
The Project Gutenberg EBook of The Biotic Associations of Cockroaches, by
Louis M. Roth and Edwin R. Willis
This eBook is for the use of anyone anywhere in the United States and most
other parts of the world at no cost and with almost no restrictions
whatsoever. You may copy it, give it away or re-use it under the terms of
the Project Gutenberg License included with this eBook or online at
www.gutenberg.org. If you are not located in the United States, you'll have
to check the laws of the country where you are located before using this ebook.
Title: The Biotic Associations of Cockroaches
Author: Louis M. Roth
Edwin R. Willis
Release Date: September 7, 2014 [EBook #46802]
Language: English
Character set encoding: ISO-8859-1
*** START OF THIS PROJECT GUTENBERG EBOOK BIOTIC ASSOCIATIONS OF COCKROACHES ***
Produced by Dianna Adair, Matthias Grammel, Bryan Ness and
the Online Distributed Proofreading Team at
http://www.pgdp.net (This file was produced from images
generously made available by The Internet Archive)
SMITHSONIAN
MISCELLANEOUS COLLECTIONS
VOL. 141

"EVERY MAN IS A VALUABLE MEMBER OF SOCIETY WHO, BY HIS OBSERVATIONS, RESEARCHES,
AND EXPERIMENTS, PROCURES KNOWLEDGE FOR MEN"—JAMES SMITHSON
(Publication 4470)
CITY OF WASHINGTON
PUBLISHED BY THE SMITHSONIAN INSTITUTION
1961
PORT CITY PRESS, INC.
BALTIMORE, MD., U. S. A.
The Smithsonian Miscellaneous Collections series contains, since the suspension in 1916 of the Smithsonian Contributions to Knowledge, all the publications issued directly by the Institution except the Annual Report and occasional publications of a special nature. As the name of the series implies, its scope is not limited, and the volumes thus far issued relate to nearly every branch of science. Papers in the fields of biology, geology, anthropology, and astrophysics have predominated.
Leonard Carmichael,
Secretary, Smithsonian Institution.
SMITHSONIAN MISCELLANEOUS COLLECTIONS
VOLUME 141 (WHOLE VOLUME)
(With 37 Plates)
By
LOUIS M. ROTH
AND
EDWIN R. WILLIS
Pioneering Research Division, United States Army
Quartermaster Research and Engineering Center
Natick, Mass.

(Publication 4422)
CITY OF WASHINGTON
PUBLISHED BY THE SMITHSONIAN INSTITUTION
DECEMBER 2, 1960
 PL. 1
PL. 1SMITHSONIAN MISCELLANEOUS COLLECTIONS
VOLUME 141 (WHOLE VOLUME)
THE BIOTIC ASSOCIATIONS OF
COCKROACHES
(With 37 Plates)
By
LOUIS M. ROTH
AND
EDWIN R. WILLIS
Pioneering Research Division, United States Army
Quartermaster Research and Engineering Center
Natick, Mass.

(Publication 4422)
CITY OF WASHINGTON
PUBLISHED BY THE SMITHSONIAN INSTITUTION
DECEMBER 2, 1960
THE LORD BALTIMORE PRESS, INC.
BALTIMORE, MD., U. S. A.
People having only casual interest in insects usually express amazement when they learn how much is known about this most numerous group of animals. However, while entomologists have good reason to take pride in the accomplishments of their contemporaries and predecessors, they are more likely to be appalled by how much remains to be learned. We are indeed ignorant of even the identity of fully half and probably much more than half the total number of insect species. Of those that have been described, we have reasonably complete information about the behavior and basic environmental relationships for only a comparative few. The great majority of the remainder are known only from specimens found in museum collections. Such information as we have about these species usually amounts to no more than date and locality of collection.
This is true of the cockroaches, which now include approximately 3,500 described species. Conservative estimates based on partially studied museum collections and the percent of new species found in recent acquisitions, particularly from tropical and subtropical countries, indicate that at least 4,000 species remain unnamed. Although the group is well known in general terms to nearly all entomologists, there is an almost complete void of information about all except the few domestic species and, to a progressively diminishing degree, some 400 others. Many details about the lives of even those that share man's habitations are not fully understood. This then is a rough measure of how little is known about cockroaches.
With the exception of mosquitoes and a few other comparatively small groups of insects on which work has been concentrated, it is doubtful if any other comparable segment of the world's insect fauna is better known. Already an estimated 800,000 kinds of insects have been described, and since this figure is generally regarded as less than half the actual total, think what this means in terms of knowledge yet to be assembled. No wonder entomology is a growing science with a promising future, but the magnitude of the task also presents a serious obstacle to progress. Progress can continue only if the scattered literature resulting from the diversified labors of hundreds of contributors is brought together and summarized in thorough and well-organized compilations that can serve as a solid basis for future research.
The present work is such a compilation, for it assembles what has been gleaned from approximately 1,700 sources, including correspondence with a large number of other workers. Original observations during some eight years of concentrated effort in U. S. Army Quartermaster research laboratories are a valuable supplement to what others have done, and with this background of experience the authors are especially well qualified to appraise previous work. Seldom has a compilation been done so thoroughly or a single large group of insects been the subject of such uninterrupted effort.
The contents gives the categories of subject matter treated and the introduction discusses the value of this assembled information and offers suggestions for future study. No longer are cockroaches regarded only as disagreeable pests; many species appear to be important, actually or potentially, as carriers of disease. Recognition of this importance has grown considerably, even in the period since World War II. Consequently, anything that increases our knowledge of the basic bionomics of cockroaches will be consulted widely for factual information and for clues to new approaches.
In spite of this extensive compilation, the limitations of present information about cockroach bionomics must be kept in mind. The cited observations of many writers were fragmentary, or their conclusions disagreed. But it is fundamental to scientific inquiry that we should know and attempt to evaluate the results of previous study, and that is what Drs. Roth and Willis have done. Fortunately, their review is readily available. Sometimes, a piece of work fails to be of maximum value because the results are not generally accessible to later students. For this reason I am especially glad that the Smithsonian Institution, by disseminating the results of the authors' labors, has this opportunity to exercise one of its traditional functions—that of diffusing knowledge.
Throughout the period of research by Drs. Roth and Willis at Natick, I was in frequent correspondence with them, and I admire their many accomplishments. Our warmest commendations should go not only to them personally but also to those in administration who encouraged their fundamental research and who aided in the financial support of this publication.
Ashley B. Gurney
Entomology Research Division
United States Department of Agriculture
| Page | ||||||
| Foreword | iii | |||||
| List of Plates | vii | |||||
| List of Illustrations | viii | |||||
| I. | Introduction | 1 | ||||
| Historical | 2 | |||||
| Methods | 4 | |||||
| Future work | 5 | |||||
| Illustrations | 7 | |||||
| II. | Species of cockroaches | 7 | ||||
| III. | Ecological relationships | 14 | ||||
| Cave habitats | 16 | |||||
| Cavernicolous cockroaches | 17 | |||||
| Cockroaches from burrows | 23 | |||||
| Desert habitats | 25 | |||||
| Desert cockroaches | 27 | |||||
| Aquatic habitats | 30 | |||||
| Amphibious cockroaches | 31 | |||||
| Outdoor habitats | 33 | |||||
| Cockroaches from outdoor habitats | 35 | |||||
| Structural habitats | 70 | |||||
| Land-based structures | 73 | |||||
| Cockroaches associated with land-based structures | 74 | |||||
| Ships | 82 | |||||
| Cockroaches associated with ships | 85 | |||||
| Aircraft | 87 | |||||
| Cockroaches associated with aircraft | 88 | |||||
| IV. | Classification of the associations | 91 | ||||
| V. | Mutualism | 96 | ||||
| Bacteroids | 96 | |||||
| Cockroaches in which bacteroids have been found | 99 | |||||
| Bacteria | 100 | |||||
| Protozoa | 101 | |||||
| VI. | Viruses associated with cockroaches | 103 | ||||
| VII. | Bacteria associated with cockroaches | 104 | ||||
| VIII. | Fungi and yeasts | 127 | ||||
| Fungi associated with cockroaches | 129 | |||||
| IX. | Higher plants associated with cockroaches | 139 | ||||
| Damage to plants by cockroaches | 162 | |||||
| X. | Protozoa associated with cockroaches | 166 | ||||
| XI. | Helminths associated with cockroaches | 190 | ||||
| Helminths for which cockroaches serve as primary hosts | 192 | |||||
| Helminths for which cockroaches serve as intermediate hosts | 203 | |||||
| Helminths whose eggs have been carried by cockroaches | 208 | |||||
| XII. | Arthropoda associated with cockroaches | 210 | ||||
| Arachnida | 211 | |||||
| Chilopoda | 222 | |||||
| [Pg vi] | Insecta | 224 | ||||
| Hymenoptera | 234 | |||||
| Predators and parasites of cockroach eggs | 234 | |||||
| Host selection by egg parasites | 254 | |||||
| Cockroach-hunting wasps | 255 | |||||
| Ants predaceous on cockroaches | 266 | |||||
| XIII. | Vertebrata associated with cockroaches | 268 | ||||
| Pisces | 268 | |||||
| Amphibia | 269 | |||||
| Reptilia | 272 | |||||
| Aves | 276 | |||||
| Mammalia | 283 | |||||
| XIV. | Checklist of cockroaches and symbiotic associates | 290 | ||||
| XV. | Cockroaches as commensals | 310 | ||||
| Hosts of commensal cockroaches | 310 | |||||
| Checklist of commensal cockroaches with their hosts | 315 | |||||
| Obscure associations | 316 | |||||
| XVI. | Cockroaches as predators | 319 | ||||
| Interspecies predation | 319 | |||||
| Intraspecies predation | 322 | |||||
| XVII. | Associations among cockroaches | 324 | ||||
| Familial associations | 325 | |||||
| Gregariousness | 330 | |||||
| Intraspecies fighting | 336 | |||||
| Interspecies compatibility | 337 | |||||
| Interspecies antagonism | 341 | |||||
| XVIII. | Defense of cockroaches against predators | 343 | ||||
| XIX. | The biological control of cockroaches | 348 | ||||
| Invertebrates | 349 | |||||
| Vertebrates | 353 | |||||
| Acknowledgments | 354 | |||||
| References | 356 | |||||
| Index | 441 | |||||
| Plate | Page | |
| 1 | Blaberus craniifer, c. X 2. 1. (Photograph by Jack Salmon, Philadelphia Quartermaster Depot.). | a |
| 2 | Blaberus craniifer, nymph. (Photograph by Jack Salmon.) | A-3 |
| 3 | Blaberus giganteus, c. X 2.2. (Photograph by Jack Salmon.) | A-4 |
| 4 | Blatta orientalis, c. X 3.8. A, Male. B, Female. (Photographs by Jack Salmon.) | A-5 |
| 5 |
A-B, Blattella germanica, c. X 5.2. A, Male. B, Female. C-D, Blattella vaga, c. X 5.2. C, Male. D, Female with oötheca. |
A-6 |
| 6 | Byrsotria fumigata, c. X 2. A, Brachypterous male. B, Macropterous male. C, Female. | A-7 |
| 7 | A and B, Cariblatta lutea minima, X 10. A, Male. B, Female with partly formed oötheca. C, Ectobius pallidus, female with completely formed oötheca, X 8. (C, From Roth and Willis [1957].) | A-8 |
| 8 | A, Cryptocercus punctulatus, c. X 4.6. (Photograph by Jack Salmon.) B, Panesthia australis, X 2.8. | A-9 |
| 9 | Cutilia sp. near sedilloti, c. X 5. A, Male. B, Female. | A-10 |
| 10 | Diploptera punctata, c. X 5. A, Male. B, Female. | A-11 |
| 11 | Eurycotis floridana, c. X 2.8. A, Male. B, Female. (Photographs by Jack Salmon.) | A-12 |
| 12 | A-B, Gromphadorhina portentosa, c. X 1.5. A, Male nymph. B, Adult female. C, Coleolaelaps (?) sp., a mite from G. portentosa, c. X 32. (Glycerine jelly preparation and photograph of C by Dr. Barbara Stay.) | A-13 |
| 12A | Ischnoptera deropeltiformis, c. X 5.3. A, Male. B, Female. | A-14 |
| 13 | Leucophaea maderae, c. X 2.2. A, Male. B, Female. (Photographs by Jack Salmon.) | A-15 |
| 14 | Nauphoeta cinerea, c. X 3.4. A, Male. B, Female. | A-16 |
| 15 | Neostylopyga rhombifolia, c. X 3.4. A, Male. B, Female with partially formed oötheca. | A-17 |
| 16 | Panchlora nivea, X 4.5. A, Dead individual showing normal, pale green coloration. B, Dead individual showing the bright red coloration (very dark areas) characteristic of infection with Serratia marcescens. C, Living male. D, Living female. | A-18 |
| 17 | A, Parcoblatta pensylvanica, female with completely formed oötheca, X 4. B, Parcoblatta virginica, female with partly formed oötheca, X 7.3. | A-19 |
| 18 | Parcoblatta uhleriana, c. X 5.5. A, Male. B, Female with oötheca. | A-20 |
| 19 | Periplaneta americana, c. X 3. A, Male. B, Female. (Photographs by Jack Salmon.) | A-21 |
| 20 | Periplaneta australasiae, c. X 3.2. A, Male. B, Female. (Photographs by Jack Salmon.) | A-22 |
| 21 | Periplaneta brunnea, c. X 2.9. A, Male. B, Female. | A-23 |
| 22 | Periplaneta fuliginosa, c. X 2.9. A. Male. B. Female. | A-24 |
| 23 | Platyzosteria novae seelandiae, c. X 2.9. A. Male. B, Female with oötheca. | A-25 |
| 24 | Pycnoscelus surinamensis, c. X 3.7. A, Male from Hawaii. B, Macropterous parthenogenetic female from Florida. C, Brachypterous nonparthenogenetic female from Hawaii. D, Late instar nymph. (Photograph of nymph D, by Jack Salmon.) | A-26 |
| 25 | Supella supellectilium, c. X 6.3. A, Male. B, Female. | A-27 |
| 26 | Bacteroids from Blattella germanica. A, Part of abdomen showing mycetocytes in fat body, X 225. B, Lobe of fat body showing 3 mycetocytes, X 750. C, Single mycetocyte; bacteroids appear hollow as result of fixation in Carnoy's fluid, X 1725. D, Smear of fat body showing bacteroids in various stages, X 1800. (All preparations and photographs through the courtesy of Dr. Marion A. Brooks.) | A-28 |
| 27 | Fungi parasitic on cockroaches. A, Herpomyces arietinus growing on antennae, legs, body, and cerci of a nymph of Parcoblatta virginica, X 7. B, Herpomyces stylopygae on antenna of Blatta orientalis, X 35. (Reproduced from Richards and Smith [1955, 1956].) C, Herpomyces sp. [probably H. stylopygae] on antenna of B. orientalis, X 132. (Photographs B and C through the courtesy of Dr. A. G. Richards.) | A-29 |
| 28 | A-B, Gregarines (Diplocystis sp.?) from Blaberus craniifer. A, Organisms removed from intestine, X 50. B, Organisms removed from hemocoele, X 32. C, Gregarine cysts in feces of Leucophaea maderae, X 12. | A-30 |
| 29 | A, Undetermined mermithid that parasitizes Ectobius pallidus. X 9. The worm has partly emerged from the neck region of the cockroach. (Reproduced from Roth and Willis [1957].) B, Undetermined gordian worm that parasitized Eurycotis floridana shown beside its host, X 1.8. (Specimen courtesy of Dr. T. Eisner.) | A-31 |
| 30 | A, Heteropoda venatoria, a cockroach-hunting spider, slightly less than natural size, on bananas. (Reproduced from a Kodachrome transparency through the courtesy of Dr. B. J. Kaston.) B to E, Lycosa sp. (avida?) capturing and feeding on a nymph of Supella supellectilium in the laboratory, X 1.4. | A-32 |
| 31 | The centipede Scutigera coleoptrata capturing and feeding on cockroaches in the laboratory. A to E, Pursuit, capture, and eating of a nymph of Supella supellectilium, c. X 1.2. F, Centipede feeding on adult of Blattella germanica, X 1.8. | A-33 |
| 32 | The mantid Hierodula tenuidentata (?) devouring a nymph of Periplaneta australasiae, c. X 1.5. | A-34 |
| 33 | A, Prosevania punctata ♂ beside an oötheca of Periplaneta americana, X 5. B, Hyptia harpyoides with oötheca of Parcoblatta uhleriana from which it had emerged, X 5. C, Larva of a lampyrid beetle feeding on Parcoblatta virginica in the laboratory, X 4. | A-35 |
| 34 | Chalcid parasites of cockroach eggs. A, Anastatus floridanus ovipositing into an oötheca which is still being carried by Eurycotis floridana, c. X 4. B, Comperia merceti ovipositing into an oötheca of Supella supellectilium, c. X 13. C, Tetrastichus hagenowii ovipositing into an oötheca of Periplaneta americana, c. X 10. (C from Roth and Willis [1954b].) | A-36 |
| 35 | A, The wasp finds a cockroach. B, She stings the prey in the thorax. C, She then leads the disabled cockroach (antennae clipped) to her nest. D, The wasp's egg was placed on the coxa of the cockroach's right mesothoracic leg where it hatched. E, Portion of the host's abdomen removed to show feeding larva. F, New adult wasp emerging from dead host. (Reproduced from F. X. Williams [1942] from the color paintings of the late W. Twigg-Smith, through the courtesy of F. A. Bianchi.) | A-37 |
| 36 | Chemical defense of Diploptera punctata against predators; the spray pattern is displayed on KI-starch indicator paper. A, Spray pattern after right mesothoracic leg was pinched. B, Cumulative spray pattern after left mesothoracic leg of the same insect was pinched. C, The defensive glands of the cockroach on the left had been excised, and it is under persistent attack by ants from a laboratory colony of Pogonomyrmex badius (Latreille). The intact cockroach on the right was also attacked by the ants, but it discharged a spray of quinones and repelled the attackers. (From Eisner [1958], through the courtesy of Dr. T. Eisner.) | A-38 |
| Fig. | Page | |
| 1 | Diagram illustrating the relationship between a mature plant of Herpomyces stylopygae and the integument of Blatta orientalis. (Reproduced from Richards and Smith [1956], through the courtesy of Dr. A. G. Richards.) | 128 |
| 2 | Representative Protozoa associated with cockroaches. A, Monocercomonoides melolonthae, X 3094 (after Grassé). B, Coelosporidium periplanetae, X 1310 (after Sprague); trophozoite with spores and chromatoid bodies. C, Endamoeba blattae, X 273 (after Kudo); trophozoite. D, Lophomonas striata, X 330 (after Kudo). E, Lophomonas blattarum, X 660 (after Kudo). F, Retortamonas blattae, X 3094 (after Wenrich). G, Nyctotherus ovalis, X 175 (after Kudo). H, Gregarina rhyparobiae, c. X 52: mature trophozoite attached to intestinal wall of Leucophaea maderae. (Redrawn from J. M. Watson [1945].) I, Diplocystis schneideri, c. X 14.4 (after Kunstler). J, Gregarina blattarum, c. X 57 (after Kudo). K, Protomagalhaesia serpentula, X 36 (after Pinto). L, Gamocystis tenax, magnification not known (after Schneider). (All figures except H redrawn from Kudo [1954] after sources indicated.) | 168 |
| 3 | Protozoa from the gut of the wood-feeding cockroach Cryptocercus punctulatus. A, Eucomonympha imla, female above, male below, c. X 375. (From Cleveland [1950c].) B, Barbulanympha sp. (From Cleveland [1953].) C, Urinympha talea, c. X 712. (From Cleveland [1951a].) D, Rhynchonympha tarda, c. X 450. (From Cleveland [1952].) E, Trichonympha okolona or T. algoa, c. X 390. (From Cleveland [1949].) (All drawings reproduced through the courtesy of Dr. L. R. Cleveland.) | 175 |
| 4 | The cockroach mite, Pimeliaphilus podapolipophagus. (From Baker et al. [1956]; reproduced through the courtesy of Dr. E. W. Baker and the National Pest Control Association.) | 221 |
| 5 | Evania appendigaster. Left, dorsal view, X 8. Right, side view, X 5. (Reproduced with permission. British Museum [Natural History], 1951, figs. 1A and 1B.) | 238 |
| 6 | Ampulex canaliculata attacking Parcoblatta virginica. A, Female wasp stinging her prey, c. X 4.8. B, Wasp's egg attached to the coxa of the mesothoracic leg of the cockroach. C, Larva of A. canaliculata (about three-quarters grown) feeding on the internal organs of the host from the exterior, c. X 4. (Reproduced from F. X. Williams [1929], through the courtesy of Dr. F. X. Williams and F. A. Bianchi, Hawaiian Sugar Planters' Association.) | 258 |
| 7 | Cockroach-hunting wasps. A, Dolichurus stantoni leading a nymph of Blattella lituricollis to her nest, c. X 4. (Reproduced from F. N. Williams [1919].) B, Podium haematogastrum attaching her egg to an Epilampra sp. while on the side of a termite mound that contains the wasp's nest, c. X 1.6. C, Epilampra sp. parasitized by P. haematogastrum showing the wasp's egg attached to the right fore coxa, c. X 3.2. (B and C reproduced from Williams [1928], through the courtesy of Dr. F. X. Williams and F. A. Bianchi.) | 263 |
By Louis M. Roth and Edwin R. Willis[2]
Pioneering Research Division, United States Army
Quartermaster Research and Engineering Center
Natick, Mass.
(With 37 Plates)
With most of us collectors the life history of an insect begins in the net and ends in the bottle.
Hanitsch (1928)
Recently we brought together much of the literature linking cockroaches with the transmission of certain organisms that cause disease in man and other vertebrates. In that paper (1957a) we concluded that cockroaches, being potential vectors of pathogenic agents, should not be regarded simply as minor annoyances. Obviously the associations of cockroaches with agents of vertebrate diseases are of more immediate importance than their relations with pathogens of lower animals or with nonpathogens. On the other hand, cockroaches are of general economic as well as medical importance, and their control is sought by many who are unaware of their medical significance. That the control of domiciliary cockroaches is far from satisfactory may be inferred from current entomological and pest-control journals in which new insecticides are continually advocated to replace others found to be inadequate. Possibly new approaches to the control of cockroaches are needed. Whether these lie in the direction of increased use of parasites and predators for the biological control of these insects remains to be seen. In any event, the more we know about any insect, especially its ecology, the greater the likelihood of achieving satisfactory control. In order to advance knowledge in any field of science, new research should proceed from the results of prior investigations when these exist. We hope that the observations and experiments cited herein may suggest areas for future research and exploitation.
To the best of our knowledge no previous publication has brought together the vast literature on the parasites, predators, commensals, and other symbiotic associates of the Blattaria. For this reason, we have tried to assemble observations on all such known associations. Undoubtedly we have overlooked some records, as, for example, those buried in papers dealing with other phases of cockroach biology. We hope that such inadvertent omissions will not seriously impair the usefulness of this compilation. Whatever its defects, this review should be a unified source of information for all who are interested in the biotic associates of cockroaches.
In addition to previously published information, this monograph also contains original records and observations on the associations of cockroaches that are reported here for the first time. Although some of the observations were made by us, others were made by colleagues who have graciously made their knowledge available to us in private communications.
Chopard (1938) in his book La Biologie des Orthoptères reviewed much of the literature on cockroaches, but of the many biotic associations that exist he discussed only the commensal cockroaches, gregariousness, and familial associations. Asano (1937), who reviewed the natural enemies of cockroaches, mentioned about 10 groups of animals that attack cockroaches. Thompson (1951) in his Parasite Host Catalogue, which was based mainly on papers abstracted or noted in the Review of Applied Entomology, listed only 19 insect parasites of cockroaches. Eighteen of these were Hymenoptera which attack only cockroach eggs; the single dipteron listed (Sarcophaga lambens Wiedemann, supposedly parasitic on Pycnoscelus surinamensis) is not a parasite in this case, but deposits its eggs on the dead insects (see p. 229). Cameron (1955) listed as parasites and predators of the cockroach 24 species of hymenopterous egg parasites, 7 species of Ampulex which hunt nymphs and adults, 17 Protozoa, 13 nematodes, 5 bacteria, 2 mites, and a few other miscellaneous predators. In his classified list of the protozoan parasites of the Orthoptera of the world, Semans (1943) listed about 26 species from cockroaches. Linstow (1878, 1889) recorded 14 species of helminths from cockroaches. Van Zwaluwenburg (1928) listed 33 names of roundworms which [Pg 3] are commensals or secondary parasites of cockroaches, but some of these names are synonyms. La Rivers (1949) extended this list with 13 additional species. Chitwood (1932) recognized 24 species of nematodes which are primary parasites (probably commensals) of blattids. Steinhaus (1946) gave many instances of biological relationships between cockroaches and bacteria, fungi, and yeasts, but the cockroaches were not discussed as an entity and the information is scattered throughout the book.
In surveying the literature on this subject we have collected a far more extensive list of animals and plants associated with cockroaches than one might have expected from an examination of any one of the previous papers on this subject. In our review of the medically important organisms associated with the Blattaria, we pointed out that in addition to many experimental associations cockroaches have been found to harbor, naturally, 4 strains of poliomyelitis virus, about 40 species of pathogenic bacteria, the eggs of 7 species of pathogenic helminths, and to serve as intermediate hosts of 12 other species of helminths pathogenic for vertebrates; cockroaches have also been found to carry, on occasion, 3 species of Protozoa that are pathogenic to man and 2 species of fungi which are sometimes found associated with pathological conditions.
In addition to the above organisms of medical importance, we have compiled records of other organisms, nonpathogenic to vertebrates, which are naturally associated in some way with cockroaches. None of the following numbers can be considered absolute because some names may be synonyms. However, we believe that these figures are very close to the actual numbers of species that have been isolated because we have attempted to refer all obvious synonyms to the currently accepted name for each organism. On this basis there are about 45 species of bacteria, 40 fungi, 6 yeasts, 90 Protozoa, and 45 helminths that have been found associated naturally with cockroaches. Of the arthropods there are about 2 species of scorpions, 4 spiders, 15 mites, 4 centipedes, and 90 insects. Of vertebrates there are 4 species of fish, 16 amphibians, 12 reptiles, 20 birds, and 27 mammals. Besides these there are many records of experimental associations that have been contrived in the laboratory.
Some idea of the increase in our knowledge of the biotic associations of cockroaches, during the last 70 years, may be gathered from a comparison of the above figures with those of Miall and Denny (1886) who presented " ... a long list of parasites which infest the Cockroach." This list included 2 bacteria, 6 Protozoa (some of the names are synonyms), 7 nematodes (some of these names are also [Pg 4] synonyms), 1 mite, 1 wasp, and 1 beetle. In addition, they mentioned as other foes of the cockroach: monkeys, hedgehogs, polecats, cats, rats, birds, chameleons, and frogs.
We have listed the organisms known to be associated with cockroaches systematically by phylum, class, order, and family. Within each family the organisms are listed alphabetically by genus and species. Under each organism the associated cockroaches are listed as natural or experimental hosts, vectors, or prey. Identified cockroaches are listed by the currently accepted name. Unidentified cockroaches are indicated by the word "Cockroaches." The name of each cockroach is followed by the country in which the observation was made, the authority for the record, and with a few exceptions[3] pertinent biological information, where this is known. Question marks following the names of organisms or countries indicate tentative or questionable identifications.
Records of predators capturing and feeding on cockroaches in zoos and on shipboard we consider natural, even though it is very likely that these particular predators would not normally have access to this prey in nature.
Experimental prey are cockroaches that were fed to predators in the laboratory. Although these predators may have little, if any, access to these cockroaches in nature, we have included such records to indicate the relative acceptability of cockroaches as food by a wide variety of animals.
Records of presumed or known cockroach associates that give no information about an associated cockroach are not included in this review, even though certain of these (e.g., species of Ampulex, Evania, Podium) probably prey upon or parasitize cockroaches exclusively.
The validity of a host-parasite or predator-prey record is dependent upon the accuracy and knowledge of the observer. In assembling these records we have had to accept, in most instances, the identifications of species made by the original authors. However, as a result of our studies on the biology of various species of cockroaches, including some work on their hymenopterous parasites, we have questioned certain records in the literature. Other dubious records which have been perpetuated from one publication to the next, but which apparently were not based on fact, have also been questioned or have been clarified with the aid of specialists in particular groups.
Because the records cover a period of many years, the names of many of the organisms as well as the names of some of the cockroaches have been changed. Although it would have been comparatively simple to list the names as they appeared in the original references, this would have resulted in misleading redundancy with the same organism being catalogued under several synonyms. We have attempted to list each organism by its currently accepted name. However, no attempt was made to prepare complete taxonomic synonymies; the only synonyms given are those that identify the organisms by the names used by the authors of the papers cited. The synonyms under which the cockroaches may have been cited originally are listed in section II. The synonyms of associated organisms are listed with each organism. Although authorities for the name changes of the cockroaches are given, these workers are not necessarily those who were initially responsible for the synonymies. Various sections have been checked by specialists in the particular groups. Although we have accepted name changes suggested by these reviewers, we assume full responsibility for the names.
After having examined thousands of references on cockroaches, we are impressed by how little is known about the biology of most species. As a conservative estimate there are 3,500 described species of Blattaria (J. W. H. Rehn, 1951). In our literature survey we found records of biotic associations for about 400 species. Unfortunately, many of these records contain only a sentence or two of biological information. Our detailed knowledge of cockroaches is based on studies of the few domiciliary pests that man attempts to eradicate. Comparable studies of the bionomics of the less-well-known species should add much valuable information to our knowledge of this ancient group.
Our understanding of most predator-prey and parasite-host relationships has barely progressed beyond the taxonomic stage. The total effect of predators and parasites in limiting natural populations of cockroaches remains to be determined. It is still not known how, for example, predatory or parasitic wasps select specific cockroaches from [Pg 6] among all other insects. Secretions produced by certain cockroaches (e.g., 2-hexenal by Eurycotis floridana) will ward off certain predators. The identities and biological activities of most cockroach secretions are unknown, but the use of protective chemicals against predators may be widespread among cockroaches. If so, how effective are these repellents in protecting the individual or the species? It is not known whether cockroaches are protected by apparent mimetic resemblances to other arthropods. There is no experimental proof that insect parasites can successfully attack the eggs of cockroaches that incubate their eggs while they are being carried by the female.
It is conceivable that biological control of cockroaches might be achieved in limited areas such as man-made structures or sewers, but this possibility has not been thoroughly explored. It would be informative to know what effects, if any, organisms such as bacteria, Protozoa (e.g., gregarines), intestinal nematodes, or other helminths have on cockroaches. Possibly pathogenic microorganisms can be used for biological control of cockroaches; this approach seems to have been little explored.
Associations of colonial cockroaches (e.g., Cryptocercus spp.) may be truly familial or they may merely result from gregariousness. Newly hatched nymphs of species that carry their oöthecae until the eggs hatch cluster near the mother. This may be a response to the mother as such, a search for shelter beneath the nearest object (thigmotaxis? or negative phototaxis?), or there may well be yet another explanation. Tepper in 1893 stated that the native cockroaches of Australia are almost wholly carnivorous; little supporting evidence for this claim has been brought forward since that time. The apparent supersedure of one species of domiciliary cockroach by another may result from antagonism between different species, or it may result from more rapid breeding and more effective utilization of available food and space; but which? Several species of cockroaches are frequently found associated with certain plants (e.g., bromeliads and bananas); the ecological relations in these associations remain to be determined. Many of the obscure associations between cockroaches and other insects, spiders, birds, and burrowing animals have never been adequately defined. The factors influencing cannibalism have never been thoroughly investigated experimentally. These are only a few ideas for future work that have occurred to us during the preparation of this review. We hope that these suggestions as well as other questions that may occur to readers will stimulate further research in areas where it is obviously needed.
Unless otherwise credited, the illustrations were prepared from photographs taken by the authors. Except where otherwise stated, all photographs were taken of unposed living specimens.
The cockroaches referred to in this paper are listed below. The currently accepted name for each species is given alphabetically by genus and species irrespective of its taxonomic affinities. Synonyms used by certain authors whose work we have quoted are given in brackets under the respective species; the synonymy is supported by the reference citation that follows each synonym. References to illustrations of certain species (e.g., Blaberus craniifer) that appear in the paper follow the names of the describers.
Agis orientalis Chopard
Aglaopteryx absimilis Gurney
diaphana (Fabricius) [Ceratinoptera diaphana Fabricius; Rehn and Hebard (1927)]
facies (Walker) [Aglaopteryx devia Rehn; Princis (1929). A. diaphana (Fabricius) in records from Puerto Rico only; Rehn (1932b); Gurney (1937)]
gemma Hebard [In Florida records = Ceratinoptera diaphana R. and H.; Hebard (1917)]
vegeta Rehn
ypsilon Princis
Allacta similis (Saussure) [Phyllodromia obtusata Brunner; Zimmerman (1948)]
Alluaudellina cavernicola (Shelford) [Alluaudella cavernicola Shelford; Chopard (1932)]
Amazonina emarginata Princis
Anaplecta asema Hebard
azteca Saussure
decipiens Saussure and Zehntner
fallax Saussure
hemiscotia Hebard
lateralis Burmeister
mexicana Saussure
Aneurina tahuata Hebard
viridis Hebard
Apotrogia angolensis Kirby [Acanthogyna deplanata Chopard; Princis (1957)]
Aptera fusca (Thunberg) [Aptera cingulata (Burmeister); Gurney (personal communication, 1957)]
Apteroblatta perplexa Shelford
Archiblatta hoevenii Vollenhoven
Archimandrita marmorata (Stoll)
tessellata Rehn
Arenivaga apacha (Saussure)
bolliana (Saussure)
erratica (Rehn)
floridensis Caudell
grata Hebard
roseni (Brancsik) [Heterogamodes roseni; Bei-Bienko (1950). "Polygamia" roseni is undoubtedly an erroneous citation of Polyphaga roseni, as there is no genus Polygamia (Gurney, personal communication, 1957)].
tonkawa Hebard
Aristiger histrio (Burmeister) [Plumiger histrio (Burm.); Bruijning (1948). Hemithyrsocera histrio Burm.; Hebard (1929)]
Aspiduchus boriquen J. W. H. Rehn [In Puerto Rico records = Aspiduchus deplanatus R. and H.; Rehn, J. W. H. (1951a)]
cavernicola J. W. H. Rehn[Pg 8]
deplanatus (Saussure)
Attaphila aptera Bolívar
bergi Bolívar
flava Gurney
fungicola Wheeler
schuppi Bolívar
sexdentis Bolívar
Atticola mortoni Bolívar
Audreia bromeliadarum Caudell
jamaicana Rehn and Hebard
Balta godeffroyi (Shelford)
patula (Walker)
platysoma (Walker) [Temnopteryx platysoma (Walker); Hebard (1943)]
quadricaudata Hebard
scripta (Shelford)
torresiana Hebard
verticalis Hebard
Bantua stigmosa (Krauss) [Derocalymma stigmosa Krauss; Princis (1957)]
Blaberus atropos (Stoll) [Blabera fusca Brunner; Hebard (1917)]
boliviensis Princis
craniifer Burmeister (pls. 1, 2)
discoidalis Serville [Blaberus cubensis Saussure; Hebard (1916)]
giganteus (Linnaeus) (pl. 3)
Blaptica dubia (Serville) [Blaberus clarazianus Saussure; Rehn, J. W. H. (1951)]
Blatta orientalis Linnaeus (pl. 4) [Periplaneta orientalis; Hebard (1917)]
(Shelfordella) lateralis (Walker) [Shelfordella tartara (Saussure); Princis (1957). Periplaneta tartara Saussure; Bei-Bienko (1950)]
Blattella germanica (Linnaeus) (pls. 5, A, B; 31, F) [Blatella germanica; Gurney (1952). Phyllodromia germanica; Hebard (1917). Ectobius germanicus; Gurney (personal communication, 1957)]
humbertiana (Saussure) [Blatta humbertiana; Phyllodromia humbertiana; Hebard (1929)]
lituricollis (Walker) (fig. 7, A) [Blattella bisignata (Brunner); Bei-Bienko (1950)]
schubotzi Shelford
vaga Hebard (pl. 5, C, D)
Buboblatta armata (Caudell) [Latindia armata Caudell; Hebard (1920)]
Byrsotria cabrerae Rehn and Hebard
fumigata (Guérin) (pl. 6)
Cahita borero Rehn
nahua (Saussure)
Capucinella delicatula Hebard
Cariblatta antiguensis (Saussure and Zehntner)
cuprea Hebard
delicatula (Guérin) [Blattella delicatula Guérin; Cariblatta punctulata (Beauvois); Rehn and Hebard (1927)]
hylaea Rehn
imitans Hebard
insularis (Walker)
landalei Rehn and Hebard
lutea lutea (Saussure and Zehntner)
lutea minima Hebard (pl. 7, A, B)
nebulicola Rehn and Hebard
orestera Rehn and Hebard
punctipennis Hebard
reticulosa (Walker)
stenophrys Rehn and Hebard
Cariblattoides instigator Rehn and Hebard
suave Rehn and Hebard
Ceratinoptera picta Brunner
Chorisoneura barbadensis Rehn and Hebard
flavipennis Saussure and Zehntner
formosella Rehn and Hebard
parishi Rehn
specilliger Hebard
texensis Saussure and Zehntner [Chorisoneura plocea Rehn; Rehn and Hebard (1916)]
translucida (Saussure)
Choristima sp.[Pg 9]
Choristimodes sp.
Chromatonotus infuscatus (Brunner)
notatus (Brunner)
Compsodes schwarzi (Caudell)
Comptolampra liturata (Serville) [Compsolampra liturata; Comptolampra is the original spelling, which is followed by Dr. K. Princis, according to Gurney (personal communication, 1959)]
Cosmozosteria lateralis (Walker)
Cryptocercus punctulatus Scudder (pl. 8, A)
relictus Bei-Bienko
Cutilia nitida (Brunner)
soror (Brunner)
sp. near sedilloti (Bolívar) (pl. 9) [Determined by Dr. A. B. Gurney from photographs.]
Cyrtotria capucina (Gerstaecker)
Dendroblatta sobrina Rehn
Derocalymma cruralis (Stål) [Homalodemas cruralis (Stål); Gurney (personal communication, 1957)]
lampyrina Gerstaecker
porcellio Gerstaecker
Deropeltis autraniana Saussure
erythropeza Adelung
melanophila (Walker)
nigrita Saussure
Diploptera punctata (Eschscholtz) (pls. 10, 36) [Diploptera dytiscoides (Serville); Princis (1950). Eleutheroda dytiscoides (Serville); Zimmerman (1948)]
Dryadoblatta scotti (Shelford) [Homalopteryx scotti Shelford; Rehn (1930)]
Ectobius africanus Saussure
albicinctus (Brunner)
duskei Adelung
lapponicus (Linnaeus) [Ectobius perspicillaris Herbst, as used by Lucas (1920); Blair (1934)]
lucidus Hgb.
nicaeensis (Brisout)
pallidus (Olivier) (pls. 7, C; 29, A) [Ectobius lividus (Fabricius); Ectobius livens (Turton); Kevan (1952); Princis (in Roth and Willis, 1957)]
panzeri Stephens [Ectobius ericetorum (Wesmaël); Ramme (1923)]
panzeri var. nigripes Stephens
semenovi Bei-Bienko
sylvester (Poda) [Ectobius sylvestris (Poda); Ramme (1951)]
tadzihicus Bei-Bienko
vittiventer (Costa) [Ectobius vittiventris (Costa); Ramme (1951)]
Ellipsidion Saussure [Apolyta Brunner; Hebard (1943)]
affine Hebard
australe Saussure [Ellipsidion pellucidum (Brunner); Hebard (1943)]
bicolor (Tepper)
simulans Hebard
variegatum (Fabricius) [Ellipsidion aurantium Saussure; Hebard (1943)]
Epilampra abdomen-nigrum (De Geer)
annandalei Shelford
azteca Saussure
conferta Walker
conspersa Burmeister
grisea (De Geer)
maya Rehn
mexicana Saussure
mona Rehn and Hebard
notabilis Walker
sodalis Walker
tainana Rehn and Hebard
wheeleri Rehn
sp. (fig. 7, B, C)
Eremoblatta subdiaphana (Scudder)
Ergaula capensis (Saussure) [Dyscologamia capensis Saussure; Dyscologamia wollastoni Kirby; Princis (1957)]
scarabaeoides Walker [Dyscologamia piolosa (Walker); Princis (1957). Parapolyphaga erectipilis Chopard; Princis (1950). Dyscologamia chopardi Hanitsch; Bruijning (1948). Miroblatta silphoides Chopard; Hebard (1929)].
Escala sp.[Pg 10]
Euandroblatta palpalis Chopard
Eublaberus posticus (Erichson)
Eudromiella bicolorata Hebard
calcarata Bei-Bienko
Euphyllodromia angustata (Latreille)
liturifera [Euphyllodromia decastigmata Hebard; Princis (1959)]
Eurycotis bananae Bei-Bienko
biolleyi Rehn [Eurycotis carbonaria Biolley; Rehn (1918)]
caraibea (Bolívar)
decipiens (Kirby)
dimidiata (Bolívar)
ferrum-equinum Rehn and Hebard
floridana (Walker) (pl. II) [Platyzosteria ingens Scudder; Platyzosteria sabalianus Scudder and hence, by inference, Eurycotis sabalianus (Scudder); Hebard (1917)]
galeoides Rehn and Hebard
improcera Rehn
kevani Princis
lixa Rehn
manni Rehn
opaca (Brunner)
Euthlastoblatta abortiva (Caudell)
Euthyrrhapha nigra Chopard
pacifica Coquebert
Geoscapheus robustus Tepper
Graptoblatta notulata (Stål) [Blatta notulata Stål; Hebard (1929). Phyllodromia hieroglyphica Brunner; Kirby (1904)]
Gromphadorhina laevigata S. and Z.
portentosa (Schaum) (pl. 12, A, B)
Gyna kazungulana Giglio-Tos
maculipennis (Schaum) [Gyna vetula Brunner; Shelford (1909b)]
tristis Hanitsch
Hebardina concinna (Haan) [Blatta concinna Haan; Blattina concinna (Haan); Bei-Bienko (1950)]
Hemiblabera brunneri (Saussure)
Henicotyle antillarum (Brunner)
Heterogamodes krügeri (Salfi)
rugosa (Schulthess)
Holocompsa azteca (Saussure)
cyanea (Burmeister)
fulva (Burmeister)
metallica Rehn and Hebard
nitidula (Fabricius)
zapoteca Saussure
Hololampra bivittata (Brullé)
chavesi (Bolívar)
maculata (Schreber) [Aphlebia maculata Schreber; Harz (1957); Gurney (personal communication, 1959)]
marginata (Schreber)
punctata (Charpentier) [Aphlebia punctata Charpentier; Ramme (1951)]
Hololeptoblatta sp.
Homalopteryx laminata Brunner
Hoplosphoropyga babaulti Chopard
Hormetica apolinari Hebard
laevigata Burmeister
ventralis Burmeister
Ignabolivaria bilobata Chopard
Ischnoptera deropeltiformis (Brunner) (pl. 12A) [Temnopteryx deropeltiformis Brunner; Hebard (1917)]
panamae Hebard
podoces Rehn and Hebard
rufa occidentalis Saussure
rufa rufa (De Geer)
schenklingi Karney
Karnyia discoidalis (Brunner)
Kuchinga hemerobina (Gerstaecker) [Phyllodromia hemerobina Gerstaecker; Rehn (1932)]
remota Hebard
Lamproblatta albipalpus Hebard
meridionalis (Brunner)
Latiblattella chichimeca (Saussure and Zehntner) [Blattella chichimeca S. and Z.; Hebard (1932)]
lucifrons Hebard[Pg 11]
rehni Hebard
vitrea (Brunner)
zapoteca (Saussure)
Leucophaea maderae (Fabricius) (pl. 13) [Rhyparobia maderae; Hebard (1917). Panchlora maderae; Kirby (1904). Very probably "Blaberus" maderae is a careless reference to this species; Gurney (personal communication, 1957)]
Leurolestes pallidus (Brunner)
Litopeltis biolleyi (Saussure)
bispinosa (Saussure) [Audreia marginata Caudell; Hebard (1920)]
deianira Rehn
musarum Rehn
Lobolampra subaptera Rambur
Loboptera decipiens (Germar)
thaxteri Hebard
Lobopterella dimidiatipes (Bolívar) [Loboptera dimidiatipes (Bolívar); Princis (1957a). Loboptera sakalava (Saussure); Hebard (1933a). Loboptera extranea Perkins; Hebard (1922). Princis (1957a) in erecting Lobopterella pointed out that only the nontypical variety of sakalava is identical with dimidiatipes.]
Lophoblatta arawaka Hebard
Macropanesthia rhinocerus Saussure
Mareta acutiventris Chopard
Maretina uahuka Hebard
Megaloblatta blaberoides (Walker) [Megaloblatta rufipes Dohrn; Hebard (1920)]
Megamareta verticalis Hebard
Melanosilpha capensis Saussure and Zehntner
Methana canae Pope
curvigera (Walker)
marginalis (Saussure)
Moluchia (?) dahli Princis
Monastria biguttata (Thunberg)
Muzoa madida Rehn
Myrmeblattina longipes (Chopard)
Myrmecoblatta rehni Mann
wheeleri Hebard
Namablatta bitaeniata (Stål)
Nauclidas nigra (Brunner) [Poroblatta nigra Brunner; Rehn (1930)]
Nauphoeta cinerea (Olivier) (pl. 14) [Nauphoeta bivittata Burmeister; Zimmerman (1948)]
flexivitta (Walker) [Nauphoeta brazzae (Bolívar); Rehn (1937)]
punctipennis Chopard
Nelipophygus ramsdeni Rehn and Hebard
Neoblattella brunneriana (Saussure) [Blattella brunneriana; Gurney (personal communication, 1959)]
carcinus Rehn and Hebard
celeripes Rehn and Hebard
detersa (Walker)
dryas Rehn and Hebard
eurydice Rehn and Hebard
fratercula Hebard
fraterna (Saussure and Zehntner)
grossbecki Rehn and Hebard
laodamia Rehn and Hebard
nahua (Saussure) [Blattella nahua Saussure and Zehntner of Caudell (1914); Hebard (1920)]
proserpina Rehn and Hebard
semota Rehn and Hebard
tridens Rehn and Hebard
vatia Rehn and Hebard
Neostylopyga rhombifolia (Stoll) (pl. 15) [Dorylaea rhombifolia; Rehn (personal communication, 1956)]
Nesomylacris cubensis Rehn and Hebard
relica Rehn and Hebard
Nocticola bolivari Chopard
caeca Bolívar
decaryi Chopard
simoni Bolívar
sinensis Silvestri
termitophila Silvestri
Nothoblatta wasmanni (Bolívar)[Pg 12]
Notolampra antillarum Shelford
Nyctibora azteca Saussure and Zehntner
brunnea (Thunberg)
laevigata (Beauvois)
lutzi Rehn and Hebard
mexicana Saussure
noctivaga Rehn
obscura Saussure
sericea Burmeister
stygia Walker
tomentosa Serville [Nyctibora latipennis Burmeister; Hebard (1917, p. 263)]
Oniscosoma granicollis (Saussure)
Opisthoplatia maculata Shiraki
orientalis (Burmeister)
Oulopteryx meliponarum Hebard
Oxyhaloa buprestoides (Saussure)
deusta (Thunberg)
Panchlora antillarum Saussure
exoleta Burmeister
fraterna Saussure and Zehntner
nivea (Linnaeus) (pl. 16) [Panchlora cubensis Saussure; Gurney (1955). Pycnosceloides aporus Hebard; Hebard (1921c)]
peruana Saussure
sagax Rehn and Hebard
virescens (Thunberg)
Panesthia angustipennis (Illiger) [Panesthia javanica Serville; Hebard (1929)]
australis Brunner (pl. 8, B)
laevicollis Saussure
lobipennis Brunner
spadica (Shiraki)
Parahormetica bilobata (Saussure)
Parcoblatta americana (Scudder)
bolliana (Saussure and Zehntner) [Kakerlac schaefferi Rehn; Hebard (1917)]
caudelli Hebard [♀♀ of Ischnoptera insolita R. and H.; Ischnoptera uhleriana fulvescens S. and Z. (in part); Hebard (1917)]
desertae (Rehn and Hebard) [♂♂ of Ischnoptera insolita R. and H.; Hebard (1917)]
divisa (Saussure and Zehntner) [Ischnoptera divisa S. and Z.; Hebard (1917)]
fulvescens (Saussure and Zehntner) [Ischnoptera uhleriana fulvescens S. and Z. (in part); Hebard (1917)]
lata (Brunner) [Ischnoptera couloniana R. and H. (not Saussure); Ischnoptera major R. and H. (not S. and Z.); Hebard (1917)]
notha Rehn and Hebard
pensylvanica (De Geer) (pl. 17, A) [Ischnoptera pennsylvanica Saussure; Hebard (1917)]
uhleriana (Saussure) (pl. 18) [Ischnoptera uhleriana Saussure; Hebard (1917)]
virginica (Brunner) (pls. 17, B; 27, A; 33, C; fig. 6) [Ischnoptera borealis Brunner; Hebard (1917)]
zebra Hebard
Pelmatosilpha coriacea Rehn
kevani Princis
marginalis Brunner
purpurascens (Kirby)
rotundata Scudder
vagabunda Princis
Periplaneta americana (Linnaeus) (pls. 19, 35) [Stylopyga americana; Blatta americana L.; Hebard (1917)]
australasiae (Fabricius) (pls. 20, 32)
brunnea Burmeister (pl. 21)
cavernicola Chopard
fuliginosa (Serville) (pl. 22)
ignota Shaw
lata (Herbst)
Perisphaerus armadillo Serville
glomeriformis (Lucas)
Phaetalia pallida (Brunner)
Phidon (?) dubius Princis
Phlebonotus pallens (Serville)
Pholadoblatta inusitata (Rehn)
Phorticolea boliviae Caudell
testacea Bolívar
"Phyllodromia" treitliana Werner
Phyllodromica brevipennis (Fischer) [Pg 13]
graeca (Brunner)
irinae (Bei-Bienko)
maculata (Schreber)
megerlei (Fieber)
polita (Krauss)
pygmaea (Bei-Bienko)
tartara (Saussure)
tartara nigrescens Bei-Bienko
Platyzosteria analis (Saussure) [Polyzosteria analis Saussure; Kirby (1904)]
armata Tepper
bifida (Saussure)
castanea (Brunner)
novae seelandiae (Brunner) (pl. 23) [Periplaneta fortipes Walker; Shelford (1912); Platyzosteria novae-zealandiae]
scabra (Brunner)
Plectoptera dorsalis (Burmeister)
infulata (Rehn and Hebard)
lacerna Rehn and Hebard
perscita Rehn and Hebard
poeyi (Saussure) [Plectoptera floridana Hebard; Rehn and Hebard (1927)]
porcellana (Saussure)
pygmaea (Saussure)
rhabdota (Rehn and Hebard)
vermiculata Rehn and Hebard
Polyphaga aegyptiaca (Linnaeus) [Blatta aegyptiaca L.; Bei-Bienko (1950). Heterogamia aegyptiaca (L.); Gurney (personal communication, 1957). "Polygamia" aegyptiaca; according to Gurney (p. c.), there is no genus Polygamia and almost surely the reference is to Polyphaga aegyptiaca.]
indica Walker [Polyphaga pellucida (Redtenbacher); Princis (1957)]
saussurei (Dohrn)
Polyzosteria limbata Burmeister
melanaria (Erichson)
Pseudoderopeltis aethiopica (Saussure) [Blatta aethiopica Saussure; Gurney (personal communication, 1957)]
Pseudomops cincta (Burmeister) [Thyrsocera cincta Scudder; Hebard (1917)]
laticornis Perty
septentrionalis Hebard
Pseudophoraspis nebulosa (Burmeister)
Pycnoscelus niger (Brunner)
striatus (Kirby) [Leucophaea striata Kirby; Gurney (personal communication, 1957)]
surinamensis (Linnaeus) (pl. 24) [Leucophaea surinamensis (L.); Hebard (1917). Blatta melanocephala Stoll; Kirby (1904)]
Rhicnoda natatrix Shelford
Rhytidometopum dissimile Princis
Riatia fulgida (Saussure) [Lissoblatta fulgida (Saussure); Gurney (personal communication, 1959)]
orientis Hebard
Robshelfordia circumducta (Walker) [Escala circumducta (Walker); Gurney (personal communication, 1957)]
longiuscula (Walker) [Escala longiuscula (Walker); Gurney (personal communication, 1957)]
Salganea morio (Burmeister)
Sibylloblatta panesthoides (Walker)
Simblerastes jamaicanus Rehn and Hebard
Spelaeoblatta gestroi Bolívar
Sphecophila polybiarum Shelford
ravana Fernando
termitium Shelford
Steleopyga (?) sinensis Walker [Dr. Gurney (personal communication, 1957) could not find a reference to this species. Walker described species named sinensis in three different genera of cockroaches, and it is uncertain which one this combination represents.]
Stictolampra buqueti concinula (Walker)
Styphon bakeri Rehn
Supella hottentotta (Saussure)
supellectilium (Serville) (pls. 25; 30, B-E; 31, A-E) [Phyllodromia supellectilium (Serv.); Bei-Bienko (1950)]
Symploce breviramis (Hanitsch) [Pg 14]
cavernicola (Shelford) [Ischnoptera cavernicola (Shelford); Phyllodromia nigrocincta Chopard; Hebard (1929)]
curta Hanitsch
flagellata Hebard
hospes (Perkins) [Symploce lita Hebard; Hebard (1922)]
jamaicana (Rehn)
kevani Chopard
parenthesis (Gerstaecker) [Phyllodromia parenthesis Gerstaecker; Rehn (1932)]
remyi (Hanitsch) [Ischnoptera remyi Hanitsch; Chopard (1938)]
ruficollis (Fabricius) [Symploce bilabiata Rehn and Hebard; Princis (1949a)]
Tartaroblatta karatavica Bei-Bienko
Temnopteryx obliquetruncata Chopard
phalerata (Saussure)
Theganopteryx straminea Chopard
Therea nuptialis (Gerstaecker) [Corydia nuptialis Gerstaecker; Princis (1950)]
Tivia australica Princis
brunnea (Chopard)
fulva (Burmeister)
macracantha Chopard
obscura (Chopard)
Typhloblatta caeca (Chopard) [Spelaeoblatta caeca Chopard; Chopard (1924b)]
Typhloblattodes madecassus Chopard
Xestoblatta festae (Griffini)
immaculata Hebard
The ecology of extinct cockroaches is necessarily a highly speculative subject. From the coexistence of fossil cockroaches and fossil plants in the same geological stratum, one might conclude that there had been intimate associations between them during prehistoric life. Heer (1864) and Goldenberg (1877) suggested that Carboniferous cockroaches fed on the plants with which they have been found as fossils. Scudder (1879) concurred with this hypothesis. However, Bolton (1911), remarking on the noticeable associations of blattoid wings with vegetable remains, suggested that the cockroaches may have been partly carnivorous, feeding on the snails Spirorbis pusillus, which were attached to the leaves of Cordaites. Yet the proximity of fossil insects and plants in the same geological formation is hardly proof of a similar association during life. In fact, Sellards (1903), Bolton (1921), and Laurentiaux (1951) have all pointed out that the cockroach remains, particularly the more resistant wings, may have been washed into streams by heavy rains and transported with drifting plant material to places where permanent deposits were accumulating.
Some species of fossil cockroaches have long, well-developed ovipositors, very unlike present-day cockroaches whose ovipositors are small and nonprotruding. Brongniart (1889) and Zalesskii (1939, 1953) have suggested that certain Permian and Carboniferous cockroaches [Pg 15] with long ovipositors may have inserted their eggs singly into trees and other plants, rather than protecting the eggs with an oötheca. However, Laurentiaux (1951), although conceding the possibility of egg laying in vegetable material, suggested that oviposition into the earth is more probable because of the unbending nature of the ovipositor.
Although the ecological associations of modern cockroaches should be well known from direct observation, actually most species are still little more than names on museum specimens, and our knowledge of them is fragmentary. All too frequently ecological observations have been only incidental to taxonomic or faunistic studies; yet the biological information that is contained in such papers is all that we know of many species. For this reason we have cited these observations in some detail, especially when they were brief; longer accounts of cockroach bionomics, of necessity, have been abstracted.
Very few exclusively ecological studies of insects have included cockroaches. The native woodroaches (Parcoblatta pensylvanica, P. uhleriana, and P. virginica) of the northern United States were included in ecological studies of the Orthoptera by Hubbell (1922), Strohecker (1937), and Cantrall (1943). Fifteen species of cockroaches were included in an ecological study of the Orthoptera of northern Florida by Friauf (1953). The original papers should be consulted for detailed descriptions of the habitats and accounts of the associated plants and other Orthoptera.
In this chapter the cockroaches are grouped into those that have been found in man-made structures and those that occur in other habitats. Certain species may appear in several categories because they live both indoors and out. The structural pests are divided into cockroaches that occur in land-based structures, those on ships, and those in aircraft. The nonstructural cockroaches are divided into those that occur in quite specific habitats (caves, water, and deserts) and those that occur generally out of doors. Nests of various arthropods serve as microhabitats of commensal cockroaches; these latter associations are discussed on pages 310-318.
In this chapter our discussion is limited to the physical environment and specific habitats of cockroaches, and only very general references are made to associated organisms. The relationships of cockroaches to the biota are examined in detail in subsequent chapters. To show the full extent of the associations, the associates, from bacteroids to vertebrates, are arranged phyletically. These associate-centered classifications serve admirably to relate various species of cockroaches within common bounds, but fail to give an integrated account of the [Pg 16] total biotic relationships in the ecology of each species. Although physically separated in this monograph, the many associates of each species of cockroach should all be considered in appraising the ecology of that species. To assist the reader to achieve this end, we have included a checklist (p. 290) which serves as a convenient index to certain organisms associated with particular species of cockroaches.
Caves, mines, and animal burrows are somewhat similar habitats that provide many species of cockroaches with shelter and frequently with food. The microclimates of these cockroach habitats have not been described in detail in the papers cited, but it seems rather obvious that natural caves, man-made caves (mines), and burrows offer relatively stable temperatures and humidities and protection from adverse climatic conditions. Although such cavernicolous animals as birds and bats periodically leave caves to search for food, cockroaches find the accumulated guano and animal and plant detritus an entirely adequate dietary (Chopard, 1938). Cockroaches in mines presumably subsist on the food and feces dropped by man and mine animals (e.g., pit ponies). Food stored in their nests by burrowing animals is undoubtedly utilized by the associated cockroaches.
Cavernicolous cockroaches show varying degrees of dependence on and adaptation to these specialized habitats. Some of the common domiciliary species (Blatta orientalis, Blattella germanica, and Periplaneta americana) may have accompanied man into caves and remained there after he left (Chopard, 1929a, 1936, 1938). Other species, from the paucity of records noting their occurrence in caves, are undoubtedly accidental inhabitants that may never become established. Besides these, however, many other species of cockroaches have established large breeding colonies in caves. Although some of the latter species show very pronounced morphological adaptations to a cave life, many others resemble their noncavernicolous relatives. The possible origin of cavernicolous Orthoptera has been discussed by Chopard (1938).
Cavernicolous cockroaches have been segregated into four groups according to their ability to adapt to their environment and the degree of their specialized evolution (Chopard, 1936, 1938): (1) Trogloxenes: Cockroaches that occur in caves in a sporadic fashion (the domiciliary cockroaches and accidentals such as Ectobius and Heterogamodes). (2) Troglophiles: Cockroaches found habitually in caves (Symploce, Periplaneta cavernicola). (3) Guanobies: Cockroaches [Pg 17] that live in the guano of cavernicolous vertebrates (Gyna, Acanthogyna, Dyscologamia, Pycnoscelus). (4) Troglobies: Cockroaches that apparently cannot live outside of caves and which show very marked adaptive characters (Alluaudellina, Nocticola, Spelaeoblatta, Typhloblatta). For complete discussions of these groups including descriptions of the adaptive characters shown by certain genera, the original sources should be consulted.
Although we know very little of the ethology of most of the cavernicolous cockroaches, it is intriguing that three of the six known species of Nocticola are cave dwellers, two are inhabitants of termite nests (p. 315), and one (N. bolivari) was found under stones and cement blocks (Chopard, 1950b). In the rather extensive list of cavernicolous cockroaches only two (Arenivaga grata and Parcoblatta sp.) were taken from caves in North America north of Yucatan. All other records are from Africa, Asia, Central America, Europe, West Indies, East Indies, and the Philippine Islands. This we find puzzling. Packard (1888) in his extensive study of the cave fauna of North America listed no cockroaches. Dearolf (1941) found only the above-mentioned Parcoblatta in one of 37 caves in Pennsylvania. Kohls and Jellison (1948) listed no cockroaches among the arthropods from six bat caves in Texas. We would expect Periplaneta americana to inhabit mines in North America, but we have found no such records. Have cockroaches been ignored in fauna collections from North American caves, or has our cave fauna been less extensively studied than that of other parts of the world?
The two species of cockroaches found in mines (Blattella germanica and Periplaneta americana) are also found in caves. For this reason we have included them in the list headed Cavernicolous Cockroaches. On the other hand, the cockroaches found in animal burrows are generally different species from those found in caves, so we have grouped these together in a second list.
Tanganyika.—From Kulumusi caves, near Tanga. The eyes of this cockroach are reduced to a pair of slender streaks (Shelford, 1910a; Chopard, 1932a).
East Africa.—Chopard (1936).
Belgian Congo.—A troglophile without well-marked adaptive characters. Collected in moist sand on floor of a sandstone grotto inhabited [Pg 18] by bats (Chopard, 1927, 1950a). Taken in many caves in Bas Congo (Leleup, 1956).
East Africa.—Accidental inhabitant of cave (Chopard, 1936).
Arizona.—"A female and many nymphs were taken by Flock in the guano in a bat cave in the Tucson Mountains" (Ball et al., 1942).
Puerto Rico.—In limestone cavern by thousands in grass and on walls (Rehn and Hebard, 1927; Rehn, J. W. H., 1951a).
Puerto Rico.—In limestone cave, in caves inhabited by bats, and apparently seen in other caves well removed from entrance. "In this latter situation great numbers were seen on the side walls and roof" (Rehn, J. W. H., 1951a).
Yucatan.—Found once, in Xmahit cave (Pearse, 1938).
Yucatan.—Collected within three caves, near the entrances (Pearse, 1938).
Panama.—Two males and several nymphs were taken under rocks in the second chamber of the Chilibrillo cave; some also were on the walls (Caudell, 1924).
Turkmen S.S.R.—All stages, but more often females and nymphs, were found in the middle and back part of Bakharden cavern, which was inhabited by tens of thousands of bats (Vlasov, 1929).
Turkmen S.S.R.—All stages found in front part of Bakharden bat cave. This cave was uninhabited by man but supported a variety of other animals (Vlasov, 1929).
South Africa.—Numerous in a gold mine on the Witwatersrand (Porter, 1930).
Tonkin.—Chopard (1929a); Colani (1952).
Cuba.—Cueva de las Cucarachas, La Pantana, Baracoa, Oriente Province: 21 specimens, "It is evident ... that the species is also a cave inhabitant" (Rehn and Hebard, 1927).
East Africa.—Found at entrance of cave; not a strictly cavernicolous form according to Chopard (1936).
France.—Nymph in cave in Basses-Pyrénées, accidental inhabitant (Chopard, 1936).
Italy.—In detritus at base of entrance shaft of Acquaviva cave in the Venezia Tridentina (Conci, 1951).
Italy.—Found in the heap of saprophytic detritus at the base of the entrance shaft in the Acquaviva cave (Conci, 1951).
Sumatra.—West coast (Hebard, 1929).
Malaya.—Found burrowing in bat guano among stones at entrance to caves in Selangor (Chopard, 1919, 1929).
Madagascar.—Three males and six females in guano in Antsinomy grotto (Chopard, 1949a).
East Africa.—This species is especially found in caves although it shows no special adaptive characters. It is a typical guanobe (Chopard, 1936).
Belgian Congo.—Troglophile, guanophile. Found in two caves in Lualaba (Leleup, 1956).
Belgian Congo.—In three caves in Uele (Leleup, 1956).
North Africa.—An accidental inhabitant of caves (Chopard, 1938).
Yucatan.—Common throughout rather dry, dusty caves in southern Yucatan (Pearse, 1938).
Stated to be a troglophile by Chopard (1938).
Philippine Islands.—Bolívar (1892).
Madagascar.—A true troglobite according to Chopard (1945).
Philippine Islands.—Bolívar (1892).
Pennsylvania.—Found in Merkle cave, Berks County (Dearolf, 1941).
East Africa.—Its presence in the cave at Shimoni was thought to indicate that man had sought refuge there and brought the cockroaches in with baggage or provisions (Chopard, 1936).
India.—Many present in cave at Vengurla, the floor of which was covered with bird guano (Abdulali, 1942).
Madagascar.—Thought to have been introduced into the cave entrance by man (Chopard, 1945, 1949a).
Great Britain.—In a coal mine at Pontewydd where they had been established for some years (Lucas, 1916). In the Pentre Pit mine where they were abundant (Lucas, 1918). Abundant in a Welch mine 2,166 feet below the surface (Lucas, 1925). This species was [Pg 21] found quite commonly in a number of South Wales coal mines; in one deep mine a white-eyed mutant form comprised about 5 percent of the cockroach population for the preceding 11 years (Jefferson, 1958).
India, western Bengal.—Very numerous in coal mines where the sole food apparently was human faeces (Chandler, 1926).
South Africa.—Numerous in four deep-level gold mines on the Witwatersrand.
Sumatra.—Numerous males and females from Sawah Lunto "'from a coal mine where they lived in great numbers on the faeces of miners'" (Hanitsch, 1929).
Sarawak.—Found swarming on walls of caves and in soft bird guano in company with Symploce cavernicola (Moulton, 1912).
Tonkin.—Chopard (1929a); Colani (1952).
Malaya.—Taken on walls of inner caverns, where they were particularly abundant (Chopard, 1919).
Tonkin.—Chopard suggested that its presence in caves is probably linked with man (Chopard, 1929a; Colani, 1952).
Malaya.—From a cave in Jalor (Annandale et al., 1913).
Malaya.—The wingless females and nymphs mined in bats' guano in a cavern of the Jalor caves (Annandale, 1900).
Turkmen S.S.R.—Females found in front part of Bakharden bat cave on several occasions (Vlasov, 1929).
Turkey.—At Magharadjik and Arab Dede, found in caves with various other animals (Lindberg, 1954).
Burma.—Hsin Dawng Cave, S. Shan States, 1 immature male under stone in complete darkness (Chopard, 1924b).
Tonkin.—Apparently not an accidental inhabitant as nymphs were present (Chopard, 1929a; Colani, 1952).
Malaya.—Found burrowing in bats' guano at entrance to caves in Selangor, where it was very abundant 50 to 600 feet from entrance; also on walls of inner cavern (Chopard, 1919, 1929). In the absence of other evidence, the presence of P. striatus in a cave indicates that bats also inhabit the cave (Chopard, 1929a).
Assam.—Found 300 to 400 feet from entrance of Siju cave in the Garo Hills (Chopard, 1924b).
South Celebes.—Hanitsch (1932).
Burma.—Chopard stated that this species shows marked characteristics of adaptation to a life in darkness (Bolívar, 1897; Annandale, 1913; Chopard, 1919).
South Celebes.—Hanitsch (1932).
Sarawak, Borneo.—Swarming on walls of caves and in soft bird guano on the cave floor (Moulton, 1912). Hanitsch (1931) noted that this species was first recorded by Shelford from a cave in Sarawak and that there is a series from a cave in the Oxford University Museum, taken by Banks in 1928.
Malaya.—On the walls of the inner cavern of a cave at Biserat; the insects covered the walls in places (Chopard, 1919).
Sumatra.—From Baso cavern, on the west coast (Hebard, 1929).
South Celebes.—Hanitsch (1932).
Tonkin.—This seems to be a true cavernicolous species (Chopard, 1929a; Colani, 1952).
Belgian Congo.—A troglophile without well-marked adaptive characters (Chopard, 1950a). At Haut-Katanga, troglophile and guanophile (Leleup, 1956).
Madagascar.—Last-stage nymphs captured in guano in Antsinomy grotto (Chopard, 1949a).
India, Assam.—An eyeless species with noticeably elongated appendages (Chopard, 1945).
Madagascar.—Unpigmented integument and reduced eyes (Chopard, 1945).
Panama.—Found under rocks on guano-covered floor of the Chilibrillo bat caves (Caudell, 1924).
Malaya.—The walls of a cave were covered by dense groups of a species of "Blatta" (Annandale, 1900).
England.—"The chief insect pests of the mines are cockroaches, which often swarm in hot mines and those with pit pony stables...." (Hardy, 1941).
Arizona.—In the nests of wood rats, Neotoma sp. (Hebard, 1917).
Texas.—In the nests of wood rats, Neotoma sp. (Hebard, 1917; 1943a).
Arizona.—The wingless females were commonly found in burrows of Dipodomys spectabilis spectabilis Merriam, the kangaroo rat. The winged males were never found in the burrows (Vorhies and Taylor, 1922). Found most commonly in wood-rat and ground-squirrel dens in the desert regions (Ball et al., 1942).
Florida.—Found in a burrow of Peromyscus polionotus rhoadsi (Bangs), the white-footed mouse (Young, 1949).
Turkmen S.S.R.—Occasionally found in burrows of Rhombomys opimus Lichtenstein; in the burrows of the desert turtle, Testudo horsfieldi Gray; and frequently in burrows of the ground squirrel, Spermophilopsis leptodactylus Lichtenstein (Vlasov, 1933; Vlasov and Miram, 1937).
Texas.—An immature specimen was found in a prairie-dog hole (Hebard, 1943a).
Florida.—It has been taken in burrows of the pocket gopher, Geomys sp. (Hubbell and Goff, 1940).
Texas.—In the nests of wood rats, Neotoma sp. (Hebard, 1917).
Texas.—In the nests of wood rats, Neotoma sp. (Hebard, 1917).
Turkmen S.S.R.—Nymphs and adult females were often found in burrows of the sand mouse, Rhombomys opimus (Vlasov, 1933).
Turkmen S.S.R.—This species prefers sandy soils where it can be found in burrows of Spermophilopsis leptodactylus and Pallasiomys meridionalis pennicilliger Heptner (Vlasov and Miram, 1937).
Tadzhikistan.—Found in burrows of turtles and rodents (Zmeev, 1936).
Turkmen S.S.R.—Nymphs and adult females are common in burrows of Rhombomys opimus and in burrows of Testudo horsfieldi. Its principal habitat is rodent burrows in loess dust, where it is not infrequently found in the food stores of the host (Vlasov and Miram, 1937).
Texas.—In the nests of wood rats, Neotoma sp. (Hebard, 1917).
There is relatively little ecological information about cockroaches that live in deserts, even though certain species, notably Polyphaga aegyptiaca, have long been known to inhabit arid zones. In fact, so little is known about the ecology of arid-zone insects in general that it is more a subject for research than for review (Pradhan, 1957). In their account of the cockroaches of Northern Kenya and Jubaland, Kevan and Chopard (1954) describe in some detail the vegetational areas of this arid desert or semidesert country, which averages only about 10 inches of rain per year. The other sources that are cited below contain very little more biological information than the abstracted material that is given under each species.
Nearly all the Polyphaginae are said to be marked xerophiles whose distribution coincides with that of the deserts (Bei-Bienko, 1950). With the exception of Arenivaga floridana, the species of Polyphaginae in the United States all occur in the Southwest, where they are (with a few exceptions) the only cockroaches that inhabit the desert regions proper (Hebard, 1917). The Polyphaginae reach their greatest diversity in the deserts of Northern Africa and Anterior and South-Central Asia (Bei-Bienko, 1950). Some of the desert-inhabiting species have also been found under nondesert conditions. This only further exemplifies the plasticity of cockroaches in adapting to different environments.
The ability of desert insects to live under what appear to be extremely unfavorable conditions has been abundantly illustrated by Pradhan (1957). Uvarov (1954) has pointed out that a desert "covers a great variety of landscapes, which provide desert animals with a wide range of habitats, some of them offering very favorable conditions for life." Pradhan (1957) stated that many desert animals avoid the extremes of desert climates by choosing suitable microclimates for diurnal resting places, that a permanent or temporary underground existence is very common among insects in arid zones, and that many nocturnal Orthoptera burrow into the soil or hide under stones where temperatures are lower. For example, the type of Parcoblatta desertae was found under a boulder on the bare desert (Rehn and Hebard, 1909).
Symbiosis with burrowing animals is another solution to the problem [Pg 26] of existence in the desert; in fact, symbiosis is a mode of life adopted by nearly half of the desert cockroaches about which we have any information. Vlasov and Miram (1937) found Polyphaga indica, Polyphaga saussurei, and Arenivaga roseni in the burrows of rodents and desert turtles. In the desert regions of Arizona, females of Arenivaga erratica were found commonly in burrows of the kangaroo rat (Vorhies and Taylor, 1922) and in dens of wood rats and ground squirrels (Ball et al., 1942). Arenivaga apacha and Arenivaga bolliana have also been found inhabiting the nests of wood rats (Hebard, 1917; 1943a). Bei-Bienko (1950) has suggested that the adaptation of desert-inhabiting cockroaches to rodent burrows might enable these insects to survive in the severe climatic conditions of deserts in summer.
Under desert conditions in southern Arizona, the relative humidity outside of the burrows of the kangaroo rat is 1 to 15 percent during the day and 15 to 40 percent at night; but inside the burrows the relative humidity is 30 to 50 percent, and the temperature, even during the day, is below 30° C. (Schmidt-Nielsen, 1949). Thus by living in rodent burrows during the day and going outside at night, the desert cockroaches could avail themselves of the most favorable microclimates obtainable. Presumably whatever food these insects eat provides them with sufficient water to enable them to survive under desert conditions. Bodenheimer (1953) has suggested that the extent of utilization of dew, which is sometimes heavy in the desert, should be investigated; he stated that tenebrionid beetles have been seen in the early morning eating dry [dead?] herbs that were still wet with dew. It is obvious that there is a need for additional detailed information without which we can only guess about the ecology of desert cockroaches.
In the following list we have cited only those species that were stated to have been found under desert conditions. Undoubtedly, related species that have been taken in similar localities are also desert-inhabiting forms, as, for example, other species of Arenivaga that were collected in Texas by Hebard (1943a). In the absence of specific information linking such other species with deserts, we have arbitrarily relegated those forms to the section on outdoor habitats. In addition to the species listed below, desert cockroaches are said to be found in the following genera: Anisogamia, Mononychoblatta, and Nymphytria (Chopard, 1938).
Northern Kenya.—In desert-grass and thorn-bush country; scattered, dry tufts of grasses interspersed among acacia bush and scattered trees (Kevan and Chopard, 1954).
U.S.A.—Inhabits desert regions of the Southwest, has been found in nests of wood rats (Hebard, 1917).
U.S.A.—On gravelly hillocks, in scattered scrub, and in the nests of wood rats in Texas. It is a desert inhabitant in the Southwest (Hebard, 1917; 1943a).
U.S.A.—Inhabits desert regions of the Southwest (Hebard, 1917). In Arizona it has been found in rodent burrows in the desert (Vorhies and Taylor, 1922; Ball et al., 1942).
Turkmen S.S.R.—Predominantly found in burrows in sand; all stages "swim" in sand and loess dust (Vlasov and Miram, 1937).
Arizona.—Found in small numbers on the dry desert (Flock, 1941a).
U.S.A.—Occurs in the Southwest where it is confined to the desert and semidesert mountainous areas, rarely being found on the desert floor (Hebard, 1917). Taken in an ant nest in mountains of Arizona (Ball et al., 1942).
Eastern Africa.—"Commonly met with under débris, the apterous females being most frequent." Thorn-bush country (Kevan and Chopard, 1954).
Northern Kenya.—Very abundant; both sexes under débris in desert-grass and thorn-bush country (Kevan and Chopard, 1954).
Northern Kenya.—Taken in upland grassland and bush (Kevan and Chopard, 1954).
Northern Kenya.—In thorn-bush country (Kevan and Chopard, 1954).
Northern Kenya.—"Very commonly found at the base of tufts of grass and other débris, the apterous female particularly in the latter situation"; in upland grassland near forest; in thorn-bush country (Kevan and Chopard, 1954).
Northern Kenya.—Taken in upland grassland and bush (Kevan and Chopard, 1954).
U.S.A.—Apparently found in greatest abundance in the extreme desert conditions of the southwestern United States (Hebard, 1917). Two small groups of males were observed in the midst of the sandy desert north of Yuma, Ariz.; these insects alternately flew and ran over the sand in the hot sun while headed in a southwesterly direction (Wheeler, 1911).
Northern Kenya.—In desert-grass and thorn-bush country (Kevan and Chopard, 1954).
Northern Kenya.—"All from desert grass and thorn bush (on sand)." It was stated (under discussion of Tivia fulva) that Heterogamodes females live more or less buried in the sand (Kevan and Chopard, 1954).
Southwestern Africa.—Limited in distribution to the more arid portions, being peculiar to extreme desert conditions (Rehn, 1937).
Northern Kenya.—In desert grass and thorn bush; "probably the commonest of all the medium-sized cockroaches occurring in the area [Pg 29] under discussion, coming very freely to light" (Kevan and Chopard, 1954).
U.S.A.—In the desert and semidesert mountainous areas of the Southwest; it is rarely found on the desert floor (Hebard, 1917). Found under boulder on bare desert (Rehn and Hebard, 1909).
Caucasus.—The wingless female was found buried in sand and dust (Burr, 1913).
Turkmen S.S.R.—Although this species is secondarily encountered in dwellings and courtyards, it is a very characteristic insect of the Trans-Caspian deserts; the females are encountered fairly frequently as inhabitants of sand, where they run slowly over the surface, or dig themselves into the sand to continue their forward motion not far below the surface (Fausek, 1906). Uvarov (in Chopard, 1929b) indicated that females of this genus are found in various desert localities, particularly where vegetative debris occurs, but they are not strictly attached to sandy terrain.
Turkmen S.S.R.—This species prefers sandy soils where the nymphs, alate males, and wingless females "swim" readily through the sand; they can also be found in the burrows of desert animals (Vlasov and Miram, 1937).
Turkmen S.S.R.—Its principal habitats are rodent burrows in loess dust and burrows of the desert turtle (Vlasov and Miram, 1937).
Northern Kenya.—Taken in bushes by dry river bed and in desert-grass and thorn-bush country at several stations (Kevan and Chopard, 1954). " ... taken with light at night running on bark of a large acacia tree" (Rehn, 1947).
Northern Kenya.—In desert grass and thorn-bush country (Kevan and Chopard, 1954).
Northern Kenya.—Taken at three stations in desert grass and thorn bush (Kevan and Chopard, 1954).
Northern Kenya.—In open sandy, riverine bush (scanty ground cover among acacia trees and doum palms) (Kevan and Chopard, 1954).
Northern Kenya.—In desert grass and thorn bush; distributed in semidesert areas south of Sahara; the apterous females probably live buried in sand (Kevan and Chopard, 1954).
Northern Kenya.—In desert grass and thorn bush (Kevan and Chopard, 1954).
The so-called aquatic or amphibious cockroaches are all members of the subfamily Epilamprinae (Chopard, 1938). These forms are not nearly as aquatic as water beetles or aquatic Hemiptera, but in their relations to water they behave differently from nonamphibious cockroaches, which tend to avoid water except for drinking. There are apparently no special morphological characteristics that distinguish amphibious cockroaches (Shelford, 1907, 1909a; Chopard, 1938), although Takahashi (1926) listed several characters that he considered made Opisthoplatia maculata adapted for an aquatic life: (1) Back of body easily wetted; (2) long hairs on underside of thorax trap air; (3) terminal abdominal spiracles open into tubes that extend rearward; (4) long hairs on ventral surfaces of cerci "protect" terminal abdominal spiracles. Annandale (1906) also suggested that the position of the posterior abdominal spiracles, at the base of tubes that project rearward from beneath the seventh tergite, are an adaptation to an aquatic life. However, as Shelford (1907) and Chopard (1938) have pointed out, this same feature may be observed in many terrestrial cockroaches. The legs of amphibious cockroaches are similar to those of nonaquatic species and are not modified for swimming (Shelford, 1909a; Takahashi, 1926).
Biological observations have been made on relatively few species, but representatives of at least six genera occur in quasi-aquatic habitats. Strictly speaking, these cockroaches live on land at the edges of streams or pools and spend relatively brief periods in the water. A [Pg 31] few species are found in water-filled bromeliads. The behavior of the known amphibious species of cockroaches in relation to their habitats is discussed below.
Panama.—These insects when disturbed would dive into the water that had collected in the base of the bromeliad; they would disappear beneath the surface and remain submerged for some considerable time (Caudell, 1914).
Trinidad.—This species was taken from the leaf bases of Tillandsia sp. at 3,100 feet; water had collected between the leaves and the insect was presumed to be more or less amphibious (Scott, 1912). Subaquatic in the bromeliad Glomeropitcairnia erectiflora: "This large and handsome species [D. scotti] is very common in the larger, water-filled, epiphytic bromeliads of the rain forest. Within these plants it is usually to be found, often in considerable numbers, just above the surface of the water or partly immersed in it. The cockroaches will descend rapidly into the water when alarmed and probably obtain their nourishment from the accumulated organic matter in the water. Floating material is probably taken and it seems less likely that they feed below the surface. They appear to be ovoviviparous." (Princis and Kevan, 1955.)
Puerto Rico.—Abundant in wet "malojillo" meadows. The nymphs swim easily and remain under water for long periods, as do the adults (Seín, 1923; Wolcott, 1950).
Panama.—A swimming nymph, captured in a dipper with mosquito larvae in a lagoon of the Rio Chilibre, was kept under observations in an aquarium. If disturbed, the insect dived into the water from floating vegetation and swam rapidly below the surface for a minute or two. Finally becoming quiescent, the cockroach would then cling to submerged roots; twice it remained still for 15 minutes before climbing to the surface, where it remained for five or more minutes before emerging completely (Crowell, 1946).
Lower Burma.—One male and three nymphs were collected in the Dawna Hills by Annandale who made the following observations: "The wingless specimens were under stones in a jungle stream and [Pg 32] behaved just as the one I obtained in Chota, Nagpur, did [Annandale, 1906]. The winged specimen was under a stone at the edge of the stream, but swam readily. It did not seem so much at home in the water, however, and apparently could not, owing to the wings, raise the tip of its abdomen above the surface." (Shelford, 1909a.)
Siamese Malay States.—Wingless females rested on floating logs from which they would dive into the water upon the least disturbance; they remained under water for several minutes, then surfaced beneath the shelter of the log. In the jungle all females were taken either in the water or among matted roots on the sides of the stream. Winged males were seen rising from the surface of the water (Annandale, 1900).
Sarawak.—All specimens were immature; they swam and dived well, but were soon drowned if prevented from rising to the surface to breathe. "When at rest the body of the cockroach is almost entirely submerged, the tip of the abdomen alone projecting above the surface of the water; the abdomen moves gently up and down and every 30-40 seconds a bubble of air issues from the prothoracic spiracle on each side." (Shelford, 1901, 1916.)
India.—A nymphal female, found in a jungle stream at Chota Nagpur, could swim with belly or back upward. When held under water it drowned in a few minutes. The tip of the abdomen was held out of water (Annandale, 1906).
Shelford (1907) has suggested that the immature stages of terrestrial species of Epilampra may well be amphibious. This is an area that could profit by more field observations.
Formosa.—Invariably found under or between rocks near mountain streams. The wingless adult and the nymph have similar habits. Normally the cockroach lives on land, and when it goes into the water it returns to land within a few minutes. This cockroach rarely swims, but when it does, it maintains its body in a horizontal position just below the surface of the water. Ordinarily, it walks on the river bottom or on water-covered rocks. This insect feeds on decayed leaves and, according to Shikano, it will eat human feces. (Takahashi, 1926.)
This species has a large number of long hydrophobic hairs on the ventral sides of the thorax and anterior abdominal segments. When the insect submerges, air is trapped in these hairs. The thoracic and [Pg 33] one pair of abdominal spiracles open into the bubble of trapped air. However, the insect apparently does not use this plastron of air to replenish its tracheal air supply, but, like Rhicnoda natatrix (see below), it inspires air while at the surface through its posterior abdominal spiracles and expires air into the bubble under the thorax. While the insect is submerged, the air bubble increases in volume until part of it breaks away and floats to the surface. (Takahashi, 1926.)
Formosa.—Lives on or in swampy ground (Takahashi, 1924).
Sarawak.—Immature cockroaches were found in sodden leaves at the edge of a pool, where they rested for hours at a time. Generally the fore part of the body was in the water but the tip of the abdomen was always in air. When disturbed the insects dived into the water and hid under sticks and stones on the bottom. Air is inspired through the posterior abdominal spiracles, when they projected above the water surface, and expired through the thoracic spiracles. In experiments in which the insects' abdomens were held immersed in water, with the thorax exposed, the insects died in 6 to 12 hours or less. (Shelford, 1907.)
Westsumba.—Found under moist fallen leaves on gravelly shore of Melolo River. The nymphs distinguished themselves through their amphibious mode of life and were often good swimmers (Princis, 1957a).
Brazil.—These cockroaches were found under stones at the side of a rocky stream at Ouro Preto. When disturbed they ran down under the surface of the water and hid under stones at the bottom. When thrown on the water surface, they were helpless, and to get beneath the water surface they had to walk down some object. When they had penetrated the surface film they could swim freely. Specimens kept in jars lived several days with only a portion of their abdomens exposed to the air. (Bristowe, 1925.)
This category is a catchall for all cockroaches that are not limited to the more circumscribed habitats that have been previously considered. [Pg 34] Some cockroaches in this section select specific microhabitats (e.g., Cryptocercus spp., which live exclusively in rotten logs; and Neoblattella dryas, N. eurydice, and N. grossbecki in bromeliads). Others are found in a wide variety of habitats (e.g., Ischnoptera deropeltiformis and Parcoblatta spp.). But some species are so little known that their actual habitats are barely suggested in the collection data.
Williams (1941) made an ecological study of the floor fauna of the Panama rain forest. He found Orthoptera (nearly all were unidentified nymphal cockroaches) in the litter of dead leaves, twigs, and other plant products in over 90 percent of the quadrats he examined. These insects represented about 0.25 percent of the total animal population.
Delamare Deboutteville (1948) made a quantitative study of the animal population in suspended soil that had accumulated between the roots of forest epiphytes of the lower Ivory Coast. He analyzed 2 dm.3 samples of soil from an epiphyte located 45 meters above ground on a main branch of Parinarium, with these results: Horizon A.—Superficial zone of large rootlets, 6 cm. deep: 2 cockroaches, 4 arachnids, and 4 beetles. Horizon B.—Zone of fine rootlets, 6 cm. deep: 6 cockroaches and numerous other arthropods. Horizon C.—Humid zone, 8 cm. deep: 7 cockroaches and numerous other arthropods. Plants, such as Palissota, were also living in this very original biotype.
The species of cockroaches listed below have been found in the following kinds of outdoor microhabitats: In jungle, forest, and woodlands they have been found in rotten wood; under bark of living, dead, and fallen trees; in decay cavities in trees; burrowing in living bark; on foliage of trees, shrubs, bushes, and low herbage; on vines and in bromeliads and epiphytic ferns; under signs on trees and stumps; in piles of logs and firewood; under dead leaves and debris; in and under decaying fruit on the ground. Cockroaches have been found between the leaves and under leaf sheaths of sugarcane, corn, and other grasses; under dry fibers and fronds of coconut trees; in hollow stems and bases of tree-fern fronds; under bracts of banana blossoms and in bunches of bananas (p. 146). Cockroaches also inhabit abandoned cocoons and larval tents, wasp nests, ant nests, termite nests, bird nests, rat nests, and burrows of other rodents (pp. 23-25, 310-319). Cockroaches have been found in rock crevices and under rocks; under boards and other objects on ground; under seaweed, drift, and other debris on beaches; burrowing in soil and under clods of earth; in marshes and swamps; in dumps and rubbish heaps.
The above list does not exhaust the available outdoor microhabitats that cockroaches find suitable for their continued existence, but it is fairly representative. Although we have no measurements to substantiate this conclusion, we suggest that the microhabitats cited above have a more constant temperature and a relatively higher humidity than is provided by the surrounding macrohabitats. We would expect insects such as cockroaches, whose water balance is dependent on a continuous supply of fluid water or moist food, to seek moist environments or to avoid situations in which their transpiration might increase. Deviations, presumably brief, from this expected behavior must occur to account for the cockroaches that are found under relatively unfavorable environmental conditions. Despite the apparent preference for cryptic habitats, some cockroaches are found in hot sunlight (Ellipsidion spp.; Tepper, 1893); Rehn (1945) has stated that many kinds are diurnal rather than nocturnal. Movement of cockroaches between habitats may be assumed to occur; but movement from an unfavorable environment to a more favorable one, following a shift in water balance, has not been observed in nature; however, laboratory experiments suggest that the mechanism for mediating such behavior is present in some species of cockroaches (Gunn and Cosway, 1938; Roth and Willis, 1952a). Obviously, additional research is needed on the bionomics of all species. Further conclusions based on current limited knowledge can only be speculative and possibly misleading.
(Except Amphibious, Desert, and Cavernicolous Forms)
Puerto Rico.—Living in rotten, wooden fence; living between leaves of Samanea saman and in abandoned cocoons of Megalopyge krugii on bucare trees (Wolcott, 1950).
Leeward Islands.—On coconut tree (Princis and Kevan, 1955).
Puerto Rico.—As diaphana, in dead branch 10 feet above the ground on Mona Island (Hebard, 1917). In trunks of trees under bark and very often in abandoned cocoons of the "plumilla" (Seín, 1923). On rotten, wooden fence; in empty cocoons of Megalopyge krugii on trunks of bucare trees, Erythrina glauca; on trunk of Inga laurina; in larval tents of Tetralopha scabridella on Inga vera (Wolcott, 1936). In large numbers in nests of the gray kingbird (Wolcott, 1950).
West Indies.—In Cuba, under corky bark of large tree in open; Jamaica, under loose bark of shade trees and in bracts of banana blossoms; in bromeliads and hollow bases of dead tree-fern fronds (Rehn and Hebard, 1927).
Florida.—On Long Key, under coquina boulder in heavy scrub; under loose, dry fibers near head of standing coconut palm (Rehn and Hebard, 1912). Climbing on roots of red mangrove, Rhizophora mangle, in swamp; under loose bark on trunk of Exothea paniculata in dense jungle; under limestone boulder in keys scrub; under signs on oaks, sweet gum, and longleaf pines in southeastern and southern States (Hebard, 1917). Infrequent in the shrub growth of the Sandhills habitat (Friauf, 1953).
Texas.—In undergrowth of pine forest; under sign on oak near river; in Tillandsia sp. (Hebard, 1917). Usually in hiding places on trees; only once found under a stone on ground (Hebard, 1943a).
Hawaii.—Common in hollow stems and under bark (Swezey and Williams, 1932).
Trinidad.—On low herbage, on hibiscus at night, and in banana bunch (Princis and Kevan, 1955).
Panama.—Under dead leaves in jungle (Hebard, 1920).
Costa Rica.—In decayed leaves (Rehn, 1906).
Costa Rica.—Under stones on borders of Surubres River (Rehn, 1906).
Panama.—Under rubbish at edge of jungle and in overgrowth of heavy vines on low bushes (Hebard, 1920).
Panama.—Under drift on edge of coral-sand beach (Hebard, 1920).
Texas.—In dense jungle brush of the river plain; on gravelly hillocks in scattered scrub; under debris and leaf mold under mesquite trees; in rat's nests, Neotoma sp. (Hebard, 1917). In dry earth under bush; inhabits litter on ground and nests of rats (Hebard, 1943a).
Florida.—Male on ground under leaves of cabbage palmetto (Blatchley, 1920). Females in sand under boards and debris along lake shore (Friauf in Cantrall, 1941). Infrequent on bare soil and ground under vegetation in the longleaf-pine flatwoods habitat (Friauf, 1953). In rodent burrow (Young, 1949).
Texas.—Under stones in upper canyon; under rocks in pine-oak forest; from oak-manzanita forest along dry stream bed (Hebard, 1943a).
Malaya.—Lives freely on bushes and flowers of Passiflora sp. (Karny, 1924).
Puerto Rico.—"Apparently the species [as deplanatus] is locally numerous in suitable locations, such as caves, rock crevices and the shelter of large stones." (Rehn and Hebard, 1927).
Panama.—Perfectly at home in bromeliads (see p. 31) (Caudell, 1914).
Jamaica.—In bromeliads; under dead wood in dense forest (Rehn and Hebard, 1927).
Australia.—Under bark (Hebard, 1943).
Australia.—From sugarcane (Hebard, 1943).
Australia, Queensland.—On leaves, grass, and sugarcane (Hebard, 1943).
Australia.—From leaves, under bark, from sugarcane (Hebard, 1943).
Australia.—In leaves, from tree, from sugarcane (Hebard, 1943).
Trinidad.—Female in rotting log (Princis and Kevan, 1955).
Jamaica.—Under dead coconut petioles in open spot. Gundlach found it under stones in a field in Cuba (Rehn and Hebard, 1927).
Trinidad.—Nymph in rotten palm tree (Princis and Kevan, 1955).
Venezuela.—Only taken in the forests of the Orinoco near the trunks of rotten trees at night (Doumerc in Blanchard, 1837).
Panama.—Among dead leaves and debris on floor of rain forest (E. C. Williams, Jr., 1941).
U.S.S.R.—Found among rocks at 2,000 or more meters elevation. It is found in cultivated areas as well as in mountainous landscapes and in semideserts (Bei-Bienko, 1950).
Great Britain.—One female nymph under bark of tree 10 feet above the ground (Burr, 1900). Swarming within a rubbish heap in February (Lucas, 1912). In refuse tip under old sacks and sheets of linoleum (Hallett in Lucas, 1922). Male under bark of oak far from houses (Donisthorpe, 1918). One adult female and nymph in prone dead elm 50 yards from house (Burr, 1937). An immature male at the roots of Ballota nigra (Buck in Gardner, 1954). Four additional records of this species outdoors away from houses (Lucas, 1920).
Southern Crimea.—Under stones, dead leaves, and detritus in small copses of Quercus pubescens, Carpinus orientalis, Cornus mas, Paliurus aculeatus, and Dictamnus fraxinella; 19 specimens, apparently breeding outdoors (Adelung, 1907).
North-central U.S.—Observations since 1950 indicate a marked increase in frequency and duration of infestations outdoors; observed in bare soil, vegetation, debris, alongside foundations in sodded areas, along sidewalks, and at edge of parking areas throughout the year; in some urban residential areas, the yards of whole blocks of houses were "alive" with this species on warm summer nights; in winter they have been found under stones, leaf debris, and soil near structures (Shuyler, 1956).
Algeria.—Under moist leaves in woods (Lucas, 1849).
California.—Under rubbish and on date palms (Herms, 1926).
Connecticut.—In city dump under loose material, very numerous (Walden, 1922). Additional infestations of dumps by this species have been reported in New York (Felt, 1926, 1928) and New Jersey (Hansens, 1949, 1950)
England.—Swarming within a rubbish heap in February (Lucas, 1912).
Formosa.—Lives among fallen leaves on the ground (Takahashi, 1924).
North-central U.S.—Reported living outdoors near buildings and in soil under basementless buildings from early summer to late fall (Shuyler, 1956).
India.—Common among decaying vegetation and on trees (Chopard and Chatterjee, 1937).
Formosa.—Normally found in sugarcane fields, pineapple fields, and grasslands where it feeds on decayed leaves and other decayed vegetable matter and dead insects. It lies concealed among and under fallen leaves and clods of earth on or close to ground and never on the upper parts of plants, except pineapple where it is found among the leaves (Takahashi, 1940).
Arizona.—Typically an inhabitant of irrigated fields and yards, it is found in fewer numbers on the dry desert. It is found under stones, plant debris, and clumps of earth; found in greatest numbers around decaying dates on ground (Flock, 1941a).
Texas.—Beneath duff under athel trees; rather abundant in clumps of Rhodes grass (Riherd, 1953).
Cuba.—In sea-coast woods: "The species [this and Byrsotria fumigata] are ground-dwelling, hiding under stones and other shelter" (Rehn and Hebard, 1927).
Cuba.—Ground dwelling, hiding under stones, etc.; also a cave inhabitant (Rehn and Hebard, 1927).
Brazil, Matto Grosso.—Beaten from tree foliage in dry scrub, from tree foliage at edge of dry riverine tangle, and from undergrowth in a dry forest area (Rehn, 1937a).
Honduras.—All beaten from foliage along roads or in thickets, during rainy season (Rehn, 1937a).
Virgin Islands, St. Croix.—Common under heaps of rubbish (Beatty, 1944).
Trinidad.—On herbage below bananas; all stages on Hibiscus at night; in grass at dusk; on low herbage under old coconut (Princis and Kevan, 1955).
Jamaica.—In leaves on leaf mold in hillside forest (Hebard, 1916a).
West Indies.—In debris in short grass in open, Cuba. Under dead petioles of coconut palms, San Domingo. In leaves on leaf mold in hillside forest, Jamaica (Hebard, 1916a).
Honduras.—Found at foot and on lower slopes of first ridges of the Sierra Pija, from 75 to at least 800 feet above sea level, where vegetation ranged from abandoned banana patches overgrown with Heliconia and Cecropia and interspersed with forest trees, at the foot of the hills, to primeval lowland forest (ceibas, figs, palms, etc.) on the slopes. In the banana patches C. hylaea was found on hanging dead banana and Cecropia leaves; on the slopes it was found on undergrowth foliage, hanging dead leaves, and in dead leaves on ground (Rehn, 1945a).
Panama.—Among loose leaves on leaf mold in heavy jungle (Hebard, 1916a).
Jamaica.—One of the most frequently encountered orthopterous insects in bromeliads on trees (Hebard, 1916a, 1917; Rehn and Hebard, 1927).
Jamaica.—In decaying herbage (Rehn and Hebard, 1927).
Jamaica.—All specimens taken from under drying bracts of banana blossoms (Rehn and Hebard, 1927).
North Carolina.—Under pine straw on ground in woods (Brimley, 1908).
Southeastern U.S.—Under dead oak leaves; under dead needles in longleaf-pine woods; in wire grass; under refuse; beaten from undergrowth in pine and oak woods (Rehn and Hebard, 1916). In undergrowth of shortleaf-pine, longleaf-pine, and oak woods; in heavy scrub in damp spot of sand dune area; from high bushes, Ilex coriacea [= lucida] along inland swampy area (Hebard, 1916a). "The species is in large part terrestrial, being usually found among dead leaves and litter on the ground. Occasional specimens are, however, sometimes beaten from bushes. Individuals are decidedly active and are usually to be found in the greatest numbers in sandy situations" (Hebard, 1917).
Florida.—Throughout winter and spring they are frequent beneath leaves and other debris on ground, especially in dry, sandy locations (Blatchley, 1920). Friauf (1953) found this species under debris, fallen leaves, leaf mold, or decaying wood in these habitats: Dry, ruderal grassland (infrequent), scrub (frequent), sandhills (dominant), xeric hammock (infrequent), mesic hammock (dominant), pond margin (infrequent), longleaf-pine flatwoods (frequent), bayhead (occasional), low hammock (frequent), and alluvial hammock (occasional). In the shrub stratum in these habitats: Scrub (frequent), sandhills (dominant), and xeric hammock (infrequent). In herbaceous stratum in these habitats: Sandhills (dominant), mesic hammock (dominant), and black-pine flatwoods (infrequent). On [Pg 42] bare soil or bare sand under vegetation in these habitats: Sandhills (dominant), pond margin (infrequent), longleaf-pine flatwoods (frequent), and slash-pine flatwoods (frequent) (Friauf, 1953).
Florida.—Series of specimens captured on Long Key under dead petioles of coconut palm on moist ground at edges of pools of brackish water. Specimens from Key West were in dry dead grass under boards (Rehn and Hebard, 1912). Nymphs frequent under bark on decaying pine logs in pine woods; occasional in leaf mold in heavy junglelike scrub (Rehn and Hebard, 1914). In water-soaked leaves in heavy red-mangrove swamp (Hebard, 1915). Under dead petioles of coconut palm on sandy soil in grapefruit grove (Hebard, 1916a). Numerous at bases of tufts of coarse grass growing just back of sea beach (Blatchley, 1920). Friauf (1953) found this species in leaf duff, leaf mold, debris, or decaying wood in these habitats: Dry, ruderal grassland (occasional), scrub (infrequent), sandhills (infrequent), mesic hammock (infrequent), pond margin (occasional), longleaf-pine flatwoods (occasional), and low hammock (infrequent). On bare soil or bare sand under vegetation in these habitats: Longleaf-pine flatwoods (occasional) and slash-pine flatwoods (occasional). Dominant in the spartina marsh habitat in the grass stratum and duff around clumps. Frequent in the saw-grass marsh habitat in the grass stratum and, during the dry season, in decaying vegetation on the marsh floor.
Jamaica.—Adults in dead leaf litter alongside the trail in dense forest of tree ferns, Podocarpus, Cyrilla, and other trees; the forest was bathed in fog much of the time (Rehn and Hebard, 1927).
Jamaica.—In leaves on leaf mold in hillside forest (Hebard, 1916a). Moderately numerous in leaf litter in mangrove swamp; in decaying herbage (Rehn and Hebard, 1927).
Puerto Rico.—Between the leaves and under the leaf sheaths of corn (Sein, 1923; Wolcott, 1936).
West Indies.—The tropical species of this genus inhabit heavy forest, living among the fallen leaves resting on the leaf mold, in [Pg 43] epiphytic bromeliads, and in dead agaves (Hebard, 1916a; Rehn and Hebard, 1927).
Cuba.—In siftings from under sea grapes, other shrubs, and low trees (Rehn and Hebard, 1927).
Puerto Rico.—On dry limestone hills (Rehn and Hebard, 1927).
Trinidad.—Under bark of old cacao tree (Princis and Kevan, 1955).
Costa Rica.—Under stones on borders of Surubres River (Rehn, 1906).
Jamaica.—Swept from huckleberry trees (Vaccinium meridionale) (Rehn and Hebard, 1927).
Panama.—From jungle undergrowth (Hebard, 1920).
Panama.—In grass (Hebard, 1920).
Florida.—"The almost impenetrable jungle on Key Largo was examined, and in its depths the two specimens of this species were secured by beating the lower branches of gumbo limbo, other trees and the lower bushes and shrubs, among which latter are to be found such tropical forms as Ocotea catesbyana [= Nectandra coriacea] and Citharexylum villosum" (Rehn and Hebard, 1912). In nests of webworm and beaten from bushes of bayberry, Myrica cerifera, along edge of pine woods (Rehn and Hebard, 1916). Beneath dead leaves in oak woods and beaten from foliage of oak and bayberry (Blatchley, 1920). Infrequent in the tall shrub stratum of the xeric hammock habitat (Friauf, 1953).
Texas.—The great majority of specimens were beaten from foliage of bushes (Hebard, 1943a).
Southeastern and southern U.S.—In undergrowth in pine woods; [Pg 44] beaten from shrubbery, from bayberry bushes, from lower branches of gumbo limbo and other trees, from lower bushes and shrubs in jungle, and from low oaks on hills. In Texas, beaten from tall weeds in opening in river-plain jungle scrub (Hebard, 1917).
Panama.—In jungle vegetation, including vines covering low bushes (Hebard, 1920).
Trinidad.—Males on low herbage under old cacao tree (Princis and Kevan, 1955).
Trinidad.—Males in orchard on low herbage at night; females under refuse and in grass (Princis and Kevan, 1955).
Malaya.—Often found between dry foliage in the beakers of the epiphytic fern, Asplenium nidus, although the species lives mainly in bamboo bushes (Karny, 1924).
North Carolina.—"They were never found except in parts of the logs [chestnut] where the decayed wood was soft, punky and wet" (Rehn and Hebard, 1910).
Oregon.—In fir logs where sap wood was soggy (Hebard, 1917).
Virginia.—In decaying chestnut and pine logs; taken six times in chestnut and once in pine (Hebard, 1917). In rotten logs in deep ravines of moist woods (Davis, 1926).
Appalachian Mountains, U.S.—In southern Virginia and eastern Tennessee, it is usually quite abundant in well-forested areas at elevations from 3,000 to 5,000 feet; "sometimes even a majority of the dead logs on a mountain side have roaches in them" (Cleveland et al., 1934). This cockroach not only lives in rotten, dead logs but also in sound logs that have been down only a few years. In Virginia it is found more often in chestnut and hemlock. "It occurs fairly often in oak, and has been found in pine, spruce, and arbor vitae.... There is little evidence that they ever leave the log and enter the ground" (Cleveland et al., 1934).
Eastern Manchuria.—In great numbers under rotting fallen trees and in rotten dead wood (Bei-Bienko, 1950).
Marquesas Islands.—Males under stones and dead log (Hebard, 1933a).
Hawaii.—In soil about roots of pineapple (Illingworth, 1927). Often found about roots of grasses and weeds and other debris (Williams et al., 1931). Under stones and pineapple mulching paper (Fullaway and Krauss, 1945).
Wake Island.—Numerous, some from rotten logs. Found in bunch grass on Ocean Island (Bryan, 1926).
Australia.—Frequent woods where they leave shelter soon after sunset and run actively on ground or ascend shrubs and trees in quest of prey (Tepper, 1893).
Panama.—Colony on tree trunk; on surface of trunk of fallen tree (Hebard, 1920).
Hawaii.—"Crowds of these insects in various stages of development sometimes gather in cypress trees, in suitable chinks, in old flowerhead sheaths of palms, etc., and even more or less openly on leafy twigs, in bunch grass, and the species is at times locally abundant behind the older leaf bases of sugar cane" (Williams et al., 1931). Williams also lists the following as food plants: Cryptomeria, algaroba, lime trees, ripening mangoes, papayas, and oranges. However, Bianchi (personal communication, 1954) doubted that any of the above are the main dietary, because the largest populations he had seen "were found in the fairly dry litter of Star Jasmine (Jasminum pubescens Willd.), well removed from any of the plants mentioned by Williams."
Raiatea, Society Islands.—Beaten out of bracken (Cheesman, 1927).
Uahuka, Marquesas Islands.—Under bark (Hebard, 1933a).
Trinidad.—Very common in water-filled, epiphytic bromeliads in the rain forest (see p. 31) (Princis and Kevan, 1955).
Belgian Congo.—Females in forest margin and in forest undergrowth (Rehn, 1931).
South France.—Females and young beneath stones (Blair, 1922).
U.S.S.R.—In the steppe belt, it is a very characteristic member of feather-grass steppes, where it is found in associations of typically steppe vegetation, with feather grasses at the head (Stipa lessingiana and others), and on rocky slopes; it occurs frequently in cultivated fields of young crops and also in young geological strata in sections with virgin soil. The populations of this steppe cockroach average 6 to 8 individuals per square meter from the middle to the end of July. By the end of summer most individuals were observed at the bases of straw stacks with a canopy, having their south sides sheltered. This is the only species of Ectobius adapted to a purely steppe biocenose. (Bei-Bienko, 1950.)
Southeastern Europe.—Numerous under stones on Trebovic (Burr, 1898).
U.S.S.R.—Found in wooded communities and peat bogs (in northern part of its range); males occur predominantly on herbaceous plants and bushes, but females hide under fallen leaves, moss, etc. (Bei-Bienko, 1950). It populated about 25 percent of the aspen trees in an experimental plot, feeding in galleries in the bark of young branches; there were 25 or more individuals per tree (Stark in Bei-Bienko, 1950).
Germany.—Abundant in woods; in pine woods in company with Stenobothrus vagans and Tettix kraussi. Numerous in low aspen bushes in forest. Numerous in deciduous and coniferous forests on trees and underbrush; under fallen leaves and moss; on oaks (Zacher, 1917). In foliage of young oak on top of mountain (Ramme, 1923).
Great Britain.—Under moss and dry leaves, among woodland undergrowth, and, generally, on vegetation close to the ground; occasional on bushes and trees (Lucas, 1920). Nymphs in heather in February and later; adults among rushes fringing pond in July (Lucas, 1925). Nymphs and males on rushy vegetation; unusually abundant on low herbage in dried-up swamp (Lucas, 1930).
France.—In dry woods, on bushes, and at the base of trees (Chopard, 1947).
Algeria.—Under stones; in moist places that are shaded and covered with plants (Lucas, 1849).
England.—Very abundant on sand dunes and among bracken in July (Buxton, 1914).
Germany.—In deciduous and coniferous forests; at edge of forest, from bare woods and bushes; numerous under leaves in oak woods and under moss (Zacher, 1917). In forest well lighted by the sun (Ramme, 1923).
Massachusetts.—Under loose lichens and bark on oak trees; under boxes, baskets, paper, etc., near houses; on Swiss chard (Flint, 1951). On roofs of houses, in shrubbery (Gurney, 1953). We have collected this species for several summers in a fairly dense, wooded area near dwellings, among fallen leaves and climbing on the erect stems and undersides of the leaves of periwinkle. Oöthecae were found on the ground under leaves and debris.
England.—Abundant on sandhills along shoreline among roots of grass (Burr, 1908). Under dead seaweed and other rubbish a few yards from shore on ground that would be washed by the sea (Lucas, 1896). Nymphs found among marram grass (Buxton, 1914). On sandhills near coast and covered with marram grass; often found on heather and low herbage; under old bark and rotten wood on posts; in decayed stump (Lucas, 1920). Swarming on Beta maritima and other plants in July (Lucas, 1920a). Very common in all stages in August, being frequently found under stones (Lucas, 1925). Common on sand dunes especially under stems of dead marram grass. Viable oöthecae found buried in sand (Brown, 1952).
Germany.—In beech woods and in pine woods (Zacher, 1917).
Kazakhstan.—Along the shores of the Syr-daria it is found on and around living willows and on Populus euphratica; under loose bark of dying and dead trees (Bei-Bienko, 1950).
U.S.S.R.—In wooded steppe zones; probably only occurs in association with forests (Bei-Bienko, 1950).
Tadzhikistan.—Great numbers at the roots of Eleagnus shrubs on the banks of reservoirs and frequently under the bark of old trees (Bei-Bienko, 1950).
South France.—One male beneath stone (Blair, 1922).
Australia.—From leaves, from scrub (Hebard, 1943). Collected in trees (Pope, 1953a).
Australia.—On eucalyptus leaves, on wattle, under bark (Hebard, 1943). Collected in trees (Pope, 1953a).
Australia.—In corn and from tree (Hebard, 1943).
Australia.—From sugarcane (Hebard, 1943).
Australia.—All stages are diurnal moving about the foliage of shrubs and small trees in bright sunlight on hottest summer days (Tepper, 1893).
Trinidad.—In dried-up drain; among grass; in debris under old cacao tree; under old leaves (Princis and Kevan, 1955).
Puerto Rico.—Abundant in damp lowlands (Seín, 1923). Under dead leaves in wet malojillo meadow (Wolcott, 1936).
This species is amphibious (p. 31). Shelford (1907) suggested that immature stages of other species of the genus may be aquatic, which would place them in moist situations on the shores of rivers and other bodies of water.
Panama.—Very scarce, under palm trees in decaying leaf mold and litter; one found under decaying bark of a log (Hebard, 1921a).
Mona Island, Puerto Rico.—One specimen under bark of dead tree (Ramos, 1946).
Cuba.—Under dead leaves on stream bank (Rehn and Hebard, 1927).
Puerto Rico.—In siftings from high-altitude primeval forest (Rehn and Hebard, 1927).
Australia.—By day the insects live under bark, stones, logs, dead vegetable debris, or buried in loose dust or soil. After sunset females wander in grass or ascend low objects (Tepper, 1893).
Uganda.—In open bush and short grass (Princis, 1955).
Panama.—Under rubbish on edge of jungle (Hebard, 1920).
Colombia.—In brushwood (Princis, 1946).
Costa Rica.—Numbers of individuals were found in the large bromeliads of the temperate localities (Picado, 1913).
Trinidad.—In old, rotten coconut stump (Princis and Kevan, 1955).
Cuba.—"This species was recorded from under stones in the fields ... by Gundlach" (Rehn and Hebard, 1927).
Cuba.—Under stones in woods (Rehn and Hebard, 1927).
Florida.—Moderately common under bark of dead pine stumps and logs; at Key West it fairly swarmed under coquina boulders in the woods (Rehn and Hebard, 1905). Many specimens under palmetto leaves on ground (Caudell, 1905). In pine woods under dry bark of dead logs; on Long Key in dry fibers at the base of the heads of [Pg 50] coconut palms; "at Key West, a large colony was discovered among boards lying on dry grass in a field, and several were captured upon turning over coquina boulders in the dense bush" (Rehn and Hebard, 1912). Particularly numerous in tree cavities and under bark along the edge of hammock areas (Hebard, 1915). Abundant between basal leaves of Tillandsia utriculata; beneath loose bark of logs and stumps; in and beneath decaying palmetto trunks and leaves; under rubbish (Blatchley, 1920). On ground in heavy tangle after dark; in decaying log of Sabal palmetto; in bromeliads; common under debris and bark in jungle; under signs on Pinus caribaea; in almost every sheltered outdoor place (Hebard, 1917). It moves about at night and hides under bark of logs and in other recesses during the day; where pines are present it almost invariably hides under bark of dead logs and stumps (Rehn and Hebard, 1914). Friauf (1953) found this species in leaf duff, leaf mold, or decaying wood in these habitats: Sandhills (infrequent), xeric hammock (dominant), mesic hammock (frequent), and low hammock (dominant); on tree trunks in sandhills habitat (infrequent) and mesic hammock (frequent); infrequent in saw-grass marsh habitat in the grass stratum and, during the dry season, in decaying vegetation on floor of marsh. Under the bark of logs and beneath logs in the woodpile habitat (Friauf, 1953).
Cuba.—Under stones in deep woods (Rehn and Hebard, 1927).
Trinidad.—Under debris, trash, and vegetable refuse (Princis and Kevan, 1955).
Cuba.—In pine and palmetto region (Rehn and Hebard, 1927).
Texas.—Under dense tangle of bushy vegetation, palms, and vines near Rio Grande; in leaves and dry litter on ground; on dead petiole hanging from palm tree (Hebard, 1917). Under bark of dead hackberry; abundant in dead leaves, dry litter, and rats' (Neotoma sp.) nests in heavy scrub (Hebard, 1943a).
Tahiti.—On foliage in sun or concealed among dead leaves that collect between the fronds of tree ferns (Cheesman, 1927).
Hawaii.—Quite active during the day, occurring on sugarcane, etc., in the wetter districts; it is also a household insect (Williams et al., 1931).
Puerto Rico.—Under bark of tamarind tree (Rehn and Hebard, 1927). Under the bark on a fence post (Wolcott, 1950).
Dominica.—From rotting wood and wood soil (Rehn and Hebard, 1927).
Dominican Republic.—Along railroad through jungle and swamp (Rehn and Hebard, 1927).
Canary Islands.—Found in numbers among pine needles; nymphs were in the majority, adults rare (Burr, 1911).
Azores.—Very common in the hedges, particularly in brambles. Contrary to most species of this genus, which live on the ground under stones, this species is exclusively dendricolous and is only captured by beating the bushes on which it abounds (Chopard, 1932).
Germany.—Abundant in deciduous forest in grass and under fallen leaves; in pine forests under lichens and between fallen needles; in edge of coniferous forest; under stones (Zacher, 1917).
Macedonia.—Usually found crawling on the flowers and stems of giant thistles in May; common on thistles in June (Burr, 1923).
Caucasus.—Numerous beneath dry leaves in a garden (Burr, 1913).
Seychelles.—Apparently only inhabits Pandanus between the leaf bases (Scott, 1910, 1912).
St. Vincent.—In decaying leaves in forest (Rehn and Hebard, 1927).
Trinidad.—In forest debris and debris under old cacao trees; it is not uncommon under dry leaves; it feigns death when disturbed (Princis and Kevan, 1955).
Brazil.—From crown of palm between leaf bases (Hancock, 1926).
U.S.S.R.—Under rocks and on the edges of woods in the lowlands in the north and in the mountains in the south (Bei-Bienko, 1950).
North Carolina.—Under pine straw on ground in woods (Brimley, 1908).
Georgia.—Under dead oak leaves; under debris in garden; running on ground in pine and oak woods (Rehn and Hebard, 1916).
Indiana.—It is "a ground-frequenting, forest-loving insect, hiding beneath cover or about the edges of deep woodland, more frequently in damp places, and rarely taken beneath bark, signs, or at lights" (Blatchley, 1920).
Missouri.—Twenty to 30 males found resting on heads of wild oats on successive evenings (Rau, 1947).
Texas.—It preferred damp, open woodlands (Hebard, 1943a).
Eastern and southeastern U.S.—Under stone in heavy deciduous forest; under damp, dead leaves on edges of forests; under bark of pine log; in wire grass and sphagnum bordering stream thicket; in leaf mold and rubbish about pothole in pine woods, Pinus caribaea; under debris and leaf mold in hammock; under dead oak leaves in heavy deciduous forest (Hebard, 1917).
Florida.—"This species is distinctly geophilous and appears to prefer damp surroundings" (Rehn and Hebard, 1912). Under boards on very wet ground in everglades; in debris and leaf mold in heavy, junglelike areas of trees, bushes, and vines (Rehn and Hebard, 1914). Adults and numerous nymphs beneath weeds, grass, and other debris washed up on beach of Lake Okeechobee (Blatchley, 1920). Friauf (1953) found this species in leaf duff, leaf mold, and/or decaying wood on ground in these habitats: Dry, ruderal grassland (infrequent), scrub (infrequent), sandhills (occasional), xeric hammock [Pg 53] (frequent), mesic hammock (dominant), shrubby, longleaf-pine flatwoods (infrequent), bayhead (dominant), and low hammock (dominant). On open bare soil or bare sand under vegetation in these habitats: Dry, ruderal grassland (infrequent), mesic hammock (dominant), moist, ruderal grassland (infrequent), pond margin (occasional), longleaf-pine flatwoods (infrequent), slash-pine flatwoods (infrequent), and low hammock (dominant). Infrequent in the herbaceous stratum of these habitats: Dry, ruderal grassland, moist, ruderal grassland, and longleaf-pine flatwoods. Infrequent in the shrub stratum of the dry, ruderal grassland habitat. (Friauf, 1953.)
Tennessee.—Taken in traps baited with cantaloupe in a parklike stand of oak, gum, hickory, and tulip trees in a creek bottom, and in a stand of oak on a dry ridge (Walker, 1957).
Panama.—Under rubbish at edge of jungle and under drift on edge of coral-sand beach (Hebard, 1920).
Jamaica.—In dead leaf litter along side trail through mountain forest (Rehn and Hebard, 1927).
Virgin Islands, St. Croix.—Common under rubbish and on shrubbery at night (Beatty, 1944).
Barbados.—Occasionally found in cane fields (Tucker, 1952).
West Indies.—In Puerto Rico, under stones in cultivated area, under debris on alkalie flat. In Jamaica, under dry petioles of coconut palm in grassy area; under logs, logwood on docks, and litter on limestone and near beach. In Panama, under drift on edge of coral-sand beach; under rubbish at edge of jungle (Hebard, 1916c).
Jamaica.—Under limbs and leaf litter in mangrove swamp (Rehn and Hebard, 1927).
Panama.—Under drift on edge of coral-sand beach. Several under decayed banana stem (Hebard, 1920).
Trinidad.—Under debris in forest and debris under old cacao trees (Princis and Kevan, 1955).
Costa Rica.—Very common in the bromeliads of all Costa Rica (Picado, 1913).
Arizona.—"Most commonly seen feeding on pollen and dead insects on the flower stalks of Yucca elata in June in the Santa Rita Mountains" (Ball et al., 1942).
Florida.—Widely distributed throughout pine woods (Pinus caribaea); under signs on Pinus clausa and Pinus caribaea (Hebard, 1917). Beneath bark of dead pine tree; beating Spanish moss; they seldom attempt flight when disturbed, but hide in crevices or drop to ground (Blatchley, 1920).
Costa Rica.—Under stones on borders of Surubres River (Rehn, 1906).
Barbados.—In cane fields (Tucker, 1952).
Dominica.—In vegetation of royal palms, guava, etc.; under loose bark and banana sheaths. In Jamaica, on logwood docks (Rehn and Hebard, 1927).
Costa Rica.—Under bark of tree in forest; in epiphytic bromeliads (Rehn, 1928).
Panama Canal Zone.—About 80 specimens from rotting banana stalks at bases of leaves; boring in decaying banana stem (Hebard, 1920).
Costa Rica.—In tree stump on edge of mountain forest; in dead wood on ground (Rehn, 1928).
Costa Rica.—Shaken from dead banana leaves. Footnote to specific name: "In relation to the liking of species of this genus for bananas (Musa) as shelter and possibly food" (Rehn, 1928).
France.—Under stones and dead leaves, always rare (Chopard, 1947).
France.—All stages common beneath stones (Blair, 1922). Under stones and dead leaves (Chopard, 1947).
Maltese Islands.—Quite common in open country under stones (Valletta, 1955).
Dalmatia.—On seashores under rocks and seaweed cast up on shore (Bei-Bienko, 1950).
Argentina.—Common in rubbish and leaf litter in small woodlot (Hebard, 1932).
Hawaii.—Abundant in wet districts, both in lowlands and to a considerable altitude in the forests, under trash, stones, boards, etc. (Williams et al., 1931). Often it is found with nymphs of Periplaneta australasiae (Fullaway and Krauss, 1945).
Trinidad.—On grass, maize, and cut sugarcane fodder; under vegetable and garden refuse; under old cacao (Princis and Kevan, 1955).
Australia.—Infrequently seen during dry season from March to October. "They burrow quite deeply, about two feet below the surface of the sandy soil in stands of cypress pine (Callitris sp.). They make a nest of dead leaves, grass roots, etc., frequently among the pine roots. The young nymphs rarely appear above ground, but following rain the adults burrow to the surface, especially at night.... This species is also found in the brigalow (Acacia harpophylla) scrub about 70 miles west of Rockhampton, Queensland, and on Fraser Island off the Coast of Queensland" (Henson in Day, 1950).
Panama.—Under bark on tree (Hebard, 1920).
Ecuador.—Under a dense pile of dead leaves around base of tree (Campos R., 1926).
Australia.—In sugarcane (Hebard, 1943).
Australia.—Under loose bark on dead upright tree (Pope, 1953a).
Australia.—Under loose bark on trees and logs; many specimens on wattle trees where in strong sunlight they hid in curled-up leaves; oöthecae attached to underside of loose bark and leaves (Pope, 1953a).
Australia.—Under loose bark of trees and logs (Pope, 1953a).
Chile.—Collected from lichens and mosses on tree trunks (Princis, 1952).
Costa Rica.—Under dead wood in dense second-growth forest; in thick mat of hanging dead vegetation in dense forest; under leaves in forest (Rehn, 1930).
St. Vincent.—Under rotten fruit (Rehn and Hebard, 1927).
Cuba.—Under rotten bark (Rehn and Hebard, 1927).
Jamaica.—Under dried leaves of coconut palm; in dry leaves under acacia on hillside; in debris on beach; under stones on coral rock; in leaf mold under dense brush on hillside; under bracts of banana blossoms (Rehn and Hebard, 1927).
Jamaica.—In bases of dead tree-fern fronds; numerous in bromeliads; nearly all collected specimens were taken in these plants (Rehn and Hebard, 1927).
Jamaica.—Nearly all collected specimens taken in bromeliads (Rehn and Hebard, 1927).
Jamaica.—In epiphytic bromeliads and hollow bases of dead tree-fern fronds; nearly all collected specimens taken in bromeliads (Rehn and Hebard, 1927).
Jamaica.—Under bark of huckleberry; nearly all collected specimens taken in bromeliads (Rehn and Hebard, 1927).
Jamaica.—All specimens collected from under drying bracts of banana blossoms (Rehn and Hebard, 1927).
Cuba.—In dry region of palmettos and pines (Rehn and Hebard, 1927).
Jamaica.—Widely distributed from sea level to 5,700 feet elevation; in bromeliads in mountain forest; among dead leaves in heavy leaf mold under dense hillside scrub; under stones and in ground litter about banana trees; under bark of tree in dense ridge-type forest; in dead agave in scrub forest (Rehn and Hebard, 1927).
Ethiopia.—Always found under stones or cement blocks, but not necessarily deeply buried in the ground (Chopard, 1950b).
Jamaica.—In cracks in dead stump of mimosa; in bromeliads (Rehn and Hebard, 1927).
Puerto Rico.—Possibly to be found most often in rotten tree trunks in the highest mountains; found in rotten stump with termites, ants, and beetle grubs (Wolcott, 1950).
Trinidad.—Under pile of cornstalks (Princis and Kevan, 1955)
Haiti.—Under loose dead bark of mesquite tree, 52 specimens (Rehn and Hebard, 1927).
Australia.—The females bury themselves in loose soil or dust (Tepper, 1893).
Formosa.—On or in swampy ground or under rotten trees on the ground (Takahashi, 1924).
Dominican Republic.—In cultivated grounds, palms, fruits, etc. (Rehn and Hebard, 1927).
Panama.—As Pycnosceloides aporus, in jungle under decaying banana stem in which were boring individuals of Litopeltis bispinosa (Hebard, 1920).
Texas.—Lives in foliage and in the green sheaths of plants (Hebard, 1943a).
Cuba.—On cane leaves; according to Gundlach this genus lives under the loose bark of trees (Rehn and Hebard, 1927).
Puerto Rico.—In rotting trunks of coconut palms (Seín, 1923). Most specimens have been collected from the very rotten interior of coconut palms (Wolcott, 1950).
Trinidad.—On corn; under old log; flies readily to lights (Princis and Kevan, 1955).
Dominica.—In decaying stump in banana patch and in rotting wood. In Puerto Rico, in rotten coconut palm (Rehn and Hebard, 1927).
Australia.—In burrows under the thick bark of fallen and rotting trees (Shaw, 1914). In loose detritus, beneath clods of earth, and in fissures at foot of cliffs along the seashore beyond direct action of the waves (Tepper, 1893).
Australia.—Under decayed logs in coastal scrub. It burrows into the soft part of the log (Froggatt, 1906).
North Carolina.—Under pine straw on ground in pine woods (Brimley, 1908).
Texas.—Under dry cow dung in pine woods (Hebard, 1917).
Nebraska.—Under pile of old boards (Hauke, 1949).
North Carolina.—From under the bark of dead trees (Rehn and Hebard, 1910).
Virginia.—At night on shrubbery. In South Carolina, under sign on tree (Hebard, 1917).
Tennessee.—In traps baited with cornmeal, cantaloupe, or fish in a stand of oak on dry ridge, and in abandoned rocky field on a south-facing slope (Walker, 1957).
Texas.—From mountains, arid, and semi-arid regions; under small boulder on desert (Hebard, 1917). On ground in dry-creek bed through scrub oak, pine, and juniper forest (Hebard, 1943a).
Eastern and southeastern U.S.—All specimens taken from under signs on red oaks and longleaf and shortleaf pines in Georgia and Virginia (Rehn and Hebard, 1916). Trapped in molasses-baited jar in oak forest in New Jersey; under signs on red and white oaks, sweet gum, and other deciduous trees; under signs on shortleaf and longleaf pines and pine stumps (Hebard, 1917). Widespread in southeastern U.S. in habitats as diverse as dry pine lands, oak scrub, moist hammocks in northern Florida, and deep, cool ravines along Apalachicola River (Hebard, 1943a).
Eastern and southeastern U.S.—Trapped in molasses jars: in heavy, barrier-beach forest; in typical pine-barrens undergrowth; in pine barrens with heavy, grassy undergrowth; on border of pine barrens and on edge of swamp; in heavy deciduous forest; in heavy oak woods. Found under debris in dead, shortleaf-pine needles; under dead leaves on edge of oak and shortleaf-pine woods; under bark of pine log; among dead leaves under live oaks; under sign on Pinus caribaea (Hebard, 1917).
Georgia.—From under bark of pine log, among dead leaves under [Pg 60] live oaks, and under leaves on edge of oak and shortleaf-pine woods (Rehn and Hebard, 1916).
Florida.—Very common among dead leaves, under logs, beneath loose bark, and wanders about at night in pinelands, hammock, turkey oak, and sand-scrub habitats (Hubbell and Goff, 1940). Beneath drift, cow dung, leaves, boards, bark of logs, and other debris, usually in open pine woods in sandy areas; frequent at the base of thistle leaves (Blatchley, 1920). Friauf (1953) found this species in leaf duff, debris, or decaying wood in these habitats: Scrub (dominant), sandhills (dominant), xeric hammock (dominant), mesic hammock, longleaf-pine flatwoods (infrequent), low hammock (infrequent), and alluvial hammock (infrequent). In the shrub stratum in these habitats: Scrub (dominant), sandhills (dominant), xeric hammock (dominant), and longleaf-pine flatwoods (infrequent). In the herbaceous stratum of the longleaf-pine flatwoods habitat, and under bark and beneath logs in the woodpile habitat.
Southeastern and southern U.S.—Under bark of pine logs and stumps; in sweet-gum logs and stumps; moderately numerous under bark of dead shortleaf pines; under bark of longleaf-pine stumps; under signs on red oak and longleaf pines; in dead oak. In Texas, under bark of pine stumps (Hebard, 1917).
North Carolina.—All stages under loose bark of dead pines, both prostrate and upright, and stumps. "It seems to prefer the space under the bark to be rather damp" (Brimley, 1908). Under bark of dead pine trees (Rehn and Hebard, 1910).
Florida.—Infrequent in leaf duff and decayed wood of low hammock habitat (Friauf, 1953).
Indiana.—Beneath rocks on sides and tops of high hills, in limestone glades where cedar abounds (Blatchley, 1920).
Missouri.—In leaf stratum of oak-hickory forest (Dowdy, 1951). Earlier, Dowdy (1947) reported finding numerous immature Pseudomopinae [presumably Parcoblatta sp.] in soil and leaf strata of oak-hickory forest.
Texas.—Captured in molasses-baited traps in low, wet, oak woods and in dry woodlot on hillside (Hebard, 1943a).
Eastern and southeastern U.S.—Trapped in molasses-baited jars; in oak and in chestnut forests, and on knoll with high deciduous trees. [Pg 61] Found in oak and pine woods, under bark of decaying chestnut log and dead chestnut stump, and under signs on trees including oaks (Hebard, 1917).
North Carolina.—In all stages under loose bark of upright, dead pines, when the space under the bark was dry (Brimley, 1908).
Virginia, North and South Carolina.—Under signs on trees (white and red oaks); under bark of dead shortleaf-pine and sweet-gum logs and stumps (Rehn and Hebard, 1916).
Indiana.—Beneath bark of logs and stumps; empty oöthecae common beneath loose bark of logs, especially shellbark hickory (Blatchley, 1920). Under loose bark on logs in January (Blatchley, 1895).
Illinois.—In pine forest associes, in black oak forest on sand, in oak-hickory forest on clay, and in climax forest; it evidently moved into the pine associes nightly, great numbers of oöthecae were found under bark of pine logs, where, in October and November, hibernating nymphs were found (Strohecker, 1937). In nests of Vespula maculata (Balduf, 1936; McClure, 1936).
Missouri.—Usually in hollow trees, under loose bark, in woodpiles, and in cracks in rural buildings (Rau, 1940).
Michigan.—Common in oak-dune and beech-maple forests, under loose bark on dead trees and fallen logs, and under debris on forest floor (Hubbell, 1922). "A characteristic inhabitant of the low shrub-terrestrial and probably the terrestrial-hypogeic stratum." It occurred throughout the upland forests; groups were found established in and under logs 100 to 200 feet from the nearest forest (Cantrall, 1943).
Ontario.—Very abundant in rocky, sparsely-wooded country, where it occurred in rotten logs and under loose bark; on tree trunk at night on rocky island in lake (Walker, 1912).
North Carolina.—Under pine straw on ground in woods (Brimley, 1908). Under bark of dead trees; 92 males attracted to lights (Rehn and Hebard, 1910).
Virginia.—Resting on woods foliage; at night on road (Rehn and Hebard, 1916).
Eastern and southeastern U.S.—Trapped in molasses-baited jars: in oak and pine woods, in heavy barrier-beach forest, in both scant and typical undergrowth on pine barrens, in heavy grassy undergrowth on pine barrens, on border of pine barrens, on edge of swamp, in heavy deciduous forest, in heavy oak woods, in upland oak and chestnut forest, in chestnut forest, in forested ravine, and on ridge [Pg 62] with heavy oak, chestnut, and maple forest. Found under damp leaves on edge of forest, under bark of decayed chestnut log, inside decaying chestnut log with Cryptocercus punctulatus, under palmetto roots, under bark of pine stump, and in dry leaves under live oaks (Hebard, 1917).
Tennessee.—In traps baited with cornmeal or cantaloupe in maple-gum-oak forest in a mesic valley, and in a stand of oak on a dry ridge (Walker, 1957).
Indiana.—Beneath cover on slopes of high wooded hills. "This is essentially a forest-loving species; usually occurring beneath leaves and other debris on or along the borders of heavy hardwood timber." (Blatchley, 1920.)
Illinois.—In oak-hickory forest on clay and in climax forest (Strohecker, 1937).
Michigan.—In oak-dune woods (Hubbell, 1922). Restricted to woodlands, where it inhabited piles of moist dead leaves and rotten logs in oak-hickory forest (Cantrall, 1943).
U.S.A.—This species, P. uhleriana, and P. virginica were attracted at night to honeydew secreted by aphids on Pyrus sp. (Davis, 1918).
New England.—Females under loose stones, boards, and other debris on ground; beneath loose bark (Morse, 1920).
North Carolina.—Under debris in dead shortleaf-pine needles (Rehn and Hebard, 1916).
Florida.—Infrequent in the shrub stratum of the scrub habitat. This was the only habitat of 25 studied in which this species was found (Friauf, 1953).
Eastern and southeastern U.S.—Trapped in molasses-baited jars: in pine and oak woods, in pine barrens, in pine woods with heavy grass undergrowth, in oak forest, in heavily forested ravine, on rocky slope with few deciduous trees, on knoll with high deciduous trees, in lofty chestnut forest, and in heavy low chestnut and oak forest on high ridge; under bark of decaying chestnut log and stump; under stones in chestnut forest; under bark of pine stumps (Hebard, 1917).
Indiana.—Frequents borders of open woods and fields; under debris, loose bark, and half-buried logs (Blatchley, 1920).
Illinois.—In black-oak forest on sand, in oak-hickory forest on clay, and in climax forest (Strohecker, 1937).
Michigan.—Common in oak-dune and beech-maple forests; under loose bark on dead trees and fallen logs and under debris on forest floor (Hubbell, 1922). Restricted to woodlands, where it inhabited [Pg 63] piles of moist dead leaves and rotten logs in oak-hickory forest (Cantrall, 1943).
Texas.—Captured in molasses traps in moist woods of maple, oak, and pine with much undergrowth and a heavy layer of duff; in open, rather dry woodlot of Spanish oak and other trees; and in low wet woods of willow and oak along creek (Hebard, 1943a).
Indiana.—Beneath log in cypress swamp (Blatchley, 1920).
Louisiana and Mississippi.—In decay cavity in sweet gum; under sign on shortleaf pine (Hebard, 1917).
Alabama.—In the dry wall of a sweet-gum stump together with serropalpid and tenebrionid beetles (Snow, 1958).
Ohio.—Oöthecae under loose bark of fallen trees, where as many as 184 oöthecae were found within a few feet of each other; others found under boards and in piles of firewood (Edmunds, 1952).
Puerto Rico.—Mona Island, under bark of dead trees and under guava leaves (Ramos, 1946). Under bark of Sideroxylon foetidissimum (Wolcott, 1941). Common along the coast and in mountains. "Very much at home" under the loose bark of Sideroxylon foetidissimum (Wolcott, 1950).
Trinidad.—Under debris in bush (Princis and Kevan, 1955).
Dominica.—In decaying logs in forest (Rehn and Hebard, 1927).
Bermuda.—Among and under decaying debris, just above high-tide line (Verrill, 1902).
Johnson Island.—Nocturnal, coming out at night in great numbers about Tribulus blossoms. Under timbers on French Frigate Shoals (Bryan, 1926).
United States.—Alleyways and yards may be overrun during the summer; adults and hundreds of nymphs found in decaying maple trees along residential street (Gould and Deay, 1938, 1940). Around [Pg 64] fumaroles where a railroad fill was burning internally (Davis, 1927). Common in palm trees along the gulf coast of Texas, where they often fly around street lights at night (Zimmern in Gould and Deay, 1940).
Bermuda.—Very abundant under stones (Rehn, 1910).
Jamaica.—Under bark of dead tree and under bases of leaves of coconut palms (Rehn and Hebard, 1927).
Virgin Islands, St. Croix.—Common in sugarcane fields and in woodlands (Beatty, 1944).
Florida.—Juveniles under bark of dead logs of Pinus caribaea (Hebard, 1915). Frequently found under signs on trees near borders of towns; under bases of dead petioles of cabbage palmetto (Hebard, 1917). Beneath logs, burlap bags, and other cover in old orange orchards (Blatchley, 1920).
Marquesas Islands.—Under coconut fronds and grass (Hebard, 1935).
Nihoa Island.—Nymphs only, on Sida, Pritchardia, bunch grass, and about camp (Bryan, 1926).
Georgia.—Under signs on oaks (Rehn and Hebard, 1916).
Florida.—Beneath bark of stump (Blatchley, 1920).
Southeastern and southern U.S.—"This species is usually encountered out of doors, in or near towns. Over its range it is frequently found under signs on trees" (Hebard, 1917).
Chile.—Collected from mosses and lichens on tree trunks (Princis, 1952).
Brazil and Guiana.—In grasslands, plantations of maize, sugarcane, and other plants on the borders of forests; the cockroaches were always found between the leaves which form the branches of the plants (Doumerc in Blanchard, 1837).
Asia Minor and western Europe.—On ground among grasses; under moss and brushwood in mountain meadows (Bei-Bienko, 1950).
U.S.S.R., western Georgia.—In pine forest mixed with deciduous trees (Bei-Bienko, 1950).
U.S.S.R., Turan Lowland.—Along margins of "tugas" under half-fallen bushes of Salsola kali that overhang the ground (Bei-Bienko, 1950).
Central Europe and western U.S.S.R.—On the edges of forests of the central-European type that are lighted by the sun; under fallen leaves; on bushes and conifers (Bei-Bienko, 1950).
U.S.S.R.—Among fallen leaves under bushes; on oak branches; under mown hay (Bei-Bienko, 1950).
Caucasus.—Under fallen leaves on slopes of mountains covered by forest or brushwood (Bei-Bienko, 1950).
U.S.S.R.—In the sands of Un-dzhal-kum and Zhety-konur it is found in the dense turf of Aristida pennata (Bei-Bienko, 1950).
Central Asia.—In lowlands and in mountains up to 2,500 meters; in fruit orchards under trap rings fastened to trees to combat lesser apple worm (Bei-Bienko, 1950).
Southern Uzbekistan.—Under bark of Juniperus sp., under stones and on flowers of Scorzonera acanthoclada (Bei-Bienko, 1950).
Australia.—Under loose wood or bark (Shaw, 1914).
New Zealand.—Swarms under loose dry bark and logs (Walker, 1904).
Dominica.—On moss-covered lime trees. "The species of the genus Plectoptera are all foliage and flower frequenters, generally secured by beating low arborescent vegetation, or are attracted to light" (Rehn and Hebard, 1927).
Puerto Rico.—In caladium, grass, weeds, coffee, and bananas; in flowers of Ipomoea tiliasea (Rehn and Hebard, 1927). "... living in trees between leaves, or in 'butterfly-nests' of Tetralopha scabridella in leaves of Inga vera, or of Pilocrocis secernalis in the leaves of 'capá blanco' (Petitia domingensis) in the mountains. Along the coast they have been found under the bracts of cotton squares or bolls, and under the leaf-sheaths of sugar cane, in curled-up leaves of grapefruit, or in the dry flower clusters of 'espino rubial' (Zanthoxylum caribaeum)." These observations apply also to Plectoptera infulata and P. rhabdota (Wolcott, 1950).
Florida.—On fringe of tall bushes at edge of mangrove swamp (Rehn and Hebard, 1914). Rehn and Hebard (1927) stated that on the Keys it frequented dry scrubby vegetation, particularly Ilex cassine.
Puerto Rico.—See Wolcott's (1950) comments under Plectoptera dorsalis above.
Cuba.—In grasses, sedges, etc., about a waterhole; on grass, pines and oak (Rehn and Hebard, 1927).
Dominica.—On moss-covered lime trees (Rehn and Hebard, 1927).
Cuba.—Taken on flowers of "Júcaro" (Gundlach in Rehn and Hebard, 1927).
Jamaica.—In relatively dense forest foliage; in shrubbery (Rehn and Hebard, 1927).
Puerto Rico.—In mixed vegetation; on grapefruit tree and guava (Psidium guajava); on bushes and shrubs (Rehn and Hebard, 1927). On coffee trees; on Spondias; on sugarcane; in caterpillar nests of Tetralopha scabridella on Inga vera; in old cotton bolls; on grapefruit (Wolcott, 1936). See also Wolcott's (1950) comments under Plectoptera dorsalis.
Cuba.—On pine in palmetto region (Rehn and Hebard, 1927).
Algeria.—Nymphal females under decaying leaves at the end of November (Lucas, 1849).
Transcaucasia.—In burrows in argillaceous cliffs along ravines. Females often covered by attached clay particles, an indication, according to Bei-Bienko, that this species is ecologically connected to compact clay soils or at least does not avoid them (Bei-Bienko, 1950).
See also the section on desert habitats (p. 29).
South-central Asia.—Occupies compact clay soils; distributed in drier regions than P. aegyptiaca; frequently found near dwellings, in yards, stables, and houses (Bei-Bienko, 1950).
Australia.—Common, usually "resting among the foliage or sunning itself on a fence or stumps, seldom or never hiding under bark or logs like most of the species" (Froggatt, 1906).
Tropical America.—"The species of Poroblatta apparently live as borers in stumps and logs in a manner similar to those of Cryptocercus Scudder in the United States" (Gurney, 1937).
Texas.—In dead-brush pile; not scarce in heavy weeds, sunflowers, etc., in openings of river-plain jungle scrub (Hebard, 1917). It lives largely in herbage (Hebard, 1943a).
Florida.—Under stones and rubbish; very abundant under coquina boulders in woods at Key West (Rehn and Hebard, 1905). "This [Pg 68] species is common under planks, stones, and other debris on the ground ... also found at Long Key in the dry fibres at the base of the petioles of a coconut palm" (Rehn and Hebard, 1912). At Musa Isle, found burrowing in sand (Hebard, 1915). In fallen leaves and decaying wood in xeric and mesic hammock habitats (Friauf, 1953).
Hawaii.—The soil swarmed with young of various stages during the summer (Illingworth, 1915). In soil about roots of pineapple under mulching paper; feeding on pineapple roots (Illingworth, 1927, 1929).
Fakarava, Tuamoto Archipelago.—Numerous among dead leaves in tree holes (Cheesman, 1927).
West Indies.—Under decayed stalks of sugarcane and in siftings from mangrove swamps, Cuba. Under manure, bases of leaves of coconut palm, litter, logs, and stones on coral rock and in bromeliads, Jamaica. Under wood, tiles, and boards in stable yards; immature individuals bored into the soil, Puerto Rico. (Rehn and Hebard, 1927.)
Barbados.—Frequents cane fields (Tucker, 1952).
Puerto Rico.—"Altho primarily a xerophytic species: collected among dry stones on Mona Island, under dry cow dung at Boquerón, and under boxes at Guánica, it is reasonably common in the more humid parts of Puerto Rico" (Wolcott, 1950).
Virgin Islands, St. Croix.—Common under rubbish; frequently seen feeding on chicken feces around chicken roosts (Beatty, 1944). By feeding on chicken feces it may become the vector of the chicken eyeworm, Oxyspirura mansoni, as described in the references cited on page 204.
Egypt.—Large numbers were found in moist soil at the site of a manure pile (Chakour, 1942).
Germany.—Under greenhouse conditions the depth to which P. surinamensis penetrated the soil was determined; 21 dug down to a depth of 8 to 10 cm., 3 dug down 10 to 12 cm., but only one dug 13 cm. below the surface. Often the tubes in the soil ended in a chamber which the cockroach might not leave for several days; nymphs molted in such chambers and females bore their young there (Roeser, 1940).
Trinidad.—Male on low herbage in orchard at night; under sacking; on Hibiscus at night (Princis and Kevan, 1955).
Trinidad.—Numerous specimens of both sexes at night on roadside Hibiscus rosa-sinensis or low herbage in orchard (Princis and Kevan, 1955).
Jamaica.—Numerous in fragmentary debris of an abandoned termite nest on ground in the dry Liguanea Plain; a specimen was also taken under a stone in a field of short grass (Rehn and Hebard, 1927).
Costa Rica.—Among humus and rubble in crevices and large cavities in rocks of the Tertiary limestone rim and the metamorphosed and igneous rocks of the interior of the islands (Baker in Rehn, 1930).
Virgin Islands, St. Croix.—Under rubbish heaps; in sugarcane straw (Beatty, 1944).
Africa.—"A cosmotropical species which occurs both out of doors and as a household pest in many warmer parts of the world. It is apparently endemic to non-forested areas in much of Africa north of the Equator." (Kevan and Chopard, 1954.)
Puerto Rico.—Under low trees on hillside and dead leaves in thicket of sea grape (Hebard, 1916c).
Hawaii.—Under stones and rubbish (Illingworth, 1915).
Virgin Islands, St. Croix.—Under rubbish and on shrubbery at night (Beatty, 1944).
Jamaica.—In dead leaves under acacia and other shrubs in desert tract; under log and rubbish in open on limestone sand near beach (Hebard, 1916c). Very common in short dry grass in roadside gutter at night, often clustered together; under beach trash in stony wash of Hope River (Rehn and Hebard, 1927).
Virgin Islands, St. Croix.—Under rubbish and on shrubbery at night (Beatty, 1944).
Puerto Rico.—In siftings from sea-grape thicket on sandy soil (Rehn and Hebard, 1927). Often living under leaf-sheaths of sugarcane (Wolcott, 1950).
Asia, Kara-tau Mountains.—Many hundreds of individuals found only under stones on moist earth and not where ground seemed dry; found on very stony slopes with sparse vegetation, often with undergrowth present (Bei-Bienko, 1950).
In this category we include all man-made structures, whether inhabited by man or not, that may become infested with cockroaches. A nonexhaustive list of such structures would include dwellings, restaurants, mess halls, barracks, groceries, markets, bakeries, dairies, drug stores, department stores, hotels, hospitals, warehouses, mills, factories, packing houses, animal houses, breweries, incinerators, privies, sewers, sewage treatment plants, ships, aircraft, etc. Although dwellings are only one of the many kinds of structures that are colonized by cockroaches, the several species that have adopted this mode of life are generally referred to as domiciliary cockroaches. This term is adequate only if we remember that these cockroaches are not restricted to domiciles but are pests in other structures as well.
Associations between man and certain species of cockroaches possibly started as casually as the short-lived association that Beebe (1953) observed when he discovered three cockroaches in the newly built couch of an orang-utan. Obviously, when man came down from the trees, his fellow travelers found his cave dwellings and other abodes particularly favorable habitats. From such primitive beginnings, domiciliary cockroaches have spread into every kind of structure that man has since devised. We predict that when man develops a suitable vehicle, cockroaches will someday accompany him into space. Yet despite the apparent predilection of certain species of cockroaches for man, man is only incidental to these associations. Only the shelter and food that man unwittingly provides for these unwelcome guests attract cockroaches to him; man's physical presence is unnecessary.
Most, if not all, of the common domiciliary cockroaches apparently originated in the Tropics or sub-Tropics from whence they have spread, through normal commercial channels, into most of the inhabited world. At least eight domiciliary cockroaches originated in Africa (Rehn, 1945): Blatta orientalis, Blattella germanica, Leucophaea maderae, Nauphoeta cinerea, Oxyhaloa buprestoides, Periplaneta americana, P. australasiae, and Supella supellectilium; and, perhaps, Periplaneta brunnea as well; Neostylopyga rhombifolia was probably of Indo-Malayan origin; Pycnoscelus surinamensis was of [Pg 71] oriental origin; and Leurolestes pallidus was endemic in the West Indies (Rehn, 1945). Princis (1954a) rejected Africa as the original home of Blatta orientalis and advanced reasons for placing its origin in Central Asia.
Several domiciliary species have become well established in temperate zones and some even in the Arctic. Bei-Bienko (1950) listed the following 10 species as sinanthropes in the Palearctic zone: Blatta lateralis, B. orientalis, Blattella germanica, Leucophaea maderae, Periplaneta americana, P. australasiae, Polyphaga saussurei, Pycnoscelus surinamensis, and Supella supellectilium. In the warmer parts of the temperate regions, as in their native Tropics, certain domiciliary species breed outdoors as well as indoors. In the less temperate extensions of their ranges most domiciliary species are nearly always found indoors. In regions with low winter temperatures these cockroaches do not survive in unheated structures; but in heated buildings Blattella germanica, for example, has been able to withstand the rigorous climate of Alaska, where it has caused severe infestations (Chamberlin, 1949).
The limiting factors that determine whether man-made structures will provide suitable habitats for cockroaches are favorable temperature and availability of water and food. The range of temperatures that man provides for his own comfort and protection fosters the rapid increase of cockroach populations indoors. Gunn (1934, 1935) has demonstrated that the preferred temperature range (zone of indifference) of Blatta orientalis is 20-29° C. The upper limit of the preferred temperature of Blattella germanica and Periplaneta americana is 33° C. (Gunn, 1935). The lower limits of temperature tolerance were not sharply defined in Gunn's work. However, less than optimum temperatures, if they last for only short periods, are not necessarily lethal. The 24-hour mortality for P. americana that had been held for one hour at 0° C. was only 2±2 percent (Knipling and Sullivan, 1957). Gunn (1934) observed that Blatta orientalis would not settle at temperatures above 33° C. and would react violently against higher temperatures (e.g., 39° C.) by running away; thus the thermotactic behavior of cockroaches might be presumed to bring them into favorable environments within structures. Thermal death points have been determined for the above three species by Gunn and Notley (1936).
It is common knowledge among those who rear cockroaches experimentally that, unless the water content of the food is high, fluid water is essential in the insects' dietary. Ten species of domiciliary cockroaches have been shown to be unable to survive as long on dry food alone as they could on food and water at 36-40 percent relative [Pg 72] humidity (Willis and Lewis, 1957). Blatta orientalis, when in a state of normal water balance, usually spent more time in the drier part of a humidity gradient; but desiccated insects tended to become hygropositive (Gunn and Cosway, 1938). We presume that other domiciliary species behave similarly. If water is available nearby, it may be presumed that partially desiccated cockroaches could locate a source through the mediation of a humidity sense. Hygroreceptors have been demonstrated on the antennae of Blattella germanica (Roth and Willis, 1952a) and suggested for Blatta orientalis (Gunn and Cosway, 1938).
Drinking water is available to cockroaches in the traps of sinks, wash basins, tubs, and toilet bowls; in flush tanks; as condensation on cold pipes, flush tanks, and windows; around leaking pipes and faucets; as spillage; in miscellaneous water-filled containers, such as pet drinking dishes, aquaria, vases; empty beverage bottles; and drainage from ice boxes. Soft, juicy fruits and vegetables can provide both moisture and food. There seems to be a tendency for certain species (Blatta orientalis and Blattella germanica) to become established in the more humid parts of structures, such as basements, around sinks, and in bathrooms. Whether this is a reaction to a preferred humidity or merely a fortuitous aggregation near sources of drinking water and food has never been clearly demonstrated. The rather widespread dissemination of these species into zones of low as well as high humidity suggests that detailed studies of the microclimatic conditions of structural microhabitats will be needed before meaningful conclusions can be drawn about the stratification of cockroaches within structures according to species.
In nearly all structures infested by cockroaches, food of some kind is available, either in the structure itself or nearby. This may be the food stored by man for his own use or the use of kept animals; it may be crumbs, food spillage, garbage, or excreta; glues and pastes on cartons, boxes, stamps, envelopes, labels, and wall paper; sizing on cloth and book covers; various dried animal and plant products; dead insects; living plants; etc. In fact, it is almost impossible, despite good housekeeping, to keep any structure used by man free of all food suitable for cockroaches.
That the requisite temperature, water, and food are provided, more or less adequately, by a variety of structures is attested by the innumerable infestations of cockroaches that develop when control measures are relaxed. Within structures the accessibility of certain harborages to cockroaches probably depends on the habits of the species and to some extent on their size. Similar types of harborages [Pg 73] in different structures may be used by the same species, although there seems to be some overlapping by different species into the same kinds of daytime shelters. The comparative ecology of domiciliary cockroaches has not been thoroughly investigated, so any interpretation of observational data is necessarily speculative and inconclusive at this time. Our discussion on pages 324 to 343 is also pertinent to this section.
Dwellings provide a variety of microhabitats that are acceptable to cockroaches. It has been stated that old houses, or houses that have many cracks and crevices, or have basement kitchens that are not kept clean and in good repair are particularly liable to invasion by cockroaches (Laing, 1946; British Museum [Nat. Hist.], 1951). Although this statement is undoubtedly true, it has been our personal experience, as well as the experience of others, that new, clean, and well-planned houses and apartments are also easily and sometimes quickly invaded by cockroaches. Mallis (1954) has cited the following places that are frequently infested by cockroaches in homes. In the kitchen, cockroaches are found in and around sinks, in cupboards above and below sinks, under tables and chairs, in stoves, around breadboards, in utility cabinets, in kitchen closets, under linoleum, behind, under, and inside refrigerators and iceboxes. In living rooms cockroaches are found in furniture, studio couches, sewing machines, closets, and bookshelves; behind picture frames, pennants, calendars, and other wall ornaments. In bathrooms cockroaches are found in and behind utility cabinets and toilets; they may be found in wicker clothes hampers, in brooms and mops, and in door hinges. Ordinarily, cockroaches are not found in bedrooms unless they are abundant elsewhere in the dwelling. Additional harborages are cited under specific cockroaches in the list below.
In markets DeLong (1948) found the German cockroach in bags of potatoes and onions, in crates of citrus fruits, in pads and shredded papers in banana boxes, and in cases of bottled beverages. The insects were attracted by coffee and crawled into the folds of coffee bags. They were found in cartons of canned goods; in bread and baked goods; in cartons of packaged cookies, cakes, and crackers. Packaged cereals were attractive, and cockroaches were sometimes found in packages of cigarettes. The insects occurred in scales (by the hundreds) and in cash registers. Rather heavy infestations were found under stainless-steel cappings that covered wooden arms on the fish cleaning stand. The insects were numerous in display cases where [Pg 74] they were warm and sheltered. They were also found behind mirrors above produce racks, in electrical switch boxes and conduits, and in telephone boxes, as well as generally in cracks and behind loose moldings or loose wall boards. Enclosed boxlike tables were frequently heavily infested.
In restaurants cockroaches may be found in the following places: Crevices in wood, plaster, concrete, and metal; in the bar; in the kitchen and in the associated equipment; in cupboards, lavatories, and garbage storage areas; and on the undersides of chairs and tables (Mallis, 1954).
In drug stores Frings (1948) found cockroaches behind the mirror and between the sink and the cooler. Thousands were found in hollow ornamental shelf edging. The hollow bases of malted-milk dispensers and drink mixers were cockroach havens.
In a hospital Frings (1948) found cockroaches in decorative trim around doorways, by the thousands in wicker laundry baskets, and in incubators for premature babies. In military hospitals we have seen cockroaches (Blattella germanica) in kitchens and dining halls in the usual hiding places mentioned above and on the undersides of stainless-steel serving tables.
In department stores cockroaches have been found in food departments, beauty salons, rest rooms, dressing rooms, linen departments, and stationery departments (Anonymous, 1952). The infestation in the linen department was traced to clean towels which, when returned from the laundry, contained at least 500 cockroaches per bundle. The insects were carried into the rest rooms and beauty salon when the towels were distributed.
The microhabitats of cockroaches in privies and sewers have not been studied. These habitats are particularly important in view of the demonstrated migrations of cockroaches from sewers into dwellings and the possible dissemination of pathogenic microorganisms from feces to food. The reader is referred to our 1957(a) paper for a summary of the known information on cockroach dispersal from sewers.
Most of the cockroaches listed below are either known domiciliary species or they have been found one or more times in houses or other man-made structures. The known structural pests breed within the building. Certain other species, which have been observed only infrequently in structures and are not known to breed there, may possibly [Pg 75] be incipient pests; these latter species may attain future economic importance if they establish breeding colonies within a structure. A few species have undoubtedly wandered indoors by accident. It is difficult to decide whether a particular species was an accidental invader or whether it was attracted indoors in response to some stimulus. Only additional information will provide the desired answers.
Trinidad.—Male found indoors (Princis and Kevan, 1955).
Hawaii.—Found only indoors at Nauhi. Otherwise this is apparently an outdoor species (Swezey and Williams, 1932).
Cuba.—Household pest (Deschapelles, 1939). Particularly abundant in houses in Santiago and Havana (Rehn and Hebard, 1927).
Florida.—Under boards in woodshed (Rehn and Hebard, 1912, 1914).
Ecuador.—In eating places (Campos R., 1926).
Hispaniola.—In houses (Rehn and Hebard, 1927).
New Jersey.—In greenhouse (Weiss, 1917).
Puerto Rico.—In homes (Seín, 1923). In fruit stores (Wolcott, 1950).
Central Asia.—Household pest, often found in homes with clay floors (Bei-Bienko, 1950).
Turkmen S.S.R.—Males and females occurred in dwellings (Vlasov, 1929).
This species is a cosmopolitan domiciliary pest (Hebard, 1917; Rehn, 1945). It is reported to occur particularly in basements and crawl spaces under basementless houses (Mallis, 1954). In damp basements where food is available large colonies are not unusual, but it also may infest offices and apartments several floors off the ground (Gould and Deay, 1940). The number encountered on upper floors is seldom large, but the frequency of occurrence may reach 30 percent of the observations (Spear et al., in Shuyler, 1956). In supermarkets this species hides during the day inside concrete blocks or cracks in [Pg 76] the foundation, under furniture, or behind cartons; it is conspicuous on the floors of the markets at night (De Long, 1948). In Great Britain the kitchen is preferred by this pest (and by Blattella germanica); they shelter beneath steam radiators and gas stoves, behind hot-water pipes, underneath furniture and floor coverings, sinks and baths; basements and underground kitchens are especially likely to be infested (Laing, 1946; British Museum [Nat. Hist.], 1951). Goodliffe (1958) noted that B. orientalis may travel long distances to find food.
This species is a cosmopolitan domiciliary pest (Hebard, 1917; Rehn, 1945). It is one of the commonest insects in homes and restaurants (Gould and Deay, 1940). It is found in kitchens, larders, bathrooms, furnace rooms, and storage rooms of bakeries, breweries, hospitals, barracks, as well as dwellings, where, during daylight, it hides behind cupboards, furniture, hanging pictures, panels and skirting boards, in cracks around drains, water pipes, electric wires, and hot-water and steam heating units (Wille, 1920). The German cockroach may be found in cracks around baseboards, pipes, conduits, sinks, and drawers; behind cabinets; inside switch boxes and refrigerators; on under surfaces of tables, chairs, and shelves; between stacks of stored goods, and in almost every place that is not readily observed (Kruse, 1948). We have also seen this species packed in electric-clock cases and loud-speaker baffles, in cash registers, and clinging to the undersurface of stainless-steel steam tables. The infestation of markets by this species has been described above. Very narrow cracks provide refuges for the German cockroach. Wille (1920) found first-instar nymphs in cracks 0.5 mm. wide and adult males and females without oöthecae in cracks 1.6 mm. wide.
Shuyler (1956) has observed extensions into relatively new structural habitats by Blattella germanica in the north-central area of the United States. A few German cockroaches are now being encountered in living rooms, bedrooms, clothes closets, bedroom furniture, lobbies, entrance halls, checkrooms, nonfood storerooms, nonfood warehouses, and coin-vending machine repair shops. In these situations this species is behaving much like the brown-banded cockroach, Supella supellectilium.
Cameroon.—Five specimens in a house (Princis, 1955).
U.S.A.—Although this is mostly an outdoor species, during dry seasons it may temporarily enter houses in great numbers, occasionally breeding indoors in Arizona (Flock, 1941a). Two adults were collected indoors in Texas (Riherd, 1953).
Trinidad.—A male was found indoors (Princis and Kevan, 1955).
Hawaii.—Almost as common in houses as Neostylopyga rhombifolia (Hebard, 1922).
U.S.S.R.—Frequently occurs in living apartments in farming localities as an accidental inhabitant (Bei-Bienko, 1950).
Massachusetts.—A summertime pest in houses along coast (Gurney, 1953; E. R. Willis, personal observation). Generally an outdoor species.
Trinidad.—Male, indoors (Princis and Kevan, 1955). An outdoor species generally.
Cameroon.—A male taken in a house (Princis, 1955).
Trinidad.—Indoors, feeding on bat feces (Princis and Kevan, 1955).
Florida.—Occasionally found in homes (Creighton, 1954; Roth and Willis, 1954a).
Hawaii.—Found outdoors and indoors where it breeds in neglected cupboards and in rubbish (Fullaway and Krauss, 1945).
Mexico.—Household pest (Ball et al., 1942).
Costa Rica.—One specimen in house (Rehn, 1906).
Apparently domiciliary in American Tropics (Hebard, 1917). In houses under chests, etc., Cuba (Gundlach, 1890-1891); Puerto Rico (Gundlach, 1887). In folds of burlap bag, Florida (Rehn and Hebard, 1914).
Panama.—Thrives about human habitations under litter, though not domiciliary (Hebard, 1920).
Jamaica.—In hotel. "While hardly a domiciliary form it would seem to frequent environments where man has considerably disturbed natural conditions, as under debris, docks, under logs and stones in cultivated areas" (Rehn and Hebard, 1927).
West Indies.—In habitations, warehouses, and other structures; "At times it is a very abundant and serious pest" (Rehn, 1945). In Puerto Rico it was also found in fruit stores, markets, and inns (Seín, 1923; Wolcott, 1950).
Reported as a domiciliary pest in Madeira (Heer, 1864); Windward Islands (Marshall, 1878); Tropics and sub-Tropics (Rehn, 1937); Philippine Islands (Uichanco, 1953); New York City (Anonymous, 1953; Gurney, 1953); Trinidad (Princis and Kevan, 1955). This species is also established in coastal Brazil, Central America, all the Greater Antilles, several other tropical islands, and tropical Africa, where it probably originated (Rehn, 1945).
Hispaniola, Grenada.—Largely domiciliary (Rehn and Hebard, 1927).
Cuba.—All over island, in houses, under lockers, etc. (Rehn, 1945; Gundlach, 1890-1891).
Florida.—Rehn and Hebard (1914).
This species has been recorded from various islands in the West Indies, from Mexico, Guatemala, and Brazil (Rehn, 1945).
Australia.—Reported entering houses (Pope, 1953a).
Australia.—In hospital (Mackerras and Mackerras, 1948). In dwellings, grain stores, and fowl-feeding pens (Pope, 1953).
Sudan.—Domiciliary in huts of the Shilluk natives; fairly widely distributed in eastern Africa (Rehn, 1945).
Hawaii.—In feed rooms of poultry plants (Illingworth, 1942).
Florida.—Established in feed mills around Tampa (Gresham, 1952; Gurney, 1953).
The wide distribution of this species from East Africa, where it originated, to the Orient and the New World was undoubtedly mediated by shipping (Rehn, 1945).
Puerto Rico.—Observed [as Blatta caraibea, Rehn and Hebard (1927)] in houses (Gundlach, 1887).
Domiciliary in Indo-Malaya and New World Tropics (Rehn, 1945); Philippine Islands (Uichanco, 1953); and Hawaii (Hebard, 1922).
Presumably to some extent domiciliary, as it evidently spread from Africa to the New World via slave ships (Rehn and Hebard, 1927; Rehn, 1945).
Colombia.—A male and a female taken in a dwelling (Princis, 1946). This is primarily an outdoor species which is frequently taken indoors as an adventive on bananas (see p. 150 for references).
Florida.—Males found in laboratory and dormitory buildings, ostensibly attracted by lights (Friauf, 1953).
Connecticut.—Domiciliary pest (Moore, 1957). Generally an outdoor species.
Arizona.—It may occasionally be a nuisance in houses (Ball et al., 1942).
U.S.A.—Country houses often badly infested, Indiana (Blatchley, 1920). Frequently taken in houses in wooded areas, Michigan (Hubbell, 1922). Infestation by males, females, and nymphs on fourth floor of building, South Dakota (Severin, 1952). Houses in wooded areas infested by nymphs and occasionally by adults (Gould and Deay, 1940).
Canada.—Pest in summer cottages in Ontario (Walker, 1912).
This is a cosmopolitan domiciliary pest (Hebard, 1917; Rehn, 1945). It is common in restaurants, grocery stores, bakeries, and where food is prepared or stored; it was trapped regularly in the basement and upper floors of store buildings, and it was also found in all heated parts of an old meat-packing plant (Gould and Deay, 1938). P. americana was numerous in latrines in Iran (Bei-Bienko, 1950) and in privies in Texas (Dow, 1955) and Georgia (Haines and Palmer, 1955). Large numbers of this species also occur in sewers adjacent to human habitations (Roth and Willis, 1957a).
Generally domiciliary, but also occurs outdoors in the West Indies (Rehn and Hebard, 1927). It is a very abundant domiciliary pest in tropical Africa and tropical America (Rehn, 1945); Ecuador (Campos R., 1926); Puerto Rico (Sein, 1923); Philippine Islands (Uichanco, 1953); Australia (Pope, 1953).
Also occurs as a pest in greenhouses in Pennsylvania (Thilow and Riley, 1891); France (Giard, 1900); Italy (Boettger, 1930); Great Britain (Laing, 1946; British Museum [Nat. Hist.], 1951).
Circumtropical domiciliary pest which is apparently more nearly peculiar to the Tropics and adjacent regions than P. americana (Hebard, 1917). This species has been trapped in significant numbers in privies and dwellings in Georgia (Haines and Palmer, 1955). It is the species of Periplaneta present in homes in San Antonio, Texas (Mallis, 1954).
U.S.A.—Frequently encountered out of doors, but has been reported common after dark about a hotel in Alabama and was captured in a house in Louisiana; it was also extremely abundant on the wharves at night in Jacksonville, Florida (Hebard, 1917). As a domiciliary pest it was, next to Blattella germanica, the most common cockroach inside homes in southwest Georgia, where it was also the most common cockroach in privies (Haines and Palmer, 1955). This species has become a very common domiciliary pest in Texas (Eads, personal communication, 1955). It infested a greenhouse for five years in Indiana (Gould and Deay, 1940).
Australia.—It occurs in dwellings occasionally (Pope, 1953).
Colombia.—Three specimens from three dwellings (Princis, 1946).
Trinidad.—Male indoors; male and female at light (Princis and Kevan, 1955).
Puerto Rico.—According to Gundlach (1887) it enters houses at night attracted by light (Rehn and Hebard, 1927).
Iraq.—Common in houses (Weber, 1954).
Caucasus.—Winged male in kitchen (Burr, 1913).
U.S.S.R.—Listed as a sinanthrope (Bei-Bienko, 1950).
South-central Asia.—One of the commonest domiciliary species (Bei-Bienko, 1950).
East Indies.—This species is sometimes difficult indoors (Karny, 1925).
A household pest in the East Indies (Karny, 1925); Philippine Islands (Uichanco, 1953); Tanganyika (Smith, 1955); Trinidad, eight records indoors (Princis and Kevan, 1955). It is also a greenhouse pest (Hebard, 1917; Zappe, 1918; Doucette and Smith, 1926; [Pg 82] Saupe, 1928; Roeser, 1940). Common in or around chicken batteries and yards in Hawaii (Schwabe, 1949).
Domiciliary wherever distributed (Rehn, 1945), this species is especially difficult to control because of its apparently nonselective dispersal throughout dwellings. For example, Mallis (1954) observed in Texas that it was widely distributed throughout the apartment and was probably the most common cockroach seen in the bedroom; its favorite harborages were beneath and behind corner braces on kitchen chairs, underneath tables, behind pictures and other objects on walls, and in shower stalls; its oöthecae were commonly fastened on walls and ceilings throughout the house. Gould and Deay (1940) reported that this species prefers high locations, such as shelves in closets, behind pictures, and picture molding; oöthecae were found about kitchen sink, desks, tables, and other furniture, and even in bedding. Hafez and Afifi (1956) stated that the adult wanders in nearly all rooms of the house and only visits the kitchen when searching for food; it hides in cupboards, pantries, closets, bookshelves, drawers, and behind picture frames; the nymphs normally hide in the corners of drawers, behind frames, and in similar situations.
Puerto Rico.—In houses, Sardinera Beach, Mona Island (Ramos, 1946).
North American Tropics.—Domiciliary, but not exclusively so, and apparently widely distributed (Hebard, 1917). In Florida, as Ischnoptera rufescens, found in a greasy cupboard (Rehn and Hebard, 1914).
Hawaii.—Illingworth (1915).
Sailing ships have long been notorious for their unwelcome hordes of cockroaches, and it was by ship that at least 11 domiciliary species migrated from their centers of origin to other parts of the world (Rehn, 1945). Over 40 nondomiciliary species have been carried by ship from the American Tropics to other parts of the world in cargoes of bananas (p. 146). In addition to these, other adventive cockroaches appear from time to time in ports to which they have been carried by ships. Yet by far the most numerous cockroaches on shipboard are [Pg 83] the breeding populations of a few common domiciliary pests. Except in the most rigorously disinsectized ships, this commerce in cockroaches has continued to the present day.
Cockroaches undoubtedly infested the first ships that sailed the Mediterranean; of these we have no records. The earliest recognizable record of cockroaches on shipboard is Moffett's (1634) statement that when Drake captured the ship Philip, he found it overrun with cockroaches [Blattarum alatarum]. Bligh (1792) described disinfesting H.M.S. Bounty with boiling water to kill cockroaches. Chamisso (1829) reported that he had seen ships casks, in which rice or grain had been stored, that were found to be filled with Blattella germanica when opened. During a voyage from England to Van Diemen's Land, Lewis (1836) was greatly annoyed by hundreds of cockroaches flying about his cabin at night; the most numerous resembled Periplaneta americana and another was similar to Ectobius lapponicus. This latter was undoubtedly B. germanica, which is the only ship-infesting species that resembles the feral E. lapponicus. Lewis continued, P. americana "were in immense profusion, and had communication with every part of the ship, between the timbers or skin. The ravages they committed on everything edible were very extensive; not a biscuit but was more or less polluted by them, and amongst the cargo 300 cases of cheeses, which had holes in them to prevent their sweating, were considerably damaged, some of them being half devoured and not one without some marks of their residence."
Kingsley (1870), Kellogg (1908), Gates (1912), Heiser (1936), and Bronson (1943) have all reported that cockroaches were so numerous on ships that they gnawed the skin and nails of the men on board. These are all independent observations of what may well have been a common occurrence on ships. We have discussed in detail the subject of cockroaches biting man in our 1957(a) paper.
Mosely (1892) reported, "At the time that England was left the ship [H.M.S. Challenger] seemed nearly free of animals, other than men, dogs, and livestock required for food. The first cockroaches apparently came on board at St. Vincent, Cape Verdes.... Cockroaches soon became plentiful on board, and showed themselves whenever the ship was in a warm climate.
"At one period of the voyage, a number of these insects established themselves in my cabin, and devoured parts of my boots, nibbling off all the margins of leather projecting beyond the seams on the upper leathers."
Sir Edmund Freemantle (1904) recalled some of his experiences in the British Navy. "Cockroaches in the tropics were also terrible scourges. One saw little of them in fine, dry weather, but in damp, wet weather they seemed to come from every hidden corner ... our remedy in the 'Spartan' was to make the boys catch them—on pain of being caned.... One brig, the 'Lily,' was so overrun by cockroaches that the officers' clothes smelt of them."
The quotation from Sonan (1924) on page 348 describes similar conditions in the Japanese Navy.
On modern cargo ships cockroaches are reported to be extremely numerous in the galley, the crew's quarters, and sometimes in the holds; they dwell in hot, humid environments such as the casing around steam pipes (Monro, 1951). Williams (1931) reported that Blattella germanica was the most important cockroach pest on ships seen at New York. Although often numerous in the holds, the cockroaches as a rule congregated in living quarters. They were also frequently found between tarpaulins covering the hatches. It was not unusual to kill 20,000 to 50,000 in the forecastle, and more than 20,000 have been taken from a single stateroom. Simanton (1946) inspected the S.S. William Kieth when it berthed at San Francisco from a 10-month voyage to the South Pacific. The holds were infested with thousands of Periplaneta americana, but in the crew's quarters, mess halls, and storerooms B. germanica predominated. After insecticidal treatment about 2,000 P. americana were seen in each hold and as many as 24 B. germanica in each cabin. Richardson (1947) reported that in Army transports inspected between 1943 and 1946 at New York, P. americana was found in the galleys and messes, and occasionally heavy infestations were found deep in the holds; B. germanica was found in the galleys and messes; Blatta orientalis was found only in the hold.
Additional references indicating the presence of Blattella germanica on ships may be found in the account of its parasite Ripidius pectinicornis (p. 232). Although Rice (1925) and Williams (1931) cite B. germanica as the most numerous cockroach on ships, Brooke (1920) stated that the great majority of ship cockroaches were Periplaneta americana. In addition to citing cockroach infestations on ships, the following authors reported various methods for disinfecting ships: Canalis (1916), Pryor (1918), Brooke (1920), Rice (1925), Williams (1931), Simanton (1946), Richardson (1947), and Anonymous (1951, 1954).
In the following list we include some previously unpublished data on cockroaches that were recovered from ships at the Miami, Fla., Quarantine Station for the periods November 1945 through May 1946; May, June, August, and September 1950; and 17 July 1957 (Porter, personal communication, 1958). These data were lumped, without breakdown to species, under the entry Orthoptera in Porter (1958).
Certain of the species listed below occur only accidentally on shipboard and will probably never establish breeding colonies on ships or become pests on shipboard or elsewhere; some were merely passengers between one land-based colony and another. Others, the truly domiciliary pests, are as likely to be pests on shipboard as they are in land-based structures.
Hispaniola.—On board ship (Rehn and Hebard, 1927).
U.S.A.—At Port of New York (Richardson, 1947).
At sea?—In ships casks (Chamisso, 1829).
U.S.A.—Port of New York (Williams, 1931; Richardson, 1947). San Francisco (Simanton, 1946). At Miami, 7,852 live specimens recovered from ships (Porter, personal communication, 1958). Most numerous species on ships (Rice, 1925).
At sea.—One male and one female found dead on S.S. Tenadores (Hebard, 1917).
Florida.—One dead specimen, Miami (Porter, personal communication, 1958).
Florida.—Five live and one dead specimen, Miami (Porter, personal communication, 1958).
At sea.—One female alive in hold of S.S. Tenadores (Hebard, 1917).
Brought from West Africa to West Indies and Brazil by slave ships (Rehn, 1945).
Widely disseminated by sailing ships (Rehn, 1945).
At sea.—Two females found dead on S.S. Tenadores (Hebard, 1917).
At sea.—One male found dead in hold of S.S. Tenadores (Hebard, 1917).
At sea.—One female dead in hold of S.S. Tenadores (Hebard, 1917).
Florida.—Five dead specimens recovered from ships, Miami (Porter, personal communication, 1958).
Widely distributed by sailing ships (Rehn, 1945).
England.—Captured on a sugar vessel from Java (Lucas, 1920).
At sea.—One male and one female nymph found dead on S. S. Tenadores (Hebard, 1917).
Florida.—Two dead specimens recovered from ships at Miami (Porter, personal communication, 1958).
Spread from Africa to New World by ships (Rehn and Hebard, 1927; Rehn, 1945).
At sea.—One female dead in hold of S.S. Tenadores (Hebard, 1917).
Florida.—Fifteen dead specimens taken from ships, Miami (Porter, personal communication, 1958).
From the numerous records of this species as an adventive taken on bananas (p. 150), it may be presumed to be a frequent traveler on banana boats.
At sea.—Lewis (1836). Hebard (1933a) stated that this is "often a serious pest on the smaller ships sailing the South Seas."
U.S.A.—San Francisco (Simanton, 1946). Port of New York (Richardson, 1947). At Miami, 62 live and 123 dead specimens (Porter, personal communication, 1958).
Migrated from West Africa to America in slave ships (Rehn, 1945).
Probably in part reached the New World by way of Africa in slave ships (Rehn, 1945).
Reached America from West Africa by slave ship (Rehn, 1945).
At sea.—One female found dead in hold of S.S. Tenadores (Hebard, 1917).
Michel (1935) stated that the development of air transportation brought the same insect dispersal problems that exist in land and water transportation; in addition, the problem of cockroach infestation had become a very serious one, quite aside from the hygienic point of view, because it had been discovered that these insects seek out the wings of airplanes, where they subsisted on the glue and dope used in airplane construction. However, Dethier (1945) found no cockroaches in dismantled or wrecked wing and tail structures of metal aircraft in central Africa. In fact, all-metal aircraft would seem to provide little in the way of food or water for stowaway cockroaches.
Laird (1951, 1952, 1956a) found living specimens of Blattella germanica, Periplaneta americana, and Periplaneta australasiae in baggage compartments and/or kitchens in aircraft. Other species which have been recovered from undisclosed spaces in aircraft are listed below. Some of the cockroaches that were reported as dead may not have died from exposure during flight but may have been killed by insecticide applied by inspecting personnel at the airports.
In the following list we include some previously unpublished data on cockroaches that were recovered from aircraft in Miami, Fla., International Airport from 1 July 1956 through 30 June 1957 (Porter, personal communication, 1958). These data were lumped under the entry Orthoptera without breakdown to species in Porter (1958).
Species reported by Hughes (1949), and cited below as from southern United States, were recovered from aircraft that arrived at Brownsville, Fort Worth, Miami, New Orleans, and San Juan. There was no way of linking a specific record with any particular city.
The comments we made above about species that are infrequently encountered on ships apply with equal validity to similar species found on aircraft.
U.S.A.—One live and 15 dead specimens recovered from 16 aircraft at Miami (Denning et al., 1947).
U.S.A.—Six live and four dead specimens recovered from six aircraft at Miami (Denning et al., 1947). Southern U.S. (Hughes, 1949).
Hawaii.—Williams (1946a).
Khartoum.—Whitfield (1940).
New Zealand.—Laird (1951, 1952, 1956a).
U.S.A.—At Miami 193 live and 184 dead specimens were recovered from 141 aircraft (Denning et al., 1947). Recovered at airports in southern U.S. (Hughes, 1949). Recovered at Miami, 51 live and 24 dead specimens (Porter, personal communication, 1958). Exposed experimentally in jet aircraft (Sullivan et al., 1958).
Khartoum.—Whitfield (1940).
Southern U.S.—Hughes (1949).
U.S.A.—One live and three dead specimens recovered from three aircraft at Miami (Denning et al., 1947). Southern U.S. (Hughes, 1949).
U.S.A.—One dead specimen, Miami (Denning et al., 1947). Southern U.S. (Hughes, 1949).
Southern U.S.—Hughes (1949).
U.S.A.—Two dead specimens recovered from two aircraft at Miami (Denning et al., 1947).
U.S.A.—Two live and one dead specimen recovered from three aircraft, Miami (Denning et al., 1947). Southern U.S. (Hughes, 1949). One dead specimen, Miami (Porter, personal communication, 1958).
U.S.A.—Three dead specimens recovered from three aircraft at Miami (Denning et al., 1947). Southern U.S. (Hughes, 1949).
U.S.A.—One live and one dead specimen, Miami (Denning et al., 1947).
U.S.A.—One dead specimen recovered at Miami (Denning et al., 1947).
U.S.A.—Two dead specimens recovered from two aircraft, Miami (Denning et al., 1947).
U.S.A.—Five live and three dead specimens recovered from seven aircraft, Miami (Denning et al., 1947). Southern U.S. (Hughes, 1949). Three live and five dead, Miami (Porter, personal communication, 1958). Experimentally exposed in jet aircraft, U.S. (Sullivan et al., 1958).
New Zealand.—Laird (1951, 1952).
U.S.A.—Two dead specimens recovered from two aircraft, Miami (Welch, 1939). Five live and three dead from five aircraft, Miami [Pg 90] (Denning et al., 1947). Southern U.S. (Hughes, 1949). Three live and two dead, Miami (Porter, personal communication, 1958).
New Zealand.—Laird (1952).
U.S.A.—One live and three dead specimens from four aircraft, Miami (Denning et al., 1947). Southern U.S. (Hughes, 1949). One live and six dead, Miami (Porter, personal communication, 1958).
U.S.A.—Two live and three dead specimens from five aircraft, Miami (Denning et al., 1947). Southern U.S. (Hughes, 1949).
New Zealand.—Laird (1956a).
U.S.A.—Two live and one dead specimen from one aircraft, Miami (Denning et al., 1947). Southern U.S. (Hughes, 1949).
Khartoum.—Whitfield (1940).
U.S.A.—Two live specimens from two aircraft, Miami (Welch, 1939). Southern U.S. (Hughes, 1949).
U.S.A.—Two live and one dead specimen from three aircraft, Miami (Denning et al., 1947). Southern U.S. (Hughes, 1949).
Anglo-Egyptian Sudan.—At Khartoum (Whitfield, 1940).
Brazil.—From flying boats, 62 specimens; from land planes, 45 specimens (Carneiro de Mendonça and Cerqueira, 1947).
Central Africa.—Dethier (1945).
Kenya.—At Kisumu (Symes in Whitfield, 1940).
New Zealand.—Laird (1956a).
U.S.A.—Four live and 14 dead specimens from 4 aircraft, Miami (Welch, 1939). One live and 15 dead specimens (adults?) from 16 aircraft; 8 oöthecae from 7 planes; 147 live and 83 dead nymphs from 108 planes, Miami (Denning et al., 1947). Southern U.S. (Hughes, 1949). Five live and 7 dead specimens, Miami (Porter, personal communication, 1958).
Asano (1937) classified the natural enemies of cockroaches into two types as follows:
1. Enemies that feed mainly on cockroaches (certain ripiphorid beetles and certain chalcid, evaniid, and ampulicid wasps).
2. Organisms which, in their search for food, devour cockroaches that may be encountered (certain species of scorpions, spiders, ticks, centipedes, Strepsiptera, ants, birds, rats, and "parasitic bacteria").
Cameron (1955) arranged the associates of cockroaches in two groups as follows:
Group A. Parasites and predators.
1. Parasites: Hymenoptera (Evaniidae, Eulophidae, Eupelmidae, Encyrtidae, Pteromalidae, Cleonymidae) and Coleoptera (Ripiphoridae).
2. Predators: Hymenoptera (Ampulicidae), Hemiptera (Reduviidae), Coleoptera (Dermestidae), Arachnida (Araneae, Acarina).
Group B. Parasites and symbionts.
1. Protozoa (including examples of both parasites and commensals).
2. Nematoda (including both primary "parasites" and secondary parasites).
3. Bacteria (including the mutualistic bacteroids).
4. Algae [Arthromitis (= Hygrocrocis) intestinalis; see p. 124].
Asano's arrangement, although essentially true, is limited; Cameron's system is divided into arthropods (group A) and lower forms (group B), but does not include higher animals. Both attempts at classification need amplification; this we have endeavored to do below.
In classifying the biotic associates of cockroaches, we were immediately confronted with a problem in semantics. The concepts parasitism, predatism, and symbiosis have all been used with various shades of meaning by different authors. The problem is not solved merely by accepting as authoritative specific definitions, however apt they seem to be, because, unfortunately, these concepts are not mutually exclusive. For example, among the entomophagous insects, as Sweetman (1936) has pointed out, there can be no definite line of separation between parasitism and predatism: the two intergrade, only the extremes being quite distinct. In fact, Andrewartha and Birch (1954) generalized these relationships by calling both categories predatism. These authors divided natural populations of associated organisms into nonpredators and predators. Although this simplifies their presentation of the general principles of ecology, for our purpose more narrowly defined terms have proved useful.
In the main we have followed Sweetman (1936) and Allee et al. (1949) in arriving at the following definitions:
Symbiosis is the living together in more or less intimate association of organisms of different species; it includes virtually all relationships between cockroaches and other organisms, such as parasitism, predatism, commensalism, and mutualism. Allee et al. (1949) apparently do not include predatism in symbiosis.
Mutualism is symbiosis in which both members benefit by the association. The smaller partner has commonly been called a symbiont or symbiote by authors.
Commensalism includes associations in which neither party appears to benefit or be harmed. One partner may live on the surplus food or wastes of the other; shelter and transport may be involved.
Parasitism is the state of symbiosis in which one of the members feeds upon the other during the whole of either the immature or mature feeding stage; the host is harmed in some way and may be killed.
Predatism is an association in which one member attacks and feeds upon, or stores as food for its progeny, one or more other organisms; the predator spends less than the immature or mature feeding period on the prey. This category includes a few invertebrates and all the vertebrates that capture, kill, and feed on cockroaches. This association may be divided into interspecies predatism, in which the predator preys upon a different species, and intraspecies predatism (cannibalism) in which the predator preys upon its own species.
Although we have attempted to adhere to these definitions throughout this discussion, we realize that in doing so we may have tended to oversimplify complex relations. Some questionable interpretations stem from insufficient knowledge of the basic relationships between cockroaches and their associates. Only further study will clarify these relationships. Some of the problems are discussed below.
Probably many of the so-called parasites (e.g., Protozoa like Nyctotherus, and intestinal nematodes of the family Thelastomatidae), which do not invade the host's tissues and seem to have no effect on the activity and vitality of the host, are commensals. Although we consider these forms to be commensals, we realize that they might actually affect the host in some way even though this has not been shown. It is possible that Rothschild and Clay's (1957) statement about bird parasites may well apply to the apparently harmless organisms found in the cockroach. These authors wrote, "It cannot be too strongly emphasized that the effect of all types of parasites on the host is detrimental. If we find that a bird seems little, if at all, inconvenienced by the presence of Protozoa or worms or lice, or a cuckoo in the nest, we can nevertheless assume that it would be better [Pg 93] off without them.... Small effects such as lack of vitality, loss of voice, excessive blinking, or perverted habits like dirt eating are extremely difficult to gauge. Nevertheless, it is only a question of degree. Potentially all parasites are harmful." It should also be pointed out that some workers would consider certain of our commensals of cockroaches to be parasites. Thus Faust (1955) stated that "A truly successful parasite is one which has developed a state of equilibrium with its host, so that no detectable damage is produced which endangers the health or life of the host. In a suitable host the parasite may obtain food and shelter without any evidence of trauma or toxicity. The damage produced may be so slight that repair and functional readjustment keep pace with the injury." Faust's successful parasite would be indistinguishable from a commensal, but there is undeniably a difference between an organism causing slight, and undetectable, damage to a host and one causing none. Certain of the organisms we list as commensals may eventually be shown to be parasites.
Certain organisms which live in cockroaches appear to have no effect on the vitality of the host even though the tissues of the host are invaded. Gregarines may penetrate the intestinal wall of the cockroach without seeming to injure the host. Fungi of the genus Herpomyces invade the cuticle of cockroaches producing pathological changes; yet the insects' behavior is apparently unaffected (see p. 129). We consider these organisms to be parasites because the host's tissues are invaded and, as far as we know, no benefit to the host results.
In the literature certain insects have been considered to be either parasites or predators or both. Among these are the ensign wasps (Evaniidae), whose larvae feed on the eggs of cockroaches within the oötheca, and the ampulicid wasps, which capture, paralyze, and store in their nests (as food for their larvae) nymphs and adults of cockroaches. Clausen (1940) claimed that the evaniid Zeuxevania splendidula is a true egg parasite when it destroys the first egg in a cockroach oötheca; but after the wasp larva molts and proceeds to devour the other eggs, he considered it to be a predator. Clausen's definition of an entomophagous parasite is an insect that in its larval stage develops either internally or externally upon a single host which is eventually killed; with few exceptions the adults are free-living and their food is usually different from that of the larvae. A predatory insect, by Clausen's definition, is principally free-living in the larval as well as adult stage, kills the host immediately by direct attack, and requires a number of victims to reach maturity; the predator is [Pg 94] of greater size than the prey, and the food sources of the adults and immature stages are frequently the same.
It is apparent, as Clausen and other writers have pointed out, that there are instances of a particular species showing characteristics which fit both the definitions for predator and parasite. Thus, among the evaniids one wasp larva destroys all the eggs in an oötheca, but in spite of this the larva has more of the characteristics of a parasite than of a predator; the adult wasp does not utilize the same food as the larva (adults have been taken on flowers and on honeydew from scale insects). It is questionable whether the evaniid larva kills the cockroach egg outright. The wasp larva, being restricted to the inside of the oötheca, is not free-living. Probably the only criterion by which the evaniid could be judged to be a predator, by Clausen's definition, is that more than a single egg is devoured by the maturing wasp larva.
Among the other wasp parasites (Encyrtidae, Eulophidae, Eupelmidae) of cockroach eggs many individuals develop in a single oötheca. When a hundred or more wasps emerge from an oötheca which contained less than 20 eggs, it is obvious that a single cockroach egg supported more than one wasp, yet it is possible that one particular wasp larva may have fed upon more than one cockroach egg before becoming an adult. We consider all entomophagous wasps that develop in cockroach oöthecae to be parasites rather than predators.
On the other hand, even though Anastatus floridanus, A. tenuipes, and Tetrastichus hagenowii are egg parasites as larvae, the adult females are, in a sense, predators when they sometimes eat part of the cockroach egg that oozes through the oviposition puncture (Roth and Willis, 1954a, 1954b). Williams (1929) has seen the female of Ampulex canaliculata imbibe blood that oozed from the cut ends of the cockroach's antennae after she had clipped them off before leading the prey to her nest. Yet despite this evident predatism on the part of the adult, the larva feeds as a parasite on the stored cockroaches in accordance with Sweetman's (1936) (though not Clausen's 1940) definition of parasitism, which is "that form of symbiosis in which one symbiont lives in or on the host organism and feeds at its expense during the whole of either the immature or mature feeding stage." The ampulicid larva, as the evaniid, is not free-living and does not kill the host immediately by direct attack, even though it may require more than one victim to reach maturity. Thus, within one individual both parasitic and predatory behavior are operant during different stages of its life history.
With the above discussion in mind we have summarized below the various biotic associations of cockroaches. Only a few examples are [Pg 95] given for each section, but all organisms with similar habits presumably would be classified in the same categories.
Class A. Associations in which cockroaches serve as hosts, vectors, or prey for other organisms.
Type I. Obligate associates. Animals and plants that normally develop only on or in the cockroach; in general, these organisms depend entirely upon the cockroach for survival.
Group 1. Mutuals (symbiotes or symbionts of authors).
(a) Bacteria-like organisms (bacteroids which are found in the fat body of all cockroaches that have been examined; p. 96).
(b) Bacteria (wood-digesting forms in Panesthia, and possibly certain bacteria in the intestines, of other cockroaches; p. 100).
(c) Protozoa (several genera and species found in Cryptocercus; p. 101).
Group 2. Commensals.
(a) Protozoa (Nyctotherus, Herpetomonas, Lophomonas, etc.; p. 172).
(b) Nematodes (Thelastomatidae; p. 193).
Group 3. Parasites.
(a) Fungi (Laboulbeniales; p. 134).
(b) Protozoa (gregarines, Plistophora, etc.; p. 181).
(c) Helminths.
(1) Primary parasites (mermithids and gordian worms; p. 201).
(2) Secondary parasites (Gongylonema neoplasticum, Oxyuris mansoni, Moniliformis spp.; p. 206).
(d) Arthropods.
(1) Mites (Pimeliaphilus podapolipophagus; p. 219).
(2) Insects (larvae of ripiphorids, evaniids, and ampulicids; p. 231).
Type II. Facultative associates. Animals and plants that prey on cockroaches or are incidentally or accidentally picked up by the cockroach, but which can survive or propagate readily on some other host or prey. Steinhaus (1946) emphasized the importance of the environment in determining the type of microbial flora associated with the cockroach which may carry one type of flora in an area which is exposed to filth and a different type in other areas. Because many of these organisms survive passage through or on the cockroach, the blattid may act as a vector of these animals and plants.
Group 1. Commensals.
(a) Viruses (strains of poliomyelitis virus; p. 103).
(b) Bacteria (Enterobacteriaceae, Pseudomonadaceae, Micrococcaceae, etc.; p. 111).
(c) Fungi (Aspergillus; p. 130).
(d) Protozoa (Iodamoeba, Dobellina, and cysts of Entamoeba coli and Entamoeba histolytica; p. 179).
(e) Helminths (cysts of various helminths parasitic in vertebrates; p. 208).
(f) Arthropods.
(1) Mites (Tyrophagus lintneri; p. 218). [Pg 96]
Group 2. Parasites.
(a) Bacteria (Serratia marcescens; p. 117).
(b) Helminths (Protospirura spp.; p. 206).
(c) Arthropods.
(1) Mites (Locustacarus sp.; p. 219).
(2) Insects (Melittobia chalybii; p. 248).
Group 3. Predators, active.
(a) Arthropods.
(1) Spiders (p. 214).
(2) Scorpions (p. 212).
(3) Centipedes (p. 222).
(4) Mites (Rhizoglyphus tarsalus; p. 218).
(5) Insects (dermestids, reduviids, and on occasion adult females of Tetrastichus, Anastatus, Ampulex; p. 234).
(b) Vertebrates.
(1) Amphibia (p. 269).
(2) Reptilia (p. 272).
(3) Aves (p. 276).
(4) Mammalia (p. 283).
Group 4. Predators, passive: Pitcher plants (p. 154).
Class B. Associations in which cockroaches serve as commensals or predators.
Group 1. Commensal cockroaches.
(a) Associates of social insects (Attaphila spp., etc.; p. 315).
(b) Obscure associates (p. 316).
Group 2. Predatory cockroaches.
(a) Interspecies predators (p. 319).
(b) Intraspecies predators (p. 322).
Class C. Associations of cockroaches with other cockroaches.
Group 1. Intraspecies associations.
(a) Familial associations (p. 325).
(b) Other conspecific associations (aggregations and fighting) (p. 336).
Group 2. Interspecies associations.
(a) Compatible associations (p. 337).
(b) Antagonistic associations (p. 329).
Class D. Ecological associations of cockroaches with higher plants.
Group 1. Benign associations (p. 139).
Group 2. Associations detrimental to plants (p. 162).
Blochmann (1887, 1888) discovered intracellular particles (the bacteroids or symbiotes of authors) that resembled bacteria in the fat body of males and females of Blatta orientalis and Blattella germanica (pl. 26), in the ova of these insects, and in their embryos. Bacteroids have since been found in at least 25 species and 19 genera of cockroaches. Presumably such microorganisms are universally distributed throughout the Blattaria. General reviews of the bacteroids [Pg 97] of cockroaches and other insects have been published by Glaser (1930b), Schwartz (1935), Steinhaus (1946, 1949), Buchner (1952, 1953), Brooks (1954), and Richards and Brooks (1958). The reader is referred to these papers, and those of authors cited in the list at the end of this section, for discussions of the morphology of the bacteroids, their distribution within the host, and attempts to culture them in vitro.
It has long been assumed, without proof, that cockroaches and their bacteroids form a mutually beneficial association. As it has not been possible to cultivate bacteroids apart from their cockroach hosts, it may be assumed that the host is essential to the continued existence of the microorganism, which also derives from the association other obvious benefits as well. Experiments to show that the host also benefits from the association have centered around rendering cockroaches bacteroid free. Starvation, parasites, electromagnetic radiation, heat or cold, or chemicals have all been used in attempts to eliminate the bacteroids. Of these, only chemical treatment has provided a satisfactory technique.
Few chemicals other than antibiotics have proved to be useful in the elimination or reduction of bacteroids. Yetwin (1932) injected various dilutions of 22 compounds into Blattella germanica. He observed decreases in the bacteroids of the fat body only following injection of methylene blue, but did not pursue this lead further. Gier (in Steinhaus, 1946) observed reduction in the numbers of bacteroids after cockroaches were injected with crystal violet, hexylresorcinol, or metaphen. Bode (1936) reported that injection of irritants such as lithium salts or quinine hydrochloride had no apparent effect on the symbiotes.
Brooks (1957) reared Blattella germanica on diets containing different concentrations of inorganic ions. On a manganese-deficient diet the cockroaches grew poorly and some of their progeny lacked normal bacteroids; about 10 percent of the aposymbiotic generation grew and reproduced on a diet fortified with yeast. Varying the concentrations of other salts in the diets gave results in which the progeny were either aposymbiotic or the fat body was abnormal but the mycetocytes were abundant; all these cockroaches soon died even on fortified diets.
The administration of certain antibiotic drugs has produced cockroaches very nearly free of bacteroids. Brues and Dunn (1945) found that although sulfa drugs had no effect on the bacteroids, penicillin in large doses reduced the number of bacteroids in Blaberus craniifer, [Pg 98] but the cockroaches died within a few days. Death was attributed to lack of bacteroids rather than effect of the drug; this is perhaps an unwarranted conclusion in view of the survival of aposymbiotic cockroaches that have been produced by other drugs (Brooks, 1954; Brooks and Richards, 1955). Glaser (1946) found that the bacteroids of Periplaneta americana could be adversely affected or destroyed by sulfathiazole, or sodium or calcium penicillin. Fraenkel (1952) questioned the conclusions of Brues and Dunn (1945) and of Glaser (1946) because of the high mortality in their experiments, and he suggested that the described phenomena "were due rather to direct toxic effects on the host than to loss of the symbionts." Noland (in Brooks, 1954; Brooks and Richards, 1955) confirmed Glaser's results with penicillin and extended sulfa treatments to include Blattella germanica. Every female whose bacteroids were reduced to the vanishing point resorbed her ovaries and was incapable of reproduction. Brooks (1954; Brooks and Richards, 1955) found that administration of several antibiotics did not eliminate the bacteroids from the fat body of B. germanica unless the dose was so high that it caused excessive mortality. Frank (1955, 1956) was able to eliminate bacteroids from Blatta orientalis by injecting or feeding chlortetracycline, oxytetracycline, or penicillin; survival of treated insects was not good and reproduction was poor; the aposymbiotic individuals were smaller than normal. As Richards and Brooks (1958) have pointed out, it is uncertain how much of this difference was the result of loss of bacteroids and how much the effect of the drug. It is obvious that in all these experiments the action of the drugs on the bacteroids was accompanied by equivocal side effects which confused interpretation of the results. The effect on the cockroach of a loss of bacteroids cannot be separated from a possible toxic effect of the drug.
Fortunately Brooks (1954; Brooks and Richards, 1955) obtained completely aposymbiotic offspring from Blattella germanica that had been reared on aureomycin. These bacteroid-free nymphs were practically incapable of growth on a natural diet that was adequate for nymphs with symbiotes. However, the addition of large amounts of dried yeast to the diet enabled aposymbiotic nymphs to mature in two to three times the period required by normal nymphs. Final proof of the function of the bacteroids was obtained by reestablishing them in aposymbiotic cockroaches. The insects that received implants of normal fat body of B. germanica showed a slow, steady gain in weight over the controls (Brooks, 1954; Brooks and Richards, 1956). Obviously, the intracellular symbiotes subserve the normal nutrition of the cockroach. Whether the bacteroids produce only vitaminlike substances, [Pg 99] as suggested by Keller (1950), or function in some other way is still to be determined. Brooks (1954) concluded that the amount of vitamin-containing food required for increased growth by aposymbiotic cockroaches is much greater than the known vitamin requirements; hence the factor(s) needed is unknown and present in low concentration, or it serves as a precursor of a second factor(s) whose synthesis is aided by the bacteroids. Brooks (in Richards and Brooks, 1958) has since found that the bacteroids of Blattella germanica "can supply the insect with B vitamins, amino acids and some larger protein fragment."
Gier (1947) stated that the symbiotes of cockroaches are generically all the same. However, as the symbiotes are presumed to have been associated with cockroaches for over 300,000,000 years (Buchner, 1952) they may be assumed to have developed specific differences that link them inseparably to their respective hosts. Ries (1932) transplanted symbiote-containing fat body from Blatta orientalis into the mealworm and larva of Ephestia kühniella, and from Blattella germanica and Stegobium paniceum (=Sitodrepa panicea) into B. orientalis. The implants did not become established in the new host, although most of the transplantations were successful in that the hosts survived and the implants remained intact for some time before they were encapsulated by host tissue. Brooks (1954; Brooks and Richards, 1956) transplanted fat body of Periplaneta americana and B. orientalis into aposymbiotic B. germanica. The growth of the cockroaches injected with foreign tissue was not different from that of aposymbiotic controls. Sections of host insects did not contain mycetocytes and no bacteroids were found. Haller (1955a) injected bacteroids or implanted mycetocytes of B. germanica into gryllids, acridids, and locustids. These implants and innoculations were rapidly destroyed by the hosts. But as Richards and Brooks (1958) have pointed out, none of these experiments provide information about the specificity of the bacteroids themselves.
Bantua stigmosa. Fraenkel (1921)
Blaberus craniifer. Brooks (1954); Brues and Dunn (1945); Hoover (1945).
Blaberus giganteus. Blochmann (1892).
Blatta orientalis. Blochmann (1887, 1888, 1892); Bode (1936); Brooks (1954); Buchner (1912); Cuénot (1896); Fraenkel (1921); Frank (1955, 1956); Gier (1936, 1947); Glaser (1920); Gropengiesser (1925); Gubler (1948); Heymons (1895); Hollande and Favre (1931); Hoover (1945); Hovasse (1930); Javelly (1914); Keller (1950); Koch (1949); Menel (1907)?; Mercier (1906a, 1907, 1907b, 1907c); Ries (1932); Ronzoni (1949); Tacchini (1946); Wolf (1924, 1924a). [Pg 100]
Blattella germanica. Blochmann (1887, 1888, 1892); Bode (1936); Borghese (1946, 1948, 1948a); Brooks (1954); Brooks and Richards (1954, 1955, 1955a, 1956); Fraenkel (1921); Gier (1936, 1947); Glaser (1920, 1930a); Gropengiesser (1925); Haller (1955, 1955a); Heymons (1892, 1895); Hoover (1945); Koch (1949); Lwoff (1923); Milovidov (1928); Neukomm (1927, 1927a, 1932); Pérez Silva (1954, 1954a); Ries (1932); Rizki (1954); Ronzoni (1949); Tacchini (1946); Wollman (1926); Yetwin (1932, 1953).
Cryptocercus punctulatus. Gier (1936, 1947); Hoover (1945).
Derocalymma cruralis. Fraenkel (1921).
Ectobius lapponicus. Heymons (1892); Cuénot (1896); Koch (1949).
Ectobius pallidus. Heymons (1892, 1895); Cuénot (1896).
Epilampra grisea. Fraenkel (1921).
Eurycotis floridana. Gier (1936, 1947).
Loboptera decipiens. Buchner (1930).
Nauphoeta cinerea. Fraenkel (1921).
Nyctibora noctivaga. Gier (1947).
Parcoblatta lata. Gier (1936, 1947).
Parcoblatta pensylvanica. Brooks (1954); Gier (1936, 1947).
Parcoblatta uhleriana. Gier (1936, 1947); Hoover (1945).
Parcoblatta virginica. Glaser (1920); Gier (1947).
Periplaneta americana. Baudisch (1956); Bode (1936); Cuénot (1896); Gier (1936, 1937, 1947); Glaser (1920, 1930, 1946); Gubler (1948); Hertig (1921); Hoover (1945); Ketchel and Williams (1953).
Periplaneta australasiae. Gier (1936, 1947); Koch (1949).
Platyzosteria armata. Fraenkel (1921).
Polyphaga aegyptiaca. Fraenkel (1921).
Pseudoderopeltis aethiopica. Fraenkel (1921).
Pycnoscelus surinamensis. Bode (1936); Koch (1949).
Supella supellectilium. Brooks (1954).
Evidence showing that intestinal bacteria contribute to the nutrition of cockroaches is meager. Cleveland et al. (1934) isolated a bacterial organism from the foregut of the wood-feeding cockroach Panesthia angustipennis. The bacterium digested cellulose rapidly in vitro and these workers believe that this cockroach and other related wood-feeding species are dependent on symbiotic bacteria for the digestion of their food.
Mencl (1907) described cell nuclei in "symbiotic," not closely defined types of bacilli that he found in abundance in the digestive tract of the Küchenschabe, Periplaneta (presumably Blatta orientalis). Unfortunately, he was more concerned about the morphology of the bacteria than the stated mutualistic relationship, so nothing is known of their physiology.
The growth rates of Periplaneta americana and Blattella germanica were retarded when the insects were reared aseptically, which suggests that microorganisms normally found in the digestive tract supply certain necessary dietary constituents (Gier, 1947a; House, 1949). Noland et al. (1949) suggested that microorganisms in the digestive tract of B. germanica synthesized riboflavin since the nymphs reared on a low riboflavin diet accumulated more of the vitamin than could have been ingested in the diet. However, Metcalf and Patton (1942) found little or no bacterial synthesis of riboflavin in P. americana. Noland and Baumann (1951) suggested that methionine, one of the amino acids essential for rapid growth of B. germanica, was synthesized by intestinal microorganisms in the insects.
It is probable that with few exceptions protozoa found in the digestive tract are not necessary for survival of the cockroach. However, very few experiments have been performed to determine the importance, if any, of these microorganisms to the host. Cleveland (1925) removed the protozoa from the cockroach (possibly Periplaneta americana) by oxygenation at 3.5 atmospheres. The ciliates Nyctotherus and Balantidium, flagellates Lophomonas and Polymastix, the amoeba Endamoeba blattae, and three unidentified protozoa were killed by this treatment, yet the insects lived normally after defaunation.
Armer (1944) studied the effects of high-carbohydrate, high-fat, and high-protein diets, as well as starvation, on the intestinal protozoa (Nyctotherus ovalis, Endamoeba blattae, Endolimax blattae, Lophomonas striata, and Lophomonas blattarum) in Periplaneta americana. Starvation of the host lowered the incidence or eliminated most of the protozoa, but a high-carbohydrate diet maintained them at a relatively high level. Lophomonas blattarum was eliminated by a high-protein diet, and practically eliminated by a high-fat diet. Lophomonas striata was eliminated from some hosts that were kept on high-fat and high-protein diets. Endamoeba blattae showed a decrease in infection rate when the cockroaches were maintained on high-fat and high-protein diets. The effects of diets on Endolimax blattae were not uniform.
It has been shown by Cleveland (1930, 1948) and Cleveland et al. (1931, 1934) that the wood-feeding cockroach Cryptocercus punctulatus depends upon certain intestinal protozoa for survival; these protozoa utilize as food the wood ingested by this cockroach. The [Pg 102] wood is broken down into compounds the cockroach can utilize by the protozoa which elaborate a cellulase and possibly a cellobiase (Trager, 1932). Only molting nymphs of Cryptocercus can pass the protozoa on to the newly hatched young, so that molting and hatching must happen concurrently each year or the young die.
The sexual cycles in species of protozoa in the genera Trichonympha, Saccinobaculus, Oxymonas, Monocercomonoides, Hexamita, Eucomonympha, Leptospironympha, Urinympha, Rhynchonympha, Macrospironympha, and Barbulanympha (fig. 3, B) are induced by hormones produced by Cryptocercus only during its molting period (Cleveland, 1931, 1947, 1947a, 1949-1956a). Perhaps the prothoracic gland hormone of the host may be responsible for initiation of the flagellate sexual cycles (Cleveland and Nutting, 1955). The protozoan sexual cycles may be used as indicators of the onset of molting in Cryptocercus; thus different species of protozoa begin their sexual cycles from 35 days before to 2 days after molting of the cockroach (Cleveland and Nutting, 1954). Hollande (1952) and Grassé (1952) have reviewed the roles and the evolution of the flagellates in Cryptocercus and in termites.
The protozoa of cockroaches and termites are clues to the relationship between these two groups of insects. Kirby (1927) pointed out similarities between Endamoeba blattae of Periplaneta and the amoebae of the termite Mirotermes, suggesting that these protozoans were probably derived from an amoeba in an ancestor common to both blattid and termite. Kirby (1932, in Kidder, 1937) found a species of Nyctotherus in Amitermes that resembles Nyctotherus ovalis from domestic cockroaches. The belief that the termites and cockroaches had a common origin is also strengthened by the similarities between the hypermastigotes of both Cryptocercus and termites (Cleveland et al., 1934).
The cockroaches Cryptocercus and Panesthia both feed on wood, but the protozoa found in Panesthia resemble more closely the species in domestic cockroaches than those in Cryptocercus. The Clevelandellidae (from Panesthia) are closely related to Nyctotherus and have probably evolved from common ancestors. However, the separation of the Clevelandellidae from Nyctotherus must have taken place at a later date than the divergence of their hosts, otherwise representatives of that family would probably also be found in Periplaneta and Blatta (Kidder, 1937).
The protozoa of Cryptocercus can be transferred from one individual to another (Nutting and Cleveland, 1954). They can also be [Pg 103] transferred to the termite Zootermopsis where they survive only until the host molts; the reverse is also true, Zootermopsis Protozoa can survive in Cryptocercus until the cockroach molts (Nutting and Cleveland, 1954a).
Annotations on some of the following observations may be found in Roth and Willis (1957a). Use of asterisk is explained in footnote 3, page 4.
Experimental vectors.—Blattella germanica, U.S.A. (Hurlbutt, 1949, 1950).
Periplaneta americana, U.S.A. (Hsiang et al., 1952).
Experimental vectors.—Periplaneta americana, U.S.A. (Fischer and Syverton, 1951; Syverton et al., 1952).
Experimental vectors.—Periplaneta americana, Great Britain? (Findlay and Howard, 1951): Results with Blattella germanica were negative.
Natural vectors.—Blattella germanica and/or Blattella vaga, Periplaneta americana and/or Periplaneta brunnea, and Supella supellectilium, U.S.A. (Syverton et al., 1952; Dow, 1955; Dow in Roth and Willis, 1957a).
Experimental vectors.—Periplaneta americana, U.S.A. (Fischer and Syverton, 1951a, 1957): Recently Fischer and Syverton (1957) found that after feeding a single meal of Coxsackie virus to Periplaneta americana, the gastrointestinal tracts of the insects, which were removed at 5-day intervals up to 20 days, contained sufficient virus to paralyze and kill test mice. Cockroach salivary glands, removed 5 days after the insects had fed, contained the virus which caused paralysis and death in test mice; mice were also infected by virus obtained from salivary glands removed from the insects 10 and [Pg 104] 20 days after the cockroaches had fed once on the virus. The virus was also isolated from the cockroaches' feces and rarely from the fat bodies and reproductive organs. Fischer and Syverton concluded that it is possible that cockroaches could acquire the virus, by feeding on mammalian excreta, maintain it for a period of time, and transmit it by contamination of food. The virus could also be transmitted through the feces of wild mice if the mice happened to feed on virus-infected cockroaches.
Experimental vectors.—Periplaneta americana, U.S.A. (Syverton and Fischer, 1950).
Experimental vectors.—Blattella germanica, Great Britain? (Findlay and MacCallum, 1939).
Classification of the bacteria follows Breed et al. (1948). Synonymy in most cases was taken from the same source. Names of bacteria preceded by the symbol † are either not listed by Breed et al. or are stated by them to be insufficiently characterized for definite classification. Use of asterisk is explained in footnote 3, page 4.
Phylum SCHIZOPHYTA
Class SCHIZOMYCETES
Order EUBACTERIALES
Family PSEUDOMONADACEAE
Natural vectors.—Blaberus craniifer, U.S.A. (Wedberg et al., 1949).
Blatta orientalis, U.S.A. (Olson and Rueger, 1950).
Blattella germanica, U.S.A. (Olson and Rueger, 1950; Janssen and Wedberg, 1952).
Periplaneta americana, U.S.A. (Bitter and Williams, 1949, 1949a; Olson and Rueger, 1950).
Experimental vectors.—Blattella germanica, U.S.A. (Herms and Nelson, 1913).
Cockroaches, U.S.A. (Longfellow, 1913).
Natural and experimental vectors.—Blatta orientalis, Italy (Cao, 1906).
Natural and experimental vectors.—Blatta orientalis, Italy (Cao, 1898, 1906; Spinelli and Reitano, 1932).
Periplaneta americana, U.S.A. (Gier, 1947): The organism was pathogenic to the cockroach when injected.
Habitat.—Periplaneta americana, France? (Kunstler and Gineste, 1906): From intestinal tract.
Habitat.—Blatta orientalis, England (Dobell, 1911, 1912): From hind gut.
Habitat.—Blatta orientalis, U.S.S.R. (Zasukhin, 1930): From intestinal tract.
Cockroaches, Venezuela (Tejera, 1926): From digestive tract.
Experimental vectors.—Blatta orientalis, Italy (Cao, 1898; Spinelli and Reitano, 1932).
Blattella germanica, Orient (Toda, 1923); Germany (Jettmar, 1927).
Periplaneta americana, Philippine Islands (Barber, 1914); Netherlands (Akkerman, 1933); Formosa (Morischita and Tsuchimochi, 1926).
Periplaneta australasiae, Formosa (Morischita and Tsuchimochi, 1926).
Experimental vectors.—Blatta orientalis, Italy (Cao, 1898, 1906).
Synonymy.—Vibrio of Deneke.
Experimental vectors.—Blatta orientalis, Italy (Cao, 1898): The organism passed through the intestinal tract unchanged.
Habitat.—Water.
Natural vectors.—Cockroaches, India (Pasricha et al., 1938): The vibrios were found in 16 or 17 percent of 94 cockroaches and resembled Vibrio comma in their morphology and their main biochemical reactions; however, serum-agglutination reactions differed.
Habitat.—Blatta orientalis, U.S.A. (Leidy, 1853): From intestine.
Family RHIZOBIACEAE
Synonymy.—B. violaceus.
Habitat.—Water.
Experimental vectors.—Cockroach, U.S.A. (Longfellow, 1913): Recovered from outer part of body and intestinal tract.
Family MICROCOCCACEAE
Natural vectors.—Blattella germanica, U.S.A. (Janssen and Wedberg, 1952).
Natural vectors.—Cockroaches, U.S.A. (Longfellow, 1913).
Experimental vectors.—Blatta orientalis, Italy (Cao, 1898).
Natural vectors.—Blattella germanica, U.S.A. (Janssen and Wedberg, 1952).
Source.—Diseased June beetle larvae.
Experimental infection.—Periplaneta americana, U.S.A. (Northrup, 1914): Three of four adults were infected by feeding them bread saturated with a broth culture of the Micrococcus. After 11 days the tarsi of the cockroaches became infected, and the hind legs split and broke off. Antennae and setae also were affected and micrococci were recovered from the feces.
Natural vectors.—Blaberus craniifer, U.S.A. (Wedberg et al., 1949).
Blatta orientalis, U.S.A. (Tauber, 1940; Tauber and Griffiths, 1942).
Blattella germanica, U.S.A. (Herms and Nelson, 1913; Herms, 1939; Janssen and Wedberg, 1952).
Experimental vectors.—Blatta orientalis, Italy (Cao, 1898); U.S.A. (Tauber and Griffiths, 1942).
Micrococcus pyogenes var. albus (=Staphylococcus albus) and an unidentified short rod form were found by Tauber (1940) in the hemolymph of B. orientalis. These microorganisms were never found together in the same insect and caused loss of appetite, sluggishness, irregular respiratory movements, and paralysis in the cockroach; in the final stages of the disease the legs were folded under the body, the head was tucked beneath the forelegs, the whole insect became arched and maintained this position until death. In some cockroaches infected with the rod pathogen, conjunctival folds, particularly those between the dorsal abdominal sclerites, and the joints of the metathoracic legs ruptured liberating thick white hemolymph filled with bacteria. Tauber suggested that the infection might be spread by contact, especially to newly molted individuals or by actual ingestion of the bacteria by the cockroaches feeding on dead or dying individuals. All the roaches died after successful inoculation with the Micrococcus. The bacterial infection was associated with high total hemocyte counts and high percentages of mitotically dividing hemolymph cells (Tauber, 1940); these responses of the insect were interpreted as a mechanism whereby the number of hemocytes increases resulting in an increase in the number of phagocytes for combating the bacteria (Tauber and Griffiths, 1942).
Natural vectors.—Blaberus craniifer, U.S.A. (Wedberg et al., 1949).
Blatta orientalis, Italy (Cao, 1906).
Blattella germanica, U.S.A. (Herms, 1939).
Cockroaches, U.S.A. (Longfellow, 1913).
Habitat.—Stale urine and soil containing urine.
Natural vectors.—Blattella germanica, U.S.A. (Janssen and Wedberg, 1952): From intestinal tract and feces.
These organisms were obtained from pus or were designated as staphylococci [i.e., pathogenic micrococci (Blair in Dubos, 1948)].
Natural vectors.—Blatta orientalis, Italy (Spinelli and Reitano, 1932); Germany (Jettmar, 1935).
Blattella germanica, Germany (Jettmar, 1935).
Experimental vectors.—Blattella germanica, on shipboard (Morrell, 1911); Germany (Vollbrechtshausen, 1953).
Natural vectors.—Periplaneta americana? ("Blatella americana"), England (Shrewsbury and Barson, 1948): From intestinal tract.
Habitat.—Water.
Natural and experimental vectors.—Blatta orientalis, Italy (Cao 1898, 1906): From intestinal contents.
Habitat.—Air and water.
Experimental vectors.—Blatta orientalis, Italy (Cao, 1906): Intestinal contents.
Habitat.—Air, soil, water, skin surfaces.
Experimental vectors.—Blatta orientalis, Italy (Cao, 1906): From intestinal contents.
Habitat.—Oöthecae of Blatta orientalis and/or Blattella germanica, Germany (Gropengiesser, 1925): It was described as "eine gelbe Sarcina"; Pribram (1933) named the organism.
Habitat.—Garden soil, dust, mud; isolated from a diseased stomach.
Natural vectors.—Blatta orientalis, Poland (Nicewicz et al., 1946): From intestinal tract.
Natural and experimental vectors.—Periplaneta americana, U.S.A. (Gier, 1947): Organism not pathogenic to the cockroach when injected.
Synonymy.—Sarcina "blanche" of Sartory and Clerc.
Natural vectors.—Blatta orientalis, France (Sartory and Clerc, 1908): Isolated from intestinal tract.
Synonymy.—Sarcina alba "patogena" of Cao.
Natural vectors.—Blatta orientalis, Italy (Cao, 1898, 1906).
Synonymy.—Sarcina "bianca" and "gialla" of Cao.
Experimental vectors.—Blatta orientalis, Italy (Cao, 1898).
Family NEISSERIACEAE
Experimental vectors.—Cockroaches, U.S.A. (Longfellow, 1913).
Natural vectors.—Periplaneta americana, U.S.A. (Hatcher, 1939).
Family LACTOBACTERIACEAE
Experimental vectors.—Blatta orientalis, Italy (Cao, 1898).
Cockroaches, U.S.A. (Longfellow, 1913).
Natural vectors.—Blatta orientalis, Italy (Spinelli and Reitano, 1932): From intestinal tract.
Habitat.—Fermenting plant or animal products.
Natural vectors.—Blatta orientalis, Poland (Nicewicz et al., 1946): From intestinal canal.
Experimental vectors.—Blattella germanica, Germany (Vollbrechtshausen, 1953).
Natural vectors.—Blatta orientalis, Poland (Nicewicz et al., 1946).
Blattella germanica, U.S.A. (Steinhaus, 1941).
Periplaneta americana? ("Blatella americana"), England (Shrewsbury and Barson, 1948).
Cockroaches [presumably any or all of the above three species], Egypt (El-Kholy and Gohar, 1945).
Natural vectors.—Blatta orientalis, Poland (Nicewicz et al., 1946).
Natural vectors.—Blatta orientalis, Poland (Nicewicz et al., 1946).
Natural vectors.—Periplaneta americana? ("Blatella americana"), England (Shrewsbury and Barson, 1948).
Natural vectors.—Blatta orientalis, Italy (Cao, 1906).
Experimental vectors.—Cockroaches, U.S.A. (Longfellow, 1913).
Experimental vectors.—Blatta orientalis, Germany (Jettmar, 1935).
Experimental vectors.—Blatta orientalis, Germany (Jettmar, 1935).
Natural vectors.—Blatta orientalis, Germany (Jettmar, 1935).
Blattella germanica, Germany (Jettmar, 1935); U.S.A. (Janssen and Wedberg, 1952).
Cockroaches, U.S.A. (Longfellow, 1913).
Experimental vectors.—Blatta orientalis, Italy (Cao, 1898).
Family CORYNEBACTERIACEAE
Experimental vectors.—Blatta orientalis, Italy (Cao, 1898).
Cockroaches, U.S.A. (Longfellow, 1913).
Family ACHROMOBACTERIACEAE
Natural vectors.—Periplaneta americana, U.S.A. (Hatcher, 1939): Isolated from feces.
Natural vectors.—Blattella germanica, U.S.A. (Janssen and Wedberg, 1952).
Synonymy.—Bacillus faecalis alkaligenes; Bacillus alcaligenes faecalis; B. alcaligenes faecalis.
Habitat.—Intestinal canal of man. Has been isolated from feces, abscesses related to intestinal canal, and occasionally in the bloodstream. However, it is generally considered nonpathogenic.
Natural vectors.—Blaberus craniifer, U.S.A. (Wedberg et al., 1949): Isolated from feces.
Blatta orientalis, Poland (Nicewicz et al., 1946): Isolated from intestinal tract.
Blattella germanica, U.S.A. (Janssen and Wedberg, 1952): From intestinal tract and feces.
Periplaneta americana, U.S.A. (Bitter and Williams, 1949): Isolated from intestinal tract.
Cockroaches [presumably Blatta orientalis, Blattella germanica and/or Periplaneta americana], Egypt (El-Kholy and Gohar, 1945): From suspensions of macerated whole insects.
Synonymy.—B. alcaligenes recti.
Habitat.—Intestinal canal.
Natural vectors.—Blatta orientalis, Poland (Nicewicz et al., 1946): Isolated from intestinal tract.
Habitat.—Water, dairy utensils; produces ropiness in milk.
Natural vectors.—Blattella germanica, U.S.A. (Janssen and Wedberg, 1952): Isolated from intestine and feces.
Family ENTEROBACTERIACEAE
Synonymy.—Bacillus lactis aerogenes.
Habitat.—Grains, plants, intestinal tract of man and other animals.
Natural vectors.—Blaberus craniifer, U.S.A. (Wedberg et al., 1949): Isolated from feces.
Blatta orientalis, Poland (Nicewicz et al., 1946): Isolated from intestinal tract.
Blattella germanica, on shipboard (Morrell, 1911): Isolated from feces.
Periplaneta americana, U.S.A. (Bitter and Williams, 1949): Isolated from intestinal tract.
Cockroaches [presumably Blatta orientalis, Blattella germanica and/or Periplaneta americana], Egypt (El-Kholy and Gohar, 1945): Isolated from outer surface of body, intestinal tract, and suspensions of macerated whole insects.
Synonymy.—Bacillus cloacae.
Habitat.—Sewage, soil, water, human and other animal feces.
Natural vectors.—Blattella germanica, on shipboard (Morrell, 1911): From feces. U.S.A. (Janssen and Wedberg, 1952; Steinhaus, 1941): From intestinal tract, feces, and oötheca.
Periplaneta americana, U.S.A. (Bitter and Williams, 1949): From intestinal tract.
Natural vectors.—Periplaneta americana, U.S.A. (Bitter and Williams, 1949): Isolated from intestines.
Habitat.—Intestinal canal.
Natural vectors.—Periplaneta americana, U.S.A. (Bitter and Williams, 1949): Intestinal tract.
Natural vectors.—Blatta orientalis, Italy (Cao 1898, 1906; Spinelli and Reitano, 1932); France (Sartory and Clerc, 1908); Europe (Jettmar, 1935); Poland (Nicewicz et al., 1946).
Blattella germanica, U.S.A. (Steinhaus, 1941).
Periplaneta americana, U.S.A. (Bitter and Williams, 1949, 1949a).
Cockroaches [presumably one or all of the above three species], Egypt (El-Kholy and Gohar, 1945).
Cockroaches, U.S.A. (Longfellow, 1913).
Experimental vectors.—Blattella germanica, Germany (Vollbrechtshausen, 1953): When injected anally or orally the bacteria invaded the intestinal cells and in heavy infections killed the cockroaches.
Synonymy.—Bacillus acidi lactici.
Source.—Diseased nun moth larvae.
Experimental vectors.—Blatta orientalis, Europe (Filatoff, 1904): Organism pathogenic to cockroach when injected but not when fed.
Natural vectors.—Blaberus craniifer, U.S.A. (Wedberg et al., 1949): From feces.
Habitat.—Soil, water, intestinal canal of man and other animals.
Natural vectors.—Blaberus craniifer, U.S.A. (Wedberg et al., 1949): From feces.
Blattella germanica, U.S.A. (Janssen and Wedberg, 1952): From intestinal canal and feces.
Periplaneta americana, U.S.A. (Bitter and Williams, 1949): From intestinal tract.
Habitat.—Soil, water, intestinal canal of man and other animals.
Natural vectors.—Periplaneta americana, U.S.A. (Bitter and Williams, 1949): From intestinal canal.
Experimental vectors.—Blatta orientalis, Italy (Cao, 1898).
Natural vectors.—Blattella germanica, U.S.A. (Janssen and Wedberg, 1952).
Periplaneta americana, U.S.A. (Bitter and Williams, 1949).
Natural vectors.—Blattella germanica, U.S.A. (Janssen and Wedberg, 1952).
Periplaneta americana, U.S.A. (Bitter and Williams, 1949).
Natural vectors.—Blatta orientalis, U.S.A. (Olson and Rueger, 1950).
Blattella germanica, U.S.A. (Olson and Rueger, 1950).
Periplaneta americana, U.S.A. (Bitter and Williams, 1949; Olson and Rueger, 1950).
Natural vectors.—Cockroaches [presumably Blatta orientalis, Blattella germanica and/or Periplaneta americana], Egypt (El-Kholy and Gohar, 1945).
Natural vectors.—Periplaneta americana, U.S.A. (Bitter and Williams, 1949, 1949a).
Natural vectors.—Periplaneta americana, U.S.A. (Bitter and Williams, 1949, 1949a).
Cockroaches [presumably Blatta orientalis, Blattella germanica, and/or Periplaneta americana], Egypt (El-Kholy and Gohar, 1945).
Natural vectors.—Periplaneta americana, U.S.A. (Bitter and Williams, 1949).
Natural vectors.—Blaberus craniifer, U.S.A. (Wedberg et al., 1949).
Blatta orientalis, Italy (Spinelli and Reitano, 1932).
Periplaneta americana, U.S.A. (Bitter and Williams, 1949, 1949a).
Cockroaches, U.S.A. (Longfellow, 1913).
Natural vectors.—Blatta orientalis, U.S.A. (Olson and Rueger, 1950).
Periplaneta americana, U.S.A. (Bitter and Williams, 1949; Olson and Rueger, 1950).
Natural vectors.—Periplaneta americana, U.S.A. (Eads et al., 1954).
Experimental vectors.—Polyphaga saussurei, U.S.S.R. (Zmeev in Pavlovskii, 1948).
Experimental vectors.—Blatta orientalis, U.S.S.R. (Rozengolts and I͡udina in Pavlovskii, 1948).
Blattella germanica, U.S.A. (Olson and Rueger, 1950); U.S.S.R. (Rozengolts and I͡udina in Pavlovskii, 1948).
Polyphaga saussurei, U.S.S.R. (Zmeev in Pavlovskii, 1948).
Natural vectors.—Periplaneta americana, Australia (Mackerras and Mackerras, 1948, 1949).
Experimental vectors.—Nauphoeta cinerea, Periplaneta australasiae, Periplaneta ignota, and Supella supellectilium, Australia (Mackerras and Pope, 1948).
Experimental vectors.—Polyphaga saussurei, U.S.S.R. (Zmeev in Pavlovskii, 1948).
Natural vectors.—Periplaneta americana, U.S.A. (Bitter and Williams, 1949, 1949a).
Experimental vectors.—Periplaneta americana, Gold Coast Colony (Macfie, 1922).
Polyphaga saussurei, U.S.S.R. (Zmeev in Pavlovskii, 1948).
Experimental vectors.—Nauphoeta cinerea, Periplaneta australasiae, Periplaneta ignota, and Supella supellectilium, Australia (Mackerras and Pope, 1948).
Natural vectors.—Periplaneta americana, U.S.A. (Eads et al., 1954).
Natural vectors.—Periplaneta americana, U.S.A. (Bitter and Williams, 1949, 1949a).
Experimental vectors.—Nauphoeta cinerea, Periplaneta australasiae, and Supella supellectilium, Australia (Mackerras and Pope, 1948).
Natural vectors.—Periplaneta americana, U.S.A. (Eads et al., 1954).
Experimental vectors.—Periplaneta australasiae, Australia (Mackerras and Pope, 1948).
Natural vectors.—Periplaneta americana, U.S.A. (Eads et al., 1954).
Experimental vectors.—Periplaneta americana, U.S.A. (Jung and Shaffer, 1952).
Natural vectors.—Periplaneta americana, U.S.A. (Eads et al., 1954).
Natural vectors.—Periplaneta americana, U.S.A. (Bitter and Williams, 1949, 1949a; Eads et al., 1954).
Experimental vectors.—Blatta orientalis, Blattella germanica, and Periplaneta americana, U.S.A. (Olson and Rueger, 1950).
Natural vectors.—Periplaneta americana, U.S.A. (Eads et al., 1954).
Natural vectors.—Periplaneta americana, U.S.A. (Eads et al., 1954).
Natural vectors.—Periplaneta americana, U.S.A. (Eads et al., 1954).
Natural vectors.—Blattella germanica, Belgium (Graffar and Mertens, 1950).
Nauphoeta cinerea, Australia (Mackerras and Mackerras, 1948).
Experimental vectors.—Blaberus craniifer, U.S.A. (Wedberg et al., 1949).
Blatta orientalis, U.S.S.R. (Rozengolts and I͡udina in Pavlovskii, 1948).
Blattella germanica, Belgium (Graffar and Mertens, 1950); U.S.A. (Olson and Rueger, 1950; Janssen and Wedberg, 1952; Beck and Coffee, 1943); U.S.S.R. (Rozengolts and I͡udina in Pavlovskii, 1948).
Nauphoeta cinerea, Australia (Mackerras and Pope, 1948).
Periplaneta americana, U.S.A. (Beck and Coffee, 1943; Jung and Shaffer, 1952).
Periplaneta australasiae and Supella supellectilium, Australia (Mackerras and Pope, 1948).
Polyphaga saussurei, U.S.S.R. (Zmeev in Pavlovskii, 1948).
Natural vectors.—Blatta orientalis, Italy (Antonelli, 1930, 1943).
Experimental vectors.—Blaberus craniifer, U.S.A. (Wedberg et al., 1949).
Blatta orientalis, Italy (Spinelli and Reitano, 1932); U.S.A. (McBurney and Davis, 1930); U.S.S.R. (Rozengolts and I͡udina in Pavlovskii, 1948).
Blattella germanica, U.S.A. (Janssen and Wedberg, 1952); Germany (Jettmar, 1927); U.S.S.R. (Rozengolts and I͡udina in Pavlovskii, 1948).
Periplaneta americana, Gold Coast Colony (Macfie, 1922); Netherlands (Akkerman, 1933); Formosa (Morischita and Tsuchimochi, 1926); U.S.A. (Olson in Roth and Willis, 1957a).
Periplaneta australasiae, Formosa (Morischita and Tsuchimochi, 1926).
Polyphaga saussurei, U.S.S.R. (Zmeev in Pavlovskii, 1948).
Cockroaches [presumably Blatta orientalis, Blattella germanica, and/or Periplaneta americana], Egypt (El-Kholy and Gohar, 1945).
Synonymy.—Bacillus prodigiosus, Bacterium prodigiosum.
Habitat.—Water, soil, milk, foods, and various insects.
Natural hosts.—Blatta orientalis, Poland (Nicewicz et al., 1946): From intestinal tract. Italy (Spinelli and Reitano, 1932).
Blattella germanica, Canada (Heimpel and West, 1959).
Diploptera punctata, Nauphoeta cinerea, Neostylopyga rhombifolia, Panchlora nivea, Pycnoscelus surinamensis, and Supella supellectilium, U.S.A. (Roth and Willis, unpublished data, 1958): The organism was isolated and identified by Dr. Hillel Levinson, Quartermaster bacteriologist, from dead specimens found in our laboratory colonies which showed the red coloration characteristic of insects that have died with infections of S. marcescens (pl. 16, A, B).
Leucophaea maderae, U.S.A. (Levinson, personal communication, 1958): The organism was isolated from the hemolymph of living insects while attempting to determine the cause of unexplained mortality in our laboratory colony of this insect.
Leucophaea maderae or Periplaneta americana, Philippine Islands (Barber, 1912): From hemolymph.
Periplaneta americana, U.S.A. (Gier, 1947; Steinhaus, 1959).
Periplaneta australasiae and Periplaneta brunnea (Roth and Willis, unpublished data [1954]): In laboratory colonies. Isolated from suspensions of ground insects. In 1954 we received a culture of Periplaneta brunnea from the Department of Public Health, University of Minnesota. These insects began to die off rapidly and the normally lightly pigmented parts of the body became red. Dr. Hillel Levinson, Quartermaster bacteriologist, cultured Serratia marcescens from several moribund individuals. The Department of Public Health of Minnesota had at times in the past cultured S. marcescens but had discarded the cultures and was unaware that it might be surviving in the cockroach colonies (Richards, personal communication, 1954).
Periplaneta sp., U.S.A. (Olson in Roth and Willis, 1957a): Isolated from an undetermined species of Periplaneta, received in a shipment from the South, a strain of S. marcescens which was toxic to mice when administered intraperitoneally.
Experimental hosts.—Blaberus craniifer, U.S.A. (Wedberg et al., 1949): When fed in small numbers, S. marcescens increased to such an extent that the insect's extremities and upper halves of their bodies turned deep red. The insects died after this color appeared and practically pure cultures of Serratia were recovered from the reddened areas.
Blatta orientalis, Italy (Cao, 1898): Isolated from intestinal contents. Passed unchanged through the gut.
Blattella germanica, Canada (Heimpel and West, 1959): Not normally pathogenic per os; LD50 by injection, is approximately 38,000 bacteria per insect.
Periplaneta americana, U.S.A. (Gier, 1947): Organism toxic to the cockroach when injected.
Cockroaches, U.S.A. (Longfellow, 1913): Isolated from legs and viscera after feeding experiments.
Natural vectors.—Periplaneta americana, U.S.A. (Bitter and Williams, 1949, 1949a).
Experimental vectors.—Blatta orientalis, Italy (Spinelli and Reitano, 1932).
Periplaneta americana, Formosa (Morischita and Tsuchimochi, 1926).
Polyphaga saussurei, U.S.S.R. (Zmeev in Pavlovskii, 1948).
Natural vectors.—Blatta lateralis, Tadzhikistan (Zmeev, 1940).
Experimental vectors.—Periplaneta americana, Gold Coast Colony (Macfie, 1922).
Polyphaga saussurei, U.S.S.R. (Zmeev in Pavlovskii, 1948).
Cockroaches, Venezuela (Tejera, 1926).
Family PARVOBACTERIACEAE
Habitat.—Probably intestinal canal of mammals; from human feces.
Natural vectors.—Blatta orientalis, Poland (Nicewicz et al., 1946): From intestinal canal.
Experimental vectors.—Periplaneta americana, U.S.A. (Ruhland and Huddleson, 1941).
Habitat.—Surface of a flagellate (Lophomonas striata) which lives in the intestine of cockroaches (Breed et al., 1948; Grassé 1926, 1926a).
Experimental vectors.—Blatta orientalis, Italy (Cao, 1898).
Experimental vectors.—Blatta orientalis, Germany (Küster, 1902, 1903); Italy (Cao, 1906).
Natural vectors.—Blatta orientalis, Hongkong (Hunter, 1906).
Experimental vectors.—Blatta orientalis, Italy (Cao, 1898); Germany (Küster, 1903).
Blattella germanica, Germany (Jettmar, 1927).
Leucophaea maderae and Periplaneta americana, Philippine Islands (Barber, 1912).
Family BACTERIACEAE
Natural vectors.—Periplaneta americana? ("Blatella americana"), England (Shrewsbury and Barson, 1948): From intestinal tract.
Source.—Diseased fly larvae.
Experimental infection.—Cockroach, France (Roubaud and Descazeaux, 1923): Organism pathogenic to cockroach when injected.
Habitat.—Diseased larvae of Mamestra oleracea.
Experimental infection.—Blatta orientalis and Blattella germanica, Germany (Pfeiffer and Stammer, 1931): Organism pathogenic, when injected, to eight B. orientalis and two B. germanica.
Experimental host.—Blatta orientalis, France (Picard and Blanc, 1913): The organism was pathogenic to B. orientalis when injected.
Family BACILLACEAE
Experimental vectors.—Blatta orientalis, Italy (Cao, 1898, 1906); Germany (Küster, 1903).
Habitat.—Blatta orientalis, Germany (Schaudinn, 1902): Isolated from intestinal tract. Three percent of the cockroaches from Berlin bakeries were infected.
Synonymy.—Bacillus albolactis.
Habitat.—Soil, dust, milk, plants.
Natural vectors.—Blaberus craniifer, U.S.A. (Wedberg et al., 1949): From feces.
Periplaneta americana, U.S.A. (Hatcher, 1939): In feces.
Experimental host.—Periplaneta americana, U.S.A. (Babers, 1938): The cockroaches died within 96 hours after being injected with 10-3 ml. of a 24-hour broth culture.
Habitat.—Soil, water, dust.
Natural vectors.—Blattella germanica, U.S.A. (Janssen and Wedberg, 1952): From intestine and feces.
Source.—Diseased nun moth larvae.
Experimental infection.—Blatta orientalis, Europe (Filatoff, 1904): The organism was not pathogenic when fed to the cockroach, but killed the insects when injected into the body cavity; after the insects died Filatoff reisolated this pathogen together with another bacillus from the cadavers. He succeeded in culturing the new microorganism and found it to be pathogenic when injected into, but not when fed to, the cockroaches. The diseased insects became sluggish, failed to eat or drink, turned over on their backs, their extremities became totally paralyzed, and they finally died.
Habitat.—Soil, water, decomposing materials.
Natural vectors.—Periplaneta americana? ("Blatella americana"), England (Shrewsbury and Barson, 1948): From intestinal tract.
Experimental vectors.—Blatta orientalis, Italy (Cao, 1898): Organism recovered, apparently unchanged, from intestinal contents.
Synonymy.—Bacterium monache.
Source.—Diseased larvae of nun moth, Lymantria monacha.
Experimental infection.—Blatta orientalis, Europe (Filatoff, 1904): Organism pathogenic to the cockroach when injected but not when fed.
Habitat.—Blatta orientalis, U.S.S.R.? (Tichomiroff, 1870[?], in Filatoff, 1904): The infected insects suffered from a diarrhea and the liquid feces were yellow-brown.
Natural infection.—Blatta orientalis, France (Hollande, 1934): Organism observed regularly in the intestine (especially rectum). Extensive description given.
Experimental vectors.—Blatta orientalis, Italy (Cao 1898): Organism recovered, apparently unchanged, from intestinal contents.
Natural vectors.—Blaberus craniifer, U.S.A. (Wedberg et al., 1949).
Blatta orientalis, Italy (Cao, 1898, 1906; Spinelli and Reitano, 1932); France (Sartory and Clerc, 1908); Poland (Nicewicz et al., 1946).
Cryptocercus punctulatus, U.S.A. (Hatcher, 1939).
Periplaneta americana? ("Blatella americana"), England (Shrewsbury and Barson, 1948).
Cockroaches, U.S.A. (Longfellow, 1913).
Experimental vectors.—Blatta orientalis, Italy (Cao, 1898, 1906).
Natural infection.—Blatta orientalis, Italy (Ronzoni, 1949): Isolated from oöthecae.
Habitat.—Isolated from feces (man?).
Natural vectors.—Blatta orientalis, Poland (Nicewicz et al., 1946): Isolated from intestinal tract.
Experimental vectors.—Blatta orientalis, Italy (Cao, 1898).
Habitat.—Soil, intestinal tract of man.
Natural vectors.—Blatta orientalis, Poland (Nicewicz et al., 1946): Isolated from intestinal tract.
Natural and experimental vectors.—Blatta orientalis, Italy (Cao, 1898).
Natural vectors.—Cockroaches [presumably Blatta orientalis, Blattella germanica, and/or Periplaneta americana], Egypt (El-Kholy and Gohar, 1945).
Experimental vectors.—Blatta orientalis, Italy (Cao, 1898).
Natural vectors.—Periplaneta americana? ("Blatella americana"), England (Shrewsbury and Barson, 1948).
Order ACTINOMYCETALES
Family MYCOBACTERIACEAE
Experimental vectors.—Blatta orientalis, U.S.S.R. (Ekzempliarskaia in Pavlovskii, 1948).
Habitat.—Parasitic in turtles and possibly sparingly distributed in soils.
Natural vectors.—Periplaneta americana, U.S.A., Texas (Micks, in Roth and Willis, 1957a): Organism isolated from batches of intestinal tracts of cockroaches collected at random.
Natural vectors.—Periplaneta americana, U.S.A. (Leibovitz, 1951).
Natural vectors.—Blattella germanica, Southern Rhodesia and Kenya (Moiser, 1945, 1946, 1946a; Anonymous, 1946).
Periplaneta americana and Periplaneta australasiae, Formosa (Arizumi, 1934, 1934a).
Cockroaches, Venezuela (Tejera, 1926); Belgian Congo (Radna, 1939).
Experimental vectors.—Blatta orientalis, Europe (Paldrock in Klingmüller, 1930); Nyasaland (Lamborn, 1940).
Blattella germanica, Europe (Paldrock in Klingmüller, 1930); Southern Rhodesia and Kenya (Moiser, 1945, 1946, 1946a, 1947; Anonymous, 1946).
Nauphoeta cinerea, Nyasaland (Lamborn, 1940).
Periplaneta americana, Gold Coast Colony (Macfie, 1922); Formosa (Arizumi, 1934, 1934a).
Periplaneta australasiae, Formosa (Arizumi, 1934, 1934a).
Cockroaches, Belgian Congo (Radna, 1939); Venezuela (Tejera, 1926).
Experimental vectors.—Cockroaches, Belgian Congo (Radna, 1939).
Natural vectors.—Periplaneta americana, U.S.A. (Leibovitz, 1951; Micks in Roth and Willis, 1957a).
Natural vectors.—Periplaneta americana, U.S.A. (Leibovitz, 1951).
Experimental vectors.—Blatta orientalis, U.S.S.R. (Ekzempliarskaia in Pavlovskii, 1948).
Experimental vectors.—Blatta orientalis, Italy (Cao, 1898); Germany (Küster, 1903); U.S.S.R. (Ekzempliarskaia in Pavlovskii, 1948).
Blattella germanica, on shipboard (Morrell, 1911).
Periplaneta americana, Gold Coast Colony (Macfie, 1922).
Cockroaches, Venezuela (Tejera, 1926); U.S.A. (Read, 1933).
Natural vectors.—Periplaneta americana, U.S.A. (Leibovitz, 1951; Micks in Roth and Willis, 1957a).
Family ACTINOMYCETACEAE
Natural vectors.—Periplaneta americana, U.S.A. (Leibovitz, 1951).
Family STREPTOMYCETACEAE
Habitat.—Surface of the nematodes Hammerschmidtiella diesingi and Leidynema appendiculata in Periplaneta americana, U.S.A. (Hoffman, 1952, 1953): Eighteen percent of 192 nematodes found in 52 adult cockroaches were infected with the bacterium.
Order CARYOPHANALES
Family ARTHROMITACEAE
Synonymy.—Hygrocrocis intestinalis.
Habitat.—Blatta orientalis, Europe (Valentin, 1836; Robin, 1847, 1853; Peshkoff, 1940): Isolated from intestinal tract. The organism appears as fragments in fecal masses or as fibers adhering to the mucous membrane of the large intestine (Robin, 1853).
Cockroach, France? (Chatton and Pérard, 1913).
Order SPIROCHAETALES
Family SPIROCHAETACEAE
Habitat.—Blaberus atropos, Venezuela (Tejera, 1926): Isolated from intestinal tract.
Habitat.—Blatta orientalis, France (Laveran and Franchini, 1920a).
Cockroaches, Venezuela (Tejera, 1926): Tejera reported finding "Spirochaeta blatarum Laveran et Franchini" which may have been a lapsus.
Family TREPONEMATACEAE
Habitat.—Blatta orientalis, England (Dobell, 1912); U.S.S.R.? Zasukhin (1930): From intestinal tract.
Synonymy.—Spirochaeta stylopygae Zuelzer.
Habitat.—Blatta orientalis, England (Dobell, 1912); U.S.S.R.? Zasukhin (1930): From intestinal tract.
Habitat.—Blatta orientalis, U.S.S.R. (Yakimov and Miller, 1922): Spirochaetes and spirilla were found in the intestines of 70 percent of 124 specimens collected in Petrograd.
Periplaneta americana, Gold Coast Colony (Macfie, 1922).
Natural vectors.—Blatta orientalis, Italy (Cao, 1906).
Natural vectors.—Blatta orientalis, Poland (Nicewicz et al., 1946): Isolated from intestinal tract.
Natural vectors.—Blatta orientalis, Italy (Cao, 1906).
Natural and experimental vectors.—Blatta orientalis, Italy (Cao, 1898, 1906).
Experimental vectors.—Blatta orientalis, Italy (Cao, 1898).
Natural and experimental vectors.—Blatta orientalis, Italy (Cao, 1898, 1906).
Natural and experimental vectors.—Blatta orientalis, Italy (Cao, 1898, 1906).
Natural infection.—Blatta orientalis, Germany (Heinecke, 1956): Disease organism found in the hemolymph of infected cockroaches. It can be spread by mouth and through wound infection. The animals died with symptoms of paralysis in 85-90 days. The organism has been isolated and is in the culture collection of the Institute for Microbiology and Experimental Therapy, Jena, under the numbers SG 896, Strain A; SG 897, Strain B; SG 898, Strain C.
Experimental infection.—Blattella germanica, Germany (Heinecke, 1956): Infected animals died in 26-30 days.
Periplaneta americana was unaffected even by heavy inoculations of the pathogen.
Source.—(I) Diseased silkworm larvae. (II) Diseased Ocneria dispar larvae and blood of Blatta orientalis.
Experimental infection.—(I)(II) Blatta orientalis, Europe (Filatoff, 1904): Organism pathogenic when injected, nonpathogenic when ingested.
(I) Cockroach, U.S.A. (Glaser, 1925): Organism pathogenic to cockroach when injected.
Natural infection.—Blatta orientalis, France (Hollande, 1934): Organism described morphologically.
Natural vectors.—Cockroaches [presumably Blatta orientalis, Blattella germanica, and/or Periplaneta americana], Egypt (El-Kholy [Pg 127] and Gohar, 1945): From the outer surface, intestinal tract, and suspensions of macerated insects.
Natural vectors.—Blatta orientalis, Germany (Jettmar, 1935): From intestinal tract.
Blattella germanica, Germany (Jettmar, 1935): From outer surface of body.
Source.—Periplaneta americana.
Natural and experimental infections.—Periplaneta americana, U.S.A. (Gier, 1947): Pathogenicity to the cockroach variable when organism injected.
Source.—Feces of Blattella germanica.
Experimental vector.—Blattella germanica, Germany (Vollbrechtshausen, 1953): Nonpathogenic to the insect when injected into the mouth or anus.
Cockroaches that were inoculated with living cultures succumbed in a few days (Glaser, 1925).
Habitat.—Blatta orientalis, France (Hollande, 1934; Hollande and Hollande, 1946): Organism found in hind intestine. This spirillum was stated to be related in external morphology to Spirillum periplaneticum Kunstler and Gineste, but it was believed that S. blattae should be in the Spirochaetaceae rather than the Spirillaceae.
Habitat.—Blatta orientalis, France (Hollande, 1934): Two kinds described but not named.
Natural and experimental infections.—Periplaneta americana, U.S.A. (Gier, 1947): Pathogenicity to the cockroach variable when organism injected.
By far the greatest number of fungi known to be associated with cockroaches belong to the Laboulbeniaceae, genus Herpomyces, the species of which are restricted to parasitizing cockroaches (Thaxter, [Pg 128] 1908). Most species are hyaline, small and inconspicuous (Thaxter, 1931) and are usually, but not exclusively, found on the insects' antennae. Species of Herpomyces are highly, but not completely, host specific (Richards and Smith, 1954). While attached to the host, these fungi appear like minute dark-colored, yellow, or white (e.g., H. arietinus) bristles or bushy hairs (pl. 27, A).
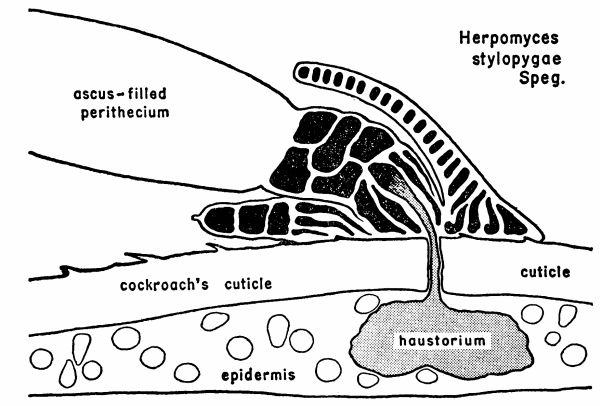 Fig. 1.—Diagram illustrating the relationship between a mature plant of
Herpomyces stylopygae and the integument of Blatta orientalis. (Reproduced
from Richards and Smith [1956], through the courtesy of Dr. A. G. Richards.)
Fig. 1.—Diagram illustrating the relationship between a mature plant of
Herpomyces stylopygae and the integument of Blatta orientalis. (Reproduced
from Richards and Smith [1956], through the courtesy of Dr. A. G. Richards.)
Richards and Smith (1955, 1955a) have studied the life history of Herpomyces stylopygae on the oriental cockroach. The plants grow only on living cockroaches, and the infection is disseminated by contact. The mature plants are found mostly on the antennae (pl. 27, B), either on setae or on hard or soft cuticle. Spores are ejected from perithecia singly or in groups of 2 to 4 spores, although groups as large as 12 spores have been found. The presence of single, paired, or multiple spore groups on the surface of the host was correlated with the presence of single, paired, or multiple plants on infected cockroaches. Development from spore to mature perithecia takes about two weeks. The plant obtains nutriment from the host by means of a tubular haustorium that extends through the cockroach's cuticle and expands into a large bulb in the underlying epidermal cells (fig. 1). Infections on nymphs are lost when the nymph moults, but infections on adults persist throughout life. However, nymphs which have lost the fungus upon moulting are readily reinfected. [Pg 129] Collart's (1947) statement that nymphs are never infected with Herpomyces is not true.
Richards and Smith (1956) concluded that there is no evidence of pathogenicity in Herpomyces infections because heavily infected cockroaches appear fully active in laboratory colonies; they can run at the same speed as uninfected cockroaches; they reproduce normally and do not appear to die prematurely. These workers stated that the infections cause a dermatitis which is neither pathogenic nor debilitant. So far as we know there are no comparative data on longevity and reproductive performance of fungus-infected versus normal cockroaches. However, Gunn and Cosway (1938) have shown that the presence of these fungi (identified as Stigmatomyces sp.; see p. 138) on the antennae seemed to interfere with the humidity reactions of Blatta orientalis. Although Richards and Smith (1956) admit that humidity receptors and other sense organs on the antennae may be destroyed by the fungus, they state that "insects possess such a large number of sensilla that the result may well be more distressing to the sensory physiologist than to the insect." Yet it seems to us that the loss of sense organs from fungal infection and concomitant shortening of the antennae (pl. 27, A) might be considerably more of a handicap to free-living cockroaches than those in laboratory colonies.
Bode (1936) studied the flora of Periplaneta americana and cultured Aspergillaceae and Mucorinae from the insect's body surface and intestinal contents; he also found nonsporulating yeasts in P. americana. To prevent fungal growth on oöthecae of P. americana, Griffiths and Tauber (1942a) autoclaved their rearing containers and dipped the oöthecae in 70-percent alcohol for 10 seconds.
Mercier (1906) isolated and cultured a pathogenic yeastlike parasite which had invaded the fat body and blood of Blatta orientalis. The abdomens of the infected insects became swollen, distended, and soft. McShan (unpublished MS., 1953) consistently isolated Saccharomycetes from the feces of Periplaneta americana.
The use of the asterisk (*) is explained in footnote 3, page 4.
Phylum THALLOPHYTA
Class FUNGI IMPERFECTI
Order MONILIALES
Family PSEUDOSACCHAROMYCETACEAE
Natural host.—Oötheca of Blatta orientalis, Italy (Ronzoni, 1949).
Natural host.—Oötheca of Blatta orientalis, Italy (Ronzoni, 1949).
Family MONILIACEAE
Natural host.—Ischnoptera rufa rufa, Puerto Rico (Wolcott, 1950): A dead specimen of this cockroach was found stuck to a leaf and covered with this fungus.
Natural hosts.—Oöthecae of Blattella germanica and Eurycotis floridana, U.S.A., Pennsylvania (Roth and Willis, unpublished data, 1952): On outer surface. Determination by Miss Mary Downing.
Oöthecae of Periplaneta americana, U.S.A., Pennsylvania (Roth and Willis, unpublished data, 1952): Inside oöthecae. Determination by Miss Mary Downing.
Natural vector.—Blatta orientalis, France (Sartory and Clerc, 1908): From intestine.
Natural vector.—Periplaneta americana, U.S.A., Texas (McShan in Roth and Willis, 1957a): From feces.
Experimental vector.—Blatta orientalis, Italy (Cao, 1898): Organism passed unchanged through the gut of the insects.
Natural host.—Oötheca of Eurycotis floridana, U.S.A., Pennsylvania (Roth and Willis, unpublished data, 1952): On outer surface. Determination by Miss Mary Downing.
Natural host.—Oöthecae of Blattella germanica, U.S.A., Pennsylvania (Roth and Willis, unpublished data, 1952): On exterior surface. Determination by Miss Mary Downing.
Natural and experimental vector.—Blattella germanica, on shipboard (Morrell, 1911): Isolated from feces. Experimentally Morrell also showed that the spores of the fungus could be recovered from feces of cockroaches that had fed on them.
Natural vector.—Periplaneta americana, England (Bunting, 1956): The fungus was isolated mostly from imperfectly excreted feces.
Experimental host.—Blattella germanica and Periplaneta americana, U.S.A. (Dresner, 1949, 1950): The nymphs of American cockroaches became infected when they (1) were injected with a 1-percent suspension of spores, (2) ate rat pellets sprayed with the spore suspension, or (3) were dusted with the fungus spores. The symptoms of the fungus infection were paralysis followed by death; some of the infected insects liquefied, others dried up after the appearance of a subcuticular blackening.
Natural vector.—Periplaneta americana, U.S.A., Texas (McShan, unpublished MS., 1953): From feces of cockroaches collected in the basement of a grain elevator at the docks in Galveston.
Experimental vector.—Blatta orientalis, Italy (Cao, 1898): Organism retained its pathogenicity after passing through the insect's gut.
Natural vector.—Blaberus craniifer, U.S.A. (Wedberg et al., 1949): From feces.
Periplaneta americana, England (Bunting, 1956): Mostly from imperfectly excreted feces.
Natural hosts.—Blattidae, Seymour (1929); Charles (1941).
Panesthia australis, U.S.A., Massachusetts (Roth and Willis, unpublished data, 1957): Growing on adult specimens that were found dead in a laboratory colony. Determination by Miss Dorothy Fennell.
Periplaneta americana, England (Bunting, 1956): Growing on genitalia of females where it prevented oöthecal formation.
Cockroach, Puerto Rico (Johnston, 1915): From a "small roach" in the pathological collection at Rio Piedras (no data).
Family DEMATIACEAE
Natural host.—Oötheca of Blattella germanica, U.S.A., Pennsylvania (Roth and Willis, unpublished data, 1952): On material that had oozed from a damaged oötheca. Determination by Miss Mary Downing.
Natural host.—Periplaneta americana, U.S.A. (Owen and Mobley, 1948): The digestive tract of this cockroach is the normal habitat of this yeast which was transmitted to sirup by the insects. The yeast superimposed a foreign taste, suggestive of malic acid, upon the original flavor of the sirup.
Natural host.—Blatta orientalis, Germany (Gropengiesser, 1925; Lodder, 1934): Isolated from fat body and oöthecae. Gier (1947) is of the opinion that the so-called yeasts that supposedly may displace the bacteroids in the fat body (Mercier, 1907b; Gropengiesser, 1925) may actually represent poorly fixed and insufficiently stained bacteroids.
Experimental host.—Blaberus craniifer, U.S.A. (Wedberg et al., 1949): Upon repeated feeding of massive doses of this yeast to the cockroach, these workers were able to isolate the organism from the feces up to six days thereafter. There was no evidence that T. rosea was pathogenic for B. craniifer.
Class PHYCOMYCETES
Order MUCORALES
Family MUCORACEAE
Natural host.—Periplaneta americana, U.S.S.R. (Nadson and Filippov, 1925; Filippov, 1926): Isolated and cultured from intestine.
Natural host.—Oötheca of Periplaneta americana, U.S.A., Pennsylvania (Roth and Willis, unpublished data, 1952): Inside oötheca. Determination by Miss Mary Downing.
Pycnoscelus surinamensis, Germany (Bode, 1936): Isolated from fat body which it had stained red.
Natural vector.—Blaberus craniifer, U.S.A. (Wedberg et al., 1949): From feces.
Natural vector.—Periplaneta americana, U.S.A., Texas (McShan, unpublished MS., 1953): From feces.
Natural vector.—Periplaneta americana, U.S.A., Texas (McShan, unpublished MS., 1953): From feces.
Order ENTOMOPHTHORALES
Family BLASTOCYSTIDACEAE
Natural vector.—Blatta orientalis, U.S.S.R. (Zasukhin, 1930): In hind gut in 40 percent of over 3,000 cockroaches.
Natural vectors.—Blatta orientalis, U.S.S.R. (Yakimov and Miller, 1922): Found in the intestinal contents of 29 percent of 124 B. orientalis.
Cockroaches, Venezuela (Tejera, 1926).
The placement of the following fungus is problematic.
Natural host.—Blatta orientalis, Germany (Avrech, 1931): Found in cells lining the lumen of midgut and caeca. The whole upper part of the epithelium was filled with sporangia and spores.
Class ASCOMYCETES
Order ENDOMYCETALES
Family SACCHAROMYCETACEAE
Natural vector.—Blaberus craniifer, U.S.A. (Wedberg et al., 1949): In feces.
Natural vector.—Blattella germanica, U.S.A. (Janssen and Wedberg, 1952): Found consistently in alimentary tract of B. germanica fed sucrose solutions.
Order HYPOCREALES
Family HYPOCREACEAE
Natural host.—Cockroaches, British Honduras (Mains, 1940).
Natural host.—Blattella germanica, Ceylon (Petch, 1924): Collected at Hakgala twice. A slight covering of brown mycelium overran the insect and fastened it to the underside of a living leaf.
Order LABOULBENIALES
Family LABOULBENIACEAE
Natural host.—Nyctibora obscura, Brazil, Natal (Thaxter, 1931): On antennae.
Natural hosts.—Anaplecta sp., Venezuela, Caracas (Thaxter, 1905, 1908); Trinidad (Thaxter, 1931): On antennae.
Cockroach, Sumatra (Thaxter, 1931).
Natural host.—Platyzosteria scabra, Australia, N.S.W. (Thaxter, 1931): On antennae.
Natural hosts.—Ischnoptera sp., U.S.A., Georgia (Thaxter, 1908).
Parcoblatta uhleriana, U.S.A., Massachusetts (Roth, unpublished data, 1957): The nymphs were in a culture of Parcoblatta virginica which was infected with this fungus; it is possible that these P. uhleriana became infected by contact with P. virginica. Fungus identified by Dr. R. K. Benjamin.
Parcoblatta virginica, U.S.A., Massachusetts (Roth, unpublished data, 1957): Fungus determined by Dr. R. K. Benjamin. Fungus found on antennae, palpi, legs, body surface (pl. 27, A).
Parcoblatta sp., U.S.A., Kentucky, Massachusetts (Thaxter, 1902, 1908): On antennae.
It is likely that Thaxter's host records (certainly those assigned to Temnopteryx and possibly those assigned to Ischnoptera) were species of Parcoblatta. Hebard (1917) has shown that all the species [Pg 135] referred to Ischnoptera in the United States, except I. deropeltiformis, now belong in the genus Parcoblatta. All species originally referred to the genus Temnopteryx in the United States are now synonymized with species of Parcoblatta.
Natural hosts.—Periplaneta americana, Brazil (Thaxter, 1931).
Periplaneta sp., Zanzibar and Mauritius (Thaxter, 1902, 1908): On spines of legs, antennae, and cerci.
Natural host.—Cockroach, Chile (Thaxter, 1918): On antennae.
Natural hosts.—Diploptera punctata, Ascension Island (Thaxter, 1902, 1908): On antennae. This species also was infected experimentally (Richards and Smith, 1954).
Cockroach, Fiji (Thaxter, 1931).
Natural hosts.—Blattella germanica, U.S.A., Massachusetts (Thaxter, 1902, 1908); Burma, Tenasserim (Spegazzini, 1915); Argentina, Buenos Aires (Spegazzini, 1917): On antennae. U.S.A., Minnesota (Richards and Smith, 1955): Scattered over entire body, wings. France? (Picard, 1913): On tibial spines. Chile and Philippine Islands (Thaxter, 1931).
"Ectobia" spp., Zanzibar and Saint Kitts, B.W.I. (Thaxter, 1902, 1908): Possibly on species that are now in the genus Blattella rather than in the genus Ectobius as it is known today, because Thaxter also used the synonym Ectobia germanica for the German cockroach, Blattella germanica.
Experimental hosts.—Blattella germanica and Blattella vaga, U.S.A. (Richards and Smith, 1954).
Natural hosts.—Cockroaches, Mauritius? and Fiji (Thaxter, 1902, 1908, 1931): On antennae.
Natural host.—Blattella humbertiana, Philippine Islands, Luzon (Thaxter, 1931): On antennae.
Natural host.—Cockroach, Grenada, B.W.I. (Thaxter, 1931): On antennae of a "brown wingless blattid."
Natural host.—Leurolestes pallidus, British Guiana and Trinidad (Thaxter, 1931): On antennae.
Natural host.—Loboptera sp., Argentina (Thaxter, 1931): On antennae.
Natural host.—Blaberus sp.?, Argentina (Spegazzini, 1917).
Cockroaches, Peru, Puerto Rico, Ecuador, and Haiti (Spegazzini, 1915, 1917): Material previously assigned by Spegazzini (1915) to H. paranensis was also placed by him in this new species. However, Thaxter (1931) believed that H. macropus may be synonymous with H. paranensis, but he provisionally retained H. macropus because he had not seen Spegazzini's material.
Natural hosts.—Nyctibora tomentosa, U.S.A., Texas (Thaxter, 1905, 1908): On antennae. This cockroach is not established in Texas, and the specimen may have been misidentified (Gurney, personal communication, 1958).
Nyctibora sp., Argentina (Spegazzini, 1917): On antennae.
Natural hosts.—Panchlora nivea, Trinidad (Thaxter, 1931): On antennae.
Natural host.—Panesthia lobipennis, Ceylon (Thaxter, 1915): On antennae.
Natural hosts.—Blaberus sp.? Brazil (Thaxter, 1902, 1908): On antennae.
Blaberus sp., Brazil and Argentina (Spegazzini, 1917): On antennae.
Cockroaches, Trinidad and Argentina (Thaxter, 1931).
Natural hosts.—Blaberus sp.?, Argentina (Spegazzini, 1917).
Blatta orientalis, U.S.A., Massachusetts (Thaxter, 1902, 1908); Locality? (Spegazzini, 1915); France? (Picard, 1913).
Periplaneta americana, Bermuda and U.S.A., Massachusetts (Thaxter, 1902, 1908); Plains of Biajar, Italian Somaliland, and Argentina (Spegazzini, 1915, 1917).
Periplaneta australasiae, Bermuda (Thaxter, 1902, 1908).
Periplaneta brunnea, Brazil (Thaxter, 1931).
Periplaneta sp., Mexico, West Indies, Panama, Brazil, Africa, South Seas, and China (Thaxter, 1902, 1908).
Cockroaches, Belgium (Collart, 1947).
Additional locality records: Grenada, Trinidad, B.W.I., and Tangier (Thaxter, 1931).
The fungus was found growing on spines, tegmina, integument, and antennae, at times abundantly.
Experimental hosts.—All the following data are from Richards and Smith (1954):
Blatta orientalis: A few plants matured.
Neostylopyga rhombifolia: Some development but no mature plants.
Periplaneta americana: Fungus developed prolifically with a density equal to that on original host.
Periplaneta australasiae: Some development but no mature plants.
Periplaneta brunnea: Fungus developed prolifically with a density equal to that on original host.
Natural host.—"Phyllodromia" sp., Abyssinia (Thaxter, 1905, 1908): On antennae.
Natural host.—"Eurycotis floridana," Mexico (Thaxter, 1905, 1908): On antennal setae.
Since this cockroach is not found in Mexico (J. A. G. Rehn, personal communication, 1957), E. floridana is undoubtedly not the host for this fungus. W. B. Brown (personal communication, 1957) searched the cockroach collection at the Museum of Comparative Zoology but was unable to find Thaxter's insect for reidentification.
Natural hosts.—Blatta orientalis, Argentina (Spegazzini, 1917); U.S.A. (Richards and Smith, 1955a).
Experimental hosts.—Neostylopyga rhombifolia (Richards and Smith, 1954): A few plants matured.
Pycnoscelus surinamensis (Richards and Smith, 1954): Some development but no mature plants.
The fungus (fig. 1) is found on antennae (pl. 27, B, C), palpi, cerci, and femurs. Thaxter (1931) believed H. stylopygae to be synonymous with H. periplanetae. However, Richards and Smith (1954) concluded that H. stylopygae would not grow on P. americana under their laboratory conditions although H. periplanetae would grow on B. orientalis. This indicated a strain or species difference between the two fungi. Gunn and Cosway (1938) reported a species of Stigmatomyces on the antennae of B. orientalis; this fungus was probably H. stylopygae (Richards and Smith, 1956).
Natural host.—Supella supellectilium, Trinidad (Thaxter, 1931): On antennal spines.
Natural hosts.—Blaberus craniifer, U.S.A., Key West (Richards and Smith, 1955).
Blaberus sp. and Epilampra? sp., Panama (Thaxter, 1902, 1908).
Epilampra sp., Saint Kitts, B.W.I., and Haiti (Thaxter, 1902, 1908).
Leucophaea maderae, Fernando Po (Spegazzini, 1915).
Nauphoeta cinerea, Brazil (Thaxter, 1931).
Cockroaches, China? (Thaxter, 1902); Philippine Islands, Mindanao (Thaxter, 1931).
Experimental hosts.—Blaberus craniifer, U.S.A. (Richards and Smith, 1955).
Infections on the antennae. Richards and Smith (1954) were unable to secure experimental infections in L. maderae with H. tricuspidatus. Experiments with N. cinerea showed some development but no mature plants although identification of the growing fungus was uncertain because of simultaneous exposure to H. ectobiae, H. stylopygae, and H. tricuspidatus.
Natural hosts.—Eurycotis manni, Brazil (Thaxter, 1931): On antennae.
Gyna sp.?, Isle of Nias (Spegazzini, 1915): On antennae.
Cockroach, Zanzibar (Thaxter, 1902): On antennae.
According to Dr. R. K. Benjamin (personal communication, 1957) and Dr. E. G. Simmons (personal communication, 1957), the phylogenetic position of the following genus is uncertain.
Natural hosts.—Cockroaches, Grenada, B.W.I. (Thaxter, 1920): On the axis of the antennae of a dark wingless and a pale winged blattid.
Natural host.—Cockroach, Grenada, B.W.I. (Thaxter, 1920): On antennal setae.
The significance of many observed associations between cockroaches and the higher plants is still obscure. Undoubtedly many associations are ecological, but lack of adequate supporting evidence makes this conclusion somewhat tentative. The ecological aspects are covered in Section III (p. 14). Other associations may be accidental (e.g., certain unique observations that have never again been confirmed). In the absence of contrary evidence, most associations are presumed to be benign; exceptions to this conclusion are found among the cockroaches that feed on living plants (p. 162) and those allegedly captured as prey by the carnivorous pitcher plants (Sarracenia and Nepenthes). In all the records cited below the cockroaches were stated to have been on, in, or feeding on the plant.
The plants are listed below by family according to the taxonomic arrangement of Lawrence (1951). Botanical nomenclature follows Bailey (1925), Fernald (1950), or Dr. R. A. Howard (personal communications, 1958, 1959). We take full responsibility for referring to appropriate taxa certain plants that were reported by common name only in the cited literature.
Division PTERIDOPHYTA
Family CYATHEACEAE
Associate.—Pycnoscelus surinamensis, Louisiana (Anonymous, 1893): Feeding on heart of tree fern.
Family POLYPODIACEAE
Associate.—Comptolampra liturata, Malaya (Karny, 1924): Often found between dry foliage of the beakers of this fern.
Division EMBRYOPHYTA SIPHONOGAMA
Family PINACEAE
Associates.—Aglaopteryx gemma and Parcoblatta lata, Alabama (Hebard, 1917): The former species was common under signs on longleaf pines, and P. lata was occasional.
Parcoblatta divisa, Georgia (Rehn and Hebard, 1916): Under signs.
Associates.—Eurycotis floridana, Latiblattella rehni, and Parcoblatta fulvescens, Florida (Hebard, 1917): Many records under signs on the tree trunks.
Associate.—Latiblattella rehni, Florida (Hebard, 1917): Under sign on tree.
Associates.—Parcoblatta divisa, Virginia (Rehn and Hebard, 1916): Under signs on shortleaf pine.
Parcoblatta zebra, Mississippi (Hebard, 1917): Under sign.
Associate.—Ectobius pallidus, England (Milton, 1899; Burr, 1899b): On Scotch fir.
Associates.—Plectoptera lacerna and Plectoptera vermiculata, Cuba (Rehn and Hebard, 1927).
Latiblattella rehni, Florida (Rehn and Hebard, 1905): Under signs. Cuba (Rehn and Hebard, 1927).
Family TAXODIACEAE
Associate.—Diploptera punctata, Hawaii (Pemberton and Williams, 1938; Zimmerman, 1948).
Family CUPRESSACEAE
Associate.—Diploptera punctata, Hawaii (Hebard, 1922): "The species is common and injurious in the territory infesting particularly the Monterey cypress trees ... and doing particular damage by gnawing away the bark." Similar injury has been cited by Pemberton (1934), Fullaway and Krauss (1945), and Zimmerman (1948).
Associate.—Phyllodromica tartara nigrescens, Southern Uzbekistan (Bei-Bienko, 1950): Under bark.
Family PANDANACEAE
Associate.—Graptoblatta notulata and Kuchinga remota, Tahiti (Hebard, 1933).
Associate.—Hololeptoblatta sp., Seychelles (Scott, 1910, 1912).
Family GRAMINEAE
Associate.—Phyllodromica pygmaea, U.S.S.R. (Bei-Bienko, 1950): Found in the dense turf.
Associate.—Comptolampra liturata, Malaya (Karny, 1925).
Associate.—Blattella vaga, Texas (Riherd, 1953): This field cockroach was rather abundant in clumps of Rhodes grass.
Synonymy.—Panicum barbinode [Hitchcock, 1936].
Associate.—Epilampra abdomen-nigrum, Puerto Rico (Seín, 1923; Wolcott, 1936): Abundant in "malojillo" meadow.
Associates.—Balta quadricaudata, Balta scripta, Balta torresiana, Balta verticalis, Ellipsidion simulans, and Megamareta verticalis, Australia, Queensland (Hebard, 1943): All collected by J. F. Illingworth on sugarcane.
Blattella humbertiana, Ischnoptera schenklingi, and Pycnoscelus surinamensis, Formosa (Box, 1953).
Cariblatta stenophrys, Puerto Rico (Seín, 1923; Wolcott, 1936): Between the leaves and under the leaf sheaths.
Panchlora nivea, Cuba (Rehn and Hebard, 1927): On the leaves.
Pelmatosilpha coriacea, Puerto Rico (Wolcott, 1936).
Phoraspis spp., Brazil and Guiana (Doumerc in Blanchard, 1837).
Plectoptera dorsalis, Plectoptora infulata, and Plectoptera rhabdota, Puerto Rico (Wolcott, 1950): Under the leaf sheaths.
Symploce ruficollis, Puerto Rico (Wolcott, 1950): Often found living under the leaf sheaths.
Cockroaches, Philippine Islands (Uichanco in Williams et al., 1931): Between cane leaf sheaths.
Synonymy.—Chaetochloa verticillata (Linnaeus) [Howard, personal communication, 1958].
Associate.—Diploptera punctata, Hawaii (Severin, 1911): The cockroach was caught on the barbed awns of this grass.
Associate.—Ischnoptera deropeltiformis, Missouri (Rau, 1937).
Associates.—Cariblatta stenophrys, Puerto Rico (Seín, 1923; Wolcott, 1936).
Ellipsidion bicolor, Australia, Queensland (Hebard, 1943).
Lophoblatta arawaka, Trinidad (Princis and Kevan, 1955).
Phoraspis sp., Brazil and Guiana (Doumerc in Blanchard, 1837).
Supella supellectilium, New Caledonia (Cohic, 1956).
Family CYPERACEAE
Associate.—Maretina uahuka, Marquesas Islands, Uahuka (Hebard, 1933a).
Family PALMAE
Associate.—Pycnoscelus surinamensis, Trinidad (Princis and Kevan, 1955): On "gru-gru" fruits.
Associates.—Aglaopteryx gemma, Florida (Rehn and Hebard, 1912).
Cariblatta lutea minima, Florida, and Cariblatta delicatula, San Domingo (Hebard, 1916a).
Eurycotis floridana, Florida (Rehn and Hebard, 1912; Hebard, 1917).
Periplaneta australasiae, Jamaica (Rehn and Hebard, 1927).
Pycnoscelus surinamensis, Florida (Rehn and Hebard, 1912; Hebard, 1917). Jamaica (Rehn and Hebard, 1927).
Associate.—Blattella germanica, California (Herms, 1926): On date palms.
Associate.—Periplaneta australasiae, Nihoa Island (Bryan, 1926). Hawaii (Zimmerman, 1948).
Associate.—Cariblatta punctulata, San Domingo (Hebard, 1916a).
Associate.—Eurycotis floridana, Florida (Scudder, 1879).
Periplaneta australasiae, Florida (Hebard, 1917).
Associates.—Euthlastoblatta abortiva, Texas (Hebard, 1917).
Hormetica laevigata, Brazil (Hancock, 1926).
Panchlora antillarum, Dominican Republic (Rehn and Hebard, 1927).
Periplaneta americana, Texas (Zimmern in Gould and Deay, 1940).
Family ARACEAE
Associate.—Latiblattella vitrea, Mexico (Hebard, 1921b): In flower shaft.
Associate.—Plectoptera dorsalis, Puerto Rico (Rehn and Hebard, 1927).
Family BROMELIACEAE
Associate.—Dryadoblatta scotti, Trinidad (Princis and Kevan, 1955).
Associates.—Pycnoscelus surinamensis, Hawaii (Illingworth, 1927, 1929): Feeding on roots of pineapple.
Blattella humbertiana, Formosa (Takahashi, 1940): Imago and grown nymphs occasionally lie concealed in the leaves.
Associates.—Cockroaches, Costa Rica (Calvert and Calvert, 1917).
Associate.—Dryadoblatta scotti, Trinidad (Princis and Kevan, 1955).
Associate.—Notolampra antillarum, Trinidad (Princis and Kevan, 1955): One male only.
Associate.—Eurycotis floridana, Florida (Rehn and Hebard, 1914; Hebard, 1917).
Associates.—Parcoblatta sp., Louisiana (Rainwater, 1941).
Latiblattella rehni, Florida (Blatchley, 1920): By beating.
Cockroaches, Louisiana (Rosenfeld, 1911, 1912): One mature and 39 immature blattids were collected from 8 of 12 samples of Spanish moss.
Associate.—Epilampra mona, Mona Island, West Indies (Rehn and Hebard, 1927): The type and one paratypic female of E. mona were collected in this bromeliad.
Eurycotis floridana, Florida (Blatchley, 1920).
Associates.—Aglaopteryx gemma, Texas (Hebard, 1917).
Dryadoblatta scotti, Trinidad (Scott, 1912): Found in the leaf bases.
Associates.—Aglaopteryx diaphana, Jamaica (Hebard, 1917; Rehn and Hebard, 1927).
Anaplecta azteca and Anaplecta sp., Costa Rica (Picado, 1913).
Anaplecta mexicana, Costa Rica (Calvert and Calvert, 1917).
Audreia bromeliadarum, Panama (Caudell, 1914).
Audreia jamaicana, Jamaica (Rehn and Hebard, 1927).
Blattella sp., Costa Rica (Picado, 1913).
Buboblatta armata, Panama (Caudell, 1914): "Probably not a typical bromeliadicolous species."
Cariblatta insularis, Jamaica (Hebard, 1916a, 1917; Rehn and Hebard, 1927).
Cariblatta nebulicola, Jamaica (Rehn and Hebard, 1927); One immature male.
Dryadoblatta scotti, Trinidad (Princis and Kevan, 1955).
Epilampra conspersa, Dominica (Scott, 1912).
Epilampra maya, Panama (Hebard, 1920).
Epilampra sodalis, Panama (Caudell, 1914).
Epilampra sp. and Hormetica laevigata, Brazil (Hancock, 1926).
Eurycotis biolleyi, Costa Rica (Picado, 1913).
Ischnoptera rufa occidentalis, Mexico (Caudell, 1914).
Latiblattella chichimeca, Costa Rica (Picado, 1913).
Litopeltis biolleyi, Costa Rica (Rehn, 1928).
Litopeltis bispinosa, Panama (Caudell, 1914).
Neoblattella brunneriana, Costa Rica (Calvert and Calvert, 1917).
Neoblattella dryas, Neoblattella eurydice, Neoblattella grossbecki, and Neoblattella proserpina, Jamaica (Rehn and Hebard, 1927).
Neoblattella fratercula, Mexico (Hebard, 1921b).
Neoblattella nahua, Mexico (Caudell, 1914).
Nesomylacris relica, Jamaica (Rehn and Hebard, 1927).
Nyctibora brunnea(?), Panama (Caudell, 1914): According to Hebard (1920) Caudell's specimen was almost certainly incorrectly identified. It may have been Nyctibora noctivaga or a smaller species of the genus. Brazil (Hancock, 1926).
Nyctibora laevigata, Jamaica (Hebard, 1917; Rehn and Hebard, 1927).
Nyctibora lutzi, Puerto Rico (Rehn and Hebard, 1927): "in epiphytes with pencil-like leaves."
Pelmatosilpha rotundata, Panama (Caudell, 1914).
Pseudomops laticornis, Costa Rica (Picado, 1913).
Pycnoscelus surinamensis, Costa Rica (Picado, 1913). Mexico (Caudell, 1914). Jamaica (Rehn and Hebard, 1927).
"Rhicnoda" sp., Costa Rica (Picado, 1913). This genus is now recognized as not being in the New World fauna. Probably the specimen was a species of Epilampra or Hyporhicnoda as suggested by Gurney (personal communication, 1959) and confirmed by Rehn (p.c., 1959).
Cockroaches, Costa Rica (Calvert, 1910): Cockroaches were said to be common in bromeliads on the moist Atlantic slope.
Family LILIACEAE
Associate.—Latiblattella lucifrons, Arizona (Ball et al., 1942).
Associate.—Pycnoscelus surinamensis, Connecticut (Zappe, 1918).
Family MUSACEAE
Cockroaches have been captured in bunches of bananas, in bracts of banana flowers, under banana leaves, and burrowing in rotten banana stalks. Although many of the species associated with bananas are indigenous to the banana-growing areas of the American Tropics, most of the specimens cited below were captured elsewhere as adventitious insects that had been imported with the fruit. It is obvious that many of these insects must have been closely associated with bananas on the plantations, where, undoubtedly, the growing plants provided attractive ecological niches. Bunting (1956) deduced, from the presence of healthy cockroaches on bananas allegedly sprayed with copper arsenate, that the insects did not feed on stems or fruit but hid among the bananas and foraged elsewhere; however, certain reports are of cockroaches actually feeding on bananas. Some of the records cited by Hebard (1917) were compiled from earlier reports not all of which we have seen. Numbers in parentheses following certain citations indicate the number of times the association had been observed. Known or suspected adventive material is so indicated.
Aglaopteryx diaphana, Jamaica (Rehn and Hebard, 1927): Found in bracts of banana blossoms. England (Bunting, 1955): Adventive, on bananas from Dominica.
Aglaopteryx vegeta, Finland (Princis, 1947): Adventive, in banana box.
Amazonina emarginata, Trinidad (Princis and Kevan, 1955): In banana bunch.
Archimandrita marmorata, Denmark (Henriksen, 1939): Adventive (2), in bananas from Jamaica(?). As Princis (1947) and Gurney (personal communication, 1959) point out, this is a Central American species, so Jamaica may be an error.
Archimandrita tessellate, Sweden (Princis, 1947): Adventive, from Honduras.
Blaberus atropos(?), Denmark (Henriksen, 1939): Adventive, from Jamaica. Princis (1947) pointed out that this species was more likely to have been Blaberus craniifer or Blaberus discoidalis, which are West Indian species, than B. atropos which is a South American species.
Blaberus boliviensis, Ecuador (Princis, 1952): In a shipment of bananas from near Puna.
Blaberus discoidalis, Puerto Rico (Rehn and Hebard, 1927): From banana ripening room. Great Britain (Pearce, 1929): Adventive. England (Bunting, 1955, 1956): Adventive, from Dominica.
Capucinella delicatula, California (Caudell, 1931): Adventive.
Cariblatta delicatula, Cuba (Rehn and Hebard, 1927).
Cariblatta hylaea, Honduras (Rehn, 1945a): Shaken from hanging dead banana leaves.
Cariblatta insularis, Finland (Frey, 1948): Adventive.
Cariblatta landalei, Jamaica (Rehn and Hebard, 1927): All specimens taken from under drying bracts of banana blossoms.
Cariblatta punctipennis and Chorisoneura barbadensis, England (Bunting, 1956): Adventive, from Dominica.
Epilampra abdomen-nigrum and Epilampra sp., England (Bunting, 1955): Adventive, from Dominica.
Epilampra maya, Massachusetts (Hebard, 1917): Adventive.
Epilampra mexicana(?), Denmark (Henriksen, 1939): Adventive (2), from Danish West Indies. Princis (1947) suggested that this should be Epilampra sp., because E. mexicana is not a West Indian species.
Eudromiella calcarata and Eurycotis bananae, U.S.S.R., Leningrad (Bei-Bienko, 1947): Adventive, from Colombia.
Euphyllodromia angustata, Sweden (Princis, 1947): Adventive.
Eurycotis caraibea, New York (Hebard, 1917): Adventive.
Eurycotis dimidiata, Washington, D. C. (Caudell, 1931): Adventive.
Eurycotis lixa, New York (Rehn, 1930): Adventive, on banana ship from Jamaica.
Graptoblatta notulata, Marquesas Islands, Uahuka (Hebard, 1933a): In banana leaves.
Holocompsa nitidula, Trinidad (Princis and Kevan, 1955): Eating banana pulp.
Hormetica laevigata, Wales (Sandemann, 1934): Adventive, in pile of banana sacks.
Hormetica ventralis, Sweden (Princis, 1947): Adventive, in local warehouse of banana company.
Hormetica spp., Europe and North America (Bei-Bienko, 1950): Adventive, introduced with bananas and other tropical fruits.
Ischnoptera rufa rufa, Puerto Rico (Wolcott, 1950): Brought into houses on bunches of bananas.
Kuchinga remota, Society Islands, Moorea (Hebard, 1933a): In dead banana leaves.
Lamproblatta albipalpus, Panama Canal Zone (Hebard, 1920): Several under decayed banana stem.
Latiblattella sp., Finland (Frey, 1948): Adventive.
Leucophaea maderae, New York (Hebard, 1917): Adventive. Dominica (Rehn and Hebard, 1927): Under banana sheaths. England (Palmer, 1928): Adventive, captured at railroad station after bananas had been unloaded. England (Bunting, 1955): Adventive, from Dominica. Trinidad (Princis and Kevan, 1955): Nymph, eating bananas in cupboard. Puerto Rico (Seín, 1923): Seín stated that bananas are the favorite food of L. maderae.
Litopeltis bispinosa, Panama Canal Zone (Hebard, 1920): From rotting banana stalks at bases of leaves.
Litopeltis musarum, Costa Rica (Rehn, 1928): Shaken from dead banana leaves.
Nauclidas nigra, England (Bunting, 1955, 1956): Adventive, from Dominica.
Nauphoeta flexivitta, Denmark (Vestergaard, 1958): Adventive.
Neoblattella carcinus, Neoblattella celeripes, and Neoblattella laodamia, England (Bunting, 1956): Adventive, from Dominica. Bunting (1955) first reported these as Neoblattella spp. and stated that they were common.
Neoblattella detersa, Jamaica (Rehn and Hebard, 1927): From under the bracts of banana blossoms. Sweden (Princis, 1947): Adventive.
Neoblattella detersa and Neoblattella tridens, Finland (Frey, 1948): Adventive.
Neoblattella fratercula, Nebraska (Hebard, 1916b): Adventive.
Neoblattella semota, Jamaica (Rehn and Hebard, 1927): From under drying bracts of banana blossoms.
Neoblattella vatia, Cuba (Rehn and Hebard, 1927).
Neoblattella sp., Finland (Princis, 1947): Adventive, from Jamaica.
Nyctibora azteca, England (Bunting, 1955): Adventive, from Dominica. Bunting reported this species as Nocticola azteca. Dr. A. B. Gurney called our attention to the fact that Nocticola is an Old World genus, presumably combined in error with the New World species azteca. The true identity of the specimen was confirmed by Dr. D. Ragge (personal communication, 1958), who examined it at the British Museum (Natural History).
Nyctibora holoserica, Canada (Walker, 1912): Adventive.
Nyctibora laevigata, Canada, Maine, Massachusetts, Pennsylvania (2) (Hebard, 1917): Adventive. Taken from banana boat Annetta at Philadelphia (Rehn and Hebard, 1927). England (Bunting, 1956): Adventive, from Dominica. Sweden, Denmark (Princis, 1947): Adventive.
Nyctibora mexicana(?), Denmark (Henriksen, 1939): Adventive (5), from Jamaica and West Indies. Princis (1947) suggested that these specimens were probably the West Indian Nyctibora noctivaga, because N. mexicana is not a West Indian insect.
Nyctibora noctivaga, Canada, Idaho, Illinois, Massachusetts, Nebraska (4), Virginia (Hebard, 1917): Adventive. Nebraska (Hauke, 1949): Adventive (2). Panama Canal Zone (Hebard, 1920): From banana stalks. England (Blair in Turner, 1930): Adventive, from Costa Rica. Washington (Hatch, 1938): Adventive. Sweden (Princis, 1947): Adventive (2). Finland (Princis, 1947): Adventive, from Jamaica.
Nyctibora obscura, Trinidad (Princis and Kevan, 1955): In banana bunch.
Nyctibora sericea, Canada (Stevenson, 1905; Walker, 1912): Adventive; Hebard (1917) synonymized Walker's specimen under N. laevigata. Isle of Wight (Meade-Waldo, 1910): Adventive, from Jamaica. England (Tulloch, 1939): Adventive, in banana crates from Brazil.
Nyctibora sp., England (Welch, 1935): Adventive, in railway truck that had carried bananas. England (Tulloch, 1939): Adventive, from Brazil.
Oxyhaloa deusta, U.S.S.R., Leningrad (Bei-Bienko, 1947): Adventive, from Colombia.
Panchlora antillarum, England (Bunting, 1955): Adventive, from Dominica.
Panchlora exoleta, Scotland (Distant, 1902): Adventive. Great Britain (Shaw, 1902): Adventive. England (Coney, 1918): Adventive. Sweden, Norway (Princis, 1947): Adventive, Norwegian specimen from Brazil. Germany (Zacher, 1917): Adventive, from Jamaica.
Panchlora nivea, Colorado, Nebraska, New Jersey, New York (2), Utah (Hebard, 1917): Adventive. Nebraska (Hauke, 1949): Adventive. Washington (Hatch, 1938): Adventive. Massachusetts (Roth and Willis, 1958): Adventive. England (Bunting, 1955): Adventive, from Dominica. U.S.S.R. (Bei-Bienko, 1947): Adventive, from Colombia. Sweden (13), Norway (3), Finland (3) (Princis, 1947): Adventive, mostly females; origin (where known) Jamaica.
Panchlora fraterna(?) and Panchlora peruana(?), Denmark (Henriksen, 1939): Adventive; origin (where known) Danish West Indies and Jamaica; Princis (1947) suggested that both species were probably Panchlora nivea.
Panchlora sagax, Puerto Rico (Wolcott, 1936).
Panchlora virescens, Canada (Walker, 1912): Adventive; this was probably P. nivea as we now know it (Gurney, personal communication, 1959).
Panchlora sp., Canada (Walker, 1912): Adventive. England (Tulloch, 1939): Adventive, from Brazil.
Pelmatosilpha coriacea, Puerto Rico (Wolcott, 1936).
Pelmatosilpha marginalis and Pelmatosilpha purpurascens, England (Bunting, 1955, 1956): Adventive, from Dominica; both species common.
Pelmatosilpha vagabunda, New Zealand (Princis, 1954): Adventive, probably from South America.
Periplaneta americana, Belgium (Schepdael, 1931): Adventive, on bananas from the American Tropics.
Periplaneta americana and Periplaneta brunnea, England (Bunting, 1955, 1956): Adventive, from Dominica.
Periplaneta americana and Periplaneta australasiae, England (Watson, 1907): Adventive; they ate ripening bananas in the tropical plant house of the Royal Botanic Gardens, Kew, where they hid in "the sheathing bases of palm, banana and pandanus leaves." Sweden (Princis, 1947): Adventive.
Periplaneta australasiae, Canada (Walker, 1912): Adventive. Denmark (Henriksen, 1939): Adventive (9); origin mostly Jamaica. England (Tulloch, 1939): Adventive, from Brazil. England (Bunting, 1955, 1956): Adventive, from Dominica; common.
Platyzosteria bifida, Nebraska (Hebard, 1917): Adventive.
Plectoptera dorsalis, Puerto Rico (Rehn and Hebard, 1927): Captured by beating banana plants.
Pycnoscelus surinamensis, Canada (Walker, 1912; Hebard, 1917): Adventive: Marquesas Islands, Nukuhiva (Hebard, 1933a): In banana leaves. England (Goodliffe, 1958): Adventive, doing considerable damage to banana plants growing in a conservatory.
Sibylloblatta panesthoides, Massachusetts (Rehn, 1937a): Adventive, from Jamaica.
Family ZINGIBERACEAE
Associate.—Cariblatta orestera, Jamaica (Rehn and Hebard, 1927): The male was taken in a head of wild ginger.
Family CANNACEAE
Associate.—Periplaneta americana, Hawaii (Zimmerman, 1948).
Family ORCHIDACEAE
Associates.—Periplaneta americana, U.S.A. (Rau, 1940a).
Periplaneta australasiae, England (Lucas, 1918).
Associates.—Periplaneta americana, U.S.A. (Rau, 1940a).
Periplaneta australasiae, England (Lucas, 1918).
Associates.—Blaberus discoidalis, Blatta orientalis, Periplaneta americana, Hawaii (Swezey, 1945).
Blatta orientalis, americana, cinerea, maderae, unidentified cockroaches, England, in bulb from Ecuador (Westwood, 1876).
Graptoblatta notulata, Hawaii (Swezey, 1945): On orchid from India.
Homalopteryx laminata and Hormetica apolinari, New York (Hebard, 1912c): In orchids shipped from Colombia.
Pelmatosilpha coriacea, Puerto Rico (Wolcott, 1936).
Periplaneta americana, Germany (Tashenberg, 1884).
Periplaneta australasiae, England (Wainwright, 1898). Pennsylvania (Skinner, 1905). Massachusetts (Morse, 1920).
Pycnoscelus surinamensis, England (Westwood, 1869). Germany (Zacher, 1920). Massachusetts (Morse, 1920). Hawaii (Swezey, 1945).
Family CASUARINACEAE
Associate.—Diploptera punctata, Hawaii (Zimmerman, 1948).
Family SALICACEAE
Synonymy.—Populus diversifolia Schrenk. [Howard, personal communication, 1959].
Associate.—Ectobius semenovi, Kazakhstan (Bei-Bienko, 1950).
Associate.—Ectobius lapponicus, U.S.S.R. (Stark in Bei-Bienko, 1950): On aspen.
Associate.—Ectobius semenovi, Kazakhstan (Bei-Bienko, 1950): On willow.
Family MYRICACEAE
Associate.—Chorisoneura texensis, Florida (Rehn and Hebard, 1916): On bayberry. Florida (Blatchley, 1920): Beaten from foliage.
Family FAGACEAE
Associate.—Parcoblatta pensylvanica, Virginia (Rehn and Hebard, 1916): Under signs on white oaks.
Associates.—Parcoblatta divisa, Virginia, and Parcoblatta pensylvanica, North Carolina (Rehn and Hebard, 1916): Under signs on red oak.
Parcoblatta lata, North Carolina (Hebard, 1917): Under sign.
Associate.—Eurycotis floridana, Georgia (Rehn and Hebard, 1916): Under dead bark on live-oak tree. Georgia (Hebard, 1917): In cavity in tree.
Associates.—Aglaopteryx gemma, Alabama, Georgia, Florida, Louisiana, Texas (Hebard, 1917): Under signs on oaks.
Blatta orientalis, England (Donisthorpe, 1918): Under bark.
Cariblatta lutea lutea, Mississippi (Hebard, 1916a): By beating low oaks on hills.
Chorisoneura texensis, Mississippi (Hebard, 1917). Florida (Blatchley, 1920): By beating.
Ectobius pallidus, England (Milton, 1899; Burr, 1899b). Massachusetts (Flint, 1951): Under loose lichens and bark.
Parcoblatta divisa, Georgia, Louisiana, and Parcoblatta pensylvanica, Georgia (Hebard, 1917): Under signs.
Periplaneta australasiae, Florida (Rehn and Hebard, 1905): Ten specimens taken from under a tin sign.
Periplaneta brunnea, Georgia (Rehn and Hebard, 1916): Under signs.
Phyllodromica megerlei, U.S.S.R. (Bei-Bienko, 1950): By shaking oak branches.
Plectoptera lacerna, Cuba (Rehn and Hebard, 1927).
Family MORACEAE
Associate.—Cariblatta hylaea, Honduras (Rehn, 1945a).
Family CHENOPODIACEAE
Associate.—Ectobius panzeri, England (Lucas, 1920a).
Associate.—Ectobius pallidus, Massachusetts (Flint, 1951): Many specimens collected in the bases of Swiss chard plants.
Family LAURACEAE
Synonymy.—Ocotea catesbyana Sarg. [Howard, personal communication, 1959].
Associate.—Chorisoneura texensis, Florida (Rehn and Hebard, 1912).
Family SARRACENIACEAE
Only a few records have been found of cockroaches being trapped in the pitchers of carnivorous plants of this and the following family. The insects drown in the fluid within the pitcher where they are apparently digested by proteinases secreted by the plant (Meyer and Anderson, 1939; Lloyd, 1942).
Natural prey—Cariblatta lutea lutea, Ischnoptera deropeltiformis, Parcoblatta lata, and nymphs of Parcoblatta sp., North Carolina (Wray and Brimley, 1943): Most of the cockroaches seemed to have been trapped accidentally with the possible exception of C. lutea lutea, 11 of which were found in Sarracenia pitchers.
Natural prey.—Cariblatta lutea lutea, North Carolina (Wray and Brimley, 1943).
Synonymy.—Sarracenia variolaris Michx. [Howard, personal communication, 1958].
Natural and experimental prey.—Periplaneta australasiae, Florida (Treat, 1876): After the insect imbibed some of the fluid in the pitcher it became docile; others became highly active and rushed wildly about before becoming quiescent. See also Treat in Scudder (1877).
Cockroaches, U.S.A. (Riley, 1875).
Family NEPENTHACEAE
Natural prey.—Cockroaches, Singapore (Dover, 1928).
Natural prey.—Cockroaches, Singapore (Dover, 1928).
Natural prey.—Cockroach, Old World Tropics? (Hooker, 1874): The insect was apparently attracted into the pitcher, where it drowned, by a piece of cartilage placed there by Hooker.
Family CUNONIACEAE
Associates.—Aneurina viridis, Marquesas Islands, Nukuhiva and Fatuhiva (Hebard, 1933a).
Maretina uahuka, Marquesas Islands, Uahuka (Hebard, 1933a).
Family HAMAMELIDACEAE
Associate.—Parcoblatta divisa, Georgia (Rehn and Hebard, 1916): Under sign on sweet gum.
Parcoblatta zebra, Louisiana (Hebard, 1917): In decay cavity.
Family ROSACEAE
Associates.—Plectoptera dorsalis, Plectoptera infulata, Plectoptera rhabdota, Puerto Rico (Wolcott, 1950): In the dry flower clusters of "espino rubial."
Associate.—Pycnoscelus surinamensis, Connecticut (Zappe, 1918); Rhode Island and Pennsylvania (Caudell, 1925); Pennsylvania (Doucette and Smith, 1926): Feeding on canes in greenhouses.
Associate.—Hololampra chavesi, Azores (Chopard, 1932): This species is exclusively dendricolous and was found only by beating the bushes on which it abounds. It was very common in hedges, particularly on brambles (ronces).
Family LEGUMINOSAE
Associate.—Diploptera punctata, Hawaii (Bridwell and Swezey, 1915; Zimmerman, 1948): Feeding on pods.
Associates.—Ellipsidion australe, Australia, New South Wales (Hebard, 1943).
Methana curvigera, Australia, Queensland (Pope, 1953a).
Associate.—Diploptera punctata, Hawaii (Pemberton and Williams, 1938): Damaging algarroba.
Associates.—Aglaopteryx absimilis, Puerto Rico (Wolcott, 1950): In abandoned cocoon.
Aglaopteryx facies, Puerto Rico (Wolcott, 1936): In empty cocoons.
Associate.—Aglaopteryx facies, Puerto Rico (Wolcott, 1936): On trunk.
Associates.—Aglaopteryx facies, Puerto Rico (Wolcott, 1936): In larval tents.
Cariblatta stenophrys, Puerto Rico (Wolcott, 1936): On leaves.
Plectoptera dorsalis, Plectoptera infulata, and Plectoptera rhabdota, Puerto Rico (Wolcott, 1950): In "butterfly nests" in leaves.
Associate.—Nyctibora stygia, Haiti (Rehn and Hebard, 1927).
Associate.—Aglaopteryx absimilis, Puerto Rico (Wolcott, 1950).
Associate.—Hemiblabera brunneri, Puerto Rico (Rehn and Hebard, 1927).
Family GERANIACEAE
Associate.—Diploptera punctata, Hawaii (Zimmerman, 1948).
Family ZYGOPHYLLACEAE
Associates.—Periplaneta americana and Pycnoscelus surinamensis, Johnston Island (Bryan, 1926). Zimmerman (1948) lists Tribulus as a host plant for these cockroaches.
Family RUTACEAE
Associates.—Plectoptera dominicae and Plectoptera perscita, Dominica (Rehn and Hebard, 1927): Beaten from moss-covered lime trees.
Associates.—Plectoptera dorsalis, Plectoptera infulata, Plectoptera rhabdota, Puerto Rico (Wolcott, 1950).
Plectoptera rhabdota, Puerto Rico (Rehn and Hebard, 1927).
Associate.—Diploptera punctata, Hawaii (Bridwell and Swezey, 1915; Zimmerman, 1948): Feeding on oranges on tree.
Associates.—Diploptera punctata, Hawaii (Zimmerman, 1948).
Riatia [= Lissoblatta] fulgida, Panama, Rio Trinidad (Hebard, 1920).
Plectoptera porcellana, Puerto Rico (Sein, 1923).
Associates.—Plectoptera dorsalis, Plectoptera infulata, Plectoptera rhabdota, Puerto Rico (Wolcott, 1950): In the dry flower clusters.
Family BURSERACEAE
Associate.—Chorisoneura texensis, Florida (Rehn and Hebard, 1912; Hebard, 1917): Beaten from the lower branches of gumbo limbo.
Family EUPHORBIACEAE
Associate.—Pycnoscelus surinamensis, Connecticut (Zappe, 1918): Ate bark of greenhouse plants.
Family ANACARDIACEAE
Associate.—Diploptera punctata, Hawaii (Bridwell and Swezey, 1915; Zimmerman, 1948): Feeding on mangoes on the tree.
Associates.—Plectoptera dorsalis, Plectoptera infulata, Plectoptera rhabdota, Puerto Rico (Wolcott, 1950): Living on leaves of "jobo."
Associate.—Eurycotis biolleyi, Costa Rica (Rehn, 1918): In the crown of dry jocoto.
Family AQUIFOLIACEAE
Associate.—Plectoptera poeyi, Florida (Rehn and Hebard, 1912, 1914; Hebard, 1917).
Synonymy.—Ilex lucida [Fernald, 1950].
Associate.—Cariblatta lutea lutea, Florida (Hebard, 1916a).
Family SAPINDACEAE
Associate.—Aglaopteryx gemma, Florida (Hebard, 1917).
Family MALVACEAE
Associates.—Graptoblatta notulata, Marquesas Islands, Tahuata (Hebard, 1933a).
Periplaneta australasiae, St. Kitts, B.W.I. (Ballou, 1916).
Periplaneta fuliginosa and Plectoptera poeyi, Florida (Rainwater, 1941).
Plectoptera dorsalis, Plectoptera infulata, Plectoptera rhabdota, Puerto Rico (Wolcott, 1950).
Associate.—Riatia orientis, Trinidad (Princis and Kevan, 1955).
Associates.—Amazonina emarginata, Cariblatta antiguensis, Eurycotis kevani, and Rhytidometopum dissimile, Trinidad (Princis and Kevan, 1955).
Associate.—Periplaneta australasiae, Nihoa Island (Bryan, 1926). Hawaii (Zimmerman, 1948).
Family STERCULIACEAE
Associate.—Ceratinoptera picta, Trinidad (Princis and Kevan, 1955).
Family BIXACEAE
Associate.—Notolampra antillarum, Trinidad (Princis and Kevan, 1955): Nymphs in dry fruits on "annato" tree.
Family FLACOURTIACEAE
Associate.—Graptoblatta notulata, Marquesas Islands, Uahuka (Hebard, 1933a).
Family PASSIFLORACEAE
Associate.—Aristiger [= Plumiger] histrio, Malaya (Karny, 1924).
Family CARICACEAE
Associate.—Diploptera punctata, Hawaii (Bridwell and Swezey, 1915; Zimmerman, 1948): Feeding on papaya fruit on tree.
Family RHIZOPHORACEAE
Associate.—Aglaopteryx gemma, Florida (Hebard, 1917).
Family COMBRETACEAE
Associate.—Plectoptera poeyi, Florida (Rehn and Hebard, 1914): Running on leaves.
Family MYRTACEAE
Associate.—Ellipsidion australe, Australia, New South Wales (Hebard, 1943).
Synonymy.—Syzygium aromaticum [Bailey, 1925].
Associate.—Plectoptera dorsalis, Puerto Rico (Wolcott, 1936): On flowers of "pomarrosa."
Associates.—Aneurina viridis, Marquesas Islands: Nukuhiva, Fatuhiva, and Tahuata (Hebard, 1933a)
Aneurina tahuata, Marquesas Islands, Tahuata (Hebard, 1933a).
Graptoblatta notulata, Marquesas Islands, Nukuhiva (Hebard, 1933a).
Associate.—Plectoptera rhabdota, Puerto Rico (Rehn and Hebard, 1927).
Family ONAGRACEAE
Associate.—Epilampra abdomen-nigrum, Panama (Crowell, 1946): In an aquarium the cockroach fed on leaves of this aquatic plant which had been collected in the lagoon where the insect was captured.
Family ERICACEAE
Associates.—Ectobius lapponicus, England (Lucas, 1925): "Nymphs of varying size were beaten out of heather ... on 9 February and later."
Ectobius panzeri, England (Lucas, 1927): "numerous imagines of both sexes were swept from heather."
Associates.—Chorisoneura formosella, Neoblattella dryas, Neoblattella proserpina, Jamaica (Rehn and Hebard, 1927).
Family SAPOTACEAE
Associate.—Pelmatosilpha coriacea, Puerto Rico (Wolcott, 1941): Under bark.
Family APOCYNACEAE
Associate.—Ectobius pallidus, Massachusetts (Willis, unpublished observation, 1958).
Family CONVOLVULACEAE
Associate.—Plectoptera dorsalis, Puerto Rico (Rehn and Hebard, 1927).
Family BORAGINACEAE
Synonymy.—Calyptracordia alba [Howard, personal communication, 1958].
Associates.—Cariblatta antiguensis, Ischnoptera rufa rufa, Supella supellectilium, Symploce ruficollis and Symploce hospes, St. Croix, Virgin Islands (Beatty, 1944): On fruits of C. dentata except S. supellectilium which was found at night on the flowers.
Family VERBENACEAE
Associate.—Chorisoneura texensis, Florida (Rehn and Hebard, 1912).
Family SOLANACEAE
Associate.—Pycnoscelus surinamensis, Sumatra (Roeser, 1940).
Associate.—Pycnoscelus surinamensis, Haiti (Hoffman, 1927): Feeding on tubers in field.
Family GESNERIACEAE
Associate.—Aneurina viridis, Marquesas Islands, Nukuhiva (Hebard, 1933a).
Family RUBIACEAE
Associate.—Graptoblatta notulata, Marquesas Islands, Uahuka (Hebard, 1933a).
Associate.—Periplaneta americana, Puerto Rico (Plank and Winters, 1949): In greenhouse.
Associate.—Plectoptera porcellana, Puerto Rico (Seín, 1923).
Family COMPOSITAE
Associate.—Eurycotis floridana, Florida (Hebard, 1917): "Climbing about on top of goldenrod at night."
Associate.—Pseudomops septentrionalis, Texas (Hebard, 1917).
Associate.—Phyllodromica tartara nigrescens, Southern Uzbekistan (Bei-Bienko, 1950): On the flowers.
Cockroaches characteristically feed on dead plant and animal material. Damage to living plants occurs principally in the Tropics or under subtropical conditions in greenhouses in temperate regions. Among the depredations attributed to cockroaches in text books, damage to plants is seldom emphasized. This is surprising in view of the many records cited below.
Capt. William Bligh (1792), while collecting breadfruit trees in Tahiti to take to the West Indies, wrote in his log during January 1789: "This morning, I ordered all the chests to be taken on shore, and the inside of the ship to be washed with boiling water, to kill the cockroaches. We were constantly obliged to be at great pains to keep the ship clear of vermin, on account of the plants."
Westwood (1869) stated that Pycnoscelus surinamensis was very destructive in orchid houses feeding on buds and young shoots. Later Westwood (1876) exhibited the bulb of an orchid from Ecuador which contained six species of cockroaches: Blatta orientalis, [Periplaneta?] americana, [Nauphoeta?] cinerea, [Leucophaea?] maderae, and two others unknown to him. Fullaway (1938) stated that cockroaches damage root tips, buds, and flowers of orchids. Periplaneta americana has been said to eat the root tips and blossoms of orchids (Taschenberg, 1884) and to devour the open flower petals of Cattleya [Pg 163] orchids as well as the aerial roots and flower spikes of Vanda orchids (Rau, 1940a). Wainwright (1898) stated that Periplaneta australasiae had been observed in an orchid house in Perthshire where over a period of three years it had caused a good deal of damage. Skinner (1905) reported that P. australasiae in greenhouses in Pennsylvania showed no preference for any one plant but ate both plants and flowers of orchids, roses, and carnations. Lucas (1918) received specimens of P. australasiae which had played havoc with orchids especially Cattleya and Vanda. Morse (1920) reported that both P. australasiae and Pycnoscelus surinamensis were obnoxious in a conservatory in Massachusetts where they gnawed the tips of the aerial roots of orchids. Swezey (1945) in Hawaii stated that the following cockroaches have been reported as occasional minor pests on orchids: Blatta orientalis, Blaberus discoidalis, P. americana, and P. surinamensis; he further stated that Graptoblatta notulata had been intercepted at Honolulu on orchids from India.
Watson (1907) stated that Blatta orientalis, Periplaneta americana, and Periplaneta australasiae were injurious in the tropical plant houses at Kew: "at night they come out and run or fly about among the plants, devouring flowers and leaves like rabbits. Such plants as Eucharis, Crinum and Alpinia, when in flower, have little chance in the palm house, where the cockroaches are most abundant; they also find out the ripening bananas and soon devour them." Raffill (1910) stated that in plant houses in England B. orientalis, P. americana, and P. australasiae commonly, and Nauphoeta cinerea, Nauphoeta flexivitta, and Pycnoscelus surinamensis more rarely, are extremely destructive to plants. Flowers having a strong perfume, such as orchids, Eucharis, Crinum, and Hedychium, were often attacked while other flowers nearby were left uninjured.
Plank and Winters (1949) reported that in Puerto Rico the species of Orthoptera most injurious under greenhouse conditions was Periplaneta americana. Large nymphs destroyed 25 to 30 percent of freshly planted seed of Cinchona pubescens. In Hawaii the host plants of P. americana are blossoms of Canna and Tribulus, and the host plants of Periplaneta australasiae are Pritchardia and Sida (Zimmerman, 1948). On St. Kitts, B.W.I., young cotton plants were severely attacked by P. australasiae; this caused loss of the stand on a considerable area and necessitated replanting (Ballou, 1916). P. australasiae was reported damaging the Polystichum aristatum Presl [= Lastrea aristata variegata] in a greenhouse (Thilow and Riley, 1891). Laing (1946; British Museum [Natural History], 1951) stated that P. australasiae abounds in greenhouses and forcing pits [Pg 164] where it may do considerable damage to the plants. Periplaneta fuliginosa is also troublesome in greenhouses because of its tendency to feed on seedlings and succulent plants (Dodge and Rickett, 1943).
Ectobius lapponicus has been observed feeding in galleries in the thick skin of young aspen in 25 percent of the trees examined (Stark in Bei-Bienko, 1950). The aquatic cockroach Epilampra abdomen nigrum fed on the leaves of Jussiaea natans in an aquarium (Crowell, 1946). Ischnoptera deropeltiformis has been taken while it was feeding on a fleshy fungus (Agaricus sp.) in dense woods in Indiana (Blatchley, 1920).
Diploptera punctata, the cypress roach or beetle roach, has been found in Hawaii feeding on ripening mangoes and papayas, oranges on the tree, and the outer covering of the pods of Acacia farnesiana (Bridwell and Swezey, 1915). Pemberton (1934) stated that D. punctata "disfigures our cypress trees by eating the bark from the young branches, often giving them a dead appearance over much of their leaf area." Fullaway and Krauss (1945) added, "This injury [to cypress] is so severe that sometimes areas of leaves die and turn brown. The Japanese cedar, ironwood, citrus and algaroba (kiawe) trees are attacked in a similar manner." Similar injury to cypress was described by Hebard (1922). In addition to girdling Cupressus, D. punctata injures Cryptomeria in the same fashion and also attacks algaroba, lime, and other plants (Pemberton and Williams, 1938). Zimmerman (1948) cited the following host plants for D. punctata in Hawaii: "Cupressus macrocarpa, Casuarina, Cryptomeria, Citrus, geraniums, Acacia farnesiana pods, mango fruits, orange fruits, papaya fruits."
In the reports of damage to plants by cockroaches, Pycnoscelus surinamensis has been implicated most often. This species is undoubtedly one of the economically most important cockroaches, being the vector of the chicken eyeworm as well as feeding on plants. In addition to the few reports of damage caused by this species that have already been mentioned, P. surinamensis has been reported to be very destructive in New Orleans to palms and ferns, attacking large alsophilas avidly, eating out the hearts (Anonymous, 1893). Zappe (1918) in Connecticut reported damage in a greenhouse to roses valued, at that time, at several hundred dollars; P. surinamensis had girdled the rose bushes, done much damage to Easter lilies, and in another greenhouse had eaten the bark from the stems of poinsettias. In Germany this species bit off the tips of the aerial roots and ate the petals of orchids (Zacher, 1920). Lucas (1923) reported damage to cucumber plants in a greenhouse in Surrey. Damage by P. surinamensis [Pg 165] to the stems of rose bushes has been reported in Rhode Island and Pennsylvania; the canes were attacked both under and above ground (Caudell, 1925). Doucette and Smith (1926) reported a heavy infestation of P. surinamensis in a range of greenhouses in Philadelphia: "The roaches were present literally by the millions.... Although the roaches had been observed in cabinets and trash barrels for several months, it was not until the manager had occasion to go through the house one evening that he discovered that roaches were the cause of the troubles previously attributed to soil condition, watering, fungus, and other agencies.... About 30,000 to 35,000 rose plants from a total of 200,000 in the three more heavily infested houses were so badly injured by the gnawing off of the bark, young buds, and shoots of the main stems, that they were not in condition to be kept in the beds for another season."
In Haiti Pycnoscelus surinamensis damaged the tubers of growing potatoes (Hoffman, 1927). Illingworth (1927, 1929) reported that in Hawaii P. surinamensis was a minor pest of pineapples, feeding on the roots. This species was very plentiful in a propagating pit in England where it did much damage to various seeds and seedlings (Lucas, 1930). Roeser (1940) summarized some of the above-mentioned damage caused by P. surinamensis and added damage to chrysanthemums in Hawaii and tobacco in Sumatra where this cockroach destroyed 300,000 plants in a few days. Roeser was of the opinion that living plants were eaten only as a substitute when the earth became poor in food material. Zimmerman (1948) listed as host plants of P. surinamensis in Hawaii: "blossoms of Tribulus; reported feeding at roots of pineapples, and unconfirmed reports of damage to underground parts of some other plants." Goodliffe (1958) reported damage by this species to banana plants in a conservatory in northern England. Cohic (1956) implied that in New Caledonia "Racines de légumes" were attacked by P. surinamensis and that Zea mays Linnaeus was attacked by Supella supellectilium. Wolcott (1924a) reported that P. surinamensis damaged transplanted tobacco plants in Puerto Rico by eating the interior of the stalks. Tobacco planters in Cuba consider P. surinamensis injurious to the roots of tobacco plants (Bruner and Scaramuzza, 1936); this belief was confirmed in the laboratory, where adults and nymphs destroyed the roots and stems of tobacco plants two inches high and ate into the edges of the leaves. Dammerman (1929) reported that in Malaya this species often appeared in large numbers in gardens where it gnawed at the underground parts of vegetables and ornamental plants. Lever (1947) listed it as a pest on the leaves of pineapple.
Blattella vaga may occasionally damage seedlings in the laboratory (Flock, 1941a), but no damage has been reported in the field (Ball et al., 1942). Heer (1864) reported receiving a shipment of cycads from Cuba with all stages of Periplaneta americana living in holes in the branches, apparently subsisting on the starchy tissues. Goldenberg (1877) stated that sago trees provide cockroaches with their favorite nourishment. Scudder (1879) found Eurycotis floridana living in the tops of the cabbage palmetto, on which he presumed it fed. Parcoblatta americana has been observed feeding on an apple 6 feet above ground (Fulton, 1930).
The classification of the Protozoa follows that of Kudo (1954). The use of the asterisk (*) is explained in footnote 3, page 4.
Phylum PROTOZOA
Class MASTIGOPHORA
Order EUGLENOIDINA
Family EUGLENIDAE
Experimental host.—Periplaneta americana, U.S.A. (Hegner, 1929): When fed to the insects in concentrated culture, Euglena could withstand conditions in the crop up to 5 hours and were passed into the stomach in a viable state up to 6 hours. However, the majority were killed in the crop within 2 hours and very few reached the stomach alive.
Order PROTOMONADINA
Family OIKOMONADIDAE
Natural host.—Cockroach, Venezuela (Tejera, 1926).
Natural host.—Blatta orientalis, U.S.S.R. (Yakimov and Miller, 1922): Oikomonas sp. and Monas sp. were found in the intestines of 83 percent of 124 cockroaches.
Cockroach, Venezuela (Tejera, 1926).
Family TRYPANOSOMATIDAE
Natural host.—Blaberus sp., Venezuela (Tejera, 1926).
Natural hosts.—Parcoblatta lata, Parcoblatta pensylvanica, Parcoblatta virginica, U.S.A., Ohio (Semans, 1939, 1941): Hind intestine. Of 70 specimens examined, 86 percent harbored Leptomonas sp.
Natural host.—Blatta orientalis, Italy, France (Laveran and Franchini, 1920, 1920a).
Family MONADIDAE
Natural host.—Blatta orientalis, U.S.S.R. (Yakimov and Miller, 1922): Monas sp. and Oikomonas sp. were found in the intestines of 83 percent of 124 cockroaches examined.
Cockroach, Venezuela (Tejera, 1926).
Family BODONIDAE
Natural host.—Blatta orientalis, England (Lankester, 1865).
Natural host.—Blattella germanica and/or Periplaneta americana, South Africa (Porter, 1930).
Synonymy.—Embadomonas blattae Bishop [Wenrich, 1932].
Natural host.—Blatta orientalis, England (Bishop, 1931): Hind intestine. The organism occurred in about 40 percent of the cockroaches examined. L. G. Feo (in Wenrich, 1932) successfully cultured this protozoan (fig. 2, F).
Natural host.—Leucophaea maderae, Philippine Islands (Hegner and Chu, 1930).
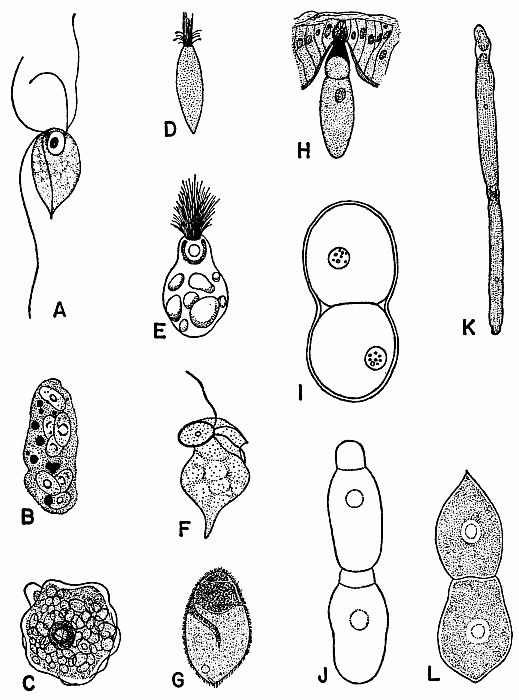 Fig. 2.—Representative Protozoa associated with cockroaches. A, Monocercomonoides
melolonthae, X 3094 (after Grassé). B, Coelosporidium periplanetae, X 1310 (after Sprague);
trophozoite with spores and chromatoid bodies. C, Endamoeba blattae, X 273 (after Kudo);
trophozoite. D, Lophomonas striata, X 330 (after Kudo). E, Lophomonas blattarum, X 660
(after Kudo). F, Retortamonas blattae, X 3094 (after Wenrich). G, Nyctotherus ovalis,
X 175 (after Kudo). H, Gregarina rhyparobiae, c. X 52: mature trophozoite attached to
intestinal wall of Leucophaea maderae. (Redrawn from J. M. Watson [1945].) I, Diplocystis
schneideri, c. X 14.4 (after Kunstler). J, Gregarina blattarum, c. X 57 (after Kudo). K,
Protomagalhaesia serpentula, X 36 (after Pinto). L, Gamocystis tenax, magnification not
known (after Schneider). (All figures except H redrawn from Kudo [1954] after sources
indicated.)
Fig. 2.—Representative Protozoa associated with cockroaches. A, Monocercomonoides
melolonthae, X 3094 (after Grassé). B, Coelosporidium periplanetae, X 1310 (after Sprague);
trophozoite with spores and chromatoid bodies. C, Endamoeba blattae, X 273 (after Kudo);
trophozoite. D, Lophomonas striata, X 330 (after Kudo). E, Lophomonas blattarum, X 660
(after Kudo). F, Retortamonas blattae, X 3094 (after Wenrich). G, Nyctotherus ovalis,
X 175 (after Kudo). H, Gregarina rhyparobiae, c. X 52: mature trophozoite attached to
intestinal wall of Leucophaea maderae. (Redrawn from J. M. Watson [1945].) I, Diplocystis
schneideri, c. X 14.4 (after Kunstler). J, Gregarina blattarum, c. X 57 (after Kudo). K,
Protomagalhaesia serpentula, X 36 (after Pinto). L, Gamocystis tenax, magnification not
known (after Schneider). (All figures except H redrawn from Kudo [1954] after sources
indicated.)
Order POLYMASTIGINA
Family CHILOMASTIGIDAE
Experimental vectors.—Blatta orientalis and Periplaneta americana, South Africa (Porter, 1918): The cockroaches were fed human excrement that contained cysts of C. mesnili. The cysts passed unharmed through the insects' digestive tract. Rats became infected with this protozoan on eating food that had been contaminated with feces from these cockroaches.
Family POLYMASTIGIDAE
Synonymy.—Trichomastix [Kudo, 1954].
Natural host.—Blattella germanica and/or Periplaneta americana, South Africa (Porter, 1930).
Natural host.—Cryptocercus punctulatus, U.S.A., Appalachian and Pacific coast areas (Cleveland et al., 1934): Organism occurs in practically all hosts.
(Fig. 2, A)
Natural host.—Platyzosteria novae seelandiae, New Zealand (Laird, 1956): Found in the intestinal tracts of the adult cockroaches, and of other species of insects.
Synonymy.—Trichomonas (Trichomastix) orthopterorum Parisi; Monocercomonas orthopterorum [Bělǎr, 1916]; Trichomastic orthopterum? [Zasukhin, 1930]; Monocercomonoides orthopterorum [Travis, 1932; Cleveland et al., 1934]; Retortamonas orthopterorum [Semans, 1943].
Natural hosts.—Blatta orientalis, Italy (Parisi, 1910); U.S.S.R. (Zasukhin, 1930).
Ectobius lapponicus, Italy (Parisi, 1910).
Periplaneta americana, Philippine Islands (Hegner and Chu, 1930).
"Küchenschaben," Austria (Bělǎr, 1916).
The protozoan is found in the hind gut. Zasukhin (1930) found [Pg 170] the organism in 85 percent of over 3,000 B. orientalis. Parisi (1910) found the flagellate present in very large numbers.
Natural host.—Panesthia angustipennis, Philippine Islands (Kidder, 1937): In hind gut.
Natural hosts.—Blatta orientalis, Blattella germanica, Periplaneta americana, U.S.A. (Young, 1935): The organism when present occurs in large numbers in the posterior part of the intestine near the anus. The protozoan was successfully cultivated in a hemoglobin-saline medium.
Family OXYMONADIDAE
Synonymy.—Saccinobaculus doroaxostylus Cleveland et al. [Cleveland, 1950].
Natural host.—Cryptocercus punctulatus, U.S.A., Appalachian and Pacific coast areas (Cleveland et al., 1934).
Synonymy.—Saccinobaculus minor Cleveland et al. [Cleveland, 1950].
Natural host.—Cryptocercus punctulatus, U.S.A., Appalachian and Pacific coast areas (Cleveland et al., 1934).
Family DINENYMPHIDAE
Natural host.—Cryptocercus punctulatus, U.S.A., Appalachian and Pacific coast areas (Cleveland et al., 1934).
Natural host.—Cryptocercus punctulatus, U.S.A. (Cleveland, 1950b): There are at least two other species of Saccinobaculus in C. punctulatus that have not been described.
Natural host.—Cryptocercus punctulatus, U.S.A., Appalachian and Pacific coast areas (Cleveland, 1950b).
Family TRICHOMONADIDAE
Experimental vectors.—Blatta orientalis, South Africa (Porter, 1918); Italy (Mariani and Besta, 1936).
Periplaneta americana, South Africa (Porter, 1918); U.S.A. (Hegner, 1928).
Natural vector.—Cockroach, Venezuela (Tejera, 1926): Organism found in digestive tract of the cockroach.
Family HEXAMITIDAE
Natural hosts.—Cryptocercus punctulatus, U.S.A. (Cleveland et al., 1934).
Panesthia angustipennis, Philippine Islands (Kidder, 1937).
Synonymy.—Octomitus periplanetae Bĕlăr [Kudo, 1954].
Natural hosts.—Blatta orientalis, U.S.S.R. (Zasukhin, 1930): Organism is found in the hind gut. Eighty-five percent of over 3,000 B. orientalis contained this organism.
Periplaneta americana, Philippine Islands (Hegner and Chu, 1930).
"Küchenschaben," Austria (Bĕlăr, 1916).
Natural host.—Leucophaea maderae, Philippine Islands (Hegner and Chu, 1930): The flagellates were present in large numbers.
Experimental vectors.—Blatta orientalis, South Africa (Porter, 1918).
Blattella germanica, Brazil (Pessôa and Corrêa, 1927).
Eurycotis floridana, U.S.A. (Young, 1937).
Leucophaea maderae, Brazil (Pessôa and Corrêa, 1927).
Periplaneta americana, South Africa (Porter, 1918); Gold Coast Colony (Macfie, 1922); Brazil (Pessôa and Corrêa, 1927); U.S.A. (Young, 1937).
Periplaneta brunnea, U.S.A. (Young, 1937).
Cockroaches, Venezuela (Tejera, 1926); Argentina (Bacigalupo, in Tejera, 1926).
Natural vectors.—Cockroaches, Venezuela (Tejera, 1926).
Order HYPERMASTIGINA
Family HOLOMASTIGOTIDAE
Natural host.—Cryptocercus punctulatus, U.S.A., Appalachian area (Cleveland et al., 1934).
Natural host.—Cryptocercus punctulatus, U.S.A., Appalachian area (Cleveland et al., 1934).
Natural host.—Cryptocercus punctulatus, U.S.A., Appalachian area (Cleveland et al., 1934).
Natural host.—Cryptocercus punctulatus, U.S.A., Appalachian area (Cleveland et al., 1934).
Family LOPHOMONADIDAE
Natural hosts.—Blatta orientalis, Czechoslovakia (Stein, 1860); Germany (Bütschli, 1878; Schubotz, 1905; Chen, 1933); U.S.A. (Leidy, 1879a; Kudo, 1922, 1925, 1926, 1926b; McAdow, 1931); Europe (Janicki, 1908); U.S.S.R. (Yakimov and Miller, 1922; Zasukhin, 1930); Poland (Lorenc, 1939).
Blattella germanica, U.S.A., Ohio (McAdow, 1931).
Blattella germanica and/or Periplaneta americana, Egypt (DeCoursey and Otto, 1956, 1957).
Periplaneta americana, England (Schuster, 1898); Europe (Janicki, 1910); U.S.A. (Kudo, 1926b; McAdow, 1931; Hatcher, 1939; Armer, 1944); Philippine Islands (Hegner and Chu, 1930).
Periplaneta sp., Goa (Mello and Lima Ribeiro, 1924, 1925).
"Küchenschaben," Austria (Bělǎr, 1916).
The protozoan (fig. 2, E) is found in the host's colon, particularly anterior portion; encysted stages of organism are found throughout hind gut. Of 1,400 B. orientalis studied, 32 percent harbored this organism (Kudo, 1925, 1926). Yakimov and Miller (1922) found 7 [Pg 173] percent of 124 B. orientalis infested. Zasukhin (1930) found 10 percent of over 3,000 B. orientalis infested. The flagellate does not harm the host and is never present in the host tissue; it should be considered a commensal (Kudo, 1926).
Synonymy.—Lophomonas sulcata Schuster is most probably identical with L. striata (Kudo, 1926b).
Natural hosts.—Blatta orientalis, Germany (Bütschli, 1878; Schubotz, 1905); Europe (Janicki, 1908, 1910); U.S.A. (Kudo, 1922, 1926, 1926b; McAdow, 1931); U.S.S.R. (Yakimov and Miller, 1922; Zasukhin, 1930); Poland (Lorenc, 1939).
Blattella germanica, U.S.A., Ohio (McAdow, 1931).
Blattella germanica and/or Periplaneta americana, South Africa (Porter, 1930).
Periplaneta americana, Indochina (Weill, 1929); Philippine Islands (Hegner and Chu, 1930); U.S.A. (Kudo, 1926b; McAdow, 1931; Armer, 1944).
Cockroach, Venezuela (Tejera, 1926); England or U.S.A.? (Lucas, 1928).
"Küchenschaben," Austria (Bělǎr, 1916).
Found in the host's colon, particularly the anterior portion. L. striata (fig. 2, D) was found in 29 percent of 1,400 B. orientalis and in 2 of 30 P. americana (Kudo, 1926, 1926b). Yakimov and Miller (1922) found the organism in 9.6 percent of 124 specimens of B. orientalis. Zasukhin (1930) found 8.6 percent of over 3,000 B. orientalis infested.
Grassé (1926, 1926a) identified corrugations on the surface of L. striata as a bacterial parasite which he named Fusiformis lophomonadis.
Natural host.—Cryptocercus punctulatus, California, Oregon, Virginia, West Virginia (Cleveland et al., 1934): Not abundant.
Family HOPLONYMPHIDAE
Natural host.—Cryptocercus punctulatus, U.S.A., Appalachian and Pacific coast areas (Cleveland et al., 1934).
Barbulanympha coahoma (Cleveland et al., 1934) represents the diploid form of B. estaboga (Cleveland, 1953).
Natural host.—Cryptocercus punctulatus, U.S.A., Appalachian and Pacific coast areas (Cleveland et al., 1934): This species, B. ufalula, and Rhynchonympha tarda occur in all parts of the colon, especially in the enlarged, flexed part near the ileum.
Natural host.—Cryptocercus punctulatus, U.S.A., Appalachian and Pacific coast areas (Cleveland et al., 1934).
Natural host.—Cryptocercus punctulatus, U.S.A., Pacific coast area (Cleveland, 1953).
(Fig. 3, D)
Natural host.—Cryptocercus punctulatus, U.S.A., Pacific coast area (Cleveland et al., 1934): Fairly abundant in every specimen examined from Pacific coast.
(Fig. 3, C)
Natural host.—Cryptocercus punctulatus, U.S.A., Appalachian and Pacific coast areas (Cleveland et al., 1934): Present in fairly great numbers in every cockroach examined.
Family STAUROJOENINIDAE
Natural host.—Cryptocercus punctulatus, U.S.A., Appalachian area (Cleveland et al., 1934): Present in only a few specimens.
Family TRICHONYMPHIDAE
Natural host.—Cryptocercus punctulatus, U.S.A., Appalachian and Pacific coast areas (Cleveland et al., 1934).
(Fig. 3, E)
Natural host.—Cryptocercus punctulatus, U.S.A., Appalachian and Pacific coast areas (Cleveland et al., 1934): Fairly abundant and present in most specimens.
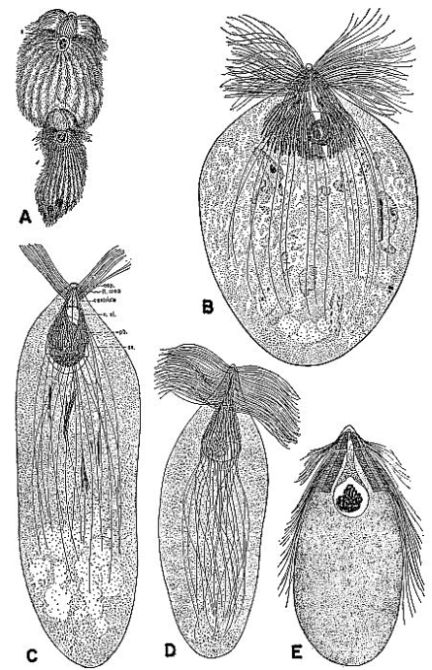 Fig. 3.—Protozoa from the gut of the wood-feeding cockroach Cryptocercus
punctulatus. A, Eucomonympha imla, female above, male below, c. X 375. (From
Cleveland [1950c].) B, Barbulanympha sp. (From Cleveland [1953].) C, Urinympha
talea, c. X 712. (From Cleveland [1951a].) D, Rhynchonympha tarda,
c. X 450. (From Cleveland [1952].) E, Trichonympha okolona or T. algoa, c.
X 390. (From Cleveland [1949].) (All drawings reproduced through the courtesy
of Dr. L. R. Cleveland.)
Fig. 3.—Protozoa from the gut of the wood-feeding cockroach Cryptocercus
punctulatus. A, Eucomonympha imla, female above, male below, c. X 375. (From
Cleveland [1950c].) B, Barbulanympha sp. (From Cleveland [1953].) C, Urinympha
talea, c. X 712. (From Cleveland [1951a].) D, Rhynchonympha tarda,
c. X 450. (From Cleveland [1952].) E, Trichonympha okolona or T. algoa, c.
X 390. (From Cleveland [1949].) (All drawings reproduced through the courtesy
of Dr. L. R. Cleveland.)
Natural host.—Cryptocercus punctulatus, U.S.A., Appalachian and Pacific coast areas (Cleveland et al., 1934).
Natural host.—Cryptocercus punctulatus, U.S.A., Pacific coast areas (Cleveland et al., 1934): Fairly abundant in all specimens from Pacific area.
Natural host.—Cryptocercus punctulatus, U.S.A., Appalachian and Pacific coast areas (Cleveland et al., 1934).
(Fig. 3, E)
Natural host.—Cryptocercus punctulatus, U.S.A., Appalachian and Pacific coast areas (Cleveland et al., 1934): Found in only a few specimens, never abundant.
Natural host.—Cryptocercus punctulatus, U.S.A., Appalachian and Pacific coast areas (Cleveland et al., 1934): This organism is smaller than any known species of Trichonympha; it is more resistant to warm weather than the other hypermastigotes.
Family EUCOMONYMPHIDAE
Natural host.—Cryptocercus punctulatus, U.S.A., Appalachian and Pacific coast areas (Cleveland et al., 1934): Organism (fig. 3, A) sometimes becomes attached to the intestinal wall; attached individuals were seen in 2 to 3 percent of the cockroaches examined.
Natural host.—Pycnoscelus surinamensis, Hawaii (Schwabe, 1950): A small flagellate was found in the digestive tract and malpighian tubules.
Class SARCODINA
Order MYCETOZOA
INCERTAE SEDIS
Synonymy.—Peltomyces blattellae. Sprague (1940a) synonymizes Peltomyces periplanetae, with Coelosporidium periplanetae.
Natural hosts.—Blatta orientalis, France (Debaisieux, 1927).
Blattella germanica, France (Léger, 1909; Debaisieux, 1927).
The organism inhabits the malpighian tubules of cockroaches. Léger and Debaisieux concluded that their organism was a mycetozoan, but they may have erred in synonymizing Plistophora periplanetae with the organism they studied. Debaisieux found intracellular stages of Peltomyces periplanetae that have not been found in Plistophora periplanetae or Coelosporidium periplanetae.
Order AMOEBINA
Family AMOEBIDAE
Natural host.—Blatta orientalis, Yugoslavia (Ivanić, 1937): Found in the hind gut.
Family ENDAMOEBIDAE
In the following classification we have accepted the conclusions of Kirby (1945), Kudo (1954), and others that species of Endamoeba are generically different from species of Entamoeba and that the latter genus is not a homonym of Endamoeba.
Natural vectors.—Blattella germanica and/or Periplaneta americana, Egypt (DeCoursey and Otto, 1956, 1957): Thirty out of 261 cockroaches examined contained this protozoan.
Synonymy.—Amoeba blattae, Entamoeba blattae, Entamoeba blattarum.
Natural hosts.—Blatta orientalis, Germany (Bütschli, 1878; Schubotz, 1905; Chen, 1933); U.S.A. (Leidy, 1879a, 1880; Kudo, 1922, 1925a, 1926a; Kirby, 1927; McAdow, 1931; Meglitsch, 1938, 1940); France (Mercier, 1907a, 1908, 1909, 1910); Europe? (Janicki, 1908, 1909); U.S.S.R. (Yakimov and Miller, 1922; Zasukhin, 1929, 1930); England (Thomson and Lucas, 1926; Lucas, 1927, 1927a, 1928); Yugoslavia (Ivanić, 1926a).
Blattella germanica and/or Periplaneta americana, South Africa (Porter, 1930); Egypt (DeCoursey and Otto, 1956, 1957): Seven out of 217 cockroaches examined harbored the protozoan.
Periplaneta americana, Philippine Islands (Hegner and Chu, 1930); U.S.A. (Morris, 1936; Armer, 1944); Gold Coast Colony (Macfie, 1922).
Periplaneta australasiae, U.S.A. (Morris, 1936).
Cockroaches, Paraguay? (Elmassian, 1909); Austria (Bělǎr, 1916); U.S.A. (Morris, 1935, 1936; Balch, 1932); Venezuela (Tejera, 1926).
The habitat of E. blattae (fig. 2, C) is the hind intestine and rectum of the cockroach. The incidence of infection varies: Kudo (1925a) found in 1,255 oriental cockroaches infections in 5 percent in March and 50 percent in the summer; Schubotz (1905) found 5 to 20 percent of the examined cockroaches to be infested; Yakimov and Miller (1922) found 4 percent of 124 oriental cockroaches infested; Zasukhin (1930) found up to 50 percent of over 3,000 B. orientalis infested; Meglitsch (1938, 1940) found almost 100 percent infection in B. orientalis kept in a crowded culture for several weeks. Chen (1933) developed two synthetic media in which E. blattae could be grown for 45 to 50 days.
Mercier (1907a) observed a fungus, Nucleophaga sp., hyperparasitic in the nucleus of Endamoeba blattae.
Natural hosts.—Panesthia angustipennis, Philippine Islands, and Panesthia spadica, Japan (Kidder, 1937): Occurred in 50 percent of P. angustipennis examined and in one of four P. spadica. The endoplasm of this amoeba contains large amounts of wood and cellulose fibers.
Natural host.—Panesthia angustipennis, Philippine Islands (Kidder, 1937): Occurred in about 10 percent of the Panesthia examined. The food vacuoles contained bacteria, no wood.
Synonymy.—Endamoeba coli, Amoeba coli [Kirby, 1945].
Natural vectors.—Blaberus atropos, Venezuela (Tejera, 1926): In a lot of 60 cockroaches captured in latrines, two were found that carried apparently live cysts similar to cysts of E. coli.
Blattella germanica or Periplaneta americana, Egypt (DeCoursey and Otto, 1956, 1957): One out of 44 cockroaches collected in a village harbored E. coli.
Experimental vector.—Periplaneta americana, Gold Coast Colony (Macfie, 1922): In nine experiments cysts of E. coli were fed to the [Pg 179] cockroaches. In seven of the experiments cysts of E. coli were found in the feces. Cysts were observed in the feces for only one to three days, and eventually disappeared completely. The cysts appeared to be unharmed. No amoebae were found.
Natural vectors.—Blatta orientalis, Blattella germanica, Periplaneta americana, Periplaneta australasiae, and/or Supella supellectilium, Peru (Schneider and Shields, 1947).
Blattella germanica and/or Periplaneta americana, Egypt (DeCoursey and Otto, 1956, 1957).
Cockroaches, Venezuela (Tejera, 1926).
Experimental vectors.—Blatta orientalis, Italian Somaliland (Mariani and Besta, 1936).
Periplaneta americana, Gold Coast Colony (Macfie, 1922); U.S.A. (Frye and Meleney, 1936).
Cockroaches, Venezuela (Tejera, 1926).
Experimental vector.—Periplaneta americana, Formosa (Morischita and Tsuchimochi, 1926): Eleven of 15 cockroaches fed feces of a monkey [Macaca cyclopis (Swinhoe)] containing cysts of the amoeba voided live cysts in their own feces.
Synonymy.—Endamoeba thomsoni [Kudo, personal communication, 1957].
Natural hosts.—Blatta orientalis, England (Lucas, 1927a, 1928); U.S.A. (Taliaferro, 1928; McAdow, 1931); U.S.S.R. (Zasukhin, 1930); Germany (Chen, 1933).
Blattella germanica, U.S.A. (McAdow, 1931).
Periplaneta americana, England (Lucas, 1927a); U.S.A. (Smith and Barret, 1928; McAdow, 1931); Philippine Islands (Hegner and Chu, 1930).
The organism is found in the hind intestine and rectum of the cockroach. Smith and Barret (1928) developed a synthetic medium in which cultures of E. thomsoni were carried through successive transfers for 24 months.
Natural vector.—Periplaneta americana, Gold Coast Colony (Macfie, 1922): Under the heading "Entamoeba histolytica and E. coli" [Pg 180] Macfie (p. 445) stated, "The cockroaches used in these experiments had previously been carefully examined for amoebic infections a precaution which was doubly necessary, because some of these insects at Accra had been found naturally infected."
Experimental vectors.—Periplaneta americana, Gold Coast Colony (Macfie, 1922): Entamoeba, resembling E. coli, from feces of the monkey [Erythrocebus patas patas (Schreber)] were fed to cockroaches, and on the second to fourth days thereafter apparently healthy cysts were recovered in the cockroach feces.
Natural hosts.—Blatta orientalis, England (Lucas, 1927, 1927a); U.S.S.R. (Zasukhin, 1930); Germany (Chen, 1933).
Periplaneta americana, England (Lucas, 1927, 1927a); Indochina (Weill, 1929); U.S.A. (Armer, 1944).
Periplaneta australasiae, U.S.A. (Steinhaus, 1946).
Organism is found in the hind gut of the cockroach. Zasukhin (1930) found 3-percent infestation in over 3,000 B. orientalis examined.
Synonymy.—Entamoeba nana.
Natural host.—Blaberus atropos, Venezuela (Tejera, 1926): A small amoeba greatly resembling E. nana was found in the intestinal contents of the cockroach. In inoculations this amoeba was not pathogenic.
Natural hosts.—Blatta orientalis, U.S.S.R. (Zasukhin, 1930): This organism was found in the hind gut of 0.3 percent of over 3,000 cockroaches examined.
Blattella germanica and/or Periplaneta americana, Egypt (DeCoursey and Otto, 1956, 1957): Seventy-four out of 261 cockroaches examined harbored this protozoan.
Natural vectors.—Blattella garmanica and/or Periplaneta americana, Egypt (DeCoursey and Otto, 1956, 1957): Fifty-nine of 261 cockroaches examined contained this protozoan. Iodamoeba sp. was common in human feces in villages in which the cockroaches were collected.
Natural host.—Panesthia angustipennis, Philippine Islands. (Kidder, 1937): Found in only one specimen.
Class SPOROZOA
Order GREGARINIDA
Family DIPLOCYSTIDAE
(Fig. 2, I)
Natural hosts.—Blatta orientalis, England (Woodcock, 1904; Jameson, 1920).
In body cavity of host. Cysts containing spores are ingested during cannibalistic feeding on infected cockroaches. Sporozoites penetrate the gut wall which later ruptures, freeing the gregarines into the coelom. There is no apparent pathogenic effect. Jameson (1920) found 81 percent of P. americana infested with D. schneideri.
Natural host.—Periplaneta americana, U.S.A. (Hertig, 1921): Heavy infections in body cavity.
Cockroach, India (Ray and Dasgupta, 1955): A large number of cockroaches, both adults and nymphs, collected in Calcutta were all infected.
Natural host.—Blaberus craniifer, U.S.A. (Nutting, 1953): From 1 to 12 or more paired trophozoites or cysts may be found in the hemocoele and occasionally in the thorax.
Family STENOPHORIDAE
Natural host.—Blatta orientalis, India (Bal and Rai, 1955): Organism found in the midgut of the cockroach.
Family GREGARINIDAE
Synonymy.—Gregarina blattae orientalis; Clepsidrina blattarum.
Natural hosts.—Blatta orientalis, Germany (Siebold, 1837, 1839; [Pg 182] Stein, 1848; Bütschli, 1881; Wolters, 1891; Marshall, 1892; Wellmer, 1910, 1911; Foerster, 1938; Schubotz, 1905); U.S.A. (Leidy, 1853a; Crawley, 1903; Watson, 1917; Kudo, 1922; McAdow, 1931; Sprague, 1940, 1941); England (Lankester, 1863); France (Schneider, 1875; Cuénot, 1901; Laveran and Franchini, 1920a); Brazil (Magalhães, 1900; Pinto, 1919); U.S.S.R. (Zasukhin, 1929, 1930).
Blattella germanica, U.S.A. (Crawley, 1903); South Africa (Fantham, 1929; Porter, 1930: these appear to be the same record).
Blattella germanica and/or Periplaneta americana, Egypt (DeCoursey and Otto, 1956, 1957).
Periplaneta americana, Brazil (Magalhães, 1900); U.S.A. (Crawley, 1903; 1907; McAdow, 1931); South Africa (Fantham, 1929; Porter, 1930: these appear to be the same record); Gold Coast Colony (Macfie, 1922).
Parcoblatta pensylvanica, U.S.A., Michigan (Ellis, 1913a).
Cockroaches, Germany (Schiffmann, 1919: probably used the oriental cockroach); Venezuela (Tejera, 1926).
Organism usually found in the intestinal tract of cockroaches where it is attached to the gut cells. Cysts are passed in the feces. Occasionally, G. blattarum (fig. 2, J) is found in the body cavity (Leidy, 1853a; Hall, 1907). Though this is considered to be one of the commonest of the Sporozoa encountered in cockroaches, DeCoursey and Otto (1956) found only 10 of 217 P. americana and B. germanica, collected in restaurants in Egypt, infested with this species. Watson (1917) found a dozen or more in one specimen of Blatta orientalis. Zasukhin (1929, 1930) found 2.6 percent of 3,000 oriental cockroaches infected with this parasite.
Natural host.—Aptera fusca, South Africa (Harrison, 1955): All mature females were heavily infected; in all specimens there were over 100 parasites in the gut. All nymphs were infected, the earlier instars more lightly than the later instars. Gregarines were found in all parts of the gut except the crop and gizzard.
Natural host.—Temnopteryx phalerata, South Africa (Harrison, 1955): Although the cockroaches were found in groups, only 32 percent were infected and only 10 percent heavily. The gregarines were found in the anterior mesenteron but none in the hepatic caeca. All cysts were found in the hind gut or rectum.
Natural host.—Parcoblatta pensylvanica, U.S.A., Illinois (Watson, 1915, 1916): The intestine of one cockroach was found to contain 25 of these gregarines.
Natural host.—Melanosilpha capensis, South Africa (Harrison, 1955): All specimens of this gregarine were found in the anterior mesenteron of the host.
Natural host.—Periplaneta americana, Brazil (Pinto, 1918, 1918a, 1919): Intestinal canal.
Natural host.—Periplaneta americana, Brazil (R. de Almeida Cunha in Pinto, 1919; Cunha, 1919).
Natural host.—Parcoblatta virginica, U.S.A., Ohio (Semans, 1939): The protozoan was present in large numbers in the insect's midgut.
Natural host.—Panchlora exoleta, Argentina (Frenzel, 1892): Midgut.
Natural hosts.—Parcoblatta pensylvanica and Parcoblatta uhleriana, U.S.A., Ohio (Semans, 1939): Midgut.
Natural host.—Leucophaea maderae, Uganda (Watson, 1945): Midgut. Trophozoites could be seen in sections attached to cells of the intestinal wall (fig. 2, H).
Natural host.—Melanosilpha capensis, South Africa (Harrison, 1955): This gregarine was found in the anterior and middle parts of the mesenteron and in the hepatic caeca.
Natural host.—Parcoblatta pensylvanica, U.S.A., Ohio (Semans, 1939): Enteric caeca and midgut.
Synonymy.—Gregarina serpentula [Pinto, 1918a, 1919; Semans, 1943].
Natural host.—Periplaneta americana, Brazil (Magalhães, 1900): In the coelom and alimentary canal. The host of this parasite (fig. 2, K) was incorrectly cited as Blatta orientalis by Watson (1916).
(Fig. 2, L)
Natural hosts.—Ectobius lapponicus, France (Schneider, 1875); Germany (Wellmer, 1910, 1911; Foerster, 1938).
Ectobius pallidus, Germany (Foerster, 1938): In intestine.
Family ACTINOCEPHALIDAE
Synonymy.—Gregarina blaberae [Watson, 1916].
Natural hosts.—Blaptica dubia and related forms, Argentina (Frenzel, 1892): In midgut.
Natural hosts.—Blaberus craniifer, U.S.A. (Roth and Willis, unpublished data, 1953): Possibly Diplocystis sp. (pl. 28, A, B).
Cryptocercus punctulatus, U.S.A. (Cleveland et al., 1934).
Leucophaea maderae, Philippine Islands (Hegner and Chu, 1930): In intestines of host. U.S.A. (Roth and Willis, unpublished data, 1958): Cysts in feces (pl. 28, C).
Gromphadorhina portentosa, U.S.A., in laboratory colony (Roth and Willis, unpublished data, 1958): In intestine of adult female.
Pycnoscelus surinamensis, Hawaii (Schwabe, 1950): A cephaline gregarine was found in the cockroach's digestive tract; it was also claimed to be present in new-born nymphs.
Order COCCIDIA
Family ADELEIDAE
Natural host.—Cryptocercus punctulatus, U.S.A. (Yarwood, 1937): This intracellular parasite was found in the fat body in light [Pg 185] infestations. In heavy infections the coccidia were found in the head, antennae, mouthparts, muscles, legs, salivary glands, nerve cord, as well as fat body. Infection in freshly collected specimens was about 3 percent; when large numbers of cockroaches were kept together in culture, the rate of infection increased because the insects ate their dead companions.
Cleveland et al. (1934) mentioned a coccidium which was sometimes generally distributed through the body (head, legs, antennae, etc.) of C. punctulatus; this parasite was probably the species described by Yarwood.
Order HAPLOSPORIDIA
Natural host.—Blatta orientalis, Yugoslavia (Georgévitch, 1953): This organism was described from the malpighian tubules of the cockroach where it apparently occurred in a mixed infection with the microsporidian Plistophora periplanetae. See synonymy under Plistophora periplanetae.
Synonymy.—Nosema periplanetae, Coelosporidium blattellae, Bertramia blatellae [after Semans, 1943]. Some of the observations cited under Plistophora periplanetae may pertain to C. periplanetae (see Sprague, 1940). See also Haplosporidium periplanetae.
Natural hosts.—Blatta orientalis, U.S.S.R. (Epshtein, 1911); U.S.A. (Kudo, 1922; Sprague, 1940); Yugoslavia (Ivanić, 1926).
Blattella germanica, U.S.A. (Crawley, 1905); Germany (Wellmer, 1910, 1911).
Periplaneta americana, Brazil (Lutz and Splendore, 1903).
This organism (fig. 2, B) passes its life cycle living free in the lumina of the malpighian tubules of cockroaches. The elongate trophozoite is firmly attached to the wall of the tubule as are clusters of immature spores. Mature spores are freed into the lumina of the tubules from whence they pass to the exterior. Sprague (1940) examined about 200 wild-caught B. orientalis and found them to be practically 100 percent infected.
Order MICROSPORIDIA
Family NOSEMATIDAE
Natural host.—Blatta orientalis, U.S.A., Illinois, West Virginia, Kentucky (Sprague and Ramsey, 1941, 1942): Found in the epithelial [Pg 186] cells of caeca and midgut. Considerable damage is done to these cells. Seventy-five percent of 52 B. orientalis harbored the parasite.
Synonymy.—Nosema periplanetae, Pleistophora periplanetae [after Semans, 1943]. Georgévitch (1953) has pointed out that one may find in the malpighian tubules of cockroaches a mixed infection of Microsporidia, Haplosporidia, and Mycetozoa, and that some of the discrepancies in the earlier literature may be attributed to attempts to combine in one organism disparate stages belonging to different orders. See also comments under Coelosporidium periplanetae, Haplosporidium periplanetae, and Peltomyces periplanetae.
Natural hosts.—Blatta orientalis, France (Mercier, 1906a; Debaisieux, 1927); England (Perrin, 1906, 1906b); U.S.S.R. (Zhivago, 1909); Yugoslavia (Georgévitch, 1925, 1926, 1926a, 1927); Germany (Wellmer, 1910, 1911).
Blattella germanica, France (Léger, 1909; Debaisieux, 1927); U.S.S.R. (Zhivago, 1909).
Periplaneta americana, Brazil (Lutz and Splendore, 1903).
This organism lives in the lumen of the malpighian tubules of cockroaches. The cited authors appear to have been convinced that this organism was a microsporidian. Georgévitch (1927, 1953) described the polar capsule and filament characteristic of this order.
Natural host.—Blatta orientalis, France (Mercier, 1908a): The organism parasitized the fat body of the cockroach. Mitoses, often abnormal, were induced in the fat cells. Infected cockroaches were easily recognizable by their distended abdomens. The fat body became chalky white and showed through the intersegmental membranes.
Porter (1930) reported finding an unidentified microsporidian in the fat bodies of Blattella germanica and Periplaneta americana collected in South Africa. It may or may not have been a species of Plistophora.
Class CILIATA
Order HOLOTRICHA
Family PARAMECIIDAE
Natural associate.—Cockroaches, U.S.A., Maryland (Cleveland, 1927): Three of 30 cockroaches collected in the basement of a department [Pg 187] store had paramecia in their stomachs but none in the rectum.
Experimental associate.—Periplaneta americana, U.S.A. (Hegner, 1929): Paramecia fed to the cockroaches were recovered from the crop at intervals from one-half to six and one-half hours. In no case were the protozoa recovered from the stomach alive.
Cockroaches, U.S.A., Maryland (Cleveland, 1927). About 200 starved cockroaches were fed a culture of Paramecium. Few, if any, of the protozoa were killed in the stomach during the first two hours, but all were killed within 5 to 6 hours after ingestion.
Family ISOTRICHIDAE
Natural host.—Periplaneta americana, Indochina (Weill, 1929): Alimentary canal.
Order SPIROTRICHA
Family BURSARIIDAE
Natural host.—Periplaneta americana, India (Ghosh, 1922; Bhatia and Gulati, 1927); Gold Coast Colony (Macfie, 1922): Intestinal tract.
Experimental vector.—Cockroach, Venezuela (Tejera, 1926).
Natural host.—Periplaneta americana, India (Ghosh, 1922a; Bhatia and Gulati, 1927); Indochina, Saigon (Weill, 1929): Intestinal tract.
Natural host.—Blatta orientalis, U.S.A., Illinois (Kudo and Meglitsch, 1938; Meglitsch, 1940): This protozoan is found in the lumen of the anterior region of the colon in association with several other species of protozoa. Only 7.6 percent of 500 cockroaches examined contained B. praenucleatum. The largest number encountered in a single host was 59, but as a rule each host harbored a smaller number.
Natural host.—Periplaneta americana, Brazil (Magalhães, 1900): These organisms were numerous in the intestine.
Family SPIROSTOMIDAE
Natural host.—"Barata sylvestre," Brazil (Pinto, 1926): Organism found in the cockroach's intestine.
Synonymy.—Bursaria blattarum; Plagiotoma blattarum.
Natural hosts.—Blatta orientalis, U.S.A. (Leidy, 1850, 1853, 1853b, 1879a; Kudo, 1922, 1926, 1936; McAdow, 1931; Kudo and Meglitsch, 1938; Meglitsch, 1940); Germany (Stein, 1860; Schubotz, 1905; Chen, 1933); England (Lankester, 1865; Schuster, 1898; Lucas, 1927a, 1928); Spain (Zulueta, 1916); U.S.S.R. (Yakimov and Miller, 1922; Zasukhin, 1928, 1930; Ostroumov, 1929); Portugal (Lima Ribiero, 1924); Brazil (Pinto, 1926); Venezuela (Tejera, 1926).
Blattella germanica, South Africa (Porter, 1930); U.S.A. (Balch, 1932; McAdow, 1931).
Parcoblatta pensylvanica, U.S.A. (Semans, 1939, 1941).
Periplaneta americana, India (Bhatia and Gulati, 1927); Indochina (Weill, 1929); Philippine Islands (Hegner and Chu, 1930); South Africa (Porter, 1930); U.S.A. (McAdow, 1931; Hatcher, 1939; Meglitsch, 1940; Armer, 1944); Goa (Mello et al., 1934); China (Pai and Wang, 1947); Czechoslovakia (Low, 1956).
"Barata sylvestre," Brazil (Pinto, 1926).
"Küchenschaben," Austria (Bělǎr, 1916).
Nyctotherus ovalis (fig. 2, G) inhabits the hind gut of cockroaches, where it occurs almost always in the anterior half of the colon in association with other species of Protozoa (Kudo, 1936). Ninety percent of 500 B. orientalis contained N. ovalis (Kudo and Meglitsch, 1938). Yakimov and Miller (1922) found N. ovalis in 68 percent of 124 B. orientalis. Zasukhin (1930) found this organism in 63 percent of over 3,000 B. orientalis. Zasukhin (1928, 1934) found a fungus and possibly a bacterium hyperparasitic in the cytoplasm of N. ovalis. N. ovalis has been cultured outside the cockroach in several media (Lucas, 1928; Balch, 1932; Chen, 1933; Low, 1956).
Natural hosts.—Panesthia angustipennis, Philippine Islands, and Panesthia spadica, Japan (Kidder, 1937): About 90 percent of all P. angustipennis harbored this ciliate in their hindguts.
Natural host.—"Barata sylvestre," Brazil (Pinto, 1926): In the intestine of the cockroach.
Family CLEVELANDELLIDAE
Most of the Clevelandellidae are parasitized by rod-shaped or spherical bacteria-like organisms usually in clusters (Kidder, 1937).
Synonymy.—Clevelandiidae (Kidder, 1938).
Genus CLEVELANDELLA
Synonymy.—The generic name Clevelandia Kidder (1937) is preoccupied; it was therefore changed to Clevelandella by Kidder in 1938. All of the following species of Clevelandella were originally described as Clevelandia.
Natural hosts.—Panesthia angustipennis, Philippine Islands, and Panesthia spadica, Japan (Kidder, 1937): In the posterior end of hindgut.
Natural hosts.—Panesthia angustipennis, Philippine Islands, and Panesthia spadica, Japan (Kidder, 1937).
Natural host.—Panesthia angustipennis, Philippine Islands (Kidder, 1937).
Natural host.—Panesthia angustipennis, Philippine Islands (Kidder, 1937): Common in hindgut.
Natural host.—Panesthia spadica, Japan (Kidder, 1937).
Natural hosts.—Panesthia angustipennis, Philippine Islands, and Panesthia spadica, Japan (Kidder, 1937): In the hindgut. This protozoan is commonly parasitized by the microorganism Sphaerita.
Natural host.—Panesthia angustipennis, Philippine Islands (Kidder, 1937).
Natural hosts.—Panesthia angustipennis, Philippine Islands, and Panesthia spadica, Japan (Kidder, 1937): Present in 100 percent of P. angustipennis and in nearly all P. spadica.
Natural hosts.—Panesthia angustipennis, Philippine Islands (Kidder, 1937, 1938): Incidence of infection about 50 percent.
Panesthia spadica, Japan (Kidder, 1937).
Natural host.—Pycnoscelus surinamensis, Hawaii (Schwabe, 1950): A large ciliate was found in the digestive tract and malpighian tubules.
In a recent experimental study Schmidtke (1955) failed to demonstrate a host-parasite relationship between Periplaneta americana and the haemosporidian Toxoplasma gondii Nicolle and Manceaux. This protozoan is a blood parasite in a rodent in North Africa (Kudo, 1954).
Intestinal nematodes of the family Thelastomatidae have no apparent pathological effect on their cockroach hosts. Galeb (1878) has shown experimentally that oxyurids eat the same food as the host insect and that if one starves them, by withholding food from the host, the oxyurids die and disappear. In other words, these worms are not parasites, in the sense that we use the term in this paper, but commensals. Dobrovolny and Ackert (1934) stated that "all observations seemed to indicate that the health, fertility and activity of the heavily infested cockroaches were comparable with those of the non-parasitised specimens."
Very few papers have dealt with the ecology of the oxyurid parasites of cockroaches. According to Galeb (1878), usually one species of nematode is found in a single cockroach, but sometimes two species live together in the same host (e.g., in Blatta orientalis and Polyphaga aegyptiaca) where they compete for food. Galeb claimed that Hammerschmidtiella diesingi would replace Leidynema appendiculata; he observed that H. diesingi surpassed L. appendiculata in numbers and the latter became uncommon in the intestines of the cockroaches. On the other hand, Sobolev (1937) found that 48 percent of his oriental [Pg 191] cockroaches were infected with both of the above species of nematodes. The average number of both species was 7.5, and the maximum number was 97; the mean number of H. diesingi was 5.1 and the maximum 64; the mean number of L. appendiculata was 2.4 and the maximum 33. More than 40 nematodes were found in each cockroach of 1.3 percent of those examined. These results apparently contradict Galeb's conclusions inasmuch as the number of each species in mixed infections was essentially the same as the number found in cockroaches infected by only one species (see pp. 195 and 197). Dobrovolny and Ackert (1934) found that 29 percent of 222 Periplaneta americana contained both of the above species of nematodes; whereas 40 percent contained L. appendiculata only, and 21 percent contained H. diesingi only. The infestation ranged from 1 to 36 worms per cockroach with averages of 3.8 per male, 5.1 per female, and 2.7 per nymph.
The eggs of some helminths pass unharmed through the guts of cockroaches that serve as vectors of these ova and have no effect on the insect. However, helminths that are secondary parasites in cockroaches damage the insect to varying degrees depending upon the extent of the infection. Thus the larvae of Moniliformis moniliformis pass through the gut wall and some may become embedded in the fat tissue (Moore, 1946). First stage larvae of Oxyspirura mansoni also burrow through the midgut wall into the fat body; Sanders (1929) believed that Pycnoscelus surinamensis could be killed if at one time a sufficient number of migrating larvae of O. mansoni penetrated the cockroach's intestinal wall. Gongylonema neoplasticum migrates through the digestive tract and encysts in the muscles of the thorax and legs of the host (Fibiger and Ditlevsen, 1914). Infective larvae of Protospirura muricola, after hatching from ingested eggs, pass through the cockroach's gut wall and encyst mainly in the thorax, around the crop, and at the bases of the large muscles of the prothoracic legs (Foster and Johnson, 1939). It is probably generally true that nematodes which are secondary parasites in cockroaches do some damage to the host's intestinal tract at least, and they probably also damage other organs in which they may encyst.
Cockroach tissues may react defensively to infections by parasitic nematodes. For example, encysted third-stage larvae of Physaloptera turgida have been found enclosed in a thin, brown, chitinous substance that was undoubtedly deposited by the tissue of the cockroach (Alicata, 1937). Cysts of similar appearance have been found in cockroaches infected with Physaloptera rara, P. maxillaris, P. hispida (Petri, 1950; Hobmaier, 1941; Schell, 1952), and Gongylonema [Pg 192] pulchrum (Schell, 1952a); in the latter species the deposit eventually completely surrounded the nematode larva which was killed and "chitinized." Apparently these pigmented cysts surround unhealthy or dead larvae and are secreted as a defensive mechanism by the host (Schell, 1952a). Oswald (1958) has reported finding similar pigmented cysts in Blatta orientalis and Periplaneta americana that were experimentally infected with Rictularia coloradensis.
Our classification of the helminths follows Hyman (1951, 1951a).
Phylum ASCHELMINTHES
Class NEMATODA
Order MERMITHOIDEA
Family MERMITHIDAE
Natural Hosts.—Ectobius pallidus, U.S.A., Plymouth, Massachusetts (Roth and Willis, 1957): This mermithid lies coiled in the body cavity of the host and one end may extend into the thorax. Apparently, the host is eventually killed and the worms may leave the cockroach ventrally between the thorax and abdomen (pl. 29, A) or thorax and head.
Periplaneta americana, Germany (Bode, 1936): Attacked by "Mermis" or "Gordius." It has been suggested that the name Mermis is often applied without critical identification to immature Nematoda found in insects (Buxton, 1955).
Order RHABDITOIDEA
Family DIPLOGASTERIDAE
Synonymy.—Lycolaimus [Goodey, 1951].
Experimental Host.—Blattella germanica, U.S.A. (Christie and Crossman, 1933).
Family STEINERNEMATIDAE
Experimental hosts.—Blattella germanica, Nauphoeta cinerea, and Periplaneta americana. U.S.A. (Dutky and Hough, 1955): This nematode, found in codling moth larvae, is close to Neoaplectana chresima [Pg 193] Steiner but apparently is a new species. Nauphoeta cinerea was very susceptible to infection; B. germanica and P. americana were less susceptible.
Order OXYUROIDEA
Family THELASTOMATIDAE
These nematodes are found in the intestinal tract of cockroaches.
Natural host.—Panesthia angustipennis, Philippine Islands (Chitwood and Chitwood, 1934).
Synonymy.—Periplaneticola mirzaia Basir, 1940.
Natural host.—Periplaneta americana, India, Aligarh (Basir, 1940).
Natural host.—Blattella germanica, India, Aligarh (Basir, 1940).
Synonymy.—Oxyuris blattae Graeffe, 1860; Oxyuris blatticola Galeb, 1878; Blatticola blatticola (Galeb, 1877) Schwenck, 1926 [Chitwood, 1930, 1932].
Natural hosts.—Blattella germanica, Brazil (Pessôa and Corrêa, 1926; Schwenck, 1926); U.S.A. (Chitwood, 1930; Bozeman, 1942); Egypt? (Galeb, 1877, 1878); U.S.S.R. (Sobolev, 1937; Sondak, 1935); Czechoslovakia (Groschaft, 1956).
Ectobius lapponicus, Ectobius pallidus, Egypt? (Galeb, 1877, 1878).
Polyphaga aegyptiaca, France (Graeffe, 1860).
The life cycle has been studied by Bozeman (1942): He found never more than four worms in the large intestine of each cockroach. Embryos developed to "resting" stage in vitro. The resting stage was infective while the active stage was not. The worms seemed to have no effect on the vital activities of the host. Alicata (1934b) found that the embryo undergoes a molt before hatching.
Chitwood (1930) found 75 percent of the German cockroaches examined from houses in Washington infected. As a rule, one adult female, one or two males, and possibly two larval females are found in a single individual, apparently only in the rectum.
Sobolev (1937) found 72 percent of Blattella germanica collected in Gorkov (U.S.S.R.) infected with Blatticola blattae. The mean number of worms per host was 1.8, the maximum 5. Sondak (1935) found about 30 percent of 788 B. germanica collected in Leningrad to be infected with B. blattae. Groschaft (1956) regularly found only single worms in B. germanica, collected in a laboratory in Prague, except for two females that contained 2 and 3 worms each.
Synonymy.—Aorurus sphaerolaima (Cobb, 1920) Travassos, 1929. Although Chitwood (1932) indicated that the taxonomic position of this nematode is questionable, Chitwood and Chitwood, 1934, apparently accepted it as a valid species in describing the variety javanica.
Natural host.—Panesthia laevicollis [Cobb recorded the host as Panesthia brevicollis, but no such cockroach exists. Van Zwaluwenburg (1928) and Caudell (in Chitwood, 1932) believed that Cobb meant Panesthia laevicollis. According to Gurney (personal communication, 1957) Caudell's notes show that in 1933 he wrote to Dr. Chitwood and explained that he had compared Cobb's figure of the cockroach with laevicollis Saussure (figures and description) and had found them the same.] Australia, New South Wales (Cobb, 1920).
Natural host.—Panesthia angustipennis, Philippine Islands (Chitwood and Chitwood, 1934).
Natural host.—Supella supellectilium, India, Aligarh (Basir, 1941).
Synonymy.—Thelastoma brevicaudatum Leidy, 1851 [Christie, 1933]. Thelastoma indiana Basir, 1940 [Basir, 1949].
Natural host.—Leucophaea sp., India, Aligarh (Basir, 1940, 1949).
Synonymy.—Bulhỡesi magalhāesi Schwenck, 1926; Thelastoma magalhāesi (Schwenck, 1926) Travassos, 1929 [Basir, 1956].
Natural host.—"Barata selvagem," Brazil, São Paulo (Schwenck, 1926).
Natural host.—Eurycotis floridana, U.S.A., Florida (Chitwood, 1932).
Synonymy.—Oxyuris aegyptiaca Galeb, 1878; Blatticola aegyptiaca (Galeb, 1878) Schwenck, 1926 [Chitwood, 1932].
Natural hosts.—Blattella germanica, Brazil (Schwenck, 1926).
Polyphaga aegyptiaca, Egypt? (Galeb, 1878).
Synonymy.—Anguillula macrura Diesing, 1851; Aorurus diesingi (Hammerschmidt, 1838) Travassos, 1929; Streptostomum gracile Leidy, 1850; Oxyuris diesingi Hammerschmidt, 1838; Oxyuris blattae orientalis Hammerschmidt, 1838 [Chitwood, 1932]. Oxyuris macrura of Lankester (1865).
Natural hosts.—Blatta orientalis, Europe (Hammerschmidt 1838, 1847; Bütschli, 1871); Egypt? (Galeb, 1878); England (Lankester, 1865; Lee, 1958); U.S.A. (Leidy, 1850a); U.S.S.R. (Yakimov and Miller, 1922; Sobolev, 1937; Sondak, 1935); Brazil (Travassos, 1929); China (Chitwood, 1932); Czechoslovakia (Groschaft, 1956).
Leucophaea maderae, Brazil (Pessôa and Corrêa, 1926).
Periplaneta americana, Brazil (Magalhães, 1900; Pessôa and Corrêa, 1926). U.S.A.: Texas (Todd, 1943); Kansas (Dobrovolny, 1933; Dobrovolny and Ackert, 1934); North Carolina (Hatcher, 1939); Iowa, North Dakota, Michigan (Hoffman, 1953). China (Chitwood, 1932). India (Basir, 1940). Czechoslovakia (Groschaft, 1956). England (Lee, 1958).
Periplaneta australasiae, Brazil (Pessôa and Corrêa, 1926).
Polyphaga aegyptiaca (Linstow, 1878).
Cockroaches (Blatta orientalis, Blattella germanica, and/or Periplaneta americana), U.S.A. (McAdow, 1931).
Cockroach, Venezuela (Tejera, 1926).
According to Hammerschmidt (1847) this worm may be found throughout the intestinal canal but especially in the small intestine. It is frequently found in adults and seldom in the nymphs. There were seldom more than 5 to 10 worms in one cockroach and female worms were found more frequently than males; the male worms were found only in winter and spring while the females were present at all times of the year. Bütschli (1871) stated that all stages from those just hatching to mature males and females are found.
Yakimov and Miller (1922) found H. diesingi in 50.8 percent of 124 B. orientalis collected in Petrograd. Sobolev (1937) found 96 percent of B. orientalis infected with H. diesingi with a mean number of 5.6 and maximum number of 22 in one cockroach. Groschaft (1956) found 18 in one specimen of B. orientalis. Dobrovolny and [Pg 196] Ackert (1934) found about 50 percent of 222 P. americana infected with H. diesingi. Sondak (1935) found about 36 percent of 412 B. orientalis infected with either or both H. diesingi and Leidynema appendiculata.
Two molts occur during development of the eggs; the first takes place outside the host resulting in a resting or infective stage. After the egg in the infective stage is eaten by the host, the second molt occurs before the egg hatches. Completion of the second molt and hatching perhaps are connected with ammonia present in the digestive tract; the ammonia seems to arise from the bacteria present in the gut. There appears to be a relationship between the intestinal bacteria of the cockroach and development and hatching of nematode eggs (Todd, 1944).
At the time of oviposition the nematode eggs are in the very earliest stages of cleavage. In 36 hours a motile, tadpole-like stage is reached and in a few days the embryo becomes quiescent and nonmotile. This nonmotile stage is infective whereas the motile embryonic stage is not. Feeding experiments proved that transmission of the nematode is direct. The worm reaches sexual maturity in 20 or 30 days after being ingested by the cockroach (Dobrovolny, 1933).
The bacterium Streptomyces leidynematis Hoffman grows on the cuticle of H. diesingi (Hoffman, 1953). The bacterium apparently is only anchored to the nematode and probably obtains its food from the intestinal contents of the cockroach. See notes under Leidynema appendiculata.
Synonymy.—Hammerschmidtiella neyrae Serrano Sánchez, 1947. [According to M. B. Chitwood, personal communication, 1957, Serrano Sánchez's emendation is apparently an error.]
Natural host.—Blatta orientalis, Spain, Grenada (Serrano Sánchez, 1947): Of 2,943 specimens examined, 1,143 were parasitized by oxyurids and of these 45 percent contained H. neyrai.
Synonymy.—Oxyuris blattae orientalis Hammerschmidt, 1847, of Bütschli, 1871, and Oxyuris blattae-orientalis of Magalhães, 1900; Oxyuris blattae Hammerschmidt, 1847, of Galeb, 1878; Aorurus (Thelastoma) appendiculatus Leidy, 1850. [Chitwood, 1932.] Serrano Sánchez (1947) has divided this species into three geographical varieties as follows: L. appendiculata (Leidy, 1852) (Dobrovolny [Pg 197] and Ackert, 1934) var. indiana; L. appendiculata (Leidy, 1852) (Chitwood, 1932) var. americana; L. appendiculata (Serrano Sánchez, 1947) var. hispana. However, Basir (1956) does not recognize these varieties. The Russians recognize hispana (M. B. Chitwood, personal communication, 1957).
Natural hosts.—Blaberus atropos, South America (Chitwood, 1932).
Blatta orientalis, Egypt? (Galeb, 1878); Europe (Bütschli, 1871); U.S.S.R. (Sobolev, 1937; Sondak, 1935); U.S.A., Nebraska (Todd, 1944); Spain (Serrano Sánchez, 1947): Recorded as var. hispana. Czechoslovakia (Groschaft, 1956). England (Lee, 1958a).
Blatta orientalis or Periplaneta americana, Brazil (Magalhães, 1900).
Periplaneta americana, U.S.A.: Texas (Todd, 1943); Nebraska (Todd, 1944); Kansas (Dobrovolny, 1933; Dobrovolny and Ackert, 1934); North Carolina (Hatcher, 1939); Iowa, North Dakota, Michigan (Hoffman, 1953). Czechoslovakia (Groschaft, 1956). England (Lee, 1958a).
Cockroach, Venezuela (Tejera, 1926).
Cockroaches (Blatta orientalis, Blattella germanica, and/or Periplaneta americana), U.S.A. (McAdow, 1931).
Chitwood (1932) also listed China for distribution of the worm, but we could not tell which host was involved.
The worms are found in the colon and rectum of the host. Galeb (1878) found as many as 20 individuals in a single B. orientalis. Sobolev (1937) found 52 percent of B. orientalis infected with L. appendiculata; the mean number of worms per roach was 1.5 and the maximum 2. Dobrovolny and Ackert (1934) found 69 percent of 222 P. americana infected with this species.
Two molts occur within the egg during development of the larva. The first molt occurs outside the host resulting in the formation of an infective resting stage. The second molt occurs inside the cockroach (Todd, 1941, 1944).
Transmission of the nematode is direct, eggs in the resting embryonated stage being infective (Dobrovolny and Ackert, 1934).
Hoffman (1953) described a filamentous bacterium, Streptomyces leidynematis Hoffman, which grows on the cuticle of L. appendiculata in P. americana. Leidy (1853) noted the presence of simple, inarticulate, amorphous filaments, growing from nematodes infecting B. orientalis. Bütschli (1871) and Magalhães (1900) described similar filaments adhering to the surface of oxyurids from cockroaches.
Natural host.—Eurycotis floridana, U.S.A., Massachusetts (Roth and Willis, unpublished data, 1955): Determined by Dr. G. Steiner who wrote us, "In Eurycotis floridana there were ten specimens of the nematode Leidynema appendiculata (Leidy, 1850). This cockroach is obviously a new host for this nematode. I am not sure that the nematode exactly agrees with the description as given in the literature."
Natural hosts.—Blaberus craniifer, U.S.A., Florida (Chitwood, 1932); Massachusetts (Roth and Willis, unpublished data, 1955). Determined by Dr. G. Steiner.
Blaberus atropos?, U.S.A., Florida (Chitwood, 1932): B. craniifer has generally been recorded as B. atropos of Stoll which is a closely related but distinct South American species (Rehn and Hebard, 1927).
Natural host.—Leucophaea maderae, Cuba, Havana (Chitwood, 1932).
Natural host.—Panesthia angustipennis, Philippine Islands (Chitwood and Chitwood, 1934).
Natural hosts.—Panesthia laevicollis?, Philippine Islands (Cobb in Chitwood and Chitwood, 1934).
Panesthia angustipennis, Philippine Islands (Chitwood and Chitwood, 1934).
Synonymy.—Oxyuris panesthiae Galeb, 1878, in part; Thelastoma panesthiae (Galeb, 1878) Travassos, 1929. [Chitwood, 1932; Chitwood and Chitwood, 1934.]
Natural host.—Panesthia sp., New Guinea (Galeb, 1878): About 40 nematodes may be found in one insect.
Natural host.—Panesthia angustipennis, Philippine Islands (Chitwood and Chitwood, 1934).
Synonymy.—Thelastoma heterogamiae (Galeb, 1878) Travassos, 1929. The taxonomic position of this species is questionable; it might possibly belong in Blatticola or Protrellina (Chitwood, 1932). Basir (1956) placed it in an appendix to the family Thelastomatidae.
Natural host.—Polyphaga aegyptiaca, Egypt? (Galeb, 1878).
Natural host.—Blaberus craniifer, U.S.A., Florida (Chitwood, 1932).
Synonymy.—The taxonomic position of this nematode is questionable (Chitwood, 1932).
Natural host.—Polyzosteria melanaria?, Australia, New South Wales (Cobb, 1920). [Caudell (in Chitwood, 1932) stated that this host was probably Platyzosteria analis.]
Synonymy.—Protrellina aurifluus Chitwood, 1932.
Natural hosts.—Parcoblatta lata, U.S.A., North Carolina, Maryland (Chitwood, 1932).
Parcoblatta uhleriana, North Carolina (Hatcher, 1939).
Synonymy.—Oxyuris australasiae Pessôa and Corrêa, 1926; Protrellina australasiae (Pessôa and Corrêa, 1926) Chitwood, 1932 [Chitwood, 1933].
Natural host.—Periplaneta australasiae, Brazil (Pessôa and Corrêa, 1926, 1927).
Synonymy.—Protrellina galebi (Schwenck, 1926) Chitwood, 1932 [Chitwood, 1933].
Natural host.—"Barata selvagem," Brazil (Schwenck, 1926).
Synonymy.—Oxyuris künckeli Galeb, 1878; Protrellina künckeli (Galeb, 1878) Chitwood, 1932 [Chitwood, 1933].
Natural hosts.—Periplaneta americana, Egypt? (Galeb, 1877, 1878.) [Chitwood (1932) questioned the determination of this host [Pg 200] because he failed to find this nematode in a large number of specimens from U.S.A. and China.] Brazil (Pessôa and Corrêa, 1926).
Periplaneta australasiae, Brazil (Pessôa and Corrêa, 1926).
Synonymy.—Protrellina manni Chitwood, 1932.
Natural host.—Aglaopteryx diaphana, Cuba (Chitwood, 1932).
Synonymy.—Protrellina phyllodromi Basir, 1942.
Natural host.—Blattella humbertiana, India, Aligarh (Basir, 1942): Found in the rectum.
Natural host.—Eurycotis floridana, U.S.A., Florida (Chitwood, 1932).
Synonymy.—Bulhõesia icemi Schwenck, 1926; Thelastoma icemi (Schwenck, 1926) Travassos, 1929; Thelastoma aligarhica Basir, 1940. [Basir, 1956.]
Natural hosts.—"Barata selvagem," Brazil, São Paulo (Schwenck, 1926).
Periplaneta americana, India, Aligarh (Basir, 1940); U.S.A., Nebraska (Todd, 1943).
Periplaneta brunnea, U.S.A., Louisiana (Todd, 1943).
Natural host.—"Blattidae sylvestres," Brazil (Pereira, 1935).
Synonymy.—Bulhõesia severianoi Schwenck, 1926 [Travassos, 1929].
Natural hosts.—"Baratas de pau podre," Brazil (Schwenck, 1926).
Pycnoscelus surinamensis, U.S.A., Florida (Chitwood, 1932).
Natural host.—Steleopyga? sinensis, Asia: Suifu, Szchuen, China (Chitwood, 1932).
Synonymy.—Oxyuris bulhõesi de Magalhães, 1900; Bulhõesia bulhõesi (Magalhães, 1900) Schwenck, 1926 [Travassos, 1929; Chitwood, [Pg 201] 1932]; Thelastoma bulhõesi (Magalhães, 1900) Travassos, 1929; although this last combination (from Chitwood, 1932) is not given by Basir (1956), it is implied by the synonymy that he does cite under T. pachyjuli.
Natural hosts.—Blatta orientalis, Czechoslovakia (Groschaft, 1956).
Periplaneta americana, Brazil (Magalhães, 1900); North America (Chitwood, 1932); U.S.A., North Carolina (Hatcher, 1939).
Natural host.—Panesthia angustipennis, Philippine Islands (Chitwood and Chitwood, 1934).
Natural host.—Periplaneta sp., Cuba (Chitwood, 1932).
Natural host.—Cutilia sp. near sedilloti, U.S.A. (hosts imported from New Zealand) (Roth, unpublished data, 1957).
Class NEMATOMORPHA
Order GORDIOIDEA
The immature stages of the following gordian worms have been found in the body cavity of cockroaches.
Family CHORDODIDAE
Synonymy.—Chordotes [sic] puerilis Montgomery, 1898 [Ward, 1918].
Natural host.—Cockroach, U.S.A. (Montgomery, 1898); Pennsylvania, Maryland, Michigan, Ohio, Florida, Iowa, Nebraska (Ward, 1918).
Family GORDIIDAE
Natural host.—Blatta sp., U.S.A. (Stiles and Hassall, 1894).
Leidy (1879) identified a 9-inch-long nematode which came from a cockroach (Blatta orientalis?) as probably being Gordius aquaticus. [Pg 202] Ransom (in Pierce, 1921) states that G. aquaticus may be an accidental parasite of man. Faust (1955) summarizes the few reported cases of human parasitism. Dorier (1930) reported that the regurgitated liquid of Blatta orientalis had no effect on cysts of G. aquaticus after one hour.
Synonymy.—Gordius orientalis of Lankester (1865).
Natural host.—Blatta orientalis, Germany (Siebold, 1842; Linstow, 1878): Found in abdomen. Von Siebold called this "Filarien" but did not otherwise name or describe the worm.
Synonymy.—Chordodes pilosus Möbius, 1855 [Diesing, 1861.]
Natural host.—Blaberus giganteus, Venezuela (Möbius, 1855): From the insect's abdomen.
Natural hosts.—Periplaneta americana, South Africa (Porter, 1930); Germany (Bode, 1936): Bode's record may have referred to a Mermis or other nematode.
Cockroaches, Venezuela (Miall and Denny, 1886; Burr, 1899a; Tejera, 1926).
Synonymy.—Gordius raphaelis Camerano, 1893 [Camerano, 1897].
Natural hosts.—Symploce parenthesis and Kuchinga hemerobina, French Equatorial Africa (Camerano, 1893, 1897).
Natural hosts.—Eurycotis floridana, Florida (T. Eisner, personal communication, 1958): See plate 29, B.
Parahormetica bilobata, Brazil (Pessôa and Corrêa, 1929): Worm referred to as "gordiaceo."
Cockroaches, Australia (E. F. Riek, personal communication, 1953): Three undescribed gordian worms were found in undetermined cockroaches of the subfamily Blattinae.
The use of the asterisk (*) is explained in footnote 3, page 4.
Phylum ACANTHOCEPHALA
Order ARCHIACANTHOCEPHALA
Family OLIGACANTHORHYNCHIDAE
Natural host.—Blattella germanica, France (Brumpt and Urbain, 1938, 1938a; Brumpt et al., 1939).
Experimental hosts.—Blaberus atropos and Leucophaea maderae, France (Brumpt and Desportes, 1938).
Natural host.—Blattella germanica, France (Brumpt and Urbain, 1938, 1938a; Brumpt et al., 1939); Netherlands (Thiel and Wiegand Bruss, 1946).
Experimental hosts.—Blattella germanica, Netherlands (Thiel and Wiegand Bruss, 1946).
Blaberus atropos and Leucophaea maderae, France (Brumpt and Desportes, 1938).
Family MONILIFORMIDAE
Natural hosts.—Periplaneta americana, Brazil (Magalhães, 1898; Travassos, 1917); Gold Coast (Southwell, 1922); India (Pujatti, 1950); U.S.A. (Burlingame and Chandler, 1941; Moore, 1946).
Periplaneta australasiae, India (Pujatti, 1950).
Experimental hosts.—Blattella germanica, Japan (Yamaguti and Miyata, 1942).
Periplaneta americana, U.S.A. (Chandler, 1941; Moore, 1946); Japan (Yamaguti and Miyata, 1942).
Natural host.—Blattella germanica, India (Meyer, 1931, 1932).
Natural hosts.—Periplaneta americana, Argentina (Bacigalupo, 1927, 1927a, 1928); Brazil (Pessôa and Corrêa, 1929); Algeria (Seurat, 1912); Burma (Subramanian, 1927); South Africa (Porter, 1930); Madras (Sita, 1949).
Periplaneta spp., New Caledonia (Rageau, 1956).
Cockroaches, Venezuela (Tejera, 1926).
Experimental hosts.—Blaberus atropos, Blatta orientalis, Blattella germanica, Leucophaea maderae, France (Brumpt and Urbain, 1938a).
Periplaneta americana, Japan (Yamaguti and Miyata, 1942); France (Brumpt, 1949); Madras (Sita, 1949).
Phylum ASCHELMINTHES
Class NEMATODA
Order OXYUROIDEA
Family SUBULURIDAE
Synonymy.—Subulura jacchi (Marcel, 1857) [Dr. J. T. Lucker, personal communication, 1957].
Experimental host.—Blaberus atropos, France (Chabaud and Larivière, 1955).
Order SPIRUROIDEA
Family THELAZIIDAE
Natural hosts.—Pycnoscelus surinamensis, Australia (Fielding, 1926, 1927, 1928, 1928a); U.S.A. (Sanders, 1927, 1928, 1929; Shealy, 1927); Formosa (Kobayashi, 1927); Antigua (Hutson, 1938, 1943); Hawaii (Illingworth, 1931; Schwabe, 1950, 1950a, 1950b, 1951); New Caledonia (Rageau, 1956).
We have recently found (Roth and Willis, 1960) that two strains of Pycnoscelus surinamensis exist; a parthenogenetic strain (from Florida), and a bisexual strain (from Hawaii) which does not reproduce parthenogenetically. The parthenogenetic strain is undoubtedly the form that has been shown to be the host of O. mansoni in the United States and Antigua, because only this form is found in the New World. Probably the parthenogenetic strain was the form involved in most Pacific areas. However, from internal evidence in his [Pg 205] papers, we concluded that Schwabe, in Hawaii, may well have been working with the bisexual strain and possibly also with the parthenogenetic strain; if this is true, then both parthenogenetic and bisexual strains of Pycnoscelus surinamensis may serve as intermediate hosts of the eyeworm.
Experimental hosts.—Periplaneta americana, Antigua (Hutson, 1943).
Pycnoscelus surinamensis, U.S.A. (Sanders, 1929); Australia (Fielding, 1927, 1928a); Hawaii (Schwabe, 1951).
Natural hosts.—Parcoblatta pensylvanica and Parcoblatta virginica, U.S.A., Ohio (Oswald, 1958): Of 49 wood roaches collected, one of each species contained a single larva each.
Experimental hosts.—Blatta orientalis, Blattella germanica, Parcoblatta pensylvanica, Parcoblatta virginica, Periplaneta americana, and Supella supellectilium, U.S.A. (Oswald, 1958): The larvae underwent normal development in all species of cockroaches except B. orientalis and P. americana in which cysts developed that contained a reddish-brown pigment; larvae in such cysts were dead or dying. Eggs of R. coloradensis hatched in the midgut of B. germanica and first-stage larvae entered the hindgut epithelium within 24 hours. The larvae underwent two molts within a cyst formed by tissues of the host's gut, the second molt occurring during the twelfth or thirteenth day. In Parcoblatta, cysts were found free in the body cavity as well as attached to the hindgut. In B. germanica and S. supellectilium the cysts remained attached to the hindgut. Usually over 20 cysts developed in each infected Parcoblatta; fewer than 10 per insect developed in the other species. Larvae became infective to the definitive host, the white-footed mouse [Peromyscus leucopus noveboracensis (Fischer)], as early as the tenth day.
Family SPIRURIDAE
Natural host.—Parahormetica bilobata, Brazil (Pessôa and Corrêa, 1929).
Experimental host.—Blattella germanica, U.S.A. (Cram, 1935).
Natural hosts.—Blatta orientalis, Netherlands (Baylis, 1925).
Blattella germanica, U.S.A. (Hitchcock and Bell, 1952).
Periplaneta americana, Denmark and St. Croix (Fibiger, 1913, 1913a; Fibiger and Ditlevsen, 1914); Netherlands (Baylis, 1925); Argentina (Bacigalupo, 1930); England (Leiper, 1926); South Africa (Porter, 1930); U.S.A. (Hitchcock and Bell, 1952); Formosa (Yokagawa, 1924, 1925, 1925a).
Periplaneta australasiae, Formosa (Yokagawa, 1924, 1925, 1925a).
Experimental hosts.—Blattella germanica, Denmark (Fibiger and Ditlevsen, 1914); U.S.A. (Hitchcock and Bell, 1952); France (Brumpt, 1949).
Blatta orientalis, Denmark (Fibiger and Ditlevsen, 1914).
Periplaneta americana, Denmark and St. Croix (Fibiger, 1913; Fibiger and Ditlevsen, 1914); U.S.A. (Hitchcock and Bell, 1952).
Experimental hosts.—Blattella germanica, U.S.A. (Ransom and Hall, 1915, 1916, 1917; Stiles and Baker, 1927; Schwartz and Lucker, 1931; Lucker, 1932; Alicata, 1934a, 1935); Europe (Baylis et al., 1925, 1926, 1926a; Sambon, 1926).
Parcoblatta sp., Alicata (1934, 1935).
Natural host.—Periplaneta americana, Brazil (Magalhães, 1900); Algeria (Seurat, 1916); England? (Leiper, 1926).
Experimental host.—Blattella germanica, U.S.A. (Cram, 1934).
Natural host.—Leucophaea maderae, Venezuela (Brumpt, 1931).
Experimental hosts.—Blatta orientalis, Blattella germanica, Leucophaea maderae, France (Brumpt, 1931).
Experimental host.—Blattella germanica, U.S.A. (Cram, 1926).
Natural host.—Leucophaea maderae, Panama (Foster and Johnson, 1938, 1939).
Experimental host.—Blattella germanica, U.S.A. (Cram, 1931, 1931a, 1933a).
Natural hosts.—Blatta orientalis, Europe? (Deslongchamps, 1824, in Seurat, 1911); Italy (Grassi, 1888); Algeria (Seurat, 1916).
Periplaneta americana, Brazil (Pessôa and Corrêa, 1929).
Cockroach, Venezuela (Tejera, 1926).
Experimental hosts.—Blatta orientalis, France (Galeb, 1878a); "Cafards," Algeria (Roger, 1906, 1907).
Natural host.—Blattella germanica, U.S.A. (Cram, 1931b, personal communication, 1956); Hawaii (Alicata, 1938, 1947).
Experimental host.—Blattella germanica, U.S.A. (Cram, 1931b).
Experimental host.—Blattella germanica, U.S.A. (Cram, 1933).
Family PHYSALOPTERIDAE
Experimental host.—Blattella germanica, U.S.A. (Schell, 1952, 1952a).
Experimental host.—Blattella germanica, U.S.A. (Hobmaier, 1941).
Experimental host.—Blattella germanica, U.S.A. (Petri and Ameel, 1950).
Experimental host.—Blattella germanica, U.S.A. (Petri and Ameel, 1950; Petri, 1950).
Experimental host.—Blattella germanica, U.S.A. (Alicata, 1937; Schell, 1952).
The use of the asterisk (*) is explained in footnote 3, page 4.
Phylum PLATYHELMINTHES
Class TREMATODA
Order DIGENEA
Family SCHISTOSOMATIDAE
Experimental vector.—Periplaneta americana, Gold Coast Colony (Macfie, 1922).
Class CESTODA
Order TAENIOIDEA
Family HYMENOLEPIDIDAE
Natural vectors.—Periplaneta americana, Formosa (Morischita and Tsuchimochi, 1926).
Polyphaga saussurei, Tadzhikistan (Zmeev, 1936).
Family TAENIIDAE
Experimental vector.—Periplaneta americana, Gold Coast Colony (Macfie, 1922).
Synonymy.—Taenia echinococcus (Zeder, 1803) [Faust, 1939].
Experimental vector.—Periplaneta americana, Uruguay (Pérez Fontana, 1955): Eggs were recovered from the feces of artificially infested cockroaches under "natural" conditions.
Family Unknown
Natural vector.—Polyphaga saussurei, Tadzhikistan (Zmeev, 1936).
Phylum ASCHELMINTHES
Class NEMATODA
Order OXYUROIDEA
Family OXYURIDAE
Natural vectors.—Blatta orientalis and Blattella germanica, U.S.S.R. (Sondak, 1935).
Order ASCAROIDEA
Family ASCARIDAE
Natural vector.—Periplaneta americana, South Africa (Porter, 1930): The eggs may have been those of A. suum Goeze, 1782.
Experimental vectors.—Periplaneta americana, Gold Coast Colony (Macfie, 1922); India (Chandler, 1926).
Periplaneta americana, Periplaneta australasiae, Neostylopyga rhombifolia, Formosa (Morischita and Tsuchimochi, 1926).
Natural vector.—Blatta orientalis, Italian Somaliland (Mariani and Besta, 1936).
Experimental vector.—Periplaneta americana, Uruguay (Pérez Fontana, 1955): Eggs recovered from the insects' feces.
Order STRONGYLOIDEA
Family ANCYLOSTOMIDAE
Experimental vector.—Periplaneta americana, Netherlands (Akkerman, 1933).
Experimental vector.—Periplaneta americana, Gold Coast Colony (Macfie, 1922); Netherlands (Akkerman, 1933).
Natural vector.—Periplaneta americana, South Africa (Porter, 1929, 1930).
Experimental vector.—Periplaneta americana, Gold Coast Colony (Macfie, 1922).
Natural vector.—Periplaneta americana, India (Chandler, 1926).
Experimental vector.—Periplaneta americana, Gold Coast Colony (Macfie, 1922).
Experimental vectors.—Periplaneta americana, Periplaneta australasiae, Neostylopyga rhombifolia, Formosa (Morischita and Tsuchimochi, 1926).
Family TRICHOSTRONGYLIDAE
Natural vector.—Blatta orientalis, Italian Somaliland (Mariani and Besta, 1936).
Order TRICHUROIDEA
Family TRICHURIDAE
Experimental vector.—Blatta orientalis, Italy? (Giordano, 1950).
Natural vectors.—Blatta orientalis, Italian Somaliland (Mariani and Besta, 1936); U.S.S.R. (Sondak, 1935).
Blattella germanica, U.S.S.R. (Sondak, 1935).
Periplaneta americana, Gold Coast Colony (Macfie, 1922); Formosa (Morischita and Tsuchimochi, 1926).
Experimental vectors.—Periplaneta americana, Gold Coast Colony (Macfie, 1922); India (Chandler, 1926); Uruguay (Pérez Fontana, 1955).
Periplaneta americana, Periplaneta australasiae, and Neostylopyga rhombifolia, Formosa (Morischita and Tsuchimochi, 1926).
The classification follows Brues et al. (1954) with the following exceptions. The Acarina are arranged according to Dr. J. H. Camin (personal communication, 1955). Family Eupelmidae of the Hymenoptera follows the classification of Peck (1951).
Class ARACHNIDA
In this class, representatives of at least four orders have utilized cockroaches as food: the whip scorpions, scorpions, spiders, and mites. Apparently none of these feed exclusively on cockroaches, but the Philippine forest scorpion Heterometrus (=Palamnaeus) longemanus seems to prefer blattids to other insects (Schultze, 1927).
Order PEDIPALPIDA
Family THELYPHONIDAE
Synonymy.—Thelyphonus giganteus Lucas [Dr. R. E. Crabill, personal communication, 1958].
Experimental prey.—Cockroaches, U.S.A. (Marx, 1892, 1894): Immature whip scorpion captured and fed on one or two cockroaches a week. It lived on this diet for about two years.
Common name.—Whip scorpion.
Experimental prey.—Blattella germanica, U.S.A., Florida [Dr. B. J. Kaston, personal communication, 1953].
Order SCORPIONIDA
Pocock (1893) noticed that a scorpion whose pectines had come in contact with a cockroach immediately turned back and ate the insect. He concluded that the scorpion detected the cockroach by means of the pectines. However, Cloudsley-Thompson (1955) has demonstrated that the main function of the pectines is probably the detection of ground vibrations. He accounted for Pocock's observation by the presence of sensory spines (presumably tactile) which project from beneath the pectines. In a house in Arizona, Lyon (1951) observed over 60 scorpions living in a kitchen cabinet that enclosed a sink. They were apparently thriving on a heavy infestation of cockroaches. Stahnke (1953) stated that he used Periplaneta americana as the principal food for scorpions at the Poisonous Animals Research Laboratory of Arizona State College. Cloudsley-Thompson (1955a) cited cockroaches as one of the arthropods that scorpions feed upon.
Family BUTHIDAE
Synonymy.—Androctonus australis [Crabill, personal communication, 1957].
Experimental prey.—Cockroaches, England (Cloudsley-Thompson, 1955a): This African species ate at least one cockroach per week during the summer months. It can, however, survive four months' starvation and is particularly adapted to a dry climate (Cloudsley-Thompson, personal communication, 1956).
Experimental prey.—Periplaneta americana, U.S.A. (Roth, unpublished data, 1953): Scorpion collected in Florida by Roth and identified by Dr. M. H. Muma.
Experimental prey.—Periplaneta australasiae and Pycnoscelus surinamensis, U.S.A. (Muma, personal communication, 1953): This scorpion occurs in large numbers in the Florida citrus groves, together with P. australasiae which is probably an important natural prey.
Natural prey.—Parcoblatta pensylvanica (?), U.S.A., Florida (Muma, personal communication, 1953). This may have been another species of this genus, possibly P. divisa, as P. pensylvanica is not known from Florida (Rehn, personal communication, 1958).
Experimental prey.—Blatta orientalis, Blattella germanica, Periplaneta americana, and Pycnoscelus surinamensis, U.S.A., Florida (Muma, personal communication, 1953).
Experimental prey.—"Common house-cockroach" (Pocock, 1893): The scorpions were collected in Cape Town, South Africa.
Family CHACTIDAE
Synonymy.—Euscorpius carpathicus [Cloudsley-Thompson, 1955a].
Experimental prey.—Blattella germanica, England? (Pocock, 1893).
Periplaneta americana, nymphs, England? (Cloudsley-Thompson, personal communication, 1956): This scorpion is found naturally in southern Europe and North Africa.
Experimental prey.—Cockroaches including nymphs of Periplaneta, England? (Cloudsley-Thompson, 1951): The cockroaches had to be disabled before the scorpion would feed on them. Prey is apparently detected by tactile and auditory senses, sight being poorly developed and not used. The scorpion is found in southern Europe and North Africa.
Family VEJOVIDAE
Experimental prey.—Periplaneta americana, U.S.A. (Stahnke, 1949): This record is a photograph showing the scorpion eating the cockroach.
Family ISCHNURIDAE
Experimental prey.—Cockroach, Australia (McKeown, 1952): This record is a photograph showing the scorpion feeding on a cockroach.
Family SCORPIONIDAE
Synonymy.—Palamnaeus longimanus [Cloudsley-Thompson, personal communication, 1956].
Natural prey.—"Large wood cockroach," Philippine Islands (Schultze, 1927): On several occasions Schultze found fragments of wings and legs of the large wood cockroach in a scorpion cavity, under a rotten log.
Experimental prey.—Leucophaea maderae, Periplaneta americana, and other species of Blattidae, Philippine Islands (Schultze, 1927): Blattids seemed to be the favored food. This scorpion is usually found in humid, damp places in forest and jungle. Schultze describes in detail feeding behavior of the scorpion and method of capturing its prey.
Experimental prey.—Periplaneta americana, Australia (Glauert, 1946): An injured cockroach was accepted at once by the scorpion, which held the insect in its claws and tore it with the alternately moving chelicerae. The scorpion ate all the soft parts and most of the sclerotized exoskeleton.
Order ARANEIDA
Many observations of spiders feeding on cockroaches are quite general, and many observers have failed to identify either the spider or its prey. Belt (1874) stated that "the cockroaches that infest houses in the tropics ... have numerous enemies—birds, rats, scorpions, and spiders." When Belt tried to drive a cockroach toward a large cockroach-eating spider, the insect rushed away from him until it came within a foot of the spider when it would double back, never advancing nearer.
Beebe (1925) watched a giant "wood roach," which was in the grasp of a 2-inch ctenid spider, fly through the window of his British Guiana laboratory. While the spider ate the cockroach, the insect gave birth to 51 nymphs. Sonan (1924) reported that large gray spiders devour nymphs and adults of Periplaneta americana and P. australasiae in Formosa; this spider also occurs on Hiyakejima Island and Okinawa. Passmore (1936), who has produced some excellent photographs of tarantulas, stated that they destroy cockroaches. Rau (1940) stated that American and oriental cockroaches were the principal item of diet of a friend's pet tarantula for several years. Kaston (personal communication, 1953) successfully fed a tarantula with Periplaneta americana.
Bristowe (1941) found that the British species of Ectobius are readily accepted by Xysticus, Clubiona, Drassodes, Zelotes, Tarantula, and the web-builders Ciniflo and Aranea. The British domestic cockroaches were accepted by Tegenaria and Ciniflo, spiders large enough to overpower them, and were useful as food for tropical avicularids, ctenids, and sparassids in captivity.
Family THERAPHOSIDAE
Common name.—Bird-eating spider.
Natural prey.—Periplaneta americana, Trinidad (Main, 1924, 1930): The remains of the host were compressed into globular form by the spider after it had extracted the nutritive parts.
Experimental prey.—Cockroach, West Indies (Wolcott, 1953).
Family SPARASSIDAE
Synonymy.—Heteropoda regia Fabricius.
Common names.—Banana spider (Comstock, 1912); huntsman spider (Gertsch, 1949); big brown house spider (Bryan, 1915).
Natural and experimental prey.—Cockroaches, Bermuda (Verrill, 1902); Puerto Rico (Sein, 1923; Wolcott, 1924a; Petrunkevitch, 1930a); Hawaii (Bryan, 1915, Williams et al., 1931); British Guiana (Moore in Williams et al., 1931); Panama (Gertsch, 1949); New Zealand (adventive) (Parrott, 1952); England (Cloudsley-Thompson, 1953); Comstock (1912); Hawaii (Pemberton, 1917).
This (pl. 30, A) is a tropical species frequently imported into northern localities with bunches of bananas (Comstock, 1912; Cloudsley-Thompson, 1953). Adults measure 3 to 4 inches across with bodies over an inch long. They seldom leave their resting places during the day, but are active at night and search for food. The female does not spin a web (Bryan, 1915; Gertsch, 1949). The spider turns the cockroach over onto its back at the instant of seizure and holds it firmly against the substrate. The cockroach dies in 10 minutes and is gradually rolled up by the spider as it sucks out the nutriment (Moore in Williams et al., 1931). The spider does not attempt to bite when captured, but if it does, its bite is said to be painful but not dangerous (Cloudsley-Thompson, 1953). Zimmerman (1948) found scores of Periplaneta australasiae breeding in rock piles in Hawaii; also present were large numbers of these spiders and centipedes which presumably preyed upon the cockroaches.
Family THERIDIIDAE
Common name.—Button spider.
Natural prey.—Karnyia discoidalis, South Africa, Western Cape Province (Hesse, 1942): The nest is constructed on the ground among grass stems or other vegetation. Preferred sites are slight hollows, hoof imprints, etc. Nests are roughly tubular. The remains of insects are entangled in the walls of the nest where they form dense accumulations. Predatory activities of the spider are limited to an area close to the tubular entrance to the nest and do not extend beyond the trapping strands near the entrance. Capture is dependent upon accidental contact of the insect with sticky threads surrounding the entrance. This spider apparently attacks any insect or arachnid that becomes [Pg 216] entangled in the nest. In an examination of 40 nests, remains of 6 K. discoidalis were found.
Common names.—Black widow, hourglass, or shoe-button spider.
Natural prey.—Cockroaches, Puerto Rico (Petrunkevitch, 1930). U.S.A., Florida, on shipboard (Anonymous, 1939): This is a presumptive host record, as the spiders were not reported as having been seen eating cockroaches; however, heavy infestations of both were found together.
Family LYCOSIDAE
Experimental prey.—Young nymphs of Diploptera punctata, U.S.A. (Eisner, 1958): Larger nymphs and adults repelled the spider by ejecting a repellent secretion, which has been identified as a mixture of p-benzoquinone and its derivatives by Roth and Stay (1958).
Experimental prey.—Supella supellectilium, U.S.A. (Roth and Willis, unpublished data, 1953): The lycosid (pl. 30, B-E) was probably L. avida Walckenaer (tentatively identified by Dr. B. J. Kaston from a photograph).
Order ACARINA
Family PHYTOSEIIDAE
Synonymy.—Blattisocius triodons Keegan [Baker and Wharton, 1952].
Natural host.—Blattella germanica, U.S.A. (Keegan, 1944): Three mites found on 238 cockroaches examined; others taken in debris from floor of cockroach cage (Keegan, 1944). Members of this family are predaceous (Baker and Wharton, 1952).
Family LAELAPTIDAE
Natural host.—Nauphoeta cinerea, Australia, Brisbane (Womersley, 1956).
Natural host.—Gromphadorhina portentosa, the hosts were imported into U.S.A. from Madagascar via Europe (Roth and Willis, [Pg 217] unpublished data, 1958): The mites (pl. 12, C) were tentatively determined by Dr. E. W. Baker.
Natural host.—Panesthia australis, imported into U.S.A. from Australia (Roth and Willis, unpublished data, 1955): Cockroach determined by J. A. G. Rehn. Generic determination of mite made by Dr. R. W. Strandtmann (Camin, personal communication, 1955).
Family UROPODIDAE
Natural host.—Blattella humbertiana, Formosa (Takahashi, 1940). Nymphs of the cockroach may be destroyed (Takahashi, 1940). Uropodids frequently attach themselves to insects, especially in nymphal stages but probably are harmless (Baker and Wharton, 1952).
Family DIPLOGYNIIDAE
Natural host.—Panesthia australis, imported into U.S.A. from Australia (Roth and Willis, unpublished data, 1955): Cockroach determined by J. A. G. Rehn. According to Dr. J. H. Camin (personal communication, 1955) this is a new genus and new species in the subfamily Diplogyniinae, and is most closely related to the genus Lobogynioides. Mites of this family live as ectoparasites and commensals on beetles and possibly other insects (Baker and Wharton, 1952).
Family ANOETIDAE
Natural host.—Pycnoscelus surinamensis, Germany (Roeser, 1940): Though not parasitic, the mites at times became so numerous that the insects were hindered in their movement, were unable to feed, and died. The mites were introduced with soil and leaves and had originally been attached to millipedes, waterfleas, and sowbugs.
The deutonymphs, hypopial forms, or travelers are found on insects; the other stages are found in decaying organic matter (Baker and Wharton, 1952).
Family ACARIDAE
Natural host.—Pycnoscelus surinamensis, Germany (Roeser, 1940): See notes following Histiostoma feroniarum above.
Natural hosts.—Blattella germanica and Periplaneta americana, U.S.A. (Piquett and Fales, 1952): Mite feeds on organic matter but can reduce the vigor of a cockroach colony.
Common name.—Mushroom mite.
Associate.—Pycnoscelus surinamensis, U.S.A. (Roth and Willis, unpublished data, 1953): Mite determined by Dr. E. W. Baker (personal communication, 1953). Although this mite was found on the cockroach, it is a known pest in stored foods (Baker and Wharton, 1952) and probably was brought into the culture with food. Rau (1924) reported that the food of Blatta orientalis often became infested with this species, but it did not affect the health or mortality of the cockroaches in his culture.
Natural host.—Periplaneta americana, U.S.A. (Roth and Willis, unpublished data, 1953): Mite determined by Dr. E. W. Baker (personal communication, 1953). Mites were found in the oöthecal cavity of a female cockroach that had been isolated for her entire adult life. The mites were in a closely packed mass behind a plug of what appeared to be feces, disintegrated eggs, and dried blood; none of the mites were visible until this plug was removed. Baker (personal communication, 1953) stated that the mite is probably not parasitic and that species of the genus feed on organic matter.
Natural host.—Periplaneta americana, U.S.A. (Rau, 1940a): Not normally parasitic on cockroaches, but the mites became so numerous at times they would attack living as well as dead and dying cockroaches.
Family GLYCIPHAGIDAE
Synonymy.—Trichotarsus sp. [Baker and Wharton, 1952].
Natural host.—Leucophaea maderae, Puerto Rico (Seín, 1923): Mites found on cockroach's thorax and particularly among the folds of the wings (Seín, 1923). Mites of this genus are found infesting organic matter (Baker and Wharton, 1952).
Family PODAPOLIPODIDAE
Natural hosts.—Diploptera punctata and Nauphoeta cinerea, U.S.A. (Roth and Willis, unpublished data, 1954): Mite genus determined by Dr. E. W. Baker (personal communication, 1954). The mites cluster thickly on intersegmental membranes, particularly around the coxae and neck. Despite a heavy infestation, the colony of Nauphoeta thrived for several years. This mite was found first on N. cinerea and possibly transferred to D. punctata when the latter was brought into the laboratory from Hawaii.
Family IOLINIDAE
Natural hosts.—Blaberus craniifer (originally from a culture at Harvard University) and Diploptera punctata (originally from Hawaii), U.S.A., Pennsylvania (Roth and Willis, unpublished data, 1953; Pritchard, 1956): The mites usually attached near the wing bases of the insects. Morphologically, the species is intermediate between certain predaceous and phytophagous mites (Pritchard, 1956).
Family PTERYGOSOMIDAE
Common name.—Cockroach mite.
Natural hosts.—Parcoblatta sp., U.S.A. (Edmunds, 1953a).
Periplaneta americana, U.S.A. (Piquett and Fales, 1952).
Cockroaches. U.S.A. (Baker and Wharton, 1952).
Experimental hosts.—Blatta orientalis, Blattella germanica, and Periplaneta americana, U.S.A. (Cunliffe, 1952).
Eggs of this mite (fig. 4) are usually laid indiscriminately in the rearing cages, rarely on the host. Eggs are coated with a sticky secretion which enables those laid on the host to adhere. Hatching occurs in 6-11 days at 90-95° F., and in 9-11 days at 80° F. The newly hatched larva starts to feed immediately on the cockroach. Larval stage lasts 4-6 days, rests 2-3 days, and molts. During the single nymphal instar, the mite feeds on the host and moves about for 6-7 days. The mite then rests 3-4 days before molting. Entire life cycle covers a period of 28-32 days. Adult mite lives 2-3 weeks, during which time it can produce 2-3 batches of from 1 to 20 eggs; the usual batch is about 12 eggs. The mites are unable to live on cockroach feces, cast skins, or dead cockroaches. Mites died within 4-5 days [Pg 220] unless live cockroaches were supplied. Parasitism was proved by detecting radioactivity in mites that had fed on cockroaches which had been previously fed radioactive NaCl (Cunliffe, 1952).
The mites can destroy laboratory cultures of cockroaches (Piquett and Fales, 1952; Edmunds, 1953a). A cockroach attacked by 25 mites succumbed after about an hour, falling on its back; it died after 5 hours (Cunliffe, 1952).
When found in homes and offices, these mites are an indication of the presence of cockroaches; the mite has been twice accused of biting people (Baker et al., 1956).
Natural hosts.—Aglaopteryx facies, Puerto Rico (Seín, 1923): Four red "tick" nymphs found under wings of female.
Blaberus craniifer, U.S.A., Florida (Hebard, 1917): "A number of lice [mites] are present on many of these specimens [28♀♀]."
Blaberus discoidalis, adventive from West Indies, taken in Scotland (Stewart, 1925): A considerable number of mites were all over the body and hind wings.
Blatta orientalis, Germany (Cornelius, 1853): Ex sexual organs of male.
Blattella germanica, U.S.A., in laboratory (Parker, 1939): Under conditions of high humidity, the cockroaches became heavily infested with mites. In cages where the infestation was heavy, an abnormally large number of females dropped their oöthecae, and the percentage of eggs hatching was low.
Parcoblatta uhleriana, U.S.A., North Carolina (Hatcher, 1939): Hypopi of mites were found deeply embedded in the fat body of two individuals.
Mites in the hypopial stage attach to insects by which they are dispersed. Hypopi have been found in the gill chambers of a mollusk and in the gonads of a millipede (Baker and Wharton, 1952).
Periplaneta americana, U.S.A., in laboratory (Fisk, 1951): The insects were sluggish and molted with difficulty. Gold Coast Colony (Macfie, 1922): Larvae of a tarsonemid mite were found in the feces.
Pycnoscelus surinamensis, Hawaii (Illingworth, 1915): During the summer the soil was literally swarming with young of various stages. Early in September most of the adults were dead and all were covered with mites. U.S.A., Connecticut, in laboratory (Zappe, 1918a). Hawaii, in laboratory (Schwabe, 1950): Some of the cockroaches apparently died from mite infestations.
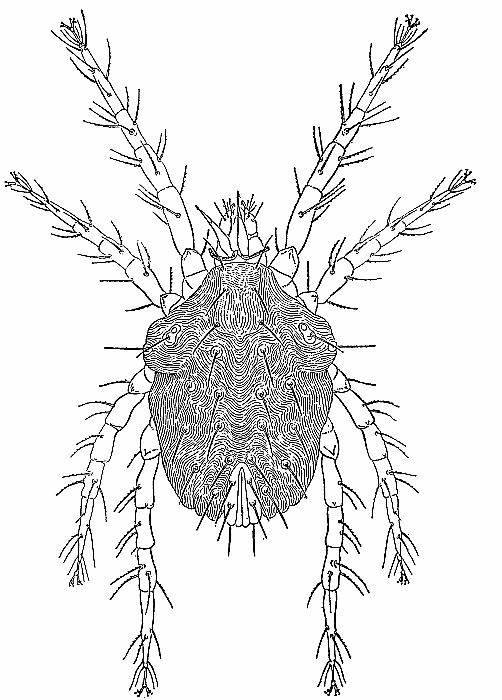 Fig. 4.—The cockroach mite, Pimeliaphilus podapolipophagus. (From Baker
et al. [1956]; reproduced through the courtesy of Dr. E. W. Baker and the National
Pest Control Association).
Fig. 4.—The cockroach mite, Pimeliaphilus podapolipophagus. (From Baker
et al. [1956]; reproduced through the courtesy of Dr. E. W. Baker and the National
Pest Control Association).
Cockroach, England? (Ealand, 1915): Cockroaches may carry the hypopial stage of the cheese mite.
Fisk (1951) eliminated the mites [possibly Pimeliaphilus podapolipophagus (Baker et al., 1956)] in his cockroach colony by using a 5-percent spray and a 5-percent dust of p-chlorophenyl, p-chlorobenzene sulfonate. The exterior of the cockroach containers were sprayed with the solution and the interior, including the insects, were dusted. Within a month the mites had disappeared and the vigor of the cockroach colony improved. Piquett and Fales (1952) used flowers of sulfur and general sanitary procedures for eliminating the mites in laboratory colonies of Blatta orientalis; they cleaned the dishes every few days and applied grease around the edges of the containers to prevent new mite invasions. Qadri (1938) employed similar control measures.
Class CHILOPODA
Large centipedes which entered houses in India probably sought out cockroaches (Maxwell-Lefroy, 1909). In Puerto Rico, centipedes entered homes to which they were attracted by cockroaches (Seín, 1923). In Hawaii, centipedes preyed on insects generally but especially on cockroaches (Bryan, 1915). Sonan (1924) reported that in Formosa and Okinawa Islands a species of centipede 5 to 6 inches long comes into the houses and devours both adults and nymphs of Periplaneta americana or P. australasiae. Zimmerman (1948) found P. australasiae breeding by scores in rock piles in Hawaii accompanied by large numbers of Scolopendra and large spiders that probably preyed upon the cockroaches.
Order SCUTIGEROMORPHA
Family SCUTIGERIDAE
Synonymy.—Scutigera forceps Rafinesque [Crabill, 1952].
Common name.—House centipede.
Natural prey.—Cockroaches, U.S.A. (Felt, 1909; Back, 1947; Auerbach, 1951; Crabill, 1952; and others): This predator-prey relationship seems to be based on good circumstantial evidence (Crabill, personal communication, 1953).
Experimental prey.—Blattella germanica, newly hatched nymphs [Pg 223] and adult female, U.S.A. (Snodgrass, 1930; Roth and Willis, unpublished data, 1953).
Periplaneta americana, U.S.A. (Roth and Willis, unpublished data, 1953).
Supella supellectilium, U.S.A. (Roth and Willis, unpublished data, 1953): See plate 31.
Our specimen caught a small American cockroach nymph that we placed in its jar. Before it had finished its meal, it caught and held two other nymphs with its legs while it continued to feed on the first. The body of this centipede reaches a maximum length of 27 mm. and it is usually found in basements, dark corners, or in spaces in the walls (Auerbach, 1951). Introduced from Europe, this species is now widespread in the United States (Crabill, 1952).
Synonymy.—Scutigera maculata [Crabill, personal communication, 1957].
Natural prey.—Cockroaches, Malay peninsula, Batu caves (Ridley in Annandale et al., 1913): This is a presumptive host record.
Order SCOLOPENDROMORPHA
Family SCOLOPENDRIDAE
Experimental prey.—Cockroaches, England (Cloudsley-Thompson, 1955): After capture in France, this specimen was kept for four weeks without food. She was then fed medium-sized nymphal cockroaches of which she ate an average of about one per week throughout the summer. Adult cockroaches were attacked only after they had been disabled.
Natural prey.—Cockroaches, Guadeloupe (Lherminier, 1837).
Experimental prey.—Cockroaches, India, Nagpur (Jangi, 1955): As soon as the centipede became aware of its prey, it rapidly embraced the cockroach within its legs and with its fangs gripped the insect's thorax. The predator continued to hold the prey with its fangs while its mouth parts prodded the victim's body. After feeding on an adult cockroach, the centipede is not inclined to kill another for 2-3 days.
Natural prey.—Cockroaches, Hawaii (Williams et al., 1931): This is a common species with a body length of 6 or more inches. It is reported to be a great enemy of cockroaches.
Natural prey.—Ectobius panzeri, England (Lucas, 1911, 1920): When captured, the centipede was holding a live cockroach which it had apparently just caught. The insect was held beneath its captor's body, ventral surface upward, by several of the anterior legs while the centipede fed.
Class INSECTA
We have found representatives of only 10 orders that have preyed on or parasitized cockroaches: Beetles, flies, bugs, ants, wasps, stylops, and cockroaches occurred in nature; the others resulted from feeding cockroaches to captive insects or were laboratory observations.
Order ODONATA
Family AESHNIDAE
Common name.—Giant Hawaiian dragonfly.
Experimental prey.—Cockroaches, Hawaii (Williams, 1936): The dragonfly nymph was fed with medium large cockroaches and other insects.
Order BLATTARIA
In this chapter the relations of other arthropods to cockroaches are either as parasites or as predators. Certain cockroaches have turned the tables on their adversaries and become predators themselves. This aspect of cockroach behavior is discussed in chapter XVI. Other associations of cockroaches, as commensals with other insects and as associates of other cockroaches, are discussed in chapters XV and XVII.
Order ORTHOPTERA
Family MANTIDAE
(Pl. 32)
Experimental prey.—Blatta orientalis, Diploptera punctata, Eurycotis floridana, Leucophaea maderae, Nauphoeta cinerea, Neostylopgya [Pg 225] rhombifolia, and Periplaneta americana, U.S.A. (Rilling, personal communication, 1957): Mrs. Rilling wrote us that with the exception of N. rhombifolia, all the above cockroaches were readily eaten. All the mantids initially rejected N. rhombifolia after grasping and making a brief attempt to chew the cockroaches. However, if specimens of N. rhombifolia were left in the jars with the mantids, the cockroaches were usually eaten within the next 24 hours. N. rhombifolia ejects an odorous substance when seized and the mantids probably ate these insects after most of this secretion had been depleted. It is highly probable that the secretion of N. rhombifolia may deter the mantid's attack, but it should be pointed out that, with the possible exception of N. cinerea, all the other species fed to these mantids give off odorous substances when seized or disturbed. Apparently, certain naturally repellent compounds will deter this mantid, whereas others that are presumed to be repellent will not; however, the nutritional state of the mantid is undoubtedly a factor which may limit the effectiveness of certain repellent secretions against this predator.
Byrsotria fumigata, teneral males, and Periplaneta australasiae, nymphs, U.S.A. (Roth and Willis, unpublished data, 1958).
Diploptera punctata, U.S.A. (Eisner, 1958).
Common name.—European mantis.
Experimental prey.—Nauphoeta cinerea, and Periplaneta americana, U.S.A. (Rilling, personal communication, 1957).
Experimental prey.—Cockroaches, Borneo (Shelford, 1916).
Synonymy.—Sphodromantis bioculata Burmeister [Gurney, personal communication, 1958].
Experimental prey.—Blatta orientalis, Egypt (Adair, 1923): This species of cockroach was apparently used regularly as food for the mantid in the laboratory.
Common name.—Carolina mantis.
Experimental prey.—Blattella germanica and Periplaneta americana, U.S.A. (Breland, 1941): The mantids were fed 1-2 German cockroaches daily. One female mantid consumed 10 adult German cockroaches plus one oötheca and part of another in 2.5 hours. An [Pg 226] adult German cockroach was consumed in an average of 8.5 minutes (range 5.5-15 minutes).
Blatta orientalis, nymphs, and Diploptera punctata, U.S.A. (Roth and Willis, unpublished data, 1953).
Experimental prey.—Cockroaches, South Africa (Faure, 1940).
Common name.—Chinese mantis.
Experimental prey.—Nauphoeta cinerea and Periplaneta americana, U.S.A. (Rilling, personal communication, 1957).
Family GRYLLACRIDIDAE
and
Natural prey.—Cockroach eggs, Japan (Asano, 1937): These are questionable records. Asano found D. apicalis and D. japanica beneath his house near several empty cockroach oöthecae which appeared to have been eaten into. He assumed from the condition of the oöthecae and the proximity of the stone crickets that the insects had devoured the cockroach eggs.
Experimental prey.—Eggs of Blattella germanica and Periplaneta japanica, Japan (Asano, 1937): Seven eggs of B. germanica (obtained from an oötheca being carried by a female) and eggs of P. japanica (presumably in oöthecae) were fed to both species of stone crickets in the evening. The eggs were devoured by the next morning.
Order DERMAPTERA
Family FORFICULIDAE
Experimental prey.—Cockroaches, France (Chopard, 1938): According to Chopard, Brisout de Barneville in 1848 indicated that earwigs in captivity can be fed small cockroaches.
Order HEMIPTERA
Family LYGAEIDAE
Natural prey.—Cockroach, Hawaii (Illingworth, 1917): This predaceous bug is commonly found about buildings. Illingworth says that [Pg 227] Kirkaldy suspected that it fed on small blattids and that Dr. Perkins saw it feeding on a dead cockroach.
Family REDUVIIDAE
Natural prey.—Periplaneta americana, Brazil, Matto Grosso (Pinto, 1927, 1927a): This bug preys principally on cockroaches and was observed infesting the walls of dwellings where it preyed on P. americana.
Natural prey.—Monastria sp., Brazil, Minas Gerais (Martins, 1941): This bug probably feeds on cockroaches of this genus, as well as on rodents.
Natural prey.—Arenivaga roseni and Polyphaga saussurei, Turkmen S.S.R. (Vlasov and Miram, 1937): These desert cockroaches are found in burrows of rodents and desert turtles around Ashkhabad. Reduviids are their main enemies. Vlasov (1933) found nymphs of Reduvius christophi Jak. and R. fedtschenkianus Osch. in similar burrows in this same area, although he did not specifically cite them as enemies of the desert cockroaches.
Family NEPIDAE
Experimental prey.—Cockroaches, U.S.A. (Hoffman, 1924).
Order NEUROPTERA
Family ASCALAPHIDAE
Experimental prey.—Blattella germanica, Kenya Colony (Someren, 1924).
Order DIPTERA
From the few observations that have come to our attention, it seems that flies are comparatively rare parasites in cockroaches.
Family PHORIDAE
Host.—Eggs of Parcoblatta sp., Ohio (Edmunds, 1952a).
Family CONOPIDAE
Hosts.—Cockroaches, Brazil (Souza Lopes, 1937): L. Travassos was quoted as having observed this species pursue cockroaches that were escaping columns of the army ant Eciton sp. Souza Lopes (1937) stated that the female deposits eggs on the cuticle of the host near the end of the body; the egg is barely inserted and two recurrent hooks prevent it from falling off. Souza Lopes (1937) also observed other species of Stylogaster pursue Orthoptera, but he was unable to devote proper attention to the behavior of the flies.
Hosts.—Chorisoneura sp., Brazil (Souza Lopes, 1937): An adult specimen was found in a museum collection with an egg of Stylogaster attached to the posterior end of its abdomen.
Cockroaches, Panama (C.W. Rettenmeyer, personal communication, 1959): "Seven species were collected hovering over army ant swarms and a few flies were seen apparently attacking cockroaches that had been flushed by the ants."
Family LARVAEVORIDAE
Hosts.—Periplaneta americana, Brazil (Souza Lopes, 1937): Obtained complete evolution of the parasite in this host. This may have been an experimental host.
Hosts.—Cockroaches, Panama (Rettenmeyer, personal communication, 1959): Swarms of army ants are accompanied by about 20 species of Calodexia. These flies larviposit on the cockroaches, crickets, and possibly other arthropods that are flushed from cover by the ants. Larvae were found in one(?) cockroach. Larvae from an adult of Calodexia were introduced experimentally into a cockroach and successfully reared.
Hosts.—Eurycotis floridana, from Florida (Roth, unpublished data, 1953): Three larvae (det. by W.W. Wirth) were found in a living adult male.
Panesthia australis, from Australia (Roth, unpublished data, 1957): Reared from a wild-caught cockroach that was maintained in a laboratory colony.
Cockroaches, Australia (E. F. Riek, personal communication, 1955): Reared from some of the larger species.
Family MUSCIDAE
Host.—Eggs of Parcoblatta sp., Ohio (Edmunds, 1952a).
Family SARCOPHAGIDAE
Host.—Arenivaga bolliana, Texas (Wirth, personal communication, 1953): Specimens in U.S. National Museum.
Synonymy.—Sarcophaga sternodontis (Towns.).
Hoffman (1927) claimed that approximately 40 percent of some specimens of Pycnoscelus surinamensis collected in southern Haiti were parasitized by S. lambens. However, according to entomologists at the University of Puerto Rico Agricultural Experiment Station, Hoffman was incorrect in his observations: S. lambens was never reared from a living insect and had been recovered only from dead cockroaches and other dead insects and was considered saprophytic rather than parasitic (Schwabe, 1950b).
Sanjean (1957) reared various species of sarcophagid larvae on Periplaneta americana which were freshly killed or chopped up; first instar larvae were also introduced into the body cavity of cockroaches which had their heads and legs removed. Adult sarcophagids were collected and freshly killed American cockroaches used as bait.
Order COLEOPTERA
Family CARABIDAE
Experimental prey.—Cryptocercus punctulatus, U.S.A. (Cleveland et al., 1934): This beetle is often found in the galleries of C. punctulatus in nature. In the laboratory it killed and devoured cockroaches as large as itself.
Family DYTISCIDAE
Experimental prey.—Cockroaches, disabled, Hawaii (Williams, 1936): This beetle, which is common in mountain streams, located wounded cockroaches in an aquarium by sense of smell or taste rather than sight.
Family LAMPYRIDAE
Experimental prey.—Parcoblatta virginica, adult female (pl. 33, C), U.S.A. (Roth and Willis, unpublished data, 1953).
Family RIPIPHORIDAE[5]
Natural host.—Cockroach (undescribed genus belonging to the Pseudomopinae), Australia Capital Territory (Riek, 1955).
Natural host.—Robshelfordia longiuscula or Robshelfordia circumducta, Australia Capital Territory (Riek, 1955).
Natural host.—Oniscosoma granicollis, Australia Capital Territory (Riek, 1955).
Natural host.—Balta patula, Australia, Victoria (Riek, 1955): Pupal stage lasted only 3 days.
Natural host.—Escala sp. or an undescribed genus of Pseudomopinae, Australia, Victoria (Riek, 1955).
Natural host.—Ellipsidion affine, Australia, New South Wales (Riek, 1955).
Natural host.—Robshelfordia longiuscula or Robshelfordia circumducta, Australia Capital Territory (Riek, 1955).
Natural host.—Robshelfordia longiuscula or Robshelfordia circumducta, Australia Capital Territory (Riek, 1955).
Natural host.—Choristima sp. and Choristimodes sp., Australia Capital Territory (Riek, 1955).
Synonymy.—Nephrites australis Riek [Selander, 1957].
Natural host.—Cutilia sp., Australia Capital Territory (Riek, 1955): Two females emerged from one host.
Synonymy.—Nephrites nitidus of Riek not Shuckard [Selander, 1957].
Natural host.—Platyzosteria sp., Tasmania (Riek, 1955).
Synonymy.—Nephrites sp. [Selander, 1957].
Natural host.—Platyzosteria castanea, Australia Capital Territory (Riek, 1955).
Biology of Australian Ripidiini.—The Australian species of Ripidiini are parasites of apparently endemic, ground-dwelling species of cockroaches. There is some correlation between host subfamily and parasite genus: Riekella spp. [= Nephrites] have only been bred from Blattinae. Rhipidioides spp. occur only in the closely related Ectobiinae and Pseudomopinae. Neonephrites and Neorhipidius also occur in the Pseudomopinae. Paranephrites occurs in the Panchlorinae. There is some evidence that the parasitized cockroaches migrate onto trees when the larval parasite is mature, as pupae have only been found on the trunks of eucalyptus trees. In all species the larva leaves the host dorsally through an intersegmental membrane. The host continues to live for a few days after the parasite emerges. The larva attaches itself to bark on the tree trunk by a few strands of silk before pupating. The larviform, wingless female remains near the pupal skin and is sought out by the winged male. The eggs are laid in a mass around the pupal skin (Riek, 1955).
Balduf (1935) lists Ripidius boissyi as parasitic on nymphs of Ectobius pallidus giving Abeille de Perrin (1909) as a source for this [Pg 232] information. However, Abeille de Perrin simply presumed that R. boissyi parasitized E. pallidus because he collected this cockroach in the same habitat as the beetle. Abeille de Perrin suggested that the species of the genus Ripidius lived in the bodies of cockroaches, but there are no rearing records, as far as we know, of R. boissyi from cockroach hosts.
Chobaut (1919), in France, collected both R. denisi and Ectobius pallidus when beating an oak tree. Because of the known association of other species of Ripidius with cockroaches, he presumed that this beetle was parasitic on E. pallidus, a cockroach common in this beetle's habitat.
Synonymy.—Symbius blattarum Sundevall [Leng, 1920].
Natural hosts.—Blattella germanica, on shipboard (Sundevall, 1831); Germany (Aclogue and Fowler, in Burr, 1899a); on steamship "Samui" (Stamm, 1936); on cruiser "Duguay-Trouin" (Barbier, 1947); Hawaii (Williams, 1946a): This last record was based on a specimen dissected from an adult German cockroach collected on an airplane from the South Pacific. The parasite was reported as Ripidius sp. by Williams, but Weber (1948) made the specific identification.
Ectobius pallidus? Abeille de Perrin (1909) stated that R. pectinicornis was first described by Sunders [sic] as blattarum because it had been captured in the body of Ectobia livida. We presume that Abeille de Perrin was referring to Sundevall's work in which the host was given as Blattella germanica.
Periplaneta americana, on shipboard (Sundevall, 1831): One nymph only.
With the exception of the single nymph of P. americana, R. pectinicornis apparently attacks only adult females and nymphs of B. germanica. Barbier (1947) found only B. germanica parasitized, although both Blatta orientalis and Supella supellectilium were prevalent on board the ship. Primary larvae of the parasite failed to parasitize Supella.
Adult behavior.—The winged male is relatively active compared to the apterous female; it runs around, flies well, and jumps on the female when in her vicinity. The female remains stationary and lays eggs around her by bending her long ovipositor (Sundevall, 1831). The eggs (50-100) are laid among a network of silk fibers secreted by [Pg 233] the female. The female dies after completing oviposition (Barbier, 1947).
Development.—The eggs hatch after 14 days, and the primary (triungulin) larvae ascend the host's legs to its body; the larvae then cut the intersegmental membrane between the metasternum and first abdominal segment of the cockroach, in order to enter the host's abdomen (Barbier, 1947). Chobaut (1892) first suggested this method of attack by the ripiphorid larva. As the parasites develop, the abdomen of the host becomes swollen. Developing larvae apparently eat the host's fat body, leaving the vital organs until the last. Parasitized female hosts were sterile and the eggs, when formed, never hatched. Development of the oötheca was also inhibited. There were usually two larval parasites per host, but three or four were found several times (Barbier, 1947). Sundevall (1831) found only one larva per cockroach except one host which, when crushed, yielded five. Stamm (1936) found three hosts infested with five larvae each. In a little over 100 cockroaches, Stamm found 10 that were parasitized.
The day before the parasite leaves the host, the cockroach shows an abrupt uneasiness and runs about, finally falling over on its back. The parasite larva emerges from the host through an opening it makes in the membrane between penultimate and last tergite. The host dies a few hours after the larva has left. The larva seeks a sheltered area and pupates within 48 hours. Adults emerge in 9 days (females) and 13 days (males) (Barbier, 1947).
Distribution.—Adult males have been collected in light traps in Hawaii (Van Zwaluenburg, 1946), and the first female was reported by Weber (1948); the parasite is now established in the islands around Pearl Harbor (Dr. F. X. Williams, personal communication, 1953). The U. S. National Museum has specimens of R. pectinicornis from England, Guatemala, Hawaii, Panama, and from Florida and Georgia in the U. S. (Dr. E. A. Chapin, personal communication, 1953). Kono (in Asano, 1937) reported two species in Japan. It is noteworthy that all these records are from localities adjacent to oceans and on ships; none are from interiors of continents. The only biological data were obtained from parasites found on board ships. Sundevall (1831) believed that the parasites boarded his ship with their hosts during loading in Calcutta, since before that not any were seen on board. Barbier (1947) suggested that the parasite must be spread very easily in ports between neighboring ships by parasitized cockroaches in baskets or sacks of provisions.
Natural hosts.—Blattidae, Philippine Islands (Schultze, 1925).
Family DERMESTIDAE
Common name.—Black larder beetle.
Natural prey.—Blatta orientalis, U.S.A. (Roth and Willis, unpublished data, 1953): Dermestes ater is generally a scavenger, but we have seen adult beetles, which had developed in our cockroach colony, clinging to and feeding on living oriental cockroaches, eventually killing them; the beetles probably attack only the weakened or injured cockroaches in a culture. This was a natural infestation of a laboratory culture by a predator.
Experimental prey.—Blattella germanica, oöthecae, U.S.A. (Roth and Willis, 1950): The beetle larvae can penetrate unhatched oöthecae of the German but not those of the American or oriental cockroaches.
Natural prey.—Blatta orientalis, oöthecae, U.S.A., Missouri: Rau (1924) stated that Dermestes larvae often infest the egg cases of this cockroach; it is probable that Rau was referring to cockroaches in laboratory cultures.
Order STREPSIPTERA
Pierce (1909) predicted that the Blattoidea and the Grylloidea would be the only groups of the Orthoptera which would be parasitized by Strepsiptera. Essig (1926) made the statement that certain cockroaches are among the hosts of Strepsiptera. E. F. Riek (personal communication, 1952) found a strepsipteron in a late nymph of Cutilia sp. from Waroona, Western Australia; he wrote us, "The female parasite is extruded between a pair of sternites towards the base of the abdomen and appears to belong to the family Halictophagidae." This is the only record that we have been able to find of a strepsipteron parasitizing cockroaches.
Order HYMENOPTERA
Wasps from at least six families of Hymenoptera have been recorded as developing on cockroach eggs. All the Evaniidae are presumed to be parasitic in the egg capsules of cockroaches (Clausen, 1940; Townes, 1951), although hosts for many of the described species have yet to be discovered. The presence of evaniids in dwellings indicates the presence of cockroaches (Gross, 1950). At times [Pg 235] these wasps may become a nuisance; a family in Worthington, Ohio, complained of the evaniid wasps that they found on the windows and in other areas of their home, but they were apparently not annoyed by the oriental cockroaches in the basement (Edmunds, 1953).
The known parasites of cockroach eggs are listed below with summaries of their biology.
Family EVANIIDAE
Synonymy.—Evania princeps [Dr. H. Townes, personal communication, 1956].
Natural host.—Cockroach eggs, Australia (Froggatt, 1906).
Synonymy.—Evania minuta Olivier [Kieffer, 1920].
Natural hosts.—Blattella germanica, Europe? (Schletterer, 1889; Kiefer, 1912; Crosskey, 1951).
Ectobius lapponicus, Europe? (Schletterer, 1889; Kieffer, 1912; Crosskey, 1951).
Ectobius panzeri var. nigripes? Great Britain (Blair, 1952): This is a presumptive record. The wasp was collected at Niton and Headon Hill, Isle of Wight, an area in which this variety of E. panzeri was the only species of cockroach known to occur.
Ectobius sp., England (Cameron, 1955, 1957): Natural History Museum records.
Adult wasps have been collected on Asparagus officinalis Linnaeus (Schmiedeknecht in Schletterer, 1889; Crosskey, 1951). Thompson's (1951) citation of records of B. minutus and Evania appendigaster from Blatta orientalis and Blattella germanica, and Cameron's (1957) citation of these records and one from Ectobius lapponicus, all attributed to Kadocsa (1921), are almost certainly in error. Kadocsa (1921, p. 33) listed these wasps as egg parasites of cockroaches but not necessarily in Hungary and did not name specific cockroach hosts.
The present writers have found no information, other than host reports, on the biology of Brachygaster minutus. The records of this wasp parasitizing B. germanica may trace back to Schletterer, but his listing may not have been an original observation. Since the female of B. germanica carries its oötheca attached to the abdomen until or just before the eggs hatch, it would seem that the female of B. minutus (if the host records are valid) must oviposit into the oötheca of this species while it is still being carried by the female; this would not [Pg 236] necessarily be true for the other hosts which drop the egg case long before the eggs hatch.
Distribution.—Europe: Sweden, Russia, England, France, Germany, Austria, Hungary, Switzerland, Italy (Kieffer, 1920).
Synonymy.—Evania desjardinsii Bordage, Evania laevigata Latreille [Dalla Torre, 1901-1902].
Natural hosts.—Blatta, "exotic species" (Westwood, 1854, 1954a).
Blatta orientalis, Europe? (Schletterer, 1886; Howard, 1888, Kieffer, 1912); Egypt? (Alfieri, 1914; Adair, 1923). [Girault (1907, 1914) erroneously attributed another record to Marlatt (1902);[6] . See also notes under Brachygaster minutus with respect to Kadocsa.]
Blattella germanica? (Girault 1907, 1914). [This record is obviously an error. Girault attributed the record to Marlatt (1902); see footnote 6.]
Cutilia soror, Hawaii (Swezey, 1929; Zimmerman, 1948).
Leucophaea maderae (Schletterer, 1889; Bordage, 1896; Kieffer, 1912): These records are probably erroneous inasmuch as this cockroach incubates its eggs internally (Roth and Willis, 1954). Later, after finding that L. maderae is ovoviviparous, Bordage (1913) admitted having misidentified a parasitized oötheca from some other species; he concluded that the developing eggs of this species are protected against egg parasites because they are carried within the female. Clausen (1940), in classifying the placement of parasitic wasp eggs in relation to the host, erected the category: Egg placed in the embryo while the latter is still within the parent. He stated that although this behavior was not definitely known to occur, it probably could occur. However, the records cited above do not indicate that the alleged parasitization followed this pattern.
Neostylopyga rhombifolia, Hawaii (Swezey, 1929).
Periplaneta americana, Europe (Schletterer, 1889; Bordage, 1896; Kieffer, 1912); Réunion Island (Bordage, 1913); Puerto Rico (Seín, 1923); Jamaica (Gowdey, 1925); Hawaii (Swezey, 1929); Palestine (Bodenheimer, 1930); U.S.A., Florida (Ashmead, 1900); Maryland (Piquett and Fales, 1952); Saudi Arabia, Jedda (Cameron, 1957); Canton Island and Samoa (Dumbleton, 1957).
Periplaneta americana or P. australasiae, Formosa (Sonan, 1924).
Periplaneta australasiae, U.S.A., Florida (Ashmead, 1900); Hawaii (Swezey, 1929; Zimmerman, 1948). [Girault (1914) erroneously attributed another record to Marlatt (1902); see footnote 6, above.]
Experimental host.—Blatta orientalis, U.S.A. (Haber, 1920).
Relatively little detailed information was known about this wasp (fig. 5), one of the earliest parasites of cockroach eggs to be discovered, until Cameron (1957) studied its biology. Arnold (Kirby and Spence, 1826) discovered that the genus Evania parasitized Blatta, but did not know whether the wasp developed on the cockroach eggs or in the nymphs. MacLeay (Westwood, 1843) determined that Evania developed within the oöthecae of cockroaches. Westwood (1854a) found the larvae, pupae, and adults of E. appendigaster in egg cases of an unidentified species of cockroach found on orchids received from Calcutta.
Adult behavior.—Adult wasps visited flowers of parsley, Petroselium crispum, and fennel, Foeniculum vulgare (Margretti in Schletterer, 1886; Crosskey, 1951). In Hawaii the adult wasps have been seen resting on leaves coated with honey dew (Williams et al., 1931); Evania sp. were attracted to the honey dew secreted by a diaspine scale insect (Williams, 1931). Adults lived two to three weeks in captivity with ample food and water (Cameron, 1957).
Oviposition.—Shelford (1912, 1916) erroneously supposed that Evania, by means of her cleaverlike abdomen, opened the oötheca at the crista and then deposited her egg or eggs on the eggs of the cockroach. Haber (1920) observed and described oviposition. The female [Pg 238] wasp crawled over the surface of the oötheca, actively vibrating her antennae, and settled with the axis of her body parallel to the axis of the egg case as it lay upon its right side. Lying on her right side, the wasp extended her ovipositor and punctured the oötheca in the fifth cell on the left side; she remained in this position for about 15 minutes. Cameron (1957) described similar oviposition behavior that lasted about half an hour. Kieffer (1912) and Crosskey (1951) stated that the female deposits her eggs before the walls of the oötheca harden.
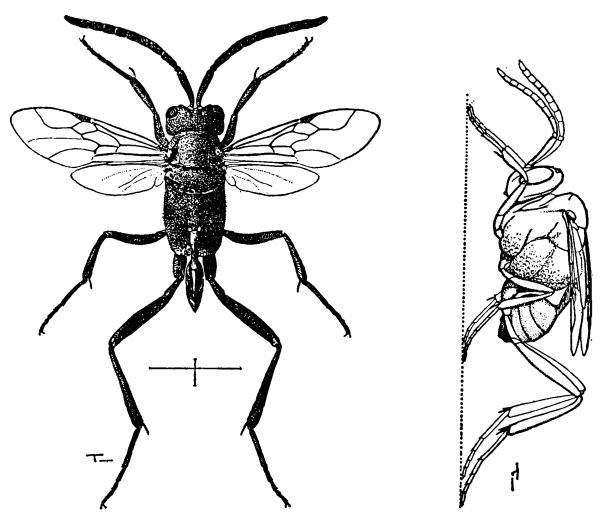 Fig. 5.—Evania appendigaster. Left, dorsal view, X 8. Right, side view, X 5.
(Reproduced with permission. British Museum [Natural History], 1951,
figs. 1A and 1B).
Fig. 5.—Evania appendigaster. Left, dorsal view, X 8. Right, side view, X 5.
(Reproduced with permission. British Museum [Natural History], 1951,
figs. 1A and 1B).
Development.—Kieffer (1912) stated that the larvae in this family eat the cockroach eggs and pupate in the oötheca without forming a cocoon. Smith (1945) stated that the larva feeds on one cockroach egg after another until all are destroyed; by that time it is full grown and it pupates within the oötheca. Cameron (1957) found that there are five larval instars and that in material from Saudi Arabia there are three or possibly four generations a year.
Distribution.—Tropical and subtropical parts of the world as far north as New York City, and all of Europe except the northern part (Kieffer, 1920; Townes, 1949). The wide distribution of Evania has been attributed to the abundance of host cockroaches on ships between the Tropics (Haldeman, 1847). Kieffer (1903) appears to have shown some correlation between the numbers of species of cockroaches found in various geographical regions and the numbers of species of evaniids found in similar regions. However, the number of blattids he listed is small.
Synonymy.—Evania abyssinica Westwood [Schletterer, 1889].
Natural host.—Blatta orientalis, Egypt? (Alfieri, 1914).
Natural host.—Periplaneta sp., Fiji (Lever, 1946): Although Lever (1946) listed this species as a cockroach-egg parasite, he did not state that he actually reared it from Periplaneta oöthecae.
Synonymy.—Dr. H. Townes, (personal communication, 1956) believes that this wasp was probably either H. reticulata, H. harpyoides, or H. thoracica; it is not possible to tell which without reexamining Ashmead's specimens; these apparently have been lost.
Natural host.—Parcoblatta pensylvanica, U.S.A., Mississippi (Ashmead, 1900).
Natural hosts.—Parcoblatta virginica, U.S.A., Ohio (Edmunds, 1952a, 1953a, 1954).
Parcoblatta pensylvanica, U.S.A. (Muesebeck, 1958).
Parcoblatta uhleriana, U.S.A., Natick, Mass.: Oötheca collected by L. Roth, May 17, 1956; wasp emerged June 12, 1956 (pl. 33, B); determined by Dr. H. Townes. The keel region of the oötheca of P. uhleriana (pl. 18, B) is different from that of any other species of Parcoblatta (Hebard, 1917; Lawson, 1954) so there can be no doubt as to the species of cockroach parasitized by this wasp.
Development.—The last instar larva overwinters inside the cockroach oötheca (Edmunds, 1954). Five oöthecae yielded one parasite each (Edmunds, 1953a).
Distribution.—Canada, Ontario. U.S.A.: New Hampshire and Minnesota to South Carolina, Mississippi, Texas, and Kansas. Upper and Lower Austral Zones (Townes, 1951).
Natural host.—Parcoblatta pensylvanica, U.S.A., Missouri (Rau, 1940).
Adult wasps have been taken on parsnip, Pastinaca sativa (Robertson, 1928).
Distribution.—U.S.A.: Pennsylvania to Florida and Louisiana. Mexico. Upper Austral to Tropical Zones (Townes, 1951).
Natural host.—Parcoblatta pensylvanica, U.S.A., Ohio (Edmunds, 1952a, 1953a, 1954).
Adult behavior.—Copulation was rapid, lasting only a few seconds. Blooms of Asmorrhiza longistylis were placed in a cage with adult wasps. The insects were attracted to and fed on the flowers (Edmunds, 1954).
Development.—Entire contents of oötheca are eaten by the single larva. Last instar larva overwinters inside the oötheca. Emergence in Ohio was around the middle of June. The emergence hole made by this genus was about 2 mm. in diameter. The hole was made at the top side of the oötheca near one end. Adult took about 65 minutes to emerge from the time its mandibles first broke through the oöthecal wall. (Edmunds, 1954.)
Distribution.—Canada, Ontario, U.S.A.: Connecticut to Wisconsin, south to Florida and Texas. Upper Austral to Tropical Zones. (Townes, 1951.)
Natural host.—Cariblatta delicatula, Cuba (Hebard, 1916a); Parasite identified by Ashmead.
Natural host.—Parcoblatta sp., U.S.A., Ohio (Edmunds, 1952a).
Synonymy.—Evania punctata Brullé [Townes, 1949].
Natural and experimental hosts.—Blatta orientalis, Istrian Peninsula (Fahringer, 1922); Algeria (Cros, 1942); U.S.A., Ohio (Edmunds, 1954).
Blattella germanica? Europe? (Girault 1907, 1914); Europe (Fahringer, 1922). [The records on this host are extremely doubtful. Girault erroneously cited Marlatt (1902) as the source of this record; see footnote 6, page 236. Fahringer, however, claimed that he obtained [Pg 241] seven female parasites from oöthecae of Blattella germanica. He placed female parasites with adults of B. germanica in a glass cage. As soon as oöthecae could be seen between folds of a woolen rag, he removed all the larger cockroaches and held the oöthecae until the parasites emerged. Fahringer may have been dealing with a different species of cockroach, because placing oöthecae in crevices (or between folds of rag) is a habit foreign to B. germanica, the female of which usually carries her oötheca until hatching or until about a day before. Edmunds (1953b) could not induce this wasp to parasitize eggs of B. germanica.]
Periplaneta americana, Istrian Peninsula (Fahringer, 1922); Palestine (Bodenheimer, 1930); U.S.A., Ohio (Edmunds, 1952, 1953b, 1954).
Adult behavior.—The wasps (pl. 33, A) are very active; they walk about a great deal and fly short distances. They are often found in abundance in buildings infested with the larger domiciliary cockroaches where they may reproduce for many generations without leaving the premises. Specimens have also been collected outdoors. (Edmunds, 1953, 1954.) As the adult walks about, the laterally compressed abdomen moves up and down like a waving flag; because of this behavior, these insects are commonly known as ensign-flies. Cros (1942) maintained adults 17 days without food. Edmunds (1954) fed adults on unidentified flowers in the laboratory. He also maintained them for 20 days after capture on a 5-percent honey solution.
Oviposition.—A female P. punctata selected oöthecae of P. americana for oviposition and ignored those of B. orientalis and Parcoblatta pensylvanica in the same cage. Oviposition was accomplished as described for Evania appendigaster. One oötheca was turned over onto its right side by the wasp before she oviposited. (Edmunds, 1952.) Although there seemed to be a "preferred" position for oviposition, it was not obligatory. The usual position was for the female to face the keel of the oötheca, but she also oviposited from the opposite side or, rarely, directly down into the side of the oötheca. The average time spent by females in 10 ovipositions was 29 minutes (range 16-62 minutes). The wasp apparently could not determine whether the eggs had been previously parasitized. The wasp laid her egg between the cockroach eggs rather than in them and she oviposited into oöthecae that had just been dropped and those two weeks old or older. On three occasions nymphal cockroaches emerged within a few hours after the wasp had oviposited. (Edmunds, 1954.) Apparently, for successful parasitization the wasp must oviposit before the cockroaches have reached the final stages of preemergence development. [Pg 242] Edmunds (1954) placed females of Periplaneta americana that were carrying oöthecae, into cages with Prosevania; some of the female wasps showed considerable interest in the attached oöthecae, but he observed oviposition only into egg cases that had been dropped by the cockroaches.
Cros (1942) described an interesting reaction that he called "instinctive hostility" of the oriental cockroach toward Prosevania. A wasp was placed in a jar in which a cockroach had just deposited its oötheca. The wasp tried to oviposit into the egg case but was upset and pursued by the cockroach. The cockroach placed herself over the oötheca, standing high on her legs, and remained there motionless. The wasp then approached from the rear, slipped under the cockroach, and, unnoticed by the cockroach, climbed on the oötheca and oviposited successfully.
Development.—In Blatta orientalis: The developmental period was completed in 40-57 days in summer and fall (Cros, 1942). Time from oviposition to emergence of adult varied from 45-177 days; three parthenotes from an oviposition by an unfertilized female wasp developed in 45-53 days (Edmunds, 1954). In Blattella germanica: Almost 4 weeks spent in development (Fahringer, 1922). In Periplaneta americana: Three wasps developed in 127 days (Edmunds, 1952). Only one parasite develops in each oötheca. There were three generations a year in Ohio. (Edmunds, 1954.) In Algeria there were two to three generations per year. The adult emerged from the oötheca through a hole 4 mm. in diameter. (Cros, 1942.) Parthenogenesis exists; the unfertilized eggs produced only males (Edmunds, 1954).
Distribution.—Eastern U.S.A., from New York and Ohio south to Georgia (Townes, 1949). Europe, Syria, Palestine (Kieffer, 1920).
Synonymy.—Evania sericea Cameron [Townes, 1949, personal communication, 1956]. Evania impressa Schletterer [Townes, p. c., 1956].
Natural hosts.—Cutilia soror and Neostylopyga rhombifolia, Hawaii (Swezey, 1929).
Periplaneta americana and Periplaneta australasiae, Hawaii (Swezey, 1929; Zimmerman, 1948).
Periplaneta sp., Fiji (Lever, 1943, 1946).
Adults are sometimes found resting on leaves covered with honey dew (Williams et al., 1931).
Natural hosts.—Loboptera decipiens, France (Lavagne, 1914; Genieys, 1924).
Picard (1913) believed that Z. splendidula parasitized L. decipiens and not its eggs; however, Lavagne (1914) explained the true relationship by dissecting two specimens of Z. splendidula from oöthecae of L. decipiens.
The following information is taken from Genieys (1924): Oviposition.—Wasp egg is introduced into the still-soft oötheca before the wall hardens. Some oöthecae had four oviposition scars but never contained more than two parasite eggs. Development.—Larva commences development in July or August. Only one larva completes development, but it eats all the eggs in the oötheca. The wasp passes the winter as a last instar larva and pupates in the spring; the adult emerges during the spring or in June. Hyperparasitism.—About 10 percent of the oöthecae of Loboptera decipiens that were parasitized by Z. splendidula were also hyperparasitized by an eulophid (see Syntomosphyrum ischnopterae, p. 249).
Family CLEONYMIDAE
Natural hosts.—Ellipsidion australe, Australia, Queensland (Dodd, 1917): "the parasite when ready to emerge fully occupies the whole space of the destroyed eggs."
Cockroach, Australia, New South Wales (Dr. B. D. Girault, 1915a).
Family ENCYRTIDAE
Natural host.—Cockroach eggs on orange leaves, Australia, New South Wales (Gahan and Peck, 1946). According to Dr. A. B. Gurney the oötheca associated with the type specimens of the wasps in the United States National Museum is possibly Balta sp. (Burks, personal communication, 1956).
Synonymy.—Blatticida ashmeadi. Blatticida Girault, 1915, is preoccupied by Blatticida Ashmead, 1904. In 1923 Gahan and Fagan renamed Blatticida Girault, Blatticidella. [Burks, p. c., 1956.]
Natural host.—Cockroach, Australia, Queensland (Girault, 1915).
Synonymy.—Cristatithorax Girault = Cheiloneurus Westwood [Mercet, 1921].
Natural host.—Ellipsidion australe, Australia, Queensland (Dodd, 1917).
Synonymy.—Comperia merceti var. falsicornis Gomes [Peck, 1951].
Natural hosts.—Blattella germanica, Brazil, Distrito Federal (Gomes, 1941): In the English summary of his paper, Gomes states that C. merceti var. falsicornis was reared from B. germanica. However, in the body of the paper, he states that the supposed origin of the parasite was the oötheca of B. germanica. Burks (personal communication, 1956) does not believe that this wasp parasitizes the eggs of B. germanica. We (unpublished data, 1957) exposed six oöthecae of B. germanica to C. merceti. In order to retard water loss the oöthecae were removed from the females by cutting the insects in two so that each oötheca remained attached to the posterior part of the abdomen. No wasps developed in these oöthecae.
Supella supellectilium, U.S.A., Kansas (Lawson, 1954a); Hawaii (Zimmerman, 1944; Compere, 1946; Keck, 1951).
Adult behavior.—Males and nonovipositing females showed a flea-like jumping tendency. Adults were attracted to light and were found near windows. Both sexes pursued an erratic course in walking and continually touched the surface with their antennae. (Lawson, 1954a.)
Oviposition.—The wasp (pl. 34, B) selected a site on an oötheca with the sheath of her ovipositor; it was uncertain whether there was a definite preference for oviposition sites. Wasp tended to choose a nearly horizontal position for oviposition. She preferred to oviposit into eggs about 2 weeks old, although she would place eggs in oöthecae less than a week old and in embryos in the green band stage. There were 1-50 oviposition punctures per oötheca. (Lawson, 1954a.)
Development.—If enough wasp larvae were present, they ate all eggs in an oötheca. Occasionally wasps developed in one end of an oötheca while cockroaches developed in the other; when this occurred, the cockroach nymphs always emerged last. The developmental period was 30-41 days at room temperature. There were 5-25 parasites per oötheca. The single exit hole in the oötheca varied from 0.6 to 0.9 mm. in diameter. (Lawson, 1954a.)
Distribution.—U.S.A.: New Jersey south to Florida, west to Illinois, Kansas, and Arizona. West Indies; Central and South America; Hawaii. (Burks, personal communication, 1956.)
Natural hosts.—"Phyllodromia" sp., Egypt (Mercet, 1930): According to Mercet, Dr. Alfieri claimed that this wasp parasitized one of the species of "Phyllodromia" found in Egypt, namely, Phyllodromia [= Blattella] germanica, Phyllodromia [= Supella] supellectilium and/or Phyllodromia treitliana. We do not know to which modern genus the host of this wasp belonged.
Cockroach, Egypt? (Mercet in Compere, 1938.)
Natural host.—Cockroach, Australia, Queensland (Girault, 1915).
Family EUPELMIDAE[7]
Natural host.—Cockroach, Australia, Queensland (Girault, 1915).
Natural host.—Eurycotis floridana, U.S.A., Florida (Roth and Willis, 1954a).
Experimental hosts.—Blatta orientalis, Eurycotis floridana, and Periplaneta americana, U.S.A. (Roth and Willis, 1954a).
Adult behavior.—Female wasps are sexually receptive almost immediately on leaving the oötheca. Mating takes 3-4 seconds. Males mate repeatedly and may fertilize several females; females may also mate more than once. At about 80° F. the female wasps lived 2-4 days, males one day.
Oviposition.—The female wasp first probes the oötheca with her sheathed ovipositor until she finds an acceptable spot; she then drills through the wall of the oötheca with her ovipositor. One female oviposited for 5 hours, but briefer periods were more usual. We have seen six or more females ovipositing simultaneously into an oötheca of Eurycotis floridana. One female was seen to feed on material that oozed from the oviposition puncture. The wasp (pl. 34, A) may oviposit into the oötheca of E. floridana while it is still being carried by the female, as well as in oöthecae that have been dropped and which have hard walls. Eggs 36 days old were successfully parasitized.
Development.—In Eurycotis floridana: In the laboratory, development was completed in 34-36 days at about 85° F. This time was regulated to some extent by the number of parasites in the oötheca.
There is evidence that larvae eat unhatched wasp eggs or other larvae. In 34 oöthecae exposed to many female wasps, the maximum number of parasites to emerge was 306; yet an average of 601 wasp larvae were dissected from four oöthecae that had each been exposed to 50 female wasps one week earlier. The larvae usually eat all the host eggs. Cockroach eggs that were not eaten by the wasp larvae sometimes developed but usually failed to hatch. Adult wasps made one to six emergence holes in the oötheca; the average number in 42 oöthecae was two holes.
Number of parasites per oötheca.—In Blatta orientalis: One of 111 oöthecae exposed to female wasps yielded 48 parasites. In Eurycotis floridana: One oötheca parasitized in the field yielded 68 parasites; 8 oöthecae exposed to single wasps for their entire lifespan yielded an average of 50 ± 6 parasites (range 23-81); 34 oöthecae exposed to many wasps for their entire lifespan yielded an average of 198 ± 8 parasites (range 93-306). In Periplaneta americana: Nine oöthecae of 152 exposed to the wasps were found to be parasitized when dissected; 11 adults emerged from one oötheca; no parasites emerged from the other 8 oöthecae.
Sex ratio.—4 ♀♀:1 ♂ from ovipositions by isolated females. In the one oötheca collected in the field, the ratio was 21.6 ♀♀:1 ♂. Parthenogenesis exists; the unfertilized eggs produced only males.
Synonymy.—Anastatus blattidarum Ferrière. Dr. C. Ferrière (personal communication, 1957) is of the opinion that his A. blattidarum is a synonym of A. tenuipes. He stated "I have never been able to see the unique type of A. tenuipes B. y P., which is in Madrid, but the description agrees with A. blattidarum. I had not yet knowledge of Bolívar's description, when describing my species. The parasite of cockroaches eggs [Supella supellectilium] should be called A. tenuipes Bol." Mani (1938) synonymized Solindenia blattiphagus Mani with Anastatus blattidarum.
Natural hosts.—Supella supellectilium, Anglo-Egyptian Sudan (Ferrière, 1930, 1935); U.S.A., Arizona (Flock, 1941); Egypt (Alfieri, in Hafez and Afifi, 1956). Ohio (Hull and Davidson, 1958).
Periplaneta americana, India (Burks in Roth and Willis, 1954a).
Cockroach, Hawaii (Weber, 1951); India (Mani, 1936).
The following is based on parasites that developed on eggs of Supella supellectilium (Flock, 1941): Adult behavior.—Wasp may be seen running rapidly on walls in buildings infested with the cockroach host. The wasp rarely flies but hops proficiently; when disturbed [Pg 247] it can hop from several inches to several feet. The female licks up the drop of fluid that oozes from the oviposition puncture. Females die in a few days, but if fed honey and water may live two weeks. Oviposition.—The female selects an oötheca by feeling with her antennae. Flock stated, without citing experimental evidence, that the age of the egg case was apparently the chief factor determining choice. The wasp took 15-45 minutes to oviposit. Three females oviposited simultaneously into a single oötheca; a single female repeatedly oviposited into one oötheca at intervals. Development.—Completed in an average of 32.6 days at a constant temperature of 82° F.
Number of parasites per oötheca.—Average about 10.7 (range 4-16) (Flock, 1941); 15 (Ferrière, 1935).
Sex ratio.—4 ♀♀:1 ♂ (Ferrière, 1935); average of 6 ♀♀:1 ♂ (Flock, 1941). Parthenogenesis occurs; the unfertilized eggs produced only males (Flock, 1941).
Distribution.—U.S.A.: Maryland, south to Florida, west to Illinois, Kansas, and Arizona. Guatemala; Hawaii; India; Egypt; Sudan. (Burks, personal communication, 1956).
Natural host.—Blattella germanica, Australia, Queensland (Girault, 1924).
Natural host.—"Tree cockroach," U.S.A., Florida (Howard, 1892).
Natural hosts.—Allacta similis, Hawaii (Perkins, 1906, 1913; Timberlake, 1924; Swezey, 1929; Zimmerman, 1948).
Other species of cockroaches, Hawaii (Perkins, 1913).
Family PTEROMALIDAE
Natural host.—Leucophaea maderae?, Jamaica (Westwood, 1839; Sells, 1842). [This host is undoubtedly an error. Sells stated that the oötheca which contained 96 unidentified chalcids had 16 dentations at the edge; the description fits the oötheca of an oviparous cockroach and not that of L. maderae (see Roth and Willis, 1954). Westwood (1839, footnote p. 423) stated that at the meeting of the Entomological Society in 1838 Mr. Sells exhibited 94 specimens of a small [Pg 248] Pteromalus (apparently identified by Westwood) obtained from one cockroach oötheca. This same record of Sells was published posthumously in 1842, although in this paper he identified the host oötheca as "Blaberus" maderae. Cameron (1955) lists a European record of Pteromalus sp. from Periplaneta americana citing Girault (1914) as the source of the record. Girault's record was apparently taken from Westwood's footnote mentioned above.]
Natural hosts.—Blatta orientalis, U.S.A., Illinois (Gahan, 1917).
Parcoblatta pensylvanica, Canada, Ontario (Judd, 1955).
Parcoblatta sp., U.S.A., Ohio (Edmunds, 1952a, 1953a).
"Blattid," U.S.A., Maryland (Gahan, 1917).
One oötheca of P. pensylvanica yielded 14 parasites with a sex ratio of 2.5 ♀♀: 1♂ (Judd, 1955). The average number of parasites in 11 oöthecae of Parcoblatta sp. collected in 1950-51 was 27 wasps (Edmunds, 1952a, 1953a). The adults made two to three emergence holes in the oötheca (Edmunds, 1953a; Judd, 1955).
Family EULOPHIDAE
Natural host.—Periplaneta americana, U.S.A., Missouri (Rau, 1940a): M. chalybii is normally a parasite of Coleoptera and Hymenoptera (Peck, 1951). This is the only record from cockroach eggs. Burks (personal communication, 1956) stated that this species will attack any insect to which it is exposed and can be a serious pest in insect cultures of practically any insect order. In nature it seems to prefer the nests of aculeate Hymenoptera; Rau suggested that the parasites were probably brought into his laboratory with mud nests of Sceliphron caementarium (Drury).
Natural host.—Ellipsidion australe, Australia, Queensland (Dodd, 1917).
Natural hosts.—Parcoblatta sp., U.S.A., Ohio (Burks, 1952; Edmunds, 1952a, 1953a): Ten oöthecae yielded an average of 92 wasps (Edmunds, 1952a). Five oöthecae, collected a year later, yielded an average of 74 wasps; adults sometimes made two to three exit holes in the oötheca (Edmunds, 1953a).
Cockroach, U.S.A., West Virginia (Burks, 1952).
Synonymy.—Epomphaloides ischnopterae Girault [Peck, 1951].
Parker and Thompson (1928) called their hyperparasite Tetrastichus sp. However, Dr. B. D. Burks (personal communication, 1955) has examined the teneral specimens which Parker and Thompson deposited in the U.S. National Museum; he stated that the species is apparently Syntomosphyrum ischnopterae. In view of the experimental work by Parker and Thompson (see below), this wasp may prove to be a hyperparasite on evaniids in cockroach oöthecae rather than a primary parasite on cockroach eggs. (See Zeuxevania splendidula, p. 243.)
Natural hosts.—Ischnoptera sp. [probably Parcoblatta sp. (Rehn, personal communication, 1958)]. U.S.A., Maryland (Girault, 1917).
Zeuxevania splendidula Costa (an evaniid in the oöthecae of Loboptera decipiens), France (Parker, 1924; Parker and Thompson, 1928).
The following information is from Parker and Thompson (1928): Adult behavior.—Courtship and mating were accomplished as soon as adults emerged, and in a manner similar to that in other chalcids. The females oviposited only into oöthecae that were parasitized by Zeuxevania, never into normal, nonparasitized oöthecae. Oviposition.—Oviposition occurred two days after mating. The female wasp stroked the oötheca with her antennae, selected a site, and bored into the oötheca with her ovipositor. She inserted the ovipositor deeply and oviposited for 10-30 minutes. The eggs were deposited randomly on the evaniid larva, some upright and others lying down. Development.—Eggs of the hyperparasite hatched within 3 days and the larvae commenced feeding on the host larva. There were 30 and 50 hyper-parasites in two oöthecae. Sex ratio.—5 ♀♀:1 ♂ (from 3 oöthecae).
Distribution.—U.S.A., District of Columbia, Maryland (Burks, 1952).
Natural host.—Periplaneta australasiae, Sumatra (Gahan, 1923).
Synonymy.—Entedon hagenowii Ratzeburg, Blattotetrastichus hagenowii (Ratzeburg) [Burks, 1943]. Tetrastichodes asthenogmus Waterston. G. J. Kerrich (personal communication, 1957) compared the type of Tetrastichodes asthenogmus Waterston with authentically determined material of Tetrastichus hagenowii and concluded that T. asthenogmus is only a weakly developed specimen of T. hagenowii. [Pg 250] He stated, "The longitudinal dorsal grooves of the scutellum, which are strongly developed in normal hagenowii, are only rather faintly developed in Waterston's type and also the second specimen, which was dissected and mounted on a series of ten microscope slides. No doubt it was this faint development that caused Waterston to describe the species in Tetrastichodes, a segregate that has since been recognized by Dr. Burks (Proc. U. S. Nat. Mus., 1943) as being not truly generically distinct from Tetrastichus."
Natural hosts.—Blatta orientalis, Seychelles (Ratzeburg, 1852); India (Usman, 1949).
Blatta sp., U.S.A., Louisiana (Gahan, 1914).
Blattella germanica (Burks, 1943; Peck, 1951). [In personal communications, Burks and Peck cite Howard (1892) and Marlatt (1902, and the 1908 revision of 1902) as sources for this host record. However, B. germanica is not mentioned specifically as a host of T. hagenowii in the sources cited nor in the 1915 revision of Marlatt's 1902 paper cited by Burks (1943); see footnote 6, p. 236.]
Neostylopyga rhombifolia, Hawaii (Pemberton, 1941): This record is based on one parasitized oötheca. We have exposed, at three different times, groups of 10 to 20 oöthecae of N. rhombifolia to many newly emerged T. hagenowii, but none of the eggs was parasitized (Roth and Willis, unpublished data, 1957).
Parcoblatta sp., U.S.A., Ohio (Edmunds, 1953a).
Periplaneta americana, Africa (Crawford, 1910; Nash, 1955): Nash's record was incorrectly attributed to Syntomosphyrum glossinae Wtstn., a parasite of tse-tse fly pupae (Jordan, 1956), Formosa (Takahashi, 1924; Sonan, 1924); Palestine (Bodenheimer, 1930); Puerto Rico (Seín, 1923; Plank, 1947, 1950; Wolcott, 1951); St. Croix, Virgin Islands (Beatty, 1944); Hawaii (Schmidt, 1937); U.S.A.: Missouri (Rau, 1940a); Ohio (Edmunds, 1955); Florida (parasitized oöthecae were collected near Orlando by members of the Orlando Laboratory, Entomology Research Branch, U.S. Department of Agriculture; the parasites were identified by Burks, personal communication, 1955). Fiji (Lever, 1943); India (Mani, 1936; Usman, 1949); Trinidad and Saudi Arabia (Cameron, 1955). Westwood (1839) stated that 70 parasites belonging to the genus Eulophus emerged from an oötheca of P. americana collected on shipboard. Burks (personal communication, 1955) stated that the wasp was probably T. hagenowii.
Periplaneta australasiae, Australia (Shaw, 1925); India (Usman, 1949); Saudi Arabia, Trinidad (Cameron, 1955); Formosa (Sonan, 1924).
Periplaneta brunnea, U.S.A., Florida (parasitized oöthecae were collected near Orlando, by members of the Orlando Laboratory, Entomology Research Branch, U.S. Department of Agriculture. The parasites were identified by Burks, p. c., 1955).
Cockroach eggs, Formosa (Maki, 1937); Ceylon (Waterston, 1914): Taken on an oötheca.
"Domestic cockroaches," U.S.A., Louisiana (Girault, 1917).
"Roach egg cases," Panama Canal Zone (Rau, 1933).
Evania sp., Hawaii (Ashmead, 1901; Perkins, 1913); Guam (Fullaway, 1912); Fiji (Lever, 1946); Europe, Cuba, Florida (Marlatt, 1902, 1915).
Experimental hosts.—Blatta orientalis, Eurycotis floridana, and Periplaneta americana, U.S.A. (Roth and Willis, 1954b): We have maintained T. hagenowii for over two years through more than 30 generations on eggs of both B. orientalis and P. americana.
Periplaneta fuliginosa, U.S.A., Pennsylvania (Roth and Willis, 1954b); Massachusetts (Roth and Willis, unpublished data, 1957).
Schmidt (1937) deduced that T. hagenowii was a primary parasite of eggs of P. americana because the parasitized oötheca was obtained from a cage covered with screen too fine to permit entry of a larger parasite, such as an evaniid. As noted above, we have reared T. hagenowii for more than 30 generations on cockroach eggs, none of which was ever exposed to parasitization by an evaniid. If T. hagenowii were ever hyperparasitic on Evania, this relationship would be accidental, the eulophid happening to oviposit into an oötheca already containing an evaniid, or vice versa.
Adult behavior.—The male mates soon after becoming adult; he mounts the female from behind, grasps her antennae with his own antennae, and vibrates his wings during copulation. Mating is accomplished in from "several" to 20 seconds (Takahashi, 1924; Edmunds, 1955). The adults are positively phototactic and are capable of hopping for some distance (Edmunds, 1955). The females feed on material that oozes through the oviposition puncture (Roth and Willis, 1954b). Females lived 10 days (Seín, 1923). Without food, females lived 7.8 days and males 3.4 days, but when fed dilute honey females lived 12.5 days (Usman, 1949). Females lived 5-11 days (Roth and Willis, 1954b). Fed water and sugar, the wasps lived 2-6 weeks at 65°F. (Cameron, 1955). Without food, 9 females lived an average of 3.5 days and 9 males an average of 1.7 days, but when fed on raisins, 9 females lived an average of 25 days and 9 males 15 days (Edmunds, 1955). In Formosa there were six generations from April to December (Maki, 1937).
In Hawaii, Severin and Severin (1915) caught 571 T. hagenowii in 10 kerosene traps that were set up to sample populations of Mediterranean fruitfly. Apparently the parasite is attracted by the odor of kerosene.
Oviposition.—The female wasp explores the surface of the oötheca with vibrating antennae (Edmunds, 1955). She bends her abdomen ventrad and repeatedly touches the surface of the oötheca with her valvae; when she finds an acceptable oviposition site, the wasp unsheathes her ovipositor and bores through the wall of the oötheca (Roth and Willis, 1954b). The wasp deposited her eggs in 2-5 minutes (Edmunds, 1955). Wasps oviposited (pl. 34, C) into young or old eggs of P. americana (Roth and Willis, 1954b). A single wasp parasitized more than one oötheca and more than one wasp oviposited into the same oötheca (Roth and Willis, 1954b; Edmunds, 1955). We found freshly laid wasp eggs in 34 empty but previously parasitized oöthecae from which the wasps had emerged (Roth and Willis, 1954b).
Development.—In Periplaneta americana: Development is completed in an average of 36 days (range 29-58 days) (Maki, 1937); 29-40 days (Lever, 1943); average of 23.6 days (range 22-26 days) at 62°-85° F. (Usman, 1949); about 3 months at 60°-65° F. (Cameron, 1955); 31-60 days at 70°-80° F. (Edmunds, 1955). We found that the wasps completed development in 23-56 days at about 85° F., but the period depended on the number of wasps in the oötheca; the larger the number of wasps (up to an average of about 70 wasps per oötheca), the shorter the time required to complete development. Wasps in oöthecae containing 70 or more parasites developed in an average of about 32 days (Roth and Willis, 1954b). Wasp larvae eat the contents of the cockroach egg in which they start development, then rupture the chorion and attack adjoining eggs (Cameron, 1955; Edmunds, 1955). All eggs are consumed when the parasite density is high, but if too few larvae develop per oötheca, some cockroach eggs survive and the embryos complete development (Roth and Willis, 1954b). However, a certain number of cockroach nymphs must complete development to enable the survivors to force open the crista and emerge from the oötheca; fewer than this number of surviving nymphs will be trapped and killed as effectively as if they had been eaten by the parasite. The adult parasites emerge from one to three holes cut through the wall of the oötheca (Usman, 1949; Roth and Willis, 1954b).
Number of offspring per female.—In Blatta orientalis: In the laboratory, 5 oöthecae were left with each of 25 female wasps for their [Pg 253] entire lifespans; of the 125 oöthecae, 32 were parasitized. The average number of offspring per female was 66 (range 5-164) (Roth and Willis, 1954b). In Periplaneta americana: Each of 206 oöthecae was exposed to a single female wasp for 24 hours; the average number of offspring per female was 103 (range 50-139). Five oöthecae were left with each of 38 females for their entire lifespans; of the 190 oöthecae, 81 were parasitized. The average number of offspring per female was 94 (range 45-168 [from original data]) (Roth and Willis, 1954b).
Number of parasites per oötheca.—In Eurycotis floridana: In the laboratory, 3 oöthecae that had been exposed to 20 female wasps yielded an average of 648 parasites (range 606 [from original data] to 685) (Roth and Willis, 1954b). In Neostylopyga rhombifolia: One oötheca yielded 73 parasites (Pemberton, 1941). In Parcoblatta sp.: Two oöthecae yielded an average of 100 parasites (Edmunds, 1953a). In Periplaneta americana: 100 parasites per oötheca (Seín, 1923); 140 (Schmidt, 1937); 25 (Rau, 1940a); 7-38, average 33 (Usman, 1949); 71 (Wolcott, 1951); 4 oöthecae exposed to 20 female wasps yielded an average of 204 wasps (range 164 [from original data] to 261) (Roth and Willis, 1954b); average of 30-40 (Cameron, 1955); 39 oöthecae yielded an average of 93 parasites (range 12-187) (Edmunds, 1955). In Periplaneta australasiae: Oöthecae yielded an average of 40-50 adult parasites (Cameron, 1955); about 50 (Shaw, 1925).
Sex ratio.—3 ♀♀:1 ♂ (Usman, 1949); 4 ♀♀:1 ♂ (Cameron, 1955); 2-8 ♀♀:1 ♂ (Roth and Willis, 1954b); 1.2 ♀♀:1 ♂ (Edmunds, 1955). Parthenogenesis exists; the unfertilized eggs produced only males (Roth and Willis, 1954b; Edmunds, 1955).
Distribution.—Probably worldwide. Eastern and southern U.S.A.; Central and South America; Europe; Arabia; Africa; India; Formosa; Hawaii.
Natural hosts.—Periplaneta americana, Mozambique (Crawford, 1910); Union of South Africa (parasites reared from oöthecae collected in Durban, Natal, by the City Health Department): The parasites were identified by Burks (personal communication, 1956). Jamaica (Gowdey, 1925); Réunion Island (Bordage, 1913).
"Domestic cockroach," Puerto Rico (Wolcott, 1951).
Taxonomy.—Burks (personal communication, 1956) stated that this species (specimens of which are in the U.S. National Museum) is very close to T. hagenowii.
Natural hosts.—Periplaneta americana, Union of South Africa (parasites reared from oöthecae collected in Durban, Natal, by the City Health Department [Burks, p. c., 1956]).
Periplaneta australasiae, Manila, Philippine Islands (Burks, p. c., 1956).
Synonymy.—Because of the war, Cros (1942) could not determine this insect specifically. He designated it provisionally and with reserve under the name Eulophus sp. However, Burks (p. c., 1956) stated that the species is most certainly a Tetrastichus from the description given; but, it is apparently not T. hagenowii because of its brilliant steel-blue color.
Natural host.—Blatta orientalis, Algeria (Cros, 1942): Adult behavior.—Mating began as soon as wasps emerged from an oötheca. Males mated repeatedly. Adults lived up to 5 days in summer and up to 12 days in fall. There were up to four generations per year in the laboratory. Oviposition.—Wasps oviposited into oöthecae 6, 22, 40, and 43 days old, and the parasites developed successfully. More than one female oviposited into the same oötheca. Oviposition was of long duration. Development.—From egg to eclosion took an average of 34 days in summer (range: 30-38 days, 5 oöthecae), and an average of 67 days in fall (range: 58-73 days, 3 oöthecae). An average of 55 parasites developed per oötheca (range 21-105, 5 oöthecae); over 130 wasps emerged from a sixth oötheca. Sex ratio.—10-20 ♀♀:1 ♂.
The nature of the oviposition stimulus(i) for the wasp parasites of cockroach eggs is unknown. Edmunds (1954) noted that Prosevania punctata showed more interest in oöthecae that had been cemented to the substrate than in clean oöthecae that had simply been dropped. Cros (1942) experimented with two females of P. punctata to see if the wasps could find oöthecae that had been buried in sand by the oriental cockroach. After prospecting the sand with their antennae, the wasps dug deep excavations with their front legs but always mistook the location of the oöthecae. Cros suggested that the wasps were misled by the odor left in the jar by the cockroaches. It is quite possible that odor helps the wasp find the host oötheca.
The extent of host selection varies among these parasites; some species will oviposit into the eggs of more than one species of cockroach, but others show some degree of host specificity. Positive selection of specific hosts by certain parasites appears in correlative data from different investigators on pages 235 to 254. There is a small body of data that shows nonacceptance of certain hosts by some of these wasps. For example, Comperia merceti would not parasitize eggs of Blatta orientalis or Periplaneta americana in the laboratory (Lawson, 1954a). We (unpublished data, 1957) exposed a soft oötheca, recently removed from Eurycotis floridana, to C. merceti; no wasps developed; we had similar negative results with C. merceti and oöthecae of B. germanica. We (1954b) could not induce Tetrastichus hagenowii to parasitize eggs of Blattella germanica, B. vaga, or Parcoblatta virginica in the laboratory. In our experiments, T. hagenowii oviposited into eggs of Supella supellectilium, but the wasp eggs either failed to hatch, or if they hatched, the larvae died before completing development. Neither would T. hagenowii parasitize eggs of N. rhombifolia (Roth and Willis, unpublished data, 1957). Anastatus tenuipes would not parasitize the eggs of Latiblattella lucifrons Hebard, Periplaneta americana, B. germanica, or B. vaga (Flock, 1941). Anastatus floridanus would not oviposit into eggs of S. supellectilium and only rarely into eggs of P. americana or B. orientalis (Roth and Willis, 1954a); in the laboratory, this wasp could not be maintained beyond one generation on the eggs of P. americana. Edmunds (1953b) could not induce Prosevania punctata to parasitize eggs of B. germanica. Cros (1942) induced P. punctata to oviposit into a mantid oötheca, but neither mantids nor parasite developed.
A number of wasps of the families Ampulicidae, Sphecidae, and a very few species of Pompilidae have been found to provision their nests with nymphal or adult cockroaches. This habit of preying on cockroaches is primitive (Leclercq, 1954); Leclercq (personal communication, 1955) stated that this habit is always associated with the conservation of a number of structures considered as archaic from a purely morphological point of view.
The records of wasps of the genus Astata capturing cockroaches (e.g., Sickmann, 1893; St. Fargeau in Sharp, 1899) "all trace back to a questionable record by Lepeletier (1841) which probably was a misidentification of the predator" (K. V. Krombein, personal communication, 1956). Marshall (1866) suggested that the braconid Paxylomma buccata Bréb., which he found frequenting cockroach [Pg 256] runs in Pembrokeshire, was parasitic on Ectobius nigripes Stephens; however, this wasp is undoubtedly parasitic on ants, probably on ant larvae (Donisthorpe and Wilkinson, 1930).
The wasps that are known to capture cockroaches, and summaries of their biology, are listed below.
Family POMPILIDAE
Natural hosts.—Cockroaches, India (Bingham, 1900).
Natural host.—Cockroach, Nyasaland (Lamborn in Poulton, 1926): The wasp was collected leading a nymph of the cockroach by its antenna. The cockroach was in a stupefied state, and its antennae were bitten off to about half their length.
Natural hosts.—Cockroaches, India (Bingham, 1900).
Family AMPULICIDAE
The species of Ampulex do not appear to make special nests in which to lay their eggs but drag their prey to any convenient hole, or crack in the ground (Arnold, 1928). Although many species of Ampulex have been described, the prey of only a small number of species have been discovered, but the known prey are all cockroaches.
Synonymy.—Ampulex novarae Saussure [Krombein, personal communication, 1957].
Natural hosts.—Periplaneta americana and Periplaneta australasiae, both as small nymphs, Formosa (Sonan, 1924, 1927): The wasp stings a nymph about one inch long and carries it to a suitable place, such as bamboo pipes, folds of newspaper, or books (in houses), for oviposition.
Periplaneta picea, Japan (Kamo, 1957; Kohriba, 1957).
Experimental hosts.—Periplaneta picea, Japan (Kamo, 1957; Kohriba, 1957).
Kamo (1957) observed that in the field both males and females sucked juices from wounds they made in the stems of Clerodendron [Pg 257] trichotomum Thunberg or Ilex rotunda Thunberg. Kohriba (1957), on the other hand, found both sexes sucking sap of Abies sp. and other trees from points injured by the rostrum of cicadas. Kamo (1957) observed that the female wasp grasped the cockroach by a tergum and stung it several times in the thorax. The wasp always amputated the antennae of the prey and sucked up the fluid oozing from the cut antennae. The wasp egg was placed on the mesocoxa of the cockroach. In the laboratory as many as three cockroaches, each with a wasp egg, were stored in artificial nests per day. Kohriba (1957) observed similar behavior in the laboratory and made these additional notes. The paralyzed cockroach could move its legs and was led to the nest by the wasp which seized its antennae. The egg hatched in 2 days, and after sucking up body fluid for 2 days the larva began to devour the prey. Three days later the larva spun its cocoon, and about one month after spinning a female wasp emerged.
Natural hosts.—Blatta lateralis, wingless females, Iraq (Hingston, 1925): Nesting sites are holes in palm trees, galleries of beetles, or tunnels in ground. The wasp first seizes a cockroach by the edge of its thorax and stings it in the thoracic region, then seizes the cockroach by an antenna and pulls and leads it to the nest. The wasp deposits her egg on the outer surface of the femur of the cockroach's midleg. The nest is closed with debris; later the cockroach recovers from the sting. The wasp larva first feeds externally, then bores into the cockroach and devours the internal organs. Pupation occurs inside the exoskeleton of the cockroach.
Synonymy.—Rhinopsis caniculatus.
Natural hosts.—Ischnoptera sp., U.S.A. (Krombein, 1951).
Lobopterella dimidiatipes, Hawaii (Williams, 1928a, 1929).
Parcoblatta pensylvanica? MacNay (1954) referred to a rare sphecoid wasp in eastern Canada which provisioned its nest with nymphs and adults of P. pensylvanica. Dr. W. R. M. Mason (personal communication, 1957) wrote us that although this wasp was Ampulex canaliculata, it was not reared from the cockroach but was swept from a pine tree. There are no positive records linking A. canaliculata with P. pensylvanica.
Experimental host.—Parcoblatta virginica, females, U.S.A., Missouri (Williams, 1928a, 1929): figure 6.
Nesting sites are in twigs (Krombein, 1951). The adult behavior is similar to that of A. compressa; the female wasp imbibes blood that oozes from the amputated antennae of the cockroach; the egg hatches in 2-3 days, and the development of one male was completed in 33 days (Williams, 1929).
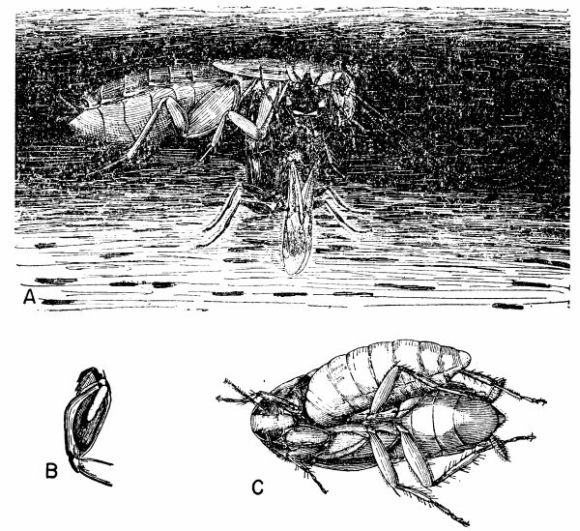 Fig. 6. Ampulex canaliculata attacking Parcoblatta virginica. A, Female
wasp stinging her prey, c. X 4.8. B, Wasp's egg attached to the coxa of the
mesothoracic leg of the cockroach. C, Larva of A. canaliculata (about three-quarters
grown) feeding on the internal organs of the host from the exterior,
c. X 4. (Reproduced from F. X. Williams [1929], through the courtesy of Dr.
F. X. Williams and F. A. Bianchi, Hawaiian Sugar Planters' Association.)
Fig. 6. Ampulex canaliculata attacking Parcoblatta virginica. A, Female
wasp stinging her prey, c. X 4.8. B, Wasp's egg attached to the coxa of the
mesothoracic leg of the cockroach. C, Larva of A. canaliculata (about three-quarters
grown) feeding on the internal organs of the host from the exterior,
c. X 4. (Reproduced from F. X. Williams [1929], through the courtesy of Dr.
F. X. Williams and F. A. Bianchi, Hawaiian Sugar Planters' Association.)
Distribution.—U.S.A.: Connecticut south to Georgia; Ohio, Wisconsin, Missouri, Kansas; in open woods (Krombein, 1951).
(Pl. 35)
Synonymy.—Guĕpe ichneumon of Réaumur [Williams, 1929]; Chlorion (Ampulex) compressum.
Natural hosts.—Periplaneta americana, New Caledonia (Lucas, 1879); India (Dutt, 1912); Reunion (Bordage, 1912).
Periplaneta australasiae, Hawaii (Swezey, 1944).
Periplaneta sp., India (Maxwell-Lefroy, 1909).
Cockroach. Mauritius (Réaumur, 1742); Burma (Bingham, 1897).
Experimental hosts.—Neostylopyga rhombifolia, Periplaneta americana, and Periplaneta australasiae, Hawaii (Williams, 1942, 1942a). Zimmerman's (1948) listings probably were taken from Williams.
Nesting sites.—Holes in walls; holes in banyan and fig trees; in houses in drawers and cartons. Behavior.—Similar to that of A. assimilis. Bordage (1912) gives a complete description of capture of prey. The female wasp cuts off part of the cockroach's antennae, legs, and wings; she sticks her egg onto the host's mesothoracic coxa. The wasp frequents houses in search of prey. Five ♀♀, supplied with a cockroach per day, stored an average of 57±14 cockroaches; 8 ♀♀ stored an average of 45±3 cockroaches; these latter wasps were not supplied with a cockroach per day throughout (mean values computed from Williams, 1942). This wasp will not attack Nauphoeta cinerea (Williams, 1942a) or Pycnoscelus surinamensis (Schwabe, 1950b). On one occasion, A. compressa stung Diploptera punctata, but did not oviposit (Williams, 1942a). Development.—Minimum 34 days, maximum 140 days (Williams, 1942). About 6 weeks (Swezey, 1944). Longevity of adults.—13 ♀♀ lived an average of 110±11 days (minimum 31, maximum 159); several ♂♂ lived 2 months (Williams, 1942).
Natural host.—Ectobius pallidus, France (Picard, 1911, 1919): Nesting sites are in brier or bramble stems, or in crevices in fig trees; the female possibly uses old nests of leaf-cutter bees. The feeding of the wasp larva is similar to that of other Ampulex. Adult wasp emerges by cutting open a passage through its cocoon and through the anus of the cockroach.
Natural hosts.—Cockroaches, Oriental region (Rothney in Sharp, 1899): Nesting sites are in crevices in bark. The female grasps the cockroach by an antenna to drag it to her nest.
Synonymy.—Perkins referred to this species as Ampulex sibirica. Williams (1942a), referring to Perkins's observations, mentions the [Pg 260] species as "A. compressiventris Guérin (=A. siberica Sauss.)." Krombein (personal communication, 1956) has commented upon this synonymy as follows: Ampulex siberica Sauss. is apparently a misidentification by Saussure of sibirica Fab. Kohl (1893) in his revision of the genus Ampulex considered A. compressiventris Guérin to be the correct name for this common African species and that sibirica, described from Siberia, must be another species. However, Turner (1912) stated that he had seen Fabricius's type specimen and that it was identical with what had been called compressiventris; he considered the Siberian locality given by Fabricius as an error. Krombein suggested that Williams's use of the combination siberica Sauss. was a lapsus and that the valid name, if Turner is correct, is sibirica Fab.
Natural hosts.—Cockroaches, West Africa (Perkins in Sharp, 1899): Nesting sites are keyholes. Enters apartments in search of cockroaches. Wasp cocoon protrudes from dead body of cockroach.
Synonymy.—"La mouche bleue" of Sonnerat (Kohl, 1893).
Natural host.—"Kakkerlac," Philippine Islands (Sonnerat, 1776): Nesting sites are readymade crevices. The wasp seizes the cockroach by an antenna and stings the host many times in the "abdomen." She drags the cockroach by an antenna to the nest, and, after depositing her egg, plugs the opening with moistened earth.
Synonymy.—Schulz (1912) considered this to be Dolichurus corniculus. Berland (1925) stated that this is possibly a color variety of D. corniculus. Soyer (1947), from a study of the behavior of the wasps, believed that both D. bicolor and D. haemorrhous are varieties of D. corniculus. Krombein (personal communication, 1956) stated that D. corniculus and D. bicolor differ in characters other than color alone and that D. bicolor is considered a valid species today.
Natural host.—Cockroach, France (Benoist, 1927): The wasp was observed closing the entrance to its burrow. Its egg was attached to the coxa of the midleg of the cockroach.
Maneval (1932) stated that D. bicolor is found at the edge of dry woods along with D. corniculus and that the wasp will also accept the prey of D. corniculus if presented to it.
Synonymy.—Dolichurus haemorrhous Costa [Schulz, 1912]. Berland (1925) listed D. haemorrhous separately but stated that it is perhaps a color variety of D. corniculus.
Natural hosts.—Blattella germanica, France (Benoist, 1927).
Ectobius lapponicus, Germany (Sickmann, 1893); Denmark (Nielsen, 1903); Sweden (Adlerz, 1903); Italy (Grandi, 1931, 1954); France (Benoist, 1927; Maneval, 1928).
Ectobius pallidus, France (Maneval, 1932; Soyer, 1947).
Ectobius panzeri, France (Soyer, 1947).
Ectobius sp., Italy (Grandi, 1954).
Hololampra punctata, Pitten (Handlirsch, 1889).
Loboptera decipiens, France (Ferton, 1894).
Cockroach, Netherlands (Bouwman, 1914).
Nesting sites.—The wasp uses already-made cavities such as rotting dead branches on ground, fissures in the earth, abandoned ant holes, chinks in stone, or the empty cocoon of the ichneumon Ophion luteus (Ferton, 1894; Maneval, 1932).
Behavior.—The prey is immobile while being dragged to the nest but recovers sufficiently from the sting so that if dug up it will run around (Ferton, 1894; Bouwman, 1914; Benoist, 1927; Grandi, 1954). The wasp cuts off about two-thirds of the cockroach's antennae prior to putting its prey in its nest (Adlerz, 1903; Bouwman, 1914; Soyer, 1947). One cockroach is placed in the nest and the wasp's egg is attached to the midcoxa (Ferton, 1894). Oviposition takes 5 to 6 minutes (Maneval, 1939). Wasp fills and seals its nest with bits of earth and stones (Ferton, 1894; Grandi, 1954). The wasp larva feeds externally and devours the entire cockroach, including its exoskeleton (Ferton, 1894).
Development.—Hatching occurs in 3 to 4 days (Ferton, 1894) or longer during cooler weather (Maneval, 1939). Larval development takes 6 days (Grandi, 1954), 8 days (Ferton, 1894), or 10 to 25 days depending on season (Maneval, 1939).
Natural hosts.—"Small Blattidae," India (Turner, 1917).
Natural host.—Parcoblatta sp., U.S.A., Virginia (Krombein, 1951, 1955): Nesting sites are under leaf litter. The prey was a paralyzed third-instar nymph. Distribution.—Ontario. U.S.A. from Canadian border south to Florida in coastal States (Krombein, 1951).
Natural hosts.—Cockroaches, Natal and Southern Rhodesia (Arnold, 1928): The wasp is "usually seen running up and down the [Pg 262] trunks of trees searching for small cockroaches in the crevices of the bark."
Natural hosts.—Allacta similis, nymphs, Hawaii (Williams et al., 1931; Zimmerman, 1948).
Blattella lituricollis, usually nymphs, Philippine Islands, Hawaii (Williams, 1919).
Cutilia soror, nymphs, Hawaii (Williams et al., 1931; Zimmerman, 1948).
"Phyllodromia" sp., Philippine Islands, Hawaii (Williams, 1918; Bridwell, 1920).
Experimental hosts.—"Field cockroaches," Philippine Islands (Williams, 1944).
Nesting site.—Readymade crevices or holes in ground; porosity in lava. Behavior.—The wasp seizes the cockroach by a cercus or leg and stings it in the thorax. She (fig. 7, A) then drags the cockroach to the nest by the base of an antenna. Wasp bites off distal part of host's antennae. She deposits her egg on one of the host's midcoxae. Nest is plugged with lumps of soil. The larva eats the entire host. Development.—Eggs hatched in about a day and a half. Adults emerged about 3 weeks later. About five generations per year. (Williams, 1918, 1919; Williams et al., 1931.)
Natural hosts.—Cockroaches, nymphs, South Africa (Bridwell, 1917). Adult female cockroach carrying an oötheca, France (Deleurance, 1943).
Nesting site.—Plant stem, or in ground possibly an old abandoned nest of Ammophile. Behavior.—Bridwell noted that one wasp larva ate two cockroach nymphs before pupating; the adult emerged about 4 months after cocoon formation. Deleurance observed the wasp close its nest with small pebbles, balls of earth, and small dead branches. The wasp egg was placed on the femur of the midleg. The prey in the nest is alert when disturbed. Deleurance believed the wasp was a variety of D. corniculus.
Natural hosts.—Periplaneta americana and Periplaneta australasiae, Formosa (Sonan, 1924): The wasp stings a nymph about one inch long and carries it to a suitable place (bamboo pipe) for oviposition.
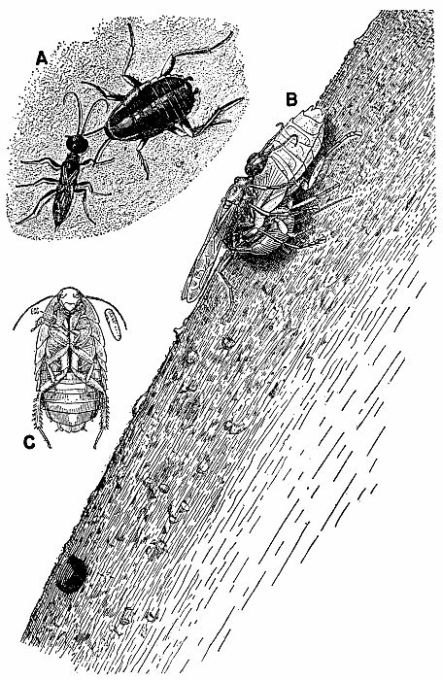 Fig. 7.—Cockroach-hunting wasps. A, Dolichurus stantoni leading a nymph
of Blattella lituricollis to her nest, c. X 4. (Reproduced from F. N. Williams
[1919].) B, Podium haematogastrum attaching her egg to an Epilampra sp.
while on the side of a termite mound that contains the wasp's nest, c. X 1.6. C,
Epilampra sp. parasitized by P. haematogastrum showing the wasp's egg attached
to the right fore coxa, c. X 3.2. (B and C reproduced from Williams
[1928], through the courtesy of Dr. F. X. Williams and F. A. Bianchi.)
Fig. 7.—Cockroach-hunting wasps. A, Dolichurus stantoni leading a nymph
of Blattella lituricollis to her nest, c. X 4. (Reproduced from F. N. Williams
[1919].) B, Podium haematogastrum attaching her egg to an Epilampra sp.
while on the side of a termite mound that contains the wasp's nest, c. X 1.6. C,
Epilampra sp. parasitized by P. haematogastrum showing the wasp's egg attached
to the right fore coxa, c. X 3.2. (B and C reproduced from Williams
[1928], through the courtesy of Dr. F. X. Williams and F. A. Bianchi.)
Natural hosts.—Cockroaches, Oriental region (Williams, 1918, 1928): As far as is known species of this genus of wasps hunt cockroaches.
Family SPHECIDAE
Natural hosts.—Chorisoneura sp., adults, Trinidad, St. Augustine (Callan, 1942): The wasps nest gregariously in sandy places. The wasp itself is parasitized by the mutillid Timulla (Timulla) eriphyla Mickel.
Cockroaches, Trinidad (Williams, 1941a; Callan, 1950).
Natural hosts.—Cockroaches, Italy (Beaumont, 1954).
Natural hosts.—Graptoblatta notulata, Society Islands (Cheesman, 1927, 1928).
Cockroach ("except for its smaller size [it] much resembles Graptoblatta notulata."), New Caledonia (Williams, 1945).
Nesting sites.—Patches of dry soil (Cheesman, 1928); coarse sand at base of a bank (Williams, 1945). Behavior.—The female wasp pounces on the cockroach and stings it into immobility; she carries her prey in flight to the nest. Two to 13 cockroaches may be found in one nest; and one or more wasp eggs may be deposited in one nest. The egg is attached at one end to the host's thorax behind a forecoxa. Nest is sealed with dry pellets of soil. The cockroaches apparently do not recover from the wasp's sting.
Natural hosts.—Ectobius lapponicus, adults, Sweden (Adlerz, 1906); France (Maneval, 1932).
Ectobius pallidus, nymphs, France (Ferton, 1894, 1901; Maneval, 1932; Deleurance, 1946); Italy (Grandi, 1928).
Ectobius panzeri, Netherlands (Bouwman, 1914).
Ectobius sp., Denmark (Nielsen, 1933).
Ferton (1914) stated that he had reported in 1912 that this species hunted Hemiptera, but that this observation was a lapsus. Nesting site.—In the ground of sandy woodlot or border of dry woods; the nest is a hole 5.5 to 8 cm. long ending in a horizontal cell. Grandi (1928) stated that the entrance to the nest descended obliquely for 5 [Pg 265] to 6 cm. and ended 4 cm. below the surface of the ground. Behavior.—Two cockroaches, either sex, adults or nymphs, were stored in the cell (Adlerz, 1903; Grandi, 1928). The wasp laid her egg on the first prey brought, attaching it behind the front coxa. The cockroaches were not excitable and their antennae had not been injured. Grandi (1928) stated that the claws of the hind tarsi of the victims may be amputated. The hatched larva may consume one of its victims in four days leaving only the head, pronotum, tegmina, wings, and the urosternum.
Synonymy.—Trigonopsis abdominalis Perty [Kohl, 1902].
Natural hosts.—Cockroaches, nymphs, Ecuador (Williams, 1928): These wasps are apparently mainly arboreal mud daubers. The female wasp constructs a mud nest on underside of a palm leaf. Wasp egg is attached behind one of the forecoxae of the cockroach. Several cockroaches are stored in each nest. The prey is not immobilized as a result of the sting, and its antennae are left intact.
Natural host.—Parcoblatta pensylvanica, nymphs (Rau, 1937): Nesting sites are mud nests of Sceliphron caementarium (Drury). One to three cockroach nymphs are stored per nest; mud partitions are placed in tube; the nest is plugged with mud which is coated with resin. Distribution.—U.S.A., New York to North Carolina (Murray, 1951); Florida (Krombein and Evans, 1955).
Natural hosts.—Epilamprine cockroaches, Brazil (Williams, 1928): Burrows, lenticular in cross section, are found on shaded trails. The wasp's habits are similar to those of P. flavipenne and P. haematogastrum.
Natural host.—Epilampra abdomen-nigrum, British Guiana (Williams, 1928): Nesting site.—Burrows, about 2 inches deep and lenticular in cross section, are dug in the ground in well-drained, partly sheltered areas; also old Podium nests are used. Behavior.—The wasp stings the cockroach to helplessness and flies with it back to her nest where the host may recover from the sting; one or more cockroaches are stored per nest; the egg is deposited behind the forecoxa while the cockroach is still outside the burrow. The nest is sealed with mud. The larva feeds on most of the cockroach and leaves only some [Pg 266] heavily sclerotized portions in the cell. In 153 nests examined, there was an average of 2.2 ± 0.08 [standard error computed from cited data] cockroaches per cell; four nests contained five cockroaches apiece. Of the 331 cockroaches in the nests, only 6 percent were adults. Development.—Egg hatches in about 2 days; larva feeds about 4 days and pupates about 2 weeks later; adult emerges about 10-12 days later.
Natural host.—Epilampra sp., Brazil, Pará (Williams, 1928): The female wasp (fig. 7, B) burrows into the surface of termite mounds, in banks, and in level ground. This wasp's behavior is similar to that of P. flavipenne. There was an average of 1.6 cockroaches (fig. 7, C) per cell in 74 nests examined. Of the 121 cockroaches collected, 28 percent were adults. Under artificial conditions, the life cycle varied from about a month to 45 days or more.
Natural host.—Parcoblatta virginica, female, U.S.A., New York (Pate, 1949).
Distribution.—U.S.A.: New York to Texas (Murray, 1951).
Natural hosts.—"Wood roaches," British Guiana (Howes, 1917, 1919); Brazil (Williams, 1928): Nesting sites were clay column nests on houses, sides of stumps, or forest trees; banks; termite mound. Variable numbers of cockroaches were placed in the nests with one wasp egg attached behind forecoxa of the last host. The egg hatches in 2 days, the larva pupates about 2 weeks later, and the adult emerges 24 days later.
Natural host.—Epilampra conferta, Brazil (Poulton, 1917): The burrow contained several cockroaches of the same species.
A large roach endeavored to escape by crossing the main front of the army. The creature made several powerful jumps, but each time it touched the ground ... its legs were grasped by the fearless ants.... In the end it fell ... and was instantly torn to bits and carried to the rear.... Another ant with the body of a wood roach was assisted by a worker who held the carrier's abdomen high in the air out of the way of her burden, all the way to the nest.
Howes (1919)
Family FORMICIDAE
From the known entomophagous habits of the lower ants (Wheeler, 1928), we wonder that there are not more records of ants feeding on cockroaches, because this act must occur frequently. Kirby and Spence (1822) stated that R. Kittoe had observed in Antigua that ants which nested in the roofs would seize a cockroach by the legs so it could not move, kill it, and carry it up to their nest. Hotchkiss (1874) observed ants kill cockroaches on shipboard. Cockroaches attracted to sugar in the pantry were killed and carried off by the ants. The destruction of cockroaches by army ants has been recorded by Bates (1863), Wallace (1891), Beebe (1917, 1919), Howes (1919), and others. Dead and mutilated specimens of Ischnoptera sp. [undoubtedly Parcoblatta americana (Gurney, personal communication, 1958)] are common in the nests of species of Formica in California (Mann, 1911).
Natural prey.—Ectobius pallidus, U.S.A., Massachusetts (Roth and Willis, 1957).
Common name.—Carpenter ant.
Natural prey.—Parcoblatta pensylvanica, U.S.A. (Rau, 1940): The ants entered traps set up to capture the cockroach and carried off about a dozen adults of both sexes.
Natural prey.—Small cockroach, Belgian Congo (Raignier and van Boven, 1955).
Natural prey.—Small cockroaches, Belgian Congo (Raignier and van Boven, 1955).
Common name.—"Safari ant."
Natural prey.—Cockroaches, Africa, Lake Victoria (Carpenter, 1920): When the "Safari ants" were hunting, many species of cockroaches were driven from hiding among dead leaves in the forest. The cockroaches rushed about but easily fell prey to the ants which tore them to bits.
Common name.—Army ant.
Natural prey.—Cockroaches, Panama Canal Zone (Johnson, 1954; Schneirla, 1956).
Synonymy.—The identity of this form is unknown. There are no species of Formica on Ceylon. There was another Formica omnivora described from tropical America, whose identity is also unknown (W. L. Brown, personal communication, 1956).
Natural prey.—Cockroaches, Ceylon (Kirby and Spence, 1822).
Common name.—Argentine ant.
Natural prey.—Cockroaches, injured individuals only (Ealand, 1915).
Natural prey.—Ectobius pallidus, U.S.A., Massachusetts (Roth and Willis, 1957).
Common name.—Big-headed ant.
Natural prey.—Holocompsa fulva, Hawaii (Illingworth, 1916).
Nauphoeta cinerea and Pycnoscelus surinamensis, Hawaii (Illingworth, 1914, 1942): The ants followed and killed N. cinerea and P. surinamensis as they burrowed in moist soil and attacked and destroyed N. cinerea in breeding cages.
Class PISCES
In British Guiana, Beebe (1925a) found undetermined cockroach remains in the stomachs of four species of fish belonging to three families, as follows:
Family POTAMOTRYGONTIDAE
(= Potamotrygon hystrix)
Family PIMELODIDAE
Family CHARACIDAE
(= Cynopotamus essequibensis)
The only other records of cockroaches being eaten by fish pertain to the use of cockroaches as bait.[8] Captain William Owen (in Webster, 1834) stated that the Chinese used cockroaches as bait in their fishing excursions. At Reelfoot Lake, Tennessee, Blatta orientalis were kept in large numbers by bait dealers and were sold to fishermen who used them for catching Lepomis pallidus, a sunfish locally known as bream, blue bream, or bluegill (Rau, 1944). In Indiana, oriental cockroaches were collected at a city dump by fishermen (Gould, 1941). Peterson (1956) states that cockroaches are satisfactory bait for bluegills, crappies, channel cat, blue heads, and large mouth black bass.
Class AMPHIBIA[9]
Order CAUDATA
Family PLETHODONTIDAE
Natural prey.—Cryptocercus punctulatus, U.S.A. (Honigberg, 1953): Protozoa which are normally only found in C. punctulatus were present in the intestine of the salamander indicating that this cockroach had been eaten by the amphibian.
Order SALIENTIA
Family BUFONIDAE
Natural prey.—Cockroaches, Belgian Congo (Noble, 1924): The stomachs of 62 out of 72 specimens contained food; this included 3 cockroaches.
Natural prey.—Cockroaches, Brazil (Valente, 1949): Stomach contents revealed the prothorax, legs, and wings of cockroaches, and fragments of wood-cockroaches. This toad frequently feeds at night.
Common name.—Giant toad, marine toad, Surinam toad.
Natural prey.—Epilampra abdomen-nigrum, Trinidad (Weber, 1938): Found in the stomachs of two toads.
Diploptera punctata, Hawaii (Pemberton and Williams, 1938).
Periplaneta sp., Fiji (Lever, 1939): Many householders in Suva have seen the toad eat considerable numbers of these cockroaches.
Pycnoscelus surinamensis, Hawaii (Alicata, 1938; Illingworth, 1941).
Cockroaches, Nicaragua (Noble, 1918): Stomach contents of toads captured at street lamps in Rio Grande consisted chiefly of large cockroaches. Puerto Rico (Wolcott, 1937).
Experimental prey.—Periplaneta americana, U.S.A. (Moore, 1946): Cockroaches containing infective acanthellas of Moniliformis dubius were fed to three toads.
Family HYLIDAE
Common name.—Green tree frog.
Natural prey.—Ischnoptera deropeltiformis, Periplaneta americana, and undetermined cockroaches, U.S.A., Georgia (Haber, 1926): Cockroaches were found in 11 of 100 stomachs.
Family RANIDAE
Natural prey.—Cockroaches, Belgian Congo (Noble, 1924): Of 52 specimens examined, the stomach contents of 17 contained food, including 3 cockroaches.
Natural prey.—Cockroach, Belgian Congo (Noble, 1924): The stomachs of 12 of 56 specimens examined contained food, including one cockroach.
Natural prey.—Cockroach, Puerto Rico (Schmidt, 1920): One of 25 stomachs contained a medium-sized cockroach.
Common name.—"Smoky jungle frog" or "pepper frog."
Natural prey.—Cockroaches, Nicaragua (Noble, 1918): Cockroach wings were found in the stomach of a frog caught around human habitation. Brazil (Valente, 1949).
Natural prey.—Cockroaches, Belgian Congo (Noble, 1924): The stomachs of 35 specimens were examined of which 13 contained food, including 2 cockroaches.
Natural prey.—Cockroaches, Belgian Congo (Noble, 1924): Forty-five of 83 stomachs examined contained food, including 2 cockroaches.
Natural prey.—Cockroaches, Belgian Congo (Noble, 1924): The stomachs of 3 of 40 specimens contained food, including 2 cockroaches.
Common name.—Bullfrog.
Natural prey.—Cockroaches, Puerto Rico (Derez, 1949).
Natural prey.—Cockroaches, Belgian Congo (Noble, 1924): The stomach contents of 138 specimens were examined, 39 of which contained food, including 2 cockroaches.
Common name.—Leopard frog.
Experimental prey.—Periplaneta americana, U.S.A. (Moore, 1946): Cockroaches containing infective acanthellas of Moniliformis dubius were fed to two frogs.
Neostylopyga rhombifolia, U.S.A. (Dr. T. Eisner, personal communication, 1958.)
Natural prey.—Blatta orientalis, U.S.A. (Rau, 1924): Frogs which escaped from a tank in the cellar consumed quantities of this cockroach.
Parcoblatta pensylvanica, U.S.A. (Frost, 1924): One adult specimen recovered from alimentary canal of a frog, probably Rana sp.
Experimental prey.—Blattella germanica, Germany, Frankfurt am Main, Zoological Garden (Lederer, 1952): These insects were preferred by all the insect eaters in the zoo.
Periplaneta americana, Germany, Frankfurt am Main, Zoological Garden (Lederer, 1952): Newly molted individuals were accepted as food, but others were usually passed by or consumed unwillingly.
Class REPTILIA[10]
Order CHELONIA
Family EMYDIDAE
Common name.—Painted turtle.
Natural prey.—Periplaneta australasiae, England (Lucas, 1916, 1920): The cockroach, apparently injured, fell into water in the tortoise house, Zoological Gardens, Regent's Park, and the terrapin ate it.
Order SAURIA
Family GEKKONIDAE
Natural prey.—Cockroaches, Philippine Islands, Laguna (Villadolid, 1934): The geckos frequent holes in trees and underside of bark which are favorable haunts of cockroaches. Stomach contents mostly Blattidae and "Locustidae."
Common name.—House lizard.
Natural prey.—Cockroaches, Philippine Islands, Laguna (Villadolid, 1934): Bulk of stomach contents of 22 lizards consisted of Orthoptera, mostly cockroaches.
Natural prey.—Cockroaches, British Guiana (Beebe, 1925a): The above lizard is found in houses.
Natural prey.—Cockroaches, British Guiana (Beebe, 1925a): The above lizard is found in houses.
Natural prey.—Cockroaches, Australia, Flinders River (Froggatt, 1906): The lizard lived in the walls of the hut and hunted cockroaches upon the roof at night. Arno Atoll (Usinger and La Rivers, 1953).
Family IGUANIDAE
Experimental prey.—Diploptera punctata, U.S.A. (Eisner, 1958).
Natural prey.—Blattella sp., Cariblatta delicatula, Epilampra wheeleri, Periplaneta americana, Periplaneta australasiae, and Symploce flagellata, Puerto Rico (Wolcott, 1924): The last-named cockroach may have been S. ruficollis Rehn and Hebard, the females of which are hard to distinguish from flagellata. Rehn and Hebard (1927) stated that in all probability flagellata does not occur on the island of Puerto Rico. Wolcott (1950) stated that Symploce ruficollis [= bilabiata] serves as food for the crested lizard.
Cockroaches, Puerto Rico (Schmidt, 1920): Of 100 stomachs examined, 16 contained Orthoptera, including cockroaches. Puerto Rico (Wolcott, 1924): One hundred A. cristatellus had eaten 8 cockroaches, 4.14 percent of the total food, or 25 percent of the food for 8 lizards.
Natural prey.—Cockroaches, Puerto Rico (Wolcott, 1924): Two small cockroaches found in 50 lizards examined.
Experimental prey.—Neostylopyga rhombifolia, U.S.A. (Eisner, personal communication, 1958.)
Natural prey.—Periplaneta spp. and Blattidae, Bermuda (Simmonds, 1958): Stomachs of 176 lizards yielded 6 cockroaches.
Natural prey.—Periplaneta spp. and Blattidae, Bermuda (Simmonds, 1958): Stomachs of 46 lizards yielded 31 cockroaches.
Natural and experimental prey.—Pycnoscelus surinamensis, Cuba (Darlington, 1938): This species was eaten both in captivity and in nature. The lizard ate most readily soft, immature cockroaches. Pycnoscelus surinamensis is probably a staple food of the lizard in nature, as Darlington observed wild lizards catch the nymphs.
Natural prey.—Aglaopteryx facies, Puerto Rico (Wolcott, 1924): One cockroach was found in 50 lizards examined.
Cockroach, Puerto Rico (Schmidt, 1920): One of 25 stomachs contained a cockroach.
Natural prey.—"Wood roaches," British Guiana (Beebe, 1925a): The above lizard is arboreal on foliage in low jungle.
Family SCINCIDAE
Common name.—Brown skink.
Natural prey.—Woodroaches, U.S.A., Louisiana (Slater, 1949): Analysis of stomach contents of 84 adult skinks showed that nymphal and adult woodroaches comprised the majority of Orthoptera.
Common name.—Spiny lizard.
Natural prey.—Cockroaches, Philippine Islands, Laguna (Villadolid, 1934): Food of this species was mostly Blattidae.
Natural prey.—Cockroaches, Arno Atoll (Usinger and La Rivers, 1953).
Family AGAMIDAE
Experimental prey?—Cockroaches, Australia (Lee and Mackerras, 1955): A general statement was made that in captivity Agamidae were observed feeding avidly on cockroaches and other insects. Three agamids studied by these workers were Amphibolurus barbatus (Gray), Physignathus lesueurii Gray, and Chlamydosaurus kingii Gray.
Family CHAMAELEONTIDAE
Experimental prey.—Cockroaches, Amsterdam (Portielje, 1914): Large cockroaches were fed to these lizards in the reptile house of Artis.
Family TEIIDAE
Common name.—Iguana, ground lizard.
Natural prey.—Cockroach (nymph), Epilampra wheeleri, and Periplaneta americana, Puerto Rico (Wolcott, 1924): Stomach contents of 15 lizards were analyzed. E. wheeleri formed 30 percent of the food of one lizard. The cockroach nymph formed 5 percent of the food of one lizard. One P. americana formed 20 percent of the food of one lizard; another formed 50 percent of the food of a second lizard.
Experimental prey.—Cockroach nymphs, Puerto Rico (Wolcott, 1924).
Natural prey.—Cockroaches, British Guiana (Beebe, 1925a): The above lizard is terrestrial and found near clearings. The stomach contents of 18 out of 40 reptiles contained cockroach remains.
Natural prey.—Cockroaches, British Guiana (Beebe, 1925a): This is a terrestrial lizard found near clearings. The stomach contents of 4 out of 40 lizards contained cockroaches.
Natural prey.—Cockroaches, West Indies (H., 1800).
Experimental prey.—Blatta orientalis, U.S.A. (Rau, 1924): Rau called the predator a common gray lizard.
Periplaneta americana, Germany, Frankfurt am Main, Zoological Garden (Lederer, 1952): Newly molted cockroaches were accepted as food, but others were usually passed by or consumed unwillingly.
Order SERPENTES
Family COLUBRIDAE
Synonymy.—Heterodon contortrix [Dr. Doris M. Cochran, personal communication, 1957].
Common name.—Hog-nosed snake.
Experimental prey.—Periplaneta americana, U.S.A. (Moore, 1946): Cockroaches containing infective acanthellas of Moniliformis dubius were fed to one snake.
Experimental prey.—Blatta orientalis, U.S.A. (Rau, 1924).
Class AVES
The cockroach is always wrong when arguing with a chicken.
Spanish proverb (Hartnack, 1939)
Arboreal cockroaches hidden in and under bark are much more likely to be encountered by birds than by other predators, and insectivorous birds undoubtedly consume many more cockroaches than the few records would indicate. Most of the records we have located identify the birds at least by common name. Where possible we have given the scientific names for those birds whose common names are recognizably specific. We have followed the systematic classification of Wetmore (1940).
Figuier (1869) stated that poultry and owls are very fond of cockroaches. Perkins (1913) made the general statement that some of the native birds of Hawaii are partial to the endemic Allacta similis. Asano (1937) stated that in Japan natural enemies of cockroaches may be found in the Galliformes, Strigiformes, Passeriformes, and Piciformes. Although Lederer (1952) successfully fed newly molted Periplaneta americana to insectivorous birds in the Zoological Garden, Frankfurt am Main, Blattella germanica were preferred by these birds. The following records are of specific birds feeding on cockroaches.
Order ANSERIFORMES
Family ANATIDAE
Natural prey.—Pycnoscelus surinamensis, Australia (Fielding 1926): The ducks became infected with Manson's eye worm of which P. surinamensis is the only known intermediate host.
Cockroach, Bermuda (Jones, 1859): "All kinds of poultry feed greedily upon the cockroach; tame ducks spending entire moonlight nights in their capture."
Order GALLIFORMES
Family PHASIANIDAE
Common name.—Kojukei.
Natural prey.—Cockroaches, Japan (Asano, 1937).
Common name.—Jungle fowl.
Natural prey.—Periplaneta australasiae, Hawaii (Schwartz and Schwartz, 1949).
Common name.—Korean pheasants.
Experimental prey.—Blattella germanica and Periplaneta picea, Japan (Asano, 1937): Adults of these cockroaches were devoured at once when they were fed with the heads cut off.
Common name.—Pheasant.
Natural prey.—Blattidae, unidentified (below 1 percent of the diet), Cutila soror (below 1 percent of the diet), Diploptera punctata (above 6 percent of the diet), and Pycnoscelus surinamensis (6 percent of the diet), Hawaii (Schwartz and Schwartz, 1949).
Common name.—Japanese quail.
Natural prey.—Blattidae (unidentified) and Lobopterella dimidiatipes, Hawaii (Schwartz and Schwartz, 1949).
Natural and experimental prey.—Blaberus craniifer, U.S.A., Key West, Florida. J.A.G. Rehn in 1912 (personal communication) observed chickens feeding on nymphs of B. craniifer which had dropped to the ground from among stacked coffins in an undertaker's shack.
Blatta orientalis, U.S.A. (Rau, 1924): The chickens ate cockroaches that were caught in traps.
Hebardina concinna, Japan (Asano, 1937): Experimental feeding to white Leghorn chickens.
Periplaneta americana, Surinam (Stage, 1947): Several cockroaches ran off the floor of a house, which was being sprayed with DDT, and were eaten by chickens. Although some chickens had DDT tremors the next day, all appeared normal two days later.
Pycnoscelus surinamensis, Australia (Fielding, 1926); Formosa, experimental feeding (Kobayashi, 1927); Australia, experimental feeding (Fielding, 1927, 1928); U.S.A., Florida, experimental feeding (Sanders, 1928); Antigua (Hutson, 1943); Hawaii (Illingworth, 1931; Schwabe, 1949, 1950a, 1950b). This cockroach is the intermediate host of Oxyspirura mansoni, the chicken eye worm.
Cockroaches, Guadeloupe (Dutertre, 1654); Africa (Moiser, 1947): "Poultry" ate cockroaches which had been killed by DDT and sodium fluoride. Hawaii (Zimmerman, 1948).
Natural prey.—Cockroaches, British Guiana (Beebe, 1925a): The food of two small species of leaf-colored partridges that lived on the jungle floor, consisted chiefly of cockroaches and beetles.
Family MELEAGRIDIDAE
Common name.—Turkey.
Natural prey.—Pycnoscelus surinamensis, Antigua (Hutson, 1943): Turkeys were found heavily infected with Manson's eye worm of which P. surinamensis is the only known intermediate host. These turkeys therefore were presumed to have fed on this cockroach.
Order COLUMBIFORMES
Family COLUMBIDAE
Common name.—Chinese dove.
Natural prey.—Pycnoscelus surinamensis, Hawaii (Schwabe, 1950b).
Experimental prey.—Pycnoscelus surinamensis, Australia (Fielding, 1927); U.S.A., Florida (Sanders, 1928).
Order STRIGIFORMES
Family STRIGIDAE
Common name.—Bare-legged owl.
Natural prey.—Epilampra sp., Puerto Rico (Wetmore, 1916): One specimen identified in stomach of a wild-caught owl.
Cockroaches, Puerto Rico (Wetmore, 1916): These insects were found in stomachs of five owls.
Order CORACIFORMES
Family TODIDAE
Common name.—Porto Rican tody.
Natural prey.—Plectoptera poeyi?, Puerto Rico (Wetmore, 1916): The stomachs of 89 birds were examined; a single bird had eaten the above cockroach. According to Wolcott (1950) P. poeyi could be Plectoptera dorsalis, P. rhabdota, or P. infulata.
Family BUCEROTIDAE
Synonymy.—Lophocerus birostris [Dr. H. Friedmann, personal communication, 1957].
Common name.—Common gray hornbill.
Natural prey.—Cockroaches, India, Central Provinces (D'Abreu, 1920).
Order PICIFORMES
Family PICIDAE
Synonymy.—Liopicus mahrattensis [Friedmann, p. c. 1957].
Common name.—Yellow-fronted pied woodpecker.
Natural prey.—Cockroaches, India, Central Provinces (D'Abreu, 1920).
Common name.—Puerto Rican woodpecker.
Natural prey.—Pycnoscelus surinamensis, Puerto Rico (Wetmore, 1916): One specimen found in 59 bird stomachs examined.
Order PASSERIFORMES
Family FORMICARIIDAE
Common name.—Bicolored antbird.
Natural prey.—Cockroaches, Panama Canal Zone (Johnson, 1954): This bird feeds on small cockroaches, and other arthropods, which are flushed from their hiding places by swarms of the army ant, Eciton burchelli.
Family ORIOLIDAE
Common name.—Puerto Rican oriole.
Natural prey.—Cockroaches, Puerto Rico (Wetmore, 1916): Cockroaches and oöthecae found in the birds' stomachs.
Family CORVIDAE
Common name.—Florida jay.
Experimental prey.—Pycnoscelus surinamensis, U.S.A., Florida (Sanders, 1928).
Common name.—Blue jay.
Experimental prey.—Diploptera punctata, U.S.A. (Eisner, 1958).
Eurycotis floridana, Neostylopyga rhombifolia, and Periplaneta americana, U.S.A. (Eisner, personal communication, 1958): E. floridana was only eaten after the odor of 2-hexenal, which was released by the insect on being attacked by the bird, had dissipated.
Family PARADISEIDAE
Experimental prey.—Cockroaches, Malaya and on shipboard (Wallace, 1869): Two adult males fed voraciously on rice, bananas, and cockroaches. Wallace collected cockroaches every night on board ship to feed the birds. "At Malta ... I got plenty of cockroaches from a bakehouse, and when I left, took with me several biscuit-tins full, as provision for the voyage home."
Family TROGLODYTIDAE
Common name.—House wren.
Natural prey.—Cockroaches, U.S.A. (Greenewalt and Jones, 1955): The wren carried three small cockroaches to nestlings; the records probably represent incidental captures.
Common name.—Cucarachero.
Natural prey.—Cockroach (called Chilicabra by Peruvian Indians), Peru (Tschudi, 1847): The bird seized the cockroach and bit off its head then devoured the body discarding the wings.
Family LANIIDAE
Common name.—Loggerhead shrike.
Experimental prey.—Pycnoscelus surinamensis, U.S.A., Florida (Sanders, 1928).
Family STURNIDAE
Common name.—Myna, mynah.
Natural prey.—Cockroaches, Hawaii, Lanai (Illingworth, 1928): Illingworth reported that he had never seen as many cockroaches anywhere else in Hawaii. The birds followed tractors that were destroying cactus and kept close to the chain that turned over the stumps. The following species were collected: Allacta similis, Blattella germanica, Cutilia soror, Diploptera punctata, Leucophaea maderae, Periplaneta americana, Periplaneta australasiae, Pycnoscelus surinamensis. Illingworth did not state whether the birds ate all these species indiscriminately.
Pycnoscelus surinamensis, Hawaii (Williams et al., 1931; Schwabe, 1950b): In many places this species forms an important fledgling food for mynah birds.
Family VIREONIDAE
Common name.—Latimer's vireo.
Natural prey.—Periplaneta sp., Puerto Rico (Wetmore, 1916): Cockroaches were found in one of 43 stomachs examined.
Family ICTERIDAE
Common name.—Yellow-shouldered blackbird.
Natural prey.—Cockroaches, Puerto Rico (Wetmore, 1916): Oöthecae and remains of adult cockroaches found in stomachs.
Common name.—Bobolink.
Experimental prey.—Pycnoscelus surinamensis, U.S.A., Florida (Sanders, 1928).
Common name.—Puerto Rican blackbird.
Natural prey.—Cockroaches, Puerto Rico (Wetmore, 1916): A few eggs (oöthecae) of cockroaches in stomachs.
Experimental prey.—Pycnoscelus surinamensis, U.S.A., Florida (Sanders, 1928).
Family FRINGILLIDAE
Common name.—English sparrow.
Natural prey.—Pycnoscelus surinamensis, Hawaii (Illingworth, 1931; Schwabe, 1950b): Remains of this cockroach were found in the stomach of the sparrow.
Natural prey.—Periplaneta americana, England (Lucas, 1908, 1920).
Cockroaches, Japan (Asano, 1937).
Common name.—Carib grassquit.
Natural prey.—Cockroaches, Puerto Rico (Wetmore, 1916): Animal food was found in 5 of 72 stomachs examined; one bird had eaten two cockroaches among other insects.
Class MAMMALIA[11]
Order MARSUPIALIA
Family DIDELPHIDAE
Natural prey.—Cockroaches, British Guiana (Beebe, 1925a): The above opossum is nocturnal and arboreal but nests on the ground in grass.
Order INSECTIVORA
Family ERINACEIDAE
Experimental prey.—Blattella germanica, France (Brumpt and Urbain, 1938): Two hedgehogs were fed cockroaches infested with Prosthenorchis elegans and P. spirula.
Common name.—Hedgehog.
Natural prey.—Cockroaches, England (Samouelle, 1841; Cowan, 1865).
Order CHIROPTERA
Family MOLOSSIDAE
Natural prey.—Cockroaches, British Guiana (Beebe, 1925a): The above bat is a common house bat of the area.
Order PRIMATES
Family LEMURIDAE
Natural prey.—Blattella germanica, France (Brumpt and Urbain, 1938): The monkey apparently became infested naturally with Prosthenorchis spirula for which B. germanica was the intermediate host in the monkey house.
Natural prey.—Blattella germanica, France (Brumpt and Urbain, 1938): See comment under Lemur coronatus.
Family LORISIDAE
Synonymy.—Lemur tardigradus [Dr. D. H. Johnson, personal communication, 1957].
Natural prey.—Cockroaches, on board ship (Cowan, 1865).
Common name.—Potto.
Natural prey.—Blattella germanica, France (Brumpt and Urbain, 1938): See comment under Lemur coronatus.
Experimental prey.—Blattidae, East Africa (Pitman, 1931): Both sexes of the potto ate freely of all types of cockroaches.
Family TARSIIDAE
Experimental prey.—Cockroaches, Borneo (Shelford, 1916).
Family CEBIDAE
Synonymy.—Aotus [Simpson, 1945].
Common name.—Canal Zone night monkey.
Natural prey.—Leucophaea maderae, Panama (Foster and Johnson, 1939): Captive monkeys became naturally infested with Protospirura muricola by eating cockroaches that contained infective larvae of the worm.
Common name.—Darien black spider monkey.
Natural prey.—Leucophaea maderae, Panama (Foster and Johnson, 1939): See comment under Aotes zonalis.
Natural prey.—Blattella germanica, France (Brumpt and Urbain, 1938): See comment under Lemur coronatus.
Common name.—White-faced monkey.
Natural prey.—Leucophaea maderae, Panama (Foster and Johnson, 1939): Favorite item of food in the laboratory. See comment under Aotes zonalis.
Natural prey.—Blattella germanica, France (Brumpt and Urbain, 1938): See comment under Callithrix chrysoleucos.
Family CALLITHRICIDAE
Synonymy.—Callithrix chrysolevea [Johnson, personal communication, 1957].
Natural prey.—Blattella germanica, France (Brumpt and Urbain, 1938): The monkey apparently became infested naturally with Prosthenorchis elegans for which B. germanica was the intermediate host in the monkey house.
Synonymy.—Simia jacchus.
Natural prey.—Blattella germanica, France (Brumpt and Urbain, 1938): See comment under Lemur coronatus.
Cockroaches, on board ship (Neill, 1829; also cited by Samouelle, 1841, and Cowan, 1865): "It was quite amusing to see it at its meal. When he had got hold of one of the largest cockroaches, he held it in his fore paws, and then invariably nipped the head off first; he then pulled out the viscera and cast them aside, and devoured the rest of the body, rejecting the dry elytra and wings, and also the legs of the insect, which are covered with short stiff bristles. The smaller cockroaches he eat[s] without such fastidious nicety."
Natural prey.—Blattella germanica, France (Brumpt and Urbain, 1938): See comment under Callithrix chrysoleucos.
Synonymy.—Midas rosalia [Simpson, 1945].
Natural prey.—Blattella germanica, France (Brumpt and Urbain, 1938): See comments under Lemur coronatus and Callithrix chrysoleucos.
Natural prey.—Blattella germanica, France (Brumpt and Urbain, 1938): See comments under Callithrix chrysoleucos.
Family CERCOPITHECIDAE
Experimental prey.—Cockroaches, East Africa (Carpenter, 1921, 1925): The monkey rarely tasted and usually ignored cockroaches offered to it. In one experiment the monkey had to be deprived of food before it would eat the cockroach.
Synonymy.—Macaca rhesus [Johnson, personal communication, 1957].
Experimental prey.—Blattella germanica, France (Brumpt and Urbain, 1938, 1938a): The macaque was fed cockroaches infested with Prosthenorchis elegans and P. spirula.
Synonymy.—Inuus sylvanus [Simpson, 1945].
Common name.—Macaque.
Natural prey.—Blattella germanica, France (Brumpt and Urbain, 1938): See comment under Lemur coronatus.
Experimental prey.—Blattella germanica, France (Brumpt and Urbain, 1938, 1938a): This baboon was fed cockroaches infested with Prosthenorchis elegans and P. spirula.
Family PONGIDAE
Common name.—Chimpanzee.
Natural prey.—Blattella germanica, Netherlands (Thiel and Wiegand Bruss, 1946): Indirect evidence for this relationship was shown by these workers who found two animals heavily infected with Prosthenorchis spirula in a zoo in Rotterdam; the intermediate host of the worm was shown to be B. germanica.
Family HOMINIDAE
Natural prey.—Oöthecae of Blatta orientalis and Neostylopyga rhombifolia, Thailand (Bristowe, 1932).
Periplaneta americana, Formosa (Takahashi, 1924).
Periplaneta americana and Periplaneta australasiae, Australia, China, and Japan (Bodenheimer, 1951).
Cockroaches, Annam and French Guinea (Brygoo, 1946).
In addition to the above records of cockroaches being used as food by man these insects have also been eaten for medicinal purposes (see Roth and Willis, 1957a).
Order EDENTATA
Family DASYPODIDAE
Synonymy.—Tatu novemcinctum [Johnson, personal communication, 1958].
Natural prey.—Ischnoptera deropeltiformis, Texas (Hebard, 1917): A specimen of this cockroach in the U. S. National Museum was taken from the stomach of the armadillo.
Order RODENTIA
Family MURIDAE
Experimental prey.—Diploptera punctata, U.S.A. (Eisner, 1958).
Synonymy.—Mus decumanus; Epimys norvegicus.
Natural prey.—Leucophaea maderae, Venezuela (Brumpt, 1931): Rats infested with Protospirura bonnei presumably ate this cockroach which is the intermediate host of the worm.
Periplaneta americana, Brazil (Magalhães, 1898): Remains found in the stomachs of brown rats. Denmark (Fibiger and Ditlevsen, 1914): This cockroach was found to be the intermediate host of Gongylonema neoplasticum, a parasite of rats.
Natural prey.—Periplaneta americana, Denmark (Fibiger and Ditlevsen, 1914): See comment after these authors under Rattus norvegicus.
Natural prey.—Cockroaches, India (Maxwell-Lefroy, 1909); Burma (Subramanian, 1927).
Family CAVIIDAE
Experimental prey.—Blattella germanica, U.S.A. (Hobmaier, 1941): Guinea pigs were fed cockroaches infested with Physaloptera maxillaris.
Order CARNIVORA
Family CANIDAE
Experimental prey.—Blattella germanica, U.S.A. (Hobmaier, 1941): Dogs were fed cockroaches infested with Physaloptera maxillaris. U.S.A. (Petri and Ameel, 1950): Cockroaches infested with Physaloptera rara were fed to a dog.
Experimental prey.—Blattella germanica, U.S.A. (Petri and Ameel, 1950): Cockroaches infested with Physaloptera rara were fed to a coyote.
Experimental prey.—Blattella germanica, France (Brumpt and Urbain, 1938a): A fox was successfully infected when fed cockroaches infested with Prosthenorchis elegans and P. spirula.
Family PROCYONIDAE
Common names.—Cacomistle, ring-tailed cat.
Natural prey.—Cockroaches, U.S.A., Arizona (Dr. H. Stahnke, personal communication, 1953): The ring-tailed cat enters dwellings located on the desert and feeds on cockroaches and other arthropods.
Natural prey.—Blattella germanica, France (Brumpt and Urbain, 1938): The coati apparently became infested naturally with Prosthenorchis spirula for which B. germanica was the intermediate host in the laboratory.
Natural prey.—Blattella germanica, on board ship (Myers, 1931): This insect was eaten when other insects were absent.
Cockroach, a small outdoor species, Trinidad (Myers, 1931).
Natural prey.—Cockroaches, British Guiana (Beebe, 1925a).
Family MUSTELIDAE
Experimental prey.—Blattella germanica, France (Brumpt and Urbain, 1938a): A badger was successfully infected when fed cockroaches infested with Prosthenorchis elegans and P. spirula.
Family VIVERRIDAE
Natural prey.—Epilampra wheeleri, Eurycotis improcera, Panchlora nivea, Pycnoscelus surinamensis, and others unidentified to species, St. Croix and Puerto Rico (Wolcott, 1953): Based on 37 or more cockroaches obtained from stomachs of 42 mongooses collected in St. Croix (by Seaman) and 56 collected in Puerto Rico (by Pimentel).
Pimentel (personal communication, 1958) has given us the following percentage occurrence of cockroach species in the total number of mongoose stomachs that he examined in Puerto Rico: Epilampra wheeleri 1.8, Ischnoptera rufa rufa 3.6, Panchlora nivea 1.8, Periplaneta americana 1.8, and Pycnoscelus surinamensis 19.6.
Natural prey.—Periplaneta americana and Periplaneta australasiae, Hawaii (Perkins, 1913): Large numbers of these cockroaches are devoured.
Cockroach, East Africa (Loveridge, 1923): Cockroach remains found in stomach of mongoose.
Family FELIDAE
Natural prey.—Periplaneta americana, Hawaii (Williams et al., 1931).
Cockroaches, U.S.A., Arizona (Stahnke, personal communication, 1953).
Experimental prey.—Blattella germanica, U.S.A. (Hobmaier, 1941): Cats were fed cockroaches infested with Physaloptera maxillaris. U.S.A. (Petri and Ameel, 1950): Cockroaches infested with Physaloptera rara were fed to a kitten. France (Brumpt and Urbain, [Pg 290] 1938a): A young cat was fed cockroaches infested with Prosthenorchis elegans and P. spirula.
Natural prey.—Cockroaches, Panama (Dr. H. L. Sweetman, personal communication, 1958): An ocelot was seen collecting and feeding on cockroaches, possibly Blaberus sp. "The ocelot was quite efficient and seemed to relish the roaches."
Only naturally occurring associations are included in this list. Commensal cockroaches are listed on page 315. Bacteroids are not listed because they undoubtedly occur in all species. The higher plants were excluded because most of the associations may be too casual to constitute symbiosis; however, many of the plant associations were included in the chapter on ecology. The cockroaches and the associates within each category are arranged alphabetically by genus and species. Page references are to citations in the classified sections where details of the associations and/or sources of the records are given.
Aglaopteryx facies
Mite: Undetermined, p. 220.
Reptile: Anolis stratulus, p. 274.
Aglaopteryx diaphana
Nematode: Protrellus manni, p. 200.
Allacta similis
Insects:
Dolichurus stantoni, p. 262.
Solindenia picticornis, p. 247.
Anaplecta sp.
Fungus: Herpomyces anaplectae, p. 134.
Aptera fusca
Protozoan: Gregarina fastidiosa, p. 182.
Arenivaga bolliana
Insect: Sarcophaga omani, p. 229.
[Pg 291]
Arenivaga roseni
Insects: Undetermined reduviids, p. 227.
Balta patula
Insect: Rhipidioides ableptus, p. 230.
Blaberus atropos
Bacterium: Spirochaeta blattae, p. 125.
Protozoa:
Endolimax nana, p. 180.
Entamoeba coli, p. 178.
Nematodes:
Leidynema appendiculata, p. 197.
Leidynema cranifera, p. 198.
Blaberus craniifer
Bacteria:
Aerobacter aerogenes, p. 111.
Alcaligenes faecalis, p. 111.
Bacillus cereus, p. 120.
Bacillus subtilis, p. 121.
Escherichia coli var. communior, p. 113.
Escherichia freundii, p. 113.
Micrococcus pyogenes var. albus, p. 106.
Micrococcus pyogenes var. aureus, p. 107.
Proteus vulgaris, p. 114.
Pseudomonas aeruginosa, p. 104.
Fungi:
Herpomyces tricuspidatus, p. 138.
Penicillium sp., p. 131.
Rhizopus nigricans, p. 133.
Saccharomyces cerevisiae, p. 133.
Protozoan: Diplocystis (?) sp., pp. 181, 184.
Nematodes:
Leidynema cranifera, p. 198.
Protrelleta floridana, p. 199.
Mite: Iolina nana, p. 219.
Undetermined, p. 220.
Bird: Chicken, p. 278.
Blaberus discoidalis
Mite: Undetermined, p. 220.
Blaberus giganteus
Hair worm: Gordius pilosus, p. 202.
Blaberus sp.
Fungi:
Herpomyces macropus, p. 136.
Herpomyces paranensis, p. 136.
Herpomyces periplanetae, p. 137.
Herpomyces tricuspidatus, p. 138.
Protozoan: Leptomonas blaberae, p. 167.
[Pg 292]
Blaptica dubia
Protozoan: Pileocephalus blaberae, p. 184
Blatta (Shelfordella) lateralis
Bacterium: Shigella paradysenteriae, p. 119.
Insect: Ampulex assimilis, p. 257.
Blatta orientalis
Bacteria:
Aerobacter aerogenes, p. 111.
Alcaligenes faecalis, p. 111.
Alcaligenes recti, p. 111.
Arthromitus intestinalis, p. 124.
B. aerobio del pseudoedema maligno, p. 125.
B. alcaligenes beckeri, p. 125.
B. del pseudoedema maligno, p. 125.
Bacillo proteisimile, p. 126.
Bacillo similcarbonchio, p. 126.
Bacillo similtifo (Bacillo tifosimile), p. 126.
Bacillus bütschlii, p. 120.
Bacillus periplanetae, p. 121.
Bacillus stellatus, p. 121.
Bacillus subtilis, p. 122.
Bacillus tritus, p. 122.
Bacteroides uncatus, p. 119.
Clostridium lentoputrescens, p. 122.
Clostridium novyi or Clostridium sporogenes, p. 122.
Enterococcus sp., p. 109.
Escherichia coli, p. 112.
Lactobacillus fermenti, p. 109.
Micrococcus pyogenes var. albus, p. 107.
Micrococcus pyogenes var. aureus, p. 107.
Micrococcus sp., p. 108.
Paracolobactrum sp., p. 113.
Pasteurella pestis, p. 119.
Proteus sp., p. 114.
Proteus vulgaris, p. 114.
Pseudomonas aeruginosa, p. 104.
Pseudomonas eisenbergii, p. 105.
Pseudomonas fluorescens, p. 105.
Salmonella typhosa, p. 117.
Sarcina alba, p. 108.
Sarcina sp., pp. 108, 109.
Sarcina symbiotica (host may have been B. germanica), p. 108.
Sarcina ventriculi, p. 108.
Serratia marcescens, p. 117.
Spirillochaeta blattae, p. 127.
Spirillum α, β and γ, p. 105.
Spirillum sp., p. 105.
Spirochaeta periplanetae, p. 125.
Streptococcus faecalis, p. 109.
Streptococcus liquefaciens, p. 110.
Streptococcus microapoika, p. 110.
Streptococcus pyogenes, p. 110.
Streptococcus sp., p. 110.
Treponema parvum, p. 125.
Treponema stylopygae, p. 125.
Vibrio sp., p. 106.
Fungi:
Aspergillus fumigatus, p. 130.
Blastocystis hominis, p. 133.
Blastocystis sp., p. 133.
Candida zeylanoides, p. 129.
Coccidioides periplanetae, p. 133.
Herpomyces periplanetae, p. 137.
Herpomyces stylopygae, p. 137.
Torula gropengiesseri, p. 132.
Torulopsis sp., p. 130.
Protozoa:
Balantidium praenucleatum, p. 187.
Bodo blattae, p. 167.
Coelosporidium periplanetae, p. 185.
Diplocystis schneideri, p. 181.
Endamoeba blattae, p. 177.
Entamoeba thomsoni, p. 179.
[Pg 293]
Endolimax blattae, p. 180.
Endolimax sp., p. 180.
Gregarina blattarum, p. 181.
Haplosporidium periplanetae, p. 185.
Hartmannella blattae, p. 177.
Herpetomonas periplanetae, p. 167.
Hexamita periplanetae, p. 171.
Lophomonas blattarum, p. 172.
Lophomonas striata, p. 173.
Monas sp., p. 167.
Monocercomonoides orthopterorum, p. 169.
Nyctotherus ovalis, p. 188.
Oikomonas sp., p. 166.
Peltomyces periplanetae, p. 177.
Plistophora kudoi, p. 185.
Plistophora periplanetae, p. 186.
Plistophora sp., p. 186.
Retortamonas blattae, p. 167.
Stenophora sp., p. 181.
Tetratrichomastix blattidarum, p. 170.
Helminths:
Ascaris sp., p. 209.
Enterobius vermicularis, p. 209.
Gongylonema neoplasticum, p. 206.
Gordius blattae orientalis, p. 202.
Hammerschmidtiella diesingi, p. 195.
Hammerschmidtiella neyrai, p. 196.
Leidynema appendiculata, p. 197.
Spirura gastrophila, p. 207.
Thelastoma pachyjuli, p. 201.
Trichostrongylus sp., p. 210.
Trichuris trichiura, p. 210.
Mite: Undetermined, pp. 220, 222.
Insects:
Dermestes ater, p. 234.
Dermestes sp., p. 234.
Evania appendigaster, p. 236.
Evania dimidiata, p. 239.
Prosevania punctata, p. 240.
Systellogaster ovivora, p. 248.
Tetrastichus hagenowii, p. 250.
Tetrastichus sp., p. 254.
Bird: Chicken, p. 278.
Mammal: Homo sapiens, p. 286.
Blatta sp.
Hair worm: Gordius aquaticus, p. 201.
Insects:
Evania appendigaster, p. 236.
Tetrastichus hagenowii, p. 250.
Blattella germanica and/or Blattella vaga
Viruses: Unspecified strains of poliomyelitis virus, p. 103.
Blattella germanica
Bacteria:
Achromobacter sp., p. 110.
Aerobacter aerogenes, p. 111.
Aerobacter cloacae, p. 112.
Alcaligenes faecalis, p. 111.
Alcaligenes viscosus, p. 111.
Bacillus circulans, p. 120.
Escherichia coli, p. 112.
Escherichia freundii, p. 113.
Micrococcus aurantiacus, p. 106.
Micrococcus epidermidis, p. 106.
Micrococcus pyogenes var. albus, p. 107.
Micrococcus pyogenes var. aureus, p. 107.
Micrococcus ureae, p. 107.
Micrococcus sp., p. 108.
Mycobacterium leprae, p. 123.
Paracolobactrum aerogenoides, p. 113.
[Pg 294]
Paracolobactrum coliforme, p. 113.
Paracolobactrum sp., p. 113.
Pseudomonas aeruginosa, p. 104.
Sarcina symbiotica (host may have been B. orientalis), p. 108.
Salmonella typhimurium, p. 116.
Serratia marcescens, p. 117.
Streptococcus faecalis, p. 109.
Streptococcus sp., p. 110.
Fungi:
Aspergillus flavus, p. 130.
Aspergillus tamarii, p. 130.
Aspergillus sp., p. 130.
Cordyceps blattae, p. 134.
Herpomyces ectobiae, p. 135.
Memnoniella echinata, p. 132.
Saccharomyces sp., p. 133.
Protozoa:
Bodo sp. (host may have been P. americana), p. 167.
Coelosporidium periplanetae, p. 185.
Dobellina sp. (host may have been P. americana), p. 177.
Endamoeba blattae (host may have been P. americana), p. 177.
Endolimax sp. (host may have been P. americana), p. 180.
Entamoeba coli (host may have been P. americana), p. 178.
Entamoeba histolytica (host may have been P. americana), p. 179.
Entamoeba thomsoni, p. 179.
Eutrichomastix sp. (host may have been P. americana), p. 169.
Gregarina blattarum, p. 182.
Iodamoeba sp. (host may have been P. americana), p. 180.
Lophomonas blattarum, p. 172.
Lophomonas striata, p. 173.
Nyctotherus ovalis, p. 188.
Peltomyces periplanetae, p. 177.
Plistophora periplanetae, p. 186.
Tetratrichomastix blattidarum, p. 170.
Helminths:
Blattelicola blattelicola, p. 193.
Blatticola blattae, p. 193.
Enterobius vermicularis, p. 209.
Galebia aegyptiaca, p. 195.
Gongylonema neoplasticum, p. 206.
Moniliformis kalahariensis, p. 203.
Prosthenorchis elegans, p. 203.
Prosthenorchis spirula, p. 203.
Tetrameres americana, p. 207.
Trichuris trichiura, p. 210.
Mites:
Blattisocius tineivorus, p. 216.
Caloglyphus sp., p. 218.
Undetermined, p. 220.
Insects:
Brachygaster minutus, p. 235.
Eupelmus atriflagellum, p. 247.
Dolichurus corniculus, p. 261.
Ripidius pectinicornis, p. 232.
Mammals:
Callithrix chrysoleucos, p. 285.
Callithrix jacchus, p. 285.
Cebus apella, p. 284.
Lemur coronatus, p. 283.
Lemur fulvus, p. 283.
Leontocebus oedipus, p. 285.
Leontocebus rosalia, p. 285.
Leontocebus ursulus, p. 285.
Macaca sylvanus, p. 286.
Nasua narica, p. 288.
Nasua nasua, p. 288.
Pan sp., p. 286.
Perodicticus potto, p. 284.
Saimiri sciurea, p. 285.
Blattella humbertiana
Fungus: Herpomyces gracilis, p. 135.
Nematode: Protrellus phyllodromi, p. 200.
Mite: Uropoda sp., p. 217.
[Pg 295]
Blattella lituricollis
Insect: Dolichurus stantoni, p. 262.
Blattella sp.
Reptile: Anolis cristatellus, p. 273.
Cariblatta delicatula
Insect: Hyptia sp., p. 240.
Reptile: Anolis cristatellus, p. 273.
Cariblatta lutea lutea
Plants:
Sarracenia flava, p. 154.
Sarracenia purpurea, p. 154.
Chorisoneura sp.
Insects:
Stylogaster sp., p. 228.
Tachysphex blatticidus, p. 264.
Choristima sp.
Insect: Rhipidioides rubricatus, p. 231.
Choristimodes sp.
Insect: Rhipidioides rubricatus, p. 231.
Cryptocercus punctulatus
Bacterium: Bacillus subtilis, p. 122.
Protozoa:
Adelina cryptocerci, p. 184.
Barbulanympha estaboga, p. 173.
Barbulanympha laurabuda, p. 174.
Barbulanympha ufalula, p. 174.
Barbulanympha wenyoni, p. 174.
Eucomonympha imla, p. 176.
Hexamita cryptocerci, p. 171.
Idionympha perissa, p. 174.
Leptospironympha eupora, p. 172.
Leptospironympha rudis, p. 172.
Leptospironympha wachula, p. 172.
Macrospironympha xylopletha, p. 172.
Monocercomonoides globus, p. 169.
Notila proteus, p. 170.
Oxymonas doroaxostylus, p. 170.
Oxymonas nana, p. 170.
Prolophomonas tocopola, p. 173.
Rhynchonympha tarda, p. 174.
Saccinobaculus ambloaxostylus, p. 170.
Saccinobaculus lata, p. 170.
Trichonympha acuta, p. 174.
Trichonympha algoa, p. 174.
Trichonympha chula, p. 176.
Trichonympha grandis, p. 176.
Trichonympha lata, p. 176.
Trichonympha okolona, p. 176.
Trichonympha parva, p. 176.
Urinympha talea, p. 174.
Undetermined gregarine, p. 184.
Amphibian: Plethodon glutinosus, p. 269.
[Pg 296]
Cutilia soror
Insects:
Dolichurus stantoni, p. 262.
Evania appendigaster, p. 236.
Szepligetella sericea, p. 242.
Bird: Phasianus sp., p. 277.
Cutilia sp.
Nematode: Undetermined, p. 201.
Insects:
Riekella australis, p. 231.
Undetermined strepsipteron, p. 234.
Diploptera punctata
Bacterium: Serratia marcescens, p. 117.
Fungus: Herpomyces diplopterae, p. 135.
Mites:
Iolina nana, p. 219.
Locustacarus sp., p. 219.
Insect: Nauphoeta cinerea, p. 324.
Amphibian: Bufo marinus, p. 270.
Bird: Phasianus sp., p. 277.
Ectobius lapponicus
Protozoa:
Gamocystis tenax, p. 184.
Monocercomonoides orthopterorum, p. 169.
Nematode: Blatticola blattae, p. 193.
Insects:
Brachygaster minutus, p. 235.
Dolichurus corniculus, p. 261.
Tachysphex lativalvis, p. 264.
Ectobius pallidus
Protozoan: Gamocystis tenax, p. 184.
Helminths:
Blatticola blattae, p. 193.
Undetermined mermithid, p. 192.
Insects:
Ampulex fasciata, p. 259.
Aphaenogaster picea, p. 267.
Dolichurus corniculus, p. 261.
Lasius alienus, p. 268.
Ripidius boissyi (presumptive record), p. 231.
Ripidius denisi (presumptive record), p. 232.
Tachysphex lativalvis, p. 264.
Ectobius panzeri
Centipede: Scolopendra sp., p. 224.
Insects:
Dolichurus corniculus, p. 261.
Tachysphex lativalvis, p. 264.
[Pg 297]
Ectobius sp.
Insects:
Brachygaster minutus, p. 235.
Dolichurus corniculus, p. 261.
Tachysphex lativalvis, p. 264.
Ellipsidion affine
Insect: Rhipidioides fuscatus, p. 230.
Ellipsidion australe
Insects:
Agamerion metallica, p. 243.
Cheiloneurus viridiscutum, p. 244.
Mestocharomyia oophaga, p. 248.
Epilampra abdomen-nigrum
Insect: Podium flavipenne, p. 265.
Amphibian: Bufo marinus, p. 270.
Epilampra conferta
Insect: Podium sp., p. 266.
Epilampra sp.
Fungus: Herpomyces tricuspidatus, p. 138.
Insect: Podium haematogastrum, p. 266.
Bird: Gymnasio nudipes, p. 279.
Epilampra wheeleri
Reptiles:
Ameiva exsul, p. 275.
Anolis cristatellus, p. 273.
Mammal: Herpestes javanicus auropunctatus, p. 289.
Escala (?) sp.
Insect: Rhipidioides adynatus, p. 230.
Eurycotis floridana
Fungi:
Aspergillus flavus, p. 130.
Aspergillus sydowi, P. 130.
Helminths:
Euryconema paradisa, p. 194.
Leidynema appendiculata(?), p. 198.
Protrelloides paradoxa, p. 200.
Undetermined gordian worm, p. 202.
Insects:
Anastatus floridanus, p. 245.
Undetermined tachinid, p. 228.
Eurycotis improcera
Mammal: Herpestes javanicus auropunctatus, p. 289.
[Pg 298]
Eurycotis manni
Fungus: Herpomyces zanzibarinus, p. 138.
Graptoblatta notulata
Insect: Tachysphex fanuiensis, p. 264.
Gromphadorhina portentosa
Protozoan: Undetermined gregarine, p. 184.
Mite: Coleolaelaps ? sp., p. 216.
Gyna sp.(?)
Fungus: Herpomyces zanzibarinus, p. 138.
Holocompsa fulva
Insect: Pheidole megacephala, p. 268.
Hololampra punctata
Insect: Dolichurus corniculus, p. 261.
Ischnoptera deropeltiformis
Plant: Sarracenia flava, p. 154
Amphibian: Hyla cinerea, p. 270.
Mammal: Dasypus novemcinctus, p. 287.
Ischnoptera rufa rufa
Fungus: Spicaria prasina, p. 130.
Mammal: Herpestes javanicus auropunctatus, p. 289.
Ischnoptera sp.
Fungus: Herpomyces arietinus, p. 134.
Insects:
Ampulex canaliculata, p. 257.
Syntomosphyrum ischnopterae, p. 249.
Karnyia discoidalis
Spider: Latrodectus indistinctus, p. 215.
Kuchinga hemerobina
Hair worm: Parachordodes raphaelis, p. 202.
[Pg 299]
Leucophaea maderae
Bacterium: Serratia marcescens, p. 117.
Fungus: Herpomyces tricuspidatus, p. 138.
Protozoa:
Gregarina rhyparobiae, p. 183.
Hexamita (?) sp., p. 171.
Retortamonas (?) sp., p. 167.
Undetermined gregarine, p. 184.
Nematodes:
Hammerschmidtiella diesingi, p. 195.
Leidynema delatorrei, p. 198.
Protospirura bonnei, p. 206.
Protospirura muricola, p. 206.
Mite: Chaetodactylus sp., p. 218.
Mammals:
Aotes zonalis, p. 284.
Ateles dariensis, p. 284.
Cebus capucinus, p. 284.
Rattus norvegicus, p. 287.
Leucophaea sp.
Nematode: Cephalobellus brevicaudatum, p. 194.
Leurolestes pallidus
Fungus: Herpomyces leurolestis, p. 136.
Loboptera decipiens
Insects:
Dolichurus corniculus, p. 261.
Zeuxevania splendidula, p. 243.
Loboptera sp.
Fungus: Herpomyces lobopterae, p. 136.
Lobopterella dimidiatipes
Insect: Ampulex canaliculata, p. 257.
Bird: Coturnix coturnix japonica, p. 277.
Melanosilpha capensis
Protozoa:
Gregarina impetuosa, p. 183.
Gregarina sandoni, p. 183.
Monastria sp.
Insect: Triatoma arthurneivai, p. 227.
Nauphoeta cinerea
Bacteria:
Salmonella typhimurium, p. 116.
Serratia marcescens, p. 117.
[Pg 300]
Fungus: Herpomyces tricuspidatus, p. 138.
Mites:
Blattilaelaps nauphoetae, p. 216.
Locustacarus sp., p. 219.
Insect: Pheidole megacephala, p. 268.
Neostylopyga rhombifolia
Bacterium: Serratia marcescens, p. 117.
Insects:
Evania appendigaster, p. 237.
Szepligetella sericea, p. 242.
Tetrastichus hagenowii, p. 250.
Mammal: Homo sapiens, p. 286.
Nyctibora obscura
Fungus: Herpomyces amazonicus, p. 134.
Nyctibora sp.
Fungus: Herpomyces nyctoborae, p. 136.
Nyctibora tomantosa
Fungus: Herpomyces nyctoborae, p. 136.
Oniscosoma granicollis
Insect: Paranephrites xenus, p. 230.
Panchlora exoleta
Protozoan: Gregarina panchlorae, p. 183.
Panchlora nivea
Bacterium: Serratia marcescens, p. 117.
Fungus: Herpomyces panchlorae, p. 136.
Mammal: Herpestes javanicus auropunctatus, p. 289.
Panesthia angustipennis
Protozoa:
Clevelandella constricta, p. 189.
Clevelandella contorta, p. 189.
Clevelandella elongata, p. 189.
Clevelandella hastula, p. 189.
Clevelandella panesthiae, p. 189.
Clevelandella parapanesthiae, p. 189.
[Pg 301]
Endamoeba javanica, p. 178.
Endamoeba philippinensis, p. 178.
Hexamita cryptocerci, p. 171.
Monocercomonoides panesthiae, p. 170.
Nyctotherus uichancoi, p. 188.
Paraclevelandia brevis, p. 190.
Paraclevelandia simplex, p. 190.
Undetermined amoeba, p. 181.
Nematodes:
Aorurus philippinensis, p. 193.
Blattophila sphaerolaima var. javanica, p. 194.
Leidynema nocalum, p. 198.
Leidynemella fusiformis, p. 198.
Leidynemella paracranifera, p. 198.
Thelastoma palmettum, p. 201.
Panesthia australis
Fungus: Metarrhizium anisopliae, p. 131.
Mite:
Hypoaspis sp., p. 217.
Undetermined diplogyniid, p. 217.
Insect: Undetermined tachinid, p. 228.
Panesthia laevicollis
Nematode: Blattophila sphaerolaima, p. 194.
Panesthia laevicollis (?)
Nematode: Leidynemella fusiformis, p. 198.
Panesthia lobipennis
Fungus: Herpomyces panesthiae, p. 136.
Panesthia spadica
Protozoa:
Clevelandella constricta, p. 189.
Clevelandella contorta, p. 189.
Clevelandella nipponensis, p. 189.
Clevelandella panesthiae, p. 189.
Endamoeba javanica, p. 178.
Nyctotherus uichancoi, p. 188.
Paraclevelandia brevis, p. 190.
Paraclevelandia simplex, p. 190.
Panesthia sp.
Nematode: Leidynemella panesthiae, p. 198.
Parahormetica bilobata
Helminths:
Agamospirura parahormeticae, p. 205.
Undetermined gordian worm, p. 202.
Parcoblatta lata
Plant: Sarracenia flava, p. 154.
Protozoan: Leptomonas sp., p. 167.
Nematode: Protrellus aurifluus, p. 199.
[Pg 302]
Parcoblatta pensylvanica
Protozoa:
Gregarina blattarum, p. 182.
Gregarina illinensis, p. 183.
Gregarina parcoblattae, p. 183.
Gregarina thomasi, p. 184.
Leptomonas sp., p. 167.
Nyctotherus ovalis, p. 188.
Nematode: Rictularia coloradensis, p. 205.
Scorpion: Centruroides vittatus (?), p. 212.
Insects:
Camponotus pennsylvanicus, p. 267.
Hyptia dorsalis, p. 239.
Hyptia harpyoides, p. 239.
Hyptia reticulata, p. 240.
Hyptia thoracica, p. 240.
Podium carolina, p. 265.
Systellogaster ovivora, p. 248.
Amphibian: Rana (?) sp., p. 272.
Parcoblatta uhleriana
Fungus: Herpomyces arietinus, p. 134.
Protozoan: Gregarina parcoblattae, p. 183.
Nematode: Protrellus aurifluus, p. 199.
Mite: Undetermined, p. 220.
Insect: Hyptia harpyoides, p. 239.
Parcoblatta virginica
Fungus: Herpomyces arietinus, p. 134.
Protozoa:
Gregarina ohioensis, p. 183.
Leptomonas sp., p. 167.
Nematode: Rictularia coloradensis, p. 205.
Insects:
Hyptia harpyoides, p. 239.
Podium luctuosum, p. 266.
Parcoblatta sp.
Fungus: Herpomyces arietinus, p. 134.
Plant: Sarracenia flava, p. 154.
Mite: Pimeliaphilus podapolipophagus, p. 219.
Insects:
Coenosia basalis, p. 229.
Dolichurus greenei, p. 261.
Hyptia sp., p. 240.
Megaselia sp., p. 227.
Syntomosphyrum blattae, p. 248.
Systellogaster ovivora, p. 248.
Tetrastichus hagenowii, p. 250.
Periplaneta americana
Viruses: Unspecified strain(s) of poliomyelitis virus (host may have been P. brunnea), p. 103.
[Pg 303]
Bacteria:
Achromobacter hyalinum, p. 110.
Aerobacter aerogenes, p. 111.
Aerobacter cloacae, p. 112.
Aerobacter sp., p. 112.
Alcaligenes faecalis, p. 111.
Bacillus cereus, p. 120.
Bacillus megaterium, p. 121.
Bacillus subtilis, p. 122.
Bacterium alkaligenes, p. 119.
Clostridium sp., p. 122.
Eberthella oedematiens, p. 112.
Escherichia coli, p. 112.
Escherichia freundii, p. 113.
Escherichia intermedium, p. 113.
Mycobacterium friedmannii, p. 123.
Mycobacterium lacticola, p. 123.
Mycobacterium leprae, p. 123.
Mycobacterium phlei, p. 123.
Mycobacterium piscium, p. 124.
Mycobacterium sp., p. 124.
Nocardia sp. (?), p. 124.
Paracolobactrum aerogenoides, p. 113.
Paracolobactrum coliforme, p. 113.
Paracolobactrum sp., p. 113.
Proteus mirabilis, p. 114.
Proteus morganii, p. 114.
Proteus rettgeri, p. 114.
Proteus vulgaris, p. 114.
Proteus sp., p. 114.
Pseudomonas aeruginosa, p. 104.
Pseudomonas fluorescens, p. 105.
Salmonella anatis, p. 114.
Salmonella morbificans, p. 115.
Salmonella schottmuelleri, p. 115.
Salmonella sp. (Type Bareilly), p. 115.
Salmonella sp. (Type Bredeny), p. 115.
Salmonella sp. (Type Kentucky), p. 115.
Salmonella sp. (Type Meleagris), p. 116.
Salmonella sp. (Type Newport), p. 116.
Salmonella sp. (Type Oranienburg), p. 116.
Salmonella sp. (Type Panama), p. 116.
Salmonella sp. (Type Rubislaw), p. 116.
Salmonella sp. (Type Tennessee), p. 116.
Sarcina sp., p. 108.
Serratia marcescens, p. 118.
Shigella alkalescens, p. 118.
Spirillum periplaneticum, p. 105.
Streptococcus faecalis, p. 109.
Streptomyces leidynematis, pp. 196, 197.
Tetragenous sp., p. 127.
Veillonella parvula, p. 109.
Fungi:
Aspergillus flavus, p. 130.
Aspergillus niger, p. 130.
Aspergillus sp., p. 131.
Cephalosporium sp., p. 131.
Herpomyces chaetophilus, p. 135.
Herpomyces periplanetae, p. 137.
Metarrhizium anisopliae, p. 131.
Mucor guilliermondii, p. 132.
Mucor sp., p. 132.
Penicillium sp., p. 131.
Rhizopus sp., p. 133.
Syncephalastrum sp., p. 133.
Torula acidophila, p. 132.
Protozoa:
Balantidium blattarum, p. 187.
Balantidium ovatum, p. 187.
Balantidium sp., p. 187.
Bodo sp. (host may have been B. germanica), p. 167.
Coelosporidium periplanetae, p. 185.
Diplocystis schneideri, p. 181.
Diplocystis sp., p. 181.
Dobellina sp. (host may have been B. germanica), p. 177.
Endamoeba blattae, p. 177.
Endolimax blattae, p. 180.
Endolimax sp. (host may have been B. germanica), p. 180.
Entamoeba coli (host may have been B. germanica), p. 178.
Entamoeba histolytica (host may have been B. germanica), p. 179.
Entamoeba sp., p. 179.
Entamoeba thomsoni, p. 179.
[Pg 304]
Eutrichomastix sp. (host may have been B. germanica), p. 169.
Gregarina blattarum, p. 182.
Gregarina légeri, p. 183.
Gregarina neo-brasiliensis, p. 183.
Hexamita periplanetae, p. 171.
Iodamoeba sp. (host may have been B. germanica) p. 180.
Isotricha caulleryi, p. 187.
Lophomonas blattarum, p. 172.
Lophomonas striata, p. 173.
Monocercomonoides orthopterorum, p. 169.
Nyctotherus ovalis, p. 188.
Plistophora periplanetae, p. 186.
Protomagalhaesia serpentula, p. 184.
Tetratrichomastix blattidarum, p. 170.
Helminths:
Ancylostoma duodenale, p. 209.
Ascaris lumbricoides or Ascaris suum, p. 209.
Binema mirzaia, p. 193.
Gongylonema neoplasticum, p. 206.
Gongylonema sp., p. 206.
Gordius sp., p. 202.
Hammerschmidtiella diesingi,p. 195.
Hymenolepis sp., p. 208.
Leidynema appendiculata, p. 197.
Moniliformis dubius, p. 203.
Moniliformis moniliformis, p. 204.
Necator americanus, p. 210.
Protrellus künckeli, p. 199.
Schwenkiella icemi, p. 200.
Spirura gastrophila, p. 207.
Thelastoma pachyjuli, p. 201.
Trichuris trichiura, p. 210.
Mites:
Caloglyphus sp., p. 218.
Pimeliaphilus podapolipophagus, p. 219.
Rhizoglyphus tarsalus, p. 218.
Tyrophagus noxius, p. 218.
Undetermined, p. 220.
Spiders:
Avicularia avicularia, p. 214.
Avicularia sp., p. 214.
Centipedes: Undetermined, p. 222.
Insects:
Ampulex amoena, p. 256.
Ampulex compressa, p. 259.
Anastatus tenuipes, p. 246.
Calodexia (?) venteris, p. 228.
Evania appendigaster, p. 237.
Melittobia chalybii, p. 248.
Prosevania punctata, p. 241.
Ripidius pectinicornis, p. 232.
Spiniger domesticus, p. 227.
Szepligetella sericea, p. 242.
Tetrastichus hagenowii, p. 250.
Tetrastichus periplanetae, p. 253.
Tetrastichus sp., p. 254.
Trirhogma caerulea, p. 262.
Amphibian:
Hyla cinerea, p. 270.
Reptiles:
Ameiva exsul, p. 275.
Anolis cristatellus, p. 273.
Birds:
Chicken, p. 278.
Sparrow, p. 282.
Mammals:
Felis catus, p. 289.
Herpestea javanicus auropunctatus, p. 289.
Herpestes sp., p. 289.
Homo sapiens, p. 286.
Rattus norvegicus, p. 287.
Rattus rattus, p. 287.
Periplaneta australasiae
Bacteria:
Mycobacterium leprae, p. 123.
Serratia marcescens, p. 118.
[Pg 305]
Fungus: Herpomyces periplanetae, p. 137.
Plant: Sarracenia minor, p. 154.
Protozoa:
Endamoeba blattae, p. 177.
Endolimax blattae, p. 180.
Nematodes:
Gongylonema neoplasticum, p. 206.
Hammerschmidtiella diesingi, p. 195.
Moniliformis dubius, p. 203.
Protrellus australasiae, p. 199.
Protrellus künckeli, p. 200.
Centipedes: Undetermined, p. 222.
Insects:
Ampulex amoena, p. 256.
Ampulex compressa, p. 259.
Evania appendigaster, p. 237.
Szepligetella sericea, p. 242.
Tetrastichus australasiae, p. 249.
Tetrastichus hagenowii, p. 250.
Tetrastichus, sp., p. 254.
Trirhogma caerulea, p. 262.
Reptiles:
Anolis cristatellus, p. 273.
Chrysemys picta, p. 272.
Bird: Gallus sp., p. 277.
Mammals:
Herpestes sp., p. 289.
Homo sapiens, p. 287.
Periplaneta brunnea
Viruses: Unspecified strain(s) of poliomyelitis virus (host may have been P. americana), p. 103.
Bacterium: Serratia marcescens, p. 118.
Fungus: Herpomyces periplanetae, p. 137.
Nematode: Schwenkiella icemi, p. 200.
Insect: Tetrastichus hagenowii, p. 251.
Periplaneta fuliginosa
Insect: Ampulex amoena, p. 256.
Periplaneta sp.
Bacterium: Serratia marcescens, p. 118.
Fungi:
Herpomyces chaetophilus, p. 135.
Herpomyces periplanetae, p. 137.
Protozoan: Lophomonas blattarum, p. 172.
Helminths: Thelastoma riveroi, p. 201.
Moniliformis moniliformis, p. 204.
Insects:
Ampulex compressa, p. 259.
Evania subspinosa (presumptive record?), p. 239.
Szepligetella sericea, p. 242.
Amphibian: Bufo marinus, p. 270.
[Pg 306]
Reptiles:
Anolis grahami, p. 274.
Anolis leachi, p. 274.
Bird: Vireo latimeri, p. 281.
"Phyllodromia" sp.
Fungus: Herpomyces phyllodromiae, p. 137.
Insects:
Dicarnosis alfierii, p. 245.
Dolichurus stantoni, p. 262.
Platyzosteria castanea
Insect: Riekella sp., p. 231.
Platyzosteria novae seelandiae
Protozoan: Monocercomonoides melolonthae, p. 169.
Platyzosteria scabra
Fungus: Herpomyces appendiculatus, p. 134.
Platyzosteria sp.
Insect: Riekella nitidioides, p. 231.
Plectoptera sp.
Bird: Todus mexicanus, p. 279.
Polyphaga aegyptiaca
Nematodes:
Blatticola blattae, p. 193.
Galebia aegyptiaca, p. 195.
Hammerschmidtiella diesingi, p. 195.
Oxyuris (?) heterogamiae, p. 199.
Polyphaga saussurei
Helminths:
Hymenolepis sp., p. 208.
Undetermined tapeworm ova, p. 208.
Insects: Undetermined reduviids, p. 227.
Polyzosteria melanaria (?) or Platyzosteria analis
Nematode: Protrellus aureus, p. 199.
Pycnoscelus surinamensis
Bacterium: Serratia marcescens, p. 117.
Fungus: Mucor sp., p. 132.
Protozoa:
Undetermined ciliate, p. 190.
Undetermined flagellate, p. 176.
Undetermined gregarine, p. 184.
[Pg 307]
Nematodes:
Oxyspirura mansoni, p. 204.
Severianoia severianoi, p. 200.
Mites:
Caloglyphus spinitarsus, p. 217.
Histiostoma feroniarum, p. 217.
Tyrophagus lintneri, p. 218
Undetermined, p. 220.
Insects:
Pheidole megacephala, p. 268.
Undetermined ants, p. 350.
Amphibian: Bufo marinus, p. 270.
Reptile: Anolis sagrei, p. 274.
Birds:
Acridotheres tristis, p. 281.
Ducks, p. 277.
Melanerpes portoricensis, p. 280.
Meleagris gallopavo, p. 278.
Passer domesticus, p. 282.
Phasianus sp., p. 277.
Streptopelia chinensis, p. 278.
Mammal: Herpestes javanicus auropunctatus, p. 289.
Robshelfordia circumducta or Robshelfordia longiuscula
Insects:
Neorhipidius neoxenus, p. 230.
Rhipidioides helenae, p. 230.
Rhipidioides mollis, p. 231.
Steleopyga (?) sinensis
Nematode: Suifunema caudelli, p. 200.
Supella supellectilium
Viruses: Unspecified strain(s) of poliomyelitis virus, p. 103.
Bacterium: Serratia marcescens, p. 117.
Fungus: Herpomyces supellae, p. 138.
Nematode: Blattophila supellaima, p. 194.
Insects:
Anastatus tenuipes, p. 246.
Comperia merceti, p. 244.
Symploce flagellata or Symploce ruficollis
Reptile: Anolis cristatellus, p. 273.
Symploce parenthesis
Hair worm: Parachordodes raphaelis, p. 202.
Temnopteryx phalerata
Protozoan: Gregarina gibbsi, p. 182.
[Pg 308]
Undetermined Cockroaches
Bacteria:
Arthromitus intestinalis, p. 124.
Bacillus subtilis, p. 122.
Clostridium perfringens, p. 122.
Escherichia coli, p. 112.
Fusiformis lophomonadis, p. 119.
Micrococcus citreus, p. 106.
Micrococcus pyogenes var. aureus, p. 107.
Mycobacterium leprae, p. 123.
Paracolon bacilli, p. 113.
Proteus morganii, p. 114.
Proteus vulgaris, p. 114.
Spirillum sp., p. 105.
Spirochaeta periplanetae, p. 125.
Streptococcus sp., p. 110.
Vibrio Types I and II Heiberg, p. 106.
Fungi:
Amphoromorpha blattina, p. 139.
Amphoromorpha sp., p. 139.
Blastocystis sp., p. 133.
Cordyceps amazonica, p. 134.
Herpomyces anaplectae, p. 134.
Herpomyces chilensis, p. 135.
Herpomyces diplopterae, p. 135.
Herpomyces forficularis, p. 135.
Herpomyces grenadinus, p. 136.
Herpomyces macropus, p. 136.
Herpomyces paranensis, p. 136.
Herpomyces periplanetae, p. 137.
Herpomyces platyzosteriae, p. 137.
Herpomyces tricuspidatus, p. 138.
Herpomyces zanzibarinus, p. 138.
Metarrhizium anisopliae, p. 131.
Plants:
Nepenthes ampularia, p. 154.
Nepenthes gracilis, p. 154.
Nepenthes sp., p. 154.
Protozoa:
Diplocystis sp., p. 181.
Endamoeba blattae, p. 177.
Entamoeba histolytica, p. 179.
Giardia sp., p. 172.
Gregarina blattarum, p. 182.
Hexamita periplanetae, p. 171.
Lophomonas blattarum, p. 172.
Lophomonas striata, p. 173.
Monas sp., p. 167.
Monocercomonoides orthopterorum, p. 169.
Nyctotherus buissoni, p. 188.
Nyctotherus ovalis, p. 188.
Nyctotherus viannai, p. 189.
Oikomonas blattarum, p. 166.
Oikomonas sp., p. 166.
Paramecium sp., p. 186.
Trichomonas sp., p. 171.
Helminths:
Cephalobellus magalhãesi, p. 194.
Chordodes morgani, p. 201.
Gordius sp., p. 202.
Hammerschmidtiella diesingi, p. 195.
Leidynema appendiculata, p. 197.
Moniliformis moniliformis, p. 204.
Protrellus galebi, p. 199.
Schwenkiella icemi, p. 200.
Severianoia magna, p. 200.
Severianoia severianoi, p. 200.
Spirura gastrophila, p. 207.
Undetermined gordian worms, p. 202.
Scorpion: Heterometrus longimanus, p. 213.
Spiders:
Ctenid, p. 214.
Heteropoda venatoria, p. 215.
Latrodectus mactans, p. 216.
Mite: Pimeliaphilus podapolipophagus, p. 219.
Centipedes:
Allothereua maculata (circumstantial evidence), p. 223.
Scolopendra morsitans, p. 223.
Scolopendra subspinipes, p. 224.
Scutigera coleoptrata (circumstantial evidence), p. 222.
[Pg 309]
Insects:
Acanthinevania princeps, p. 235.
Agamerion metallica, p. 243.
Ampulex compressa, p. 259.
Ampulex ruficornis, p. 259.
Ampulex sibirica, p. 260.
Ampulex sonnerati, p. 260.
Anastatus blattidifurax p. 245.
Anastatus tenuipes, p. 246.
Blatticida pulchra, p. 243.
Blatticidella ashmeadi, p. 243.
Calodexia spp., p. 228.
Clerada apicicornis, p. 226.
Dicarnosis alfierii, p. 245.
Diestrammena apicalis, p. 226.
Diestrammena japonica, p. 226.
Dolichurus bicolor, p. 260.
Dolichurus corniculus, p. 261.
Dolichurus gilberti, p. 261.
Dolichurus ignitus, p. 261.
Dolichurus sp., p. 262.
Dorylus nigricans sjöstedi, p. 267.
Dorylus sp., p. 267.
Dorylus wilverthii, p. 267.
Eciton burchelli, p. 268.
Eupelmus sp., p. 247.
Eutrichosomella blattophaga, p. 245.
"Formica omnivora," p. 268.
Iridomyrmex humilis, p. 268.
Neonephrites partiniger, p. 230.
Podium abdominale, p. 265.
Podium dubium, p. 265.
Podium rufipes, p. 266.
Pompilus bracatus, p. 256.
Pompilus sp., p. 256.
Salius verticalis, p. 256.
Ripidius scutellaris, p. 233.
Solindenia picticornis, p. 247.
Stylogaster spp., p. 228.
Stylogaster stylata, p. 228.
Syntomosphyrum blattae, p. 248.
Systellogaster ovivora, p. 248.
Tachysphex blatticidus, p. 264.
Tachysphex coriaceus, p. 264.
Tachysphex fanuiensis, p. 264.
Tetrastichus hagenowii, p. 251.
Tetrastichus periplanetae, p. 253.
Trirhogma sp., p. 264.
Undetermined tachinid, p. 229.
Fish:
Chalceus macrolepidotus, p. 269.
Cyrtocharax magdalenae essequibensis, p. 269.
Potamotrygon humboldti, p. 268.
Rhamdia sebae, p. 268.
Amphibians:
Arthroleptis variabilis, p. 270.
Bufo ictericus, p. 270.
Bufo funereus, p. 270.
Bufo marinus, p. 270.
Hyla cinerea, p. 270.
Hyperolius picturatus, p. 271.
Leptodactylus albilabris, p. 271.
Leptodactylus pentadactylus, p. 271.
Leptopelis calcaratus, p. 271.
Leptopelis rufus, p. 271.
Megalixalus fornasinii, p. 271.
Rana catesbeiana, p. 271.
Rana mascareniensis, p. 271.
Tree frogs, p. 351.
Reptiles:
Ameiva exsul, p. 275.
Ameiva sp., p. 275.
Anolis cristatellus, p. 273.
Anolis grahami, p. 274.
Anolis leachi, p. 274.
Anolis pulchellus, p. 273.
Anolis sp., p. 274.
Anolis stratulus, p. 274.
Cnemidophorus sp., p. 275.
Geckos, p. 273.
Gekko gecko, p. 272.
Hemidactylus frenatus, p. 272.
Leiolopisma laterale, p. 274.
Lizards, p. 275.
Skinks, p. 274.
Sphaerodactylus sp., p. 273.
Thecadactylus sp., p. 273.
Tropidophorus grayi, p. 274.
Birds:
Acridotheres tristis, p. 281.
Agelaius xanthomus, p. 282.
Bambusicola thoracica, p. 277.
Chickens, p. 278.
[Pg 310]
Coturnix coturnix japonica, p. 277.
Dendrocopus mahrattensis, p. 279.
Ducks, p. 277.
Gymnasio nudipes, p. 279.
Gymnopithys leucaspis, p. 280.
Holoquiscalus brachypterus, p. 282.
Icterus portoricensis, p. 280.
Owl, p. 351.
Partridge, p. 278.
Phasianus sp., p. 277.
Sparrow, p. 282.
Tiaris bicolor omissa, p. 282.
Tockus birostris, p. 279.
Troglodytes aedon, p. 281.
Troglodytes audax, p. 281.
Mammals:
Bassariscus astutus, p. 288.
Callithrix jacchus, p. 285.
Erinaceus sp., p. 283.
Felis catus, p. 289.
Felis paradalis mearnsi, p. 290.
Herpestes javanicus auropunctatus, p. 289
Herpestes sp., p. 289.
Homo sapiens, p. 287.
Loris tardigradus, p. 284.
Molossus sp., p. 283.
Monodelphis sp., p. 283.
Nasua nasua, p. 288.
Nasua sp., p. 289.
Rattus sp., p. 287.
These particular associations may well have been accidental and due to a predilection for the same type of nesting site. But this fact in no way detracts from the interest of such records. Chance must play a very considerable part in first bringing symbiotic or commensal partners together. Once such a partnership between species has been firmly established, it is on the whole, fairly obvious, ... On the other hand, in the early stages before the relationship has become fixed as a specific habit, individual cases are generally dismissed as coincidences. It is, however, unwise to disregard such isolated observations or dismiss them lightly.
Rothschild and Clay (1957)
The following social insects have been found harboring cockroaches in a state of commensalism in which the cockroaches presumably benefit by acquiring food from their hosts. Benefits accruing to the hosts are not apparent. Unfortunately, biological details are not always sufficient to substantiate the suspected association. However, it seems significant that the cockroach commensals of the insects listed below have been found only in association with their hosts and, so far as we know, have never been found apart from them. Chopard (1938) has pointed out that the myrmecophilous cockroaches are all small, being only a very few millimeters long; they are apterous or subapterous; their eyes are reduced; and they are all of American origin.
HOSTS OF COMMENSAL COCKROACHES
Order ISOPTERA
Family RHINOTERMITIDAE
Commensal.—Sphecophila ravana, Ceylon (Fernando, 1957): Six females, 50 males, and nymphs of both sexes were found among decaying [Pg 311] timber in the ground in association with a colony of this termite. The antennae of most specimens were mutilated unsymmetrically.
Family TERMITIDAE
Commensal.—Nocticola sinensis, Kowloon (Silvestri, 1947): Among specimens of termites collected from a nest.
or
Synonymy.—Termes bellicosus [Snyder, 1949].
Commensal.—Sphecophila termitium, Kibonoto, East Africa (Shelford, 1910): Two males were collected in a termite mound.
Synonymy.—Termes malaccensis Haviland [Snyder, 1949].
Commensal.—Nocticola termitophila, Tonkin (Silvestri, 1946): The cockroach was found in the termite nest.
Commensals.—Nocticola sinensis, Kowloon (Silvestri, 1946): In termite nest.
Nocticola termitophila, Penang (Silvestri, 1946): In termite nest.
Commensal.—Nocticola sinensis, Repulse Bay, Australia(?) (Silvestri, 1946): In a termite gallery.
Commensal.—Ergaula capensis [= Dyscologamia wollastoni] French Equatorial Africa, Brazzaville (Rehn, 1926; Chopard, 1949).
Order HYMENOPTERA
Family FORMICIDAE
Subfamily FORMICINAE
Commensal.—Phorticolea boliviae, Bolivia, Cachuela Esperanza (Caudell, 1923): Three males collected in the joint nests of C. femoratus and Crematogaster limata.
Note.—Dr. W. L. Brown (personal communication, 1957) states that this ant is an Old World species only. So presumably Mann's record pertains to a different species.
Commensal.—Myrmecoblatta rehni, Mexico (Mann, 1914): "They were very abundant, several occurring in almost every nest, where they are no doubt very efficient scavengers."
Commensals.—Atticola mortoni, Nothoblatta wasmanni, and Phorticolea testacea, Brazil, San Leopoldo (Bolívar, 1905): Found in the formicaries of C. rufipes.
and
Commensal.—Myrmecoblatta rehni, Mexico (Mann, 1914): "They were very abundant, several occurring in almost every nest."
Subfamily MYRMICINAE
Commensal.—Attaphila bergi, or possibly a variety of this species, Huasán, Argentina? (Bruch, 1916).
Synonymy.—Atta lundi [Brown, personal communication, 1957].
Commensal.—Attaphila bergi, Argentina and Uruguay (Bolívar, 1901): The cockroach was found in the nests of the ants sitting on the back, neck, or head of sexual individuals. It remains attached to the ant during swarming. The antennae seem always to be mutilated. Bruch (1916) stated that in La Plata A. bergi is encountered by hundreds in every nest of A. lundi.
Synonymy.—Atta nigra Schupp [Brown, p.c., 1957].
Commensal.—Attaphila schuppi, Brazil, Porto Alegre (Bolívar, 1905): Found outside the nest of the ant and mixed in the columns of ants on the march.
Synonymy.—Atta octospinosa [Brown, p.c., 1957].
Commensals.—Attaphila fungicola, Panama (Wheeler, 1928): Taken in the fungus gardens of the ant.
Attaphila aptera, Esperanza, Dibulla, Colombia (Bolívar, 1905).
Commensal.—Attaphila bergi, or possibly a variety of this species, San Luis Province, Argentina (Bruch, 1916): According to Bruch, the behavior of this species of Attaphila is identical with the one encountered in Huasán in the nests of Acromyrmex lobicornis Emery; it differed from A. bergi in size and color.
Commensal.—Attaphila fungicola, British Guiana (Wheeler, 1928): Taken in the fungus gardens of the ant.
Attaphila sp., British Guiana (Beebe, 1921): 7 of 12 queens in one nest had cockroaches hanging on them.
Commensal.—Attaphila sexdentis, Brazil, San Leopoldo (Bolívar, 1905): Found in nests of the ant.
Synonymy.—Atta fervens Say [Wheeler, 1910].
Commensal.—Attaphila fungicola, U.S.A., Texas (Wheeler, 1900, 1910): The cockroach does not feed on the fungus in the ants' nest, as Wheeler (1900) first supposed, but mounts the back of the soldiers and licks their surfaces. It is tolerated by the ants with no signs of hostility. The antennae of the cockroach are clipped short. Although Wheeler (1910) stated that this is probably accidental or unintentional, it is peculiar that Bolívar (1905) noticed the same invariable mutilation of the antennae of Attaphila bergi. Wheeler (1900) had originally suggested that the antennae were probably clipped off by the ants which are continuously trimming the fungus hyphae. Louisiana (Moser, personal communication, 1959): Numerous specimens were encountered in some nests of A. texana. This cockroach is the most closely associated inquiline in the nest and maintains very intimate terms with the ants. It is found living in the fungus cavities and tunnels.
Commensal.—Phorticolea boliviae, Bolivia, Cachuela Esperanza (Caudell, 1923): Collected in joint nests of C. limata and Componotus femoratus.
Commensal.—Myrmecoblatta wheeleri, Guatemala (Hebard, 1917a): Collected from a colony of this ant under a stone on the shores of Lake Atitlan, altitude 11,719 feet.
Cockroach.—Attaphila flava, British Honduras (Gurney, 1937): Because the known hosts of the other five species of Attaphila are ants, we presume that this species also lives in the nest of some myrmecine ant.
Subfamily PONERINAE
Commensal.—Myrmeblattina longipes, Brazil, Rio de Janeiro (Chopard, 1924, 1924a; Hancock, 1926): Originally described as Phileciton longipes by Chopard (1924) from the nest of an ant mistakenly identified as Eciton sp.
Family VESPIDAE
Commensal.—Sphecophila polybiarum, French Guiana (Shelford, 1906a): Shelford stated that it was probable that the cockroaches living on the floor of the paper nest fed on small fragments of insects and spiders that were dropped by the wasp larvae feeding in the cells above.
Family MEGACHILIDAE
Commensal.—Oulopteryx meliponarum, Brazil (Hebard, 1921): According to Hebard, this cockroach is the first one to be known to inhabit the nests of bees. Nothing is known of the relationship between the cockroach and the bees. [See comment by Sonan (1924) on page 318.]
The cockroaches are arranged alphabetically by genus and species. The page references are to citations in the classified section above, where details and/or sources of the records are given.
Attaphila aptera
Ant: Acromyrmex octospinosus, p. 313.
Attaphila bergi
Ants: Acromyrmex lobicornis, p. 312.
Acromyrmex lundi, p. 312.
Acromyrmex silvestrii, p. 313.
Attaphila flava
Host unknown, presumably an ant, p. 314.
Attaphila fungicola
Ants: Acromyrmex octospinosus, p. 313.
Atta cephalotes, p. 313.
Atta texana, p. 313.
Attaphila schuppi
Ant: Acromyrmex niger, p. 312.
Attaphila sexdentis
Ant: Atta sexdens, p. 313.
Attaphila sp.
Ant: Atta cephalotes, p. 313.
Atticola mortoni
Ant: Camponotus rufipes, p. 312.
Ergaula capensis
Termites, p. 311.
Myrmeblattina longipes
Ant: Odontomachus affinis, p. 314.
Myrmecoblatta rehni
Ants: Camponotus maculatus(?), p. 312.
Formica rufibarbis, p. 312.
Formica subcyanea, p. 312.
Myrmecoblatta wheeleri
Ant: Solenopsis geminata, p. 314.
Nocticola sinensis
Termites: Macrotermes barneyi, p. 311.
Odontotermes sp., p. 311.
Nocticola termitophila
Termites: Macrotermes malaccensis, p. 311.
Odontotermes sp., p. 311.
Termes sp., p. 311.
Nothoblatta wasmanni
Ant: Camponotus rufipes, p. 312.
Oulopteryx meliponarum
Bee: Melipona nigra, p. 314.
Phorticolea boliviae
Ants: Camponotus femoratus, p. 311.
Crematogaster limata parabiotica, p. 312.
Phorticolea testacea
Ant: Camponotus rufipes, p. 312.
Sphecophila polybiarum
Wasp: Polybia pygmaea, p. 314.
Sphecophila ravana
Termite: Coptotermes ceylonicus, p. 310.
Sphecophila termitium
Termite: Macrotermes bellicosus or Macrotermes natalensis, p. 311.
Cockroaches that are sometimes found in the nests of, or in association with, other animals are not necessarily commensals. This is particularly true of cockroaches that normally are found unassociated with other animals or that merely occupy the same habitat with the other animals because of similar microclimatic requirements (see Chopard, 1924c).
McCook (1877) excavated in February a nest of Formica rufa in Pennsylvania. A hundred or more lively cockroaches occupied a part of the nest that contained few ants. Near the cockroaches McCook also found a colony of Termes flavipes. Ischnoptera deropeltiformis has been found in the company of ants, but it is probably not myrmecophilous (Donisthorpe, 1900). Mann (1911) found an "Ischnoptera" sp. (probably a species of Parcoblatta) abundant in the nests of, and tolerated by, Camponotus maccooki Forel in California. Dead and mutilated specimens of this cockroach were common in the nests of "Formicas." "Ischnoptera" sp. was also common in the nests of Veromessor andrei (Mayr) [= Stenamma andrei]. Hebard (1917) reported that W. M. Wheeler collected Eremoblatta subdiaphana in Arizona as an ant guest. Rehn (1906a; Rehn and Hebard, 1927) reported that Pholadoblatta inusitata had also been taken by Wheeler from the galleries of a jumping ant, Odontomachus clarus Roger [= O. haematodes insularis Guérin var. pallens Wheeler; Brown (personal communication, 1958)], on Andros Island, Bahamas; Rehn and Hebard (1927) stated that "This genus and species is the only blattid, which is presumably a myrmecophile, known from the West Indies." Rehn (1932a) reported Dendroblatta sobrina as taken in an ant nest in a tree in the Amazon Basin. Tivia australica was taken in an ant nest in Australia (Princis, 1954). The male of Compsodes schwarzi was taken in an ant nest in the Santa Rita Mountains of Arizona (Ball et al., 1942). A male and female of Stilpnoblatta minuta were taken in a migrating column of the ant Myrmicaria natalensis Sm. subsp. eumenoides Gerst. in Nyasaland (Princis, 1949). Princis cautioned that it is premature to derive any inference from this, possibly accidental, association. Four females of Parcoblatta desertae were taken about a nest of an ant, Ischnomyrmex sp. (Hebard, 1943a). A nymph of Parcoblatta virginica was found in a nest of Formica sp. (Hauke, 1949).
Chorisoneura texensis has been found in nests of webworm in Florida (Rehn and Hebard, 1916). Karny (1924) in Malaya found an oötheca of Aristiger histrio (sp.?) between leaves (Costus sp.) [Pg 317] that had been stuck together by a thysanopteron, Anaphothrips sp. He pointed out that the oötheca would not adhere to leaves that were not stuck together but would fall to the ground.
Seín (in Rehn and Hebard, 1927) in Puerto Rico found Aglaopteryx facies in abandoned cocoons of Megalopyge krugii (Dewitz) and in leaves webbed together by caterpillars and in abandoned spiders nests. Wolcott (1950; and in Rehn and Hebard, 1927) also found A. facies in the empty cocoons of M. krugii and in the larval tents of Tetralopha scabridella Ragonot on Inga vera (coffee shade tree); and "Where there are no butterfly-nests, it lives in abandoned spider-nests on the leaves of other forest trees." Cotton (in Wolcott, 1950) found the type of Aglaopteryx absimilis also living in the abandoned cocoon of M. krugii on bucare trees in Puerto Rico. Wolcott (1950) reported that Plectoptera dorsalis, Plectoptera infulata, and Plectoptera rhabdota have been found living in trees between leaves or in "butterfly-nests" of Tetralopha scabridella in leaves of Inga vera, or nests of Pilocrocis secernalis (Möschler) in the leaves of Petitia domingensis in the mountains of Puerto Rico. Seín (in Rehn and Hebard, 1927) had collected P. rhabdota in the nest of larvae of T. scabridella.
Wolcott (1950) reported that Nyctibora lutzi had been found in a large rotten stump associating with "'comején' termites [Nasutitermes costalis (Holmgren)], yellow wood-ants and rhinoceros beetle grubs." Rehn and Hebard (1927) found Simblerastes jamaicanus in numbers in the debris of an abandoned termites' nest in Jamaica: "To what extent the species is dependent upon the protection of the termite or other structures remains to be determined."
In Virginia Cryptocercus punctulatus has been found living in the same galleries with Reticulitermes sp., and on the Pacific Coast it has been found occupying the same log with Termopsis sp. (Cleveland et al., 1934).
Shelford (1909) found one male and one female of Balta platysoma in a nest of a spider of the genus Phryganoporus and assumed a symbiotic association. Chopard (1924) recorded Mareta acutiventris from empty nests of spiders on Barkuda Island, India; nothing is known of the relationships, if any, between these cockroaches and spiders.
Chopard (1924c) found Margattea sp. in the nest of the ant Acropyga acutiventris Roger; he also found Margattea sp., Periplaneta sp., Polyphaga indica, and Temnopteryx obliquetruncata in deserted termite mounds in India. However, he believed that none of these species were more than accidental associates of the host insects; he considered them hygrophilous cockroaches which had found a retreat in the nests.
McClure (1936) obtained a large nest of Vespula maculata (Linnaeus) [= Vespa maculata] in March in Illinois. In it were living 65 nymphs of Parcoblatta pensylvanica, 3 spiders (Philodromus pernix Blackwall), 2 immature spiders (Drassus sp.), and 6 mites. Balduf (1936) observed four individuals of Parcoblatta pensylvanica in a nest of Vespula maculata; he suggested that they probably fed on dead bodies and organic wastes of the wasps. However, Rau (1940) has observed this cockroach devour a Polistes larva in its cell. Although we do not imply that a commensal relationship exists between Parcoblatta and the wasp, it is well to recall a statement by Rothschild and Clay (1957): "A commensal relationship is potentially even more dangerous than a merely social tie, for by nature it is more intimate. The closer the association, the more easily is the balance upset. One partner can then suddenly take a mean advantage of the other."
Cockroach nymphs may enter bees' nests where, according to Imamura (in Sonan, 1924), they do not feed on honey or pollen but presumably feed on excreta of bees or anything scattered by bees in their nest; the bees are not disturbed by the cockroaches.
Cockroaches that have been found in the burrows of vertebrates are listed on pages 23-25.
Paulian (1950) found immature cockroaches in the nests of birds (Ploceinae) in Madagascar and Ivory Coast. All nests of Fondia sp. examined in Madagascar contained many cockroaches, and Paulian believed that the blattid was a species peculiar to the nests of birds. Three nests of Ploceus sp. in Ivory Coast yielded one or two cockroaches each in association with more numerous mites, Psocoptera, Heteroptera, beetles, and lepidopterous larvae (Delamare, Deboutteville and Paulian, 1952). These last cited workers also found four cockroaches in a nest of Estrildine sp., and two in a nest of an undetermined bird, all in association with other arthropods. Moulton (1912) observed large numbers of Symploce cavernicola and Periplaneta australasiae swarming in soft bird guano on the floor of caves in Borneo. Abdulali (1942) found in India many Periplaneta americana in caves containing the edible-nest swift; there was no indication of association of the cockroaches with the birds. Danforth (in Wolcott, 1950) reported finding large numbers of Aglaopteryx facies "in the nests of the grey kingbird, in the region of the Cartagena Lagoon [Puerto Rico], 'living among the twigs.'" In Trinidad, Kevan found a male of Blaberus discoidalis in a bird's nest (Princis and Kevan, 1955).
Davis (in Rehn and Hebard, 1914a) stated that "At Punta Gorda [Florida] there was a vacant house at the end of the town frequented at night by a Nanny and Billy goat, and on warm evenings many Periplaneta australasiae would run about on the piazza floor and on the sides of the house. They were seen feeding on the excrement of the goats and were no doubt to a great degree dependent upon them." This is another example of a coprophagous insect that has taken advantage of a particular situation favorable to its survival. Similar associations exist in which many of the domiciliary cockroaches feed on the feces of man and domestic and other animals (Roth and Willis, 1957a).
Tepper (1893) made the broad statement that the majority of Australian and Polynesian cockroaches appear to be wholly carnivorous, eating other insects, eggs, and larvae. He stated that, because of their voracity and cannibalistic tendencies, the carnivorous species lead more or less solitary lives so that one rarely meets several in close proximity; they are never very numerous at any time because the stronger devour the weaker in the absence of other prey. Tepper stated that Australian species of Ischnoptera hunt for their prey among the foliage of shrubs, and that Australian species of Cutilia [= Drymaplaneta, Hebard (1943)] run about actively on the surface, or ascend shrubs and trees in quest of living insects and therefore are highly beneficial. Tepper (1894) also stated that Geoscapheus robustus ate earthworms, grubs, and caterpillars. Froggatt (1906) and Marlatt (1915) attributed to Tepper the statement that cockroaches, like Epilampra notabilis, which are found out-of-doors in Australia, are carnivorous and feed on caterpillars and other soft-bodied insects; but Froggatt (1907) believed that this alleged behavior needed confirmation.
A number of observations have been recorded which indicate that sometimes cockroaches may be predatory. According to Ealand (1915), nymphs of the cockroach Pseudomops cincta fed on the Argentine ant Iridomyrmex humilis. In the laboratory, Eurycotis floridana has been observed to catch and devour the wasp Anastatus floridanus which parasitizes the eggs of Eurycotis (Roth and Willis, 1954a). Parcoblatta pensylvanica was observed devouring a larva of Polistes sp. in its cell in a deserted wasps' nest (Rau, 1940). Brigham (1866) saw a cockroach kill and eat a centipede four or five inches [Pg 320] long. Annandale (1910) described the destruction in Calcutta of termites by Periplaneta americana. During a heavy rain storm many termites flew into the dining room and were set upon by the cockroaches which seized them with their mandibles and began to gnaw their abdomens. If disturbed, the cockroaches carried the termites away in their mandibles without using their legs to seize, hold, or carry the prey. Sometimes only the abdomen, but other times the whole body with the exception of the wings, was devoured. Perhaps this observation led Allyn (Anonymous, 1937) to theorize that, first, cockroaches could eradicate termites from houses, and then the blattids in turn could be eliminated. Falls (1938) has pointed out the unfeasibility of this idea. Blattella vaga has shown some tendency to eat plant lice (Flock, 1941a). Certain small cockroaches found beneath cane leaf-sheaths, in the Philippine Islands, preyed in part upon leafhoppers (Uichanco, in Williams et al., 1931).
Takahashi (1924) stated that the American cockroach will eat the eggs of the hemipteron Cantao ocellatus (Thunberg). Cunliffe (1952) observed mite-infested cockroaches (Blatta orientalis, Blattella germanica, and/or Periplaneta americana) dislodge and eat the mite Pimeliaphilus podapolipophagus. Sonan (1924) reported that cockroaches (P. americana and P. australasiae) devoured the egg clusters and first instar larvae of Prodenia litula and the first instar larvae of Attacus atlas which were being reared in the laboratory. Lederer (1952) stated that Periplaneta americana ate reptile eggs in the aquarium at Frankfurt am Main. Pettit (1940) stated that cockroaches "are said to have destroyed a large colony of dermestids used to skeletonize carcasses at the University of Kansas."
DeFraula (1780) believed that his silent "gryllon" [obviously Blatta orientalis from his drawings; see Willemet (1784)] was the enemy of the chirping species of cricket, because after the cockroach became established in his home he no longer heard crickets chirping. Gilbert White (1905 ed.), writing in England in the late 18th century, stated that "Poda says that these [Blatta orientalis] and house crickets will not associate together; but he is mistaken in that assertion"; however, in August 1792 White noted that "Since the blattae have been so much kept under, the crickets have greatly increased in number." For several years Jolivet (1950) had observed changes in a mixed population of Blatta orientalis and Acheta domesticus in an old kitchen in France. He suggested that the cyclical fluctuations in the relative abundance of the cockroaches and crickets might be caused by reciprocal predatism with one species more susceptible than the other [Pg 321] at certain stages. Mallis (1954) has stated that crickets prey on other insects as well as on one another. Lhéritier (1951) had also observed crickets becoming rare in bakeries in France, having been superseded everywhere by B. orientalis; however, he doubted that Jolivet's hypothesis was the correct explanation and suggested that the higher optimum temperature requirements of crickets might be the regulating factor. Lederer (1952) stated that the number of crickets decreased in the aquarium buildings at Frankfurt am Main as the population of American cockroaches increased.
Platyzosteria novae seelandiae was found under the bark of trees in New Zealand devouring bugs (Walker in Shelford, 1909b).
For years it has been believed that cockroaches feed on bedbugs (Cimex lectularius L.) and this statement has been repeated in many reference works and articles. Ealand (1915) stated that cockroaches devour bedbugs with avidity. Even today similar statements are to be found in the literature. "In the old sailing ship days, they [cockroaches] were often welcomed by crews because of the belief that they would eradicate a population of bedbugs. This belief was based on scientific fact, as cockroaches are known as predators of bedbugs" (Monro, 1951). Cockroaches will often "help rid a house of bedbugs by devouring all the little parasites they can capture" (Gaul, 1953). The basis for this belief may have originated with a statement by Webster (1834) who wrote that bedbugs disappeared aboard "H.M. Sloop Chanticleer" when cockroaches made their appearance. Newman (1855) reported the observations of a friend who claimed to have seen a cockroach seize a bedbug in an infested boardinghouse in London. In 1920 Purdy reintroduced cockroaches into a house from which they had been exterminated, in order to control the bedbugs which had become established. According to a popular account by Lillingston (1934) African natives are said to ask sailors for a cockroach or two to be used to hunt bedbugs.
In Siberia, Burr (1926, 1939) found Blattella germanica and bedbugs inhabiting the same room. Mellanby (1939) studied the populations of an animal house in which bedbugs and cockroaches occurred in large numbers; the bugs apparently were not attacked and their numbers increased greatly over a period of a few weeks (Johnson and Mellanby, 1939). Wille (1920) placed starved B. germanica with bedbugs for 20 days, but the cockroaches failed to attack the bugs. In India, captive adults and nymphs of two species of house cockroaches would not touch living bedbugs or their eggs (Cornwall, 1916). In laboratory experiments Gulati (1930) found that Periplaneta americana [Pg 322] ate young bedbugs which had soft, blood-filled abdomens; adult bedbugs with harder exoskeletons sometimes were rejected. The maximum number of bedbugs eaten by a cockroach was 3 out of 12 during a period of 48 hours. Johnson and Mellanby (1939), also in laboratory experiments, were unable to show that bedbugs can be controlled by Blatta orientalis or that bedbugs are eaten to any extent by them. The existing evidence indicates that there is little basis for the often repeated statement that cockroaches destroy bedbugs in nature. As Lorando (1929) pointed out, assassin bugs, cockroaches, and red ants can hardly be considered as practical factors in bedbug control, though he did recommend the use of spiders.
According to Martini (1952), cockroaches prey on mosquitoes and sand flies but we have been unable to find any original sources for these statements; the only reference we have found in which cockroaches and Phlebotomus are mentioned together is a paper by Whittingham and Rook (1923); they fed ground-up cockroaches to larvae of Phlebotomus papatasii. Wharton (1951) reported that cockroaches and other predators attacked mosquitoes knocked down by insecticides and affected the number recovered.
Cockroaches will on occasion attack and bite animals other than insects. In an earlier paper (1957a) we discussed about 20 reports of cockroaches biting man. The injury is usually confined to abrasion of the callused portions of hands and feet but may result in small wounds in the softer skin of the face and neck. We failed to include the following reference in the above-mentioned paper. Sonan (1924) had his toes and breast nibbled by cockroaches on Hiyakejima Island during sleep. He had previously learned from a policeman that Periplaneta americana and P. australasiae nibbled people on that island, but he had hardly believed it before he experienced the biting himself.
Those who have reared cockroaches in the laboratory have undoubtedly seen cannibalism occur in the cultures. Cannibalism has been observed among the common domiciliary species of cockroaches as well as laboratory colonies of Leucophaea maderae (Scharrer, 1953), and Blaberus craniifer[12] (Saupe, 1928). Edmunds (1957) [Pg 323] reported that cannibalism was common in a laboratory colony of Periplaneta brunnea and that egg capsules deposited by a female were often eaten by the other cockroaches.
Periplaneta americana occasionally ate other cockroaches and their oöthecae and also attacked members of their own species (Lederer, 1952). Griffiths and Tauber (1942) recorded the killing of male American cockroaches by females of the species: "One female was especially vicious and attacked each new male as he was introduced into the container. Most of such males had molted less than 2 days previously. Older males were more capable of defending themselves against attacks of these cannibalistic females." Even though adequate food may be present, females of Periplaneta americana may eat their own eggs (Klein, 1933). Some females may regularly eat their oöthecae as soon as they are dropped (Griffiths and Tauber, 1942). To be completely eaten an oötheca generally must be attacked before it has hardened. If a hole is eaten in one side of the capsule, the cockroach may devour the eggs and leave a portion of the oötheca. Frequently only the keel or a part of the keel is eaten and when this occurs the eggs fail to hatch and usually do not complete development because of the rapid loss of water (Roth and Willis, 1955). When adults of P. americana and P. australasiae were deprived of food, both males and females ate newly deposited eggs and, finally, the females ate the males (Sonan, 1924).
Parcoblatta virginica in laboratory cultures also may eat part of its oöthecae; in this species only the soft end of the recently deposited oötheca was eaten (Roth, unpublished data, 1957).
Cros (1942) observed oöthecae-bearing females of Blatta orientalis attack and kill males of the same species which were attempting to mate; these males were then eaten by the females. Cros also observed injured and recently molted nymphs of B. orientalis to be eaten by others of the same species.
Pettit (1940) noted that cannibalism in his culture of Blattella germanica occurred only when the insects were molting. Adult insects attacked the molting cockroaches more often than did the nymphs. However, nymphs after the fourth instar occasionally set upon other molting nymphs. First-to third-instar nymphs rarely victimized their mates. The victims were all older than third instar; the later stadia were progressively more subject to attack, and molting adults suffered the greatest mortality. No direct correlation was noted between population density and cannibalism.
German cockroaches may attack newly molted nymphs of their own kind and cause them to deflate (Gould and Deay, 1938). Lhéritier (1951) has observed the hatching nymphs of B. germanica being devoured by their congeners even before they have left the oötheca.
Nauphoeta cinerea in laboratory cultures will eat newly hatched young of the same species (Roth and Willis, 1954; Willis et al., 1958). In Hawaii, in nature, N. cinerea may kill and eat the cypress cockroach, Diploptera punctata (Illingworth, 1942; Fullaway and Krauss, 1945).
Bunting (1956) stated that species of Neoblattella are omnivorous with carnivorous and cannibalistic tendencies. An adult female Panchlora sp. was killed and eaten by Neoblattella sp. in captivity. A male, provisionally identified as N. celeripes, was killed and partly eaten by two other males of the same species.
The factors influencing the extent of cannibalism among cockroaches are not completely known. According to Wille (1920) hunger was not the cause of cannibalism in Blattella germanica. Wille claimed that the tendency toward cannibalism increased at high temperatures and decreased at low temperatures. Pettit (1940) also noted this effect. Gould and Deay (1938) stated that under crowded laboratory conditions, when there was a scarcity of food, cannibalism among Periplaneta americana was common. The injured cockroaches and those unable to molt were often eaten. Adair (1923) made similar observations. Undoubtedly, conditions of crowding, availability of food, temperature and other factors all influence cannibalism, but practically no experimental work has been done on this subject.
It is interesting, in comparison with the above positive examples of cannibalism, that both Saupe (1928) and Roeser (1940) observed no cannibalism during extensive studies with Pycnoscelus surinamensis. In fact, Roeser stated that there was never a case of cannibalism in spite of long hunger periods imposed on both nymphal and adult insects.
Besides preying on their own species or on other blattids, cockroaches exhibit additional symbiotic relationships among themselves. These relationships are (1) the familial associations of parent and offspring, (2) gregariousness, (3) intraspecies fighting, (4) interspecies compatibility, and (5) interspecies antagonism. There are some inconsistencies between observations made on the same species [Pg 325] by different workers, which only further observation and experimentation will explain. Some of the reported observations are unique; this is especially true for the feral species. Because of the paucity of information, it is impossible at this time to make valid generalizations about some of these interesting relationships.
The females of many species of cockroaches insure varying degrees of protection to the developing young in their ways of disposing of the oötheca after it has been formed. The extent of this association between the mother and her developing progeny varies from the minimum amount of time spent by oviparous females in concealing their oöthecae, to the duration of embryogenesis in the so-called viviparous species, a period of over a month or more.
Haber (1920a) observed a female of Periplaneta americana chew a groove in a piece of pasteboard into which she attempted to deposit her oötheca. The oötheca failed to adhere to the shallow hole and fell to the floor. After several futile attempts to replace the oötheca in the hole, the female finally left the egg case on the floor of the cage and coated it with an oral secretion to which she attached bits of trash. During this operation she chased other females away when they ventured near the site. Qadri (1938) described the behavior of the female of Blatta orientalis in concealing her oötheca in a hole that she dug in sand; she deposited the egg case in the hole, coated it with saliva and sand, and then refilled the pit. Rau (1943) described in detail how females of P. americana and B. orientalis covered their oöthecae with wood dust or sand in holes they had prepared in the substrate. Both species placed a sticky oral secretion in the holes and then deposited their oöthecae therein. After coating the oöthecae with more sticky secretion, the females adjusted the oöthecae so that the keels were uppermost and then carefully concealed the oöthecae with the excavated debris. Both females spent over an hour in the act. Rau (1924) previously reported that of 90 oöthecae deposited by B. orientalis in jars containing earth and trash, 36 were placed in crevices or excavated holes, and 38 were hidden by being covered with dirt stuck to them with saliva; only 16 were left uncovered.
Edmunds (1957) described oviposition by Periplaneta brunnea. Some females spent from 30 to 40 minutes secreting from the mouth a frothy substance that was smeared on the substrate; the egg capsule was deposited in the secretion and covered with additional froth, which hardened into a very strong cement. Some females spent as [Pg 326] long as two hours coating the capsule after it was deposited. It was not stated whether the oötheca was otherwise concealed. The female remained with her body over the oötheca for several hours and drove away other cockroaches which approached.
Sonan (1924) observed that Periplaneta americana and Periplaneta australasiae spent from 40 minutes to an hour covering their oöthecae, and that if the females were frightened away from this activity, they returned again to complete it. As well as excavating holes in the substrate in which to deposit its oöthecae, P. americana also avails itself of readymade crevices of appropriate size (Ehrlich, 1943). Species of Epilampra in Malaya were said by Annandale (1900) to deposit their oöthecae in crevices in floating logs just above the water line. However, Shelford (1906) stated that four genera (including Epilampra) of the subfamily Epilamprinae are "viviparous," in which event the females would carry their oöthecae within their bodies during embryogenesis and would not place the oöthecae in crevices in logs.
The female of Cryptocercus punctulatus was observed to make a groove in a piece of wood, then carry her oötheca 6 inches from where she had dropped it and place it in the groove; she covered the oötheca so that only a portion was visible (Cleveland in Cleveland et al., 1934). Dr. W. L. Nutting (personal communication, 1954) collected a number of oöthecae of C. punctulatus in the field and found each one almost completely sealed off with bits of wood in a deep groove in the roof of a chamber in a log. The keel of the oötheca was visible but the rest was well camouflaged. He stated that "The adult pair usually frequents the chamber at this time, while their broods of previous years occupy neighboring galleries."
Berland (1924) observed a female of Loboptera decipiens filling a hole (the abandoned nest of a hymenopteron) with earth that she carried in her mouth; he later found her oötheca behind the earthen barricade which she had erected.
In summary, the following species of oviparous cockroaches have been observed concealing their oöthecae (only those references not previously cited are given): Blatta orientalis; Cryptocercus punctulatus; Ectobius sylvester (Harz, 1956, 1957); Epilampra sp.; Eurycotis floridana (Roth and Willis, 1954a); Loboptera decipiens; Balta scripta, Methana curvigera, Methana marginalis, and Methana caneae (Pope, 1953a); Pelmatosilpha marginalis, Pelmatosilpha purpurascens, and Nauclidas nigra (Bunting, 1956); Periplaneta americana (Haber, 1919; Adair, 1923; Seín, 1923; Nigam, 1933; Gould and Deay, 1938; Rau, 1940a); Periplaneta australasiae (Girault, 1915b; Spencer, 1943; Pope, 1953); Periplaneta brunnea (Roth and Willis, [Pg 327] unpublished data, 1958); Periplaneta fuliginosa (Gould and Deay, 1940); Periplaneta ignota (Pope, 1953); Supella supellectilium (Flock, 1941). Undoubtedly other oviparous species that drop their oöthecae long before the eggs hatch also make some attempt to conceal the oöthecae by placing them in crevices or covering them with debris.
Sometimes the oöthecae are deposited but not concealed. Hafez and Afifi (1956) reported that in Egypt Supella supellectilium attaches its oötheca to a suitable substrate with a gummy oral secretion but leaves the egg capsule otherwise exposed. We (1954) have noticed similar behavior in laboratory colonies of this species and of Blatta orientalis, as have Gould and Deay (1940). Cornelius (1853) stated that the female of B. orientalis takes care of the safety of her offspring to the extent of usually dropping her oöthecae in places which are dry and raised above the ground, although rarely one also may find some oöthecae scattered on the ground. For lack of suitable material females of Periplaneta americana sometimes did not conceal their oöthecae (Nigam, 1933). Frequently in laboratory colonies P. americana merely drops the oöthecae loosely in sand or food "in contrast to P. australasiae, which almost always went to considerable trouble to fasten their eggs securely and to conceal them with debris" (Pope, 1953). If conditions under which Nauclidas nigra is kept are not suitable, the female will drop her oötheca anywhere (Bunting, 1956). Rau (1940) stated that the female of Parcoblatta pensylvanica does not conceal her oötheca. However, Gould and Deay (1940) stated that this species deposits its oöthecae loosely behind bark. Ellipsidion affine and Ellipsidion australe attach their oöthecae to bark or the underside of leaves but apparently make no attempt to conceal them (Pope, 1953a).
The females of most of the above species have no further familial association with their offspring. The eggs hatch with no attention from the mother who is probably not even in the vicinity at that time. The young apparently do not react to the presence of the parent, as such, after hatching. This is not unexpected, as several additional oöthecae may have been deposited by these oviparous females before the eggs of the first oöthecae hatch. However, a different behavior is encountered among species that do not form a second oötheca until after the eggs of the first have hatched (see below) and in the so-called colonial species.
Shaw (1925) reported that in Australia both Panesthia australis and Panesthia laevicollis appear to live in families, and that one usually finds a pair of adults associated with from 12 to 20 nymphs in [Pg 328] different stages of development; he continued, "it is only where the molts are very abundant that one loses sight of this familial habit." Tillyard (1926) also stated that the Australian species of Panesthia live in burrows in soil in strict family communities of a pair of adults and 10 to 20 nymphs. A related colonial species, Cryptocercus punctulatus, lives in both sound and rotten logs in colonies consisting of a pair of adults and 15 or 20 nymphs, probably representing two or three broods (Cleveland et al., 1934; Cleveland, 1948). Chopard (1938) has cited this association as an example of gregariousness, which it may well be; however, the presence of only one pair of adults in each colony suggests a more intimate relationship.
Among species of Blattella and certain other genera with similar reproductive habits the female carries her oötheca clasped in her genital cavity with the posterior portion projecting behind her. Each normal oötheca is carried for approximately the duration of embryogenesis and is not dropped until, or shortly before, hatching. We have seen (1954, fig. 65) newly hatched nymphs of Blattella vaga crawl over the body of the mother who stood quietly near the dropped oötheca; this female raised her wings and some of the nymphs crawled under them onto the dorsal surface of her abdomen. The nymphs seemed to feed on the grease covering the mother's body. The association was short-lived, however, and soon the nymphs scattered. Pettit (1940) stated that when hatching of Blattella germanica occurs in the open (on a table top), the nymphs may remain near the capsule only a few minutes. Ledoux (1945) found that newly hatched nymphs of B. germanica remained together without shelter in a single, sparse group. If the nymphs were separated by blowing on them, the group quickly reassembled, usually in the same spot. Ledoux showed that this gregarious grouping of first-instar nymphs was not necessarily a familial association by placing nymphs from two oöthecae together. In groups of 8 to 12 nymphs there was a perfect intermingling of the offspring from the two different females.
It is among the so-called viviparous cockroaches that the greatest number of observations have been made of postparturient associations between female cockroaches and their offspring. The females of these species carry their oöthecae in brood sacs within their bodies until embryogenesis has been completed. This behavior ensures protection of the young from desiccation and attack by parasites (Roth and Willis, 1955a). (See Roth and Willis, 1958a, for an analysis of oviparity and viviparity in the Blattaria.) Shelford (1906, 1916) reported that he had captured a female of Pseudophoraspis nebulosa in Borneo with numerous young nymphs clinging to the undersurface of [Pg 329] her abdomen. He also recalled that there was in the Hope Museum (Oxford) a female of Phlebonotus pallens to which the following label was attached: "'Ceylon ... carries its young beneath its wing covers. 1878.'" Pruthi (1933) found in South India another female of P. pallens which was carrying over a dozen young nymphs on her back beneath her wings. In his paper Pruthi reproduced a photograph of this specimen with the light-colored nymphs in place on the back of the female. Hanitsch (1933) reported having seen a museum specimen from Luzon, Philippine Islands, of the apterous female of Perisphaerus glomeriformis with nymphs still clinging to her undersurface; he also reported having seen a museum specimen of a female of Ellipsidion variegatum from Australia with four young clinging to the upper side of the apex of her tegmina and six to the oötheca which projected beyond her body. Presumably this specimen was giving birth when captured. Gurney (1954; personal communication, 1958) stated that specimens of Perisphaerus sp. from Mindanao and Luzon have been found with young nymphs clinging to the middle and hind coxae. The first-instar nymph has an elongate face and specialized galeae. Karny (1925) also observed that at the slightest alarm the young of some species of Phoraspidinae creep under the dome-shaped front wings of the mother.
The newly hatched young of Leucophaea maderae have also been seen congregated under the mother on several occasions. Seín (1923) stated that after being born, the nymphs of this species gather under the mother and accompany her at night in her excursions in search of food. Pessôa and Corrêa (1928) reported that "During the first days the free larvae hide under the adult cockroach which becomes restless and active in contrast to its usual slow gait." Wolcott (1950) stated that "They are not only gregarious, but the mother broods over her young, and together they sally forth at night in search for food, until they are of such a size as to mingle with their elders."
The African mountain cockroach Aptera fusca has been observed during late summer and early winter in familial groups beneath loose bark, under stones, and in dead leaves (Skaife, 1954): "Each party consists of a number of black young ones, together with one, two or more adult females and perhaps a winged male or two. Later on they scatter and live more or less solitary lives." In Malaya Karny (1924) often found phoraspidine females between leaves surrounded by about 20 young nymphs. He stated that one also often found females of Perisphaerus armadillo surrounded by pale, yellowish-white young; similarly he had observed that Archiblatta hoevenii was found [Pg 330] mostly in colonies made up of mothers and their young. The duration of these associations is not known.
Saupe (1928) noticed that the newly hatched nymphs of Blaberus craniifer (see footnote 12, p. 322) collected together under the body of their mother and stated that this is as pronounced a case of brood care as Zacher had observed with Pycnoscelus surinamensis. Nutting (1953) stated that "A degree of maternal solicitude is exhibited by this roach [B. craniifer], for many times I have observed the female to remain motionless for an hour or more with her unpigmented brood clustered around and beneath her body." We, too, have observed similar behavior in laboratory colonies of B. craniifer and Leucophaea maderae.
Chopard (1950) noted that after hatching the young of Gromphadorhina laevigata remained grouped around the female for some time; the mother stood motionless, high on her legs, with her thorax curved up to make room for the brood which hid under her body. We (unpublished data, 1958) have seen young nymphs of Gromphadorhina portentosa also stay near their mother for some time after birth; the mother at this time produced a characteristic hissing sound when she was only slightly disturbed by the movement of our hand near her and her brood. The sound is produced as air is expelled through the second abdominal spiracle. We have seen recently hatched nymphs of Nauphoeta cinerea crawl beneath the mother, even under her wings, where they remained about an hour (Willis et al., 1958). Bunting (1956) observed a female of Blaberus discoidalis collect a mound of debris into which she inserted the tip of her abdomen; he found young in the mound later the same day. This female showed no maternal care for the young after birth. Whole families of cockroaches may be found in bromeliads in Brazil (Ohaus, 1900). Hebard (1920) observed a colony of adults and young of Dendroblatta sobrina on a tree trunk in the Panama Canal Zone.
Whether any of the above associations exemplify maternal care for the newly hatched young is questionable. The behavior of the mother, beyond placing her eggs in a suitable location, seems to be entirely passive. The first-instar nymphs are the active partners in these associations, and they may merely be seeking shelter under the nearest convenient object rather than under the mother as such. More extensive studies of some of these relationships will be needed before claims for maternal care, as suggested by Scott (1929), can be substantiated.
Casual statements that cockroaches are gregarious are often encountered in the literature. There has been some argument to the [Pg 331] effect that large numbers of these insects seeking the same environment in a limited space would appear to be gregarious, whereas there is probably no true social tendency (Rau, 1924). Reactions of cockroaches to certain stimuli in the environment undoubtedly do result in aggregations of individuals. However, as Chopard (1938) has pointed out, it is difficult to assign the respective parts played in assembling by the attraction of the milieu and by gregarious instincts. Chopard (1938) also stated that Orthoptera with a gregarious tendency are found rarely isolated; one finds them, on the contrary nearly always collected in the same shelters, close together, as if conscious of a need for contact between themselves. He continued further that one can be tempted to attribute the assembling to taxes but that interattraction equally plays an important role; for example, if one places a large number of cockroaches in a container and offers them similar shelters composed of cardboard tubes, one finds that nearly all the individuals will assemble in one of the tubes, ignoring the others. Pettit (1940) claimed that in Blattella germanica gregariousness seemed to depend on the mutual attractiveness of body secretions as well as a thigmopositive behavior and love of warmth.
Ledoux (1945) has studied experimentally gregariousness and social interattraction in Blatta orientalis and Blattella germanica. He also found that the cockroaches tended to collect in shelters containing other cockroaches. He concluded that group formation is not the result of chance, but is a social phenomenon, and that interattraction is mainly olfactory, conditioned by (1) positive chemotaxis to odors emitted by the cockroaches themselves, (2) positive hygrotaxis, and (3) thigmotaxis. He found also that large groups are not stable and tend to break into smaller groups.
Gregariousness in the Orthoptera varies in intensity according to the species and within a species according to the age or physiological state of the insects (Chopard, 1938). This is well exemplified by several of the blattid species discussed below.
Gregarious groupings of cockroaches have been observed most frequently among the domiciliary species. A few examples will suffice. Gal'kov (1926) observed heavy infestations of undetermined cockroaches in workers' living quarters in the Ural region: "In the corners near the stove, the cockroaches covered the walls in a dense carpet." After fumigating he collected about 135,000 dead cockroaches from one barracks and about 475,000 from another. We have reviewed a few other examples of heavy infestations in our 1957(a) paper.
Periplaneta americana was observed by Gould and Deay (1938) in an old meat-packing building in Indiana. Adult cockroaches were present in large numbers between closely placed beams, but the nymphs were more common in cracks between bricks. Clusters of several hundred cockroaches were seen on the open walls of the cold, dark hide room. Gould and Deay stated "American roaches of all sizes live together in perfect harmony. Young nymphs have been noted in clusters underneath adults and crawling over the adults as they wander about in rearing jars." In the monkey house of the Hamburg zoo, P. americana spent most of the day in the cellars resting on the walls in groups of about 200 individuals (Brecher, 1929). Lederer (1952) noted that in closed, dark, heated spaces under the aquarium at Frankfurt am Main, P. americana rested in groups of 20 to 30 individuals; he stated that it was remarkable that the "herd" divided itself into groups each of which usually contained insects of the same age or stage of development. Eads (1954) found P. americana in 40 percent of 762 sewer manholes in Tyler, Tex.; 13 percent of 670 of these manholes were heavily infested with 100 or more cockroaches in each. Other heavy sewer infestations have been reviewed in our 1957(a) paper.
Ehrlich (1943) has stated that Periplaneta americana exhibits social behavior. For instance, cockroaches of various ages inhabit a fairly large space jointly; the adults and older nymphs sense approaches with their antennae and warn and protect the young by a beating of wings and by body movements. There is complete utilization of the available living space; the imagos drive older nymphs from their resting places, and the older nymphs drive out the younger ones, until all cracks, depending on their size, are occupied by various age groups of different sizes. In his experiments Ehrlich observed that in cages with no hiding places the cockroaches would group together; when given a choice of small and large shelters, P. americana hid only under the larger ones that could shelter more insects. Finally, the cockroaches ceased to bite and fight each other when they crowded together in the face of danger.
Of Blatta orientalis Marlatt (1915) stated "This species is notably gregarious in habit, individuals living together in colonies in the most amicable way, the small ones being allowed by the larger ones to sit on them, run over them, and nestle beneath them without any resentment being shown." Haber (1919) also observed that this species is often noticed "huddled together, the younger ones crawling over, around, and beneath the older ones."
Wille (1920) observed that nymphs of Blattella germanica remained [Pg 333] almost constantly in groups during the first and second instars, but less so during the third instar. He believed that the aggregations of young occurred because they could occupy narrow crevices where the larger insects could not penetrate. At usual room temperatures the older nymphs and adults lived completely isolated, but at certain temperatures they gathered together in large, tightly pressed groups.
Supella supellectilium is said to be gregarious (Gould and Deay, 1940). The smaller nymphs aggregate in small groups in rearing containers, but the older ones remain separate from one another (Hafez and Afifi, 1956). Leucophaea maderae is sociable and rarely found alone; in their favorite hiding places, hills of these cockroaches can be seen hanging together (Seín, 1923). Wolcott (1950) also stated that L. maderae is gregarious. Annandale (1900) observed that in the "Siamese Malay States" large colonies of Periplaneta australasiae conceal themselves in hollows of bamboo logs from which houses are built. Moulton (1912) stated that he was astonished at the large numbers of P. australasiae and Symploce cavernicola that he saw swarming on the sides of caves of Mt. Jibong, Borneo.
Rehn and Hebard (1905) stated that in Key West, Fla., Eurycotis floridana fairly swarmed under the coquina boulders in the woods, in groups of a dozen containing both young and adults; Pycnoscelus surinamensis was very abundant in the same type of habitat. Caudell (1905) also found the young of E. floridana with the mature individuals. Hebard (1917) in his discussion of Lattiblattella rehni again mentioned finding frequent colonies of E. floridana in Florida. He also found many specimens of Blaberus craniifer under boards on the ground at Key West. He found Parcoblatta lata numerous under bark of dead pine trees in Alabama. However, Dowdy (1955), in an ecological study of oak-hickory forest in Missouri, stated that "Parcoblatta [sp.] were never recorded as being gregarious, in fact they were mostly solitary. However, in some cases two were found together." Yet Blatchley (1895) stated of Parcoblatta pensylvanica that in the winter in Indiana "One cannot pull the loose bark from an old log without dislodging a colony of from ten to a hundred of the nymphs of various sizes." Males of Parcoblatta virginica were said to be often gregarious beneath loose bark and under chunks and rubbish (Blatchley, 1920).
Rehn and Hebard (1927) quoted observations made earlier by Hebard on Byrsotria fumigata in Cuba: "I found the specimens under flat stones, sometimes in colonies of 3 or 4 mature specimens and numbers of immature individuals in all stages of development." These observers also reported that Aspiduchus borinquen was found in Puerto [Pg 334] Rico in a limestone cavern by thousands in the grass and on the walls. J. W. H. Rehn (1951a) stated that a related species, apparently Aspiduchus cavernicola, was seen in great numbers on the side walls and roof of a cave in Puerto Rico, but it was not possible to collect any of these and, we infer, confirm the species. Rehn and Hebard (1927) in their account of Simblerastes jamaicanus reported finding it in numbers in a termite nest. Pemberton and Williams (1938) stated that Diploptera punctata is of gregarious habits in Hawaii. Saupe (1928) observed a strong "herd instinct" in all age groups of Blaberus craniifer. Bunting (personal communication, 1956) stated that large nymphs and adults of Blaberus discoidalis "congregate in narrow cracks or on the underside of some low object. The younger nymphs keep in close communities of approximately the same age." Sonan (1924) stated that in Formosa(?) Salganea morio is usually found in groups of six or seven in decayed trees. Species of the genus Litopeltis may be found in small groups as they are somewhat gregarious (Rehn, 1928).
The physiological or psychological effects of gregariousness, or lack of it, are interesting aspects of the basic phenomenon. Landowski (1937) studied in Blatta orientalis the effect on development and growth of the transition from life in complete isolation to life in groups. He kept nymphs in groups of 1, 2, 4, 8, and 16 in jars of identical size and shape. Landowski found that (1) mortality increased with the size of the group and with age, as each animal occupied more of the available space. [Presumably these factors are less detrimental in nature where the group is unconfined.] He further found that (2) life in complete isolation extended the time required to produce an adult insect; and (3) the mean weight of the adult insect was, generally, in inverse proportion to the number of nymphs raised together; isolated insects usually attained the greatest adult weight.
Similarly, Griffiths and Tauber (1942a) found that isolation extended the period of nymphal development in Periplaneta americana. As most of their isolates died before reaching maturity, these workers concluded that the American cockroach does not thrive when individually isolated and that several individuals must be together for optimum development to occur. Pettit (1940, 1940a) observed that isolated nymphs of Blattella germanica take longer to mature than those reared in groups. Wallick (1954) found indications in B. germanica that there is an inverse relationship between population density and individual weight; as the population decreased the weight [Pg 335] increased. He also noted an inverse relationship between population density and life expectancy in this species.
We (Willis et al., 1958) have confirmed the above observations that Blattella germanica, Blatta orientalis, and Periplaneta americana complete nymphal development in less time when reared in groups rather than individually. We (loc. cit.) also found that nymphs of the following additional species matured more quickly when reared in groups: Eurycotis floridana, Periplaneta fuliginosa, Supella supellectilium, Nauphoeta cinerea, and Pycnoscelus surinamensis; only a very slight decrease in the average length of the developmental period was found in grouped nymphs of Leucophaea maderae.
Wharton et al. (1954) observed that virgin adult males of Periplaneta americana that had been individually isolated upon emergence were almost wholly unresponsive to the sexually stimulating, female odor for a test period of four weeks. Similar males of comparable age that were kept in groups reacted strongly from the sixth day on. Removal of reactive males from the group inhibited the reaction in these isolates, but the response returned when the insects were regrouped. We (1952) had similarly observed that no isolated male of Blattella germanica was ever seen to give a courting response without having received some form of external stimulation. Yet when numbers of males were kept together isolated from females, on several occasions the males became active and a few individuals gave a courting response. As the sexual stimulus is received by the male of B. germanica through contact rather than odor, as in P. americana, presumably it was mutual contact between the grouped males that released the courting activity.
Cloudsley-Thompson (1953a), in his studies of diurnal rhythms in Periplaneta americana, observed a steady decline in total activity in successive 24-hour cycles: "When two cockroaches, even of different species (P. americana and P. australasiae) were kept together, this depression did not appear to set in so readily." The associates apparently kept each other active.
Isolated females of Periplaneta americana can be conditioned to run a simple maze with less time and fewer errors per trial than when paired or when a member of a group of three (Gates and Allee, 1933). There was less activity, and accordingly fewer errors per minute, among cockroaches tested as pairs and groups of three than as isolated individuals. This observation should not be contrasted with that of Cloudsley-Thompson (1953a), cited above, because the intervals during which activity was observed were quite different.
In the above account we have presumed that aggregations of some [Pg 336] species are indications of gregariousness. However, until gregariousness has been proved experimentally for each species, we concede that reactions to environmental stimuli might be sufficient to bring about some of the observed groupings without any interaction between individuals.
In concluding this section we note that Tepper (1893) stated that carnivorous cockroaches in Australia lead more or less solitary lives, and that one rarely meets several together in close proximity. Takahashi (1940) observed that in Formosa Blattella humbertiana does not have a tendency to throng together. Rau (1947) stated that the adults of Ischnoptera deropeltiformis showed no tendency toward gregariousness, but in the laboratory newly hatched young lived close together under bark and remained together throughout the nymphal stages. We wonder whether this gregariousness was not imposed by the restricted quarters of the cage. As mentioned above, Dowdy (1955) did not find Parcoblatta sp. to be gregarious in the field.
Fighting occurs among cockroaches of the same species over food or shelter or between males. Saupe (1928) observed late-instar nymphs of Blaberus craniifer attack each other and even adults. Additional records cited in the section on intraspecies predation (p. 322) imply fighting within a species. Rau (1924) saw a male of Blatta orientalis attack another male in copula and bite away a large portion of its wing. Two other males in the container had their wings badly torn overnight, presumably as a result of fighting.
Ehrlich (1943) stated that individuals of Periplaneta americana that are feeding will ward off intruders by spreading their wings and pushing with their hind legs. However, the intruder will approach again and again biting the feeder in the legs and wings. Frequently the odor of approaching food was sufficient to cause the cockroaches to fight and bite each other. Biting and fighting also occurred when individuals of this species defended their daytime hiding places. A position of attack is assumed when two antagonistic individuals of P. americana meet (Ehrlich, 1943, fig. 14). The insects raise their bodies slightly above the ground, by extending their legs, and they stretch their heads forward horizontally so that their mouth parts protrude; when the insects jump at each other, they may wound each other severely in the soft parts of the body. Fighting between sexually excited males resulted in injury to their legs, wings, cerci, and other parts of the body. Frequently an insect that could no longer defend [Pg 337] itself was killed. Lederer (1952) also made similar but less extensive observations on fighting in this species.
Pettit (1940) quoted Woodruff as stating that nymphs of Blattella germanica, apparently healthy and perfectly normal, would do battle for no apparent cause other than a chance meeting, and that occasionally the fight was to the finish, the loser being eaten. Pettit could not substantiate such voracious attacks, although he saw nymphs engage in fights lasting about two seconds during which one would be driven off by vigorous bites on legs or cerci. Small nymphs of B. germanica tended to ignore each other, but third-and later-instar nymphs would engage in "quarrels" of short duration when two met. Pettit noted that males of B. germanica that were crowded together quickly set upon, but did not always kill, other cockroaches introduced into their cage. When he isolated a dozen males in a small cage, they became quarrelsome and three of the group were killed and partly eaten. After several days the surviving males had taken positions so that each was equidistant from his neighbors. Some of these males attacked other males and a female that were introduced, by biting their legs and cerci. Females under similar conditions were much less aggressive, although Pettit saw some females that roved about biting all large members of the group that were within easy reach.
We have frequently observed aggressive behavior between males of Nauphoeta cinerea, which resulted in torn wings. The males would wrestle with each other rolling over and over.
We agree in essence with Chopard (1938) who stated that it is improper to speak of associations apropos of the ecological distribution of Orthoptera. He continued that it is clearly evident that different species of Orthoptera, which are found grouped on a territory more or less narrowly limited, have no interdependence among them. Their grouping results uniquely from almost similar reactions to the different factors which characterize this limited milieu. There is neither interdependence nor interaction; the grouping is a false biocoenose, born under the action of the environment, and does not survive a modification of this milieu.
However, as there are numerous examples of mutual toleration between different species as well as examples of incompatibility, the subject has more than academic interest even if no true ecological significance. On the other hand, further study may show that certain of these associations are definitely ecological, particularly among the [Pg 338] feral species. As might be expected, most of the following examples pertain to domiciliary cockroaches.
Dozier (1920) occasionally found Periplaneta americana with Eurycotis floridana in decaying stumps, beneath loose bark of decayed trees, and beneath corded wood. Adair (1923) stated that in his house in Egypt Periplaneta americana, Blatta orientalis, and Blattella germanica were found together in a cupboard. Sambon (1925) found B. orientalis and B. germanica side by side but not fraternizing in a home in Italy. Gould and Deay (1938) observed that apartments over stores were infested with both B. germanica and P. americana, but did not indicate whether these occupied the same microhabitat. Gould and Deay (1940) observed that in the Purdue University greenhouse Periplaneta fuliginosa was found "under benches, boxes, pots and other objects in association with the American roach." Dr. L. A. Hetrick (personal communication, 1954) wrote us that several summers before he had had a mixed infestation of cockroaches, which included Periplaneta australasiae, Periplaneta fuliginosa, and Pycnoscelus surinamensis, in his chicken shed.
Eads (personal communication, 1955), in response to our inquiry about the mixed populations of cockroaches that he had reported infesting sewers in Texas (Eads et al., 1954), stated that "Each of the ten colonies of B. orientalis found in Tyler manholes were associated with larger colonies of P. americana. True breeding colonies of B. orientalis appeared to be present since all the developmental stages were taken. The same situation existed with the P. fuliginosa and the two species of Parcoblatta. Larger colonies of P. americana were associated with the other species in each case. From our limited observations the two species always appeared to be perfectly compatible." Eads et al. (1954) had found Periplaneta fuliginosa in three manholes, Parcoblatta bolliana in one manhole and Parcoblatta pensylvanica in one manhole. We assume that the groups of each species were spacially discrete so that they were recognizable as colonies. Dr. T. A. Olson (personal communication, 1958) has observed two or more species of cockroaches in a single structure but never in mixed colonies. Each species was separated physically from the others. Olson concluded that cockroaches of different species do not mingle freely unless forced to do so by some special environmental condition. Pettit (1940) found B. germanica and P. americana similarly separated in the same building or even in the same basement laboratory.
Perkins (1899) found Lobopterella dimidiatipes generally living in company with the young of Periplaneta australasiae in Hawaii. Rehn and Hebard (1914) in Florida found P. australasiae abundant with [Pg 339] Periplaneta americana on a quarter-boat. They also noted that the forficulid Marava [= Prolabia] arachidis (Yersin) appeared in numbers in a kitchen after dark accompanied by swarms of P. americana. These workers also found Leurolestes pallidus in a fruit store in Key West "where the species was common in a pile of old burlap bags and in cracks under the stands which it shared with one fairly large colony of Blattella germanica, occasional specimens of Holocompsa nitidula, a few specimens of Periplaneta americana, and one specimen of Supella supellectilium." They also found H. nitidula with Blaberus craniifer "between old boards in a woodshed, where nymphs were more numerous than adults."
Rehn and Hebard (1914) stated of Supella supellectilium in Florida that "The females were all taken in cupboards where Blattella germanica was found in swarms." The association in human habitations of S. supellectilium and B. germanica has been reported also by Sein (1923), Puerto Rico; Shaw (1924), Australia; Mallis (1954): "German and brown-banded roaches were often found in the same crevice."; Anonymous (1958), Texas; and Anonymous (1958a), Georgia. Gould and Deay (1940) stated that other species of cockroaches, especially B. germanica, may be found with S. supellectilium in the same part of a building. Yet Shaw (1925) stated that "when Supella supellectilium Serv. invades places already occupied by Blattella germanica L., it tends to oust the latter."
Blaberus discoidalis has been found in homes or in fruit debris in Puerto Rico in company with the more common, domiciliary species Leucophaea maderae, but never in abundance (Sein, 1923; Wolcott, 1950). Illingworth (1915) in Hawaii found Symploce hospes associated with Nauphoeta cinerea, Graptoblatta notulata, and Diploptera punctata.
Hebard (1917) found Aglaopteryx diaphana in a bromeliad on a forest tree in Jamaica together with Nyctibora laevigata and numerous Cariblatta insularis. He also found numerous Aglaopteryx gemma under signs on longleaf pines in Alabama with occasional specimens of Parcoblatta lata. In Virginia he found Parcoblatta uhleriana in a decaying chestnut log with Cryptocercus punctulalus. In Florida he found Latiblattella rehni with Eurycotis floridana and, more rarely, with Periplaneta australasiae under bark of pine trees. In Key West he found Symploce hospes in the cupboard of a hotel with swarms of Blattella germanica and a few Supella supellectilium.
Rehn and Hebard (1927) in their study of West Indian blattids reported finding Neoblattella proserpina in epiphytic bromeliads in [Pg 340] Jamaica in company with Neoblattella eurydice and Neoblattella dryas. They also list most of the associations cited by Hebard (1917).
Ramme (1923) reported that he found in Germany four species of Ectobius (lapponicus, lucidus, pallidus, and sylvester) living together in an area about 50 m. by 200 m. Although he had stated that his specimens of E. lucidus were a distinct species in 1923, Ramme (1951) later decided that they were a form of E. sylvester, E. sylvester f. lucidus.
Dow (1955) reported trapping Blattella germanica, Periplaneta americana, and Periplaneta brunnea in houses and privies in south Texas. At our request Dr. Dow (personal communication, 1958) analyzed his records to determine whether there were indications of associations between these species, with the following results:
As stated in my published note, the roaches were at first classified to genus only. The 83 Periplaneta subsequently identified to species represented 28 different collections, 11 from houses and 17 from privies, all in Pharr, Texas. Tabulation of the data shows first that P. americana was taken only once in a house and that P. brunnea was taken only 4 times in privies. Of course this distribution greatly reduces the probability that they would be caught together, and it is not surprising that P. americana was trapped alone in the single house collection. P. brunnea, however, was trapped with P. americana 2 of the 4 times it occurred in privy collections.
To investigate the occurrence of Periplaneta with and without Blattella, an analysis has been made of 560 trap collections taken in 40 houses and 40 associated privies in Pharr, Texas, in weekly intervals (from May 14 to June 23 [1948]). In the houses, Periplaneta and Blattella were caught in the same jar 26 times, Periplaneta alone 12 times, Blattella alone 83 times, and neither genus 159 times. In a fourfold table, the value of chi-square (14.7) is significant and indicates that the frequencies are not proportional. The number of times Periplaneta and Blattella actually occurred together (26) is, however, much larger than the expected number calculated from the row and column frequencies (14.8). In the privies, Periplaneta and Blattella were caught in the same jar 9 times, Periplaneta alone 50 times, Blattella alone 18 times, and neither genus 203 times. In a fourfold table, the value of chi-square (1.95) is not significant but the same type of disproportion is evident and the expected frequency of both genera in one trap is 5.7, lower than the actual frequency of 9. Both immature and adult roaches are included in this analysis.
The above evidence would be more satisfactory if based on more extensive data. There is also a possible objection in that the traps were operated for at least overnight, during which time one species could theoretically supplant another. Of course, it is doubtful that there is anything involved here like territory (in the ornithologists' sense). On the other hand, it is well to consider that Periplaneta and Blattella are both likely to be more abundant in the same type of favorable location and that this factor might offset in part some direct antagonism between the species.
The only known specimen of Ischnoptera podoces was captured in company with the type series of Cariblatta nebulicola, in dead leaf [Pg 341] litter in Jamaica (Rehn and Hebard, 1927). In Florida Periplaneta australasiae was often taken in company with Pycnoscelus surinamensis and Eurycotis floridana (Blatchley, 1920).
In contrast to the presumably amicable associations mentioned above, other observations in the literature seem to indicate that some species of cockroaches are incompatible when they attempt to occupy the same habitat niche. Marlatt (1915) stated "Rarely do two of the domestic species occur together in the same house. Often, also, of two neighboring districts one may be infested with one species, while in the other a distinct species is the commoner one. The different species are thus seemingly somewhat antagonistic, and it is even supposed that they may prey upon one another, the less numerous species being often driven out." Phelps (1924) stated "Roaches of different species are rarely found together, although roaches of the same species live together on very amicable terms."
In 1859 Darwin (1887) stated that "In Russia the small Asiatic cockroach [Blattella germanica?] has everywhere driven before it its great congener [Blatta orientalis?]." Yet in France Girard (1877) suggested that the oriental cockroach be introduced into a restaurant infested with the German cockroach as the best way to expel the latter, because the more robust species drives away cockroaches of smaller size. Wille (1920) in Germany found usually only one species of cockroach in a house. Yet when he placed B. orientalis and B. germanica together, there were no reciprocal attacks even by hungry individuals. Wille concluded that because of their greater speed, smaller size, greater number of eggs, and faster development, the German cockroaches eat the available food and so make the environment unfavorable for the oriental. However, he noted that cases may be seen in which the opposite is also possible. Laing (1946; British Museum [Natural History], 1951) observed that in the British Isles B. orientalis seems to have lost its dominant position to B. germanica in recent years; it was stated that these species are not as a rule found together and that the greater rapidity of breeding and ability to climb of B. germanica, as well as the layout of modern buildings, are some of the factors that favor the spread of B. germanica. Ledoux (1945) found that first-instar nymphs of B. germanica and fourth-instar nymphs of B. orientalis, adults of B. germanica and sixth-instar nymphs of B. orientalis, as well as adults of both species, did not form mixed groups. However, when he combined fifth-and sixth-instar [Pg 342] nymphs of B. germanica with fourth-and fifth-instar nymphs of B. orientalis, which are all practically of equal size, sometimes he would find mixed groups, but generally the groups were distinct. Lucas (1912) stated that Burr had found B. germanica and B. orientalis swarming within a rubbish heap in England; presumably both colonies were breeding and multiplying and one species was not detrimental to the presence of the other.
Shaw (1925) claimed that Supella supellectilium tended to oust Blattella germanica, but Pope (1953) thought it doubtful in Queensland. Wolcott (1950) stated that "The larger and more powerful domestic cockroaches, Periplaneta americana (L.), P. australasiae (F.) and P. brunnea Burmeister have very definitely fallen behind in Puerto Rico in competition with the little German roach." Pessôa and Corêa (1928) observed that other species of cockroaches were rare in Brazil in houses that were infested with Leucophaea maderae. Lederer (1952) noticed that in the reptile house of the aquarium at Frankfort am Main Blatta orientalis was obviously kept down by Blattella germanica, even before the appearance of P. americana. However, B. germanica was not driven out of the reptile house by P. americana although the populations of each fluctuated for about 22 years after the American cockroach had settled there; both species occupied separate resting places. Lederer further observed that within four years of the introduction of P. americana into the crocodile house, none of the original infestation of B. orientalis could be found; a small colony of Pycnoscelus surinamensis in the reptile house was apparently also driven out by P. americana. Chopard (1932, 1938) stated that the oriental cockroach does not exist in company with P. americana which very probably destroys it. Pettit (1940) kept B. germanica and P. americana together in a cage for several weeks but neither species gave any indication of feeding on the other.
Froggatt (1906) stated that "It is probable that the advent of the larger and more formidable American cockroach into Australia has led to the retirement or destruction of our indigenous species" [presumably Periplaneta australasiae]. Tillyard (1926) noted that this statement is incorrect as neither species is native to Australia. Yet Shaw (1925) stated that in Australia "When both species live together in the same places, australasiae Fabr. will probably be found gradually to displace americana L." Local fluctuations in the relative abundance of these species could be a basis for such dissimilar observations. However, MacDougall (1925) observed that in the plant houses of the Royal Botanical Garden, Edinburgh, the Australian cockroach [Pg 343] seemed to have overcome the American which had been more numerous in former years.
In conclusion, we emphasize that many of the above observations are merely tentative impressions gathered by workers who have watched many species of cockroaches in nature. Obviously, additional observations coupled with appropriate experimentation will be needed to disclose the true structure of each presumed association and to resolve apparent discrepancies. Although we are greatly indebted to the cited authors for their contributions to the known information, we anticipate that future results of cleverly designed laboratory experiments will do much to dispel the uncertainty that still surrounds our knowledge of the relations of the Blattaria to each other.
Irritating or repellent secretions provide many animals belonging to widely unrelated groups with a more or less potent means of defence....
It will be seen that this method of defence does not rest merely upon a passive unpalatable attribute, but upon an active emission of the unpalatable substance which, since it occurs when the animal is seized or threatened by an enemy, enforces its effectiveness. In its highest development we find different forms whose specialized habits and modified structure enables them to project secretion at the enemy, and thus to discourage attack.
Cott (1940)
There are very few records indicating that cockroaches are unaccepted as food by other animals. Hutson (1943) found that the duck, guinea fowl, and pigeon would not normally eat Pycnoscelus surinamensis, and in his experiments with the chicken eye worm he had to force-feed his birds with infected cockroaches. Lederer (1952) found that insectivorous birds in the Zoological Garden, Frankfurt am Main, either refused hardened (as opposed to teneral) American cockroaches or ate them unwillingly. Carpenter (1925) reported that a monkey (Cercopithecus) failed to feed on cockroaches and suggested that the insects' odor made them repugnant; however, there are a number of positive records of monkeys feeding on cockroaches (see pp. 284-286).
Cockroaches may escape capture by predators through evasive behavior, concealment, protective coloration, mimicry, or secretion of malodorous materials. Nocturnal cockroaches may avoid predators that are active during the day (Crawford, 1934), but nocturnal predators are apparently quite successful in capturing cockroaches. Some [Pg 344] cockroaches may be protected by their swiftness, others by their resemblance to vegetation (Williams, 1928). The habit of squeezing into narrow cracks may afford cockroaches some protection.
Burrowing forms such as Pycnoscelus may spend much time in underground cells (Roeser, 1940). Polyphagids rapidly burrow into sand (Fausek, 1906), where they may be protected from predators. Tepper (1893) discovered that a very large Australian cockroach, Geoscapheus robustus, had its fore legs, especially the tibiae, adapted for digging. He observed this species in captivity and in 1894 reported that it appeared to sink into the soil without raising any considerable amount above the surface and that it did not form an unobstructed tunnel. Another large Australian cockroach, Macropanesthia rhinocerus, burrows about two feet below the surface of sandy soil; it also makes nests among pine roots and the nymphs rarely appear above ground (Henson in Day, 1950). Tepper (1893) observed that Australian cockroaches of the genera Epilampra and Oniscosoma buried themselves in loose soil and dust. Baker (in Rehn, 1930) observed that Styphon bakeri is found in humus and rubble in the Dutch West Indies where "It is sluggish in the open, but wedges into the humus quite quickly."
Therea nuptialis, found in India, conceals itself at the roots of fig trees, etc. The small hairs on its elytra retain sufficient dust to conceal it, or at any rate to render it inconspicuous, when not on the wing (Annandale, in Chopard, 1924c). Rehn and Hebard (1914) observed that the nymphs of Blaberus craniifer[13] at Key West, Fla., "were usually found half buried in loose damp earth under boards, where they remained motionless, looking much like lumps of earth (with which they were usually much dusted) until disturbed." Hebard (1917) reported of Monastria biguttata from Brazil that "All of the juveniles are heavily coated with foreign particles" which adhere "to a multitude of closely placed, minute and usually curved spines, which cover the dorsal surface and marginal portions of the ventral surface."
It is apparent from the numbers of predators reported herein that many animals are not deterred by the odorous secretions of cockroaches; these secretions, because they may seem repugnant to man, are often claimed to be repellent to predators. However, Cott (1940) points out that "There are many instances in which protective devices and associated warning colours are known to be ineffectual against certain enemies. But this does not necessarily imply that they are not on the whole beneficial to the species attacked." Certain cockroach secretions may well be repellent to many predators, but as this is a purely negative aspect of the predator-prey relationship little thus far has been observed or published. Potential prey that successfully defends itself against attack is never found in a predator's stomach.
Cockroaches have a variety of glands which secrete odorous materials. Certain secretions, produced by tergal or dorsal glands in males, are involved in sexual behavior; the females feed on the secretion from these glands prior to copulating (Roth and Willis, 1954). However, other secretions which are produced by both sexes are ejected or given off when the insect is disturbed; undoubtedly these are defensive weapons that are used against predators. Very few experiments or observations are on record to show how effective these secretions may be in protecting the cockroach. Although the morphology of some of the glands has been described, relatively little is known about the chemistry of their secretions.
Many species of Australian cockroaches have been reported to emit "disgusting" odors, though the glands producing these secretions have not been described, nor is the chemistry of the compounds known. Cosmozosteria lateralis exposed two orange-red spots on the abdomen while emitting a pungent odor which deterred a collector from capturing it (Shelford, 1912). Another Australian species, Platyzosteria castanea, when disturbed on barren ground tilts forward on the vertex and straddles out the posterior legs, supporting itself in a vertical position on the head and tarsi; in assuming this attitude it will squirt a foetid fluid as a fine spray for a distance of 6 or 7 inches (Shaw, 1914). Spencer (1892) mentions the pungent odor given off by a cockroach which had been accidentally cut in two. Rageau (1956) stated that in the New Hebrides and New Caledonia Cutilia nitida emits, when disturbed, a corrosive liquid with an extremely disagreeable odor.
The adults of Eurycotis floridana emit an odorous fluid when seized (Rehn and Hebard, 1905). The fluid, which may irritate sensitive skin areas, may be ejected as a spray for a distance of several inches. This secretion has been identified as 2-hexenal (Roth et al., 1956), and the ventral abdominal glands which produce it have been described (Stay, 1957). Eisner (personal communication, 1958) has found that the toad Bufo marinus and the frog Rana pipiens invariably spit out adults of E. floridana which they have seized. The odor of 2-hexenal was strongly apparent after these attacks, and the insect was never damaged. However, the lizard Anolis equestris [Pg 346] seized and crushed E. floridana before releasing its hold and dropping the insect 5 to 10 minutes later. The blue jay Cyanocitta cristata readily attacked adults of E. floridana and killed them but did not eat the insects until after the odor had dissipated; however, the bird carried nymphs of E. floridana to its perch and ate them. Nymphs of this species do not secrete 2-hexenal (Roth et al., 1956). Recently, 2-hexenal has been tested for its antibacterial activity and has been found to be active against seven species of pathogenic bacteria (Valcurone and Baggini, 1957). Eurycotis decipiens from Trinidad also ejects a fluid which may produce toxic symptoms such as vertigo and nausea (Bunting in Roth and Willis, 1957a).
Large reservoirs of glands similar in appearance and position to those of Eurycotis floridana are present in the adults of both sexes of Neostylopyga rhombifolia and Platyzosteria novae seelandiae. Walker (1904) and Longstaff (in Shelford, 1912) noted that the latter species had a strong odor. Roth (unpublished data, 1957) found that the secretion of P. novae seelandiae when ejected is grayish or milky in color. In the reservoirs of the ventral gland of this insect the secretion is a milky liquid containing floating greenish globules. Both infrared and mass spectrographic analyses show that the secretion is a mixture containing 2-hexenal, the aldehyde that is found in E. floridana. Eisner (personal communication, 1958) observed that the lizard Anolis carolinensis immediately released Neostylopyga rhombifolia without injury, but that Bufo marinus, Anolis equistris, and Cyanocitta cristata ate the insect despite the secretion; several unidentified spiders and the ant Pogonomyrmex badius were not repelled by the secretion of N. rhombifolia.
Dorsal and ventral glands have been found in both sexes of Blatta orientalis and Periplaneta americana (Minchin, 1888, 1890; Kul'vets, 1898; Oettinger, 1906; Harrison, 1906; Liang, 1956). The ventral glands are found in the same general region as those of Eurycotis. We have also found similar ventrally located glands in both Periplaneta australasiae, and P. brunnea. The reservoirs which store the secretion of the ventral glands are smaller in Blatta and Periplaneta spp. than those found in Eurycotis, Neostylopyga, or Platyzosteria.
In Blatta orientalis the dorsal glands can be everted by pressure on the abdomen; the secretion in these glands, according to Haase (1889), has the typical oriental cockroach odor. Although the dorsal glands of the oriental cockroach are usually given a defensive role (Haase, 1889, 1889a; Kul'vets, 1898; Oettinger, 1906; Konček, 1924), the functions of secretions of these nonepigamic dorsal glands and the ventral glands are still open to question. It is possible that [Pg 347] some of the odors produced by cockroaches have functions other than defense or sex attraction. For example, Ledoux (1945) showed that the species odor is largely responsible for the gregarious behavior shown by Blatta orientalis and Blattella germanica. The olfactory stimulus acts over a short distance only, and the source of this odor in the insect is unknown. By washing Blattella germanica in warm chloroform Dusham (1918) extracted a wax which had the odor of the German cockroach. However, there is no evidence to show that cockroaches respond to the same cockroach odors that are detected by man.
Certain cockroaches have recently been found to have odorous secretions which are produced in tracheal glands. In Diploptera punctata the tracheae leading to the second abdominal spiracles of nymphs and adults are modified into odoriferous glands which produce a mixture of 2-ethyl-1,4-benzoquinone; 2-methyl-1,4-benzoquinone; and para benzoquinone; this material is ejected as a means of defense. The offensive odor emitted by adults and nymphs of Leucophaea maderae also issues from the second abdominal spiracles (Roth and Stay, 1958).
Diploptera is capable of ejecting its quinones from either its right or left tracheal gland according to which side of the insect is attacked (pl. 36, A-B). Eisner (1958) found that the secretion repelled the ant Pogonomyrmex badius (Latreille) (pl. 36, C) and the beetle Galerita janus Fabricius when they attacked the cockroach. The spider Lycosa helluo Walckenaer was repelled by large nymphs and adults of D. punctata but young nymphs were usually eaten promptly (Eisner, 1958).
Bordas (1901, 1908) believed that the "conglobate" gland (Miall and Denny, 1886), found in males of Periplaneta americana and Blatta orientalis, was an odoriferous gland used for defense, but Gupta (1947) has shown that in all probability this gland (the phallic gland) secretes the outermost covering of the spermatophore.
What appears to be mimicry occurs in some species of Blattaria. The nymphs of many Panchlorini and Blaberinae vaguely resemble sow bugs (Chopard, 1938). Certain members of the Perisphaerini (e.g., Perisphaerus glomeriformis) from the Malayan region which resemble sow bugs (Annandale, 1900; Hanitsch, 1915) can roll themselves up into a ball thus hiding their antennae and legs (Lucas, 1862). Although these cockroaches are found among dead leaves or under stones, in places in which sow bugs are also found, the benefit to either or both forms is questionable; Annandale (1900) believed [Pg 348] that the crustacean and the cockroach, living under similar conditions, developed the same general body shape. Rolling up into a ball is nothing more than an exaggeration of a reflex common to many young cockroaches, that is, an arched position which these insects assume when they immobilize themselves in response to certain stimuli (Chopard, 1938).
There are cockroaches that resemble various Coleoptera and Hemiptera (Belt, 1874; Shelford, 1912; Hanitsch, 1915). Some look like cerambycids, lampyrids, coccinellids, pentatomids, etc. Perhaps the most striking examples are the resemblances of cockroaches in the genus Prosoplecta of the Epilamprinae to beetles of the family Coccinellidae; Shelford (1912) has figured a number of species of Prosoplecta together with the species of beetles which they seem to have taken for models. Williams (1928) mentioned diurnal cockroaches which by a combination of markings, shape, posture, and active flight about vegetation suggest certain wasps.
Unfortunately, practically nothing is known about the behavior of these so-called mimics and models or their relationships with predators in the field. For the most part, the examples are based on a comparison of pinned insects from museum collections (Burr, 1899); for this reason Chopard (1938) believed that not much value should be placed on superficial resemblances of this kind. However, we believe that a lack of knowledge of cockroach mimicry is not a valid reason for rejecting the idea that mimicry, if it occurs, may be of some benefit in the survival of mimetic species. Certainly Cott's (1940) voluminous compilation of the literature on adaptive coloration should make the most skeptic hesitate to conclude dogmatically that these instances of mimicry are merely accidental and meaningless.
In the Navy [Japanese] a seaman who has captured 300 cockroaches will be granted one day special shore leave. They call it "shore leave for cockroaches." The purpose is to promote extermination of cockroaches in a warship because, on the one hand, any warship suffers from numerous cockroaches, and, on the other hand, any seaman likes shore leave.... The formalities for a shore leave for cockroaches are as follows. A seaman keeps cockroaches which he captured (mainly B. germanica, because P. americana and P. australasiae are seldom found in Japan) in a bottle or in a bag until the number reaches 300. Then he brings them to the deck officer to get the confirmation that he has actually captured more than 300 cockroaches. If the deck officer confirms it, the seaman goes to a cabin where a petty officer reports that the
deck officer confirmed the number of cockroaches. The petty officer signs the seaman's name, name of division, rank, and date to be on shore leave in the log book for cockroach shore leaves. The petty officer brings the log book again to the deck officer to get his approval and then goes to the commander for the final approval. In the Navy, they have another special shore leave for rats. In this system, a seaman gets one day shore leave for one rat. The formalities for the latter are the same as for the former, and there is a log book for the rat shore leave in the petty officer's quarters. The author took advantage of these systems frequently.
Sonan (1924)
Little is known of the effects of predatism and parasitism on natural populations of cockroaches. Many statements in the literature are very general; yet there are a few data on egg parasites (e.g., Tetrastichus hagenowii) which suggest that, in the absence of parasites, populations of domestic cockroaches might be much larger than they are in certain areas. We have summarized the literature on natural control and also that on the use by man of predators and parasites in the biological control of cockroaches. However, because of the paucity of information, we have been unable to evaluate the effectiveness of biological control in reducing the numbers of pest cockroaches. This is an area that might reward further investigation.
Scorpions.—In Puerto Rico, cockroaches are probably the principal food of the scorpions which live in old houses, on tree trunks, etc. (Seín, 1923). The staple diet of scorpions in Arizona is the small cockroach commonly known as the water bug (Stahnke, 1949); in the part of Arizona where he resides, Stahnke (personal communication, 1953) says that the "water-bug" is most generally Supella supellectilium although Blattella germanica is also found, but less abundantly.
Spiders.—Jefferys (1760) mentioned a large spider which was protected in the Antilles and especially on Guadeloupe because it hunted down and fed on cockroaches; the spider was reputed to be common in every house. Sir Hans Sloane (1725, in Cowan, 1865) reported that residents of Jamaica kept spiders in their houses to destroy cockroaches. Takahashi (1924) reported that, in the Taihoku area of Formosa, human habitations contained large numbers of spiders which caught and ate cockroaches. Smith (in Marlatt, 1915) reported that Brazilians encourage large house spiders because they tend to rid the house of "other insect pests." In British Guiana tarantulas were kept in a bungalow to control Periplaneta and Pycnoscelus (Beebe, 1925a).
Ants.—A Madam Merian noticed that ants cleared houses of cockroaches (Kirby and Spence, 1822). A small reddish-yellow ant, called Pucchuçiçi by Peruvian Indians, pursued and destroyed a cockroach called Chilicabra which was a pest in native huts (Tschudi, 1847). Schwabe (1950b) found swarms of ants attacking living Pycnoscelus surinamensis and stated that ants are probably the chief enemy of this cockroach in Hawaii. Wallace (1891) stated that in Africa a band of driver ants may enter a house and clear it of cockroaches and other arthropods. In British Guiana, Beebe, (1925) found that several times a year army ants cleared the laboratory of all cockroaches and tarantulas.
Wasp egg parasites.—Matsumura (1917, in Asano, 1937) proposed that parasitic wasps such as Evania and Brachygaster be protected in Japan as the natural enemies of cockroaches. In one area in France, 20 percent of the oöthecae of Loboptera decipiens were parasitized by Zeuxevania splendidula (Genieys, 1924). Edmunds (1952a) found that 12 percent of 459 oöthecae of Parcoblatta collected during December through April of 1950-51 in Ohio were parasitized; evaniids accounted for about 7 percent of the parasitization. Additional collection data in 1951-52 Edmunds (1953a) showed that 8.7 percent of 320 wood-cockroach oöthecae were parasitized; 2.8 percent of these parasites were evaniids; almost 13 percent of the egg capsules collected showed evidence of previous parasite emergence. Cameron (1957) reported that oöthecae of Periplaneta americana collected in Saudi Arabia were 29 percent parasitized in March and 25 percent parasitized in October by Evania appendigaster. Sonan (1924) found 1 of 65 oöthecae of P. americana and P. australasiae parasitized by E. appendigaster in Formosa.
Cottam (1922) stated that the increase of Supella supellectilium in Khartoum was checked by a wasp egg-parasite that was later identified as Anastatus tenuipes (see p. 246) (Ferrière, 1930, 1935). In this country, this wasp seemed to be effective in decreasing the numbers of Supella in certain areas in Arizona (Flock, 1941).
In Formosa, Tetrastichus hagenowii was an important parasite of cockroach eggs (Maki, 1937). Sonan (1924) reported 30 percent parasitization of 65 oöthecae of Periplaneta americana and P. australasiae collected in Formosa. In Bangalore, India, the natural parasitization of randomly collected oöthecae of P. americana varied from 21 percent (of 495 oöthecae), July 1947-June 1948, and 43 percent (of 288 oöthecae), July-December 1948, to 57 percent (of 178 oöthecae), July-October 1949 (Usman, 1949). Cameron (1955) obtained T. hagenowii from oöthecae collected in Trinidad, B.W.I., and [Pg 351] Saudi Arabia; some 15 percent of the oöthecae of P. americana and P. australasiae collected in October in Trinidad were parasitized; a later collection (March) was 34 percent parasitized; a small sample of P. americana oöthecae was 65 percent parasitized. The oöthecae collected in Saudi Arabia in March were 20 percent parasitized. Plank (1947) found that the eggs of the American cockroach in Puerto Rico (probably in laboratory cultures) were so heavily parasitized by T. hagenowii that he had to use P. australasiae for experimental purposes; in 1950 Plank stated that more than 50 percent of American cockroach oöthecae were parasitized.
Fahringer (1922) stated that Prosevania punctata could be used to eradicate cockroaches, but he did not test his hypothesis. Marlatt (1902) felt that the usefulness of Evania appendigaster in biological control was impaired by Tetrastichus acting as a hyperparasite (see footnote 6, p. 236). However, Wolcott (1951) stated that in Puerto Rico E. appendigaster is quite abundant and is a factor of considerable importance in controlling cockroaches. Kadocsa (1921) stated that Brachygaster minutus and Evania appendigaster were not important in the biological control of cockroaches. These general statements are not supported by experimental evidence.
It is likely that the smaller wasp egg parasites are more effective than the evaniids in controlling cockroaches. Only one evaniid develops in a parasitized oötheca, but many individuals of the other wasps develop in one oötheca and the number of females that emerge is usually large. However, Cameron (1957) concluded that, with a parasitism rate of 25 to 29 percent and three to four generations a year, against one or less for the host, Evania appendigaster in the areas where it is established is a valuable control agent.
The use of specific egg parasites to control cockroaches has not been attempted extensively. Cros (1942) liberated a species of Tetrastichus (=Eulophus sp.; see p. 254) in his home in Algeria to control the oriental cockroach; as far as we know, he did not report the parasite's effectiveness in reducing the cockroach population. According to Zimmerman (1948) Comperia merceti, when accidentally imported, practically wiped out Supella supellectilium in parts of Hawaii; he claimed to have controlled the brown-banded cockroach in a store building with this parasite. In some parts of Honolulu, almost 100 percent of the oöthecae of this cockroach were parasitized (Zimmerman, 1944). We (1954b) ran some simulated field tests in which we liberated Tetrastichus hagenowii in rooms artificially seeded with oöthecae; from 28 to 83 percent of American cockroach oöthecae and [Pg 352] 56 percent of oriental cockroach oöthecae were parasitized during these tests.
Evania appendigaster was introduced from Hawaii into Canton Island in 1940 against Periplaneta americana, and it has become established (Dumbleton, 1957). This parasite was also successfully introduced into Samoa (Dumbleton, 1957).
Cockroach-hunting wasps.—An earnest attempt has been made to establish in Hawaii wasps that prey on cockroaches. Just how effective these wasps are in controlling cockroaches is still unknown. Dolichurus stantoni was introduced from the Philippines in 1917 and spread to several of the Islands (Swezey, 1920, 1921; Williams, 1944). Bridwell (1920) stated that as a result of this introduction there was a great decrease in cockroaches of the genus "Phyllodromia." A number of Podium haematogastrum from Brazil were liberated in Honolulu (Williams, 1925) but did not become established (Williams, 1928). The effectiveness of Podium was questioned by Williams (1928) who observed that Podium "destroyed innumerable Blattidae, which nonetheless swarmed in their neighborhood, and I must confess from my observations on the various cockroach-hunting wasps that the blattid more than holds its own alongside its enemy."
Introductions of Ampulex have proved more successful. Ampulex canaliculata was introduced into Hawaii from the United States (Williams, 1928a, 1929). Williams also introduced A. compressa into Hawaii in 1940, and the species was reared in large numbers for distribution (Pemberton, 1942). A. compressa has since become established on most of the Islands (Pemberton, 1945a, 1947; Williams, 1946; Van Zwaluwenburg, 1950). The thousand of A. compressa now found in the Hawaiian Islands are all descendants of three wasps captured in Noumea, New Caledonia (Williams, 1944). According to Williams (1941), the number of cockroaches was noticeably reduced at the University of Hawaii poultry farm, where some A. compressa were released. Pemberton (1953) believed that this wasp has become sufficiently abundant to be of definite value. Simmonds (1941) recommended importing A. compressa into Fiji for cockroach control. A. compressa was introduced from Hawaii into Guam in 1954 against Periplaneta americana and into the Cook Islands in 1955 against Periplaneta spp.; it is not yet known whether the parasite became established in either place (Dumbleton, 1957).
... on conserve avec soin les crapauds dans les maisons, et que les dames les tolèrent, même sous leurs robes, en raison de leurs continuels services, car ils se promènent sans cesse à la recherche des Kakerlacs.
Girard (1877)
Toads.—Bufo marinus was first introduced into Puerto Rico from Barbados in 1920 to reduce several major insect pests including cockroaches (Leonard, 1933). It was introduced from Puerto Rico into Hawaii by C. E. Pemberton in 1932 where it rapidly became established; it has since been distributed throughout the Pacific area. B. marinus is one of the world's largest toads; it attains a body length (exclusive of the hind legs) of 7 to 9 inches (Oliver, 1949) and has been kept alive for more than 11 years in captivity (Pemberton, 1945). Alicata (1938) placed giant toads in a fenced area in Hawaii containing an infestation of Pycnoscelus surinamensis; after 24 hours the toads were dissected and each was found to have eaten from 11 to 25 cockroaches. Illingworth (1941) found that 40 to 90 percent of 53 stools of this toad in Hawaii contained remains of P. surinamensis. Alicata (1947) recommended the maintenance of B. marinus in poultry yards to reduce the population of P. surinamensis, the vector of the chicken eye worm.
Toads have also been recommended for controlling cockroaches in houses (Meech, 1889; Sweetman, 1936). Girard (1877) cited a note in a French newspaper which stated that toads were kept in houses in Cuba to control the American cockroach.
Tree frogs.—Tree frogs enclosed in a room overnight were said to effectively clear it of cockroaches (Marlatt, 1915); on sugar plantations in Australia, these amphibians were encouraged in houses and kept as pets because they hunted and devoured large brown cockroaches (Froggatt, 1906).
Birds.—In Guadeloupe, Dutertre (1654) claimed that all the fowls of the country were fond of small cockroaches and lived on practically nothing else. In Hawaii (Zimmerman, 1948) and in the Lesser Antilles (Ballou, 1912) cockroaches are eaten by poultry whenever the birds can find them. In Puerto Rico, Wetmore (1916) stated that owls kept in houses feed extensively on cockroaches; the stomach of one owl which had been kept in a native house was filled entirely with cockroaches. In British Guiana, Beebe (1925) found that cockroaches were eaten by 27 species of birds.
Reptiles.—H. (1800) claimed that two lizards cleared his house of the "true brown cockroach" and suggested that lizards be used for [Pg 354] cockroach control because the reptiles are docile and harmless. On Arno Atoll geckos and night-feeding skinks eat large numbers of cockroaches (Usinger and La Rivers, 1953). According to Wolcott (1924) the number of cockroaches eaten by lizards is surprisingly large considering the nocturnal habits of these insects. Beebe (1925a) kept geckos in a bungalow to help control Periplaneta and Pycnoscelus.
Mammalia.—Cowan (1865) stated that in England hedgehogs were often kept domesticated in kitchens to destroy cockroaches. This writer also stated that a lemur was kept on board ship to destroy cockroaches.
Large numbers of the American and Australian cockroaches were eaten by the mongoose in Hawaii (Perkins, 1913).
We would have been unable to complete this review without the help of many people who have generously given us their time and the benefit of their special experience. We are exceedingly grateful to these individuals for they have contributed much to whatever merit this work possesses; we alone are responsible for the deficiencies and inaccuracies that remain in the text.
Dr. A. B. Gurney, Entomology Research Division, U. S. Department of Agriculture, and J. A. G. Rehn, Academy of Natural Sciences of Philadelphia, have given us much help and advice throughout the preparation of this monograph. Both have patiently answered our many queries, and Mr. Rehn allowed us free access to his large collection of cockroach literature. We are especially pleased to thank them for their many favors.
Many persons have determined at our request the identity of specific organisms. These individuals are cited in the text and to them we extend our thanks. We thank our colleagues, cited in the text, who have made their unpublished observations available to us. We also thank the individuals and organizations for the use of photographs and/or drawings for which they are credited in the accompanying legends.
We thank the following individuals for supplying us with living specimens of the species indicated: T. Campbell, Commonwealth Scientific and Industrial Research Organization, Canberra, New South Wales (Panesthia australis); Dr. L. R. Cleveland, Harvard University (Cryptocercus punctulatus); Dr. N. T. Davis, University of Connecticut (Byrsotria fumigata); Dr. F. Englemann, Albert Einstein Medical School (Gromphadorhina portentosa); Dr. F. A. [Pg 355] Lawson, Kansas State College (Comperia merceti); Dr. K. D. Roeder, Tufts University (Hierodula tenuidentata (?)); Dr. E. O. Wilson, Harvard University (Ischnoptera deropeltiformis).
We thank the following individuals for checking the taxonomy of the following organisms or for reading the indicated sections of the manuscript: Fungi.—Dr. R. K. Benjamin, University of California; Dr. E. G. Simmons, Quartermaster Research Laboratories. Protozoa.—Dr. R. R. Kudo, Professor Emeritus, University of Illinois. Helminths.—Mrs. May Belle Chitwood and Dr. J. T. Lucker, U. S. Department of Agriculture. Centipedes and whipscorpions.—Dr. R. E. Crabill, Jr., U. S. National Museum. Scorpions.—Dr. J. L. Cloudsley-Thompson, University of London; Dr. R. E. Crabill, Jr. Spiders.—Dr. B. J. Kaston, University of Connecticut; Dr. R. E. Crabill, Jr. Mites.—Dr. J. H. Camin, Chicago Academy of Sciences; Dr. E. W. Baker, U. S. National Museum. Cockroaches.—Dr. A. B. Gurney and J. A. G. Rehn. Ants.—Dr. W. L. Brown, Jr., Museum of Comparative Zoology, Harvard College. Hymenopterous parasites of cockroach eggs.—Dr. B. D. Burks, U. S. National Museum; Dr. H. K. Townes, University of Michigan. Cockroach-hunting wasps.—Dr. K. V. Krombein, U. S. National Museum. Lepidoptera.—Dr. J. F. G. Clarke, U. S. National Museum; Dr. E. L. Todd, U. S. Department of Agriculture. Miscellaneous insects.—Dr. R. S. Beal, Dr. A. B. Gurney, C. W. Sabrosky, Dr. R. I. Sailer, and J. T. Spilman, U. S. National Museum. Fishes.—Dr. L. P. Schultz, U. S. National Museum. Amphibians and reptiles.—Dr. Doris M. Cochran, U. S. National Museum. Birds.—Dr. Herbert Friedmann, U. S. National Museum. Mammals.—Dr. D. H. Johnson, U. S. National Museum.
We thank the following individuals for reading the entire manuscript: J. A. G. Rehn; Dr. A. B. Gurney; Maj. Gordon Field, U. S. Army; and Dr. H. L. Sweetman, University of Massachusetts. The monograph has profited by the friendly criticism of these entomologists.
We thank Dr. R. A. Howard, Harvard University, for checking lists of plant names; Mrs. Maria E. W. Torok, formerly of the Quartermaster Technical Library, for assistance in obtaining obscure literature; Miss Louise Bercaw, U. S. Department of Agriculture Library, for identifying the journal containing the paper by Vlasov and Miram; the individuals who translated foreign language articles, for which they are credited in the bibliography; and Miss G. Lillian Fede, Quartermaster Research Laboratories, for typing the manuscript.
Abdulali, H.
1942. The terns and edible-nest swifts at Vengurla, West Coast, India. Journ. Bombay Nat. Hist. Soc., vol. 43, pp. 446-451.
Abeille de Perrin, E.
1909. Étude d'un Rhipidius nouveau de Provence, R. Boissyi Abeille. Compt. Rend. Soc. Biol., Paris, vol. 67, pp. 854-858.
Adair, E. W.
1923. Notes sur Periplaneta americana L. et Blatta orientalis L. (Orthop.). Bull. Soc. Ent. Égypte, vol. 7, pp. 18-38.
Adelung, N. von.
1907. Beiträge zur Orthopterenfauna der südlichen Krim. Mus. Zool., Akad. Nauk, St. Petersburg, vol. 12, pp. 388-413. [Extract translated by H. L. Middleton.]
Adlerz, G.
1903. Lefnadsförhållanden och instinkter inom familjerna Pompilidae och Sphegidae. K. Svenska Vet.-Akad. Handl., Stockholm, vol. 37, pp. 1-181.
1906. Lefnadsförhållanden och instinkter inom familjerna Pompilidae och Sphegidae. II. K. Svenska Vet.-Akad. Handl., Stockholm, vol. 42, pp. 1-48.
Akkerman, Klaasje.
1933. Researches on the behaviour of some pathogenic organisms in the intestinal canal of Periplaneta americana with reference to the possible epidemiological importance of this insect. Acta Leiden., vol. 8, pp. 80-120.
Alfieri, A.
1914. Un Hyménoptère parasite des oothèques d'un blattide. Bull. Soc. Ent. Égypte, 1913, vol. 3, pp. 14-15.
Alicata, J. E.
1934. New intermediate hosts for some heteroxenous nematodes. Proc. Helminthol. Soc. Washington, vol. 1, p. 13.
1934a. Observations on the development to egg-laying maturity of Gongylonema pulchrum (Nematoda: Spiruridae) in the guinea pig. Proc. Helminthol. Soc. Washington, vol. 1, pp. 51-52.
1934b. Observations on the period required for Ascaris eggs to reach infectivity. Proc. Helminthol. Soc. Washington, vol. 1, p. 12.
1935. Early developmental stages of nematodes occurring in swine. U. S. Dept. Agr. Techn. Bull. No. 489, 97 pp.
1937. Larval development of the spirurid nematode, Physaloptera turgida, in the cockroach, Blatella germanica. In Papers on Helminthology, All-Union Lenin Acad. Agr. Sci., Moscow, pp. 11-14.
1938. Studies on poultry parasites. Hawaii Agr. Exp. Stat. Ann. Rep. for 1937, pp. 93-96.
1938a. The life history of the gizzard-worm (Cheilospirura hamulosa) and its mode of transmission to chickens, with special reference to Hawaiian conditions. Livro jubilar do Professor Lauro Travassos, Rio de Janeiro, pp. 11-19, 5 pls.
1947. Parasites and parasitic diseases of domestic animals in the Hawaiian Islands. Pacific Sci., Honolulu, vol. 1, pp. 69-84.
Allee, W. C.; Emerson, A. E.; Park, O.; Park, T.; and Schmidt, K. P.
1949. Principles of animal ecology. 837 pp. Philadelphia.
Andrewartha, H. G., and Birch, L. C.
1954. The distribution and abundance of animals. 782 pp. Chicago.
Annandale, N.
1900. Notes on Orthoptera in the Siamese Malay States. Ent. Rec. and Journ. Var., vol. 12, pp. 75-77.
1906. Notes on the freshwater fauna of India. No. III. An Indian aquatic cockroach and beetle larva. Journ. Proc. Asiatic Soc. Bengal, n. s., vol. 2, pp. 105-107.
1910. Cockroaches as predatory insects. Rec. Indian Mus. 5 pts., vol. 3, pp. 201-202.
Annandale, N.; Brown, J. C.; and Gravely, F. H.
1913. The limestone caves of Burma and the Malay Peninsula. Journ. Asiatic Soc. Bengal., n.s., vol. 9, pp. 391-424, 5 pls.
Anonymous.
1893. A tropical cockroach in a New Orleans greenhouse. Insect Life, vol. 5, p. 201.
1937. Roaches combat termites. Soap and San. Chem., vol. 13, No. 6, p. 123.
1939. Unusual infestation of a ship with black widow spiders. U. S. Publ. Health Rep., vol. 54, pp. 2195-2196.
1946. [Leprosy and cockroaches.] East African Med. Journ., vol. 23, pp. 293-294.
1951. Pest destruction in ships. Shipbuilding and Shipping Rec., vol. 78, pp. 145-146.
1952. Department stores have pest control problems, too. Pest Control, vol. 20, pp. 9-10, 50.
1953. West Indies roach still rare in city. New York Times, August 11, Sect. C, p. 24.
1954. New insecticidal lacquer. Chem. Age, vol. 71, p. 373.
1958. Infestation report. Pest Control, vol. 26, No. 1, p. 51.
1958a. Infestation report. Pest Control, vol. 26, No. 4, p. 44.
Antonelli, G.
1930. La blatta nella igiene domestica. Riv. Soc. Ital. Igiene, Milan, vol. 52, pp. 132-142. [Translated by M. DiPaolo.]
1943. La blatta—veicolo di malattie infettive nei rurali. Mutual. Rurale Fasciata, vol. 7, pp. 206-220. [Translated abstract prepared by College of Physicians, Philadelphia.]
Arizumi, S.
1934. On the potential transmission of B. leprae by certain insects. Internat. Journ. Leprosy, vol. 2, pp. 470-472.
1934a. On the potential transmission of Bacillus leprae by certain insects. (In Japanese.) Journ. Med. Assoc. Formosa, vol. 33, pp. 634-661. English summary, pp. 54-55.
Armer, Sister Joseph Marie.
1944. Influence of the diet of Blattidae on some of their intestinal protozoa. Journ. Parasitol., vol. 30, pp. 131-142.
Arnold, G.
1928. The Sphegidae of South Africa. Part IX. Ann. Transvaal Mus., vol. 12, pp. 191-232.
Asano, I.
1937. On the natural enemies of cockroaches. (In Japanese.) Konchusekai (Insect World), vol. 41, pp. 43-47, 90-98. [Translated by Associated Technical Services.]
Ashmead, W. H.
1900. Classification of the ichneumon flies, or the superfamily Ichneumonoidea. Proc. U. S. Nat. Mus., vol. 23, pp. 1-220.
1901. Hymenoptera parasitica. In Sharp, D., ed., Fauna Hawaiiensis, vol. 1, pp. 277-364, 2 pls.
Auerbach, S. I.
1951. The centipedes of the Chicago area with special reference to their ecology. Ecol. Monogr., vol. 21, pp. 97-124.
Avrech, V. V.
1931. Zur frage der Parasiten der Brotschabe. Arch. Protistenk., vol. 74, pp. 236-248.
Babers, F. H.
1938. A septicemia of the southern army worm caused by Bacillus cereus. Ann. Ent. Soc. Amer., vol. 31, pp. 371-373.
Bacigalupo, J.
1927. Periplaneta americana Fabr., hôte intermédiare du Gigantorhyncus moniliformis Bremser, dans la Republique Argentine. Compt. Rend. Soc. Biol., Paris, vol. 97, p. 1251.
1927a. La Periplaneta americana Fabr. huésped intermediario del Gigantorhyncus moniliformis Bremser en nuestro páis. Rev. Soc. Argentina Biol. y Fil. Soc. Biol. Litoral, vol. 3, pp. 486-490.
1928. Moniliformis moniliformis (Bremser, 1811). Su evolución en la Periplaneta americana (Fabr.) en nuestro páis. Semana Méd., vol. 35, pp. 537-546.
1930. Contribución al estudio dei Gongylonema neoplasticum (Fibiger-Ditlevsen) en la Argentina. Rev. Chilena Hist. Nat., 1929, vol. 33, pp. 414-417.
Back, E. A.
1947. Centipedes and millipedes in the house. U. S. Dept. Agr., Leafl. No. 192, 6 pp.
Baer, J. G.
1956. The taxonomic position of Taenia madagascariensis Davaine, 1870, a tapeworm parasite of man and rodents. Ann. Trop. Med. Parasitol., vol. 50, pp. 152-156.
Bailey, L. H.
1925. Manual of cultivated plants. 851 pp. New York.
Baker, E. W.; Evans, T. M.; Gould, D. J.; Hull, W. B.; and Keegan, H. L.
1956. A manual of parasitic mites of medical or economic importance. 170 pp. Techn. Publ. Nat. Pest Control Assoc., Inc., New York.
Baker, E. W., and Wharton, G. W.
1952. An introduction to acarology. 465 pp. New York.
Bal, C., and Rai, B. P.
1955. On the occurrence of Stenophora sp. in the midgut of Blatta orientalis. Proc. Indian Sci. Congr. Assoc., vol. 42, p. 281. (Abstract.)
Balch, H. E.
1932. The cultivation of Nyctotherus ovalis and Endamoeba blattae. Science, vol. 76, p. 237.
Balduf, W. V.
1935. The bionomics of entomophagous Coleoptera. 185 pp. St. Louis.
1936. Observations on Podalonia violaceipennis (Lep.) (Sphecidae) and Vespula maculata (Linn.) (Vespidae). Canadian Ent., vol. 68, pp. 137-139.
Ball, E. D.; Tinkham, E. R.; Flock, R.; and Vorhies, C. T.
1942. The grasshoppers and other Orthoptera of Arizona. Univ. Arizona Coll. Agr. Exp. Stat. Techn. Bull. No. 93, pp. 257-373.
Ballou, H. A.
1912. Insect pests of the Lesser Antilles. Imp. Dept. Agr., West Indies, pamphlet ser., No. 71, 210 pp.
1916. Report on the prevalence of some pests and diseases in the West Indies during 1915. Part I. Insect pests. West Indian Bull., Barbados, vol. 16, pp. 3-16.
Barber, H. S.
1939. A new parasitic beetle from California (Ripiphoridae). Bull. Brooklyn Ent. Soc., vol. 34, pp. 17-20.
Barber, M. A.
1912. The susceptibility of cockroaches to plague bacilli inoculated into the body cavity. Philippine Journ. Sci., vol. 7, sect. B, pp. 521-524.
1914. Cockroaches and ants as carriers of the vibrios of Asiatic cholera. Philippine Journ. Sci., vol. 9, sect. B, pp. 1-4.
Barbier, J.
1947. Observations sur les moeurs de Rhipidius pectinicornis Thunbg. et description de sa larve primaire. (Col. Rhipiphoridae.) Entomologiste, Paris, vol. 3, pp. 163-180.
Basir, M. A.
1940. Nematodes parasitic in Indian-cockroaches. Proc. Indian Acad. Sci., ser. B., vol. 12, pp. 8-16.
1941. A new species of the Nematode genus Blattophila Cobb, 1920, from a cockroach. Current Sci., Bangalore, vol. 10, pp. 443-445.
1942. Protrellina phyllodromi sp. nov. A new nematode parasite of the cockroach Phyllodromia humbertiana Sauss. [Letter to Editor.] Current Sci., Bangalore, vol. II, pp. 195-197.
1949. A redescription of Cephalobellus brevicaudatum (Leidy, 1851) Christie, 1933 (Nematoda), with comments on other species of the genus Cephalobellus. Canadian Journ. Res., vol. 27 D, pp. 31-36.
1956. Oxyuroid parasites of Arthropoda. A monographic study. 1. Thelastomatidae. 2. Oxyuridae. Zoologica, Orig. Abhandl. Gesamt. Zool., Stuttgart, Bd. 38, Lief 2, Heft 106, pp. 1-79, 13 pls.
Bates, H. W.
1863. The naturalist on the river Amazons. Vol. 1, 351 pp.; vol. 2, 423 pp. London.
Baudisch, K.
1956. Zytologische Beobachtungen an den Mycetocyten von Periplaneta americana L. Naturwissenschaften, vol. 43, p. 358.
Baylis, H. A.
1925. Some notes on nematode parasites found by Dr. Wassink in rats and mice. Journ. Trop. Med. Hyg., vol. 28, pp. 316-317.
Baylis, H. A.; Pan, T. C.; and Sambon, J. E. B.
1925. Some observations and experiments on Gongylonema in northern Italy. A preliminary note. Journ. Trop. Med. Hyg., vol. 28, pp. 413-419.
Baylis, H. A.; Sheather, A. L.; and Andrews, W. H.
1926. Further experiments with the Gongylonema of cattle. Journ. Trop. Med. Hyg., vol. 29, pp. 194-197.
1926a. Further experiments with the Gongylonema of cattle. II. Journ. Trop. Med. Hyg., vol. 29, pp. 346-349.
Beatty, H. A.
1944. Fauna of St. Croix, V. I. Journ. Agr., Univ. Puerto Rico, vol. 28, pp. 103-185.
Beaumont, J. de.
1954. Sphecidae de l'institut d'Entomologie de l'Université de Bologne. II. Larrinae. Boll. Ist. Ent., Bologna, vol. 20, pp. 53-64.
Beck, O., and Coffee, W. B.
1943. Observations on Salmonella typhimurium. Journ. Bact., vol. 45, p. 200. (Abstract.)
Beebe, W.
1917. With army ants 'somewhere' in the jungle. Atlantic Month., vol. 119, pp. 514-522.
1919. The home town of the army ants. Atlantic Month., vol. 124, pp. 454-464.
1921. Edge of the jungle. 303 pp. New York.
1925. Jungle days. 201 pp. New York and London.
1925a. Studies of a tropical jungle. One quarter of a square mile of jungle at Kartabo, British Guiana. Zoologica, vol. 6, pp. 5-193.
1953. Unseen life of New York as a naturalist sees it. 165 pp. New York.
Bei-Bienko, G. Ia.
1947. On Blattodea introduced with bananas in Leningrad. (In Russian, English summary.) Ent. Obozrenie, vol. 29, pp. 44-48.
1950. Fauna of the U.S.S.R. Insects. Blattodea. (In Russian.) Zool. Inst. Akad. Nauk, S.S.S.R., Moskva, n. s., No. 40, 343 pp. [Translated by Ruth Ericson.]
Bělǎr, K. J.
1916. Protozoenstudien. II. Arch. Protistenk., vol. 36, pp. 241-302, 9 pls.
Belt, T.
1874. The naturalist in Nicaragua. London. (Everyman's Library ed., 306 pp.)
Benoist, R.
1927. Sur la biologie des Dolichurus corniculus (Hymen. Sphegidae). Ann. Soc. Ent. France, vol. 96, pp. 111-112.
Berland, L.
1924. Observations biologiques sur les Orthoptères. Bull. Soc. Ent. France, No. 4, pp. 70-72.
1925. Hyménoptères Vespiformes. I. Faune de France, vol. 10, pp. 1-364.
Bhatia, B. L., and Gulati, A. N.
1927. On some parasitic ciliates from Indian frogs, toads, earthworms, and cockroaches. Arch. Protistenk., vol. 57, pp. 85-120.
Bingham, C. T.
1897. Fauna of British India, including Ceylon and Burma. Hymenoptera I. 579 pp. (especially pp. 123, 148, 253-254), 4 pls. London.
1900. Account of a remarkable swarming for breeding purposes of Sphex umbrosus, Christ, with notes on the nests of two other species of Sphex and of certain of the Pompilidae. Journ. Bombay Nat. Hist. Soc., vol. 13, pp. 177-180.
Bishop, Ann.
1931. A description of Embadomonas n. spp. from Blatta orientalis, Rana temporaria, Bufo vulgaris, Salamandra maculosa; with a note upon the "cyst" of Trichomonas batrachorum. Parasitology, vol. 23, pp. 286-300.
Bitter, Ruth S., and Williams, O. B.
1949. Enteric organisms from the American cockroach. Journ. Infect. Dis., vol. 85, pp. 87-90.
1949a. Enteric organisms from the American cockroach. Soc. Amer. Bact., Abstracts, 49th Gen. Meet., p. 80.
Blair, K. G.
1922. An entomological holiday in S. France. Entomologist, vol. 55, pp. 147-151.
1934. A note on the British species of Ectobius Steph. Ent. Month. Mag., vol. 70, pp. 157-159.
1952. Some further records of British Evanioidea (Hym.). Ent. Month. Mag., vol. 88, p. 7.
Blanchard, E.
1837. Monographie du genre Phoraspis, de la famille des blattiens; précédée de quelques observations sur les blattes des anciens. Ann. Soc. Ent. France, vol. 6, pp. 271-298.
Blatchley, W. S.
1895. Notes on the winter insect fauna of Vigo County, Indiana. Psyche, vol. 7, pp. 247-250.
1920. Orthoptera of North-Eastern America. 784 pp. Indianapolis.
Bligh, W.
1792. His narrative of the voyage of Otaheite; with an account of the mutiny and of his boat journey to Timor. 283 pp. Cover title: Bligh and the Bounty. Unabridged 1936 ed. of Bligh's narrative first published in 1792. New York.
Blochmann, F.
1887. Bakterienähnliche Körperchen in den Geweben und Eiern. Biol. Centralbl., vol. 7, pp. 606-608.
1888. Ueber das regelmässige Vorkommen von bakterienähnlichen Gebilden in den Geweben und Eiern verschiedener Insecten. Zeitschr. Biol., vol. 24, pp. 1-15. [Pertinent sections translated by P. Bernhardt.]
1892. Ueber das Vorkommen von bakterienähnlichen Gebilden in den Geweben und Eiern verschiedener Insecten. Centralbl. Bakt. Parasitol., vol. 11, pp. 234-240.
Bode, H.
1936. Untersuchungen über die Symbiose von Tieren mit Pilzen und Bakterien. V. Die Bakteriensymbiose bei Blattiden und das Verhalten von Blattiden bei aseptischer Aufzucht. Arch. Mikrobiol., vol. 7, pp. 391-403. [Translated by Medical Literature Service.]
Bodenheimer, F. S.
1930. Die Schädlingsfauna a Palästinas. Monogr. Angew. Ent., Zeitschr. Angew. Ent., vol. 16. No. 10. pp. 1-439.
1951. Insects as human food. 352 pp. The Hague.
1953. Problems of animal ecology and physiology in deserts. In Desert Research. Res. Counc. of Israel, Spec. Publ. No. 2. pp. 205-229.
Boettger, C. R.
1930. Untersuchungen über die Gewächshausfauna Unter-und Mittelitaliens. Zeitschr. Morph. Ökol., vol. 19, pp. 534-590.
Bolívar, I.
1892. Orthoptères. In Voyage de M. E. Simon aux Iles Philippines. Ann. Soc. Ent. France, vol. 61. pp. 29-34.
1897. Viaggio de Leonardo Fea in Birmania e regioni vicine. 78. Nouvelle espèce cavernicole de la famille des Blattaires. Ann. Mus. Civico di Storia Nat., Genova, ser. 2, vol. 18, pp. 32-36.
1901. Un nuevo ortóptero mirmecófilo Attaphila Bergi. Com. Mus. Nac. Buenos Aires, vol. 1, pp. 331-336, 1 pl.
1905. Les blattes myrmécophiles. Schweiz. Ent. Ges. Mitt., vol. 11, pp. 134-141.
Bolton, H.
1911. On a collection of insect-remains from the South Wales coalfield. Quart. Journ. Geol. Soc. London, vol. 67, pp. 149-174. pls. 7-10.
1921. A monograph of the fossil insects of the British coal measures. Part I. Paleontographical Soc., London, pp. 1-80, pls. 1-4.
Bordage, E.
1896. Sur les moeurs de l'Evania desjardinsii, Blanch. Compt. Rend. Acad. Sci., Paris, vol. 123. pp. 610-613.
1912. Notes biologique recueillies à l'Ile de la Réunion. Bull. Sci. France et Belgique, vol. 46, pp. 29-92.
1913. Notes biologiques recueillies à l'Ile de la Réunion. Chap. II-IV. Bull. Sci. France et Belgique, vol. 47, pp. 377-412.
Bordas, L.
1901. Les glandes défensives ou odorantes des blattes. Compt. Rend. Acad. Sci., Paris, vol. 132, pp. 1352-1354.
1908. Produit de sécrétion de la glande odorante des blattes. Bull. Soc. Zool. France, vol. 33, pp. 31-32.
Borghese, E.
1946. Richerche sul batterio simbionte della Blattella germanica (L.). Bol. Soc. Ital. Biol. Sper., vol. 22, pp. 106-107. [Translated by M. Di Paolo.]
1948. Il ciclo del batterio simbionte di Blattella germanica (L.). Mycopathologia, vol. 4, pp. 85-102, 3 pls.
1948a. Osservazioni sul batterio simbionte della Blattella germanica (L.). Monitore Zool. Ital., Florence, vol. 56, Suppl., pp. 252-253. [Translated by J. J. Buckley.]
Bouwman, B. E.
1914. Kakkerlakken en wespen. De Levende Natur. Tijdschr. Natuur., vol. 18, pp. 385-395. [Translated by J. H. Vanderbie.]
Box, H. E.
1953. List of sugarcane insects. 101 pp. Commonwealth Institute of Entomology, London.
Bozeman, W. B., Jr.
1942. An experimental investigation into the life history of Blatticola blattae, a nematode found in Blattella germanica. Trans. Kansas Acad. Sci., vol. 45, pp. 304-310.
Brecher, G.
1929. Beitrag zur Raumorientierung der Schabe Periplaneta americana. Zeitschr. Vergl. Physiol., vol. 10, pp. 497-526. [Translated by Ruth V. Judson.]
Breed, R. S.; Murray, E. G. D.; and Hitchens, A. P., Eds.
1948. Bergey's manual of determinative bacteriology. 6th ed. 1,529 pp. Baltimore.
Breland, O. P.
1941. Notes on the biology of Stagmomantis carolina (Joh.) (Orthoptera, Mantidae). Bull. Brooklyn Ent. Soc., vol. 36, pp. 170-177.
Bridwell, J. C.
1917. Dolichurus n. sp. Proc. Hawaiian Ent. Soc., vol. 3, p. 268.
1920. Dolichurus stantoni. Proc. Hawaiian Ent. Soc., vol. 4, pp. 277, 438.
Bridwell, J. C., and Swezey, O. H.
1915. Entomological notes. Proc. Hawaiian Ent. Soc., vol. 3, pp. 55-56.
Brigham, W. T.
1866. [Cockroach attacking centipede.] Proc. Boston Soc. Nat. Hist., vol. 11, p. 100.
Brimley, C. S.
1908. Notes on the Orthoptera of Raleigh, North Carolina. Ent. News, vol. 19, pp. 16-21.
Bristowe, W. S.
1925. Notes on the habits of insects and spiders in Brazil. Trans. Ent. Soc. London, 1924. vol. 4, pp. 475-504.
1932. Insects and other invertebrates for human consumption in Siam. Trans. Ent. Soc. London, vol. 80, pp. 387-404.
1941. The comity of spiders. Ray Soc., London, vol. 2, pp. 229-560, 3 pls.
British Museum (Natural History).
1951. The cockroach, its life-history and how to deal with it. 5th ed. Economic Series No. 12, 26 pp. [Revision of Laing (1946).]
Brongniart, C.
1889. Les blattes de l'époque houillère. Compt. Rend. Acad. Sci., Paris, vol. 108, pp. 252-254.
Bronson, W. S.
1943. The grasshopper book. 127 pp. New York.
Brooke, G. E.
1920. Marine hygiene and sanitation. 409 pp. London.
Brooks, Marion A.
1954. Certain aspects of the histochemistry and metabolic significance of the intracellular bodies (bacteroids) of cockroaches (Blattariae). Ph. D. thesis, University of Minnesota. 63 pp., 10 pls.
1957. Investigation of possible antagonism between host and symbiote. Anat. Rec., vol. 128, pp. 527-528. (Abstract.)
Brooks, Marion A., and Richards, A. G.
1954. The necessity for intracellular bacteroids (symbiotes) in growth and reproduction of cockroaches (Blattariae). Anat. Rec., vol. 120, p. 742. (Abstract.)
1955. Intracellular symbiosis in cockroaches. I. Production of aposymbiotic cockroaches. Biol. Bull., vol. 109, pp. 22-39.
1955a. Intracellular symbiosis in cockroaches. II. Mitotic division of mycetocytes. Science, vol. 122, p. 242.
1956. Intracellular symbiosis in cockroaches. III. Re-infection of aposymbiotic cockroaches with symbiotes. Journ. Exp. Zool., vol. 132, pp. 447-466.
Brown, E. B.
1952. Observations on the life-history of the cockroach Ectobius panzeri Stephens (Orth., Blattidae). Ent. Month. Mag., vol. 88, pp. 209-212.
Bruch, C.
1916. Contribución al estudio de los hormigas de la provincia de San Luis. Rev. Mus. de la Plata, vol. 23, pp. 291-357.
Brues, C. T., and Dunn, R. C.
1945. The effect of penicillin and certain sulfa drugs on the intracellular bacteroids of the cockroach. Science, vol. 101, pp. 336-337.
Brues, C. T.; Melander, A. L.; and Carpenter, F. M.
1954. Classification of insects. Bull. Mus. Comp. Zool., Harvard College, vol. 108, pp. 1-917.
Bruijning, C. F. A.
1948. Studies on Malayan Blattidae. Zool. Meded., Leiden, vol. 29, pp. 1-174.
Brumpt, E.
1931. Némathelminthes parasites des rats sauvages (Epimys norvegicus) de Caracas. I. Protospirura bonnei. Infections expérimentales et spontanées. Formes adultes et larvaires. Ann. Parasitol. Hum. et Comp., vol. 9, pp. 344-358.
1949. Précis de parasitologie. 6th ed., vol. 1, 1,042 pp.; vol. 2, 2,120 pp. Paris.
Brumpt, E., and Desportes, C.
1938. Hôtes intermédiaires expérimentaux de deux espèces d'acanthocéphales (Prosthenorchis spirula et P. elegans) parasites des lémuriens et des singes. Ann. Parasitol. Hum. et Comp., vol. 16, pp. 301-304.
Brumpt, E., and Urbain, A.
1938. Une curieuse épizootie vermineuse à acanthocéphales, devenue endémique à la singerie du Muséum. Mesures prophylactiques efficaces prises pour en arrêter les méfaits. Compt. Rend. Acad. Sci., Paris, vol. 206, pp. 1927-1930.
1938a. Épizootie vermineuse par acanthocéphales (Prosthenorchis) ayant sévi a la singerie du Museum de Paris. Ann. Parasitol. Hum. et Comp., vol. 16, pp. 289-300.
Brumpt, L.; Dechambre, E.; and Desportes, C.
1939. Prophylaxie et traitement utilisés pour combattre les acanthoceplales parasites des makis de la menagerie du Jarden des Plantes. Bull. Acad. Vét. France, vol. 12, pp. 198-202.
Bruner, S. C., and Scaramuzza, L. C.
1936. Reseña de los insectos del tobaco en Cuba. Estac. Exp. Agron. Santiago de Las Vegas Prov. Habana, Circ. No. 80, 50 pp.
Bryan, E. H., Jr.
1926. Insects of Hawaii, Johnston Island and Wake Island. Bernice P. Bishop Mus. Bull., vol. 31, pp. 1-94 (especially p. 89).
Bryan, W. A.
1915. Natural history of Hawaii. 596 pp. Honolulu.
Brygoo, E.
1946. Essai de bromatologie entomologique. Les insectes comestibles. Thèse de Doctorate, Bergerac. 73 pp. Imprimerie Générale du Sud-Ouest, Bergerac.
Buchner, P.
1912. Studien an intracellularen Symbionten. I. Die intracellularen Symbionten der Hemipteren. Arch. f. Protistenk., vol. 26, pp. 1-116, 12 pls. [Pertinent sections translated by H. L. Middleton.]
1930. Tier und Pflanze in Symbiose. 900 pp. Berlin.
1952. Historische Probleme der Endosymbiose bei Insekten. Tijdschr. Ent., vol. 95, pp. 143-165. [Pertinent section translated by K. Gingold.]
1953. Endosymbiose der Tiere mit pflanzlichen Mikroorganismen. 771 pp. Basel/Stuttgart.
Bunting, W.
1955. Orthoptera imported into Britain with bananas from Dominica (Leeward Isles). Ent. Month. Mag., vol. 91, p. 134.
1956. Preliminary notes on some Orthoptera imported with bananas from Dominica. Ent. Month. Mag., vol. 92, pp. 284-286.
Burks, B. D.
1943. The North American parasitic wasps of the genus Tetrastichus—a contribution to biological control of insect pests. Proc. U. S. Nat. Mus., vol. 93, pp. 505-608.
1952. The North American species of Syniomosphyrum (Hymenoptera, Chalcidoidea). Proc. Ent. Soc. Washington, vol. 54, pp. 258-264.
Burlingame, P. L., and Chandler, A. C.
1941. Host-parasite relations of Moniliformis dubius (Acanthocephala) in albino rats, and the environmental nature of resistance to single and superimposed infections with this parasite. Amer. Journ. Hyg., vol. 33, sect. D, pp. 1-21.
Burr, M.
1898. Orthoptera collected in South-Eastern Europe. Ent. Rec. and Journ. Var., vol. 10, pp. 1-5.
1899. Mimicry in Orthoptera. Ent. Rec. and Journ. Var., vol. 11, pp. 48-50.
1899a. Parasites of Orthoptera. Ent. Rec. and Journ. Var., vol. 11, pp. 186-187.
1899b. Local Orthoptera in 1899. Ent. Rec. and Journ. Var., vol. 11, p. 333.
1900. On the British Orthoptera in the Hope Museum, Oxford. Ent. Rec. and Journ. Var., vol. 12, pp. 97-99.
1908. Orthoptera in east Kent. Ent. Rec. and Journ. Var., vol. 20, pp. 275-278.
1911. Orthoptera in the Canary Islands. Ent. Rec. and Journ. Var., vol. 23, pp. 92-95, 175-178, 193-195.
1913. Collecting Orthoptera in the Caucasus and Transcaucasus. Ent. Rec. and Journ. Var., vol. 25, pp. 12-15, 37-41.
1923. A contribution to our knowledge of the Orthoptera of Macedonia. Trans. Ent. Soc. London, 1923, pp. 110-130.
1926. In Northeast Siberia. Ent. Rec. and Journ. Var., vol. 38, pp. 97-101.
1937. Periplaneta orientalis L. in the open. Ent. Rec. and Journ. Var., vol. 49, p. 115.
1939. The insect legion. 321 pp. London.
Bütschli, O.
1871. Untersuchungen über die beiden Nematoden der Periplaneta (Blatta) orientalis L. Zeitschr. Wiss. Zool., vol. 21, pp. 252-293, 2 pls.
1878. Beiträge zur Kenntniss der Flagellaten und einiger verwandten Organismen. Zeitschr. Wiss. Zool., vol. 30, pp. 205-281, 5 pls.
1881. Kleine Beiträge zur Kenntnis der Gregarinen. Zeitschr. Wiss. Zool., vol. 35, pp. 384-409, 2 pls.
Buxton, P. A.
1914. British Blattidae. Ent. Rec. and Journ. Var., vol. 26, p. 185.
1955. The natural history of tse-tse flies. London School Hyg. Trop. Med., Mem. No. 10, 816 pp.
Callan, E. McC.
1942. A note on Timulla (Timulla) eriphyla Mickel (Hym., Mutillidae), a parasite of Tachysphex blatticidus F. X. Williams (Hym., Larridae), from Trinidad, B. W. I. Proc. Roy. Ent. Soc. London, ser. A, vol. 17, p. 18.
1950. Observations on tropical wasps in Trinidad. Proc. 8th Internat. Congr. Ent., Stockholm, 1948, pp. 204-206.
Calvert, Amelia S., and Calvert, P. P.
1917. A year of Costa Rican natural history. 577 pp. New York.
Calvert, P. P.
1910. Zoological researches in Costa Rica. Old Penn Weekly Rev., Univ. Pennsylvania, vol. 9, pp. 165-170.
Camerano, L.
1893. Sur quelques gordiens nouveaux ou peu connus. Bull. Soc. Zool. France, vol. 18, pp. 213-216.
1897. Monografia dei Gordii. Mem. Reale Accad. Sci., Torino, ser. 2, vol. 47, pp. 339-419.
Cameron, Ewen.
1955. On the parasites and predators of the cockroach. I.—Tetrastichus hagenowii (Ratz.). Bull. Ent. Res., vol. 46, pp. 137-147.
1957. On the parasites and predators of the cockroach. II.—Evania apppendigaster (L.). Bull. Ent. Res., vol. 48, pp. 199-209.
Campos R., F.
1926. Catalogo preliminar de los blatidos (cucarachas) del Ecuador. Rev. Col. Nac. Vicente Rocafuerte, vol. 8, pp. 41-57.
Canalis, P.
1916. Note de M. le Professeur P. Canalis, relative à quelques expériences sur l'action insecticide du gaz Clayton. Bull. Mens. Off. Internat. Hyg., Paris, vol. 8, pp. 457-463.
Cantrall, I. J.
1941. Notes on collecting and preserving Orthoptera. Part 2 of Compendium of entomological methods. 28 pp. Ward's Natural Science Establishment, Rochester, N. Y.
1943. The ecology of the Orthoptera and Dermaptera of the George Reserve, Michigan. Misc. Publ. Mus. Zool., Univ. Michigan, No. 54, 182 pp., 10 pls.
Cao, G.
1898. Sul passagio dei microrganismi attraverso l'intestino di alcuni insetti. L'Uff. San. Riv. Igiene Med. Prat., Naples, vol. II, pp. 337-348, 385-397.
1906. Nuove osservazioni sul passaggio dei microrganismi a traverso l'intestino di alcuni insetti. Ann. Igiene Sper., vol. 16, pp. 339-368.
Carneiro de Mendonça, F., and Cerqueira, N. L.
1947. Insects and other arthropods captured by the Brazilian Sanitary Service on landplanes or seaplanes arriving in Brazil between January 1942 and December 1945. Bol. Ofic. Sanataria Panamer., vol. 26, pp. 22-30.
Carpenter, G. D. H.
1920. A naturalist on Lake Victoria with an account of sleeping sickness and the tse-tse fly. 333 pp. London.
1921. Experiments on the relative edibility of insects, with special reference to their coloration. Trans. Ent. Soc. London, 1921, pp. 1-105.
1925. A naturalist in East Africa. 187 pp., 8 pls., 1 map. Oxford, England.
Castellani, A., and Chalmers, A. J.
1919. Manual of tropical medicine. 3d ed., 2,436 pp. New York.
Caudell, A. N.
1905. Notes on some Florida Orthoptera. Ent. News, vol. 16, pp. 216-219.
1914. Some bromeliadicolous Blattidae from Mexico and Central America. Insecutor Inscitiae Menstruus, vol. 2, pp. 76-80.
1923. Phorticolea boliviae, a new myrmecophilous cockroach from South America. Psyche, vol. 30, pp. 28-30.
1924. Some insects from the Chilibrillo bat caves of Panama. Insecutor Inscitiae Menstruus, vol. 12, pp. 133-135.
1925. Pycnoscelus surinamensis Linnaeus (Orthoptera); on its nymphs and the damage it does to rose bushes. Proc. Ent. Soc. Washington, vol. 27, pp. 154-157.
1931. Notes on Blattidae, adventive to the United States (Orthop.). Ent. News, vol. 42, p. 204.
Chabaud, A. G., and Larivière, M.
1955. Cycle évolutif d'un ascaride: Subulura jacchi (Marcel 1857) parasite de primates, chez la blatte Blabera fusca. Compt. Rend. Soc. Biol., Paris, vol. 149, pp. 1416-1419.
Chakour, E.
1942. Notes diverses sur Leucophaea surinamensis (L.) et Nauphoeta cinerea (Oliv.). Bull. Soc. Ent. Fouad 1er Ent., vol. 26, pp. 21-23.
Chamberlin, J. C.
1949. Insects of agricultural and household importance in Alaska with suggestions for their control. U. S. Dept. Agr., Alaska Agr. Exp. Sta., Circ. No. 9, 59 pp.
Chamisso, A. von.
1829. Ein Zweifel und zwei Algen. Verhandl. Ges. Naturf. Freunde, Berlin, vol. 1, pp. 173-180.
Chandler, A. C.
1921. Notes on the occurrence of Moniliformis sp. in rats in Texas. Journ. Parasitol., vol. 7, pp. 179-183.
1922. Species of Hymenolepis as human parasites. Journ. Amer. Med. Assoc., vol. 78, pp. 636-639.
1926. Some factors affecting the propagation of hookworm infections in the Asansol Mining Settlement with special reference to the part played by cockroaches in mines. Indian Med. Gaz., vol. 61, pp. 209-212.
1941. The specific status of Moniliformis (Acanthocephala) of Texas rats, and a review of the species of this genus in the Western Hemisphere. Journ. Parasitol., vol. 27, pp. 241-244.
1949. Introduction to parasitology. 756 pp. New York.
Charles, Vera K.
1941. A preliminary check list of the entomogenous fungi of North America. Insect Pest Survey Bull., vol. 21, Suppl. to No. 9, pp. 707-785.
Chatton, E., and Pérard, C.
1913. Schizophytes du caecum du cobaye. I. Oscillospira Guilliermondi n. g., n. sp. Compt. Rend. Soc. Biol., Paris, vol. 74, pp. 1159-1162.
Cheesman, L. E. [Miss].
1927. A contribution towards the insect fauna of French Oceania. Part I. Trans. Ent. Soc. London, vol. 75, pp. 147-161.
1928. A contribution towards the insect fauna of French Oceania. Part II. Ann. Mag. Nat. Hist., ser. 10, vol. 1, pp. 169-194.
Chen, L.
1933. Züchtungsversuche an parasitischen Protozoen von Periplaneta orientalis. (Endamoeba blattae, Nyctotherus ovalis, Lophomonas blattarum.) Zeitschr. Parasitenk., vol. 6, pp. 207-219. [Translated by Medical Literature Service.]
Chitwood, B. G.
1930. A recharacterization of the nematode genus Blatticola Schwenk, 1926. Trans. Amer. Micr. Soc., vol. 49, pp. 178-183, 1 pl.
1932. A synopsis of the nematodes parasitic in insects of the family Blattidae. Zeitschr. Parasitenk., vol. 5, pp. 14-50.
1933. A note on the genera Protrellus Cobb, 1920, and Protrellina Chitwood, 1932. Journ. Parasitol., vol. 19, p. 253.
Chitwood, B. G., and Chitwood, May B.
1934. Nematodes parasitic in Philippine cockroaches. Philippine Journ. Sci., vol. 52, pp. 381-393.
Chobaut, A.
1892. Un nouveau "Rhipidius" du Mont-Ventoux. Mem. Acad. Vaucluse, vol. II, pp. 213-221. [Translated by Ruth V. Judson.]
1919. Description des deux sexes, de l'oeuf et de la larve primaire d'un nouveau Rhipidius de Provence (Col. Rhipiphoridae). Bull. Soc. Ent. France, 1919, No. 11, pp. 200-206.
Chopard, L.
1919. Zoological results of a tour in the far east. Les Orthoptères cavernicoles de Birmanie et de la Peninsule Malaise. Mem. Asiatic Soc. Bengal, vol. 6, pp. 341-396, 3 pls.
1921. On some cavernicolous Dermaptera and Orthoptera from Assam. Rec. Indian Mus., Calcutta, vol. 22, pp. 511-527.
1924. Description d'un blattide myrmécophile nouveau (Orth.) Bull. Soc. Ent. France, 1924, Nos. 11-12, pp. 131-132.
1924a. Rectification à propos d'un blattide myrmécophile (Orth.). Bull. Soc. Ent. France, 1924, No. 17, p. 186.
1924b. On some cavernicolous Orthoptera and Dermaptera from Assam. Rec. Indian Mus., Calcutta, vol. 26, pp. 81-92.
1924c. The fauna of an island in the Chilka Lake. The Dermaptera and Orthoptera of Barkuda Island. Rec. Indian Mus., Calcutta, vol. 26, pp. 165-191.
1927. Description d'une blatte cavernicole du Congo Belge. Rev. Zool. Afrique, vol. 15, pp. 123-126.
1929. Fauna of the Batu caves, Selangor. XII. Orthoptera and Dermaptera. Journ. Fed. Malay States Mus., vol. 14, pp. 366-371.
1929a. Note sur les Orthoptères cavernicoles du Tonkin. Bull. Soc. Zool. France, vol. 54, pp. 424-438.
1929b. Orthoptera palearctica critica. VII. Les polyphagièns de la faune paléarctique (Orth., Blatt.). Eos, vol. 5, pp. 223-358, pls. 8-9.
1932. Voyage de MM. L. Chopard et A. Méquignon aux Açores (Aout-Septembre 1930). I. Orthoptères. Ann. Soc. Ent. France, vol. 101, pp. 55-68, pls. 5-6.
1932a. Un cas de microphthalmie liée à l'atrophie des ailes chez une blatte cavernicole. Soc. Ent. France, Livre Centenaire, pp. 485-496, pl. 26.
1936. Biospeleologica. LXIII. Orthoptères et Dermaptères. Premiere série. Arch. Zool. Exp. et. Gén., vol. 78, pp. 195-214.
1938. La biologie des Orthoptères. Encyl. Ent., Paris, sér. A, vol. 20, 541 pp.
1945. Note sur quelques Orthoptères cavernicoles de Madagascar. Rev. Franç. Ent., vol. 12, pp. 146-155.
1947. Atlas des aptérygotes et orthoptèroides de France. 111 pp. Paris.
1949. Ordre des Dictyoptère Leach, 1818. In Grassé, P. P., Traite de zoologie, Paris, vol. 9, pp. 355-407.
1949a. Les orthoptèroides cavernicoles de Madagascar. Mem. Inst. Sci. Madagascar, sér. A, vol. 3, pp. 41-56.
1950. Sur l'anatomie et le développement d'une blatte vivipare. Eighth Internat. Congr. Ent., Stockholm, 1948, pp. 218-222.
1950a. Orthoptèroides cavernicoles du Congo Belge. Rev. Zool. Bot. Africa, vol. 43, pp. 244-250.
1950b. Les blattes cavernicoles de genre Nocticola Bol. Eos, spec. No., pp. 301-310. [Translated by Ruth V. Judson.]
Chopard, L., and Chatterjee, N. C.
1937. Entomological investigations on the spike disease of sandal. (31) Dermaptera and Orthoptera. Indian Forest Rec., vol. 3, pp. 1-30.
Christie, J. R.
1933. The generic names Cephalobellus Cobb, 1920 and Scarabanema Christie, 1931 (Nematoda). Journ. Washington Acad. Sci., vol. 23, P. 358.
Christie, J. R., and Crossman, L.
1933. On the dissemination of a diplogasterid. Journ. Parasitol., vol. 20, pp. 69-70.
Clausen, C. P.
1940. Entomophagous insects. 688 pp. New York.
Cleveland, L. R.
1925. Toxicity of oxygen for protozoa in vivo and in vitro: Animals defaunated without injury. Biol. Bull., vol. 48, pp. 455-468.
1927. Natural and experimental ingestion of Paramoecium by cockroaches. Science, vol. 66, p. 222.
1930. The symbiosis between the wood-feeding roach, Cryptocercus punctulatus Scudder, and its intestinal flagellates. Anat. Rec., vol. 47, pp. 293-294. (Abstract.)
1931. The effects of molting on the protozoa of Cryptocercus. Anat. Rec., vol. 51, Suppl., p. 84. (Abstract.)
1947. Sex produced in the protozoa of Cryptocercus by molting. Science, vol. 105, pp. 16-17.
1947a. The origin and evolution of meiosis. Science, vol. 105, pp. 287-289.
1948. An ideal partnership. Sci. Month., vol. 67, pp. 173-177.
1949. Hormone-induced sexual cycles of flagellates. I. Gametogenesis, fertilization, and meiosis in Trichonympha. Journ. Morph., vol. 85, pp. 197-296.
1950. Hormone-induced sexual cycles of flagellates. II. Gametogenesis, fertilization, and one-division meiosis in Oxymonas. Journ. Morph., vol. 86, pp. 185-214.
1950a. Hormone-induced sexual cycles of flagellates. III. Gametogenesis, fertilization, and one-division meiosis in Saccinobaculus. Journ. Morph., vol. 86, pp. 215-228.
1950b. Hormone-induced sexual cycles of flagellates. IV. Meiosis after syngamy and before nuclear fusion in Notila. Journ. Morph., vol. 87, pp. 317-348.
1950c. Hormone-induced sexual cycles of flagellates. V. Fertilization in Eucomonympha. Journ. Morph., vol. 87, pp. 349-368.
1951. Hormone-induced sexual cycles of flagellates. VI. Gametogenesis, fertilization, meiosis, oöcysts, and gametocysts in Leptospironympha. Journ. Morph., vol. 88, pp. 199-244.
1951a. Hormone-induced sexual cycles of flagellates. VII. One-division meiosis and autogamy without cell division in Urinympha. Journ. Morph., vol. 88, pp. 385-440.
1952. Hormone-induced sexual cycles of flagellates. VIII. Meiosis in Rhynchonympha in one cytoplasmic and two nuclear divisions followed by autogamy. Journ. Morph., vol. 91, pp. 269-323.
1953. Hormone-induced sexual cycles of flagellates. IX. Haploid gametogenesis and fertilization in Barbulanympha. Journ. Morph., vol. 93, pp. 371-403.
1954. Hormone-induced sexual cycles of flagellates. X. Autogamy and endomitosis in Barbulanympha resulting from interruption of haploid gametogenesis. Journ. Morph., vol. 95, pp. 189-211.
1954a. Hormone-induced sexual cycles of flagellates. XI. Reorganization in the zygote of Barbulanympha without nuclear or cytoplasmic division. Journ. Morph., vol. 95, pp. 213-236.
1954b. Hormone-induced sexual cycles of flagellates. XII. Meiosis in Barbulanympha following fertilization, autogamy, and endomitosis. Journ. Morph., vol. 95, pp. 557-619.
1956. Hormone-induced sexual cycles of flagellates. XIV. Gametic meiosis and fertilization in Macrospironympha. Arch. Protistenk., vol. 101, pp. 99-169.
1956a. Brief accounts of the sexual cycles of the flagellates of Cryptocercus. Journ. Protozool., vol. 3, pp. 161-180.
Cleveland, L. R.; Hall, S. R.; Sanders, E. P.; and Collier, J.
1934. The wood-feeding roach Cryptocercus, its protozoa, and the symbiosis between protozoa and roach. Mem. Amer. Acad. Arts Sci., vol. 17, pp. 185-342, 60 pls.
Cleveland, L. R., and Nutting, W. L.
1954. Protozoa as indicators of developmental stages in molting of the roach Cryptocercus. Anat. Rec., vol. 120, pp. 785-786. (Abstract.)
1955. Suppression of sexual cycles and death of the protozoa of Cryptocercus resulting from change of hosts during molting period. Journ. Exp. Zool., vol. 130, pp. 485-513.
Cleveland, L. R.; Sanders, E. P.; and Hall, S. R.
1931. The relation of the protozoa of Cryptocercus to the protozoa of termites and the bearing of this relationship on the evolution of termites from roaches. Anat. Rec., vol. 51, Suppl., p. 92. (Abstract.)
Cloudsley-Thompson, J. L.
1951. Notes on Arachnida. 16. The behavior of a scorpion. Ent. Month. Mag., vol. 87, p. 105.
1953. Notes on Arachnida. 19. Biological observations, etc. Ent. Month. Mag., vol. 89, pp. 88-89.
1953a. Studies in diurnal rhythms. III. Photoperiodism in the cockroach Periplaneta americana (L.). Ann. Mag. Nat. Hist., ser. 12, vol. 6, pp. 705-712.
1955. On the function of the pectines of scorpions. Ann. Mag. Nat. Hist., ser. 12, vol. 8, pp. 556-560.
1955a. Some aspects of the biology of centipedes and scorpions. Naturalist, 1955, pp. 147-153.
Cobb, N. A.
1920. One hundred new nemas. (Type species of 100 new genera.) Contributions to a science of nematology, IX, pp. 217-343. Baltimore.
Cohic, F.
1956. Parasites animaux des plantes cultivées en Nouvelle-Calédonie et dépendances. Inst. Franç. Océanie, Nouméa, 92 pp.
Colani, Mlle.
1952. Grottes du Tonkin. Ann. de Spéléologie, vol. 7, pp. 143-146.
Collart, A.
1947. A la découverte des Laboulbéniales. Bull. Ann. Soc. Ent. Belgique, vol. 83, pp. 21-35.
Compere, H.
1938. A report on some miscellaneous African Encyrtidae in the British Museum. Bull. Ent. Res., vol. 29, pp. 315-337.
1946. Identity of the new immigrant parasite of cockroach eggs. Proc. Hawaiian Ent. Soc., vol. 12, pp. 464-465.
Comstock, J. H.
1912. The spider book. 721 pp. Garden City, New York.
Conant, N. F.; Smith, D. T.; Baker, R. D.; Callaway, J. L.; and Martin, D. S.
1954. Manual of clinical mycology. 2d ed. 456 pp. Philadelphia.
Conci, C.
1951. Contributo alla conoscenza della speleofauna della Venezia Tridentina. Mem. Soc. Ent. Ital., vol. 30, pp. 5-76.
Coney, B. A.
1918. Panchlora exoleta. Entomologist, London, vol. 51, p. 188.
Coplin, W. M. L.
1899. The propagation of diseases by means of insects, with special consideration of the common domestic types. Philadelphia Med. Journ., vol. 3, pp. 1303-1307.
Cornelius, C.
1853. Beiträge zur nähern Kenntniss von Periplaneta (Blatta) orientalis Linné. (Morgenländische Küchenschabe, auch Schwabe genannt.) 40 pp., 2 pls., Elberfeld, R. L. Friderichs. [Translated by K. Gingold.]
Cornwall, J. W.
1916. A contribution to the study of kala-azar (II). Indian Journ. Med. Res., vol. 4, pp. 105-119.
Cott, H. B.
1940. Adaptive coloration in animals. 508 pp. London.
Cottam, R.
1922. Observations on the phyllodromine cockroach, Blattella supellectilium Serv., in Khartoum. Ent. Month. Mag., vol. 58, pp. 156-158.
Cowan, F.
1865. Curious facts in the history of insects; including spiders and scorpions. 396 pp. Philadelphia.
Crabill, R. E., Jr.
1952. The centipedes of northeastern North America. Ph.D. thesis, Cornell University, 450 pp., 9 pls.
Cram, Eloise B.
1926. A new nematode from the rat, and its life history. Proc. U. S. Nat. Mus., vol. 68, art. 15, pp. 1-7.
1929. [Note on life history of the gizzard worm of ruffed grouse and bobwhite quail.] Proc. Helminthol. Soc. Washington, Journ. Parasitol., vol. 15, pp. 285-286.
1931. Internal parasites and parasitic diseases of the bobwhite. Methods and general findings. In Stoddard, H. L., The bobwhite quail: its habits, preservation and increase. pp. 229-296. New York.
1931a. Developmental stages of some nematodes of the Spiruroidea parasitic in poultry and game birds. U. S. Dept. Agr. Techn. Bull. No. 227, pp. 1-28.
1931b. The cockroach, Blattella germanica, as an intermediate host of Tetrameres americana of poultry. Journ. Parasitol., vol. 18, p. 52.
1933. Observations on the life history of Tetrameres pattersoni. Journ. Parasitol., vol. 20, pp. 97-98.
1933a. Observations on the life history of Seurocyrnea colini. Journ. Parasitol., vol. 20, p. 98.
1934. Orthopterans and pigeons as secondary and primary hosts, respectively, for the crow stomach-worm, Microtetrameres helix (Nematoda: Spiruridae). Proc. Helminthol. Soc. Washington, vol. 1, p. 50.
1935. New avian and insect hosts for Gongylonema ingluvicola (Nematoda: Spiruridae). Proc. Helminthol. Soc. Washington, vol. 2, p. 59.
1937. A species of Orthoptera serving as intermediate host of Tetrameres americana of poultry in Puerto Rico. Proc. Helminthol. Soc. Washington, vol. 4, p. 24.
Crawford, J. C.
1910. Two new species of African parasitic Hymenoptera. Canadian Ent., vol. 42, pp. 222-223.
Crawford, S. C.
1934. The habits and characteristics of nocturnal animals. Quart. Rev. Biol., vol. 9, pp. 201-214.
Crawley, H.
1903. List of the polycystid gregarines of the United States. Proc. Acad. Nat. Sci. Philadelphia, vol. 55, pp. 41-58. 3 pls.
1905. Coelosporidium blattellae, a new sporozoan parasite of Blattella germanica. Proc. Acad. Nat. Sci. Philadelphia, vol. 57, pp. 158-161.
Creighton, J. T.
1954. Household pests. Florida Dept. Agr. Bull., n.s., No. 156, 72 pp.
Cros, A.
1942. Blatta orientalis et ses parasites. I. Evania punctata Brullé; II. Eulophus sp. Étude Biologique. Eos, vol. 18, pp. 45-67.
Crosskey, R. W.
1951. Part II. The taxonomy and biology of the British Evanioidea. Trans. Roy. Ent. Soc. London, vol. 102, pp. 282-301.
Crowell, H. H.
1946. Notes on an amphibious cockroach from the Republic of Panama. Ent. News, vol. 57, pp. 171-172.
Cuénot, L.
1896. Études physiologiques sur des Orthoptères. Arch. Biol., vol. 14, pp. 293-341, 2 pls.
1901. Recherches sur l'evolution et la conjugaison des grégarines. Arch. Biol., vol. 17, pp. 581-652, 5 pls.
Cunha, R. de Al.
1919. Sobre a Gregarina neo-brasiliensis Al. Cunha, 1919. Trabalho apresentado à Faculdade de Medicina de Bello Horizonte, Estado de Minas Gerais. Brazil. [Original not seen.]
Cunliffe, F.
1952. Biology of the cockroach parasite, Pimeliaphilus podapolipophagus Trägårdh, with a discussion of the genera Pimeliaphilus and Hirstiella. (Acarina, Pterygosomidae.) Proc. Ent. Soc. Washington, vol. 54, pp. 153-169.
D'Abreu, E. A.
1920. Some insect prey of birds in the central provinces. Proc. 3d Ent. Meeting, Pusa, 1919, vol. 3, pp. 859-871.
Dalla Torre, C. G. de
1901/02. Catologus hymenopterorum. Vol. 3, p. 1076. Lipsiae.
Dammerman, K. W.
1929. The agricultural zoology of the Malay Archipelago. 473 pp. Amsterdam.
Darlington, P. J., Jr.
1938. Experiments on mimicry in Cuba, with suggestions for future study. Trans. Roy. Ent. Soc. London, vol. 87, pp. 681-695.
Davis, W. T.
1918. The honeydew of aphids very attractive to moths and roaches. Journ. New York Ent. Soc., vol. 26, p. 227.
1926. An annotated list of the Dermaptera and Orthoptera collected in mid-summer at Wingina, Virginia, and vicinity. Journ. New York Ent. Soc., vol. 34, pp. 27-41.
1927. [Periplaneta americana outdoors.] In Proceedings of the Society, Meeting of December 16, 1926. Bull. Brooklyn Ent. Soc., n.s., vol. 22, p. 179.
Darwin, C.
1887. The origin of species by means of natural selection. 458 pp. New ed., from 6th English ed., with additions and corrections. New York.
Day, M. F.
1950. The histology of a very large insect, Macropanesthia rhinocerus Sauss. (Blattidae.) Australian Journ. Sci. Res., B, vol. 3, pp. 61-75.
Dearolf, K.
1941. The invertebrates of 37 Pennsylvania caves. Proc. Pennsylvania Acad. Sci., vol. 15, pp. 170-180.
Debaisieux, P.
1927. A propos des cnidosporidies des blattides. Compt. Rend. Soc. Biol., Paris, vol. 96, pp. 1404-1406.
DeCoursey, J. D., and Otto, J. S.
1956. Some protozoan organisms in cockroaches in the Cairo, Egypt area, with special reference to Endamoeba histolytica. U. S. Nav. Med. Res. Unit No. 3, Res. Rep. NM 005 050.60.01, 7 pp. 2 pls.
1957. Endamoeba histolytica and certain other protozoan organisms found in cockroaches in Cairo, Egypt. Journ. New York Ent. Soc., 1956, vol. 64, pp. 157-163.
DeFraula, T. F. J.
1780. Mémoire sur la génération singulière d'une espèce de gryllon qui découvre un fait de plus, de l'analogie, qui existe entre les règnes animal et végétal. Mem. Acad. Roy. Sci. Let. Beaux Arts Belgique, vol. 3, pp. 219-225. [Same article in Journ. de Physique (1783), vol. 22, pp. 130-133.]
Delamare Deboutteville, C.
1948. Étude quantitative du peuplement animal des sols suspendu et des épiphytes en forêt tropicale. Compt. Rend. Acad. Sci., Paris, vol. 226, pp. 1544-1546.
Delamare Deboutteville, C., and Paulian, R.
1952. Recherches sur la faune des nids et des terriers en Basse Côte d'Ivoire. Encycl. Biogeogr. et Ecol., No. 8, 116 pp. Paris.
Deleurance, E. P.
1943. Notes sur la biologie de quelques prédateurs de la région de Montignac (Dardogne). Bull. Mus. Hist. Nat. Marseille, vol. 3, pp. 56-73.
1946. Études sur quelques éléments de la faune entomologique du bois des Rièges (Camargue). Ann. Soc. Ent. France, 1944, vol. 113, pp. 31-70.
DeLong, D. M.
1948. The supermarket's roach problem. Soap. San. Chem., vol. 24, No. 8, pp. 143, 145, 147, 149.
Denning, D. G.; Pratt, J. J., Jr.; Staebler, A. E.; and Wirth, W. W.
1947. A tabulation of insects recovered from aircraft entering the United States at Miami, Florida, during the period July 1943 through December 1944. Foreign Plant Quar. Mem. No. 474, 41 pp. (Processed.)
Derez, M. E.
1949. The food of Rana catesbeiana Shaw in Puerto Rico. Program 61st Ann. Meet. Amer. Assoc. Econ. Ent., No. 74, pp. 35-36. (Abstract.)
Deschapelles, J. B.
1939. Las cucarachas. Rev. Agr., Cuba, vol. 23, pp. 41-47. [Translated by J. Gotlob.]
Dethier, V. G.
1945. The transport of insects in aircraft. Journ. Econ. Ent., vol. 38, pp. 528-531.
Diesing, C. M.
1851. Systema helminthum. Vol. 2, 591 pp. Vindobonae.
1861. Revision der Nematoden. Sitzb. K. Akad. Wiss., Wien, Math. Nat. Cl., 1860, vol. 42, pp. 595-736, 1 pl.
Distant, W. L.
1902. Panchlora exoleta, Klug (Blattidae), imported into Scotland. Ent. Month. Mag., vol. 38, p. 247.
Dobell, C. C.
1911. Contributions to the cytology of the bacteria. Quart. Journ. Micr. Sci., n.s., vol. 56, pp. 395-506, 4 pls.
1912. Researches on the spirochaets and related organisms. Arch. Protistenk., vol. 26, pp. 117-240.
Dobrovolny, C. G.
1933. Studies on the nematode parasites of the American cockroach, Periplaneta americana. Trans. Kansas Acad. Sci., vol. 36, p. 213.
Dobrovolny, C. G., and Ackert, J. E.
1934. The life history of Leidynema appendiculata (Leidy), a nematode of cockroaches. Parasitology, vol. 26, pp. 468-480.
Dodd, A. P.
1917. Record and descriptions of Australian Chalcidoidea. Trans. and Proc. Roy. Soc. South Australia, vol. 41, pp. 344-368.
Dodge, B. O., and Rickett, H. W.
1943. Diseases and pests of ornamental plants. 638 pp. Lancaster, Pa.
Dodge, C. W.
1935. Medical mycology. Fungous diseases of man and other mammals. 900 pp. St. Louis.
Donisthorpe, H. St. J. K.
1900. Myrmecophilous Orthoptera. Ent. Rec. and Journ. Var., vol. 12, pp. 162-163.
1918. A fortnight in the New Forest in July. Ent. Rec. and Journ. Var., vol. 30, pp. 170-173. [The same record was reported by Donisthorpe in this same journal in 1919, vol. 31, p. 116, and in 1937, vol. 49, p. 136. It was also reported by him in Trans. Ent. Soc. London in 1919, vol. for 1918, pts. 3/4, p. clvii.]
Donisthorpe, H. St. J. K., and Wilkinson, D. S.
1930. Notes on the genus Paxylomma (Hym. Brac.), with the description of a new species taken in Britain. Trans. Ent. Soc. London, vol. 78, pp. 87-93.
Dorier, A.
1930. Recherches biologiques et systématiques sur les Gordiacés. Trav. Lab. Hydrobiol. Piscicult. Univ. Grenoble, vol. 22, pp. 1-183, 4 pls.
Doucette, C. F., and Smith, F. F.
1926. Control experiments on the Surinam cockroach (Pycnoscelus surinamensis L.). Journ. Econ. Ent., vol. 19, pp. 650-656.
Dover, C.
1928. Notes on the fauna of pitcher-plants from Singapore Island. I. General. Journ. Malayan Branch Roy. Asiatic Soc., vol. 6, pp. 1-9, 5 pls.
Dow, R. P.
1955. A note on domestic cockroaches in south Texas. Journ. Econ. Ent., vol. 48, pp. 106-107.
Dowdy, W. W.
1947. An ecological study of the Arthropoda of an oak-hickory forest with reference to stratification. Ecology, vol. 28, pp. 418-439.
1951. Further ecological studies on stratification of the Arthropoda. Ecology, vol. 32, pp. 37-52.
1955. An hibernal study of Arthropoda with reference to hibernation. Ann. Ent. Soc. Amer., vol. 48, pp. 76-83.
Dozier, H. L.
1920. An ecological study of hammock and piney woods insects in Florida. Ann. Ent. Soc. Amer., vol. 13, pp. 325-380.
Dresner, E.
1949. Culture and use of entomogenous fungi for the control of insect pests. Contr. Boyce Thompson Inst., vol. 15, pp. 319-335.
1950. The toxic effects of Beauveria bassiana (Bals.) Vuill., on insects. Journ. New York Ent. Soc., vol. 63, pp. 269-278.
Dubos, R. J., Editor.
1948. Bacterial and mycotic infections of man. 785 pp. Philadelphia.
Dumbleton, L. J.
1957. Parasites and predators introduced into the Pacific Islands for the biological control of insects and other pests. South Pacific Comm., Techn. Pap. No. 101, 40 pp. [This paper appeared erroneously under the name of C. P. Hoyt. See Kroon, A. H. J., 1957, South Pacific Commission Technical Paper No. 101. Erratum, 1 p. (multigraphed). Nouméa. (Rev. Appl. Ent., vol. A 46, p. 155, 1958.)]
Dusham, E. H.
1918. The wax glands of the cockroach (Blatta germanica). Journ. Morph., vol. 31, pp. 563-581.
Dutertre, J. B.
1654. Histoire generale des Iles de S. Christophe, de Guadeloupe, de la Martinique, et autres dans l'Amerique. Paris.
Dutky, S. R., and Hough, W. S.
1955. Note on a parasitic nematode from codling moth larvae, Carpocapsa pomonella (Lepidoptera, Olethreutidae). Proc. Ent. Soc. Washington, vol. 57, p. 244.
Dutt, G. R.
1912. Life histories of Indian insects—IV (Hymenoptera). Mem. Dept. Agr. India, vol. 4, pp. 183-267.
Eads, R. B.; Von Zuben, F. J.; Bennett, S. E.; and Walker, O. L.
1954. Studies on cockroaches in a municipal sewerage system. Amer. Journ. Trop. Med. Hyg., vol. 3, pp. 1092-1098.
Ealand, C. A.
1915. Insects and man. An account of the more important harmful and beneficial insects, their habits and life-histories, being an introduction to economic entomology for students and general readers. 343 pp. London.
Edmunds, L. R.
1952. The oviposition of Prosevania punctata (Brullé): A hymenopterous parasite of cockroach egg capsules. Ohio Journ. Sci., vol. 52, pp. 29-30.
1952a. Some notes on the habits and parasites of native woodroaches in Ohio (Orthoptera: Blattidae). Ent. News, vol. 63, pp. 141-145.
1953. Some notes on the Evaniidae as household pests and as a factor in the control of roaches. Ohio Journ. Sci., vol. 53, pp. 121-122.
1953a. Collecting and culturing of native wood roaches in Ohio, with some additional notes on their parasites. Ent. News, vol. 64, pp. 225-230.
1953b. A study of the biology and life history of Prosevania punctata (Brullé) with notes on additional species. (Hymenoptera: Evaniidae). Ph.D. thesis, Ohio State University.
1954. A study of the biology and life history of Prosevania punctata (Brullé) with notes on additional species (Hymenoptera: Evaniidae). Ann. Ent. Soc. Amer., vol. 47, pp. 575-592.
1955. Biological notes on Tetrastichus hagenowii (Ratzeburg), a chalcidoid parasite of cockroach eggs (Hymenoptera: Eulophidae; Orthoptera: Blattidae). Ann. Ent. Soc. Amer., vol. 48, pp. 210-213.
1957. Observations on the biology and life history of the brown cockroach Periplaneta brunnea Burmeister. Proc. Ent. Soc. Washington, vol. 59, pp. 283-286.
Ehrlich, H.
1943. Verhaltensstudien an der Schabe Periplaneta americana L. Zeitschr. Tierpsychol., vol. 5, pp. 497-552. [Translated by Medical Literature Service.]
Eisner, T.
1958. Spray mechanism of the cockroach Diploptera punctata. Science, vol. 128, pp. 148-149.
El-Kholy, S., and Gohar, M. A.
1945. Cockroaches as possible carriers of disease. Journ. Roy. Egyptian Med. Assoc., vol. 29, pp. 82-87.
Ellis, M. M.
1913. A descriptive list of the cephaline gregarines of the New World. Trans. Amer. Micros. Soc., vol. 32, pp. 259-296, 4 pls.
1913a. Gregarines from some Michigan Orthoptera. Zool. Anz., Leipzig, vol. 43, pp. 78-84.
Elmassian, M.
1909. Sur l'Amoeba blattae. Morphologie génération. Arch. Protistenk., vol. 16, pp. 143-163.
Epshtein, G. V.
1911. Beiträge zur Kenntnis von Pleistophora periplanetae (Lutz und Splendore). Biol. Centralbl., vol. 31, pp. 676-682.
Essig, E. O.
1926. Insects of western North America. 1,035 pp. New York.
Fahringer, J.
1922. Beiträge zur Kenntnis der Lebensweise einiger Schmarotzer-wespen unter besonderer Berücksichtigung ihrer Bedeutung für biologischen Bekämpfung von Schädlingen. Zeitschr. Angew. Ent., vol. 8, pp. 325-388.
Falls, O.
1938. Cockroach vs. termite idea. Exterminators Log, vol. 6, No. 2, p. 18.
Fantham, H. B.
1929. Some parasitic Protozoa found in South Africa—XII. South African Journ. Sci., vol. 26, pp. 386-395, 1 pl.
Faure, J. C.
1940. Maternal care displayed by mantids (Orthoptera). Journ. Ent. Soc. South Africa, vol. 3, pp. 139-150.
Fausek, V.
1906. Biological investigations in the Transcaspian Region. Orthoptera. (In Russian.) Imp. Russk. Geo. Obshch., Zapiski Obshch. Geo., vol. 27, pp. 35-37. [Translated by D. Kraus.]
Faust, E. C.
1928. The life cycle of Spirocerca sanguinolenta—a natural nematode parasite of the dog. Science, vol. 68, pp. 407-409.
1939. Human helminthology. A manual for physicians, sanitarians and medical zoologists. 2d ed., 780 pp. Philadelphia.
1955. Animal agents and vectors of human disease. 660 pp. Philadelphia.
Felt, E. P.
1909. Twenty-fourth report of the state entomologist 1908. Education Dept., New York State Mus. Bull. 455, pp. 22-23, 26-27.
1926. Entomology. In Twenty-first report of the director of the State Museum and Science Department. New York State Mus. Bull. 267, pp. 37-48.
1928. Observations and notes on injurious and other insects of New York State. New York State Mus. Bull. 274, pp. 145-176.
Fernald, M. L.
1950. Gray's manual of botany. 8th ed., 1,632 pp. New York.
Fernando, W.
1957. Sphecophila ravana, sp. n., a new termitophilous cockroach from Ceylon. Ann. Mag. Nat. Hist., ser. 12, vol. 10, pp. 81-84.
Ferrière, C.
1930. On some egg-parasites from Africa. Bull. Ent. Res., vol. 21, pp. 33-44.
1935. Notes on some bred exotic Eupelmidae (Hym. Chalc.). Stylops, vol. 4, pp. 145-153.
Ferton, C.
1894. Sur les moeurs de Dolichurus haerorrhous Costa. (Hyménoptère). Act. Soc. Linné. Bordeaux, vol. 47, pp. 215-221.
1901. Notes détachées sur l'instinct des Hyménoptères mellifères et ravisseurs avec la description de quelques espèces (sér. 1). Ann. Soc. Ent. France, vol. 70, pp. 83-148.
1914. Notes détachées sur l'instinct des Hyménoptères mellifères et ravisseurs ([8e] série). Ann. Soc. Ent. France, vol. 83, pp. 81-119, 3 pls.
Fibiger, J. A. G.
1913. Ueber eine durch Nematoden (Spiroptera sp. n.) hervorgerufene papillomatöse und carcinomatöse Geschwulstbildung im Magen der Ratte. Berliner Klin. Wochenschr., vol. 50, pp. 289-298.
1913a. Untersuchungen über eine Nematode (Spiroptera sp. n.) und deren Fähigkeit, papillomatöse und carcinomatöse Geschwulstbildungen im Magen der Ratte hervorzurufen. Zeitschr. Krebsforsch., vol. 13, pp. 217-280.
Fibiger, J. A. G., and Ditlevsen, H.
1914. Contributions to the biology and morphology of Spiroptera (Gongylonema) neoplastica n. sp. Mindeskr. Japetus Steenstrups Fødsel, Copenhagen, No. 25, 28 pp.
Fielding, J. W.
1926. Preliminary note on the transmission of the eye worm of Australian poultry. Australian Journ. Exp. Biol. Med. Sci., vol. 3, pp. 225-232.
1927. Further observations on the life history of the eye worm of poultry. Australian Journ. Exp. Biol. Med. Sci., vol. 4, pp. 273-281.
1928. Observations on eye worms of birds. Queensland Agr. Journ., vol. 30, pp. 37-41.
1928a. Additional observations on the development of the eye worm of poultry. Australian Journ. Exp. Biol. Med. Sci., vol. 5, pp. 1-8.
Figuier, L.
1869. The insect world; being a popular account of the orders of insects, etc. 519 pp. New York.
Filatoff, E. D.
1904. Ueber das Verhalten einiger Bakterienarten zu dem Organismus der Bombyx mori (L.) und der Periplaneta orientalis (L.) bei artifizieller Infektion derselben. Centralbl. Bakt., Parasitenk. und Infekt., Abt. 2, vol. 11, pp. 658-686, 748-762. [Pertinent sections translated by P. Bernhardt.]
Filippov, G. S.
1926. Oplesnevykh gribkakb gruppy Mucorinae. [Mold fungi of the Mucorinae.] Bolezni Rastenii (Morbi-Plantarum, Leningrad), vol. 15, pp. 175-186. [Pertinent section translated by K. Gingold.]
Findlay, G. M., and Howard, E. M.
1951. Observations on Columbia SK virus. Journ. Path. and Bact., vol. 63, pp. 435-443.
Findlay, G. M., and MacCallum, F. O.
1939. Epidemiology of yellow fever. Nature, London, vol. 143, p. 289.
Fischer, R. G., and Syverton, J. T.
1951. The cockroach as an experimental vector of viruses. Bact. Proc., May 27-31, p. 95.
1951a. The cockroach as an experimental vector of Coxsackie virus. Amer. Journ. Trop. Med., vol. 31, pp. 238-242.
1957. Distribution of Coxsackie virus in experimentally infected cockroaches, Periplaneta americana. Proc. Soc. Exp. Biol. Med., vol. 95, pp. 284-287.
Fisk, F. W.
1951. Use of a specific mite control in roach and mouse cultures. Journ. Econ. Ent., vol. 44, p. 1016.
Flint, O. S.
1951. A new cockroach record for the United States. Bull. Brooklyn Ent. Soc., vol. 46, P. 53.
Flock, R. A.
1941. Biological control of the brown-banded roach. Bull. Brooklyn Ent. Soc., vol. 36, pp. 178-181.
1941a. The field roach Blattella vaga. Journ. Econ. Ent., vol. 34, p. 121.
Foerster, H.
1938. Gregarinen in Schlesischen Insekten. Zeitschr. Parasitenk., vol. 10, pp. 157-209.
Foster, A. O., and Johnson, C. M.
1938. Protospiruriasis, a new nematode disease of captive monkeys. Journ. Parasitol., vol. 24, p. 32. (Abstract.)
1939. A preliminary note on the identity, life-cycle, and pathogenicity of an important nematode parasite of captive monkeys. Amer. Journ. Trop. Med., vol. 19, pp. 265-277.
Fox, C.
1925. Insects and disease of man. 349 pp. Philadelphia.
Fraenkel, G.
1952. The role of symbionts as sources of vitamins and growth factors for their insect hosts. Tijd. Ent., vol. 95, pp. 183-195.
Fraenkel, Helene.
1921. Die Symbionten der Blattiden im Fettgewebe und Ei insbesondere von Periplaneta orientalis. Zeitschr. Wiss. Zool., vol. 119, pp. 53-66.
Frank, W.
1955. Einwirkung verschiedener Antibiotica auf die Symbionten der Küchenschabe Blatta orientalis L. und die dadurch bedingten Veränderungen am Wirtstier. Zool. Anz., 18 Supplementband, pp. 381-388.
1956. Entfernung der intrazellulären Symbioten der Küchenschabe (Periplaneta orientalis L.) durch Einwirkung verschiedener Antibiotica, unter besonderer Berücksichtigung der Veränderungen am Wirtstier und an den Bakterien. Zeitschr. Morph. u. Ökol. Tiere, vol. 44, pp. 329-366.
Freemantle, Sir Edmund, R.
1904. The Navy as I have known it. 472 pp. London.
Frenzel, J.
1892. Über einige Argentinische Gregarinen. Ein Beitrag zur Organisation und Physiologie der Gregarinen überhaupt. Zeitschr. Naturwiss., vol. 27 (N.F. 20), pp. 233-336.
Frey, R.
1948. [Cockroaches found in banana shipments.] (In Finnish.) Notulae Ent., vol. 28, p. 118.
Friauf, J. J.
1953. An ecological study of the Dermaptera and Orthoptera of the Welaka area in northern Florida. Ecol. Monogr., vol. 23, pp. 79-126.
Frings, H.
1948. An ounce of prevention. Pests and Their Control, vol. 16, No. 2, pp. 24, 26.
Froggatt, W. W.
1906. Domestic Insects: Cockroaches (Blattidae). Agr. Gaz. New South Wales, vol. 17, pp. 440-447.
1907. Australian insects. 449 pp. Sydney.
Frost, S. W.
1924. Frogs as insect collectors. Journ. New York Ent. Soc., vol. 32, pp. 174-185.
Frye, W. W., and Meleney, H. E.
1936. The viability of Endamoeba histolytica cysts after passage through the cockroach. Journ. Parasitol., vol. 22, pp. 221-222.
Fullaway, D. T.
1912. Entomological notes. Ann. Rep. Guam Agr. Exp. Stat., 1911, pp. 26-35.
1938. Orchid insects. Proc. Hawaiian Ent. Soc., vol. 10, pp. 45-49.
Fullaway, D. T., and Krause, N. L. H.
1945. Common insects of Hawaii, 228 pp. Honolulu.
Fulton, B. B.
1930. Notes on Oregon Orthoptera with descriptions of new species and races. Ann. Ent. Soc. Amer., vol. 23, pp. 611-641.
Gahan, A. B.
1914. Descriptions of new genera and species, with notes on parasitic Hymenoptera. Proc. U. S. Nat. Mus., vol. 48, pp. 155-168.
1917. Descriptions of some new parasitic Hymenoptera. Proc. U. S. Nat. Mus., vol. 53, pp. 195-217.
1923. Report on a small collection of parasitic Hymenoptera from Java and Sumatra. Treubia, vol. 3, pp. 47-52.
Gahan, A. B., and Peck, O.
1946. Notes on some Ashmeadian genotypes in the hymenopterous superfamily Chalcidoidea. Journ. Washington Acad. Sci., vol. 36, pp. 314-317.
Galeb, O.
1877. Sur l'anatomie et les migration des oxyuridés, parasites des insectes du genre Blatta. Compt. Rend. Acad. Sci., Paris, vol. 85, pp. 236-239.
1878. Recherches sur les entozoaires des insectes. Organisation et développement des oxyuridés. Arch. Zool. Exp. et Gén., Paris, vol. 7, pp. 283-390, 10 pls.
1878a. Observations et expériences sur les migrations du Filaria rytipleurites, parasite des blattes et des rats. Compt. Rend. Acad. Sci., Paris, vol. 87, pp. 75-77.
Gal'kov, V. P.
1926. An experiment with hydrocyanic acid to control insect parasites in dwellings. (In Russian.) Zashchita Rastenii (Défense des Plantes), vol. 3, pp. 98-100. [Translated by D. Kraus.]
Gallardo, E.; Forgash, A. J.; and Beaudette, F. R.
1957. An exploratory investigation of the effect in vivo and in vitro of cockroach tissues on the viability of Newcastle disease virus. Ann. Ent. Soc. Amer., vol. 50, pp. 192-197.
Gardner, A. E.
1954. Blatta orientalis out-of-doors. Entomologist, vol. 87, p. 167.
Gates, M. F.
1912. Roaches and their extermination by the use of sodium fluorid (NaF). U. S. Nav. Med. Bull., vol. 6, pp. 212-214.
Gates, Mary F., and Allee, W. C.
1933. Conditioned behavior of isolated and grouped cockroaches on a simple maze. Journ. Comp. Psychol., vol. 15, pp. 331-358.
Gaul, A.
1953. The wonderful world of insects. 290 pp. New York.
Genieys, P.
1924. Contribution a l'étude des Evaniidae, Zeuxevania splendidula Costa. Bull. Biol. France et Belgique, vol. 58, pp. 482-494.
Georgévitch, J.
1925. Sur la structure de la spore de Pleistophora periplanetae. Compt. Rend. Acad. Sci., Paris, vol. 181, pp. 1191-1194.
1926. Sur le cycle évolutif de Pleistophora periplanetae. Compt. Rend. Acad. Sci., Paris, vol. 182, pp. 102-104.
1926a. Prouchavanje razvoya Pteistophora periplanetae Lutz et Splend. [Study of the development of Pleistophora periplanetae Lutz and Splend.] Glas Srpske Kral. Acad., vol. 122, 1. Razred, No. 56, pp. 1-33, 3 double pls. (German summary in Zool. Berichte, 1929, vol. 20, pp. 392-393.)
1927. Recherches sur Pleistophora periplanetae Lutz et Splend. Arch. Zool. Exp. et Gén., Paris, vol. 66, pp. 1-21, 3 pls.
1953. Étude de cycle évolutif de Haplosporidium periplanetae nov. spec. Bull. Acad. Serbe des Sci., Cl. des Sci. Math. et Nat., Sci. Nat., n. s., vol. 12, pp. 98-102, 1 pl.
Gertsch, W. J.
1949. American spiders. 285 pp. New York.
Ghosh, E.
1922. On a new ciliate, Balantidium blattarum, sp. nov., intestinal parasite in the common cockroach (Blatta americana). Parasitology, vol. 14, pp. 15-16.
1922a. On a new ciliate, Balantidium ovatum, sp. nov., an intestinal parasite in the common cockroach (Blatta americana). Parasitology, vol. 14, p. 371.
Giard, A.
1900. Captures. Bull. Soc. Ent. France, vol. for 1900, No. 20, pp. 390-391.
Gier, H. T.
1935. The "intracellular symbionts" of some Blattidae, their nature and behavior. Anat. Rec., vol. 64, Suppl. 1, pp. 126-127. (Abstract.)
1936. The morphology and behavior of the intracellular bacteroids of roaches. Biol. Bull., vol. 71, pp. 433-452.
1937. Growth of the intracellular symbionts of the cockroach, Periplaneta americana. Anat. Rec., vol. 70, Suppl. No. 1, p. 69. (Abstract.)
1947. Intracellular bacteroids in the cockroach (Periplaneta americana Linn.). Journ. Bact., vol. 53, pp. 173-189.
1947a. Growth rate in the cockroach Periplaneta americana (Linn.). Ann. Ent. Soc. Amer., vol. 40, pp. 303-317.
Giordano, M.
1950. Patologia, parassitologia ed igiene dei paesi caldi. 3d ed., vol. 1, 812 pp. Italy.
Girard, M.
1877. La domestication des blattes. Bull. Soc. Acclimatation, sér. 3, vol. 4, pp. 297-309.
Girault, A. A.
1907. Hosts of insect egg-parasites in North and South America. Psyche, vol. 14, pp. 27-39.
1914. Hosts of insect egg parasites, in Europe, Asia, Africa and Australasia, with a supplementary American list. Zeitschr. Wiss. Insektenbiol., Berlin, vol. 10, pp. 87-91, 135-139, 175-178, 238-240.
1915. Australian Hymenoptera Chalcidoidea—VII. The family Encyrtidae with descriptions of new genera and species. Mem. Queensland Mus., vol. 4, pp. 1-184.
1915a. Australian Hymenoptera Chalcidoidea—IX. The family Cleonymidae with descriptions of new genera and species. Mem. Queensland Mus., vol. 4, pp. 203-224.
1915b. New fragments on some well-known insects (Col., Orth., Hem.). Ent. News, vol. 26, pp. 53-56.
1917. New chalcid flies from Maryland, II (Hym.). Ent. News, vol. 28, pp. 255-258.
1924. Lése majestè, new Insecta and robbery. Gympie, Australia, 1 p. (Privately printed.)
Glaser, R. W.
1920. Biological studies on intracellular bacteria. No. 1. Biol. Bull., vol. 39, pp. 133-145.
1925. Specificity in bacterial disease with special reference to silkworms and tent caterpillars. Journ. Econ. Ent., vol. 18, pp. 769-771.
1930. On the isolation, cultivation and classification of the so-called intracellular "symbiont" or "rickettsia" of Periplaneta americana. Journ. Exp. Med., vol. 51, pp. 59-82.
1930a. Cultivation and classification of "bacteroides," "symbionts," or "rickettsiae" of Blattella germanica. Journ. Exp. Med., vol. 51, pp. 903-907.
1930b. The intracellular "symbionts" and the "rickettsiae." Arch. Pathol., vol. 9, pp. 79-96.
1946. The intracellular bacteria of the cockroach in relation to symbiosis. Journ. Parasitol., vol. 32, pp. 483-489.
Glauert, L.
1946. Scorpions. Australian Mus. Mag., vol. 9, pp. 93-98.
Goldenberg, F.
1877. Die fossilen Thiere aus der Steinkohlenformation von Saarbrücken. Fauna Saraepontana Fossilis, II tes-Heft. 54 pp., 2 double pls. Saarbrücken.
Gomes, J. G.
1941. Subsídios a sistemática dos calcidídeos Brasileíros. Bol. Escola Nac. Agron., Rio de Janeiro, No. 2, pp. 9-53.
Goodey, T.
1951. Soil and freshwater nematodes. A monograph. 390 pp. New York.
Goodliffe, E. R.
1958. Current methods of roach control. Ann. Appl. Biol., vol. 46, pp. 102-105.
Gould, G. E.
1941. The effect of temperature upon the development of cockroaches. Proc. Indiana Acad. Sci., vol. 50, pp. 242-248.
Gould, G. E., and Deay, H. O.
1938. The biology of the American cockroach. Ann. Ent. Soc. Amer., vol. 31, pp. 489-498.
1940. The biology of six species of cockroaches which inhabit buildings. Purdue Univ. Agr. Exp. Stat. Bull. No. 451, 31 pp.
Gowdey, C. C.
1925. Report of government entomologist. Ann. Rep. Dept. Sci. and Agr., Jamaica, 1924, pp. 17-20.
Graeffe, E.
1860. Beobachtungen über Radiaten und Würmer in Nizza. Denkschr. Allgem. Schweiz. Ges. Gesammt. Naturwiss., Zurich, vol. 17, pp. 1-59, 10 pls.
Graffar, M., and Mertens, S.
1950. Le rôle des blattes dans la transmission des salmonelloses. Ann. Inst. Pasteur, vol. 79, pp. 654-660.
Grandi, G.
1928. Contributi alla conoscenza biologica e morfologica degli Imenotteri melliferi e predatori. VII. Boll. Lab. Ent. R. Ist. Sup. Agrar. Bologna, vol. 1, pp. 259-326. [Pertinent sections translated by J. J. Buckley.]
1931. Scoperta di un nuova braconide (Perilitus morimi Ferr.) parassita degli adulti del Morimus asper Sulz. (Coleoptera-Cerambycidae) e descrizione della sua larva. Boll. Lab. Ent. R. Ist. Sup. Agrar. Bologna, vol. 4, pp. 1-72. [Pertinent sections translated by J. J. Buckley.]
1954. Contributi alla conoscenza degli Imenotteri Aculeati. XXVI. Boll. Ist. Ent. Univ. Bologna, vol. 20, pp. 81-255. [Pertinent sections translated by J. J. Buckley.]
Grassé, P. P.
1926. Sur la nature des cotes cuticulaires des Polymastix et du Lophomonas striata. Compt. Rend. Soc. Biol., Paris, vol. 94, pp. 1014-1016.
1926a. Protistologica. V. Contribution à l'étude des flagellés parasites. Arch. Zool. Exp. et Gén., vol. 65, pp. 345-602, 12 pls.
1952. Rôle des flagellés symbiotiques chez les blattes et les termites. Tijd. Ent., vol. 95, pp. 70-80.
Grassi, G. B.
1888. Beiträge zur Kenntniss des Entwicklungscyclus von fünf Parasiten des Hundes (Taenia cucumerina Goeze; Ascaris marginata Rud.; Spiroptera sanguinolenta Rud.; Filaria immitis Leidy und Haematozoon Lewis). Centralbl. Bakt. Parasitol., vol. 4, pp. 609-620.
Greenewalt, C. H., and Jones, F. M.
1955. Photographic studies of the feeding of nestling house wrens. Amer. Sci., vol. 43, pp. 541-549.
Gresham, W. B.
1952. [Occurrence of Nauphoeta cinerea in Florida.] Florida Ent., vol. 35, p. 77.
Griffiths, J. T., and Tauber, O. E.
1942. Fecundity, longevity and parthenogenesis of the American roach, Periplaneta americana L. Physiol. Zool., vol. 15, pp. 196-209.
1942a. The nymphal development for the roach, Periplaneta americana L. Journ. New York Ent. Soc., vol. 50, pp. 263-272.
Gropengiesser, C.
1925. Untersuchungen über die Symbiose der Blattiden mit niederen pflanzlichen Organismen. Centralbl. Bakt. Parasitol. Infekt., Abt. 2, vol. 64, pp. 495-511.
Groschaft, J.
1956. Nálezy roupovitých (Oxyuroidea) u laboratorně chovaných švábu (Blattodea). [Oxyuroidea in laboratory rearings of Blattidae.] (In Czech.) Ceskoslovenska Parasit., vol. 3, pp. 67-74.
Gross, J. R.
1950. Entomological ballistics. Pest Control, vol. 18, No. 2, pp. 16, 50.
Gubler, H. U.
1948. Versuche zur Züchtung intrazellulärer Insektensymbionten. Schweiz. Zeitschr. Path. Bact., vol. 11, pp. 489-493. [Translated by H. L. Middleton.]
Gulati, A. N.
1930. Do cockroaches eat bed bugs? Nature, vol. 125 p. 858.
Gundlach, J.
1887. Apuntes para la fauna Puerto-Riquena. Anal. Soc. Españ. Hist. Nat., vol. 16, pp. 115-199.
1890-1891. Contribución a la entomología Cubana. Vol. 2, pt. 4, Ortópteros, pp. 289-396.
Gunn, D. L.
1934. The temperature and humidity relations of the cockroach (Blatta orientalis). II. Temperature preference. Zeitschr. Vergl. Physiol., vol. 20, pp. 617-625.
1935. The temperature and humidity relations of the cockroach. III. A comparison of temperature preference, and rates of desiccation and respiration of Periplaneta americana, Blatta orientalis, and Blattella germanica. Journ. Exp. Biol., vol. 12, pp. 185-190.
Gunn, D. L., and Cosway, C. A.
1938. The temperature and humidity relations of the cockroach. V. Humidity preference. Journ. Exp. Biol., vol. 15, pp. 555-563.
Gunn, D. L., and Notley, F. B.
1936. The temperature and humidity relations of the cockroach. IV. Thermal death-point. Journ. Exp. Biol., vol. 13, pp. 28-34.
Gupta, P. O.
1947. On the structure and formation of spermatophore in the cockroach, Periplaneta americana (Linn.). Indian Journ. Ent., vol. 8, pp. 79-84.
Gurney, A. B.
1937. Studies in certain genera of American Blattidae (Orthoptera). Proc. Ent. Soc. Washington, vol. 39, pp. 101-112.
1951. Praying mantids of the United States, native and introduced. Ann. Rep. Smithsonian Inst. for 1950, pp. 339-362.
1952. The correct spelling of the generic name of the German cockroach. Journ. Econ. Ent., vol. 45, p. 752.
1953. Distribution, general bionomics, and recognition characters of two cockroaches recently established in the United States. Proc. U. S. Nat. Mus., vol. 103, pp. 39-56.
1954. [Habits of nymphs of Perispherus.] Proc. Ent. Soc. Washington, vol. 56, p. 46. (Abstract.)
1955. Notes on the Cuban cockroach, Panchlora nivea (L.) (Orthoptera, Blattidae). Proc. Ent. Soc. Washington, vol. 57, pp. 285-286.
H., W.
1800. An effectual remedy against black-beetles. Gentleman's Magazine, vol. 70, pp. 933-934.
Haase, E.
1889. Zur Anatomie der Blattiden. Zool. Anz., vol. 12, pp. 169-172. [Translated by P. Bernhardt.]
1889a. Stinkdrüsen der Orthopteren. Sitzb. Ges. Naturf. Freunde, Berlin, No. 2, pp. 57-58. [Pertinent section translated by P. Bernhardt.]
Haber, V. R.
1919. Cockroach pests of Minnesota with special reference to the German cockroach. Univ. Minnesota Agr. Exp. Stat. Bull. No. 186, 16 pp.
1920. Oviposition by an evaniid, Evania appendigaster Linn. Canadian Ent., vol. 52, p. 248.
1920a. Oviposition by a cockroach, Periplaneta americana Linn. (Orth.). Ent. News, vol. 31, pp. 190-193.
1926. The food of the Carolina treefrog, Hyla cinerea Schneider. Journ. Comp. Psychol., vol. 6, pp. 189-220.
Hafez, M., and Afifi, A. M.
1956. Biological studies on the furniture cockroach Supella supellectilium Serv. in Egypt [Orthoptera: Blattidae]. Bull. Soc. Ent. Égypte, vol. 40, pp. 365-396.
Haines, T. W., and Palmer, E. C.
1955. Studies of the distribution and habitat of cockroaches in southwestern Georgia, 1952-1953. Amer. Journ. Trop. Med. Hyg., vol. 4, pp. 1131-1134.
Haldeman, S. S.
1847. [Distribution of Evania.] Amer. Assoc. Geol. and Nat. 1847, Emmons Amer. Journ. Sci. and Agr., vol. 6, pp. 211-212.
Hall, M. C.
1907. A study of some gregarines with especial reference to Hirmocystis rigida n. sp. Univ. Stud., Univ. Nebraska, vol. 7, pp. 149-174, 1 pl.
1916. Nematode parasites of mammals of the orders Rodentia, Lagomorpha, and Hyracoidea. Proc. U. S. Nat. Mus., vol. 50, pp. 1-258.
1929. Arthropods as intermediate hosts of helminths. Smithsonian Misc. Coll., vol. 81, No. 15, 77 pp.
Haller, G. de.
1955. L'isolement du symbiote intracellulaire de la blatte (B. germanica) (note preliminaire). Rev. Suisse Zool., vol. 62, pp. 164-170.
1955a. La symbiose bacterienne intracellulaire chez la blatte B. germanica. Arch. Sci., vol. 8, pp. 229-306.
Hammerschmidt, K. E.
1838. Helminthologische Beiträge. Isis (Oken), vol. 5, pp. 351-358.
1847. Beschreibung einiger Oxyuris-Arten. Naturwiss. Abh., Wien., vol. 1, pp. 279-288.
Hancock, G. L. R.
1926. A winter entomological visit to Central Brazil. Entomologist, vol. 59, pp. 49-52, 131-137.
Handlirsch, A.
1889. Ueber die Lebensweise von Dolichurus corniculus Spinola. Verh. Zool.-Bot. Ges. Wien., vol. 39, pp. 81-83.
Hanitsch, R.
1915. Malayan Blattidae. Journ. Roy. Asiatic Soc., Straits Branch, No. 69, pp. 17-178, 7 pls.
1928. Spolia mentawiensia. Bull. Raffles Mus. Singapore, Straits Settlements, No. 1, pp. 1-44.
1929. Fauna Sumatrensis. (Beitrag No. 63). Blattidae. Tijdschr. Ent., Amsterdam, vol. 72, pp. 263-302.
1931. Résultats scientifiques du voyage aux Indes Orientales Néerlandaises de LL. AA. RR. le Prince et la Princesse Léopold de Belgique. Blattidae. Mém. Mus. Roy. Hist. Nat. Belgique, hors sér., vol. 4, pp. 39-62, pl. 1.
1932. On some cave-dwelling blattids from Celebes. Tijdschr. Ent., Amsterdam, vol. 75, suppl., pp. 264-265.
1933. Notes by Dr. R. Hanitsch, Ph.D., on female cockroaches (Blattidae) which carry their young. Proc. Roy. Ent. Soc. London, vol. 8, pp. 18-19.
Hansens, E. J.
1949. Insects associated with dumps and their control. Public Health News, New Jersey State Health Dept., vol. 30, pp. 335-337.
1950. How to control insects in refuse dumps. Public Works, vol. 81, pp. 57-58.
Hardy, E.
1941. Colliery insect pests. Colliery Eng., vol. 18, pp. 156-157.
Harrison, A. D.
1955. Four new species of gregarines from mountain cockroaches of the Cape Peninsula. Ann. South African Mus., vol. 41 pp. 387-405.
Harrison, Ruth M.
1906. Preliminary account of a new organ in Periplaneta orientalis. Quart. Journ. Micr. Sci., n.s., vol. 50, pp. 377-382.
Hartnack, H.
1939. 202 common household pests of North America. 319 pp. Chicago.
Harz, K.
1956. Ablage der Oothek bei Ectobius sylvestris (Poda). Aschaffenburg, Naturwiss. Mus., Nachr. 1956, No. 53, p. 51.
1957. Zur Biologie der Waldschabe Ectobius sylvestris (Poda). Nachr. Bayer. Ent., vol. 6, No. 3, pp. 30-31.
Hatch, M. H.
1938. Note on the Blattariae or cockroaches of western Washington. Pan-Pacific Ent., vol. 14, p. 120.
Hatcher, Elizabeth.
1939. The consortes of certain North Carolina blattids. Journ. Elisha Mitchell Sci. Soc., vol. 55, pp. 329-334.
Hauke, H. A.
1949. An annotated list of the Orthoptera of Nebraska. Part 1. The Blattidae, Mantidae and Phasmidae. Bull. Univ. Nebraska State Mus., vol. 3, pp. 63-75.
Hebard, M.
1915. Dermaptera and Orthoptera found in the vicinity of Miami, Florida, in March, 1915. Ent. News, vol. 26, pp. 397-408.
1916. Critical notes on certain species of Blaberus (Orthoptera, Blattidae). Ent. News, vol. 27, pp. 289-296.
1916a. A new genus, Cariblatta, of the group Blattellites (Orthoptera, Blattidae). Trans. Amer. Ent. Soc., vol. 42, pp. 147-186.
1916b. A new species of the genus Neoblattella from Costa Rica (Orthoptera, Blattidae). Ent. News, vol. 27, pp. 159-161.
1916c. Studies in the group Ischnopterites (Orthoptera, Blattidae, Pseudomopinae). Trans. Amer. Ent. Soc., vol. 42, pp. 337-383.
1917. The Blattidae of North America north of the Mexican boundary. Mem. Amer. Ent. Soc., No. 2, 284 pp., 10 pls.
1917a. A new species of myrmecophilous blattid (Orthoptera, Blattidae, Corydiinae). Ent. News, vol. 28, pp. 360-363.
1920. The Blattidae of Panama. Mem. Amer. Ent. Soc., No. 4, 148 + vi pp., 6 pls.
1921. South American Blattidae from the Museum National d'Histoire Naturelle, Paris, France. Proc. Acad. Nat. Sci. Philadelphia, vol. 73, pp. 193-304, 6 pls.
1921a. A note on Panamanian Blattidae, with the description of a new genus and two new species (Orth.). Ent. News, vol. 32, pp. 161-169.
1921b. Mexican records of Blattidae (Orthoptera). Trans. Amer. Ent. Soc., vol. 47, pp. 199-220.
1921c. Studies in the Dermaptera and Orthoptera of Colombia. Second paper. Trans. Amer. Ent. Soc., vol. 47, pp. 107-169.
1922. Dermaptera and Orthoptera of Hawaii. Occ. Pap., Bernice P. Bishop Mus., vol. 7, pp. 305-378.
1929. Studies in Malayan Blattidae (Orthoptera). Proc. Acad. Nat. Sci. Philadelphia, vol. 73, pp. 1-109, 6 pls.
1932. New species and records of Mexican Orthoptera. Trans. Amer. Ent. Soc., vol. 58, pp. 201-371.
1933. Dermaptera and Orthoptera from the Society Islands. Bernice P. Bishop Mus. Bull. No. 113, pp. 57-65. (Pacific Ent. Surv. Publ. 6, art. 11.)
1933a. The Dermaptera and Orthoptera of the Marquesas Islands. Bernice P. Bishop Mus. Bull. No. 114, pp. 105-140. (Pacific Ent. Surv. Publ. 7, art. 8.)
1943. Australian Blattidae of the subfamilies Chorisoneurinae and Ectobiinae (Orthoptera). Acad. Nat. Sci. Philadelphia Monogr. No. 4, 129 pp., 14 pls.
1943a. The Dermaptera and orthopterous families Blattidae, Mantidae, and Phasmidae of Texas. Trans. Amer. Ent. Soc., vol. 68, pp. 239-311.
Heer, O.
1864. Ueber die fossilen Kakerlaken. Naturf. Ges. Zürich, Vierteljahrssch., vol. 9, pp. 273-302, 1 pl.
Hegner, R.
1928. Experimental studies on the viability and transmission of Trichomonas hominis. Amer. Journ. Hyg., vol. 8, pp. 16-34.
1929. The viability of paramecia and euglenae in the digestive tract of cockroaches. Journ. Parasitol., vol. 15, pp. 272-275.
1936. College zoology. 4th ed. 742 pp. New York.
Hegner, R., and Chu, H. J.
1930. A survey of protozoa parasitic in plants and animals of the Philippine Islands. Philippine Journ. Sci., vol. 43, pp. 451-482.
Heimpel, A. M., and West, A. S.
1959. Notes on the pathogenicity of Serratia marcescens Bizio for the cockroach Blattella germanica L. Canadian Journ. Zool., vol. 37, pp. 169-172.
Heinecke, H.
1956. Über einen pathogenen Sporenbildner in der Haemolymphe von Blatta orientalis L. Zentralbl. Bakt. Parasitenk. Infekt., Abt. 2, vol. 109, pp. 524-535.
Heiser, V.
1936. An American doctor's odyssey. 544 pp. New York.
Henriksen, K. L.
1939. Fremmede gaester i vor orthopterfauna. Ent. Medd., København, vol. 20, pp. 195-211.
Herms, W. B.
1926. Hippelates flies and certain other pests of the Coachella Valley, California. Journ. Econ. Ent., vol. 19, pp. 692-695.
1939. Medical entomology. 3d ed., reprinted 1946, 582 pp. New York.
Herms, W. B., and Nelson, Y.
1913. The Croton bug (Ectobia germanica) as a factor in bacterial dissemination. Amer. Journ. Publ. Health, vol. 3, pp. 929-934.
Hertig, M.
1921. Attempts to cultivate the bacteroids of the Blattidae. Biol. Bull., vol. 41, pp. 181-187.
Hesse, A. J.
1942. The insect-food and Hymenopterous parasites of the South African poisonous "button spider," Latrodectus indistinctus Camb. Journ. Ent. Soc. South Africa, vol. 5, pp. 45-63.
Heymons, R.
1892. Die Entwicklung der weiblichen Geschlechtsorgane von Phyllodromia (Blatta) germanica L. Zeitschr. Wiss. Zool., vol. 53, pp. 434-536. [Pertinent sections translated by P. Bernhardt.]
1895. Die Embryonalentwickelung von Dermapteren und Orthopteren unter besonderer Berücksichtigung der Keimblätterbildung. 136 pp., 12 pls. Jena. [Pertinent sections translated by P. Bernhardt.]
Hingston, R. W. G.
1925. Nature at the desert's edge. Studies and observations in the Bagdad Oasis. 299 pp. Boston.
Hitchcock, A. S.
1936. Manual of the grasses of the West Indies. U. S. Dept. Agr. Misc. Publ. No. 243, 439 pp.
Hitchcock, C. R., and Bell, E. T.
1952. Studies on the nematode parasite, Gongylonema neoplasticum (Spiroptera neoplasticum), and avitaminosis A in the fore-stomach of rats: Comparison with Fibiger's results. Journ. Nat. Cancer Inst., vol. 12, pp. 1345-1387.
Hobmaier, M.
1941. Extramammalian phase of Physaloptera maxillaris Molin, 1860 (Nematoda). Journ. Parasitol., vol. 27, pp. 233-235.
Hoffman, G. L.
1952. A filamentous bacterium growing on two nematodes (Oxyuroidea: Theleostomidae) of the cockroach. Journ. Parasitol., vol. 38, p. 27. (Abstract.)
1953. Streptomyces leidynematis n. sp., growing on two species of nematodes of the cockroach. Trans. Amer. Micr. Soc., vol. 72, pp. 376-378.
Hoffman, W. A.
1927. Damage to potato by Pycnoscelus surinamensis. Journ. Econ. Ent., vol. 20, p. 237.
Hoffman, W. E.
1924. Biological notes on Lethocerus americanus (Leidy). Psyche, vol. 31, pp. 175-183.
Hollande, Andre.
1952. L'evolution des flagellés symbiotiques, hôtes du Cryptocercus et des termites inférieurs. Tijdschr. Ent., vol. 95, pp. 81-110.
Hollande, Augustin Charles.
1934. Contribution a l'etude cytologique des microbes (Coccus, Bacillus, Vibrio, Spirillum, Spirochaeta). Arch. Protistenk., vol. 83, pp. 465-608, 8 pls.
Hollande, A. C., and Favre, R.
1931. La structure cytologique de Blattabacterium cuenoti (Mercier) n. g., symbiote du tissu adipeux des blattides. Compt. Rend. Soc. Biol., Paris, sér. 3, vol. 107, pp. 752-754.
Hollande, A. C., and Hollande, G. (Mme.)
1946. La structure cytologique des bactéries et des cyanophycées. Arch. Zool. Exp. et Gén., vol. 84, pp. 375-441, 3 pls.
Honigberg, B. M.
1953. A transient infection of a salamander with flagellates of the wood-feeding roach, Cryptocercus punctulatus. Soc. Protozool., Proc. Ann. Meet., vol. 4, pp. 16-17. (Abstract.)
Hooker, C. B.
1874. The carnivorous habits of plants. Nature, London, vol. 10, pp. 366-372.
Hoover, S. C.
1945. Studies on the bacteroids of Cryptocercus punctulatus. Journ. Morph., vol. 76, pp. 213-225.
Hotchkiss, T.
1874. The ant's instinct. Sci. Amer., n. s., vol. 31, p. 132.
House, H. L.
1949. Nutritional studies with Blattella germanica (L.) reared under aseptic conditions. II. A chemically defined diet. Canadian Ent., vol. 81, pp. 105-112.
Hovasse, R.
1930. Bacillus cuenoti Mercier, bactéroide de Periplaneta orientalis, à la morphologie d'une bactérie. Arch. Zool. Exp. et Gén., vol. 70, pp. 93-96.
Howard, L. O.
1888. A commencement of a study of the parasites of cosmopolitan insects. Proc. Ent. Soc. Washington, vol. 1, pp. 118-136.
1892. The biology of the hymenopterous insects of the family Chalcididae. Proc. U. S. Nat. Mus., vol. 14, pp. 567-588.
Howes, P. G.
1917. Part III Entomological. In Beebe, W.; Hartley, G. I.; and Howes, P. G., Tropical wild life in British Guiana, pp. 371-450, 2 pls. New York Zoological Society.
1919. Insect behavior. 176 pp. Boston.
Hoyt, C. P. (See Dumbleton, L. J.)
Hsiang, C. M.; Pollard, M., and Micks, D. W.
1952. The possible role of the cockroach in the dissemination of poliomyelitis virus. Texas Rep. Biol. Med., vol. 10, pp. 329-335.
Hubbell, T. H.
1922. The Dermaptera and Orthoptera of Berrien County, Michigan. Occ. Pap. Mus. Zool. Univ. Michigan, No. 116, 77 pp.
Hubbell, T. H., and Goff, C. C.
1940. Florida pocket-gopher burrows and their arthropod inhabitants. Proc. Florida Acad. Sci., 1939, vol. 4, pp. 127-166.
Hughes, J. H.
1949. Aircraft and Public Health Service foreign quarantine entomology. Public Health Rep., suppl. 210, 38 pp.
Hull, G., Jr., and Davidson, R. H.
1958. The biology of the brown-banded cockroach and its relative susceptibility to five organic insecticides. Journ. Econ. Ent., vol. 51, pp. 608-610.
Hunter, W.
1906. The spread of plague infection by insects. Centralbl. Bakt., Parasitenk. Infekt., vol. 40, pp. 43-55.
Hurlbut, H. S.
1949. The recovery of poliomyelitis virus after parenteral introduction into cockroaches and houseflies. Naval Med. Res. Inst., Nat. Naval Med. Cent., Bethesda, Md., Rep. No. 8, Proj. NM 005 007, 4 pp.
1950. The recovery of poliomyelitis virus after parenteral introduction into cockroaches and houseflies. Journ. Infect. Dis., vol. 86, pp. 103-104.
Hutson, L. R.
1938. Some observations on Manson's eyeworm of poultry in Antigua, B.W.I., and a suggested method of control. Trop. Agr., Trinidad, vol. 15, pp. 66-68.
1943. Miscellaneous veterinary research in Antigua, British West Indies. Part 1. Studies on Manson's eyeworm of poultry. Ph.D. thesis, University of Toronto, 21 pp.
Hyman, Libbie H.
1951. The invertebrates: Platyhelminthes and Rhynchocoela. The acoelomate Bilateria. Vol. 2, 550 pp. New York.
1951a. The invertebrates: Acanthocephala, Aschelminthes, and Endoprocta. The pseudocoelomate Bilateria. Vol. 3, 572 pp. New York.
Illingworth, J. F.
1914. [Ant kills cockroach.] Proc. Hawaiian Ent. Soc., vol. 3, p. 85.
1915. Notes on Hawaiian cockroaches. Proc. Hawaiian Ent. Soc., vol. 3, pp. 136-140.
1916. A new cockroach to the Hawaiian Islands (Holocompsa fulva Burmeister). Proc. Hawaiian Ent. Soc., vol. 3, pp. 254-255.
1917. Clerada apicicornis sucking blood. Proc. Hawaiian Ent. Soc., vol. 3, pp. 274-276.
1927. A report on insects and other animal organisms collected in the pineapple growing section at Mauna Loa, Molokai, June, 1926. Proc. Hawaiian Ent. Soc., vol. 6, pp. 390-397.
1928. Insects collected in the pineapple growing section on the island of Lanai, August, 1927. Proc. Hawaiian Ent. Soc., vol. 7, pp. 42-46.
1929. Pests of pineapple in Hawaii. Proc. Hawaiian Ent. Soc., vol. 7, pp. 254-256.
1931. Manson's eyeworm distributed by English sparrows. Proc. Hawaiian Ent. Soc., vol. 7, p. 461.
1941. Feeding habits of Bufo marinus. Proc. Hawaiian Ent. Soc., vol. 11, p. 51.
1942. An outbreak of cockroaches, Nauphoeta cinerea (Olivier), in Honolulu. Proc. Hawaiian Ent. Soc., vol. 11, pp. 169-170.
Ivanić, M.
1926. Zur Kenntnis der Entwicklungsgeschichte von Coelosporidium periplanetae (Lutz u. Splendore). Arch. Protistenk., vol. 56, pp. 63-89, 2 pls.
1926a. Ispitivańa o slabodnim i parazitskim amebama. VII. Prilozi za poznavańe istoriye razvića entamebe iz tsreva buba-shvabe (Entamoeba blattae Bütschli). (Untersuchungen über die freilebenden und parasitischen Amoeben. VII. Beiträge zur Kenntnis der Entwicklungsgeschichte von Entamoeba blattae (Bütschli)). Glasnik Tsentral. Khig. Zavoda, Beograd, year 1, 1(1-4), pp. 162-201, pls. 1-4. German summary, pp. 194-198.
1937. Körperbau, Ernährung und Vermehrung einer im Enddarme der Küchenschabe [Blatta (Periplaneta, Stylopyga) orientalis L.] lebenden Hartmannella-Art (Hartmannella blattae spec. nov.). Arch. Protistenk., vol. 88, pp. 339-352, 1 pl.
Jameson, A. P.
1920. The chromosome cycle of gregarines, with special reference to Diplocystis schneideri Kunstler. Quart. Journ. Micr. Sci., n.s., vol. 64, pp. 207-266, 4 pls.
Jangi, B. S.
1955. On the ecology of the centipedes Scolopendra morsitans L. (Scolopendridae) in Nagpur. Ent. Month. Mag., vol. 91, pp. 211-213.
Janicki, C.
1908. Contribuzione alla conoscenza di alcuni protozoi parassiti della Periplaneta orientalis. (Lophomonas blattarum Stein, L. striata Bütschli, Amoeba blattae Bütschli). Atti Reale Accad. Lincei, Roma. Rendic. Cl. Sc. Fis., Mat. e Nat., an. 305, 5th ser., vol. 17, pp. 140-151.
1909. Über Kern und Kernteilung bei Entamoeba blattae Bütschli. Biol. Centralbl., vol. 29, pp. 381-393.
1910. Untersuchungen an parasitischen Flagellaten. I. Teil. Lophomonas blattarum Stein, L. striata Bütschli. Zeitschr. Wiss. Zool., vol. 95, pp. 243-315, 5 pls. [Pertinent section translated by P. Bernhardt.]
Janssen, W. A., and Wedberg, S. E.
1952. The common house roach, Blattella germanica Linn., as a potential vector of Salmonella typhimurium and Salmonella typhosa. Amer. Journ. Trop. Med. Hyg., vol. 1, pp. 337-343.
Javelly, E.
1914. Les corps bactéroides de la blatte (Periplaneta orientalis) n'ont pas encore été cultivés. Compt. Rend. Soc. Biol., Paris, vol. 77, pp. 413-414.
Jefferson, G. T.
1958. A white-eyed mutant form of the American cockroach, Periplaneta americana (L.) Nature, London, vol. 182, p. 892.
Jefferys, T.
1760. The natural and civil history of the French dominions in North and South America. Part II. Containing part of the islands of St. Domingo and St. Martin, St. Bartholomew, Guadaloupe, Martinico, La Grenade, and the island and colony of Cayenne. 246 pp. London.
Jettmar, H. M.
1927. Beiträge zum Studium der Pest unter den Insekten. II. Mitt. Zeitschr. Hyg., vol. 107, pp. 498-509. [Pertinent sections translated by P. Bernhardt.]
1935. Küchenschaben als Krankheitsüberträger. Wiener Klin. Wochenschr., vol. 48, pp. 700-704. [Pertinent sections translated by P. Bernhardt.]
Johnson, C. G., and Mellanby, K.
1939. Bed-bugs and cockroaches. Proc. Roy. Ent. Soc. London, vol. 14, p. 50.
Johnson, R. A.
1954. The behavior of birds attending army ant raids on Barro Colorado Island, Panama Canal Zone. Proc. Linn. Soc. New York, 1951-1953, Nos. 63-65, pp. 41-70.
Johnston, J. R.
1915. The entomogenous fungi of Porto Rico. Board of Comm. Agr., Río Piedras, Puerto Rico, Bull. No. 10, 33 pp. 9 pls.
Jolivet, P.
1950. Fluctuations dans une population de blattes et de grillons. Entomologiste, Paris, vol. 6, p. 139.
Jones, J. M.
1859. The naturalist in Bermuda. 200 pp. London.
Jordan, A. M.
1956. A note on the parasitisation of the oöthecae of Periplaneta americana (L.) by the chalcid, Syntomosphyrum glossinae Wtstn.—A correction. Bull. Ent. Res., vol. 47, pp. 683-684.
Joyeux, C. E.
1920. Cycle évolutif de quelques cestodes. Recherches Experimentales. Bull. Biol. France et Belgique, Suppl. 2, 219 pp., 7 pls.
Joyeux, C. E., and Baer, J. G.
1936. Helminthes des rats de Madagascar. Contribution à l'étude de Davainea madagascariensis (Dav., 1869). Bull. Soc. Path. Exot., Paris, vol. 29, pp. 611-619.
Judd, W. W.
1955. Systellogaster ovivora Gahan (Hymenoptera: Pteromalidae) reared from egg capsules of the wood-roach, Parcoblatta pensylvanica (DeGeer), collected at Rondeau Park, Ontario. Canadian Ent., vol. 87, pp. 98-99.
Jung, R. C., and Shaffer, M. F.
1952. Survival of ingested Salmonella in the cockroach Periplaneta americana. Amer. Journ. Trop. Med. Hyg., vol. 1, pp. 990-998.
Kadocsa, G.
1921. A csótányokról. [On cockroaches.] Allattani Közlemények, Budapest, vol. 20, pp. 27-37. (Abstract in Rev. Appl. Ent., 1923, vol. B11, p. 87.) [Pertinent sections translated by G. Susich.]
Kamo, T.
1957. On the habits of a cockroach hunting wasp (Ampulex amoena Stål) in Japan. (In Japanese with English summary.) Kontyû, vol. 25, pp. 94-98.
Karny, H. H.
1924. Beiträge zur Malayischen Orthopterenfauna. V. Bemerkungen ueber einige Blattoiden. Treubia, vol. 5, pp. 3-19. [Extract translated by P. Bernhardt.]
1925. Een en ander over kakkerlakken (Blattoidea). De Trop. Natuur, No. 12, pp. 185-192, 1 pl. [Pertinent portions translated by J. H. Vanderbie.]
Keck, C. B.
1951. [Comperia falsicornis at Pearl Harbor.] Proc. Hawaiian Ent. Soc., vol. 14, p. 207.
Keegan, H. L.
1944. On a new genus and species of parasitid mite. Journ. Parasitol., vol. 30, pp. 181-183.
Keller, H.
1950. Die Kultur der intrazellularen Symbionten von Periplaneta orientalis. Zeitschr. Naturf., vol. 5b, pp. 269-273. [Pertinent sections translated by P. Bernhardt.]
Kellogg, V. L.
1908. American insects. 694 pp. New York.
Ketchel, M. M., and Williams, C. M.
1953. Isolation of the intracellular symbiont of the roach. Anat. Rec., vol. 117, p. 650. (Abstract.)
Kevan, D. K. McE.
1952. A summary of the recorded distribution of British orthopteroids. Trans. Soc. British Ent., vol. 11, pp. 165-180.
Kevan, D. K. McE., and Chopard, L.
1954. Blattodea from Northern Kenya and Jubaland. Ann. and Mag. Nat. Hist., ser. 12, vol. 7, pp. 166-187.
Kidder, G. W.
1937. The intestinal protozoa of the wood-feeding roach Panesthia. Parasitology, vol. 29, pp. 163-205.
1938. Nuclear reorganization without cell division in Paraclevelandia simplex (Family Clevelandellidae), endocommensal ciliate of the wood-feeding roach, Panesthia. Arch. Protistenk., vol. 91, pp. 69-77.
Kieffer, J. J.
1903. Les evaniides. In André, E., Species des Hyménoptères d'Europe et d'Algérie, vol. 7 bis, pp. 347-469. Paris.
1912. Das Tierreich. Evaniidae. 431 pp. Berlin.
1920. Hymenoptera. Fam. Evaniidae. Genera insectorum, Fasc. 2, pp. 1-13.
Kingsley, C.
1870. Cockroaches. Nature, London, vol. 3, p. 148.
Kirby, H.
1927. Studies on some Amoebae from the termite Mirotermes, with notes on some other protozoa from the Termitidae. Quart. Journ. Micr. Sci., n. s. (282), vol. 71, pp. 189-222, 5 pls.
1945. Entamoeba coli versus Endamoeba coli. Journ. Parasitol., vol. 31, pp. 177-184.
Kirby, W., and Spence, W.
1822. An introduction to entomology: or elements of the natural history of insects. 4th ed., vol. 1, 518 pp. London.
1826. An introduction to entomology: or elements of the natural history of insects. Vol. 4, 572 pp. London.
Kirby, W. F.
1904. A synonymic catalogue of Orthoptera. London.
Klein, H. Z.
1933. Zur Biologie der amerikanischen Schabe (Periplaneta americana L.) Zeitschr. Wiss. Zool., vol. 144, pp. 102-122.
Klingmüller, V.
1930. Die Lepra. Handb. Haut-und Geschlechtskrank., Berlin, vol. 10, pp. 1-805.
Knipling, E. B., and Sullivan, W. N.
1957. Insect mortality at low temperature. Journ. Econ. Ent., vol. 50, pp. 368-369.
Kobayashi, H.
1927. On the life-history of the Oxyspirura mansoni and pathological changes in the conjunctiva and the ductus lacrymalis caused by this worm. Trans. Japanese Path. Soc., vol. 17, pp. 239-242.
Koch, A.
1949. Die Bakteriensymbiose der Küchenschaben. Mikrokosmos, vol. 38, pp. 121-126. [Translated by H. L. Middleton.]
Kohl, F. F.
1893. Ueber Ampulex Jur. (s. l.) und die damit enger verwandten Hymenopteren-Gattungen. Ann. K. K. Naturhist. Hofmus., Wien, vol. 8, pp. 455-516.
1902. Die Hymenopterengruppe der Sphecinen. II. Monographie der neotropischen Gattung Podium Fabr. Abh. K. K. Zool. Bot. Ges. Wien, vol. 1, pp. 1-101.
Kohls, G. M., and Jellison, W. L.
1948. Ectoparasites and other arthropods occurring in Texas bat caves. Bull. Nat. Speleological Soc., No. 10, pp. 116-117.
Kohriba, O.
1957. Some biological notes on Ampulex amoena Stål. (In Japanese with English summary.) Kontyû, vol. 25, pp. 99-101.
Konček, S. K.
1924. Zur Histologie der Rückendrüse unserer einheimischen Blattiden. Zeitschr. Wiss. Zool., vol. 122, pp. 311-322.
Krombein, K. V.
1951. Family Ampulicidae. In Muesebeck, C.F.W.; Krombein, K. V.; Townes, H. K.; and others, U. S. Dept. Agr. Agr. Monogr. No. 2, pp. 937-938.
1955. Miscellaneous prey records of solitary wasps. I. (Hymenoptera: Aculeata). Bull. Brooklyn Ent. Soc., vol. 50, pp. 13-17.
Krombein, K. V., and Evans, H. E.
1955. An annotated list of wasps collected in Florida, March 20 to April 3, 1954. (Hymenoptera, Aculeata.) Proc. Ent. Soc. Washington, vol. 57, pp. 223-235.
Kruse, C. W.
1948. Roach control. Soap San. Chem., vol. 24, No. 11, pp. 131, 133, 135, 137, 139, 169.
Kudo, R. R.
1922. The parasitic Protozoa of Blatta orientalis and their value as the material for use in a class of parasitic Protozoa. Anat. Rec., vol. 23, p. 113. (Abstract.)
1925. A study of Lophomonas blattarum, a flagellate inhabitant in the colon of Blatta orientalis. Anat. Rec., vol. 31, pp. 308-309. (Abstract.)
1925a. Division of Endamoeba blattae. Anat. Rec., vol. 31, p. 309. (Abstract.)
1926. Observations on Lophomonas blattarum, a flagellate inhabiting the colon of the cockroach, Blatta orientalis. Arch. Protistenk., vol. 53, pp. 191-214, 2 pls.
1926a. Observations on Endamoeba blattae. Amer. Journ. Hyg., vol. 6, pp. 139-152, 4 pls.
1926b. A cytological study of Lophomonas striata Bütschli. Arch. Protistenk., vol. 55, pp. 504-515, 2 pls.
1936. Studies on Nyctotherus ovalis Leidy, with special reference to its nuclear structure. Arch. Protistenk., vol. 87, pp. 10-42, 3 pls.
1954. Protozoology. 4th ed., 968 pp. Springfield, Ill.
Kudo, R. R., and Meglitsch, P. A.
1938. On Balantidium praenucleatum n. sp., inhabiting the colon of Blatta orientalis. Arch. Protistenk., vol. 91, pp. 111-124.
Kul'vets, K. V.
1898. The cuticular glands of Orthoptera and Hemiptera-Heteroptera. (In Russian.) Raboty iz Lab. Zool. Kab. Imper. Varshanskago Univ., 1897, pp. 49-82. (Same article abstracted in Zool. Anz., 1898, vol. 21, pp. 66-70, and Zool. Centralbl., 1899, vol. 6, p. 90.) [Translated by D. Kraus.]
Kunstler, J.
1884. Sur une forme aberrante du phylum Sporozoa. Compt. Rend. Acad. Sci., Paris, vol. 98, pp. 633-634.
1887. Diplocystis schneideri (nov. gen. nov. sp.). Tabl. Zool. Poitiers, vol. 2, pp. 25-66, 1 pl.
Kunstler, J., and Gineste, C.
1906. Spirillum periplaneticum, nov. spec. Compt. Rend. Soc. Biol., Paris, vol. 61, p. 135.
Küster, H. A.
1902. Ueber den Durchgang von Bakterien durch den Insektendarm. Inauguraldissertation, Heidelberg. [From Jettmar, H. M., 1935.]
1903. Die Uebertragung bakterieller Infektionen durch Insekten. Centralbl. Bakt., Parasitol. u. Infekt., vol. 33, pp. 90-94.
Laing, F.
1946. The cockroach, its life-history and how to deal with it. 5th ed. British Mus. (Nat. Hist.), Economic Ser., No. 12, 28 pp.
Laird, M.
1951. Insects collected from aircraft arriving in New Zealand from abroad. Zool. Publ. Victoria Univ. Coll., No. 11, 30 pp.
1952. Insects collected from aircraft arriving in New Zealand during 1951. Journ. Aviation Med., vol. 23, pp. 280-285.
1956. Intestinal flagellates from some New Zealand insects. Trans. Roy. Soc. New Zealand, vol. 84, pp. 297-308.
1956a. Wartime collections of insects from aircraft at Whenuapai. New Zealand Journ. Sci. and Techn., B, Gen. Res. Sect., vol. 38, pp. 76-84.
Lamborn, W. A.
1940. Annual report of the medical entomologist for 1939. Ann. Med. Sanit. Rep. Nyasaland, 1939, pp. 26-31.
Landowski, J.
1937. Influence of isolation and co-habitation on the development and growth of the larvae of Periplaneta orientalis L. (In Polish.) Sprawoz. Towarz. Nauk. Warszaw., Cl. IV, vol. 30, pp. 190-203. [Translated by D. Kraus.] [German summary in Biol. Zentralbl., 1938, vol. 58, pp. 512-515.]
Lankester, E. R.
1863. On our present knowledge of the Gregarinidae, with descriptions of three new species belonging to that class. Quart. Journ. Micr. Sci., n. s., vol. 3, pp. 83-96, 1 pl.
1865. The parasites of the cockroach. Intellectual Observer: Rev. Nat. Hist., Micros. Res. and Recreative Sci., London, vol. 6, pp. 337-343, 1 pl.
La Rivers, I.
1949. Entomic nematode literature from 1926 to 1946, exclusive of medical and veterinary titles. Wasmann Coll., vol. 7, pp. 177-206.
Laurentiaux, D.
1951. La problème des blattes Paléozoïques à ovipositeur externe. Ann. de Paléontol., vol. 37, pp. 185-196, 2 pls.
Lavagne, H.
1914. Note rectificative sur les moeurs de Zeuxevania splendidula Costa (Hym. Evaniidae). Bull. Soc. Ent. France, 1914, pp. 362-363.
Laveran, A., and Franchini, G.
1920. Contribution à l'étude des flagelles des culicides, des muscides, des phlébotomes et de la blatte orientale. Bull. Soc. Path. Exot., Paris, vol. 13, pp. 138-147.
1920a. Herpetomonas et Spirochaeta de la blatte orientale. Bull. Soc. Path. Exot., Paris, vol. 13, pp. 331-333.
Lawrence, G. H. M.
1951. Taxonomy of vascular plants. 2d printing, 1955. 823 pp. New York.
Lawson, F.
1954. Structural features of cockroach egg capsules IV. The oötheca of Parcoblatta uhleriana (Orthoptera: Blattidae). Journ. Kansas Ent. Soc., vol. 27, pp. 14-20.
1954a. Observations on the biology of Comperia merceti (Compere). (Hymenoptera: Encyrtidae). Journ. Kansas Ent. Soc., vol. 27, pp. 128-142.
Leclercq, J.
1954. Monographie systèmatic, phylogénetique et zoogéographique des Hyménoptères Crabroniens. 371 pp. Liège.
Lederer, G.
1952. Ein Beitrag zur Ökologie der amerikanischen Schabe Periplaneta americana (Linné 1758). Anz. Schadlingsk., vol. 25, pp. 102-104. [Translated by H. L. Middleton.]
Ledoux, A.
1945. Étude expérimentale du grégarisme et de l'intérattraction sociale chez les blattides. Ann. Sci. Nat. Zool., sér. 7, vol. 11, pp. 75-104. [Translated by Ruth V. Judson.]
Lee, D. L.
1958. On the morphology of the male, female and fourth-stage larva (female) of Hammerschmidtiella diesingi (Hammerschmidt), a nematode parasitic in cockroaches. Parasitology, vol. 48, pp. 433-436.
1958a. Digestion in Leidynema appendiculata (Leidy, 1850), a nematode parasitic in cockroaches. Parasitology, vol. 48, pp. 437-447.
Lee, P. E., and Mackerras, I. M.
1955. Salmonella infections of Australian native animals. Australian Journ. Exp. Biol. Med. Sci., vol. 33, pp. 117-125.
Lee, S. H.
1955. The mode of egg dispersal in Physaloptera phrynosoma Ortlepp (Nematoda: Spiruroidea), a gastric nematode of Texas horned toads, Phrynosoma cornutum. Journ. Parasitol., vol. 41, pp. 70-74.
Léger, L.
1909. Sur un mycétozoaire nouveau endoparasite des insectes. Compt. Rend. Acad. Sci., Paris, vol. 149, pp. 239-240.
Leibovitz, A.
1951. The cockroach, Periplaneta americana, as a vector of pathogenic organisms. I. The acid-fast organisms; a preliminary report. Bull. Ofic. San. Panamer., vol. 30, pp. 30-41.
Leidy, J.
1849. [Some new genera and species of Entozoa.] Proc. Acad. Nat. Sci. Philadelphia, 1848-49, vol. 4, pp. 229-233.
1850. Two new species of infusorial Entozoa. Proc. Acad. Nat. Sci. Philadelphia, 1850-51, vol. 5, p. 100.
1850a. Description of some nematoid Entozoa infesting insects. Proc. Acad. Nat. Sci. Philadelphia, 1850-51, vol. 5, pp. 100-102.
1851. Contribution to helminthology. Proc. Acad. Nat. Sci. Philadelphia, 1850-51, vol. 5, pp. 205-209.
1851a. Corrections and additions to former papers on helminthology published in the Proceedings of the Academy. Proc. Acad. Nat. Sci. Philadelphia, 1850-51, vol. 5, pp. 284-290.
1853. A flora and fauna within living animals. Smithsonian Contr. Knowl., vol. 5, Art. 2, pp. 1-67, 10 pls.
1853a. On the organization of the genus Gregarina of Dufour. Trans. Amer. Philos. Soc., n. s., vol. 10, pp. 233-240, 2 pls.
1853b. Some observations on Nematoidea imperfecta, and descriptions of three parasitic Infusoriae. Trans. Amer. Philos. Soc., n. s., vol. 10, pp. 241-244, 1 pl.
1879. Notices of Gordius in the cockroach and leech. Proc. Acad. Nat. Sci. Philadelphia, vol. 30, pp. 383-384. (Abstract.)
1879a. On Amoeba blattae. Proc. Acad. Nat. Sci. Philadelphia, vol. 31, pp. 204-205. (Abstract.)
1880. Endamoeba blatta. Amer. Month. Micr. Journ., vol. 1, p. 17.
Leiper, R. T.
1926. Gongylonema larvae in cockroach. Trans. Roy. Soc. Trop. Med. Hyg., vol. 19, p. 279.
Leleup, N.
1956. La faune cavernicole du Congo Belge et considerations sur les Coleoptères reliques d'Afrique intertropicale. Ann. Mus. Roy. Congo Belge, Tervuren (Belgique), sér. in-8°, Zool., vol. 46, 171 pp., 5 pls.
Leng, C. W.
1920. Catalogue of the Coleoptera of America, North of Mexico. 470 pp. Mount Vernon, N. Y.
Leonard, M. D.
1933. Notes on the giant toad, Bufo marinus (L.), in Puerto Rico. Journ. Econ. Ent., vol. 26, pp. 67-71.
Leprosy Research Department, School of Tropical Medicine, Calcutta.
1948. Cockroaches and transmission of leprosy. Leprosy in India, vol. 20, pp. 197-198.
Lever, R. J. A. W.
1939. Entomological notes. Agr. Journ., Fiji, vol. 10, pp. 17-20.
1943. Entomological notes. Agr. Journ., Fiji, vol. 14, pp. 40-44.
1946. Entomological notes. 6. Additions and corrections to insect past records. Agr. Journ., Fiji, vol. 17, pp. 11-12.
1947. Insect pests of some economic crops in Fiji. No. 2. Bull. Ent. Res., vol. 38, pp. 137-143.
Lewis, R. H.
1836. Notes made during a voyage from England to Van Diemen's Land, with a sketch of the entomology of the Cape of Good Hope. Trans. Ent. Soc. London, Journ. of Proc., vol. 1, pp. lxxix-lxxxi.
Lhéritier, G.
1951. Blattes et grillons. Entomologiste, Paris, vol. 7, pp. 29-30.
Lherminier (Monsieur).
1837. Observations sur les habitudes des insectes de la Guadeloupe. Ann. Soc. Ent. France, vol. 6, pp. 497-513.
Liang, C.
1956. The dorsal glands and ventral glands of Periplaneta americana (L.) (Blattidae, Orthoptera). Ann. Ent. Soc. Amer., vol. 49, pp. 548-551.
Lillingston, C.
1934. Our parasites. Hygeia, vol. 12, pp. 516-518, 520.
Lima Ribeiro, Josina.
1924. Morphologie et cycle évolutif du Nictotherus ovalis Leidy. Bull. Soc. Portugaise Sci. Nat., 1922-1924, vol. 9, pp. 91-96.
Lindberg, K.
1954. Notes sur quelques grottes de la Turquie avec liste des Cyclopides, Blattides, Gryllacrides et Lépidoptères récoltés dans ces grottes. Ann. Spéléologie, vol. 9, pp. 1-9.
Linstow, O. F. B. von.
1878. Compendium der Helminthologie. 382 pp. Hannover.
1889. Compendium der Helminthologie. Nachtrag. Die Litterature der Jahre 1878-1889. 151 pp. Hannover.
Lloyd, F. E.
1942. The carnivorous plants. 352 pp. Waltham, Mass.
Lodder, J.
1934. Die hefesammlung des Centraal-Bureau voor Schimmel-cultures. Beiträge zu einer monographie der hefearten. II Teil. Die Anaskosporogenen Hefen. Erste Hälfte. Verh. K. Akad. Wet. Amsterdam, ser. 2, vol. 32, pp. 1-256.
Lom, J.
1956. Experiments with the cultivation of our three species of the genus Nyctotherus and of Balantidium entozoon and B. coli. Vestnik Cs. Zool. Spol., vol. 20, pp. 16-61, 1 pl.
Longfellow, R. C.
1913. The common house roach as a carrier of disease. Amer. Journ. Publ. Health, vol. 3, pp. 58-61.
Lorando, N. T.
1929. A biological method for destroying bedbugs. Sci. Month., vol. 29, pp. 265-268.
Lorenc, W.
1939. Badania nad wiciowcami z radzaju Lophomonas: 1. Hodowanie Lophomonas blattarum Stein poza organizmem zywiciela, 2. Geneza axostylu u Lophomonas blattarum Stein i Lophomonas striata Bütschli.—[Untersuchungen an Flagellaten aus dem Genus Lophomonas: 1. Zucht von Lophomonas blattarum Stein ausserhalb des Wirtstieres, 2. Genese des Achsenstabes bei Lophomonas blattarum Stein und Lophomonas striata Bütschli.] Zoologica Poloniae, vol. 3, pp. 225-250, 2 pls. German summary pp. 244-246.
Loveridge, A.
1923. Notes on East African insects collected 1915-1922. Proc. Zool. Soc. London, 1923, pp. 1013-1041.
Lucas, Catherine L. T.
1927. The intestinal amoebae of the cockroach. Journ. Parasitol., vol 13, p. 220.
1927a. Two new species of amoeba found in cockroaches: with notes on the cysts of Nyctotherus ovalis Leidy. Parasitology, vol. 19, pp. 223-235.
1928. A study of excystation in Nyctotherus ovalis. With notes on other intestinal protozoa of the cockroach. Journ. Parasitol., vol. 14, pp. 161-175.
Lucas, H.
1849. Histoire naturelle des animaux articulés. Troisieme partie. Insectes. 527 pp. In Exploration scientifique de l'Algerie pendant les années 1840, 1841, 1842. Paris.
1862. Note sur la Perisphoera glomeriformis. Ann. Soc. Ent. France, sér. 4, vol. 2, p. 130.
1879. Une note relative à la vie évolutive d'un Hyménoptère du genre Chlorion Fabricius, sous-genre Ampulex Jurine. Ann. Soc. Ent. France, sér. 5, vol. 9, p. CLIX.
Lucas, W. J.
1896. Notes on Orthoptera. Entomologist, vol. 29, pp. 366-367.
1908. Orthoptera in 1907. Entomologist, vol. 41, pp. 186-188.
1911. Notes on British Orthoptera in 1910. Entomologist, vol. 44, pp. 208-211.
1912. British Orthoptera in 1911. Entomologist, vol. 45, pp. 114-117.
1916. British Orthoptera in 1914. Entomologist, vol. 49, pp. 16-19.
1918. British Orthoptera in 1917. Entomologist, vol. 51, pp. 229-231.
1920. British Orthoptera. 264 pp. London.
1920a. Notes on British Orthoptera, 1919. Entomologist, vol. 53, pp. 128-131.
1922. Notes on British Orthoptera in 1921. Entomologist, vol. 55, pp. 200-203.
1923. Leucophaea surinamensis Linn., etc. (Orthoptera). Entomologist, vol. 56, p. 141.
1925. Notes on British Orthoptera (including Dermaptera) in 1924. Entomologist, vol. 58, pp. 81-86.
1927. Notes on British Orthoptera (including Dermaptera) in 1926. Entomologist, vol. 60, pp. 265-268.
1930. Notes on British Orthoptera in 1929. Entomologist, vol. 63, pp. 145-147.
Lucker, J. T.
1932. Some cross transmission experiments with Gongylonema of ruminant origin. Journ. Parasitol., vol. 19, pp. 134-141.
Lutz, A., and Splendore, A.
1903. Ueber Pebrine und verwandte Mikrosporidien. Ein Beitrag zur Kenntnis der brasilianischen Sporozeen. Centralbl. Bakt., Abt. 1 Orig., vol. 33, pp. 150-157.
Lwoff, A.
1923. Nature et position systématique du bactéroïde des blattes. Compt. Rend. Soc. Biol., Paris, vol. 89, pp. 945-948.
Lyon, S. R.
1951. More about scorpions. Pest Control, vol. 19, No. 9, pp. 11-12, 14.
MacDougall, R. S.
1925. Insect pests of 1924. Trans. Highland and Agr. Soc. Scotland, ser. 5, vol. 37, pp. 167-193.
Macfie, J. W. S.
1922. Observations on the rôle of cockroaches in disease. Ann. Trop. Med. Parasitol., vol. 16, pp. 441-448.
Mackerras, I. M., and Mackerras, M. J.
1949. An epidemic of infantile gastroenteritis in Queensland caused by Salmonella bovis-morbificans (Basenau). Journ. Hygiene, vol. 47, pp. 166-181.
Mackerras, I. M., and Pope, P.
1948. Experimental Salmonella infections in Australian cockroaches. Australian Journ. Exp. Biol. Med. Sci., vol. 26, pp. 465-470.
Mackerras, M. J., and Mackerras, I. M.
1948. Salmonella infections in Australian cockroaches. Australian Journ. Sci., vol. 10, p. 115.
MacNay, C. G.
1954. Summary of important insect infestations, occurrences, and damage in Canada in 1954. Ent. Soc. Ontario, Ann. Rep., vol 85, pp. 61-91.
Madel, W.
1942. Ueber Lebensweise, Schaden und Bekämpfung der in Häusern und Betrieben auftretenden Schaben. Prakt. Desinfektor, vol. 34, pp. 1-5.
Magalhães, P. S. de.
1898. Notes d'helminthologie brèsilienne. 7. Du Gigantorhyncus moniliformis Bremser chez le Mus decumanus Pallas et de sa larve chez Periplaneta americana Fabr. comme hôte intermédiare. Arch. Parasitol., Paris, vol. 1, pp. 361-368.
1900. Notes d'helminthologie brèsilienne. 10 [i.e., 11] Matériaux pour servir à l'histoire de la flore et de la faune parasitaire de la Periplaneta americana Fabricius.—Une Nouvelle espéce d'Oxyuris, O. Bulhõesi. Arch. Parasitol., Paris, vol. 3, pp. 34-69.
Main, H.
1924. A living example of a bird-eating spider. Proc. Ent. Soc. London, in Trans. Ent. Soc. London, 1924, pts. 1, 2, p. xvii.
1930. A living example of a large spider from Trinidad. Proc. Ent. Soc. London, vol. 5, pt. 2, p. 21.
Mains, E.B.
1940. Cordyceps species from British Honduras. Mycologia, vol. 32, pp. 16-22.
Maki, T.
1937. The generation number of Tetrastichus hagenowi Ratz., an egg parasite of cockroaches, at Taihoku. (In Japanese.) Kagaku No Taiwan, vol. 5, pp. 307-309. [Translated by Associated Technical Services.]
Mallis, A.
1954. Handbook of pest control. 2d ed., 1,068 pp. New York.
Maneval, H.
1928. Notes sur quelques Hyménoptères fouisseurs. Bull. Soc. Ent. France, 1928, pp. 29-32.
1932. Notes recueillies sur les Hyménoptères fouisseurs. Ann. Soc. Ent. France, vol. 101, pp. 85-110.
1939. Notes sur les Hyménoptères (6e Série). Ann. Soc. Ent. France, vol. 108, pp. 49-108.
Mani, M. S.
1936. A new encyrtid parasite (Chalcidoidea: Hymenoptera) of a cockroach from India. Rec. Indian Mus., vol. 38, pp. 131-132.
1938. Catalogue of Indian insects. Part 23. Chalcidoidea. 174 pp. Delhi, India.
Mann, W. M.
1911. Notes on the guests of some California ants. Psyche, vol. 18, pp. 27-31.
1914. Some myrmecophilous insects from Mexico. Psyche, vol. 21, pp. 171-184.
Mariani, G., and Besta, B.
1936. La blatta orientale serbatoio di protozoi ed elminti. Arch. Ital. Sci. Med. Colon. e Parassitol., vol. 17, pp. 177-183. [Pertinent sections translated by J. J. Buckley.]
Marlatt, C. L.
1902-1915. Cockroaches. U. S. Dept. Agr. Div. Entomol. Circ. No. 51, 2d ser., 15 pp. [Revised 1908 as Circ. 51. Revised 1915 as Farmers' Bull. No. 658.]
Marquis, D.
1931. Archy and Mehitabel. 196 pp. Garden City, N. Y.
Marshall, T. A.
1866. Hemiptera and Hymenoptera of Freshwater Bay, Pembrokeshire. Ent. Month. Mag., vol. 3, p. 92.
1878. Notes on the entomology of the Windward Islands. Proc. Ent. Soc. London, 1878, pp. xxvii-xxxviii.
Marshall, W. S.
1892. Beiträge zur Kenntnis der Gregarinen. Arch. Naturges., Berlin, 1893, 59 J., vol. 1, pp. 25-44, 1 pl.
Martini, E.
1952. Lehrbuch der medizinischen Entomologie. 4th rev. ed., 694 pp. Jena.
Martins, A. V.
1941. Infeccao experimental do "Triatoma arthurneivai" Lent e Martins, 1940, pelo Schizotrypanum cruzi. Brasil-Medico, vol. 55, p. 131.
Marx, G.
1892. Contributions to the knowledge of the life history of Arachnida. Proc. Ent. Soc. Washington, 1891, vol. 2, pp. 252-256.
1894. Continuation of the life-history of the whiptail scorpion. Proc. Ent. Soc. Washington, 1893, vol. 4, pp. 54-55.
Maxwell-Lefroy, H., assisted by Howlett, F. M.
1909. Indian insect life. A manual of the insects of the plains. (Tropical India.) 786 pp. Calcutta and Simla; London.
McAdow, Clarice M.
1931. Observations on some protozoa parasitic in cockroaches. M.A. thesis, Ohio State University.
McBurney, R., and Davis, H.
1930. Common cockroach as a host-carrier of Bacillus typhosus (preliminary report). Trans. Med. Assoc. Alabama, vol. 63, pp. 307-325.
McClure, H. E.
1936. An odd hibernaculum. Psyche, vol. 43, p. 19.
McCook, H.C.
1877. Mound-making ants of the Alleghenies, their architecture and habits. Trans. Amer. Ent. Soc., vol. 6, pp. 253-296, 6 pls.
McKeown, K. C.
1952. Australian spiders. 274 pp. Sydney-London.
McShan, Oleta B.
A preliminary study of the role of the cockroach in the dissemination of certain pathogenic fungi. (Unpublished manuscript, 1953.)
Meade-Waldo, G.
1910. Occurrence of Nyctibora sericea Burm., a West-Indian cockroach, in the Isle of Wight. Entomologist, vol. 43, p. 354.
Meech, W. W.
1889. The toad vs. cockroaches. Insect Life, vol. I, p. 341.
Meglitsch, P. A.
1938. Cytological observations on Endamoeba blattae from the cockroach, Blatta orientalis. Ph.D. thesis, University of Illinois, pp. 1-10. (Abstract.)
1940. Cytological observations on Endamoeba blattae. Illinois Biol. Monogr., vol. 17, pp. 1-146.
Mellanby, K.
1939. The physiology and activity of the bedbug (Cimex lectularius L.) in a natural infestation. Parasitology, vol. 31, pp. 200-211.
Mello, I. F. de, and Lima Ribeiro, Josina.
1934. Cytological studies on Nyctotherus ovalis with special reference to its morphological types. Proc. Indian Acad. Sci., Sect. B, vol. 1, pp. 249-257.
Mello, I. F. de, and Lima Ribeiro, Josina.
1924. Morphologie et cycle évolutif de Lophomonas blattarum Stein. Bull. Soc. Portugaise Sci. Nat., 1922-1924, vol. 9, pp. 74-80.
1925. Morphologie et phénomènes divisionnels de Lophomonas blattarum Stein. Arq. Indo-Portugaise Med. Hist. Nat., vol. 2, pp. 178-184, 5 pls.
Mencl, E.
1907. Eine Bermerkung zur Organisation der Periplaneta-symbioten. Arch. Protistenk., vol. 10, pp. 188-198, 1 pl. [Translated by Maria E. W. Torok.]
Mercet, R. G.
1921. Fauna Ibérica. Himenópteros. Fam. Encirtidos. 732 pp. Mus. Nac. Cien. Nat., Madrid.
1930. Algunos calcididos de Africa. Eos, vol. 6, pp. 221-228.
Mercier, L.
1906. Un organisme à forme levure parasite de la blatte (Periplaneta orientalis L.). Levure et Nosema. Compt. Rend. Soc. Biol., Paris, vol. 60, pp. 1081-1083.
1906a. Les corps bactéroides de la blatte (Periplaneta orientalis): Bacillus Cuenoti (n. sp. L. Mercier). Compt. Rend. Soc. Biol., Paris, vol. 61, pp. 682-684.
1907. Cellules à Bacillus Cuenoti dans la paroi des gaines ovariques de la blatte. Compt. Rend. Soc. Biol., Paris, vol. 62, pp. 758-759.
1907a. Un parasite du noyau d'Amoeba blattae Bütschli. Compt. Rend. Soc. Biol., Paris, vol. 62, pp. 1132-1134.
1907b. Sur la mitose des cellules à Bacillus cuenoti. Compt Rend. Acad. Sci., Paris, vol. 145, pp. 833-835.
1907c. Recherches sur les bactéroides des blattides. Arch. Protistenk., vol. 9, pp. 346-358.
1908. La schizogonie simple chez Amoeba blattae Bütschli. Compt. Rend. Acad. Sci., Paris, vol. 146, pp. 942-945.
1908a. Néoplasie du tissu adipeux chez des blattes (Periplaneta orientalis L.) parasitées par une microsporidie. Arch. Protistenk., vol. 11, pp. 372-381, 1 pl.
1909. Le cycle évolutif d'Amoeba blattae Bütschli. (Note préliminaire.) Arch. Protistenk., vol. 16, pp. 164-168.
1910. Contribution à l'étude de l'amibe de la blatte (Entamoeba blattae Bütschli). Arch. Protistenk., vol. 20, pp. 143-175, 3 pls.
Metcalf, R. L., and Patton, R. L.
1942. A study of riboflavin metabolism in the American roach by fluorescence microscopy. Journ. Cell. Comp. Physiol., vol. 19, pp. 373-376.
Meyer, A.
1931. Neue Acanthocephalen aus dem Berliner Museum. Begründung eines neuen Acanthocephalensystems auf Grund einer Untersuchung der Berliner Sammlung. Zool. Jahrb., vol. 62, pp. 53-108.
1932. Acanthocephala. Bronn's Klassen und Ordnungen des Tierreichs ... Vermes, Askhelminthen. Akad. Verlags., Leipzig, Bd. 4, Abt. 2, Buch 2, Lief. 1, pp. 1-332.
Meyer, B. S., and Anderson, D. B.
1939. Plant physiology. 696 pp. New York.
Miall, L. C., and Denny, A.
1886. The structure and life-history of the cockroach (Periplaneta orientalis). An introduction to the study of insects. 224 pp. London.
Michel, C.
1935. Destruction des moustiques et autre insectes à bord des aeroplanes. Off. Internat. Hyg. Publ., Bull. Mens., vol. 27, pp. 553-557. [Translated by Ruth V. Judson.]
Milovidov, P. F.
1928. À propos des bactéroïdes des blattes (Blattella germanica). Compt. Rend. Soc. Biol., Paris, vol. 99, pp. 127-128.
Milton, F.
1899. Local Orthoptera in 1899. Ent. Rec. and Journ. Var., vol. 11, p. 333.
Minchen, E. A.
1888. Note on a new organ, and on the structure of the hypodermis, in Periplaneta orientalis. Quart. Journ. Micr. Sci., vol. 29, pp. 229-233.
1890. Further observations on the dorsal gland in the abdomen of Periplaneta and its allies. Zool. Anz., vol. 13, pp. 41-44.
Möbius, K.
1855. Chordodes pilosus, ein Wurm aus der Familie der Gordiaceen. Zeitschr. Wiss. Zool., vol. 6, pp. 428-431, 1 pl.
Moffett, T.
1634. Insectorum sive minimorum animalium theatrum. 326 pp. Thomas Cotes, Londini. [Translation: 1658. The theater of insects: or, lesser living creatures. 889-1130 pp. London.]
Moiser, B.
1945. Modes of transmission of Hansen's disease (leprosy). Leprosy Rev., vol. 16, pp. 63-66.
1946. Leprosy: A new outlook. East African Med. Journ., vol. 23, pp. 295-300.
1946a. Transmission of Hansen's disease (leprosy). Acta Med. Scandinav., vol. 126, pp. 347-350.
1947. Hansen's disease (leprosy) and cockroaches. East African Med. Journ., vol. 24, pp. 230-236.
Monro, H. A. U.
1951. Insect pests in cargo ships. Canada Dept. Agr. Publ. No. 855, 45 pp.
Montgomery, T. H., Jr.
1898. The Gordiaceae of certain American collections with particular reference to the North American fauna. Bull. Mus. Comp. Zool., Harvard College, vol. 32, pp. 23-59.
Moore, C. E.
1957. Wood roaches in New England. Pest Control, vol. 25, No. 10, p. 6.
Moore, D. V.
1946. Studies on the life history and development of Moniliformis dubius Meyer, 1933. Journ. Parasitol., vol. 32, pp. 257-271.
Morischita, K., and Tsuchimochi, K.
1926. Experimental observations on the dissemination of diseases by cockroaches in Formosa. (In Japanese.) Taiwan Igakki Zasshi, Journ. Med. Assoc. Formosa, No. 255, pp. 566-599. English summary, pp. 2-6.
Morrell, C. C.
1911. The bacteriology of the cockroach. British Med. Journ., vol. 2, pp. 1531-1532.
Morris, S.
1935. Life cycle of Endamoeba blattae (Bütschli, 1878). Anat. Rec., vol. 64, Suppl. 1, p. 102. (Abstract.)
1936. Studies of Endamoeba blattae (Bütschli). Journ. Morph., vol. 59, pp. 225-263, 3 pls.
Morse, A. P.
1920. Manual of the Orthoptera of New England ... Proc. Boston Soc. Nat. Hist., vol. 35, pp. 197-556.
Moseley, H. N.
1892. Notes by a naturalist. An account of observations made during the voyage of H.M.S. Challenger round the world in the years 1872-1876. 540 pp. London.
Moulton, J. C.
1912. "Where Wallace trod": Being an account of an entomological trip to Mt. Serambu, Sarawak, Borneo. Entomologist, vol. 45, pp. 213-217, 246-251, 2 pls.
Muesebeck, C. F. W.
1958. Superfamily Proctotrupoidea. In Krombein, K. V., and others, Hymenoptera of America north of Mexico. Synoptic catalog. U.S. Dept. Agr. Agr. Monogr. No. 2, First suppl., pp. 88-94.
Murray, W. D.
1951. Tribe Podiini. In Muesebeck, C. F. W.; Krombein, K. V.; Townes, H. K., and others, Hymenoptera of America north of Mexico. U.S. Dept. Agr. Agr. Monogr. No. 2, pp. 979-980.
Myers, J. G.
1931. Observations on the insect food of the coati. Proc. Ent. Soc. London, vol. 5, pp. 69-74.
Nadson, G. A., and Filippov, G. S.
1925. Une nouvelle Mucorinée Mucor guilliermondii nov. sp. et ses formes levures. Rev. Gen. de Bot., vol. 37, pp. 450-461, 1 pl.
Nash, T. A. M.
1955. A note on the parasitisation of the oöthecae of Periplaneta americana (L.) by the chalcid, Syntomosphyrum glossinae Wtstn. Bull. Ent. Res., vol. 46, pp. 111-112.
Neill, P.
1829. Account of the habits of a specimen of the Simia jacchus Lin. or Jacchus vulgaris Geoff. now in the possession of Gavin Milroy, Esq., Edinburgh. Mag. Nat. Hist., vol. 1, pp. 18-20.
Neukomm, A.
1927. Sur la structure des bactéroïdes des blattes (Blattella germanica). Compt. Rend. Soc. Biol., Paris, vol. 96, pp. 306-308.
1927a. Action des rayons ultra-violets sur les bactéroïdes des blattes (Blattella germanica). Compt. Rend. Soc. Biol., Paris, vol. 96, pp. 1155-1156.
1932. Le réaction de la fixation du complément appliquée à l'étude des bactéroides des blattes (Blattella germanica). Compt. Rend. Soc. Biol., Paris, vol. III, pp. 928-929.
Neveu-Lemaire, M.
1933. Les arthropods hôtes intermédiaires de helminthes parasites de l'homme. (Suite.) Ann. Parasitol. Hum. et Comp., vol. 11, pp. 303-319.
1938. Traite d'entomologie médicale et vétérinaire. 1,339 pp. Paris.
Newman, E.
1855. A word for the cockroach. Proc. Ent. Soc. London, n. s., vol. 3, p. 77.
Nicewicz, N.; Nicewicz, W.; and Kowalik, R.
1946. Description of microorganisms supported on the bacteriological analysis in the alimentary tracts of the bed bug, house fly, and cockroach. (In Polish.) Ann. Univ. M. Curie Sklodowska Lublin, C, vol. 1, pp. 35-38. English summary. [Translated by D. Kraus.]
Nielsen, E. T.
1933. Sur les habitudes des Hyménoptères aculéates solitaires. III. (Sphegidae.) Ent. Medd. København, vol. 13, pp. 259-348.
Nielsen, J. C.
1903. Iagttagelser over nogle danske Gravehvespes Biologi. Ent. Medd., København, vol. 2, pp. 110-114.
Nigam, L. N.
1933. The life-history of a common cockroach (Periplaneta americana Linnaeus). Indian Journ. Agr. Sci., vol. 3, pp. 530-543.
Noble, G. K.
1918. The amphibians collected by the American Museum expedition to Nicaragua in 1916. Bull. Amer. Mus. Nat. Hist., vol. 38, pp. 311-347.
1924. Contributions to the herpetology of the Belgian Congo based on the collection of the American Museum Congo Expedition. Part III. Amphibia. Bull. Amer. Mus. Nat. Hist., vol. 49, pp. 147-347, 20 pls.
Noland, J. L., and Baumann, C. A.
1951. Protein requirements of the cockroach Blattella germanica (L.). Ann. Ent. Soc. Amer., vol. 44, pp. 184-188.
Noland, J. L.; Lilly, J. H.; and Baumann, C. A.
1949. A laboratory method for rearing cockroaches, and its application to dietary studies on the German roach. Ann. Ent. Soc. Amer., vol. 42, pp. 63-70.
Northrup, Z.
1914. A bacterial disease of the larvae of the June beetle Lachnosterna spp. Centralbl. Bakt., Parasitenk. Infekt., Abt. 2, vol. 41, pp. 321-339.
Nutting, W. L.
1953. A gregarine, Diplocystis, in the haemocoele of the roach, Blaberus craniifer Burm. Psyche, vol. 60, pp. 126-128.
Nutting, W. L., and Cleveland, L. R.
1954. Effects of transfaunation on the sexual cycles of the protozoa of the roach Cryptocercus. Anat. Rec., vol. 120, p. 747. (Abstract.)
1954a. Effects of reciprocal transfaunations on protozoa of the roach Cryptocercus and the termite Zootermopsis. Anat. Rec., vol. 120, p. 786. (Abstract.)
Oettinger, R.
1906. Über die Drüsentaschen am Abdomen von Periplaneta orientalis und Phyllodromia germanica. Zool. Anz., vol. 30, pp. 338-349.
Ohaus, F.
1900. Bericht über eine entomologische Reise nach Centralbrasilien. Ent. Zeitung, vol. 61, pp. 193-274.
Oliver, J. A.
1949. The peripatetic toad. Nat. Hist., New York, vol. 58, pp. 29-33.
Olson, T. A., and Rueger, M. D.
1950. Experimental transmission of Salmonella oranienburg through cockroaches. Publ. Health Rep., vol. 65, pp. 531-540.
Ostroumov, V. G.
1929. K Morfologii i biologii Nyctotherus ovalis Leidy. (Ueber den Bau und Biologie von Nyctotherus ovalis Leidy.) Russk. Arkh. Protistenk., vol. 8, pp. 25-50, 2 pls. German summary pp. 49-50.
Oswald, V. H.
1958. Studies on Rictularia coloradensis Hall, 1916 (Nematoda: Thelaziidae). 1. Larval development in the intermediate host. Trans. Amer. Micr. Soc., vol. 77, pp. 229-240.
Owen, W. L., and Mobley, R. L.
1948. A new species of Torulae occurring in and transmitted by the American cockroach Periplaneta americana (Linn.). Food Res., vol. 13, pp. 281-290.
Packard, A. S.
1888. The cave fauna of North America, with remarks on the anatomy of the brain and the origin of the blind species. Mem. Nat. Acad. Sci., vol. 4, pp. 3-156.
Pai, Kuo-tung, and Wang, Chia-Chi.
1947. The variation of Nyctotherus ovalis Leidy, and its fibrillar system. Sinensia, Shanghai, vol. 18, pp. 43-58.
Palmer, R.
1928. Cockroaches introduced with bananas. Entomologist, vol. 61, p. 19.
Parisi, B.
1910. Su alcuni flagellati endoparassiti. Arch. Protistenk., vol. 19, pp. 232-238, 1 pl.
Parker, Barbara M.
1939. Effects of liquid household insecticides on the oöthecae of the German cockroach. Ph.D. thesis, Ohio State University.
Parker, H. L.
1924. Recherches sur les formes post-embryonaires des chalcidiens. Ann. Soc. Ent. France, vol. 93, pp. 261-379, 39 pls.
Parker, H. L., and Thomson, W. R.
1928. Contribution à la biologie des chalcidiens entomophages. Ann. Soc. Ent. France, vol. 97, pp. 425-465.
Parrott, A. W.
1952. The banana spider (Heteropoda venatoria Linn.) recorded from New Zealand. New Zealand Sci. Rev., vol. 10, pp. 129-130.
Pasricha, C. L.; Chatterjee, D. N.; and Das, P. C.
1938. The distribution and characteristics of vibrios isolated from certain non-human sources in Calcutta. Indian Journ. Med. Res., vol. 26, pp. 33-37.
Passmore, L.
1936. Introducing the tarantula. Nature Mag., vol. 27, pp. 90-94.
Pate, V. S. L.
1949. A minute on Podium luctuosum (Hymenoptera: Sphecidae). Ent. News, vol. 60, p. 174.
Patton, W. S.
1931. Insects, ticks, mites and venomous animals of medical and veterinary importance. Part II. Public health. 740 pp. Croydon, England.
Paulian, R.
1950. Observations sur la faune entomologique des nids de Ploceinae. Proc. 8th Internat. Congr. Ent., Stockholm, 1948, pp. 454-456.
Pavlovskii, E. N.
1948. Handbook on the parasitology of man together with a treatise on the vectors of transmissible diseases. (In Russian.) 5th ed., vol. 2, pp. 527-1022. Moscow. [Pertinent sections translated by M. Ycas.]
Pearce, E. J.
1929. An introduced blattid. Ent. Month. Mag., vol. 65, p. 276.
Pearse, A. S.
1938. Insects from Yucatan caves. In Pearse, A. S., and others, Fauna of the caves of Yucatan. Carnegie Inst. Washington Monogr. No. 491, 304 pp.
Peck, O.
1951. Superfamily Chalcidoidea. In Muesebeck, C. F. W.; Krombein, K. V.; Townes, H. K.; and others, Hymenoptera of America north of Mexico. U.S. Dept. Agr. Agr. Monogr. No. 2, pp. 410-594.
Pemberton, C. E.
1917. Heteropoda regia. Proc. Hawaiian Ent. Soc., vol. 3, p. 273.
1934. Some future work for the entomologist in Hawaii. Proc. Hawaiian Ent. Soc., vol. 8, pp. 505-514.
1941. Tetrastichus hagenowii (Ratz.). Proc. Hawaiian Ent. Soc., vol. II, p. 19.
1942. Entomology. Hawaiian Sugar Planters' Assoc., R. 61, Exp. Stat. Rep., 1940/41, pp. 21-27.
1945. Entomology. Rep. Comm. Exp. Stat. Hawaiian Sugar Planters' Assoc., 1943/1944, pp. 17-21.
1945a. Ampulex compressa (Fabr.). Proc. Hawaiian Ent. Soc., vol. 12, p. 228.
1947. Cockroach parasite. Proc. Ent. Hawaiian Sugar Planters' Assoc., Exp. Stat. Rep., 1945/1946, pp. 22-26.
1953. The biological control of insects in Hawaii. Proc. 7th Pacific Sci. Congr., Pacific Sci. Assoc., Auckland and Christchurch, New Zealand, 1949, vol. 4 (Zoology), pp. 220-223.
Pemberton, C. E., and Williams, F. X.
1938. Some insect and other animal pests in Hawaii not under satisfactory biological control. Hawaiian Planters' Rec., vol. 42, pp. 211-230.
Pereira, C.
1935. Sobre um Lepidonemidae Trav., 1919 e urn Rhabdiasidae Raillet, 1915 (Nematoda) novos. Rev. Biol. Hyg., São Paulo, vol. 6, pp. 19-21.
Pérez Fontana, V.
1955. Ciclo colateral de la hidatidosis. Arch. Internac. Hidatidosis, Madrid, vol. 14, pp. 119-121.
Pérez Silva, J.
1954. La naturaleza de los simbiotes intracelulares de Blattella germanica. Microbiol. Españ., vol. 7, pp. 51-58, 2 pls.
1954a. Cultivo "in vitro" de simbiotes intracelulares de insectos. Microbiol. Españ., vol. 7, pp. 187-241, 9 pls. [Conclusions translated by J. J. Buckley.]
Perkins, R. C. L.
1899. Orthoptera. Part I. In Fauna Hawaiiensis, vol. 2, pp. 1-30. Cambridge, England.
1906. Insects at Kilauea, Hawaii. Proc. Hawaiian Ent. Soc., vol. 1, pp. 89-99.
1913. Introduction. Being a review of the land-fauna of Hawaii. In Fauna Hawaiiensis, vol. 1, pp. xv-ccxxviii. Cambridge, England.
Perrin, W. S.
1906. Preliminary communication on the life-history of Pleistophora periplanetae, Lutz and Splendore. Proc. Cambridge [England] Philos. Soc., vol. 13, pp. 204-208.
1906a. Notes on a hitherto undescribed parasite (?) of Periplaneta orientalis. Proc. Cambridge [England] Philos. Soc., vol. 13, p. 208.
1906b. Observations on the structure and life-history of Pleistophora periplanetae Lutz and Splendore. Quart. Journ. Micr. Sci., n. s., vol. 49, pp. 615-633, 2 pls.
Peshkoff, M. A.
1940. Phylogenesis of new microbes Caryophanon latum and Caryophanon tenue—organisms which are intermediate between blue-green algae and the bacteria. (In Russian with English summary.) Zhurn. Obsh. Biol., vol. 1, pp. 597-618, 7 pls.
Pessôa, S. B., and Corrêa, C.
1926. Notas sobre os Oxyurus parasitas das baratas domesticas, com a descripção de uma nova especie: Oxyurus australasiae n. sp. Mem. Inst. Butantan, São Paulo, vol. 3, pp. 71-74. (French summary, pp. 75-76.) [Summary in Rev. Biol. e Hyg., São Paulo, 1927, vol. 1, pp. 105-106.]
1927. Sobre a disseminação de cystos de Giardia intestinalis (Lambl.) pelas baratas. Rev. Biol. e Hyg., São Paulo, vol. 1, pp. 90-93. (Abstract in Trop. Dis. Bull., 1927, vol. 24, p. 871.)
1928. Nota sobre a biologia da Rhyparobia maderae Fabr. Rev. de Biol. e Hyg., São Paulo, vol. 1, No. 3, pp. 83-87. (English summary.)
1928. Nota sobre a biologia da Rhyparobia maderae, Fabr. Rev. Biol. e Sci. Med., Rio de Janeiro, vol. 7, pp. 304-305.
Petch, T.
1924. Studies in entomogenous fungi. IV. Some Ceylon Cordyceps. Trans. British Mycol. Soc., vol. 10, pp. 28-45, 1 pl.
Peterson, A.
1956. Fishing with natural insects. An angler's guide to useful and interesting information about many common insects and a few imitation lures that fishermen use for bait. 176 pp. Columbus, Ohio.
Petri, L. H.
1950. Life cycle of Physaloptera rara Hall and Wigdor, 1918 (Nematoda: Spiruroidea) with the cockroach, Blatella germanica, serving as the intermediate host. Trans. Kansas Acad. Sci., vol. 53, pp. 331-337.
Petri, L. H., and Ameel, D. J.
1950. Studies on the life cycle of Physaloptera rara Hall and Wigdor, 1918, and Physaloptera praeputialis Linstow, 1889. Journ. Parasitol., vol. 36, No. 6, sect. 2, p. 40.
Petrunkevitch, A.
1930. The spiders of Porto Rico. Part II. Trans. Connecticut Acad. Arts, Sci., vol. 30, pp. 159-355.
1930a. The spiders of Porto Rico. Part III. Trans. Connecticut Acad. Arts, Sci., vol. 31, pp. 1-191.
Pettit, L. C.
1940. The roach, Blattella germanica (Linn.): its embryogeny, life history, and importance. Ph.D. thesis, Cornell University, 121 pp., 2 pls.
1940a. The effect of isolation on growth in the cockroach Blattella germanica (L.) (Orthoptera Blattidae). Ent. News, vol. 51, p. 293.
Pfeiffer, H., and Stammer, H. J.
1931. Pathogenes Leuchten bei Insekten. Zeitschr. Morph. u. Ökol. Tiere, vol. 20, pp. 136-171.
Phelps, J. R.
1924. Eradication of vermin on board ship. U.S. Naval Med. Bull., vol. 20, pp. 247-268.
Picado, C.
1913. Les broméliacées épiphytes. Considérées comme milieu biologique (1). Bull. Sci. France et Belgique, vol. 47, pp. 215-360, pls. VI-XXIV.
Picard, F.
1911. Sur les moeurs et le genre de proie de l'Ampulex fasciatus Jurine (Hym. Sphegidae). Bull. Soc. Ent. France, 1911, No. 6, pp. 113-116.
1913. Sur le genre Zeuxevania Kieffer et sur les moeurs du Zeuxevania splendidula Costa. (Hym. Evaniidae). Bull. Soc. Ent. France, 1913, pp. 301-304.
1913a. Contribution à l'étude des Laboulbéniacées d'Europe et du nord de l'Afrique. Bull. Soc. Mycol. France, vol. 29, pp. 503-571, 4 pls.
1919. Contribution à l'étude du peuplement d'un vegetal. La faune entomologique du figuier. Ann. Serv. Epiphyties, vol. 6, pp. 34-174.
Picard, F., and Blanc, G. R.
1913. Les infections à coccobacilles chez les insectes. Compt. Rend. Acad. Sci., Paris, vol. 157, pp. 79-81.
Pierce, W. D.
1909. A monographic revision of the twisted winged insects comprising the order Strepsiptera Kirby. U.S. Nat. Mus. Bull. 66, xii+232 pp., 15 pls.
Pierce, W. D., Editor.
1921. Sanitary entomology. 518 pp. Boston.
Pinto, C. F.
1918. Sobre as eugregarinas parasitos dos artrópodos brazileiros. (6a nota prévia.) Brazil-Medico, vol. 32, pp. 113-114.
1918a. Estudos sobre as gregarinas Monocystis perforans n. sp. Formação dos esporozoitos observada in vitro. (12a nota prévia.) Brazil-Medico, vol. 32, pp. 321-322.
1919. Contribuição ao estudo das gregarinas. 113 pp., 6 pls. Tese, Rio de Janeiro.
1926. Nyctotherus dos blattideos do Brasil. Bol. Biol., São Paulo, No. 1, pp. 14-16.
1927. Crithidia spinigeri n. sp. parasita do apparelho digestivo de Spiniger domesticus (Hemiptero Reduviidae). Bol. Biol., No. 7, pp. 86-87.
1927a. Spiniger domesticus, n. sp. hemiptère suceur d'insectes (famille des Reduviidae, sous-famille des Reduviinae). Compt. Rend. Soc. Biol., Paris, vol. 97, pp. 833-834.
Piquett, P. G. and Fales, J. H.
1952. Rearing cockroaches for experimental purposes. U.S. Dept. Agr., Bur. Ent. and Plant Quar., ET-301, 12 pp.
Pitman, C. R. S.
1931. Further experiments with insect-food on the African lemur Perodicticus potto Lesson. Proc. Ent. Soc. London, vol. 5, Part 3, pp. 91-92.
Plank, H. K.
1947. Plant toxicological studies. Federal Exp. Stat. Rep., Mayagüez, Puerto Rico, 1946, pp. 11-13.
1950. Insecticidal properties of some plants growing in Puerto Rico. Federal Exp. Stat., Mayagüez, Puerto Rico, Bull. No. 49, 17 pp.
Plank, H. K., and Winters, H. F.
1949. Insect and other animal pests of Cinchona and their control in Puerto Rico. Fed. Exp. Stat. Puerto Rico, Mayagüez, Bull. No. 46, 16 pp.
Pocock, R. I.
1893. Notes upon the habits of some living scorpions. Nature, London, vol. 48, pp. 104-107.
Pope, Pauline.
1953. Studies of the life histories of some Queensland Blattidae (Orthoptera). Part 1. The domestic species. Proc. Roy. Soc. Queensland, vol. 63, pp. 23-46.
1953a. Idem. Part 2. Some native species. Proc. Roy. Soc. Queensland, vol. 63, pp. 47-59.
Porter, Annie.
1918. A survey of the intestinal entozoa, both protozoal and helminthic, observed among natives in Johannesburg, from June to November, 1917. South African Inst. Mem., No. 11, pp. 1-58.
1929. Some remarks on the hookworm problem in South Africa. South African Journ. Sci., vol. 26, pp. 396-401.
1930. Cockroaches as vectors of hookworms on gold mines of the Witwatersrand. Journ. Med. Assoc. South Africa, vol. 4, pp. 18-20.
Porter, J. E.
1958. Further notes on Public Health Service quarantine entomology. Florida Ent., vol. 41, pp. 41-44.
Portielje, A. F. V.
1914. Uit het reptielenhuis van "Artis." (In Dutch.) De Levende Natuur, vol. 18, pp. 557-563. [Pertinent sections translated by J. H. Vanderbie.]
Poulton, E. B.
1917. Predaceous reduviid bugs and fossors, with their prey, from the S. Paulo district of south-east Brazil. Proc. Ent. Soc. London, 1917, pp. xxxiv-xli.
1926. A Nyasaland pompilid with unusual prey. Complete recovery of prey after being stung. Proc. Ent. Soc. London, 1926, vol. 1, pp. 12-13.
Pound, C. J.
1907. Bacteriological report. II. Interesting observations in connection with the possible accidental transmission of plague from rat to rat by the agency of cockroaches. In Ham, B. Burnett, Report on plague in Queensland, 1900-1907. Queensland Dept. Publ. Health, Brisbane, p. 161.
Pradhan, S.
1957. The ecology of arid zone insects (excluding locusts and grasshoppers). In Human and animal ecology, arid zone research, vol. 8, pp. 199-240.
Pribram, E.
1933. Klassifikation der Schizomyceten (Bakterien). 143 pp. Leipzig and Wien.
Princis, K.
1946. Colombianische Blattodeen, gesammelt von Herrn G. Dahl und Frau M. Althén-Dahl in den Jahren 1936-1939. Kungl. Fysiograf. Sällskap. Lund Förh., vol. 16, pp. 1-15.
1947. Beitrag zur Kenntnis der adventiven Blattarien Skandinaviens und Finnlands. Notulae Ent., vol. 27, pp. 8-13.
1949. Kleine Beiträge zur Kenntnis der Blattarien und ihrer Verbreitung. I. Opusc. Ent., vol. 14, pp. 68-70.
1949a. Kleine Beiträge zur Kenntnis der Blattarien und ihrer Verbreitung. II. Ent. Medd., København, vol. 25, pp. 361-364.
1950. Indomalaiische und australische Blattarien aus dem Entomologischen Museum der Universität in Lund. Opusc. Ent., vol. 15, pp. 161-188.
1952. Reports of the Lund University Chile Expedition 1948-1949. 8. Blattariae. Lunds Univ. Årsskr., N.F., Avd. 2, vol. 48, No. 9, 11 pp.
1954. Report from Professor T. Gislén's Expedition to Australia 1951-1952. 10. Australian Blattariae. Lunds Univ. Årsskr., N.F., Avd. 2, vol. 50, No. 13, 49 pp.
1954a. Wo ist die Urheimat von Blatta orientalis L. zu suchen? Opusc. Ent., vol. 19, pp. 202-204. [Translated by Maria E. W. Torok.]
1955. Contributions à l'étude de la faune entomologique du Ruanda-Urundi (Mission P. Basilewsky 1953) XLIX. Blattariae. Ann. Mus. Congo, Tervuren, in 8°, Zool., vol. 40, pp. 15-42.
1957. Revision der Walker'schen und Kirby'schen Blattarientypen in British Museum of Natural History, London. Opusc. Ent., vol. 22, pp. 87-116.
1957a. Zur Kenntnis der Blattarien der Kleinen Sundainseln. Verhl. Naturf. Ges. Basel, vol. 68, pp. 132-159.
1959. Revision der Walkerschen und Kirbyschen Blattarientypen im British Museum of Natural History, London. III. Opusc. Ent., vol. 24, pp. 125-150.
Princis, K., and Kevan, D. K. McE.
1955. Cockroaches (Blattariae) from Trinidad, B.W.I., with a few records from other parts of the Caribbean. Opusc. Ent., vol. 20, pp. 149-169.
Pritchard, A. E.
1956. A new superfamily of trombidiform mites with the description of a new family, genus, and species (Acarina: Iolinoidea: Iolinidae: Iolina nana). Ann. Ent. Soc. Amer., vol. 49, pp. 204-206.
Pruthi, H. S.
1933. An interesting case of maternal care in an aquatic cockroach Phlebonotus pallens Serv. (Epilamprinae). Current Sci., Bangalore, vol. 1, p. 273.
Pryor, J. C.
1918. Naval hygiene. 507 pp. Philadelphia.
Pujatti, D.
1950. Acanthellae di Moniliformis dubius Meyer 1932 (Acanthocephala) nella Periplaneta australasiae F. (Blattidae) in sud-India. Mem. Soc. Ent. Italiana, vol. 29, pp. 110-111.
Purdy, J. S.
1920. The control of insect vectors of disease in war and peace. Australian Med. Congr., Trans. 11th Sess., Brisbane, Queensland, 21st-28th August 1920, pp. 298-306.
Qadri, M. A. H.
1938. The life-history and growth of the cockroach Blatta orientalis, Linn. Bull. Ent. Res., vol. 29, pp. 263-276.
Radna, R.
1939. Contribution au problème de la transmission de la lèpre. Les formes de la lèpre dans la région de Pawa et leur infectiosité. Deuxième note: La transmission du bacille de Hansen. Ann. Soc. Belge Méd. Trop., vol. 19, pp. 201-225.
Raffill, C. P.
1910. Cockroaches in plant houses. Gardeners' Chronicle, ser. 3, vol. 47, p. 43.
Rageau, J.
1956. Les arthropodes parasites de l'homme et des animaux domestiques dans les territoires Français du Pacifique. Inst. Franç. Océanie, Nouméa, 56 pp.
Raignier, A., and Boven, J. van.
1955. Étude taxonomique, biologique et biométrique des Dorylus du sous-genre Anomma (Hymenoptera Formicidae). Ann. Mus. R. Congo Belge, Tervuren, n. s., vol. 2, pp. 1-359.
Railliet, A.
1889. Cycle évolutif du Spiroptera sanguinolenta. Rec. Méd. Vét., Paris, vol. 6, pp. 127-128.
Rainwater, C. F.
1941. Insects and spiders found in Spanish moss, gin trash, and woods trash, and on wild cotton. U.S. Bur. Ent. and Plant. Quar., E-528, 20 pp.
Ramme, W.
1923. Vorarbeiten zu einer Monographie des Blattidengenus Ectobius Steph. Arch. Naturg., Berlin, Abt. A, vol. 89, pp. 97-145, 2 pls. [Translated by K. Gingold.]
1951. Zur Systematik Faunistik und Biologie der Orthopteren von Südost-Europa und Vorderasien. Mitt. Zool. Mus. Berlin, 1950, vol. 27, 431 pp., 39 pls.
Ramos, J. A.
1946. The insects of Mona Island (West Indies). Journ. Agr., Univ. Puerto Rico, vol. 30, pp. 1-74, 2 pls.
Ransom, B. H., and Hall, M. C.
1915. The life history of Gongylonema scutatum. Proc. Helminthol. Soc. Washington, in Journ. Parasitol., vol. 1, p. 154. (Abstract.)
1916. The life history of Gongylonema scutatum. Journ. Parasitol., 1915, vol. 2, pp. 80-86.
1917. A further note on the life-history of Gongylonema scutatum. Journ. Parasitol., vol. 3, pp. 177-181.
Ratzeburg, J. T. C.
1852. Die Ichneumonen der Forstinsecten ... vol. 3, p. 211.
Rau, P.
1924. The biology of the roach, Blatta orientalis. Trans. Acad. Sci., St. Louis, vol. 25, pp. 57-79.
1933. Jungle bees and wasps of Barro Colorado Island, pp. 196-197. Kirkwood, Mo.
1937. A note on the nesting habits of the roach-hunting wasp, Podium (Parapodium) carolina Rohwer (Hym). Ent. News, vol. 48, pp. 91-94.
1940. The life history of the wood-roach, Parcoblatta pennsylvanica DeGeer (Orthoptera: Blattidae). Ent. News, vol. 51, pp. 4-9, 33-35.
1940a. The life history of the American cockroach, Periplaneta americana Linn. (Orthop.: Blattidae). Ent. News, vol. 51, pp. 121-124, 151-155, 186-189, 223-227, 273-278.
1943. How the cockroach deposits its egg-case; a study in insect behavior. Ann. Ent. Soc. Amer., vol. 36, pp. 221-226.
1944. Another use for the cockroach, Blatta orientalis. Ent. News, vol. 55, pp. 49-50.
1947. Life history notes on the wood-roach, Ischnoptera deropeltiformis Brunner. Ent. News, vol. 58, pp. 1-4.
Ray, H. N., and Dasgupta, B.
1955. Occurrence of Diplocystis sp. as a parasite in the haemocoele of cockroach. Indian Sci. Congr. Assoc. Proc., vol. 42, pp. 281-282. (Abstract.)
Read, H. C., Jr.
1933. The cockroach as a possible carrier of tuberculosis. Amer. Rev. Tuberc., vol. 28, pp. 267-272.
Réaumur, R. A. F. de.
1742. Memoires pour servir à l'histoire des insectes. Vol. 6, 608 pp., 48 pls. Paris.
Rehn, J. A. G.
1906. Notes on the Orthoptera of Costa Rica, with descriptions of new species. Proc. Acad. Nat. Sci. Philadelphia, 1905, vol. 57, pp. 790-843.
1906a. The Orthoptera of the Bahamas. Bull. Amer. Mus. Nat. Hist., vol. 22, pp. 107-118.
1910. On the Orthoptera of Bermuda. Proc. Acad. Nat. Sci. Philadelphia, vol. 62, pp. 3-11.
1918. Descriptions of one new genus and fifteen new species of tropical American Orthoptera. Trans. Amer. Ent. Soc., vol. 44, pp. 321-371.
1926. Zoological results, of the Swedish Expedition to Central Africa 1921. Insecta. 18. Blattidae. Ark. Zool., vol. 18A, No. 18, 24 pp.
1928. New or little known Neotropical Blattidae (Orthoptera). Number one. Trans. Amer. Ent. Soc., vol. 54, pp. 125-194.
1930. New or little known Neotropical Blattidae (Orthoptera). Number two. Trans. Amer. Ent. Soc., vol. 56, pp. 19-71.
1931. African and Malagasy Blattidae (Orthoptera), part I. Proc. Acad. Nat. Sci. Philadelphia, vol. 83, pp. 305-387.
1932. African and Malagasy Blattidae (Orthoptera), part II. Proc. Acad. Nat. Sci. Philadelphia, vol. 84, pp. 405-511, 4 pls.
1932a. Wissenschaftliche Ergebnisse der Schwedischen entomologischen Reisen des Herrn Dr. A. Roman 1914-15 und 1923-24 in Amazonas. 16. Blattidae. (In English.) Arch. Zool. K. Svenska Vetensk., vol. 24, pp. 1-73.
1932b. New or little known Neotropical Blattidae (Orthoptera). Number three. Trans. Amer. Ent. Soc., vol. 58, pp. 103-137.
1937. African and Malagasy Blattidae (Orthoptera), part III. Proc. Acad. Nat. Sci. Philadelphia, vol. 89, pp. 17-123, 3 pls.
1937a. New or little known Neotropical Blattidae (Orthoptera). Number four. Trans. Amer. Ent. Soc., vol. 63, pp. 207-258, pls. XIV-XVII.
1945. Man's uninvited fellow traveler—the cockroach. Sci. Month., vol. 61, pp. 265-276.
1945a. Three new species of the reticulosa group of the blattid genus Cariblatta (Orthoptera, Blattidae, Pseudomopinae). Notulae Naturae, No. 149, 15 pp.
1947. African and Malagasy Blattidae (Orthoptera). Part IV. Proc. Acad. Nat. Sci. Philadelphia, vol. 99, pp. 59-92.
Rehn, J. A. G., and Hebard, M.
1905. A contribution to the knowledge of the Orthoptera of south and central Florida. Proc. Acad. Nat. Sci. Philadelphia, vol. 57, pp. 29-55.
1909. An orthopterological reconnaissance of the southwestern United States. Part II: New Mexico and Western Texas. Proc. Acad. Nat. Sci. Philadelphia, vol. 61, pp. 111-175.
1910. Preliminary studies of North Carolina Orthoptera. Proc. Acad. Nat. Sci. Philadelphia, vol. 62, pp. 615-650.
1912. On the Orthoptera found on the Florida Keys and in extreme southern Florida. Proc. Acad. Nat. Sci. Philadelphia, vol. 64, pp. 235-276.
1914. On the Orthoptera found on the Florida Keys and in extreme southern Florida. II. Proc. Acad. Nat. Sci. Philadelphia, vol. 66, pp. 373-412.
1914a. Records of Dermaptera and Orthoptera from west central and southwestern Florida, collected by William T. Davis. Journ. New York Ent. Soc., vol. 22, pp. 96-116.
1916. Studies in the Dermaptera and Orthoptera of the Coastal Plain and Piedmont Region of the southeastern United States. Proc. Acad. Nat. Sci. Philadelphia, vol. 68, pp. 87-314.
1927. The Orthoptera of the West Indies. Number 1. Blattidae. Bull. Amer. Mus. Nat. Hist., vol. 54, pp. 1-320.
Rehn, J. W. H.
1951. Classification of the Blattaria as indicated by their wings (Orthoptera). Mem. Amer. Ent. Soc., No. 14, 134 pp. 13 pls.
1951a. The genus Aspiduchus (Orthoptera: Blattidae: Blaberinae). Notulae Naturae, No. 231, pp. 1-7.
Rice, C. E.
1925. Destruction of cockroaches and devitalization of their eggs by cyanogen-chloride mixture. Public Health Rep., vol. 40, pp. 1808-1811.
Richards, A. G.
1954. Similarities in histochemical differentiation of insect cuticle and the walls of parasitic fungi. Science, vol. 120, pp. 761-762.
Richards, A. G., and Brooks, Marion A.
1958. Internal symbiosis in insects. In Steinhaus, E. A., and Smith, R. F., eds., Ann. Rev. Ent., vol. 3, pp. 37-56. Palo Alto, Calif.
Richards, A. G., and Smith, Myrtle N.
1954. Infection of cockroaches with Herpomyces (Laboulbeniales). III. Experimental studies on host specificity. Bot. Gaz., vol. 116, pp. 195-198.
1955. Infection of cockroaches with Herpomyces (Laboulbeniales). I. Life history studies. Biol. Bull., vol. 108, pp. 206-218.
1955a. Infection of cockroaches with Herpomyces (Laboulbeniales). IV. Development of H. stylopygae Spegazzini. Biol. Bull., vol. 109, pp. 306-315.
1956. Infection of cockroaches with Herpomyces (Laboulbeniales). II. Histology and histopathology. Ann. Ent. Soc. Amer., vol. 49, pp. 85-93.
Richards, O. W., and Hamm, A. H.
1939. The biology of the British Pompilidae (Hymenoptera). Trans. Soc. British Ent., vol. 6, pp. 51-114.
Richardson, H. H.
1917. Methyl bromide delousing, DDT insecticides, and rodent control problems at New York Port of Embarkation. Pest Control, vol. 15, No. 9, pp. 20, 22, 24.
Riek, E. F.
1955. The Australian rhipidiine parasites of cockroaches (Coleoptera: Rhipiphoridae). Australian Journ. Zool., vol. 3, pp. 71-94.
Ries, E.
1932. Experimentelle Symbiosestudien. I. Mycetomtransplantationen. Zeitschr. Wiss. Biol., Abt. A, vol. 25, pp. 184-234.
Riherd, P. T.
1953. The occurrence of Blattella vaga Hebard in Texas (Orthoptera, Blattidae). Proc. Ent. Soc. Washington, vol. 55, pp. 39-40.
Riley, C. V.
1875. On the insects more particularly associated with Sarracenia variolaris (Spotted trumpet-leaf). Proc. Amer. Assoc. Adv. Sci., Pt. II. Sect. B., 23d meeting 1874, pp. 17-25.
Riley, W. A., and Johannsen, O. A.
1938. Medical entomology. 2d ed. 483 pp. New York.
Rizki, M. T. M.
1954. Desoxyribose nucleic acid in the symbiotic microorganisms of the cockroach, Blattella germanica. Science, vol. 120, pp. 35-36.
Robertson, C.
1928. Flowers and insects. 221 pp. Lancaster, Pa.
Robin, C.
1847. Des végétaux qui croissent sur l'homme et sur les animaux vivants. viii+120 pp., 3 pls. Libraire de l'Académie Royale de Médecine, Paris.
1853. Histoire naturelle des végétaux parasites qui croissent sur l'homme et sur les animaux vivants. 702 pp. Libraire de l'Académie Impériale de Médecine, Paris.
Roeser, G.
1940. Zur Kenntnis der Lebensweise der Gewächshausschabe Pycnoscelus surinamensis L. Die Gartenbauwissenschaft, vol. 15, pp. 184-225. [Translated by P. Bernhardt.]
Roger, J.
1906. Spiroptérose canine. Bull. Soc. Cent. Méd. Vét., vol. 60, pp. 378-379.
1907. Spiroptérose canine. Rev. Vét., Toulouse, vol. 64, pp. 241-251.
Ronzoni, Maria G.
1949. Funghi lievitiformi isolati dalle ovoteche di Periplaneta orientalis L. (English summary.) Mycopathologia, vol. 4, pp. 333-341.
Rosenfeld, A. H.
1911. Insects and spiders in Spanish moss. Journ. Econ. Ent., vol. 4, pp. 398-409.
1912. Insects and spiders in Spanish moss. Journ. Econ. Ent., vol. 5, pp. 338-339.
Roth, L. M.; Niegisch, W. D.; and Stahl, W. H.
1956. Occurrence of 2-hexenal in the cockroach Eurycotis floridana. Science, vol. 123, pp. 670-671.
Roth, L. M., and Stay, Barbara.
1958. The occurrence of para quinones in some arthropods, with emphasis on the quinone-secreting tracheal glands of Diploptera punctata (Blattaria). Journ. Insect Physiol., vol. 1, pp. 305-318.
Roth, L. M., and Willis, E. R.
1950. The oviposition of Dermestes ater DeGeer, with notes on bionomics under laboratory conditions. Amer. Midl. Nat., vol. 44, pp. 427-447.
1952. A study of cockroach behavior. Amer. Midl. Nat., vol. 47, pp. 66-129.
1952a. Possible hygroreceptors in Aedes aegypti (L.) and Blattella germanica (L.). Journ. Morph., vol. 91, pp. 1-14.
1954. The reproduction of cockroaches. Smithsonian Misc. Coll., vol. 122, No. 12, pp. 1-49, 12 pls.
1954a. Anastatus floridanus (Hymenoptera: Eupelmidae), a new parasite on the eggs of the cockroach Eurycotis floridana. Trans. Amer. Ent. Soc., vol. 80, pp. 29-41, 3 pls.
1954b. The biology of the cockroach egg parasite, Tetrastichus hagenowii (Hymenoptera: Eulophidae). Trans. Amer. Ent. Soc., vol. 80, pp. 53-72, 3 pls.
1955. Water relations of cockroach oöthecae. Journ. Econ. Ent., vol. 48, pp. 33-36.
1955a. Water content of cockroach eggs during embryogenesis in relation to oviposition behavior. Journ. Exp. Zool., vol. 128, pp. 489-509.
1957. Observations on the biology of Ectobius pallidus (Olivier) (Blattaria, Blattidae). Trans. Amer. Ent. Soc., vol. 83, pp. 31-37, 3 pls.
1957a. The medical and veterinary importance of cockroaches. Smithsonian Misc. Coll., vol. 134, No. 10, 147 pp. 7 pls.
1958. The biology of Panchlora nivea with observations on the eggs of other Blattaria. Trans. Amer. Ent. Soc., vol. 83, pp. 195-207.
1958a. An analysis of oviparity and viviparity in the Blattaria. Trans. Amer. Ent. Soc., vol. 83, pp. 221-238.
Study of the bisexual and parthenogenetic strains of Pycnoscelus surinamensis (Blattaria: Epilamprinae). Ann. Ent. Soc. Amer. (In press, 1960.)
Rothschild, Miriam, and Clay, Theresa.
1957. Fleas, flukes and cuckoos; a study of bird parasites. 3d ed. 305 pp. New York.
Roubaud, E., and Descazeaux, J.
1923. Sur un agent bactérien pathogène pour les mouches communes: Bacterium delendae-muscae n. sp. Compt. Rend. Acad. Sci., Paris, vol. 177, pp. 716-717.
Ruhland, H. H., and Huddleson, I. F.
1941. The rôle of one species of cockroach and several species of flies in the dissemination of Brucella. Amer. Journ. Vet. Res., vol. 2, pp. 371-372.
Sambon, L. W.
1925. Researches on the epidemiology of cancer made in Iceland and Italy (July-October, 1924). Journ. Trop. Med. Hyg., London, vol. 28, pp. 39-71.
1926. Observations and researches on the epidemiology of cancer made in Holland and Italy (May-September, 1925). Journ. Trop. Med. Hyg., London, vol. 29, pp. 233-287.
Samouelle, G.
1841. The entomological cabinet; being a natural history of British insects. (Blatta germanica, Linn., pp. 1-3.) 2d ed., 340 pp. London.
Sandemann, R. G.
1934. An exotic cockroach in S. Wales. Entomologist, vol. 67, p. 66.
Sanders, D. A.
1927. Control of eye worm in chickens. Tests show cockroaches carry parasite. Florida Grower, vol. 35, No. 12, p. 23.
1928. Manson's eyeworm of poultry. Journ. Amer. Vet. Med. Assoc., vol. 72 (n.s. vol. 25), pp. 568-584.
1929. Manson's eyeworm of poultry. Florida Agr. Exp. Stat. Bull., No. 206, pp. 567-585.
Sanjean, J.
1957. Taxonomic studies of Sarcophaga larvae of New York, with notes on the adults. Cornell Univ. Agr. Exp. Stat., Mem. No. 349, 115 pp.
Sartory, A., and Clerc, A.
1908. Flore intestinale de quelques Orthoptères. Compt. Rend. Soc. Biol., Paris, vol. 64, pp. 544-545.
Saupe, R.
1928. Zur Kenntnis der Lebensweise der Riesenschabe Blabera fusca Brunner und der Gewächshausschabe Pycnoscelus surinamensis L. Zeitschr. Angew. Ent., vol. 14, pp. 461-500. [Pertinent sections translated by P. Bernhardt.]
Scharrer, B.
1953. Insect tumors induced by nerve severance: Incidence and mortality. Cancer Res., vol. 13, pp. 73-76.
Schaudinn, F.
1902. Beiträge zur Kenntnis der Bakterien und verwandter Organismen. I. Bacillus bütschlii n. sp. Arch. Protistenk., vol. 1, pp. 306-343.
Schell, S. C.
1952. Studies on the life cycle of Physaloptera hispida Schell (Nematoda; Spiruroidea) a parasite of the cotton rat (Sigmodon hispidus littoralis Chapman). Journ. Parasitol., vol. 38, pp. 462-472.
1952a. Tissue reactions of Blattella germanica L. to the developing larva of Physaloptera hispida Schell, 1950 (Nematoda; Spiruroidea). Trans. Amer. Micr. Soc., vol. 71, pp. 293-302.
Schepdael, J. van.
1931. Note sur quelques insectes exotiques recueillis vivants à Hal (Brabant) en 1931. Lambillionea, Rev. Mens. Union Ent. Belges, vol. 31, pp. 167-169.
Schiffman, Olga.
1919. Über die Fortpflanzung von Gregarina blattarum und Gregarina cuneata. Arch. Protistenk., vol. 40, pp. 76-96, 1 pl.
Schletterer, A.
1886. Ueber die Hymenopteren-Gattung Evania Fabr. Verhandl. K. K. Zool. Bot. Ges., Wien, vol. 36, pp. 1-46, 1 pl.
1889. Die Hymenopteren-Gruppe der Evaniiden. Ann. K. K. Naturhist. Hofmus., Wien, vol. 4, pp. 107-180.
Schmidt, C.
1937. Exhibition and discussion of local material. Proc. Hawaiian Ent. Soc., vol. 9, pp. 356-358.
Schmidt, K. P.
1920. Contributions to the herpetology of Porto Rico. Ann. New York Acad. Sci., vol. 28, pp. 167-200.
Schmidtke, Liselotte.
1955. Histologische Untersuchungen an toxoplasmainfizierten Insekten (Calliphora erythrocephala, Periplaneta americana). Zentralbl. Bakt., Abt. 1, Orig., vol. 164, pp. 508-513.
Schmidt-Nielsen, Bodil and K.
1949. The water economy of desert mammals. Sci. Month., vol. 69, pp. 180-185.
Schneider, A. C. J.
1875. Contributions à l'histoire des grégarines des invertébrés de Paris et de Roscoff. Arch. Zool. Exp. et Gén., Paris, vol. 4, pp. 493-604, 8 pls.
Schneider, R. F., and Shields, G. W.
1947. Investigation on the transmission of E. histolytica by cockroaches. Med. Bull., Standard Oil Co. New Jersey, vol. 7, pp. 119-121.
Schneirla, T. C.
1956. The army ants. Ann. Rep. Smithsonian Inst. for 1955, pp. 379-406, 2 pls.
Schubotz, H.
1905. Beiträge zur Kenntnis der Amoeba blattae (Bütschli) und Amoeba proteus (Pall.). Arch. Protistenk., vol. 6, pp. 1-46, 2 pls.
Schultze, W.
1925. Macroxenos piercei (order Strepsiptera), a new genus and species of wasp parasites of the Philippine Islands. Philippine Journ. Sci., vol. 27, pp. 235-241, 1 pl.
1927. Biology of the large Philippine forest scorpion. Philippine Journ. Sci., vol. 32, pp. 375-389, 4 pls.
Schulz, W. A.
1912. Zweihundert alte Hymenopteren. Zool. Ann., Zeitschr. Ges. Zool., vol. 4, pp. 1-220.
Schuster, E. H. J.
1898. On a new flagellate protozoon of the genus Lophomonas. Proc. Zool. Soc. London, 1898, No. 16, pp. 242-244.
Schwabe, C. W.
1949. Observations on the life history of Pycnoscelus surinamensis (Linn.), the intermediate host of the chicken eyeworm in Hawaii. Proc. Hawaiian Ent. Soc., vol. 13, pp. 433-436.
1950. Note on the survival of eyeworm larvae. Proc. Hawaiian Ent. Soc., vol. 14, p. 4.
1950a. Studies on Oxyspirura mansoni, the tropical eyeworm of poultry. III. Preliminary observations on eyeworm pathogenicity. Amer. Journ. Vet. Res., vol. 11, pp. 286-290.
1950b. Studies on Oxyspirura mansoni, the tropical eyeworm of poultry. IV. Methods for control. Proc. Hawaiian Ent. Soc., vol. 14, pp. 175-183.
1951. Studies on Oxyspirura mansoni, the tropical eyeworm of poultry. II. Life history. Pacific Sci., vol. 5, pp. 18-35.
Schwartz, B., and Lucker, J. T.
1931. Experimental transmission of Gongylonema scutatum to pigs. Journ. Parasitol., vol. 18, p. 46.
Schwartz, C. W., and Schwartz, E. R.
1949. A reconnaissance of the game birds in Hawaii. Board of Comm. Agr. and Forest., Territory of Hawaii, Honolulu, 168 pp.
Schwartz, W.
1935. Untersuchungen über die Symbiose von Tieren mit Pilzen und Bakterien. IV. Mitteilung: Der Stand unserer Kenntnisse von den physiologischen Grundlagen der Symbiosen von Tieren mit Pilzen und Bakterien. Arch. Mikrobiol., vol. 6, pp. 369-460.
Schwenck, J. M.
1926. Fauna parasitologica dos blattideos do Brasil. Sci. Med., Rio de Janeiro, vol. 4, pp. 491-504.
Scott, H.
1910. Eight months' entomological collecting in the Seychelles Islands, 1908-1909. Trans. Linn. Soc. London, ser. 2, Zool., vol. 14, pp. 21-39.
1912. A contribution to the knowledge of the fauna of Bromeliaceae. Ann. Mag. Nat. Hist., ser. 8, vol. 10, pp. 424-438, pl. 10.
1929. On some cases of maternal care displayed by the cockroaches and their significance. Ent. Month. Mag., vol. 65, pp. 218-222.
Scudder, S. H.
1877. The Florida Orthoptera collected by Mr. J. H. Comstock. Proc. Boston Soc. Nat. Hist., vol. 19, pp. 80-94.
1879. Palaeozoic cockroaches: A complete revision of the species of both worlds, with an essay toward their classification. Mem. Boston Soc. Nat. Hist., vol. 3, pp. 23-134, pls. 2-6.
Seín, F., Jr.
1923. Cucarachas. Puerto Rico Insular Exp. Stat. Circ. No. 64, 12 pp.
Selander, R. B.
1957. The systematic position of the genus Nephrites and the phylogenetic relationships of the higher groups of Rhipiphoridae (Coleoptera). Ann. Ent. Soc. Amer., vol. 50, pp. 88-103.
Sellards, E. H.
1903. Some new structural characters of Paleozoic cockroaches. Amer. Journ. Sci., vol. 15, pp. 307-315, 2 pls.
Sells, W.
1842. Note respecting the egg-cases of Blattae. Trans. Ent. Soc. London., vol. 3, pp. 103-104.
Semans, F. M.
1939. Protozoan parasites of the Orthoptera, with special reference to those of Ohio. II. Description of the protozoan parasites recognized in this study. Ohio Journ. Sci., vol. 39, pp. 157-181.
1941. Protozoan parasites of the Orthoptera, with special reference to those of Ohio. III. Protozoan parasites in relation to the host and to host ecology. Ohio Journ. Sci., vol. 41, 457-464.
1943. Protozoan parasites of the Orthoptera, with special reference to those of Ohio. IV. Classified list of the protozoan parasites of the Orthoptera of the world. Classes Mastigophora, Sarcodina, and Sporozoa. Ohio Journ. Sci., vol. 43, pp. 221-234, 271-276.
Serrano Sánchez, Amparo.
1945. Hammerschmidtiella neyrai n. sp. en Periplaneta orientalis L., en Granada. Rev. Ibérica Parasitol., Tomo Extraordinario, Homenaje al C. R. López-Neyra, pp. 213-215, 1 pl.
1947. Nematodes parásitos intestinales de los artrópodos en España. Rev. Ibérica Parasitol., vol. 7, pp. 279-332.
Seurat, L. G.
1911. Sur l'habitat et les migrations du Spirura talpae Gmel. (=Spiroptera strumosa Rud.). Compt. Rend. Soc. Biol., Paris, vol. 71, pp. 606-608.
1912. La grande blatte, hôte intermédiaire de l'echinorhynque moniliforme en Algérie. Compt. Rend. Soc. Biol., Paris, vol. 72, pp. 62-63.
1916. Contributions a l'étude des formes larvaires des nématodes parasites hetéroxènes. Bull. Sci. France et Belgique, sér. 7, vol. 49, pp. 297-377.
Severin, H. C.
1952. An unusual infestation of wood cockroaches. Journ. Econ. Ent., vol. 45, p. 1079.
Severin, H. H.
1911. [Insects trapped by awns of a grass.] Proc. Hawaiian Ent. Soc., vol. 2, p. 195.
Severin, H. H. P., and Severin, H. C.
1915. Kerosene traps as a means of checking up the effectiveness of a poisoned bait spray to control the Mediterranean fruitfly (Ceratitis capitata Wied.) with a record of beneficial insects captured in the kerosene. Journ. Econ. Ent., vol. 8, pp. 329-338.
Seymour, A. B.
1929. Host index of the fungi of North America. 732 pp. Cambridge, Mass.
Sharp, D.
1895. Insects. Part I. Chap. IX. In The Cambridge Natural History, vol. 5, pp. 220-241. London.
1899. Insects. Part II. Hymenoptera (Tubulifera and Aculeata), Coleoptera, Strepsiptera, Lepidoptera, Diptera, Aphaniptera, Thysanoptera, Hemiptera, Anoplura. In The Cambridge National History, vol. 6, 626 pp. London.
Shaw, E.
1902. Two cockroaches new to Britain. Ent. Rec. and Journ. Var., vol. 14, pp. 295-296.
1914. Australian Blattidae. Part I. Notes and preliminary descriptions of new species. Victorian Nat., vol. 31, pp. 103-108.
1924. Supella supellectilium Serville: a cockroach not before recorded for Australia. Queensland Nat., vol. 4, p. 115.
1925. New genera and species (mostly Australasian) of Blattidae, with notes, and some remarks on Tepper's types. Proc. Linn. Soc. New South Wales, vol. 1, pp. 171-213.
Shealy, A. L.
1927. On Manson's eye worm in poultry. Science, vol. 66, pp. 426-427.
Shelford, R.
1901. Notes on some Bornean insects. British Assoc. Adv. Sci., Rep. 71st Meet., pp. 689-691.
1906. Studies of the Blattidae. VI. Viviparity amongst the Blattidae. Trans. Ent. Soc. London, 1906, pp. 509-514.
1906a. Studies of the Blattidae. VII. A new genus of symbiotic Blattidae. Trans. Ent. Soc. London, 1906, pp. 515-519.
1907. Aquatic cockroaches. Zoologist, ser. 4, vol. 11, pp. 221-226.
1909. Blattidae. In Michaelsen, W., and Hartmeyer, R., Die Fauna Südwest-Australiens. Ergebnisse der Hamburger südwest-australischen Forschungsreise 1905, vol. 2, pp. 129-142.
1909a. Notes on some amphibious cockroaches. Rec. Indian Mus., vol. 3, pp. 125-127.
1909b. Studies of the Blattidae. X. A revision of the Old-World Blattinae belonging to the Polyzosteria group. Trans. Ent. Soc. London, 1909, pp. 253-327, pls. VII-IX.
1910. Orthoptera. 2. Blattodea. Wiss. Ergeb. Schwed. Zool. Exped. Kilimandjaro, Meru Deutsch-Ostafrikas 1905-06 (Sjöstedt), vol. 3, pp. 13-48.
1910a. A new cavernicolous cockroach. Ann. Mag. Nat. Hist., ser. 8, vol. 6, pp. 114-116.
1912. Mimicry amongst the Blattidae; with a revision of the genus Prosoplecta Sauss., and the description of a new genus. Proc. Zool. Soc. London, 1912, pp. 358-376.
1912a. The oöthecae of Blattidae. Ent. Rec. and Journ. Var., vol. 24, pp. 283-287.
1916. A naturalist in Borneo. E. B. Poulton, ed. (2d impress., 1917.) 331 pp. London.
Shrewsbury, J. F. D., and Barson, G. J.
1948. A study of the intestinal flora of Blatella americana. Journ. Path. Bact., vol. 60, pp. 506-509.
Shuyler, H. R.
1956. Are German and oriental roaches changing their habits? Pest Control, vol. 24, No. 9, pp. 9-10.
Sickmann, F.
1893. Die Hymenopterenfauna von Iburg und seiner nächsten Umgebung, mit biologischen und kritischen Bemerkungen. I. Die Grabwespen. Jahresb. Naturwiss. Vereins Osnabrück, 1891, vol. 9, pp. 39-112.
Siebold, C. T. von.
1837. Fernere Beobachtungen über die Spermatozoen der wirbellosen Thiere. Arch. Anat. Physiol. Wiss. Med., 1837, pp. 381-439, 1 pl.
1839. Beiträge zur Naturgeschichte der wirbellosen Thiere. 4. Ueber die zur Gattung Gregarina gehörigen Helminthen. Neueste Schrift. Naturf. Ges. Danzig, vol. 3, pp. 56-71, 1 pl.
1842. Ueber die Fadenwürmer der Insekten. Ent. Zeitschr., Stettin, vol. 3, pp. 146-161.
Silvestri, F.
1946. Prima nota su alcuni termitofili dell' Indocina. Boll. Lab. Ent. Agr. Portici, vol. 6, pp. 313-330.
1947. Seconda nota su alcuni termitofili dell' Indocina con una appendice sul Macrotermes Barneyi Light. Bol. Lab. Ent. Agr. Portici, vol. 7, pp. 13-40.
Simanton, W. A.
1946. Insect control on ships. Pacific Mar. Rev., vol. 43, No. 6, pp. 68-69.
Simmonds, F. J.
1958. The effect of lizards on the biological control of scale insects in Bermuda. Bull. Ent. Res., vol. 49, pp. 601-612.
Simmonds, H. W.
1941. A predatorial wasp of cockroaches. Agr. Journ. Fiji, vol. 12, p. 26.
Simpson, G. G.
1945. The principles of classification and a classification of mammals. Bull. Amer. Mus. Nat. Hist., vol. 85, pp. 1-350.
Sita, E. (Mrs.).
1949. The life-cycle of Moniliformis moniliformis (Bremser, 1811), Acanthocephala. Current Sci., Bangalore, vol. 18, pp. 216-218.
Skaife, S. H.
1954. African insect life. 387 pp., 70 pls. New York.
Skinner, H.
1905. Destructiveness of the Australian roach Periplaneta australasiae. Ent. News, vol. 16, p. 183.
Slater, J. A.
1949. Food habits of the brown skink, Leiolopisma laterale Say. Herpetologica, vol. 5, pp. 79-80.
Smith, A.
1955. The transmission of Bancroftial filariasis on Ukara Island, Tanganyika. I. Bull. Ent. Res., vol. 46, pp. 419-436, pl. XV.
Smith, D. T.; Martin, D. S.; Conant, N. F.; Beard, J. W.; Taylor, G.; Kohn, H. I.; and Poston, M. A.
1948. Zinsser's textbook of bacteriology. 9th ed. 992 pp. New York.
Smith, J. H.
1945. Useful parasitic insects. Queensland Agr. Journ., vol. 61, pp. 340-351.
Smith, Nannie M., and Barret, H. P.
1928. The cultivation of a parasitic amoeba from the cockroach. Journ. Parasitol., vol. 14, pp. 272-273, 1 pl.
Snodgrass, R. E.
1930. Insects, their ways and means of living. Smithsonian Sci. Ser., vol. 5, 362 pp.
Snow, W. E.
1958. Stratification of arthropods in a wet stump cavity. Ecology, vol. 39, pp. 83-88.
Snyder, T. E.
1949. Catalog of the termites (Isoptera) of the world. Smithsonian Misc. Coll., vol. 112, 490 pp.
Sobolev, A. A.
1937. Helminthofauna of Blattidae of U.S.S.R. Papers on Helminthology published in commemoration of the 30-year jubileum of K. J. Skrjabin and of the 15th anniversary of the All-Union Institute of Helminthology. (In Russian.) Raboty 32-ĭ 38-ĭ Soi͡uznykh Gel'mintologicheskikh Ekspedsiĭ na Territorii Severo-Dvinskoĭ Gubernii v 1926 i 1927 godakh, pp. 663-670. [Pertinent sections translated by M. Ycas.]
Someren, V. G. L. van.
1924. Notes on the life-history and insect-food-preferences of a lichen-like ascalaphid larva at Nairobi. Proc. Ent. Soc. London, in Trans. Ent. Soc. London, 1924, pp. lix-lxv.
Sonan, H.
1924. Observations upon Periplaneta americana, Linnaeus, and Periplaneta australasiae, Fabricius. (In Japanese.) Trans. Nat. Hist. Soc. Formosa, vol. 14, pp. 4-21. [Translated by Associated Technical Services.]
1927. A taxonomic study together with observations of various families of egg-laying wasps of Formosa. (In Japanese.) Trans. Nat. Hist. Soc. Formosa, vol. 17, pp. 121-138. [Pertinent sections translated by Mary Watanabe.]
Sondak, V. A.
1935. Cockroaches as carriers and hosts of parasitic worms in Leningrad and its environs. (In Russian, English summary.) In Parazity, Perenoschiki i Iadovitye Zhivotnye. Sbornik rabot, posviash-chennyi dvadtsatipiatiletiiu nauchnoi deiatel' nosti Professora E. N. Pavlovskogo, 1909-1934, pp. 316-327. [Pertinent sections translated by D. Kraus.]
Sonnerat, M.
1776. Voyage à la Nouvelle Guinée, dans lequel on trouve la description des lieux, des observations physiques et morales, et des détails relatifs à l'histoire naturelle dans le regne animal et le regne végétal. 208 pp. Paris.
Southwell, T.
1922. Notes on the larvae of Moniliformis moniliformis (Brems.) found in African cockroaches. Journ. Parasitol., vol. 9, pp. 99-101.
Souza Lopes, H. de.
1937. Contribuiçao ao conhecimento do genero "Stylogaster" Marquart 1835, (Dipt. Conopidae). Arch. Inst. Biol. Veg., Rio de Janeiro, vol. 3, pp. 257-293.
Soyer, B.
1947. Notes sur les Sphégiens et les Pompiles. VI. Le Sphex albisectus Lepeletier; les Dolichurus de la faune française. Bull. Mens. Soc. Liné., Lyon, vol. 16, pp. 117-121.
Spegazzini, C.
1915. Laboulbeniali ritrovate nelle collezioni di alcuni musei italiani. An. Mus. Nac. Hist. Nat. Buenos Aires, vol. 26, pp. 451-511.
1917. Revisión de las Laboulbeniales Argentinas. An. Mus. Nac. Hist. Nat. Buenos Aires, vol. 29, pp. 445-688.
Spencer, B.
1892. A trip to Queensland in search of Ceratodus. Nature, London, vol. 46, pp. 305-310.
Spencer, G. J.
1943. On the oviposition habits of the Australian cockroach, Periplaneta australasiae (Fab.). Proc. Ent. Soc. British Columbia, vol. 40, pp. 29-30.
Spinelli, A., and Reitano, U.
1932. Ricerche sulle blatte, quali agenti di diffusione dei germi del colera, della febbre tifoide e della dissenteria bacillare. Ann. Igiene, vol. 42, pp. 745-755.
Sprague, V.
1940. Studies on Gregarina blattarum with particular reference to the chromosome cycle. Journ. Parasitol., vol. 26, No. 6, Suppl., pp. 26-27.
1940a. Observations on Coelosporidium periplanetae with special reference to the development of the spore. Trans. Amer. Micr. Soc., vol. 59, pp. 460-474.
1941. Studies on Gregarina blattarum with particular reference to the chromosome cycle. Illinois Biol. Monogr., vol. 18, pp. 1-57.
Sprague, V., and Ramsey, J.
1941. A preliminary note on Plistophora kudoi n. sp., a microsporidian parasite of the cockroach. Anat. Rec., vol. 81, Suppl., pp. 132-133. (Abstract.)
1942. Further observations on Plistophora kudoi, a microsporidian of the cockroach. Journ. Parasitol., vol. 28, pp. 399-406.
Stage, H. H.
1947. DDT to control insects affecting man and animals in a tropical village. Journ. Econ. Ent., vol. 40, pp. 759-762.
Stahnke, H. L.
1949. Scorpions. 23 pp. Tempe, Ariz.
Stamm, R. H.
1936. A new find of Rhipidius pectinicornis Thbg. (Symbius blattarum Sund.) (Col. Rhipiphor.). Ent. Medd., vol. 19, pp. 286-297.
Stay, Barbara.
1957. The sternal scent gland of Eurycotis floridana (Blattaria: Blattidae). Ann. Ent. Soc. Amer., vol. 50, pp. 514-519, 1 pl.
Stein, F. von.
1848. Ueber die Natur der Gregarinen. Arch. Anat., Physiol. Wiss. Med., 1848, pp. 182-223, 1 pl.
1860. Ueber die neue Gattung Lophomonas. Sitzungsb. K. Böhm. Ges. Wiss. Prag, 1860, pp. 49-50.
Steinhaus, E. A.
1941. A study of the bacteria associated with thirty species of insects. Journ. Bact., vol. 42, pp. 757-790.
1946. Insect microbiology. 763 pp. Ithaca, N. Y.
1949. Principles of insect pathology. 757 pp. New York.
1959. Serratia marcescens Bizio as an insect pathogen. Hilgardia, vol. 28, pp. 351-380.
Stevenson, C.
1905. The Blattidae of Montreal. Ent. News, vol. 16, p. 98.
Stewart, A. M.
1925. Blaberus cubensis (Orthoptera) and its oötheca. Entomologist, vol. 58, pp. 57-58.
Stiles, C. W., and Baker, C. E.
1927. Gongylonema scutatum in relation to cancer. Journ. Parasitol., vol. 14, p. 67.
Stiles, C. W., and Hassall, A.
1894. A preliminary catalogue of the parasites contained in the collections of the United States Bureau of Animal Industry, United States Army Medical Museum, Biological Department of the University of Pennsylvania (Coll. Leidy) and in Coll. Stiles and Coll. Hassall. Vet. Mag., vol. 1, pp. 331-354.
1926. Key-catalogue of the worms reported for man. U. S. Publ. Health Serv. Hyg. Lab. Bull. No. 142, pp. 69-196.
1928. Key-catalogue of insects of importance in Public Health. U. S. Publ. Health Serv. Hyg. Lab. Bull. No. 150, pp. 291-408.
Strohecker, H. F.
1937. An ecological study of some Orthoptera of the Chicago area. Ecology, vol. 18, pp. 231-250.
Subramanian, K. A.
1927. On a small collection of Acanthocephala from Rangoon. Ann. Mag. Nat. Hist., ser. 9, vol. 19, pp. 645-650.
Sullivan, W. N.; Duchanois, F. R.; and Hayden, D. L.
1958. Insect survival in jet aircraft. Journ. Econ. Ent., vol. 51, pp. 239-241.
Sundevall, J.
1831. Einer neuen Coleopteren Gattung, Symbius blattarum. Isis (Oken), vol. 24, pp. 1222-1228.
Sweetman, H. L.
1936. The biological control of insects. 461 pp. Ithaca, N. Y.
Swezey, O. H.
1920. Dolichurus stantoni. Proc. Hawaiian Ent. Soc., 1919, vol. 4, p. 286.
1921. Kauai insect notes and records. Proc. Hawaiian Ent. Soc., vol. 4, pp. 521-523.
1929. Notes on the egg-parasites of insects in Hawaii. Proc. Hawaiian Ent. Soc., vol. 7, pp. 282-292.
1944. Ampulex compressa (Fabr.). Proc. Hawaiian Ent. Soc., vol. 12, p. 19.
1945. Insects associated with orchids. Proc. Hawaiian Ent. Soc., vol. 12, pp. 343-403.
Swezey, O. H., and Williams, F. X.
1932. Some observations on forest insects at the Nahui nursery and vicinity on Hawaii. Proc. Hawaiian Ent. Soc., vol. 8, pp. 179-190.
Syverton, J. T., and Fischer, R. G.
1950. The cockroach as an experimental vector of the virus of spontaneous mouse encephalomyelitis (Theiler). Proc. Soc. Exp. Biol. Med., vol. 74, pp. 296-298.
Syverton, J. T.; Fischer, R. G.; Smith, S. A.; Dow, R. P.; and Schoof, H. F.
1952. The cockroach as a natural extrahuman source of poliomyelitis virus. Fed. Proc., vol. 11, p. 483.
Swarczewsky, B.
1914. Über den Lebenscyclus einiger Haplosporidien. Arch. Protistenk., vol. 33, pp. 49-108, 5 pls.
Tacchini, I.
1946. Ricerca sui batteri simbionti intracellulari della blatte. Boll. Soc. Ital. Biol. Sper., vol. 22, pp. 113-114. [Translated by M. Di Paolo.]
Takahashi, R.
1924. Life-history of Blattidae. (In Japanese.) Dôbuts. Zasshi, Zool. Mag., Tokyo, vol. 36, pp. 215-230.
1926. Observations on the aquatic cockroach Opisthoplatia maculata. (In Japanese.) Dôbuts. Zasshi, Tokyo, vol. 38, pp. 89-92.
1940. Habits and nymph of Phyllodromia humbertiana de Sauss. (Blattidae, Orthoptera). (In Japanese.) Trans. Nat. Hist. Soc. Formosa, vol. 30, pp. 26-29.
Taliaferro, W. H.
1928. A note on the amoeba of the cockroach cultivated by Smith and Barret. Journ. Parasitol., vol. 14, p. 274.
Taschenberg, E. L.
1884. Die Insekten, Tausendfüssler, und Spinnen. In Brehms Thierleben, Bd. 1, Abt. 4, 711 pp.
Tauber, O. E.
1940. Mitotic response of roach hemocytes to certain pathogenes in the hemolymph. Ann. Ent. Soc. Amer., vol. 33, pp. 113-119.
Tauber, O. E., and Griffiths, J. T., Jr.
1942. Isolation of Staphylococcus albus from hemolymph of the roach, Blatta orientalis. Proc. Soc. Exp. Biol. Med., vol. 51, pp. 45-47.
Tejera, E.
1926. Las cucarachas como agentes de diseminación de gérmenes patógenos. Rev. Soc. Argentina Biol., vol. 2, pp. 243-256, 1 pl. [Translated by J. Gotlob.] [French summary in Compt. Rend. Soc. Biol., Paris, vol. 95, pp. 1382-1384.]
Tepper, J. G. O.
1893. The Blattariae of Australia and Polynesia. Trans. Roy. Soc. South Australia, vol. 17, pp. 25-126.
1894. The Blattariae of Australia and Polynesia. Supplementary and additional descriptions and notes. Trans. Roy. Soc. South Australia, vol. 18, pp. 169-189.
Thaxter, R.
1896. Contribution towards a monograph of the Laboulbeniaceae. Mem. Amer. Acad. Arts, Sci., n. s., vol. 12, pp. 187-429.
1902. Preliminary diagnoses of new species of Laboulbeniaceae. V. Proc. Amer. Acad. Arts, Sci., vol. 38, pp. 9-57.
1905. Preliminary diagnoses of new species of Laboulbeniaceae. VI. Proc. Amer. Acad. Arts, Sci., vol. 41, pp. 303-318.
1908. Contribution toward a monograph of the Laboulbeniaceae. Part II. Mem. Amer. Acad. Arts, Sci., n. s., vol. 13, pp. 219-469.
1915. New Indo-Malayan Laboulbeniales. Proc. Amer. Acad. Arts, Sci., vol. 51, pp. 3-51.
1918. New Laboulbeniales from Chile and New Zealand. Proc. Amer. Acad. Arts, Sci., vol. 54, pp. 207-232.
1920. Second note on certain peculiar fungus-parasites of living insects. Bot. Gaz., vol. 69, pp. 1-27.
1931. Contribution towards a monograph of the Laboulbeniaceae. Part V. Mem. Amer. Acad. Arts, Sci., n. s., vol. 16, pp. 1-435.
Thiel, P. H. van, and Wiegand Bruss, C. J. E.
1946. Présence de Prosthenorchis spirula chez les chimpanzés. Son rôle pathogène et son développement dans Blattella germanica. Ann. Parasitol. Hum. et Comp. (1944-45), vol. 20, pp. 304-320. [Reprinted in 1948 in Acta Leidensia, 1947, vol. 18, pp. 200-218.]
Thilow, J. O., and Riley, C. V.
1891. A southern roach in a northern greenhouse. Insect Life, vol. 3, pp. 406-407.
Thompson, W. R.
1951. A catalogue of the parasites and predators of insect pests. Section 1. Parasite host catalogue. Part II. Neuroptera, Odonata, Orthoptera, Psocoptera, Siphonaptera, Thysanoptera. 35 pp. Commonwealth Bureau of Biological Control, Ottawa.
Thomson, J. G., and Lucas, Catherine L. T.
1926. A preliminary note on the study of Endamoeba blattae (Bütschli, 1878) Leidy, 1879, found parasitic in the intestine of Blatta orientalis in England, with some remarks on its generic status. Journ. Trop. Med. Hyg., vol. 29, pp. 41-43, 1 pl.
Tillyard, R. J.
1926. The insects of Australia and New Zealand. 560 pp.
Timberlake, P. H.
1924. Records of the introduced and immigrant chalcid flies of the Hawaiian Islands (Hymenoptera). Proc. Hawaiian Ent. Soc., vol. 5, pp. 418-449.
Toda, T.
1923. Cholera and the ship "cockroach." Journ. Hyg., vol. 21, pp. 359-361.
Todd, A. C.
1941. An addition to the life history of Leidynema appendiculatum (Leidy, 1850) Chitwood, 1932, a nematode parasitic in cockroaches. Journ. Parasitol., vol. 27, Suppl, pp. 34-35.
1943. Thelastoma icemi (Schwenck), a nematode of cockroaches. Journ. Parasitol., vol. 29, pp. 404-406.
1944. On the development and hatching of the eggs of Hammerschmidtiella diesingi and Leidynema appendiculatum, nematodes of roaches. Trans. Amer. Micr. Soc., vol. 63, pp. 54-67.
Townes, H.
1949. The nearctic species of Evaniidae (Hymenoptera). Proc. U. S. Nat. Mus., vol. 99, pp. 525-539.
1951. Family Evaniidae. Genus Astata Latreille. In Muesebeck, C. F. W.; Krombein, K. V.; Townes, H. K., and others, Hymenoptera north of Mexico. U. S. Dept. Agr. Agr. Monogr. No. 2, pp. 655-656, 939-940.
Trager, W.
1932. A cellulase from the symbiotic intestinal flagellates of termites and of the roach, Cryptocercus punctulatus. Biochem. Journ., vol. 26, pp. 1762-1771.
Travassos, L. P.
1917. Contribuções para o conhecimento da fauna helmintológica brazileira. VI. Revisão dos acantocefalos brazileiros. Parte I. Fam. Gigantorhynchidae Hamann, 1892. Mem. Inst. Oswaldo Cruz, vol. 9, pp. 5-61, 24 pls.
1929. Contribuição prelíminar á systemática dos nematóides dos artrópodes. Mem. Inst. Oswaldo Cruz, Suppl. No. 5, pp. 19-25.
Travis, B. V.
1932. A discussion of synonymy in the nomenclature of certain insect flagellates, with the description of a new flagellate from the larvae of Ligyrodes relictus Say (Coleoptera-Scarabaeidae). Iowa State Coll. Journ. Sci., vol. 6, pp. 317-323.
Treat, Mary.
1876. Carnivorous plants of Florida. Harper's Month. Mag., vol. 53, pp. 710-714.
Tryon, H.
1926. Poultry—Manson's eye worm. Queensland Agr. Journ., vol. 25, p. 272.
Tschudi, J. J. von
1847. Travels in Peru, during the years 1838-1842. 506 pp. London.
Tucker, R. W. E.
1952. The insects of Barbados. Journ. Agr., Univ. Puerto Rico, vol. 36, pp. 330-363.
Tulloch, J. B. G.
1939. Insects and other things which arrive by steamer from overseas. Entomologist, vol. 72, pp. 114-116.
Turner, H. J.
1930. South London Entomological Society. Entomologist, vol. 63, p. 143.
Turner, R. E.
1912. Notes on fossorial Hymenoptera.—X. On new species from the Oriental and Ethiopian Regions. Ann. Mag. Nat. Hist., ser. 8, vol. 10, pp. 361-377.
1917. On a collection of Sphecoidea sent by the Agricultural Research Institute, Pusa, Bihar. Mem. Dept. Agr. India, Ent. ser., vol. 5, pp. 173-205.
Uichanco, L. B.
1953. Some features of the distribution of insect life on Philippine mountains. Proc. 7th Pacific Sci. Congr., Pacific Sci. Assoc., Auckland and Christchurch, New Zealand, 1949, vol. 4 (Zoology), pp. 350-357.
Usinger, R. L., and LaRivers, I.
1953. The insect life of Arno. Atoll Res. Bull. No. 15, Pacific Science Board, Nat. Acad. Sci., Nat. Res. Council, Washington, D. C., 28 pp. (Processed.)
Usman, S.
1949. Some observations on the biology of Tetrastichus hagenowii, Ratz.—An egg parasite of the house-cockroach (Periplaneta americana, L.). Current Sci., Bangalore, vol. 18, pp. 407-408.
Uvarov, B. P.
1954. The desert locust and its environment. In Cloudsley-Thompson, J.L., ed., Biology of deserts. Inst. Biol., London, pp. 85-89.
Valcurone, Maria L., and Baggini, A.
1957. Sulle sostanze antibatteriche di origine entomologica. Boll. 1st. Sieroterap. Milanese, vol. 36, pp. 283-305.
Valente, D.
1949. Feeding habits of some Brazilian amphibians. Bol. Fac. Filos. Univ. São Paulo, Zool., 1949, pp. 335-337.
Valentin, G.
1836. Hygrocrocis intestinalis, eine auf der lebendigen und ungestört functionirenden Schleimhaut des Darmkanales vegetirende Conferve. Rep. Anat. Physiol., vol. 1, pp. 110-114, 1 pl.
Valletta, A.
1955. Second contribution to a list of the Orthoptera of the Maltese Islands. Ent. Month. Mag., vol. 91, pp. 55-56.
Van Cleave, H. J.
1946. Remarques sur le genre Moniliformis (Acanthocéphales) et particulièrement sur les espèces parasites des rats. Ann. Parasitol., vol. 21, pp. 142-147.
Van Zwaluwenburg, R. H.
1928. The interrelationships of insects and roundworms. Bull. Exp. Stat. Hawaiian Sugar Planters' Assoc., Ent. ser., No. 20, 68 pp.
1946. Recent immigrant insects. Hawaiian Planters' Rec., vol. 50, pp. 11-17.
1950. New wasp records. Proc. Hawaiian Ent. Soc., vol. 14, p. 16.
Verrill, A. E.
1902. The Bermuda Islands: Their scenery, climate, productions, physiography, natural history, and geology; with sketches of their early history and changes due to man. Trans. Connecticut Acad. Arts and Sci., vol. 11, pp. 413-956.
Vestergaard, Karen.
1958. Cockroaches. In Annual Report 1955-1956, Government Pest Infestation Lab., Springforbi, Denmark, p. 14.
Villadolid, D. V.
1934. Food habits of six common lizards found in Los Baños, Laguna, Philippine Islands. Philippine Journ. Sci., vol. 55, pp. 61-67.
Vlasov, ȊȂ. P.
1929. Biology of Phlebotomus sergenti Parrot. (In Russian.) Russek. Zhurn. Trop. Med., Med. Vet. Parazitol., vol. 7, pp. 688-692. [Translated by D. Kraus.]
1933. Die Fauna der Wohnhöhlen von Rhombomys opimus Licht. und Spermophilopsis leptodactylus Licht. in der Umgebung von Aschhabad. Zool. Anz., vol. 101, pp. 143-158.
Vlasov, ȊȂ. P., and Miram, E. F.
1937. Cockroaches and Orthoptera from the burrows around Ashkhabad. (In Russian.) Trudy Sov. Izuch. Proizvod. Sil, Akad. Nauk, S.S.S.R., Ser. Turkmenskaia, vol. 9, pp. 259-262. [Pertinent sections translated by M. Ycas.]
Vollbrechtshausen, R.
1953. Bakterien als Symbioten, Synöken und Parasiten bei Phyllodromia germanica. Zeitschr. Parasitenk., vol. 15, pp. 437-456. [Translated by H. L. Middleton.]
Vorhies, C. T., and Taylor, W. P.
1922. Life history of the kangaroo rat, Dipodomys spectabilis spectabilis Merriam. U. S. Dept. Agr. Dept. Bull. 1091, 40 pp.
Wainwright, C. J.
1898. Birmingham Entomological Society. Entomologist, vol. 31, p. 304.
Walden, B. H.
1922. Abundance of the German roach in a city dump. Blattella germanica, Linn. Connecticut Agr. Exp. Stat. Bull. No. 234, pp. 188-189.
Walker, E. M.
1912. The Blattidae of Ontario. Canadian Ent., vol. 44, pp. 171-172.
Walker, J. J.
1904. Antipodan field notes. II. A year's insect hunting in New Zealand. Ent. Month. Mag., vol. 40, p. 70.
Walker, T. J., Jr.
1957. Ecological studies of the arthropods associated with certain decaying materials in four habitats. Ecology, vol. 38, pp. 262-276.
Wallace, A. R.
1869. The Malay Archipelago: The land of the orang-utan and the bird of paradise. 638 pp. New York.
1891. Natural selection and tropical nature. 492 pp. London.
Wallick. L. S.
1954. The effects of overcrowding upon Blattella germanica with respect to weight and longevity. M.S. thesis, Ohio State Univ., 33 pp.
Ward, H. B.
1918. Parasitic roundworms. In Ward, H. B., and Whipple, G. C., Freshwater biology, pp. 506-552. New York.
Waterston, J.
1914. New species of Chalcidoidea from Ceylon. Bull. Ent. Res., vol. 5, PP. 325-342.
Watson, J. M.
1945. A new sporozoon, Gregarina rhyparobiae n. sp., from a tropical cockroach,
Rhyparobia maderae. Parasitology, vol. 36, pp. 195-198.
Watson, Minnie, E.
1915. Some new gregarine parasites from Arthropoda. Journ. Parasitol., vol. 2, pp. 27-36, 2 pls.
1916. Studies on gregarines. Including descriptions of twenty-one new species and a synopsis of the eugregarine records from the Myriapoda, Coleoptera and Orthoptera of the world. Illinois Biol. Monogr., vol. 2, pp. 213-468, 15 pls.
1917. Observations on polycystid gregarines from Arthropoda. Journ. Parasitol., 1916, vol. 3, pp. 65-75.
Watson, W.
1907. Tropical cockroach. In Lucas, W. J., Additions to the wild fauna and flora of the Royal Botanic Gardens, Kew: VI. Roy. Bot. Gard., Kew, Bull. Misc. Inform., No. 10, pp. 402-403, 1 pl.
Weber, N. A.
1938. The food of the giant toad, Bufo marinus (L.), in Trinidad and British Guiana with special reference to the ants. Ann. Ent. Soc. Amer., vol. 31, pp. 499-503.
1954. The insect fauna of an Iraq oasis, the city of Baghdad. Ent. News, vol. 65, pp. 178-182, 203-206.
Weber, P. W.
1948. Rhipidius pectinicornis Thunberg. Proc. Hawaiian Ent. Soc., vol. 13, p. 202.
1951. Anastatus blattidarum Ferrière. Proc. Hawaiian Ent. Soc., vol. 14, p. 223.
Webster, W. H. B.
1834. Narrative of a voyage to the southern Atlantic Ocean in the years 1828, 29, 30, performed in H. M. Sloop Chanticleer, under the command of the late Captain Henry Foster, F. R. S. &c. by order of the Lords Commissioners of the Admiralty. Vol. 1, 399 pp. London.
Wedberg, S. E.; Brandt, C. D.; and Helmboldt, C. F.
1949. The passage of microorganisms through the digestive tract of Blaberus craniifer mounted under controlled conditions. Journ. Bact., vol. 58, pp. 573-578.
Weill, R.
1929. 2. Notes protistologiques indochinoises (première série). La présence d'un infusoire du genre Isotricha (I. caulleryi n. sp.) chez un insecte (Periplaneta americana Forbes) et sa signification possible. Arch. Zool. Exp. et Gén., Notes et Rev., vol. 69, pp. 21-26.
Weiss, H. B.
1917. Undesirable insect immigration into New Jersey. Canadian Ent., vol. 49, pp. 293-298, pl. XIV.
Welch, E. V.
1939. Insects found on aircraft at Miami, Fla., in 1938. Publ. Health Rep., vol. 54, pp. 561-566.
Welch, F. D.
1935. Large foreign cockroach in Kent. Entomologist, vol. 68, p. 122.
Wellmer, L.
1910. Beitrag zur Kenntnis der Sporozoenfauna Ostpreussens. Zool. Anz., Leipzig, vol. 35, pp. 530-534.
1911. Sporozoen ostpreussicher Arthropoden. Schrift. Phys.-Ökonom. Ges. Königsberg i. Pr., vol. 52, pp. 103-164, 1 pl.
Wenrich, D. H.
1932. The relation of the protozoan flagellate, Retortamonas gryllotalpae (Grassi, 1879) Stiles, 1902 to the species of the genus Embadomonas Mackinnon, 1911. Trans. Amer. Micr. Soc., vol. 51, pp. 225-238.
Westwood, J. O.
1839. An introduction to the modern classification of insects ... vol. 1, 462 pp. London.
1843. On Evania and some allied genera of hymenopterous insects. Trans. Ent. Soc. London., vol. 3, pp. 237-278.
1854. Economy of Evania. Proc. Ent. Soc. London, 1854, in Trans. Ent. Soc. London, n. s., vol. 3, pp. 21-22.
1854a. The cockroach parasite. Gardener's Chron. and Agr. Gaz., No. 33, p. 533.
1869. Exhibitions, etc. Proc. Ent. Soc. London, April 5, 1869, in Trans. Ent. Soc. London, 1869, p. x.
1876. Exhibitions, etc. Meeting of November 1, 1876. Trans. Ent. Soc. London, 1876, p. xxxii.
Wetmore, Alexander.
1916. Birds of Porto Rico. U. S. Dept. Agr. Dept. Bull. 326, 140 pp.
1940. A systematic classification for the birds of the world. Smithsonian Misc. Coll., vol. 99, No. 7, pp. 1-11.
Wharton, D. R. A.; Miller, G. L.; and Wharton, M. L.
1954. The odorous attractant of the American cockroach, Periplaneta americana (L.). I. Quantitative aspects of the response to the attractant. Journ. Gen. Physiol., vol. 37, pp. 461-469.
Wharton, R. H.
1951. The behaviour and mortality of Anopheles maculatus and Culex fatigans in experimental huts treated with DDT and BHC. Bull. Ent. Res., vol. 42, pp. 1-20, 1 pl.
Wheeler, W. M.
1889. The embryology of Blatta germanica and Doryphora decimlineata. Journ. Morphol., vol. 3, pp. 291-386.
1900. A new myrmecophile from the mushroom gardens of the Texas leaf-cutting ant. Amer. Nat., vol. 34, pp. 851-862.
1910. Ants. Their structure, development and behavior. 663 pp. New York.
1911. A desert cockroach. Journ. New York Ent. Soc., vol. 19, pp. 262-263.
1928. The social insects. Their origin and evolution. 378 pp. New York.
White, G.
1905. The natural history and antiquities of Selborne in the County of Southampton. 476 pp. London.
Whitfield, F. G. S.
1940. Air transport, insects and disease. Bull. Ent. Res., vol. 30, pp. 365-442.
Whittingham, H. E., and Rook, A. F.
1923. Observations on the life-history and bionomics of Phlebotomus papatasii. British Med. Journ., Dec. 15, pp. 1144-1151.
Wille, J.
1920. Biologie und Bekämpfung der deutschen Schabe (Phyllodromia germanica L.). Monogr. Angew. Ent., Nr. 5, Zeitschr. Angew. Ent., Beiheft 1, Band 7, 140 pp.
Willemet, R.
1784. Lettre aux auteurs du Journal de Physique. Journ. Physique, Paris, vol. 24, p. 62.
Williams, C. L.
1931. Effect of fumigation on cockroaches on ships. Publ. Health Rep., vol. 46, pp. 1680-1694.
Williams, E. C., Jr.
1941. An ecological study of the floor fauna of the Panama rain forest. Bull. Chicago Acad. Sci., vol. 6, pp. 63-124.
Williams, F. X.
1918. Dolichurus stantoni Ashmead, a Philippine wasp parasitic on cockroaches. Hawaiian Planters' Rec., vol. 19, pp. 251-255.
1919. Philippine wasp studies. Part 2. Descriptions of new species and life history studies. Rep. Exp. Stat. Hawaiian Sugar Planters' Assoc., Ent. Ser., Bull. No. 14, pp. 19-186.
1925. Wasps from tropical countries. Proc. Hawaiian Ent. Soc., vol. 6, pp. 16-17.
1928. Studies in tropical wasps—their hosts and associates (with descriptions of new species). Bull. Exp. Stat. Hawaiian Sugar Planters' Assoc., Ent. Ser., Bull. No. 19, 179 pp.
1928a. [Rhinopsis caniculatus Say.] Proc. Hawaiian Ent. Soc., vol. 7, p. 227.
1929. Notes on the habits of the cockroach-hunting wasps of the genus Ampulex, sens. lat., with particular reference to Ampulex (Rhinopsis) caniculatus Say. Proc. Hawaiian Ent. Soc., vol. 7, pp. 315-329.
1931. [Evania attracted to honey dew.] Proc. Hawaiian Ent. Soc., vol. 7, p. 334.
1936. Biological studies in Hawaiian water-loving insects. Part I. Coleoptera or beetles (pp. 235-273). Part II. Odonata or dragonflies (pp. 273-352). Proc. Hawaiian Ent. Soc., vol. 9, pp. 235-352.
1941. Blitzkrieg on cockroaches will be shown at Maui fair. Hawaii Farm and Home, vol. 4, p. 9.
1941a. An apparently undescribed Tachysphex (Hymenoptera, Larridae) from Trinidad, B.W.I. Proc. Roy. Ent. Soc. London, ser. B, vol. 10, pp. 197-199.
1942. The New Caledonian cockroach wasp (Ampulex compressa) in Hawaii. Hawaiian Planters' Rec., vol. 46, pp. 43-48.
1942a. Ampulex compressa (Fabr.), a cockroach-hunting wasp introduced from New Caledonia into Hawaii. Proc. Hawaiian Ent. Soc., vol. II, pp. 221-233.
1944. Cockroaches are their dish. Hawaii Farm and Home, vol. 7, p. 18.
1945. The aculeate wasps of New Caledonia, with natural history notes. Proc. Hawaiian Ent. Soc., vol. 12, pp. 407-452.
1946. Entomology. Rep. Comm. Exp. Stat. Hawaiian Sugar Planters' Assoc., 1944/45, pp. 16-22.
1946a. Rhipidius sp. in Hawaii. Proc. Hawaiian Ent. Soc., vol. 12, p. 485.
Williams, F. X.; Van Zwaluwenburg, R. H.; and Swezey, O. H.
1931. Handbook of the insects and other invertebrates of Hawaiian sugar cane fields. Exp. Stat. Hawaiian Sugar Planters' Assoc., 400 pp.
Willis, E. R., and Lewis, N.
1957. The longevity of starved cockroaches. Journ. Econ. Ent., vol. 50, pp. 438-440.
Willis, E. R.; Riser, G. R.; and Roth, L. M.
1958. Observations on reproduction and development in cockroaches. Ann. Ent. Soc. Amer., vol. 51, pp. 53-69.
Wilson, G. S., and Miles, A. A.
1955. Topley and Wilson's principles of bacteriology and immunity. 4th ed., vol. 1, 1,106+xlviii pp. Baltimore.
Wolcott, G. N.
1924. The food of Porto Rican lizards. Journ. Dept. Agr. Porto Rico, vol. 7, pp. 5-37.
1924a. Entomologica económica Puertorriqueña. Estac. Exp. Insular, Río Piedras, Bol. No. 32, 176 pp.
1936. "Insectae borinquensis." Journ. Agr. Univ. Puerto Rico, vol. 20, pp. 1-627.
1937. What the giant Surinam toad, Bufo marinus L., is eating now in Puerto Rico. Journ. Agr. Univ. Puerto Rico, vol. 21, pp. 79-84.
1941. A supplement to "Insectae borinquensis." Journ. Agr. Univ. Puerto Rico, vol. 25, pp. 22-158.
1950. The insects of Puerto Rico. Journ. Agr. Univ. Puerto Rico, 1948, vol. 32, pp. 1-224.
1951. The insects of Puerto Rico: Hymenoptera, acknowledgements, addenda et corrigenda and index. Journ. Agr. Univ. Puerto Rico, 1948, vol. 32, pp. 749-975.
1953. The food of the mongoose (Herpestes javanicus auropunctatus Hodgson) in St. Croix and Puerto Rico. Journ. Agr. Univ. Puerto Rico, vol. 37, pp. 241-247.
Wolf, J.
1924. Contribution à la morphologie des bacteroïdes des blattes (Periplaneta orientalis L.). Compt. Rend. Soc. Biol., Paris, vol. 91, pp. 1180-1182.
1924a. Contribution à localisation des bactéroïdes dans les corps adipeux des blattes (Periplaneta orientalis L.) Compt. Rend. Soc. Biol., Paris, vol. 91, pp. 1182-1183.
Wollman, E.
1926. Observations sur une lignée aseptique de blattes (Blattella germanica) datant de cinq ans. Compt. Rend. Soc. Biol., Paris, vol. 95, pp. 164-165.
1927. Le rôle des mouches dans le transport de quelques germes importants pour la pathologie tunisienne. Arch. Inst. Pasteur, Tunis, vol. 16, pp. 347-364.
Wollman, E.; Anderson, C.; and Colas-Belcour, J.
1928. Recherches sur la conservation des virus hémophiles chez les insectes. Arch. Inst. Pasteur, Tunis, vol. 17, pp. 229-232.
Wolters, M.
1891. Die Conjugation und Sporenbildung bei Gregarinen. Arch. Mikros. Anat., vol. 37, pp. 99-138, 4 pls.
Womersley, H.
1956. On some new Acarina—Mesostigmata from Australia, New Zealand and New Guinea. Journ. Linn. Soc. London, vol. 42, pp. 505-599.
Woodcock, H. M.
1904. On Cystobia irregularis (Minch.) and allied "neogamous" gregarines. (Preliminary note.) Arch. Zool. Exp. et Gén., ser. 4, vol. 2, Notes et Rev. No. 8, pp. cxxv-cxxviii.
Wray, D. L., and Brimley, C. S.
1943. The insect inquilines and victims of pitcher plants in North Carolina. Ann. Ent. Soc. Amer., vol. 36, pp. 128-137.
Yakimov, V. L., and Miller, G. A.
1922. Les protozoaires de l'intestin de l'homme en dehors de l'organisme de l'homme. L'examen de l'intestin du Periplaneta orientalis. Bull. Soc. Path. Exot., Paris, vol. 15, pp. 8-11. [Essentially the same article appeared in Russian in Arkh. Russk. Protist. Obsh., vol. 1, pp. 131-132; German summary p. 133.]
Yamaguti, S., and Miyata, I.
1942. Über die Entwicklungsgeschichte von Moniliformis dubius Meyer, 1933 (Acanthocephala) mit besonderer Berücksichtigung seiner Entwicklung im Zwischenwirt. 32 pp. Kyoto.
Yarwood, E. A.
1937. The life cycle of Adelina cryptocerci sp. nov., a coccidian parasite of the roach Cryptocercus punctulatus. Parasitology, vol. 29, pp. 370-390, 4 pls.
Yetwin, I. J.
1932. A study of the intracellular symbionts in the fat body of Blatella germanica Linn. M. S. thesis, Univ. Chicago, 56 pp.
1953. The cytology of the fat body of the common roach, Blatella germanica, with emphasis on intracytoplasmic inclusion bodies. Mil. Surgeon, vol. 113, pp. 111-113.
Yokogawa, S.
1924. On the cancroid growths caused by Gongylonema-orientale n. sp. in the rat. "Gann," Japanese Journ. Cancer Res., vol. 18, pp. 48-69, 2 pls.
1925. On a new species of nematode, Gongylonema orientale, found in Formosa. Journ. Parasitol., vol. 11, pp. 195-200, 1 pl.
1925a. On the cancroid growths caused by Gongylonema orientale n. sp. in the rat. Taiwan Igakki Zasshi, Journ. Med. Assoc. Formosa, No. 240, pp. 1-20.
Young, F. N.
1949. Insects from burrows of Peromyscus polionotus. Florida Ent., vol. 32, p. 77.
Young, M. D.
1935. Description, incidence, and cultivation of Tetratrichomastix blattidarum n. sp. from the cockroach. Journ. Parasitol., vol. 21, pp. 309-310.
1937. Cockroaches as carriers of Giardia cysts. Journ. Parasitol., vol. 23, pp. 102-103.
Zacher, F.
1917. Die Geradflüger Deutschlands und ihre Verbreitung. 287 pp. Jena.
1920. Schaben als Schädlinge in Gewächshäusern. Gartenflora, Berlin, No. 13/14, pp. 165-168. [Translated by H. L. Middleton.]
Zalesskii, ǏǓ. M.
1939. Sur une nouvelle blatte permienne portant un oviscapte. [Par Georges Zalessky.] Ann. Soc. Géol. du Nord, vol. 64, pp. 85-94.
1953. New Permian cockroaches from the family Spiloblattinidae. (In Russian.) Ent. Obozrenie, vol. 33, pp. 266-272. [Pertinent sections translated by M. Ycas.]
Zappe, M. P.
1918. A cockroach pest of greenhouses, Pycnoscelus (Leucophaea) surinamensis Linn. Connecticut Agr. Exp. Stat., Bull. No. 203, pp. 302-313, 4 pls.
1918a. Life history and development of the greenhouse cockroach. Pycnoscelus surinamensis Linn. Connecticut Agr. Exp. Stat., Bull. No. 211, pp. 311-313.
Zasukhin, D. N.
1928. Zur Frage über die Parasiten der Protozoen. Parasiten von Nyctotherus ovalis Leidy. Arch. Protistenk., vol. 64, pp. 61-70, 2 pls.
1929. Usloviia obitaniia, stroenie i razvitie Endamoeba blattae (Bütschli) Leidy (1879.) [Lebensbedingungen, Cytologie und Entwicklung von Endamoeba blattae Büt. (Leidy) 1879.] Russk. Arkh. Protistenk., vol. 8, pp. 163-244, 4 pls. (German summary pp. 240-244.)
1930. Lebensbedingungen, Cytologie und Entwicklung von Endamoeba blattae Büt. (Leidy) 1879. Arch. Protistenk., vol. 70, pp. 681-686.
1934. Hyperparasitism in protozoa. Quart. Rev. Biol., vol. 9, pp. 215-224.
Zhivago, P.
1909. Ueber Vermehrung bei Pleistophora periplanetae Lutz und Splendore. Zool. Anz., vol. 34, pp. 647-654.
Zimmerman, E. C.
1944. New cockroach egg parasite from Honolulu. Proc. Hawaiian Ent. Soc., vol. 12, p. 20.
1948. Insects of Hawaii. Vol. 2, Apterygota to Thysanoptera, inclusive. 475 pp. Honolulu.
Zmeev, G. I.
1936. Les insectes synanthropes comme hôtes intermédiaires et hôtes vecteurs des helminthes au Tadjikistan. (In Russian, French summary.) Trudy Tadzhiksk. Bazy Akad. Nauk SSSR, vol. 6, pp. 241-248. [Russian text translated by M. Ycas.]
1940. Certain points in the epidemiology of dysentery and its endemic foci in Central Asia connected with the cockroach Shelfordella tartara Sauss. (In Russian.) Second conference on Parasitological problems. November 1940, Leningrad, p. 35. Izd. Akad. Nauk SSSR, Moscow. [From Rev. App. Ent., 1946, ser. B., vol. 34, p. 110.]
Zulueta, A. de.
1916. Sobre la estructura y bipartición de Nyctotherus ovalis Leidy. Trab. Mus. Nac. Cienc. Nat., Madrid, ser. Zool., No. 26, pp. 1-16.
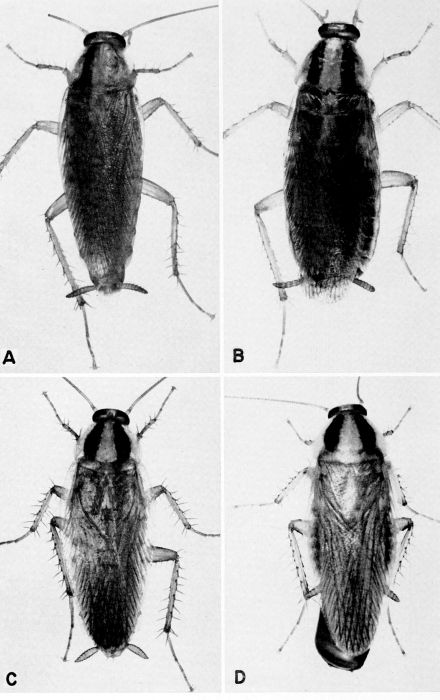 Plate 5
Plate 5 Plate 7
Plate 7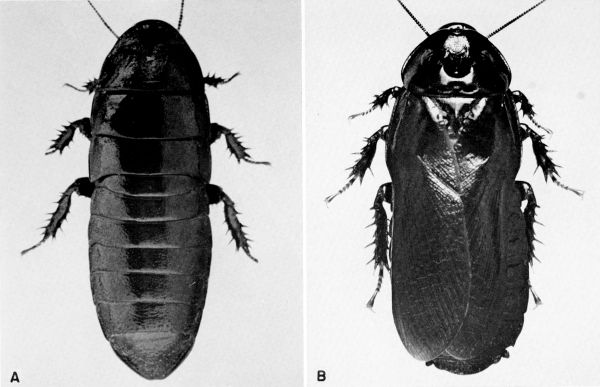 Plate 8
Plate 8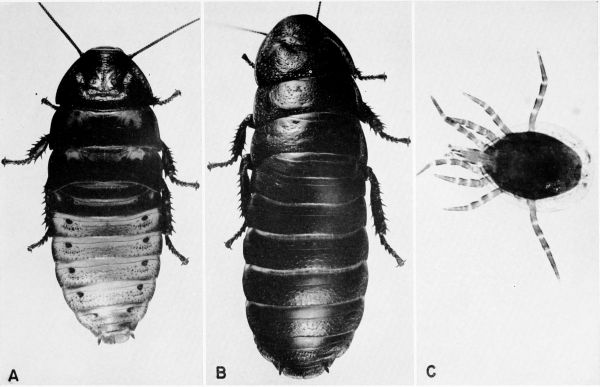 Plate 12
Plate 12 Plate 16
Plate 16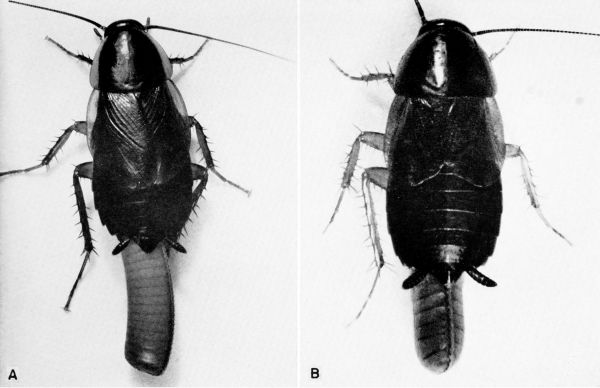 Plate 17
Plate 17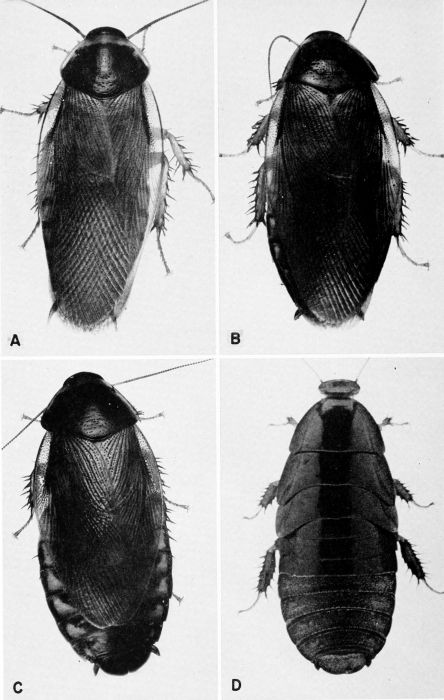 Plate 24
Plate 24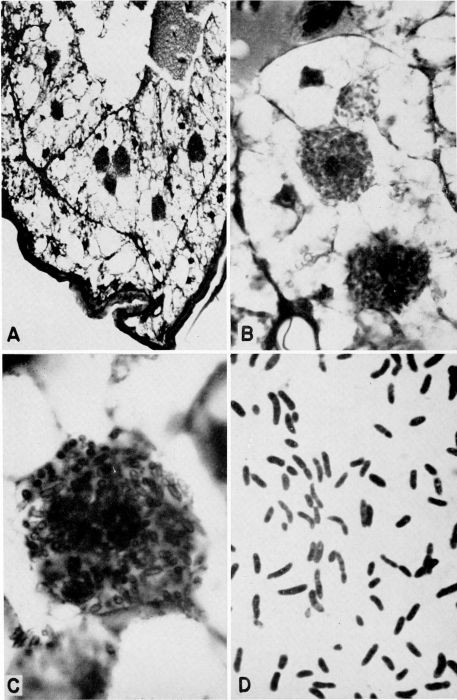 Plate 26
Plate 26 Plate 27
Plate 27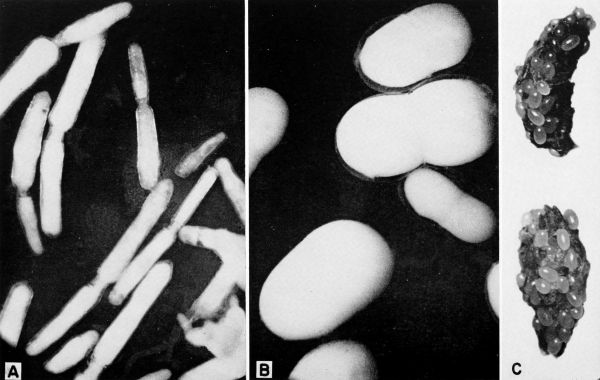 Plate 28
Plate 28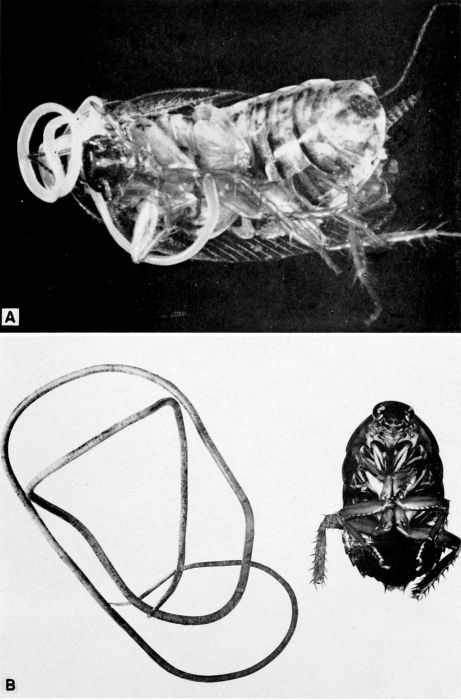 Plate 29
Plate 29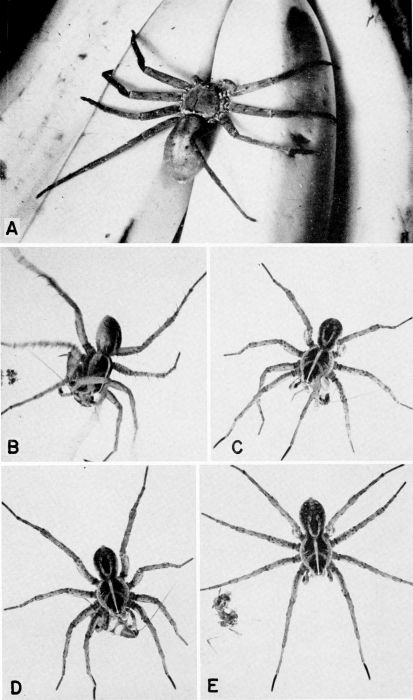 Plate 30
Plate 30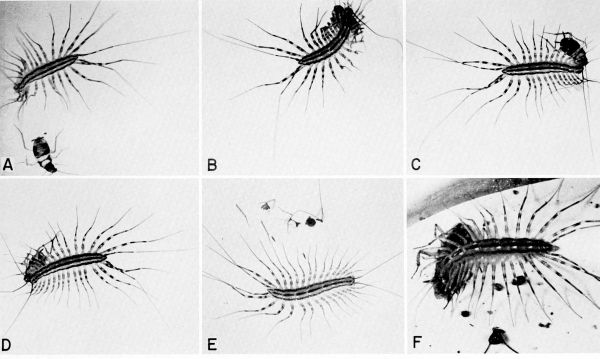 Plate 31
Plate 31 Plate 32
Plate 32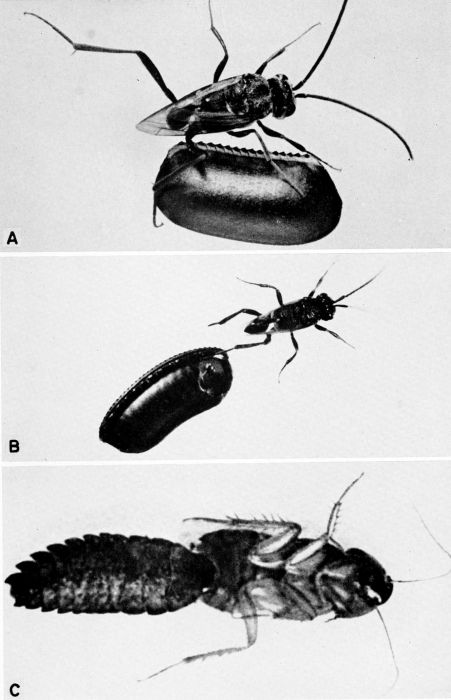 Plate 33
Plate 33 Plate 34
Plate 34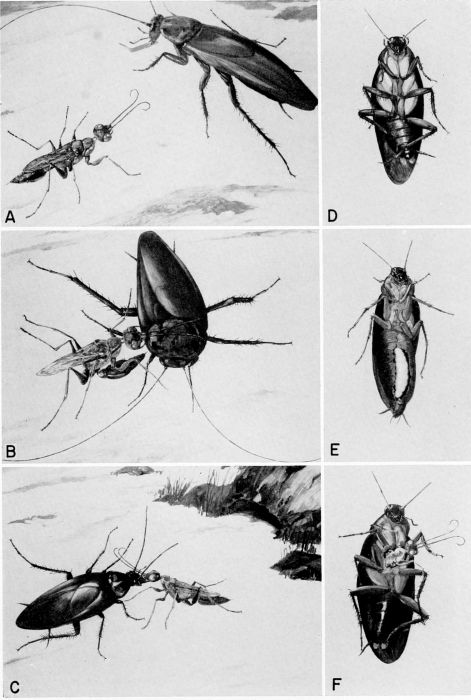 Plate 35
Plate 35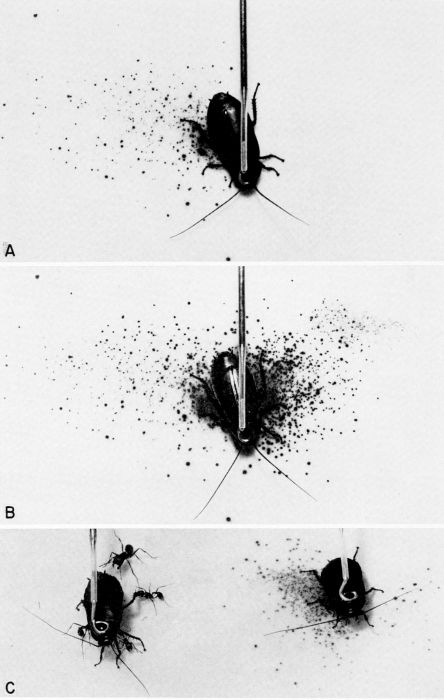 Plate 36
Plate 36Plate and page numbers in boldface type indicate illustrations. In general, entries are placed in the index as unmodified substantives except where a modifier contributes significantly to the identification of the item (e.g., blue heads, prairie dog). This index should be used in conjunction with the indexed check list of natural associations (pages 290 to 310) because these are not repeated here. All experimental associations are indexed below except those involving Blatta orientalis, Blattella germanica, and Periplaneta americana that are cited on pages that also contain references to the natural associations of these three species.
A
Abies sp., 257
Acacia, 56, 69
Acacia farnesiana, 155, 164
harpophylla, 55
sp., 155
Acanthinevania princeps, 235
Acanthocephala, 203
Acanthogyna deplanata, 7
Acaridae, 217
Acarina, 210, 216
Acheta domestica, 320
Achromobacter hyalinum, 110
sp., 110
Achromobacteriaceae, 110
Acridotheres tristis, 281
Acrocomia aculeata, 142
Acromyrmex lobicornis, 312, 313
lundi, 312
niger, 312
octospinosus, 312
silvestrii, 312
Acropyga acutinventris, 317
Actinocephalidae, 184
Actinomycetaceae, 124
Actinomycetales, 123
Adeleidae, 184
Adelina cryptocerci, 184-185
Aechmaea porteoides, 144
Aerobacter aerogenes, 111
cloacae, 112
sp., 112
Aeschnidae, 224
Agamerion metallica, 243
Agamidae, 275
Agamospirura parahormeticae, 205
Agaricus, 164
Agave, 43, 57
Agelaius xanthomus, 282
Agis orientalis, 7, 27
Aglaopteryx absimilis, 7, 35, 156, 317
devia, 7
diaphana, 7, 35, 36, 145, 146, 290, 339
facies, 7, 35, 156, 290, 317, 318
gemma, 7, 36, 140, 143, 144, 153, 158, 159, 339
vegeta, 7, 146
ypsilon, 7, 75
Aircraft, 87-90
Alcaligenes faecalis, 111
recti, 111
viscosus, 111
Algaroba, 45, 156, 164
Allacta similis, 7, 36, 75, 276, 290
Allothereua maculata, 223
Alluaudellina cavernicola, 7, 17
Alluaudella cavernicola, 7
Alpinia, 163
Alsophila sp., 139
Alsophilas, 164
Amazonina emarginata, 7, 36, 146, 158
Ameiva exsul, 275
sp., 275
Amitermes, 102
Amoeba blattae, 177
coli, 178
undetermined sp., 181
Amoebidae, 177
Amoebina, 177
Amphibia, 269-272
Amphibians, 3, 353
Amphibolurus barbatus, 275
Amphoromorpha blattina, 139
sp., 139
Ampulex, 2, 256
amoena, 256-257
assimilis, 257
canaliculata, 94, 257-258, 352
compressa, 258-259, 352; pl. 35
compressiventris, 260
fasciata, 259
novarae, 256
ruficornis, 259
siberica, 260
sibirica, 259
sonnerati, 260
Ampulicidae, 256
Anacardiaceae, 157
Ananas comosus, 144
Anaphothrips sp., 317
Anaplecta asema, 7, 36
azteca, 7, 145
decipiens, 7, 36
fallax, 7, 36
hemiscotia, 7 36
lateralis, 7, 36
mexicana, 7, 145
spp., 88, 145, 290
Anastatus blattidarum, 246
blattidifurax, 245
floridanus, 94, 245, 255, 319; pl. 34
tenuipes, 94, 246, 255, 350
Anatidae, 277
Anax strenuus, 224
Ancylostoma caninum, 209
ceylanicum, 209
duodenale, 209
Ancylostomidae, 209
Androctonus australis, 211
Aneurina tahuata, 7, 160
viridis, 7, 155, 160, 161
Anguillula macrura, 195
Anisogamia, 26
Annato, 159
Annetta, Steamship, 149
Anoetidae, 217
Anolis carolinensis, 273, 346
cristatellus, 273
equestris, 273, 345, 346
grahami, 274
leachi, 274
pulchellus, 273
sagrei, 274
sp., 274
stratulus, 274
Anseriformes, 277
Antagonism, interspecies, 341-343
Antbird, 280
Antibiotics, 97-98, 346
Ants, 57, 266-268, 316, 317, 350
Argentine, 268, 319
army, 228, 267, 268, 280, 350
big-headed, 268
carpenter, 267
hosts of cockroaches, 311-314
predaceous, 266
safari, 267
wood, 317
Aorurus (Thelastoma) appendiculatus, 196
diesingi, 195
philippinensis, 193
sphaerolaima, 194
Aotes zonalis, 284
Aotus, 284
Aphaenogaster picea, 267
Aphelocoma coerulesens, 280
Aphids, 62
Aphlebia maculata, 10
punctata, 10
Apocynaceae, 161
Apolyta, 9
Apotrogia angolensis, 7, 17
Apple, 166
Aptera cingulata, 7
fusca, 7, 290, 329
Apteroblatta perplexa, 7, 18
Aquifoliaceae, 158
Arachnida, 211
Arachnids, 34
Araceae, 143
Aranea, 214
Araneida, 214
Arbor vitae, 44
Archiacanthocephala, 203
Archiblatta hoevenii, 7, 329
Archimandrita marmorata, 7, 147
tessellata, 7, 147
Arenivaga apacha, 7, 23, 26, 27
bolliana, 7, 23, 26, 27, 37, 290
erratica, 7, 23, 26, 27
floridensis, 7, 24, 37
grata, 7, 17, 18, 37
roseni, 7, 24, 26, 27, 291
tonkawa, 7, 24
Aristida pennata, 65, 141
Aristiger histrio, 7, 37, 159, 316
Armadillo, 287
Army transports, 84
Arthroleptis variabilis, 270
Arthromitaceae, 124
Arthromitus intestinalis, 124
Arthropoda, 210-268
Arum sp., 143
Ascalaphidae, 227
Ascaridae, 209
Ascaris lumbricoides, 209
sp., 209
suum, 209
Ascaroidea, 209
Aschelminthes, 192, 204, 209
Ascomycetes, 133
Asmorrhiza longistylis, 240
Asparagus officinalis, 235
Aspen, 46, 152, 164
Aspergillaceae, 129
Aspergillus flavus, 130
fumigatus, 130
niger, 130
sp., 130, 131
sydowi, 130
tamarii, 130
Aspiduchus borinquen, 7, 18, 37, 333-334
cavernicola, 8, 18, 334
deplanatus, 7, 8, 37
Asplenium nidus, 44
Assembling of cockroaches, 331-334
Associations, 6, 15, 91-96
among cockroaches, 324-343
biotic, 2, 91, 94-96
classification of, 91, 95-96
ecological, 14-90, 324-343
facultative, 95-96
familial, 325-330
gregarious, 330-336
interspecies, 337-343
intraspecies, 336-337
mutualism, 96-102
number of, 3, 5
obligate, 95
obscure, 96, 316-319
with man, 70-90
Astata, 255
Ateles dariensis, 284
Athel, 39
Atta cephalotes, 313
fervens, 313
lundi, 312
nigra, 312
octospinosa, 313
sexdens, 313
texana, 313
Attacus atlas, 320
Attaphila aptera, 8, 313
bergi, 8, 312, 313
flava, 8, 314
fungicola, 8, 313
schuppi, 8, 312
sexdentis, 8, 313
sp., 313
Atticola mortoni, 8, 312
Attraction, intraspecies, 328-334
to decaying material, 53, 59-63
to honeydew, 62
to lights, 52, 61, 64, 66, 79, 81
Audreia bromeliadarum, 8, 31, 37, 145
jamaicana, 8, 37
marginata, 11
Aves, 276-282
Avicularia avicularia, 214
sp., 214
B
"B. aerobia del pseudoedema maligno," 125
B. alcaligenes beckeri, 125
faecalis, 111
recti, 111
"B. del pseudoedema maligno," 125
B. violaceus, 106
Baboon, 286
Bacillaceae, 120
Bacilli, colon, 126
paracolon, 113
"Bacillo del barbone dei bufali," 126
"proteisimile," 126
"similcarbonchio," 126
"similtifo," 126
"tifosimile," 126
"Bacillus," 126
"subtilis group," 122
Bacillus acidi lactici, 112
albolactis, 120
alcaligenes faecalis, 111
anthracis, 120
bütschlii, 120
cereus, 120
circulans, 120
cloacae, 112
faecalis alkaligenes, 111
flacheriae, 121
lactis aerogenes, 111
megaterium, 121
monachae, 121
periplanetae, 121
prodigiosus, 117
radiciformis, 121
stellatus, 121
subtilis, 121
tritus, 122
Bacteria, 2-3, 100-101, 104-127, 346
"spirochaetoid," 127
Bacteriaceae, 119
"Bacterium," 126
Bacterium alkaligenes, 119
delendae-muscae, 120
haemophosphoreum, 120
monache, 121
prodigiosum, 117
Bacteroides uncatus, 119
Bacteroids, 96-100; pl. 26
Badger, 289
Balantidium, 101
blattarum, 187
coli, 187
ovatum, 187
praenucleatum, 187
sp., 187
Ballota nigra, 38
Balta godeffroyi, 8, 37
patula, 8, 291
platysoma, 8, 317
quadricaudata, 8, 37, 141
scripta, 8, 37, 141, 326
torresiana, 8, 38, 141
verticalis, 8, 38, 141
Bamboo, 44, 141
Bambusicola thoracica, 277
Banana, 36, 40, 53, 54, 58, 66, 82, 146-151, 163, 165, 215
blossoms, 36, 41, 56, 57, 146-148
Bantua stigmosa, 8, 99
"Barata selvagem," 194, 199, 200
"sylvestre," 188, 189
"Baratas de pau podre," 200
Barbulanympha, 102
coahoma, 173
estaboga, 173
laurabuda, 174
sp., 175
ufalula, 174
wenyoni, 174
Bark, 36-38, 42, 43, 45-65, 141, 152, 153, 160, 164, 165
Bass, large-mouth black, 269
Bassariscus astutus, 288
Bat, 77, 283
caves, 17-18, 21-23
Batrachians, unidentified, 272
Bayberry, 43, 44, 152
Beauveria bassiana, 131
Bedbug, 321-322
Beech, 47, 61, 62
Bees, 314, 318
Beetles, 26, 34, 57, 63, 91, 229-234, 318, 347, 348
black (see Blatta orientalis)
black larder, 234
rhinocerus, 317
Bertramia blatellae, 185
Beta maritima, 47, 153
vulgaris cicla, 153
Binema mirsaia, 193
Birds, 3, 4, 276-282, 353
nests, 35, 318
Bites, cockroach, 83, 322, 336-337
mite, 220
spider, 215
Bixa sp., 159
Bixaceae, 159
Blabera fusca, 8, 322
Blaberus atropos, 8, 18, 38, 125, 147, 203, 204, 291, 322, 344
boliviensis, 8, 147
clarazianus, 8
craniifer 8, 18, 75, 97, 99, 116-118, 132, 138, 291, 322, 330, 333, 336, 339, 344; pls. 1, 2, 28
cubensis, 8
discoidalis, 8, 38, 75, 85, 147, 151, 163, 291, 318, 330, 334, 339
giganteus, 8, 18, 38, 99, 291; pl. 3
"maderae," 11, 248
spp., 38, 290, 291
Blackbird, 282
Puerto Rican, 282
yellow-shouldered, 282
Blaptica dubia, 8, 292
Blastocystidaceae, 133
Blastocystis hominis, 133
sp., 133
"Blatella americana," 108, 110, 119, 121, 122
Blatta aegyptiaca, 13
aethiopica, 13
americana, 12
caraibea, 79
concinna, 10
humbertiana, 8
(Shelfordella) lateralis, 8, 18, 38, 71, 75, 292
melanocephala, 13
notulata, 10
orientalis, 8, 18, 38, 70-72, 75-76, 84, 85, 88, 96, 99, 100, 118, 123, 128, 129, 131, 151, 153, 162, 163, 190, 204, 205, 212, 219, 222, 224-226, 232, 235, 237, 245, 255, 269, 275, 276, 292-293, 320-323, 325-327, 331, 332, 334-336, 338, 341, 342, 346, 347, 352; pls. 4, 27
sp., 292
Blattaria, 3, 224
Blattarum alatarum, 83
Blattelicola blattelicola, 193
Blattella bisignata, 8
brunneriana, 11
chichimeca, 10
delicatula, 8
germanica, 8, 17, 18, 39, 70-72, 76 83-85, 87, 88, 96-101, 103-105, 114, 118, 119, 124, 126, 131, 143, 171, 192, 204, 205, 211, 212, 219, 222, 225-227, 234-236, 240, 241, 255, 272, 276, 277, 283, 293-294, 320, 321, 323, 324, 328, 331, 332, 334, 335, 337-342, 347-349; pls. 5, 26, 31
humbertiana, 8, 39, 142, 144, 294, 336
lituricollis, 8, 263, 295
nahua, 11
schubotzi, 8, 76
spp. 88, 145, 295
vaga, 8, 27, 39, 77, 135, 141, 166, 255, 293, 320, 328; pl. 5
Blatticida ashmeadi, 243
pulchra, 243
Blatticidella ashmeadi, 243
Blatticola, 199
aegyptiaca, 195
blattae, 193-194
blatticola, 193
"Blattidae sylvestres," 200
Blattilaelaps nauphoetae, 216
Blattina concinna, 10
Blattisocius tineivorus, 216
triodons, 216
Blattophila sphaerolaima, 194
sphaerolaima var. javanica, 194
supellaima, 194
Blattotetrastichus hagenowii, 249
Bluegills, 269
Blue heads, 269
Blue jay, 280, 346
Bobolink, 282
Bodo Blattae, 167
sp., 167
Bodonidae, 167
Bog, peat, 46
Boraginaceae, 161
Bounty, H. M. S., 83
Brachygaster, 350
minutus, 235-236, 351
Bracken, 45, 47
Brambles, 51, 155
Breadfruit, 162
Bream, 269
Bromeliaceae, 144
Bromeliads, 31, 36, 37, 41, 42, 45, 49, 50, 54 56, 57, 68, 144-146, 320
Brucella abortus, 119
Buboblatta armata, 8, 145
Bucerotidae, 279
Bufo funereus, 270
ictericus, 270
marinus, 270, 345, 346, 353
valliceps, 270
Bufonidae, 270
Bulhõesia bulhõesi, 200
icemi, 200
magalhãesi, 194
severianoi, 200
Bullfrog, 271
Burrows, 27
cockroach, 55, 58, 67, 68, 328, 344
relative humidity in, 26
vertebrate, 23-24, 26, 27, 29
Bursera simaruba, 157
Burseraceae, 157
Busaria blattarum, 188
Busariidae, 187
Buthidae, 211
Buthus australis, 211-212
Butterfly nests, 66, 156, 317
Byrsotria cabrerae, 8, 40
fumigata, 8, 19, 40, 225, 333, 354; pl. 6
C
Cacao, 43, 52, 53, 55
Cacomistle, 288
Cafards, 207
Cahita borero, 8, 40
nahua, 8, 40
Caladium, 66, 143
Callithricidae, 285
Callithrix chrysoleucos, 285
chrysolevea, 285
jacchus, 285
Callitris sp., 55
Calluna vulgaris, 160
Calodexia venteris, 228
spp., 228
Caloglyphus spinitarsus, 217
sp., 218
Calyptracordia alba, 161
Camouflage, 344
Camponotus femoratus, 311, 314
maccooki, 316
maculatus, 312
pennsylvanicus, 267
rufipes, 312
Candida zeylanoides, 129
Canidae, 288
Canis familiaris, 288
latrans, 288
Canna, 151, 163
Cannaceae, 151
Cannibalism, 322-324
Cantao ocellatus, 320
Canthium barbatum, 161
Capá blanco, 66
Capillaria hepatica, 210
Capucinella delicatula, 147
Carabidae, 229
Carboniferous, 14
Care, maternal, 325-330
Cariblatta antiguensis, 8, 40, 158, 161
cuprea, 8, 40
delicatula, 8, 40, 143, 147, 295
hylaea, 8, 40, 147, 153
imitans, 8, 41
insularis, 8, 41, 145, 147, 339
landalei, 8, 41, 147
lutea, 23
lutea lutea, 8, 41, 153, 158, 295
lutea minima, 8, 42, 143; pl. 7
nebulicola, 8, 42, 145, 340
orestera, 8, 151
punctipennis, 8, 147
punctulata, 8, 143
reticulosa, 8, 42
spp., 42, 88
stenophrys, 8, 42 142, 156
Cariblattoides instigator, 8, 43
suave, 8, 43
Carica papaya, 159
Caricaceae, 159
Carnations, 163
Carnivora, 288
Carpinus orientalis, 38
Caryophanales, 124
Casuarina, 152, 164
Casuarinaceae, 152
Cat, 4, 289
ring-tailed, 288
Caterpillars, 319
Catopsis fulgens, 144
Cattleya, 151, 162-163
Caudata, 269
Caves, 16-17
Cavia sp., 288
Caviidae, 288
Cebidae, 284
Cebus apella, 284
capucinus, 284
Cecropia sp., 40, 153
Cedar, Japanese, 164
Centipedes, 3, 222-224, 319
house, 222
Centruroides gracilis, 212
hentzi, 212
vitattus, 212
Cephalobellus brevicaudatum, 194
magalhãesi, 194
Cephalosporium sp., 131
Ceratinoptera diaphana, 7
picta, 8, 43, 159
Ceratonia siliqua, 156
Cercopithecidae, 286
Cercopithecus sp., 286, 343
Cestoda, 208
Chactidae, 212
Chaetochloa verticillata, 142
Chaetodactylus sp., 218
Chalceus macrolepidotos, 269
Challenger, H. M. S., 83
Chamaeleon chamaeleon, 275
oustaleti, 275
Chamaeleontidae, 275
Chameleon, 275
Channel cat, 269
Characidae, 269
Cheiloneurus viridiscutum, 244
Chelonia, 272
Chemotaxis, 331
Chenopodiaceae, 153
Chestnut, 44, 60-61
Chicken, 68, 82, 276, 278
Chilicabra, 281
Chilomastigidae, 169
Chilomastix mesnili, 169
Chilopoda, 222
Chimpanzee, 286
Chiroptera, 283
Chlamydosaurus kingii, 275
Chlorion compressum, 258
Chloris gayana, 141
Chordodes morgani, 201
pilosus, 202
puerilis, 201
Chordodidae, 201
"Chordotes" puerilis, 201
Chorisoneura barbadensis, 8, 147
flavipennis, 8, 43
formosella, 8, 43, 160
parishi, 8, 43
plocea, 8
sp., 295
specilliger, 8, 43
texensis, 8, 43, 152, 153, 157, 161, 316
translucida, 8, 44
Choristima sp., 9, 295
Choristimodes sp., 9, 295
Chromatonotus infuscatus, 9, 44
notatus, 9, 44, 77
Chromobacterium violaceum, 106
Chrysanthemums, 165
Chrysemys picta, 272
Cicadas, 257
Ciliata, 186
Ciliate, unidentified, 190
Cimex lectularius, 321
Cinchona pubescens, 162, 163
Ciniflo, 214
Citharexylum villosum, 43, 161
Citrus aurantifolia, 157
maxima, 157
sinensis, 157
sp., 157, 164
Cleonymidae, 243
Clepsidrina blattarum, 181
Clerada apicicornis, 226
Clerodendron trichotomum, 256-257
Clevelandella constricta, 189
contorta, 189
elongata, 189
hastula, 189
nipponensis, 189
panesthiae, 189
parapanesthiae, 189
Clevelandellidae, 102, 189
Clevelandia, 189
Clevelandiidae, 189
Clostridium feseri, 122
lentoputrescens, 122
novyi, 122
perfringens, 122
sporogenes, 122
spp., 122
tetani, 122
Clubiona, 214
Cnemidophorus sp., 275
Coati, 288
Coccidia, 184
Coccidioides periplanetae, 133
Coccinellidae, 348
"Coccobacillus," 126
Coccobacillus cajae, 120
Cockroach, American (see Periplaneta americana)
amphibious, 30-33, 48
aposymbiotic, 97-99
Australian (see Periplaneta australasiae)
beetle (see Diploptera punctata)
brown (see Periplaneta brunnea)
brown-banded (see Supella supellectilium)
burrowing, 27, 29, 30, 55, 58, 67, 68, 328, 344
carnivorous, 6, 14, 319-324, 336
cavernicolous, 16-25, 37
checklists of, 290-310, 315
cinereous (see Nauphoeta cinerea)
colonies, 75, 327-334
commensal, 310-315
control, 1, 6, 84, 162, 331, 348
Cuban (see Panchlora nivea)
cypress (see Diploptera punctata)
dendricolous, 51
defenses, 343-348
desert, 25-30
disease relations, 1, 3, 74
domiciliary, 51, 70-90, 322, 331, 338, 349
enemies, 2-4, 91, 95-96
field (see Blattella vaga)
Florida (see Eurycotis floridana)
German (see Blattella germanica)
harlequin (see Neostylopyga rhombifolia)
Key West (see Blaberus craniifer)
Madeira (see Leucophaea maderae)
myrmecophilous, 310, 311-314
odor, 6, 84, 280, 344-347
oriental (see Blatta orientalis)
oviparous, 325-327
phytophagous, 68, 162-166
predaceous, 319-324
predators, escape from, 343
ship, 82-87
smoky brown (see Periplanet fuliginosa)
species, list of, 7-14
number of known, iii, 5
spotted Mediterranean (see Ectobius pallidus)
structural habitats of, 70-90
Surinam (see Pycnoscelus surinamensis)
undetermined, 308-310
viviparous, 31, 326, 328-330
"wood" (except here, usually Parcoblatta spp.), 214, 266, 274
Coconut, 35, 38, 49, 143
Cocos nucifera, 143
Coelosporidium blattellae, 185
periplanetae, 168, 176, 177, 185
Coenosia basalis, 229
Coffea sp., 162
Coffee, 66, 67
Coleolaelaps sp., 216; pl. 12
Coleoptera, 229-234, 348
"Colon bacilli," 126
Colonies, cockroach, 75, 327-334
Colubridae, 276
Columbidae, 278
Columbiformes, 278
Combretaceae, 159
Commensalism, 92
Commensals, 2-3, 95, 173, 310-314
cockroach, check list of, 315
Compatibility, interspecies, 337-341
Comperia merceti, 244, 255, 351, 355; pl. 34
merceti var. falsicornis, 244
Compositae, 162
Compsodes schwarzi, 9, 27, 316
Compsolampra, 9
Comptolampra liturata, 9, 44, 140, 141
Conocarpus erectus, 159
Conopidae, 228
Control, biological, 1, 6, 348-354
Convolvulaceae, 161
Coptotermes ceylonicus, 310
Coraciformes, 279
Cordaites, 14
Cordia dentata, 161
Cordyceps amazonica, 134
blattae, 134
Corn, 42, 48, 57, 58
Cornus mas, 38
Corvidae, 280
Corydia nuptialis, 14
Corynebacteriaceae, 110
Corynebacterium diphtheriae, 110
Cosmozosteria lateralis, 9, 345
Costus sp., 316
Cotton, 66, 67, 158, 163
Coturnix coturnix japonica, 277
Coxsackie viruses, 103-104
Coyote, 288
Crappies, 269
Crataegus sp., 155
Crickets, 320-321
stone, 226
Crinum, 163
Cristatithorax, 244
Cryptocercus punctulatus, 9, 44, 62, 67, 100-103, 175-229, 295, 317, 326, 328, 339, 354; pl. 8
relictus, 9, 44
spp., 6, 34
Cryptomeria, 45, 140 164
Cucarachero, 281
Cucumber, 164
Cunoniaceae, 155
Cupressaceae, 141
Cupressus, 164
macrocarpa, 141, 164
Cutilia nitida, 9, 345
soror, 9, 45, 77, 296
sp. near sedilloti, 9; pl. 9
spp., 45, 296, 319
Cyanocitta cristata, 280, 346
Cyatheaceae, 139
Cycads, 166
Cynopotamus essequibensis, 269
Cyperaceae, 142
Cyperus sp., 142
Cypress, 45, 63, 164
Cypress pine, 55
Cyrilla, 42
Cyrtandra sp., 161
Cyrtocharax magdalenae essequibensis, 269
Cyrtotria capucina, 9, 27
D
Dasypodidae, 287
Dasypus novemcinctus, 287
Dates, 39
DDT, 278
Defenses, cockroach, 343-348
Dematiaceae, 132
Dendroblatta sobrina, 9, 45, 316, 330
Dendrocopus mahrattensis, 279
Dermaptera, 226
Dermestes ater, 234
sp., 234
Dermestidae, 234
Dermestids, 320
Derocalymma cruralis, 9, 100
lampyrina, 9, 27
porcellio, 9, 28
stigmosa, 8
Deropeltis autraniana, 9, 28
erythropeza, 9, 19
melanophila, 9, 28
nigrita, 9, 28
Deserts, 25-30
Dicarnosis alfierii, 245
Dictamnus fraxinella, 38
Didelphidae, 283
Diestrammena apicalis, 226
japonica, 226
Digenea, 208
Dinenymphidae, 170
"Diphtheroid I and II," 127
"Diplococci," 127
Diplococcus pneumoniae, 109
Diplocystidae, 181
Diplocystis schneideri, 168, 181
sp., 181; pl. 28
Diplogaster, 192
Diplogasteridae, 192
Diplogyniidae, 217
Diplogyniid, undetermined, 217
Diploptera dytiscoides, 9
punctata, 9, 45, 140-142, 152, 155-157, 159, 164, 216, 224-226, 259, 273, 280, 287, 296, 324, 334, 339, 347; pls. 10, 36
Dipodomys spectabilis spectabilis, 23
Diptera, 227
Disease organisms transmitted by cockroaches, 3
Dobellina, 177
Dog, 288
Dolichonyx oryzivorus, 282
Dolichurus bicolor, 260
corniculus, 260-261
gilberti, 261
greenei, 261
haemorrhous, 260
ignitus, 261-262
stantoni, 262, 263, 352
sp., 262
Dorylaea rhombifolia, 11
Dorylus (Anomma) nigricans subsp. sjostedi, 267
sp., 267
wilverthi, 267
Dove, Chinese, 278
Dragonfly, giant Hawaiian, 224
Drake (Francis), 83
Drassodes, 214
Drassus sp., 318
Dryadoblatta scotti, 9, 31, 45, 144, 145
Drymaplaneta, 319
Ducks, domestic, 277, 343
Duguay-Trouin, Cruiser, 232
Dyscologamia capensis, 9
chopardi, 10
pilosa, 9
wollastoni, 9
Dytiscidae, 230
E
Earthworms, 319
Earwigs, undetermined, 226
Eberthella oedematiens, 112
Echinococcus granulosis, 208
Eciton burchelli, 268, 280 sp., 228
Ecology, 14-96, 324-343
"Ectobia" spp., 135
Ectobia livida, 232
Ectobius africanus, 9, 45
albicinctus, 9, 46
duskei, 9, 46, 77
ericetorum, 9
germanicus, 8
lapponicus, 9, 46, 83, 100, 152, 160, 164, 296, 340
livens, 9
lividus, 9
lucidus, 9, 340
nicaeensis, 9, 46
nigripes, 256
pallidus, 9, 19, 47, 77, 100, 140, 153, 161, 296, 340; pls. 7, 29
panzeri, 9, 47, 153, 160, 296
panzeri var. nigripes, 9
perspicillaris, 9
semenovi, 9, 47, 152
spp., 19 214, 297
sylvester, 9, 47, 326, 340
sylvestris, 9
tadzihicus, 9, 48
vittiventer, 9, 19, 48
vittiventris, 9
Edentata, 287
Eleagnus, 48
Eleutheroda dytiscoides, 9
Ellipsidion affine, 9, 48, 297, 327
aurantium, 9
australe, 9, 48, 155, 160, 297, 327
bicolor, 9, 48, 142
pellucidum, 9
simulans, 9, 48, 141
spp., 35, 48
variegatum, 9, 329
Elm, 38
Embadomonas blattae, 167
Embryophyta siphonogama, 140
Emydidae, 272
Encephalomyelitis virus, mouse, 104
Encyrtidae, 94, 243, 245
Endamoeba blattae, 101, 102, 168, 177-178
coli, 178
javanica, 178
philippinensis, 178
thomsoni, 179
versus Entamoeba, 177
Endamoebidae, 177
[Pg 451] Endolimax blattae, 101, 180
nana, 180
sp., 180
Endomycetales, 133
Entamoeba blattae, 177
blattarum, 177
coli, 178, 179
histolytica, 179
nana, 180
pitheci, 179
sp., 179-180
thomsoni, 179
Entedon hagenowii, 236, 249
Enterobacteriaceae, 111
Enterobius vermicularis, 209
Enterococcus, 109
Entomophthorales, 133
Ephestia kühniella, 99
Epilampra abdomen-nigrum, 9, 31, 48, 77, 141, 147, 160, 164, 297
annandalei, 9, 31-32
azteca, 9, 48
conferta, 9, 297
conspersa, 9, 145
grisea, 9, 100
maya, 9, 85, 145, 147
mexicana, 9, 147
mona, 9, 48, 144
notabilis, 9, 319
sodalis, 9, 145
spp., 9, 32, 85, 89, 145-147, 263, 297, 326, 344
tainana, 9, 49
wheeleri, 9, 49, 297
Epilamprinae, 30, 326, 348
unidentified, 33
Epimys norvegicus, 287
Epiphytes, 34, 44 (see also Bromeliads)
Epomphaloides ischnopterae, 249
Eremoblatta subdiaphana, 9, 28, 316
Ergaula capensis, 9, 49, 77, 311
scarabaeoides, 9, 19
Ericaceae, 160
Erinaceidae, 283
Erinaceus europaeus, 283
sp., 283
Erythrina glauca, 35, 156
Erythrocebus patas patas, 180
Escala circumducta, 13
longiuscula, 13
sp., 10, 297
Escherichia coli, 112
coli var. acidilactici, 112
coli var. communior, 113
freundii, 113
intermedium, 113
"Espino rubial," 66, 155
Estrildine sp., 318
Euandroblatta palpalis, 10, 28
Eubacteriales, 104
Eublaberus posticus, 10, 77, 89
Eucalyptus, 48, 231
Eucalyptus sp., 160
Eucharis, 163
Eucomonympha, 102
imla, 175, 176
Eucomonymphidae, 176
Eudromiella bicolorata, 10, 49
calcarata, 10, 147
Eugenia aromatica, 160
Euglena sp., 166
Euglenidae, 166
Euglenoidina, 166
Eulophidae, 94, 248
Eulophus, 250, 351
Eupelmidae, 94, 210, 245
Eupelmus atriflagellum, 247
sp., 247
Euphorbiaceae, 157
Euphyllodromia angustata, 10, 147
liturifera, 10, 49
decastigmata, 10
Euryconema paradisa, 194
Eurycotis bananae, 10, 47
biolleyi, 10, 49, 145, 158
caraibea, 10, 147
carbonaria, 10
decipiens, 10, 49, 346
dimidiata, 10, 49, 147
ferrum-equinum, 10, 49
floridana, 6, 10, 49-50, 77, 100, 140, 143, 144, 152, 162, 166, 171, 224, 255, 280, 297, 319, 326, 333, 334, 338, 339, 341, 345, 346; pls. 11, 29, 34
galeoides, 10, 50
improcera, 10, 297
kevani, 10, 50, 158
lixa, 10, 147
manni, 10, 298
opaca, 10, 50
sabalianus, 10
Euscorpius carpathicus, 212
germanus, 212
italicus italicus, 213
Euthlastoblatta abortiva, 10, 24, 50, 143
Euthyrrhapha nigra, 10, 19
pacifica, 10, 77
Eutrichomastix sp., 169
Eutrichosomella blattophaga, 245
Evania, 237, 350
abyssinica, 239
appendigaster, 235, 236-237, 238, 239, 350-352
desjardinsii, 236
dimidiata, 239
impressa, 242
laevigata, 236
minuta, 235
princeps, 235
punctata, 240
sericea, 242
sp., 237, 251
subspinosa, 239
Evaniidae, 93, 234-243
Exothea paniculata, 36, 158
Eyeworm, chicken, 68, 164, 204-205, 277, 278, 343
F
Fagaceae, 152
Felidae, 289
Felis catus, 289
pardalis mearnsi, 290
Fennel, 237
Fern, 44, 140, 164
Fig, 344
Fighting, intraspecies, 323, 336-337
Filarien, 202
Fir, Scotch, 140
Fish, 3, 268-269
Flacourtiaceae, 159
Flagellate, unidentified, 176
Flies, conopid, 228
ichneumon, 236
muscid, 229
phorid, 227
sand, 322
sarcophagid, 229
tachinid, 228
Foeniculum vulgare, 237
Fondia sp., 318
Food, cockroaches as human, 286-287
contamination of, 83
of cockroaches, 16, 21, 31, 32, 45, 68, 72, 77, 83, 87, 162-166, 314, 318, 319-324
Forficulid, 339
Forficulidae, 226
Formica, 267
omnivora, 268
rufa, 316
rufibarbis, 312
sp., 316
subcyanea, 312
Formicariidae, 280
Formicas, 316
Formicidae, 267, 311
Formicinae, 311
Fossils, 14
Fowl, guinea, 343
jungle, 277
Fox, 288
Freycinetia sp., 141
Fringillidae, 282
Frogs, 4, 270-272
tree, 270, 353
Fungi, 3, 127-139
hyperparasitic in protozoa, 178, 188
Imperfecti, 129
Fungus gardens, 313
Fusiformis lophomonadis, 119, 173
G
Galebia aegyptiaca, 195
Galerita janus, 347
Galliformes, 277
Gallus sp., 277
Gamocystis tenax, 168, 184
Geckos, 273, 354
Gekko gecko, 272
Gekkonidae, 272
Geomys sp., 24
Geoscapheus robustus, 10, 319, 344
Geotrichum candidum, 131
Geraniaceae, 156
Geraniums, 156, 164
Gesneriaceae, 161
Giardia intestinalis, 171
sp., 172
Ginger, wild, 151
Glands, secretory, 345-347
Glomeropitcairnia erectiflora, 31, 144
Glyciphagidae, 218
Goat, 319
Goldenrod, 162
Gongylonema ingluvicola, 205
neoplasticum, 191, 206, 287
pulchrum, 192, 206
sp., 206
Gopher, pocket, 24
Gordiaceo, 202
Gordian worms, undetermined, 202; pl. 29
Gordiidae, 201
Gordioidea, 201
Gordius, 192
aquaticus, 201-202
blattae orientalis, 202
orientalis, 202
pilosus, 202
raphaelis, 202
sp., 202
Gossypium spp., 158
"Gram positive rods," 127
Gramineae, 141
Grapefruit, 66, 67
Graptoblatta notulata, 10, 50-51, 141, 147, 151, 158-161, 163, 298, 339
Grass, 37, 40, 42-53, 55, 59-62, 64-66, 69, 141-142
bunch, 64
desert, 27-30
feather, 46
marram, 47
Rhodes, 39, 141
saw, 42, 50
wire, 41, 52
Grassquit, Carib, 282
Greenhouses, 68, 80, 81, 155, 157, 162-165, 342
Gregarina blaberae, 184
blattae orientalis, 181
blattarum, 168, 181-182
fastidiosa, 182
gibbsi, 182
illinensis, 183
impetuosa, 183
légeri, 183
neo-brasiliensis, 183
ohioensis, 183
panchlorae, 183
parcoblattae, 183
rhyparobiae, 168, 183
sandoni, 183
serpentula, 184
thomasi, 184
Gregarine, 181; pl. 28
Gregarinida, 181
unidentified, 184
Gregarinidae, 181
Gregariousness, 328, 330-336
physiological effects, 334-335
Grevisia, 144
Gromphadorhina laevigata, 10, 330
portentosa, 10, 298, 330, 354; pl. 12
Gru-gru, 142
Grubs, 317, 319
Gryllacrididae, 226
Guano, 16, 18-23
Guanobies, 16-17, 19
Guava, 54, 63, 67
Guêpe ichneumon, 258
Guinea pigs, 288
Gum tree, 36, 53, 59, 61, 63, 155
Gumbo limbo, 43, 44, 157
Gymnasio nudipes, 279
Gymnopithys leucaspis, 280
Gyna kazungulana, 10, 19
maculipennis, 10, 19
sp., 298
tristis, 10, 20
vetula, 10
H
Habitats, 15
aquatic, 30-33, 48
beach, 36, 42, 47, 52, 53, 58, 63, 69
cave, 16-17
cultivated areas, 38, 39, 53, 58
desert, 25-30, 39, 59, 65, 67, 69
dumps and rubbish, 38-40, 50, 53, 55, 67, 69
flatwoods, 37, 41, 42, 53, 60
forest, 36-38, 40-47, 49-57, 59-66
grassland, 41, 42, 52, 53, 64
hammock, 41-43, 50, 52, 53, 59, 60, 68
marsh, 42, 50
outdoor, 33-70
rotten wood, 34, 35, 38, 44, 45, 47, 49-52, 56-63
sand dune, 47, 61
sandhills, 36, 41, 42, 47, 50, 52, 60
scrub, 36, 37, 40-42, 48, 50, 52, 55, 57-60, 62, 66, 67
soil, 37, 39, 49, 53, 55 58, 60, 67, 68, 344
steppe, 46, 47
stones and rock, 29, 31-33, 36-40, 43, 45-57, 59, 60, 62, 64, 67-70
structures, 70-90
aircraft, 87-90, 232
land-based, 73-82
ships, 82-87, 232-233
swamp, 41, 42, 46, 51, 53, 58, 61, 63, 66, 68
winter, 38-39, 61, 71
Hackberry, 50
Hadrurus arizonensis, 213
Hamamelidaceae, 155
Hammerschmidtiella diesingi, 124, 190, 191, 195-196
neyrae, 196
neyrai, 196
Haplosporidia, 185
Haplosporidium periplanetae, 185
Harpalus pennsylvanicus, 229
Hartmannella blattae, 177
Heather, 46, 47, 160
Hebardina concinna, 10, 278
Hedgehog, 4, 283, 354
Hedychium, 163
Helianthus sp., 162
Heliconia, 40
Helminths, 2-3, 190-210
cockroaches as intermediate hosts, 203-207
cockroaches as primary hosts, 192-202
ova carried by cockroaches, 208-210
Hemiblabera brunneri, 10, 51, 156
Hemidactylus frenatus, 272
Hemiptera, 226, 348
Hemithyrsocera histrio, 7
Hemlock, 44
Henicotyle antillarum, 10, 51
Herpestes javanicus auropunctatus, 289
sp., 289
Herpetomonas periplanetae, 167
Herpomyces, 93, 127-129
amazonicus, 134
anaplectae, 134
appendiculatus, 134
arietinus, 128, 134-135; pl. 27
chaetophilus, 135
chilensis, 135
diplopterae, 135
ectobiae, 135
forficularis, 135
gracilis, 135
grenadinus, 136
leurolestis, 136
lobopterae, 136
macropus, 136
nyctoborae, 136
panchlorae, 136
panesthiae, 136
paranensis, 136
periplanetae, 137, 138
phyllodromiae, 137
platyzosteriae, 137
stylopygae, 128, 137-138; pl. 27
supellae, 138
tricuspidatus, 138
zanzibarinus, 138
Heterodon contortrix, 276
platyrhinos, 276
Heterogamia aegyptiaca, 13
Heterogamodes krügeri, 10, 20
roseni, 7
rugosa, 10, 28
Heterometrus longemanus, 211, 213
Heteropoda regia, 215
venatoria, 215; pl. 30
Heteroptera, 318
Hexamita, 102
cryptocerci, 171
periplanetae, 171
sp., 171
Hexamitidae, 171
2-hexenal, 6, 280, 345-346
Hibiscus, 36, 40, 68, 158
Hibiscus rosa-sinensis, 68, 158
Hickory, 53, 60, 61, 63
Hierodula tenuidentata, 224-225, 355; pl. 32
Histiostoma feroniarum, 217
Holocompsa azteca, 10, 77
cyanea, 10, 78
fulva, 10, 298
metallica, 10, 51
nitidula, 10, 78, 148, 339
zapoteca, 10, 20
Hololampra bivittata, 10, 51
chavesi, 10, 51, 155
maculata, 10, 51
marginata, 10, 51
punctata, 10, 298
sp., 51
Hololeptoblatta sp., 51, 141
Holomastigotidae, 172
Holoquiscalus brachypterus, 282
Holotricha, 186
Homalodemas cruralis, 9
Homalopteryx laminata, 10, 52, 151
scotti, 9
Hominidae, 286
Homo sapiens, 286
Honeydew, 237, 242
Hookworm, ova, 210
Hoplonymphidae, 173
Hoplosphoropyga babaulti, 10, 20
Hormetica apolinari, 10, 51
laevigata, 10, 52, 143, 145, 148
spp., 148
ventralis, 10, 148
Hormurus caudicula, 213
Hornbill, gray, 279
Huckleberry, 43, 57
Humidity reactions, 35, 72, 129
Hygrocrocis intestinalis, 124
Hygroreceptors, 72
Hygrotaxis, 72, 331
Hyla cinerea, 270
Hylidae, 270
Hymenolepididae, 208
Hymenolepis diminuta, 203
sp., 208
Hymenoptera, 2, 234, 311
cockroach-hunting wasps, 255-266
host selection by egg parasites, 254-255
hosts of commensal cockroaches, 311-314
predators and parasites of cockroach eggs, 234-254
Hypermastigina, 172
Hyperolius picturatus, 271
Hypoaspis sp., 217
Hypocreaceae, 134
Hypocreales, 134
Hyporhicnoda, 146
Hyptia dorsalis, 239
harpyoides, 239; pl. 33
reticulata, 239, 240
sp., 240
thoracica, 239, 240
I
Icteridae, 282
Icterus portoricensis, 280
Idionympha perissa, 174
Ignabolivaria bilobata, 10, 52
Iguanidae, 273
Ilex cassine, 66, 158
coriacea, 41, 158
lucida, 41, 158
rotunda, 257
Incertae sedis, fungi, 139
protozoa, 176
Inermicapsifer madagascariensis, 203
Infestations of cockroaches, 331
Inga laurina, 35, 156
vera, 35, 66, 67, 156, 317
Insecta, 224-268
Insectivora, 283
Inuus sylvanus, 286
Invertebrates in biological control, 349-352
Iodamoeba sp., 180
Iolina nana, 219
Iolinidae, 219
Ipomoea tiliasea, 66, 161
Iridomyrmex humilis, 268, 319
Ironwood, 164
Ischnomyrmex sp., 316
Ischnoptera, 134-135, 319
borealis, 12
cavernicola, 14
couloniana, 12
deropeltiformis, 10, 52, 142, 164, 298, 316, 336, 355; pl. 12A
divisa, 12
insolita, 12
major, 12
panamae, 10, 53
pennsylvanica, 12
podoces, 10, 53, 340
remyi, 14
rufa occidentalis, 10, 78, 145
rufa rufa, 10, 53, 78, 89, 148, 161, 298
rufescens, 82
schenklingi, 10, 142
spp., 85, 89, 267, 298, 316
uhleriana, 12
uhleriana fulvescens, 12
Ischnuridae, 213
Isoptera, 310
Isotricha caulleryi, 187
Isotrichidae, 187
J
Jasmine, 45
Jasminum pubescens, 45
Jay, blue, 280
Florida, 280
Júcaro, 66
Juniper, 59
Juniperus sp., 65, 141
Jussiaea natans, 160, 164
K
Kakerlac schaefferi, 12
Kakkerlac, 260
Karnyia discoidalis, 10, 298
Kiawe tree, 164
Kingbird, grey, 35, 318
Klebsiella pneumoniae, 113
Kojukei, 277
Kuchinga hemerobina, 10, 298
remota, 10, 141, 148
Küchenschaben, 100, 169, 171-173, 188
L
Laboulbeniaceae, 127, 134
Laboulbeniales, 134
Lactobacillus fermenti, 109
Lactobacteriaceae, 109
Laelaptidae, 216
Lamproblatta albipalpus, 10, 53, 148
meridionalis, 10, 53
Lampyrid, undetermined, 230; pl. 33
Lampyridae, 230
Laniidae, 281
Lanius ludovicianus, 281
Larvaevoridae, 228
Lasius alienus, 268
Lastrea aristata, 163
Latiblattella chichimeca, 10, 54, 145
lucifrons, 11, 54, 146, 255
rehni, 11, 54, 140, 144, 339
spp., 85, 148
vitrea, 11, 143
zapoteca, 11, 54
Latindia armata, 8
Latrodectus indistinctus, 215-216
mactans, 216
Lauraceae, 153
Leafhoppers, 320
Legumes, 165
Leguminosae, 155
Leidynema appendiculata, 124, 190, 191, 196-198
appendiculata var. americana, 197
appendiculata var. hispana, 197
appendiculata var. indiana, 197
cranifera, 198
delatorrei, 198
nocalum, 198
Leidynemella fusiformis, 198
panesthiae, 198
paracranifera, 198
Leiolopisma laterale, 274
Lemur coronatus, 283
fulvus, 283
tardigradus, 284
Lemuridae, 283
Leontocebus oedipus, 285
rosalia, 285
ursulus, 285
Lepidoptera, 316-318
Lepomis pallidus, 269
Leptodactylus albilabris, 271
pentadactylus, 271
Leptomonas blaberae, 167
sp., 167
Leptopelis calcaratus, 271
rufus, 271
Leptospironympha, 102
eupora, 172
rudis, 172
wachula, 172
Lesser apple worm, 65
Leucophaea maderae, 11, 54, 70, 71, 78, 86, 89, 119, 148, 162, 168, 171, 203, 204, 213, 224, 236, 247, 299, 322, 329, 330, 333, 335, 339, 342, 347; pls. 13, 28
sp., 299
striata, 13
surinamensis, 13
Leurolestes circumvagans, 11, 78
pallidus, 11, 71, 78, 299, 339
Lice, plant, 320
Lichens, 47, 51, 56, 64, 153
Liliaceae, 146
Lily, Brig, 84
Lily, Easter, 146, 164
Lime, 45, 66, 157, 164
Liopicus mahrattensis, 279
Liquidambar styraciflua, 155
Lissoblatta fulgida, 13, 157
Litopeltis biolleyi, 11, 54, 145
bispinosa, 11, 54, 58, 145, 148
deianira, 11, 54
musarum, 11, 54, 148
spp., 334
Lizards, 272-276, 353-354
Lobogynioides, 217
Lobolampra subaptera, 11, 55
Loboptera decipiens, 11, 55, 100, 299, 326, 350
dimidiatipes, 11
extranea, 11
sakalava, 11
sp., 299
thaxteri, 11, 55
Lobopterella dimidiatipes, 11, 55, 299, 338
Locustacarus sp., 219
Lophoblatta arawaka, 11, 55, 142
Lophocerus birostris, 279
Lophomonadidae, 172
Lophomonas, 101
blattarum, 101, 168, 172-173
striata, 101, 119, 168, 173
sulcata, 173
Loris tardigradus, 284
Lorisidae, 284
Lycolaimus, 192
Lycosa avida, 216
helluo, 216, 347
sp., 216; pl. 30
Lycosidae, 216
Lygaeidae, 226
Lymantria monacha, 121
M
Macaca cyclopis, 179
mulatta, 286
rhesus, 286
sylvanus, 286
Macaque, 286
Macropanesthia rhinocerus, 11, 55, 344
Macrospironympha, 102
xylopletha, 172
Macrotermes barneyi, 311
bellicosus, 311
malaccensis, 311
natalensis, 311
Maize, 55, 64, 142
Malleomyces mallei, 119
Malojillo, 31, 48, 141
Malpighian tubules, parasites in, 177, 185, 186
Malvaceae, 158
Mamestra oleracea, 120
Mammalia, 283, 354
Mammals, 3, 283
Mangifera indica, 157
Mangoes, 45, 157, 164
Mangrove, 36, 42, 53, 66
Mantidae, 224
Mantis, Carolina, 225
Chinese, 226
European, 225
Mantis religiosa, 225
Maple, 61-63
Marava arachidis, 339
Mareta acutiventris, 11, 317
Maretina uahuka, 11, 142, 155
Margattea sp., 317
Marsupialia, 283
Mastigophora, 166
Mastigoproctus giganteus, 211
sp., 211
Maze, 335
Mediterranean fruitfly, 252
Megachilidae, 314
Megalixalus fornasinii, 271
Megaloblatta blaberoides, 11, 55
rufipes, 11
Megalopyge krugii, 35, 317
Megamareta verticalis, 11, 56, 141
Megaselia sp., 227
Melanerpes portoricensis, 280
Melanosilpha capensis, 11, 299
Meleagrididae, 278
Meleagris gallopavo, 278
Meles sp., 289
Melipona nigra, 314
Melittobia chalybii, 248
Memnoniella echinata, 132
Mermis, 192, 202
Mermithidae, 192
Mermithids, undetermined, 192; pl. 29
Mermithoidea, 192
Mesquite, 37, 58, 156
Mestocharomyia oophaga, 248
Metallyticus semiaeneus, 225
Metarrhizium anisopliae, 131
Metrosideros collina, 160
Methana canae, 11, 56, 326
curvigera, 11, 56, 155, 326
marginalis, 11, 56, 79, 326
Methionine, 101
Micrococcaceae, 106
Micrococcus aurantiacus, 106
citreus, 106
epidermidis, 106
nigrofaciens, 106
pyogenes var. albus, 106-107
pyogenes var. aureus, 107
spp., 107, 108
ureae, 107
Microhabitats, 16-17, 25-26, 30-31, 33-35, 70-74
Microsporidia, 185
Microtetrameres helix, 206
Midas rosalia, 285
Mimicry, 6, 347-348
Mimosa, 57
Mines, 16, 19-21, 23
Miroblatta silphoides, 10
Mirotermes, 102
Mites, 2, 3, 211, 216-222, 318, 320
cheese, 222
cockroach, 219, 221
control, 222
mushroom, 218
unidentified, 220
Molossidae, 283
Molossus sp., 283
Moluchia (?) dahli, 11, 56
Monadidae, 167
Monas sp., 167
Monastria biguttata, 11, 344
sp., 299
Mongoose, 289, 354
Monilaceae, 130
Moniliales, 129
Moniliformidae, 203
Moniliformis dubius, 203, 270, 271, 276
kalahariensis, 203
moniliformis, 191, 204
Monkey, 4, 284-286, 343
Canal Zone night, 284
Darien black spider, 284
white-faced, 284
Monocercomonas orthopterorum, 169
Monocercomonoides, 102
globus, 169
melolonthae, 168, 169
orthopterorum, 169-170
panesthiae, 170
Monodelphis sp., 283
Mononychoblatta, 26
Moraceae, 153
Mosquitoes, 322
Moss, 46, 47, 56, 64
Spanish, 54, 144
"Mouche bleue, La," 260
Mouse, 103-104
sand, 24
white-footed, 24 205
Mucor guilliermondii, 132
sp., 132
Mucoraceae, 132
Mucorales, 132
Muridae, 287
Murorinae, 129
Mus decumanus, 287
musculus, 287
Musa (see banana)
Musaceae, 146
Muscidae, 229
Mustelidae, 289
Mutant, white-eyed, 21
Mutualism, 92, 96-102
Mutuals, 95
Muzoa madida, 11, 56
Mycetozoa, 176
Mycobacteriaceae, 123
Mycobacterium avium, 123
friedmannii, 123
lacticola, 123
leprae, 123
lepraemurium, 123
phlei, 123
piscium, 124
spp., 124
tuberculosis, 124
Myna (mynah), 281
Myrica cerifera, 43, 152
Myricaceae, 152
Myrmeblattina longipes, 11, 314
Myrmecoblatta rehni, 11, 312
wheeleri, 11, 314
Myrmecophiles, 311-314, 316
Myrmicaria natalensis, 316
Myrmicinae, 312
Myrtaceae, 160
N
Namablatta bitaeniata, 11, 28
Nasua narica, 288
nasua, 288
sp., 289
Nasutitermes costalis, 317
Nauclidas nigra, 11, 56, 148, 326, 327
Nauphoeta bivittata, 11
brazzae, 11
cinerea, 11, 70, 79, 86, 89, 100, 115, 116, 123, 162, 163, 192, 224-226, 259, 299, 324, 330, 335, 337, 339; pl. 14
flexivitta, 11, 148, 163
punctipennis, 11, 28
Necator americanus, 210
Nectandra coriacea, 43, 153
Neisseria meningitidis, 109
Neisseriaceae, 109
Nelipophygus ramsdeni, 11, 56
Nematoda, 192, 204, 209
Nematodes, 2-3, 190-192, 204, 209
undetermined, 201
Nematomorpha, 201
Neoaplectana chresima, 192-193
sp., 192
Neoblattella brunneriana, 11, 145
carcinus, 11, 148
celeripes, 11, 148, 324
detersa, 11, 56, 148
dryas, 11, 56, 145, 160, 340
eurydice, 11, 57, 145, 340
fratercula, 11, 86, 145, 148
fraterna, 11, 86
grossbecki, 11, 57, 145
laodamia, 11, 148
nahua, 11, 86, 145
proserpina, 11, 57, 145, 160, 339
semota, 11, 57, 148
spp., 79, 86, 89, 148, 149, 324
tridens, 11, 148
vatia, 11, 148
Neonephrites partiniger, 230
Neorhipidius neoxenus, 230
Neostylopyga rhombifolia, 11, 70, 77, 79, 86, 138, 209, 210, 224, 255, 259, 272, 273, 280, 300, 346; pl. 15
Neotoma sp., 23-25, 37, 50
Nepenthaceae, 154
Nepenthes ampularia, 154
gracilis, 154
sp., 154
Nephrites australis, 231
nitidus Riek not Shuckard, 231
sp., 231
Nepidae, 227
Nesomylacris cubensis, 11, 57
relica, 11, 57, 145
Nests, ant, 27, 311-314, 316
bird, 35, 318
insect, 35, 43, 66, 67, 69, 156, 317, 318
rodent, 23-25, 37, 50
spider, 215-216, 317
Neuroptera, 227
Nicotiana sp., 161
Nocardia sp., 124
"Nocticola" azteca, 149
Nocticola, 17, 149
bolivari, 11, 17, 57
caeca, 11, 20
decaryi, 11, 20
simoni, 12, 20
sinensis, 12, 311
termitophila, 12, 311
Nosema periplanetae, 185, 186
Nosematidae, 185
Nothoblatta wasmanni, 12, 312
Notila proteus, 170
Notolampra antillarum, 12, 144, 159
Nucleophaga sp., 178
Nyctibora azteca, 12, 149
brunnea, 12, 145
holosericea, 12, 149
laevigata, 12, 57, 145, 149, 339
latipennis, 12
lutzi, 12, 57, 145, 317
mexicana, 12, 149
noctivaga, 12, 86, 100, 145, 149
obscura, 12, 57, 149, 300
sericea, 12, 149
spp., 86, 149, 300
stygia, 12, 58, 156
tomentosa, 12, 300
Nyctotherus, 92, 101, 102
buissoni, 188
ovalis, 101, 102, 168, 188
uichanci, 188
viannai, 189
Nymphytria, 26
O
Oak, 36-38, 41, 43, 44, 46, 47, 52, 53, 59-66, 152-153
Oats, wild, 52, 142
Ocelot, 290
Ocneria dispar, 126
Ocotea catesbyana, 43, 153
Octomitus periplanetae, 171
Odonata, 224
Odontomachus affinis, 314
clarus, 316
haematodes insularis var. pallens, 316
Odontotermes sp., 311
Oikomonadidae, 166
Oikomonas blattarum, 166
sp., 166, 167
Oligacanthorhynchidae, 203
Onagraceae, 160
Oniscosoma granicollis, 12, 300
spp., 58, 344
Oöthecae, concealment of, 47, 61, 63, 325-327
Ophion luteus, 261
Opisthoplatia maculata, 12, 30, 32-33
orientalis, 12, 33, 58
Orange, 45, 157, 164
Orangutan, 70
Orchidaceae, 151
Orchids, 151, 162-163, 164, 237
Origins of domiciliary cockroaches, 70-71
Oriole, Puerto Rican, 280
Oriolidae, 280
Orthoptera, 2, 15, 16, 25, 34, 85, 88, 224
Oulopteryx meliponarum, 12, 314
Ovipositor, cockroach, 15
Owls, 276, 353
bare-legged, 279
Oxyhaloa buprestoides, 12, 70, 79, 86
deusta, 12, 149
Oxymonadidae, 170
Oxymonas, 102
doroaxostylus, 170
nana, 170
Oxyspirura mansoni, 68, 191, 204-205, 278
Oxyuridae, 209
Oxyuris aegyptiaca, 195
australasiae, 199
blattae, 193, 196
blattae orientalis, 195, 196
blatticola, 193
bulhõesi, 200
diesingi, 195
(?) heterogamiae, 199
künckeli, 199
macrura, 195
panesthiae, 198
Oxyuroidea, 193, 204, 209
P
Palamnaeus, 211
Palissota, 34
Paliurus aculeatus, 38
Pallasiomys meridionalis pennicilliger, 24
Palmae, 141
Palmetto, 37, 49, 50, 57, 62, 64, 67, 166
Palms, 45, 50, 52, 58, 64, 143, 150, 164
coconut, 36, 40, 42, 50, 53, 56, 58, 64, 68, 143
date, 143
royal, 54, 143
sago, 166
Pan sp., 286
Panchlora antillarum, 12, 58, 143, 149
cubensis, 12
exoleta, 12, 150, 300
fraterna, 12, 150
maderae, 11
nivea, 12, 58, 79, 86, 89, 142, 150, 300; pl. 16
peruana, 12, 150
sagax, 12, 58, 150
spp., 150, 324
virescens, 12, 150
Panchlorinae, 231
Pandanaceae, 141
Pandanus, 51, 141, 150
Panesthia, 102
angustipennis, 12, 100, 300
australis, 12, 58, 301, 327, 354; pl. 8
"brevicollis," 194
javanica, 12
laevicollis, 12, 58, 301, 327
lobipennis, 12, 301
spp., 301, 328
spadica, 12, 301
Panicum barbinode, 141
purpurascens, 141
Papayas, 45, 159, 164
Papio papio, 286
Parabuthus capensis, 212
Parachordodes raphaelis, 202
Paraclevelandia brevis, 190
simplex, 190
Paracolobactrum aerogenoides, 113
coliforme, 113
spp., 113
Paradisea papuana, 280
Parahormetica bilobata, 12, 301
Parameciidae, 186
Paramecium sp., 186-187
Paranephrites xenus, 230
Parapolyphaga erectipilis, 9-10
Parasites, 91, 95-96; pls. 16, 27, 29, 35
cockroach egg, 2, 234-254, 350-352; pls. 33, 34
host selection by, 254-255
Parasitism, 91-94
Parcoblatta, 350
americana, 12, 267
bolliana, 12, 59, 338
caudelli, 12, 59
desertae, 12, 25, 29, 59, 316
divisa, 12, 59, 140, 152, 153, 155
fulvescens, 12, 24, 59-60, 79, 140
lata, 12, 60, 79, 100, 140, 152, 301, 333, 339
notha, 12, 80
pensylvanica, 12, 15, 61, 80, 100, 152, 153, 257, 302, 318, 319, 327, 333, 339; pl. 17
spp., 17, 20, 63, 144, 206, 302, 316, 333, 336
uhleriana, 12, 15, 61-62, 100, 302, 339; pls. 18, 33
virginica, 12, 15, 62-63, 100, 230, 255, 257, 258, 302, 316, 323, 333; pls. 17, 27, 33
zebra, 12, 63, 140, 155
Parinarium, 34
Parsley, 237
Parsnip, 240
Partridge, 278
Parvobacteriaceae, 119
Passer domesticus, 282
Passeriformes, 280
Passiflora sp., 37, 159
Passifloraceae, 159
Pasteurella multocida, 119
pestis, 119
Pastinaca sativa, 240
Paxylomma buccata, 255
p-benzoquinone, 216, 347
p-chlorophenyl, p-chlorobenzene sulfonate, 222
Pedipalpida, 211
Pelmatosilpha coriacea, 12, 63, 142, 150, 151, 160
kevani, 12, 63
marginalis, 12, 150, 326
purpurascens, 12, 63, 150, 326
rotundata, 12, 145
vagabunda, 12, 150
Peltomyces blattellae, 176
periplanetae, 176-177
Penicillium sp., 131
Periplaneta americana, 12, 17, 20, 63-64, 70, 71, 80, 83, 84, 87, 89, 98-101, 103, 104, 106, 126, 129, 143, 150, 151, 56, 162, 163, 166, 190, 191, 205, 211-213, 223, 225, 226, 229, 245, 255, 271, 272, 276, 280, 302-304, 318, 320, 321, 323-327, 332, 334-336, 338-340, 342, 346-348, 350-352, 354; pls. 19, 33, 34, 35
australasiae, 12, 21, 55, 64, 70, 71, 80, 87, 89, 100, 105, 115, 117, 123, 143, 150, 151, 153, 158, 159, 163, 209, 210, 214, 225, 304-305, 318-320, 323, 326, 327, 333, 335, 338, 339, 341, 342, 348, 350, 351, 354; pls. 20, 32
brunnea, 12, 64, 70, 80, 150, 153, 171, 305, 323, 325-326, 340, 346; pl. 21
cavernicola, 12, 16, 21
fortipes, 13
fuliginosa, 12, 64, 81, 158, 164, 251, 277, 305, 327, 335, 338; pl. 22
ignota, 12, 81, 115, 327
lata, 12, 21
orientalis, 8
picea, 12
spp., 21, 90, 305-306, 317, 352
tartara, 8
Periplaneticola mirzaia, 193
Perisphaerus armadillo, 12, 329
glomeriformis, 12, 329, 347
sp., 21, 329
Periwinkle, 47
Permian, 14
Perodicticus potto, 284
Peromyscus leucopus noveboracensis, 205
polionotus rhoadsi, 24
Petitia domingensis, 66, 317
Petroselium crispum, 237
Phaetalia pallida, 12, 81
Phasianidae, 277
Phasianus calchicus karpowi, 277
sp., 277
Pheasants, 277
Pheidole megacephala, 268
Phidon (?) dubius, 13, 64
Phileciton longipes, 314
Philip, Ship, 83
Philodromus pernix, 318
Phlebonotus pallens, 13, 329
Phlebotomus papatasii, 322
Phoenix dactylifera, 143
Pholadoblatta inusitata, 13, 316
Phoraspidinae, 329
Phoraspis sp., 64, 142
Phoridae, 227
Phormictopus cancerides, 214
Phorticolea boliviae, 13, 311, 314
testacea, 13, 312
Phryganoporus, 317
Phycomycetes, 132
Phyllodromia germanica, 8
hemerobina, 10
hieroglyphica, 10
humbertiana, 8
nigrocincta, 14
obtusata, 7
parenthesis, 14
spp., 306, 352
supellectilium, 13-14
treitliana, 13, 245
Phyllodromica brevipennis, 13, 64
graeca, 13, 65
irinae, 13, 65
maculata, 13, 65
megerlei, 13, 65, 153
polita, 13, 65
pygmaea, 13, 65, 141
tartara, 13, 65
tartara nigrescens, 13, 65, 141, 162
Physaloptera hispida, 191, 207
maxillaris, 191, 207, 288, 289
praeputialis, 207
rara, 191, 207, 288, 289
turgida, 191, 207
Physalopteridae, 207
Physignathus lesueurii, 275
Phytoseiidae, 216
Picidae, 279
Piciformes, 279
Pigeon, 279, 343
Pileocephalus blaberae, 184
Pilocrocis secernalis, 66, 317
Pimeliaphilus podapolipophagus, 219-221, 320
Pimelodidae, 268
Pinaceae, 140
Pine, 36, 41-44, 46, 47, 49-54, 57, 59-67, 140
Pineapple, 39, 45, 68 144, 165
Pinus australis, 140
caribaea, 50, 52, 53, 59, 64, 140
clausa, 53, 140
echinata, 140
spp., 40
sylvestris, 140
Pisces, 268
Plagiotoma blattarum, 188
Plants, carnivorous, 154
damage to, by cockroaches, 162-166
higher, 139-162
pitcher, 154
Platyhelminthes, 208
Platyzosteria analis, 13, 306
armata, 13, 100
bifida, 13, 151
castanea, 13, 65, 306, 345
ingens, 10
novae seelandiae, 13, 65, 306, 321, 346; pl. 23
novae-zealandiae, 13
sabalianus, 10
scabra, 13, 306
sp., 306
Plectoptera dominicae, 13, 66, 157
dorsalis, 13, 66, 67, 81, 142, 143, 151, 155-158, 160, 161, 317
floridana, 13
infulata, 13, 66, 142, 155, 156-158, 317
lacerna, 13, 66, 140, 153
perscita, 13, 66, 157
poeyi, 13, 66, 158, 159
porcellana, 13, 66, 157, 162
pygmaea, 13, 66
rhabdota, 13, 66, 67, 142, 155-158, 160, 317
sp., 306
vermiculata, 13, 67, 140
Pleistophora periplanetae, 186
Plethodon glutinosus, 269
Plethodontidae, 269
Plistophora kudoi, 185-186
periplanetae, 177, 185, 186
sp., 186
Ploceinae, 318
Ploceus sp., 318
Plumiger histrio, 7
Plumilla, 35
Pneumococcus, 109
Podapolipodidae, 219
Podium abdominale, 265
carolina, 265
dubium, 265
flavipenne, 265-266
haematogastrum, 263, 266, 352
luctuosum, 266
rufipes, 266
sp., 266
Podocarpus, 42
Pogonomyrmex badius, 346, 347; pl. 36
Poinsettia sp., 1575
Poinsettias, 164
Polecat, 4
Poliomyelitis viruses, 3, 103
Polistes, 318, 319
Polybia pygmaea, 314
"Polygamia" aegyptiaca, 13
roseni, 7
Polymastigidae, 169
Polymastigina, 169
Polymastix, 101
Polyphaga aegyptiaca, 13, 21, 24, 25 29, 67, 81, 100, 190, 306
indica, 13, 24, 29, 317
pellucida, 13
saussurei, 13, 24, 29, 67, 71, 81, 114, 115, 117-119, 306
sp., 21
Polyphagids, 344
Polyphaginae, 25
Polypodiaceae, 140
Polystichum aristatum, 163
Polyzosteria analis, 13
limbata, 13, 67
melanaria, 13, 306
Pomarrosa, 160
Pompilidae, 256
Pompilus bracatus, 256
sp., 256
Ponerinae, 314
Pongidae, 286
Ponies, pit (mine), 16, 23
Populus diversifolia, 152
euphratica, 47, 152
sp., 152
Poroblatta nigra, 11
spp., 67
Potamotrygon humboldti, 268
hystrix, 268
Potamotrygontidae, 268
Potato, 165
Potto, 284
Poultry, 276-278, 353
Prairie dog, 24
Predation, interspecies, 92, 319-322
intraspecies, 92, 322-324
Predatism, 91-95
Predators, 91, 96, 319
defense against, 343-348
of cockroach eggs, 234
Prey, experimental, 4
natural, 4
Primates, 283
Pritchardia, 64, 163
Privies (latrines), cockroaches in, 74, 80, 81, 178, 340
Procyonidae, 288
Prodenia litula, 320
Prolabia, 339
Prolophomonas tocopola, 173
Prosevania punctata, 240-242, 254-255, 351; pl. 33
Prosoplecta, 348
Prosthenorchis elegans, 203, 283, 285, 286, 288-290
spirula, 203, 283, 286, 288-290
Proteus mirabilis, 114
morganii, 114
rettgeri, 114
spp., 114
vulgaris, 114
Protomagalhaesia serpentula, 168, 184
Protomonadina, 166
Protospirura bonnei, 206
columbiana, 206
muricola, 191, 206, 284
Protozoa, 2, 92, 101-103, 166-190
cultured in vitro, 170, 178, 179, 188
Protrelleta floridana, 199
Protrellina, 199
aurifluus, 199
australasiae, 199
galebi, 199
künckeli, 199
manni, 200
phyllodromi, 200
Protrelloides paradoxa, 200
Protrellus aureus, 199
aurifluus, 199
australasiae, 199
galebi, 199
künckeli, 199
manni, 200
phyllodromi, 200
Pseudoderopeltis aethiopica, 13, 100
Pseudomonadaceae, 104
Pseudomonas aeruginosa, 104
eisenbergii, 105
fluorescens, 105
Pseudomopinae, 60, 230, 231
Pseudomops cincta, 13, 319
laticornis, 13, 145
septentrionalis, 13, 67, 162
Pseudophoraspis nebulosa, 13, 81, 328
Pseudosaccharomycetaceae, 129
Psidium guajava, 67, 160
Psocoptera, 318
Pteridophyta, 139
Pteromalidae, 247
Pteromalus sp., 247, 248
Pterygosomidae, 219
Pycnosceloides aporus, 12, 58
Pycnoscelus niger, 13, 22
striatus, 13, 22
surinamensis, 2, 13, 22, 25, 67-68, 70, 71, 81, 87, 90, 100, 138, 139, 142-146, 151, 152, 155-157, 161-165, 191, 212, 229, 259, 279, 280, 282, 306-307, 324, 330, 333, 335, 338, 341-343, 350, 353; pl. 24
Pyrus sp., 62
Q
Quail, Japanese, 277
Quercus alba, 152
pubescens, 38
rubra, 152
spp., 153
virginiana, 152
Quinones, 347
R
Rana catesbeiana, 271
mascareniensis, 271
pipiens, 271, 345
Ranatra sp., 227
Ranidae, 270
Rats, 4, 287
kangaroo, 23, 26
Norway, 287
wood, 23, 26
Rattus norvegicus, 287
rattus, 287
spp., 287
Reduviidae, 227
Reduviids, undetermined, 227
Reduvius christophi, 227
fedtschenkianus, 227
Renealmia sp., 151
Repellents, 345-347
Reptiles, 3, 272-276, 353
eggs of, 320
Reptilia, 272-276
Reticulitermes sp., 317
Retortamonas blattae, 167, 168
orthopterorum, 169
sp., 167
Rhabditoidea, 192
Rhamdia sebae, 268
Rhantus pacificus, 230
Rhicnoda natatrix, 13, 33
sp., 146
Rhinopsis caniculatus, 257
Rhinotermitidae, 310
Rhipidioides ableptus, 230
adynatus, 230
fuscatus, 230
helenae, 230
mollis, 231
rubricatus, 231
Rhipidius, 230
Rhipiphoridae, 230
Rhizobiaceae, 106
Rhizoglyphus tarsalus, 218
Rhizophora mangle, 36, 159
Rhizophoracea, 159
Rhizopus nigricans, 133
sp., 133
Rhombomys opimus, 24
Rhynchonympha, 102
tarda, 174, 175
Rhyparobia maderae, 11
Rhythms, diurnal, 335
Rhytidometopum dissimile, 13, 68, 158
Riatia fulgida, 13, 157
orientis, 13, 68, 158
Riboflavin, 101
Rictularia coloradensis, 192, 205
Riekella australis, 231
nitidioides, 231
sp., 231
Ripidiini, biology of Australian, 231
Ripidius, 230
boissyi, 231-232
denisi, 232
pectinicornis, 84, 232-233
scutellaris, 233
sp., 232
Ripiphoridae, 230
Roach (see cockroach)
Robshelfordia circumducta, 13, 307
longiuscula, 13, 307
Rodentia, 287
Rosa sp., 155
Rosaceae, 155
Rose, 155, 163-165
Roundworms, 2 (see Helminths)
Roystonea regia, 143
Rubiaceae, 161
Rubus spp., 155
Rushes, 46
Rutaceae, 157
S
Sabal palmetto, 50
Saccharomyces cerevisiae, 133
sp., 133
Saccharomycetaceae, 133
Saccharomycetes, 129
Saccharum officinarum, 141-142
Saccinobaculus, 102
ambloaxostylus, 170
doroaxostylus, 170
lata, 170
minor, 170
undescribed spp., 170
Saimiri sciurea, 285
Salamander, 269
Salganea morio, 13, 334
Salicaceae, 152
Salientia, 270
Salius verticalis, 256
Salix sp., 152
Salmonella anatis, 114
choleraesuis, 114
enteritidus, 114
morbificans, 115
paratyphi, 115
schottmuelleri, 115
typhimurium, 116
typhosa, 117
Salmonella sp.
(Type Adelaide), 115
(Type Bareilly), 115
(Type Bredeny), 115
(Type Derby), 115
(Type Kentucky), 115
(Type Kottbus), 115
(Type Meleagris), 116
(Type Montevideo), 116
(Type Newport), 116
(Type Oranienburg), 116
(Type Panama), 116
(Type Rubislaw), 116
(Type Tennessee), 116
Salsola kali, 65
Samanea saman, 35, 156
Samui, Steamship, 232
Sapindaceae, 158
Sapotaceae, 160
Sarcina alba, 108
aurantiaca, 108
lutea, 108
spp., 108-109
symbiotica, 108
ventriculi, 108
Sarcodina, 176
Sarcophaga lambens, 2, 229
omani, 229
sternodontis, 229
spp., 229
Sarcophagidae, 229
Sarracenia flava, 154
minor, 154
purpurea, 154
variolaris, 154
Sarraceniaceae, 154
Sauria, 272
Scale, diaspine, 237
Sceliphron caementarium, 248
Schistosoma haematobium, 208
Schistosomatidae, 208
Schizomycetes, 104
Schizophyta, 104
Schwenkiella icemi, 200
Scincidae, 274
Scolopendra, 222
cingulata, 223
morsitans, 223
subspinipes, 224
sp., 224
Scolopendridae, 223
Scolopendromorpha, 223
Scorpionida, 211
Scorpionidae, 213
Scorpions, 3, 211, 349
Scorzonera acanthoclada, 65, 162
Scutigera coleoptrata, 222-223; pl. 31
forceps, 222
maculata, 223
Scutigeridae, 222
Scutigeromorpha, 222
Sea grape, 43, 69
Seaweed, 47, 55
Secretions, repellent, 6, 216, 225
sexual, 235
Sedge, 66
Serpentes, 276
Serratia marcescens, 117-118; pl. 16
Setaria verticillata, 142
Seurocyrnea colini, 207
Severianoia magna, 200
severianoi, 200
Sewers, cockroaches in, 74, 332, 338
Shelfordella tartara, 8
Shigella alkalescens, 118
dysenteriae, 118
paradysenteriae, 119
Ships, 82-84
cockroaches on, 79, 82-87
slave, 79, 86, 87
Shrike, 281
Sibylloblatta panesthoides, 13, 151
Sida, 64, 159, 163
Sideroxylon foetidissimum, 63, 160
Silkworm, bacterium from, 126
"disease bacillus," 127
Simblerastes jamaicanus, 13, 69, 317, 334
Simia jacchus, 285
Sinanthropes, 71, 81
Skinks, 274, 354
Snakes, 276
Sodium fluoride, 278
Solanaceae, 161
Solanum tuberosum, 161
Solenopsis geminata, 314
Solindenia picticornis, 246, 247
Sound, cockroach, 330
Sow bugs, 347
Sparassidae, 215
Sparrow, 282
English, 282
Spartan, Ship, 84
Spartina marsh, 42
Spelaeoblatta caeca, 14
gestroi, 13, 22
Spermophilopsis leptodactylus, 24
Sphaerita, 189
Sphaerodactylus sp., 273
Sphagnum, 52
Sphecidae, 264
Sphecophila polybiarum, 13, 314
ravana, 13, 310-311
termitium, 13, 311
Sphodromantis bioculata, 225
viridis, 225
Spicaria prasina, 130
Spiders, 3, 211, 214-216, 317, 318, 347, 349; pl. 30
banana, 215
big, brown house, 215
bird-eating, 214
black widow, 216
button, 215
huntsman, 215
nests, 215-216
Spiniger domesticus, 227
Spirillochaeta blattae, 127
Spirillum α, β, and γ, 105
periplaneticum, 105, 127
sp., 105
Spirocerca sanguinolenta, 203
Spirochaeta blattae, 125
blattarum, 125
periplanetae, 125
stylopygae, 125
Spirochaetaceae, 125
Spirochaetales, 125
Spirochaetes, unidentified, 125
"Spirochaetoid bacteria," 127
Spirorbis pusillus, 14
Spirostomidae, 188
Spirotricha, 187
Spirura gastrophila, 207
Spiruridae, 205
Spiruroidea, 204
Spondias, 67
mombin, 158
purpurea, 158
Sporozoa, 181
Spruce, 44
Squirrel, ground, 23, 24, 26
Stagmomantis carolina, 225-226
Staphylococci, 107
Staphylococcus albus, 107
Staurojoeninidae, 174
Stegobium paniceum, 99
Steinernematidae, 192
Steleopyga sinensis, 13, 307
Stenamma andrei, 316
Stenobothrus vagans, 46
Stenophora sp., 181
Stenophoridae, 181
Sterculiaceae, 159
Stictolampra buqueti concinula, 14, 33
Stigmatomyces sp., 129, 138
Stilpnoblatta minuta, 316
Stipa lessingiana, 46
Strepsiptera, 234
Streptococcus faecalis, 109
liquefaciens, 110
microapoika, 110
non-hemolyticus, 110
pyogenes, 110
spp., 110
Streptomyces leidynematis, 124, 196, 197
Streptomycetaceae, 124
Streptopelia chinensis, 278
Streptostomum gracile, 195
Strigidae, 279
Strigiformes, 279
Strongyloidea, 209
Sturnidae, 281
Stylogaster spp., 228
stylata, 228
Stylopyga americana, 12
Styphon bakeri, 14, 69, 344
Subulura jacchi, 204
Subuluridae, 204
Sugarcane, 37-39, 45, 48, 51, 53-56, 58, 64, 66-69, 141-142
Suifunema caudelli, 200
Sulfur, flowers of, 222
Sunfish, 269
Sunflower, 67
Supella hottentotta, 14, 29
supellectilium, 14, 69, 70, 71, 76, 82, 87, 89, 100, 115, 117, 142, 161, 165, 205, 216, 223, 232, 255, 307, 327, 333, 335, 339, 342, 349-351; pls. 25, 30, 31, 34
sp., 90
Swift, edible-nest, 318
Swiss chard, 47, 153
Symbiosis, 25-26, 92
Symbius blattarum, 232
Symploce bicolor, 14, 82
bilabiata, 14
breviramis, 14, 22
cavernicola, 14, 21, 22, 318, 333
curta, 14, 22
flagellata, 14, 69, 307
hospes, 14, 82, 161, 339
jamaicana, 14, 69
kevani, 14, 29
lita, 14
parenthesis, 14, 307
remyi, 14, 22
ruficollis, 14, 69, 142, 161, 307
sp., 90
Syncephalastrum sp., 133
Synonyms, 5
Syntomosphyrum blattae, 248
glossinae, 250
ischnopterae, 249
Systellogaster ovivora, 248
Syzygium aromaticum, 160
Szepligetella sericea, 242
T
Tachinid, undetermined, 228
Tachysphex blatticidus, 264
coriaceus, 264
fanuiensis, 264
lativalvis, 264-265
Taenia echinococcus, 208
saginata, 208
Taeniidae, 208
Taenioidea, 208
Tamarind, 51
Tamarindus indica, 156
Tapeworm, undetermined, 208
Tarachodes maurus, 226
Tarantula, 214
Tarantula, 214
Tarsiidae, 284
Tarsius, 284
Tartaroblatta karatavica, 14, 70
Tatu novemcinctum, 287
Taxodiaceae, 140
Tegenaria, 214
Teiidae, 275
Temnopteryx, 134-135
deropeltiformis, 10
obliquetruncata, 14, 317
phalerata, 14, 307
platysoma, 8
Temperature preference, 25, 71
Tenadores, Steamship
Tenodera aridifolia sinensis, 226
Termes bellicosus, 311
flavipes, 316
malaccensis, 311
sp., 311
Termites, 57, 69, 102, 310-311, 317, 320
comején, 317
Termitidae, 311
Termopsis sp., 317
Testudo horsfieldi, 24
Tetragenous sp., 127
Tetralopha scabridella, 35, 66, 67, 317
Tetrameres americana, 207
pattersoni, 207
Tetrastichodes asthenogmus, 249
Tetrastichus australasiae, 249
hagenowii, 94, 249-253, 255, 349-351; pl. 34
periplanetae, 253
sp., 249, 254, 351
Tetratrichomastix blattidarum, 170
Tettix kraussi, 46
Thallophyta, 129
Thecadactylus sp., 273
Theganopteryx straminea, 14, 30
Thelastoma aligarhica, 200
brevicaudatum, 194
bulhõesi, 201
heterogamiae, 199
icemi, 200
indiana, 194
magalhãesi, 194
pachyjuli, 200-201
palmettum, 201
panesthiae, 198
riveroi, 201
Thelastomatidae, 190, 193
Thelaziidae, 204
Thelyphonidae, 211
Thelyphonus giganteus, 211
Theobroma cacao, 159
Theraphosidae, 214
Therea nuptialis, 14, 344
Theridiidae, 215
Thistle, 60
giant, 51
Thyrsocera cincta, 13
Tiaris bicolor omissa, 282
Tillandsia fasciculata, 144
usneoides, 144
uttriculata, 50, 144
sp., 31, 36, 144
Timulla eriphyla, 264
Tivia australica, 14, 316
brunnea, 14, 30
fulva, 14, 28, 30
macracantha, 14, 23
obscura, 14, 30
sp., 23
Toads, 270, 353
Tobacco, 165
Tockus birostris, 279
Todidae, 279
Todus mexicanus, 279
Tody, 279
Torula acidophila, 132
gropengiesseri, 132
rosea, 132
Torulopsis, 130
Toxoplasma gondii, 190
Trapping, 53, 59-63
Treeferns, 36, 42, 50, 56, 57, 139
Trees (see under common or scientific name)
in general, 35-68
Trematoda, 208
Treponema parvum, 125
stylopygae, 125
Treponemataceae, 125
Triatoma arthurneivai, 227
Tribulus sp., 63, 156, 163, 165
Trichomastic orthopterum, 169
Trichomastix, 169
Trichomonadidae, 171
Trichomonas hominis, 171
orthopterorum, 169
sp., 171
Trichonympha, 102
acuta, 174
algoa, 174, 175
chula, 176
grandis, 176
lata, 176
okolona, 175, 176
parva, 176
Trichonymphidae, 174
Trichostrongylidae, 210
Trichostrongylus sp., 210
Trichotarsus, 218
Trichuridae, 210
Trichuris trichiura, 210
Trichuroidea, 210
Trigonopsis abdominalis, 265
Trirhogma caerulea, 262
sp., 264
Troglobies, 17, 20
Troglodytes aedon, 281
audax, 281
Troglodytidae, 281
Troglophiles, 16, 17, 19, 20, 23
Trogloxenes, 16
Tropidophorus grayi, 274
Trypanosomatidae, 167
Tulip tree, 53
Turkey, 278
Turtle, desert, 24, 26
painted, 272
Typhloblatta caeca, 14, 23
Typhloblattodes madecassus, 14, 23
Tyrophagus lintneri, 218
noxius, 218
U
Urinympha, 102
talea, 174, 175
Urodacus novaehollandiae, 213
Uropoda sp., 217
Uropodidae, 217
V
Vaccinium meridionale, 43, 160
Vanda, 151, 163
Veillonella parvula, 109
Vejovidae, 213
Verbenaceae, 161
Veromessor andrei, 316
Vertebrata, 268-290
Vertebrates, 3, 268
in biological control, 353-354
Vespa maculata, 318
Vespidae, 314
Vespula maculata, 61, 318
Vibrio comma, 105
Deneke's, 105
metschnikovii, 105
sp., 106
types I and II, 106
tyrogenus, 105
Vinca minor, 161
Vireo latimeri, 281
Vireonidae, 281
Viruses, 103-104
Coxsackie, 103-104
encephalomyelitis, mouse, 104
poliomyelitis, 3, 103
Brunhilde type, 103
Columbia SK, 103
Lansing strain, 103
unspecified strains, 103
yellow-fever, 104
Viverridae, 289
Vulpes sp., 288
W
Wasps, 91, 94, 314, 318, 348
cockroach-egg parasites, 234-254, 350, 352
cockroach-hunting, 255-266, 352
Water, balance, 72
drinking, 72
need for, 71
Water bug, 349
Wattle, 49
Webworm, 316
Weinmannia sp., 155
Whipscorpion, 211
William Kieth, Steamship, 84
Willow, 47, 63, 152
Woodpeckers, 279-280
Wren, 281
X
Xerophiles, 25
Xestoblatta festae, 14, 87
immaculata, 14, 23
Xylosma suaveolens, 159
Xysticus, 214
Y
Yeasts, 3, 127, 129, 132
Yellow-fever virus, 104
Yucca elata, 54, 146
Z
Zanthoxylum caribaeum, 66, 157
Zea mays, 142, 165
Zelotes, 314
Zeuxevania splendidula, 93, 243, 249, 350
Zingiberaceae, 151
Zootermopsis, 103
Zygophyllaceae, 156
[1] Part of the cost of publication of this monograph was borne by the Office of Naval Research, Department of the Navy (through the American Institute of Biological Sciences), and by the Quartermaster Research and Engineering Center, Department of the Army.
[2] Present address of both authors, Central Research Laboratories, United Fruit Co., Upland Road, Norwood, Mass.
[3] Names of organisms preceded by an asterisk (*) are known or suspected pathogens of vertebrates. These records were presented with annotations in our 1957a paper on the medical and veterinary importance of cockroaches. For that reason the annotations have not been repeated herein, although the records have been included to make the listing of the biotic associates of cockroaches substantially complete.
[4] The following helminths also have been stated in the literature to pass their intermediate stages in cockroaches: Hymenolepis diminuta (Rudolphi, 1819) [Blanchard (1891)]; Inermicapsifer madagascariensis (Davaine in Grenet, 1870) [Baer (1956)]; Spirocerca sanguinolenta (Rudolphi, 1819) [Seurat (1913)]. These doubtful records are discussed in Roth and Willis (1957a).
[5] Barber (1939) has shown that the correct spelling of the name of the type genus is Ripidius and not Rhipidius, as it is frequently written, and that, consequently, the family should be Ripiphoridae and not Rhipiphoridae.
[6] Page 11 of Marlatt (1902) has been cited erroneously so many times in support of host records for T. hagenowii and Evania appendigaster that we are quoting the pertinent section below. In the section preceding the quoted material Marlatt discusses the American, Australian, oriental, and German cockroaches. There is nothing in the paper to connect any of these cockroaches specifically with the parasites mentioned below:
NATURAL ENEMIES AND PARASITES
"In Europe the egg capsules of the cockroach are often parasitized by an ichneumon fly (Evania appendigaster). This insect has become widely distributed over the world following its host insect, and has been redescribed under a great many different names. It was found in Cuba as early as 1829, and has been several times collected in the United States. Unfortunately, its usefulness as a means of keeping the roach in check by destroying the egg capsules is greatly impaired by the occurrence of another ichneumon fly (Entedon hagenowi), which is parasitic upon the first. This is also a European species which has been brought over with its host parasite. If the true egg capsule parasite of the roach could have been introduced into this country without this secondary parasite, its usefulness would doubtless have been very much greater. The secondary parasite, however, seems to have been introduced early, and has been found in Cuba and Florida, and probably occurs as widely as its host and prevents the latter from multiplying very greatly."
[7] Brues et al. (1954) include this family in the Encyrtidae.
[8] archy (Marquis, 1931) was living in a dream world when he typed:
"there is always
something to be thankful
for you would not
think that a cockroach
had much ground
for optimism
but as the fishing season
opens up i grow
more and more
cheerful at the thought
that nobody ever got
the notion of using
cockroaches for bait"
[9] Classification of Amphibia and Reptilia follows Hegner (1936).
[10] Classification of Amphibia and Reptilia follows Hegner (1936).
[11] Classification of Mammalia follows Simpson (1945).
[12] The West Indian "Blabera fusca Brunner" of Saupe (1928) is obviously Blaberus craniifer as can readily be seen from a comparison of Saupe's figures of the pronotal shields of his species with the descriptions by Hebard (1917) of B. craniifer and Blaberus atropos (Stoll). The Chilean B. fusca Brunner is a junior synonym of B. atropos (Stoll), a South American insect (Hebard, 1917).
[13] The species was recorded by these authors as Blaberus atropos. (Hebard, 1917 p. 204, footnote 327.)
End of the Project Gutenberg EBook of The Biotic Associations of Cockroaches, by
Louis M. Roth and Edwin R. Willis
*** END OF THIS PROJECT GUTENBERG EBOOK BIOTIC ASSOCIATIONS OF COCKROACHES ***
***** This file should be named 46802-h.htm or 46802-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/4/6/8/0/46802/
Produced by Dianna Adair, Matthias Grammel, Bryan Ness and
the Online Distributed Proofreading Team at
http://www.pgdp.net (This file was produced from images
generously made available by The Internet Archive)
Updated editions will replace the previous one--the old editions will
be renamed.
Creating the works from print editions not protected by U.S. copyright
law means that no one owns a United States copyright in these works,
so the Foundation (and you!) can copy and distribute it in the United
States without permission and without paying copyright
royalties. Special rules, set forth in the General Terms of Use part
of this license, apply to copying and distributing Project
Gutenberg-tm electronic works to protect the PROJECT GUTENBERG-tm
concept and trademark. Project Gutenberg is a registered trademark,
and may not be used if you charge for the eBooks, unless you receive
specific permission. If you do not charge anything for copies of this
eBook, complying with the rules is very easy. You may use this eBook
for nearly any purpose such as creation of derivative works, reports,
performances and research. They may be modified and printed and given
away--you may do practically ANYTHING in the United States with eBooks
not protected by U.S. copyright law. Redistribution is subject to the
trademark license, especially commercial redistribution.
START: FULL LICENSE
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full
Project Gutenberg-tm License available with this file or online at
www.gutenberg.org/license.
Section 1. General Terms of Use and Redistributing Project
Gutenberg-tm electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or
destroy all copies of Project Gutenberg-tm electronic works in your
possession. If you paid a fee for obtaining a copy of or access to a
Project Gutenberg-tm electronic work and you do not agree to be bound
by the terms of this agreement, you may obtain a refund from the
person or entity to whom you paid the fee as set forth in paragraph
1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this
agreement and help preserve free future access to Project Gutenberg-tm
electronic works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the
Foundation" or PGLAF), owns a compilation copyright in the collection
of Project Gutenberg-tm electronic works. Nearly all the individual
works in the collection are in the public domain in the United
States. If an individual work is unprotected by copyright law in the
United States and you are located in the United States, we do not
claim a right to prevent you from copying, distributing, performing,
displaying or creating derivative works based on the work as long as
all references to Project Gutenberg are removed. Of course, we hope
that you will support the Project Gutenberg-tm mission of promoting
free access to electronic works by freely sharing Project Gutenberg-tm
works in compliance with the terms of this agreement for keeping the
Project Gutenberg-tm name associated with the work. You can easily
comply with the terms of this agreement by keeping this work in the
same format with its attached full Project Gutenberg-tm License when
you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are
in a constant state of change. If you are outside the United States,
check the laws of your country in addition to the terms of this
agreement before downloading, copying, displaying, performing,
distributing or creating derivative works based on this work or any
other Project Gutenberg-tm work. The Foundation makes no
representations concerning the copyright status of any work in any
country outside the United States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other
immediate access to, the full Project Gutenberg-tm License must appear
prominently whenever any copy of a Project Gutenberg-tm work (any work
on which the phrase "Project Gutenberg" appears, or with which the
phrase "Project Gutenberg" is associated) is accessed, displayed,
performed, viewed, copied or distributed:
This eBook is for the use of anyone anywhere in the United States and
most other parts of the world at no cost and with almost no
restrictions whatsoever. You may copy it, give it away or re-use it
under the terms of the Project Gutenberg License included with this
eBook or online at www.gutenberg.org. If you are not located in the
United States, you'll have to check the laws of the country where you
are located before using this ebook.
1.E.2. If an individual Project Gutenberg-tm electronic work is
derived from texts not protected by U.S. copyright law (does not
contain a notice indicating that it is posted with permission of the
copyright holder), the work can be copied and distributed to anyone in
the United States without paying any fees or charges. If you are
redistributing or providing access to a work with the phrase "Project
Gutenberg" associated with or appearing on the work, you must comply
either with the requirements of paragraphs 1.E.1 through 1.E.7 or
obtain permission for the use of the work and the Project Gutenberg-tm
trademark as set forth in paragraphs 1.E.8 or 1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any
additional terms imposed by the copyright holder. Additional terms
will be linked to the Project Gutenberg-tm License for all works
posted with the permission of the copyright holder found at the
beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including
any word processing or hypertext form. However, if you provide access
to or distribute copies of a Project Gutenberg-tm work in a format
other than "Plain Vanilla ASCII" or other format used in the official
version posted on the official Project Gutenberg-tm web site
(www.gutenberg.org), you must, at no additional cost, fee or expense
to the user, provide a copy, a means of exporting a copy, or a means
of obtaining a copy upon request, of the work in its original "Plain
Vanilla ASCII" or other form. Any alternate format must include the
full Project Gutenberg-tm License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works
provided that
* You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is owed
to the owner of the Project Gutenberg-tm trademark, but he has
agreed to donate royalties under this paragraph to the Project
Gutenberg Literary Archive Foundation. Royalty payments must be paid
within 60 days following each date on which you prepare (or are
legally required to prepare) your periodic tax returns. Royalty
payments should be clearly marked as such and sent to the Project
Gutenberg Literary Archive Foundation at the address specified in
Section 4, "Information about donations to the Project Gutenberg
Literary Archive Foundation."
* You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or destroy all
copies of the works possessed in a physical medium and discontinue
all use of and all access to other copies of Project Gutenberg-tm
works.
* You provide, in accordance with paragraph 1.F.3, a full refund of
any money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days of
receipt of the work.
* You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project
Gutenberg-tm electronic work or group of works on different terms than
are set forth in this agreement, you must obtain permission in writing
from both the Project Gutenberg Literary Archive Foundation and The
Project Gutenberg Trademark LLC, the owner of the Project Gutenberg-tm
trademark. Contact the Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
works not protected by U.S. copyright law in creating the Project
Gutenberg-tm collection. Despite these efforts, Project Gutenberg-tm
electronic works, and the medium on which they may be stored, may
contain "Defects," such as, but not limited to, incomplete, inaccurate
or corrupt data, transcription errors, a copyright or other
intellectual property infringement, a defective or damaged disk or
other medium, a computer virus, or computer codes that damage or
cannot be read by your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH 1.F.3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium
with your written explanation. The person or entity that provided you
with the defective work may elect to provide a replacement copy in
lieu of a refund. If you received the work electronically, the person
or entity providing it to you may choose to give you a second
opportunity to receive the work electronically in lieu of a refund. If
the second copy is also defective, you may demand a refund in writing
without further opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS', WITH NO
OTHER WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT
LIMITED TO WARRANTIES OF MERCHANTABILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of
damages. If any disclaimer or limitation set forth in this agreement
violates the law of the state applicable to this agreement, the
agreement shall be interpreted to make the maximum disclaimer or
limitation permitted by the applicable state law. The invalidity or
unenforceability of any provision of this agreement shall not void the
remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in
accordance with this agreement, and any volunteers associated with the
production, promotion and distribution of Project Gutenberg-tm
electronic works, harmless from all liability, costs and expenses,
including legal fees, that arise directly or indirectly from any of
the following which you do or cause to occur: (a) distribution of this
or any Project Gutenberg-tm work, (b) alteration, modification, or
additions or deletions to any Project Gutenberg-tm work, and (c) any
Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of
computers including obsolete, old, middle-aged and new computers. It
exists because of the efforts of hundreds of volunteers and donations
from people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need are critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future
generations. To learn more about the Project Gutenberg Literary
Archive Foundation and how your efforts and donations can help, see
Sections 3 and 4 and the Foundation information page at
www.gutenberg.org Section 3. Information about the Project Gutenberg
Literary Archive Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Contributions to the Project Gutenberg Literary
Archive Foundation are tax deductible to the full extent permitted by
U.S. federal laws and your state's laws.
The Foundation's principal office is in Fairbanks, Alaska, with the
mailing address: PO Box 750175, Fairbanks, AK 99775, but its
volunteers and employees are scattered throughout numerous
locations. Its business office is located at 809 North 1500 West, Salt
Lake City, UT 84116, (801) 596-1887. Email contact links and up to
date contact information can be found at the Foundation's web site and
official page at www.gutenberg.org/contact
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
[email protected]
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To SEND
DONATIONS or determine the status of compliance for any particular
state visit www.gutenberg.org/donate
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including checks, online payments and credit card donations. To
donate, please visit: www.gutenberg.org/donate
Section 5. General Information About Project Gutenberg-tm electronic works.
Professor Michael S. Hart was the originator of the Project
Gutenberg-tm concept of a library of electronic works that could be
freely shared with anyone. For forty years, he produced and
distributed Project Gutenberg-tm eBooks with only a loose network of
volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as not protected by copyright in
the U.S. unless a copyright notice is included. Thus, we do not
necessarily keep eBooks in compliance with any particular paper
edition.
Most people start at our Web site which has the main PG search
facility: www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.