Red. Orange. Yellow. Green.
Blue. Indigo. Violet.
The Project Gutenberg EBook of The Dyer's Guide, by Thomas Packer This eBook is for the use of anyone anywhere in the United States and most other parts of the world at no cost and with almost no restrictions whatsoever. You may copy it, give it away or re-use it under the terms of the Project Gutenberg License included with this eBook or online at www.gutenberg.org. If you are not located in the United States, you'll have to check the laws of the country where you are located before using this ebook. Title: The Dyer's Guide Author: Thomas Packer Release Date: December 23, 2016 [EBook #53797] Language: English Character set encoding: ISO-8859-1 *** START OF THIS PROJECT GUTENBERG EBOOK THE DYER'S GUIDE *** Produced by Deaurider, Chris Pinfield and the Online Distributed Proofreading Team at http://www.pgdp.net (This file was produced from images generously made available by The Internet Archive)
Transcriber's Note.
Apparent typographical errors have been corrected. The use of hyphens has been rationalised.
THE DYER'S GUIDE.
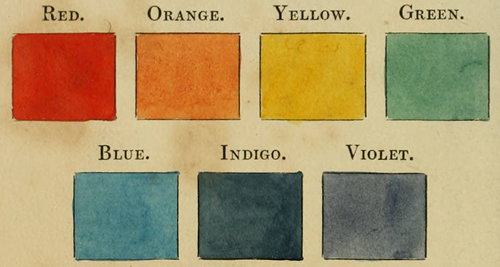
Colours obtained by Sir Isaac Newton's method of decomposing the rays of light, the least refrangible being placed first, the most refrangible last. See p. 18.

Red. Orange. Yellow. Green.
Blue. Indigo. Violet.
THE DYER'S COLOURS AND THEIR CHIEF COMPOUNDS.
SIMPLE COLOURS.
Blue, Yellow, Red, Black[1].
Red includes Crimson, Scarlet, Maroon, Pink, &c.
COMPOUND COLOURS.
Green is made with Blue and Yellow.
Orange with Red and Yellow.
| Purple | ⎫ | |
| Violet | ⎬ | with Blue and Red. |
| Lilac | ⎭ |
Greys with Black, Blue, and Red.
| ⎧ | Blue, Yellow, and Black; | |
| Olives with | ⎨ | or |
| ⎩ | Blue, Yellow, and Red; |
[1] Black according to the theory of Newton, denotes the absence, and White the presence of all colours.
BEING A
COMPENDIUM OF THE ART OF DYEING
LINEN, COTTON, SILK, WOOL, MUSLIN, DRESSES,
FURNITURE, &c. &c.
WITH THE METHOD OF
SCOURING WOOL, BLEACHING COTTON, &c.
AND
DIRECTIONS FOR UNGUMMING SILK, AND FOR WHITENING
AND SULPHURING SILK AND WOOL.
AND ALSO
AN INTRODUCTORY EPITOME OF THE LEADING FACTS IN CHEMISTRY,
AS CONNECTED WITH THE ART OF DYEING.
By THOMAS PACKER,
DYER AND PRACTICAL CHEMIST.
SECOND EDITION,
CORRECTED AND MATERIALLY IMPROVED.
LONDON:
PRINTED FOR SHERWOOD, GILBERT, AND PIPER,
PATERNOSTER-ROW.
1830.
To insist on the utility of the present Manual is, assuredly, superfluous. The favourable reception of the first edition, sometime since out of print, has stimulated the author to revise the work throughout, and to render it more deserving the public approbation. The Appendix to the first edition now forms a part of the Introductory Chapter, to which it naturally belongs; to the whole have been added such improvements as the present advanced state of knowledge, and particularly chemical knowledge, has rendered absolutely necessary; and which the practical dyer will find of considerable importance and much utility.
The following letter from the late Sir Humphry Davy, the first chemist of the age, appeared in the Preface to the first edition; it is here again reprinted as some proof of the sufficiency of that learned man's judgment, at least concerning the chemical theory of the art of dyeing.
I am very much obliged to you for your liberal communication on a subject of my Lectures: I will attend to the information you are so good as to give me in the next Edition.
The author has only to add, that an Index is now appended to the work, by which every article may be most readily and conveniently found.
Page 22, line 3, for proximate read ultimate.
| CHAPTER I. INTRODUCTORY. |
PAGE |
| On the different branches of dyeing— On the drugs used in dyeing—On vegetable and animal substances—On substantive and adjective colours, and mordants—And on the leading facts of chemical science as connected with the art of dyeing—On the calico printer's mordant for yellow and red, and on compound colours—On bleaching—On the theory of fast and fugitive colours—On dye-houses and water—Miscellaneous observations. | 1 |
| CHAPTER II. ON DYEING COTTON. |
|
| To dye cotton a Saxon or chemic blue—Sulphate of Indigo—Saxon or chemic green—To set a cold indigo vat—Another Indigo vat—To dye cotton a fast green with the cold indigo vat and weld—Another cold blue vat for linen and cotton—solution of indigo for penciling printed muslin, &c.—To dye cotton a fast buff—To dye cotton pink. | 47 |
| CHAPTER III. ON DYEING SILK. |
|
| To alum silk—The blue vat of indigo for silk—Another blue vat for silk—To dye silk violet, royal purple, &c.—To dye silk lilac—Another process for lilac—Another process for dyeing muslin, &c. lilac—To dye silk a violet or purple with logwood—To dye silk violet with Brazil wood and logwood—To dye silk violet or purple with Brazil wood and archil. | 63 |
| CHAPTER IV. ON SCOURING AND DYEING WOOL. |
|
| On the action of alum and tartar upon wool—A pastil or woad vat for blue—To prepare the indigo mentioned in the preceding directions—Rules to judge of the state of the vat—Indications when a vat has had too much or too little lime—To work a vat which is in proper order—On the putrefaction of the woad vat—Methods of dyeing blues—To dye wool with lac-dye, scarlet, or crimson—To dye worsted yarn a crimson—A preparation of archil to finish the crimson—on dyeing wool scarlet—To dye wool maroon—To dye wool yellow—To dye wool brown or of a fawn colour—To dye wool purple, &c.—To dye wool green—A chemic vat for green woollen—A chemic vat for blue woollen—To dye wool orange, gold colour, &c.—To dye wool black—Another process for black without a blue ground—To dye wool a grey—Mixture of black or grey with red and blue—On browns, fawns, greys, &c.—On the yellow of Quercitron bark—On a full bright yellow from the same bark—Bancroft's murio-sulphate of tin—To dye wool buff—To dye wool peach—To set an indigo vat for worsted, serge, &c. | 70 |
| CHAPTER V. ON DYEING SILK AND COTTON BLACK. |
|
| To dye silk black for velvets—To dye silk black London process—On dyeing cotton black at Rouen—To dye cotton black, London process—For dyeing black, particularly cotton velvets, at Manchester—On dyeing silk and cotton black with a blue ground—Another iron liquor—To dye cotton black by using the preceding solution—to dye cotton violet—To dye cotton red—To dye cotton an Adrianople or Turkey red—Miscellaneous observations relative to Adrianople red. | 105 |
| CHAPTER VI. ON DYEING COTTON AND SILK. |
|
| To dye skein cotton yellow—On dyeing and re-dyeing cotton furniture yellow—to dye cotton skein a duck's wing green and olive—Of browns, maroons, coffee colours, &c.—Observations on silk—On ungumming and boiling silk—Whitening—Sulphuring—On aluming silk—Skein silk for yellow—Preparation of annatto for aurora or orange, moidore, gold colour, and chamois—To dye silk aurora or orange—To dye moidore—Process for orange—To dye silk poppy or coquelicot—A cheaper poppy with annatto and Brazil wood—On dyeing, silk a fine crimson—Composition for dyeing silks scarlet or crimson with cochineal—Another process for crimson—Another process for crimson by Brazil wood—Of fine violet—Observations on crimson and scarlet upon silk—On dyeing silk green—On olives—On dyeing silk grey—Nut grey—Black greys—Iron greys—On dyeing silk of a Prussian blue colour—Chromate of lead for yellow on silk or cotton—Conclusion. | 123 |
| Index | 153 |
THE
DYER'S GUIDE.
On the different branches of dyeing—On the drugs used in dyeing—On vegetable and animal substances—On substantive and adjective colours and mordants, and on the leading facts of chemical science, as connected with the art of dyeing—On the Calico-Printers' mordant for yellow and red, and on compound colours—On bleaching—On the theory of fast and fugitive colours—On dye-houses and water—Miscellaneous observations.
The trade of a Dyer is, in this country, subdivided into several distinct branches. Thus we have woollen dyers, who are occupied solely in the colours obtained from cochineal, such as scarlet, crimson, orange, buff, &c.; likewise purple, or royal purple, obtained from cochineal and indigo. They are called, also, grain dyers, from the circumstance of the colouring material, cochineal, {2} being in small grains[2]. Yet it ought to be observed, that the term dyed in grain is applied by the public generally in a very different sense, namely, to those cloths the raw material of which is dyed previously to being spun into thread, or at least before woven into cloth; and hence such dyes are usually more permanent than those which are dyed after the materials are woven into cloth. This class of dyers generally dye cloth in the piece, or a number of pieces of cloth tacked together, and worked over a winch in a suitable copper.
There are dyers who likewise dye worsted and woollen yarn of those grain colours, but they are generally a distinct branch. The yarn is dyed in hanks, upon sticks; and, when in the copper, the hanks are changed end for end, so that they may be kept even; such changing being performed five or six times to each turning in.
There are also silk dyers who are grain dyers. These dye in the skein, chiefly for new goods. Some silk, and some mixed silk and worsted goods, are dyed in the piece.
In dyeing cotton, the Adrianople or Turkey Red is, in many cases, a branch of itself, and comes the nearest to what may be called grain or scarlet dyeing upon cotton, because cochineal cannot be applied to cotton to any advantage; yet cotton is occasionally dyed with this material.
In woollen another branch consists of the woad dyers. These often superintend the black dye on woollen cloth, {3} as well as the blue from woad and indigo. There is the same distinction among worsted yarn dyers, they having likewise to do slates, greys, &c. Nearly the same may be said of the silk skein dyers.
In many places, particularly in the country, browns, drabs, stone-colours, &c. constitute a branch in woollen. The same colours form also a branch in calico and muslin; but black, in calico and muslin, is a distinct branch.
The dyers (whether in London or provincial towns) who keep shops, and take in garments, furniture, &c. to be dyed, are termed by the trade Rag-dyers.
There are a few dyers in the metropolis who dye black on woollen, silk, cotton, &c. for the dye-shops, many of these putting all their black out to be dyed.
There are one or two dyers famous for dyeing silk stockings black; these constitute a particular branch. Dyeing bombasins black is also another branch.
The following constitute also particular branches: black hats,—hats of fancy colours,—fur,—chip and straw,—feathers,—leather, Morocco and Spanish, and kid leather for shoes and gloves. Many other branches of the dye-trade might be enumerated, but more detail does not appear necessary.
Concerning all these different branches, one general observation will suffice; namely, that those who are concerned in them have, for the most part, obtained, their knowledge of the art of dyeing, not from theories adapted to explain the different processes, but from practice in that branch in which they are occupied. They usually, therefore, perform those processes which {4} they have been shewn and told, without any inquiry into the causes which produce the results. There are, it is admitted, exceptions to this, men of general information and knowledge being occasionally found in the various branches of dyeing, but they are so few, that it may be questioned, when compared with the great body employed in the art, whether they amount to one in a thousand. This is not, however, to be attributed to any indifference in such persons to acquire a correct knowledge of the art, but is chiefly owing to a deficiency of the ready means of acquiring such information; which information it is the design of the present Treatise to supply; there not being, as far as the present writer knows, any such work, at a moderate price, to be obtained in the English language.
It is true many of the Cyclopędias furnish us with much useful information on the subject of dyeing: one of these, Jennings's Family Cyclopędia, may be particularly mentioned as containing such; but it is scattered about in these dictionaries in various ways, at once troublesome and unpleasant to obtain. Dr. Bancroft's work on the philosophy of Permanent Colours, in two octavo volumes, will also supply much valuable information; so also will the edition, some time since published, of Berthollet's Elements of the art of Dyeing, with the addition of valuable Notes by Dr. Ure. Dr. Ure's Chemical Dictionary is also very useful to the dyer, us well as many detached papers in several of our English publications. A Treatise on Printing and Dyeing Silks, &c. lately published by H. M'Kernan, is also valuable, and should be consulted by the curious in this {5} art. But all these works are expensive, and such as few dyers will be disposed to obtain; hence the necessity of the present Manual, the author of which has not servilely followed the directions or recommendations of any previous writer; but from his own practice, a practice of more than thirty years, has laid down such rules as he knows to be at once practical and efficient. At the same time he thinks it right to state, that he has not only consulted all the works mentioned above, but also Hellot, Macquer, &c. adopting all that appeared essential in these, and giving such additions as accord with the present improved state of chemistry and dyeing; and, as far as was possible, in the limits prescribed for this work, so that it may be within the reach of every dyer in the kingdom, as well as every journeyman and apprentice in all the various branches of this truly extensive and mysterious art, as carried on in London, Norwich, Yorkshire, Gloucestershire, and various other parts of the British dominions.
The author has, in treating of the various matters to be dyed, adopted nearly the same arrangement as that which appears in the Title, taking Cotton first, in consequence of its having the least affinity for dyeing bodies. He has taken Silk next, which has a greater affinity for many dyes, and, when dyed, yields colours more permanent than cotton.
Wool he has not placed entirely last, although many of the colours which it receives from the dyer are complex. The black dyeing of cotton and silk is placed after the processes of black for wool, as likewise the Turkey red, &c. these being naturally difficult to perform.
{6} White and black have been considered colours by dyers, and with propriety, black forming a part of slate, grey, &c. White is seldom pure; in proportion to its clearness and purity will the colours be with which it is dyed.
In regard to black dye, and particularly cotton black dye, the author does not know any simple and concise theory, consistent with chemical principles. He flatters himself, however, that from his extensive experience, his observations are founded on interesting facts. Cotton, for instance, will take fast blues from the cold indigo vat; this vat, with the combination of iron, and in a heat no greater than the hand can bear, will easily produce all shades of grey, slate, &c. Many of these colours may be done by logwood instead of the blue vat, and in the same heat of the dye bath; so cotton likewise, whether in pieces or skeins, may be dyed brown, fawn, drab, &c. in consequence of the great affinity which cotton has for acetate and sulphate of iron.
With respect to black, it should be also observed, that few substances are known which yield by themselves a good black. The juice of the cashew nut communicates, however, a black colour, which resists not only washing, but even boiling with soap and alkaline leys. It is used for marking linen. The Toxicodendron yields a juice which produces nearly the same effect. Some other vegetables also produce black dyes, but all of them in such small quantities as not to be available for the purposes of art; nor do they, besides, produce blacks equal to those formed in the dye-house.
Blue, red, and yellow are admitted to be three distinct {7} colours. In many of the browns, red and yellow are combined naturally in the drugs from which they are produced, and so they are in logwood. Blue, red, and yellow, are developed by iron, whether in the state of an acetate or sulphate.
It may be useful, before we proceed any farther in noticing the theories of dyeing, to give a brief description of the
Alum, or Potash-sulphate of alumina, is a concrete salt, composed of alumina or clay, potash, and sulphuric acid. It is found native in some places; but the greatest part of the alum of commerce is prepared by a peculiar management of schistose pyritic clays, usually denominated alum ores. Alum is made at Civita Vecchia, in Italy, and also at many other places on the continent; at Hurlett near Glasgow, at Whitby in Yorkshire, &c. Its form and appearance are both too well known to need being described. Its chemical composition is as follows: sulphate of alumina, 36.70; sulphate of potash, 18.88; and water, 44.42—together 100. The alum called in commerce Roch alum, said to be obtained from Roccha, in Syria, is in smaller crystals than common alum, and has a reddish hue, but does not appear to be essentially different from the common alum. Common alum requires sixteen parts of water, at a temperature of 60°. to dissolve one of it; but there is another kind not generally made or known, containing soda instead of potash, and hence with propriety named soda-sulphate of alumina, which is soluble in less than its {8} own weight of water, and which, on this account, may become valuable in some processes of dyeing.—Ure.
Acetate of Alumina is prepared in large quantities for the calico printers, by decomposing alum with acetate of lead, or more economically with aqueous acetate of lime, having a specific gravity of about 1.050, a gallon of which, equivalent to nearly half a pound avoirdupoise of dry acetic acid, is employed for every 2½ lbs. of alum. A sulphate of lime is formed by complex affinity which precipitates, and an acetate of alumina floats.—Ure.
Archil, Archilla, Rocella, Orseille, or Litmus, is said to be a whitish lichen growing upon rocks in the Canary and Cape Verd islands, which yields a rich purple tincture, fugitive, but extremely beautiful. It is brought to this country as it is gathered; it is prepared here for the dyer, by grinding it between stones, so as thoroughly to bruise but not to reduce it into powder; it is moistened occasionally with a strong spirit of urine, or urine itself, mixed with quicklime; in a few days it acquires a purplish red, and at length a blue colour; in the first state it is called archil, in the latter lacmus or litmus. The dyers rarely employ this drug by itself, on account of its dearness and the perishableness of its beauty. Its chief use is to give a bloom to other colours, as pinks, &c.
Cudbear is also manufactured in this country from archil, and is in repute for dyeing various shades, from pink and crimson to a mazarine blue; it is said these colours are very permanent.
Argol, or Tartar, is a crystalline substance deposited in wine casks during the fermentation of the wine, {9} from the juice of the grape, in which it exists in considerable abundance. It is an impure supertartate of potash; that is, potash combined with a superabundant quantity of tartaric acid. Algol is found in commerce of two colours, white and red. Cream of tartar is the same substance freed from colouring and other extraneous matter.
Blood. See Adrianople red.
Bran acts in some peculiar way on colouring matter, but scarcely on the mordants. It seems to loosen and remove the colouring matter; as also to alter its hue in some cases, an effect obvious in the bran pinks.—Ure.
Chlorine. See Oxymuriatic acid.
Cochineal is the female insect of the coccus cacti found on the cactus coccinellifer and cactus opuntia, Prickly pear or Indian fig, natives of South America, the West Indies, and other tropical regions. The female of the insect is the true cochineal; in her full sized, pregnant, and torpid state, she bears so small a proportion to her former or creeping state, that her antennę, legs and proboscis are scarcely discernible; her whole appearance is that of a whitish berry, and so it was formerly regarded. This insect is found in a wild state in Mexico, Georgia, South Carolina, and some of the West India Islands, feeding on several species of the cactus; but in some of the Spanish settlements, as well as in Mexico, the insect is domesticated, and fed on the cactus coccinellifer, which is cultivated for the purpose, on which it attains a much larger size than in its wild state. Cochineal is also obtained from the East Indies; but East Indian cochineal has not yet attained the quality of that produced {10} in the West Indies and America. Its use, as a colour for dyeing many shades of red, &c. is great and important.
Copper is also used in dyeing, in the state of a sulphate or blue copperas, a nitrate, and also as an acetate. See Verdigris.
The Gall or Bile of ANIMALS consists of a saponaceous bitter, yellowish fluid, secreted by the liver, and found in the sac usually called the gall-bladder. It is sometimes preferred to soap for cleansing cloths by the dyer and the scourer.
Galls are excrescences produced on the quercus infectoria, a species of oak growing throughout Asia Minor. The gall grows on the shoots of the young boughs, and is produced by an insect, the cynips quercusfolii; this insect punctures the tender shoot with its sting and deposits its egg in the puncture; the egg is soon hatched, and the irritation of the maggot feeding on the plant produces the wen or gall-nut. When the nuts are gathered before the worm within changes to a fly, and not yet having eaten its way out, they are of a dusky green colour, and are called in commerce blue galls, and are by far the best. Those collected after the fly has eaten its way out have a hole in each, are of a whitish yellow colour, considerably lighter than the blue-galls, and of an inferior quality: they are brought to this country chiefly from Aleppo. They are used in large quantities in the arts, principally for dyeing, and making ink. They contain a large quantity of Tannin and Gallic acid.
Indigo is a well known deep blue substance, obtained {11} from the Indigofera tinctoria or Indigo bearing plant, a native of the East Indies, which is propagated by seed and will thrive in most tropical climates; hence we have good indigo from South America, the East Indies, Carolina, &c. The chief criterion of the goodness of indigo is, if, when cut with a knife, it exhibits a reddish copper-like appearance; where this shade is not, or only very slight, the indigo is of inferior value. It is prepared by macerating the leaves in water, whence is obtained the blue feculence or indigo. Indigo is insoluble in water, but soluble in sulphuric acid, hence a solution of it in this acid, forming a sulphate of indigo, is well known in the art of dyeing.
The best indigo is that called Flora, which floats in water, all the other kinds sink in that fluid.
The constituent parts of indigo are Carbon, 73.22, Nitrogen 11.26, Oxygen 12.60, and Hydrogen, 2.92, = 100.
When indigo is digested in concentrated sulphuric acid, it is converted into a peculiar blue substance, commonly called sulphate of indigo; this colouring matter has been, however, lately named Cerulin, by Mr. W. Crum, who has made many experiments on it; (see notes to Bertholet, vol. ii. p. 357. et seq.) he observes that cerulin dissolves more abundantly in sulphuric acid than water; but this does not prove the formation of a compound entitled to be called sulphate of indigo; that, such a solution differs in no respect from that of resins in acids or in alcohol. Another substance has been also obtained from indigo by Mr. Crum, of a purple colour, which he calls Phenicin; it dissolves both in water and alcohol.
{12} Iron rarely in its metallic state enters into the manipulations of dyeing, but its sulphate, muriate, acetate, &c. as well as its oxides contribute largely to the dyer's art.
Sulphate of Iron, or green copperas, as it is commonly called, is too well known to need description; it is in green crystals of different sizes, and is used for various purposes in dyeing, &c.
Peracetate of Iron, or Acetate of Iron, forms a reddish-brown uncrystallizable solution, much used by the calico printers, and is prepared by keeping iron turnings or pieces of old iron for six months immersed in redistilled pyrolignous acid. It may be also prepared in a more expeditious way by boiling filings of iron with the acid.
Lac dye and LAC LAKE are two articles now regularly imported from the East Indies, and employed for dyeing scarlet. They both appear to be the colouring matter of seed-lac, obtained from it in India by a process not generally known. Both these articles are in lumps or cakes of a dark-reddish or blackish colour.
Muriatic acid, or spirit of salt, as it was formerly called, is obtained from common salt or muriate of soda, by distillation with sulphuric acid. When this acid is pure it is perfectly colourless, but it generally has a yellow hue arising from a little iron. It gives out, at all temperatures, a large quantity of a fuming suffocating gas of a peculiar smell. Its usual specific gravity is about 1.160. For the basis of this acid see Oxymuriatic acid.
Nitric acid is composed of oxygen and nitrogen: it is usually obtained from nitre, (the chemical name of which {13} is nitrate of potash,) by distilling three parts of it with two of sulphuric acid. When pure, nitric acid is a colourless, extremely sour, and corrosive liquor. Its specific gravity is 1.42; it always contains more or less water, which modifies its specific gravity. It is usually coloured with nitrous acid gas. It forms a variety of compounds with numerous other bodies. Aqua fortis is this acid diluted more or less with water; when strong it is called double, when weak single aqua fortis. For Nitrogen, see forwards.
Nitro-muriatic acid, or AQUA REGIA, is a mixture of nitric and muriatic acids. It is usually made by dissolving sal ammoniac or common salt in nitric acid. When the former is employed the usual proportion is one of the salt to four of the acid; but equal parts will be necessary to dissolve platinum. Aqua regia is the only menstruum which will dissolve gold.
Orpiment, REALGAR, or SULPHURET of ARSENIC has been lately applied to the purposes of dyeing a yellow colour. Sulphur may be combined with arsenic in different proportions. Realgar is red, and occurs native in Germany and Switzerland; it is also produced by art. Orpiment is commonly produced by art and is of a yellowish colour; native orpiment is also occasionally found; it is of a bright lemon colour.
Oxymuriatic acid, or as it is now more correctly termed CHLORINE, from its yellowish green colour, is an elastic gaseous fluid of a pungent disagreeable smell, and highly injurious to animal life, even when largely diluted with atmospheric air. Mixed with hydrogen, and exposed to light, they combine and produce a sour compound {14} called muriatic acid gas; this gas is greedily absorbed by water, which takes up 480 times its bulk, and has its specific gravity increased from 1 to 1.210. Thus dissolved in water it forms the liquid muriatic acid mentioned in a preceding article.
Chlorine forms combination, besides, with several other bodies; many of its combinations are termed oxymuriates, or more properly, chlorides: some of these are extremely useful in bleaching, dyeing, &c. The muriatic acid appears to be the only acid of any consequence into which oxygen does not enter.
Oxide is the combination of oxygen with some base, without being in the state of acid; it is most commonly applied to the combination of oxygen with metals; most of the different rusts of metals are oxides. As oxygen combines with the metals and other bodies in different proportions, its combinations are distinguished by different prefixes, thus: protoxide denotes an oxide containing the least quantity of oxygen: deutoxide the next larger quantity; tritoxide the next; and peroxide the largest possible quantity of oxygen in the compound when it is not acid. For Oxygen see forwards.
Pot-ashes and Pearl-ashes (one of the fixed alkalies) are both impure carbonates of potash obtained from the ashes of innumerable vegetables, over which water is poured which dissolves the salts, and by evaporating the water leaving the salt, a dry powdery white mass is obtained. The chief difference between pot-ashes and pearl-ashes consists in the superior whiteness of the latter, and in the former being of a more dirty colour, and more caustic than the latter; hence it is not so highly {15} saturated with carbonic acid. For many purposes in the arts such caustic potash is to be preferred.
Quercitron, or American-bark is obtained from the quercus nigra or black oak, a native of North America. It is used for dyeing yellow, and was brought into notice by Dr. Bancroft, who obtained the exclusive privilege of using it as a dye by an Act of Parliament, passed in the 25th year of the reign of George III.
Safflower, bastard-saffron or carthamus, is obtained from one or two plants, species of the carthamus genus, natives of the South of Europe and the Mediterranean coasts. This dyeing material consists of two colouring substances, a yellow and a red. The former is of little value, the latter which is soluble in alkalies forms, by precipitation with acids, a beautiful red pigment sometimes used for silk dyeing, but more commonly in the preparation of rouge.
Soda, called sometimes mineral alkali, is another of the fixed alkalies; it forms the basis of common salt, that being a muriate of soda; soda, under the name of barilla, is used in making soaps, and also in dyeing.
Sulphur, or Brimstone, is scarcely used for dyeing in its crude state, but when combined with oxygen forming sulphuric acid, as well as when that acid is combined with various bases, as iron, alumina, &c. it becomes of great importance in this art; see Sulphuric acid.
Sulphate of Iron, see Iron above.
Sulphuric Acid was for many years, and still is called by the vulgar, oil of vitriol, because it was formerly obtained from green vitriol or sulphate of iron, but the more simple and ingenious processes of modern chemistry {16} have superseded the old methods; sulphuric acid is now obtained by burning sulphur with a certain portion of saltpetre in large leaden cisterns. The acid fumes sink into the water placed at the bottom of the cistern, the water being afterwards boiled away: the acid is afterwards purified by retorts, placed in a sand heat. The specific gravity of good sulphuric acid should be 1.85.
Sumach is the production of the rhus coriaria, a shrub which grows naturally in Syria, Palestine, Spain, and Portugal. It is cultivated in the two last countries with great care. Its shoots are cut down every year quite to the root, and after being dried are reduced to powder, and thus prepared for the purposes of dyeing, &c. Sumach bears a great resemblance, as an astringent, to galls. Sumach alone gives a brown and a fawn colour, but cotton stuffs impregnated with acetate of alumina take a durable yellow from it.
Tartar, see Argol.
Tin, dissolved in nitric or muriatic acid, forms solutions of great importance in many processes of dyeing, particularly scarlet. These solutions are called respectively nitrate and muriate of tin.
Turmeric is a root obtained from a plant growing both in the East and West Indies. The root is used chiefly for dyeing yellow; but it is a fugacious colour.
Verdigris is a crude acetate of copper, obtained by exposing copper plates to the husks, &c. of grapes, which containing considerable acetic acid, the acid combines with the surface of the copper plates, forming a blueish green rust, which is scraped off, and forms the verdigris of commerce. A still more complete acetate of {17} copper is obtained in distilled verdigris, which is in elegant green crystals. The best verdigris is made in France; some is now also made in this country.
Weld, sometimes called improperly Woulds, dyer's-weed, or Reseda luteola, is a plant found wild, in this country, but cultivated for the purposes of the dyer; it is much used for yellows.
Woad, or Pastel, is obtained from a plant growing in various parts of Europe and also in this country; it is the Isatis tinctoria, and is cultivated with care for the dyeing matter which it affords, and which is obtained from the leaves of the plant, collected and prepared in a particular manner. Woad gives a full-bodied and fast blue to wool, yet not very bright, so that it is usually mixed with indigo[3].
Besides the preceding substances we may mention that annatto is used for dyeing several colours; kermes, madder, and Brazil wood for reds; logwood for purple and black; peach-wood for maroon, &c.; fustic, dyer's-broom, saw-wort, French-berries, &c. for yellow; walnut-root, and the outside green shell of the nuts for browns. We may also mention prussiate of potash, acetate of lead, commonly called sugar of lead, and oxide of manganese, as occasional articles used for various purposes by the dyer. Several other substances are also used in dyeing, which we cannot enumerate; some are mentioned in the subsequent pages. We may, however, name cam-wood, bar-wood, redsanders, and myrobolans. We ought also {18} to observe that how desirable soever it may be to have all woods for dyeing, in powder, in order to obtain the greatest quantity of colouring matter from them by decoction or otherwise, yet, as in a powdered state they are much more likely to be adulterated than in chips, it is most advisable to purchase them in this last state; logwood in particular ought never to be purchased in powder.
In order more correctly to understand the theory and practice of dyeing, it is essential that the pupil should become acquainted with the nature of the substances upon which and with which he must necessarily operate. We shall not enter into the theories of light and of colours, as propounded by Sir Isaac Newton, as well as many illustrious chemists, who have already done so much for the art of dyeing, but shall simply refer to such writers as Ure, Bancroft, Berthollet, Brande, &c. from whom may be learnt what is of most importance to be known concerning this curious subject.
We may just add, however, in regard to light, that Sir Isaac Newton proved it consists of rays differing from each other in their relative refrangibilities. By causing light to pass through a hole in a window-shutter into a darkened room, and receiving that light on a glass prism, the rays, in passing through the prism, not only became refracted, that is, thrown out, of the rectilinear direction, but also separated into seven distinct colours, namely, red, orange, yellow, green, blue, indigo, and {19} violet. The red being the least refracted and violet the most. If these prismatic, or primary colours, as they are usually called, be divided into 360 equal parts, the red rays will occupy 45 of these parts, the orange 27, the yellow 48, the green 60, the blue 60, the indigo 40, and the violet 80, and, what is very remarkable, these colours, when mixed in the proportions here set down, produce white. This may be readily proved by mixing seven powders of the colours and quantity mentioned, or by painting a wheel with the same proportions of the different colours and making it revolve rapidly. But it should be noted, that, in either case, the white will not be so pure and delicate, as that produced by the mixture of the rays of light. Upon these phenomena is founded the Newtonian theory of colours. Thus green bodies reflect the green rays and absorb the others. All the rays are reflected by white bodies, and absorbed by those which are black.
It is, notwithstanding, highly necessary that the learner should know that portion of modern chemistry which will lead him to the best secrets of his art, and hence assure him of that which was only before conjecture. And it cannot be sufficiently impressed upon him, that if our theory be not true, we work from wrong data; we may, it is true, approach the truth; be right in some things and wrong in others, and our uncertainty and mistakes will be accordingly; yet the most complete dyer must be he, who with extensive practice combines a knowledge of the true principles of his art, to which modern chemistry is, doubtless, the key.
It is scarcely necessary to insist further on the importance {20} of a knowledge of the constituent parts of vegetable and animal bodies, as well as those inorganic substances with which chemistry has so largely to deal; but it will be seen, in the course of our subsequent observations, what difficulty there is in dyeing cotton of a red colour, similar to that produced by cochineal on wool; how, in dyeing cotton yarn an Adrianople red, the intestinal liquor of the sheep, and the dung and the blood of the same animal are used, and have been found so important by the dyers of Asia; hence the colour is called the Adrianople or Turkey red.
It is found by experience, and particularly in hot climates, that substances containing ammonia (volatile alkali) quite developed, have the property of raising and rendering more intense the red colours. It has been found, too, that the bones of animals retain the colour of madder very strongly, when they have been given that colouring material; and the vivacity of the colour has been attributed in such cases, it is presumed with truth, to the ammonia which the bones contain.
There are, therefore, in regard to vegetables in particular, some things, the nature and properties of which it is absolutely necessary that the dyer should understand: for want of a knowledge of one of them, it is a fact that losses are very often sustained to a serious amount. It may seem surprising, but the author has not seen in any writer on dyeing or chemistry, a proper method of working the pastil or woad vat; nor how to renew and work it down, again and again, with an assurance that it will be neither decomposed nor spoiled; and which, for want of a proper knowledge, it has often {21} been. We shall therefore endeavour to give some directions by which those fatal and expensive disasters may be avoided.
Although, at first sight, it seems easy to distinguish the three kingdoms of nature from each other, yet there is such an imperceptible transition from one to the other, that it will be difficult to give such a definition as shall embrace all the individuals of each, and, at the same time, exclude those of the other kingdoms. On examination, indeed, we do find that there is in fact no natural distinction of this kind; and that there is scarcely a function common to vegetables and minerals which some of the animal tribe do not enjoy, and vice versā. Yet it must, however, be noted, that most animals have the power of voluntary loco-motion, and are thus rendered peculiarly different from all other bodies which we find upon or in the earth.
The substances constituting vegetable differ from those constituting mineral bodies, in their being of a more complex kind; and though vegetables are extremely susceptible of decomposition in various ways, not one can be, by any art, synthetically produced. Yet, although what are called by chemists the proximate constituents of vegetables are numerous, such are water, starch, sugar, gum, gluten, wax, oil, camphor, resins, colouring matter, extractive matter, several acids, &c. &c. all of which are capable of being decomposed, the ultimate constituents of vegetables are very few; the chief are carbon, hydrogen, and oxygen; some afford nitrogen; in some are traces of sulphur, potassa, lime, soda, magnesia, {22} silica, &c.; in nearly all vegetables are traces of iron; in many manganese.
As the ultimate principles of vegetables are chiefly carbon, hydrogen, and oxygen, it will be useful to inquire how vegetables obtain these materials. Water, which is composed of hydrogen and oxygen, is a ready source whence both its constituents may be obtained; and it is concluded that it is decomposed in the glands of vegetables, assisted by solar light, and becomes fixed in them in the state of oil, extract, mucilage, &c. The greatest part, however, of vegetables consists of carbon, or, to make ourselves more intelligible, pure charcoal; the carbon, notwithstanding its solidity in the shape of charcoal, most readily combines with oxygen, and hence it forms, as carbonic acid, a small portion of atmospheric air, from which source the carbon of plants is in part at least derived. Another source from which plants derive their carbon is the earth, and decaying vegetable matters; the dung of animals supplies also some of the constituents of vegetables. Indeed, in the application of dung and other matters, so as to promote the healthy and vigorous growth of vegetables, does the science of agriculture chiefly consist. It appears, however, that nourishment is received principally, if not entirely, by plants in a liquid or gaseous form. It should be noticed too, that few, if any, healthy vegetables will grow any where except in light, a powerful stimulant at all times, not only to plants but to animals; such are its effects, that many dyes in cloth are materially altered, nay, sometimes destroyed by it.
{23} Animal substances thus differ from vegetables: they afford a considerable quantity of ammonia, (which is, it is now known, a compound body consisting of hydrogen and nitrogen), and very fetid products, either by the action of fire, or by the putrid fermentation. They also putrify more readily and speedily than vegetables, and give out a very disagreeable smell. They also contain a considerable quantity of nitrogen, the presence of which constitutes the most striking peculiarity of animal compared with vegetable bodies; but as some vegetables contain nitrogen, so there are certain animal principles into the composition of which nitrogen does not enter. The chief ultimate principles then of animal matter are carbon, hydrogen, oxygen, and nitrogen; but phosphorus and sulphur are also often contained in it. Lime also exists in animal bones and shells in considerable quantity, usually, however, in combination with the phosphoric and the carbonic acid. The chief proximate principles of animal matter are blood, albumen, gelatine, colouring matter, milk, bile, lymph, urine, skin, muscle, horn, hair, fat, cerebral substance, shell, and bone, &c.
The differences between vegetable and animal bodies appear to depend upon animal matter containing nitrogen in much greater abundance than it is found in vegetables; and hence the decomposition of animal matter by destructive distillation is characterized by the presence of ammonia, which is formed by the union of the hydrogen with the nitrogen; and it is sometimes so abundantly generated as to be the leading product: thus when horns, hoofs, or bones are distilled by themselves, a quantity of solid carbonate of ammonia and of the same substance {24} combined with a fœtid oil, and dissolved in water, are obtained. Hence the preparations called salt and spirit of hartshorn and animal oil.
The principal animal fluids are blood, milk, and bile. The blood, soon after it is taken from the living animal, separates into two parts, one called the crassamentum, which is red, and the other serum, which is a fluid, and of a pale straw-colour; the crassamentum is a more firm and consistent mass than the serum, by which it is usually, when cool, surrounded. Milk consists of serum or whey, butter, which while floating on the milk is called cream, and curd or cheese, which has the leading properties of coagulated albumen. The bile, as has been before stated, is a saponaceous fluid consisting chiefly of albumen, soda, a bitter resin, water, and some other saline matter. Fat, in the dead animal, is merely animal oil in a concrete or hardened state.
The principal animal solids besides bone, are albumen, gelatine, and fibrin. These substances, in certain states of concretion and combination, form all the solids of animals, and are separable from each other by easy analysis.
By whatever means we deprive animal substances of their nitrogen, we reduce them to a state similar to that of vegetables. The muscular fibre, or flesh as it is usually called, when excluded from the air, but particularly if in contact with water, parts with its nitrogen, and is converted into a substance resembling spermaceti, which in its analysis agrees with vegetable expressed oil.
When vegetables and animals are deprived of life, their various parts, and especially their fluids, sooner or {25} later, spontaneously assume processes which terminate in their total decomposition. The earlier stages which lead to their decomposition are termed fermentation. Of this there are three kinds; the first, or vinous fermentation, takes place in vegetable juices which contain a considerable quantity of sugar, such are the juices of the grape forming wine, of the apple forming cyder, &c. In this fermentation a considerable quantity of carbonic acid gas is disengaged; this gas is very destructive to animal life, no one can live for a minute in it. If, after the vinous fermentation is completed, the liquor be exposed for some time to atmospheric air, another fermentation takes place, oxygen is absorbed, and the liquor becomes vinegar, hence called the acetous fermentation. The putrid fermentation generally takes place in animal bodies very soon after death, so that neither of the other processes, certainly not the vinous, the acetous rarely, becomes a condition of animal matter.
The chief product of the vinous fermentation is an intoxicating, colourless, volatile, and highly inflammable liquor called alcohol; in common language rectified spirits of wine. It may be obtained by distillation from wine, cyder, perry, brandy, &c. &c.; and from whatever liquor it be obtained, when freed from extraneous matter, it is in every case the same. Alcohol consists of hydrogen, carbon, and oxygen. Its usual specific gravity is 825, water being 1000.
After vegetables have passed through these fermenting processes, the decomposition continuing, unless checked by extraneous means, the remainder of their constituents become separated, many of them being volatilized in the {26} form of gas, and nothing remains but a black or brown residuum called mould, consisting of carbon, some salts, a little oil, and extractive matter.
In the decomposition of animal substances, we perceive the union of hydrogen and nitrogen forming ammonia; the combination of carbon with oxygen produces carbonic acid; and nitric acid arises from the union oxygen and nitrogen. A quantity of hydrogen is also extricated in the form of gas, carrying off with it sulphur and phosphorus, which produce together the disagreeable smell arising from animal putrefaction. Nothing now remains but a portion of carbon mixed with phosphate of soda and phosphate of lime.
Hence we see that, by the processes of fermentation, complex bodies are converted into more simple substances, and that nature restores, in the new combinations, the principles which she had borrowed from the atmosphere for the formation of both animals and vegetables; and that she accomplishes a perpetual circle of ever-changing being, at once demonstrating the fecundity of her resources, and the grandeur and simplicity of her operations.
The substances commonly dyed are either animal, as wool, silk, hair, leather, and skins of all kinds; or vegetable, as cotton, flax, hemp, &c. Great differences exist between the affinities for colouring matter possessed by {27} these substances, so that a process which perfectly succeeds in dyeing wool may fail when applied to cotton. Wool has generally the strongest affinity for colour; silk and other animal substances come next; cotton next, and hemp and flax last.
Of the numerous known dyes, few can be applied to either animal or vegetable fibre without some preparation beyond that of cleansing the stuff, and immersing it in the dyeing liquor. When colours can be fixed on cloth without any previous preparation, they are called substantive colours, such is indigo; when they cannot be so fixed, but require to be saturated with some preparation, such as acetate of alumina, or a metallic oxide, &c. they are called adjective colours; of this kind are madder, cochineal, &c. The substances with which cloths are impregnated, previously to being dyed, are called mordants, because they are supposed to bite or lay hold of the colour which is applied.
The chief difference between vegetable and animal substances is, that animal (as for instance wool) contains a small portion of carbon, and a large quantity of hydrogen and nitrogen; while vegetables contain a very large proportion of carbon, less hydrogen, and, in general, no nitrogen.
It is the interest of every dyer to acquire as much information as possible concerning the nature of alum, iron, carbon, nitrogen, hydrogen, the alkalies, acids, &c. in order to prevent or obviate the consequences of an incorrect application of these agents in the various departments of his art, and also to apply them with the greatest success. We shall, therefore, enter a little into {28} the nature and combinations of some of these bodies, and state some of the leading facts with which the modern discoveries in chemistry have made us acquainted.
Carbon, or charcoal, is considered an elementary body, because, as yet, no means have been found adequate to decompose it; it forms the skeleton of vegetables or their woody fibre.
We must now direct the attention of the reader to oxygen gas, the discovery of which was made by Dr. Priestley in the year 1774, and by him called dephlogisticated air; the most important discovery that was, perhaps, ever made in chemistry. When a metal is exposed to atmospheric air, at almost every temperature, it loses its metallic lustre, and acquires the form and appearance of an earthy substance. If this change be produced in a given quantity of air, the oxidation can only be carried on to a certain degree; and on examining the air which remains, we shall find that it has lost the whole of its oxygen, and that nothing remains but nitrogen gas. What was formerly called the calcination of metals is nothing but the process of their union with oxygen, which is now therefore properly called their oxidation.
If charcoal be mixed with the metallic oxide, and a suitable heat be applied to the mixture, it will unite with the oxygen and form carbonic acid, which will fly off in the form of gas, while the metal will assume its metallic form. From this we learn that oxygen is a part of atmospheric air, and that nitrogen constitutes another portion of the same air. Ammonia is a combination of nitrogen and hydrogen. Combustion, or the burning of any combustible body, cannot take place, at least under {29} ordinary circumstances, without the presence of oxygen. Nitrogen gas, (called by its discoverers azotic gas), constitutes about three fourths of atmospheric air; the other fourth consists of oxygen, besides a small fraction of carbonic acid gas. Oxygen decomposes and destroys all fugitive colours. Oxygen is, besides, the basis of almost all the acids, and hence is one of the most universal agents in nature.
Hydrogen, formerly called inflammable air, was discovered by Mr. Cavendish in 1767; it is called hydrogen, because it is one of the component parts of water; or, more properly, it is the base of water. It is obtained in the most pure state from the decomposition of water by means of metals, thus: pass one hundred parts of water through a red hot iron tube, a gun barrel for instance, fifteen parts of hydrogen gas will be produced, while the inside of the tube will be found converted into an oxide, and to have gained eighty five parts in weight.
Again, when eighty five parts of oxygen gas are burned with fifteen of hydrogen gas, both gases vanish, and one hundred parts of water are the result. Hydrogen gas, when in a pure state, is about fifteen times lighter than atmospheric air; hence its use for inflating balloons. Hydrogen, if inhaled, destroys animal life; combined with nitrogen, it forms ammonia, or the volatile alkali, as we have before stated.
We have mentioned the fixed alkalies in a preceding section. We may add here, that by the discoveries of Sir Humphry Davy, in the year 1807, the base of caustic, or pure potash, is now known to consist of a light, white metallic substance, to which the name of potassium has been {30} given; it is of the consistence of soft wax; at a freezing temperature it is hard, brittle, and solid; when thrown upon water it instantly takes fire, hydrogen gas escapes, and an oxide of potassium, or caustic pot-ash, is produced. The potash and pearl-ash of the shops we must not forget, are combinations of carbonic acid and pot-ash, hence they effervesce with all the acids; but caustic pot-ash, containing no carbonic acid, combines with any of the acids without effervescence.
The SODA, as obtained from barilla, is a carbonate of soda; pure soda, or caustic soda, was, till the discoveries of Sir Humphry Davy, supposed to be, as well as potash, a simple substance. It is now, however, known to consist of a metallic substance of the colour of lead, but, nevertheless, lighter than water; upon which, when thrown, it produces, like potassium, violent action, yet does not, in general, like potassium, inflame. It is called sodium; pure soda consists therefore of sodium and oxygen, hence it is an oxide of sodium. These discoveries of the composition of the fixed alkalies are of infinite importance in the arts. The alkalies contain some very striking properties:
Their taste is acrid, burning and urinous. They generally change the blue colours of vegetable infusions green. When mixed with silex or flint, by exposure to great heat they form glass, and they render oils miscible with water, and hence combine with them forming soaps. They effervesce (when combined with carbonic acid,) with many other acids, and form neutral salts with all the acids. The volatile alkali or ammonia, on exposure to air, flies entirely away. Pot-ash, either in its caustic {31} state, or in that of a carbonate, absorbs moisture from the air, and liquifies. While soda, on the contrary, and many of its combinations, effloresce in the air; they, nevertheless, effervesce, and combine with the acids in a similar way to pot-ash.
We have mentioned how pot-ash is obtained in a preceding section. Soda is commonly procured from the ashes of marine plants; the barilla of commerce is obtained, it is said, in Spain, chiefly from many species of the salsola, or salt-wort. Barilla is an impure subcarbonate of soda, it is used largely in the manufacture of soap.
We now proceed to notice the nature of acids.
They excite a particular sensation on the palate, which we call sour. They change the blue colour of vegetables red. All of them, except the carbonic acid, effervesce with the volatile as well as the fixed alkalies when in the state of carbonates, as they are most commonly found in commerce. Oxygen is the principle of almost all acids; their difference depends upon the base combined with the oxygen: thus oxygen combined with carbon or pure charcoal, forms carbonic acid; with nitrogen the nitric acid; with sulphur the sulphuric acid, &c. &c.
Gas is a term implying the same as air; but as the term air, when used, is liable to be misunderstood for the air of the atmosphere, which is, as we have seen, a compound body, the term gas is more appropriately applied to all elastic fluids of a specific kind. Thus we say carbonic acid gas, oxygenous gas. The difference between carbonic acid and carbonic acid gas, and oxygen and oxygenous gas, consists in the latter being combined {32} with heat only, and in the state of air, while in the former they are fixed in some body, as in carbonate of pot-ash and oxide of lead, in both which cases the carbonic acid exists in a fixed state, or combined with the pot-ash, and the oxygen is in a fixed state, or combined with the lead.
We may now treat of carbonic acid gas, which is thus produced, as well as in many other ways: when charcoal is burned in oxygen gas, exactly sufficient for its combustion, both the charcoal and oxygen disappear, and an elastic fluid is found in the vessel, which is equal in weight to both. This air or gas is carbonic acid gas; it combines with lime, the alkalies, and pure or burnt magnesia: it constitutes a considerable portion of the weight of chalk, limestone and marble, as is readily seen by comparing these bodies before and after their conversion into quicklime. It is frequently combined with hydrogen. The gas with which the streets are now lighted is chiefly carburetted hydrogen.
Carbonic acid gas has the following properties. It extinguishes flame, and, like nitrogen and hydrogen, kills animals immersed in it. It is heavier than common air, and may therefore be poured out of one vessel into another like water. Cider, wine, beer and other fermented liquors owe their briskness to the carbonic acid which they contain; soda-water also owes its briskness entirely to the quantity of carbonic acid gas which it contains, a small quantity of heat being sufficient to give the acid the gaseous state.
Sulphur has been mentioned before; it is well known to be a very combustible substance; it is found in great quantities {33} throughout nature; the sulphur of commerce comes either from Italy or Sicily; or from the isle of Anglesea, where it is obtained from the smelting of sulphuret of copper; the best, however, comes from Sicily. It is, sometimes, found pure; but often combined with some of the metals, forming sulphurets. It is also frequently obtained by the decomposition of animal and vegetable substances; it is sometimes found combined with hydrogen (hence called sulphuretted hydrogen), in the human stomach, more frequently in the intestines. Sulphur combined with a small dose of oxygen, forms a volatile suffocating acid, called the sulphureous acid; with a large dose it forms sulphuric acid, or oil of vitriol.
For the nitric and muriatic acids, see a preceding section. We may, however, mention here, that nitric acid has the peculiar property of staining the scarf skin of the human body a dull yellow, of such permanence, that it can scarcely, by any means, be destroyed, it usually remaining till the skin wears or peels off.
The principal vegetable acids are the tartaric and the acetic. The tartaric acid exists in superabundance in tartar, and particularly in cream of tartar, which is nothing more than a purified tartar. See argol in a preceding section.
The acetic acid constitutes the vinegar both common and distilled; it is found in a very concentrated state in the shops, under the name of aromatic vinegar. It is also now obtained in large quantities, and of great strength from wood by distillation, or burning, in vessels, adapted for the purpose, hence called the pyrolignous acid, but essentially the acetic acid. This last is now used by {34} Calico-Printers to make acetate of iron. See a preceding section.
Alumina, or earth of alumina, sometimes called argil, is soft to the touch, adheres to the tongue, and hardens in the fire, contracting its dimensions: it constitutes the greatest part of clays. With sulphuric acid and pot-ash, it forms the common alum of the shops. Alum dissolves in about sixteen times its weight of cold water. For acetate of alum see alum in a preceding section.
Agriculturists and agricultural chemists know that alumina constitutes three eighths or more of a fruitful soil; some vegetables, likewise, contain this earth in their composition. Iron is also a component part of many soils, particularly those in which a red colour is predominant; hence it is, probably, a component part of all drugs used for browns, fawns, and blacks. It will be seen what affinity cotton has for iron in the dye of buff[4] upon cotton; and it seems reasonable to conclude that this metal not only produces the black, grey, and brown hues, but, with lime, forms a component part of the drugs themselves which give the brown dyes. It may be here also mentioned, that the red colour of the blood has been by many chemists supposed to arise from the iron which it contains; Mr. Brande, however, does not, from his own experiments, conclude this to be the fact. The blood of animals is, nevertheless, occasionally used for dyeing, as will be seen under Adrianople red. See {35} Kirwan on Manures, &c. and Davy's Agricultural Chemistry.
From the acids or oxygen combined with alkalies, earths, or metals, almost innumerable mordants, as we have seen, are formed; and upon the correct and proper application of these to the cloth or other matters to be dyed, depends the goodness and permanence of the colours. The dyer cannot, therefore, be too scrupulously attentive to this portion of his art.
In dyeing the student ought also to remember, that the material to be dyed combines intimately, in numerous instances, with alumina or other mordants; in the case of alumina it, in some instances, takes up from one twelfth to one fourth of its weight of alum, leaving the alum bath nearly tasteless. So also will rich extract of American bark, or even weld, when the proportion of weld is in weight more than two to one of the wool, form a triple compound with the cloth and alum, of permanent duration.
All these preliminaries the author considers of the first importance to be understood, and he has, therefore, mentioned them again and again. For so doing he is sure that he shall be excused in the dye-house, although not perhaps by the critics, whose candour he nevertheless respectfully solicits.
We now proceed to the application of mordants. In regard to muslins and calicoes, the alum is to be mixed with gum and carried to the piece, as will be described below in the Calico-Printers' mordant, and then immersed in the dye-bath: it thus receives the base or mordant. If the base be alum and the dye-bath madder, then, where the block strikes the pattern with the alumine {36} base, the colour will come out red; the other parts will clean and bleach white. If alum and iron form the base, the colour will be purple; if iron alone be applied, and galls, sumach, logwood, &c. are the component parts of the dye-bath, then it will be black.
Take one gallon of soft and pure water, of a heat of 150°, three pounds of common alum, one pound and a half of sugar (acetate) of lead; mix these together, and let them stand for two or three days, so that they may incorporate, often stirring them during that interval; then add two ounces of pearl-ash, and the same quantity of clean powdered chalk or whiting. After a time the clear liquor, now become an acetate of alum, must be drawn off. When used, it is thickened either with paste, flour, or gum arabic, or senegal; four pounds of either of the gums to each gallon of liquor[5]. A block or a press similar to a copper-plate press for paper, but much larger, and having the copper plates in proportion, is employed to spread the acetate of alum from a utensil {37} called a sieve, which is, however, not porous, while a boy or girl called a Teerer, works it smooth; when smooth on the sieve, the printer applies his block, and charges it with the acetate of alum; the block thus charged, is correctly put on the cotton cloth, which is laid upon a blanket spread upon a table; it is then struck with a mallet once or twice, by which, or by the pressure of the rolling-press, if copper-plate, the acetate of alum is driven into the pores of the cloth. The cloth thus prepared, is hung up in a hot stove, and dried by a high degree of heat. The goods are now ready, if for red, for the madder; and if for yellow, for the weld copper. Sometimes, however, lately, the colour is previously prepared, and applied at once in more instances than are prudent. To the above mordant, M'Kernan adds three ounces of sulphate of copper, omitting the potash; and he adds, "When the colour is wanting on the scarlet cast, omit the sulphate of copper."
Wool readily takes the alum at a boiling heat; common alum is in many instances proper for wool; and in others, where it might be improper, it is corrected by the use of argol or cream of tartar.
Yellow and red produce orange; red and blue, purple; but upon cotton, a scarlet, purple, or crimson cannot be produced in any way equal to those colours in wool or silk. Yellow and blue form the green.
We cannot enter with much minuteness into this part of the subject, more especially as the art of bleaching is {38} usually a separate one from that of dyeing. Yet as in fact the arts of dyeing and of bleaching depend in a great degree on the same principles, some notice of bleaching, in a treatise on dyeing, seems absolutely necessary.
Linen, cotton, and other cloths, were for ages deprived of their colour, in other words, bleached, rendered white, by a tedious process. Thus, the article to be bleached being boiled in a solution of pot-ash, was washed, and then spread on the grass in a field, watered occasionally, and, thus exposed to the atmosphere for two or three months, became white. This method is, however, in part, if not now entirely, dispensed with. M. Berthollet, an ingenious French chemist, to whose valuable work on dyeing we have before alluded, employed what was then called oxygenated muriatic acid, now chlorine, to perform in a few days what before took months to accomplish. His method was as follows. To six pints of powdered oxide of manganese he added sixteen of muriate of soda, (common salt) and twelve of concentrated sulphuric acid diluted with an equal quantity of water. These were placed in a leaden retort and distilled: the product was oxygenated muriatic acid, or chlorine, which being conducted to a vessel containing the material to be dyed, produced the same effects as the former tedious process, and bleached as much, in two or three days, as was before done in two or three months. This process has been since much further improved by the use of a combination of chlorine with lime, called chloride, or oxymuriate of lime. This article is at present used in almost all the bleaching grounds in the United Kingdom. It appears, therefore, that upon the use of the agent, chlorine, does the expedition {39} and whiteness of modern bleaching principally depend. Yet it ought, nevertheless, to be stated, that although, in the hands of scientific and judicious persons, chlorine is one of the most powerful agents in bleaching that ever was discovered, still, in the hands of bungling and avaricious persons, it may contribute greatly to the destruction of the cloth; and therefore, even now, a demand is occasionally heard for the old method of bleaching.
These processes constitute the art of the bleacher; the dyer has seldom any thing to do with them except in piece-goods or rough cambric, which he has sometimes to dye black as they come from the bleacher's in a state which they call once boucked; and sometimes he has them just as they come from the weaver; in which case, if for black, they need not be bleached white, but should be boiled in pot-ash, to take out the grease, &c.
Many attempts have been made by chemical philosophers to account for the permanence or want of permanence of various colours, when imparted to cloths and other bodies as a dye. Among these, Hellot, D'Apligny, and others of the old, and Berthollet, Bancroft, Henry, and others of the modern school, may be mentioned.
The power of resisting vegetable acids, alkalies, and soap, and, above all, the action of air and light, constitutes the durability of a colour. But this property has a very unequal standard, according to the nature of the colour and the species of the stuff.
{40} There is no obscurity in the action of water, alkalies, acids, and soap: for a solution is effected by means of these agents, or a small portion of acid or alkali unites to the combination, which forms the colour. But this is not the case with the action of light and air. Till lately, however, it was not known in what this action consisted.
Of the two principles which compose atmospheric air, it is only the oxygen gas which acts on the colouring particles. It combines with them, and thus impairs their colour or makes them fade. But its action is soon chiefly exerted on the hydrogen which enters into their composition, and it thereby forms water. This effect may be compared to a feeble combustion. Hence the carbon, which enters into the composition of the colouring particles, becomes predominant, and the colour usually passes to yellow, dun, or brown, or other appearances.
Light promotes this decomposition of the colouring particles, which frequently takes place only with its concurrence, and thus it contributes to the destruction of the colour. Heat also favours the same result, but less efficaciously so, unless it have a certain intensity.
It is concluded, therefore, that colours are more or less fixed in the air, according to the greater or less tendency which the colouring particles have to undergo this change[6]. Hence the utility of mordants in rendering fugitive colours fast.
The natural proofs of a dye's being effectual, are exposure to the air, to the sun, or to rain. If the colour be not changed by such exposure after twelve or fourteen days, it may be considered as fixed. These proofs are not, however, adapted to every colour: for some resist the action of air, light, and rain, yet are nevertheless injured by certain acids. There are also colours which do not resist the natural proofs and yet remain unchanged by acids.
Colours may be arranged in this respect in three classes: the first class is tried with alum, the second with soap, the third with tartar. For the proof with alum, half an ounce of this salt must be dissolved in a pint of water in an earthen pipkin, and into this liquor is to be put half a quarter of an ounce of the dyed thread or stuff, the whole being boiled about five minutes; it is then to be washed clean with water. Thus are tried crimson, scarlet, flesh-colour, violet, ponceau, peach-blossom, different shades of blue, and other colours bordering on these.
The next proof consists in boiling a quarter of an ounce of soap in a pint of water, with half a quarter of an ounce of the dyed stuff or thread for five minutes. With this proof all sorts of yellow, green, madder-red, cinnamon, and similar colours are to be tried.
The proof with tartar consists in boiling one ounce of that salt, previously powdered very fine, with a quarter of an ounce of dyed thread or stuff, in a pint of water for five minutes. This proof is used for all colours bordering {42} upon fallow, or hair-brown.—Journal of Science, vol. xxii. 219.
But notwithstanding these general rules may be given for dye-tests, yet so many are the niceties in this art, that, after all, nothing but long practice combined with scientific knowledge, will enable the dyer to become in this respect, a complete and successful artist.
The dye-house should be as spacious as possible, according to the quantity of work intended to be done in it; it should be also as near as possible to a clear running stream. The floor should be a mixture of lime and cement, and sufficiently inclining, so that water, the old contents of the vats, &c. &c. may run off freely when thrown down.
A dyer cannot be too particular in regard to the water which he uses. Some pump, well, and other spring waters, contain iron; this is injurious to many colours, while for black, brown, slates, and grey, it is very advantageous. It has been supposed that some dyers succeed in dyeing even the very same colour in a superior manner, in consequence of the peculiar purity or other properties of the water which they use.
To discover whether water contains iron or not, a little tincture of galls or prussiate of potash must be dropped into it; if a purple or blue tinge be produced in the water, we may be assured that it does contain iron.
For dyeing delicate colours, the water, which ought to be chosen for such purpose the purest and best, should {43} be heated with bran in a bag, when much of the contents of the water inimical to dyeing will rise to the top in the form of a scum, and should be taken off just before the water boils. Instead of bran, a little alum will answer the same purpose when it is not inimical to the colour intended to be dyed.
The boiling point of water is at the degree of 212° of Fahrenheit's thermometer; the freezing point is at 32° of the same instrument; blood heat is at 98°.
The limits and price of this manual preclude the possibility of our giving plates to explain some of the machinery and utensils which are now employed in dyeing. To inform a dyer what kind of coppers, casks, and vats are necessary, would seem to be superfluous; and the pupil may soon acquire such knowledge in the dye-house. Should a dyer find it his interest to undertake a branch of his art of which he has not any previous knowledge, he had better engage a man who understands it; if, however, he thinks himself competent to manage it, but is unacquainted with the best modern utensils appropriated to that particular branch, he had better get a dyer's labourer who has been used to it; a man of sufficient intelligence may be found with due encouragement to perform this part. It may just be added, that Ure's Berthollet and Mr. M'Kernan's work, both contain numerous explanatory plates of the utensils and machinery which are described and recommended in those works.
{44} All solutions and decoctions of Brazil wood, logwood, fustic, &c. should always be prepared in the same quantity and proportion, and one measure be invariably set apart for each. This observation is meant more particularly to apply to drugs in stock, always kept in a state of preparation ready for any process or work which may occur. The drugs just named may be kept in a prepared state; but weld boiled will not keep, nor will some others which are mentioned in the body of the work.
Weld, as it will not keep, should be used thus: a copper in proportion to the size of the work should always be used; and as weld will bear boiling and re-boiling, it can be boiled by the half bundle or more according as it may be wanted, whether you work little or much. If you are exact and near in your estimate, practice will soon render you perfect in any branch. It should be observed too, that to dye to pattern cannot be the result of a receipt, without a great latitude be left for the judgment.
The most difficult part of dyeing is that of light drabs, stone drabs, &c.
Nothing but practice will qualify you for this and all pattern dyeing: the way, and the only good way to obtain practice, is to work with all possible regularity. In the dyeing of fancy cloths in the clothing districts of Yorkshire, Gloucestershire, and other fine cloth manufactories, the manufacturers who dye their own cloths, as well as dyers of the greatest eminence, always number, measure, weigh, and time all the component parts of their various processes of dyeing. Such in fact ought {45} to be the universal practice; and then a person of ordinary abilities may soon be able to perfect his processes and obtain the best results.
Hence, however, it is very necessary that the dyer should have a competent knowledge of chemistry and drugs, that he may be able to judge of the goodness of the articles which he uses, and of the numerous and extraordinary combinations into which they enter. To chemistry, in particular, every able and scientific dyer must be largely indebted; for this reason it is that we have endeavoured, in this introductory chapter, to sketch some of the most important facts in this universal and interesting science.
In possession of these qualifications, and working upon the above plan, the dyer can never be far from the desired result in all his processes. His deviations, if any, will be few, as from his knowledge, he will soon perceive the first approach of any incorrectness, and be able to adjust it generally without much inconvenience.
The chemical terms now introduced into treatises on dyeing are chiefly taken from the Greek language, and are used in such a manner as to convey, by their etymology, an idea of the nature of the substances to which they are applied. Oxygen implies the producer of acid: hydrogen, the producer of water; nitrogen, the producer of nitre, &c. The term gas has been explained above. Caloric is a term used by chemists for heat; but caloric is used in a more extensive signification than the term heat, thus: although a gas might possess no sensible heat, yet being in a gaseous state, it is assumed to contain a certain portion of caloric which keeps it in its {46} gaseous state; the same observation will apply to liquids whether aqueous, oleous, or metallic.
All the measures mentioned in this work unless otherwise described, are those usually called in this country WINE MEASURE, and not those which have been introduced by a late act of parliament, called IMPERIAL MEASURES.
[2] Cochineal was at first supposed to be a grain, which name it still retains by way of eminence among dyers. Ure.
[3] For the cultivation of Woad in England, see Parish's paper in vol. xii. of the Bath Society's Report, or Tilloch's Mag. vol. xxxviii.
[4] What are called iron moulds in cotton, linen, &c. are, it is well known, nothing but the marks of a buff colour, usually left by ink and other matters which contain iron: acids, of course, dissolve, and discharge these buff colours; the oxalic acid does so without decomposing the cloth.
[5] "Acetate of Alumina is now most frequently made for the Calico-Printers by dissolving alum in a solution of crude acetate of lime, (pyrolignite); a gallon of the acetate, of specific gravity, 1.050 or 1.060, being used with two pounds and three-quarters of alum. A sulphate of lime is formed, which precipitates, while an acetate of alumina mixed with some alum floats above. The acetate of alumina employed as a mordant for chintz, is still commonly made by the mutual decomposition of alum and acetate of lead."—Ure's Berthollet, vol. ii. p. 331.
[6] Berthollet.
To dye cotton a Saxon or chemic blue—Sulphate of indigo—Saxon or chemic green—To set a cold indigo vat—Another indigo vat—To dye cotton a fast green with the cold indigo vat and weld—Another cold blue vat for linen and cotton—Solution of indigo for penciling printed muslin, &c.—To dye cotton a fast buff—To dye cotton pink.
We refer the reader to the preceding chapter for many observations relative to cotton, with which, in order to understand correctly the best method of dyeing this material, it is necessary that he should become acquainted: indeed, the whole of that chapter ought to be well studied by every one desirous of becoming an expert dyer.
This is performed with the sulphate of indigo thus:—put into a brown stone glazed earthen pot four pounds of good sulphuric acid, add to it twelve ounces of good indigo finely powdered, stirring the mixture very quickly {48} and frequently: break the lumps, if it should get lumpy before it is thoroughly mixed, with a glass rod, or with a stick, the bark of which has been taken off: if for wool or silk, the solution will be fit for use in forty-eight hours, but if for cotton it will not be fit for use till the acid is neutralized by an alkali. Some persons, however, use whiting, but this precipitates and wastes the indigo; others use magnesia, but this is expensive: some, again, use pure or caustic potash prepared thus—take American pot or pearl-ash about seven pounds, put some of it into a brown stone glazed jar, or rather an open pan; upon the ashes put some quicklime recently burnt, and then alternately ashes and lime, slacking the lime with water as it is put on the ashes; let the whole stand together for about two hours: provide now another brown stone earthen vessel with a hole in the bottom, of larger dimensions than the other, put into this a piece of coarse linen to prevent the lime, the impurities, or any foreign body from running through the hole, then upon the bottom put some of the previously mixed lime and ashes, well incorporated, and placed gently upon the linen so as to be sure of its keeping its place and letting the liquor pass through clear. As the mixture is put in add some water occasionally, so as to keep it just covered, and leave room at the top for the swelling of the materials, as the lime especially will increase in bulk. Water must fill the whole, and cover the lime, &c. which will be known by the bubbles ceasing to rise. When it has stood twelve or fourteen hours, water being occasionally added as it is absorbed, some may be drawn out.
To determine whether the carbonic acid has entirely {49} quitted the potash, (and for which purpose the quicklime, having a greater affinity with the carbonic acid than potash has, is specifically applied,) take some of the fluid in a wine glass and drop a drop of sulphuric acid into it; if the carbonic acid has entirely combined with the lime, the sulphuric acid will enter the fluid in the glass quietly, and without any other appearance than so much water; if you still doubt add more drops of the sulphuric acid successively. If the carbonic acid has not entirely left the potash when the sulphuric acid is dropped into the liquor, an effervescence or fermentation will be seen in it. Whenever this is the case the liquor must be returned to the mixture for a longer time, and, if necessary, more lime be added.
When the liquor or ley is fit for use, all of it is to be drawn off, and more water may be added and remain on the ingredients till it is wanted. It is best to keep it close from the air, because as the air contains a certain portion of carbonic acid, the liquor would in time absorb it, and the ley, instead of containing caustic potash, would become a solution of carbonate of potash, and consequently not answer the end designed.
To know when the alkali of the mixture is exhausted, take a piece of paper stained with the juice of the blue flowers of violets, or the blossom of the mallow, which is thus prepared—pound the blossoms in a glass mortar with a glass pestle, and squeeze the juice into a tea-cup, then, with a small hair pencil, cover a sheet of white paper with the juice, and dry it for use. All acids will turn it red, and all alkalies will turn it green; and, therefore, as long as any of the alkali remains in the liquor, the {50} paper thus prepared will, when immersed in it, be stained green.
The comparative strength of such solutions may also be ascertained thus: take a wine-glass full of the liquor, drop into it a few drops of sulphuric acid, stirring it with a glass rod or clean bit of tobacco-pipe, and then apply a bit of test paper; if it appear green more acid must be added and stirred again; apply the test paper a second time, if it be still stained green, a few drops more of the acid must be added, and thus continue till the colour of the paper is neither altered to green nor red: the liquor will then be neither acid nor alkaline, but contain a neutral salt consisting of a combination of the acid and the alkali. By adding, however, a few more drops of the acid, this last will be found predominant, and the test paper, being immersed in the liquor, will be stained red.
By treating different leys in this manner, and counting the number of drops necessary to neutralize each, the strongest ley will always be found that which requires the greatest quantity of acid for the purpose.
Alkaline leys are also to be judged of by their weight compared with that of water; a wine pint of water usually weighs about sixteen ounces avoirdupoise; all alkaline leys are heavier than water, and the heavier they are the more alkali they necessarily contain. A wine pint of some of them will weigh more than seventeen ounces.
To return to the dyeing of cotton a chemic blue: (to which a knowledge of these chemical processes, as well as of other processes in our work, is essentially necessary,) take some of the blue liquid prepared with indigo and sulphuric acid, as before directed, and put it into a vessel {51} large enough to hold two or three times as much as is intended to be put in, in order that there may be room to stir it; add some of the potash, or alkaline liquor, by degrees till, after several trials, the mixture ceases to be sour; or, if you do not like to taste it, take a small slip of cotton or muslin and dip it in, after having wetted it out in warm water. If the acid be neutralized the cotton will be sound, if not it will be tender when dried: if the acid predominates much the cotton will be as rotten as tinder; when the cotton is perfectly strong and sound after being dried, the liquid is in a proper state to dye both cotton and muslin.
The goods to be dyed must first be wetted out and wrung, then work them in the flat tub with water, with a little of this blue added, and well stirred in proportion to the shade wanted. From half a pint to a pint of the liquid blue is sufficient for two pieces of twenty-four or twenty-eight yards each, if not of a very full pattern.
Blue, when dyed, should be dried in a cool stove, and if book-muslins, framed; furniture should be stiffened, glazed, or calendered.
The preceding are essentially the same directions for preparing and dyeing with the chemic blue which were given in the first edition of this work, and which we see no reason to alter. As, however, for silk in particular, another method has been given in the late work of Mr. M'Kernan, we give his processes below.
"Take one pound of the best flora indigo in very fine powder, put this into a stone-ware or lead vessel, then {52} add gradually three pounds of the best sulphuric acid, specific gravity 1.800; mix well and stir often, and in twenty-four hours the indigo will be dissolved. Adding three ounces of sulphur to the acid, and heating it to 180°; then, when cooled to 100°, pouring the acid off the sediment, and then adding to it the indigo, is considered the best way of opening or dissolving the indigo. When the indigo and acid have been mixed twenty-four hours, add three pints of boiling water; stir often; when cold it will be fit for use."
"Take six pounds of alum and dissolve it in two gallons of water at 120°, when dissolved add, by degrees, five pounds of pearl-ashes until the acid of the alum is neutralized and the alumine formed, then put the whole on a piece of calico that has been hooked in a square frame, or tied over a vessel; when the liquor has run off then add one gallon of boiling water on the alumine and stir it up well. When the water has gone through the calico; the alumine is fit for use. Then add a part of this alumine to some of the sulphate of indigo until the acid is neutralized."
The same blue vat will do for green; but it is best to make another by putting only eight ounces of indigo instead of twelve to four pounds of sulphuric acid. If the preparation has been made two or three months it is the {53} better, having been often stirred before it was neutralized with the alkali.
Prepare a strong decoction[7] of old fustic, which should always be ready at hand as a store, keeping plenty according to the work to be done, including cotton, silk, and worsted goods.
Mix some of the chemic blue with the decoction of fustic in the following manner: put into a tub six pails of soft clear water, to which add a pint of the neutralized blue; and six pails of the decoction of fustic; stir all well together. Some dyers add a little weak alum liquor till it just tastes before they put in the blue; it should be but little, otherwise it will precipitate the fustic. This mixture should stand two hours to settle.
The muslin or calico, say two pieces of twenty-four yards each, should, with the usual precautions, be passed through a strong decoction of old fustic or turmeric as hot as the hand will bear. They are then to be taken out and submitted to a quantity of the green mixture above, described, in proportion to the fulness of the green required. When finished, whether for the calenderer or glazer, they should be dried in a moderately warm stove.
These two colours are very fugitive, especially upon cotton goods; but sometimes the customer will not go to the price of the fast green or blue, hereafter to be described.
Put three pounds of slacked lime, sifted, into six quarts or more of boiling water; stir the mixture well for some time, and after it has settled, draw off the clear liquid, to which add three pounds of sulphate of iron, stirring it well till all is dissolved; let it settle till the next day; have ready a deal cask, because one made of oak would blacken and otherwise injure the dye, in consequence of the affinity between the tannin, &c. of the oak and the sulphuric acid. Put into the cask seventy-five gallons of water, to which add the mixture of lime and sulphate of iron; take now three pounds of indigo, well ground and ready at hand, dissolved in three pints of strong solution of potash, such as was directed to be prepared for neutralizing the chemic blue. Put this solution of indigo and potash into the tub with the water, lime, &c.; after it is well stirred, and left to settle, it produces a deal of froth; but the liquor takes a fine green colour, which turns to blue when exposed to the air.
Soda may be used instead of potash, if treated the same way. Soda, it may be observed, forms the usual ley of the soap manufacturer; and answers for soap much better than potash, because its combinations do not usually absorb moisture from the air: potash, and several of its combinations, do so.
Take five hundred quarts of water; indigo seven or right pounds. The boiler must be iron.
{55} Boil the indigo with sixteen pounds of a liquor made with potash and eight pounds of lime. After the lime and potash have been in contact, as in all these instances they should be, from twelve to twenty-four hours, to take away the carbonic acid from the potash, the clear liquor of this mixture is what must be used. The indigo must be previously powdered, and ground extremely fine in water before it is put into the alkaline liquor. The mixture must now be added to the five hundred quarts of water, and the whole boiled till the indigo rises to the surface like cream, and till, in striking the bottom of the boiler with a stick, it is found to contain no solid substance.
While the indigo is boiling, another eight pounds of lime must be slacked in about twenty quarts of warm water; dissolve in this lime-water sixteen pounds of sulphate of iron. The vat being half filled with water, the solution of lime and sulphate of iron is to be put into it; the indigo solution is now also to be added. The vat, being thus filled to within about three or four inches of the edge, must be stirred two or three times a day till it is fit for dyeing, which it will be in about forty-eight hours, and sometimes sooner, according to the temperature of the air, by which the completion of the process is more or less accelerated.
When the strength of the vat is exhausted, it must be, of course, replenished. If the liquor becomes black, it wants sulphate of iron; if yellow, lime is required. When the indigo is far spent, more must be added in the same manner as at first.
In this vat, as respects the blue dye, if it be for muslin, {56} calico, &c., the form should be square, about two yards long, one yard to one and a half wide, and from seven to eight feet deep; the pieces of cloth are to be hooked into a frame.
Where much work is done, it will be necessary to have two or three such vats, in order that they may be worked in succession: by stirring them some hours previously to working, the weaker will do for the lighter shades, the stronger for the fuller colours. If the vat is in proper order, the goods always come out green, and turn blue in the air. This should be ascertained by small patterns previously to working the whole. When any goods are dyed in these vats, if not full enough at one dip, they may be left a certain time, and then be dipped again, once or more, as they appear to require it.
When they are blue enough, and fully aired, they must be taken from the hooks, and well washed off in two or three fresh clean waters, or at a wash wheel in a clear running stream. When perfectly clean, they are ready for the calenderer or glazer.
After dyeing muslin blue in the blue vat, weld must be boiled in the same manner as for fast yellow. The quantity of weld to be used, must be according to the fulness of the blue ground, and of what shade the green is to be; a proportion of alum must also be used. The goods, after being worked in the same manner by the selvage, must be washed off, and stiffened, if for the glazer, but not if for dress, but be framed by the muslin dresser.
The indigo is to be powdered, and put into warm water with sulphate of iron, in quantity twice the weight of the indigo, to which is also to be added, the same weight of fresh-burnt lime. The water should be only sufficient to mix it thick at first; keep it stirred; and, as it becomes dissolved and green under the surface, increase the water, often stirring and trying the mixture, by putting in a pattern between the stirrings, at some hours distance, between each pattern, and increasing the water: in twenty-four hours it will be fit for use.
To twenty-five gallons of water, add sixteen pounds of indigo, and thirty pounds of carbonate of potash; when mixed, and placed over the fire, as soon as the mixture begins to boil, add quicklime, by a little at a time, to render the alkali caustic; then twelve pounds of red orpiment, and boil till it will give a yellow colour to transparent glass.
This form is from Haussman. Were the author to make this solution of indigo, he would first make the alkali caustic with lime, and then put the clear liquor to the other materials.
Mr. M'Kernan gives another form for pencil blue with indigo: the principal differences between which and the above, consist in adding equal parts of brown sugar and gum senegal to it, which, in regard to the addition of the gum, is, we presume, a great improvement.
{58} Dr. Ure (Notes to Berthollet, vol. ii. page 437.) gives a similar form from Vitalis, for topical or pencil blue; but he adds, it was much used formerly. Another blue, of less permanence but more brilliance, is now preferred; it is made thus:—
Into an earthen pot four ounces of finely ground and sifted Prussian blue are to be put. Over this must be slowly poured, stirring all the while, sufficient muriatic acid to bring it to the consistence of syrup. The mixture is to be stirred every hour for a day, and afterwards thickened with from four to eight pots (of two litres each; a litre French contains about two wine pints;) of gum-water, according to the shade wanted.
Take a brown stone pan or pipkin, glazed. It must not be the common glazed wares, because these are glazed with lead, and the acids will dissolve the lead; if, therefore, such are used, the lead being dissolved, will be mixed with the dyeing materials, and sometimes totally spoil the dye. Stone-ware, if used with care, will bear the fire: such ware is usually glazed with muriate of soda or common salt.
Having a proper vessel capable of holding from two quarts to a gallon, fill it half-full of strong nitric acid, to which add, in small quantities at a time, either old horse-shoe nails from the farrier, they being the purest iron, or the cuttings of tin-plate from the tin-man's, for this is also very pure iron, although covered with tin; but the small portion of tin in the iron is not {59} inimical to the dye. Be careful not to put too much in at a time, nor to stoop near to it while the solution is going on, as the red fumes arising are very noxious; and if the iron be added in large quantity, so much effervescence would be produced, that a considerable part of the liquor would be thrown over the top of the vessel.
When this solution is prepared in haste the air is greatly contaminated, and therefore it is best to prepare it long before it is wanted, and slowly, by dropping hourly small quantities of the iron into the acid, and then little if any perceptible fumes will arise. Continue this process till, by stirring with a glass rod or tobacco-pipe, you find the iron dissolves more slowly: by keeping a little iron at the bottom, and occasionally adding the acid, you may always have this preparation at hand.
It is to be used thus:—having a copper of hot water ready, put a part of it to some cold in a flat tub till the mixture is as hot as the hand will bear; then, according to the paleness or fulness of your pattern, add some of the solution of iron, and mix it well by stirring: begin with about half a pint for two pieces of twenty-four yards each: it is best to add a smaller quantity than is necessary first, as you can make another addition as you please, but, if you add the solution in excess, to diminish it is by no means so easy. Now, having ready a flat tub with water of as hot a temperature as the hand will bear, put into it a clear solution of pearl-ash, and have also ready another tub of clear cold water to wash off in; then pass the pieces (always taking care to have them well wetted out in one of the tubs of hot water before either the solution of iron or pearl-ash is put into either {60} of them) through the solution of iron six or seven times, edging them over by the selvage to keep them even; next, folding them first even upon a board, wring them out, wash them off and pass them through the solution of pearl-ash; lastly, wash them off again in fresh and clean water: a permanent and bright buff will be found, and as good a colour as can be dyed upon cotton.
We here see what an affinity iron and cotton have for each other when the iron is combined with an acid and the combination in a liquid state. Although the colour is not so beautiful in every instance, yet, be the acid which dissolves the iron what it may, cotton imbibes the iron from it.
What is left of the solutions of iron and the pearl-ash may each be kept in a separate deal tub for use.
Take safflower in proportion to its goodness and the quantity of work to be dyed; put it into pure and clear water; tread it in the water till the water becomes fully charged with a kind of extractive yellow colour. It is best to put the safflower into a strong linen bag or sack; a sack containing sixty pounds will take a man two days to wash it clean: if done in a clear running stream the yellow colour will of course run away; if you have a small quantity in a tub it must be let out at a plughole, which every flat tub should have. The safflower must be worked or trod till all the yellow colour is got out of it, or the pink to be obtained from it afterwards will not be bright.
{61} When the safflower is thoroughly washed, take it out of the bag and put it into a deal tub or trough, and add to it pearl-ash in the proportion of six pounds to one hundred pounds of the safflower, which should be weighed before it is wetted. Let the potash be well dissolved in water; pour part of the clear solution off, and mix it thoroughly with the safflower; after having stood for some time strain the liquor through a cloth or sieve into another deal trough. The whole solution of pearl-ash should not be put in at once, but at different times. If there should be reason to believe that the safflower will yield more colouring matter by a farther addition of the solution of pearl-ash, such additional solution may be made. The water for the solution and the solution to the safflower should both be applied cold. Carbonate or mild potash is better than the caustic. By putting the solution of pearl-ash on the safflower at different times it will be readily seen when the fluid passes through the cloth or sieve free from colour.
The colour is of a cherry hue, and is resinous, therefore the water dissolves but little of it; the carbonate of potash is added to dissolve this resin.
To overcome the influence of the pearl-ash, which tinges the red of a yellow colour, some cream of tartar must be finely powdered and dissolved in boiling water, and added to the liquor when it is nearly cold. In the South of France lemon juice is used.
The colour, being thus raised by the cream of tartar, is now to be mixed with cold water in proportion to the fulness of the pattern desired, and the cloth must be worked six or seven times in it, as in other colours.
{62} What is left of the colour must be taken up with some skein cotton, and dried; this may be added to water upon another occasion by saturating the acid with a solution of pearl-ash, which will abstract the dye from the cotton.
The solution of tartar will again redden the colour from the yellow of the pearl-ash; this must be done if any remain, for it will not keep in a fluid state.
We shall not here describe any other process with cotton till we have treated of wool and silk.
For dyeing cotton black, and some other colours, see the chapters V. and VI.
[7] The difference between decoction and infusion should be always carefully observed: a decoction is made by boiling the ingredient or ingredients in any liquor; an infusion is that in which the ingredients are put but not boiled.
To alum silk—The blue vat of indigo for silk—Another blue vat for silk—To dye silk violet, royal purple, &c.—To dye silk lilac—Another process for lilac—Another process for dyeing muslin, &c. lilac—To dye silk a violet or purple with logwood—To dye silk violet with Brazil wood and logwood—To dye silk violet or purple with Brazil wood and archil.
Forty or fifty pounds of alum being dissolved in a copper of hot water, the solution is to be poured into a tub containing forty or fifty pails of cold water; during the mixing of the solution of alum with the water it should be well stirred lest the cold water should crystallize the alum and spot the silk; when, however, this happens, dipping silk in warm water will dissolve the alum. The silk should be alumed cold, for, if hot, the lustre of the silk will be injured. Alum is used for certain reds and yellows but not for blue. See also chapter VI.
When silk is deprived of its gum so as to acquire the greatest possible degree of whiteness, it is still necessary {64} to have different shades of white, some yellow, some blue, and others reddish; these are known under five denominations, namely, China white, India white, thread or milk white, silver and azure white. All these whites, although differing from each other by very slight shades, are nevertheless apparent, especially when compared with each other, which will be seen in the processes of dyeing silk.
For ungumming and boiling, whitening and sulphuring silk, see chapter VI.
We have described M'Kernan's method of preparing and neutralizing sulphate of indigo in pages 51 and 52, to which the reader will be kind enough to refer: the following blue vat is from Macquer.
This should be so contrived that heat may be applied to it, which it now mostly is, by steam, as well for woollen woad vats as for indigo vats. For silk, take eight pounds of the finest indigo and six pounds of the best pearl-ash, and from three to four ounces of madder for every pound of ash, besides eight pounds of bran for the whole, washed in several waters to take the flour out. When washed, and the water squeezed out, the bran is to be put at the bottom of the vat; the pearl-ash and the madder being mixed by bruising them roughly together, are now to be boiled a quarter of an hour in a copper containing two-thirds of the vat; the fire being damped, the liquor is then suffered to rest. Two or three days previous to this the indigo is to be steeped in a bucket of warm water, {65} and washed well, the water being changed once or more. Some dyers begin by boiling the indigo in a ley made with one pound of pearl-ash and two buckets of water; they afterwards pound it in a mortar quite wet, and, when it becomes like paste, fill the mortar with the liquor before boiled, and still hot, stirring it for some time. It is then suffered to stand a few moments, and then the clear is poured off into a separate boiler or into the vat. The same quantity of the mixture is then poured upon the indigo remaining in the mortar and mixed as before; again the clear is poured off into the boiler, and the operation is repeated till the whole of the indigo is dissolved in the liquor. The whole of the liquor in the boiler is now to be gradually poured into the vat on the bran at the bottom, adding afterwards the remainder of the composition, grounds and all.
After stirring and raking for some time, the mixture is left to cool till it will bear the hand in it, when a little heat is added to keep it in this state, and so continued till it begins to turn green, which is easily known by trying it with a little silk. When the green begins to appear it should be stirred with the rake, then suffered to stand till the brown and coppery scum which rises upon the surface shews that the vat is come to; or, in other words, the preparation of this part of the process is complete. But as it is necessary to be very certain of this, the scum should be well examined; and if, when blown aside, a fresh scum is immediately formed it is as it ought to be. In this state it is to remain for three or four hours, when a new composition is thus made:—
Put as much water as is requisite to fill the vat into a {66} copper, boiling it with two pounds of pearl-ashes and four ounces of madder as at first. This new liquor is to be poured into the vat, raked and mixed, and being left to stand for four hours it is then ready for dyeing.
When a vat or vats are set for green, double the quantity of madder must be added. (See Chap. VI.)
The size of the vat for the above quantity of indigo, should be about five feet deep, two feet or two feet and a half in diameter at the top, and one foot and a half or two feet in diameter at the bottom: the form of an inverted frustum of a cone; or of a sugar loaf inverted, with the pointed top cut off.
In order to produce different shades of blue, the silk intended for the darkest, should be first dipped in the fresh vat and so on to the lightest; as the vat weakens the silk should be kept in longer, till the vat, being exhausted, serves only for the lightest shades. When it begins not only to be weak but dull, it is then necessary to feed the vat with the following composition:
Take of the decoction of pearl-ash with indigo one pound; of madder, two ounces; and a handful of washed bran; boil them together for a quarter of an hour, either in water or a portion of the same vat if yet sufficiently full to afford it; after this mixture is added, it should be well raked and suffered to rest two or three hours, more or less, before the dyeing is resumed.
For the finest blues, however, a fresh vat is the best; and if only pale blues are required, a vat set on purpose with less indigo will answer better than a strong vat which has been weakened, because though weak it will give more vivid colours.
Take fifty pounds of good indigo in fine powder; fifty pounds of fresh slacked stone lime; one hundred pounds of sulphate of iron; and five pounds or more of pearl-ashes. Stir often for three or four days till there is a fine copper-colour scum on the top of the liquor in the vat. The vat is of course to be set with water in the usual way.
The substance of this form is from M'Kernan; we cannot, however, avoid thinking, that his directions for this vat are very vague.
Boil archil with water in a copper; the quantity of archil according to the colour required must be from two to four times the weight of the silk. When the archil has boiled about ten minutes the fire must be damped, the archil left to subside, and the clear liquor put into a vessel of a convenient size, in which the silk is to be immersed and worked with care.
You must have a small corresponding pattern that you intend for purple, which at times you must put into the blue vat to regulate the depth of the archil ground, as the purple is a compound colour, arising from the blue of the indigo and the red of the archil. When the red of the archil is deep enough, you must wash it off and put it into the blue vat with proper precaution. The fulness of the archil ground and the depth of the blue, {68} must be regulated according to the patterns which are to be matched.
Lilac is and should be a bright light shade of violet or purple; to give it the blue requires great management. The vats being generally too strong, it is best to mix a little of the new rich vat with some pearl-ash in clean cold water, and so prepare a liquor on purpose, by which the lilacs may be blued or reddened at pleasure. When this liquor is first mixed it becomes of a green colour; the silks therefore should not be dipped till the liquor begins to lose its green colour and inclines to blue. Pearl-ash added to this liquor helps to blue the archil, because the effect of the alkali upon red is to render it violet.
Consists in simply using the chemical blue with archil according to the shade required.
This is accomplished by mixing the neutralized chemic blue for cotton with the pink dye of safflower, according to the shade required.
The silk should be alumed and washed. The logwood should be boiled in large quantities like fustic, as {69} as directed for green; but it should not be kept longer than two or three weeks; it is far better used cold than hot.
The silk must be alumed and cooled as usual; it is then to be alumed and dyed in a liquor made of Brazil wood of the common heat, then in the cold logwood liquor, and lastly, a solution of pearl-ash must be added to the liquor in which the silk is last dyed. It is afterwards to be washed and dried; but for some shades it is best to have fresh liquor, particularly for the warm Brazil, the cold logwood, and the solution of pearl-ash: in this case the quantity of each may be much better regulated.
The silk when alumed is to be dyed in the decoction of Brazil wood according to the shade required; it is then to be washed and dyed in archil: and it is afterwards washed a second time. After this it is dipped in the blue vat, and wrung and dried with the same accuracy used in greens and blues.
For dyeing silk black and some other colours, see Chapters V. and VI.
On the action of alum and tartar upon wool—A pastil or woad vat for blue—To prepare the indigo mentioned in the preceding directions—Rules to judge of the state of the vat—Indications when a vat has had too much or too little lime—To work a vat which is in proper order—On the putrefaction of the woad vat—Methods of dyeing blues—To dye wool, with lac-dye, scarlet, or crimson—To dye worsted yarn a crimson—A preparation of archil to finish the crimson—On dyeing wool scarlet—To dye wool maroon—To dye wool yellow—To dye wool brown or of a fawn colour—To dye wool purple, &c.—To dye wool green—A chemic vat for green woollen—A chemic vat for blue woollen—To dye wool orange, gold colour, &c.—To dye wool black—another process for black without a blue ground—To dye wool grey—Mixture of black or grey with red and blue—On browns, fawns, greys, &c.—On the yellow of quercitron bark—On a full bright yellow from the same bark—Bancroft's murio-sulphate of tin—To dye wool buff—To dye wool peach—To set an Indigo vat for worsted, serge, &c.
Wool is usually scoured for being dyed with stale urine, the staler the better: it is used in the proportion of one {71} part to three parts of water, full as hot as the hands can bear when the wool is worked about in the fluid.—If the wool be in the fleece, what is called its natural yolk, and which is said to preserve the wool from the moth, is of a greasy nature, and is scoured out by the volatile alkali in the urine. If the wool be in the state of spun yarn it has gallipoli, or rape oil, in its thread, the spinner, or rather the comber, using it to render the wool more flexible, &c. It is absolutely necessary that wool, (as indeed every other material to be dyed) should be made very clean and white, if any brilliant or bright colour is to be imparted to it. For this reason it is that the wool is passed two, three, and in some instances even four times through fresh scouring liquors; in the last, and sometimes in that which precedes the last, soap is used in the proportion of from seven to fourteen pounds, and in some instances to twenty one pounds or more, to, a pack of two hundred and forty pounds of wool, according as it is fine or coarse: for superfine colours more than common is used. Worsted requires less than coarse yarn, having less grease and dirt in it.
It ought, however, to be known, that boiling wool for a long time in any alkaline liquors, or a liquor made of soap, tends greatly to the decomposition of the cloth; indeed long boiling in any strong alkaline ley converts wool into a kind of soap, and, hence, it is easy to see why such processes injure wool or cloths made with it.
The preceding observations apply to the alkalies in a caustic state, or in the state of carbonate, not when they are neutralized by powerful acids: for wool, when fit to receive the dyes, and if it is designed to be dyed {72} yellow, should boil two hours with one-twelfth or one-tenth of its weight, of sulphate of alum (common alum) observing proper precautions, and the use of a sufficient quantity of prepared weld plant boiled, &c.: or of quercitron bark, as will be shown in the processes of the different yellows. If it were yarn, and the threads cut in two, it would be found dyed throughout, and of a body and richness in proportion to the correct application of the various ingredients, and with due regard to time, weight, measure, &c.
In the process just mentioned, we may observe, that the quantity of alum and of the weld plant used will be found very considerable: from one twelfth to a fourth of alum, and, according to the French method, four or five times more weld than the quantity of the wool.
When a process of dyeing has been scientifically conducted, the wool will take so much of the alum that the bath will hardly taste of it; and afterwards take the colour of the dye bath out of it; so that the remaining liquor put into a glass will be nearly like water.
From the experiments of Dr. Ure, (Notes to Berthollet, vol. ii. p. 323.) it appears that alum has the property of increasing the solubility of cream of tartar; that as, in using alum and tartar, the wool is impregnated with alum and a large quantity of tartaric acid, these two salts should never be employed together, except when the colour is susceptible of being heightened and rendered brighter by acids, as is the case with cochineal, madder, {73} and kermes. On the contrary, alum should never be employed for wools intended to be dyed with woad, or Brazil wood, the colour of which is easily destroyed or altered by acids.
To conclude these preliminary observations, wool has a strong and powerful affinity for all dyeing materials; and, therefore, the processes for dyeing wool are, in general, by no means so complicated as those for dyeing cotton, silk, &c.; although some colours, even to these, are readily, and without a complication of processes, imparted.
Take, upon as small a scale as can conveniently be tried, a copper vessel, which will contain about twelve gallons, two thirds full of soft water, and one ounce of madder. Fix this small copper in a larger copper of water, so that the heat may be applied to keep the liquor in the smaller copper at a proper temperature; it will be then, in fact, a water bath.
Having kindled the fire in the afternoon, put in a good handful of bran and five pounds of woad; at five o'clock in the evening let it be well stirred and covered over, the liquor being about blood-warm; let the same heat be continued as nearly as possible, at least so as not to be lower than summer heat by the thermometer, nor higher than fever heat by the same instrument. The vat must again be well stirred at seven, at nine, at twelve at night, at two in the morning, and at four.
{74} Hellot, describing this process, observes, that "the woad then working, some air bubbles began to rise pretty large, but few in number, and of a very faint colour; it had then two ounces of lime added, and was stirred; this was four o'clock in the morning; at five a pattern was put in, and at six it was taken out and the vat stirred. This pattern had received some colour. At seven o'clock another pattern was put in, and at eight it was stirred again. The second pattern was tolerably bright. An ounce of prepared indigo, (see p. 75.) was then added; at nine o'clock another pattern was put in; at ten it was stirred again, taking the pattern out, and putting in an ounce of lime because it began to smell sweetish; at eleven another pattern; at twelve at noon it was stirred again. This process was continued till five o'clock in the evening; then were added three ounces of prepared indigo; at six another pattern was tried, and at seven it was stirred again; the last pattern came out of a very good green, and became a bright blue. One ounce of lime was added to sustain it till nine o'clock the next morning; patterns were put in from time to time: the last was very beautiful. The vat was then filled up with water and a small quantity of bran and stirred; after which patterns were tried every hour till five o'clock in the evening, when, being in a proper state, it was immediately worked. Some lime was then added to preserve it; it was stirred and left to another opportunity to reheat."
Boil, in a gallon of water, for three quarters of an hour, two ounces of pot-ash, three quarters of an ounce of madder, and one ounce of bran; then let the whole settle for half an hour. After all is settled and taken out of the boiler, and put into another copper with four ounces of indigo finely powdered, the liquor should be kept stirred, and very hot, but not be boiled. At intervals some lixivium of lime should be put into it, and that being cold will keep the liquor from boiling, and render the pot-ash more active.
As soon as the indigo is dissolved and properly diluted, damp the fire and cover over the solution; after it is settled put in a pattern, which, when taken out, will turn blue on being exposed to the air; if it does not, more clear lixivium must be added. Of this solution of indigo such proportions are to be added to the woad vat as are directed in the preceding process.
The vat is ready for working, and to dye blue, when the sediment at the bottom, on being taken out of the vat changes to a fine brown-green. When the froth which rises in great bubbles on the surface is of a fine Prussian-blue, and when the pattern which has been steeped an hour, comes out of a dark grass-green, and changes in the air to a blue; when the liquor is clear and reddish, and the drops which stick to the rake are {76} brown; when the sediment changes colour on being taken out of the liquor, and becomes brown on exposure to the open air: when the liquor is neither harsh nor greasy to the feel, and neither smells of lime nor of ley, the vat is known to be in a proper state for working.
These extremes ought to be carefully avoided. When the lime is deficient, or a pattern comes out of a dirty grey, and the sediment does not change its colour, there is scarcely any effervescence on the vat; the liquor smells only of lime, or of the lixivium of lime.
If the vat be not too far gone, after the addition of a little bran, madder, and some woad, then try the patterns from hour to hour; thus you will be enabled to judge.
A deficiency of lime is evident when there is no effervescence on the liquor; and when, by dashing about the surface of the liquor, it makes a hissing noise, and by the bursting of a number of small air bubbles, which as soon as they are formed break, and appear tarnished, and are not large, nor of a fine colour; the liquor too has an offensive smell, like rotten eggs; it is harsh and dry to the feel, and the sediment, as has been before observed, does not change colour when taken out of the liquor.
Sometimes such a condition of the vat is absolutely irremediable; but when not gone too far, sprinkle some {77} lime into the liquor, and stir it. If you can thus remedy the defect, and bring the liquor to smell of lime, and to feel soft, cover the vat, and let it stand. If, at the expiration of an hour and a half, the effervescence begin, you may put in a pattern; in an hour afterwards, it may be taken out, and regulate your process by the degree of green which the pattern has imbibed; but, in general, when vats are thus out of order, they are not so soon recovered.
The vat being in a proper state, the cross suspended, and thirty ells of cloth ready, or scoured wool in proportion, designed for black, by dyeing it of a blue grey; and having passed and repassed the cloth through the liquor for a full half-hour, it is to be wound round the winch, and thrown off into the barrow, and aired by the listings to change the green to blue. After this, a second piece may be dyed by the same process.
Having made this overture, or first stirring, as it is also called, the vat must be stirred afresh, adding lime; but not so much as to destroy the proper smell and feel. If the vat be in a good state, on the first day, it may be stirred three or four times; but it must not be overworked, particularly on the second day.
Concerning the colours to be obtained to the best possible advantage from a fresh vat on the first day,—the first is for black, the next for royal blue, and the third a brown green. On the second day, violet, purple, and Turkey blues in the last stirring. On the third day, if the liquor be too much diminished, it must be filled up {78} with hot water. At the end of the week light blues may be done, and on Saturday night add rather more lime, to preserve the vat till Monday morning. On Monday morning add more indigo, and stir the paste; keep the vat liquor at a proper distance from the top. Cover it for two hours; then put in a pattern, and in an hour take it out; add lime according to the green shade of the pattern, and in an hour or two, if your vat has not suffered, you may begin working it afresh.
To keep the cloth, &c. from the sediment, there is always let down into the vat, before the work is begun, an iron circle, with cords fastened from the circumference to the centre.
Whatever be the cause, most certain it is, that the woad vat, even when prepared in the most careful and scientific manner, is soon disposed, if not used, to go into the putrid fermentation; of this we may be satisfied, when it smells like rotten eggs, as stated above.
The loss of a woad vat to dyers is extremely serious, both from the quantity of woad, as well as of indigo, which it contains: these articles being always expensive. The woad vat being worked by heat directly applied from an open fire, (the old method of heating it,) was much more liable to be lost than if it remained cold, or was worked continually, as it usually now is in London; added to which, the more equable application of heat by steam, there is not now the danger which there was in cessation, at uncertain times, and in uncertain states of the vat, as to richness or poorness of woad or of indigo.
{79} But a dyer in the country, whose business is barely sufficient to keep a vat going, will find more difficulty in this respect. If, therefore, he does a small batch of work on Monday, but has not half worked down his vat, and has no prospect for two or three days of doing any more work, he may possibly try to keep it with lime for a day or two: he may do so, and in the issue, in some instances, too much lime is the consequence. We consider, however, that when the vat can be worked daily, and replenished as it is worked down, as is the case in London, with care and attention, there is no danger of the loss of a woad vat: in London, such an accident now seldom happens. The author is, notwithstanding, persuaded that all the art of man cannot always keep a vat from the state of having either too much or too little lime, when heated but seldom, under a short course of work: for when a vat is in order, it is like a ripe vegetable; you must gather it, or it passes the time of its perfection; it may even be rotten ripe. We say, therefore, WORK THE VAT: withdraw from it, upon your cloth, its colour, which, as soon as you expose it to the atmosphere, will combine with its oxygen,—the oxygen with the carbon of the indigo and the woad. If you play with it too long, the putrid fermentation will begin, and the vat will be spoiled. The smell of rotten eggs always proclaims the approach of the mischief.
No one, therefore, should attempt to have a woad vat or vats, unless he can keep them nearly always at work. When worked down in a moderate time, and replenished with lime, woad, indigo, &c., working out and replenishing in, there can be no danger. On the other hand, in {80} proportion as the vat is out of condition, although partially recovered, it must always be with more or less loss.
Whether the goods be cloth, or skeins of yarn, they must, in all cases, be first wetted out and wrung, and then put into the vat, worked in it, taken out and aired, that they may turn from green to blue; and, if necessary, they must be put in again.
There is no difficulty in dyeing dark blues, by repeated dippings; but if light blues be dyed in vats which are nearly exhausted, they will not be bright.
Blue vats, upon a large scale, are now mostly heated by steam; they are then, with little trouble, always in a state for working, without the necessity of re-heating. They are very convenient for light colours, even after they become very weak. In some instances, in order to dye light colours to the best advantage, it would be advisable to set a vat on purpose, which should be strong in woad and weak in indigo; because the colour would be given more slowly, and the light colour obtained from them with much more facility.
We have mentioned lac-lake and lac-dye in page 12. Lac-lake is of very uncertain quality, having many heterogeneous substances mixed with it. Lac-dye is very superior to lac-lake. Lac-dye is much used for dyeing woollen yarn scarlet and crimson, for carpets and hearthrugs. {81} It is used with a peculiar spirit, which may be purchased of the dry-salters. Some think that this colouring material is nearly equal to cochineal; the author has, however, never seen any thing dyed with it equal to the colour obtained from cochineal, although it affords, nevertheless, a good scarlet.
Lac-dye is used by being powdered and put into a stone pan, (the quantity must be in proportion to what is likely to be used), with a portion of the above-named lac-spirit sufficient to make it about as fluid as treacle; it must be stirred with a glass-rod or a tobacco-pipe. Some use alum and tartar as a preparation, and some not. After putting the mixture of lac-lake and spirit in the copper with a proper quantity of water, add the goods and work them at a boiling heat. For scarlet add quercitron bark, for crimson, archil.
Lac-dye may be, however, prepared for dyeing, by submitting it, in powder, in a leaden vessel, to the action of sulphuric acid, in the proportion of not more than one part to two of the dye; and after the lac-dye is dissolved, the acid may be neutralized by carbonate of soda. With suitable mordants to the cloth or yarn, the colour may be then applied. Other processes for the employment of this dye are also adopted, but we have no room to detail them. (See Ure's Notes on Berthollet.)
Proportion of wool, one pound; of alum, two ounces and a half; of white tartar in powder, one ounce and a half. Having the water properly cleared by bran, let the {82} alum and tartar be boiled in it; when it begins to boil, stir the mixture well, and put in the worsted, which boil in the liquor for two hours; then prepare a fresh liquor for the cochineal, one ounce of which, in powder, is to be used for every pound of wool; when it begins to boil, stir it well, put in the worsted, and boil it till the liquor in the vessel is free from colour, it having parted with the colouring matter of the cochineal, which should now all be upon the worsted. If a series of shades be required, less quantities of cochineal, alum, and tartar, must be used; the lightest shade is dyed first.
Put as much archil as the goods may require, and according to the deepness or lightness of the shades of the crimson required, into a copper of water of a suitable size, and boil it, (the best canary archil will bear boiling); damp the fire, let the archil settle, and then have a fresh liquor for the goods to be put in, to receive a proportion of archil according to the pattern desired to be matched. Begin with the lightest and end with the deepest, reserving the remains of the archil liquor, if it be not all spent, for common compound colours of such shades as it will be advantageous to use it in. (See the next article.)
Scarlet owes its beauty to a solution of tin in muriatic acid. For this purpose some use muriate of ammonia, commonly called sal-ammoniac, others use common salt. {83} It is of little consequence whether common salt or sal-ammoniac be used: different preparations are employed by different persons. The author has found the following to answer every expectation.
Melt an ounce of grain tin in an iron ladle, till an oxide is formed on the surface; then pour it from a height or distance into cold water. Pour the water from it, and it is fit for use, being then called feathered tin. Put this tin into a glass vessel or stone jar, and add to it eight ounces of nitric acid, eight ounces of water, half an ounce of sal-ammoniac, and two drachms of nitrate of potash. This preparation is better if made some time before it is used; it is a compound of nitrate and muriate of tin.
Should any one prefer a pure muriate of tin, the method of making it will be found in the last chapter, in observations on crimson and scarlet upon silk.
Into a copper of cleared boiling water, the heat being reduced, and having the worsted wetted out ready; for every pound of which (dry) put two ounces of cream of tartar or white tartar in powder, and one drachm and a half of cochineal in powder. When the liquor is ready to boil, add two ounces and a half of the first-mentioned solution of tin, which immediately changes the colour; stir it well: as soon as the liquor boils put in the worsted, and boil it till the colour of the cochineal is taken up by it. The worsted must now be taken out, when it will be of a flesh-colour, the water in the copper having lost its colouring matter. To finish the worsted, another quantity of clean water is made warm, into which six drachms and a half of cochineal are to be put; just before it boils, two ounces of the same solution of tin are to be {84} poured in, the liquor undergoing a similar change as before. The worsted is again put in, and boiled till it has imbibed the colour; it is then taken out, wrung, and rinsed in clean water, when the scarlet is in perfection.
One ounce of cochineal to a pound of wool, will impart a colour sufficiently deep, if managed according to the method above described, no colour being left in the remaining liquor.
For many shades of scarlet it will be, however, necessary, and, in a fresh liquor, to add either a certain portion of turmeric or young fustic, to give the scarlet that fiery red which some scarlets have. If not in an entire fresh liquor, a part of the old liquor must be taken out before the yellow is added.
When it is wished to dye a regular series of scarlet shades in worsted, half the quantity or less, for some of the lightest, will be sufficient of the solution of tin, the tartar, the cochineal, &c. The worsted should be separated into divisions corresponding with the shades required; the lightest is of course to be done first: if any deficiency be in the shade, it may have another dip. This deficiency is easily perceived, and a very little practice will enable the operator to assort them perfectly.
It should be noted, that the vessel most proper to dye scarlet in ought to be made of block tin; such as are used by the scarlet dyers for the East India Company.
When woollen cloth is to be dyed scarlet, to every hundred pounds of cloth put six pounds of tartar and eighteen pounds of the solution of tin at first; the same quantity in the completion; and in each operation, six pounds and a quarter of cochineal.
{85} For the accommodation of those who would make small experiments, one ounce of cream of tartar, six ounces of solution of tin, and one ounce of cochineal, may be used for every pound of worsted or cloth, putting two-thirds of the solution of tin and the tartar, and a quarter of the cochineal, into the preparation, and the remainder to the completion.
Observe, that although we have given processes for dyeing woollen cloth crimson as well as scarlet, yet crimson may be obtained in another way: for alum, the salts in general with an earthy base, and the fixed and volatile alkalies, possess the property of changing the colour of scarlet into crimson, the natural colour of the cochineal. The cloth which is dyed scarlet has only to be boiled, therefore, for about an hour, in a solution, more or less charged with alum, according as a deeper or lighter crimson is wanted. When a piece of scarlet has any defects, it is set apart for crimson. Soap and potash will also produce crimson from scarlet, but not of so bright a colour as from alum. Hence also we learn the necessity, in, at any time, working scarlet cloth, to avoid boiling it with soap or pot-ash, &c. if we desire the scarlet to remain.
The worsted or yarn must be boiled for an hour or two in one twelfth its weight of alum and the same quantity of white argol. It is best, when there is a large quantity of yarn, to do this on the preceding day: if your copper hold a pack of two hundred and forty pounds, it {86} will be cold enough to handle after remaining with the fire out during the night.
When the skeins, &c. are taken out and arranged upon poles or sticks, have a fresh water ready in the copper, into which put about thirty pounds of chipped peach-wood, and when it has boiled half an hour, pour in some water to cool it down, and add fifteen pounds of crop madder; work the yarn in this liquor rather under a boiling heat. When it is full enough, for some shades you must add archil. As the whole pack is dyed at four or five turnings in, some of the parcels may be varied in the hues instead of confining them all to one shade. The various turnings will take the greater part of the day to perform. When you choose to have as many shades as there are turnings in, you divide the drugs into different portions for different periods of the time, to be used according to the patterns required. The most economical method of using the drugs being to follow the patterns one after the other: practice will teach the operator to do this most advantageously.
More madder than peach-wood gives a lively red; more peach-wood than madder gives a bright maroon red, bordering on crimson, but more so without any madder; with the addition of archil it gives a crimson, but by no means to be compared with the crimson of cochineal. Urine with the archil renders a less quantity of archil necessary.
The proportion of alum used by dyers in these processes varies from one-fourth down to one-twelfth, of tartar one-sixteenth is used, for every pound of cloth. Equal parts of alum and tartar are used for worsted and yarn, each of which (alum and tartar) is only from one-twelfth to one-tenth of the weight of the material to be dyed.
The shades of yellow are straw yellow, pale yellow, lemon yellow, and full yellow.
In order that the cloth should be properly impregnated with the mordants of alum and tartar, according to what is allotted to the shade, whether light or full, it should be boiled in the preparation at least one hour; two hours for a full yellow; then a fresh liquor is to be made to receive the weld, which must be previously boiled: for a full yellow four or five pounds of weld will be required to one pound of cloth or worsted; for the lighter shades less of course: but a sufficient quantity only of weld should be used, and this should be boiled and re-boiled, as it will keep but a very little time after boiling. If you have a gradation of shades you will save drugs and expense by dyeing the fullest shades first, and the lightest last; but by this method the lightest will not be so bright as if they were done first, and the liquor renewed with fresh boiled weld, and so on to the fullest shade. At last you must have for the goods a preparation weak or strong according to the light or full colour of which they are to be. The last dyeing, whether of cloth or yarn, will assuredly {88} take all the colour out of the liquor of any consequence.
While expense is not an object, it is best, not only for yellows, but for all other colours, to have the preparation and the dye proportioned to the shade, the colour done at once, and the remaining liquor thrown away; but as the price usually paid for dyeing will not enable the dyer so to do, he commonly dyes his shades in succession, as above, and with the utmost economy.
These shades are extremely various, and are dyed without any preparation with alder-bark, red sanders, sumach, galls, madder, &c. and under a boiling heat, although it is occasionally necessary to boil some of the ingredients together previous to the dyeing: for instance, red sanders will give its colour out best when boiled with galls, alder-bark, sumach, &c. Cam-wood, bar-wood, walnut rinds, roots, &c. are used in some of these shades, the varieties of which are almost infinite. Practice is required in this branch of dyeing equal to or beyond any other.
Pass the goods through archil, next through the blue vat, with the usual precautions, then through hot water. For some shades they should be alumed, and then dyed with cochineal for the crimson part of the purple. Blue and crimson make purple, violet, &c. according to the patterns required.
The shades of this colour are very numerous, as yellow green, pale green, bright green, grass green, laurel green, olive green, sea green, parrot green, cabbage green, duck's-wing green, &c.
The goods must first have a blue ground from the woad vat, light or full according to the pattern, they are afterwards to be prepared with alum and tartar, weak or strong according to the lightness or fulness of the pattern, and are afterwards dyed in weld liquor. Many of the shades of green are more readily done by dyeing the wool first yellow with old fustic, with a preparation of alum and tartar, and using the chemic blue vat made with sulphuric acid and indigo. See page 47.
Prepare in the manner described for cotton (page 52.), eight ounces of indigo and four pounds of sulphuric acid. This preparation need not, however, be neutralized for wool as described for cotton. In some instances the preparation is to be for the yellow of fustic one-twelfth of alum, the same quantity of tartar, and in some cases one-twelfth of alum only.
This is to be made the same as for green; it need not be neutralized as for cotton. For blue, however, twelve ounces of indigo are necessary to four pounds of sulphuric {90} acid. In dyeing the heat must be much under boiling, or the using of a high heat would give the blue a green tinge. This blue colour is very bright, yet not fast, but no preparation is of any advantage to either its fastness or brightness. Some put alum and tartar, and some use one, and some the other, to prevent a green cast: if, however, the wool be fine, white, and worked much below the boiling point of heat, it will not turn green although neither be used.
The processes of crimson, scarlet, and of yellow united produce the various shades of these colours, leaving archil out. See buff, peach, &c. on wool.
Black includes a prodigious number of shades, beginning from the lightest grey or pearl colour to the most intense shade of black. On account of these shades it is classed by dyers among their chief or primitive colours[8]; for the greater number of browns, of whatsoever shade they be, are finished in the same dye as would dye white wool a grey more or less dark. This operation is called browning. The best superfine black should have a full ground of mazarine blue previously to being finished black.
{91} A great quantity of cloth and other articles have, however, no indigo ground, but a ground of logwood, or of logwood and alder-bark, or of logwood and old fustic, or of logwood, alder-bark, and old fustic, all boiled together, and sometimes they are boiled in a decoction of oak saw-dust.
Indigo for the ground is the richest drug, in carbon, that is or can be used; logwood is next to it: too much logwood, however, whether indigo be used with it or not, gives the black a foxy hue; alder-bark and old fustic modify this effect, and are used in small quantities for this purpose, because the dye from these, as well as that from oak saw-dust, will produce a soot or dead black.
A jet black is required full and rich, therefore old fustic and oak saw-dust are only used to modify the richness of the ground as it regards the blue, whether of indigo or of logwood; for logwood especially, without these, if overcome with sumach and sulphate of iron only, would be foxy, purplish, or have a reddish cast.
So many different grounds being used for blacks, and every dyer thinking his own the best, is the occasion of such a great variety of hues, even of black, being found in the market. It is, therefore, thought unnecessary to describe the various methods of dyeing black which are pursued by different dyers, and which would be, in fact, impossible. But the author has done what is of much greater importance to the student, who, after a little practice, let him have a pattern of black to dye, will know how to do it, let who may have dyed it.
Even a blue-ground is, according to some, dyed afterwards {92} in a decoction of logwood and galls, or logwood and sumach, and two pounds of verdigris for a hundred pounds of cloth. Thus, ten pounds of logwood and ten of galls, are to form the decoction, and are boiled previously for twelve hours. One third of it with the verdigris is used first, and then the cloth, after boiling in it for two hours, is aired; it is then passed through one third more of the decoction of logwood and galls, having previously had eight pounds of sulphate of iron dissolved in it, and the scum arising from the solution taken off. The goods are to be worked in this one hour at a boiling heat, then aired again by turning them about on a stone floor. The remainder of the decoction of logwood and galls is then added, with fifteen or twenty pounds of sumach; boil it some time, and then add five pounds of sulphate of iron; scum it, and let the liquor cool down, then put the goods in, and work them at a boiling heat an hour or two, taking them out once or twice, at least, in the time, to air and cool; they are then to be well washed, and passed through a decoction of weld liquor, to soften the black, which will be very fine. This process is chiefly from Hellot; but the quantity of sulphate of iron is more by three pounds than he directs.
When the cloth is blue, it is usually boiled two hours in a decoction of galls, then washed and aired, when sulphate of iron and logwood are added to the liquor, and the goods worked in it for two hours, and then washed.
The above have been the processes in practice for a century past in France, where the galls were not so dear as they now are in England: sumach is here, therefore, now most commonly used as a substitute for galls.
To dye one hundred weight of cloth, take thirty pounds of chipped logwood, half a bushel of alder-bark, and six pounds of sumach, and boil them together in a proper quantity of water for half an hour; then cool the decoction down with cold water, enter the cloth, turning it on the winch; bring it to a boil, having the sumach in a bag; boil and keep the cloth turning for one hour and a half: this is the ground. Have now ready fourteen pounds of sulphate of iron dissolved in water, which is to be laded into the copper by one man, while another turns the cloth for an hour at a boiling heat; it is then to be taken out, cooled, and aired, returned to the copper, and boiled gently for two hours, and then cooled again.
While the cloth is cooling, six pounds of logwood, ten pounds of alder-bark, two pounds of argol, ten of soda, or common pot-ashes, and three pounds of sulphate of iron, are to be added to the liquor in the copper, and boiled one hour, when the goods must be turned and worked one hour; and, lastly, taken out and aired. This black is said to be of the hue of a raven's feather. This process is from Heigh.
The argol is professed to be put in to counteract the sulphuric acid of the sulphate of iron; the alkali is said to cause the logwood to retain its natural violet colour: and if too great a quantity of logwood be not used, the result would be as above stated. But the author presumes that such a black would not be at this time much esteemed. We object to the introduction of so much, {94} indeed of any alkali or argol, as the time employed in performing the process is wasted. Alkali is good, however, where a chemic green is to be dyed black.
Wool will take up whatever the copper contains necessary to dye black; but, for the beauty of the colour and the durability of the cloth, it is best to let it have most of its ground of vegetable colour before it has the sulphate of iron, which blackens that ground, with sumach instead of galls; and even in some instances, dyeing some goods without the sumach.
Were the author, however, to direct the dyeing of black cloth, such as should be of the best kind, he would have an indigo ground with logwood and alder-bark, without old fustic or oak saw-dust; and to finish the cloth he would use sumach, sulphate of iron, and a small quantity of verdigris. He would give it the blue ground first; then the logwood, alder-bark, and verdigris; and then finish it with sumach and sulphate of iron.
If the blue ground were omitted, he should dye the cloth twice, giving it more of logwood and alder-bark, but verdigris the same; and finish it with sumach and sulphate of iron. Nevertheless, when we dye to a pattern, the pattern must be our guide.
Different goods will require different quantities of drugs. Logwood should be about one-fourth of the weight of the goods; the sulphate of iron about one-fifth of the logwood; alder-bark, when used, about the same quantity as sulphate of iron; but for some yarns this bark is not used, nor is it necessary; and where fustic or oak saw-dust is used, there is the less necessity for using alder-bark. The sumach must be about the {95} same quantity as sulphate of iron. Remember that carbon is generally considered as that which makes the richness of a dye. That it is the iron in the sulphate of iron, combined with the tannin and gallic acid which are assumed to be in the sumach and logwood, that produces the blackness of the dye; but this theory is questionable. See below.
The way to ascertain when the quantities of drugs are most appropriate for producing the desired effect is as follows:—
First, ground with different quantities of drugs, from three to five or seven patterns, and use from one third to one fifth of sulphate of iron and sumach to the grounding; afterwards finish with the remainder of the sulphate of iron and sumach: the fuller the ground the richer will be the black, if the logwood be not in excess, and the quantities be used as thus stated.
We ought also to state here (from Berthollet, vol. ii. p. 4.) that commonly more simple processes than any of those above described are employed for black. Thus the blue cloth is simply turned through a bath of gallnuts, when it is boiled for two hours. It is next passed through a bath of logwood and sulphate of iron for two hours without boiling, after which it is washed and fulled.
A black may also be dyed without a blue ground with walnut rinds or the roots of the walnut tree; in this case the cloth receives a dun ground from the walnut husks or roots, and is afterwards made black in the manner above described, with logwood and sulphate of iron.
{96} The blacks, however, without the blue ground are only given in general to inferior cloths.
The colouring principle of logwood is called hematin; it is crystalline, of a rosy-white, and, viewed through a lens, very brilliant; its taste is slightly astringent, bitter and acrid; exposed to the action of fire in a retort it affords all the products of animal substances, and also a small quantity of ammonia, which proves that it contains nitrogen. It dissolves easily in boiling water; on adding some acid very gradually, it changes to yellow and then red. Potash and ammonia give the solution of hematin a purple red; if a great excess of these alkalies be added, the colour becomes violet-blue, then brown-red, and finally yellow-brown. In this state it is decomposed and cannot be recovered by any acids. Protoxide of lead, protoxide of tin, hydrate of tritoxide of iron, hydrate of copper, oxide of zinc and its hydrate, flowers of antimony and oxide of bismuth combine with hematin and give it a blue colour, with the loss of the violet shade. See notes to Ure's Berthollet, vol. ii. p. 420. See the explanation of protoxide, &c. under OXIDE in Chapter I.
The above facts concerning logwood may, by the ingenious dyer, be applied on many occasions with great success.
All greys, from the darkest to the lightest, are composed of black in varying proportions. They are of great use in dyeing, not only for their own colours, but {97} also when applied to other colours, which operation is called saddening or darkening.
Some greys have a woad ground of blue, then of logwood, sumach or sulphate of iron, of which decoctions of the three last, for expedition, should be in readiness when wanted. When a succession of light shades, in particular, is required, in some instances the chemic blue is used: when we treat of the mixture of black, or rather grey with red and blue, the utility of grey will be seen.
These produce an infinite number of all shades of grey as sage grey, slate and lead colour, and others still darker.
Browns and Fawns owe, in all probability, their colour to the iron which their dyes contain. Iron is so universally diffused throughout nature, that it, very likely, enters into the composition of many other colours; it exists in blood, in water, and in innumerable vegetable and animal substances, as well as in earths and many minerals. Hence we ought not to be surprised that blue, red, and fawn produce olives from the darkest to the lightest; as well as slate and lavender when the shade is very light.
Fawn and yellow produce the feuille-morte or dead-leaf.
Fawn and red produce cinnamon, tobacco, chestnut, &c.
{98} Fawn and black produce coffee, maroon, &c.
Blue, yellow, and black produce all the dark greens, even to black.
Blue, fawn, and black produce dark olives and greenish greys. Red, yellow, and fawn produce orange, gold colour, withered-leaf, carnation, burnt cinnamon and tobacco colours of all kinds.
Yellows, fawn, and black produce hair colour, nut-brown, &c.
This enumeration is meant only to give a general idea of the ingredients proper for the production of shades composed of several colours.
Where red forms a component part of the colour wanted, the goods must have a preparation of alum and argol, strong or weak, according to the fulness or weakness of the red which forms a part of the compound dye, such as the half or quarter of the quantity which is required for a full colour of red; the same as to yellow, and, in proportion, when red and yellow are joined.
The quercitron bark is said to yield from eight to ten times more colour than weld, and about four times more than old fustic; this was, however, Dr. Bancroft's account, who had a strong interest in this dyeing drug, as stated in the first chapter. He also asserts, that one pound of bark with muriate of tin, will dye forty pounds of woollen a bright golden yellow, which afterwards becomes a beautiful and durable scarlet, with a fourth part less cochineal than is usually employed on other occasions for such a colour. But Bancroft did not succeed in {99} doing away the old method of saving tartar and cochineal.
His fullest yellow upon cloth, the author has, however, often tried and found it rich and golden; the process is as follows:
Cloth one hundred pounds; bark in powder, and in a bag, ten pounds; muriate of tin, or murio-sulphate of tin, (for which see forward,) ten pounds. The bark in the bag must be first immersed in the proper sized vessel for six or eight minutes; then add the solution of tin and stir it well for two or three minutes, when the cloth must be put in, and kept in motion by two or three men working over the winch from end to end; then proceed to boil; and, in fifteen minutes boiling, the highest yellow is produced; a longer time would turn the yellow brown.
When a very bright yellow, approaching less to orange, is wanted, seven or eight pounds of solution of tin, five pounds of alum, and ten pounds of bark, will do for a hundred pounds of cloth. In this process, boil the bark first in a bag for a few minutes, then add the solution of tin and the alum, and the cloth afterwards, as before directed; less body requires less quantities of course.
Use eight pounds of quercitron bark, to six of muriate of tin, six pounds of alum, and four pounds of white tartar, for cloth as before. The alum and tartar render the yellow more delicate, and give it more of the lemon or greenish tinge; where this is wanted in the greatest perfection proceed as follows:
{100} Take ten pounds of bark, ten of muriate of tin, or murio-sulphate of tin, ten of alum, and ten of tartar. For cloth three or four times the quantity of the preceding processes may be taken, namely three or four hundred pounds.
In this process the bark must be boiled fifteen minutes in water only, and then the other ingredients be added and mixed in the liquor by stirring. The cloth is next to be put into it, the liquor being first cooled a little; it is then immediately to be turned briskly on the winch till the colour is sufficiently raised.
When a variety of shades are wanted, in working the bark, (contrary to the processes for many other colours) the higher shades should, in this colour, be dyed first, and the weaker afterwards. When about two-thirds of the quantity of the cloth have been dyed, it will be generally found that the liquor, by continuing to extract colouring matter from the bark, has acquired an over proportion, and wants a small quantity of muriate of tin, of alum, and of tartar, perhaps a pound of each, to enable the bark at last, as well as at first, to give the same delicate, pale and greenish tinge. A surer way, however, is to boil the bark in a small quantity of water, separately, for six or eight minutes; and then to add to it the solution of tin, alum, and tartar, and boil them with the bark together for fifteen minutes, and then damp the fire; then have the cloth in a proper sized vessel, supplied with boiling water, and the cloth moving on the winch; after it has gone a few turns round, and is thoroughly wetted out (which it should be before, and now again) lest any part should be dry, add the supplies of the yellow liquor above {101} described, by little and little as they may be wanted: in this way expectation is surpassed by the beauty produced.
is made thus:—Take of muriatic acid, three pounds; of feathered tin, as described in the process of dyeing wool scarlet, fourteen ounces; to the tin add gradually the muriatic acid; afterwards, with due and great precaution, by degrees, in the course of a day or two, two pounds of sulphuric acid. Care must be taken that the vessel in which this operation is conducted, be of stone ware or of glass. These acids being mixed with the tin, should be left to saturate themselves with it, which they will do in time, without artificial heat; but the dissolution of the tin will be rapidly promoted by a sand heat. This murio-sulphuric solution of tin, thus made, will be perfectly transparent and colourless, and will probably remain so for years, without suffering any precipitation of the metal.
This is done with the most economy after scarlet, and, in such case, requiring very little addition (in some cases none) of cochineal. The wool, having an alum preparation, it may be requisite to add some fresh prepared decoction of young fustic or weld. See the next article.
This process is the same as the last; that is, after scarlet; but the wool is not to be alumed: in some cases, a little tartar and cochineal is added.
{102} Observe, that the cochineal and tartar being added, the previous preparation must be according to the fulness or faintness of the shade wanted, whether of buff, peach, or flesh, all of which require, essentially, the same process. By such means, a pattern of any shade, compounded of red and yellow, from scarlet to the weakest buff and flesh, may be produced.
The vat being five feet high, and two feet in diameter at top, you may use for it from two to six pounds of indigo, according as you set it light or full.
Boil two pounds of potash, two ounces of madder, and a handful of bran, in fifteen gallons of clear soft water, for half an hour.
The indigo must be powdered; after which it must be levigated in a peculiar circular cast-iron mill, having a contrivance for two large round stones, or cast iron balls, which are kept in a perpetual circular motion while the indigo is ground. Water it, and put it into the mill, and as the balls run round, the indigo in the water is reduced to a fine flowery paste. There are mills more convenient than these, but, perhaps, none more simple for a small concern.
When the indigo is thus prepared, boil it in the copper with the grounds of the madder and the potash, which fell to the bottom; it is all, then, to be put into the vat at the same time with the indigo; the whole is to be stirred, the vat covered, and heat applied to make it more than blood warm, and to keep it so. The vat {103} should be stirred twice, slightly, both the second and third day, the heat remaining the same; when a brassy scum, divided and interrupted in many places, begins to appear on the surface. On the fourth day, the heat being continued, the scum becomes more perfect and less broken, the froth which rises, upon stirring, is more blue, and the vat a deep green.
When it becomes green in this manner, it is an indication that it must be filled; to do which, boil half an ounce of madder, and one pound of potash, in five gallons of water; put in this liquor, and stir it; if it produce much froth, stir it again, and the next day it will be fit for working; which, however, will be sufficiently known by the quantity of froth, and by the brassy and scaly crust on the surface of the liquor, on blowing or stirring which, the liquor beneath is green, although the surface appears brown or blue.
When the vat has worked about forty or fifty pounds of serge or worsted, it may be necessary to replenish it with one pound of potash, half an ounce of madder, and a handful of bran; these being boiled a quarter of an hour, are added to the vat.
When this vat wants replenishing with indigo, which may be known by the liquor being no longer green, but brown, blue, or almost black, two-thirds of it must be put into a copper; when ready to boil, the scum on the top must be taken off by a sieve, after which it should be suffered to boil, with the addition of two handfuls of bran, a quarter of a pound of madder, and two pounds of potash; soon after it has boiled, it is to be put into the vat with one pound of indigo, prepared as before; the {104} vat being again stirred, and covered, the heat always remaining between blood and fever heat.
When an indigo vat has been several times re-heated, it should be emptied out entirely, and set anew, because the colour becomes dull. The preceding process is from Hellot.
[8] It is necessary that the student should not confound the terms primitive colours here with the prismatic or primary colours, for the discovery of which we are indebted to Sir Isaac Newton. See the Introductory Chapter.
To dye silk black for velvets—To dye silk black, London process—On dyeing cotton black at Rouen—To dye cotton black, London process—For dyeing black, particularly cotton velvets, at Manchester—On dyeing silk and cotton black, with a blue ground—Another iron liquor—To dye cotton black, by using the preceding solution—To dye cotton violet—To dye cotton red—To dye cotton an Adrianople or Turkey red—Miscellaneous observations relative to Adrianople red.
Some of the more simple and less difficult processes of dyeing both cotton and silk, are described in the preceding chapters; we shall now describe those, not only for black, but for some other colours, which require more care and attention. For ungumming and boiling silk, &c. see Chap. VI.
Silk has a strong affinity for galls, and advantage is sometimes taken of this: for silk, being a valuable article, is often galled to excess, merely to increase its weight.
{106} Cotton has a strong affinity for iron, and iron has the same for gallic acid, wherever it may be found; therefore, in sumach, alder-bark, &c., iron unites with the acid, whenever both are connected by the medium of water. Tannin, doubtless, has also some share in such dyeing processes, although what does not even now appear to be well understood.
Black, Macquer observes, is rather difficult to be dyed upon silk; or, at least, there is reason to think so, from the numberless experiments which have been found necessary to the attainment of a good black, as well as from the multitude of heterogeneous ingredients which Macquer admitted into the composition of his various processes for this dye, some of which consisted of arsenic, corrosive sublimate, litharge, antimony, plumbago, and about ten other ingredients! we shall not, therefore, detail such preposterous mixtures; one, however, we may just put down by way of showing what the art was in Macquer's time.
Take twenty quarts of strong vinegar, one pound of black nut galls pounded, and five pounds of iron filings; these ingredients are to be mixed in one vessel.
The silk should be ungummed by boiling it four hours with a quarter of its weight of white soap, and afterwards to be well cleared from the soap.
Take, for every hundred pounds of silk, twenty pounds of galls in powder, and boiled one hour; two pounds {107} of sulphate of iron; twelve pounds of iron filings; and twenty pounds of gum arabic or senegal.
This process is very simple: here are the gallic acid and tannin of the galls, and the iron of the sulphate and the filings. But we must proceed to a more modern process.
Take of wove silk, twilled sarsenet, one hundred and fifty yards. Boil, for three hours, of alder bark one bushel and a half; of logwood fourteen pounds; and of iron filings one pound. Then let the fire be damped; dissolve four ounces of sulphate of copper in water; wet out the silk in hot water; after which put the solution of sulphate of copper into the liquor and stir it only; then put the silk into the copper, and work it from end to end four times; after which take it out in the air; now put it in again and work it as before; take it out again and let it be aired on the floor, opening it from time to time till it is cold; repeat the same thing twice more, in all four times. This is termed four wets. While the last wet is cooling and airing, dissolve and put into the copper three pounds of sulphate of iron, and then give the silk two more wets, which make the number of wets six. The drugs are now left to boil as much as they will during the night, being left so to do, because in a large business, this part of the process would close the day's work.
The next morning give the silk four or five wets more, and leave it in the copper all the following night, observing {108} when it is left in, and always when it is worked in, that the heat, must be considerably under the boiling point, and the silk kept covered by the liquor: for if any part be exposed to the air it will be marked.
Take one hundred quarts of sour wine, bad vinegar, or small beer; put to either of these twenty-five pounds of old iron hoops rusted by the air or dew; twelve pounds of rye meal or coarse bran; put the whole into a copper and heat it rather more than blood warm. In the summer it would do exposed to the sun and air with a porous cloth over it, to let in the air, but keep out dirt, &c.; the older this solution is the better; but it should be at least two months old.
Cotton skeins are galled by being worked in a solution of galls; alumed and then dyed in weld liquor; this in the result is yellow; they are then passed through a decoction of logwood, and after that of sulphate of iron, a quarter of a pound to every pound of cotton; they are then dyed in madder, half a pound to every pound of cotton.
We cannot recommend this process, although we give it, as much better methods are now known.
Cotton cambric piece-goods are passed through a blotching machine to receive a mordant of acetate of iron, {109} and galled slightly; sumach is used instead when galls are dear; the cotton is then passed through logwood, or logwood and fustic, and then through sumach; so that it is possible thus to give them the mordant sufficiently in proportion to the iron liquor at first; proceed as in dyeing afterwards, at a heat approaching boiling or even boiling. You may now proceed by adding first the galling or sumach slightly; afterwards the logwood, &c.; and then the remainder of the galling or sumach may be used to finish it; and thus dye the goods black by the quickest possible process.
It should be observed respecting the last process and the process which precedes it, that in dyeing black alum is inimical to the colour. Therefore D'Apligny's is not now esteemed. Alum for black is as improper, as it is proper and essential for red and yellow.
In regard to giving the acetate of iron for black at once, as the second, or London process directs, it may be done by having the proportions full; by full is meant that the mordant should be full enough; then, after the slight galling, as directed in giving the logwood and alder bark decoction, or logwood and fustic, be sure to have that decoction strong enough. This might be called the ground; and the most perfect judgment might be formed of it by having a part of a piece, or one piece of a batch dried in the stove: for, according to the fullness of the ground, so will the black be rich and perfect or otherwise.
The alder bark and fustic are used only to prevent the hue of the logwood from being predominant. If the ground be a full and rich brown, the second full galling {110} or sumaching will bring it to a full and rich black; but, if the ground be poor, these processes will cut or destroy the ground, and the black will be foxy, nasty, and poor; and not only so, but the material dyed will soon wear rotten, because having an over-dose of iron, the iron will tend to decompose the cotton. Therefore the following process is most esteemed.
In a large dye-house where much business is done, a great many wine-pipes or other large tubs, or any substitutes are arranged in an appropriate place. Into these are put old iron hoops, rusted in the air, and cut into short pieces; a layer of the iron pieces and a layer of the alder bark, again a layer of iron and a layer of the bark, and so on in succession from the bottom to the top. When the pipes are all thus filled, water is poured into them till they are filled up; they remain in this state for six weeks or two months according to the season, whether summer or winter.
The same process will do for any other cotton goods as well as velvets, such as calicoes, cambric, and jaconot muslins, cotton in the skein, &c.
In some cases there are persons who pass the goods through the liquor of the aforesaid black vat. The colour of this liquor when it is fit for use is purplish, particularly after being once used and returned to the vat again, which it always is. Others begin by passing the goods through a decoction of logwood and sumach, {111} then through sulphate of iron, then wash off through logwood only; then through sulphate of iron; always washing off from this last; the goods are then dried, and this is called the first time of saddening.
They are next passed through logwood, then through sulphate of iron, then washed off, then again through logwood, then sulphate of iron, and then washed off; and then dried. This is called the second time of saddening.
Supposing the goods to consist of a hundred or a thousand pieces, after drying the second time they are brought in lots to the foreman for examination, and assorted into lots one, two, and three. All that is fit for lot one is full enough and has ground enough, and is of a rich full-bodied brown, ready for galling or sumaching: sumach being the substitute for galls, this process is termed in the dye-house, macing. Lot two is not full enough, and must pass through logwood, then sulphate of iron, and then be washed off. Lot three is still more deficient; this must be passed through logwood and the sulphate of iron twice and then washed off, and both lots two and three dried again.
Lot one is now to be sumached for the first time: that is, passed through a decoction of sumach, then through sulphate of iron, and then washed off: if the decoction of sumach be kept up strong after all of them are once sumached, they may be dried. Lots two and three, when they are dry, are also to be sumached the same as lot one, and dried.
As soon as any of them are dry they are ready to be sumached the second time by passing them through the {112} decoction as before; but instead of sulphate of iron, some of the alder bark and iron liquor are used; or as we shall term it, the liquor of the black vat. They are then to be washed off and dried. If the black liquor and the sumaching be powerful, some of the goods will be finished when dry. Such are examined by the foreman; those which are not finished must go through the last process again. The finished goods are well and repeatedly washed off in fresh clear soft water two or three times and then dried.
The cambric muslins are sent to be calendered to imitate silk sarsenets.
Book-muslins must be sent to the muslin dressers, except where, in some cases, they sarsenet and dry their own goods.
By the above method the ground is secured, and so is the black, and also the strength of the goods.
It is remarkable, that although an indigo ground for wool enriches the black, yet for silk and cotton it is not generally considered necessary. Latterly, however, we believe the dyers of black on cotton do first give it an indigo ground before the black is given. This is, nevertheless, not a new method, for D'Apligny describes the process in his Art of Dyeing, for linen and cotton yarns; these are first dyed sky-blue in the vat, then wrung out and set to dry. They are galled in the proportion of one part galls to four of yarn, being left twenty-four hours in the gall liquor, wrung out anew, and set to dry: about {113} ten pints of iron liquor to every pound of yarn are then poured into a tub, in this the yarn is turned on sticks, and worked with the hand for a quarter of an hour, it is then wrung out and aired. This operation is twice repeated, adding each time a new dose of the iron liquor; the yarn is aired once more, then wrung out, well washed, and dried. To complete the dyeing of the yarn a weight of alder-bark equal to that of the yarn is boiled with a sufficient quantity of water for an hour; to this is added one half of the bath which has served for the galling and sumach. The whole is boiled for two hours. When cold the yarn is put in and worked, aired occasionally, and then left in the bath for twenty-four hours, when it is wrung out and dried. To complete it, it is steeped and worked in the residue of a bath of weld, to which a little logwood is added; it is then taken out, wrung, and immediately passed through a tub of warm water, into which one part of olive oil to sixteen of yarn has been poured. It is finally wrung out and dried. See Ure's Berthollet, vol. ii. page 18.
Although we have described an iron liquor in a preceding section, it may be useful to give the following process for another here. Fill a cast-iron boiler with pyrolignous acid, add to it old iron well oxidized, and boil. The solution of the oxide will take place rapidly. When the iron grows clean, and the solution black as ink, throw the whole into a cask, to be employed as occasion shall require.
Prepare the cotton as usual, by giving it a blue ground; gall it, and pass it through a bath of the solution of pyrolignite of iron diluted with lukewarm water. Renew the gallings and the passings through the bath of pyrolignite of iron till a deep and brilliant black is obtained. Finish by passing the cotton through olive oil thus: throw on some lukewarm water a little olive oil, pass the cotton through it; the cotton absorbs the oil, but it must be worked a long time in the bath to diffuse the oil equally. Dry in the shade. The cotton is now a perfect and very durable black.
Every time the bath of pyrolignite is used, what remains must be thrown away; the old baths are never added to the cask.
The application of oil, which heightens the black, and imparts softness to the stuffs, is given to such articles as cotton velvet by means of brushes, which are slightly imbued with it. Berthollet.
We may add here, that an iron liquor called tar-iron liquor, prepared from the acid obtained from tar, (the acetic acid we presume) is now well known in commerce, but we have not room, nor does it appear necessary, to describe the method of making it; it is much used in preparing mordants for black and other colours by the dyers and printers of silk. This iron liquor may be obtained of Blake, North Street, Back Church Lane, St. George's in the East, London. See M'Kernan.
Pass the skeins through the black vat and dry them, then pass them through a decoction of galls and dry them again, then through a decoction of logwood, then of alum and verdigris, washed off, and dried.
Or thus: by the black vat liquor, that is, the liquor of old iron and alder bark in some cases. Let the vat liquor be prepared from the iron hoops, vinegar, rye, or coarse bran, described in page 108. By this liquor it is easy to procure all the violet shades from the pansy flower up to the lilac and violet.
The goods must be first blue-vatted and dried, then galled and dried, then passed through the iron liquor, then maddered, then washed off, and dried; the liquor must always be kept much below a boiling heat, as this heat makes the colour obtained from madder brown: whatever drugs require boiling must be prepared by a decoction previously made.
For some shades sulphate of copper is used; for others verdigris, saltpetre, and alum.
To dye to the pattern the preparations should be always of one given strength, and all solutions of mordants the same. The time of working the goods in the dye must be regulated by the fulness or lightness of the pattern; and the quantities of the various drugs, &c. used much or little accordingly, reserving patterns of processes, with the particulars of such processes noted down. In proportion to the number of these upon record, and with strict attention to the subject, a good pattern dyer is formed. Time and practice are, however, {116} absolutely necessary, with a delight in the business: for without a pleasure in dyeing no one can become a good or an eminent dyer. In many of the branches of this art there are, it is true, labour and pains in abundance; but there is also a portion, and that not a small one, of pleasure in others, which will counterbalance the care, anxiety, labour, and fatigue inseparable from this useful and important occupation, and which so strikingly exhibits the science and ingenuity of man.
If the cotton skein has not been cleansed since it was spun it must be cleansed by being boiled in a solution of potash, one ounce of which, if good, to a pail of water may be enough, or more than enough. The cotton must be put into bags when boiled, then washed off and passed through clean water, scoured with a little sulphuric acid, and then washed off again; then galled, washed off, and dried. The galls should be white galls: for twenty pounds of cotton five pounds of bruised galls are boiled in about one hundred and twenty quarts of water for two hours.
After galling, the cotton must be alumed: four ounces of Roche alum for every pound of cotton. When alumed it must be washed off and dried.
The cotton is now to be dyed in a copper containing six pounds and a quarter of best crop madder, with a sufficiency of water. The heat is kept under that of boiling for three quarters of an hour. After being aired, washed, &c. it is put in, worked, and boiled for twelve {117} or fifteen minutes. Some dye it again two days after, because the longer to a certain degree between aluming, dyeing, and drying, and between one dyeing and another, the better. The second time of dyeing eight ounces of madder are used for every pound of cotton. Some dyers gall it twice, and consequently dry it as often, then dye it at once in the madder, having a proportion accordingly. This is a red full-bodied colour.
For one hundred pounds of unbleached cotton, take the following articles and pursue the described processes.
Lixivium No. 1. Dissolve one hundred and fifty pounds of alicant soda, (barilla) in three hundred quarts of river water. There must be no more water than enough to dissolve the salt. An egg must float on it or it will not be strong enough.
Lixivium No. 2. One hundred and fifty pounds of fresh wood ashes, and three hundred quarts of water.
Lixivium No. 3. Seventy-five pounds of quicklime, and three hundred quarts of water.
The cotton is to be boiled three hours in a liquor composed of equal parts of each of the above solutions, taken from them when clear and in a settled state. The liquor must be replenished occasionally, so that it shall always cover the cotton during the whole time it is boiling; after which it must be taken out, washed, and dried in the air.
Into one hundred and thirty quarts of a mixture consisting of equal parts of the above three lixiviums, put {118} twenty five pounds of sheep's dung and part of the intestinal liquor, previously well mixed by means of a wooden pestle, and the whole strained through a hair sieve. Then twelve pounds and a half of good olive oil is poured into the mixture, when it instantly forms a soapy liquor.
Into this liquor the cotton should be worked hank by hank, often stirring it; the cotton, after all the hanks have been worked separately first, is then left in the liquor for twelve hours; it is then taken out, lightly wrung and dried. The liquor is put by for brightening. This process is repeated three times during the working; and by the time the solution is all worked four hundred quarts might be used, but that will not injure the clear of it from being applied in brightening; and it must be reserved for that purpose.
When the cotton has been three times dipped in this soapy water, and three times dyed, the same process is repeated, except that the sheep's dung is left out; the liquor is also preserved for brightening. The cotton, having gone through these processes, should be as white as if it had been bleached.
When dry it is to be galled, using a quarter of a pound of galls to every pound of cotton; after this it is dried, then take six ounces of alum for the first aluming; it is then to be dried again, and to hang three or four days in the air, and then, when dry, to be alumed again; four ounces of alum, and four of the lixivium may be added to the last alum water.
The madder used for this red is called lizary, which furnishes a dye incomparably finer than that produced {119} by any other madder. Of lizary madder, therefore, take two pounds for every pound of cotton, and twenty pounds of liquid sheep's blood well mixed with the water in the copper before the madder is put in. The butcher should stir the blood to prevent its coagulating; the copper should be carefully skimmed; the madder should not boil, but be brought during the process from blood-heat to within a few degrees of the boiling point: if it boil at last, as some prefer it, it should only be for a few minutes.
In order to brighten the colour, the cotton is dipped in a lixivium of fresh wood ashes, and five pounds of white soap: yellow or mottled soap is improper. When the cotton has been well worked in this liquor, it is, with the liquor itself, put into a copper sufficiently large to hold it with some addition of water, and made to boil over a slow fire, for three, four, or more hours. The liquor must be covered with coarse white linen cloths, to keep as much steam in as possible.
Some of the skeins of cotton must be taken out from time to time, and washed perfectly; when the red is judged perfect and sufficiently bright, the fire is withdrawn.
If instead of the wood-ash lixivium and soap, the two reserved liquors and soap are used, the red will be much brighter than the finest Adrianople carnation.
In regard to the above processes, we may observe, that those given for Adrianople red in Ure's Berthollet, are {120} more numerous, being regularly numbered to the seventeenth, or last operation called brightening. After a careful attention to those processes we see no reason to alter our own, yet we nevertheless advise the dyer to become acquainted with what is stated in that work, many details being there given for which we have not room, particularly for making different shades of the colour. We add, however the following from vol. ii. p. 140.
"Cotton dyed red, may, moreover, be made to pass through all the shades, down to the palest orange, thus: pure nitric acid is diluted with two-fifths of water; chips of tin are oxidized in it till the liquor grows opal; the solution is employed at different strengths; the colour varies according to the concentration of the solution: when it is strong, shades are obtained which have some relation to those of scarlet.
"In general, when brilliant colours are desired, we must not charge them too much with oil; we must give feeble leys long repeated, charge little with alum, employ the best madders, and, at last, brighten powerfully without sparing soap."
We have directed good olive oil; but M. Vitalis directs fat oil, (gallipoli) to be used in the processes for dyeing Adrianople red, and Berthollet says, it must not be a fine oil, but one containing a strong portion of the extractive principle.
A factory for dyeing this red was first established in this country in 1790, by M. Papillon, who obtained a premium from the Commissioners and Trustees for Manufactures in Scotland, for communicating the details of it on condition it should not be divulged for a term of years, {121} during which M. Papillon was to have the sole use of his secret. This term being expired the process was published. See vol. xviii. of Tilloch's Magazine.
M. Vitalis, (in his work on Dyeing published in 1823) has given, at length, the mode of dyeing Turkey red at Rouen. It differs in many particulars from Berthollet and others. We learn from him that two systems for imparting this colour are in use at Rouen. The first is called the grey course from the cotton being subjected to the maddering immediately after it has received the oily preparations, and the mordants of galls and alum which give it a grey colour. The yellow course, is so called from the cotton, after having received a first time the oily preparations, as well as the mordants of galls and alum, not being exposed to the maddering till it has passed a second time through the same preparations, and the same mordants which give it a yellow colour. This second manner of working the Turkey red is called, in the dye-house, remounting on the galls. Dr. Ure, in a note to Berthollet, vol. ii. p. 378, has detailed these two courses, and made, besides many valuable observations on them, and the dyeing of Adrianople red generally, for which we must refer to the work, as our limits prevent the possibility of any further notice of them here, except to add, that a process for dyeing cotton of a smoke red; and another for dyeing cotton a cherry red, is well deserving the attention of the dyer.
In regard to the blood used in dyeing Adrianople red, Dr. Ure decidedly affirms, that "it adds no colouring matter to the madder in the dyeing operation;" in this he is countenanced by the observations of Chaptal, see {122} Berthollet, vol. ii. p. 141. "To the use of blood in the madder copper," says Dr. Ure, "I attribute nothing, as from the rancid and putrid state in which I have seen it used, were it not for the prejudice of the operator, it might be safely dispensed with." A very eminent calico manufacturer, whom Dr. Ure consulted, assured him, that in the Turkey red process the only essential mordants were oil and alumina; and that bright and fast reds, equal to any produced by the complicated processes of sheep's dung, galls and blood may be obtained without those articles.
We make no comments on these observations, but leave them to the good sense and intelligence of the dyer: they deserve the utmost attention.
Linen yarn takes a colour almost as brilliant as that of cotton, but it must be passed through a double number of oils and leys. The latter must even be very strong, otherwise the oil flows out at the surface. The greatest attention must be bestowed on the scouring out first: for the yarn mingles and entangles by the heat to such a degree, that it sometimes can be neither dipped nor unravelled.
It should be mentioned that the large dyers of Adrianople red, now obtain their soda for lixivium No. 1, by using common salt in solution, to which is added a solution of pearl-ashes. On boiling these together a muriate of potash is formed, which is taken out of the liquor with a skimmer; a carbonate of soda remains dissolved in the liquor, and is, of course, applied to the same purpose as, and at a much cheaper rate than, the Alicant soda.
To dye skein cotton yellow—On dyeing and re-dyeing cotton furniture yellow—To dye cotton skein a duck's wing green and olive—Of browns, maroons, coffee colours, &c.—Observations on silk—On ungumming and boiling silk—Whitening—Sulphuring—On aluming silk—Skein silk for yellow—Preparation of annatto, for aurora, orange, moidore, gold colour, and chamois—To dye silk aurora or orange—To dye moidore—Process for orange—To dye silk poppy or coquelicot—A cheaper poppy with annatto and Brazil wood—On dyeing silk a fine crimson—Composition for dyeing silk scarlet or crimson, with cochineal—Another process for crimson—Crimson by Brazil wood—Of fine violet—Observations on crimson and scarlet upon silk—On dyeing silk green—On olives—On dyeing silk grey—Nut-Grey—Black greys—Iron greys—On dyeing silk of a Prussian blue colour—Chromate of lead for yellow on silk or cotton—Conclusion.
We have in several preceding chapters treated of both cotton and silk; we shall here treat of certain processes and {124} colours relative to both these substances, which are most conveniently arranged in this chapter.
The simpler processes for cotton will be found in the second chapter, the more complex in the fifth; the simpler processes for silk are given in the third chapter, the more complex in the fifth; the remaining processes for both in the present chapter, will conclude the work.
The same operations as those in the first common red dye are to be used here; to one pound of cotton four ounces of roche alum, and from one to four pounds of weld.
When dyed the cotton is to be worked in hot, but not boiling, liquor, consisting of four ounces of sulphate of copper to every pound of cotton; it is then to be boiled for three hours in a solution containing four ounces of soap to every pound of cotton.
When a dark or jonquil colour is wanted, no alum is used; of weld take two pounds and a half, very little verdigris, or a little alum in its stead, but nothing else. For brightening, however, boiling in a solution of soap is in all cases necessary.
If the furniture, such as rough or finished cotton or cambric, intended for yellow linings for bed or window curtains, be in a perfectly bleached state, which is now generally the case, according to the number of the {125} pieces, so must the size of the copper be to boil the weld in for the yellow dye. A small copper holding four or five pails would do for three pieces of twenty eight yards each. The weld may be purchased by the half bundle, the bundle, or the load. Half a bundle would be enough for the above quantity of cotton, if a moderate yellow is wanted. The weld must be increased or decreased according as the pattern approaches a straw, a canary, a lemon, or towards a gold colour or orange.
The weld must be boiled about twenty minutes, the liquor then strained off into a proper tub, and the weld boiled again. While the boilings are going on, three tubs, being wine pipes cut in two, must be got ready and made particularly clean, being also previously seasoned for the work. One is to receive the boiled weld with some cold water to regulate it to the heat which the hand will bear; the other is for water, and as much alum liquor as will colour it and make it taste strong; and the third is to contain clear water to wash the furniture off.
Whatever yellow is in fashion (or indeed any fashionable colour,) has commonly a fashionable name. But if the dyer can, by his experience, proportion his drugs to the weakest, and from that to the strongest shade, let the name be what it may, after he has a set of patterns of his own dyeing, he will see, upon the first sight of any colour, how to set about it.
In the present instance let the pattern be a moderately pale colour of yellow; then put all the first boiling of the weld in the first tub, and cool down as above directed. Two or three persons should then work the pieces quick from end to end by the selvages, that they may be {126} even, two may do this; one of whom must be an expeditious hand to work them and keep them even. When they have been edged over six or seven times, they are to be folded out upon a board laid over the tub, and wrung as dry as possible by two persons. When they are all out, they are passed in the same manner through the tub of alum, and, after six or seven turns, they are to be taken out of the alum liquor, wrung as before, and then washed off.
By this time the second weld liquor will be boiled; some of the first must be thrown away, and the second weld liquor added in its place. The goods are then passed through as before, and wrung out; the alum liquor being strengthened, they are passed through it, wrung out as before, and then washed off: the water in the wash tub having been changed.
In some instances verdigris is used instead of alum; and in other cases it is used in addition to the alum. For some shades old fustic is used instead of weld, and sulphate of copper instead of verdigris.
The alum solution, and the sulphate of copper, and the verdigris, or acetate of copper should be always ready. It is necessary to have a tub for each, in size proportioned to the work to be done; but larger for the alum than for the other two.
Sulphate of iron is also used in some dark greys, browns, slates, and in all blacks; this will require a tub as large or larger than that for alum.
When the yellows are dyed and wrung as dry as possible, they should be taken into a close room or stove to dry, particularly in London, because of the smoke, {127} especially in winter. A German, or other stove, should be placed in the room, the size of which, as well as the number of the stoves, must be regulated by the quantity of the work. When the goods are dry they must be sent to the callenderers, if directed to be callendered; but the general and better way is to stiffen them with starch after they are dyed, and before they are dry; and when dry they should be sent to the glazers, instead of the callenderers, except when both branches are carried on by the same person.
When furniture, originally yellow, has become faded, it may be re-dyed thus: In this case it should be dyed rather of a fuller shade than the original. A large flat tub, such as described above, is to be filled three parts full of water, to which sufficient sulphuric acid must be added to make it taste strongly sour. After being well stirred, the pieces are to be put in, and worked in this sour liquor; and the yellow dye in consequence is stripped off. If the acid liquor be not strong enough more acid must be added, with the precaution of well mixing it with the water, and the goods must be passed through the liquor again: by these means the yellow is discharged. They are then to be taken out on a board upon the tub and wrung by two persons; then to be washed off and wrung, washed and wrung again, when they are fit to be dyed.
It is still to be remembered that any faded or worn out colour, or that goods more or less decayed, seldom become so bright as the colour which a new piece of goods receives from the same dye.
Some cloths for re-dyeing require the application of {128} oxymuriate or chloride of lime to discharge their colours, particularly when madder or galls, &c. form the constituent parts of the dye. In this case if a bleacher be near it might be best to let him perform the process with the oxymuriate of lime; not only from the pernicious nature, but also from the expense of it, which, unless the business be upon a large scale, will not pay the dyer for his trouble.
However, if the dyer thinks proper to perform this operation, then the oxymuriate of lime or bleacher's ashes, &c. may be obtained at the dry salters and dissolved in a cask, and the clear liquor used in proportion to the quantity of goods, the colour of which is intended to be discharged, which, when done, should be washed off in two waters at least before they are dyed.
This is performed by a blue ground, next galling, dipping in the black vat, then in the weld dye, then in verdigris, remembering to wash off previously to performing each process.
Olive is to be performed with weld or old fustic, verdigris, and Brazil wood.
It would answer little purpose to enlarge this treatise with a detail of all the possible methods of producing the various shades of these several colours, the whole {129} consisting in the use of galls, verdigris, sulphate of copper, weld, and madder.
By welding a stuff previously maddered for red you may produce a gold colour; and by dipping the same red in a blue vat you obtain a plum colour.
Silk, as it is obtained from the cocoons of the worm, is generally of an orange or yellow colour, more or less dark; in the South of France it is generally very dark: its natural shade is unfavourable to almost all other colours. It is also imbued with a kind of varnish or gum, which makes it stiff and hard; this stiffness is improper in the fabrication of most silk stuff, it is therefore ungummed, as it is called, by the following processes.
Observe, that throughout the following processes for silk white soap is directed to be used; and, generally speaking, we believe it will be found the best, more especially for the more delicate operations. Yet Mr. M'Kernan, in his process for ungumming silk, directs yellow soap and soft soap in equal parts, and of the same weight as the silk to be used: he adds, however, that different sorts of silk require more or less soap; the best rule he finds, nevertheless, is the same weight of soap as of silk: and he says also, that yellow soap and soft soap of the best quality he finds the best for this purpose.
The silk is divided into hanks, each hank is tied with {130} a string, several of these are tied together (a handful of them) by putting a piece of string through each separate skein, and tying the piece of string in a long tie to slip easily when they are wanted to be untied.
A liquor is prepared of thirty pounds of white soap to a hundred pounds of silk; the soap is cut into small pieces and boiled in water, when it is dissolved the fire is damped.
While the liquor is preparing the skeins of silk are put on rods; as soon as the soap liquor becomes a little below boiling heat (for it should not boil, as boiling would tangle the silk) the silk is to be put into it in an oblong copper, being nearly full; it is to remain in the liquor till its gummy matter has left it, which will be seen by its whiteness and flexibility. It is then turned end for end on the rods, that the part above the liquor may undergo the same operation. As soon as this is accomplished the silk is taken out of the copper, the hanks which were first turned being soonest done.
The hanks are now to be taken from the rods to the peg, disentangled, and nine or ten of them put on one cord, this cord passing through the string that tied each hank. When the whole is corded it is put into pockets of coarse strong white linen fifteen inches wide and five feet long, closed at each end and on one side; when the silk is put in, the pocket is sewed all along the other side with packthread, and fastened with a knot; four pockets will hold the whole hundred pounds.
The pockets being thus ready another liquor is prepared like the first. When ready, and the boiling checked with cold water, the pockets are put in and {131} boiled well for a quarter of an hour, checking with cold water in order to prevent its boiling over; it is necessary also to turn the bags about often with a pole, or rather let two persons have a pole each for this purpose. This operation is called boiling.
In addition to the processes of boiling with soap, as above directed, Mr. M'Kernan recommends that the silk should be winched through a copper of water at the heat of 160°, having two pounds of soda (barilla) dissolved in it, then winch or wash in water, and wring and dry.
In the boiling of silks for common colours twenty pounds of soap will do for a hundred weight of silk; but, as in this case, the silk is not ungummed, it should boil for three hours and a half, adding water to supply the evaporation.
The silks intended for the greatest degree of white, either to remain white, or for the fabrication of white stuff, are boiled twice in soap and water; those that are to be dyed of different colours are boiled but once, and with a smaller quantity of soap, because the little remaining redness is by no means prejudicial to many colours. Different quantities of soap are, however, necessary for different colours.
Silk designed for blue, iron grey, brimstone, or any other colour requiring a very white ground, should be done according to the preceding process, and have thirty pounds of soap.
When the silk is boiled it is taken out of the copper by two men with poles, and placed in a clean barrow; they are then taken to a long shallow trough, from which the water may run away, the pockets are opened, and {132} the silks examined; such as have yellow or lemon colour spots remaining are boiled again for some time, till the spots are removed. After unpocketing, the whole is dressed on the pegs.
Silk loses from twenty-five to twenty-eight per cent. of its weight in ungumming and whitening. The bags of silk should never be suffered to lie long together before they are emptied after being boiled, as their doing so would make the silk hard.
White silk, as before observed, is distinguished into five principal shades, namely, China white, India white, thread or milk white, silver white, and azure white.
The three first are prepared and boiled as has already been shewn. Silver and azure white in the preparation or ungumming thus: take fine powdered indigo, put it into water boiling hot, when settled the liquor is called azure.
To azure the silk it is taken from the ungumming copper after it is dressed and put into a trough of water; after it is worked, drained, and again dressed, it is ready for the
Put into a copper with thirty pails of water half a pound of soap; when it boils, and the soap dissolved, add for China white a little prepared annatto, (of which hereafter.) The silk, being on rods, is now to be put into the copper and kept turning end for end without intermission till the shade is uniform. For India white a little azure is added, to give the blue shade: for thread white and others a little azure is also to be added.
{133} Observe, the liquor should be very hot, but not boiling; the turnings five times repeated, by which the shade is made even. When finished it is taken out, wrung, Spread on poles to dry, and that part of it required for sulphuring must be put upon rods or slight poles.
The hanks, being upon poles seven or eight feet from the ground, in an appropriate room, one pound and a half or two pounds of roll brimstone will sulphur a hundred weight of silk.
Put the brimstone, coarsely powdered, into an earthen pipkin with a little charcoal or small coal at bottom. Light one of the bits with a candle, which will kindle all the rest.
The room should be close, the chimney, if any, being closed up; the sulphur should burn under the silk all night. The next morning the windows should be opened to let out the smoke and admit the air, which, in summer, will be sufficient to dry the silk; but in winter, as soon as the sulphurous fumes are dissipated, the windows must be shut and a fire kindled in the stove or stoves to dry the silk.
Observe, if the room for sulphuring does not admit of openings sufficient for the dissipation of the sulphuric fumes, the work-people will be in danger of suffocation.
When the sulphur is consumed it leaves a black crust which will light the future sulphur like spirit of wine.
If, in dressing, the silk sticks together, it is not sufficiently dry.
{134} Silk, which has been sulphured, has a rustling, which, for some things, is esteemed; but this would not do for silk to be watered. If silk, which has been sulphured is to be dyed, it must, for many colours, be unsulphured.
Silks for lace, gauze, &c. are neither boiled nor ungummed; silks which are naturally the whitest are the best for those articles. It is sufficient to dip the silks in warm water, and wring them; then sulphur them, afterwards azure them, again wring them, sulphur them a second time, or soak them in soap and water, those for whitening hot enough to bear the hand, adding azure, if necessary, and turning and re-turning the silk in this liquor.
The fine silk of Nankin requires no whitening.
We have treated of this before at the commencement of the third chapter, but a few more observations may be useful.
The silk being first well washed and beetled, and the hanks tied loose so that every thread may take alike, should be turned and re-turned in the alum liquor and worked, cooled in it, at intervals, from morning till night, afterwards taken out, beetled, and rinsed.
The above proportion of alum will do for a hundred and fifty pounds of silk, before you need replenish it; when this is necessary add twenty-five pounds more of alum, as at first directed in Chapter III., and so continue to replenish it till it gets a bad smell. When this is the {135} case you may dip for browns, maroons, &c.; and afterwards throw the liquor away; the trough is then to be rinsed for a fresh liquor.
Remember always to alum cold or you will spoil the lustre of the silk.
This is to be boiled with about twenty pounds of soap for every hundred pounds of silk. When boiled it is to be washed and alumed, and again washed, dressed, and put on the rod, seven or eight ounces to a rod, and then dipped and re-turned in the yellow liquor, in the proportion of two pounds of weld to one pound of silk.
The liquor is not to be hotter than the hand can bear while the silk is in it. The silk, when in the vessel for dyeing, should cause the liquor to float within two inches of the edge. The silk must be taken out and the liquor strengthened, if the pattern is to be very full; when full enough, one pound of pearl-ash for every twenty pounds of silk must be dissolved in some warm water; about a quarter of this liquor is put into the dye bath: take the silk out while you put in the liquor, stir the mixture well. Put in the silk and work it, turning and re-turning it as at first. After seven or eight re-turns, one of the hanks is to be taken out, wrung, and tried at the peg, and, if sufficiently full and bright, all is well; if not enough so, some more pearl-ash liquor must be added, and the silk worked as before, till the shade required is obtained.
For jonquil it may be necessary to add some annatto when you put in the pearl-ash.
{136} To make the light shades, such as canary or lemon, perfectly white, they must be boiled with thirty pounds of soap to a hundred of silk; and if these be not azured to be dyed, they must have a little of the blue vat, and a little of the weld liquor in water, (the whole mixture being as hot, but no hotter than the hand will bear,) and the silk ready on rods, must be quickly worked through and out. For deeper lemons the same process must be used as for the fuller yellows; only less weld, and twenty pounds of soap will do for a hundred pounds of silk in whitening it.
The blue of the vat is only used for such articles as are to have a green cast, and that extremely light; the aluming also should be in a weaker alum liquor: for light lemons it should be prepared in a separate liquor.
You must have a colander proportioned to the size of the copper in which you boil the annatto. To every pound of annatto put from twelve ounces to one pound of pearl-ashes, which last dissolve in water, and add the solution, by degrees, to the solution of annatto as it boils and dissolves, for which purpose the annatto must be suspended in the colander over the copper by a flat stick about six inches broad, run through a flat handle on each side of the colander, by which means the colander is kept sunk in the water with the annatto in it, till it is all dissolved, except some little foreign matters. The holes in the colander should be moderately small.
{137} Dissolved in this manner the annatto, if kept clean, will keep as long as you please.
These require but twenty pounds of soap for boiling white. To dye aurora the silk must be prepared the same as for yellow.
Annatto prepared (as directed in the last article) and settled, is then put into a copper of hot water, in quantity according to the shade required; having mixed it well, the liquor being as hot as the hand will bear, put the silk into it; when one hank is tried, as in the yellow, if it be not full enough, the liquor must be strengthened till the colour is brought to the shade required. When finished the whole must be washed twice and beetled. The aurora serves as a ground for moidore.
As fustic and logwood are to form part of this dye upon the annatto ground, the silk must be alumed, then washed from the alum, in order that the superflux of the alum may not render the dye uneven. A fresh liquor is then prepared, rather hot, to which must be added a little of the decoction of logwood, and of the decoction of young fustic. The silk is re-turned in this liquor, but if apparently too red, you may put in a very little of solution of sulphate of iron, which will make it sufficiently yellow.
When the silk is dyed with the gum, in the raw state, the annatto must be used nearly cold, or the elasticity of the silk will be destroyed.
After dyeing aurora with annatto, it is necessary to redden the annatto ground with vinegar, alum or lemon juice.
For the brightest oranges, and up to scarlets and poppy, &c. silk should have an annatto ground three or four shades under that of aurora. There is no occasion for alum when the silk has been grounded and washed off. If for orange a liquor which has been used for poppy will be sufficiently strong to finish it, or for light cherry, rose, &c. For flesh, the lightest of these colours is so delicate that a little of the soap water used for boiling should be added to the liquor, to prevent the silk from taking the colour too quickly or unevenly.
Liquors having safflower or weld in their composition, require to be immediately worked, as by keeping they lose their colour, that is, the safflower and its compounds, and are entirely spoiled. They are also always used cold, as the safflower cannot bear heat.
The safflower preparation has been before described in Chapter II. where the process of cotton pink is performed by its solution.
When the silk has received the annatto ground three shades less than for aurora, the safflower preparation must be ready, and turned by the solution of tartar as before described; the silk must also be well washed from the annatto ground; that the alkali used with the annatto {139} may not counteract the tartar of the safflower, a bath of which must be prepared as strong as possible, through which the silk must be worked six or seven times: for a full poppy it is necessary to pass the silk through four or five such liquors. Poppy is the deepest colour which can be done with the safflower. It has been before observed, that the liquors from the poppy, if used directly, will serve for orange, cherry, flesh, &c.
Archil, as described for crimson, with cochineal for wools as before described, is to be used on some occasions. In other cases some patterns have no ground of annatto.
The silk is to be grounded with annatto as before; when well washed off it must be alumed and washed off again; then passed through the decoction of Brazil wood, washed off again, again passed through a fresh decoction of Brazil wood; and every time that goods are passed through the dye, as has been before stated, they must be worked from end to end of the skeins, from five to seven times, to have them even, and to give them a full opportunity of combining with the colouring materials of the dye.
These repetitions must of course be in number proportionate to the slightness or intensity of the colour wanted. With the Brazil decoction it is necessary to mix well a little soap liquor, about five quarts to thirty pounds of silk. This keeps the alum used to receive the Brazil {140} decoction not only from producing a stiffness, but, on the contrary, preserves the silk soft and pliant.
The above poppy serves for a ground for brown red colours, by the addition of logwood. A decoction of logwood, Brazil wood, and old fustic, as has been before observed, should always be kept ready boiled.
Silk intended for the crimson of cochineal should have only twenty pounds of soap to one hundred pounds of silk, and no azure, because the natural yellow of the silk which remains is favourable to the intended colour.
The silk is to be strongly alumed and left in the alum from seven to eight hours, then washed and twice beetled at the river. Remember how the alum is to be worked, as to the manual part.
While this is doing, a liquor is to be thus got ready: take of blue and white galls from one to two ounces to each pound of silk, let them be well powdered and sifted; of fine cochineal, also well powdered and sifted, from two to three ounces, for every pound of silk; put these articles into pure soft water, and in a boiler made of grain-tin, (and not in what is commonly called tin, which is iron covered with tin, and which would utterly spoil the dye.) Neither would copper or brass suit as well as grain-tin. This has been observed before, (page 84.) in the article on dyeing wool scarlet. It ought, nevertheless, to be stated, that such tin boilers are difficult to be made of a certain size, and being liable, besides, to be melted without {141} great care. Many dyers therefore, still use copper boilers. When the cochineal and galls have boiled you add to the liquor for every pound of cochineal, about one ounce of solution of tin, which is called composition, and is made in the following manner:
Take one pound of nitric acid, two ounces of muriate of ammonia, six ounces of fine tin, prepared as mentioned under dyeing wool scarlet, water twelve ounces.
The muriate of ammonia, the prepared tin, and the water, are put into a stone jar, to which the nitric acid, is added, and the whole left to dissolve.
This composition contains much more tin and sal-ammoniac than is used for the scarlet of cochineal upon wool; it is, however, absolutely necessary.
An ounce of this composition, for every pound of silk, is to be added to the galls and cochineal when boiling. The boiler is then cooled down a little, the fire-door thrown open, the silk put in and worked from five to seven times, when the silk will have become pretty even as far as it is dyed. The copper is now again to be brought to boil; it should continue boiling, and the silk kept turning, for two hours; the fire is then taken from under the copper, and the silk is immersed entirely and left all night, or for seven or eight hours at least; it thus takes a full half shade. In the morning it is washed, twice beetled, wrung as usual, and hung up to dry.
The least tincture of sulphate of iron in the water saddens {142} the crimsons, takes off their yellow, and gives the violet cast; but if too much of the yellow is carried off, it may be restored by fustic. Nothing but sulphate of iron will sadden grain scarlets, logwood being quite useless for this purpose; sulphate of iron darkens greatly with galls. Macquer.
When the silk is boiling in the soap-liquor, add one ounce of annatto, for every pound of silk, working it through the colander as directed, (page 136.) but without the composition or tartar: in some shades, however, both composition and tartar are admitted. The solution applied to cochineal with worsted has a considerable effect, changing it from a crimson, its natural colour, to a very bright fire colour; but it produces only a crimson when applied to silk; it gives, however, this colour a very beautiful tint; for, uniting with the tartar, it increases the effect without impoverishing the colour, and saving the annatto ground. Macquer.
The silk should be first alumed, and then passed through a strong decoction of Brazil wood, half a pail to a pound of silk, which is to be worked, and put through an additional and strengthened dye of Brazil wood, and then washed off: if in hard water this will generally crimson the Brazil wood sufficiently; but if in soft water a little pearl-ash must be added; about one pound of the clear {143} solution of pearl-ash, or rather the clear solution of a pound of pearl-ash, as one pound of water will not, we believe, dissolve a pound of pearl-ash: this is enough for forty pounds of silk.
The decoction of Brazil wood is prepared thus: one hundred and fifty pounds of Brazil wood chips are put into a copper which holds about sixty buckets of water; the copper is then filled with water and boiled for three hours, the waste by evaporation being occasionally supplied. The fire is now damped, the clear liquor drawn off, the copper filled again, and again boiled for three hours more. This process is repeated four times in all, when the dye of the wood will be fully extracted.
Logwood and old fustic are treated in the same manner, but only two boilings are required for these.
In regard to crimson generally, see forward, observations on dyeing silk crimson and scarlet, and also some observations on the dyeing of wool scarlet, page 85.
For this colour the common boiling is enough, the silk is alumed the same as for fine scarlet, washed and twice beetled. Thus prepared, two ounces of cochineal are given to it, with the same precaution as usual, but no composition nor tartar. Being worked moderately warm, in working it must be expeditiously turned; after a quarter of an hour the liquor should be brought to boil, when the turning need not be so expeditious, but it should, nevertheless, be continued for two hours. After being washed the silk is dipped in the vat, more or less strong, according to the shade required.
{144} Washing and drying are done in the same manner as for blues and greens, and in general for all colours dipped in the vat, namely, a small quantity at a time, in order that the silk may be kept open to the air, and that the greening of the vat may pass correctly and equally to blue. For some shades archil forms a part of this dye. For other violets on silk see Chapter III.
Crimson upon silk is produced at Norwich, London, and many other places, by using a much larger quantity of cochineal than that which is directed by Macquer: for in some cases, as much as a guinea a pound, has, it is said, been paid for dyeing silk crimson at Norwich. Archil has been used, likewise, in crimson, and the time of boiling is not so long. In some shades a little of the composition and tartar may be admitted, but in a small degree. It should be stated, however, that scarlet upon silk, is often done by annatto and safflower.
Observe, that although we have given the preceding processes for crimson and scarlet, yet many others might be mentioned. What has been said in regard to dyeing scarlet on woollen, (page 85.) should also be carefully attended to, particularly relative to the conversion of scarlet into crimson by alum, soap, and the alkalies. And though we have given directions for the preparation of a nitro-muriate of tin, yet pure
is now very often used for dyeing silk red. Mr. M'Kernan, gives us the following process for preparing it:
{145} Take of fine muriatic acid, of the specific gravity of 1.120, two quarts; add by degrees, one ounce at a time, of feathered tin, for twenty-four hours. Put the vessel in a sand heat and bring it gently to boil, observing to add more tin as that in the acid becomes dissolved. There should be some tin left undissolved when the liquor is cold, thus indicating that the acid is perfectly neutralized by the tin. Bottle for use.
This colour is composed of blue and yellow. It is with difficulty produced on silk, because the blue vat is liable to spot and give a party colour, an inconvenience to which green is more liable than blue, and more perceptible. The boiling of silk for greens is the same as for common colours.
The silk being alumed as usual rather strongly, is washed off and divided on the sticks into small hanks of about four or five ounces, that it may be equally and easily managed in the working, from the yellow to green, in the blueing from the blue vat.
Weld is then boiled as stated in the article concerning yellow; when boiled, a liquor of it is prepared strong enough to give a lemon ground; the silk is then turned with all the expedition, care, and caution possible, that it may be even. When it appears full enough, some of the threads are to be separated and dipped in the vat, to determine this. If not full enough, more of the weld liquor must be added to the dye bath, and the silk returned and tried again, and so on; when the colour is {146} right, the silk is washed off and beetled. It is then wrung and formed into hanks, and dipped skein by skein in the blue vat, the same as the blue and the purple should be; it must be wrung with equal care and dispatch.
This green is a kind of sea-green, of which there are upwards of twenty shades. The lighter shades, when taken out of the vat, are not washed but the silk must be worked in the hands by clapping it between them, and then be carefully opened and aired. A few threads are then washed, or rinsed; if the colour be right the whole is washed.
For the dark shades, when the weld is exhausted a little logwood is added to the liquor; in some cases, old fustic, in some annatto.
For very dark-wing or bottle-green shades, a little sulphate of iron is required.
Proceed in aluming, &c. the same as for other colours; the weld liquor being stronger, some logwood must be added. When the weld and logwood are exhausted a very small quantity of each must be added, which green the liquor, when the silk being passed through, a greenish olive is produced.
A reddish olive requires fustic, instead of logwood and pearl-ash, both of these being omitted.
Fustic gives a colour commonly called drab-olive upon cloth, because generally made to match with olive, this is commonly redder than the preceding.
All the greys, namely, nut-greys, thorn-greys, black and iron-greys, and others of the same hue, black-grey excepted, are produced without aluming. The silk being washed from the soap and drained on the peg, a liquor is made of fustic, archil, logwood and sulphate of iron: fustic gives the ground, archil the red, logwood darkens, and the sulphate of iron softens all these colours, turns them grey, and, at the same time, serves instead of alum as a mordant.
As there is an infinite variety of greys, without any positive names, produced by the same methods, it would be endless to enter into details, which would prolong this treatise to little purpose.
For reddish-grey the archil should predominate; for those more grey, the logwood; and for those rather greenish, the fustic.
Care should be taken not to use the logwood too much, as with the sulphate of iron it darkens more than most drugs: therefore the black vat, made either with alder-bark, or the other preparation mentioned in dyeing cotton, is preferable to the sulphate of iron.
The fustic decoction, archil, and a little logwood are put into water moderately hot, the silk is then returned, and when the liquor is exhausted, the silk is taken out, and to soften the colour the solution of sulphate of iron, or the black vat, is used. The silk is then returned once more, and if the colour does not appear sufficiently even, {148} some red spots still remaining, it may be concluded that it requires a little more sulphate of iron.
Observe that, as sulphate of iron is the general base of all greys, if this be deficient in quantity, the colour is apt to change in dyeing, and to become rough and uneven.
To know whether the colour be sufficiently softened, it should be examined, and if it wet easily, after having been wrung on the peg, it wants sulphate of iron. On the contrary, if it wets with a little difficulty, the colour is sufficiently softened.
Too much sulphate of iron stiffens the silk considerably, making it harsh, and even depriving it of a part of its lustre; to remedy this it must be extra washed and wrung at the peg; this process carries off the sulphate of iron.
These are alumed and welded as for yellow, and, when the liquor is exhausted, part of it is thrown away, and some logwood is added; when the logwood is exhausted, sulphate of iron is added, sufficient to blacken the colour, the silk is then washed, wrung, and finished in the usual way.
For iron-grey it is necessary to boil the same as for blues: this colour is much more beautiful when laid on a very white ground.
By having the drugs made into decoctions before-hand, greys either in woollen, silk, or cotton, may be dyed at a heat not much above what the hand will bear; and in {149} a rotation of shades from light to dark, and varied, blue, red, yellow, brown, &c. with ease and with pleasure; so may, likewise, many stone-drabs, and other light brown drabs, as the mixture of yellow, fawn, and black, produces nut-browns, &c.
The application of colours derived from the mineral kingdom to dyeing is one of the most striking modern improvements in our art. Mr. Raymond received from the French government in 1801, eight thousand francs, (more than three hundred pounds sterling,) as a reward for communicating to the public his process for dyeing silk of a uniform fast and bright Prussian-blue colour by the application of that well known pigment. His process is as follows.
He first converts, by a gentle calcination, sulphate of iron into a red sulphate of iron: this he dissolves in sixteen times its weight of warm water and filters. The silk, prepared as for indigo dye, is put into the solution of iron, and left there for a shorter or longer time, according to the shade of blue that is wanted; it is then taken out and wrung very dry over a pole placed above the vat. It is then thoroughly cleansed by being twice beetled, plunging and agitating it each time in running water. Dissolve in pure water heated to 167°, and put into a deal vat, one ounce of ferroprussiate of potash, for every twelve ounces of silk to be dyed. When the prussiate is dissolved add one part, or even rather more, of muriatic acid, stirring the mixture well. When the liquor has {150} acquired a greenish colour, and about 144° of heat, the silk must be immediately plunged into it and stirred about for some minutes. The silk having received the dye in an equal manner, it is taken out of the vat, well wrung on a pole above the vat, and then taken to the stream to receive two or three beetlings, and be plunged and agitated in the water, in order that it may be entirely freed from any portion of the prussiate of iron not truly combined with it.
Lastly, the silk being well washed in the stream, and thoroughly wrung, is to be placed loosely on the poles, as in the preceding operations; after which it must be well agitated in a large vessel three-fourths filled with cold water, to which must be added, for a hundred pounds of silk, two pounds of water of ammonia. The blue colour immediately becomes many shades deeper, of a much richer and brighter tint, and at the same time is fixed more perfectly in the silks. This change is effected in a few minutes. The silk must then be wrung by the hand and rinsed in the running water without beating. After this, it is dried on the poles in the same manner as other dyed silks. It need not be left on the poles more than twenty-four hours: but, nevertheless, this colour so far from fading in the drying, as is the case with many colours, is improved by it.
The solution of a little soap added cold to the ammonia bath, improves it, giving also softness to the silk, and rendering it more easy to separate. The soap should be uniformly dissolved.
For the substance of the above process, we are indebted {151} to Dr. Ure's notes on Berthollet, vol. ii. p. 422. The prussiate of potash is now to be obtained as a regular article of trade from the dry-salters in this country.
Woollen cloth takes also the above dye, but it must be left longer than silk in the iron mordant.
Chromate of lead, as a pigment has been for some time in use; M. Lassaigne, in 1820, made public a process for dyeing cloth with this article, which has since become pretty common in this country.
Immerse hanks of scoured silk for a quarter of an hour in a weak solution of acetate of lead at the ordinary temperature; take them out and wash them in a great deal of water: then dip them into a weak solution of chromate of potash. They immediately take a fine yellow colour; at the end of ten minutes the effect is complete. From this colour being decomposed in part by soap and water, it is chiefly applicable to silks. But by applying, however, a mordant of acetate or nitrate of lead, and passing the goods through bichromate of potash, a very beautiful and sufficiently fast yellow is now given to cotton goods in this country.
We cannot conclude our work without observing, that from the researches continually going on in botany and other branches of natural history, and, more especially, from those in chemistry, there can be no doubt that discoveries, {152} which will materially improve the art of dyeing, must, from time to time, be made. Some of these, not yet generally known, in the hands of a few persons, have already been found useful; but individual interest is, of course, a great enemy to their being made public. Others, although public, are, as yet, of too doubtful a utility to be noticed here.
If we have not given forms for the employment of some articles in use by certain dyers, such as kermes for reds; French Berries, (rhamnus infectorius,) the Canada golden rod (solidago Canadensis,) the Barberry (Berberis vulgaris,) and the French marygold, (Tagetes patula,) for yellows, &c. &c.; it is not to be concluded that such are not good in their kind, and might not be used occasionally with advantage. But as our object has been to give the best methods of dyeing the various colours, it would be impossible to notice many others in a manual of this kind, and in the limits within which we are necessarily confined. To mention those substances recently introduced into dyeing, the utility of which is not confirmed by extensive practice, would be injudicious, and tend to lead the young dyer astray; those, however, who have leisure and inclination, and are, besides, able to run the risk of the failure of new processes, may, and no doubt will, make experiments with them by which our art must be eventually served and improved.
THE END.
Printed by R. Gilbert, St. John's-Square, London.
End of the Project Gutenberg EBook of The Dyer's Guide, by Thomas Packer
*** END OF THIS PROJECT GUTENBERG EBOOK THE DYER'S GUIDE ***
***** This file should be named 53797-h.htm or 53797-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/5/3/7/9/53797/
Produced by Deaurider, Chris Pinfield and the Online
Distributed Proofreading Team at http://www.pgdp.net (This
file was produced from images generously made available
by The Internet Archive)
Updated editions will replace the previous one--the old editions will
be renamed.
Creating the works from print editions not protected by U.S. copyright
law means that no one owns a United States copyright in these works,
so the Foundation (and you!) can copy and distribute it in the United
States without permission and without paying copyright
royalties. Special rules, set forth in the General Terms of Use part
of this license, apply to copying and distributing Project
Gutenberg-tm electronic works to protect the PROJECT GUTENBERG-tm
concept and trademark. Project Gutenberg is a registered trademark,
and may not be used if you charge for the eBooks, unless you receive
specific permission. If you do not charge anything for copies of this
eBook, complying with the rules is very easy. You may use this eBook
for nearly any purpose such as creation of derivative works, reports,
performances and research. They may be modified and printed and given
away--you may do practically ANYTHING in the United States with eBooks
not protected by U.S. copyright law. Redistribution is subject to the
trademark license, especially commercial redistribution.
START: FULL LICENSE
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full
Project Gutenberg-tm License available with this file or online at
www.gutenberg.org/license.
Section 1. General Terms of Use and Redistributing Project
Gutenberg-tm electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or
destroy all copies of Project Gutenberg-tm electronic works in your
possession. If you paid a fee for obtaining a copy of or access to a
Project Gutenberg-tm electronic work and you do not agree to be bound
by the terms of this agreement, you may obtain a refund from the
person or entity to whom you paid the fee as set forth in paragraph
1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this
agreement and help preserve free future access to Project Gutenberg-tm
electronic works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the
Foundation" or PGLAF), owns a compilation copyright in the collection
of Project Gutenberg-tm electronic works. Nearly all the individual
works in the collection are in the public domain in the United
States. If an individual work is unprotected by copyright law in the
United States and you are located in the United States, we do not
claim a right to prevent you from copying, distributing, performing,
displaying or creating derivative works based on the work as long as
all references to Project Gutenberg are removed. Of course, we hope
that you will support the Project Gutenberg-tm mission of promoting
free access to electronic works by freely sharing Project Gutenberg-tm
works in compliance with the terms of this agreement for keeping the
Project Gutenberg-tm name associated with the work. You can easily
comply with the terms of this agreement by keeping this work in the
same format with its attached full Project Gutenberg-tm License when
you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are
in a constant state of change. If you are outside the United States,
check the laws of your country in addition to the terms of this
agreement before downloading, copying, displaying, performing,
distributing or creating derivative works based on this work or any
other Project Gutenberg-tm work. The Foundation makes no
representations concerning the copyright status of any work in any
country outside the United States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other
immediate access to, the full Project Gutenberg-tm License must appear
prominently whenever any copy of a Project Gutenberg-tm work (any work
on which the phrase "Project Gutenberg" appears, or with which the
phrase "Project Gutenberg" is associated) is accessed, displayed,
performed, viewed, copied or distributed:
This eBook is for the use of anyone anywhere in the United States and
most other parts of the world at no cost and with almost no
restrictions whatsoever. You may copy it, give it away or re-use it
under the terms of the Project Gutenberg License included with this
eBook or online at www.gutenberg.org. If you are not located in the
United States, you'll have to check the laws of the country where you
are located before using this ebook.
1.E.2. If an individual Project Gutenberg-tm electronic work is
derived from texts not protected by U.S. copyright law (does not
contain a notice indicating that it is posted with permission of the
copyright holder), the work can be copied and distributed to anyone in
the United States without paying any fees or charges. If you are
redistributing or providing access to a work with the phrase "Project
Gutenberg" associated with or appearing on the work, you must comply
either with the requirements of paragraphs 1.E.1 through 1.E.7 or
obtain permission for the use of the work and the Project Gutenberg-tm
trademark as set forth in paragraphs 1.E.8 or 1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any
additional terms imposed by the copyright holder. Additional terms
will be linked to the Project Gutenberg-tm License for all works
posted with the permission of the copyright holder found at the
beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including
any word processing or hypertext form. However, if you provide access
to or distribute copies of a Project Gutenberg-tm work in a format
other than "Plain Vanilla ASCII" or other format used in the official
version posted on the official Project Gutenberg-tm web site
(www.gutenberg.org), you must, at no additional cost, fee or expense
to the user, provide a copy, a means of exporting a copy, or a means
of obtaining a copy upon request, of the work in its original "Plain
Vanilla ASCII" or other form. Any alternate format must include the
full Project Gutenberg-tm License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works
provided that
* You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is owed
to the owner of the Project Gutenberg-tm trademark, but he has
agreed to donate royalties under this paragraph to the Project
Gutenberg Literary Archive Foundation. Royalty payments must be paid
within 60 days following each date on which you prepare (or are
legally required to prepare) your periodic tax returns. Royalty
payments should be clearly marked as such and sent to the Project
Gutenberg Literary Archive Foundation at the address specified in
Section 4, "Information about donations to the Project Gutenberg
Literary Archive Foundation."
* You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or destroy all
copies of the works possessed in a physical medium and discontinue
all use of and all access to other copies of Project Gutenberg-tm
works.
* You provide, in accordance with paragraph 1.F.3, a full refund of
any money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days of
receipt of the work.
* You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project
Gutenberg-tm electronic work or group of works on different terms than
are set forth in this agreement, you must obtain permission in writing
from both the Project Gutenberg Literary Archive Foundation and The
Project Gutenberg Trademark LLC, the owner of the Project Gutenberg-tm
trademark. Contact the Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
works not protected by U.S. copyright law in creating the Project
Gutenberg-tm collection. Despite these efforts, Project Gutenberg-tm
electronic works, and the medium on which they may be stored, may
contain "Defects," such as, but not limited to, incomplete, inaccurate
or corrupt data, transcription errors, a copyright or other
intellectual property infringement, a defective or damaged disk or
other medium, a computer virus, or computer codes that damage or
cannot be read by your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH 1.F.3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium
with your written explanation. The person or entity that provided you
with the defective work may elect to provide a replacement copy in
lieu of a refund. If you received the work electronically, the person
or entity providing it to you may choose to give you a second
opportunity to receive the work electronically in lieu of a refund. If
the second copy is also defective, you may demand a refund in writing
without further opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS', WITH NO
OTHER WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT
LIMITED TO WARRANTIES OF MERCHANTABILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of
damages. If any disclaimer or limitation set forth in this agreement
violates the law of the state applicable to this agreement, the
agreement shall be interpreted to make the maximum disclaimer or
limitation permitted by the applicable state law. The invalidity or
unenforceability of any provision of this agreement shall not void the
remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in
accordance with this agreement, and any volunteers associated with the
production, promotion and distribution of Project Gutenberg-tm
electronic works, harmless from all liability, costs and expenses,
including legal fees, that arise directly or indirectly from any of
the following which you do or cause to occur: (a) distribution of this
or any Project Gutenberg-tm work, (b) alteration, modification, or
additions or deletions to any Project Gutenberg-tm work, and (c) any
Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of
computers including obsolete, old, middle-aged and new computers. It
exists because of the efforts of hundreds of volunteers and donations
from people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need are critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future
generations. To learn more about the Project Gutenberg Literary
Archive Foundation and how your efforts and donations can help, see
Sections 3 and 4 and the Foundation information page at
www.gutenberg.org
Section 3. Information about the Project Gutenberg Literary Archive Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Contributions to the Project Gutenberg Literary
Archive Foundation are tax deductible to the full extent permitted by
U.S. federal laws and your state's laws.
The Foundation's principal office is in Fairbanks, Alaska, with the
mailing address: PO Box 750175, Fairbanks, AK 99775, but its
volunteers and employees are scattered throughout numerous
locations. Its business office is located at 809 North 1500 West, Salt
Lake City, UT 84116, (801) 596-1887. Email contact links and up to
date contact information can be found at the Foundation's web site and
official page at www.gutenberg.org/contact
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
[email protected]
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To SEND
DONATIONS or determine the status of compliance for any particular
state visit www.gutenberg.org/donate
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including checks, online payments and credit card donations. To
donate, please visit: www.gutenberg.org/donate
Section 5. General Information About Project Gutenberg-tm electronic works.
Professor Michael S. Hart was the originator of the Project
Gutenberg-tm concept of a library of electronic works that could be
freely shared with anyone. For forty years, he produced and
distributed Project Gutenberg-tm eBooks with only a loose network of
volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as not protected by copyright in
the U.S. unless a copyright notice is included. Thus, we do not
necessarily keep eBooks in compliance with any particular paper
edition.
Most people start at our Web site which has the main PG search
facility: www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.