Project Gutenberg's On the Existence of Active Oxygen, by Edward H. Keiser
This eBook is for the use of anyone anywhere in the United States and most
other parts of the world at no cost and with almost no restrictions
whatsoever. You may copy it, give it away or re-use it under the terms of
the Project Gutenberg License included with this eBook or online at
www.gutenberg.org. If you are not located in the United States, you'll have
to check the laws of the country where you are located before using this ebook.
Title: On the Existence of Active Oxygen
Thesis Presented for the Attainment of the Degree of Doctor
of Philosophy at the Johns Hopkins University
Author: Edward H. Keiser
Release Date: May 25, 2016 [EBook #52162]
Language: English
Character set encoding: UTF-8
*** START OF THIS PROJECT GUTENBERG EBOOK ON THE EXISTENCE OF ACTIVE OXYGEN ***
Produced by Paul Marshall and the Online Distributed
Proofreading Team at http://www.pgdp.net (This file was
produced from images generously made available by The
Internet Archive)
Thesis
presented for the attainment of
the degree of Doctor of Philosophy
at the Johns Hopkins University.
by
Edward H. Keiser.
Baltimore,
1884
That a gaseous element can exist in an allotropic condition was first clearly shown by a careful study of the properties of ozone. Although discovered by Schönbein in 1840, chemists were for a long time unable to determine its true nature, and it was not until seven years later that Marignac[1] succeeded in proving that it was oxygen in an allotropic condition. [Pg 4] [Pg 5] Marignac’s work was confirmed by De la Rive, and subsequently the elaborate researches of Andrews and Tait, and Soret, as well as those of von Bato and Claus have established beyond all question that ozone is an allotropic modification of oxygen, and that its density is one and a half times that of ordinary oxygen.
The possibility of the existence of allotropic modifications of oxygen having been thus established it is not surprising that attempts should have been made to find other forms in which this element might occur. As early as 1855 Houzeau[2] stated that when barium superoxide was decomposed with concentrated [Pg 6] [Pg 7] sulphuric acid, at low temperatures, a colorless gas was evolved which oxidized metals and ammonia. It had a penetrating odor and possessed the power of bleaching litmus paper, and liberated iodine from potassium iodide. By heating the gas to a temperature of 75°C it was completely converted into ordinary oxygen. He calls the gas nascent oxygen and further states that it is probable that whenever oxygen is set free from any of its compounds at low temperatures it is in the nascent or active state.
Clausius[3] at one time supposed that free atoms of oxygen might exist in an uncombined [Pg 8] [Pg 9] state, and his hypothesis on the nature of ozone was that this substance consisted of a mixture of molecules and free atoms of oxygen. In a later paper[4], however, he abandoned this view and regarded ozone as consisting of one or more atoms of oxygen feebly united, (lose verbunden) with molecules of ordinary oxygen.
The idea that a third form of oxygen existed also obtained support from the fact that certain organic substances when exposed to the light in the presence of oxygen or air, acquire oxidizing properties. In 1850 Schönbein[5] stated that ether turpentine, and oil of lemons if allowed [Pg 10] [Pg 11] to stand in diffused light in contact with the air acquires the power of decomposing potassium iodide, and decolorizing indigo. In a subsequent paper[6] he shows that methyl and ethyl alcohols, tartaric and citric acids and even sulphuretted and arsenuretted hydrogen in the presence of sun light can decolorize indigo. These studies led Schönbein to publish a theory on the different modifications of oxygen. In this paper[7] he states that besides ordinary oxygen there are two other conditions in which it may exist one of these is ozone, or positively electrified oxygen, the other antozone or negatively electrified oxygen. The [Pg 12] [Pg 13] union of ozone and antozone gives ordinary oxygen. He also stated that antozone was formed by the action of light on turpentine and air, and subsequently in 1862[8] he claimed that antozone was identical with the gas obtained when barium superoxide is treated with acids. Meissner[9] also supported the views of Schönbein and claimed that antozone was formed in two ways:—1st, By treating barium dioxide with concentrated sulphuric acid, 2nd, By the electrification of oxygen; being produced simultaneously with the ozone. [Pg 14] [Pg 15]
These statements remained unquestioned for a number of years and are found in the text books of the period, (for example Graham-Otto, and Gorup-Besauez) but in 1870 Engler and Nasse[10] undertook a thorough investigation to determine whether antozone existed. By treating barium dioxide with strong sulphuric acid they find a gas to be given off which is a mixture of ozone and hydrogen dioxide, and they also show that the stronger the acid the greater the quantity of ozone produced. Secondly, Meissner had stated that by the electrification of oxygen ozone and antozone were formed. The evidence of the existence of antozone being this; when the ozonized oxygen was passed through a solution of potassium iodide to destroy the ozone, the residual gas gave white fumes when brought into contact with water, and [Pg 16] [Pg 17] after a time hydrogen dioxide could be detected in the water. Schönbein and Meissner held that the ozone having been destroyed by the potassium iodide the antozone passed on and oxidized the water to hydrogen dioxide. Now Engler and Nasse show that when ozone is decomposed by easily oxidisable substances in the presence of water hydrogen dioxide also is formed, and it was the vapor of this compound which had been regarded as antozone. It is known that ozone cannot oxidize water, but that it is to a slight extent oxidized when other oxidisable substances are present is not surprising, as other phenomena of a similar kind are [Pg 18] [Pg 19] known. Thus when nitric acid acts on an alloy of silver with gold or platinum, containing a certain proportion of silver, some of the gold or platinum are dissolved although by themselves they are insoluble. When ammonia burns some of the nitrogen as well as the hydrogen is oxidized. Engler and Nasse therefore conclude that there is no basis for the assumption of a third form of oxygen having the properties attributed to antozone.
Berthelot[11] and Houzeau[12] conclude from their investigations that the oxidizing properties which turpentine and other organic compounds acquire under certain conditions is due to the formation of unstable oxygenated compounds which readily decompose giving up oxygen. [Pg 20] [Pg 21]
Fudakowski[13] has described experiments showing that benzene can become active, i.e. acquire oxidizing properties, but states that he is unable to explain the phenomenon. Loew[14], however, believes active turpentine to contain atomic oxygen or antozone in solution.
After Engler and Nasse had demonstrated the nonexistence of antozone all discussion on the subject ceased for a number of years, and it was not until 1878 that Hoppe-Seyler[15] again opened the question. In studying the processes of putrefaction he observed that free hydrogen is given off in those cases in which oxygen [Pg 22] [Pg 23] is not present, and that whenever oxygen has access to decaying liquids, not only is all the hydrogen oxidized but energetic oxidation processes are observed as well. The simplest explanation of this seemed to be that the nascent hydrogen has the power of splitting up the oxygen molecule, uniting with one atom and setting the other free, and these free atoms he imagined brought about the strong oxidations which take place in decaying bodies. [Pg 24] [Pg 25]
To test this hypothesis he made experiments with palladium hydrogen. Graham has described the energetic reducing power of this compound but that it can also cause oxidations Hoppe-Seyler showed by bringing some strips of palladium charged with hydrogen into a solution of indigo in the presence of air. The solution soon became yellow and after a time the indigo was completely destroyed. If palladium hydrogen be brought into a neutral solution of potassium iodide and starch, the liquid becomes blue in a few minutes, after which the starch is slowly destroyed. In a similar way benzene was oxidized to phenol. “These experiments and others of a similar nature,” he asserts, “admit of no explanation other than that the active hydrogen renders the oxygen [Pg 26] [Pg 27] active, and since the former unites with oxygen we cannot well conceive of the process without supposing that the hydrogen in uniting with one atom of the molecule O2 sets the remaining atom free, thus making it active.” “Just as the hydrogen atom cannot exist in a free state so the active oxygen, if no oxidizable material is present, unites with water to form hydrogen dioxide, or with inactive oxygen to form ozone.”
This theory has been taken up and developed by Baumann, who in 1881 published a paper[16] entitled “Contribution to the knowledge of Active Oxygen.” The paper [Pg 28] [Pg 29] begins with the statement that besides ordinary inactive oxygen and ozone there is a third modification known as active or nascent oxygen. He states that this active oxygen cannot be isolated, and its formation can only be recognized by its action on other bodies. “Active oxygen (O) is the most powerful oxidizing substance known and can unite with inactive oxygen (O2) to form ozone (O3). The production of ozone is always preceded by the formation of active oxygen,” but he states “active oxygen can be formed under conditions when no ozone can be formed, this is the case when easily oxidized substances are in contact with the active oxygen in such a way that the [Pg 30] [Pg 31] latter is completely consumed in oxidizing those substances.” “Thus, for instance, ozone is formed when oxygen is rendered active by the slow combustion of moist phosphorus in air, but no ozone is formed if the atmosphere surrounding the phosphorus contains the vapor of alcohol, ether and similar substances.” The fallacy of this reasoning becomes apparent on referring to the work of Müller[17] on the luminosity of phosphorus who shows that substances which prevent the luminosity also prevent its oxidation and if the phosphorus is not oxidized we have no reason for assuming the formation of active oxygen. [Pg 32] [Pg 33]
He then compares active oxygen with the antozone of Schönbein and says that several of the properties of antozone can be ascribed to active oxygen above all that property of antozone of oxidizing water to hydrogen dioxide. The only difference between the two being that active oxygen has but a momentary existence while antozone was supposed to be capable of isolation.
Baumann then describes results obtained by himself which enable us to clearly distinguish between active oxygen and ozone. Starting from the observation of Remsen[18] and Southworth that carbon monoxide, at ordinary temperatures, is not oxidized by ozone, he suspected that active oxygen would readily effect [Pg 34] [Pg 35] its oxidation. Palladium hydrogen was therefore sealed up in a capacious glass tube with a few cubic centimeters of clear lime water and a mixture of carbon monoxide and oxygen free from carbon dioxide. At first the lime water remained clear, but after several hours a cloudiness in the lime water became visible and after several days a precipitate of calcium carbonate settled to the bottom of the tube. He then repeated the experiment in a modified form. From a gasometer, containing a mixture of three volumes of oxygen and one of carbon monoxide free from carbon dioxide a slow current of gas was passed, [Pg 36] [Pg 37] first into a wash bottle containing a clear solution of baryta water, then into a tube containing palladium hydrogen. Then the gases were again passed through a wash bottle containing baryta water. After the current had been passing for four hours, the first baryta water was still perfectly clear, but the second showed a distinct cloudiness of barium carbonate, which slowly increased in the course of twelve hours. The baryta water in the first wash bottle remained clear even to the end of the experiment.
The different behavior of ozone and active oxygen was then shown by the following experiment:—“A slow current of air free from carbon dioxide was passed into a flask containing moist phosphorus, from there into a [Pg 38] [Pg 39] second flask where the ozonized air came in contact with a somewhat slower current, consisting of a mixture of three volumes of oxygen to one of carbon monoxide, carefully purified from carbon dioxide. From the second flask the gases were passed through a clear solution of baryta water.” “After all carbon dioxide had been removed from the apparatus the baryta water remained perfectly clear (völlig klar) after the gases had passed through for six hours.” “But, on the other hand, if the mixture of carbon monoxide and oxygen was passed into the first flask, containing the moist phosphorus and in which according to our [Pg 40] [Pg 41] theory active oxygen must occur, then the result is quite different, the baryta water becomes cloudy in a short time and in the course of an hour there is formed an abundant precipitate (‘reichlicher Niederschlag’) of barium carbonate.”
From these results he concludes that active oxygen may be detected by its power of oxidizing carbon monoxide, and states that this fact enables us to decide whether in oxidations effected by ozone there occur free atoms of oxygen.
Closely related to these experiments of Baumann are those of Professor Remsen[19] on the transformation of ozone into oxygen by heat. Now if atoms of oxygen can exist in the free state, it is difficult to see why [Pg 42] [Pg 43] transformation some of the oxygen atoms should not be in the free condition, and the statements of Baumann being true, if carbon monoxide is also present this should be oxidized. To test the question a gasometer was filled with carbon monoxide made from potassium ferrocyanide and sulphuric acid. Before entering the gasometer the gas was purified by passing through four wash bottles containing concentrated sodium hydroxide. Another gasometer was filled with pure oxygen. The ozone was produced by the silent electric discharge in a Wright’s tube connected with a Stoltz electrical machine. In detail the experiments were conducted as follows:—[Pg 44]
[Pg 45] A slow current of oxygen from the gasometer was passed through three woulfe bottles containing a concentrated solution of caustic soda and then into the ozonizer, the ozonized oxygen was then passed into a U tube, rubber joints between the ozonizer and U tube were found to be rapidly perforated and were replaced by a device of this kind:—

A, the tube from the ozonizer was introduced several inches into B, the tube leading to the U tube, and the joint C was closed by a cement composed of beeswax and paraffin. The carbon monoxide was passed [Pg 46] [Pg 47] through wash bottles containing caustic soda and finally through baryta water. The two gases were then brought together in a U tube placed in an air bath. After leaving the U tube the gases passed through perfectly clear lime water. Under these conditions the current of the gases was continued for an hour, and no precipitate was formed in the lime water.
“Separate experiments were made for the purpose of determining how readily the ozone was destroyed, and it was found that, even when the thermometer in the U tube indicated a temperature considerably below that stated as the decomposition temperature of ozone, and when highly [Pg 48] [Pg 49] ozonized oxygen was certainly entering the U tube, no ozone passed out, whether carbon monoxide was present or not in the tube at the same time.” The experiment as thus described was repeated several times, but always with the same result. “One modification of the experiment should also be mentioned in this connection. In order to get as good ozone as possible the ionizer was filled with oxygen and the current of gas stopper, the silent discharge was allowed to continue for a few minutes, then the gas was slowly passed into the heated U tube containing carbon monoxide. This interrupted current of oxygen was [Pg 50] [Pg 51] continued for about an hour but no oxidation of carbon monoxide to dioxide could be detected.” The conclusion that must necessarily be drawn from the result is that if carbon monoxide is a test for active oxygen, then when ozone is decomposed by heat there is no nascent or active oxygen formed.
The negative result obtained in the preceding investigation, naturally called in question the accuracy of Baumann’s statements in regard to the formation of active oxygen by the slow oxidation of phosphorus, and of palladium hydrogen in the presence of oxygen and water. The two [Pg 52] [Pg 53] experiments upon which he had based his conclusion have been described on pages 16 and 18. The first of these was that palladium hydrogen in the presence of oxygen and water effected the oxidation of carbon monoxide, the second, that when carbon monoxide was brought in contact with moist phosphorus and air oxidation was observed.
In regard to the first of these experiments Traube[20] has carefully investigated what takes place when palladium hydrogen is allowed to remain in contact with water and oxygen. Hoppe-Seyler had noticed that under these conditions small quantities of hydrogen dioxide were formed, but he attributed this to the union of the active [Pg 54] [Pg 55] oxygen with the water. Traube, on the other hand, finds that in the formation of hydrogen dioxide under these circumstances there is nothing formed which has oxidizing properties, not even indigo sulphuric acid is oxidized. He shows by direct experiments that nascent hydrogen can not by its action on oxygen produce active oxygen or ozone. He finds the action of palladium hydrogen to be analogous to that of zinc and other metals, which when allowed to oxidize slowly in contact with air and water give rise to the formation of hydrogen dioxide. The process is to be regarded rather as a reduction of molecules of oxygen than as an [Pg 56] [Pg 57] oxidation of water. Traube represents the action by the following equations:—
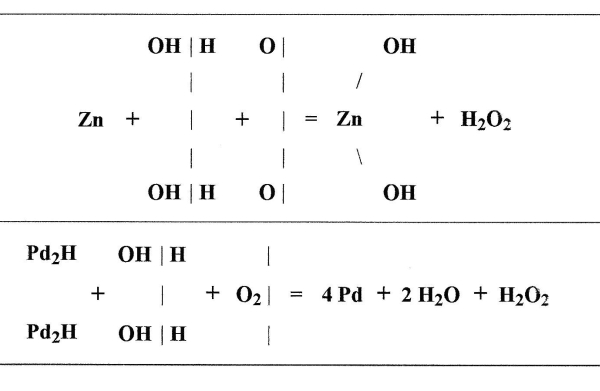
He proves by direct experiments that no active oxygen is formed during this process, and points out that the oxidations observed by Hoppe-Seyler and Baumann must have been brought about by the hydrogen dioxide. But this would not account for the oxidation of carbon monoxide, for it has been previously shown by Remsen[21] [Pg 58] [Pg 59] that hydrogen dioxide cannot oxidize carbon monoxide, not even when it is heated to its point of decomposition. Traube[22] therefore repeats Baumann’s experiment, he finds that palladium hydrogen in the presence of water and oxygen does oxidize carbon monoxide; but as he had shown that no active oxygen was formed during the process, and as the hydrogen dioxide could not cause the oxidation, he concluded that the palladium itself must play an important role in the reaction. By further experiments he soon became convinced that there are two stages in the process. 1st the palladium hydrogen acting on water and oxygen forms hydrogen dioxide, 2nd the hydrogen dioxide in [Pg 60] [Pg 61] the presence of palladium oxidizes the carbon monoxide. Traube[23] introduced into a glass flask containing carbon monoxide a dilute solution of hydrogen dioxide and a small piece of palladium foil, previously ignited, the action was allowed to continue for 22 hours after which the CO was replaced by air free from carbon dioxide. After leaving the flask the gas passed through a solution of barium hydroxide; an abundant precipitate was formed, showing that in this case the quantity of carbon dioxide formed was greater than in the first experiment, in which he used palladium hydrogen, water, oxygen and carbon monoxide. [Pg 62] [Pg 63]
Traube’s conclusion is as follows:—
“The carbon dioxide obtained in Baumann’s experiments is not formed during the oxidation of the hydrogen of the palladium hydrogen, (there being formed by this action merely hydrogen dioxide and palladium free from hydrogen) but by the combined action of these last two substances on carbon monoxide.” “Therefore, the proof is given that the act of slow combustion (Autoxidation) has not in itself the power of making oxygen active.”
The conclusion reached by Traube was tested by Professor Remsen and myself in the following way: A current of carbon monoxide was passed [Pg 64] [Pg 65] through several wash bottles containing solutions of caustic soda, then through a wash bottle containing clear baryta water, then into a flask containing a solution of hydrogen dioxide, containing a slight excess of hydrochloric acid. The flask also contained a small piece of palladium foil free from hydrogen. After leaving the flask the gas passed through a solution of baryta water, which was protected from the air by a U tube containing solid KOH. It was only necessary to pass the carbon monoxide through for a very short time to obtain an abundant precipitate of barium carbonate. We also noticed that under these conditions the palladium foil was dissolved. [Pg 66] [Pg 67]
From these experiments it is clear that the oxidation phenomena which Hoppe-Seyler and Baumann attributed to active oxygen are really due to the combined action of palladium and hydrogen dioxide, and to suppose that atomic oxygen exists in the free state at any time during the process is entirely gratuitous.
It remained to test the second of Baumann’s statements; namely that carbon monoxide in the presence of moist phosphorus and air is oxidized to carbon dioxide by the active oxygen formed by the slow combustion of the phosphorus. Leeds[24] also has on record an experiment on this subject, in which he claims [Pg 68] [Pg 69] that under these conditions oxidation takes place. In taking up the subject, therefore, Prof. Remsen[25] and myself have taken the greatest care to avoid all sources of error. We tried the effects of passing air alone freed from carbon dioxide over moist phosphorus and then into clear baryta water, Baumann[26] states that this can be done, and the baryta water remains perfectly clear, even if the current of air is passed for six hours. We obtained a precipitate immediately. Thinking this might be caused by the white vapors which are formed during the process, we passed the gas after its exit from the vessel containing the phosphorus through a layer of previously [Pg 70] [Pg 71] ignited asbestos. The layer of asbestos was between two and three feet in length and the air after having traversed it no longer contained any white fumes. From the asbestos tube the air passed into a solution of clear baryta water. A precipitate was formed at once and increased in quantity the longer the current continued. “It was tested for phosphoric acid and phosphorus in general but not a trace could be detected. The current of air over the phosphorus was continued for several days in order to obtain enough of the precipitate for examination and analysis. It proved to be nothing but barium carbonate.” [Pg 72] [Pg 73]
“The carbon dioxide must have come from one of two sources, either from some carbonaceous substance contained in our phosphorus, or as the result of the action of ozone on the cork stoppers used to make connections. The use of rubber was avoided as far as possible, and every precaution was taken as in the earlier experiments on the carbon monoxide and ozone. It did not appear improbable therefore that the difficulty arose from the use of impure phosphorus. Phosphorus was, therefore, obtained from as many different sources as possible, and with each of these the above described experiment was repeated, using [Pg 74] [Pg 75] the same apparatus. In every case the precipitate of barium carbonate was obtained and as far as could be estimated in about the same quantity. Attempts were then made to purify the phosphorus. One specimen was placed in hot water under the receiver of an air pump and the air exhausted, for the purpose of recovering any gases which might be contained in the phosphorus. Other specimens were distilled in an atmosphere of pure hydrogen and the vapor condensed in cold water. No matter what process of purification had been adopted the phosphorus acted in the same way afterwards as before.” [Pg 76] [Pg 77]
“We then constructed an apparatus in which the gases could at no point come in contact with cork stoppers or rubber joints. This consisted of a flask of from three to four litres capacity, provided with a doubly perforated cork stopper. Through this there passed one glass tube reaching to the bottom of the flask, and another reaching only half way. Outside the flask the shorter tube was connected with the wash bottles used to purify the air from carbon dioxide, while the longer tube was bent twice at right angles, and passed through the stopper of a U tube about 8 in. high. In the flask there were placed two or three [Pg 78] [Pg 79] sticks of phosphorus, each three or four inches long, and enough distilled water to somewhat more than fill the neck when the flask was inverted. The U tubes were filled with moistened asbestos which had been previously ignited. There was then added some mercury, so that when the tubes were inverted in which position the entire apparatus was placed when in use the mercury covered the corks with a layer from three quarters to an inch in thickness.
The last U tube was connected with the vessel containing the baryta water by means of a mercury joint. The baryta water was protected from [Pg 80] [Pg 81] the action of the air by placing before it a small U tube containing solid potassium hydroxide, this was in connection with an aspirator. Before connecting the bulbs containing the baryta water, air free from carbon dioxide was drawn through the apparatus. On now connecting the baryta water bulbs no precipitate was formed. About one third of the air in the flask was replaced by pure carbon monoxide, the mixture was allowed to remain several hours in contact with the moist phosphorus and then drawn through the baryta water bulbs. No precipitate was formed. This experiment was frequently repeated with the same result.” [Pg 82] [Pg 83]
“In some cases the air & carbon monoxide were drawn together slowly for several hours over the phosphorus, but this made no difference in the result.”
Having found, therefore, no evidence of the oxidation of carbon monoxide, we have no right to assume that when phosphorus oxidizes slowly in the presence of water and air that there is formed an active condition of oxygen distinct from ozone.
To this paper both Baumann[27] and Leeds[28] replied. The former recognizing the necessity of avoiding all connections of rubber or organic matter, describes a new form of apparatus, in which the joints are all made of ground glass. With this [Pg 84] [Pg 85] new apparatus he finds that he can pass air over phosphorus at the rate of from two to three bubbles per second, then through 10 cubic centimetres of water, and finally into baryta water, and claims that only a slight turbidity of phosphate and phosphite of barium is formed in the course of several days! This statement to us is incomprehensible and as will be evident from what follow, unless Baumann had phosphorus absolutely free from carbon (of which he makes no mention and which as far as we know it is impossible to obtain) he has described an impossibility. On introducing 100 cubic centimetres of carbon monoxide [Pg 86] [Pg 87] into the air every two hours he soon obtained a distinct cloudiness which constantly increased, until in 10 hours the inlet tube in the baryta water became stopped up and the experiment was discontinued. He then determined the percentage of oxidation; his results are as follows—
700 cubic centimetres of carbon monoxide mixed with enough air to require 15 hours to pass through the apparatus gave 366 milligrams CO2 or 2.6% of oxidation. In another experiment a mixture consisting of thirty litre of air and 2.45 litres of carbon monoxide, requiring 12 hours in passing the phosphorus, gave 466 milligrams of CO2, or 1.3% of the original quantity of monoxide was oxidized. [Pg 88] [Pg 89]
Baumann, in the arrangement of his apparatus, has taken no precautions to prevent the air from coming in contact with organic connections before it is introduced into the flask containing the phosphorus. Now Karsten[29] has shown that air alone when it comes in contact with the organic matter of corks and connectors forms carbon dioxide; it is, therefore, highly probable that in the course of from 12 to 15 hours a portion of his precipitate was due to this cause. The reminder came, as will appear presently, from carbon contained in the phosphorus. [Pg 90] [Pg 91]
Leeds conducted his experiment as follows:—A ten litre flask provided with a glass stopper, was filled with a mixture of equal parts of carbon monoxide and air, and allowed to stand in contact with moist phosphorus for six days. The glass stopper was then removed and replaced by a cork; and the mouth of the vessel being placed under mercury, the gases were displaced and passed through baryta water. A precipitate containing 15.5 mg of carbon dioxide was obtained. It is evident that in the course of six days, in a tightly closed vessel, the oxygen of the air must have been completely used up so that the mixed [Pg 92] [Pg 93] gases were necessarily under diminished pressure. Then in taking out the glass stopper for the purpose of introducing the cork, no precautions were taken to prevent the access of ordinary air, and a considerable volume of the air of the laboratory must have entered; enough, certainly, to account for some of the precipitate he obtained. The rest of the carbon dioxide must have come as in Baumann’s experiment from the oxidation of carbon contained in the phosphorus.
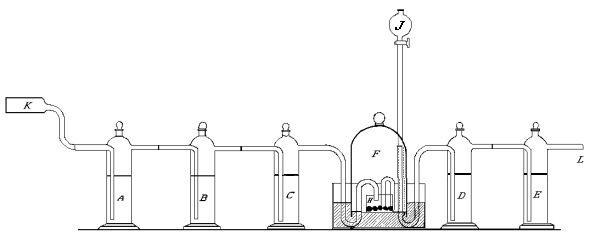
That ordinary commercial sticks of phosphorus contain carbon was shown by us in the following way[30]:—Air was passed from a gasometer into a [Pg 94] [Pg 95] hard glass tube containing copper oxide heated to redness, represented by K in the drawing. Then through a series of wash bottles A, B, C, so constructed that the connecting tubes were fitted into each other by means of ground glass joints. A and B contained a concentrated solution of caustic soda, C a solution of baryta water. The air then passed into an ordinary bell jar, having a capacity of about a litre and a quarter. This was held in position on mercury contained in a crystallizing dish. The inlet tube was bent downward into a small dish containing the phosphorus, represented by H in the figure. The gas after leaving the bell jar passed through two wash bottles D and E, similar to A, B, C. D [Pg 96] [Pg 97] [Pg 98] [Pg 99] contained 30-40 c.c. of ordinary distilled water. E contained a clear solution of baryta water, and was connected with a tube containing solid caustic potash to protect it from the air. The outlet tube from the wash bottle C is bent so as to pass beneath the edge of the bell jar, then up into the closed space above the mercury, and then down towards the phosphorus. A long funnel tube J served to introduce or remove water from the dish containing the phosphorus. The air therefore after having entered the tube K came at no point in contact with organic matter, and yet we found that after all ordinary air had been displaced by purified air, and clear baryta water introduced into [Pg 100] [Pg 101] the wash bottle E, a precipitate was found. Ten litres of air were sufficient to cause a distinct turbidity, while 20 to 30 gave a precipitate. As there is no possible source of error it follows that the carbon dioxide must have come from the oxidation of carbon contained in the phosphorus.
That carbon should be present in phosphorus is not surprising considering its method of manufacture. Whether the carbon existing in the phosphorus is in chemical combination or not we are unable to say. The specimens of phosphorus used by us were perfectly homogenous. There was no evidence of the presence of [Pg 102] [Pg 103] particles in them, and the solution in carbon bisulphide was perfectly clear, and on standing nothing whatever was deposited. Even distilled phosphorus acted in the same way, showing that this also contained carbon.
A simple way to show the presence of carbon in any sample of phosphorus is to burn a small piece of the latter in a small porcelain dish, floating in water under a bell jar fitted with a glass stop cock. After the combustion is over the vessel is allowed to stand some time until the white fumes have entirely disappeared. The gas is then passed through water and finally into baryta water where a precipitate is [Pg 104] [Pg 105] invariably formed. The air in the bell jar must of course be free from carbon dioxide. As the bell jar is only lifted far enough to permit the introduction of the dish with the phosphorus, and this operation is performed instantaneously, the amount of carbon dioxide thus introduced can only be infinitesimal.
We now made some experiments with the object of determining whether changes in the amount of phosphorus exposed in the bell jar F of our ozonizing apparatus had any effect upon the amount of barium carbonate formed in the wash bottle E. We found that the amount of precipitate is [Pg 106] [Pg 107] plainly influenced by the rate of passage of the gases, the temperature and the amount of phosphorus exposed, but that if the temperature is between 20 and 25°C, the rate of passage of the air about two or three bubbles per second, and the amount of phosphorus exposed from 20 to 30 grams a slight precipitate is always formed by 10 litres of air, and that 25 to 30 litres give a decided precipitate.
Having therefore demonstrated the presence of carbon in all the specimens of phosphorus at our disposal, and knowing that purified air [Pg 108] [Pg 109] alone when passed over phosphorus would give a precipitate when passed into baryta water, we next determined whether if carbon monoxide being present in the air passing over the phosphorus, and all other conditions the same, the amount of precipitate is increased. For this purpose parallel experiments under as nearly the same conditions as possible were made one with air alone, the other with air and carbon monoxide. In the first experiment about 25 litres of air were passed through the apparatus, the conditions being carefully noted. The wash bottle containing the precipitate was removed at the end of the operation, instantly stoppered and set aside for comparison. [Pg 110] [Pg 111]
The water was then removed from the wash bottle D and replaced by fresh distilled water, a new bottle attached in the place of E and after passing about a litre of pure air through the apparatus, the necessary quantity of baryta water filtered rapidly through a plaited filter into the wash bottle.
Now the experiment was repeated, with the difference that during the passage of twenty-five litres of air, a very slow current of carefully purified carbon monoxide (made from pure sulphuric and formic acids) was passed through three wash bottles, like those used for the air, and [Pg 112] [Pg 113] containing the same substances, and then into the bell jar containing phosphorus and air. The rate of the current was so regulated that during the time of the experiment, which varied in different cases from three to eight hours, three litres of carbon monoxide were used. The same slow formation of a precipitate was noticed when the carbon monoxide was used as in the case of air alone. At the end of the operation we were unable to distinguish any difference between the amounts of the small precipitates formed. They did not appear to be as great as that found by Baumann, they were too small to permit of [Pg 114] [Pg 115] accurate filtering and weighing, if we consider the nature of the liquid in which they were present.
The only conclusion which we can draw is, as is stated in the first paper on this subject, that carbon monoxide is not oxidized by air in the presence of moist phosphorus.
That in our first experiments we did not obtain evidence of the presence of carbonic of phosphorus is due to the fact that we worked with small volumes of the gases. In those cases in which relatively large volumes were used the slight cloudiness produced was disregarded as the same result was obtained with air alone. [Pg 116] [Pg 117]
Having, therefore, been unable to obtain any evidence of the oxidation of carbon monoxide when phosphorus undergoes slow combustion in the presence of air and water, the second and last of Baumann’s arguments for the existence of active oxygen becomes untenable. Whether oxygen ever does occur in the so called active condition still remains to be shown.
That the nascent state of an element should be due to the momentary existence of free atoms is entirely hypothetical. Tommasi[31] has shown that the properties of nascent hydrogen vary according to the [Pg 118] [Pg 119] method by which it is formed. He regards nascent hydrogen as ordinary molecular hydrogen plus varying quantities of heat, and he shows that as the heat of the reaction varies so the activity of the hydrogen varies. The same is undoubtedly true of oxygen, for it is known that oxygen evolved by some reactions is more powerful than by others. That we shall ever be able to show that this heat in some cases is sufficient to dissociate the molecules of oxygen seems improbable.
Baumann[32] has recently published another paper, but has failed to contribute either new facts or ideas on the subject. [Pg 120] [Pg 121]
Having found carbon present in all varieties that we examined, we naturally attempted its quantitative determination. Our first experiments did not prove successful. Chromic acid was tried, but this gave unsatisfactory results for the reason that it was impossible to control the action and at the same time secure complete oxidation of the phosphorus. With concentrated solutions the action is liable to become violent unless the temperature is kept low. [Pg 122] [Pg 123]
We also arranged an apparatus similar to that used in making phosphorus pentoxide on the small scale. The combustion took place in a bell jar filled with pure air, and after being thoroughly washed the gases were passed through baryta water. The operation was imperfect owing to the formation of red phosphorus, and to incomplete oxidation.
Finally we succeeded in obtaining satisfactory results by using nitric acid of 1.2 sp gr. The phosphorus was oxidized in a retort of 500 c.c. capacity. The retort was inclined so that any liquid condensing in the neck would run back. A glass tube fitted to the neck of the retort by means of gypsum served to convey the evolved [Pg 124] [Pg 125] gases into a wash bottle containing pure water. The latter was connected with a combustion tube containing in one end a layer of metallic copper about six inches in length, this served to decompose the oxides of nitrogen. The remainder of the tube was filled with copper oxide, which served to oxidize any carbon compound, which might be formed by the oxidation of the phosphorus, to carbon dioxide. After leaving the combustion tube the gases passed, first through a wash bottle containing water, then into one containing clear barium hydroxide, which was protected from the action of the air. All joints which were not of ground glass were made by means [Pg 126] [Pg 127] of gypsum. The operation was conducted as follows:—After 200 to 300 cubic centimetres of nitric acid (sp gr 1.2) and the weighted quantity of phosphorus had been introduced into the retort, a slow current of air free from carbon dioxide was drawn through the apparatus. The tubulus of the retort was then closed by means of a glass stopper, the combustion tube containing the metallic copper and copper oxide heated to a red heat, and a solution of baryta water rapidly filtered into the last wash bottle. The retort was then [Pg 128] [Pg 129] heated gently, after a time a regular evolution of gas takes place, and a precipitate gradually forms in the baryta water. At the end of the operation, air free from carbon dioxide is again drawn through the apparatus to remove all of the oxidation products. The precipitate is allowed to settle, the clear liquid is rapidly decanted through a filter. The precipitate is then washed, and quickly brought upon the filter paper. The filtering is done by means of a pump and is very rapid. The precipitate is then dissolved in dilute hydrochloric acid and the solution heated to boiling and the barium precipitated by sulphuric acid in the usual way. From the [Pg 130] [Pg 131] weight of barium sulphate obtained, the quantity of carbon in the phosphorus is readily calculated.
In some instances the experiment was varied by using a large quantity of phosphorus and allowing the action to continue for two or three hours, then weighing the phosphorus which remained unacted upon. In two instances the carbon dioxide was weighed directly by replacing the wash bottle containing the baryta water by weighed potash bulbs. [Pg 132] [Pg 133]
The following are the results obtained
I. 6.2272 grams Phosphorus gave .0300 gr BaSO4 = .0057 grm CO2 = .0016 gr C = .026%C.
II. 7.9545 grm Phosphorus gave .0324 gr CO2 = .0088 gr Carbon = .111% Carbon
III. 8.8041 grm Phosphorus gave .0134 gr CO2 = .00365 gr Carbon = .042% Carbon
IV. 9.0650 grm Phosphorus gave .0540 BaSO4 = .0101 grm CO2 = .00278 gr C = .031% C.
V. 16.4633 grm Phosphorus gave .1303 gr BaSO4 = .0246 gr CO2 = .0067 C = .041% C.
VI. 11 grams Phosphorus gave .0929 grams BaSO4 = .0175 gr CO2 = .00478 C = .043% C.
FOOTNOTES:
[1] Annales de Chim. et de Phys. 14-252
[2] Poggendorf. Ann. 95-484
Journ. f. prakt. Chemie. 65-499
[3] Pogg. Ann. 103-644
[4] Pogg. Ann. 120-250
[5] Journ. f. prakt Chem. 52-135
[6] Journ f. prakt. Chem. 53-65
[7] Journ f. prakt. Chem. 77-129
[8] Journ f. prakt. Chemie 86-65
[9] Untersuchungen über Sauerstoff. Hanover 1863
[10] Liebig’s Annalen 154-215.
[11] Annales de Chim. et de Physiologie 3-58
[12] Compt. Rendus 50-829
[13] Berichte der Deutschen Chem. Gesellschft. 6-108
[14] Zeitschrift f. Chemie (1870) 6-611
[15] Zeitschrift f. Physiol. Chemie 2-22
[16] Zeitschrft. Physiol Chem. 5—244
[17] Berichte der Deutsch. Chem. Gesell. 3-84
[18] Berichte der Deutsch. Chem. Gesell. 8-1415
[19] American Chemical Journal 4-50
[20] Berichte d. Deutsch. Chem. Gesell. 15-2421
[21] Berichte der Deutsch. Chem. Gesell. 15-222
American Chem. Journ. 4-53
[22] Berichte der Deutsch. Chem. Gesell. 16-123
[23] Berichte der Deutsch. Chem. Gesell. 16-126
[24] Journal Am. Chem. Soc. 1-232
[25] American Chem. Journal 4-454
[26] Zeitsch. f. phys. Chem. 5-250
[27] Berichte der Deutsch. Chem. Gesell. 16-2146
[28] Chemical News 48-25
[29] Poggendorff’s Annalen 115-348.
[30] American Chemical Journal 5-426.
[31] Bulletin Soc. Chim. 38-148
[32] Berichte d. Deutsch. Chem. Gesell. 7-283
Transcriber Notes:
Uncertain or antiquated spellings or ancient words were not corrected.
The illustrations have been moved so that they do not break up paragraphs and so that they are next to the text they illustrate.
Errors in punctuation and inconsistent hyphenation were not corrected unless otherwise noted.
Typographical errors have been silently corrected but other variations in spelling and punctuation remain unaltered.
In the original text, the volume number for the journals was double underlined for emphasis. In this book this has been replaced with the use of bold numerals.
End of the Project Gutenberg EBook of On the Existence of Active Oxygen, by
Edward H. Keiser
*** END OF THIS PROJECT GUTENBERG EBOOK ON THE EXISTENCE OF ACTIVE OXYGEN ***
***** This file should be named 52162-h.htm or 52162-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/5/2/1/6/52162/
Produced by Paul Marshall and the Online Distributed
Proofreading Team at http://www.pgdp.net (This file was
produced from images generously made available by The
Internet Archive)
Updated editions will replace the previous one--the old editions will
be renamed.
Creating the works from print editions not protected by U.S. copyright
law means that no one owns a United States copyright in these works,
so the Foundation (and you!) can copy and distribute it in the United
States without permission and without paying copyright
royalties. Special rules, set forth in the General Terms of Use part
of this license, apply to copying and distributing Project
Gutenberg-tm electronic works to protect the PROJECT GUTENBERG-tm
concept and trademark. Project Gutenberg is a registered trademark,
and may not be used if you charge for the eBooks, unless you receive
specific permission. If you do not charge anything for copies of this
eBook, complying with the rules is very easy. You may use this eBook
for nearly any purpose such as creation of derivative works, reports,
performances and research. They may be modified and printed and given
away--you may do practically ANYTHING in the United States with eBooks
not protected by U.S. copyright law. Redistribution is subject to the
trademark license, especially commercial redistribution.
START: FULL LICENSE
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full
Project Gutenberg-tm License available with this file or online at
www.gutenberg.org/license.
Section 1. General Terms of Use and Redistributing Project
Gutenberg-tm electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or
destroy all copies of Project Gutenberg-tm electronic works in your
possession. If you paid a fee for obtaining a copy of or access to a
Project Gutenberg-tm electronic work and you do not agree to be bound
by the terms of this agreement, you may obtain a refund from the
person or entity to whom you paid the fee as set forth in paragraph
1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this
agreement and help preserve free future access to Project Gutenberg-tm
electronic works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the
Foundation" or PGLAF), owns a compilation copyright in the collection
of Project Gutenberg-tm electronic works. Nearly all the individual
works in the collection are in the public domain in the United
States. If an individual work is unprotected by copyright law in the
United States and you are located in the United States, we do not
claim a right to prevent you from copying, distributing, performing,
displaying or creating derivative works based on the work as long as
all references to Project Gutenberg are removed. Of course, we hope
that you will support the Project Gutenberg-tm mission of promoting
free access to electronic works by freely sharing Project Gutenberg-tm
works in compliance with the terms of this agreement for keeping the
Project Gutenberg-tm name associated with the work. You can easily
comply with the terms of this agreement by keeping this work in the
same format with its attached full Project Gutenberg-tm License when
you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are
in a constant state of change. If you are outside the United States,
check the laws of your country in addition to the terms of this
agreement before downloading, copying, displaying, performing,
distributing or creating derivative works based on this work or any
other Project Gutenberg-tm work. The Foundation makes no
representations concerning the copyright status of any work in any
country outside the United States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other
immediate access to, the full Project Gutenberg-tm License must appear
prominently whenever any copy of a Project Gutenberg-tm work (any work
on which the phrase "Project Gutenberg" appears, or with which the
phrase "Project Gutenberg" is associated) is accessed, displayed,
performed, viewed, copied or distributed:
This eBook is for the use of anyone anywhere in the United States and
most other parts of the world at no cost and with almost no
restrictions whatsoever. You may copy it, give it away or re-use it
under the terms of the Project Gutenberg License included with this
eBook or online at www.gutenberg.org. If you are not located in the
United States, you'll have to check the laws of the country where you
are located before using this ebook.
1.E.2. If an individual Project Gutenberg-tm electronic work is
derived from texts not protected by U.S. copyright law (does not
contain a notice indicating that it is posted with permission of the
copyright holder), the work can be copied and distributed to anyone in
the United States without paying any fees or charges. If you are
redistributing or providing access to a work with the phrase "Project
Gutenberg" associated with or appearing on the work, you must comply
either with the requirements of paragraphs 1.E.1 through 1.E.7 or
obtain permission for the use of the work and the Project Gutenberg-tm
trademark as set forth in paragraphs 1.E.8 or 1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any
additional terms imposed by the copyright holder. Additional terms
will be linked to the Project Gutenberg-tm License for all works
posted with the permission of the copyright holder found at the
beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including
any word processing or hypertext form. However, if you provide access
to or distribute copies of a Project Gutenberg-tm work in a format
other than "Plain Vanilla ASCII" or other format used in the official
version posted on the official Project Gutenberg-tm web site
(www.gutenberg.org), you must, at no additional cost, fee or expense
to the user, provide a copy, a means of exporting a copy, or a means
of obtaining a copy upon request, of the work in its original "Plain
Vanilla ASCII" or other form. Any alternate format must include the
full Project Gutenberg-tm License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works
provided that
* You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is owed
to the owner of the Project Gutenberg-tm trademark, but he has
agreed to donate royalties under this paragraph to the Project
Gutenberg Literary Archive Foundation. Royalty payments must be paid
within 60 days following each date on which you prepare (or are
legally required to prepare) your periodic tax returns. Royalty
payments should be clearly marked as such and sent to the Project
Gutenberg Literary Archive Foundation at the address specified in
Section 4, "Information about donations to the Project Gutenberg
Literary Archive Foundation."
* You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or destroy all
copies of the works possessed in a physical medium and discontinue
all use of and all access to other copies of Project Gutenberg-tm
works.
* You provide, in accordance with paragraph 1.F.3, a full refund of
any money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days of
receipt of the work.
* You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project
Gutenberg-tm electronic work or group of works on different terms than
are set forth in this agreement, you must obtain permission in writing
from both the Project Gutenberg Literary Archive Foundation and The
Project Gutenberg Trademark LLC, the owner of the Project Gutenberg-tm
trademark. Contact the Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
works not protected by U.S. copyright law in creating the Project
Gutenberg-tm collection. Despite these efforts, Project Gutenberg-tm
electronic works, and the medium on which they may be stored, may
contain "Defects," such as, but not limited to, incomplete, inaccurate
or corrupt data, transcription errors, a copyright or other
intellectual property infringement, a defective or damaged disk or
other medium, a computer virus, or computer codes that damage or
cannot be read by your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH 1.F.3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium
with your written explanation. The person or entity that provided you
with the defective work may elect to provide a replacement copy in
lieu of a refund. If you received the work electronically, the person
or entity providing it to you may choose to give you a second
opportunity to receive the work electronically in lieu of a refund. If
the second copy is also defective, you may demand a refund in writing
without further opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS', WITH NO
OTHER WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT
LIMITED TO WARRANTIES OF MERCHANTABILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of
damages. If any disclaimer or limitation set forth in this agreement
violates the law of the state applicable to this agreement, the
agreement shall be interpreted to make the maximum disclaimer or
limitation permitted by the applicable state law. The invalidity or
unenforceability of any provision of this agreement shall not void the
remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in
accordance with this agreement, and any volunteers associated with the
production, promotion and distribution of Project Gutenberg-tm
electronic works, harmless from all liability, costs and expenses,
including legal fees, that arise directly or indirectly from any of
the following which you do or cause to occur: (a) distribution of this
or any Project Gutenberg-tm work, (b) alteration, modification, or
additions or deletions to any Project Gutenberg-tm work, and (c) any
Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of
computers including obsolete, old, middle-aged and new computers. It
exists because of the efforts of hundreds of volunteers and donations
from people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need are critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future
generations. To learn more about the Project Gutenberg Literary
Archive Foundation and how your efforts and donations can help, see
Sections 3 and 4 and the Foundation information page at
www.gutenberg.org Section 3. Information about the Project Gutenberg
Literary Archive Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Contributions to the Project Gutenberg Literary
Archive Foundation are tax deductible to the full extent permitted by
U.S. federal laws and your state's laws.
The Foundation's principal office is in Fairbanks, Alaska, with the
mailing address: PO Box 750175, Fairbanks, AK 99775, but its
volunteers and employees are scattered throughout numerous
locations. Its business office is located at 809 North 1500 West, Salt
Lake City, UT 84116, (801) 596-1887. Email contact links and up to
date contact information can be found at the Foundation's web site and
official page at www.gutenberg.org/contact
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
[email protected]
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To SEND
DONATIONS or determine the status of compliance for any particular
state visit www.gutenberg.org/donate
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including checks, online payments and credit card donations. To
donate, please visit: www.gutenberg.org/donate
Section 5. General Information About Project Gutenberg-tm electronic works.
Professor Michael S. Hart was the originator of the Project
Gutenberg-tm concept of a library of electronic works that could be
freely shared with anyone. For forty years, he produced and
distributed Project Gutenberg-tm eBooks with only a loose network of
volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as not protected by copyright in
the U.S. unless a copyright notice is included. Thus, we do not
necessarily keep eBooks in compliance with any particular paper
edition.
Most people start at our Web site which has the main PG search
facility: www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.