The Project Gutenberg EBook of American Weasels, by E. Raymond Hall
This eBook is for the use of anyone anywhere at no cost and with
almost no restrictions whatsoever. You may copy it, give it away or
re-use it under the terms of the Project Gutenberg License included
with this eBook or online at www.gutenberg.org
Title: American Weasels
Author: E. Raymond Hall
Release Date: July 21, 2013 [EBook #43272]
Language: English
Character set encoding: ISO-8859-1
*** START OF THIS PROJECT GUTENBERG EBOOK AMERICAN WEASELS ***
Produced by Chris Curnow, Richard Tonsing, Joseph Cooper
and the Online Distributed Proofreading Team at
http://www.pgdp.net
University of Kansas Publications
Museum of Natural History
Vol. 4, pp. 1-466, plates 1-41, 31 figures in text
December 27, 1951
BY
E. RAYMOND HALL
University of Kansas
Lawrence
1951
University of Kansas Publications, Museum of Natural History
Editors: E. Raymond Hall, Chairman, A. Byron Leonard,
Edward H. Taylor, Robert W. Wilson
Vol. 4, pp. 1-466, plates 1-41, 31 figures in text
December 27, 1951
University of Kansas
Lawrence, Kansas
PRINTED BY
FERD VOILAND, JR., STATE PRINTER
TOPEKA, KANSAS
1951
23-3758

Plate 1.Coloration of head and foreparts in ten
subspecies of long-tailed weasel, Mustela frenata. All figures
are of males, approximately × 1/2.
In regions of heavy rainfall (see figs. 2 and 3) there is an increase
in pigmentation and extent of blackish color backward over the neck and
a decrease in extent of the white facial markings. In regions
progressively more arid (see figs. 3 to 7) there is a decrease in
pigmentation and extent of blackish color and an increase in extent of
the white facial markings.
As shown by rearing mammals from humid regions in arid regions, and
vice versa, the color is not visibly altered in one or a few
generations; the color is an hereditary character. Beginning with the
southernmost subspecies (fig. 1) and continuing northward to the
northern subspecies (fig. 10) there is a darkening, next a lightening,
and finally a darkening closely conforming to amounts of precipitation
in the geographic regions concerned. A fuller discussion of this
correlation is given on page 51.
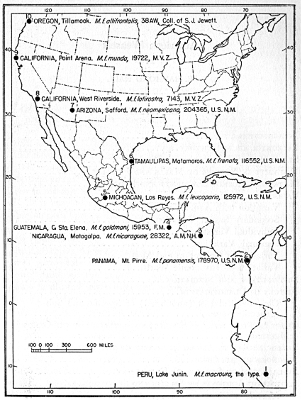
Fig. 1. Map showing localities of capture of
specimens depicted in plate 1.
American Weasels
BY
E. RAYMOND HALL
|
PAGE |
| Introduction |
7 |
| Paleontological History |
10 |
| Skeleton and Dentition |
12 |
| Disparity in Numbers of Males and Females |
19 |
| Materials, Acknowledgments and Methods |
21 |
| Variation |
24 |
| Variation with Age |
24 |
| Secondary Sexual Variation |
26 |
| Individual Variation |
28 |
| Seasonal Variation |
30 |
| Variation in Coloration and Molt |
30 |
| Variations of Taxonomic Worth |
44 |
| Distribution and Speciation |
54 |
| History of Classification |
69 |
| Chronological List (annotated) of Specific and Subspecific Names Applied to American Weasels |
71 |
| Check-List of American Species and Subspecies of the Genus Mustela |
81 |
| Artificial Key to American Species of the Genus Mustela |
83 |
| Diagnosis of the Genus |
83 |
| Explanation of Systematic Treatment |
84 |
| Systematic Accounts of Species and Subspecies |
87 |
| Mustela erminea |
87 |
| Mustela rixosa |
168 |
| Mustela frenata |
193 |
| Mustela africana |
406 |
| Explanation of Cranial Measurements |
417 |
| Table of Cranial Measurements |
418 |
| Literature Cited |
442 |
| Index |
461 |
American Weasels
By E. Raymond Hall
The weasel's agility and speed take it in and out of retreats, over
obstacles and across open places in amazingly rapid fashion and are
responsible for the animal's actions being described as "quick as a
flash." The common long-tailed weasel of the United States measures
approximately a foot and a half in length, of which the tail comprises
a third; but the round, slender body is scarcely more than an inch and
a half in diameter. Brown above and whitish below in summer dress, the
animal is sleek as well as lithe and graceful. It is easy to
understand, therefore, why the Bavarian name Schönthierlein
(pretty little creature) and the Italian name donnola (little lady)
were bestowed upon it. The Spanish name is comadreja (godmother).
In the winter, in temperate and northern regions, the coat becomes pure
white except for the black tail-tip. In this dress the correct name for
the animal is ermine, a mammal whose fur is known to all and justly
esteemed, especially for its luster in artificial light, where it is
scarcely excelled in enhancing the beauty of gems and their feminine
wearers.
In relation to its weight, the weasel is thought to be unsurpassed, and
perhaps it is unequalled among mammals, in the effectiveness with which
it exercises its carnivorous heritage; it kills with speed and strength
a wide variety of animals including many much larger than itself; and
it has been known to attack even man himself when he stood between the
weasel and its intended prey. In structure and temperament it is so
highly specialized for offense that, when opportunity affords, it
sometimes kills, for storage in its larder, far more than enough to
meet its immediate needs. After speaking of this tendency, Elliott
Coues (1877:129) has said:
"A glance at the physiognomy of the weasels would suffice to betray
their character. The teeth are almost of the highest known raptorial
character; the jaws are worked by enormous masses of muscles covering
all the side of the skull. The forehead is low and the nose is sharp;
the eyes are small, penetrating, cunning, and glitter with an angry
green light. There is something peculiar, moreover, in the way that
this fierce face surmounts a body extraordinarily wiry, lithe, and
muscular. It ends in a remarkable long and slender neck in such a way
that it may be held at right angle with the axis of the latter. When
the creature is glancing around, with the neck stretched up, and flat
triangular head bent forward, swaying from one side to the other, we
catch the likeness in a moment—it is the image of a serpent." Although
Coues' colorful description more closely links the weasel with the
symbol of evil than pleases me, his description does emphasize the
raptorial character of the weasel.
Even though most weasels are intractable as pets, they have a value to
man, as, for instance, when he is plagued by mice. In a field where
mice and other small rodents are so abundant as to damage cultivated
crops, the weasel is the farmer's best friend. A weasel may inhabit one
den until the rodents thereabouts are almost exterminated in an area
two or three hundred yards across; in this way the weasel acts as a
control, locally, as well as a check more widely, on the increase in
size of populations of kinds of rodents upon which it preys. The
smaller species are mousers of remarkable efficiency and can, if
necessary, follow a mouse to the end of the mouse's burrow. The slender
body allows the weasel to pass through any burrow or hole into which it
can thrust its head. This ability in an organism as highly specialized
for killing other animals as is the weasel, has earned for it a bad
name in connection with poultry yards. Authentic instances are recorded
in which a weasel, gaining entrance through a knot-hole to a coop of
young chickens, killed several dozen of the fowls. In other instances,
however, weasels have lived under buildings close by a poultry yard
without even molesting the birds in the slightest; in the latter
instances the weasels probably were present because there was an
abundant supply of rats and mice. At least three poultry raisers (see
page 214) have encouraged weasels to live in their poultry yards
feeling that the good they do by destroying rats outweighs the damage
caused by the occasional weasel which turns to the fowls; the idea is
that the individual weasel can be eliminated if he becomes destructive.
Although tending to be nocturnal, weasels are almost as active by day
as by night. Their young, numbering 4 to 9, are born in a nest in a
burrow and as with other members of the Order Carnivora, are blind, and
incapable of looking after themselves at the time of birth. In Mustela
frenata of Montana, breeding occurs in July and August, and the young
are born in the following April and May. Wright (1948A:342) showed that
the gestation period could not have been less than 337 days in one
individual and that it averaged 279 (205-337) days in 18 instances.
Findings of the same author (1942B:109) showed that the embryos are
implanted only 21 to 28 days before the young are born. In the
preceding part of the "long gestation period, the embryos lie dormant
in the uterus as un-implanted blastocysts. The young female weasel [of
M. frenata] mates when 3 or 4 months old." Consequently, in the
spring, all females of this species may produce young (Wright,
1942A:348). The circumboreal species Mustela erminea likewise has
been shown to have a delayed implantation of the ova. Each of these two
species, M. frenata and M. erminea, has only one litter per year;
but the weasel, Mustela nivalis, of the Old World seems to lack the
delayed implantation, in this respect resembling the ferret (subgenus
Putorius) as it does also in its ability to have more than one litter
per year (see Deanesly, 1944). The manner of reproduction in the South
American species M. africana and the circumboreal species M. rixosa
at this writing is unknown.
The genus Mustela includes the true weasels, the ferrets and minks.
The ferrets commonly are treated as a subgenus, Putorius, along with
the Old World polecat. The minks usually are accorded subgeneric
distinction under the name Lutreola, and the true weasels comprise
the subgenus Mustela, the three subgenera together, along with some
other subgenera which are mostly monotypic, comprising the genus
Mustela. Considered in this way, the group of true weasels, subgenus
Mustela, has a geographic range roughly coextensive with that of the
genus Mustela. This range includes Asia and Europe, Northern Africa,
North America and northern South America. Java has its weasel.
Australia and nearly all the oceanic islands lack weasels, and the
animals are absent from roughly the southern half of Africa and the
southern half of South America. Other small mustelids, weasellike in
shape and with corresponding habits and dentition, take the place of
true Mustela in the southern half of Africa and in the corresponding
part of South America.
In America the subgenus Mustela occurs from the northernmost land in
Arctic America southward to Lake Titicaca in the Andes of South
America, a distance of approximately 6900 miles. Felis, I think, is
the only other genus of land mammals in the western hemisphere that has
a geographic range as extensive from north to south. Felis does not
range so far north but does range farther south. The one species,
Mustela frenata, ranges from Lake Titicaca northward to about 57° N
in British Columbia or for approximately 5000 miles in a north to south
direction and from within the Alpine Arctic Life-zone through the
Tropical Life-zone. In North America, weasels occur in almost every
type of habitat, being absent only in the extremely desert terrain of
western Arizona and western Sonora and in adjoining parts of California
and Baja California. Even this area, along the Colorado River, may
support some weasels; evidence suggesting that it does so is given in
the account of Mustela frenata neomexicana.
The paleontological record fails to show the precise ancestry of
Mustela. The genus has been found in deposits of Pleistocene age,
but, so far as I can ascertain, not in deposits of earlier times. The
Pleistocene remains are not specifically distinct from Recent (living)
species, and in only a few instances (see M. f. latirostra and M. e.
angustidens) are they even subspecifically distinct from the Recent
weasel living in the same area today. It is true that fossil remains
from deposits of several stages of the Tertiary beds have in the past
been identified in the literature as Mustela, but most of these
identifications were made many years ago when the generic name
Mustela was used in a far broader and more inclusive sense than it is
today and much of the fossil material was so fragmentary that the
generic identity could not be ascertained, at least at that time.
Because the generic identity could not be ascertained, the fossil
material was tentatively assigned to the genus Mustela, the "typical"
genus of the family Mustelidae instead of to some other more
specialized or less well-known genus of the family. To satisfy my
curiosity about these species of "Mustela" of a geological age
earlier than the Pleistocene I have personally studied nearly all of
the original specimens from North America and have found each to be of
some genus other than Mustela. Also, such study as I have been able
to make of the Old World fossils themselves that have been referred to
the genus Mustela up to 1938, and my study of the illustrations and
descriptions of the others from there lead to the same conclusion; that
is to say, none that is true Mustela is known up to now from deposits
older than the Pleistocene.
When, in 1930 (pp. 146-147), I wrote about the taxonomic position of
three American genera of fossils (known only from lower jaws), each of
which had been previously referred to the genus Mustela, I said that
they pertained "to that section of the weasel family (Mustelidae)
which comprises the polecats, true weasels, ferrets, minks and martens.
The fossil specimens . . . are smaller than any other later Tertiary
members of the group yet described, and are more primitive than any of
the above mentioned Recent relatives. Of the three extinct genera . . .
Miomustela [Lower Pliocene or Upper Miocene of the Lower Madison
Valley, Montana] is the most primitive and Martinogale [Pliocene, 18
mi. SE Goodland, Sherman County, Kansas] is the most advanced. This
view rests largely on the character of M=1 which in Miomustela has
a deeply basined, short, narrow talonid with a thick, high metaconid
situated partly posterior to the protoconid. In Martinogale the
talonid is incipiently trenchant, long, broad, and it has a lesser
developed metaconid which is situated more anterior [ly]. Pliogale
[Lower Pliocene, Humboldt County, Nevada] is intermediate in this
respect.
"These three forms are of special interest as possible ancestors of the
subgenus Mustela, true weasels. No members of this subgenus, nor
related forms which can with any degree of certainty be regarded as
directly ancestral to them, have yet been described from Miocene or
Pliocene deposits. Palaeogale of the Old World and Bunaelurus of
North America, each of Oligocene age, have been placed by Schlosser
(1888, p. 116) and Matthew (1902, p. 137) as members of the primitive
group of mustelids ancestral to Mustela. This course seems logical;
and with no truly intermediate links between these forms of the
Oligocene on the one hand, and Mustela which first appears in the
Pleistocene, on the other, more definite statements about ancestral
positions of the small Oligocene forms can hardly be made. The deciding
considerations for authors who placed Palaeogale and Bunaelurus as
ancestral to Mustela were the absence of a metaconid on M1 and the
trenchant talonid of that tooth. These characters are found also in
Mustela. On the other hand certain structures in the basicranial
region of Palaeogale and more especially of Bunaelurus indicate
that these genera possibly are not close to the ancestral form of
Mustela . . . Martinogale may stand near the ancestral form of
Mustela and . . . Pliogale may be ancestral to Martinogale.
Pliogale, in turn, may have had an ancestor similar to Miomustela.
If this should prove to be the case, Palaeogale and Bunaelurus
might be regarded as an independent branch which displays merely a
parallelism to Mustela in the loss of the metaconid on M1 and the
development of a trenchant talonid on that tooth. The writer would make
it clear that he does not hold such to be the case. The ancestral
relation of Martinogale to Mustela is presented merely to show the
possibility, and not the special probability, of such an origin for
Mustela. Knowledge of the tympanic bullae and other structures of the
basicranial region would go far toward answering the question and until
these structures are known [in mustelids of the Later Tertiary,] some
uncertainty will remain."
At the present writing I can add to the above statement only a few
facts. The discovery of better material of Bunaelurus than was
available to previous workers led Simpson (1946), correctly I think, to
synonymize Bunaelurus with Palaeogale. Simpson figures the cranial
foramina in Palaeogale. The differences, between Palaeogale and
Mustela, in cranial foramina, possibly are only the result of the
elongation of the tympanic bullae. The bullae of the subgenus Mustela
are seen to be much elongated posteriorly if comparison is made with
the bullae of earlier mustelids. Consequently, it might be concluded
that there is nothing in the arrangement of the cranial foramina which
would preclude the derivation of Mustela from Palaeogale. However,
the anterior situation of the carotid foramen—well forward along the
medial margin of the tympanic bulla—is a character typical of other
mustelids and the posterior location of this foramen in Palaeogale
might indicate that it was not ancestral to Mustela.
The outstanding features of a weasel's skeleton are its length and
slenderness. Whereas the length of the vertebral column measured from
the atlas (the first cervical vertebra) to the last sacral vertebra is
175 per cent of the length of the hind leg (as measured from the head
of the femur to the tip of the longest claw), the corresponding
percentage is only 116 in the raccoon. Stated in another way, the
vertebral column and the hind leg are of approximately equal length in
a raccoon, but in a weasel the vertebral column is one and
three-fourths times as long as the hind leg.
VERTEBRAE
The vertebral column consists of 7 cervicals, and ordinarily 14
thoracics, 6 lumbars, 3 sacrals and, depending on the species, 11 to 23
caudals. For the three species of which skeletons were examined,
variations from the normal number of vertebrae are noted in the
following table:
Table 1
Data on vertebrae in three species of the subgenus Mustela
(Numerals in parentheses indicate number of specimens)
| |
Mustela erminea |
Mustela rixosa |
Mustela frenata |
| Number of cervical vertebrae |
7(75) |
7(12) |
7(65) |
| Number of thoracic vertebrae |
14(71) |
14(12) |
14(54) |
| 15(4) |
|
15(13) |
| The dorsal vertebraconstituting the
anticlinal |
11th(18) |
11th(12) |
11th(40) |
| 12th(7) |
|
12th(27) |
| Number of lumbar vertebrae |
5(2) |
|
5(11) |
| 6(73) |
6(12) |
6(54) |
| Number of sacral vertebrae |
2(9) |
|
2(3) |
| 3(65) |
3(10) |
3(67) |
| 4(1) |
4(2) |
|
| Number of pseudosacral vertebrae |
0(73) |
0(12) |
0(57) |
| 1(2) |
|
1(6) |
| |
11(1) |
|
| |
14(3) |
|
| 15(2) |
15(7) |
|
| 16(3) |
16(1) |
|
| 17(9) |
|
|
| Number of caudal vertebrae |
18(28) |
|
|
| 19(11) |
|
19(6) |
| |
|
20(14) |
| |
|
21(14) |
| |
|
22(7) |
| |
|
23(1) |
Variation according to the species is evident in the number of caudal
vertebrae, but in the other categories of vertebrae no consistent
difference in number according to species was found in the material
examined. Apparently there is also some geographic variation in the
number of caudal vertebrae within a species. For example, the one
skeleton seen of Mustela rixosa eskimo (no. 219036, U. S. Nat. Mus.,
from St. Michaels, Alaska) has only 11 caudal vertebrae, whereas in the
11 Mustela rixosa rixosa from Roseau County, Minnesota, the usual
number is 15 with extremes of 14 and 16. Similarly specimens of
Mustela frenata from Idaho and California almost always have 1 or 2
more caudal vertebrae than do individuals of the shorter-tailed
subspecies of the same species from eastern Kansas.
Of the vertebrae, only the cervicals, of which there are 7, were found
to be constant in number. In M. erminea, two of the seven individuals
in which the anticlinal vertebra was the 12th (instead of the 11th) had
15 instead of the customary 14 thoracic vertebrae. In M. frenata,
seven of the twenty-seven individuals in which the anticlinal vertebra
was the 12th (instead of the 11th) had 15 instead of 14 thoracic
vertebrae. The one M. erminea with a pseudosacral vertebra had only
two instead of the customary 3 sacral vertebrae but the same individual
had 15 thoracic vertebrae. Of the six M. frenata with a pseudosacral
vertebra, two animals had only two instead of three sacral vertebrae.
Conceivably, therefore, the pseudosacral vertebra in each of the three
instances mentioned may represent merely an unfused sacral vertebra,
instead of a true pseudosacral as occurs in four individuals of M.
frenata.
TEETH
In American weasels, for example in Mustela frenata, the permanent
dentition normally is
I 3 C 1 P 3 M 1
-, -, -, -, -, -, -, -
i 3 c 1 p 3 m 2
or 34 teeth in all. In most respects the dentition is typical for
post-Tertiary mustelids but in several parts is highly specialized for
a diet of flesh, the degree of this specialization being second only to
that of the cats, family Felidae. The outstanding specialization is in
the first lower molar, in which, as in the cats, the internal cusp
(metaconid) is completely suppressed and the heel (talonid) forms an
elevated blade for cutting food rather than a basin for crushing it. In
one sense the tooth is simplified since it owes its distinctive form to
a reduction in number of parts; nevertheless, the distinctive form of
the lower molar clearly is correlated with a diet of flesh, and
the tooth is correctly to be thought of as the lower blade of a
pair of shears; the upper blade is the fourth upper premolar. The
reduction in size of the second (last) lower molar and small size of
the inner lobe of the one remaining upper molar probably are additional
modifications for a diet of flesh.
The absence of the last two upper molars and last molar in the lower
jaw would be expected in any mammal as highly specialized for a diet of
flesh as is the weasel, but these teeth are absent also in other
Quaternary members of the family Mustelidae, many of which are
substantially less specialized for a diet of flesh than is the weasel.
Therefore, in the weasel, it is reasonable to regard the absence of
these teeth more as a heritage than as an indication of a special
adaptation. The absence of a first premolar above and below, as in the
weasel, is to be expected in any carnivore that has the first lower
molar and fourth upper premolar highly specialized for shearing, but
the loss of these premolars and the small size of the second premolars
may be as much the result of a slight shortening of the face as it is a
result of a lengthening of the third and especially the fourth
premolars. The lengthening of these more posteriorly-situated teeth
would appear to be an adaptation to a diet of flesh. The cause of the
lengthening of the mentioned teeth and the reason for the absence of
the first premolars probably will be unknown until the fossil record is
more complete.
The teeth of American species vary little except in size. The absence
of P2 in Mustela africana is the only difference of a qualitative
(presence or absence) nature that was detected. Also, the Central
American subspecies of Mustela frenata exhibit a tendency to early
loss of P2 and thus foreshadow the condition typical of M. africana.
As a whole the dentition of the weasel exhibits a high degree of
specialization for a diet of flesh and this specialization is fully as
evident in the deciduous dentition as in the permanent dentition.
The deciduous, or milk, dentition, of Mustela frenata, as known from
immature specimens of Mustela frenata noveboracensis and Mustela
frenata frenata available for this study, is comprised of canines, one
on each side above and below, and 3 cheek teeth on each side above and
below. See figures 2-9. The upper cheek teeth from anterior to
posterior are: a minute peglike tooth in general similar to the first
premolar of the permanent dentition; a shearing tooth in general
similar to P4 of the permanent dentition; and an anteroposteriorly
compressed tooth in general similar to M1 of the permanent dentition.
In the lower jaw, behind the canine, there is first a minute peglike
tooth, second a two-rooted tooth similar in general outline to a
permanent third premolar, and finally a shearing tooth corresponding in
function to m1 of the permanent dentition.
No postnatal specimens which show deciduous incisors have been
examined.
Selected, outstanding differences between the permanent teeth and the
deciduous teeth are as follows: In the deciduous teeth the canine above
has on the posterior face a well-defined ridge extending from the tip
to the cingulum. This ridge is absent or at most faintly indicated in
the permanent tooth. The lower deciduous canine, in cross section is
seen to have a marked indentation on the anteromedial border in the
region of the cingulum; this indentation is lacking in the permanent
tooth. The anterior one of the deciduous cheek teeth, both above and
below, is single rooted and its crown-surface is only about
one-fifteenth as much as that of the anterior premolar of the permanent
dentition. The second deciduous cheek tooth below has two roots,
usually fused, and differs from p4 of the permanent dentition in having
the tip of the principal cusp more recurved, in having the anterior
basal cusp better developed and the posterior heel less well developed.
The second deciduous cheek tooth above corresponds in function and
general plan of construction to P4 of the permanent dentition but
differs from that tooth in the more pronounced protostyle, longer
tritocone, more posteriorly located deuterocone and as noted by Leche
(1915:322) separation of the protocone and tritocone by a notch. The
third upper deciduous tooth has a single cusp internally and two cusps
laterally. Thus it reverses the relation of parts seen in M1 where the
internal moiety is larger than the lateral or buccal moiety. The third
deciduous tooth below differs from m1 in very much shorter talonid and
separation of the paraconid from the protoconid by a deeper notch.
All the features in which the last two deciduous teeth, both above and
below, are described as differing from their functional counterparts in
the permanent dentition, are features found in the permanent teeth of
primitive fossil mustelids and certain fossil and Recent viverrids.
Even so, taking into account Leche's (1915) work, which shows that the
milk teeth of some carnivores have structures lacking in the
corresponding permanent teeth of the same individual animal and also in
the teeth of genera that seem to be ancestral, a person suspects that
some of the structural features mentioned above are not inheritances
of ancestral conditions but rather specializations of the milk
dentition.
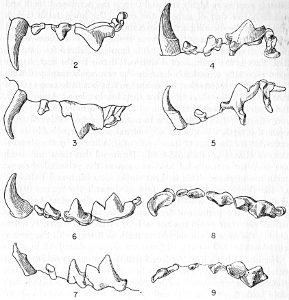
Figs. 2-9. Views of permanent and deciduous teeth of
Mustela frenata nigriauris. Incisors not shown. In each instance
teeth are of the left side.
Permanent dentition × 3. No. 32421, Mus. Vert. Zoöl., ♂, adult;
Berkeley, Alameda County, California; obtained October 4, 1921, by D.
D. McLean.
Deciduous dentition × 5. No. 132158, U. S. Nat. Mus., ♂, juvenile;
Stanford University, Santa Clara County, California; obtained May 7,
1898, by W. K. Fisher.
Figs. 2-3. Lateral views of upper teeth, of adult and juvenile
respectively.
Figs. 4-5. Occlusolingual views of upper teeth of adult and juvenile
respectively.
Figs. 6-7. Lateral views of lower teeth of adult and juvenile
respectively.
Figs. 8-9. Occlusolingual views of lower teeth of adult and juvenile
respectively.
In other deciduous teeth there is clearer evidence of more
specialization for a diet of flesh in the deciduous teeth than in the
permanent teeth. For example, the upper carnassial of the milk
dentition is even more highly sectorial than is the permanent tooth
and strikingly like that of some of the cats. The lower tooth that is
effective in the shearing action bears no more trace of the metaconid
than does the permanent first lower molar. These features of the
deciduous dentition suggest that it is more specialized for a diet of
flesh than is the permanent dentition. If this be the fact, it may seem
especially remarkable because the commonly employed term "milk teeth"
suggests that the animal makes but little or no use of these teeth in
the short time that they are in place. Accordingly, the student may
credit the form of these teeth more to some indirect effects of
inheritance than to natural selection acting directly upon the teeth.
But, after all, natural selection probably is responsible for the form
of these teeth as is indicated by the observations of Hamilton
(1933:318-325). He found that these milk teeth are used for eating
solid food as soon as the principal shearing teeth are in place. This
is three weeks after birth and before all of the deciduous teeth have
broken through the gums. These shearing teeth are used for almost two
months before being replaced by the permanent teeth and it is,
therefore, evident that natural selection could operate to fully as
great a degree in determining the form of the deciduous teeth as it may
with the permanent teeth.
Hamilton (1933:325-326) found that the permanent dentition was complete
at 75 days after birth in captive specimens of Mustela frenata
noveboracensis. In the same subspecies, he noted 28 days after birth
that the canines and carnassial teeth [second deciduous cheek tooth
above and third below] had erupted through the gums. Animals 45 days
old, Hamilton found, were losing the milk dentition, and had the gums
broken through by several of the permanent cheek teeth.
Study of the cleaned skulls available of juveniles indicates that the
deciduous teeth which persist longest are, on each side of the mouth,
the second cheek tooth above and the third cheek tooth below. These
teeth persist until after the permanent P4 and m1 have come into use.
These permanent teeth are situated immediately behind their functional
counterparts of the milk dentition. P3 and p4 are the teeth of the
permanent dentition which ultimately push out the last milk teeth to be
lost. Accordingly, in the permanent dentition, P4 and M1 appear before
P3 does, and m1 and m2 make their appearance before p4.
The question has frequently been asked why twice as many male as female
weasels are captured. This is the proportion in research collections,
as may be seen from table no. 2, and I am convinced that the specimens
in these collections are saved in approximately the same proportion as
that in which they are caught. Although it might be assumed, upon first
consideration, that there are twice as many males as females in nature,
selective factors enter into the catch. For example, because a male
weasel is approximately twice as heavy as a female, it may be necessary
for him, in a given length of time, to travel twice as far as the
female to obtain the required amount of food with the result that a
given number of traps or snares will catch twice as many males as
females. Indeed, Glover (1943B:8) shows that, on the average, in
Mustela frenata noveboracensis in Pennsylvania, the male actually
does travel slightly more than twice as far as the female (704 feet
versus 346 feet). From table no. 2, it may be seen that in most winter
months the ratio is 3 males to one female. This ratio is reasonable
enough, in view of what has been said, if it is considered also that
the lighter weight of the female permits her safely to step on the pans
of traps that would be sprung by heavier males.
If in the breeding season, which is April through August in M.
frenata, the female is passive and if the male is restlessly searching
for her, he may thus increase still more his chances of being caught in
traps set for weasels.
My own studies of live weasels in nature indicate that in the season
when females are attending young which are half grown, or larger, the
adult male weasels live singly in dens of their own, separate and apart
from the females and their young (Hamilton, 1933:328, records adult
males living with the female and her young, but possibly this was when
the young were less than half grown). Perhaps these males at that time
travel no farther than is necessary to obtain food for themselves.
Females, at this time, forage not only to meet their own needs, but for
food to supply their young as well. At this time, in May and June, as
may be seen from table no. 2, almost as many adult females as adult
males are caught. The reason why only relatively more females than in
other months, instead of actually more females than males, are caught
at this time probably is that the adult males also are extraordinarily
active at this time because they are in breeding condition. Perhaps
the explanation in part is to be found in the lesser weight of the
female (approximately half of the male's weight) which, as indicated
above, permits her to step on the pan of a steel trap without springing
it whereas the heavier male does spring the trap and as a consequence
is caught. Hamilton (1933:299-300), who mentions this selective factor,
found an equal number of males and females in the three newly born
litters that came under his observation.
Table 2
Specimens of Mustela frenata (north of the range of M. f. frenata)
arranged by sex and under each sex by age
|
Male |
Female |
total number of ♂ and ♀ |
total number of adults, ♂ and ♀ |
|
adult ♂ |
♂ ad., % of total adults |
subadult ♂ |
young ♂ |
juvenal ♂ |
total number of ♂ |
♂, % of total |
adult ♀ |
♀ ad., % of total adults |
subadult ♀ |
young ♀ |
juvenal ♀ |
total number of ♀ |
♀, % of total |
| May |
29 |
55 |
4 |
14 |
7 |
54 |
59 |
24 |
45 |
1 |
9 |
3 |
37 |
41 |
91 |
53 |
| June |
42 |
53 |
14 |
40 |
8 |
97 |
59 |
38 |
47 |
4 |
25 |
2 |
69 |
41 |
166 |
80 |
| July |
59 |
70 |
18 |
55 |
2 |
130 |
59 |
25 |
30 |
5 |
58 |
2 |
90 |
41 |
220 |
84 |
| August |
40 |
77 |
23 |
55 |
.. |
113 |
74 |
12 |
23 |
2 |
25 |
.. |
39 |
26 |
152 |
52 |
| September |
15 |
79 |
25 |
12 |
1 |
51 |
75 |
4 |
21 |
4 |
9 |
.. |
17 |
25 |
68 |
19 |
| October |
11 |
58 |
46 |
7 |
.. |
43 |
66 |
8 |
42 |
13 |
1 |
.. |
22 |
34 |
65 |
19 |
| November |
41 |
70 |
48 |
1 |
.. |
88 |
73 |
18 |
30 |
12 |
2 |
1 |
33 |
27 |
121 |
59 |
| December |
59 |
69 |
43 |
1 |
.. |
108 |
73 |
26 |
31 |
15 |
... |
.. |
41 |
27 |
149 |
85 |
| January |
80 |
69 |
32 |
2 |
1 |
126 |
72 |
36 |
31 |
14 |
... |
.. |
50 |
28 |
176 |
116 |
| February |
45 |
66 |
19 |
5 |
.. |
82 |
73 |
23 |
34 |
4 |
3 |
.. |
30 |
27 |
112 |
68 |
| March |
38 |
72 |
2 |
... |
.. |
57 |
70 |
15 |
28 |
8 |
1 |
.. |
24 |
30 |
81 |
53 |
| April |
30 |
67 |
2 |
4 |
3 |
39 |
67 |
15 |
33 |
.. |
2 |
2 |
19 |
33 |
58 |
45 |
| Totals |
489 |
67 |
281 |
196 |
22 |
988 |
68 |
244 |
33 |
82 |
135 |
10 |
471 |
32 |
1,459 |
733 |
I suppose that in nature there are approximately equal numbers of male
and female weasels and further suppose that the selective factors which
cause more males than females to be caught are the greater distances
traveled by the males and their greater weight.
At a late stage in the preparation of this manuscript a total of 5,457
specimens had been examined. For the most part these were conventional
study-specimens; that is to say, they were stuffed skins with the
skulls separate and each was accompanied by the customary data as to
locality of capture, date of capture, name of collector, external
measurements and sex recorded on the labels by the collectors. Skulls
unaccompanied by skins, nevertheless, comprised a large share of the
total and a small proportion was made up of skins unaccompanied by
skulls, mounted specimens, skeletons, and entire animals preserved in
liquid.
It was the recognition of this need for specimens from extensive
areas from which no specimens previously had been collected that
influenced me, approximately a year after the study was begun, to
allot for it a long span of time. The procedure adopted, in
general, was to study the weasels of one species from a given
geographic area in so far as the material warranted, then lay this
aside until additional critical material could be obtained, and
finally, some months or a year later, complete the account. In
this fashion the manuscript of the American weasels received my
attention in each of the past twenty-five years (September, 1926
to date of publication). This is a confession of fact rather than
a recommendation of procedure. This type of procedure unduly
delays the diffusion of knowledge and for a variety of reasons
justifiably annoys other students of the subject. Nevertheless,
many gaps have been filled that otherwise would have remained
open. Although specimens to solve several problems still remain to
be collected and studied, it seems that a point of diminishing
returns has now been reached, which, in fairness to all concerned,
calls for publication of the results so far obtained.
For assistance in the entire undertaking, I am more indebted to
Miss Annie M. Alexander than to any other one person; she provided
the means by which specimens from critical areas were obtained,
made it possible to examine the European collections, and assisted
in other ways. The late Professor Joseph Grinnell and Mr. Charles
D. Bunker, among others, gave truly valuable encouragement and
assistance.
Collections containing weasels which were examined in the study
here reported upon were as follows:
The largest single collection is in the United States National
Museum, where the specimens of the National Museum proper and the
United States Biological Surveys Collection, together, provide
essential materials including a large share of the holotypes.
Specimens in all of the North American collections including
Canada and México have been made available, by loan, and in 1937
materials were examined in the principal collections of northern
and central Europe. After the materials in North American
collections were assembled, special effort, with considerable
success, was made in each of several winters, to obtain specimens
from areas not previously represented in collections.
To the many persons who were in charge of the collections
consulted, to those who at my request sought critical specimens,
and to those who assisted in various stages of assembling data and
in preparation of the manuscript, I am grateful indeed. Likewise,
I am deeply appreciative of the grants-in-aid received from the
Carnegie Institution of Washington, the University of California
Chapter of Sigma Xi, the John Simon Guggenheim Memorial Foundation
and the Kansas University Endowment Association. I am mindful also
of an obligation to those who appropriated funds, by legislative
action, for research use by The University of California and The
University of Kansas.
For assistance with the illustrations I am indebted to the late
Major Allan Brooks for Plate 1 , to Mrs. Mary Blos for figures
25-31, to Miss Ann Murray for figures 11-13, to Mr. W. C. Matthews
for all the photographs, to Mrs. Freda L. Abernathy for figures
2-9, 18-22, 24, and for retouching all the photographs except the
following which were retouched by Mrs. Virginia Unruh: figs. d
of plates 2, 3, 4, 9, 10, 11, 16, 17; figs. i of plates 5, 6, 7;
figs. h, j, k of plate 7; figs. f and g of plates 12 and
13; and figs. c and d of plate 14. To Mrs. Unruh I am further
indebted for figures 1, 16, 17 and 23 and for much terminal
assistance with preparing most of the illustrations for the
engraver.
The methods of study, after specimens were assembled, included first
comparisons of specimens of like age and sex from each of several
localities to ascertain the constant features by which full species
were distinguishable, one from the other. For example, it was found
that in every individual from Trout Lake, Washington, of the species
here designated Mustela erminea, the postglenoidal length of the
skull amounted to more than 47 per cent of the condylobasal length
whereas it was less than 47 per cent in all individuals here designated
as Mustela frenata, from the same locality. Testing of specimens from
other localities by means of this and other selected characters
permitted the outlining of the geographic ranges of the full
"species-groups." By comparing specimens of other nominal species and
by examining specimens from localities geographically intermediate
between the nominal species, I found intergradation and therefore
arranged the nominal species as subspecies of a single species.
Intergradation here is understood to be the result of crossbreeding in
nature between two kinds of animals in the area where the geographic
ranges of the two kinds meet. Presence of intergradation between two
kinds of weasels was basis for according them subspecific rank. Absence
of intergradation in nature at every place where the geographic ranges
of two kinds met or overlapped, and absence of intergradation by way of
some other kind, or chain of kinds, was basis for according each of the
two kinds full specific rank. By thus applying the test of
intergradation, or lack of it, I found that there were four full
species of weasels, of the subgenus Mustela, in all of the Americas.
Next, the specimens of one species were arranged in trays in a
geographic sequence. The specimens from any one locality were
segregated by sex and under one sex from one place were arranged from
oldest to youngest, that is to say by age. The four series with the
largest numbers of individuals of a given age were selected. Seventeen
cranial measurements and three external measurements were recorded for
each individual of each of these four series. For each measurement, the
coefficient of variation, standard deviation and probable error were
computed. The four samples subjected to such analysis were a series of
adult males, one of adult females, one of subadult males and one of
subadult females. Also, studies of each sex were made to ascertain
seasonal changes in pelage. After data were obtained on ontogenetic
(age) variation, secondary sexual variation, seasonal variation, and
degree of individual variation by studying specimens in the manner
described above, tests were made for subspecific (geographic) variation
by comparing series of specimens of like sex, age and season, from
different localities. For each one of several geographically variable
features noted, a map was prepared for animals of each sex. When all
the data thus obtained were codified, subspecific ranges were, in a
sense automatically, obtained. On the resulting map showing geographic
ranges of subspecies for a species, a type locality was accurately
plotted for each name that had been applied to the species, and names
then were applied in accordance with the international rules of
zoölogical nomenclature.
Variation with Age
The kind of variation which results from increasing age has been dealt
with extensively for the skull (of the Old World Mustela erminea) by
Hensel (1881) and for the external features and to some extent for the
skull by Hamilton (1933) in the North American forms M. erminea
cicognanii and M. frenata noveboracensis.
The young of both erminea and frenata are hairless and blind at
birth. In M. frenata noveboracensis, the eyes open on approximately
the 37th day. When 2 to 4 months old, the tail is pointed at the tip.
This is because the terminal hair of the tail, including the black tip,
is short and lies flat on the tail. In subadults and adults the hair on
the terminal part of the tail is as long as that on the basal part, and
the tail appears to be of uniform diameter all the way out to the end.
In the western subspecies of M. frenata, and in its tropical
subspecies, animals so young as to have pointed tails commonly have the
underparts of the body more intensely colored than do adults. The young
may have salmon-colored instead of yellowish fur on the underparts.
Otherwise, in animals that have attained approximately adult
proportions—which appears to be at approximately 6 months of age in
males—there are no variations which are ascribable to increasing age
in the color-pattern or pelage that cause the systematist to confuse
species or subspecies.
Of the several parts of the skull in juvenal animals, the braincase and
width of the posterior part of the palate are most nearly of the size
attained in the adult, the facial part of the skull at birth is the
least developed, and the interorbital region is, in relation to its
ultimate adult size, intermediate in stage of development. The
permanent teeth are acquired when the animal is approximately eleven
weeks old.
Four age groups, based on characters of the dentition and skull, have
been recognized. They are:
Juvenile.—One or more deciduous (milk) teeth present. Birth to
three months of age.
Young.—Sutures widely open between the maxillae and nasals and
between the premaxillae and nasals. Three to seven and a half
months of age.
Subadult.—Sutures between maxillae and nasals visible but
indistinct. Seven and a half to ten months of age.
Adult.—Bones of rostrum coalesced with no traces of sutures
visible to the naked eye. More than ten months old.
The skull as a whole increases in size until the animal is two-thirds
of the way through the stage designated as young. After this time the
width of the rostrum, as measured across the hamular processes of the
lacrimals, increases until approximately a third of the way through
adulthood. The interorbital breadth decreases from late subadulthood to
adulthood and even in adults there appears to be a slight decrease in
this part of the skull with increasing age.
The average zoölogist will readily distinguish skulls of juveniles and
young from adults but usually fails to distinguish subadults from
adults. Nevertheless, subadults must be distinguished from adults if
geographic variation is to be measured accurately. The reason for this
is that such differences in the form (not size) of the skull as result
from increasing age equal and often exceed the differences of a
geographic sort which serve for distinguishing subspecies that have
adjoining geographic ranges. All sutures in the skull, except those
between the tympanic bulla and the braincase, and those on the dorsal
face of the rostrum, are obliterated while the animal is a subadult.
Most kinds of mammals retain sutures throughout life or until the
animals are well into adulthood. Therefore, skulls of weasels offer
fewer features for estimating age than do those of most mammals and the
skulls of weasels that are subadults or older are more difficult to
classify accurately as to age than are the skulls of most other
mammals. More reliance on shape of entire skull and less reliance on
extent and shape of any individual bone is necessary in estimating the
age of a weasel. Wright (1947:344) shows that the weight of the baculum
(os penis) is a certain means of differentiating adults from males of
lesser age. When approximately eleven months old, Mustela frenata
oribasus of western Montana molts from the white winter coat into the
brown summer coat. At that time spermatogenesis starts for the first
time and the weight of the baculum increases from less than 30
milligrams to more than 52 milligrams.
In the autumn and early winter, most of the specimens are subadults.
Ordinarily the few adults obtained in these seasons can easily be
segregated from the subadults because ontogenetic development in the
twelve additional months of life of each of the older animals has
obliterated the sutures on the rostrum, heightened (vertically) and
lengthened (anteriorly) the sagittal crest, widened the rostrum, and
produced still other changes in form that are revealed by direct
comparison of specimens of the two ages.
Secondary Sexual Variation
The secondary sexual variation, which has been detected, is in size of
the animal, relative length of the tail and shape of the skull. The
female is the smaller. In the small Mustela rixosa and apparently in
Mustela africana the secondary sexual difference in size is
relatively slight. In Mustela frenata and Mustela erminea, males
are approximately twice as heavy as females, the degree of difference
very definitely depending upon the subspecies. For example, in M. e.
richardsonii the recorded weights are 175 and 69 grams as opposed to
81 and 54 grams in M. e. cicognanii. In general, within one species
the greatest difference in size of males and females is in those
subspecies in which the animals are of large size. The secondary sexual
variation in size is much more than the individual variation in either
sex. The same is not true of secondary sexual difference in length of
the tail (relative to the length of the head and body), which in
eighteen subspecies of M. erminea is from 1 to 7 per cent longer in
males than in females. In two subspecies, M. e. haidarum and M. e.
olympica, the tail is a fraction of a per cent the longer in females
if we may rely upon the few specimens for which collectors'
measurements are available.
In both M. erminea and M. frenata the skull of the female is
approximately 45 per cent lighter than that of the male, or put in the
opposite way, the skull of the male is 83 per cent heavier than the
skull of the female. The difference in this respect varies greatly
depending on the subspecies. For example, the skull of the male is 127
per cent heavier than that of the female in M. e. richardsonii but
only 33 per cent heavier in M. e. anguinae. In Mustela frenata, the
subspecies noveboracensis shows most sexual dimorphism in weight of
skull (3.6 and 1.7 grams) and olivacea the least (5.3 and 3.8 grams).
In general, the difference in this respect is less in subspecies the
individuals of which are of small size.
Therefore, as might be expected, the secondary sexual variation in
weight of the skull is less in M. rixosa, individuals of which are of
small size, than in M. erminea or than in M. frenata, in general of
larger size. Nevertheless, in M. africana, in which the individuals
are of large size, there appears to be less sexual dimorphism in weight
of the skull than in M. frenata or than in M. erminea, although it
should be remarked that there are too few data for M. africana to
allow of forming a trustworthy conclusion concerning the amount of
secondary sexual variation in that species.
The secondary sexual variation in shape of the skull consists of a
slenderness in the female. In relation to the basilar length the spread
of the zygomatic arches is more in males and, except in the one
subspecies M. f. altifrontalis, the rostrum is broader. Also the
interorbital region is relatively broader in males of most subspecies.
In most subspecies of both M. frenata and M. erminea the tympanic
bullae are relatively (to the basilar length) longer in females. The
maximum sexual dimorphism occurs in M. erminea arctica and the
minimum dimorphism in M. e. haidarum, M. e. anguinae and M. e.
muricus. Taking into account all of the subspecies of each of the
North American species, the shape of the skull differs most in M.
erminea and least in M. frenata. In the latter species the greatest
difference in shape of the skull, as was true also of its weight, is in
the subspecies M. f. noveboracensis. In these two subspecies, M. f.
noveboracensis and M. e. arctica, in addition to the secondary
sexual variation already mentioned in the skull, females have the
braincase smoother and more rounded, the postorbital-, mastoid-, and
lacrimal-processes relatively smaller, and the ventral face of the
tympanic bulla at its anterior margin more nearly flush with the floor
of the braincase.
In the weasels, subgenus Mustela, the disparity in size of the two
sexes is almost or quite as much as in any other fissiped carnivore. It
is because of this large degree of difference that the skulls of the
two sexes are described separately in the following systematic
accounts. The need for such treatment was recognized by Reinhold Hensel
(1881:127) more than sixty years ago when he wrote in the introduction
to his "Craniologische Studien," of Mustela, as follows: ". . . die
Geschlechtsdifferenzen am Schädel vieler Säugethiere . . . so gross
sind, dass man diese wie Schädel verschiedener species behandeln muss,
während in anderen Ordnungen (Rosores, Edentaten) die Schädel solche
Unterschiede nichtzeigen." In the past, failure to appreciate the large
amount of secondary sexual variation has resulted in erroneous
deductions as regards characters of certain geographic races and has
been the cause of some nomenclatural confusion, as for example, in
Mustela frenata macrura, where the female was named as a separate
species (Mustela jelskii).
Individual Variation
Individual variation is here considered to be the variation in one
species which can occur between offspring of a single pair of parents,
after variation ascribable to differences in age, sex, and season is
excluded. Individual variation, therefore, is a term here used in a
composite sense; it includes variations which probably represent
different genetic strains within certain populations and variations
induced within one generation by environmental factors.
In skulls of weasels, the individual variation in size is more than it
is in relative proportions. Hensel (op. cit.) has stressed that
weasels, like other carnivores, produced "dwarfed" individuals more
than do herbivorous mammals. I cannot vouch for the accuracy of this
view, but can say that individual variation is not greater than in some
other fissiped carnivores. Impressions to the contrary probably result
largely from failure to recognize age-variation. When skulls of a large
series from any one locality are arranged first by sex, and under each
sex according to probable age on the basis of extension anteriorly of
the sagittal crest and of degree of postorbital constriction,
individual variation is seen to be less than a cursory examination,
even of only one sex, would suggest.
Study of a large series of one age of one sex of one species from one
locality shows that some parts, of the skull for example, vary more
than other parts. In illustration, among 22 male topotypes of Mustela
frenata washingtoni the least interorbital breadth varied 25 per cent
(9.0 mm. to 12 mm.) whereas the length of the tooth-rows varied only
13.3 per cent (15.6 mm. to 18.0 mm.). In color the individual variation
definitely is more in areas of intergradation between subspecies than
in other areas. Details of one such instance of intergradation are
given in the account of Mustela frenata spadix.
Statements to the effect that there is much individual variation in the
color of weasels, were made mostly fifty years or so ago by writers who
had but few specimens from widely separated localities. Where marked
climatic differences exist between localities only a few miles apart,
marked differences occur in coloration of the weasels from the
different localities. Much of what formerly was mistaken for individual
variation now proves to be geographic variation. Individual variation
actually is of slight amount in comparison with that in mammals
generally. Differences in size and relative proportions of parts
usually are correlated with geographic differences in color. The color
does fade slightly in the period between molts. Also as a result of the
seasonal color change, in autumn along the upper margin of the Austral
Life-zone, some individuals become white whereas others become white on
only the underparts, the upper parts changing only to lighter brown.
Probably it would be correct to say that this variation was a
combination of seasonal and individual variation rather than either one
alone.
As might be supposed, individual variation is not the same in all
species or subspecies. For example, p2 is always absent in Mustela
africana and always present in certain subspecies of M. frenata. In
some other subspecies of M. frenata, p2 is absent approximately as
often as present. In the writer's experience, when only a few specimens
are available for comparison, individual variation is more difficult to
distinguish from specific and subspecific (geographic) variation than
is age-variation or secondary sexual variation.
Among the larger series of specimens examined, only one instance of
what might be called a mutation in the old sense of a large, sudden
change, was detected. That was the loss of the second lower molar in
many (less than a third) of the specimens from Newfoundland. The six
instances of abnormal coloration described on pages 41 to 43, might be
regarded as mutations of large magnitude but no evidence was found of
repetition of an abnormality in any one population. Otherwise, in
every instance where plotted, the manifestations of a variation
arranged themselves about the mean in such a way as to form a smooth,
unimodal curve.
Seasonal Variation
When subspecific and specific variations are the objectives of study,
seasonal variation must be understood, in order to be excluded from
consideration, in the same way that variations ascribable to age, sex
and individualism must be understood in order to be excluded from
consideration. In weasels, change in color of the pelage is the
seasonal variation most important for the systematist to understand.
Other seasonal variations in the pelage are hairiness versus nakedness
of the pads of the feet, length of the pelage on the body, and possibly
the density of the pelage on the body. In the northern half of North
America, roughly speaking, seasonal change in color is so pronounced
(white in winter and brown in summer) as to be easily recognized. South
of this area, in the Austral and Sonoran life-zones, the color of the
winter pelage differs only slightly from that of the summer pelage. In
these more southern latitudes the winter pelage in almost all
subspecies is of lighter color than the summer pelage and has a smoky
suffusion. With material of the two seasons in hand for comparison,
close attention to the variation will permit the systematist to
recognize the difference in shade of brown as seasonal variation and
not geographic or specific variation. Farther south still, in the
Tropical Life-zone, seasonal difference in color was not detected in
the material studied. Seasonal change in color is discussed in the
section immediately following.
Variation in Coloration and Molt
In all American weasels (subgenus Mustela) the color, at least in
summer, is brown with more or less white or whitish on the underparts.
In one species, Mustela africana, there is a longitudinal stripe of
brown on the middle of the light-colored underparts; this stripe is
absent in each of the other three American species. Two species, M.
erminea and M. frenata, always have a black tip on the tail. Of the
other two species, M. africana lacks the black tip and M. rixosa
may or may not have a few black hairs in the tip of its tail. White or
light yellowish facial markings occur in subspecies of M. frenata
from the southwestern United Stated to Central America. Subspecies
having the most extensive light-colored facial markings have the
remainder of the upper part of the head black. In weasels without light
facial markings the upper parts of the head all are brown. In the two
species, M. erminea and M. frenata, the extent to which the light
color of the underparts extends down the insides of the legs and out on
the underside of the tail, or the absence of light color on these
parts, is a matter of geographic variation. The same can be said for
M. rixosa except that first its tail is unicolored and second
individual variation as well as geographic variation accounts for the
color pattern on the underparts and legs in animals from the
southeastern part of the range of the species.
The most remarkable feature of the coloration of weasels is the winter
whitening. This occurs in the northern part of North America in each of
the three species of weasels found on that continent. The black tip of
the tail in M. erminea and M. frenata remains black in winter. If
an individual of M. rixosa has black hairs on the tip of its tail in
summer, there are thought to be black hairs there also in winter.
Otherwise the winter pelage is all white in northern areas in each of
the three species. In this white winter coat the animal is known as
ermine.
The underlying cause seems to be protective coloration. At any rate,
weasels are always white in winter if they are from areas where snow
lies on the ground all winter, every winter, or almost every winter;
and they are always brown if from areas where there is never, or
rarely, snow in winter. The changes in color are effected by molt, one
in autumn and one in spring. Animals that are brown in winter undergo
the same two molts as do those that are white in winter. The capacity
to acquire a white coat or a brown coat in winter is an hereditary
matter just as one man grows red hair and another grows black hair. In
the weasels, however, all individuals in the north turn white in winter
and if one that was born there is kept through successive winters in
the warmer south where there is no snow, he will still turn white each
winter. A weasel born in a southern area, where all are brown in
winter, molts into a brown (not white) winter coat even when kept in a
cold, snowy, northern area where native weasels of the same species all
turn white. Obviously, therefore, neither snow nor temperature is an
immediate cause and, as we have said, the color in winter is a matter
of heredity. The time of the molt, we now know, is determined by the
amount of light. When nights grow longer and days shorter, a point is
reached at which the lesser light received through the eyes causes the
pituitary gland to cease producing a gonadotropic hormone. Directly or
indirectly, the lack of this hormone stimulates molt and, probably
enzyme action, or the lack of it, causes the melanoblasts of the cells
in the hair follicle to be without pigment. Hence the hair grown from a
follicle under such conditions lacks pigment (melanin) and is white. In
spring, as the days grow longer and the nights shorter, the increasing
amount of light received day by day through the eyes stimulates the
pituitary gland to produce the gonadotropic hormone which directly or
indirectly, stimulates molt and, probably by enzyme action, the
melanoblasts are caused to be present in cells of the hair follicle and
the melanoblasts provide granules of melanin pigment which are
incorporated in cells of the growing hair. These granules of pigment
give the hair its color.
Evidence in support of this hypothesis is given below.
Along the Pacific Coast from British Columbia southward, M. erminea
(see fig. 25 on page 95) is brown in winter. This is an area where snow
rarely falls and the temperature in winter ordinarily is above
freezing. In the remaining part of the American range of this species
the temperature in winter is below freezing much of the time and snow
remains throughout the winter or for long periods. In this colder part
of the animal's range, only white coats occur in winter. M. frenata
likewise has a white coat in winter in the part of its geographic range
where snow and freezing temperatures prevail throughout most of the
winter and a brown coat in warmer, snowless areas to the southward and
along the Pacific Coast. The third species, M. rixosa, exhibits a
corresponding correlation between coat color and climate. On the
Asiatic continent, several species, including M. erminea, provide
parallel correlations and nowhere are there any exceptions for the
subgenus Mustela. These data are an important part of the material on
which we have based the induction that the underlying cause of seasonal
change in color is a need for protective coloration.
As regards molt, most naturalists who have written upon the subject
regard it as responsible for the change from the white winter coat to
the brown summer coat. However, the change from brown summer coat to
white winter coat has been thought by several writers to be effected by
change in coloration of the individual hairs. Among those holding this
opinion there may be cited Bell (1874:197) in reference to Mustela
erminea, and Coues (1877:123) in reference to American specimens to
which he applied the same name. More lately Hadwen (1929) has taken
this same view, and Gunn (1932) also discusses the possibility of the
hairs changing color. Bachman (1839:228-232), Macgillivary
(1843?:158), Audubon and Bachman (1851 (vol. 2):62), Schwalbe
(1893:538), Pearson et al. (1913:447), Miller (1930, 1931A), Hamilton
(1933:300) and Rothschild (1942), among others, have been inclined to
the opinion, or positively affirm, that the color change in autumn is
the result of a molt. The papers cited above contain, in turn,
references to many other printed accounts dealing with this question.
To my mind, it has not so far been demonstrated that the change in
color of weasels in autumn is accomplished without a molt. Also so far
as I am aware, no explanation has been given of how the pigment may
disappear from the hair of weasels. Metchnikoff's (1901:156) idea that
the senile whitening of the hair in man is accomplished by phagocytes
which remove the pigment granules would hardly seem to explain the
relatively sudden and complete autumnal change occurring in weasels.
Anyhow, Danforth (1925:108), and some other students have thought that
the action of these phagocytes was at most a factor of slight
importance in the whitening of hair. Whatever be the complete answer to
the question of how the weasel changes color in autumn, at least one
specimen of long-tailed weasel, which is in process of color change in
autumn, presents clear evidence of molt of the overhairs. This specimen
of M. f. longicauda is no. 188408, U. S. Nat. Mus., taken on November
12, 1897, at Rapid City, South Dakota. Other specimens of M. erminea
which were taken in autumn similarly show molt to be in progress. For
these and other reasons, I am inclined to the opinion that the autumnal
change in color, like the one in spring, is effected by molt. During
the period of the autumnal color change, Noback (1935:27) had a captive
M. f. noveboracensis and, each morning, found clumps of brown hair on
the floor of its cage; this was strong indication that molt was
responsible for the color change in this instance.
However, I freely admit that the evidence does not prove that the
change from brown to white can be accomplished only by molt; in the
present state of knowledge it would be unscientific to deny that the
change were possible of accomplishment by other means. Also, it is true
that the fifteen specimens before me of Mustela frenata, subspecies
included, in process of change from brown to white, with the exception
of the one from Rapid City, South Dakota, if taken individually, do
not, in macroscopic examination, show definite molt lines or other
absolutely convincing evidence of molt. However, these same specimens,
insofar as examined microscopically, do show overhairs all white, or
overhairs pigmented throughout. The lighter color of the proximal parts
of the overhairs in itself should not be accepted as evidence of color
change, for in the fresh summer pelage, the same condition exists.
Also, careful macroscopic examination suffices to show that in the
transitional pelage of autumn, the brown overhairs generally are longer
than the intermixed white overhairs.
Whether the underfur behaves in exactly the same way as the overhair, I
have not myself definitely ascertained, but I assume that the underfur
is molted twice each year, at least in the northern populations of
Mustela frenata and in the other species of more northern
distribution. Schwalbe's (1893) work, including sectioning of the skin
and study of the hair follicles, led him to conclude that the underfur
was molted twice each year in Mustela erminea.
In Mustela frenata noveboracensis, M. f. nevadensis, and M. f.
nigriauris, measurements taken on adult males show the overhairs to be
longer in the winter pelage than in the summer pelage of specimens from
the same locality. For example, in M. f. nigriauris from Berkeley,
California, the overhairs of the summer coat (July and August) average
8 millimeters in length on the hinder back and 7 mm. on the belly, but
average 9.5 mm. and 8 mm. respectively in January-taken specimens
possessing the full winter coat. At Ann Arbor, Michigan, in the summer
coat, the longest hairs on the hinder back average approximately 12
mm., and those on the belly, 9.5 mm., against 13 mm. and 9.5 mm.
respectively in winter. Although general observations initially led me
to believe that the black, terminal hairs of the tip of the tail are
longer in the winter pelage than in the summer pelage, actual
measurements fail to show a difference in length.
The change from one coat to the other in the long-tailed weasel has
been described among others by Miller (1930, 1931A), Hamilton (1933)
and Glover (1942) on the basis of captive specimens. In a general way,
the progress of the molt in their specimens agrees with that which I
have been able to make out from examination of skins taken in the wild.
There is, however, this difference: Their specimens show a more spotted
pattern when in process of hair-change than do specimens taken in the
wild. Probably the more or less unnatural conditions under which these
captive animals lived modified the normal progress of molt.
In wild-taken specimens of the species Mustela frenata, subspecies
included, the spring molt begins on the mid-dorsal line and proceeds
laterally, producing, at almost any given time, a relatively sharp
molt line separating the white winter hair from the incoming brown
summer coat. However, in autumn the change takes place first on the
belly, then on the sides, and finally makes its appearance over all the
upper parts at about the same time, with the result that the upper
parts have a salt-and-pepper appearance without at this time any
sharply defined molt lines. In general, the molt pattern can be said to
be reversed in the two seasons; in spring, it begins on the back and in
autumn, on the belly. The difference in spring and autumn color pattern
is better illustrated on plate 39 than by additional description.
Swanson and Fryklund (1935:123) have observed that the "spring molt
proceeds differently" than the fall one in Mustela rixosa, and
Barrett-Hamilton (1903:309) in commenting on the European hare (and the
stoat?) remarks, "In spring the moult, and with it the brown colour,
progresses in exactly the opposite order . . ." as compared with the
white color of autumn, which that particular writer thought resulted
from removal of pigment from the hairs rather than from molt.
The tail, excepting the black tip, lags in the molt in many instances,
with the result that, especially in spring, it may retain a few white
hairs as late as does the belly. In autumn it is less tardy and so far
as I have observed, becomes white at about the same time that the
general area of the back changes color. On the tail, the black tip
itself, as clearly shown in more than a score of specimens, is molted
at approximately the same time in autumn as is the pelage of the body.
However, the long black hairs, which appear in, say, November, appear
to increase in length until January. In spring, the long black hairs of
the tip of the tail seem not to be shed at the same time as the rest of
the winter pelage, but remain approximately six weeks longer and then
are replaced by long black hairs of the summer coat. At any rate, this
is the picture presented by a half dozen specimens of M. f.
nevadensis and M. f. longicauda which do show a spring molt to be in
progress on the black tip of the tail. Schwalbe similarly
(1893:536-537) has suggested that the black tip of the tail in Mustela
erminea in spring is not molted until about two months after the
pelage on the rest of the body is changed. Schwalbe (loc. cit.)
thinks also that in M. erminea studied by him, the black tip of the
tail in autumn is replaced approximately one month in advance of the
pelage on the rest of the body. As indicated above, my specimens of
Mustela frenata, subspecies longicauda and nevadensis, do not
show this discrepancy in autumn. I have considered the possibility that
the black tip of the tail, in some species of Mustela, is molted only
once while the remainder of the coat was undergoing two molts. My
inconclusive data lend but little support to this possibility.
The difference in pattern of color between specimens taken in autumn
and spring is known to some fur-trappers of my acquaintance who have
suggested that molt occurs in spring, whereas the individual hairs
change color in autumn. Reference to plate 39 will show how gross
comparisons might lead one to this erroneous explanation of the color
change.
As to time of molt: In eight subspecies of Mustela frenata, namely,
noveboracensis, occisor, primulina, spadix, longicauda,
arizonensis, nevadensis and effera, material is available to
indicate that the autumnal molt begins in October and is completed in
November, and that the spring molt occurs in March or April. A
condensed list of specimens providing basis for this statement is as
follows:
M. f. noveboracensis: 26 specimens in transitional pelage taken
in autumn and 14 taken in spring; M. f. occisor: One topotype
has acquired one-fifth of the winter pelage on October 22, 1896;
M. f. primulina: 2 in November, one in March, and 2 in April are
in process of change; M. f. spadix: 6 autumnal specimens and one
in April show pelage change; M. f. longicauda: 7 autumnal
specimens and one in April show pelage change; M. f.
arizonensis: 12 specimens in autumn and 3 in spring are in
process of molt; M. f. effera: One November-taken male has
acquired four-fifths of the winter coat and another taken on April
21 at Fort Rock, Oregon, is half finished with the spring molt.
It may be added that no marked difference in time of either autumnal or
spring molt is apparent as between the more northern and more southern
localities from which the mentioned specimens come. With more complete
material I would expect to find a difference in this regard.
The material of the other, more southern, subspecies of Mustela
frenata has not been adequate to show the time of molting or the
number of molts which occur in one year.
Animals in the northern part of the range of Mustela frenata acquire
a white winter coat, whereas those in the southern part acquire a brown
winter coat, and in an intervening area the winter coat may be either
brown or white. By plotting on a map the localities of capture of all
specimens examined in the winter coat, it was possible to outline this
intervening area as shown in figure 10 on page 37. However, Dearborn
(1932:36) shows that in Michigan some animals have a brown coat in
winter at places farther north than figure 10 shows to be the case.
Hamilton's (1933-306) map for New York shows the same to be true in
that state. Accordingly, the boundaries of the area shown in figure 10,
in which both brown and white long-tailed weasels occur in winter, are
known to be only approximate; with full information available the belt
would be represented as wider.
Fig. 10. Map showing the region (in black) where both
the brown and white winter pelage is found in the long-tailed weasel,
Mustela frenata.
Hamilton (1933:302) has pointed out that "Where half of the weasels
remain brown, these brown winter specimens are always males." The
results of my own examination of specimens not studied by Hamilton, in
a general way provide confirmatory data. More exactly, my examination
reveals that at the most northern localities where brown specimens
occur, only males are in this coat. In explanation, it may be said that
in plotting on a map localities of capture of specimens in the winter
coat, thirteen places were found where both sexes were represented and
where both brown and white winter coats were found. With the two sexes,
it is theoretically possible to have nine different combinations of
coat color. With males all brown, there might occur females (1) all
brown, (2) all white, or (3) some brown and some white. In addition to
these three combinations, we might have three more by finding the
mentioned types of female coat color repeated where all males are
white, and three more, or nine in all, by substituting a population of
males some of which were brown and some of which were white. Seven of
these possible combinations actually were found. The two combinations
not found were all white males with all brown females, and all white
males with females both brown and white. In the three instances where
the males all were brown and the females all were white, the localities
of capture were in the northern part of the variable area. This
indicates that where the brown winter coat occurs at northern
localities, the brown individuals are all males. Farther south, of
course, the females, too, acquire the brown winter coat.
Stated in another way, there is a broad belt across North America from
the Atlantic to the Pacific in which males of Mustela frenata at any
one locality may be either brown or white in winter. Inside this broad
belt there is a narrower one, approximately half as wide, in which
females at any one locality may be either brown or white.
In support of the idea that color of the winter coat is an hereditary
matter and that it is not dependent on temperature, the following
evidence derived from my transplanting specimens of Mustela frenata
supports the idea that color of the winter pelage is dependent on
heredity and not on temperature or snowfall.
A male captured on June 24, 1937, in the brown summer coat in Salt Lake
City, Utah, was received by me at Berkeley, California, five days later
and kept in captivity almost six months. On November 17, 1937, half the
pelage was white and on December 27, 1937, when next examined, the
animal was in the full, white, winter coat as it was on January 25,
1938, when it died. Native weasels all turn white in winter in Salt
Lake City, but in Berkeley native weasels always are brown in winter.
A juvenile or young animal, a male, captured in May, 1936, at
Lafayette, Contra Costa County, California, was kept there until August
13, 1936, when transferred to Calneva at the north end of Lake Tahoe,
California. The weasel was kept at Calneva until its death on December
23, 1937. In both the winter of 1936-'37 and in that of 1937-'38, the
winter coat was brown as in animals from its place of origin (Contra
Costa County) and unlike weasels of the Tahoe region nearly all of
which turn white in winter.
Two females, each approximately two months old, captured on May 1,
1936, at James Landing, 4 miles northwest of San Pablo, Contra Costa
County, California, were kept in Berkeley, California, until August 13,
1936, when they were transferred to the mouth of Blackwood Creek, on
the west side of Lake Tahoe, California. On October 25, 1936, both
weasels escaped. On December 25, 1936, the headless body of one of
these was found approximately 300 yards south of the mouth of Blackwood
Creek. The animal had been dead at most a few days when found and was
in the brown winter coat. At the place of its origin all weasels are
brown in winter but at the mouth of Blackwood Creek only 2 of 60
weasels caught there in the winter coat were brown; the other 58 were
white. The headless weasel was identified, as one of the two formerly
in captivity, by means of certain short toes, the ends of which had
been clipped off when the animal was a captive. No trace of the second
female was found.
A female of unknown age, in white winter pelage, captured 4 miles
southeast of Tahoe City, California, and kept there until April 3,
1937, on which date it was brought to Berkeley, California, molted to
brown in the spring. The first signs of the brown coat were noted on
April 14. On May 24 or 25 she gave birth to 4 young which lived less
than ten days. In the following winter this animal acquired a white
coat. As previously noted, weasels native to the Berkeley area, where
this female was kept, have brown coats in winter.
The weasels were in every instance kept in cages out-of-doors. The
sides of the cages were open to the elements. A nest box in each cage
provided shelter. All were of the species Mustela frenata.
The significant results, it seemed to me, were that the winter coat was
the kind found in the area where the weasel originated instead of the
kind found in weasels native to the areas in which the specimens were
held in captivity.
That the time of molt is determined by the amount of light has clearly
been shown by Bissonnette (1944:223) for American weasels of the two
species Mustela erminea and M. frenata. In his words (op.
cit.:246) "Reducing the daily periods of light induced molting and
regrowth of new fur. . . . In the Bonaparte weasels [Mustela
erminea], white replaced brown. . . . Increasing daily light-periods
caused molting and change to dark brown. . . . Incomplete molts in both
directions (toward white or toward brown) were produced as a result of
early reversal of increase or decrease of daily light-time. . . . That
this stimulus is received through the eyes and acts through the
anterior pituitary gland is indicated by Bissonnette's [1935:159]
studies on ferrets, a nearly related animal. That the thyroids and
sex-glands are not essential is at least suggested . . . by Lyman's
(1942) study on the varying hare [Lepus americanus]." It can be added
that Lyman (1943:451) demonstrated in Lepus americanus that the
effect of light is received through the eyes. He demonstrated this by
masking the animals. To Wright (1942B:109) who studied the two American
weasels, M. erminea and M. frenata, it seemed likely that the
pituitary produced or released gonadotropic hormone at about the time
of the spring molt and that this molt and the spring changes in the
reproductive tracts of the weasels might be caused by a stimulus from a
common source. Later, Wright (1950:130) injected a gonadotropic hormone
into long-tailed weasels which had recently acquired their white winter
pelage and thereby caused them to lose the white pelage and acquire the
brown pelage. It is Lyman (1943:450) who says, in relation to Lepus
americanus, "When in the physiologically white condition, the
melanoblasts of the regenerating guard- and pile-hair follicles contain
no melanin-forming enzyme (dopa-oxidase), which may be the reason for
the lack of pigment." Schwalbe (1893) by sectioning the skin and
microscopically examining the hair-follicles of M. erminea learned
that the basal cells producing hairs lacked pigment granules in autumn
when the European ermine (M. erminea) was acquiring its white winter
coat and that the cells contained granules of pigment in spring when,
as we know, the granules are incorporated in the growing hair and give
it its color.
The above material, then, is basis for the account on pages 31 and 32
of what causes the weasel of northern areas to have a white coat in
winter. The discerning student will instantly perceive that although
some parts of the account on pages 31 and 32 are precisely accurate,
other parts are the result of inferences which need to be proved. More
careful work of the kind that Schwalbe (1893) and Wright (1942B) did is
needed. The account on pages 31 and 32 is merely the best that can be
given with the information now available.
Many writers have commented on the yellowish color, sometimes with a
greenish tinge, found on the fur of weasels in the white winter coat.
The stain is more often found on the tail and hinder-parts of the body
than elsewhere. Possibly, partly on this account, some have ascribed
this color to the smearing of the fur with urine. Still others have
thought it resulted from the smearing of the fur with secretions from
the anal scent glands. Schumacher (1928) takes this point of view, and
while it may be that he has not proved his point, still his conclusions
fit the known facts and seem sound to me. Schumacher points out that
the same soiling of the fur is present in summer as well as in winter,
but that on the summer pelage the stain can be detected only on the
light-colored underparts. It is from this point of view that he
criticizes the systematic worth of white versus yellowish-white
underparts in the summer pelage of geographic races of Mustela
erminea and Mustela nivalis. Although in the long-tailed weasels
(Mustela frenata) the underparts of all the races are pigmented with
some form of red, orange or yellow, it seems probable to me that the
additional color resulting from the soiling effect of this glandular
secretion explains the greater variation, found at a single locality,
in the color of underparts than of upper parts in the summer pelage.
I have neither seen nor heard of a black weasel in any part of the New
World or of the Old World. I have found only one albino among American
specimens. It is an adult female, no. 121424, American Museum of
Natural History, of Mustela erminea richardsonii, taken on August 30,
1935, at Hot Springs, Northwest Territory. This place, I am told by G.
G. Goodwin who obtained the animal, is on the "Nahanni River where the
rugged mountain ridges rise abruptly from the low mud flat lands,
latitude 61, longitude 125." The shortness and coarseness of the hair
corresponds to that of the summer pelage and not winter pelage. The
pelage is everywhere white, even the tip of the tail. True, all except
the nape and top and sides of the head has a faint yellowish-green
tinge which has been supposed to result from staining by secretion of
the anal scent glands but there is no pigment in the hair as in
erythristic specimens. From the Old World, Farurick (1873:17) has
recorded what he regards as an albino of Mustela vulgaris since it
had no black hairs on the tip of its tail. Flintoff (1935:228, 229)
records what may have been an albino Mustela vulgaris from Yorkshire
and an albino M. erminea from an unstated locality. Jäckel (1873:459)
mentions specimens of Mustela erminea and Mustela vulgaris, which
were partly "albinistic" or "erythristic." Among the American specimens
of M. erminea I have not recorded any which appeared to be either
partly or wholly erythristic or only partly albinistic. Among the 1550
skins of M. frenata which were in summer pelage or brown winter
pelage, five, described below, show marked abnormalities in color.
Two of these five are partly albinistic. One is an adult male, no.
223880, U. S. Nat. Mus., from Billy's Island, Okefinokee Swamp,
Georgia, which has the nose as well as the area between the eyes white.
Also there is a tuft of white hairs at the anterodorsal margin of each
ear, scattering white hairs suggesting a postorbital bar on each side
of the head, and a patch of white hairs on the mid-dorsal line behind
the ears. Markings of this kind are not abnormal in M. f. peninsulae,
the subspecies adjoining on the south, except for the white nose which
clearly is an instance of partial albinism. The second specimen is a
subadult male, of M. f. noveboracensis, no. 177679, U. S. Nat. Mus.,
in process of acquiring the brown winter coat, taken on November 27,
1911, at Gaylordsville, Connecticut. It has white markings on the nose,
on the right side of the neck, on the right hind foot and right
forefoot, and on the tip of the tail. The white area of the nose on the
left side extends back to the eye, but on the right side barely
encircles the nose-pad. On the right side of the neck, all that area
between the foreleg and ear is white from the mid-dorsal line
(including 7 or 8 millimeters to the left of the mid-dorsal line) down
to the throat, which is white as it is also in normal individuals. The
toes of the right hind foot are more extensively white than in normal
specimens of noveboracensis, and all of the right forefoot as well as
the wrist is white. The tail is of striking appearance because of its
tricolor pattern. The proximal part is of the normal brown color. The
black terminal part commences proximally at the usual place, but the
distal 11 millimeters of the fleshy part of the tail bear only pure
white hairs producing a terminal white pencil 35 millimeters long.
The three other specimens abnormally colored are erythristic
individuals. An adult male of M. f. latirostra, no. 7574, coll. D. R.
Dickey, taken on April 14, 1918, at Covina, Los Angeles County,
California, has the color of the upper parts greatly restricted, and,
in addition, has spots and blotches of the color of the underparts
distributed over the back and rump. A spot of this same color occurs
above each ear. Incidentally, this and other subspecies of Mustela
frenata from the Pacific Coast of North America obviously have the
factor for erythrism operating over a larger part of the body than it
does in M. erminea or than in M. f. noveboracensis, where the
underparts sometimes are white. In M. f. latirostra and in other
subspecies from the Pacific Coast the light color of the underparts
always is tinged with this reddish color.
Another erythristic specimen is a young male of M. f. nevadensis, no.
23493, U. S. Nat. Mus., taken on August 6, 1890, at Birch Creek, Idaho.
It has all of each foreleg, the axillary regions, and a saddle-shaped
area over the shoulders of the same buff-yellow color as the
underparts.
The third erythristic specimen is a subadult female, of M. f.
oregonensis, no. 47149, Mus. Vert. Zoöl., taken on December 20, 1930,
at Carlotta, Humboldt County, California. This specimen appears to be
white and initially was thought to be merely an individual in the white
winter coat. Closer examination, however, shows that it has a light
wash of ochraceous or faint reddish color. Also, other specimens taken
in winter at Carlotta show that weasels there do not acquire a white
winter coat. The only normally brown area is approximately three
millimeters in diameter at the anterodorsal margin of the pinna of the
right ear. The tip of the tail is black as in a normal specimen. The
specimen in question is actually pure white only on top of the head
from a short distance behind the ears on over the forehead nearly to
the eyes, and on the inside of the ears. In a normally colored animal
this area is the dark area of the head. In this freak, the other parts
of the head, which, in individuals of normal coloration are the white
or light orange facial markings, have the reddish cast of the remainder
of the body, although the color is less intense than on the back. The
collector noted that the specimen had eyes of normal color. A possible
explanation for the coloration of this specimen is that this species
has three factors for color, one for the black tail tip, one for the
reddish color, and a third, missing in the specimen in question, for
the blackish brown.
For some more exact knowledge concerning this erythristic type of
coloration, we are indebted to Pitt (1921:99), who describes a
population of polecats, Mustela putorius, in Cardiganshire, England,
in which this erythristic variation is maintained in a state of nature.
In ferrets, Mustela furo, Pitt (op. cit.:114) notes that ". . .
erythrism is certainly dependent on a Mendelian factor, being dominant
to albinism and recessive to the black-brown coloration. Both in the
ferret and polecat, erythrism seems to be correlated with increased
size, and certainly in the ferret is usually accompanied by a quick
temper and general increase in vitality."
Variations of Taxonomic Worth
Variations of taxonomic worth usually are referred to as characters.
For example, shortness of the tympanic bulla is a character, and the
opposite condition, long tympanic bulla, is another character. Specific
variations, that is to say specific characters, are provided by the
color-pattern, length of tail, number of premolar teeth, shape of the
tympanic bullae, and length of the braincase in relation to the length
of the tooth-bearing parts of the skull. Subspecific characters are
provided by color-pattern, color itself, size as measured by weight of
the animal, and its linear measurements, size of the skull, and size
and shape of parts of the skull. The characters distinguishing
subspecies from one another are not of a different nature from those
distinguishing species from one another.
Given any one of the above structural features, say, dorsal outline of
the skull, several characters may be provided by it. For example,
weasels of the species Mustela frenata have the dorsal outline of the
skull convex in southern Louisiana, straight in Missouri and concave in
North Dakota, thus providing three characters. This is geographic
variation. These variations, characters in zoölogical parlance, when
plotted on maps, reveal the geographic occurrence of, say, the convex
shape of the skull. In combination with other characters, for example,
dark color and short tail, basis is provided for recognizing a
subspecies, in this instance Mustela frenata arthuri of Louisiana.
Because the change from convex to flat skull takes place geographically
at about the same place (in eastern Texas) as does the change from
short tail to long tail, and the change from dark color to light color,
it is easy to draw a line there marking the western geographic limit of
occurrence of the M. f. arthuri. This same line marks also the
eastern margin of the geographic range of the subspecies Mustela
frenata frenata, the subspecies next adjacent to the westward. On this
line and for several miles to either side of it weasels show varying
combinations of these three characters or an intermediate condition as
regards one or more of the characters, or both. For example, from a
locality in eastern Texas a weasel may have (1) a facial pattern
exactly intermediate between that of the unicolored face of arthuri
and that of the bicolored face of frenata, (2) the long tail of
frenata and (3) the convex skull of arthuri. In the sum of its
characters this specimen is exactly intermediate between typical
arthuri and typical frenata. Another specimen from the same place
may differ from the first specimen only in having the tail slightly
shorter. The total "score" for the two specimens is, therefore, by a
very slight margin in favor of arthuri. Let us suppose that we obtain
a third specimen from the same place and that it has the face marked
like that of arthuri but the tail fully as long, and the skull as
lacking in dorsal convexity, as in frenata. Now the score is
definitely for frenata. For convenience of handling, the population
is referred to frenata, providing that the average of specimens from
a nearby locality to the westward is not in favor of arthuri. In
event the average of specimens from a locality next adjacent to the
westward is in favor of M. f. arthuri, the total evidence from the
two localities may be weighed together and appropriate decision as to
subspecific status of weasels from the area is made according to what
the average is for the area as a whole.
The three individual animals of an intermediate sort are ordinarily
termed intergrades. This implies that their characters are the result
of mixed parentage—perhaps a female of M. f. arthuri and a male of
M. f. frenata but probably each parent itself was an intergrade and
the offspring, of which we examined three, owe their characters to
reproductive processes operating in obedience to Mendelian laws of
inheritance.
The two kinds of animals, Mustela frenata arthuri and Mustela
frenata frenata, are identified as subspecies because of the
intergradation between them. If at this and all other places where the
geographic ranges of arthuri and frenata met there was no
crossbreeding (no intergrades), the two kinds would be treated as
distinct species. Intergradation, and the lack of it, are accepted as
the criteria of subspecies and species, respectively.
These criteria suffice for animals, in this instance weasels, which
have a continuous geographic distribution. Some kinds of weasels are
confined to islands, as for example the islands off the coast of Alaska
and British Columbia. Because weasels are land animals, crossbreeding
in nature between the weasels of two islands is, of course, impossible.
A modified test (used in the study here reported upon) in deciding on
specific versus subspecific status in these instances can be made as
follows: On the adjacent mainland, ascertain the degree of difference
between two subspecies whose geographic ranges meet (for example, M.
e. richardsonii and M. e. alascensis). Next ascertain the degree of
difference between the insular kind of animal and the kind on the
mainland. If the degree of difference is greater when the insular kind
is compared than when only the kinds of the mainland are compared, the
insular kind is to be regarded as a species. If the degree of
difference is no greater between the insular kind and the mainland kind
than it is between the two adjacent mainland kinds, the insular kind is
to be regarded as a subspecies. In short, for insular kinds, the
criterion is degree of difference, with the limitation of geographic
adjacency, rather than intergradation.
The geographic variation (subspecific characters) found could be spoken
of as two kinds: First, there is the variation which is expressed in a
general trend for a long distance, producing, in general, a cline of
even slope; and second, that of inconstant trend in any one direction.
In his "The Rabbits of North America" Nelson (1909:34-35) has commented
on the latter type of variation as follows: "While studying series of
specimens from all parts of the vast range occupied by the geographic
races of such species as Sylvilagus floridanus and S. auduboni, I
have been impressed with evidences of fluctuation of both external and
skull characters. These fluctuations are somewhat wavelike in character
and rise to central points of extreme development and then sink away to
intermediate borders beyond which new waves rise. Where the waves of
differentiation are pronounced they mark recognizable geographic races.
Within the area covered by the larger or geographically broader waves
of differentiation (recognized as of subspecific value), smaller waves
of differentiation are included, which may represent local variations
in intensity of characters of the subspecies, or these characters may
diminish and the variation tend in other directions, sometimes even
closely reproducing the characters of another subspecies occupying a
distinct area." In Mustela frenata, much of the geographic variation
at first inspection appears to be of this nature. Closer scrutiny,
however, reveals that the repetition, at geographic intervals, of
several features of color and structure are closely correlated with
environmental features which are repeated only at these same places.
In Mustela erminea, much of the variation is of the first kind,
namely, that which can be expressed as long clines of relatively even
slope. As several authors have said, zoölogical classification based on
this kind of variation is like dividing the spectrum and depends
largely upon the standards set, for, theoretically, the possibilities
of subdivision are unlimited. Actually, however, none of the clines has
an even slope and the possibilities for subdivision therefore are
limited. Also, when several features are used, instead of only one
feature, the classification is more satisfactory even if the basis is
more complex.
Some features of structure which provide subspecific characters are
mentioned below.
Total length, of males, ranges from 598 to 360 mm. in M. frenata and
from 336 to 228 mm. in M. erminea. There is no cline of sustained
slope in M. frenata but in M. erminea there is a progressive
decrease in total length from north to south.
Length of tail varies from as little as a half to as much as
seven-tenths of the length of the head and body in M. frenata, the
subspecies neomexicana having the long tail and the two subspecies
arthuri and primulina having short tails. The geographic ranges of
primulina and neomexicana are contiguous. In M. erminea there is
likewise no variation of a clinal nature in length of tail and
furthermore the variation is much less than in M. frenata.
In length of hind foot, which in males varies from 49 mm. in northern
populations of M. erminea to 28 mm. in southern populations, the same
cline is seen as in the total length of animals of this species. In M.
frenata, however, there are several decreases and increases along any
straight line which can be drawn through the geographic range of the
species. The range of variation in males is 41 mm. (M. f.
arizonensis) to 59 mm. (M. f. macrophonius).
Weight of the entire animal is an excellent measure of size but weights
are unavailable for many subspecies. In M. frenata, the two
subspecies texensis and macrophonius probably are the heaviest and
effera, arizonensis and helleri probably are the lightest.
Geographically the variation in weight behaves in approximately the
same way as does the measurement of total length. In M. erminea the
variation in weight of males is from 206 grams in northern animals to
58 grams in southernmost populations, there being a relatively constant
gradient geographically.
Degree of hairiness of the foot-soles in M. frenata clearly is linked
with the temperature; in regions of high average temperature the
hairiness is least and in regions of low average temperature it is
most. The decrease in hairiness is accomplished in two ways, namely,
smaller breadth and decreased length of individual hairs and decrease
in number of hairs on a given area of dermal surface. This correlation
holds throughout the entire north to south range of the species.
Corresponding differences are found on the same latitude where
topographic diversity in an east to west direction produces northern
conditions at high altitudes and southern conditions at low altitudes.
The conclusion seems unavoidable that climate, directly or indirectly,
determines the degree of hairiness. Less careful observations were made
on the hairiness of the soles of the feet in other species but it is
clear that the northern species M. erminea has the most hair on the
foot-soles and that M. africana, the tropical weasel, has the least.
In this regard, M. frenata is intermediate as it is also in
geographic position.
Figs. 11-15. Dorsal views of adult skulls of each sex of
five subspecies of the ermine, Mustela erminea, to show secondary
sexual variation and geographic variation in size of the skull. Males
on the left and females on the right. All × 1.
Note especially the geographic variation in decreasing size of the
skull from north to south in each sex, and that the secondary sexual
variation in size of skull is less in ermines with small skulls than in
those with large skulls.
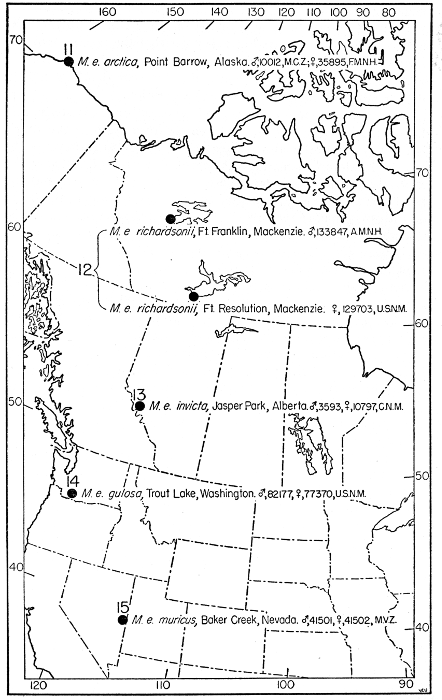
Fig. 16. Map showing the localities where the skulls,
represented in figures 11-15, were obtained.
The maximum length of facial and carpal vibrissae is attained in M.
erminea in the far north. In weasels from north of the Arctic Circle
the longest facial vibrissae extend posteriorly beyond the posterior
border of the ear. In the tropical weasel, M. africana, the facial
vibrissae do not extend posteriorly beyond the ear and the carpal
vibrissae are not so long as the distance between their bases and the
apical pad of the first digit. The correlation of long vibrissae with
low temperature, is mentioned here merely because length and density of
pelage were under consideration.
The most obvious and most exact correlation between change in climate
and change in the animal is furnished by color. This is well shown in
the one species, Mustela frenata, to which the following remarks
apply unless indication is given to the contrary. The color of the
upper parts varies from bay (blackish brown) in M. f. panamensis to
buckthorn brown (light brown) in M. f. neomexicana. The color of the
head varies from solid brown (white chin excepted) to contrasting black
and white markings.
Dark color of the upper parts is associated with a large area of this
color; the enlargement of this area is at the expense of the area of
light color on the underparts. In the weasels of darkest color the
upper parts occupy four-fifths of the circumference of the body (as
measured in the anterior lumbar region) but in the lightest-colored
weasels the upper parts comprise only two-thirds of the total
circumference. In these light-colored animals the color of the
underparts extends onto the underside of the tail and down the insides
of the legs and over the feet whereas in the animals with the darkest
upper parts the entire tail, feet, and legs below the knees ordinarily
are of the same dark color as the upper parts. The length of the black
tip on the tail varies inversely with the length of the tail, probably
because the lightest-colored weasel has the longest tail. In some
subspecies the black brush is almost half as long as the tail-vertebrae
but in others is less than a fourth as long as the tail-vertebrae.
The extent of the color of the head, as well as the intensity of the
color there, varies markedly and is correlated with climatic
conditions. The extent and intensity of this dark color is greater in
weasels inhabiting regions of heavy rainfall than in those inhabiting
regions of sparse rainfall. Considering the geographic range of each
subspecies of Mustela frenata, that of M. f. panamensis has the
maximum of rainfall. Reference to the colored plate (1) will show that
in M. f. panamensis (2) the black of the head is extended over all of
the upper parts. M. f. macrura (1) of Perú, to the southward, is from
an area of lesser rainfall and is correspondingly lighter colored.
Returning to panamensis (2) as a starting point and proceeding
northward to the range of nicaraguae (3), which also has lesser
rainfall, thence another step northward to Guatemala, which has still
less rainfall, the weasel there, M. f. goldmani (4) has the black
extending posteriorly only to the shoulders. M. f. leucoparia (5)
from Michoacán, and M. f. frenata (6) from Tamaulipas are from
progressively more northern and also progressively drier regions. In
M. f. frenata (6) the dark color extends posteriorly only to the ears
and is blackish rather than black. In M. f. neomexicana (7) of the
extremely arid parts of Durango, Arizona, and New Mexico the dark
marking of the head is confined to a brown spot on the nose. Its
geographic range is the most arid of those of all of the subspecies.
The contrast between neomexicana (7) and panamensis (2) illustrates
the great range of geographic variation in color which occurs in the
one species. Continuing from the geographic range of neomexicana
(specimen from Safford, Arizona) northwesterly 480 miles to Riverside,
California (see 8, latirostra), 430 miles north to Point Reyes,
California (see 9, munda), and finally 570 miles north to Tillamook,
Oregon (see 10, altifrontalis), each place with more rainfall than
the one farther south, another correlation of increasingly dark
coloration with increasing amount of rainfall is illustrated.
This geographic variation, it should be remembered, is all within one
species. It is the more significant still when we remember that the
same correlation, with never an exception, occurs at hundreds of places
within the geographic range of the species. A particular feature of
climate, namely rainfall, and possibly therefore humidity, is concerned
in this correlation. The same correlation, heavy rainfall and dark
color, is shown also in the other species of North American weasels.
The conclusion is unavoidable that climate, directly or indirectly,
determines or influences the color of weasels.
The light facial markings appear in American weasels in two separate
geographic areas. One is the southwestern United States, México and
northern Central America. The second area is in the same latitude, in
Florida and adjoining parts of Georgia and Alabama. In the western
weasels the markings are white south of latitude 32° N. North of this
latitude, the facial markings, if at all extensive, usually are of the
same yellowish color as the underparts of the body. Weasels of southern
California and its interior valley usually have these yellowish instead
of white facial markings. The light facial markings, in this instance,
white markings, attain their maximum extent in M. f. leucoparia of
the southwestern margin of the tableland of México, at latitude 19° N.
A gradual decrease in area of the light facial markings occurs both to
the north and south; they disappear at 10° N in M. f. costaricensis
and at 35° N at approximately the southern limits of range of M. f.
arizonensis and M. f. nevadensis. In the mild climate of California
the light (yellowish) facial markings are found at still higher
latitudes. These light facial markings crop up as vestiginal remnants,
consisting of a few white hairs, in some individuals of nearly all
races of weasels.
In certain parts of the skull there are trends, in size and shape,
which continue for long distances geographically. In other words,
clines can be recognized. Changes in size and shape in some other parts
of the skull are wavelike; change toward narrower rostrum, for example,
is not progressive in a given geographic direction for any great
distance. Length of the upper tooth-rows and zygomatic breadth, when
expressed as percentages of the basilar length, and also the actual
length of individual teeth vary geographically in the same wavelike
fashion as does the width of the rostrum.
Size of the skull, on the other hand, shows a sustained trend for a
long distance; it becomes progressively smaller from the southern
United States southward to Columbia, South America. This clinal
variation can be demonstrated by plotting on a graph, the basilar
length, the zygomatic breadth, or the weight of the skull. Beginning at
Mérida, Venezuela, and proceeding southward to increasing elevations in
the mountains of South America, there is a reversal of the direction of
the variation in this cline; weight of skull, for example, increases to
the southward from Mérida for a considerable distance. A cline of
decreasing width of the postorbital constriction of the skull is
evident from Panamá north into Texas.
Variations in the tympanic bullae provide many characters useful in
distinguishing weasels from different localities. Most of these
characters have to do with degree of inflation of the bullae.
Indirectly correlated with degree of inflation is first the extent of
removal of the anterior margin of the bulla from the glenoid fossa and
foramen ovale, and second the form (convex, flat, or concave) of the
part of the squamosal bone between the foramen ovale and the anterior
margin of the tympanic bulla. As one proceeds southward from, say,
southwestern Kansas through the geographic range of the species
Mustela frenata, there is a progressive deflation of the bulla, an
increase in length of the space between its anterior margin and the
foramen ovale, and the floor of the braincase in front of the bulla
changes from ventrally concave to ventrally convex. (See figs. e and
h of pl. 24 and figs. e and f of pl. 27.)
One extreme of this variation in bulla is shown in Mustela frenata
neomexicana (fig. e of pl. 24), in which the anterior margin of the
bulla (viewed from the ventral side) rises vertically from the floor of
the braincase to form a 90-degree angle. The other extreme, the
uninflated bulla, is in Mustela frenata panamensis (fig. e of pl.
27), in which the anterior margin of the bulla is not raised above the
floor of the braincase. This variation is remarkable because it occurs
within a single species. Otherwise, in the family Mustelidae,
differences in the tympanic bullae as great as that between the two
subspecies M. f. neomexicana and M. f. panamensis, occur only
between genera. The need for caution in inferring the limits of
variation for a particular structure in one species or genus, on the
basis of variation in another group, is therefore obvious.
Speaking now of full species, the most inflated tympanic bullae in
American weasels are in Mustela frenata, and more restrictedly in
those subspecies of it which occur in the temperate region. Subspecies
of M. frenata in Central and South America, as already noted, have
less inflated bullae. The tropical weasel, Mustela africana, of the
Amazon drainage of South America has the bullae still less inflated
(see fig. i of pl. 39 and fig. f of pl. 40). The bullae are less
inflated even than in the mink, subgenus Lutreola. In M. africana
the cleidomastoideus, omotrachelian, levator scapulae, and rhomboideus
profundus muscles take origin from a fossa on the mastoid bone, whereas
in the forms with greatly inflated bullae these muscles take origin
from a raised ridge or tubercle. Using Mustela frenata of the
temperate region as a starting point and proceeding northward, a
reduction in inflation of the tympanic bulla is seen also in that
direction in that Mustela erminea has less inflated bullae. The
bullae are less inflated in southern than in far northern (arctic)
populations of Mustela erminea. In erminea the lesser inflation is
real enough but at the same time there appears to be less inflation
than actually exists, for the squamosal floor of the braincase is
"pushed down." This places the anterior end of the tympanic bulla
farther in the braincase than it otherwise would be. Although the
anterior end of the bulla is flattened to the extent that it resembles
the sharp edge of a splitting-wedge, inspection of the lateral and
medial edges shows that in its central part the bulla is more inflated
than it is in the weasels of Central and South America.
For reasons set forth later, M. erminea is judged to resemble the
ancestral stem form more closely than does any one of the other three
American species of weasels. If this judgment is correct, the shape of
the tympanic bullae of the American weasels may be explained as
follows: In the subspecies of Mustela frenata of the temperate
regions of North America the bullae have most nearly been pushed out of
the braincase and at the same time have undergone some enlargement. The
subspecies of this same species in Central and South America represent
an earlier stage in the evolution of American weasels and retain less
inflated bullae—less inflated even than those of the southern
subspecies of erminea. M. africana probably separated from the stem
form at a still earlier time if we may judge by the lesser inflation of
its tympanic bullae. There are other reasons for thinking that
africana separated from the stem form earlier than M. frenata did.
During the time that elapsed since the separation of M. frenata from
the stem form, the tympanic bullae of M. erminea probably increased
slightly in size, as probably also did the brain but without shoving
the auditory complex forward from its former position.
Weasels of the subgenus Mustela are known from the Pleistocene but
not from deposits laid down at an earlier time (see page 10). The
Pleistocene weasels from Rancho La Brea of southern California and from
Potter Creek Cave and Samwel Cave, both of northern California, are
subspecifically indistinguishable from the weasels living in those same
localities today. The other notable occurrence of weasels in the
Pleistocene is in the Conard Fissure of Arkansas. Brown (1908:181, 182,
pl. 17) names two kinds from the Fissure. One is an extinct subspecies
(Mustela frenata gracilis) possibly of the species which occurs in
the same region today and the other, Mustela erminea? angustidens,
is an extinct subspecies of a species which occurs only farther north
today. M. erminea came south, probably in front of one of the ice
sheets, as did several other species of American mammals, now of more
northern distribution, that left their remains in Conard Fissure.
Mustela rixosa is not recorded as a fossil in America although it is
known from the "Diluvial" deposits of the Old World; see Woldrich
(1884:1000), who employs the name "Foetorius minutus n. sp.," and see
also Zimmerman (1943:295-296).
The ermine, Mustela erminea, is the most generalized of the full
species. For example, the number of teeth is as large as in any other
species and greater than in certain species. The teeth are
sharp-pointed, uncrowded, and individually less specialized than in any
other American weasel. M1 has the inner half, or lobe, of approximately
the same size as the outer lobe instead of much larger than the outer
lobe (the outer lobe is the larger in several other species). The
tympanic bullae are less inflated and less protruded from the
braincase. The skull is rounded, and has no marked crests and ridges
whereas the skulls of the other species are more pronouncedly modeled
and sculptured. Therefore, it is possible to think of these other
species as derived from M. erminea. A derivation in the reverse
direction would be more difficult. From the foot soles of an ermine, or
a weasel closely resembling an ermine, the more complex soles of
Mustela africana could have been derived by a decrease in hairiness,
although it would be necessary to suppose that the thenar pad has been
retained in africana and has been lost in the living erminea. The
alternate possibility, namely, that the thenar pad was a relatively
recent acquisition in the africana line seems less probable. The tail
of erminea is of "average" length and in size of entire animal
erminea is intermediate between the other American weasels.
Structurally, Mustela erminea appears to be nearest the stem form
from which all of the living weasels ascended. Its present holarctic
distribution is in harmony with the view that it is a direct descendant
from the stem form because the stem forms of most of the known kinds of
mustelids appear to have lived in the holarctic region. To be sure,
Mustela erminea is regarded as having undergone some progressive
change in structure, but less than the other weasels, in the period of
time when the weasels were evolving from the stem form.
The least weasel, Mustela rixosa, seems to be an ancient type and to
judge from the size and proportions of its parts, was differentiated
from the erminea stem at a time earlier than were the other American
Recent species of weasels. In size, in reduction of the tail, and in
proportions of the skull, M. rixosa is, in each instance, the most
aberrant of all the weasels, Mustela nivalis of Europe and western
Asia included. This aberrancy results from the retention of certain
primitive features, in the teeth and basicranial region, and from
specialization in proportions of the skull. The skull is long, deep,
and narrow. These proportions probably are adaptations permitting the
animal to follow the smaller kinds of mice into their burrows. In most
of that part of North America where erminea and rixosa occur
together, erminea is a much larger animal and takes as prey almost
all kinds of land vertebrates that it is powerful enough to kill. These
include varying hares and ptarmigans. The least weasel, rixosa, can
hardly manage such large prey and lives on the smaller rodents.
Mustela rixosa may eat numbers of insects (see page 176 beyond),—a
kind of food which Mustela erminea is not known to eat. Apparently
the two species are able to live in the same areas because each eats a
somewhat different kind of food than does the other and hence they do
not compete to the point where one is crowded out by the other. This is
the case in the latitudes where the two species of weasels are of
different bodily size, but in the southernmost latitudes where these
two species occur, erminea becomes almost as small as rixosa and
only one of the species, to the exclusion of the other, occurs in a
given area. All through the Rocky Mountains, south of Montana and in
the territory west of these mountains all the way to the Pacific Coast,
only the small subspecies of erminea is to be found. In the
Alleghenies of the eastern United States only rixosa occurs. In New
England where erminea approaches the size of rixosa, the latter is
unknown. Probably this exclusiveness results from competition for food,
although competition for dens, safe breeding places and other
requirements of life may be involved.
The species erminea invaded the western United States and in the
process of invasion probably developed there the small size appropriate
to permit erminea to live in that latitude before it could do the
same thing in the Appalachian region. Later than erminea, the least
weasel, Mustela rixosa, which was small to begin with, also spread
southward from the holarctic region, stopped short in the western
United States at the northern boundary of the area in which erminea
was of small size, but in the Appalachian region of the eastern United
States continued on southward to the limits of temperature tolerant for
it because erminea had not yet penetrated into that region and no
other small carnivore was there to offer competition.
The long-tailed weasel, Mustela frenata, occurs mostly south of the
regions inhabited by the ermine, and mostly south of the region
inhabited by the least weasel which appears to live as well with
frenata as with erminea. It is true that erminea and frenata
occur in the same region, but this is a relatively narrow belt across
the United States; and from within it a person cannot go far either
north or south without reaching a region in which only one of the two
species occurs. Exception has to be made for the Rocky Mountains and
the Sierra Nevada, where erminea is of exceptionally small size. In
these mountains and in the boreal mountainous parts of the intervening
region of the United States, erminea and the large-sized frenata
occur together over a wide area. Presumably the two occupy different
ecologic niches, much as rixosa and frenata probably do where they
occur together.
Most of the geographic range of the long-tailed weasel, M. frenata,
is in the temperate region. Structurally, this species is the most
advanced of the American weasels. Its dentition is the most highly
specialized for cutting. M1 is relatively small and the inner lobe is
slightly larger than the outer lobe. The skull, throughout, is more
modeled than in the other species; the rostrum, the lower jaws and the
teeth—all parts of the offensive equipment—are well developed
relative to the corresponding structures in other weasels; the
basicranial region exhibits an advanced stage of development in that
the tympanic bullae show the maximum degree of inflation. Also, they
are thrust far out of the braincase, thereby providing more room for
the relatively larger brain which is protected by a more solidly built
braincase than in erminea.
Several subspecies of Mustela frenata occur in the tropics, that is
to say, south of the Mexican tableland and on the coastal plain to the
east of it. Each is structurally more primitive than subspecies of the
temperate region. As compared with Mustela frenata frenata of the
temperate Mexican tableland the size in these tropical subspecies is
smaller; the tail is shorter; the braincase and entire skull are less
modeled; the postorbital breadth is more; the teeth are smaller; the
deuterocone of P4 is not so far anterior to the protocone; the tympanic
bullae are less inflated, are farther removed from the foramen ovale,
and a larger proportion of each bulla is contained within the
braincase. These features serve to set off from northern races of
frenata all those subspecies of frenata which occur from southern
México southward to the northern and western limits of the Amazon
drainage of South America. The Amazon Basin is inhabited by another
species, Mustela africana, having more primitive characters.
In the species frenata, the explanation for this abrupt change in
characters between the animals of the temperate highlands and those of
the tropical lowlands may be this: In the early Pleistocene, after the
emergence of much or all of Central America took place, weasels
distributed themselves over the Isthmus and into South America. These
weasels were more generalized in structure than those now inhabiting
the uplands of México. Failure of this stock of weasels often to cross
some still-persisting water barrier, or failure of this stock to cross
some water barrier that was widened or reformed because of a rise in
sea level in some one of the interglacial periods of the Pleistocene
cut the frenata stock into two or more parts. After the land
connection was established or re-established and when the necessary
precedent plants and rodents again had established themselves, the two
groups of weasels, one from the northern tableland of México, and the
other from the southern area of tropical complexion, met. The weasels
of the frenata stock that reinvaded the area from the north probably
did so by following along the chain of high volcanic cones and narrow
uplifts. If and when a subsequent inundation occurred in some part of
Central America, weasels were stranded on the adjacent
mountains—converted into islands—only the higher parts of which were
above water. Mustela frenata costaricensis and Mustela frenata
goldmani may be examples of a northern stock of weasel that pushed
southward in the highlands and became stranded for a short time.
Following the latest emergence of land to provide a continuous highway
between the two continents, weasels from the south and the insular
populations, as for example, M. f. costaricensis, were the first to
invade the low tropical areas most recently under water. When the
Pleistocene history of Central America is better known, the facts will
provide a useful means of testing the hypothesis that has been outlined
immediately above.
As explained above, fossil specimens of M. frenata from deposits of
the last half of Pleistocene time show that no appreciable change
occurred in some areas, for example, in the vicinity of Hawver Cave and
Samwel Cave of California, and that but slight change occurred in other
areas, for example, in southern California (fossils from Rancho La
Brea) and probably in the central United States (fossil from Conard
Fissure). It is possible to imagine, therefore, that the two groups of
weasels, one occurring southward only as far as the highlands of
Central America and the other occurring in northern South America, had
not differentiated sufficiently in the period of their isolation to
prevent crossbreeding when they last came into contact. If the
separation of the two groups had been maintained for a longer period,
the two groups, tropical weasels and austral weasels, probably would
have been so different when the two met as to prevent crossbreeding and
they would have constituted two full species instead of only one.
Mustela africana is the most primitive of the American weasels. Some
of the most important structural features that mark it as such are in
the basicranial region. The tympanic bullae are less inflated than in
other weasels, are pointed anteriorly and posteriorly, and do not have
the lateral margins carried outward to the outer margins of the
braincase. The mastoid sinus is not involved, by inflation or marked
modification in the production of the auditory complex. Between the
alisphenoid and the squamosal there is a clear demarcation posteriorly
from a point directly lateral to the foramen ovale. This demarcation
permits a transverse rounding of the alisphenoid to form a longitudinal
ridge between the anterior margin of each bulla and the base of the
pterygoid of the same side. Nevertheless, there is no such
specialization of this primitive, structural feature such as occurs in
some African and Asiatic mustelids in which the tympano-pterygoid part
of the alisphenoid fuses with the tip of the hamulus of the pterygoid.
However, the tympano-pterygoid eminence has not been obliterated in M.
africana as it has in the other American weasels. Another primitive
feature in the basicranial region of M. africana is the tendency
toward separation of the paroccipital processes from the tympanic
bullae. The thenar pad of the foot probably is an inheritance from a
primitive ancestor since the pad is present in the viverrids and in a
majority of mustelids judged to be more primitive than Mustela.
Some specializations are obvious in Mustela africana. One is the
reduction in number of premolars; p2 is absent whereas it is normally
present in the other weasels; P2 has one instead of two roots; and, in
relation to the other teeth, m2 is smaller. The shortness of the
preorbital part of the skull in relation to the length of the skull as
a whole may reflect the mentioned reduction of the premolars or
retention of a primitive shape of skull, or both. Also, certain
features which denote immaturity in other weasels are retained in
adults of this species, as for example, sutures on the dorsal face of
the preorbital region of the skull.
Figs. 17-22. Views of the feet of American weasels
(subgenus Mustela) to show differences in number and arrangement of
the pads and variation in degree of hairiness of the soles. × 1-1/2. In
each figure, left-forefoot on left, and left hind foot on right.
Fig. 17. M. rixosa rixosa, Halifax, N.S.; juv., ♀, 7425 U.S.N.M.
Fig. 18. M. erminea richardsonii, Ft. Chimo; ad. ♀, 14866 U.S.N.M.
Fig. 19. M. frenata noveboracensis, Mich., July 7, 1913; ad. ♂,
44689 M.Z.
Fig. 20. M. f. frenata, Brownsville, June 1, 1892; yg. ♂, 34043
U.S.N.M.
Fig. 21. M. frenata panamensis, Panamá, February 17, 1911; sad. ♀,
type.
Fig. 22. M. a. africana, Pará, Brazil, Sept., 1908; yg. ♂, 37475
A.M.N.H.
Figs. 17, 18 and 19. Drawn from specimens preserved in alcohol.
Figs. 20, 21 and 22. Drawn from relaxed feet of dried skins.
Mustela africana, all characters considered, is the most aberrant of
the American weasels. That is to say, greater difference prevails
between M. africana and any other American weasel than exists between
any other two American weasels. The distinctive cranial and dental
characters, excepting the reduction in number of premolars, are of a
primitive nature. For example, the relatively wide postorbital region,
the large braincase that is inflated anteriorly, and the flattened
tympanic bullae are points of resemblance to the holarctic Mustela
erminea, the species which is regarded as most closely resembling the
stem form. Also, the mentioned characters in adults of M. africana
resemble ontogenic stages passed through by other weasels.
Consequently, it is thought that M. africana crossed the
filter-barrier from North America to South America, remained isolated
from the original stock for a length of time sufficient to permit
africana to differentiate from North American weasels and vice
versa to such a degree that crossbreeding with the frenata stock was
prevented when frenata, at a later time, pushed southward over the,
then zoölogically less-effective, water barrier, or continental bridge
if it was by this time in existence.
Fig. 23. Diagram indicating probable relationships of
the species of American weasels.
The four full species of American weasels may well be thought of as
having the same stem form of which erminea is the most nearly direct
descendant. Geographic and climatic changes may have operated to
isolate, and then to foster morphologic differentiation of, first
rixosa in Eurasia, next africana, third the tropicalis section of
M. frenata, and finally M. frenata itself, leaving M. erminea as
a modern version, somewhat altered to be sure, of the stem form. Some
of these ideas are expressed in figure 16. The climate is different in
the ranges of the several species and the climate has changed through
time in the ranges of at least many subspecies. Natural selection of
morphological features best adapted to a particular kind of climate
probably has altered some species more than others. M. erminea in
almost every one of its characteristics is generalized and potentially
progressive whereas africana retains more characters which are truly
primitive along with a few which are specializations. M. africana is
potentially the least progressive of any of the American weasels. The
most specialized weasels are the North American races of Mustela
frenata. A progressive series of increasing specialization is
comprised in (1) M. africana, (2) the M. tropicalis (Central
American, lowland) section of M. frenata, and (3) the races of M.
frenata in North America.
Considering now features of the environment which have obviously
influenced the distribution and speciation of weasels, water barriers
are important. Bering Strait, Carquinez Strait (along with San
Francisco Bay) which opens through the Golden Gate, and the channels
between the islands of southeastern Alaska, have contributed to the
formation of subspecies. The difference is really slight on the two
sides of Bering Strait and San Francisco Bay and is slightly more on
two sides of each of several of the channels between the islands of
southeastern Alaska. The differences between the weasels on the two
sides of one of these water barriers supposedly result from the
preservation in animals on one side, or on one island, of small
mutations, which would be swamped by crossbreeding if the water barrier
were not present. The effect of this isolation is easily seen if
ermines from the Queen Charlotte Islands are compared with those of the
opposite mainland. The degree of morphological difference is great.
Isolationwise, the Queen Charlotte Islands are the seaward end of a
chain, beginning with Admiralty Island in southeastern Alaska, and are
farther from the mainland, zoölogically, than the distance in actual
miles across the water channel would suggest. Between any two islands
that are geographically consecutive, however, and between the mainland
and the first island of the chain, the difference in the ermines is
small. In other places, water barriers of equal or greater width have
contributed little if anything to the differentiation from one another
of weasels on the two sides of the water barrier. The strait between
eastern Canada and Newfoundland is an example.
The absence of water, or scarcity of it to a degree that closely
approaches absence, in any large area appears to prevent weasels from
living there. At any rate, the one sizeable region of North America
from which weasels are unknown is the desert of the southwestern United
States and adjoining part of northwestern México. More precisely, in
western Arizona, the Mohave Desert and the desert of northwestern
Sonora, collectors of mammals have repeatedly sought small carnivores
without ever finding any weasels.
Degree of moisture is closely correlated with color in weasels.
Humidity and cloudiness as well as actual precipitation seem to be
involved. Even if we take into account average annual rainfall alone,
the darkest-colored weasels are found in the areas of heaviest rainfall
and the lightest-colored weasels in areas of lightest rainfall (extreme
type of desert where no weasels occur being excepted). In any large
region where there is a geographic gradient in rainfall, the transition
from light to dark color almost exactly parallels the increase in
amount of rainfall. Within a given species the same color reappears in
widely separated areas that have the same amount and seasonal
distribution of rainfall. This correlation is repeated so often that
one can almost certainly say that heavy rainfall, or the associated
phenomena of high humidity and cloudiness, acting separately or
together, causes an increase in intensity of color. Relative extent of
the color of the upper parts and underparts and presence and absence of
light facial markings seem also to be correlated, in a more general
way, with differences in rainfall. A fuller discussion of the nature
and amount of the variation in color is given on page 51.
Temperature seems not to be an important factor in directly limiting
the distribution of weasels, since M. frenata occurs from the hottest
to some of the coldest parts of the Americas. Do M. erminea and M.
rixosa range no farther south, than they do at present, because high
temperatures constitute a barrier? No evidence is known to me which
provides an answer, one way or the other, to this question. Granting
that temperature is unimportant in limiting the distribution of
weasels, it seems to cause geographic variation. Increase in mean
annual temperature is correlated with decreased size in M. erminea
and with increased size in M. rixosa. Temperature, it seems, causes
the hair to vary; the pelage is harsher and sparser in weasels from
tropical regions than in those from boreal regions. Difference in
number of hairs is especially well shown on the soles of the feet. In
the weasels from the far north, the pads are concealed by hair and in
the weasels from the tropical regions the soles are mostly bare. Also,
the hair on the soles of the feet is longer in northern than in
southern weasels. Furthermore there is seasonal change in length of the
hair on the soles of the feet; at a given locality in southern Canada
the hair of the white winter coat is so long on the soles of the feet
as to obscure completely the palmar and plantar pads whereas the hair
of the brown summer coat is shorter and leaves these pads boldly
exposed to view. This seasonal change, as would be expected, is most
marked in animals of northern regions and is not perceptible in those
from the tropics; it is correlated with increase in seasonal change as
the distance from the equator increases.
Temperature and moisture acting together may cause extensive white
facial markings, that neither alone would cause. In Mustela frenata
these markings occur where there is heavy rainfall and high mean annual
temperature. Where there is heavy rainfall and a low mean annual
temperature they do not occur and where there is high mean annual
temperature and light rainfall the markings are not pure white but are
of the same color as the underparts. Plate 1 and the description of
color on page 51 may be consulted in this connection. Extremely high
mean annual temperature together with extremely heavy rainfall may
inhibit the development of light facial markings. M. f. meridana,
panamensis and costaricensis are cases in point. In either
direction, north or south, from the territory inhabited by these three
subspecies a similar combination of temperature and rainfall is found
and similar light facial markings appear there.
Considering the delicate response of structure to climate, a person
naturally questions whether or not natural selection accounts for all
of the differences between subspecies. To show that natural selection
determines the color of Mustela frenata, it would be necessary to
assume that climate, color, and utility of color are positively
correlated. Although climate (rainfall) and color are correlated in
such a manner that three subspecies of weasel in places as far apart as
New England, Perú, and the state of Washington are colored alike, other
features of the three environments are unlike. Kinds of animals which
the weasel catches for food, and flora in which the weasel finds
concealment, are dissimiliar. If natural selection alone determined the
color, some difference in color would be expected between the weasel
which needed to be obliteratively colored, that is camouflaged, the
better to catch a Phyllotis in Perú and the weasel in Washington
which needed nature's aid in catching Microtus. Mustela frenata
goldmani of the highlands of southern México, which is known to attack
the huge pocket gophers, Orthogeomys and Cratogeomys, has a weaker
dental armature than Mustela frenata texensis which does not have to
overcome prey so formidable as does goldmani. Equally formidable
enemies endanger M. f. goldmani and texensis. Examples of this
nature could be multiplied. Without actually proving anything
concerning selection, these examples give reason for us to suppose that
some characters are not determined by natural selection.
Another question upon which data obtained from a study of Mustela has
some bearing, is this: Where the geographic ranges of two subspecies
meet, why does not the swamping effect of crossbreeding cause one
subspecies to disappear? Although swamping may have occurred in some
instances, it does not occur in the majority of instances. Witness the
long-continued existence of the living subspecies Mustela frenata
nevadensis of which skulls are available from Pleistocene deposits.
Therefore, its distinctive characters, cranially at least, have been
maintained for a long time. Furthermore, these characters are
maintained over a large geographic region more than a thousand miles
across. On the eastern margin of its range, at the eastern base of the
Rocky Mountains in Colorado, M. f. nevadensis intergrades in a
relatively narrow belt with the lighter-colored, longer-tailed and
cranially different Mustela frenata longicauda, which has a
geographic range almost equally extensive. M. f. longicauda also is
uniform in its characters over a large area but at approximately 400
miles east of the base of the Rocky Mountains, it begins to intergrade
with the darker-colored, shorter-tailed and cranially different
Mustela frenata primulina and does so over a belt of 100 miles or
more in width. At any given locality within this wide belt of
intergradation the range of individual variation ordinarily does not
exceed that in animals from a given locality well within the geographic
range of M. f. longicauda. In the narrow belt of intergradation along
the eastern base of the Rocky Mountains, the range of individual
variation at several places is greater than in animals from a given
locality well within the geographic range of M. f. longicauda or for
that matter from well within the geographic range of M. f.
nevadensis.
Considering the dominance and recessiveness of genes and the genetic
mechanism in general by which characteristics of offspring are
inherited from their parents, it would seem that M. f. longicauda and
for that matter M. f. nevadensis and M. f. primulina would lose
their distinctive characteristics because of the crossbreeding that is
every year going on between longicauda and nevadensis on the one
hand and between longicauda and primulina on the other hand.
Sumner (1932:84) suggests that homogeneity is prevented by population
pressure. Applying his suggestion to the species Mustela frenata we
could say that the subspecies longicauda pressing westward meets
strong pressure from the subspecies nevadensis pressing eastward and
that the width of the zone of intergradation between the two subspecies
varies inversely with the strength of the population pressure from the
two sides. Sumner recognizes that according to his hypothesis the two
contiguous races would remain distinct only so long as there was a
preponderance of centrifugal movement from both of the centers of
dispersal. Sumner (op. cit.:85) recognizes that an abrupt change of
environmental conditions could account in part for the boundaries of
the ranges of the two subspecies and finally that his hypothesis does
not certainly answer the question of why crossbreeding does not result
in homogeneity between two subspecies with contiguous geographic
ranges.
The hypothesis of harmoniously stabilized complexes of genes was
offered by Timofeeff-Ressovsky (1940:124) to explain why the swamping
effect of crossbreeding does not obliterate subspecies. The hypothesis
takes into account that any one of several characters of a subspecies
may be caused by several genes. Some characters of this kind may be
favored by natural selection more than others. In the belt of
intergradation between two subspecies, where two of these favored
characters meet, a "biological tension" as Huxley (1939:415) terms it
"will result, which will produce partial discontinuity between the
two groups. Each group will evolve a gene-complex which is not only
broadly adapted to the external environment of the central area of its
range, but is also harmoniously stabilized, in adaptation to the
internal genetic environment, by the selection of modifiers." Crosses,
that is to say intergrades, between the two subspecies will lack this
stabilization and will therefore be at a selective disadvantage. The
zone of intergradation will therefore remain narrow; intermediates are
constantly being brought into existence there by crossing but are as
constantly being extinguished by selection.
These two hypotheses are the best that geneticists yet have offered.
Neither has been tested and both, as originally proposed, would hardly
apply everywhere because there are some contradictions.
I can offer no better explanation—in fact no original one as good—but
would emphasize that under similar climate, weasels remain constant in
character, or at most do not vary beyond certain limits. Crossing at
the margins of ranges of two subspecies does not result in homogeneity
of weasels. There is, therefore, some stabilizing influence, or
influences, that maintain, and even develop, structural characteristics
of weasels in opposition to the contrary tendency of crossing.
That this influence not only maintains uniform characters over areas of
large extent, but also permitted their development over large
geographic areas, must logically be supposed, for otherwise,
considering the swamping effect of crossing, such variations would not
have made their appearance in more than a few individuals. Also, if the
races had been formed in response to some kind of physiological
differentiation, or other non-climatic cause, the characters of the
population in the belt of intergradation probably would disappear in a
short time. In any event the close correlation between degree of change
in weasels and degree of change in climate, at once makes one suspect
that climate has been the deciding factor. Finally, when one recalls
that in certain parts of the animal, certain characters invariably
appear under similar climates and never under dissimilar climates, the
evidence is almost conclusive that, given long enough time, the animals
vary in response to climate. The variations (characters) may be induced
indirectly, but are no less exactly reproduced than if they can be
shown to be induced directly.
In considering how the species and subspecies of American weasels were
formed and in attempting to account for some of the individual
characters, it is profitable to view the facts in the light of some of
the theories of species-formation—theories that are accessory to that
of organic descent and that are concerned with the modus operandi of
organic descent.
In any group of closely related species some of them, by the laws of
chance, are almost certain to be more primitive than others. Mustela
is no exception and the more primitive species closely match, in
several characters, ontogenetic stages passed through by more advanced
species. Jaeckel's (1902) theory of metakinesis, therefore, is to be
considered since it postulates that many cases of epistasis occur; that
is to say, that many sexually adult animals are arrested in
development in early otogenetic stages and undergo no further
development. Although this theory is appealing upon initial
consideration, it is less so when we recall that in Mustela there is
a direct correlation of increasingly primitive structure with
decreasing latitude as one proceeds from the steppe of North America
southward to the equator. It follows that the conditions seen in
Mustela can be explained even better than by metakinesis, by assuming
that the several species have differentiated from a stem form at
different times, have developed at different rates, have developed in
different directions and that ontogeny recapitulates phylogeny.
The theory of Age and Area (see Willis, 1922) holds that the species of
widest distribution are, on the average, the oldest, and that the
species which are distributed over small areas are, in general, of
recent origin. So far as the weasels are concerned, little support is
given to this theory. The same can be said of any one of the teological
theories, including the orthogenesis of post-Darwinian writers. All of
these imply a determinate line of variation controlled by the inherent
qualities of the organism. The idea that the several species of
Mustela result from mutations of large degree and sudden appearance
is contrary to the evidence accumulated. In fact the evidence rather
clearly indicates that the mutations which may have occurred were of
small degree and in most instances owe their preservation to natural
selection.
The data obtained by the study of weasels accords almost exactly with
the theory of species-forming embodied in Matthew's (1915) "Climate and
Evolution." Although the essential features of this theory were made
out from a study of families and orders and therefore would not be
expected to apply to members of only a genus or subgenus, the facts
known about the present distribution of American Mustela,
nevertheless, are strikingly in accord with the ideas advanced by
Matthew. In the first place, climate is an important factor in the
evolution of the weasels. In the second place, the line of migration
seems to have been outward from the holarctic region. In the third
place, the geographic changes necessary to explain the present
distribution of the species of Mustela are not extensive and do not
affect the permanency of oceans as defined by the continental shelf.
These three statements are, almost verbatim, those made in the first
three of the five points of Matthew's (1915:172-173) thesis. The
remaining two points of Matthew's thesis have to do with
generalizations based on evidence obtained from sources outside the
scope of the present study.
Furthermore, the relative degrees of specialization of the different
species and subspecies in relation to their geographic distribution are
in accord with the ideas elaborated by Matthew. For instance, the most
primitive species is farthest south from the probable center of
dispersal, the holarctic region. Also the full species become
progressively more primitive as one proceeds southward from the
holarctic, or at least from the northern half of the nearctic, region.
Although, in view of the known geological changes that have occurred in
the Caribbean region, we cannot say that the more primitive species owe
their positions entirely to having been pushed farther south from the
center of dispersal by actual and continuous contact and competition
with the more advanced species, this seems to have been the case in a
general way. At any rate the more primitive kinds seem to have been
prevented from pushing northward by the more advanced kinds which
developed there and the latter have actually pushed southward.
Additionally and in review: There is strong indication that the
American species of weasels were formed by gradual and slow change.
Much of this change probably is the result of natural selection
operating on fortuitous variations of a minor nature, but, also,
particular features of the environment, especially climate, and more
especially amount of rainfall, seem to compel variations that
differentiate subspecies and that characterize full species—compel
some of them without the direct operation of natural selection, or at
least compel them within limits so wide that natural selection exerts
no exact control.
In the earlier accounts of American weasels, from the time of Linnaeus
and before, up until 1890, names then in use for European weasels
frequently were applied also to those in North America. For the next 50
years, and almost without exception after 1896, the American weasels
were regarded as specifically distinct from those in the Old World. In
this 50-year period many new names were proposed, usually as full
species, although now that material from more localities has been
brought together and studied, geographic intergradation is evident
between many of the named kinds and most of these names now therefore
take only subspecific rank. In 1933 Glover M. Allen showed that
Mustela rixosa occurred also in the Old World, and in 1943 I
emphasized that a second American species, Mustela erminea, was
circumpolar in distribution. In neither rixosa, nor erminea,
however, were the subspecies the same in the two continents. To this
general outline of the nomenclature, exception must be made for weasels
of the southwestern United States, México and Central America, and
South America, because as early as 1813 a distinctive name was given to
one of these and weasels from the three areas mentioned were, so far as
I know, never given names of Old World kinds.
The first paper that could be regarded as revisionary in nature was
"Remarks on the species of the genus Mustela" by the zoölogist and
world-traveler, Charles L. Bonaparte, in Charlesworth's Magazine of
Natural History, for 1838. In that paper three new names, Mustela
cicognanii, M. richardsonii and M. longicauda, all still valid,
were proposed for American weasels.
Audubon and Bachman in their "Quadrupeds of North America," which
appeared in parts from 1845 to 1853, recognized 5 species. Actually
they were dealing with only 3 taxonomically valid kinds. For one of
these, Mustela frenata noveboracensis, they were misled by the
difference in size between males and females, and in the males by the
presence of a brown coat in some and a white coat in others. The male
that was white in winter they regarded as Putorius ermineus of the
Old World; the male that was brown in winter they designated by their
earlier proposed name P. fuscus, and the female they named P.
agilis. The ermine, subspecies M. erminea cicognanii, they called
P. pusillus. Their fifth name, P. frenatus, included at least some
animals that today are assigned to the subspecies M. frenata frenata.
Each of three and perhaps four of the five names employed by Audubon
and Bachman embraced individuals of more than one species and in that
sense the names were composite.
Only five years later, in 1858, Professor Spencer Fullerton Baird's
great work, "The Mammals of North America," made it clear that no
American weasel was identical (in the modern subspecific sense) with
any Old World weasel, and he applied most of his names in a correct
zoölogical sense. It is true that he thought that the female weasel of
the eastern United States was specifically different from the male,
misapplied to it the name richardsonii, and did not correctly
allocate every one of the few poor specimens available to him of the
little ermine (M. e. streatori) of the Pacific Coast; but he did
recognize that the least weasel was a distinct kind and his treatment
in general was excellent.
After Baird came a period of great confusion in which most writers did
no better than had Audubon and Bachman, ordinarily confusing the two
sexes as different species, and, in 1877 in his "Fur-bearing Animals,"
Elliot Coues went rather to the other extreme and allowed only 4 kinds
to all of the Americas, regarding two of these, for purposes of
zoölogical nomenclature, as identical with the European species.
But, in 1896 Outram Bangs published "A Review of the Weasels of Eastern
North America" in which he correctly recognized eight kinds. Although
some of these were treated by him as full species, whereas the material
accumulated since 1896 has shown that subspecific status is in order,
his names, still in use, were correctly applied in every instance, save
probably one. This was his use of Putorius richardsonii for the
animal now known as M. e. arctica. Unlike the earlier, excellent
treatment by Baird, this accurate one by Bangs was heeded and followed
by subsequent writers. For example, Dr. C. Hart Merriam in the same
year, 1896, accepted Bangs' conclusions except for correcting the
application of the name richardsonii. The principal contributions of
Merriam's paper "Synopsis of the Weasels of North America" were first,
the wider geographic scope and second, the naming as new of several
kinds outside the geographic area studied by Bangs. Otherwise the work
was not up to Dr. Merriam's usual standard and the internal evidence of
haste in its preparation and the superficial study of some of the
material at his disposal explain why the weasels of North America since
that time have been but little better understood than in 1896. Baird
and Bangs, then, unquestionably did the best systematic work on the
American weasels.
In 1916 Dr. Joseph A. Allen published a valuable paper on the South
American weasels. The material available to him was inadequate and
prevented a thoroughly satisfactory treatment. There are too few
specimens even today to permit of a thorough treatment of the South
American weasels in the present paper; nevertheless the material today
is more nearly adequate than it was in 1916 and it is hoped that the
systematic arrangement is correspondingly improved.
Chronological List (annotated) of Specific and Subspecific Names
Applied to American Weasels
At least eighty-seven specific and subspecific names have been proposed
for American weasels. Of these sixty-nine are now regarded as valid
designations of recognizable subspecies. The average is 1.2 names per
subspecies. Some names in the following chronological list were a
second time applied wholly or in part to some other kind of weasel. In
general, mention of the second or any other later application is
omitted from the following list but two usages of agilis (1844 and
1853) and of americana (1865) are recorded.
1734. javonica (Mustela) Seba, Locupletissimi Rerum naturalium
Thesauri ..., 1:77, 78, pl. 48, fig. 4. The weasel to which this
name was applied was said to have come from Java. Since no animal
answering to the description has again been found in Java, and
because specimens from Central America or possibly some from
northern India, may do so, it is conceivable that Seba was the
first to distinguish by name an American weasel from those in the
Old World. My attempts to locate the specimen concerned in places
where it might have been preserved along with some of the other
specimens thought to have belonged to Seba have been fruitless.
Since it is impossible positively to link Seba's description with
any known weasel, no further use is made of the name javonica in
the present account.
1772. erminea (Mustela) Forster [= Mustela erminea
richardsonii], Philos. Trans., London, 1772:373. Forster's use of
the name is one of the earliest applications of it to American
animals. The name dates from Linnaeus, Syst. Naturae, (10th ed.)
1:46, 1758, with type locality in Europe. In the subspecific sense
the name applies to the ermine which occurs over most of the
Scandinavian Peninsula, if Miller (1912:387) be followed in
regarding the type locality as Upsala, Sweden. If, instead,
Cabrera (1913A:394-396) be followed in regarding the type locality
as in Switzerland, the name, in the subspecific sense, will apply
to the ermine of continental Europe. As the earliest available
name applied to the circumpolar species concerned, it is used now
as the name of the species in the New World as well as in the Old
World. From the time of Forster until approximately 1890 the name
erminea by many, but not by all, authors was applied to the
American weasels in the belief that they were zoölogically
indistinguishable from those in the Old World. From 1896 to 1943
the name was not used by American authors at all because the
ermine of America was in 1896 treated nomenclaturally by Merriam
as specifically distinct from the animal in the Old World. Since
1943 erminea has been used in the specific sense for American
animals in recognition of the circumpolar distribution of the
species. Some of the early allocations of American specimens to
erminea probably resulted in a composite use of the name in that
one or another subspecies of the American species Mustela
frenata may also have been included with individuals truly of the
species erminea.
1772. nivalis (Mustela), Forster, Philos. Trans., London,
1772:373. This is one of the early applications of this name to
American weasels of small size, made in the belief that they were
taxonomically the same in America and Europe. Linnaeus, Syst. Nat.
(12th ed.) 1:69, 1766 is the authority for the name [Mustela]
nivalis, and the Province of Vesterbotten, Sweden, is regarded
as the type locality. The name is in use today for the common
weasel of Europe and parts of Asia. Animals of the species
nivalis are intermediate in size between Mustela erminea and
Mustela rixosa. The name as used for American animals by some
authors who wrote later than Forster did, probably was composite
in that these authors may have applied the name to the small
weasels of North America and thus may have intended it to apply
not only to Mustela erminea cicognanii but also to females of
Mustela frenata noveboracensis, and conceivably to both sexes of
Mustela rixosa of any American subspecies.
1813. Brasiliensis (Mustela) Sevastianoff, Mem. Acad. Imp. Sci.
St. Petersburg, 4:356-363, table (= plate) 4. This name was
proposed for a weasel brought to St. Petersburg by Capt.
Krusenstern on his return from a voyage around the world. The
animal was said to have come from Brazil, but to judge from the
description, came instead from México, Central America, or west of
the Andes in South America, and was based on some one of the
subspecies of Mustela frenata. Although the name was in use for
more than 60 years it was shown by Merriam (1896:27) to be
unavailable because it was preoccupied by Mustela brasiliensis,
a name earlier used by Gmelin (Syst. Nat., ed. 13, p. 93, 1788)
for a South American otter.
1815. vulgaris (Mustela), Ord, Guthrie's Geography as reprinted
by Rhoads in 1894, vol. 2, p. 291. This use by Ord is one of the
earliest applications of this name to American weasels, in the
belief that the smaller weasels of North America and Europe were
zoölogically the same; [Mustela] vulgaris seems originally to
have been proposed in 1777 by Erxleben on p. 471 of vol. 1 of his
Syst. Regni Anim., for the weasel of the temperate part of Europe
and to be a synonym of Mustela nivalis Linnaeus (1766). Probably
the name as used by Ord was composite in the sense that he may
have intended it to apply to females of Mustela frenata
noveboracensis as well as to one or both sexes of Mustela
erminea cicognanii and, if he ever saw them, to the two sexes of
Mustela rixosa (one or several subspecies).
1818. africana (Mustela) Desmarest [= Mustela africana
africana], Nouv. Diction. d. Hist. Nat., 19:376. In 1808 E.
Geoffroy St.-Hilaire visited Portugal and was given several
African primates and the specimen of Mustela named by Desmarest
in 1818 who wrongly supposed that it, like most of the primates,
came originally from Africa. After the name had been misapplied
for 95 years Angel Cabrera showed that it pertained instead to the
tropical weasel of Brazil. Of distinctive names applied to
American weasels today, this is the one first proposed.
1832. frenata (Mustela) Lichtenstein [= Mustela frenata
frenata], Darstellung neuer oder wenig bekannter Säugethiere, pl.
42 and corresponding text unpaged. This name is the first one
available for the long-tailed weasel and therefore applies to the
species as a whole.
1838. Cicognanii (Mustela) Bonaparte [= Mustela erminea
cicognanii], Charlesworth's Mag. Nat. Hist., 2:38. The name
erroneously spelled Cigognanii was correctly spelled on page 39.
For a detailed consideration of this name see the account of the
subspecies cicognanii on page 120.
1838. Richardsonii (Mustela) Bonaparte [= Mustela erminea
richardsonii], Charlesworth's Mag. Nat. Hist., 2:39. Until 1896
the name sometimes was applied to the subspecies now known as M.
e. arctica and sometimes to part of the subspecies now designated
as M. e. cicognanii under the principal treatment of which see
(page 120) for a detailed account of the basis of the name
richardsonii, and the reasons for regarding Fort Franklin as the
type locality.
1838. longicauda (Mustela) Bonaparte [= Mustela frenata
longicauda], Charlesworth's Mag. Nat. Hist., 2:39. The type
locality appears to be Carlton House, Saskatchewan, and the name
always seems to have been applied to the long-tailed weasel of the
Great Plains, although in some earlier accounts the name was used
in a more inclusive sense to refer also to animals now of
subspecies closely allied to longicauda. As with the two
preceding names, a detailed consideration of the basis for, and
application of, this name is given on pages 120-123 in the account
of Mustela erminea cicognanii.
1840. Noveboracensis (Putorius) Emmons [= Mustela frenata
noveboracensis], Quadrupeds of Mass., p. 45. This name was
credited by Emmons to De Kay who in the same year published it in
his report on the "Zoology of New York" but without a description
and De Kay's name is a nomen nudum. Emmons' was the first use of
the name accompanied by a recognizable description and therefore
the name must date from Emmons although this obviously was not his
intent since he credited the name to De Kay.
1842. fuscus (Putorius) Audubon and Bachman [= Mustela frenata
noveboracensis], Jour. Acad. Nat. Sci., Philadelphia, 8: (pt. 2)
288.
1842. pusilla (Mustela) De Kay [= Mustela erminea cicognanii],
Nat. Hist. of New York, Zool., Pt. 1, Mammalia, p. 34. This name
was proposed for small weasels of 12 to 13 inches in length of
which the tail amounted to a fourth of the same and although
obviously applying in considerable part to the earlier named M.
e. cicognanii seems to have included some individuals of the also
earlier named M. f. noveboracensis.
1843. xanthogenys (Mustela) Gray [= Mustela frenata
xanthogenys], Ann. and Mag. Nat. Hist., 11:118, February, 1843,
was applied to all of the long-tailed weasels of California that
had light-colored facial markings. Merriam in 1896 suggested that
San Diego was the type locality and in 1899 Bangs proposed the
name mundus for the California weasel north of San Francisco Bay
thus restricting the application of the name xanthogenys. In
1936 Hall further restricted the application of the name and
applied it to the long-tailed weasel of the big interior valley of
California, pointing out that the name was correctly applied to
this weasel of the big interior valley or possibly instead to the
race named munda.
1844. agilis (Mustela) Tschudi [= Mustela frenata agilis],
Untersuch. ü. die Fauna Peruana, p. 110, is a name applied today
to the race of weasel of the Temperate Zone of the western Andes
and intermountain valleys of Perú.
1851. nigripes (Putorius) Audubon and Bachman [= Mustela
nigripes], Quadr. N. Amer., 2:297, 1851, applies to the
black-footed ferret of North America.
1853. agilis (Putorius) Audubon and Bachman [= Mustela frenata
noveboracensis], Viv. Quadrupeds N. Amer., 3:184, pl. 140. This
name was proposed for the female in the mistaken belief that it
was specifically distinct from the larger male for which several
names already were available. Also Tschudi in 1844 had already
used the name Mustela agilis for a South American weasel.
1864. aureoventris (Mustela) Gray [= Mustela frenata
aureoventris], Proc. Zoöl. Soc. London, 1864:55, pl. 8, February
9, 1864, is the name applicable to the dark-colored weasel of the
Pacific coastal region of Ecuador and Columbia.
1865. americana (Mustela erminea Var. 3) Gray, Proc. Zoöl. Soc.
London, 1865:111. The larger individuals of American weasels of
both Mustela erminea and Mustela frenata from the Atlantic
Coast to as far west as Carlton House, Saskatchewan, were lumped
under this name because Gray desired more information than he then
had before recognizing as different from one another several
species proposed for America up to the time concerned. The name is
unavailable because it is preoccupied by Mustela americana
Turton (1806) the name for the American marten.
1865. americana (Mustela vulgaris Var.) Gray, Proc. Zoöl. Soc.
London, 1865:113. Under this name the smaller weasels of the
northern and northeastern part of North America were lumped by
Gray but the name is preoccupied and can be ignored.
1874. affinis (Mustela) Gray [= Mustela frenata affinis], Ann.
and Mag. Nat. Hist., 14 (ser. 4):375, 1874, from New Granada [=
Colombia], had the type locality restricted to Bogotá, Colombia,
by Allen in 1916, and is applied to the long-tailed weasel of the
tropical and temperate zones of the eastern Andes of Colombia.
1874. macrura (Mustela) Taczanowski [= Mustela frenata
macrura], Proc. Zoöl. Soc. London, for 1874, p. 311, pl. 48, May
19, 1874, applies to the long-tailed weasel of central Perú and
northern Ecuador.
1877. culbertsoni (Putorius) Coues [= Mustela frenata
longicauda], Fur-bearing animals ..., p. 136, 1877, is based on
specimens from Fort Laramie, Wyoming. In the past the name has
been regarded as a nomen nudum but there is some reason for
regarding it as having nomenclatural status. In either event it is
here arranged as pertaining to the long-tailed weasel of the Great
Plains which takes the prior name longicauda. See the account of
longicauda for a more detailed account of the name
culbertsoni.
1877. aequatorialis (Putorius (Gale) brasiliensis) Coues [=
Mustela frenata aureoventris], Fur-bearing animals ..., p. 142.
Proposed "merely as a substitute for Gray's [supposedly]
preoccupied name," aureoventris.
1881. stolzmanni (Mustela) Taczanowski [= Mustela africana
stolzmanni], Proc. Zoöl. Soc. London, for 1881, p. 835, November
15, 1881, is applied to the tropical weasel of the Upper Amazon
Basin.
1881. jelskii (Mustela) Taczanowski [= Mustela frenata
macrura], Proc. Zoöl. Soc. London, for 1881, p. 647, May 17,
1881, was proposed for the female in the mistaken opinion that it
was specifically distinct from the larger male which the same
author previously had named macrura.
1891. arizonensis (Putorius) Mearns [= Mustela frenata
arizonensis], Bull. Amer. Mus. Nat. Hist., 3:234, June 5, 1891,
until 1936 was applied to long-tailed weasels of most of the
western United States west of the Great Plains but by restriction
since 1936 has been applied only to the animals in parts of
Arizona and New Mexico.
1894. peninsulae (Putorius) Rhoads [= Mustela frenata
peninsulae], Proc. Acad. Nat. Sci. Philadelphia, 1894:152, June
19, 1894, applies to the weasel of central and southern Florida.
1896. alascensis (Putorius richardsonii) Merriam [= Mustela
erminea alascensis], N. Amer. Fauna, 11:12, June 30, 1896, with
type locality at Juneau, Alaska, has been used for the ermine of
southeastern Alaska ever since it was proposed. In 1944 separate
subspecific rank was accorded ermines on several of the islands of
southeastern Alaska which proportionately restricted the range
assigned to alascensis.
1896. streatori (Putorius) Merriam [= Mustela erminea
streatori], N. Amer. Fauna, 11:13, June 30, 1896, applies to the
ermine of the Pacific Coast from Puget Sound, Washington, south
nearly to the Golden Gate of California.
1896. arcticus (Putorius) Merriam [= Mustela erminea arctica],
N. Amer. Fauna, 11:15, June 30, 1896. Ever since it was proposed,
this name has been applied to the subspecies of ermine of Alaska
and the northern parts of Canada.
1896. kadiacensis ([Putorius arcticus]) Merriam [= Mustela
erminea kadiacensis], N. Amer. Fauna, 11:16, June 30, 1896, is a
valid name applied to the ermine of Kodiak Island, Alaska.
1896. washingtoni (Putorius) Merriam [= Mustela frenata
washingtoni], N. Amer. Fauna, 11:18, June 30, 1896, applies to the
long-tailed weasel of the southern Cascades of Washington and the
northern Cascades of Oregon.
1896. saturatus (Putorius) Merriam [= Mustela frenata
saturata], N. Amer. Fauna, 11:21, June 30, 1896, was little used
until 1936 but applies to long-tailed weasel of limited region in
northern California and southern Oregon.
1896. alleni (Putorius) Merriam [= Mustela frenata alleni], N.
Amer. Fauna, 11:24, June 30, 1896, applies to weasel of Black
Hills region.
1896. oregonensis (Putorius xanthogenys) Merriam [= Mustela
frenata oregonensis], N. Amer. Fauna, 11:25, June 30, 1896,
applies to long-tailed weasel of parts of western Oregon and
northern California.
1896. goldmani (Putorius frenatus) Merriam [= Mustela frenata
goldmani], N. Amer. Fauna, 11:28, June 30, 1896, applies to the
long-tailed weasel of Chiapas, and parts of Guatemala and
Salvador.
1896. leucoparia (Putorius frenatus) Merriam [= Mustela frenata
leucoparia], N. Amer. Fauna, 11:29, June 30, 1896, applies to the
long-tailed weasel of Michoacán and Nayarit.
1896. tropicalis (Putorius) Merriam [= Mustela frenata
tropicalis], N. Amer. Fauna, 11:30, June 30, 1896, applies to the
long-tailed weasel of the Tropical Life-zone of Veracruz.
1896. spadix (Putorius longicaudus) Bangs [= Mustela frenata
spadix], Proc. Biol. Soc. Washington, 10:8, February 25, 1896,
applies to the long-tailed weasel of Minnesota and adjoining
areas.
1896. rixosus (Putorius) Bangs [= Mustela rixosa rixosa],
Proc. Biol. Soc. Washington, 10:21, February 25, 1896, applies to
the least weasel of Saskatchewan and adjoining areas and as the
first available name for the species has been used as the specific
name for the species in America since 1896.
1897. paraensis (Putorius (Mustela) braziliensis) Goeldi [=
Mustela africana africana], Zool. Jahrb., abt. f. systematik,
geogr. u. biol., 10:560, pl. 21, September 15, 1897, a synonym for
the weasel of the lower Amazon area.
1898. neomexicanus (Putorius frenatus) Barber and Cockerell [=
Mustela frenata neomexicana], Proc. Acad. Nat. Sci.
Philadelphia, p. 188, May 3, 1898, applies to the long-tailed
weasel of New Mexico, Arizona, Durango and adjoining areas.
1898. haidarum (Putorius) Preble [= Mustela erminea haidarum],
Proc. Biol. Soc. Washington, 12:169, August 10, 1898, applies to
the ermine of the Queen Charlotte Islands, British Columbia.
1899. notius (Putorius noveboracensis) Bangs [= Mustela frenata
noveboracensis], Proc. New England Zoöl. Club, 1:53, June 9,
1899, was applied to the long-tailed weasel of the Carolinas until
1936 since which time it has been regarded as a synonym of
noveboracensis.
1899. occisor (Putorius) Bangs [= Mustela frenata occisor],
Proc. New England Zoöl. Club, 1:54, June 9, 1899, applies to the
long-tailed weasel of central and northern Maine. Until 1936,
occisor was ordinarily used as the name of a full species but
since then has been arranged as a subspecific name under Mustela
frenata.
1899. mundus (Putorius xanthogenys) Bangs [= Mustela frenata
munda], Proc. New England Zoöl. Club, 1:56, June 9, 1899, is now
applied, and generally has been since 1899, to the long-tailed
weasel of the coastal district of California north of San
Francisco Bay.
1899. muricus (Putorius (Arctogale)) Bangs [= Mustela erminea
muricus], Proc. New England Zoöl. Club, 1:71, July 31, 1899,
applies to the diminutive ermine, often erroneously designated
least weasel, of the western United States.
1899. oribasus (Putorius (Arctogale) longicauda) Bangs [=
Mustela frenata oribasus], Proc. New England Zoöl. Club, 1:81,
December 27, 1899, applies to the long-tailed weasel of the Rocky
Mountains northward from Yellowstone National Park.
1900. eskimo (Putorius rixosus) Stone [= Mustela rixosa
eskimo], Proc. Acad. Nat. Sci. Philadelphia, 1900:44, March 24,
1900, is applied to the least weasel of Alaska and adjacent parts
of boreal North America.
1901. allegheniensis (Putorius) Rhoads [= Mustela rixosa
allegheniensis], Proc. Acad. Nat. Sci. Philadelphia, 1900:75,
March 25, 1901, applies to the least weasel of the eastern United
States.
1902. perdus (Putorius tropicalis) Merriam [= Mustela frenata
perda], Proc. Biol. Soc. Washington, 15:67, March 22, 1902,
applies to the long-tailed weasel of the Lower Tropical Life-zone
from southern Veracruz into Guatemala.
1903. microtis (Putorius) Allen [= Mustela erminea
richardsonii], Bull. Amer. Mus. Nat. Hist., 19:563, October 10,
1903, is a name applied to an individual ermine of small size from
Shesley, British Columbia, which Allen thought was specifically
distinct from the ermine of the Hudsonian Life-zone and adjacent
territory. Now the name is arranged as a synonym of
richardsonii.
1904. audax (Putorius) Barrett-Hamilton [= Mustela erminea
arctica], Ann. and Mag. Nat. Hist., ser. 7, 13:392, May, 1904. In
the original description the type locality, Discovery Bay, was
erroneously stated to be in Greenland and the name audax until
1945 was applied to the kind of weasel occurring in northern
Greenland whereas the type specimen was taken instead in northern
Ellesmere Island and because the weasel there is subspecifically
indistinguishable from ermines from farther west, audax is a
synonym of Putorius arcticus.
1904. imperii (Putorius arcticus) Barrett-Hamilton [= Mustela
erminea richardsonii], Ann. and Mag. Nat. Hist., ser. 7, 13:392,
May, 1904, based on an animal from Fort Simpson, Mackenzie,
Canada, proves to be inseparable from richardsonii which has
priority.
1904. polaris (Putorius arcticus) Barrett-Hamilton [= Mustela
erminea polaris], Ann. and Mag. Nat. Hist., ser. 7, 13:393, May,
1904, is the name used for the ermine of eastern Greenland and
since 1945 has been used for the weasel of Greenland as a whole.
1905. macrophonius (Putorius) Elliott [= Mustela frenata
macrophonius], Proc. Biol. Soc. Washington, 18:235, December 9,
1905, applies to the long-tailed weasel of the mountains along the
eastern border of Veracruz.
1906. leptus (Putorius streatori) Merriam [= Mustela erminea
murica], Proc. Biol. Soc. Washington, 16:76, May 29, 1903, until
1945 was applied to the diminutive ermine of the Rocky Mountains
from Wyoming south to northern New Mexico but proves to be a
synonym of muricus with type locality in the Sierra Nevada of
California.
1908. angustidens (Putorius cicognanii) Brown [= Mustela
erminea angustidens], Mem. Amer. Mus. Nat. Hist., 9(pt. 4):181,
pl. 17, is applied to an extinct subspecies known from fossil
remains of Pleistocene age from northern Arkansas.
1908. gracilis (Putorius) Brown [= Mustela frenata gracilis],
Mem. Amer. Mus. Nat. Hist., 9(pt. 4):182, 1908, applies to a
Pleistocene weasel known from a single skull from northern
Arkansas.
1912. costaricensis (Mustela) Goldman [= Mustela frenata
costaricensis], Proc. Biol. Soc. Washington, 25:9, January 23,
1912, applies to the long-tailed weasel of Costa Rica.
1913. primulina (Mustela) Jackson [= Mustela frenata
primulina], Proc. Biol. Soc. Washington, 26:123, May 21, 1913,
applies to the long-tailed weasel of the central part of the
United States in eastern Kansas and adjoining areas.
1913. campestris (Mustela) Jackson [= Mustela rixosa
campestris], Proc. Biol. Soc. Washington, 26:124, May 21, 1913,
applies to the least weasel of the Great Plains region.
1913. olivacea (Mustela peninsulae) Howell [= Mustela frenata
olivacea], Proc. Biol. Soc. Washington, 26:139, May 21, 1913,
applies to the long-tailed weasel of the southeastern United
States excepting most of Florida.
1914. meridana (Mustela) Hollister [= Mustela frenata
meridana], Proc. Biol. Soc. Washington, 27:143, July 10, 1914,
applies to the long-tailed weasel of northern South America.
1916. nicaraguae (Mustela tropicalis) Allen [= Mustela frenata
nicaraguae], Bull. Amer. Mus. Nat. Hist., 35:100, April 28, 1916,
applies to the long-tailed weasel of Nicaragua.
1927. arthuri (Mustela noveboracensis) Hall [= Mustela frenata
arthuri], Proc. Biol. Soc. Washington, 40:193, December 2, 1927,
applies to the long-tailed weasel of Louisiana and adjoining
areas.
1932. semplei (Mustela arctica) Sutton and Hamilton [= Mustela
erminea semplei], Ann. Carnegie Mus., 21(2):79, February 13,
1932, originally was applied to the ermine of Southampton Island
but after 1945 was applied also to the ermine of Baffin Island,
Melville Peninsula and the west side of Hudsons Bay as far south
as Eskimo Point.
1932. panamensis (Mustela frenata) Hall, Proc. Biol. Soc.
Washington, 45:139, September 9, 1932, applies to the long-tailed
weasel of Panamá.
1932. anguinae (Mustela cicognanii) Hall [= Mustela erminea
anguinae], Univ. California Publ. Zoöl., 38:417, November 8,
1932, applies to the ermine of Vancouver Island, British Columbia.
1935. labiata (Mustela arctica) Degerbøl [= Mustela erminea
semplei], Rept. 5th Thule Exped., 1921-1924, vol. 2, no. 4, p.
25, 1935. When Degerbøl wrote his description and proposed this
name he was unaware that Sutton and Hamilton had three years
before based a new name on weasels from Southampton Island.
Because the two names apply to the same subspecies, Degerbøl's
name, labiata, must fall as a synonym of semplei which has
priority.
1935. helleri (Mustela frenata) Hall, Proc. Biol. Soc.
Washington, 48:143, August 22, 1935, applies to the long-tailed
weasel of eastern Perú.
1936. nevadensis (Mustela frenata) Hall, Carnegie Inst.
Washington, publ. no. 473, p. 91, November 20, 1945, applies to
the long-tailed weasel of the western United States. For many
years, animals of this subspecies were referred to longicauda
and from 1891 until 1936 to arizonensis.
1936. effera (Mustela frenata) Hall, Carnegie Inst. Washington,
publ. no. 473, p. 93, November 20, 1945, applies to the
long-tailed weasel of the Blue Mountains region. From 1891 until
1936 this animal was referred to under the name arizonensis.
1936. altifrontalis (Mustela frenata) Hall, Carnegie Inst.
Washington, publ. no. 473, p. 94, November 20, 1936, applies to
the long-tailed weasel of the humid coastal district from Puget
Sound southward into Oregon.
1936. nigriauris (Mustela frenata) Hall, Carnegie Inst.
Washington, publ. no. 473, p. 95, November 20, 1936, applies to
the long-tailed weasel of the coastal district of California from
San Francisco Bay southward to Point Concepcion. Previous to 1936,
xanthogenys was the name applied to this race of weasel.
1936. latirostra (Mustela frenata) Hall, Carnegie Inst.
Washington, publ. no. 473, p. 96, November 20, 1936, applies to
the long-tailed weasel of southern California which previously had
borne the name xanthogenys.
1936. pulchra (Mustela frenata) Hall, Carnegie Inst. Washington,
publ. no. 473, p. 98, November 20, 1936, is applied to the
long-tailed weasel of the southern end of the San Joaquin Valley
of California.
1936. inyoensis (Mustela frenata) Hall, Carnegie Inst.
Washington, publ. no. 473, p. 99, November 20, 1936, is applied to
the long-tailed weasel of Owens Valley, California.
1936. texensis (Mustela frenata) Hall, Carnegie Inst.
Washington, publ. no. 473, p. 99, November 20, 1936, applies to
the long-tailed weasel of central Texas which previously had been
assigned to the subspecies frenata.
1936. perotae (Mustela frenata) Hall, Carnegie Inst. Washington,
publ. no. 473, p. 100, November 20, 1936, applies to long-tailed
weasel of the mountains along the Puebla-México boundary.
1938. boliviensis (Mustela frenata) Hall, Proc. Biol. Soc.
Washington, 51:67, May 18, 1938, applies to the southernmost known
long-tailed weasel which is in the Lake Titicaca region in Perú
and Bolivia.
1944. salva (Mustela erminea) Hall, Proc. Biol. Soc. Washington,
57:35, June 28, 1944, applies to the ermine of Admiralty Island,
southeastern Alaska.
1944. initis (Mustela erminea) Hall, Proc. Biol. Soc.
Washington, 57:37, June 28, 1944, applies to the ermine of Baranof
and Chichagof islands, southeastern Alaska.
1944. celenda (Mustela erminea) Hall, Proc. Biol. Soc.
Washington, 57:38, June 28, 1944, applies to the ermine of Prince
of Wales, Dall and Long islands, Alaska.
1944. seclusa (Mustela erminea) Hall, Proc. Biol. Soc.
Washington, 57:39, June 28, 1944, applies to the ermine of Suemez
Island, southeastern Alaska.
1945. invicta (Mustela erminea) Hall, Jour. Mamm., 26:75,
February 27, 1945, applies to the ermine of the Rocky Mountains
for several hundred miles both north and south of the United
States-Canadian boundary.
1945. fallenda (Mustela erminea) Hall, Jour. Mamm., 26:79,
February 27, 1945, applies to the ermine of the coastal mainland
in southern British Columbia and northern Washington.
1945. olympica (Mustela erminea) Hall, Jour. Mamm., 26:81,
February 27, 1945, applies to the diminutive ermine of the Olympic
Peninsula, state of Washington.
1945. gulosa (Mustela erminea) Hall, Jour. Mamm., 26:84,
February 27, 1945, applies to the diminutive ermine of the
Cascades in Washington.
1945. bangsi (Mustela erminea) Hall, Jour. Mamm., 26:176, July
19, 1945, is the name applied today to the ermine of the western
Great Lakes region.
In 1925 when this study was begun, the American weasels (subgenus
Mustela proper) were arranged as belonging to 47 kinds (including
subspecies) of 29 full species. In the present account a total of 68
kinds, belonging to 4 full species are recognized in the subgenus
Mustela. The increase in number of subspecies and the decrease in
number of species are the nomenclatural results ordinarily obtained in
this decade from a systematic study of a genus of American mammals.
|
Subgenus MUSTELA
Linnaeus |
|
|
|
PAGE |
| Mustela erminea |
87 |
|
Mustela erminea arctica
(Merriam) |
96 |
|
Mustela erminea polaris
(Barrett-Hamilton) |
103 |
|
Mustela erminea semplei
Sutton and
Hamilton |
105 |
|
Mustela erminea
kadiacensis
(Merriam) |
108 |
|
Mustela erminea
richardsonii
Bonaparte |
110 |
|
Mustela erminea
cicognanii
Bonaparte |
118 |
|
Mustela erminea bangsi
Hall |
124 |
|
Mustela erminea invicta
Hall |
128 |
|
Mustela erminea
alascensis
(Merriam) |
131 |
|
Mustela erminea salva
Hall |
135 |
|
Mustela erminea initis
Hall |
136 |
|
Mustela erminea celenda
Hall |
139 |
|
Mustela erminea seclusa
Hall |
141 |
|
Mustela erminea
haidarum
(Preble) |
142 |
|
Mustela erminea
anguinae
Hall |
145 |
|
Mustela erminea
fallenda
Hall |
148 |
|
Mustela erminea
olympica
Hall |
153 |
|
Mustela erminea
streatori
(Merriam) |
155 |
|
Mustela erminea gulosa
Hall |
159 |
|
Mustela erminea muricus
(Bangs) |
161 |
|
Mustela erminea
angustidens
(Brown) |
165 |
| Mustela rixosa |
168 |
|
Mustela rixosa eskimo
Stone |
181 |
|
Mustela rixosa rixosa
Bangs |
184 |
|
Mustela rixosa
allegheniensis
Rhoads |
187 |
|
Mustela rixosa
campestris
Jackson |
190 |
| Mustela frenata |
193 |
|
Mustela frenata
noveboracensis
(Emmons) |
222 |
|
Mustela frenata occisor
(Bangs) |
230 |
|
Mustela frenata
primulina
(Jackson) |
232 |
|
Mustela frenata arthuri
Hall |
241 |
|
Mustela frenata
olivacea
Howell |
244 |
|
Mustela frenata
peninsulae
Rhoads |
250 |
|
Mustela frenata spadix
(Bangs) |
252 |
|
Mustela frenata
longicauda
Bonaparte |
262 |
|
Mustela frenata
oribasus
(Bangs) |
270 |
|
Mustela frenata alleni
(Merriam) |
274 |
|
Mustela frenata
arizonensis
(Mearns) |
276 |
|
Mustela frenata
nevadensis
Hall |
280 |
|
Mustela frenata effera
Hall |
291 |
|
Mustela frenata
washingtoni
(Merriam) |
294 |
|
Mustela frenata
saturata
(Merriam) |
297 |
|
Mustela frenata
altifrontalis
Hall |
300 |
|
Mustela frenata
oregonensis
(Merriam) |
304 |
|
Mustela frenata munda
(Bangs) |
309 |
|
Mustela frenata
xanthogenys
Gray |
315 |
|
Mustela frenata
nigriauris
Hall |
319 |
|
Mustela frenata
latirostra
Hall |
323 |
|
Mustela frenata pulchra
Hall |
328 |
|
Mustela frenata
inyoensis
Hall |
331 |
|
Mustela frenata
neomexicana
(Barber
and Cockerell) |
333 |
|
Mustela frenata
texensis
Hall |
338 |
|
Mustela frenata frenata
Lichtenstein |
341 |
|
Mustela frenata
leucoparia
(Merriam) |
347 |
|
Mustela frenata perotae
Hall |
351 |
|
Mustela frenata
goldmani
(Merriam) |
355 |
|
Mustela frenata
macrophonius
(Elliot) |
360 |
|
Mustela frenata
tropicalis
(Merriam) |
363 |
|
Mustela frenata perda
(Merriam) |
366 |
|
Mustela frenata
nicaraguae
Allen |
370 |
|
Mustela frenata
costaricensis
Goldman |
372 |
|
Mustela frenata
panamensis
Hall |
375 |
|
Mustela frenata
meridana
Hollister |
379 |
|
Mustela frenata affinis
Gray |
384 |
|
Mustela frenata
aureoventris
Gray |
387 |
|
Mustela frenata helleri
Hall |
391 |
|
Mustela frenata agilis
Tschudi |
393 |
|
Mustela frenata macrura
Taczanowski |
398 |
|
Mustela frenata
boliviensis
Hall |
402 |
|
Mustela frenata
gracilis
(Brown) |
404 |
| Subgenus Grammogale
Cabrera |
|
| Mustela africana |
406 |
|
Mustela africana
africana
Desmarest |
409 |
|
Mustela africana
stolzmanni
Taczanowski |
413 |
| Subgenus Putorius
Cuvier |
| (Black-footed Ferret-not treated in present work) |
|
Mustela nigripes
(Audubon
and Bachman) |
|
|
| Subgenus Lutreola
Wagner |
| (Minks-not treated in present work) |
| Mustela vison |
|
Mustela vison vison
Schreber |
|
Mustela vison mink
Peale and
Beauvois |
|
Mustela vison lutensis
(Bangs) |
|
Mustela vison
evergladensis
Hamilton |
|
Mustela vison vulgivaga
(Bangs) |
|
Mustela vison letifera
Hollister |
|
Mustela vison lacustris
(Preble) |
|
Mustela vison
energumenos
(Bangs) |
|
Mustela vison evagor
Hall |
|
Mustela vison
aestuarina
Grinnell |
|
Mustela vison
nesolestes
(Heller) |
|
Mustela vison
melampelus
(Elliot) |
|
Mustela vison ingens
(Osgood) |
|
Mustela macrodon
(Prentiss) |
|
PAGE |
| A Length of upper
tooth-rows
less than 20 mm. in males and 17.8 mm. in females. |
|
| B
Postglenoid length of
skull more than 47 per cent of condylobasal length. |
|
| C
Tail without a
black pencil and with at most a few black hairs at extreme tip; in both
sexes mastoid breadth ordinarily exceeds breadth of braincase, |
|
| Mustela
rixosa, least
weasel, p. |
168 |
| C'
Tail with a black
pencil; in females mastoid breadth ordinarily exceeded by breadth of
braincase, |
|
| Mustela
erminea, ermine,
p. |
87 |
| B'
Postglenoid length of
skull less than 47 per cent of condylobasal length. |
|
| D
Tail with distinct
black tip; midventral line white, yellowish, orange, not same color as
upper parts; p2 present; thenar pad on forefoot absent, |
|
| Mustela
frenata,
long-tailed weasel, p. |
193 |
| D'
Tail without
black tip; midventral line same color as upper parts; p2 absent; thenar
pad on forefoot present, |
|
| Mustela
africana,
tropical weasel, p. |
406 |
| A' Length of upper tooth-rows
more than 20 mm. in males and 17.8 mm. in females. |
|
| E
Abdomen all white; face
with blackish mask; m1 lacking even a trace of a metaconid; distance
between upper canines more than width of basioccipital as measured
between foramina situated midway along medial sides of tympanic bullae, |
|
| Mustela
nigripes, black-footed ferret. |
|
| E'
Abdomen dark brown,
like back; face uniformly brown without blackish mask; m1 with
incipient metaconid; distance between upper canines less than width of
basioccipital as measured between foramina situated midway along medial
sides of tympanic bullae, |
|
| Mustela
vison, mink, American mink. |
|
Genus Mustela Linnaeus
Weasels, Ferrets, Polecats, Minks
Genotype.—Mustela erminea Linnaeus.
Diagnosis.—Legs short; body relatively long; adults 190 mm. to 700
mm. in total length; skull ranging in basilar length from 16 to 70 mm.;
facial angle slight; tympanic bullae greatly inflated (moderately in
Lutreola), cancellous, and with paroccipital processes closely
appressed to bullae; palate behind upper molars; dental formula:
I 3 C 1 P 2-3 M 1
-, -; -, -; -, ---; -, -;
i 3 c 1 p 3-2 m 2
inner moiety of M1 larger than outer; P4 with simple deuterocone; in m1 trigonid longer than
talonid, metaconid absent (incipiently developed in Lutreola), and
talonid trenchant.
For many years prior to 1911, the name Mustela was applied to
martens, and Putorius was regarded as the first available generic
name for the weasels. In 1911 Thomas (1911:139) showed that M.
erminea (Mustela of Gesner) by tautonymy was the type of Mustela
and subsequently the generic name Mustela has been used for the true
weasels which include the American weasels to which we now apply the
specific names erminea, rixosa and frenata. The mink, Mustela
(Lutreola) vison, and the black-footed ferret, Mustela (Putorius)
nigripes, since 1911 also have been referred by most American authors
to the genus Mustela, the names Lutreola and Putorius being
regarded by these authors as having no more than subgeneric status.
European writers, on the other hand, accord greater taxonomic weight to
the zoölogical differences between ferrets and weasels and, therefore,
accord full generic rank to Putorius. Consequently, for the
black-footed ferret, Europeans today write Putorius nigripes and
Americans write Mustela nigripes. For the same reasons, the name of
the mink is written by some European zoölogists Lutreola vison and by
American zoölogists Mustela vison.
For each full species there will be found under the account of it the
following information: Type, statement of geographic range, selected
characters for ready recognition, other characters of the species, a
summary of geographic variation, and information on habits, in the
order mentioned.
For each subspecies, information is presented in the following order:
earliest available zoölogical name, synonyms, type, geographic range,
zoölogical characters for ready recognition, description (mentioning
size, certain external features including color, the skull and teeth)
historical material when warranted, remarks which may elaborate on
points made in preceding paragraphs, and other information thought to
be useful, and finally a list of specimens examined.
In explanation of certain of these categories it should be said that in
the synonymy no attempt is made to list every published reference to
the subspecies concerned. It is aimed, however, to include at least one
citation to each name-combination that has been applied, to the
subspecies concerned, along with other especially important references.
Mere records of occurrence are not regarded as especially important and
citations to them ordinarily are omitted in the synonymy. No comma is
placed between the zoölogical name and the name of the author who
coined and first used the name in accordance with the rules of
zoölogical nomenclature. Otherwise a comma is interposed between the
zoölogical name and the name of the user (author). When the accepted
(earliest available) name of a subspecies at the head of any one of the
following accounts is combined with a generic name different from that
with which
it originally was placed, the authority for the name is set
in parentheses. The same rule is followed with the name of a full
species when it is written without any subspecific name following.
Parentheses in such situations, therefore, denote that for the terminal
part of the scientific name there has been a change in generic name
with which the terminal part of the scientific name is here associated.
In the paragraph headed "characters for ready recognition," only a few
characters, namely, those regarded as most useful for identification
when the student has limited time, are mentioned. Other features useful
for distinguishing the kind of animal in question from its near
relatives are to be found in the description and comparisons.
In the description, external measurements, unless otherwise indicated,
are those recorded by the collector on the label attached to the skin.
Total length is the distance from the tip of the pad on the nose to the
tip of the fleshy part of the tail when the relaxed animal is laid out
straight, not stretched. This measurement does not include the hairs
that project beyond the end of the fleshy part of the tail. Length of
tail is the distance from the base of the tail, when it is bent at
right angles to the long axis of the body, to the tip of the fleshy
part of the tail excluding the hairs that project beyond the fleshy
part of the tail. Length of tail and length of tail-vertebrae are
synonymous. Length of hind foot is measured from the proximal end of
the calcaneum to the tip of the longest claw.
Capitalized color terms, unless otherwise indicated, refer to Ridgway's
(1912) Color Standards and Color Nomenclature. Some use is made of
color terms taken from Oberthür and Dauthenay (1905) because those
authors show a much larger number of shades between dark brown and
black than does Ridgway (1912). The colors of the upper parts of most
weasels are some shade or other of dark brown. Color terms that do not
have the initial letter capitalized do not refer to any one standard
and consequently are used in a general sense.
Relative extents of the color of the upper parts and underparts are
computed from measurements of the circumference of the body at the
place where the color of the underparts is narrowest. Ordinarily this
place is in the lumbar region rather than in the thoracic region.
An explanation of how cranial measurements were taken is given on page
417. In designating teeth, capital letters are used for teeth in the
upper jaw and lower case letters are used for teeth in the lower jaw.
For example: I2 denotes the second incisor tooth in the upper jaw and
i2 denotes the second incisor tooth in the lower jaw; C1 and c1 refer
to the canine tooth of the upper jaw and lower jaw, respectively; P3
and p3 refer to the third premolar of the upper jaw and lower jaw,
respectively, bearing in mind that the first (anterior) premolar is
absent in the lower jaw and upper jaw of weasels (see fig. 31 on page
416), as also, in some kinds of weasels, is the second premolar; M1 and
m1 refer to the first molar of the upper jaw and lower jaw
respectively.
In describing the skull and teeth the two sexes are treated separately
because differences in shape as well as size are the rule. Unless
otherwise indicated, the skulls on which descriptions are based are of
adults. Weights of skulls include the weight of the lower jaws. In
general, every second subspecies is described. For a subspecies
geographically next adjacent to the
one described, only the
differences between the two are enumerated. This method of description
indicates also likenesses and is more economical of words than some
other methods of description. Also, by use of this method, cross
reference is reduced to one other subspecies. Following this formal
description, there is a comparison of the cranial and dental characters
with those of geographically adjacent subspecies.
In the paragraph headed "Remarks" the two words "character" and
"structure" frequently appear. The word structure here is used to mean
some part of an animal, as for example, a hair, a muscle, a bone, or an
internal organ. A structure is not a system, as for example, the
digestive system or osseous system. A character is some weight, linear
dimension, volume, shape, color, or other perceptible attribute of a
structure, of a system, or of an entire organism.
In recording the localities of capture of specimens examined, effort
has been made to be exactly as precise as the locality data on the
labels of the specimens permit. The word "County" is written out in
full when the name of the county is written on the label of each
specimen listed from that county. When one specimen, or more, here
assigned to a given county lacks the name of the county on the label,
then the abbreviation "Co." is used. The surprising frequency with
which the same place name is repeated in a given state or province
makes it desirable for the collector to write the name of the county,
or corresponding minor political subdivision, on labels of study
specimens at the time they are prepared.
MUSTELA ERMINEA Linnaeus
Ermine
(Synonymy under subspecies)
Type.—Mustela erminea Linnaeus, Systema Naturae, 10th ed., p.
46, 1758.
Range.—From the British Isles and Atlantic Coast of Europe
across Eurasia and North America including Greenland, from the
northernmost land, south, in North America, to the lower margin of
the Canadian Life-zone; geographically south to Connecticut, New
York, Pennsylvania, northeastern Ohio, southern Michigan,
Wisconsin, northern Iowa, Minnesota, North Dakota, in the Rocky
Mountains to northern New Mexico, in the Sierra Nevada to Mono
County, California, and on the Pacific Coast to the Golden Gate.
Characters for ready recognition.—Differs from Mustela rixosa in
presence of black pencil on tail, tail-vertebrae more than a fourth of
length of head and body, and in regions where the two species occur
together, basilar length of skull more than 32.5 in males and more than
31.0 in females; from Mustela frenata, in regions where the two
species occur together, by tail less than 44 per cent of length of head
and body and by postglenoidal length of skull more than 46 per cent of
condylobasal length in males and more than 48 per cent in females.
Characters of the species.—Size medium to small (total length 225 to
340 mm. in males and 190 to 290 mm. in females); tail 30 to 45 per cent
of length of head and body, with distinct black pencil; caudal
vertebrae 16 to 19; skull with long braincase and short precranial
portion; postglenoidal length, when expressed as a percentage of the
condylobasal length, more than 48 in females and ordinarily more than
46 in males; upper parts brown; underparts whitish, ordinarily
continuous from chin to inguinal region but in subspecies in the humid
region along the Pacific Coast interrupted in some individuals by brown
of upper parts encircling body in the abdominal region. The soles of
the feet in each of the subspecies are densely haired in winter and
have only a relatively small area of the foot-pads exposed in summer,
the intervening areas being well haired even at that season. The
uniformity throughout the species as regards hairiness of the
foot-soles and also the character of the vibrissae makes it unnecessary
to describe these features in the accounts of the subspecies of
erminea.
Geographic variation.—In the Old World 16 or more subspecies are
currently recognized and there are 20 in North America. The features in
which geographic variation is especially prominent are:
First, size,
as expressed by external measurements and weight, second, color
pattern, depending on the extent, in relation to one another, of the
dark-colored upper parts and light-colored underparts, and third,
breadth and depth of the rostral region of the skull. Except in size,
the variation in the skull is less than in M. frenata. Likewise in
tone and shade of upper parts and hue or tint of underparts, erminea
is less variable than frenata and has the face all of one color
without the contrasting color-pattern of the face and head seen in many
subspecies of frenata. M. erminea exceeds frenata as regards
variation of the size of the area occupied by the light-colored
underparts. At one extreme is the subspecies arctica in which the
area of the light color extends well up on the sides of the body, down
the insides of the legs, over the feet and far out on the lower side of
the tail whereas at the other extreme are the races streatori and
olympica in which the light-colored underparts are restricted to two
areas, one on the chin, throat and chest, and the other on the inguinal
region. These areas may or may not be connected by a thin line of white
color along the midline of the underparts. In size of animal, erminea
probably exhibits the maximum variation among American species of
weasels; an average-sized male of the race arctica weighs 4 times as
much as one of the race muricus, and in the species frenata I doubt
that the difference is quite as great between individuals of the
smallest race, effera, on the one hand, and either of the largest
races, texensis or macrophonius, on the other hand although actual
weights are not available for these races of frenata. As elsewhere
indicated, the small-sized individuals of M. erminea are of the
southern races and the large-sized individuals are of the northern
races. This decrease in size southward occurs both in Asia and in
America.
Natural History.—Habitat and numbers.—Along the International
Boundary east of the Turtle Mountains, Soper (1946:136) found this
species present only in timbered areas and absent from many untimbered
areas. Of the same species to the westward he comments "so far as I
know at present, there is no evidence to show that any short-tailed
weasels inhabit a broad strip of treeless territory immediately north
of the International Boundary in Canada from southwestern Alberta to
southeastern Saskatchewan." The same author (1942) reports that in the
general area of Wood Buffalo Park, Northwest Territory, south of Great
Slave Lake, the ermine is uncommon on pine-grown sand ridge and rolling
upland and common in lower spruce-aspen parklands, stream-side
coniferous belts, and grassy, semi-wooded swamplands.
Nine ermines per square mile is the number that Soper (1919:46-47)
estimated at Edmonton on the basis of the numbers that he trapped there
in the winters of 1912-13 and 1913-14 and on the basis of the tracks of
remaining ermines. From corresponding data he estimated the population
in the winter of 1913 on the Hay River, north of Jasper Park, to be
nine per square mile. In each of these instances he estimated ten
weasels per square mile but he inclined to the view that one-tenth of
the animals involved in his counts were long-tailed weasels (Mustela
frenata). Osgood (1909B:30) and his field companion in the period July
31 to September 3, 1903, took a series of 42 specimens within a radius
of 500 yards of their camp at the head of Seward Creek, Alaska, all
caught in four traps, in one month. Of the 42 specimens, 28 are males
and 14 are females.
Fluctuations of a multiannual nature are marked in this species. Bailey
(1929:156) observes that in Sherburne County, Minnesota, when meadow
mice are abundant for two or three years these weasels become abundant
but that when the mice are scarce the weasels also become scarce.
Manning (1943:56), on Southampton Island, noted "that the maximum and
minimum points of the weasel cycle are much more sharply marked than
those of the fox cycle and the increase and decrease are more rapid."
How far an ermine will travel in a given length of time has seldom been
recorded but Hamilton (1933:293), on March 20, 1932, "followed the
track of a small weasel, presumably a male cicognanii, for four miles
in the fresh snow," and Ingles (1942) observed a diminutive ermine of
the subspecies M. e. muricus, at Woods Lake, California, 286 yards
from its den.
Behavior
As regards locomotion, Soper (1919:46), in reference to Mustela
cicognanii, presumably in Ontario, Canada, writes that in the bounding
gait the hind feet register almost, if not exactly, in the front-foot
impressions, with the right front and hind feet lagging slightly
behind. "The distance normally is about 19 inches, representing a
regular rate of travel. . . . In traversing open spaces they resort to
long, graceful leaps upwards of six feet in length. . . . I measured a
record . . . of 8 feet, 2 inches."
Of M. e. arctica, Dice (1921:22) writes that when it runs "the tail
is carried off the ground usually at an angle of about 45 degrees."
Seton (1929 (2):598) states that "At Carberry [Manitoba] I have often
seen this energetic little creature seeking for Mice in the deep,
soft
snow. Its actions are much like those of an Otter pursuing salmon.
Sometimes it gallops along a log, or over an icy part of the drift;
then plunges out of sight in a soft place, to reappear many yards
away. . . ."
Little is recorded concerning swimming but on this score Seton (1929
(2):602) does quote J. W. Curran, who in July, 1899, at Lake
Couchiching, Ontario, watched an ermine pursue a chipmunk into the
water and for 100 yards before giving up the chase and wheeling around
and making for shore. In swimming "The Weasel, I think, showed more of
his body, and seemed to exert himself more" than the chipmunk.
As to voice, Dice (1921:22), at Tanana, Alaska, heard the ermine, when
excited, bark somewhat like a mink but not so loud and Seton (1929
(2):606) quotes Manley Hardy to the effect that the species has a
purring note.
Sense of smell was used by an M. e. muricus that Dixon (1931:72)
watched as the ermine followed a three-fourths-grown pika. Concerning
the ermine at Carberry, Manitoba, Seton (1929 (2):598-599) writes that
"The smell of blood must be as far-reaching as it is attractive to
these sanguinary little creatures. I have frequently hung new-killed
Rabbits and partridges temporarily in trees, and, after an absence, in
some cases of a few minutes only, have found an Ermine mauling the
game, though there was no sign of such a visitor when the cache was
made."
Enemies
George Measham, of Winnipeg, found sign in the snow indicating that a
great snowy owl had killed an ermine and T. McIlwraith shot a bald
eagle at Hamilton Bay which had the bleached skull of a weasel
(probably of this species) clinging to the throat (Seton, 1929
(2):603).
A. B. Howell (1943:98) likens mustelid mammals to domestic cats in
their manner of crossing roads and thinks that mustelids loiter at the
side of the road until the stimulus of the approaching car causes them
to make a dash whereupon they are caught by the wheels and killed.
Three of four weasels seen to cross the road were killed, one even
having apparently crossed the road before turning back and being killed
under the car. One weasel killed was Mustela erminea cicognanii.
Dalquest (1948:190) in writing of this species in the state of
Washington, says "I have seen only one abroad in the daytime. It dashed
from a roadside thicket . . . and was crushed beneath the wheels of a
car."
Food
The killing of prey is described by Hamilton (1933:332) as follows: "A
rapid dash, and the bird or mouse is grabbed over the back of the
skull, the fore legs encircle the animal as though hugging it, and the
hind legs are brought up to scratch wildly at the captive. . . . If
[the prey is] a large animal, as a rat, the weasel usually lies on its
side, while the diminishing struggles of the rodent continue, but if a
mouse or a small bird [is the object of attack], the weasel is apt to
crouch over its prey. Little time is lost over the first [mouse] . . .
if two mice are present [;] a strong bite through the brain case . . .
[is] sufficient. If only one animal is present, the weasel dawdles over
its kill some time after life has departed."
Hamilton's (1933:333) study of the contents of the digestive tracts of
bodies of ermines obtained from fur trappers and fur buyers in New York
enabled him to publish the following "Frequency Indices of Mammal
Genera in Fall and Winter Food of 191 Mustela cicognanii": Microtus,
35.7 per cent; mammals undetermined to genus but principally mice,
16.3; Blarina, 15.1; Peromyscus, 11.4; Sylvilagus, 9.0; Sorex,
4.9; Rattus, 4.4; Tamias, 3.6. Close correspondence is shown by the
following data of Aldous and Manweiler (1942) for the ermine from Lake
of the Woods, Minnesota: mice, 58.7 per cent by number and 54.5 by
volume; shrews 22.5 and 21.8 per cent; birds, 2.7 and 5.0 per cent. Of
the mice in stomachs, 40 per cent were microtines, 15 per cent were
Peromyscus and 45 per cent were unidentified as to kind. Fragments of
a small fish were found in one stomach. Summed up, the dominant winter
foods were mice and shrews. Trapping of the mammal populations was done
to see what the available food was and it was found that the small
mammals were eaten in direct ratio to their relative abundance.
Snowshoe rabbits and red squirrels were not eaten. The Minnesotan data
were from 60 stomachs and 53 intestinal tracts recovered from 129
weasels trapped by use of scent (not bait) mostly from January 1 to
February 7, 1939, although a few were trapped in 1938. Analyses of
contents from stomachs gave approximately the same results as those
from intestines. In 1939 at Lake of the Woods, weasels were
concentrated where food was abundant but no such concentration was
noted in the following winter.
Big short-tailed shrew (Blarina brevicauda).—In New York State, the
ermine preys on Blarina as shown by Hamilton's (1933:330) seeing one
being carried by a male ermine on May 6, 1931, and another being
carried by a female on May 13, 1932. The same
author (1928:249) found
the remains of a Blarina in a small female from Malone, New York.
Kirk (1921) observed, however, that the ermine (M. e. cicognanii)
avoided the shrew, Blarina, caught in a trap and that Blarina
avoided the weasel caught in a trap.
Chipmunk (genus Tamias).—Remains were found in a male ermine in New
York on May 14, 1932 (Hamilton, 1933:330), and Seton (1929 (2):602)
records a chipmunk at Lake Couchiching, Ontario, that was pursued into
the water by an ermine.
Deer mice (genus Peromyscus).—As shown by Hamilton (1933:33) and
Aldous and Manweiler (1942), Peromyscus was second only to microtines
in numerical abundance among the food items of ermines in New York and
Minnesota. Peromyscus and microtine rodents were brought to a den of
the diminutive M. e. muricus in early August, in Fresno County,
California, according to Ingles (1942). He observed that an Alpine
chipmunk was active under and around the tree and that juncos reared
young 40 feet from the den but that the chipmunk and juncos were
unmolested by the ermines.
Lemming (genus Lemmus).—One was recovered from a female ermine (with
milk in her glands) at Laurier Pass, British Columbia (Sheldon,
1932:201).
Red-backed mouse (genus Clethrionomys).—Criddle and Criddle
(1925:146) record that on "May 31, 1921.—Saw a Bonaparte's weasel
capture a Red-backed Vole after a long hunt during which the pursuer
never once lost track of its victim."
Meadow mice (genus Microtus).—As shown by the data of Hamilton
(1933:333) and Aldous and Manweiler (1942) recorded above, Microtus
is the item of first importance in the diet of the ermine in New York
and Minnesota. Criddle and Criddle (1925:146) write concerning the
vicinity of Treesbank, Manitoba, that "October, 1918.—Following a
severe outbreak of mice in 1916-17, Bonaparte's weasel increased
enormously and very soon reduced the rodents to comparative rarity.
This resulted in a scarcity of food for the weasels, which in their
turn became greatly reduced in numbers."
Old World rat (Rattus).—Bishop (1923) found two headless rats near a
nest of this species in Albany, New York.
Pika (Ochotona).—Dixon (1931:72) at Milner Pass, Colorado, on July
20, 1931, saw an ermine, of the subspecies muricus, following a
three-fourths grown pika by scent and outrunning the pika. The pikas
worked a relay system and the weasel abandoned the trail when the
fourth pika became the object of the chase.
Cottontail (genus Sylvilagus).—Hamilton (1933:33), as noted above,
found remains of cottontail in the digestive tracts of ermine that had
been trapped for fur in winter. Possibly these remains were bait that
had been placed at traps.
Snowshoe rabbit (Lepus americanus).—Morse (1939:210) in a study of
predation on hares and grouse in the period of notable decimation of
these two game species in 1935-1936 in the Cloquet Valley State Forest,
in St. Louis County, Minnesota, found that "weasel predation on hares
appeared to be of very low incidence or altogether lacking."
Wild birds (Class Aves).—Aldous and Manweiler (1942), as noted above,
found that the remains of birds constituted five per cent by volume of
the food of the ermine in winter in Minnesota.
Chicken (genus Gallus).—Criddle and Criddle (1925:145), who
published relatively extensive data on the three species of weasels of
Manitoba, write that: "We have no record of Bonaparte's weasel killing
poultry, and we doubt whether it ever does so." However, Soper
(1919:46) investigated the excited cackling of a hen brooding chicks at
night and found a solitary ermine that had killed three chicks and that
had the remainder under very active scrutiny.
Leopard frog (Rana pipiens).—One frog was found in a male ermine on
November 20, 1931, in New York by Hamilton (1933:300).
Fish (Class Pisces).—Aldous and Manweiler (1942) found fragments of a
small fish in one of 60 stomachs of ermine from Minnesota.
Earthworm (Phylum Annelida).—Osgood (1936:64), presumably at Rutland,
Vermont, observed a pair of weasels from 2:15 P.M. to 5:00 P.M., in a
barn and saw the female in that time make many trips for food for her
young. Only earthworms were brought. Fifty traps in an adjacent, swampy
field caught only one bull frog and no mice indicating that mice had
been eliminated from the foraging territory of the ermine.
In handling food, Dice (1921:22) noted that the Alaskan ermine did not
use the feet but only the mouth.
Reproduction
Litters of 4, 4, 7, 7, and 8, yielding an average of 6 young per litter
have been recorded from the northeastern United States by Hamilton
(1933:327). He (op. cit.:321-325) described animals one day old from
New York State as being flesh-colored, having the long neck of the
adult and a fine growth of white hair two milli
meters in length, on
the dorsal surface of the neck, that foreshadows the mane or pompadour
that is prominent from the 14th to the 21st day of life. Six animals,
when one day old averaged 1.7 grams in weight, which was three per cent
of the weight of an adult female and one and one half per cent of the
weight of an adult male. At two weeks of age the heavy brown mane stood
out in marked contrast to the rest of the scantily, white-furred
animal. The eyes opened on the thirty-fifth day of life.
Fig. 24. Mustela erminea richardsonii, adult female,
Catalogue Number 14866, U. S. Nat. Mus., Fort Chimo, Ungava. × 1/2.
Ventral view of body of a pregnant female to show details of mastology.
Note the five pairs of mammae characteristic of weasels, and the uneven
arrangement of mammae of the two sides which is also common among
weasels.
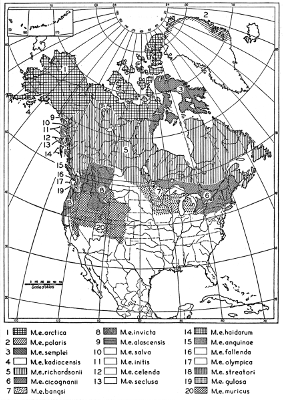
Fig. 25. Map showing geographic ranges of the subspecies
of Mustela erminea in the New World.
For rearing their young, ermines live in burrows. Bishop (1923), in
Albany, New York, found a burrow occupied by four young and a pair of
adults. The burrow had many galleries and contained a nest constructed
of rat fur, fine grass and fragments of leaves.
At Woods Lake, Fresno
County, California, in early August, Ingles observed (1942) some young
and at least one adult at their den which was in a burrow beneath a
hollow tree. The ermines used the hollow root and the hollow tree as
well as the burrow beneath. Seton (1929 (2):591) quotes S. Eldon
Percival, of Barretts Rapids, Ontario, as finding the living quarters
of an ermine in unthreshed grain stacked in a barn and says (op.
cit.:590) that John Burroughs dug out a nest, composed of leaves and
the fur of mice and moles, two or three handfuls in bulk, from a cavity
the size of a hat, arched over with a fine network of tree roots.
Four instances in which the male as well as the female was present at a
den containing young are cited by Hamilton (1933:328) and he gives some
evidence, although not at all conclusive, that "adults customarily
pair, or at least run together, at times other than the breeding
season." No other writers remark on this matter. I doubt that adult
ermines are associated in pairs for most of the year but such may be
the case.
Mustela erminea arctica (Merriam)
Ermine
Plates 2, 3, 4, 9, 10, 11 and 41
Putorius arcticus Merriam, N. Amer. Fauna, 11:15, pl. 2, figs. 1,
1a, and pl. 5, figs. 6, 6a, June 30, 1896.
Putorius (Gale) erminea, Coues, Fur-bearing animals, p. 109,
1877 (part).
Putorius richardsonii, Bangs, Proc. Biol. Soc. Washington, 10:16,
pl. 1, figs. 3, 3a, pl. 2, figs. 3, 3a, and pl. 3, figs. 6, 6a,
February 25, 1896 (part).
Putorius cicognanii alascensis, Osgood, N. Amer. Fauna, 19:43,
October 6, 1900.
Putorius kadiacensis, Osgood, N. Amer. Fauna, 21:69, September
26, 1901.
Putorius audax Barrett-Hamilton, Ann. and Mag. Nat. Hist.,
13(ser. 7):392, May, 1904, type from Discovery Bay, Ellesmere
Island.
Putorius alascensis, Heller, Univ. California Publ. Zoöl., 5:345,
March 5, 1910.
Mustela arctica arctica, Miller, U. S. Nat. Mus. Bull., 79:97,
December 31, 1912; Dice, Journ. Mamm., 2:22, February 10, 1921.
Mustela arctica, Hall, Univ. California Publ. Zoöl., 30:420,
March 19, 1929.
Mustela erminea arctica, Ognev, The mammals of U.S.S.R. and
adjacent countries, 3:31, 1935; Hall, Proc. California Acad. Sci.,
23:559, August 22, 1944; Hall, Journ. Mamm., 26:179, July 19,
1945.
Type.—Male, adult, skull and skin; no. 14062/23010, U. S. Nat.
Mus.; Point Barrow, Alaska; July 16, 1883; obtained by John
Murdock, original no. 1672.
The skull has a fracture, on the dorsal surface, extending from
the anterior nares to the interorbital constriction and another
fracture on the left margin of the nasal bone. The middle of the
left zygomatic arch is broken away. Otherwise the skull is
complete. Right incisor one, above and below, are missing.
Otherwise the teeth are present and entire. The skin is in the
brown summer pelage, well made, in a good state of preservation,
and shows no obvious signs of fading.
Range.—Arctic regions of Alaska and western Canada from the
Pacific Ocean to Smith Sound; from the northern limit of land
south approximately to a line from Skagway through Ft. Goodhope,
north shore of Great Bear Lake, south shore of Clinton Colden
Lake, north shore of Baker Lake, west end of Wagner Bay to south
end of Committee Bay. See figure 25 on page 95.
Characters for ready recognition.—Differs from M. e. polaris
in darker upper parts (Raw Umber rather than Buckthorn Brown) and
less intensely colored underparts that are Sulphur Yellow,
Colonial Buff or Primrose Yellow rather than Buff Yellow; from M.
e. semplei, in males, in that hind foot more than 44 and basilar
length more than 41 and in that females average larger, the skulls
of females being only about 11 per cent heavier; from M. e.
kadiacensis in hind foot more than 33 in females, zygomatic
breadth amounting to more, rather than less, than distance between
last upper molar and jugular foramen irrespective of sex; from M.
e. richardsonii, alascensis, salva and initis, both sexes
so far as known, by proximal two-thirds of under side of tail
colored same as underparts rather than same as upper parts, and by
zygomatic breadth amounting to more, rather than less, than
distance between last upper molar and jugular foramen.
Description.—Size.—Male: Six adults from Tanana, Alaska, yield
average and extreme measurements as follows: Total length, 336
(310-350); length of tail, 93 (84-105); length of hind foot, 49
(45-51).
Female: Five adults, one each from Alatna River, mountains near
Eagle, Kamarkak in Alaska, Arctic Red River and Baillie Island in
Canada, yield average and extreme measurements as follows: Total
length, 285 (272-304); length of tail, 77 (68-95); length of hind
foot, 39 (34-43).
Weight of 5 subadult males from Tanana is 206 (163-248) grams;
adults would be heavier.
Color.—Winter pelage all white except tip of tail. Summer
pelage with upper parts uniform in color and Raw Umber or darker
(16n) of Ridgway and about tones 2 to 3 of Chocolate of Oberthür
and Dauthenay, pl. 343, but in autumn some specimens have more
light red than tones 2 or 3. Underparts Sulphur Yellow, Colonial
Buff, or Primrose Yellow, often white on chin and insides of
forelegs; color of underparts extends narrowly over upper lips,
distally on posterior sides of forelegs onto antipalmar surface of
forefeet, onto proximal two-thirds or three-fourths of underside
of tail as length of tail is measured along tail-vertebrae, on
medial sides of hind legs to a point between knee and ankle but
reappears on antiplantar faces of toes and in some individuals is
narrowly continuous onto toes; rim of ear in some specimens with
short, white or pale hairs giving ears distinct whitish border;
least width of color of underparts averaging, in adult males from
Alaska, 65 (46-93) per cent of greatest width of color of upper
parts. Black tip of tail in 5 males in winter pelage from Tanana
averaging 84 (70-93) mm. which is 91 (75-107) per cent of length
of tail-vertebrae.
Skull.—Male (based on 5 adult topotypes): See measurements and
plates 2-4. As described in Mustela erminea richardsonii except
that: Weight, 3.5 (3.1-3.9) grams; basilar length 42.5
(41.8-43.3); length of tooth-rows more than length of tympanic
bulla; breadth of rostrum measured across lacrimal processes
averaging more than a third of basilar length; interorbital
breadth
more than distance between glenoid fossa and posterior
border of external auditory meatus; zygomatic breadth more than
distance between last upper molar and jugular foramen.
Female (based on 2 adult topotypes and 2 adults and 4 subadults
from central Alaska): See measurements and plates 9-11. As
described in Mustela erminea richardsonii except that: Weight,
1.5 (1.2-2.0) grams; basilar length, 35.7 (34.5-37.0); length of
tooth-rows more than length of tympanic bulla; breadth of rostrum
more than 30 per cent of basilar length; interorbital breadth more
than distance between glenoid fossa and posterior border of
external auditory meatus; zygomatic breadth more than distance
between last upper molar and jugular foramen (except in specimens
from Ellesmere Island where two distances are approximately
equal).
Cranial differences from Mustela erminea kaneii (which occurs on the
Asiatic side of Bering Strait), in both males and females, are: larger
size relatively as well as actually, broader except in mastoidal region
where relatively (to basilar length) the width is less; preorbital part
of skull broader as well as longer.
From kadiacensis differences in the skull of the male are: size less;
13 per cent heavier, relatively (to basilar length) narrower across
interorbital region and zygomatic arches; tympanic bullae relatively as
well as actually narrower. Judging by the single available adult female
of kadiacensis, the skull of female arctica is larger in all parts
measured, a fourth heavier, has tympanic bullae of almost twice the
volume and the interorbital and preorbital regions, relative to the
braincase, are much reduced in whatever plane measured.
Differences from richardsonii, additional to those noted above in the
formal description of the skull, between the males, are: larger in all
parts measured except length of tympanic bulla which is about the same;
42 per cent heavier; relative to basilar length, skull broader with
preorbital part longer as well as broader; tympanic bullae more
inflated posteriorly. The same differences prevail between females
except that the skull is 36 per cent heavier and in arctica the
length of the bulla is actually more (although relative to the basilar
length less) and its greater inflation posteriorly is hardly
perceptible. Differences from alascensis, additional to those
indicated in the formal descriptions of the skulls of the two, in
males, are: larger in every part measured; 95 per cent heavier;
relative to the basilar length, skull broader with preorbital part
longer as well as broader; measured at a point opposite the foramen
lacerum anterius, the width of the pterygoid space is more, rather than
less, than 40 per cent of its length. Excepting this difference in
width of interpterygoid space, the same differences prevail between
females, those of arctica being 56 per cent heavier.
Comparison with semplei is made in the account of that subspecies.
Skull indistinguishable from that of polaris.
Remarks.—The person who studies specimens of this subspecies finds
labels inscribed with the names of naturalists well known to all
readers of literature on the Arctic. Sir John Franklin, R. McFarlane,
R. Kennicott, E. W. Nelson and R. M. Anderson are names which appear
commonly. Of Alaskan specimens prepared according to modern methods, a
large share was obtained by O. J. Murie and L. R. Dice.
The ermine was observed in the far north by early explorers and was
mentioned in the literature, almost always under the name then used for
the ermine of northern Europe and Asia. In 1896 Bangs misapplied to it
the name richardsonii but Merriam in the same year corrected the
application of this name and proposed as new for this weasel the name
arctica, the name in use today. For almost 50 years after Merriam and
Bangs wrote about it, arctica was treated, nominally at least, as a
species distinct from its other relatives in both the Old- and
New-World. The subspecific status of arctica was emphasized in 1944
(555) by the present writer in reporting in detail upon the specimens,
of Mustela erminea, from Eastern Asia which were made available on
loan by Professor B. S. Vinogradov and the late Anatol I. Argyropulo of
the Leningrad Academy of Sciences. Specimens of Mustela erminea
kaneii from the Asiatic side of Bering Strait and Mustela erminea
arctica from the American side are distinguishable by slight cranial
characters but in coloration and external measurements I can detect no
differences. Merriam's (1896:16) mention of more golden-colored upper
parts and darker underparts in American specimens than in erminea was
the result of his comparison of Alaskan and northern European
specimens. When Old World specimens from eastern Siberia, instead of
from Europe, are used the differences mentioned by Merriam do not
apply. Incidentally, many Siberian specimens have the white border, on
the ear, which Merriam (loc. cit.) noted as a distinguishing feature
of arctica. When Merriam named arctica he said (1896:15, 16)
"Putorius arcticus . . . has heretofore been confounded with
erminea or richardsonii. . . . It is interesting to find in this
country an Arctic circumpolar weasel which, though specifically
distinct, is strictly the American representative of the Old World
erminea." Bearing in mind that Merriam's concept of species and
subspecies (see Merriam, 1919:6) differed from that of nearly all
modern systematists it is clear from his statement quoted
above that
he correctly understood the zoölogical relationship obtaining between
the ermines of the Old and New Worlds.
Ognev (1935:31) seems to have been the first to use the name
combination Mustela erminea arctica for Alaskan specimens. Thereby he
expresses the view adopted here, namely that the American ermine is
subspecifically but not specifically distinct from the Old World
animal. Whether actual intergradation (crossbreeding) ever takes place
across the narrow Bering Strait I do not know. I doubt that
crossbreeding occurs but considering the Diomedes (islands), that might
serve as a half way stopping point, and remembering Mr. Charles
Brower's oral statement to me that he had seen tracks of ermine as far
as 10 miles from the northern shore of Alaska out on the ice, the
possibility must be granted of an occasional individual crossing from
one side to the other of Bering Strait on the ice in winter or of being
carried across when the ice broke up and drifted. If transfers of this
kind occurred often one would expect ermines to occur also on Saint
Lawrence Island where apparently they do not. The one skin (U. S. Nat.
Mus. no. 259046) seen as labeled from there, my friend, Otto William
Geist ascertained was imported as a skin with other furs from Siberia.
Ognev (op. cit.) who used the name combination Mustela erminea
arctica for Alaskan specimens, applied it also to animals from
Kamchatka. At the same time he recognized the animal from the eastern
mainland of Siberia (as opposed to the peninsula of Kamchatka) under
the name Mustela erminea orientalis Ognev 1928. Hall (1944:556)
applied the earlier proposed name Putorius kaneii Baird 1857, to the
animal on the eastern mainland of Asia and proposed the new name
Mustela erminea digna for the ermine of Kamchatka. In comparing
material of these two Asiatic races with topotypes and other specimens
of M. e. arctica from Alaska, it seemed to me that the degree of
relationship, one with the other, was about the same. M. e. digna has
a slightly larger preorbital region than M. e. kaneii, and the skull
is longer. In both of these particulars digna approaches closer to
arctica. M. e. kaneii has longer tympanic bullae and a wider skull
than digna and therein approaches more towards arctica than toward
digna. As nearly as I can make out, digna and kaneii show a
nearly equal degree of resemblance to arctica. Also the degree of
difference between digna and kaneii is about the same as between
either one of them and arctica. In view of the above considerations
the ermines of the New and Old worlds are here regarded as only
subspecifically distinct.
In the original description of Putorius audax (here regarded as
inseparable from Putorius arcticus Merriam) Barrett-Hamilton
erroneously designated the type locality as "Discovery Bay, North
Greenland" whereas he should have written Grinnell Land [= Ellesmere
Island of modern terminology] in place of Greenland. As reference to
Nares (1877 and 1878) will readily reveal, Discovery Bay is near 65° W
and 81° 40´ N, across Robeson Channel, to the west, from Greenland. The
label on the type specimen and the specimen register in the British
Museum of Natural History each designates the locality for this
specimen, the type of audax, as Discovery Bay without mention of
Greenland. The published accounts of Feilden (1878) and Nares (1877 and
1878) state that specimens of ermine were obtained at Discovery Bay.
Probably H. C. Hart is the collector of the specimen; he was the
naturalist attached to H. M. S. Discovery which wintered at Discovery
Bay while H. W. Feilden was the naturalist attached to H. M. S. Alert
which wintered a few miles southeast of Cape Sheridan, also on the
eastern coast of Ellesmere Island.
It is true that from these ships a trip was made into Greenland and an
ermine (only one individual it seems) was obtained there, but this
individual was the type specimen of Mustela erminea polaris, in the
account of which race something of the history of this specimen is
given.
With the material available—and it is not entirely adequate—I can
detect no features by which animals from the type locality of audax
can be distinguished from typical arctica which latter name has
priority.
Intergradation with richardsonii probably occurs completely across
the continent. Intergrades here referred to arctica include those
from Fort Goodhope. The one defective specimen from Lake Lebarge,
Yukon, is not certainly identified as arctica and how far west of
Teslin Lake the boundary-line between arctica and richardsonii
should be drawn remains to be ascertained. The one specimen available
from Hinchenbrook Island, no. 912 Mus. Vert. Zoöl., an adult female, is
doubtfully referred to arctica because the damaged tympanic bullae
appear to be no larger than in alascensis, and the size of the skull
is more as in alascensis although intermediate between that race and
arctica. Shape of the skull is more as in arctica. Possibly more
nearly adequate material would show the existence on Hinchenbrook
Island of an insular race differing in about the same degree from
arctica of the mainland as does the insular kadiacensis.
Nevertheless, the males from farther south at Cape Yakataga are in all
respects arctica and this argues against near
relationship to
alascensis of the animal on Hinchenbrook Island. The three animals
seen from Yakutat Bay are so young as not to display clearly the
cranial characters of the subspecies but the extension of the color of
the underparts onto the underside of the tail in them and also in the
skin without corresponding skull from Glacier Bay, Alaska, is as in
arctica, the race to which they are referred, and gives substantial
basis for showing the geographic range of arctica as extending this
far south along the Pacific Coast.
Specimens examined.—Total number, 281, arranged alphabetically
by Districts and from north to south in each District. Unless
otherwise indicated, specimens are in the collection of the United
States National Museum.
Alaska. Point Barrow, 22 (1[1], 1[2], 1[75], 4[1], 7[60], 6[74]);
Flaxman Island, 3; Collinson Point, 1[77]; Salirochet River,
1[77]; Hulahula River, 1[2]; 69°20´ & 141°, 1; Rampart House, 1;
Yukon River, mouth of Porcupine River, 18; Alatna River, 30 mi.
from mouth, 1; Koyakuk Riv., 16 mi. below Bettles, 4; Shelton,
1[75]; Kruzamepa, 1[75]; Tanana, 6; Boulder Creek, Chena River, 3;
Fort Reliance, 4; Yukon River, 20 miles above Circle, 2; Mts. near
Eagle, 42 (1[60]); Snake River, Nome, 1[9]; Nulato, 3;
No[e]wikakat Riv., 1; Kantishna, 3; Fairbanks, 5 (1 20 mi. E and 1
33 mi. E); Richardson, 1; N. Fk. Kuskokwim R. at base of Mt.
Sischo, 1; N. Fk. Kuskokwim R. at Junction with McKinley Fk., 1;
Nenana Riv., mouth of Maurice Cr., 1; Ober Cr., trib. of Jarvis
Cr., Delta Riv. region, 1; head of Savage Riv., near Jennie Cr.,
1; Wonder Lake, 1[74]; Bear Cr., 3; Unlakleet, 3; St. Michaels,
11; 125 mi. E and a little N of Knik, Cook Inlet, on S side
Matanuska Range, 1[60]; Hope, Cook Inlet, 1; Iak Lake, 1[68]; head
of Behring Riv., 1; Bethel, 2; Kenai Lake, 8; Kenai Peninsula, 13
(2[2]); He[i]nchenbrook Island, 1200 ft., 1[74]; Sunshine Point,
Kaliekh River, Yakataga Dist., 1[8]; Cape Yakataga, 3[8]; Yakutat
Bay, 3[74]; Seward, 7; Seldovia, 22 (4[2]); Homer, 1[2]; Cape
Elizabeth, 18; Akchookuk Lake, 1; Lake Weelooluk, 1; Kokwok Riv.,
80 mi. up, 4; Nushagak, 1; Nushagak Riv., 1; Kolukuk, 1; Egooshik
River at mouth, 1; Glacier Bay, 1; Becharof Lake, between Portage
Bay and Becharof Lake, 1; Ugashik Riv., 4; Chignik, 7; East base
Frosty Peak, 1; Pavlov Bay, 1[100]; Mt. Pavlof, 1[75]; Unimak
Island, 2 (1[75]).
District of Franklin. Cape Sheridan, 1[2]; Discovery Bay,
Ellesmere Island, 1[7] (type specimen of Putorius audax
Barrett-Hamilton); Axel Heiberg Island, 1[95]; Bache Peninsula,
Ellesmere Island, 1[77]; Bedford Pims Island, 4[75]; Craig
Harbor, 2[77]; Cape Kellett, Banks Island, 1[77]; Franklin
Isthmus, 1[95]; King William Island, 2[95].
District of Keewatin. Ualiak, Ogden Bay, 2[95].
District of Mackenzie. Baillie Island, 1[75]; Franklin Bay, 1;
Langton Bay, arm of Franklin Bay, 15 mi. S of, 1[2]; Cockburn
Point, 69°N, 115°W, 2[77]; Dolphin and Union Strait, 1[77];
Bernard Harbor, 2[77]; Kent Peninsula, 4[95]; Horton Riv., near
Fort Anderson, 1; Fort Anderson, 6; Anderson River, 3; Barry
Island, Bathurst Inlet, 1[77]; Fort McPherson, 1; Peels River, 2;
Arctic Red River, 8[75]; Fort Good Hope, 6; Clinton Colden, 1[2].
Yukon. Kamarkak, 1[77]; Herschel Island, 1[75]; Lapierres House,
2; Forty Mile, L. T. Coal Cr., 4[74]; head of Coal Cr., 1;
Macmillan River, Forks, 1; 20 mi. W. Ft. Selkirk, 1; Slims River,
near Kluane, 1[75]; head of Lake Lebarge, 1.
Mustela erminea polaris (Barrett-Hamilton)
Ermine
Putorius arcticus polaris Barrett-Hamilton, Ann. and Mag. Nat.
Hist., 13 (ser. 7):393, May, 1904.
Mustela erminea, Manniche, Meddelelser om Grønland, 45:80-85, 1
fig., 1910.
Mustela arctica polaris, Miller, U. S. Nat. Mus. Bull., 79:97,
December 31, 1912.
Mustela erminea polaris, Hall, Journ. Mamm., 26:179, July 19,
1945.
Type.—Probably female, skin only; no. 78. 6. 19. 11, Brit. Mus.
Nat. Hist.; Gap Valley, 7-1/4 miles northeast Cape Brevoort, 82°
N, 59° 20´ W, Northwestern Greenland; June 15 or 16, 1876;
obtained by Lewis A. Beaumont.
The skin is in full, fresh summer pelage, fairly well stuffed
except for the tail which is unstuffed; the whole is in a good
state of preservation.
Range.—North coast, and east coast as far south as Turner Sound
(between 69 and 70 degrees) of Greenland. See figure 25 on page
95.
Characters for ready recognition.—Differs from M. e. arctica
in lighter upper parts (near [j] Buckthorn Brown rather than Raw
Umber or darker) and more intensely-colored underparts that are
Buff Yellow rather than Sulphur Yellow, Colonial Buff, or Primrose
Yellow; from M. e. semplei in color in same fashion as from
arctica and in larger size of skull.
Description.—Size.—Male: One subadult and two adults (one
ad. from Scøresby Sound and other two from Ymer Island) measure as
follows, the average being given first: Total length, 318 (301,
320, 315); length of tail, 72 (69, 70, 73); length of hind foot,
46.5 (44, 46, 47).
Female: No measurements taken in the flesh available but hind
foot, measuring 33.5 in the dried state and therefore
approximately 35 in life.
Color.—As described in Mustela erminea arctica except that
upper parts in summer near (j) Buckthorn Brown and tone 4 of Dark
Fawn of plate 307 to tone 1 of Raw Umber of plate 301 of Oberthür
and Dauthenay. Underparts Buff-Yellow. Least width of color of
underparts averaging, in 3 males, 66 (57-72) per cent of greatest
width of color of upper parts. Black tip of tail in same males
averaging 71 (70-72) mm. which is 99 (99-104) per cent of length
of tail-vertebrae.
The lighter-colored upper parts and more intensely yellow
underparts are the distinguishing features of the subspecies
polaris in comparison with other races of American M. erminea.
Skull.—Male (based on 5 adults from eastern Greenland): See
measurements. As described in Mustela erminea richardsonii
except that: Weight more (not recorded); basilar length, 41.3
(39.0-42.4); length of tooth-rows more than length of tympanic
bulla; breadth of rostrum measured across lacrimal processes
averaging more than a third of basilar length; interorbital
breadth more than distance between glenoid fossa and posterior
border of external auditory meatus; zygomatic breadth more than
distance between last upper molar and jugular foramen.
Female (based on 2 adults, Turner Sund and Kap Hoeg): See
measurements. As described in Mustela erminea arctica except
that basilar length 36.8 (35.9, 37.8), and length of tooth-rows
not more than length of tympanic
bulla. Skulls of females not in
hand when this comparison is written; only the recorded
measurements are available.
To me the skull of polaris is indistinguishable from that of
arctica. Therefore the comparisons made of the skull of arctica
with those of other subspecies will apply also for polaris.
Remarks.—In view of the heretofore erroneous assignment of the type
locality of Mustela erminea audax to Greenland, pains were taken to
verify the statement by Barrett-Hamilton (1904:393) relative to the
type specimen of polaris. Taking pains thus seemed the more
worthwhile because in the specimen register at the British Museum of
Natural History, there is written to the right of catalogue numbers
78-6 = 19 nos. 1-11, "Discovery Bay Presented by Mr. Hart Arctic
Collection." This refers to no. 78.6.19.1. There are no ditto marks
below but by implication this data applies also to nos. 1-11, which
include the holotype of polaris. A label attached to the specimen
does however give the locality as "Hall Land" "N Greenland" and another
label has on it "Ermine, procured by Mr. Beaumont Greenland Lat 89°
Long W 59-20." The 89° is obviously a mistake (on the label or in my
transcription of it) for 82°.
Reference to Nares (1877:385) reveals that Lieutenant Lewis A.
Beaumont, under date of June 15 and 16, 1876, wrote in his field
journal as follows: "I shot an ermine." In the daily accounts of his
journey from Discovery Bay on Grinnell Land [= Ellesmere Island],
across Robeson Channel and along the north coast of Greenland to the
west base of Mount Farragut near 50° 30´ W he mentions the ermine only
this once. For several other kinds of animals, Beaumont mentions
individuals seen or shot, often with the notation that this is the
second, or third seen. This mention of a kind of animal whenever seen
was in accordance with orders. On page 39 of the Discovery Report (op.
cit., 1877) in "General orders to sledging parties" by Captain G. S.
Nares, Commanding the Expedition, we find ". . . note daily: IV State
the animals seen and those shot." Reference to the map facing page 358
of the (op. cit.) report reveals that on the 15th and 16th, camps
were made by Beaumont in Gap Valley, each 7-3/4 miles northeast of Cape
Brevoort, one camp on either side of the 82° line, and separated from
each other by a distance of only 2-1/4 air line miles or 4-1/2 miles
march according to his journal.
These several data, then, are the bases for designating the type
locality of M. e. polaris, in the way that I have stated it at the
beginning of this account of the subspecies.
The light-colored upper parts and more intensely yellow underparts well
differentiate this subspecies from arctica or semplei.
Intergradation is suggested by a skin, no. 1462, Copenhagen Zoological
Museum, from Axel Heibergs Land, the color of the underparts of which
agrees with that of specimens from Greenland. Also the color of the
upper parts is decidedly nearer that of animals from Greenland than to
that of specimens from Ponds Inlet, Tulican and Gifford River. No other
specimens west or south of Greenland suggest intergradation. In
Greenland itself, one adult, a female from Turner Sund, East Greenland,
has the underparts no more yellowish than in some specimens from
Melville Peninsula. This female is darker on the back than any one of
the other 10 specimens from Greenland in summer pelage examined at the
same time, but even so is not so dark colored as animals from Baffin
Island or other islands to the west of Greenland.
The final summation of information about this subspecies would have
been more precise if I had been able to have actually in hand, at the
time of writing, specimens preserved in the Copenhagen Zoological
Museum. The war made it impractical to secure the loan of these as
previously planned. Even so, the measurements and notes on color that I
obtained from this material, in 1937, in Copenhagen, suffice to prove
that the subspecies polaris is well set off in color from the other
American subspecies of Mustela erminea.
The best material of this subspecies is in the University Zoological
Museum at Copenhagen, Denmark.
Specimens examined.—Total number, 35, arranged by locality from
the western end of the north coast of Greenland, eastward and then
southward down the east coast. Unless otherwise indicated,
specimens are in the Universitetets Zoologisk Museum, Købnhavn,
Danmark.
Gap Valley, 7-1/4 mi. NE Cape Brevoort, 82° N, 59° 20´ W, 1 (British
Mus.); Dragon Point, 1; Danmarks Havn (Fjeldene ved Baadskjeret,
1; lille Fjeld, 1; Lyservig, 1; harefjeldets, 4; Rypefjeldet, 1;
Baadskjeret, 1; Danmarkshavn, 3) 12; Christians Havn, 1 (not found
on map); Shannon Island, 4; Germania Havn, 2; Claveringoen, 1;
Carls Havn, 1; Myggbukta, 2 (British Mus.); Ymer[s] Island, 2
(Mus. Comp. Zool.); Kap Hoegh, Jamesonsland, 1 (Berlin Zool.
Mus.); Scoresby Sund, 3; Turner Sund, 4.
Mustela erminea semplei Sutton and Hamilton
Ermine
Plates 2, 3, 4, 9, 10 and 11
Mustela arctica semplei Sutton and Hamilton, Ann. Carnegie Mus.,
21:79, February 13, 1932.
Mustela arctica labiata Degerbøl, Rept. 5th Thule Exped., 2 (no.
4):25, 1935, type from Malugsitaq, Melville Peninsula, Canada.
Mustela erminea semplei, Hall, Journ. Mamm., 26:179, July 19,
1945.
Type.—Male, subadult, skull and skin; no. 6470, Carnegie Mus.;
Coral Inlet,
South Bay, Southampton Island, Canada; October 8,
1929; obtained by George Miksch Sutton, original no. 3M.
The skull has two holes in it: one is immediately above the left
canine, and the other (2 × 5.5 mm.) is 3 millimeters to the left
of the median line at the juncture of the frontal and parietal
bones. From this last mentioned hole a fracture extends back
halfway to the lambdoidal crest. The tip of the left upper canine
is broken off. Otherwise the skull is complete, and the teeth all
are present and entire. The skin is well made and in fresh white
winter pelage except for a trace of the old brown summer pelage on
the back, on the tail, on the anterior borders of the ears, and in
a spot 11 mm. long and 8 mm. wide on the nose.
Range.—Baffin and Southampton islands, Melville Peninsula and
west side of Hudsons Bay as far south as Eskimo Point. See figure
25 on page 95.
Characters for ready recognition.—Differs from M. e. arctica,
in that, in males, hind foot less than 44 and basilar length less
than 41 and in that females average smaller, their skulls being
only about 10 per cent lighter; from M. e. polaris in darker
upper parts (Raw Umber rather than Buckthorn Brown) and
less-intensely-colored underparts that are Sulphur Yellow,
Colonial Buff or Primrose Yellow rather than Buff Yellow, and in
lesser size in the same fashion as from arctica; from M. e.
richardsonii, of both sexes, in that proximal two-thirds of under
side of tail colored same as underparts rather than same as upper
parts and by least interorbital breadth amounting to more, instead
of less, than distance between glenoid fossa and posterior border
of external auditory meatus.
Description.—Size.—Male: Ten adults and subadults, from
Southampton Island, yield average and extreme measurements as
follows: Total length, 282 (267-318); length of tail, 77 (59-87);
length of hind foot, 40 (38-43).
Female: Four subadults from Southampton Island yield average and
extreme measurements as follows: Total length, 271 (256-288);
length of tail, 71 (69-74); length of hind foot, 35 (33-38).
Color.—As described in M. e. arctica except that least width
of color of underparts averaging, in 7 males, 59 (45-81) per cent
of greatest width of color of upper parts. Black tip of tail in 19
male topotypes averaging 72 (64-83) mm. which is 91 (75-122) per
cent of length of tail-vertebrae.
Skull.—Male (based on 2 adults and 10 subadults from
Southampton Island): See measurements and plates 2-4. As described
in Mustela erminea richardsonii except that: Weight, 2.0 (in one
subadult) grams; basilar length, 37.5 (35.7-39.9); length of
tooth-rows more than length of tympanic bulla; breadth of rostrum
more than a third of basilar length; interorbital breadth more
than distance between glenoid fossa and posterior border of
external auditory meatus; zygomatic breadth more than distance
between last upper molar and jugular foramen.
Female (based on 1 adult and 4 subadults from Southampton Island):
See measurements and plates 9-11. As described in Mustela erminea
richardsonii except that: Weight, 1.35 (in one adult) grams;
basilar length, 34.2; breadth of rostrum more than 30 per cent of
basilar length; interorbital breadth more than distance between
glenoid fossa and posterior border of external auditory meatus;
zygomatic breadth more or less than (approximately same as)
distance between last upper molar and jugular foramen.
In comparison with richardsonii, the skulls of males averaged smaller
in every measurement taken except breadth of rostrum and interorbital
breadth which are more, and zygomatic breadth and length of inner lobe
of M1 which are approximately the same; skull about 20 per cent
lighter; in relation to basilar length, preorbital region longer and
broader in every part measured. Female averages larger, in every part
measured; 23 per cent heavier; in relation to basilar length, every
other measurement more. It is noteworthy that the skull of the male is
smaller and the skull of the female larger than in richardsonii.
Differences from arctica are: Size less, in each sex; males about 40
per cent and females 10 per cent lighter; in males, skull more rounded
in outline as viewed from above because zygomatic arches arise less
abruptly from skull; in males tympanic bullae do not project so far
ventrally from squamosal floor of braincase; with these exceptions,
skull of semplei can be said to be a smaller edition of that of
arctica.
From polaris, semplei differs, cranially, in the same way as from
arctica.
Remarks.—There is a slight increase in size of ermines toward the
north which probably is the result of intergradation between semplei
and arctica. Specimens from the northern part of Baffin Island are
larger than those from farther south. Specimens from the mainland west
of Southampton Island may owe their smaller (than in arctica) size to
intergradation with richardsonii almost as much as to intergradation
with semplei.
Degerbøl's name Mustela arctica labiata was applied to specimens,
which to me are indistinguishable from topotypes of Mustela arctica
semplei, which latter name has three years priority. Degerbøl
(1935:34) states that Malugsitaq, Melville Peninsula, is the type
locality. He did not designate a type specimen. Reference to his
account (op. cit.:26) shows that he lists five specimens from the
type locality, or more precisely as "Malugsitaq, Lyon Inlet. 5 summer
skins. ♂ ♂ June-July 1922. P. F., CN. 2262-2266." On labels
attached to these specimens, "Lyon Inlet" is replaced with "Melville
Peninsula." On July 28, 1937, Degerbøl and I together examined these
specimens in his laboratory. Because no. 2262 is first mentioned I
regard it as the type. It is a juvenal male, skull and skin, no. 2262
(20.5 1931.8), Univ. Zool. Mus. Copenhagen, obtained in June or July of
1922 by Peter Freuchen whose original number was / s 2324. The specimen
is one of 5 males taken at the same locality by the same collector and
they bear identical data as
to date. They look to be of the same
litter for all are roughly of the same size and each retains milk
teeth.
Additional females, with external measurements carefully taken, are
much needed from Southampton Island, because the available females are
insufficient to show the degree of sexual dimorphism. If the meager
data available be accepted, the difference in size between the two
sexes is less than in other subspecies. My own feeling is that a better
sample of females would show the secondary sexual difference in size to
be more than available data indicate.
Specimens examined.—Total number, 183, arranged from north to
south by islands, or regions attached to the mainland, and from
north to south in each region or island. Unless otherwise
indicated, specimens are in the Zoological Museum, University of
Copenhagen, Denmark.
Baffin Island. Pond[s] Inlet, 8; (5[77]); Tulukan (sometimes
spelled Tulukat), 6; Cape Eglinton, 1[7]; Gifford River, 2; Clyde,
3[86]; head of Cumberland Sound, 1[91]; Pangnirtung, 2[77];
Kingnait Fiord, 1[91]; Kikkulin Island, Cumberland Sound, 1[7];
Blacklead Island, Cumberland Gulf, 1; merely Cumberland Gulf,
1[7]; merely east Baffin Island, 34[7]; Cape Dorset, 2[2]; SW
coast of Baffin Island, 1[75].
Melville Peninsula. Iglulik, 3; Pingerqalik, 2; Kingadjuaq,
Amitsog, 3; Rae Isthmus, 3; Lyons Inlet, 13(9[2]); M[N?]
alugsitaq, Lyon Inlet, 5; Itibdjeriang, 2; Repulse Bay, 27 (22[2],
2[19]); Drichetts Cove, Hurd Channel, 1[2]; Gore Bay, 1; Haviland
Bay, 1; Cleveland Harbor, Frozen Strait, 1.
Southampton Island and adjacent islands. Danish Island, 11;
Vansittart Island, 4. Southampton Island: Coral Inlet, 19 (1[77],
18[9]); Prairie Point, 1[9]; Munnimunnek Point, South Bay, 5[9];
Native Point, 1[9]; Ranger Rim, 1[9]; Koodloatok (not found on
map), 1[77]; merely Southampton Island, 1[77]; Gore Bay, 1[2];
Fox Channel, 2[2].
Mainland to west of Southampton Island. Cape Fullerton, 3 (1[77],
2[2]); Chesterfield Inlet, 4 (1[77], 1[9]); Tavane, 1[77]; N of
Wagner Inlet, 1; Eskimo Point, 1[86].
Mustela erminea kadiacensis (Merriam)
Ermine
Plates 2, 3, 4, 9, 10 and 11
[Putorius arcticus] subspecies kadiacensis Merriam, N. Amer.
Fauna, 11:16, June 30, 1896.
Putorius kadiacensis, Preble, Proc. Biol. Soc. Washington,
12:169, August 10, 1898.
Mustela kadiacensis, Miller, U. S. Nat. Mus. Bull., 79:97,
December 31, 1912.
Mustela erminea kadiacensis, Hall, Journ. Mamm., 26:179, July 19,
1945.
Type.—Male, subadult, skull and skin; no. 65290, U. S. Nat.
Mus., Biol. Surv. Coll.; Kodiak Island, Alaska; April 25, 1894;
obtained by B. J. Bretherton, original no. 304.
The skull lacks the basioccipital, part of the basiphenoid, the
occipital region on the right side and the posterior part of the
right tympanic bulla. The third, upper, left incisor is missing.
Otherwise the teeth all are present and entire.
The white, winter skin is only moderately well stuffed but in a
good state of preservation. The spring coat is appearing along the
back. This coat is visible at only two places unless the hair be
parted when the new brown pelage, which is coming in, can be seen
all along the midline of the back.
Range.—Kodiak Island, Alaska. See figure 25 on page 95.
Characters for ready recognition.—Differs from M. e. arctica
in hind foot less than 33 in females and in zygomatic breadth
amounting to less, instead of more, than distance between last
upper molar and jugular foramen irrespective of sex.
Description.—Size.—Male: One adult and 3 subadults yield
average and extreme measurements as follows: Total length, 341
(318-360); length of tail, 93 (86-102); length of hind foot, 47
(44-49).
Female: An adult measures: Total length, 258; length of tail, 70;
length of hind foot, 31.
Color.—As described in M. e. arctica, except that least width
of color of underparts averaging 54 (40-83) per cent of greatest
width of color of upper parts. Black tip of tail in 3 males in
summer pelage averaging 80 (70-90) mm. which is 85 (69-96) per
cent of length of tail-vertebrae.
Skull.—Male (based on 2 adults): See measurements and plates
2-4. As described in Mustela erminea richardsonii except that:
Weight 3.1 grams; basilar length, 42.6 (42.1-43.2); length of
tooth-rows more than length of tympanic bulla; breadth of rostrum
measured across lacrimal processes averaging more than a third of
basilar length; interorbital breadth more than distance between
glenoid fossa and posterior border of external auditory meatus.
Female (based on one adult, no. 98042): See measurements and
plates 9-11. As described in Mustela erminea richardsonii except
that: Weight, 1.2 grams; basilar length, 33.0; length of
tooth-rows more than length of tympanic bulla.
Comparison with arctica has been made in the account of that
subspecies. Although richardsonii and kadiacensis are described as
having the zygomatic breadth less than the distance between the last
upper molar and jugular foramen, the zygomatic breadth is considerably
more in kadiacensis than in richardsonii; consequently the two
dimensions are more nearly equal than in richardsonii. Except for
being slightly narrower, the skull of kadiacensis is only a slightly
smaller edition of that of arctica.
Remarks.—When naming the weasel from the mainland of Alaska as new,
under the name Putorius arcticus, Merriam (1896:16) wrote: "A small
form of arcticus occurs on Kadiak Island. . . . It is probably worthy
of recognition as subspecies kadiacensis." The informality of this
description possibly was in part due to the describer's recognition of
the fact that the degree of difference between arcticus and the
insular kadiacensis was slight. Specimens collected after Merriam
proposed the name for the weasel of Kodiak Island show the animal there
to be less different from arctica of the adjacent mainland than he
thought; small size is the most pro
nounced distinction of
kadiacensis and Merriam's male type specimen is smaller than any of
the five additional males saved from Kodiak Island since that time.
Even so the differences fully warrant subspecific recognition, in my
opinion, although kadiacensis is not a strongly differentiated race.
More adult females are needed to ascertain the norm of form and size
for that sex. If the one female known is typical, the difference from
arctica is more pronounced in females than in males. The lesser size
of kadiacensis can hardly be credited entirely to the effect of
insularity, for animals from the southern part of the mainland, on
Kenai Peninsula for example, are smaller than those from central and
northern Alaska and provide evidence of intergradation of a sort
between kadiacensis and arctica.
Specimens examined.—Total number, 9, all from Kodiak Island,
Alaska, and unless otherwise indicated in the U. S. National
Museum.
Karluk, 1 (Stanford Univ.); Kodiak, 7; Kodiak Island, 1 (Field
Mus. Nat. Hist.).
Mustela erminea richardsonii Bonaparte
Ermine
Plates 2, 3, 4, 9, 10 and 11
Mustela richardsonii Bonaparte, Charlesworth's Mag. Nat. Hist.,
2:38, 1838.
Putorius cicognanii, Baird, Mamm. N. Amer., p. 161, 1858 (part).
Putorius richardsonii, Baird, Mamm. N. Amer., p. 164, 1858
(part-Halifax, N. S.).
Putorius (Gale) erminea, Coues, Fur-bearing animals, p. 109,
1877 (part).
Putorius richardsoni, Bangs, Proc. Biol. Soc. Washington, 10:16,
February 25, 1896.
Putorius cicognani richardsoni, Merriam, N. Amer. Fauna, 11:11,
June 30, 1896.
Putorius (Arctogale) cicognanii cicognanii, Bangs, Proc. New
England Zoöl. Club, 1:18, February 28, 1899.
Putorius microtis Allen, Bull. Amer. Mus. Nat. Hist., 19:563,
October 10, 1903. Type from Shesley, British Columbia.
Putorius arcticus imperii Barrett-Hamilton, Ann. and Mag. Nat.
Hist., 13(ser. 7):392, May, 1904. Type from Fort Simpson,
Mackenzie, Canada.
Putorius cicognanii richardsoni, Preble, N. Amer. Fauna, 27:231,
October 26, 1908.
Mustela microtis, Miller, U. S. Nat. Mus. Bull., 79:96, December
31, 1912.
Mustela cicognanii mortigena Bangs, Bull. Mus. Comp. Zoöl.,
54:511, July, 1913. Type from Bay St. George, Newfoundland.
Mustela cicognanii, Sheldon, Journ. Mamm., 13:201, August 9,
1932.
Mustela cicognanii richardsonii, Miller, U. S. Nat. Mus. Bull.,
79:95, December 31, 1912; Hall, Univ. California Publ. Zoöl.,
40:368, November 5, 1934.
Mustela cicognanii cicognanii, Hall, Canadian Field-Nat., 52:108,
October, 1938.
Mustela erminea richardsonii, Hall, Journ. Mamm., 26:77, February
27, 1945; Hall, Journ. Mamm., 26:180, July 19, 1945.
Type.—Male, age unknown, skin; no. 43.3.3.4, British Museum of
Natural History; probably from Fort Franklin, Canada; presented to
British Museum on or before March 3, 1843; may be the type.
In September, 1937, when I searched in the British Museum for the
skull, I found no trace of it nor mention of it in catalogues. The
skin is in white, winter pelage, mounted on a pedestal. See under
remarks for Mustela e. cicognanii for reasons for and reasons
against regarding this specimen as the holotype.
Range.—Hudsonian and Canadian life-zones of the greater part of
Canada from the Atlantic to the Pacific. See figure 25 on page 95.
Characters for ready recognition.—Differs from M. e. arctica,
polaris, semplei and haidarum, in both sexes, by proximal
two-thirds of under side of tail colored same as upper parts
rather than same as underparts, and interorbital breadth less,
rather than not less, than distance between glenoid fossa and
posterior border of external auditory meatus; from M. e. bangsi,
in that, in both sexes, least width of color of underparts
averages two-fifths rather than about a third of greatest width of
color of upper parts, and in that skulls of males are a fourth
heavier, basilar length averaging more than 40; from M. e.
cicognanii, in both sexes, in that least width of color of
underparts averages two-fifths instead of less than a third of
greatest width of color of upper parts, in females by 20 per cent
heavier skull (1.1 versus 0.92), in males by skull more, rather
than less, than 1.9 grams, and basilar length more, instead of
less, than 38; from M. e. invicta, in males, by skull more,
instead of less, than 1.9 grams; mastoid breadth more, instead of
less, than 19.9 mm.; depth of skull at anterior margin of
braincase more, instead of less, than 12.4 mm.; in females, by
same measurement of depth more, instead of less, than 10.1, and
weight of skull averaging more, instead of less, than one gram;
from M. e. fallenda in both sexes upper lips white rather than
brown, in males, hind foot more than 41, basilar length more than
38.3, in females hind foot more than 29, basilar length more than
31.4, and breadth of rostrum amounting to less, instead of more,
than 30 per cent of basilar length; from M. e. alascensis in
males in that black tip of tail more than 43, total length more
than 320, tympanic bullae more than 14 and longer than tooth-row
rather than less than 14 mm. and sometimes shorter than tooth-row,
females not individually distinguishable.
Description.—Size.—Male: Four adults (Fort Franklin, Fort
Simpson, Mts. W Fort Nelson, and Govt. Hay Camp, Wood Buffalo
Park) yield average and respective measurements as follows: Total
length, 331 average (340, 325, 330, 328); length of tail, 93 (102,
91, 93, 87); length of hind foot, 45 (48, 43, 45, 44). Weight of 4
adults from the Belcher Islands is 175 (135-180) grams. Of 10
subadults from Belcher Islands it is 119 (92-137) grams.
Female: Three adults from Great Slave Lake (Willow River,
Fairchild Point, and Fort Resolution) yield average and respective
measurements as follows: Total length, 252 (237, 238, 282); length
of tail, 69 (63, 60, 85); length of hind foot, 32 (31, 32, 34).
Corresponding, average measurements for three adults from Glacier
Lake are 240, 60, 32 and for 3 adults from the Athabasca Delta,
243, 65, 30. Weight of 8 subadults from the Belcher Islands is 69
(64-78) grams. Weight of adults would be more.
Color.—Winter pelage all white except tip of tail. Summer
pelage with upper parts uniform in color and darker (16n) than
Raw Umber, and about tones 3 to 4 of Chocolate of Oberthür and
Dauthenay, pl. 343. Underparts Sulphur Yellow, Colonial Buff, or
Primrose Yellow, often nearly white on chin and insides of
forelegs; color of underparts extends narrowly over upper lips,
distally on posterior sides of forelegs onto antipalmar faces of
toes and sometimes over most of antipalmar surfaces of forefeet,
on medial sides of hind legs to a point between knee and ankle but
reappears on antiplantar faces of toes and in some individuals is
narrowly continuous onto toes. Least width of color of underparts
averaging, in a series of 12 males from the Athabasca Lake Region,
40 (25-54) per cent of greatest width of color of upper parts.
Black tip of tail averaging 56 (45-63) mm. in 5 adult males from
same region and thus 60 (48-70) per cent of length of
tail-vertebrae.
From arctica, polaris, semplei and kadiacensis,
richardsonii differs in: Color darker; ventral side of tail same
color as upper parts; light-colored underparts a fifth narrower;
black tip of tail by actual measurement a fifth shorter and
averaging less than two-thirds rather than more than four-fifths
of length of tail-vertebrae. From cicognanii, richardsonii
differs in that the underparts are a fourth wider and in some
specimens more brightly colored. The width of the underparts is
likewise a fourth more than in bangsi. In invicta the
underparts are not so brightly colored as in some specimens of
richardsonii. From fallenda, richardsonii differs in that
the upper parts often are lighter colored, upper lips white rather
than colored like upper parts, and underparts as wide again. In
comparison with alascensis, the black tip of the tail averages
three-fifths rather than a half of length of tail-vertebrae.
Skull.—Male (based on 6 adults from 3 miles south of Big
Island, Great Slave Lake): See measurements and plates 2-4;
weight, 2.5 (2.1-2.9) grams; basilar length, 40.9 (39.6-43.7);
length of tooth-rows less than length of tympanic bulla; breadth
of rostrum measured across lacrimal processes less than a third of
basilar length; interorbital breadth less than distance between
glenoid fossa and posterior border of external auditory meatus;
zygomatic breadth less than distance between last upper molar and
jugular foramen.
Female (based on 4 adults: from Willow River, 1; Fort Resolution,
1; Athabasca Delta, 2; and 2 subadults, one from 3 mi. S Big
Island and one from 15 mi. above Smith Landing): See measurements
and plates 9-11; weight, 1.1 (0.9-1.4) grams; basilar length, 33.1
(31.5-34.2); length of tooth-rows less than length of tympanic
bulla; breadth of rostrum less than 30 per cent of basilar length;
interorbital breadth less than distance between glenoid fossa and
posterior border of external auditory meatus; zygomatic breadth
less than distance between last upper molar and jugular foramen.
The skull of the female averages 56 per cent lighter than that of
the male.
Comparison of the skull with that of arctica, polaris, semplei,
kadiacensis, haidarum, cicognanii, bangsi, invicta,
fallenda, and alascensis is made in the accounts of those
subspecies.
Remarks.—M. e. richardsonii has the most extensive geographic
range of any American race of erminea, is centrally located with
respect to the other races, is more abundantly represented by study
specimens in zoölogical collections than any other race, and is a sort
of average for the species as a whole in most structural features.
Therefore richardsonii is used as a standard of comparison and
accordingly is more fully described than any one of the other races
each of which by reference to richardsonii is described in
comparative fashion. This comparative description has the virtue of
more clearly indicating differences between subspecies and also makes
for brevity.
John Richardson, Bernard R. Ross, and names of their companions, as
written on the labels of the older specimens recall to the student's
mind early explorations of the north country. Edward A. Preble obtained
important specimens at several places and in recent years J. Kenneth
Doutt and G. G. Goodwin have made the reviser's work easier by
preparing specimens in series from areas not previously well
represented.
The nomenclatural history of this subspecies begins with references in
the literature that identify the animal as the Old World species,
Mustela erminea—an identification which the study here reported upon
shows to have been correct in the specific, although not in the
subspecific, sense. Richardson, for example, in his "Fauna
Boreali-Americana" published in 1829 so identified the animal. In 1838,
Bonaparte, basing his description on Richardson's account of 1829,
proposed the new name richardsonii. Richardson himself, the following
year in the "Zoology of Beechey's Voyage," accepted Bonaparte's name
and it has been applied to the animal in the central part of the
northern timber-belt of North America ever since, except as authors
used the name Mustela erminea in the belief that richardsonii was
not distinct from erminea.
The north and south boundaries of the range assigned to richardsonii
varied according to the notions of the particular writer who was
employing the name. Until Merriam in 1896 named arctica as distinct,
animals from the far north were generally included under the name
richardsonii along with populations to which the latter name now is
applied. Because richardsonii grades gradually into the smaller
cicognanii of more southern occurrence the boundary between the two
has been set farther north by one writer and farther south by another,
depending probably upon what the writer felt was the halfway point in
size. This point of course depended upon the samples selected as
typical of richardsonii on the north and cicognanii on the south.
Because Bangs, in 1896, took as representative of richardsonii the
far northern and hence large-sized animals (now separated as M. e.
arctica), his halfway point in size between them and the small
cicognanii of New England naturally fell farther north than it would
have had he used as representative of richardsonii specimens from
places south of the range of arctica.
In 1903 J. A. Allen proposed the name Putorius microtis for a
specimen from Shesley, northwestern British Columbia, a place
approximately 50 miles northwest of Telegraph Creek. Considering the
great disparity in size between this one specimen and the other larger
specimens of normal size, from the general region, available to Allen
at that time, it is not surprising that he thought two full species
were represented. In 1943 when G. G. Goodwin called to my attention two
males, as small as the type of microtis and taken by him
approximately 300 miles east of Shesley, in the valley between the
Musqwa and Prophet rivers, I for a second time examined all available
specimens and data with the possibility in mind that microtis was a
species or subspecies distinct from M. e. richardsonii, but again
concluded that only one subspecies was involved because no character
except size was found to distinguish the large from the small
individuals of a given sex and there are, preserved from northern
British Columbia, individuals of intermediate size. Putorius microtis
Allen seems to have been based on an individual of M. e. richardsonii
near the lower limit of size for that subspecies and microtis is
regarded as a synonym.
Barrett-Hamilton in 1904 named the animal at "Fort Simpson, British
Columbia" Putorius arcticus imperii. Preble (1908:232) pointed out
that Fort Simpson on the Mackenzie undoubtedly was the place intended,
and arranged imperii as a synonym of M. e. richardsonii. The type
specimen of imperii was stated to have been received from B. M. Ross
who is known to have collected specimens, including specimens of this
species (now in U. S. Nat. Mus.), at Fort Simpson on the Mackenzie. I
know of no Fort Simpson in British Columbia. If, as seems improbable,
Port Simpson, British Columbia, was the place that Barrett-Hamilton
intended to designate (where so far as I know Ross did not collect),
the name imperii still would seem to be a synonym of richardsonii
because richardsonii seems to be the race of weasel at Port Simpson.
In proposing the name Putorius arcticus imperii, Barrett-Hamilton
stressed that the weasel, which he was naming, was a subspecies of P.
arcticus, gave characters which applied perfectly to richardsonii
but made no reference to richardsonii. Barrett-Hamilton did not refer
to richardsonii possibly because he relied on Merriam's
classification of 1896 wherein richardsonii is treated as a species
distinct from arctica. Merriam, it will be remembered, held that
slight degree of morphological difference rather than intergradation
was the criterion for subspecies. Although I have no record of having
examined the type specimen of imperii I have but little hesitancy in
treating it as a synonym, and would have no hesitancy at all in so
doing if the type was certainly known to have been obtained at Fort
Simpson on the Mackenzie.
The name Mustela cicognanii mortigena Bangs, 1913, proposed for the
ermine of Newfoundland, is placed as a synonym of richardsonii only
after repeated, detailed comparisons. In advance of study I supposed
that the isolation of the ermine, in Newfoundland, had contributed to
its differentiation, which, however, the original describer, Bangs,
indicated was slight. Bangs was a careful worker and I am confident
that the differences he described really existed between his specimens.
Material more nearly adequate than he had from the mainland, shows the
males, so far as my measurements and comparisons go, to be in nowise
different from those in Newfoundland. Females in Newfoundland may have,
on the average, slightly longer hind feet than on the opposite mainland
but I am not certain that they do and even if there is a slight
difference in this regard as suggested by available data, I think it
insufficient basis, alone, for according subspecific status to the
insular animal.
The name richardsonii was based by Bonaparte on Richardson's
description which in turn was drawn from a specimen taken at Fort
Franklin, that thus becomes the type locality. It is fortunate that
Preble, in 1903, succeeded in taking specimens there because the place
is near the belt of intergradation between arctica and
richardsonii. Of Preble's two adult males (see Preble, 1908:232) I
have examined no. 133847, which is in transitional pelage and therefore
gives no clue in so far as coloration is concerned, as to affinities
with arctica versus richardsonii. Specimens in the summer pelage
are much to be desired from Fort Franklin. Regardless of what their
coloration may be, specimen no. 133847, in external measurements and
most certainly in cranial features is of the race to the south and not
the race that Merriam named arctica. Because all specimens from
localities to the south of Fort Franklin likewise differ from arctica
of the barren grounds, considerable additional confidence is felt in
allocating the name richardsonii to the animal which ranges from Fort
Franklin southward rather than to the one, here designated arctica,
that occurs to the northward of Fort Franklin.
Although in most structural features richardsonii is a sort of
average for the American races of the species, it is the extreme in
high degree of sexual dimorphism. The difference in size between the
males and females is greater than in any other race except possibly M.
e. kadiacensis in which so little is known of the female that the
difference between the two sexes cannot be accurately judged. It will
aid in understanding the high degree of secondary sexual difference in
richardsonii to visualize two
kinds of weasels distributed over the
northern half of the continent, thinking now of the geographic area in
America occupied by the whole species Mustela erminea of which the
subspecies richardsonii is only a part. One of the two kinds of
weasel is the male ermine and the other the female. The decrease in
size of the male, as measured by the weight of the skull, is in the
ratio of 7 in the north to 2 in the south. This decrease is gradual
whereas the corresponding decrease from 3 to 1 in the female is not
gradual; half of the decrease in the female occurs in the short north
to south distance comprised in the belt of intergradation, along the
northern boundary of richardsonii, between it and arctica. As a
result richardsonii is composed of females with medium sized skulls
and males with relatively large skulls, the ratio by weight being
approximately 5 to 2. The disproportion in races of ermines both to the
north and to the south is less. Actually in the north (arctica) the
approximate ratio by weight is 2-1/3:1; in richardsonii, 2-1/2:1; in
the south (muricus), 1-2/5:1. Indicated in still another way in
richardsonii the skull of the female is 56 per cent lighter than that
of the male and the skull of the male is 127 per cent heavier than that
of the female. Intergradation with races whose ranges border on that of
richardsonii is complete. On the northern boundary of the range of
richardsonii along the western shore of Hudsons Bay for perhaps a
hundred miles north of Eskimo Point, there are intergrades with
arctica. As judged by their lesser size, individuals of this
population are influenced by the semplei-stock. Otherwise,
intergradation on the northern boundary, with arctica, is abrupt
whereas intergradation at the south, between richardsonii and
cicognanii, is gradual. Intergradation is similarly gradual between
richardsonii on the one hand and bangsi and invicta on the other.
By speaking of the intergradation as abrupt, it is intended, in this
instance, to indicate that in a relatively narrow belt, between the
geographic ranges of arctica and richardsonii, ermines intermediate
in color-pattern, shape of skull, and size, bridge the gap between the
ermine of the tundra (arctica) and that in the forest belt
(richardsonii). It may be added that the degree of difference between
the two subspecies just mentioned is approximately twice as much as
between richardsonii and cicognanii. The intergradation between
cicognanii and richardsonii is gradual. By gradual it is meant that
the change from one kind to the other is achieved in a wider area where
ermines from locality A do not differ appreciably from those taken at,
say, locality B, 50 miles farther south, although ermines from A and
those from a third locality, C, say, 130 miles south, clearly show
differences indicative of geographic variation.
Specimens examined.—Total number, 1035, as follows. Arranged
alphabetically by provinces and districts and from north to south
in each province or district. Unless otherwise indicated,
specimens are in the United States National Museum.
Alberta. 15 mi. above Smith Landing, 2; Fort Smith, 2 (1[77]);
Smith Landing, 2; LaButte, Fitzgerald, 1[77]; Egg Lake, 15 mi. NW
Ft. Chippewyan, 4 (2[75]); Lobstick Island, near Ft. Chippewyan,
1; Athabasca Delta, 9 mi. above mouth of main branch, 1; Athabasca
Delta, Long Creek, 1 mi. W of main branch, 2; Ft. Chippewyan, 1;
Peace Point, 1[75]; 18 mi. below Peace Point, 1; Embarass River, 7
(4[75]); Athabasca River, 1[2]; Ft. McMurray, 1; Athabasca River,
Middle Rapid, 2; 60 mi. above Grand Rapids, 1; Boiler Rapid, 1;
Entrance, 3[2]; St. Albert, 2.
British Columbia. Fort Halket, 1; Shesley, 1[2]; Dorothy Lake,
Mts. W of Ft. Nelson, 4000 ft., 3[2]; valley between Musqwa and
Prophet rivers, 3800 ft., SW of Ft. Nelson, 2[2]; Sikanni Chief
Riv., 1; Telegraph Creek, 7 (6[2]); head of Bad River, 2350 ft.,
on lake, 1; Six Mile, 5[74]; Tuchodi Lake, 2[2]; Iskoot River,
2[14]; Level Mtn., 1[2]; head of Tatletuey Lake, 12 mi. W Thudade
Lake, 2; Robb Lake District, 5[2]; Ft. Grahame, 12 (2[77]);
Sustut Mts., on trib. Sustu Riv., 25 mi. SE Thudade Lake, 2;
Laurier Pass, 1; Omineca Mts., 1[85]; Point Creek and Clearwater
River, 2; Kispiox Valley, 23 mi. N Hazelton, 5[74]; Hazelton,
3[77]; NW arm Tacla Lake, 7; N end Babine Lake, 1; Pt. Simpson,
1; Metlakatla, 1; Stuart Lake, 27; S Fk. Salmon Riv., 1[77];
mouth Salmon Riv., 1[77]; Vanderhoof, 4[77]; Wistaria P. O.,
near Burns Lake, 1[77]; Kruger Lake, 9[74]; Indianpoint Lake,
23[74]; Quesnel, 1; Ahbau Lake, 3[74]; Isaacs Lake, 6[74]; Beaver
Pass, 56[74]; Lightning Creek, 54[74]; LaFontaine, 16[74];
Barkerville, 1[74]; Barkerville District, 34[74]; Swift River,
27[74]; Cunningham Creek, 34[74]; Itcha Mts., 1[31]; Anahim Lake,
1[74]; Chezacut Lake, 8[31]; Kleena Kleene, 18[74]; 158 mi. House
(Cariboo on labels), 3[60]; Rivers Inlet, 6 (5[94]; 1[77]); Horse
Lake, 4[22]; Kingcome Inlet, 8[77]; Loughborough Inlet, 7[77];
McGillivary Creek, 1; Camel Back, Pemberton Meadows, 1[31]; Arrow
Rapids, mainland opposite Stuart Island, 1[77]; Butte Inlet,
9[77]; Green Lake, 1[31]; Mt. Whistler, 1[86]; Alta Lake, 2
(1[31]; 1[21]); Mons, 1[31].
Keewatin. Foot of Baker Lake, 1.
Labrador. Okak, 3[75]; Nain, 22 (11[75]; 11[60]); Hopedale,
24[75]; Kippokak Bay, 7[75]; Ailik, 1; Makkovik, 26[75]; Labrador,
55° N, 3; Hamilton Inlet, 2[75]; NW River Post, interior Labrador,
5[1]; Cartwright, 5; Paradise, 12; Sandwich Bay (Muddy Bay, 6;
North River, 6), 12; Battle Harbor, 1[7]; St. Marys River, 3[7];
Black Bay, 16 (15[75]; 1[76]); Lanceau Loup, 17 (1[75]).
Mackenzie. Ft. Franklin, 1[2]; Ft. Rae, 12; Fairchild Point, 6[9];
Fort Simpson, 10 (2[2]); Hot Springs (61°, 125°), 1[2]; Willow
River, near Ft. Providence, 1; 35 mi. N Big Island, 7; Big Island,
9; 3 mi. S Big Island, 7; Ft. Resolution, 9; 100 mi. N Ft. Smith,
2; 75 mi. NW Ft. Smith, 1; Ft. Liard, 2; Sucker Creek, 4[77];
Govt. Hay Camp, Wood Buffalo Park, 2[77].
Manitoba. Egg Is., Rabbit Point, 1; Ft. Churchill, 1; Ft. York, W
Hudsons Bay 57° N, 1[7]; Oxford House, 11; Gypsumville, 1[86];
Lake St. Martin.
New Brunswick. Restigouche County: Bird Bait, north Camp, 6 mi.
NE Nictau Lake, 2[59]; Red Brook, Tobique River, 1[59]. Victoria
County: Trousers Lake, 3[2]. Glouchester County: Youghall,
1[77]; Miramichi Road, 15 mi. from Bathurst, 13[77]. York
County: Scotch Lake, 2.
Newfoundland. Nicholsville, 3[75]; Bay St. George, 48 (26[75];
2[7]; 1[9]); Codroy, 9 (7[75]; 2[60]).
Nova Scotia. Victoria County: Cape North, 2[77]. Inverness
County: Fizzleton, 3[77]. Richmond County: St. Peters, 1[77].
Pictou County: Glengary, 1[4]. Guysborough County: East Roman
Valley, 5[77]. Kings County: Wolfville, 5 (3[74], 2[77]); near
Wolfville, 1[77]. Halifax County: Hammond Plains, 1. Annapolis
County: Annapolis Royal, 1. Digby County: Digby, 3. No locality
more definite than Nova Scotia, 3.
Ontario. Severn River, 1[77]; R. C. Mission, Yellow Creek, near
mouth of Albany, 2[86]; Ft. Albany, 4; Charlton Island, 1; Moose
Factory, 10 (7[9]; 3[77]); Abitibi, 1[4].
Quebec. Fort Chimo, 10[77]; Ungava Forks, 1; Belcher Islands,
Hudsons Bay (Tukarak Island, 29; Eskimo Harbor, 2; Innetalling
Island, 1; S tip Gibson Peninsula, 2; Flaherty Island, 1), 35[9];
Cairn Island, Richmond Gulf, 2[9]; Manitounuk Sound, 4[9]; about
15 mi. S Great Whale River, 1[9]; Ft. George, 1[9]; Charlton
Island, 1[9]; Waswonaby Post, 1[77]; Mistassinnay Post, 3[77];
Godbout, 36; Mt. Albert, 7 (4[78]; 3[2]); St. Anne River, 1500
ft., 1[77]; Ste. Anne des Monts, 3[2]; "Federal Mine," 1[77];
Berry Mountain Camp, 1[77]; Berry Mountain Brook, 1[2];
Cascapedia River (Middle Camp, 2; Tracadie, 2; Square Forks, 1),
5[2].
Saskatchewan. Poplar Point, Athabasca Lake, 1[75]; Fair Point,
Athabasca Lake, 1[75]; Emma Lake, 1[74]; Harper Lake, 2[77];
Livelong, 3[55]; Fairholme, 2[74]; Touchwood Hills, 2[7]; Indian
Head, 1[86].
Yukon. Hoole Canyon, 1; Teslin Lake (30 mi. N of, 1; Lake itself,
1; "near" the lake, 1; Mts. "near," 2; Snowden Mts., 2; Teslin
Post, 2; Eagle Bay, 1; Morley Bay, 2; Nisutlin River, 1; Nisutlin
Flats, 2; Wolf River, 1; Wolf Lake, 5), 21[77].
Mustela erminea cicognanii Bonaparte
Ermine
Plates 2, 3, 4, 9, 10 and 11
Mustela cigognanii [sic.] Bonaparte, Charlesworth's Mag. Nat.
Hist., 2:37, 1838.
Putorius vulgaris, Emmons, Quadrupeds of Massachusetts, p. 44,
1840.
Mustela pusilla DeKay, Zool. of New York, Pt. 1, Mammalia, p. 34,
pl. 14, fig. 1, 1842. Type from New York State.
Putorius pusillus, Audubon and Bachman, Vivip. Quadrupeds of N.
Amer., 2:100, pl. 64, 1851 (pl. 1846) and erroneously labeled
Mustela fusea, as pointed out on page 102 of text.
Putorius cicognanii, Baird, Mamm. N. Amer., p. 161, 1858.
Putorius richardsoni cicognani, Bangs, Proc. Biol. Soc.
Washington, 10; 18, figs. 4, 4a of pls. 1 and 2, and pl. 3, figs.
2, 2a, February 25, 1896 (part).
Putorius cicognani, Merriam, N. Amer. Fauna, 11:10, pl. 2, figs.
3, 3a, 4, 4a and pl. 5, figs. 2, 2a, June 30, 1896.
Mustela cicognanii cicognanii, Miller, U. S. Nat. Mus. Bull.,
79:95, December 31, 1912; Bishop, Journ. Mamm., 4:26, February 9,
1923.
Mustela cicognanii, Jackson, Journ. Mamm., 3:15, February 8,
1922.
Mustela erminea cicognanii, Hall, Journ. Mamm., 26:77, February
27, 1945; Hall, Journ. Mamm., 26:180, July 19, 1945.
Type.—No type specimen designated; type locality, eastern
United States.
The restriction of the type locality from the general region of
northeastern North America, as given by Merriam (1896:10) to the
less inclusive area of the eastern United States as earlier given
by Bangs (1896:18) is supported by Bonaparte's remarks in
connection with the proposal of the name cicognanii. He says
(1838:37-38) "During my stay in the United States, I only saw a
small species of Mustela, very common throughout the
Union . . . ." This animal constituted basis for the name
cicognanii which name, he points out, is bestowed in order that
the Americans ". . . should have constantly under their eye, this
very common little animal, as the perpetual memorial . . ." to the
Italian Governmental representative ". . . who, for upwards of
fourteen years had served, in diplomatic and commercial
concerns, . . . two countries, . . . so different . . . as the
Roman and the United States. . . ." Clearly he had in mind
principally, if not exclusively, the animal of the United States.
Range.—Transition and higher life-zones of northeastern United
States south to Connecticut, central Pennsylvania and extreme
northeastern Ohio; in Quebec and Ontario westward from the
latitude of central Maine to Lake Nipigon and Lake of the Woods.
See figure 25 on page 95.
Characters for ready recognition.—Differs from M. e.
richardsonii of both sexes, in that least width of color of
underparts averages less than a third rather than two-fifths of
greatest width of color of upper parts, in males skull less,
instead of more, than 1.9 grams and basilar length less than 38,
in females by 16 per cent lighter skull (0.92 versus 1.1 grams);
from M. e. bangsi, in males hind foot less instead of more than
40, linear measurements of skull averaging 11 per cent less (depth
of skull at plane of molars 10.0 versus 11.4), in females
averaging smaller, hind foot 30 versus 32 and depth of skull at
plane of molars 8.6 versus 9.1.
Description.—Size.—Male. Seven adults and subadults from New
York and Pennsylvania, yield average and extreme measurements as
follows: Total length, 266 (240-295); length of tail, 74 (66-80);
length of hind foot, 36 (33-39). Hamilton (1933:294) gives the
weight of 31 adults from New York as 81 (66-105) grams.
Female: Twelve adults and subadults from Maine and the area south
to central Pennsylvania, yield average and extreme measurements as
follows: Total length, 243 (225-260); length of tail, 63 (55-72);
length of hind foot, 29.8 (26-32). Hamilton (1933:294) gives the
weight of 15 adults from New York as 54 (45-71) grams.
Color.—As described in Mustela erminea richardsonii except
that underparts in summer Marguerite Yellow or even more whitish;
least width of color of underparts averaging, in adult males from
New York and Pennsylvania, 29 (27-32) per cent of greatest width
of color of upper parts. Black tip of tail in same series
averaging 42 (30-51) mm. which is 57 per cent of length of
tail-vertebrae.
Skull.—Male (illustrated by 4 adults in table of cranial
measurements, which see): See plates 2-4. As described in Mustela
erminea richardsonii except that: Weight, 1.5 (1.2-1.7) grams;
basilar length, 35.7 (33.8-37.6).
Female (illustrated by adult and subadults recorded in table of
cranial measurements, which see): See plates 9-11. As described in
Mustela erminea richardsonii except that: Weight of 2 subadults,
0.92 (0.86-0.98) grams; basilar length, 32.4 (31.4-33.3).
The skull of the male, in linear measurements, is approximately 13
(12-16) per cent smaller and 40 per cent lighter than in M. e.
richardsonii. In relation to the basilar length, the skull averages
slightly narrower, slightly shallower as measured in the vertical plane
touching the posterior borders of the last upper molars, and the
preorbital part is slightly longer. In skulls of females of
cicognanii, linear measurements average 3 (0-6) per cent less, the
weight is 16 per cent less and the teeth are 5 per cent shorter. In
relation to the basilar length, measurements of the skull are
approximately the same or slightly less in cicognanii.
In comparison with bangsi, the male sex in linear measurements of the
skull and teeth averages 11 per cent less than in bangsi from Aitkin,
Minn., and 6 per cent less than in bangsi from Elk River, but in
relation to the basilar length the preorbital region is larger. The
weight is approximately a fourth less. In females the measurements
average less, some being the same, and in relation to the basilar
length, the bullae are shorter and the skull is shallower. The weight
is about the same.
Remarks.—In January, 1838, in Charlesworth's Magazine of Natural
History, C. L. Bonaparte proposed for three kinds of American weasels
the names Mustela cicognanii, Mustela richardsonii and Mustela
longicauda.
In this paper Bonaparte indicates that he previously had written (for
his Iconografia della Fauna Italica ...) an account of Mustela
cicognanii using this same name. Fasciola XXII of the Iconogr. d.
Fauna Italica, presenting his account of Mustela, like the English
paper was published in the year 1838. In his article in Charlesworth's
Magazine, Bonaparte refers to his book published [used the past tense]
in Rome but whether it actually appeared first I am unable to determine
and hence am uncertain which of the two constitutes the original
description.
Reference to the Italian account suggests as basis for the name M.
cicognanii, (1) specimens possibly seen in the United States by
Bonaparte, or (2) Godman's published account of the animal.
In the English publication, however, Bonaparte actually says that (1)
he saw the small species in the Union [= United States]. Also, he (2)
mentions his earlier written Italian account, (3) mentions that "all
the [American?] naturalists" used the name M. vulgaris for this
animal, (4) incidentally mentions Godman's account, and (5) in naming
two other American species cites accounts of them by Richardson. Also,
Bonaparte in this English article makes clear that when he wrote [not
necessarily published] his Italian paper he did not know of the
existence of two of the three American species.
In the register of mammals at the British Museum of Natural History,
there appears:
43.3.3.3 Mustela longicauda Bonap N Amer. presented by Dr. J. Richardson
4 Mustela Richardsonii Bonap "
5 " Cicognanii Bonap "
To the right of these entries there appears, in three lines, the
notation: "The three specimens examined by Prince Canino on which he
established the three species."
Every part of each of the above entries is in the hand writing of J. E.
Gray, in charge of the collections from 1824 to 1840 and associated
with them as Keeper until 1875. The three specimens are in good
condition considering their age. The catalogue or register number
shows, among other things, that they were entered in the register on
March 3, 1843.
Questions which might occur to anyone are:
(1) Was there a type specimen of Mustela Cicognanii Bonaparte? If so
is it no. 43.3.3.5?
(2) If there was no type specimen was there a type locality? If so what
is it?
Among other things that may have bearing on these questions, are these:
Bonaparte in Charlesworth's Magazine appears to base the two names
Mustela Richardsonii and Mustela longicauda on Richardson's
published account of Mustela erminea. At any rate immediately
following each of the two names, Bonaparte writes "Nob. (M. erminea
Rich. F. Bor. Amer.)." Bonaparte's other, first newly proposed name,
Mustela Cicognanii, in Charlesworth's Magazine has following it only
"Nob. North America," although in a paragraph above he did point out
that this was the animal which all naturalists, at the time he was in
America, considered as M. vulgaris.
Turning to Richardson's account (Fauna Boreali Americana, ...
Quadrupeds, pp. 45-47. 1829) one finds that he recognized two species,
M. vulgaris and M. erminea. Of the first he gives measurements "of
an old female killed at Carlton House." Of the second species he
distinguishes two varieties, the first represented by a specimen, of
which he gives measurements, "killed at Fort Franklin, Great Bear Lake"
and, the second variety "of a larger size, having a longer tail and
longer fore-claws" he indicates the size of by giving measurements of
a specimen taken "in the neighborhood of Carlton House."
The last variety is clearly the basis of Bonaparte's M. longicauda.
The specimen from which Richardson took his measurements I have been
unable to locate [no. 43.3.3.3 in the British Museum, appears to be
another specimen, although of the same subspecies and provided by
Richardson].
The first variety of Richardson's Mustela erminea, clearly is the
basis of Bonaparte's M. Richardsonii. The specimen from which
Richardson took his measurements may well be no. 43.3.3.4 now preserved
in the British Museum of Natural History, but I could not be certain
about this.
Richardson's M. vulgaris is accompanied by measurements of a female
which I have ascertained to my full satisfaction is the identical
specimen now bearing catalogue number 43.3.3.5 said by Gray to be the
specimen on which Bonaparte based his name Mustela cicognanii.
Gray probably saw his guest, Bonaparte, at work on these weasels and
Gray's own written indication perhaps should be accepted at its face
value. I found only 4 Richardson specimens of North American weasel in
the British Museum in 1937 and it is conceivable that Bonaparte, 100
years before, actually had at hand only one specimen each of two kinds
and 2 specimens of the third. This I think is not an important
consideration, though, for Gray says just which specimens did serve as
basis for Bonaparte's names and there is only one specimen for each
name according to Gray.
But I wonder if a type specimen can be made in this way? That is to
say, after a name is published in a manner which makes it available,
and if two or more specimens of the kind of animal involved, were, or
may have been, available to the describer, can a person, even the
author, himself, make a type specimen by saying that one particular
specimen is beyond doubt the specimen on which a given name was
established even though no particular specimen was designated in the
original description? I incline to the view that a specimen so
designated would at most be only a lectotype, unless it were a cotype.
However, if a holotype can be made by action such as Gray took, then
(1) is no. 43.3.3.3 the type specimen of Mustela longicauda Bonaparte
and, (2) is no. 43.3.3.4 the type specimen of Mustela Richardsonii
Bonaparte?
Incidentally, Mustela longicauda Bonaparte whether based on no.
43.3.3.3 or on Richardson's account will continue in its present
application. The same is true of Mustela richardsonii. If the basis
of Mustela cicognanii Bonaparte [the diagnosis in the Iconografia d.
Fauna Italica ... makes it clear that the name applies to the
short-tailed species] was a weasel from the eastern United States or
a description of a weasel or weasels from there, the name will continue
in its present application. If, instead, the name is based on no.
43.3.3.3 (from Carlton House, Saskatchewan) or on Richardson's account
of M. vulgaris, the name will apply to a different subspecies (now
called richardsonii and richardsonii will fall as a synonym of
cicognanii) and the ermine of the eastern United States will take the
next available name. Bonaparte probably named (in manuscript at least)
cicognanii before he ever saw the specimen in the British Museum.
This is indicated by his statement in Charlesworth's Magazine (1838:37)
that "I have now [Italics mine] found two [other] American species. . . ."
Whereas the names richardsonii and longicauda are based on
Richardson, the name cicognanii, even if it dates from the account in
Charlesworth's Magazine, appears to have a composite basis composed at
the very least of (1) animals seen by Bonaparte in the United States,
and (2) those called vulgaris by some other authors. Conceivably the
specimen no. 43.3.3.3 in the British Museum, was part of the basis.
From the nature of the case it can be argued that there could be no
type and that if someone should bring to light a specimen in, say,
Philadelphia, bearing the notation "this is the specimen seen in the
United States by Bonaparte" it would immediately become as important as
the one in London. Any American weasel or weasels (then alive or
preserved in a zoölogical collection) that Bonaparte saw in the United
States probably were of the eastern United States. Bangs (1896:18-21),
for one, previous to the present consideration of the name
cicognanii, restricted it to the ermine of the eastern United States.
Consequently, the name cicognanii, in the present account is applied
to the ermine of the eastern United States. In my opinion there was and
is no type. Almost certainly there was no type if the Fauna Italica
appeared before the account in Charlesworth's Magazine did.
Specimens examined.—Total number, 172, arranged alphabetically
by provinces and states, then (except where indication is given to
the contrary) by counties from north to south within each state or
province. Unless otherwise indicated, specimens are in the U. S.
National Museum.
Connecticut. Windham County: S. Woodstock, Woodstock Lake, 1[2].
Hartford County: Windsor, 1[5]. New London County: Liberty
Hill, 3[75].
Maine. Aroostook County: Quimby, 1[75]; Ashland 2[75].
Piscataquis County: tableland on top of Mt. Katahdin, 1; Chimney
Pond, 3; T. 5, R. 13, 3[5]; "vicinity of Chesnucook," 1[5]; T. 4,
R. 13, 1[5]; Moosehead Lake, 7[75]; Grenville, 10
[75]; Barnard, 3
(1[86]). Penobscot County: South Twin Lake, 1[2]; Lincoln, 11
(7[1], 2[14], 2[50]). Franklin County: Seven Pond Township,
7[75]. Oxford County: Umbago Lake, 1[75]; Upton, 4[86]; Bethel,
1[75]. Hancock County: Bucksport, 17[75]; Naskeag, 1. Lincoln
County: Booth Bay, 1[5].
Massachusetts. Middlesex County: Wilmington, 2; Burlington, 6
(1[75]); Worcester County: Cambridge, 5 (1[5], 3[75]); Sterling,
1[5]. Plymouth County: Middleboro, 7 (1[75]).
New Hampshire. Carroll County: Ossipee, 5. Rockingham County:
Greenland, 1[76]. Cheshire County: Dublin, 1.
New York. St. Lawrence County: Ogdensburg, 1[74]. Franklin
County: Malone, 1[58]. Lewis County: Locust Grove, 1. Warren
County: Lake George, 1. Montgomery County: Amsterdam, 1.
Albany County: Albany, 1[80]. Rensselaer County: Berlin, 2[2];
Schoharie, 1[2]. Thompkins County: Cascadilla Creek, Ithaca,
1[58]. Allegany County: Ford Brook, Wellsville, 1[58]. Ontario
County: Phelps, 1[50]. Cattaraugus County: Cattaraugus, 1[5].
Ontario (localities locally north to south, then west to east).
Thunder Bay Dist.: Grand Bay, Lake Nipigon, 5[86]; Macdiarmid,
2[86]; Oscar, 2[14]; 20 mi. SW Fort Williams, 1[76]; Michipicoten
Island, 3[102]. Algoma Dist.: Michipicoten, 1; Franz, 1[74];
Pancake Bay, 2[77]. Parry Sound Dist.: French River, Georgia
Bay, 1[2]; Seguin Falls, Twp. Montieth, 1[86]. Sudbury Dist.:
Casselman, Rathbun Twp., 1[86]. Nipissing Dist.: Smoky Falls,
near Kapuskasing, 4[86]; Franks Bay, Lake Nipissing, 1[86].
Haliburton County: Gooderham, 1[60]. Simcoe County: Orillia,
1[2]; no locality more definite than county, 1[60]. Carleton
County: Britannia, 5 mi. W Ottawa, 1[77]; Ottawa, 1[77];
Constant Bay, NE? of Ottawa, 1[77]. Wellington County: Mt.
Forest, 2[75]; Guelph, 1[31]. Addington County: Buckshot Lake,
Abinger Twp., 1[86]. Fontenac County: Clear Lake, Arden, 1[77].
Pennsylvania (by counties from west to east). Crawford County:
North Shenango Township, Pymatuning Swamp, 2[9]; Linesville (3 mi.
NW, 1; 3-1/2 mi. W, 2; 3 mi. W, 1; 2 mi. SW, 1; 7-1/2 mi. SW, 1)
6[9]. Potter County: Cherry Springs Farm, Abbott Township, 1; 3
mi. S Inez, South Fork Sinnamahoning Creek, 1[9]. Sullivan
County: Lopez, 1[74]. Lackawanna County: Scranton, 1[1]. Wayne
County: Waymart, 1.
Quebec (west to east). Labelle County: Kamika [= Kiamika] Lake,
2[77]; Lacoste, 2[77]; Trout Lake, probably in this county,
2[77]. Megantic County: Black Lake, 1[77].
Rhode Island. Newport County: Middletown, 2[5].
Vermont. Lamoille County: Mt. Mansfield, 1. Windsor County:
Barnard, 1[5].
Mustela erminea bangsi Hall
Ermine
Plates 2, 3, 4, 9, 10 and 11
Mustela erminea bangsi Hall, Journ. Mamm., 26:176, July 19, 1945.
[Putorius] cicognani, Mearns, Bull. Amer. Mus. Nat. Hist.,
3:235, June 5, 1891.
Putorius richardsoni cicognani, Bangs, Proc. Biol. Soc.
Washington, 10:18, February 25, 1896 (part).
Putorius cicognanii, Cory, Mamm. Illinois and Wisconsin, p. 375,
1912.
Mustela cicognanii, Aldous and Manweiler, Journ. Mamm., 23:250,
August 13, 1942.
Mustela cicognanii cicognanii, Bailey, N. Amer. Fauna, 49:169,
January 8, 1927; Leraas, Journ. Mamm., 23:344, August 13, 1942.
Type.—Male, subadult, skull and skin; no. 11541, D. R. Dickey
Coll.; Elk River, Sherburne County, Minnesota; November 1, 1925;
obtained by Bernard Bailey, original no. A 606.
The skull is complete and the teeth all are present and entire.
The skin is well made and in a good state of preservation.
Range.—Southern Manitoba, northeastern North Dakota, the whole
of Minnesota, Wisconsin and Michigan and northern Iowa. See figure
25 on page 95.
Characters for ready recognition.—Differs from M. e.
richardsonii, in that, in both sexes, least width of color of
underparts averages about a third, instead of two-fifths, of
greatest width of color of upper parts, and in that skulls of
males are a fifth or more lighter, basilar length averaging less
than 40; from M. e. cicognanii, in that hind foot more than 40
in males, averaging 32 versus 30 in females, and in larger skull,
depth of skull at plane of molars being 11.4 versus 10.0 in males
and 9.1 versus 8.6 in females.
Description.—Size.—Male: Twelve adult and subadult males
from Aitkin, Minnesota, yield average and extreme measurements as
follows: Total length, 316 (291-341); length of tail, 87 (70-101);
length of hind foot, 43 (40-44). Two adults from Aitkin each weigh
170 grams.
Four adult and subadult females from Elk River and Fort Snelling,
Minnesota, yield average and extreme measurements as follows:
Total length, 249 (240-260); length of tail, 61 (55-65); length of
hind foot, 32 (30-33).
Color.—As described in Mustela erminea richardsonii except
that, least width of color of underparts averaging, in males from
Minnesota, 32 (19-51) per cent of greatest width of color of upper
parts. Black tip of tail in 12 male topotypes in white winter
pelage averaging 52 (45-58) mm. which is 60 (53-66) per cent of
length of tail-vertebrae.
Skull.—Male (based on adults from Aitkin): See measurements and
plates 2-4. As described in Mustela erminea richardsonii except
that: Weight of 2 adults from Aitkin, 2.2, 2.3 grams (9 subadults
from T. 61 N, R. 26 W, average 1.95 grams); basilar length, 39.7
(38.5-40.7); length of tooth-rows rarely more (usually less) than
length of tympanic bulla.
Female (based on adults from Minnesota as listed in table of
cranial measurements, which see): See plates 9-11. As described in
Mustela erminea richardsonii except that: Weight, of a subadult
from T. 61 N, R. 26 W, 0.91 grams; basilar length, 32.8
(31.8-33.6); breadth of rostrum rarely equal to as much as 30 per
cent of basilar length.
From richardsonii, topotypes of bangsi differ in that cranial
measurements in males are approximately 7 (5-9) per cent less, linear
measurements of teeth are 10 (9-11) per cent less and the skull is a
fifth lighter. In relation to basilar length the tympanic bullae of
bangsi are longer. Skulls of females are individually
indistinguishable, those of bangsi averaging approximately 1 per cent
less in linear measurements. Comparison with the smaller cicognanii is
made in the account of that subspecies.
Remarks.—Before the subspecific name bangsi was proposed,
individuals of this subspecies ordinarily were recorded in the
literature as Mustela cicognanii. The best single lot of material is
in the zoölogical collection of the University of Wisconsin. The late
naturalist Albert Lano preserved a large share of the material from
Minnesota. The large series from Elk River of that same state was
mostly collected by Bernard Bailey although his Aunt, Anna (Bailey)
Mills, and her brother the late Vernon Bailey, at an earlier time saved
some specimens from Elk River. The name bangsi was proposed in
recognition of the superior work done on American weasels by the late
Outram Bangs.
From the range of M. e. invicta in the Rocky Mountains, that of
bangsi is separated by the Great Plains from a large part of which
region the species is unknown. M. e. bangsi differs from invicta in
greater degree of sexual dimorphism in size, and in each sex by larger
size, narrower light-colored underparts, and deeper braincase as
measured at the anterior margin of the basioccipital. In bangsi the
braincase is deeper relative to the length of the skull as well as, of
course, actually deeper.
Of the two subspecies whose ranges do meet that of bangsi, it more
closely resembles richardsonii than cicognanii. From
richardsonii, especially from southeastern populations of the same in
which the skull is of the same size as in bangsi, the latter differs
in longer hind feet. This is an average difference and by one
interpretation the animals here referred to bangsi might be lumped
with some of the populations from the southeastern part of the range of
richardsonii and the whole lot treated as intergrades between
richardsonii and cicognanii. Nevertheless, the animals here
referred to bangsi are not geographically intermediate between
richardsonii and cicognanii and this consideration had much to do
with the decision to recognize as a separate subspecies the animals
here named bangsi.
Within the range of the subspecies there is some geographic variation;
the hind feet of animals from Iowa average slightly shorter than those
of animals from Minnesota and Wisconsin but are nowhere nearly so short
as in cicognanii at the same latitude in the eastern United States.
It is noteworthy that the few specimens seen from Isle Royal have the
long hind feet of bangsi and not the short hind feet of cicognanii
which occurs all along the northern mainland.
Because an oft cited record of occurrence even though erroneous, has a
way of being repeated in later works, attention is here called to the
alleged occurrence of this ermine in northwestern Ohio at New Bremen.
Henninger (1921:239) published the original account of the supposed
occurrence but as I pointed out in 1937 (p. 304), the specimen
concerned proved upon examination to be a female of Mustela frenata
noveboracensis. Henninger was misled probably by the short tail; the
end of the tail had been lost and healed over before the animal's
death. The present study has revealed that M. erminea everywhere east
of the Cascade Mountains assumes a white winter coat. Had this been
known when Henninger obtained his specimen he probably would not have
wrongly identified the animal from New Bremen which was in the brown,
winter pelage.
Specimens examined.—Total number, 222, arranged alphabetically
by provinces and states and, arranged from north to south, by
counties in each state. Unless otherwise indicated, specimens are
in the University of Wisconsin Museum of Zoölogy.
Iowa. Dickinson County: W side Lake Okobogie, 1[48]. Winnebago
County: Lake Mills, 7[65]. Worth County: Northwood, 1[65].
Clay County: "Dewey's Pasture, near Ruthven," 1[76].
Manitoba. Aweme, 4[47]; Red River Settlement, 1[91].
Michigan. Isle Royal: Tobin Harbor, 1[76]; Bell Isle, 1[76];
Washington Harbor, 3[76]. Ontonagon County: Ontonagon, 2 (1[76],
1[14]); T. 51N, R. 43W, S. 17, Porcupine Mts., 1[76]. Gogebic
County: Little Girls Point, 5[76]; Ironwood, 1[76]. Iron
County: no locality more definite than county, 1[76]. Luce
County: Tahquamenon River Falls, 1[91]. Chippewa County: Sault
Ste. Marie, 2[76]. Emmet County: Wilderness State Park, 2[76].
Cheboygan County: Univ. Mich. Biol. Station, 1[76]. Washtenaw
County: Ann Arbor, 1[76].
Minnesota. Kittson County: no locality more definite than
county, 1[2]. Roseau County: Deer Township, 1[14]; Falun
Township, 2[14]. Marshall County?: Moose River, 5[93]; Warren,
definitely in Marshall County, 1[93]. Cook County: Grand Marais,
3 (2[76], 1[14]). St. Louis County: 2 mi. E Babbitt, 14[93];
Burntside [= Burnside] Lake, 1[91]. Itasca County: T. 61N, R.
26W, 23. Clay County: Moorhead, 3[9]. Aitkin County: Aitkin,
13 (11[60], 1[7], 1[4]). Otter Tail County: Arthur, 3[60]; Ten
Mile Lake, 1[76]; Parkers Prairie, 2[75]. Chisago County: North
Branch, 1[60]. Sherburne County: Elk River, 42 (16[91], 5[14],
20[59], 1[74]). Hennepin County: Lake Minnetonka, 1[75];
Minneapolis, 1[91]; Fort Snelling, 5 (4[2], 1[60]).
North Dakota. Pembina County: Walhalla, 1[91]. Nelson County:
Stump Lake, 1[91]. Eddy County: Brantford, 2[76].
Wisconsin. Douglas County: T. 44N, R. 13W, 1; Gordon, 1.
Bayfield County: Brinks Camp, Washburn, 1[2]; "near Cable," 1.
Ashland County: Bear Lake, 2. Iron County: Fisher Lake, 4;
Mercer, 5. Vilas County: Mamie Lake, 16[91]; Ox Bow Lake, 1[91].
Oneida County: Tomahawk Lake, 1[60]. Langlade County: T. 34N,
R. 11E, 3. Rush County: Ladysmith, 1. Dunn County: Colfax, 2.
Door County: Mink River, Ellison Bay, 1[76]. Dodge County: Fox
Lake, 1[50]; Beaver Dam, 12[50].
Mustela erminea invicta Hall
Ermine
Plates 2, 3, 4, 9, 10, 11 and 41
Mustela erminea invicta Hall, Journ. Mamm., 26:75, February 27,
1945; Hall, Journ. Mamm., 26:180, July 19, 1945.
Putorius cicognanii, Preble, N. Amer. Fauna, 27:230, October 26,
1908.
Type.—Male, subadult, skull and skin; no. 101122, Mus. Vert.
Zoöl.; Benewah, Benewah County, Idaho; October 24, 1926; obtained
by William T. Shaw.
The skull has a hole in the right squamosal bone on the floor of
the braincase, and lacks the hamular process of the left
pterygoid. The postmolar part of the right lower jaw is missing.
The teeth all are present and entire. The skin is in white, winter
pelage, well made, and in a good state of preservation.
Range.—Central Rocky Mountain region from Jasper Park south
over Alberta, southeastern British Columbia, Washington east of
the Cascades, and north and central Idaho and northwestern
Montana. See figure 25 on page 95.
Characters for ready recognition.—Differs from M. e.
richardsonii, in males, by skull lighter than 1.9 grams, mastoid
breadth less than 19.9, depth of skull at anterior margin of
basioccipital less than 12.4, in females by corresponding
measurement of depth less than 10.1, and weight of skull less than
one gram; from M. e. fallenda, in both sexes, by upper lips
white (not brown), in males by skull averaging longer (37.0 versus
35.7), in females by breadth of rostrum less, instead of more,
than 30 per cent of basilar length; from M. e. streatori,
gulosa, and muricus by hind foot more than 36 and basilar
length more than 35 in males and by hind foot more than 29.5 and
basilar length more than 30.5 in females; further distinguished
from streatori by white (not brown) upper lips and from gulosa
by black tip of tail more than half length of tail-vertebrae.
Description.—Size.—Male: Ten adults and subadults from
central Idaho County yield average and extreme measurements as
follows: Total length, 291 (272-328); length of tail, 86 (75-100);
length of hind foot, 39.9 (38-44).
Female: Five adults and subadults from the same locality yield
average and extreme measurements as follows: Total length, 255
(245-270); length of tail, 71 (68-76); length of hind foot, 32.3
(32-33).
Color.—As described in Mustela erminea richardsonii except
that underparts in summer Marguerite Yellow or more whitish; least
width of color of underparts averaging, in four females from Idaho
and Montana, 38 (33-43) per cent of greatest width of color of
upper parts. Black tip of tail in same specimens 38 (31-42) mm.
which is 57 (52-65) per cent of length of tail-vertebrae.
Skull.—Male (5 adults from Idaho County): See measurements and
plates 2-4. As described in Mustela erminea richardsonii except
that: Weight, 1.5 (1.4-1.7) grams; basilar length, 37.0
(35.8-39.8).
Female (illustrated by adult and 4 subadults in table of cranial
measurements, which see): See plates 9-11. As described in
Mustela erminea richardsonii except that: Weight, 0.72 (0.7-0.9)
grams; basilar length, 32.2 (31.6-32.8).
From fallenda, invicta differs in that the skull of the male has a
relatively narrower rostrum and relatively shallower braincase. Females
show the same differences but the degree of difference is about as
great again as in males. The teeth are almost exactly the same size in
the two subspecies. The weight is the same in males but in females
invicta is 18 per cent heavier.
From streatori, invicta differs in that males average larger in
every measurement taken except that the anteroposterior diameter of the
inner moiety of M1 is less; 36 per cent heavier; linear measurements of
the skull are about 5 per cent larger and those of the teeth, with the
one exception noted, about 6 per cent larger; relative to the basilar
length the tympanic bullae are longer and the rostrum is relatively
narrower. In females, measurements of the skull average 8 per cent more
and those of the teeth 7 per cent more except that, as in males, the
inner lobe of M1 is actually shorter. Females of invicta are 12 per
cent heavier; relative to the basilar length the skull is narrower
throughout and the tooth-rows are shorter than in streatori.
From gulosa, invicta differs in that males average larger (about 12
per cent) in every measurement taken, excepting the anteroposterior
diameter of M1 which is the same; 50 per cent heavier; relative to the
basilar length the length of the tooth-rows and interorbital breadth
are less. In females the inner lobe of M1 is smaller but every other
measurement taken of the skull and teeth is more, invicta averaging
about 8 per cent larger and 22 per cent heavier; relative to the
basilar length, the tooth-rows are shorter and the skull is narrower
interorbitally, through the rostrum and across the zygomata.
From murica, invicta of corresponding sex differs in being larger
in every measurement taken; males average 17 per cent larger in cranial
measurements, 13 per cent larger in dental measurements and are 83 per
cent heavier; corresponding percentages for females are 11, 9 and 20.
Exception must again be made for the anteroposterior diameter of the
inner lobe of the last upper molar which is less in females, and only
slightly more in males. In males of invicta the tympanic bullae are
longer in relation to the basilar length.
From the geographically remote cicognanii, skulls of both males and
females of invicta are to me individually indistinguishable. There
is, nevertheless, an average difference not apparent to the eye between
skulls of males. If the length of the tooth-rows be taken as a standard
(100 per cent), the rostrum, of invicta, as measured across the
lacrimal processes is broader (89 rather than 84 per cent) but the
width across the fourth upper premolars is less, 94 rather than 97 per
cent of the length of the tooth-rows.
Since the skull of invicta closely resembles that of cicognanii, it
follows that invicta differs from richardsonii and bangsi in
about the manner described in the account of cicognanii.
Remarks.—Animals of this subspecies in advance of the present study
generally were recorded in the literature under the name Mustela
cicognanii. The difficulty in distinguishing individual specimens of
invicta on morphological grounds from those of the geographically
remote M. e. cicognanii should not be taken to indicate that the
populations do not differ appreciably. Actually they differ in several
characters although in no one of these is the degree of difference
sufficient to allow of using it alone as a certain means of diagnosis.
In invicta, as compared with cicognanii, the light-colored
underparts are wider in relation to the dark-colored upper parts and
the tail is longer by 4 per cent relative to the head and body. Given a
population of each of the two subspecies, in which the skull is of the
same mass, the hind feet are longer in invicta, there is more sexual
dimorphism in size, and the anterior part of the skull differs in some
particulars as just described in the comparison of the skull of
invicta with other forms. Nevertheless, each of these differences is
of an average sort. Therefore, and because overall size is about the
same in the two subspecies concerned, one or a few specimens from, say,
central Idaho, can be distinguished from animals from western
Pennsylvania only with difficulty, if at all. The close resemblance of
skulls of invicta and cicognanii may be a function of their living
at approximately the same latitudinal position in a climate that has
marked seasonal variation.
Intergradation with richardsonii is complete and gradual; in one
sense invicta is but little more than a small richardsonii.
Intergradation with fallenda is shown by several specimens. These two
races differ in large degree in color, and in size and shape of the
skull of females. Although the geographic area where intergradation in
color occurs is fairly wide, the area in which intergradation in
cranial characters in females occurs, appears, from the inadequate
material available, to be much narrower. Intergradation occurs freely
in Washington with streatori but with muricus so far as known only
in the Bitterroot and nearby mountains of northwestern Montana. The
Snake River Plains and low country along much of the Columbia River
appears to be uninhabited by weasels of the species erminea and hence
there is opportunity for intergradation only in the mentioned area of
Montana.
Specimens examined.—Total number, 177, as follows. Arranged
alphabetically by provinces and states then by localities from
north to south in each province and by counties from north to
south in each state. Unless otherwise indicated, specimens are in
the United States National Museum.
Alberta. Jasper House, 4[77]; Shovel Pass, 2[77]; Jasper Park,
10[77]; head of Smoky River, 9; Henry House, 2 (1[77]); Blindman
River, 1[2]; forks of Blindman River and Red Deer River, 2 (1[60],
1[75]); "near Red Deer, Red Deer River," 1[77]; Red Deer River, 2
(1[2], 1[60]); Red Deer, 2[60]; Rosebud, 2[77]; Prairie, 3000
ft., 1; Didsbury, Little Red Deer River, 1; Canadian Nat'l Park,
1[60]; Canmore, 1; Banff, 1[60]; High River, 1[86]; "Waterton Lake
Park" in Alberta, 6[77].
British Columbia. Grand Forks of Fraser River, 1; Canoe River,
1[77]; Field, 1; Glacier, 1[58]; E side Beaverfoot Range,
4000-4500 ft., 6 mi. SE Fraser Creek, 8[74]; Wentworth Lake,
1[31]; Revelstoke, 2 (1[77], 1[60]); Spillimacheen[e]en River,
2[2]; Sicamous, 2; Albert River, 7000 ft., 1[2]; Lumby, Creighton
Valley, 1[31]; Okanagan, 4 (2[75], 1[94], 1[2]); Kettle River
Lake, Gold Range, 4000 ft., 1; Crows Nest Station, 1[74]; Yale
District, 3; Fort Hope, 1; Chilliwack Lake, 1[77]; Skagit, 2
(1[77], 1[31]); Skagit Valley, 1[77]; Skagit Summit, 1[77];
Lightning Lakes, 2 mi. N International Boundary, 3;
Osoyoos-Bridesville Summit, 2; Westbridge, 1[77]; Rossland,
5[77]; Creston, mouth Goat Creek, 3[77]; Yahk, 4[77].
Idaho. Bonner County: Coolin, 4. Benewah County: Benewah,
1[55]. Idaho County: "Pete Kings, Lochsa River," 1[97]; 2 mi.
SSE Selway Falls, 1900 ft., 1[8]; 4 mi. SW Selway Falls, 5800 ft.,
3[8]; Newsome Cr., 12 mi. above jct. with S Fk. Clearwater River,
2[74]; Iron Mt., to 14 mi. W thereof, 24[74]; Pilot Cr., 3/4 to
2-1/2 mi. above Newsome Cr., 4[74]; Sawmill Cr., 1-1/4 mi. W
Newsome, 1[74]; between Selway River and S. Fk. Clearwater R.,
4[74].
Montana. Teton County (of old arrangement of counties): Many
Glacier, 4900 ft., 1[74]; Duck Lake, 6 mi. NE St. Marys Lake, 1;
St. Marys, Glacier Park, 1[76]; Lower St. Marys Lake, 1[2].
Flathead County: Stanton Lake, 5. County in question: Bitter
Root Mts., 1. Ravalli County: Tin Cup District, 1[74]; Bass
Creek, 6800 ft., NW of Stevensville, 1; Capitan Peak, 7000 ft., 1;
Darby, 2[74]; Girds Creek, 1[74]; Charlos Heights, 2[74].
Washington. Whatcom County: Twin Lakes, Winchester Mts., 3
(1[10]); Chilliwack River, 2600 ft., 2; Cooper Creek, near head,
4500 ft., Hannegan Pass, 1; Cooper Cr., 4300 ft., Hannegan Pass,
1[10]; Beaver Creek (2500 ft., and at McMillan Ranch, 1700 ft.),
2; Barron, Bornite Mine, 5000 ft., 1. Okanogan County: Tungsten
Mine, 6800 ft., Bauerman Ridge, 4; Hidden Lakes, 4100 ft., 1; West
Fork Pasayten River, 4700 ft., 1. Stevens County: Orin, 1[51].
Pend Oreille County: Ione, 2[51]. Chelan County: Lake Chelan,
1[46].
Mustela erminea alascensis (Merriam)
Ermine
Plates 2, 3, 4, 9, 10 and 11
Putorius richardsoni alascensis Merriam, N. Amer. Fauna, 11:12,
pl. 2, figs. 2, 2a, June 30, 1896.
Putorius cicognanii alascensis, Miller, U. S. Nat. Mus. Bull.,
79:96, December 31, 1912; Swarth, Univ. California Publ. Zoöl.,
7:140, January 12, 1911.
Mustela erminea alascensis, Hall, Proc. Biol. Soc. Washington,
57:36, June 28, 1944; Hall, Journ. Mamm., 26:180, July 19, 1945.
Type.—Male, adult, skull and skin; no. 74423, U. S. Nat. Mus.,
Biol. Surv. Coll.; Juneau, Alaska; August 22, 1895; obtained by
Clark P. Streator, original no. 4806.
The skull shows malformation of the frontal sinuses due to
parasites and lacks osseous tissue where the parasitic infestation
was localized. The left exoccipital condyle and adjacent region is
less developed than the right and the posterior part of the skull
is bent slightly to the left. Otherwise the skull is unbroken. The
teeth all are present and entire. The skin is in the brown summer
coat, fairly well made and in a good state of preservation. A few
white hairs persist where the proximal line of the black hair of
the tip of the tail meets the distal line of the brown hair.
Range.—Mainland of southeastern Alaska from Lynn Canal south to
include Mitkof, Zarembo, Wrangel and Revillagigedo islands. See
figures 25, 26 on pages 95 and 134.
Characters for ready recognition.—Differs from M. e. arctica
and haidarum, in both sexes, by proximal two-thirds of under
side of tail colored same as upper parts rather than same as
underparts and interorbital breadth less, instead of more, than
distance between glenoid fossa and posterior border of external
auditory meatus; from M. e. salva, in males, by overall depth of
braincase including tympanic bullae less than 89 per cent of
orbitonasal length, females not individually distinguishable but
averaging shallower through the braincase; from M. e. initis,
celenda and seclusa by interorbital breadth less than distance
between glenoid fossa and posterior border of external auditory
meatus (females of initis, celenda and seclusa unknown);
further from initis by total length less than 317 and black tip
of tail less than 57 per cent of length of tail-vertebrae; further
from celenda by chest white, not mostly covered by brown patch.
Description.—Size.—Male: Eight adults from Windham, Alaska,
yield average and extreme measurements as follows: Total length,
298 (288-315); length of tail, 88 (84-94); length of hind foot,
41.3 (37-44).
Female: Two adults from Juneau and Helm Bay measure, respectively,
as follows: Total length, 258, 258; length of tail,——, 76;
length of hind foot, 32, 34.
Color.—As described in Mustela erminea richardsonii except
that least width of color of underparts averaging, in five
females, 42 (35-53) per cent of greatest width of color of upper
parts. Black tip of tail in same specimens averaging 36 (30-40)
mm. which is 49 (48-53) per cent of length of tail-vertebrae.
Skull.—Male (based on 8 adults from Windham): See measurements
and plates, 2-4. As described in Mustela erminea richardsonii
except that: Weight, 1.8 (1.5-2.6) grams; basilar length, 37.5
(36.5-38.9); length of tooth-rows more or less than (about same
as) length of tympanic bulla.
Female (based on 5 adults, from localities listed in the table of
cranial measurements): See measurements and plates 9-11. As
described in Mustela erminea richardsonii except that: Weight,
0.96 (0.7-1.1) grams; basilar length, 32.7 (31.9-33.2); breadth of
rostrum more or less than (about equal to) 30 per cent of basilar
length.
From richardsonii, alascensis differs in that the skull of the male
averages smaller in every measurement taken and is 28 per cent
lighter. Relative to the basilar length, the orbitonasal length is more
and the braincase is shallower as measured at the anterior end of the
basioccipital. The four adult females seen of alascensis are more
variable than those of richardsonii and average smaller in some
measurements and larger in others but give no proof of any consistent
difference.
From haidarum, alascensis differs in that the rostrum and entire
preorbital part of the skull is actually as well as relatively much
smaller in both sexes. In males of alascensis the length of the
skull, and other cranial measurements of length, is more. In males, the
mastoid breadth and zygomatic breadth are about the same as in
haidarum, as also is the weight. M1 is larger but m1 and P4 are
smaller. In females the anteroposterior extent of the inner moiety of
M1 and length of tympanic bulla are about the same in the two
subspecies but all other cranial and dental measurements in
alascensis are less. It is 29 per cent lighter. The difference in the
preorbital region is of about the same degree as in the males.
Comparisons of the skull with those of arctica, salva, initis,
celenda, and seclusa are made in the accounts of those subspecies.
Remarks.—The relatively few specimens known of this race seem always
to have been referred to in the literature by the name alascensis and
the nomenclatural history is therefore simple. The original materials
were obtained by the collector Clark P. Streator and the additional
series of skeletons, one with skin, from Windham were procured by
Stanton Price, a resident there.
The subspecies is well differentiated from both arctica and
richardsonii. Although actual intergrades are lacking between
alascensis and the two races just mentioned I have no doubt that
intergradation occurs with richardsonii and think it probably does
also with arctica.
The assignment of the three females from Mitkof Island, Zarembo Island,
and Loring on Revillagigedo Island, is tentative because each is so
young as not to show diagnostic cranial characters. The two other
specimens from Revillagigedo Island (Carroll Inlet), labeled as males,
are in white winter pelage. Only one, no. 136358, a subadult, is
accompanied by a skull. The small size of each specimen, and its
cranial characters which are intermediate between those of males and
females of alascensis of the adjacent mainland, indicate the
existence of a distinct race of weasel on Revillagigedo Island. On the
chance that the one specimen with a skull is a dwarf, or is wrongly
sexed as seems improbable, the population is tentatively referred to
alascensis.
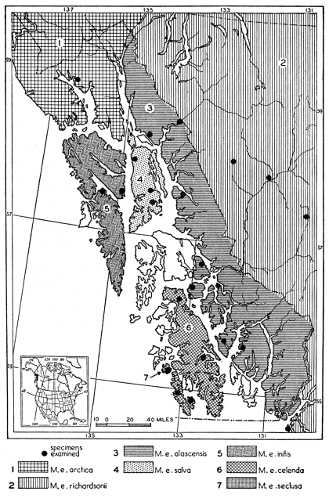
Fig. 26. Map showing known occurrences and probable
geographic ranges of the subspecies of Mustela erminea in
southeastern Alaska.
Specimens examined.—Total number, 24, arranged by localities
from north to south. Unless otherwise indicated, specimens are in
the Museum of Vertebrate Zoölogy, University of California.
Alaska. Juneau, 5[91]; Taku River, 1; Windham, 9; Mitkof Island,
1; St. John Harbor, Zarembo Island, 1; Wrangel, 1[91]; Helm Bay,
Cleveland Peninsula, 1; Cleveland Peninsula, 2[91]; Revillagigedo
Island, Carroll Inlet, 2[91]; Loring, 1[91].
Mustela erminea salva Hall
Ermine
Plates 2, 3, 4, 9, 10 and 11
Mustela erminea salva Hall, Proc. Biol. Soc. Washington, 57:35,
June 28, 1944; Hall, Journ. Mamm., 26:180, July 19, 1945.
Type.—Male, adult, skull only; no. 74641, Mus. Vert. Zoöl.;
Mole Harbor, Admiralty Island, Alaska, December 27, 1936; obtained
by A. Hasselborg.
The skull (plates 2-4) shows malformation of the frontal sinuses
owing to parasites and lacks osseous tissue where the parasitic
infestation was localized. The skull is unbroken. The teeth all
are present and entire.
Range.—Admiralty Island, Alaska. See figures 25, 26 on pages
95, 134.
Characters for ready recognition (known only from
skulls).—Differs from males of M. e. alascensis in overall
depth of braincase which is more than 89 per cent of orbitonasal
length; from M. e. initis, in males, in that orbitonasal length
and mastoid breadth total less than 35 mm., weight of skull and
lower jaws less than 2.1 grams; from M. e. celenda, in males, in
that breadth of rostrum measured across lacrimal processes less
than a third of basilar length.
Description.—Size.—Male: An adult from Gambier Bay measures:
Total length, 320; length of tail, 95; length of hind foot, 45 (41
in dry skin).
Female: A subadult from Hawk Inlet, measures: Total length, 250;
length of tail, 70; length of hind foot, 33.
Color.—As described in Mustela erminea richardsonii except
that least width of color of underparts in four individuals 40
(38-43) per cent of greatest width of color of upper parts. Black
tip of tail, in two individuals for which external measurements
are given, amounting to 50 and 40 mm. respectively which is 53 and
57 per cent of length of tail-vertebrae.
Skull.—Male (type and 4 adult topotypes): See measurements and
plates 2-4. As described in Mustela erminea richardsonii except
that: Weight, 1.7 (1.5-1.9) grams; basilar length, 37.8
(36.4-39.5, extremes are in subadults); length of tooth-rows more
or less (usually more) than length of tympanic bulla; interorbital
breadth rarely more than distance between glenoid fossa and
posterior border of external auditory meatus.
Female (2 ad. and 1 ad.-sad. topotypes): See measurements, and
plates 9-11. As described in Mustela erminea richardsonii except
that: Weight, 0.9 (0.8-1.0) grams; basilar length, 33.0
(32.0-33.6); length of tooth rows approximately same as length of
tympanic bulla; breadth of rostrum approximately 30 per cent of
basilar length.
From alascensis, salva differs in that males have the preorbital
region slightly wider in relation to the length of the tympanic bulla;
also the braincase is smaller, actually as well as in comparison with
the preorbital part of the skull. The tympanic bullae do not project so
far below the squamosals and the braincase itself is shallower, in
adults averaging only 11.5 mm. as against 12.5 mm. The overall depth of
the braincase, including the tympanic bullae, when divided into the
orbitonasal length gives an average of 93 (90-97) per cent whereas in
alascensis the figure is only 85 (78-88) per cent. On this basis
alone, everyone of the adult skulls of the two races can be
distinguished. The females and subadult males show the same tendency to
reduction in depth of braincase but not every individual among them can
be surely distinguished. By weight the skull of salva of
corresponding sex is only about 6 per cent smaller. Comparisons with
initis and celenda are made in the accounts of those subspecies.
Remarks.—Most of the specimens seen were collected by Allen E.
Hasselborg, resident on Admiralty Island. On the basis of skulls—few
skins, and measurements taken in the flesh, are available—salva more
closely resembles alascensis than does any other subspecies so far
known from southeastern Alaska. The race on Admiralty Island is only
slightly differentiated from alascensis of the adjacent mainland.
Specimens examined.—Total number, 26, all from Admiralty
Island, Alaska, arranged in general by localities from north to
south, and unless otherwise indicated in the Museum of Vertebrate
Zoölogy, University of California.
Alaska. Admiralty Island: Hawk Inlet, 2; Seymour Canal, 4; Mole
Harbor, 18 (skulls only); Gambier Bay, 1; no locality more
definite than Admiralty Island, 4 (1 in U. S. Nat. Mus.).
Mustela erminea initis Hall
Ermine
Plates 4, 5 and 6
Mustela erminea initis Hall, Proc. Biol. Soc. Washington, 57:37,
June 28, 1944; Hall, Journ. Mamm., 26:180, July 19, 1945.
Type.—Male, adult, skull and skin; no. 289, Mus. Vert. Zoöl.;
Saook Bay, Baranof Island, Alaska; October 9, 1907; obtained by A.
Hasselborg, original no. 4.
The top of the skull is fractured on the left side from the
anterior nares posteriorly through the postorbital process to the
posterior root of the zygomatic arch. On the left lower jaw the
canine and three incisors are missing; otherwise the teeth all are
present and entire.
The skin is in process of molt, approximately nine-tenths of the
incoming white pelage being in place. The skin is well made and in
a good state of preservation.
Range.—Chichagof and Baranof islands, Alaska. See figures 25,
26 on pages 95, 134.
Characters for ready recognition (only males known).—Differs
from M. e. arctica, in that proximal two-thirds of under side of
tail colored same as upper parts rather than same as underparts,
zygomatic breadth less than distance between last upper molar and
jugular foramen; from M. e. salva in that orbitonasal length and
mastoid breadth total more than 35 mm., weight of skull and lower
jaws more than 2.1 grams; from M. e. alascensis, by total length
more than 317, black tip of tail more than 57 per cent of length
of tail-vertebrae, interorbital breadth more than 10.3 and equal
to, instead of less than, distance between glenoid fossa and
posterior border of external auditory meatus; from M. e. celenda
by chest white (not mostly covered by brown patch), breadth of
rostrum measured across lacrimal processes less than a third of
basilar length; from M. e. seclusa in zygomatic breadth more
than distance between last upper molar and jugular foramen.
Description.—Size.—Male: The type and an adult topotype
measure, respectively, as follows: Total length, 330, 320; length
of tail, 95, 95; length of hind foot, 45, 45.
Female: No external measurements available.
Color.—As described in Mustela erminea richardsonii except
that least width of color of underparts averages, in two young
female topotypes, 50 (49, 50) per cent of greatest width of color
of upper parts. Black tip of tail in three young female topotypes
averaging 54 (52-55) mm. which is 67 (63-69) per cent of length of
tail-vertebrae.
Skull.—Male (illustrated by type and 1 ad. topotype): See
measurements and plates 4-6. As described in Mustela erminea
richardsonii except that: Weight, 2.3 and 2.5 grams; basilar
length, 39.6, and 40.5; interorbital breadth equal to distance
between glenoid fossa and posterior border of external auditory
meatus.
Female: No adults available.
From salva, initis differs in that skulls of males average larger
in every measurement taken, being 41 per cent heavier. Relative to the
basilar length, the interorbital and preorbital parts of the skull are
larger; the relatively greater interorbital and mastoid breadths are
particularly noticeable. Although the depth of the braincase, including
the tympanic bullae, is both relatively as well as actually more than
in salva, the depth is relatively less than in alascensis which
otherwise differs from initis in about the same way that salva
differs from initis. Whereas the interorbital breadth in initis is
about equal to the distance between the glenoid fossa and the posterior
border of the external auditory meatus, the interorbital breadth is
uniformly less than this distance in both salva and alascensis. In
comparison with seclusa the teeth are of the same size but all
measurements of the skull are larger. The skull of initis is 25 per
cent heavier. In relation to the basilar length, the interorbital and
preorbital parts of the skull are much less in initis. The preorbital
and interorbital regions in initis are relatively smaller in
comparison also with arctica. The one measurement of interorbital
breadth in initis is greater in relation to the basilar length than
in kadiacensis but the rostral region, and all that part of the skull
anterior to the braincase, is relatively smaller in initis.
Remarks.—The two adult males, nos. 286 and 289 from Saook Bay,
provide convincing evidence of the existence of a distinct race of
weasel on Baranof Island. Three other young specimens, almost subadult,
from the same place are labeled as males although the basilar lengths
of these skulls are only 35.5, 35.9 and 37.3 millimeters as against
39.6 and 40.5 in the two adult males. The difference in size is too
great to be age-variation. The fact that 3 are definitely of one
category and 2 of the other makes it doubtful that individual variation
accounts for the differences. The small size of these 3 specimens and
the fact that in each the anterior margin of the tympanic bulla is
flush with the squamosal rather than protruded from the braincase,
suggests that the three are females. If they are females, the amount of
secondary sexual variation is rather less than would be expected by
analogy with the amount obtaining in alascensis on the mainland and
in salva on Admiralty Island. Another possibility that I can not
disprove is that two stocks of weasels persist on Baranof Island, the
two larger specimens being descendants of the stock which first became
established on the island and the three smaller specimens being
descendants of an individual ermine, or of ermines, that were rafted or
otherwise transported to the island at a considerably later date.
Assuming for the moment that there are two stocks, it must be admitted
that each one differs from any stock known from elsewhere. Therefore,
each stock would be presumed to have been long resident on the island.
But—two stocks as closely related as the two in question would not be
expected to persist for long in an area as small as that of Baranof
Island because competition would give one the ascendancy. Therefore,
the first suggestion, namely that the three smaller animals are really
females, seems the more probable. The feasible way to clear up the
present uncertainty is, of course, to obtain additional specimens,
carefully labeled as to sex. Yet another reason why additional
collecting is desirable in this area is to ascertain whether there is
subspecific differentiation between the ermines of Baranof and
Chichagof islands. The one specimen available from the latter island,
although in general like the three smaller animals from Baranof Island,
differs in the fuller (less scooped out) medial side of the tympanic
bulla and to a slight degree in each of some other features. This
specimen from Chichagof Island is labeled as a male also.
Specimens examined.—Total number, 6, arranged by localities
from north to south, and in the Museum of Vertebrate Zoölogy,
University of California.
Alaska. Chichagof Island, Freshwater Bay, 1. Baranof Island, Saook
Bay, 5.
Mustela erminea celenda Hall
Ermine
Plates 5, 6 and 7
Mustela erminea celenda Hall, Proc. Biol. Soc. Washington, 57:38,
June 28, 1944; Hall, Journ. Mamm., 26:181, July 19, 1945.
Type.—Male, adult, skull and skin; no. 130987, U. S. Nat. Mus.,
Biol. Surv. Coll.; Kasaan Bay, Prince of Wales Island, Alaska;
June 16, 1903; obtained by Cyrus Catt; original no. 4407X.
The skull has a piece 1.5 mm. long broken out of the left
zygomatic arch. P2 is absent on both sides. The right I1, and the
left I1 and I2 are missing. The skin, in summer pelage, is fairly
well made. A scrotal pouch attests to the correctness of the sex
recorded on the label. The rostral part of the skull is smaller
than in average-sized males of corresponding age.
Range.—Prince of Wales, Dall, and Long islands, Alaska. See
figures 25, 26 on pages 95, 134.
Characters for ready recognition (only males known).—Differs
from M. e. alascensis and initis in chest mostly covered by
brown patch, not white, and breadth of rostrum measured across
lacrimal processes more than a third of basilar length, which
cranial character serves to distinguish also salva; from M. e.
seclusa in zygomatic breadth less than distance between last
upper molar and jugular foramen; from M. e. haidarum in chest
white (not mostly covered by brown patch), proximal two-thirds of
underside of tail colored like upper parts rather than underparts,
basilar length more than 38.2 mm.
Description.—Size.—Male: Seven adults and subadults from
Prince of Wales Island, yield average and extreme measurements as
follows: Total length, 286 (277-304); length of tail, 77 (74-85);
length of hind foot, 36 (35.5-40.5).
Female: No specimen available.
Color.—As described in Mustela erminea richardsonii except
that upper parts about tone 3 of dark Chocolate Brown of Oberthür
and Dauthenay, pl. 342; lower throat and chest covered by a large
patch of same color as upper parts; color of underparts extending
to toes but in interrupted fashion on both fore- and hind-feet;
least width of color of underparts averaging, in four males from
Prince of Wales Island, 41 (38-49) per cent of greatest width of
color of upper parts. Black tip of tail averaging, in 8 males in
winter pelage, 65 (59-78) mm. which is 84 (69-92) per cent of
length of tail-vertebrae.
From its geographic neighbors alascensis and initis, celenda
differs in darker color of upper parts, presence rather than
absence of patch of dark color on lower throat and chest, and
longer black tip on tail. From haidarum, celenda differs in
darker color of upper parts, presence rather than absence of
patch of dark color on lower throat and chest, narrower
light-colored under parts, black tip of tail averaging less rather
than more than nine-tenths of length of tail-vertebrae and ventral
face of tail colored like upper parts rather than like underparts.
Skull.—Male (illustrated by 5 adults): See measurements and
plates 5-7. As described in Mustela erminea richardsonii except
that: Weight, 2.3 (2.2-2.5) grams; basilar length, 39.5
(38.9-40.7) mm.; length of tooth-rows more than length of tympanic
bulla; breadth of rostrum measured across lacrimal processes more
than a third of basilar length; interorbital breadth more than
distance between glenoid fossa and posterior border of external
auditory meatus; zygomatic breadth more or less than (about equal
to) distance between last upper molar and jugular foramen.
Female.—Complete skull of adult unavailable.
Differences from richardsonii are indicated in the formal description
just given. Additional to differences therein noted, celenda differs
from initis in larger interorbital and preorbital parts of the skull
although dimensions of other parts of the skull and the teeth are about
the same or even less. From salva, celenda differs in larger
average size in every measurement taken, except for the inner moiety of
M1 which is about the same. The skull of celenda is 35 per cent
heavier. In relation to the basilar length the skull of celenda is
wider, especially in the interorbital and preorbital regions. In
comparison with alascensis the tympanic bullae are of approximately
the same length; otherwise essentially the same differences obtain as
are noted in comparison with salva and the zygomatic breadth is
relatively more in celenda. From seclusa, in which the teeth are of
comparable size, celenda differs in that every cranial measurement is
more and the skull is 28 per cent heavier. Because the skull of
celenda is so much longer, its dimensions in other planes are less in
relation to the length than in seclusa. M. e. celenda is larger in
every part measured than haidarum, 21 per cent heavier, and in
relation to the basilar length the interorbital, and preorbital, parts
of the skull are smaller, the braincase is shallower, and the skull is
relatively wider across the zygomata and mastoid processes. In
comparison with kadiacensis, differences are: 26 per cent lighter,
skull shorter; in relation to the basilar length, braincase shallower
as measured at the anterior end of the basioccipital, tooth-rows
shorter but orbitonasal length more. In comparison with arctica all
parts measured of the teeth and skull of celenda are smaller and its
skull is 34 per cent lighter. In relation to the basilar length, the
interorbital breadth of celenda is only slightly less but its skull
is narrower across the rostrum and zygomata, the tooth-rows are
shorter, and the braincase is shallower.
Remarks.—The late George Willett in the course of his work in Alaska
collected most of the known specimens of this strongly differentiated
subspecies. In both coloration and cranial characters the
distinguishing features are so well marked that the zoölogist could
with reason accord full specific rank to celenda. Nevertheless it
obviously is an ermine. Also, races from other islands of southeastern
Alaska tend to bridge the gap, as regards cranial features, between
celenda and the mainland ermine. The specimen from Dall Island agrees
in all respects with topotypes. The specimen from Howkan on Long Island
is in white winter pelage and the skull has suffered shrinkage from
some chemical solution; the reference of this specimen to celenda is
tentative.
Specimens examined.—Total number, 25, as follows: Arranged by
localities from north to south. Unless otherwise indicated, in U.
S. National Museum.
Alaska. Prince of Wales Island: Craig, 18 (10 in Mus. Vert. Zoöl.,
and 8 in Los Angeles Mus. Hist. Art and Sci.); Kasaan Bay, 2; no
locality more definite than the Island itself, 3; Dall Island,
Otter Harbor, 1 (Los Angeles Mus. Hist. Art and Sci.). Long
Island, Howkan, 1 (Field Mus. Nat. Hist.).
Mustela erminea seclusa Hall
Ermine
Plates 5, 6 and 7
Mustela erminea seclusa Hall, Proc. Biol. Soc. Washington, 57:39,
June 28, 1944; Hall, Journ. Mamm., 26:181, July 19, 1945.
Type.—Male, adult, skull alone; no. 31232, Mus. Vert. Zoöl.;
Port Santa Cruz, Suemez Island, Alaska; March 24, 1920; obtained
by George Willett.
The skull (plates 5-7) is complete and unbroken. Of the upper
incisors only right I3 is present. Otherwise the teeth are present
and unbroken.
Range.—Known only from the type locality. See figures 25, 26 on
pages 95, 134.
Characters for ready recognition (only the male known).—Differs
from M. e. celenda in basilar length less than 38.2, from M. e.
salva, initis and haidarum in zygomatic breadth more than
distance between last upper molar and jugular foramen.
Description.—Size and Color.—No external measurements or
skins available.
Skull.—Male: See measurements and plates 5-7. As described in
Mustela erminea richardsonii except that: Weight, 1.8 grams;
basilar length, 34.3; length of tooth-rows about the same as
length of tympanic bulla; breadth of rostrum measured across
lacrimal processes more than a third of basilar length;
interorbital breadth more than distance between glenoid fossa and
posterior margin of external auditory meatus; zygomatic breadth
more than distance between last upper molar and jugular foramen.
Female.—Skull not available.
From alascensis and salva, seclusa differs in larger teeth,
shorter skull, much larger preorbital and interorbital regions,
actually as well as in relation to basilar length. Excepting the teeth,
which are of about the same size, the same general differences obtain
in comparison with initis which, however, is 29 per cent heavier.
From celenda, seclusa differs in smaller skull in all parts
measured, being 22 per cent lighter. The teeth are about the same size.
In relation to its length the skull of seclusa is much broader and
deeper. From haidarum, seclusa differs in: teeth larger; skull
shorter and more convex in dorsal outline along median longitudinal
axis; in relation to basilar length, skull broader, deeper and
braincase relatively shorter.
Remarks.—The characters shown in the one available skull are far
outside the limits of individual variation for other known subspecies.
Other specimens are much to be desired to ascertain what the "average"
individual is like and to learn the characters of the female.
Specimen examined.—One, the holotype.
Mustela erminea haidarum (Preble)
Ermine
Plates 5, 6, 7, 11, 12 and 13
Putorius haidarum Preble, Proc. Biol. Soc. Washington, 12:169,
August 10, 1898.
Mustela haidarum, Miller, U. S. Nat. Mus. Bull., 79:97, December
31, 1912.
Mustela erminea haidarum, Hall, Proc. Biol. Soc. Washington,
57:38, June 28, 1944; Hall, Journ. Mamm., 26:181, July 19, 1945.
Type.—Male, adult, skull, skeleton and skin; no. 94430, U. S.
Nat. Mus., Biol. Surv. Coll.; Massett, Queen Charlotte Islands,
British Columbia; March 17, 1898; obtained by J. H. Keen; original
no. 1800x.
The skull is unbroken and complete except for osseous tissue
destroyed in the region of each postorbital process; this is the
result of infestation of the frontal sinuses by parasites. The
skeleton is complete down to the distal ends of the tibiae; the
more distal bones are in the skin. The first, right, upper incisor
is missing. Otherwise the teeth all are present and entire.
The skin is in the white, winter pelage but the new under fur is
visible along the back and on the head although mostly covered
with white hair.
Range.—Queen Charlotte Islands. See figure 25, page 95.
Characters for ready recognition.—Differs from M. e. celenda
in chest white (not mostly covered by brown patch), proximal
two-thirds of under side of tail colored like underparts instead
of upper parts, in males basilar length less than 38.2; from M.
e. seclusa, in male, in zygomatic breadth less than distance
between last upper molar and jugular foramen; from M. e.
richardsonii and alascensis, in both sexes, in proximal
two-thirds of under side of tail colored like underparts instead
of upper parts, interorbital breadth not less than distance from
glenoid fossa to posterior margin of external auditory meatus;
from M. e. anguinae and fallenda, in both sexes, in
light-colored underparts more than half the width of dark-colored
upper parts, proximal two-thirds of under surface of tail colored
like underparts instead of upper parts, interorbital breadth equal
to or more than distance between glenoid fossa and posterior
margin of external auditory meatus.
Description.—Size.—Male: Two adults, U.S.N.M., no. 100622,
from Cumsheva Inlet, and Amer. Mus. N. H., no. 37411, and the
type, measure, respectively, as follows: Total length, 283, 290,
275; length of tail, 70, 75, 60; length of hind foot, 39, 40, 37.
Female: Corresponding measurements of an adult, no. 100624, and a
young individual, no. 100623, each from Cumsheva Inlet, are: 252,
250; 63, 61; 31, 32.
Color.—As described in Mustela erminea richardsonii except
that underparts not Sulphur Yellow but ranging from near (e)
Colonial Buff through Marguerite Yellow to almost pure white;
color of underparts extends distally on posterior sides of
forelegs and onto toes but in many specimens interrupted at wrist
by color of upper parts; color of underparts extends onto proximal
three-fourths of under side of tail as length of tail is measured
along tail-vertebrae; least width of color of underparts
averaging, in 5 males, 79 (66-130) per cent of greatest width of
color of upper parts. Black tip of tail in same males averaging 62
(60-70) mm. which is 92 (83-115) per cent of length of
tail-vertebrae.
The close correspondence in color-pattern of this weasel with the
Arctic races, arctica, polaris, semplei and kadiacensis is
noteworthy, and distinguishes it from weasels on the adjacent
mainland and adjoining islands to the north and south. The color
of the upper parts is darker than in the four Arctic races named.
Skull.—Male (7 adults): See measurements and plates 5-7. As
described in Mustela erminea richardsonii except that: Weight,
1.9 (1.7-2.0) grams; basilar length, 36.7 (35.6-37.5); length of
tooth-rows more than length of tympanic bullae; breadth of rostrum
measured across lacrimal processes more than a third of basilar
length; interorbital breadth more than distance between glenoid
fossa and posterior margin of external auditory meatus; zygomatic
breadth barely less than distance between last upper molar and
jugular foramen.
Female (2 adults): See measurements and plates 11-13. As described
in Mustela erminea richardsonii except that: Weight, 1.3 and 1.4
grams; basilar length, 34.2; length of tooth-rows more or less
than (about equal to) length of tympanic bulla; breadth of rostrum
more than 30 per cent of basilar length; interorbital breadth not
less than distance between glenoid fossa and posterior margin of
external auditory meatus.
From richardsonii, haidarum differs in that skull of the male is
actually larger in its anterior part (breadth of rostrum, interorbital
breadth and orbitonasal length) but all measurements of other parts
average less. In relation to the basilar length, the tympanic bulla is
shorter but all other measurements are more. In the skull of the
female, which is 23 per cent heavier, the width of the tympanic bulla
and anteroposterior extent of the inner lobe of M1 are the same; in all
other measurements the female of haidarum is larger, and in relation
to the basilar length all measurements are more except the depth of the
skull at the anterior margin of the basioccipital and the width of the
tympanic bulla, which are less. By actual weight the skull of the male
is 25 per cent lighter and the skull of the female 24 per cent heavier
than in richardsonii. From fallenda and anguinae, haidarum
differs in that measurements of the skulls of both sexes either average
more, or are uniformly more, with two exceptions. These are the lesser
length and breadth of the tympanic bulla, in comparison with males of
fallenda, and the dimensions of M1 which are about the same in all
three races concerned. The pre- and interorbital parts are larger in
relation to the remainder of the skull. The postorbital breadth is
actually a third more than in fallenda. In relation to the basilar
length, the tympanic bulla is shorter and the braincase deeper than in
males of anguinae. The skull of the male is 27 per cent heavier than
that of fallenda and 58 per cent heavier than that of anguinae. The
skull of the female is 59 and 50 per cent heavier than those of
fallenda and anguinae, respectively. Comparison of the skull with
those of alascensis, celenda and seclusa has been made in the
accounts of those subspecies.
Remarks.—The available specimens of this ermine were obtained by J.
H. Keen in 1898, Wilfred H. Osgood and E. A. Lewis in 1900, W. W. Brown
in 1914, J. A. Munro in 1917 and 1918, and Allan Brooks in 1920. M. e.
haidarum has more claim to full specific status than any other race of
ermine because the diagnostic structural features are numerous and
individually of relatively great degree. Indeed, individual variation
appears not to bridge the gap between any population of haidarum and
other subspecies and strong reasons could be advanced for according
haidarum the status of a full species. It differs from the subspecies
of erminea on the adjoining mainland and adjoining islands to the
north and south and agrees with the Arctic races (arctica, polaris,
semplei and kadiacensis) in great extent of the color of the
underparts, extension of this color onto the underneath side of the
tail, long black tip of the tail and general form of the skull
including the relatively heavy preorbital region. The color although
darker than in the Arctic subspecies, is lighter than in the insular
races immediately to the north and south. In combination, the features
mentioned could be taken as indication that haidarum is a relict
population from a former glacial period. Assuming that it is a relict
population, the color may have become slightly darker since that period
but the main response appears to have been a decrease in size for this
is a much smaller animal than the Arctic ermines. The size is about
what would be expected if one were to judge by the slightly larger
ermines on the islands of southeastern Alaska to the north and the
smaller ermine on Vancouver Island to the south.
The ermines of the islands of southeastern Alaska, excepting possibly
the incompletely known seclusa, have fewer characters of the Arctic
races and more characters of the races of the adjoining mainland.
Therefore, a possible inference is that the distinctive characters of
ermines of the Alaskan islands developed with the aid of isolation from
stocks which reached the islands after the glacial period. M. e.
haidarum may have found its way to the Queen Charlotte Islands in the
glacial period.
Specimens examined.—Total number, 17, as follows. Arranged by
locality from north to south. Unless otherwise indicated,
specimens are in the U. S. National Museum.
British Columbia. Queen Charlotte Islands. Masset, 7 (4[74], 1[2],
1[59]); Skidegate, 1; Graham Island, 5 (2[94], 1[77], 1[2]);
Cumsheva Inlet, 3; no locality more definite than Queen Charlotte
Islands, 1[2].
Mustela erminea anguinae Hall
Ermine
Plates 5, 6, 7, 11, 12 and 13
Mustela cicognanii anguinae Hall, Univ. California Publ. Zoöl.,
38:417, November 8, 1932.
Putorius cicognanii, Baird, Mamm. N. Amer., p. 161, 1858 (part).
Putorius streatori, Swarth, Univ. California Publ. Zoöl., 10:102,
February 13, 1912.
Mustela erminea anguinae Hall, Journ. Mamm., 26:79, February 27,
1945; Hall, Journ. Mamm., 26:181, July 19, 1945.
Type.—Male, adult, complete skeleton (no skin); no. 12482, Mus.
Vert. Zoöl., French Creek, Vancouver Island, British Columbia;
found as a desiccated carcass on May 1, 1910; obtained by Harry S.
Swarth.
Range.—Vancouver Island, British Columbia. See figures 25, 27
pages 95, 149.
Characters for ready recognition.—Differs from M. e.
haidarum, in both sexes, in light-colored underparts less than
half the width of dark-colored upper parts, proximal two-thirds of
under surface of tail colored like upper parts instead of
underparts, interorbital breadth less than distance between
glenoid fossa and posterior margin of external auditory meatus;
from M. e. fallenda, in both sexes, anterior margin of tympanic
bullae flush with squamosal rather than projecting from floor of
braincase, in males by sagittal crest absent, in females by total
length more than 238 and tooth-rows about same length as, instead
of longer than, tympanic bulla; from M. e. streatori, in male,
by sagittal crest absent and hind foot ordinarily more than 33.5,
in female by hind foot more than 27.5, basilar length more than
30.2; from M. e. olympica, in males, by greater average size,
hind foot ordinarily more than 33.4 and interorbital breadth
ordinarily more than 8.5, in females by larger size, total length
more than 235, tail more than 65, hind foot more than 27.5,
basilar length more than 30.2.
Description.—Size.—Male: Sixteen adults and subadults yield
average and extreme measurements as follows: Total length, 272
(261-284) mm.; length of tail, 81 (74-86); length of hind foot,
35.0 (33.5-36).
Female: Five adults and subadults have corresponding measurements
as follows: 247 (241-257); 69 (66-73); 30.0 (28.0-32.0).
Color.—As described in Mustela erminea streatori except that:
occasionally white in winter; upper parts about tone 2 of Dark
Chocolate of Oberthür and Dauthenay; least width of color of
underparts averaging, in 7 adult males, 6 (0-15) per cent of
greatest width of color of upper parts. Black tip of tail in same
series averaging 37 (26-46) mm. which is 46 (32-54) per cent of
length of tail-vertebrae.
Skull.—Male (based on 13 adults): See measurements and plates
5-7. As described in Mustela erminea richardsonii except that:
Weight, 1.2 (1.0-1.3) grams; basilar length, 34.0 (32.5-35.6);
length of tooth-rows more or less (usually less) than length of
tympanic bulla.
Female (based on 5 adults): See measurements and plates 11-13. As
described in Mustela erminea richardsonii except that: Weight
0.9 (0.77-1.06) grams; basilar length, 31.5 (30.9-31.8) grams;
length of tooth-rows more or less than (approximately same as)
length of tympanic bulla; breadth of rostrum more than 30 per cent
of basilar length.
The sexual dimorphism in the skull is slight, the skull of the male
being only a third heavier than that of the female. In fallenda of
the adjacent mainland to the east the male is three-fourths heavier
than the female. In comparison with fallenda, males are smaller,
averaging less in every cranial and dental measurement taken and by
weight are a fifth lighter; sagittal crest absent rather than present;
tympanic bullae flush with squamosal rather than projecting below floor
of braincase; in relation to basilar length, tympanic bullae smaller,
braincase deeper and broader, skull wider interorbitally and across
zygomata. Females are larger than in fallenda, and with one exception
average larger in every cranial and dental measurement taken, being 6
per cent heavier. The one exception mentioned is the lesser actual
length of the tympanic bulla in anguinae, in which the length of the
tooth-rows is about the same as, rather than less than, the length of
the tympanic bulla. The postorbital breadth is greater than in
fallenda and the anterior edges of the tympanic bullae are flush with
the squamosals rather than projecting below the floor of braincase. In
relation to the skull as a whole the preorbital and interorbital parts
are larger.
In comparison with streatori, skulls of males are of about the same
size, anguinae being only 9 per cent heavier. The length of the
tooth-rows is ordinarily less than, rather than about equal to, the
length of the tympanic bulla; sagittal crest wanting rather than
present since in anguinae the temporal muscles meet usually only at
the posterior end of the braincase instead of all along the midline on
its top; tympanic bullae narrower and more nearly flush with squamosal
(less protruded from braincase). Relative to the basilar length, the
zygomatic breadth is more, the tympanic bullae are narrower, and the
braincase is deeper at the anterior end of the basioccipital. The
female is 41 per cent heavier than streatori, there being no overlap
in most cranial and dental measurements. M1, however, is approximately
the same size in each subspecies. The tooth-rows and tympanic bulla are
of almost equal length whereas in streatori the length of the
tooth-rows is less than that of the bulla.
Differences from olympica, in males, are: M1 shorter; all other
measurements of teeth and parts of skull averaging larger; skull 20 per
cent heavier; tooth-rows averaging shorter than tympanic bulla rather
than about the same; relative to basilar length, braincase deeper at
anterior end of basioccipital and tooth-rows shorter. The skull of the
female is 64 per cent heavier, larger in every measurement taken
without overlap; temporal ridges meeting, rather than separated, at
lambdoidal crest; length of tooth-rows about equal to, rather than
shorter than, tympanic bulla; in relation to basilar length, skull
deeper, orbitonasal length more, mastoid and zygomatic breadths more,
and tympanic bullae shorter.
Remarks.—References in the literature to this insular race mostly
were under the name streatori until 1932 when in the course of the
present study the name anguinae was proposed. A few specimens have
been taken by nearly every student of small mammals who has collected
on Vancouver Island. Arthur Peake and Herbert Laing have probably
collected more specimens than any other two zoölogists.
M. c. anguinae is noteworthy for the slight secondary sexual
variation in size; the disparity between the two sexes is less than in
any other American subspecies of erminea. By linear measurement the
body of the female is only 7 per cent shorter than in the male (178
versus 191 mm.). Linear measurements and weights of the skulls of the
two sexes are further indicative of this approximation in size. By
weight the skull of the female is only a fourth lighter than that of
the male, or, stated in another way, the male's skull is only a third
heavier (1.2 versus 0.9 grams).
No geographic variation has been detected between lots of specimens
from different parts of Vancouver Island. The one specimen available
from Salt Spring Island presents no obvious differences from selected
individuals from Vancouver Island.
The winter pelage is more often brown than white. Of 17 specimens seen
in winter pelage or in transition pelage, only 6 are white. These 6 are
from Comox, Stamp River, Hilliers, Jeune Landing and Port Alice. Of the
34 specimens in brown pelage, 7 have the dark color of the upper parts
meeting on the abdomen. Six of the 34 have brown color on the pectoral
region. In two, this is a separate patch but in the other four the dark
color is a continuation of the upper parts and extends in front of each
foreleg over part of the pectoral region, but the two extensions, one
from either side, do not meet on the underparts. The color of the lips
was recorded in 22 individuals: one had both the upper and lower-lips
white; 7 had the upper lips brown and the lower lips white; in 14 both
the upper and lower-lips were brown.
Specimens examined.—Total number, 40, listed by localities from
north to south as follows. Unless otherwise indicated, specimens
are in the National Museum of Canada.
British Columbia. Vancouver Island: Cape Scott, 4; Shushartie, 1;
Quatsino, 1[74]; Jeune Landing, 1[74]; Port Alice, 5[15]; Marble
Creek, Quatsino Sound, 1[22]; Port Hardy, 5; Sayward, 2; Bear
Lake, 4; Bear River, 1; Comox, 4(3[85]); Stamp River, Alberni,
1[31]; Errington, 1[74]; French Creek, 1[74]; Hilliers, 1[74];
Craigs Crossing, 1[74]; Nanaimo, 2[22]; Cowichan Lake, 1[22];
Duncan, 2[85]; Salt Spring Island, 1[85].
Mustela erminea fallenda Hall
Ermine
Plates 5, 6, 7, 11, 12 and 13
Mustela erminea fallenda Hall, Journ. Mamm., 26:79, February 27,
1945; Hall, Journ. Mamm., 26:181, July 19, 1945.
Putorius streatori Merriam, N. Amer. Fauna, 11:13, June 30, 1896
(part-Sumas).
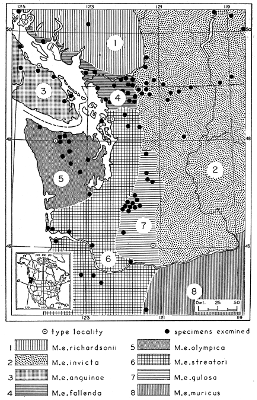
Fig. 27. Map showing known occurrences and probable
geographic ranges of the subspecies of Mustela erminea in Washington
and parts of British Columbia and Oregon.
Type.—Male, adult, skull and skin; no. 7096, Nat. Mus. Canada;
Huntingdon, British Columbia; May 21, 1927; obtained by C. H.
Young, original no. 317.
The brown summer skin is well made. The skull (plates 5-7) is
complete. Right p2 has the crown broken away; otherwise the teeth
all are present and entire.
Range.—On mainland in immediate vicinity of coast from probably
opposite Texada Island, British Columbia, south to Lake Whatcom,
Washington, and east to Mount Baker Range on International
boundary. See figures 25, 27 on pages 95, 149.
Characters for ready recognition.—Differs from M. e.
haidarum, in both sexes, in light-colored underparts less than
half the width of dark-colored upper parts, proximal two-thirds of
under surface of tail colored like upper parts instead of
underparts, interorbital breadth less than distance between
glenoid fossa and posterior margin of external auditory meatus;
from M. e. richardsonii in both sexes, by upper lips brown
rather than white, in males hind foot less than 41 and basilar
length less than 38.3, in females hind foot less than 29, basilar
length less than 31.4 and breadth of rostrum more, instead of
less, than 30 per cent of basilar length; from M. e. invicta, in
both sexes, by upper lips brown (not white); in males by skull
averaging shorter (basilar length 35.7 versus 37.0); in females by
breadth of rostrum more, instead of less, than 30 per cent of
basilar length; from M. e. anguinae, in both sexes, by anterior
margin of tympanic bulla projecting from floor of braincase rather
than flush with squamosal (the difference is slight in females),
in males by sagittal crest present, in females by total length
less than 238 and tooth-rows longer than, instead of about same
length as, tympanic bulla; from M. e. streatori, in both sexes,
by black tip of tail more than half of length of tail-vertebrae,
in males hind foot more than 33.7, tympanic bulla longer than,
instead of about same length as, upper tooth-rows; weight of skull
more than 1-1/4 grams, in females weight of skull more than 0.7
grams, length of lateral side of P4, 4 mm. or more; from M. e.
olympica, in males, length of hind foot more than 33, black tip
of tail more than 36.5 mm., weight of skull more than 1.2 grams,
basilar length more than 33.5, in females length of hind foot more
than 25.5, weight of skull more than 0.66 grams, basilar length
more than 28.4; from M. e. gulosa, in both sexes, by anterior
margin of tympanic bulla projecting below floor of braincase
rather than flush with squamosal (the difference is slight in
females), in males hind foot more than 33.5, weight of skull more
than 1-1/4 grams, basilar length more than 33.9, in females by
total length more than 222, hind foot longer than 26, weight of
skull more than 0.7 grams, basilar length more than 29.
Description.—Size.—Male: Seven adult topotypes yield average
and extreme measurements as follows: Total length, 278 (249-305);
length of tail, 77 (69-81); length of hind foot, 36.5 (34-40). A
male topotype of unknown age weighed 113 grams.
Female: Two adult topotypes, with actual measurements in
parentheses, average as follows: Total length, 232 (228-236);
length of tail, 60 (57-62); length of hind foot, 27 (27-27). An
adult from Morovitz Guard Station, Wash., weighed 54 grams.
Color.—Winter pelage rarely white, brown pelage
indistinguishable from summer pelage except for slightly more
smoky tinge in winter in specimens from some localities; otherwise
as described in Mustela erminea streatori except that least
width of color of underparts averaging, in seven adult topotypes,
18 (0-37) per cent of greatest width of color of upper parts.
Black tip of tail averaging, in same series, 45 (38-52) mm. which
is 58 (53-65) per cent of length of tail-vertebrae.
In comparison with richardsonii and invicta, fallenda
differs in darker color of upper parts and their extension at the
expense of the light-colored underparts which are narrower by a
half. In correlation with this restriction in area of the
light-colored underparts, the upper lips are brown instead of
white. In comparison with anguinae, olympica and streatori,
the longer black tip on the tail is the principal difference in
color. From gulosa, fallenda differs in slightly darker color
of upper parts and in narrow underparts, the width of the same
being only about a fifth instead of a third of the width of the
dark-colored upper parts.
Skull.—Male (based on 7 adults): See measurements and plates
5-7. As described in Mustela erminea richardsonii except that:
Weight, 1.5 (1.3-1.7) grams; basilar length, 35.7 (34.3-38.2).
Female (based on 6 ads.): See measurements and plates 11-13. As
described in Mustela erminea richardsonii except that: Weight,
0.85 (0.73-1.0) grams; basilar length, 30.6 (29.4-31.7); breadth
of rostrum more than 30 per cent of basilar length.
In comparison with richardsonii, skulls of males differ as follows:
averaging smaller in every measurement taken with no overlap in several
dimensions; 40 per cent lighter; in relation to basilar length, rostrum
(orbitonasal length) longer and skull slightly broader interorbitally.
Females average smaller in every cranial and dental measurement taken
with no overlap in basilar length, length of tooth-rows and length of
tympanic bulla; 22 per cent lighter; breadth of rostrum more, rather
than less, than 30 per cent of basilar length; in relation to basilar
length, pre- and interorbital parts of skull larger, and mastoid breadth
more.
Differences from males of olympica are: size larger with no overlap
in most measurements; 50 per cent heavier; tympanic bullae longer than
upper tooth-rows rather than of about equal length; in relation to
basilar length, rostrum shorter, braincase wider and deeper, zygomata
more expanded. Females are larger with no overlap in most measurements;
35 per cent heavier; in relation to basilar length, pre- and
interorbital regions narrower, braincase deeper and wider across
mastoids.
Differences from streatori, in males, are: skull averaging larger in
every cranial and dental measurement taken; 36 per cent heavier;
tympanic bulla longer than, instead of about same length as, upper
tooth-rows. In females the inner lobe of M1 is shorter
anteroposteriorly; otherwise all measurements of fallenda average
larger and it is 33 per cent heavier; rostrum and interorbital region
broader in relation to remainder of skull.
In comparison with gulosa, skulls of males differ as follows:
averaging larger in every measurement taken with no overlap in several
dimensions; 50 per cent heavier; tympanic bullae with anterior margins
projecting slightly below squamosals rather than flush with same;
length of bulla more than, rather than about same as, that of upper
tooth-rows. Considering the great difference in size, the relative
proportions are remarkably alike. In females, length of inner lobe of
M1 about the same; otherwise averaging larger in every measurement
taken; 44 per cent lighter; relative to basilar length, tooth-rows
longer, skull wider across zygomata and mastoids, rostrum and
interorbital regions slightly narrower, skull shallower in plane of
last upper molars.
Comparisons with haidarum, invicta and anguinae are made in
accounts of those subspecies.
Remarks.—Until the name fallenda was proposed in the course of the
present study, most of the specimens of this race were assigned to
streatori.
Intergradation with streatori is complete as it is also with
invicta and richardsonii, in other words with each of the
subspecies whose ranges meet that of fallenda. In color and in size
the difference is least between streatori and fallenda. As between
fallenda and invicta the size is not greatly different and the
intergradation in color is gradual. Between fallenda and
richardsonii intergradation is somewhat different and to fully
appreciate its nature we should remember that the color of fallenda
resembles that of the saturate coastal races, streatori, anguinae
and olympica although the black tip of the tail is longer. In this
latter feature and in several cranial details, as well as in greater
degree of secondary sexual variation in size, fallenda resembles
richardsonii. Because the two differ more than do most subspecies of
ermine whose ranges meet, some of the intergrades at first inspection
appear to be widely different from either parent stock. For example,
specimens from Alta Lake, British Columbia, may give this impression
because the combination of large size and dark color suggests a kind of
ermine different from either fallenda or richardsonii. In no
instance, however, has there been found in these intergrades any
character other than those occurring in one or the other of the two
parent races.
Along the coast in the north part of the geographic range assigned to
fallenda, some specimens nearly typical of richardsonii have been
taken so near to the place where fairly typical fallenda was
obtained that I have doubted whether there is intergradation in the
usual fashion in this area; more specimens will have to be obtained
from this coastal area to resolve the doubt one way or the other.
The winter pelage is brown in all specimens at most localities. The
only white pelage seen was in each of three specimens from Glacier,
Whatcom County, Washington. A fourth specimen from there is in brown
winter pelage. At any one locality there is much variation in the
degree to which the dark color of the upper parts encroaches on the
area that in most other races is light-colored. An extreme degree of
encroachment is shown by a specimen taken on December 1, 1935, by R. A.
Cummings, at Vancouver, British Columbia, in which the light color
occurs only in three restricted areas, the chin, the throat and the
lower breast; otherwise the coat is brown. There are other specimens,
for instance from the type locality, which differ mainly in having an
additional white spot in the inguinal region. The opposite extreme, in
a specimen also from the type locality, is where the least width of the
light-colored underparts on the abdominal region is a third of the
circumference of the body. The two extremes are connected by a dozen
intermediate stages. Of 64 specimens in which the color of the lips was
carefully examined, one, from Vancouver, has both the upper and
lower-lips brown; 9 have both the upper and lower-lips white; and 54
have the upper lips brown and the lower lips white.
Specimens examined.—Total number, 72, arranged by localities
from north to south. Unless otherwise indicated, specimens are in
the National Museum of Canada.
British Columbia. Horseshoe Lake, Stillwater, 2; Vancouver, 1[74];
Point Grey, 1[31]; Port Moody, 5[91]; Chilliwack, 8 (2[75], 4[91],
1[60]); Sumas, 19 (18[75], 1[60]); Thurstons Ranch, 2; Cultus
Lake, 2; Mt. Baker Range, 5[75]; Lihumption Park, 1; Huntingdon,
14; Tami Hy Creek, 1.
Washington. Whatcom County: Semiahmoo, 1[91]; New Whatcom,
1[68]; Lake Whatcom, 2[91]; 5 mi. W Glacier, 1[51]; Glacier (3 at
900 ft.), 4[91]; E Side Easton Glacier, Mt. Baker, 1[55]; Morovitz
Guard Station, 831 ft., 1[55].
Mustela erminea olympica Hall
Ermine
Plates 5, 6, 7, 12, 13 and 14
Mustela erminea olympica Hall, Journ. Mamm., 26:81, February 27,
1945; Hall, Journ. Mamm., 26:181, July 19, 1945.
Mustela rixosa, Svihla and Svihla, Murrelet, 13:24, January,
1932.
Mustela rixosa rixosa, Svihla and Svihla, Murrelet, 14:39, May,
1933.
Type.—Male, adult, skull and skin; no. 90738, U. S. Nat. Mus.,
Biol. Surv. Coll.; near head of Soleduc River, 4500 ft., Olympic
Mountains, Clallam County, Washington; April 28, 1897; obtained by
Vernon Bailey, original no. 6213.
The skin is well prepared and in good condition. The skull (plates
5-7) is unbroken and the teeth all are present and entire.
Range.—Olympic Peninsula, Washington, south to Olympia. See
figures 25, 27 on pages 95, 149.
Characters for ready recognition.—Differs from M. e.
anguinae, in males, by lesser average size, hind foot ordinarily
less than 33.4, and interorbital breadth ordinarily less than 8.5,
in females by smaller size, total length less than 235, tail less
than 65, hind foot less than 27.5, basilar length less than 30.2;
from M. e. fallenda, in males, by length of hind foot less than
33, black tip of tail less than 36.5, weight of skull less than
1.2 grams, basilar length less than 33.5, in females length of
hind foot less than 25.5, weight of skull less than 0.6 grams,
basilar length less than 28.4; from M. e. streatori by smaller
size, in males hind foot less than 33.0, basilar length ordinarily
less than 32.5, in females by hind foot ordinarily not longer than
24, by breadth of rostrum less than 8.6, depth of braincase at
posterior border of upper molars less than 7.6.
Description.—Size.—Male: Twelve individuals of adult
proportions yield average and extreme measurements as follows:
Total length, 243 (205-269); length of tail, 65 (60-74); length of
hind foot, 31 (29-32).
Female: Corresponding measurements of six females are: 196
(188-208), 52 (45-60?), 23.4 (22.7-24.0). An adult weighs 30
grams.
Color.—As described in Mustela erminea streatori except that
least width of color of underparts averaging, in 12 males of adult
proportions, 5 (0-11) per cent of greatest width of color of upper
parts. Black tip of tail averaging, in same series, 26 (20-35)
mm., which is 40 (31-58) per cent (average the same as in
streatori) of length of tail-vertebrae.
Skull.—Male (based on 5 adults): See measurements and plates
5-7. As described in Mustela erminea richardsonii except that:
Weight, 1.0 (0.9-1.1) grams; basilar length, 31.8 (30.6-32.5);
length of tooth-rows more or less than (about equal to) length of
tympanic bulla.
Female (illustrated by 3 adults): See measurements and plates
12-14. As described in Mustela erminea richardsonii except that:
Weight, 0.55 (0.52-0.58) grams; basilar length, 27.1 (26.7-27.5);
breadth of rostrum more than 30 per cent of basilar length.
In comparison with streatori, skulls of corresponding sex average
smaller in every measurement taken with no overlap in most of those of
females. Exception is to be made for the inner lobe of M1 in males
where the size is the same. By weight males are smaller by 10 per cent
and females by 14 per cent. In relation to other parts of the skull the
tympanic bullae are narrower and in females they are shorter as well.
Comparison with anguinae and fallenda has been made in the accounts
of those subspecies.
Remarks.—The smaller size, especially of females, is the principal
feature distinguishing this race from streatori. On the basis of
available data the female of olympica is smaller than that of any
other race and hence is the smallest adult weasel of the species
erminea, in either the Old World or in America.
Intergradation with streatori is indicated by specimens from the
southern end of Puget Sound. These specimens are intermediate in size
between typical examples of the two races concerned.
The color of the upper parts is uniform and the color pattern varies
less than in geographically adjoining races. The white color of the
underparts is restricted to a thin line on the abdominal region, but
widens out posteriorly in the inguinal region and anteriorly over the
pectoral region, throat, chin and lower lips. The upper lips are brown.
The brown of the upper parts extends around in front of each foreleg,
the two brown areas not quite meeting on the lower throat. The above
description applies to each of the 19 specimens examined with regard to
these details. Every specimen seen in the winter coat was brown, not
white.
Specimens examined.—Total number, 20, arranged by counties from
north to south. Unless otherwise indicated, specimens are in the
U. S. National Museum.
Washington. Clallam County: Clallam Bay, 2 (1[74], 1[94]);
Elwha, 2[10]; Johnsons Ranch, 1[60]; Happy Lake, 1[60]; Boulder
Lake, 2[60]; near head of Soleduc River, 4500 ft., 1; 12 mi. S
Port Angeles, 1[10]. Jefferson County: Hayes Cr., 2000 ft.,
Elwha River, 2; head N Fork Quinault River, 4000 ft., 1;
Duckabush, 3; N Fork Skokomish River, 1. Mason County: Lake
Cushman, 2[76]; 4 mi. S Olympia, 1.
Mustela erminea streatori (Merriam)
Ermine
Plates 5, 6, 7, 12, 13 and 14
Putorius streatori, Merriam, N. Amer. Fauna, 11:13, pl. 2, figs.
5, 5a, 6, 6a, June 30, 1896.
Putorius cicognanii, Baird, Mamm. N. Amer., p. 161, 1858 (part
unless no. 2395 was a female of M. frenata).
Putorius pusillus, Baird, Mamm. N. Amer., p. 159, 1858 (part).
Putorius (Gale) vulgaris, Coues, Fur-bearing animals, p. 102,
1877 (part).
Mustela streatori streatori, Miller, U. S. Nat. Mus. Bull.,
79:96, December 31, 1912; Grinnell, Univ. California Publ. Zoöl.,
40:101, September 26, 1933.
Mustela cicognanii streatori, Hall, Murrelet, 12:22, January,
1931; Hall, Univ. California Publ. Zoöl., 38:417, November 8,
1932.
Mustela erminea streatori, Hall, Journ. Mamm., 26:77, February
27, 1945; Hall, Journ. Mamm., 26:181, July 19, 1945.
Mustela rixosa, Beer, Journ. Mamm., 29:296, August 31, 1948.
Type.—Male, adult, skull and skin; no. 76646, U. S. Nat. Mus.,
Biol. Surv. Coll.; Mount Vernon, Skagit Valley, Skagit County,
Washington; February 29, 1896; obtained by D. R. Luckey, original
no. 3.
The skull is unbroken and the teeth all are present and entire.
The skin, in brown winter pelage, is stuffed and in good
condition.
Range.—Western Washington along eastern side of Puget Sound,
western Oregon from the Cascades to the coast, and northwestern
California south in the humid coastal district nearly to the
Golden Gate. See figures 25, 27 on pages 95, 149.
Characters for ready recognition.—Differs from M. e.
anguinae, in male, by sagittal crest present and hind foot
ordinarily less than 33.5, in female by hind foot less than 27.5,
basilar length less than 30.2; from M. e. fallenda, in both
sexes, by black tip of tail less than half of length of
tail-vertebrae, in males hind foot less than 33.7, tympanic bulla
about same length as, instead of longer than, upper tooth-rows;
weight of skull less than 1-1/4 grams, in female weight of skull
less than 0.7 grams, length of lateral side of P4 less than 4 mm.;
from M. e. olympica, by larger size, in males hind foot more
than 33.0, basilar length ordinarily more than 32.5, in females by
hind foot ordinarily longer than 24, by breadth of rostrum more
than 8.6, depth of braincase at posterior border of upper molars
more than 7.6; from M. e. gulosa and muricus, in both sexes,
by upper lips brown (not white), light color of underparts
extending down hind leg no farther than knee, depth of skull at
posterior border of upper molars more than 7.7 in females and
ordinarily more than 9.6 in males, further from muricus by tail
more than 62 in males and more than 49 in females; from M. e.
invicta by upper lips white (not brown), in males hind foot more
than 36 and basilar length more than 35, in females hind foot more
than 29.5 and basilar length more than 30.5.
Description.—Size.—Male: Twelve adults from Blaine and
Tillamook, Oregon, yield average and extreme measurements as
follows: Total length, 255 (245-275); length of tail, 72 (64-80);
length of hind foot, 31.5 (30.0-33.5).
Female: Seven adults from Blaine and Tillamook, Oregon, yield
average and extreme measurements as follows: Total length, 214
(193-230); length of tail, 55 (50-63); length of hind foot, 25
(24-27).
Color.—Winter and summer pelages indistinguishable; upper parts
uniform and ranging from Raw Umber to slightly darker (16n), and
about tones 1 to 3 of Dark Chocolate of Oberthür and Dauthenay,
pl. 342; underparts white, in summer rarely with a faint buffy
suffusion in pectoral region; color of underparts extends from
chin, and often lower lips, posteriorly to inguinal region,
distally on posterior sides of forelegs onto antipalmar faces of
toes (sometimes interrupted at and above wrist) and on medial
sides of hind legs hardly to knee. Least width of color of
underparts averaging, in twelve adults from Blaine and Tillamook,
10 (0-47) per cent of greatest width of color of upper parts.
Black tip of tail, in same series, averaging 28 (24-33) mm. which
is 40 (34-47) per cent of length of tail-vertebrae.
Skull.—Male (based on 12 adults): See measurements and plates
5-7. As described in Mustela erminea richardsonii except that:
Weight, 1.1 (1.0-1.2) grams; basilar length, 33.2 (32.5-33.8);
length of tooth-rows more or less than (about same as) length of
tympanic bulla.
Female (based on 7 adults): See measurements and plates 12-14. As
described in Mustela erminea richardsonii except that: Weight,
0.64 (0.60-0.67) grams; basilar length, 28.5 (27.6-29.5); breadth
of rostrum more than 30 per cent of basilar length.
Comparison with anguinae, fallenda, olympica, gulosa and
muricus is made in accounts of those subspecies.
Remarks.—This weasel is rare in collections and the best material of
it was obtained by Alex Walker in Tillamook County, Oregon, where he
resides. The almost ideal series of 30 specimens showed the range of
secondary sexual, age, and individual variation expectable in the small
ermines of the Pacific Coast of the United States and was the means of
allowing satisfactory decision on questions of classification in the
related subspecies in which individuals are of comparable size.
Intergradation with each of the geographically adjoining subspecies,
olympica, fallenda, invicta, gulosa and muricus is shown by
specimens examined. With the last mentioned subspecies, intergradation
is shown by two specimens from as far south as Siskiyou County,
California, assigned to muricus.
The application of the name streatori is difficult because it was
based on a specimen from a place where two clines cross. The
north-south cline is one of size which decreases to the south. The
east-west cline is one of intensity of color, the westernmost (coastal)
population being the most intensely colored. The type locality of
streatori is at the place where two lines perpendicular to one
another, and representing the two clines, cross. This intersection is
near the place where the ranges of several subspecies meet. The
nomenclatural question is, to which one of 6 subspecies should the name
streatori apply. Specimens from barely within the geographic
boundaries of four of these subspecies so closely resemble topotypes of
streatori that a student with material at his disposal from only the
area about Puget Sound naturally would apply the name streatori to
all of his specimens, and knowing even of the arrangement adopted in
the present account the student will have difficulty in identifying his
specimens according to it. Not only will the student find the
arrangement difficult, but probably unsatisfactory if he thinks of
streatori as being the kind of animal represented by topotypes. I
conceive of topotypes of streatori as being nontypical of the
subspecies; they are intergrades with fallenda. My aim was initially
to work out the geographic ranges of subspecies and only subsequently
to apply names, according to which type localities fell within the
previously determined geographic ranges. By this procedure no greater
weight was given to a holotype and to topotypes than to specimens from
any other locality.
Of the 40 specimens seen in winter pelage, only one is white. It is
from Darrington in the Cascade Mountains of Washington. The 39 others
are brown and I doubt that the white pelage ever occurs in the low
coastal territory included within the geographic range of streatori.
This subspecies resembles anguinae and olympica in the great
extension of area of the dark-colored upper parts at the expense of the
area of the light-colored underparts. The usual arrangement is one
where the brown of the two sides nearly meets on the midventral line
leaving a sizable, inguinal area of light color connected by a thin
line to the sizable area of light color on the pectoral region. The
light color of the pectoral area ordinarily is continuous with the
light-colored area of the throat and chin but the dark color of the
upper parts extends around in front of each foreleg. These extensions
of dark color meet on the chest in only 2 of the 56 specimens examined
in this regard. Across the abdomen the dark color is continuous in 4 of
the 56 specimens. The lower lips are brown instead of white in only 3
individuals and in 2 of these the lip of one side is brown and its
opposite is white. The variation in color-pattern is less than in
anguinae or than in fallenda.
Specimens examined.—Total number, 63, arranged alphabetically
by states, then by counties from north to south in each state.
Unless otherwise indicated, specimens are in the U. S. National
Museum.
California. Humboldt County: 10 mi. NE Carlotta, 1[74].
Mendocino County: Russian Gulch State Park, 1[74]. Sonoma
County: Mouth of Gualala River, 1[74].
Oregon. Clatsop County: Astoria, 1. Tillamook County:
Tillamook, 16 (14[14], 1[59]); Blaine, 12 (7[14], 2[59], 1[93],
2[76]). Washington County: Beaverton, 1[60]; Forest Grove,
1[36]. Clackamas County: Oregon City, 1[46]. Lincoln County:
Newport, 1. Linn County: Sico, 1[46]. Lane County: Vida Fish
Hatchery, 2[101]; McKenzie Bridge, 1[101]; Mercer, 1[75]. Klamath
County: Deschutes River, 6 mi. E Crescent Lake, 1[101]. Douglas
County: Gardiner, 1[60]. Curry County: Port Orford, 1; Gold
Beach, 2[60].
Washington. Skagit County: N end Whidby Island opposite
Deception Pass, 1; Hamilton, 4; Mt. Vernon, 3. Snohomish County:
Oso, 550 ft., 1; Darrington, 600 ft., 1. Pacific County:
Wallicut River, 2 mi. E Ilwaco, 1[74]. Wahkiakum County: 4 mi.
E. Skamokawa, 3[74]. Cowlitz County: 4 mi. E mouth Kalama River,
2[74]; 6 mi. E mouth Kalama River, 1[74]. Skamania County: 15
mi. N Govt. Springs, 1300 ft., 1.
Mustela erminea gulosa Hall
Ermine
Plates 5, 6, 7, 12, 13 and 14
Mustela erminea gulosa Hall, Journ. Mamm., 26:84, February 27,
1945; Hall, Journ. Mamm., 26:181, July 19, 1945.
Putorius streatori Merriam, N. Amer. Fauna, 11:14, June 30, 1896.
Type.—Male, subadult, skull and skin; no. 81998, U. S. Nat.
Mus., Biol. Surv. Coll.; Trout Lake, Klickitat County, Washington;
February 3, 1897; obtained by P. Schmid, original no. 147.
The skin is in brown winter pelage, and appears to have been made
up from a skin split along the midventral line from the anus to
the forelegs. It probably was dried by a trapper, is well made,
and lacks a patch of hair on the left flank but otherwise is in
good condition. The skull lacks the central part of the left
zygomatic arch and the posterior two-thirds of the right one. The
right m2 is represented only by an abortive stump or the broken
root, and i1 and i2 on each side are absent; otherwise, the teeth
all are present and entire.
Range.—Cascades of Washington from northeastern King County
south to Mount Adams. See figures 25, 27 on pages 95, 149.
Characters for ready recognition.—Differs from M. e. invicta
and fallenda, in both sexes, by anterior margin of tympanic
bulla flush with squamosal rather than projecting below floor of
braincase (difference slight in females), in males hind foot less
than 33.5, weight of skull less than 1-1/4 grams, basilar length
less than 33.9, in females by total length less than 222, hind
foot shorter than 26, weight of skull less than 0.7 grams, basilar
length less than 29; from M. e. muricus, in both sexes, by upper
parts darker, tone 4 of Chocolate or darker (see description of
color), least width of light-colored underparts averaging one-third
instead of approximately two-thirds of greatest width of
dark-colored upper parts, in males, on the average, tail more than
65, weight of skull more than 0.90 grams, basilar length more than
30.8 mm.; from M. e. streatori, in both sexes, by upper lips
white (not brown), light color of underparts extending down hind
legs below knee, depth of skull at posterior border of upper
molars less than 7.7 in females and ordinarily less than 9.6 in
males.
Description.—Size.—Male: One adult and four subadults from
Mount Rainier yield average and extreme measurements as follows:
Total length, 253 (238-266); length of tail, 75 (70-83); length of
hind foot, 31.5 (30-33). Corresponding measurements of 9 subadults
from Trout Lake are: 257 (233-282); length of tail, 76 (56-83);
length of hind foot, 30.2 (26-33).
Female: Of adults, 2 from Mount Rainier and 2 from Trout Lake
measure as follows: Total length, 202, 203, 216, 210; length of
tail, 54, 52, 57, 51; length of hind foot, 24, 24, 25, 24. The
averages for these females are 208, 54, 24.3.
Color.—As described in Mustela erminea richardsonii except
that color sometimes brown in winter (with more smoky tinge than
summer coat); upper parts ranging from tone 2 through tones 3 and
4 of Dark Chocolate (pl. 342) into tone 4 of Chocolate (pl. 343)
of Oberthür and Dauthenay; underparts (always white in winter) in
summer Sulphur Yellow or more whitish; least width of color of
underparts averaging, in 5 males from Mount Rainier, 31 (18-45)
per cent of greatest width of color of upper parts. Black tip of
tail, in same series, averaging 34 (29-40) mm., which is 45
(41-50) per cent of length of tail-vertebrae.
Skull.—Male (based on 2 ad. and 13 sad.): See measurements and
plates 5-7. As described in Mustela erminea richardsonii except
that: Weight, 1.0 (0.95-1.16) grams; basilar length, 32.3
(30.9-33.4); length of tooth-rows more or less than (about equal
to) length of tympanic bulla.
Female (illustrated by 5 adults): See measurements and plates
12-14. As described in Mustela erminea richardsonii except that:
Weight, 0.59 (0.53-0.65) grams; basilar length, 28.1 (27.8-28.4);
breadth of rostrum ordinarily more than 30 per cent of basilar
length.
In comparison with streatori, skulls of males and females average
smaller in every cranial measurement taken. Teeth of about same size
and males 9 per cent, and females 8 per cent, lighter. In relation to
basilar length, skull of female shallower, tympanic bullae slightly
shorter and, on the average, zygomata less expanded.
In comparison with muricus, males average larger in every measurement
taken; 23 per cent heavier; in relation to other dimensions, braincase
shallower at anterior end of basioccipital. Females are of about equal
size; in relation to other dimensions, braincase shallower and mastoid
and zygomatic breadths less.
Comparisons with invicta and fallenda have been made in the
accounts of those subspecies.
Remarks.—This is not a strongly marked race and in most of the
characters used for differentiating it from other races it resembles
either streatori to the west or muricus to the southeast.
Nevertheless, there is a geographic area, the southern Cascades of
Washington, throughout which individual characters are combined in
essentially the same way and there are a few features, for instance,
smaller skull of the female, in which gulosa differs from either of
its close relatives. In view of these circumstances and because the
animals can not well be included in the subspecies streatori or
muricus, gulosa is recognized as distinct. The races gulosa and
olympica are what might be termed weakly differentiated subspecies in
contrast to the strongly differentiated subspecies streatori and
muricus.
Of the 21 specimens in winter pelage, 17 are white and four are brown.
The brown winter coat is distinctly paler, with more of a smoky tinge,
than the brown summer pelage. The light-colored underparts are narrower
than in the subspecies immediately to the east but are wider than in
the coastal forms to the west. The dark color of the upper parts
extends onto the chest in front of the forelegs, as in the coastal
forms, in only one of the 13 specimens in summer pelage and in it on
one side only. The black tip of the tail is short as in the coastal
forms. One specimen is in transitional pelage. It has acquired
approximately half of the white winter pelage and was taken on October
12, 1897, at Keechelus Lake.
Specimens examined.—Total number, 38, arranged by counties from
north to south. Unless otherwise indicated, specimens are in the
U. S. National Museum.
Washington. King County: 2 mi. E Skykomish, 2[51]. Kittitas
County: Keechelus Lake, 3 (1[1]); Martin, 1[1]; Easton, 3.
Pierce County: James Lake, 4370 ft., Mt. Rainier, 1; Glacier
Basin, 5935 ft., Mt. Rainier, 1; Meslers Ranch, 2000 ft., 1 mi. W
Rainier Park, 1. Lewis County: Mt. Rainier Nat'l Park, 5 (1 each
from: Paradise Park, 5400 ft.; Reflection Lakes, 4900 ft.;
Ohanapecosh [Hot] Springs, 2000 ft.; Tahoma Creek, 1[72]; Bear
Prairie); also in Mt. Rainier Nat'l Park, Longmire, 3 (1[72],
1[94]). Skamania County: Mt. St. Helens, 6000 ft., 1. Klickitat
County: Trout Lake, 18.
Mustela erminea muricus (Bangs)
Ermine
Plates 7, 8, 12, 13, 14 and 41
Putorius (Arctogale) muricus Bangs, Proc. New England Zoöl. Club,
1:71, July 31, 1899.
Putorius streatori leptus Merriam, Proc. Biol. Soc. Washington,
16:76, May 29, 1903. Type from Silverton, San Juan County,
Colorado.
Putorius muricus, Stephens, California Mammals, p. 248, 1906.
Putorius cicognani, Taylor, Univ. California Publ. Zoöl., 7:298,
June 24, 1911.
Mustela streatori leptus, Miller, U. S. Nat. Mus. Bull., 79:96,
December 31, 1912; Bailey, N. Amer. Fauna, 35:48, September 5,
1913; Dixon, Journ. Mamm., 12:72, February 12, 1931; Whitlow and
Hall, Univ. California Publ. Zoöl., 40:246, September 30, 1933.
Mustela muricus, Miller, U. S. Nat. Mus. Bull., 79:96, December
31, 1912; Kellogg, Univ. California Publ. Zoöl., 12:358, January
27, 1916.
Mustela cicognanii lepta, Dice, Journ. Mamm., 1:12, November 28,
1919; Hall, Mamm. Nevada, p. 184, July 1, 1946.
Mustela rixosa, Seton, Journ. Mamm., 14:70, February 14, 1933.
Mustela cicognanii leptus, Miller, Journ. Mamm., 14:368, November
13, 1933; Bailey, N. Amer. Fauna, 55:293, August 29, 1936.
Mustela erminea murica, Hall, Journ. Mamm., 26:84, February 27,
1945; Hall, Journ. Mamm., 26:181, July 19, 1945.
Type.—Male, young, skull and skin; no. 9146, collection of E.
A. and O. Bangs in Mus. Comp. Zoöl.; Echo, 7500 ft., El Dorado
County, California; July 15, 1897; obtained by W. W. Price and E.
M. Nutting.
The skull has a fracture along the sagittal suture and fractures
on the left side of the braincase but these have been glued, and
no part of the skull is missing except in the region of the right
P4 which part has been shot away. On the left side m2 never
developed. Excepting this tooth and the right P4, all the teeth
are present and entire. The skin is well made but has the soles of
the hind feet turned up.
Range.—Near 5300 feet (Denver) to 11000 feet (Santa Fe Baldy);
typically boreal but taken in Upper Sonoran Life-zone in winter at
Denver; from central and southwestern Montana, southern Idaho, and
Blue Mountains of southeastern Washington southward east of the
Cascade Divide through the Salmon River Mountains and Sierra
Nevada at least into Fresno County of California, in the Great
Basin to central Nevada, in the Rocky Mountains into northern New
Mexico; eastward to the Black Hills. See figure 25 on page 95.
Characters for ready recognition.—Differs from M. e. invicta
by hind foot less than 36 and basilar length less than 35 in males
and by hind foot less than 29.5 and basilar length less than 30.5
in females; from M. e. gulosa, in both sexes, by upper parts
lighter, tone 2 of Chocolate or lighter (see description of
color), least width of light-colored underparts averaging about
two-thirds instead of one-third of greatest width of dark-colored
upper parts, in males, on the average, tail less than 65, weight
of skull less than 0.90 grams, basilar length less than 30.8
grams; from M. e. streatori, in both sexes, by upper lips white
(not brown), light color of underparts extending down hind leg
below knee, depth of skull at posterior border of upper molars
less than 7.7 in females and ordinarily less than 9.6 in males,
tail less than 62 in males and less than 49 in females.
Description.—Size.—Male: An adult from Black Butte,
California, measures: Total length, 227; length of tail, 55;
length of hind foot, 27. Corresponding measurements of another
from Wheeler Peak, Nevada, are: 220, 56, 26. Two subadults from
Colorado, one from Crested Butte and another from Coventry,
measure, respectively, as follows: 238, 227; 66, 60; 30, 30. An
adult from Wheeler Peak, Nevada, weighs 57.7 grams and another
from 2 mi. W Black Butte, Calif., 54.5 grams.
Female: Two adults from Teton County, Wyoming, measure: Total
length, 205, 200; length of tail 52,—; length of hind foot, 23,
23.7. A subadult from 9-1/2 mi. E Pocatello, Idaho, measures: 197,
50, 25. An adult from Wheeler Peak, Nevada, has corresponding
measurements of 190, 42, 23, and weighs 33.8 grams.
Color.—As described in Mustela erminea richardsonii except
that upper parts tone 2 or lighter of Chocolate of plate 343 of
Oberthür and Dauthenay; underparts white, Pale Buff or with faint
wash of Sulphur Yellow; least width of color of underparts in male
from Black Butte and one from Wheeler Peak, amounting to 65 and 59
per cent of greatest width of color of upper parts. Black tip of
tail, respectively, 28 and 33 mm., which amounts to 51 and 59 per
cent of length of tail-vertebrae. In two adult females, one from
Teton County, Wyoming, and one from Wheeler Peak, Nevada, the
least width of the underparts amounts to 55 and 60 per cent of the
greatest width of color of upper parts. Black tip of tail,
respectively, 23 and 19 mm., which amounts to 44 and 45 per cent
of length of tail-vertebrae.
From the other subspecies of small-sized weasels of more
northwestern occurrence, namely anguinae, fallenda,
olympica, streatori and gulosa, muricus differs in lighter
color of upper parts, wider light-colored underparts and
relatively longer black tip of tail.
Skull.—Male (illustrated by 5 adults in table of measurements,
which see): See plate 7. As described in Mustela erminea
richardsonii except that: Weight, 0.78 (Wheeler Peak) and 0.85
(Black Butte) grams; basilar length, 30.6 (29.8-31.2); length of
tooth-rows more or less than (approximately equal to) length of
tympanic bulla.
Female (illustrated by 6 adults in table of measurements, which
see): See plates 12-14. As described in Mustela erminea
richardsonii except that: Weight, 0.60 (0.575-0.645); basilar
length, 28.0 (27.3-29.4); breadth of rostrum approximately 30 per
cent of basilar length.
In comparison with streatori, males average smaller in every
measurement taken with no overlap in most dimensions; 25 per cent
lighter; anterior margin of tympanic bulla more nearly flush with
squamosal, that is to say less protruded from braincase; in relation to
other dimensions of skull, braincase shallower anteriorly (at plane of
last molars) and deeper posteriorly (at anterior end of basioccipital).
Females average smaller in every measurement taken except mastoid and
zygomatic breadths which are actually more; 6 per cent lighter; in
relation to other parts of skull, preorbital and interorbital parts
slightly smaller; in relation to length of skull, braincase shallower.
Comparison with invicta and gulosa is made in the accounts of those
subspecies.
Remarks.—The smallest males of the entire species are of this
subspecies and the females of it are barely larger than those of
olympica and gulosa and hence are among the three smallest. The
material now available consists only of one or a few specimens from
each of several widely separated localities. If as many specimens per
unit area were available as there are of the species M. erminea from
southern British Columbia, geographic variation warranting the division
of muricus into more than one subspecies might be revealed. Evidence
pointing in this direction is comprised in the pale color and small
size of the pair of adults from Wheeler Peak on the eastern border of
Nevada; the suggestion is that there is a distinct pale race of small
individuals in the isolated spots of boreal life-zone in the mountains
of the desert. The color and size of the specimens from the Toyabe
Mountains, and that from the Pine Forest Mountains, both places also in
Nevada, nevertheless, lend no support to this suggestion. Comparison of
specimens from the Rocky Mountains of Colorado with those from the
Sierra Nevada of California gives no basis for recognizing more than
one subspecies. Therefore, Putorius streatori leptus Merriam with
type locality at Silverton, San Juan County, Colorado, falls as a
synonym of the earlier named Putorius (Arctogale) muricus Bangs with
type locality at Echo, El Dorado County, California. Furthermore,
specimens from northern New Mexico, the southernmost known area of
occurrence for the subspecies (and for the species), are as large as
specimens from far north in the range of the subspecies, say, in
northwestern Wyoming; there is therefore no evidence of progressive
decrease in size to the southward as in advance of study I supposed
existed in muricus. This erroneous supposition was held because I
knew that there was a decrease in size to the southward in the species
as a whole and also in each of the subspecies richardsonii and
invicta directly to the north of muricus.
Intergradation with invicta is shown by specimens from southwestern
Montana. Where the margins of the geographic ranges of invicta and
muricus approach one another elsewhere, low-lying territory, zonally
unsuited to the existence of the species, occurs along the Snake and
Columbia rivers, and precludes any chance of intergradation except
around the head of the Snake River Plains. Two specimens, here referred
to muricus, from Siskiyou County, California, in both color and
cranial characters, are intergrades with streatori and might be
referred with almost equal propriety to streatori.
Specimens examined.—Total number, 52, arranged alphabetically
by states, then by counties from north to south within each state.
Unless otherwise indicated, specimens are in the Museum of
Vertebrate Zoölogy, University of California at Berkeley.
California. Siskiyou County: head of Rush Creek, 6400 ft., 1;
Castle Lake, 5434 ft., 1. Tehama County: 2 mi. W Black Butte,
6800 ft., 1. Placer County: ridge W of Tahoe Pines, Lake Tahoe,
1; Blackwood Creek, 6250 ft., near Tahoe Pines, 1. El Dorado
County: Fallen Leaf Lake, 6500 ft., 1[33]; Echo, 1[75]. Tuolumne
County: Ten Lakes, 9200 ft., Yosemite Park, 1. Mariposa County:
Vogelsang Lake, 10350 ft., Yosemite Park, 1. Mono County:
Mammoth, 1[59].
Colorado. Rio Blanco County: Marvine, 1. Boulder County: Camp
Albion, 10600 ft., 1[60]; Boulder, 1[91]. Denver County: Denver,
1[57]. Park County: Jefferson, 1[57]. Gunnison County: near
Placita in Gunnison County, 1[26]; Crested Butte, 9000 ft., 3
(1[91], 2[19]). El Paso County: Turkey Creek, SW Colorado
Springs, 6000 ft., 1[19]. Chaffee County: Arbourville, 1[91];
Hancock, 1. Montrose County: Coventry, 6800 ft., 1[19]. San
Juan County: Silverton, 1[91]; in San Juan County above
timberline, 1[87].
Idaho. Bannock County: West Fork of Rapid Creek, 9-1/2 mi. E
Pocatello, 1.
Montana. Meagher County: Camas Creek, Big Belt Mts., 4 mi. S Ft.
Logan, 1[91]. Beaverhead County: Donovan, 1[91]. County in
question: Yellowstone Park, 1[75].
Nevada. Humboldt County: Alder Creek, 6000 ft., Pine Forest
Mts., 1. Ormsby County: 1/2 mi. S Marlette Lake, 8150 ft., 1.
Nye County: South Twin River, Toyabe Mts., 1[91]. White Pine
County: Baker Creek (8500 ft., 8675 ft., 11100 ft.), 3.
New Mexico. Taos County: Twining, 10700 ft., 1[91]. Sandoval
County: 9 mi. E Cuba, 9000 ft., 1. Santa Fe County: Saddle S of
Santa Fe Baldy, 11000 ft., Santa Fe Range, 1[1].
Oregon. Wasco County: Mill Creek, 20 mi. W Warmsprings, 1[91].
Klamath County: Fort Klamath, 1[91].
South Dakota. Pennington County: 4 mi. SE Hill City, 5300 ft.,
2[76]; Pfander's Ranch, 3 mi. SSE Hill City, 5300 ft., 1[76];
Palmer Gulch, 3 mi. SE Hill City, 5300 ft., 1[76]; Spring Creek, 2
mi. W Oreville, 5500 ft., 1[76]. Custer County: 1/2 mi. E Sylvan
Lake, 6250 ft., 1[76].
Washington. Columbia County: Butte Creek, 1; Stayawhile Spring,
5150 ft., 1.
Wyoming. Crook County: 5 mi. NW Sundance, 5900 ft., 1[93].
Teton County: Whetstone Creek, 2[76]; 1/4 mi. E Moran, 6700 ft.,
1[93]. Sublette County: 1/2 mi. NE Pinedale, 7500 ft., 1[93].
Albany County: 30 mi. N and 10 mi. E Laramie, 6560 ft., 1[93]; 26
mi. N and 4-1/2 mi. E Laramie, 6960 ft., 1[93]. Carbon County: 8
mi. N and 19-1/2 mi. E Savery, 8800 ft., 2[93].
Mustela erminea? angustidens (Brown)
Plates 7, 12, 13 and 14
Putorius cicognanii angustidens Brown, Mem. Amer. Mus. Nat.
Hist., 9 (pt 4):181, pl. 17, 1908.
Mustela cicognanii angustidens, Hay, Iowa Geol. Surv. Bull.,
23:32, 1914; Hay, Carnegie Inst. Washington, Pub. no. 322A:252,
October 15, 1924; Hay, ibid., Pub. no. 390 (vol. 2): 528, 1930;
Hall, ibid., Pub. no. 473:111, 112, November 20, 1936.
Type.—Female, adult, skull and lower jaws lacking zygomata,
right P2 and incisors, no. 12432, Amer. Mus. Nat. Hist.; from
Conard Fissure, four miles west of Willcockson, Newton County,
Arkansas; obtained sometime in the period 1903 to 1905 inclusive
(see plates 8, 14).
Range.—Known only from the Pleistocene deposit in Conard
Fissure, at the type locality in northern Arkansas.
Description.—Skull.—Male (based on nos. 12437, 12441 and
12444): See measurements and plates 7 and 8; weight, unknown;
basilar length, 38.1 (36.6-39.2); length of tooth-rows more than
length of tympanic bulla; breadth of rostrum measured across
lacrimal processes less than a third of basilar length;
interorbital breadth ordinarily equal to distance between glenoid
fossa and posterior border of external auditory meatus; zygomatic
breadth probably averaging approximately the same as distance
between last upper molar and jugular foramen.
Female (based on nos. 11766 and 12435): See measurements and plates
8, 12-14; weight, unknown; basilar length, 34.0 (32.5-35.1);
length of tooth-rows more than length of tympanic bulla; breadth
of rostrum about equal to (more or less than) 30 per cent of
basilar length; interorbital breadth less than distance between
glenoid fossa and posterior border of external auditory meatus;
zygomatic breadth probably less than distance between last upper
molar and jugular foramen.
Comparison of the cranial description given above with those of the
American races of erminea from the far north will show that
many characters are held in common—more than with more southern
subspecies of erminea.
Remarks.—The ten specimens studied by the writer fall into two
groups of six larger individuals and four smaller. Upon comparing these
with each sex of the three species of American Recent weasels,
frenata, erminea and rixosa, it is seen that size, and to some
degree shape, rule out of consideration both sexes of rixosa and also
males of frenata. Thus we are left with females of frenata and
males and females of erminea. So far as size is concerned, it can be
assumed that the larger specimens are females of frenata and that the
smaller are males of erminea. This assumption has in its favor also,
the fact that the postglenoidal length of the skull accords with that
in Recent specimens. The difference in this regard in Recent animals is
that the postglenoidal length of the skull, expressed as a percentage
of the total (condylobasal) length of the skull, amounts to:
| in frenata |
in erminea |
| ♂ ordinarily less than 46 |
♂ ordinarily more than 46 |
| ♀ less than 47 |
♀ more than 48 |
In the fossils the percentage for the larger skulls is 46; for the
smaller skulls it is 48.
It may be that the ten fossil skulls are six female frenata and four
male erminea but I think not. In the first place a skull of different
shape, seemingly of the frenata stock, is known from the deposit and
it is almost certain that two subspecies of the same species would not
occur at the same place at the same time. It is possible, of course,
that parts of the deposits were laid down at times so far apart that a
shift in geographic range of two subspecies had occurred. This one
skull, seemingly of the frenata stock, is the type of Putorius
gracilis Brown (see p. 404) and was regarded as the only known
specimen of gracilis. Regardless of the specific identity of this one
specimen named gracilis, the chances of obtaining otherwise from a
deposit, like that in Conard Fissure, six females of frenata and four
males of erminea without a male frenata or a female of erminea
coming to light are so slight as strongly to incline me to the view
that the six larger specimens are males of the same species to which
the 4 smaller specimens belong. By either this interpretation, or the
one initially considered (of female frenata and male erminea), the
animals from the fissure are at least subspecifically distinct from any
American Recent weasel. Furthermore, by this latter interpretation each
sex of this weasel, angustidens, is intermediate between the
frenata and erminea stocks in the feature of postglenoidal length
which feature, at any place where the two Recent species occur
together, serves to distinguish one from the other. In the northernmost
subspecies of erminea (arctica for example) the postglenoidal
length in some males is no longer than in males of frenata.
Considering general size, angustidens agrees better with erminea
than with frenata and this circumstance has influenced me to place
angustidens as a subspecies of erminea.
Today, erminea is not known to occur nearer Conard Fissure than
northern Iowa, more than 400 miles to the northward. In comparison with
the race there, bangsi, males of angustidens are of approximately
the same size but in the shorter distance between the glenoid fossa and
anterior margin of the tympanic bulla, and also in the lesser
postglenoidal length of the skull, angustidens resembles the
northernmost American subspecies of erminea. Females of angustidens
differ more from any living weasel than the males do. The females are
much larger than those of bangsi, and among living American races of
erminea most closely resemble intergrades between arctica and
richardsonii which intergrades are found approximately 1700 miles to
the north of Conard Fissure. In females, the preorbital part of the
skull in M. e. arctica is broader and in M. e. richardsonii
narrower than in angustidens. If it seems strange that females of
angustidens resemble one subspecies whereas males, in size, resemble
another subspecies almost a thousand miles distant, it should be
remembered that the degree of sexual dimorphism varies much from one
subspecies to another in the Recent animals. An example is furnished by
Mustela erminea fallenda and Mustela erminea invicta.
The assemblage of mammals from Conard Fissure includes several species
of boreal predilections which, like Mustela erminea, now occur only
much farther north than Arkansas. At one time the edge of the sheet of
ice was only about 200 miles north of Arkansas. It may be significant
that the cranial characters of the female ermine from the Fissure, and
qualitative cranial characters of males from there, are most nearly
approximated among Recent weasels by those which live along the
southern edge of the frozen tundra.
In view of what has been said, the possibility should be considered
that the distinctive cranial features of angustidens may be the
result of evolutionary change in time as well as of geographic
variation resulting from horizontal placement.
MUSTELA RIXOSA (Bangs)
Least Weasel
(Synonymy under subspecies)
Type.—Putorius rixosus Bangs, Proc. Biol. Soc. Washington,
10:21, February 25, 1896.
Range.—From Norway and Switzerland eastward through Siberia and
all the way across North America, but unknown from Iceland,
Greenland and the Arctic islands west of Greenland; in North
America, from the Arctic Life-zone south to Central British
Columbia, Montana and into parts of the Upper Austral Life-zone as
in the eastern half of the continent.
The southern extension of range in the Appalachians (to North
Carolina) is not duplicated in the Rocky Mountains of western
North America probably because the region there suitable for
rixosa south of Central British Columbia and Montana is occupied
by the almost equally small Mustela erminea muricus and related
subspecies which seem to fill the ecological role that rixosa
plays where it occurs. The small size of females of M. erminea
cicognanii in New England may similarly account for the absence
of rixosa there.
Characters for ready recognition.—Differs from both Mustela
erminea and Mustela frenata by tail a fourth or less of length of
head and body and without a black tip (at most a few black hairs at
extreme tip in rixosa), and from M. frenata and from M. erminea in
regions where it and rixosa occur together, by basilar length of
skull less than 32.5 in males and less than 31.0 in females.
Characters of the species.—Size small: Total length less than 250 in
males and 225 in females; tail a fourth or less of length of head and
body, and without a black pencil and at most with a few black hairs at
extreme tip; caudal vertebrae 11 to 16, normally 15 in M. r. rixosa,
and 11 in one M. r. eskimo examined; skull with long braincase and
short precranial portion, thus essentially same shape as in M.
erminea but the largest males of M. rixosa always with a lesser
basilar length that even the smallest females of M. erminea or M.
frenata of the same geographic area. In fact no specimens of M.
frenata have skulls so small as the largest M. rixosa, and skulls of
equal size of M. erminea and M. rixosa, for example, M. erminea
muricus of Colorado and M. rixosa eskimo of Alaska, differ in that
when the skulls are viewed from directly above those of rixosa have
the mastoid processes more prominent, or the braincase is higher in
relation to its width or both differences together prevail. Stated in
another way, comparison of skulls of equal size of rixosa and
erminea shows that in the latter the braincase is more nearly flat
and is wider above and in front of the mastoid processes; therefore,
the greatest breadth of the braincase equals or exceeds the mastoid
breadth, whereas the reverse is ordinarily true of rixosa.
Geographic variation.—In the Old World four subspecies are currently
recognized (see Allen, 1933:316) and the same number is here recognized
in North America. Length of the tail, length of head and body and hind
foot, breadth of the rostral part of the skull in relation to its
length, and position on the side of the head of the line of demarcation
between the dark color of the upper parts and the white underparts, are
the features in which geographic variation has been detected. The
general impression is that the amount of geographic variation is much
less than in Mustela frenata and only slightly less than in Mustela
erminea of the same geographic area.
Nomenclature.—It is exceptional for a species which occurs in both
the Old- and New-World to take its specific name from New World
material, especially if the name was proposed as recently as 1896; most
circumboreal species take their names from descriptions of European
specimens. Although the least weasel, Mustela rixosa (Bangs) 1896,
seems now to be an exception, it may yet turn out that the first
available name was based on European material. Zimmermann (1943) shows
that the least weasel actually was named on the basis of European
material long before 1896 and concludes that the name Putorius
minutus Pomel, 1853, based on a specimen from France, is the first
available name.
Because Putorius nowadays is relegated to subgeneric rank under the
generic name Mustela, we have for consideration the name-combination
Mustela minuta (Pomel). Unfortunately for Zimmermann's conclusion,
Mustela minuta Pomel is not available because it is preoccupied by
Mustela minuta Gervais [= Palaeogale minuta (Gervais),
1848-1852—see Simpson, 1946: 2, 12], a name applied to another species
of small mustelid from the Oligocene or lower Miocene deposits of
Europe.
Some other early names thought by Zimmermann (1943:290) to have been
based on the dwarf weasel of Europe are judged to be nomina nuda and
therefore are to be ignored.
The name Mustela minor Nilsson 1820 was thought by Miller (1912:402)
to be a renaming, and hence a synonym, of Mustela nivalis Linnaeus.
If that is the case the name does not apply to the dwarf weasel. If the
name Mustela minor Nilsson was instead based on the dwarf weasel, the
name might still be unavailable, depending on rulings on secondary
homonyms, because the name might be preoccupied by [Lutra] minor
Erxleben 1777 which is a synonym of [Mustela] lutreola Linnaeus 1766.
Two names seemingly available for weasels, and in use for them today,
which might replace rixosa as the name of the species, are, first,
Mustela boccamela Bechstein, 1801, of Sardinia [= Mustela nivalis
boccamela of Miller, 1912, 405] and second, Putorius numidicus
Pucheran, 1855, of Morocco and Algeria [= Mustela numidica of Allen,
G. M., 1939, 183]. As they stand in the current literature, Mustela
numidica is a species distinct from the dwarf weasel and the other
name, Mustela nivalis boccamela, is an insular subspecies of the
mouse weasel. Zimmermann (1943:292), however, implies that M.
numidica may belong to the dwarf weasel group when he says "Ob auch
iberica Barr.-Ham. als Unterart zu minuta Pom. zu stellen ist, soll
hier nicht untersucht werden, ebensowenig die von Cabrera vermutete
Zugehörigkeit der grossen nordafrikanischen M. numidica Puch. zur
'iberica-Gruppe'." The answer to this problem requires a taxonomic,
rather than a nomenclatural, decision. Whether either M. numidica or
M. boccamela are conspecific with the dwarf weasel I cannot at this
time ascertain for want of adequate specimens. Because these two names,
M. boccamela, and M. numidica, are assigned to kinds of weasels
which are currently regarded as specifically distinct from the dwarf
weasel, and because all the other names which certainly have been
assigned to Old World populations of the dwarf weasel before 1896, so
far as I know, are nomina nuda or are preoccupied, the next available
name, Mustela rixosa (Bangs, 1896), is here employed.
Remarks.—This species may have a wider geographic range in
northeastern North America than is now known. Strong (1930:7) writes
that the Naskapi Indians of the interior country of Labrador between
Hamilton Inlet and Ungava Bay "have only one name for weasel,
mé-tah-kwut, but they say there are three kinds in their territory, a
large, an intermediate, and a very small weasel. The latter suggests
the least weasel . . . which has not been recorded from northern
Labrador."
In the northern part of the range of the species, the winter pelage is
white and the summer pelage is brown. In the southern part of the
range, that is in the range of the subspecies allegheniensis, the
winter pelage is either brown or white and the time of the molt into
winter pelage is irregular; each of eleven individuals from
Pennsylvania, Michigan and Ohio, taken in December, January, February
and March is mostly white but retains some considerable part of the
brown pelage of the previous coat on top of the head and usually also
along the midline of the entire dorsum. These eleven animals include
individuals of each sex. Of each sex, some are adults and some are
subadults. Therefore, the delayed or incomplete fall molt, at present,
cannot be correlated with either sex or with any particular age. No
wild-taken specimens of M. erminea or of M. frenata of the same
region show this delayed or incomplete molt.
Possibly this delay or incompleteness of molt is the result of the same
cause that lies behind the birth of some M. rixosa in midwinter. As
listed below, several litters of young have been found in midwinter. In
fact it appears that in the United States, young may be born in every
month of the year although, according to existing information, more
litters are produced in spring and in winter than in summer and autumn.
Many juveniles and young of allegheniensis examined in study
collections clearly were born in spring but about as many seem to have
been born in midwinter as at any other time (in the light of present
knowledge) and this is in contrast to what we know of the two other
species of American weasels since their young, so far as known, are
born in spring.
One instance is worthy of detailed comment. An adult female, no. 783
Ohio State Museum, taken on January 31, 1931, at Vinton, Meigs County,
Ohio, bears the following notation on the attached label "nest plowed
out of ground. Very small young escaped—marked like parent. ♀ was
nursing." The enlarged mammae on the dried skin substantiate the
statement that the female was nursing young. She has a brown mask
continuous from one ear through the eye, across the forehead and
through the other eye to the opposite ear. On each side of the body a
stripe of brown 5 to 10 mm. wide extends from the upper part of the
foreleg back to the thigh and base of the tail, uniting there with its
opposite and covering the tail. There are a few spots of brown on the
shoulders, and rump and one on the middle of the back. Otherwise the
specimen is white. One implication of the statement on the label that
the young which escaped were marked like the parent (presumably this
female parent) is that this female is a partial albino. I am more
inclined, however, to the view that there was an unseasonable activity
of the particular glands of internal secretion the hormones of which
promote embryonic growth and that these glands, or others controlled
by them, were in some way responsible for an abnormal progress of molt,
or for a reversal of molt in that one molt began before the previous
molt had been completed.
Excepting this one specimen, no. 783 from Vinton, Ohio, all of those in
transitional pelage indicate that the direction of the molt pattern is
the same as in M. frenata and M. erminea. That is to say, the
autumnal molt begins on the midventral line and the molt in spring
begins on the mid-dorsal line. Furthermore, the normal progress of each
molt appears to follow the same pattern that has been described above
for Mustela frenata.
A possible explanation of unseasonal molt in the southeastern area of
occurrence of the species Mustela rixosa, and a possible explanation
of the abnormal molt of the female from Vinton, Ohio, is that the
species has only relatively recently invaded the area, and has had
insufficient time to adjust the physiology of its molting mechanism to
the longer periods of daylight that obtain later in autumn and earlier
in spring than farther north. In the other two species of American
weasels, the change in length of periods of light, it will be recalled,
is known to indirectly control both molt and some changes in the sexual
cycle. Wright (1942B:109) has shown that molt in spring precedes by one
or two months the birth of young in M. frenata, that the two
phenomena are correlated in a way that is statistically significant,
and recognizes that progressively longer periods of daylight may be the
causal stimulus. The suggestion made above that M. rixosa does not
live in New England or in the Rocky Mountains of the western United
States because each of the two areas already is inhabited by weasels of
almost equally small size, is in line with the idea that rixosa is a
recent immigrant to America, or more precisely that rixosa arrived
later than erminea.
Natural History.—Habitat and Numbers.—Soper (1946:136) recounts
that near the junction of the Antler and Souris rivers, Manitoba, this
species occurs "both in the river valleys and on the upper prairies,"
and later (1948:55), with reference to the Grand Prairie of the Peace
River region of Alberta, writes that the least weasel "inhabits both
parklands and mixed wood forest environments."
At most times, wherever found, the least weasel is regarded as rare.
Not only mammalogists regard it as rare and as a desirable catch, but
Indians likewise value it, probably because of its rarity. For example,
Osgood (1901:69-70), who caught a female least weasel at Tyonek,
Alaska, writes that: "The natives regard the capture of one of these
rare animals as a piece of great good fortune. One old Indian who
frequently visited our cabin told us that his brother who had caught
one when a small boy had in consequence become a 'big chief'; and he
assured me that since I had caught one I must surely be destined to
become a man of great wealth and power."
Swenk's (1926:313-330) account of the species in Clay County, Nebraska,
shows, however, that the animal was far more abundant in 1916 and 1917
than subsequently and inferentially than it was before 1916. Clearest
proof of multiannual fluctuation is provided by P. O. Fryklund's
(Swanson and Fryklund, 1935:120-126) receipt of weasels from Roseau
County, Minnesota. From 1895 to 1932 he had approximately equal
opportunity to receive least weasels each year. Those which came to his
attention were distributed by years as follows: 1895-1927, 7
individuals in all; winter of 1927-28, 3 individuals; winter of
1928-29, 59 individuals; 1929-1930, 84 individuals; 1930-1935, 3
individuals. "These records indicate a very definite increase in the
abundance of least weasels in the Roseau region [in] the two years from
the autumn of 1928 to the spring of 1930. Mr. Fryklund has handled 166
least weasels in his 40 years in Roseau County, and of these, 143 were
taken in the two years mentioned."
The maximum home range of the least weasel is two acres and a weasel
seldom travels farther than ten rods from its burrow according to
Polderboer (1942:146) who, in the period December 20, 1939, to January
2, 1940, studied four least weasels and one long-tailed weasel on a 144
acre farm in Butler County, Iowa.
Behavior
Of the voice, Llewellyn (1942:441) records that his captive specimen
taken in Virginia uttered a shrill shriek when seizing prey or when
teased. When excessively annoyed the weasel also emitted musk.
The sense of smell is used in hunting as was witnessed by George L.
Fordyce; he observed a least weasel following the scent of a
Peromyscus and saw the least weasel overtake and kill the mouse
(Seton, 1929 (2):637).
At a nest in a clover stack, in Manitoba, Criddle (1947:69), on
December 27, 1946, found the least weasel "to have been rather remiss
in its sanitary habits as its pile of dung was almost, or quite,
touching the nest and only just to the side of its entrance." There
were 117 voids.
Enemies
The great-horned owl, barn owl and long-tailed weasel are to be counted
as enemies since Nelson (1934:252) found the fur, skull and other
fragments of the skeleton of a least weasel in one of 26 pellets of the
great-horned owl in Wisconsin; Handley (1949:431) found the skull and
other skeletal remains of a least weasel in one of 22 pellets of the
barn owl in Virginia; and Polderboer, Kuhn and Hendrickson (1941), in
Iowa, found the remains of a least weasel in the den and scats of a
Mustela frenata. A domestic cat in Michigan killed a least weasel
(Dearborn, 1932B:277).
Food
Mice are killed by the least weasel biting into the back of the head
and neck according to Allen (1940:460) who reported upon the growth of
five young, from Michigan, that he had in captivity. He further states
that a weasel was able to kill a mouse in 30 seconds. One large
Microtus introduced into the cage slept with a weasel for several
days and ate parts of the mice that the weasel killed but then the
weasel killed this mouse! Llewellyn (1942:440-441), in writing of a
captive from Virginia, says: "When a live mouse was placed in the cage,
the weasel sprang upon it almost instantly. Grasping the mouse by the
back of the head, the weasel bit its victim through the skull several
times in rapid succession and held on with its sharp teeth. The sound
of the teeth piercing the bone was distinctly audible at a distance of
several feet. During this interval the weasel hugged the mouse closely
with its fore legs and pressed it firmly to its belly through a kicking
motion of the hind legs. The hold on the back of the head was not
relinquished until the mouse was dead. The killing took only a few
seconds. Upon releasing the mouse the weasel usually came to the front
of the cage and inspected the observer for an interval of several
seconds after which it returned to its prey and began its meal at once.
Sometimes the blood would be licked from the wound in the back of the
head or perhaps an ear would be chewed a bit and the blood licked off,
but never did the weasel 'cut the throat' of its prey and 'suck the
blood.'
"The weasel ate the head and brain first, beginning at the back of the
head and working forward. Just before reaching the nose the process was
reversed and eating then proceeded from the base of the skull toward
the tail of the mouse. The tip of the nose, maxilla with teeth, and the
tail seemed to be the parts least preferred; they were not eaten when
an abundance of food was present. At no time did the weasel place its
front feet on the mouse in an attempt to hold it. A second or third
mouse was killed immediately upon being placed in the cage even though
the first one had not been consumed. The weasel, however, usually
returned to the partially eaten mouse and finished it before starting
on a new one. Upon completing a meal, especially if the meal had been
particularly bloody, the weasel rubbed its chin on the bottom of the
cage, scooting along and appearing more snakelike than normal. Whenever
I attempted to remove a mouse, or partially eaten one, from the cage,
the weasel hung to the mouse tenaciously, and often allowed itself to
be lifted up in this manner.
"In the six days that the weasel was kept in captivity it was fed 10
house mice having a total weight of 118 grams. As no food was given on
one day, the amount of food eaten is probably slightly below the actual
capacity of the animal. Since the weasel weighed only about 32 grams,
the average amount of food eaten a day was slightly in excess of
one-half the weight of the animal."
Polderboer (1942:146-147) found in three dens, in Iowa, bits of
Reithrodontomys (harvest mice) and Peromyscus maniculatus (deer
mouse), and in the digestive tract of one least weasel there was a bone
fragment and a few hairs of a deer mouse. In the account, given beyond,
of a nest, Criddle (1947:69) records the Pennsylvania meadow mouse
(Microtus pennsylvanicus drummondi) and the Gapper red-backed vole
(Clethrionomys gapperi) as prey at Treesbank, Manitoba. The same
author, concerning the same place, earlier (1926:199-200) wrote that in
1922 the meadow mouse, Microtus minor, "went into winter quarters in
great numbers and its homes were well stocked with provisions . . . all
went well until the middle of February, 1923. Then, within a few days,
each was taken possession of by a least weasel (Mustela rixosa) and
the inhabitants were quickly destroyed. One dwelling was occupied by
one of these weasels for about two weeks during which time I observed
that it had dragged several mice over the snow to its temporary home.
This residence was examined in April, and in it were discovered six
dead Microtus minor, one Evotomys, the head of another, and at
least six or eight remnants of small rodents including Microtus
drummondi, these last remains being chiefly indicated by the
hair-lined nest of the weasel.
"The homes of 27 other vole communities examined at this time were all
found to have been entered by weasels, the inhabitants having been
killed and partly eaten. Moreover, the weasels had made the homes
temporary centers from which they raided other rodent habitations in
the vicinity. Thus from being an abundant animal this vole was reduced
to insignificance in the course of a few weeks, while all other kinds
of mice had suffered severely from the same enemy."
An instance of predation on Peromyscus, revealing some of the methods
of capturing prey, is recounted by Seton (1929 (2):636-637) who quotes
a letter to him from George L. Fordyce, of Youngstown, Ohio, as
follows: "While out in the field this morning (Dec. 26), walking along
the bank of a ravine at the edge of our golf course, I saw a
Field-mouse run out of the bushes into the rough grass that is just
outside of the fair-green of the course. In another instant, what I
thought at first to be a white Mouse came out at the same place. The
Mouse ran into a wheel track, and disappeared under the grass, coming
out about 6 feet from where it went in. The white animal followed
through the same course, and when it came out, I saw that it was a
small Weasel, very little larger than the Mouse, and that it was
following the trail of the Mouse by scent.
"For a time the Mouse ran in circles, and zigzagged about, often . . .
within 4 or 5 feet of the Weasel; but the latter seemed so intent on
the trail, that it did not notice the Mouse to one side. After a time
the latter started toward the open golf course; and when the Weasel
reached the point where the trail was straight, it sighted the prey,
made a sudden dash forward, and, although 25 feet behind, overtook the
Mouse while it was going 3 or 4 feet.
"For a few seconds, they seemed to fight, until the Weasel got the
Mouse by the throat, and started for the bushes, dragging the body.
When it came to within about three feet of me, I moved a little to see
what it would do. It dropped its victim, and ran into the ravine. The
Mouse had a drop of bright red blood in the center of its white throat.
I waited near by for 15 or 20 minutes, thinking the Weasel might come
back, but it did not show up again; even an hour later, the Mouse had
not been disturbed."
There are two suggestions, but no proof that I know of, in the
literature that the least weasel eats insects. Abbott (1884:27-32—1st
ed., 1884) gives considerable information on the food (some insects
included) of the "little weasel" which he describes (op. cit.; 27) as
having "a little pointed tail of a uniform brown color." Although this
suggests Mustela rixosa, Abbott mentions on the next page (page 28)
that a specimen of the smaller weasel measured six and a half inches
from the tip of the snout to the base of the tail and that the tail
itself measured two and a fourth inches to the tip of the last caudal
vertebra. These measurements indicate that Mustela erminea was
involved. Because of the uncertainty as to the species of Mustela
involved, Abbott's interesting data on food, nest and behavior are not
recorded in the present work. Seton (1929 (2):636) says that of several
least weasels brought to D. Nicholson at Morden, Manitoba, most of them
decayed so quickly that they could not be saved as specimens. To Seton
this indicated that insects were an important part of the food of the
weasels.
In summary: Least weasels are known to eat harvest mice, deer mice,
meadow mice and red-backed mice; it is suspected that they eat also
insects.
Reproduction
Polderboer (1948:296) has taken six specimens in "northeastern Iowa
[in] . . . January and December—all males in winter pelage. None of
these males showed signs of sexual activity; in all, the testes were
retracted and diminutive in size. . . . A male least weasel in brown
pelage was taken November 17, 1945, at Marion, Iowa. This specimen had
large testes that had descended into the scrotum. The testes, when
removed, were about the size of medium-sized garden peas. Microscopic
examination of the testes and the vasa deferentia showed mature sperms
to be present. . . ."
On July 1, 1917, in Clay County, Nebraska, a nest with four young was
found (Swenk, 1926:321). On July 29, 1939, an adult and five young were
plowed out of the ground in Allegan County, Michigan; one of the two
young males weighed 40.5 grams two days after capture (Allen,
1940:459-460). On August 12, 1932, ten young with the mother, were
found in Roseau County, Minnesota (Swanson and Fryklund, 1935:125).
September appears to have been the month of birth of a specimen, no.
8472 in the Carnegie Museum, taken on November 24 in Pittsburgh,
Pennsylvania. In October, a young least weasel is recorded from
Pennsylvania (Winecoff, 1930:313). Early December was the time of birth
of a specimen, approximately 10 weeks old, no. 88077, University of
Michigan, taken on February 21 in Allegan County. On December 25, 1927,
in Washington County, Pennsylvania, "five full-sized, though young . . .
animals were caught under the same pile of corn fodder" (Sutton,
1929:253). The first week of January seems to have been the time of
birth of a juvenile, no. 88080, University of Michigan, taken in
Livingston County, Michigan, on March 27, 1943, since the specimen is
approximately seven weeks old. On January 15, 1929, in Washington
County, Pennsylvania, four young with the eyes yet unopened were
obtained from a nest (Sutton, 1929:254). On January 25, 1928, young,
the eyes of which may not yet have been open, were taken from a den in
Washington County, Pennsylvania, by Winecoff (1930:313), who records
other young having been taken in the same month as well as in February.
On March 10, a female from North Portal, Saskatchewan, gave birth to
four young (Dunk, 1946:392). On April 18, 1916, four young, half grown,
were taken in Nebraska (Swenk, 1926:317). On April 2, 1929, three young
were found in Roseau County, Minnesota, according to Swanson and
Fryklund (1935:125) who remark that: "The Pennsylvania and Minnesota
records show that least weasels may be born any time from July to early
February in the northern states." Now, with all of the above records
available, it turns out that November, May and June are the only months
in which young least weasels have not been reported. Of course some of
the young, for which the ages were not specified, were born in
preceding months. Even so, the data now available suggests that, in the
United States, young least weasels may be born in every month of the
year. The number per litter is 3, 4, 4, 4, 5, 5, and 10, yielding an
average of 5.
The rate of growth of the young has not been studied enough to allow of
judging if it differs significantly from that of other species of the
genus. Allen (1940:459-460), however, tells us that of the three young
females and two young males captured on July 29, 1939, in Allegan
County, Michigan, one male that was killed on July 31, 1939, weighed
40.5 grams. The male remaining alive increased from 46 grams (August 5)
to 62.5 grams on September 20, having eaten 63 mice while in captivity.
The females in the period of August 5 to September 4 increased in
weight as follows: 41 up to 49 grams; 44 to 50 grams; and 47 to 58
grams, having eaten, by September 26, 60, 64 and 65 mice.
Concerning a nest in which young were found, Sutton (1929:254) writes
that on January 15, 1929, near Burgettstown, Washington County,
Pennsylvania, an animal was seen to enter a small hole in a creek bank.
After the observer dug in a distance of approximately six inches an
adult, female least weasel was seen and obtained. Back of the animal,
the hole, which turned sharply downward, was full of water. The weasel
first seen was a female nursing young. A chamber, to the side of the
hole, filled with dead grass, comprised a nest containing four young
with the eyes yet unopened. Several nests occupied by adult least
weasels or by least weasels that were old enough to shift for
themselves have been found. Polderboer (1942:145-147) in the winter of
1939-40, on a 144 acre farm in Butler County, Iowa, found four least
weasels living, singly, in burrows dug by moles and pocket gophers. The
nests therein made by mice were used by the least weasels. Winecoff
(1930:312-313) mentions one den in Pennsylvania that contained the
remains of only mice, "and not a hint of a feather." Above, in the
account of food of the least weasel, Criddle's (1926:199-200) account
of the havoc wrought by least weasels among the meadow mice (Microtus
ochrogaster minor) has been given. In this account he mentions the
fur-lined nests of the weasels that had appropriated the homes of the
Microtus. Criddle's (1947:69) later account of a nest at Treesbank,
Manitoba, is as follows: "A Nest of the Least Weasel.—When a least
weasel finds its way into a locality that has a large number of mice in
it, it selects for its home one of their nests that has been made in a
well concealed place. This it immediately starts to improve by lining
it with hair plucked from its victims before eating them; and as long
as sufficient numbers of mice remain in the district the weasel
continues adding their hair to the nest, so that the thickness of its
walls give one a good idea of the length of time it has been in use.
The nest is not only used for sleeping in, as most of the food is
consumed in it. Frozen mice are taken in to be thawed out and the
weasel carries those it has recently killed in to prevent them getting
frozen, or perhaps to have them warm for its next meal.
"On January 27, 1946, my son Percy called my attention to a nest that
he had just uncovered in a clover stack that we were using. This nest
had originally been made by a Drummond's vole, Microtus pennsylvanicus
drummondii, and taken from it by the least weasel, Mustela rixosa,
the tracks of which had been noticed about the stack yard since the
first snow in early November.
"The nest had evidently been in use for at least three months and the
continual additions made to its walls had been so great that they were
nearly an inch thick of hair matted together so closely that it
appeared to be felt. The hair alone weighed nearly 22 gm., so that with
this for protection the weasel must have been warm and comfortable
through the severest winter weather.
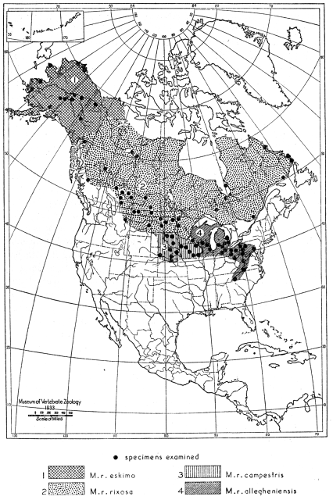
Fig. 28. Map showing occurrences and probable geographic
ranges of the subspecies of Mustela rixosa in North America.
"In the nest were two red-backed mice, Clethrionomys gapperi, one of
which had the base of its skull eaten out. No hair had been removed
from either of them, but a Microtus lying in a side tunnel some feet
away had the long hair plucked from its back and sides. In and close
about the nest were found forty-three front parts of mice skulls which
had evidently been discarded because of the sharp teeth in the
maxillaries. Seven full stomachs and eleven hind feet of adult
Microtus with parts of leg bones were disclosed in, or under, the
weasel's bed and a few small bits of skin with hair attached were
scattered among the plucked hair of the nest.
"This weasel seems to have been rather remiss in its sanitary habits as
its pile of dung was almost, or quite, touching the nest and only just
to the side of its entrance. It was composed of 117 voids all of which
contained much hair and broken bone.
"Six other mouse nests found in the same stack, or others adjoining it,
had been thinly lined with hair. One of these had two mice in it, a
red-backed with its brain eaten out and a Microtus with some hair
plucked from its neck. Another nest contained the front part of a skull
with teeth and the hind feet and tail of a red-back. Besides the mice
found in the nests seven others were discovered tucked away in side
tunnels. One of these mice had most of the hair plucked from its back.
Whether all these mice and nests belonged to the same weasel or not I
am unable to say, but it is usual for them to have several nests in the
area surrounding the one that is used as their headquarters or home."
Mustela rixosa eskimo (Stone)
Least Weasel
Plates 14 and 15
Putorius rixosus eskimo Stone, Proc. Acad. Nat. Sci.
Philadelphia, 1900:44, March 24, 1900.
Putorius (Gale) vulgaris, Coues, Fur-bearing animals, p. 102,
1877 (part).
Putorius rixosus, Bangs, Proc. Biol. Soc. Washington, 10:21,
February 25, 1896 (part); Merriam, N. Amer. Fauna, 11:14, June
30, 1896 (part).
Mustela rixosa eskimo, Miller, U. S. Nat. Mus. Bull., 79:96,
December 31, 1912; Swenk, Journ. Mamm., 7:327, November 23, 1926;
Hall, Univ. California Publ. Zoöl., 30:421, March 19, 1929.
Type.—Female, age in question, no. 848 in Acad. Nat. Sci.
Philadelphia; Point Barrow, Alaska; July 25, 1898; obtained by E.
A. McIlhenny. Type not seen by me.
Range.—Alaska and Yukon Territory. See figure 28 on page 180.
Characters for ready recognition.—Differs from M. r. pygmaea
of eastern Asia in longer tail, averaging 11 rather than 16 per
cent of length of head and body, and in study skins reaching only
to heel instead of to point between heel and toes; from M. r.
rixosa in shorter tail averaging 16 rather than 19 per cent of
length of head and body and not extending beyond outstretched hind
feet in study skins; white of underparts extending dorsally as a
reëntrant angle from upper lip to behind eye, rather than
delimited dorsally by a boundary between white and brown color
that extends straight across cheeks from upper lip to side of body
well below eye and ear; breadth of rostrum measured across
lacrimal processes more, instead of less, than 85.5 per cent of
orbitonasal length; from M. erminea of same region by basilar
length of skull less than 32; tail less than 50 and lacking black
pencil.
Description.—Size.—Male: The original describer lists
measurements of topotypes as follows: Total length, 204, 230;
length of tail, 28, 31; length of hind foot, 20, 22. Allowing 5
per cent for shrinkage, the hind feet of 5 topotypes yield an
average measurement of 23 for the hind foot.
Female: Measurements of two topotypes are: Total length, 184, 180;
length of tail, 25, 25; length of hind foot, 24, 18. In four other
topotypes the hind feet, allowing 5 per cent for shrinkage, yield
an average of 21.
Color.—Winter pelage all white, rarely with few white hairs in
tip of tail but no black pencil; summer pelage with upper parts
about Raw Umber and tone 3 of Chocolate pl. 343 of Oberthür and
Dauthenay; underparts white, extending over upper lip, insides of
limbs and over all four feet. Line of demarcation between
underparts and upper parts extends from upper lip posterodorsally
to behind eye down to base of ear, up behind ear for a third or
more of its height, and back along side of body. Tail unicolor all
around and same color as upper parts. Least width of color of
underparts averaging 83 per cent of greatest width of color of
upper parts.
Skull.—Based on topotypes; see measurements and plates 14 and
15; weight, 0.82 (0.74-0.93) grams in males, and 0.80 and 0.84 in
two females; basilar length, 29.5 (27.6-30.1) in males and 27.8
(27.1-28.8) in females; otherwise as described in M. e.
richardsonii.
Remarks.—Among the earliest specimens preserved was one by Edward W.
Nelson in the course of his explorations of the Upper Yukon, and one in
1874 by L. T. Turner from St. Michaels, Alaska. Bangs, in 1896 (p. 22)
mentioned the occurrence of the species in Alaska, but it was not until
1900 (p. 44) that Stone named the subspecies, and then principally on
the basis of specimens obtained two years before by E. A. McIlhenny.
The large size, broad skull, light color and short tail are the
distinguishing subspecific characters of the race eskimo, and the
three characters first mentioned are distinguishing features also of
the subspecies of Mustela erminea, namely arctica, which inhabits
the same region. Possibly eskimo also will be found on Banks Island
and the other Arctic islands between Alaska and Greenland, as is M. e.
arctica; at the present time no specimens of Mustela rixosa are
known from these islands although some race of rixosa would be expected
to occur there.
Animals from southern Alaska average slightly smaller than those from
northern Alaska, and this decrease in size toward the south probably
represents intergradation with M. r. rixosa. Further evidence of
intergradation is furnished by the short tail of the specimen from 15
miles east of Atlin; in other particulars this specimen agrees with the
subspecies rixosa to which it is here referred. Nevertheless, the
short tail, and color pattern, namely reëntrant angle of white behind
the eye, is to be seen in all Alaskan specimens examined in the brown
pelage, even in no. 107591, from Tyonek on Cook Inlet, which Osgood
(1901:69) and Swenk (1926:323) thought might not differ from the
subspecies M. r. rixosa.
Each of four male topotypes, hardly subadult in age, probably of a
single litter, is much larger than any other specimen seen from Point
Barrow. The basilar length, for example, is 31.9 as against 29.5, and
the weight of the skull (with lower jaws) is as much as 1.5 grams, as
against 0.93 in the heaviest of the other males. Initial examination of
materials from Point Barrow raised the suspicion that two distinct
species were represented—rixosa and a larger one possibly allied to
M. nivalis of the Old World. Nevertheless, further study almost
completely allayed the suspicion because the only difference
discernible is one of size, and it is supposed that additional
specimens will bridge the gap in size and show that M. r. eskimo at
Point Barrow averages larger than the adult specimens now available
indicate. The four large males of subadult age are nos. 42814-42816 and
42818 of the American Museum of Natural History.
Of the fourteen adult and subadult skulls examined, two display lesions
resulting from infestation of the frontal sinuses by nematode
parasites. None of the young skulls show such infestation.
Specimens examined.—Total number, 42 as follows. Arranged
alphabetically by Territory and District and unless otherwise
indicated in the United States National Museum.
Alaska. Barrow and Point Barrow, 19 (8[2], 7[74], 2[1], 1[50]);
Wainwright, 1[57]; Mts. back of Icy Cape, 1[77]; west of Beechey
Point, 1[2]; west edge of Colville River Delta, 1[2]; Koyukuk
River, 16 mi. above Beetles, 1; upper Yukon, 1; Fort Yukon, 1;
Stephens Village, 1; Wales, 1[57]; McDonald Creek, tributary of
Salcha Slough, 1; near head of Toklat River, 1; head of Kantishna
River, 1; St. Michael, 4 (2[74]); Tyonek [= Tyonek], 1; Bethel, 3;
vic. Bristol Bay, 1.
Yukon. La Pierre's House, 1; Klotassin River, tributary of White
River, 1.
Mustela rixosa rixosa (Bangs)
Least Weasel
Plates 14 and 15
Putorius rixosus Bangs, Proc. Biol. Soc. Washington, 10:21, pl.
1, fig. 6, pl. 2, fig. 6, pl. 3, fig. 4, February 25, 1896;
Merriam, N. Amer. Fauna, 11:14, pl. 2, figs. 7, 7a, June 30,
1896.
Putorius pusillus, Baird, Mamm. N. Amer., p. 159, 1858.
Putorius (Gale) vulgaris, Coues, Fur-bearing animals, p. 102,
1877.
Mustela rixosa, Thomas, Proc. Zoöl. Soc. London, p. 168, March,
1911.
Mustela rixosa rixosa, Miller, U. S. Nat. Mus. Bull., 79:96,
December 31, 1912; Swenk, Journ. Mamm., 7:327, November 23, 1926.
Type.—Female, adult, skin and skull; no. 642 Bangs Coll. in
Mus. Comp. Zoöl.; Osler, Saskatchewan; July 15, 1893; obtained by
W. C. Colt; original no. 79 according to describer.
The skull lacks the basioccipital, basisphenoid, and left
zygomatic arch. The "crowns" of the lower canines are missing;
otherwise the teeth are present and entire. The skin is fairly
well made, with soles of hind feet up, in good condition and in
summer pelage.
Range.—From northern British Columbia and Great Slave Lake
south on the west side of the Rocky Mountains to Ootsa Lake,
British Columbia, and on the east side of the Rocky Mountains,
south to central Montana, North Dakota and Minnesota; eastward in
Canada, entirely north of St. Lawrence River, to Atlantic Ocean.
See figure 28 on page 180.
Characters for ready recognition.—Differs from M. r. eskimo
in longer tail averaging 19 rather than 16 per cent of length of
head and body and extending beyond outstretched hind feet in study
skins, rather than to a point short of tips of toes; boundary
between brown upper parts and white underparts extending straight
across cheeks from upper lip to side of body well below eye and
ear, rather than with reëntrant angle from upper lip carrying
white upward to point behind eye, and with breadth of rostrum
less, instead of more, than 85.5 per cent of orbitonasal length;
from M. r. campestris by smaller size: hind foot less than 25 in
males and ordinarily less than 22 in females; in males total
length less than 216 and tail averaging less than 34, and in
females total length averaging less than 182 and tail averaging
less than 29; color said to average darker; from M. r.
allegheniensis by three average differences, namely lighter
color, longer tympanic bullae and larger size of males; from M.
frenata and M. erminea of same region by basilar length of
skull less than 32; tail less than 50, and lacking black pencil.
Description.—Size.—Male: Six adults and subadults from
Shaunavon, Saskatchewan, yield average and extreme measurements as
follows: Total length, 202 (188-208); length of tail, 32.5
(31.5-34.0); length of hind foot, 22.8 (21-24).
Female: One adult and 3 subadults from the same area yield average
and extreme measurements as follows: Total length, 172
(162-190.5); length of tail, 27.4 (24-34); length of hind foot,
19.6 (17.5-22).
Color.—Winter pelage all white, rarely brown; as described in
M. r. eskimo except that line of demarcation on side of head
between upper parts and underparts passes almost straight back
without the dorsally directed reëntrant area of white behind the
eye and ear; least width of color of underparts averaging 52 per
cent of greatest width of color of upper parts.
Skull (Based on those from Shaunavon, Sask.)—See measurements
and plates 14 and 15; weight, 0.88 (0.70-0.98) grams in males and
0.55 (0.54-0.56) in females; basilar length, 29.5 (28.4-30.4) in
males and 26.1 (24.7-27.0) in females; otherwise as described in
M. e. richardsonii.
Remarks.—As early as 1858 (p. 159) Baird recognized an individual of
this race from Pembina, Minnesota, as pertaining to a distinct species.
Although he used for it the specific name pusillus originally
proposed by DeKay for a small weasel from the state of New York, Baird
wisely noted that the specimen he described "may be different from the
New York species. . . ." After preparing this account, Baird included a
second specimen, from Fort Steilacoom, Washington Territory, which he
thought might be the same, but the differences that he was careful to
point out, in the light of later knowledge, show it to be of the
species Mustela erminea. Only a few other naturalists followed Baird
in distinguishing the least weasel as a separate species until Bangs in
1896 (p. 21) clearly differentiated it and proposed for it the name
Putorius rixosus, which continues in use today and applies to the
species.
The accumulation at the National Museum of Canada, through the energy
of Dr. R. M. Anderson, of a good series of specimens from Saskatchewan
in the general vicinity of the type locality allows for the first time
an adequate conception of the amount of secondary sexual variation and
individual variation and permits recognition of subspecific characters
to differentiate between M. r. rixosa and the subspecies eskimo and
campestris. In comparison with the subspecies allegheniensis the
basis for segregation is less clear and will remain somewhat in doubt
until additional adults of allegheniensis from, say, Pennsylvania,
become available with accurate external measurements taken in the flesh
and especially with complete skulls.
Intergradation with the subspecies eskimo is suggested by the short
tail of the specimen from fifteen miles east of Atlin, British
Columbia; in other particulars that specimen, a skin-alone, agrees with
the subspecies rixosa. Intergradation with campestris is indicated
by increased size of some specimens from North Dakota, and is suggested
with allegheniensis by the color of specimens from Wisconsin and
Illinois. Three specimens from Winona County, in southeastern
Minnesota, unfortunately are skulls-alone without external
measurements. Also, two of these skulls are of young animals. The one
adult, unsexed, is from Crystal Springs. Selected cranial measurements
are: basilar length, 28.5; length of tympanic bulla, 10.9. These
measurements accord with those of males of the subspecies rixosa to
which the specimens from Winona County, therefore, are here assigned.
The possibilities have not been excluded, however, that the adult is an
unusually large female of the subspecies campestris or a male of
allegheniensis that has tympanic bullae longer than average for that
subspecies.
Some hesitation is felt in assigning the specimens, 8 in all, from
eastern Canada to the subspecies rixosa. The skin-alone from Eagle
River and the skin, with part of the skull, from St. Michael Bay, are
in transitional pelage and are of no help in appraising subspecific
characters. The one adult specimen which does have a complete skull is
from an island south of the Comb Hills. This animal in all respects
agrees with selected individuals of M. r. rixosa from Saskatchewan,
but each of the five other skins in summer pelage has spots of dark
brown color on the breast. Only about one specimen in three of rixosa
from Saskatchewan is similarly marked. Furthermore, on some of the
specimens from eastern Canada the spots are larger than on any of the
animals from farther west. The greater frequency of brown spots on the
breast, the larger average size of these spots, and the darker average
coloration of the upper parts are suggestive of geographic variation,
the existence of which has to be proved by additional and more complete
specimens from eastern Canada. For the time being, specimens from there
are tentatively assigned to the race rixosa.
Of 56 subadult and adult skulls only 3 (1 North Dakota; 1 Calgary,
Alberta; and 1 Island S Comb Hills, Queb.) display lesions resulting
from infestation of the frontal sinuses by nematode parasites. None of
the young skulls shows such infestation.
Specimens examined.—Total number, 87 as follows. Arranged
alphabetically by provinces and states and within each from north
to south. Unless otherwise indicated, specimens are in the United
States National Museum.
Alberta. Miette River, 1[77]; 5 mi. NW Camrose, 1[77]; Camrose,
2 (1[77], 1[31]); "near Camrose," 2[77]; Forks Blindman and Red
Deer rivers, 1[60]; Innisfail, 1[86]; Veteran, 1[93]; Diddsbury [=
Didsbury], 1; Calgary, 2 (1[93], 1[2]); Shepard, 1[86].
British Columbia. Clarks Ranch, Halfway River, Peace River Dist.,
1[85]; 15 mi. E Atlin, 1[8]; Wistaria, P. O., 3 (2[77], 1[85]);
Ootsa Lake, 1[85].
Labrador. Davis Inlet, 1[60]; 30 mi. upriver and 20 mi. toward
Groswater Mts., Eagle River, 1; St. Michael Bay, 1.
Mackenzie. Old Fort Reliance, 1[2]; Fort Resolution, 2; Fort
Smith, 1.
Manitoba. Gypsumville, 1[86]; Lake St. Martin Reserve, 1[86].
Minnesota. Roseau County: Cedarbend, 2[14]; Grimstad, 1[14];
America, 2 (1[14], 1[74]); Malung, 1[74]; Norland, 1[41]; Falun, 3
(1[14], 1[74], 1[41]); Palmville, 1[41]; Spruce, 1[74]; Stokes,
1[74]. No locality more definite than Marshall County, 1[14].
Clay County: Moorhead, 1[36]. Winona County: "near" Whitman,
1[34]; Altura, 1[98]; Crystal Springs, 1[98].
Montana. Sun River Valley, 1; Wibaux in Wibaux County, 1.
North Dakota. Walsh County: Grafton, 15 (3[60], 1[93], 5[2],
2[14], 1[74], 1[1], 1[76]). McHenry County: 4 and 4-1/2 mi. N
Upham, 2. Wells County: 1[36]. Morton County: Mandan, 1[60].
Ontario. Algoma Dist.: Tatnall, near Oba, 1[86]. Moose Factory,
1[75].
Quebec. Island S of Comb Hills, James Bay, 1[9]. Saguenay
County: Natashkwan, 1.
Saskatchewan. Osler, 1[75]; "near Regina," 1[77]; Dollard, 2[31];
Shaunavon (and "near" and 1 mi. NE), 9[77]; Klintowel P. O.
(about 15 mi. N of Eastend), 1[77]; Eastend and "near" Eastend,
2[77].
Mustela rixosa allegheniensis (Rhoads)
Least Weasel
Plates 14, 15 and 41
Putorius allegheniensis Rhoads, Proc. Acad. Nat. Sci.
Philadelphia, 1900:751, March 25, 1901.
Putorius rixosus allegheniensis, Cory, Mamm. Illinois and
Wisconsin, p. 378, 1912.
Mustela allegheniensis, Miller, U. S. Nat. Mus. Bull., 79:96,
December 31, 1912.
Mustela rixosa allegheniensis, Swenk, Journ. Mamm., 7:328,
November 23, 1926.
Type.—Probably male adult, skin and skull, no. 6195, Acad. Nat.
Sci. Philadelphia; near Beallsville, Washington Co., Pa.; about
1885 or 1886; obtained by Robert Hawkins.
Type not seen by me.
Range.—Wisconsin, northern Illinois, northern Indiana,
Michigan, Ohio, Pennsylvania east to Dauphin County and south in
the mountains to northwestern North Carolina. See figure 28 on
page 180.
Characters for ready recognition.—Distinguished from M. r.
rixosa by three average differences, namely, darker color,
shorter tympanic bullae, and smaller size of males; from M. r.
campestris in smaller size: hind foot less than 25 in males and
less than 22 in females; in males total length less than 216 and
tail averaging less than 34, color averaging darker; from M.
frenata and M. erminea of same region by basilar length less
than 31, tail less than 45, and lacking black pencil.
Description.—Size.—Male: An adult or subadult from Fair
Oaks, Pa., a subadult from Finleyville, Pa., and an adult from
Huttonsville, W. Va., measure, respectively as follows: Total
length, 206, 194, 191 (average 197); length of tail, 37, 32, 28
(32); length of hind foot, 23 in each. An adult from Roanoke,
Indiana, weighs 40.6 grams.
Female: Two young from Leasuresville, Pa., and Middle Paxton
Twp., Pa., measure, respectively, as follows: Total length, 188,
172; length of tail, 33, 30; length of hind foot, 20.5, 21. An
adult from Monroeville, Ohio, weighs 40.5 grams and a young
individual from Middle Paxton Twp., Pa., 39.3 grams, and a
subadult from Swan Creek Exp. Station, Allegan Co., Mich., weighs
49 grams.
Color.—Winter pelage either all white, or brown as in summer;
upper parts about Raw Umber, or tone 2 of Carbo Brown of pl. 342
of Oberthür and Dauthenay. Underparts white at least on thoracic
region; approximately three-fourths of specimens with brown rictal
spot at angle of mouth or with this area covered by brown upper
parts which extend down on each side and meet on the underparts in
about one specimen out of three; upper lips and hind feet
ordinarily brown; toes of forefeet ordinarily white (see under
remarks for details of color pattern). Least width of color of
underparts in the specimens in which the dark color of the upper
parts does not encircle the body averages 60 per cent of greatest
width of color of upper parts, or including all specimens the
percentage is 42.
Skull (based on specimens from Pa. listed in table of cranial
measurements, which see and plates 14 and 15).—Basilar length
29.7 and 28.6 in male and 28.0 in female; weights unavailable;
otherwise as described in M. e. richardsonii. The length of the
tympanic bullae seems to be actually less, and less in relation to
the basilar length, than in other American subspecies of M.
rixosa.
Remarks.—Robert Kennicott's mention in 1859 (p. 245) of what seems
to be this subspecies is the earliest reference to it that I can
identify in the literature. He used the specific name pusillus and it
was not until 1900 that Samuel N. Rhoads proposed the name Putorius
allegheniensis. Since 1900, several records of occurrence have been
published which have made the geographic range of this race better
known.
An adequate number of specimens has been gathered only from Ohio and
from western Pennsylvania. Many from Ohio are without accurate external
measurements taken in the flesh. The majority of the specimens from
Pennsylvania owe their preservation to the willingness of local
officials, who pay bounties on weasels, to save the skins of Mustela
rixosa. These specimens ordinarily comprise the skin with locality but
because the feet, external measurements in the flesh, and skulls are
unavailable, the material is far from adequate and to give an accurate
notion of the usual or average cranial characters of allegheniensis
in Pennsylvania, skulls from there are especially desirable.
A smaller percentage of the specimens from Ohio than from Pennsylvania
have the brown color of the upper parts meeting on the underparts.
Also, more of the specimens from Ohio are lighter colored and this
suggests intergradation with the subspecies campestris and rixosa
to the westward.
From Pennsylvania 23 animals in brown pelage are available. In 5 there
is a rictal spot at the angle of the mouth; in 5 the area is white and
in 13 the brown color of the upper parts is continuous over the area in
question. Only 2 of 23 have the upper lips white. Eight have the color
of the upper parts meeting on the venter thus restricting the white of
the underparts to the chin, throat, and pectoral region, and 6 of these
have a white area in the inguinal region as well. The toes of the
forefeet are white in 3 of 4 animals suitable for examination in this
regard and the hind feet are marked with white in 3 of the 8 animals
which have the hind feet preserved. Mustela rixosa in Pennsylvania
parallels the species Mustela frenata in that in this relatively
humid area of the northeastern United States the color of the upper
parts is darker and the area of the dark-colored upper parts is
increased at the expense of the area of the light-colored underparts.
Also Mustela erminea in this same region (range of the subspecies
Mustela cicognanii) shows the same tendency to darker color of upper
parts and their extension in area at the expense of the area of the
light-colored underparts, or was mentioned above.
It is difficult to account for the seeming absence of the species from
New England and all that part of Canada and the United States south of
the St. Lawrence River and northeastward from Pennsylvania. The size of
females of M. erminea cicognanii in that territory is so little more
than in rixosa that the latter possibly cannot successfully compete
with the erminea stock which may already occupy the ecologic niche to
which rixosa is adapted. It will be remembered that in western North
America in territory seemingly climatically suitable for rixosa it
occurs no farther southward than the line below which M. erminea has
become reduced to a size comparable with that of M. rixosa.
Of 41 subadult and adult skulls assigned to this subspecies 24 have
obvious lesions in the frontal sinuses evidently resulting from
infestation by nematodes. More in detail, none of the specimens from
Illinois (3 individuals), Pennsylvania (3 barely subadult), or West
Virginia (2) displays lesions. From Wisconsin, Indiana, Virginia and
North Carolina there is one specimen each and each specimen displays
lesions. From Ohio, 17 of 23 specimens display lesions. From Michigan 3
of 8 specimens display lesions; 2 adults and one subadult have lesions
and 5 subadults do not have lesions.
Specimens examined.—Total number, 102 as follows: Arranged
alphabetically by states and within each state by counties from
north to south. Unless otherwise indicated, specimens are in the
United States National Museum.
Indiana. Huntington County: Roanoke, 1. Wells County: Harrison
Township, 1[76].
Illinois. Lake County: Deerfield, 3[60]; no locality more
definite than county, 1[60]. Cook County: Northfield, 1[60]; La
Grange, 1[18].
Michigan. Tuscola County: 8 mi. N Caro, 1[76]. Santilac
County: Deckerville, 1[76]. Allegan County: Swan Creek Exp.
Station, 1[76]; Swan Creek Farm, 1[76]; T. 2N, R. 14W, 1[76];
Allegan, 1[76]. Livingston County: George Reserve, 1[76]; 1/2
mi. N Unadilla, 1. Oakland County: Rochester, 1[76]. Macomb
County: Romeo, 1[76]. Washtenaw County: 5 mi. SW Ann Arbor,
1[76]. Branch County: vic. Coldwater, 1[76].
North Carolina. "near Marshall," 1.
Ohio. Northern part of state, 1[81]. Williams County: Stryker,
1[60]. Lucas County: Monclova, 1[60]. Erie County: Sandusky,
2[76]; marsh near Sandusky, 1[76]; Berlin Heights, 1[76]; no
locality more definite than county, 1[2]. Wood County: 10 mi. NE
Bowling Green, 1[76]; Bowling Green, 4[76]; 3 mi. E Bowling Green
1[76]; Plain Township, 1[2]; Portage Township, 1[60]. Loraine
County: Wellington, 1[81]. Huron County: west of Monroeville,
1[76]. Summit County: Ira, 3[81]. Portage County: Suffield,
1[81]. Hancock County: Vanburen, 1[76]; Findlay, 1[81]; 9 mi. S
Findlay, 1[76]; no locality more definite than county, 7 (2[76],
2[81], 3[2]). Mahoning County: Ellsworth, 1. Crawford County:
"near Crestline," 1[81]. Delaware County: Sunbury, 1[2]; Lewis
Center, 1[81]; no locality more definite than county, 1[81].
Licking County: Johnstown, 1[2]. Fairfield County: Baltimore,
1[81]; Violet Township, 1[81]. Meigs [= Gallia?] County: Vinton,
1[81].
Pennsylvania. Erie County: McKeen Twp. 1. Crawford County:
Springboro, 1[1]; Pymatuning Swamp, between Hartstown and
Shermansville, Sadsbury Twp., 3[9]. Mercer County: Shenango
Twp., 1. Lawrence County: Little Beaver Twp., 1. Butler
County: Leasuresville, 1[9]; Clearfield Twp., 1; Valencia, 1[9].
Armstrong County: Ford City, Burrell Twp., 1. Indiana County:
Smicksburg, 1; N. Mahoning Twp., 2; White Twp., 1. Allegheny
County: South Hills, Pittsburgh, 1[9]; "near Pittsburgh," 1[9];
Fair Oaks, 1[9]. Westmoreland County: Bolivar, 1. Dauphin
County: Middle Paxton Twp., 1. Washington County: Finleyville,
1; Rea, 5; Beallsville, 1[1]; Claysville, 1. Green County: Deep
Valley, 1; Waynesburg, 1; Jefferson, 1; Cumberland Twp., 1.
Fayette County: Acme, 1[9]. Somerset County, 1. Lancaster
County, 1.
West Virginia. Randolph County: Huttonsville, 1.
Wisconsin. Sauk County: Sumpter Twp., 1[60]. Dodge County:
Beaver Dam, 1[50]. Dane County: Madison, 1; McFarland (=
MacFarland), 1.
Mustela rixosa campestris Jackson
Least Weasel
Plates 14 and 15
Mustela campestris Jackson, Proc. Biol. Soc. Washington, 26:124,
May 21, 1913.
Putorius pusillus, Aughey, Sketches of the physical geography and
geology of Nebraska, p. 119, 1880, Omaha.
Mustela rixosa campestris, Swenk, Journ. Mamm., 7:329, Nov. 23,
1926.
Type.—Female, adult, skin and skull; no. 171490, U. S. Nat.
Mus., Biol. Surv. Coll.; Beemer, Cuming County, Nebraska; April
18, 1911; obtained by G. Sharp; x catalogue no. 8440.
The skull is unbroken. On the left side, C1 and P2 are missing;
the other teeth are present and entire. The skin is excellently
made and in a good state of preservation.
Range.—South Dakota, Nebraska and Iowa. See figure 28 on page
180.
Characters for ready recognition.—Differs from M. r. rixosa
and M. r. allegheniensis in larger size: Hind foot more than 25
in males and ordinarily more than 22 in females; in males total
length more than 216 and tail averaging more than 34; color
possibly slightly paler than in M. r. rixosa and averaging paler
than in M. r. allegheniensis; from M. frenata and M. erminea
of the same region by basilar length less than 32; tail less than
50, and lacking black pencil.
Description.—Size.—Male: Four adults from Nebraska yield
average and extreme measurements as follows: Total length, 231
(225-237); length of tail, 36 (32-39); length of hind foot, 29
(28-31).
Female: Six adults from Nebraska yield average and extreme
measurements as follows: Total length, 192 (184-225); length of
tail, 35 (28-40); length of hind foot, 23 (20.5-26).
Color.—Winter pelage ordinarily white; as described in M. r.
eskimo except possibly paler and certainly with line of
demarcation on side of head between upper parts and underparts
passing almost straight back without the dorsally directed
reëntrant angles of white behind the eye and ear; least width of
color of underparts in four specimens from Nebraska averaging 80
(49-89) per cent of greatest width of color of upper parts, but in
a fifth animal in summer pelage the brown color of the upper parts
encircles the body.
Skull.—See measurements in table and plate 15; weight 1.1 grams
(male from Brown Co., S. D.); basilar length, 30.7 in male from
Clay Co., Neb., and 28.8 in female from same county; otherwise as
described in M. e. richardsonii.
Remarks.—In his revisionary treatment of the American races of
Mustela rixosa, Myron H. Swenk (1926:313) credits Samuel Aughey with
recording this animal, M. r. campestris, from Nebraska, as early as
1880, under the name Putorius pusillus. In 1908, Swenk recorded the
animal from the same state under the name rixosus and in 1913 the
race campestris was formally named by H. H. T. Jackson.
On the testimony of a friend who had previously obtained several
specimens for him, Swenk (1926:321) records the least weasel from
Oshkosh, Garden County, Nebraska, which is a marginal record of
occurrence to the southwest for M. r. campestris.
At an early stage in the study of American weasels the writer examined
the specimens from Nebraska saved by Mr. Myron H. Swenk and recorded
measurements of them. However, at the time of writing this account the
specimens were not available for examination and the account of
coloration is accordingly incomplete.
The large size, particularly the large external measurements, comprises
the principal distinguishing character of this subspecies of the least
weasel.
Of the four adults examined from Iowa and South Dakota one exhibits
lesions such as result from infestation of the frontal sinuses by
nematodes.
Specimens examined.—Total number, 21 as follows. Arranged
alphabetically by states and by counties, from north to south in
each state. Unless otherwise indicated, specimens are in the
United States National Museum.
Iowa. Howard County: Chester, 1[12]. Palo Alto County:
Emmetsburg, 1[65]. Kassuth County: Algona, 1[65]. Clayton
County: National, 1. Storey County: Nevada, 1[65]. Wapello
County: Ottumwa, 1[65]. Henry County: Mount Pleasant, 1[66].
Nebraska. Holt County: Page, 1[35]. Madison County: Norfolk
1[35]. Cuming County: Beemer, 1. Hamilton County: Chapman,
1[35]. Clay County: Inland to 1 mi. east thereof, 7[35].
South Dakota. Brown County: shore of Sand Lake, S. 15 T. 126N,
R. 62W, 1. Day County: Waubay Migratory Waterfowl Refuge, 1.
McCook County: Salem, 1[102].
MUSTELA FRENATA Lichtenstein
Long-tailed Weasel
(Synonymy under subspecies)
Type.—Mustela frenata Lichtenstein, Darstellung neuer oder
wenig bekannter Säugethiere, pl. 42 and corresponding text
unpaged. 1832.
Range.—From southern Canada southward over all of the United
States, México, Central America, Venezuela, and the republics of
western South America to southern Perú and extreme northern
Bolivia. All the life-zones from Alpine Arctic to Tropical are
inhabited. In the extremely desert region of southeastern
California and western Arizona the species is scarce or possibly
absent although recovery of a skull (see under account of M. f.
neomexicana) from near the center of this region at Potholes on
the Colorado River, and a reported occurrence in the mountains of
Baja California, México, indicate that a few individuals of the
species live in favorable habitat even in this desert region.
Characters for ready recognition.—Differs from Mustela erminea, in
regions where the two species occur together, by tail more than 44 per
cent of length of head and body and by postglenoidal length of skull
less than 46 per cent of condylobasal length in males and less than 48
per cent in females (see under characters of the species); from
Mustela rixosa by presence of black pencil on tail, caudal vertebrae
more than a fourth (2/5-3/4) of length of head and body, basilar length
of skull more than 34 mm.; from Mustela africana by absence of thenar
pad on forefoot, underparts without longitudinal, median, abdominal
stripe of same color as upper parts, upper lips narrowly (rather than
broadly) edged with color of underparts, longest facial vibrissae
extending to or behind posterior margin of ear; presence of p2; more
inflated (see pls. 23 and 30) tympanic bullae.
Characters of the species.—Size large: Total length 300 to 550 mm.;
tail two-fifths to seven-tenths of length of head and body, with
distinct black pencil at end; caudal vertebrae 19 to 23; skull with
long precranial portion; postglenoidal length, expressed as a
percentage of the condylobasal length, less than 47 in females and
ordinarily less than 46 in males; upper parts brown; light-colored
underparts, in summer pelage, tinged with buffy or yellowish and
continuous from chin to inguinal region; some subspecies (southwestern
United States, México, Central America, and Florida) with white or
yellowish facial markings which do not occur in any other American
species of the genus Mustela.
Geographic variation.—Forty-two subspecies are recognized, and the
species is geographically more variable than any of the other 3
American species. Color, color-pattern especially on the head, relative
proportions of the tail, hind feet, body including the head, and shape
and size of the skull are the principal features in which geographic
variation has been noted. The variation in the skull extends to the
basicranial region (shape and size of tympanic bullae and related
structures), interorbital region and preorbital region.
Natural History.—Habitat and Numbers.—As has already been remarked,
the long-tailed weasel is absent from the extreme desert of the
southwestern United States and northwestern México. Possibly the
absence of water to drink is the limiting factor. In southern Nevada
the finding of weasels only in places that were well watered, even
though small rodents suitable as food for weasels were even more
abundant in the surrounding desert, supports this possibility that the
absence of water to drink is the limiting factor. Also at Berkeley,
California, in early December of 1927 in the canyon at the head of
Dwight Way and in the autumn and winter of 1928 in Strawberry Canyon on
the campus of the University of California, I trapped extensively for
this species in different habitats and obtained, in all, four
individuals no one of which was farther than 10 feet from water. The
lesser cruising range of the individual weasel than of, say, the
coyote, probably explains why, in an arid region, for example
Pahranagat Valley, Nevada, only the meadow mice and their riparian
associates are preyed upon by the long-tailed weasel whereas the coyote
preys upon these riparian rodents and also upon the kangaroo rats and
other rodents which are so abundant in adjoining habitats that are
devoid of water.
In areas where water is available every few hundred yards, no
particular habitat seems to be avoided in summer providing there is
food for the long-tailed weasel. In winter (January and March) there
obviously was a choice of habitat, possibly occasioned by more abundant
food or more satisfactory shelter, or both, in Centre County,
Pennsylvania, where Glover (1943B) found the population density in the
chestnut-oak habitat to be one weasel per 6.5 acres in areas of tree
cuttings and slash and one weasel per 13.3 acres in the open forest. In
the scrub oak-pitch pine forest type the population was one weasel per
26.4 acres in tree cuttings and slash and one weasel per 38.2 acres in
the open forest. No weasel was found in an area of 9.6 acres comprising
a wood lot, the edge of the forest, abandoned fence rows and an
abandoned orchard. The two types of forest in which he did find
weasels, 25 in all, comprised 381.6 acres. Glover's (op. cit.) data
is the only precise information known to me on actual numbers of
long-tailed weasels in a given area of any considerable size.
Fluctuations which I elsewhere (1946:57) have designated as multiannual
fluctuations occur in this species but seemingly not in the degree that
they do in Mustela erminea. This difference between the two species
is to be expected because M. frenata does not range so far northward
toward the polar regions as does M. erminea and populations of most
kinds of animals in the polar, at least in the arctic, regions are
subject to more extreme and more regular fluctuations than are kinds of
animals in temperate or tropical regions. Indication of the means by
which decrease in the weasel population is brought about is afforded by
Osgood's (1935:156) observations around Rutland, Vermont. In the late
winter of 1934, tracks indicated that weasels left their usual haunts
and hunted cross lots, vainly trying to find food. Testing of the small
mammal population in the spring and summer of 1934 showed that it was
at low ebb. In the fall of 1934 mice and shrews were abundant again but
weasels seemed to be entirely absent. The decrease in the population of
weasels lagged behind the decrease in the population of the herbivorous
prey as did the subsequent increase; this, of course, is the normal
relation of carnivorous species of mammals and their prey, at least in
and above the Transition Life-zone.
The average distance away from the central den which four weasels (sex
unspecified) traveled in a single night at Ames, Iowa, was 312 feet;
the maximum distance was 642 feet. These data were obtained in the
winter of 1939 by Polderboer, Kuhn and Hendrickson (1941:115) who
studied the tracks in the snow. In Manitoba, Criddle and Criddle
(1925:143) noted that a female which lived in their basement often
wandered more than half a mile away in search of food. In Michigan,
Quick (1944:75) found the maximum distance traveled in one day (=
night?) by a large male to be 3.43 miles although two miles was the
average distance traveled by this individual. In 1942, from January 4
to March 4, in Centre County, Pennsylvania, Glover (1943B) studied
tracks of 11 males and 10 females, in newly fallen snow, and
ascertained that the distance traveled in a single night averaged 704
(60-2535) feet for the male and 346 (20-1420) feet for the female. The
weasels in the open timber traveled farther per trip than those in the
brushland and dense stands of trees.
Behavior
An adult female (now the holotype of Mustela frenata nevadensis) seen
running across a field, and, I think, unaware of my presence, at every
bound bent her back up so far that she reminded me of a measuring worm.
For part of the time when running, the tail was held off the ground
straight out behind, and then, for a while, inclined upward at an angle
of about 45°. Another weasel that I saw in the daytime, and that I
think was unaware of my presence, was bounding along among the
Baccharis bushes on the south-facing slope of Dwight Way Canyon,
Berkeley, California. This individual, at each bound, arched the back
up so high as to remind me, again, of a measuring worm.
The long-tailed weasel is a land mammal and unlike its close relative,
the mink, is seldom seen in the water. That it can swim, however, is
attested by the capture of one while it was swimming across the Río
Ramos in México (Davis, 1944:381). Also, Green (1936), in May, in
Gratiot County, Michigan, saw a weasel, running with a Peromyscus in
its mouth. The weasel dropped the mouse, entered the water and swam to
a hole among stones.
More instances of climbing, than of swimming, have been reported in the
literature for the long-tailed weasel. Seton (1929 (2):625) quotes
William M. Graffius of Pennsylvania as having seen a weasel closely
pursue a red squirrel nearly to the topmost branch of a large hemlock.
When the squirrel loosed its hold and dropped into a stream, the weasel
descended to the ground and caught and killed the squirrel when it
emerged from the water. Pearce (1937:483), in central New York State,
on July 29, 1931, watched a weasel chase a chipmunk up a black cherry
tree ten inches in diameter, and noted that the first rush carried the
weasel "straight up the trunk for approximately 10 feet, where it
hesitated momentarily before continuing. Then, instead of climbing
vertically, it made progress by traveling in short ascending spirals
around the trunk, scarcely making 3 feet in height for each circuit of
the tree. Upon reaching the limb by which the chipmunk escaped, the
weasel followed out along this in the same spiral manner. This limb had
a diameter of about 4 inches at its base and extended upward at an
angle of perhaps 20 degrees above the horizontal . . . it made its way
head first almost down to the ground, using the same spiral mode of
progress, but at a leisurely pace. . . . While traveling down the side
limb it appeared practically to wrap its sinuous body around the limb."
A male long-tailed weasel, from Colorado, which I kept captive was
often fed freshly killed mice. These I thrust through one of the small
openings in the wire mesh. The weasel quickly learned to seize any part
of a mouse thus introduced and his tugging aided in getting the mouse
into the cage. Occasionally a mouse too large to be got through the
mesh had to be withdrawn. In such an instance, if the weasel had
already had hold of the mouse, he would screech frightfully. I have
heard no other vocal sounds from a weasel except a kind of purring.
The sense of smell apparently is well developed; at any rate it is keen
enough to allow the weasel to follow the trail of an intended victim by
the scent left by the latter. Murie's (1935:321-322) account, for
example, of a weasel pursuing a snowshoe rabbit gives clear evidence
that the weasel relied on scent in following the rabbit.
A captive male weasel obtained at Gainesville, Florida, stamped his
hind feet when annoyed (Moore, 1945:259).
A male from Colorado that I kept for months in a cage at Lafayette,
California, was several times found in a sleep so deep that he was
awakened with difficulty. Seton (1929 (2):629-630) writes: "In my small
menagerie, I have had half-a-dozen Weasels of the New York species.
Their sleeping dens are arranged so as to be easily and silently
opened. Several times I have lifted the lid to find the weasel in a
deep sleep—a sleep so profound that I had to poke him vigorously with
a stick before he awoke, looked up, and rushed forth with a little puff
of wrath, and a little puff of smell."
Feces and urine were ordinarily deposited in one particular place by
each of the captive weasels that I have observed. Hamilton (1933:294)
records that a large male M. f. noveboracensis, in a week, averaged
10 evacuations every twenty-four hours, that urination immediately
precedes defecation, and describes the feces as black or brown, long
and narrow and often spiral-shaped owing "to the matted fur of some
rodent that had been eaten." Quick (1944:77) writes, concerning four
winter dens in Michigan, that "The latrines of weasels were in the
entries of used dens and scats could be collected there by the
handful." Polderboer, Kuhn and Hendrickson (1941:116) in the spring of
1939 at Ames, Iowa, gathered scats "from latrines found at the
entrances of burrows and from latrine chambers found within burrows."
Scats were found by them in the linings of some nests.
Courage of a high order might be credited to the long-tailed weasel
because individuals have attacked animals much larger than the weasels.
Actually, however, in few if any of these instances was the motive for
attack known. That a hawk was attacked is suggested by Soper's
(1919:45) account of Mustela frenata noveboracensis wherein he
repeats a story told to him of a hawk observed in unsteady flight, and
obviously in distress, which when it plummeted to earth was with a
weasel which escaped from the observer. Charles Tatham, Jr., of
Cambridge, Massachusetts, according to Seton (1929 (2): 630, 631)
observed one that attacked his dog.
Persons and long-tailed weasels have figured in some rather strange
encounters. For example, Oehler (1944:198) recounts that in the autumn
of 1940 at Cincinnati, Ohio, an animal, mistakenly thought to be a
chipmunk, was seen to dash into a hollow log whereupon pounding on the
log brought out the weasel which bit and clung to the hand of one man
whose companion was bitten when he attempted to free the man that was
bitten first.
Seton (1929 (2): 631) writes that on the night of September 5, 1897, on
Roosevelt's old ranch, near Medora, North Dakota, a man turned over his
saddle (which was lying on the ground) to dislodge what was thought to
be a pack-rat. The animal was a long-tailed weasel which attacked him.
It ran up his legs a number of times aiming at his throat before being
killed by a dog.
Criddle and Criddle (1925:146) wrote: "August 20, 1919.—A longicauda
in the Insectary ran at me this morning apparently with a view to
intimidating. It uttered a shrill cry while making the attack, but
retreated after advancing within two feet." The same authors (op.
cit.: 147) further write that a "Long-tailed Weasel was caught in a
trap set for gophers, and, on being released by Miss M. Criddle, at
once turned upon its liberator and bit savagely at her boot. It then
moved a short distance away to a tub of water, where it drank
thirstily, merely glancing at the observer from time to time while
doing so, and then ran off out of sight.
"Mr. T. Criddle records a similar experience. After liberating a large
weasel from a trap, it immediately rushed at him and persisted in its
attack with such ferocity that it was three times picked up and thrown,
on each occasion to a greater distance, before it finally abandoned its
offensive.
"We have no record of a weasel making an unprovoked attack upon
anyone."
Wight (1932: 164) in Michigan, detected a weasel attacking a hen. The
weasel fled at Wight's approach but returned and attacked him several
times. Finally the weasel went around Wight to reach the hen. In
Wight's words "There was no evidence of infuriation, but rather a well
directed offense at the one object, regardless of its size, which stood
between the weasel and an opportunity to satisfy its desire to kill,
which was probably based upon the uncontrollable urge of hunger pangs."
Weasels of each of the three North American species have been
successfully kept in captivity. A type of cage satisfactory for keeping
the animals in the laboratory is described by Bissonnette and Bailey
(1940:761-763). Some of the captives used their teeth to break glass
water-containers and to gnaw slivers of wood from the cages. Ingested
slivers of wood and bits of broken glass caused the deaths of some of
the captives. Weasels kept by me all were of the species Mustela
frenata. They thrived on a meat diet but I was always careful to give
them, every few days, if not each day, some small rodents entire,
thinking that the bits of bone and fur ingested might, in some way
unknown to me, keep the digestive tract in better condition than would
flesh devoid of hair and bone.
Three young weasels approximately the size of mice, in the Okefinokee
Swamp of Georgia, were obtained by a hunter who, according to Harper
(1927:303), raised them by feeding "milk for a few days, and then fresh
meat." Litters of young born in captivity have been successfully raised
by the mothers (Hamilton, 1933) and success in getting the animals to
breed in captivity and to rear their young is recorded by Wright
(1948A). He has found, however, that the majority of his captive adult
males show no interest in mating when placed with females in heat. He,
therefore, uses only selected males and when a female in heat is to be
bred, he places one of his responsive males with her one day, another
of his responsive males with her the second day and thus alternates a
couple of males for three or four days. Even so, slightly fewer than
half of the females which were thus bred produced young.
A weasel in the white winter coat was used by Audubon and Bachman
(1856:177, Quarto edit.) to drive rabbits out of their burrows in the
same fashion that ferrets commonly are used. Although these naturalists
refer to their animal as an ermine it probably was Mustela frenata
noveboracensis, the long-tailed weasel. The animal's teeth (probably
canines) were blunted and a long cord tied on its neck. With the aid of
this weasel 12 rabbits were caught in one morning and more than 50 in
four weeks.
Enemies
Little is recorded concerning enemies of weasels and it may be that
other vertebrates are not an important factor in removing the annual
increase. Errington (1935:195-198), in Iowa, found four, putrid
weasels about dens of red foxes, Vulpes fulvus. No remains of weasels
were found in the feces of the foxes and it appears that the foxes do
not eat the weasels. The label on an adult female specimen of M. f.
spadix from Boone County, Iowa, bears the date May 10, 1938, and the
annotation, by T. G. Scott, "fox-killed." Bailey (1931:328) recounts
that "Weller saw a coyote carrying one in its mouth" at an elevation of
11,500 feet in the Pecos Mountains of New Mexico. The type specimen, a
young female, of M. f. peninsulae from Hudsons, Florida, according to
Rhoads (1894:155) ". . . was caught in the woods by a cat." Barber and
Cockerell (1898:189) mention one that was killed by a dog in Mesilla
Park, New Mexico. Moore (1945:258) records the death of a weasel in
Florida. Circumstantial evidence indicated that it was killed by the
bite of a water moccasin. In the Biological Surveys Collection of
mammals in the United States National Museum, the label with the skull
of an adult male weasel, No. 160663, from Banning, California, carries
the information that the skull was taken from the stomach of a
Crotalus (rattlesnake).
In reporting on a study of owl predation in Delaware County,
Pennsylvania, Pearson and Pearson (1947:143) mention that "weasels are
found throughout the county but . . . were never eaten by the owls."
The Uinta spermophile at some places and times probably is a prey
sought by the long-tailed weasel but Warren (1924:265) records
Citellus armatus repeatedly chasing weasels in August, at Camp
Roosevelt, Yellowstone National Park, and how the ground squirrels at
one time ignored the weasel even when it came within a few inches of a
squirrel.
Warren (1932:71), on August 2, 1931, at Grand Mesa, Colorado, obtained
a large male weasel with two porcupine quills in it; one was near the
mouth and another "in the skull." Osgood (1935:156) writes that near
Rutland, Vermont, a male weasel "taken in April, was heavily
parasitized and had several short porcupine quills embedded in its
neck, head, and shoulders." The remainder of Osgood's account implies
that the weasel may have turned to porcupine because the normal food
for weasels was scarce at the time. Porcupine quills, then, are a
hazard for weasels although it is unlikely that the porcupine is ever
to be classed as an enemy of the weasel.
An accident of another sort, which must at the very least have been
annoying to the weasel that suffered it, was recorded by Soper
(1921:37). The animal had a stick lodged crosswise between the fourth
upper premolar teeth.
The recorded actions of several kinds of animals which are too small to
be dangerous to the weasel suggest that they recognize that the weasel
is a danger to them. Borell and Ellis (1934:21) mention that a weasel
in Nevada caused a great disturbance among the chipmunks. Long
(1938:250) heard pikas give evidence of terror by a peculiar cry when a
weasel was in a rock slide occupied by the pikas. Seton (1929 (2):629)
writes "On June 14, 1915, as I prowled around the south side of the
lake on my homeland at Greenwich, Conn., my attention was called to a
pair of song sparrows and a male towhee that were noisily mobbing a
Weasel, twittering around and darting at him, as though they knew full
well his evil ways. The weasel paid little heed, but soon dived from
sight in a stone wall."
No account has been found of an American weasel or ermine rolling,
tumbling and frolicking in a manner that aroused the curiosity of birds
to a degree which permitted the weasel to come within leaping distance
of the birds. Accounts of such behavior are on record for the English
stoat (ermine).
Food and Hunting
Weasels are active both in the daytime and at night. Whether the time
of activity varies with the season, with the locality, with the sex or
with other conditions, I do not know. Adult, live, free-living,
actively moving weasels that I recall having seen all were observed in
the daytime: two were in Alameda County, California, two were in White
Pine County, Nevada, one was in Scotts Bluff County, Nebraska, and one
was in Laramie County, Wyoming. I recall ten adults, from the same
three states, and one from Washington State, that got into my traps;
two of these certainly got in the traps in the night; one certainly got
in the trap in the daytime; the other eight were found in traps which
may have caught the weasels either in the night or in the daytime.
Soper (1946:136) in speaking of M. f. longicauda north of the
International Boundary in Canada remarks that it has the "habit to some
extent of hunting at all times of day." Criddle and Criddle (1925:144)
in writing of Mustela frenata longicauda in Manitoba record that "The
shrill cry of a rabbit [Lepus americanus] in the dark is nearly
always due to the weasel's attack. Indeed, we have often watched the
latter at work during the twilight hours. First would come the almost
noiseless run of the small rabbit with its characteristic dodging and
this would be followed by the appearance of the agile foe which, at
times, would leap high over obstacles and at others move swiftly
beneath them. Then there would follow intermittent cries of the rabbit
as the weasel secured a temporary hold of its quarry, for be it noted
that this hunter apparently bites anywhere to begin with and it is
probable that the blood made to flow acts as an aid to tracking as well
as weakening the prey. Several similar close encounters might occur
before the rabbit would be finally overcome, but weasels are very
persistent when they once get into contact with their victims and it is
therefore very seldom that the latter escape. In killing, they either
penetrate the brain with their teeth, or dislodge the vertebrae behind
the head." These and more than two score other observations which
record the time when weasels were seen make it clear that some were
active at night and that some were active in the daytime.
As to the routes traveled while the weasels are hunting, Quick
(1944:77) says of four individuals that he studied in Washtenaw County,
Michigan: "The weasels appeared to prefer hunting certain coverts with
noticeable regularity, but rarely cruised the same area on two
consecutive nights."
The killing technique of fifteen captive Mustela frenata
noveboracensis was studied by Glover (1943A). For the weasels he
released 19 mice, 3 brown rats, 6 cottontails and 4 ring-necked
pheasants. Most of the mice were killed by a bite on the back of the
head, with the body and legs of the weasel hugging the back of the
victim. "The weasel shoved the prey in close to the stomach with the
hind legs, and the kill was made in a reclining semi-curled-up
position." On each of the rats (Rattus) an initial grip was secured
at the base of the ear. When the rat rested, a new hold was taken by
the weasel. Finally the weasel secured a hold at the base of the skull
and near the ear, and a light crushing sound followed. Four of the six
cottontails were killed by bites on top of the head and ear; two
cottontails succumbed from neck wounds. In three instances, neither of
two weasels could be induced to make a determined attack on the
cottontails or to kill them. At times the cottontails proved to be able
opponents for weasels by striking out with their front feet and by
kicking with their strong hind legs. In killing the pheasants the teeth
of the upper jaw of the weasel pierced the top of the braincase and the
teeth of the lower jaw entered the region of the auditory process. The
forelegs hugged the neck of the pheasant, the body of the weasel was
extended in a riding position on the back of the bird and no amount of
kicking or rolling dislodged the weasel.
Polderboer, Kuhn and Hendrickson (1941) describe a cottontail cached by
a weasel as having the muscles of the neck severed from the region
behind the right mastoid process and noted "that hemorrhage in the
region of the right jugular vein had occurred."
Concerning the methods of killing mammals smaller than cottontails, the
accounts by Nichols and Nichols (1935:297-299) and that by Svihla
(1931) corroborate Glover's (1943A) account, as do also the accounts of
Miller (1931B:164) and Moore (1945:257). The latter says that his
captive male, from Gainesville, Florida, customarily bit its rodent
prey at the base of the skull and used the feet to manipulate the live
prey. Miller (loc. cit.) emphasized that his male weasel (M. f.
longicauda) grasped where it could, used its snakelike body to coil
over the prey and shifted the grip of its teeth to the nape of the neck
or back of the skull. The captives that I have had [one from Salt Lake
City, Utah; three from Contra Costa County, California; and the same
individual reported upon by Miller (1931:150)] customarily employed the
techniques of killing small rodents that were described by Glover and
Miller (loc. cit.).
Allen (1938:225-229) experimented with the ability of four different
males of M. f. noveboracensis from Michigan to kill adult
cottontails. The method used was to place the weasel in a cage of
quarter-inch hardware cloth approximately three feet long, two feet
wide, and two feet high. The bottom of the box was covered with several
inches of straw. One cottontail was offered to each weasel. In two
instances the weasel attacked and bit the cottontail, was struck by the
hind feet of the cottontail, retired from the attack and died a few
hours later as a result of the blows of the cottontail's hind feet. In
the other two instances the weasel rendered the cottontail helpless by
severing the neck muscles from the skull. Subsequently an incision made
by the weasel, in each of the two instances gave access to blood on
which the weasel fed until it was full, in one instance by licking
"blood as a cat laps milk." One rabbit was subdued in 10 minutes and
the other in 15 minutes. Allen (op. cit.) points out that cottontails
form a considerable portion of the weasel's food and thinks that they
are killed in burrows more easily than they were in the cage.
In writing of the three species of weasels, including Mustela
frenata, found at Treesbank and vicinity, Manitoba, Norman Criddle and
Stuart Criddle (1925:143, 144), in my opinion, correctly explain the
killing of more prey than weasels need. "The fact that weasels
frequently kill many more animals than they require for immediate use
has been universally interpreted as a lust for killing—a supposition
which we believe to be quite erroneous. It is true that weasels often
kill more than they need, but the surplus is not necessarily wasted
because the animals always store it for future use, in much the same
way as do badgers, minks or skunks, and with the same object in view as
squirrels have in gathering nuts. We have observed many such stores,
but as far as our observations go, the habit of killing in excess
occurs much more prominently in the late summer and autumn months than
in the spring. Indeed, we have no records of excessive spring slaughter
and this indicates that the supposedly blood-thirsty habit of weasels
is no more a lust for killing than is the woodsman's foresight in
providing his larder with meat for the winter months. It should be
noted in this connection that members of the weasel family, when
undisturbed, do not leave their victims scattered about, but carefully
store them away, and in many instances the bodies are buried with earth
or taken under ground to preserve them. We suspect that this instinct
for preserving food for future use accounts for most of the excessive
killing by carnivorous animals instead of this latter indicating an
aimless desire for slaughter which would unnecessarily deplete the food
supply of the future. This instinct, however, does not seem to be as
definite as that of some rodents, and there is no doubt that much of
the stored meat decays before it can be utilized."
Criddle and Criddle (1925:146) note that a weasel in the vicinity of
Treesbank was carrying a rat [Rattus] and that "Two small punctures
in the throat were the only evidence of the manner in which its death
had been brought about."
Considerable information has been recorded concerning the food of
Mustela frenata and a little information is on record as to kinds of
foods not taken that could have been taken. For example, Ingles
(1939:253, 254) on May 14, 1938, near Shasta City, California, noted
that nestlings of russet-backed thrushes were ignored by an adult
weasel and four young weasels which were feeding instead on meadow mice
and a mole. Howard (1935:322, 323) records that a weasel in Michigan
which carried bits of meat from beef bones on a porch ignored a red
squirrel which drew on the same food supply but which retreated to the
end of the porch when the weasel appeared. Quick (1944) records that in
the winter of 1940 on a 640 acre area in Washtenaw County, Michigan,
four resident weasels did not kill any of the 10 rabbits or several
pheasants but subsisted on smaller animals. Glover (1943A) thought that
M. frenata kills only a few adult cottontails in the wild. To judge
from these observations, M. frenata chooses small mammals as prey in
greater measure than it does birds or larger mammals.
Records of prey taken, attacked or pursued by Mustela frenata include
the following:
Broad-footed mole (Scapanus latimanus).—One was fed on by an adult
M. frenata and four young, on May 14, 1939, "near Shasta City,"
California (Ingles, 1939:253, 254).
Dusky shrew (Sorex cinereus).—A female weasel, at Majestic, Long
Island, N. Y., was shot when carrying a Sorex cinereus that had a
small hole in the top of its head (Nichols and Nichols, 1935:297-299).
Big short-tailed shrew (Blarina brevicauda).—One was taken from the
stomach of a weasel (Hamilton, 1928:249).
Townsend ground squirrel (Citellus townsendii).—Alcorn saw a weasel
five miles west of Fallon, Nevada, carrying a squirrel (Hall,
1946:192).
Richardson ground squirrel (Citellus richardsonii).—The attempted
capture of one of these squirrels in Saskatchewan is recorded by Seton
(1929 (2):625).
Belding ground squirrel (Citellus beldingi).—Grinnell, Dixon and
Linsdale (1937:233) recount that at Tuolumne Meadows, California, a
weasel killed a ground squirrel of this species.
Thirteen-lined ground squirrel (Citellus
tridecemlineatus).—Errington (1936:406, 407) found a den in Palo Alto
County, Iowa, on June 22, 1934, where he collected 32 fecal pellets.
Sixteen samples contained thirteen-lined ground squirrels, 9 contained
rabbits, 9 contained mice (7 Microtus, 1 Peromyscus and 1
unidentified); red-winged blackbirds and unidentified fringillids were
represented as also were ground beetles, grasshoppers and other
insects. One red-winged blackbird lay near the entrance of the den.
Franklin ground squirrel (Citellus franklinii).—Sowls (1948:126)
records that at Delta, Manitoba, a weasel was observed killing one of
these squirrels and that "the weasel had taken the squirrel from its
hibernating burrow as evidenced by tracks in the snow." On July 19,
1917, in the vicinity of Treesbank, Manitoba, T. Criddle saw a weasel
attacking one of these ground squirrels which was in mortal terror and
squeaking continuously. Eventually the squirrel was thrown on its back
"and would have been speedily killed but for an interruption" (Criddle
and Criddle, 1925:146).
Golden-mantled ground squirrel (Citellus lateralis).—On August 15,
1941, along the Kaweah River in Sequoia National Park, Boyer (1943:99,
100) saw a weasel chasing a Citellus lateralis; three or four times
the weasel grasped the back of the neck of the squirrel which each time
threw off the weasel until the two, weasel after the squirrel, plunged
into the river. The squirrel, bleeding at the base of the skull, was
rescued and entered a hole; the weasel got out of the water and under a
rotting log. Follett (1937:365) at 2 p.m. in Plumas County, California,
saw a weasel have hold of the lower jaw of a golden-mantled ground
squirrel near its throat. Alcorn watched a weasel chase a
golden-mantled ground squirrel in Nevada (Hall, 1946:192) and Grinnell
and Dixon (1919:681) record that on August 4, 1911, near Monache
Meadows in eastern Tulare County, California, a weasel pursued,
captured and killed a golden-mantled ground squirrel.
Eastern chipmunk (Tamias striatus).—Pearce (1937:483) in central New
York State, on July 29, 1931, saw a chipmunk scamper up a tree pursued
by a weasel.
Chipmunk (subgenus Neotamias).—Stanford (1931:363) on November 11,
1931, at Fish Lake, Utah, saw a weasel pursuing a chipmunk. On August
5, 1910, "near Independence Lake," Nevada County, California, Louise
Kellogg recorded that a weasel seized and ran off with a chipmunk
(Grinnell, Dixon and Linsdale, 1937:233). Allen (1938:228) observed
that a chipmunk (whether Tamias striatus or T. minimus not
specified) was killed in 30 seconds whereas 10 to 15 minutes were
required by the caged, male Mustela frenata noveboracensis to kill a
cottontail.
Red squirrel (Tamiasciurus).—Seton (1929 (2):625) records the
capture of one in Pennsylvania, and Grinnell, Dixon and Linsdale
(1937:232), at Cisco, California, saw one closely pursued by a weasel.
Flying squirrel (Glaucomys).—Burroughs (1900:77, 78) records remains
of one of these squirrels along with the remains of other animals in a
food cache of a Mustela but his account does not make clear whether
Mustela frenata or Mustela erminea was the species of weasel
involved.
Northern pocket gopher (Thomomys).—In "July, 1939, near Stillwater
[Nevada], Alcorn pursued . . . [a] weasel and caused it to drop . . .
a pocket gopher [Thomomys bottae] which was about two-thirds grown"
(Hall, 1946:192). Grinnell, Dixon and Linsdale (1937:233) write that
"at least twice, weasels in the [Yosemite] Valley were seen carrying
pocket gophers." Relative to Thomomys talpoides in the vicinity of
Treesbank, Manitoba, Criddle and Criddle (1925:146) record that on
September 11, 1918, an individual of Mustela frenata longicauda took
seven pocket gophers dead. . . . It seized the rodents by the middle of
their back and held them high while carrying them away. They were
stored in a gopher burrow some two hundred yards distant. On February
17, 1921, "Came across the marks of a weasel carting some object over
the snow. An investigation revealed a recently-killed pocket gopher
with its captor still in possession." Criddle (1930:279), at Aweme,
Manitoba, "frequently observed this weasel [M. f. longicauda] . . .
carrying a pocket gopher to its larder, and twice it has been
encountered in mid winter with freshly killed gophers in its
possession." The evidence already presented that weasels levy heavily
on pocket gophers is strengthened by the many references in the
literature to weasels having been caught in traps set for pocket
gophers in the burrows of those rodents and by the many statements, not
quoted here, that living quarters of weasels are in burrows made
originally by pocket gophers. For example, the present writer, in an
account of the Mammals of Nevada (Hall, 1946:191, 192), has said of the
long-tailed weasel, Mustela frenata nevadensis, that "All the three
dens that were excavated . . . were originally burrows of pocket
gophers. . . . Although we have found weasels in many situations in
Nevada, . . . they most often were obtained from the burrows of pocket
gophers." Excluding the weasels taken by Alcorn, more specimens of the
remaining lot were caught in traps set in the burrows of pocket gophers
than by all other means combined. All of the 22 weasels taken by Alcorn
[within a radius of 10 miles of Fallon] were obtained in gopher traps.
Mexican pocket gopher (Cratogeomys).—At Chalchicomula, 8000 feet,
Puebla, Nelson (1918:470 and letter dated March 9, 1928) saw a weasel
fastened to a pocket gopher. Nelson obtained the pocket gopher and
found that its neck muscles were torn loose from the skull.
Grasshopper mouse (Onychomys).—Barber and Cockerell (1898:189) found
remains of this mouse in the stomach of a weasel at Mesilla Park, New
Mexico.
White-footed mice (Peromyscus).—Green (1936) saw a weasel in Gratiot
County, Michigan, in May, carrying a Peromyscus. Quick (1944:76), in
winter, in Michigan, found one dead, probably killed by a weasel. From
Washtenaw County, Michigan, Quick (1944:77) examined 294 scats of
free-living weasels and found Peromyscus in 189 scats, Microtus in
83, small birds in 20, red squirrel in 3, and hair of weasels in small
quantities (probably from the animals which deposited the scats) in 36.
He concludes (op. cit., 78) that the winter food was 65 to 70 per
cent Peromyscus, 23 to 33 per cent Microtus, and 2 to 7 per cent
small birds.
Wood rats (Neotoma).—A female long-tailed weasel weighing 250 grams
was taken one mile north of Kent, Texas, while eating a Neotoma
albigula (Davis and Robertson, 1944:263). A wood rat house under
observation by Vestal (1937:364) in Contra Costa County, California,
was invaded by one weasel which ate two adult wood rats (Neotoma
fuscipes) and one young. In the same area he saw a weasel in a wood
rat nest some months later (Vestal, 1938:5). Three miles east of Reno,
Nevada, on May 13, 1936, W. B. Richardson watched a long-tailed weasel
carrying a half-grown round-tailed wood rat (Neotoma lepida) across a
rock slide (Hall, 1946, 192). Harper (1927:303) records three wood rats
[Neotoma floridana] and two cotton rats [Sigmodon hispidus] found
dead in the den of a female weasel and her three young in the
Okefinokee Swamp of Georgia. Another female and three young
approximately half grown were found in the swamp in a hollow pine log.
Contents of the den as described to Harper were nearly a peck of wood
rats, whole and in pieces; remains of several kinds of birds including
robins and quail, and a piece of joint snake (Ophisaurus ventralis).
Meadow mice (Microtus).—Polderboer, Kuhn and Hendrickson (1941), in
1939, at Ames, Iowa, identified "A total of 118 items . . . in 97
winter scats and 48 in the 38 spring scats." Their combined data are as
follows:
| |
Frequency |
Percentage |
| Meadow mouse |
71 |
42.85 |
| Harvest mouse |
36 |
21.75 |
| Deer mouse |
17 |
10.23 |
| Mearns cottontail |
14 |
8.42 |
| Short-tailed shrew |
9 |
5.42 |
| House mouse |
3 |
1.86 |
| Tree sparrow |
2 |
1.02 |
| Grasshopper |
1 |
.60 |
| Shaw pocket gopher |
1 |
.60 |
| Least weasel |
9 |
5.40 |
| Unidentified material |
3 |
1.85 |
Polderboer, Kuhn and Hendrickson divide their data into two categories,
winter and spring. Items recorded in winter but not in spring are house
mouse, tree sparrow, and grasshopper. Items recorded only in spring
were pocket gopher and least weasel. The samples of cottontail and
least weasel all were from the scats of one large male weasel. Of a
total of 14 pheasants, 24 quail and 35 cottontails on the 160 acres
involved in the study only two cottontails appear to have been killed
by the weasels—really by one weasel of four which lived on the area.
Food items taken from the nests (3) and adjacent caches of food in the
dens, were as follows: meadow mouse, 30; short-tailed shrew, 4; pocket
gopher, 2; deer mouse, 2; least weasel, 1; tree sparrow, 1. The authors
remark that the abundance of several prey species does not cause the
weasels to ignore the shrews which are said to be distasteful to
carnivores.
Two horned larks, apparently killed by weasels, were found on the 160
acre area studied; the horned larks were not in caches of food, nor
were remains of horned larks found in scats.
Dearborn (1932:34, 37) for Michigan, on the basis of contents of (37?)
intestinal tracts and "feces collected partly in winter and partly in
summer" found that, by frequency of occurrence, mammals comprised 83
per cent of the food, birds 10 per cent and insects 7 per cent.
Frequency indices for the genera of mammals in percentages of food
items of all kinds were as follows: Microtus, 31 per cent;
Peromyscus, 24 per cent; Sylvilagus, 14 per cent; Sorex, 7 per
cent; Blarina, 5 per cent; Scalopus, 2 per cent.
Criddle and Criddle (1925:146), for the vicinity of Treesbank,
Manitoba, record that on October 3, 1913, a weasel was seen to take a
field mouse down a hole. They add (op. cit.:147) that "Once while
ploughing, we observed a Long-tailed Weasel carrying a field mouse. . . ."
Ingles (1939:253, 254), in June, 1938, near Mt. Shasta City,
California, found an adult and four young weasels which fed on several
Microtus montanus montanus. Green (1936) in May, in Gratiot County,
Michigan, in the vicinity of a nest in which there were four young
weasels, found "several" dead Microtus. Hamilton (1933:330) records
that in New York State a male weasel, on April 5, 1932, at Ithaca, had
eaten a Microtus and that in May, 1927, a female weasel was seen
carrying a Microtus in its mouth.
Hamilton's (1933:333) study of the contents of the digestive tracts of
bodies of weasels obtained from fur trappers and fur buyers enabled him
to publish the following "Frequency Indices of Mammal Genera in Fall
and Winter Food of 163 Mustela noveboracensis": Microtus, 33.6 per
cent; Sylvilagus, 17.3; mammals undetermined to genus but principally
mice, 17.1; Peromyscus, 11.3; Rattus, 9.1; Blarina, 5.9;
Sciurus, 2.7; Tamias, 1.0; Condylura, 0.8; Ondatra, 0.8.
Grinnell, Dixon and Linsdale (1937:233, 234) quote W. Fry concerning a
weasel which reared six young at Giant Forest, California, in 1919, as
follows: "This parent weasel, after the birth of her young, remained at
the premises for a period of thirty-seven days; during which time, from
actual count, the following numbers of mammal species fell victim to
her: mice [genera not specified] 78; gophers 27; moles 2; chipmunks 34;
wood rats 3; ground squirrels 4. This is a total of 148 animals for
the . . . thirty-seven days . . . not a bird was captured during the
period."
Rats (Rattus).—Criddle and Criddle (1925:146), on the farm at
Treesbank, Manitoba, record a long-tailed weasel, on July 2, 1918,
running away from the farm buildings carrying a rat; July 11, 1919,
"Two longicaudas . . . have been seen running off with rats on
several occasions."; July 11, 1920, "There are two large weasels about
the buildings[;]. . . . Each has been noted with rats and this
afternoon one of them was seen running into the woods carrying a rat,
followed by two excited swallows." The authors (op. cit.:147) add "In
the fall of 1924, Mr. A. Cooper, a prominent poultryman of Treesbank,
observed a large weasel carrying a freshly killed rat which it stored
below ground and then returned towards the poultry-house, causing no
little apprehension to the owner. Within a short time, however, the
weasel reappeared with another rat which it hid as before. In this way
several rodents were accounted for during the afternoon, and Mr. Cooper
assures us that the weasel 'kept up the good work for some days'."
Hamilton (1933:330) in New York State in May, 1927, saw a male weasel
in possession of a rat.
Big jumping mouse (Zapus major).—In the Warner Mountains of
California, on Parker Creek, H. C. Bryant frightened a weasel that
dropped a freshly killed jumping mouse (Grinnell, Dixon and Linsdale,
1937:232).
Snowshoe rabbit (Lepus americanus).—Adolph Murie (1935:321-322)
writes that: "Four miles north of Funkley, Minnesota, early on the
morning of November 13, 1921, . . . watched from the top of a 30-foot
spruce a weasel. .. hunting a varying hare. . . . The ground was
covered with six inches of fresh snow . . . both animals . . . [had]
their [white] winter pelage.
"My attention was first attracted to the hare as it came hopping
steadily but unhurriedly from the north. Directly in front of me, about
75 feet from the tree I had climbed, the hare crisscrossed back and
forth at various angles over an open area about 20 feet in diameter.
After producing a maze of tracks, the hare 'froze' near one edge of the
pattern. In a few minutes the weasel appeared, all his faculties
focused on the warm trail. Expertly he followed its convolutions,
passing at times within a few feet of the watching hare. Not until the
weasel had followed every turn of the trail to within three feet of its
termination did the hare skip off. It came out to the road almost
directly below me, turned at right angles northward and was soon out of
sight. At the road the weasel lost the trail, . . . and then ran
parallel with it, once more in hot pursuit.
"Ten minutes later the hare emerged from the north as before, came on
directly to the tracked-up area, and continuing its stratagem,
leisurely hopped about to leave its zigzag trail. Then it sat down
quietly to wait. . . . The weasel['s] . . . nose led him through the
network with little trouble. He was almost upon the hare before it
jumped off and followed the same path [as] . . . before. . . .
"The hare had to show his big heels [a third time] . . . as the weasel
approached him. This time the weasel failed to follow. . . . After
examining a few brush heaps he vanished into the woods behind me."
Seton (1929 (4):723, 724) writes that in December of 1886 in the
sandhills northeast of Carberry, Manitoba, he saw a weasel chasing a
snowshoe rabbit which took refuge near his feet under the sleigh and so
escaped the weasel. Thurber (1940:356) mentions a month-old varying
hare that was rescued from a weasel and of approximately the same size
as the weasel.
Criddle and Criddle (1925:146) for the vicinity of Treesbank, Manitoba,
record "August 21, 1921.—Heard cries of a small rabbit at dusk
to-night, which investigation showed was being attacked by a large
weasel. The rabbit was later carried to the weasel's store chamber
below ground." They record further (op. cit., 146, 147): "November 8,
1924.—Shot a bush rabbit and left it lying. Two hours later [it] . . .
was found to have been dragged beneath a brush pile and partly eaten.
Innumerable weasel tracks left no doubt as to the identity of the
thief." In describing a weasel that wintered in a nest in a threshing
machine, the same authors (op. cit.:143) say that no bird remains
were found in the pile of approximately three pounds of droppings
adjacent to the nest. In a store chamber some 140 yards away from the
nest, two bush rabbits (Lepus americanus) had been dragged to the
entrance and numerous smaller rodents were taken below ground. The
rabbits were buried beneath the snow and eaten as necessity arose.
Narrow selectivity on the part of the weasel in choosing food is almost
always shown in instances where the food of weasels has been studied.
For example, the weasel which lived in the threshing machine ate
rodents and rabbits and not poultry although the weasel had ready
access to the poultry building. The weasel which lived in the bag of
feathers in the basement of Stuart Criddle's house ignored grouse,
approximately 20 in number, in favor of other non-avian food.
Cottontail (Sylvilagus).—Polderboer, Kuhn and Hendrickson (1941)
mention that one of 4 weasels which they studied on a 160 acre area at
Ames, Iowa, in 1939, had a cache of food in a pocket gopher burrow 10
rods distant from the weasel's den. The cache contained only two
cottontails, one partly eaten. Leopold (1937) records seeing a
Mustela (probably a long-tailed weasel but possibly an ermine) kill a
third-grown cottontail by biting it at the base of the skull. Leopold
describes the blood sucking or licking, suggesting that he shared the
popular misconception that weasels suck blood. The supposition that
weasels suck blood has been refuted by many observers, for example by
Svihla (1931). My own observation of captives makes me think that
weasels do not suck blood. Seton (1929 (2):626) quotes B. H. Warren as
seeing a weasel dragging a freshly killed, still warm, rabbit that
contained nine embryos almost ready for birth. A young rabbit was seen
being carried by a weasel in Hidalgo County, Texas, in March, 1935
(Mulaik, 1938:104). An instance of a cottontail being chased in June in
South Carolina is recorded by Hamilton (1933:330). Addy (1939:372,
373), in Virginia, on August 14, 1939, shot a large weasel which was
pursuing a Sylvilagus that was only a foot and a half ahead of the
weasel. The rabbit stopped when a shot was fired and permitted itself
to be stroked and petted. Tracking showed that the weasel had chased
the rabbit for a half mile. On November 20, 1942, at Lake James,
Indiana, a weasel was seen by Grosjean (1942:443) attacking a "young
rabbit" in the throat of which the weasel had made five large holes
from which there was no obvious bleeding. Seton (1929 (4):798) recounts
that in 1910 at Base Lake, Michigan, F. C. Hicks saw a cottontail with
a weasel hanging to its legs rush to the cottage. When only four feet
from Hicks the weasel loosed its hold and the cottontail escaped under
the cottage. Burroughs (1939:253) on May 14, 1939, in Saginaw County,
Michigan, records that a young cottontail weighing between 200 and 250
grams was carried from the nest and killed. Burroughs was attracted
first by the "hissing scream" of the weasel, strode toward the sound,
flushed an adult cottontail, and discovered the empty nest from which
the weasel had taken the young cottontail.
Brush rabbit (Sylvilagus bachmani).—Vestal (1937:364) in Contra
Costa County, California, found a brush rabbit that appeared to have
been killed by a weasel.
Reptiles.—Grinnell, Dixon and Linsdale (1937:234) recount that in
July, 1889, in Wilson Canyon, near Pasadena, California, a weasel
killed a red racer by severing the backbone of the snake. In April,
1935, in Hidalgo County, Texas, a half grown bull snake (Pituophis
sayi sayi) was regurgitated by a young weasel. Russell (1930:504, 505)
has recorded finding in California a male weasel and a king snake
(Lampropeltis getulus boylii) three feet five inches long in mortal
combat. The weasel killed the snake but the weasel, incapacitated by
the conflict, was easily picked up by hand and was also saved as a
specimen.
Wild birds.—In the spring of 1940, in Washtenaw County, Michigan, one
bobwhite, of 10 bobwhite living on a 640 acre area, was killed by one
of four weasels that lived on the area. No other quail was killed
there. The one unfortunate bird was killed in the mouth of an abandoned
den where the quail roosted (Quick, 1944:76). A male weasel, subspecies
M. f. effera, was seen by Booth (1946:439) attempting to enter the
nesting hole of a pair of flickers, Colaptes. One song sparrow
(Melospiza melodia), and one slate-colored junco (Junco hyemalis)
were recorded by Quick (1944:76) as killed by weasel in Michigan.
Chicken (genus Gallus).—Quick (1944:78) writes that in one year
(1938-1939) weasels were reported to have killed 1.03 per cent of all
chickens in one township of Washtenaw County, Michigan, and that of the
total damage to all kinds of poultry, 59 per cent was done by weasels.
Weasels entered 19 per cent of the chicken coops on the study area.
Farmers killed 68 per cent of the weasels seen in barn yards. Spring
and summer were the seasons in which most of the weasels were observed
in barn yards. Internal evidence in Quick's (op. cit.) account leads
me to suspect that some losses of poultry were charged to weasels when
Rattus was actually responsible.
Criddle and Criddle (1925:146), quote a neighbor in the vicinity of
Treesbank, Manitoba, as recording that on October 29, 1917, "A weasel
last night made its way into our fowl-house, the door being
inadvertently left open. The weasel killed eleven fowl, some of which
were dragged into the yard. All the largest fowls were selected, the
pullets remaining untouched though they were in the majority. Next
night the weasel dug a hole beneath the building and killed a hen and
two cocks, returning for another during the day, making a total of
fourteen in all." Criddle and Criddle (1925:146) remark that the weasel
proved to be a large one, probably an old male. The same authors (op.
cit.:147) record that at their farm at Treesbank, Manitoba, on January
31, 1925, "A Long-tailed Weasel killed three hens last night, and
rather severely bit a cock about the neck. This, or another weasel, had
been around the farm-yard for sometime (The specimen was a large
male). . . .
"In the fall of 1924, Mr. A. Cooper, a prominent poultryman of
Treesbank, observed a large weasel carrying a freshly killed rat which
it stored below ground and then returned towards the poultry-house,
causing no little apprehension to the owner. Within a short time,
however, the weasel reappeared with another rat which it hid as before.
In this way several rodents were accounted for during the afternoon,
and Mr. Cooper assures us that the weasel 'kept up the good work for
same days'.
"Being a farmer of many years' standing, Mr. Cooper has naturally lost
some poultry through the agency of weasels, but while he remarks that
'there are good as well as bad actors among weasels', he has the
practical good sense to recognize the value of an efficient ratter even
though it be a weasel.
"Our sister, Maida Criddle, writes under date of March 4, 1925:
"'There is another weasel (longicauda) in the fowl-house, a
well-behaved one this time. It came and took a piece of meat out of my
hand quite nicely, which it carried down a hole and then came and
sniffed all over my mitt to see if there was any more. I thought it had
been killed when I visited the farm buildings next day as there was a
strong smell of musk on the cat and in the fowl house, but the weasel
was there as cheeky as ever. It got hold of my skirt twice and tried to
pull me down its hole. I think it wanted the cloth for a bed, as it was
taking straw and other material down the burrow. The poultry were very
frightened at first, but they are getting used to the weasel's presence
now'."
In commenting on the economic role of the long-tailed weasel in
Manitoba, Criddle and Criddle (1925:145) write as follows: "Supply and
demand are prominent factors in governing our weasels' food habits. The
two smaller species, as we have already pointed out are so dependent
upon mice for a living that they increase or diminish with the
fluctuation of these creatures. The Long-tailed Weasel, however, is not
so easily checked by the temporary disappearance of any particular kind
of game. If mice are scarce it devotes greater attention to gophers or
bush rabbits and if these are not in sufficient numbers to satisfy its
appetite, the animal raids a poultry house as a last resource. In nine
years out of ten, this weasel will find sufficient food about the
fields and woods, but on the tenth it may be obliged to temporarily
turn to domestic animals. It is at such times that the weasel is seen
and its deeds recorded. A thousand mice may have been killed in the
meantime, but the destruction of half a dozen hens is alone used as
evidence of the weasel's economic standing.
"In the last twenty years we have permitted weasels to frequent the
farm buildings at will and the poultry house has been no exception. In
that time rats and mice suffered severely from the weasels, while the
total number of poultry taken were six. Many times that number,
however, have been killed by rats.
"When we review our experiences of the past, we are astonished to find
what few poultry have been killed by weasels. Our own losses in
forty-two years have not exceeded fifteen birds and even these were
usually eatable. There have been reports of losses from time to time
from neighbors, but on looking into details we find that there are very
few farmers who have experienced more than three separate occasions of
weasel depredation and the total loss per farmer in the last thirty
years does not, we are sure, exceed ten birds. This is surely a
remarkably small payment to weasels in general for the great good done
by them in killing rodents.
"We wish to point out, too, that only the exceptional weasel becomes a
poultry killer. In most cases apparently it is a fully-grown male that
does the killing. There are exceptions, of course, but when we see a
large weasel actively engaged in rodent hunting within a few feet of a
brood of newly hatched chickens and not even looking at them, we must
at least pause to ask if this animal is the enemy that we were taught
to believe it to be."
A suggestion that weasels sometimes obtain the prey killed by hawks is
offered by Criddle and Criddle (1925:147) who write: "Hawks are not
always the aggressors, as is shown by an incident reported by Mr. H.
L. Seamans, of Lethbridge, Alberta. Mr. Seamans noted a large buzzard
suddenly fly straight upwards from a fence post, and then alight upon
another one some distance away. A little while afterward this bird once
more arose in the same manner as before, and presently repeated the
performance again. An investigation then followed and revealed that a
Long-tailed Weasel was following the hawk from post to post.
"We should hardly expect a weasel to attempt to capture a bird of the
above type. On the other hand, it is possible that these animals might
be able to startle a hawk sufficiently to cause it to drop its prey,
which would thus provide food for the weasel."
The following frequency index is compiled from the foregoing data on
prey of Mustela frenata.
- Moles (family Talpidae), 5
- Shrews (family Soricidae), 26
- Pigmy weasel (Mustela rixosa), 1
- Ground squirrels (genus Citellus), 23
- Chipmunks (genus Tamias), 38
- Tree squirrel (possibly all Tamiasciurus), 8
- Flying squirrel (genus Glaucomys), 1
- Pocket gophers (family Geomyidae), 34
- Mice (order Rodentia), 96
- Harvest mice (genus Reithrodontomys), 36
- Grasshopper mouse (genus Onychomys), 1
- Deer mice (genus Peromyscus), 235
- Cotton rat (genus Sigmodon), 2
|
- Wood rats (genus Neotoma), 14
- Meadow mice (genus Microtus), 248
- Muskrat (genus Ondatra), 1
- Old World rats (genus Rattus), 19
- House mouse (genus Mus), 1
- Jumping mouse (genus Zapus), 5
- Varying hare (Lepus americanus), 5
- Rabbits (genus Sylvilagus), 48
- small birds, 32
- chickens, 17
- lizard, 1
- snakes, 4
- insects, 3
|
More significant than the above compilation, of course, are the results
of careful studies of the food of the long-tailed weasel in restricted
areas. Examples of such studies are those of Polderboer, Kuhn and
Hendrickson (1941) and Hamilton (1933:333).
According to Hamilton's (1933:332) observations on captive weasels,
"There seems to be little relative difference in the amount they eat,
regardless of their activities.
"In general, more food is taken in summer than in winter. Usually about
a third their weight every 24 hours is eaten, but a growing young
weasel will consume much more. A young male noveboracensis, weighing
145 grams, consumed an entire chipmunk, fur and bones, weighing 85
grams, in 24 hours. A day later it ate all of a partly grown rat, 105
grams, in the same length of time."
Moore (1945:253) records that a captive male that he obtained at
Gainesville, Florida, consumed, on the average, between 63 and 70 grams
of flesh and blood per day. The weasel itself weighed approximately 320
grams.
Sanderson (1949:413), concerning seven young weasels from Manitoba,
that he raised in captivity, writes: "From the fifth to the seventh
week of age, they consumed approximately 22 per cent of their body
weight per day; from the eighth to the tenth week (just before reaching
mature size) they consumed approximately 24 per cent; but after
reaching maturity they consumed only 18 per cent. When given all the
food they would take in one day, they ate as much as 40 per cent of
their body weight."
Criddle and Criddle (1925:143, 146) say that weasels drinking at a bird
trough "held their mouths very close to the water and as far as we
could see, lapped the liquid up with rapid movements of the tongue. As
a rule, after drinking, they would merely spring to the ground and
vanish amid a bunch of scolding birds, but occasionally we have seen an
animal slowly drag itself through the water and follow this performance
by some rapid gambols, or a quick run, a method of drying which most of
us have practiced in our youth." According to Hamilton's (1933:332)
observations on captives, "Weasels are great drinkers, and while they
take but little at a time, about 25 c.c. is drunk by a large animal
during a day. . . ."
Reproduction
Philip L. Wright's several papers (1942A, 1942B, 1947, 1948A, and
1948B) reporting on his detailed studies of Mustela frenata
(subspecies oribasus and longicauda) in captivity have yielded a
large share of the precise information that we have concerning breeding
and reproduction in this species. He has found that a single litter, of
up to 9 young is born in the spring, usually in April. At three months
of age the females "are full grown." The young males remain sexually
immature during the first summer but the young females, as well as the
females which are more than a year old, come into heat in the midsummer
and are bred by the adult males. After a long period of quiescence
lasting for several months, the embryos resulting from these matings
become active in early spring and develop to full term in less than 27
days after they become implanted. The adult males are sexually active
from April into August, when the testes are at maximal size and are
conspicuous in the scrotum. A gradual regression takes place starting
in August and extending into September. By October the testes may be
fully regressed and the molt to white may start in this month. The
white winter weasel, of either sex, is sexually inactive. The testes of
the sexually active male in early spring and late summer are seven to
eight times the size of the fully regressed testes. Females which had
borne and suckled young were first found to be in oestrus 65 to 104
days after birth of the young. Lactation lasts for approximately 5
weeks. In 18 litters the length of the gestation period varied from 220
to 337 days with an average of 279 days. The female in heat has the
vulva much swollen and she will remain in this condition for several
weeks if not bred. Wright (1948A) describes the actual mating as
beginning with a scuffle after which the male grabs the female by the
scruff of the neck with his teeth and holds her until she becomes
subdued when he clasps her lower abdomen with his front feet and arches
his back over her posterior regions. The two animals remain locked in
this position usually for two hours and sometimes for longer than three
hours. If the animals are left together, copulation may take place
again on the same day or upon succeeding days.
Hamilton (1933:316-321) writes of a freshly born M. f. noveboracensis
that it ". . . was pink and much wrinkled. The wetness . . . did not
entirely obscure a few sparse, rather long, white hairs . . . over its
back and head. It had the pronounced and extraordinarily long neck of
the adult." At one day of age the average weight of six individuals in
the litter was 3.1 grams, which is 3 per cent of the weight of the
adult female and 1-1/2 per cent of the weight of an adult male. At two
weeks of age "The silky white hair . . . obscures the general flesh
color of the skin, evident a week earlier. The hair on the back of the
head and neck, also over the shoulders, is slightly longer than that of
the back . . ." but there is no crest or mane or pompadour at this or
any other age such as characterizes the juvenal ermine. When 21 days
old one young male "hurried from the nest chamber and commenced to eat
some meat." At three and a half weeks "They are all eating small pieces
of meat. . . . The canine teeth have made their appearance in both the
upper and lower jaw, but just a hint of the incisors show. Some of the
cheek teeth are through, as the meat appears to be thoroughly
masticated by the little ones." On the 36th and 37th days the eyes
opened. Sanderson (1949:415) found that a litter of seven young of
Mustela frenata longicauda, from Manitoba, raised in captivity,
"reached the peak of their growth" at approximately ten weeks of age.
Several nests have been found. In Manitoba, Sanderson (1949:412)
excavated a burrow at the mouth of which he had trapped the adult
female and in which he found eight young approximately five weeks old.
The "burrow was about three inches in diameter, with two chambers at a
depth of twelve inches. One of these was empty, the other contained the
young. The two surface-openings were but two feet apart and the entire
burrow was no more than three feet long. . . . The meager nest
material consisted entirely of finely chopped grass. There was no mouse
hair present, no accumulation of fecal material, and no storehouse
containing food."
Charles O. Handley has written me that on January 25, 1929, on the
Sinkola Plantation, Thomas County, Georgia, he investigated the living
quarters of a family of five weasels, four of which had been shot five
days before by a hunter. According to the hunter each of the four which
had been killed was approximately two-thirds the size of one which
escaped into a hole in the ground. Handley found that the weasels had
been using as headquarters a burrow in the trunk of an old uprooted oak
as well as a nearby gopher burrow. The burrow in the oak was
approximately ten feet long and had been excavated in the rotten wood.
In a distance of fifty feet along the gopher tunnel there were several
used openings with pathways leading away from each. On February 6,
Handley, with the help of a friend, trapped a large male weasel near
this place.
Criddle and Criddle (1925:143) describe a female which, one winter,
slept in a bag of feathers in a basement of a house occupied by one of
the authors; another weasel in winter made its headquarters in a
threshing machine. The nest of the latter "was somewhat roughly
constructed and consisted of a convenient bunch of straw and chaff
under the cylinder."
Harper (1927:303) in the Okefinokee Swamp of Georgia dislodged a weasel
from the house of a wood rat and was told of a den found in the swamp
"in the trunk of a hollow cypress tree" from which a mother weasel and
three young "about the size of mice" were obtained. "The bed contained,
I suppose, a bushel or more of rabbit hair, rat hair, and squirrel
hair. It looked like it must have been used as a den for several years,
although there was no stink that I could detect except the musk from
the old Weasel." Another female and three young approximately half
grown were found in a hollow pine log.
Between January 6 and April 12, 1940, on 640 acres of land, in
Washtenaw County, Michigan, four weasels were studied and each weasel
used only one den in this period (Quick, 1944:78). Criddle (1930:279)
remarks that M. f. longicauda at Aweme, Manitoba, often makes its
temporary headquarters in the burrows of pocket gophers (Thomomys). A
female and three young weasels were found by Shaw (1921:167) using a
nest of a mountain beaver in the burrow of that animal. Green (1936),
in May, in Gratiot County, Michigan, saw a weasel enter a hole under a
decayed log and investigated finding four young weasels in a nest
mostly of Microtus fur.
In the early part (winter and spring) of 1939, at Ames, Iowa,
Polderboer, Kuhn and Hendrickson (1941) studied four weasels living in
four separate dens on 160 acres typical of Iowa farmland and excavated
three of the dens. One den was in a weed patch in an old mole run. The
nest chamber, approximately nine inches in diameter and six inches
below the surface of the ground "was filled with grasses packed in a
layer-like formation. In the center of this mass was a nest hollow
lined with patches of mouse and shrew fur. Beneath this layer of fur
and at the sides of the nest were skins, various bones, and skulls of
partially eaten mice and shrews . . . scats [were in the nest]. . . .
At intervals, layers of clean grass had been laid over the filth of the
former bed, thus giving the nest a stratified appearance." A second
den, of a large male, was in a field of sweet clover two feet high in
the former burrow of a Franklin's ground squirrel. The nest cell, seven
inches in diameter and nine inches below the surface of the ground,
"was lined with grasses mixed with much rabbit and mouse fur. Some
scats, and bones and fur of mice and shrews were matted together in
layers at the bottom of the nest." When this den was abandoned the male
weasel occupied, for a month, another burrow, 20 rods distant, of a
Franklin ground squirrel, in the field of sweet clover. The nest cell
measured 11 by nine inches and was 11 inches below the surface of the
ground. "Two nest layers were present. The first, composed chiefly of
coarse straw and grass, had apparently been occupied at some time by a
spotted skunk. . . . On top of the skunk nest was the weasel nest
composed of fine grasses, mouse fur, and skeletal remains of mice."
Relation of the Sexes to each other and to the young
Quick (1944:75) writes that on March 28, in Michigan, he found the
tracks of a male and those of a smaller animal, supposedly a female,
meeting. The two "then led along the fence for about 18 chains and both
entered the den of the male. . . . Only the tracks of the smaller
weasel left the den on the same date. Observation on April 12 showed
that the large male still occupied the den." I am at a loss to explain
this behavior since breeding would not be expected to occur in late
March and since I suppose that the male and female do not live
together except in the breeding season. Consequently, I wonder if the
sign was wrongly read.
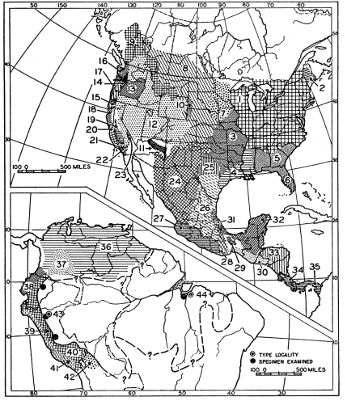
Fig. 29. Map showing the geographic ranges of the
subspecies of Mustela frenata and Mustela africana.
1. M. f.
noveboracensis
2. M. f. occisor
3. M. f. primulina
4. M. f. arthuri
5. M. f. olivacea
6. M. f. peninsulae
7. M. f. spadix
8. M. f. longicauda
9. M. f. oribasus
10. M. f. alleni
11. M. f. arizonensis
12. M. f. nevadensis
13. M. f. effera
14. M. f. washingtoni
15. M. f. saturata
16. M. f. altifrontalis
17. M. f. oregonensis
18. M. f. munda
19. M. f. xanthogenys
20. M. f. nigriauris
21. M. f. latirostra
22. M. f. pulchra
|
23. M. f. inyoensis
24. M. f. neomexicana
25. M. f. texensis
26. M. f. frenata
27. M. f. leucoparia
28. M. f. perotae
29. M. f. macrophonius
30. M. f. goldmani
31. M. f. tropicalis
32. M. f. perda
33. M. f. nicaraguae
34. M. f. costaricensis
35. M. f. panamensis
36. M. f. meridana
37. M. f. affinis
38. M. f. aureoventris
39. M. f. helleri
40. M. f. macrura
41. M. f. agilis
42. M. f. boliviensis
43. M. a. africana
44. M. a. stolzmanni
|
Hamilton (1933:328), however, writes that M. f. noveboracensis is to
"be found in pairs when caring for the young. During mid-May, 1927, I
several times saw a male of this species carrying food to a den of
young ones." Green (1936), in May in Gratiot County, Michigan, remarks
that while he was uncovering and examining a nest of four young
weasels, two adults ran about excitedly and one removed a young weasel.
In instances where several nearly full-grown young have been obtained
from one den it has been my experience (Hall, 1946:191) that the only
adult trapped there was the female; no adult male was found or in the
one instance when found he was living alone in a den 200 yards away
from the den of the female and her young. Data are too few to warrant a
definite conclusion about the extent to which the male aids in rearing
the young, but I have wondered if he might not do so when the young
were less than half grown and then live alone when they were more than
half grown.
Mustela frenata noveboracensis (Emmons)
Long-tailed Weasel
Plates 16, 17, 18, 31, 32 and 33
Putorius Noveboracensis Emmons, Quadrupeds of Massachusetts, p.
45, 1840.
Mustela fusca DeKay, Zool. of New York, Pt. 1, Mammalia, p. 34,
1842.
Putorius fuscus Audubon and Bachman, Journ. Acad. Nat. Sci.
Philadelphia, 8 (Pt. 2):288, 1842; Audubon and Bachman, Vivip.
quadrupeds of N. Amer., 3:234, pl. 148, 1853 (pl. 1848).
Putorius noveboracensis, DeKay, Zool. of New York, Pt. 1,
Mammalia, p. 34, 1842; Baird, Mamm. N. Amer., p. 166, 1858;
Merriam, N. Amer. Fauna, 11:16, pl. 4, figs. 1, 1a, 2, 2a, pl. 5,
figs. 3, 3a, text figs. 4-6, 30, June 30, 1896; Bangs, Proc.
Biol. Soc. Washington, 10:13, pl. 1, figs. 2, 2a, pl. 2, figs. 2,
2a, and pl. 3, figs. 3, 3a, February 25, 1896; Cory, Mamm.
Illinois and Wisconsin, p. 366, plates, 1912.
Putorius erminea, Audubon and Bachman, Vivip. quadrupeds of N.
Amer., 2:56, pl. 59, 1851.
Putorius agilis Audubon and Bachman, Vivip. quadrupeds of N.
Amer., 3:184, pl. 140, 1853.
Putorius richardsonii, Baird, Mamm. N. Amer., p. 164, 1858
(part).
Putorius (Gale) erminea, Coues, Fur-bearing animals, p. 109,
1877 (part).
Putorius noveboracensis notius Bangs, Proc. New England Zool.
Club, 1:53, June 9, 1899. Type from Weaverville, Buncombe County,
North Carolina.
Mustela noveboracensis noveboracensis, Miller, U. S. Nat. Mus.
Bull., 79:97, December 12, 1912; Soper, Journ. Mamm., 4:251,
November 1, 1923.
Mustela cicognanii, Henninger, Journ. Mamm., 2:239, November 29,
1921; Seton, Lives of game animals, 2:584, 1929 (part, Ohio);
Hamilton, Amer. Midland Nat., 14:290, July, 1933 (part, Ohio);
Lyon, Amer. Midland Nat., 17:109, January, 1936 (part, Ohio).
Mustela noveboracensis, Jackson, Journ. Mamm., 3:15, February 8,
1922.
Mustela frenata noveboracensis, Hall, Carnegie Instit. Washington
Publ., 473:104, November 20, 1936; Hall, Amer. Midland Nat.,
18:304, March, 1937.
Type.—Williamstown, Berkshire County, Massachusetts. Type
specimen not known to be in existence.
Range.—Altitudinally, sea level to highest parts of mountains
of eastern United States; Canadian Life-zone of Ontario and Quebec
southward through eastern United States in Canadian, Transition
and Upper Austral life-zones to and including upper edge of Lower
Austral Life-zone in the Carolinas and northern parts of Georgia,
Alabama, and Mississippi; westward from the Atlantic Coast to St.
Croix and Mississippi rivers. See figure 29 on page 221.
Characters for ready recognition.—Differs: From M. f.
olivacea, in males, by width of tympanic bulla which is less than
rather than more than 8.5 mm., and in adult females by total
length which is less than rather than more than 345 mm. and by
mastoid breadth which is less than rather than more than distance
between articular faces of exoccipital condyle and glenoid fossa;
from M. f. occisor by a number of average differences including
smaller size, relatively shorter tail and relatively narrower
skull (see measurements); from M. f. spadix by least width of
color of underparts amounting to less than 41 per cent of greatest
width of color of upper parts, absence of color of underparts on
ankles and feet, adults with hind foot less than 50 in males and
40 in females, orbitonasal length less than 15.5 in males and 13.5
in females, length of tooth-rows less than 18.0 in males and 15.7
in females, mastoid breadth less than 25.5 in males and 22.0 in
females; from M. f. primulina in males by interorbital breadth
averaging more than 24 per cent of basilar length, orbitonasal
length averaging more than 34 per cent of basilar length or 64 per
cent of mastoid breadth, tympanic bullae less inflated
anteromedially, than posteromedially, and in females by
orbitonasal length amounting to more than two-thirds of mastoid
breadth, by zygomatic breadth averaging less than 21, and by
anterolateral margin of tympanic bullae not projecting below
squamosal; from M. f. arthuri in males, by zygomatic breadth
more than distance between anterior palatine foramen and anterior
margin of tympanic bulla and by convex dorsal outline of skull in
longitudinal axis.
Description.—Size.—Male and
Female:
| Locality |
Number of specimens averaged |
Total length |
Length of tail |
Per cent of body- length |
Length of hind foot |
| Massachusetts |
8 ad. ♂ |
415 (390-432) |
146 (127-159) |
54% |
46.0 (41.0-48.0) |
| 4 ad. ♀ |
311 (298-321) |
104 (95-114) |
50% |
33.9 (31.5-37.0) |
| Liberty Hill, Conn. |
10 ad. ♂ |
411 (379-438) |
141 (124-155) |
52% |
47.1 (43.0-51.5) |
| 6 ad. and sad. ♀ |
318 (303-338) |
105 (80-123) |
49% |
33.0 (31.7-36.0) |
| Beaver Dam, Wisc. |
10 ad. ♂ |
407 (372-431) |
130 (113-143) |
47% |
46.0 (42.0-50.0) |
| 4 ad. ♀ |
326 (303-338) |
99 (86-108) |
43% |
35.6 (34.6-38.0) |
| Washtenaw Co., Mich. |
10 ad. ♂ |
371 (350-405) |
130 (115-140) |
54% |
45.0 (40.0-50.0) |
| 10 ad. ♀ |
306 (290-335) |
97 (90-120) |
46% |
34.0 (30.0-40.0) |
The length of the hind foot averages more than the basal length in
males whereas the reverse is true in females. The tail, relative
to the length of the body, is longer in males than in females. The
average differences in external measurements of the two sexes in
Massachusetts, are: total length, 104; length of tail, 42; length
of hind foot, 12.1. In Michigan, where the males are smaller,
corresponding differences are only, 65, 33, and 11. Weight of 19
adult males from New York (Hamilton, 1933:294), 225 (196-267)
grams and in 13 adult females, 102 (72-126) grams. Weights of 2
adults from Michigan are: ♂ 258; ♀ 101 grams.
Externals.—Longest facial vibrissae black, brown, or white
(often all three colors in same specimen) and extending beyond
ear; carpal vibrissae same color as underparts and extending to
apical pad of fifth digit; hairiness of foot-soles as shown in
figure 19.
Color.—Upper parts, in summer, Vandyke Brown or darker than
tone 4 of Burnt Umber of Oberthür and Dauthenay, pl. 304.
Sometimes approaching tone 2 of Warm Sepia of Oberthür and
Dauthenay, pl. 305. Underparts, in summer, ranging from white
through Napthalene Yellow (Peterboro, N. Y.), Pale Orange Yellow
(eastern Mass.), near Primuline Yellow (unusual specimen from
Leelanau Co., Mich.) to near (c) Deep Chrome (no. 19053, U. S.
Nat. Mus., Roan Mts., N. C.) In winter, all white except tip of
tail, or upper parts near (12" 1) Rood's Brown and tone 2 of Raw
Umber of Oberthür and Dauthenay, pl. 301, with underparts white or
sometimes tinged with yellowish. Tip of tail at all times black.
Upper parts of uniform color except for occasional slight
darkening of nose. Color of underparts extends distally on
posterior sides of forelegs to foot and sometimes over upper sides
of toes and on medial sides of hind limbs only to knees. Least
width of color of underparts averaging, in a series of twenty-two
males, mostly in full winter pelage, from Liberty Hill,
Connecticut, 21 (11-40) per cent of greatest width of color of
underparts. In eleven females from the same place, corresponding
percentages are 20 (14-29). Black tip of tail in same series of
males, most of which are in full winter pelage, 70 (60-75) mm.
long; thus longer than hind foot and averaging 50 per cent of
length of tail-vertebrae.
Skull and teeth.—Male (based on ten adults from Massachusetts):
See measurements and plates 16-18; weight, 3.6 (3.3-4.4) grams;
basilar length, 44.6 (43.3-46.0); zygomatic breadth less than
distance between condylar foramen and M1 or than between anterior
palatine foramen and anterior margin of tympanic bulla; mastoid
breadth less than postpalatal length; postorbital breadth more or
less than length of upper premolars and greater than width of
basioccipital measured from medial margin of one foramen lacerum
posterior to its opposite; interorbital breadth more or less
(usually more) than distance between foramen opticum and anterior
margin of tympanic bulla; breadth of rostrum less than length of
tympanic bulla; least width of palate less than length of P4;
anterior margin of tympanic bulla as far posterior to foramen
ovale as width of 3 to 6 upper incisors; height of tympanic bulla
more or less than distance from its anterior margin to foramen
ovale; length of tympanic bulla more than length of lower molar
and premolar tooth-row and longer or shorter than rostrum;
anterior margin of masseteric fossa behind or directly below
posterior fourth of m1.
Female (based on five adults from Mass.): See measurements and
plates 31-33; weight, 1.7 (1.2-2.1) grams; basilar length, 36.5
(35.2-38.1); zygomatic breadth less than distance between condylar
foramen and M1 or than between anterior palatine foramen and
anterior margin of tympanic bulla; postorbital breadth more or
less than length of upper premolars and more than width of
basioccipital measured from medial margin of one foramen lacerum
posterior to its opposite; least width of palate more or less
(usually less) than greatest length of P4; tympanic bulla as far
posterior to foramen ovale as width of 4 to 5-1/2 upper incisors;
height of tympanic bulla less than distance from its anterior
margin to foramen ovale; length of tympanic bulla more than length
of lower molar and premolar tooth-row and longer than rostrum.
The skull of the female averages 53 per cent lighter than that of
the average male.
Comparisons of the skull with those of M. f. olivacea, M. f.
spadix, M. f. primulina, and M. f. arthuri, are made in the
accounts of those subspecies. As compared with that of M. f. occisor
the skull of adult male noveboracensis, is of smaller average size
with relatively (to basilar length of Hensel) lesser mastoid and
zygomatic breadths. In addition to the zygomatic arches of
noveboracensis being less widely bowed outward they seem to be more
rounded posteriorly. Comparisons of subadult females indicate that
these differences exist in the females as well as in the adult males.
Remarks.—The earliest of the post-Linnaean references to this weasel
mostly were under the specific name erminea in the belief that the
American animal was the same as the larger of the two common species of
weasel in the Old World. The name noveboracensis, now in use for this
subspecies, was applied in 1840 and since that time the males usually
have borne that name; the females, because they are smaller, were more
frequently confused with some other species. Audubon and Bachman in
1853 even proposed the name agilis for the female in the mistaken
belief that it was a species distinct from the male. After 1896, when
Bangs correctly classified the weasels of the eastern United States,
the males have been correctly identified and the females, except by a
few authors, likewise have been correctly named. Because many early
American naturalists did their first collecting of mammals in the
geographic range of noveboracensis, the person who examines labels of
specimens of this subspecies can find data written in the hand of
Spencer Fullerton Baird, Theodore Roosevelt, and other naturalists
famous for their work as scientists or accomplishments otherwise. The
material is more nearly adequate than is that of many other subspecies
and the number of specimens is exceeded—and only slightly—by that of
the subspecies nevadensis, which like noveboracensis has a
relatively large geographic range.
Intergradation with Mustela frenata spadix is indicated by subadult
males from western Wisconsin, namely, one from Gordon, three from
Colfax and one from Meridean. Linear measurements of the teeth of these
specimens are exactly intermediate between those of spadix from Elk
River, Minnesota, to the west, and noveboracensis from, say, Beaver
Dam, Wisconsin, to the east. The specimens from western Wisconsin show
approach to spadix also in that the length of the tooth-rows and
breadth of the rostrum are slightly greater than in noveboracensis
from farther east, say, Beaver Dam, Wisconsin.
Indeed, animals from as far east as Beaver Dam itself might be thought
of as showing some approach to spadix. Although, along the eastern
seaboard, the upper lips, with rare exceptions, are the same color as
the underparts, farther west, in Michigan and Wisconsin, the lips more
often than not are white. Animals from Beaver Dam have slightly shorter
black tips on the tails, broader extent of the light color of the
underparts and females average slightly larger than typical
noveboracensis, say, those from Massachusetts. Each of these
differences reflects characters found better developed in the
spadix-longicauda stock to the west.
Toward the southern part of its range where noveboracensis meets M.
f. olivacea there is a marked increase in yellowness of the
underparts. This coloration of the underparts, since it is not so well
marked in the northern part of the range of noveboracensis, might be
regarded as showing intergradation with olivacea and primulina,
each of which has far more intensely colored underparts than does
noveboracensis. Excepting this increase of yellow on the underparts,
however, there are few if any characters of noveboracensis which
undergo marked change as approach to the range of olivacea is made.
Indeed, the characters of noveboracensis remain constant to within a
relatively short distance of the geographic range of olivacea.
Notwithstanding the state of affairs described above, intergradation
seems to take place. Three specimens referred to noveboracensis but
which at the same time are regarded as intergrades with olivacea are
as follows: No. 28.300, Charleston Museum, from five miles east of
York, South Carolina, is an adult female with a badly crushed skull. In
external measurements the specimen agrees with noveboracensis. The
underparts, as regards color and width, are intermediate. The general
proportions of the skull and tympanic bullae agree with those of
noveboracensis but the skull is larger than in any female of true
noveboracensis and approaches that of olivacea. The same can be
said of a young female, no. 80, Ohio State Museum, from Roswell,
Georgia.
Another female, no. 171559, U. S. Nat. Mus., from Lookout Mountain,
1500 ft., Fort Payne, Alabama, is barely subadult. The external
measurements are nearer those of olivacea. The color and narrowness
of the underparts are typical of noveboracensis. The proportions and
especially size of the skull show approach to olivacea, though they
are nearer to noveboracensis when all features are taken into
account. In the northern part of its range individuals of
noveboracensis attain larger size than farther south. This tendency
reaches its extreme, in males at least, in M. f. occisor of Maine.
Specimens of noveboracensis from the Adirondacks of New York average
larger (see cranial measurements on page 418) than those from farther
south, and thus approach occisor in size as well as in geographic
position. Also, occasional individuals which strongly show characters
of occisor are found even farther south than the Adirondacks of New
York. This is true of no. 96518, U. S. Nat. Mus., ♂ ad., from
Lunenburg; Massachusetts. The animal has a large skull of relatively
great width much as in occisor, although its external measurements,
relative length of tail and long, terminal, black brush place it with
noveboracensis rather than with occisor. Of a pair of specimens
from Ossipee, New Hampshire, the male, no. 77108, U. S. Nat. Mus., has
a long (175 mm.) tail, and short (60 mm.) black pencil as in occisor,
although otherwise it is referable to noveboracensis. Still another
specimen, a subadult male, no. 4193, Mus. Comp. Zoöl., from Upton,
Maine, has a longer (51 mm.) hind foot than noveboracensis although
it otherwise agrees with that subspecies. As remarked by Bangs
(1899:55), other than fully adult specimens from the range of occisor
are "troublesome," and would not be selected as distinct from
noveboracensis if placed in a series of that subspecies, say, from
New York State. In view of the facts that several specimens from
intermediate localities combine the characters of noveboracensis and
occisor, that noveboracensis in the northern part of its range
averages larger than it does farther south and thus approaches
occisor in size, and that occasional large specimens resembling
occisor in several, but not all, features sometimes crop up in the
northern part of the range of noveboracensis, it appears that
noveboracensis and occisor intergrade. Therefore they are treated
as two subspecies of the single species, Mustela frenata.
Intergradation with M. f. primulina has been commented on in the
discussion of that subspecies. Female, no. 159980, U. S. Nat. Mus.,
from Golconda, Illinois, has many characters of primulina but two
young males from there agree better with noveboracensis.
Examination of 283 adult and subadult skulls for malformation of the
frontal sinuses revealed only ten that were not obviously malformed.
Two were from New York, one from Massachusetts, one from Pennsylvania,
and six from the 52 specimens from Michigan and Wisconsin. In addition,
skulls of many young and even juveniles were malformed.
Specimens examined.—Total number, 555, arranged alphabetically
by states and provinces and, unless otherwise noted, from north to
south by counties in each state. Except as otherwise noted
specimens are in the United States National Museum.
Alabama. DeKalb County: Fort Payne, 1.
Connecticut. Litchfield County: Riverton, 1[5]; Gaylordsville,
1. Hartford County: East Hartford, 4 (3[5]); Glastonbury, 2[5];
South Glastonbury, 4[5]. Windham County: Plainfield, 2 (1[14]).
Fairfield County: Greenwich, 2[2]. New London County: Liberty
Hill, 35 (33[75], 2[7]).
District of Columbia. Washington, 3; near Washington, 1; Eastern
Branch, 1; Congress Heights, 1; Benning, 1; no definite locality,
1.
Georgia. Towns County: Young Harris, 1. Cherokee County:
Canton, 1. Cobb County: Roswell, 1[81].
Indiana. St. Joseph County: Notre Dame, 2[99]. Porter County:
Hebron, 1. Miami County: Denver, 5 (4[75], 1[4]). Wells
County: Bluffton, 1. Howard County: Russiaville, 1. Jay
County: Salamonia, 1[2]. Boone County, 1[2]. Knox County:
Bicknell, 3.
Illinois. Lake County: Camp Logan, 3[60]; Fort Sheridan, 1[60].
Cook County: W Northfield, 2; Flossmoor, 1[60]; no locality more
definite than county, 1. Du Page County: Bloomingdale Spg.,
1[60]. Carroll County: Savanna, 1[87]. McLean County: Normal,
1[7]. Champaign County: Harwood Township, 1[7]. Pike County?:
Milton Spring, 1[60]. Pope County: Golconda, 3.
Kentucky. Woodford County: Midway, 1. Hancock County:
Hawesville, 1.
Maine. Oxford County: Upton, 1[75]; Bethel, 1[74].
Maryland. Howard County: Long Corner, 1; Hanover, 1. Montgomery
County: Gaithersburg, 1; Garret Park, 1; Chevy Chase, 1;
Bethesda, 1. Prince Georges County: Laurel, 18; Plummer Island,
3; Oxon Hill, 1. Talbot County: Easton, 1. Dorchester County:
Cambridge, 5[40].
Massachusetts. Middlesex County: Wilmington, 6; Burlington, 6;
Lexington, 1[75]; Wayland, 2[75]. Berkshire County: New
Marlboro, 1[5]. Worcester County: Lunenburg, 2; Lancaster,
1[75]; Princeton, 2[75]. Norfolk County: So. Weymouth, 1[75].
Plymouth County: Wareham, 5[75].
Michigan. Marquette County: Michigamme, 1. Charlevoix County:
Thumb Lake, 1[76]; 1/2 mi. N Thumb Lake, 1[76]. Leelanau County:
Leland, 3[76]; Duck Lake, 2 mi. S Leland, 1[76]; Lost Pond, 8-1/2
mi. S Leland, 1[76]. Osceola County: Le Roy, 2[76]. Huron
County: Rush Lake, 1[76]. Saginaw County: East Saginaw, 1.
Oakland County: Royal Oak, 4[76]; South Lyon, 1[76]. Livingston
County: Portage Lake, 1[76]. Washtenaw County: Portage Lake,
6[76]; Waterloo, 2[14]; Lima, 1[76]; Ann Arbor, 11[76]; 3 mi. E
Ann Arbor, 1[76]; 2 mi. SE Ann Arbor, 1[76]; 2 mi. S Ann Arbor,
1[76]; 3 mi. S Ann Arbor, 1[76]; Dixboro, 1[76]; Pittsfield, 3
(2[76]); Saline, 1[76]; near Saline, 2[76]; 1 mi. S Saline, 2[76];
York, 2[76]; Manchester, 2[76]. Lenawee County: Morenci, 1[76].
Cass County: Marcellus Township, 1[76]. Berrien County:
Harbert, 1[76]; Warren Wood Preserve, 1[76]; Warren Woods, 1[76].
New Hampshire. Grafton County: Franconia, 1[2]. Carroll
County: South Chatham, 4 (3[5]); Ossipee, 2; Intervale, 1[5].
Merrimack County: Webster, 2[75].
New Jersey. Morris County: Morristown, 1. Essex County: West
Orange, 1[2]. Mercer County: Princeton, 1[1]. Ocean County:
Point Pleasant, 1[2]. Camden County: Haddonfield, 1[1].
Cumberland County: Millville, 2[74].
New York. St. Lawrence County: Ogdensburg, 1[74]. Clinton
County: Rouses Point, 1[80]. County?: Adirondacks, 12. Essex
County: Elizabethtown, 1; Schroon Lake, 1; no locality more
definite than county, 1. Lewis? County: Locust Grove, 4; Lyons
Falls, 1. Warren County: Lake George, 6; Caldwell, 1. Hamilton
County: Beaver Brook, 1/2 mi. above mouth Indian Lake, 1[80].
Oswego County: Scriba, 2[74]; Palermo, 1[74]. Monroe County:
Penfield, 3. Madison County: Peterboro, 6 (2[75]). Schoharie
County: Schoharie, 1[2]. Rensselaer County: East Shodack,
1[80]. Tompkins County: Taughannock Falls, 2[58]; Ithaca, 4
(3[58]); Glenside, Ithaca, 1[58]; 6 mi. Creek, Ithaca, 1[58].
Green County: Lanesville, 1[2]. Orange County: Poplopen's
Pond, 1[2]; Highland Falls, 1[2]. Putnam County, 1[19].
Westchester County: Sing Sing, 4; Armonk, 1[2]; Hastings, 3
(2[2], 1[19]). Nassau County: Flushing Meadows, 1[2]; Flushing,
1[58]; near Flushing, 1[2]; Oyster Bay, 2. Long Island: Cold
Spring Harbor, 1; Bridgehampton, 1[2]. County in question:
Severance, 3; Lake Grove (Long Island?), 1.
North Carolina (east to west by counties). Wake County: Raleigh,
4 (1[2], 1[75], 2[76]). Mitchell County: Magnetic City, foot of
Roan Mountain, 6; Roan Mt., 1; Roan Mt., 6000 ft., 3. Buncombe
County?: Valley of Black Mts., 1[2]. Madison County, 2[11].
Ohio. Trumbull County: Warren, 1[93]. Seneca County: Tiffin,
1[81]. Summit County: Ira, 2[81]. Crawford County: Galion,
1[81]. Ashland County: Loudonville, 1[76]. Auglaize County:
New Bremen, 3[81]. Franklin County: 3 mi. N Columbus, 1[81];
Minerva Park, Columbus, 5[81]. Fairfield County: Sec. 32,
Pleasant Twp., 1[81]; Lancaster, 1[81]. Clinton County:
Reesville, 1; 1/2 mi. S and 1/2 mi. W Wilmington, 2[74]. Pike
County: Waverly, 1[81].
Ontario. Sudbury District: Metagama, 2[86]. Carleton County:
Ottawa, 2[77]. Muskoka County: Lake of Bays, 1; Bracebridge, 1.
Haliburton County: Gooderham, 1[60]. Simcoe County: Orillia, 4
(2[2], 2[60].) Prince Edward County: Bloomfield, 1[77]. York
County: Toronto, 1[2]. Waterloo County: Branchton, 3[60];
Preston, 2[77]; no locality save county, 1[60]. Welland County:
Ridgway, 1[14]. Elgin County: St. Thomas, 1[77]. Essex County:
Kingsville, 1[77]; Point Pelee, 1[77].
Pennsylvania (east to west by counties). Crawford County:
Pymatuning Swamp, 3-1/2 mi. W Linesville, 1[9]; Meadville, 2[9].
Beaver County: Beaver, 1[9]; Raccoon Creek, 1[9]. Butler
County: Mars, 1[9]; Leasuresville, 4[9]. Allegheny County:
Allegheny, 1. Warren County: Bear Lake, 2[9]. Westmoreland
County: Kingston, 1[9]; Laughlinstown, 2[9]. Somerset County:
Confluence, 1[9]; Tub Mill Run, 2 mi. N Springs, 1[9]. Jefferson
County: Siegel, 1[9]. Clearfield? County: Penfield, 1[9].
Cambria County: Cresson, 1[9]. Fulton County: Well's Tannery,
1[9]. Clinton County: near Round Island, 2[1]. Cumberland
County: Carlisle, 1. Snyder County: 5 mi. S Selinsgrove, 1.
Northumberland County: Pottsgrove, 1. Union County:
Mifflinburg, 1. Sullivan County: Lopez, 7 (4[1], 3[74]).
Chester County: Westtown, 1[1]; Valley Forge, 1[1]; W Bradford
Township, 1[1]; no locality more definite than county, 3.
Philadelphia County: Holmsburg, 2[1]. Bucks County: 1[1].
Pike County: Milford, 1.
Rhode Island. Providence County: Chepachet, 1. Washington
County: Lake Warden, 2.
Quebec. Megantic County: Black Lake 1[77]. County in question:
Meach Lake, 1[77].
South Carolina. York County: 5 mi. E York, 1[11]. Laurens
County: Laurens, 1[39].
Tennessee. Campbell County: Highcliff, 1. Carter? County:
Roan Mts., 1[2]. Hamilton County: Walden Ridge, near Soddy, 3.
Vermont. Windsor County: Hartland, 1[2].
Virginia. Shenandoah County: Toms Brook, 1. Arlington County:
Arlington, 1; Ballston, 1; Alexandria, 1. Fairfax County: Falls
Church, 3; Mt. Vernon, 2; no locality more definite than county,
1. Prince William County: Occoquan, 1. Essex County: Montague,
1. Prince George County, 1. Norfolk County: Wallaceton, 1.
Grayson County: Mt Rogers, 3. County in question: Dismal
Swamp, 1; Massanutten Mt., 1.
West Virginia. Hardy County, 1. Pendleton County: radius of 2
mi. Smoke Hole, 1[74]. Greenbriar County: White Sulphur, 2[60].
Wisconsin. Douglas County: Gordon, 1[102]. Vilas County: Mamie
Lake, 4. Dunn County: Colfax, 4[102]; Meridean, 1[102]. Door
County: state game farm, 17[102]; no locality more definite than
county, 1[102]. Dodge County: Rolling Prairie, 1[50]; Beaver
Dam, 52[50]. Dane County: Wingra Lake, 1[102]. Waukesha
County: Pewaukee, 2[102]. Racine County: Waterford Township,
2[102]. Rock County: Milton, 1[102]; Bowers Lake, 1[102].
Walworth County: Lane's Mill, 8 mi. N Elkhorn, 7 (1[102],
6[54]); Delavan, 7.
Mustela frenata occisor (Bangs)
Long-tailed Weasel
Plates 16, 17, 18, 31, 32 and 33
Putorius occisor Bangs, Proc. New England Zool. Club, 1:54, June
9, 1899.
Mustela occisor, Miller, U. S. Nat. Mus. Bull., 79:98, December
31, 1912.
Mustela frenata occisor, Hall, Carnegie Instit. Washington Publ.
473:104, November 20, 1936.
Type.—Male, adult, skull and skin; no. 9102, coll. of E. A. and
O. Bangs in Mus. Comp. Zoöl.; Bucksport, Hancock County, Maine;
January 15, 1899; obtained by Alvah G. Door but measured and sexed
by O. Bangs.
The skin is well made and in good condition. It is in full, white
winter-dress with black-tipped tail. The skull has the posterior
half of the left zygomatic arch broken away; otherwise the skull
is unbroken and complete. Left I3 and right P3 are missing. The
teeth otherwise all are present and entire.
Range.—Maine; possibly north locally to south side of St.
Lawrence River in Quebec and possibly occurring in western New
Brunswick. Zonal range Canadian and probably Transition. See
figure 29 on page 221.
Characters for ready recognition.—Differs from M. f.
noveboracensis by a number of average differences including
larger size, relatively longer tail and relatively wider skull
(see page 225, and measurements on pages 418, 419).
Description.—Size.—Male: Five adults yield average and
extreme measurements as follows: Total length, 443 (430-465);
length of tail, 163 (154-175); length of hind foot, 50 (47-54).
Tail averages 58 per cent as long as head and body. Length of hind
foot averages more than basal length.
Female: Measurements of two subadult female topotypes are as
follows: Total length, 346, 318; length of tail, 116, 110; length
of hind foot, 39, 35.5.
Tail amounts to 50 per cent and 54 per cent of body-length
respectively. Length of hind foot more or less than (about equal
to) basal length.
The average differences in external measurements of the two sexes
are: Total length, 111; length of tail, 50; length of hind foot,
12.5.
Externals.—As described in Mustela frenata noveboracensis.
Color.—As described in Mustela frenata noveboracensis except
that black tail-tip in series of 10 males in full winter pelage 60
(45-80) mm. long; thus averaging 39 per cent of length of tail
vertebrae.
Skull and teeth.—Male (based on 3 adults): See measurements and
plates 16-18. As described in Mustela frenata noveboracensis
except that: Weight, 4.2 (4.1-4.3) grams; basilar length, 45.7
(44.9-46.9); zygomatic breadth more or less than (about equal to)
distance between anterior palatine foramen and anterior margin of
tympanic bulla; least width of palate rarely less than length of
P4; anterior margin of masseteric fossa behind or directly below
posterior half of m2.
Female (based on 2 subadults): See measurements and plates 31-33.
As described in Mustela frenata noveboracensis except that:
Weight, 2.0 (1.9-2.1) grams; basilar length, 37.3, 38.2.
Comparison of the skull with that of M. f. noveboracensis is
made in the account of that subspecies.
Remarks.—Excepting a specimen in the Academy of Natural Sciences of
Philadelphia, obtained in 1893, and two in the Boston Society of
Natural History, obtained in 1925, I have seen no material of this
subspecies in addition to that examined by Bangs at the time he
prepared the original description in 1899.
Anderson (1945:56, 57) records a specimen, Canadian National Museum
Catalogue Number 18426, from Kamouraska County, Quebec, as of this
subspecies and thinks that occisor occurs north of Maine "locally to
south side of lower St. Lawrence River in Quebec; probably also in
western New Brunswick."
So far as the available material of occisor permits one to judge, it is
distinguished from noveboracensis by a combination of characters no
one of which invariably can be relied upon as diagnostic. Employing
adult males, average differences indicate that M. f. occisor is
larger in each of the external and cranial measurements; tail
relatively longer; black tip of tail relatively shorter; mastoid and
zygomatic breadth relatively greater and zygomatic arches more nearly
square posteriorly.
Considering the large number of specimens of noveboracensis which are
available in comparison with the few of occisor it is not surprising
that some noveboracensis should be found which exceed in size those
of occisor. This is the case as regards the basilar length of a very
old male, no. 96518, U. S. Nat. Mus., from Lunenburg, Massachusetts.
Also, the skull is actually broader than any of those of occisor.
However, this specimen is much older than any occisor examined. In a
female, no. 4260, Mus. Comp. Zoöl., from Liberty Hill, Connecticut, the
skull is longer (but narrower) than in either of the two available
females of occisor.
The average differences pointed out above which characterize this
extreme northern population of noveboracensis-like weasel in
comparison with true noveboracensis without much question are
geographic variations. Whether or not these variations are of a degree
sufficient to warrant nomenclatural recognition is debatable. With
equally scanty material from other regions I have not named variations
seemingly as great as those shown by occisor. The paucity of material
of occisor is a handicap in making a decision in this instance.
Each of the adult and subadult specimens, except the one from Perry,
shows malformation resulting from the infestation of the frontal
sinuses with parasites.
Specimens examined.—Total number, 18, listed by counties from
west to east and unless otherwise indicated in the Museum of
Comparative Zoölogy.
Maine. Oxford County: South Andover, 2 (Boston Soc. Nat. Hist.);
Umbagog Lake, 1. Franklin County: Seven Pd. Township, 1.
Piscataquis County: Grenville, [= Greenville?], 1. Hancock
County: Bucksport, 10. Washington County: 3rd Mopang Lake, 1
(Acad. Nat. Sci. Phila.); Perry, 1 (Boston Soc. Nat. Hist.).
County in question: Moosehead Lake, 1.
Mustela frenata primulina Jackson
Long-tailed Weasel
Plates 16, 17, 18, 31, 32 and 33
Mustela primulina Jackson, Proc. Biol. Soc. Washington, 26:123,
May 21, 1913.
Putorius noveboracensis, Baird, Mamm. N. Amer., p. 166, 1858
(part).
Mustela longicauda longicauda, Dice, Journ. Mamm., 4:108, May 9,
1923.
Mustela longicauda primulina, Linsdale, Journ. Mamm., 9:141, May
9, 1928.
Mustela frenata primulina, Hall, Carnegie Instit. Washington
Publ. 473:104, November 20, 1936.
Mustela frenata, Leopold and Hall, Journ. Mamm., 26:143, July 19,
1945.
Type.—Male?, young, skull and skin; no. 168006, U. S. Nat.
Mus., Biol. Surv. Coll.; 5 miles northeast of Avilla, Jasper
County, Missouri; May 11, 1905; obtained by Hartley H. T. Jackson;
original no. 7869X.
The skin lacks the distal part of the tail—the part which bears
the black tip. Otherwise the skin is complete and well preserved.
The teeth of the permanent dentition all are present and entire.
The lower jaws are complete and unbroken. The skull is broken
transversely through the interorbital region, transversely through
the braincase and longitudinally through the basioccipital. Both
zygomatic arches are gone. The type is judged to be a male rather
than a female as stated by the original describer, Jackson
(1913:123), whose measurements of hind foot, interorbital
constriction, maxillary tooth-row, and mandibular tooth-row agree
with those of males and are larger than those of any female seen
of this subspecies.
Range.—Upper and Lower Austral life-zones west of the
Mississippi River in Missouri and Arkansas, the southeastern half
of Iowa, eastern half of Kansas and Oklahoma, northern Louisiana
and northeastern Texas. Southern and southwestern limits of range
undetermined. See figure 29 on page 221.
Characters for ready recognition.—Differs from M. f.
noveboracensis in males by interorbital breadth averaging less
than 24 per cent of basilar length, orbitonasal length averaging
less than 34 per cent of basilar length or 64 per cent of mastoid
breadth, tympanic bullae as much inflated anteromedially as
posteromedially, and in females by orbitonasal length amounting to
less than two-thirds of mastoid breadth, by zygomatic breadth
averaging more than 21 mm., and by anterolateral margin of
tympanic bulla projecting below squamosal; from M. f. spadix by
least width of color of under parts amounting to less than 40 per
cent of greatest width of color of upper parts, by absence of
color of underparts on hind leg below knee, and by smaller size
(hind foot less than 50 in males and 40 in females; orbitonasal
length less than 15.5 in males and 13.5 in females; length of
tooth-rows less than 18 in males and 15.7 in females; mastoid
breadth less than 25.5 in males and 22 in females); from M. f.
longicauda by Brussels Brown rather than near (h) Clay Color of
upper parts, least width of underparts less than 40 per cent of
greatest width of color of upper parts, absence of color of
underparts on hind leg below knee, zygomatic breadth less than
28.8 in males and 24.1 in females; from M. f. neomexicana by
Brussels Brown rather than Buckthorn Brown color of upper parts,
in absence of white frontal spot and broad white bands on sides of
head, in anterolaterally rounded, rather than "square," tympanic
bullae and in zygomatic breadth of less than 30 in males and 24 in
females; from M. f. frenata and M. f. texensis by absence of
white facial markings and smaller size (basilar length of adult
males less than 47, tail-length less than 155 in males, and hind
foot less than 40 in females); from M. f. arthuri by less evenly
spreading zygomatic arches (see pls. 16, 17 and 18), greater
inflation of the tympanic bullae anteromedially and more nearly
straight (less convex) dorsal outline of skull.
Description.—Size.—Male: Eighteen adults and subadults from
Douglas County, Kansas, yield average and extreme measurements as
follows: Total length, 397 (371-440); length of tail, 133
(120-147); length of hind foot, 43 (40-47). Tail averages 50 per
cent as long as head and body. Length of hind foot averages less
than basal length. Corresponding measurements, originally taken in
inches and fractions thereof, of 9 adults and subadults from Boone
County, Arkansas, are as follows: 413 (384-438); 138 (127-155); 41
(38-44).
Female: Six adults and subadults from Douglas County, Kansas,
yield average and extreme measurements as follows: Total length,
339 (317-355); length of tail, 107 (95-115); length of hind foot,
35 (34-37). Tail averages 46 per cent of length of head and body.
Length of hind foot less than basal length. Corresponding
measurements, originally taken in inches and fractions thereof, of
5 adults and subadults from Boone County, Arkansas, are as
follows: 355 (332-397); 113 (101-127); 33 (29-38).
The average differences in external measurements of the two sexes,
in Douglas County, Kansas, are: Total length, 58; length of tail,
26; length of hind foot, 8. An adult male from Boone Co., Iowa,
weighed 293 grams.
Externals.—Longest facial vibrissae black or dark brown (often
both colors in same specimen) and extending beyond ear; carpal
vibrissae colored either like underparts or upper parts and
extending to apical pad of fifth digit; hairiness of foot-soles as
shown in figure 20.
Color.—Upper parts, in summer, Brussels Brown to near (14 n)
Brussels Brown, or tones 2 to 4 of Raw Umber of Oberthür and
Dauthenay, pl. 301. Chin and rarely upper lips white. Remainder of
underparts Picric Yellow to Primuline Yellow. In winter, color
essentially the same except for smoke-gray effect in upper parts
and more whitish in underparts. Tip of tail at all times black.
Upper parts of uniform color except for occasional darkening of
nose and mid-dorsal region. Color of underparts extends distally
on posterior sides of forelegs onto antipalmar faces of toes, on
medial sides of hind legs only to a point between knee and ankle.
Least width of color of underparts averaging, in a series of 21
males from Lawrence, Kansas, 23 (9-35) per cent of greatest width
of color of upper parts. Black tip of tail in same series, most of
which are in full winter pelage, 52 (40-70) mm. long; thus longer
than hind foot and averaging 39 per cent of length of
tail-vertebrae.
Skull and teeth.—Male (based on ten adults from Douglas County,
Kansas): See measurements and plates 16-18; weight, 3.7 (3.3-4.2)
grams; basilar length, 44.8 (43.8-46.0); zygomatic breadth more or
less (less in 80 per cent) than distance between condylar foramen
and M1 or than between anterior palatine foramen and anterior end
of tympanic bulla (less in 70 per cent); mastoid breadth more or
less (less in 80 per cent) than postpalatal length; postorbital
breadth less than length of upper premolars and, except in one
specimen, more than width of basioccipital measured from medial
margin of one foramen lacerum posterior to its opposite;
interorbital breadth more or less (less in 70 per cent) than
distance between foramen opticum and anterior margin of tympanic
bulla; breadth of rostrum less than length of tympanic bulla;
least width of palate less than outside length of P4 (except in
one specimen); anterior margin of tympanic bulla as far posterior
to foramen ovale as width of 2-1/2 to 5 upper incisors; height of
tympanic bulla more (except in one specimen) than distance from
its anterior margin to foramen ovale; length of tympanic bulla
more than length of lower molar and premolar tooth-row and longer
(except in one specimen) than rostrum; anterior margin of
masseteric fossa behind or just below posterior eighth of m1.
Female (based on 5 adults and subadults from Douglas County,
Kansas): See measurements and plates 31-33; weight, 2.2 (2.0-2.4)
grams; basilar length, 38.9 (37.6-40.7); zygomatic breadth less
than distance between condylar foramen and M1 or than between
anterior palatine foramen and anterior margin of tympanic bulla;
postorbital breadth less than length of upper premolars and except
in one specimen, more than width of basioccipital measured from
medial margin of one foramen lacerum posterior to its opposite;
least width of palate less than greatest length of P4; tympanic
bulla as far posterior to foramen ovale as width of 3 to 5 upper
incisors; height of tympanic bulla more or less than distance from
its anterior margin to foramen ovale; length of tympanic bulla
more than length of lower molar and premolar tooth-row and longer
than rostrum.
The skull of the female averages 41 per cent lighter than that of
the male.
Compared with the skull of M. f. noveboracensis from Massachusetts,
that of the male of primulina, in dorsal view, is seen to be shorter
anteriorly to the postorbital processes and to have a more marked
postorbital constriction. In lateral view the dorsal outline of the
skull of primulina is less concave in the postorbital region. In
ventral view the skull of primulina is seen to be wider across the
mastoid processes and zygomatic arches but the most pronounced
difference is in the tympanic bullae. In noveboracensis each bulla is
scooped out on the anterior part of the medial face and appears to be
narrower anteriorly than posteriorly whereas in primulina the
anterior part of the medial face is not scooped out but is moderately
inflated and the bulla appears to be of uniform breadth anteriorly and
posteriorly. By actual measurement the breadth of the bulla averages 59
per cent of its length in primulina but only 50 per cent in
noveboracensis. Other respects in which the skull of the male of
primulina differs from that of noveboracensis are as follows:
Linear measurements of teeth more; relative to the basilar length, the
length of the tooth-rows averages more, whereas the interorbital
breadth and orbitonasal length are less.
When skulls of females are compared, each of the differences mentioned
above is found to apply, except that the degree of difference is in
some parts greater, for example, in the tympanic bullae. In
primulina, the bulla is in general like that of the male
noveboracensis, whereas in the female noveboracensis it is less
inflated, especially anteromedially, shorter, relatively narrower, and
in ventral view projects little or none below the squamosal floor of
the braincase. The breadth of the bulla averages 51 per cent of its
length in primulina but only 47 per cent in noveboracensis. The
bullae project below the basioccipital on the average, for a distance
of 2.9 millimeters in female primulina and only 2.3 millimeters in
female noveboracensis. In primulina the temporal ridges are well
developed and fuse to form a low sagittal crest, but in
noveboracensis the ridges are absent. Also, in primulina the
mastoid processes project farther laterally beyond the braincase. The
skull of female noveboracensis is much lighter than that of
primulina. Average weights of the two are 1.7 and 2.2 grams. The
skulls of females of primulina and noveboracensis differ more than
do the skulls of males.
Compared with the skull of spadix, that of the male, and the female,
of primulina averages smaller in every part measured. Expressed in
percentages of the basilar length, the two depth measurements of the
skulls are not significantly different, but, excluding the measurements
of the bullae and teeth, the other cranial measurements are less. The
main difference in relative proportions is in the tympanic bullae which
average only a half millimeter shorter in males of primulina and one
and one-tenth millimeters shorter in females. The bullae are,
therefore, relative to the basilar length, longer in primulina. The
skull of primulina, then, differs from that of spadix mainly in
smaller size and relatively longer tympanic bullae, especially in
males.
Compared with the skull of M. f. longicauda, that of both sexes of
primulina averages smaller in every part measured, except in males
where the length of the tympanic bulla, and breadth and length of M1
are the same or slightly larger in primulina. Relative to the basilar
length, the length of the tympanic bullae, and in females only, the
depth measurements are greater in primulina but all the others, in
both sexes, are less. These ratios reflect the relative narrowness of
the skull of primulina. Upon direct comparison the narrowness is
especially noticeable in the interorbital region, mastoid region,
tympanic bullae, and across the zygomata.
Compared with the skull of M. f. neomexicana that of both sexes of
primulina averages smaller in every part measured. Excepting the
measurements of the teeth, most of the other measurements are
constantly larger. Relative to the basilar length, the length of
tooth-rows and length of tympanic bulla are more, but excepting the
depth measurements, the others are less. Still other differences are,
in primulina, less well-developed sagittal crest, anterolateral
corner of bulla rounded rather than "square," and in males a
transversely convex rather than flat interorbital region.
Compared with M. f. frenata and M. f. texensis, the skulls of males
of primulina differ in being smaller in every part measured but
relative to the basilar length, have longer tooth-rows, a lesser
zygomatic breadth and are less constricted interorbitally.
Compared with the skull of M. f. olivacea, those of both sexes of
primulina average smaller in every part measured, have shallower
(dorsoventrally) tympanic bullae, a lower sagittal crest and slightly
weaker postorbital processes on the frontals. Relative to the basilar
length, the several cranial measurements are about the same.
Comparison of the skull with that of M. f. arthuri has been made in
the account of that subspecies.
Remarks.—The first specimens of this race known to have been
preserved in study collections are one in the United States National
Museum, taken at Bridge, Carroll County, Missouri, many years ago by J.
Burbage, and less than a dozen specimens preserved before 1900 from
eastern Kansas in the University of Kansas Museum of Natural History.
In 1913 Hartley H. T. Jackson bestowed a name on this animal on the
basis of two specimens taken by him in southwestern Missouri. Later,
through the efforts of Charles D. Bunker, and his associates at the
University of Kansas, nearly 100 specimens were saved from eastern
Kansas, principally from Douglas County. In the course of the present
study, Lawrence V. Compton obtained a topotype for the California
Museum of Vertebrate Zoölogy, and with the assistance of Mr. B. G.
Roberts, a good series of specimens from Boone County, Arkansas, was
preserved in the same museum. In the early years of the 20th Century,
the late B. H. Bailey at Coe College, Iowa, collected specimens from
that state. The specimens from these several sources suffice to give a
relatively clear idea of the characters of this subspecies.
Mustela frenata primulina is closely related to M. f.
noveboracensis, from which, on the average, it differs in the lighter
color of the upper parts of the summer coat, in the more intense
coloring of the underparts, and in certain cranial features pointed out
above. In the southern part of its range, however, noveboracensis has
the underparts only a little less intensely colored with yellow than
primulina. Also, the skull of the one topotype from 7-1/2 miles
southeast of Carthage, a subadult male in brown, winter pelage, is
almost exactly intermediate between that of noveboracensis from
Massachusetts and primulina of Douglas County, Kansas, and Boone
County, Arkansas. M. f. primulina often has the underparts white in
winter, as does this topotype which agrees with the average of
noveboracensis in small size of teeth and narrowness across the
mastoid processes and zygomatic arches. However, it agrees with
primulina in shape and relative size of the rostrum. It is almost
exactly intermediate in shape and width of the tympanic bullae.
Three other males, but no females, all in winter pelage, are available
from eastern Missouri. Of the two from Silex, Lincoln County, one is
nearer noveboracensis and the other nearer primulina on the basis
of cranial characters. The third specimen, from four miles south of
Lesterville, so far as I can determine by examination of individual
cranial characters and tabulation of results, is exactly intermediate.
Final decision on the proper allocation of specimens from the parts of
Missouri represented can best be made when skulls of females are
available. From the fact that the skull of the female referred to
noveboracensis from Golconda, Illinois, shows almost as many
characters of primulina as of noveboracensis, it is judged that
females from as far west as Silex and Lesterville, Missouri, will show
even more characters of primulina and so be referable to that form.
If this supposition be correct, the present reference of the almost
exactly intermediate males, from eastern Missouri, will stand;
otherwise, it may not.
Additional intergrades with noveboracensis are available from eastern
Iowa. Of five specimens from Hillsboro, Iowa, two males and a female
have tympanic bullae like those of primulina but the other two males
have bullae like those of noveboracensis. The female is smaller than
primulina and in this small size and in general configuration of the
skull, viewed dorsally, is more nearly like noveboracensis. As a
whole, the population averages almost exactly intermediate. The same is
true of 3 males and one female from Muscatine. The subadult male from
Keosaqua, to my eye, resembles noveboracensis in the greater length
of the skull anteriorly to the postorbital processes, and in the
relative narrowness across the mastoidal region, but otherwise is more
like that of primulina. Two males and one female from Tipton,
although in each instance variously intermediate, are as a whole nearer
primulina, No. 2865, Coe College, male adult, from Cedar Rapids, has
characters of the three races, spadix, noveboracensis and
primulina. In the skull, the width suggests spadix, the narrow
mastoid region, noveboracensis, and the tympanic bullae are as in
spadix or primulina. One male, no. 12, Coe College, from Dubuque,
is as narrow across the mastoid region as is noveboracensis although
the bullae are well inflated as in primulina. The skull, without
corresponding skin, of a female, no. 140a, Iowa State College, from
Green's Island, also resembles noveboracensis in narrowness of the
mastoidal region, and in small size of skull, but in larger teeth,
broader tympanic bullae, and sagittal crest is referable to
primulina. Of two females from Vinton, one adult is typical of
primulina but the other, a subadult, is practically indistinguishable
from female noveboracensis, from Ann Arbor, Michigan. Three males
from Vinton agree well with primulina except that the interorbital
region is wider than average and thereby suggests spadix or
noveboracensis. An adult female from New Hartford also is typical of
primulina except for the broader interorbital region. Three males
from Fayette are typical of primulina.
Other specimens from Iowa are intergrades with spadix, or if not with
spadix, with the animal of northwestern Iowa which in some ways
combines the characters of longicauda and spadix. For example, no.
2665, Coe College, an adult male from Davenport, has the anterior part
of the skull (all that is preserved) heavily ridged as in spadix and
in addition, the underparts are marked with the shade of reddish
displayed by topotypes of spadix and with some yellowish as seen in
longicauda. The color pattern, however, is as in primulina. A young
male, no. C-51, Iowa State College, from Kelley, Story County, has
anteriorly truncate bullae as are more frequently found in the
longicauda-spadix stock of northwestern Iowa, than in primulina. In
other respects, the animal, in so far as can be judged from the broken
skull, agrees with primulina as it certainly does in color, color
pattern, and external measurements. An adult male, no. 499a, Iowa State
College, from 2 miles east of Ledges St. Park, in Boone County, in
short body, size of teeth, and size of skull, in so far as the broken
parts can be measured, resembles primulina more closely than it does
any other subspecies. The long tail, long hind foot, wide extent of the
light-colored underparts, and extension of the color of the underparts
onto the hind feet are more as in spadix. Other intergrades with
spadix from Iowa are mentioned in the account of spadix.
The specimen from Swartz, Louisiana, suggests intergradation with
arthuri in that the anteromedial part of the tympanic bulla is less
inflated than in typical primulina.
Intergrades with longicauda are available from Riley and Pratt
counties, Kansas. No. 7182, Univ. Kans., subadult male in winter
pelage, from near Winkler, has a skull of larger size as in
longicauda with which race it seems to agree in large size of body,
tail and hind foot, although the collector's measurements are lacking.
Color pattern and relative proportions of the skull throughout are as
in primulina. The young male, no. 3495, Univ. Kans., from Pratt,
Kansas, agrees in external measurements and large size of skull with
longicauda, but has the color and color pattern precisely as in
primulina. The teeth are smaller as in primulina. Immaturity
prevents judging of its relationships on the basis of relative
proportions of the skull.
The two specimens, skins only, available from Oklahoma, are
provisionally referred to primulina. These are remarkable for the
restriction of the color of the underparts and for the intensity of the
yellow coloration of the underparts. The specimen from Norman has the
color of the underparts entirely absent from the hind legs and not
extending posteriorly to the penis. On the chest and lower throat,
large spots of color of the upper parts are present and the yellow area
of the underparts on the belly is narrower than in any other specimen
of primulina examined. The specimen from 8 miles northwest of
Stillwater has the color of the underparts only a little less
restricted although this color does extend over the inguinal region
almost to the knees. The skin of the posterior part of the body of a
weasel is available from 10 miles south of Sulphur Springs, Texas. It,
likewise, is only provisionally referred to primulina. The coloration
is about as in the specimens from Oklahoma but the distribution of the
color of the underparts cannot be made out.
The dark color of the upper parts occurs far westward in animals which
otherwise display characters of longicauda. Among these intergrades,
the larger size of longicauda generally is combined with this dark
color. This geographic behavior of the dark color of the upper parts is
analogous to the condition described in M. f. spadix. Stated in
another way, the dark color of the upper parts is the character, of the
eastern animal, last to disappear as one goes westward across the
Mississippi Valley toward the range of longicauda which is a
subspecies of markedly different size, shape of skull, and coloration.
Only two of 29 specimens from Kansas show infestation of the frontal
sinuses. All four of the specimens from Missouri have the frontal
sinuses malformed as do 9 of the 14 from Arkansas examined in this
respect.
An adult female from Boone County, Iowa, bears the date May 9, 1938,
and the annotation by T. G. Scott, "Fox-killed."
Specimens examined.—Total number, 131, arranged alphabetically
by states and from north to south by counties in each state.
Except as otherwise indicated, specimens are in the University of
Kansas, Museum of Natural History.
Arkansas. Boone County: 3 mi. E Bergman, 4[74]; 3 mi. SE
Bergman, 1[74]; 3 mi. S Bergman, 1[74]; 3 mi. SW Bergman, 1[74]; 4
mi. SE Bergman, 2[74]; 5 mi. SE Bergman, 1[74]; 4-1/2 mi. SE
Bergman, 3[74]; 5 mi. SE Bergman, 1[74]; 5 mi. S Bergman, 2[74]; 5
mi. SW Bergman, 2[74]. Washington County: Fayetteville, 1[96].
Crawford County: 10 mi. S Winslow, 1. Sebastian County: Fort
Smith, 1[91].
Iowa. Fayette County: Fayette, 3[12]. Dubuque County: Dubuque,
1[12]; Green's Island, 1[65]. Butler County: New Hartford,
1[12]. Hardin County: Union, 1[65]. Benton County: Vinton,
5[12]. Linn County: Cedar Rapids, 1[12]. Boone County: Worth
Township, Sec. 21, 1[65]; 2 mi. E Ledges St. Park, 1[65]. Story
County: Kelley, 1[65]. Cedar County: Tipton, 3[12]. Scott
County: Davenport, 2[12]. Muscatine County: Muscatine, 4[12].
Henry County: Hillsboro, 5[91]. Van Buren County: Keosaqua,
1[65]; no locality more definite than county, 1[50]. Taylor
County, 1.
Kansas. Riley County: near Winkler, 1. Pottawatomie County:
Onaga, 1[83]. Atchison County: Doniphan Lake, 1; 5 mi. NE
Muscotah, 1; no locality more definite than county, 1. Douglas
County: Lawrence, 8; 6 mi. NW Lawrence, 1; 1-1/2 mi. W Lawrence,
1; 6 mi. S Lawrence, 1; 7 to 7-1/2 mi. SW Lawrence, 14; 10 mi. W
Lawrence, 1; Clinton, 4; Baldwin, 1; no locality more than county,
29 (2[74]). Woodson County: 1-1/2 mi. S Neosho Falls, 1[59].
Greenwood County: 8 mi. SW Toronto, 2. Pratt County: Pratt, 1.
Louisiana. Quachita Parish: Swartz, 1[71].
Missouri. Carroll County: Bridge Creek, 1[91]. Lincoln County:
Silex, 1[74]; 1 mi. E Silex, 1[74]. Reynolds County: 4 mi. S
Lesterville, 1[74]. Jasper County: 5 mi. NE Avilla, 1[91]; 7-1/2
mi. SE Carthage, 1[74].
Oklahoma. Payne County: 8 mi. NW Stillwater, 1[82]. Cleveland
County: Norman, 1[100].
Texas. Hopkins County: 10 mi. S Sulphur Springs, 1[43].
Mustela frenata arthuri Hall
Long-tailed Weasel
Plates 16, 17 and 18
Mustela noveboracensis arthuri Hall, Proc. Biol. Soc. Washington,
40:193, December 2, 1927.
Mustela frenata arthuri Hall, Carnegie Instit. Washington Publ.
473:105, November 20, 1936.
Type.—Male, subadult, skull and skin; no. 37515, Mus. Vert.
Zoöl.; Remy, St. James Parish, Louisiana; December 15, 1926;
obtained by Stanley C. Arthur.
The skin is stuffed and well preserved. The skull (plates 16-18)
is unbroken. The teeth all are present and entire. The presence of
a well-developed scrotal pouch shows the specimen to be a male.
Contrary to what was stated in the original description the
specimen was taken in 1926 and not in 1925.
Range.—Lower Austral Life-zone of southeastern Texas,
Louisiana, and into Mississippi. See figure 29 on page 221.
Characters for ready recognition (of males).—Differs from M.
f. olivacea in smaller size (adult males with hind foot and
basilar length less than 45), depth of skull at anterior margin of
basioccipital, ignoring sagittal crest, amounting to more than 63
per cent of mastoid breadth, and greater convexity of dorsal
outline of skull in longitudinal axis (see pls. 16-18); from M.
f. noveboracensis, in males, by zygomatic breadth not less than
distance between anterior palatine foramen and anterior margin of
tympanic bulla and by convex dorsal outline of skull in
longitudinal axis; from M. f. primulina by evenly spreading
zygomatic arches, lesser inflation of tympanic bullae
anteromedially than posteromedially, and convex dorsal outline of
skull in longitudinal axis; from M. f. texensis and M. f.
frenata by absence of white facial markings and postorbital
breadth more than distance between posterior borders of P4 and P2.
Description.—Size.—Male: The type, a subadult male, measures
(inches and quarter fractions thereof, transposed into
millimeters) as follows: Total length, 390; length of tail, 113;
length of hind foot, 44. Tail is 41 per cent as long as head and
body. Length of hind foot less than basal length.
Typical female unknown.
Externals.—Longest facial vibrissae black, or dark brown (both
colors in the type) and extending beyond ear; carpal vibrissae
same color as underparts and extending to within 3.5 millimeters
of apical pad of fifth digit. Hairiness of foot-soles in type
slightly less than shown in figure 20.
Color.—Upper parts in summer tone 4 of Burnt Umber of Oberthür
and Dauthenay, pl. 304; underparts as described in M. f.
olivacea. In winter, upper parts (based on type) near (1)
Brussels Brown or grayer than tone 4 of Burnt Umber of Oberthür
and Dauthenay, pl. 304, darker on top of head from nose to a line
connecting posterior margins of ears. Chin and posterior third of
each upper lip white. Remainder of underparts white with wash of
Warm Buff. Tip of tail black. Color of underparts extends distally
on posterior sides of forelegs over toes but represented on
antipalmar faces of feet by only a few scattered hairs. Color of
underparts extends distally on medial sides of hind limbs only to
knees. Least width of color of underparts amounting to 15 per cent
of greatest width of color of upper parts. Black tip of tail 50
mm. long; thus longer than hind foot and 44 per cent as long as
tail-vertebrae.
Skull and teeth.—Male (based on type and 2 subadults): See
measurements and plates 16-18. As described in M. f.
noveboracensis except that: Weight, 4.0 (3.7-4.3) grams; basilar
length, 43.5 (43.3-43.6); zygomatic breadth not less than distance
between anterior palatine foramen and anterior margin of tympanic
bulla; postorbital breadth more than length of upper premolars;
interorbital breadth more than distance between foramen opticum
and anterior margin of tympanic bulla; least width of palate more
or less than length of P4; tympanic bulla longer than rostrum.
Female: Typical skull unknown. The skull from 12 miles east of
Eagle Lake, Texas, lacks the convexity in the dorsal longitudinal
axis and the skull agrees with those of larger individuals of
primulina except that the anteromedial faces of the tympanic
bullae are less inflated, and the mastoid and zygomatic breadths
are greater than in any female seen of primulina. Probably this
greater breadth is the result of intergradation with M. f.
frenata to the westward.
Compared with the skull of M. f. olivacea that of arthuri differs
as follows: Averaging smaller in every part measured; basilar length 5
mm. less; by weight a fourth lighter; relative to basilar length,
interorbital breadth greater and zygomatic and especially mastoid
breadth less; dorsal outline of skull more convex in longitudinal axis;
tympanic bullae narrower and less inflated especially on anteromedial
faces. Compared with the skull of noveboracensis that of arthuri
has the zygomatic breadth equal to or exceeding the distance from the
anterior palatine foramen to the anterior margin of the tympanic bulla,
whereas the zygomatic breadth is less than this distance in
noveboracensis. Also, in arthuri, the rostrum is relatively
shorter, the braincase is more inflated anteriorly, the zygomatic
arches are more uniformly spreading, and the dorsal outline of the
skull is distinctly convex, both transversely and longitudinally,
whereas it is transversely more nearly flat in noveboracensis and
longitudinally is concave in the interorbital region.
Compared with M. f. primulina, arthuri has narrower bullae, which
are much less inflated on their anteromedial faces, a less marked
postorbital constriction, a braincase which is narrower across the
mastoid region and broader anteriorly, and a skull, which, in
longitudinal axis, has the dorsal outline markedly more convex.
Compared with the skull of M. f. texensis that of arthuri is
smaller in every part measured; length one-fifth less; one-half as
heavy; postorbital constriction less marked; braincase relatively
narrower posteriorly and tympanic bullae less inflated especially
anteromedially. Compared with the skull of M. f. frenata that of
arthuri is smaller in every part measured; basilar length 6 mm. less;
a third lighter; postorbital constriction less marked; relative to the
basilar length the rostrum is broader, longer and deeper; the zygomatic
expanse and breadth of the braincase across the mastoids is less; the
dorsal profile of the skull is more convex in longitudinal axis;
zygomata evenly spreading rather than abruptly protruding from skull
posteriorly; tympanic bullae less inflated anteromedially.
Remarks.—In 1926, Stanley C. Arthur, then Director of the Division
of Wild Life, for the Louisiana State Department of Conservation,
obtained specimens of this weasel. Some of them were mounted and the
remainder were placed in the collections of the United States National
Museum and the Museum of Vertebrate Zoölogy. In 1938 to 1940 George H.
Lowery saved specimens from Baton Rouge, which showed the color of the
summer pelage and revealed that the size of males was more than was
indicated by the original materials. In 1940 and 1941 Rollin H. Baker
obtained specimens from eastern Texas which greatly extended the known
geographic range.
In addition to the localities represented by specimens examined, Arthur
(1928:117) has recorded specimens from Greensburg, St. Helena Parish;
Braithwaite, Plaquemines Parish; Geismar, Assumption Parish; Laurel
Hill, West Feliciana Parish; French Settlement, Livingston Parish; and
Kentwood, Tangipahoa Parish. All these localities lie within the
eastern half of southern Louisiana. A skin-only, no. 38902, Mus. Vert.
Zoöl., obtained from a fur buyer by Stanley C. Arthur, was taken in
Mississippi "south of Jackson." Possibly it is of the subspecies
arthuri.
Intergradation with M. f. olivacea is indicated by a specimen from
Mobile County, Alabama, commented on in the account of olivacea.
Intergradation with primulina is indicated by the shape of the
anteromedial part of the bullae of the specimen from Swartz, Louisiana,
that is referred to primulina. The lack of specimens from the
northern two-thirds of Mississippi and from western Tennessee, prevents
any definite statement as to the limits of range of arthuri in those
areas. In comparison with animals from the type locality, the slightly
larger size of the adult male from Baton Rouge, and the still larger
size of the adult male of primulina from Swartz, Louisiana, suggests
that the olivacea "influence" may extend farther west in the
latitude of northern Louisiana than anywhere else.
None of the skulls examined shows malformation of the frontal sinuses
such as results from infestation by parasites in some races. Arthur
(1928:115) speaks of the ". . . cut-over swamp land, where the tupelo
and cypress have been removed, . . ." as constituting suitable habitat
for this animal.
Specimens examined.—Total number, 13, as follows:
Texas. Colorado County: 12 mi. N Eagle Lake, 1[43]; 5 mi. W
Eagle Lake, 1[43]; 3 mi. S Garwood, 1[43].
Louisiana. East Baton Rouge Parish: Baton Rouge, 4[71].
Livingston Parish: Springville, 1[74]. Saint James Parish:
Convent, 1[91]; Remy, 2 (1[74], 1[45]). Assumption Parish: near
Lake Verret, 1[45].
Mississippi. Harrison County: Saucier, 1[71].
Mustela frenata olivacea Howell
Long-tailed Weasel
Plates 16, 17, 18, 31, 32 and 33
Mustela peninsulae olivacea Howell, Proc. Biol. Soc. Washington,
26:139, May 21, 1913.
Mustela frenata olivacea, Hall, Carnegie Instit. Washington Publ.
473:104, November 20, 1936.
Type.—Male, adult, skull and skin; no. 180802, U. S. Nat. Mus.,
Biol. Surveys Coll.; Autaugaville, Autauga County, Alabama;
December 22, 1912; obtained by L. S. Golsan.
The skull (plates 16-18), although cracked at two places in the
interorbital region, is in one piece and not warped out of shape.
The teeth all are present and entire. The skin is exceptionally
well made and in perfect condition except for the extreme tip of
the tail which is broken off.
Range.—Lower and Upper Austral life-zones in eastern
Mississippi, Alabama, Georgia, South Carolina, and northern
Florida. See figure 29 on page 221.
Characters for ready recognition.—Differs from M. f.
peninsulae in finer, softer pelage and shorter (less than 15.8 in
ad. ♀) tympanic bullae; from M. f. noveboracensis, in adult
males by wider tympanic bulla which is more than rather than less
than 8.5, in adult females by total length which is more than
rather than less than 345, and by mastoid breadth which is more
than rather than less than distance between articular faces of
exoccipital condyle and glenoid fossa; from M. f. arthuri in
larger size (adult males with hind foot and basilar length each
more than 45); depth of skull at anterior margin of basioccipital,
ignoring sagittal crest, amounting to less than 63 per cent of
mastoid breadth, and lesser convexity of dorsal outline of skull
in longitudinal axis (See pls. 16-18).
Description.—Size.—Male and female: External measurements
of adults are available as follows:
| Catalogue No. |
Sex |
Locality |
Total length |
Length of tail |
Length of hind foot |
|
| 47165 |
♂ |
Box Springs, Talbot Co., Georgia |
454 |
160 |
48 |
|
| 47166 |
♂ |
Box Springs, Talbot Co., Georgia |
435 |
147 |
47 |
|
| 47167 |
♂ |
Box Springs, Talbot Co., Georgia |
422 |
145 |
45 |
|
| 41023 |
♂ |
Thomas Co., Georgia |
443 |
140 |
47 |
|
| 41025 |
♂ |
Grady Co., Georgia |
395 |
142 |
47 |
|
| 223880 |
♂ |
Okefinokee Swamp, Georgia |
416 |
145 |
49 |
|
| 198 |
♂ |
Okefinokee Swamp, Georgia |
425 |
140 |
48 |
|
| Average 7 |
♂ |
|
427 |
146 |
47 |
|
| 49385 |
♀ |
Gainesville, Alachua Co., Florida |
396 |
124 |
45 |
[not typical] |
| 41024 |
♀ |
Thomas Co., Georgia |
380 |
125 |
41 |
|
| 51527 |
♀ |
Talbot Co., Georgia |
376 |
128 |
43 |
|
The length of the hind foot averages less than the basal length in
both males and females. The tail averages 52 per cent as long as
the head and body in males and 51 per cent in females. Average
differences in measurements of the two sexes are: Total length,
49; length of tail, 19; length of hind foot, 5. An adult male, no.
41023, and an adult female, no. 41024, each taken in February,
1929, on the Sinkola Plantation, Thomas County, Georgia, weighed
15 ounces (425 grams) and 7 ounces (198 grams) respectively
according to Charles O. Handley.
Externals.—As described in Mustela frenata noveboracensis,
except that hairiness of foot-soles slightly less than shown in
figure 19.
Color.—Upper parts, in summer, near tone 4 of Burnt Umber of
Oberthür and Dauthenay, pl. 304. In winter lighter, between tones
3 and 4 of Raw Umber of Oberthür and Dauthenay, pl. 301. Dark spot
at each angle of mouth present or absent. Underparts ranging from
Massicot Yellow to Cream Buff except on chin and upper lips which
are white. Tip of tail black. Upper parts of uniform color. Color
of underparts extends distally on posterior sides of forelegs over
antipalmar faces of toes and on medial sides of hind limbs to
ankles. Least width of color of underparts averaging, in a series
of five males from Talbot Co., Georgia, 29 (extremes 24-34) per
cent of greatest width of color of upper parts. Black tip of tail
in same series, averaging 65 (extremes 60-70) mm. long, thus
longer than hind foot and averaging 43 per cent of length of
tail-vertebrae.
The spot at the angle of the mouth is absent in one-third of the
specimens examined. The upper lips are white in specimens from the
southern part of the range of olivacea but in the northern part
of the range of the subspecies the upper lips are dark colored as
in noveboracensis.
Skull and teeth.—Male (based on 5 adults from Talbot Co.,
Georgia): See measurements and plates 16-18; weight, 5.3 (5.0-6.4)
grams; basilar length, 48.3 (45.8-50.1); zygomatic breadth more or
less (usually less) than distance between condylar foramen and M1
and more or less (usually more) than distance between anterior
palatine foramen and anterior margin of tympanic bulla; mastoid
breadth more or less than (averaging about equal to) postpalatal
length; postorbital breadth less than length of upper premolars
and more or less than (about equal to) width of basioccipital
measured from medial margin of one foramen lacerum posterior to
its opposite; interorbital breadth more or less than (about equal
to) distance between foramen opticum and anterior margin of
tympanic bulla; breadth of rostrum less than length of tympanic
bulla; least width of palate less than greatest length of P4;
anterior margin of tympanic bulla as far posterior to foramen
ovale as width of 3 to 5 upper incisors; height of tympanic bulla
not less than distance from its anterior margin to foramen ovale;
length of tympanic bulla more than length of lower molar and
premolar tooth-row and longer than rostrum (one exception);
anterior margin of masseteric fossa below posterior half of m2.
Female (based on 2 adults from Thomas Co., Ga., and one from
Talbot Co., Ga.): See measurements and plates 31-33; weight, 3.8
(3.5-4.0) grams; basilar length, 43.4 (42.7-44.0); zygomatic
breadth less than distance between condylar foramen and M1 or than
between anterior palatine foramen and anterior margin of tympanic
bulla; postorbital breadth less than length of upper premolars and
more or less (usually more) than width of basioccipital measured
from medial margin of one foramen lacerum posterior to its
opposite; least width of palate less than greatest length of P4;
tympanic bulla as far posterior to foramen ovale as width of 3 to
4 (including I3) upper incisors; height of tympanic bulla not less
(usually more) than distance from its anterior margin to foramen
ovale; length of tympanic bulla more than length of lower molar
and premolar tooth-row and longer than rostrum.
The skull of the female averages 28 per cent lighter than that of
the male.
Compared with the skull of M. f. peninsulae, of which only one good
skull, and that a female, is available, that of M. f. olivacea
averages smaller and has relatively and actually smaller and less
inflated bullae. As compared with the skull of M. f. noveboracensis,
that of olivacea in the case of males is larger in every part
measured and relative to the basilar length is broader across the
zygomatic arches and mastoids. However, the rostrum and interorbital
region are relatively narrower. The orbitonasal length is relatively
less. The tympanic bullae are broader and more inflated. The same
differences hold as between females of noveboracensis and olivacea.
Indeed, the females of these two races differ more than do the males.
Additional, selected differential cranial characters in the females
are, in olivacea, as follows: Weight averaging 3.8 grams rather than
1.7 grams; braincase with, rather than without, sagittal crest;
anterior border of tympanic bulla separated from foramen ovale by
breadth of less than, rather than breadth of more than, 4 upper
incisors (including I3); height of tympanic bulla not less than, rather
than less than, distance from its anterior margin to foramen ovale;
squamosal bone, between anterior margin of tympanic bulla and foramen
ovale, ventrally concave rather than ventrally convex. Comparisons of
the skulls with those of M. f. arthuri and M. f. primulina are made
in the accounts of those subspecies.
Remarks.—Excepting two young specimens from South Carolina in the
Charleston Museum, no specimens of this race of large weasel seem to
have been preserved until Arthur H. Howell, in the course of his study
of the mammals of Alabama, procured specimens on which his name,
olivacea, was based. Later, Francis Harper obtained three instructive
specimens from Okefinokee Swamp. Really adequate material, for the
localities represented, owes its preservation to the alertness of
Charles O. Handley, when he resided at Thomasville, Georgia, and to
Hallie E. Fuller of Geneva, Talbot Co., Georgia.
The distinctness of M. f. olivacea from M. f. peninsulae is not
satisfactorily established due to inadequate material of peninsulae.
Differences shown by the specimens seen indicate that, as compared with
olivacea, peninsulae is larger, has transversely wider
light-colored underparts which possess more yellow, and a larger skull
with more inflated tympanic bullae. In each of these characters,
olivacea is intermediate between noveboracensis on the north and
peninsulae on the south. The question arises, therefore, whether the
animals here recognized under the name olivacea really constitute a
recognizable subspecies or instead are only representatives of a
subspecies which reaches its extreme development in Florida. In the
latter event, the name peninsulae would apply to all. Examination of
more material from Florida, especially from the southern half of
Florida, will be necessary to answer this question.
This large weasel of the southeastern United States is remarkably
different from noveboracensis. Indeed, were it not for actual
intergrades such as the two from Fort Payne, Alabama, and York, South
Carolina, which are described in the account of M. f. noveboracensis,
and the six specimens from northwestern Alabama, which are referred to
olivacea, the systematist, I believe, would have little or no
hesitancy in designating the two as distinct species, especially on the
basis of differences to be seen in the skull.
Not only are the two forms structurally more different than usually is
the case but between two geographically, adjacent subspecies of the
same species of mammal, but the belt where intergradation occurs
appears to be narrow. Nevertheless, when material of the two races is
laid out in geographic order, and examined in mass, certain features
are seen to undergo gradual change as a person's eye travels from
specimens from, say, the center of the range of noveboracensis to
specimens from southern localities adjoining the territory occupied by
olivacea. One of these features subject to gradual change is the
color of the underparts. Beginning at the Adirondacks of New York
where a large number of the specimens have white underparts, the
underparts become more intensely yellowish southward through the range
of noveboracensis into that of olivacea. Indeed, this progressive
trend seems to continue right on southward through the range of
olivacea into that of peninsulae. Turning in the opposite direction
we find that the least width of the underparts decreases gradually
northward toward the range of noveboracensis. There is, likewise, a
decrease to the northward in length of the skull and relative, as well
as actual, narrowing of the braincase and tympanic bullae. However, in
least width of color of underparts and the mentioned cranial features,
the trend stops relatively abruptly at the southern boundary of the
geographic range of noveboracensis and does not continue on,
northward, into the range of noveboracensis as is the case with the
change in intensity of yellowness of the underparts.
Two males, in the United States National Museum, Biological Surveys
Collection, from near Leighton, Alabama, no. 178386 from the Tennessee
River nine miles north [of Leighton?] and no. 180240 from La Grange
Mountain, although clearly referable to olivacea on the basis of
cranial characters, show some approach to noveboracensis in lesser
size of the skull and agree with noveboracensis in the narrowness of
the color of the underparts. Also, these specimens, like others from
the northern part of the range of olivacea, for instance, no 31.227,
Charleston Museum, from Mayesville, South Carolina, have the color of
the underparts extended only part way out on the hind limb toward the
foot. In specimens of olivacea from the southern part of its range
the color of the underparts is extended onto the hind feet and this
trend reaches its extreme in peninsulae, specimens of which have the
feet and larger parts of the limbs marked with the light color of the
underparts.
An adult female, no. 32.32, Charleston Museum, although typical of
olivacea in most respects, is nevertheless an intergrade. The teeth
are as small as in some specimens of noveboracensis. The size of the
skull is only slightly nearer that of olivacea than it is to that of
noveboracensis. The proportions of the skull, however, are distinctly
those of olivacea.
Five other specimens, from northwestern Alabama, namely two from eight
miles north of Nauvoo, two from Shoal Creek, and one from White Creek,
also show intergradation between noveboracensis and olivacea. The
remarks concerning color and color pattern of the specimens from
Leighton apply equally well to the five from northwestern Alabama. In
cranial characters, no. 51658 from Shoal Creek is referable to
olivacea, as also is no. 51677 from the same place, providing it is a
female rather than a male as sexed by the collector. No. 57146 from
White Creek also is referable to olivacea although the skull shows
some approach to that of noveboracensis. Of the two males from near
Nauvoo, no. 51652 is to me indistinguishable from noveboracensis, but
no. 51653 does have some characters of olivacea, although on the
whole, the latter, too, seems to be a little nearer noveboracensis
than olivacea. However, because the mean of these seven specimens
from northwestern Alabama is nearer olivacea than noveboracensis
the former name may be applied.
Another specimen from "Souinlonie" Creek, Clark County, Mississippi,
has the coloration and rostral configuration of primulina, narrow
mastoidal breadth and smaller teeth of noveboracensis and skull of
large size with "full" braincase as in olivacea. No. 235364, U. S.
Nat. Mus., from the Mobile River at the "L. and N. RR. Crossing,"
Mobile County, Alabama, although definitely olivacea, shows approach
to arthuri in that the dorsal outline of the skull is longitudinally
more convex and the tympanic bullae are less inflated than in
olivacea and in that the color of the underparts is almost exactly as
in the type specimen of arthuri. The young specimen labeled as from
"Silver Springs," Florida, has large tympanic bullae (17 mm. long) and
several characters that show its relationship to peninsulae as that
race is now understood. Because the sex is unknown the identification
as olivacea is tentative and is made on the assumption that the
specimen is a male. If it is instead a female, the animal is referable
to peninsulae.
An adult, female specimen in the Charleston Museum, no. 27.239.1, taken
at St. Matthews, South Carolina, on December 8, 1927, contained four
embryos which averaged 19 mm. in length and 47.75 centigrams in weight.
Another adult female, in the Charleston Museum, no. 32.32, taken on
February 21, 1932, at the same place, has prominent mammae, and the
collector has noted that two were slightly active.
Sixteen of twenty-nine adults examined show infestation of the frontal
sinuses by parasites. However, in none is the malformation of the
frontal region so great as frequently occurs in M. f. noveboracensis.
Specimens examined.—Total number, 52, arranged alphabetically
by states and from north to south by counties in each state.
Except as otherwise indicated specimens are in the University of
California Museum of Vertebrate Zoölogy.
Alabama. Lawrence County: White Creek, 1; Little Sand Mt., Shoal
Creek, 2. Winston County: 7-1/2 mi. N Nauvoo, 1; 8 mi. N Nauvoo,
1. Lauderdale County: near Leighton, 9 mi. N Tennessee River,
1[91]. Colbert County: Leighton, 1[91]. Autauga County:
Autaugaville, 1[91]. Dale County: Midland City, 1[91]. Mobile
County: Mobile River, 12 mi. NE Mobile, 1[91].
Florida. Alachua County: Gainesville, 4[61]. Marion County:
"Silver Springs," 1.
Georgia. Spalding County, 1. Lamar County, 1. Talbot County:
southwest part of county, 1; Box Springs, near Geneva, 3; Upatoie
Creek, 1 mi. SW Box Springs, 2; 3 mi. SE Geneva, 1; 4 mi. W
Geneva, 1; 5 mi. W Geneva, 1; 2 mi. E Geneva, 1. Chattahoochee
County, 2. Grady County: Beachton, 3[91]; locality no more
definite than county, 4. Thomas County: Sinkola Plantation, 2;
locality no more definite than county, 2. Charlton County: 1/2
mi. E Chesser's Island, Okefinokee Swamp, 1[58]. County in
question: Billy's Island, Okefinokee Swamp, 1[91]; Okefinokee
Swamp, 1[58].
Mississippi. Clark County: Souinlonie Creek, 1.
South Carolina. Darlington County: Society Hill, 1[91]. Sumter
County: Mayesville, 1[11]. Calhoun County: St. Matthews, 2[11].
Georgetown County: Sampit, 1[11]. Charleston County: Rantowles,
1[11]; 8 mi. N Charleston, 1[11]. Beaufort County: Yemassee,
1[2].
Mustela frenata peninsulae (Rhoads)
Long-tailed Weasel
Plates 16, 17 and 18
Putorius peninsulae Rhoads, Proc. Acad. Nat. Sci. Philadelphia,
1894:152, June 19, 1894; Bangs, Proc. Biol. Soc. Washington,
10:10, February 25, 1896.
Mustela peninsulae, Miller, U. S. Nat. Mus. Bull., 79:98,
December 31, 1912.
Mustela p. peninsulae, Bailey, Bailey Mus. and Library Nat.
Hist., 1(no. 5):1, December 1, 1930.
Mustela frenata peninsulae, Hall, Carnegie Instit. Washington
Publ. 473:105, September 20, 1936.
Type.—Female, young, part skull and skin; no. 8515, Acad. Nat.
Sci. Philadelphia; Hudson's, Pasco County [14 miles north of
Tarpon Springs], Florida; before 1895; obtained by W. S.
Dickinson.
The skull has been cut vertically in two at the plane of the
glenoid fossae. These fossae and all the cranium posterior to them
are missing. In addition to the part of the cranium anterior to
the glenoid fossae, the lower jaws are preserved complete. The
teeth all are present and entire. The prominent sutures on the
rostrum and palate show the specimen to be young and its small
size leaves but little doubt that the animal was a female. The
light facial markings are more extensive than in any of the
referred specimens. In the type these light facial markings
consist of a median isolated spot immediately in front of the
ears, a larger one on the nose, with an interrupted bar on each
side extending posteroventrally in front of and anterior to the
eye, a wider bar, on each side, extending anterodorsally between
the ear and eye and finally an isolated spot at the anterior
border of each ear. The skin is stuffed and in fair condition
except that the vertebrae remain in the tail.
Range.—Austral and probably Tropical life-zones of Florida
south of latitude 29°. See figure 29 on page 221.
Characters for ready recognition.—Differs from M. f.
olivacea in coarser pelage and larger tympanic bullae.
Description.—Size.—Male: No external measurements available.
Female: The type a young animal and no. 2379, an adult from Tarpon
Springs, measure respectively as follows: Total length, 375, 378;
length of tail, 100, 130; length of hind foot, 40, 44.5.
Externals.—As described in Mustela frenata noveboracensis
except that hairiness of foot-soles as shown in figure 20.
Color.—Upper parts (in winter) near tone 3 of Burnt Umber of
Oberthür and Dauthenay, pl. 304. Dark spot at each angle of mouth
present or absent. Tip of tail black. Underparts Reed Yellow
except on chin and usually on legs where white. Upper lips white
entirely around. Upper parts of uniform color. Color of underparts
extends distally on legs over both sides of feet and on front legs
over wrists. Proximal part of tail slightly lighter below than
above. Least width of color of underparts, in seven specimens,
averaging 41 (extremes 31-52) per cent of greatest width of color
of upper parts. Black tip of tail, in each of two females, 45 mm.
long; thus slightly longer than hind foot and amounting to 36 per
cent of length of tail-vertebrae.
The spot at the angle of the mouth is absent in four of the ten
specimens and is present on both sides in the other six.
Skull and teeth.—Male (based on an adult from Apopka and the
anterior part of an adult from Enterprise): See measurements and
plates 16-18. As described in Mustela frenata olivacea except
that: Weight, 7.0 grams; basilar length, 49.8.
Female (based on an adult from Tarpon Springs, Florida): See
measurements. As described in Mustela frenata olivacea except
that: Weight, 4.7 grams; basilar length, 44.2; zygomatic breadth
more than distance between anterior palatine foramen and anterior
margin of tympanic bulla.
In comparison with M. f. olivacea, the insufficient material of M.
f. peninsulae suggests that its skull averages larger and has
relatively as well as actually larger and more inflated tympanic
bullae.
Remarks.—The first published mention of this weasel seems to have
been the original description which appeared in 1894. This description
was based on a single specimen sent to Samuel N. Rhoads by W. S.
Dickinson, who, in the following year, procured another specimen at
Tarpon Springs. So far as known only eight other specimens, as listed
under "Specimens examined," have found their way into collections of
study specimens.
H. H. Bailey (1930:1) credits the range of this subspecies as extending
south "to the shores of Florida Bay and the Gulf of Mexico, where ever
high ground occurs."
Evidence of intergradation between M. f. peninsulae and M. f.
olivacea is provided by specimens of olivacea from Gainesville,
Florida, and the Okefinokee Swamp, Georgia. These specimens, on the
average, have the color of the underparts wider, the skull larger, and
the tympanic bullae relatively larger than do specimens of olivacea
from farther north. In these features, approach to M. f. peninsulae
is shown.
Light facial markings occur in this subspecies. They are similar to
those possessed by weasels which occur at the same latitude and under
corresponding climatic conditions on the Pacific Coast. The type
specimen and one from Tarpon Springs have white facial markings. Two of
the three specimens from Apopka also show white facial markings,
although in reduced amount. One of the four specimens of M. f.
olivacea from Gainesville, Florida, has well-developed light (white)
facial markings. Also of the four specimens of M. f. olivacea
examined from Okefinokee Swamp, Georgia, one has prominent white facial
markings. However, in it the pattern is so unusual as to suggest that
it is an instance of partial albinism rather than an outcropping of a
racial tendency, or a pattern of coloration induced by climatic
factors.
None of the eight available skulls show any infestation of the frontal
sinuses by parasites.
Specimens examined.—Total number, 10, arranged by counties from
west to east.
Florida. Pasco County: Hudson's, 1[1]. Pinellas County: Tarpon
Springs, 1[1]. Hernando County, 1[91]. Polk County:
Auburndale, 1[91]; no locality more definite than county, 1[91].
Orange County: Apopka, 3[61]. Volusia County: Enterprise,
1[60]. Seminole County: Osceola, 1[2].
Mustela frenata spadix (Bangs)
Long-tailed Weasel
Plates 16, 17, 18, 31, 32 and 33
Putorius longicauda spadix Bangs, Proc. Biol. Soc. Washington,
10:8, February 25, 1896; Merriam, N. Amer. Fauna, 11:21, figs.
10, 11, June 30, 1896; Cory, Mamm. Illinois and Wisconsin, p.
374, 1912.
Mustela longicauda spadix, Miller, U. S. Nat. Mus. Bull., 79:98,
December 31, 1912; Bailey, Journ. Mamm., 10:156, May 9, 1929.
Mustela longicauda, Johnson, Journ. Mamm., 11:439, November 11,
1930.
Mustela noveboracensis, Murie, Journ. Mamm., 16:321, November 15,
1935.
Mustela frenata spadix, Hall, Carnegie Instit. Washington Publ.
473:105, November 20, 1936.
Type.—Male, young, skull and skin; no. 3265/1786, Amer. Mus.
Nat. Hist.; Fort Snelling, Hennepin County, Minnesota; June 25,
1889; obtained by Edgar A. Mearns; original no. 812.
The skull is complete although there are fractures on the top of
the braincase, on the right side of the braincase and at the
middle of the right zygomatic arch. The teeth all are present and
entire. The skin, although overstuffed, is complete, well
preserved, and in summer pelage.
Range.—Upper Austral and Transition life-zones of Minnesota,
northern and western Iowa, southeastern North Dakota, eastern part
of South Dakota, and northeastern Nebraska. See figure 29 on page
221.
Characters for ready recognition.—Differs from M. f.
noveboracensis and M. f. primulina in that specimens of all
ages have least width of color of underparts amounting to more
than 41 per cent of greatest width of color of upper parts, and
have light color of underparts extended onto hind foot rather than
stopped short of ankle; adults with hind feet more than 50 in
males and 40 in females; orbitonasal length more than 15.5 in
males and 13.5 in females; length of tooth-rows more than 18.0 in
males and 15.7 in females; mastoid breadth more than 25.5 in males
and 22.0 in females. From M. f. longicauda by color darker than
near (h) Clay Color, in males by a flattened occiput in which
the depth of the skull, exclusive of the sagittal crest and taken
at the anterior border of the basioccipital, amounts to less than
58 per cent of the mastoid breadth.
Description.—Size.—Male: Three adults from Elk River,
Minnesota, yield average and extreme measurements as follows:
Total length, 458 (444-467); length of tail, 154 (140-165); length
of hind foot, 55 (52-59). Tail averages 51 per cent as long as
head and body. Length of hind foot averages more than basal
length. Corresponding measurements of three subadults from
Madison, Minnesota, are as follows: 453 (438-469); 157 (152-165);
50 (47-51). Tail averages 53 per cent as long as head and body.
Female: Three adults from Elk River, Minnesota, yield average and
extreme measurements as follows: Total length, 387 (380-391);
length of tail, 131 (121-138); length of hind foot, 44 (43-46).
Tail averages 51 per cent as long as head and body. Length of hind
foot more or less than (approximately equal to) basal length.
Corresponding measurements of two adults and one subadult from
Madison, Minnesota, are as follows: 385 (379-396); 137 (119-159);
42 (38-44). Tail averages 55 per cent as long as head and body.
The average differences in external measurements of the two sexes
from Elk River, are: Total length, 71; length of tail, 23; length
of hind foot, 11. At Madison, corresponding differences are 68,
20, and 8. Two adult females from Elk River, Minnesota, weigh 205
and 210 grams.
Externals.—Longest facial vibrissae black, brown, or white
(often all three colors in same specimen) and extending beyond
ear; carpal vibrissae same color as underparts and extending to
apical pad of fifth digit; hairiness of foot-soles (in summer
pelage) as shown in figure 19.
Color.—Winter pelage all white except tip of tail. In southern
part of range sometimes assumes a brown winter coat. Summer pelage
with upper parts ranging from near (16´) Cinnamon Brown to Vandyke
Brown. Chin and upper lips white. Remainder of underparts ranging
from near (a) Olive Ocher to Ochraceous Buff and Pale Orange
Yellow. Tip of tail at all times black. Upper parts of uniform
color except for occasional slight darkening of nose. Color of
underparts extends distally on posterior sides of forelegs over
toes onto antipalmar faces of feet and ankles, on medial sides of
hind limbs to ankle, over antiplantar faces of toes and
distomedial fourth of each tarsus, and over proximal fifth to
third of under side of tail. Least width of color of underparts
averaging (in 3 specimens from Elk River) 54 (47-59) per cent of
greatest width of color of upper parts. Black tip of tail
averaging same length as hind foot and 28 per cent of length of
tail-vertebrae. Save for the greater width of the light-colored
underparts and relatively short black tip of the tail, both
features of M. f. longicauda, spadix is variously
intermediate, depending on locality, as between noveboracensis
and longicauda.
Skull and teeth.—Male (based on 3 adults from Elk River,
Minn.): See measurements and plates 16-18. As described in
Mustela frenata longicauda except that: Weight, 5.6 (5.0-6.5);
basilar length, 49.0 (48.7-49.2); zygomatic breadth sometimes less
than distance between anterior palatine foramen and anterior
margin of tympanic bulla; postorbital breadth more or less (about
equal to) width of basioccipital measured from medial margin of
one foramen lacerum posterior to its opposite; interorbital
breadth more or less than distance between foramen opticum and
anterior margin of tympanic bulla; anterior margin of tympanic
bulla as far posterior to foramen ovale as width of 4 to 5 upper
incisors; height of tympanic bulla more or less than distance from
its anterior margin to foramen ovale; length of tympanic bulla
less than length of rostrum; anterior margin of masseteric fossa
below talonid of m1.
Female (based on 4 adults from Elk River, Minn.): See measurements
and plates 31-33. As described in Mustela frenata longicauda
except that: Weight, 3.5 (3.3-4.0) grams; basilar length, 42.9
(42.3-43.2); least width of palate more or less than greatest
length of P4; tympanic bulla as far posterior to foramen ovale as
width of 3 to 5 upper incisors.
The skull of the female averages 33 per cent lighter than that of
the male.
Skulls of adult males of spadix from Elk River, Minnesota, as
compared with those of longicauda from Alberta, are larger in every
part measured. Relative to the basilar length these skulls of spadix
are broader across the mastoid region, narrower across the zygomata,
deeper through the plane of the postorbital processes, shallower
through the braincase and have relatively shorter tympanic bullae.
Whereas the tympanic bullae of longicauda are, on the average,
approximately as long as the rostrum (orbitonasal length), in spadix
the rostrum is longer than the bulla. Viewed posteriorly, the braincase
of spadix is seen to be much shallower and wider than that of
longicauda. Indeed, the depth of the braincase, measured at the
anterior end of the basioccipital, amounts to only 56 per cent of the
mastoid breadth in spadix as against 61 per cent in longicauda. The
longer, waistlike, postorbital constriction, relatively smaller
braincase, and especially the relatively narrower zygomatic expanse in
spadix imparts to its skull a more slender appearance than has the
skull of longicauda. These differences are not shown by the skulls of
females. To be sure, spadix, in most of its cranial measurements,
averages slightly larger, has a relatively shallower braincase and is
relatively deeper through the postorbital processes, but these
differences are so slight that inclusion of one more specimen, of
slightly different proportions, in the average might cause the average
measurements to read as they do in longicauda.
Compared with noveboracensis, from Massachusetts, adult skulls of
spadix, taking sex into account, are larger in every part measured
and are relatively as well as actually wider and deeper throughout.
Also, in spadix: Sagittal and lambdoidal crests higher, especially in
females; anterior margin of tympanic bulla projecting up sharply from
squamosal; occiput more flattened in posterior view; tooth-rows
relatively and actually longer but orbitonasal length relatively
shorter; postorbital processes more robust; zygomatic arches widely
bowed outward rather than evenly rounded; canines larger; squamosal
less swollen ventrally, especially in females. Between noveboracensis
and spadix, the differential cranial characters are greater in number
and degree between females than between males. Comparison of the skull
with that of M. f. primulina is made in discussion of that
subspecies.
Remarks.—Edgar A. Mearns in 1889 and the early nineties took several
specimens of this weasel and it was principally on these that Bangs in
1896 (p. 8) based his description. The best material, however, is that
from Elk River, Minnesota, collected in later years by Bernard Bailey,
and supplemented by one specimen taken in 1885 by Vernon Bailey and
another by his sister Anna Bailey in 1891 at the same place.
Mustela frenata spadix has just one structural feature of a "unique"
kind which serves to differentiate it from the geographically adjoining
subspecies. This feature is large size. The other diagnostic characters
ascribed to spadix are of an intermediate sort—intermediate as
between two extremes, one found to the westward in longicauda and the
other to the eastward in noveboracensis. For example, the
dark-colored upper parts are merely darker than in longicauda and
merely lighter than in noveboracensis. The color is not "different";
it is only "intermediate." Furthermore, each of the characters ascribed
to spadix, including large size itself, undergoes change from one
part of its geographic range to another; the characters are not
constant over a wide area. Indeed, excepting the large size which
remains relatively uniform over the northern two-thirds of the range,
no two localities have been found from which the specimens can be said
really to agree in characters.
By way of illustration, the coloration of the upper parts may be cited.
Near the range of noveboracensis the average coloration of
individuals from one locality is only a little lighter than in
noveboracensis. Farther westward the average coloration is a little
lighter and farther westward yet, toward the range of the extremely
light colored longicauda, the average coloration is lighter still.
Although all these animals are darker than longicauda and lighter
than noveboracensis, those from the three places do not agree among
themselves. Because of the lack of more than one character of a
"unique" kind and because of the inconstancy, geographically, of other
characters, and for that matter, lack of constancy geographically in
combination of characters, the writer regards spadix as a barely
recognizable subspecies.
Examination of the specimens of spadix shows that the individual
variation in a single species is greater in a region of intergradation
than it is some distance inside the borders of the geographic range of
a well-marked subspecies. This is illustrated by three specimens of M.
f. spadix in fresh summer pelage from the single locality, Elk River,
Minnesota. In these, the color of the upper parts varies from a little
darker than Cinnamon Brown to Vandyke Brown. At any one locality well
within the range of longicauda, or noveboracensis, there is nowhere
nearly so much variation in color, even in much larger series of
specimens.
Study of the specimens here assigned to spadix reveals that some
features regarded as of diagnostic value for one or the other of the
two races, longicauda and noveboracensis, behave differently. For
example, the dark coloration of the upper parts, which is
characteristic of noveboracensis, manifests itself far westward
within the range of spadix whereas the wider extent of the
light-colored underparts, which is characteristic of longicauda, and
the Olive Ocher, rather than Pale Orange Yellow, color of these
underparts, are seen in varying degree all the way across the range of
spadix. Thus, these animals are colored above like noveboracensis
and below like longicauda, but not vice versa. In these animals,
then, the longicauda type of underparts is dominant, in one sense of
the word, over the noveboracensis type of underparts, and the
noveboracensis type of upper parts is dominant over the longicauda
type of upper parts. Each of these features is subject to actual
intergradation and does not always behave as a "unit character," that
is to say, one which is either present or absent. However, the
noveboracensis type of upper parts is carried much farther west
before being diluted than is the noveboracensis type of underparts.
Indeed, within the range of noveboracensis itself, the broad extent
of the longicauda type of underparts is manifest. This is, of course,
near the western margin of the range of noveboracensis.
The large size of males of spadix, as exemplified by specimens from
Elk River (see measurements on page 421), seems to be retained across the
northern part of the range here assigned to the subspecies. This larger
size than is found in longicauda from Alberta, is shown also by some
specimens from eastern North Dakota which are assigned to longicauda.
However, the average of these Dakotan specimens, all characters
considered, is nearer to my concept of longicauda.
Inspection of the cranial measurements of spadix shows also that in
addition to its large size it is distinguishable from any one of the
geographically adjoining races by its relatively (to basilar length)
greater, as well as actually greater, mastoidal breadth. This might be
included with size as a unique character distinguishing spadix from
longicauda and noveboracensis. However, it is not clear whether or
not this greater mastoidal breadth is more than a function of the large
size.
Excepting the greater mastoidal breadth and generally larger size of
the skull, the cranial features distinguishing males of spadix from
longicauda are features in which spadix shows approach to
noveboracensis. This is true, in spadix, of the relatively longer
(in comparison with longicauda) rostrum, relatively lesser zygomatic
breadth, relatively shallower braincase measured at the anterior end of
the basioccipital, and relatively deeper skull as measured at the
posterior borders of the last upper molars. This same approach to
noveboracensis already has been pointed out with respect to color of
the upper parts and is evident also in the relative shortness of the
tail which averages only 51 per cent of the length of the head and body
rather than 55 per cent as in longicauda.
Because the longicauda type of animal previously has been regarded as
specifically distinct from the noveboracensis type of animal, comment
is offered below on selected specimens, referred to spadix, which are
regarded as intergrades with noveboracensis or with other subspecies.
No. 8722, Univ. Wisconsin, adult male, in the white winter coat, from
north central Itasca County, Minnesota, obviously has characters of M.
f. spadix or longicauda that occur to the west and M. f.
noveboracensis of the east. Selected outstanding characters of
longicauda are its long tail, anteriorly truncate tympanic bullae and
large teeth. Characters indicating its affinities with
noveboracensis are smaller size of skull, general narrowness of
skull, and relatively low tympanic bullae. The skull is intermediate as
regards several individual structural features. For example, although
long and narrow and in this feature more nearly approaching
noveboracensis, the skull is wider than usual in that subspecies and
thus approaches that of longicauda or spadix. The hind foot, in the
dried state, measures 47 millimeters. This large hind foot, obviously
long tail (the specimen lacks external measurements), and anteriorly
truncate bullae constitute basis for here referring the specimen to
spadix. However, the seemingly small size of the body and the narrow
skull clearly show relationship to noveboracensis.
Specimens, referred to spadix, from northern Iowa, are instructive as
showing what happens where the ranges of noveboracensis, primulina,
spadix, and perhaps longicauda, meet. No. 47167, Univ. Mich. Mus.
Zoöl., a nearly adult female, taken on November 22, 1915, at Island,
Clay County, and in process of assuming a brown winter pelage, retains
enough of the dark summer pelage to show that the color was slightly
lighter than average for spadix. The color pattern, white lips, and
extension of light color of the underparts onto the feet, agrees with
spadix or longicauda as does also the long tooth-row. The overall
length of the skull is intermediate between that of spadix and
primulina. The proportions of the anterior part of the skull and of
the tympanic bullae resemble those found in primulina. A subadult
male skull only, no. 123846, American Museum of Natural History, from
Webb, Clay County, shows approach to primulina in the narrowness of
the rostrum.
A young male from Ruthven, Iowa, no. 48340, Univ. Michigan, has a large
skull approaching in size that of spadix, has the longicauda-spadix
type of light-colored underparts and color pattern, and is slightly
darker above than true longicauda. Another subadult male in the white
winter coat from Palo Alto County, no. 35756, Univ. Michigan, has a
large skull, which shows approach to primulina in its narrowness
anteriorly and in some other features. Although the tail is of moderate
length, the body is large as in spadix or longicauda, and the
length of the hind foot suggests spadix or longicauda.
A subadult male, no. 425a, Iowa State College, from Manson, Iowa, in
brown winter pelage, agrees with primulina in the restriction of the
area of the light color of the underparts and in less expanded
zygomatic arches. The teeth are intermediate in size between those of
noveboracensis and primulina on the one hand and those of spadix
and longicauda on the other. In other respects it agrees with, or is
more nearly like, spadix.
An adult female, no. 426a, Iowa State College, from Barnum, in the
brown winter coat, agrees with primulina except that the orbitonasal
length of the skull is more as in spadix and the presence of some
light color on the lower part of the hind legs suggests spadix. The
skull only, no. 440a, Iowa State College, labeled merely Webster
County, Iowa, is almost a duplicate of no. 426a. A subadult male, no.
427a, Iowa State College, from Moorland, Iowa, only about six miles
southeast of Barnum, likewise is indistinguishable from primulina
except for having a white winter coat and in being relatively broad in
the mastoidal region. Nevertheless, both of these animals are here
referred to spadix because the average of specimens from this general
area is nearer that of spadix. No. 497a, Iowa State College, an adult
female in white winter pelage, from Ames, approaches primulina in the
narrow rostrum and smaller teeth but otherwise approaches or even
agrees with spadix.
Two adult males, without external measurements, from Pilot Mound, Iowa,
have skulls quite like males of longicauda from Alberta. The only
approach noted to eastern forms is the restricted color of the
underparts on no. 2856, Coe College, which has a brown winter coat. The
color of the underparts is not extended so far out on the feet as in
longicauda. Also the tympanic bullae of this specimen are a trifle
narrower. The other male, no. 2652, is in the white winter coat. The
one female from the same place, no. 2660, Coe College, in brown winter
pelage, has a skull notably unlike that of longicauda or spadix;
the skull is narrower and practically indistinguishable from that of
the largest female skull of primulina available from Lawrence,
Kansas, save that the tooth-row is much longer. The color pattern also
agrees with that of primulina or noveboracensis in that the color
of the underparts extends only as far as the knee on the hind legs and
is narrow on the belly. Nevertheless, another adult female, no. 120a
from Amaqua Township, some 6 miles southwest of Pilot Mound, is in all
respects typical of spadix. This is the more remarkable because
another comparable specimen from less than 20 miles to the southwest in
Worth Township is equally typical of primulina.
Two young females from Chester, Iowa, nos. 2656 and 2874/2873, Coe
College, have skulls larger than those of corresponding age of
primulina or noveboracensis. The color is as in spadix. The color
pattern of the underparts also is as in spadix or longicauda except
that the width of the area of light color on the belly is restricted
somewhat although not so much as in noveboracensis or primulina.
Of four males from the same place, also in the collection of Coe
College, no. A2874 is a white skin only and does not provide diagnostic
characters. The three other males, each in summer pelage, are marked
and colored as are the two females from the same place except that male
no. 2861 has the color of the underparts so much attenuated on the hind
legs that it barely, uninterruptedly, extends to the feet. No. 2658 is
young, or perhaps barely subadult. The skull is large and referable to
spadix. The two adults, nos. 2861 and 2657, differ cranially from
typical (Elk River, Minn.) spadix only in being slightly narrower
across the mastoids and in having the bullae a little narrower. In
these departures they show some approach to primulina and to
noveboracensis. Another male, subadult, no. 2867, Coe College, from
Decorah, which has acquired half of the white winter coat, agrees with
the males from Chester except that the preorbital part of the skull is
shortened much as in some specimens of primulina.
From Lansing, in extreme northeastern Iowa, a large subadult male, no.
2864, Coe College, of 453 mm. in total length and half through with
acquiring the white winter coat, agrees with the males previously
described from Chester except in having the palate narrower as in
noveboracensis. The adult female available from Lansing, no.
2863/2862, Coe College, in white winter pelage except for the top of
the head, although a large skin, has a skull smaller than that of any
spadix or longicauda and of about the same size as that of no.
3838, Univ. Kansas Mus. Nat. Hist., of primulina, from Lawrence,
Kansas, except that the skull of no. 2863/2862 is much narrower across
the mastoids. This specimen, then, shows approach to noveboracensis
in narrowness of the mastoidal region, to primulina in other respects
and to spadix.
Many of these instructive specimens from Iowa, made available to the
present writer by Mr. W. F. Kubichek, were brought together at the Coe
College Museum by the late B. H. Bailey. Most of them were obtained
from trappers who did not supply the conventional external measurements
taken in the flesh. Even though these are lacking, the specimens
clearly show that actual intergradation occurs where the ranges of M.
f. longicauda, spadix, noveboracensis and primulina meet.
The dark color of the upper parts, restriction of the color of the
underparts on the ankles with the result that the color reaches the
toes in interrupted fashion, and large skull, of no. 18912 of the
Museum of the University of South Dakota, from Roberts County, South
Dakota, clearly place this specimen with spadix, rather than with
longicauda. Likewise, male, subadult, no. 11376, Univ. South Dakota,
from Clay County, South Dakota, is referable to spadix. Although
without external measurements, the specimen obviously is large. The
patch of summer pelage on its head and neck is darker than the summer
pelage of longicauda, and the orbitonasal length is greater than the
length of the tympanic bullae; all these features are characters of
spadix. The adult male from Fort Sisseton, South Dakota, no. 188407,
United States National Museum, figured by Merriam (1896, p. 20, figs.
7-9), is almost exactly intermediate between longicauda and spadix,
although here referred to the latter.
Five specimens, nos. 147375, 147432, 147762, 148720 and 148721, U. S.
Nat. Mus., including 3 skulls only from Beemer, Cuming County,
Nebraska, are intergrades between M. f. longicauda, M. f. primulina
and M. f. spadix. One skin is in white winter pelage and the other, a
female, is in summer pelage which in coloration and color pattern
agrees with that of spadix. External measurements of the male agree
with those of longicauda. Measurements of the female agree with those
of spadix except that the tail is shorter as in primulina. The
skulls are as long as in longicauda but are more slender than in
either longicauda or spadix although nearer the latter in this
respect. In dorsal aspect, the skulls especially posteriorly to the
orbital region, resemble primulina. All points considered, the
animals seem best referred to spadix.
Although the degree of development of certain morphological features
has been settled upon as indicative of the race spadix, some doubt
remains as to where the western boundary of its range should be shown.
This results from the fact that color has been taken into account as
one diagnostic feature and this feature is lacking in the white winter
specimens which, from the following places, are all that are available:
Kittson County, Minnesota; Moorhead, Minnesota; Casselton and Valley
City in North Dakota; Armour, South Dakota and Clay County, South
Dakota. In summary, more specimens in the summer coat will be required
to establish definitely the boundary between the ranges of longicauda
and spadix.
Surber (1932:49) has referred to additional specimens of this weasel in
the University of Minnesota Museum as from Winona, Hennepin and Isanti
counties of that state.
At Elk River, Minnesota, B. Bailey (1929:156) found this species to be
about half as abundant as Mustela cicognanii and that it is "more
often found in the open timber and about the dry ridges and fields."
Of seventeen adult or subadult skulls of this race from Minnesota, ten
have obvious marks of infestation of the frontal sinuses. In no skull,
however, has the infestation resulted in so much malformation, as
occurs in noveboracensis.
Specimens examined.—Total number, 76, arranged alphabetically
by states and from north to south by counties in each state.
Iowa. Lyon County: Granite, 1[65]. Howard County: Chester,
6[12]. Winneshiek County: Decorah, 1[12]; 8 mi. NE Ossian,
1[76]. Allamakee County: Lansing, 2[12]. Clay County: Island,
1[76]; Webb, 1[2]. Palo Alto County: Ruthven, 1[76]; no locality
more definite than county, 1[76]. Calhoun County: Manson, 1[65].
Webster County: Barnum, 1[65]; Moorland, 1[65]; no locality more
definite than county, 1[65]. Boone County: Pilot Mound, 3[12];
Amaqua Township, Sec. 19, 1[65]. Story County: Ames, 1[65].
Minnesota. Kittson County, 1[2]. Roseau County: 2-1/2 mi. SW
Roseau, Jadis Township, 1[14]. Itasca County: T. 61N, R. 26W,
1[102]. Clay County: Moorhead, 2[9]. Atkin County: Atkin,
1[50]. Otter Tail County: Lake Lizzie, 1[9]; Parkers Prairie,
1[57]. Grant County: 3 mi. NW Barrett, 1[76]. Benton (now
Mille Lacs?) County: Princeton, 1[91]. Sherburne County: Elk
River, 14 (6[59], 4[14], 3[91], 1[74]). Hennepin County: Fort
Snelling, 6 (5[2], 1[91]). Carver? County: Chaska, 1[60]. Lac
qui Parle County: Madison, 5 (3[91], 2[1]); no locality more
definite than county, 2 (1[68], 1[75]). Yellow Medicine County:
Wood Lake, 1[2]. Blue Earth County: Rapidan, 1[64]. County in
question: Moore Lake, 1[91].
Nebraska. Cuming County: Beemer, 5[91].
North Dakota. Cass County: Fargo, 1[91]; Casselton, 1[91].
Dickey County: Oakes, 1[91].
South Dakota. Roberts County, 1[102]. Marshall County: Fort
Sisseton, 1[91]. Douglas County: Armour, 1[14]. Clay County,
1[102].
Mustela frenata longicauda Bonaparte
Long-tailed Weasel
Plates 16, 17, 18, 31, 32 and 33
Mustela longicauda Bonaparte, Charlesworth's Mag. Nat. Hist.,
2:38, 1838.
Putorius longicauda, Baird, Mamm. N. Amer., p. 169, 1858; Coues,
Fur-bearing animals, p. 136, 1877; Bangs, Proc. Biol. Soc.
Washington, 10:7, figs. 1, 1a of pls. 1, 2 and 3, February 25,
1896; Merriam, N. Amer. Fauna, 11:19, pl. 3, figs. 3, 3a, 4, 4a,
pl. 5, figs. 1, 1a, text figs. 7-9, June 30, 1896.
Mustela longicauda longicauda, Bailey, N. Amer. Fauna, 49:166,
January 8, 1927.
Mustela frenata longicauda, Hall, Carnegie Instit. Washington
Publ. 473:105, November 20, 1936; Hall, Canadian Field-Nat.,
52:108, October, 1938.
Mustela frenata, Sowls, Journ. Mamm., 29:126, May 14, 1948.
Type.—Possibly not in existence. No. 43.3.3.3 [from Carlton
House, Saskatchewan] in the British Museum of Natural History has
been regarded by several zoölogists as the type. It is a subadult
female, skull and skin, from North America. See the account of M.
erminea cicognanii for reasons for and reasons against regarding
this specimen as the holotype.
No. 43.3.3.3 from the collection of Dr. John Richardson is in the
white winter coat and now (Sept. 24, 1937) is prepared as a study
skin. Evidences of its once having been mounted are: holes in the
soles of the hind feet for supporting-wires, large straight wire
in the tail, folds in the skin of the now backward-projecting hind
feet, and unevenness of the skin on the back resulting from
straightening out the specimen. The tip of the tail and some skin
from the middle of the belly are missing. Otherwise the skin is
intact. The skull is that of an animal in its first year, lacks
the zygomatic arch on each side, but otherwise is complete and
unbroken. The teeth all are present and entire except that p2 on
the right side is missing from its alveolus.
Range.—Transition and Upper Sonoran life-zones of the Great
Plains, southward from central Alberta, Saskatchewan and southern
Manitoba through eastern Montana, the Dakotas and Nebraska into
southeastern Wyoming, northeastern Colorado and western Kansas.
See figure 29 on page 221.
Characters for ready recognition.—Differs from M. f.
primulina in near (h) Clay Color rather than Brussels Brown of
upper parts, least width of color of underparts more than 40 per
cent of greatest width of color of upper parts, color of
underparts extended onto hind foot rather than stopped short of
ankle, zygomatic breadth more than 28.8 in adult males and more
than 24.1 in adult females; from M. f. spadix in lighter color
being near (h) Clay Color, in males by deeper occiput in which
the depth of the skull, exclusive of the sagittal crest and taken
at the anterior border of the basioccipital amounts to more than
59 per cent of the mastoid breadth; from M. f. oribasus in near
(h) Clay Color rather than near (14n) Brussels Brown color of
the upper parts and in males by deeper occiput in which the depth
of the skull, exclusive of the sagittal crest and taken at the
anterior border of the basioccipital, amounts to more than 59 per
cent of the mastoid breadth; from M. f. alleni in larger size,
adult males having a total length of more than 400 millimeters,
hind foot more than 45, basilar length more than 43.5, and females
having a total length of more than 375 and basilar length not less
than 40.0; from M. f. nevadensis in near (h) Clay Color rather
than near (14n to 1) Brussels Brown of upper parts, basilar
length more than 40 in females and averaging more than 45 in
males; from M. f. neomexicana by near (h) Clay Color rather
than Buckthorn Brown color of upper parts, absence of white and
Argus Brown facial markings, and length of tooth-rows amounting to
more than 37 per cent of basilar length.
Description.—Size.—Male: Five adults from Alberta yield
average and extreme measurements as follows: Total length, 438
(418-473); length of tail, 158 (140-193); length of hind foot, 50
(46-54). Tail averages 56 per cent as long as head and body.
Length of hind foot averaging more than basal length.
Corresponding measurements of five adults and subadults from North
Dakota are as follows: 465 (445-516); 164 (150-179); 51 (50-54).
Tail averages 55 per cent as long as head and body.
Female: Six adults (Alberta, 4; Saskatchewan, 1; Manitoba, 1)
yield average and extreme measurements as follows: Total length,
401 (383-425); length of tail, 145 (141-159); length of hind foot,
43 (41-44). Tail averages 57 per cent as long as head and body.
Length of hind foot more or less than (approximately equal to)
basal length.
The average differences in external measurements of the two sexes
are: Total length, 37; length of tail, 13; length of hind foot, 7.
General comparisons indicate that the Alberta-taken males may not
attain so large a size as those from some other areas. Thus the
differences in external measurements might be some greater
elsewhere, say, in North Dakota.
Externals.—Longest facial vibrissae black, brown or white
(often all three colors in same specimen) and extending beyond
ear; carpal vibrissae same color as underparts and extending to
apical pad of fifth digit; hairiness of foot-soles (in summer
pelage) only slightly greater than shown in figure 20.
Color.—Winter pelage all white except tip of tail. Summer
pelage with upper parts near (h) Clay Color or near tone 3 and 4
of Snuff Brown of Oberthür and Dauthenay, pl. 303. Chin and upper
lips white. Remainder of underparts ranging from near (a) Olive
Ocher to near (16´) Ochraceous Buff. Upper parts of uniform color
except for occasional darkening of head in front of ears. Color of
underparts extends distally on posterior sides of forelegs over
toes onto antipalmar faces of feet and wrists, on medial sides of
hind limbs to ankles over antiplantar faces of toes and
distomedial third of each tarsus, and over proximal fourth to
third of under side of tail. Least width of color of underparts
averaging, in a series of 10 males from Alberta, 58 (45-60) per
cent of greatest width of color of upper parts. Corresponding
figures for 10 females from the same place are 57 (50-74). Black
tip of tail in same series of males, most of which are in full
summer pelage, averaging 43 (35-60) mm. long. Thus, averaging
shorter than hind foot and 27 per cent of length of
tail-vertebrae.
As compared with M. f. neomexicana, longicauda lacks the white
facial markings, black ears, black forehead and nose, but
otherwise is similarly colored. As compared with M. f.
nevadensis, M. f. oribasus and M. f. spadix, each of color
pattern similar to longicauda, selected differences of
longicauda are its much lighter color, especially of the upper
parts, with less conspicuous darkening on the nose. From M. f.
primulina, longicauda differs in lighter color of upper parts,
reddish rather than yellowish underparts, and light rather than
dark-colored hind feet.
Skull and teeth.—Male (based on 5 adults from Alberta): See
measurements and plates 16-18; weight, 4.7 (4.6-4.9) grams;
basilar length, 46.0 (44.7-46.8); zygomatic breadth more than
distance between condylar foramen and M1 or than between anterior
palatine foramen and anterior margin of tympanic bulla; mastoid
breadth more than postpalatal length; postorbital breadth less
than length of upper premolars and more than width of
basioccipital measured from medial margin of one foramen lacerum
posterior to its opposite; interorbital breadth greater than
distance between foramen opticum and anterior margin of tympanic
bulla; breadth of rostrum more or less (usually less) than length
of tympanic bulla; least width of palate less than greatest length
of P4; anterior margin of tympanic bulla as far posterior to
foramen ovale as width of 3 to 4 (including I3) upper incisors;
height of tympanic bulla more than distance from its anterior
margin to foramen ovale; length of tympanic bulla more than length
of lower molar and premolar tooth-row and longer or shorter than
rostrum; anterior margin of masseteric fossa below talonid of m1
or anterior half of m2.
Female (based on 5 adults: Alberta, 3; N. D., 1; Sask., 1.): See
measurements and plates 31-33; weight, 3.1 (2.8-3.5) grams;
basilar length, 42.3 (40.0-43.7); zygomatic breadth more or less
(approximately equal to) than distance between condylar foramen
and M1 or that between anterior palatine foramen and anterior
margin of tympanic bulla; postorbital breadth less than length of
upper premolars and more or less than width of basioccipital
measured from medial margin of one foramen lacerum posterior to
its opposite; least width of palate not more than greatest length
of P4; tympanic bulla as far posterior to foramen ovale as width
of 3 to 4 (including I3) upper incisors; height of tympanic bulla
not less than distance from its anterior margin to foramen ovale;
length of tympanic bulla more than length of lower molar and
premolar tooth-rows and longer or shorter than rostrum.
The skull of the female averages 34 per cent lighter than that of
the male.
Comparisons of the skull with those of M. f. primulina, M. f.
spadix, M. f. oribasus, M. f. alleni, M. f. nevadensis, and
M. f. neomexicana are made in accounts of those subspecies.
Remarks.—Richardson's (1829:47) account on which Bonaparte may be
said to have based his name, records measurements in inches and lines
which I transpose into millimeters as follows: Total length, 440 mm.;
length of head and body, 305; length of tail (vertebrae), 135; length
of tail (including fur), 164 mm. Specimen no. 43.3.3.3 in the British
Museum, which has by some persons been regarded as the type, yields
measurements as follows: Total length, 408 (which allows for 15 mm.
loss of the fleshy part of the end of the tail); length of head and
body, 272; length of tail (vertebrae), 136 (= 121 + 15); length of tail
(including fur), 162 (142 + 20 mm. that appears to have been lost).
Richardson's specimen would appear to have been of unusual proportions
and to have been larger than no. 43.3.3.3. Some reasons for and reasons
against regarding this specimen as the holotype are given in the
account of M. erminea cicognanii.
The name longicauda was applied to practically all long-tailed
weasels of the western United States at one time but as one after
another of the geographic variants in the mountainous regions were
designated as separable, the name longicauda came to be restricted to
the light-colored, relatively large, animal of the Great Plains.
The intergradation of longicauda with spadix and oribasus has
been commented on in the discussions of those subspecies. The larger
size and darker color of specimens referred to longicauda from Devils
Lake and Grafton, North Dakota, are features indicative of
intergradation there with spadix. Two young females from Waterton
Lake Park, Alberta, by their darker than average color, suggest
intergradation with oribasus, as, for that matter, does the specimen
from Waterton Lake [= Chief Mountain Lake, in Montana] itself, which,
however, is even darker than the two specimens taken on the Canadian
side of the line and hence is referred to oribasus. An adult female,
no. 175586, U. S. Nat. Mus., from Moose Pass, Alberta, examined after
the above was written, is larger than any other female seen of
longicauda and in this respect may show approach to oribasus, which
in the northern part of its range is of large size as judged by males
from the Bowron Lake region.
One male, no. 8564, Nat. Mus. Canada, from Max Lake, Turtle Mountain,
Manitoba, presents puzzling characters. The external measurements of
465, 170, and 57, are in keeping with the great length of the skull
which has a basilar length of 48.8. The tooth-rows are 19.3 in length
and the mastoid breadth, 25.4. The relative narrowness indicated by the
mastoid breadth is maintained throughout the skull. The only other
specimens relating to the Turtle Mountains that have been seen are two
male, skins without measurements or corresponding skulls, nos. 38902
and 38903, Amer. Mus. Nat. Hist., labeled as from either "Stump Lake or
Turtle Mts.," North Dakota. One of these, no. 38902, is much darker
than the other. Possibly it is from the Turtle Mountains and the other,
lighter-colored one, is from Stump Lake. Study of additional specimens
from the Turtle Mountains might show the existence there of a distinct
race.
Four specimens, in the collection of Myron Swenk, from Inland, Clay
County, Nebraska, are instructive as showing how intergradation occurs
between primulina and longicauda. A subadult male, no. 10, is
intermediate in external measurements and in color but in each instance
is nearer primulina. The same is true of the least width of the color
of the underparts. The color of the underparts extends uninterruptedly
over the hind legs to the toes as in longicauda, but is absent from
the underside of the tail as in primulina. In the skull, the basilar
length, breadth of bulla, and size of teeth are nearer longicauda, as
are also the ratios to the basilar length of the length of tooth-rows,
breadth of the rostrum, length of the tympanic bulla, and depth of the
braincase at the anterior margin of the basioccipital. Ratios to the
basilar length of the interorbital breadth, mastoid breadth, zygomatic
breadth, and depth of the skull at the posterior borders of the upper
molars are nearer to those of primulina. The relatively long rostrum,
as represented by the orbitonasal length, is nearest to that of
spadix. A young, almost subadult, female, no. 7, agrees with
primulina in color, color pattern, and length of hind foot. The other
external measurements are intermediate, but nearer those of
primulina. Size of skull and teeth are as in longicauda. Relative
proportions of parts of the skull are not diagnostic in specimens as
young as this female. An adult female, skull only, no. 8, agrees with,
or approaches nearer to, longicauda in size of skull and teeth and in
relative proportion of every part studied. A juvenile, skull only, of
questionable sex, no. 9, provides no diagnostic characters. On the
basis of color, these specimens from Inland are distinctly nearer
primulina. On the basis of cranial characters they are distinctly
nearer longicauda. External measurements are intermediate and are a
little nearer those of primulina. By placing the most weight on the
cranial characters, the animals may be referred to longicauda. The
same may be said of 2 skins, one skin with a skull, from Hastings,
Nebraska. In each skin the color-pattern is as in primulina; in one
the under side of the tail is nevertheless lighter-colored more as in
longicauda and the skull, adult male 121651 American Museum of
Natural History, approaches nearer to primulina in narrowness but has
the large teeth of longicauda.
Intergradation with neomexicana is suggested by one specimen, no.
7936, Univ. Kans., from Thomas County, Kansas, which has well-developed
white facial markings.
The specimen, no. 180, Kansas Agric. College, from Glasco, is mounted,
of large size, in white winter pelage, and lacks external measurements.
On the basis of its obvious large size, and a hind foot measurement of
49 millimeters obtained from the mounted skin, the animal is
provisionally referred to longicauda rather than to primulina.
Putorius culbertsoni is a name now credited to Coues (1877:136).
Although Coues probably intended only to indicate that Baird wrote this
name on the labels of two specimens in the mammal collection of the
Smithsonian Institution, Coues gave an "indication" of the application
of the name by publishing at the same time the catalogue numbers of
specimens whose labels bore the name and thus, in accordance with
article 21 of the International Rules of Zoölogical Nomenclature,
himself becomes the author of the name. Of the two specimens mentioned
by Coues, only the first recorded by him, no. 4320 (with skull no.
37995, U. S. Nat. Mus.), can now be found.
Fortunately, the skull of this specimen labeled (see Lyon and Osgood,
1909:218) as taken at Fort Laramie, Wyoming, is well preserved. Its
only defects are a fracture in the left zygomatic arch and the absence
of parts of each of the first lower molars. In deciding on the
subspecific application of the name Putorius culbertsoni Coues, the
skull of the type must be principally relied upon, for there is
available only one other specimen, a skin only (no. 12596, U. S. Nat.
Mus.), from the same place, and it, like the type, is in white winter
pelage and lacks flesh measurements.
The ranges as now known of three subspecies of Mustela frenata
approach near to Fort Laramie. These are M. f. longicauda, M. f.
alleni, and M. f. nevadensis. The skull of the type of culbertsoni
is not typical of any one of the three mentioned races. The small size
of its teeth and relative (to basilar length) shallowness of the
frontal region of the skull through the postorbital processes of the
frontal are as in nevadensis. The zygomatic arches are not so greatly
expanded as in some specimens of longicauda and are more like the
average for nevadensis or alleni, as also is the relatively (to
basilar length) long orbitonasal length. However, each of these
characters is subject to variation and alone is not surely diagnostic,
especially toward the margin of the range of any one of the subspecies
concerned. The same may be said of the relatively great breadth of the
skull interorbitally—a feature typically found in longicauda. More
important, in my estimation, is the large size of the skull; all parts
measured (excepting the teeth, the depth at the posterior border of the
last upper molars, the zygomatic breadth, and the depth of the tympanic
bullae) equal or approach nearest to the average for males of
longicauda of similar age.
The small size of alleni prevents its identification with
culbertsoni. The question of application lies between nevadensis
and longicauda. If the long-tailed weasel at Fort Laramie is found to
be referable to the race earlier named longicauda, no change in
current nomenclature will be effected. If, on the other hand, the
long-tailed weasel from Fort Laramie is found to be referable to
nevadensis this name will have to fall before the earlier proposed
name culbertsoni. There is, however, a third possibility, namely,
that the long-tailed weasel of the Transition and Upper Sonoran zones
of southern Wyoming and northern Colorado, as for example, at Lay,
Colorado, may represent a recognizable race characterized by size about
as in longicauda, relative proportions of skull about as in
nevadensis and coloration intermediate, to which the name
culbertsoni may apply. For more detailed discussion of this
possibility, see remarks under M. f. nevadensis.
Satisfactory application of the name Putorius culbertsoni Coues
requires an adequate series of adult specimens, of both sexes in the
summer coat with external measurements taken in the flesh, from the
type locality and like material from elsewhere in southern Wyoming. On
the evidence furnished by the skull of the type of culbertsoni, that
name tentatively is placed in the synonomy of longicauda.
Only 2 of 25 adults examined for malformation of the frontal sinuses by
parasites showed evidence of disease.
Specimens examined.—Total number, 138, arranged alphabetically
by provinces and states and further by districts or counties from
north to south except as otherwise indicated. Unless otherwise
indicated specimens are in the collection of the United States
National Museum.
Alberta. St. Albert, 1; S. Edmonton, 3; Islay, 4[77]; Battle
River, south of Camrose, 1[77]; Daysland, 1[77]; Moose Pass, 1;
Blindman River, 2 (1[75], 1[2]); Red Deer, 3 (2[2], 1[60]);
Bearberry Creek near Sundre, 1[77]; Canad. Nat. Park, N.W.
Territory, 1[60]; Red Deer River, Didsbury, 1; Canmore, 1;
Calgary, 11 (6[60], 2[1], 1[86]); Red Deer River, 3[2]; Little
Sandhill Creek, Red Deer River, 1[77]; Waterton Lake Park, 2[77];
Sweetgrass Hills, 1[77]; Alberta, 1[14].
Colorado. Yuma County: Wray 4 (1[88], 3[74]).
Kansas. Rawlins County: 7 mi. N, 3 mi. W Beardsley, 1[74]; 6 mi.
S and 2 mi. E Atwood, 1[74]; 15 mi. SE Atwood, 1[74]. Thomas
County: near Brewster, 2[93]; no locality more definite than
county, 2[93]. Trego County, 2 (1[2]). Cloud County: Glasco,
1[67].
Manitoba. Portage la Prairie, 3[75]; Carberry, 2 (1[2], 1[1]);
Carman, 1[60]; Max Lake, Turtle Mt., 1[77].
Montana. Glacier County: St. Marys Lake, 1; Blackfoot, 1:
Blackfoot Agency, 1. Blaine County: 6 mi. east Chinook, 1[74].
Pondera County: 1/2 mi. SE Conrad, 1[74]. Toole County: Shelby
Junction, 1. Hill County: Havre, 1. Fergus County: Moccasin
Mts., 5 mi. NW Hilger, 1; 7 mi. NE Hilger, 1. Rosebud County:
3/4 mi. N Ingomar, 1. County in question, Milk River, 2.
Nebraska. Dawes County: Chadron, 2[35]. Cherry County:
Kennedy, 1; no locality more definite than county, 1. Brown
County: Long Pine, 1[68]. Antelope County: Neligh, 1[35].
Adams County: Hastings, 2[2]. Clay County: Inland, 4[35].
North Dakota (arranged by counties from west to east). Divide
County: Crosby, 1. Mountrail County: Lostwood, 1. Little
Missouri River, 1. Golden Valley County: Sentinel Butte, 1.
Billings County: Medora, 1[60]. McLean County: 3 mi. W
Elbowoods, 1. Oliver County: Ft. Clark, 2. Morton County:
Mandan, 1. Sioux County: 3 mi. N Cannonball, 1. Logan County:
6 mi. SW Napoleon, 1. Rolette County: Turtle Mts., 1[76]; Fish
Lake, 1. Benson County: Ft. Totten, 3[14]; Sully Hill Nat. Park,
1. Ramsey County: Devils Lake, 2. Stump Lake or Turtle Mts.,
2[2]. Nelson County: Stump Lake, 1. Grand County: Larimore, 1.
Walsh County: Grafton, 11 (4[76], 3[74], 2[2]). Stutsman
County: Jamestown, 1. Barnes County: Valley City, 1.
Saskatchewan. Wingard, 5; Osier, 2[75]; Simpson, 1[2]; Touchwood
Hills, 4[7]; South arm Last Mountain Lake, 1[77]; Rush Lake
(Assiniboia, N.W.T.), 2[75].
South Dakota. Pennington County: Rapid City, 1.
Wyoming. Goshen County: Fort Laramie, 2.
Mustela frenata oribasus (Bangs)
Long-tailed Weasel
Plates 16, 17, 18, 31, 32, 33 and 40
Putorius (Arctogale) longicauda oribasus Bangs, Proc. New England
Zoöl. Club, 1:81, December 27, 1899.
Putorius longicauda, Coues, Fur-bearing animals, p. 136, 1877
(part).
Mustela longicauda oribasus, Miller, U. S. Nat. Mus. Bull.,
79:98, December 31, 1912.
Mustela longicauda oribasa, Hall, Univ. California Publ. Zoöl.,
40:368, November 5, 1934.
Mustela frenata oribasa, Hall, Carnegie Instit. Washington Publ.
437:105, November 20, 1936.
Type.—Female, adult, skull and skin; no. 9058, collection of E.
A. and O. Bangs, but now in collection of Mus. Comp. Zoöl.; source
of Kettle River, 7500 feet [the summit between middle fork of
Kettle River and Cherry Creek at Pinnacles—oral information from
the collector, Feb. 12, 1936], British Columbia; September 10,
1898; obtained by Allan Brooks; original no. 1368.
The skull (plate 40) is complete and unbroken. The teeth all are
present and entire except right I3 which has the anterior half
broken away. The skin is complete, fairly well made, and in summer
pelage.
Range.—Canadian and Hudsonian life-zones from near 56°N in the
Rocky Mountains of British Columbia and Alberta and Ootsa Lake
along the Fraser and Chilcotin rivers south to Alta Lake, in the
Caribou and Monashee mountains, probably in the Selkirks and
Rockies, and through the Rockies of Montana into extreme northern
Wyoming. See figure 29 on page 221.
Characters for ready recognition.—Differs from M. f.
longicauda by near (14 n) Brussels Brown rather than near (h)
Clay Color of upper parts and in males by relatively shallower
occiput in which the depth of the skull, exclusive of the sagittal
crest and taken at the anterior border of the basioccipital,
amounts to less than 59 per cent of the mastoid breadth; from M.
f. nevadensis by greater average size, see measurements.
Description.—Size.—Male: Two adults from Florence, Montana,
measure as follows: Total length, 440, 440; length of tail, 165,
161; length of hind foot, 47, 49. Corresponding measurements of an
adult male from Quesnel, British Columbia, are: 443; 168; 55. Tail
amounts to 60, 58, and 61 per cent as long as head and body.
Length of hind foot averages more than basal length.
Female: The type specimen, the only typical adult or subadult
specimen of this sex of which external measurements are available,
measures: Total length, 392, length of tail, 150, length of hind
foot, 46. Tail is 63 per cent as long as head and body. Length of
hind foot amounts to more than basal length.
The differences in external measurements, between the one female
and the average of the three males are: Total length, 49; length
of tail, 15; length of hind foot, 4.
Externals.—Longest facial vibrissae brown or white (often both
colors in same specimen) and extending beyond ear; carpal
vibrissae same color as underparts and extending to or beyond
apical pad of fifth digit; hairiness of foot-soles (in summer
pelage) slightly less than shown in figure 19.
Color.—Upper parts, in summer, near (14 n) Brussels Brown,
more blackish and less reddish than tone 4 of Burnt Umber of
Oberthür and Dauthenay, pl. 304; in type near tone 4, pl. 301 of
Oberthür and Dauthenay. Underparts, in summer, Buff Yellow or near
(20 c) Amber Yellow. In winter, all white except tip of tail
which is at all times black. Upper parts of uniform color except
for occasional slight darkening of top of head and along
mid-dorsal line of back. Color of underparts extends distally on
posterior sides of forelegs over feet, on medial sides of hind
limbs over antiplantar faces of toes and over proximal two-thirds
of ventral side of tail. Least width of color of underparts
amounting to 43 per cent of greatest width of color of upper
parts, 75 per cent in male from 4 miles northeast of Quesnel,
British Columbia, and 52 (33-66) in four males from Montana. Black
tip of tail in four males from Montana averaging 50 (44-60) mm.
long. Thus averaging approximately as long as hind foot and 33 per
cent of length of tail-vertebrae.
Color not different than in many specimens of M. f. nevadensis.
Color comparison with M. f. longicauda has been made in the
account of that subspecies.
Skull and teeth.—Male (based on 5 adults and 2 subadults from
British Columbia and 4 adults from Montana): See measurements and
plates 16-18. As described in Mustela frenata longicauda except
that: Weight, 5.0 (3.8-6.0) grams; basilar length, 46.7
(43.6-48.8); postorbital breadth in one of nine instances less
than width of basioccipital measured from medial margin of one
foramen lacerum posterior to its opposite; interorbital breadth
more or less than distance between foramen opticum and anterior
margin of tympanic bulla; breadth of rostrum less than length of
tympanic bulla; anterior margin of tympanic bulla as far posterior
to foramen ovale as width of 2-1/2 to 5 upper incisors; length of
tympanic bulla not less than length of lower molar and premolar
tooth-row and shorter than rostrum.
Female (based on the type, specimen): See measurements and plates
31-33, 40. As described in Mustela frenata longicauda except
that: Weight, 3.5 grams; basilar length, 41.6 mm.; zygomatic
breadth more than distance between anterior palatine foramen and
anterior margin of tympanic bulla; postorbital breadth more than
width of basioccipital measured from medial margin of one foramen
lacerum posterior to its opposite; least width of palate more than
outside length of P4; tympanic bulla as far posterior to
foramen ovale as width of 4-1/2 upper incisors; height of tympanic
bulla less than distance from anterior margin of tympanic bulla to
foramen ovale; length of tympanic bulla less than length of
rostrum. If more than one skull were available of the female of
oribasus it is believed that the description would agree with
that of longicauda in nearly all features.
The skull of the female is 30 per cent lighter than that of the
average male.
Comparison with longicauda reveals that, on the average, skulls of
males are larger, relative to the basilar length broader across the
mastoids, shallower through the braincase as measured at the anterior
end of the basioccipital exclusive of the sagittal crest, with longer
rostrum. Compared with nevadensis, the skull averages larger in all
measurements taken, and has a relatively broader rostrum, relatively
greater mastoid breadth and a braincase which is shallower relative to
the basilar length. By weight, the skull of nevadensis is a fourth
lighter, and in linear measurements 5 to 18 per cent smaller.
Remarks.—Some of the specimens from Montana, which here are referred
to oribasus, more than half a century ago were listed by Coues
(1877:138) under the name longicauda. It was not until 1899 that this
race was given a name by Bangs, who at that time (1899B:81) accurately
made out the distinctive color features. Distinctive cranial characters
cannot be described with assurance even now because there still are too
few specimens.
The type specimen, at one time examined by the present writer, has on
the stuffed skin no well-developed mammae, scrotal pouch, or other
visible sexual part. Probably the collector's sex mark for female is
correct.
As judged by the two skulls of subadult males from the Barkerville
region, individuals of this race attain larger size than do those of
longicauda. On the basis of larger size than either longicauda or
nevadensis, the specimens from the Rocky Mountains of Montana and two
from northern Wyoming are referred to this race. The short, wide, flat,
tympanic bullae, relatively great mastoidal breadth, and some other
features of the specimen from Donovan, Montana, point toward
oribasus, whereas nearly as many more cranial features, in this
instance mainly differences in size, are indicative of nevadensis to
which race the specimen might almost equally well be referred. Another
male from Darby, in the Bitterroot Valley of Montana, has a slightly
longer hind foot than those from Florence, but a female from Hamilton,
agrees more nearly with nevadensis. The average of all the specimens
from the Bitterroot Valley is a little nearer oribasus. Four skulls
from Buffalo, Wyoming, here referred to nevadensis show approach to
oribasus in size of skull. The specimens from Big Snowy Mountains,
and the Highwood Mountains of Montana are too young clearly to show
size of the adult skull, but are distinctly darker colored than
longicauda of the plains country proper. Of two subadult females from
Tacy, Montana, the color of the one in summer pelage is distinctly
nearer that of oribasus and nevadensis than it is to that of
longicauda to which some approach in color might be expected. The
reduced size of both of the specimens is further suggestive of
nevadensis and it may be that adult specimens from these more eastern
mountainous areas in Montana will show that nevadensis is the name
proper to apply to animals of this region.
Intergradation with nevadensis is suggested by specimens collected
from along the upper reaches of Okanagan Lake, British Columbia, by
Major Allan Brooks and Mr. J. A. Munro and by a series of skulls from
Ione, Pend Orielle County, Washington, lent me by Mr. Walter Dalquest.
At each place, the average of all specimens is nearest to that of
nevadensis.
Specimens from near Waterton Lake show several steps in the transition
from the light-colored longicauda type of coloration to the darker
coloration characterizing oribasus. One taken here, at a time when
the body of water referred to seems to have been known as Chief
Mountain Lake, is barely dark enough to be placed with oribasus. Two
other specimens from across the Canadian Border labeled as "Waterton
Lake Park" are slightly lighter colored above, and on this account are
placed with longicauda.
The two adult males from Lillooet, British Columbia, are referable to
oribasus although neither is quite typical. One has a saturated
coloration suggestive of that of altifrontalis and the skull is
shorter and broader than in other specimens of oribasus. The female
from Lillooet, skin alone, no. 916, Prov. Mus., B. C. is small for
oribasus. The female, no. 1539, collection of Kenneth Racey, from
Alta Lake, in brown winter pelage, in almost every measurement falls
nearly midway between altifrontalis and oribasus but slightly
nearer the latter. The skull from Chezacut and 3 animals from Wistaria,
British Columbia, probably are females and show a greater average size
than specimens from farther to the southeast. For example, the basilar
length of the skull, 44.8 (44.3 to 45.1), exceeds that of the type
specimen. The animals from Wistaria on Ootsa Lake furnish the
northwesternmost station of occurrence of which I have record for this
subspecies.
The northernmost records of occurrence, at "Clearwater River, Peace
River, B. C," and at Little Prairie, are furnished by a white skin
without skull, no. 257450, U. S. Nat. Mus., purchased on August 2,
1932, at the place mentioned by W. H. Sheldon and Richard Borden, and a
skull with white winter skin, no. 3585, Provincial Museum, British
Columbia, respectively. The characters distinguishing longicauda and
oribasus are not shown by white winter skins; the skull shows some
features of longicauda, and the reference of these specimens to
oribasus rather than longicauda is tentative.
Only the skull from Little Prairie shows evidence of infestation of the
frontal sinuses by parasites. In the Barkerville area of British
Columbia, Mr. and Mrs. Thomas T. McCabe obtained only 2 skulls of this
subspecies from a total of 238 weasel skulls gathered by local
trappers. The others were Mustela erminea.
Specimens examined.—Total number, 46, listed by localities from
north to south and unless otherwise indicated, in the United
States National Museum.
British Columbia. West of Hudson Hope, 1[7]; Clearwater River,
tributary to Peace River, 1; Little Prairie, a few miles south of
Peace River and about 40 miles west of the main highway between
Dawson Creek and Fort St. John, 1[85]; Wistaria, 3[85]; Four Mile
Creek, 4 mi. NE Quesnel, 1[21]; Isaacs Lake, 3200 ft., 1[74];
Barkerville region, 1[74]; Clear River, 4800 ft., 1[74]; Chezacut,
1[31]; Lillooet 3 (2[77], 1[85]); Alta Lake, 1[31]; source of
Kettle River, 7500 ft., 1[75]; E side Beaverfoot Range, 4000 to
4500 ft. between Fraser Creek and 6 mi. SE of Fraser Creek, 1[74];
Cranbrook, 1[86]; head of Cross River, 10 mi. below Assiniboine
Pass, 1[7]; camp east of "Kootanie," 1[7]; camp east of Kootanie
River, 1[7].
Alberta. Thoral Creek, 7000 ft., 50 mi. NE Jasper, 1[2].
Montana. Glacier? County: Chief Mt. Lake (= Waterton Lake), 1.
Flathead County: Columbia Falls, 1. Chouteau? County: Highwood
Mts., 1. Fergus? County: Big Snowy Mts., 1. Wheatland County:
Harlowton, 1[74]. Ravalli County: Florence, 2; Hamilton, 1[56];
Darby, 1[56]; Carlos [= Charlos] Heights, 2[74]; Tin Cup District,
2[74]; no locality more definite than county, 2[74]. Beaverhead
County: Donovan, 1. Madison County: Sheridan, 1[74]. Gallatin
County: Ranch 7-11, Eldridge, 1[60]. Stillwater County: Tacy,
2[76]. County in question: Gallatin Valley, 1; Yellowstone Park,
1[75].
Wyoming. Glen Creek, Mammoth Hot Springs, 1. Park County: Four
Bears, 1[2].
Mustela frenata alleni (Merriam)
Long-tailed Weasel
Plates 18, 19, 20, 31, 32 and 33
Putorius alleni Merriam, N. Amer. Fauna, 11:24, June 30, 1896.
Mustela alleni, Miller, U. S. Nat. Mus. Bull., 79:99, December
31, 1912.
Mustela frenata alleni, Hall, Carnegie Instit. Washington Publ.
473:106, November 20, 1936.
Type.—Male, adult, skull and skin; no. 186451, U. S. Nat. Mus.
(formerly 4485/5120, collection of Dr. C. Hart Merriam); Custer,
South Dakota; obtained by Vernon Bailey; original no. 90.
The skull is complete and unbroken. The upper incisors are
missing. All the other teeth are present although the premolars,
and especially the canines, are much worn, possibly as the result
of the animal's efforts to free itself from a trap. The skin is
fairly well made, in a good state of preservation, and entire.
Range.—Canadian, Transition and Upper Sonoran life-zones of the
Black Hills of South Dakota and adjacent semi-bad-land territory
of Wyoming and Nebraska southward to Mitchell, Scottsbluff County.
See figure 29 on page 221.
Characters for ready recognition.—Differs from M. f.
longicauda in smaller size, adult males having a total length of
less than 400, hind foot less than 45, basilar length less than
43.5, and in adult females total length less than 375, and basilar
length less than 40; from M. f. nevadensis in near Clay Color
rather than near (14 n to l) Brussels Brown of upper parts in
summer.
Description.—Size.—Male: External measurements of the type
specimen are: Total length, 372; length of tail, 137; length of
hind foot, 44. Tail is 58 per cent as long as head and body.
Length of hind foot more than basal length.
Female: No external measurements for typical adults are available.
No. M1 #41 from Mitchell, Scottsbluff Co., Nebraska, an adult
female which is an intergrade with the larger M. f. longicauda,
measures as follows: Total length, 367; length of tail, 120;
length of hind foot, 41.
Externals.—Longest facial vibrissae dark brown or white and
extending beyond ear; carpal vibrissae same color as underparts
and extending to apical pad of fifth digit; hairiness of
foot-soles (in summer pelage) as shown in figure 20.
Color.—Winter pelage unknown; probably white except, of course,
tip of tail. Summer pelage as described in Mustela frenata
longicauda except that: Least width of color of underparts
averaging, in 3 males from Black Hills, 54 (38-62) per cent of
greatest width of color of upper parts. Black tip of tail
averaging 43 (40-45) mm. long. Thus, averaging approximately same
length as hind foot and in type specimen amounting to 33 per cent
of length of tail-vertebrae.
Skull and teeth.—Male (based on the type and no. 7440 Amer.
Mus. Nat. Hist., from Hill City, S. Dak.): See measurements and
plates 18-20. As described in Mustela frenata longicauda except
that: Weight, 3.1 (3.0-3.2) grams; basilar length, 41.0
(40.9-41.0); mastoid breadth not less than postpalatal length;
breadth of rostrum more than length of P4; anterior margin of
tympanic bulla as far posterior to foramen ovale as width of 4 to
5 upper incisors; height of tympanic bulla more or less than
distance from its anterior margin to foramen ovale.
Female (based on no. 7441, American Mus. Nat. Hist., from Black
Hills, S. Dak.): See measurements and plates 31-33. As described
in Mustela frenata longicauda except that: Weight, 2.0 grams;
basilar length 37.6. The skull of the female is 35 per cent
lighter than the average for the two males.
Comparison with M. f. longicauda and M. f. nevadensis reveals
that the tympanic bullae average more nearly flat and that the
skull is smaller.
Remarks.—Animals of this subspecies were described and named by
Merriam in 1896 as a distinct species on the basis of two or possibly
three specimens from the Black Hills of South Dakota and the name seems
never to have been applied to specimens from other regions. Vernon
Bailey obtained only the one specimen, the type, on his trip in 1888,
but two more were obtained for the American Museum of Natural History
by Walter Granger in 1894.
Mustela frenata alleni combines the light coloration of M. f.
longicauda with the small size of M. f. nevadensis. Indeed, the size
may average less than that of nevadensis. M. f. alleni seems to
reach its extreme of small size in the Black Hills of South Dakota.
Specimens from Mitchell, Scottsbluff County, Nebraska, here referred
to alleni are of larger size and in this respect are intermediate
between the subspecies alleni and longicauda. Of the two specimens
available from Chadron, Nebraska, and here referred to as longicauda,
the female, M1 #6, is almost exactly intermediate in size between
alleni and longicauda, whereas the male, M1 #11, is as large as the
average-sized longicauda.
None of the nine skulls (5 adults) shows malformation resulting from
the infestation of the frontal sinuses with parasites.
Specimens examined.—Total number, 10, as follows.
Wyoming. Crook County: Sundance, 1[91].
South Dakota. Pennington County: Hill City, 1[2]; 20 mi. N Elk
Mt, 1[91]. County in question: Black Hills, 1[2]. Custer
County: Custer, 2 (1[91], 1[2]).
Nebraska. Scottsbluff County: Mitchell, 4[35].
Mustela frenata arizonensis (Mearns)
Long-tailed Weasel
Plates 19, 20, 21, 31, 32 and 33
Putorius arizonensis Mearns, Bull. Amer. Mus. Nat. Hist., 3:234,
June, 1891; Merriam, N. Amer. Fauna, 11:22, fig. 12, June 30,
1896.
Mustela arizonensis, Miller, U. S. Nat. Mus. Bull., 79:99,
December 31, 1912.
Mustela frenata arizonensis, Hall, Carnegie Instit. Washington
Publ. 473:106, November 20, 1936.
Type.—Female, adult, skull and skin; no. 2490/1886, Amer. Mus.
Nat. Hist.; San Francisco Forest [then (1886?), Yavapai County],
Arizona; June 20, 1886; obtained by Edgar A. Mearns.
The skull (plates 31-33) is complete and unbroken save for a small
puncture in the right squamosal. The incisors above and below and
M2 and P2 on each side are missing. Four canines are preserved
separately. Otherwise the teeth are in place. The skin has been
taken down from a mount. Some hair has been lost from in front of
the ears. Seven mammae are evident and show the animal to have
been nursing young. The slightly faded color was mentioned by
Mearns in the original description. He says (1891:234): "The
memorandum of the colors was made before skinning, the specimen
having been subsequently preserved in a solution of alum and salt,
which extracted much of the coloring matter."
Range.—Transition to Hudsonian life-zones of Arizona and
extreme western New Mexico, along the Colorado River, and south of
the Little Colorado River, from San Francisco Mountain region
along Mogollon Plateau to extreme western New Mexico. See figure
29 on page 221.
Characters for ready recognition.—Differs from M. f.
neomexicana by near (14 n) Brussels Brown rather than Buckthorn
Brown color of upper parts, in absence rather than presence of
white frontal spot continuous with color of underparts, in basilar
length of less than 44 in males and 39.3 in females; from M. f.
nevadensis in that total length averages less than 375 in males
and 330 in females, basilar length averaging less than 41 in males
and less than 36.7 in females.
Description.—Size.—Male: No. 24679/32071, from
Springerville, and no. 248993 from the Kaibab Plateau, measure
respectively, as follows: Total length, 363, 367; length of tail,
140, 143; length of hind foot, 41.5, 41.0. Tail is 63, and 64 per
cent as long as head and body. These males, the only specimens of
that sex of which external measurements are available, probably
are grading toward nevadensis and therefore are nontypical.
Female: Three specimens, one young from Little Spring, a subadult
from Deadmans Flat and the type specimen, measure respectively as
follows: Total length, 323, 296, 302; length of tail, 110, 101,
109; length of hind foot, 38, 33, 36. These average, 307, 107, 36.
Tail averages 53 per cent as long as head and body.
Differences in external measurements of the two sexes are: Total
length, 56; length of tail, 39; hind foot, 5.5.
Externals.—Longest facial vibrissae black, brown or white
(often all three colors in same specimen) and extending beyond
ear; carpal vibrissae same color as underparts and extending to
apical pad of fifth digit; hairiness of foot soles (in summer
pelage) about as shown in figure 19.
Color.—Winter pelage unknown. Summer pelage with upper parts
near (14 n) Brussels Brown or tone 2 of Raw Umber of Oberthür and
Dauthenay, Pl. 301, darker on top of head from nose to line
connecting posterior margins of ears. Tip of tail always black.
Chin and upper lips white. Remainder of underparts Buff Yellow to
Straw Yellow and rarely Ochraceous Buff. Color of underparts
extends distally on posterior sides of forelegs over toes onto
antipalmar faces of feet and wrists, on medial sides of hind legs
to ankles and over antiplantar faces of toes, medial third of
tarsus, and over proximal fifth to fourth of ventral side of tail.
Least width of color of underparts averaging, in 8 specimens, 44
(29-54) per cent of greatest width of color of upper parts. Black
tip of tail, in four females averaging 35 (33-38) mm. long. Thus,
averaging shorter than hind foot and 32 per cent of length of
tail-vertebrae. Three of the eight specimens before me (no. 242671
from 25 mi. SE Flagstaff, not available at time of this
accounting) have the dark spot near the angle of the mouth faintly
indicated, whereas the other five lack the spots. The color is as
in M. f. nevadensis.
Skull and teeth.—Male (based on 55211, 65231, and 248993; see
page 422): See measurements and plates 19-21; weight 2.7 and 3.1
grams; basilar length, 40.4; zygomatic breadth more than distance
between condylar foramen and M1 or than between anterior palatine
foramen and anterior margin of tympanic bulla; mastoid breadth
more than postpalatal length; postorbital breadth less than length
of upper premolars and more than width of basioccipital measured
from medial margin of one foramen lacerum posterior to its
opposite; interorbital breadth more or less than distance between
foramen opticum and anterior margin of tympanic bulla; breadth of
rostrum less than length of tympanic bulla; least width of palate
more or less than medial length of P4; anterior margin of tympanic
bulla as far posterior to foramen ovale as width of 3-1/2
(including I3) upper incisors; height of tympanic bulla more than
distance from its anterior margin to foramen ovale; length of
tympanic bulla more than length of lower molar-premolar tooth-row
and longer or shorter than rostrum; anterior margin of masseteric
fossa below talonid of m1.
Female (based on the type specimen): See measurements and plates
31-33; weight, 1.6 grams; basilar length, 35.5; zygomatic breadth
less than distance between condylar foramen and M1 and more than
distance between anterior palatine foramen and anterior margin of
tympanic bulla (nearly equal in each instance); postorbital
breadth less than length of upper premolars and greater (7.1-8.4)
than width of basioccipital measured from medial margin of one
foramen lacerum posterior to its opposite; least width of palate
equal to inside length of P4; tympanic bulla as far posterior to
foramen ovale as width of 3 (including I3) upper incisors; height
of tympanic bulla more than distance from its anterior margin to
foramen ovale; length of tympanic bulla more than length of lower
molar-premolar tooth-row and greater than length of rostrum.
The skull of the female averages 41 per cent lighter than that of
the male.
Compared with the skull of M. f. nevadensis, that of arizonensis is
smaller, less heavily ridged and has more inflated tympanic bullae and
a relatively greater mastoid breadth. Comparison with the skull of M.
f. neomexicana is made in the account of that subspecies.
Remarks.—In 1891 Mearns (234-235) named this weasel as a full
species on the basis of two individuals taken by him in 1886 and 1887.
Since that time only a few additional specimens have been preserved.
Only four are adults. Although this material does not permit of a
definition of the subspecies as precise as could be wished, still, it
clearly shows that the animals from the plateau region of Arizona are
recognizably different from those farther north in the Sierra Nevada of
California and those of the Rocky Mountains and Great Basin region
northward to the Canadian border. These more northern animals have gone
by Mearns' name, arizonensis, since the date of its proposal until
1939 when the name nevadensis was proposed.
The smaller size, especially of the skull, and the greater inflation of
the tympanic bullae are the outstanding characters which distinguish
arizonensis from the similarly marked nevadensis. The bullae are
relatively much inflated throughout but especially so on the
posteromedial parts.
Although the three adult males and two subadult females available of
this subspecies are smaller in most parts measured than any of the
scores of nevadensis of similar age that have been measured, overlap
in size probably will be found as additional specimens of arizonensis
become available. A young female, no. 18513, coll. D. R. Dickey, from
Little Spring, does have certain cranial measurements as large as are
found in the minimum-sized nevadensis from farther north.
Intergradation with the two subspecies whose geographic ranges adjoin
that of arizonensis is indicated by specimens at hand. One of these
is the adult male from 25 miles southeast of Flagstaff, which shows
decided approach to neomexicana, in color and in possessing white
facial markings less well developed than in neomexicana. Even better
developed white facial markings, with intervening blackish coloration,
are displayed by no. 148271, U. S. Nat. Mus., from 8500 feet altitude
on Willow Creek, New Mexico. This subadult female shows approach to
neomexicana also in larger size of the skull and entire animal. The
great inflation of the posterior part of each of its bullae and the
dark color of the upper parts are characters of arizonensis. The
color of the underparts stops at the ankles leaving the hind feet dark
colored, in which respect the specimen is unlike either neomexicana
or arizonensis. If additional specimens showing the same characters
as this one be found at other nearby localities they probably should be
given recognition as a separate subspecies. For the present it seems
best to regard the specimen merely as an intergrade. Although it might,
with almost equal propriety, be referred to either neomexicana or
arizonensis, the specimen is here placed with the latter. The
subadult male from Springerville, Arizona, is of larger size than the
topotypical male of arizonensis and in this respect shows slight
approach to nevadensis. The narrower mastoidal breadth and slightly
less inflated tympanic bullae of the male from the Kaibab Plateau may
reflect merely individual variation or may represent intergradation in
these features with nevadensis.
The statement made by Merriam (1896:22) that, "The type specimen . . .
is an immature female and is of unusually small size. A male obtained
by him [Mearns] near the same place is of the normal size, as is
another male in the Department collection from Springerville, Ariz.,
collected by E. W. Nelson," needs correction. The female is not
immature. The specimen obtained by Mearns near the same place probably
refers to Amer. Mus. No. 2489, from Quaking Asp Settlement, which lacks
both the skull and external measurements. As stuffed it is of small
size for a male. The male from Springerville, as shown by the external
and cranial measurements, is not of normal (i. e. average) size, but
is smaller than the average for the other populations of similarly
colored weasels referred to by Merriam (op. cit.) as arizonensis
but here described under the name nevadensis.
None of the skulls shows signs of infestation of the frontal sinuses by
parasites.
Specimens examined.—Total number, 17, arranged alphabetically
by states and from north to south by counties in each state.
Unless otherwise indicated specimens are in the collection of the
United States National Museum.
Arizona. Coconino County: VT Park, Kaibab Plateau, 1; Deadman
Flat, 6400 ft., 1[74]; Little Spring, 1[59]; Government Prairie,
near Parks, 1[74]; Coconino? County: San Francisco Forest
(Yavapai Co., in 1886), 1[2]; 25 mi. SE Flagstaff, 1; Quaking Asp
Settlement, 1[2]. Apache County: Springerville, 1; North Fork
White River, White Mts., 8200 ft., 4[87]; head San Francisco
River, Judd Ranch, Alpine, 1[74]; 2 mi. SE Big Lake Knoll, 8700
ft., 24 mi. S Springerville, 1[74]. Greenlee County: S end Blue
Range, 9000 ft., Prieto Plateau, 1; Beaver Creek, 7000 ft., 1[74].
New Mexico. Grant County: Mogollon Mts., Willow Creek, 8500 ft.,
1.
Mustela frenata nevadensis Hall
Long-tailed Weasel
Plates 19, 20, 21, 33, 34, 35 and 39
Mustela frenata nevadensis Hall, Carnegie Instit. Washington
Publ. 473:91, November 20, 1936.
Putorius longicauda, Coues, Fur-bearing animals, p. 136, 1877
(part); Merriam, N. Amer. Fauna, 5:83, July 30, 1891.
Putorius (Gale) brasiliensis frenatus, Coues, Fur-bearing
animals, p. 142, 1877 (part).
Putorius arizonensis, Merriam, N. Amer. Fauna, 11:22, figs. 13,
14, June 30, 1896 (part); Stephens, Mammals of California, p.
247, 1906.
Mustela arizonensis, Grinnell and Swarth, Univ. California Publ.
Zoöl., 10:376, October 31, 1913; Whitlow and Hall, Univ.
California Publ. Zoöl., 40:247, September 30, 1933.
Mustela arizonensis arizonensis, Grinnell, Univ. California Publ.
Zoöl., 40:102, September 26, 1933.
Mustela frenata, Boyer, Journ. Mamm., 24:99, February 20, 1943.
Type.—Female, adult, skull and skin; no. 41053, Mus. Vert.
Zoöl.; three miles east Baker, White Pine County, Nevada; May 30,
1929; obtained by E. R. Hall and W. C. Russell; original no. 2674,
E. R. H.
The skull (plates 33-35) is complete and unbroken. The teeth all
are present and entire. The skin is fairly well made. Eight mammae
are evident and show the animal to have been nursing young.
Range.—Altitudinally, 700 feet at Wenatchee, Washington, to the
highest parts of the mountains of the western United States; Upper
Sonoran Life-zone to Arctic Alpine Life-zone; southern British
Columbia in the Cascades and territory west to Monashee Mountains,
and Nelson, southward in the Cascades of northern Washington, over
western Washington, Idaho, Utah, and Nevada to northeastern
Arizona and northern New Mexico; westward from the eastern base of
the Rocky Mountains in Colorado to the western base of the Sierra
Nevada and Cascades of California and to the Cascades of southern
Oregon. See figures 29 and 30 on pages 221 and 314.
Characters for ready recognition.—Differs from M. f. oribasus
by smaller average size, see measurements; from M. f. longicauda
by near (14 n to l) Brussels Brown rather than near (h) Clay
Color of the upper parts, and in males by a shallower occiput in
which the depth of the skull, exclusive of the sagittal crest, and
taken at the anterior border of the basioccipital, amounts to
less than 59 per cent of the mastoid breadth; from M. f. alleni
by near (14 n to l) Brussels Brown rather than near (h) Clay
Color of upper parts in summer; from M. f. neomexicana by near
(14 n to l) Brussels Brown rather than Buckthorn Brown color
of upper parts, in absence of white frontal spot continuous with
color of underparts, in basilar length of less than 46 in males
and 40 in females; from M. f. arizonensis by total length
averaging more than 375 in males and 330 in females, basilar
length averaging more than 41 in males and 36.7 in females; from
M. f. inyoensis by absence of white facial markings; from M. f.
pulchra by absence of light facial markings, near (14 n to l)
Brussels Brown rather than near (16 j) Buckthorn Brown color of
upper parts, and lesser size, hind foot less than 40 in females
and basilar length averaging less than 46.0 in males; from M. f.
xanthogenys by absence of light facial markings and near (14 n
to l) Brussels Brown rather than Buckthorn Brown color of upper
parts; from M. f. munda by absence of white facial markings,
presence of color of underparts on ventral face of proximal third
of tail, and hind foot of less than 50 in males; from M. f.
saturata by presence of light color of underparts on tail and
ankle and in lesser average breadth across mastoid processes of
skull (see measurements); from M. f. oregonensis by absence of
nasofrontal white patch, presence of light color of underparts on
ventral face of tail, and shorter skull, which, relative to its
length in males, is deeper through the braincase; from M. f.
washingtoni by presence of light color of underparts on ventral
face of tail, by skull which in male relative to basilar length is
shorter in the preorbital region and wider across the zygomata and
mastoid processes, and in female has longer preorbital region and
larger bullae (see measurements); from M. f. altifrontalis by
lighter colored upper parts which are tones 1 to 3 of Raw Umber,
pl. 301, rather than tone 4 of Brownish Drab, pl. 302, of Oberthür
and Dauthenay, by Buff-Yellow to Straw Yellow rather than near
(14´ a to 16´ c) Ochraceous-Buff color of underparts, by least
width of color of underparts amounting to more than 37 per cent of
greatest width of color of upper parts, by presence of color of
underparts on ventral side of tail and on hind leg over ankle, and
by lesser depth of skull through frontal region; from M. f.
effera by larger size, males averaging 12-1/2 per cent larger in
external measurements, 8 per cent larger in linear measurements of
skull, and 22 per cent heavier in weight of skull, total length
averaging 400 rather than 360, basilar length averaging 43.6
rather than 40.5.
Description.—Size.—Male: Twenty-one adults from the southern
half of the Sierra Nevada of California yield average and extreme
measurements as follows: Total length, 400 (356-428); length of
tail, 150 (125-178); length of hind foot, 46.1 (42-50). Tail
averages 60 per cent as long as head and body. Length of hind foot
averaging more than basal length. Corresponding measurements of
twelve adults from extreme southern and southwestern Colorado are
as follows: 407 (355-431); 150 (133-170); 46.0 (42-49).
Female: Ten adults from the Sierra Nevada of California yield
average and extreme measurements as follows: Total length, 349
(336-362); length of tail, 127 (120-133); length of hind foot,
36.3 (32-39). Tail averages 57 per cent as long as head and body.
Length of hind foot less than basal length. Corresponding
measurements of ten adults from the Rocky Mountains of central
Colorado are as follows: 347 (325-375); 123 (111-141); 40
(32-43).
The average differences in external measurements of the two sexes,
in the Sierras of California are: Total length, 51; length of
tail, 23; length of hind foot, 9.8. Weight of 7 adult males from
California is 267 (226-345) grams. Two adult females from there
weigh 148 and 115 grams and 3 from White Pine County, Nevada, 134,
122 and 124, giving an average of 129 grams.
Externals.—Longest facial vibrissae black, brown or white
(often all three colors in same specimen) and extending beyond
ear; carpal vibrissae same color as underparts and extending to
apical pad of fifth digit; hairiness of foot-soles (in summer
pelage) about as shown in figure 19.
Color.—Upper parts, in summer, near (14 n to l) Brussels
Brown or tones 1 to 3 of Raw Umber of Oberthür and Dauthenay, pl.
301, darker on top of head from nose to line connecting posterior
margins of ears. Chin and upper lips white. Remainder of
underparts Buff-Yellow to Straw Yellow and sometimes
Ochraceous-Buff especially in young, and in some adults from
southern Colorado. In winter, all white, except tip of tail, or
upper parts near (j) Snuff Brown or lighter than Brussels Brown
with a smoked effect, and underparts white. Tip of tail at all
times black. Color of underparts extends distally on posterior
sides of forelegs over toes onto antipalmar faces of feet and
wrists, on medial sides of hind legs to ankles, over antiplantar
faces of toes, medial third of tarsus and usually over proximal
tenth to three-fourths of ventral side of tail. Least width of
color of underparts averaging, in a series of twenty males from
the southern half of the Sierra Nevada of California, 59 (37-76)
per cent of greatest width of color of upper parts. In seven males
from southern Colorado corresponding percentages are 55 (37-71).
Black tip of tail in series from Sierra Nevada averaging 50
(40-60) mm. long; thus longer than hind foot and averaging 33-1/3
per cent of length of tail-vertebrae.
Skull and teeth.—Male (based on 25 adults, from Sierra Nevada
of California): See measurements and plates 19-21; weight, 3.7
(2.9-4.9) grams; basilar length, 43.6 (40.6-46.1); zygomatic
breadth more than distance between condylar foramen and M1 (save
in four instances) and more than distance between anterior
palatine foramen and anterior margin of tympanic bulla (save in
two specimens); mastoid breadth more (80 per cent of specimens) or
less (20 per cent) than postpalatal length; postorbital breadth
less than length of upper premolars and more or less than width of
basioccipital measured from medial margin of one foramen lacerum
posterior to its opposite; interorbital breadth more or less than
distance between foramen opticum and anterior margin of tympanic
bulla; breadth of rostrum less than length of tympanic bulla;
least width of palate less than medial length of P4 (except in two
specimens); anterior margin of tympanic bulla as far posterior to
foramen ovale as width of 3 to 5 upper incisors; height of
tympanic bulla more than distance from its anterior margin to
foramen ovale; length of tympanic bulla more than length of lower
molar and premolar tooth-row and longer or shorter than rostrum;
anterior margin of masseteric fossa not carried farther forward
than point directly below hypoconid of m1.
Female (based on ten adults from Sierra Nevada of California): See
measurements and plates 33-35; weight, 2.2 (1.8-2.4) grams;
basilar length, 38.2 (36.7-39.5); zygomatic breadth more (except
in one specimen) than distance between condylar foramen and M1 and
more (save in two specimens) than distance between anterior
palatine foramen and anterior margin of tympanic bulla;
postorbital breadth less than length of upper premolars and less
than (except in one specimen) width of basioccipital measured from
medial margin of one foramen lacerum posterior to its opposite;
least width of palate more or less than either outside or inside
length of P4 but generally less than inside length; tympanic bulla
as far posterior to foramen ovale as width of 3 to 5-1/2 upper
incisors; height of tympanic bulla more or less (usually more)
than distance from its anterior margin to foramen ovale; length of
tympanic bulla more than length of lower molar and premolar
tooth-row and more or less than length of rostrum.
The skull of the female averages 41 per cent lighter than that of
the average male.
Compared with the skull of M. f. longicauda, that of both sexes
averages smaller in every measurement taken. Males of nevadensis, on
the average, relative to the basilar length, are narrower in the
interorbital region and across the zygomata but have the orbitonasal
length greater. Stated in another way, the rostrum of longicauda
appears to be shorter and broader and the zygomata are more expanded.
Females of nevadensis, on the average, relative to the basilar length
are narrower across the mastoid processes and zygomata and have the
braincase deeper at the anterior margin of the basioccipital. Also in
nevadensis the mastoid processes do not project so far laterally
beyond the braincase, the lambdoidal crest and postorbital processes
are less well developed and except in the interparietal region, the
temporal ridges hardly meet and they form a sagittal furrow rather than
a low sagittal crest which characterizes adult females of longicauda.
Each of these differences separating the females of longicauda from
those of nevadensis are of the same nature, although not necessarily
of the same degree, as those which appear in longicauda with
increasing age. The differences mentioned above are readily appreciable
when series of specimens are compared. However, none of the differences
is of great degree, and most parts of the skulls of the two subspecies
are of similar relative proportions. Even so, there is but little
overlap in actual size. Comparisons with the skulls of M. f.
oribasus, alleni, neomexicana, arizonensis, inyoensis,
pulchra, xanthogenys, munda, saturata, oregonensis,
washingtoni, altifrontalis, and effera are made in the accounts
of those subspecies.
Remarks.—The populations to which the name nevadensis at present
is assigned have gone by the name arizonensis since Mearns proposed
this name in 1891. Before that time Coues (1877:141) had included
individuals of this race under the name Putorius longicauda.
Among the populations here assigned to M. f. nevadensis, there is
some geographic variation but it is of lesser degree than in most other
species of mammals which range over the same region. Comparison of 20
adult males from the Rocky Mountains of Colorado with 25 adult males
from a place as far distant as the Sierra Nevada of California shows
that the two populations closely resemble each other. The specimens
from Colorado average a trifle wider across the zygomata, have a longer
body and therefore relatively shorter tail, and, except in southern
Colorado, a slightly longer hind foot. Comparison of ten adult females
from each of the two areas reveals that those from Colorado have a
markedly longer hind foot, and a tail somewhat shorter relative to the
length of the body. The mentioned differences are the only ones found
among the great number of points investigated, except that as remarked
by Merriam (1896:23) the Sierran animal has the yellow of the
underparts reaching farther up under the chin, the underside of the
tail on the average is more suffused with yellowish and the white on
the upper lip is more extensive. As regards the last mentioned feature,
my check of 34 skins from Colorado reveals that the white extends all
the way around the upper lip in every specimen but one, whereas in 69
specimens from the Sierra Nevada the white extends all the way around
the upper lip in only 39. However, as further remarked by Merriam
(loc. cit.), not only this but the other color features are
inconstant in addition to being slight. When the occurrence of the dark
spots near the angles of the mouth are tabulated, it is found that in
33 Colorado-taken specimens they are absent in 19, faintly indicated in
13, and well developed in 1. In 62 California-taken specimens they are
absent in 37, faintly indicated in 20, and well developed in 5.
In northwestern Colorado, southern Wyoming, and possibly through the
Bear River Divide into southeastern Idaho, long-tailed weasels here
referred to nevadensis approach longicauda in large size and
occasionally in other features, more closely than do specimens of
nevadensis from most other places in its range. This tendency is
thought to be significant for much of the area in question lies in or
below the Transition Life-zone, the same life zones in which farther to
the eastward true longicauda occurs.
One specimen that illustrates this approach to longicauda is an adult
male, no. 2334, collection of E. R. Warren, from 6160 feet, Lay, Routt
[now Moffat] County, Colorado. In large size and, relative to the
basilar length, shorter rostrum and shorter tympanic bullae, it agrees
with longicauda but the darker color and, relative to the basilar
length, narrowness of the rostrum, interorbital region, zygomatic
expanse and the shallowness through the region of the postorbital
processes place it with nevadensis. Of two other specimens from
Steamboat Springs, Routt County, a young male, no. 4010, in the
collection of E. R. Warren, has a hind foot (50 mm.) as long as in
longicauda; and the other, no. 138195, U. S. Nat. Mus., an adult
male, agrees well enough in size and proportions with nevadensis but
has the coloration typical of longicauda.
From Wyoming, one subadult female, no. 177553, U. S. Nat. Mus., from
Garrett, is intermediate in size and coloration but is nearer to
nevadensis in these particulars, as it is in all other points
considered except size of the molar teeth which are as large as in
longicauda and larger than in any female nevadensis from Colorado
or California. Another female, an adult, no. 179304, U. S. Nat. Mus.,
from Lonetree, Wyoming, agrees with longicauda in size of skull.
Indeed, ten of seventeen cranial measurements exceed the maximum for
Colorado-taken nevadensis. Where differences exist in relative
proportions of the skull as expressed in percentages of the basilar
length, the specimen approaches nevadensis in 5 instances and
longicauda in only 3. The color is intermediate but much nearer that
of nevadensis with which the animal agrees also in external
measurements. Ten subadults (5 of each sex) from within 12 miles of
Laramie (not Fort Laramie) show greater resemblance to nevadensis but
definitely approach longicauda. Average external measurements are:
♂, 408, 155, 44; ♀, 361, 134, 40. The two other specimens examined
from this general locality, a young female, no. 2711, Mus. Vert. Zoöl.,
from Fort Bridger, and a subadult female, no. 188377, U. S. Nat. Mus.,
from Bridger Pass, show no departures from nevadensis of similar age.
The specimens from scattered localities in the Transition Life-zone of
northwestern Colorado and southern Wyoming are larger than nevadensis
is elsewhere, and also in certain other features resemble longicauda
of the plains to the eastward. Everything considered, the animals in
question are much more like nevadensis than longicauda. Study of
more specimens, especially from Wyoming, might provide grounds for
recognizing as a different subspecies the animals in this large area
comprising parts of Colorado and Wyoming from which so few specimens
now are available. Possibly the name Putorius culbertsoni Coues would
apply. Decision on that point will require adequate material from the
type locality, Fort Laramie. See discussion of this name under M. f.
longicauda.
In southeastern Idaho males are larger than they are at most other
places within the range of nevadensis. An average of 7 adults and
subadults from Pegram, Montpelier, Springfield, and the vicinity of
Pocatello, reveals, when compared with the average of nevadensis from
Colorado and that of longicauda from the Great Plains, that this
population from southeastern Idaho is nearest to longicauda in linear
measurements of the orbitonasal length, mastoid breadth, length of
tympanic bullae, and as expressed in percentage of the basilar length,
length of tooth-row, breadth of rostrum, and zygomatic breadth. In all
other points of size, relative proportions and color, the animals
approach nearer to, or actually agree with, nevadensis.
The specimens commented upon clearly show intergradation between
nevadensis and longicauda. Similarly, the specimens from
Scottsbluff County, Nebraska, here referred to M. f. alleni, by their
larger size suggest intergradation of that subspecies with the larger
nevadensis-longicauda stock although the approach is more toward
longicauda than nevadensis. Between oribasus and nevadensis,
however, there is no lack of material showing intergradation. As set
forth in the account of oribasus, specimens from Montana are truly
intermediate structurally as well as geographically.
Intergradation with washingtoni is shown by specimens from the
northern part of the Cascade Range in Chelan and Okanogan counties,
Washington. The adult male, U. S. Nat. Mus., no. 235183, from Bald
Mountain, is referable to washingtoni on the basis of cranial
characters but all the other adult and subadult specimens examined from
Chelan and Okanogan counties are nearer nevadensis on the basis of
cranial characters. Indeed, some show no approach to washingtoni in
cranial characters. As might be expected on geographic grounds, the
specimen from Easton, U. S. Nat. Mus., male subadult, no. 116870, shows
approach to washingtoni. This is true of the coloration of the hind
limbs, small size of the tympanic bullae, and relatively greater length
of the preorbital part of the skull. However, the greater width of the
light color of the underparts and relatively great breadth across the
mastoid processes and zygomatic arches are points of agreement with
nevadensis. Similarly, a series of 7 specimens from the Entait River,
20 miles above its mouth, in tone of color is nearer to washingtoni,
as is one of the two skulls of adult males in length of the preorbital
region. However, in greater breadth of the skull otherwise, and in the
relatively great width of the light color of the underparts, the
animals are nearer to nevadensis, to which they are here referred.
Some of these characters mentioned above in which departure is shown
from typical nevadensis are characters that show approach to
altifrontalis. This is especially true of the more intense coloration
and restriction of the color of the underparts.
Complete intergradation with effera is shown by specimens from
southern Oregon. The change from small effera to the larger
nevadensis here is gradual; consequently in northeastern California
and southern Oregon the size increases gradually to the northward.
Specimens showing complete intergradation with oregonensis and
saturata are wanting. However, one specimen from Crescent Lake
suggests oregonensis in having near (18) apricot yellow underparts
such as occur frequently in oregonensis. Also some specimens from
northern California approach saturata in having the color of the
underparts reduced in the extent to which it reaches out on the under
side of the tail. This fact and the consideration that the two races
are less different from one another than are other kinds which
definitely are known to intergrade leave no doubt but that material
from the intervening localities would show complete intergradation.
Intergradation between nevadensis and munda is indicated by
specimens from South Yolla Bolly Mountain, Trinity County, which are
commented on at greater length in the account of M. f. munda. M. f.
inyoensis is so closely related to nevadensis as to leave no doubt
that specimens from suitable localities will show actual
intergradation. That intergradation occurs directly with the bridled
weasel of the interior valleys of California, M. f. xanthogenys, is
shown by specimens from along the west-facing flank of the southern
part of the Sierra Nevada. Probably intergradation occurs all along the
Sierra Nevada on the western slope but specimens are lacking to show
this. Weasels are known to occur in the foothill territory and the
lesser attention given to this region by mammal collectors than to the
higher parts of the mountains may explain the lack of preserved
specimens. Individual specimens, here referred to nevadensis, but,
showing varying degrees of approach to xanthogenys are as follows: A
female from Hume; a male and a female from 8000 feet elevation, Monache
Meadows; a male from 9800 feet elevation on the east fork of the Kaweah
River; and 7 specimens, probably one family, from one-half mile south
of Mineral King, 7850 feet. Of the specimens from 7850 feet, the adult
male has no light facial markings and the head is only slightly darker
than the back. The adult female has much restricted, light facial
markings and the intervening areas are darker than in the male. The
five juveniles trapped in the same burrow as the female, each has more
extensive light facial markings than the adult female although the area
of this varies from only slightly more than in the female to as much as
in typical specimens of xanthogenys. Also, the dark color of the head
in these five specimens averages darker than in nevadensis and more
as in weasels to the southwestward especially latirostra. One of the
five juveniles is lighter colored over all of the upper parts than
nevadensis and is suggestive of xanthogenys in this respect.
Finally, the adult male has on the underparts small spots of ochraceous
orange suggestive of latirostra and some individuals of pulchra.
No. 30655/42628, U. S. Nat. Mus., taken on Mount Whitney, also shows
white facial markings and some other features of the valley-inhabiting
xanthogenys. A suggestion of intergradation with arizonensis is
furnished by specimens, referred to that race, from Springerville and
the Kaibab Plateau. No specimens happen to be available from the region
in which intergradation would be expected between nevadensis and
neomexicana. Since neomexicana and arizonensis intergrade it is
probable that nevadensis also will be found to intergrade with
neomexicana. In summary, nevadensis is judged to intergrade with
each of the subspecies of Mustela frenata whose range adjoins that of
nevadensis.
This subspecies is remarkably free from injury to the frontal sinuses
such as result from the presence of parasites. In 98 adults from
Oregon, California, Nevada, and Colorado, no malformation was noted.
Only 1 of the 26 specimens from Washington was malformed and it was an
intergrade with washingtoni. The single adult from New Mexico was
diseased, as were 3 of the 6 from British Columbia, 1 of the 20 from
Idaho, and 1 of the 7 from Utah.
Specimens examined.—Total number, 568, arranged alphabetically
by provinces and states and from north to south by counties in
each state. Unless otherwise indicated specimens are in the
collection of the United States National Museum.
Arizona. Apache County: 15 mi. E Luka Chu Kai Navajo School,
8000 ft., 2.
British Columbia. Monte Cr., 20 mi. E Kamloops, 1[21]; Sicamous,
2; Okanagan, 18 (7[2], 6[85], 1[75], 1[86]); Monashee Pass, 1[31];
Swan Lake, near Okanagan Landing, 1[22]; Okanagan Landing, 11
(2[74], 3[31], 3[86], 3[22]); Vernon, 1[74]; Hope-Princeton
Summit, 5600 ft., 1[77]; Hope, 1[20]; Similkameen, 1[77];
Osoyoos-Bridesville Summit, 1[77]; Anarchist Mt., Osoyoos, 1[31];
Myer's Creek, 1[77]; Rossland, Mt. Glory, 7000 ft., 1[77]; Cascade,
1[77]; Nelson, 1.
California. Siskiyou County: Hornbrook, 1; Tule Lake Refuge,
5[74]; Upper Mud Creek, 6700 ft., Mt. Shasta, 3; Mt. Shasta, 1.
Modoc County: Goose Lake, 1[20]; Joseph Creek, 1[74]; 5280 ft.,
Parker Creek, near Alturas, 1[74]; Warner Mts., near Alturas,
1[8]; 5 mi. NW Eagle Peak, 7000 ft., 2[74]; Shields Creek, 5000
ft., 1[74]; Jess Valley, 1[8]. Shasta County: Cassel, 1. Lassen
County: 3 mi. W Eagle Lake, 5800 ft., 1[74]; 4 mi. S Eagle Lake,
6000 ft., 2[74]; Mill Creek, 5000 ft., S base Mt. Lassen, 1; 6 mi.
SW Calneva, 1. Tehama County: Dale's, 600 ft., on Paines Creek,
1[74]. Plumas County: Kelly's, 2 mi. S Willow Lake, 5200 ft.,
3[74]; Quincy, 4[68]; Beckwith, Sierra Valley, 1. Butte County:
Jonesville, 1[74]. Sierra County: Little Truckee River, 6500
ft., 3 mi. N Independence Lake, 2[42]. Nevada County:
Independence Lake, 1[74]. Placer County: Donner, 3; 2 mi. W Soda
Springs Station, 6500 ft., 1[74]; Blue Canyon, 5000 ft., 2
(1[74]); 4 mi. S Tahoe City, 1[74]. Eldorado County: 5 mi. S
Tallac, 6300 ft., 1; Gilmore Lake, Mt. Tallac, 2[74]; Mt. Tallac,
1[68]; Phillips, 1[59]. Alpine County: 8000 ft., Hope Valley, 1;
8000 ft., Silver Creek, 1. Tuolumne County: Strawberry, 5200
ft., 1[74]; 9300 ft., Ten Lakes, Yosemite Park, 1[74]; Tuolumne
Meadows, 8600 ft., Yosemite Park, 1[74]; Tuolumne Meadows (Soda
Springs), 1; Tuolumne Meadows, 8500 ft., Yosemite Park, 1[74];
Sequoia, 1. Mariposa County: Chinquapin, 6256 ft., 2[74]; Merced
Grove Big Trees, 5400 ft., 1[74]; Wawona, 1; no locality more
definite than county, 1. Madera County: Bass Lake, 1[74]. Mono
County: Tioga Crest, near Tioga Pass, 4[74]; Warren Creek, 1[74];
Tioga Lake, 1[74]; Ellery Lake, 9600 ft., 1[74]; Mono Lake P. O.,
Mono Lake, 1[74]; Walker Lake, 8000 ft., 2[74]; Pine City, 1;
Mammoth, 13 (12[59], 1[14]); 10300 ft., near Big Prospector
Meadow, White Mts., 2[74]. Inyo County: Little Onion Valley,
7500 ft., 1[74]; N Fork Bishop Cr., 10500 ft., 1[74]; S fork
Bishop Cr., Andrews Camp, 8000 ft., 1[74]; South Lake, S Fk.
Bishop Cr., 9750 ft., 1[74]; Lamarck Cr., 9900 ft., 15 mi. SW
Bishop, 1[74]. Fresno County: Hume, 1. Tulare County: Mt.
Whitney, 2; Whitney Meadow, 9800 ft., 1[74]; Monache Meadow, 8000
ft., 3[74]; E fork Kaweah River, 9800 ft., 1; 1/2 mi. S Mineral
King, 7850 ft., 7[52]; Quaking Aspen Meadow, 7500 ft., 1[52].
Colorado. Moffat County: Lay, 1[19]. Routt County: Steamboat
Springs, 2 (1[19]); no locality more definite than county, 1[57].
Jackson County: Higho, North Park, 8400 ft., 1; Buffalo or
Illinois Creek, "near Rand," 6[74]. Washington County: 6 mi. NE
Hillrose, 1[74]. Larimer County: Estes Park, 2 (1[2], 1[7]);
Pinewood, 1; Loveland, 2 (1[57]); no locality more definite than
county, 1[7]. Rio Blanco County: Compass Creek, 9000 ft., 1[2];
White River, 6200 ft., 1[21]; Piceance Creek, 6200 ft., 1[2]; Dry
Fork, 6200-6600 ft., 4[2]; Meeker, 1; Marvine, 1[74]. Grand
County: Crembling [= Kremmling?], 1[50]; Middle Park, 1[57].
Boulder County: Foot Mt. Meeker, 8700 ft., 1[2]; Silver Lake
Mine, 1[60]; Boulder, 1[60]; Dixie Lake, 2 (1[2], 1[57]); Caribou,
1[2]; no locality more definite than county, 1. Clear Creek
County?: Grays Peak, 1[93]. Jefferson County: 7000 ft., Mt.
Parks, 1[57]; 6 mi. W Denver, 1[57]. Adams County: Barr, 1[2];
near East Lake, 2[57]. Denver County: Denver, 2 (1[2], 1[74]).
Arapahoe County: Littleton, 1[19]. Summit County:
Breckenridge, 1[57]. Eagle County: Eagle, 9500 ft., 1[102].
Park County: Jefferson, 4 (1[2]); 12800 ft., Mt. Bross, 1[57].
Mesa County: Tunnel, 1. Montrose County: near Crawford, Clear
Fork of Smiths Fork, 1[19]; Coventry, 3 (1[19]); Naturita, 1;
Paradox, 1[94]; West Paradox Valley, 1[57]. Pitkin County:
Placita, 2[26]. Gunnison County: Marble, 1[26], Crested Butte,
2[19]; Deckers Ranch, Crested Butte, 2[19]; Sapinero, 7245 ft.,
1[19]. Chaffee County: Buena Vista, 1[76]; Hancock, 1[16];
Salida, 5[19]. Teller County: Glencore, Pikes Peak, 1[76]. El
Paso County: Monument, 1[76]; Seven Lakes, 1[19]; Lake Moraine,
10250 ft., 1[19]; Colorado Springs, 6000 ft., 1[19]; 5 mi. E Sand
Creek, Colorado Springs, 1[19]; no locality more definite than
county, 1[50]. Saguache County: Villa Grove, 1[19]; Pierce
Place, Cochetopa Nat. Forest, 1; Houselog Creek, Cochetopa Nat.
Forest, 1; P. Tevebaugh's Ranch, near Cochetopa Pass, 1; P.
Tevebaugh's Ranch, 9 mi. S Cochetopa Pass, 1. Rio Grande County:
between Monte Vista and Del Norte, 1[88]. Archuleta County:
Upper Navajo River, 2[57]; Navajo River, 5 (4[57], 1[2]); Chromo,
2[57]. Conejos County: Osier, 3[57]. Montezuma County: Ure
Peak, 1[57]. County in question: Del Norte Peak, 1[76]; no
locality more definite than state, 4[75].
Idaho. Latah County: Cedar Mt., 4000 ft., 12 mi. NE Moscow,
1[55]; Moscow and 1/2 mi. W, 2[97]. Idaho County: Lochsa River
(= Locksaw Fork), 1; between Selway Riv., and S Fork Clearwater
Riv., 8[74]; Selway Divide, 8[74]; Pilot Creek, 2[74]; Newsome
Cr., 1[74]. Lemhi County: Salmon River Mts., (now Lemhi Mts.),
8000 ft., 5; Leadore, 3. Adams County: summit Smith Mt., 7500
ft., 1[41]. Washington County: Midvale, 2. Custer County:
Pahsimeroi Mts., 1; Double Springs, 16 mi. NE Dickey, 1[74];
Mackay?, 1; Stanley Lake, 1. Payette County: 2 mi. S Payette,
1[74]. Fremont County: 17 mi. E, 4 mi. N Ashton, 6275 ft.,
2[74]. Teton County: 3 mi. S Victor, 1[74]. Jefferson County:
20 mi. W Camas, 1. Blaine County: Sawtooth City, 1; Ketchum, 5
(3[50], 2[75]). Canyon County: Nampa 3. Clark County: Dry
Creek, Targhee Nat. Forest, 1[2]; Birch Creek, 2. County in
question: North fork of Teton River, 1. Bingham County: Shelley,
1; Alridge, 2; Springfield, 1. Lincoln County: Shoshone, 1.
Minidoka County: 1/4 mi. E Heyburn Bridge, 1[74]. Power
County: 4 mi. NW American Falls, 1[74]. Bannock County: 3 mi. N
Schutt's Mine, Ross Creek, 1[74]; 3 mi. N Pocatello, 1[74]; near
(within 10 miles of) Pocatello, 1[74]; 3 mi. S Pocatello, 1[74]; 1
mi. E Portneuf, 1[74]; 2 mi. up Mink Creek, 2 (1[74], 1[41]);
Inkom, 2; Swan Lake, 1. Owyhee County: 5 mi. SE Riddle, 1; Three
Creek, 2. Cassia County: Elba, 1[52]. Bear Lake County:
Geneva, 6171 ft., 1[74]; Montpelier, 1; Paris, 6000 ft., 1[6];
Pegram, 2.
Nevada. Humboldt County: Alder Creek, 7000 ft., Pine Forest
Mts., 1[74]; head of Big Creek, 8000 ft., Pine Forest Mts., 1[74];
Cottonwood Range, 1; Calico Mt., Little Owyhee R., 1; Mahogany,
Little Owyhee R., 2; Sulphur, 1. Pershing County: Lovelocks, 1.
Elko County: Mountain City, 3; Three Lakes, Ruby Mts., 1[41].
Washoe County: Pyramid Lake, 1; 3 mi. E Reno, 1[74]; Incline
Creek, 7100 ft., 1[74]; 2-1/2 mi. S Incline, 6250 ft., 1[74]; E
side Marlette Lake, 8000 ft., 1[74]; Marlette Lake, 8000 ft.,
1[74]. Ormsby County: 1/2 mi. S Marlette Lake, 8150 ft., 1[74].
Churchill County: 4 mi. W Fallon, 1[74]; 3 mi. W Fallon, 1[74];
2 mi. W Fallon, 1[74]; Fallon, 3970 ft., 1[74]. 5 mi. S Fallon,
4000 ft., 1[74]; 8 mi. S and 3 mi. E Fallon, 1[74]. Douglas
County: Mt. Siegel, 1[60]. Mineral County: Lapon Cañon, 8900
ft., Mt. Grant, 1. Nye County: Arc Dome, 1; 10700 ft., 1/2 mi.
SW Jefferson Peak, Toquima Range, 1[74]. White Pine County: 3
mi. E Baker, 1[74]; Baker Creek, 6600 ft., 4[74]; Baker Creek,
8400 to 8450 ft., 4[74]; Gleason Creek, 7500 ft., 1[74].
Esmeralda County: Arlemont, 4850 ft., Fish Lake Valley, 1[74].
Lincoln County: 3 mi. S Crystal Spring, 3900 ft., Pahranagat
Valley, 1[27].
New Mexico. Taos County: 2 mi. N Twining, 10500 ft., 1; Taos, 2.
Santa Fe County: 11600 ft., Pecos Baldy, 1. San Miguel County:
8000 ft., above Willis, Pecos River, Forest Reserve, 2[75];
Ribera, 1.
Oregon (by counties from west to east). Jackson County: Rustler
Peak, Crater Nat. Forest, 1[46]; Siskiyou (probably south of), 2.
Klamath County: 20 mi. W Crescent, 1[101]; Anna Creek, Mt.
Mazama, 2; S Boundary Crater Lake Nat. Park, 1[74]; Fort Klamath,
15; Upper Klamath Lake, 2[4]; Klamath Falls, 1[75]. Lake County:
Dog Lake Ranger Station, 30 mi. SW Lakeview, 1. Harney County:
Camp Harney, 2[75]; Burns, 2 (1[101]); 20 mi. S Burns, 1[46];
Narrows, 1[59]; Voltage, 1; Shirk P. O., 2; Keiger Gorge, Steen
Mts., 4. Malheur County: Riverside, 1; 2 mi. NW Riverside, 2;
Barren Valley, Cord, 1; Cedar Mts., 2; Cow Creek Lake, 1; Jordan
Valley, 1. County in question: Sageview, 1.
Utah. Cache County: Logan, 1[74]. Rich County: 8000 ft., near
Laketown, 1. Boxelder County: Willard, 1[101]. Salt Lake
County: Salt Lake City, 1[74]; Barclay, 6500 ft., Wasatch Mts.,
1; Mill Creek, 1[101]. Utah County: Provo Bench, 2[6]; Aspen
Grove, Mt. Timpanogos, 1[6]; Payson, 1[6]. Juab County: between
Santaquin and Starr, 1[103]. Uinta County: Dry Fork Canyon, 20
mi. NW Vernal, 1[9]. Carbon County: Sunnyside, 1[44]; Range
Creek, 1[44]. Millard County: Deseret, 1[74]. Sevier? County:
Fish Lake Plateau, 1. Grand County: Warner Ranger Station, La
Sal Mts., 1[6]. Beaver County: Britts Meadows, 11000 ft., Beaver
Range, 1[2]; Britts Meadows, Beaver Range, 1; Puffer Lake, 1[44].
Garfield County: Boulder, 2[6]. Washington County: Pine
Valley, 1[44]; St. George, 1. San Juan County: Geyser Pass, La
Sal Mts., 2[6]. County in question: Salt Lake, 2; Wasatch Mts.,
1; La Sal Mts., 11000 ft., 1.
Washington. Okanogan County: Bald Mt., 6800 ft., 1; Bauerman
Ridge, 6800 ft., Tungsten Mine, 1; Hart Pass, Methow River Trail,
1[46]; Conconully, 2 (1[51], 1[49]); 5 mi. NW Loomis, 1; Molson,
3800 ft., 1; Tunk Mt., 3500 ft., 1. Whatcom County: Barron, 5000
ft., 1. Stevens County: Colville, 1; Orin, 11[51]. Pend Oreille
County: Ione, 6[51]. Chelan County: Chelan Mts., 1[2]; Lake
Chelan, 1[46]; Manson, 1; Entiat River, 1680 ft., 20 mi. from
mouth, 7; Dryden, 2[49]; Wenatchee, 1. Kittitas County: Easton,
2 (1[51]); Ellensburg, 1[51]; 4 mi. E Ellensburg, 1[51]. Grant
County: Neppel, 1[51]. Lincoln County: Sprague, 1. Spokane
County: Spokane, 1[94]; Cheney 2[89]. Whitman County: Pullman,
11 (6[55], 1[68], 1[10]); 6 mi. S Pullman, 1. Garfield County:
Snake River, 1. Yakima County: Yakima, 1[74]; 1 mi. W Moxee,
1[74].
Wyoming. NW Wyoming, 1[75]. Yellowstone National Park: Lamar
River, 1; Yellowstone Lake, 1. Park County: Greybull River,
1[80]. Teton County: Crystal Creek, 2; Jackson, 1; Whetstone
Creek, 2[76]. Johnson County: Buffalo, 4 (2[93]). Fremont
County: Continental Divide, 20 mi. NW Dubois, 1[75]. Sublette
County: Bronx, 1[75]. Carbon County: Medicine Bow Mts., 1[75];
15 mi. SE Parco, 1[74]. Albany County: Garrett, 1; 12 mi. W
Laramie, 1[74]; 7 mi. W Laramie, 2[74]; 5 mi. W Laramie, 4[74];
"near" Laramie, 1[74]; 3 mi. SW Laramie, 1[74]; 12 mi. S Laramie,
1[74]. Uinta County: Fort Bridger, 6800 ft., 1[74]; Lonetree, 1;
Bridger Pass, 1. County in question: Laramie River, 2. No
locality more definite than state, 1.
Mustela frenata effera Hall
Long-tailed Weasel
Plates 19, 20 and 21
Mustela frenata effera Hall, Carnegie Instit. Washington Publ.
473:93, November 20, 1936.
Mustela arizonensis, Dice, Journ. Mamm., 1:12, November 28, 1919.
Type.—Male, adult, skull and skin; no. 33637, Amer. Mus. Nat.
Hist.; Ironside, 4000 ft., Malheur County, Oregon; September 8,
1912; obtained by H. E. Anthony; original no. 267.
The skull (plates 19-21) is complete and unbroken. The teeth all
are present and entire. The skin, in summer pelage, is well made.
Range.—Upper Sonoran to Arctic Alpine life-zones of northern
two-thirds of Oregon east of the Cascades, and southeastern
Washington, south of the Snake River. See figure 29 on page 221.
Characters for ready recognition.—Differs from M. f.
nevadensis in small size, males averaging 12-1/2 per cent smaller
in external measurements, 8 per cent smaller in linear
measurements of skull, and 22 per cent in weight of skull, total
length averaging 360 rather than 400, condylobasal length
averaging 40.5 rather than 43.6; from M. f. oregonensis in
absence of frontonasal white patch, presence of light color of
underparts on ventral face of tail and smaller skull with basilar
length averaging less than 41.7 in males; from M. f. washingtoni
in presence of light color of underparts on ventral face of tail,
in male skull by linear measurements averaging 7 (5-12) per cent
shorter and relative to basilar length shorter in preorbital
region and broader across mastoid processes and zygomatic arches.
Description.—Size.—Male: Eight (6 adult and 2 subadult)
males from northeastern Oregon yield average and extreme
measurements as follows: Total length, 360 (340-378); length of
tail, 129 (122-136); length of hind foot, 42 (40-44). Tail
averages 56 (52-59) per cent as long as head and body. Length of
hind foot more or less than (about same as) basal length.
Female: No. 212423 from Vale, and no. 566 V. B. Scheffer, from 15
mi. E Ukiah, measure, respectively: Total length, 312, 306; length
of tail, 113, 114; length of hind foot, 35, 35. Tail averages 57
per cent as long as head and body.
Differences in external measurements between the one adult female
and the average of the males are: Total length, 51; length of
tail, 16; length of hind foot, 7.
Externals.—Longest facial vibrissae black, brown or white
(often all three colors in same specimen) and extending beyond
ear; carpal vibrissae same color as underparts and extending to
apical pad of fifth digit; hairiness of foot-soles (in summer
pelage) about as shown in stage 4 of figure 19.
Color.—Upper parts, in summer, near (14 n to l) Brussels
Brown or tones 1 to 3 of Raw Umber of Oberthür and Dauthenay, pl.
301, darker on top of head from nose to, or slightly behind, line
connecting posterior margins of ears. Chin and usually all of
upper lips white. Remainder of underparts Buff-Yellow to Straw
Yellow. In winter all white except tip of tail or upper parts near
(j) Snuff Brown or lighter than Brussels Brown with a smoked
effect, with underparts white. Tip of tail at all times black.
Color of underparts extends distally on posterior sides of
forelegs over toes onto antipalmar faces of toes and wrists, on
medial sides of hind legs to ankles over antiplantar faces of
toes, distomedial third of tarsus and usually over proximal fourth
to three-fourths of ventral side of tail. Least width of color of
underparts averaging, in 15 males, 53 (36-69) per cent of greatest
width of color of upper parts. Black tip of tail averaging 47
(38-67) mm. long. Thus averaging longer than hind foot, and 36 per
cent of length of tail-vertebrae.
Skull and teeth.—Male (based on 6 adults from northeastern
Oregon): See measurements and plates 19-21. As described in
Mustela frenata nevadensis except that: Weight, 2.9 (2.5-3.4)
grams; basilar length, 40.5 (39.3-41.8).
Female (based on no. 212423, adult from Vale): In so far as parts
of the broken skull permit a person to judge, the skull is as
described in M. f. nevadensis except that: Smaller; lighter;
postorbital breadth more than width of basioccipital measured from
medial margin of one foramen lacerum posterior to its opposite.
As compared with the skull of M. f. nevadensis that of effera
seems, on the average, to have the preorbital part relatively smaller.
Otherwise, the skull is a miniature of the skull of nevadensis,
averaging about eight per cent smaller in linear measurements and
weighs twenty-two per cent less. Comparisons of the skull with those of
M. f. washingtoni and M. f. oregonensis are made in accounts of
those subspecies.
Remarks.—This geographic race has long borne the name of Mustela
arizonensis (Mearns). Small size differentiates effera from
nevadensis and specimens have been allocated to one or the other
subspecies on the basis of size, or average size when several
individuals are available from one locality. Complete intergradation
with each adjoining subspecies is indicated by numerous specimens, more
of which are assigned to these adjoining subspecies than to effera
itself.
The minimum of size in M. f. effera is found in the Blue Mountain
region of northeastern Oregon. Specimens from the area intervening
between these mountains and the Cascades average larger but are nearer
the mean of typical effera than they are to the means of
washingtoni, oregonensis or nevadensis.
Two males, nos. 204883, adult, and 204884, young, from Sisters, Oregon,
near the eastern base of the Cascades, show approach structurally to
M. f. washingtoni as it is represented at the nearby locality,
Permilia Lake, at the west base of Mount Jefferson. Everything
considered, however, the two specimens from Sisters are nearer to
effera. A male from Condon, Oregon, shows approach to the Cascade
race in slightly increased size.
No perfect skulls of adult females are available from the part of
northwestern Oregon in which effera reaches its typical state of
development as judged by the small size of the skull of the adult male.
Skulls of adult females are available, however, from more nearly
marginal localities. These, though smaller than in nevadensis, show
relatively less difference in size when compared with nevadensis than
do skulls of males. Even so the females at these marginal localities
are smaller than those of nevadensis of comparable age and adequate
material of adult female effera from the region where the males
attain their extreme of small size probably will show about the same
relative difference in size between nevadensis and effera as is
known to exist between the adult males of these two subspecies. The
small size of a subadult female, no 74631, U. S. Nat. Mus., from
Asotin, Washington, constitutes partial basis for this opinion.
Of 14 adults examined none showed malformation of the frontal sinuses
due to infestation by parasites.
Specimens examined.—Total number, 53, arranged within each
state by counties from north to south. Unless otherwise indicated
specimens are in the collection of the United States National
Museum.
Oregon. Wasco County: 4 mi. S The Dalles, 1[74]; Wapinita, 1;
Antelope, 2; 7 mi. E Antelope, 5. Gilliam County: Condon, 1[46].
Morrow County: 10 mi. S Hardman, 1. Umatilla County: Umatilla,
2; 15 mi. E Ukiah, 4000 ft., 1[49]. Union County: Elgin, 1; 20
mi. E Lehman, 1[46]. Wallowa County: Horse Creek, 15 mi. N
Paradise, 1; Enterprise, 1[46]; Wallowa Lake, 1[46]; Wallowa
Mts., 8300 ft., 1. Baker County: Haines, 1[49]; Anthony, 3[2];
Bourne, 2. Grant County: Long Creek, 1[46]; Canyon Creek, 1[46];
Strawberry Mts., 2; Silvies, 1[14]. Crook County: Prineville, 4.
Deschutes County: Sisters, 2; Bend, 1. Lake County: 3 mi. W
Stauffer, 1; Fort Rock, 1[46]. Harney County: 25 mi. NW Burns,
1. Malheur County: 4000 ft., Ironside, 2[2]; 1-1/2 mi. S Vale,
2.
Washington. Walla Walla County: Prescott, 4 (2[76], 1[60],
1[74]); Ft. Walla Walla, 2 (1[75]); Wallula, 1[76]. Asotin
County: Asotin, 1.
Mustela frenata washingtoni (Merriam)
Long-tailed Weasel
Plates 19, 20, 21, 34, 35 and 36
Putorius washingtoni Merriam, N. Amer. Fauna, 11:18, pl. 4, figs.
3, 3a, 4, 4a, June 30, 1896.
Mustela washingtoni, Miller, U. S. Nat. Mus. Bull., 79:98,
December 31, 1912.
Mustela frenata washingtoni, Hall, Carnegie Instit. Washington
Publ. 473:106, November 20, 1936.
Type.—Male, adult, skin and skull; no. 76322, U. S. Nat. Mus.,
Biol. Surv. Coll.; Trout Lake, Mt. Adams, Klickitat (?) County,
Washington; December 15, 1895; obtained by D. N. Kaegi; original
no. 2.
The skull is unbroken. The left incisors above are missing.
Otherwise the teeth are present and entire. The skin is well made,
in brown winter pelage, lacks collector's measurements, has no
bones in the feet, but by large size is judged to be a male.
Range.—Altitudinally from near 2000 feet at Trout Lake up to
the highest parts of the Cascade Range from Mount Jefferson,
Oregon, north to Mount Rainier, Washington; Upper Sonoran
Life-zone to Arctic Alpine Life-zone. See figure 29 on page 221.
Characters for ready recognition.—Differs from M. f.
altifrontalis in lighter color of upper parts and underparts,
latter ranging from Buff-Yellow to Naples Yellow rather than near
(14 a to 16 c) Ochraceous-Buff, in shallower skull in both
sexes (see measurements), in males, a longer preorbital region,
narrower skull with shorter bullae, and in females, a smaller
skull with interorbital breadth averaging less than 24 per cent of
basilar length; from M. f. nevadensis in absence of light color
of underparts on ventral face of tail, in skulls of males, by
longer preorbital region and narrower skull across mastoid
processes and zygomatic arches, in skulls of females, by shorter
preorbital region, and smaller bullae (see measurements); from M.
f. effera in absence of light color of underparts on ventral face
of tail, in skulls of males, by linear measurements averaging 7
(5-12) per cent larger, and relative to basilar length, longer in
the preorbital region and narrower across mastoid processes and
zygomatic arches; from M. f. oregonensis in absence of
frontonasal white patch, longer skull in males, which in
percentage of basilar length has, on the average, orbitonasal
length amounting to more than 35, mastoid breadth less than 55,
and zygomatic breadth less than 63, and in females, smaller skull
with least width of palate less than length of P4, upper
tooth-rows less than 38-1/2 per cent of basilar length, bullae
smaller, averaging less than 13.4 in length.
Description.—Size.—Male: Fifteen subadult topotypes yield
average and extreme measurements as follows: Total length, 400
(357-437); length of tail, 149 (122-171); length of hind foot,
47.6 (42-59). Tail averages 59 per cent as long as head and body.
Length of hind foot averaging more than basal length.
Corresponding measurements of one adult and 3 young from Mount
Rainier are: 415 (405-423); 155 (145-164); 51 (50-53).
Female: Five adult topotypes yield average and extreme
measurements as follows: Total length, 349 (330-393); length of
tail, 124 (114-133); length of hind foot, 38 (36-39). Tail
averages 55 per cent as long as head and body. Length of hind foot
averaging about same as basal length. Corresponding measurements
of two adults and 6 young from Mount Rainier are: 338 (320-360);
121 (115-132); 36 (34-40).
The average differences in external measurements of the two sexes,
from Mount Adams, are: Total length, 51; length of tail, 25;
length of hind foot, 9.6. Corresponding differences between the
specimens from Mount Rainier are: 77; 34; 15.
Externals.—Longest facial vibrissae black or brown (often both
colors in same specimen) and extending beyond ear; carpal
vibrissae same color as underparts and extending to or beyond
apical pad of fifth digit; hairiness of foot-soles slightly less
than shown in figure 19.
Color.—Upper parts in summer near (14 n) Argus Brown or tone
4 of Burnt Umber of Oberthür and Dauthenay, pl. 304; one topotype
Buckthorn Brown or tone 3 to 4 of Snuff Brown of Oberthür and
Dauthenay, pl. 303. Dark spot at each angle of mouth present or
absent, and when present, often fused with color of upper parts,
which rarely covers lower lips. Chin, and usually lower lips,
white. Remainder of underparts Buff-Yellow to Naples Yellow. In
winter, all white except tip of tail which is at all times black,
or upper parts near (14) Brussels Brown to near (j) Snuff Brown
with smoked effect and underparts white, rarely with trace of
yellowish. Color of underparts extends distally on posterior sides
of forelegs over toes onto antipalmar faces of feet and usually
all of wrists, on medial sides of hind legs anywhere from knee to
tips of toes. Least width of color of underparts averaging in ten
topotypes, 24 (10-37) per cent of greatest width of color of upper
parts. Black tip of tail in same series averaging 55 (45-60) mm.
long, thus longer than hind foot and averaging 37 per cent of
length of tail-vertebrae.
The color of the underparts is not so narrow in the specimens from
Mount Rainier and it is believed that the slender bodies used in
stuffing the topotypes has accentuated in them the appearance of
narrowness of the light-colored underparts.
Skull and teeth.—Male (based on 22 adult topotypes): See
measurements and plates 19-21; weight, 3.5 (2.8-4.7) grams;
basilar length, 43.7 (40.0-47.7); zygomatic breadth more or less
than distance between condylar foramen and M1 or than between
anterior palatine foramen and anterior margin of tympanic bulla;
mastoid breadth more or less than postpalatal length; postorbital
breadth less than length of upper premolars and greater than width
of basioccipital measured from medial margin of one foramen
lacerum posterior to its opposite; interorbital breadth more or
less than distance between foramen opticum and anterior margin of
tympanic bullae; breadth of rostrum less (except in no. 82180)
than length of tympanic bulla; least width of palate more (except
in no. 81954) than length of P4; anterior margin of tympanic bulla
as far posterior to foramen ovale as width of 2 to 5 upper
incisors; height of tympanic bulla more or less than distance from
its anterior margin to foramen ovale; length of tympanic bulla
more (except in two instances) than length of lower molar and
premolar tooth-row and shorter (except in two instances) than
rostrum; anterior margin of masseteric fossa below m2.
Female (based on 11 ad. topotypes): See measurements and plates
34-36; weight, 2.0 (1.8-2.2) grams; basilar length, 37.6
(37.0-38.9); zygomatic breadth less (except in no. 70945) than
distance between condylar foramen and M1 or than between anterior
palatine foramen and anterior margin of tympanic bulla;
postorbital breadth less than length of upper premolars and more
than width of basioccipital measured from medial margin of one
foramen lacerum posterior to its opposite; least width of palate
less (except in one specimen) than greatest length of P4; tympanic
bulla as far posterior to foramen ovale as width of 3-1/2 to 5-1/2
upper incisors; height of tympanic bulla more or less than
distance from its anterior margin to foramen ovale; length of
tympanic bulla more than length of lower molar and premolar
tooth-row and longer or shorter than rostrum.
Compared with M. f. nevadensis, the skull of the male of
washingtoni averages more slender, as shown by the mastoid and
zygomatic breadths and has the preorbital part longer, on the average,
as shown by the greater ratio (to the basilar length) of the length of
the tooth-rows and orbitonasal length. Also, on the average, the
postorbital constriction is longer than in nevadensis and the
tympanic bullae are smaller. In females, the skull is lighter, the
tooth-rows are shorter, the tympanic bullae are smaller, and the
preorbital part of the skull is shorter and narrower as shown by the
orbitonasal length and interorbital breadth. Except that the tympanic
bullae are actually, although not relatively, smaller in males of
effera, it differs from washingtoni in the same way as does
nevadensis as regards relative proportions, but, of course, the
actual difference in size is greater since effera is smaller than
nevadensis. Comparison of the skull with that of oregonensis is
made in the account of that subspecies.
Remarks.—M. f. washingtoni was described and named in 1896 by
Merriam as a distinct species. Subsequently, specimens which here are
regarded as intergrades between altifrontalis and nevadensis, were
classified as washingtoni.
The external measurements given for the specimens from Mount Adams are
those recorded on the labels in inches and fractions thereof. Instead
of total length there sometimes is written "tip to tip." In the series
of 19 winter-taken topotypes the hairs project beyond the end of the
caudal vertebrae for an average distance of 28 (19-40) millimeters. If
the hairs on the end of the tail were included in the measurements, 28
millimeters should be subtracted from the averages. Probably the
measurements should stand as given, since an adult male topotype, no.
226758, U. S. Nat. Mus., taken subsequently by Walter P. Taylor
measures 405; 152; 51.
Mustela frenata washingtoni is not a strongly marked geographic race.
In many features it is intermediate between M. f. altifrontalis and
M. f. nevadensis. This is especially true of coloration. In the
series from Mount Adams and that from Mount Rainier, some individuals
have the light color of the underparts extended down the hind legs over
the feet and over the proximal face of the ventral third of the tail as
in nevadensis, whereas others from the same place have the light
color of the underparts absent from the tail and extending no farther
down the hind limbs than the knees. The light color of the underparts
in the series of topotypes is so restricted that the transverse extent
at the narrowest place amounts to only 24 (10-37) per cent of the
greatest width of the color of the upper parts. This narrowness of the
color of the underparts has been likened by Merriam (1896:18) to the
condition in Mustela frenata noveboracensis. So it is, but it is
similar to the condition found also in the geographically adjoining M.
f. altifrontalis.
Of the 37 skulls of subadults and a few adults, 11 had the frontal
sinuses malformed as a result of infestation by parasites.
Specimens examined.—Total number, 56, arranged within each
state by localities from north to south. Unless otherwise
indicated specimens are in the collection of the United States
National Museum.
Oregon. Mt. Jefferson, Permilia Lake, 1.
Washington. Pierce County: 5500 ft., Spray Park, Mt. Rainier, 1;
Spray Park, 1[74]; 5935 ft., Glacier Basin, Mt. Rainier, 5
(1[10]); 5051 to 5100 ft., Owyhigh Lakes, Mt. Rainier, 7 (1[10]),
Tahoma Creek, 1[72]; Nisqually entrance, 1[72]; Longmire, 1[72];
Mt. Rainier Nat'l Park, 2[72]. Klickitat County: Trout Lake, S
Base Mt. Adams, 35; 3500 ft., Gotchen Creek, Mt. Adams, 1.
Mustela frenata saturata (Merriam)
Long-tailed Weasel
Plates 19, 20, 21 and 30
Putorius saturatus Merriam, N. Amer. Fauna, 11:21, June 30, 1896.
Mustela saturata, Miller, U. S. Nat. Mus. Bull., 79:98, December
31, 1912.
Mustela arizonensis saturata, Grinnell, Univ. California Publ.
Zoöl., 40:102, September 26, 1933.
Mustela frenata saturata, Hall, Carnegie Instit. Washington Publ.
473:106, November 20, 1936.
Type.—Male, adult, skull and skin; no. 65930, U. S. Nat. Mus.,
Biol. Surv. Coll.; Siskiyou, Jackson County, Oregon; June 6, 1894;
obtained by C. P. Streator; original no. 3905.
The skull (plates 19-21, 30) lacks the middle part of each
zygomatic arch. The teeth all are present although much worn,
probably from gnawing at the trap which captured the animal. The
skin, in fresh summer pelage, is fairly well made.
Range.—Transition and Boreal life-zones of Siskiyou and Trinity
mountains in southern Oregon and northwestern California. See
figures 29 and 30 on pages 221 and 314.
Characters for ready recognition.—Differs from M. f.
nevadensis in lacking light color of underparts on tail and ankle
and in greater average breadth across mastoid processes of skull
(see measurements); from M. f. oregonensis in lacking white
nasofrontal spot, in having color of underparts interrupted at
ankle; from M. f. munda in lacking white nasofrontal spot, in
smaller and relatively deeper skull of males and smaller skull of
the female.
Description.—Size.—Male: Four adult males (the type, 1 from
Mt. Ashland and 2 from Jackson Lake) yield average and extreme
measurements as follows: Total length, 414 (402-437); length of
tail, 150 (136-160); length of hind foot, 46 (43-50). Tail
averages 57 (49-62) per cent as long as head and body. Length of
hind foot more or less than basal length.
Female: One young from the summit of the Trinity Mountains east of
Hoopa and one nontypical adult from 5500 feet elevation on South
Fork Mountain, Humboldt County, measure respectively as follows:
Total length, 330, 325; length of tail, 115, 123; length of hind
foot, 37, 37. Tail is 53 and 61 per cent as long as head and body.
Length of hind foot less than basal length.
Average differences in external measurements between the two
sexes, indicated by the unsatisfactory material available, are:
Total length, 86; length of tail, 31; length of hind foot, 9.
Externals.—Longest facial vibrissae black or dark brown and
extending beyond ear; carpal vibrissae same color as underparts
and extending as far as apical pad of fifth digit; hairiness of
foot-soles, in summer pelage, as shown in figure 19.
Color.—Upper parts, in summer, Brussels Brown to near (n)
Brussels Brown or lighter than tone 3 of Raw Umber of Oberthür and
Dauthenay, pl. 301, usually darkest on nose and forehead. Chin
white. Remainder of underparts Buff-Yellow to Warm Buff. Tip of
tail black. Winter pelage unknown. Color of underparts extends
distally on posterior sides of forelegs over toes onto antipalmar
faces of feet and sometimes wrists, on medial sides of hind legs
only to ankles, but toes sometimes with isolated white markings.
Least width of color of underparts in the type and 2 adults from
Jackson Lake averaging 35 (30-40) per cent of greatest width of
color of upper parts. Black tip of tail averaging 54 (53-55) mm.
long; thus longer than hind foot and averaging 37 per cent of
length of tail-vertebrae.
Skull and teeth.—Male (based on 4 adults: Type, Mt. Ashland, 1;
Jackson Lake, 2): See measurements and plates 19-21, 30. As
described in Mustela frenata nevadensis except that: Weight, 3.8
(3.5-4.3) grams; basilar length, 44.4 (42.6-45.8); zygomatic
breadth more or less than distance between condylar foramen and M1
or than distance between anterior palatine foramen and anterior
margin of tympanic bulla; mastoid breadth more than postpalatal
length; least width of palate less than medial length of P4
(except in one specimen).
Female (based on one adult possibly not typical, from 5500 ft.,
South Fork Mt.): See measurements. As described in Mustela
frenata nevadensis except that: Weight, 2.2 grams; basilar
length, 38.1; zygomatic breadth less than distance between
condylar foramen and M1 and less than distance between anterior
palatine foramen and anterior margin of tympanic bulla;
postorbital breadth more than width of basioccipital measured from
medial margin of one foramen lacerum posterior to its opposite.
The skull of the male of saturata, relative to the basilar length, is
broader across the mastoids and narrower across the rostrum and
interorbital region than that of nevadensis. Skull not known
certainly to differ from that of oregonensis. Compared with the skull
of munda, that of the male of saturata is smaller in every part
measured except depth of tympanic bullae which averages 3.6
millimeters, rather than 3.5 as in munda. Also, the skull of
saturata has a less-marked postorbital constriction, is less heavily
ridged, less angular, does not have the impressions of the temporal
muscles carried so far forward on the frontal bones and is relatively
much narrower across the zygomatic arches.
Remarks.—In 1896, Merriam named M. f. saturata as a distinct
species on the basis of one specimen, taken by Clark P. Streator at
Siskiyou, Oregon, and a second specimen taken the year previously by
Allan C. Brooks at Chilliwack, British Columbia. On the basis of these
two specimens, Merriam (1896:22) ascribed to the race a range ". . . on
the Cascade and Siskiyou mountains of Oregon and Washington, reaching a
short distance into British Columbia." Since that time, this name,
saturata, has been employed for the dark-colored weasels, of the
coastal region of Oregon, Washington, and extreme southwestern British
Columbia, which here are arranged under the name M. f. altifrontalis.
M. f. saturata proves to be restricted to the humid mountainous
region inland from the coast in northern California and in the Siskiyou
Mountains of southern Oregon. Its range is separated by that of M. f.
oregonensis from the range of the darker-colored, deeper-skulled, M.
f. altifrontalis of the humid costal region proper.
On May 5, 1933, Mr. Clark P. Streator, informed the writer that he
remembered taking the type specimen of Mustela frenata saturata
(Merriam) in the town of Siskiyou, Oregon. The exact place, he said,
was reached, at the time of his work there, by going one or two blocks
east of the depot, then through a garden into the thick woods where
there were springs and numerous burrows of the rodent, Aplodontia.
Two other weasels labeled as taken at Siskiyou, on September 28 and
29, 1893, by Mr. Streator, are much lighter colored than the type of
saturata and have the color of the underparts extended distally on
the hind legs to the tips of the toes and in other features of
coloration are more like nevadensis, the subspecies to which they are
referred, than saturata. Probably these did not come from exactly the
same place that the type specimen of saturata did. Although Mr.
Streator does not remember the taking of these particular specimens in
1893, he does remember that on this visit to Siskiyou, he walked
southward through the railroad tunnel and collected on the opposite
side of the ridge from Siskiyou. Here on more southern exposures, the
country was markedly different than in the thick forest at Siskiyou.
Probably these two specimens taken in 1893, and referred to
nevadensis, came from a little way south of Siskiyou and from a
different habitat and life-zone than the type specimen of M. f.
saturata.
Of the 6 specimens examined, only one, the type, shows malformation of
the frontal sinuses such as result from infestation by parasites.
Specimens examined.—Total number, 6, as follows:
California. Siskiyou County: Jackson Lake, 5900 ft., 2, Mus.
Vert. Zoöl. Humboldt County: South Fork Mt., 5500 ft., 1, Mus.
Vert. Zoöl. County in question, Trinity Mts., summit east of
Hoopa, 5800 ft., 1, U. S. Nat. Mus.
Oregon. Jackson County: Mt. Ashland, 1, Univ. Oreg.; Siskiyou,
1, U. S. Nat. Mus.
Mustela frenata altifrontalis Hall
Long-tailed Weasel
Plates 1, 19, 20, 21, 34, 35 and 36
Mustela frenata altifrontalis Hall, Carnegie Instit. Washington
Publ. 473:94, November 20, 1936.
Putorius (Gale) brasiliensis frenatus, Coues, Fur-bearing
animals, p. 142, 1877 (part).
Putorius saturatus Merriam, N. Amer. Fauna, 11:21, June 30, 1896
(part).
Mustela saturata, Miller, U. S. Nat. Mus. Bull., 79:98, December
31, 1912.
Type.—Male, adult, skull and skin; no. 42093, Mus. Vert. Zoöl.;
Tillamook, Tillamook County, Oregon; July 10, 1928; obtained by
Alex Walker; original no. 717.
The skull is complete and unbroken. P3 on the left side is
missing; otherwise the teeth all are present and entire. The skin
is well made and the enlarged scrotal pouch shows the collector's
sexing of the specimen to have been correct.
Range.—Altitudinally from sea level up to at least 4800 feet
(Mount Baker) in the Transition Life-zone of the humid, coastal
region of Oregon, Washington and extreme southwestern British
Columbia. See figure 29 on page 221.
Characters for ready recognition.—Differs from M. f.
nevadensis in tone 4 of Brownish Drab, pl. 302, rather than tones
1-3, of Raw Umber, pl. 301, of Oberthür and Dauthenay of upper
parts, in near (14 a´ to 16 c´) Ochraceous-Buff rather than
Buff-Yellow to Straw Yellow of underparts, in that least width of
color of underparts amounts to less than 37 per cent of greatest
width of color of upper parts, in absence of color of underparts
on ventral side of tail and on hind leg distal to knee, and in
greater depth of skull through frontal region; from M. f.
washingtoni in darker color of upper parts and underparts, latter
near (14 a´ to 16 c´) Ochraceous-Buff rather than ranging from
Buff-Yellow to Naples Yellow, in deeper skull in both sexes (see
measurements), in males a shorter preorbital region, broader skull
with longer bullae and in females a larger skull with interorbital
breadth averaging more than 24 per cent of basilar length; from
M. f. oregonensis in frontonasal white patch absent, color above
darker (tone 4 of Brownish Drab, pl. 302, rather than tone 2 to 3
of Raw Umber, pl. 301 of Oberthür and Dauthenay), light-colored
underparts narrower and not extended distally beyond knee, in
females tooth-row shorter, amounting to less than 38 per cent of
basilar length.
Description.—Size.—Male: Eight adult topotypes yield average
and extreme measurements as follows: Total length, 426 (392-445);
length of tail, 160 (148-170); length of hind foot, 47 (42-53).
Tail averages 60 per cent as long as head and body. Length of hind
foot averages more than basal length.
Female: Five adults from Tillamook and Blaine, Oregon, yield
average and extreme measurements as follows: Total length, 347
(320-370); length of tail, 125 (114-131); length of hind foot, 38
(35-44). Tail averages 56 per cent as long as head and body.
Length of hind foot less than basal length.
The average differences in the external measurements are: Total
length, 79; length of tail, 35; length of hind foot, 9.
Externals.—Longest facial vibrissae black, brown or white
(often all three colors in same specimen) and extending beyond
ear; carpal vibrissae same color as underparts and extending to or
beyond apical pad of fifth digit; hairiness of foot-soles (in
summer pledge) slightly less than shown in figure 19.
Color.—Upper parts, in summer, near (n) Argus Brown or tone 4
of Brownish Drab of Oberthür and Dauthenay, pl. 302. Dark spot at
each angle of mouth well developed; often fused with color of
upper parts which sometimes covers lower lips. Chin white.
Remainder of underparts near (14 a´ to 16 c´) Ochraceous-Buff.
In winter, upper parts near (14) Argus Brown with smoked effect
and Warm Buff to Naples Yellow below. Tip of tail at all times
black. Color of underparts extends distally on posterior sides of
forelegs over toes onto antipalmar faces of feet and usually all
of wrists, on medial side of hind legs typically only to knee but
sometimes to ankle. Tips of toes of hind feet almost always marked
with color of underparts. Least width of color of underparts
averaging in a series of 14 males from Blaine, Oregon, 23 (14-36)
per cent of greatest width of color of upper parts. Black tip of
tail in 8 adult males from Blaine, Oregon, averaging 59 (47-70)
mm. long; thus longer than hind foot and averaging 37 per cent of
length of tail-vertebrae.
Skull and teeth.—Male (based on 9 adults from Blaine, Tillamook
Co., Oregon): See measurements and plates 19-21; weight, 4.4
(3.3-5.3) grams; basilar length, 45.6 (42.4-47.7); zygomatic
breadth more or less (usually more) than distance between condylar
foramen and M1 or than between anterior palatine foramen and
anterior margin of tympanic bulla; mastoid breadth more or less
(usually more) than postpalatal length; postorbital breadth less
(except in some instances of malformations of frontal sinuses
which result from infestation by parasites) than length of upper
premolars and more or less than width of basioccipital measured
from medial margin of one foramen lacerum posterior to its
opposite; interorbital breadth more or less than distance between
foramen opticum and anterior margin of tympanic bulla; breadth of
rostrum less than length of tympanic bulla; least width of palate
more or less than length of P4; anterior margin of tympanic bulla
as far posterior to foramen ovale as width of 3 to 4 (including
I3) upper incisors; height of tympanic bulla more than distance
from its anterior margin to foramen ovale; length of tympanic
bulla more than length of lower molar and premolar tooth-row and
more or less than orbitonasal length; anterior margin of
masseteric fossa directly below m2.
Female (based on 4 adults): See measurements and plates 34-36;
weight, 2.2 (2.2-2.3) grams; basilar length, 38.1 (37.8-39.7);
zygomatic breadth more or less (less in three of four specimens)
than distance between condylar foramen and M1 or than between
anterior palatine foramen and anterior margin of tympanic bulla;
relation of postorbital breadth to other measurements in doubt
because of malformation of frontal sinuses by parasites; least
width of palate not less than greatest length of P4; tympanic
bulla as far posterior to foramen ovale as width of 3-1/2 to 5-1/2
upper incisors; height of tympanic bulla more than distance from
its anterior margin to foramen ovale; length of tympanic bulla
more than length of lower molar and premolar tooth-row and longer
or shorter than rostrum.
Compared with the skull of M. f. washingtoni that of each sex of
altifrontalis averages slightly larger in every measurement taken,
except measurements of teeth which are approximately the same, and is
relatively deeper through the frontal region and through the braincase
as measured at the anterior margin of the basioccipital. Skulls of
females of altifrontalis have a relatively broader interorbital
region. Skulls of males of altifrontalis further differ in having
relatively, as well as actually, longer tympanic bullae, relatively
lesser orbitonasal length and a greater relative breadth across the
mastoids and across zygomata. Compared with M. f. nevadensis, the
skull of the male of altifrontalis averages slightly larger and
heavier although the skulls of females are of approximately the same
size and weight. Relative to the basilar length, the skulls of both
sexes are deeper through the braincase and narrower across the
mastoids; the rostrum is broader, especially in males; the tooth-rows
are shorter and the interorbital breadth less, especially in females.
Comparison with the skull of oregonensis is made in the account of
that subspecies.
Remarks.—Until the present study was begun, animals of this race
have gone under the name Mustela saturata (Merriam). The United
States National Museum has a juvenile taken, in 1858, by Wayne at
Astoria, O. T.; the Samuel N. Rhoads collection contained one specimen
taken in 1891, at Tacoma, Washington; one in the Bangs' collection was
taken at Chilliwack, British Columbia, in 1895, and the Field Museum
has one taken on the Olympic Peninsula in 1898. The best material is
that collected by Alex Walker, at Tillamook, Oregon.
Intergradation with nevadensis is indicated by several specimens. The
coloration of the one adult female, no. 90, Chas. R. Conner Mus., from
Swamp Creek, Washington, has the color of the underparts extended down
the hind legs over the feet, and over the proximal third of the ventral
face of the tail as in nevadensis although the other two specimens
from the same place have the color pattern of altifrontalis. Of the
four specimens from British Columbia referred to this subspecies, only
the specimen from Chilliwack is typical as regards color pattern. The
one from Cultus Lake has the color pattern of nevadensis and might be
referred to that race almost as well as to altifrontalis. The two
specimens from Lihumption Park are intermediate between the two races
in tone of color. Neither has the color of the underparts extended onto
the tail or continuously over the hind feet as in nevadensis but each
does have the color of the underparts less restricted and of lighter
hue than in altifrontalis. Only one of the specimens, no. 7848 Canad.
Nat. Mus., from Lihumption Park is adult and it has a skull which
agrees with that of altifrontalis rather than nevadensis.
After writing the above, a good representation of the weasel population
along the eastern side of Puget Sound was made available by friends in
that area. Study of the weasels from there shows that their color is
intermediate between that of altifrontalis and nevadensis. On the
whole, they (specimens from Bellingham, for example) resemble one
subspecies about as much as the other. In cranial characters some
specimens, in certain features, approach nevadensis but most
specimens agree with altifrontalis and all are more nearly like
altifrontalis to which race all are referred.
The color of these animals is to me indistinguishable from that of
washingtoni. The color of washingtoni is merely intermediate
between that of nevadensis and altifrontalis. Nevertheless, the
race washingtoni has cranial characters (long narrow skull) which set
it off from both altifrontalis and nevadensis. This shape of skull
is not found in the specimens from along the eastern side of Puget
Sound; these animals have skulls like that of altifrontalis and when
departures from this occur they are in the direction of nevadensis
and not washingtoni.
The above, then, explains why specimens which are colored like those
of washingtoni are not referred to that race but instead to the race
altifrontalis.
Of 23 adult skulls examined, 19 have the frontal sinuses malformed as
the result of infestation by parasites.
Specimens examined.—Total number, 80, arranged within states by
counties from north to south. Unless otherwise indicated specimens
are in the United States National Museum.
British Columbia. Chilliwack, 1[74], Lihumption Park, 4750 ft.,
2[77]; Cultus Lake, 1[77].
Oregon. Clatsop County: Old Fort Clatsop, 1[74]; Astoria, 1.
Tillamook County: Tillamook, 12 (7[14], 2[74], 2[2], 1[46]);
Netarts, 1[46]; Blaine, 16 (13[14], 1[93], 1[76], 1[59]). Lane
County: Reed, 1; Mercer, 1[46]. Curry County: Langlois, 1[46].
Washington. Whatcom County: Nooksack River, 2000 ft., 14 mi. E
Glacier, 1; Swamp Creek, 2050 ft., Nooksack River, 3[10]; Lookout,
4800 ft., Mt. Baker, 2[10]; Bellingham, 8[25]; 5 mi. S Bellingham,
1[49]. Skagit County: Rockport, 300 ft., 1. King County:
Bothell, 2[94]; N Seattle 1[51]; Seattle, 1[49]; Tye, 1[51], 2 mi.
E Skykomish, 1[51]; 7 mi. E Kent, 1[76]; Auburn, 3[94]. Pierce
County: Tacoma, 1[1]. Clallam County: Sequim, 1[49]; Soleduc
Riv., near [sic.] Sappho, 1[49]; Happy Lake, 1[60]; mouth of
Boulder Creek, Elwha River, 560 ft., Olympic Mts., 1; Hume's
Ranch, 1000 ft., Elwha River, 1; Bogachiel Riv., 1[49]. Mason
County: Lake Cushman, 2; 4 mi. N Shelton, 1[51]. Thurston
County: Olympia, 2[49]; Tenino, 1[51]. Pacific County: 2-1/2
mi. SE Chinook, 3[74].
Mustela frenata oregonensis (Merriam)
Long-tailed Weasel
Plates 19, 20, 21, 30, 34, 35 and 36
Putorius xanthogenys oregonensis Merriam, N. Amer. Fauna, 11:25,
June 30, 1896; Bangs, Proc. New England Zoöl. Club, 1:57, June 9,
1899.
Mustela xanthogenys oregonensis, Miller, U. S. Nat. Mus. Bull.,
79:99, December 31, 1912.
Mustela xanthogenys munda, Grinnell, Univ. California Publ.
Zoöl., 40:102, September 26, 1933 (part).
Mustela frenata oregonensis, Hall, Carnegie Instit. Washington
Publ. 473:107, November 20, 1936.
Type.—Male, subadult, skull and skin; no. 32019/43828, U. S.
Nat. Mus., Biol. Surv. Coll.; Grants Pass, Rogue River Valley,
Josephine County, Oregon; December 19, 1891; obtained by C. P.
Streator; original no. 1404.
The skull (plates 19-21, 30) is complete and unbroken. P3 on the
left side is missing. Otherwise the teeth all are present although
worn probably as a result of gnawing at the trap which captured
the specimen. The skin, in brown, winter pelage, is fairly well
made.
Although the label on the skin and the label in the skull vial
each give the sex of the specimen as female, and although Merriam
(1896:25) regarded the specimen as a female, the present writer
regards the specimen as a male.
It is as large as other undoubted males and larger than any known
female of this subspecies. The labels with the skull and skin give
the locality as "Rogue River Valley, Oregon." The listing here of
the more restricted locality, Grants Pass, is made on the basis of
Merriam's (1896:25) original description of the subspecies.
Range.—Transition and Canadian life-zones along coast of
northern California and southern Oregon from Humboldt County,
California, north through Curry County, Oregon, thence inland,
west of the Cascades, north to the Columbia River. See figures 29
and 30 on pages 221 and 314.
Characters for ready recognition.—Differs from M. f.
altifrontalis in presence of frontonasal white patch, lighter
color above (tone 2 to 3 of Raw Umber, pl. 301, rather than tone 4
of Brownish Drab, pl. 302, Oberthür and Dauthenay), wider extent
of light color of underparts which is extended distally beyond
knee, and in females, longer tooth-row which amounts to more than
38 per cent of basilar length; from M. f. munda in shorter hind
foot of males which is less than 50, and in both sexes, smaller,
less rugose skull (see measurements and plates); from M. f.
saturata in presence of frontonasal white patch, in having color
of underparts extended uninterruptedly over ankle onto foot; from
M. f. nevadensis in presence of frontonasal white patch, lack of
light color of underparts on ventral face of tail, and longer
skull, which relative to its length in males, is shallower through
braincase; from M. f. effera in presence of frontonasal white
patch, lack of light color of underparts on ventral face of tail,
and larger skull with basilar length averaging more than 41.7 in
males; from M. f. washingtoni in presence of frontonasal white
patch, shorter skull in males, which in percentage of basilar
length has, on the average, orbitonasal length amounting to less
than 35, mastoid breadth more than 55, and zygomatic breadth more
than 63; and in females larger skull with least width of palate
more than length of P4, upper tooth-rows more than 38-1/2 per cent
of basilar length, bullae larger and averaging more than 13.4
long.
Description.—Size.—Male: Five males (3 adults and 2
subadults from Eureka, Ferndale, and Carlotta, California) yield
average and extreme measurements as follows: Total length, 392
(347-430); length of tail, 138 (110-160); length of hind foot, 46
(43-50). Tail averages 54 (46-61) per cent as long as head and
body. Length of hind foot more or less than basal length. The type
specimen, and an adult from Goldbeach measure, respectively, as
follows: Total length, 412, 386; length of tail, 155, 137; length
of hind foot, 44, 46.
Female: Three adults (2 from Fortuna and 1 from Carlotta,
California) yield average and extreme measurements as follows:
Total length, 367 (360-374); length of tail, 130 (123-134); length
of hind foot, 40 (39-40). Tail averages 55 (52-57) per cent as
long as head and body. Length of hind foot less than basal length.
A subadult from Goldbeach, an adult from 13 mi. SW Grants Pass,
and an adult from Medford, measure, respectively, as follows:
Total length, 316, 344, 294; length of tail, 114, 120, 122; length
of hind foot, 36, 40, 38.
The average differences in external measurements of the two sexes
in the vicinity of Carlotta, are: Total length, 25; length of
tail, 8; length of hind foot, 6. Corresponding differences, at
Goldbeach, are: 70, 23, 10. Probably the females at Fortuna
reflect the large size of munda more than do the males at
Carlotta and the differences between the measurements of the two
sexes probably, therefore, are actually more than are indicated by
the figures above.
Externals.—Longest facial vibrissae black, brown or white
(often all three colors in same specimen) and extending beyond
ear; carpal vibrissae same color as underparts and extending to
apical pad of fifth digit; hairiness of foot-soles, in summer
pelage, as shown in figure 20.
Color.—Upper parts, in summer, near (16 l) Brussels Brown or
tone 2 of Raw Umber of Oberthür and Dauthenay, pl. 301, to
slightly darker than tone 3 of same plate. Darker on nose and top
of head, usually with frontonasal white patch but lacking white
bar in front of each ear, except in the type and 2 specimens from
Salem. Chin, lower lips, angle of mouth, and usually posterior
seventh of upper lip white. Remainder of underparts Pale
Orange-Yellow. In winter usually lighter above with underparts
Warm Buff to Straw Yellow. Tip of tail at all times black. Color
of underparts extends distally on posterior sides of forelegs over
toes onto antipalmar faces of feet and wrists, on medial side of
hind leg, typically over ankle in extremely narrow line which
widens out over distal phalanges of antiplantar faces of toes but
sometimes interrupted at ankle. Least width of color of underparts
averaging, in twenty available specimens, 39 (27-54) per cent of
greatest width of color of upper parts. Black tip of tail in five
adults averaging 50 (43-60) mm. long; thus averaging longer than
hind foot and 33 per cent of length of tail-vertebrae.
Skull and teeth.—Male (based on 4 adults and subadults from
Eureka, Requa, Goldbeach, and Grant Pass): See measurements and
plates 19-21, 30. As described in Mustela frenata nevadensis
except that: Weight, 3.5 (3.5-4.1) grams; basilar length, 42.9
(41.8-44.0); least width of palate more or less than medial length
of P4.
Female (based on 2 adults, one from Carlotta and one from 13 mi.
SW Grants Pass): See measurements and plates 34-36. As described
in Mustela frenata nevadensis except that: Weight, 2.4 (2.2-2.6)
grams; basilar length, 37.7 and 39.5; zygomatic breadth less than
distance between condylar foramen and M1 and less than distance
between anterior palatine foramen and anterior margin of tympanic
bulla. See under "Remarks" for additional data on variation in
size of skulls of females.
The skulls of the female averages 31 per cent lighter than that of
the average male.
Because there is much geographic variation between specimens here
referred to oregonensis, the person who is guided by the present
account should keep in mind that results, here reported, of comparisons
of the skull with those of other races, were obtained by employing
specimens of oregonensis from Carlotta and Eureka, California. These
specimens from California are judged to have more of the characters of
the subspecies munda than do specimens of oregonensis from more
northern localities.
Compared with that of M. f. washingtoni the skull of the male is
shorter, especially in the preorbital region and is relatively broader
across the mastoidal processes and zygomatic arches. The skull of the
female is longer in the preorbital region, has a less cylindrical
braincase and differs less from the male skull than is the case in M.
f. washingtoni. Compared with M. f. effera, the skull of the male is
smaller in every part measured and relative to the basilar length is
broader across the mastoids and has relatively shorter tympanic bullae.
From M. f. nevadensis the skull of the male differs in the same way
except that size is about the same. The skull of the female
oregonensis is more heavily ridged and is relatively broader across
the mastoids than that of effera. From M. f. saturata,
oregonensis is not surely known to differ in cranial characters. From
M. f. munda, oregonensis differs in having the skull of both sexes
smaller, and on the average, in all parts measured, has a less marked
postorbital constriction, relatively narrower interorbital region and
relatively more expanded zygomata. From M. f. altifrontalis, males of
oregonensis differ on the average, in having larger teeth, and
relative to the basilar length, a greater mastoid breadth and a
shallower braincase as measured at the anterior margin of the
basioccipital. Females of oregonensis differ in larger average size
of skull, except for breadth of rostrum and interorbital breadth which,
therefore, are relatively less in oregonensis, as also is the
relative depth of the skull measured at the posterior borders of the
upper molars and at the anterior margin of the basioccipital. However,
skulls of females of oregonensis have relatively longer tooth-rows
and are relatively broader across the zygomata and mastoidal processes.
Remarks.—In 1896, Merriam named oregonensis as a subspecies of the
California bridled weasel on the basis of a single specimen taken by
Clark P. Streator. Three additional specimens were acquired in later
years, by workers of Dr. Merriam's bureau, from near the type locality
and specimens from farther north in Oregon have been accumulated at the
University of Oregon. The most satisfactory material is that saved from
Humboldt County by the late H. E. Wilder, which, when brought together,
is adequate to give some idea of the range of variation that can be
expected in a given population.
Of two specimens from Goldbeach, one shows approach to altifrontalis
in that the color of the underparts stops at the ankle, and in one, the
angle of the mouth is dark colored. Specimens from Eugene and vicinity
lack the white facial markings, and in this feature approach the
adjoining washingtoni-effera-nevadensis stock. A specimen from 6
miles south of Medford shows approach to saturata in the
interruption, on the ankle and lower tibial region, of the color of the
underparts. One adult female, no. 1413, Univ. Oregon, from the Rogue
River Valley, 13 miles southwest of Grants Pass, stands out
prominently, among the other specimens from extreme southern Oregon and
northwestern California, by reason of the near (18) Apricot Yellow
color of the underparts, but this same color occurs in specimens from
the more northerly localities of Buchanan, Eugene, Vida Fish Hatchery,
and McKenzie Bridge, as well as in no. 2178, Univ. Oregon, from Cresent
Lake. The last mentioned specimen is here referred to nevadensis.
Two females referred to oregonensis from southern Oregon differ so
greatly in size of skull that they challenge one's imagination in any
attempt to provide an explanation for so wide a range of variation in
one subspecies. One of these, no. 244520, U. S. Nat. Mus., is an adult
female from Medford. The other, no. 224034, U. S. Nat. Mus., is a
subadult female (though labeled male) from 43 miles northeast of Grants
Pass. The skull of the adult from Medford has a basilar length of 41.5,
upper tooth-rows, 16.1 in length, and a weight of 2.75 grams, whereas
corresponding figures for the subadult are only 33.8, 12.9, and 1.4.
Two other adult females are intermediate in size: No. 1413, Univ.
Oregon, from 13 miles southwest of Grants Pass, Oregon, approaches the
specimen from Medford in size, and the second specimen, no. 34325, Mus.
Vert. Zoöl., from Carlotta, California, is smaller.
Not only is there a difference in length between the skulls of the two
extremes of the females but this difference extends to all other
dimensions of their skulls, and is most pronounced in the preorbital
region. The differences in breadth of the braincase and other parts of
the skull are relatively less than the differences in length.
Differences of the same nature, although of lesser degree than found in
the females, are to be seen in two males. The skull of an adult no.
51590, Mus. Vert. Zoöl., from 6 miles south of Medford, has a basilar
length of 46.4, upper tooth-rows, 17.6 mm. long, and a weight of 4.0
grams, whereas corresponding figures for the subadult type specimen
from Grants Pass, are only 43.0, 16.2, and 3.3.
The wide range of variation in size of skull of both sexes, together
with the considerable variation in color pattern of the specimens here
referred to oregonensis raises the suspicion that we are using the
name in a composite sense; nevertheless, to recognize more than one
subspecies with the material now available would be unwise.
A subadult female, of abnormal color, no. 47149, Mus. Vert. Zoöl.,
taken by Mr. H. E. Wilder at Carlotta, California, on December 20,
1930, in a region where weasels do not turn white in winter, is white,
except for the black tip of the tail, but has a suffusion of orange.
This specimen, discussed at greater length on page 43, is instructive
in that it suggests that there are separate determiners for the brown
and red elements of the pelage. It is interesting also as suggesting
how natural selection may tend to eliminate from the population a
conspicuous color-variation of this kind. At any rate, Mr. Wilder (Ms.)
states: "This specimen was picked up in a field, where it evidently had
been dropped by a hawk or an owl." The braincase of the skull is
crushed in three places as though by a raptor's beak. None of the
several other weasels, all normally colored, saved by Mr. Wilder from
this general locality gives evidence of having fallen a victim to a
raptor.
Only 2 skulls of the 12 adults and subadults examined show malformation
of the frontal sinuses such as results from the presence of parasites.
Specimens examined.—Total number, 29, arranged within states
from north to south by counties. Unless otherwise indicated
specimens are in the collection of the United States National
Museum.
California. Del Norte County: Requa, 1[8]. Humholdt County:
Eureka, 2 (1[74], 1[75]); Ferndale, 1[74]; Fortuna, 2[63];
Carlotta, 6 (3[74], 3[59]); 12 mi. E Bridgeville, 1[59]; 2 mi. W
Bridgeville, 1[59].
Oregon. Washington County: Forest Grove, 1. Marion County:
Salem, 2. Benton County: Buchanan, 1. Lane County: McKenzie
Bridge, 1[101]; Vida Fish Hatchery, 1[101]; Eugene, 1[101].
Douglas County: Anchor, 1. Curry County: Gold Beach, 2[60].
Josephine County: Rogue River Valley (Grants Pass), 1; 13 mi. SW
Grants Pass, 1[101]. Jackson County: Medford, 2; 6 mi. S
Medford, 1[74].
Mustela frenata munda (Bangs)
Long-tailed Weasel
Plates 1, 19, 20, 21, 22, 23, 30, 34, 35, 36 and 40
Putorius xanthogenys mundus Bangs, Proc. New England Zoöl. Club,
1:56, June 9, 1899; Stephens, California mammals, p. 247, 1906.
Mustela frenata, Audubon and Bachman, Journ. Acad. Nat. Sci.
Philadelphia, 8 (Pt. 2):291, 1842 (North California about 40°
latitude).
Mustela xanthogenys munda, Miller, U. S. Nat. Mus. Bull., 79:99,
December 31, 1912.
Mustela frenata munda, Hall, Carnegie Instit. Washington Publ.
473:107, November 20, 1936.
Type.—Male, adult, skull, os penis and skin; no. 5459,
collection of E. A. and O. Bangs, but now in collection of Mus.
Comp. Zoöl.; Point Reyes, Marin County, California; June 19,
1896; obtained by C. A. Allen; original no. 931. (See comments
under "Remarks," below, on places in California to which the name
Point Reyes has been applied.)
The skull (pls. 19-21, 30) is complete and unbroken. I1 on each
side and right I2 are broken away; p2 and p3 on each side have
been aborted and the only alveoli remaining are two for the right
p3. Otherwise all teeth are present and entire. The skin is fairly
well made and in good condition.
Cranially, the type is a "runt"; its small size and the
circumstance that the tympanic bulla is longer than the lower
molar and premolar tooth-row and longer than the rostrum are
features which differentiate the type from any other specimen seen
of this race.
Range.—Sea level to at least 6,000 feet (South Yolla Bolly
Mountain, Trinity County, California); Upper Sonoran and
Transition life-zones of the coast and Coast Range of northwestern
California from the Golden Gate northward into southern Humboldt
and Trinity counties. See figures 29 and 30 on pages 221 and 314.
Characters for ready recognition.—Differs from M. f.
oregonensis in longer hind foot of males which is more than 50
mm., and in both sexes, larger, more prominently ridged skull (see
measurements and plates); from M. f. saturata by presence of
nasofrontal white spot, larger and relatively shallower skull of
males and larger skull of female; from M. f. nevadensis by
presence of well-developed, white, facial markings; absence of
color of underparts on ventral face of proximal third of tail; and
hind foot of males more than 50; from M. f. xanthogenys by near
(l) Sudan Brown to near (l) Antique Brown rather than
Buckthorn Brown colors of upper parts and greater size, and in
adult male basilar length more than 45 and hind foot more than 47;
from M. f. nigriauris by having inside of ears same color as
back rather than much darker than back.
Description.—Size.—Male: Three adults and two young from
Point Arena and Gualala, Mendocino County, yield average and
extreme measurements as follows: Total length, 447 (434-470);
length of tail, 167 (150-185); length of hind foot, 53 (50-60).
Corresponding measurements of three adults from 5 and 6 miles west
of Inverness, Marin County, are: 430 (420-440), 154 (141-160), 48
(48-49). Corresponding measurements of four individuals (3 adults
and 1 young of large size) from South Yolla Bolly Mountain,
Trinity County, are: 383 (374-400), 134 (130-138); 44 (43-44). The
tail averages 60 per cent as long as the head and body in the
series from Point Arena, 56 per cent in the series from Point
Reyes, and 53 per cent in the series from South Yolla Bolly
Mountain. In every specimen except two, length of hind foot less
than basal length. The two exceptions are no. 19720, M.V.Z., male
adult from Point Arena in which the hind foot is recorded as 60
(probably an error in measurement), and no. 19721, M.V.Z., from
the same place, in which the skull has not yet attained its full
growth.
Female: One adult from Point Arena measures as follows: Total
length, 383; length of tail, 134; length of hind foot, 43.
Corresponding measurements of an adult from seven miles north of
Laytonville, Mendocino County, are: 336, 121, 33 (= 36 on dried
skin). Corresponding measurements of an adult from South Yolla
Bolly Mountain, Trinity County, are, 326, 113, 37. In these three
specimens, the tail is, in the order given, 54, 56, and 53 per
cent as long as the head and body. Length of hind foot more than
basal length.
Differences in external measurements of the two sexes as indicated
by the five males and one female from Point Arena, are: Total
length, 64; length of tail, 33; length of hind foot, 10. Weights
of 2 adult males are 265 and 221 grams and of one adult female 155
grams.
Externals.—As described in Mustela frenata nigriauris.
Color.—Spot between eyes, narrow band or spot confluent with
color of underparts on each side of head anterior to each ear,
chin, lower lips, and rarely posterior third or less of each upper
lip white; dark spot posterior to each angle of mouth uniformly
present and of large size; tip of tail black; remainder of upper
parts near (14 l) Sudan Brown and tone 4 of Raw Umber of
Oberthür and Dauthenay, pl. 301; occasionally, slightly darker
brown on forehead, nose, and about eyes. Underparts near (a to
c) Ochraceous-Buff and sometimes Orange-Buff. Color of
underparts extends distally on posterior sides of forelegs over
toes onto antipalmar faces of feet and wrists, on medial sides of
hind limbs over antiplantar faces of toes. Least width of color of
underparts averaging, in a series of 5 males from Mendocino
County, 57 (46-67) per cent of greatest width of color of upper
parts; 38 (35-40) in 3 males from Point Reyes, Marin County. Black
tip of tail in Mendocino County series averaging 53 (46-60) mm.,
which is same length as hind foot and 32 per cent of length of
tail. In Point Reyes males, black tip of tail averages 44 (34-52)
mm., which is less than length of hind foot and 45 per cent as
long as tail-vertebrae.
Several specimens of the smaller, inland variant (see under
"Remarks") are near (l) Antique Brown rather than near (14
l) Sudan Brown above and hence do not differ in this respect
from nigriauris.
Skull and teeth.—Male (based on 3 adults from Mendocino
County): See measurements and plates 19-23, 30. As described in
Mustela frenata nigriauris except that: Weight, 6.0 (5.4-6.3)
grams; basilar length, 47.6 (46.5-48.2); length of tympanic bulla
more than length of lower molar and premolar tooth-row.
Female (based on no. 19723, M.V.Z., from Point Arena): See
measurements and plates 34-36, 40. As described in M. f.
nigriauris except that: Weight, 3.0 grams; basilar length, 42.3.
The skull of the female is 50 per cent lighter than that of the
average male.
Compared with the skull of the male of nevadensis that of munda
averages larger in every part measured and specimens from Point Arena
are nearly as heavy again, have relatively more expanded zygomata and
mastoid processes but are relatively narrower anteriorly as shown by
the breadth of the rostrum, interorbital breadth and postorbital
breadth. Also the braincase is less inflated anteriorly, the tympanic
bullae are lower and the skull is more angular. Females show the same
differences although in different degree. Compared with the skull of
the male of M. f. nigriauris, that of munda from Point Arena
averages larger in every part measured except for the length of the
upper tooth-rows. Relative to the basilar length, the skull of munda
averages broader across the mastoids and across the zygomata, is deeper
through the braincase at the anterior end of the basioccipital, and
has a greater development of the lambdoidal crest.
Remarks.—The skin and part skull, no. 536/1849, U. S. Nat. Mus.,
taken by Lieutenant W. P. Trowbridge at San Pablo Bay, is the first
specimen known to have been saved of this subspecies. Since 1899 when
O. Bangs diagnosed munda as of small size, the weasel of the humid
costal belt north of San Francisco Bay has been regarded as smaller
than bridled weasels from farther south in the State. Actually,
however, the weasel of the humid costal belt shares with M. f.
pulchra the distinction of being one of the two largest weasels in
California.
M. f. munda may be a composite subspecies, for the variation in
facial markings, in coloration otherwise, in external measurements and
in size and shape of skull is great. At one time in the course of the
present study, manuscript accounts of two subspecies were prepared for
the animals now all called munda and there is still much
justification for recognizing two subspecies, one, along the coast
proper, the larger, darker-colored animal with reduced white facial
markings and large, wide, heavily ridged skull from Point Arena, and 6
miles south of Laytonville, Mendocino County, along with the specimens
from 5 and 6 miles west of Inverness, Marin County, and the other, an
inland race, which is a smaller, lighter-colored animal with more
extensive white facial markings and a smaller, narrower, skull, known
by specimens from Point Reyes [station?], Nicasio, 15 mi. north of San
Rafael, Freestone, Vallejo, and Mount Sanhedrin. The differences
between these two lots of specimens are of great degree. However, a
female from Fort Bragg proves to be no larger than three females
labeled as from Point Reyes. Also, a male from 2 miles south and one
mile east of Stewarts Point on the coast has a skull no larger than the
animal from Vallejo, whereas the skin alone of an adult female from 3
miles south of Stewarts Point is large and agrees with the specimens
from Point Arena. Consequently, no logical ranges can be worked out for
the two variants with the material now available.
Finally, the type specimen of munda is a "runt," smaller than any
other male seen. This specimen, purchased by E. A. and O. Bangs from C.
A. Allen, who collected and sold specimens widely, was labeled as from
Point Reyes. So far as this place-name is concerned, it might refer to:
(1) The point of land by that name which projects out into the Pacific
Ocean, (2) an abandoned ranch house bearing that name at the head of
Drakes Bay, 6 miles north and 3-3/4 miles east of the actual point, or
(3) the railway station by the same name at the head of Tomales Bay,
12 miles east and 4-3/4 miles north of the actual point. Allen,
himself, lived near San Geronimo (then Nicasio) about nine miles
southeast of the Point Reyes railway station. All these places are in
Marin County, but differ markedly as regards climate and flora. The
first two are treeless, windswept and have much fog, whereas Point
Reyes Station is more often sunny, and is situated in a shallow valley,
inland, where the open grass-covered west-facing slopes meet the
east-facing wooded ones. From which one of these three places the type
specimen came, I do not know. The same may be said of the three female
specimens labeled Point Reyes; two of these are in the United States
National Museum and one in the Field Museum.
The specimens in the Museum of Vertebrate Zoölogy from 5 and 6 miles
west of Inverness and those from near the same place in the collection
of John Cushing come from within a couple of miles or less of the Point
Reyes represented by the abandoned ranch house. These specimens, as
remarked above, agree with those from Point Arena in large size,
reduced facial markings and wide skull. These are points of difference
from the smaller variant suspected of being a recognizable subspecies.
It is the smaller variant which the type specimen approaches in size,
and with which it agrees in relatively well-developed white facial
markings. This suggests that the type specimen came from Point Reyes
Station rather than from either of the two other places bearing the
name "Point Reyes," from one of which, as just stated, the variant of
large size is known. The three females labeled "Point Reyes" also have
well-developed white facial markings and are of lesser size than the
female of similar age from Point Arena, Mendocino County. The
presumption is that these three females also came from Point Reyes
Station.
The smaller, inland variant seems to agree in size, cranial characters,
and coloration with M. f. nigriauris to the southward of San
Francisco Bay, but lacks the black on the head which characterizes
nigriauris. The larger variant, on which the description here used
for munda is based, comprises animals which differ from nigriauris
in larger size, darker color, reduced white facial markings, and
larger, relatively wider skull. Both of the variants mentioned above
are sharply distinct from nigriauris on the basis of coloration of
the inside of the ear which is blackish in nigriauris like the dark
facial markings, and in munda is colored like the back. M. f. munda
lacks the dark facial markings; an occasional specimen has at most, a
trace of the markings but this does not extend back so far as the ears.
This difference, blackish versus non-blackish face, persists eastward
of San Francisco Bay to at least as far as the Carquinez Straits, where
a specimen of munda is available from 4 miles north of Vallejo and
one of nigriauris from Glen Frazer Station on the south shore
opposite Vallejo.
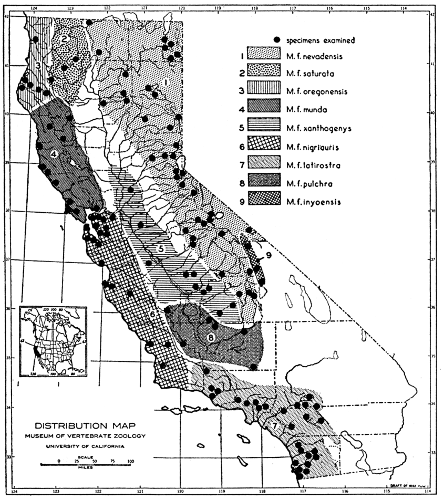
Fig. 30. Map showing the geographic distribution of
subspecies of Mustela frenata in California
Intergradation with M. f. nevadensis and possibly with M. f.
saturata is indicated by specimens from South Yolla Bolly Mountain,
Trinity County. In them the external measurements and measurements of
the skull are intermediate. Also the white frontal spot is much reduced
in size. The white bars in front of the ears are absent in three
specimens, and weakly developed in the other two. The relative
proportions of the skulls as a whole are nearer those of nevadensis
or saturata than munda. The skull of one of the three adult males
and the skull of the adult female suggests M. f. oregonensis in
certain features; for example, the dorsal outline of the skull in
longitudinal axis is slightly convex as it is in oregonensis.
None of the specimens shows malformation of the frontal sinuses such as
results from infestation by parasites.
Specimens examined.—Total number, 37, arranged by counties from
north to south. Unless otherwise indicated specimens are in the
Museum of Vertebrate Zoölogy.
California. Trinity County: S. Yolla Bolly Mt., 3[91]; 1/2 mi. S
S. Yolla Bolly Mt., 1. Tehama County: 2 mi. S S. Yolla Bolly
Mt., 1. Mendocino County: 6 mi. N Laytonville, 1; Mt. Sanhedrin,
1[87]; Ft. Bragg, 1; Gualala, 1; Point Arena, 5. Sonoma County:
2 mi. S and 1 mi. E Stewarts Point, 1; 3 mi. S Stewarts Point P.
O., 1; Freestone, 1. Napa County: 6 mi. SSW, Napa, 1; 4 mi. N
Vallejo, 1. County in question: San Pablo Bay, 1[91]. Marin
County: 6 mi. W Inverness, 2; 5 mi. W Inverness, 2(1[28]); Point
Reyes, 4 (2[91] 1[60], 1[75]); Nicasio, 2 (1[60], 1[75]); Kehoes
Ranch, Pierce Point, 1[28]; Drakes Bay, 1[28]; Tomales Point,
about 1/2 mi. SW White Gulch, 1; Point Reyes School, 3-3/4 mi. W
Inverness, 1; 15 mi. (by road) N San Rafael, 1[52]; Hurley Ranch,
2 mi. W Tomales, 1. No locality more definite than California,
1[7].
Mustela frenata xanthogenys Gray
Long-tailed Weasel
Plates 21, 22, 23, 28, 30, 34, 35 and 36
Mustela xanthogenys Gray, Ann. and Mag. Nat. Hist., 11:118, 1843.
Putorius (Gale) brasiliensis frenatus, Coues, Fur-bearing
animals, p. 142, 1877 (part).
Putorius xanthogenys, Merriam, N. Amer. Fauna, 11:25, June 30,
1896; Bangs, Proc. New England Zoöl. Club, 1:56, June 9, 1899.
Mustela xanthogenys xanthogenys, Miller, U. S. Nat. Mus. Bull.,
79:99, December 31, 1912.
Mustela frenata xanthogenys, Hall, Carnegie Instit. Washington
Publ. 473:107, November 20, 1936.
Type.—Male, adult, skull and skin; skull no. 197a-43.6.4.55,
skin no. 234a-42.11.21.4, British Museum (Nat. Hist.); from the
bank of Sacramento River below mouth of Feather River, or from
north shore of San Francisco Bay, California; taken in "1837 or
1838"; presented by Captain Edward Belcher.
The skull (plate 28) lacks the occiput, the right mandible
posterior to m1, and the right pterygoid; the right zygomatic arch
is fractured. The teeth are not greatly worn. The skin was
originally mounted for exhibition (R. I. Pocock in Litt.) but in
1937 when I saw the skin, it was prepared as a conventional study
skin. The skin is in fairly good condition; some hair is missing
on the hind quarters and the skin of the tail is torn at one
place.
Range.—Altitudinally, less than 600 feet (Fair Oaks); Lower
Sonoran and Upper Sonoran life-zones of all but southern end of
the San Joaquin Valley, and probably Sacramento Valley,
California. See figures 29 and 30 on pages 221 and 314.
Characters for ready recognition.—Differs from M. f.
nevadensis by presence of light facial markings and Buckthorn
Brown rather than near (14n to l) Brussels Brown color of
upper parts; from M. f. munda by Buckthorn Brown rather than
near (l) Sudan Brown, or near (l) Antique Brown color of upper
parts and lesser size, in adult males basilar length less than 45
and hind foot less than 47; from M. f. nigriauris by lighter
color in same way as from munda and also by having inside of
ears same color as back rather than much darker than back; from
M. f. pulchra in hind foot of males less than 46 and narrower
skull, in males having breadth of rostrum less than 13.9 and
mastoid breadth less than 26.0, see comparison of skulls in the
account of pulchra.
Description.—Size.—Male: Three adults, from Fresno, Selma
and Los Banos, measure, respectively as follows: Total length,
425, 417, 450; length of tail, 152, 154, 180; length of hind
foot,—, 43, 44. Tail averages 61 per cent as long as head and
body. Length of hind foot less than basal length.
Female: Adults from Selma, Los Banos, and 4 mi. SW Turlock,
measure respectively as follows: Total length, 357, 365, 395;
length of tail, 133, 132, 145; length of hind foot, 40, 38, 41.
Tail averages 58 per cent as long as head and body. Length of hind
foot less than basal length.
The average differences in external measurements between the two
sexes, as represented by these six specimens, are: Total length,
65; length of tail, 25; length of hind foot, 3.5. One adult male
weighs 274 grams and 2 adult females 182 and 214 grams.
Externals.—As described in Mustela frenata nigriauris.
Color.—Spot between eyes, band confluent with color of
underparts on each side of head extending anterodorsally anterior
to each ear, and posterior half to third of each upper lip white,
or whitish tinged with some shade of yellowish; chin and lower lip
white; dark spot posterior to each angle of mouth of varying size
but uniformly present; tip of tail black; remainder of upper parts
Buckthorn Brown of Ridgway or a trifle browner than tone 4 of
Brown Pink of Oberthür and Dauthenay, pl. 297. Upper parts of
uniform color except for slight darkening of head-markings
anterior to ears. Underparts Ochraceous-Buff to Warm Buff. Color
of underparts extends distally on posterior sides of forelegs over
toes onto antipalmar faces of feet and wrists, on medial sides of
hind limbs over antiplantar faces of toes and sometimes tarsal
region. Least width of color of underparts averaging, in 9
specimens from Fresno, Selma and Los Banos, 54 (32-74) per cent of
greatest width of color of upper parts. Black tip of tail in three
males (one subadult and 3 adults) averages 55 (50-60) mm. long.
Thus longer than hind foot and averaging 34 per cent of length of
tail-vertebrae.
Skull and teeth.—Male (based on 2 adults from Fresno and one
from Selma): See measurements and plates 21-23, 30. As described
in M. f. nigriauris except that: Weight 3.8 grams; basilar
length, 43.7 (43.4-43.9); least width of palate more or less than
lateral length of P4; length of tympanic bulla more than length of
lower molar and premolar tooth-rows.
Female (no. 2626 W. E. Snyder, from Selma): See measurements and
plates 34-36. As described in M. f. nigriauris except that:
Weight, 2.5 grams; basilar length, 39.4.
The skull of the female is 34 per cent lighter than the average
for the three males.
Compared with skulls of nevadensis from the Sierra Nevada, those of
the two adult males from Fresno differ as follows: M1 wider
(transversely); tympanic bullae narrower; preorbital part of skull
smaller. Comparison with pulchra is made in the account of that
subspecies. Compared with skulls of adult males of nigriauris, from
Santa Clara County, the two skulls from Fresno are generally smaller
and in basilar length, length of tooth-rows and measurements of the
teeth fall below the minimum for nigriauris. Relative proportions of
the skulls are approximately the same. Comparison with munda reveals
essentially the same differences as does comparison with nigriauris
except that the difference in size is greater.
Remarks.—The name Mustela xanthogenys Gray was long applied to all
the weasels of the interior valleys of California and of the coast of
that state south of San Francisco Bay. Gray, when he named the species
and when referring to it in later accounts, never defined the locality
whence the specimen came more definitely than "California." In 1896,
Merriam (1896:25) gave the type locality as "Southern California,
probably vicinity of San Diego" and later writers have not contradicted
him. The type specimen was obtained in the course of the voyage of the
British ship Sulphur, under command of Sir Edward Belcher. Examination
of Belcher's (1843, vol. 1, p. 129) narrative of the voyage indicates
the following places in California at which the specimen of weasel,
described by Gray, could have been obtained: Fort Ross, Bodega,
vicinity of San Francisco Bay and up Sacramento River to the mouth of
the Feather River, Monterey, Santa Barbara, Buenaventura, San Pedro,
San Juan, and San Diego.
Reginald I. Pocock has kindly compared the type specimen in the British
Museum with several specimens sent for that purpose. In the first
place, comparison of skulls shows that the type specimen is a member of
one of the races north of San Diego. In the second place, comparison of
skins shows that the inside of the ears are not blackish but similar in
color to the back. In fact, Pocock writes under date of February 12,
1929, regarding the type specimen, that "It is practically uniformly
colored from the snout to the base of the tail, there being scarcely a
trace of the darkening of the head, or muzzle, observable in your
specimens [those sent for comparison]." This character of coloration of
the ear excludes all the weasels of the Coast region of California from
San Francisco Bay southward, namely, M. f. latirostra and M. f.
nigriauris. My own examination of this type specimen at a date later
than that on which Pocock compared it satisfies me as to the accuracy
of his statement above.
Accordingly, the name xanthogenys would seem to apply to one of the
two subspecies here called munda and xanthogenys. Perusal of
Belcher's narrative of the voyage (loc. cit.) shows that little, if
any, opportunity was afforded to obtain vertebrate specimens at Fort
Ross or Bodega, both localities within the range of the subspecies here
called munda. Furthermore, the type specimen is smaller than
individuals of munda from 5 to 6 miles west of Inverness and from
Point Arena with which the animals from Fort Ross and Bodega would be
expected to agree in size. Weasels from along the north shore of San
Francisco Bay are smaller than those on the coast north of the bay.
Possibly the type specimen of xanthogenys came from the north side of
San Francisco Bay but probably it came from the bank of the Sacramento
River and almost certainly not farther up stream from San Francisco Bay
than the junction of the Sacramento and Feather rivers. The statement
of Belcher (1843, vol. 1, p. 129), regarding the trip up the Sacramento
River as far as Point Victoria, lat. 38°46´47" north, and return to
San Francisco Bay, that "Cuyote or jackal—fox, racoon, land otter,
weasel, and squirrel were obtained" lends strong probability to the
idea that this type specimen was taken along the Sacramento River,
possibly in the vicinity of the existing city of Sacramento.
Unfortunately no specimens are available from the Sacramento Valley. If
some were available, a comparison of them and specimens of munda from
along the north side of San Francisco Bay and Carquinez Straits with
the type specimen of xanthogenys should determine the correct
application of the name. For the present it seems best to retain the
name munda and apply the name xanthogenys to the weasels inhabiting
the northern part of the San Joaquin Valley and presumably the southern
part of the Sacramento Valley.
Efforts to obtain specimens of weasels from the Sacramento Valley have
been in vain. A juvenal specimen taken five miles south of Fair Oaks,
Sacramento County, by Mr. John Fitzgerald, Jr., in December, 1927, was
examined at his home and found to agree in coloration with specimens
from farther south. Geographically, this specimen probably is more
nearly a topotype than any other examined.
Most of the specimens examined are immature and adequate adult cranial
material has not been seen. Two adults, one of each sex, from Los Banos
have skulls of large size which agree with those of nigriauris. The
same is true of one adult and one young female from 4 miles southwest
of Turlock, which, unlike the animals from Los Banos, show a darkening
of the head extending in reduced degree even to the inside of the ears,
as in nigriauris. The slightly darker than average (for
xanthogenys) color on the back may indicate intergradation with
nevadensis. Intergradation with M. f. nevadensis is shown by
specimens, from the southern part of the Sierra Nevada, mentioned in
the account of nevadensis.
None of the skulls shows malformation of the frontal sinuses such as
result from infestation by parasites.
Specimens examined.—Total number 30, arranged by counties from
north to south.
California. Sacramento County: Bank of Sacramento River, 1[7]; 5
mi. S Fair Oaks, 1[29]. San Joaquin County: 4 mi. W Stockton,
1[74]. Merced County: Tegner School, 4 mi. SW Turlock, 2; Los
Banos, 4 (2[74], 1[91] 1[87]). Fresno County: Mendota, 1[74];
Biola, 1[30]; Clovis, 1[55]; Fresno, 5 (1[74], 1[91], 2[55],
1[1]); 5 mi. W Fresno, 1[14]; Selma, 3 (2[50], 1[104]); 4 mi. NW
Sanger, 1[55]; 5 mi. S Selma, 1[62]. Tulare County: Monson,
1[74]; 1-1/2 mi. N Goshen, 1[74]; Milo, 1[91]; 2 mi. N Tipton,
1[74]; Poplar, 2[53]. No locality more definite than California,
1[4].
Mustela frenata nigriauris Hall
Long-tailed Weasel
Plates 22, 23, 24, 34, 35, 36 and 41
Mustela frenata nigriauris Hall, Carnegie Instit. Washington
Publ. 473:95, November 20, 1936.
Putorius xanthogenys, Baird, Mamm. N. Amer., 1858, p. 176 (part).
Mustela xanthogenys Gray, Ann. and Mag. Nat. Hist., 14(ser.
4):375, 1874 (part?).
Putorius (Gale) brasiliensis frenatus, Coues, Fur-bearing
animals, p. 142, 1877 (part).
Putorius xanthogenys xanthogenys, Grinnel, Proc. California Acad.
Sciences, fourth series, 3:292, August 28, 1913.
Mustela xanthogenys xanthogenys, Miller, U. S. Nat. Mus. Bull.,
79:99, December 31, 1912; Grinnell, Univ. California Publ. Zoöl.,
40:102, September 26, 1933.
Type.—Male, adult, skeleton and skin; no. 32820, Mus. Vert.
Zoöl.; Half Moon Bay, San Mateo County, California; received at
Museum of Vertebrate Zoölogy, May 4, 1922, through A. L.
Hagedoorn, after having been in captivity a few days where death
occurred owing to injuries received in trap; original no. 1590.
The skull has each of the zygomatic arches and the anterior end of
the nasals broken through. The only part missing is the central
two millimeters of the left zygomatic arch. The teeth all are
present and entire. The skeleton appears to be complete except for
the bones of the feet, which are preserved within the skin. The
skin is well made and in good condition.
Range.—Altitudinally, sea level to more than 4000 feet; Sonoran
and Transition life-zones of Coast Range and coast of California
from San Francisco Bay south to Point Conception, Santa Barbara
County, California. See figures 29 and 30 on pages 221 and 314.
Characters for ready recognition.—Differs from M. f. munda,
xanthogenys, and pulchra by having inside of ears darker than
back rather than same color as back, and from xanthogenys and
pulchra in near (l) Antique Brown color of upper parts rather
than Buckthorn Brown or near (16 j) Buckthorn Brown to near
(h) Yellow Ocher respectively; from M. f. latirostra by
postorbital breadth, of adult males and females, less, rather than
more, than width of basioccipital measured from medial margin of
one foramen lacerum posterior to its opposite.
Description.—Size.—Male: Five adults from Palo Alto, Santa
Clara County, yield average and extreme measurements as follows:
Total length, 447 (412-465); length of tail, 167 (147-175); length
of hind foot, 46 (45-47). Corresponding measurements of four
adults from San Francisco are: 412 (394-435); 153 (145-160); 43.5
(41-46). Corresponding measurements of five adults and subadults
from Berkeley, Alameda County, are: 419 (390-448); 148 (135-160);
44 (42-47). Tail averages 59 per cent as long as head and body in
series from Palo Alto and in one from San Francisco. The average
of 55 for the Berkeley series probably reflects a lesser average
age. Length of hind foot less than basal length. The type specimen
measures, 415, 150, 43. It is smaller than the mean.
Female: A subadult from Palo Alto measures: Total length, 368;
length of tail, 126; length of hind foot, 39. An adult and two
subadults from Berkeley measure, respectively, as follows: Total
length, 347, 365, 340; length of tail, 134, 123, 125; length of
hind foot, 37, 38.4, 36.5. In these four females the tail averages
55 per cent as long as head and body. Length of hind foot less
than basal length.
The average differences in external measurements of the two sexes,
as represented by specimens from Berkeley, Alameda County, are:
Total length, 68; length of tail, 21; length of hind foot, 7.
Eight adult males weigh 249 (217-335) grams and one adult female
123 grams.
Externals.—Longest facial vibrissae brownish like dark color of
head and extending beyond ear; carpal vibrissae mostly color of
underparts and extending to apical pad of fifth digit; hairiness
of foot-soles slightly more than shown in figure 20.
Color.—Spot between eyes, band, confluent with color of
underparts, on each side of head extending anterodorsally anterior
to ear, and posterior third of each upper lip tinged with color of
underparts or, less often, pure white; chin and lower lips white;
remainder of sides and top of head posteriorly to, or a little
behind, a line connecting posterior margins of ears, blackish;
inside of pinna of ear, and sometimes outside of pinna, blackish;
dark spot posterior to each angle of mouth present on each side in
three-fourths of specimens; tip of tail black; remainder of upper
parts near (l) Antique Brown, and with more yellow than tone 3
of Raw Umber of Oberthür and Dauthenay, pl. 301. Often with more
blackish and red in winter. Underparts near (a to c)
Ochraceous-Buff or Ochraceous-Salmon. Ochraceous-Salmon in some
juveniles. Color of underparts extends distally on posterior sides
of forelegs over toes onto antipalmar faces of feet and wrists,
and on medial sides of hind limbs over antiplantar faces of toes.
Least width of color of underparts averaging, in 17 adult males
(Berkeley, 5; San Francisco, 5; Palo Alto, 7), 55 (40-73) per cent
of greatest width of color of upper parts. Black tip of tail in
same series of males averaging 51 (35-60) mm., thus averaging
longer than hind foot and 33 per cent of length of tail (Palo Alto
and San Francisco, 31 per cent; Berkeley, 35 per cent). In 8 adult
females, least color of underparts amounts to 55 (47-62) per cent
of greatest width of color of upper parts. Black tip of tail
averages 41.5 (28-50) mm., thus averaging longer than hind foot
and 32 per cent of length of tail-vertebrae.
Skull and teeth.—Male (based on six adults from Stanford Univ.
and vicinity): See measurements and plates 22-24; weight (four
adults), 5.4 (5.0-5.9) grams; basilar length, 47 (46.1-48.1);
zygomatic breadth more than distance between condylar foramen and
M1, or than between anterior palatine foramen and anterior margin
of tympanic bulla; mastoid breadth more than postpalatal length;
postorbital breadth less than length of upper premolars (less than
distance between posterior borders of P4 and P2) and less than
width of basioccipital measured from medial margin of one foramen
lacerum posterior to its opposite; interorbital breadth not
greater than distance between foramen opticum and anterior margin
of tympanic bulla; breadth of rostrum less than length of tympanic
bulla; least width of palate less than lateral length of P4;
anterior margin of tympanic bulla as far posterior to foramen
ovale as width of 3 or 4 (including I3) upper incisors; height of
tympanic bulla more than distance from its anterior margin to
foramen ovale; length of tympanic bulla more or less than (about
equal to) length of lower molar and premolar tooth-row and longer
or shorter (usually shorter) than rostrum; anterior margin of
masseteric fossa below anterior half of m2.
Female (based on three adults, Hayward, Palo Alto, and Morro): See
measurements and plates 34-36; weight (no. 43574, from Morro) 2.7
grams; basilar length, 41.2 (40.2-42.2); zygomatic breadth more or
less than distance between condylar foramen and M1 and more or
less than distance between anterior palatine foramen and anterior
margin of tympanic bulla; postorbital breadth less than length of
upper premolars and less than width of basioccipital measured from
medial margin of one foramen lacerum posterior to its opposite;
least width of palate less than lateral length of P4; tympanic
bulla as far posterior to foramen ovale as width of 3 (including
I3) upper incisors; height of tympanic bulla more than distance
from its anterior margin to foramen ovale; length of tympanic
bulla more than length of lower molar and premolar tooth-row and
longer or shorter than rostrum.
The skull of the female averages 50 per cent lighter than that of
the average male.
Comparisons of the skull of the male with those of M. f.
latirostra, pulchra, xanthogenys, and munda are made in the
accounts of those subspecies.
Remarks.—Like M. f. latirostra, nigriauris long bore the name
xanthogenys. The fairly adequate lot of specimens is divided between
the collections of several institutions. The most satisfactory material
in any one collection is in the Stanford University Natural History
Museum where local specimens have been accumulated over a period of
many years.
No actual intergrade between nigriauris and xanthogenys has been
seen, although the specimens from Los Banos, referred to xanthogenys,
have large skulls as in nigriauris. Intergradation with latirostra
is shown by specimens, referred to latirostra, from the Los Angeles
area. Also the one adult male from 5 miles southeast of Santa
Margarita, San Luis Obispo County, is of small size and in this respect
approaches latirostra. The range of nigriauris is separated from
that of munda by San Francisco Bay, Carquinez Straits, and I suppose
by the lower part of the San Joaquin River. On the basis of color of
the inside of the pinna of the ear, the two subspecies are uniformly
distinct. Intergradation is assumed to occur through the subspecies
xanthogenys.
None of the 26 adult and subadult specimens examined for evidences of
infestation of the frontal sinuses by parasites shows malformation of
the sinuses.
Specimens examined.—Total number, 103, arranged by counties
from north to south. Unless otherwise indicated specimens are in
the Museum of Vertebrate Zoölogy.
California. Contra Costa County: Glen Frazer Station, 1; 2 mi. W
Pinole, 1[13]; 1 mi. E Pinole, 1; Richmond, 1[13]; Lafayette, 1; 7
mi. E Clayton, 1; Moraga Valley, 1; Pinehurst, Redwood Canyon, 1;
Concord, 1. Alameda County: Berkeley, 11; Oakland, 1; Piedmont,
1; Haywards, 2; near Haywards, 2; 10 mi. E Haywards, 1[91];
Redwood Canyon, 1; Calaveras Dam, 1. San Francisco County: San
Francisco, 11 (5[8], 2[91], 1[60], 1[7]); Ocean View, 1[68];
Visitation Valley, 1. San Mateo County: Moss Beach, 1; Half Moon
Bay, 1; Redwood City, 1[87]; Menlo Park, 9 (5[87], 2[68]); no
locality more definite than county, 1[8]. Santa Clara County:
1/4 mi. N Milpitas, 1; 1/4 mi. S Milpitas, 1; Stanford University,
6 (4[68], 2[91]); Palo Alto, 11 (6[41], 2[60], 1[75], 1[87]).
Santa Cruz County: 3 mi. E Santa Cruz, 1; 2-1/2 mi. E Santa
Cruz, 1; Santa Cruz, 6 (2[91], 1[68], 1[4]). Monterey County: 1
mi. E mouth Salinas River, 10 ft., 1[37]; Pacific Grove, 1[8];
Monterey, 2 (1[7]); Carmel, 1[8]; Carmel Valley, 1[68]; Point
Lobos, 1; Gonzales, 1. San Luis Obispo County: 5 mi. SE Santa
Margarita, 1; Morro, 1[91]; 3-1/2 mi. S Oceano, 6. Santa Barbara
County: Santa Maria, 1[87]; 5 mi. N Las Cruces, 1; 7 mi. W
Gaviota, 1; Gaviota, 1.
Mustela frenata latirostra Hall
Long-tailed Weasel
Plates 1, 22, 23, 24, 34, 35 and 36
Mustela frenata latirostra Hall, Carnegie Instit. Washington
Publ. 473:96, November 20, 1936.
Putorius xanthogenys, Baird, Mamm. N. Amer., p. 176, 1858 (part);
Stephens, California mammals, p. 246, 1906; Merriam, N. Amer.
Fauna, 11:25, June 30, 1896 (part).
Putorius (Gale) brasilianus frenatus, Coues, Fur-bearing animals,
p. 142 (part).
Mustela xanthogenys xanthogenys, Miller, U. S. Nat. Mus. Bull.,
79:99, December 31, 1912; Grinnell, Univ. California Publ. Zoöl.,
40:102, September 26, 1933.
Mustela arizonensis, Grinnell and Swarth, Univ. California Publ.
Zoöl. 10, 376, October 31, 1913.
Type.—Male, adult, skull and skin; no. 3257, Mus. Vert. Zoöl.;
San Diego, San Diego County, California; May 20, 1907; obtained by
Frank X. Holzner.
Right M1 is missing and the part of the jaw bearing this tooth is
broken away. With this exception the skull is complete and
unbroken and the teeth are all present and entire. The skin is
fairly well made and in good condition except that it is slightly
soiled.
Range.—Altitudinally sea level to 8000 feet (Tahquitz Valley,
San Jacinto Mountains); Sonoran and Transition life-zones of coast
and mountains west of Mohave and Imperial deserts of southern
California from Point Conception and Cuyama Valley southward at
least to Mexican boundary. See figures 29 and 30 on pages 221 and
314.
Characters for ready recognition.—Differs from M. f.
nigriauris by having postorbital breadth of adult males and
females, more, rather than less, than width of basioccipital
measured from medial margin of one foramen lacerum posterior to
its opposite; from M. f. pulchra by having tympanic bulla longer
than rostrum (orbitonasal length) and by near (l) Antique Brown
rather than near (16 j) Buckthorn Brown to near (h) Yellow
Ocher color of upper parts.
Description.—Size.—Male: Six adults and subadults from San
Diego yield average and extreme measurements as follows: Total
length, 439 (428-449); length of tail, 153 (142-160); length of
hind foot, 45 (40-47). Corresponding measurements for a series of
eight adult males from the vicinity of Los Angeles are: 416
(394-428); 158 (151-166); 44 (40-47). In the series from San Diego
the tail averages 54 per cent as long as head and body. In the
series from Los Angeles the average is 61 per cent. Length of hind
foot in each series, less than basal length. The type specimen
measures, 435, 142, 42.
Female: No. 5070, adult, from San Diego, measures 367, 141, 38.
Nos. 22 and 6748 from Santa Ysabel, measure: 359, 380; 130, 140;
39, 35. No. 7194 from Jamacha measures, 358, 125, 35. Three adult
females from Los Angeles yield the following: Total length, 373,
345, 368; length of tail, 150, 122, 134; length of hind foot,—,
41, 41. In no. 5070 the tail is 62 per cent as long as the head
and body and in the three from Los Angeles it averages 60 (55-67)
per cent. Length of hind foot, in each case, less than basal
length.
The average differences in external measurements of the two sexes
as shown by the six males from San Diego and the four females
from San Diego County are: Total length, 73; length of tail, 19;
length of hind foot, 8. Corresponding differences shown by the
eight males and three females from Los Angeles are: 54, 23, 3.
Externals.—Longest facial vibrissae brownish, like dark color
of head and extending beyond ear; carpal vibrissae mostly color of
underparts and extending to apical pad of fifth digit; hairiness
of foot-soles slightly more than shown in figure 20.
Color.—Spot between eyes, band confluent with color of
underparts on each side of head extending anterodorsally anterior
to ear, and posterior third of each upper lip tinged with color of
underparts or, less often, white; chin and lower lips white;
remainder of sides and top of head posteriorly to near line
connecting posterior margins of ears, blackish; inside of pinna of
ear, and sometimes outside of pinna, blackish; dark spot posterior
to each angle of mouth present on each side in three-fourths of
specimens; tip of tail black; remainder of upper parts near (l)
Antique Brown, and with more yellow than tone 3 of Raw Umber of
Oberthür and Dauthenay, pl. 301. Underparts Ochraceous-Buff to
Warm Buff and in some specimens from Los Angeles and Ventura
counties Ochraceous-Orange, especially in young and juveniles.
Color of underparts extends distally on posterior sides of
forelegs over toes onto antipalmar faces of feet and wrists and on
medial sides of hind limbs over antiplantar faces of toes. Least
width of color of underparts averaging, in 15 adult and subadult
males from San Diego County, 54 (35-75) per cent of greatest width
of color of upper parts. Black tip of tail in same series of males
averaging 54.5 (46-60) mm. long. Thus averaging longer than hind
foot and 35 per cent of length of tail-vertebrae.
Skull and teeth.—Male (based on 6 adults from San Diego
County). See measurements and plates 22-24. As described in M. f.
nigriauris except that: Weight (4 specimens), 3.9 (3.8-4.0)
grams; basilar length 43.8 (41.9-47.0); postorbital breadth more
than width of basioccipital measured from medial margin of one
foramen lacerum posterior to its opposite; interorbital breadth
not less than distance between foramen opticum and anterior margin
of tympanic bulla; anterior margin of tympanic bulla as far
posterior to foramen ovale as width of 2 to 2-1/2 (including I3)
upper incisors; length of tympanic bulla more than length of lower
molar and premolar tooth-row and longer than rostrum; anterior
margin of masseteric fossa below m2.
Female (based on 4 adults from San Diego County): See
measurements. As described in M. f. nigriauris except that:
Weight, 2.6 (2.2-2.8) grams; basilar length, 40.0 and 40.1;
postorbital breadth more than width of basioccipital measured from
medial margin of one foramen lacerum posterior to its opposite;
length of tympanic bulla more than length of rostrum.
The skull of the female averages 34 per cent lighter than that of
the average male.
The skull of the male of latirostra, compared with that of
nigriauris, is by weight, more than one-fourth lighter, has a lesser
basilar length, a lesser mastoid breadth, a lesser zygomatic breadth
and a narrower M1. In these features no overlap has been observed
between adults from the general vicinities of the type localities of
the two forms. In adult males of latirostra the postorbital breadth,
with one exception, is more than the combined length of P4 and P3
whereas the reverse is true in adult males of nigriauris. Both males
and females of latirostra have a generally smaller skull with
relatively broader interorbital and postorbital parts and the tympanic
bullae are relatively larger, rounder and more inflated.
Compared with the skull of the male of pulchra that of latirostra
is, by weight, more than one-fourth lighter, has a lesser basilar and
orbitonasal length, lesser zygomatic and mastoid breadth and a more
nearly flat braincase. In these features no overlap has been observed
between adults from the general vicinities of the type localities of
the two subspecies. Also, in latirostra the tympanic bulla is longer
than the rostrum whereas the opposite is true in pulchra. The skull
of latirostra is generally smaller and relatively, on the average,
has the preorbital part of the skull deeper and broader with longer
tooth-rows, although with shorter rostrum, while the zygomatic and
mastoid breadths are less. Study of skulls of subadult females of
pulchra indicate that females of latirostra and pulchra differ in
the same fashion as do males.
Remarks.—This subspecies long has gone by the name M. xanthogenys
and the type locality was generally supposed to be in the vicinity of
San Diego. This supposition seems to have originated with Merriam's
(1896:25) statement that the type locality was "Southern California,
probably vicinity of San Diego." Nevertheless, as set forth in the
account of M. f. xanthogenys the type specimen concerned now is
thought to have come from much farther north.
Although 76 Recent specimens are available from southern California,
additional adults are needed to understand the geographic variation
there. M. f. latirostra may be a composite—made up of more than one
geographic race. Specimens from San Diego County differ so much in
relative length of the tail that at one stage in the present study it
was thought that a difference in this respect existed between the
coastal animals and those from farther inland. Material received later
did not wholly substantiate this view and because of the uniformly
small size of all of the skulls from that county, the animals were
later regarded as of the same subspecies. Eventually, even this
supposed common feature proved to be inconstant for an adult male from
Jamacha, no. 7098, of the San Diego Society of Natural History, and
another adult male from San Marcos, no. 8869, collection of Ralph
Ellis, were later examined and found to have skulls as large as those
of average-sized, adult males of nigriauris.
Despite these puzzling local variations, it is established that the
long-tailed weasels of southern California are smaller than those from
farther north. Also, the southern animal averages smaller in weight and
size of skull, and the skull is differently proportioned. Specimens in
series from Los Angeles County definitely are intermediate in size and
shape of skull between latirostra from San Diego County and
nigriauris from, say, Santa Clara County, but definitely more closely
resemble latirostra from San Diego County than they do nigriauris.
A skull of a young animal, not here identified to subspecies, from
Potholes, in the Colorado River Valley, 10 miles northeast of Bard,
Imperial County, California, may have closest relationship to M. f.
latirostra. Additional comment on this specimen is offered in the
account of M. f. neomexicana.
From the asphalt pits of Rancho La Brea, in Los Angeles County, a total
of 57 skulls have been examined, more than half of which are reasonably
complete. I have been unable to learn whether these came from pits
regarded by students of the deposit as wholly Recent, from pits
regarded as of Pleistocene age, or from both. Suffice to say that only
two specimens were found which could be distinguished from skulls of
the subspecies of weasel living in that area today.
These two specimens, lent to me by Professor Chester Stock, were with
other skulls received from the Los Angeles Museum of History, Art and
Science and bore identifying numbers as follows: 16/20-27, the anterior
part of the skull of an adult, and 16, the skull posterior to the
cribiform plate of a subadult or possibly young individual. The latter
has a mastoid breadth of 28.0 millimeters, a tympanic bulla 16.1 long
and other measurements in proportion. It is larger than any specimen of
weasel, of any subspecies, seen from California and in the subgenus
Mustela seems to be exceeded in size only by certain individuals of
M. f. texensis. M. f. neomexicana attains relatively large size and
comparisons were made with individuals of that subspecies. However, the
young specimen from Rancho La Brea differs from neomexicana in that
the tympanic bullae rise less steeply on the medial sides and the
inferior lip of the external auditory meatus is less developed
laterally. Age considered, the sagittal crest is less developed and the
mastoid processes project more abruptly from the skull. The anterior
part of the skull of the adult, no. 16/20-27 is larger than any
specimen seen of M. f. latirostra or adjoining subspecies, and among
California-taken specimens is equaled in size only by the largest males
of M. f. munda from the northwest coastal district in Mendocino
County. This adult from Rancho La Brea differs from neomexicana, sex
and age taken into account, in greater postorbital breadth, lesser
rostral width in comparison with the interorbital breadth, and in
having the temporal ridges at the anterior end of the sagittal crest
spread out into a Y-shaped, rather than a T-shaped, pattern. All these
differences from neomexicana are features of agreement with the
California bridled weasels of the subspecies latirostra,
nigriauris, and munda. The same is true of the characters which set
apart the young specimen from neomexicana. In summary: of 57 weasel
skulls examined from the asphalt pits at Rancho La Brea, Los Angeles
County, all but two are indistinguishable from the skulls of the Recent
weasel living in that region today. These two skulls agree in
qualitative characters with animals of the California coastal
subspecies now living from Los Angeles northward to Humboldt County,
but are larger. For the time being these two may be thought of as
giants of the same type of animal inhabiting the Los Angeles region
today.
Only one of 41 adult and subadult skulls examined for malformation of
the frontal sinuses shows infestation by parasites.
Specimens examined.—Total number, 142, listed by counties from
north to south. Unless otherwise indicated specimens are in the
Museum of Vertebrate Zoölogy.
California. Santa Barbara County: Rincon Point, 1. Ventura
County: Cuyama Valley, 2200 ft., 1[91]; Nordhoff, 3[59]; Santa
Paula, 1[59]; Ventura, 7. Los Angeles County: near Owensmouth,
1[24]; Cahuenga, 1[91]; Llano, 10 mi. E Littlerock, 1; Flint
Ridge, Pasadena, 1[59]; Pasadena, 3; Lankershim, 1[24]; 1 mi. S
Lankershim, 1[24]; Duarte, 1[59]; Covina, 1[59]; Claremont, 1[91];
El Monte, 4 (2[75], 1[24]); Montebello, 1; Alhambra, 6 (5[2],
1[91]); El Nogal, 2[8]; Gardena, 1[26]; Palos Verdes Estate, 3;
Rancho La Brea asphalt deposits, 57[70] and [92]. San
Bernardino County: San Bernardino Valley, 1[75]; San Bernardino,
4 (2[20], 1[91]); Redlands, 2 (1[38]); Bluff Lake, 2 (1[59],
1[33]). Riverside County: West Riverside, 1; Arlington, 800 ft.,
1[17]; 3-1/2 mi. E and 1/2 mi. N Beaumont, 2600 ft., 1; Banning,
1[91]; Cabazon, 1[91]; San Jacinto Plain, 1[20]; Tahquitz Valley,
8000 ft., 1; Elsinore, 1[1]. San Diego County: Twin Oaks, 1[91];
San Marcos, 2 (1[87], 1[41]); Escondido, 1; Witch Creek, 1[91];
Ballena, 1[20]; Santa Ysabel, 3 (2[20], 1[87]); Julián, 1; La
Jolla, 1; Lakeside, 1[91]; El Cajon, 1[91]; El Vido (not found on
map), 1[91]; San Diego, 9 (1[91], 1[20], 1[87], 1[32]); Jamacha,
2[87]; Chula Vista, 1[20].
Mustela frenata pulchra Hall
Long-tailed Weasel
Plates 22, 23 and 24
Mustela frenata pulchra Hall, Carnegie Instit. Washington Publ.
473:98, November 20, 1936.
Type.—Male, adult, skeleton and skin; no. 16668, Mus. Vert.
Zoöl.; Buttonwillow, Kern County, California; April 30, 1912;
obtained by J. Grinnell; original no. 1953.
The skull (plates 22-24) is complete and unbroken (a fracture in
the right jugal has healed). All teeth are present and entire. The
skeleton lacks the os penis, left fibula, shaft of left tibia and
the distal three or four caudal vertebrae. Some of the bones of
the feet distal to the radius and tibia are with the skeleton, and
the remainder probably are in the skin. The skin is fairly well
made and in good condition, except for the left hind leg which was
torn when the animal was captured. A well-developed scrotal pouch
shows the specimen to have been a male.
Range.—Altitudinally around 300 feet in San Joaquin Valley to
2500 feet at Isabella; Upper Sonoran and Lower Sonoran life-zones
of southern end of San Joaquin Valley and in mountains at southern
end of Valley, California. See figures 29 and 30 on pages 221 and
314.
Characters for ready recognition.—Differs from M. f.
nevadensis in presence of light facial markings, and from M. f.
nevadensis and M. f. inyoensis in near (16 j) Buckthorn Brown
to near (h) Yellow Ocher rather than near (14 n to l)
Brussels Brown color of upper parts, and greater size with hind
foot more than 40 in females and basilar length averaging more
than 46.0 in males; from M. f. latirostra in having rostrum
(orbitonasal length) longer than tympanic bulla and from M. f.
latirostra and M. f. nigriauris by color of upper parts as
stated above rather than near (l) Antique Brown, and by having
inside of ears same color as back rather than much darker than
back; from M. f. xanthogenys in hind foot of males more than 46
and broader skull which in males has breadth of rostrum more than
13.9 and mastoid breadth more than 26.0.
Description.—Size.—Male: The type specimen and five other
adults yield average and extreme measurements as follows: Total
length, 454 (428-477); length of tail, 178 (153-184); length of
hind foot, 50 (47-55). Tail averages 65 per cent as long as head
and body. Length of hind foot approximately equal to basal length.
The type specimen measures, 460, 184, 49.
Female: Three subadult topotypes yield average and extreme
measurements as follows: Total length, 399 (383-411); length of
tail, 154 (140-161); length of hind foot, 42 (41-42). Tail
averages 63 per cent as long as head and body. Length of hind foot
less than basal length.
The average differences in external measurements of the two sexes
are: Total length, 55; length of tail, 24; length of hind foot, 8.
Externals.—As described in Mustela frenata nigriauris.
Color.—Spot between eyes, band confluent with color of
underparts, on each side of head extending anterodorsally anterior
to each ear, posterior third of each upper lip, lower lips and
chin white or more often darker than Ochraceous-Buff and
therefore same color as belly; dark spot posterior to each angle
of mouth present but small; tip of tail black; remainder of upper
parts near (16 j) Buckthorn Brown to near (h) Yellow Ocher and
from tone 2 to 4 of Brown Pink of Oberthür and Dauthenay, pl. 297,
but with a trifle more reddish brown. Upper parts of uniform color
except for occasional slight darkening of nose, forehead, and
areas around eyes. Underparts darker (a) than Ochraceous-Buff.
Color of underparts extends distally on posterior sides of
forelegs over toes, onto antipalmar faces of feet and wrists, on
medial sides of hind limbs over antiplantar faces of toes, tarsal
region and sometimes in diluted fashion on proximal third of
underside of tail. Least width of color of underparts averaging,
in 6 male topotypes, 55 (43-81) per cent of greatest width of
color of upper parts. Black tip of tail in same series of males
averaging 58 (53-63) mm. long; thus averaging longer than hind
foot and 33 per cent of length of tail-vertebrae.
Skull and teeth.—Male (based on 6 ads., type and 5 topotypes):
See measurements and plates 22-24. As described in M. f.
nigriauris except that: Weight (6 ads.), 5.3 (4.5-6.1) grams;
basilar length, 47.6 (46.0-48.6); (one skull, no. 335, with
postorbital breadth more than distance between posterior borders
of P4 and P2); interorbital breadth more or less than distance
between foramen opticum and anterior margin of tympanic bulla;
anterior margin of tympanic bulla as far posterior to foramen
ovale as width of 2 to 3-1/2 (including I3) upper incisors; length
of tympanic bulla more than length of lower molar and premolar
tooth-row and shorter than rostrum.
Female: Adult skull of typical female not seen.
As compared with the skull of the type specimen of inyoensis, skulls
of adult males of pulchra are larger throughout, relatively broader,
especially in the preorbital part of the skull, have more inflated
tympanic bullae, and are less convex in dorsal outline. Comparison of
the skull with that of latirostra has been made in discussion of that
subspecies. Comparison of skulls of adult males of nigriauris and
pulchra shows that those of pulchra average larger in every
measurement taken except those of m1, M1, P4, and depth of skull at
posterior borders of upper molars. The basilar length is only slightly
more and it follows that, relative to this length, other measurements
of the skull are relatively, as well as actually, larger. In no one
measurement is there an entire lack of overlap, but the skulls of adult
males, and probably adult females, may be distinguished from those of
nigriauris by the combination of the following mentioned, average
differences: Tympanic bullae larger in each of three dimensions;
preorbital and interorbital parts of skull broader and notably heavier;
interorbital breadth greater; zygomatic arches more expanded laterally;
mastoid processes more prominent. As compared with xanthogenys,
differences of similar nature, but of greater degree, distinguish
pulchra. As compared with those of nevadensis (represented by
specimens from Mono Co., Calif.), skulls of adult males of pulchra
average larger in every measurement taken and no overlap exists in
basilar length, orbitonasal length, mastoid breadth, zygomatic breadth,
length of tympanic bulla, or depth of skull at either the anterior
margin of the basioccipital or at the posterior margins of the upper
molars. Relatively, the preorbital portion is about the same size in
the two forms.
Remarks.—The best material of this big weasel was obtained in 1910
and 1911 by John Wimmer and forwarded to the California Academy of
Sciences through John R. Rowley, although in 1905, one specimen had
been obtained by A. S. Bunnell for the collections of the United States
Bureau of Biological Survey, another by J. Grinnell for the Museum of
Vertebrate Zoölogy in 1912, and in 1933, another by L. M. Huey, for the
San Diego Society of Natural History.
The males from the type locality are relatively uniform in size and
shape of skull. The one exception is no. 137935, U. S. Nat. Mus.,
slightly younger than the others. Its skull is relatively more slender
than any of the others and does not display several of the differential
characters. The male, no. 127566, U. S. Nat. Mus., from Alila (=
Earlimart) is intermediate in cranial features between pulchra and
xanthogenys as known from specimens taken in the vicinity of Fresno.
The skull of the female, no. 127565, from the same place, is too young
to provide diagnostic characters. Since the skull of an adult female of
topotypical pulchra is unknown, doubt attaches to the identification
of the adult, female specimen, no. 115895, U. S. Nat. Mus., from
Delano. It has a relatively broad skull in comparison with the adult
female of xanthogenys from Los Banos. The adult female, no. 9998, San
Diego Soc. Nat. Hist., from 2 mi. SW Simmler, shows approach to
nigriauris in slightly reduced size. The skin alone from Coalinga, a
male, taken on April 10, 1935, measures 462, 179, 47. The adult female,
with crushed skull, from 4 miles east of Coalinga, measures 350, 129,
40. In size, these specimens agree better with pulchra than with
xanthogenys. The skin alone from 3 miles south of Coalinga is unsexed
and without external measurements. Skulls of adults from Coalinga are
needed to permit of more positive identification of the subspecies
found there. The female from 4 miles east of Coalinga, taken on
February 21, 1936, is in process of molt on the underparts, and the
longer hair which is near (20´) Naples Yellow contrasts strongly with
the incoming shorter hair which is near (10 c) Salmon-Orange. The
skin alone, no. 16270, Mus. Vert. Zoöl., from Isabella, was made up
from a decayed animal and is of but little use. It is referred to
pulchra purely because of geographic nearness of Isabella to the type
locality of pulchra. The most that can be told from the specimen is
that it is a relatively light-colored, bridled weasel. The fact that
the color is slightly darker than in pulchra may or may not indicate
intergradation with nevadensis. No. 54103/41042, U. S. Nat. Mus.,
consisting of crushed bits of skull and the skin of the head, is from
Willow Spring, Kern County. This marginal locality is really in the
Mojave Desert rather than in the San Joaquin Valley. The light color of
the skin of the head suggests pulchra, but it is realized that a
complete specimen might show the animal there to be unlike pulchra.
None of the skulls shows evidence of having had the frontal sinuses
infested by parasites.
Specimens examined.—Total number, 18, listed by counties from
north to south. Unless otherwise indicated, specimens are in the
Museum of Vertebrate Zoölogy.
California. Fresno County: Coalinga, 1[23]; 4 mi. E Coalinga, 1;
3 mi. S Coalinga, 1[8]. Tulare County: Alila (= Earlimart),
2[91]. Kern County: Delano, 1[91]; Buttonwillow, 9 (6[8],
2[91]); Isabella, 1; Willow Spring, 1[91] San Luis Obispo
County: 2 mi. SW Simmler, 1[87].
Mustela frenata inyoensis Hall
Long-tailed Weasel
Plates 22, 23 and 24
Mustela frenata inyoensis Hall, Carnegie Instit. Washington Publ.
473:99, November 20, 1936.
Putorius xanthogenys, Merriam, N. Amer. Fauna, 11:25, June 30,
1896 (part).
Mustela xanthogenys xanthogenys, Miller, U. S. Nat. Mus. Bull.,
79:99, December 31, 1912.
Type.—Male, adult, skull (with skeleton) and skin; no. 25907,
Mus. Vert. Zoöl.; Carl Walter's Ranch, 2 mi. N Independence, Inyo
County, California; June 26, 1917; obtained by A. C. Shelton;
original no. 3143.
The skull (plates 22-24) is complete and unbroken. All teeth are
present and entire. The skin is well made and in good condition.
Range.—From 3700 feet (Lone Pine) to at least 4000 feet
(Alvord); Lower Sonoran Life-zone of the floor of Owens Valley in
Inyo County, California. See figures 29 and 30 on pages 221 and
314.
Characters for ready recognition.—Differs from M. f.
nevadensis in presence of white facial markings; from M. f.
pulchra in near (l) Brussels Brown rather than near (16 j)
Buckthorn Brown to near (h) Yellow Ocher color of upper parts
and basilar length of less than 45 in males; from M. f.
latirostra in brownish rather than blackish color of inside of
ear and orbitonasal length of more than 15.
Description.—Size.—Male: Two adults, the type specimen and
no. 25392/32805, measure, respectively, as follows: Total length,
423 and 390; length of tail, 170 and 145; length of hind foot, 42
and 44. Tail is 67 and 59 per cent as long as head and body.
Length of hind foot less than basal length.
Female: No. 12400, Field Mus. Nat. Hist., which is young, has the
following measurements: Total length, 390; length of tail, 150;
length of hind foot, 39. Tail is 63 per cent as long as head and
body. Length of hind foot less than basal length.
The differences in external measurements between the two sexes, as
represented by the male type specimen and by the young female,
are: Total length, 33, length of tail, 20; length of hind foot, 3.
Externals.—Longest facial vibrissae black or dark brown and
reaching beyond ear; carpal vibrissae same color as underparts and
extending to apical pad of fifth digit; hairiness of foot-soles
(in summer pelage) slightly less than shown in figure 19.
Color.—Large spot between eyes, band confluent with color of
underparts, on each side of head extending anterodorsally anterior
to each ear, upper throat, chin, lower lips and in some specimens
part or all of upper lips white; patch between eyes and bars in
front of ears tinged with some shade of yellowish in one specimen;
dark spot posterior to each angle of mouth present in four of five
specimens; tip of tail black; remainder of upper parts, in summer,
near (l) Brussels Brown or tones 1 to 2 of Raw Umber of Oberthür
and Dauthenay, pl. 301; slightly darker brown on forehead, nose
and about eyes. In winter near (j) Snuff Brown or lighter than
Brussels Brown with a smoked effect. Underparts Buff-Yellow,
winter and summer. Color of underparts extends distally on
posterior sides of forelegs over toes onto antipalmar faces of
feet and wrists and on medial sides of hind legs over antiplantar
faces of toes. Least width of color of underparts averaging, in 5
available specimens 34 (24-42) per cent of greatest width of color
of upper parts. Black tip of tail, in two adult males, averaging
53 (45 and 60) mm. Thus longer than hind foot and averaging 34 per
cent of length of tail-vertebrae.
Skull and teeth.—Male (based on the type): See measurements and
plates 22-24. As described in M. f. nigriauris except that:
Weight, 4.4 grams; basilar length, 44.7; postorbital breadth not
less than width of basioccipital measured from medial margin of
one foramen lacerum posterior to its opposite; length of tympanic
bulla less than length of lower molar and premolar tooth-row.
Female: Adult unknown.
Compared with the skull of the male of nevadensis, no single
difference not covered by individual variation in nevadensis has been
detected. Selected differences of inyoensis in comparison with
latirostra are larger size, less inflated tympanic bullae and
relative narrowness of the postorbital, interorbital and preorbital
parts of the skull. Comparison of the skull with that of M. f.
pulchra is made in the account of that subspecies.
Remarks.—Although two specimens of this subspecies were taken during
the Death Valley Survey conducted by Dr. C. Hart Merriam, only three
additional individuals are known to have been saved as study specimens
since that time.
M. f. inyoensis as now known may be thought of as closely similar to
M. f. nevadensis except for the presence of well-developed white
facial markings like those found in the weasels of the San Joaquin
Valley and coastal region of California south of San Francisco Bay. The
nonwhite areas of the head are almost the same color as the back and
not distinctly blackish as in M. f. latirostra and M. f.
nigriauris. The one specimen in the winter coat, no. 25392/32805, U.
S. Nat. Mus., from Lone Pine, is brown rather than white. The brown has
the pale smoke-tinge common in the winter pelage of subspecies whose
members are either brown or white in winter. The range of this
subspecies is thought to include the floor and lower elevations of
Owens Valley although it may occur in limited numbers southwestward
along the base of the Sierra Nevada and through the mountains in places
of low elevation like Walker Pass its range may meet that of pulchra.
The type specimen was taken in an alfalfa field by ranch hands, who,
according to A. C. Shelton (MS), stated that the species was common at
the type locality. None of the five specimens shows infestation of the
frontal sinuses by parasites.
Specimens examined.—Total number, 5, listed by localities from
north to south.
California. Inyo County: Alvord, 4000 ft., 1 (U. S. Nat. Mus.);
2 mi. N Independence, 1 (Mus. Vert. Zoöl.); Lone Pine, 3 (2 in
Field Mus. Nat. Hist. and 1 in U. S. Nat. Mus.).
Mustela frenata neomexicana (Barber and Cockerell)
Long-tailed Weasel
Plates 1, 22, 23, 24, 34, 35 and 36
Putorius frenatus neomexicanus Barber and Cockerell, Proc. Acad.
Nat. Sci. Philadelphia, 1898:188; Lantz, Trans. Kansas Acad.
Sci., 19:178, 1905.
Mustela frenata neomexicana, Miller, U. S. Nat. Mus. Bull.,
79:100, December 31, 1912; Bailey, Animal Life of Carlsbad
Cavern, p. 97, 1928; Hall, Carnegie Instit. Washington Publ.
473:108, November 20, 1936.
Mustela frenatus neomexicanus, Bailey, N. Amer. Fauna, 35:19,
September 5, 1913.
Type.—Male, adult, skull and skin; no. 10475, Mus. Comp. Zoöl.;
Armstrongs Lake, Mesilla Park, Dona Ana County, New Mexico;
February 1, 1898; obtained by A. C. Tryson; original no. 58 of C.
M. Barber.
The skull is imperfectly cleaned but unbroken. The right upper
incisors, right P2 and left p3 are broken away. The skin is
indifferently stuffed but in a good state of preservation except
that the distal part of the tail is missing. The animal's coat is
ragged, and this imperfect appearance is heightened by injury to
the posterior part of the body, probably at the time of capture.
Range.—From 3800 feet (type locality) to 9000 feet (Cloudcroft,
N. Mex.); Upper Sonoran and Lower Sonoran life-zones of northern
México, southeastern Arizona, New Mexico and western Texas,
panhandle of Oklahoma, southeastern Colorado and southwestern
Kansas. See figure 29 on page 221.
Characters for ready recognition.—Differs from M. f. frenata
and M. f. texensis by Buckthorn Brown rather than Brussels Brown
color of upper parts, mastoid breadth of adult males ordinarily
more, rather than less, than postpalatal length; from M. f.
leucoparia by Buckthorn Brown rather than Argus Brown color of
upper parts, distance from anterior margin of tympanic bulla to
foramen ovale less, rather than more, than four-fifths height of
tympanic bulla; from M. f. arizonensis and M. f. nevadensis by
Buckthorn Brown, rather than near (14 n) Brussels Brown or, in
winter, white color of upper parts, in presence of white frontal
spot continuous with color of underparts, in basilar length of
more than 46 mm. in males and 40 mm. in females; from M. f.
longicauda by Buckthorn Brown rather than near (h) Clay Color
of upper parts, by presence of white facial markings on Argus
Brown head, and by length of tooth-rows amounting to less than 37
per cent of basilar length; from M. f. primulina by Buckthorn
Brown rather than Brussels Brown color of upper parts, in presence
of white frontal spot and broad white bands on side of head, in
anteriorly truncate rather than anterolaterally rounded bullae and
zygomatic breadth of more than 30 in males and 24 in females.
Description.—Size.—Male: The type specimen (see Barber and
Cockerell, 1898:188) measured: Total length, 500; length of tail,
205; length of hind foot, 50. Tail 70 per cent as long as head and
body. Length of hind foot less than basal length.
Female: No. 21779 from Tombstone, Arizona, measured: Total length,
419; length of tail, 165; length of hind foot from dried skin, 41
(probably 43 in flesh). Tail 65 per cent as long as head and body.
Length of hind foot less than basal length.
The average differences in external measurements of the two sexes,
as known from these two individuals, are: Total length, 81; length
of tail, 40; length of hind foot, 7.
Compared with M. f. frenata, the size, proportions of parts and
difference in size of the two sexes, appears to be about the same.
Externals.—Longest facial vibrissae colored like upper parts
[in the type specimen some of the "long bristles of the upper lip"
are white as pointed out by Barber and Cockerell (op. cit.:
188)] and extending beyond ear; carpal vibrissae colored like
underparts and extending to apical pad of fifth digit; hairiness
of foot-soles as shown in figure 20.
Color.—Broad white bands on sides of head, extending
anterodorsally anterior to each ear, confluent with white spot
between eyes and with color of underparts; posterior half or all
of each upper lip edged with white; usually few white hairs on top
of head between ears; remainder of top of head near Argus Brown of
Ridgway and Chocolate, tone 4, of Oberthür and Dauthenay; dark
spot posterior to each angle of mouth usually absent; tip of tail
black; remainder of upper parts varying, in different specimens,
from Buckthorn Brown to Dresden Brown of Ridgway, and Brown Pink
(tones 3 to 4, pl. 297, of Oberthür and Dauthenay); underparts
Antimony Yellow or near (c) Warm Buff of Ridgway, and Brown Pink
(tone 1, pl. 297, of Oberthür and Dauthenay); color of underparts
extends distally on legs over forefeet and hind feet. Least width
of color of underparts averaging 46 (41-55) per cent of greatest
width of color of upper parts; black tip of tail 35 to 45 mm. long
in females; 43 to 68 mm. long in males and averaging 21 (20-36)
per cent as long as tail-vertebrae.
No specimen of this subspecies in the white winter coat has been
seen. Animals taken in midwinter are available from Mesilla Park,
Willcox, and 10 miles east of Roswell.
From M. f. frenata, neomexicana differs in: upper parts and
underparts much lighter colored; white facial markings more
extensive; color of underparts more extended onto feet. From M.
f. leucoparia, neomexicana differs as follows: above and below,
much lighter colored, but white facial markings less extensive and
color of underparts less extended onto feet and legs.
Skull and teeth.—Male (based on adults: the type; no. 131582
from Berino, New Mexico; and no. 1485 from Seward Co., Kansas):
See measurements and plates 22-24. As described in Mustela
frenata frenata except that: Weight, 6.2 (4.9 and 7.5); basilar
length, 49.3 (48 and 50.5); mastoid breadth more than postpalatal
length; least width of palate less than length of P4; anterior
margin of masseteric fossa directly below m2 or heel of ml.
Female (based on three adults): See measurements and plates 34-36.
As described in Mustela frenata frenata except that: Weight, 3.1
(2.6-3.5) grams; basilar length, 42.7 (40.8-45.5); zygomatic
breadth less than distance between condylar foramen and M1 and
more or less than distance between anterior palatine foramen and
anterior margin of tympanic bulla.
The skull of the female averages 50 per cent lighter than that of
the male.
As compared with the skull of the male of M. f. frenata, that of
neomexicana is decidedly more angular and ridged. The postorbital
constriction is narrower, the mastoid breadth greater (it is less than
the postpalatal length in some subadult males), the sagittal crest much
higher with impressions of the temporal and masseter muscles carried
farther forward on the frontals, rostrum shorter and tympanic bullae
wider and more inflated. Similar, though less marked, differences exist
between the females. As compared with M. f. leucoparia and perotae,
the same differences as noted above between frenata and neomexicana
exist. In addition the tympanic bullae are so far removed from the
foramen ovale that the distance from the anterior end of each bulla to
the foramen ovale, instead of being less than the height of tympanic
bullae, is in leucoparia more than four-fifths this height and in
perotae more than the entire height. Also, in perotae, the
squamosal, anterior to each tympanic bulla, is ventrally convex rather
than ventrally concave as in neomexicana. Compared with M. f.
longicauda, neomexicana is relatively narrower in the interorbital
region, has relatively shorter tooth-rows, a V-shaped rather than a
U-shaped interpterygoid space and in males has the interorbital region
flat rather than convex and the sagittal crest is higher. The same
differences are to be noted in comparison with nevadensis but here
the difference in relative length of tooth-row is less. The same
differences exist also in comparison with M. f. arizonensis except
that its interorbital breadth, relative to the rest of the skull, is
about the same. Difference in size is especially marked here; even
females of neomexicana average larger than males of arizonensis.
Remarks.—When Barber and Cockerell named this subspecies in 1898,
they had three specimens. Only two others are known to have been taken
before this time. These are a skeleton, without corresponding skin,
taken at Lozier, Texas, in 1890 by Wm. Lloyd, and no. 21779/36482, U.
S. Nat. Mus., taken on April 6, 1893, by R. D. Lusk at Tombstone,
Arizona. On the back of a label recently attached to the last mentioned
specimen the name C. K. Worthen appears and probably signifies that the
specimen was purchased from this dealer in vertebrate specimens.
M. f. neomexicana has a large geographic range. The old male from
Liberal, Seward County, Kansas, extends the known range far to the
northeast. Geographically, this occurrence is logical for the
southwestern desertlike conditions extend to this part of Kansas.
Probably the subspecies occurs in southeastern Colorado and in the
panhandle of Oklahoma where conditions are similar. Bailey (1905:198)
lists neomexicana as a member of the mammalian fauna of Texas. As
stated by him (loc. cit.:198) this inclusion is based on geographic
grounds and not on actual specimens. Strecker (1926:13) also includes
neomexicana in his list of Texas mammals but writes me, under date of
January 9, 1928, that "I included Mustela frenata neomexicana as a
Texas mammal on the strength of its being mentioned by Bailey. . . ."
On better ground, Bailey (1928:97) lists the subspecies as occurring in
southeastern New Mexico at Carlsbad Cavern. However, Bailey (loc.
cit.) knew of the existence of weasels just below El Paso and at
Langtry, Texas. An unsexed skeleton, no. 167891, in the United States
National Museum, from Lozier, Texas, is not certainly identifiable to
subspecies. If, as I think, the animal is a female, its skull is
intermediate between that of frenata and neomexicana although when
all features are considered it is seen to be nearest the latter. The
large size (basilar length of 46.5 mm.) may reflect some relationship
to texensis. The field notes of the collector furnished me by Dr. H.
H. T. Jackson (MS), describe the color as brownish yellow above and
sulphur below. The admission of this subspecies to the list of mammals
of Texas is made certain by the female (no. 1572, Texas Cooperative
Research Collection) taken on July 28, 1940, 1-1/2 mi. NW Kent, Texas,
by C. E. Scull.
The skull alone from Durango (City of), extends the known range far to
the south. This skull is typical of neomexicana. Skins from the same
place would be especially interesting as showing the approach, if any,
in color, of neomexicana to M. f. leucoparia.
Mr. D. D. Stone of Casa Grande, Arizona, writes, under date of February
2, 1927, that a weasel was seen by an acquaintance of his in a field
near Chandler, Maricopa County, Arizona. Probably this was
neomexicana. If so, its range extends much farther west than
collected specimens show.
Actual intergradation with M. f. frenata is not shown by the material
at hand. The two females from Albuquerque, although typically
neomexicana as regards color, have smaller, less prominently ridged
skulls than females of neomexicana of the same age from farther south
and approach M. f. nevadensis.
Probably the geographic ranges of M. f. neomexicana and M. f.
latirostra do not meet; the only evidence of the existence of weasels
in all of the large area, comprising western Arizona and the deserts of
eastern California, which intervenes between the ranges of the two
subspecies is the skull of a young individual, no. 68842, Mus. Vert.
Zoöl., from 10 miles northeast of Bard, Imperial County, California.
There, on December 29, 1932, Jack C. vonBloeker, Jr., retrieved the
weathered skull with some of the vertebrae attached, from the top of a
wood rat's nest beneath a mesquite tree near the west bank of the
Colorado River.
The idea that the carcass may have been washed down the river from far
upstream gains no support from a comparison of the specimen itself for
the tympanic bullae are larger than in nevadensis and the skull is
larger than the largest males seen of arizonensis, the two upriver
races. On the basis of size the skull could be either a male of
latirostra or a female of neomexicana. These two subspecies, like
arizonensis and the skull in question, have much inflated bullae.
However, the immaturity of the specimen conceals any other diagnostic
cranial features, and prevents referring it certainly to either
neomexicana or latirostra. In any event the specimen provides no
evidence of intergradation between the two forms last mentioned.
Speculating on its identity, I should say that it might be either an
intergrade between arizonensis and nevadensis, from southern Utah
or northwestern Arizona, or a member of an unnamed race resident in the
lower part of the valley of the Colorado River.
Whereas M. f. panamensis and M. f. aureoventris are the
darkest-colored weasels and occur in regions of heavy rainfall, M. f.
neomexicana is the lightest-colored American weasel and occurs in an
extremely arid region where the rainfall and humidity are slight.
According to Barber and Cockerell (1898:189) "The type specimen was
shot in the grass on the shore of Armstrong's Lake. . . ." Bailey
(1928:97) found the tracks of one of these animals "in the great pit at
the west entrance to" Carlsbad Cavern and supposes they "hunt the cave
walls for mice and other small game." Data on the label attached to no.
230973 states that the specimen was taken, two miles west of Willcox,
Arizona, in a prairie dog town.
Only two of the 23 skulls show evidence of infestation of the frontal
sinuses by parasites.
Specimens examined.—Total number, 28, arranged alphabetically
by states and from north to south by counties in each state.
Unless otherwise indicated specimens are in the United States
National Museum.
Arizona. Graham County: Safford, 1. Cochise County: 2 mi. W
Willcox, 1; Willcox, 1; 8000 ft., Chiricahua Mts., 1; 6000 ft.,
Pinery Canyon, Chiricahua Mts., 1[33]; Tombstone, 1; Sulphur
Spring Valley, 1[74].
Durango. "Durango City," 1.
Kansas. Seward County: Liberal, 1[93].
New Mexico. Bernalillo County: 3 mi. NW Albuquerque, 2. Lincoln
County: 7800 ft., South Fork Eagle Creek, White Mts., 1. Chaves
County: Pecos River, 10 mi. E Roswell, 8[74]; Dexter, 1[74].
Otero County: Cloudcroft, 9000 ft., 1[90]. Dona Ana County:
Mesilla Park, 2 (1[75], 1[7]); Berino, 2.
Texas. Culberson County: 1-1/2 mi. NW Kent, 1[90]. Terrel
County: Lozier, 1.
Mustela frenata texensis Hall
Long-tailed Weasel
Plates 22, 23 and 24
Mustela frenata texensis Hall, Carnegie Instit. Washington Publ.
473:99, November 20, 1936.
Mustela frenata, Strecker, The Baylor Bull., 27:14, September,
1924.
Mustela frenata frenata, Strecker, The Baylor Bull., 27:12,
August, 1926 (part).
Type.—Male, adult, skull with skin of head, neck and tail; no.
14821, Amer. Mus. Nat. Hist.; Kerr County, Texas; September 17,
1897; obtained by H. P. Attwater.
The skull (plates 22-24) and dentition are complete and unbroken.
The preserved parts of the skin are not stuffed.
Range.—Lower Sonoran and possibly Upper Sonoran life-zones of
central Texas. See figure 29 on page 221.
Characters for ready recognition.—Differs from Mustela frenata
arthuri in possessing white facial markings and postorbital
breadth less than distance between posterior borders of P4 and P2;
from M. f. frenata in larger size of body and skull, the basilar
length of which in adult males is more than 52.5; from M. f.
neomexicana in Brussels Brown rather than Buckthorn Brown color
of upper parts and basilar length of skull more than 52.5.
Description.—Size.—Male: Measurements taken from the dried
skins of a young male, no. 15476, Mus. Comp. Zoöl., from Kerr
County, Texas, and a subadult male, no. 2017, Baylor Univ. Mus.,
from 5 mi. N Waco, Texas, are, respectively, as follows: Total
length, 600 and more than 510; length of tail, 200 and 225; length
of hind foot, 52 and 52.
Female: Skins unknown.
Externals.—As described in Mustela frenata frenata.
Color.—As described in Mustela frenata frenata.
Skull and teeth.—Adult male: See measurements and plates 22-24.
As described in Mustela frenata frenata except that: Weight, 8
grams; basilar length 54; least width of palate less than length
of P4; anterior margin of masseteric fossa anterior to middle of
m2.
Female: Skull unknown.
Remarks.—The type specimen, taken by the veteran collector of Texan
mammals, H. P. Attwater, appears to have been the first one of these
animals to find its way into the collection of any museum or
naturalist. The second specimen from Kerr County was secured by, or
through, the well-known commercial collector, F. B. Armstrong. Two
trade skins, from Kerr County, taken on December 10, 1938, are in the
Texas Cooperative Research Collection, as is also the skeleton of a
young animal from Fredericksburg. The two other specimens from McLennan
County (both males contrary to the statement of Strecker, 1924:14), owe
their preservation to the alertness of John K. Strecker, Curator of the
Baylor University Museum, who has given a complete account of their
history.
The range of this subspecies is thought to include much of central
Texas.
The preserved parts of the skin of the type specimen show it to have
been generally large. The part of the tail preserved measures 226
millimeters and the skin of the head and neck is correspondingly large.
The skin alone, no. 427, from near Waco, Texas, has, as now stuffed, a
body 365 millimeters long. Individuals of this race attain larger size
than those of any other American member of the subgenus Mustela with
the possible exception of Mustela frenata macrophonius from Veracruz,
México. In addition to large size, texensis and macrophonius are
analogous in that each has a small geographic range at the northern end
of an extensive range of its similarly colored southern relative from
which it differs mainly in size. Each of the two groups, goldmani and
macrophonius on the one hand and perotae, frenata and texensis
on the other, has relatively uniform color, color pattern and body
proportions over a large region but at its northern extremity develops
a "giant" population, M. f. macrophonius and M. f. texensis,
respectively. The skull of the type specimen of M. f. texensis is the
largest one seen of any American weasel. The type specimen of M. f.
macrophonius has a basilar length that is greater by one-tenth of a
millimeter but in every other measurement taken the skull of M. f.
texensis is the larger. Its weight, 8 grams, also shows it to be
larger.
The broad, white bands in front of the ears are confluent with the
white patch between the eyes on both sides in two specimens and on one
side only in one other specimen. A white patch between the ears is
present in four specimens. The dark spot at each angle of the mouth is
absent on both sides in four specimens and on one side only in one
other specimen. Thus out of a possible twelve cases, the broad bands in
front of the ears are confluent with the spot between the eyes in five
cases. Four of the six specimens have a white spot between the ears.
The dark spot at each angle of the mouth is present three out of a
possible twelve times.
The skull of no. 2017, from five miles north of Waco, is smaller than
either of the two skulls seen from Kerr County and in this respect
approaches M. f. frenata. There is no actual evidence of
intergradation with any other subspecies but intergradation probably
does take place with M. f. neomexicana and possibly with M. f.
arthuri and M. f. primulina.
Strecker (1924:14) remarks that of the two specimens obtained near
Waco, one was taken in a trap baited for mink and the other was shot in
a hen house. None of the four skulls had the frontal sinuses infested
with parasites.
Specimens examined.—Total number, 7, arranged by counties from
north to south.
Texas. McLennan County: 5 mi. N Waco, 1[3]; Erath, 1[3].
Gillespie County: Fredericksburg, 1[90]. Kerr County: 4[75];
1[2]; and 2[90] trade skins.
Mustela frenata frenata Lichtenstein
Long-tailed Weasel
Plates 1, 22, 23, 24, 36, 37, 38 and 40
Mustela frenata Lichtenstein, Darstellung neuer oder wenig
bekannter Säugethier, 1832, pl. 42, and corresponding text,
unpaged; Seton, Lives of game animals, 2:576, 1929.
Mustela brasiliensis Sevastianoff, Mem. de l'Acad. Imp. Sci. St.
Petersburg, 4:356-363, tab. 4, 1813, name on plate only, the
description being in the text (not of Gmelin, 1788); Gray, Proc.
Zoöl. Soc. London, 1865:114.
Putorius frenatus, Baird Mamms. N. Amer., p. 173, 1858; Merriam,
N. Amer. Fauna, 11:26, pl. 3, figs. 1, 1a, 1b, June 30, 1896;
Bailey, N. Amer. Fauna, 25:198, October 24, 1905.
Putorius (Gale) brasiliensis aequatorialis Coues, Fur-bearing
animals, p. 142, 1877, part? ("merely as a substitute for Gray's
[supposedly] preoccupied name" that is, aureoventris).
Putorius (Gale) brasiliensis frenatus, Coues, Fur-bearing
animals, p. 142, 1877 (part).
Putorius mexicanus Coues, Fur-bearing animals, p. 142, 1877,
[nomen nudum, cited by Coues in synonymy as "Putorius
mexicanus, Berlandier, MSS. ic. ined. 4 (Tamaulipas and
Matamoros)"].
Putorius brasiliensis frenata, Allen, Bull. Amer. Mus. Nat.
Hist., 3:219, April 17, 1891.
Putorius brasiliensis frenatus, Allen, Bull. Amer. Mus. Nat.
Hist., 6:197, May 31, 1894; Bangs, Proc. Biol. Soc. Washington,
10:9, February 25, 1896; Allen, Bull. Amer. Mus. Nat. Hist.,
8:74, April 22, 1896.
Mustela frenata frenata, Strecker, The Baylor Bull., 27:12,
August, 1926; Hall, Carnegie Instit. Washington Publ. 473:108,
November 20, 1936.
Type.—Female, adult, skull and skin; no. 991, Berlin Zool.
Mus., México City, México; June, 1829; obtained by F. Deppe.
The specimen once mounted, now is remade into a study skin and
lacks the distal part of the tail. The skull (plates 36-38, 40)
lacks the basicranial region.
Range.—Altitudinally, sea level (Brownsville, Texas) to 7600
feet (Tlalpam, México); from southern Texas as far south as México
City; Lower Sonoran to at least Transition life-zone. See figure
29 on page 221.
Characters for ready recognition.—Differs from M. f. perotae
in nonextension of blackish over anterior fourth of neck, least
width of color of underparts more than 37 per cent of greatest
width of color of upper parts; height of tympanic bulla more than
distance from its anterior margin to foramen ovale; from M. f.
leucoparia by restricted white facial markings that cover less
than half surface of head in front of ears, by nonextension of
black of head onto anterior half of neck and by wider (more than
7.8) tympanic bullae; from M. f. neomexicana by Brussels Brown
rather than Buckthorn Brown color of upper parts and mastoid
breadth less than postpalatal length; from M. f. texensis by
smaller size of body and skull (basilar length in adult males less
than 52.5); from M. f. arthuri by white facial markings and
postorbital breadth less than distance between posterior borders
of P4 and P2; from M. f. tropicalis by nonextension of blackish
over anterior fourth of neck, least width of underparts more than
37 per cent of greatest width of upper parts, postorbital breadth
of adult males less than distance between posterior borders of P4
and P2.
Description.—Size.—Male: Fifteen adults and subadults from
Brownsville, Texas, yield average and extreme measurements as
follows: Total length, 485 (430-556); length of tail, 202
(165-250); length of hind foot, 48 (40-55). Averages believed to
be reliable but extremes probably are not. Tail averages 71 per
cent as long as head and body. Length of hind foot less than basal
length. Corresponding measurements of an adult male (topotype, no.
50826) from Tlalpam, México, are: 505, 203, 53. Another adult
male, from Miquihuana, Tamaulipas, México, measures: 520, 215, 52.
Female: Six adults, subadults and young from Brownsville, Texas,
yield average and extreme measurements as follows: Total length,
420 (362-456); length of tail, 173 (126-200); length of hind foot,
41 (40-46). Tail averages 70 per cent as long as head and body.
Length of hind foot more (with possible exception of no.
36362/48732 U. S. Nat. Mus.) than basal length.
The average differences in external measurements of the two sexes
are: Total length, 65; length of tail, 29; length of hind foot, 7.
Externals.—Longest facial vibrissae black and reaching beyond
ear; carpal vibrissae same color as underparts and extending to
apical pad of fifth digit; hairiness of foot-soles as shown in
figure 20.
Color.—Spot between eyes, broad band, confluent with color of
underparts, on each side of head extending anterodorsally anterior
to each ear, and posterior two-thirds to one-half of each upper
lip, white; remainder of sides and top of head, posteriorly to
line connecting posterior margins of ears, blackish; dark spot
posterior to angle of mouth present on both sides in about half
the specimens; tip of tail black; remainder of upper parts
Brussels Brown; chin white; remainder of underparts near (16´a)
Ochraceous-Buff (same color in juveniles and young), which color
extends distally on posterior sides of forelegs over forefeet and
on medial sides of hind legs to feet and sometimes onto upper
sides of toes. Least width of color of underparts averaging, in a
series of seventeen males from Brownsville, Texas, 47 (extremes
38-53) per cent of greatest width of color of upper parts. Black
tip of tail, in same series, averaging 49 (extremes 40-55) mm.
long, thus about equal to length of hind foot and averaging 24 per
cent of length of tail-vertebrae.
Skull and teeth.—Male (based on ten adults from Brownsville):
See measurements and plates 22-24; weight (three adults, one
topotype and two from Brownsville, Texas), 6.2 (5.3-7.2) grams;
basilar length, 49.8 (48.2-51.3); zygomatic breadth more than
distance between condylar foramen and M1 or than between anterior
palatine foramen and anterior margin of tympanic bulla; mastoidal
breadth less than postpalatal length; postorbital breadth less
than length of upper premolars (less than distance between
posterior borders of P4 and P2) and not greater (usually less)
than width of basioccipital measured from medial margin of one
foramen lacerum posterior to its opposite; interorbital breadth
not greater than distance between foramen opticum and anterior
margin of tympanic bulla; breadth of rostrum less than length of
tympanic bulla; least width of palate more or less than length of
P4; anterior margin of tympanic bulla as far posterior to foramen
ovale as width of 3 or 4 (including I3) upper incisors; height of
tympanic bulla more than distance from its anterior margin to
foramen ovale; length of tympanic bulla more than length of lower
molar and premolar tooth-row and longer or shorter (usually
longer) than rostrum; anterior margin of masseteric fossa just
behind m2.
Female (based on two adults from Brownsville, Texas): See
measurements and plates 36-38, 40; weight, 3.4 (3.3-3.5) grams;
basilar length (six, adult to young) 43.3 (41.3-47.3); zygomatic
breadth more or less than distance between condylar foramen and M1
and more than distance between anterior palatine foramen and
anterior margin of tympanic bulla; postorbital breadth less than
length of upper premolars and more or less than (about equal to)
width of basioccipital measured from medial margin of one foramen
lacerum posterior to its opposite; least width of palate less than
outside length of P4; tympanic bulla as far posterior to foramen
ovale as width of 2 to 3-1/2 (including I3) upper incisors; height
of tympanic bulla more than distance from its anterior margin to
foramen ovale; length of tympanic bulla more than length of lower
molar and premolar tooth-row and longer or shorter than rostrum.
The skull of the female averages 45 per cent lighter than that of
the average male.
Comparison of the skull with those of M. f. arthuri, tropicalis,
perotae, leucoparia and neomexicana has been made in accounts of
those subspecies. As compared with M. f. texensis (known only from
males), the only difference detected is smaller size.
Remarks.—As Merriam (1896:27) has said: "In 1813 a Russian
naturalist, Sevastianoff, gave the name 'Mustela brasiliensis' to a
weasel brought to St. Petersburg by Capt. A. J. Krusenstern on his
return from a voyage around the world. The animal was said to have come
from Brazil, but no definite locality was given." This name was long
applied by many European naturalists to American weasels which had
white facial markings, and several American naturalists adopted the
name. However, Lichtenstein in 1832 applied the name Mustela frenata
to the weasels of the vicinity of México City and that name was used
for bridled weasels from México and the southwestern United States by
most subsequent German writers and by several Americans. In 1896
Merriam (1896:27) showed that Sevastianoff's Mustela brasiliensis,
1813, although probably the same as Mustela frenata, was preoccupied
by Gmelin's Mustela brasiliensis, 1788, applied to an otter and that
Lichtenstein's name must be used as the next available one. Since that
time, 1896, frenata has been the name applied to the large
bridled-weasels of Texas and the high table land of México south to
México City. It may be added that in 1937 search by the writer among
the specimens and records at the Russian Academy of Sciences, in
Leningrad, failed to reveal any trace of the type specimen of
Sevastianoff's Mustela brasiliensis.
The geographic range of this subspecies is relatively large and, as
might therefore be expected, specimens show geographic variation. The
specimens from Tlalpam, which Merriam (op. cit.:27) regards as
topotypes, differ in certain respects from specimens from Texas. The
skull of the adult male "topotype," no. 50826, differs from any other
adult male seen in that the basilar length, the length of the upper
tooth-rows, the orbitonasal length, the ratio of the same to the
basilar length, the mastoidal breadth, the zygomatic breadth, the depth
of the skull at the posterior margins of the upper molars, and the
length and breadth of M1, are greater. The height of the tympanic
bullae is less than the average height for these structures in more
northern specimens. The specimens from Tlalpam have also larger
external measurements than the average of more northern specimens. All
of these features show an approach to the subspecies of more southern
distribution. On the other hand, the blackish of the head is not more
intense or more extended posteriorly onto the neck than in specimens
from Brownsville, Texas. The skin, with skull crushed, no. 767, in the
Paris Museum, from 3200 meters elevation near Toluca, does have the
black color of the head extended 30 millimeters posteriorly to the
ears. In this feature, and also in the extensively white face on which
the white bar in front of each ear connects with the frontal spot, as
well as with the color of the underparts, the specimen resembles
leucoparia. Better material from the western part of the state of
México may show the range of leucoparia to extend eastward almost or
quite to Toluca.
An adult male, taken on July 15 at Miquihuana, Tamaulipas, is unique in
several respects. The top of its head is black, rather than blackish,
and this color extends posteriorly on the top and sides of the neck
almost halfway to the shoulders. All of the upper parts are much more
darkly colored than in other specimens of this race. The least width of
the color of the underparts is 63 per cent of the greatest width of the
color of the upper parts; thus the color of the underparts is
considerably more extensive than in any other specimen seen. The
underparts are more intensely colored than in the average specimen. The
mastoidal breadth is greater than in any other adult male and amounts
to more than the postpalatal length. On available maps the elevation of
Miquihuana is given as 1892 meters (about 6200 feet). Thus the dark
colors can hardly be ascribed to more tropical conditions than those
under which animals from Brownsville, Texas, live. Brownsville is only
a few feet above sea level and only 235 miles farther north. The
difference noted, therefore, seems to be of geographic significance.
However, there is from Alvarez, San Luis Potosí, approximately 115
miles south of Miquihuana, a young (nearly subadult) female, no. 21968,
which is as light colored as specimens from Brownsville, Texas, or
Tlalpam, México. The only distinctive feature of this specimen is the
much greater extent of its white facial markings; they are more
extensive even than in the specimen from Miquihuana.
Finally, the series from Brownsville, Texas, indicates that the animal
there is smaller than frenata from the vicinity of México (city). The
skull is similarly proportioned except that relative to the basilar
length the orbitonasal length is more. Several other measurements of
the skull of the adult male from Tlalpam, as pointed out above, are
actually, although not relatively, greater than in any specimen from
Brownsville. The similarities between specimens from the two
localities, Tlalpam and Brownsville, are striking; since the two
localities lie at opposite, extreme ends of the range more geographic
variation would be expected. All that is known of the characters of
populations from intermediate localities is that the one specimen from
Alvarez shows no peculiarities whereas the one from Miquihuana suggests
the existence there of a geographic variant.
None of the specimens seen shows actual intergradation with M. f.
neomexicana or with M. f. arthuri but it is supposed that frenata
intergrades with each of these subspecies. The difference between
frenata and arthuri is greater than between frenata and
neomexicana. Bailey (1905:198) records tracks of a weasel seen just
below El Paso which he supposed had been made by a weasel of the
neomexicana type. He also cited the taking of a weasel at Langtry
which suggested to him (op. cit.) ". . . a continuous range from the
country of frenatus up the Rio Grande to the type locality of
neomexicanus at Mesilla Valley," New Mexico. Other records of
occurrence in Texas cited by Bailey, in addition to those provided by
specimens examined by the writer, are San Diego, Beeville, and Port
Lavaca. The Port Lavaca record is the easternmost one assigned to the
subspecies frenata; possibly specimens from there would be referable
to arthuri.
The series of thirty-four specimens from Brownsville, Texas, permits
measuring the amount of individual and age variation in several
features. For instance, the material is sufficient to show that
external measurements of subadults and those that fall in the upper
part of the category designated as "young" may be included with the
measurements of adults, because the mentioned measurements are not
appreciably greater in adults. The series of skulls, although not
providing more than six of any one age, shows the range of variation in
size and proportion of certain parts and enables the student the better
to evaluate cranial characters of nearby races known from only a few
specimens. For example, not one of the twenty skulls of males from
Brownsville and immediate vicinity is as large as either of the two
specimens of texensis from Kerr County.
The white facial markings vary much in size and shape. In the series of
thirty-four skins from Brownsville the broad white bands in front of
the ears are confluent with the white patch between the eyes on both
sides in three specimens and on one side only in six other specimens.
These bands are confluent with the color of the underparts in all but
two specimens. In one specimen the connection is lacking on both sides
and in the other on one side only. A white patch between the ears is
present in two specimens. The dark spot at each angle of the mouth is
absent on both sides in eleven specimens and absent on one side only in
ten others.
In six other specimens from parts of Texas north of Brownsville, the
broad white bands in front of the ears are confluent with the white
patch between the eyes on both sides in one specimen. A white spot
between the ears is present in one specimen. The dark spot at each
angle of the mouth is absent on both sides in six specimens and on one
side only in three other specimens.
In eleven specimens from México, the broad white bands in front of the
ears are confluent with the white spot between the eyes on both sides
in two specimens and on one side only in one other specimen. The white
spot between the ears is present in one specimen. The dark spot at each
angle of the mouth is absent on both sides, in six specimens, and on
one side only in one other specimen.
Thus, in 51 specimens the broad bands (one in front of each ear) are
confluent with the white patch between the eyes in nineteen out of 100
instances, and not with the color of the underparts in three instances.
A white spot between the ears is present in four specimens. The dark
spot at each angle of the mouth is present 47 out of a possible 98
times.
Four juvenal specimens from Brownsville, Texas, with their dates of
capture and probable age, are as follows: no. 58574, ♀, three weeks
old, taken on February 15; no. 17318/24239, ♂, four weeks old, taken
on March 16; no. 45899, ♀, forty days old, taken on May 21; no.
21778/36481, ♂, thirty days old, taken on October 20. In the order
given, the dates of birth of these four juveniles would be
approximately as follows: January 25, February 15, April 1, and
September 20. The dates of birth of other specimens less than three
months old as judged by the stage of development of the skull, and
reckoning backward from the dates of capture, are as follows: April 1,
April 30, May 25, October 12, and December 21. Thus, young appear to be
brought forth at Brownsville, Texas, in the fall, winter and spring,
that is to say from the latter part of September until the latter part
of May.
Mustela frenata frenata is either free of the parasites that infest
the frontal sinuses of most weasels, or withstands their presence
remarkably well, for only one skull shows a definite pathological
condition of the frontal sinuses.
Allen (1896:74) quotes H. P. Attwater, with respect to this species in
Bexar County, Texas, as follows: "Not common, but occasionally met
within the chaparral and cactus lands, where Wood Rats, Rabbits and
Quail abound. They were frequently met with around San Antonio during
the great 'Tramp Rat' [= Sigmodon hispidus texianus, see Bailey
(1905:116)] invasion of 1889-90."
Specimens examined.—Total number, 63, arranged by counties, and
in México by states, from north to south. Unless otherwise
indicated specimens are in the collection of the United States
National Museum.
Texas. Bexar County: San Antonio, 2 (1[2]). Goliad County:
Charco, 1. Nueces County: Corpus Christi, 1[2]. San Diego
County (not found), 1. Hidalgo County: La Hacienda, 1. Duval
County: San Diego, 2[7]. County in question: Lower Rio Grande,
1. Cameron County: Brownsville, 34 (3[2], 4[1], 3[93], 2[75],
1[59], 1[60], 1[4]); no locality more definite than county, 2.
Nuevo León. Río Ramis, 20 mi. NW Montemorelos, 1[90].
Tamaulipas. Matamoros, 6; Miquihuana (now in Nuevo León), 1[75].
San Luis Potosí. Alvarez, 1[75].
México: Region montagneuse des environs de Toluca, Nevada Toluca,
3200 M., 1[84]
Distrito Federal. City of México, 2 (1[4]); Tlalpam, 2. No
locality more definite than México, 4 (1[4], 3[7]).
Mustela frenata leucoparia (Merriam)
Long-tailed Weasel
Plates 1, 24, 25, 26, 29, 30, 36, 37 and 38
Putorius frenatus leucoparia Merriam, N. Amer. Fauna, 11:28, June
30, 1896.
Putorius brasiliensis frenatus, Allen, Bull. Amer. Mus. Nat.
Hist., 2:165, October 21, 1889.
Putorius frenatus frenatus, Allen, Bull. Amer. Mus. Nat. Hist.,
22:259, July 25, 1906.
Mustela frenata leucoparia, Miller, U. S. Nat. Mus. Bull.,
79:100, December 31, 1912; Hall, Carnegie Instit. Washington
Publ. 473:108, November 20, 1936.
Type.—Male, adult, skull and skin; no. 34914/47179, U. S. Nat.
Mus., Biol. Surv. Coll.; Pátzcuaro, Michoacán, México; July 27,
1892; obtained by E. W. Nelson; original no. 2960.
The skull (plates 29 and 30) lacks most of the braincase; a
fragment, consisting of the supraoccipital and the coalesced
frontals and parietals remains. The rostrum, left zygomatic arch,
palate, left pterygoid, left glenoid fossa and right postorbital
process are intact. The teeth all are present and entire. The
lower jaw lacks the right coronoid process and the lateral part of
the articular condyle. The skin is well made and in good
condition. It differs from an adult male topotype (36855, U. S.
Nat. Mus.) and other referred specimens in having: the black of
the head extended farther posteriorly on the neck, the maximum
amount of white on the head, and a white stripe 50 mm. long
extending down the middle of the nape from a point between the
ears more than half way to the shoulders.
Range.—Sonoran and Transition life-zones of mountains west of
México (city) in Michoacán and Nayarit. See figure 29 on page 221.
Characters for ready recognition.—Differs from M. f. goldmani
in least width of color of underparts more than 47 per cent of
greatest width of color of upper parts, hind feet colored like
underparts rather than like upper parts; postorbital constriction
less than, rather than more than, combined length of upper
premolars; from M. f. macrophonius by same details of coloration
as from goldmani and by ventrally concave rather than ventrally
convex pretympanic part of squamosal; from M. f. perotae by
least width of color of underparts more than 40 per cent of
greatest width of color up upper parts; height of tympanic bulla
more than three-fifths distance from its anterior margin to
foramen ovale; from M. f. frenata by white facial markings that
cover half of surface of head in front of ears, by extension of
black of head onto neck halfway to shoulders and by narrower (less
than 7.8) tympanic bullae; from M. f. neomexicana by Argus Brown
rather than Buckthorn Brown color of upper parts and distance from
anterior margin of tympanic bulla to foramen ovale more, rather
than less, than four-fifths of height of tympanic bulla.
Description.—Size.—Male: Two adults and one young from Los
Reyes and Pátzcuaro, Michoacán, yield average and extreme
measurements as follows: Total length, 514 (510-523); length of
tail, 206 (196-215); length of hind foot, 55 (52-58). Tail
averages 67 per cent as long as head and body. Length of hind foot
more than basal length.
Female: One adult from Artenkiki, Jalisco, and one subadult from
Pátzcuaro, Michoacán, measure, respectively, as follows: Total
length, 412, 400; length of tail, 159, 159; length of hind foot,
41, 42. Tail averages 64 per cent as long as head and body. Length
of hind foot equal to or greater than basal length.
The average differences in external measurements of the two sexes
are: Total length, 108; length of tail, 47; length of hind foot,
13.
Mustela frenata leucoparia has a greater total length and length
of tail than either M. f. frenata or goldmani. The hind foot
is longer than that of frenata and approximately the same as in
goldmani. Relative to the body length, the tail averages longer
than that of goldmani and shorter than that of frenata.
Externals.—As described in Mustela frenata frenata.
Color.—Broad white bands on sides of head, extending
anterodorsally anterior to each ear, confluent with white spot
between eyes and with color of underparts; posterior third of each
upper lip white; remainder of sides and top of head, and neck
posteriorly to point halfway to shoulders from ears, black; no
dark spots at angles of mouth; tip of tail black; remainder of
upper parts Argus Brown; chin white and sometimes also chest, neck
and medial sides of hind legs; remainder of underparts near (16´)
Ochraceous-Buff (near (a) Ochraceous-Buff in juvenal female),
which color extends distally over all of each foreleg (except its
lateral face proximally from about middle of forearm) and on
medial side of hind leg and over most of upper side of each foot.
Least width of color of underparts averaging, in eight specimens,
54 (extremes 44-61) per cent of greatest width of color of upper
parts; black tip of tail averaging, in four males, 52 (extremes
38-78) mm. long, thus averaging 25 per cent of length of
tail-vertebrae.
As compared with M. f. frenata and goldmani: white facial
markings more extensive; color of underparts less restricted and
more extended on legs; black tip of tail relatively of about same
extent as in frenata and thus much less than in goldmani;
black color of head extending farther posteriorly than in
frenata but not so far as in goldmani.
Skull and teeth.—Male (adult): See measurements and plates
24-26, 29, 30. As described in Mustela frenata frenata except
that: Weight (no. 128972) 6.3 grams; basilar length, 51.2;
interorbital breadth less than distance between foramen opticum
and anterior margin of tympanic bulla; anterior margin of tympanic
bulla as far posterior to foramen ovale as width of 4 or 5 upper
incisors; height of tympanic bulla more or less than (about equal
to) distance from its anterior margin to foramen ovale; anterior
margin of masseteric fossa anywhere from slightly anterior, to
slightly posterior, to m2.
Female (based on no. 26153): See measurements and plates 37-39. As
described in Mustela frenata frenata except that: Weight, 3.6
grams; basilar length, 44.5; zygomatic breadth less than distance
between condylar foramen and M1, or than between anterior palatine
foramen and anterior margin of tympanic bulla; tympanic bulla as
far posterior to foramen ovale as width of 4 or 5 upper incisors;
height of tympanic bulla not more than distance from its anterior
margin to foramen ovale; length of tympanic bulla more than length
of lower molar and premolar tooth-row or than length of rostrum.
The skull of the female is 43 per cent lighter than that of the
male.
Comparison of the skull with those of M. f. perotae, goldmani and
neomexicana has been made in the accounts of those subspecies. As
compared with that of frenata the main difference is the less
inflated tympanic bulla, the height of which is approximately equal to,
rather than decidedly more than, distance from its anterior margin to
foramen ovale.
Remarks.—The first specimen known to have been preserved is the
alcoholic in the British Museum of Natural History, taken in September,
1891, on the Río Santiago in Jalisco, by D. A. C. Buller. The other
known specimens of this white-faced weasel are divided between the
American Museum and the United States National Museum. The two referred
specimens from Jalisco were the last of several helpful ones collected
in México and Central America by J. H. Batty, and these two were taken
less than three months before Batty's tragic death in Chiapas (see
Allen, J. A., 1906:191). The five specimens from Michoacán were taken
by Nelson or Nelson and Goldman together. Merriam had only three of
these when he named the subspecies and remarked (1896:29) that "This
form is the poorest subspecies described in the present paper."
Although the form is not strongly marked, the two additional specimens
from Michoacán and better comparative material than Merriam had confirm
several of the differential characters ascribed to it by him and
indicate the existence of still other characters.
M. f. leucoparia occurs in the Sonoran and Transition life-zones. No.
27258 from Los Masos, and no. 26153 from Artenkiki (see specimens
examined for other spellings) approach true frenata in coloration.
Each of these specimens has a few white hairs between the ears and the
white patch between the eyes is confluent on one side only with the
lateral white bands on the side of the head. No. 27258 from Los Masos
has a dark spot at each angle of the mouth. The 7 other specimens are
relatively uniform in coloration. Each has the white spot between the
eyes confluent on both sides with the extensive white areas on each
side of the face. None has a dark spot at either angle of the mouth. Of
these 7 specimens, the type specimen and three others have white hairs
forming a median line between the ears and a fifth specimen has a white
spot behind each ear.
M. f. leucoparia is most like M. f. frenata. Unlike frenata,
leucoparia has tympanic bullae that are less inflated, narrower and
less projected, at their anterior margins, from the cranium. In these
characters leucoparia is intermediate between M. f. frenata and M.
f. goldmani. The latter subspecies has the least inflated, narrowest
and least projecting tympanic bullae of the three. The black color of
the head extends, on the average, farther posteriorly than in M. f.
frenata but not so far as in M. f. goldmani. The general color, too,
is intermediate between that of M. f. frenata and that of the much
darker M. f. goldmani. The white facial markings are more extensive
than in either M. f. frenata or M. f. goldmani. This applies to
both the white area between the eyes and the one on each side of the
head between the ear and eye. M. f. neomexicana, whose range possibly
meets that of M. f. leucoparia, also has more extensive white facial
markings than M. f. frenata but less extensive markings than M. f.
leucoparia.
On the basis of skulls alone, specimens of frenata from Tlalpam and
those of leucoparia from Los Reyes can hardly be distinguished. This
fact, and the circumstance that the specimens from the northern part of
the range of leucoparia closely resemble frenata in color,
constitute sufficient evidence for regarding the two as only
subspecifically distinct. The female, no. 26153 from Artenkiki, as
mentioned above, approaches true frenata in coloration. On this
account it is not to be regarded as typical and it was because no other
skulls of adult females were available that this one was used for
comparison with females of allied races.
M. f. leucoparia is, then, a subspecies of the large, temperate-zone
group and is unique in possessing the maximum extent of white facial
markings.
None of the seven skulls shows signs of having had the frontal sinuses
infested with parasites.
Specimens examined.—Total number, 8, all from México.
Localities are listed by states from north to south. Specimens
from Michoacán are in the United States National Museum; one from
Río Santiago is in the British Museum of Natural History; all
others are in the American Museum of Natural History.
Nayarit. Tepic, 1.
Jalisco. Río Santiago, 1; Los Masos, 1; "Artenkiki" (J. A. Allen,
1906, p. 238, writes "Artenkikil" and, on p. 259, "Artenkiki."),
1.
Michoacán. Zamora, 1; Los Reyes, 1; Pátzcuaro, 3.
Mustela frenata perotae Hall
Long-tailed Weasel
Plates 36, 37 and 38
Mustela frenata perotae Hall, Carnegie Instit. Washington Publ.
473:100, November 20, 1936.
Putorius frenatus, Merriam, N. Amer. Fauna, 11: pl. 3, fig. 2,
June 30, 1896.
Type.—Female, adult, skull and skin; no. 54278, U. S. Nat.
Mus., Biol. Surv. Coll.; 12,500 feet, Cofre de Perote, Veracruz,
México; May 26, 1893; obtained by E. W. Nelson; original no. 4864.
The skull (plates, 37-39) lacks the right zygomatic arch. Left p2
is missing. The skin is fairly well made and in good condition
except that the extreme tip of the tail has been broken off and
there are two holes in the right hind leg. The underparts show the
beginning of a spring molt.
Range.—From 7500 (?) feet (Perote) to 13,500 feet
(Popocatépetl), Upper Sonoran, Transition and Boreal life-zones of
mountains along Puebla-México boundary, eastward to western
central Veracruz and south into Oaxaca. See figure 29 on page 221.
Characters for ready recognition.—Differs from M. f. frenata,
its nearest relative, in extension from head of blackish onto
anterior fourth of neck; restriction of color of underparts (least
width of same less than 37 per cent of greatest width of color of
upper parts), height of tympanic bulla less than distance from its
anterior margin to foramen ovale; from M. f. macrophonius and
M. f. goldmani in presence of, rather than absence of, color of
underparts on hind feet; upper parts (black) Brussels Brown rather
than Argus Brown or darker; from M. f. tropicalis in larger size
(adult female with total length more than 400, basilar length more
than 40, weight of skull more than 3 grams); postorbital breadth
less than combined length of upper premolars; m1 more than 5.4
long; from M. f. leucoparia in white facial markings so
restricted that spot between eyes is not confluent with white
stripe in front of ear, or, if so, narrowly (less than 4 wide)
confluent; color of upper parts extending onto antipalmar face of
forefoot, least width of color of underparts not more than 40 per
cent of greatest width of color of upper parts; height of tympanic
bulla not more than three-fifths distance from its anterior margin
to foramen ovale.
Description.—Size.—Male: A nontypical specimen from Cerro
San Felipe, Oaxaca, measures: Total length, 500; length of tail,
205; length of hind foot, 52.
Female: The type specimen, measures: Total length, 418; length of
tail, 160; length of hind foot, 45.
In this male the tail is 70, and in the female, 62 per cent as
long as the head and body. In each the hind foot is longer than
the basal length.
The differences in external measurements between these two
specimens, representing the two sexes, are: Total length, 82;
length of tail, 45; length of hind foot, 7.
Externals.—As described in Mustela frenata frenata.
Color (based on type specimen).—Color and color pattern as
described in Mustela frenata frenata except that: blackish of
sides and top of head extends one-fourth of way back to shoulders
from ears; throat and breast as well as chin white; remainder of
underparts near (16´ c) Ochraceous-Buff; least width of color of
underparts equals 36 per cent of greatest width of color of upper
parts; black tip of tail equal to 28 per cent of length of
tail-vertebrae.
Skull and teeth.—Male (based on a referred specimen from Cerro
San Felipe which certainly is nontypical): See measurements. As
described in Mustela frenata frenata except that: Weight, 4.9
grams; basilar length, 49.2; postorbital breadth more than
distance between posterior borders of P4 and P2; tympanic bulla as
far posterior to foramen ovale as width of 5 upper incisors;
height of tympanic bulla less than distance from its anterior
margin to foramen ovale; zygomatic breadth less than distance
between condylar foramen and M1 or than between anterior palatine
foramen and anterior margin of tympanic bulla.
Female (based on type specimen, an adult): See measurements and
plates 37-39. As described in Mustela frenata frenata except
that: Weight 3.4 grams; basilar length, 43.5; zygomatic breadth
less than distance between condylar foramen and M1 or than between
anterior palatine foramen and anterior margin of tympanic bulla;
postorbital breadth less than width of basioccipital measured from
medial margin of one foramen lacerum posterior to its opposite;
tympanic bulla as far posterior to foramen ovale as width of 5 or
6 upper incisors; height of tympanic bulla one-half to
three-fifths distance from its anterior margin to foramen ovale;
length of tympanic bulla more than length of lower molar and
premolar tooth-row and longer than rostrum.
The skull of the female is 33 per cent lighter than that of the
nontypical (and smaller than average) male from Cerro San Felipe.
Comparison of the skull with that of M. f. tropicalis is made in the
account of that subspecies. Compared with the skull of M. f.
macrophonius, that of the female of perotae is more flattened, has
the longitudinal dorsal outline distinctly concave rather than flat
just behind the postorbital processes, and much wider tympanic bullae.
Accordingly, the basioccipital is slightly narrower in perotae. The
more marked postorbital constriction of the type specimen of perotae
possibly is due to its relatively greater age. As compared with the
skull of M. f. leucoparia, that of the female of perotae has less
inflated tympanic bullae, the height of each being half as great as
distance from its anterior margin to foramen ovale, whereas, in
leucoparia (as represented by no. 26153) the two distances are equal.
As compared with that of M. f. frenata, the skull of the female of
perotae differs mainly in the lesser inflation of the tympanic bullae
and their relative position. The height of each bulla is in perotae
only half as much as, but in frenata more than, the distance from its
anterior margin to foramen ovale. The anterior margin of the bulla is
much less projected from the floor of the braincase in perotae. The
squamosal anterior to each bulla is convex ventrally in perotae but
flat or concave ventrally in frenata.
Remarks.—The type specimen and a juvenal female from the town of
Perote were taken in the spring of 1893 by E. W. Nelson. Of these two,
the type specimen was mentioned and figured by Merriam (1898:30, fig.
16 [= fig. 15], pl. 3, fig. 2) as Putorius frenatus. The referred
nontypical specimen from Cerro San Felipe, Oaxaca, was referred by
Merriam (op. cit.:29) to Putorius frenatus goldmani with the comment
that it was intermediate ". . . both in coloration and cranial
characters, between typical frenatus and goldmani;. . . ." No other
published references to this subspecies, or specimens of it, have been
seen. In 1941 and 1942, W. B. Davis and associates took four specimens
along the boundary between the states of Puebla and México.
Although the specimen from Cerro San Felipe, Oaxaca, is referred to
Mustela frenata perotae, to the description of which it answers best,
that specimen, on account of its structural characters and geographic
position relative to adjacent races, is in reality an intergrade
between several of the adjacent races. Some of its intermediate
characters are pointed out in the discussion of M. f. goldmani. In
the specimens from 45 and 55 kilometers ESE of México (city) the black
color of the top of the head does not extend so far behind the ears as
in the holotype of M. f. perotae and in this feature the two
specimens show intergradation between the two subspecies, perotae and
frenata.
The type specimen taken on May 26, is acquiring new hair on the belly
and lower sides which appears to be the result of a normal molt.
As would be expected from its geographic position, M. f. perotae
resembles M. f. frenata of northern México and the high mountain
forms of southern México more than it does the lowland tropical forms.
This is true as regards size of entire animal, proportions of its
parts, and size, general angularity and major proportions of its skull.
The marked postorbital constriction, convex supralacrymal face of
rostrum, width of tympanic bullae and angularity of the braincase place
it nearest M. f. frenata as does also the color and color pattern.
The ventrally convex squamosal anterior to each tympanic bulla and the
slight degree of projection from the cranium of the anterior margin of
each tympanic bulla are intermediate in degree between the condition in
M. f. macrophonius and that in M. f. frenata. Thus M. f. perotae
combines several characters of M. f. frenata on the one hand with
some of M. f. macrophonius on the other and in some features, for
instance in the size, shape and degree of inflation of the tympanic
bullae, presents intermediate stages of development.
On the eastern plain below the high mountain, Cofre de Perote, there
ranges the similarly colored, smaller, tropical weasel, Mustela
frenata tropicalis. Between M. f. perotae and M. f. tropicalis
there is marked differentiation in the skulls with much less
differentiation in coloration. The differences in typical skulls of the
two subspecies are so pronounced that one would, at first glance,
hardly believe it possible for direct intergradation to occur between
them on the sides of this mountain. Merriam (1896:30) thought that it
did not. The two skulls figured by him (op. cit.:31) are a topotype
of M. f. tropicalis from Jico and the one which now is the type
specimen of M. f. perotae. They show the great difference in size and
proportions and are females of comparable ages, not of different ages
as I suspected before examining the skulls. However, despite this
marked difference in the skulls, there is some, although not
conclusive, evidence of intergradation furnished by a young female from
Xuchil, Veracruz. This specimen is described in connection with M. f.
tropicalis (see page 366).
None of the seven skulls shows marked deformity of the interorbital
region, but two of the three adults appear to have had these parts
infested with nematodes.
Specimens examined.—Total number, 7, all from México, listed by
localities from north to south. Specimens from Veracruz and Oaxaca
in the United States National Museum; remainder in Texas
Cooperative Research Collection.
México: Monte Río Frío, 45 Km. ESE México City, 1; 55 Km. ESE
México City, 1; N slope Mt. Popocatépetl, 13,555 ft., 1.
Puebla. Río Otlati, 8700 ft., 1.
Veracruz. Cofre de Perote, 12,500 ft., 1; Perote, 1.
Oaxaca. Cerro San Felipe, 10,000 ft., 1.
Mustela frenata goldmani (Merriam)
Long-tailed Weasel
Plates 1, 24, 25, 26 and 30
Putorius frenatus goldmani Merriam, N. Amer. Fauna, 11:28, June
30, 1896; Elliot, Proc. Biol. Soc. Washington, 18:236, December
9, 1905.
Mustela frenata goldmani, Miller, U. S. Nat. Mus. Bull., 79:100,
December 31, 1912; Hall, Carnegie Instit. Washington Publ.
473:109, November 20, 1936.
Type.—Male, adult, skull and skin; no. 77519, U. S. Nat. Mus.,
Biol. Surv. Coll.; Pinabete, Chiapas, México; February 10, 1896;
obtained by E. A. Goldman (on attached label collectors recorded
as Nelson and Goldman); original no. 9279.
The skull (plates 24 and 30) has the rostrum badly injured. All
the right, and part of the left nasal, the upper part of the right
maxilla, the postorbital process and intervening area of frontals
are missing. Each zygomatic arch is broken but the parts are
present and attached to the skull. The frontal and interorbital
regions are greatly malformed owing to parasites that infested the
sinuses. Right I2 and I3, right and left i3, and the medial parts
of the paraconid and protoconid of right m1 are missing. The light
facial markings are less extensive than in any of the referred
specimens. These markings consist of a separate spot between the
eyes and a white line, confluent with the color of the underparts,
on each side of the head, that extends from the base of the ear to
above the eye. The dark color of the underparts is represented at
the angles of the mouth by a spot on the left side and a similar
dark area, confluent with the dark color of the face, on the right
side. The large size, characters of the skull, and scrotal pouch
on the skin prove the specimen to be a male as stated on the
label.
Range.—Two thousand five hundred feet (El Cipres, Guatemala) to
9500 feet (near Tecpám, Guatemala), Upper Tropical Life-zone of
mountains and western coasts of southern México, Guatemala and
Salvador. See figure 29 on page 221.
Characters for ready recognition (characters based on
males).—Differs from M. f. nicaraguae and M. f. perda by
larger size (total length of adult males more than 489), least
width of color of underparts not less than 26 per cent of greatest
width of color of upper parts, weight of skull of adult male more
than 5 grams; from M. f. macrophonius by smaller size (total
length of adult males less than 540), skull of male with basilar
length less than 52.5 and weight less than 6 grams; from M. f.
perotae (typical specimens of same sex not available) by darker
color of upper parts which are Argus Brown or darker rather than
Brussels Brown; nonextension of color of underparts onto hind
feet; from M. f. leucoparia in least width of color of
underparts not more than 37 per cent of greatest width of color of
upper parts; color of underparts not extended onto hind feet;
black tip of tail two-fifths rather than one-fourth as long as
tail-vertebrae; height of tympanic bulla less than four-fifths
distance from its anterior margin to foramen ovale.
Description.—Size.—Male: Four adults yield average and
extreme measurements as follows: Total length, 508 (500-512);
length of tail, 196 (185-207); length of hind foot, 55.5 (54-58).
Tail averages 63 (59-67) per cent as long as head and body. Length
of hind foot more than basal length.
Female: Typical specimen unknown.
Externals.—Longest facial vibrissae black and reaching beyond
ear; carpal vibrissae wholly or in part of same color as upper
parts and reaching as far as hypothenar pad; hairiness of
foot-soles distinctly less than that shown in figure 20 on page
60.
Color.—Spot between eyes, band, confluent with color of
underparts, on each side of head extending anterodorsally anterior
to each ear and posterior third of each upper lip, white;
remainder of sides and top of head and neck posteriorly to or
slightly behind shoulders, black; dark spots at angles of mouth
usually absent; tip of tail black; remainder of upper parts Argus
Brown or near (n) Argus Brown; chin, throat and breast white;
remainder of underparts near (16' c) Ochraceous-Buff; color of
underparts extending distally on posterior sides of forelegs onto
medial toes and on hind legs to points between knees and heels.
Least width of color of underparts, in five adult males, averaging
28 (extremes 26-33) per cent of greatest width of color of upper
parts; black tip of tail, in four adult males, averaging 40 per
cent of length of tail-vertebrae.
Skull and teeth.—Male (based on five adults): See measurements
and plates 24-26, 30; weight, 5.4 (5.3-5.5) grams; basilar length,
49.9 (49.6-51.3); zygomatic breadth (except in no. 12523 from
Salvador) more than or equal to distance between condylar foramen
and M1 or between anterior palatine foramen and anterior margin of
tympanic bulla. Mastoid breadth less than postpalatal length;
postorbital breadth more or less than length of upper premolars
and greater than width of basioccipital measured from median
margin of one foramen lacerum posterior to its opposite;
interorbital breadth less than distance between foramen opticum
and anterior margin of tympanic bulla; breadth of rostrum less
than length of tympanic bulla; least width of palate more or less
than length of P4; anterior margin of tympanic bulla as far
posterior to foramen ovale as width of five upper incisors; height
of tympanic bulla less than distance from its anterior margin to
foramen ovale; length of tympanic bulla more than length of lower
molar and premolar tooth-row and shorter than or equal to length
of rostrum; anterior margin of masseteric fossa immediately behind
m2.
Female: Typical skull unknown.
Comparison of male skull with that of M. f. perda made in discussion
of that form. Comparison with that of M. f. nicaraguae shows similar
differences, some of which are more pronounced. For example, squamosals
anterior to tympanic bullae more convex ventrally and these bullae
project less from braincase than in M. f. perda; thus the difference
in these features is greater between goldmani and nicaraguae than
between goldmani and perda.
As compared with the skull of the male of M. f. macrophonius, each
one of the skulls of the adult males of M. f. goldmani is smaller in
every measurement taken, with two exceptions. The width of the tympanic
bullae was more in three specimens of M. f. goldmani as was also the
depth of the same in three specimens. Relative to the basilar length
all but two of these measurements average less in goldmani; the
exceptions are the zygomatic breadth and depth of the skull at the
anterior margin of the tympanic bullae which average more. Relative to
the basilar length, the orbitonasal length and depth of the skull at
the posterior margin of M1 are less in each skull of goldmani. Thus,
excepting the width and height of the tympanic bullae and the relative
zygomatic breadth and relative depth of the braincase posteriorly, the
skull of goldmani is shorter and relatively as well as actually
narrower and lighter throughout.
As compared with the skull of the male of M. f. leucoparia, that of
M. f. goldmani averages a trifle shorter and no skull of goldmani
equals that of leucoparia in actual or relative zygomatic and mastoid
breadths or length or height of tympanic bullae. In depth, the skull of
goldmani averages actually and relatively greater. Its teeth are
smaller. The squamosal anterior to each tympanic bulla is convex
ventrally whereas it is concave ventrally in leucoparia as in
frenata.
Remarks.—When Merriam (1896:28) named this subspecies, he had only
one specimen but he called attention to the more important diagnostic
characters, which additional specimens show pertain to the race as a
whole.
M. f. goldmani in typical form occurs in high mountains of the Upper
Tropical Life-zone and is most closely related to M. f. frenata and
M. f. macrophonius. The altitude at which the two specimens were
taken, twenty miles southeast of Teopisca in Chiapas, is not known.
Merriam (1896:28) states that the type specimen was obtained at "about
8200 feet." The specimen taken by Stirton in Salvador comes from 8000
feet and the one obtained by Barber in Guatemala from 9500 feet. The
specimen from Dueñas, the skin alone of a young animal, is not
instructive.
As regards size, goldmani is larger than the immediately adjacent
subspecies from the Lower Tropical Life-zone but is smaller than M. f.
leucoparia or macrophonius. As compared with M. f. frenata,
goldmani is longer, has an actually as well as relatively shorter
tail, and a much longer hind foot.
The most outstanding difference in externals from frenata is the
naked foot soles.
Molting probably takes place twice each year although actual proof of
this is lacking. In number 133254 from twenty miles southeast of
Teopisca, taken on May 12, the molt is well advanced. Another specimen
from the same place still retains the winter coat.
In color, goldmani is much darker than frenata, has less extensive
white facial markings, longer black tip on tail, more restricted color
of underparts, and lacks the extension of color of the underparts onto
the hind feet.
Of the adult males from the high mountains, the type specimen from
Chiapas is lightest, and the one from Salvador is darkest. This
progressively darker color to the southward probably is geographic
variation.
In total length and relative and actual length of tail, the specimen
from Salvador is the smallest of the five adult males from the higher
mountains. In addition to its darker color and smaller size, no. 12523
from Salvador shows certain distinctive cranial characters. The
zygomatic breadth is less than, rather than more than, or equal to, the
distance between the condylar foramen and M1 or than that between the
anterior palatine foramen and the anterior margin of the tympanic
bulla. This difference appears to be correlated with geographic
position, since no. 15953 from Guatemala has the three distances about
equal and therefore is intermediate in this respect between the
specimen from Salvador and those from Chiapas, in which the zygomatic
breadth is greater than the other two measurements. Also in the greater
depth of the skull and smaller size of the teeth, this specimen from
Salvador approaches the subspecies of the Lower Tropical Life-zone. It
has, however, the longest, highest and widest tympanic bullae of any of
the five specimens. The amount of ventral convexity of the squamosal in
front of each tympanic bulla appears not to be greater than in the
other specimens.
As indicative of intergradation with perotae, leucoparia and
possibly frenata, there is the specimen from Cerro San Felipe,
Oaxaca. The degree of restriction of the color of the underparts is
intermediate between that of goldmani and leucoparia. The same is
true as regards the amount of projection from the braincase of the
anterior margins of the tympanic bullae. The squamosal immediately
anterior to each tympanic bulla is flat instead of ventrally convex as
in goldmani or ventrally concave as in leucoparia and frenata. In
accordance with the custom adopted in this paper of referring every
specimen to some one subspecies, this specimen from Cerro San Felipe is
referred to Mustela frenata perotae, to the description of which it
most nearly answers.
Possibly goldmani, as here constituted, is a composite form. The
specimens from the high mountains closely resemble one another.
However, a specimen, no. 68541 from "Finca El Cipres," Guatemala, which
place Mr. G. Goodwin tells me is at an elevation of 2500 feet,
approximately 5 miles north of Retalhuleu, has a basilar length of 47.3
and is correspondingly small in other parts. This suggests the
existence of a small, lowland race on the western side of the central
divide corresponding to perda and tropicalis on the eastern side.
From only a few miles away, at San Sebastian, there is available, the
adult skull of a still smaller animal. This skull only, no. 41026, in
the Berlin Zoological Museum, has a basilar length of 46.1, zygomatic
breadth of 27.4, and other cranial measurements notably smaller than
those of specimens from the high mountains. A skin-only, no. 12038,
collection of Donald R. Dickey, from La Cebia, altitude 2150 feet, near
the city of San Salvador, seemingly represents an animal smaller than
typical goldmani. This specimen from La Cebia has the light color of
the underparts extended distally on the hind legs to the tips of the
toes as in M. f. tropicalis. However, the upper parts are darker and
resemble those of M. f. goldmani. A fourth specimen from only 3500
feet elevation, on the south side of Volcano Tajumulco, Guatemala, no.
41768, Field Museum of Natural History, a subadult male, measures only
490 in total length and has the least color of the underparts so
restricted as to amount to only 22 per cent of the greatest width of
the color of the upper parts. Both these features are suggestive of the
lowland races.
These four specimens indicate that the lowland population on the
western side of the divide is smaller than the mountain population. The
juvenile from Carolina and a young male from Finca Cipres, however,
both closely resemble individuals of goldmani from the higher
mountains. All these animals here are referred to goldmani. More
specimens may reveal an amount and a pattern of geographic variation in
weasels of this region that will require application of another
subspecific name.
The female, no. 68540, from Puebla agrees remarkably well with the
skull of the female, no. 132528, of macrophonius. Differences
displayed by the specimen from Puebla are its slightly narrower
braincase and longer space between the foramen ovale and anterior end
of the tympanic bulla. Considering the far eastern location of Puebla
(just north of Río Motagua, at 89° W, according to a sketch map
provided by Mr. G. G. Goodwin), this specimen might be expected to show
some approach to the small lowland races. Actually, however, it
displays the characters of goldmani better than does the subadult
female from Volcano San Lucas, which is nearer the metropolis of
goldmani, and I assume at a higher elevation than Puebla.
Concerning this weasel Merriam (1896:29) says: "Mr. E. W. Nelson writes
me that this fine weasel is found sparingly in the forest about
Pinabete, Chiapas, at an altitude of 7000 to 8000 feet (2100 to 2500
meters). The type specimen was shot in the afternoon while hunting on a
heavily wooded hill slope. It was heard making long, slow leaps over
the dry, crisp leaves. Coming to a log, it stood up and rested its fore
feet on the log, in which position it was shot by Mr. Goldman."
The specimen taken by R. A. Stirton in Salvador comes from an elevation
of 8000 feet in the rain forest of the Upper Tropical Life-zone. Mr.
Stirton tells me that one morning on visiting his traps set for small
rodents, he found in one the partly eaten remains of a Heteromys.
Leaving these remains as found he placed a steel trap beside them and
on the following morning found the male weasel in the trap.
At least three of the ten specimens had the frontal sinuses infested
with parasites.
Specimens examined.—Total number, 15, listed by localities from
north to south, and unless otherwise indicated in the American
Museum of Natural History.
México: Chiapas: 20 mi. SE Teopisca, 2[91]; Pinabete, 1[91].
Guatemala: Puebla, 1; Finca Porvenir, 3500 ft., S slope Volcan
Tajumulco, 1[60]; Sierra [= ? Cerro] Santa Elena, 9500 ft. (near
Tecpám), 1[60]; Carolina, 1; Volcano San Lucas, 1; "Finca El
Cipres," 1; "Finca Cipres," 2500 ft., 1; Finca San Isidro, San
Sebastión, Dept. Retalhuleu, 1[4]; Dueñas, 1[7]; no locality more
definite than Guatemala, 1[7].
El Salvador: Los Esesmiles, 8000 ft., Chalatenango, 1[59]; La
Cebia, 2150 ft., near San Salvador, 1[59].
Mustela frenata macrophonius (Elliot)
Long-tailed Weasel
Plates 24, 25, 26, 30, 37, 38 and 39
Putorius macrophonius Elliot, Proc. Biol. Soc. Washington,
18:235, December 9, 1905.
Mustela macrophonius, Miller, U. S. Nat. Mus. Bull., 79:100,
December 31, 1912.
Mustela frenata macrophonius, Hall, Carnegie Instit. Washington
Publ. 473:109, November 20, 1936.
Type.—Male, adult, skull and skin; no. 14063, Field Mus. Nat.
Hist.; Achotal, Veracruz, México; January 15, 1904; obtained by
Edmund Heller and Charles M. Barber; original no. 3424.
The skull (plates 24-26, 30) is complete and unbroken. Excepting
right P2, which has been aborted or broken away, all the teeth are
present. The skin is well made and in good condition. As shown by
the scrotal pouch, the specimen is a male.
Range.—Tropical Life-zone, probably into Boreal life-zones, of
mountains along eastern border of southern Veracruz. See figure 29
on page 221.
Characters for ready recognition.—Differs from M. frenata
frenata and M. f. perotae and M. f. leucoparia in lacking
color of underparts on hind feet and in larger skull (skulls of
adult males with basilar length more than 52.5); from M. f.
goldmani by larger size of skull (see above) and entire animal
and wider tympanic bullae; from M. f. tropicalis and M. f.
perda by larger size (total length of adult males more than 510),
postorbital breadth amounting to less than combined length of
upper premolars.
Description.—Size.—Male: External measurements of the type
specimen, an adult, are: Total length, 598; length of tail, 246;
length of hind foot, 59. Tail 70 per cent as long as body; length
of hind foot more than basal length.
Female: The skin, without field collector's measurements, of an
adult female from Pérez, Veracruz, shows this sex to be
correspondingly large. Because the skin is understuffed and
because the hind feet are skinned out, reliable measurements can
not be obtained from the dried skin.
Externals.—As described in Mustela frenata goldmani except
that all carpal vibrissae are of same color as upper parts and
that hairiness of foot-soles is halfway between that shown in
figures 20 and 21.
Color.—As in darkest individuals of M. f. goldmani, thus,
color of upper parts on posterior part of back near (n) Argus
Brown. Color of underparts near (12) Mikado Orange in a juvenile,
extending distally on posterior sides of forelegs onto inner toes
and on hind legs to points between knees and heels. Least width of
color of underparts 28 per cent of greatest width of color of
upper parts. Black tip of tail 34 per cent of length of
tail-vertebrae.
Skull and teeth.—Male (based on type specimen): See
measurements and plates 24-26, 30. As described in Mustela
frenata frenata except that: Weight, 6.9 grams; basilar length,
54.1; zygomatic breadth less than distance between condylar
foramen and M1 or that between anterior palatine foramen and
anterior margin of tympanic bulla; interorbital breadth less than
distance between foramen opticum and anterior margin of tympanic
bulla; anterior margin of tympanic bulla as far posterior to
foramen ovale as width of 4 to 6 upper incisors; height of
tympanic bulla less than distance from its anterior margin to
foramen ovale; anterior margin of masseteric fossa below posterior
half of m2.
Female (based on no. 132528): See measurements and plates 37-39.
As described in Mustela frenata frenata except that: Weight, 3.6
grams; basilar length, 43.5; zygomatic breadth less than distance
between condylar foramen and M1 and more or less than (in the
single specimen, equal to) that between anterior palatine foramen
and anterior margin of tympanic bulla; least width of palate more
or less than (about equal to) outside length of P4; tympanic bulla
as far posterior to foramen ovale as width of 4 or 5 upper
incisors; height of tympanic bulla less than distance from its
anterior margin to foramen ovale.
The skull of the female is 48 per cent lighter than that of the
male.
Comparison of the skull with that of M. f. goldmani is made in the
account of that subspecies. Similar differences probably exist between
males of perotae and macrophonius. As compared with skulls of males
of M. f. tropicalis and perda, the skull of the male of
macrophonius is larger in every measurement taken. The postorbital
constriction is less, rather than more, than the combined length of the
upper premolars. Relative to the basilar length, the following
measurements are less than in any specimen of tropicalis or perda:
length of tooth-rows; orbitonasal length; depth of skull at posterior
border of upper molars; and depth of skull at anterior margin of
basioccipital.
Remarks.—This large weasel appears to have escaped the notice of
naturalists until the spring of 1903 when J. Friesser obtained an adult
female and juvenal male at Pérez for the collection of the United
States Bureau of Biological Survey. These specimens were tentatively
referred to Mustela tropicalis. In the following January, Edmund
Heller and Charles M. Barber obtained the adult male that was made the
type specimen by Elliot who did not see, or if he did, did not mention,
the specimens from Pérez. He did, however, refer a young female from
Xuchil, Veracruz, to his Putorius macrophonius. This young female is
here referred to Mustela frenata tropicalis.
The extent of the geographic range of this subspecies is not well
known.
Mustela frenata macrophonius and M. f. texensis are the largest
American weasels. The basilar length in the type specimen is greater by
one-tenth of a millimeter than in the type specimen of M. f.
texensis. The other cranial measurements taken are greater in M. f.
texensis. The skull of the female from Pérez is one of the largest
skulls examined of that sex. The juvenal male has teeth as large as
those of the type specimen and the skull is the largest for its age of
any seen. Although the skin of the female is understuffed and hence
does not provide reliable measurements, it shows that the female is
also large.
The white bands in front of the ears are confluent with the white patch
between the eyes on one side only in one specimen. It is the juvenal
male. These bands are not confluent with the color of the underparts
on either side in the female and on one side only in the adult male.
None of the specimens has a white patch between the ears. The dark spot
at each angle of the mouth is present only in the juvenile where it
occurs on each side. Of the three specimens, the juvenile is the
darkest and the adult male the lightest. The white facial markings are
most extensive in the juvenal male and the least extensive in the adult
female.
M. f. macrophonius most closely resembles M. f. goldmani but in the
relatively flattened braincase, deep constriction of the postorbital
region and general angularity of the skull approaches M. f. perotae
and M. f. frenata.
Only one of the three skulls, that of the female, shows evidence of
infestation of the frontal sinuses by parasites, and this did not
result in malformation of the interorbital region.
Specimens examined.—Total number, 3, all from México, listed by
localities from north to south.
Veracruz. Achotal, 1 (Field Mus. Nat. Hist.); Pérez, 2 (U. S. Nat.
Mus.).
Mustela frenata tropicalis (Merriam)
Long-tailed Weasel
Plates 25, 26, 27, 30, 37, 38 and 39
Putorius tropicalis Merriam, N. Amer. Fauna, 11:30, pl. 3, figs.
5, 5a, 6, 6a, text fig. 16, June 30, 1896; Merriam, Proc. Biol.
Soc. Washington, 15:68, March 22, 1902.
Putorius frenatus, Merriam, N. Amer. Fauna, 11:27, June 30, 1896.
Mustela tropicalis tropicalis, Miller, U. S. Nat. Mus. Bull.,
79:100, December 31, 1912; Allen, Bull. Amer. Mus. Nat. Hist.,
35:99, April 28, 1916.
Mustela frenata tropicalis, Hall, Carnegie Instit. Washington
Publ. 473:109, November 20, 1936.
Type.—Male, adult, skull and skin; no. 54994, U. S. Nat. Mus.,
Biol. Surv. Coll.; Jico, Veracruz, México; July 9, 1893; obtained
by E. W. Nelson; original no. 5195.
The skull (plates 25-27, 30) is complete. All the upper incisors,
except the second and third on the left side, are missing. The
right upper canine is broken. The skin is well made and in good
condition.
Range.—Up to 5000 feet (as now known) in Tropical Life-zone of
Veracruz, México. See figure 29 on page 221.
Characters for ready recognition.—Differs from M. f. frenata
and M. f. perotae in least width of color of underparts not
exceeding 36 per cent of greatest width of color of upper parts
and in postorbital breadth exceeding length of upper molar and
premolar tooth-rows; from M. f. macrophonius and M. f. perda
in least width of color of underparts averaging more than 29 per
cent of greatest width of color of upper parts; and from M. f.
perda by longer tympanic bullae which in males are more than
14.9; and from M. f. macrophonius by lesser basilar length (not
more than 48) and in postorbital breadth exceeding length of upper
molar and premolar tooth-row.
Description.—Size.—Male: The type specimen and no.
12764/11058, a subadult, from Jalapa, Veracruz, measure,
respectively, as follows: Total length, 444, 442; length of tail,
175, 160; length of hind foot, 50, 47. The tail is 65 and 57 per
cent as long as the head and body. The hind foot is more or less
than (approximately equal to) the basilar length.
Female: Merriam (1896:31) gives the measurements of a female
topotype (probably no. 54993, U. S. Nat. Mus., which has no
measurements written on the attached label) as: Total length, 333;
length of tail, 121; length of hind foot, 37. The length of the
tail amounts to 57 per cent of the length of the body. The length
of the hind foot of no. 54993, U. S. Nat. Mus. is the same as the
basal length.
The differences in external measurements between the male and the
female topotypes are: Total length, 111; length of tail, 54;
length of hind foot, 13.
Externals.—As described in Mustela frenata frenata except
that carpal vibrissae do not reach apical pad of fifth digit and
hairiness of foot soles is less.
Color.—As described in M. f. frenata except that: Blackish of
head extends half way or more from ears to shoulders; upper parts
near (14) Brussels Brown or slightly faded tone 2 of Maroon of
Oberthür and Dauthenay, pl. 341; underparts of juvenal pelage near
(a) Ochraceous-Buff. Least width of color of underparts
averaging (in three specimens from Jico and one from Jalapa) 34
(extremes 30-37) per cent of greatest width of color of
underparts. Black tip of tail, in two male topotypes, 57.5 (55 and
60) mm. long; thus longer than hind foot and in each individual
comprising 34 per cent of length of tail-vertebrae.
As compared with M. f. frenata: White facial markings slightly
less extensive; blackish (not black) of head extending onto neck;
upper parts slightly darker; ventral side of tail noticeably
darker; color of underparts more restricted, averaging
approximately one-third rather than nearly one-half width color of
upper parts; black tip of tail one-third rather than one-fourth
length of tail and much longer than hind foot. Similar differences
of lesser amount exist between perotae and tropicalis. M. f.
perda, macrophonius and goldmani bear the opposite relation
to tropicalis. That is to say, in the latter: White facial
markings slightly more extensive; blackish of head less extended
over neck; upper parts markedly lighter; color of underparts less
restricted and black tip of tail shorter.
Skull and teeth.—Male (based on type specimen and a subadult,
no. 11058, from Jalapa): See measurements and plates 25-27, 30. As
described in Mustela frenata perda except that: Weight 4.7 (4.6
and 4.7) grams; basilar length 46.7 (45.5 and 47.8); zygomatic
breadth more or less than distance between condylar foramen and M1
or than between anterior palatine foramen and anterior margin of
tympanic bulla; least width of palate more than length of P4;
anterior margin of tympanic bulla as far posterior to foramen
ovale as width of 4 (including I3) upper incisors; anterior margin
of masseteric fossa below middle of m2 or posterior to that tooth.
Female (based on no. 54993 and no. 1060): See measurements and
plates 37-39. As described in Mustela frenata perda except that:
Weight (of 54993) 2.2 grams; basilar length, 37.5 (36.0-39.0);
zygomatic breadth more or less than distance between anterior
palatine foramen and anterior margin of tympanic bulla; least
width of palate more than greatest length of P4; height of
tympanic bulla equal to one-third to three-fourths of distance
from its anterior margin to foramen ovale.
The skull of the adult female is 53 per cent lighter than that of
the type specimen, a male.
Comparison of the skulls of males and females with those of M. f.
perda, the nearest relative, has been made in the discussion of that
subspecies. Some of the features that readily distinguish skulls of M.
f. tropicalis from those of M. f. frenata, perotae and
macrophonius are as follows: Weight less than 4.8 grams; basilar
length less than 48; postorbital breadth more than length of upper M-Pm
tooth-row. The skulls of male frenata, perotae and macrophonius
are much larger, heavier, and are decidedly more angular with more
constricted postorbital region the least width of which is less than
the length of the upper premolars. In frenata the anterior margins of
the tympanic bullae are protruded much farther from the braincase. The
skull of the female of M. f. tropicalis is smaller, weighing less
than 3 grams; basilar length less than 41; postorbital breadth more
than length of upper molar and premolar tooth-row.
Remarks.—This subspecies was originally described by Merriam as a
full species. Later he described Putorius tropicalis perdus as
another subspecies. Allen (1916) placed P. t. perdus in synonymy but
named Mustela tropicalis nicaraguae as new. In the present paper all
three forms are recognized but are regarded as only subspecifically
distinct from the other bridled weasels of México and Central America.
The limits of the geographic range of tropicalis are fairly well
known on the south and west but the only specimen available from the
tropical coastal region north of Jico, is a young female from a point
50 miles south of Victoria. Thus, how far north along the coast it
ranges toward Matamoros, where M. f. frenata occurs, is not known.
The three specimens from Jico, a young female from Jalapa and another
adult collected by J. Potts and labeled as coming from México City, are
assumed to be typical. The latter specimen certainly came from an
elevation lower than that of México City because M. f. frenata occurs
there. Although the female from Jalapa, agrees well with specimens from
Jico, a male, no. 12764/11058, from Jalapa, has a relatively broader
skull, as in perda, although the tympanic bullae are short as in
tropicalis. The resemblances to perda in features of coloration
are: slightly darker upper parts, and the termination just below the
knees of the color of the underparts. There are three specimens labeled
as from Orizaba that indicate intergradation with perotae as does
also the coloration of the juvenal female from 5 kilometers north of
Jalapa. The specimens labeled as from Orizaba are old, poorly-prepared
skins, only two of which have partial skulls. The size and coloration
of the skins suggest perotae as do also the partial skulls in some
respects although the skulls show greater resemblance to those of
tropicalis.
The topotype, female, no. 54993, was figured by Merriam (1896, fig. 16,
p. 31) along with that of what now is the type specimen of M. f.
perotae. Merriam called attention to the great difference in size
between the skulls of the two sexes of M. f. tropicalis and compared
the condition to that found in noveboracensis. Although the skull of
the female from Jico is fully adult, it probably is exceptionally
small.
The young female from Xuchil is indistinguishable in coloration from
the juvenal female of M. f. perotae from Perote, but in size of skull
and size of teeth is intermediate between the female of tropicalis from
Jalapa and the females from Cofre de Perote.
There is then, indication of intergradation with M. f. perotae as
well as with M. f. perda. M. f. tropicalis differs from M. f.
perotae and M. f. frenata in about the same way that M. f. perda
differs from M. f. goldmani and M. f. macrophonius. M. f.
tropicalis and perda each is smaller and more intensely colored than
goldmani and macrophonius, and inhabits the lowland to the east of
their highland relative.
At least five of the nine skulls have the frontal sinuses infested by
parasites.
Specimens examined.—Total number, 13, all from México, listed
by localities from north to south. Unless otherwise indicated
specimens are in the collection of the United States National
Museum.
Tamaulipas. 50 mi. S Victoria, 1[71]. Near? México City, 1.
Veracruz. Jico, 3; 5 km. N Jalapa, 1[90]; Jalapa, 2 (1[2], 1[75]);
Xuchil, 1[60]; Orizaba, 4 (2[75], 1[4]).
Mustela frenata perda (Merriam)
Long-tailed Weasel
Plates 25, 26, 27, 30, 37, 38 and 39
Putorius tropicalis perdus Merriam, Proc. Biol. Soc. Washington,
15:67, March 22, 1902.
Putorius (Gale) brasiliensis frenatus, Coues, Fur-bearing
animals, p. 142, 1877 (part).
Mustela tropicalis perda, Miller, U. S. Nat. Mus. Bull., 79:100,
December 31, 1912.
Mustela tropicalis tropicalis, Allen, Bull. Amer. Mus. Nat.
Hist., 35:99, April 28, 1916.
Mustela frenata perda, Hall, Carnegie Instit. Washington Publ.
473:109, November 20, 1936.
Type.—Male, subadult, skull and skin; no. 100041, U. S. Nat.
Mus., Biol. Surv. Coll.; Teapa, Tabasco, México; March 31, 1900;
obtained by E. W. Nelson and E. A. Goldman; original no., 14074.
The skull (plates 25-27, 30) is unbroken and all the teeth are
present and entire. The skin is well made and in good condition.
Range.—Fifty feet (Chichén Itzá) to 4000 feet (San Vicente) in
Lower Tropical Life-zone south from southern Veracruz through
southern México into Guatemala. See figure 29 on page 221.
Characters for ready recognition.—Differs from M. f.
nicaraguae in lesser extent of color of underparts (not more than
22 per cent of greatest extent of color of upper parts), black tip
of tail more than 38 per cent of length of tail, and broader skull
(in adult males, mastoid breadth more than 23.9 and zygomatic
breadth more than 27.4); from M. f. tropicalis in more
restricted color of underparts (least width of color of underparts
less than 28 per cent of greatest width of color of upper parts)
and shorter tympanic bullae, which in males are less than 15; from
M. f. goldmani by total length not exceeding 489, least width of
color of underparts not exceeding 24 per cent of greatest width of
color of upper parts, weight of adult skull less than 5 grams and
basilar length less than 48.5.
Description.—Size.—Male: The type specimen and another
subadult from San Vicente, Chiapas, measure, respectively, as
follows: Total length, 473 and 443; length of tail, 184 and 169;
length of hind foot, 51 and 51.5. The tail is 62 and 64 per cent
as long as the head and body. The length of the hind foot is
greater than the basal length.
Female: Estimates made from the dried skin of no. 218036 are:
Total length, 375; length of tail, 140; length of hind foot, 40.
The hind foot of no. 65422 from Catemaco also measures 40.
The average differences in external measurements of the two sexes
are: Total length, 83; length of tail, 37; length of hind foot,
11.
Externals.—As described in Mustela frenata goldmani except
that hairiness of foot soles is slightly less.
Color.—As described in Mustela frenata goldmani except that:
back near (n) Argus Brown or Carbon Brown, tone 3, of Oberthür
and Dauthenay, pl. 342; underparts Ochraceous-Buff. Least width of
color of underparts, in four specimens, averaging 20 (extremes
18-22) per cent of greatest width of color of upper parts; black
tip of tail, in two subadult males, averaging 48 (extremes 46-49)
per cent of length of tail-vertebrae.
Skull and teeth.—Male (based on type specimen and subadult no.
132997 from San Vicente): See measurements and plates 25-27, 30;
weight 4.4 grams (same for each); basilar length 45.7 (45.3 and
46.1); zygomatic breadth less than distance between condylar
foramen and M1 or than between anterior palatine foramen and
anterior margin of tympanic bulla; mastoid breadth less than
postpalatal length; postorbital breadth more or less than
(approximately equal to) length of upper premolars and greater
than width of basioccipital measured from medial margin of one
foramen lacerum posterior to its opposite; interorbital breadth
less than distance between foramen opticum and anterior margin of
tympanic bulla; breadth of rostrum not greater than length of
tympanic bulla; least width of palate less than length of P4;
anterior margin of tympanic bulla as far posterior to foramen
ovale as width of 4-1/2 to 5-1/2 upper incisors; height of
tympanic bulla less than distance from its anterior margin to
foramen ovale; length of tympanic bulla more than length of lower
molar and premolar tooth-row and longer or shorter than rostrum;
anterior margin of masseteric fossa below middle of m2.
Female (based on two subadults, nos. 65422 and 218036): See
measurements and plates 36-39; weight, 2.4 (2.3-2.5) grams;
basilar length, 40.5 (40.4-40.6); zygomatic breadth less than
distance between condylar foramen and M1 or than between anterior
palatine foramen and anterior margin of tympanic bulla;
postorbital breadth more than length of upper premolars or than
width of basioccipital measured from medial margin of one foramen
lacerum posterior to its opposite; least width of palate more than
outside length of P4 and less than inside length of same; anterior
margin of tympanic bulla as far posterior to foramen ovale as
width of 5 or 6 upper incisors; height of tympanic bulla equal to
one-third to one-half distance from its anterior margin to foramen
ovale; length of tympanic bulla more than length of lower molar
and premolar tooth-row and more or less than (about equal to)
length of rostrum.
The skull of the female averages 48 per cent lighter than that of
the male.
Comparison of the skull of the male with that of M. f. nicaraguae has
been made in the account of that subspecies. The skull of the male as
compared with that of M. f. tropicalis has shorter tympanic bullae,
deeper braincase at anterior margin of basioccipital, lesser zygomatic
and palatal breadth and smaller P4 and m1. The skull of the female is
larger in every measurement taken except those reflecting width of the
preorbital portion. This part is actually narrower but probably mainly
because the females of perda are younger than those of tropicalis.
Features in which three skulls of subadults of M. f. perda differ
from the five adults of M. f. goldmani and show no overlap are:
lesser basilar length, lesser weight, greater relative length of upper
tooth-rows, greater relative width of rostrum, greater relative length
of rostrum, lesser mastoid and zygomatic breadths, lesser width, length
and height of tympanic bullae; lesser outside length of P4 and greater
relative depth of braincase at anterior margin of basioccipital and at
posterior margin of M1. Features in which perda averages less are:
length of tooth-rows, interorbital breadth, orbitonasal length,
relative zygomatic breadth, length of m1, outside and inside lengths of
P4, width and length of M1, and depth of skull at posterior margin of
M1. Features in which perda averages more than goldmani are:
relative interorbital breadth, relative mastoid breadth and depth of
skull at anterior margin of basioccipital. The length of the inner
half of M1 averages the same. As compared with goldmani, the skull of
the male of perda is shorter, otherwise generally smaller, but
relatively broader except across the zygomatic arches, and relatively
deeper. The anterior margins of the tympanic bullae project slightly
less from the braincase and the squamosals immediately in front of
these bullae are slightly more convex ventrally.
Remarks.—Described by Merriam in 1902 as a subspecies of Putorius
tropicalis, the form perda was regarded by Allen (1916:99) as not
subspecifically distinct from P. t. tropicalis.
This is the eastern, lowland subspecies of the Tropical Life-zone,
corresponding to M. f. goldmani of the higher mountains just as M.
f. tropicalis corresponds to M. f. frenata and perotae of the high
mountains and table land. The difference in size between perda and
nicaraguae and between perda and tropicalis is slight. M. f.
perda is slightly less richly colored than M. f. nicaraguae but has
the color of the underparts more restricted and has a longer black tip
on the tail. In these respects it is second only to M. f. panamensis
among Central American weasels. Evidence of intergradation with
goldmani is furnished by the specimens from Cobán, Guatemala, and the
nearby locality San Cristóbal in Verapaz, Guatemala. Reduced size as
compared with goldmani suggests affinity with perda but the greater
width of the light-colored underparts, which averages 24 (extremes
18-32) per cent of the greatest width of the color of the upper parts,
shows approach to goldmani. Farther north, in Chiapas, however,
specimens of perda from San Cristóbal and San Vicente are readily
distinguishable from those of goldmani taken a few miles away at
Pinabete and near Teopisca. The latter two localities are, however,
several thousand feet higher than San Cristóbal (Chiapas) and San
Vicente.
Two of the nine skulls (only 3 adult) examined for malformation of the
frontal sinuses reveal infestation by parasites.
Specimens examined.—Total number, 18, listed by localities from
north to south, and unless otherwise indicated in the United
States National Museum.
Veracruz. Catemaco, 1.
Tabasco. Teapa, 2 (1[7]).
Chiapas. San Cristóbal, 1; San Vicente, 1; no locality more
definite than state, 1.
Yucatán. Chichén-Itzá, 1[76].
Guatemala: Cobán, 2 (1[7], 1[4]); Finca la Providenci, S.
Cristóbal, Verapaz, 3[76]; central Guatemala, 1; no locality more
definite than Guatemala, 5 (2[7]).
Mustela frenata nicaraguae Allen
Long-tailed Weasel
Plates 1, 25, 26, 27 and 30
Mustela tropicalis nicaraguae Allen, Bull. Amer. Mus. Nat. Hist.,
35:100, April 28, 1916.
Putorius tropicalis, Allen, Bull. Amer. Mus. Nat. Hist., 24:661,
1908.
Mustela frenata nicaraguae, Hall, Carnegie Instit. Washington
Publ. 473:109, November 20, 1936.
Type.—Male, subadult, skull and skin; no. 30754, Amer. Mus.
Nat. Hist., Matagalpa, Nicaragua; April 16, 1910; obtained by W.
B. Richardson; original no., 712.
The skull (plates 25-27, 30) of the type specimen lacks the entire
right zygomatic arch. Otherwise it is complete. The teeth all are
present and unbroken. The skin is complete and unfaded but only
partly stuffed.
Range.—Honduras and Nicaragua. Altitudinal and zonal limits
unknown. See figure 29 on page 221.
Characters for ready recognition.—Differs from M. f.
costaricensis and M. f. goldmani in shorter black tip of tail
(not more than 35 per cent of length of tail) and lesser width
(usually not more than 7 mm.) of tympanic bulla; from M. f.
perda in greater extent of color of underparts (22 or more per
cent of greatest width of color of upper parts), shorter black tip
on tail (not more than 35 per cent as long as tail) and narrower
skull, the mastoid breadth in adult males being less than 23.9 and
the zygomatic breadth less than 27.
Description.—Size.—Male: Average and extreme measurements of
five subadults and one young (four from Matagalpa and one from San
Rafel del Norte) are: Total length, 450 (420-480); length of tail,
178 (150-190); length of hind foot, 48 (46-50). Tail averages 65
(extremes 56-69) per cent as long as head and body. Length of hind
foot (measurements from dried skins) more than basal length.
Female: Measurements unrecorded.
Externals.—As described in Mustela frenata goldmani, except
that hairiness of foot soles (between that shown in figures 20 and
21) is less, slightly less even than in M. f. perda.
Color.—As described in Mustela frenata goldmani except that:
Back near (n) Argus Brown, or Carbon Brown, tone 4 of Oberthür
and Dauthenay, pl. 342. Underparts Ochraceous-Buff. Least width of
color of underparts, in four males, young, subadult and adult, 24
(extremes 22-26) per cent of greatest width of color of upper
parts; the corresponding per cent in one female is 32; black tip
of tail, in two subadult males, averaging 29 (extremes, 28-30) per
cent of length of tail-vertebrae; corresponding per cent in one
female, 36.
Skull and teeth.—Male (based on type specimen, one adult
topotype [?] and one subadult from San Rafel del Norte): See
measurements and plates 25-27, 30. As described in Mustela
frenata perda except that: Weight, 4.2 grams (estimated for
adults); basilar length 45.0 (44.8-45.5); interorbital breadth
more or less than distance between foramen opticum and anterior
margin of tympanic bulla; anterior margin of tympanic bulla as far
posterior to foramen ovale as width of four to five upper
incisors; length of tympanic bulla not less than length of lower
molar and premolar tooth-row; anterior margin of masseteric fossa
below anterior margin of m2 or posterior to that tooth.
Female: Skull unknown.
Comparison of the skull of the male with that of M. f. costaricensis
is made in the account of that subspecies. As compared with that of M.
f. perda, which it most closely resembles, the skull of the male has a
narrower, shorter rostrum, lesser interorbital breadth, lesser mastoid
and zygomatic breadths and slightly shallower braincase, measured at
anterior margin of basioccipital. The tympanic bullae are slightly less
projected, at their anterior margins, from the braincase and the
squamosal, directly anterior to each, is a little more convex
ventrally. The skull of M. f. nicaraguae is, then, slightly shorter
than that of M. f. perda and relatively narrower.
Remarks.—When naming this form, Allen (1916:100) characterized it as
"Similar to M. tropicalis tropicalis but general coloration much
darker and the white face markings somewhat reduced in area." In the
sentence preceding the one quoted, Putorius tropicalis perdus was
placed as a synonym of Putorius tropicalis tropicalis. M. f.
nicaraguae and M. f. perda are nearly alike in color and color
pattern but differ in cranial characters. M. f. perda and M. f.
tropicalis are widely different in color and more especially in color
pattern but differ only slightly in cranial characters. The aggregate
difference between perda and nicaraguae is less than that between
perda and tropicalis. All three are lowland forms and each is
smaller than the adjacent highland forms, namely, M. f. goldmani,
macrophonius, perotae and frenata.
The weasels from Honduras definitely are not typical of nicaraguae as
it is known from the specimens from Nicaragua itself. The specimens
from the state of Tegucigalpa, Honduras, are larger. Some are darker
than topotypical nicaraguae. The dorsal outline of the skull is more
nearly flat (less convex) in some. In these and several other
differential features studied, the average of specimens from
Tegucigalpa is intermediate toward goldmani, but everything
considered the animals seem best placed with nicaraguae rather than
with goldmani or perda, to which latter also, they show some
resemblance. With better material from Nicaragua and additional
specimens from Salvador (here referred to goldmani) a restudy of all
the material now referred to the three races named would be profitable.
Aims of this restudy might be to determine if a highland race
additional to goldmani should be recognized and if the lowland races
perda and nicaraguae differ from one another in the way that the
existing specimens indicate.
In the five males from Matagalpa, the narrow white band in front of
each ear is confluent with the color of the underparts on one side only
in one specimen and on both sides in two specimens. None of these bands
is confluent with the white patch between the eyes. A dark spot at the
angle of the mouth is present on one side in one specimen. The
corresponding area is dark colored in all other specimens but not
separated from the color of the upper parts. In the specimen from San
Rafel del Norte the white bands are not confluent with the color of the
underparts. The female from Mambacho has the mentioned bands confluent
with the color of the underparts. This female approaches M. f.
costaricensis in the dark color of the upper parts but has more
extensive white facial markings than some specimens from much farther
north. Like a female seen of M. f. costaricensis, this one has a
"frosted" nape but the white hairs on the back of the neck are less
numerous than in the female of M. f. costaricensis.
M. f. nicaraguae in typical form, then, is thought of as a small,
lowland, tropical subspecies only slightly differentiated from M. f.
perda. By reason of its intermediate characters, it constitutes a link
between the lowland forms, and the larger animals called M. f.
goldmani and M. f. costaricensis.
None of the four skulls from Nicaragua shows signs of infestation of
the frontal sinuses by parasites.
Specimens examined.—Total number, 16, listed by localities from
north to south. Specimens are in the American Museum of Natural
History, unless otherwise indicated.
Honduras: Alto Cantoral, 2; Cerro Grande La Paz, 1. La Flor
Archaga, 1[75]; Comayagüela, 1[75]; vicinity of Tegucigalpa, 2; no
locality more definite than Honduras, 1[4].
Nicaragua: San Rafel del Norte, 1; Matagalpa, 6; Ma[o]mbacho, 1.
Mustela frenata costaricensis Goldman
Long-tailed Weasel
Plates 25, 26, 27, 28, 29 and 30
Mustela costaricensis Goldman, Proc. Biol. Soc. Washington, 25:9,
January 23, 1912.
Mustela brasiliensis, Gray, Ann. and Mag. Nat. Hist., 14(ser.
4):374, 1874.
Putorius (Gale) brasiliensis frenatus, Coues, Fur-bearing
animals, p. 142, 1877 (part).
Putorius affinis, Merriam, N. Amer. Fauna, 11:31, June 30, 1896
(part).
Mustela affinis costaricensis, Allen, Bull. Amer. Mus. Nat.
Hist., 35:101, April 28, 1916; Lönnberg, Arkiv för Zool., 14(no.
4):16, 1921.
Mustela frenata costaricensis, Hall, Carnegie Instit. Washington
Publ. 473:109, November 20, 1936.
Type.—Male, young, skull and skin; no. 13770/37149, U. S. Nat.
Mus.; San José, Costa Rica; obtained by C. H. Van Patten.
The skull (plates 28-30) is complete and unbroken. All teeth are
present and unworn. The skin apparently has been remade. It lacks
the distal two-thirds of the tail. The head is somewhat shrunken.
The color is possibly faded but if so only to a slight degree.
Otherwise, the skin is in good condition. The orange color of the
underparts is so intense as to suggest that the full, adult pelage
has not been acquired. No white markings are present on the face.
There is no sex mark on the label attached to the skin but the
size and proportions of the skull and the scrotal pouch on the
skin prove that the specimen is a male. The presence of sutures on
the dorsal face of the rostrum and the short, wide, and low
sagittal crest show the specimen to be young.
Range.—Costa Rica. Altitudinal and zonal range unknown. See
figure 29 on page 221.
Characters for ready recognition.—Differs from M. f.
panamensis in lighter color of upper parts (tone 2 rather than
tone 4 of Reddish Black of Oberthür and Dauthenay, pl. 344) and
longitudinally flat interorbital region of skull; from M. f.
nicaraguae in darker color of upper parts (of Oberthür and
Dauthenay, tone 2 of pl. 344 rather than tone 4 of pl. 342) and
greater width (more than 7) of tympanic bulla.
Description.—Size.—Male: No collector's measurements
available of fully grown animals. Estimated measurements of adult
males: Total length, 470; length of tail, 165; length of hind foot
(taken from dried skins of 3 adults), 52 (50-52). Tail estimated
to average 55 per cent as long as head and body. Length of hind
foot more or less than (about equal to) basal length.
Female: A subadult or adult, from the Candelaria Mountains, and
a subadult from Irazú, measure, respectively: Total length, 370,
385; length of tail, 130, 150; length of hind foot, 40, 31. Tail
59 per cent as long as head and body. Length of hind foot probably
about equal to basal length.
The estimated differences in external measurements of the two
sexes are: Total length, 92; length of tail, 25; length of hind
foot, 16 (probably average difference is less).
Externals.—As described in M. f. panamensis (figure 21)
except that foot soles are slightly more hairy.
Color.—As described in Mustela frenata panamensis except
that: back is near Reddish Black, tone 2 of Oberthür and
Dauthenay, pl. 344; chin, lips, and throat white or whitish;
remainder of underparts near (c) Ochraceous-Buff; color of
underparts rarely extending distally onto toes of forefeet. Least
width of color of underparts, in eleven specimens, averaging 23
(10-36) per cent of greatest width of color of upper parts; black
tip of tail, in six specimens, averaging 36 (31-38) per cent of
length of tail-vertebrae.
Skull and teeth.—Male (based on 2 adults, no. 3.2.1.6. from
vicinity of San José and no. 11408, U. S. Nat. Mus., from "Costa
Rica"): See measurements and plates 25-30; weight, 5.9 grams;
basilar length 49 +; zygomatic breadth more than distance between
condylar foramen and M1 or than between anterior palatine foramen
and anterior margin of tympanic bulla; mastoid breadth less than
postpalatal length; postorbital breadth in undiseased skulls less
than length of upper premolars (less than distance between
posterior borders of P2 and P4) and less than width of
basioccipital measured from medial margin of one foramen lacerum
posterior to its opposite; interorbital breadth more or less than
distance between foramen opticum and anterior margin of tympanic
bulla; breadth of rostrum more or less (about equal to) length of
tympanic bulla; least width of palate less than length of P4;
anterior margin of tympanic bulla as far posterior to foramen
ovale as width of 5 upper incisors; height of tympanic bulla less
than distance from its anterior margin to foramen ovale; tympanic
bulla longer or shorter than (about equal to) lower molar and
premolar tooth-row and longer or shorter than (about equal to)
rostrum; anterior margin of masseteric fossa directly below
posterior border of m2.
Female: Skull of adult unknown.
Comparison of the skull of the male with that of M. f. panamensis has
been made in the account of that subspecies. As compared with that of
M. f. nicaraguae the skull of M. f. costaricensis is heavier and in
every measurement taken is larger. The skull is generally more massive
and it follows that most measurements of depth and width are greater in
relation to the basilar length as well as actually greater. The
individual teeth are larger and the tympanic bullae wider and at their
anterior ends are more projected from the braincase. Indeed the skull
is more like that of M. f. goldmani than like that of M. f.
nicaraguae.
Remarks.—The half dozen ill-prepared skins, with partial skulls
inside, of this form in the United States National Museum long were
referred either to Mustela brasiliensis or Mustela affinis. It was
not until 1912 when Goldman studied these specimens that the
distinctive characters of the Costa Rican weasel were recognized and
made the basis of the name costaricensis.
M. f. costaricensis is well differentiated from M. f. nicaraguae
and M. f. goldmani which occur to the northward and from M. f.
panamensis which occurs to the southward and is a large,
heavy-skulled, dark-colored animal with white facial markings
restricted or absent. In the type specimen and the female from the
Candelaria Mountains the white facial markings are only narrow facial
bars or a few white hairs, but in the young male from Cervantes there
is a well developed bar 6 millimeters wide on each side of the face and
a separate nasofrontal spot, 10 x 12 mm. The young female from Cachí
has a V-shaped frontonasal spot, on the right side of the face a white
bar 5 mm. wide and 17 mm. long connected with the color of the
underparts, and on the left side a white spot in front of the ear and
another between the ear and eye. White facial markings were not
recorded in the other specimens. The color of the upper parts is only a
little less dark than those of M. f. panamensis. Owing to the
numerous white hairs on the dorsal side of the neck, the nape of the
female from the Candelaria Mountains has a frosted appearance not
present in other specimens.
M. f. costaricensis is a large animal and among its geographic
neighbors is approached in size only by a specimen of panamensis from
Boquete, Panamá. Also the young male from Cervantes suggests
panamensis in the less flattened interorbital region, but even so is
more like costaricensis. The small size of two young males, one from
Navarro and the other from the vicinity of San José, is suggestive of
M. f. nicaraguae. However, the large size of most of the specimens
and the configuration of the skull are more as in M. f. goldmani than
in M. f. nicaraguae and thus suggest that the known specimens are of
high mountain subspecies. The long black tip of the tail is another
point of resemblance to M. f. goldmani, the high mountain subspecies
to the north. Perhaps in the lowlands of Costa Rica, there are weasels
of another subspecies.
Of the eight skulls examined for malformation of the frontal sinuses,
each of the two adults and two subadults shows signs of having the
frontal sinuses infested with parasites.
Specimens examined.—Total number, 14, listed by localities from
north to south.
Costa Rica: Irazú (Frasu or Irasu on label), 3000 M., 1[4];
Cervantes, 1[2]; San José, 1[91]; vicinity of San José, 2[7];
Azahar Cartago, 1[78]; Tucurrique, 1[7]; Cachí, 1[7]; El Muñco [=
Muñeco?] (Río Nivarro [= Navarro?]), 4000 ft., 10 mi. S Cartago,
Caribbean Slope, 1[76]; Navarro, 1[91]; Candelaria Mts., 1[75]; no
locality more definite than Costa Rica, 3[91].
Mustela frenata panamensis Hall
Long-tailed Weasel
Plates 1, 25, 26, 27, 28, 29 and 30
Mustela frenata panamensis Hall, Proc. Biol. Soc. Washington,
45:139, September 9, 1932; Hall, Carnegie Instit. Washington
Publ. 473:109, November 20, 1936.
Mustela brasiliensis, Alston, Biol. Cent. Amer., Mammalia, p. 78,
1879.
Putorius affinis, Bangs, Bull. Mus. Comp. Zoöl., 39:49, April,
1902; Hollister, Proc. Biol. Soc. Washington, 28:143, July 10,
1914.
Mustela affinis, Goldman, Proc. Biol. Soc. Washington, 25:10,
January 23, 1912; Hollister, Proc. Biol. Soc. Washington, 28:143,
July 10, 1914.
Mustela affinis costaricensis, Allen, Bull. Amer. Mus. Nat.
Hist., 35:101, April 28, 1916; Goldman, Smithsonian Miscel. Col.,
69 (no. 5): 161, 1920.
Type.—Female, subadult, skull and skin; no. 170970, U. S. Nat.
Mus., Biol. Surv. Coll.; Río Indio, Canal Zone, near Gatún,
Panamá; February 17, 1911; obtained by E. A. Goldman; original no.
20897.
The skull is complete and unbroken. The left lower incisor is
broken off but all the other teeth are present and entire. The
skin is well made and seems to be in faded, worn, first, adult
pelage.
Range.—Sea level (type locality) to 5800 feet (Boquete, see
Bangs [1902:49]); Upper Tropical and Lower Tropical life-zones of
Panamá. See figure 29 on page 221.
Characters for ready recognition.—Differs from both M. f.
meridana and M. f. costaricensis in darker tone (tone 4 of
Oberthür and Dauthenay, pl. 344) of color of upper parts and in
convex dorsal outline of skull (Compare figures of mentioned
subspecies on plates 25-27).
Description.—Size.—Male: Two adults from Boquete in the
Museum of Comparative Zoölogy, nos. 10112 and 10113, measure,
respectively, as follows: Total length, 480 and 400; length of
tail, 170 and 143; length of hind foot, 52 and 43. Hind feet of
two other adult males measure 46 on dried skins. Tail, in two
specimens mentioned above, is 55 and 56 per cent as long as head
and body. Length of hind foot, in each of three adults, slightly
longer than basal length. Corresponding measurements of no. 178970
from Mt. Pirre are: 422, 164, 50. Tail 64 per cent (same per cent
as in young male, no. 137514 from Boquete) as long as head and
body, and hind foot longer than basal length.
Female: An adult and a young from Chiriquí, nos. 18434 and 18435
(Acad. Nat. Sci. Philadelphia), measure, respectively: Total
length, 372, 389; length of tail, 138, 144; length of hind foot,
42, 41. The type specimen measures: Total length, 408; length of
tail, 159; length of hind foot, 46.5. Tail 64 per cent as long as
head and body, and hind foot longer than basal length.
The average differences in external measurements of the two sexes
from the vicinity of Boquete are: Total length, 59; length of
tail, 15; length of hind foot, 6.
Externals.—Longest facial vibrissae black and extending beyond
posterior border of ear; carpal vibrissae wholly, or in part,
black and extending as far as hypothenar pad; hairiness of
foot-soles as shown in figure 21.
Color.—Usually, posterior fourth of each upper lip and
sometimes few hairs in front of ear, white; sides and top of head
and neck posteriorly to, or behind, shoulders, black; dark areas
at angles of mouth confluent with color of upper parts; tip of
tail, black; remainder of upper parts near (n) Bay of Ridgway
and Reddish Black, tone 4, pl. 344 of Oberthür and Dauthenay; chin
and lips, whitish; remainder of underparts Warm Buff or near (16´
c) Ochraceous-Buff; near (12) Salmon-Orange in juveniles and
small young; color of underparts extending distally on posterior
sides of forelegs to wrists, but not to soles, and on hind legs to
or slightly below knees. Least width of color of underparts, in
seven specimens, averaging 18 (extremes 11-28) per cent of
greatest width of color of upper parts; black tip of tail, in five
adults and subadults, averaging 45 (extremes 41-50) per cent of
length of tail-vertebrae.
Skull and teeth.—Male (based on three adults from Boquete): See
measurements and plates 25-30; weight, 5 (4.5-5.4) grams; basilar
length, 45.2 (42.8-48.3); zygomatic breadth more or less than
distance between condylar foramen and M1 or than between anterior
palatine foramen and anterior margin of tympanic bulla; mastoid
breadth less than postpalatal length; postorbital breadth more
than length of upper premolars and more than width of
basioccipital measured from medial margin of one foramen lacerum
posterior to its opposite; interorbital breadth not less than
distance between foramen opticum and anterior margin of tympanic
bulla; breadth of rostrum approximately same (more or less than)
length of tympanic bulla; least width of palate less than length
of P4; anterior margin of tympanic bulla as far posterior to
foramen ovale as width of 4-1/2 to 5-1/2 upper incisors; height of
tympanic bulla less than distance from its anterior margin to
foramen ovale; length of tympanic bulla more or slightly less than
(approximately equal to) length of lower molar and premolar
tooth-row or length of rostrum; anterior margin of masseteric
fossa directly below posterior fourth of m2.
Female (based on subadult, type specimen and one adult from
Siola): See measurements; weight, 3.3 and 2.1 grams; basilar
length, 41.3 and 39.3; zygomatic breadth more than distance
between condylar foramen and M1 and more or less than (about equal
to) that between anterior palatine foramen and anterior margin of
tympanic bulla; postorbital breadth more than combined length of
upper premolars or than width of basioccipital measured from
medial margin of one foramen lacerum posterior to its opposite;
least width of palate more than length of P4 (less in the adult);
anterior margin of tympanic bulla as far posterior to foramen
ovale as width of five upper incisors; height of tympanic bulla
less than (about half) distance from its anterior margin to
foramen ovale; length of tympanic bulla less than length of lower
molar and premolar tooth-row or than rostrum.
The skull of the one adult female from Chiriquí is 58 per cent lighter
than the average of the two adult males.
The skull of the male of M. f. panamensis as compared with that of
M. f. meridana, is heavier and averages larger in nearly every
measurement taken. Relative to basilar length, tooth-rows, orbitonasal
length, interorbital breadth and zygomatic breadth averaging narrower.
Mastoid breadth always narrower. Tympanic bullae longer, narrower, and
usually slightly less protruded. P4 and m1 larger. Dorsal outline of
skull, viewed laterally, more convex. Postorbital breadth actually and
relatively greater. Postorbital processes, mastoid processes, and
sagittal crest not so well developed. Differences between skulls of
females, in so far as known, similar to those described between males.
As compared with M. f. costaricensis, M. f. panamensis has a
lighter skull averaging smaller in every measurement taken except
interorbital breadth, which is greater. Relative to basilar length,
width of rostrum, interorbital breadth and depth of skull at plane of
upper molars, less. Tympanic bullae shorter, narrower, less protruded.
P4, M1, and m1 larger. Dorsal outline of skull, viewed laterally, more
convex. Postorbital breadth relatively and actually greater.
Postorbital processes, mastoid processes, sagittal crest and lambdoidal
crest less developed. No skull of an adult female of M. f.
costaricensis is available for comparison.
Remarks.—This subspecies had not been recognized by previous workers
because specimens from Panamá were supposed to be Mustela affinis
Gray up until 1916, when Allen (1916:100) restricted the type locality
of M. affinis to Bogotá, Colombia. At that time Allen referred
specimens from Panamá to Mustela affinis costaricensis, and Goldman
(1920:161) followed Allen.
The specimens examined show much variation. Part of this is geographic
variation. For instance the specimens from Boquete approach M. f.
costaricensis in size more than do those from farther south. Too few
adult females have been seen to ascertain the amount of secondary
sexual variation. Bangs (1902:49) suggested that the sex of no. 10113
was wrongly recorded and that it was not really a male. If so, this
would reduce the range of apparent variation in size of males from
Boquete by half and bring it into accord with the amount normally
existing in adult males from one locality. No. 10113 is adult but the
skin shows no mammae which would prove it to be a female instead of a
pigmy male. Although even smaller than 10113, the type specimen is so
much larger than females of M. f. meridana that I have wondered if it
is correctly sexed. However, the fact that it was sexed by E. A.
Goldman, a collector of wide experience, lessens the possibility that a
mistake was made.
The color of the underparts is more restricted in panamensis than in
any other subspecies of the species. Excluding the specimen from Mt.
Pirre, the least width of color of the underparts averages 16 (extremes
6-24) per cent of greatest width of the color of the upper parts. This
feature, together with the black color, imparts an appearance to the
Panamá weasel that is strikingly like that of a mink. M. f.
panamensis is one of the two blackest weasels; M. f. aureoventris is
the other. Each of these subspecies occurs in a region of heavy
rainfall and there clearly is a positive correlation between high
humidity and intensity of color. The black tip of the tail, as regards
extent, here reaches the maximum attained among Central and South
American weasels. The foot soles are less hairy than in any other
member of the subgenus Mustela. The tympanic bullae are lower and
less inflated than in any other subspecies of the species.
Adequate specimens from central and southern Panamá may reveal the
existence of one or more additional subspecies since animals from each
of the three localities now represented differ from those from the
other two and some of these differences are correlated with geographic
position. However, specimens from all three localities agree in
several features. For example all of them have the dorsal outline of
the skull highly convex, transversely, and, more especially,
longitudinally. In this respect they are sharply differentiated from
any other American weasel. Nevertheless, M. f. panamensis is clearly
a link between the North and South American subspecies and panamensis
intergrades with the adjacent subspecies. The large size of the skull
and teeth and the slightly more ventrally projected tympanic bullae of
no. 10112 from Boquete approach features seen in M. f. costaricensis.
The smaller size of skull and teeth of no. 178970 from Mt. Pirre are
points of resemblance to M. f. meridana.
The type specimen was selected from a region where M. f. panamensis
is thought to have its distinctive characters well developed. The
specimen is not adult and, therefore, does not show as many
differential characters as does a nontypical adult from Boquete.
Nevertheless, the majority of the above mentioned differential
characters are shown by the type specimen and an adult from the same
place would, it is judged, show all the differential characters better
than would an adult from Boquete.
Of the 11 skulls examined, 6 show no signs of having had the frontal
sinuses infested with parasites.
Specimens examined.—Total number, 19, listed by localities from
north to south and unless otherwise indicated in the United States
National Museum.
Panamá: Boquete, 10 (3[75], 1[8], 1[2], 3[4], 1[7]); Río Gariche
[é], 5300 ft., 1[1]; Siola, 1[1]; Chiriquí, 1[7]; Río Indio, near
Gatún, 1; Mt. Pirre, 3 (2[1]); Calovebora, 1[7] (locality not
found, possibly misspelling of Calovébora); no locality more
definite than Panamá, 1[4].
Mustela frenata meridana Hollister
Long-tailed Weasel
Plates 25, 26, 27, 37, 38 and 39
Mustela meridana Hollister, Proc. Biol. Soc. Washington, 28:143,
July 10, 1914.
Putorius affinis, Robinson and Lyon, Proc. U. S. Nat. Mus.,
24:147, October, 1901; Allen, Bull. Amer. Mus. Nat. Hist.,
30:256, December 2, 1911.
Mustela affinis, Osgood, Field Mus. Nat. Hist. Publ. 155, zoöl.
ser. 10:61, January 10, 1912.
Putorius macrurus, Allen, Bull. Amer. Mus. Nat. Hist., 31:92,
April 19, 1912.
Mustela affinis affinis, Allen, Bull. Amer. Mus. Nat. Hist.,
35:100, April 28, 1916 (part).
Mustela affinis costaricensis, Allen, Bull. Amer. Mus. Nat.
Hist., 35:101, April 28, 1916 (part).
Mustela frenata meridana, Hall, Carnegie Instit. Washington Publ.
473:110, November 20, 1936; Hall, Physis, 16:175, 1939.
Type.—Male, subadult, skull and skin; no. 123341, U. S. Nat.
Mus., 1630 meters elevation, Montes de Mérida, near Mérida,
Venezuela; August 14, 1903; obtained by S. Briceno.
The skull (plates 25 and 26) lacks the right exoccipital condyle
and posterior half of the right zygomatic arch. The teeth all are
present, unworn and entire. The skin is well made and complete.
Range.—Near sea level (San Julián) to 8500 feet (Montes de
Culata, Mérida, Venezuela), and 9000 feet (Santa Elena, Colombia).
Temperate to Subtropical life-zones of Venezuela and northern and
western Colombia. See figure 29 on page 221.
Characters for ready recognition.—Differs from Mustela
africana stolzmanni by absence of median, longitudinal, abdominal
stripe of same color as upper parts, presence of p2 and two roots,
rather than one root, on P2; from M. frenata panamensis in
lighter color of upper parts (tone 3 rather than tone 4, pl. 344,
Reddish Black, Oberthür and Dauthenay), flat rather than convex
dorsal outline of skull immediately behind postorbital processes
(see pl. 27); from M. f. affinis, in males, by lesser average
breadth and length of skull and greater actual and relative size
(see measurements) of facial part of skull; from M. f.
aureoventris, in males, by lighter upper parts (tone 3 rather
than tone 4, pl. 344, Reddish Black, Oberthür and Dauthenay) and
by smaller skull and teeth (basilar length less than 45, length of
m1 less than 6.3, width of M1 less than 4.8, outside length of P4
less than 5.7).
Description.—Size.—Male: Average and extreme measurements of
topotypes (as recorded by collectors on labels, and so uniform as
to show them not to be accurate to within more than 5 mm.) are as
follows: Total length, 434 (410-460); length of tail, 164
(150-180); length of hind foot, 50 (no variation in collectors'
measurements). Tail averages 61 per cent as long as head and body.
Length of hind foot more than basal length. Corresponding
measurements of no. 22191, a young male from Mérida, measured by
Osgood or Conover, are 439, 165, 54. The adult male no. 18703,
from Páramo de Tama (eastern boundary of Venezuela) has the
following measurements written on the label by Osgood: 404, 150,
47.
Female: Average and extreme measurements of topotypes (as recorded
by collectors on labels and so uniform as to show them not to be
accurate to within more than 5 mm.) are as follows: Total length,
347 (320-370); length of tail, 128 (120-130); length of hind foot,
40 (no variation in collectors' measurements). Tail averages 57
per cent as long as head and body. Length of hind foot more than
basal length. Two females, adult no. 11034 and young no. 11033
from Cincinnati, Santa Marta, Colombia, measured by M. A.
Carriker, Jr., measure, respectively, as follows: 371, 330; 140,
140; 38, 36. No. 14463, adult, from Río Zapata, Colombia, measured
(by J. H. Batty), 315, 138, 39. No. 32182, adult, from Mira
Flores, Cauca, Colombia, measured (by W. B. Richardson), 375, 150,
43.
The average differences in external measurements of the two sexes,
at Mérida, are: Total length, 87; length of tail, 36; length of
hind foot, 10.
Externals.—Longest facial vibrissae black (few rarely white)
and extending beyond ear; carpal vibrissae colored like underparts
or upper parts, and not extending beyond apical pad of fifth
digit; hairiness of foot soles slightly greater than shown in
figure 21.
Color.—As described in Mustela frenata panamensis except
that: Posterior fourth of each upper lip rarely, and small spot in
front of ear usually, white; black of head proper not extending
back of ears and grading insensibly into color of upper parts;
anterior half of upper parts of adults "frosted" with numerous
white hairs (tick bites?), upper parts near (n) Bay or tone 2 of
Reddish Black (pl. 344, Oberthür and Dauthenay) or tone 3 in
freshest, unfaded pelage. Least width of color of underparts (in
ten males from Mérida) 20 (17-23) per cent of greatest width of
color of upper parts. Black tip of tail, in same series, 60 to 75
mm. long, thus longer than hind foot and 41 (40-44) per cent as
long as tail-vertebrae.
Skull and teeth.—Male (based on type specimen and seven
topotypes, five adults and three subadults): See measurements and
plates 25-27; weight, 4.1 (3.8-4.3) grams; basilar length, 43.6
(42.3-44.3); zygomatic breadth more than distance between condylar
foramen and M1 or than between anterior palatine foramen and
anterior margin of tympanic bulla; mastoid breadth less than
postpalatal length; postorbital breadth greater than length of
upper premolars or than width of basioccipital measured from
medial margin of one foramen lacerum posterior to its opposite;
interorbital breadth not less than distance between foramen
opticum and anterior margin of tympanic bulla; breadth of rostrum
greater than length of tympanic bulla; least width of palate
greater than length of P4; anterior margin of tympanic bulla as
far posterior to foramen ovale as width of 4 to 5 upper incisors;
height of tympanic bulla less than distance from its anterior
margin to foramen ovale; length of tympanic bulla more or less
than (approximately equal to) alveolar length of lower molar and
premolar tooth-row and shorter than rostrum; anterior margin of
masseteric fossa posterior to m2 and confined to posterior third
(34 per cent average, 32 minimum, 37 maximum) of mandible.
Female (based on four adult topotypes): See measurements and
plates 37-39; weight (no. 143665), 2.3 grams; basilar length 37.2
(36.3-38.2); zygomatic breadth more or less than distance between
condylar foramen and M1 or than distance between anterior palatine
foramen and anterior margin of tympanic bulla; postorbital breadth
(sinuses badly infested with parasites) more than length of upper
premolars or width of basioccipital measured from medial margin of
one foramen lacerum posterior to its opposite; least width of
palate more than length of P4; tympanic bulla as far posterior to
foramen ovale as combined width of 4 to 5 upper incisors; height
of tympanic bulla less than (one half to three fourths of)
distance from its anterior margin to foramen ovale; length of
tympanic bulla more than length of lower molar and premolar
tooth-row.
The skull of the female is 44 per cent lighter than that of the
average male.
Comparisons of the skull with those of M. f. panamensis and affinis
have been made in the accounts of those subspecies. As compared with
the skull of the male of M. f. aureoventris, that of meridana
averages smaller in every measurement taken. Indeed, none of the skulls
of meridana equals that of aureoventris in basilar length, length
of tooth-rows, length of tympanic bulla, depth of skull at anterior
margin of basioccipital or at posterior margin of upper molars, or
measurements of teeth. Relative to the basilar length, most of the
measurements are greater in meridana. Exceptions are the relative
length of the tooth-rows, and the two measurements of depth of the
skull which average less.
Remarks.—In 1914 when Hollister named this weasel he compared it
with M. f. affinis and most of the differential characters which he
ascribed to meridana were merely "more than" or "less than" in
affinis. In affinis, Hollister included specimens from Chiriquí,
Panamá, and the coast of Venezuela. The specimens from these three
places were referred by Allen (1916:101) to Mustela affinis
costaricensis, and he restricted (op. cit.:100) the type locality of
Mustela affinis affinis to Bogotá, Colombia, and synonymized Mustela
meridana with M. a. affinis. Hollister probably would not have named
meridana had he had specimens from Bogotá for comparison and had he
regarded them as topotypes of affinis for the difference is slight.
Nevertheless, within the large geographic range of M. f. meridana
there is some geographic variation. There is more of such variation in
the color of the pelage than in shape and size of the skull. The
specimen from San Julián is darker than the average and in this respect
approaches true panamensis. San Julián is situated at a relatively
low elevation on the coast of Venezuela.
M. f. meridana so closely resembles M. f. affinis that the writer
has no quarrel with anyone who would synonymize meridana. However, as
represented by topotypes, the two races unquestionably are, on the
average, different, and specimens from the southeastern part of the
range of affinis probably are individually distinguishable from
topotypes of meridana.
Variation in the skulls of the series from Mérida is relatively small.
This applies to both males and females. The external measurements
recorded by native collectors are not accurate to within more than five
millimeters but, considering this, variation in external measurements
also seems to be slight. The difference in size of the two sexes
appears to be uniformly greater than in weasels from Central America.
The twenty-six topotypes show that the color and color pattern are
relatively uniform. All are of nearly the same tone except juveniles or
young which are, as in the case of panamensis, much brighter colored
on the underparts. Also, the young have darker-colored upper parts. The
adults, without exception, have numerous white hairs scattered over the
back of the head, neck and between the shoulders. I have no
trustworthy evidence to support the suggestion that these white hairs
are the results of tick bites or that they are caused by other
parasites which damage the hair follicles. The white facial markings
vary relatively little in the 45 specimens carefully examined in this
regard. Also, the variation in color pattern of the two sides of the
head is small. Indeed, within rather narrow limits, the color of the
two sides of the head is the same in every specimen except two. In
these two the white spots anterior to the ears are confluent with the
color of the underparts. Only one specimen, no. 21342, has a white spot
between the eyes and this spot is small. Ten of the twenty-six
specimens have a definite white spot or band in front of each ear. Two
specimens have such a spot on one side only. The dark spots at the
angles of the mouth are present on two sides in three specimens and on
one side only in three others. The mentioned spots are, then, present
nine out of a possible fifty-two times. When the spots are absent, dark
color usually is present in the required area but is confluent with the
color of the upper parts.
A young male from San Julián, Robinson and Lyon (1901:147) state ". . .
was shot . . . as it ran over some bowlders in a ravine. Its eyes shone
with the same greenish light as do the eyes of our common weasel, and
it emitted the same strong odor." No. 14463, Am. Mus. Nat. Hist., from
Río Zapata, Colombia, according to data on the label, was "taken in
timber belt in valley in balk hills" and the native name is Cosonebi.
Two specimens taken on the Páramo de Tama, head of Tachira River,
Venezuela and Colombia are commented on by Osgood (1912:61) as follows:
"One . . . was caught in a steel trap baited with birds and set by the
side of a rushing mountain stream. . . . The other was shot in midday
as it came prowling about our 'house' in the clearing. . . ."
Of the thirty-three skulls before me, twelve have the frontal sinuses
malformed by parasites. These twelve include most of the adults for few
of the subadults and fewer of the young show pathologic conditions in
the frontal region.
Note on localities.—Several of the localities in Colombia mentioned
in "Specimens examined" are described and located by Chapman
(1917:640-656, pl. 41) in his "Distribution of Bird-life in Colombia."
Place names for Colombia on labels, not found on any map, or duplicated
names of which I can not certainly select one, are Río Barrotow, Río
Oscuro, Río Zapata, Río Japata, Guasca and El Baldro. Sonson may or
may not be the town of that name situated some eighty miles northwest
of Bogotá and on the east flank of the Central Andes west of the
Magdalena River on the drainage of the Cauca River. In Venezuela most
of the specimens from Mérida are labeled 1630 meters, Montes de Mérida.
San Julián is some seven miles east of La Guaira (see Robinson and
Lyon, 1901:136). San Esteban is located a little way back from the
coast between Puerto Cabello and Valencia. Páramo de Tama is on the
Venezuelan-Colombian border near the source of the Tachira River (see
Osgood, 1912:35). Mt. Duida is shown as at 3° 30´ N and 65° 40´ W by
Chapman (1931:13) and Mt. Auyán-tepui as near 5° 15´ N and 62° 50´ W by
Chapman (1937:760).
Specimens examined.—Total number, 78, arranged by localities
from north to south and unless otherwise indicated in the British
Museum of Natural History.
Venezuela: San Julián, 1[91]; Carácas, 2; Galipare, Cerro del
Avila, 6500 feet, 1; San Esteban, 1[2]; Mérida, 45 (10[91], 14[2],
10[4], 2[60], 2[14], 1[78]); Páramo de Tama, 1[60].
Colombia: Páramo de Tama, 1[60]; Cincinnati, 3[9]; Valdiva, 3800
ft., 1; Medellín, 2; 7200 ft., Barro Blanco, 1[2]; Santa Elena,
9000 ft., 1[2]; Santa Elena, 1[2]; Sonson, 2 (1[91], 1[2]); Mt.
Auyan-tepuy, 1[2]; Pueblo Rico, 5200 ft., 1[91]; Mira Flores,
1[2]; Jerico, near Cauca River, 1; Tornel, 20 mi. NE Quitichao, 1;
Mt. Duida, 1[2]; El Tambo, Cauca, 1[78]; El Baldro, 1[2]; Río
Japata, 2[2]; Río Zapata, 4500 ft., 1; Río Oscuro, 3300 ft., 1;
Río Barrotow, 3300 ft., 1; Guasca, 1[75]; no locality more
definite than Colombia, 1.
Mustela frenata affinis Gray
Long-tailed Weasel
Plate 30
Mustela affinis Gray, Ann. and Mag. Nat. Hist., 14(ser. 4):375,
1874.
Putorius (Gale) brasiliensis frenatus, Coues, Fur-bearing
animals, p. 142, 1877 (part).
Putorius affinis, Merriam, N. Amer. Fauna, 11:31, June 30, 1896.
Mustela affinis affinis, Allen, Bull. Amer. Mus. Nat. Hist.,
35:100, April 28, 1916; Allen, Bull. Amer. Mus. Nat. Hist.,
35:220, May 31, 1916.
Mustela frenata affinis, Hall, Carnegie Instit. Washington Publ.
473:110, November 20, 1936; Hall, Physis, 16:175, 1939.
Type.—Male, adult, skull with skin; no. 54.1.11.3 (skull
originally numbered 195d, later 54.6.3.4), Brit. Mus. Nat. Hist.;
Colombia [given as new Granada in original description]; purchased
from Mr. S. Stevens. Type locality restricted by Allen (1916:99)
to Bogotá, Colombia.
The skin is in a good state of preservation and has been made over
into a conventional study specimen from a mount on exhibition.
Exposure to light when mounted probably accounts for the faded
color. The skull (plate 30) lacks the middle 9 mm. of the right
zygomatic arch, occiput, basioccipital and posterior two-thirds
of the left tympanic bulla. The teeth all are present and entire.
Range.—Four thousand six hundred feet (Quetame) to 9154 feet
(El Carmen), Tropical to Temperate life-zones of eastern Andes of
Colombia. See figure 29 on page 221.
Characters for ready recognition.—Differs from Mustela
africana stolzmanni by absence of median, longitudinal, abdominal
stripe of same color as upper parts, by presence of p2 and by two
roots rather than one root on P2; from M. frenata meridana, in
case of males, by, on average, greater breadth and length of skull
and lesser actual and relative size (see measurements) of facial
part of skull; from M. f. aureoventris by lighter-colored upper
parts (tone 2 rather than tone 4, pl. 344, Reddish Black of
Oberthür and Dauthenay); from M. f. macrura by darker color
(Reddish Black, tone 2, pl. 344, Ober. and Dauth., rather than
Chocolate, tone 3, pl. 343, Ober. and Dauth.).
Description.—Size.—Male: Measurements in life, estimated
from dried skins, are: Total length, 455; length of tail, 175;
length of hind foot, 52. Proportions of parts supposedly as
described in Mustela frenata meridana.
Female: Estimates from two dried skins: Total length, 365; length
of tail, 135; length of hind foot, 43. Proportions of parts
supposedly as described in Mustela frenata meridana.
The estimated differences in external measurements of the two
sexes are: Total length, 90; length of tail, 40; length of hind
foot, 9.
Externals.—As described in Mustela frenata meridana.
Color.—As described in Mustela frenata panamensis except
that: posterior fourth of each upper lip and spot in front of each
ear white in approximately half of the specimens; black of head
proper not extending back of ears and grading insensibly into
color of upper parts; upper parts near (n) Bay, or tone 2 of
Reddish Black (pl. 344, Oberthür and Dauthenay). Least width of
color of underparts (in five males from vicinity of Bogotá) 24
(15-29) per cent of greatest width of color of upper parts. Black
tip of tail, in same series, 60 to 75 mm. long, thus longer than
hind foot and averaging 38 per cent as long as tail-vertebrae.
Skull and teeth.—Male (based on three adults and two subadult
topotypes): See measurements and plate 30. As described in
Mustela frenata meridana except that: Weight, 4.5 grams
(estimated); basilar length 45.8±; interorbital breadth not
greater than distance between foramen opticum and anterior margin
of tympanic bulla (type as in meridana where interorbital
breadth is more than distance between foramen opticum and anterior
margin of tympanic bulla); least width of palate not less than
length of P4; masseteric fossa confined to posterior two-fifths
(38 to 40 per cent; average 39 per cent) of mandible and not
extended anteriorly to middle of m2.
Female: No adults examined.
As compared with M. f. meridana the skull of the male is larger, to
the average amount of 2.2 mm. in basilar length and 1.2 mm. in
zygomatic breadth of adults; length of tooth-rows and mastoid breadth
average greater but relatively less; breadth of rostrum, interorbital
breadth and orbitonasal length average actually and relatively less.
Thus the skull of affinis is longer and broader, but the facial
region is actually, as well as relatively, smaller. As compared with
the skull of the male of M. f. aureoventris, that of M. f. affinis
is about the same in basilar length. However, in no specimen of
affinis are the measurements of length of tooth-rows or breadth of
rostrum, actually, or relatively, as great as in aureoventris. The
same is true of all measurements taken of M1, P4 and m1. The specimens
from the vicinity of Quito and north of there, although referred to
macrura, are nearly as dark as typical affinis, approach affinis
in cranial characters, and indicate intergradation of affinis with
macrura.
Remarks.—Mustela affinis was named by John Edward Gray in 1874 (p.
375) on the basis of a specimen from New Granada. Although usually
synonymized with Mustela brasiliensis by later authors until 1896
when Merriam (1896:31) applied the name to weasels from Costa Rica,
nearly all the South American and several of the Central American
weasels have, at one time or another, had Gray's name, affinis,
applied to them. Gray, in 1865 (p. 115) when giving measurements of
Mustela aureoventris, probably mentioned the specimen, that later
became the holotype. In 1916 (p. 98) Allen restricted the type locality
to Bogotá, Colombia. Allen's action was a necessary procedure in
clearing up the systematics of South American weasels and was based on
good grounds. As set forth by Allen (loc. cit.), and more in detail
by Chapman (1917:642), Bogotá has long been the shipping point for
Colombian vertebrate specimens, many of which were obtained in the
mountains to the east. Allen (1916A:220) quotes Thomas as saying that
the type specimen was purchased from Stevens at about the same time
that a number of Colombian birds were purchased from the same dealer.
Also, specimens from Bogotá agree with Gray's description of the type
specimen.
Mustela frenata affinis, as here defined, constitutes one of the
several slight geographic variants met with, on the sides of, and
between, the three north and south mountain chains of Colombia. The
others are lumped under the name Mustela frenata meridana. M. f.
affinis, in common with specimens from the northern part of the range
of macrura has large teeth. Weasels of all of the region from Quito
to Bogotá have large teeth. To the north there is the smaller-toothed
meridana and to the south the smaller-toothed macrura grading into
the still smaller-toothed agilis, and boliviensis.
Two skins, without corresponding skulls, from Caqueta are lighter
colored than any others of affinis; possibly the skins are faded by
exposure to light. Since they probably come from an elevation of less
than 1000 feet in the Amazonian region, they may pertain to another
subspecies.
Complete, unbroken, skulls of affinis are needed to ascertain the
degree to which affinis and meridana differ in cranial features.
The several specimens from the immediate region of Bogotá show well the
color and the color pattern but lack collectors' measurements.
None of the ten skulls examined shows malformation of the frontal
region due to infestation of the frontal sinuses by parasites. Possibly
three of the four adults were infested, although not severely.
Specimens examined.—Total number, 27, arranged by localities
from north to south and unless otherwise indicated in the United
States National Museum.
Colombia: El Carmen, 1[2]; W. Cundinamarca, 1[7]; Muzzo [= Muzo?],
1[4]; Bogotá, 1; Castillo, near Bogotá, 1[7]; Fambrias, near
Bogotá, 1[75]; Bogotá district, 1[2]; Choachí, 9 (1[75], 2[7],
1[84]); Páramo de Choachí, 2 (1[2], 1[84]); Laguna del Verjón (=
City of Bogotá), 1[75]; Quetame, 2[2]; Fusagasuga, 1; Caqueta,
2[2]; no locality more definite than Colombia, 3 (1[7]).
Mustela frenata aureoventris Gray
Long-tailed Weasel
Plates 27, 28 and 29
Mustela aureoventris Gray, Proc. Zoöl. Soc. London, 1864:55, pl.
8, 1864; Gray, Proc. Zoöl. Soc. London, 1865:115, 1865.
Putorius (Gale) brasiliensis var. aequatorialis Coues,
Fur-bearing animals, p. 142, 1877, part? ("merely as a substitute
for Gray's [supposedly] preoccupied name," that is,
aureoventris).
Mustela affinis costaricensis, Allen, Bull. Amer. Mus. Nat.
Hist., 35:101, April 28, 1916 (part).
Mustela macrura, Lönnberg, Arkiv för Zool., 14 (no. 4):11, 1921
(part ?).
Mustela frenata aureoventris, Hall, Carnegie Instit. Washington
Publ. 473:110, November 20, 1936; Hall, Physis, 16:175, 1939.
Type.—Probably female, juvenile, skull with skin, no. 64.6.6.3
(formerly 1432a), British Mus. Nat. Hist.; probably Subtropical
Life-zone of western Ecuador (locality given as Quito, probably
because received from that place).
The skin, once exhibited as a mount, has lost some hair from the
back and other parts of the body and is not suitable for remaking
into a conventional study specimen. The skull lacks the occiput,
basioccipital, premaxillae, upper incisors, two of the lower
incisors, all of the canines, premolars 2/2 on both sides, right
P3, left p3, and has the left jugal mesially defective. The
premolars present are not all fully emerged.
Range.—Pacific coastal regions of Ecuador and Colombia;
Subtropical and Tropical life-zones. See figure 29 on page 221.
Characters for ready recognition.—Differs from Mustela
africana stolzmanni by absence of median, longitudinal, abdominal
stripe of same color as upper parts, by presence of p2 and by two
rather than one root on P2; from Mustela frenata macrura by
Reddish Black, tone 4, plate 344 rather than Chocolate, tone 3,
pl. 343 (of Oberthür and Dauthenay), or slightly darker color of
upper parts; from M. f. affinis and M. f. meridana by darker
color (tone 4 rather than tone 2, Reddish Black of Ober. and
Dauth.) of upper parts and larger size of teeth (M1 with length
more than 2.4 and breadth more than 4.7; P4 with outside length
more than 5.9; length of m1 more than 6.2).
Description.—Unless otherwise stated, information concerning
this subspecies is derived from the one referred specimen
available, a young male, no. 34677, Amer. Mus. Nat. Hist.
Size.—Male: Total length, 470; length of tail, 160; length of
hind foot, 50. Tail 51 per cent as long as head and body.
Female: Not known.
Externals.—Longest facial vibrissae black and reaching beyond
ear. Carpal vibrissae reaching to or beyond apical pad of fifth
digit; hairiness of foot soles slightly less than shown in figure
20.
Color.—Sides and top of head and neck posteriorly to shoulders
black; white facial markings represented by only five white hairs
anterior to right ear, one anterior to left ear and three far back
on forehead; dark areas at angles of mouth confluent with color of
upper parts; tip of tail black; remainder of upper parts near
(n) Bay or Reddish Black, tone 4 of Oberthür and Dauthenay, pl.
344; chin whitish; remainder of underparts Warm Buff, deep orange
in juvenile, type specimen, according to Gray (1864, pl. 8); color
of underparts extending distally on posterior sides of forelegs to
wrists but not reaching foot soles and on hind legs to or slightly
below knees. Least width of color of underparts equal to 15 per
cent of greatest width of color of upper parts. Black tip of tail
equal to 27 per cent of length of tail-vertebrae.
In color, no. 34677 is, to me as it was to Allen (1916:101),
indistinguishable from the darkest specimens (nos. 178970 and
10112) of M. f. panamensis. Therefore, M. f. aureoventris is
one of the two darkest subspecies of weasels.
Skull and teeth.—Male: See measurements and plates 27-29;
weight, 4.3 grams; basilar length, 45.8; zygomatic breadth
approximately equal to distance between condylar foramen and M1
and to distance between anterior palatine foramen and anterior
margin of tympanic bulla; mastoid breadth less than postpalatal
length; postorbital breadth more than length of upper premolars
and greater than width of basioccipital measured from medial
margin of one foramen lacerum posterior to its opposite;
interorbital breadth greater than distance between foramen opticum
and anterior margin of tympanic bulla; breadth of rostrum less (at
least in young specimen) than length of tympanic bulla; least
width of palate seldom if ever greater than length of P4; anterior
margin of tympanic bulla as far posterior to foramen ovale as
width of three (including I3) upper incisors; height of tympanic
bulla not greater than distance from its anterior margin to
foramen ovale; length of tympanic bulla more than length of lower
molar and premolar tooth-row and shorter than orbitonasal length;
anterior margin of masseteric fossa below anterior half of m2.
Skulls of males of M. f. aureoventris, and Mustela frenata
macrura from the vicinity of Quito so closely resemble one
another as not to be distinguished with the material now
available, although the teeth of aureoventris are larger.
Comparisons of the skulls of males with those of M. f. meridana
and affinis, which are readily distinguishable from those of
aureoventris, have been made in the accounts of those
subspecies.
Female: Skull of adult unknown.
Remarks.—This subspecies of the Tropical Life-zone, or at least the
Subtropical Life-zone, of Ecuador, in certain cranial characters
resembles Mustela frenata macrura of the Temperate Life-zone. The two
differ markedly in color. Nevertheless, a large number of the specimens
collected in Ecuador are intermediate in color as well as in zonal
distribution.
The type specimen is young or a juvenile. The measurements of no. 34677
from Gualea indicate an animal similar in size to M. f. affinis. Gray
(1864:55) states that the type specimen measures "Length of body and
head 6 inches, of tail 4-1/2 inches." The plate (pl. 8) accompanying
Gray's original description (loc. cit.) is marked one-half natural
size and represents the animal as having a head and body length of
eight and one-half inches. One year later Gray (1865:115) gives the
measurements of this species as "Length of body and head 12, tail 8
inches." Since he had at this time another specimen, larger than the
type specimen (which specimen later, probably, became the type of
Mustela affinis Gray), the larger measurements probably were taken
from it.
Geographically, and as regards cranial characters, Mustela frenata
aureoventris is most closely related to M. f. affinis and to the
northern section of M. f. macrura, but in color to M. f.
panamensis. M. f. aureoventris and M. f. panamensis are the two
darkest-colored subspecies and each occurs in a region of extremely
heavy rainfall. There is a skin only, no. 32620, Amer. Mus. Nat. Hist.,
from Munchique, obtained on June 1, 1911, which is appreciably darker
than specimens of M. f. affinis in corresponding pelage and is
intermediate between M. f. affinis and M. f. aureoventris in color
as it is geographically. The specimen measures 495, 202, 52.
The name Mustela aureoventris Gray has been regarded by most authors
as preoccupied by Mustela auriventer Hodgson (1841:909). However, the
writer is not of this opinion and agrees with Thomas (1920:224) that
"The name aureoventris is not invalidated by the auriventer of
Hodgson, as, apart from 'one-letterist' differences, its first half
comes from the adjective aureus, while Hodgson's name is based on the
substantive aurum, so that not only the spellings but the derivations
are different." The spelling of Gray's name should be aureoventris
for this is the spelling in the original description which in
pagination precedes the colored plate of the animal that is labeled
Mustela aureoventris. Putorius brasiliensis var. aequatorialis
Coues (1877:142) is the only name known to the writer that has been
proposed as a substitute for Mustela aureoventris Gray.
Thomas (1920:224) treats Mustela macrura Taczanowski as a synonym of
Mustela aureoventris Gray. Allen (1916:101) also treats the two names
as applying to the same kind of weasel but regards aureoventris as
preoccupied and therefore uses the name macrura. Taczanowski's
original description (1874:311) and plate of Mustela macrura indicate
an animal that is lighter colored than M. f. affinis. Gray's original
description (1864:55) and plate of aureoventris indicate an animal
that is darker colored than M. f. affinis. Indeed Gray (1865:115) in
speaking of the type of aureoventris as compared with an adult from
New Granada [= Colombia] that probably later became the type specimen
of Mustela affinis, states: "The young from Quito is much darker than
the adult;. . . ." Comparison of the plates accompanying the original
descriptions of aureoventris and macrura well illustrate the
difference stated in the written descriptions. My examination of the
type specimens of M. macrura and M. f. aureoventris shows them to
have been fairly accurately portrayed in the plates accompanying the
original descriptions. Accordingly the two names are used for the two
kinds of animals which appear, however, to be only subspecifically
distinct.
Comparison of Gray's plate (1864, pl. 8) with the available specimens
from South America indicates that the name aureoventris is based on
an individual that is lighter colored than no. 34677 Amer. Mus. Nat.
Hist., from Gualea, Ecuador, but on one which resembles no. 34677 more
than it does the lighter-colored specimens from the Temperate Zone of
Ecuador and northern Perú. Because Quito, Ecuador, is in the Temperate
Life-zone and because the available specimens from this zone in Ecuador
and northern Perú are distinctly lighter colored than Gray's plate
representing the type of aureoventris shows this specimen to be, it
is judged to have come from an altitude lower than that of Quito (9350
feet, according to Chapman, 1926:717); probably it came from the
Subtropical Life-zone of Ecuador. Indeed Gray (1864:55) did not say
that the specimen was collected or obtained at Quito but that it was ". . .
received from Quito. . . ." Chapman (1926:717) has pointed out that
Quito, since 1846 has been the distributing point for bird skins which
specimens ". . . come from the vicinity of the city, from the 'Napo'
region on the Amazonian slopes of the Andes, and from Nanegal, Gualea,
and other localities on the Pacific side rarely below the Subtropical
Zone." It is also pointed out that only some of the specimens are
labeled with their approximate place of capture and that even then
these localities cannot be accepted as definite; they indicate mainly
whether the specimen is from the eastern or western side of the Andes.
The above mentioned considerations and information gained by study of
the specimens cause me to think that the type is an intergrade tending
toward the lighter-colored Mustela f. macrura of the Temperate Zone
although sufficiently dark to be referred to the dark subspecies
represented by no. 34677 Amer. Mus. Nat. Hist., from Gualea, Ecuador.
The skull of no. 34677 shows no infestation of the frontal sinuses by
parasites.
Specimens examined.—Total number, 3, as follows:
Ecuador: Gualea, 1, Amer. Mus. Nat. Hist.
Colombia: 8325 ft., Munchique, 1, Amer. Mus. Nat. Hist. In the
British Museum of Natural History, the type, (1).
Mustela frenata helleri Hall
Long-tailed Weasel
Plates 27, 28 and 29
Mustela frenata helleri Hall, Proc. Biol. Soc. Washington,
48:143, August 22, 1935; Hall, Carnegie Instit. Washington Publ.
473:110, November 20, 1936; Hall, Physis, 16:175, 1939.
Type.—Male, adult, skull and skin; no. 24133, Field Mus. Nat.
Hist.; 3000 feet, Hacienda San Antonio, Río Chinchao, Perú; August
22, 1922. Obtained by Edmund Heller. Original no. 6589.
The skull (plates 27-29) is complete and unbroken. The teeth all
are present, entire and but slightly worn. The skin is well made,
unfaded, and in good condition.
Range.—Three thousand feet (type locality) to 6700 feet (Ambo),
Tropical and Subtropical life-zones of eastern Perú. See figure 29
on page 221.
Characters for ready recognition.—Differs from Mustela
africana stolzmanni by absence of median, longitudinal, abdominal
stripe of same color as upper parts, presence of p2 and two roots
rather than one root on P2; from Mustela frenata macrura by
darker color (Carbon Brown, tone 3, pl. 342 rather than Chocolate,
tone 3, pl. 343, Oberthür and Dauthenay) of upper parts.
Description.—Size.—Male: Measurements of the type specimen
and topotype, no. 24132, are, respectively, as follows: Total
length, 382, 418; length of tail, 152, 164; length of hind foot,
52, 48. Tail 66 and 65 per cent as long as head and body. Hind
foot more than basal length.
Female: Measurements of two referred females, no. 24134 from Ambo
and no. 24136 from Huanuco, are, respectively, as follows: Total
length, 328 and 303; length of tail, 118 and 103; length of hind
foot, 39 and 38.5. Tail 56 and 51 per cent as long as head and
body. Hind foot shorter than basal length.
The average differences in external measurements of the two sexes
are: Total length, 85; length of tail, 49; length of hind foot,
11.
Externals.—Longest facial vibrissae black and extending beyond
ear; carpal vibrissae same color as upper parts and extending to
apical pad of fifth digit; hairiness of foot-soles as shown in
figure 20.
Color.—Rarely a few white hairs anterior to each ear; posterior
fifth of each upper lip white; top of head, posteriorly to
slightly behind ears, black, grading into color of upper parts of
body; dark spots at angles of mouth absent; tip of tail black;
remainder of upper parts near (n) Argus Brown and Carbon Brown,
tone 3 (pl. 342, Oberthür and Dauthenay); chin whitish; remainder
of underparts Warm Buff; color of underparts extends distally on
posterior sides of forelegs to wrists but not reaching foot-soles
and on hind legs to slightly below knees. Least width of color of
underparts 24 per cent of greatest width of color of upper parts
in each of two males and 19 to 30 per cent in three females. Black
tip of tail longer than hind foot and averaging 40 (39-42) per
cent of length of tail-vertebrae.
Skull and teeth.—Male (based on type specimen and adult no.
24132): See measurements and plates 27-29. As described in
Mustela frenata macrura except that: Weight, 4.5 (4.2 and 4.8);
basilar length, 44.6 (44.0-45.3); zygomatic breadth more than
distance between condylar foramen and M1 or than between anterior
palatine foramen and anterior margin of tympanic bulla; breadth of
rostrum more or less than (approximately equal to) length of
tympanic bulla; height of tympanic bulla less than distance from
its anterior margin to foramen ovale; length of tympanic bulla
less than length of rostrum; anterior margin of masseteric fossa
posterior to m2 by length of that tooth.
Female (based on nos. 24134 to 24136): See measurements. As
described in Mustela frenata macrura except that: Weight, 1.7
(1.5-1.9) grams; basilar length, 36.5 (35.3-38.1); zygomatic
breadth less than distance between anterior palatine foramen and
anterior margin of tympanic bulla; least width of palate more or
less than (approximately equal to) outside length of P4; length of
tympanic bulla less than length of rostrum.
The skull of the female averages 62 per cent lighter than that of
the male.
The skull of the male is generally large and heavy as are the teeth.
Comparison with macrura is made in the account of that subspecies.
From males of affinis those of helleri differ in: skull shorter;
breadth of rostrum and interorbital breadth actually and relatively
greater.
Remarks.—The five specimens examined of this subspecies were taken
by Edmund Heller for the Field Museum of Natural History in 1922 and
1923. It is to honor his contributions to mammalogy that the subspecies
is named helleri. No. 24135 is the specimen carried as a pet for some
time by Mr. and Mrs. Heller and of which Mrs. Heller (1924:481) has
given an account.
This subspecies is insufficiently known, especially as to geographic
range; probably it occupies a considerable range in the Tropical
Life-zone along the eastern base of the Andes. The three females, two
from Ambo and one from Huanuco, come from a much higher altitude than
do the two males and the climate is said to be arid at Ambo and
Huanuco. The skulls of the females are 62 per cent lighter and
correspondingly smaller in measurements, than those of males. This
difference is more than that found in any other South American weasel
and it may be that the females are of a subspecies other than
helleri.
The type specimen has a broad skull with major proportions strikingly
like those of Mustela stolzmanni. Possibly the similar climatic
conditions under which the two live have left their impress in similar
fashion in this part of each of the two species. The teeth, tympanic
bullae, and certain other parts of the skull are, however, so
differently proportioned as to show that the skulls represent two
species. The referred male has a much longer skull than the type
specimen and the relative proportions of breadth and depth of the two
skulls differ widely. Judging from large series of weasels examined
from localities outside the range of M. f. helleri, the two skulls
probably represent almost the maximum of individual variation occurring
in one subspecies.
The dark color is as might be expected since helleri inhabits the
humid Tropical Zone.
None of the five skulls shows signs of having had the frontal sinuses
infested by parasites.
Specimens examined.—Total number, 5, all in the Field Museum of
Natural History.
Perú: 3500 ft., Hacienda Buena Vista, Río Chinchao, 1; 3000 ft.,
Hacienda San Antonio, Río Chinchao, 1; Huanuco, 1; Ambo, 2.
Mustela frenata agilis Tschudi
Long-tailed Weasel
Plates 27, 28, 29, 39 and 40
Mustela agilis Tschudi, Fauna Peruana, p. 110, 1844; Gray, Proc.
Zoöl. Soc. London, 1865:113, 1865; Taczanowski, Proc. Zoöl. Soc.
London, 1874:311, 1874; Taczanowski, Proc. Zoöl. Soc. London,
1881:648, 1881; Allen, Bull. Amer. Mus. Nat. Hist., 35:104; April
28, 1916; Thomas, Proc. U. S. Nat. Mus., 58-224, 1920.
Mustela macrura, Allen, Bull. Amer. Mus. Nat. Hist., 35:103,
April 28, 1916.
Mustela frenata agilis, Hall, Carnegie Instit. Washington Publ.
473:110, November 20, 1936; Hall, Physis, 16:176, 1939.
Type.—No type specimen, or type locality more restricted than
cold, barren highlands of the Cordillera [referring to Perú]
designated.
Range.—High, barren Cordillera of Perú (see Tschudi, orig.
descr.); as here restricted, Temperate Life-zone and higher in
western Andes and intermountain valleys of Perú. See figure 29 on
page 221.
Characters for ready recognition.—Differs from Mustela frenata
macrura by lighter color (Chocolate, tone 2 rather than 3, pl.
343, Oberthür and Dauthenay) of upper parts; length of upper
tooth-rows, in females, less than 13; inside length of P4 more
than 4.6; from M. f. aureoventris by smaller teeth (maximum size
just given for agilis); from M. f. boliviensis by lighter
color, upper parts being Chocolate, tone 2, pl. 343, rather than
tone 4 or darker of Carbon Brown, pl. 342 (Oberthür and
Dauthenay).
Description.—Size.—Male: The stuffed skin of an adult, from
Lima, measures: Total length, 460; length of tail, 125; length of
hind foot, 45.7. A skin alone from Huarochirí has a body, as now
stuffed, 277 mm. long. The tail is missing and the bones of the
hind feet have been removed.
Female: The mounted specimen, no. 565, Mus. Polonais d'Hist. Nat.,
yields measurements, taken by me, as follows: Total length, 250;
length of tail, 75; length of hind foot, 32.5. The female, no.
21147, from Macate, measures, 300, 102, 34.
Externals.—Longest facial vibrissae, either dark-or
light-colored and extending beyond ear; carpal vibrissae either
dark-or light-colored and extending to apical pad of fifth digit;
hairiness of foot soles as shown in figure 20.
Color.—Tschudi's description of the color is, in substance, as
follows: Head, back and tail reddish gray; base of hair gray,
followed by broader grayish-yellow ring and then reddish-brown
tip; nose simply dark brown or upper lips edged with white;
throat, breast, belly and higher parts of inner sides of
extremities whitish gray, at times wholly gray, bases of hairs
always gray; feet darker than body, almost chestnut brown; tail
darker on tip than at base; ears externally dark brown, internally
whitish.
No. 565 possibly somewhat faded from exposure to light, has all
the upper parts near (14´ j) Ochraceous-Tawny or Cinnamon, and
tone 4 of Oberthür and Dauthenay, plate 323; posterior half of
each upper lip white; no other white facial markings present; dark
spot at each angle of mouth (one spot confluent with color of
upper parts); tip of tail probably black (tip missing); underparts
white, belly probably originally with slight tinge of yellow or
allied color; color of underparts extending distally on forelegs
to feet and onto upper sides of toes and on hind legs to just
above heels. Least width of color of underparts equal to about
one-fourth of greatest width of color of upper parts.
No. 21147, subadult, from Macate, has a white band confluent with
the underparts extending anterodorsally anterior to each ear and
the posterior third of each upper lip white. Top of head near
(n) Mars Brown, and Carbon Brown, tone 3 (pl. 342, Ober. and
Dauth.); tip of tail black; remainder of upper parts near (16"
j) Tawny-Olive, and Chocolate (tone 2, of pl. 343 of Ober. and
Dauth.) or Raw Umber (tone 3 of pl. 301 of Ober. and Dauth.);
anterior half of underparts, including posterior sides of forelegs
and antipalmar faces of forefeet, white; remainder of underparts
tinged with Warm Buff and extended on posterior legs almost to
ankles.
No. 8.1.10.1., male adult, from Lima, is also light colored, and
as described in no. 21147, except that left side of head has a
white spot rather than bar; posterior eighth of each upper lip
white; white frontonasal spot present, 11 x 11 mm.; antipalmar
faces of forefeet spotted with brown color of upper parts; color
of underparts extending distally on hind legs along medial side of
foot to point halfway between heel and tip of inner toe.
No. 13257 from Huarochirí in color and color pattern closely
resembles no. 21147. It differs from no. 21147 in slightly lighter
color of upper parts, entirely white underparts, less extension of
color of underparts onto forefeet, few white hairs instead of
white band in front of each ear; color of underparts more
restricted.
In each of the four specimens, the least width of the underparts,
expressed as a percentage of the upper parts, is as follows: no.
13257, 11 per cent; no. 21147, 29 per cent; no. 565, 31 per cent;
no. 8.1.10.1., nineteen per cent.
Skull and teeth.—Male (based on no. 8.1.10.1.): See
measurements and plates 27-29. As described in Mustela frenata
macrura except that: Weight 4.1 grams; basilar length, 42.5;
zygomatic breadth more than distance between anterior palatine
foramen and anterior margin of tympanic bulla; mastoid breadth
less than postpalatal length; tympanic bullae shorter than
rostrum.
Female (based on no. 21147): See measurements and plates 39 and
40. As described in Mustela frenata macrura except that: Weight
(no. 21147, subadult), 1.5 grams; basilar length, 35.2; least
width of palate less than outside length of P4; tympanic bulla as
far posterior to foramen ovale as combined width of five upper
incisors; no. 565 answers to the same description but differs from
no. 21147 in greater basilar length and larger tympanic bullae
which are slightly more projected, at their anterior margins, from
the braincase.
To judge from the skull of the female from Macate and the skull of the
male from Lima, the skull and teeth of agilis are smaller than in any
other South American subspecies of Mustela frenata, except M. f.
boliviensis.
Remarks.—Tschudi almost certainly used the name Mustela agilis in
a composite sense. His statement (see quoted matter below) about the
marked variation in color of this species, as represented by the skins
carried by the Indian women as purses, indicates that the forms here
designated as Mustela macrura, M. helleri and possibly others
additional to the one here called agilis were included by him under
the name Mustela agilis. Taczanowski took account of Mustela agilis
when he described other species from Perú. Allen (1916:104) and Thomas
(1920:224) were not convinced that Mustela agilis and Mustela
macrura were distinct species or subspecies.
Search on August 28, 1937, in the Musée d'Histoire Naturelle, at
Neuchatel, Switzerland, by Mr. Théodore Delachaux, assistant there, and
the writer, revealed no trace of weasels from Tschudi's collection,
although some other specimens of mammals that he figured in the "Fauna
Peruana" are preserved in that Museum. Not only were the collections of
specimens examined but the new catalogue and old catalogue of mammals
were vainly searched for mention of weasels deposited by Tschudi.
Later, at the British Museum of Natural History, on p, 105 of a
personal notebook, of the late Mr. Oldfield Thomas, record was found of
his fruitless search for the same specimens of Mustela in May, 1902,
at Neuchatel.
Although Tschudi certainly used the name Mustela agilis in a
composite sense, as subspecies are at present understood, his
description most nearly applies to the light-colored animals from
western Perú—the lightest colored of any South American weasels seen.
They are of approximately the same color as North American subspecies
inhabiting semiarid regions, for example Mustela frenata longicauda
of the Great Plains.
Another, but in my opinion less weighty, justification for applying
Tschudi's name agilis to these light-colored weasels of western Perú
is that by one line of reasoning, Taczanowski in naming macrura
(jelskii is a synonym of it) from farther eastward in Perú, and that
Hall in naming helleri from still farther eastward, and boliviensis
to the southeastward, geographically restricted the application of the
name agilis. Hall's action did this because he recognized geographic
variation and employed the subspecies concept. Taczanowski, however,
proposed his name macrura for a kind of animal which he indicated was
specifically (as opposed to subspecifically) distinct from agilis and
his account (1881:649) of jelskii indicates that he thought Mustela
agilis Tschudi might occur in the same place as the animals which he
named as new kinds. Thus, we can not credit Taczanowski with intent
to restrict the name agilis geographically, even though later authors
may choose to rule that his naming of macrura in effect did so
restrict the application of Mustela agilis Tschudi.
The equivalents in millimeters given by Allen (1916:104) for Tschudi's
measurements of 9 to 10 inches entire length, and tails of 4 inches to
4 inches and 4 lines, apparently are based on the London scale in use
today. If Tschudi employed the Rhine scale also of eight lines to the
inch, but one which has the foot longer by an amount of 20 millimeters,
or the Leipzig scale in which the foot is 22 millimeters shorter than
the London foot, the measurements recorded by Tschudi differ in one
direction or the other from those computed by Allen. However, knowledge
of which scale Tschudi employed would not help much, if any, in more
precise application of the name agilis because he does not indicate
whether his measurements are of male or female animals; animals of the
two sexes of the same subspecies differ more in external measurements
than animals of the same sex of different subspecies of Peruvian
weasels.
Specimen no. 565, in the Polish Museum of Natural History, without
definite locality, is provisionally referred to this subspecies. The
specimen is intermediate in several respects between the female from
Macate and the one of macrura from Cutervo.
Tschudi (1844:111-112) has given the following account:
"Lebensweise und geographische Verbreitung. Das peruanische
Wiesel lebt auf den kalten, öden Hochebenen der Cordillera an
sonnigen Steinhaufen und Felsen gewöhnlich in Gesellschaft von
8-12 Stücken. Diese Thierchen sind so ausserordentlich behende und
scheu, dass bei dem leisesten Geräusche die ganze Schaar mit
Blitzesschnelle verschwindet. Es ist uns auch nie gelungen, eines
derselben zu erlegen. Die Indianer aber verstehen es, dieselben
lebendig einzufangen und zu zähmen. Ein sehr zahmes sahen wir bei
einer uns befreundeten Dame in Tarma; gegen alle Fremden biss es
mit Wuth und liess sich nicht anfassen, während es sich von seiner
Herrin Alles gefallen liess; sie öffnete ihm den Mund und steckte
ihm den Finger hinein, ohne dass es eine böse Miene dazu machte,
während es bei der geringsten Bewegung, die wir machten, es zu
ergreifen, grimmig auf uns lossprang. Wenn es eingeschüchtert
wurde, versteckte es sich in den Busen seiner Gebieterin und kroch
ihr bald nachher zum Aermel heraus. An den Wänden und Meublen
kletterte es mit grosser Behendigkeit und schlüpfte durch so
kleine Ritzen und Löcher, dass wir fast an der Möglichkeit dieses
Hindurchdringens gezweifelt haben würden, wenn wir es nicht selbst
mit angesehen hätten. Wenn es unartig war, wurde es mit einer
Schnur an seinem kleinen Halsbande festgebunden; dadurch vermehrte
sich sein Zorn, so dass es zuweilen gegen die Dame auffuhr.
Mehrmals verschwand es während 8-10 Tagen und kam dann plötzlich
wieder zum Vorschein. Seine Nahrung bestand in Gemüse und Fleisch,
besonders aber liebte es Zuckerbrod in Milch aufgeweicht; einmal
machte es sich an einen Kanarienvogel, den es auch tödtete. Es
erhielt seine Strafe und verschwand dann für immer. Die Indianer
sollen dieses Wiesel zum Fange der Viscacha abrichten (davon
weiter unten). Sie nennen es Comadreja, auch Ardilla. ([footnote]
Ardilla ist spanisch und heisst Eichhörnchen. Mit diesem Namen werden
sehr verschiedene Thiere bezeichnet; ausser dem Sc. variabilis und der
Galictis agilis auch noch mehrere Nager und einige Didelphysarten.) Die
Indianerinnen verfertigen sich aus dem kleinen Felle Geldbeutel. Des
Sonntags trifft man unter den vielen tausend Punaindianerinnen die nach
den grossen Dörfern der Sierra kommen, um ihre Einkäufe zu machen, kaum
ein halbes Dutzend, die nicht solche Börsen mit sich führten, und dann
kann man auch die verschiedensten Farbennuancen, die bei dieser Species
vorkommen, beobachten."
None of the three skulls referred to this subspecies shows infestation
of the frontal sinuses by parasites.
Specimens examined.—Total number, 4.
Perú: Macate, 1 (Field Mus. Nat. Hist.); Huarochirí, 1 (Mus. Comp.
Zool.); Lima, 1 (British Mus. Nat. Hist.); no locality more
definite than Perú, 1 (Mus. Polonais d'Hist. Nat.).
Mustela frenata macrura Taczanowski
Long-tailed Weasel
Plates 1, 27, 28, 29, 30, 37, 38, 39 and 40
Mustela macrura Taczanowski, Proc. Zoöl. Soc. London, 1874:311,
pl. 48, May 19, 1874; ibid., 1881:647, May 17, 1881; ibid.,
835, November 15, 1881; Lönnberg, Arkiv för Zool., 8 (no. 1):21,
1913 (?); Hollister, Proc. Biol. Soc. Washington, 28:143, July
10, 1914; Allen, Bull. Amer. Mus. Nat. Hist., 35:101, April 28,
1916; Lönnberg, Arkiv för Zool., 14 (no. 4):11, 1921.
Putorius (Gale) braziliensis frenatus, Coues, Fur-bearing
animals, p. 142, 1877.
Mustela jelskii Taczanowski, Proc. Zoöl. Soc. London, 1881:647,
May 17, 1881.
Mustela affinis, Lönnberg, Arkiv för Zool., 8 (no. 1):21, July
12, 1913.
Mustela aureoventris, Thomas, Proc. U. S. Nat. Mus., 58:224,
1920.
Mustela frenata macrura, Hall, Carnegie Instit. Washington Publ.
473:110, November 20, 1936; Hall, Physis, 16:176, 1939.
Type.—Male, adult, mounted skin, with skull separate; no. 561,
Mus. Polonais d'Hist. Nat. (Warsaw, Poland); Lake Junín, central
Perú; 1873; obtained by M. Jelski.
The skull (plates 27-29, 30), mounted with the skin but removed by
me for study, lacks the right jugal, the basisphenoid, the
basioccipital and parts of each exoccipital bearing the
exoccipital condyles. The right tympanic bulla, although detached
from the skull, is preserved separately. The teeth all are present
and entire. The skin is fairly-well mounted, in a good state of
preservation, and shows no fading due to exposure to light.
Range.—Altitudinally, 3200 (Guainche) to at least 12000 feet
(Pichincha); Upper Subtropical and Temperate life-zones of central
Perú and Ecuador north from the states of Apurimac and Cuzco,
Perú, to San Antonio, northern Ecuador. See figure 29 on page 221.
Characters for ready recognition.—Differs from Mustela
africana stolzmanni by absence of median, longitudinal, abdominal
stripe of same color as upper parts; presence of p2 and two roots
rather than one root on P2; from Mustela frenata helleri, M. f.
affinis and M. f. aureoventris by lighter color of upper parts
which are Chocolate tone 3, pl. 343, Oberthür and Dauthenay,
whereas, with reference to the same color standard, the colors
are: in helleri, Carbon Brown, tone 3, pl. 342; in affinis,
Reddish Black, tone 2, pl. 344; in aureoventris, Reddish Black,
tone 4, pl. 344; from M. f. agilis by darker color (Chocolate,
tone 3 rather than 2, pl. 343, Oberthür and Dauthenay) of upper
parts, length of upper tooth-rows, in females, more than 13,
inside length of P4 more than 4.6; from M. f. boliviensis by
lighter color of upper parts which are as above rather than tone 4
of Carbon Brown, pl. 342 of Oberthür and Dauthenay, and larger
size (in males, hind foot more than 45 and m1 more than 5.6).
Description.—Size.—Male (measurements as recorded by
Taczanowski in the original description, for two specimens, type
and topotype, with correction of the length of tail of his
"female" [= male]): Total length, 420, 415; length of tail, 150,
145; length of hind foot, 51, 51. An adult from Yana Mayo, Río
Tarma, was measured by Hendee as 394, 134. Hind foot relaxed
measures, 47. Tail 55 per cent as long as head and body. Length
of hind foot more than basal length.
Female (based on measurements given by Taczanowski (1881:647) of
no. 564): Total length, 323; length of tail, 120; length of hind
foot, 37. Tail 59 per cent as long as head and body. Length of
hind foot approximately equal to basal length.
Differences in external measurements of the two sexes are: Total
length, 87; length of tail, 23; length of hind foot, 13.
Externals.—Longest facial vibrissae extending beyond ear;
carpal vibrissae color of either upper parts or underparts;
hairiness of foot-soles as shown in figure 20.
Color.—(Based on specimens from Cutervo and south thereof).
Rarely few white hairs between eyes and in front of ears; top of
head posteriorly to slightly behind eyes, near (n)
Chestnut-Brown (Ridgway) and Carbon Brown, tone 2 or darker (pl.
342, Oberthür and Dauthenay); posterior half of upper lip rarely
white; dark spots at angles of mouth absent; tip of tail black;
remainder of upper parts near (l) Russet (Ridgway) and
Chocolate, tone 3 (pl. 343, Ober. and Dauth.); underparts white or
whitish on medial sides of forelegs, otherwise cream color with
tinge of Ochraceous-Buff; color of underparts extended distally on
posterior sides of forelegs to just below elbow (in type specimen)
or onto forefeet (in specimen from Yana Mayo) and on medial sides
of hind legs to points between knees and ankles. Least width of
color of underparts averages (in six skins) 17 (14-21) per cent of
greatest width of color of upper parts. Black tip of tail longer
than hind foot and averaging 36 (32-49) per cent of length of
tail-vertebrae.
Skull and teeth.—Male (based on type specimen and no. 562): See
measurements and plates 27-30; weight, not known; basilar length,
43.2 (40.8 and 45.5); zygomatic breadth more or less than distance
between condylar foramen and M1 and more than that between
anterior palatine foramen and anterior margin of tympanic bulla;
mastoid breadth more or less than postpalatal length; postorbital
breadth more than length of upper premolars and greater than width
of basioccipital, measured from medial margin of one foramen
lacerum posterior to its opposite; interorbital breadth more than
distance between foramen opticum and anterior margin of tympanic
bulla; breadth of rostrum less than length of tympanic bulla;
least width of palate more than inside length of P4; anterior
margin of tympanic bulla as far posterior to foramen ovale as
width of 4 (including I3) upper incisors; height of tympanic bulla
not more than distance from its anterior margin to foramen ovale;
length of tympanic bulla more than length of lower molar and
premolar tooth-row and longer or shorter than rostrum; anterior
margin of masseteric fossa below or behind m2.
Female (based on no. 564, from Cutervo, Perú, type specimen of
Mustela jelskii Taczanowski): See measurements and plates 37-40;
weight, not known; basilar length, 38±; zygomatic breadth less
than distance between condylar foramen and M1 and not greater than
that between anterior palatine foramen and anterior margin of
tympanic bulla; postorbital breadth more than alveolar length of
upper premolars and (probably) more than width of basioccipital
measured from medial margin of one foramen lacerum posterior to
its opposite; least width of palate more than inside length of P4;
tympanic bulla as far posterior to foramen ovale as width of at
least 5-1/2 upper incisors; height of tympanic bulla less than
distance from its anterior margin to foramen ovale; length of
tympanic bulla more than length of lower molar and premolar
tooth-row and longer or shorter than rostrum.
As compared with that of helleri, the skull of the male of macrura
from Junín southward has a lesser mastoid breadth, notably smaller
teeth, and a flatter skull which averages lighter throughout. The
skulls of females available indicate that the skull and teeth are
larger than in agilis.
Remarks.—Seven years after Taczanowski named this subspecies, he
applied the name jelskii to a female taken farther north than the
original examples of macrura. As indicated in synonymy, various other
names have been applied to animals included by the present author in
this subspecies.
Mustela frenata macrura intergrades with M. f. affinis as shown by
practically all the referred specimens from north of Junín. As one
proceeds northward the color of the weasels becomes progressively
darker and the teeth become larger until the conditions found in
affinis are met with near the northern border of Ecuador. From the
material available it appears that the light-colored upper parts found
in macrura characterize weasels of, at least, the Temperate Zone,
from Marcapata, Perú, to near Quito, Ecuador. West of the range of
macrura there exists the still lighter-colored subspecies, M. f.
agilis. Immediately adjacent on the north, east, and south,
darker-colored weasels occur. So far as color is concerned, the
geographic range of the subspecies M. f. macrura is not difficult to
define. However, the small size of the teeth characterizes only that
part of this light-colored subspecies from Junín southward including
the subspecies boliviensis at the southern extremity of the range of
the species. From Cutervo northward the light-colored weasels of the
Temperate Zone have teeth similar in size to those of the darker, more
northern affinis. To designate the slightly larger-toothed,
light-colored animals from Ecuador as a subspecies distinct from
affinis and macrura is one solution but at present it seems best to
refer all of these light-colored animals to macrura.
The type specimen and topotype no. 562 differ more in the amount of
inflation of the tympanic bullae than adult males of comparable ages
from a given locality usually do. In other respects, the differences
between the two skulls are not greater than those ordinarily found in
specimens from the same locality. No. 562 has the tympanic bullae
greatly, relative to the other South American weasels, inflated
posteriorly. Otherwise, the bullae agree with those of the type
specimen.
Specimens from southwestern Ecuador, average large, and include the
largest specimens of the species Mustela frenata seen from South
America. A subadult male, no. 61406, in the American Museum of Natural
History, is the largest. Its external measurements are 482, 191, 56.
The basilar length of the skull is 48.2 and the zygomatic breadth is
30.3. Although not so large as this specimen, the corresponding
measurements of specimens from Alamor, El Chiral, and even from as far
away as Sigsig also are distinctly large.
The skull of the female from Ollantaytambo and that of the male from
Marcapata have teeth equally as small as do the specimens from Lake
Junín.
The skin alone, no. 194328, from Ollantaytambo has the color of the
underparts extended over the entire upper sides of the forefeet. The
male from Marcapata has less of this color on the forefeet and is in
this respect intermediate between the specimens from Lake Junín and the
one from Ollantaytambo.
In size of teeth the female, type specimen of M. jelskii, from
Cutervo, shows an approach to the larger-toothed weasels of the
northern part of the range of macrura.
The specimens in the Riksmuseum from the vicinity of Quito, Ecuador,
have been rather fully described by Lönnberg (1921:11-17) and need
little comment here, except to say that they show, as he suggested,
that the weasel of the Temperate Zone of Ecuador is an intermediate
link between M. f. macrura and M. f. affinis.
The adult female and juvenal male labeled as from Ambato have little
left of the skulls except some of the teeth and the assignment of the
specimens to the subspecies macrura is made mainly on geographic
grounds. These two specimens probably are part of the shipment of birds
and mammals of which Chapman (1926:703) speaks as follows: "A small
collection of native-made skins purchased by the American Museum from a
commission merchant in New York City as from 'Ambato' proved to be from
the eastern slope of the Andes." Another skin in the same Museum,
labeled by a native collector as from "Baeza arriba" [= above Baeza] is
so dark colored and has the color of the underparts so much restricted,
as to suggest that it belongs to the race aureoventris. Possibly,
therefore, it was taken not at Baeza, Ecuador, which I find to the
eastward of Quito at 77° 55' W and O° 25' S, but at some place of the
same name on the Pacific Slope, unless the locality has been altogether
wrongly recorded on the label. If the specimen was taken near the Baeza
above referred to, then it gives evidence of an unnamed race of
Mustela on the eastern slope of the Andes, characterized by its dark
color. Unfortunately the specimen is young and its skull therefore
offers insufficient basis for the judging of its subspecific
relationships.
Other specimens, in the British Museum of Natural History, recorded as
taken "near Quito" and here tentatively listed under macrura, mostly,
include specimens so dark colored as to lead me to think they came from
country, lower than Quito, adjacent to the range of aureoventris.
Nematodes taken from the right frontal sinus of no. 562 from Junín
proved to belong to the superfamily Oxyuriodea according to Professor
W. B. Herms and Mr. O. L. Williams, who have independently identified
them. Because these worms had been dried fifty-five years in the
mounted specimen and were later boiled in cleaning the skull, a more
accurate determination was impossible and whether or not they pertain
to the same species found in North American weasels cannot be said. Of
18 adult skulls examined for this type of infestation, 13 were found
affected as judged by the evident malformation of the frontal region.
Specimens examined.—Total number, 74, arranged by localities
from north to south and unless otherwise indicated in the American
Museum of Natural History.
Ecuador: Ibarra, 6600 ft., and 7500 ft., 2[7]; San Antonio,
8000-8500 ft., 5 mi. N Quito, 4 (2[7], 2[78]); Nono, 10000 ft., 1;
Mindo, 1[78]; Zambiza, 8000-8100 ft., NE Quito, 4 (2[78], 2[95]);
Carapungo, 8500 ft., NE Quito, 1[78]; Panecillo, 10000 ft., near
Quito, 2[78]; Guapulo, 8800 ft., 3 mi. E of Quito, 1[78];
Pichincha, 10500 ft., and 12000 ft., 2 (1[78], 1[95]); San
Ignacio, 11500 ft., Pichincha, 1; Santa Rosa, 9600 ft., Río Pita,
2; near Santa Rosa, 9000 ft., 1; Río San Rafel, 9000 ft., 1; N
side Quito, 9000 ft., 1[78]; Quito, 1[4]; near Quito, 5[7]; Nára
Papallacta, 11000 ft., 1[78]; below Papallacta, 9000 ft., 1[78];
Chillo Valley, 1[78]; "Hacienda Hda," 10000 ft., Pintag, Valencia,
1; Baeza arriba, 1; Ambato, 2; San Francisco, 8000 ft., E of
Ambato, 1; Chunchí, Pagma Forest, 6400 ft., 1[1]; Canar, 2600 M.,
1[7]; Malletura, 7600 ft., 1; Contrayerbas, 11000 ft., 1; Sisig,
8500 ft., 3[7]; El Chiral, 1; Almor, 1; Guainche, 3200 ft., 1; no
locality more definite than Ecuador, 4[95]; "Received from Quito,"
1[7]; Quisaya, 6000 ft. (locality not found), 1[7]; La Carolina
(locality not found), 1[78].
Perú: La Lejía, 1; Huancabamba, 4 (2[75]); Cutervo, 9000 ft.,
1[73]; Condechacha, 7000 ft., Río Utcubamba, 1[7]; San Pedro,
8600-9400 ft., S of Chachapoyas, 1; Celendín, 1[7]; Junín, 2[73];
Yana Mayo, Río Tarma, 1[7]; Ollantaytambo, 9000 ft., 3 (1[7],
2[91]); Ocabamba, 1[7]; Anta Cuzco, 3400 and 3500 M., 2[4];
Marcapata, 1[91].
Mustela frenata boliviensis Hall
Long-tailed Weasel
Plates 28, 29 and 30
Mustela frenata boliviensis Hall, Proc. Biol. Soc. Washington,
51:67, March 18, 1938.
Mustela frenata macrura, Hall, Carnegie Instit. Washington Publ.
473:110, November 20, 1936; Hall, Physis, 16:176, 1939 (part).
Type.—Male, adult, skull and skin; no. 72587, Amer. Mus. Nat.
Hist.; Nequejahuira, 8000 feet, Bolivia; May 19, 1926; obtained by
G. H. H. Tate; original no. 4135 (see plates 28-30).
Range.—As now known 8000 to 9500 feet in the Andes from
Limbaní, Perú, south to Nequejahuira, Bolivia; upper Subtropical
and Temperate life-zones. See figure 29 on page 221.
Characters for ready recognition.—Differs from Mustela
africana stolzmanni by absence of median, longitudinal, abdominal
stripe of same color as upper parts; presence of p2 and two roots
rather than one root on P2; from Mustela frenata macrura by
darker color of upper parts (tone 4 or darker of Carbon Brown, pl.
342 rather than tone 3 of Chocolate, pl. 343, Oberthür and
Dauthenay) and lesser size (in males hind foot less than 45 and m1
less than 5.6); from Mustela frenata agilis by darker color of
upper parts (as given above rather than tone 2 of Chocolate, pl.
343, of Oberthür and Dauthenay).
Description.—Size.—Male: The type and two young specimens
from Limbaní, Perú, measure respectively, as follows: Total
length, 383, 368, 304; length of tail, 140, 132, 115; length of
hind foot, 43, 44, 41. Tail 55 per cent as long as head and body.
Length of hind foot approximately equal to basal length.
Female: Unknown.
Externals.—As described in Mustela frenata macrura.
Color.—Top of head blackish posteriorly to behind ears; upper
lips same color as upper parts of head; dark area at angle of
mouth not separated from upper parts as a distinct spot; tip of
tail black; remainder of upper parts near (n) Mars Brown of
Ridgway and tone 4 or darker of Carbon Brown (pl. 342, Oberthür
and Dauthenay); underparts Cream-Colored with strong wash of
Ochraceous-Buff; whitish on insides of forelegs to just below
elbow; color of underparts extended distally on forelegs over
ankles onto antipalmar faces of inner toes, and on hind legs to
knees. Least width of color of underparts averages 15 (11-19) per
cent of greatest width of color of upper parts. Black tip of tail
in type longer than hind foot and amounting to 36 per cent of
length of tail-vertebrae.
Skull and teeth.—Male (based on the type): See measurements and
plates 28-30. As described in Mustela frenata macrura except
that: Weight, 2.8 grams; basilar length, 41.6; zygomatic breadth
less than distance between anterior palatine foramen and anterior
margin of tympanic bulla; anterior margin of tympanic bulla as far
posterior to foramen ovale as width of 5 upper incisors.
Female: Skull unknown.
Remarks.—Apparently the first specimens of this race to find their
way into a zoölogical collection were the two young males taken on
February 17, 1904, at Limbaní, by Geo. Ockenden [sic.].
M. f. boliviensis is smaller than any other South American weasel
except possibly agilis. Better material of the two races probably
will show even agilis to be larger.
Early in my study of Mustela after examination of the one young
specimen, from Limbaní, in the United States National Museum, an
account of this race was drawn up, but the account was discarded for
want of satisfactory material and the animal was referred to macrura.
Then, in 1937, when the two other specimens were studied, the race was
formally characterized as different from previously recognized kinds.
The collector has noted on the labels of the two young from Limbaní
that they were shot in the afternoon when running together beneath
bushes. The frontal sinuses of the type are malformed as a result of
infestation by parasites.
Specimens examined.—Total number, 3, as follows:
Perú: Carabaya, Limbaní, 2 (one in U. S. Nat. Mus. and one in
Berlin Zool. Mus.).
Bolivia: Nequejahuira, 1 Amer. Mus. Nat. Hist.
Mustela frenata (?) gracilis (Brown)
Plates 39 and 40
Putorius gracilis Brown, Mem. Amer. Mus. Nat. Hist., 9(pt.
4):182, pl. 17, 1908.
Mustela gracilis, Hay, Iowa Geol. Surv. Bull., 23:32, 1914; Hay,
Carnegie Instit. Washington Publ. 322A:252, October 15, 1924;
Hay, Carnegie Instit. Washington Publ. 390(vol. 2):528, 1930.
Mustela frenata gracilis, Hall, Carnegie Instit. Washington Publ.
473:112, November 20, 1936.
Type.—Adult skull without lower jaws, probably of a female, no.
12431, Amer. Mus. Nat. Hist.; from Conard Fissure, four miles west
of Willcockson, Newton County, Arkansas; obtained sometime in the
period 1903 to 1905 inclusive. (See plates 39 and 40.)
Range.—Known only from the Pleistocene deposit in Conard
Fissure, at the type locality in northern Arkansas.
Description.—Skull. Probably female (based on the type): See
measurements and plates 39 and 40; weight unknown; basilar length,
38.1; least width of palate less than greatest length of P4;
tympanic bulla as far posterior to foramen ovale as width of 3 to
5 upper incisors; height of tympanic bulla less than distance from
its anterior margin to foramen ovale; length of tympanic bulla
less than length of rostrum.
Comparison and remarks.—The type specimen was the only individual
referred by Brown (1908) to this species. The remaining material of
weasels from this deposit was referred by Brown to his Putorius
cicognanii angustidens. Examination of the original materials
convinces the writer, too, that the specimens, except no. 12431, are
of the species erminea [= cicognanii of Brown]. No. 12431 itself
may possibly be erminea but is far more probably of the species
frenata. The uncertainty is due to the fact that an occasional skull
alone of a subadult male erminea is extremely difficult certainly to
distinguish from a skull alone of an adult female frenata. This is
true among Recent specimens in the northern Mississippi Valley today;
more exactly in Iowa and southern Minnesota the females of frenata,
oftentimes intergrades between the subspecies Mustela frenata
longicauda, M. f. noveboracensis and M. f. primulina, by only the
skulls are next to indistinguishable from certain, unusually slender
skulls of male erminea. At other places where the ranges of the two
species meet, this difficulty is not so often encountered. Also, the
type of gracilis has the skull broken in such a way that the
postglenoid length in relation to the length of the skull as a whole
could not be accurately determined in this particular skull.
The type specimen of gracilis surely is an adult and because of its
small size is thought to be a female. Of known long-tailed weasels of
the species frenata, gracilis is structurally nearest to M. f.
primulina which occurs in the same region today and to M. f.
noveboracensis, the long-tailed weasel of the eastern United States.
M. gracilis differs from noveboracensis and agrees with primulina
in possessing well-marked temporal ridges which fuse to form a low
sagittal crest, in having the mastoid processes projecting farther,
laterally, beyond the braincase, in having the anterior ends of the
tympanic bullae produced below the squamosal rather than on the same
plane with the squamosal, and in having the bullae more inflated
anteromedially. M. gracilis differs from both noveboracensis (97
♂ and 56 ♀ with skulls of comparable age) and primulina (64 ♂
and 24 ♀ with skulls of comparable age) in that the zygomatic breadth
amounts to less than 58 per cent of the basilar length. Another
difference from any one of the skulls of females of primulina is the
longer rostrum, which, when measured from the posterior base of the
postorbital process of the frontal to the anterior end of the nasal on
the same side, amounts to more than 35 per cent of the basilar length.
As pointed out by Brown (1908:182) this specimen represents the extreme
of slender skull among known kinds of American weasels.
Selected measurements of no. 12431, the type specimen of Mustela
gracilis, are as follows: Basilar length of Hensel, 38.1 mm.;
length of upper tooth-rows, 14.3 to 14.4; breadth of rostrum,
11.0; interorbital breadth, 8.5; orbitonasal length, 13.6; mastoid
breadth, 18.2; length of tympanic bulla, 13.0; breadth of tympanic
bulla, 6.3; depth of tympanic bulla, 3.25; outside length of P4,
4.5; inside length of P4, 4.7; breadth of M1, 3.4; length of inner
moiety of M1, 1.8; depth of skull at anterior margin of
basioccipital, 12.2; depth of skull at posterior borders of last
upper molars, 11.3; distance from foramen ovale to tympanic bulla,
3.6 mm.
MUSTELA AFRICANA Desmarest
Tropical Weasel
(Synonymy under subspecies)
Type.—Mustela africana Desmarest, Nouv. Dict. d'Hist. Nat.,
vol. 19, p. 376. 1818.
Range.—Known from the headwaters of the Amazon in eastern Perú
and from near the mouth of the same river, on its southern side in
Brazil, all within the Tropical Life-zone. See figure 29 on page
221.
Characters for ready recognition.—Differs from Mustela frenata,
the only geographically adjacent species of the genus, by: presence of
thenar pad on forefoot; presence of a longitudinal, median, abdominal
stripe of same color as upper parts; upper lips being broadly edged,
entirely round, with color of underparts; failure of longest facial
vibrissae to reach posterior margin of ear; absence of p2; relative
flatness (see pl. 29, fig. i and pl. 39, fig. h) of tympanic bullae.
Characters of the species.—Size large (total length of adults
approximately 500 mm.); foot-soles naked; thenar pad present on
forefoot; length of claws, measured on concave sides, less than one and
one-fourth times depth of claws measured at bases; longest facial
vibrissae not reaching posterior margin of ear; tail relatively long
haired; tail at all ages terminating in point as is characteristic of
only juveniles and very young of Mustela frenata and M. erminea;
tip of tail, and muzzle, only slightly darker than remainder of upper
parts; upper lips broadly edged with color of underparts; pelage
coarse, harsh and sparse; longitudinal, median, abdominal stripe of
same color as upper parts present; skull broad and deep; braincase
large, rounded, and much inflated anteriorly; palatal region wide;
tympanic bullae less inflated than in any other American species of the
subgenus; angle of lower jaw reduced; dental formula
3 1 2-3 1
-, -, ---, -;
3 1 2 2
teeth heavy; medial lobe of M1 but slightly larger than lateral lobe.
See plates 28, 29, 30, 39 and cranial measurements.
Geographic variation.—The reddish versus chocolate color of the
upper parts constitutes the only variation of a geographic nature so
far detected.
Remarks.—One of the most noteworthy of the several unique characters
of this large, tropical weasel is the longitudinal, median, abdominal
band. The species exhibits the minimum degree of development of certain
features that become progressively less apparent as one proceeds
southward from Central America. The relative uniformity of the
coloration of the upper parts (reduction in intensity of black color on
the muzzle and tip of the tail) and the reduction of the tympanic
bullae are two cases in point. Viewed dorsally the general outline of
the skull is most nearly matched by that of the skull of Mustela
frenata meridana from Venezuela or that of M. f. helleri from Perú.
However, the resemblance is not close. The tympanic bullae, although
unique among American weasels, are more like those of M. f. meridana
from Venezuela than like those of any other kind. The great postorbital
width (relatively less in M. africana than in several South American
subspecies of Mustela frenata) and small angular process of the
mandible are characters, in varying degrees, also common to all South
American weasels. Structurally M. africana clearly is more nearly
like other subspecies of M. frenata from South America than it is
like any species or subspecies from North America.
Mustela africana is the most primitive of the American weasels. The
distinctive cranial and dental characters, excepting the reduction in
number of premolars, are of a primitive nature. For example, the
relatively wide postorbital region, the large braincase that is
inflated anteriorly, and the flattened, tympanic bullae, are points of
resemblance to the holarctic Mustela erminea, which species is
regarded as nearest the original stem form; also the mentioned
characters correspond to ontogenetic stages passed through by other
weasels. Mostly on these accounts, one is led to look upon M.
africana as a migrant from North America. It may have become isolated
from its original stock, by a water barrier in the Central American
region, for a length of time sufficient to permit of a degree of
differentiation to develop between it and the North American weasels
which prevented crossbreeding with the frenata stock when that stock,
at a later time, reached South America. This assumption is suggested
only by evidence from the Recent specimens. No remains of true weasels
(subgenus Mustela) have been recorded from deposits in South America
older than the Recent period. The alternate possibility, that M.
africana intergrades with some race of M. frenata in western or
northern South America, has been considered and regarded as highly
improbable.
Cabrera (1940:15) has made the distinctive structural characters of
Mustela africana basis of the generic name Grammogale to include
the one species africana. I am inclined to accord Grammogale only
subgeneric rank.
It is possibly significant that Mustela africana is intermediate in
several respects between Lyncodon and typical Mustela. The median,
longitudinal, abdominal band of the same color as the upper parts in
M. africana and the relative uniformity of the coloration of its
upper parts might be considered as an intermediate stage between the
dark, bicolored (black muzzle and tail tip and brown body) upper parts
and light-colored underparts of the North American weasels on the one
hand and the light, unicolored upper parts and dark-colored underparts
of the Patagonian weasel (Lyncodon) on the other hand. The number of
premolars,
2-3
---,
2
is also intermediate between the numbers
3 2
- and -
3 2
of the North American Mustela and the Patagonian Lyncodon,
respectively. The American Mustela and the Patagonian Lyncodon,
respectively. The more medially, as opposed to anteriorly, directed
medial cusp of P4 (characteristic of approximately half of the
specimens examined), and the structure of the skull in general of M.
africana also seem to be morphologically intermediate between those
parts of Mustela and Lyncodon.
On the chance that Lyncodon is closely enough related to Mustela,
to be included in a group with Mustela rather than in a group with
Grisonella, it is worth noting that Lyncodon lujanensis Ameghino
(1889:324, 325), from the villa of Lujan and at the city of Córdoba, at
each place in the Pampean [= Pleistocene] formation of Argentine (see
also Cabrera, 1928:263) is the first and only fossil form of this group
recorded from the whole of South America. Actually, however, Lyncodon
seems to me to be as nearly related to Grisonella, if not more so,
than to Mustela. If Lyncodon is more closely related to
Grisonella and Grison than to Mustela, then the above remarked
intermediacy in characters of M. africana has more of interest as a
tendency to parallelism than it has of phylogenetic import. Appraisal
of phylogenetic relationships would require appraisal of the ancestral
stem forms of the Grison stock and the Mustela stock. None of
either is known from deposits of the Pliocene, the period of time
immediately preceding the Pleistocene.
None of the skulls of Mustela africana seen or figured has the
nasomaxillary sutures entirely obliterated and the specimens would,
judged on this character alone and by analogy with North American
species, be regarded as young and subadult. However, the sutures close
at what seems to be a later age than in M. frenata and M. erminea.
The condition of the mammae in the type specimen of M. stolzmanni and
in the specimen from Moyobamba, indicate that they have borne young.
North American weasels old enough to bear young lack visible traces of
the nasomaxillary sutures. I have examined no skulls of africana
with greatly worn teeth and hence cannot say if the sutures are
obliterated in advanced age.
If available data be correct, this species is unique within the genus
in that the two sexes are of approximately the same actual size and of
the same relative proportions in the body and in the skull. There was
no difference between individuals said to be of different sexes from
Pará, described and figured by Goeldi (1904:61-62, pls. 1, 2). The
undoubted female, type specimen of Mustela africana stolzmanni, is as
large as the undoubted male, no. 37475, of the same species, but of a
different subspecies, from Pará, Brazil. All the specimens of M. a.
africana that I have handled are labeled male and those of M. a.
stolzmanni female. More material may show that the female is smaller
than the male, as is the case in all near relatives of M. africana.
Little has been recorded concerning the habits of this species. Tate
(1931:254) states that a live individual which he saw in a cage at Pará
had been captured "swimming in the salt water of the estuary about half
a mile away from the shore." On the label of the specimen from
Moyobamba, there appears: "caught in Willow tree."
Subspecies examined.—All described forms, of which there are two.
Mustela africana africana Desmarest
Tropical Weasel
Plates 28, 29, 30 and 41
Mustela africana Desmarest, Nouv. Diction. d'Hist. Nat., 19:376,
1818; Cabrera, Bol. Real Soc. Españ. de Hist. Nat., 13:429,
November, 1913; Cabrera, Bol. Real Soc. Españ. de Hist. Nat.,
14:175, pl. 1, March, 1914.
Putorius (Mustela) brasiliensis paraensis, Goeldi, Zool. Jahrb.
abt. f. systematik, geogr. u., Biol., 10:556, pl. 21, September
15, 1897, type from Pará, Brazil, near Pará, Ward of Marco da
Legoa, Brazil; Goeldi, Bol. do Mus. Paraense, 3:195 [translation
of orig. descr.], August, 1901.
Putorius paraensis, Goeldi, Bol. do Museu Goeldi, 4:61, pls. 1,
2, 1904.
Mustela affinis paraensis, Hollister, Proc. Biol. Soc.
Washington, 28:143, July 10, 1914.
Mustela paraensis, Allen, Bull. Amer. Mus. Nat. Hist., 35:105,
April 28, 1916; Tate, Journ. Mamm., 12:253, August 24, 1931.
Mustela stolzmanni paraensis, Hall, Carnegie Instit. Washington
Publ. 473:111, November 20, 1936; Hall, Physis, 16:167, pl. 1,
figs. 1-4, 1939.
Type.—Male, adult or subadult, mounted; no. 848, Paris Museum;
from the "Cabinet de Lisbonne 1808," originally from South America
as determined from the characters of the animal; probably came
from Brazil, and for the present assumed to be from Pará.
On August 25, 1937, the skull was in the mounted skin and the
specimen was in the position shown in the figure published by
Cabrera (1914, pl. 1). Except for the loss of the distal part of
the tail, and fading because of exposure to light, the specimen
was in good condition. See also under remarks.
Range.—Known from the south side of the Amazon River, near its
mouth at Pará and Cametá, Río Tocantins, in the Tropical Life-zone
of Brazil. See figure 29 on page 221.
Characters for ready recognition.—Differs from Mustela
frenata, the only other geographically adjacent species of the
genus, in presence of median, longitudinal, abdominal stripe of
same color as upper parts and naked foot-soles, in absence of p2
and in reduced size of tympanic bullae (see pls. 29 and 30) and
from Mustela africana stolzmanni by lighter color of upper parts
which although near Chestnut-Brown are in adults 10' l (darker
in yg. M. C. Z., no. 30802), instead of 11' n as in M. a.
stolzmanni.
Description.—Size.—This is a relatively large weasel. Goeldi
(1897:559) gives the total length of the type specimen of his P.
b. paraensis, a female, as 520 mm. (495 in the flesh) and, by
computation from his figures, the length of the tail as 200 (205
in the flesh). These measurements probably include the hairs on
the tip of the tail as probably also do the measurements given of
two other specimens (see Goeldi, 1904:62). One of these specimens,
a female, measured: Total length, 520; length of tail, 200. The
other specimen, a male, measured: Total length, 510; length of
tail, 200. The skin of no. 37475, Amer. Mus. Nat. Hist., a male,
has the following measurements written on the attached label:
Total length, 548; length of tail, 234; length of hind foot, 56.
The hairs project 20 mm. beyond the tip of the last vertebra of
the tail and probably are included in the measurements of total
length and length of tail. Collectors' measurements of a young
male from Cametá, and a subadult labeled as male, from Pará
Murutucu, are respectively as follows: 500, 430; 210, 190; 50 and
54.
Externals.—Foot-soles naked, except for a few scattered hairs
on ventral sides of interdigital membranes; length of claws,
measured on concave sides, not more than one and one-fifth times
depth of claws measured at bases; carpal vibrissae not extending
beyond apical pad of first digit (not beyond hypothenar pad except
in one young specimen); longest facial vibrissae not extending to
posterior margin of ear; superior genal tuft not found, hairiness
of foot-soles as shown in figure 22.
Color.—Upper parts near (10 l) Chestnut-Brown and relatively
uniform since tip of tail and muzzle are only slightly darker than
remainder; underparts with longitudinal stripe of same color as
upper parts extending along median line of belly from throat or
breast posteriorly to within 40 to 50 millimeters of anus.
Underparts otherwise near (20" a) Olive-Ocher (lips and chin
whiter in one young specimen). Color of underparts extends
distally on median sides of forelegs to bare foot-soles and on
median side of hind legs two-thirds of distance from knee to
ankle. Upper lips broadly edged with whitish, which color passes
posteriorly below and not touching eye to ventral margin of concha
of ear. An inverted, basally broad, V-shaped extension passes
upward 4 millimeters, just posterior to the eye.
Skull and teeth.—See measurements (plates 28-30). Male: (based
on 3 adult and subadult topotypes and figures and descriptions
published by Goeldi, 1897 and 1904.) Weight, 7.0 grams; basilar
length, 45.8 (44.6-47.8); skull broad and deep; braincase large,
rounded, and much inflated anteriorly; distance from postorbital
process to anterior, nasal notch approximately equal to breadth
across exoccipital condyles; palatal region wide; tympanic bullae
less inflated than in any other species; mastoid bone, laterally,
concave; length of upper tooth-rows in adults and subadults less
than breadth of palate measured between two outer margins of
fourth upper premolars; alveolar distance between C1 and P4 less
than length of P4; teeth heavy; medial lobe of M1 only slightly
larger than lateral lobe; deuterocone of P4 heavy and often
inclined mesially; p2 absent (P2 present above on both sides in
only one of seven specimens seen or described); lower jaw heavy;
masseteric fossa not extending anteriorly to posterior fourth of
talonid of m1; paraconid of m1 low and base of cleft between it
and protoconid relatively low on tooth.
Female: No skull examined but from figures published by Goeldi
(1904, pl. 2), apparently as described in the male.
Remarks.—Desmarest in 1818 gave a remarkably good description of
this animal which he named as a new species, Mustela africana, but
mistakenly indicated that the single specimen known to him came
originally from Africa. Until 1913 the name was applied, wrongly, to
weasels of northern Africa or to those of the Azores Islands and St.
Thomas Island. In that year Cabrera (1913:429) identified the species
with the one later named Putorius (Mustela) brasiliensis paraensis by
Goeldi (1897:556, pl. 21) from Pará, Brazil. Despite Cabrera's clear
identification in 1913, and his later mention of the correct
application of the name Mustela africana, it was not correctly
employed by other authors, including myself who even as late as 1936
(p. 111) instead used Goeldi's name. In 1937 Mr. Cabrera called my
attention to his published account of Mustela africana and so
permitted me to examine the type specimen in the Paris Museum, whither
I was bound when I received Mr. Cabrera's letter. My own examination of
the specimen fully confirmed the conclusions published by Cabrera
(1913:429).
As a matter of historical interest, however, it is worth noting that
Cabrera (1913) originally supposed the type specimen to have been taken
as booty of war from Portugal by the French and that Cabrera later, at
the request of P. Trouessart, pointed out (1914:176) that the specimen
had been acquired in exchange ("a cambio") since according to Dr.
Trouessart the Museum register showed that offer had been made to
Portugal to return this and other specimens but that Portugal had
replied that it had nothing to reclaim. Dr. P. Rode in August, 1937, at
the Paris Museum, gave it to me as his opinion that the specimen had
been an outright gift from the "Cabinet de Lisbonne" to E. Geoffroy
St.-Hilaire on his trip to Portugal in 1808 when he was given also from
the same cabinet several primates, all from Brazil. Of the labels
attached to the pedestal on which the specimen is mounted, that of most
ancient appearance is glued to the bottom of the stand and bears in a
hand apparently written before Trouessart's entries on the same label,
the information "Du Cabinet de Lisbonne 1808" and "J. H. S. 1809."
The opened mouth of the mounted specimen permits one to determine that
P2 is absent on each side above. The stuffed scrotal pouch and hair
projecting downward about the preputial opening clearly show the animal
to have been a male. The least faded portions of the mounted specimen,
its sides, are of the same reddish color as characterizes adults from
Pará and not of the darker chocolate color of specimens of M.
stolzmanni from Perú. The specimen is indistinguishable from topotypes
of P. paraensis of Goeldi and his name will have to fall as a synonym
of Mustela africana Desmarest.
Goeldi gave an extended description, with figures of the skull, head,
and entire animal, when he named paraensis. As his account shows, he
was unaware that Taczanowski had described a similar weasel from the
headwaters of the Amazon, or for that matter that any weasel excepting
Mustela affinis Gray, had been found in South America. Goeldi's later
account of additional specimens (1904:61, pls. 1, 2) gives much useful
information about the animal. Photographs of several specimens and
photographs and detailed measurements of several skulls are presented
by him.
Pará, and Cametá, Brazil, places from which Mustela africana africana
is known, are nearly 2000 miles from the localities in eastern Perú and
eastern Ecuador from which M. a. stolzmanni is known, and no
specimens, from intermediate localities, are available to show actual
intergradation of the two. However, the similarity in structure of the
two weasels is so great as to indicate close affinity. Furthermore, it
is understood that environmental conditions at and between the two
localities are similar. These considerations, in the light of our
knowledge of actual intergradation of geographic races of weasels in
other places, cause me to treat, with a feeling of assurance, M.
africana [= P. paraensis Goeldi] and M. stolzmanni Taczanowski as
subspecies of a single species. M. Rodolpho Legueira Rodríguez writes
me, under date of June 16, 1928, that the type specimen of Putorius
(Mustela) brasiliensis paraensis Goeldi is stuffed and preserved in a
"vitrine" at the Museum Goeldi (Museum Paraense) De Historia Natural e
Ethnographia, Pará, Brazil.
The one young specimen seen, that from Cametá, is darker colored than
any of the four older specimens examined. It is almost exactly the
Chestnut Brown of Ridgway (1912) and therefore approaches closely in
color the adult specimens of M. a. stolzmanni. This same tendency to
greater richness of color in young than in adults is seen also in
Mustela frenata.
Specimens examined.—Total number, 5, all from eastern Brazil,
as follows: Pará, 2 (1[2], 1[7]); Pará Murutucu, 1[7]; Río
Tocantins, Cametá, 1[75]; type specimen, 1[84].
Mustela africana stolzmanni Taczanowski
Tropical Weasel
Plates 39 and 40
Mustela stolzmanni Taczanowski, Proc. Zoöl. Soc. London,
1881:835, November 15, 1881; Allen, Bull. Amer. Mus. Nat. Hist.,
35:105, April 28, 1916.
Mustela stolzmanni stolzmanni, Hall, Carnegie Instit. Washington
Publ. 473:111, November 20, 1936; Hall, Physis, 16:167, pl. 1,
figs. 5, 6, 1939.
Type.—Female, adult, mounted skin, with skull separate; no.
563, Mus. Polonais d'Hist. Nat. (Warsaw, Poland); Yurimaguas,
Perú; 1880; obtained by J. Stolzmann.
The skull (plate 40), mounted with the skin but removed by me for
study, consists of the premaxillae, maxillae, two halves of the
lower jaw and dentition. Of these parts, right m2, left coronoid
process, right P4 and M1 and adjacent part of maxilla are lost.
The skin is well mounted, in a good state of preservation and
shows no fading due to exposure to light. Inguinal mammae are
distinctly shown on the skin and prove that the specimen is a
female. Except for a few scattered hairs on the lower throat, a
spot six by eight millimeters on the medial side of the region of
the olecranon of the left foreleg and another of similar size in
the left axilla, the underparts are, excepting the ventral
longitudinal, abdominal stripe, unmarked by color of the upper
parts.
Range.—Known from the Tropical Life-zone of eastern Ecuador and
Perú from Jatun Yacu south to Valle del Perené. See figure 29 on
page 221.
Characters for ready recognition.—Differs from Mustela
frenata, the only other geographically adjacent species of the
genus, in presence of median, longitudinal, abdominal stripe of
same color as upper parts and naked foot soles, in absence of p2
and in reduced size of tympanic bullae (see pls. 28, 29, 30, 39
and 40) and from Mustela africana africana by darker color of
upper parts which, although near Chestnut Brown, are 11' n
instead of 10' l as in M. a. africana.
Description.—Size.—Male: unknown.
Female: Taczanowski (1881:836) gives, among others, the following
measurements of the type specimen: Total length, 523, length of
body, 260; length of tail without hair, 190 (with hair 224);
length of hind foot, 54. Whether or not the measurements were
taken from the animal when in the flesh I do not know. Allowing
for shrinkage of hind feet and changes due to the posture of the
now mounted specimen, I get from it essentially the same
measurements. Collectors' measurements of a subadult from
Moyobamba and a young female from Valle del Perené, are
respectively, as follows: 469, 415; 184, 160; 57, 52. My own
measurements of the dry hind feet on the skins are respectively,
48 and 49.
Externals.—As described in M. a. africana except that the
length of the concave sides of the claws are approximately one and
one-fourth times the depth; thus the claws are relatively longer
than in M. a. africana.
Color.—As described in M. a. africana with the following
noted exceptions: Upper parts near (11' n) Chestnut-Brown; area
of lighter ventral coloration on the throat and sides of head less
strongly tinged with yellow; pelage more dense, finer and softer
than in M. a. africana.
Skull and teeth.—Male: Skull unknown.
Female: See measurements and plate 39 and 40. As described in male
of Mustela africana africana except that: Weight, 4.7 grams. As
contrasted with M. a. africana, the dentition of the lower jaw
is lighter; the transverse diameter of m2 is 1.2 mm. in the type
and also in the specimen from Moyobamba as against 1.5 to 1.7 in
three male topotypes of M. a. africana.
Remarks.—After the Polish naturalist, Stolzmann, in the course of
his explorations in Perú, obtained the single specimen which was made
the type, no other naturalist, so far as known, visited the type
locality until thirty-two years later when Wilfred H. Osgood and M. P.
Anderson spent more than a month collecting at Yurimaguas (see Osgood,
1914:147), but secured no topotypes of this little-known weasel. C. O.
Schunke took the second specimen in the Valle del Perené in April,
1921; L. Rutter on January 25, 1924, took the third specimen, and W.
Clark-MacIntyre took the fourth specimen on the Jatun Yacu. This
obscure place name is shown on the map (fig. 4, p. 827) published by
Brown (1941) and is the stream flowing from the west to the town of
Napo. Napo is situated at approximately 1° 2' S and 77° 49' W.
In the female from Moyobamba there are only 3 pairs of mammae. One pair
is inguinal and two pairs are on the posterior part of the abdomen.
Taczanowski (1881:836) relates that this species was taken in the
forest to which it appears to be restricted since the inhabitants of
the village did not know of the animal. He points out also that the
previously known Peruvian species [M. f. macrura and M. f. agilis]
live in the treeless territory of eight to eleven thousand feet
altitude whereas M. stolzmanni was found in the humid forest of the
great plain of the Maynas at an elevation of 500 feet or less above sea
level. The frontal sinuses of the specimens seen reveal no malformation
as a result of infestation by parasites.
Specimens examined.—Total number, 4, as follows:
Ecuador: R. Tatun [= Jatun] = Yacu, 1, Mus. Comp. Zoöl.
Perú: Yurimaguas, 1 in Mus. Polonais d'Hist. Naturelle, Warsaw;
Moyobamba, 2700 ft. [6° S, 77° W], 1 in Brit. Mus. Nat. Hist.;
Valle del Perené, 1200 meters, 1 in Amer. Mus. Nat. Hist.
EXPLANATION OF CRANIAL MEASUREMENTS APPEARS ON PAGE 417
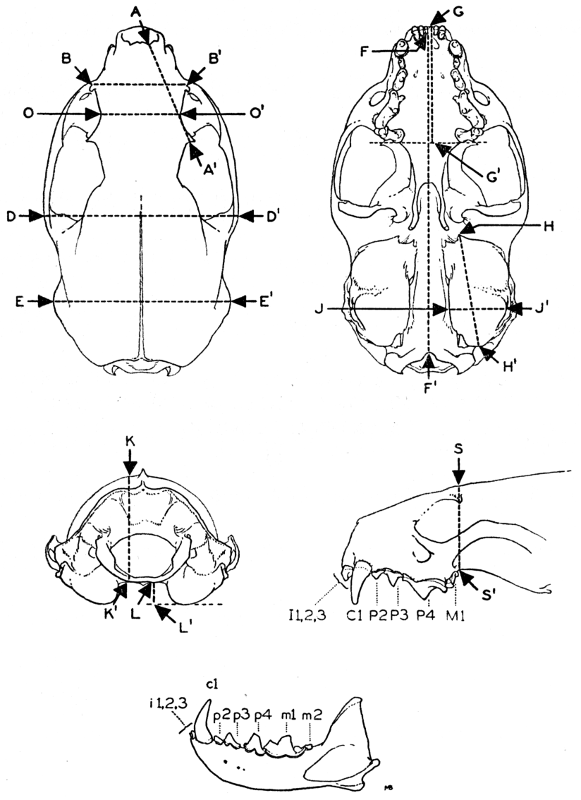
Fig. 31. Four views of the skull and a lateral view of
the left lower jaw to show points between which measurements of the
skull were taken. Based on M. f. primulina, from 3 mi. E Bergman,
Boone County, Arkansas, obtained December 12, 1933, by B. G. Roberts;
ad. ♀. 62854 Mus. Vert. Zoöl. × 1-2/5.
Basilar length (of Hensel).—From the anteriormost border of the
foramen magnum to a line connecting the posterior margins of the
alveoli of the first upper incisors. F to F' on fig. 31.
Condylobasal length.—Least distance from a line connecting the
posteriormost parts of the exoccipital condyles to a line
connecting the anteriormost projections of the premaxillary
bones.
Length of tooth-rows.—Least distance between a line connecting
posterior borders of upper molars and a line connecting anterior
faces of middle upper incisors. G to G' on fig. 31.
Breadth of rostrum.—Least distance from lateral base of hamular
process of lacrimal bone to corresponding point on opposite side
of skull. B to B' on fig. 31.
Interorbital breadth.—Least distance across top of skull between
orbits (eye sockets). O to O' on fig. 31.
Orbitonasal length.—Distance on anterior part of skull from
posterior margin of base of postorbital process of frontal bone
to posteriormost part of anterior border of nasal bone on same
side of skull. A to A' on fig. 31.
Mastoid breadth.—Greatest distance across mastoid bones
perpendicular to long axis of skull. E to E' on fig. 31.
Zygomatic breadth.—Greatest distance across zygomatic arches of
cranium perpendicular to long axis of skull. D to D' on fig. 31.
Tympanic bulla:
Length.—From posterior face to most anterior part of anterior
border. H to H' on fig. 31.
Breadth.—From bottom of pit immediately posterior to external
auditory meatus to medial face of bulla at right angle with
longitudinal axis of skull. J to J' on fig. 31.
Depth.—Least distance from ventral face of basioccipital,
excluding median ridge, to line touching ventralmost points of
the two bullae. L to L' on fig. 31.
m1, Length.—Greatest length which rarely or never is alveolar length.
P4.—
Lateral.—Length from posterior margin of tooth to anteriormost
part of the protocone (anterolateral cusp).
Medial.—Length from the posterior margin of tooth to
anteriormost part of the deuterocone (anterointernal cusp).
M1.—
Breadth.—Distance from medial edge of crown to lateral margin of
crown, approximately at a right angle with longitudinal axis of
the skull.
Length.—Greatest diameter, anteroposteriorly, of the inner lobe
or inner half of the tooth.
Depth of skull at anterior margin of basioccipital.—Measured from
anterior end of ventral face of basioccipital, excluding median
ridge, vertically to dorsal face of parietal excluding sagittal
crest. K to K' on fig. 31.
Depth of skull at posterior borders of Ms1.—Measured from ventral
face of palatine bones at posterior edge of upper molars to
dorsal face of frontals in plane of postorbital processes of
frontals. S to S' on fig. 31.
| Collection |
Catalog
Number |
Sex
and age |
Locality |
Basilar length (of
Hensel) |
Length of tooth
rows |
Breadth of rostrum |
Interorbital
breadth |
Orbitonasal length |
Mastoid breadth |
Zygomatic breadth |
Length |
Breadth |
Depth |
Length m1 |
Lateral |
Medial |
Breadth |
Length |
Depth of Skull at
Ant. margin of basioccipital |
Depth of Skull at
posterior borders of Msl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata
noveboracensis |
| average} |
|
♂ ad. 10 |
Adirondacks, New
York |
{45.1 |
16.6 |
13.7 |
10.9 |
15.3 |
23.3 |
26.7 |
15.4 |
7.8 |
3.5 |
5.9 |
5.3 |
5.5 |
4.3 |
2.4 |
14.1 |
12.7 |
| maximum} |
{47.0 |
17.4 |
14.6 |
11.9 |
15.9 |
24.7 |
28.0 |
16.2 |
8.4 |
4.0 |
6.2 |
5.7 |
5.9 |
4.5 |
2.7 |
14.5 |
13.3 |
| minimum} |
{43.2 |
15.9 |
13.0 |
10.4 |
14.8 |
22.0 |
25.3 |
14.6 |
7.0 |
3.1 |
5.5 |
4.9 |
4.9 |
4.0 |
1.9 |
13.5 |
12.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♂ ad. 10 |
Massachusetts |
{44.7 |
16.3 |
13.7 |
11.3 |
15.7 |
23.2 |
26.5 |
15.4 |
7.7 |
3.4 |
5.9 |
5.2 |
5.5 |
4.4 |
2.4 |
14.1 |
12.7 |
| maximum} |
{47.0 |
16.8 |
14.9 |
12.0 |
16.7 |
25.6 |
28.2 |
16.4 |
8.2 |
3.7 |
6.3 |
5.5 |
5.9 |
4.7 |
3.0 |
14.7 |
13.0 |
| minimum} |
{43.3 |
15.9 |
13.1 |
10.5 |
14.8 |
21.8 |
25.1 |
14.3 |
7.3 |
3.1 |
5.6 |
5.0 |
5.2 |
4.0 |
2.0 |
13.5 |
12.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♂ ad. 10 |
Liberty Hill,
Connecticut |
{44.0 |
16.3 |
13.2 |
10.9 |
15.3 |
22.4 |
26.3 |
15.0 |
7.5 |
3.3 |
5.8 |
5.1 |
5.4 |
4.2 |
2.2 |
14.3 |
12.6 |
| maximum} |
{46.0 |
17.4 |
14.4 |
12.0 |
16.4 |
23.5 |
28.0 |
15.9 |
8.0 |
3.6 |
6.3 |
5.6 |
6.1 |
5.0 |
2.5 |
15.2 |
13.2 |
| minimum} |
{41.5 |
15.2 |
12.1 |
9.7 |
14.2 |
20.8 |
24.0 |
14.4 |
6.9 |
3.0 |
5.3 |
4.6 |
5.0 |
3.7 |
2.0 |
13.4 |
11.7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♂ ad. 10 |
Beaver Dam,
Wisconsin |
{44.4 |
16.5 |
13.3 |
10.6 |
15.6 |
23.0 |
26.3 |
15.2 |
8.0 |
|
5.8 |
5.3 |
5.5 |
4.3 |
2.4 |
13.9 |
12.6 |
| maximum} |
{46.1 |
17.7 |
14.1 |
11.3 |
16.4 |
23.8 |
27.4 |
16.2 |
8.2 |
|
6.1 |
5.7 |
5.8 |
4.6 |
2.7 |
14.5 |
13.2 |
| minimum} |
{40.6 |
15.6 |
12.3 |
9.8 |
13.7 |
22.1 |
23.9 |
14.2 |
7.7 |
|
5.6 |
4.7 |
5.1 |
3.9 |
2.1 |
13.0 |
11.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♂ ad. 10 |
Washtenaw Co.,
Michigan |
{43.0 |
15.6 |
13.0 |
10.2 |
14.9 |
22.0 |
24.9 |
14.7 |
7.6 |
|
5.6 |
5.0 |
5.3 |
4.2 |
2.2 |
13.4 |
11.9 |
| maximum} |
{45.4 |
16.5 |
13.4 |
11.0 |
15.7 |
22.8 |
26.1 |
15.2 |
7.9 |
|
6.0 |
5.3 |
5.6 |
4.5 |
2.5 |
14.2 |
12.3 |
| minimum} |
{39.7 |
14.7 |
11.6 |
9.6 |
13.8 |
21.1 |
23.5 |
14.1 |
7.3 |
|
5.0 |
4.7 |
4.9 |
3.8 |
1.9 |
12.6 |
11.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♀ ad. 8 |
Adirondacks, New
York |
{37.5 |
13.4 |
10.7 |
8.7 |
12.9 |
18.7 |
20.9 |
13.5 |
6.5 |
2.8 |
4.8 |
4.3 |
4.6 |
3.5 |
1.7 |
11.4 |
10.2 |
| maximum} |
{39.8 |
14.5 |
11.2 |
9.3 |
13.4 |
19.3 |
22.1 |
14.3 |
7.0 |
2.9 |
5.2 |
4.7 |
5.0 |
3.7 |
1.9 |
12.1 |
10.8 |
| minimum} |
{36.4 |
12.8 |
9.8 |
7.9 |
12.1 |
17.5 |
20.0 |
12.6 |
5.9 |
2.5 |
4.5 |
4.0 |
4.2 |
3.2 |
1.7 |
10.3 |
9.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♀ ad. 5 |
Massachusetts |
{36.5 |
13.4 |
10.5 |
8.5 |
12.9 |
18.5 |
20.1 |
13.3 |
6.3 |
2.3 |
5.0 |
4.3 |
4.5 |
3.5 |
1.8 |
11.4 |
10.0 |
| maximum} |
{38.1 |
14.0 |
11.0 |
8.6 |
13.3 |
19.6 |
20.8 |
13.9 |
6.9 |
2.7 |
5.1 |
4.5 |
4.7 |
3.7 |
2.0 |
11.6 |
10.4 |
| minimum} |
{35.2 |
13.0 |
10.0 |
8.3 |
12.1 |
17.2 |
19.3 |
12.8 |
5.6 |
2.0 |
4.9 |
4.2 |
4.4 |
3.3 |
1.4 |
11.0 |
9.6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♀ ad. 6 |
Maryland |
{37.3 |
13.5 |
10.8 |
8.6 |
13.0 |
18.8 |
21.0 |
13.1 |
6.6 |
|
4.9 |
4.4 |
4.7 |
3.6 |
1.9 |
11.9 |
10.9 |
| maximum} |
{37.9 |
14.1 |
11.2 |
8.9 |
13.5 |
19.6 |
21.7 |
13.5 |
6.8 |
|
5.2 |
4.6 |
4.9 |
4.1 |
2.3 |
12.6 |
11.4 |
| minimum} |
{36.8 |
13.0 |
10.4 |
8.5 |
12.8 |
18.2 |
20.1 |
13.0 |
6.4 |
|
4.7 |
4.2 |
4.5 |
3.1 |
1.4 |
11.6 |
10.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♀ ad. 5 |
Beaver Dam,
Wisconsin |
{37.2 |
13.4 |
10.6 |
8.8 |
13.0 |
18.7 |
21.0 |
13.5 |
6.5 |
|
4.9 |
4.5 |
4.6 |
3.5 |
1.8 |
11.6 |
10.2 |
| maximum} |
{37.6 |
13.7 |
11.3 |
9.7 |
13.5 |
19.5 |
21.7 |
13.8 |
6.8 |
|
5.0 |
4.5 |
4.8 |
3.7 |
2.1 |
12.3 |
10.3 |
| minimum} |
{37.0 |
12.9 |
10.0 |
8.0 |
12.0 |
17.6 |
20.2 |
13.1 |
6.1 |
|
4.9 |
4.4 |
4.3 |
3.3 |
1.5 |
10.8 |
9.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♀ ad. 9 |
Washtenaw Co.,
Michigan |
{36.5 |
13.1 |
10.5 |
8.4 |
12.6 |
18.5 |
20.5 |
13.2 |
6.3 |
|
4.8 |
4.3 |
4.5 |
3.5 |
1.8 |
11.8 |
10.3 |
| maximum} |
{37.5 |
13.7 |
11.0 |
8.8 |
13.1 |
19.1 |
21.3 |
13.8 |
6.7 |
|
5.0 |
4.5 |
4.7 |
3.7 |
2.0 |
12.2 |
10.7 |
| minimum} |
{35.9 |
12.8 |
9.8 |
7.8 |
12.0 |
18.1 |
19.5 |
12.9 |
6.0 |
|
4.6 |
4.1 |
4.3 |
3.3 |
1.5 |
11.3 |
10.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata occisor
|
| A. N. S. P. |
4137 |
♂ ad. |
Washington Co., Maine |
45.8 |
16.5 |
13.3 |
11.0 |
16.2 |
24.5 |
27.5 |
15.7 |
8.5 |
3.5 |
6.2 |
5.6 |
5.9 |
4.6 |
2.8 |
13.3 |
13.0 |
| M. C. Z. |
7267 |
♂ ad. |
Moose Head Lake, Maine |
44.9 |
16.6 |
14.5 |
11.8 |
15.6 |
24.2 |
27.7 |
15.2 |
7.7 |
3.1 |
6.0 |
5.2 |
5.6 |
4.2 |
2.4 |
14.2 |
13.0 |
| M. C. Z. |
5501 |
♂ ad. |
Bucksport, Maine |
46.9 |
17.1 |
14.4 |
11.0 |
15.8 |
24.0 |
28.0 |
16.4 |
8.0 |
4.0 |
6.0 |
5.8 |
6.1 |
4.5 |
2.6 |
14.7 |
13.2 |
| M. C. Z. |
9142 |
♂ ad. |
Bucksport, Maine |
45.0 |
16.1 |
14.0 |
11.2 |
15.3 |
24.2 |
27.4 |
15.8 |
7.3 |
2.8 |
6.1 |
5.3 |
5.6 |
4.3 |
2.4 |
14.5 |
12.6 |
|
|
av |
|
45.7 |
16.6 |
14.1 |
11.3 |
15.7 |
24.2 |
27.7 |
15.8 |
7.9 |
3.4 |
6.1 |
5.5 |
5.8 |
4.4 |
2.6 |
14.2 |
13.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| M. C. Z. |
9101 |
♀ sad. |
Bucksport, Maine |
37.7 |
14.0 |
11.6 |
9.1 |
13.0 |
19.0 |
21.7 |
14.1 |
6.3 |
2.6 |
5.2 |
4.6 |
4.6 |
3.8 |
2.1 |
12.5 |
10.5 |
| M. C. Z. |
9122 |
♀ sad. |
Bucksport, Maine |
38.2 |
13.8 |
11.5 |
9.2 |
13.6 |
19.5 |
21.8 |
13.6 |
6.5 |
2.5 |
4.6 |
4.3 |
4.5 |
3.5 |
1.7 |
12.3 |
10.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata primulina |
| average} |
|
♂ ad. 10 |
Douglas Co.,
Kansas |
{44.8 |
16.8 |
13.5 |
10.6 |
14.9 |
24.0 |
27.2 |
15.5 |
8.3 |
3.4 |
6.1 |
5.5 |
5.8 |
4.6 |
2.4 |
14.2 |
12.8 |
| maximum} |
{46.0 |
17.8 |
14.1 |
11.5 |
15.4 |
24.9 |
28.2 |
16.3 |
8.8 |
3.9 |
6.6 |
5.9 |
6.1 |
4.7 |
2.6 |
14.8 |
13.2 |
| minimum} |
{43.8 |
16.2 |
12.9 |
10.0 |
14.3 |
23.2 |
26.2 |
14.4 |
8.0 |
3.0 |
5.8 |
5.2 |
5.5 |
4.3 |
2.2 |
13.6 |
12.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♂ ad. an d
sad. 8 |
Boone Co.,
Arkansas |
{44.7 |
16.5 |
13.2 |
10.5 |
15.0 |
24.1 |
26.9 |
15.5 |
8.1 |
3.6 |
6.0 |
5.5 |
5.6 |
4.3 |
2.2 |
14.2 |
12.3 |
| maximum} |
{46.5 |
17.1 |
14.0 |
11.8 |
16.3 |
24.8 |
27.7 |
16.4 |
8.8 |
4.2 |
6.5 |
6.0 |
6.2 |
4.9 |
2.5 |
14.6 |
13.0 |
| minimum} |
{42.5 |
15.6 |
12.6 |
9.7 |
14.1 |
22.8 |
26.1 |
14.9 |
7.5 |
3.1 |
5.8 |
5.2 |
5.2 |
4.0 |
2.0 |
13.9 |
11.7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♀ ad. an d
sad. 11 |
Douglas Co.,
Kansas |
{38.9 |
14.4 |
11.3 |
8.6 |
12.9 |
20.3 |
22.6 |
13.5 |
6.9 |
2.9 |
5.2 |
4.8 |
5.0 |
3.5 |
1.8 |
12.6 |
11.2 |
| maximum} |
{40.7 |
15.3 |
12.0 |
9.2 |
13.4 |
21.4 |
23.8 |
15.1 |
7.5 |
3.3 |
5.7 |
5.2 |
5.4 |
4.2 |
2.1 |
13.5 |
11.7 |
| minimum} |
{37.6 |
13.8 |
10.8 |
7.9 |
12.4 |
18.8 |
21.1 |
13.0 |
6.3 |
2.5 |
4.8 |
4.5 |
4.6 |
3.4 |
1.5 |
11.7 |
10.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♀ ad. an d
sad. 6 |
Arkansas |
{39.3 |
14.1 |
11.4 |
8.7 |
13.1 |
20.3 |
23.1 |
14.0 |
6.9 |
2.9 |
5.2 |
4.8 |
5.0 |
3.8 |
1.9 |
12.5 |
10.7 |
| maximum} |
{40.1 |
14.6 |
11.9 |
8.8 |
13.7 |
21.0 |
23.8 |
14.5 |
7.2 |
3.0 |
5.5 |
5.0 |
5.3 |
4.2 |
2.0 |
13.0 |
11.8 |
| minimum} |
{38.8 |
13.7 |
11.0 |
8.5 |
12.6 |
19.8 |
22.5 |
13.5 |
6.5 |
2.8 |
5.0 |
4.5 |
4.7 |
3.5 |
1.7 |
12.3 |
10.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata arthuri |
| U. S. N. M. |
246345 |
♂ ad. |
Convent, Louisiana |
43.4 |
15.4 |
13.7 |
11.5 |
14.5 |
22.3 |
26.6 |
15.4 |
7.7 |
3.3 |
5.4 |
4.9 |
5.1 |
3.7 |
2.0 |
14.8 |
12.4 |
| M. V. Z. |
37515 |
♂ sad. |
Remy, Louisiana |
43.8 |
16.4 |
12.7 |
10.9 |
15.0 |
22.3 |
25.5 |
15.5 |
7.5 |
3.7 |
4.9 |
5.7 |
5.8 |
4.2 |
2.3 |
14.2 |
12.4 |
| M. V. Z. |
38901 |
♂ sad. |
Springville, Louisiana |
*45.0 |
*16.5 |
|
|
15.5 |
|
|
16.0 |
8.3 |
|
5.8 |
5.5 |
5.7 |
4.6 |
2.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata olivacea |
| M. V. Z. |
47165 |
♂ ad. |
Talbot Co., Georgia |
50.1 |
18.3 |
15.0 |
12.5 |
16.5 |
26.7 |
30.8 |
17.2 |
9.2 |
4.0 |
6.8 |
6.2 |
6.2 |
4.7 |
2.5 |
15.0 |
13.8 |
| M. V. Z. |
47144 |
♂ ad. |
Talbot Co., Georgia |
49.2 |
18.3 |
13.8 |
11.7 |
16.9 |
26.0 |
29.0 |
17.9 |
9.4 |
4.2 |
6.8 |
6.0 |
6.3 |
4.8 |
2.8 |
15.5 |
13.4 |
| M. V. Z. |
47166 |
♂ ad. |
Talbot Co., Georgia |
48.5 |
17.6 |
14.6 |
11.5 |
16.5 |
27.0 |
30.9 |
17.5 |
9.8 |
4.3 |
6.8 |
5.7 |
6.0 |
4.5 |
2.5 |
15.6 |
13.2 |
| M. V. Z. |
47167 |
♂ ad. |
Talbot Co., Georgia |
45.8 |
17.3 |
14.2 |
11.5 |
16.2 |
25.7 |
29.4 |
15.9 |
8.7 |
4.1 |
6.1 |
5.6 |
6.0 |
4.9 |
2.3 |
15.4 |
13.7 |
| M. V. Z. |
47147 |
♂ ad. |
Talbot Co., Georgia |
48.1 |
18.0 |
14.0 |
10.9 |
16.9 |
26.2 |
28.9 |
17.2 |
8.8 |
4.2 |
6.1 |
5.7 |
6.2 |
4.6 |
2.3 |
14.5 |
13.2 |
|
|
av. 5 |
|
48.3 |
17.9 |
14.3 |
11.6 |
16.6 |
26.3 |
29.8 |
17.1 |
9.2 |
4.2 |
6.5 |
5.8 |
6.1 |
4.7 |
2.5 |
15.2 |
13.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| M. V. Z. |
41023 |
♂ ad. |
Thomas Co., Georgia |
48.8 |
17.5 |
14.9 |
11.9 |
16.0 |
27.3 |
31.2 |
17.9 |
8.7 |
4.2 |
6.4 |
5.9 |
6.3 |
4.9 |
2.5 |
17.0 |
13.4 |
| M. V. Z. |
41025 |
♂ ad. |
Grady Co., Georgia |
44.8 |
16.9 |
13.2 |
10.9 |
15.5 |
24.7 |
28.3 |
16.8 |
8.8 |
3.9 |
6.2 |
5.7 |
5.8 |
4.3 |
2.4 |
15.0 |
13.3 |
| M. V. Z. |
40934 |
♂ ad. |
Grady Co., Georgia |
47.8 |
17.4 |
14.1 |
12.1 |
15.5 |
25.1 |
29.1 |
17.9 |
8.8 |
4.5 |
6.6 |
6.0 |
6.0 |
4.7 |
2.6 |
15.0 |
14.2 |
| M. V. Z. |
40935 |
♂ ad. |
Grady Co., Georgia |
47.4 |
18.0 |
13.6 |
11.7 |
16.2 |
24.1 |
29.1 |
15.8 |
8.5 |
4.5 |
6.4 |
5.7 |
6.0 |
4.6 |
2.2 |
15.0 |
13.5 |
|
|
av. 4 |
|
47.2 |
17.5 |
14.0 |
11.7 |
15.8 |
25.3 |
29.4 |
17.1 |
8.7 |
4.3 |
6.4 |
5.8 |
6.0 |
4.6 |
2.4 |
15.5 |
13.6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| U. S. N. M. |
223880 |
♂ ad. |
Okefinokee Swamp |
49.0 |
18.8 |
14.2 |
11.6 |
14.6 |
26.1 |
30.4 |
16.5 |
8.6 |
4.4 |
6.1 |
5.7 |
6.0 |
4.6 |
2.4 |
15.6 |
13.2 |
| Cornell |
198 |
♂ ad. |
Okefinokee Swamp |
47.3 |
18.0 |
14.0 |
11.4 |
16.3 |
26.8 |
29.8 |
17.2 |
8.8 |
4.3 |
6.5 |
5.8 |
6.3 |
4.6 |
2.5 |
14.8 |
13.2 |
| Cornell |
652 |
♂ ad. |
Okefinokee Swamp |
47.0 |
17.0 |
13.8 |
11.8 |
16.0 |
25.8 |
30.5 |
17.3 |
8.6 |
4.5 |
6.2 |
5.8 |
6.0 |
4.5 |
2.5 |
15.5 |
13.6 |
| U. S. N. M. |
180802 |
♂ ad. |
Autaugaville, Alabama |
46.5 |
17.3 |
13.4 |
10.7 |
16.3 |
25.5 |
29.2 |
15.6 |
8.3 |
3.7 |
6.8 |
5.8 |
6.1 |
4.9 |
2.6 |
14.5 |
13.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| M. V. Z. |
51527 |
♀ ad. |
Talbot Co., Georgia |
43.5 |
16.4 |
12.5 |
9.8 |
14.5 |
22.4 |
25.7 |
15.5 |
7.8 |
3.8 |
5.8 |
5.6 |
5.8 |
4.2 |
2.2 |
13.5 |
12.3 |
| M. V. Z. |
41024 |
♀ ad. |
Thomas Co., Georgia |
42.7 |
16.0 |
12.0 |
9.6 |
14.7 |
22.8 |
26.0 |
15.8 |
8.0 |
3.9 |
5.8 |
5.0 |
5.4 |
4.1 |
2.1 |
14.4 |
12.2 |
| M. V. Z. |
41022 |
♀ ad. |
Thomas Co., Georgia |
44.0 |
16.1 |
12.8 |
10.6 |
15.3 |
23.4 |
25.8 |
15.3 |
8.2 |
3.6 |
6.1 |
5.7 |
5.8 |
4.4 |
2.1 |
13.2 |
12.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata peninsulae |
| F. S. M. |
49387 |
♂ ad. |
Apopka, Florida |
49.8 |
18.6 |
15.1 |
12.2 |
15.8 |
27.8 |
31.4 |
17.6 |
10.2 |
4.8 |
6.3 |
5.9 |
6.2 |
4.7 |
2.4 |
15.3 |
14.6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| A. N. S. P. |
9379 |
♀ ad. |
Tarpon Springs, Florida |
44.2 |
16.6 |
13.8 |
11.0 |
15.8 |
23.7 |
27.1 |
16.4 |
8.4 |
4.3 |
6.3 |
5.7 |
5.7 |
4.1 |
1.9 |
13.3 |
12.9 |
| A. N. S. P. |
8515 |
♀ yg. |
Pasco Co., Florida |
|
15.9 |
11.8 |
9.6 |
14.2 |
|
|
|
|
|
6.4 |
5.7 |
5.9 |
4.6 |
2.0 |
|
12.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata spadix
|
| M. V. Z. |
53795 |
♂ ad. |
Elk River, Minnesota |
49.2 |
19.1 |
15.9 |
11.9 |
17.1 |
28.0 |
31.7 |
15.9 |
9.5 |
3.6 |
6.9 |
6.5 |
6.7 |
5.4 |
2.7 |
15.5 |
14.0 |
| Walker |
A23 |
♂ ad. |
Elk River, Minnesota |
49.0 |
18.7 |
15.9 |
13.5 |
16.5 |
28.1 |
32.1 |
16.4 |
9.7 |
4.0 |
6.8 |
6.2 |
6.7 |
4.9 |
2.0 |
15.9 |
15.0 |
| Walker |
A37 |
♂ sad. |
Elk River, Minnesota |
48.7 |
18.9 |
15.2 |
11.9 |
16.5 |
27.0 |
29.9 |
15.5 |
9.2 |
3.8 |
6.6 |
6.1 |
6.5 |
5.0 |
3.0 |
15.0 |
13.8 |
| Dickey |
A865 |
♂ sad. |
Elk River, Minnesota |
46.8 |
17.6 |
15.4 |
|
16.7 |
26.1 |
|
15.2 |
8.4 |
3.3 |
6.2 |
5.5 |
5.9 |
4.3 |
2.3 |
|
|
| Dickey |
A846 |
♂ sad. |
Elk River, Minnesota |
46.1 |
18.0 |
14.2 |
10.6 |
15.5 |
24.9 |
28.2 |
14.8 |
8.5 |
3.7 |
6.6 |
5.9 |
6.6 |
4.8 |
2.9 |
15.1 |
12.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Dickey |
11548 |
♀ ad. |
Elk River, Minnesota |
42.3 |
16.4 |
13.1 |
10.7 |
15.0 |
22.9 |
27.0 |
13.8 |
7.7 |
3.1 |
6.6 |
5.6 |
5.6 |
4.4 |
2.1 |
13.0 |
12.3 |
| Walker |
A174 |
♀ ad. |
Elk River, Minnesota |
43.2 |
16.2 |
13.8 |
10.8 |
15.3 |
24.5 |
26.8 |
14.5 |
8.6 |
3.7 |
5.8 |
5.3 |
5.5 |
4.5 |
2.1 |
13.9 |
13.2 |
| Dickey |
9688 |
♀ ad. |
Elk River, Minnesota |
43.2 |
16.8 |
12.7 |
10.5 |
15.0 |
23.3 |
25.6 |
15.2 |
7.6 |
3.2 |
5.9 |
5.4 |
5.7 |
4.1 |
2.0 |
14.0 |
12.2 |
| U. S. N. M. |
188410 |
♀ ad. |
Elk River, Minnesota |
43.0 |
16.2 |
13.2 |
10.7 |
14.9 |
23.3 |
26.0 |
14.7 |
8.1 |
3.3 |
5.7 |
5.3 |
5.6 |
4.3 |
2.4 |
13.8 |
12.8 |
|
|
av. 4 |
|
42.9 |
16.4 |
13.2 |
10.7 |
15.1 |
23.4 |
26.4 |
14.6 |
8.0 |
3.3 |
6.0 |
5.4 |
5.6 |
4.3 |
2.2 |
13.7 |
12.6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata longicauda |
| A. M. N. H. |
15875 |
♂ ad. |
Red Deer, Alberta |
46.5 |
18.6 |
15.1 |
12.1 |
15.3 |
25.3 |
30.8 |
15.2 |
8.9 |
3.4 |
6.9 |
6.3 |
6.5 |
4.9 |
2.6 |
15.0 |
14.6 |
| N. M. C. |
8060 |
♂ ad. |
Sweet Grass Hills, Alberta |
45.5 |
18.1 |
15.5 |
12.2 |
15.5 |
|
31.0 |
|
|
|
6.9 |
6.3 |
6.6 |
5.0 |
2.5 |
|
13.8 |
| F. M. N. H. |
7021 |
♂ ad. |
Canadian Nat. Park, Alberta |
46.8 |
17.8 |
13.8 |
11.4 |
16.0 |
25.7 |
30.4 |
15.7 |
8.7 |
3.9 |
6.3 |
5.7 |
5.9 |
4.4 |
1.9 |
15.5 |
13.4 |
| F. M. N. H. |
8567 |
♂ ad. |
Calgary, Alberta |
44.7 |
17.2 |
13.9 |
11.3 |
14.8 |
24.8 |
29.7 |
15.5 |
8.7 |
3.6 |
6.0 |
5.4 |
5.6 |
4.3 |
2.4 |
15.0 |
13.4 |
| U. S. N. M. |
75725 |
♂ ad. |
St. Albert, Alberta |
46.5 |
18.3 |
15.0 |
12.4 |
15.6 |
25.0 |
29.4 |
15.2 |
8.3 |
3.4 |
6.6 |
6.2 |
6.4 |
4.6 |
2.0 |
16.0 |
13.6 |
|
|
av. 5 |
|
46.0 |
17.9 |
14.7 |
11.9 |
15.4 |
25.2 |
30.3 |
15.4 |
8.6 |
3.6 |
6.5 |
6.0 |
6.2 |
4.6 |
2.3 |
15.4 |
13.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| N. M. C. |
6968 |
♀ ad. |
Daysland, Alberta |
43.7 |
16.8 |
13.1 |
|
14.9 |
24.0 |
26.1 |
14.7 |
8.3 |
3.1 |
5.7 |
5.2 |
5.4 |
4.3 |
2.0 |
13.3 |
12.2 |
| U. S. N. M. |
68731 |
♀ ad. |
S. Edmonton, Alberta |
42.5 |
16.0 |
13.4 |
10.3 |
14.3 |
24.1 |
26.7 |
14.5 |
8.8 |
3.3 |
5.6 |
5.4 |
5.5 |
4.3 |
2.3 |
14.0 |
12.5 |
| A. M. N. H. |
16044 |
♀ ad. |
Blindman River, Alberta |
40.0 |
15.1 |
12.2 |
8.8 |
13.2 |
22.5 |
24.5 |
13.4 |
8.2 |
2.8 |
5.6 |
5.0 |
5.0 |
4.0 |
2.0 |
12.8 |
11.5 |
| M. V. Z. |
53792 |
♀ ad. |
Grafton, North Dakota |
42.8 |
16.5 |
13.6 |
10.5 |
14.9 |
23.2 |
26.1 |
15.3 |
8.0 |
2.8 |
6.1 |
5.4 |
5.6 |
4.3 |
2.4 |
14.0 |
12.3 |
| U. S. N. M. |
75483 |
♀ ad. |
Wingard, Sask. |
42.3 |
16.9 |
12.9 |
9.8 |
15.5 |
23.3 |
25.9 |
14.8 |
8.3 |
3.2 |
6.4 |
5.9 |
6.1 |
4.8 |
2.4 |
14.3 |
12.1 |
|
|
av. 5 |
|
42.3 |
16.3 |
13.0 |
9.9 |
14.6 |
23.4 |
25.9 |
14.6 |
8.3 |
3.0 |
5.9 |
5.4 |
5.5 |
4.3 |
2.2 |
13.7 |
12.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata oribasus |
| M. V. Z. |
44568 |
♂ sad. |
Barkerville reg., B. C. |
48.8 |
19.1 |
17.2 |
13.0 |
17.7 |
28.5 |
32.2 |
17.4 |
9.8 |
3.2 |
6.8 |
6.2 |
6.4 |
5.3 |
2.8 |
15.5 |
14.6 |
| M. V. Z. |
43817 |
♂ sad. |
Isaacs Lake, British Columbia |
48.8 |
19.5 |
15.8 |
12.0 |
17.3 |
28.4 |
31.7 |
15.9 |
9.7 |
4.0 |
6.9 |
6.3 |
6.4 |
5.3 |
2.9 |
15.5 |
13.7 |
| Cowan |
443 |
♂ ad. |
Quesnel, British Columbia |
46.6 |
17.7 |
14.5 |
10.1 |
15.9 |
26.4 |
30.7 |
15.8 |
9.2 |
3.9 |
6.2 |
5.8 |
6.1 |
4.6 |
2.5 |
15.0 |
13.1 |
| N. M. C. |
2676 |
♂ ad. |
Lillooet, British Columbia |
47.5 |
18.5 |
14.3 |
10.3 |
15.9 |
25.5 |
30.1 |
15.2 |
9.1 |
3.8 |
6.4 |
5.9 |
6.3 |
4.6 |
2.5 |
15.0 |
13.1 |
| N. M. C. |
2695 |
♂ ad. |
Lillooet, British Columbia |
45.0 |
17.5 |
15.9 |
11.8 |
15.9 |
25.6 |
31.0 |
15.2 |
8.9 |
3.0 |
6.2 |
5.6 |
6.1 |
4.7 |
2.9 |
15.0 |
13.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| M. C. Z. |
9058 |
♀ ad. |
Source Kettle River, B. C. |
41.7 |
16.4 |
12.8 |
10.5 |
14.8 |
24.9 |
26.7 |
14.3 |
8.2 |
2.8 |
6.1 |
5.5 |
5.7 |
4.5 |
2.5 |
14.0 |
12.6 |
| M. V. Z. |
62791 |
♀ ad. |
Beaverfoot Range, B. C. |
42.0 |
16.4 |
13.5 |
10.2 |
15.1 |
24.4 |
27.0 |
14.3 |
8.1 |
3.1 |
5.9 |
5.5 |
5.6 |
4.3 |
2.2 |
14.0 |
12.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata alleni |
| U. S. N. M. |
186451 |
♂ ad. |
Custer, South Dakota |
40.9 |
15.0 |
13.8 |
11.0 |
14.1 |
22.0 |
27.7 |
13.7 |
7.9 |
3.0 |
5.7 |
4.9 |
5.3 |
3.9 |
2.5 |
14.3 |
12.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| A. M. N. H. |
7440/9136 |
♂ ad. |
Hill City, South Dakota |
41.0 |
15.6 |
13.7 |
11.0 |
13.9 |
23.3 |
25.7 |
13.6 |
8.3 |
2.3 |
5.1 |
4.8 |
5.2 |
4.2 |
1.9 |
13.7 |
12.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| A. M. N. H. |
7441 |
♀ ad. |
Black Hills, South Dakota |
37.6 |
14.1 |
12.2 |
9.1 |
13.2 |
22.3 |
23.1 |
13.8 |
7.3 |
3.1 |
5.5 |
4.9 |
5.0 |
3.6 |
1.8 |
12.2 |
10.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata arizonensis
|
| M. V. Z. |
55211 |
♂ ad. |
near Parks, Arizona |
40.4 |
15.5 |
12.5 |
|
14.2 |
23.3 |
26.0 |
14.3 |
8.9 |
3.1 |
5.7 |
5.2 |
5.5 |
4.0 |
2.2 |
13.5 |
12.1 |
| M. V. Z. |
65231 |
♂ ad. |
Alpine, Arizona |
39.6 |
15.1 |
12.2 |
9.7 |
13.7 |
22.4 |
25.6 |
13.7 |
8.3 |
3.2 |
5.4 |
4.9 |
5.1 |
4.0 |
2.1 |
14.0 |
11.4 |
| U. S. N. M. |
248993 |
♂ ad. |
Kaibab Plat., Arizona |
40.4 |
15.6 |
12.9 |
9.8 |
14.5 |
22.9 |
26.3 |
14.1 |
8.4 |
3.2 |
5.8 |
5.5 |
5.5 |
4.0 |
1.7 |
14.8 |
12.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| A. M. N. H. |
2490/1886 |
♀ ad. |
S. F. Forest, Arizona |
35.5 |
13.8 |
10.8 |
8.6 |
12.9 |
19.9 |
21.7 |
13.3 |
7.7 |
2.9 |
4.8 |
4.6 |
4.7 |
3.6 |
1.6 |
12.3 |
10.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata nevadensis |
| average} |
|
♂ ad. 25 |
Sierra Nevada,
California |
{43.6 |
16.6 |
13.7 |
10.7 |
15.1 |
23.9 |
28.0 |
15.0 |
8.4 |
3.4 |
5.9 |
5.4 |
5.6 |
4.3 |
2.2 |
14.4 |
12.5 |
| maximum} |
{46.1 |
17.6 |
14.9 |
12.0 |
16.2 |
26.1 |
31.4 |
15.9 |
9.0 |
4.0 |
6.4 |
5.8 |
6.1 |
4.8 |
2.7 |
15.2 |
14.8 |
| minimum} |
{40.6 |
15.2 |
12.5 |
9.9 |
14.0 |
22.1 |
25.0 |
14.4 |
7.8 |
2.9 |
5.5 |
4.9 |
5.1 |
3.9 |
1.8 |
13.7 |
11.6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♂ ad. 10 |
S and SW
Colorado |
{43.7 |
16.5 |
13.9 |
10.6 |
15.0 |
24.2 |
27.9 |
15.1 |
8.6 |
3.5 |
6.1 |
5.5 |
5.7 |
4.5 |
2.1 |
14.5 |
12.6 |
| maximum} |
{44.6 |
17.3 |
14.8 |
11.1 |
16.4 |
25.3 |
29.3 |
15.8 |
9.2 |
4.0 |
6.8 |
5.9 |
6.0 |
4.8 |
2.6 |
15.6 |
13.0 |
| minimum} |
{41.6 |
16.0 |
12.8 |
9.9 |
13.8 |
23.1 |
26.5 |
14.4 |
8.2 |
3.0 |
5.7 |
5.2 |
5.3 |
4.0 |
1.9 |
13.9 |
12.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♀ ad. 10 |
California |
{38.2 |
14.7 |
11.8 |
9.2 |
13.4 |
20.9 |
23.1 |
13.4 |
7.3 |
2.9 |
5.2 |
4.8 |
5.0 |
3.8 |
1.7 |
12.3 |
11.0 |
| maximum} |
{39.5 |
15.1 |
12.4 |
9.9 |
14.2 |
21.8 |
23.4 |
14.1 |
7.9 |
3.2 |
5.6 |
5.2 |
5.4 |
4.1 |
2.0 |
13.0 |
11.8 |
| minimum} |
{36.7 |
13.9 |
11.0 |
8.6 |
12.6 |
20.1 |
22.4 |
12.7 |
6.8 |
2.6 |
4.9 |
4.5 |
4.7 |
3.6 |
1.5 |
11.4 |
10.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♀ ad. 14 |
Colorado |
{38.5 |
14.8 |
12.2 |
9.3 |
13.4 |
20.6 |
23.1 |
13.4 |
7.6 |
2.8 |
5.4 |
4.9 |
5.1 |
3.9 |
1.9 |
12.9 |
11.1 |
| maximum} |
{39.7 |
15.4 |
13.1 |
10.2 |
14.4 |
22.1 |
24.6 |
13.9 |
8.1 |
3.2 |
5.6 |
5.3 |
5.4 |
4.1 |
2.3 |
13.8 |
11.8 |
| minimum} |
{36.1 |
14.0 |
11.1 |
8.5 |
12.5 |
19.8 |
22.0 |
12.9 |
7.0 |
2.5 |
5.1 |
4.3 |
4.6 |
3.4 |
1.7 |
12.0 |
10.6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata effera |
| average} |
|
♂ ad. 6 |
NE Oregon |
{40.5 |
15.2 |
12.3 |
9.6 |
13.7 |
22.1 |
25.6 |
14.1 |
7.8 |
3.3 |
5.5 |
5.0 |
5.3 |
3.9 |
2.0 |
13.5 |
11.8 |
| maximum} |
{41.8 |
16.4 |
12.7 |
10.0 |
14.4 |
23.3 |
27.3 |
15.0 |
8.4 |
3.5 |
5.9 |
5.3 |
5.8 |
4.1 |
2.6 |
14.3 |
12.2 |
| minimum} |
{39.3 |
14.4 |
11.9 |
9.2 |
13.1 |
20.5 |
25.0 |
12.3 |
7.2 |
3.2 |
5.0 |
4.7 |
5.0 |
3.6 |
1.6 |
12.6 |
11.4 |
| U. S. N. M. |
212423 |
♀ ad. |
Vale, Oregon |
37.4 |
|
|
9.2 |
|
19.5 |
22.0 |
13.1 |
6.9 |
3.0 |
5.4 |
4.9 |
5.0 |
3.7 |
1.8 |
12.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata washingtoni |
| average} |
|
♂ ad. 22 |
Mt. Adams,
Washington |
{43.7 |
16.7 |
13.7 |
10.5 |
15.5 |
23.4 |
27.0 |
14.6 |
8.0 |
3.1 |
5.9 |
5.4 |
5.6 |
4.3 |
2.2 |
14.4 |
12.6 |
| maximum} |
{47.7 |
18.0 |
15.4 |
12.0 |
16.5 |
26.4 |
29.6 |
15.8 |
8.7 |
3.4 |
6.5 |
6.0 |
6.1 |
4.8 |
2.6 |
15.8 |
13.7 |
| minimum} |
{40.0 |
15.6 |
12.5 |
9.0 |
14.5 |
22.1 |
24.6 |
13.5 |
7.6 |
2.7 |
5.4 |
4.9 |
5.0 |
4.0 |
1.7 |
13.1 |
11.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♀ ad. 11 |
Mt. Adams,
Washington |
{37.7 |
14.3 |
11.5 |
8.8 |
12.8 |
20.2 |
22.5 |
12.9 |
7.2 |
2.8 |
5.1 |
4.7 |
4.8 |
3.9 |
1.9 |
12.2 |
10.4 |
| maximum} |
{39.0 |
14.9 |
12.0 |
9.3 |
13.2 |
21.1 |
24.5 |
13.6 |
7.7 |
3.0 |
5.6 |
4.9 |
5.0 |
4.2 |
2.1 |
13.0 |
11.0 |
| minimum} |
{37.1 |
13.3 |
10.8 |
8.2 |
12.1 |
19.4 |
21.3 |
12.3 |
6.8 |
2.3 |
4.7 |
4.4 |
4.2 |
3.4 |
1.7 |
11.2 |
9.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata saturata |
| U. O. |
3709 |
♂ ad. |
Mt. Ashland, Oregon |
45.8 |
17.2 |
14.1 |
11.1 |
15.8 |
26.0 |
27.9 |
15.1 |
8.9 |
3.9 |
6.1 |
5.5 |
5.9 |
4.3 |
2.3 |
14.2 |
12.6 |
| U. S. N. M. |
65930 |
♂ ad. |
Siskiyou, Oregon |
42.6 |
15.9 |
14.0 |
10.9 |
14.4 |
24.5 |
27.7 |
14.7 |
8.5 |
4.0 |
5.7 |
5.0 |
5.5 |
4.1 |
1.9 |
14.8 |
12.4 |
| M. V. Z. |
13778 |
♂ ad. |
Jackson Lake, California |
45.5 |
18.0 |
13.2 |
10.0 |
16.2 |
24.7 |
27.4 |
14.6 |
8.7 |
3.2 |
6.3 |
5.9 |
6.2 |
4.6 |
2.5 |
14.6 |
12.8 |
| M. V. Z. |
13779 |
♂ ad. |
Jackson Lake, California |
43.8 |
16.9 |
12.7 |
9.9 |
15.3 |
24.2 |
26.9 |
15.1 |
8.6 |
3.3 |
5.6 |
5.2 |
5.5 |
4.0 |
2.2 |
14.2 |
12.3 |
|
|
av. 4 |
|
44.4 |
17.0 |
13.5 |
10.2 |
15.4 |
24.9 |
27.5 |
14.9 |
8.7 |
3.6 |
5.9 |
5.4 |
5.8 |
4.3 |
2.2 |
14.5 |
12.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| M. V. Z. |
52144 |
♀ ad. S |
. Fork Mt., California |
38.2 |
14.7 |
10.8 |
8.1 |
13.5 |
19.8 |
21.8 |
12.4 |
7.2 |
2.7 |
5.0 |
4.6 |
4.9 |
3.7 |
1.8 |
12.2 |
10.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata altifrontalis |
| average} |
|
♂ ad. 10 |
Tillamook Co.,
Oregon |
{45.8 |
17.5 |
14.2 |
11.2 |
15.9 |
25.1 |
29.2 |
15.6 |
8.5 |
3.5 |
6.1 |
5.5 |
5.7 |
4.5 |
2.5 |
15.4 |
13.6 |
| maximum} |
{48.0 |
18.9 |
15.0 |
12.0 |
16.8 |
26.0 |
31.6 |
16.5 |
9.0 |
3.6 |
6.5 |
6.0 |
6.0 |
4.9 |
2.8 |
16.2 |
14.0 |
| minimum} |
{42.4 |
16.1 |
13.2 |
10.0 |
14.8 |
23.9 |
26.0 |
15.0 |
7.7 |
3.2 |
5.6 |
5.0 |
5.2 |
4.0 |
2.1 |
14.5 |
12.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Walker |
392 |
♀ ad. |
Blaine, Oregon |
39.7 |
15.1 |
12.8 |
10.1 |
13.7 |
21.8 |
24.0 |
13.8 |
7.7 |
|
5.2 |
5.0 |
5.0 |
3.7 |
1.9 |
13.5 |
11.8 |
| Walker |
185 |
♀ ad. |
Blaine, Oregon |
37.8 |
13.9 |
11.9 |
9.5 |
13.4 |
20.8 |
22.7 |
13.4 |
7.3 |
2.8 |
4.9 |
4.5 |
4.7 |
3.2 |
1.5 |
12.6 |
10.7 |
| Walker |
89 |
♀ ad. |
Blaine, Oregon |
38.3 |
14.3 |
11.3 |
9.5 |
12.7 |
19.8 |
22.7 |
13.2 |
7.0 |
3.1 |
|
4.4 |
4.7 |
3.8 |
1.9 |
13.4 |
11.3 |
| Walker |
45 |
♀ ad. |
Tillamook, Oregon |
37.8 |
14.1 |
11.0 |
8.6 |
12.5 |
20.1 |
23.2 |
13.0 |
7.3 |
|
4.9 |
4.7 |
4.8 |
3.8 |
2.0 |
13.5 |
11.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata oregonensis |
| M. V. Z. |
11747 |
♂ ad. |
Eureka, California |
44.0 |
16.8 |
13.2 |
10.7 |
15.3 |
24.3 |
28.0 |
14.6 |
8.3 |
3.4 |
6.0 |
5.4 |
5.8 |
4.3 |
2.2 |
13.6 |
12.7 |
| C. A. C. |
3907 |
♂ ad. |
Requa, California |
41.8 |
16.0 |
13.5 |
10.9 |
14.0 |
22.9 |
26.6 |
14.2 |
7.6 |
3.1 |
5.8 |
5.3 |
5.4 |
4.0 |
2.2 |
13.4 |
12.3 |
| F. M. N. H. |
9595 |
♂ ad. |
Gold Beach, Oregon |
43.0 |
16.9 |
13.1 |
10.5 |
15.5 |
23.4 |
26.2 |
15.1 |
8.2 |
3.3 |
5.8 |
5.2 |
5.5 |
4.4 |
2.0 |
14.7 |
12.5 |
| U. S. N. M. |
32019 |
♂ sad. |
Grants Pass, Oregon |
42.9 |
16.3 |
13.7 |
10.5 |
15.7 |
23.2 |
26.5 |
15.2 |
8.8 |
4.0 |
5.5 |
4.8 |
5.2 |
4.3 |
2.1 |
14.0 |
12.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| M. V. Z. |
34325 |
♀ ad. |
Carlotta, California |
37.8 |
14.7 |
10.8 |
8.8 |
13.0 |
20.7 |
23.2 |
13.4 |
7.5 |
2.2 |
5.3 |
5.0 |
5.5 |
4.0 |
1.8 |
13.7 |
11.0 |
| U. O. |
1413 |
♀ ad. |
13 mi. S Grants Pass, Oregon |
39.4 |
14.7 |
12.0 |
9.7 |
13.9 |
21.5 |
24.0 |
14.2 |
7.6 |
2.7 |
4.8 |
4.4 |
4.7 |
3.7 |
1.9 |
12.6 |
10.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata munda |
| M. V. Z. |
19720 |
♂ ad. |
Point Arena, California |
49.0 |
18.5 |
15.2 |
11.7 |
16.8 |
27.5 |
33.2 |
16.2 |
9.3 |
3.6 |
6.5 |
5.6 |
6.0 |
4.8 |
2.6 |
14.2 |
14.2 |
| M. V. Z. |
19722 |
♂ ad. |
Point Arena, California |
48.5 |
18.9 |
14.4 |
10.5 |
17.2 |
26.8 |
32.+ |
16.0 |
8.5 |
3.8 |
6.5 |
5.6 |
6.2 |
5.0 |
2.9 |
14.7 |
13.8 |
| M. V. Z. |
19718 |
♂ ad. |
Gualala, California |
45.7 |
17.2 |
13.7 |
10.5 |
15.5 |
26.4 |
31.7 |
15.5 |
8.5 |
3.1 |
6.5 |
5.7 |
5.8 |
4.9 |
2.3 |
13.4 |
14.2 |
|
|
av. 3 |
|
47.7 |
18.2 |
14.4 |
10.9 |
16.5 |
26.9 |
32.7 |
15.9 |
8.8 |
3.5 |
6.5 |
5.7 |
6.0 |
4.9 |
2.6 |
14.1 |
14.1 |
| M. V. Z. |
19714 |
♂ ad. |
6 mi. W Inverness, California |
48.2 |
18.4 |
13.9 |
11.3 |
17.2 |
26.2 |
30.5 |
15.7 |
8.5 |
3.5 |
6.5 |
5.6 |
6.0 |
4.8 |
2.6 |
14.2 |
14.2 |
| M. V. Z. |
19715 |
♂ ad. |
6 mi. W Inverness, California |
48.2 |
18.9 |
14.5 |
11.4 |
16.6 |
26.5 |
30.0 |
16.3 |
8.7 |
3.5 |
6.3 |
5.6 |
6.2 |
5.0 |
2.9 |
14.7 |
13.8 |
| M. V. Z. |
19716 |
♂ ad. |
5 mi. W Inverness, California |
46.5 |
17.5 |
13.8 |
11.0 |
15.9 |
25.2 |
30.3 |
15.4 |
7.9 |
3.5 |
6.1 |
5.7 |
5.8 |
4.9 |
2.3 |
13.4 |
14.2 |
|
|
av. 3 |
|
47.6 |
18.3 |
14.1 |
11.2 |
16.6 |
26.0 |
30.3 |
15.8 |
8.4 |
3.5 |
6.3 |
5.7 |
6.0 |
4.9 |
2.6 |
14.1 |
14.1 |
| F. M. N. H. |
9598 |
♂ ad. |
Nicasio, California |
46.5 |
18.5 |
14.0 |
10.5 |
15.4 |
25.2 |
30.5 |
15.0 |
8.8 |
3.5 |
6.9 |
5.9 |
6.3 |
5.2 |
2.7 |
15.2 |
13.0 |
| M. C. Z. |
8632 |
♂ ad. |
Nicasio, California |
44.2 |
17.8 |
14.0 |
11.4 |
16.9 |
24.4 |
27.6 |
15.7 |
8.2 |
3.7 |
6.4 |
5.7 |
5.9 |
5.0 |
2.6 |
12.6 |
13.7 |
| M. C. Z. |
5459 |
♂ ad. |
Point Reyes, California |
39.7 |
15.5 |
13.3 |
9.0 |
13.4 |
23.1 |
26.5 |
13.7 |
7.7 |
3.2 |
6.1 |
5.1 |
5.5 |
4.4 |
2.5 |
13.6 |
12.1 |
| M. V. Z. |
40302 |
♂ ad. |
4 mi. N Vallejo, California |
45.8 |
17.7 |
13.0 |
10.7 |
15.9 |
24.4 |
26.9 |
15.1 |
8.0 |
2.8 |
6.3 |
5.7 |
6.2 |
4.8 |
2.3 |
14.5 |
13.0 |
|
|
av. 4 |
|
44.1 |
17.4 |
13.6 |
10.4 |
15.4 |
24.3 |
27.9 |
14.9 |
8.2 |
3.3 |
6.4 |
5.6 |
6.0 |
4.9 |
2.5 |
14.0 |
13.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| M. V. Z. |
19723 |
♀ ad. |
Point Arena, California |
42.3 |
16.0 |
12.3 |
12.2 |
14.0 |
23.6 |
25.5 |
14.5 |
8.3 |
2.9 |
|
5.0 |
5.2 |
4.0 |
1.9 |
13.4 |
11.3 |
| U. S. N. M. |
135010 |
♀ ad. |
Point Reyes, California |
38.7 |
15.3 |
11.0 |
8.7 |
14.5 |
21.0 |
23.7 |
13.0 |
7.5 |
3.0 |
5.1 |
4.9 |
5.4 |
3.9 |
2.3 |
12.9 |
10.8 |
| F. M. N. H. |
9597 |
♀ ad. |
Point Reyes, California |
39.5 |
15.2 |
11.1 |
8.2 |
13.1 |
20.2 |
22.8 |
12.7 |
6.8 |
2.7 |
5.3 |
4.9 |
5.0 |
3.9 |
1.9 |
14.7 |
10.8 |
| U. S. N. M. |
91764 |
♀ ad. |
Point Reyes, California |
38.7 |
15.4 |
12.0 |
9.7 |
12.7 |
21.7 |
24.7 |
13.7 |
7.4 |
2.9 |
5.5 |
5.0 |
5.3 |
4.2 |
2.0 |
13.2 |
11.7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata xanthogenys |
| Walker |
1440 |
♂ ad. |
5 mi. W Fresno, California |
43.9 |
16.5 |
13.8 |
10.4 |
14.1 |
23.8 |
28.5 |
14.7 |
7.9 |
3.2 |
5.7 |
5.2 |
5.3 |
4.2 |
2.1 |
14.8 |
12.6 |
| A. N. S. P. |
11863 |
♂ ad. |
Fresno, California |
43.4 |
16.8 |
12.9 |
10.0 |
14.8 |
24.0 |
27.5 |
14.5 |
7.5 |
|
5.8 |
5.3 |
5.5 |
4.4 |
2.0 |
13.7 |
12.7 |
| Wisconsin U. |
4232 |
♂ ad. |
Selma, California |
43.7 |
16.2 |
13.3 |
9.9 |
14.5 |
23.7 |
27.1 |
15.2 |
8.5 |
3.7 |
5.8 |
5.3 |
5.4 |
4.2 |
2.2 |
13.8 |
12.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Snyder |
2626 |
♀ ad. |
Selma, California |
39.4 |
15.0 |
12.0 |
8.9 |
13.2 |
21.3 |
24.3 |
13.7 |
7.2 |
|
5.3 |
|
|
4.0 |
|
13.0 |
11.4 |
| M. V. Z. |
79640 |
♀ ad. |
Tegner School, California |
43.4 |
16.5 |
12.6 |
9.3 |
15.1 |
22.8 |
24.9 |
15.0 |
7.5 |
3.0 |
5.6 |
5.5 |
5.9 |
4.5 |
2.0 |
13.3 |
12.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata nigriauris |
| Stanford U. |
863 |
♂ ad. |
Palo Alto, California |
48.1 |
18.7 |
15.0 |
11.2 |
17.5 |
|
32.9 |
15.5 |
|
|
6.5 |
6.4 |
6.7 |
5.2 |
2.4 |
|
14.3 |
| F. M. N. H. |
6559 |
♂ ad. |
Palo Alto, California |
48.0 |
18.7 |
13.9 |
11.0 |
16.2 |
27.0 |
29.6 |
15.7 |
8.7 |
3.4 |
6.2 |
5.8 |
6.0 |
4.8 |
2.7 |
14.0 |
14.1 |
| Stanford U. |
1651 |
♂ ad. |
Menlo Park, California |
47.1 |
17.8 |
13.3 |
9.8 |
14.9 |
25.6 |
30.0 |
15.0 |
8.0 |
3.3 |
6.5 |
6.0 |
6.5 |
4.6 |
2.4 |
14.6 |
13.8 |
| Stanford U. |
487 |
♂ ad. |
Palo Alto, California |
46.5 |
18.3 |
13.4 |
11.0 |
16.1 |
25.2 |
31.1 |
15.1 |
8.0 |
2.9 |
6.3 |
5.7 |
6.0 |
4.9 |
2.5 |
14.5 |
14.3 |
| F. M. N. H. |
7031 |
♂ ad. |
Palo Alto, California |
46.1 |
18.1 |
14.5 |
10.7 |
15.1 |
26.0 |
29.5 |
14.9 |
8.3 |
2.4 |
6.3 |
6.0 |
6.1 |
4.6 |
2.6 |
15.0 |
13.2 |
| Stanford U. |
236 |
♂ ad. |
Menlo Park, California |
46.1 |
17.8 |
13.5 |
10.4 |
15.5 |
|
29.6 |
15.6 |
8.5 |
|
6.0 |
5.6 |
5.7 |
4.8 |
2.5 |
|
14.0 |
|
|
av. 6 |
|
47.0 |
18.2 |
13.9 |
10.7 |
15.9 |
26.0 |
30.5 |
15.3 |
8.3 |
3.0 |
6.3 |
5.9 |
6.2 |
4.8 |
2.5 |
14.5 |
14.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| M. V. Z. |
5851 |
♀ ad. |
Hayward, California |
40.7 |
15.3 |
11.8 |
8.8 |
13.9 |
21.8 |
24.9 |
14.2 |
7.9 |
3.0 |
5.2 |
4.9 |
4.9 |
4.1 |
1.8 |
12.4 |
11.0 |
| M. V. Z. |
30327 |
♀ ad. |
Palo Alto, California |
41.2 |
16.2 |
11.1 |
8.6 |
14.3 |
21.7 |
24.8 |
13.4 |
7.8 |
3.0 |
5.3 |
5.0 |
5.4 |
4.1 |
2.1 |
12.6 |
11.0 |
| U. S. N. M. |
43574 |
♀ ad. |
Morro, California |
42.2 |
16.1 |
12.2 |
8.9 |
14.2 |
22.7 |
24.3 |
15.1 |
8.1 |
2.9 |
5.5 |
5.4 |
5.6 |
4.2 |
2.0 |
13.2 |
11.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata latirostra |
| M. V. Z. |
3257 |
♂ ad. |
San Diego, California |
44.4 |
17.8 |
13.4 |
10.2 |
15.0 |
23.5 |
27.6 |
15.6 |
8.4 |
3.9 |
6.0 |
5.5 |
5.5 |
4.3 |
2.2 |
14.0 |
12.8 |
| U. S. N. M. |
52701 |
♂ ad. |
El Vido, California |
43.7 |
16.9 |
12.7 |
9.9 |
14.3 |
24.0 |
|
14.7 |
8.7 |
4.0 |
5.7 |
5.6 |
5.6 |
4.2 |
2.0 |
14.2 |
13.4 |
| U. S. N. M. |
52702 |
♂ ad. |
El Cajon, California |
43.2 |
16.7 |
13.9 |
10.0 |
15.0 |
24.0 |
27.2 |
15.1 |
8.2 |
4.2 |
5.8 |
5.3 |
5.5 |
4.2 |
2.5 |
14.0 |
12.5 |
| M. V. Z. |
3258 |
♂ ad. |
San Diego, California |
42.5 |
16.6 |
14.1 |
12.1 |
14.7 |
24.0 |
28.7 |
15.1 |
8.1 |
3.9 |
5.9 |
5.6 |
5.5 |
4.4 |
2.5 |
14.3 |
13.5 |
| Stephens |
20 |
♂ ad. |
San Jacinto Plain, California |
41.9 |
16.9 |
13.1 |
10.6 |
14.0 |
24.0 |
28.2 |
14.7 |
8.0 |
3.2 |
6.0 |
5.5 |
5.7 |
4.2 |
2.1 |
14.0 |
12.9 |
| S. D. M. |
7098 |
♂ ad. |
Jamacha, California |
47.0 |
18.5 |
15.6 |
11.3 |
16.8 |
24.8 |
29.2 |
16.7 |
8.9 |
3.8 |
6.0 |
5.8 |
6.2 |
4.8 |
2.2 |
14.0 |
13.2 |
|
|
av. 6 |
|
43.8 |
17.2 |
13.8 |
10.7 |
15.0 |
24.1 |
28.2 |
15.3 |
8.4 |
3.8 |
5.9 |
5.6 |
5.7 |
4.4 |
2.3 |
14.1 |
13.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stephens |
22 |
♀ ad. |
Santa Ysabel, California |
40.1 |
15.5 |
11.7 |
9.3 |
13.1 |
21.9 |
24.5 |
14.2 |
8.0 |
3.0 |
5.3 |
4.8 |
5.1 |
4.1 |
2.0 |
14.0 |
11.3 |
| Stephens |
19 |
♀ ad. |
Ballena, California |
40.0 |
15.0 |
12.3 |
9.3 |
13.5 |
21.6 |
23.5 |
13.7 |
8.1 |
3.2 |
5.1 |
4.9 |
5.0 |
4.0 |
2.1 |
12.0 |
11.2 |
| S. D. M. |
6748 |
♀ ad. |
Santa Ysabel, California |
42.0 |
16.1 |
12.9 |
9.4 |
14.2 |
22.0 |
24.5 |
14.0 |
7.8 |
3.0 |
5.8 |
5.3 |
5.7 |
4.5 |
2.0 |
13.4 |
11.5 |
| S. D. M. |
7194 |
♀ ad. |
Jamacha, California |
39.8 |
15.0 |
11.8 |
9.1 |
13.7 |
20.2 |
23.7 |
13.7 |
7.0 |
2.9 |
5.2 |
4.8 |
4.9 |
3.9 |
1.9 |
12.6 |
11.5 |
|
|
av. 4 |
|
40.5 |
15.4 |
12.2 |
9.3 |
13.6 |
21.4 |
24.1 |
13.9 |
7.7 |
3.0 |
5.4 |
5.0 |
5.2 |
4.1 |
2.0 |
13.0 |
11.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata pulchra |
| C. A. S. |
335 |
♂ ad. |
Buttonwillow, California |
48.6 |
18.8 |
15.2 |
11.6 |
17.2 |
27.4 |
31.4 |
16.7 |
9.1 |
3.6 |
6.6 |
5.9 |
6.4 |
5.1 |
2.7 |
16.2 |
13.7 |
| C. A. S. |
337 |
♂ ad. |
Buttonwillow, California |
48.6 |
18.8 |
15.0 |
11.8 |
17.2 |
27.1 |
32.7 |
16.6 |
9.0 |
3.5 |
6.6 |
6.3 |
6.3 |
5.0 |
2.4 |
16.5 |
14.3 |
| M. V. Z. |
16668 |
♂ ad. |
Buttonwillow, California |
48.1 |
18.8 |
14.8 |
12.0 |
17.1 |
27.7 |
31.2 |
16.4 |
9.2 |
3.6 |
6.4 |
5.7 |
5.9 |
5.1 |
2.8 |
15.2 |
14.0 |
| U. S. N. M. |
137935 |
♂ ad. |
Buttonwillow, California |
47.2 |
18.2 |
14.0 |
10.3 |
16.0 |
26.1 |
29.5 |
15.6 |
8.5 |
3.3 |
6.0 |
5.6 |
5.9 |
4.1 |
2.0 |
15.0 |
12.9 |
| C. A. S. |
336 |
♂ ad. |
Buttonwillow, California |
47.0 |
18.3 |
14.5 |
11.5 |
16.5 |
27.0 |
29.5 |
15.5 |
9.1 |
3.2 |
6.3 |
5.6 |
5.9 |
5.0 |
2.3 |
15.0 |
13.0 |
| C. A. S. |
338 |
♂ ad. |
Buttonwillow, California |
46.0 |
17.4 |
14.7 |
11.3 |
16.1 |
26.3 |
32.6 |
15.9 |
8.9 |
3.7 |
6.1 |
5.7 |
5.8 |
4.6 |
2.2 |
15.0 |
13.3 |
|
|
av. 6 |
|
47.6 |
18.4 |
14.7 |
11.4 |
16.7 |
26.9 |
31.1 |
16.1 |
9.0 |
3.5 |
6.3 |
5.8 |
6.0 |
4.8 |
2.4 |
15.5 |
13.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata inyoensis |
| M. V. Z. |
25907 |
♂ ad. |
2 mi. N Independence, Calif. |
44.7 |
17.3 |
13.3 |
10.8 |
15.9 |
25.3 |
29.5 |
15.9 |
9.0 |
3.7 |
6.0 |
5.7 |
5.9 |
4.6 |
2.4 |
15.0 |
13.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata texensis |
| A. M. N. H. |
14821 |
♂ ad. |
Kerr Co., Texas |
54.0 |
19.2 |
16.0 |
12.6 |
17.2 |
28.6 |
35.1 |
17.5 |
8.0 |
3.5 |
6.9 |
6.5 |
6.7 |
5.0 |
2.5 |
16.7 |
15.1 |
| M. C. Z. |
15476 |
♂ yg. |
Kerr Co., Texas |
53.3 |
18.9 |
16.3 |
12.8 |
18.3 |
28.2 |
34.8 |
18.0 |
8.3 |
4.0 |
6.4 |
6.3 |
6.5 |
4.8 |
2.4 |
16.0 |
15.0 |
| Baylor U. |
2017 |
♂ sad. |
5 mi. N Waco, Texas |
52.0 |
18.3 |
16.5 |
13.5 |
16.7 |
29.2 |
|
18.0 |
9.1 |
|
6.6 |
6.3 |
6.4 |
4.9 |
2.6 |
16.3 |
15.6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata neomexicana |
| Kansas U. |
1485 |
♂ ad. |
Liberal, Kansas |
50.5 |
17.9 |
15.4 |
12.4 |
16.4 |
28.3 |
35.0 |
17.0 |
9.0 |
4.0 |
6.3 |
5.9 |
6.0 |
4.9 |
2.4 |
15.5 |
14.3 |
| U. S. N. M. |
131582 |
♂ ad. |
Berino, New Mexico |
47.7 |
17.5 |
15.0 |
11.1 |
15.9 |
26.3 |
31.2 |
16.5 |
7.9 |
3.4 |
6.4 |
5.7 |
6.3 |
4.7 |
2.5 |
15.8 |
13.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| U. S. N. M. |
36482 |
♀ ad. |
Tombstone, Arizona |
45.5 |
16.5 |
12.8 |
9.7 |
15.5 |
22.3 |
26.6 |
15.2 |
7.5 |
3.3 |
5.9 |
5.6 |
5.9 |
4.5 |
2.2 |
13.8 |
12.0 |
| U. S. N. M. |
230973 |
♀ ad. |
Willcox, Arizona |
42.5 |
15.1 |
12.9 |
9.9 |
14.0 |
22.6 |
26.5 |
14.5 |
7.0 |
3.1 |
5.5 |
5.3 |
5.6 |
4.1 |
1.8 |
14.0 |
12.0 |
| U. S. N. M. |
225629 |
♀ ad. |
Albuquerque, New Mexico |
40.8 |
15.0 |
12.4 |
9.4 |
13.3 |
21.6 |
24.5 |
14.5 |
7.5 |
3.0 |
5.2 |
4.7 |
5.0 |
3.9 |
1.8 |
13.0 |
11.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata frenata |
| M. C. Z. |
240 |
♂ ad. |
Brownsville, Texas |
49.4 |
17.4 |
14.9 |
11.6 |
15.4 |
25.9 |
33.0 |
16.7 |
7.9 |
4.3 |
5.9 |
5.9 |
6.1 |
4.5 |
2.1 |
15.0 |
13.8 |
| A. N. S. P. |
724 |
♂ ad. |
Brownsville, Texas |
50.1 |
17.9 |
15.5 |
12.3 |
15.5 |
27.0 |
32.2 |
16.5 |
8.5 |
4.1 |
6.5 |
6.2 |
6.3 |
4.8 |
2.7 |
16.0 |
14.0 |
| U. S. N. M. |
58684 |
♂ ad. |
Brownsville, Texas |
48.2 |
17.3 |
14.2 |
11.0 |
15.3 |
27.2 |
31.0 |
16.6 |
8.3 |
4.2 |
6.3 |
5.9 |
6.1 |
4.8 |
2.7 |
15.5 |
13.5 |
| U. S. N. M. |
63857 |
♂ ad. |
Brownsville, Texas |
48.6 |
18.0 |
13.7 |
11.1 |
16.5 |
26.0 |
31.0 |
16.0 |
8.2 |
4.8 |
6.5 |
5.7 |
6.1 |
4.8 |
2.6 |
15.0 |
13.6 |
| U. S. N. M. |
44976 |
♂ ad. |
Brownsville, Texas |
50.9 |
18.0 |
|
|
16.9 |
26.9 |
|
16.6 |
7.9 |
3.4 |
6.5 |
5.6 |
6.1 |
4.7 |
2.3 |
16.0 |
13.4 |
|
|
av. 5 |
|
49.4 |
17.7 |
14.6 |
11.5 |
15.9 |
26.6 |
31.8 |
16.3 |
8.2 |
4.2 |
6.4 |
5.9 |
6.2 |
4.7 |
2.5 |
15.5 |
13.7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| A. M. N. H. |
24405 |
♀ ad. |
Brownsville, Texas |
47.3 |
16.2 |
12.5 |
10.0 |
14.6 |
|
|
|
|
|
5.9 |
5.4 |
5.4 |
4.1 |
2.1 |
|
12.2 |
| U. S. N. M. |
58685 |
♀ sad. |
Brownsville, Texas |
41.3 |
15.0 |
11.8 |
9.5 |
12.8 |
22.7 |
27.0 |
14.0 |
6.9 |
3.3 |
5.5 |
5.2 |
5.4 |
4.1 |
2.0 |
14.0 |
11.7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| U. S. N. M. |
36362/4873 |
2 ♀ sad. |
Brownsville, Texas |
42.5 |
15.4 |
12.5 |
10.0 |
14.3 |
23.8 |
26.7 |
14.3 |
7.5 |
3.2 |
5.9 |
5.5 |
5.7 |
4.2 |
2.0 |
13.5 |
11.8 |
|
|
av. 3 |
|
43.7 |
15.5 |
12.3 |
9.8 |
13.9 |
23.3 |
26.9 |
14.2 |
7.2 |
3.3 |
5.8 |
5.4 |
5.5 |
4.1 |
2.0 |
13.8 |
11.9 |
| B. Z. M. |
991 |
♀ ad. |
México |
|
15.5 |
|
12.2 |
13.8 |
22.8 |
27.0 |
13.7 |
|
|
5.7 |
5.2 |
5.5 |
4.4 |
2.2 |
|
12.7 |
| B. Z. M. |
992 |
♀ ad. |
México |
|
13.9 |
10.7 |
8.9 |
12.9 |
21.1 |
|
13.0 |
6.6 |
2.9 |
4.9 |
4.5 |
4.7 |
3.7 |
1.7 |
12.3 |
10.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| M. C. Z. |
20841 |
♂ ad. |
Miquihana, Nuevo León |
50.2 |
18.0 |
15.3 |
12.1 |
16.3 |
28.0 |
32.0 |
16.9 |
8.8 |
3.3 |
|
|
|
4.6 |
2.2 |
15.0 |
|
| U. S. N. M. |
50826 |
♂ ad. |
Tlalpam, D. F. |
51.3 |
18.3 |
15.1 |
12.1 |
17.5 |
27.7 |
33.5 |
16.3 |
8.4 |
3.5 |
6.7 |
5.9 |
6.4 |
4.7 |
2.7 |
15.3 |
14.6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata leucoparia |
| U. S. N. M. |
125972 |
♂ ad. |
Los Reyes, Michoacán |
51.2 |
17.5 |
15.0 |
12.0 |
16.0 |
28.3 |
32.9 |
16.0 |
7.7 |
3.5 |
5.9 |
5.7 |
5.7 |
4.3 |
2.2 |
15.5 |
14.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| U. S. N. M. |
34914/4717 |
9 ♂ ad. |
Pátzcuaro, Michoacán |
|
18.7 |
14.3 |
|
16.8 |
|
|
|
|
|
6.8 |
6.5 |
6.8 |
5.0 |
2.6 |
|
14.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| A. M. N. H. |
26153 |
♀ ad. |
Artenkiki, Jalisco |
44.5 |
16.0 |
12.7 |
10.0 |
14.4 |
22.4 |
26.3 |
15.0 |
7.0 |
3.2 |
5.9 |
5.5 |
6.0 |
4.5 |
2.1 |
14.0 |
11.9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata perotae |
| U. S. N. M. |
68197 |
♂ ad. |
Cerro San Felipe, Oaxaca |
49.2 |
17.3 |
13.9 |
11.7 |
15.9 |
25.0 |
29.2 |
15.5 |
6.8 |
2.5 |
6.1 |
5.3 |
5.8 |
4.1 |
2.1 |
15.5 |
13.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| U. S. N. M. |
54278 |
♀ ad. |
Type specimen |
43.5 |
15.5 |
12.3 |
10.3 |
14.0 |
23.2 |
25.5 |
15.0 |
7.0 |
2.0 |
5.7 |
5.2 |
5.7 |
4.1 |
2.1 |
12.0 |
13.7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata goldmani |
| U. S. N. M. |
133253 |
♂ ad. |
20 mi. SE Teopisca, Chiapas |
50.4 |
18.0 |
15.0 |
12.5 |
15.9 |
26.5 |
32.3 |
15.6 |
7.1 |
2.9 |
6.4 |
5.9 |
6.2 |
5.0 |
2.5 |
16.5 |
13.7 |
| U. S. N. M. |
133254 |
♂ ad. |
20 mi. SE Teopisca, Chiapas |
49.6 |
17.5 |
13.8 |
10.9 |
16.2 |
26.4 |
31.8 |
15.8 |
7.4 |
3.0 |
6.1 |
5.5 |
5.8 |
4.5 |
2.5 |
15.0 |
13.7 |
| U. S. N. M. |
77519 |
♂ ad. |
Pinabete, Chiapas |
50.7 |
18.3 |
|
|
|
26.5 |
31.8 |
15.3 |
7.5 |
2.9 |
6.6 |
5.8 |
6.1 |
5.0 |
2.4 |
16.0 |
|
| F. M. N. H. |
15953 |
♂ ad. |
near Tecpám, Guatemala |
50.0 |
17.0 |
13.5 |
12.0 |
16.1 |
26.2 |
31.5 |
15.5 |
7.0 |
|
5.9 |
5.6 |
6.0 |
4.5 |
2.2 |
|
|
| Dickey |
12523 |
♂ ad. |
Los Esesmiles, Salv. |
51.3 |
17.5 |
14.5 |
11.7 |
16.2 |
26.7 |
30.6 |
15.8 |
7.7 |
3.1 |
6.3 |
5.5 |
5.8 |
4.5 |
2.3 |
16.5 |
14.1 |
|
|
av. 5 |
|
50.4 |
17.7 |
14.2 |
11.8 |
16.1 |
26.5 |
31.6 |
15.6 |
7.3 |
3.0 |
6.3 |
5.7 |
6.0 |
4.7 |
2.4 |
16.0 |
13.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata macrophonius |
| F. M. N. H. |
14063 |
♂ ad. |
Achotal, Veracruz |
54.1 |
19.2 |
15.6 |
12.9 |
17.8 |
28.5 |
33.6 |
16.8 |
7.6 |
2.9 |
7.1 |
6.4 |
6.8 |
5.2 |
2.9 |
16.8 |
15.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| U. S. N. M. |
132528 |
♀ ad. |
Pérez, Veracruz |
43.5 |
15.3 |
12.5 |
10.2 |
14.5 |
23.1 |
26.5 |
15.0 |
6.5 |
2.6 |
5.5 |
5.2 |
5.7 |
4.1 |
1.9 |
14.2 |
12.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata tropicalis |
| U. S. N. M. |
54994 |
♂ ad. |
Jico, Veracruz |
47.8 |
17.2 |
13.7 |
10.7 |
15.8 |
24.5 |
28.2 |
15.5 |
6.3 |
2.9 |
6.2 |
5.6 |
5.9 |
4.5 |
2.2 |
15.5 |
13.7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| A. M. N. H. |
12764/1105 |
8 ♂ sad. |
Jalapa, Veracruz |
45.5 |
16.8 |
13.7 |
11.4 |
16.0 |
24.0 |
30.0 |
15.4 |
6.7 |
3.0 |
6.4 |
5.7 |
5.9 |
4.7 |
2.2 |
15.2 |
13.6 |
| M. C. Z. |
6514 |
♂ sad. |
Orizaba, Veracruz |
*46.0 |
16.4 |
13.2 |
|
15.5 |
|
|
15.0 |
8.0 |
|
6.3 |
5.7 |
6.0 |
5.0 |
2.5 |
16.5 |
14.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| U. S. N. M. |
54993 |
♀ ad. |
Jico, Veracruz |
36.0 |
13.0 |
11.0 |
9.2 |
12.1 |
19.8 |
22.6 |
12.4 |
6.1 |
2.5 |
5.0 |
4.5 |
4.7 |
4.0 |
1.7 |
12.5 |
11.6 |
| U. S. N. M. |
1060 |
♀ ad. |
México |
39.0 |
14.0 |
11.0 |
9.6 |
|
21.0 |
22.5 |
|
|
|
4.9 |
4.6 |
4.9 |
3.9 |
1.9 |
|
11.5 |
| M. C. Z. |
2605 |
♀ yg. |
Jalpa, Veracruz |
38.7 |
13.8 |
10.3 |
8.3 |
12.6 |
19.4 |
|
12.7 |
5.8 |
|
4.9 |
4.5 |
4.6 |
3.5 |
1.5 |
13.7 |
10.9 |
| F. M. N. H. |
14050 |
♀ yg. |
Xuchil, Veracruz |
39.0 |
14.2 |
11.6 |
9.3 |
13.0 |
20.5 |
23.5 |
13.5 |
6.1 |
|
5.3 |
4.9 |
5.3 |
4.4 |
2.0 |
15.3 |
11.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata perda |
| U. S. N. M. |
100041 |
♂ sad. |
Teapa, Tabasco |
46.1 |
17.0 |
14.4 |
11.1 |
15.3 |
24.4 |
28.5 |
14.9 |
6.6 |
2.2 |
6.4 |
5.5 |
6.0 |
4.6 |
2.5 |
16.4 |
13.8 |
| U. S. N. M. |
132997 |
♂ sad. |
San Vicente, Chiapas |
45.3 |
16.7 |
13.9 |
12.0 |
15.5 |
24.0 |
27.4 |
14.5 |
6.7 |
2.8 |
5.5 |
5.1 |
5.4 |
4.2 |
2.2 |
15.8 |
13.0 |
| U. S. N. M. |
132996 |
♂ sad. |
San Cristóbal, Chiapas |
|
16.9 |
13.3 |
10.6 |
16.0 |
|
|
|
|
|
6.4 |
5.5 |
6.0 |
4.5 |
2.5 |
|
13.0 |
|
|
av. 3 |
|
45.7 |
16.9 |
13.9 |
11.2 |
15.6 |
24.2 |
28.0 |
14.7 |
6.7 |
2.5 |
6.1 |
5.4 |
5.8 |
4.4 |
2.4 |
16.1 |
13.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| U. S. N. M. |
218036 |
♀ sad. |
State of Chiapas |
40.0 |
14.6 |
11.6 |
9.4 |
13.5 |
20.2 |
23.2 |
13.0 |
5.6 |
2.1 |
5.2 |
4.8 |
5.0 |
3.7 |
1.7 |
13.2 |
11.4 |
| U. S. N. M. |
65422 |
♀ sad. |
Catemaco, Veracruz |
40.4 |
14.5 |
11.5 |
9.1 |
13.2 |
21.2 |
23.0 |
13.7 |
6.5 |
2.1 |
4.8 |
4.7 |
5.2 |
3.8 |
1.9 |
14.2 |
10.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata nicaraguae |
| A. M. N. H. |
30754 |
♂ sad. |
Matagalpa, Nicaragua |
44.8 |
17.2 |
12.8 |
10.5 |
15.0 |
23.4 |
25.5 |
14.0 |
6.5 |
2.9 |
6.3 |
6.0 |
6.3 |
4.6 |
2.5 |
15.5 |
13.4 |
| A. M. N. H. |
28331 |
♂ ad. |
Matagalpa, Nicaragua |
44.8 |
16.7 |
13.6 |
11.5 |
15.2 |
22.7 |
26.8 |
15.2 |
6.7 |
|
5.8 |
5.1 |
5.5 |
4.4 |
2.4 |
14.7 |
13.4 |
| A. M. N. H. |
29280 |
♂ sad. |
San Rafel Del Norte |
45.5 |
17.2 |
13.5 |
11.0 |
15.5 |
23.5 |
27.4 |
14.2 |
6.8 |
|
6.3 |
6.0 |
6.3 |
4.7 |
2.5 |
15.5 |
13.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata costaricensis |
| U. S. N. M. |
11408 |
♂ ad. |
Costa Rica |
*49.0 |
18.3 |
15.2 |
9.4 |
*18.0 |
26.0 |
30.5 |
15.0 |
7.5 |
|
6.5 |
5.9 |
6.4 |
5.0 |
2.6 |
16.7 |
14.2 |
| B. M. |
3216 |
♂ ad. |
Vic. San José, Costa Rica |
49.3 |
18.6 |
15.0 |
12.7 |
16.9 |
25.9 |
31.3 |
15.3 |
7.3 |
2.9 |
6.6 |
6.0 |
6.5 |
4.9 |
2.5 |
16.8 |
15.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| U. S. N. M. |
13770/3714 |
9 ♂ yg. |
San José, Costa Rica |
48.2 |
18.0 |
14.0 |
11.7 |
16.0 |
24.8 |
29.0 |
15.0 |
7.2 |
2.7 |
7.0 |
6.0 |
6.5 |
4.8 |
2.9 |
16.5 |
14.0 |
| N. H. R. S. |
1-138 |
♂ ad. |
Azahar Cartago, Costa Rica |
47.8 |
17.4 |
14.9 |
11.7 |
15.5 |
24.3 |
*30.1 |
15.1 |
7.5 |
|
6.6 |
5.9 |
6.2 |
4.8 |
2.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| B. Z. M. |
A 59.13 |
♀ sad. |
Irazú, 3000M., Costa Rica |
38.8 |
14.4 |
12.5 |
10.1 |
14.2 |
20.0 |
23.6 |
12.8 |
6.3 |
2.9 |
4.9 |
4.7 |
5.0 |
3.7 |
1.8 |
13.7 |
11.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata panamensis |
| M. C. Z. |
10112 |
♂ ad. |
Boquete, Panamá |
48.3 |
17.1 |
15.0 |
12.5 |
15.7 |
25.1 |
28.3 |
14.1 |
7.0 |
2.7 |
6.3 |
5.7 |
5.9 |
4.5 |
2.5 |
16.7 |
14.0 |
| A. M. N. H. |
18848 |
♂ ad. |
Boquete, Panamá |
44.5 |
16.3 |
14.0 |
12.0 |
15.0 |
22.5 |
29.1 |
14.4 |
6.1 |
2.7 |
6.2 |
5.7 |
6.0 |
4.5 |
2.3 |
16.0 |
13.5 |
| M. C. Z. |
10113 |
♂ ad. |
Boquete, Panamá |
42.8 |
15.8 |
14.0 |
12.0 |
15.5 |
|
27.1 |
13.6 |
5.5 |
|
6.1 |
5.4 |
5.7 |
4.3 |
2.2 |
15.8 |
13.5 |
|
|
av. 3 |
|
45.2 |
16.4 |
14.3 |
12.2 |
15.4 |
23.8 |
28.2 |
14.0 |
6.2 |
2.7 |
6.2 |
5.6 |
5.9 |
4.4 |
2.3 |
16.3 |
13.7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| U. S. N. M. |
170970 |
♀ sad. ne |
ar Gatún, Panamá |
41.3 |
15.3 |
12.5 |
10.4 |
14.3 |
23.0 |
26.8 |
12.5 |
6.1 |
|
5.7 |
5.3 |
5.5 |
4.1 |
2.1 |
15.5 |
12.5 |
| A. N. S. P. |
18434 |
♀ ad. Si |
ola, Panamá |
39.3 |
14.2 |
11.3 |
9.4 |
12.5 |
20.1 |
22.5 |
13.0 |
6.0 |
|
5.0 |
4.6 |
5.0 |
3.7 |
2.0 |
13.2 |
10.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata meridana |
| A. M. N. H. |
33154 |
♂ ad. |
Mérida, Venezuela |
44.3 |
16.7 |
14.2 |
12.0 |
15.0 |
23.9 |
28.5 |
13.0 |
6.6 |
3.0 |
6.2 |
5.4 |
5.6 |
4.4 |
2.5 |
15.0 |
13.4 |
| U. S. N. M. |
137517 |
♂ ad. |
Mérida, Venezuela |
43.4 |
16.0 |
14.1 |
11.8 |
15.2 |
23.5 |
27.4 |
13.8 |
6.5 |
2.4 |
6.0 |
5.5 |
5.7 |
4.1 |
2.3 |
15.6 |
13.3 |
| U. S. N. M. |
172959 |
♂ ad. |
Mérida, Venezuela |
44.0 |
16.5 |
14.1 |
11.6 |
15.4 |
23.6 |
28.0 |
13.9 |
7.0 |
|
6.2 |
5.6 |
5.7 |
4.7 |
2.4 |
15.7 |
12.9 |
| A. M. N. H. |
24309 |
♂ ad. |
Mérida, Venezuela |
44.2 |
16.1 |
13.7 |
11.5 |
15.4 |
23.6 |
27.3 |
14.2 |
6.4 |
2.3 |
5.9 |
5.2 |
5.6 |
4.5 |
2.3 |
15.3 |
13.0 |
| A. M. N. H. |
33155 |
♂ ad. |
Mérida, Venezuela |
42.3 |
16.7 |
13.8 |
11.5 |
15.5 |
23.0 |
27.6 |
13.5 |
6.5 |
2.6 |
5.8 |
5.4 |
5.6 |
4.3 |
2.1 |
15.5 |
13.7 |
|
|
av. 5 |
|
43.6 |
16.4 |
14.0 |
11.7 |
15.3 |
23.5 |
27.8 |
13.7 |
6.6 |
|
6.0 |
5.4 |
5.6 |
4.4 |
2.3 |
16.0 |
13.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| U. S. N. M. |
123341 |
♂ sad. |
Mérida, Venezuela |
43.5 |
16.9 |
13.8 |
12.1 |
15.1 |
23.0 |
27.5 |
13.2 |
|
|
6.0 |
5.5 |
5.7 |
4.5 |
2.5 |
|
|
| U. S. N. M. |
137516 |
♂ sad. |
Mérida, Venezuela |
43.6 |
16.2 |
13.5 |
11.4 |
15.5 |
23.4 |
27.9 |
13.4 |
6.4 |
2.2 |
5.6 |
5.2 |
5.7 |
4.2 |
2.3 |
16.0 |
13.0 |
| U. S. N. M. |
143667 |
♂ sad. |
Mérida, Venezuela |
|
15.4 |
12.5 |
10.5 |
15.4 |
|
25.0 |
13.2 |
|
|
5.5 |
5.0 |
5.3 |
4.2 |
2.3 |
15.0 |
13.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| U. S. N. M. |
143666 |
♀ ad. |
Mérida, Venezuela |
38.2 |
14.1 |
11.9 |
9.7 |
13.2 |
20.3 |
23.0 |
12.4 |
5.9 |
1.8 |
|
|
|
|
|
|
|
| U. S. N. M. |
143665 |
♀ ad. |
Mérida, Venezuela |
36.4 |
13.4 |
11.5 |
9.9 |
13.0 |
20.1 |
23.4 |
12.1 |
5.9 |
2.0 |
|
|
|
|
|
|
|
| A. M. N. H. |
24308 |
♀ ad. |
Mérida, Venezuela |
36.3 |
13.4 |
11.4 |
9.7 |
11.8 |
18.8 |
22.0 |
11.7 |
5.5 |
2.0 |
4.9 |
4.5 |
4.6 |
3.5 |
1.6 |
|
10.5 |
| A. M. N. H. |
24311 |
♀ ad. |
Mérida, Venezuela |
37.7 |
13.9 |
11.4 |
9.8 |
13.8 |
19.5 |
|
12.1 |
5.9 |
1.8 |
4.9 |
4.7 |
4.9 |
3.7 |
1.8 |
13.0 |
11.2 |
|
|
av. 4 |
|
37.2 |
13.7 |
11.6 |
9.8 |
13.0 |
19.7 |
22.8 |
12.1 |
5.8 |
1.9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| A. M. N. H. |
21343 |
♀ sad. |
Mérida, Venezuela |
37.3 |
13.6 |
11.1 |
9.2 |
13.2 |
19.2 |
22.5 |
11.9 |
5.6 |
2.1 |
5.1 |
4.6 |
4.8 |
3.5 |
1.7 |
13.5 |
11.1 |
| A. M. N. H. |
24310 |
♀ sad. |
Mérida, Venezuela |
35.0 |
12.6 |
11.1 |
8.7 |
12.0 |
19.3 |
21.8 |
11.5 |
5.8 |
1.8 |
4.7 |
4.4 |
4.7 |
3.5 |
1.6 |
13.8 |
11.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata affinis |
| U. S. N. M. |
241314 |
♂ ad. |
Choachí, Colombia |
*46.0 |
17.4 |
13.6 |
11.8 |
15.6 |
|
29.5 |
|
|
|
6.1 |
5.9 |
6.1 |
4.4 |
2.3 |
|
13.9 |
| U. S. N. M. |
239946 |
♂ ad. |
Choachí, Colombia |
|
16.1 |
12.7 |
10.8 |
14.3 |
|
27.7 |
|
|
|
6.0 |
5.3 |
5.7 |
4.5 |
2.1 |
|
13.0 |
| A. M. N. H. |
35805 |
♂ ad. |
Quetame, Colombia |
45.5 |
16.6 |
13.7 |
11.0 |
15.3 |
|
|
|
6.4 |
|
5.8 |
5.5 |
5.7 |
4.4 |
2.0 |
|
13.3 |
|
|
av. 3 |
|
45.8 |
16.7 |
13.3 |
11.2 |
15.1 |
|
28.6 |
|
|
|
6.0 |
5.6 |
5.8 |
4.4 |
2.1 |
|
13.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| U. S. N. M. |
241313 |
♂ yg. |
Bogotá, Columbia |
44.7 |
16.7 |
13.9 |
12.3 |
15.6 |
23.9 |
29.0 |
|
|
|
5.9 |
5.7 |
5.7 |
4.4 |
2.5 |
|
|
| U. S. N. M. |
241315 |
♂ yg. |
Choachí, Columbia |
45.1 |
16.7 |
13.5 |
11.5 |
15.2 |
23.9 |
28.7 |
14.0 |
6.9 |
|
5.9 |
5.6 |
5.8 |
4.5 |
2.1 |
|
13.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata aureoventris |
| A. M. N. H. |
34677 |
♂ yg. |
Gualea, Ecuador |
45.6 |
17.6 |
14.0 |
11.8 |
15.4 |
23.8 |
28.3 |
15.1 |
6.8 |
2.7 |
6.7 |
6.0 |
6.4 |
5.0 |
3.0 |
17.0 |
14.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata helleri |
| F. M. N. H. |
24133 |
♂ ad. |
Rio Chinchao |
44.0 |
16.4 |
14.5 |
12.5 |
14.9 |
24.5 |
29.1 |
14.4 |
6.5 |
2.4 |
6.2 |
5.8 |
5.9 |
4.6 |
2.5 |
16.0 |
14.0 |
| F. M. N. H. |
24132 |
♂ ad. |
Rio Chinchao |
45.3 |
17.0 |
13.9 |
11.7 |
15.5 |
24.5 |
29.0 |
14.4 |
6.5 |
2.4 |
5.8 |
5.5 |
5.8 |
4.5 |
2.5 |
16.0 |
13.7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| F. M. N. H. |
24136 |
♀ ad. |
Huanuco, Perú |
35.3 |
13.0 |
10.5 |
8.9 |
12.1 |
17.9 |
20.8 |
11.8 |
4.7 |
2.0 |
4.7 |
4.4 |
4.6 |
3.5 |
1.6 |
11.8 |
10.0 |
| F. M. N. H. |
24135 |
♀ sad. |
Ambo, Perú |
36.1 |
13.6 |
10.9 |
9.0 |
12.4 |
18.8 |
22.4 |
11.8 |
5.0 |
1.9 |
4.7 |
4.4 |
4.6 |
3.6 |
1.7 |
12.6 |
10.5 |
| F. M. N. H. |
24134 |
♀ sad. |
Ambo, Perú |
38.1 |
14.0 |
10.6 |
9.1 |
12.6 |
19.8 |
22.2 |
12.0 |
5.4 |
2.0 |
4.7 |
4.6 |
4.6 |
3.5 |
1.5 |
13.9 |
11.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata agilis |
| B. M. 8 |
.1.10.1 |
♂ ad. |
Lima, Perú |
42.5 |
16.0 |
13.4 |
11.1 |
14.9 |
23.1 |
28.2 |
13.8 |
6.9 |
2.6 |
5.8 |
5.5 |
5.8 |
4.5 |
2.2 |
14.8 |
12.9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| F. M. N. H. |
21147 |
♀ ad. |
Macate, Perú |
35.2 |
12.7 |
10.0 |
8.4 |
12.0 |
18.0 |
20.6 |
12.4 |
5.0 |
1.7 |
4.4 |
4.1 |
4.4 |
3.2 |
1.5 |
13.0 |
10.3 |
| M. P. H. N. |
565 |
♀ ad. |
Perú |
*36.0 |
12.6 |
10.7 |
9.2 |
12.5 |
18.1 |
21.5 |
13.0 |
5.8 |
2.2 |
4.8 |
4.1 |
4.5 |
3.5 |
1.9 |
12.0 |
11.7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata macrura |
| M. P. H. N. |
561 |
♂ ad. |
Junín, Perú |
45.5 |
16.6 |
14.0 |
11.6 |
15.1 |
23.8 |
29.0 |
14.5 |
6.2 |
|
5.5 |
5.4 |
5.5 |
4.3 |
2.1 |
15.7 |
13.2 |
| M. P. H. N. |
562 |
♂ ad. |
Junín, Perú |
40.8 |
15.0 |
13.6 |
11.3 |
15.1 |
22.9 |
26.5 |
14.5 |
7.5 |
2.7 |
5.8 |
5.0 |
5.1 |
4.0 |
2.0 |
14.6 |
12.9 |
| B. M. 2 |
6.2.1.2 |
♂ ad. |
Yana Mayo, Perú |
42.6 |
15.9 |
13.0 |
10.2 |
15.0 |
21.8 |
26.2 |
13.5 |
7.0 |
2.5 |
5.8 |
5.5 |
5.6 |
4.4 |
2.3 |
14.6 |
12.3 |
| U. S. N. M. |
148528 |
♂ ad. |
Marcapata, Perú |
|
16.0 |
|
|
14.5 |
|
|
|
|
|
5.7 |
5.0 |
5.2 |
4.3 |
2.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| M. P. H. N. |
564 |
♀ ad. |
Cutervo, Perú |
*38.0 |
13.8 |
11.2 |
9.5 |
13.0 |
18.1 |
*23.0 |
|
|
|
4.9 |
4.6 |
4.9 |
3.6 |
1.9 |
|
11.7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| A. M. N. H. |
60508 |
♂ ad. |
El Chiral, Ecuador |
45.5 |
17.1 |
13.8 |
11.5 |
14.5 |
24.2 |
23.2 |
15.0 |
7.0 |
2.3 |
5.9 |
5.6 |
6.0 |
4.8 |
2.6 |
16.4 |
13.8 |
| A. M. N. H. |
61406 |
♂ sad. |
Guainche, Ecuador |
48.2 |
17.3 |
14.5 |
12.3 |
15.7 |
25.6 |
30.3 |
15.6 |
7.2 |
3.0 |
6.1 |
5.2 |
5.4 |
4.5 |
2.3 |
15.7 |
13.9 |
| N. H. R. S. |
2 |
♂ ad. |
Panecillo, Ecuador |
48.0 |
16.8 |
13.3 |
|
14.8 |
|
|
14.5 |
7.0 |
|
6.0 |
5.4 |
5.7 |
4.3 |
2.2 |
|
|
| N. H. R. S. |
5 |
♂ ad. |
San Antonio, Ecuador |
44.0 |
16.3 |
14.0 |
11.9 |
14.8 |
23.5 |
28.0 |
14.2 |
7.0 |
2.9 |
5.8 |
5.1 |
5.4 |
4.3 |
2.1 |
16.8 |
14.1 |
| N. H. R. S. |
7 |
♂ ad. |
Carapungo, Ecuador |
46.8 |
17.4 |
13.5 |
11.1 |
14.9 |
23.7 |
29.0 |
14.6 |
7.0 |
2.9 |
6.2 |
5.8 |
6.2 |
4.5 |
2.3 |
16.0 |
14.2 |
| N. H. R. S. |
14 |
♂ ad. |
Nára Papallacta, Ecuador |
45.4 |
17.3 |
14.1 |
12.6 |
15.8 |
24.5 |
29.9 |
14.3 |
7.1 |
2.6 |
6.1 |
5.4 |
5.6 |
4.3 |
2.5 |
17.5 |
9.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| N. H. R. S. |
10 |
♀ ad. |
Guapulo, Ecuador |
37.9 |
13.5 |
11.3 |
9.5 |
12.7 |
19.7 |
22.5 |
12.4 |
5.9 |
2.0 |
5.1 |
4.5 |
4.7 |
3.7 |
1.7 |
12.9 |
11.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela frenata boliviensis |
| A. M. N. H. |
72587 |
♂ ad. |
Nequejahuira, Bolivia |
41.6 |
15.3 |
12.2 |
10.0 |
14.7 |
22.2 |
25.0 |
13.4 |
7.4 |
2.2 |
5.3 |
5.0 |
5.2 |
4.4 |
2.2 |
14.8 |
12.4 |
| B. Z. M. |
602 |
♂ yg. |
Limbaní, Perú |
42.4 |
15.5 |
|
|
|
|
|
5 |
.5 |
5.0 |
5.4 |
4.2 |
2.2 |
|
|
|
|
| U. S. N. M. |
137513 |
♂ yg. |
Limbaní, Perú |
40.3 |
15.2 |
|
|
|
|
|
5 |
.1 |
4.8 |
5.1 |
3.9 |
1.9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela africana africana |
| A. M. N. H. |
374.75 |
♂ sad. |
Pará, Brazil |
47.8 |
17.2 |
14.3 |
12.9 |
15.4 |
26.4 |
32.2 |
16.9 |
8.2 |
2.8 |
6.6 |
5.8 |
6.2 |
4.7 |
2.4 |
17.8 |
14.9 |
| B. M. 5. |
1.25.1. |
♂? sad. |
Pará, Brazil |
44.9 |
16.4 |
13.0 |
10.2 |
14.2 |
23.3 |
26.8 |
14.4 |
7.0 |
2.7 |
5.9 |
5.4 |
5.5 |
4.5 |
1.8 |
16.4 |
13.7 |
| B. M. 26. |
1.8.10. |
♂? ad. |
Pará, Brazil |
44.6 |
16.4 |
13.3 |
10.9 |
14.4 |
24.4 |
29.4 |
14.4 |
6.7 |
2.5 |
6.0 |
5.6 |
5.7 |
4.5 |
1.8 |
15.5 |
13.6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela africana stolzmanni |
| B. M. 24.12 |
.12.24. |
♀ ad. |
Moyobamba, Perú |
45.8 |
16.9 |
13.5 |
12.0 |
14.6 |
23.5 |
28.8 |
14.5 |
6.2 |
2.6 |
5.9 |
5.4 |
4.9 |
4.3 |
1.8 |
16.5 |
13.9 |
| M. P. H. N. |
563 |
♀ ad. |
Yurimaguas, Perú |
|
17.5± |
|
|
|
|
|
|
|
|
6.0 |
5.8 |
5.6 |
4.4 |
2.3 |
|
|
| A. M. N. H. |
61813 |
♀ yg. |
Val. d. Perené, Perú |
44.6 |
16.0 |
13.0 |
11.0 |
15.4 |
24.1 |
28.9 |
15.7 |
7.0 |
2.8 |
6.2 |
5.6 |
5.9 |
4.5 |
2.2 |
16.8 |
13.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela erminea arctica |
| M. C. Z. |
10012 |
♂ ad. |
Pt. Barrow |
43.3 |
15.9 |
15.2 |
12.0 |
16.2 |
23.5 |
27.6 |
15.5 |
8.4 |
|
5.6 |
5.1 |
5.1 |
3.9 |
2.0 |
13.3 |
13.2 |
| F. M. N. H. |
35894 |
♂ ad. |
Pt. Barrow |
41.8 |
15.6 |
15.3 |
13.3 |
16.0 |
23.2 |
27.6 |
15.1 |
8.5 |
|
6.0 |
5.2 |
5.3 |
4.0 |
2.2 |
14.1 |
13.5 |
| A. N. S. P. |
6909 |
♂ ad. |
Pt. Barrow |
42.3 |
15.1 |
14.8 |
11.6 |
15.3 |
22.9 |
26.2 |
15.2 |
8.4 |
|
5.9 |
5.1 |
5.3 |
4.1 |
2.4 |
14.5 |
12.5 |
| A. N. S. P. |
6910 |
♂ ad. |
Pt. Barrow |
|
16.2 |
16.0 |
12.7 |
16.7 |
|
|
|
|
|
5.7 |
5.4 |
5.7 |
4.3 |
2.1 |
|
|
| N. M. C. |
2445 |
♂ ad. |
Salirochet River |
42.8 |
15.7 |
15.4 |
13.0 |
16.0 |
23.5 |
28.5 |
15.2 |
8.0 |
|
5.6 |
5.0 |
5.2 |
4.0 |
2.0 |
14.3 |
13.3 |
|
|
av. 5 |
|
42.5 |
15.7 |
15.3 |
12.5 |
16.0 |
23.3 |
27.5 |
15.3 |
8.3 |
|
5.8 |
5.2 |
5.3 |
4.1 |
2.1 |
14.1 |
13.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| F. M. N. H. |
35895 |
♀ ad. |
Pt. Barrow |
35.4 |
13.1 |
12.7 |
10.3 |
13.3 |
19.3 |
23.0 |
12.7 |
7.2 |
|
5.0 |
4.5 |
4.8 |
3.5 |
1.7 |
12.0 |
10.5 |
| M. V. Z. |
43286 |
♀ ad. |
Pt. Barrow |
35.4 |
12.9 |
12.0 |
10.0 |
13.2 |
18.8 |
21.8 |
12.6 |
6.7 |
|
4.6 |
4.2 |
4.3 |
3.2 |
1.8 |
11.4 |
10.3 |
| U. S. N. M. |
243489 |
♀ sad. |
Alatna River |
37.0 |
13.4 |
11.9 |
9.4 |
13.5 |
19.4 |
22.0 |
13.2 |
7.2 |
|
4.7 |
4.3 |
4.5 |
3.3 |
1.7 |
11.9 |
10.6 |
| U. S. N. M. |
243493 |
♀ sad. |
16 mi. below Bettles |
36.2 |
13.0 |
12.0 |
9.4 |
13.0 |
19.1 |
21.8 |
12.9 |
7.2 |
|
5.0 |
4.2 |
4.5 |
3.3 |
1.8 |
11.9 |
10.3 |
| U. S. N. M. |
180459 |
♀ ad. |
N. Fk. Kuskokim |
35.3 |
12.9 |
11.2 |
8.8 |
12.8 |
18.9 |
20.7 |
12.7 |
7.0 |
|
4.7 |
4.3 |
4.5 |
3.3 |
1.8 |
10.5 |
9.7 |
| U. S. N. M. |
242205 |
♀ ad. |
Fairbanks |
34.5 |
12.4 |
11.5 |
9.3 |
13.0 |
18.0 |
|
12.7 |
6.8 |
|
4.8 |
4.3 |
4.5 |
3.3 |
1.7 |
11.8 |
10.2 |
| U. S. N. M. |
157306 |
♀ sad. |
Bear Creek |
36.9 |
13.5 |
12.6 |
9.5 |
13.6 |
18.7 |
20.9 |
13.0 |
6.9 |
|
4.8 |
4.4 |
4.5 |
3.3 |
1.8 |
11.4 |
10.2 |
| U. S. N. M. |
157305 |
♀ sad. |
Bear Creek |
35.2 |
12.5 |
11.4 |
9.6 |
12.8 |
17.8 |
20.5 |
12.6 |
6.3 |
|
4.8 |
4.2 |
4.4 |
3.3 |
1.6 |
11.2 |
10.1 |
|
|
av. 8 |
|
35.8 |
12.9 |
11.8 |
9.3 |
13.1 |
18.7 |
21.2 |
12.9 |
6.9 |
|
4.8 |
4.3 |
4.5 |
3.3 |
1.7 |
11.5 |
10.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela erminea polaris |
| M. C. Z. |
29831 |
♂ ad. |
Ymer Is. |
41.6 |
15.6 |
15.3 |
12.0 |
13.0 |
22.3 |
26.1 |
14.8 |
8.0 |
|
5.8 |
5.2 |
5.7 |
4.1 |
2.2 |
13.7 |
12.3 |
| C. Z. M. |
1245 |
♂ ad. |
Danmarks Havn. |
40.7 |
15.0 |
14.7 |
11.9 |
14.9 |
22.3 |
25.5 |
14.6 |
7.8 |
|
5.9 |
5.1 |
5.6 |
3.9 |
2.0 |
13.4 |
12.2 |
| C. Z. M. |
1246 |
♂ ad. |
Danmarks Havn. |
39.0 |
14.1 |
13.8 |
10.9 |
14.2 |
21.3 |
24.7 |
14.5 |
7.3 |
|
5.3 |
4.8 |
5.0 |
3.7 |
1.9 |
12.0 |
11.2 |
| C. Z. M. |
1247 |
♂ ad. |
Danmarks Havn. |
41.0 |
15.2 |
14.5 |
11.5 |
14.4 |
22.3 |
26.7 |
14.4 |
7.6 |
|
5.7 |
5.1 |
5.5 |
4.1 |
2.0 |
13.1 |
12.1 |
| C. Z. M. |
1248 |
♂ ad. |
Danmarks Havn. |
42.3 |
15.5 |
15.5 |
12.0 |
15.3 |
23.2 |
27.9 |
15.1 |
8.1 |
|
5.8 |
5.5 |
5.8 |
4.0 |
2.3 |
13.8 |
12.8 |
| C. Z. M. |
1249 |
♂ ad. |
Danmarks Havn. |
42.2 |
15.8 |
15.0 |
12.2 |
15.1 |
23.2 |
28.0 |
15.2 |
8.1 |
|
5.9 |
5.2 |
5.7 |
4.2 |
2.3 |
13.7 |
12.4 |
| C. Z. M. |
1871 |
♂ ad. |
Scoresby Sd. |
42.4 |
15.3 |
15.3 |
12.3 |
15.6 |
|
|
15.4 |
8.3 |
|
5.7 |
5.1 |
5.3 |
3.9 |
2.0 |
14.4 |
12.8 |
|
|
av. 7 |
|
41.3 |
15.2 |
14.9 |
11.8 |
14.9 |
22.4 |
26.5 |
14.9 |
7.9 |
|
5.7 |
5.2 |
5.5 |
4.0 |
2.1 |
13.4 |
12.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| C. Z. M. |
1060 |
♀ ad. |
Turner Sd. |
35.9 |
13.0 |
12.5 |
10.1 |
12.9 |
19.6 |
22.6 |
13.6 |
7.1 |
|
5.0 |
4.5 |
4.7 |
3.6 |
1.7 |
12.3 |
10.3 |
| B. Z. M. |
43965 |
♀ ad. |
Kap Hoegh |
37.8 |
13.4 |
12.7 |
10.4 |
13.2 |
19.3 |
22.4 |
13.9 |
7.0 |
|
4.8 |
4.2 |
4.5 |
3.7 |
1.8 |
12.1 |
10.9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela erminea semplei |
| C. M. |
6688 |
♂ ad. |
Prairie Point |
*39.9 |
14.5 |
13.5 |
11.5 |
14.6 |
21.5 |
24.8 |
14.1 |
7.9 |
|
5.0 |
4.6 |
4.7 |
3.6 |
2.0 |
14.0 |
11.6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♂ 1 ad. and 10
sad . |
Southampton Isl. |
{37.5 |
13.8 |
13.1 |
10.6 |
13.8 |
20.2 |
23.6 |
13.6 |
7.2 |
|
5.1 |
4.7 |
4.9 |
3.6 |
2.0 |
13.4 |
11.0 |
| maximum} |
{39.9 |
14.5 |
13.5 |
11.5 |
14.8 |
21.5 |
24.8 |
15.0 |
7.9 |
|
5.3 |
4.9 |
5.0 |
3.7 |
2.3 |
14.0 |
11.6 |
| minimum} |
{35.7 |
13.1 |
12.6 |
9.7 |
12.7 |
19.2 |
22.2 |
12.9 |
6.4 |
|
4.8 |
4.4 |
4.5 |
3.4 |
1.8 |
12.3 |
10.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| C. M. |
8474 |
♀ ad. |
Minnimunnek Pt. |
32.6 |
11.9 |
11.3 |
9.2 |
12.2 |
18.5 |
21.2 |
12.2 |
6.6 |
|
4.4 |
3.9 |
4.2 |
3.0 |
1.5 |
11.9 |
9.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♀ 1 ad. and 4
sad . |
Southampton Isl. |
{34.2 |
12.4 |
11.7 |
9.5 |
12.6 |
18.8 |
20.9 |
12.8 |
6.7 |
|
4.5 |
4.1 |
4.3 |
3.1 |
1.6 |
12.1 |
10.1 |
| maximum} |
{35.1 |
13.0 |
12.7 |
9.9 |
13.1 |
19.4 |
21.5 |
13.4 |
7.0 |
|
4.3 |
4.3 |
4.5 |
3.3 |
1.7 |
12.4 |
10.4 |
| minimum} |
{32.6 |
11.9 |
11.3 |
9.2 |
12.2 |
18.1 |
20.1 |
12.2 |
6.6 |
|
4.6 |
3.9 |
4.2 |
3.0 |
1.5 |
11.9 |
9.6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela erminea kadiacensis |
| U. S. N. M. |
107496 |
♂ ad. |
Kadiak |
43.2 |
15.4 |
14.9 |
11.1 |
14.9 |
22.1 |
26.0 |
15.1 |
7.6 |
|
5.5 |
5.1 |
5.1 |
3.8 |
2.0 |
14.0 |
12.2 |
| F. M. N. H. |
7290 |
♂ ad. |
Kadiak Id. |
42.1 |
14.9 |
14.0 |
10.9 |
14.4 |
21.6 |
26.0 |
14.1 |
7.1 |
|
5.7 |
5.2 |
5.2 |
3.8 |
1.7 |
13.5 |
11.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| U. S. N. M. |
98042 |
♀ ad. |
Kadiak |
33.0 |
12.0 |
10.2 |
8.0 |
11.3 |
16.9 |
19.4 |
11.5 |
6.0 |
|
4.4 |
4.0 |
4.0 |
2.8 |
1.4 |
11.5 |
8.9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela erminea richardsonii |
| average} |
|
♂ ad. 6 |
3 mi. S Big Isl. |
{40.9 |
14.3 |
12.5 |
10.2 |
13.5 |
21.2 |
24.2 |
14.9 |
7.9 |
|
5.3 |
4.7 |
4.8 |
3.8 |
2.1 |
13.0 |
11.9 |
| maximum} |
{43.7 |
15.0 |
13.2 |
11.1 |
14.0 |
22.2 |
25.5 |
15.5 |
8.3 |
|
6.0 |
5.0 |
5.2 |
4.2 |
2.3 |
14.2 |
11.4 |
| minimum} |
{39.6 |
13.8 |
11.7 |
9.4 |
12.9 |
20.0 |
22.9 |
14.2 |
7.2 |
|
4.9 |
4.5 |
4.6 |
3.7 |
2.0 |
12.5 |
12.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♂ sad. 7 |
3 mi. S Big Isl. |
{40.2 |
14.1 |
12.2 |
9.8 |
14.0 |
20.6 |
23.3 |
14.6 |
7.7 |
|
5.2 |
4.7 |
4.9 |
3.7 |
2.1 |
13.6 |
11.8 |
| maximum} |
{41.5 |
14.7 |
12.6 |
10.4 |
14.2 |
21.5 |
24.4 |
15.1 |
8.2 |
|
5.5 |
4.9 |
5.0 |
3.8 |
2.5 |
14.5 |
12.2 |
| minimum} |
{38.4 |
13.6 |
12.0 |
9.5 |
13.7 |
20.0 |
22.0 |
13.7 |
7.0 |
|
5.0 |
4.5 |
4.7 |
3.5 |
2.0 |
12.8 |
11.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| U. S. N. M. |
136112 |
♀ ad. |
Willow River |
33.0 |
11.5 |
8.8 |
7.6 |
11.4 |
15.9 |
18.0 |
12.5 |
5.7 |
|
4.0 |
3.8 |
3.9 |
2.7 |
1.5 |
11.6 |
8.9 |
| M. C. Z. |
242866 |
♀ sad. |
3 mi. S Big Isl. |
32.3 |
11.0 |
9.0 |
7.2 |
11.7 |
16.6 |
17.7 |
12.3 |
6.5 |
|
4.0 |
3.7 |
3.9 |
3.0 |
1.5 |
10.0 |
8.8 |
| U. S. N. M. |
129703 |
♀ ad. |
Ft. Resolution |
34.2 |
12.0 |
10.4 |
8.2 |
11.5 |
17.2 |
19.7 |
12.3 |
6.5 |
|
4.2 |
4.0 |
4.2 |
3.2 |
1.7 |
12.0 |
9.6 |
| U. S. N. M. |
110682 |
♀ sad. |
15 mi. above Smith Landing |
33.8 |
11.3 |
9.8 |
7.4 |
11.0 |
16.5 |
18.3 |
12.1 |
6.0 |
|
4.2 |
3.8 |
3.9 |
2.9 |
1.5 |
11.2 |
9.3 |
| U. S. N. M. |
235959 |
♀ ad. |
Athabasca Delta |
33.1 |
11.7 |
9.3 |
7.3 |
11.0 |
15.7 |
18.1 |
11.6 |
5.6 |
|
4.0 |
3.8 |
3.8 |
3.0 |
1.6 |
10.5 |
8.6 |
| M. C. Z. |
18776 |
♀ ad. |
Athabasca Delta |
31.5 |
10.8 |
8.9 |
7.6 |
11.2 |
16.3 |
18.3 |
11.6 |
6.3 |
|
3.8 |
3.7 |
3.8 |
3.1 |
1.6 |
11.0 |
8.7 |
|
|
av. 6 |
|
33.0 |
11.4 |
9.4 |
7.6 |
11.3 |
16.4 |
18.3 |
12.1 |
6.3 |
|
4.0 |
3.8 |
3.9 |
3.0 |
1.6 |
11.1 |
9.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela erminea cicognanii |
| M. V. Z. |
53789 |
♂ ad. |
Ogdensburg, N. Y. |
36.6 |
12.9 |
11.5 |
9.1 |
13.2 |
18.5 |
21.7 |
13.3 |
7.0 |
|
4.7 |
4.4 |
4.5 |
3.4 |
1.8 |
12.3 |
10.4 |
| U. S. N. M. |
32240/4406 |
6 ♂ sad. |
Amsterdam, N. Y. |
36.7 |
12.9 |
11.5 |
9.4 |
13.3 |
19.1 |
21.5 |
13.7 |
6.9 |
|
4.6 |
4.4 |
4.5 |
3.4 |
2.0 |
13.2 |
10.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| A. M. N. H. |
67869 |
♂ ad. |
Berlin, N. Y. |
36.2 |
12.5 |
10.2 |
8.9 |
12.7 |
18.8 |
20.6 |
12.9 |
6.7 |
|
4.3 |
3.8 |
4.0 |
3.3 |
1.9 |
11.5 |
10.0 |
| A. M. N. H. |
67868 |
♂ ad. |
Berlin, N. Y. |
34.8 |
12.1 |
9.8 |
8.2 |
12.7 |
17.4 |
19.8 |
12.3 |
6.3 |
|
4.5 |
3.9 |
4.1 |
2.9 |
1.7 |
11.5 |
9.7 |
| A. M. N. H. |
15841 |
♂ ad. |
Schoharie, N. Y. |
33.8 |
11.7 |
9.7 |
7.7 |
11.9 |
17.3 |
20.0 |
11.8 |
6.4 |
|
4.2 |
3.8 |
4.0 |
2.9 |
1.5 |
11.8 |
9.5 |
| Cornell |
494 |
♂ sad. |
Cascadilla Creek, N. Y. |
34.8 |
12.2 |
10.4 |
8.3 |
12.5 |
17.9 |
19.3 |
12.4 |
6.6 |
|
4.4 |
4.1 |
4.2 |
3.0 |
1.6 |
12.2 |
10.3 |
| C. M. |
7461 |
♂ sad. |
Pymatuning Swamp |
34.2 |
12.0 |
9.6 |
8.1 |
12.8 |
17.3 |
19.0 |
11.9 |
6.4 |
|
4.3 |
3.9 |
4.2 |
3.0 |
1.9 |
11.5 |
9.2 |
| C. M. |
10264 |
♂ sad. |
3-1/2 mi. W Linesville |
37.6 |
13.2 |
11.0 |
8.9 |
12.7 |
18.8 |
20.6 |
12.9 |
7.2 |
|
4.9 |
4.3 |
4.5 |
3.5 |
2.0 |
12.6 |
10.6 |
| M. V. Z. |
53788 |
♂ sad. |
Lopez, Penn. |
36.5 |
12.6 |
10.7 |
8.6 |
12.6 |
18.6 |
20.3 |
13.1 |
6.8 |
|
4.5 |
4.1 |
4.3 |
3.2 |
1.6 |
10.9 |
9.9 |
|
|
av. 9 |
|
35.7 |
12.5 |
10.5 |
8.6 |
12.7 |
18.2 |
20.3 |
12.7 |
6.7 |
|
4.5 |
4.1 |
4.3 |
3.2 |
1.8 |
11.9 |
10.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| U. S. N. M. |
135570 |
♀ ad. |
Lake George |
33.3 |
11.8 |
9.3 |
7.8 |
11.4 |
|
|
11.8 |
5.8 |
|
4.4 |
3.8 |
4.0 |
3.0 |
1.8 |
|
9.1 |
| B. S. N. |
994 |
♀ sad. |
Cattaraugus |
31.4 |
10.8 |
9.2 |
7.2 |
10.0 |
15.3 |
|
10.7 |
5.7 |
|
3.8 |
3.7 |
3.6 |
2.7 |
1.5 |
10.3 |
8.2 |
| C. M. |
7460 |
♀ sad. |
Pymatuning Swamp |
32.0 |
10.9 |
9.2 |
7.5 |
11.0 |
15.9 |
17.5 |
11.1 |
5.9 |
|
3.7 |
3.4 |
3.6 |
2.8 |
1.5 |
10.3 |
8.2 |
| C. M. |
10252 |
♀ sad. |
3 mi. NW Linesville |
32.7 |
11.2 |
9.5 |
7.6 |
11.3 |
16.0 |
18.0 |
11.8 |
6.2 |
|
3.9 |
3.6 |
3.7 |
3.1 |
1.7 |
10.9 |
8.8 |
|
|
av. 4 |
|
32.4 |
11.2 |
9.4 |
7.5 |
10.9 |
15.7 |
17.8 |
11.4 |
5.9 |
|
3.9 |
3.6 |
3.7 |
2.9 |
1.6 |
10.5 |
8.6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela erminea bangsi |
| F. M. N. H. |
18134 |
♂ ad. |
Aitkin, Minn. |
40.3 |
14.1 |
12.7 |
10.6 |
14.6 |
20.6 |
23.5 |
14.3 |
7.7 |
|
4.9 |
4.6 |
4.9 |
3.6 |
1.9 |
13.3 |
11.8 |
| F. M. N. H. |
18135 |
♂ ad. |
Aitkin, Minn. |
|
14.1 |
12.5 |
10.4 |
14.4 |
|
24.3 |
|
|
|
5.3 |
4.5 |
4.6 |
3.7 |
2.1 |
|
12.2 |
| F. M. N. H. |
18130 |
♂ ad. |
Aitkin, Minn. |
40.7 |
15.0 |
12.2 |
9.7 |
14.6 |
20.2 |
22.8 |
14.6 |
7.5 |
|
5.2 |
5.0 |
5.1 |
3.8 |
2.6 |
13.5 |
12.0 |
| F. M. N. H. |
18133 |
♂ ad. |
Aitkin, Minn. |
39.3 |
13.9 |
11.2 |
9.0 |
13.0 |
19.5 |
22.0 |
14.3 |
7.2 |
|
5.1 |
4.5 |
4.6 |
3.5 |
2.0 |
13.0 |
11.2 |
| F. M. N. H. |
18131 |
♂ ad. |
Aitkin, Minn. |
38.5 |
13.4 |
11.2 |
9.0 |
12.6 |
18.8 |
21.0 |
13.7 |
7.3 |
|
4.8 |
4.4 |
4.5 |
3.4 |
1.8 |
12.0 |
10.5 |
| F. M. N. H. |
7222 |
♂ ad. |
Aitkin, Minn. |
|
13.6 |
12.4 |
9.5 |
13.3 |
|
|
14.4 |
7.9 |
|
4.8 |
4.4 |
4.4 |
3.5 |
1.8 |
|
11.2 |
| F. M. N. H. |
18127 |
♂ sad. |
Aitkin, Minn. |
38.4 |
13.1 |
11.1 |
9.0 |
13.0 |
19.5 |
22.0 |
14.3 |
6.9 |
|
4.6 |
4.5 |
4.5 |
3.5 |
1.9 |
13.4 |
11.0 |
| F. M. N. H. |
18129 |
♂ sad. |
Aitkin, Minn. |
39.4 |
13.7 |
11.1 |
9.1 |
13.3 |
19.0 |
21.6 |
13.6 |
7.2 |
|
4.7 |
4.3 |
4.5 |
3.4 |
2.0 |
12.8 |
11.1 |
| F. M. N. H. |
18132 |
♂ sad. |
Aitkin, Minn. |
39.3 |
14.3 |
11.5 |
9.5 |
13.2 |
20.2 |
22.8 |
14.3 |
7.2 |
|
4.9 |
4.7 |
4.7 |
3.5 |
2.1 |
12.5 |
11.2 |
| F. M. N. H. |
18441 |
♂ sad. |
Aitkin, Minn. |
40.2 |
14.0 |
12.0 |
9.9 |
14.6 |
19.6 |
22.6 |
15.0 |
7.1 |
|
5.2 |
4.7 |
4.8 |
3.7 |
2.0 |
13.3 |
11.5 |
| F. M. N. H. |
18440 |
♂ sad. |
Aitkin, Minn. |
38.8 |
13.8 |
11.3 |
9.3 |
13.2 |
19.7 |
22.0 |
13.8 |
7.6 |
|
5.1 |
4.5 |
4.7 |
3.7 |
2.0 |
13.0 |
11.1 |
| F. M. N. H. |
7219 |
♂ sad. |
Aitkin, Minn. |
|
13.9 |
11.9 |
10.0 |
13.5 |
|
24.7 |
14.6 |
7.3 |
|
5.3 |
4.6 |
4.8 |
4.0 |
2.1 |
|
11.8 |
|
|
av. 12 |
|
39.4 |
13.9 |
11.8 |
9.6 |
13.6 |
19.7 |
22.7 |
14.3 |
7.4 |
|
5.0 |
4.6 |
4.7 |
3.6 |
2.0 |
13.0 |
11.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♂ ad. 5 and
sad. 5 |
Elk River |
{37.9 |
13.2 |
11.4 |
9.1 |
13.1 |
19.3 |
21.6 |
14.4 |
7.2 |
|
4.7 |
4.3 |
4.4 |
3.4 |
1.9 |
12.7 |
10.8 |
| maximum} |
{39.5 |
13.9 |
12.6 |
9.8 |
14.2 |
20.5 |
22.8 |
15.3 |
8.0 |
|
5.1 |
4.4 |
4.6 |
3.6 |
2.0 |
14.0 |
11.7 |
| minimum} |
{34.8 |
12.0 |
10.5 |
8.5 |
12.2 |
18.0 |
20.8 |
12.4 |
6.3 |
|
4.3 |
4.1 |
4.1 |
3.1 |
1.7 |
12.0 |
10.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Walker |
377 |
♀ ad. |
Deer |
31.8 |
11.0 |
9.0 |
7.5 |
10.9 |
16.6 |
18.4 |
11.7 |
5.9 |
|
4.2 |
3.7 |
3.8 |
2.8 |
1.6 |
11.2 |
9.3 |
| Walker |
11 |
♀ ad. |
Grand Maris |
32.7 |
10.8 |
|
|
11.0 |
15.9 |
17.3 |
12.2 |
5.6 |
|
3.9 |
3.8 |
3.8 |
2.9 |
1.6 |
10.7 |
8.5 |
| Wisc. U. |
8681 |
♀ ad. |
T. 61N, R. 26W |
32.9 |
10.8 |
9.2 |
7.4 |
11.3 |
15.8 |
16.8 |
12.3 |
6.2 |
|
3.8 |
3.6 |
3.6 |
2.8 |
1.4 |
10.0 |
8.3 |
| Wisc. U. |
8679 |
♀ ad. |
T. 61N, R. 26W |
33.6 |
11.6 |
9.8 |
7.6 |
12.2 |
15.7 |
17.1 |
11.9 |
5.7 |
|
4.0 |
3.6 |
3.7 |
3.0 |
1.6 |
9.6 |
8.9 |
| Walker |
A 58 |
♀ ad. |
Elk River |
32.8 |
11.5 |
9.9 |
8.1 |
11.3 |
16.8 |
18.3 |
11.8 |
6.4 |
|
4.0 |
3.8 |
4.0 |
2.9 |
1.7 |
10.5 |
9.2 |
|
|
av. 5 |
|
32.8 |
11.1 |
9.5 |
7.7 |
11.3 |
16.2 |
17.6 |
12.0 |
6.0 |
|
4.0 |
3.7 |
3.9 |
2.9 |
1.6 |
10.4 |
8.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Wisc. U. |
8691 |
♀ ad. |
Fisher Lake |
31.5 |
10.6 |
9.5 |
7.7 |
10.8 |
15.8 |
17.4 |
11.2 |
5.6 |
|
3.8 |
3.5 |
3.7 |
2.6 |
1.4 |
10.4 |
8.5 |
| Wisc. U. |
8674 |
♀ ad. |
Gordon |
32.8 |
11.5 |
9.8 |
7.2 |
11.4 |
15.7 |
16.7 |
11.6 |
5.7 |
|
4.2 |
3.8 |
3.8 |
2.8 |
1.6 |
9.6 |
8.9 |
| Snyder |
2637 |
♀ ad. |
Beaver Dam |
32.9 |
11.0 |
9.8 |
7.8 |
11.7 |
16.9 |
18.9 |
12.2 |
6.4 |
|
4.1 |
3.8 |
3.8 |
3.2 |
1.5 |
11.3 |
9.7 |
| Snyder |
993 |
♀ ad. |
Beaver Dam |
34.1 |
11.3 |
9.4 |
7.5 |
11.8 |
16.7 |
18.0 |
12.8 |
6.1 |
|
4.4 |
3.8 |
3.8 |
3.0 |
1.7 |
10.8 |
9.0 |
| Snyder |
2999 |
♀ ad. |
Beaver Dam |
31.9 |
10.8 |
9.2 |
7.4 |
11.2 |
15.9 |
17.5 |
12.4 |
5.8 |
|
4.0 |
3.6 |
3.8 |
3.0 |
1.6 |
11.4 |
9.2 |
|
|
av. 5 |
|
32.6 |
11.0 |
9.5 |
7.5 |
11.4 |
16.2 |
17.7 |
12.0 |
5.9 |
|
4.1 |
3.7 |
3.8 |
2.9 |
1.6 |
10.7 |
9.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela erminea invicta |
| average} |
|
♂ ad. 5 |
Idaho Co |
{37.0 |
12.8 |
11.4 |
9.1 |
13.1 |
19.0 |
21.3 |
13.6 |
7.0 |
|
4.5 |
4.1 |
4.2 |
3.2 |
1.7 |
11.7 |
10.5 |
| maximum} |
{39.8 |
14.1 |
13.0 |
10.0 |
14.2 |
19.7 |
22.6 |
14.2 |
7.1 |
|
4.9 |
4.3 |
4.5 |
3.5 |
1.9 |
12.3 |
11.3 |
| minimum} |
{35.8 |
12.2 |
10.6 |
8.6 |
12.0 |
18.2 |
20.5 |
13.3 |
6.8 |
|
4.2 |
4.0 |
4.0 |
3.1 |
1.4 |
10.9 |
9.7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| M. V. Z. |
90763 |
♀ ad. |
Pilot Creek, Idaho |
31.6 |
10.8 |
9.2 |
7.0 |
10.5 |
15.6 |
16.5 |
10.9 |
5.6 |
|
4.0 |
3.6 |
3.7 |
2.9 |
1.4 |
9.0 |
8.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♀ ad. 1 and
sad. 4 |
Idaho Co |
{32.2 |
10.6 |
9.0 |
7.1 |
11.1 |
16.3 |
17.2 |
12.0 |
5.9 |
|
3.9 |
3.7 |
3.7 |
2.8 |
1.4 |
9.5 |
8.3 |
| maximum} |
{32.8 |
11.2 |
9.2 |
7.2 |
12.2 |
17.0 |
17.8 |
12.7 |
6.5 |
|
4.3 |
3.9 |
3.9 |
2.9 |
1.5 |
10.0 |
8.7 |
| minimum} |
{31.6 |
10.8 |
8.5 |
7.0 |
10.5 |
15.6 |
16.5 |
10.9 |
5.6 |
|
3.5 |
3.6 |
3.6 |
2.7 |
1.3 |
9.0 |
8.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela erminea alascensis |
| average} |
|
♂ ad. 8 |
Windham |
{37.5 |
13.1 |
11.5 |
9.4 |
13.2 |
19.4 |
21.9 |
13.2 |
6.9 |
|
4.8 |
4.2 |
4.4 |
3.5 |
1.9 |
12.5 |
10.7 |
| maximum} |
{38.9 |
13.7 |
12.3 |
10.1 |
14.3 |
20.5 |
23.7 |
13.7 |
7.4 |
|
5.0 |
4.4 |
4.7 |
3.9 |
2.2 |
13.8 |
11.6 |
| minimum} |
{36.5 |
12.3 |
11.0 |
8.6 |
12.0 |
18.5 |
20.4 |
12.9 |
6.6 |
|
4.5 |
4.1 |
4.2 |
3.3 |
1.8 |
11.8 |
10.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| U. S. N. M. |
74422 |
♀ ad. |
Juneau |
33.2 |
11.4 |
10.5 |
8.3 |
10.5 |
16.2 |
18.3 |
11.5 |
5.7 |
|
3.9 |
3.5 |
3.7 |
2.9 |
1.6 |
10.0 |
8.7 |
| M. V. Z. |
995 |
♀ ad. |
Juneau |
33.1 |
11.3 |
9.4 |
7.8 |
11.3 |
16.3 |
17.8 |
12.0 |
5.8 |
|
3.9 |
3.7 |
3.8 |
2.9 |
1.7 |
|
8.7 |
| M. V. Z. |
473 |
♀ ad. |
Helm Bay |
32.9 |
11.2 |
9.5 |
8.5 |
11.6 |
16.3 |
17.6 |
11.9 |
6.0 |
|
4.0 |
3.7 |
3.9 |
2.6 |
1.4 |
10.5 |
9.3 |
| U. S. N. M. |
74773 |
♀ ad. |
Wrangel |
32.2 |
11.3 |
9.2 |
7.5 |
|
16.0 |
17.6 |
11.9 |
5.8 |
|
4.0 |
3.8 |
4.0 |
3.2 |
1.7 |
11.5 |
8.7 |
| M. V. Z. |
78243 |
♀ sad. |
Windham |
31.9 |
11.1 |
10.1 |
7.7 |
11.5 |
16.8 |
18.0 |
11.5 |
5.7 |
|
4.0 |
3.6 |
3.8 |
3.1 |
1.7 |
10.6 |
8.8 |
|
|
av. 5 |
|
32.7 |
11.3 |
9.7 |
8.0 |
11.2 |
16.3 |
17.9 |
11.8 |
5.8 |
|
4.0 |
3.7 |
3.8 |
2.9 |
1.6 |
10.6 |
8.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela erminea salva |
| average} |
|
♂ ad. to sad.
12 |
Mole Harbor |
{37.8 |
13.0 |
11.9 |
9.6 |
13.3 |
19.2 |
22.0 |
12.8 |
6.8 |
|
4.6 |
4.3 |
4.4 |
3.5 |
1.8 |
11.7 |
10.7 |
| maximum} |
{39.5 |
13.7 |
13.0 |
10.8 |
14.2 |
20.0 |
23.2 |
13.8 |
7.2 |
|
5.0 |
4.6 |
4.8 |
3.9 |
2.0 |
13.1 |
11.4 |
| minimum} |
{36.4 |
12.5 |
10.7 |
8.4 |
12.4 |
18.0 |
20.4 |
12.0 |
6.2 |
|
4.4 |
4.0 |
4.0 |
3.1 |
1.7 |
11.2 |
10.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♀ ad. 2 and
sad. 4 |
Mole Harbor |
{33.0 |
11.3 |
9.9 |
8.1 |
11.6 |
16.5 |
18.2 |
11.5 |
5.8 |
|
4.0 |
3.8 |
3.8 |
3.0 |
1.5 |
10.1 |
9.1 |
| maximum} |
{33.6 |
11.9 |
10.2 |
8.7 |
12.3 |
17.1 |
18.7 |
12.0 |
6.2 |
|
4.2 |
3.9 |
3.9 |
3.2 |
1.6 |
10.9 |
9.4 |
| minimum} |
{32.0 |
10.9 |
9.5 |
7.5 |
11.1 |
15.4 |
17.1 |
11.0 |
5.3 |
|
3.8 |
3.6 |
3.6 |
2.9 |
1.5 |
8.9 |
8.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela erminea initis |
| M. V. Z. |
289 |
♂ ad. |
Saook Bay |
40.5 |
13.9 |
12.8 |
10.6 |
14.4 |
22.1 |
24.5 |
14.8 |
7.6 |
|
5.2 |
4.7 |
5.0 |
4.1 |
1.9 |
12.8 |
11.0 |
| M. V. Z. |
286 |
♂ ad. |
Saook Bay |
39.6 |
13.5 |
13.1 |
11.4 |
15.0 |
21.0 |
24.3 |
13.6 |
7.6 |
|
5.0 |
4.7 |
4.9 |
3.6 |
2.1 |
12.3 |
12.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela erminea celenda |
| average} |
|
♂ ad. 5 |
Prince of Wales
Id |
{39.5 |
14.0 |
13.6 |
11.5 |
14.7 |
20.9 |
24.2 |
13.6 |
7.5 |
|
5.1 |
4.7 |
4.8 |
3.7 |
1.9 |
12.9 |
11.6 |
| maximum} |
{40.7 |
14.4 |
14.5 |
12.1 |
15.6 |
21.7 |
25.8 |
14.2 |
7.9 |
|
5.1 |
4.9 |
4.9 |
3.9 |
2.2 |
13.6 |
12.5 |
| minimum} |
{38.9 |
13.9 |
13.1 |
10.9 |
13.8 |
19.9 |
23.2 |
13.2 |
7.0 |
|
5.0 |
4.6 |
4.6 |
3.6 |
1.7 |
12.3 |
10.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♂ ad. 5 and
sad. 1 5 |
Prince of Wales
Id |
{38.7 |
13.6 |
13.2 |
11.2 |
14.4 |
20.3 |
23.3 |
13.2 |
7.3 |
|
5.0 |
4.6 |
4.7 |
3.6 |
1.8 |
12.9 |
11.4 |
| maximum} |
{40.9 |
14.4 |
14.5 |
12.1 |
15.6 |
21.7 |
25.8 |
14.2 |
7.9 |
|
5.3 |
5.0 |
4.9 |
3.9 |
2.2 |
13.6 |
12.5 |
| minimum} |
{36.7 |
13.0 |
11.8 |
10.2 |
13.4 |
19.0 |
21.3 |
12.3 |
6.8 |
|
4.6 |
4.3 |
4.2 |
3.3 |
1.6 |
12.1 |
10.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| M. V. Z. |
31223 |
♀ sad. |
Prince of Wales Id |
|
12.2 |
11.5 |
9.8 |
12.6 |
|
|
|
|
|
4.5 |
4.2 |
4.2 |
3.2 |
1.5 |
|
10.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela erminea seclusa |
| M. V. Z. |
31232 |
♂ ad. |
Suemez Id |
34.3 |
12.6 |
12.6 |
10.6 |
13.9 |
20.2 |
22.7 |
12.7 |
6.9 |
|
5.1 |
4.7 |
5.0 |
3.8 |
1.8 |
12.3 |
11.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela erminea haidarum |
| U. S. N. M. |
94430 |
♂ ad. |
Massett |
36.7 |
13.4 |
12.7 |
10.5 |
13.9 |
19.3 |
22.6 |
12.4 |
6.4 |
|
5.0 |
4.3 |
4.5 |
3.3 |
1.9 |
12.4 |
11.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♂ ad. 7 |
Graham Id |
{36.7 |
13.4 |
12.7 |
10.9 |
14.3 |
18.9 |
21.8 |
12.6 |
6.8 |
|
5.0 |
4.3 |
4.6 |
3.4 |
1.7 |
12.6 |
11.3 |
| maximum} |
{37.5 |
13.6 |
12.9 |
11.2 |
14.8 |
19.6 |
22.4 |
13.0 |
7.1 |
|
4.8 |
4.4 |
4.7 |
3.4 |
1.9 |
13.1 |
11.7 |
| minimum} |
{35.6 |
13.0 |
12.2 |
10.5 |
14.0 |
18.0 |
21.1 |
12.3 |
6.4 |
|
5.1 |
4.2 |
4.4 |
3.3 |
1.6 |
12.1 |
10.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| M. V. Z. |
31209 |
♀ ad. |
Massett |
34.2 |
12.5 |
11.3 |
9.8 |
13.3 |
17.3 |
19.8 |
11.5 |
6.1 |
|
4.7 |
4.1 |
4.2 |
3.0 |
1.5 |
11.5 |
10.2 |
| U. S. N. M. |
100624 |
♀ ad. |
Cumsheva Inlet |
34.2 |
12.3 |
11.5 |
9.8 |
13.1 |
17.0 |
19.8 |
11.8 |
6.1 |
|
4.6 |
4.0 |
4.3 |
3.2 |
1.7 |
11.2 |
10.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela erminea anguinae |
| average} |
|
♂ 13 ad. |
Vancouver Id |
{34.0 |
11.7 |
10.8 |
9.0 |
12.0 |
17.1 |
19.3 |
11.9 |
6.1 |
|
4.3 |
3.8 |
4.0 |
3.1 |
1.7 |
11.6 |
10.0 |
| maximum} |
{35.6 |
12.2 |
11.3 |
9.6 |
12.5 |
17.9 |
20.6 |
12.5 |
6.7 |
|
4.6 |
4.0 |
4.1 |
3.3 |
1.9 |
12.5 |
10.5 |
| minimum} |
{32.5 |
11.0 |
10.1 |
8.5 |
11.3 |
16.5 |
18.8 |
11.2 |
5.7 |
|
4.0 |
3.6 |
3.7 |
2.9 |
1.6 |
10.7 |
9.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♀ 5 ad. |
Vancouver Id |
{31.5 |
10.9 |
9.8 |
8.2 |
11.5 |
15.8 |
17.5 |
10.8 |
5.5 |
|
4.0 |
3.6 |
3.8 |
2.9 |
1.6 |
10.5 |
9.3 |
| maximum} |
{31.8 |
11.1 |
10.0 |
8.8 |
12.4 |
16.1 |
17.8 |
11.1 |
5.7 |
|
4.2 |
3.8 |
4.0 |
3.0 |
1.6 |
11.5 |
10.0 |
| minimum} |
{30.9 |
10.5 |
9.6 |
7.9 |
10.6 |
15.6 |
17.3 |
10.4 |
5.4 |
|
3.8 |
3.5 |
3.6 |
2.8 |
1.5 |
10.0 |
8.9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela erminea fallenda |
| average} |
|
♂ ad. 7 |
Topotypes |
{35.7 |
12.6 |
11.1 |
9.2 |
12.5 |
18.3 |
20.8 |
13.1 |
6.8 |
* |
*4.7 |
**4.2 |
**4.4 |
**3.4 |
**1.9 |
11.7 |
10.5 |
| maximum} |
{38.2 |
13.1 |
11.6 |
9.9 |
13.0 |
19.6 |
22.8 |
14.1 |
7.6 |
|
5.1 |
4.6 |
4.7 |
3.6 |
2.1 |
12.4 |
11.0 |
| minimum} |
{34.3 |
12.0 |
10.5 |
8.3 |
12.0 |
17.0 |
19.4 |
12.2 |
6.2 |
|
4.3 |
3.9 |
3.9 |
3.2 |
1.7 |
11.0 |
10.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| N. M. C. |
7284 |
♀ ad. |
Topotype |
29.4 |
10.1 |
9.1 |
7.1 |
10.5 |
15.4 |
17.4 |
11.1 |
5.5 |
|
3.7 |
3.5 |
3.6 |
2.8 |
1.5 |
10.5 |
8.6 |
| N. M. C. |
7516 |
♀ ad. |
Topotype |
31.1 |
10.1 |
9.6 |
8.0 |
11.0 |
16.0 |
18.5 |
11.5 |
5.6 |
|
4.1 |
3.7 |
3.8 |
2.9 |
1.5 |
10.0 |
9.0 |
| M. C. Z. |
6852 |
♀ ad. |
Sumas |
31.3 |
10.3 |
9.2 |
7.3 |
10.1 |
15.8 |
17.2 |
11.0 |
5.3 |
|
3.8 |
3.5 |
3.7 |
2.7 |
1.5 |
11.0 |
8.7 |
| M. C. Z. |
3645 |
♀ ad. |
Sumas |
29.4 |
10.2 |
8.6 |
7.3 |
10.7 |
14.7 |
15.4 |
10.5 |
5.2 |
|
3.8 |
3.5 |
3.8 |
2.7 |
1.5 |
9.5 |
8.3 |
| M. C. Z. |
10728 |
♀ ad. |
Sumas |
31.7 |
10.2 |
9.1 |
7.1 |
10.7 |
15.7 |
17.4 |
11.5 |
5.5 |
|
3.8 |
3.4 |
3.7 |
2.6 |
1.2 |
9.3 |
8.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela erminea olympica |
| U. S. N. M. |
90738 |
♂ ad. |
Type |
31.9 |
11.6 |
10.0 |
7.9 |
11.9 |
15.3 |
17.9 |
11.6 |
5.3 |
|
4.0 |
3.6 |
3.9 |
2.9 |
2.1 |
9.9 |
8.9 |
| U. S. N. M. |
241941 |
♂ ad. |
N. Fk. Quinault River |
32.5 |
11.7 |
10.2 |
8.2 |
12.0 |
16.3 |
18.2 |
11.7 |
5.6 |
|
4.2 |
3.6 |
3.7 |
2.9 |
1.8 |
10.0 |
9.2 |
| U. S. N. M. |
231829 |
♂ ad. |
Duckabush |
30.6 |
10.9 |
9.2 |
7.4 |
10.3 |
15.0 |
17.2 |
10.7 |
5.2 |
|
4.2 |
3.5 |
3.7 |
2.7 |
1.7 |
10.0 |
8.6 |
| U. S. N. M. |
231830 |
♂ ad. |
Duckabush |
32.1 |
11.1 |
10.0 |
8.2 |
12.3 |
16.6 |
18.6 |
11.2 |
5.9 |
|
4.0 |
3.7 |
3.9 |
3.0 |
1.7 |
10.2 |
9.3 |
| M. Z. |
53700 |
♂ ad. |
Lake Cushman |
32.0 |
11.4 |
9.8 |
8.0 |
11.0 |
16.9 |
18.8 |
11.4 |
6.3 |
|
4.3 |
3.8 |
3.9 |
3.1 |
1.7 |
10.3 |
9.2 |
|
|
av. 5 |
|
31.8 |
11.3 |
9.8 |
7.9 |
11.5 |
16.0 |
18.1 |
11.3 |
5.7 |
|
4.2 |
3.7 |
3.9 |
2.9 |
1.8 |
10.1 |
9.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| C. R. C. M. |
96 |
♀ ad. |
Elwha River |
27.5 |
9.4 |
8.3 |
6.9 |
9.3 |
13.2 |
15.3 |
10.2 |
4.6 |
|
3.4 |
3.0 |
3.3 |
2.5 |
1.3 |
8.5 |
7.2 |
| C. R. C. M. |
1164 |
♀ ad. |
12 mi. S Port Angeles |
26.7 |
9.0 |
8.1 |
6.7 |
9.0 |
13.1 |
14.4 |
9.7 |
4.8 |
|
3.4 |
3.1 |
3.4 |
2.3 |
1.3 |
9.0 |
7.3 |
| U. S. N. M. |
242133 |
♀ ad. |
Hayes Creek |
27.2 |
9.2 |
8.4 |
7.2 |
9.2 |
13.7 |
15.4 |
9.5 |
4.8 |
|
3.3 |
3.0 |
3.1 |
2.1 |
1.2 |
8.3 |
7.2 |
|
|
av. 3 |
|
27.1 |
9.2 |
8.3 |
6.9 |
9.2 |
13.3 |
15.0 |
9.8 |
4.7 |
|
3.4 |
3.1 |
3.3 |
2.3 |
1.2 |
8.6 |
7.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela erminea streatori |
| average} |
|
♂ ad. 12 |
Tillamook Co |
{33.2 |
11.7 |
10.5 |
8.5 |
11.7 |
17.0 |
19.2 |
11.8 |
6.4 |
|
4.3 |
3.8 |
3.9 |
3.1 |
1.8 |
10.8 |
9.8 |
| maximum} |
{33.8 |
12.1 |
11.1 |
9.1 |
12.5 |
18.0 |
19.8 |
12.6 |
6.9 |
|
4.4 |
4.0 |
4.1 |
3.5 |
2.1 |
11.4 |
10.3 |
| minimum} |
{32.5 |
11.3 |
10.0 |
8.2 |
11.1 |
16.1 |
18.5 |
11.1 |
6.0 |
|
4.1 |
3.6 |
3.8 |
2.9 |
1.6 |
10.3 |
9.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♀ ad. 7 |
Tillamook Co |
{28.5 |
9.9 |
8.9 |
7.3 |
10.1 |
14.3 |
15.9 |
10.6 |
5.2 |
|
3.6 |
3.2 |
3.5 |
2.6 |
1.6 |
9.4 |
8.1 |
| maximum} |
{29.5 |
10.2 |
9.2 |
7.6 |
10.2 |
14.8 |
16.3 |
11.2 |
5.4 |
|
3.7 |
3.3 |
3.6 |
2.7 |
1.7 |
10.0 |
8.4 |
| minimum} |
{27.6 |
9.6 |
8.7 |
7.0 |
9.8 |
14.1 |
15.5 |
10.0 |
5.0 |
|
3.5 |
3.1 |
3.3 |
2.5 |
1.5 |
9.2 |
7.9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela erminea gulosa |
| U. S. N. M. |
82177 |
♂ ad. |
Trout Lake |
32.0 |
11.3 |
10.1 |
8.5 |
11.2 |
16.3 |
18.3 |
11.3 |
5.9 |
|
4.2 |
3.8 |
4.1 |
2.9 |
1.6 |
10.5 |
9.4 |
| U. S. N. M. |
64768 |
♂ ad. |
Trout Lake |
33.3 |
12.0 |
9.9 |
8.3 |
12.2 |
16.9 |
18.5 |
11.8 |
5.9 |
|
4.2 |
3.7 |
3.7 |
2.8 |
1.8 |
10.6 |
9.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♂ ad. 2, sad.
13 |
Trout Lake |
{32.3 |
11.5 |
10.0 |
8.3 |
11.5 |
16.4 |
18.4 |
11.5 |
5.9 |
|
4.2 |
3.7 |
3.9 |
2.9 |
1.7 |
10.3 |
9.2 |
| maximum} |
{33.4 |
12.0 |
10.7 |
8.8 |
12.4 |
17.2 |
19.3 |
12.1 |
6.3 |
|
4.5 |
3.8 |
4.1 |
3.2 |
2.0 |
11.2 |
9.7 |
| minimum} |
{30.9 |
10.8 |
9.0 |
7.4 |
10.8 |
15.6 |
17.8 |
10.8 |
5.5 |
|
3.9 |
3.5 |
3.6 |
2.7 |
1.6 |
9.6 |
8.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| U. S. N. M. |
232741 |
♀ ad. |
Reflection Lakes |
28.4 |
9.6 |
8.7 |
7.4 |
9.3 |
14.1 |
15.8 |
10.9 |
5.4 |
|
3.7 |
3.2 |
3.5 |
2.7 |
1.6 |
8.4 |
7.4 |
| U. S. N. M. |
90727 |
♀ ad. |
Mt. St. Helens |
28.0 |
9.7 |
8.1 |
6.9 |
9.5 |
13.6 |
15.6 |
10.0 |
4.8 |
|
3.6 |
3.3 |
3.5 |
2.5 |
1.6 |
9.0 |
7.6 |
| U. S. N. M. |
81919 |
♀ ad. |
Trout Lake |
28.1 |
9.7 |
8.8 |
7.0 |
10.0 |
13.8 |
15.4 |
10.1 |
4.9 |
|
3.6 |
3.2 |
3.3 |
2.3 |
1.4 |
8.1 |
7.3 |
| U. S. N. M. |
87039 |
♀ ad. |
Trout Lake |
28.4 |
9.8 |
8.7 |
7.1 |
*10.8 |
14.5 |
15.6 |
10.0 |
5.0 |
|
3.5 |
3.2 |
3.4 |
2.5 |
1.4 |
8.6 |
7.5 |
| U. S. N. M. |
77370 |
♀ ad. |
Trout Lake |
27.8 |
9.6 |
8.6 |
6.6 |
*9.3 |
13.6 |
15.2 |
9.7 |
4.7 |
|
3.8 |
3.4 |
3.5 |
2.7 |
1.5 |
8.1 |
7.2 |
|
|
av. 5 |
|
28.1 |
9.7 |
8.6 |
7.0 |
9.8 |
13.9 |
15.5 |
10.1 |
5.0 |
|
3.6 |
3.3 |
3.4 |
2.5 |
1.5 |
8.4 |
7.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela erminea muricus |
| U. S. N. M. |
231397 |
♂ ad. |
Donovan, Mont |
*31.2 |
10.5 |
9.5 |
8.0 |
11.3 |
*16.0 |
17.5 |
|
5.7 |
|
4.0 |
3.6 |
3.7 |
2.8 |
1.6 |
11.2 |
8.8 |
| U. S. N. M. |
206991 |
♂ ad. |
Mill Creek, Oreg |
*30.9 |
10.8 |
9.0 |
7.2 |
11.0 |
|
17.4 |
11.3 |
5.6 |
|
4.3 |
3.7 |
4.0 |
3.0 |
1.6 |
|
8.7 |
| M. V. Z. |
34746 |
♂ ad. |
Black Butte, Calif |
30.8 |
11.1 |
9.4 |
7.7 |
11.1 |
15.9 |
17.5 |
10.8 |
5.6 |
|
4.2 |
3.5 |
3.6 |
2.8 |
1.8 |
9.6 |
8.4 |
| M. V. Z. |
41501 |
♂ ad. |
Wheeler Peak, Nev |
29.8 |
10.4 |
9.3 |
7.2 |
10.6 |
15.1 |
17.1 |
10.7 |
5.4 |
|
4.0 |
3.5 |
3.5 |
2.7 |
1.5 |
9.8 |
8.0 |
| E. R. W. |
3050 |
♂ ad. |
Crested Butte, Colo. |
*30.4 |
11.1 |
9.8 |
7.7 |
11.2 |
16.2 |
18.3 |
10.8 |
5.7 |
|
4.3 |
3.6 |
3.9 |
2.9 |
1.6 |
|
8.5 |
|
|
av. 5 |
|
30.6 |
10.8 |
9.4 |
7.6 |
11.0 |
15.8 |
17.6 |
10.9 |
5.6 |
|
4.2 |
3.6 |
3.7 |
2.8 |
1.6 |
10.2 |
8.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| M. Z. |
62111 |
♀ ad. |
Teton Co., Wyoming |
28.0 |
9.7 |
8.3 |
6.7 |
10.0 |
14.9 |
16.0 |
10.9 |
5.3 |
|
3.6 |
3.2 |
3.3 |
2.7 |
1.7 |
8.8 |
7.2 |
| M. Z. |
62112 |
♀ ad. |
Teton Co., Wyoming |
27.3 |
9.7 |
8.1 |
6.5 |
9.2 |
14.0 |
15.6 |
10.1 |
5.0 |
|
3.5 |
3.2 |
3.3 |
2.5 |
1.4 |
8.3 |
7.2 |
| M. V. Z. |
13776 |
♀ ad. |
Rush Creek, California |
28.1 |
9.5 |
8.8 |
7.4 |
9.7 |
14.3 |
16.5 |
10.2 |
4.7 |
|
3.6 |
3.2 |
3.4 |
2.7 |
1.5 |
9.1 |
7.8 |
| M. V. Z. |
13777 |
♀ ad. |
Castle Lake, California |
29.4 |
9.9 |
8.8 |
7.3 |
10.8 |
15.2 |
17.1 |
10.0 |
5.3 |
|
3.6 |
3.1 |
3.4 |
2.6 |
1.5 |
|
8.2 |
| M. V. Z. |
41502 |
♀ ad. |
Wheeler Peak, Nev |
27.3 |
9.3 |
8.0 |
6.3 |
9.5 |
13.9 |
15.8 |
10.0 |
5.0 |
|
3.4 |
2.9 |
3.1 |
2.4 |
1.3 |
8.8 |
7.2 |
| F. M. N. H. |
11440 |
♀ ad. |
Camp Albion, Colo |
27.8 |
9.4 |
8.4 |
6.9 |
9.5 |
14.1 |
15.5 |
10.5 |
5.1 |
|
3.6 |
3.2 |
3.3 |
2.6 |
1.4 |
8.9 |
7.5 |
|
|
av. 6 |
|
28.0 |
9.6 |
8.4 |
6.9 |
9.8 |
14.4 |
16.1 |
10.3 |
5.1 |
|
3.6 |
3.1 |
3.3 |
2.6 |
1.5 |
8.8 |
7.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela erminea angustidens |
| A. M. N. H. |
12432 |
♀ ad. |
Conard Fissure, Ark |
35.1 |
12.7 |
10.4 |
8.1 |
12.1 |
18.7 |
|
12.1 |
6.2 |
|
|
4.5 |
4.4 |
3.3 |
1.65 |
11.0 |
9.6 |
| A. M. N. H. |
12433 |
♀ ad. |
Conard Fissure, Ark |
|
12.6 |
9.9 |
8.3 |
11.4 |
|
|
|
|
|
|
4.2 |
4.5 |
3.4 |
1.5 |
|
9.9 |
| A. M. N. H. |
12435 |
♀ ad. |
Conard Fissure, Ark |
*32.5 |
12.4 |
10.0 |
*7.5 |
12.2 |
17.1 |
*19.0 |
11.4 |
5.8 |
|
|
3.8 |
4.0 |
2.9 |
1.4 |
|
8.6 |
| A. M. N. H. |
11766 |
♀ ad. |
Conard Fissure, Ark |
34.5 |
12.3 |
10.7 |
8.4 |
11.8 |
18.2 |
|
12.5 |
6.6 |
|
|
4.1 |
4.3 |
3.2 |
1.5 |
10.0 |
9.5 |
|
|
av |
|
34.0 |
12.5 |
10.3 |
8.1 |
11.9 |
18.0 |
|
12.0 |
6.2 |
|
|
4.2 |
4.3 |
3.2 |
1.5 |
10.5 |
9.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| A. M. N. H. |
12437 |
♂ sad. |
Conard Fissure, Ark |
39.2 |
14.5 |
11.7 |
9.6 |
13.6 |
20.4 |
|
13.0 |
6.7 |
|
|
4.6 |
4.9 |
3.8 |
2.1 |
13.2 |
11.0 |
| A. M. N. H. |
12441 |
♂ ad. |
Conard Fissure, Ark |
38.5 |
13.9 |
11.3 |
9.1 |
13.0 |
20.0 |
|
13.5 |
6.9 |
|
|
4.7 |
4.9 |
3.6 |
1.5 |
12.1 |
10.7 |
| A. M. N. H. |
12436 |
♂ ad. |
Conard Fissure, Ark |
|
13.5 |
11.7 |
8.9 |
13.5 |
|
|
|
|
|
|
4.0 |
4.3 |
3.2 |
1.6 |
|
10.4 |
| A. M. N. H. |
12444 |
♂ ad. |
Conard Fissure, Ark |
|
14.3 |
11.6 |
9.2 |
13.8 |
|
|
|
|
|
|
4.7 |
4.6 |
3.2 |
1.8 |
|
10.8 |
| A. M. N. H. |
11769 |
♂ sad. |
Conard Fissure, Ark |
|
|
|
|
|
|
|
|
|
|
|
4.5 |
4.9 |
3.9 |
1.9 |
|
|
| A. M. N. H. |
12438 |
♂ yg. |
Conard Fissure, Ark |
36.6 |
13.5 |
12.2 |
9.3 |
12.8 |
|
|
13.0 |
6.6 |
|
|
4.4 |
4.6 |
3.5 |
1.7 |
13.7 |
10.9 |
|
|
av |
|
38.1 |
13.9 |
11.7 |
9.2 |
13.3 |
20.2 |
|
13.2 |
6.7 |
|
|
4.5 |
4.7 |
3.5 |
1.8 |
13.0 |
10.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela rixosa eskimo |
| average} |
|
♂ ad. 6 |
Point Barrow |
{29.5 |
10.1 |
9.1 |
7.4 |
10.1 |
15.6 |
17.8 |
11.3 |
5.4 |
|
3.8 |
3.6 |
3.6 |
2.7 |
1.4 |
9.5 |
8.4 |
| maximum} |
{30.1 |
10.6 |
9.9 |
7.8 |
10.6 |
16.3 |
18.0 |
11.9 |
5.8 |
|
4.2 |
3.9 |
3.9 |
2.9 |
1.6 |
10.0 |
8.7 |
| minimum} |
{27.6 |
9.3 |
8.6 |
7.1 |
9.3 |
14.5 |
17.0 |
10.1 |
5.0 |
|
3.5 |
3.2 |
3.3 |
2.5 |
1.1 |
8.5 |
7.7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♀ ad. an d
sad. 4 |
Point Barrow |
{27.8 |
9.3 |
8.3 |
6.9 |
9.6 |
14.1 |
15.7 |
10.5 |
5.1 |
|
3.4 |
3.2 |
3.2 |
2.5 |
1.2 |
9.5 |
7.7 |
| maximum} |
{28.5 |
9.5 |
8.5 |
7.2 |
9.7 |
15.0 |
16.5 |
11.1 |
5.6 |
|
3.7 |
3.3 |
3.4 |
2.6 |
1.3 |
10.0 |
8.0 |
| minimum} |
{27.0 |
9.0 |
7.9 |
7.0 |
9.5 |
13.6 |
15.0 |
10.2 |
4.8 |
|
3.2 |
3.0 |
3.0 |
2.3 |
1.1 |
9.2 |
7.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela rixosa rixosa |
| average} |
|
♂ ad. 2, and
sad. 4 |
Shaunavon |
{29.5 |
10.1 |
8.2 |
6.6 |
9.9 |
15.1 |
16.4 |
11.0 |
5.2 |
|
3.7 |
3.3 |
3.6 |
2.6 |
1.4 |
10.0 |
8.4 |
| maximum} |
{30.4 |
10.5 |
9.0 |
6.9 |
10.5 |
16.1 |
17.1 |
11.5 |
5.5 |
|
3.9 |
3.5 |
3.8 |
2.7 |
1.5 |
10.4 |
8.8 |
| minimum} |
{28.4 |
9.6 |
7.4 |
6.3 |
9.2 |
14.0 |
15.2 |
10.7 |
5.0 |
|
3.5 |
3.1 |
3.3 |
2.4 |
1.3 |
9.4 |
8.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| average} |
|
♀ ad. 3, and
sad. 1 |
Regina and
Shaunavon |
{26.1 |
8.9 |
7.2 |
5.5 |
8.9 |
13.1 |
14.1 |
9.7 |
4.9 |
|
3.3 |
3.0 |
3.2 |
2.3 |
1.2 |
8.6 |
7.1 |
| maximum} |
{27.0 |
9.2 |
7.5 |
5.9 |
9.5 |
13.6 |
14.6 |
10.0 |
5.0 |
|
3.5 |
3.1 |
3.3 |
2.4 |
1.3 |
9.0 |
7.2 |
| minimum} |
{24.7 |
8.5 |
6.9 |
5.2 |
8.3 |
12.3 |
13.7 |
9.5 |
4.7 |
|
3.1 |
2.8 |
2.9 |
2.3 |
1.1 |
8.2 |
7.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela rixosa campestris |
| Swenk, Mr. |
5 |
♂ ad. |
1 mi. E Inland |
|
11.6 |
8.8 |
7.6 |
11.1 |
16.1 |
18.0 |
|
|
|
4.1 |
3.6 |
3.8 |
2.7 |
1.6 |
11.5 |
9.7 |
| Swenk, Mr. |
8 |
♂ ad. |
Inland |
30.7 |
10.5 |
8.2 |
7.0 |
10.5 |
15.9 |
17.9 |
10.9 |
5.7 |
|
3.8 |
3.4 |
3.5 |
2.6 |
1.5 |
|
8.6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Swenk, Mr. |
10 |
♀ ad. |
Inland |
28.0 |
9.8 |
7.6 |
5.8 |
9.4 |
14.2 |
|
10.3 |
5.4 |
1.7 |
3.5 |
3.1 |
3.3 |
2.5 |
1.5 |
9.3 |
7.8 |
| U. S. N. M. |
171490 |
♀ ad. |
Type |
28.8 |
|
7.7 |
6.1 |
9.1 |
14.1 |
15.0 |
10.2 |
5.1 |
1.6 |
3.8 |
3.2 |
3.4 |
2.5 |
1.5 |
9.3 |
7.7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mustela rixosa allegheniensis |
| U. S. N. M. |
249285 |
♂ sad. |
Finleyville, Pa |
29.7 |
10.2 |
|
|
10.1 |
15.0 |
16.5 |
10.5 |
5.2 |
|
4.1 |
3.4 |
3.7 |
2.7 |
1.5 |
9.6 |
8.1 |
| U. S. N. M. |
203173 |
♂ sad. |
Waynesburg, Pa |
28.6 |
9.5 |
7.7 |
6.7 |
9.5 |
14.7 |
16.1 |
10.5 |
5.4 |
|
3.4 |
3.5 |
3.2 |
2.5 |
1.3 |
10.2 |
8.0 |
| U. S. N. M. |
206340 |
♂ ad. |
Huttonsville, W. Va |
28.5 |
9.9 |
8.5 |
7.1 |
10.3 |
15.1 |
16.7 |
10.2 |
5.1 |
|
3.3 |
3.0 |
3.2 |
2.4 |
1.3 |
10.5 |
8.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| C. M. |
7543 |
♀ ad. |
Pymatuning Swamp |
28.0 |
9.2 |
8.1 |
|
9.5 |
13.6 |
|
10.0 |
5.2 |
|
3.4 |
3.0 |
3.1 |
2.4 |
1.3 |
|
|
| A. N. S. P. |
11279 |
♀ ad. |
Beallville, Pa |
28.0 |
9.5 |
7.5 |
6.2 |
9.7 |
13.5 |
14.6 |
10.0 |
5.1 |
|
3.7 |
3.3 |
3.5 |
2.6 |
1.4 |
8.7 |
7.8 |
| U.
S. N. M. |
245843 |
♀
ad. |
near
Marshall, N. C |
27.5 |
9.4 |
7.3 |
6.4 |
9.4 |
|
15.0 |
9.3 |
5.0 |
|
3.6 |
3.2 |
3.4 |
2.5 |
1.3 |
|
7.7 |
(Abbreviations used for names of collections in the table of
measurements of Mustela)
| A. M. N. H. |
American Museum of Natural History |
M. C. Z. |
Museum of Comparative Zoölogy |
| A. N. S. P. |
Academy of Natural Sciences of Philadelphia |
M. P. H. N. |
Musée Polonais d'Histoire Naturelle (Warsaw, Poland) |
| Baylor U. |
Baylor University |
M. V. Z. |
Museum of Vertebrate Zoölogy, University of California |
| B. M. |
British Museum of Natural History |
M. Z. |
Museum of Zoölogy, University of Michigan |
| B. S. N. |
Boston Society of Natural History |
N. H. R. S. |
Naturhistoriska Riksmuseum |
| B. Z. M. |
Berlin Zoological Museum |
N. M. C. |
National Museum of Canada |
| C. A. C. |
California Academy of Sciences |
S. D. M. |
San Diego Society of Natural History |
| C. M. |
Carnegie Museum |
Snyder |
W. E. Snyder, Beaver Dam, Wisconsin |
| C. R. C. M. |
Charles R. Conner Museum, Washington State College |
Stan. U. |
Leland Stanford Junior University |
| C. Z. M. |
University Zoological Museum, Copenhagen, Denmark |
Stephens |
Frank Stephens, private collection |
| Cornell |
Cornell University |
Swenk, Mr. |
Myron H. Swenk, private collection |
| Cowan |
Ian McTaggart-Cowan, private collection |
U. O. |
University of Oregon, Eugene, Oregon |
| Dickey |
Donald R. Dickey (deceased), private collection |
U. S. N. M. |
United States National Museum |
| E. R. W. |
Edward R. Warren, private collection |
Walker |
Alex Walker, private collection |
| F. M. N. H. |
Field Museum of Natural History |
Wisc. U. |
University of Wisconsin |
| F. S. M. |
Florida State Museum |
* |
Approximate |
| Kans. U. |
University of Kansas, Museum of Natural History |
** |
Average of 14 |
Abbot, C. C.
1884. A naturalist's rambles about home. D. Appleton and Co., New
York, 485 pp.
Addy, E.
1939. A weasel trails a rabbit. Jour. Mamm., 20:372-373, August 14,
1939.
Aldous, S. E., and Manweiler, J.
1942. The winter food habits of the short-tailed weasel in northern
Minnesota. Jour. Mamm., 23:250-255, August 13, 1942.
Allen, D. L.
1938. Notes on the killing technique of the New York weasel. Jour.
Mamm., 19:225-229, May 14, 1938.
1940. Two recent mammal records from Allegan County, Michigan.
Jour. Mamm., 21:459-460, November 14, 1940.
Allen, G. M.
1933. The least weasel a circumboreal species. Jour. Mamm.,
14:316-319, November 13, 1933.
Allen, J. A.
1889. Notes on a collection of mammals from southern México, with
descriptions of new species of the Genera Sciurus, Tamias,
and Sigmodon. Bull. Amer. Mus. Nat. Hist., 2:165-181, October
21, 1889.
1891. On a collection of mammals from southern Texas and
northeastern México. Bull. Amer. Mus. Nat. Hist., 3:219-228,
April 17, 1891.
1894. On the mammals of Aransas County, Texas, with descriptions of
new forms of Lepus and Oryzomys. Bull. Amer. Mus. Nat. Hist.,
6:165-198, 1 map, May 31, 1894.
1896. On mammals collected in Bexar County and vicinity, Texas, by
Mr. H. P. Attwater, with field notes by the collector. Bull.
Amer. Mus. Nat. Hist., 8:47-80, April 22, 1896.
1904. Mammals from southern México and Central and South America.
Bull. Amer. Mus. Nat. Hist., 20:29-80, text figs. 1-18,
February 29, 1904.
1906. Mammals from the states of Sinaloa and Jalisco, México,
collected by J. H. Batty during 1904 and 1905. Bull. Amer.
Mus. Nat. Hist., 22:191-262, pls. 22-33, 3 figs. in text,
July 25, 1906.
1908. Mammals from Nicaragua. Bull. Amer. Mus. Nat. Hist.,
24:647-670, 12 figs. in text, October 13, 1908.
1911. Mammals from Venezuela collected by Mr. M. A. Carriker, Jr.,
1909-1911. Bull. Amer. Mus. Nat. Hist., 30:239-273, December
2, 1911.
1912. Mammals from western Colombia. Bull. Amer. Mus. Nat. Hist.,
31:71-95, April 19, 1912.
1916. The neotropical weasels. Bull. Amer. Mus. Nat. Hist.,
35:89-111, April 28, 1916.
1916A. List of mammals collected in Colombia by the American Museum
of Natural History Expeditions, 1910-1915. Bull. Amer. Mus.
Nat. Hist., 35:191-238, 1 map, May 31, 1916.
Alston, A. R.
1879-1882. Biologia Centrali-Americana. Mammalia, xx + 220 pp.,
pls. 1-22.
Ameghino, F.
1889. Contribución al conocimiento de los mamiferos fosiles de la
República Argentina. Imprenta de Pablo E. Coni É Hijos,
Especial para obras, xxxii + 1027 pp., pls. 1-94, numerous
figures in text.
Anderson, R. M.
1945. Three mammals of the weasel family (Mustelidae) added to the
Quebec list with descriptions of two new forms. Ann. Rept.
Provancher Soc., 25th Anniversary, pp. 56-61, November 2,
1945.
Arthur, S. C.
1928. The fur animals of Louisiana. State of Louisiana, Dept.
Conservation Bull., 18:1-433, illustrated, November, 1928.
Audubon, J. J., and Bachman, J.
1845-1853. The viviparous quadrupeds of North America: pls. in 3
vols., elephant folios, each of 50 pls., vol. 1, 1845; vol.
2, 1846; vol. 3, 1848. Text in 3 vols.: vol. 1, xiv + 389;
vol. 2, 1-334; vol. 3, iv + 257.
1851. The quadrupeds of North America, vol. 2, pp. 1-334, pls.
51-100. Publ. by V. G. Audubon, New York.
1856. The quadrupeds of North America, vol. 2, pp. xiii-xiv +
i-viii + 2-383, pls. 1-50. Publ. by V. G. Audubon, New York.
Bachman, J.
1839. Observations on the changes of colour in birds and
quadrupeds. Trans. Amer. Philos. Soc., 6:197-239.
Bailey, B.
1929. The mammals of Sherburne County, Minnesota. Jour. Mamm.,
10:153-164, May 9, 1929.
Bailey, H. H.
1930. Correcting inaccurate ranges of certain Florida mammals and
others of Virginia and the Carolinas. The Bailey Mus. and
Library of Nat. Hist., Bull. no. 3; 4 pages (Miami, Florida),
December 1, 1930.
Bailey, V.
1905. Biological survey of Texas. N. Amer. Fauna, 25:1-222, 16
pls., 24 figs. in text, October 24, 1905.
1928. Animal life of the Carlsbad Cavern. Monograph, Amer. Soc.
Mammalogists, no. 3, pp. xiii + 195 pp., 67 figs. Williams
and Wilkins Co., Baltimore [Md.].
1932. Mammals of New Mexico. N. Amer. Fauna, 53:1-412, 22 pls., 58
figs. in text, March 1, 1932.
Baird, S. F.
1858. General report upon the zoölogy of the several Pacific
Railroad Routes. Part I, Mammals, xlvii + 757 pp., 60 pls.,
July 14, 1858.
Bangs, O.
1896. A review of the weasels of eastern North America. Proc. Biol.
Soc. Washington, 10:1-24, 3 pls., February 25, 1896.
1899. Three new weasels from North America. Proc. New England Zoöl.
Club, 1:53-57, June 9, 1899.
1899B. Description of a new weasel from the Rocky Mountains of
British Columbia. Proc. New England Zoöl. Club, 1:81-82,
December 27, 1899.
1902. Chiriquí Mammalia. Bull. Mus. Comp. Zoöl., 39:17-51, 27 figs.
in text, April, 1902.
Barber, C. M., and Cockerell, T. D. A.
1898. A new weasel from New Mexico. Proc. Acad. Nat. Sci.
Philadelphia, 1898:188-189.
Barrett-Hamilton, G. E. H.
1903. Abstract of a physiological hypothesis to explain the winter
whitening of mammals and birds inhabiting snowy countries,
and the more striking points in the distribution of white in
vertebrates generally. Proc. Royal Irish Acad., 24:303-314.
1904. Notes and descriptions of some new species and subspecies of
Mustelidae. Ann. and Mag. Nat. Hist., 13(ser. 7):388-395,
May, 1904.
Bell, T.
1874. A history of British quadrupeds. John Van Voorst, London, xi
+ 474 pp., illustrated.
Belcher, E.
1843. Narrative of a voyage round the world, ... in her majesty's
ship Sulphur, during the years 1836-1842.... London, H.
Colburn, 2 vols.: v. 1, xxxviii + 387; v. 2, vi + 474,
illustrated.
Bishop, S. C.
1923. Note on the nest and young of the small brown weasel. Jour.
Mamm., 4:26-27, 1 pl., February 9, 1923.
Bissonnette, T. H., and Bailey, E. E.
1940. Den and runway system for weasels and other small mammals in
the laboratory. Amer. Midland Nat., 24:761-763, November,
1940.
1944. Experimental modification and control of molts and changes of
coat-color in weasels by controlled lighting. Ann. New York
Acad. Sci., 45:221-260, 7 pls., 1 fig., April 7, 1944.
Bonaparte, C. L.
1838. Remarks on the species of the genus Mustela. Charlesworth's
Mag. Nat. Hist., 2:37-38.
Booth, E. S.
1946. Account of a weasel in a tree. Jour. Mamm., 26:439, February
12, 1946.
Borell, A. E., and Ellis, R.
1934. Mammals of the Ruby Mountains region of northeastern Nevada.
Jour. Mamm., 15:12-44, 6 pls., 1 fig. in text, February 15,
1934.
Boyer, R. H.
1943. Weasel versus squirrel in Sequoia National Park. Jour. Mamm.,
24:99-100, February 20, 1943.
Brown, B.
1908. The Conard Fissure, a Pleistocene bone deposit in northern
Arkansas: with descriptions of two new genera and twenty new
species of mammals. Mem. Amer. Mus. Nat. Hist., 9(pt.
4):155-208, pls. 14-25, 3 figs. in text.
Brown, M.
1941. A gazetteer of entomological stations in Ecuador. Ann. Ento.
Soc. Amer., 34:809-851, 10 figs. in text, December 19, 1941.
Burroughs, J.
1900. Squirrels and other fur-bearers. Houghton, Mifflin and
Company, 149 pp., illustrated.
Burroughs, R. D.
1939. New York weasel preying on the cottontail rabbit. Jour.
Mamm., 20:253, May 14, 1939.
Cabrera, A.
1913. Sobre algunas formas del género "Mustela." Bol. d. l. Real
Soc. Español d. Hist. Nat., 13:429-435, November, 1913.
1914. Sobre algunas formas del género "Mustela." Bol. d. l. Real
Soc. Español d. Hist. Nat., 14:175-176, 1 pl., March, 1914.
1928. Sobre Lyncodon patagonicus con descripción de una nueva
subespecie. Revista Chilean d. Hist. Nat., 32:259-263.
1940. Sobre carnívoros Sudamericanos. Inst. d. Mus. d. l. Univ.
Nac. d. La Plata, 5(no. 29):1-22.
Chapman, F. M.
1917. The distribution of bird-life in Colombia; a contribution to
a biological survey of South America. Bull. Amer. Mus. Nat.
Hist., 36: x + 729 pp., 41 pls., 21 figs. in text.
1926. The distribution of bird-life in Ecuador. Bull. Amer. Mus.
Nat. Hist., 55: xiii + 784 pp., 30 pls., 20 figs. in text.
1931. The upper zonal bird-life of mts. Roraima and Duida. Bull.
Amer. Mus. Nat. Hist., 63:1-135, 42 figs.
1937. The Phelps Venezuela Expedition. Nat. Hist., 40(no.
5):760-761, December, 1937.
Coues, E.
1877. Fur-bearing animals: A monograph of North American
Mustelidae. Dept. Int., U. S. Geol. Surv. Terr., Miscl.
Publ., No. 8, xiv + 348 pp., 20 pls., 1 fig. in text.
Criddle, N., and Criddle, S.
1925. The weasels of southern Manitoba. Canadian Field-nat.,
39:142-148, September, 1925.
Criddle, S.
1926. The habits of Microtus minor in Manitoba. Jour. Mamm.,
7:193-200, August 9, 1926.
1930. The prairie pocket gopher, Thomomys talpoides rufescens.
Jour. Mamm., 11:265-280, 1 pl., August 9, 1930.
1947. A nest of the least weasel. Canadian Field-nat., 61:69,
April, 1947.
Dalquest, W. W.
1948. Mammals of Washington. Univ. Kansas Publ., Mus. Nat. Hist.,
2:1-444, 140 figs. in text, April 9, 1948.
Danforth, C. N.
1925. Hair with special reference to hypertrichosis. Amer. Med.
Assoc., N Dearborn St., Chicago, Illinois, 152 pp., 64 figs.
Davenport, C. B.
1905. Evolution without mutation. Jour. Exp. Zoöl., 2:139-143,
April, 1905.
1906. The mutation theory in animal evolution. Science, N. S.,
24:556-558, November 2, 1906.
Davis, W. B.
1944. Notes on Mexican mammals. Jour. Mamm., 25:370-403, 1 fig. in
text, December 12, 1944.
Davis, W. B., and Robertson, J. L., Jr.
1944. The mammals of Culberson County, Texas. Jour. Mamm.,
25:254-273, September 8, 1944.
Deanesly, R.
1944. The reproductive cycle of the female weasel (Mustela
nivalis). Proc. Zoöl. Soc. London, 114(pt. 3):339-349.
Dearborn, N.
1932. Foods of some predatory fur-bearing animals in Michigan.
Univ. Michigan School Forestry and Conservation, Bull. No.
1:1-52, 8 figs. in text, 22 charts, 10 maps.
1932B. Occurrence of the least weasel in Michigan. Jour. Mamm.,
13:277, August 9, 1932.
Degerbøl, M.
1935. Mammals. Part 1. Systematic notes. Rept. 5th Thule Exped....,
2(no. 4):1-67, 12 figs. in text.
Desmarest, A. G.
1818. Nouveau Dictionnaire d' Histoire Naturelle.... Tome XIX, 619
pp., Paris.
Dice, L. R.
1921. Notes on the mammals of interior Alaska. Jour. Mamm.,
2:20-28, February 21, 1921.
Dixon, J.
1931. Pikas versus weasel. Jour. Mamm., 12:72, February 12, 1931.
Dunk, F. A.
1946. Least weasel in Saskatchewan. Jour. Mamm., 27:392, November
25, 1946.
Elliot, D. G.
1905. Descriptions of apparently new species and subspecies of
mammals from México and San Domingo. Proc. Biol. Soc.
Washington, 18:233-236, December 9, 1905.
Errington, P. L.
1935. Food habits of mid-west foxes. Jour. Mamm., 16:192-200,
August 12, 1935.
1936. Food habits of a weasel family. Jour. Mamm., 17:406-407,
November 14, 1936.
Farurick, B.
1873. Ein Albino des kleiner Wiesels (Mustela vulgaris). Der
Zool. Garten, 14:17-18.
Feilden, H. W.
1878. See as included under Nares, G. S., 1878.
Flintoff, R. J.
1935. Stoats and weasels, brown and white. North Western Naturalist
[England], 10:214-229, September 30, 1935.
Follett, W. I.
1937. Prey of weasel and mink. Jour. Mamm., 18:365, August 14,
1937.
Fremont, J. C.
1856. A narrative of the exploring expedition to Oregon and North
California, pp. 190-493, in The Life of Col. John Charles
Fremont, and his narrative of Explorations and Adventures, in
Kansas, Nebraska, Oregon and California. By Samuel M.
Smucker, New York and Auburn.
Glover, F. A.
1942. Spring color change of the New York weasel. Pennsylvania Game
News, 13(no. 7):18, 32, October, 1942.
1943A. Killing techniques of the New York weasel. Pennsylvania Game
News, 13(no. 10):11, 23, January, 1943, issue.
1943B. A study of the winter activities of the New York weasel.
Pennsylvania Game News, 14(no. 6):8, 9, September, 1943.
Goeldi, E. A.
1897 (for 1898). Ein erstes authentisches Exemplar eines echten
Wiesels aus Brasilien. Zoöl. Jahrb., Abt. f. systematik,
geogr. u. Biol., 10:556-562, pl. 21, September 15, 1897.
1901. O primeiro exemplar authentico deuna genuina dominha do
Brazil. Bol. do Mus. Paraense, 3:195-203, August, 1901.
1904. Catalogo de mammiferos. Bol. do Mus. Goeldi, 4:41-122, 6
pls., February, 1904.
Goble, F. C.
1942. The Guinea-worm in a Bonaparte weasel. Jour. Mamm., 23:221,
June 3, 1942.
Goldman, E. A.
1912. A new weasel from Costa Rica. Proc. Biol. Soc. Washington,
25:9-10, January 23, 1912.
1920. Mammals of Panamá. Smithsonian Miscl. Coll., 69(no. 5):1-309,
pls. 1-39, 1 map.
Gray, J. E.
1844A. The zoölogy of the voyage of H. M. S. Sulphur, under the
command of Captain Sir Edward Belcher, R. N., C. B., F. R. G.
S., etc., during the years 1836-42. Mammalia, pp. 9-36, pls.
1-18.
1864. Description of a new Mustela from Quito. Proc. Zoöl. Soc.
London, 1864:55, pl. 8.
1865. Revision of the genera and species of Mustelidae contained in
the British Museum. Proc. Zoöl. Soc. London, 1865:100-154, 1
pl., 2 figs. in text.
1874. Notes on the varieties of the western-American weasels. Ann.
and Mag. Nat. Hist., 14(ser. 4):374-375.
Green, C. V.
1936. Observations on the New York weasel. Jour. Mamm., 17:247-249,
August 14, 1936.
Grinnell, J.
1913. A distributional list of the mammals of California. Proc.
California Acad. Sci., 3(ser. 4):265-390, pls. 15, 16, August
28, 1913.
1917. Field tests or theories concerning distributional control.
Amer. Nat., 51:115-128, February, 1917.
1917A. The niche-relationships of the California Thrasher. Auk,
34:427-433, October, 1917.
1922. A geographical study of the kangaroo rats of California.
Univ. California Publ. Zoöl., 24:1-124, pls. 1-7, text figs.
A-X, June 17, 1922.
1926. Geography and evolution in the pocket gopher. Univ.
California Chronicle. July, 1926, pp. 247-262, 1 pl., 3 figs.
in text.
Grinnell, J., and Dixon, J.
1919. Natural history of the ground squirrels of California.
California State Comm. Hortic., Monthly Bull., 7:4 + 597-708,
5 col. plates, 30 figs. in text, January 27, 1919.
Grinnell, J., Dixon, J., and Linsdale, J. M.
1937. Fur-bearing mammals of California.... Univ. California Press,
2 vols., xii + 375 pp., pls. 1-7, figs. 1-138, xiv + 377-777
pp., pls. 8-13, figs. 139-345, July 22, 1937.
Grinnell, J., and Swarth, H. S.
1913. An account of the birds and mammals of the San Jacinto Area
of southern California with remarks upon the behavior of
geographic races on the margins of their habitats. Univ.
California Publ. Zoöl., 10:197-406, pls. 6-10, 3 text figs.,
October 31, 1913.
Grosjean, M. S.
1942. A persistent weasel. Jour. Mamm., 23:443, December 30, 1942.
Gunn, C. K.
1932. Color and primeness in variable mammals. Amer. Nat,
66:546-559, 4 figs. in text, December, 1932.
Hadwen, S.
1929. Color changes in Lepus americanus and other animals. Pp.
1-12, pls. 1-5. Reprint, Canad. Jour. Research, 1:189-200,
July, 1929.
Hall, E. R.
1926. Systematic notes on the subspecies of Bassariscus astutus
with description of one new form from California. Univ.
California Publ. Zoöl., 30:39-50, pls. 2 and 3, September 8,
1926.
1926A. Changes during growth in the skull of the rodent
Otospermophilus grammurus beecheyi. Univ. California Publ.
Zoöl., 21:355-404, 43 figs. in text, March 9, 1926.
1927. A new weasel from Louisiana. Proc. Biol. Soc. Washington,
40:193-194, December 2, 1927.
1930. Three new genera of Mustelidae from the Later Tertiary of
North America. Jour. Mamm., 11:146-155, 2 pls., May 9, 1930.
1936. Mustelid mammals from the Pleistocene of North America with
systematic notes on some Recent members of the genera
Mustela, Taxidea and Mephitis. Carnegie Institution Publ.,
no. 473:41-119, 5 pls., 6 figs. in text, November 20, 1936.
1937. Mustela cicognanii, the short tailed weasel, incorrectly
ascribed to Ohio. Amer. Midland Nat., 18:304, March, 1937.
1944. Classification of the ermines of eastern Siberia. Proc.
California Acad. Sci., 23(ser. 4):555-560, 1 fig. in text,
August 22, 1944.
1946. Mammals of Nevada. Univ. California Press, Berkeley, xi +
710, frontispiece, colored, 11 pls., 485 figs. in text,
unnumbered silhouettes, July 1, 1946.
Hamilton, W. J., Jr.
1928. Weasels eat shrews. Jour. Mamm., 9:249-250, August 9, 1928.
1933. The weasels of New York. Amer. Midland Nat., 14:289-344, 2
maps, 3 figs., 4 pls., July, 1933.
Handley, C. O., Jr.
1949. Least weasel, prey of barn owl. Jour. Mamm., 30:431, November
14, 1949.
Harper, J.
1927. The mammals of the Okefinokee Swamp region of Georgia. Proc.
Boston Soc. Nat. Hist., 38(7):191-396, pls. 4-7, March, 1927.
Heller, H. H.
1924. Peruvian pets. Natural History, 24:479-493, 14 figs. in text,
July-August, 1924.
Henninger, W. F.
1921. Two mammals new for Ohio. Jour. Mamm., 2:239, November 29,
1921.
Hensel, R.
1881. Craniologische Studien. Nova Acta d. Ksl. Leop.-Carol.
Deutschen Akad. d. Nat., 42:127-195, pls. 6-13, tables A-T.
Hodgson, B. H.
1841. Classified catalogue of mammals of Nepal (corrected to end of
1841, first printed in 1832). Jour. Asiatic Soc. Bengal,
10:907-916.
Hollister, N.
1913. A synopsis of the American minks. Proc. U. S. Nat. Mus.,
44:471-480, April 18, 1913.
1914. Descriptions of four new mammals from tropical America. Proc.
Biol. Soc. Washington, 28:141-144, July 10, 1914.
Holmes, S. J.
1925. "Age and Area" in relation to extinction. Science, N. S.,
61:77-79, January 23, 1925.
Howard, W. J.
1935. Apparently neutral relations of weasel and squirrel. Jour.
Mamm., 16:322-323, November 15, 1935.
Howell, A. B.
1943. An apparent mustelid trait. Jour. Mamm., 24:98-99, February
20, 1943.
Huxley, J. S.
1939. Ecology and taxonomic differentiation. Jour. Ecology,
27:408-420, August, 1939.
Ingles, L. G.
1939. Observations on a nest of the long-tailed weasel. Jour.
Mamm., 20:253-254, May 14, 1939.
1942. Observations on the short-tailed weasel in California. Jour.
Mamm., 23:446-448, 1 pl., December 30, 1942.
Jackson, H. H. T.
1913. Two new weasels from the United States. Proc. Biol. Soc.
Washington, 26:123-124, May 21, 1913.
Jäckel, A. J.
1873. Ueber Leucismen unter den Mustelen. Der Zool. Garten,
14:456-459.
Jaeckel, O.
1902. Uber verschiedene Wege phylogenetischer Entwicklung [original
not seen—only pp. 289-290 of V. L. Kellogg's Darwinism
today].
Kellogg, V. L.
1907. Darwinism today. Henry Holt and Co., New York, xii + 403 pp.
Kennicott, R.
1859. The quadrupeds of Illinois, injurious and beneficial to the
farmer. Agric. Rept. for 1858, U. S. Patent Office Rept., pp.
241-256.
Kirk, G. L.
1921. Shrews and weasels. Jour. Mamm., 2:111, May 2, 1921.
Lantz, D. E.
1905. A list of Kansas mammals. Trans. Kansas Acad. Sci.,
19:171-178.
Leche, W.
1915. Zur Frage nach der stammesgeschichtlichen Bedeutung des
Milchgebisses bei den Säugetieren. Zool. Jahrb., Abt. f.
Syst., 38:275-370, 126 figs. in text.
Leopold, A.
1937. Killing technique of the weasel. Jour. Mamm., 18:98-99,
February 14, 1937.
Lichtenstein, M. H. C.
1832. Darstellung neuer oder wenig bekannter Säugethiere, pl. 42
and corresponding text (unpaged), 1832 [not seen].
Llewellyn, L. M.
1942. Notes on the Alleghenian least weasel in Virginia. Jour.
Mamm., 23:439-441, December 30, 1942.
Long, W. S.
1938. The weasel an enemy of the pika. Jour. Mamm., 19:250, May 14,
1938.
Lönnberg, E.
1913. Mammals from Ecuador and related forms. Arkiv för Zool.,
8(no. 1):1-36, 1 pl., 1 text fig., July 12, 1913.
1921. A second contribution to the mammalogy of Ecuador with some
remarks on Caenolestes. Arkiv för Zool., 14(no. 4):1-104, pl.
1, 8 figs. in text.
Lyman, C. P.
1943. Control of coat color in the varying hare Lepus americanus
Erxleben. Bull. Mus. Comp. Zoöl., 93:393-461, 11 pls.,
December, 1943.
Lyon, M. W., Jr., and Osgood, W. H.
1909. Catalogue of the type-specimens of mammals in the United
States National Museum, including the Biological Survey
Collection. U. S. Nat. Mus. Bull., 62: ix + 325.
Macgillivary, W.
1848? The naturalists library, Vol. XVII. Mammalia, pp. 1-309, J.
Ogden and Co., London.
Manniche, A. L. V.
1910. The terrestrial mammals and birds of northeast Greenland.
Meddelelser om Grønland, 45:1-200, 6 pls., 20 figs. in text,
1910.
Manning, T. H.
1943. Notes on the mammals of south and central west Baffin Island.
Jour. Mamm., 24:47-59, 1 fig. in text, February 20, 1943.
Matthew, W. D.
1902. On the skull of Bunaelurus, a musteline from the White River
Oligocene. Bull. Amer. Mus. Nat. Hist., 16:137-140, April 7,
1902.
Mearns, E. A.
1891. Description of a new species of weasel, and a new subspecies
of the gray fox, from Arizona. Bull. Amer. Mus. Nat. Hist,
3:234-238, June 5, 1891.
Merriam, C. H.
1896. Synopsis of the weasels of North America. N. Amer. Fauna,
11:1-33, 5 pls., 16 figs. in text, June 30, 1896.
1902. Five new mammals from México. Proc. Biol. Soc. Washington,
15:67-69, March 22, 1902.
1906. Is mutation a factor in the evolution of higher vertebrates.
Science, N. S., 23:241-257, February, 1906.
1919. Criteria for the recognition of species and genera. Jour.
Mamm., 1:6-9, November 28, 1919.
Metchnikoff, E.
1901. On the process of hair turning white. Proc. Royal Soc.
London, 69:156.
Miller, F. W.
1930. The spring molt of Mustela longicauda. Jour. Mamm.,
11:471-473, November 11, 1930.
1931A. The fall moult of Mustela longicauda. Jour. Mamm.,
12:150-156, 1 fig. in text, May 14, 1931.
1931B. A feeding habit of the long-tailed weasel. Jour. Mamm.,
12:164, May 14, 1931.
Miller, G. S., Jr.
1912. Catalogue of the mammals of western Europe.... British Museum
of Natural History, London, xv + 1019 pp., 213 figs. in text.
1924. List of North American Recent mammals, 1923. Bull. U. S. Nat.
Mus., 128: xvi + 673 pp., April 29, 1924.
Moore, J. C.
1945. Life history notes on the Florida weasel. Proc. Florida
Academy of Science, 7(no. 4):247-263, for 1944.
Morse, M.
1939. A local study of predation upon hares and grouse during the
cyclic decimation. Jour. Wildlife Management, 3:203-211,
July, 1939.
Mulaik, S.
1938. Notes on Mustela frenata frenata. Jour. Mamm., 19:104,
February 14, 1938.
Murie, A.
1935. A weasel goes hungry. Jour. Mamm., 16:321-322, November 15,
1935.
Nares, G. S.
1877. Arctic Expedition/1875-6/Journals and Proceedings/of
the/Arctic Expedition, 1875-6,/under the command of/Captain
Sir George S. Nares, R. N., K. C. B./[in continuation of
Parliamentary Papers C 1153 of 1875, and C 1560 of
1876.]/presented to both Houses of Parliament by Command of
her Majesty, 1877./London:/printed for her Majesty's
stationary office, by/Harrison & Sons, printers in ordinary
to her majesty,/St. Martin's Lane,/[c.-1636.] Price £1 1s.
1878. Narrative of a voyage to the Polar Sea during 1875-6 in H. M.
ships "Alert" and "Discovery" by Captn. Sir G. S. Nares, R.
N.... commander of the expedition with notes on the Natural
History edited by H. W. Feilden, F. G. S., ... naturalist to
the expedition. In 2 vols., 2 ed., London, Sampson Low,
Marston, Searle, & Rivington, 1878. Vol. 1, xl + 395 pp.;
vol. 2, vii + 378 pp., both vols. illustrated.
Nelson, A. W.
1934. Notes on Wisconsin mammals. Jour. Mamm., 15:252-253, August
10, 1934.
Nelson, E. W.
1909. The rabbits of North America. N. Amer. Fauna, 29:1-314, 13
pls., 19 figs. in text, August 31, 1909.
1918. Smaller mammals of North America. Nat'l Geographic,
33(5):371-493, with abundant illustrations, many in color,
May, 1918.
Nichols, D. G., and Nichols, J. T.
1935. Notes on the New York weasel (Mustela noveboracensis). Jour.
Mamm., 16:297-299, November 15, 1935.
Noback, C. V.
1935. Observations on the seasonal hair moult in a New York State
weasel (Mustela noveboracensis). Bull. New York Zoöl. Soc.,
38(no. 1):25-27, February, 1935.
Oberthür, R., and Dauthenay, H.
1905. Répertoire de Couleurs. Rennes, Impr. Oberthür; Paris, Librairie
horticole. 5p. 1., [9]-82p. illus., 3 pl. (2 col.) and 365 col. pl.
in 2 portfolios.
Oehler, C.
1944. Notes on the temperament of the New York weasel. Jour. Mamm.,
25:198, May 26, 1944.
Ognev, S. I.
1935. The mammals of USSR and adjacent countries, vol. 3: viii +
752 pp., 8 pls., 299 figs. in text, 19 maps, 52 tables.
Moskow.
Osgood, F. L.
1935. Fluctuations in small mammal populations. Jour. Mamm.,
16:156, May 15, 1935.
1936. Earthworms as a supplementary food for weasels. Jour. Mamm.,
17:64, February 14, 1936.
Osgood, W. H.
1901. Natural history of the Queen Charlotte Islands, British
Columbia; natural history of the Cook Inlet Region, Alaska.
N. Amer. Fauna, 21:1-87, 8 pls., 1 fig. in text, September
26, 1901.
1909. Revision of the mice of the American genus Peromyscus. N.
Amer. Fauna, 28:1-267, 8 pls., 12 figs. in text, April 17,
1909.
1909B. Biological Investigations in Alaska and Yukon Territory. N.
Amer. Fauna, 30:1-96, 5 pls., 2 figs. in text, October 7,
1909.
1912. Mammals from western Venezuela and eastern Colombia. Field
Mus. Nat. Hist., Publ. no. 155, zoöl. ser., 10:33-66, pls. 4,
5, January 10, 1912.
1914. Mammals of an expedition across northern Perú. Field Mus.
Nat. Hist., Publ. 176, zoöl. ser., 10(no. 12):143-185, April
20, 1914.
Pearce, J.
1937. A captive New York weasel. Jour. Mamm., 18:483-488, November
14, 1937.
Pearson, K., Nettleship, E., and Usher, C. H.
1913. A monograph on albinism in man. Drapers Co., Research
Memoirs, Biometric series, VIII, Dept. Applied Statistics,
Univ. College, Univ. London. Text, P II, vii + 264-524 pp.,
London.
Pearson, O. P., and Pearson, A. K.
1947. Owl predation in Pennsylvania, with notes on the small
mammals of Delaware County. Jour. Mamm., 28:137-147, June 1,
1947.
Pitt, F.
1921. Notes on the genetic behavior of certain characters in the
polecat, ferret, and in polecat-ferret hybrids. Jour.
Genetics, 11:99-115, 2 pls., 5 figs. in text, September,
1921.
Polderboer, E. B.
1942. Habits of the least weasel (Mustela rixosa) in northeastern
Iowa. Jour. Mamm., 23:145-147, June 3, 1942.
1948. Late fall sexual activity in an Iowa least weasel. Jour.
Mamm., 29:296, August 31, 1948.
Polderboer, E. B., Kuhn, L. W., and Hendrickson, G. O.
1941. Winter and spring habits of weasels in central Iowa. Jour.
Wildlife Management, 5:115-119, 1 pl., January, 1941.
Preble, E. A.
1908. A biological investigation of the Athabasca-Mackenzie region.
N. Amer. Fauna, 27:1-574, 25 pls., 13 figs. in text, October
26, 1908.
Quick, H. F.
1944. Habits and economics of the New York weasel in Michigan.
Jour. Wildlife Management, 8:71-78, January, 1944.
Rhoads, S. N.
1894. Contributions to the mammalogy of Florida. Proc. Acad. Nat.
Sci. Philadelphia, pp. 152-161, June 19, 1894.
Ridgway, R.
1912. Color standards and color nomenclature. Published by the
author, Washington, D. C., iii + 43 pp., 53 pls.
Robinson, W., and Lyon, M. W.
1901. An annotated list of mammals collected in the vicinity of La
Guaira, Venezuela. Proc. U. S. Nat. Mus., 24:135-162,
October, 1901.
Rothschild, M.
1942. Change of pelage in the stoat Mustela erminea L. Nature,
149:78, January 17, 1942.
Russell, W. C.
1930. Weasel badly injured by king snake. Jour. Mamm., 11:504-505,
November 11, 1930.
Sanderson, G. C.
1949. Growth and behavior of a litter of captive long-tailed
weasels. Jour. Mamm., 30:412-415, 1 fig. in text, November
14, 1949.
Schlosser, M.
1888. Die Affen, Lemuren, Chiropteren, Insectivoren, Marsupialier,
Creodonten und Carnivoren des Europäischen Tertiärs. Alfred
Hölder, K. K. Hof.-und Univ.-Buchhändler, Wien. Part 2, pp.
225-386, pls. 6-9.
Schumacher, S.
1928. Wie kommt die stellenweise Gelbfärbung des winterweissen
Wiesels (Mustela erminea L.) zustande? Zeitschr. f. Morph, u.
Ökologie der Tiere, 11:229-234, 1 fig., July 3, 1928.
Schwalbe, G.
1893. Ueber den Farbenwechsel winterweisser Thiere. Morphologische
Arbeiten, 2:483-600, Jena.
Seton, E. T.
1929. Lives of game animals. Vol. 2, 1929, xvii + 746 pp.,
illustrated; vol. 4, xxii + 949 pp., Doubleday, Doran & Co.,
New York.
Shaw, W. T.
1921. The nest of the Washington weasel (Mustela washingtoni).
Jour. Mamm., 2:167-168, August 19, 1921.
Sheldon, W. G.
1932. Mammals collected or observed in the vicinity of Laurier
Pass, B. C. Jour. Mamm., 13:196-203, August 9, 1932.
Simpson, G. G.
1946. Palaeogale and allied early mustelids. Amer. Mus. Novitates,
1320:1-14, 4 figs. in text, May 28, 1946.
Snyder, L. L., and Logier, E. B. S.
1930. No. 3. A faunal investigation of King Township, York County,
Ontario. Trans. Royal Canadian Institute, 17(pt. 2):167-208.
Soper, J. D.
1919. Notes on Canadian weasels. Canadian Field-nat., 33:43-47,
September, 1919.
1921. Curious palatal obstruction in Mustela longicauda. Jour.
Mamm., 2:37-38, 1 fig. in text, February 10, 1921.
1942. Mammals of Wood Buffalo Park, northern Alberta and District
of Mackenzie. Jour. Mamm., 23:119-145, 2 pls., 1 map in text,
June 3, 1942.
1946. Mammals of the northern Great Plains along the International
Boundary in Canada. Jour. Mamm., 27:127-153, 1 fig. in text,
May 14, 1946.
1948. Mammal notes from the Grand Prairie-Peace River region,
Alberta. Jour. Mamm., 29:49-64, 1 pl., 1 fig. in text,
February 13, 1948.
Sowls, L. K.
1948. The Franklin ground squirrel, Citellus franklinii (Sabine),
and its relationship to nesting ducks. Jour. Mamm.,
29:113-137, 3 pls., 3 figs. in text, May 14, 1948.
Stanford, J. S.
1931. Notes on small mammals of Utah. Journ. Mamm., 12:356-363,
November 11, 1931.
Stephens, F.
1906. California mammals. West Coast Publishing Co., San Diego,
California, pp. 1-351, numerous figs. and maps.
Strecker, J. K.
1924. The mammals of McLennan County, Texas. The Baylor Bull.,
Baylor Univ., Waco, Texas, 27(no. 1):1-20, September, 1924.
1926. A check-list of the mammals of Texas exclusive of the Sirenia
and Cetacea. The Baylor Bull., Baylor Univ., Waco, Texas,
29(no. 3):1-48, August, 1926.
Strong, W. D.
1930. Notes on mammals of the Labrador Interior. Jour. Mamm.,
11:1-10, February 11, 1930.
Sumner, F. B.
1932. Genetic, distributional, and evolutionary studies of the
subspecies of deer mice (Peromyscus). Bibliographia Genetica,
9:1-106, 24 figs. in text.
Surber, T.
1932. The mammals of Minnesota. Minnesota Dept. Conservation, Div.
Game and Fish, Saint Paul, Minnesota, pp. 1-84, illustrated.
Sutton, G. K.
1929. The Alleghenian least weasel in Pennsylvania. Jour. Mamm.,
252-254, 1 fig. in text, August 10, 1929.
Svihla, A.
1931. Habits of the New York weasel in captivity. Jour. Mamm.,
12:67-68, February 12, 1931.
Swanson, G., and Fryklund, P. O.
1935. The least weasel in Minnesota and its fluctuation in numbers.
Amer. Midland Nat., 16:120-126, figs. 1-6, 1935.
Swenk, M. H.
1926. Notes on Mustela campestris Jackson, and on the American
forms of least weasels. Jour. Mamm., 7:313-330, 1 fig. in
text, November 23, 1926.
Taczanowski, L.
1874. Description d' une nouvelle espéce de Mustela du Pérou
central. Proc. Zool. Soc. London, 1874:311-312, pl. 48, May
19, 1874.
1881. Description d' une nouvelle belette du Pérou septentrional.
Proc. Zool. Soc. London, 1881:647-649, May 17, 1881.
1881. Description d' une nouvelle espéce du genre Mustela du
Pérou nord-oriental. Proc. Zool. Soc. London, 1881:835-836,
November 15, 1881.
Tate, G. H. H.
1931. Random observations on habits of South American mammals.
Jour. Mamm., 12:248-256, August 24, 1931.
Thomas, O.
1911. The mammals of the tenth edition of Linnaeus; an attempt to
fix the types of the genera and the exact bases and
localities of the species. Proc. Zoöl. Soc. London,
1911:120-158, March, 1911.
1920. Report on the Mammalia collected by Mr. Edmund Heller during
the Peruvian expedition of 1915 under the auspices of Yale
University and the National Geographic Society. Proc. U. S.
Nat. Mus., 58:217-249, pls. 14-15.
Thurber, W. A.
1940. A weasel attacks a varying hare. Jour. Mamm., 21:356, August
14, 1940.
Tschudi, J. J.
1844. Untersuchungen über die Fauna Peruana. Therologie. Druck und
verlag von Scheitlin und Zollikofer, St. Gallen, xxx + 262
pp., 18 pls.
Vestal, E. H.
1937. Activities of a weasel at a wood rat colony. Jour. Mamm.,
18:364, August 14, 1937.
1938. Biotic relations of the wood rat (Neotoma fuscipes) in the
Berkeley Hills. Jour. Mamm., 19:1-36, figs. 1-4, February 14,
1938.
Warren, E. R.
1924. Ground squirrels and weasels. Jour. Mamm., 5:265-266,
November 15, 1924.
1932. When do weasels mate? Jour. Mamm., 13:71-72, February 9,
1932.
Wight, H. M.
1932. A weasel attacks a man. Jour. Mamm., 13:163-164, May 11,
1932.
Willis, J. C.
1922. Age and Area: A study in geographic distribution and origin
of species. University Press, Cambridge, England, x + 259
pp., illustrated.
Winecoff, T. E.
1930. Least weasel in Pennsylvania. Jour. Mamm., 11:312-313,
August, 1930.
Woldrich, J. N.
1884. Diluviale Fauna von Zuzlawitz bei Winterberg im Bohmerwalde.
Dritter Theil.... Sitzungsberich. d. K. Akad. d. Wissen.
Math.—Natur.—Classe, 88(heft 3, erste abth.):978-1057, 3
pls., 2 figs. in text.
Wright, P. L.
1942A. Delayed implantation in the long-tailed weasel (Mustela
frenata), the short-tailed weasel (Mustela cicognani), and
the marten (Martes americana). Anat. Rec., 83:341-353, 2
pls., July, 1942.
1942B. A correlation between the spring molt and spring changes in
the sexual cycle in the weasel. Jour. Exp. Zoöl., 91:103-110,
October, 1942.
1947. The sexual cycle of the male long-tailed weasel (Mustela
frenata). Jour. Mamm., 28:343-352, 1 pl., 3 figs. in text,
November 19, 1947.
1948A. Breeding habits of captive long-tailed weasels (Mustela
frenata). Amer. Midland Nat., 39:338-344, March, 1948.
1948B. Preimplantation stages in the long-tailed weasel (Mustela
frenata). Anat. Rec., 100:595-607, 2 pls., April, 1948.
1950. Effects of gonadotropic hormone on the pelage of white
winter long-tailed weasels; abstract. Anat. Rec., 106(no.
2):130, February, 1950.
Zimmermann, K.
1943. Zur Kenntnis deutscher Maus-und Zwerg-Wiesel. Zeitschr. fur
Säugetierkunde, 15(3):289-298, 2 pls., 2 figs. in text, 3
tables, January 11, 1943.
Transmitted May 22, 1950.
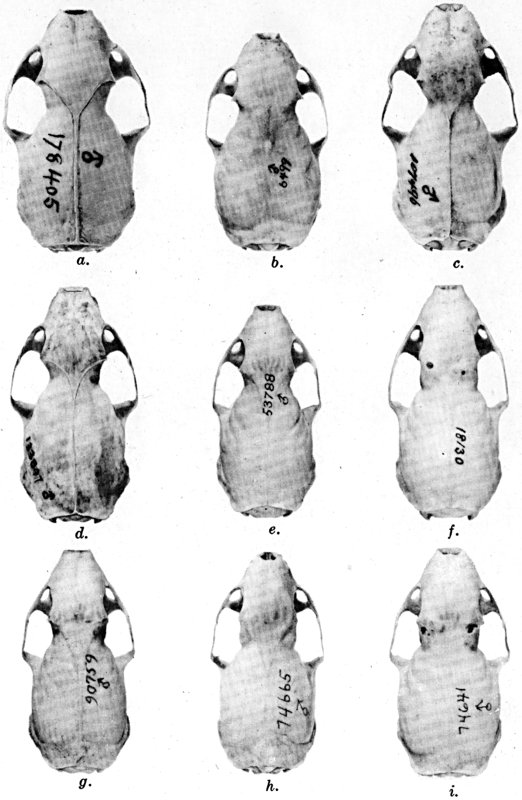
Plate 2. Photographs, retouched, of skulls in dorsal
view of nine subspecies of Mustela erminea. Natural size.
a. Mustela erminea arctica, ♂ ad., 178405, U. S. Nat. Mus.,
Tanana, Alaska.
b. Mustela erminea semplei, ♂ sad., 6499, Carnegie Mus.,
Southhampton Island.
c. Mustela erminea kadiacensis, ♂ ad., 107496, U. S. N. M.,
Kodiak Island, Alaska.
d. Mustela erminea richardsonii, ♂ ad., 133847, U. S. N. M., Ft.
Franklin, MacK.
e. Mustela erminea cicognanii, ♂ ad., 53788, Mus. Vert. Zoöl.,
Lopez, Pennsylvania.
f. Mustela erminea bangsi, ♂ ad., 18130, Field Mus. Nat. Hist.,
Aitkin, Minn.
g. Mustela erminea invicta, ♂ ad., 90759, Mus. Vert. Zoöl., Pilot
Creek, Idaho.
h. Mustela erminea alascensis, ♂ ad., 74665, Mus. Vert. Zoöl.,
Windham, Alaska.
i. Mustela erminea salva, ♂ ad., 74641, M. V. Z., Mole Harbor,
Admiralty Id., Alaska.
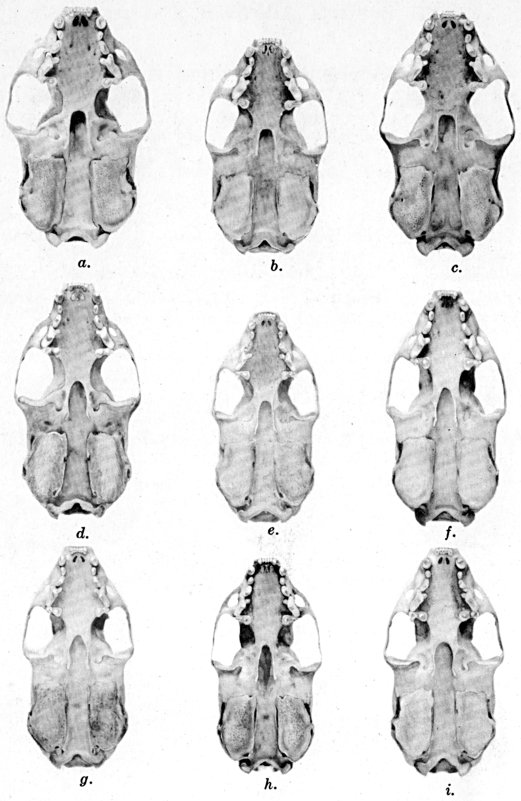
Plate 3. Photographs, retouched, of skulls in ventral
view of nine subspecies of Mustela erminea. Natural size.
a. Mustela erminea arctica, ♂ ad., 178405, U. S. Nat. Mus.,
Tanana, Alaska.
b. Mustela erminea semplei, ♂ sad., 6499, Carnegie Mus.,
Southampton Island.
c. Mustela erminea kadiacensis, ♂ ad., 107496, U. S. N. M.,
Kodiak Island, Alaska.
d. Mustela erminea richardsonii, ♂ ad., 133847, U. S. N. M., Ft.
Franklin, MacK.
e. Mustela erminea cicognanii, ♂ ad., 53788, Mus. Vert. Zoöl.,
Lopez, Pennsylvania.
f. Mustela erminea bangsi, ♂ ad., 18130, Field Mus. Nat. Hist.,
Aitkin, Minn.
g. Mustela erminea invicta, ♂ ad., 90759, Mus. Vert. Zoöl., Pilot
Creek, Idaho.
h. Mustela erminea alascensis, ♂ ad., 74665, Mus. Vert. Zoöl.,
Windham, Alaska.
i. Mustela erminea salva, ♂ ad., 74641, M. V. Z., Mole Harbor,
Admiralty Id., Alaska.
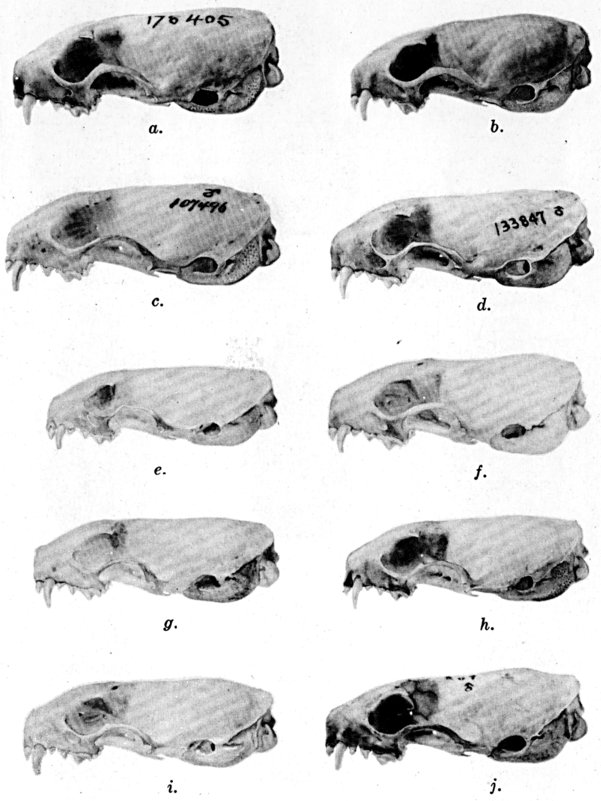
Plate 4. Photographs, retouched, of skulls in lateral
view of ten subspecies Mustela erminea. Natural size.
a. Mustela erminea arctica, ♂ ad., 178405, U. S. Nat. Mus.,
Tanana, Alaska.
b. Mustela erminea semplei, ♂ sad., 6499, Carnegie Mus.,
Southampton Island.
c. Mustela erminea kadiacensis, ♂ ad., 107496, U. S. N. M.,
Kodiak Island, Alaska.
d. Mustela erminea richardsonii, ♂ ad., 133847, U. S. N. M., Ft.
Franklin, MacK.
e. Mustela erminea cicognanii, ♂ ad., 53788, Mus. Vert. Zoöl.,
Lopez, Pennsylvania.
f. Mustela erminea bangsi, ♂ ad., 18130, Field Mus. Nat. Hist.,
Aitkin, Minn.
g. Mustela erminea invicta, ♂ ad., 90759, Mus. Vert. Zoöl., Pilot
Creek, Idaho.
h. Mustela erminea alascensis, ♂ ad., 74665, Mus. Vert. Zoöl.,
Windham, Alaska.
i. Mustela erminea salva, ♂ ad., 74641, M. V. Z., Mole Harbor,
Admiralty Id., Alaska.
j. Mustela erminea initis, ♂ ad., 289, Mus. Vert. Zoöl., Saook
Bay, Alaska.
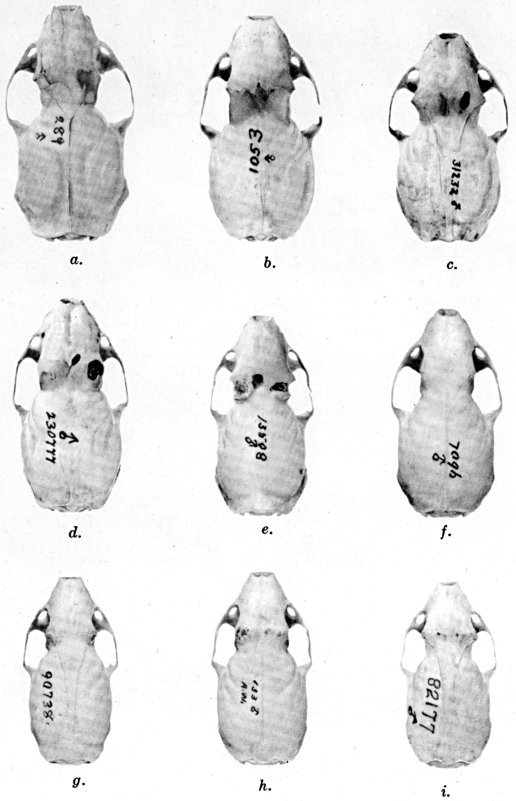
Plate 5. Photos, retouched, of skulls of 9 subspecies of
Mustela erminea, × 1.
a. Mustela erminea initis, ♂ ad., 289, Mus. Vert. Zoöl., Saook
Bay, Alaska.
b. Mustela erminea celenda, ♂ ad., 1053, Los Angeles Mus., Craig,
Alaska.
c. Mustela erminea seclusa, ♂ ad., 31232, M. V. Z., Port Santa
Cruz, Alaska.
d. Mustela erminea haidarum, ♂ ad., 230777, U. S. N. M., Graham
Island, B. C.
e. Mustela erminea anguinae, ♂ ad., 13508, Nat. Mus. Canada, Cape
Scott, V. I., B. C.
f. Mustela erminea fallenda, ♂ ad., 7096, Nat. Mus. Canada,
Huntingdon, B. C.
g. Mustela e. olympica, ♂ ad., 90738, U. S. N. M., near head of
Soleduc Riv., Wash.
h. Mustela erminea streatori, ♂ ad., 133, Coll. of Alex Walker,
Blaine, Oregon.
i. Mustela erminea gulosa, ♂ ad., 82177, U. S. Nat. Mus., Trout
Lake, Wash.
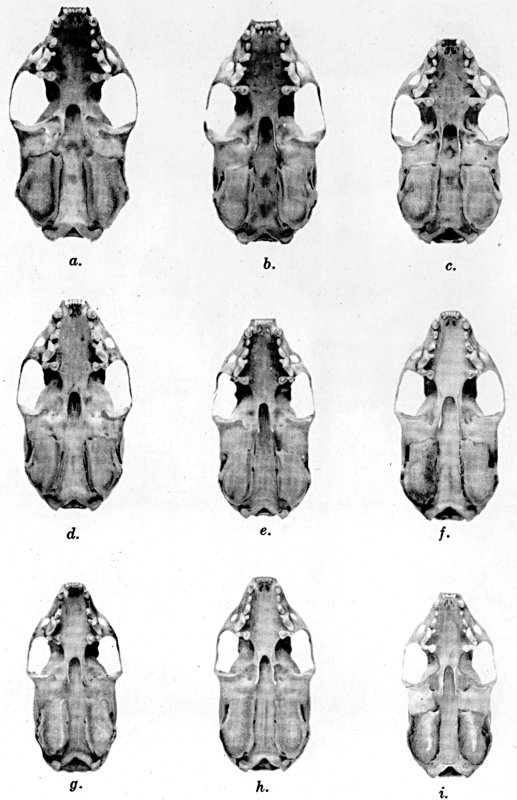
Plate 6. Photos, retouched, of skulls of 9 subspecies of
Mustela erminea, × 1.
a. Mustela erminea initis, ♂ ad., 289, Mus. Vert. Zoöl., Saook
Bay, Alaska.
b. Mustela erminea celenda, ♂ ad., 1053, Los Angeles Mus., Craig,
Alaska.
c. Mustela erminea seclusa, ♂ ad., 31232, M. V. Z., Port Santa
Cruz, Alaska.
d. Mustela erminea haidarum, ♂ ad., 230777, U. S. N. M., Graham
Island, B. C.
e. Mustela erminea anguinae, ♂ ad., 13508, Nat. Mus. Canada, Cape
Scott, V. I., B. C.
f. Mustela erminea fallenda, ♂ ad., 7096, Nat. Mus. Canada,
Huntingdon, B. C.
g. Mustela e. olympica, ♂ ad., 90738, U. S. N. M., near head of
Soleduc Riv., Wash.
h. Mustela erminea streatori, ♂ ad., 133, Coll. of Alex Walker,
Blaine, Oregon.
i. Mustela erminea gulosa, ♂ ad., 82177, U. S. Nat. Mus., Trout
Lake, Wash.
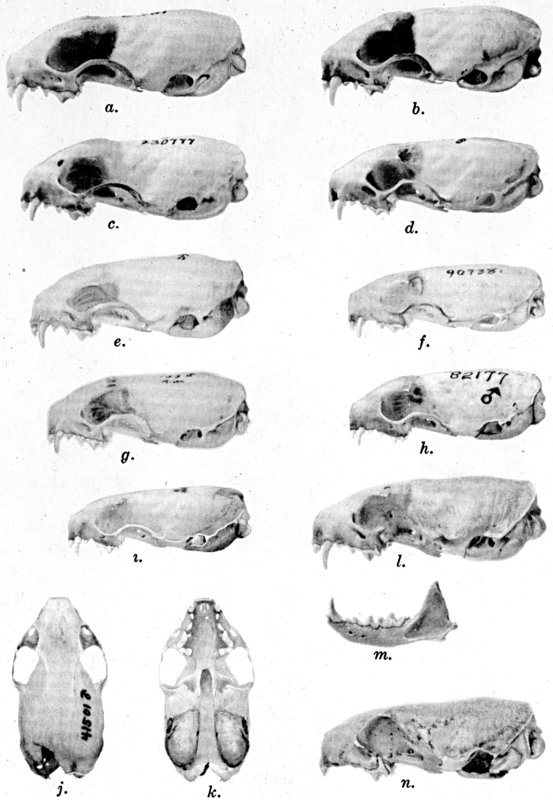
Plate 7. Photos, retouched, of skulls, of subspecies of
Mustela erminea. Natural size.
a. Mustela erminea celenda, ♂ ad., 1053, Los Angeles Mus., Craig,
Alaska.
b. Mustela erminea seclusa, ♂ ad., 31232, M. V. Z., Port Santa
Cruz, Alaska.
c. Mustela erminea haidarum, ♂ ad., 230777, U. S. N. M., Graham
Island, B. C.
d. Mustela erminea anguinae, ♂ ad., 13508, Nat. Mus. Canada, Cape
Scott, V. I., B. C.
e. Mustela erminea fallenda, ♂ ad., 7096, Nat. Mus. Canada,
Huntingdon, B. C.
f. Mustela erminea olympica, ♂ ad., 90738, U. S. N. M., near head
of Soleduc Riv., Wash.
g. Mustela erminea streatori, ♂ ad., 133, Coll. of Alex Walker,
Blaine, Oregon.
h. Mustela erminea gulosa, ♂ ad., 82177, U. S. Nat. Mus., Trout
Lake, Wash.
i, j, k. Mustela erminea muricus, ♂ ad., 41501, M. V. Z.,
Baker Creek, 8675 ft., Nev.
l, m. Mustela erminea angustidens, ♂?, sad., 12437, A. M. N.
H., Conard Fissure, Ark.
n. Mustela erminea angustidens, ♂?, ad., 12441, A. M. N. H.,
Conard Fissure, Ark.
Plate 8. Photos, retouched, of Mustela erminea
angustidens. All in Amer. Mus. Nat. Hist., from Conard Fissure,
Arkansas. Pleistocene in age, × 1.
a. Adult, probably male, 12441.
b. Subadult, probably male, 12437.
c. Adult, probably male, 12444.
d. Adult, probably male, 12441.
e. Subadult, probably male, 12437.
f. Young, probably male, 12438.
g, h. Adult, type, probably female, 12432.
i. Adult, probably female, 12433.
Plate 9. Photographs, retouched, of skulls in dorsal
view of 9 subspecies of Mustela erminea. Natural size.
a. Mustela erminea arctica, ♀ ad., 35895, Field Mus. Nat. Hist.,
Point Barrow, Alaska.
b. Mustela erminea semplei, ♀ ad., 6600, Carnegie Mus.,
Southhampton Island.
c. Mustela erminea kadiacensis, ♀ ad., 98042, U. S. Nat. Mus.,
Kadiak, Alaska.
d. Mustela erminea richardsonii, ♀ ad., 129703, U. S. N. M., Fort
Resolution, MacK.
e. Mustela erminea cicognanii, ♀ ad., 7460, Carnegie Mus.,
Pymatuning Swamp, Pa.
f. Mustela erminea bangsi, ♀ ad., 8679, Univ. Wisconsin, T. 61N,
R. 26W, Minn.
g. Mustela erminea invicta, ♀ ad., 90820, M. V. Z., 1-1/2 mi. W
Iron Mtn., Idaho.
h. Mustela erminea alascensis, ♀ ad., 74422, U. S. Nat. Mus.,
Juneau, Alaska.
i. Mustela erminea salva, ♀ ad., 74655, Mus. Vert. Zoöl., Mole
Harbor, Alaska.
Plate 10. Photographs, retouched, of skulls in dorsal
view of 9 subspecies of Mustela erminea. Natural size.
a. Mustela erminea arctica, ♀ ad., 35895, Field Mus. Nat. Hist.,
Point Barrow, Alaska.
b. Mustela erminea semplei, ♀ ad., 6600, Carnegie Mus.,
Southhampton Island.
c. Mustela erminea kadiacensis, ♀ ad., 98042, U. S. Nat. Mus.,
Kadiak, Alaska.
d. Mustela erminea richardsonii, ♀ ad., 129703, U. S. N. M., Fort
Resolution, MacK.
e. Mustela erminea cicognanii, ♀ ad., 7460, Carnegie Mus.,
Pymatuning Swamp, Pa.
f. Mustela erminea bangsi, ♀ ad., 8679, Univ. Wisconsin, T. 61N,
R. 26W, Minn.
g. Mustela erminea invicta, ♀ ad., 90820, M. V. Z., 1-1/2 mi. W
Iron Mtn., Idaho.
h. Mustela erminea alascensis, ♀ ad., 74422, U. S. Nat. Mus.,
Juneau, Alaska.
i. Mustela erminea salva, ♀ ad., 74655, Mus. Vert. Zoöl., Mole
Harbor, Alaska.
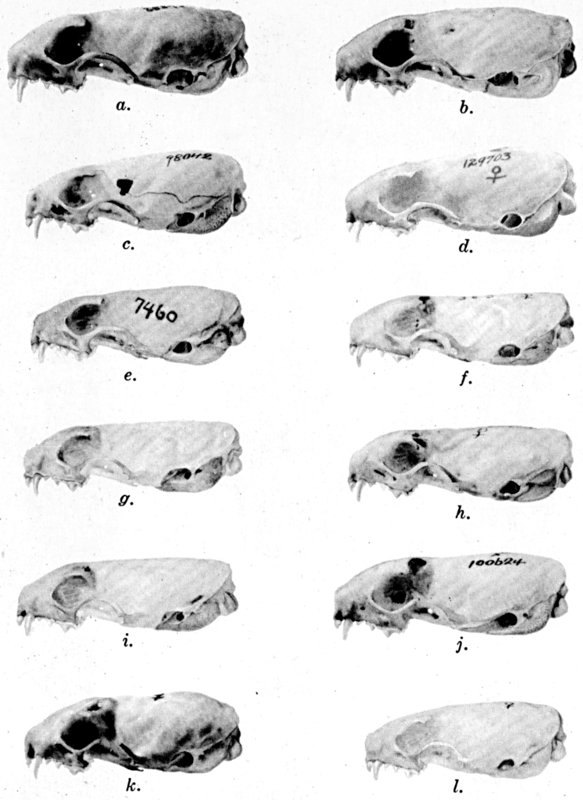
Plate 11. Photographs, retouched, of skulls in lateral
view of twelve subspecies of Mustela erminea. Natural size.
a. Mustela erminea arctica, ♀ ad., 35895, Field Mus. Nat. Hist.,
Point Barrow, Alaska.
b. Mustela erminea semplei, ♀ ad., 6600, Carnegie Mus.,
Southhampton Island.
c. Mustela erminea kadiacensis, ♀ ad., 98042, U. S. Nat. Mus.,
Kadiak, Alaska.
d. Mustela erminea richardsonii, ♀ ad., 129703, U. S. Nat. Mus.,
Fort Resolution, MacK.
e. Mustela erminea cicognanii, ♀ ad., 7460, Carnegie Mus.,
Pymatuning Swamp, Pa.
f. Mustela erminea bangsi, ♀ ad., 8679, Univ. Wisconsin, T. 61N,
R. 26W, Minn.
g. Mustela erminea invicta, ♀ ad., 90820, M. V. Z., 1-1/2 mi. W
Iron Mtn., Idaho.
h. Mustela erminea alascensis, ♀ ad., 74422, U. S. Nat. Mus.,
Juneau, Alaska.
i. Mustela erminea salva, ♀ ad., 74655, Mus. Vert. Zoöl., Mole
Harbor, Alaska.
j. Mustela erminea haidarum, ♀ ad., 100624, U. S. Nat. Mus.,
Moresby Island, B. C.
k. Mustela erminea anguinae, ♀ ad., 13673, Nat. Mus. Canada, Cape
Scott, V. I., B. C.
l. Mustela erminea fallenda, ♀ ad., 7284, Nat. Mus. Canada,
Huntingdon, B. C.
Plate 12. Photographs, retouched, of skulls in dorsal
view of eight subspecies of Mustela erminea. Natural size.
a. Mustela erminea haidarum, ♀ ad., 100624, U. S. N. M., Moresby
Island, B. C.
b. Mustela erminea anguinae, ♀ ad., 13673, N. M. Canada, Cape
Scott, V. I., B. C.
c. Mustela erminea fallenda, ♀ ad., 7284, Nat. Mus. Canada,
Huntingdon, B. C.
d. Mustela erminea olympica, ♀ ad., 242133, U. S. Nat. Mus.,
Hayes Creek, Wash.
e. Mustela erminea streatori, ♀ ad., 9040, D. R. Dickey Coll.,
Blaine, Oregon.
f. Mustela erminea gulosa, ♀ ad., 77370, U. S. Nat. Mus., Trout
Lake, Wash.
g. Mustela erminea muricus, ♀ ad., 41502, Mus. Vert. Zoöl., Baker
Creek, Nevada.
h. Mustela erminea angustidens, ♀?, ad., 12435, A. M. N. H.,
Conard Fissure, Ark.
i. Mustela erminea angustidens, ♀?, ad., 11766, A. M. N. H.,
Conard Fissure, Ark.
Plate 13. Photographs, retouched, of skulls in ventral
view of eight subspecies of Mustela erminea. Natural size.
a. Mustela erminea haidarum, ♀ ad., 100624, U. S. N. M., Moresby
Island, B. C.
b. Mustela erminea anguinae, ♀ ad., 13673, Nat. Mus. Canada, Cape
Scott, B. C.
c. Mustela erminea fallenda, ♀ ad., 7284, Nat. Mus. Canada,
Huntingdon, B. C.
d. Mustela erminea olympica, ♀ ad., 242133, U. S. Nat. Mus.,
Hayes Creek, Wash.
e. Mustela erminea streatori, ♀ ad., 9040, D. R. Dickey Coll.,
Blaine, Oregon.
f. Mustela erminea gulosa, ♀ ad., 77370, U. S. Nat. Mus., Trout
Lake, Wash.
g. Mustela erminea muricus, ♀ ad., 41502, Mus. Vert. Zoöl., Baker
Creek, Nevada.
h. Mustela erminea angustidens, ♀?, ad., 12435, A. M. N. H.,
Conard Fissure, Ark.
i. Mustela erminea angustidens, ♀?, ad., 11766, A. M. N. H.,
Conard Fissure, Ark.
Plate 14. Photographs, retouched, of M. erminea and
M. minuta. Natural size.
a. Mustela erminea olympica, ♀ ad., 242133, U. S. Nat. Mus.,
Hayes Creek, Wash.
b. Mustela erminea streatori, ♀ ad., 9040, D. R. Dickey Coll.,
Blaine, Oregon.
c. Mustela erminea gulosa, ♀ ad., 77370, U. S. Nat. Mus., Trout
Lake, Wash.
d. Mustela erminea muricus, ♀ ad., 41502, Mus. Vert. Zoöl., Baker
Creek, Nevada.
e. Mustela erminea angustidens, adult, probably female, type,
12432, Amer. Mus. Nat. Hist., with lower jaw, Conard Fissure, Ark.
f. M. e. angustidens, ♀?, ad., 12435, A. M. N. H., Conard
Fissure, Ark.
g. Mustela rixosa eskimo, ♂ sad., 43288, Mus. Vert. Zoöl.,
Barrow, Alaska.
h. Mustela rixosa eskimo, ♀ sad., 40059, Mus. Vert. Zoöl.,
Barrow, Alaska.
i. Mustela rixosa rixosa, ♂ ad., 11743, Nat. Mus. Canada,
Shaunavon, Sask.
j. Mustela rixosa rixosa, ♀ ad., 12679, Nat. Mus. Canada, south
of Shaunavon, Sask.
k. Mustela r. allegheniensis, ♂ ad., 35381, Field M. N. H.,
Portage Twp., Ohio.
l. Mustela rixosa allegheniensis, ♀ ad., 33021, Field M. N. H.,
Stryker, Ohio.
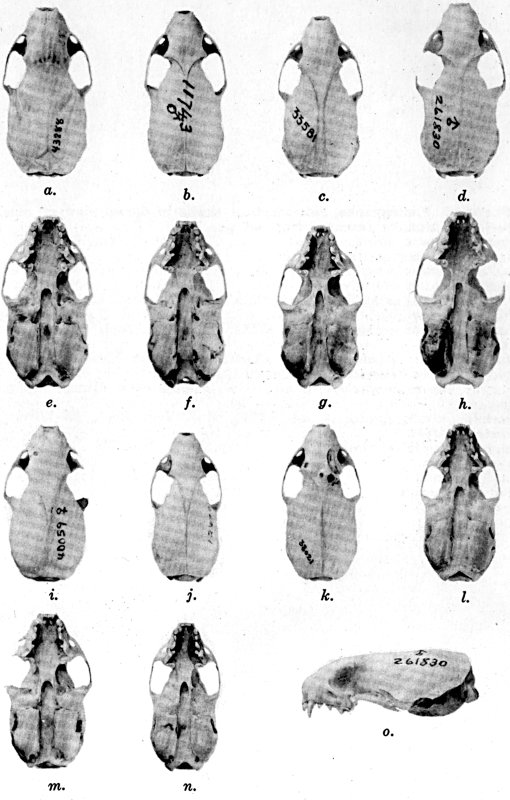
Plate 15. Photographs, retouched, of Mustela rixosa.
Natural size.
a. Mustela rixosa eskimo, ♂ sad., 43288, Mus. Vert. Zoöl.,
Barrow, Alaska.
b. Mustela rixosa rixosa, ♂ ad., 11743, Nat. Mus. Canada,
Shaunavon, Saskatchewan.
c. Mustela rixosa allegheniensis, ♂ ad., 33581, Field Mus. Nat.
Hist., Portage Township, Wood County, Ohio.
d. Mustela rixosa campestris, ♂ ad., 261830, U. S. Nat. Mus.,
shore of Sand Lake, South Dakota.
e. Mustela rixosa eskimo, ♂ sad., same specimen shown in a.
f. Mustela rixosa rixosa, ♂ same specimen shown in b.
g. Mustela rixosa allegheniensis, ♂ ad., same specimen shown in
c. c.
h. Mustela rixosa campestris, ♂ ad., same specimen shown in d.
i. Mustela rixosa eskimo, ♀ sad., 40059, Mus. Vert. Zoöl.,
Barrow, Alaska.
j. Mustela rixosa rixosa, ♀ ad., 12679, Nat. Mus. Canada, south
of Shaunavon, Saskatchewan.
k. Mustela rixosa allegheniensis, ♀ ad., 33021, Field Mus. Nat.
Hist., Stryker, Ohio.
l. Mustela rixosa allegheniensis, ♀ ad., same specimen shown in
k.
m. Mustela rixosa eskimo, ♀ sad., same specimen shown in i.
n. Mustela rixosa rixosa, ♀ ad., same specimen shown in j.
o. Mustela rixosa campestris, ♂ ad., same specimen shown in d
and h.
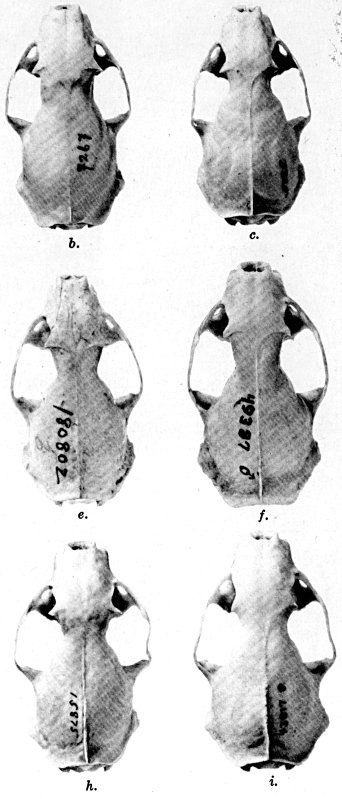
Plate 16. Photographs, retouched, of skulls in dorsal
view of nine subspecies of Mustela frenata. Natural size.
a. Mustela frenata noveboracensis, ♂ ad., 77112, U. S. Nat. Mus.,
Wilmington, Massachusetts.
b. Mustela frenata occisor, ♂ ad., 7267, Mus. Comp. Zool.,
Moosehead Lake, Maine.
c. Mustela frenata primulina, ♂ ad., 3325, Mus. Nat. Hist., Univ.
Kansas, Clinton, Kansas.
d. Mustela frenata arthuri, ♂ sad., 37515, Mus. Vert. Zoöl.,
type, Remy, Louisiana.
e. Mustela frenata olivacea, ♂ ad., 180802, U. S. Nat. Mus.,
type, Biological Surveys Collection, Autaugaville, Alabama.
f. Mustela frenata peninsulae, ♂ ad., 49387, Florida State Mus.,
Apopka, Florida.
g. Mustela frenata spadix, ♂ ad., 53745, Mus. Vert. Zoöl., Elk
River, Minnesota.
h. Mustela frenata longicauda, ♂ ad., 15875, Amer. Mus. Nat.
Hist., Red Deer, Alberta.
i. Mustela frenata oribasa, ♂ ad., 43817, Mus. Vert. Zoöl.,
Isaacs Lake, British Columbia.
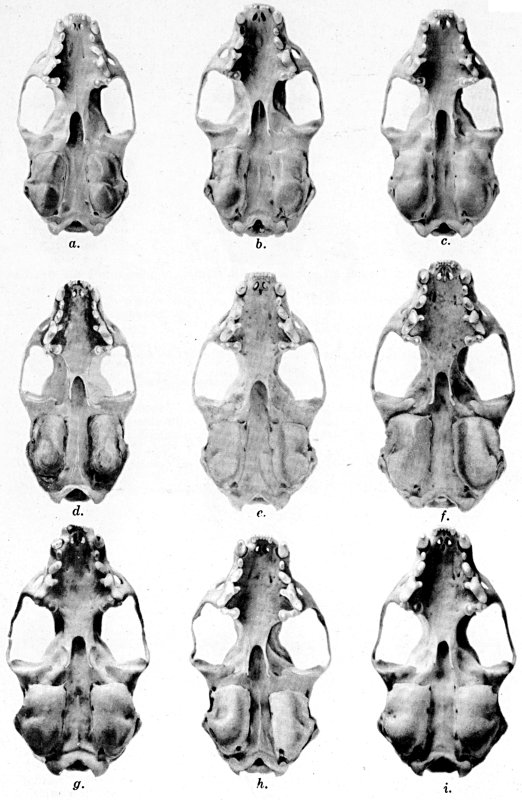
Plate 17. Photos, retouched, of skulls of males, in
ventral view, of 9 subspecies of Mustela frenata, × 1. Data for a
to i are given on Plate 18.
a. M. f noveboracensis
b. M. f. occisor
c. M. f. primulina
d. M. f. arthuri
e. M. f. olivacea
f. M. f. peninsulae
g. M. f. spadix
h. M. f. longicauda
i. M. f. oribasus
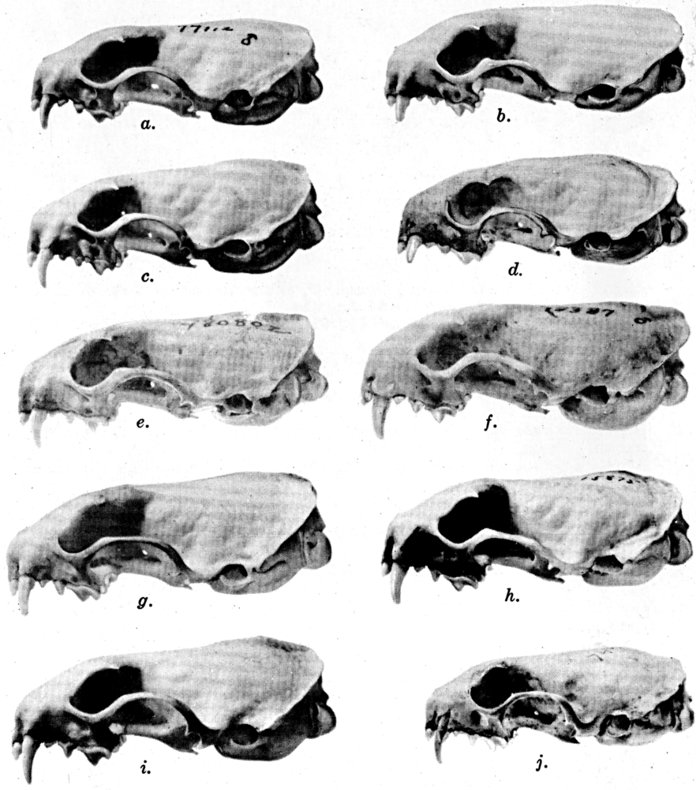
Plate 18. Photographs, retouched, of skulls in lateral
view of ten subspecies of Mustela frenata. Natural size.
a. Mustela frenata noveboracensis, ♂ ad., 77112, U. S. Nat. Mus.,
Wilmington, Mass.
b. Mustela frenata occisor, ♂ ad., 7267, M. C. Z., Moosehead
Lake, Maine.
c. Mustela frenata primulina, ♂ ad., 3325, Mus. Nat. Hist., Univ.
Kansas, Clinton, Kans.
d. Mustela frenata arthuri, ♂ ad., 37515, Mus. Vert. Zoöl., type,
Remy, Louisiana.
e. Mustela frenata olivacea, ♂ ad., 180802, U. S. Nat. Mus.,
type, Biological Surveys Collection, Autaugaville, Alabama.
f. Mustela frenata peninsulae, ♂ ad., 49387, Florida State Mus.,
Apopka, Florida.
g. Mustela frenata spadix, ♂ ad., 53795, Mus. Vert. Zoöl., Elk
River, Minnesota.
h. Mustela frenata longicauda, ♂ ad., 15875, Amer. Mus. N. H.,
Red Deer, Alberta.
i. Mustela frenata oribasus, ♂ ad., 43817, Mus. Vert. Zoöl.,
Isaacs Lake, B. C.
j. Mustela frenata alleni, ♂ ad., 7440/9136, A. M. N. H., Hill
City, S. D.
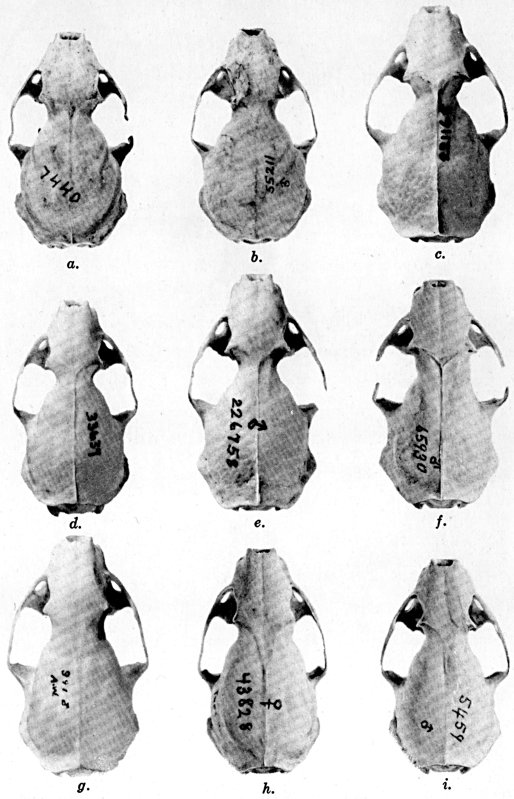
Plate 19. Photographs, retouched, of skulls in dorsal
view of nine subspecies of Mustela frenata. Natural size.
a. Mustela frenata alleni, ♂ ad., 7440/9136, Amer. Mus. Nat.
Hist., Hill City, South Dakota.
b. Mustela frenata arizonensis, ♂ ad., 55211, Mus. Vert. Zoöl.,
Government Prairie, Arizona.
c. Mustela frenata nevadensis, ♂ ad., 22116, Mus. Vert. Zoöl.,
Chinquapin, California.
d. Mustela frenata effera, ♂ ad., 33637, Amer. Mus. Nat. Hist.,
Ironside, Oregon.
e. Mustela frenata washingtoni, ♂ ad., 226758, U. S. Nat. Mus.,
Gotchen Creek, Washington.
f. Mustela frenata saturata, ♂ ad., 65930, U. S. Nat. Mus., type,
Siskiyou, Oregon.
g. Mustela frenata altifrontalis, ♂ ad., 391, Coll. Alex Walker,
Blaine, Oregon.
h. Mustela frenata oregonensis, ♂ sad., 43828/32019, U. S. Nat.
Mus., Grants Pass, Oregon.
i. Mustela frenata munda, ♂ ad., 5459, Mus. Comp. Zool., type,
Point Reyes, California.
Plate 20. Photos, retouched, of skulls of males in
ventral view of 9 subspecies of Mustela frenata, × 1. Data for a to i
on Plates 18 and 21.
a. M. f. alleni
b. M. f. arizonensis
c. M. f. nevadensis
d. M. f. effera
e. M. f. washingtoni
f. M. f. saturata
g. M. f. altifrontalis
h. M. f. oregonensis
i. M. f. munda, 5459.
Plate 21. Photographs, retouched, of skulls, in lateral
view, of nine subspecies of Mustela frenata. Natural size.
a. Mustela frenata arizonensis, ♂ ad., 55211, Mus. Vert. Zoöl.,
Government Prairie, Ariz.
b. Mustela frenata nevadensis, ♂ ad., 22116, Mus. Vert. Zoöl.,
Chinquapin, California.
c. Mustela frenata effera, ♂ ad., 33637, Amer. Mus. Nat. Hist.,
Ironside, Oregon.
d. Mustela frenata washingtoni, ♂ ad., 226758, U. S. Nat. Mus.,
Gotchen Creek, Wash.
e. Mustela frenata saturata, ♂ ad., 65930, U. S. Nat. Mus., type,
Siskiyou, Oregon.
f. Mustela frenata altifrontalis, ♂ ad., 391, Coll. Alex Walker,
Blaine, Oregon.
g. Mustela frenata oregonensis, ♂ sad., 43828/32019, U. S. N. M.,
Grants Pass, Ore.
h. Mustela frenata munda, ♂ ad., 5459, M. C. Z., type, Point
Reyes, Calif.
i. Mustela frenata munda, ♂ ad., 19722, Mus. Vert. Zoöl., Point
Arena, Calif.
j. Mustela frenata xanthogenys, ♂ ad., 1440, Coll. Alex Walker, 5
mi. W Fresno, Calif.
Plate 22. Photographs, retouched, of skulls, in dorsal
view, of nine subspecies of Mustela frenata. Natural size.
a. Mustela frenata munda, ♂ ad., 19722, Mus. Vert. Zoöl., Point
Arena, California.
b. Mustela frenata xanthogenys, ♂ ad., 1440, col. Alex Walker, 5
mi. W Fresno, California.
c. Mustela frenata nigriauris, ♂ ad., 487, Stanford Univ., Palo
Alto, California.
d. Mustela frenata latirostra, ♂ ad., 52702, U. S. Nat. Mus., El
Cajon, California.
e. Mustela frenata pulchra, ♂ ad., 16668, Mus. Vert. Zoöl., type,
Buttonwillow, California.
f. Mustela frenata inyoensis, ♂ ad., 25907, Mus. Vert. Zoöl., 2
mi. N Independence, California.
g. Mustela frenata neomexicana, ♂ ad., 1485, Mus. Nat. Hist.,
Univ., Kansas, Liberal, Kansas.
h. Mustela frenata texensis, ♂ ad., 14821, Amer. Mus. Nat. Hist.,
Kerr County, Texas.
i. Mustela frenata frenata, ♂ ad., 50826, U. S. Nat. Mus.,
Tlalpam, México, D. F.
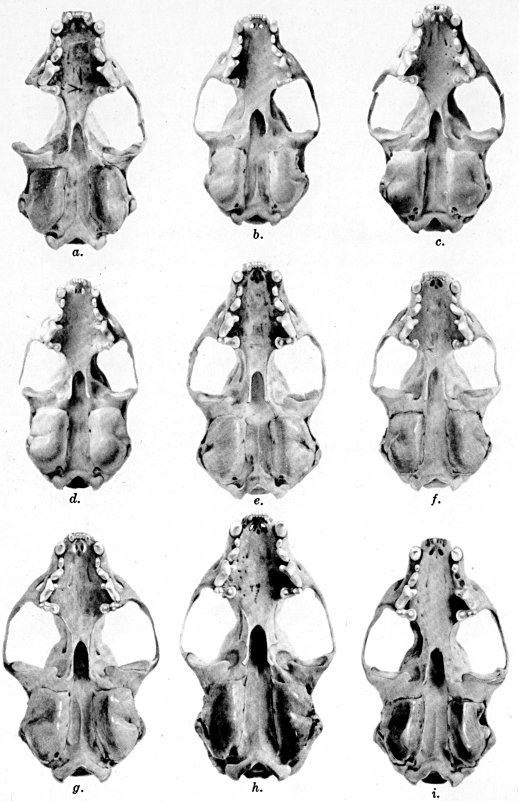
Plate 23. Ventral views of same skulls shown in Plate
22.
a. munda;
b. xanthogenys;
c. nigriauris;
d. latirostra;
e. pulchra;
f. inyoensis;
g. neomexicanus;
h. texensis;
i. frenata.
Plate 24. Photographs, retouched, of skulls, in lateral
view, of ten subspecies of Mustela frenata. Natural size.
a. Mustela frenata nigriauris, ♂ ad., 487, Stanford Univ., Palo
Alto, California.
b. Mustela frenata latirostra, ♂ ad., 52702, U. S. Nat. Mus., El
Cajon, California.
c. Mustela frenata pulchra, ♂ ad., 16668, Mus. Vert. Zoöl., type,
Buttonwillow, Calif.
d. Mustela frenata inyoensis, ♂ ad., 25907, Mus. Vert. Zoöl.,
type, 2 mi. N Independence, California.
e. Mustela frenata neomexicana, ♂ ad., 1485, M. N. H., Univ.
Kansas, Liberal, Kansas.
f. Mustela frenata texensis, ♂ ad., 14821, A. M. N. H., Kerr
County, Texas.
g. Mustela frenata frenata, ♂ ad., 50826, U. S. Nat. Mus.,
Tlalpam, México, D. F.
h. Mustela frenata leucoparia, ♂ ad., 125972, U. S. N. M., Los
Reyes, Michoacán.
i. Mustela frenata macrophonius, ♂ ad., [14063,] Field Mus. N.
H., type, Achotal, Veracruz.
j. Mustela frenata goldmani, ♂ ad., 77519, U. S. Nat. Mus.,
Pinabete, Veracruz.
Plate 25. Photographs, retouched, of skulls, in dorsal
view of nine subspecies of Mustela frenata. Natural Size.
a. Mustela frenata leucoparia, ♂ ad., 125972, U. S. Nat. Mus.,
Los Reyes, Michoacán.
b. Mustela frenata macrophonius, ♂ ad., 14063, Field Mus. Nat.
Hist., type, Achotal, Veracruz.
c. Mustela frenata goldmani, ♂ ad., 133253, U. S. Nat. Mus., 20
mi. SE Teopisca, Chiapas.
d. Mustela frenata tropicalis, ♂ ad., 54994, U. S. Nat. Mus.,
type, Jico, Veracruz.
e. Mustela frenata perda, ♂ sad., 100041, U. S. Nat. Mus., type,
Teapa, Tabasco.
f. Mustela frenata nicaraguae, ♂ sad., 30754, Amer. Mus. Nat.
Hist., type, Matagalpa, Nicaragua.
g. Mustela frenata costaricensis, ♂ ad., 3.2.1.6., British Mus.
Nat. Hist., San José, Costa Rica.
h. Mustela frenata panamensis, ♂ ad., 18848, Amer. Mus. Nat.
Hist., Boquete, Panamá.
i. Mustela frenata meridana, ♂ ad., 123341, U. S. Nat. Mus.,
type, Mérida, Venezuela.
Plate 26. Ventral views of same skulls shown in Plate
25.
a. leucoparia;
b. macrophonius;
c. goldmani;
d. tropicalis;
e. perda;
f. nicaraguae;
g. costaricensis;
h. panamensis;
i. meridana.
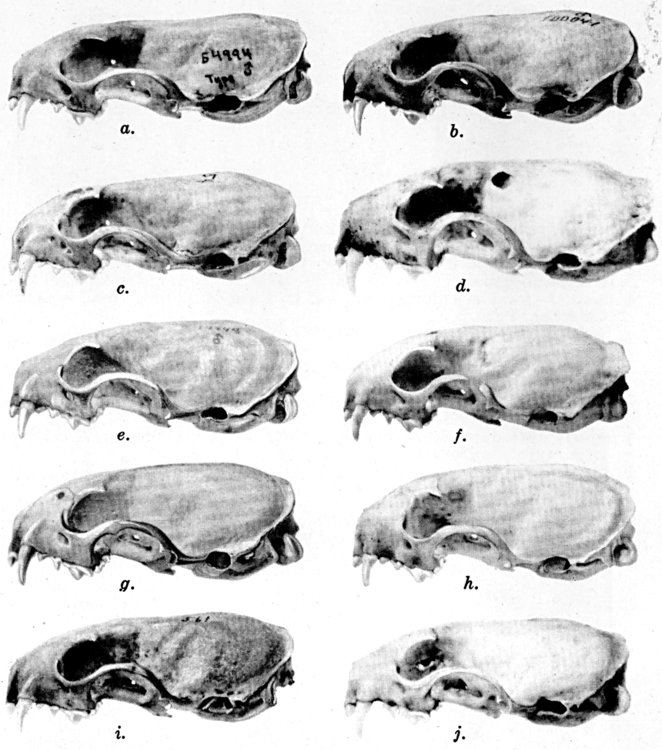
Plate 27. Photographs, retouched, of skulls, in lateral
view of ten subspecies of Mustela frenata. Natural size.
a. Mustela frenata tropicalis, ♂ ad., 54994, U. S. Nat. Mus.,
type, Jico, Veracruz.
b. Mustela frenata perda, ♂ sad., 100041; U. S. Nat. Mus., type,
Teapa, Tabasco.
c. Mustela frenata nicaraguae, ♂ sad., 30754, Amer. Mus. Nat.
Hist., type, Matagalpa, Nicaragua.
d. Mustela frenata costaricensis, ♂ ad., 3.2.1.6., British Mus.
Nat. Hist., San José, Costa Rica.
e. Mustela frenata panamensis, ♂ ad., 18848, Amer. Mus. Nat.
Hist., Boquete, Panamá.
f. Mustela frenata meridana, ♂ ad., 24309, Amer. Mus. Nat. Hist.,
Mérida, Venezuela.
g. Mustela frenata aureoventris, ♂ yg., 34677, Amer. Mus. Nat.
Hist., Gualea, Ecuador.
h. Mustela frenata helleri, ♂ ad., 24133, Field Mus. Nat. Hist.,
type, Rio Chinchao, Perú.
i. Mustela frenata macrura, ♂ ad., 561, Mus. Polonais d' Hist.,
Nat., type, Junín, Perú.
j. Mustela frenata agilis, ♂ ad., 8.1.10.1., British Mus. Nat.
Hist., Lima, Perú.
Plate 28. Photographs, retouched (except f), of
skulls, in dorsal view of nine kinds (species and subspecies) of
Mustela. Natural size.
a. Mustela frenata aureoventris, ♂ yg., 34677, Amer. Mus. Nat.
Hist., Gualea, Ecuador.
b. Mustela frenata helleri, ♂ ad., 24133, Field Mus. Nat. Hist.,
type, Rio Chinchao, Perú.
c. Mustela frenata macrura, ♂ ad., 561, Mus. Polonais d' Hist.
Nat., type, Junín, Perú.
d. Mustela frenata agilis, ♂ ad., 8.1.10.1., British Mus. Nat.
Hist., Lima, Perú.
e. Mustela frenata boliviensis, ♂ ad., 72587, Amer. Mus. Nat.
Hist., type, Nequejahuira, Bolivia.
f. Mustela frenata xanthogenys, ♂ ad., 43.6.4.55., British Mus.,
Nat. Hist., type, California.
g. Mustela frenata costaricensis, ♂ yg., 37149, U. S. Nat. Mus.,
type, San José, Costa Rica.
h. Mustela frenata panamensis, ♂ yg., 178970, U. S. Nat. Mus.,
Mt. Pirre, Panamá.
i. Mustela africana africana, ♂ yg., 37475, Amer. Mus. Nat.
Hist., Pará, Brazil.
Plate 29. Photographs, retouched, of skulls, in ventral
view, of nine kinds (species and subspecies) of Mustela. Natural
size.
a. Mustela frenata aureoventris, ♂ yg., 34677, Amer. Mus. Nat.
Hist., Gualea, Ecuador.
b. Mustela frenata helleri, ♂ ad., 24133, Field Mus. Nat. Hist.,
type, Rio Chinchao, Perú.
c. Mustela frenata macrura, ♂ ad., 561, Mus. Polonais d' Hist.
Nat., type, Junín, Perú.
d. Mustela frenata agilis, ♂ ad., 8.1.10.1., British Mus. Nat.
Hist., Lima, Perú.
e. Mustela frenata boliviensis, ♂ ad., 72587, Amer. Mus. Nat.
Hist., type, Nequejahuira, Bolivia.
f. Mustela frenata leucoparia, ♂ ad., 47179/34914, U. S. Nat.
Mus., type, Pátzcuaro, Michoacán.
g. Mustela frenata costaricensis, ♂ yg., 37149, U. S. Nat. Mus.,
type, San José, Costa Rica.
h. Mustela frenata panamensis, ♂ yg., 178970, U. S. Nat. Mus.,
Mt. Pirre, Panamá.
i. Mustela africana africana, ♂ yg., 37475, Amer. Mus. Nat.
Hist., Pará, Brazil.
Plate 30. Photographs, retouched (except
e and q) of skulls and lower jaws of Mustela.
a. Mustela frenata boliviensis, ♂ ad., 72587, Amer. Mus. Nat.
Hist., type, Nequejahuira, Bolivia.
b. Mustela frenata xanthogenys, ♂ ad., 43.4.6.55, British Mus.
Nat. Hist., type, California.
c. Mustela frenata costaricensis, ♂ yg., 37149, U. S. Nat. Mus.,
type, San José, Costa Rica.
d. Mustela frenata panamensis, ♂ yg., 178970, U. S. Nat. Mus.,
Mt. Pirre, Panamá.
e. Mustela frenata affinis, ♂ ad., 54.6.3.4, British Mus. Nat.
Hist., type, New Granada [= Colombia].
f. Mustela africana africana, ♂ yg., 37475, Amer. Mus. Nat.
Hist., Pará, Brazil.
g. Mustela frenata saturata, ♂ ad., 65930, U. S. Nat. Mus., type,
Siskiyou, Oregon.
h. Mustela frenata oregonensis, ♂ ad., 43828/32019, U. S. Nat.
Mus., type, Grants Pass, Oregon.
i. Mustela frenata munda, ♂ ad., 5459, Mus. Comp. Zool., type,
Point Reyes, California.
j. Mustela frenata leucoparia, ♂ ad., 47179/34914, U. S. Nat.
Mus., type, Pátzcuaro, Michoacán.
k. Mustela frenata macrophonius, ♂ ad., 14963, Field Mus. Nat.
Hist., type, Achotal, Veracruz.
l. Mustela frenata goldmani, ♂ ad., 77519, U. S. Nat. Mus., type,
Pinabete, Chiapas.
m. Mustela frenata tropicalis, ♂ ad., 54994, U. S. Nat. Mus.,
type, Jico, Veracruz.
n. Mustela frenata perda, ♂ sad., 100041, U. S. Nat. Mus., type,
Teapa, Tabasco.
o. Mustela frenata nicaraguae, ♂ sad., 30754, Amer. Mus. Nat.
Hist., type, Matagalpa, Nicaragua.
p. Mustela frenata costaricensis, ♂ yg., 37149, U. S. Nat. Mus.,
type, San José, Costa Rica.
q. Mustela frenata affinis, ♂ ad., 54.6.3.4, British Mus. Nat.
Hist., type, New Granada [= Colombia].
r. Mustela frenata macrura, ♂ ad., 561., Mus. Polonais d' Hist.
Nat., type, Junín, Perú.
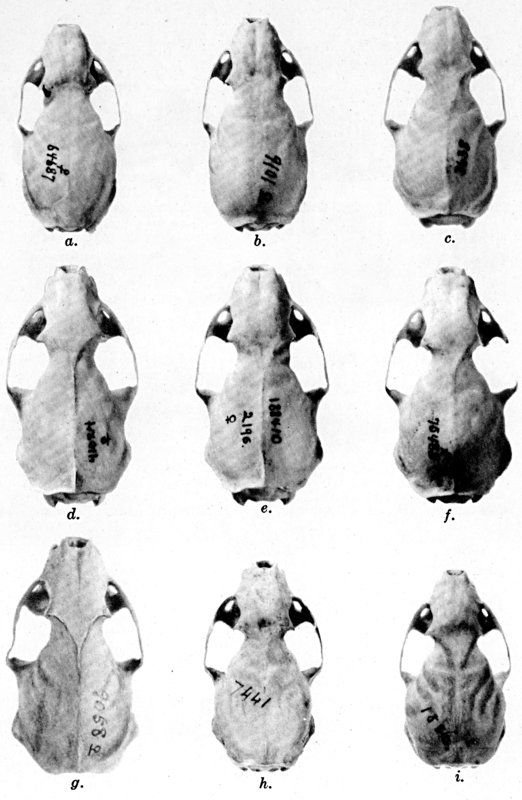
Plate 31. Photos, retouched, of skulls of 9 subspecies
of M. frenata, x 1.
a. Mustela frenata noveboracensis, ♀ ad., 64687, U. S. N. M.,
Wilmington, Mass.
b. Mustela frenata occisor, ♀ ad., 9101, Mus. Comp. Zool.,
Bucksport, Maine.
c. Mustela frenata primulina, ♀ ad., 3638, U. K. M. N. H., 7 mi.
SW Lawrence, Kans.
d. Mustela frenata olivacea, ♀ ad., 41024, Mus. Vert. Zoöl.,
Sinkola Plantation, Ga.
e. Mustela frenata spadix, ♀ ad., 188410, U. S. Nat. Mus., Elk
River, Minn.
f. Mustela frenata longicauda, ♀ ad., 75483, U. S. Nat. Mus.,
Wingard, Sask.
g. Mustela frenata oribasus, ♀ ad., 9058, M. C. Z., type, source
of Kettle River, B. C.
h. Mustela frenata alleni, ♀?, ad., 7441, A. M. N. H., Black
Hills, S. D.
i. Mustela frenata arizonensis, ♀ ad., 1886, A. M. N. H., type,
S. F. Forest, Ariz.
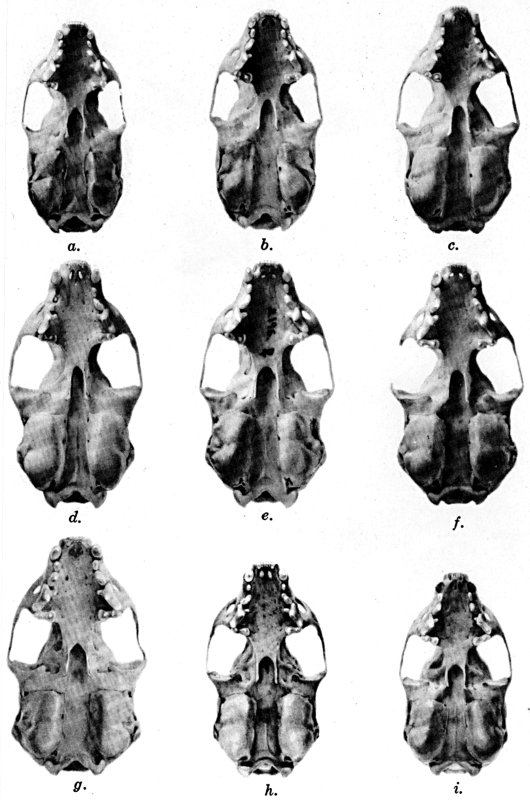
Plate 32. Photos, retouched, of skulls of 9 subspecies
of M. frenata, × 1.
a. Mustela frenata noveboracensis, ♀ ad., 64687, U. S. N. M.,
Wilmington, Mass.
b. Mustela frenata occisor, ♀ ad., 9101, Mus. Comp. Zool.,
Bucksport, Maine.
c. Mustela frenata primulina, ♀ ad., 3638, U. K. M. N. H., 7 mi.
SW Lawrence, Kans.
d. Mustela frenata olivacea, ♀ ad., 41024, Mus. Vert. Zoöl.,
Sinkola Plantation, Ga.
e. Mustela frenata spadix, ♀ ad., 188410, U. S. Nat. Mus., Elk
River, Minn.
f. Mustela f. longicauda, ♀ ad., 75483, U. S. Nat. Mus., Wingard,
Sask.
g. Mustela frenata oribasus, ♀ ad., 9058, M. C. Z., type, source
of Kettle Riv., B. C.
h. Mustela frenata alleni, ♀?, ad., 7441, A. M. N. H., Black
Hills, S. D.
i. Mustela frenata arizonensis, ♀ ad., 1886, A. M. N. H., type,
S. F. Forest, Ariz.
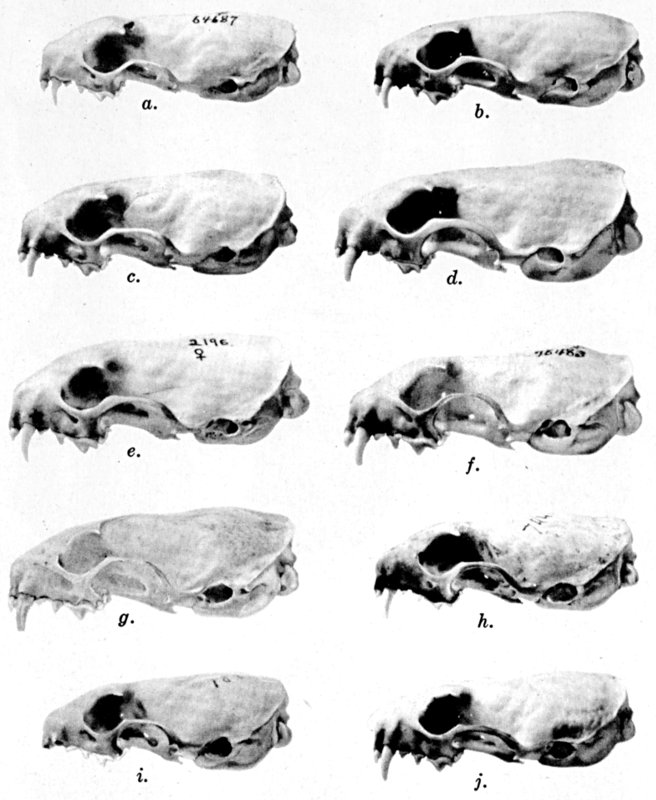
Plate 33. Photographs, retouched, of skulls in lateral
view of ten subspecies of Mustela frenata. Natural size.
a. Mustela frenata noveboracensis, ♀ ad., 64687, U. S. N. M.,
Wilmington, Mass.
b. Mustela frenata occisor, ♀ ad., 9101, Mus. Comp. Zool.,
Bucksport, Maine.
c. Mustela frenata primulina, ♀ ad., 3638, Univ. Kansas Mus. Nat.
Hist., 7 mi. SW Lawrence, Kansas.
d. Mustela frenata olivacea, ♀ ad., 41024, Mus. Vert. Zoöl.,
Sinkola Plantation, Ga.
e. Mustela frenata spadix, ♀ ad., 188410 (2196), U. S. Nat. Mus.,
Elk River, Minn.
f. Mustela frenata longicauda, ♀ ad., 75483, U. S. Nat. Mus.,
Wingard, Sask.
g. Mustela frenata oribasus, ♀ ad., 9058, Mus. Comp. Zool., type,
source of Kettle River, British Columbia.
h. Mustela frenata alleni, ♀?, ad., 7441, Amer. Mus. N. H., Black
Hills, S. D.
i. Mustela frenata arizonensis, ♀ ad., 1886, Amer. Mus. Nat.
Hist., type, San Francisco, Forest, Arizona.
j. Mustela frenata nevadensis, ♀ ad., 41503, M. V. Z., type, 3
mi. E Baker, Nev.
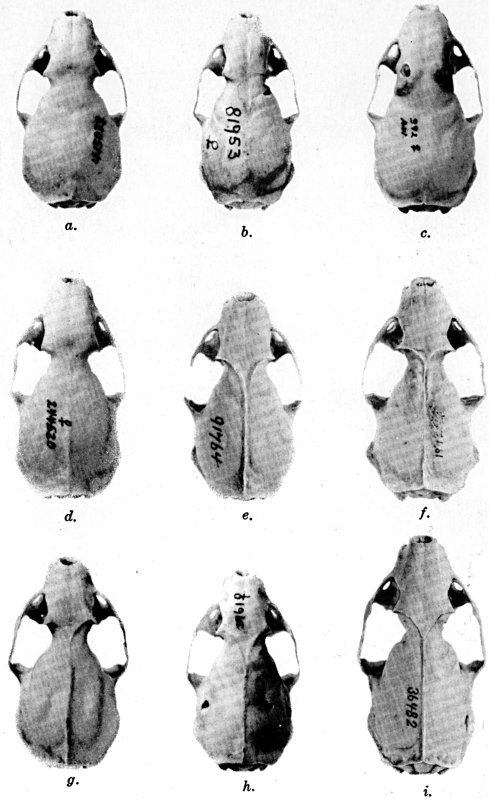
Plate 34. Photographs, retouched, of skulls in dorsal
view, of eight subspecies of Mustela frenata. Natural size.
a. Mustela frenata nevadensis, ♀ ad., 41503, M. V. Z., type, 3
mi. E Baker, Nevada.
b. Mustela frenata washingtoni, ♀ sad., 81953, U. S. N. M., Trout
Lake, Wash.
c. Mustela frenata altifrontalis, ♀ ad., 392, coll. of Alex
Walker, Blaine, Oregon.
d. Mustela frenata oregonensis, ♀ ad., 244520, U. S. Nat. Mus.,
Medford, Oregon.
e. Mustela frenata munda, ♀ ad., 91764, U. S. Nat. Mus., Point
Reyes, California.
f. Mustela frenata munda, ♀ ad., 19723, Mus. Vert. Zoöl., Point
Arena, California.
g. Mustela frenata xanthogenys, ♀ ad., 2626, coll. of W. E.
Snyder, Selma, California.
h. Mustela frenata nigriauris, ♀ ad., 3761, M. V. Z., San
Francisco, California.
i. Mustela frenata neomexicana, ♀ ad., 36482, U. S. N. M.,
Tombstone, Arizona.
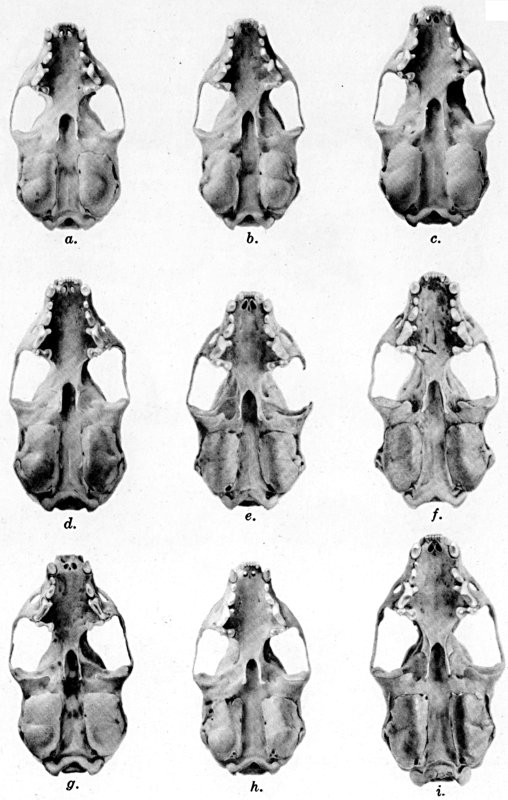
Plate 35. Photographs, retouched, of skulls in ventral
view, of eight subspecies of Mustela frenata. Natural size.
a. Mustela frenata nevadensis, ♀ ad., 41503, M. V. Z., type, 3
mi. E Baker, Nevada.
b. Mustela frenata washingtoni, ♀ sad., 81953, U. S. N. M., Trout
Lake, Wash.
c. Mustela frenata altifrontalis, ♀ ad., 392, coll. of Alex
Walker, Blaine, Oregon.
d. Mustela frenata oregonensis, ♀ ad., 244520, U. S. Nat. Mus.,
Medford, Oregon.
e. Mustela frenata munda, ♀ ad., 91764, U. S. N. M., Point Reyes,
California.
f. Mustela frenata munda, ♀ ad., 19723, M. V. Z., Point Arena,
California.
g. Mustela frenata xanthogenys, ♀ ad., 2626, coll. of W. E.
Snyder, Selma, Calif.
h. Mustela frenata nigriauris, ♀ ad., 3761, M. V. Z., San
Francisco, California.
i. Mustela frenata neomexicana, ♀ ad., 36482, U. S. N. M.,
Tombstone, Arizona.
Plate 36. Photographs, retouched, of skulls in lateral
view, of eight subspecies of Mustela frenata. Natural size.
a. Mustela frenata washingtoni, ♀ sad., 81953, U. S. N. M., Trout
Lake, Wash.
b. Mustela frenata altifrontalis, ♀ ad., 392, coll. of Alex
Walker, Blaine, Oregon.
c. Mustela frenata oregonensis, ♀ ad., 244520, U. S. N. M.,
Medford, Oregon.
d. Mustela frenata munda, ♀ ad., 91764, U. S. N. M., Point Reyes,
California.
e. Mustela frenata munda, ♀ ad., 19723, Mus. Vert. Zoöl., Point
Arena, Calif.
f. Mustela frenata xanthogenys, ♀ ad., 2626, coll. of W. E.
Snyder, Selma, Calif.
g. Mustela frenata nigriauris, ♀ ad., 3761, Mus. Vert. Zoöl., San
Francisco, Calif.
h. Mustela frenata neomexicana, ♀ ad., 36482, U. S. N. M.,
Tombstone, Ariz.
i. Mustela frenata frenata, ♀ ad., 58685, U. S. Nat. Mus.,
Brownsville, Texas.
j. Mustela frenata frenata, ♀ ad., 991, Berlin Zool. Mus., type,
México City, D. F.
k. Mustela frenata leucoparia, ♀ ad., 26153, Amer. Mus. N. H.,
Artenkiki, Jalisco.
l. Mustela frenata perotae, ♀ ad., 54278, U. S. Nat. Mus., type,
12500 ft., Cofre de Perote, Veracruz.
Plate 37. Photos, retouched, of skulls of 8 subspecies
of Mustela frenata, × 1.
a. Mustela frenata frenata, ♀ ad., 58685, U. S. Nat. Mus.,
Brownsville, Texas.
b. Mustela frenata frenata, ♀ ad., 991, Berlin Zool. Mus., type,
México City, D. F.
c. Mustela frenata leucoparia, ♀ ad., 26153, Amer. Mus. Nat.
Hist., Artenkiki, Jalisco.
d. Mustela f. perotae, ♀ ad., 54278, U. S. N. M., type, Cofre de
Perote, Veracruz.
e. Mustela frenata macrophonius, ♀ ad., 132528, U. S. Nat. Mus.,
Pérez, Veracruz.
f. Mustela frenata tropicalis, ♀ ad., 54993, U. S. Nat. Mus.,
Jico, Veracruz.
g. Mustela frenata perda, ♀ sad., 65422, U. S. Nat. Mus.,
Catemaco, Veracruz.
h. Mustela frenata meridana, ♀ ad., 143665, U. S. N. M., Mérida,
Venezuela.
i. Mustela f. macrura, ♀ ad., 564, M. P. H. N., type of Mustela
jelskii, Cutervo, Perú.
Plate 38. Photos, retouched, skulls in ventral view, 8
subspecies of M. frenata, × 1.
a. Mustela frenata frenata, ♀ ad., 58685, U. S. Nat. Mus.,
Brownsville, Texas.
b. Mustela frenata frenata, ♀ ad., 991, Berlin Zool. Mus., type,
México City, D. F.
c. Mustela frenata leucoparia, ♀ ad., 26153, Amer. Mus. Nat.
Hist., Artenkiki, Jalisco.
d. Mustela f. perotae, ♀ ad., 54278, U. S. N. M., type, Cofre de
Perote, Veracruz.
e. Mustela frenata macrophonius, ♀ ad., 132528, U. S. Nat. Mus.,
Pérez, Veracruz.
f. Mustela frenata tropicalis, ♀ ad., 54993, U. S. Nat. Mus.,
Jico, Veracruz.
g. Mustela frenata perda, ♀ sad., 65422, U. S. Nat. Mus.,
Catemaco, Veracruz.
h. Mustela frenata meridana, ♀ ad., 143665, U. S. N. M., Mérida,
Venezuela.
i. Mustela f. macrura, ♀ ad., 564, M. P. H. N., type of Mustela
jelskii, Cutervo, Perú.
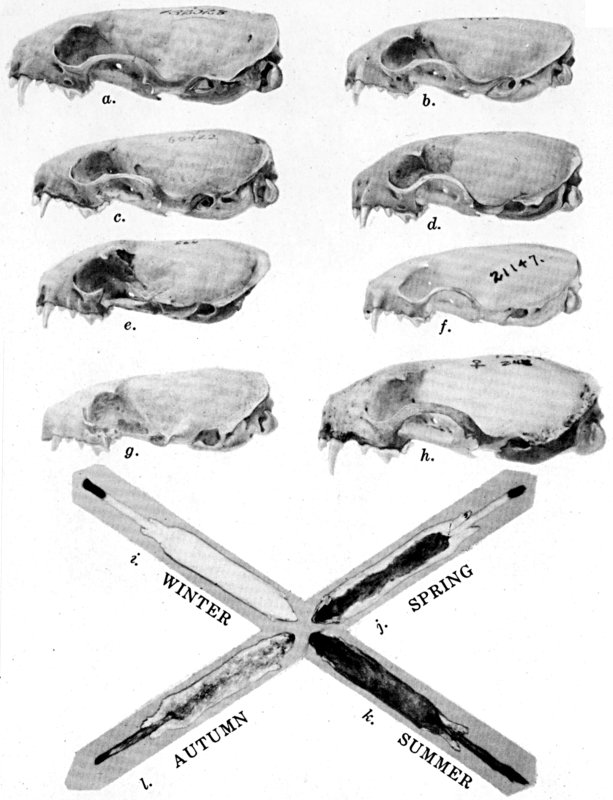
Plate 39. Figs. a-h.
Photos, retouched, of 10 kinds Mustela, × 1.
a. Mustela frenata macrophonius, ♀ ad., 132528, U. S. Nat. Mus.,
Pérez, Veracruz.
b. Mustela frenata tropicalis, ♀ ad., 54993, U. S. Nat. Mus.,
Jico, Veracruz.
c. Mustela frenata perda, ♀ sad., 65422, U. S. Nat. Mus.,
Catemaco, Veracruz.
d. Mustela frenata meridana, ♀ ad., 143665, U. S. Nat. Mus.,
Mérida, Venezuela.
e. Mustela f. macrura, ♀ ad., 564, M. P. H. N., type of Mustela
jelskii, Cutervo, Perú.
f. Mustela frenata agilis, ♀ sad., 21147, Field Mus. Nat. Hist.,
Macate, Perú.
g. Mustela frenata gracilis, ♀?, ad., 12431, Amer. Mus. Nat.
Hist., type, Conard Fissure, Arkansas, Pleistocene.
h. Mustela a. stolzmanni, ♀ sad., 24.12.12.24, Brit. M. N. H.,
Myobamba, Perú.
Figs. i-l. Mustela frenata nevadensis, all
males, from Colorado, showing seasonal change in color, × approximately
1/9. Note the sharply marked molt line in the pelage of spring and the
absence of any definite molt line in autumn.
i. No. 151415 U. S. Nat. Mus., Coventry, December 27, 1907.
j. No. 202741 U. S. Nat. Mus., Pierce Place, April 18, 1913.
k. No. 201681 U. S. Nat. Mus., Jefferson, June 23, 1913.
l. No. 41997 Amer. Mus. Nat. Hist., Navajo River, October 29, 1913.
Plate 40. Photos, retouched, of skulls and lower jaws of
Mustela, × 1.
a. Mustela frenata agilis, ♀ sad., 21147, Field Mus. Nat. Hist.,
Macate, Perú.
b. Mustela frenata gracilis, ♀?, ad., type, 12431, A. M. N. H.,
Conard Fiss., Ark.
c. Mustela africana stolzmanni, ♀ sad., 24.12.12.24, Brit. M. N.
H., Myobamba, Perú.
d. Mustela frenata agilis, ♀ sad., 21147, Field Mus. Nat. Hist.,
Macate, Perú.
e. Mustela frenata gracilis, ♀?, ad., type, 12431, A. M. N. H.,
Conard Fiss., Ark.
f. Mustela africana stolzmanni, ♀ sad., 24.12.12.24, Brit. M. N.
H., Myobamba, Perú.
g. Mustela africana stolzmanni, ♀ ad., 563, Mus. Polonais d'
Hist. Nat., type, Yurimaguas, Perú. The palate is broken longitudinally
and the two maxillae are slightly out of normal position.
h. Mustela frenata oribasus, ♀ ad., 9058, M. C. Z., type, source
of Kettle Riv., B. C.
i. Mustela frenata munda, ♀ ad., 91764, U. S. Nat. Mus., Point
Reyes, Calif.
j. Mustela frenata frenata, ♀ ad., 991, Berlin Z. M., type,
México City, D. F.
k. Mustela f. macrura, ♀ ad., 564, Mus. Polonais d' Hist. Nat.,
type of Mustela jelskii.
l. Mustela africana stolzmanni, ♀ ad., 563, Mus. Polonais d'
Hist. Nat., type, Yurimaguas, Perú. Right half of lower jaw reversed.

Plate 41. Photographs, approximately 1/2, of stuffed
study-skins of the four species of American weasels. For each pair the
male is at the left and the female at the right. Photo. by W. C.
Matthews.
Mustela erminea arctica, both in U. S. B. S., from Mts. near Eagle,
Alaska, ♂ 131256 and ♀ 131245.
Mustela erminea invicta, both in U. S. B. S., from Tungsten Mine,
Washington, ♂ 235236 and ♀ 235235.
Mustela erminea muricus, both in M. V. Z., from Baker Creek, 8675
ft., Nevada, ♂ 41501 and ♀ 41502.
Mustela frenata nigriauris, both in M. V. Z., from California, ♂
51666 from Concord and ♀ 73109 from Berkeley.
Mustela africana africana, ♂ 37475 A. M. N. H., from Pará, Brazil.
Mustela rixosa allegheniensis, both in M. Z. U. M., from Michigan,
♂ 83260 Swan Creek Farm and ♀ 88079 from Unadilla.
Principal references are in boldface type
aequatorialis,
Mustela, 75
Putorius, 75, 341, 387
aestuarina, Mustela, 82
affinis,
Mustela, 75, 375, 379, 384, 398, 409
Putorius, 372, 375, 379, 384
africana, Mustela, 73, 406, 409
agilis,
Mustela, 74, 393
Putorius, 74, 222
alascensis,
Mustela, 75, 131
Putorius, 75, 131
albigula, Neotoma, 208
allegheniensis,
Mustela, 77, 187
Putorius, 77, 187
alleni,
Mustela, 76, 274
Putorius, 76, 274
altifrontalis, Mustela, 79, 300
americana, 74, 75
americanus, Lepus, 93, 201, 210, 212, 216
anguinae, Mustela, 79, 145
angustidens,
Mustela, 78, 165
Putorius, 78, 165
Annelida, 93
arctica, Mustela, 76, 96
arcticus, Putorius, 76
arizonensis,
Mustela, 75, 276, 280, 291, 323
Putorius, 75, 276, 280
armatus, Citellus, 200
arthuri, Mustela, 78, 241
audax,
Mustela, 77
Putorius, 77
aureoventris, Mustela, 74, 387, 398
Aves, 93
bachmani, Sylvilagus, 213
bangsi, Mustela, 80, 124
barn owl, 173
Belding ground squirrel, 205
beldingi, Citellus, 205
big jumping mouse, 210
birds,
small, 216
wild, 93, 213
Blarina, 91, 205, 209, 210
brevicauda, 205
bobwhite, 213
boccamela, Mustela, 170
boliviensis, Mustela, 79, 402
bottae, Thomomys, 207
boylii, Lampropeltis, 213
brasiliensis,
Mustela, 73, 341, 372, 375
Putorius, 280, 300, 315, 319, 323, 384, 387, 398, 409
brevicauda, Blarina, 91, 205
brush rabbit, 213
bull snake, 213
Bunaelurus, 11
campestris, Mustela, 78, 190
cat, domestic, 174
celenda, Mustela, 80, 139
chicken, 93, 213, 216
chipmunk, 92, 196, 201, 206, 210, 216
cicognanii,
Mustela, 73, 110, 118, 124, 222
Putorius, 118, 124, 128, 145, 155, 161
cinereus, Sorex, 205
Citellus, 216
armatus, 200
beldingi, 205
franklini, 205
lateralis, 206
richardsonii, 205
townsendii, 205
tridecemlineatus, 205
Clethrionomys, 92
gapperi, 175, 181
Colaptes, 213
comadreja, 7
Condylura, 210
costaricensis, Mustela, 78, 372, 379, 387
cotton rat, 208
cottontail, 202, 203, 205, 212
Mearns, 208
coyote, 200
Cratogeomys, 65, 207
culbertsoni,
Mustela, 75
Putorius, 75
deer mouse, 208, 209, 216
domestic cat, 174
donnola, 7
[462]
drummondi, Microtus, 175
earthworm, 93
effera, Mustela, 79, 291
energumenos, Mustela, 82
ermine, 87
erminea,
Mustela, 71
Putorius, 222
eskimo,
Mustela, 77, 181
Putorius, 77, 181
evagor, Mustela, 82
evergladensis, Mustela, 82
Evotomys, 175
fallenda, Mustela, 80, 148
Felis, 9
ferrets, 43
fish, 91, 93
flickers, 213
floridana, Neotoma, 208
flying squirrel, 206, 216
Franklin ground squirrel, 205
franklini, Citellus, 205
frenata, Mustela, 73, 232, 252, 280, 309, 338, 341
frenatus, Putorius, 280, 300, 315, 319, 323, 341, 351, 363, 366, 372, 384, 398
frog, leopard, 93
fulvus, Vulpes, 200
furo, Mustela, 43
fusca, Mustela, 222
fuscipes, Neotoma, 208
fuscus, Putorius, 74, 222
Gallus, 93, 213
gapperi, Clethrionomys, 175, 181
Geomyidae, 216
getulus, Lampropeltis, 213
Glaucomys, 206, 216
golden-mantled ground squirrel, 206
goldmani,
Mustela, 76, 355
Putorius, 76, 355
gracilis,
Mustela, 78, 404
Putorius, 78, 404
Grammogale, 407
grasshopper, 208
grasshopper mouse, 207, 216
great-horned owl, 173
ground squirrel, 210, 216
Belding, 205
Franklin, 205
golden-mantled, 206
Richardson, 205
thirteen-lined, 205
Townsend, 205
gulosa, Mustela, 80, 159
haidarum,
Mustela, 76, 142
Putorius, 76, 142
hare, varying, 210, 216
harvest mouse, 208, 216
helleri, Mustela, 79, 391
hispidus, Sigmodon, 208
horned lark, 209
house mouse, 216
hyemalis, Junco, 213
imperii, Putorius, 77, 110
ingens, Mustela, 82
initis, Mustela, 80, 136
insects, 176, 209, 216
intergrades, 45
invicta, Mustela, 80, 128
inyoensis, Mustela, 79, 331
javonica, Mustela, 72
jelskii, Mustela, 75, 398
jumping mouse, 216
big, 210
Junco hyemalis, 213
kadiacensis,
Mustela, 76, 108
Putorius, 76, 108
king-snake, 213
labiata, Mustela, 79
lacustris, Mustela, 82
Lampropeltis,
boylii, 213
getulus, 213
lark, horned, 209
lateralis, Citellus, 205
latimanus, Scapanus, 205
latirostra, Mustela, 79, 323
least weasel, 168, 209
lemming, 92
Lemmus, 92
Leopard frog, 93
lepida, Neotoma, 208
lepta, Mustela, 161
leptus,
Mustela, 161
Putorius, 78, 161
Lepus americanus, 93, 201, 210, 211, 212, 216
letifera, Mustela, 82
leucoparia,
Mustela, 76, 347
Putorius, 76, 347
lizards, 216
longicauda,
Mustela, 73, 232, 252, 262
Putorius, 280
long-tailed weasel, 193
lutensis, Mustela, 82
Lutra minor, 170
Lutreola, 84
lutreola, Mustela, 170
[463]
Lyncodon, 407
macrodon, Mustela, 82
macrophonius,
Mustela, 78, 360
Putorius, 78, 360
macrura, Mustela, 75, 387, 393, 398, 402
macrurus, Putorius, 379
maniculatus, Peromyscus, 175
Martinogale, 11
meadow mice, 92, 208, 209, 216
Mearns cottontail, 208
melampelus, Mustela, 82
melodia, Melospiza, 213
Melospiza melodia, 213
meridana, Mustela, 78, 379
Mexican pocket gopher, 207
mexicanus, Putorius, 341
mice, meadow, 92, 208, 209, 216
microtis,
Mustela, 110
Putorius, 77, 110
Microtus, 65, 91, 92, 174, 179, 181, 208, 209, 210, 216, 220
drummondi, 175
minor, 175
montanus, 209
ochrogaster, 179
pennsylvanicus, 175
minimus, Tamias, 206
mink, Mustela, 82
minor,
Lutra, 170
Mustela, 169
minor, Zapus, 210
minuta,
Mustela, 169
Palaeogale, 169
minutus, Putorius, 169
Miomustela, 11
mole, 210
moles, 216
montanus, Microtus, 209
mortigena, Mustela, 110
mouse,
deer, 208, 209, 216
grasshopper, 207, 216
harvest, 208, 216
house, 208, 216
jumping, 210
meadow, 208, 209, 216
red-backed, 92
munda, Mustela, 77, 304, 309
mundus, Putorius, 77, 309
murica, Mustela, 161
muricus,
Mustela, 77, 161
Putorius, 77, 161
Mus, 216
muskrat, 216
Mustela, 83
aequatorialis, 75
aestuarina, 82
affinis, 75, 375, 379, 384, 398, 409
africana, 73, 406, 409
agilis, 74, 393
alascensis, 75, 131
allegheniensis, 77, 187
alleni, 76, 274
altifrontalis, 79, 300
americana, 74, 75
anguinae, 79, 145
angustidens, 78, 165
arctica, 76, 96
arizonensis, 75, 276, 280, 291, 323
arthuri, 78, 241
audax, 77
aureoventris, 74, 387, 398
bangsi, 80, 124
boccamela, 170
boliviensis, 79, 402
brasiliensis, 73, 341, 372, 375
campestris, 78, 190
celenda, 80, 139
cicognanii, 73, 118, 222
cigognanii, 118
costaricensis, 78, 372, 379, 387
culbertsoni, 75
digna, 100
effera, 79, 291
energumenos, 82
erminea, 72, 87, 103
eskimo, 77, 181
evagor, 82
evergladensis, 82
fallenda, 80, 148
furo, 43
frenata, 73, 193, 232, 252, 280, 309, 338, 341
fusca, 224
goldmani, 76, 355
gracilis, 78, 404
gulosa, 80, 159
haidarum, 76, 142
helleri, 79, 391
ingens, 82
initis, 80, 136
invicta, 80, 128
inyoensis, 79, 331
javonica, 72
jelskii, 75, 398
kadiacensis, 76, 108
kaneii, 99
labiata, 79, 105
lacustris, 82
latirostra, 79, 323
lepta, 161
leptus, 161
letifera, 82
leucoparia, 76, 347
longicauda, 73, 232, 252, 262
lutensis, 82
lutreola, 170
macrodon, 82
[464]
macrophonius, 78, 360
macrura, 75, 387, 393, 398, 402
melampelus, 82
meridana, 78, 379
microtus, 77, 110
mink, 82
minor, 169
minuta, 169
munda, 77, 304, 309
murica, 161
muricus, 77, 161
neomexicana, 76, 333
neomexicanus, 333
nesolestes, 82
nevadensis, 79, 280
nicaraguae, 78, 370
nigriauris, 79, 319
nigripes, 74
nivalis, 72
notius, 77
noveboracensis, 74, 222, 252
numidica, 170
occisor, 77, 230
olivacea, 78, 244
olympica, 80, 153
oregonensis, 76, 304
oribasa, 270
oribasus, 77, 270
orientalis, 100
panamensis, 78, 375
paraensis, 76, 409
peninsulae, 75, 250
perda, 77, 366
perotae, 79, 351
polaris, 77, 103
primulina, 78, 232
pulchra, 79, 328
pusilla, 74, 118
putorius, 43
richardsonii, 73, 110
rixosa, 76, 153, 155, 161, 168, 184
salva, 80, 135
saturata, 76, 297
seclusa, 80, 141
semplei, 78, 105
spadix, 76, 252
stolzmanni, 75, 409, 413
streatori, 76, 155
texensis, 79, 338
tropicalis, 76, 363, 367
vison, 82
vulgivaga, 82
washingtoni, 76, 294
xanthogenys, 74, 315, 331
neomexicana, Mustela, 76, 333
neomexicanus,
Mustela, 333
Putorius, 76, 333
Neotamias, 206
Neotoma, 208, 216
albigula, 208
floridana, 208
fuscipes, 208
lepida, 208
nesolestes, Mustela, 82
nevadensis, Mustela, 79, 280
nicaraguae, Mustela, 78, 370
nigriauris, Mustela, 79, 319
nigripes,
Mustela, 74
Putorius, 74
nivalis, Mustela, 72, 169
northern pocket gopher, 206
notius, Putorius, 77, 222
noveboracensis,
Mustela, 74, 222, 252
Putorius, 74, 222
numidica, Mustela, 170
numidicus, Putorius, 170
occisor,
Mustela, 77, 230
Putorius, 77
Ochotona, 92
ochrogaster, Microtus, 179
olivacea, Mustela, 78, 244
olympica, Mustela, 80, 153
Ondatra, 210, 216
Onychomys, 207, 216
oregonensis,
Mustela, 304
Putorius, 304
oribasa, Mustela, 270
oribasus,
Mustela, 77, 270
Putorius, 77
Orthogeomys, 65
owl,
barn, 173
great-horned, 173
snowy, 90
Palaeogale, 11
minuta, 169
panamensis, Mustela, 78, 375
paraensis,
Mustela, 76, 409
Putorius, 76, 409
peninsulae,
Mustela, 75, 250
Putorius, 75, 250
pennsylvanicus, Microtus, 175
perda, Mustela, 77, 366
perdus, Putorius, 77, 366
Peromyscus, 91, 92, 173, 196, 208, 209, 210, 216
maniculatus, 175
perotae, Mustela, 79, 351
pheasant, 209
ring-necked, 202
pigmy weasel, 216
pika, 92
pikas, 201
[465]
pipiens, Rana, 93
Pisces, 93
Pituophis sayi sayi, 213
Pliogale, 11
pocket gopher, 209, 216
Mexican, 207
northern, 206
Shaw, 208
polaris,
Mustela, 77, 103
Putorius, 77
polecats, 43
porcupine, quills of, 200
primulina, Mustela, 78, 233
pulchra, Mustela, 79, 323
pusilla, Mustela, 74, 118
pusillus, Putorius, 118, 155, 184, 190
Putorius, 76, 294, 300
aequatorialis, 75, 341, 387
affinis, 372, 375, 379, 384
agilis, 74, 222
alascensis, 75, 96, 131
allegheniensis, 77, 187
alleni, 76, 274
angustidens, 76, 165
arcticus, 76, 96
arizonensis, 75, 276, 280
audax, 77, 96
brasiliensis, 280, 300, 315, 319, 323, 384, 387, 398, 409
cicognanii, 96, 110, 118, 124, 128, 145, 155, 161
culbertsoni, 75
erminea, 96, 110, 222
eskimo, 77, 181
frenatus, 280, 300, 315, 319, 323, 341, 347, 351, 363, 366, 372, 384, 398
fuscus, 74, 222
goldmani, 76, 355
gracilis, 78, 404
haidarum, 76, 142
imperii, 110
kadiacensis, 76, 96, 108
kaneii, 100
leptus, 78, 161
leucoparia, 76, 347
longicauda, 262, 270, 280
macrophonius, 78, 360
macrotis, 77
macrurus, 370
mexicanus, 341
microtus, 100
minutus, 169
mundus, 77, 309
muricus, 77, 161
neomexicanus, 76, 333
nigripes, 74
notius, 77, 222
noveboracensis, 74, 222, 232
numidicus, 170
occisor, 77, 230
oregonensis, 304
oribasus, 77, 270
paraensis, 76, 409
peninsulae, 75, 250
perdus, 77, 366
polaris, 77, 103
pusillus, 118, 155, 184, 190
richardsonii, 96, 110
rixosus, 76, 181, 184
saturatus, 76, 294, 300
spadix, 76, 252
stolzmanni, 145, 148, 155, 159
streatori, 76
tropicalis, 76, 363, 370
vulgaris, 118, 155, 181, 184
washingtoni, 76, 294
xanthogenys, 315, 319, 323, 331
putorius, Mustela, 43
quail, 209, 213
rabbit,
brush, 213
snowshoe, 93, 201
racer, red, 213
Rana pipiens, 93
rat,
brown, 202
cotton, 208, 216
wood, 208
Rattus, 91, 92, 210, 216
rattlesnake, 200
red-backed mouse, 92
red
racer, 213
squirrel, 206
Reithrodontomys, 175, 216
reptiles, 213
Richardson ground squirrel, 205
richardsonii,
Citellus, 205
Mustela, 73, 110
Putorius, 222
ring-necked pheasant, 202
rixosa, Mustela, 76, 153, 155, 161, 168, 184
rixosus, Putorius, 76, 181, 184
Rodentia, 216
russet-backed thrush, 204
salva, Mustela, 80, 135
saturata, Mustela, 76, 297
sayi, Pituophis, 213
Scalopus, 209
Scapanus latimanus, 205
Schönthierlein, 7
Sciurus, 210
seclusa, Mustela, 80, 141
[466]
semplei, Mustela, 78, 105
short-tailed shrew, 208, 209
short-tailed weasel, 87
shrews, 216
Sigmodon, 216
Sigmodon hispidus, 208
slate-colored junco, 213
snake,
bull, 213
king, 213
snakes, 216
snowshoe rabbit, 93, 210
snowy owl, 90
song sparrow, 201, 213
Sorex, 91, 209
cinereus, 205
Soricidae, 216
spadix,
Mustela, 76, 252
Putorius, 76, 252
sparrow,
song, 201, 213
tree, 208, 209
spermophile, Uinta, 200
squirrel,
flying, 206
red, 206
tree, 216
stolzmanni, Mustela, 75, 409, 413
streatori,
Mustela, 76, 155
Putorius, 76, 145, 148, 155, 159
striatus, Tamias, 206
Sylvilagus, 91, 93, 209, 210, 216
bachmani, 213
Talpidae, 216
talpoides, Thomomys, 207
Tamias, 91, 92, 206, 210, 216
minimus, 206
striatus, 206
Tamiasciurus, 206, 216
texensis, Mustela, 79, 338
thirteen-lined ground squirrel, 205
Thomomys, 206, 219
bottae, 207
talpoides, 207
thrush, russet-backed, 204
towhee, 201
Townsend ground squirrel, 205
townsendii, Citellus, 205
tree sparrow, 208, 209
tridecemlineatus, Citellus, 205
tropical weasel, 406
tropicalis,
Mustela, 76, 363, 367
Putorius, 76, 363, 370
Uinta spermophile, 200
varying hare, 210, 216
vison,
Mustela, 82
Lutreola, 84
vulgaris,
Mustela, 73
Putorius, 118, 155, 181, 184
vulgivaga, Mustela, 82
Vulpes fulvus, 200
washingtoni,
Mustela, 76, 294
Putorius, 76, 294
weasel,
least, 168, 209
long-tailed, 193
Pigmy, 216
short-tailed, 87
tropical, 406
wild birds, 93, 213
wood rat, 208, 210, 216, 219
xanthogenys,
Mustela, 74, 315, 331
Putorius, 315, 319, 323, 331
Zapus, 216
minor, 210
Transcribers Notes:
Punctuation and spelling were made consistent when a predominant
preference was found in this book; otherwise they were not changed.
Simple typographical and spelling errors were corrected.
P. 162 changed Tahoma Creek, [72] to Tahoma Creek, 1[72].
Plate 24 added [14063,] for missing specimen number.
End of the Project Gutenberg EBook of American Weasels, by E. Raymond Hall
*** END OF THIS PROJECT GUTENBERG EBOOK AMERICAN WEASELS ***
***** This file should be named 43272-h.htm or 43272-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/4/3/2/7/43272/
Produced by Chris Curnow, Richard Tonsing, Joseph Cooper
and the Online Distributed Proofreading Team at
http://www.pgdp.net
Updated editions will replace the previous one--the old editions
will be renamed.
Creating the works from public domain print editions means that no
one owns a United States copyright in these works, so the Foundation
(and you!) can copy and distribute it in the United States without
permission and without paying copyright royalties. Special rules,
set forth in the General Terms of Use part of this license, apply to
copying and distributing Project Gutenberg-tm electronic works to
protect the PROJECT GUTENBERG-tm concept and trademark. Project
Gutenberg is a registered trademark, and may not be used if you
charge for the eBooks, unless you receive specific permission. If you
do not charge anything for copies of this eBook, complying with the
rules is very easy. You may use this eBook for nearly any purpose
such as creation of derivative works, reports, performances and
research. They may be modified and printed and given away--you may do
practically ANYTHING with public domain eBooks. Redistribution is
subject to the trademark license, especially commercial
redistribution.
*** START: FULL LICENSE ***
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full Project
Gutenberg-tm License available with this file or online at
www.gutenberg.org/license.
Section 1. General Terms of Use and Redistributing Project Gutenberg-tm
electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or destroy
all copies of Project Gutenberg-tm electronic works in your possession.
If you paid a fee for obtaining a copy of or access to a Project
Gutenberg-tm electronic work and you do not agree to be bound by the
terms of this agreement, you may obtain a refund from the person or
entity to whom you paid the fee as set forth in paragraph 1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this agreement
and help preserve free future access to Project Gutenberg-tm electronic
works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the Foundation"
or PGLAF), owns a compilation copyright in the collection of Project
Gutenberg-tm electronic works. Nearly all the individual works in the
collection are in the public domain in the United States. If an
individual work is in the public domain in the United States and you are
located in the United States, we do not claim a right to prevent you from
copying, distributing, performing, displaying or creating derivative
works based on the work as long as all references to Project Gutenberg
are removed. Of course, we hope that you will support the Project
Gutenberg-tm mission of promoting free access to electronic works by
freely sharing Project Gutenberg-tm works in compliance with the terms of
this agreement for keeping the Project Gutenberg-tm name associated with
the work. You can easily comply with the terms of this agreement by
keeping this work in the same format with its attached full Project
Gutenberg-tm License when you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are in
a constant state of change. If you are outside the United States, check
the laws of your country in addition to the terms of this agreement
before downloading, copying, displaying, performing, distributing or
creating derivative works based on this work or any other Project
Gutenberg-tm work. The Foundation makes no representations concerning
the copyright status of any work in any country outside the United
States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other immediate
access to, the full Project Gutenberg-tm License must appear prominently
whenever any copy of a Project Gutenberg-tm work (any work on which the
phrase "Project Gutenberg" appears, or with which the phrase "Project
Gutenberg" is associated) is accessed, displayed, performed, viewed,
copied or distributed:
This eBook is for the use of anyone anywhere at no cost and with
almost no restrictions whatsoever. You may copy it, give it away or
re-use it under the terms of the Project Gutenberg License included
with this eBook or online at www.gutenberg.org
1.E.2. If an individual Project Gutenberg-tm electronic work is derived
from the public domain (does not contain a notice indicating that it is
posted with permission of the copyright holder), the work can be copied
and distributed to anyone in the United States without paying any fees
or charges. If you are redistributing or providing access to a work
with the phrase "Project Gutenberg" associated with or appearing on the
work, you must comply either with the requirements of paragraphs 1.E.1
through 1.E.7 or obtain permission for the use of the work and the
Project Gutenberg-tm trademark as set forth in paragraphs 1.E.8 or
1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any additional
terms imposed by the copyright holder. Additional terms will be linked
to the Project Gutenberg-tm License for all works posted with the
permission of the copyright holder found at the beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including any
word processing or hypertext form. However, if you provide access to or
distribute copies of a Project Gutenberg-tm work in a format other than
"Plain Vanilla ASCII" or other format used in the official version
posted on the official Project Gutenberg-tm web site (www.gutenberg.org),
you must, at no additional cost, fee or expense to the user, provide a
copy, a means of exporting a copy, or a means of obtaining a copy upon
request, of the work in its original "Plain Vanilla ASCII" or other
form. Any alternate format must include the full Project Gutenberg-tm
License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works provided
that
- You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is
owed to the owner of the Project Gutenberg-tm trademark, but he
has agreed to donate royalties under this paragraph to the
Project Gutenberg Literary Archive Foundation. Royalty payments
must be paid within 60 days following each date on which you
prepare (or are legally required to prepare) your periodic tax
returns. Royalty payments should be clearly marked as such and
sent to the Project Gutenberg Literary Archive Foundation at the
address specified in Section 4, "Information about donations to
the Project Gutenberg Literary Archive Foundation."
- You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or
destroy all copies of the works possessed in a physical medium
and discontinue all use of and all access to other copies of
Project Gutenberg-tm works.
- You provide, in accordance with paragraph 1.F.3, a full refund of any
money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days
of receipt of the work.
- You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project Gutenberg-tm
electronic work or group of works on different terms than are set
forth in this agreement, you must obtain permission in writing from
both the Project Gutenberg Literary Archive Foundation and Michael
Hart, the owner of the Project Gutenberg-tm trademark. Contact the
Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
public domain works in creating the Project Gutenberg-tm
collection. Despite these efforts, Project Gutenberg-tm electronic
works, and the medium on which they may be stored, may contain
"Defects," such as, but not limited to, incomplete, inaccurate or
corrupt data, transcription errors, a copyright or other intellectual
property infringement, a defective or damaged disk or other medium, a
computer virus, or computer codes that damage or cannot be read by
your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH 1.F.3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium with
your written explanation. The person or entity that provided you with
the defective work may elect to provide a replacement copy in lieu of a
refund. If you received the work electronically, the person or entity
providing it to you may choose to give you a second opportunity to
receive the work electronically in lieu of a refund. If the second copy
is also defective, you may demand a refund in writing without further
opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS', WITH NO OTHER
WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO
WARRANTIES OF MERCHANTABILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of damages.
If any disclaimer or limitation set forth in this agreement violates the
law of the state applicable to this agreement, the agreement shall be
interpreted to make the maximum disclaimer or limitation permitted by
the applicable state law. The invalidity or unenforceability of any
provision of this agreement shall not void the remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in accordance
with this agreement, and any volunteers associated with the production,
promotion and distribution of Project Gutenberg-tm electronic works,
harmless from all liability, costs and expenses, including legal fees,
that arise directly or indirectly from any of the following which you do
or cause to occur: (a) distribution of this or any Project Gutenberg-tm
work, (b) alteration, modification, or additions or deletions to any
Project Gutenberg-tm work, and (c) any Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of computers
including obsolete, old, middle-aged and new computers. It exists
because of the efforts of hundreds of volunteers and donations from
people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need are critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future generations.
To learn more about the Project Gutenberg Literary Archive Foundation
and how your efforts and donations can help, see Sections 3 and 4
and the Foundation information page at www.gutenberg.org
Section 3. Information about the Project Gutenberg Literary Archive
Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Contributions to the Project Gutenberg
Literary Archive Foundation are tax deductible to the full extent
permitted by U.S. federal laws and your state's laws.
The Foundation's principal office is located at 4557 Melan Dr. S.
Fairbanks, AK, 99712., but its volunteers and employees are scattered
throughout numerous locations. Its business office is located at 809
North 1500 West, Salt Lake City, UT 84116, (801) 596-1887. Email
contact links and up to date contact information can be found at the
Foundation's web site and official page at www.gutenberg.org/contact
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
[email protected]
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To
SEND DONATIONS or determine the status of compliance for any
particular state visit www.gutenberg.org/donate
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including checks, online payments and credit card donations.
To donate, please visit: www.gutenberg.org/donate
Section 5. General Information About Project Gutenberg-tm electronic
works.
Professor Michael S. Hart was the originator of the Project Gutenberg-tm
concept of a library of electronic works that could be freely shared
with anyone. For forty years, he produced and distributed Project
Gutenberg-tm eBooks with only a loose network of volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as Public Domain in the U.S.
unless a copyright notice is included. Thus, we do not necessarily
keep eBooks in compliance with any particular paper edition.
Most people start at our Web site which has the main PG search facility:
www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.























































