated hairs. | |||
| Schizothecium cervinum | ||||
| 70(60) |
|
|||
| - | Asci with more than 4 spores. | 71 | ||
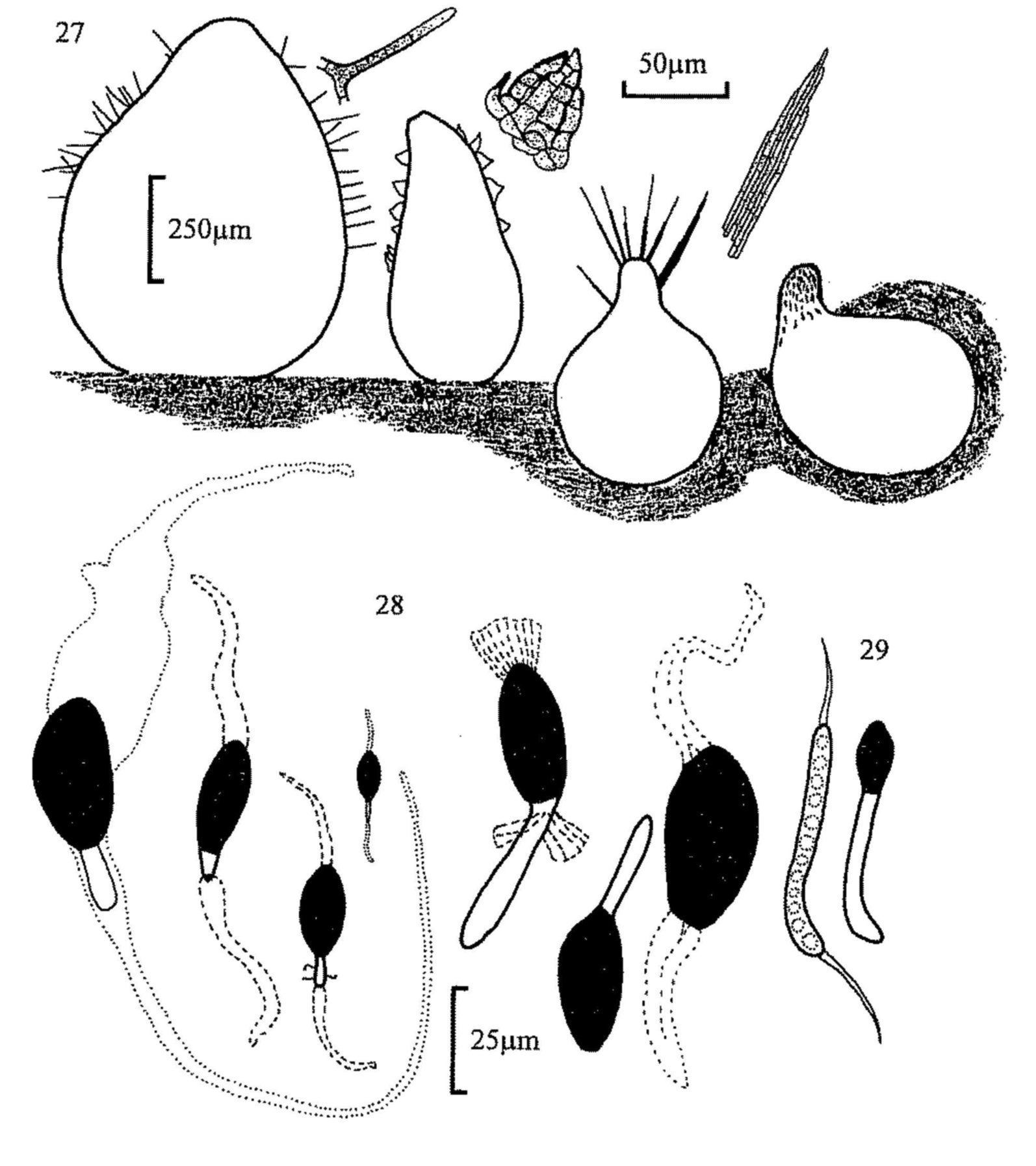
Fig. 27. Perithecia, from left, of Podospora appendiculata,
Schizothecium conicum, P. excentrica and P. decipiens, with detail of
hairs.
Fig. 28. Ascospores of, from left, Podospora excentrica, P.
appendiculata, S. vesticola, S. nanum, P. decipiens, 'P.
dagobertii' and Arnium sp.
Fig. 29. Cercophora coprophila, immature (l) and mature (r) ascospores.
| 71(70) | Asci 8-spored. | 72 | ||
| - | Asci with more than 8 spores. | 82 | ||
| 72(71) | Spores more than 45µm long. | 73 | ||
| - | Spores less than 45µm long. | 74 | ||
| 73(72) | Spores 48-60 × 27-31µm, caudae apparently striate. Perithecia superficial, covered with rigid, nonagglutinated hairs up to 120µm. | |||
| Podospora fimiseda | ||||
| - | Spores 50-68 × 22-32µm, caudae apparently segmented, with an intestine-like appearance. Perithecia immersed to superficial, with a long neck, tomentose with long flexuous hairs when young, more or less glabrous when mature. | |||
| Podospora intestinacea | ||||
| 74(72) | Perithecia superficial, ovoid to globose, covered with short (up to 100µm), sparse, radiating, hyaline tipped, hairs. Spores 24-31 × 11-15µm, with simple caudae. | |||
| Podospora appendiculata (figs 27, 28) | ||||
| - | Perithecia with base immersed in substrate, pyriform, without such hairs. | 75 | ||
| 75(74) | Perithecial neck with short tubercular hairs, up to 20µm long. Spores 32-42 × 17-22µm, with a long but withering primary appendage. Caudae in two rings, one inserted near the base of the primary appendage, the other at the spore apex. The individual filaments may be free, but often clump together to form an apparently broad appendage. | |||
| Podospora decipiens (figs 27, 28) | ||||
| - | Perithecial hairs longer. Caudae single or 4 at each end. | 76 | ||
| 76(75) | Spores with 4 caudae at each end. | 77 | ||
| - | Spores with a single cauda at each end. | 78 | ||
| 77(76) |
|
|||
| - |
|
|||
| 78(76) | Spores less than 30 × 15µm. | 79 | ||
| - | Spores larger than 30 × 15µm. | 80 | ||
| 79(78) | Spores 21-28 × 11-14µm, primary appendage 12-14 × 4µm. Perithecia 0.3-0.5mm diam., neck setose with rigid cylindrical hairs. Asci 200-250 × 22-26µm, broadest in the middle. | |
| Podospora ellisiana | ||
| - | Spores 18-23 × 11-14µm, primary appendage 4-8 × 3µm. Perithecia 0.2-0.3mm diam., neck setose with rigid hairs. Asci 140-210 × 19-25µm, broadest at the markedly rounded apex. | |
| Schizothecium pilosum | ||
| 80(78) | Perithecia ca O.9-1.4mm high × 0.6-0.7(0.85)mm diam., neck not hairy. Spores (29)36-45 × (17.5)22-27µm, caudae ephemeral and difficult to see, even in Indian ink. | |
| Podospora pyriformis | ||
| - | Perithecial neck with tufts of rigid hairs. | 81 |
| 81(80) | Perithecia 0.38-0.53mm high × 0.21-0.38mm diam., ± immersed, with hairs at the neck up to 335µm long, grouped in rigid fascicles. Spores slightly flattened on one side, 30-37 × 18-24µm, caudae invisible in water. | |
| Podospora excentrica (figs 27, 28) | ||
| - | Perithecia ca 0.8-1.4mm high × 0.4-0.7mm diam., semi-immersed, hairy all over, flexuous below, rigid and pointed at the neck up to 170µm. Spores 33-45 × 22-27µm. | |
| Podospora perplexens | ||
| 82(71) | Asci 16-32-spored. Perithecial neck with short tubercular hairs. Spores 25-36 × 15-24µm. Caudae in two rings, one inserted at the base of the primary appendage, the other at the spore apex; individual filaments may be separate or clumped to appear as a broad single appendage (cf. P. decipiens). | |
| Podospora pleiospora | ||
| - | Asci with more than 32 spores. | 83 |
| 83(82) | Perithecia with tufts of rigid hairs at neck. Asci with more than 64 spores. | 84 |
| - | Perithecia without tufts of rigid hairs. Asci 64-spored. | 87 |
| 84(83) | Spores 14-17 × 9-11µm. Asci 256-spored. Perithecia ca 500µm diam., immersed, except for the neck, which has tapered tufts of hairs up to 300µm. | |
| Podospora curvicolla | ||
| - | Spores larger. Perithecia semi-immersed. | 85 |
| 85(84) | Spores (18)20-26 × 12-16µm, caudae of 2-several filaments covered with granules. Asci 512-spored. Perithecia up to 1mm high × 0.95mm diam., neck with rigid but non-agglutinated hairs up to 130µm long. | |||
| Podospora granulostriata | ||||
| - | Caudae simple, without granular appearance. Asci 128-spored. Perithecia not larger than 750µm high × 500µm diam., with rigid, non-agglutinated hairs up to 190µm long at neck. | 86 | ||
| 86(85) |
|
|||
| - |
|
|||
| (See discussion in Lundqvist (1972) on these last three names) | ||||
| 87(83) | Spores 24-34 × 14-19µm, caudae in two rings, one inserted at the base of the primary appendage, the other at the spore apex; individual filaments may be separate or clumped to appear as a broad single appendage (cf. P. decipiens/P. pleiospora). Perithecia ca 0.6-1.1mm high × 0.4-0.5mm diam., covered with flexuous hairs or rarely smooth. | |||
| Podospora myriaspora | ||||
| - | Spores 15-20 × 10-15µm, caudae small, simple and evanescent. Perithecia 0.4-0.5mm high, covered with long flexuous hairs. | |||
| Podospora collapsa | ||||
| 88(47) | Spores with primary appendage. | 89 | ||
| - | Spores without primary appendage. | 93 | ||
| 89(88) | Spores with primary appendage directed towards base of ascus. | 90 | ||
| - | Spores with primary appendage directed towards apex of ascus. | |||
| (Anopodium) 91 | ||||
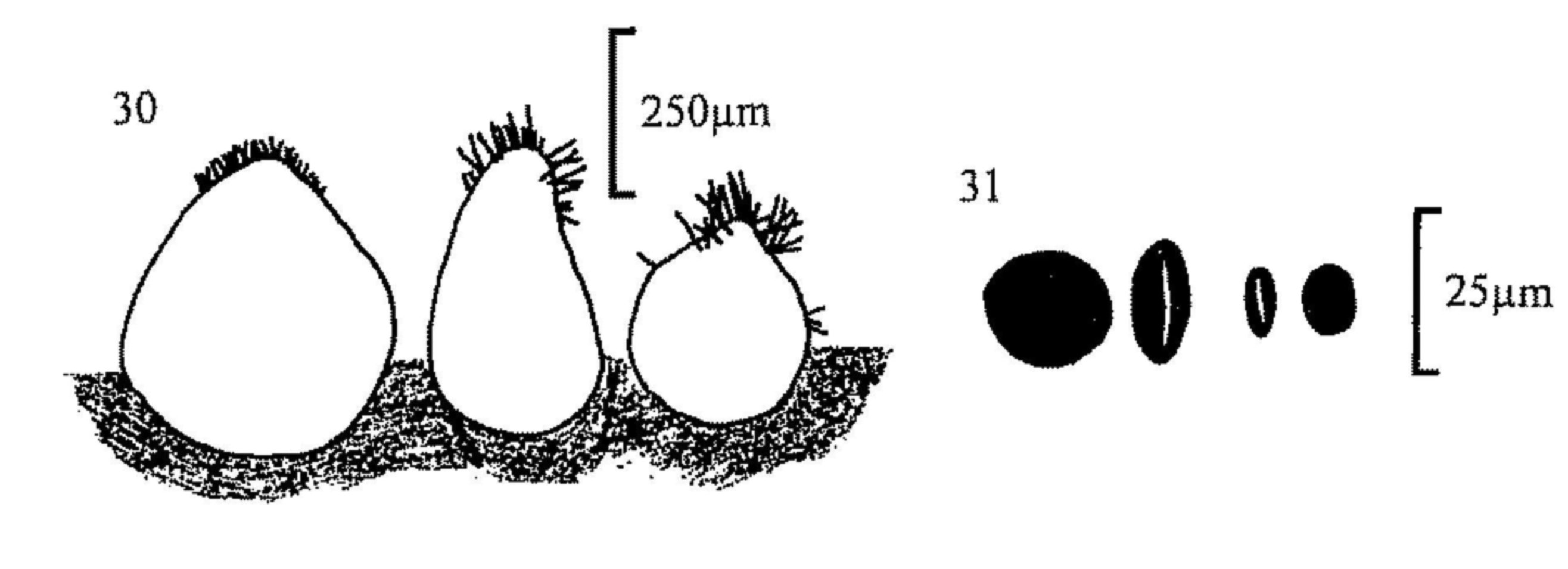
Fig. 30.. Perithecia of, from left, Coniochaeta ligniaria, C.
scatigena and C. hansenii.
Fig. 31.. Ascospores of C. scatigena (l) and C. ligniaria (r).
| 90(89) | Spores 34-45 × 19-25µm, without caudae but surrounded by a thin (ca 5µm) gelatinous sheath. Perithecia ca 0.5-0.7mm diam., ± smooth. | |
| Podospora globosa | ||
| - | Spores 17-20 × 8-9.5µm, flattened on one side, convex on the other. Perithecia 0.3-0.45µm diam., with distal cells of agglutinated hairs fimbriate. | |
| Podospora fimbriata | ||
| 91(89) | Perithecia hairy. Spores 27-32 × 16-19µm, appendage 15-18 × 2.5-3µm. | |
| Anopodium ampullaceum | ||
| - | Perithecia glabrous. | 92 |
| 92(91) | Spores 28-32 × 16-21µm, appendage 12-15 × 3-3.8µm. | |
| Anopodium epile | ||
| - | Spores 30-37 × 16-20µm, appendage 24-27 × 5µm. | |
| 'Podospora' dagobertii (fig. 28) | ||
| (The combination in Anopodium has not been made; see Lundqvist, 1964, 1972) | ||
| 93(88) | Spores flattened, disc shaped, with a germ slit around the edge. Perithecial neck with short (up to 120µm) setae. | |
| (Coniochaeta, figs 30, 31) 94 | ||
| - | Spores ellipsoid. Perithecial neck without setae or with very prominent (up to 950µm) tufts of agglutinated hairs. | 99 |
| 94(93) | Asci with numerous (64-128) spores. | 95 |
| - | Asci 8-spored. | 96 |
| 95(94) | Spores 6-10 × 5-9 × 4-7µm. Perithecial setae up to 120µm long. | |
| Coniochaeta hansenii (fig. 30) | ||
| - | Spores 13-16 × 9.5-13.5 × 5.5-8µm. Perithecial setae up to 35µm long. | |
| Coniochaeta sp. | ||
| 96(94) | Spores 7-9 × 6-8 × 5-6µm, slightly flattened. | |
| Coniochaeta leucoplaca | ||
| - | Spores larger. | 97 |
| 97(96) | Spores narrowly elliptical in face view (length more than 2 × width), ca 13-18 × 6-9 × 4-6µm. | |
| Coniochaeta saccardoi | ||
| - | Spores broadly elliptical to nearly circular in face view (length less than 2 × width). | 98 |
| 98(97) | Spores (9)10-16(20) × 7.5-10(15) × (4)5-8µm. Neck setae 20-50µm long. | |||
| Coniochaeta ligniaria (figs 30, 31) | ||||
| - | Spores (16)17-23 × (10)13-19 × 7.5-10(15)µm. Neck setae 40-80µm long. | |||
| Coniochaeta scatigena (figs 30, 31) | ||||
| 99(93) | Perithecial neck with prominent agglutinated tufts of rigid setae up to 950µm long. Spores 43-54 × 20-29µm, with apical germ pore. A gelatinous sheath which surrounds the whole spore swells in water, and appears fringed at the margin and radially striate. | |||
| Arnium macrothecium | ||||
| - | Perithecial neck without setae. Gelatinous sheaths may be clearly visible around spores, but are not complex in structure. | 100 | ||
| 100(99) | Spores with germ slit along the side. Ascus with a large and complex plug at the tip staining blue or red in KI (other genera have asci with blue staining ascus tips, but the feature is very pronounced in this genus and is unlikely to be mistaken). Perithecia form singly or severally in a stroma which is usually of limited extent, often without a definite margin. [N.B. if orange and with a stroma see Selinia, 119]. | |||
| (Hypocopra, fig. 32) 101 | ||||
| - | Spores without germ slits, but often asymmetrical, and with a small papilla at the basal end. Asci without complex apical plug. | |||
| (Sordaria, fig. 33) 107 | ||||
| 101(100) | Spores mostly less than 25µm long. | 102 | ||
| - | Spores more than 25µm long. | 104 | ||
| 102(101) |
|
|||
| - | Spores larger. | 103 | ||
| 103(102) | Stroma with a brown hyphal mat between perithecial necks. Spores 19-27 × 10-14µm. | |||
| Hypocopra equorum (fig. 32) | ||||
| - | Stroma with white hyphae between black perithecial necks, becoming smooth. Spores 23-25 × 12-14µm. | |||
| Hypocopra brefeldii | ||||
| 104(101) | Ascospores up to 15µm wide. | 105 | ||
| - | Ascospores 15µm or wider. | 106 | ||
| 105(104) | Ascospores 25-31 × 10-15µm, distinctly flattened on one side. Ascus plug blue in KI, but becoming reddish. | |||
| Hypocopra planispora | ||||
| - | Ascospores 26-32 × 13-14µm, ellipsoid and narrowed towards their ends. | |||
| Hypocopra stephanophora | ||||
| 106(104) |
|
|||
| - |
|
|||
| 107(100) | Spores up to 10µm long. | 108 | ||
| - | Spores 10µm or longer. | 109 | ||
| 108(107) |
|
|||
| - | Asci ca 128-spored. Spores 5-8 × 4-5µm. | |||
| Sordaria polyspora | ||||
| 109(107) | Spores relatively narrow, at least twice as long as wide, 22-26 × 9-12µm. Gelatinous sheath broad, distinct. | |||
| Sordaria alcina | ||||
| - | Spores relatively broad, less than twice as long as wide. | 110 | ||
| 110(109) | Spores mostly 25µm or longer. | 111 | ||
| - | Spores up to 25µm long. | 112 | ||
| 111(110) | Spores (21)23-29(30) × 14.5-17(18)µm, with apiculate base. Gelatinous sheath broad, distinct. Asci 240-300 × 20-24µm. | |||
| Sordaria superba | ||||
| - | Spores (26)28-35 × (17)18-22µm, with slightly apiculate base. Gelatinous sheath broad, distinct. Asci 280-350 × 30-35µm. | |||
| Sordaria macrospora | ||||
| 112(110) | |Spores with gelatinous sheath absent or very thin, 19.5-25 × 15.5-19µm. | |||
| Sordaria humana (fig. 33) | ||||
| - | Spores with gelatinous sheath, up to 15µm diam. | 113 | ||
| 113(112) | Spores obovoid to broadly ellipsoid, 18-23 × 12-15µm. | |||
| Sordaria lappae | ||||
| - | Spores ellipsoid, 17-25 × 10-14µm. | |||
| Sordaria fimicola (fig. 33) | ||||
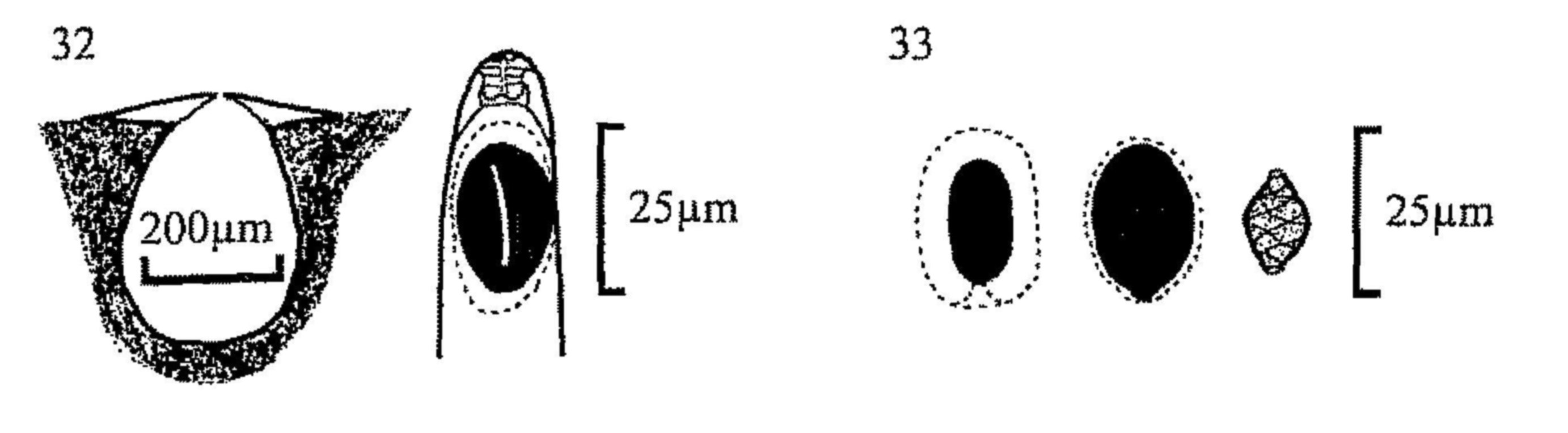
Fig. 32. Hypocopra equorum, perithecium with limited stroma, and
detail of ascus tip with blue staining plug and spore.
Fig. 33. Ascospores, from left, of Sordaria fimicola, S. humana and
Sphaerodes fimicola.
| 114(3) | Spores 20-28 × 12-16µm, with subacute ends, each with a germ pore. | |||
| Gelasinospora adjuncta | ||||
| - | Spores larger. | 115 | ||
| 115(114) | Asci 4-spored. Spores 24-29 × 15-18µm, with rounded ends and one germ pore. | |||
| Gelasinospora tetrasperma | ||||
| - |
|
|||
| 116(4) | Perithecia orange to golden, often gregarious, almost spherical, necks ca 50µm diam., 15µm high, setae at ostiole hyaline, up to 35 × 3µm. Spores limoniform, with a germ pore at each end, 15-25 × 9-16µm. | |||
| Sphaerodes fimicola (fig. 33) | ||||
| - | Perithecia yellow or reddish brown (darker when filled with mature spores), neck 50µm long, with setae at the ostiole 40-70µm long. Spores dark brown to black, limoniform, 20-34 × 11-17µm, with apical germ pore. | |||
| Melanospora brevirostris | ||||
| 117(2) |
|
|||
| - | Asci with 8 or fewer spores, or asci evanescent, not readily observed. | 118 | ||
| 118(117) | Perithecia orange/yellow, 500-1000µm diam. Spores long (over 45µm) or 2-celled if shorter. | 119 | ||
| - | Perithecia smaller, or black or with a neck. Spores shorter (less than 20µm) or septate if longer. | 120 | ||
| 119(118) | Perithecia orange, 500-1000µm diam., in small groups on a limited stroma. Spores thick walled, 48-60 × 22-26µm, with a gelatinous sheath. | |||
| Selinia pulchra | ||||
| - | Perithecia orange yellow, superficial, ca 500µm diam., with ostiole in a disc surrounded by silvery triangular tufts of hyphae ca 100µm long. Spores ellipsoid, 1-septate, 12-14 × 4-5µm. | |||
| Nectria suffulta | ||||
| 120(118) | Perithecia reddish brown or pale, hyaline, with a distinct neck. | 121 | ||
| - | Perithecia black. | 131 | ||
| 121(120) | Perithecia globose, up to 250µm diam., immersed, reddish brown, with a neck 1-3 mm long. Asci broad ellipsoid, 5-8.5µm, rapidly breaking down and difficult to see. Spores ellipsoid-allantoid. 5.5-7 × 1.5-2µm, collecting in a pearly droplet at the fringed tip of the perithecial beak. | |
| Viennotidia fimicola (fig. 34) | ||
| - | Perithecia pyriform, very pale in colour, 60-200µm diam., with a neck 60-700µm long. Asci rarely visible. Spores pointed-fusiform, 1-3 septate, often with a sheath and clumped together in fascicles. | |
| (Pyxidiophora, fig. 36) 122 | ||
| 122(121) | Neck 95-145µm long, brown, rugose, with cells arranged in 5-6 longitudinal rows visible in one view. Spores 38-52µm long. | |
| Pyxidiophora badiorostris | ||
| - | Neck not brown or rugose, composed of hyaline, irregularly arranged cylindrical cells. | 123 |
| 123(122) | Spores less than 45µm long. | 124 |
| - | Spores more than 45µm long. | 125 |
| 124(123) | Spores 35-45µm long, with brown apical or subapical patches of pigment. | |
| Pyxidiophora brunneocapitatus | ||
| - | Spores 35-43µm long, without brown apical or subapical patches of pigment. | |
| Pyxidiophora microsporus | ||
| 125(123) | Spores mostly 45-60µm long. | 126 |
| - | Spores mostly longer than 60µm. | 129 |
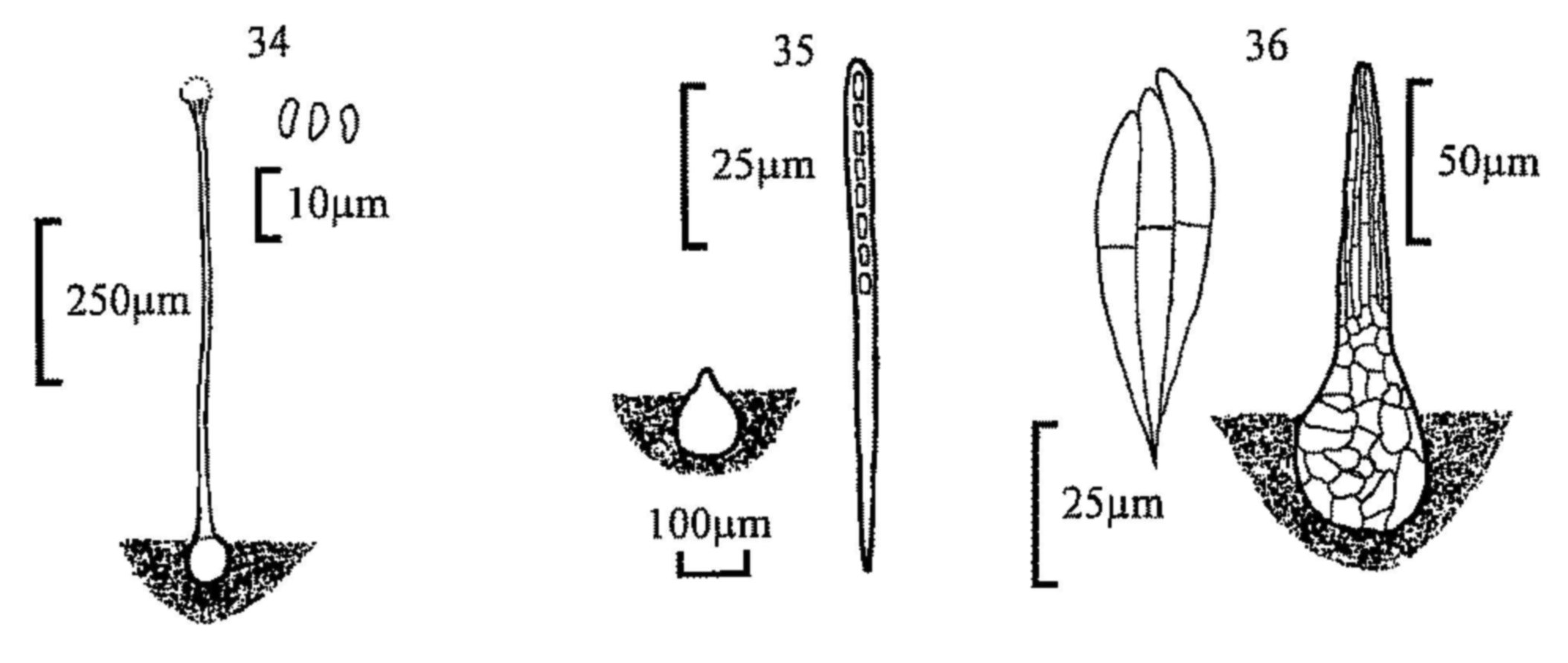
Fig. 34. Viennotidia fimicola, perithecium and spores.
Fig. 35. Phomatospora coprophila, perithecium, and ascus with spores.
Fig. 36. Pyxidiophora petchii, perithecium and spores.
| 126(125) | Perithecia 70-100µm diam., neck 100-190µm long. Spores (43)48-58(65)µm long. | |||
| Pyxidiophora grovei | ||||
| - | Perithecia usually less than 80µm diam. | 127 | ||
| 127(126) | Perithecial necks mostly less than 100µm long. Spores (45)48-57(60)µm long. | |||
| Pyxidiophora arvernensis | ||||
| - | Perithecial necks up to 200µm long. | 128 | ||
| 128(127) | Spores 45-53µm long. | |||
| Pyxidiophora petchii (fig. 36) | ||||
| - |
|
|||
| 129(125) | Spores 60-70µm long. | 130 | ||
| - | Spores (75)80-90(100)µm. Perithecia 120-160µm diam., neck 220-370µm long. | |||
| Pyxidiophora bainemensis | ||||
| 130(129) | Perithecial necks 300-700µm long. Spores 60-70µm. Perithecia 100-120µm diam. | |||
| Pyxidiophora spinuliformis | ||||
| - | Perithecial necks 225-265µm long. Spores 65-70µm. Perithecia 110-125µm diam. | |||
| Pyxidiophora marchalii | ||||
| 131(120) | Perithecia small, up to 400µm diam., with hairy necks. Spores hyaline or pale, coppery-red en masse, extruded in tendrils. | 132 | ||
| - | Perithecia larger, without hairy necks. If smaller than 200µm, with spores smaller than 5 × 3µm. | 134 | ||
| 132(131) | Spores reniform, with gelatinous sheath, 3.5 × 2-3µm, yellow, reddish brown en masse in extruded tendrils. Asci spherical, evanescent. Perithecia black, spherical, 200-400µm diam., with cylindrical neck up to 300µm long, with sparse pointed hairs. | |||
| Microascus longirostris | ||||
| - | Spores larger, not reniform. Perithecia up to 300µm diam. | 133 | ||
| 133(132) | Perithecial necks long, up to 750µm, with terminal hairs up to 1500µm, curved or circinate at tips. Spores limoniform, 7-10.5 × 5.5-7µm. | |||
| Lophotrichus ampullus | ||||
| - | Perithecial necks short, ca 50µm, with long straight tapering hairs. Spore shape limoniform/variable, 6-7.5 × 5-5.5µm, with prominent germ pores. | |||
| Lophotrichus bartletti | ||||
| 134(131) | Perithecia up to 150µm diam., immersed but for a conical neck 50-75µm high. Asci 50 × 2-2.5µm. Spores minute, cylindrical, 3.5-4.5 × 1.75-2.5µm. | |
| Phomatospora coprophila (fig. 35) | ||
| - | Perithecia more obvious, often hairy, or tomentose when young. Immature spores up to 70µm long, wavy cylindrical, with a row of globules inside and a short thin appendage at each end. | |
| (see Cercophora, 49) | ||
| 135(1) | Perithecia immersed, surrounded at the neck by a very limited flange-like stroma which is easily overlooked. | |
| see Hypocopra, 101 or if orange see Selinia, 119 |
||
| - | Stroma very conspicuous. | 136 |
| 136(135) | Perithecia in a subglobose group at the tip of the stromatic stalk. Spores with germ slit and gelatinous sheath. | |
| (Podosordaria) 137 | ||
| - | Perithecia not in a terminal head. | 139 |
| 137(136) | Stalk short, 3-5mm. Spores (12)14-19 × 6-9µm, slightly flattened on one side. | |
| Podosordaria leporina | ||
| - | Stalk long, 1-6cm. Spores larger. | 138 |
| 138(137) | Spores 21-24 × 11-12µm. Stromatic stalk hairy. | |
| Podosordaria tulasnei | ||
| - | Spores 40-60 × 20-30µm. Stromatic stalk not hairy. | |
| Podosordaria pedunculata | ||
| 139(136) | Stroma externally black, rooted or partially immersed in the dung, expanding at the surface to form a white disc up to 15mm diam., punctate with black perithecial ostioles. | |
| (Poronia) 140 | ||
| - | Stroma not as above. | 141 |
| 140(139) | Spores 18-26 × 7-12µm, bean shaped, with gelatinous sheath. Stroma deeply rooted. Especially on horse dung. | |
| Poronia punctata | ||
| - | Spores (22)25-32(35) × (12)14-18µm, oblong ellipsoid to slightly fusiform. Stroma not deeply rooted. Especially on rabbit dung near the sea. | |
| Poronia erici | ||
| 141(139) | Stroma spreading over surface of dung or filamentous. Spores ellipsoid to slightly flattened on one side, with germ slit. (Xerophilic fungi developing after long periods of relatively dry incubation). | |
| (Wawelia) 142 | ||
| - | Stroma clavate, black, partly immersed to superficial, usually aggregated in small groups, ca 1-1.5mm high × 0.6-0.7mm diam., each containing a single perithecium. Spores ellipsoid with germ pore and gelatinous sheath. | |
| (Bombardioidea) 146 | ||
| 142(141) | Stroma spreading on substrate, black brown, firm but not brittle. Ascomata globose, 0.5-1mm, with white hyphae at neck. Spores broad limoniform, 15-19 × 9-10µm. | |
| Wawelia effusa | ||
| - | Perithecia globose to pyriform, black, brown or dark grey, produced laterally along the length of fine stromatal strands growing from the dung. | 143 |
| 143(142) | Asci 4-spored. | 144 |
| - | Asci 8-spored. | 145 |
| 144(143) | Spores 15-18 × 9-12µm. Perithecia up to 400µm diam., dark grey at maturity, single or clustered, the ostiole with a crown of silvery white hyphae. Stromata up to 30 × 0.1-0.5mm. | |
| Wawelia sp. | ||
| - | Spores 6-8 × 4-6µm. Stromata conical, white, 5-12 × 1-2mm. | |
| Wawelia regia | ||
| 145(143) | Perithecia hairy, globose, 350-500µm diam., stromatal strands up to 25mm long. Spores ellipsoid, flattened on one side, 9-12 × 6-8µm. | |
| Wawelia octospora | ||
| - | Perithecia villose with conidiophores, globose, 230-420µm diam., produced laterally on stromatic filaments 20-30 × 0.1-0.3mm. Filaments pink at first, with a white pointed tip, becoming brown, velvety with conidiophores. Spores ellipsoid to flattened on one side, 7.5-9.5 × 3-4.5µm. | |
| Wawelia sp. (fig. 37) | ||
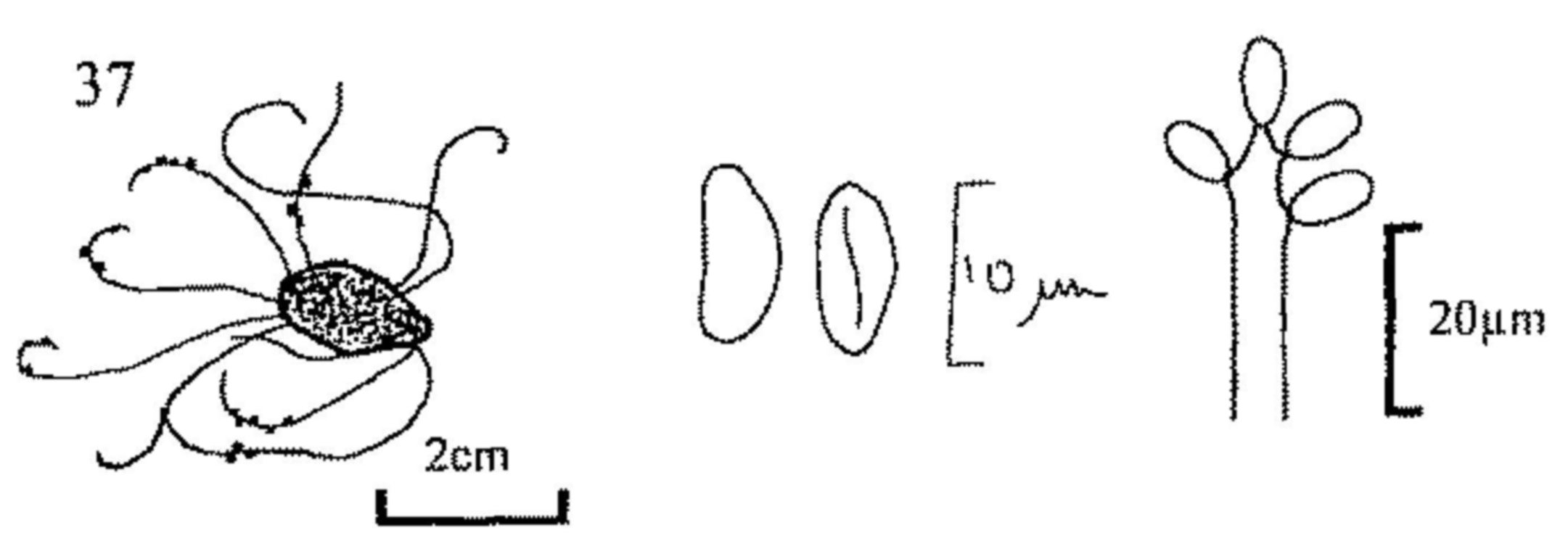
Fig. 37. Wawelia sp., stromatic filaments with perithecia growing from a rabbit pellet, ascospores, and conidiophore and conidia.
| 146(141) | Asci 8-spored. Spores 20-31 × 9.5-15µm. | |||
| Bombardioidea bombardioides | ||||
| - | Asci 4-spored. | 147 | ||
| 147(146) | Spores 24-34 × 15-19(20)µm. Basal germ pore less distinct than the apical one. | |||
| Bombardioidea serignanensis | ||||
| - | Spores 34-43 × 16-22µm. Distinct germ pore at each end of spore. | |||
| Bombardioidea stercoris | ||||
| 148 (key 1,1) |
Fruit bodies solitary or in small groups, each a subglobose, fertile, light brown head on a slender sterile stalk. Head soon bursting to expose the yellow ochraceous spore mass. On mixtures of bird droppings, cast pellets and decaying animal material. | 149 | ||
| - | Fruit bodies superficial, lacking a distinct stalk. | 150 | ||
| 149(148) | Spores 5-8 × 2-3µm. Head 1-2mm diam. | |||
| Onygena corvina (fig. 38) | ||||
| - |
|
|||
| 150(148) | Fruit bodies with an external wall of loosely anastomosing and interwoven hyphae, and with ± specialised terminal cells (gymnothecia, fig. 39). | 151 | ||
| - | Fruit bodies with a well defined parenchymatic wall (cleistothecia, fig. 46). | 161 | ||
| 151(150) | Gymnothecia with simple thin-walled, ± uniform and poorly developed hyphae constituting the outer hyphal sheath. | 152 | ||
| - | Gymnothecia with thick-walled hyphae modified at their ends into appendages, or if thin-walled then always accompanied by appendages (i.e. curled, toothed or pointed hyphae). | 155 | ||
| 152(151) | Gymnothecia red-orange to brick-red. Ascospores orange, subglobose to ellipsoid, with an equatorial furrow, smooth, 4.5-5.5 × 3.5-4.5µm. | |
| Arachniotus ruber (fig. 40) | ||
| - | Gymnothecia white or yellow, never orange or brick-red. Ascospores without an equatorial furrow. | 153 |
| 153(152) | Gymnothecia white. Ascospores hyaline, ellipsoid, smooth, 3-4 × 2-2.5µm. | |
| Arachniotus candidus | ||
| - | Gymnothecia distinctly pigmented, yellow or brown. Ascospores larger than 4µm. | 154 |
| 154(153) | Gymnothecia yellow brown. Ascospores orange to brownish, slightly lenticular, smooth or slightly roughened, 5-6.5 × 3.3-4.6µm. | |
| Arachniotus confluens | ||
| - | Gymnothecia lemon yellow. Ascospores lemon yellow, lenticular, smooth, 5-6 × 3-4.5µm. | |
| Arachniotus citrinus | ||
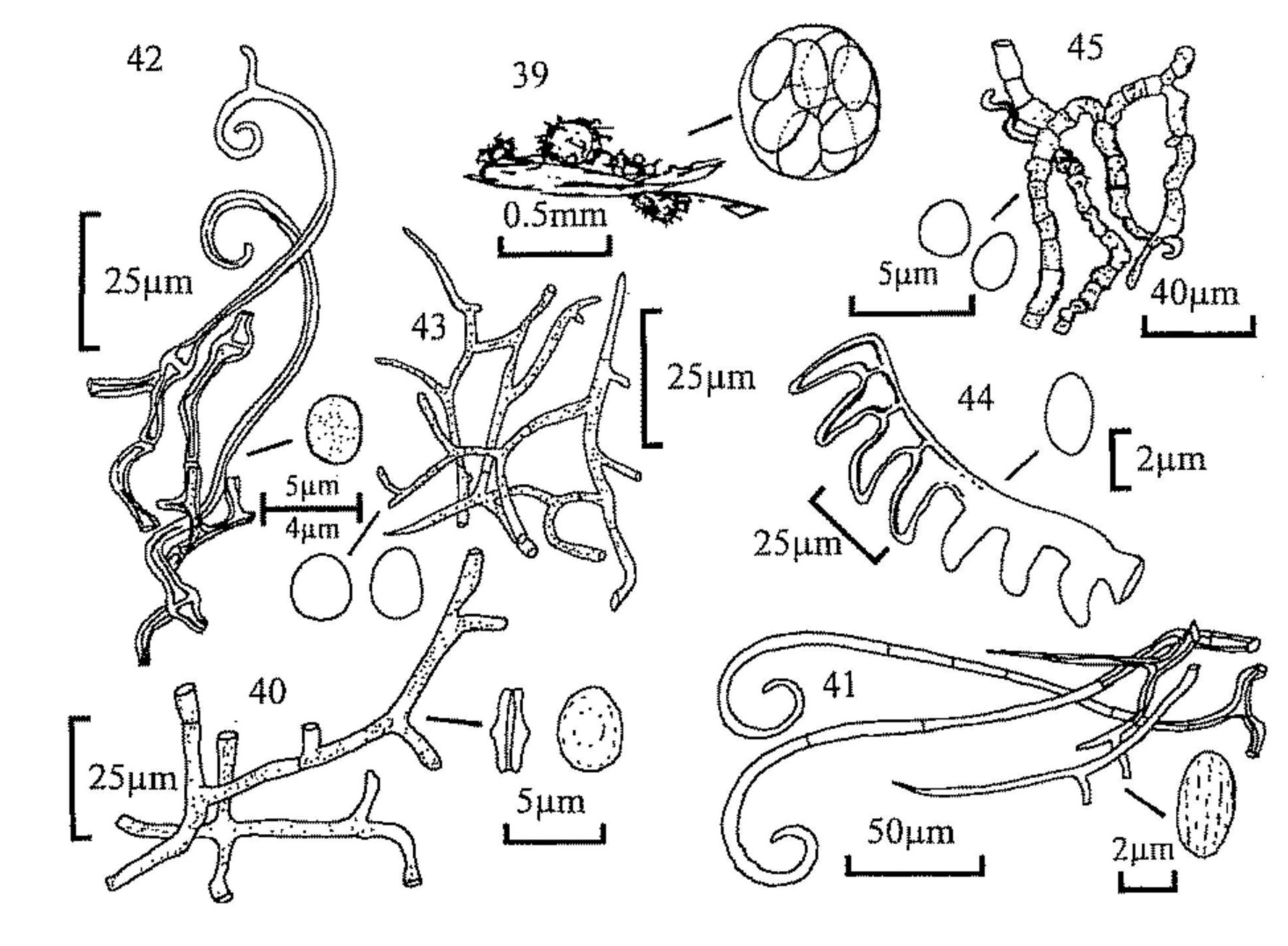
Fig. 39. Habit sketch of a gymnothecium and ascus.
Figs 40-45. Spores and peridial hyphae.
Fig. 40. Arachniotus ruber. Fig. 41. Myxotrichum
chartarum.
Fig. 42. Gymnoascus californiensis. Fig. 43. Gymnoascus
reesii.
Fig. 44. Ctenomyces serratus. Fig. 45. Arthroderma
curreyi.
| 155(151) | Gymnothecia possessing only thick pigmented hyphae. | 156 |
| - | Gymnothecia possessing ± thin, hyaline hyphae with only a few, although often distinctive, appendages (i.e. comb-shaped end cells or dumb-bell shaped asperulate cells accompanying twisted and bent hyphae). | 160 |
| 156(155) | Gymnothecia brown-black or dark greenish-grey, with external hyphae with spine-like branches and septate, hooked appendages. Ascospores orange brownish, ovate, delicately striate, 4-5.2 × 2.4-3.3µm. | |
| Myxotrichum chartarum (fig. 41) | ||
| - | Gymnothecia never black, and, if possessing thick-walled hyphae, then appendages never septate. Ascospores smooth, or if ornamented then asperulate or echinulate. | 157 |
| 157(156) | Gymnothecia rose to orange-brown or yellowish. Appendages curved or irregularly branched and pointed, never verticillately branched. Ascospores smooth, or at most asperulate. | 158 |
| - | Gymnothecia red-brown with appendages verticillately branched. Ascospores 3-4.5 × 2-2.8µm, yellowish brown, lenticular. | |
| Actinodendron verticillatum | ||
| 158(157) | Gymnothecia rosy pink when young, becoming browner, with spines and curved, non-septate hairs. Ascospores hyaline, globose to subglobose, asperulate, 3-5 × 2.5-4µm. | |
| Gymnoascus californiensis (fig. 42) | ||
| - | Gymnothecia yellow. Ascospores smooth. | 159 |
| 159(158) | Gymnothecia yellow to yellow-brown, without elongated appendages but with thick-walled branches, few of which are pointed. Ascospores globose-ellipsoid, yellow to brownish, 3-4.5 × 3.5µm. | |
| Gymnoascus reesii (fig. 43) | ||
| - | Gymnothecia golden yellow to reddish-brown, with acute-ended appendages. Ascospores lenticular, smooth, hyaline, 2.5-3.5 × 2-2.5µm. | |
| Pseudogymnoascus roseus | ||
| 160(155) | Gymnothecia orange brown, with comb-like appendages. Ascospores slightly lenticular, pale orange, 3.3-3.6 × 2-2.6µm. | |
| Ctenomyces serratus (fig. 44) | ||
| - | Gymnothecia whitish to pale ochraceous, particularly when dry, with few appendages but those present twisted and bent, and their branches constricted with regular or irregular dumb-bell shaped cells. Hyphal walls asperulate or with protuberances. Ascospores smooth, lenticular, hyaline, 2.4-3.3 × 2µm. | |
| Arthroderma curreyi (fig. 45) | ||
| 161(150) | Asci relatively large, 100-200-spored, 1-3/fruit body. 'Cleistothecia' minute, <100 (rarely <250)µm diam., immersed. | |
| see Thelebolus etc. (Key 1, 86) | ||
| - | Asci with 8 or fewer spores. | 162 |
| 162(161) | Ascospores purple at maturity, large, 50-70 × 25-35µm, epispore with a few longitudinal cracks. | |
| see Ascobolus immersus (Key 1, 48) | ||
| - | Ascospores smaller, hyaline, yellow, olivaceous, brown or black. | 163 |
| 163(162) | Ascospores olivaceous, brown or black, at least in part. | 164 |
| - | Ascospores aseptate, hyaline, yellow or other pale colours. | 174 |
| 164(163) | Ascospores 4-celled (cf. Sporormiella), with germ slits, readily fragmenting. Asci clavate, bitunicate. Cleistothecia black, shiny, up to 500µm diam. | 165 |
| - | Ascospores 1- or 2-celled. | 166 |
| 165(164) | Ascus stalk up to 20µm long. Ascospores 25-32 × 5µm. | |
| Preussia vulgaris | ||
| - | Ascus stalk 30-60µm long. Ascospores 26-38 × 5-7µm. | |
| Preussia funiculata (fig. 47) | ||
| 166(164) | Ascospores 2-celled. | 167 |
| - | Ascospores 1-celled | 170 |
| 167(166) | Spores unequally 2-celled, one brown ellipsoid, with an apical germ pore, 10-12 × 6.5-7.5µm, the other a basal hyaline, cylindrical pedicel, 6-8 × 3µm. Cleistothecia black, globose, up to 250µm diam., covered with flexuous brown hairs up to 1mm long. Asci evanescent. | |
| Zopfiella erostrata | ||
| - | Spores equally 2-celled. | 168 |
| 168(167) | Spores not constricted at the septum, ellipsoid, golden-brown, 25-30 × 10-15µm with 1-3 guttules in each cell. Cleistothecia gregarious on a mycelial mat, whitish to pale orange, up to 500µm diam. | |
| Heleococcum aurantiacum (fig. 48) | ||
| - | Spores hyaline, divided into two almost globose cells by the constricting septum. Ascomata superficial, globose, dark coloured. | |
| (Mycoarachis) 169 | ||
| 169(168) | Asci 8-spored, 5.5-11µm diam. Spores 5-5.5 × 3-3.5µm. | |
| Mycoarachis inversa | ||
| - | Asci 4-spored, 6-6.5µm diam. Spores 4.5-5 × 2-2.5µm. | |
| Mycoarachis tetraspora | ||
| 170(166) | Asci broad-clavate, (1)-2-(3)-spored, 30-50 × 13-18µm. Spores brown-black with short ridges and warts, subglobose, 12-15.5 × 11-12.5µm, with a single germ pore. | |
| Copromyces bisporus (fig. 49) | ||
| - | Asci 8-spored. | 171 |
| 171(170) | Spores globose, sooty brown, 3µm diam. Cleistothecia gregarious, with basal spirally coiled appendages, black, 100-200µm diam., partially immersed in a white to red felty hyphal mat. | |
| Pleuroascus nicholsonii | ||
| - | Spores larger, ellipsoid or limoniform. | 172 |
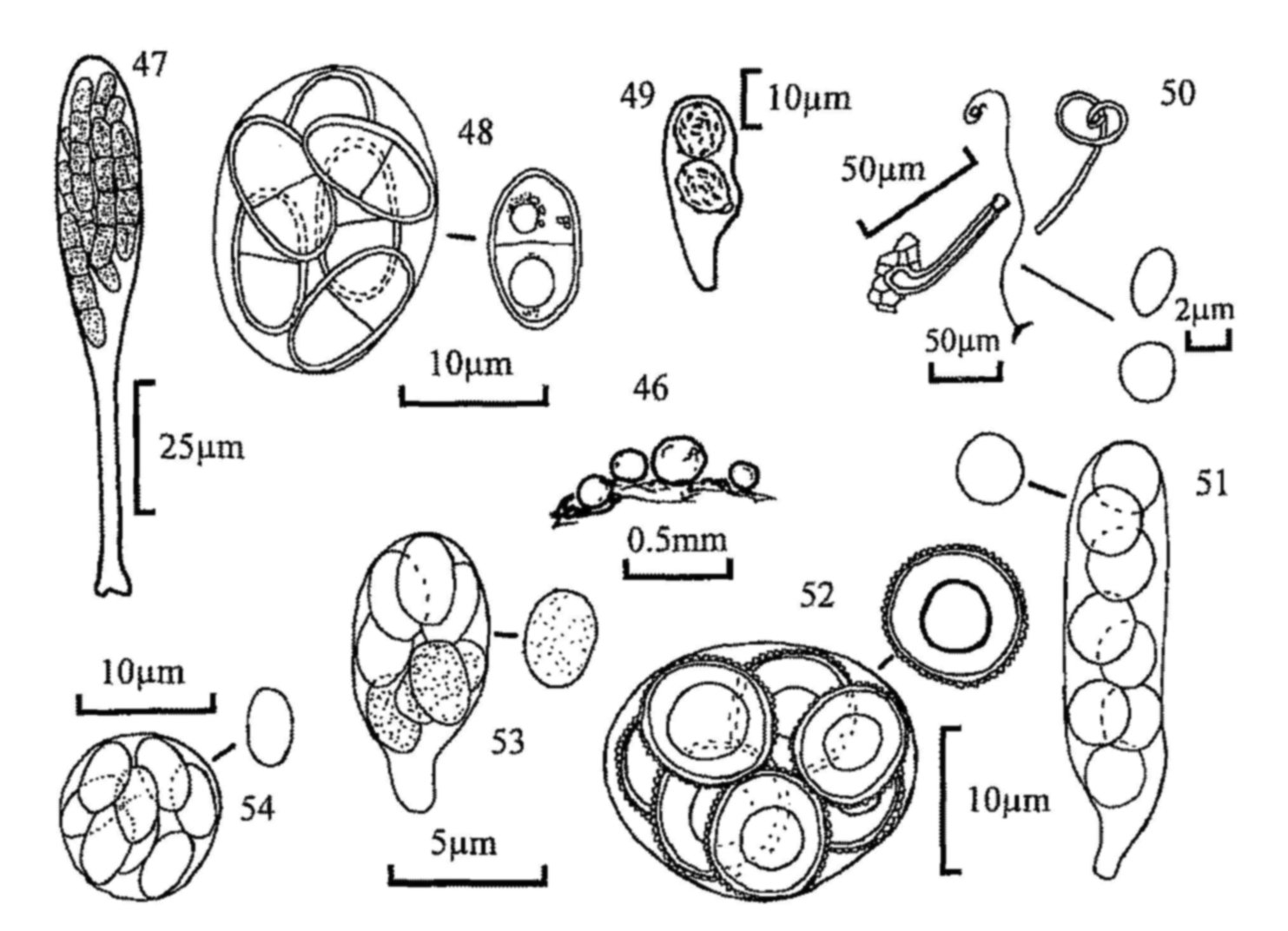
Fig. 46. Habit sketch of cleistothecia.
Figs 47-54. Asci and spores.
Fig. 47. Preussia funiculata. Fig. 48. Heleococcum
aurantiacum.
Fig. 49. Copromyces bisporus. Fig. 50. Arachnomyces
nitidus.
Fig. 51. Orbicula parietina. Fig. 52. Roumegueriella
rufula.
Fig. 53. Aphanoascus stercoraria. Fig. 54. Pseudeurotium
ovale.
| 172(171) | Spores olivaceous, limoniform, usually with an apical germ pore. Perithecia greyish or greenish, abundantly hairy, branched or simple, straight or curly. Asci pedicellate, soon disappearing. | |
| see Chaetomium at 5 | ||
| - | Spores darker, with 1 or more minute germ pores. Cleistothecia distinctly but not abundantly hairy. | 173 |
| 173(172) | Spores smoky brown, broadly ovoid, 9-14 × 6-9µm. Cleistothecial hairs short, up to 30µm. | |
| Thielavia wareingii | ||
| - | Spores dark brown, flattened limoniform, 13-16 × 10-13 × 8-9µm. Cleistothecial hairs of two types, some smooth, dark brown, arising from the base up to 3mm long, others greyish green, rough, up to ca 120µm. | |
| Thielavia fimeti | ||
| 174(163) | Cleistothecia produced within a common arachnoid mycelial mass. Spores smooth or minutely asperulate, yellow to yellow-brown, broadly ellipsoid, 4-5 × 3-5µm. | |
| Aphanoascus fulvescens | ||
| - | Cleistothecia single or gregarious, but not on or in a mycelial mass. | 175 |
| 175(174) | Cleistothecia 170-750µm diam., covered with long (several mm when extended), thick-walled, aseptate, helical appendages. Asci clavate cylindrical, evanescent, 35-62 × 12-21µm. Spores ellipsoid, hyaline, 12-17 × 9-12µm. | |
| Lasiobolidium spirale | ||
| - | Cleistothecia without coiled appendages. | 176 |
| 176(175) | Cleistothecia with hairs or appendages. | 177 |
| - | Cleistothecia smooth. | 178 |
| 177(176) | Cleistothecia black, shining, 100-200µm diam., with dark brown-black thick-walled hairs with hooked tips. Asci 8-15µm diam. Spores straw or copper coloured, ellipsoid, 4-7 × 3.5-4.5µm with de Bary bubble and a germ pore at each end. | |
| Kernia nitida | ||
| - | Cleistothecia reddish brown, less than 1mm diam., with long simple appendages curled at the tips. Spores hyaline, oblate, 3.55 × 2-3µm. | |
| Arachnomyces nitidus (fig. 50) | ||
| 178(176) | Ascospores globose, larger than 9µm. | 179 |
| - | Ascospores ellipsoid, up to 9µm. Asci always subglobose. | 180 |
| 179(178) | Ascospores, smooth, 9-13µm. | |
| Orbicula parietina (fig. 51) | ||
| - | Ascospores ornamented, 13-24µm Asci subglobose. Cleistothecia ochraceous, becoming yellowish brown or flushed cinnamon. | |
| Roumegueriella rufula (fig. 52) | ||
| 180(178) | Ascospores hyaline, then faintly yellowish, minutely spiny, 2.5-3 × 2-2.5µm. Cleistothecia pale, then dark brown. | |
| Aphanoascus stercoraria (fig. 53) | ||
| - | Ascospores hyaline, then brown, smooth, 5.5-6 × 3.5-4µm. Cleistothecia dark brown from the beginning. | |
| Pseudeurotium ovale (fig. 54) | ||
| 1 | Basidia single-celled (fig. 55). | 2 |
| - | Basidia transversely or longitudinally septate (fig. 55), or difficult to observe. | 71 |
| 2(1) | Fruit body agaricoid, i.e. mushroom-shaped with gills underneath cap (figs 56, 67). | 3 |
| - | Fruit body not agaricoid, without gills (figs 65, 66). | 69 |
| 3(2) | Spore print white or pale coloured, hyaline s.m. (Usually on straw/dung mixtures, never on raw dung except when very old). | 5 |
| - | Spore print coloured. | 4 |
| 4(3) | Spore print pinkish or pale cinnamon, honey-coloured s.m. (Usually on straw/dung mixtures, never on raw dung). | 6 |
| - | Spore print darker, in shades of brown or black. | 8 |
| 5(3) | Stem eccentric. Fruit body pure white. Spores ellipsoid, smooth. | |
| Pleurotellus s. lato | ||
| (If gills pink and spores longitudinally ridged see Clitopilus passackerianus, fig. 67) | ||
| - | Stem central. | 7 |
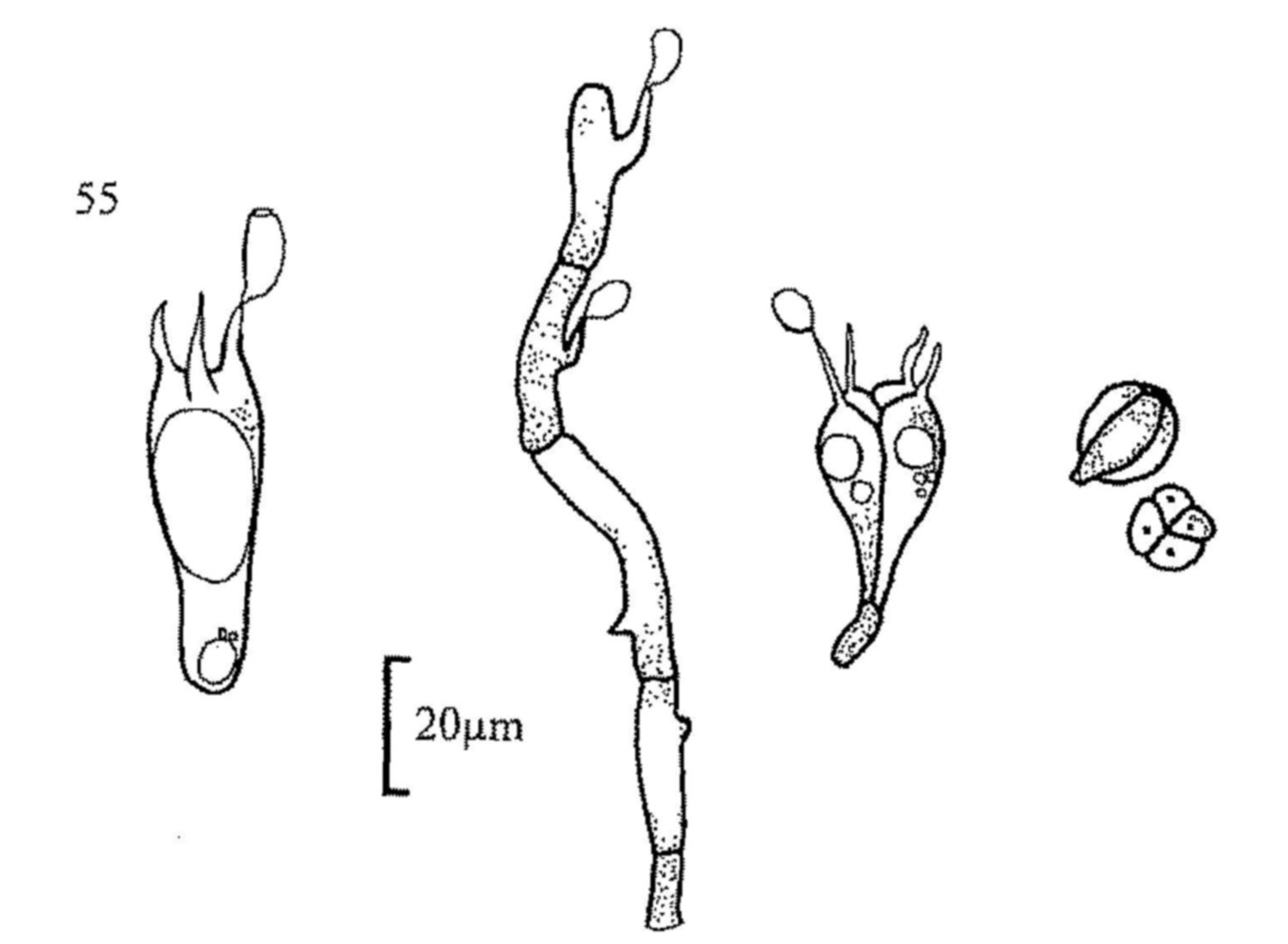
Fig. 55. From left, sketches of holobasidium, with mature basidiospore showing germ pore; auriculariaceous basidium; tremellaceous basidium, lateral view and as often seen in sections.
| 6(4) | Fruit body white, ivory or very pale tan, with a smell of cucumber. Gills decurrent. | |||
| Clitocybe augeana | ||||
| - | Fruit body yellow, with scaly cap. Gills free or just adnate. Fruit body with distinct ring and granular veil. (Commonly in plant pots. Probably associated with peaty material more than dung). | |||
| Leucocoprinus birnbaumii | ||||
| (L. cepaestipes and L. lilacinogranulosus occur in similar situations). | ||||
| 7(5) | Fruit body with amethyst/purple shades, with eccentric stem. Spores subglobose, slightly ornamented to nearly smooth. (On compost heaps in gardens). | |||
| Lepista nuda | ||||
| - | Fruit body with pink gills and distinct volva at stem base. Cap white to pale hazel. Stem white. Spores broadly ellipsoid, smooth. | |||
| Volvariella speciosa | ||||
| 8(4) | Spore print distinctly brown (fulvous, tawny, rust coloured etc.). | 9 | ||
| - | Spore print some darker shade, fuscous, fuliginous or violaceous black. | 20 | ||
| 9(8) |
|
|||
| (Has been found on straw/dung mixtures, never on raw dung). | ||||
| - | Stem lacking a veil. | 10 | ||
| 10(9) | Cap rich chrome yellow, viscid, soon reduced to a sticky mass, easily collapsing. | |||
| Bolbitius vitellinus | ||||
| - | Cap in shades of brown, never brightly coloured and if collapsing then cap elongate-cylindric and white to pale cream. | 11 | ||
| 11(10) | Spore print dull, sepia or snuff-brown. On rabbit pellets in sand dunes. | |||
| Agrocybe subpediades | ||||
| - | Spore print, brighter coloured, orange/rust brown. | |||
| (Conocybe) 12 | ||||
| 12(11) | Gill edge with irregularly fusoid cystidia with obtuse apices (lageniform). Cap viscid. | |||
| Conocybe coprophila | ||||
| - | Gill edge with distinctly capitate cells resembling a glass stoppered bottle (lecythiform). Cap never viscid, often pubescent under a lens. | 13 | ||
| 13(12) | Stem covered in long hairs. | 14 | ||
| - | Stem covered in lecythiform cells similar to those on gill edge, giving a farinaceous appearance under a lens. NEVER with long hairs. (Dung/straw mixtures). Large as in a Cortinarius. Spores smooth. | |||
| Conocybe intrusa | ||||
| (C. leucopus has been found on manured soil in gardens; C. antipus has hexagonal spores and grows on dung piles). | ||||
| 14(13) | Stem with both long hairs and lecythiform cystidia.  |
15 | ||
| - | Stem with hairs and lageniform cystidia.  |
16 | ||
| 15(14) | Spores 11-14 × 7-9µm. Taste and smell strong, of fresh meal. | |||
| Conocybe farinacea | ||||
| - | Spores large, over 15 × up to 10µm. Taste and smell none or slightly acidic. | |||
| Conocybe pubescens | ||||
| (C. subpubescens might be found on straw/dung mixtures, and differs in spores 11-13 × 6-8µm). | ||||
| 16(14) |
|
|||
| - | Basidia 4-spored. | 17 | ||
| 17(16) | Spores ellipsoid. | 18 | ||
| - |
|
|||
| 18(17) | Cap grey, contrasting with yellowish cream gills and pale stem. Spores 10.5-12.5 × 6-7µm. | |||
| Conocybe murinacea | ||||
| - | Cap pinkish brown or tawny. | 19 | ||
| 19(18) | Spores 11-12 × 7.2-7.8µm. Cap sienna. On raw dung. | |||
| Conocybe fimetaria | ||||
| - | Spores 10-12 × 6-7µm. Cap pinkish to cinnamon brown. On manured soil or sewage sludge. | |||
| Conocybe fuscomarginata | ||||
| (Conocybe siennophylla might be found on straw/dung mixtures or in soil in greenhouses. It differs in having smaller spores). | ||||
| 20(8) | Cap deliquescing to some degree at maturity. Basidia of 2 or 3 different sizes. | |||
| (Coprinus) 21 | ||||
| - | Cap not deliquescing. Basidia of one size only. | 49 | ||
| 21(20) | Veil on cap absent, cap either covered with small hairs (setules) or naked. | 22 | ||
| - | Cap covered with a granular, micaceous, powdery or fibrillar veil. | 28 | ||
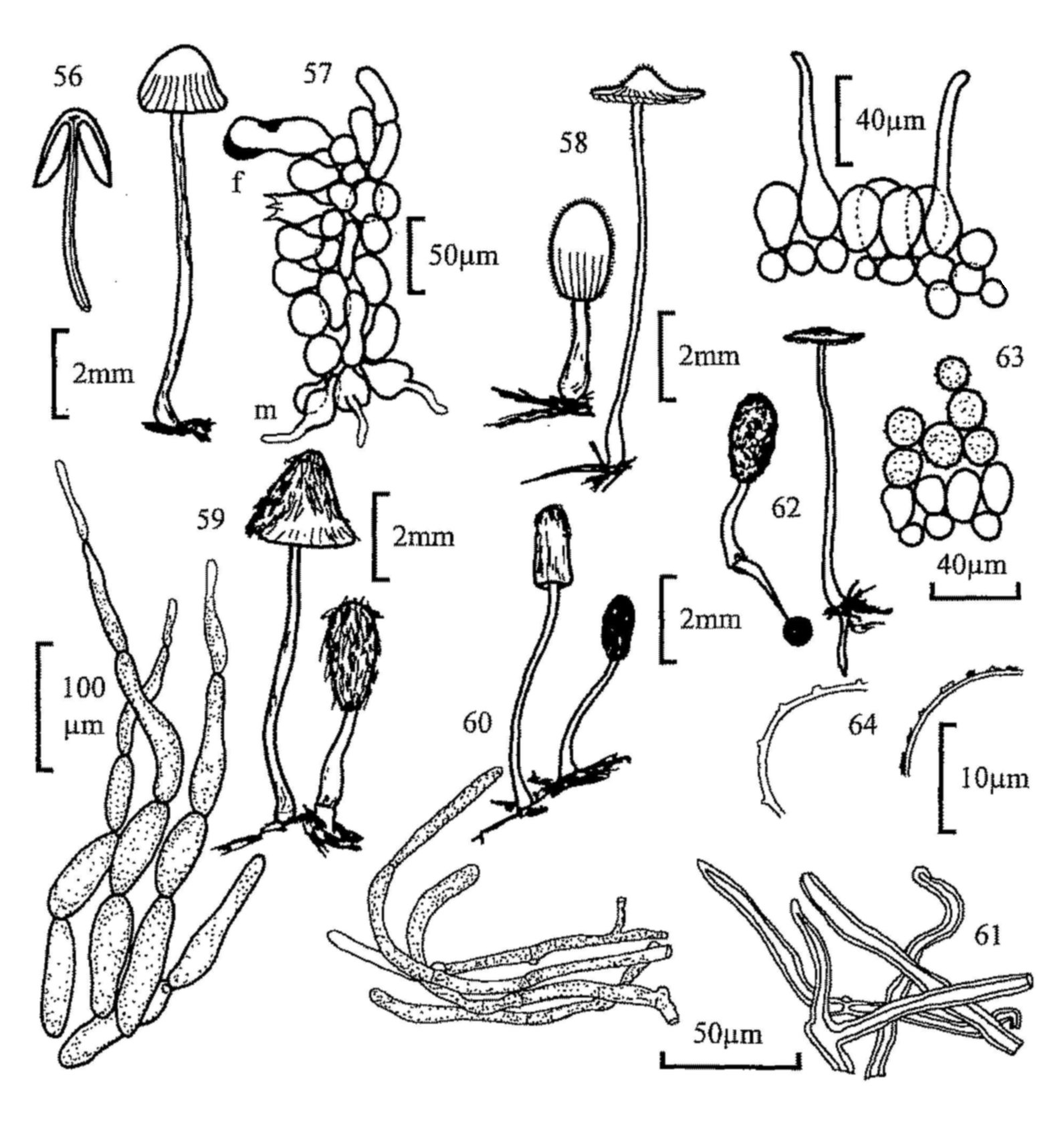
Fig. 56. Habit sketch of a stipitate agaric, Psathyrella
stercoraria, with section.
Fig. 57. Sketch of gill section of Psathyrella sp., showing position of marginal
(m) and facial (f) cystidia.
Fig. 58. Coprinus pellucidus, habit and vertical section of cap cuticle.
Fig. 59. C. pseudoradiatus, habit and veil constituents.
Fig. 60. C. vermiculifer, habit and veil constituents.
Fig. 61. C. filamentifer, veil constituents.
Fig. 62. C. stercoreus, habit.
Fig. 63. C. cordisporus, vertical section of cap showing nature of veil cells on
the cap cuticle.
Fig. 64. Veil cells with structural (l) and superficial crystalline (r)
ornamentation.
| 22(21) | Cap without setules. | 23 |
| - | Cap with setules. | 24 |
| 23(22) | Cap minute, 1-5mm high before expanding, reddish orange at first, soon fading. Basidiospores almost globose to triangular in one view, elliptic in another, 7-10 × 7-9 × 5.5-6.5µm. (2- and 4-spored forms have been found). | |
| Coprinus miser | ||
| - | Cap larger, up to 15mm when expanded. Basidiospores pip-shaped, 7.5-8.5 × 9.5-11 × 9.5-11.5µm. (4-spored). | |
| Coprinus nudiceps | ||
| 24(22) | Spores hexagonal, 10-13 × 6.5-7.5µm. Cap purplish. | |
| Coprinus hexagonosporus | ||
| - | Spores ellipsoid. Cap brown or reddish, without purplish tints. | 25 |
| 25(24) | Basidia 4-spored. | 26 |
| - | Basidia 2-spored. Spores 11-13 × 5.5-7µm. Facial cystidia absent. | |
| Coprinus bisporus | ||
| (Coprinus sassii, not yet recorded in British Isles, has 2-spored basidia with very large ellipsoid spores up to 20µm long). | ||
| 26(25) | Cap with a mixture of hyaline and brown thick-walled setules. Spores 9-10 × 5.5-6µm, with eccentric germ pore. Facial cystidia absent. | |
| Coprinus heterosetulosus | ||
| - | Cap with only one type of setule. Facial cystidia present or absent. | 27 |
| 27(26) | Facial cystidia present. Spores 7.9-13.3 × 4.4-6.4µm, with apical germ pore. | |
| Coprinus stellatus | ||
| - | Facial cystidia absent. Spores elongate and narrow, rarely greater than 5µm wide, with apical germ pore. Fruit body usually quite small, up to 6mm before expanding. | |
| Coprinus pellucidus (fig. 58) | ||
| (Several species in the group, e.g. C. congregatus and C. ephemerus have been found on straw/dung mixtures). | ||
| 28(21) | Veil strongly adhering to cap. Spores elliptic ovate, 15-20 × 8-12µm. Stem with distinct ring. Usually on buried dung. | |
| Coprinus sterquilinus | ||
| - | Veil more floccose or powdery. Stem lacking ring or, if present (C. ephemeroides), fruit body small with 5-angled spores less than 10µm long. | 29 |
| 29(28) | Veil composed of filamentous units. | 30 | ||
| - | Filamentous units, if present, masked by a preponderance of rounded cells. | 35 | ||
| 30(29) | Veil composed of strings of sausage-shaped, thin-walled, hyaline cells. | 31 | ||
| - | Veil composed of rather narrow, slightly thickened hyphae. | 32 | ||
| 31(30) | Spores large, 11-14 × 6-7µm. Cap up to 1cm before expanding. Fruit body with or without a rooting base. | |||
| Coprinus radiatus | ||||
| - | Spores smaller, up to 9µm long. Cap up to 6mm before expanding. Fruit body without a rooting base. | |||
| Coprinus pseudoradiatus (fig. 59) | ||||
| (C. cinereus is found on straw/dung mixture and C. macrocephalus, with large spores, has been recorded on raw dung). | ||||
| 32(30) | Veil citrus- or lime-yellow, or a mixture of hyaline and brown strongly coloured hyphae. | 33 | ||
| - | Veil grey or whitish. | 34 | ||
| 33(32) | Veil of yellow hyphae. Spores 10.5-12.5 × 6-7.5µm. | |||
| Coprinus luteocephalus | ||||
| - | Veil with brown hyphae. Spores 7-9 × 3.5-5µm. | |||
| Coprinus poliomallus | ||||
| 34(32) | Veil hyphae thin-walled. Spores 6.5-7.5 × 5µm, 'shouldered' about the apiculus. | |||
| Coprinus filamentifer (fig. 61) | ||||
| - | Veil hyphae thin- and thick-walled, often with clamps. Spores elliptic-oblong, 9-10 × 5-6µm. | |||
| Coprinus vermiculifer (fig. 60) | ||||
| (Coprinus flocculosus, with spores 11.5-16.5 × 6-9.5µm, can be found on straw/dung mixtures). | ||||
| 35(29) | Stem with small, distinct ring. Spores subglobose to lentiform and 5-angled, 6-9 × 6.5-8 × 5-6µm. | |||
| Coprinus ephemeroides | ||||
| - | Stem at most with fibrils, even then rarely forming a faint ring zone. | 36 | ||
| 36(35) | with setules in addition to veil. | 37 | ||
| - | Cap without setules. | 38 | ||
| 37(36) | Cap cystidia tapered. Spores 11-14 × 5-6.5µm. | |||
| Coprinus heptemerus | ||||
| - |
|
|||
| 38(36) | Veil of inflated bladder-like cells attached to filamentous units. Spores 7.5-8 × 4.5-5.5µm. | |||
| Coprinus utrifer | ||||
| - | Veil of globose and subglobose cells and filamentous units often encrusted or with minute projections found sometimes at cap margin. | 39 | ||
| 39(38) | Globose cells, if ornamented then possessing crystalline or amorphous material (dissolved by 1N HCl, fig. 64.) | 40 | ||
| - | Globose cells covered in small fine blunt projections on the walls (not removed by 1N HCl, fig. 64). | 45 | ||
| 40(39) | Basidia 2-spored. | 41 | ||
| - | Basidia 4-spored. | 42 | ||
| 41(40) |
|
|||
| - | Spores smaller, 9-11 × 6-6.5 × 8-9µm. | |||
| Coprinus cordisporus (2-spored form) | ||||
| 42(40) | Spores less than 10µm long. | |||
| Coprinus cordisporus (fig. 63) | ||||
| (C. patouillardii is known on garden refuse, and an undescribed species with lemon-shaped spores has recently been found). | ||||
| - | Spores 10µm or more long. | 43 | ||
| 43(42) | Veil soon discolouring greyish, drab or buff, Spores 11.5-14.5 × 6-8 × 7.5-9µm. | |||
| Coprinus cothurnatus | ||||
| - | Veil remaining snowy white, only slowly discolouring greyish. | 44 | ||
| 44(43) | Fruit bodies several cm tall. Spores 15-19 × 8.5-11.5 × 11-13µm. | |||
| Coprinus niveus | ||||
| - | Cap small, 5-6mm at first. Spores 14-16 x 8-9 × 10-12.5µm. | |||
| Coprinus latisporus | ||||
| 45(39) | Basidia 3-spored. | 46 | ||
| - | Basidia 4-spored. | 47 | ||
| 46(45) |
|
|||
| - | Spores broad, 9-10 x 6-6.5 × 6-7µm, slightly flattened in face view. | |||
| Coprinus trisporus | ||||
| (These are possibly a single taxon). | ||||
| 47(45) | Spores 7-8 × 4-4.5µm, perispore not visible in water or alkali mounts. | |||
| Coprinus stercoreus (fig. 62) | ||||
| - | Spores 9µm or more long. | 48 | ||
| 48(47) | Spores 9-11 × 5.5-6µm. Perisporal sac none or incomplete or indistinct. | |
| Coprinus foetidellus | ||
| - | Spores longer, 10.8-13.5 × 5.5-7µm, with distinct perispore with dark lines and inclusions. Distinctive smell of gas. | |
| Coprinus narcoticus | ||
| (C. sclerotiger is found on straw/dung mixtures, and the smaller C. tuberosus on garden refuse etc.). | ||
| 49(20) | Spores not discoloured in conc. H2SO4. | 50 |
| - | Spores discolouring in conc. H2SO4. Gills not spotted at maturity. | 66 |
| 50(49) | Cap cuticle cellular. Gills spotted at maturity. (More often on rich, 'dungy', soils. P. subbalteatus, with copper coloured cap, drying paler but retaining a dark marginal zone, occurs in gardens on mulch etc.). | |
| (Panaeolus) 51 | ||
| - | Cap cuticle filamentous. | 56 |
| 51(50) | Velar remnants very obvious, either as an appendiculate veil or as a distinct ring. | 52 |
| - | Lacking all velar remnants. | 54 |
| 52(51) | Cap distinctly pigmented, with appendiculate veil. | 53 |
| - | Cap pale coloured, smooth, semi-globate, soon cracking. Gills with marginal cystidia only. | |
| Panaeolus papilionaceus | ||
| 53(52) | Cap brown, smooth, sometimes viscid, not exceedingly wrinkled. | |
| Panaeolus campanulatus | ||
| - | Cap grey, olivaceous, even black, with contrasting white appendiculate veil. | |
| Panaeolus sphinctrinus | ||
| 54(51) | Cap with or without appendiculate veil, but always with distinct ring. | |
| Panaeolus semiovatus | ||
| - | Cap lacking veil. | 55 |
| 55(54) | Cap pinkish ochraceous to tawny-buff. Lacking facial cystidia. | |
| Panaeolus speciosus | ||
| - | Cap whitish or slightly yellowish. With facial cystidia. | |
| Panaeolus antillarum | ||
| 56(50) | Gills with facial cystidia often containing yellow amorphous material when seen in ammonia solution or deep blue with cotton blue. | |||
| (Stropharia) 57 | ||||
| (Blue-green S. cyanea & S. aeruginosa often occur in rich garden soils). | ||||
| - | Gills lacking facial cystidia. Never with yellowing cystidia in ammonia. | |||
| (Psilocybe) 58 | ||||
| (Red-capped P. aurantia can be found on straw/mulch mixtures in gardens). | ||||
| 57(56) | Cap sticky, semi-globate ± expanding at maturity. On raw dung. | |||
| Stropharia semiglobata | ||||
| - | Cap plano-convex, often broad with a central umbo, margin flaring with age. On dungy mixtures in gardens. | |||
| Stropharia stercoraria | ||||
| 58(56) | Stipe bluing, with ring. Spores ellipsoid, 11-14 × 6.5-7.5µm. Fruit body with mealy smell and taste. | |||
| Psilocybe fimetaria | ||||
| - | Stipe lacking distinct ring, or if with ring or ring zone 2-spored and/or stem not bluing. Fruit body without mealy smell and taste. | 59 | ||
| 59(58) | Stem always with distinct ring. Basidia 2-spored. Spores 15-20µm long. | |||
| Psilocybe luteonitens | ||||
| - | Stem with or without ring. Basidia 4-spored. If with ring, spores smaller. | 60 | ||
| 60(59) | With ring zone. | 61 | ||
| - | Lacking velar remnants on stem, or only appendiculate teeth at cap margin. | 62 | ||
| 61(60) | Spores slightly angular/limoniform, 11-13(14) × 7-8µm. Often on sewage sludge. | |||
| Psilocybe merdaria | ||||
| - |
|
|||
| 62(60) |
|
|||
| - | Spores smaller. | 63 | ||
| 63(62) | Spores lentiform, angled, 6-8(8.5) × 4.5-5.5 × 3.75-4.5µm. | |||
| Psilocybe bullacea | ||||
| (P. crobula, occasional on dung, differs in lacking purple colour in gills, and slightly smaller, ovoid, not angular, spores). | ||||
| - | Spores larger. | 64 | ||
| 64(63) |
|
|||
| - | Spores lentiform, angular. | 65 | ||
| 65(64) |
|
|||
| - |
|
|||
| 66(49) | Round cells on cap as a micaceous veil. (Re-examine gill face; if different sized basidia and facial cystidia separating the gills are present go to Coprinus at 21). | |||
| Psathyrella sphaerocystis | ||||
| - | Cap lacking veil, or if present then fibrillar. | 67 | ||
| 67(66) | White copious veil at margin or also covering cap centre. Spores 10-12 × 5.5-6µm. | |||
| Psathyrella coprobia | ||||
| - | Lacking copious veil. | 68 | ||
| 68(67) | With red edge to gill. Spores 12-13 × 6-6.5µm, with central germ pore. | |||
| Psathyrella stercoraria | ||||
| - | Lacking red gill edge. Spores with eccentric germ pore. | |||
| Psathyrella coprophila | ||||
| (P. fimetaria differs in spore size; there are several members of the P. prona group which grow on soil/straw mixtures). | ||||
| 69(2) | Fruit body club-shaped. | |||
| Typhula setipes (fig. 65) | ||||
| (Clavaria acuta often grows on peaty soil in pots in greenhouses). | ||||
| - | Fruit bodies effuse, resupinate | 70 | ||
| 70(69) | Fruit-body cobweb-like and greyish white. Basal hyphae 3-4.5µm wide. Spores sub-globose, 4.5µm diam. (Generally on old dung or straw/soil mixtures). | |||
| Athelia coprophila | ||||
| (If with spiny spores 5-6µm diam., see the recently recorded Tomentellopsis echinospora). | ||||
| - | Fruit-body with pores, white or flushed slightly ochraceous, brownish or greyish. (On clods of soil in dunged land). | |||
| Cristella candidissima | ||||
| 71(1) | Fruit body either a cup containing several 'eggs' or a single orange or yellowish gelatinous sphere. | 72 | ||
| - | Fruit-body effuse, without distinct shape. | 73 | ||
| 72(71) | Fruit-body whitish or pale yellow, up to 2.5mm diam., splitting at maturity to shoot away the orange/yellow spore mass. | |
| Sphaerobolus stellatus (fig. 66) | ||
| - | Fruit-body cup shaped, with silvery-grey 'eggs'. (Usually on dung and straw or attached to rabbit pellets). | |
| Cyathus stercoreus | ||
| (Cyathus vernicosus often grows in plant pots on rich soil). | ||
| 73(71) | Basidia with transverse septa. Spores 11 × 7µm. Fruit body pinkish. | |
| Platygloea fimicola | ||
| (Not British; included for completeness. Pilacrella solani, with a glistening stipitate head, has been isolated from dungy soil). | ||
| - | Basidia with longitudinal septa. Spores 14-18 × 9-10µm. Fruit body cream-white or ivory. | |
| Sebacina incrustans | ||
Fig. 65. Habit sketch of Typhula sp. Note attachment to
sclerotium.
Fig. 66. Sphaerobolus stellatus, habit.
Fig. 67. Clitopilus passackerianus, a sessile agaric—habit sketch and
section.
| 1 | Spores formed in multispored sporangia (figs 68, 70, 72, 75, 76) or in few-spored sporangioles (figs 70, 73). | 2 | |||||||||
| - | Multispored sporangia and globose sporangioles absent. Spores formed singly on terminal, lateral or intermediate vesicles (figs 74, 79, 80, 82-86), or in short chains (figs 77, 78, 81). | 11 | |||||||||
| 2(1) | Sporangiophore stout, simple, with a subsporangial swelling and a basal swelling buried in the substrate. Sporangia tough walled, black, projected some distance towards the light when mature, and sticking to whatever they hit. | ||||||||||
|
|||||||||||
| - | Sporangiophores not stout; sporangia not violently discharged. | 3 | |||||||||
| 3(2) | Sporangial wall black, tough, not readily broken when touched. Sporangia with a sticky base, becoming attached to whatever they contact after the marked elongation of the white sporangiophores at maturity. | ||||||||||
|
|||||||||||
| - | Sporangial wall diffluent, spores readily removed in a droplet, or fragile and then spores easily dispersed by external violence. | 4 | |||||||||
| 4(3) | Sporangiophores stiff and metallic in appearance, growing towards the light and often to great length (5-30cm). | ||||||||||
|
|||||||||||
| - | Sporangiophores white, not reaching extreme lengths. | 5 | |||||||||
| 5(4) | Small lateral sporangia (sporangioles) present. | 10 | |||||||||
| - | Sporangioles absent. | 6 | |||||||||
| 6(5) | Sporangiophores usually grouped, less often single, connected by stolon-like hyphae. | 7 | |||||||||
| - | Sporangiophores arising singly, or if grouped then lacking stolon-like hyphae. | 9 | |||||||||
| 7(6) | Stolons joining groups of sporangiophores often with rhizoids at the base of the group. | 8 | |||||||||
| - | Sporangiophores arising singly or in groups from stolons, which may be 'rooted' at intervals along their length, but rarely beneath the groups of sporangiophores. | ||||||||||
|
|||||||||||
| 8(7) | Sporangiophores mostly unbranched. | ||||||||||
|
|||||||||||
| - | Sporangiophores with a whorl of branches beneath the main sporangium, each with a small columellate sporangium. Spores 6-8.5µm. | ||||||||||
| Actinomucor elegans | |||||||||||
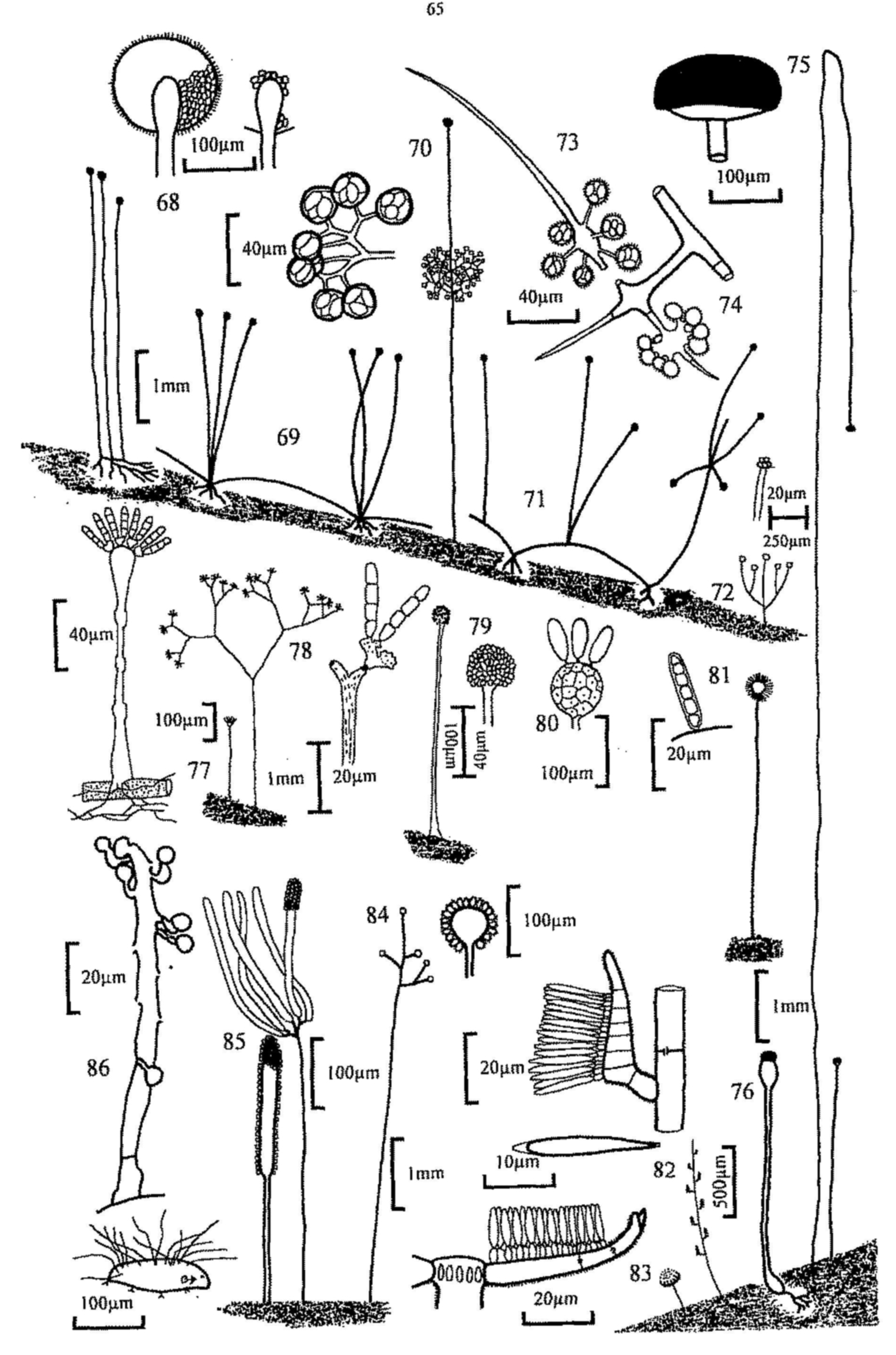
Fig. 68. Mucor, habit and detail of sporangium before and after
dehiscence.
Fig. 69. Rhizopus, habit.
Fig. 70. Thamnidium elegans, habit and detail of sporangioles.
Fig. 71. Absidia, habit.
Fig. 72. Mortierella, habit and sporangiophore tip after sporangial
dehiscence.
Fig. 73. Helicostylum, sporangioles.
Fig. 74. Chaetocladium, sporangioles.
Fig. 75. Pilaira, sporangiophores before and after elongation, and
sporangium.
Fig. 76. Pilobolus, sporangiophore.
Fig. 77. Syncephalis, habit, sporangiophore and merosporangia.
Fig. 78. Piptocephalis, habit and detail of final branch with head cell and
merosporangia.
Fig. 79. Oedocephalum, habit and sporing head.
Fig. 80. Rhopalomyces, sporing head.
Fig. 81. Syncephalastrum, habit and detail of merosporangium.
Fig. 82. Coemansia, habit, sporoclade with sporangia and sporangium with spore
inside.
Fig. 83. Kickxella, habit and sporoclade.
Fig. 84. Cunninghamella, habit and fertile head.
Fig. 85. Mycotypha (l) and Ostracoderma (r) conidiophores.
Fig. 86. Ballocephala, habit of sporangiophores growing from parasitised
tardigrade, sporangiophore and sporangia.
| 9(6) | Sporangia often with pigmented walls, yellowish when young, finally grey or black, with well marked columella left after spore dispersal. Individual sporangiophores observable with unaided eye, up to 20mm long. | |||||||||||||
|
||||||||||||||
| (N.B. Zygorhynchus would key out with Mucor. It is more often isolated from soil, and is distinguished from Mucor by the presence of zygospores with unequal suspensors) | ||||||||||||||
| - | Sporangia white, without a columella, readily becoming a spore droplet. Sporangiophores delicate, often only 200-400µm long. Fine, white, garlic-smelling mycelium often present. | |||||||||||||
|
||||||||||||||
| 10(5) | Sporangioles formed at the final tips of a densely dichotomous system of branchlets, originating some distance below a terminal sporangium (which may be absent in young specimens). Sporangioles up to 25µm diam., with up to 6 spores. Spores 8-12 × 6-8µm. | |||||||||||||
| Thamnidium elegans (fig. 70) | ||||||||||||||
| - | Sporangioles either at the curved tips of slender branches, or clustered in groups about halfway along tapering branches which radiate from the sporangiophore below the sporangium; the branch tips of the latter give the fertile portion of the sporangiophore a bristly appearance. | |||||||||||||
|
||||||||||||||
| 11(1) | Spores formed in chains. | 12 | ||||||||||||
| - | Spores formed singly. | 14 | ||||||||||||
| 12(11) | Sporangiophores regularly and repeatedly dichotomously branched. Chains of 2-10 spores produced in small groups, which may be wet or dry, on deciduous heads, 4-15µm diam. Parasitic on other fungi, mostly other Mucorales. | |||||||||||||||||||
|
||||||||||||||||||||
| - | Sporangiophores simple or irregularly branched. | 13 | ||||||||||||||||||
| 13(12) | A large conspicuous fungus, macroscopically Mucor-like, mycelium coarse. Sporangiophores with a distinct terminal swelling with crowded spore chains. Spores usually 5-10 in a chain, globose to ovoid, 2-8 × 4-6µm. | |||||||||||||||||||
| Syncephalastrum racemosum (fig. 81) | ||||||||||||||||||||
| - | Sporangiophores less conspicuous, 100-1000µm high, with a 'holdfast' at the base attaching the sporangiophore to the substrate. Mycelium very fine. Parasitic on other Mucorales. | |||||||||||||||||||
|
||||||||||||||||||||
| (N.B. Oedocephalum spp. (fig. 79), the anamorphic states of many dung fungi (esp. Ascobolaceae and Pezizaceae), Rhopalomyces (fig. 80), and some Aspergillus spp. are superficially similar to Syncephalis at first sight). | ||||||||||||||||||||
| 14(11) | Sporangia containing a single closely fitting elongated spore, produced in serried ranks on one side of a boat-shaped branch (sporoclade). | 15 | ||||||||||||||||||
| - | Single-spored sporangia ('spores') globose, produced singly or if in groups not on sporoclades. | 16 | ||||||||||||||||||
| 15(14) | Sporoclades lateral. Sporangiophores usually yellowish. (No parasitism has been demonstrated, but in culture grows much better in the presence of the white, garlic-smelling Mortierella spp.). | ||||||||||
|
|||||||||||
| - | Sporoclades produced in a terminal verticil. Sporangiophores shining white. | ||||||||||
| Kickxella alabastrina (fig. 83) | |||||||||||
| 16(14) | Spores produced in clusters below the apex of the final branches of a compound, often trifid, branching system which is given a bristly appearance by the projecting tips. Superficially similar to Thamnidium or Helicostylum. Capable of parasitising, and growing much better in association with, other Mucorales. | ||||||||||
|
|||||||||||
| - | 'Spores' not produced in subterminal clusters, but terminally on lateral vesicles, or over the surface of swollen fertile regions of the sporangiophore. | 17 | |||||||||
| 17(16) | Sporangiophores up to 250µm high. Lateral vesicles numerous, each producing a single 'spore', which is projected when mature. Parasitic on tardigiades. | ||||||||||
| Ballocephala (fig. 86) | |||||||||||
| - | Sporangiophores visible with the unaided eye. Spores produced on swollen parts of the sporangiophore. | 18 | |||||||||
| 18(17) | Sporangiophores branched, with more or less globose terminal fertile regions. Spores dry and powdery, yellowish or pinkish in mass. | ||||||||||
|
|||||||||||
| - | Sporangiophores unbranched, fertile portion 200-300 × 15-20µm. Fertile region terminal only, cylindrical. Spores smooth, greyish in mass, 2-4µm diam. | ||||||||||
| Mycotypha microspora (fig. 85) | |||||||||||
| (N.B. Ostracoderma epigea (fig. 85), the anamorph of Peziza astracoderma, which occurs on paper and sometimes dung and highly organic substrates, was originally described as Mycotypha dichotoma. The fertile regions are cylindrical but multiple as the result of several close dichotomous divisions at the base of the fertile portion). | |||||||||||
Notes
There are few reports of Ascozonus, apart from A. woolhopensis. Observed spore sizes of A. woolhopensis suggest that measurement of Renny's (1874) illustrations of spores leads to values which are too large (19-20 × 6-6.5µm). Those in parentheses are what they might be, based on the discrepancy between observed values for A. woolhopensis and Renny's illustration.
End of the Project Gutenberg EBook of Keys to Fungi on Dung, by
Mike Richardson and Roy Watling
*** END OF THIS PROJECT GUTENBERG EBOOK KEYS TO FUNGI ON DUNG ***
***** This file should be named 57291-h.htm or 57291-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/5/7/2/9/57291/
Produced by Keith Edkins, Mary Glenn Krause, Eric Lehtonen
and the Online Distributed Proofreading Team at
http://www.pgdp.net
Updated editions will replace the previous one--the old editions will
be renamed.
Creating the works from print editions not protected by U.S. copyright
law means that no one owns a United States copyright in these works,
so the Foundation (and you!) can copy and distribute it in the United
States without permission and without paying copyright
royalties. Special rules, set forth in the General Terms of Use part
of this license, apply to copying and distributing Project
Gutenberg-tm electronic works to protect the PROJECT GUTENBERG-tm
concept and trademark. Project Gutenberg is a registered trademark,
and may not be used if you charge for the eBooks, unless you receive
specific permission. If you do not charge anything for copies of this
eBook, complying with the rules is very easy. You may use this eBook
for nearly any purpose such as creation of derivative works, reports,
performances and research. They may be modified and printed and given
away--you may do practically ANYTHING in the United States with eBooks
not protected by U.S. copyright law. Redistribution is subject to the
trademark license, especially commercial redistribution.
START: FULL LICENSE
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full
Project Gutenberg-tm License available with this file or online at
www.gutenberg.org/license.
Section 1. General Terms of Use and Redistributing Project
Gutenberg-tm electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or
destroy all copies of Project Gutenberg-tm electronic works in your
possession. If you paid a fee for obtaining a copy of or access to a
Project Gutenberg-tm electronic work and you do not agree to be bound
by the terms of this agreement, you may obtain a refund from the
person or entity to whom you paid the fee as set forth in paragraph
1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this
agreement and help preserve free future access to Project Gutenberg-tm
electronic works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the
Foundation" or PGLAF), owns a compilation copyright in the collection
of Project Gutenberg-tm electronic works. Nearly all the individual
works in the collection are in the public domain in the United
States. If an individual work is unprotected by copyright law in the
United States and you are located in the United States, we do not
claim a right to prevent you from copying, distributing, performing,
displaying or creating derivative works based on the work as long as
all references to Project Gutenberg are removed. Of course, we hope
that you will support the Project Gutenberg-tm mission of promoting
free access to electronic works by freely sharing Project Gutenberg-tm
works in compliance with the terms of this agreement for keeping the
Project Gutenberg-tm name associated with the work. You can easily
comply with the terms of this agreement by keeping this work in the
same format with its attached full Project Gutenberg-tm License when
you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are
in a constant state of change. If you are outside the United States,
check the laws of your country in addition to the terms of this
agreement before downloading, copying, displaying, performing,
distributing or creating derivative works based on this work or any
other Project Gutenberg-tm work. The Foundation makes no
representations concerning the copyright status of any work in any
country outside the United States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other
immediate access to, the full Project Gutenberg-tm License must appear
prominently whenever any copy of a Project Gutenberg-tm work (any work
on which the phrase "Project Gutenberg" appears, or with which the
phrase "Project Gutenberg" is associated) is accessed, displayed,
performed, viewed, copied or distributed:
This eBook is for the use of anyone anywhere in the United States and
most other parts of the world at no cost and with almost no
restrictions whatsoever. You may copy it, give it away or re-use it
under the terms of the Project Gutenberg License included with this
eBook or online at www.gutenberg.org. If you are not located in the
United States, you'll have to check the laws of the country where you
are located before using this ebook.
1.E.2. If an individual Project Gutenberg-tm electronic work is
derived from texts not protected by U.S. copyright law (does not
contain a notice indicating that it is posted with permission of the
copyright holder), the work can be copied and distributed to anyone in
the United States without paying any fees or charges. If you are
redistributing or providing access to a work with the phrase "Project
Gutenberg" associated with or appearing on the work, you must comply
either with the requirements of paragraphs 1.E.1 through 1.E.7 or
obtain permission for the use of the work and the Project Gutenberg-tm
trademark as set forth in paragraphs 1.E.8 or 1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any
additional terms imposed by the copyright holder. Additional terms
will be linked to the Project Gutenberg-tm License for all works
posted with the permission of the copyright holder found at the
beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including
any word processing or hypertext form. However, if you provide access
to or distribute copies of a Project Gutenberg-tm work in a format
other than "Plain Vanilla ASCII" or other format used in the official
version posted on the official Project Gutenberg-tm web site
(www.gutenberg.org), you must, at no additional cost, fee or expense
to the user, provide a copy, a means of exporting a copy, or a means
of obtaining a copy upon request, of the work in its original "Plain
Vanilla ASCII" or other form. Any alternate format must include the
full Project Gutenberg-tm License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works
provided that
* You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is owed
to the owner of the Project Gutenberg-tm trademark, but he has
agreed to donate royalties under this paragraph to the Project
Gutenberg Literary Archive Foundation. Royalty payments must be paid
within 60 days following each date on which you prepare (or are
legally required to prepare) your periodic tax returns. Royalty
payments should be clearly marked as such and sent to the Project
Gutenberg Literary Archive Foundation at the address specified in
Section 4, "Information about donations to the Project Gutenberg
Literary Archive Foundation."
* You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or destroy all
copies of the works possessed in a physical medium and discontinue
all use of and all access to other copies of Project Gutenberg-tm
works.
* You provide, in accordance with paragraph 1.F.3, a full refund of
any money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days of
receipt of the work.
* You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project
Gutenberg-tm electronic work or group of works on different terms than
are set forth in this agreement, you must obtain permission in writing
from both the Project Gutenberg Literary Archive Foundation and The
Project Gutenberg Trademark LLC, the owner of the Project Gutenberg-tm
trademark. Contact the Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
works not protected by U.S. copyright law in creating the Project
Gutenberg-tm collection. Despite these efforts, Project Gutenberg-tm
electronic works, and the medium on which they may be stored, may
contain "Defects," such as, but not limited to, incomplete, inaccurate
or corrupt data, transcription errors, a copyright or other
intellectual property infringement, a defective or damaged disk or
other medium, a computer virus, or computer codes that damage or
cannot be read by your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH 1.F.3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium
with your written explanation. The person or entity that provided you
with the defective work may elect to provide a replacement copy in
lieu of a refund. If you received the work electronically, the person
or entity providing it to you may choose to give you a second
opportunity to receive the work electronically in lieu of a refund. If
the second copy is also defective, you may demand a refund in writing
without further opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS', WITH NO
OTHER WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT
LIMITED TO WARRANTIES OF MERCHANTABILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of
damages. If any disclaimer or limitation set forth in this agreement
violates the law of the state applicable to this agreement, the
agreement shall be interpreted to make the maximum disclaimer or
limitation permitted by the applicable state law. The invalidity or
unenforceability of any provision of this agreement shall not void the
remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in
accordance with this agreement, and any volunteers associated with the
production, promotion and distribution of Project Gutenberg-tm
electronic works, harmless from all liability, costs and expenses,
including legal fees, that arise directly or indirectly from any of
the following which you do or cause to occur: (a) distribution of this
or any Project Gutenberg-tm work, (b) alteration, modification, or
additions or deletions to any Project Gutenberg-tm work, and (c) any
Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of
computers including obsolete, old, middle-aged and new computers. It
exists because of the efforts of hundreds of volunteers and donations
from people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need are critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future
generations. To learn more about the Project Gutenberg Literary
Archive Foundation and how your efforts and donations can help, see
Sections 3 and 4 and the Foundation information page at
www.gutenberg.org
Section 3. Information about the Project Gutenberg Literary Archive Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Contributions to the Project Gutenberg Literary
Archive Foundation are tax deductible to the full extent permitted by
U.S. federal laws and your state's laws.
The Foundation's principal office is in Fairbanks, Alaska, with the
mailing address: PO Box 750175, Fairbanks, AK 99775, but its
volunteers and employees are scattered throughout numerous
locations. Its business office is located at 809 North 1500 West, Salt
Lake City, UT 84116, (801) 596-1887. Email contact links and up to
date contact information can be found at the Foundation's web site and
official page at www.gutenberg.org/contact
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
[email protected]
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To SEND
DONATIONS or determine the status of compliance for any particular
state visit www.gutenberg.org/donate
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including checks, online payments and credit card donations. To
donate, please visit: www.gutenberg.org/donate
Section 5. General Information About Project Gutenberg-tm electronic works.
Professor Michael S. Hart was the originator of the Project
Gutenberg-tm concept of a library of electronic works that could be
freely shared with anyone. For forty years, he produced and
distributed Project Gutenberg-tm eBooks with only a loose network of
volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as not protected by copyright in
the U.S. unless a copyright notice is included. Thus, we do not
necessarily keep eBooks in compliance with any particular paper
edition.
Most people start at our Web site which has the main PG search
facility: www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.