Museum of Natural History
JOHN M. LEGLER
Lawrence
1960
Editors: E. Raymond Hall, Chairman, Henry S. Fitch, Robert W. Wilson
Volume 11, No. 10, pp. 527-669, 16 pls., 29 figs.
Published March 7, 1960
THE STATE PRINTING PLANT
TOPEKA, KANSAS
1960
The ornate box turtle, Terrapene o. ornata Agassiz, was studied more or less continuously from September, 1953, until July, 1957. Intensive field studies were made of free-living, marked populations in two small areas of Douglas County, Kansas, in the period 1954 to 1956. Laboratory studies were made, whenever possible, of phenomena difficult to observe in the field, or to clarify or substantiate field observations. Certain phases of the work (for example, studies of populations and movements) were based almost entirely on field observation whereas other phases (for example, growth and gametogenic cycles) were carried out almost entirely within the laboratory on specimens obtained from eastern Kansas and other localities.
A taxonomic revision of the genus Terrapene was begun in 1956 as an outgrowth of the present study. The systematic status of T. ornata and other species is here discussed only briefly.
Objectives of the study here reported on were: 1) to learn as much as possible concerning the habits, adaptations, and life history of T. o. ornata; 2) to compare the information thus acquired with corresponding information on other emyid and testudinid chelonians, and especially with that on other species and subspecies of Terrapene; 3) to determine what factors limit the geographic distribution of ornate box turtles; and, 4) to determine the role of ornate box turtles in an ecological community.
The aid given by a number of persons has contributed substantially to the present study. I am grateful to my wife, Avis J. Legler, who, more than any single person, has unselfishly contributed her time to this project; in addition to making all the histological preparations and typing the entire manuscript, she has assisted and encouraged me in every phase of the study. Dr. Henry S. Fitch has been most helpful in offering counsel and encouragement. Thanks are due Professor E. Raymond Hall for critically reading the manuscript.
Special thanks are due also to the following persons: Professor A. B. Leonard for helpful suggestions dealing with photography and for advice on several parts of the manuscript; Professor William C. Young for the use of facilities at the Endocrine Laboratory, University of Kansas; Professor Edward H. Taylor for permission to study specimens in his care; Dr. Richard B. Loomis for identifying chigger mites and offering helpful suggestions on the discussion of ectoparasites; Mr. Irwin Ungar for identification of plants; and, Mr. William R. Brecheisen for allowing me to examine his field notes and for assistance with field work. Identifications of animal remains in stomachs were made by Professor A. B. Leonard (mollusks, crustaceans), Dr. George W. Byers (arthropods), and Dr. Sydney Anderson (mammals).
Miss Sophia Damm generously permitted the use of her property as a study area and Mr. Walter W. Wulfkuhle made available two saddle horses that [Pg 532] greatly facilitated field work. The drawings (with the exception of Fig. 21) are by Miss Lucy Jean Remple. All photographs are by the author.
I am grateful also to the Kansas Academy of Science for three research grants (totaling $175.00) that supported part of the work. The brief discussion of taxonomic relationships and distribution results partly from studies made by means of two research grants (totaling $150.00), from the Graduate School, University of Kansas, for which I thank Dean John H. Nelson.
Turtles of the genus Terrapene belong to the Emyidae, a family comprising chiefly aquatic and semiaquatic species. Terrapene, nevertheless, is adapted for terrestrial existence and differs from all other North American emyids in having a hinged and movable plastron and a down-turned (although often notched) maxillary beak. Emydoidea blandingi, the only other North American emyid with a hinged plastron, lacks a down-turned beak. The adaptations of box turtles to terrestrial existence (reduction of webbing between toes, reduction in number of phalanges, reduction of zygomatic arch, and heightening of shell) occur in far greater degree in true land tortoises of the family Testudinidae. Four genera of emyid turtles in the eastern hemisphere (Cuora, Cyclemys, Emys, and Notochelys) possess terrestrial adaptations paralleling those of Terrapene but (with the possible exception of Cuora) the adaptations are less pronounced than in Terrapene. A movable plastron has occurred independently in two groups of emyids in the New World and in at least three groups in the Old World.
The genus Terrapene, in my view, contains seven species, comprising 11 named kinds. Of these species, five are poorly known and occur only in Mexico. Terrapene mexicana (northeastern Mexico) and T. yucatana (Yucatan peninsula) although closely related, differ from each other in a number of characters. Similarly, Terrapene klauberi (southern Sonora) and T. nelsoni (Tepic, Nayarit—known from a single adult male) are closely related but are considered distinct because of their morphological differences and widely separated known ranges. Terrapene coahuila, so far found only in the basin of Cuatro Ciénegas in central Coahuila, is the most primitive Terrapene known; it differs from other box turtles in a number of morphological characters and is the only member of the genus that is chiefly aquatic.
Two species of Terrapene occur in the United States. Terrapene carolina, having four recognized subspecies, has a nearly continuous distribution from southern Maine, southern Michigan, and southern Wisconsin, southward to Florida and the Gulf coast and westward to southeastern Kansas, eastern Oklahoma and eastern Texas, and characteristically inhabits wooded areas.
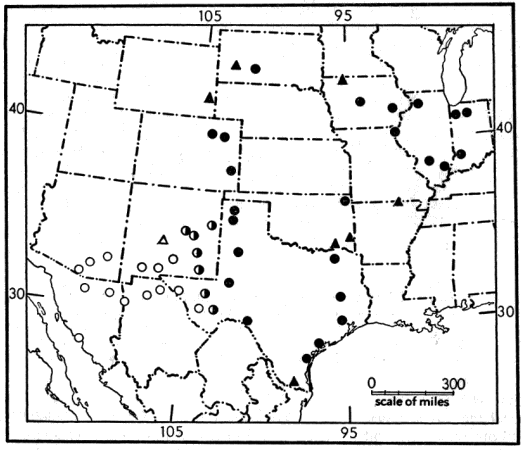
Terrapene ornata is a characteristic inhabitant of the western prairies of the United States, and ranges from western and southern Illinois, Missouri, Oklahoma, and all but the extreme eastern part of Texas, westward to southeastern Wyoming, eastern Colorado, eastern and southern New Mexico, and southern Arizona, and, from southern South Dakota and southern Wisconsin, southward to northern Mexico (Fig. 1). It is the only species of the genus that occurs in both Mexico and the United States. The northeasternmost populations of T. ornata, occurring in small areas of prairie in Indiana and Illinois, seem to be isolated from the main range of the species. The ranges of T. ornata and T. carolina overlap in the broad belt of prairie-forest ecotone in the central United States. Interspecific matings under laboratory conditions [Pg 533] are not uncommon and several verbal reports of such matings under natural conditions have reached me. Nevertheless, after examining many specimens of both species and all alleged "hybrids" recorded in the literature, I find no convincing evidence that hybridization occurs under natural conditions.
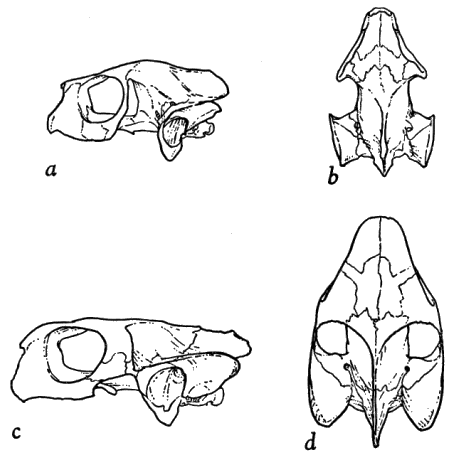
Terrapene ornata differs from T. carolina in having a low, flattened carapace lacking a middorsal keel (carapace highly arched and distinctly keeled in carolina), and in having four claws on the hind foot (three or four in carolina), the claw of the first toe of males being widened, thickened, and turned in (first toe not thus modified in carolina). Terrapene ornata is here considered to be the most specialized member of the genus by virtue of its reduced phalangeal formula, lightened, relatively loosely articulated shell, reduced plastron, and lightly built skull, which completely lacks quadratojugal bones (Fig. 2); most of these specializations seem to be associated with adaptation for terrestrial existence in open habitats.
Two subspecies of T. ornata an recognized. Terrapene o. luteola, Smith and Ramsey (1952), ranges from northern Sonora (Guaymas) and southern Arizona (southern Pima County) eastward to southeastern New Mexico and Trans-Pecos, Texas, where it intergrades with T. o. ornata; the latter subspecies is not yet known from Mexico but almost surely occurs in the northeastern part [Pg 534] of that country. The subspecies luteola differs from ornata in being slightly larger and in having more pale radiations on the shell (11 to 14 radiations on the second lateral lamina in luteola, five to eight in ornata). In individuals of luteola the markings of the shell become less distinct with advancing age and eventually are lost; shells of most old individuals are uniform straw color or pale greenish-brown; this change in coloration does not occur in T. o. ornata.
Of the several species of fossil Terrapene described (Hay, 1908b:359-367, Auffenberg, 1958), most are clearly allied to Recent T. carolina. One species, Terrapene longinsulae Hay, (1908a:166-168, Pl. 26) from "… the Upper Miocene or Lower Pliocene…." of Phillips County, Kansas, however, is closely related to T. ornata (if not identical). I have examined the type specimen of T. longinsulae. Stock and Bode (1936:234, Pl. 8) reported T. ornata from sub-Recent deposits near Clovis, Curry County, New Mexico.
Ornate box turtles, referred to as "land terrapins" or "land tortoises" over most of the range of the species, are regarded by most persons whom I have queried as innocuous. These turtles occasionally damage garden crops and [Pg 535] have been known to eat the eggs of upland game birds. Terrapene ornata is seldom used for food. A. B. Leonard told me the species was eaten occasionally by Arapaho Indians in Dewey County, Oklahoma. Several specimens in the University of Kansas Archeological Collections were found in Indian middens in Rice County, Kansas, from a culture dated approximately 1500 to 1600 A. D. The flesh of T. ornata occasionally may be toxic if the turtle has eaten toxic fungi as has been recorded for T. carolina (Carr, 1952:147).
Preliminary studies and collections of specimens were made at a number of localities in northeastern Kansas in 1953 and 1954. Two small areas were finally selected for more intensive study. One of these areas, the University of Kansas Natural History Reservation, five and one-half miles north-northeast of Lawrence in the northeasternmost section of Douglas County, Kansas, is a tract of 590 acres maintained as a natural area for biological investigations. Slightly less than two thirds (338 acres) of the Reservation is wooded; the remainder consists of open areas having vegetation ranging from undisturbed prairie grassland to weedy, partly brushy fields (Fitch, 1952). Although ornate box turtles were not numerous at the Reservation, the area was selected for study because: 1) there was a minimum of interference there from man and none from domestic animals; 2) the vegetation of the Reservation is typical of areas where T. ornata and T. carolina occur sympatrically (actually only one specimen of T. carolina has been seen at the Reservation); and, 3) availability of biological and climatological data there greatly facilitated the present study. Actual field work at the Reservation consisted of studies of hibernation and long-term observations on movements of a few box turtles.
A much larger number of individuals was intensively studied on a tract of land, owned by Sophia Damm, situated 12 miles west and one and one-half miles north of Lawrence in the northwestern quarter of Douglas County, Kansas. The Damm Farm lies on the southern slope of a prominence—extending northwestward from Lawrence to Topeka—that separates the Kansas River Valley from the watershed of the Wakarusa River to the south. The prominence has an elevation of approximately 1100 feet and is dissected on both sides by small valleys draining into the two larger river valleys.
The Damm Farm (see Pl. 15) has a total area of approximately 220 acres. The crest of a hill extends diagonally from the middle of the northern edge approximately two thirds of the distance to the southwestern corner. Another hill is in the extreme northwestern corner of the study area.
The northeastern 22 acres were wooded and had small patches of overgrazed pasture. Trees in the wooded area were Black Walnut (Juglans nigra), Elms (Ulmus americana, U. rubra), Cottonwood (Populus deltoides), and Northern Prickly Ash (Xanthoxylum americanum). The areas used as pasture had thick growths of Buckbush (Symphoricarpos orbiculatus) mixed with short grasses (Bromus japonicus, Muhlenbergia Schreberi, and Poa pratensis). Farm buildings were situated in the wooded area at the end of an entry road. The southeastern 74 acres were cultivated; corn, wheat, and milo were grown here and fallow fields had a sparse growth of weeds.
Most of the western two thirds of the study area, comprising 124 acres, was open rolling prairie (hereafter referred to as "pasture") upon which beef-cattle were grazed (Pl. 16, Fig. 1; Pl. 17, Fig. 1; Pl. 18, Fig. 2). Rock [Pg 536] fences (Pl. 17, Fig. 2) two to four feet high bordered the northern edge, southern edge, and one half of western edge of the pasture. A wagon track lead from a gate on the entry road, along the crest of the hill, to a gate in the southern fence. Except for the latter gate and for ocassional under-cut places in low areas, there were no openings in the rock fences through which box turtles could pass. A few trees—American Elm, Hackberry (Celtis occidentalis), Red Mulberry (Morus rubra), Osage Orange (Maclura pomifera), Black Cherry (Prunus serotina), Box-Elder (Acer Negundo), and Dogwood (Cornus Drummondi)—were scattered along fences at the borders of the pasture and in ravines. Larger trees in a small wooded creek-bed at the southwestern edge of the pasture were chiefly Cottonwood, American Elm, Red Mulberry, and Black Willow (Salix nigra). The only trees growing on the pasture itself were a few small Osage Orange, none of which bore fruit.
Paths were worn along fences by cattle and in several places near the fence, usually beneath shade trees, there were large bare places where cattle congregated. Vegetation near paths and bare places was weedy and in some places there were tall stands of Smooth Sumac (Rhus glabra).
Rich stands of prairie grasses occurred along the top of the hill in the pasture; bluestems (Andropogon gerardi, A. scoparius) were the dominant species and Switchgrass (Panicum virgatum) and Indian grass (Sorghastrum nutans) were scattered throughout. A number of small areas on top of the hill were moderately overgrazed, as indicated by mixture of native grasses with an association of shorter plants consisting chiefly of Ragweed (Ambrosia artemisiifolia var. elatior), Mugwort (Artemisia ludoviciana), Japanese Chess (Bromus japonicus), and Asters (Aster sp.).
The upper parts of the hillsides were overgrazed moderately to heavily. Limestone rocks of various sizes were partly embedded in soil or lay loose at the surface. Depressions beneath rocks provided shelter for box turtles as well as for other small vertebrates. Native grasses were sparse in this area and gave way to Sideoats Grama (Bouteloua curtipendula), extensive patches of Smooth Sumac, and scattered colonies of Buckbrush.
Tall grasses were dominant on the lower hillsides and small patches of Slough grass (Spartina pectinata) grew in moist areas. Ravines originated at small intermittent springs on the sides of the hill. The banks of ravines were high and steep and more or less bare of vegetation. High, dense stands of Slough grass grew at intermittent springs and along the courses of ravines; sedges (Carex, sp.) grew where small pools of water formed and created marshy conditions. Prairie grasses along the tops of ravine embankments formed a narrow overhanging canopy of vegetation that was accentuated in many places where the sod was under-cut by erosion or by the activities of burrowing animals (Pl. 18, Fig. 1). Box turtles frequently sought shelter beneath this vegetational canopy or burrowed beneath the sod.
On the highest part of the pasture near the entry road several small areas were nearly bare, presumably because of heavy overgrazing; grasses (except for scattered clumps of Bouteloua curtipendula and Setaria lutescens) were absent and dominant vegetation consisted of Buffalo-bur (Solanum rostratum), Blue Vervain (Verbena hastata), Mullein (Verbascum Thapsus), Ragweed, Asters, and a few Prickly Pear (Opuntia humifusa). Two small areas on the pasture completely lacked vegetation; these may have been wallows or the sites of old salt-licks.
Three shallow stock ponds, behind earthen dikes in ravines, were present on the pasture. The pond near the farm buildings ("House Pond") and that in the southwestern part of the pasture ("Far Pond") were present when studies of box turtles were begun. The largest pond, in a deep ravine in the northern part of the pasture, was constructed in June, 1956, and became filled in approximately one month (Pls. 16 and 18). Pond embankments were chiefly bare of vegetation because of trampling by cattle; in a few places at the edge of the water, or in places too steep for cattle to walk, there were small patches of weeds, sedges, and Slough Grass. The ponds contained some water at all times of the year. The only vertebrates permanently inhabiting the ponds in the course of my studies were Bullfrogs (Rana catesbeiana) and Leopard frogs (Rana pipiens).
The three parts of the pasture in which studies were concentrated were designated as separate subdivisions. The northwest corner area (28 acres) was triangular and bounded on two sides by rock fences and on its third side by a deep ravine. The southern ravine area (17 acres) constituted the part of the lower southern hillside drained by a series of ravines. The house pond area (seven acres) surrounded "House Pond." Habitat in these three subdivisions of the pasture was especially favorable for box turtles.
Observations were made at the Damm Farm on 102 days in the two-year period beginning in Autumn, 1954; observations were concentrated in the period from May to October although some observations were made in every month, January and February excepted. Field work was done chiefly in daylight hours but a few trips were made to the study area at night.
Routine handling of each turtle captured at the Damm Farm consisted of: marking, weighing and measuring turtle; recording the exact place of capture, body temperature and environmental temperature; and, recording miscellaneous items such as the presence of ectoparasites, injuries, distinctive markings, and in some instances, the approximate age of the turtle.
Excursions on the Damm Farm were made on foot in 1954 and 1955, and, in 1956, on horseback. By using a horse, more ground could be covered per unit of time, a better view could be obtained of immediate surroundings, and, cattle on the area, being accustomed to horses, did not become agitated as they would when unmounted persons were nearby.
The entire study area could not be inspected thoroughly in a single day. It was usually more profitable to find and mark turtles along fences, in ravines, or in other open areas, and subsequently to follow their movements away from these areas by means of trailing threads. Turtles could be observed from a distance through binoculars. Cultivated areas were regularly scanned with binoculars but turtles were seldom seen there. Behavior was observed by sitting motionless on rock fences or in a blind on top of a stepladder.
No box turtles were removed from the study area. Specimens obtained in other areas were used for studies of growth, reproduction, and food habits. Measurements, weights, and data concerning temperature and ectoparasites were obtained from specimens collected elsewhere as well as from individuals on study areas.
Turtles were obtained by hand-collecting and in unbaited traps; the number captured in a single day ranged from 12 to none. Traps, like those used by [Pg 538] Packard (1956:9) for tree squirrels, were set in the mouths of burrows and dens, or—with leads to channel animals into the trap—along ravines and rock fences. Traps set in the open were covered to prevent death of turtles from overheating in direct sunlight. Live-trapping provided much valuable data, although quail, rabbits, opossums, and box turtles were caught with about equal frequency in the traps.
Turtles were marked by notching the marginal scutes of the carapace by means of a hacksaw blade, following the code system described by Cagle (1939). Notches, one eighth to one quarter of an inch deep and wide could be cut more quickly than filed and were more evident than drilled holes which often became plugged with soil and obscured. Hatchlings and juveniles were notched with a sharp knife.
Movements of individual turtles were studied by means of a turtle-trailing device—similar to the kind first described by Breder (1927) and later modified by Stickel (1950:355-356)—a tin can, cut to fit the shell of a turtle, with an axle that bore a spool of thread (Pl. 27, Fig. 1). The device was taped to the turtle; the free end of the thread was tied to a stationary object. Thread payed out from the spool through a guide-loop and marked the course of the turtle as it moved away from the starting point. Because of its great strength and elasticity (as compared to cotton), nylon sewing thread was used in trailers. Ordinarily, turtles were unable to break the thread if it became snarled or was expended. Cattle frequently tangled the thread and displaced it but did not often break it. Ordinary spools were cut down on a lathe so they would hold 600 to 800 yards of thread. Turtle-trailing provided an accurate record of where and how far a turtle had traveled, and to a lesser extent, the sort of activity in which the turtle had been engaged (evidence of feeding, forms, or trial nest holes). Trailers seemed not to alter the normal activity of turtles.
Prominent landmarks were rare or wanting in most places on the pasture. Locations of captures (or reference points in the movements of trailer-turtles) were determined by triangulation with a Brunton compass, using trees along fences as known points of reference. Rough maps were made in the field and used later, along with compass readings and measurements, to make a more precise record of movements and captures on a large map (scale, 100 feet to one inch) of the study area. Mapped points of capture in grassy areas were accurate within ten to twenty feet; points of capture in areas where landmarks were nearby were nearly exact. Areas were measured with a planimeter; distances traveled by individuals were measured with a cartometer.
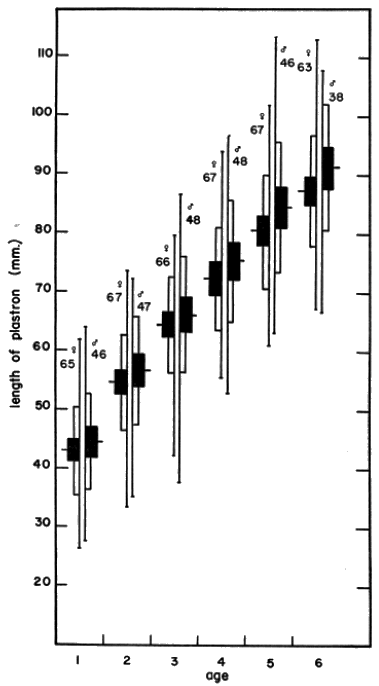
Turtles were measured in the field to the nearest millimeter with large wooden calipers (of the type used by shoe salesmen) and a clear plastic ruler. Measurements in the laboratory, especially in studies of growth, were made, to the nearest tenth of a millimeter with dial calipers. Measurements made on each specimen examined in the field were: length of carapace, width of carapace, length of plastron (sum of lengths of forelobe and hind lobe), width of plastron (at hinge), and height. All measurements were made in a straight line. A spring scale of 500 gram capacity, used in the field, gave weights accurately within three grams. A triple-beam balance was used in the laboratory. Unless otherwise noted, measurements are expressed in millimeters and weights are expressed in grams.
Body temperatures were taken by means of a quick-reading Schultheis thermometer inserted into the distal portion of the large intestine with the [Pg 539] bulb directed ventrally to avoid puncturing the bladder. Body temperature of turtles were altered little or not at all in the few seconds the turtles were held and no attempt was made (except for small juveniles) to insulate them from the warmth of my hands. Data recorded with body temperature were: air temperature (in shade, approximately one inch from turtle); ground temperature (or water temperature); behavior of turtle; weather conditions; nature of vegetation or other cover; and, time of day. Unless otherwise noted, temperatures are expressed in degrees Centigrade.
A maximum-minimum thermometer was installed near the buildings at the Damm Farm. Notes on general weather conditions were made on each visit to the study area. Additional climatological data were obtained from the U. S. Weather Stations in Topeka and Lawrence, from records at the Reservation, and from official bulletins of the U. S. Weather Bureau.
Stomachs and gonads were removed and preserved by standard techniques soon after specimens were killed. The dates given to gonads were, in all instances, the dates when the specimens were killed. Eggs were prepared for incubation in the manner described by Legler (1956). Females laying or containing eggs used in studies of incubation were preserved for further studies and comparison with young hatched from the eggs. Histological preparations were fixed in ten per cent formalin or Bouin's fluid, embedded in paraffin, and stained with hematoxalin and eosin.
Names used for the epidermal and bony parts of the shell follow the classification proposed by Carr (1952:35-39). The terms "scute," "lamina," and "scale" are used here more or less interchangeably for the epidermal parts as are the terms "plate," "bone," and "element" for the bony parts of the shell.
The term "form" is used here in the same sense that Stickel (1950:358) used it in her study of T. carolina—to indicate a depression or cavity made by a turtle in vegetation or soil. Forms correspond closely in shape and size to shape and size of the turtle. Forms of T. ornata differ from those of T. carolina chiefly in being made most often in soil, over which there is a minimum of vegetational cover. The term "den" refers to natural cavities (or cavities of unknown origin) beneath rocks, in rock fences, or in cut banks. The term "burrow," unless otherwise noted, refers to burrows made by animals other than box turtles.
The known range of T. ornata includes the southern half of the Grassland Biome, part of the Desert Biome, and that part of the Temperate Deciduous Forest Biome known as the Prairie-Forest Ecotone. The species is found in microhabitats that differ widely in food supply, temperature, moisture, and kind of soil. In spite of its relatively high degree of morphological specialization, T. ornata is remarkably versatile in regard to habitat requirements.
Ornate box turtles are relatively inconspicuous in natural surroundings and collectors seldom seek out and obtain specimens under completely natural conditions as may be done with certain [Pg 540] other reptiles and amphibians by turning rocks, tearing apart logs, or setting traps. Most series of specimens are obtained by hunting after rains on roads or other natural breaks in vegetational cover. Detailed information on habitat preferences is lacking.
Low temperature seems to be an important factor limiting the distribution of T. ornata in the northern part of its range. Box turtles, like nearly all other reptiles occurring at these latitudes, spend the winter in underground hibernacula. The depth to which the ground freezes in the coldest part of the winter is therefore a critical factor. The ground freezes to an average depth of 30 inches or less over most of the range of the species; only in the extreme northern part of the range (southern South Dakota, southeastern Wyoming) does the ground freeze to an average depth of as much as 35 inches. Average depth of freezing is, in fact, less than 15 inches over more than one half the range of the species. The average number of frost-free days per year ranges from 130 to 140 days in the northern part of the range to more than 250 days in the southwestern part of the range.
Terrapene ornata occurs from near sea level to elevations of more than 5000 feet. Both subspecies are found at both high and low elevations but luteola is more consistently taken at high elevations than ornata. The latter subspecies commonly occurs at elevations above 4000 feet on the high plains in extreme western Kansas and eastern Colorado; the highest elevation from which I have examined specimens of T. o. ornata is between 4600 and 4700 feet near Akron, Washington County, Colorado. The greater part of the known range of T. o. luteola lies above 3000 feet.
Norris and Zweifel (1950:1) observed T. o. luteola on the Jornada del Muerto, an elongate plain approximately 4500 feet high, in southeastern Socorro County, New Mexico; box turtles were abundant on the level part of the plain and on the bordering foothills but not at higher elevations where the substratum was rocky. The authors otherwise noted no preference for any kind of soil. The principal elements of the plant associations in which the turtles were found were creosote bush, yucca, mesquite, juniper, tarbush, and grasses. Lewis (1950:3) reported that T. ornata luteola inhabited the yucca-grassland zone in Dona Ana County, New Mexico; he stated (op. cit.: 10) that individuals were commonly found on roads after rains and in cloudy weather. No specimens were taken at altitudes higher than 4300 feet.
I have examined specimens of luteola from elevations of approximately 5500 feet in Cochise County, Arizona, and Lincoln County, [Pg 541] New Mexico. These localities are probably at or near the maximum elevation at which the species occurs. The texture of the substrate is the most important factor limiting vertical distribution. Ornate box turtles, like nearly all other turtles, excavate nests; T. ornata is a burrower, at least for purposes of hibernation. Populations of the species, therefore, could not survive in areas of hard unyielding substrata. Such substrata seem to be the most important factor limiting altitudinal distribution.
Most of the area in which T. ornata occurs is semiarid or arid. Average precipitation in the warm season (April through September) varies from approximately 25 inches in the northeast to less than ten inches in the southwest. In drier parts of the range, precipitation is unevenly distributed over the warm season. Long, hot, dry periods are unfavorable for reptilian activity. T. ornata, like many other reptiles inhabiting dry regions, survives long periods without water by seeking shelter (usually underground) and remaining quiescent. Populations of the subspecies luteola live under far more rigorous conditions in this respect than do the more northern populations. Specimens of luteola from Arizona that were kept for several years in the laboratory under dry conditions and fed adequately, but at infrequent intervals, were able to remain healthy and even to grow whereas examples of ornata kept under the same conditions soon languished and died; luteola seems to be physiologically adapted for existence under arid conditions, where normal activity is sometimes possible for only a few weeks in the year.
The prairies of Nebraska, Kansas, Oklahoma, and northern Texas seem to provide the most nearly optimum habitat for the species; in these regions box turtles are active on a large majority of the days from April to October in years having average or better than average precipitation and population density seems to be greater than in the more arid parts of the range.
Activities of man have probably affected the density of populations of the ornate box turtle in many parts of its range but appear not to have acted as limiting factors except in certain areas along the northern edge of the range (Blanchard, 1923:19-20, 24) where disruption of grassland through intensive cultivation probably has excluded the species. Unlike certain other reptiles of the Great Plains (Fitch, 1955:64), T. ornata seems not to have been affected—either by direct decimation of populations or by disruption of habitat—by intensive zoological collecting in restricted areas. Environmental changes such as those resulting from overgrazing and [Pg 542] erosion, or from protection of the habitat from grazing could be expected to cause long-term changes in populations of ornate box turtles.
Terrapene o. ornata is an omnivorous, opportunistic feeder, primarily insectivorous but able to subsist on nearly any sort of animal or vegetable food. The general food habits of luteola are poorly known but probably resemble those of ornata. Although kind of food available probably does not limit the distribution of T. ornata there are indications that it influences population density. In Kansas, for example, dung insects are an important staple in the diet and box turtles were found always to be more numerous in areas where domestic cattle provided an abundant supply of dung than elsewhere. A similar relationship probably existed in former times between box turtles and native ungulates. Near extinction of buffalo in the Great Plains possibly caused a decrease in populations of box turtles. Henry S. Fitch told me that the number of T. ornata at the Reservation gradually declined after cattle were removed from the area in 1948.
In summary, the distribution of T. ornata seems to be limited by: 1) Presence of a substrate too hard to permit digging of nests and forms (southwestern and western edges of range); 2) temperatures causing the ground to freeze deep enough (approximately 30 inches) to kill turtles in hibernacula (northern edge of range); and, 3) the lack of one or more relatively wet periods in the course of the warm season, preventing at least temporary emergence from quiescence (southwestern edge of range).
Clarke (1958:40-45) reported T. o. ornata in all terrestrial communities studied in Osage County; he considered the subspecies to be characteristic of the "… cultivated-field community …" and to be of frequent occurrence in (but not characteristic of) the "… Oak-Walnut Hillside Forest …, Buckbrush-Sumac …, and Prairie communities …". Brennan (1937:345) found T. o. ornata to be equally abundant in mixed prairie and prairie-streamside habitats in Ellis County; the subspecies was much rarer on rocky hillsides and in the habitat surrounding prairie ponds. Carpenter (1940:641) listed T. o. ornata as an inhabitant of "… tall and mixed-grass prairies …" (also in Oklahoma and Nebraska). Fitch (1958:99) found the order of preference for habitats at the Natural History Reservation to be grazed pasture land, woodland, open fields with undisturbed [Pg 543] prairie vegetation, and fallow fields with a rank growth of weeds.
At the Damm Farm the greatest number of box turtles was collected on the pasture, especially in three areas designated in Plate 15 as the "northwest corner," "southern ravine," and "house pond" areas. These three areas had several features in common. All contained ravines and rocky slopes that provided many places of concealment (dens, burrows of larger animals, and suitable substrate for the excavation of earthen forms). All contained water (in ponds and intermittent streams) for most of the year; and, all were frequented daily by cattle that left an abundant supply of dung in which box turtles foraged. In addition, each of the three areas contained at least one mulberry tree, under which fruit was abundant in the months of June and July.
The relative numbers of box turtles found in different areas on the Damm Farm were, of course, governed to some extent by my activity in these areas and by the relative ease with which box turtles were seen in different types of vegetational cover. Turtles were more easily seen in the pasture (especially in sparsely vegetated or denuded areas) where much of my field work was done on horseback, than in the wooded areas, where excursions were usually made on foot. It was evident, however, after mapping known ranges and studying patterns of movement in marked turtles, that concentrations in the three above-mentioned areas of pasture were an indication of actual preference by turtles for the more favorable habitat in these areas rather than the result of incomplete sampling.
Mating takes place throughout the season of activity but is most common in spring—soon after emergence from hibernation—and in autumn. Turtles frequently copulated in the laboratory in spring and autumn. Copulation was observed under natural conditions on several occasions but only once at the Damm Farm.
Norris and Zwiefel (1950:4) saw two captive individuals of T. o. luteola copulating on 12 August; copulation lasted two hours. Brumwell (1940:391-2) gave the following description of mating in T. o. ornata. A male pursued a female for nearly half an hour, first nudging the margins of her shell and later approaching her rapidly from the rear and hurling himself on her back in an attempt to mount, at the same time emitting a stream of liquid from each nostril. The liquid was presumably water; both sexes had imbibed [Pg 544] water in a pond just before courtship began. Brumwell suggested that pressure on the plastron of the male had forced the water out his nostrils. The pair remained in the coital position for 30 minutes after the male had achieved intromission. In another instance, Brumwell (loc. cit.) saw four males pursuing a single female, the males exhibiting the same behavior (nudging and lunging) outlined above. Males that attempted to mount other males were repelled by defensive snapping of the approached male. The female also snapped at some of the males that tried to mount her. One male was finally successful in mounting and was henceforth unmolested by the other males. Brumwell suggested that shell biting and tapping may be methods of sex-recognition.
In the several instances of mating that I observed, the male, after mounting the shell of the female (Pl. 28), gripped her, with the first claws of his hind feet, just beneath her legs or on the skin of the gluteal region and, with the remaining three claws, gripped the posterior edges of her plastron. In most instances the female secured the male's legs by hooking her own legs around them. The coital position of T. ornata seems to differ from that of T. carolina, at least in regard to the position of the male's legs. The coital positions of T. carolina illustrated by Cahn (1937:94, Fig. 13) are physically impossible for T. ornata.
In T. ornata the pressure exerted on the male's legs by the female probably impairs circulation and probably is painful to the male, especially after coitus, when the male falls backward but is still held by the female. The heavily developed musculature of the legs of males may be an adaptation to strengthen the legs for this temporary period of stress. Evans (1953:191) and Cahn and Conder (1932:87-88) observed the hind legs of males of T. carolina to be noticeably weakened after copulation, causing the males to remain inactive for several hours.
Evans (op. cit.) observed 72 matings of T. carolina and divided the process into three phases as follows: 1) circling, pushing and biting by the male; 2) mounting (female with shell closed); and, 3) coition (female with shell open). Penn and Pottharst (1940:26) reported that captive T. carolina in New Orleans mated chiefly under conditions of optimum temperature (21 to 27° C.) and high humidity; some matings took place in a pool of water. Males pushed females about after mating, often rolling them over several times.
Because ornate box turtles observed by me were able easily to right themselves from an inverted position on substrata of all [Pg 545] kinds, males left lying on their backs after copulation are probably in no danger of perishing in this position, as was suggested by Allard (1939) for T. carolina.
Oviducts of several females were flushed by means of a pipette to determine whether they contained sperm. Approximately half of the females captured in May, 1956, had sperm in their oviducts, but females captured in June and July did not. Sperm flushed from the oviducts were in clumps of several hundred and showed no sign of motility a few minutes after the female was anesthetized with chloroform. No sperm were found in the oviducts of immature females but one female of nearly adult size was observed in copulation with a mature male.
Thorough examination of microscopic sections of oviduct (taken at various times in the season of activity) usually revealed a few sperm lodged in the folds (Pl. 19, Fig. 8) of the cephalic as well as the caudal portion of the tube, but no specialized seminal receptacles such as occur in snakes (Fox, 1956) were present. Fertilization without reinsemination probably occurs in T. ornata. Ewing (1943) and Finneran (1948:126) reported that females of T. carolina produced fertile eggs for periods of four and two years, respectively, after being removed from all contact with males.
Testes were preserved in each month from April to October. The following description of spermatogenesis is based chiefly on material collected in 1955, although testes were preserved also in 1954. Comparison of material obtained in 1954 and 1955 revealed that spermatogenesis began earlier and was more advanced on any given date in 1955 than in 1954.
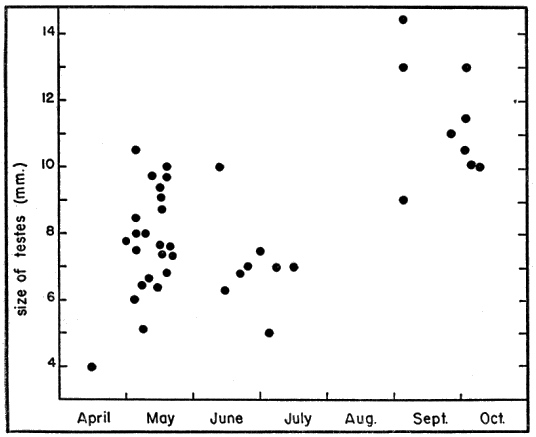
Testes of mature individuals are pale yellow and slightly oblong. The epididymis is ordinarily dark brown or black and contrasts sharply with the color of the testes. Size of testes was expressed as the average length (greatest diameter) of both testes. Testes are smallest in April, immediately after emergence from hibernation, and largest in early September (Pl. 20, Figs. 3-4). They are nearly spherical when of maximum size; increase in bulk, therefore, is relatively greater than the increase in size shown in Figure 3. They increase in size from April until early June, recede during most of June, and again increase in size in July and August. They remain [Pg 546] large from early September until hibernation is begun, becoming only slightly smaller in late September and October.
Increase in size following emergence from hibernation may be due in part to proliferation of the sustentacular cytoplasm. Decrease in size in early June is correlated with the end of the period of most active mating; maximal size is coincident with the peak of the spermatogenic cycle in early September.
Spermatogenesis (refer to Pl. 19, Figs. 1-5) begins in early May when a few spermatogonia appear in the seminiferous tubules. The histological appearance of testes preserved in April and May is much the same. Nuclei of Sertoli cells, which outnumber the spermatogonia, are evident at the periphery of the tubules and the clear cytoplasm of the cells extends into and nearly fills the lumina. The few darkly stained spermatids that are present in April are cells that probably were produced in the previous summer. Sperm are present in small groups within the sustentacular cytoplasm, but ordinarily are absent in the lumina.
Primary spermatocytes appear in the tubules from mid-May to early June. By mid-May there are practically no sperm at any place in the tubules. The sustentacular cytoplasm has a less compact arrangement in late May than in April.
Spermatogenesis is well under way by mid-June; at this time, two or three distinct layers of primary and secondary spermatocytes are present and these cells outnumber the Sertoli cells. The lumina are filled with cellular detritus and are no longer bordered by a clear ring of sustentacular cytoplasm. No sperm are present.
Spermatids appear in late June and a few of them undergo metamorphosis in early July; by mid-July, spermatids and secondary spermatocytes are the dominant cells in the seminiferous tubules, although spermatogonia are still active.
By late August, clusters of sperm and metamorphosing spermatids surround the Sertoli cells; large numbers of sperm as well as sloughed cells representing various spermatogenic stages are present in the lumina. Secondary spermatocytes are still evident near the periphery of the tubules but they are much less numerous than spermatids. The germinal epithelium is still semiactive and small groups of primary spermatocytes are present in nearly all of the tubules.
The spermatogenic cycle is completed in the latter half of October when most of the spermatozoa pass into the epididymides. A few spermatozoa and spermatids remain in the seminiferous tubules during hibernation. Although no testicular material was obtained from hibernating turtles, comparisons of sections made in October and April show that the germinal epithelium remains inactive from autumn until spring. Possibly some spermiogenesis takes place in the early phases of hibernation or in the period in late autumn when turtles are intermittently active. It is uncertain whether the reorganization of the sustentacular cytoplasm occurs in autumn, in spring, or in the course of hibernation.
The seminiferous tubules of immature males are small, lack lumina, and contain a few large but inactive spermatogonia (Pl. 19, Fig. 6). The testes of specimens that were nearly mature contained primary and secondary spermatocytes but lacked lumina; it was thought that such individuals would have matured in the following summer and bred in the following autumn.
Mature sperm were found in epididymides at all times of the year but were most numerous in spring and autumn, the period between [Pg 548] spermatogenic cycles (Pl. 19, Fig. 7). Sperm expelled from the epididymides in autumn matings are seemingly replaced by others from the seminiferous tubules; the epididymides become much smaller when their supply of sperm is nearly exhausted after spring mating.
Risley (1938:304) found the testes of the common musk turtle, Sternotherus odoratus, to be largest in August and smallest in early May. Recession of testes in spring was coincident with the period of active breeding; increase in size, later in the season, corresponded to increasing spermatogenic activity and enlargement of seminiferous tubules. Altland (1951:600-603) found the spermatogenic cycle of Terrapene carolina to be nearly like that of Sternotherus odoratus. Fox (1952) found that testes of garter snakes (Thamnophis sirtalis and T. elegans) in California reached a peak of spermatogenic activity in midsummer, regressed in the latter half of the summer, and were inactive in winter.
The spermatogenic cycle of T. ornata as here reported, differs in no important respect from those of Thamnophis, Sternotherus odoratus, or Terrapene carolina, except that in T. ornata the cycle begins and ends somewhat later in the season of activity. In most of the lizards that have been studied (Fox, 1952:492-3), spermatogenesis reaches a peak in spring (more or less coincident with the mating period and with ovulation) and the germinal epithelium remains active in winter. Sternotherus, Terrapene, and Thamnophis are alike in completing spermatogenesis late in the season and storing spermatozoa, in the seminiferous tubules or in the epididymides, during hibernation.
It is noteworthy that, in the turtles and snakes mentioned above, sperm produced in autumn are used to fertilize eggs laid in the following year, and mating [with the exception of Thamnophis elegans, (Fox, 1956)] occurs in both spring and autumn. It is not definitely known in any of these instances, whether sperm resulting from autumn or spring inseminations (or both) fertilize the eggs. Risley (1933:693) found motile sperm in the oviducts of female Sternotherus odoratus that had recently emerged from hibernation; he believed that spring mating, although it commonly occurred, was not necessary to fertilize eggs. Disadvantages, if any, of completing spermatogenesis well in advance of ovulation seem to be at least partly counteracted by two annual mating periods or by mating throughout the season of activity.
The following account of oögenesis is based on examination of preserved ovaries from 68 mature specimens. The ages of most specimens were known, inasmuch as the specimens were used in studies of growth as well as gametogenesis. Other data were obtained from adult females that were dissected but not preserved, and from immature females.
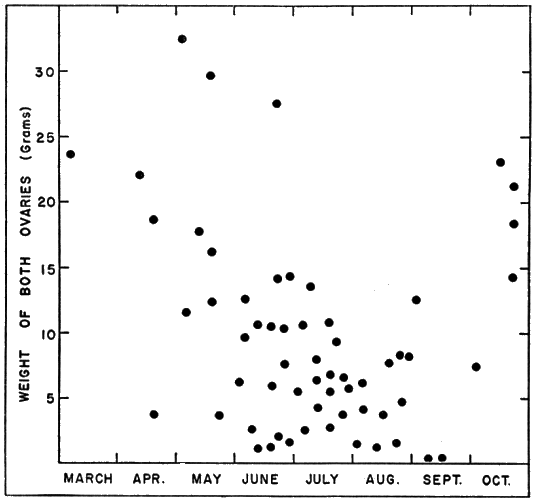
Size of ovarian follicles was determined by means of a clear plastic gauge containing notches 5, 10, 15, 20, and 25 millimeters wide. The number of follicles within a given size range could be quickly determined by finding the smallest notch into which the follicles fit. It was necessary to weigh all ovaries after preservation since some of them had not been weighed when fresh. Since all ovarian samples were preserved in the same manner, weights [Pg 550] remained relatively the same. Preserved material was lighter than fresh by an average of 13 per cent. Follicles less than one millimeter in diameter were not counted. Corpora lutea and corpora albicantia were studied under a binocular dissecting microscope. No histological studies were made of the female reproductive system.
Ovarian follicles and oviducal eggs were recorded separately for the right and left sides. Each ovary was always kept associated with the oviduct of the same side, but in some instances it was not recorded whether the organs were left or right.
Ovaries ordinarily weighed most in October, March, and April, when most females contained enlarged follicles, and least in August and September when the supply of enlarged follicles was usually exhausted (Figs. 4 and 5).
The ovarian cycle begins in July or August, after ovulation has occurred. At that time many minute follicles form on the germinal ridges of the ovaries. On the basis of the material that I examined, it seems that ovarian follicles either grow to nearly mature size in the season preceding ovulation and remain quiescent over winter or grow rapidly in the period of approximately six weeks between spring emergence and ovulation. Altland (1951:603-5) reported [Pg 551] that the former condition was the usual one in T. carolina; he suggested that possibly some of the enlarged follicles were absorbed during hibernation.
Examination of yolks of oviducal eggs revealed that follicles mature when they reach a diameter of 16 to 20 millimeters and a weight of two to two and one-half grams (Pl. 20, Fig. 1).
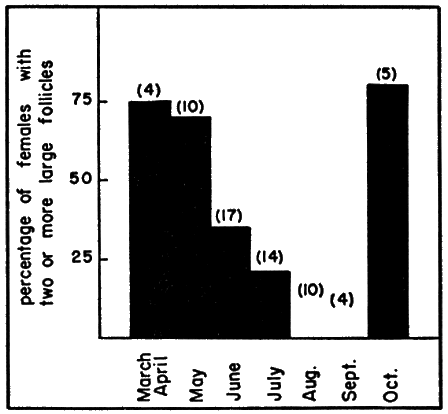
The enlarged follicles remaining on the ovaries after ovulation (excluding those smaller than six mm.) can be grouped according to diameter as: large (greater than 15 mm.), medium (11 to 15 mm.), and small (six to 10 mm.). Ten females collected in the period from June 2 to 8, after they had ovulated, all had follicles falling in at least one of these size groups, and eight had follicles falling in two or more of the groups. In females having enlarged follicles of more than one of the size groups, there were several follicles in each of two groups and no follicles, or only one follicle, in the remaining group. Enlarged follicles represent future clutches but whether the enlarged follicles will be ovulated in the same season or in a later season is questionable.
Evidence found in the present study suggested that at least a few females lay more than one clutch of eggs per year. Among 34 specimens obtained in June and July, eight (24 per cent) had corpora lutea (or easily discernible corpora albicantia) and at least two follicles more than 15 millimeters in diameter; in three specimens (9 per cent) the ovaries bore fresh corpora lutea (representing recent ovulations) and a set of older corpora lutea (representing ovulations that had occurred several weeks previously). It was thought that each of these eleven females (33 per cent of sample) had produced or would have produced two clutches of eggs in the season of its capture. The number of large follicles present after the first set of ovulations (mean, 3.5) was fewer in most instances than the average clutch-size (see below), indicating that second clutches are smaller than first clutches. Smaller second clutches were found also in T. carolina (Legler, 1958).
Further evidence for multiple clutches was the absence of enlarged ovarian follicles in some females obtained in September. Atretic follicles, ordinarily orange, brown, or purplish, were observed on the ovaries of many of the females examined; in most instances, not more than two follicles of the small or medium size groups were atretic. Atresia was in no instance great enough to account for the complete loss of enlarged follicles.
Further study probably will show that many of the females laying [Pg 552] in May and early June lay again before the end of July, and that eggs in the oviducts of females captured in the latter month frequently represent second clutches. Under favorable conditions, eggs laid by the end of July would have a good chance of hatching before the advent of cold weather in autumn; turtles hatching too late to escape from the nest could burrow into its sides and probably escape freezing temperatures.
Cagle's findings concerning Pseudemys scripta (1950:38) and Chrysemys picta (1954:228-9) suggest that these species lay more than one clutch per season, at least in the southern parts of their ranges. Carr (1952) indicated that multiple layings were known in most species of marine turtles (families Dermochelydae and Chelonidae) and strongly suspected in other species. Other turtles recorded to have produced multiple clutches in a single season (based chiefly on captive specimens or cultured populations) include: the starred tortoise, Geochelone elegans (Deraniyagala, 1939:287); the Asiatic trionychid, Lissemys punctata (op. cit.:304); the diamond-backed terrapin, Malaclemys terrapin (Hildebrand and Prytherch, 1947:2); and the Japanese soft-shelled turtle, Trionyx japonicus (Mitsukuri, 1895, cited by Cagle, 1950:38).
There is a marked alternation of ovarian activity in T. ornata, one ovary being more active than its partner in a given season. The less active ovary is more active than its partner in the following season. For example, a specimen killed in July had four corpora lutea on the right ovary and two on the left and there were five enlarged follicles (of the medium size group), representing the next set of eggs to be ovulated, four on the left ovary and one on the right. Similar alternation of ovarian activity was observed, to a greater or lesser extent, in nearly all of the females examined. Many subadult females that were approaching their first breeding season (as evidenced by the presence of large ovarian follicles but no indication of former ovulation) had but one active ovary. This may account in part for the tendency of small, young females to lay clutches smaller than average. One ovary may become senile in old females before its partner does; this may explain the occasional absence or atrophy of one ovary in large females that I have examined.
In all the specimens examined, it was evident that ovulation had occurred or would occur in two successive seasons. Senile or young females might, however, be expected to skip a laying season if only one ovary was functioning.
After ovulation, the collapsed follicle assumes a cuplike shape [Pg 553] and becomes a glandular corpus luteum (Pl. 20, Fig. 2). Corpora lutea are approximately eight millimeters in diameter and are easily discernible at least until the eggs are laid; they are somewhat less distinct after preservation. Corpora lutea undergo rapid involution following oviposition and, after two to three weeks, are little more than small puckerings on the ovarian epithelium. At this stage they are properly referred to as corpora albicantia and are discernible only after careful examination of the ovary under low magnification. Corpora albicantia remain on the ovary until April of the year following ovulation but disappear in May and are never present after the new set of eggs is ovulated. Ovaries of some sub-adults (that would have laid first in the season following capture) contained enlarged follicles and, but for their lack of corpora lutea and corpora albicantia, were indistinguishable from those of older, fully mature females.
Altland (1951:605-610) gave a histological description of the corpus luteum of Terrapene carolina. Corpora lutea were glandular and filled with lipoidal material until the eggs were laid. Atresia of corpora lutea began when eggs were laid, was completed by mid-August, and was coincident with atresia of large follicles that did not undergo ovulation. Altland did not describe the gross external appearance of the corpus albicans.
The corpus luteum of oviparous reptiles seems to be closely associated with the intrauterine life of the eggs and, in viviparous reptiles, it may be an important factor in maintaining optimum gestational environment; however, its functions in all reptiles are poorly understood (Miller, 1948:200-201).
Information gleaned from records of gravid females and known dates of nesting suggests that eggs are retained in the oviducts two to three weeks before laying. Once they are ovulated, the eggs are exposed to but few hazards until laid; counts of corpora lutea are an accurate indication of the number of eggs laid. In the gravid females examined by me, number of corpora lutea on the ovaries was equal, in all but one instance, to the number of oviducal eggs. In the single instance in which an extra corpus luteum was found, one egg had probably been laid before the specimen was captured. The high incidence of correspondence between counts of corpora lutea and counts of oviducal eggs indicates also that T. ornata deposits the entire complement of oviducal eggs at one time, not singly or in smaller groups.
Extrauterine migration of ova, whereby eggs from one ovary pass into the oviduct of the opposite side, is of common occurrence in [Pg 554] T. ornata and is known to occur also in T. carolina, Chrysemys picta, Emydoidea blandingi, Pseudemys scripta, Cnemidophorus sexlineatus, and in several mammals (Legler, 1958). This ovular migration may serve to redistribute eggs to the oviducts when the ovaries are functioning at unequal rates.
The eggs acquire shells soon after they enter the oviducts. No shell-less eggs were found in oviducts but several specimens of T. ornata had oviducal eggs, the thin, parchmentlike shells of which lacked the outer calceous layer; in these specimens the corpora lutea were fresh, probably not more than two days old. Eggs that had remained in the oviducts longer had a calceous layer on the outside of the shell. Eggs having incompletely developed shells were successfully incubated in the laboratory. Cagle (1950:38) found shelled but yolkless eggs in the oviducts of several Pseudemys scripta but found no yolkless eggs in nests. No yolkless eggs were found in specimens of T. ornata in the course of the present study.
The uterine portion of the oviducts becomes darkened (pale gray to intense black) in the breeding season. Darkening of oviducts seemed to coincide with the period when eggs were in the oviducts and it persisted for a variable length of time after the eggs were laid. Oviducts of immature females were ordinarily pale.
Ornate box turtles nest chiefly in June. Some females nest as early as the first week of May or as late as mid-July but the nesting season reaches its peak in mid-June. Eggs nearly ready to be laid were in oviducts (determined by bimanual palpation in the field or by dissection in the laboratory) of many females captured in June; nearly half of the records so obtained were in the second week of that month. Early records of shelled oviducal eggs were April 25 (specimen from Ottawa County, Oklahoma), May 5, and May 22. The two latest records are for females retaining oviducal eggs on July 2 and 11. Known dates for nesting of free-living females were distributed rather evenly through the month of June. It is worthy of note that all (four) of the nestings known to occur in July were by captive females. Females of T. ornata, like those of some other turtles (Cagle and Tihen, 1948; Risley, 1933:694), seem to retain their eggs until conditions are suitable for nesting. Most of the reports in the literature of nesting after mid-July represent records for captive females.
Nests of T. o. ornata were so well-concealed that they were difficult to find even when a gravid female had been followed to the approximate location by means of a trailing thread. Females spend one to several days seeking a site for the nest, usually traveling a circuitous route within a restricted area. Movements of nest-seeking females were more extensive than those of males and non-gravid females observed in the same periods.
Activities of one gravid female, typical in most respects of the activities of several other gravid females observed (for periods of one to 23 days) at the Damm Farm, illustrate pre-nesting behavior (Fig. 29). A trailer was attached to the female on the morning of June 7. She was recovered early on the following afternoon; her movements in the elapsed period had been restricted to a small, deep, ravine 150 feet long and 20 to 30 feet wide. She had traversed each edge of the ravine at least once and had crossed it six or seven times, keeping mostly to areas on the upper parts of south—or west—facing slopes where vegetation was sparse or lacking. In six places she had dug into the ground, probably to test the suitability of the soil for nesting. In three places she dug beneath rocks that jutted out from the bank, and in two places merely scratched away the upper crust of soil. Her most recent attempt at digging (probably late the previous evening or in early morning on the day of her capture) consisted of a flask-shaped cavity that, but for the lack of eggs and a covering of earth, was like a completed nest (Pl. 21, Fig. 1). The cavity was 55 millimeters deep, 80 millimeters wide at the bottom, and 60 millimeters wide at the opening. For several inches about the opening the earth was slightly damp. That piled on the rim of the opening was of the consistency of thick mud, indicating that the female had voided fluid first on the surface of the earth and again inside the cavity to soften the soil. Subsequently during eight days her activities were similar but not so extensive as on the day described above. It was determined by daily palpation that she laid her eggs somewhere in the general area of the ravine on June 15 but the nest could not be found.
No completed nests containing eggs were discovered at the Damm Farm but the locations of several robbed nests and partly completed nests provided some information on preferred sites. The nests found were on bare, well-drained, sloping areas and were protected from erosion by upslope clumps of sod or rocks. [Pg 556] The nest cavity illustrated in Plate 21 was at the edge of the sod-line on the upper lip of the west-facing bank of a ravine. One nest had been excavated in a shallow den beneath an overhanging limestone rock. Three nests were on west- or south-facing slopes and one was on the north-facing bank of a ravine. Box turtles presumably select bare areas for nesting because of the greater ease of digging. One female at the Damm Farm was thought to have laid her eggs in a cultivated field and William R. Brecheisen told me he discovered two nests in a wheat field being plowed in July, 1955.
The repeated excavation of trial nest cavities presumably exhausts the supply of liquid in the female's bladder. Frequent imbibing of water is probably necessary if the search for a nesting site is continued for more than a day or two. Standing water was usually available in ponds, ravines, ditches, and other low areas at the Damm Farm in June. Nesting in June, therefore, is advantageous not only because of the greater length of time provided for incubation and hatching but also because of the amount of water available for drinking. Females can probably be more selective in the choice of a nesting site if their explorations are not limited by lack of water.
Females of T. ornata, in all instances known to me, began excavation of their nests in early evening and laid their eggs after dark; Allard (1935:328) reported the same behavior for T. carolina.
William R. Brecheisen, on July 22, 1955, at his farm, two miles south and one mile west of Welda, Anderson County, Kansas, observed that a large female began digging a nest in an earth-filled stock tank at 6:00 P. M. At first she moved her body about on the surface of the earth, loosening it and pushing it aside with all four legs, making a depression approximately two inches deep and large enough to accommodate her body. At 7:30 P. M. she began digging alternately with her hind feet at the bottom of the depression. Digging continued until 10:00 P. M., at which time the nest cavity was three inches deep, and three inches in diameter, with a smaller opening at the top. Six eggs were laid in the next half-hour. Covering of the nest probably took more than one hour but observations were terminated after the final egg was laid. By the following morning the nest-site had been completely covered and was no different in appearance from the rest of the earthen floor of the tank. (Brecheisen observed more of the nesting than anyone else [Pg 557] has recorded and I am obliged to him for permission to abstract, as per the above paragraph, the notes that he wrote on the matter.)
A nest made by a captive female at the Reservation was of normal proportions except for an accessory cavity that opened from the neck of the nest, immediately below the surface of the ground. This smaller cavity contained a single egg. This peculiar nest may have resulted from the efforts of two different females since several were kept in the same outdoor pen.
Ten adult females were kept in an outdoor cage in the summer of 1955. The cage was raised off the ground on stilts and its floor was covered with 12 inches of black, loamy soil. A small pan of water was always available in the cage and the turtles were fed greens, fruit, and table scraps each evening. Nesting activity was first noted on June 21, when one of the females was digging a hole in a corner of the enclosure. She dug with alternate strokes of her fully-extended hind legs in the manner described (Legler, 1954:141) for painted turtles (Chrysemys picta bellii). Nevertheless, digging was much less efficient than in Chrysemys, because of the narrow hind foot of the female T. ornata; approximately half of the earth removed by any one stroke rolled back into the nest or was pulled back when she reinserted her leg. The female stopped digging when I made sudden movements or held my hand in front of her. Digging continued for approximately 45 minutes; then the female moved away and burrowed elsewhere in the cage. The nest cavity that she left was little more than a shallow depression. Three other females were digging nests early in the evening on July 3, 5, and 8; in each of these instances the female stopped digging to eat when food was placed in the cage and completed the nesting process, unobserved, later in the evening. In each instance where nest-digging by captive females was observed, the hind quarters of the female rested in a preliminary, shallow depression, and the anterior end of the body was tilted upward at an angle of 20 to 30 degrees. In late June and early July several eggs were found, unburied, on the floor of the cage and in the pan of water.
The excavation of a preliminary cavity by captive females may not represent a natural phenomenon. Allard (1935) made no mention of it in his meticulous description of the nesting process in T. carolina. It is worthy of mention, however, that Booth (1958:261) reported the digging of a preliminary cavity by a captive individual of Gopherus agassizi.
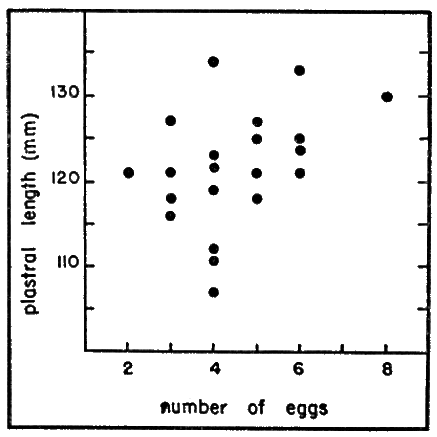
The number of eggs in 23 clutches ranged from two to eight (mean, 4.7 ± 1.37 σs]); clutches of four, five, and six eggs were most common, occurring in 18 (78 per cent) instances. The tendency for large females to lay more eggs than small females (Fig. 6) was not so pronounced as that reported by Cagle (1950:38) for Pseudemys scripta. The small size of T. ornata, in comparison with other emyid turtles, seemingly limits the number of eggs that can be accommodated internally. The number of eggs per clutch in T. carolina [2 to 7, average 4.2, Allard (1935:331)], is nearly the same as that of T. ornata.
Shells of the eggs are translucent and pinkish or yellowish when the eggs are in the oviducts. After several days outside the oviducts the shells become chalky-white and nearly opaque. Eggs incubated in the laboratory retained the pinkish color somewhat longer than elsewhere on their under-surfaces, which were in contact with moist cotton, but eventually even this part of the shell became white. Infertile eggs remained translucent and eventually became dark yellow, never becoming white; they could be distinguished from fertile eggs on the basis of color alone. Shells of infertile eggs became brittle and slimy after several weeks.
The outer layer of the shell of a freshly laid egg is brittle and cracks when the egg is dented. After a few days, when the eggs begin to expand, the shell becomes flexible and has a leathery texture. The shell is finely granulated but appears smooth to the unaided eye. The granulations are approximately the same as those illustrated by Agassiz (1857:Pl. 7, Fig. 18) for T. carolina.
Eggs are ellipsoidal. Data concerning size and weight (consisting of mean, one standard deviation, and extremes, respectively) taken from 42 eggs (representing 9 clutches) within 24 hours after they were laid, or dissected from oviducts, are as follows: length, 36.06 ± 2.77 (31.3-40.9); width, 21.72 ± 1.04 (20.0-26.3); and weight, 10.09 ± 1.31 (8.0-14.3). There was a general tendency [Pg 559] for smaller clutches to have larger eggs; the largest and heaviest were in the smallest clutch (two eggs) and the smallest were in the largest clutch (eight eggs). Risley (1933:697) reported such a correlation in Sternotherus odoratus, as did Allard (1935:331) in T. carolina. Measurements in the literature of the size of eggs of T. ornata suggest a width greater than that stated above, probably because some eggs already had begun to expand when measured.
Eggs of T. ornata expand in the course of incubation, as do other reptilian eggs with flexible shells, owing to absorption of water. In the laboratory, 48 eggs increased by an average of approximately three grams in weight and three millimeters in width over the entire period of incubation; increase in width coincided with decrease in length. Cotton in incubation dishes was kept moist enough so that some water could be squeezed from it. When the cotton was constantly moist, eggs showed a fairly steady expansion from the first week of incubation until hatching. The process could be reversed by allowing the cotton to dry. Eggs that were allowed to dry for a day or more became grossly dented or collapsed. Eggs at the periphery of the incubation dish were ordinarily more seriously affected by drying than were those at the center or in the bottom of the dish. A generous re-wetting of desiccated eggs and cotton caused the eggs to swell to their original proportions within 24 hours. Recessions occurred, however, even in the clutches that received the most nearly even amount of moisture. Increases in weight and size seemed to reach a peak in the middle of the incubation period and again immediately before hatching. Infertile eggs expanded in the same manner as fertile eggs in the first week or two of incubation, but thereafter gradually regressed in bulk or failed to re-expand after temporary periods of dryness. Fertile eggs that were in good condition had a characteristically turgid, springy feel and could be bounced off a hard surface.
Temporary lack of moisture usually did not kill embryos; prolonged dryness, combined with high temperatures, probably could not be tolerated. Lynn and Ullrich (1950), by desiccating the eggs of Chrysemys picta and Chelydra serpentina, produced abnormalities in the young ranging from slight irregularities of the shell to eyeless monstrosities; eggs desiccated in the latter half of incubation produced a higher percentage of abnormal young than eggs that were desiccated earlier.
In 1956, three fertile eggs, from clutches that were at different stages of incubation, were immersed in water for 48 hours. The eggs rested on the bottom of the bowl in the same position in which they had been placed in the incubation dishes; when turned, they returned invariably to the original position. The embryos in two of the eggs (one and 27 days old at the time of immersion) were still living ten days after the eggs were removed from the water; the embryo in the remaining egg (21 days old at the time of immersion) was dead. Eggs immersed in water increased in size and weight at the same rate as eggs in incubation dishes, indicating that absorption of water probably operates on a threshold principle, the amount absorbed being no more than normal even under wet conditions.
Natural nests usually are in well-drained areas, but water probably stands in some nests for short periods after heavy rains. Provided the nest cavity itself is not damaged, water in the nest is probably more beneficial than harmful to the eggs; however, nests that are inundated during floods probably have little chance of survival.
Eggs were examined by transmitted light in the course of incubation. At the time of laying (or removal from oviducts) no embryonic structures were discernible even in eggs that had been retained in the oviducts of captive females some weeks past the normal time of laying; a colorless blastodisc could be seen if eggs were opened. Embryonic structures first became visible at eight to ten days of incubation; at this time vascularization of the blastodisc was evident and the eyes appeared as dark spots. Heart beats were observed in most embryos by the fifteenth day but were evident in a few as early as the tenth day. The pulse of a fifteen-day-old embryo averaged 72 beats per minute at a temperature of 30 degrees. Embryos at fifteen days, measured in a straight line from cephalic flexure to posteriormost portion of body, were approximately nine to ten millimeters long and at 22 days were 14 millimeters long. At approximately 35 days the eggs became dark red; embryonic structures were discernible thereafter only in eggs that had embryos situated at one end, close to the shell.
Incubation periods for 49 eggs (representing 12 clutches) kept in the laboratory ranged from 56 to 127 days, depending on the temperature of the air during the incubation period. In 1955, eggs [Pg 561] were kept at my home in Lawrence where air temperatures were uncomfortably hot in summer and fluctuations of 20 degrees (Fahrenheit) or more in a 24-hour period were common. The following summer eggs were kept in my office at the Museum where temperatures were but slightly cooler than in my home and subject also to wide variation. In 1957 this part of the Museum was air-conditioned and kept at approximately 75 degrees. The greater lengths of incubation periods at lower temperatures are shown in Table 1. Risley (1933:698) found the incubation period of Sternotherus odoratus to be longer at lower temperatures; corresponding observations were made by Allard (1935:332) and Driver (1946:173) on the eggs of Terrapene carolina. Cagle (1950:40) and Cunningham (1939) found no distinct differences in length of incubation period for eggs of Pseudemys scripta and Malaclemys terrapin, respectively, at different temperatures within the range tolerated by the eggs.
Most nests observed in the field were in open situations where they would receive the direct rays of the sun for at least part of the day; the shorter average incubation periods (59 and 70 days, respectively), observed in 1955 and 1956, therefore, more nearly reflect the time of incubation under natural conditions than does the excessively long period (125 days at 75 degrees) observed in 1957 under cooler, more nearly even temperatures.
Table 1.—The Relationship of Temperature and Duration of Incubation Period as Determined from Laboratory Studies of 49 Eggs of T. ornata. |
|||||
| Average daily temperature (Fahrenheit) | Period of incubation (Days) | Number of clutches | Number of eggs | Remarks | |
| Mean | Range | ||||
| 91 | 59 | 56-64 | 6 | 24 | Wide daily fluctuations in temperature |
| 82 | 70 | 67-73 | 4 | 21 | Wide daily fluctuations in temperature |
| 75 | 125 | 124-127 | 2 | 4 | Temperature thermostatically controlled |
Sixty-five days seems to be a realistic estimate of a typical incu [Pg 562]bation period under natural conditions; eggs laid in mid-June would hatch by mid-August. Even in years when summer temperatures are much cooler than normal, eggs probably hatch by the end of October. Hatchlings or eggs would have a poor chance of surviving a winter in nests on exposed cut-banks or in other unprotected situations. Overwintering in the nest, hatchlings might survive more often than eggs, since hatchlings could burrow into the walls and floor of the nest cavity. Unsuitable environmental conditions that delay the nesting season and retard the rate of embryonic development may, in some years, be important limiting factors on populations of ornate box turtles.
In areas where T. ornata and T. carolina are sympatric (for example, in Illinois, Kansas, and Missouri) the two species occupy different habitats, ornata preferring open grassland and carolina wooded situations. Under natural conditions, the average incubation periods of these two species can be expected to differ, T. carolina having a somewhat longer period due to lower temperatures in nests that are shaded. In the light of these speculations, the remark of Cahn (1937:102)—that T. ornata nested later in the season (in Illinois) and compensated for this by having a shorter incubation period—is understandable.
The range of temperatures tolerated by developing eggs probably varies with the stage of embryonic development. When temperatures in the laboratory were 102 to 107 degrees Fahrenheit for approximately eight hours, due to a defect in a thermostat, the young in two eggs of T. ornata, that had begun to hatch on the previous day, were killed, as were the nearly full-term embryos in a number of eggs of T. carolina (southern Mississippi) kept in the same container. A five-day-old hatchling of T. ornata, kept in the same container, survived the high temperatures with no apparent ill effects. Cagle (1950:41) found that eggs of Pseudemys scripta could not withstand temperatures of 10 degrees for two weeks nor would they survive if incubated at 40 degrees. Cunningham (1939) reported that eggs of Malaclemys terrapin could not survive prolonged exposure to temperatures of 35 to 40.6 degrees but tolerated temporary exposure to temperatures as high as 46 degrees.
In the summer of 1955, a clutch of three eggs, all of which contained nearly full-term embryos, was placed in a refrigerator for 48 hours. The temperature in the refrigerator was maintained at approximately 4.5 degrees; maximum and minimum temperatures for the 48 hour period were 2.8 and 9.5 degrees, respectively. When the eggs were removed from the refrigerator they showed gains in [Pg 563] weight and increases in size comparable to eggs, containing embryos of the same age, used as controls. The experimental eggs began to hatch two days after they were removed to normal temperatures—approximately 24 hours later than the controls.
In the late stages of incubation, the outer layer of the shell becomes brittle and is covered with a mosaic of fine cracks or is raised into small welts. Several days before hatching, movements of the embryo disturb the surface of the shell and cause the outer layer to crumble away, especially where the head and forequarters of the embryo lie against the shell. Some embryos could be seen spasmodically thrusting the head and neck dorsally against the shell.
The role of the caruncle in opening the shell seems to vary among different species of turtles. Cagle (1950:41) reported that it was used only occasionally by Pseudemys scripta; Allard (1935:332) thought that it was not used by Terrapene carolina; and, the observations of Booth (1958:262) and Grant (1936:228) indicate that embryos of Gopherus agassizi use the caruncle at least in the initial rupturing of the shell.
In the three instances in which hatching was closely observed in T. ornata, the caruncle made the initial opening in the shell; claws of the forefeet may have torn shells in other hatchings that were not so closely observed. In all observed instances, the shell was first opened at a point opposite the anterior end of the embryo. The initial opening had the appearance of a three-cornered tear. A quantity of albuminous fluid oozed from eggs as soon as the shells were punctured.
The initial tear is enlarged by lateral movements of the front feet, and later the hind feet reach forward and lengthen the tear farther posteriorly. In many instances a tear develops on each side and the egg has the appearance of being cleft longitudinally. The young turtle emerges from the anterior end of the shell or backs out of the shell through a lateral tear.
The process of hatching, from rupture of shell to completion of emergence, extended over three to four days in the laboratory. Many hatchlings from time to time crawled back into the shell over a period of several days after hatching was completed. In a clutch of eggs kept in a pail of earth, by William R. Brecheisen, eight days elapsed between onset of hatching and appearance of the first hatchling at the surface.
A nest in an outdoor pen at the Reservation was discovered in [Pg 564] early October. The cap had been recently perforated and the hatchlings had escaped. One of them, judged to be approximately two weeks old, was found in a burrow nearby. The cavity of the nest appeared to have been enlarged by the young. The eggs were probably laid in early July. Emergence of young from the nest had been delayed for a time after hatching, until rain softened the ground in late September and early October.
Eggs were incubated in the laboratory at more nearly optimum temperature and humidity than were eggs in natural nests. Percentage of prenatal mortality probably was lower in laboratory-incubated eggs than in those under natural conditions.
Of sixty eggs studied in the laboratory, 45 (75 per cent) were fertile; 36 (80 per cent) of the fertile eggs (those in which the blastodisc was at some time discernible by transmitted light) hatched successfully. In six clutches all the eggs were fertile and five of these clutches hatched with 100 per cent success. One clutch contained eggs that were all infertile and another clutch had four infertile eggs and two fertile eggs that failed to hatch. Among nine fertile eggs that failed to survive, four casualties occurred in the late stages of incubation or after hatching had begun, indicating that these are probably critical periods.
Fertility of eggs was not correlated with size or age of female, with size of clutch, or with size of egg. Eggs laid in the laboratory had higher rates of infertility and prenatal mortality than did eggs dissected from oviducts. Handling of eggs in removing them from nests to incubation dishes, after embryonic development had begun, might have been responsible for reduced viability (Table 2).
Table 2.—Comparative Rates of Fertility and Prenatal Mortality for Eggs Dissected from Oviducts and for Eggs That Were Laid in the Laboratory and Subsequently Removed to Incubation Dishes. |
||
| Number or Percent | Eggs removed from nest | Eggs dissected from oviducts |
| Number of eggs examined | 22 | 38 |
| Percentage of fertile eggs | 64 | 82 |
| Percentage of fertile eggs hatched | 50 | 94 |
| Percentage of eggs hatched | 32 | 76 |
Assuming that 4.7 eggs are laid per season, that all eggs are fertile and all hatch, that all young survive to maturity, that half the hatchlings are females, and that females first lay eggs in the eleventh year, the progeny of a single mature female would number 699 after twenty years. Considering that infertility and prenatal mortality eliminate approximately 40 per cent of eggs laid (according to laboratory findings) the average number of surviving young per clutch would be 2.8 and the total progeny, after 20 years, would be 270, provided that only one clutch of eggs was laid per year. But it is thought that, on the average, one third of the female population produces two clutches of eggs in a single season. If the second clutch contains 3.5 eggs (resulting in 2.1 surviving young when factors of infertility and prenatal mortality are considered), the progeny of a single female, after 20 years, would number approximately 380. Postnatal mortality reduces the progeny to a still smaller number.
The small number of eggs laid each year and the long period required to reach sexual maturity make the reproductive potential of T. ornata smaller than that of the other turtles of the Great Plains, and much smaller than nearly any of the non-chelonian reptiles of the same region.
The total span of reproductive years is difficult to determine; I am unable to ascertain the age of a turtle that has stopped growing. No clearly defined external characteristics of senility were discovered in the populations studied. A male that I examined had one atrophied testis. In another male both testes were shrunken and discolored and appeared to be encased by fibrous tissue. Both males were large, well past the age of regular growth, and had smoothly worn shells. Several old females had seemingly inactive ovaries. Reproductive processes probably continue throughout life in most members of the population, although possibly at a somewhat reduced rate in later life.
Young box turtles became active and alert as soon as they hatched, and remained so until low temperatures induced quiescence. If sand or soil was available, hatchlings soon burrowed into [Pg 566] it and became inactive. Covering containers with damp cotton also induced inactivity; the hatchlings usually made no attempt to burrow through the confining layer. Desire to feed varied in hatchlings of the same brood and seemed not to be correlated with retraction of the yolk sac or retention of the caruncle. Some hatchlings actively pursued mealworms; on subsequent feedings they learned to associate my presence with food and eagerly took mealworms from forceps or from my hand. Meat, vegetables, and most other motionless but edible objects were ignored by hatchlings but some individuals learned to eat meat after several forced feedings. Hatchlings that regularly took food in the first month of life ordinarily grew faster than hatchlings that did not eat. Many of the hatchlings in the laboratory showed no areas of new epidermal growth on the shell in the time between hatching and first (induced) hibernation.
The proportions of the shell change somewhat in the first few weeks of life. At hatching the shell may be misshapen as a result of confinement in the egg. Early changes in proportions of the shell result from expansion—widening and, to a lesser degree, lengthening of the carapace—immediately after hatching. Subsequent retraction or rupture of the yolk sac and closure of the navel are accompanied by a decrease in height of shell and slight, further widening of the carapace.
The yolk sac retracts mainly between the time when the egg shell is first punctured and the time when the turtle actually emerges from the shell. When hatching is completed, the yolk sac usually protrudes no more than two millimeters, but in some individuals it is large and retracts slowly over a period of several days.
One individual began hatching on November 11 and was completely out of the egg shell next day; the yolk sac was 15 millimeters in diameter, protruded six millimeters from the umbilical opening, and hindered the hatchling's movements. The sac broke two days later, smearing the bottom of the turtle's dish with semifluid yolk. The hatchling then became more active. Twenty-six days later the turtle was still in good condition and its navel was nearly closed. A turtle that hatched with a large yolk sac in a natural nest possibly would benefit, through increased ease of mobility, if the yolk sac ruptured.
A recently hatched turtle was found at the Reservation in October, [Pg 567] 1954, and was kept in a moist terrarium in the laboratory where it died the following May. The turtle was sluggish and ate only five or six mealworms while in captivity; no growth was detectable on the laminae of the shell. Autopsy revealed a vestige of the retracted yolk sac, approximately one millimeter in diameter, on the small intestine.
The navel ("umbilical scar") of captive hatchlings ordinarily closed by the end of the second month but in three instances remained open more than 99 days. The position of the navel is marked by a crescent-shaped crease, on the abdominal lamina, that persists until the plastron is worn down in later years (Pl. 24, Fig. 1).
The caruncle ("egg tooth") (Fig. 7) remains attached to the horny maxillary beak for a variable length of time; 93 per cent of the live hatchlings kept in the laboratory retained the caruncle on the tenth day, 71 per cent on the twentieth day, and only 10 per cent on the thirtieth day of life. Few individuals retained the caruncle when they entered hibernation late in November, and none retained it upon emergence from hibernation. Activities in the first few days or weeks of life influence the length of time that the caruncle is retained; turtles that begin feeding soon after hatching probably lose the caruncle more quickly than do those that remain quiescent. The caruncles of some laboratory specimens became worn before finally dropping off. Almost every caruncle present after 50 days could be flicked off easily with a probe or fingernail. The initiation of growth of the horny maxillary beak probably causes some loosening of the caruncle. The caruncle may aid hatchlings in escaping from the nest.
After the caruncle falls off, a distinct boss remains, marking its former place on the horny beak (Pl. 25, Fig. 1); this boss is gradually obliterated over a period of weeks by wear and by differential growth, and is seldom visible in turtles that have begun their first full year of growth. The "first full year of growth" is here considered to be the period of growth beginning in the spring after hatching.
Growth of ornate box turtles was studied by measuring recaptured turtles in the field, by periodically measuring captive hatchlings and juveniles, and by measuring growth-rings on the epidermal laminae of preserved specimens. Studies of growth-rings provided by far the greatest volume of information on growth, not only for the years in which field work was done, but for the entire life of each specimen examined.
It was necessary to determine the physical nature of growth-rings and the manner in which they were formed before growth could be analyzed. Examination of epidermal laminae on the shell of a box turtle reveals that each has a series of grooves—growth-rings—on its surface. The deeper grooves are major growth-rings; they occur at varying distances from one another and run parallel to the growing borders of the lamina. Major growth-rings vary in number from one to 14 or more, depending on the age of the turtle (Pl. 22). In juvenal turtles and in young adults, major growth-rings are distinct and deep. Other grooves on the shell—minor growth-rings—have the same relationship to the borders of the laminae but are shallower and less distinct than major growth-rings. One to several minor growth-rings usually occur on each smooth area of epidermis between major growth-rings. As the shell of an adult turtle becomes worn, the minor growth-rings disappear and the major rings become less distinct. Both sets of rings may be completely obliterated in old turtles but the major rings usually remain visible until several years after puberty.
In cross section, major growth-rings are V- or U-shaped. The inner wall of each groove is the peripheral edge of the part of the scute last formed whereas the outer wall represents the inner edge of the next new area of epidermal growth. The gap produced on the surface of the lamina (the open part of the groove) results from cessation of growth at the onset of hibernation. Minor growth-rings are shallow and barely discernible in cross-section (Fig. 8). It may therefore be understood that growth-rings are compound in origin; each ring is formed in part at the beginning of hibernation and in part at the beginning of the following growing season.
The few publications discussing growth in turtles express conflicting views as to the exact mode of growth of epidermal laminae. Carr (1952:22) briefly discussed growth of turtle scutes in general and stated that eccentric growth results from an entirely new [Pg 569] laminal layer forming beneath, and projecting past the edges of the existing lamina. Ewing (1939) found the scutes of T. carolina to be the thickest at the areola and successively thinner in the following eight annual zones of growth; parts of scutes formed subsequent to the ninth year varied irregularly in thickness. He stated that epidermal growth took place at the margins of the laminae rather than over their entire under-surfaces.
It is evident that the mode of scutular growth described by Carr (loc. cit.) applies to emyid turtles that shed the epidermal laminae more or less regularly (for example, Chrysemys and Pseudemys). In these aquatic emyids a layer of the scute, the older portion, periodically becomes loose and exfoliates usually in one thin, micalike piece; since the loosened portion of the scute corresponds in size to the scute below, it must be concluded that a layer of epidermis is shed from the entire upper surface of the scute, including the area of new epidermal growth. Box turtles ordinarily do not shed the older parts of their scutes; the areola and successively younger portions of the lamina remain attached to the shell until worn off. The appearance of a single unworn scute, especially one of the centrals or the posterior laterals, closely resembles a low, lopsided pyramid.
Examination of parasagittal sections of scutes revealed that they were composed of layers, the number of layers varying with the age of the scute. A scute from a hatchling consists of one layer. A scute that shows a single season of growth has two layers; a new layer is added in each subsequent season of growth. Stratification is most evident in the part of the scute that was formed in the first three or four seasons and becomes increasingly less distinct in newer parts of the scute. It may further be understood that scutes grow in the manner described by Carr (loc. cit.).
When the epidermal laminae are removed, a sheet of tough, pale grayish tissue remains firmly attached to the bones of the shell beneath. This layer probably includes, or consists of, germinal epithelium. Contrasting pale and dark areas of the germinal layer correspond to the pattern of markings on the scute removed.
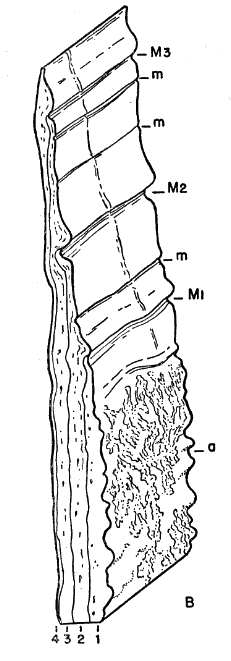 |
Fig. 8. The second central scute from
a juvenal T. o. ornata (KU 16133) in its third full season of growth.
A) Entire scute from above (× 2½); dashed line shows portion
removed in parasagittal section. B) Diagonal view of section removed from
scute in "A" (× 43∕8, thickness greatly
exaggerated) showing layers of epidermis formed in successive seasons of
growth. Each layer ends at a major growth-ring (M 1-3) that was formed
during hibernation; minor growth-rings (m), formed in the course of the
growing season, do not result from the formation of a new layer of epidermis.
Note the granular texture of the areola (a); the smooth zone between the
areola and M1 shows amount of growth in the season of hatching.
|
Growth of epidermal laminae is presumably stimulated by growth of the bony shell. As the bone grows, the germinal layer of the epidermis grows with it. When growth ceases at the beginning of hibernation, the thin edges of the scutes are slightly down-turned where they enter the interlaminal seams (Fig. 8). When growth is resumed in spring, the germinal layer of the epidermis, rather than continuing to add to the edge of the existing scute, forms an [Pg 570] entirely new layer of epidermis. The new layer is thin and indistinct under the oldest part of the scute but becomes more distinct toward its periphery. Immediately proximal to the edge of the scute, the new layer becomes greatly thickened, and, where it passes under the edge, it bulges upward, recurving the free edge of the scute above. At this time the formation of a major growth-ring is completed. The newly-formed epidermis, projecting from under the edges of the scute, is paler and softer than the older parts of the scute; the presence or absence of areas of newly formed epidermis [Pg 571] enables one to determine quickly whether a turtle is growing in the season in which it is captured. There is little actual increase in thickness of the scute after the first three or four years of growth. The epidermal laminae are therefore like low pyramids only in appearance. This appearance of thickness is enhanced by the contours of bony shell which correspond to the contours of the scutes.
Minor growth-rings differ from major growth-rings in appearance and in origin. Ewing (op. cit.: 91) recognized the difference in appearance and referred to minor growth-rings as "pseudoannual growth zones." Minor growth-rings result from temporary cessations of growth that occur in the course of the growing season, not at the onset of hibernation. They are mere dips or depressions in the surface of the scute. The occurrence of minor growth-rings indicates that interruptions in growth of short duration do not result in the formation of a new layer of epidermis. Slowing of growth or its temporary cessation may be caused by injuries, periods of quiescence due to dry, hot, or cold weather, lack of food, and possibly by physiological stress, especially in females, in the season of reproduction. Minor growth-rings that lie immediately proximal to major growth-rings (Pl. 22, Fig. 2), are the result of temporary dormancy in a period of cold weather at the end of a growing season, followed by nearly normal activity in a warmer period before winter-long hibernation is begun. Cagle (1946:699) stated that sliders (Pseudemys scripta elegans) remaining several weeks in a pond that had become barren of food would stop growing and develop a growth-ring on the epidermal laminae; he did not indicate, however, whether these growth-rings differ from those formed during hibernation.
In species that periodically shed scutes a zone of fracture develops between the old and new layers of the scute as each new layer of epidermis is formed, and the old layer is shed. Considering reptiles as a group, skin shedding is of general occurrence; the process in Pseudemys and Chrysemys differs in no basic respect from that in most reptiles. Retention of scutes in terrestrial emyids and in testudinids is one of many specializations for existence on land. Retention of scutes protects the shell of terrestrial chelonians against wear. Some box turtles were observed to have several scutes of the carapace in the process of exfoliation but no exfoliation was observed on the plastron. Exfoliation ordinarily occurred on the scutes of the carapace that were the least worn; the exfoliating portion included the areola and the three or four oldest (first formed) layers of the scute. The layer of scute exposed [Pg 572] was smooth and had yellow markings that were only slightly less distinct than those on the portion that was exfoliating.
Wear on the shell of a box turtle reduces the thickness of scutes, as does the shedding of scutes in the aquatic emyids mentioned. It is noteworthy that any of the layers in the scute of a box turtle can form the cornified surface of the scute when the layers above it wear away or are shed.
It is uncertain whether turtles that have ceased to grow at a measurable rate continue to elaborate a new layer of epidermis at the beginning of each season. Greatly worn shells of ornate box turtles, particularly those of the subspecies luteola, have only a thin layer of epidermis through which the bones of the shell and the sutures between the bones are visible. I suspect that, in these old individuals, the germinal layer of the epidermis does not become active each year but retains the capacity to elaborate new epidermis if the shell becomes worn thin enough to expose and endanger the bone beneath it. The germinal layer of old turtles loses the capacity to produce color.
Major growth-rings constitute a valuable and accurate history of growth that can be studied at any time in the life of the turtle if they have not been obliterated. They are accurate indicators of age only as long as regular growth continues but may be used to study early years of growth even in turtles that are no longer growing. Minor growth-rings, if properly interpreted, provide additional information on growing conditions in the course of each growing season.
Nichols (1939a: 16-17) found that the number of growth-rings formed in marked individuals of T. carolina did not correspond to the number of growing seasons elapsed; he concluded that growth-rings were unreliable as indicators of age and that box turtles frequently skipped seasons of growth. Woodbury and Hardy (1948:166-167) and Miller (1955:114) came to approximately the same conclusion concerning Gopherus agassizi. It is significant that these workers were studying turtles of all sizes and ages, some of which were past the age of regular, annual growth. Cagle's review of the literature concerning growth-rings in turtles (1946) suggests that, in most of the species studied, growth-rings are formed regularly in individuals that have not attained sexual maturity but are formed irregularly after puberty.
Cagle's (op. cit.) careful studies of free-living populations of Pseudemys scripta showed that growth-rings, once formed, did not change in size, that the area between any two major growth-rings [Pg 573] represented one season of growth, and that growth-rings were reliable indicators of age as long as the impression of the areola remained on the scutes studied. Cagle noted decreasing distinctness of growth-rings after each molt.
The relative lengths of the abdominal lamina and the plastron remain approximately the same throughout life in T. ornata. Measurements were made of the plastron, carapace, and abdominal lamina in 103 specimens of T. o. ornata from Kansas and neighboring states. The series of specimens was divided into five nearly equal groups according to length of carapace. Table 3 summarizes the relationship of abdominal length to plastral length, and of carapace length to plastral length. The mathematical mean of the ratio, abdominal length/plastral length, in each of the four groups of larger-sized turtles, was not significantly different from the same ratio in the hatchling group. The relative lengths of carapace and plastron are not so constant; the carapace is usually longer than the plastron in hatchlings and juveniles, but shorter than the plastron in adults, especially adult females.
| Length of Carapace | Number of Specimens | Length of abdominal as a percentage of length of plastron | Individuals having plastron longer than carapace | ||
| Mean ± σm | Extremes | Number | Percentage | ||
| Less than 50 mm. (Juveniles) | 23 | 18.3±.498 | 13.7-20.3 | 7 | 38.5 |
| 50 to 69 mm. (Juveniles) | 20 | 17.8±.303 | 15.2-20.2 | 8 | 40.0 |
| 70 to 100 mm. (Subadults) | 20 | 17.9±.445 | 14.3-20.6 | 15 | 75.0 |
| More than 100 mm. (Adult males) | 20 | 17.8±.236 | 16.4-20.6 | 13 | 65.0 |
| More than 100 mm. (Adult females) | 20 | 18.8±.510 | 15.1-25.7 | 19 | 95.0 |
The length of any growth-ring on the abdominal lamina can be used to determine the approximate length of the plastron at the time the growth-ring was formed. Actual and relative increases in length of the plastron can be determined in a like manner. For example, a seven-year-old juvenile (KU 3283) with a plastron 74.0 millimeters long had abdominal growth-rings (beginning with areola and ending with the actual length of the abdominal) 5.9, 7.8, 9.5, 10.7, 12.0, 12.5, 14.3, and 14.9 millimeters long. Using the proportion,
| [ |
|
] |
where AB is the abdominal length, PL the plastral length, AB1 the length of any given growth-ring, and X the plastral length at the time growth-ring AB^1 was formed, the plastral length of this individual was 29.3 millimeters at hatching, 38.8 at the end of the first full season of growth, and 47.2, 53.2, 59.6, 62.1, and 71.0 millimeters at the end of the first, second, third, fourth, fifth, and sixth seasons of growth, respectively. The present length of the abdominal (14.9 mm.) indicates an increment of three millimeters in plastral length in the seventh season, up to the time the turtle was killed (June 25). This method of studying growth in turtles was first used by Sergeev (1937) and later more extensively used by Cagle (1946 and 1948) in his researches on Pseudemys scripta. Because the plastron is curved, no straight-line measurement of it or its parts can express true length. Cagle (1946 and 1948) minimized error by expressing plastral length as the sum of the laminal (or growth-ring) lengths. This method was not possible in the present study because growth-rings on parts of one or more laminae (chiefly the gulars and anals) were usually obliterated by wear, even in young specimens. It was necessary to express plastral length as the sum of the lengths of forelobe and hind lobe.
The abdominal lamina was selected for study because of its length (second longest lamina of plastron), greater symmetry, and flattened form. Although the abdominal is probably subject to greater, over-all wear than any other lamina of the shell, wear is even, not localized as it is on the gulars and anals.
In instances where some of the growth-rings on an abdominal lamina were worn but other rings remained distinct, reference to [Pg 575] other, less worn lamina permitted a correct interpretation of indistinct rings.
Abdominal laminae were measured at the interlaminal seam; since the laminae frequently did not meet perfectly along the midline (and were of unequal length), the right abdominal was measured in all specimens. Growth-rings on the abdominal laminae were measured in the manner shown in Plate 22.
Data were obtained for an aggregate of 1272 seasons of growth in 154 specimens (67 females, 48 males, and 39 of undetermined sex, chiefly juveniles). Averages of calculated plastral length were computed in each year of growth for specimens of known sex (Figs. 9 and 10) and again for all specimens examined. Annual increment in plastral length was expressed as a percentage of plastral length at the end of the previous growing season (Fig. 11). Increment in plastral length for the first season of growth was expressed as a percentage of original plastral length because of variability of growth in the season of hatching; growth increments in the season following hatching are, therefore, not so great as indicated in Figure 11.
Areas of new laminal growth were discernible on laboratory hatchlings soon after they ate regularly. Hatchlings that refused to eat or that were experimentally starved did not grow. The first zone of epidermis was separated from the areola by an indistinct growth-ring (resembling a minor growth-ring) in most hatchlings, but in a few specimens the new epidermis appeared to be a continuation of the areola. Major growth-rings never formed before the onset of the first hibernation.
Growth in the season of hatching seems to depend on early hatching and early emergence from the nest. Under favorable conditions hatchlings would be able to feed and grow eight weeks or more before hibernation. Hatchlings that emerge in late autumn or that remain in the nest until spring are probably unable to find enough food to sustain growth.
Sixty-four (42 per cent) of the 154 specimens examined showed measurable growth in the season of hatching. The amount of increment was determined in 36 specimens having a first growth-ring and an areola that could be measured accurately. The average increment of plastral length was 17.5 per cent (extremes, 1.8-66.0 per cent) of the original plastral length. Ten individuals showed an increment of more than 20 per cent; the majority of these individuals (8) were hatched in the years 1947-50, inclusive.
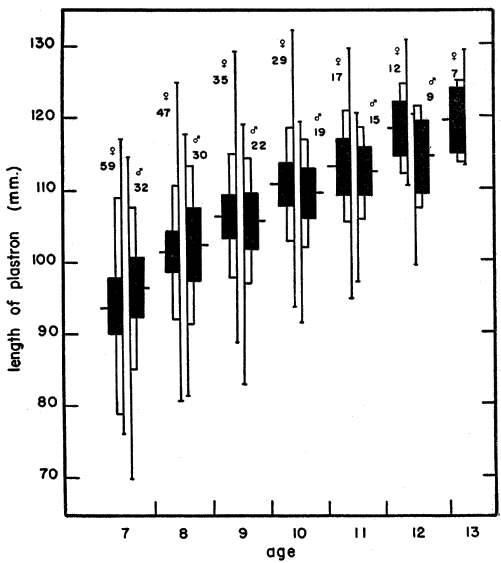
Some hatchlings that grow rapidly before the first winter are as large as one- or two-year-old turtles, or even larger, by the following summer. Individuals that grew rapidly in the season of hatching tended also to grow more rapidly than usual in subsequent seasons; 80 per cent of the individuals that increased in plastral length by 20 per cent or more in the season of hatching, grew faster [Pg 578] than average in the two seasons following hatching. Early hatching and precocious development presumably confer an advantage on the individual, since turtles that grow rapidly are able better to compete with smaller individuals of the same age. Theoretically, turtles growing more rapidly than usual in the first two or three years of life, even if they grew subsequently at an average rate, would attain adult size and sexual maturity one or more years before other turtles of the same age. A few turtles (chiefly males) attain adult size (and presumably become sexually mature) by the end of the fifth full season of growth (Figs. 9 and 10). These individuals, reaching adult size some three to four years sooner than the average age, were precocious also in the earlier stages of post-natal development.
Young box turtles reared in the laboratory grew more slowly than turtles of comparable ages under natural conditions; this was especially evident in hatchlings and one-year-old specimens. Slower growth of captives was caused probably by the unnatural environment of the laboratory. Captive juveniles showed a steady increase in weight (average, .52 grams per ten days) as they grew whereas captive hatchlings tended to lose weight whether they grew or not.
After the first year growth is variable and size is of little value as an indicator of age. Although in the turtles sampled variation in size was great in those of the same age, average size was successively greater in each year up to the twelfth and thirteenth years (for males and females, respectively), after which the samples were too small to consider mathematically.
Increments in plastral length averaged 68.1 per cent in the year after hatching, 28.6 per cent in the second year and 18.1 per cent in the third year. From the fourth to the fourteenth year the growth-rate slowed gradually from 13.3 to about three per cent (Fig. 11). These averages are based on all the specimens examined (with no distinction as to sex); they give a general, over-all picture of growth rate but do not reflect the changes that occur in growth rate at puberty (as shown in Figs. 9 and 10).
Rate of growth and, ultimately, size are influenced by the attainment of sexual maturity. Adult females grow larger than adult males. Males, nevertheless, grow faster than females and become sexually mature when smaller and younger. Examination of gonads showed 17 per cent of the males to be mature at plastral lengths of 90 to 99 millimeters, 76 per cent at 100 to 109 millimeters, [Pg 579] and 100 per cent at 110 millimeters, whereas the corresponding percentages of mature females in the same size groups were: zero per cent, 47 per cent, and 66 per cent. Of the females, 97 per cent were mature at 120 to 129 millimeters and all were mature at 130 millimeters (Fig. 13). Because growth slows perceptibly at sexual maturity, it is possible, by examination of growth-rings, to estimate the age of puberty in mature specimens.
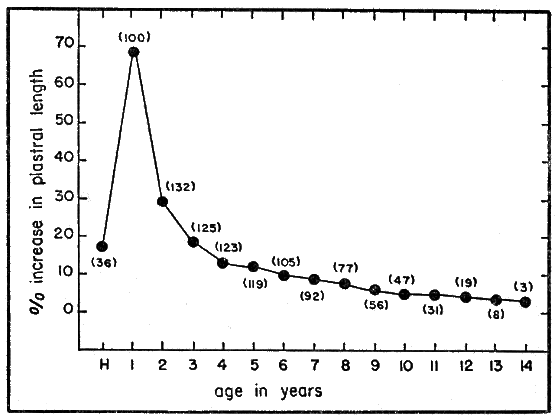
Attainment of sexual maturity, in the population studied, was more closely correlated with size than with age. For example, nearly all males were mature when the plastron was 100 to 110 millimeters long, regardless of the age at which this size was attained. The smallest mature male had a plastral length of 99 millimeters; according to the data presented in Figures 9 and 10, therefore, a few males reach sexual maturity in the fourth year, and increasingly larger portions of the population become mature in the fifth, sixth, and seventh years. The majority become mature in the eighth and ninth years. Likewise, females (smallest mature specimen, 107 mm.) may be sexually mature at the end of the sixth year but most of them mature in the tenth and eleventh years.
In growing individuals, narrow zones of new epidermis form on the laminae in spring. Nearly all the growing individuals collected in May of 1954 and 1955 had zones of new epidermis on the shell but those collected in April did not. Activity in the first week or two after spring emergence is sporadic and regular feeding may not begin until early May. Once begun, growth is more or less continuous as long as environmental conditions permit foraging. The formation of minor growth-rings and adjacent growth-zones in autumn, provides evidence that growth commonly continues up to the time of hibernation. The number of growing days per year varies, of course, with the favorableness of environmental conditions. The length of time (162 days) given by Fitch (1956b:438) as the average annual period of activity for T. ornata is a good estimate of the number of growing days per season.
Zones of epidermis formed in some years are wider or narrower than the zones bordering them (Pl. 22). Zones notably narrower or wider than the average, formed in certain years, constituted distinct landmarks in the growth-histories of nearly all specimens; for example, turtles of all ages grew faster than average in 1954 and zones of epidermis formed in this year were always wider than those formed in 1953 and 1955.
An index to the relative success of growth in each calendar year was derived. Records of growth for all specimens in each age group were averaged; the figure obtained was used to represent "normal" or average growth rate in each year of life (Fig. 12). The over-all averages for the various age groups were then compared with records of growth attained by individuals of corresponding age in each calendar year, growth in a particular year being expressed as a percentage of the over-all average. The percentages of average growth for all ages in each calendar year were then averaged; the mean expressed the departure from normal rate of growth for all turtles growing in a particular calendar year. For example, the over-all average increment in plastral length in the fifth year of life was 12.1 per cent, the increment in the sixth year was 10 per cent, and so on (Fig. 11). In 1953, turtles in their fifth and sixth years increased in plastral length by 11.4 and 9.1 per cent, or grew at 94.2 and 91.0 per cent of the normal rate, respectively. The percentages of normal growth rate for these age groups averaged with percentages of the other age groups in 1953 [Pg 581] revealed that turtles grew at approximately 86 per cent of the normal rate in 1953.
Growth rates were computed for the twelve-year period, 1943-1954, because of the concentration of records in these years. Scattered records also were available for many of the years from 1901-1942. Records for individuals in the season of hatching and the first full season of growth were not considered.
Direct correlation exists between growth rate and average monthly precipitation in the season of growth (April to September) (Fig. 12). In nine of eleven years, the curve for growth rate followed the trend of the curve for precipitation; but because other climatic conditions also influenced growth, the fluctuations in the two curves were not proportional to one another.
Grasshoppers form an important element in the diet of box turtles. Smith (1954) traced the relative abundance of grasshoppers over a period of 100 years in Kansas, and this information is of significance for comparison with data concerning growth of box turtles. In general, the growth index was higher when favorable weather and large populations of grasshoppers occurred in the same year.
In the following summary, the numbers (1 to 5) used to express the relative abundance of grasshoppers are from Smith (op. cit.). Maxima and minima refer to the twelve-year period, 1943-1954. The growth index for each year (shown as a graph in Fig. 12) appears in brackets and indicates the percentage of normal growth attained by all turtles in that year.
1954 [126.3]: Growth was better than average for turtles of all ages. Grasshopper populations were highest (4+) since 1948. Continuously warm weather, beginning in the last few days of March, permitted emergence in the first week of April; thereafter conditions were more or less continuously favorable for activity until late October. Although there was less than an inch of precipitation in September, precipitation in August and October was approximately twice normal and more or less evenly distributed. Warm weather in early November permitted an additional two weeks of activity.
1945 [125.5]: This was the second most favorable year for growth and the second wettest year. Records of growth are all from young turtles (one to four years old), all of which grew more than average. Daily maximum temperatures higher than 60 degrees Fahrenheit [Pg 582] on 18 of the last 19 days of March, combined with twice the normal amount of precipitation in the same period, stimulated early emergence. August and October were both dry (each with less than one inch of precipitation) but diurnal temperatures remained warm through the first week in November and probably prolonged activity of box turtles at least until then. Grasshoppers were more abundant (3.7) than normal.
1944 [83.1]: This was the poorest growing year for the period considered. The lack of a continuously warm, wet period in early spring probably delayed emergence until the last week in April. Temperatures remained warm enough for activity until early November, but dry weather in September and October probably curtailed activity for inducing long periods of quiescence; most of the precipitation that occurred in the latter two months fell in a one-week period beginning in the last few days of September. Grasshopper populations were higher (4.0) than normal.
1953 [85.6]: This was the second poorest growing year and the driest year in the period considered. Intermittently cold weather in spring delayed emergence until the last week in April when nearly an inch of rain fell. Temperatures were higher than normal from June to October. The period from September to the end of October was dry and the small amount of precipitation that occurred was concentrated chiefly at the beginning and end of that period. Temperatures in late October and early November were lower than normal. Grasshopper populations were low (2.2).
1952 [88.3]: Environmental conditions were poor for growth and much like the conditions described for 1953. In both years growth was much less than normal in turtles of all ages except for one group (adults that were 10 and 11 years old in 1952 and 1953, respectively) that was slightly below normal in 1952 and slightly above normal in 1953.
The small number of records for 1955 were not considered in Figure 12. Warm weather in the last half of March lengthened the growing season, and environmental conditions, as in 1954, were more or less favorable throughout the rest of the summer; 1955 probably ranks with 1954 as an exceptionally good year for growth of box turtles.
Although the number of records available for turtles hatched in the period from 1950 to 1954 is small, a few records are available for all these years except 1951. In general, small samples of turtles [Pg 583] hatched in these years reflect only the difficulty of collecting hatchlings and juveniles. In 1951, conditions for incubation and hatching were poor and the lack of records for that year actually represents a high rate of prenatal and postnatal mortality. Rainfall in the nesting season was two to three times normal and temperatures were below normal. Flooding occurred in low areas of Douglas County and many eggs may have been destroyed when nests were inundated. Cold weather probably increased the time of incubation for surviving eggs so that only a few turtles could hatch before winter. Flooding and cold, wet weather in the season of growth and reproduction, affecting primarily eggs and hatchlings, may act as checks on populations of T. ornata in certain years.
The environmental factors governing activity of terrestrial turtles seem to differ at least in respect to threshold, from the factors influencing the activity of aquatic turtles. A single month that was drier or cooler than normal probably would not noticeably affect [Pg 584] growth and activity of aquatic emyids in northeast Kansas, but might greatly curtail growth of box turtles.
Cagle (1948:202) found that growth of slider turtles (Pseudemys scripta) in Illinois paralleled the growth of bass and bluegills in the same lake; in the two years in which the fish grew rapidly, the turtles did also, owing, he thought to "lessened total population pressure" and "reduced competition for food." Growth of five-lined skinks (Eumeces fasciatus) on the Natural History Reservation paralleled growth of box turtles, probably because at least some of the same environmental factors influence the growth of both species. Calculations of departure from normal growth in E. fasciatus, made by me from Fitch's graph (1954:84, Fig. 13), show that relative success of growth in the period he considered can be ranked by year, in descending order, as: 1951, 1949, 1948, 1950, 1952. This corresponds closely to the sequence, 1951, 1948, 1949, 1950, 1952, for T. ornata.
Growth almost stops after the thirteenth year in females and after the eleventh or twelfth year in males, approximately three years, on the average, after sexual maturity is attained. The oldest individuals in which plastral length had increased measurably in the season of capture were females 14 (2 specimens) and 15 (1) years old. The age of the oldest growing male was 13 years.
The germinal layer of the epidermis probably remains semiactive throughout life but functions chiefly as a repair mechanism in adults that are no longer growing. Growth-rings continue to form irregularly in some older adults. Growth-rings formed after the period of regular growth are so closely approximated that they are unmeasurable and frequently indistinguishable to the unaided eye. If the continued formation of growth-rings is not accompanied by wear at the edges of the laminae, the laminae meeting at an interlaminal seam descend, like steps, into the seam (Pl. 22, Fig. 2). Interlaminal seams of the plastron deepen with advancing age in most individuals.
Some individuals that are well past the age of regular growth show measurable increments in years when conditions are especially favorable. The three oldest growing females were collected in 1954—an exceptionally good year for growth. Allowing some latitude for irregular periods of growth in favorable years subsequent to the period of regular, more or less steady growth, 15 to 20 years is a tenable estimate of the total growing period.
Practically nothing is known about longevity in T. ornata or in other species of Terrapene although the several plausible records of ages of 80 to more than 100 years for T. carolina (Oliver, 1955:295-6) would indicate that box turtles, as a group, are long-lived. There is no known way to determine accurately the age of an adult turtle after it has stopped growing. It was possible occasionally to determine ages of 20 to 30 years with fair accuracy by counting all growth-rings (including those crowded into the interabdominal seam) of specimens having unworn shells. Without the presence of newly formed epidermis as a landmark, however, it was never certain how many years had passed since the last ring was formed.
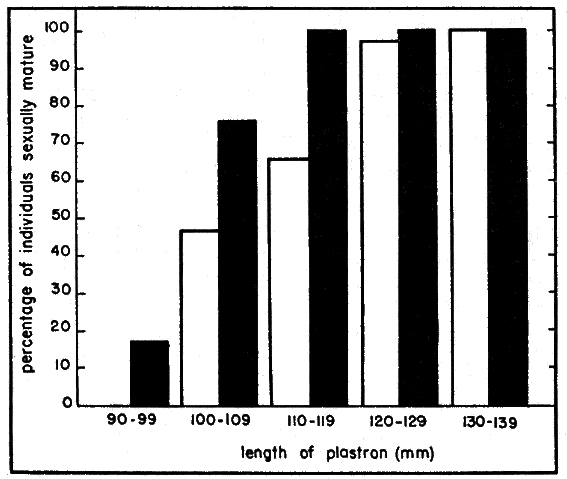
Mattox (1936) studied annual rings in the long bones of painted turtles (Chrysemys picta) and found fewer rings in younger than in older individuals but, beyond this, reached no important conclusion. [Pg 586] In the present study, thin sections were ground from the humeri and femurs of box turtles of various ages and sizes; the results of this investigation were negative. Distinct rings were present in the compact bony tissue but it appeared that, after the first year or two, the rings were destroyed by encroachment of the marrow cavity at about the same rate at which they were formed peripherally.
The only methods that I know of to determine successfully the longevity of long-lived reptiles would be to keep individuals under observation for long periods of time or to study populations of marked individuals. Both methods have the obvious disadvantage of requiring somewhat more than a human lifetime to carry them to completion. Restudy, after one or more decades, of the populations of turtles marked by Fitch and myself may provide valuable data on the average and maximum age reached by T. ornata.
Ornate box turtles probably live at least twice as long as the total period of growing years. An estimated longevity of 50 years would seem to agree with present scant information on age. Considering environmental hazards, it would be unusual for an individual to survive as long as 100 years in the wild.
Weights of ornate box turtles varied so much that no attempt was made to correlate weight with size. Absolute weights have little significance since weight is affected to a large extent by the amount of fluid in the body. Turtles that had recently imbibed were naturally heavier than those that had not; turtles brought to the laboratory and kept there for several days lost weight by evaporation and by voiding water. Weights of 22 adult females (53 records) and 10 adult males (22 records) averaged 391 and 353 grams respectively, in the period from September, 1954, to October, 1956. Females characteristically gained weight in spring and early summer and were lighter after nesting. Turtles of both sexes gained weight in September and October.
At the time of hatching, fontanelles remain where bones of the shell have not yet articulated with their neighbors. In general, the fontanelles of the shell are closed by the time sexual maturity is attained, but some remain open a year or two longer.
The fontanelles of the shell are classified as follows (see Figs. 14 to 16 and 18 to 19):
1.) Anteromedian. Rhomboidal; limited anteriorly by hyoplastral bones and posteriorly by hypoplastral bones; posterior tip of entoplastral bone may project into this fontanelle.
2.) Posteromedian. Limited anteriorly by hypoplastral bones and posteriorly by xiphyplastral bones (since hypoplastral bones do not articulate medially in hatchlings, anteromedian and posteromedian fontanelles form a single, more or less dumbbell-shaped opening).
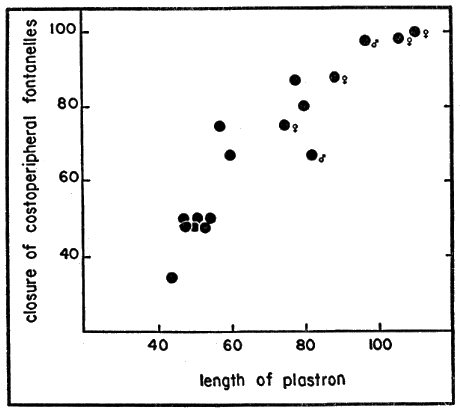
1.) Costoperipheral. Openings between the free ends of developing ribs, between nuchal bone and first rib, and, between pygal bone and last rib; limited laterally by peripheral bones; variable in shape.
2.) Costoneural. Triangular openings on either side of middorsal line between proximal ends of costal plates and developing neural plates.
The costoneural fontanelles are nearly closed in individuals [Pg 588] of the 70 millimeter (plastron length) class and seldom remain open after a length of 80 millimeters is attained (Fig. 14). Of the costoperipheral fontanelles, the anterior one (between first rib and nuchal bone) closes first and the posterior one (between last rib and pygal bone) last. It remains open in some turtles in which the plastron is longer than 100 millimeters. The remaining costoperipheral fontanelles close in varying sequence but those in the area of the bridge (nos. 2 to 5), where there is presumably greater stress on the shell, close sooner than the others.
The plastral fontanelles are closed in most specimens of the 90 millimeter (plastron length) class; the anteromedian fontanelle closes first.
The meager covering of the fontanelles makes juvenal turtles more susceptible than adults to many kinds of injuries and to predation.
Parts of the shell that are more or less movable upon one another and that function in closing the shell are found in several families of Recent turtles. African side-necked terrapins of the genus Pelusios have a movable forelobe on the plastron. Kinosternids have one or two flexible transverse hinges on the plastron. In the Testudinidae the African Kinixys has a movable hinge on the posterior part of the carapace and Pyxis arachnoides of Madagascar has a short, hinged, anterior plastral lobe. Certain trionychid turtles, such as Lissemys, utilize the flexible flaps of the carapace (the flaps of some species are reinforced with peripheral bones) to close the shell.
Movable shell-parts of turtles are, in general, protective in function; they cover parts of the soft anatomy that would otherwise be exposed.
A hinged plastron, capable of wholly or partly closing the shell, occurs in six genera of the family Emyidae (see introduction). In these emyids the plastron is divided into two lobes, which are joined to each other by ligamentous tissue at the junction of the hyoplastral and hypoplastral bones; externally, the hinge occurs along the seam between the pectoral and abdominal laminae. This junction forms a more or less freely movable hinge in adults. The plastron is attached to the carapace by ligamentous tissue. Both lobes of the plastron or only the buttresses of the hind lobe may articulate with the carapace. The former condition obtains in Emys and Emydoidea; the latter more specialized condition is found in Terrapene.
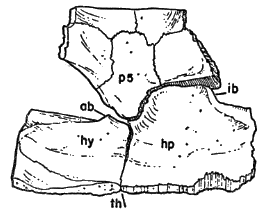
In generalized emyid turtles such as Clemmys there are no movable shell parts. The plastron is joined to the carapace by the sutures of the bridge. A long stout process, the axillary buttress, arises on each side from the hyoplastron and articulates with the tip of the first costal. A similar process, the inguinal buttress, arises from the anterior part of each of the hypoplastral elements and meets the sixth costal on each side. The buttresses form the anterior and posterior margins of the bridge. It is clear that movement of the plastron in many emyids is mechanically impossible because of the bracing effect of the buttresses.
In Terrapene the movable articulations of the shell are neither structurally nor functionally developed in juveniles. Adults of T. ornata have highly modified bony buttresses on the plastron that are homologous with those in more generalized emyids. The inguinal buttresses are low and wide, and have a sheer lateral surface forming a sliding articulation with the fifth and sixth peripheral bones of the carapace. The axillary buttresses are reduced to mere bony points near the posterolateral corners of the forelobe and do not articulate directly with the carapace (Figs. 15 and 16).
The fifth peripheral bone, constituting the lowest point of the carapace, has a medial projection that acts as a pivoting point for both lobes of the plastron; the roughened anterior corners of the hind lobe articulate with these processes. The roughened posterior corners of the forelobe of the plastron likewise articulate with these processes. The posterior process or "tail" of the entoplastron extends to, or nearly to, the bony transverse hinge.
In juveniles that have been cleared and stained, the homologues of the parts that are movable in adults are easily identifiable; the proportions of these parts and their relations to one another are, however, much different.
In juveniles (Figs. 18 and 19) the buttresses are relatively longer and narrower, and are distinct—more nearly like those of generalized emyids than those of adult T. ornata. The buttresses enclose a large open space, which in adults is filled by the fifth peripheral. The hyoplastral and hypoplastral bones are in contact only laterally. They are firmly joined by bony processes; the interdigitating nature of this articulation contrasts with its homologue in the adult, the point where the roughened corners of the forelobes and hind lobes meet. The fifth peripheral in juveniles (Fig. 19) lies dorsal to this articulation. The position of the future transverse hinge corresponds to a line passing through the articulations of the hyoplastra [Pg 590] and hypoplastra. The tail of the entoplastron ordinarily extends posterior to this line in juveniles.
The external scutellation of the plastral hinge in adults also differs from that in juveniles. In adults (Fig. 17 and Pl. 22) the transverse hinge is marked by ligamentous tissue between the pectoral and abdominal laminae; the forelobe of the plastron is distinctly narrower than the hind lobe. Two small scales lie near the corner of the hinge on each side. The larger and more anterior of these scales is the axillary; it is present in box turtles of all ages. The smaller scale (Fig. 17), to my knowledge, has never been named or mentioned in the literature; it is herein termed the apical scale. It is a constant feature in adults but is always lacking in hatchlings and small juveniles. Other scales, much smaller than the axillary [Pg 591] and apical, occur on the ligamentous tissue of the hinge of some adults.
 Fig. 18. Plastron of hatchling
(× 2), cleared and stained to show bony structure. (Abbreviations not
listed in legend for Fig. 15 are as follows: af, anteromedian fontanelle;
ep, epiplastron; pf, posteromedian fontanelle.)
|
||
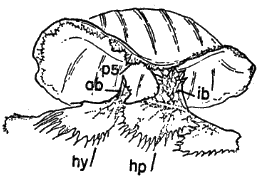 Fig. 19. Carapace of hatchling
(× 1½), cleared and stained to show bony structure; lateral view;
anterior end at left. (Abbreviations as in Fig. 15.)
|
 Fig. 20. Lateral view of hatchling
(× 1); note the lateral process of the pectoral lamina (pl) extending
posterior to the axillary scale (ax) in a position corresponding to the apical
scale of adults. There is no external indication of the transverse hinge in young
individuals. The yolk sac of this individual has been retracted but the umbilicus
(umb) has not yet closed.
|
|
In juveniles (Fig. 20) the pectoroabdominal seam contains no ligamentous tissue and is like the other interlaminal seams of the plastron. A lateral apex of the pectoral lamina projects upward behind the axillary scale on each side, in the position occupied by the apical scale of adults. Examination of a large series of specimens revealed that the apical scale of adults becomes separated from the lateral apex of the pectoral lamina at approximately the time when the hinge becomes functional as such.
Ontogenetic changes in the shell can be summarized as follows: [Pg 592] 1) Buttresses become less distinct in the first two years of life (plastral lengths of 40 to 55 mm.); 2) Interdigitating processes of the forelobes and hind lobes become relatively shorter and wider, the entoplastron no longer projects posterior to the hinge, the lateral apex of the pectoral lamina becomes creased, and some movement of the plastron can take place between the second and third years (plastral lengths of 55 to 65 mm.); 3) Plastral lobes become freely movable upon one another and upon the carapace by the end of the fourth year (plastral length approximately 70 mm.) in most individuals.
The plastron of a juvenal box turtle is not completely immovable. The bones of the shell are flexible for a time after hatching and allow some movement of the plastron; but the relatively greater bulk of the body in young box turtles would prevent complete closure of the shell even if a functional hinge were present. Hatchlings can withdraw the head and forelegs only to a line running between the anterior edges of the shell. To do so the rear half of the shell is opened and the hind legs are extended. When the head and forelegs are retracted to the maximum, the elbow-joints are pressed against the tympanic region or behind the head; the fore-limbs cannot be drawn part way across the snout, as in adults. Hatchlings can elevate the plastron to an angle of approximately nine degrees; the plastron of an adult, with shell closed, is elevated about 50 degrees. Hatchlings flex the plastron chiefly in the region of the humeropectoral seam, rather than at the anlage of the transverse hinge.
Adult box turtles, when walking, characteristically carry the forelobe of the plastron slightly flexed. This flexion of the plastron, combined with its naturally up-turned anterior edge, cause it to function in the manner of a sled runner when the turtle is moving forward. A movable plastron, therefore, in addition to its primarily protective function, seems to aid the turtle in traveling through tall grass or over uneven ground. The gular scutes, on the anterior edge of the forelobe, become worn long before other plastral laminae do.
An adult female from Richland County, Illinois, had an abnormal but functional hinge on the humeropectoral seam in addition to a normal hinge on the pectoroabdominal seam. The abnormal hinge resulted from a transverse break in which ligamentous tissue later developed. The muscles closing the plastron moved the more anterior of the two hinges; the normal hinge was not functional.
The markings of the shell change first when postnatal growth begins and again when sexual maturity is attained. They are modified gradually thereafter as the shell becomes worn.
In hatchlings the ground color ordinarily is dark brown but in some individuals is paler brown or tan. Markings on the dark background are pale yellow. Markings on the central and lateral scutes vary from a regularly arranged series of well defined spots and a middorsal stripe to a general scattering of small flecks. In some specimens the pale markings of the carapace are faint or wanting. Lateral parts of marginal scutes are always pale yellow and form a border around the carapace.
Close examination of the carapace of any hatchling shows the following basic arrangement of markings: each lateral scute has a centrally placed pale spot and four to seven smaller pale marks arranged around the edge of the scute; each central scute has a central, longitudinal mark and several (usually two, four, or six) smaller pale marks arranged around the edge of the scute, chiefly the lateral edges (Pl. 23). Variations in pattern result when some or all of the markings divide into two or more parts.
By the end of the first full season of growth, the markings have a radial pattern. At this stage, the markings of the areola, with the exception of the central spot, are obscure. The radial marks, sharply defined and straight-sided, appear only on the newly formed parts of the epidermal laminae. Each radial mark originates opposite one of the peripheral marks of the areola. Other radial marks are developed later by bifurcation of the original radiations.
The ground color of the plastron of hatchlings is cream-yellow, or less often, bright yellow. The solid, dark brown markings on the medial part of each lamina form a central dark area that contrasts sharply with the pale background (Pl. 24). The soft tissue of the navel is pale yellow or cream; when the navel closes, the dark central mark of the plastron is unbroken except for thin, pale lines along the interlaminal seams.
When growth begins, the areas of newly formed epidermal tissue on the anterior and medial borders of each areolar scute are pale. Wide, dark radial marks, usually three per scute, appear on the newly formed tissue. Subsequently, finer dark radiations appear between the three original radiations. The wide radiations later bifurcate. By the time adult or subadult size is reached, the plastron [Pg 594] appears to have a pattern of pale radiations on a dark background. In general, the markings of the plastron are less sharply defined than the markings of the carapace (Pl. 24).
There is a tendency for the dark markings of the plastron to encroach on the lighter markings, if no wear on the shell occurs. However, as the plastron becomes worn, the pale areas become more extensive and the dark markings become broken and rounded. Severely worn plastra of some old individuals lack dark markings. Wear on the carapace produces the same general effect; but markings of the carapace, although they may become blotched, are never obliterated in Terrapene o. ornata.
The top of the head in most hatchlings is dark brown, approximately the same shade as the ground color of the carapace; the part anterior to the eyes is usually unmarked but a few individuals have a semicircle of small pale spots over each eye or similar spots on much of the head. The posterior part of the head is ordinarily flecked with yellow. The skin on the top of the head, particularly between the eyes, is roughened. The granular skin of the neck is grayish brown to cream-yellow. There are one or two large pale spots behind the eye and another pale spot at the corner of the mouth. Smaller, irregularly arranged pale markings on the necks of some specimens form, with the post-orbital and post-rictal spots, one or two short, ragged stripes. The gular region is pale.
In juveniles, the yellow markings of the head and neck are larger and contrast more sharply with the dark ground color than in hatchlings. Markings above the eyes, if present, fuse to form two pale, semicircular stripes. In some older juveniles yellow marks on top of the head blend with the dark background to produce an amber color. The top of the neck darkens or develops blotches of darker color that produce a mottled effect. Spots and stripes on the side of the neck remain well defined. The skin on top of the head becomes smooth and shiny.
Adult females tend to retain the color and pattern of juveniles on the head and neck although slight general darkening occurs with age. Many adult females have the top of the head marked with bright yellow spots. In adult males, the top and sides of the head, anterior to the tympanum, are uniformly grayish green or bluish green; the mandibular and maxillary beaks are brighter, yellowish green. Markings on the head and neck of most adult males are obscure (Pl. 25) but the sides of the neck remain mottled in some individuals.
The antebrachium has large imbricated scales and is distinctly [Pg 595] set off from the proximal part of the foreleg which is covered with granular skin. The antebrachial scales of hatchlings are pale yellow; each scale is bordered with darker color. General darkening of the antebrachium occurs at puberty. In adult females each scale on the anterior surface of the antebrachium is dark brown and has a contrasting yellow, amber, or pale orange center. The anterior antebrachial scales of adult males are dark brown to nearly black and have bright orange or red centers. Old males have thickened antebrachial scales.
The iris of hatchlings and juveniles is flecked with yellow and brown; the blending of these colors makes the eye appear yellow, golden, or light brown when viewed without magnification. Adult females retain the juvenal coloration of the eye; the iris of adult males is bright orange or red. The work of Evans (1952) on T. carolina suggests that eye color in box turtles is under hormonal control.
Presence or absence of areolae on laminae of the shell indicated degree and sequence of wear. The anterior edges of carapace and plastron, and the slightly elevated middorsal line (Pl. 23) wear smooth in some individuals before the first period of hibernation. Subsequent wear on the carapace proceeds posteriorly. For example, turtles that retained the areola of the third central lamina, retained also the areolae of the fourth and fifth centrals; when only one central areola remained, it was the fifth. Lateral laminae wear in the same general sequence. The areola of the fifth central lamina, because of its protected position, persists in adult turtles that are well past the age of regular growth. Areolae that are retained in some older turtles are shed along with the epidermal layers formed in the first year or two of life. Wear on the shell is probably correlated with the habits of the individual turtle; smoothly-worn specimens varied in size and age but were usually larger, older individuals. No smoothly worn individual was still growing.
Wear on the plastron is more evenly distributed than wear on the carapace; wear is greatest on the lowest points of the plastron (the gular laminae, the anterior portions of the anal laminae, and the lateral edge of the tranverse hinge).
The claws and the horny covering of the jaws are subject to greater wear than any other part of the epidermis; presumably they continue to grow throughout life. The occasional examples of hypertrophied beaks and claws that were observed, chiefly in [Pg 596] juveniles, were thought to result from a continuous diet of soft food or prolonged activity on a soft substrate. Ditmars (1934:44, Fig. 41) illustrated a specimen of T. carolina, with hypertrophied maxillary beak and abnormally elongate claws, that had been kept in a house for 27 years.
The conformation of the maxillary beak in all species of Terrapene is influenced to a large extent by wear and is of limited value as a taxonomic character. The beak of T. ornata is slightly notched in most individuals at the time of hatching and remains so throughout life. The underlying premaxillary bone is always notched or bicuspidate. The sides of the beak are more heavily developed than the relatively thin central part. Normal wear on the beak maintains the notch (or deepens it) in the form of an inverted U or V, much in the manner of the bicrenate cutting edge on the grooved incisors of certain rodents. In a series of 34 specimens of T. ornata from Kansas, selected at random from the K. U. collections, 92 per cent had beaks that were "notched" to varying degrees, four per cent had hooked (unnotched) beaks, and four per cent had beaks that were flat at the tip (neither hooked nor notched).
Differences between adult males and females of T. ornata have been mentioned in several places in the preceding discussion of growth and development. Several sexual characteristics—greater [Pg 597] preanal length, thickened base of the tail, slightly concave plastron, and smaller bulk—are found also in males of many other kinds of emyid turtles. From females, males of T. ornata are most easily distinguished by the bright colors of their eyes, heads, and antebrachial scales. An additional, distinctive characteristic of males is the highly modified hind foot. The first toe is greatly thickened and widened; when the foot is extended, the first toe is held in a horizontal plane nearly at right angles to the medial edge of the plantar surface (Fig. 21). The hind foot of females is unmodified in this respect. Males tend to have heavier, more muscular hind legs than females.
The bright colors of males are maintained throughout the year and do not become more intense in the breeding season. Males of T. o. luteola become melanistic in old age whereas males of the subspecies ornata do not. In old males of luteola the skin becomes dark gray, bluish, or nearly black and much of the bright orange or red of the antebrachial scales and the green of the head is obliterated; the iris may also darken but in most specimens it retains some red. Females of luteola tend also to darken somewhat in old age but not so much as males; females of ornata do not. Table 4 summarizes the more important secondary sexual characters of T. ornata.
Table 4.—A Summary of Sexual Dimorphism
in Terrapene ornata |
||
| Character | Males | Females |
| Head | Snout truncate in lateral profile, top of head and front of maxilliary beak forming an angle of nearly 90°; head yellowish green to bluish green; markings on head and neck reduced; head never spotted dorsally (Pl. 19, Figs. 7 and 8). | Snout relatively round in lateral profile; front of maxillary beak not forming right angle with top of head; head dark brown, distinct pale markings on head and neck; head commonly spotted dorsally (Pl. 25, Figs. 5 and 6). |
| Iris | Red | Yellowish brown |
| Hind legs | Heavy and muscular; first toe turned in, thickened, and widened (Fig. 21). | Not especially heavy or muscular; first toe, if turned in, never thickened or widened (Fig. 21). |
| Forelegs | Centers of antebrachial scales bright orange or red. | Centers of antebrachial scales yellow, pale orange, or brown. |
| Carapace | Relatively lower, length contained in height (48 specimens) .58 times (± .005σm, range, .50 to .69). | Relatively higher, length contained in height (94 specimens) .50 times (± .005σm, range .44 to .60). |
| Plastron (hind lobe) | Ordinarily slightly concave. | Flat or convex, never concave. |
Tolerances to environmental temperatures, and reactions to thermal stimuli influence the behavior of ectothermal animals to a large extent. Terrapene ornata, like other terrestrial reptiles inhabitating open grassland, is especially subject to the vicissitudes of environmental temperature. Other species of turtles living in the same area are more nearly aquatic and therefore live in a microhabitat that is more stable as regards temperature.
Approximately 500 temperature readings in the field and many others in the laboratory were obtained from enough individuals to permit interpretation of reactions involved in basking, in seeking cover, and in emerging from temporary periods of quiescence at various times of the day.
Box turtles commonly used open places such as cow paths, ravines, and wallows, for basking as well as for feeding and as routes of travel. Burrows, dens beneath rocks, and forms, were used as shelter from high and low temperatures as well as from predators. Determining whether a turtle was truly active (moving about freely, feeding, or copulating), was basking, or was seeking shelter was difficult because the turtle sometimes reacted to the observer; for instance, basking turtles, whose body temperatures were still suboptimum, might take cover when surprised, and truly active turtles might remain motionless and appear to be basking. By scanning open areas from a distance with binoculars, an observer frequently could determine what turtles were doing without disturbing them. In the final analysis of data, temperature records accompanied by data insufficient to determine correctly the state of activity of the turtle, were discarded, as were temperature records of injured turtles and turtles in livetraps.
Cowles and Bogert (1944:275-276) and Woodbury and Hardy (1948:177) emphasized the influence of soil temperatures on body temperatures. It is thought that air temperatures played a more important role than soil temperatures in influencing body tempera [Pg 599]tures of T. ornata. Soil temperatures were taken in the present study only when the turtle was in a form, hibernaculum, or den.
Cowles and Bogert (1944:277) determined optimum levels of body temperature of desert reptiles by averaging body temperatures falling within the range of normal activity; they defined this range as, "… extending from the resumption of ordinary routine [activity] … to … a point just below the level at which high temperatures drive the animal to shelter." Fitch (1956b:439) considered optimum body temperature in the several species that he studied to be near the temperature recorded most frequently for "active" individuals; he found (loc. cit.) that of body temperatures of 55 active T. ornata, 66 per cent were between 24 and 30 degrees, and that the temperatures 27 and 28 occurred most frequently. Fitch concluded (op. cit.:473) that the probable optimum body temperature of T. ornata was 28 degrees and that temperatures from 24 to 30 degrees were preferred. Although Fitch treated all non-torpid individuals that were abroad in daytime as "active" and did not consider the phenomenon of basking, his observations on optimum body temperature agree closely with my own.
Body temperatures of 153 box turtles that were known definitely to be active, ranged from 15.3 to 35.3 degrees. The mean body temperature for active turtles was 28.8 degrees (± 3.78σ) (Fig. 22). Ninety-two per cent of the temperatures were between 24 and 30 degrees and 50 per cent were between 28 and 32; temperatures of 29 and 30 degrees occurred most frequently (22 and 21 times, respectively). The ten body temperatures below 24 degrees all were recorded before 9 A. M. on overcast days when the air was cool and humid. It is noteworthy that two of these low temperatures (18.8° and 19.0°) were from a copulating pair of turtles; two others (21.8° and 22.0°) were from individuals that were eating. The highest temperature (35.3°) was from a large female that was feeding at mid-morning in a partly shaded area.
The mean body temperature for active individuals (Fig. 22) is probably somewhat below the ecological optimum, because a few temperatures were abnormally low. The large number of body temperatures in the range of 29 to 31 degrees indicates an optimum closer to 30 degrees. Optimum body temperatures may vary somewhat with the size, sex, or individual preference of the turtle concerned.
Although basking is common in terrestrial turtles, only a few authors have mentioned it. Woodbury and Hardy (1948:177-178) did not use the term in their account of thermal relationships in Gopherus agassizi; their discussion indicates, however, that the tortoises move alternately from sunny to shady areas to regulate body temperature. Desert tortoises removed from hibernacula and placed in the sun warmed to approximately 29.5 degrees before they became active, although a few did so at temperatures as low as 15 degrees. According to Cagle (1950:45), Sergeev (1939) studied body temperature and activity in the Asiatic tortoise, Testudo horsefieldi, and found that individuals basked for as much as two hours in the morning before beginning the first activity of the day (feeding), but that tortoises did not bask after a period of quiescense from late morning to late afternoon, during which body temperatures were seemingly maintained nearer the optimum than they were during nocturnal rest; body temperatures rose to approximately 30 degrees before the tortoises became active. Since body temperatures of 23 to 24 degrees were maintained at night, the basking range of Testudo horsefieldi may be considered to be approximately 23 to 32 degrees.
Ornate box turtles basked chiefly between sunrise and 10 or 11 A. M. Body temperatures of 60 basking turtles ranged from 17.3 to 31.4 degrees (mean, 25.5 ± 3.08σ). More than two-thirds (42) of these body temperatures were higher than the air temperature near the turtle, indicating probably that body temperature rises rapidly once basking is begun. In the instances where body temperature was below air temperature, the turtles had recently begun to bask (many were known to have just emerged from forms or other cover where they had spent the night) or were warming up more slowly because of reduced sunlight. On cloudy days basking began later than on clear days and body temperatures usually remained at a suboptimum level. Turtles that basked on days that were cloudy and windy, or cold and windy, did so in sheltered places, usually on the leeward sides of windbreaks such as limestone rocks, rock fences, or ravine banks. It was evident in these instances that the turtles either sought such shelter from the wind or remained ensconced in the more complete shelter of a form or burrow, not emerging at all.
Open areas of various kinds were used as basking sites. Level ground—such as on roads, cattle pathways, and bare areas surrounding [Pg 601] farm ponds—having unobstructed morning sunlight, nearby dense vegetation, and choice opportunities for feeding (cow dung, mulberry trees) was preferred. Basking was frequently combined with feeding; in several instances box turtles were noted early in the morning at suboptimum body temperatures eating grasshoppers, berries, or dung insects. The predilection of box turtles for open areas is probably important in permitting extended activity at suboptimum temperatures. T. ornata probably carries on more nearly normal activity on cool days than do reptilian species with more sharply delimited thermal tolerances. Collared lizards (Crotaphytus collaris), for example, are chiefly inactive on days when the sky is overcast, although a few individuals having suboptimum body temperatures can be found in open situations (Fitch, 1956a:229 and 1956b:442).
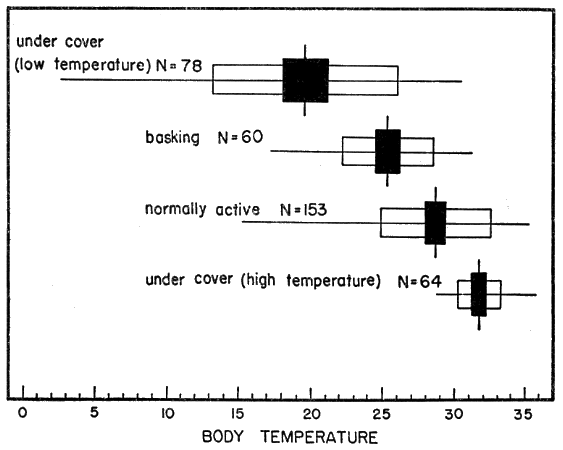
The foregoing remarks on basking indicate the approximate, normal, thermal tolerances of ornate box turtles. Many additional records of body temperature were taken from turtles that were found under cover. Turtles under cover in daylight were usually [Pg 602] seeking protection from either below-optimum or above-optimum temperatures. In avoiding low temperatures, turtles usually chose more complete and permanent cover than in avoiding high temperatures.
Body temperatures of 64 box turtles that were seeking cover or that were under cover because of high temperatures ranged from 28.9 to 35.8 degrees (mean, 31.9 ± 1.55σ). Fifty-nine of these temperatures (92 per cent) were 30 degrees or higher. Figure 22 shows this range to overlap broadly with the temperature range of active turtles and the means of the two groups are close to each other. Body temperatures below 30 degrees (5) were all recorded late in the morning on hot summer days when the air temperature was well above 30 degrees; they are somewhat misleading because they are from turtles that were under cover long enough to lower body temperature to the range of activity although the turtles remained under cover because of hazardous environmental temperatures.
The commonest retreats used by box turtles to escape heat were burrows of other animals and small dens under thick limestone rocks, where the air remained cool, even in late afternoon. Most of the burrows and dens on the Damm Farm were known to me and could be checked each day. Turtles seeking temporary refuge from high temperatures characteristically rested just inside the opening of a den or burrow. Less frequently, turtles burrowed into ravine banks or just under the sod on level ground. A number of individuals with above-optimum body temperatures were found in the shade of trees or high weeds in early afternoon on hot days. Mulberry trees provided ample shade for such activity and, in June and July, when ripe mulberries were abundant on the ground, turtles frequently fed on them at times of the day when temperatures were more hazardous in other areas.
Several turtles were found buried in mud or immersed in water at the edges of ponds in the hottest part of the day; they were discovered at first by accident and, on subsequent field trips by systematic probing. Ordinarily the turtles were covered with mud or muddy water and remained motionless, except for periodically raising the head to the surface to breath. There was little vegetation near the edges of ponds and by late morning on hot days the temperature of the shallowest water was as high as the air temperature or higher. Correspondingly, turtles found resting in mud and water had body temperatures much higher than turtles in dens, burrows, or forms at the same time of day. Box turtles that retreat [Pg 603] to mud or shallow water cool themselves less efficiently than they would in drier, better protected microhabitats. I found no evidence that turtles went into deeper water to cool themselves.
The length of time spent under cover varied; most turtles had two daily periods of activity, the second beginning in late afternoon. Some turtles moved from shelter to shelter in the time between periods of activity. Several turtles were known to remain quiescent continuously for several days in the hottest part of the summer.
The maximum temperature that a reptile can tolerate physiologically is ordinarily higher than the maximum temperature tolerated voluntarily (Cowles and Bogert, 1944:277); but, the two maxima may be separated by only a few degrees. Most poikilothormous vertebrates neither tolerate nor long survive body temperatures exceeding 40 degrees (Cowles and Bogert, op. cit.:269).
It is evident (Fig. 22) that ornate box turtles do not often tolerate body temperatures above 33 degrees and that temperatures in excess of 35 degrees are probably never tolerated under natural conditions. At 9:15 A. M. on July 5, 1955, an adult female emerged from mud where she had spent the night (body temperature 28.4°, mud 28.4°, air 30°). After foraging for 40 minutes in bright sunlight on a grassy hillside she had moved approximately 100 feet and her temperature had reached 34.6 degrees (air 33.0°). At 9:56 A. M. she moved rapidly and directly to a den under a rock nearby; 15 minutes later her body temperature had not changed but after 65 minutes it had dropped to 33.4 degrees. The temperature of air in the den was 31 degrees. This female began her activities at nearly optimum body temperature relatively late in the morning and, by foraging intensively for less than one hour, probably was able nearly to satisfy her daily food requirements; by foraging near suitable cover she could remain active until her body temperature reached a critical threshold, and she thereby saved time otherwise required for finding cover or making a form.
The following observations, extracted from field notes, indicate that body temperatures near 40 degrees are the approximate lethal maximum and are well above those temperatures voluntarily tolerated by T. ornata. On July 4, 1955, a subadult female was in the water at the edge of a pond. The temperatures of the air, water, and turtle were 32.0, 30.6, and 30.2 degrees, respectively. At 11 A. M. the turtle was tethered in direct sunlight on the hard-baked clay of the pond embankment (temperature of air 33.4°). The turtle's response to steadily rising body temperature over a period of 31 minutes is illustrated by the following notes.
| Time (A. M.) |
Body temperature |
Remarks |
| 11:00 | 33.0 | Tethered on slope. |
| 11:05 | 34.6 | Strains at tether in several directions. |
| 11:09 | 36.5 | Tries frantically to get away; draws in limbs and head rapidly and momentarily at any movement on my part, and hisses loudly. |
| 11:13 | 37.5 | Mouth held open slightly; turtle overturns in effort to escape; frantic scrambling resumed a few seconds after I right turtle. |
| 11:17 | 38.2 | Mouth now held open most of the time; white froth begins to appear around mouth. |
| 11:20 | 38.6 | Stops activities every 10 seconds or so, rests chin on ground and gapes widely; will still pull into shell when prodded with stick. |
| 11:23 | 39.2 | Still wildly active; continues to gape widely every few seconds. |
| 11:27 | 39.4 | Frothing at mouth profusely. |
| 11:30 | 39.6 | Attempts to escape are now in short feeble bursts. Turtle released; crawls toward me and immediately seeks shade of my body; when I move off, turtle seeks shade of small isolated weed on pond embankment; turtle removed to damp earth at edge of pond. |
| 11:35 | 39.5 | Attempts to burrow into mud at edge of pond. |
| 11:36 | Enters shallow water and moves slowly back to shore. | |
| 11:37 | 38.8 | Turtle thrown into center of pond where it remains motionless and drifts with wind to opposite shore; remains inactive in mud and shallow water at edge of pond; temperature of water near turtle 35.5. |
| 11:57 | 35.0 | Moves 50 ft. up slope to shade of low vegetation. |
| 1:55 P. M. | 32.5 | Turtle has not moved. |
The overheating may have incapacitated the turtle since it moved only 50 feet in the next two days; its body temperatures on the two days subsequent to the experiment were 26.8 and 20.6, respectively.
The mentioned gaping, as in higher vertebrates generally, cools the animal by evaporation from the moist surfaces of the mouth and pharynx. By keeping the mouth open for more than a few minutes at a time in hot dry weather, a turtle would surely lose body water in amounts that could not always be easily replaced. Ornate box turtles seem to utilize evaporation for cooling only in emergencies and rely for the most part on radiation and conduction [Pg 605] to lower body temperature after reaching a relatively cool, dark retreat.
Box turtles were never active at body temperatures below 15 degrees and were seldom active at temperatures below 24 degrees. The two lowest temperatures (15.3° and 16.3°) were taken from individuals crossing roads on overcast days in early May.
In 78 box turtles that were under cover because their environmental temperatures were low, the body temperatures ranged from 2.7 to 30.6 degrees (mean 19.8 ± 6.38σ). The range of body temperatures in this group is greater than in the other groups shown in Figure 22 because low body temperatures were studied over a wide range of conditions, including hibernation.
Box turtles actually seek cover because of low temperatures only in fall and spring and on occasional unseasonable days in summer when temperatures drop rapidly. Retreat to cover, in the normal cycle of daily activity, is governed usually by high temperatures at mid-day or by darkness at the end of the day. Turtles in dens, burrows, and grass forms, tended to burrow if temperatures remained low for more than a few hours.
Box turtles under cover where they cannot bask have little control over the lower range of body temperatures. The freezing temperatures of winter can be escaped by burrowing deeper into the ground. Temperatures approaching the lethal minimum, however, seldom occur during the season of normal activity. By remaining hidden in a burrow or den therefore, box turtles are fairly well protected from predators but are at a thermal disadvantage.
A number of turtles that had wet mud on their shells were found basking in early morning near ditches, ponds, and marshy areas; several others were partly buried in mud, shortly after daybreak, and another was at the edge of a pond after dark.
Eight adults, located just as they emerged from cover in early morning on sunny days, had body temperatures of 19.7, 21.9, 24.2, 24.5, 25.8, 26.6, 28.7, and 29.5 degrees. In five emerging from earth forms, body temperatures were at least a degree or two below the temperature of the air; the other three came from mud or shallow water and had body temperatures higher than the air temperature.
Temperature is probably the primary stimulus governing emergence after temporary periods of quiescence. Turtles in earthen forms are usually completely covered or are head downward with only the hind quarters exposed. Obviously, the more thoroughly [Pg 606] a turtle protects itself (beneath the insulating cover of a form, burrow, or den) against unfavorable temperatures, the longer it will take for favorable temperatures to bring about normal activity again. Turtles in forms and deep burrows have a minimum of contact with the outer environment; but in dens beneath rocks and in shallow burrows light and air can enter freely. Turtles might be influenced in their activities to some extent by the intensity of light at the opening of a burrow or den; they are surely stimulated by changes in the temperature and humidity of air coming through the opening. Shallow retreats that a turtle can enter and leave with the least effort therefore seem most efficient for purposes of thermocontrol, especially when they provide earthen surfaces into which the turtles can burrow more deeply if more severe environmental conditions develop.
In October, 1955, nine T. ornata of various sizes, collected in Douglas County, Kansas, were brought to the laboratory for observation under conditions of controlled temperature. They were kept at room temperature for several days and were fed regularly, with the exception of one hatchling that was fed nothing in this period. On October 22 the turtles were placed in a room where the temperature was maintained constantly at zero degrees. One of the nine turtles, an adult female, was killed with chloroform immediately prior to its removal to the cold room. A list of the turtles used in this experiment is given below.
| Age class |
Carapace length in mm. |
Weight in grams |
| 1) Hatchling | 33.1 | 8.4 |
| 2) Hatchling [A] | 29.9 | 6.7 |
| 3) Juvenile | 52.5 | 29.3 |
| 4) Juvenile | 50.2 | 26.1 |
| 5) Adult ♂ | 125 | 376 |
| 6) Adult ♀ | 118 | 400 |
| 7) Adult ♂ | 119 | 386 |
| 8) Adult ♀ | 110 | 325 |
| 9) Adult ♀ | 115 | —— |
| [A] Starved. | ||
Turtles were kept in the cold room for periods of 100 minutes (hatchlings and juveniles) and 200 minutes (adults). The entire experiment, including the time in which the turtles were allowed to warm after they were taken from the cold room, covered a period of nearly six hours (375 minutes) during which the turtles were under constant observation. Individual body temperatures were taken continuously in this period (39 for each juvenile and 24 for each adult) in the order that the turtles were numbered; gaps between records of the body temperature of a given individual [Pg 607] therefore represent the time required to record temperatures for the rest of the turtles in the group. The rates of rise and fall of temperature for each of the nine turtles considered are shown as a graph in Figure 23. Rate of temperature change was inversely proportional to bulk; hatchlings, for example, cooled and warmed a little more than twice as rapidly as did adults. Rate of temperature change was intermediate in juveniles but was more nearly like that of adults in the warming phase and closer to that of hatchlings in the cooling phase (Table 5).
Considering that hatchling no. 2 was smaller than no. 1, the rate of change in its temperature did not seem to be significantly altered by starvation. The adult males showed a tendency to change temperature faster than adult females even though both males were larger than any of the females. The slight difference in rate of temperature change between the sexes (Fig. 23) may have been fortuitous.
One hatchling (No. 1), when its temperature dropped below one degree, fully extended all four limbs and the body was elevated and only the anterior edge of the plastron was in contact with the confining glass dish. Raising the body from an uncomfortably cold or hot substrate is a well known phenomenon in many lizards and in crocodilians, but to my knowledge has not been reported for turtles.
Table 5.—Average Rate of Change
in Temperature (Expressed in Degrees per minute) for four Groups of Turtles
Subjected to Temperature of Zero Degrees and then Allowed to Warm at 27 Degrees
(Centigrade). |
|||
| Group | Number | Cooling phase | Warming phase (to 25°) |
| Hatchlings | 2 | .282 | .310 |
| Juveniles | 2 | .264 | .180 |
| Adult ♂ | 2 | .122 | .152 |
| Adult ♀ | 3 | .119 | .130 [B] |
| Adult (all) | 5 | .120 | .138 |
| [B] None of the females reached a temperature of 25° before the experiment was terminated. | |||
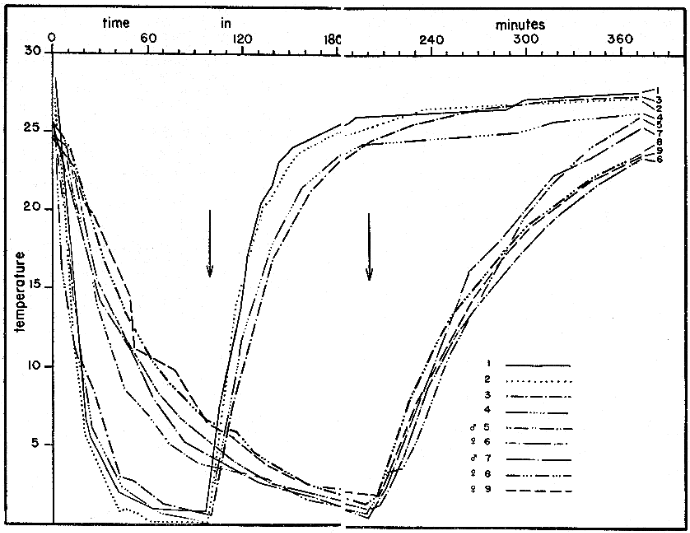
Click on image to view larger sized.
Hibernating turtles and those experimentally chilled were usually comatose but were almost never completely incapacitated even at temperatures at or near zero degrees. Experimental pinching, probing, and pulling revealed that muscles operating the neck, the limbs, and the lobes of the plastron could be controlled by the turtle at low temperatures; hissing, resulting from rapid expulsion of air through the mouth and nostrils (when the head and limbs are drawn in reflexively) occurred at all body temperatures but was sometimes barely audible in the coldest turtles. Of all living turtles observed, only two (hatchlings 1 and 2 in coldroom experiment) were completely immobile at low temperatures, failing to respond even to pinpricks at body temperatures of 0.8 and 1.7 degrees, respectively, although other turtles, under the same experimental conditions, consistently gave at least some response to the same stimulation.
Turtles chilled experimentally continued to move about voluntarily, albeit sluggishly, at temperatures much lower (2.5° for each of four adults; 10.0° and 6.2° for two juveniles) than those at which locomotion was resumed in the warming phase (13° for the adults, 21.7° and 20.1° for the juveniles). Hatchlings chilled so rapidly that it was difficult to ascertain accurately the temperature at which inactivity was induced. Juveniles became active gradually, moving slowly about when the body temperature reached approximately 20 degrees but not attempting more strenuous activities such as climbing the walls of enclosures, until body temperatures of 22 to 25 degrees were attained. Adults, on the other hand, exhibited "normal" activity as soon as they became voluntarily active.
The ability of ornate box turtles to move about when the body temperature is near the lethal minimum probably enables those caught in the open by a sudden drop in environmental temperature to find cover that keeps them from freezing to death. Prolonged chilling, on the other hand, seems to create a physiologically different situation; the temperature at which activity is resumed is higher and subject to less variation.
Juveniles were more rapidly affected by environmental temperatures, were subject to different thresholds, and were inactive over a wider range than were the adults. Indeed, the rate of chilling, rather than absolute body temperature alone, might in large measure influence the reactions of turtles to environmental temperatures. If this be so, smaller turtles, having a narrower thermal range of normal activity, must lose at least some of the advantages gained by their ability to warm up more rapidly.
Hatchlings and juveniles at the Damm Farm were always active on days when at least some adults were also active. Fitch (1956b:466) found that, in northeastern Kansas, species of small reptiles and amphibians are active earlier in the season than larger [Pg 611] species and that the young of certain species become active earlier than adults. Fitch stated, "… small size confers a distinct advantage in permitting rapid rise in body temperature by contact with warmed soil, rock or air, until the threshold of activity is attained"; he pointed out also that young animals, if able to emerge earlier than adults, would benefit from a longer growing season. Hatchlings and juveniles of T. ornata would benefit greatly from an extra period of activity of say, one or two weeks in spring and a similar period in autumn, especially if food were plentiful. The extra growth realized from such a "bonus" period of feeding would significantly increase the chance of the individual turtle to survive in the following season of growth and activity.
Ornate box turtles are active within a narrower range of temperatures than are aquatic turtles in nearby ponds and streams of the same region. Observations by William R. Brecheisen and myself on winter activity of aquatic turtles indicate that, in Anderson County, Kansas, the commoner species (Chelydra serpentina, Chrysemys picta, and Pseudemys scripta) are more or less active throughout the year; although they usually do not eat in winter, they are able to swim about slowly and in some instances (P. scripta) even to carry on sexual activity at body temperatures only one or two degrees above freezing. But, ornate box turtles hibernating in the ground a few yards away are incapable of purposeful movement at such low body temperatures.
In northeastern Kansas ornate box turtles are dormant from late October to mid-April—approximately five and one half months of the year. Individuals may be intermittently active for short periods at the beginning and end of the season, however. Once a permanent hibernaculum is selected dormancy continues until spring; unseasonably warm weather between mid-November and March does not stimulate temporary emergence. There is little movement during dormancy except for the deepening or horizontal extension of the hibernaculum.
Woodbury and Hardy (1948:171) found desert tortoises (Gopherus agassizi) in dormancy from mid-October to mid-April in southwestern Utah; some tortoises became temporarily active on warm days in winter. Cahn (1937:102) was able to compare hibernation in several individuals each of T. ornata and T. carolina, kept under the same conditions in Illinois. Individuals of T. ornata burrowed into the ground in October, two weeks before those of T. carolina [Pg 612] did, and continued to burrow to a maximum depth of 22½ inches. Some individuals of T. carolina spent the entire winter in the mud bottom of a puddle and became semiactive on warm winter days. Other individuals of T. carolina burrowed nearly as deeply as did T. ornata. Individuals of T. ornata emerged from hibernation one or two weeks later in the spring than did those of T. carolina. There are some indications that populations of T. carolina in eastern Kansas are dormant for a shorter period of time than those of T. ornata but comparative studies are needed to verify this. Richard B. Loomis gave me a large female of T. carolina that he found active beside a highway in Johnson County, Kansas, on November 23, 1954; on that date most individuals of T. ornata under my observation had already begun permanent hibernation but a few at the Reservation were still semiactive.
Fitch (1956b:438) listed earliest and latest dates on which box turtles were active at the Reservation in the years 1950 to 1954; in the five year period box turtles were active an average of 162 days per year (range, 140-187) or approximately 5.3 months of the year. It is significant that 1954, having the most days of activity was, according to my studies of growth-rings, an exceptionally good year for growth. Fitch's data indicate the approximate season of growth and reproduction but not of total activity, since he did not take into account the sporadic movements of box turtles in late fall and early spring.
Activity in autumn is characterized by movement into ravines and low areas; many turtles move into wooded strips along the edges of fields or small streams. Sites protected from wind, providing places for basking and for burrowing, are sought. Burrows of other animals, along the banks of ravines, were often used for temporary shelter; overhanging sod at the lips of ravine-banks provided cover beneath which turtles could easily burrow. After mid-October progressively fewer box turtles were found in open places and activity was restricted to a few hours in the warmest part of the day.
Low air temperature probably is the primary stimulus for hibernation. Autumn rains are usually followed by a decrease in general activity. Rain probably hastens burrowing by softening the ground.
Ornate box turtles more often than not excavate their own hibernacula. Digging begins with the excavation of a shallow form which is deepened or extended horizontally over a period of days or weeks. Such hibernacula are sometimes begun at the edges of [Pg 613] rocks or logs; the overhanging edge of an unyielding object acts as a fulcrum on the shell and hastens digging. Ornate box turtles are slow but efficient burrowers.
Forms in open grassy areas are begun at an angle of 30 to 40 degrees; an adult box turtle requires approximately one hour to burrow far enough beneath the sod to conceal itself but can dig into soft, bare earth much more rapidly. Once a hibernaculum is begun, all four feet are used for its excavation, the front feet doing most of the digging and the hind feet pushing loose earth to the rear.
Several turtles were seen entering burrows and dens in late autumn and trailing records showed that some individuals visited several of these shelters in the course of a single day.
By means of systematic probing of known hibernacula it was found that they are deepened gradually in the course of the winter. Depth seems to be governed by the temperature of the soil. Hibernacula in wooded or sheltered areas were ordinarily shallower than hibernacula in open grassland.
In the autumn of 1953-54 two pens were constructed at the Reservation in order to study hibernation; one pen was on a wooded hillside and the other was on open grassland. Turtles in the grassland pen were in newly excavated hibernacula, just beneath the sod, on October 25 and did not emerge for the remainder of the winter, whereas turtles in the woodland pen were intermittently active until November 10. Correspondingly, turtles in the grassland pen descended to depths of eight and one half and 11½ inches, respectively, whereas those in the woodland pen were covered by a scant six inches of loose earth and leaf litter. In 1954 four turtles were traced (by means of trailing threads) to hibernacula on wooded slopes at the Reservation; two entered permanent hibernacula on November 13 and two remained semiactive until sometime after November 20. All four turtles spent the winter in hibernacula that were not more than six inches deep. Temperatures of the soil at a depth of nine inches were usually slightly lower at the grassland pen than at the woodland pen on a given date. It is probably significant that individuals with trailing devices and individuals in experimental pens furnish the latest records for autumn activity. The unnatural conditions created by confining the turtles in pens restricted the number of hibernation sites that were available to them; although trailing devices did not affect the normal movements of box turtles on the surface of the ground these devices certainly hampered the turtles somewhat in digging. However, [Pg 614] it is noteworthy that box turtles are able to move about after mid-November, whether this is of general occurrence under more natural conditions or not. Depths of hibernacula at the Damm Farm were also influenced by amount of vegetation or other cover. Maximum depth of hibernacula in more or less open situations ranged from seven to 18 inches whereas a female hibernating in a ditch that was covered with a thick mat of dead grasses was four inches beneath the surface of the soil, and another female was only two and one half inches below the floor of a den.
Several T. ornata kept by William R. Brecheisen in a soil-filled stock tank on his farm in the winter of 1955-56, burrowed to maximum depths of seven to eight inches in the course of the winter. A layer of straw covered the soil. All the turtles were alive the following spring except for one juvenile, found frozen at a depth of one inch on December 30 (the lowest air temperature up to this time was approximately -12°). Three adult and 24 juvenal T. ornata hibernating in the earth of an outdoor cage at the University of Kansas in the winter of 1955-56, were all dead on December 3 after air temperatures had reached a low of -12 degrees.
Ornate box turtles are usually solitary when hibernating; in the rare instances in which more than one turtle is found in the same hibernaculum, the association has no social significance and is simply a reflection of the availability and suitability of the hibernaculum. The only communal hibernaculum—the "Tree Den"—at the Damm Farm was discovered on October 16, 1955, after a turtle was traced to it by means of a trailing thread. The flask-shaped cavity, approximately two and one-half feet deep, in the north-facing bank of a narrow ravine, had an entrance one foot wide and nine inches high, nearly flush with the bottom of the ravine. Grasses on the bank of the ravine hung over the entrance and nearly concealed it. The steep sides of the ravine protected the entrance from wind.
Seven turtles were in the den when it was discovered, and on each of five subsequent visits from October 20, 1955, to March 6, 1956, fewer turtles were found in the den. Figure 24 shows the approximate length of stay of each known occupant of the den. Only one of the turtles (an adult female) that left the den returned. Turtles found in the den on three visits in October were more or less torpid and were seen easily from the entrance but on November 6 the two remaining individuals had burrowed into the sides and floor of the den.
Three turtles (one female, one male, and one juvenile) were found in separate form-hibernacula within a few inches of one [Pg 615] another on November 6, 1955 (Pl. 21, Fig. 2). The common entrance to all three hibernacula was a shallow depression that resulted from an old post-hole. Soil in the depression was loose and moist and ideal for burrowing. The three hibernating turtles were situated, in a vertical plane, at depths of 18 (♂), 12 (juvenile), and seven (♀) inches. One of the turtles hibernating at this place on November 6 was basking on October 30 in the shelter of some tall weeds a few feet from the hibernaculum.
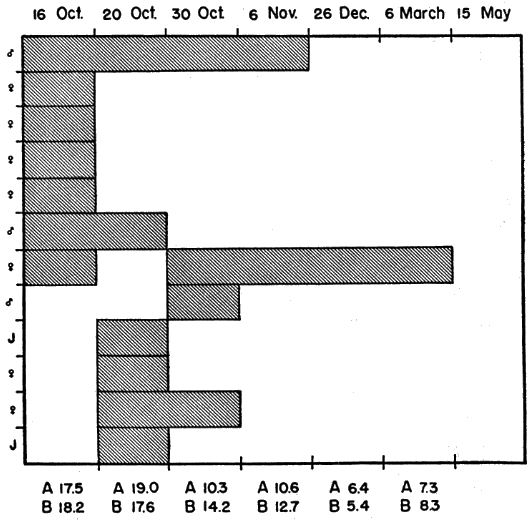
In general, body temperatures approximated the temperature of the soil around the turtle. Body temperatures tended to be slightly higher than soil temperatures in November and December but were slightly lower than soil temperatures in the months of February and March. The lowest body temperature recorded for any turtle that [Pg 616] survived a winter was 2.7 degrees, taken from an adult female on December 26, 1955. Body temperatures one to three degrees higher were common in the coldest part of the winter. Turtles in shallow hibernacula, like those observed in wooded areas at the Reservation, are probably subjected to freezing temperatures at least for short periods but I have no records of body temperatures this low, except where they were induced experimentally. Turtles exposed to temperatures of zero degrees or slightly lower would retain enough heat to survive without freezing for a period of several hours or even a day if well insulated. A temperature gradient exists within the body; cloacal temperatures, for example, differ from temperatures deep in the colon and temperatures in the dorsal and ventral parts of the body cavity (taken by manipulating the bulb of the thermometer while it was in the colon) differ from one another. Probably, therefore, some parts of some turtles—probably the top of the shell or the extremities—freeze in winter without causing the death of the turtle. Ewing (1939:91) found a female of T. carolina, just emerging from hibernation, that had lost some scutes from its carapace; he found the missing scutes in the hibernaculum and attributed their loss to severe temperatures in the winter of 1933-34.
The incidence of mortality due to freezing is unknown for most species of reptiles. The observations of Bailey (1948) on DeKay snakes (Storeria dekayi) and Legler and Fitch (1957) on collared lizards suggest that rates of mortality are high in dormant reptiles. Bailey (op. cit.) suggested that winter mortality might act as a natural check on snake populations. Neill (1948a:114) thought more box turtles (T. carolina) were killed in Georgia by cold weather in late autumn than "… by all other factors together," and that this winter mortality acted as an effective check on population levels. Neill reported that many turtles left their burrows in late autumn and began to forage; if the temperature dropped suddenly, the turtles became "… too torpid to dig" and froze.
If ornate box turtles are occasionally caught in the open by a sudden cooling of air temperature, it would occur at a time of year when temperatures would approximate freezing but would drop not far below this level; laboratory and field records show that adults could probably survive these low temperatures overnight and warm up sufficiently on the following day to seek adequate shelter. Box turtles deepening their burrows in winter do so at body temperatures somewhat lower than 10 degrees (near the minimum temperature at which co-ordinated activity was observed [Pg 617] in the laboratory); turtles found in the open in late October were known to burrow into the ground at body temperatures of approximately 15 degrees.
Emergence from hibernation usually occurs in April but in some years a few turtles may emerge as early as the first week of March. Emergence is stimulated by temperature and humidity. Fitch (1956b:438) stated that emergence was delayed until "…the ground has been sufficiently moistened and until air temperatures have reached at least 26°." Box turtles at the Reservation emerged on April 21 in 1954 and from April 16 to 17 in 1955. William R. Brecheisen found recently emerged box turtles in Anderson County on April 2, 1955, and March 6, 1956.
Turtles were found facing upward in their hibernacula in early March. As the temperature of the soil rises, they move slowly upward, usually following the route by which they entered. They remain just below the surface of the soil for a week or two before actually emerging; this final phase of emergence is probably hastened by spring rains that soften the soil. Activity may be sporadic after emergence if the weather is cold.
A number of box turtles at the Reservation emerged in a cold rain in 1954 when the temperatures of the air and ground were 16 and 13 degrees, respectively, but remained inactive for several days afterward. In 1955 the air and ground temperatures were higher (28° and 17°, respectively) on the day of emergence and box turtles became active almost immediately.
Published information on the food of T. ornata consists of a few miscellaneous observations. Cahn (1937:103) opened five stomachs that contained partly digested vegetable matter but no insects or other animal food: Ortenburger and Freeman (1930:187) noted that grasshoppers were a main part of the diet of T. ornata in Oklahoma and that turtles displayed unsuspected agility in catching them. Those authors also saw turtles eating caterpillars and robber flies. Strecker (1908:79) stated that "The natural diet of this species consists of vegetable matter and earthworms." Norris and Zweifel (1950:3) observed the feeding habits of captive T. o. luteola. Coyote melon (Cucurbita foetidissima) was eaten with reluctance but a collared lizard (Crotaphytus collaris) was quickly devoured. Tadpoles of Scaphiopus hammondi were caught in a small pool and eaten. Adults of the same species were rejected after being caught; box turtles were seen wiping their mouths [Pg 618] after rejecting adult toads. The authors suggested that T. o. luteola is an important predator of Scaphiopus hammondi, since the two species occur together in many areas and the emergence of both is controlled to a large extent by rainfall. One individual of luteola was seen eating a dead box turtle on a road.
Captive individuals of T. ornata, observed in the present study, ate nearly every kind of animal and vegetable food given to them. Table scraps, consisting chiefly of greens, various fruits and vegetables, meat, and cooked potatoes, formed the main diet of turtles kept in outdoor cages.
A number of persons have told me of ornate box turtles eating the succulent stems and leaves, and the fruits of various garden plants; similar incidents probably occur in areas of native vegetation. J. Knox Jones told me he saw an individual of T. ornata eating a spiderwort (Tradescantia sp.) in Cherry County, Nebraska.
Sight-records of foods eaten by box turtles at the Damm Farm (excluding the many records of individuals foraging in dung or eating mulberries) were for grasshoppers, caterpillars, and various kinds of carrion. Box turtles were often seen eating grasshoppers on roads in early morning; Sophia Damm told me of frequently seeing individuals catching grasshoppers in her garden. Ralph J. Donahue told me that on his farm in Bates County, Missouri, an individual of T. ornata made a circuit of the lawn each morning in summer and ate all the cicadas (Magicicada septendecim) found.
Vertebrate remains found in the stomachs of box turtles seem to result chiefly from the ingestion of carrion. One box turtle ate a white egg (unidentified) that had fallen from a nest and another was seen with a blue down feather clinging to its mouth. Several colleagues have told me of box turtles eating small mammals caught in snap-traps and Marr (1944:489) reported a similar incident. J. Knox Jones told me he once found an ornate box turtle in the nest of a blue-winged teal in Cherry County, Nebraska; the three eggs in the nest had been broken. The only authentic record of an ornate box turtle preying on a vertebrate under natural conditions was one supplied by Ralph J. Donahue who saw an adult catch and eat one of a brood of bobwhite quail. In many areas where box turtles are abundant, it is the opinion of local residents that the turtles decimate populations of upland game birds by eating the eggs and young of these birds; these opinions result probably from rare encounters such as the one described by Donahue. I believe that box turtles at the Damm Farm were sometimes able to catch young frogs and tadpoles (chiefly Rana catesbeiana and R. pipiens) [Pg 619] at the margins of ponds. In autumn literally thousands of young Rana were present in these places.
Ornate box turtles ordinarily attempt to catch and, without further examination, to eat, small objects moving on the ground, but are more critical of stationary objects. Captive turtles, for example, would immediately chase and seize a grape that was pulled or rolled slowly across a floor but a stationary grape was examined and then smelled before it was eaten. Similar observations were made a number of times with living and dead insects in the field and in the laboratory. A turtle discovering an object that is of possible value as food, approaches it closely, turns the head from side to side (presumably using the eyes alternately to examine the object), and then, with head cocked at a slight angle, momentarily presses the nostrils against the object (Pl. 28, Fig. 4). If acceptable as food, the object is then swallowed whole or taken into the mouth with a series of bites; large insects are usually broken into several pieces in the process of being bitten and swallowed. Larger objects, such as dead vertebrates, are torn to pieces with the beak and forefeet before they are swallowed. Hatchlings, when fed for the first time, ignored inanimate foods but eagerly chased mealworms, catching them usually by the anterior end. The tendency of the young of certain species of turtles (especially captives) to be more carnivorous than adults is probably due to the association of movement with food; recognition of inanimate objects as food is presumably learned by older individuals.
Mulberries (Morus rubra), when they are abundant, constitute all or an important part of the diet of ornate box turtles. On June 4, 1955, William R. Brecheisen and I drove along a road in Anderson County, Kansas, and stopped at each mulberry tree that we saw beside the road; we found at least one specimen of T. ornata under nearly every tree. Approximately twenty box turtles were collected in this manner in a little more than one hour. The heads and necks of most were stained dark-red from the fruit and, in some, nearly the entire shell was stained. Dissection of these turtles revealed that their stomachs were distended to two or three times normal size with mulberries; no other kinds of food were found in the stomachs. Some of the turtles voided purplish-black fluid from the cloaca when we handled them; the color of the fluid presumably resulted from mulberries.
Several turtles were observed through binoculars as they foraged. Individuals snapped or lunged periodically at objects on the ground along the route of travel. Upon reaching an area where cow dung [Pg 620] was abundant, a turtle would move directly to a pile of dung and begin tearing it apart with the forelegs or burrowing into it. Turtles most often foraged in cow dung that had a superficial, dried crust. The invertebrate fauna of older dung was probably greater than that of fresh dung. Adult and larval insects were eaten, along with quantities of dung, as they were uncovered. Sometimes box turtles chased and caught larger insects that ran a foot or more away from the pile of dung; the turtles could cover the distance of one foot with three or four quick steps. Depressions made by box turtles in cow dung, as well as drier cow dung that had been more completely dissected, were regarded as characteristic "sign" of T. ornata at the Damm Farm and in other areas studied (Pl. 26). Several persons have told me of box turtles "eating cow dung"; these reports, most of them made by competent observers, probably result from observations of box turtles ingesting cow dung incidentally, along with some unseen item of food.
Contents of stomachs were analyzed. Scats and contents of lower digestive tracts, although obtained in large quantity, were unsuitable for analysis because of the fragmentary nature of the foods they contained. Relative amounts of various kinds of foods in stomachs were estimated; volume was determined by displacement of water or fine shot.
Twenty-three stomachs of adults were selected at random (except for the fact that empty stomachs were discarded) from more than a hundred specimens collected in Douglas County, Kansas, in the period from June, 1954, to June, 1957; the sample included stomachs obtained in nearly all the months of the season of activity. Kinds of foods in stomachs did not differ significantly in regard to the sex of the turtles or to time of year. The stomach of each of two juveniles (included in Table 6) contained a greater variety of animal food than did the stomach of any adult, but no kind of animal was eaten by the juveniles exclusively.
Each of the 23 stomachs contained animal matter and, in addition, all but two contained at least some plant material from dung, which constituted up to 20 per cent of total stomach contents.
Insects were present in each of the 23 stomachs and constituted the bulk of the animal matter; beetles, caterpillars, and grasshoppers (ranked in descending order) were the kinds occurring most frequently and constituting the largest average percentages of total stomach-contents. Most of the beetles were scarabaeids and carabids; the bulk of the caterpillars were noctuids and arctiids. Grasshoppers, with one exception, were of a single species, Melanoplus differentialis. It is noteworthy that two of the kinds of insects frequently eaten (differential grasshoppers and noctuid caterpillars) are of economic importance in that they damage crops.
| Frequency of Occurrence | |||
| Adults | Larvae | Total | |
| Gastropoda | |||
| Helisoma sp | 1 | . . . . | 1 |
| Succinia sp | 1 | . . . . | 1 |
| Polygyra sp | 1 | . . . . | 1 |
| Retinella sp | 1 | . . . . | 1 |
| Crustacea | |||
| Procambaris gracilis | 1 | . . . . | 1 |
| Armadillidium vulgare | 4 | . . . . | 4 |
| Orthoptera (Locustidae) | |||
| Locustinae (Melanoplus differentialis) | 13 | . . . . | 13 |
| Oedipodinae | 1 | . . . . | 1 |
| Lepidoptera (unspecified) | . . . . | 1 | 1 |
| Arctiidae | . . . . | 9 | 9 |
| Noctuidae | . . . . | 10 | 10 |
| Pyralidae | . . . . | 1 | 1 |
| Sphingidae | . . . . | 1 | 1 |
| Diptera (Sarcophagidae) | . . . . | 1 | 1 |
| Coleoptera (unspecified) | 3 | . . . . | 3 |
| Cantharidae | . . . . | 1 | 1 |
| Carabidae (unspecified) | 6 | . . . . | 6 |
| Carabidae (Eumolops colossus) | 1 | . . . . | 1 |
| Cerambycidae (Prionus fissicornis) | 1 | . . . . | 1 |
| Chrysomelidae (Diabotrica 12-punctata) | 1 | . . . . | 1 |
| Curculionidae (Calendra parvulus) | 3 | . . . . | 3 |
| Lampyridae (Photinus pyralis) | 2 | . . . . | 2 |
| Lampyridae (Photuris sp.) | 1 | 1 | |
| Phengodidae | . . . . | 1 | 1 |
| Scarabaeidae | 11 | . . . . | 11 |
| Hymenoptera (Formicidae) | 2 | . . . . | 2 |
| Phalangida | 1 | . . . . | 1 |
| Araneida (Epeira) | 1 | . . . . | 1 |
| Diplopoda | 1 | . . . . | 1 |
| Vertebrata (carrion) | . . . . | . . . . | 4 |
| Insects (all) | Orthoptera | Lepidoptera (larvae) | Coleoptera | |
| Average volumetric percentage |
88.6 | 28.7 | 26.9 | 32.5 |
| Range (volumetric percentage) |
trace to 100 | 0 to 100 | 0 to 100 | 0 to 100 |
| Frequency of occurrence (percentage of total stomachs in which found) |
100 | 52 | 65 | 74 |
Snails, sowbugs, and the one individual of crayfish found in stomachs were kinds that could be expected to occur in moist grassland or in wooded stream courses. Mulberries were present in one stomach and fragments of bird's-nest fungi (Cyathus striatus) were present in another. Carrion consisted of remains of mammals and birds; the only identifiable items were bones of the eastern cottontail (Sylvilagus floridanus) and a chicken. Stones up to seven millimeters in diameter were found in many stomachs; stones constituted as much as half of total stomach-contents. Presumably the stones were accidentally swallowed when food was taken from the ground.
The few adequate reports on dietary habits of T. carolina (Allard, 1935:325-326; Carr, 1952:147, 150, 152, 153; Stickel, 1950:361; Surface, 1908:175-177) indicate that the species is omnivorous but that individuals tend to be herbivorous or carnivorous at certain times. Ornate box turtles resemble T. carolina in being opportunistic feeders but rely on insects as a staple part of the diet. In this respect the ornate box turtle seems to differ from all other kinds of box turtles in the United States and it is probably unique in its habitual utilization of dung communities as a source of food.
Ornate box turtles were probably more numerous on the Damm Farm than any other kinds of reptiles, excepting skinks (Eumeces fasciatus and E. obsoletus), and were by far the most conspicuous element of the reptilian fauna.
The 194 box turtles that were marked at the Damm Farm were captured a total of 437 times. Seventy-nine (41 per cent) individuals were recaptured at least once, 49 (25 per cent) twice, 29 (15 per cent) three times, and 20 (10 per cent) were recaptured at least four times. Only three individuals were recaptured more than eight times. The greatest number of recaptures for a single individual, an old female, was 23.
In all, 185 turtles (95 per cent of total recorded at Damm Farm) were captured on the pasture. Of these, 73 were in the northwest corner area, 44 in the house pond area, and 35 in the southern ravine area. The density of the population at the Damm Farm, considering the entire area, was .88 turtles per acre; for the woodland area alone, density was .41 turtles per acre and for the pasture alone, density was 1.49. Acreage and population density in the northwest corner, house pond, and southern ravine areas were respectively, 28 acres with 2.6 turtles per acre, 7 acres with 6.3 turtles per acre, and, 17 acres with 2.6 turtles per acre. The densities noted above for the wooded area and for the entire Damm Farm are low as a result of incomplete sampling in the wooded area. Estimates of population density for the subdivisions of the pasture seem more closely to approach the true population density in areas of favorable habitat.
Fewer unmarked turtles were captured as the study progressed, but they were still being captured occasionally when field work was terminated. In order to estimate the number of turtles in the population at the Damm Farm the "Lincoln Index" (Lincoln, 1930) was used to compare the ratio of marked individuals to total number of individuals (17:56) in collections for June, 1956, to the ratio of marked individuals as of July 31, 1955 (87) to total individuals in the population; the result was 286.
Fitch (1958:78) estimated the population of T. ornata in one area of the Reservation (including woodland and ungrazed pasture) to be .076 turtles per acre. Stickel (1950:373) estimated the population of adult T. carolina to be four to five turtles per acre in favorable habitat at the Patuxent Research Refuge, Laurel, Maryland; juveniles comprised less than ten per cent of the population.
Of the 194 turtles marked at the Damm Farm, 103 (53 per cent) were adult or subadult females, 61 (31 per cent) were mature [Pg 624] males, and 30 (16 per cent) were juveniles of undetermined sex. The ratio of males to females was then, 1.00 to 1.69, and the ratio of juveniles to adults was, 1.00 to 6.47. Eighteen of the 194 individuals were juveniles less than 90 millimeters in plastral length and only six had plastra less than 60 millimeters long (Fig. 25). The unbalanced ratio between males and females may result, in part, from sexual differences in habits. The studies of Carr (1952:9), Fitch (1954:140), Forbes (1940:132), Legler (1954:138), and Risley (1933:690), have shown, however, that unbalanced sex ratios, with females outnumbering males, are found in several species of reptiles, especially in turtles.
Records for 540 adult T. ornata collected at the Damm Farm, the Reservation, and on roads in eastern Kansas, show that females outnumber males just before and during the nesting season and again in late autumn (Fig. 26). The high incidence of females in May, June, and July, can be explained by their more extensive movements associated with nesting in these months. I have no explanation for the increased number of females captured in late autumn. In April and August, the only two months in which males were more abundant than females, the samples were small. The number of juveniles collected was too small to allow any trustworthy conclusions concerning their seasonal incidence; a few juveniles were taken in nearly all the periods in which adults were active.
Risley (1933:690), studying Sternotherus odoratus in Michigan, found an over-all sex ratio of 1.0 male to 2.3 females; the percentage of females in collections ranged from 50 to 71 per cent in April and most of May and rose to 83 and 85 per cent in late May and mid-June, respectively.
The infrequency with which hatchlings and small juveniles of ornate box turtles are observed is well known to naturalists. Several of my colleagues who are expert field observers and who have lived in areas where ornate box turtles are abundant, have never seen hatchlings; many other persons have seen only one or two. Rodeck (1949:33), noting the abundance of coleopterous insects in the scats of captives and the rarity of individuals of all age groups during dry periods in Colorado, commented, "It is possible that the young are even more subterranean than the adults. Perhaps they spend their early years in rodent or other burrows where there is a fairly abundant insect fauna. Increasing size might force them to the surface for feeding, with a daily return to a burrow for resting and protection."
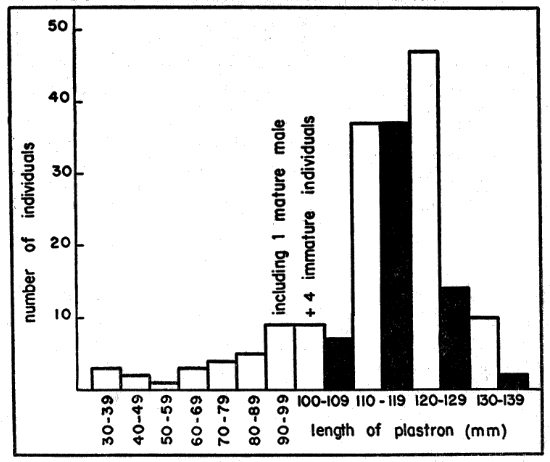
My own experience in the field has shown that small examples of T. ornata are not so rare as previous workers have believed. Small box turtles occupy the same microhabitat as do the adults and seem not to be more aquatic or subterranean in habits. Juveniles are found in burrows, in marshy areas, and in other sheltered places, but so are adults. Most of the juveniles that I found were in open situations where adults were abundant, sometimes within several inches of a place where an adult was feeding or basking. Nearly every one of the smaller turtles was discovered when I was closely scrutinizing some other object on the ground; sometimes juveniles were actually touched before being seen. Most juveniles were covered with cow dung or mud and blended so well with the substrate that they were detected only when they moved. It is likely that only a small number of the young box turtles present in an area is ever actually observed. Young are more vulnerable to predation and injury because of their small size, soft shells, and immovable plastra. They evidently rely, to a large extent, on inconspicuousness for protection.
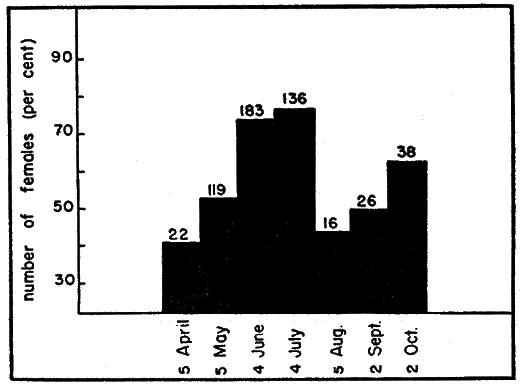
The only previous study of movements of T. ornata is that of Fitch (1958:99-101). He recovered 14 marked T. ornata at the Reservation a total of 30 times, the period between recaptures varying from one to seven years. He reported that the average radius of home range was 274 feet (for an area of approximately 5.4 acres), excluding a single (presumably gravid) female that moved 1830 feet in 53 days.
Although published information on T. ornata is scant, a considerable amount of information is available concerning its congener, T. carolina. The classic studies of Stickel (1950) on it constitute the most complete account of populations and movements for any reptile or amphibian, and probably, for any vertebrate. She found the average home range of adults to be 350 feet in diameter. Home ranges were not defended as territories and nearly all individuals were socially tolerant of one another. Movements (studied by means of a thread-trailing device) were characterized by frequent [Pg 627] travel over the same routes within the home range. Some turtles concentrated their activities in only one part of the home range, moving subsequently to another part, and some turtles had two ranges between which they traveled at varying intervals. Females ordinarily left their home ranges to nest.
Other noteworthy, but less detailed, studies of populations of T. Carolina are those of Breder (1927) who found evidence of home range and homing behavior, and of Nichols (1939b) who, after observing a marked population on Long Island over a period of twenty years, found evidence of homing behavior and estimated normal home range to be approximately 250 yards in diameter. Numerous shorter papers such as those of Schneck (1886) and Medsger (1919) document the tendency of T. carolina to remain in restricted areas over long periods.
Important studies that indicate the presence of home range and homing behavior in other chelonians are those of Cagle (1944) on Pseudemys scripta and Chrysemys picta, and of Woodbury and Hardy (1948) on Gopherus agassizi. Grant (1936) and Bogert (1937) have also indicated that movements of individuals of Gopherus agassizi are restricted to limited areas.
Ornate box turtles moving forward over even terrain hold the plastron a quarter to a half inch above the ground and keep the head and neck lowered and extended. Each foreleg is brought forward and the humerus points nearly straight ahead when the foot touches the ground. Nearly all of the palmar surface is initially in contact with the ground but as the body is brought forward and the humerus swings outward, only the claws, and finally, only the two inner claws are in contact with the ground. Of the hind feet, the medial surfaces are the principal parts that touch the ground but some traction is derived from the hind claws at the beginning of each cycle of the hind leg. Under normal conditions, box turtles move slowly and pause to rest and examine their surroundings every few feet. When resting, the plastron is in contact with the ground, the legs relaxed, and the head and neck are extended upward. Some turtles seeking shelter from the heat of sunshine walk rapidly for a hundred feet or more without pausing.
Turtles seen feeding under natural conditions displayed remarkable agility in making lunges, consisting of one or two short steps and a thrust of the head, at moving objects. Turtles kept in my [Pg 628] home were able, after being conditioned to hand-feeding, quickly to intercept a grape rolled slowly across a linoleum-covered floor.
Frederick R. Gehlbach told me that, of several species of captive turtles observed by him, T. ornata characteristically walked with the plastron held well above the substrate, as did Gopherus berlandieri, but that T. carolina (specimens from the northeastern U. S.) dragged their shells as they walked. Apparently T. carolina in Kansas (currently referred to the subspecies triunguis) differs somewhat in gait from populations in the eastern part of the range; several individuals of T. carolina from Kansas that I observed in captivity, kept their plastra raised well above the smooth, hard substrate over which they walked.
Box turtles at the Damm Farm were able easily to climb ravine banks that sloped at an angle of 45 degrees and, with some difficulty, could climb banks as steep as 65 degrees. Most individuals, however, were reluctant to walk directly downward on banks as steep as 45 degrees. Several individuals were seen to lose footing when climbing up or down a steep bank and to roll or slide to the bottom. Ordinarily, T. ornata is able to climb over a sheer surface as high as its shell is long, provided the surface is rough enough to give some traction to the foreclaws. The claws of first one, then the other forefoot are placed over the top of the barrier and then a hind foot, extended as far forward as possible, secures a hold as the turtle goes over the barrier.
A number of observations on speed were made in the field where distance traveled and time elapsed were known approximately. Speeds ranged from 20 to 100 feet per hour in the course of foraging. Higher speeds (400 or more feet in one hour) were for turtles moving along pathways or seeking shelter. Gould (1957:346) observed somewhat faster speeds in T. carolina (192 feet per hour in cloudy weather and 348 feet per hour in sunny weather); he observed individuals that had been removed from their normal home ranges.
Individuals of T. ornata that were placed in water swam moderately well but were clumsy in comparison to individuals of more aquatic emyids such as Pseudemys and Chrysemys. Box turtles were never observed to swim voluntarily, although they were frequently found in shallow water. On several occasions I confronted individuals at the edge of a pond so that the only unblocked route for their escape was through deeper water; nearly always these individuals attempted to crawl past me, to crawl away in shallow [Pg 629] water parallel to the shore, or to hide in soft mud at the edge of the water. Box turtles floated high in the water with the dorsal side upward and had little difficulty in righting themselves when turned over. The head and neck are extended and submerged when the turtle is swimming; forward progress is interrupted every few moments to elevate the head, presumably for purposes of breathing and orientation. The shell is never submerged. The swimming of T. ornata is in general like that of Pseudemys or Chrysemys that have become dehydrated after long periods out of water and cannot submerge. These more aquatic turtles, however, quickly overcome their bouyancy, whereas examples of T. ornata, even if left in water for several days, are unable to submerge. Clarke (1950) saw an ornate box turtle swim a 60-foot-wide stream in Osage County, Kansas; his description of swimming agrees with that given above.
The meager swimming ability of T. ornata is of apparent survival value under unusual conditions and enables T. ornata to traverse bodies of water that would act as geographic barriers to completely terrestrial reptiles; however, swimming is a mode of locomotion seldom used under ordinary circumstances.
Gehlbach (1956:366) and Norris and Zweifel (1950:2) observed individuals of T. o. luteola swimming in temporary rain pools and small ponds in New Mexico; the two authors last named saw an individual quickly enter a pond and dive beneath the water after being startled on the bank. Several of my colleagues, in conversation, have also reported seeing T. o. luteola in small bodies of water in the southwestern United States.
The daily cycle of T. ornata consists basically of periods of basking, foraging, and rest that vary in length depending upon environmental conditions. Turtles emerge from burrows, forms, and other places of concealment soon after dawn and ordinarily bask for at least a few minutes before beginning to forage; foraging is combined sometimes with basking, especially in open areas that are suitable for both kinds of activity. Foraging usually continues until shelter is sought sometime between mid-morning and noon. Turtles remain under cover (or continue to forage in shaded areas) until mid-afternoon or late afternoon when they again become active. They forage in both morning and afternoon. Study of travel records of a few of the turtles equipped with trailers [Pg 630] suggests that, under normal conditions, activity is slightly greater in forenoon than in afternoon, but that the converse is true of gravid females seeking nesting sites. Strecker (1908:79) reported that captive T. ornata, after developing a feeding reflex, ate and retired until feeding time next day.
As environmental temperatures rise in summer, the period of mid-day quiescence is lengthened. In the hottest part of the year, some turtles remain under cover for several days at a time. In periods of clear, cool weather at the beginning and end of the growing season, some turtles remain abroad and bask for most of the day.
Examination of thread trails showed that activity of all individuals except nesting females was terminated at dusk. Breder (1927:236), Allard (1935:336), and Stickel (1950:358) reported a corresponding lack of nocturnal activity in T. carolina. Terrapene o. ornata in Kansas, and T. o. luteola in New Mexico (Norris and Zweifel, 1950:2)—unlike desert tortoises, Gopherus agassizi, which are active at night in hot weather (Woodbury and Hardy, 1948:186)—do not utilize the hours of darkness for foraging, even in the hottest part of the year.
Data obtained by mapping the movements of turtles that were equipped with trailing devices made it possible to compare distances traveled in the course of daily activities at different times of the year. Some of these data are expressed graphically in Figure 27. It should be noted that movement at all times in the season of activity was uneven; that is to say, an individual would move several hundred feet each day for a period of several days, and then, for an interval of one to several days, move only a few feet from one shelter to another, or not move at all. Such periods of rest could not be correlated definitely with environmental conditions; some individuals were inactive on days that were probably ideal (in terms of moderately warm temperatures and high humidity) for activity of box turtles. Analagous rest periods were noted in T. carolina by Stickel (1950:358).
Two males of T. ornata that had been removed by me from their normal home ranges traveled the longest average distance per day (429 feet). Gravid females in June traveled the next longest average distance per day (363 feet). The average distances traveled per day by non-gravid females in June (226 feet) and July [Pg 631] (260 feet) and by males (within their known home ranges) in June (289 feet) were thought to approximate normal amount of movement under average environmental conditions. Average distance traveled per day by females in October (152 feet) was shortest because of frequent and extended rest periods. Nevertheless, in October actual distances traveled on days of activity tended to be longer than in any other month. A gravid female traveled farther in a single day than any other individual of T. ornata observed; she moved along a rock fence for approximately 700 feet, then left the study area and moved, in a nearly straight line, 1,200 feet across a cultivated field. Then the thread on her trailer was expended. The total distance moved, therefore, was at least 1,900 feet and probably more.
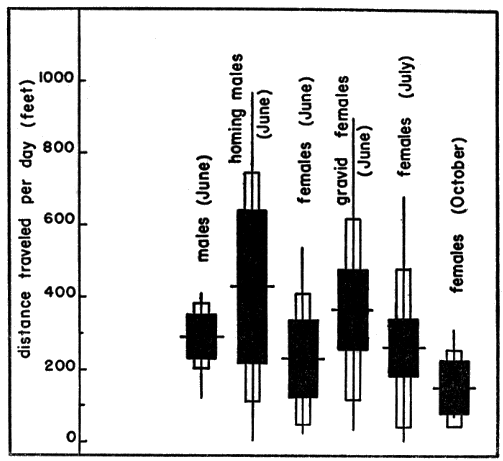
An adult male at the Reservation traveled 2,240 feet in the 36-day period from October 16 to November 20, 1954, mostly on a wooded [Pg 632] hillside. Eleven forms found along the route of the turtle's travels indicated that movement took place on roughly one out of three days in the elapsed period and demonstrated the sporadic nature of movements in autumn. The turtle remained active for an undetermined time after November 20.
Data obtained from trailing and various methods of recapture at the Damm Farm indicated that each individual used only a small part of the total study area in the course of daily activities and tended to remain within a restricted area for a long time.
The number of recaptures of no individual was great enough to permit application of refined calculations of size of home range as described by Odum and Kuenzler (1955). For individuals that were recaptured six or more times, or individuals for which adequate trailing records were available, the area enclosed by a line joining the peripheral points of capture was considered adequately representative of the home range of that individual, unless recaptures were all within a few feet of each other or lay in an approximately straight line. If less than six records of recapture were available, home range was estimated, in the manner described by Fitch (1958:73), by averaging the distance between successive points of recapture and letting this average represent the radius of home range; the actual area of home range was determined by the formula, π(R)2, for the area of a circle.
Size of home ranges of males and females did not differ significantly and data for the two sexes were combined in the final analysis. The average radius of the home ranges of 44 adults (captured a total of 146 times) was 278 feet (extremes, 71 to 913) when computed by measuring the distance between successive captures; the average area of these home ranges was 5.6 acres. Data from 10 turtles that had been recaptured only once were combined with data from 34 turtles that had been recaptured more than once when it was found that the average size of home range in these two groups did not differ significantly. Data concerning the home ranges of eight of the 44 individuals were sufficient to permit actual measurement of home ranges with a planimeter; home ranges of these eight individuals had an average area of five acres (extremes, 1.2 to 10.2).
A minimum home range could theoretically consist of the smallest area in which adequate food and shelter were available. Under favorable conditions a turtle could stay in an area ten to twenty feet in diameter. Although several such favorable small areas [Pg 633] existed on the Damm Farm, box turtles seldom stayed in one for more than a day or two. Seemingly, therefore, factors additional to food and shelter influence size of home range. At the Damm Farm these additional factors seemed to be: rock fences that acted as physical barriers; areas that were cultivated, barren, or otherwise unfavorable, acting as ecological barriers; and, cowpaths and ravines that offered relatively unobstructed routes along which box turtles tended to move.
One subdivision of the main pasture, the northwest corner area, is an example of a relatively small natural area in which many individual box turtles had home ranges. This tract of 28 acres was roughly triangular and was bordered on two sides by rock fences that contained no gates or other passageways. On its third (southeastern) side the area sloped into a deep ravine. Habitat in this subdivision of the pasture (as well as in the other two subdivisions) was especially favorable for box turtles because of permanent water, rocky slopes, ravines, and several fruit trees. Box turtles usually foraged near the rock fences and the ravine (where dung was more abundant than in other parts of the area), and tended, as they foraged, to move parallel to these barriers. Turtles crossing the area eventually came either to one of the fences or the ravine. Therefore, most of the turtles in the northwest corner area eventually completed a circuit of the area. Turtles that came to the ravine tended to move along its bottom or sides. Several turtles were known to cross the ravine and to forage in the grassy area on its southeastern side. These turtles usually re-entered the ravine by way of smaller side-ravines. Of 22 box turtles known to have home ranges in the northwest corner area, only two individuals (both gravid females) were known to leave the area in the period in which observations were made.
Two other subdivisions of the main pasture—the house pond area and the southern ravine area—although not so distinct as the northwest corner area in terms of limiting barriers, nevertheless constituted separate areas of favorable habitat, each of which contained a number of individual home ranges. Although the two areas were not far apart, but little movement was observed of turtles from one area to the other. The home range of only one turtle, an adult female, was known to include parts of both areas.
Unbroken expanses of tall grass seem not to be optimum habitat. The crest of the hill at the Damm Farm (Pl. 17, Fig. 1) was an area of more or less homogeneous grassy habitat. Turtles were seldom [Pg 634] found on the crest of the hill although this area was as thoroughly searched for turtles as any other area. Known home ranges of nearly every individual observed were on either one of the sides of the hill but not on both sides.
At several places on the border of the pasture, turtles were able to move freely into cultivated areas but seldom did so except for nesting. Trailing records show that most of the turtles that entered one of the cultivated areas returned again to the pasture.
Ornate box turtles seem to find places of shelter by trial and error along regularly used routes of travel in their home ranges. The individuals that I studied never returned to the same forms, and seldom returned to the same natural burrows and dens. Probably foraging, basking, and watering sites are found also by trial and error.
Stickel (1950:375) placed considerable importance on the occurrence of transient turtles in populations of T. carolina; in estimating population density, she added to her study area a peripheral strip, half as wide as the average, estimated home range, to account for turtles that had home ranges only partly within the study area. The study area used by Stickel had no natural boundaries, as habitat conditions on all sides were essentially the same as those of the study area itself. The pasture at the Damm Farm, on the contrary, is a relatively isolated area of natural grassland, bordered by rock fences and cultivated fields. I believe that most of the box turtles found on the pasture were permanent residents there. Individual box turtles at the Damm Farm seemingly occupied but one home range and it did not change from year to year. Populations of T. ornata in areas less isolated than the Damm Farm, like the populations of T. carolina studied by Stickel (loc. cit.), could be expected to have a higher percentage of transient individuals and individuals with multiple or changing home ranges. Henry S. Fitch told me that he considered most of the individuals of T. ornata that were captured only once at the Reservation were transients.
Several females at the Damm Farm traveled long distances from their home ranges to nest but other females nested within their known or estimated home ranges. Seemingly a complex of environmental factors, including soil texture, weather, availability of water, and possibly the urge for random wandering in the breeding season, governs the distances traveled by gravid females and the ultimate selection of a satisfactory nesting site. Females, because of their more extensive travels in the nesting season, seem more likely than [Pg 635] males to have multiple or changing home ranges. Males of T. ornata did not noticeably alter the extent or pattern of their movements in the breeding season. Hibernacula, unlike nesting sites, were within the known or estimated home ranges of all individuals studied.
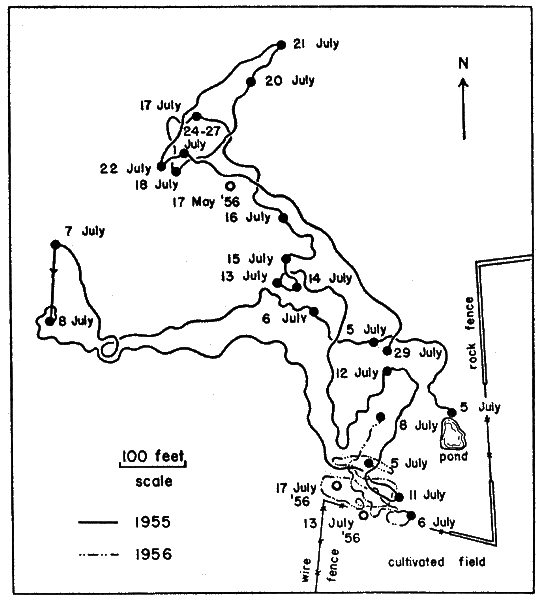
The actual home range of almost every individual studied, even of those individuals for which the most data were available, probably differed at least slightly from the observed or estimated home range. One adult female, for example, was captured six times in [Pg 636] two years within a radius of approximately 50 feet. Another female was found 2780 feet from her last point of capture. These last two records were regarded as unusual; when they were grouped with records of the 44 individuals mentioned above, the average radius of home range for the entire group was much larger (327 feet).
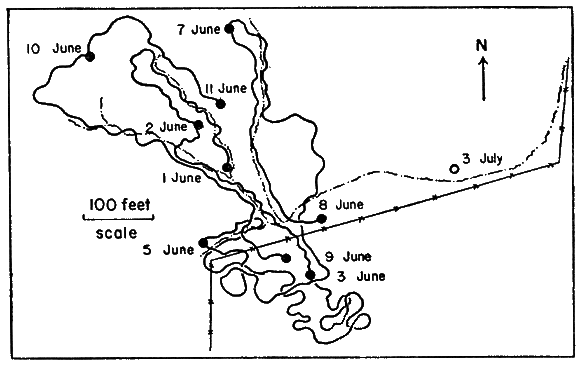
Gould (1957) reported that 22 of 43 T. carolina moved in a homeward direction when they were released in open fields up to 5.8 miles from their original points of capture. Turtles oriented themselves by the sun; homeward headings were inaccurate or lacking on overcast days and, light reflected from a mirror caused turtles to alter their courses. Seven of ten turtles released more than 150 miles from home headed in directions that corresponded most nearly to the headings last taken (at release-points near home base) and did not necessarily correspond to the direction of home. Gould's studies point out that box turtles perhaps practice a kind of "solar navigation." His work raises the question of whether the movements of box turtles are guided by the sighting of local landmarks or whether such landmarks alter the course of movement only when acting as barriers.
In the present study two experiments were made to determine the homing ability of T. ornata. An adult male, taken from his normal home range in the house pond area and released 1200 feet away in the southern ravine area, traveled a generally northward course (not northeastward in the direction of home) for five days, moving a distance of approximately 1900 feet. His detached trailer was recovered several days later 740 feet southeast of the last known point in his travels (a distance that could have been covered in two days) and 150 feet from the point of original capture; he had returned to his home range by a circuitous route in a period of approximately seven days. Another adult male, captured in the southern ravine area, and released in the house pond area 1900 feet away, traveled on a course that bore approximately 25 degrees north of true homeward direction; after five days he was approximately 600 feet north of the original capture point. He then began a northeastward course that took him back to the house pond area where he remained for several days; no further data are available for this individual. It is significant that the homing males discussed above traveled greater average distances per day (based on records for nine days of trailing) than any of the other turtles studied (Fig. 27). Fitch (1958:101) released an individual one half mile from where he captured it and, one year later, recovered the turtle near the point of release.
Ornate box turtles are solitary except during periods of mating. Meetings with other individuals in the course of foraging, basking, or seeking shelter, are fortuitous and have no social significance. A broad overlapping of home ranges of both sexes at the Damm Farm suggests that box turtles do not intimidate other individuals in the home range or exclude them from it. No instances of fighting were observed.
Allard (1935:336), Perm and Pottharst (1940:26), and Latham (1917) recorded instances of fights between individuals of T. carolina; in the latter two instances fights were between males. Stickel (1950:362) observed an incident between two males that may have been a fight; however, she was of the opinion that fights rarely occur in nature and that box turtles do not defend territories. Evans (1954:23-25) considered the behavior of T. carolina reported by Perm and Pottharst (loc. cit.) to represent "territoriality." He found "… a true hierarchy…." existing between [Pg 638] four captive males of T. carolina and another between three captive females of the same species; young individuals in the group raised their social level in the hierarchy after receiving experimental doses of male hormone. Evans (op. cit.:25) pointed out that true tortoises (family Testudinidae) have a more complex pattern of social behavior than do emyid turtles.
Observations made with binoculars from the vantage point of a blind provide the only information that I have concerning the reactions of box turtles to one another under natural conditions. Turtles foraging in a bare area were not startled by the approach of other turtles, and turtles moving across the area seemed to take no notice of turtles already there, regardless of whether these turtles were moving or not. Adults and subadults behaved in approximately the same manner.
Individuals traveling or foraging in rough terrain or in grassy areas probably are unable to see each other even when they are close to one another. Conversely, box turtles can see each other and are surely aware of each other's presence in bare, flat areas. These facts suggest that no social hierarchy exists in T. ornata. On one occasion an adult male and a juvenile (hatched the previous autumn) were found foraging next to one another on the same pile of cow dung.
When an individual became motionless in an attitude of wariness after having detected me in my blind, its behavior evoked no response on the part of other turtles, a few feet away.
Fire, freezing, molestation by predators, and trampling by cattle or native ungulates are only a few natural sources of injury to which box turtles have always been exposed. Man's civilization in the Great Plains, chiefly his automobile and other machines, have compounded the total of environmental hazards. Automobiles now constitute a major cause of death and serious injury to box turtles. Each year thousands are struck on Kansas highways alone, not to mention the many casualties resulting from mowing machines, combines, and other farm machinery.
Although grass fires usually occur in early spring or late fall when box turtles are underground, some turtles are surely killed by fires and many are injured. In early April of 1955 the pasture at the Damm Farm was burned. Similar burnings, I discovered, had occurred both intentionally and accidently in past years at [Pg 639] irregular intervals. No deaths or injuries, attributable to fire were discovered in the course of intensive field work in the spring and summer of 1955, when the new grass was short and conditions for finding and marking box turtles were ideal. Badly burned individuals, if any, may have secreted themselves until their wounds had healed. In June, 1957, an adult female, that had been burned severely, was taken from a small puddle in a ravine on the Damm Farm. The soft parts of her body, excepting her head and neck, were a nearly solid mass of smooth scar tissue, the scales and rugosities of the skin being practically obliterated. The tail was reduced to a mere knob surrounding the anus and dead, exposed bone was visible on most of the dorsal part of the carapace. Possibly this female was burned in the fire of 1955. Lack of injury to the head and neck can probably be accounted for by the additional protection afforded these parts by the folded forelegs when the turtle was withdrawn in the shell.
Turtles that are smashed flat on the highway, of course, have no chance of survival. Highway fatalities are usually the result either of "direct hits," where the tire of a vehicle passes directly over the turtle, or of repeated pummeling by subsequently passing vehicles. The writer, while driving behind other cars that struck turtles or by sitting beside roads, has observed numerous turtle casualties. Most are struck a glancing blow by a tire and are propelled some distance through the air or on the surface of the pavement, often to the side of the road. Such a blow is usually sufficient to crack or chip the shell, or at least to scuff away parts of the epidermal covering. Turtles, so injured, usually survive.
Parts of the shell do not break away easily, even when several deep cracks are present, and only a little bleeding occurs. A common injury inflicted on the highway is the wrenching and subsequent dislocation of the carapaco-plastral articulation. In such instances the ligamentous tissue joining the two parts is torn extensively. Under these circumstances the movable shell parts seem to act as a safety device, giving way under pressure that would crack the shell of a turtle with rigid, fixed buttresses. Dislocations of the carapaco-plastral articulation that have healed are characterized by abnormally heavy development of ligamentous tissue, which may elaborate a horny, scutelike substance on its outer surface.
The extent to which serious injury incapacitates a turtle is not known. Surely open wounds are susceptible to infection and to [Pg 640] various kinds of secondary injury; normal activity is probably interrupted by a period of quiescence, at least in the period of initial healing.
An injured female had a hole, slightly more than one inch in diameter, in the right side of the carapace at the level of the second lateral lamina. A tight, thin membrane stretched between the broken edges of the opening; this membrane contained no bone and was covered externally by scar tissue. It was obvious that this turtle had recovered, at least in part, from a serious injury (inflicted probably by a piece of heavy farm machinery).
Minor chips, scratches, and abrasions on the shell result from a variety of sources, some of them mentioned above. Small rounded pits in the bony shell (shell pitting) due to causes other than mechanical injury, are found in nearly all kinds of turtles according to Carpenter (1956), Hunt (1957), and my personal observation. In T. ornata, however, the condition is less common than in the specimens of T. carolina described by Carpenter and in the remaining species of Terrapene that I have examined.
Carpenter (1956:86) came to no conclusion as to the cause of shell pitting in Terrapene carolina but suggested that a variety of factors including parasitic fungi, parasitic invertebrates, and simple shell erosion, might be responsible.
According to my own observations on turtles in the University of Kansas collections, shell pits range in size and shape from shallow, barely discernible depressions to deep borings; I suspect that shell pitting for turtles in general has many causes, some of which may be of more frequent occurrence in one species than in another.
Hunt (1957:20) presumably was referring to shell pitting by a more suitable name when he wrote of, "… necrosis … of mycotic origin." Hunt (loc. cit.) stated that "Of those cases which have been recently examined, the author found all were due to the invasion of Mucorales beneath the plates of the epidermal laminae. This disease is of extremely common occurrence and has been found in all members of the order but is seldom found in marine species. Mycosis more frequently occurs on the plastron than on the carapace." Hunt presented no evidence to support his statement regarding invasion of the shell by Mucorales.
Evidence that injury to the soft parts of the body is also fairly common is seen in the many T. ornata with missing feet and legs. Stumps resulting from amputations are covered with tough, calloused skin and sometimes by horny tissue similar to that of the [Pg 641] antebrachial scales. Amputees are incapacitated only slightly in normal locomotion if a functional stump remains; probably a cripple is somewhat handicapped in other functions, such as burrowing, nest digging (females), and copulation (males). Causes of amputation are discussed in the section on predators.
Fractures of the limb bones are common. A female from Stafford County, Kansas (Pl. 29, Fig. 4), showed a typical case of fracture and subsequent repair; the right fibula had been broken and the ends dislocated; a great mass of bone joined the repaired break to the middle of the tibia, giving the entire skeleton of the leg the appearance of the letter "H." The fibula, shortened by the dislocation, no longer articulated by its proximal end with the femur; the tibia probably bore the entire load in the period of repair and the transverse connection that formed between the bones later took over the function of the fibula.
There is little doubt that ornate box turtles are stepped on or trampled by cattle, at least occasionally, but I never observed such an incident; the predilection of ornate turtles for dung insects and for moving along cattle pathways brings them to close quarters with cattle and probably did likewise with native ungulates. A steer, stepping on a box turtle, could inflict superficial damage to the shell or cause broken limbs but would probably not crush the turtle unless on a hard substrate.
Most adults and a few juveniles examined in the field and laboratory had one or more small injuries on the carapace that had healed or were undergoing repair. Such injuries almost never occurred on the plastron. In an injury that was undergoing repair, a small piece of smooth, whitened bone was exposed where a piece of epidermis was missing from the shell. One or more edges of the exposed bone characteristically projected over the surrounding epidermis, making the bone appear as though it had been driven forcefully, like a splinter, into the shell (Pl. 29, Figs. 1 and 2). Because of their curious appearance, small areas of repair were referred to in my notes as "splinter scars." The position and number of splinter scars were often recorded as supplementary means of individualizing turtles in the field.
Splinter scars result from minor abrasions that damage a few square millimeters of the shell. Larger areas of exposed bone were noted in only a few specimens. Two turtles at the Damm Farm had [Pg 642] bone exposed on more than one-half the surface area of the carapace; both of these turtles were probably burned in the grass fire of 1955. Ordinarily, a break in the shell does not induce extensive regeneration of tissues; when shells are damaged by crushing or cracking, regeneration of epidermis and bone occurs only along the lines of fracture, unless the broken parts have been dislocated. Ligamentous tissue develops in some breaks on the plastron, the broken area remaining slightly movable after healing is completed (Pl. 24).
Dissection of injured shells revealed the mode of shell regeneration to be the same whether a large or small portion of the shell had been damaged. An abrasion may gouge out a small portion of the shell; burning, freezing, or concussion may kill a portion of the epidermis and a corresponding portion of bone beneath it without actually disfiguring the shell. Dead bone and epidermis become loosened at the margin of the wound. The epidermis sloughs off soon afterward but the bone adheres to the wound. New epidermis and new bone, growing from undamaged tissues at the edges of the wound, encroach on the wound beneath the layer of dead bone. The piece of dead bone is thereby gradually isolated from the rest of the shell and is sloughed off when healing is complete. The dead bone may come off in one piece or slough off gradually at its edges as healing proceeds toward the center of the wound. The layer of dead bone protects the wound during the process of regeneration (Pl. 30). Areas of exposed bone become white and shiny, nearly enamellike in appearance, as a result of wear on the shell.
The above conclusions, in regard to T. ornata, agree basically with the findings of Woodbury and Hardy (1948:161-162) and Miller (1955:116) on regeneration of the shell in desert tortoises (Gopherus agassizi). Danini (1946:592-4, English summary) made histological studies on regeneration of the shell in specimens of Emys orbicularis; he found that new bone trabeculae formed on the surfaces of undamaged trabeculae at the edge of the wound and formed also in connective tissue at the center of the wound. Regeneration of bone was incomplete in some instances where total extirpation of a portion of the shell had occurred. Regenerated epidermis was usually thicker than the original scute.
Exposed bone on the shells of turtles that have been injured in fires, although dead, is unmarked and shows no evidence of being burned. Exposure to fire kills the growing portions of both the epidermis and the bone but seemingly does not actually char or disfigure [Pg 643] the bone (although the epidermis may be so affected) (Pl. 29, Fig. 3). Injuries from fire result probably from brief encounter with the fire itself or from more prolonged contact with some surface heated by the fire. A turtle that remained in a fire long enough to have its shell charred would presumably have little chance of survival. Grossly disfigured shells therefore do not result directly from burns but are due to the gnarled texture of the regenerated bone and epidermis remaining after the dead portions of the shell have been sloughed off. Information on injuries from fire was supplemented by examination of several badly burned specimens of T. carolina. Their shells were nearly covered with exposed bone and regenerated epidermis. One specimen was so badly damaged that the entire anterior rim of its carapace was loose and could be pulled away easily to disclose a gnarled mass of regenerating bone beneath it (Pl. 29, Fig. 3). There were areas near the posterior margin of the carapace of each specimen where regenerated epidermis was evident but where the bone was seemingly uninjured; the regenerated epidermis was nearly transparent.
Areas of regenerated epidermis on specimens of T. ornata were rough in texture and slightly paler than the surrounding scutes. Color-pattern is not reproduced in the process of regeneration but irregularly shaped light blotches sometimes occur in the places where radiations or other distinct markings formerly were present. A slight depression remains on the shell after regeneration is completed. I suspect that small injuries may be repaired in the course of a single growing season but that injuries involving a large part of the shell may take several years to heal completely. Cagle (1945:45) reported that a bullet wound in the shell of a painted turtle (Chrysemys picta) healed completely in approximately 23 months. Danini (loc. cit.) found that regeneration of the shell in Emys orbicularis was complete in as short a time as 225 days. Woodbury and Hardy (loc. cit.) stated that small injuries to the shell of Gopherus agassizi may take as long as seven years to heal.
Two kinds of ectoparasites were found on ornate box turtles in the course of the present study; larvae of chigger mites (Trombicula alfreddugesi) were abundant on specimens collected in summer and, larvae of the bot fly (Sarcophaga cistudinis) were found on specimens throughout the season of activity, and, in a few instances, on hibernating turtles. In general, these ectoparasites [Pg 644] do little or no harm to ornate box turtles, although heavy infestations may cause temporary interruption of normal activity or may even cause occasional death.
Concerning the larvae of T. alfreddugesi, Loomis (1956:1260) wrote, "In northeastern Kansas, larvae become numerous in early June (shortly after they first appear), increase in numbers to greatest abundance throughout late June and July, decrease slightly in August, become markedly reduced in September, and only a few larvae (mostly on hosts) remain in October and early November." He considered T. alfreddugesi to be the most abundant chigger mite in Kansas and stated (op. cit.:1265) that it is most common "… in open fields supporting good stands of grasses, weeds and shrubs, and where moderate to large populations of vertebrates are present." Loomis listed ornate box turtles (op. cit.:1261-2) as important hosts of Trombicula alfreddugesi but noted that box turtles are not so heavily infested as are certain other reptiles. The two other species of chigger mites that Loomis (op. cit.:1368) found on T. ornata in Kansas (T. lipovskyana and T. montanensis) were not found in the present study.
Box turtles were considered to have chigger infestations when the reddish larvae could be detected with the unaided eye. No chiggers were seen on turtles in the period from spring emergence until June 13, 1955. On the latter date a few scattered chiggers were noted on several individuals and it was on this same date that the writer received his first "chigger bites" of the year. Numbers of chiggers increased in the latter half of June and heavily infested turtles were noted throughout July. No chiggers were seen on box turtles after mid-September in 1955.
Chiggers were ordinarily found only on the soft parts of the turtles' bodies. Early in the season infestations were chiefly on the head and neck. Favorite sites of attachment were the point where the skin of the neck joins the carapace and on the skin around the eyes. Later in the season some chiggers could be found on nearly every part of the body where soft skin was present; concealed areas of skin, such as the axillary and inguinal pockets, the anal region, and the inner rim of the carapace (where it joins the skin of the body), harbored concentrations of chiggers. Juveniles were relatively more heavily infested than adults and, even early in the season, had chiggers attached along many of the interlaminal seams of the shell. Broad areas of soft, newly-formed epidermis on the shells of juveniles probably afforded a better [Pg 645] place of attachment to chiggers than did the interlaminal seams of adults. The interlaminal seams and transverse hinges of adults were not infested until the height of the season of chigger activity. Heavily infested adults, observed in early July, were literally covered with chiggers; red larvae outlined nearly all the scutes of the shell, the anus, the mouth, and the eyes. When turtles were picked up for examination, chiggers could be seen moving rapidly from one interlaminal seam to another.
Box turtles kept in outdoor pens and in the laboratory did not long maintain visible infestations of chiggers, even during the time in summer when turtles found in the field were heavily infested.
A four-year-old juvenile was found nearly immersed in the shallow water of a pond on July 4, 1955; its right eye had been damaged by an especially heavy concentration of chiggers. When I released the turtle, some 50 feet from the pond, it returned to the water and spent the next four days there. The turtle was probably in a period of quiescence induced by the eye injury and the heavy infestation of chiggers; immersion in water could be expected to help free the turtle of chiggers and to relieve trauma resulting from the injured eye. Richard B. Loomis told me that larval chiggers are able to survive under water for several days but that warm water will hasten their demise.
Infestations of larval bot flies (Sarcophaga cistudinis) were noted in several turtles at the Damm Farm and, upon closer scrutiny, were found to be common in preserved specimens from other areas. Larvae were always found in flask-shaped pockets (Pl. 27, Fig. 2) beneath the skin; the pockets opened to the outside by a small hole, the edges of which were dried and discolored. One larva sometimes protruded from the opening. The inside of the pocket is lined with smooth, skinlike tissue. Heavily infested box turtles may have four or five such pockets, each containing one to many larvae. The most frequent sites of the pockets are the skin of the axillary and inguinal regions, and the skin of the limbs and neck, especially near the bases of these members. Subadults were more heavily infested than older adults; no infestations of hatchlings or small juveniles were noted.
An adult female, infested with bot fly larvae when she was removed from her hibernaculum in late October, 1955, bore no trace of larvae or of the pocket that had contained them when she was recaptured the following June. According to Rokosky (1948), the larvae eventually fall to earth and pupate. The individuals of T. [Pg 646] carolina studied by him were not re-infested by adult bot flies; one turtle ate some of the larvae that dropped from its body.
The manner in which box turtles are infested by bot fly larvae is uncertain. Possibly the eggs are picked up accidentally or laid on the skin while box turtles are foraging in dung. Belding (1952:841) classifies the genus Scarophaga as semi-host-specific, depositing eggs in open wounds.
McMullen (1940), Rodeck (1949), and Rainey (1953), described individuals of T. ornata parasitized by S. cistudinis. Rokosky (1948) and Peters (1948:473) reported infestations in T. carolina. Infestations were the cause of death in the instances noted by Rainey and Rokosky.
Few first-hand observations on predators of T. ornata are available and I have found little direct evidence of predation in the course of this study. In general, adults of the species seem to have few natural enemies other than man. Several of my colleagues at the University of Kansas have observed dogs carrying box turtles in their mouths or chewing on them. Frank B. Cross told me his dog caught and ate young T. ornata in Payne County, Oklahoma, and A. B. Leonard once saw a badger carrying one in Dewy County, Oklahoma. At the Reservation, a freshly killed juvenile was found beneath the nest of a crow (Corvus brachyrhynchos) and remains of a hatchling were found in a scat of a copperhead (Agkistrodon contortrix).
Dr. Fred H. Dale, Director of the Patuxent Research Refuge, Laurel, Maryland, kindly furnished photostatic copies of cards, from the Division of Food Habits Research of the U. S. Fish and Wildlife Service, recording the instances in which Terrapene ornata was listed as a food-item. In one instance the stomach of each of two nestlings, in the same nest, of the White-necked Raven (Corvus cryptoleucus) in Terry County, Texas, contained remains of recently hatched ornate box turtles; the remains of one turtle made up 64 per cent of the contents of one stomach, and parts of three turtles made up 80 per cent of the contents of the other stomach. Each of two stomachs of the coyote (Canis latrans) from Quay County, New Mexico, contained a "trace" of ornate box turtle.
Wild carnivores known to occur on the Damm Farm were raccoons (Procyon lotor), striped skunks (Mephitis mephitis), badgers (Taxidea taxus), and coyotes (Canis latrans); all were suspect as predators of ornate box turtles.
On December 10, 1953, ten dead box turtles (eight adults and two juveniles) were discovered at the top of a cut bank on the Damm Farm, within a few feet of a burrow that was used at least part of the time by a striped skunk. The condition of the turtles suggested that they had lain in the open for several weeks. The heads and legs were missing from most of the turtles and tooth marks were discernible on several of the shells. A logical explanation of this occurrence is that the turtles, using the burrow as a hibernaculum, were ousted by a predator that also inhabited the burrow. Turtles moving about sporadically in late autumn may be quickly chilled by a sudden drop in temperature and therefore be more susceptible to predation than at other times of the year. Two of my colleagues at the Museum of Natural History informed me that they had observed similar concentrations of dead T. ornata in winter.
In July, 1952, H. B. Tordoff collected eight shells of juvenile T. ornata in a dry creek bed near Sharon, Barber County, Kansas. Some of the shells had small tooth-punctures. The stream bed habitat and the appearance of the tooth punctures tended to incriminate raccoons as predators. Raccoons, more than any other carnivore mentioned above, possess the manual dexterity necessary to pry open the shell of a box turtle and bite away the soft parts. Badgers and possibly coyotes are probably the only local carnivores (excluding large dogs) that could crack open the shell of an adult turtle by sheer force.
Adults of T. ornata, since they occasionally molest small juveniles, must be considered in the category of predators. When captive adults and juveniles were fed from the same container in the laboratory, the turtles occasionally bit one another accidently. Serious injury to the young was prevented by watching the adults closely and moving them away when they caught a smaller turtle by the leg or head. Similar accidents presumably occur in nature; juveniles and adults were sometimes found feeding side by side. William R. Brecheisen told me that adults kept in a stock tank at his farm in the summer of 1955 regularly and purposefully chased and bit small juveniles in the same tank. Brecheisen gave me a juvenile that had been so bitten; the right side of its head was badly damaged (the eye gone and a portion of the bony orbit broken) but was partly healed. Ralph J. Donahue told me that he saw an adult T. ornata attack a juvenal T. carolina, and provided a photograph of the incident. The juvenile was not injured.
Although small box turtles may occasionally be caught and killed by adults in nature, this seems not to constitute a major source of predation on the young.
Other animals that may prey upon young box turtles occasionally (and that were known to occur at the Damm Farm) are bullsnakes (Pituophis catenifer), red-tailed hawks (Buteo jamaicensis), marsh hawks (Circus cyaneus), crows (Corvus brachyrhynchos), and opossums (Didelphis marsupialis), and domestic cats.
Nest predators probably have greater effect on populations of T. ornata than do predators of hatchlings, juveniles, and adults. Four robbed nests were found at the Damm Farm; in each instance, striped skunks were thought to be the predators. E. H. Taylor told me that he once saw a bullsnake swallow an entire clutch of newly laid eggs before the female turtle could cover the nest.
Box turtles rely for protection on the closable shell and on inconspicuousness; defense reactions, except in the rare instances that biting is provoked, are purely passive.
Box turtles handled in the course of field work varied widely in their reactions. Many struggled violently when being measured or marked whereas others were completely passive, closing the shell tightly and making it difficult for me to examine the soft parts of the body. These differences in behavior did not seem to be correlated either with sex or with age; generally lessened activity was associated with suboptimum body temperatures. All box turtles found in the field were extremely wary. As soon as one sighted me (sometimes at a distance of 200 feet or more), it became motionless with shell raised from the ground and neck extended (Pl. 28, Fig. 5). Some turtles remained in this motionless stance for half an hour or more, finally moving slowly away if I remained motionless. Turtles made no attempt to escape until I approached them closely or until they were in danger of being trampled by my horse; they would then move away with remarkable rapidity. Box turtles seemed unaware of an intruder until he could be seen or until he touched the turtle. When a turtle was approached from the rear, whistling, finger snapping, and normal footfalls did not attract its attention. Latham (1917:16) observed corresponding behavior in T. carolina. Wever and Vernon (1956) found the ear of T. carolina to be keenly sensitive to sounds in the range of 100-600 cycles per second but progressively less sensitive to sounds of higher and lower frequen [Pg 649]cies. Surely a predator as stealthy as a coyote could approach a box turtle unseen and could quickly bite off at least one of the turtle's legs. Many of the mutilated box turtles that I observed may have survived such encounters with carnivores. The tendency of some individuals, when handled, to over-extend the limbs and neck (rather than closing the shell) in an attempt to escape, would make them easy victims for any predator.
Ornate box turtles were kept in my home, along with several cats. Initial behavior was characterized by mutual wariness; subsequently the cats would follow a turtle about the house for a time, occasionally pawing at an exposed limb. The turtles withdrew only when touched or when approached from the front. After a day or two the cats and turtles ignored each other, often eating and drinking from the same dishes without incident. Under these circumstances the cats, I believe, could easily have killed or injured the turtles. A turtle would occasionally gain the respect of a cat by biting it.
The strong odor sometimes given off by box turtles is produced by the secretions of four musk glands, two situated anteriorly on each side and opening by small, nearly invisible apertures beneath the fourth marginal scute. According to Hoffman (1890:9), two other musk glands, opening beneath the eighth marginal scute on each side, are also present in Terrapene; these posterior glands were not found in the several specimens of T. ornata that I dissected.
Strong odors were produced by nearly all small juveniles until they became accustomed to being handled. Older juveniles and adults produced strong odors only in response to pain or injury, as, for example, when they were killed in the laboratory prior to preservation or when they were being marked in the field. Young box turtles were capable of producing strong odors as soon as they hatched.
Norris and Zweifel (1950:3) considered the odor produced by T. o. luteola to issue from the "… concentrated, highly pungent urine…." voided by individuals when they were disturbed, and thought the production of odor to be a defense mechanism. Neill (1948b:130) reported that hatchlings of T. carolina with unhealed umbilical scars emitted a musky odor comparable to that of the stinkpot, Sternotherus odoratus; he thought the capacity to produce this odor was lost at about the time that the plastral hinge became functional.
The function of musk glands in Terrapene and, in all other turtles, is unknown. Since biting and nuzzling of the edges of the shell is [Pg 650] an integral part of the courtship of many turtles, odor produced by the musk glands may well be a means of social recognition or of sexual stimulation. Repellant odor may have a protective value in young box turtles but it is unlikely that larger predators would be frightened away or even discouraged by odor alone. In this respect Neill (loc. cit.) and I concur.
Most of the morphological characteristics distinguishing box turtles from other North American emyid turtles, the most notable of which is the movable plastron, are modifications that have evolved as a result of selectional pressures favoring adaptation to more or less terrestrial existence. Similar adaptations have arisen independently in several branches of the emyid stock (see introduction). The genus Terrapene seems to have departed farther from a generalized emyid form than have other kinds of box-turtle-like chelonians. In a morphological sense, Terrapene ornata is clearly the most specialized member of its genus now occurring in the United States (my own studies have revealed that populations in western Mexico now referred to as T. klauberi and T. nelsoni are as specialized as T. ornata in some respects but more generalized in others). The present ecological study has demonstrated that T. ornata is specialized in habits as well as in structure. It is concluded that these specializations (of more generalized and perhaps more primitive conditions as, for example in T. carolina) constitute adaptation for terrestrial existence in open, semiarid habitats. These adaptations in T. ornata have resulted, in a few instances, in unique habits and structures; however, in most instances the adaptations have produced slight but recognizable changes that are definable only by degree of difference from other species of box turtles.
The closable shell of box turtles is of obvious survival value in providing protection for the soft parts of the body. In most of the species of Terrapene, the lobes of the plastron completely close the openings of the shell; closure is so tightly effected in some individuals that it is difficult to insert the blade of a knife between the adpressed margins of carapace and plastron. In T. ornata nevertheless, both lobes of the plastron are deficient on their lateral margins; four narrow openings remain when the lobes are drawn shut. Emargination of the plastron has occurred at the places where the limbs rub against it during locomotion. This [Pg 651] reduction of the plastron permits the body to be held off the ground during forward locomotion and seemingly permits a generally freer range of movement for the limbs. The possible disadvantages of an imperfectly closable shell seem to be compensated for by increased mobility. Reduction of the plastron is correlated with a general lightening of the shell, probably associated with the increased vagility of this species. Lightening of the shell is evident also in the relatively thin, loosely articulated bony elements. Shells of adult T. ornata that are old and weathered, or macerated (unless they are partly co-ossified because of injury), can nearly always be disarticulated with ease, whereas the bony elements in the shells of adult T. carolina (all races) are nearly always co-ossified or separable only after prolonged maceration.
The relatively low, flattened shell of T. ornata is an adaptation associated with the tendency to seek shelter in the limited space of earthen forms, burrows, or small natural cavities in the course of the warm season and to burrow more deeply into the ground in winter. Terrapene ornata is, in fact, the only species of the genus that may be considered an habitual burrower. Individuals of T. carolina tend to seek shelter in the warm season by making forms in dense vegetation or by digging into yielding substrata such as mud or humus, although they may burrow deeply into the earth in winter. Extreme weakness or absence of the middorsal keel of T. ornata seems to be a modification associated with burrowing habits and general adaptation to terrestrial life; the keel is similarly reduced in testudinids.
Retention of epidermal laminae (as opposed to regular exfoliation of the older parts of scutes) occurs in all box turtles, in several other groups of terrestrial emyids, and in testudinids. The phenomenon is here considered to be a specialization of scute shedding—developed in terrestrial and semiterrestrial chelonians—that provides additional protection to the shell against wear and minor injuries.
General shortening of digits—the result of reduction in number of phalanges as well as in their length, and to a lesser degree the shortening of metapodial elements—has occurred in several groups of chelonians with terrestrial tendencies (the opposite—lengthening of phalanges and metapodials, and hyperphalangy—has occurred in certain groups that are highly aquatic). The pes of box turtles has remained relatively unchanged in this respect; a few [Pg 652] phalanges on the lateral digit have been lost (especially in three-toed forms), but little reduction in length has occurred. The chief modification of the pes is a general narrowing brought about by the tendency of the digits to be crowded together, one on top of the other, rather than spread in a horizontal plane. Considerably more modification is seen in the manus of Terrapene. Phalangeal formulae (expressing the number of phalanges from the first digit outward) range from 2-3-3-3-2 (primitive in Terrapene) to 2-3-3-2-2 in the races of carolina and have the same range in the species of eastern Mexico. Extreme reduction in number (2-2-2-2-2) as well as general shortening of phalanges occurs in T. ornata. The formula is the same in the one specimen of T. klauberi that has been skeletonized. This modification of the forelimb in T. ornata has produced a more rigid, stronger manus that is well adapted to the requirements of burrowing and to locomotion over unyielding substrata. Shortening of the manus (and, to a lesser extent, the pes) has been accompanied by reduction and loss of interdigital webbing. It is noteworthy that T. ornata has achieved the same reduction in number of phalanges as Gopherus, which displays the extreme of specialization in this respect among North American turtles. The manus in T. ornata is not shortened so much as in Gopherus.
The first toe in males of T. ornata is uniquely widened, thickened, and inturned. Males of some other species of Terrapene have greatly enlarged rear claws, some of which turn slightly inward, but none has the flexed first toe hooklike as it is in ornata (a modified first toe, resembling that described for T. ornata, has been observed in a live male of T. klauberi [now KU 51430] since the preparation of this manuscript). In males of T. ornata the penultimate phalanx of the first toe has a normal, vertical articular surface on its proximal end. However, the distal articular surface (when viewed from the distal end of the phalanx) has its axis rotated away from the vertical plane approximately 45 degrees in a counterclockwise direction. As the foot is pronated and extended, and as the digits are flexed, there is a concomitant inward rotation of the first metatarsal at its proximal joint; this rotation, combined with the divergent planes of the articulating surfaces on the penultimate phalanx, cause the ungual phalanx to be flexed at right angles to the inner side of foot, in a plane perpendicular to that of the other toes (Fig. 21).
The precise function of the modified first toe of males is unknown, [Pg 653] although it is reasonably safe to assume that the modification is closely associated with clasping during coition. In the matings that I observed, the inturned first claw of the male secured a hold on the female's rump or just beneath her legs, whereas the remaining three toes gripped the edge of her plastron. The combined hold, on shell and skin, clearly affords the male a more secure position during coitus (whether the female clasps his legs with hers or not) than would a hold on skin or shell alone. Possibly intromission can be maintained in this position even when the female is attempting to escape. In males the plastron is less concave in T. ornata than in T. carolina. Furthermore, males of T. ornata are, on the average, smaller than females, whereas the reverse is true in T. carolina. Possibly the ability of the male to secure an especially firm grip on the female enhances the probability of small males mounting and inseminating larger females, whereas successful matings might otherwise be limited to pairs in which the male was the larger member.
It is worthy of note that turtles of the genus Terrapene are seemingly the only North American emyids that carry out the entire process of mating on land; other, semiterrestrial emyids (for example, Clemmys insculpta and Emydoidea blandingi) return to water for actual coition, although the precoital behavior sometimes occurs on land.
Nearly all gradations from a fully developed zygomatic arch to a greatly reduced arch can be observed in skulls of the various species of Terrapene (Fig. 2) (Taylor, 1895:586, Figs. 2-7). The highest degree of reduction is achieved in T. ornata and T. klauberi, both of which lack the quadratojugal bone and have no zygomatic arch whatever (except for an occasional, poorly defined anterior vestige formed by the postfrontal, the jugal, or both). Reduction of the zygoma clearly represents modification of a more generalized, complete arch. As yet there is no clear evidence that reduction of the zygomatic arch is of adaptive value. It is noteworthy, however, that similar reduction of the arch has occurred independently in a number of emyid and testudinid groups, nearly all of which have terrestrial or semiterrestrial habits. Although discussion of phyletic lines in Terrapene is beyond the scope of this report, I tentatively suggest that reduced zygomatic arches have arisen independently in more than one group of Terrapene and that similar reduction of the arch in two species of the genus does not necessarily indicate an especially close relationship of such species.
In a recent survey of cloacal bursae in chelonians, Smith and James (1958:88) reported T. ornata and T. mexicana to be among the few emyids that lacked these structures; in the opinion of the authors (op. cit.:94) cloacal bursae evolved in chelonians that required an accessory respiratory organ for long periods of quiescence (hibernation or aestivation) under water, and were secondarily lost in terrestrial forms that hibernated on land. The assumption is a reasonable one, at least in regard to emyids and testudinids. Lack of cloacal bursae in T. ornata and in all testudinids, can be correlated with the completely terrestrial habits of those turtles. Cloacal bursae seem to be vestigial in the species of Terrapene possessing them and to be of little or no use as respiratory structures (except perhaps in T. coahuila).
In most of the species of Terrapene the carapace has a pattern of pale markings on a darker background; however, unicolored individuals are the rule in certain populations (for example, at the western edge of the range of T. carolina and in T. ornata luteola) and occur as occasional variations in other populations (in T. yucatana, T. mexicana, and, throughout the range of T. carolina, albeit more commonly in the southeastern part of the range). Personal observation of interspecific and ontogenetic variation of color patterns of box turtles has convinced me that a basic pattern of more or less linear radiations is the one from which all other patterns (including spots, blotches, rosettes, and the unicolored condition) can be derived, and, that the radial pattern is generalized and primitive for Terrapene (possibly for all emyids and testudinids as well). In the light of this conclusion, the radial pattern of T. ornata may be considered generalized. I suspect, however, that the pattern of a living species most closely approaching that of the primitive ancestral stock of Terrapene is the pattern of fine, wavy, dark radiations (on a paler background) present in young examples of T. coahuila.
Box turtles in general have lower reproductive potentials (as indicated by fewer eggs and longer prepuberal period) than do most aquatic emyids. This low potential seems to be compensated for by a lower rate of postnatal mortality (especially in adults) due to the protection afforded by the closable shell and the ability to recover from serious injury. Terrapene o. ornata and T. c. carolina are the only box turtles the life histories of which are known well enough to permit significant comparison. The reproductive potentials of T. o. ornata and T. c. carolina seem to be much the same.
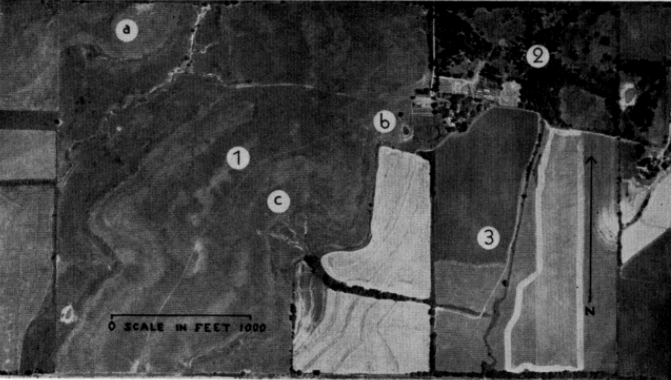






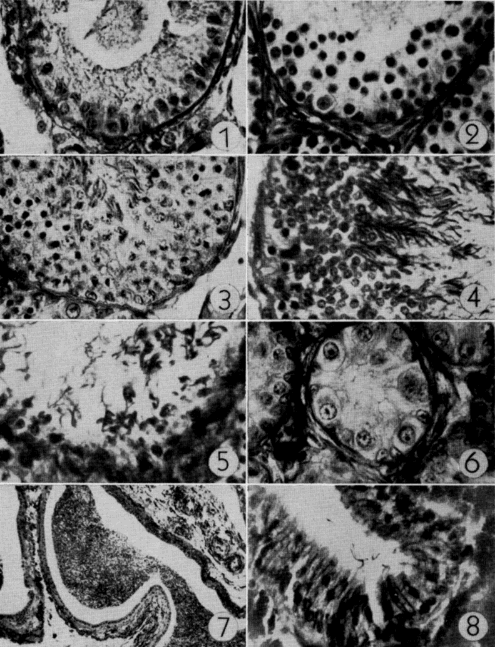
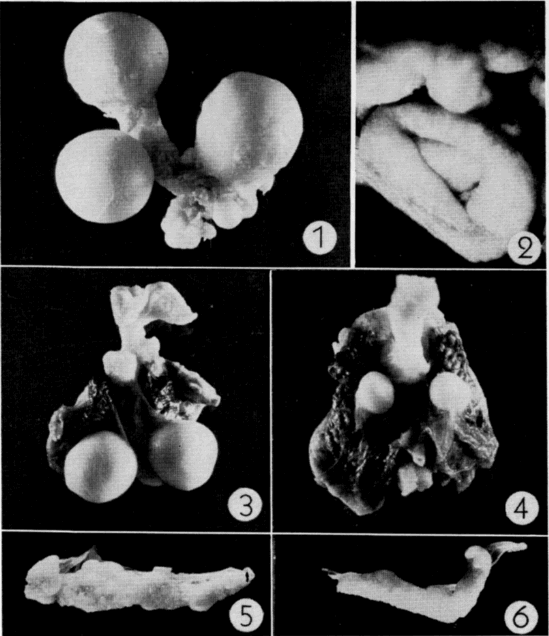


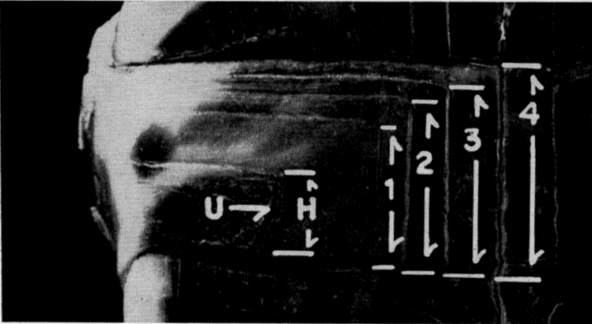
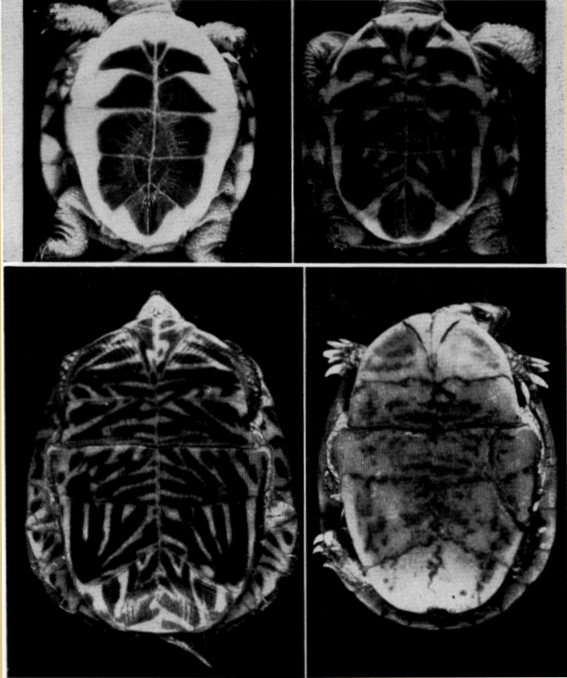
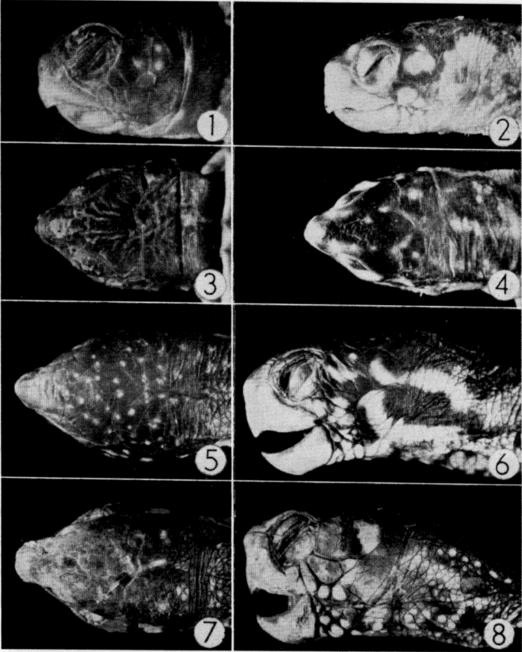


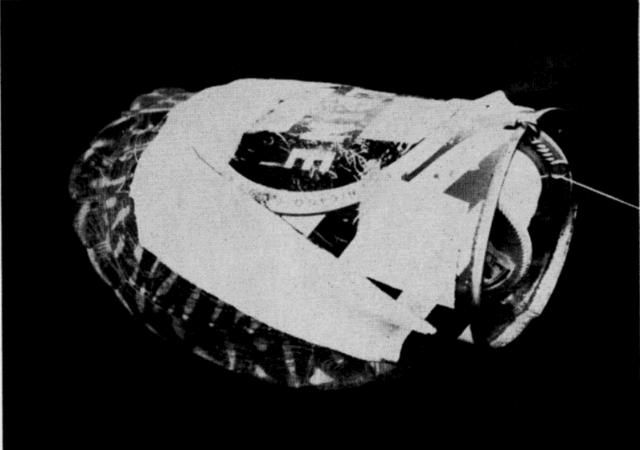
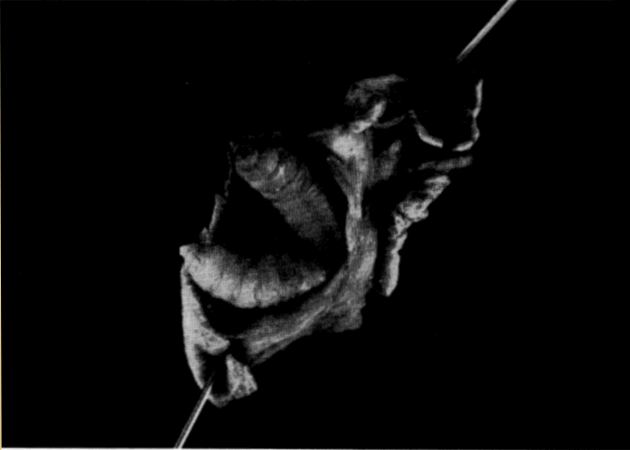
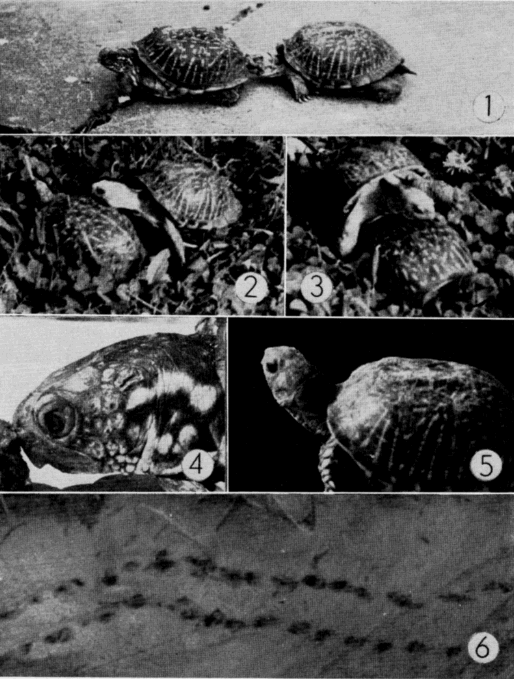
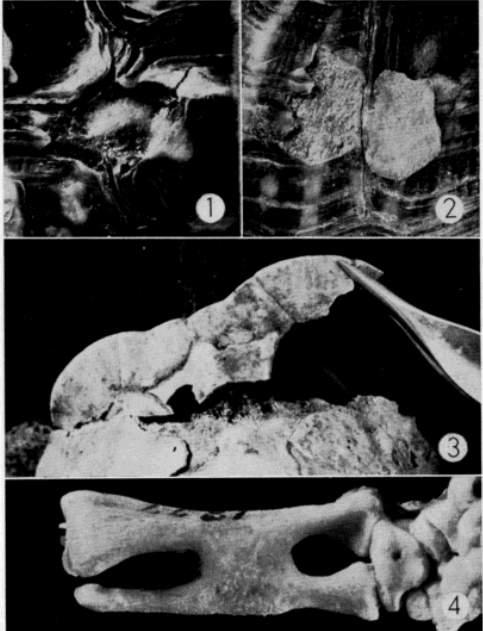
Terrapene ornata seems to concentrate its breeding season (laying, incubation, and hatching of eggs) more nearly in the middle of the warm season than does T. c. carolina. This concentration probably is an adaptation for breeding in open habitats where, under environmental temperatures less equable than in forest, eggs would develop more rapidly and hatch sooner but would be less able to survive winter temperatures.
Males of T. o. ornata become sexually mature when younger and smaller than females and rarely grow as large as females. Nichols (1939a:20) indicated the reverse to be true of T. c. carolina; Nichols further indicated that growth continued some six to eight years after puberty. Most individuals of T. o. ornata attain maximum size within two to three years after puberty.
Although it is difficult to be certain about the adaptive value of color and pattern, it seems that in box turtles, as in many other kinds of animals, patterns and colors most nearly blending with those of the habitat have some selective value in providing concealment from enemies. The pattern of linear radiations in T. o. ornata closely resembles the patterns formed by light passing through grasses and associated vegetation and camouflages the turtle. In a similar manner, partial or complete loss of radial markings in T. o. luteola seems to provide concealment in habitats where vegetation is sparse and where blending with the substrate is of survival value. The patterns of blotches and broken radiations in most of the subspecies of T. carolina likewise provide camouflage by tending to match patterns formed by the light passing through a leafy canopy.
Although ornate box turtles are omnivorous, they probably depend on insects as a dietary staple. In years when preferred kinds of insects were unusually abundant, the turtles grew more than in other years. A large proportion of the insects eaten is obtained by foraging in or near dung. Alteration of the dung community—at least in a physical sense, but presumably also by influencing the successional stages of the dung biota—is one of the few evident effects of box turtles on the environment. Although certain kinosternids (Carr, 1952:93), emyids (Deraniyagala, 1939:257; Loveridge and Williams, 1957:198), and testudinids (Loveridge and Williams, op. cit.:247) eat mammalian feces, T. ornata is seemingly the only chelonian that habitually seeks its staple diet in dung. The habit seems to be yet another specialization for terrestrial existence. The carnivorous habits of T. ornata reverse the general trend toward omnivorous and herbivorous habits in other turtles [Pg 656] that have become partly (emyids) or wholly (testudinids) terrestrial.
It seems remarkable that none of the species of true tortoises occurring in the grasslands of the world has developed insectivorous habits or utilized the unique food niche (in regard to dung-foraging) filled by ornate box turtles in the Great Plains; tortoises are, as far as is known, strictly herbivorous. The ranges of Gopherus and Terrapene are now almost mutually exclusive and the two kinds do not compete with each other for food in the few places where they occur together. It is known, however, that box turtles (T. longinsulae, ornata-like, earliest known box turtle) and true tortoises (genera Testudo and Gopherus, see Williams, 1950:25-26, Fig. 2) occurred together in what is now the Great Plains in early Pliocene times and probably for some time before and after this. Assuming that food habits of fossil representatives of these genera were somewhat like the habits of recent representatives, ornate box turtles may have developed insectivorous habits at a time when other food niches were filled by herbivorous tortoises. Box turtles possibly survived subsequent changes in habitat that made it impossible for populations of large tortoises to exist in the Great Plains.
Box turtles of the genus Terrapene are emyid turtles that are specialized for terrestrial existence. Two of the seven species now recognized—T. ornata and T. carolina—occur in the United States. Terrapene carolina inhabits forested areas in the east whereas T. ornata is characteristic of open grassy areas in the west; the ranges of the two species overlap in the broad belt of prairie-forest ecotone in the central United States. Terrapene ornata is considered to be the most specialized of living box turtles.
The natural history of T. o. ornata Agassiz was studied in the period, 1953 to 1957. Intensive field studies were made in Douglas County, northeastern Kansas, on a small area of prairie and on the University of Kansas Natural History Reservation. Field observations were made also in a number of other places in eastern Kansas. Laboratory studies supplemented field studies.
Habitats occupied are chiefly open areas; they vary in regard to food supply, temperature, moisture, and kind of soil. The grassy prairies of Nebraska, Kansas, Oklahoma, and northern Texas seem to provide optimum habitat for ornate box turtles; in these areas box turtles are active on a majority of days from April to [Pg 657] October. The subspecies luteola is adapted to the more rigorous and arid environment of the southwestern United States, where activity may be possible for only a few weeks in the year. The remainder of the year is spent in a state of quiescence. Factors limiting the distribution of T. ornata are: 1) the presence of a substrate too hard to permit digging of nests and forms (altitudinal distribution in southwestern United States and distribution at western edge of the range); 2) temperatures causing the ground to freeze deep enough (approximately 30 inches) to kill turtles in hibernacula (northern edge of range); and, 3) the lack of one or more relatively wet periods in the course of the warm season, preventing at least temporary emergence from quiescence (southwestern part of range). The activities of man probably have affected population density in local areas but limit the geographic range only in the north (Blanchard, 1923:19-20, 24) where intensive cultivation probably has excluded the species.
Preferred habitat in northeastern Kansas is open rolling grassland grazed by cattle; populations are most dense near natural breaks in the grassy vegetation such as fences, scattered rocks on hillsides, ravines, and stream-beds.
Mating occurs most commonly in spring and autumn; courtship behavior includes pushing and biting on the part of the male. In coitus the hind legs of the male are held tightly by the female; the male falls backward after coitus, still clasped by the female. A few sperm are stored in the oviducts; fertilization without reinsemination can occur. The spermatogenic cycle begins in May and reaches its peak in September, when large numbers of sperm and spermatids are present in the testes; the cycle is completed in October, when sperm pass into the epididymides. The testes are smallest in spring and largest in September. Females are inseminated with sperm produced in the preceding year. The ovarian cycle begins in midsummer, soon after ovulation, and continues up to the time of the next ovulation. Follicular growth is rapid in the period from spring emergence to ovulation. Large follicles remaining after ovulation represent, in many instances, eggs that will be laid later in the same season. Follicular atresia is never great enough to account for the destruction of all large follicles remaining after ovulation. All mature females lay at least one clutch of eggs per year. It is estimated that one-third of the females produces two clutches of eggs in a single season. Second clutches contain fewer eggs than first clutches. An alternation [Pg 658] of ovarian activity occurs, whereby one ovary is more active than its partner in one season and less active in the next season. Alternating activity of ovaries accounts in part for the reduced number of eggs in young females, breeding for the first time, and in older, nearly senile females. Extrauterine migration of ova results usually in a more even distribution of eggs in the oviducts. Corpora lutea constitute an accurate record of the number of eggs produced by the ovary as well as the number of eggs laid.
Nesting occurs from May through July but is most common in mid-June; some of the females nesting early in the season lay a second clutch of eggs in July. Nests are dug in the earth by the female using her hind legs. Preferred nesting sites are open, well-drained places with a soft substrate. The nesting site is selected after a period of wandering, in which the female tests the substrate at a number of places; some females search for a nest site for more than a week. Nest digging begins in the evening and is usually completed after dark. Captive females dug a preliminary cavity in which the body rested during the digging of the main nest cavity. The entire clutch of eggs is laid in one nest. The average number of eggs in 23 clutches was 4.7 (range, 2 to 8). The average size of eggs tends to be inversely proportional to the number of eggs in a clutch. Eggs increase in bulk by absorption of water in the course of incubation. Immersion in water for short periods does not harm eggs. The incubation period under favorable environmental conditions is approximately 65 days; cool, damp conditions prolong the incubation period and probably constitute an important factor of prenatal mortality in certain years. Eggs that do not hatch before winter probably do not survive. Emergence of hatchlings from the nest may, however, be delayed until spring if the soil is dry in autumn. Hatchlings can probably escape freezing by burrowing into the walls of the nest. Infertility and prenatal mortality account for at least 40 per cent of the eggs laid, according to laboratory findings. Progeny of a single adult female (considering factors of mortality, multiple layings, and average age of puberty) would number approximately 300 after 20 years. Reproductive processes probably continue throughout life, although possibly at a somewhat reduced rate in later life.
Young box turtles are active soon after hatching but become quiescent if allowed to burrow in soil or if they are covered with damp cotton. Some captive hatchlings take live food in the first days of life but others do not eat until the following spring; initiation [Pg 659] of growth is coincident with initiation of regular feeding. The yolk sac retracts mainly during hatching; it sometimes ruptures after hatching. The caruncle remains on the beak for a variable length of time, but never is present in the spring following hatching.
Major growth-rings on the epidermal laminae are formed regularly, one after each season of growth, in the first 10 to 14 years of life. Minor growth-rings occur between major rings and are shallower. Growth of epidermal laminae results from the formation, in spring, of a new layer of epidermis beneath the existing scute. The peripheral projection of the new layer is distinct in texture and color from the older part of the scute and is separated from it by a major growth-ring. Minor growth-rings form when growth slows or temporarily stops during periods of quiescence; no new layer of epidermis is formed. Growth-rings constitute an accurate record of growth that can be studied at any time in the life of the turtle; they are accurate indicators of age only as long as regular annual growth persists.
Growth in the season of hatching depends on early hatching and early emergence from the nest. Turtles that remain in the nest until spring probably do not grow. Slightly less than half of the free-living individuals studied grew in the season of hatching. Precociousness in early life often results in the attainment of sexual maturity at an earlier than average age.
Growth is rapid at first (increments in plastral length average 68, 29, and 18 per cent, respectively, in the first three years) and then slows gradually until puberty. Attainment of sexual maturity is more closely correlated with size than with age. Males mature when smaller (76 per cent were mature when plastron 100 to 109 mm. long) and younger (average age, eight to nine years) than females (66 per cent were mature when plastron 110 to 119 mm. long, average age at maturity, ten to eleven years) but females grow larger than males. A few individuals of each sex reach puberty three to four years sooner than average.
The average number of growing days per season is approximately 160. Amount of growth in any season depends on climatic factors that influence food supply and foraging conditions. Growth rate is directly correlated with precipitation, being highest when large populations of grasshoppers and long periods of favorable weather occur in the same year. Zones of epidermis formed in years when growth was especially slow or especially fast constituted landmarks [Pg 660] that were helpful in interpreting growth-histories. Growth stops two to three years after puberty. The total growing period is estimated to be not more than 15 to 20 years. Longevity is estimated to be approximately 50 years.
A number of changes in structure and appearance occur in the period from hatching to puberty. Fontanelles of the bony shell close at or before puberty. Movable parts of the plastron are not functional until the fourth year. Markings on the carapace change from a series of dots to distinct, straight-sided radiations, and a similar pattern develops on the plastron. Markings on the heads of females resemble those of juveniles but males have greenish heads. Males further differ from females in having a red iris, more brightly colored antebrachial scales, and a turned in first toe.
Analysis of some 500 body temperatures (Centigrade) obtained under natural conditions revealed the following: the optimum temperature for activity is near 30 degrees; box turtles emerge from cover usually when body temperature is 24 degrees or higher, and almost never when the body temperature is below 15 degrees; body temperature is raised to optimum by basking in open areas although activity begins at suboptimum temperatures if basking is impossible; cover of dens, burrows, or forms is sought when the body temperature rises above 30 degrees; and, maximum and minimum body temperatures that would be lethal to box turtles (for prolonged periods) are approximately 40 and zero degrees, respectively. Laboratory experiments showed speed of response to environmental temperature to be inversely proportional to bulk; hatchlings could be chilled or warmed more than twice as fast as adults and were active within a narrower range of temperature. Ornate box turtles in general are subject to a narrower range of thermal activity than are aquatic turtles that occur in the same areas.
Box turtles are dormant approximately five and one-half months of the year—from late October to mid-April. Warm weather in November and late March sometimes stimulates temporary activity but dormancy is uninterrupted from mid-November to early March. Forms, dens, and burrows are used as hibernacula. Depth of hibernacula is dependent on severity of temperatures and amount of vegetational cover; hibernacula in open grassland were seven to 18 inches deep whereas those in wooded areas were six inches or shallower. Box turtles are ordinarily solitary when hibernating. Injuries and deaths due to freezing probably occur in the coldest part of the winter. The lowest body temperature of a turtle that [Pg 661] survived a winter was 2.7 degrees; an individual, the temperature of which was nearly zero for several days, subsequently died. Turtles burrow upward at the end of hibernation and remain just below the surface for a week or two before emerging. The primary stimulus for emergence seems to be a period of warm moist weather.
Populations of T. ornata observed under natural conditions were chiefly carnivorous, although captives ate a variety of animal and vegetable matter. Insects, consisting chiefly of beetles, caterpillars, and one species of grasshopper, comprised approximately 89 per cent (by volume) of the food present in stomachs. Beetles (chiefly scarabaeids and carabids) are obtained in or near dung and seem to constitute the most important staple element of the diet. Piles of dung, disturbed by turtles in the course of their foragings, were characteristic "sign" of T. ornata in the areas studied.
Insects form the bulk of the diet for most of the year, although certain other foods, when especially abundant for short periods (mulberries for example), are eaten in large quantity or eaten to the exclusion of all other foods. Ornate box turtles occasionally eat the eggs and young of ground-nesting birds and slightly damage vegetables, but in no instance do these feeding habits significantly affect the economy of man. Box turtles probably benefit man by destroying large numbers of crop-damaging insects (locustids and noctuid caterpillars).
Box turtles were more numerous than most kinds of reptiles at the Damm Farm and were the most conspicuous of any kind of reptile. One hundred and ninety-four turtles were marked; one-fourth of these were recaptured at least twice. Population density in certain areas of favorable habitat ranged from 2.6 to 6.3 turtles per acre. The total number of individuals on the study area was estimated to be 286. The marked population consisted of 53 per cent adult or subadult females, 31 per cent adult males, and 16 per cent juveniles of undetermined sex. Only six individuals had plastra shorter than 60 millimeters. Small box turtles are not so rare as these samples indicate; they are infrequently obtained because their smallness and ability to blend with the substrate make them difficult to see. More females than males were found in all months of the season of activity, excepting April and August when more males were found; the preponderance of females was greatest in the nesting season (June and July).
Ornate box turtles walk with the shell held off the substrate. They are able to climb steep embankments or low barriers with [Pg 662] some facility. Swimming ability is sufficient to permit survival in water and traversal of water-barriers but ornate box turtles almost never swim voluntarily.
Daily activity consists of periods of basking, foraging, and rest, the durations of which are influenced by temperature and humidity. There is no activity after dark except that of nesting females. After several days of activity there is a period of rest; rest periods seemed not to be correlated with climatic conditions. The average distance traveled per day in summer is 200 to 300 feet. Movements of gravid females are more extensive (average, 363 feet per day) than those of other members of the population; one individual traveled approximately one-fourth of a mile in a single day. Turtles removed from their normal home ranges traveled farther per day than any other group. Movements in autumn are less extensive (average, 152 feet per day) than at other times in the season of activity.
Individual box turtles tended to remain in small areas for long periods; these areas were interpreted as home ranges. The estimated average radius of 44 home ranges was 278 feet (average area, 5.6 acres). The average area of eight home ranges that were actually measured was five acres. General suitability of habitat and certain physical features of terrain (rock fences, ravines, barren fields) that acted as barriers were thought to be the most important factors governing size of home range. Of two turtles removed more than one-fourth of a mile from their home ranges, one homed and one did not. Home ranges of turtles of all ages and sexes overlap broadly. There was no indication that territoriality or social hierarchy existed in the population studied.
Box turtles are subject to injury from natural causes that include fire, cold, molestation by predators, and trampling by cattle. Automobiles and farm machinery now constitute major causes of mortality and serious injury. Capacity to recover after serious injury is great but there is increased chance for secondary injury, infection, and predation in the period of recovery. Pits on the shell from unknown causes ("shell pitting") are less common in ornate box turtles than in other kinds of turtles.
Ectoparasites infesting T. ornata are larvae of chigger mites (genus Trombicula) and larvae of bot flies (Sarcophaga cistudinis). Ectoparasites usually have little adverse effect on the turtles, although heavy infestations cause occasional injury or death.
Few natural enemies other than man are known; however most [Pg 663] wild carnivores as well as opossums, large birds, and domestic dogs and cats are suspect as predators. The incidence of predation on eggs and small juveniles is far greater than on older juveniles and adults. Adults of T. ornata occasionally attack smaller individuals.
Ornate box turtles are able to detect the presence of intruders, by sight, from a distance of several hundred feet in open country; apparently, intruders are not detected until seen. Defensive behavior is passive; the shell is closed tightly in response to painful stimuli and, in some instances, at the sight of an intruder. Juveniles usually void odoriferous fluid from the musk glands when handled but adults do so only in response to pain or injury. The function of the musk glands is unknown; possibly the odor of musk is a means of sexual identification or stimulation. Although the musk is probably distasteful to predators, repellent odor alone seems to be of doubtful value as a defense mechanism.
Cagle, F. R., and Tihen, J.
Cahn, A. R., and Conder, E.
Cowles, R. B., and Bogert, C. M.
Hildebrand, S. F., and Prytherch, H. F.
Legler, J. M., and Fitch, H. S.[Pg 667]
Loveridge, A., and Williams, E.
Lynn, W. G., and Ullrich, M. C.
Norris, K. S., and Zweifel, R. G.
Odum, E. P., and Kuenzler, E. J.
Ortenburger, A. I., and Freeman, B.
Penn, G. H., Jr., and Pottharst, K. E.
Smith, H. M., and James, L. F.
Smith, H. M., and Ramsey, L. W.[Pg 669]
Stock, C., and Bode, F. D.
Wever, E. G., and Vernon, J. A.
Woodbury, A. M., and Hardy, R.
Transmitted August 27, 1959.
THE STATE PRINTING PLANT
TOPEKA, KANSAS
1960
MUSEUM OF NATURAL HISTORY
Institutional libraries interested in publications exchange may obtain this series by addressing the Exchange Librarian, University of Kansas Library, Lawrence, Kansas. Copies for individuals, persons working in a particular field of study, may be obtained by addressing instead the Museum or Natural History, University of Kansas, Lawrence, Kansas. There is no provision for sale of this series by the University Library, which meets institutional requests, or by the Museum of Natural History, which meets the requests of individuals. When individuals request copies from the Museum, 25 cents should be included, for each separate number that is 100 pages or more in length, for the purpose of defraying the costs of wrapping and mailing.
* An asterisk designates those numbers of which the Museum's supply (not the Library's supply) is exhausted. Numbers published to date, in this series, are as follows:
| Vol. 1. | Nos. 1-26 and index. Pp. 1-638, 1946-1950. | |
| *Vol. 2. | (Complete) Mammals of Washington. By Walter W. Dalquest Pp. 1-444, 140 figures in text. April 9, 1948. | |
| Vol. 3. | *1. | The avifauna of Micronesia, its origin, evolution, and distribution. By Rollin H. Baker. Pp. 1-359, 16 figures in text. June 12, 1951. |
| *2. | A quantitative study of the nocturnal migration of birds. By George H. Lowery, Jr. Pp. 361-472, 47 figures in text. June 29, 1951. | |
| 3. | Phylogeny of the waxwings and allied birds. By M. Dale Arvey. Pp. 473-530, 49 figures in text, 13 tables. October 10, 1951. | |
| 4. | Birds from the state of Veracruz, Mexico. By George H. Lowery, Jr., and Walter W. Dalquest. Pp. 531-649, 7 figures in text, 2 tables. October 10, 1951. | |
| Index. Pp. 651-681. | ||
| *Vol. 4. | (Complete) American weasels. By E. Raymond Hall. Pp. 1-466, 41 plates, 31 figures in text. December 27, 1951. | |
| Vol. 5. | Nos. 1-37 and index. Pp. 1-676, 1951-1953. | |
| *Vol. 6. | (Complete) Mammals of Utah, taxonomy and distribution. By Stephen D. Durrant. Pp. 1-549, 91 figures in text, 30 tables. August 10, 1952. | |
| Vol. 7. | *1. | Mammals of Kansas. By E. Lendell Cockrum. Pp. 1-303, 73 figures in text, 37 tables. August 25, 1952. |
| 2. | Ecology of the opossum on a natural area in northeastern Kansas. By Henry S. Fitch and Lewis L. Sandidge. Pp. 305-338, 5 figures in text. August 24, 1953. | |
| 3. | The silky pocket mice (Perognathus flavus) of Mexico. By Rollin H. Baker. Pp. 339-347, 1 figure in text. February 15, 1954. | |
| 4. | North American jumping mice (Genus Zapus). By Philip H. Krutzsch. Pp. 349-472, 47 figures in text, 4 tables. April 21, 1954. | |
| 5. | Mammals from Southeastern Alaska. By Rollin H. Baker and James S. Findley. Pp. 473-477. April 21, 1954. | |
| 6. | Distribution of Some Nebraskan Mammals. By J. Knox Jones, Jr. Pp. 479-487. April 21, 1954. | |
| 7. | Subspeciation in the montane meadow mouse, Microtus montanus, in Wyoming and Colorado. By Sydney Anderson. Pp. 489-506, 2 figures in text. July 23, 1954. | |
| 8. | A new subspecies of bat (Myotis velifer) from southeastern California and Arizona. By Terry A. Vaughan. Pp. 507-512. July 23, 1954. | |
| 9. | Mammals of the San Gabriel mountains of California. By Terry A. Vaughan. Pp. 513-582, 1 figure in text, 12 tables. November 15, 1954. | |
| 10. | A new bat (Genus Pipistrellus) from northeastern Mexico. By Rollin H. Baker. Pp. 583-586. November 15, 1954. | |
| 11. | A new subspecies of pocket mouse from Kansas. By E. Raymond Hall. Pp. 587-590. November 15, 1954. | |
| 12. | Geographic variation in the pocket gopher, Cratogeomys castanops, in Coahuila, Mexico. By Robert J. Russell and Rollin H. Baker. Pp. 591-608. March 15, 1955. | |
| 13. | A new cottontail (Sylvilagus floridanus) from northeastern Mexico. By Rollin H. Baker. Pp. 609-812. April 8, 1955. | |
| 14. | Taxonomy and distribution of some American shrews. By James S. Findley. Pp. 613-618. June 10, 1958. | |
| 15. | The pigmy woodrat, Neotoma goldmani, its distribution and systematic position. By Dennis G. Rainey and Rollin H. Baker. Pp. 619-624, 2 figures in text. June 10, 1955. | |
| Index. Pp. 625-651. | ||
| Vol. 8. | 1 | Life history and ecology of the five-lined skink, Eumeces fasciatus. By Henry S. Fitch. Pp. 1-156, 26 figures in text. September 1, 1954. [Pg ii] |
| 2 | Myology and serology of the Avian Family Fringillidae, a taxonomic study. By William B. Stallcup. Pp. 157-211, 23 figures in text, 4 tables. November 15, 1954. | |
| 3 | An ecological study of the collared lizard (Crotaphytus collaris). By Henry S. Fitch. Pp. 213-274, 10 figures in text. February 10, 1956. | |
| 4 | A field study of the Kansas ant-eating frog, Gastrophryne olivacea. By Henry S. Fitch. Pp. 275-306, 9 figures in text. February 10, 1956. | |
| 5 | Check-list of the birds of Kansas. By Harrison B. Tordoff. Pp. 307-359, 1 figure in text. March 10, 1956. | |
| 6 | A population study of the prairie vole (Microtus ochrogaster) in northeastern Kansas. By Edwin P. Martin. Pp. 361-416, 19 figures in text. April 2, 1956. | |
| 7 | Temperature responses in free-living amphibians and reptiles of northeastern Kansas. By Henry S. Fitch. Pp. 417-476, 10 figures in text, 6 tables. June 1, 1956. | |
| 8 | Food of the crow, Corvus brachyrhynchos Brehm, in south-central Kansas. By Dwight Platt. Pp. 477-498, 4 tables. June 8, 1956. | |
| 9 | Ecological observations on the woodrat, Neotoma floridana. By Henry S. Fitch and Dennis G. Rainey. Pp. 499-533, 3 figures in text. June 12, 1956. | |
| 10 | Eastern woodrat, Neotoma floridana: Life history and ecology. By Dennis G. Rainey. Pp. 535-646, 12 plates, 13 figures in text. August 15, 1956. | |
| Index. Pp. 647-675. | ||
| Vol. 9. | 1 | Speciation of the wandering shrew. By James S. Findley. Pp. 1-68, 18 figures in text. December 10, 1955. |
| 2 | Additional records and extensions of ranges of mammals from Utah. By Stephen D. Durrant, M. Raymond Lee, and Richard M. Hansen. Pp. 69-80. December 10, 1955. | |
| 3 | A new long-eared myotis (Myotis evotis) from northeastern Mexico. By Rollin H. Baker and Howard J. Stains. Pp. 81-84. December 10, 1955. | |
| 4 | Subspeciation in the meadow mouse, Microtus pennsylvanicus, in Wyoming. By Sydney Anderson. Pp. 85-104, 2 figures in text. May 10, 1956. | |
| 5 | The condylarth genus Ellipsodon. By Robert W. Wilson. Pp. 105-116, 6 figures in text. May 19, 1956. | |
| 6 | Additional remains of the multituberculate genus Eucosmodon. By Robert W. Wilson. Pp. 117-123, 10 figures in text. May 19, 1956. | |
| 7 | Mammals of Coáhuila, Mexico. By Rollin H. Baker. Pp. 125-335, 75 figures in text. June 15, 1956. | |
| 8 | Comments on the taxonomic status of Apodemus peninsulae, with description of a new subspecies from North China. By J. Knox Jones, Jr. Pp. 337-346, 1 figure in text, 1 table. August 15, 1956. | |
| 9 | Extensions of known ranges of Mexican bats. By Sydney Anderson. Pp. 347-351. August 15, 1956. | |
| 10 | A new bat (Genus Leptonycteris) from Coahuila. By Howard J. Stains. Pp. 353-356. January 21, 1957. | |
| 11 | A new species of pocket gopher (Genus Pappogeomys) from Jalisco, Mexico. By Robert J. Russell. Pp. 357-361. January 21, 1957. | |
| 12 | Geographic variation in the pocket gopher, Thomomys bottae, in Colorado. By Phillip M. Youngman. Pp. 363-387, 7 figures in text. February 21, 1958. | |
| 13 | New bog lemming (genus Synaptomys) from Nebraska. By J. Knox Jones, Jr. Pp. 385-388. May 12, 1958. | |
| 14 | Pleistocene bats from San Josecito Cave, Nuevo Leon, Mexico. By J. Knox Jones, Jr. Pp. 389-396. December 19, 1958. | |
| 15 | New Subspecies of the rodent Baiomys from Central America. By Robert L. Packard. Pp. 397-404. December 19, 1958. | |
| 16 | Mammals of the Grand Mesa, Colorado. By Sydney Anderson. Pp. 405-414, 1 figure in text. May 20, 1959. | |
| 17 | Distribution, variation, and relationships of the montane vole, Microtus montanus. By Sydney Anderson. Pp. 415-511. 12 figures in text, 2 tables. August 1, 1959. | |
| 18 | Conspecificity of two pocket mice, Perognathus goldmani and P. artus. By E. Raymond Hall and Marilyn Bailey Ogilvie. Pp. 513-518, 1 map. January 14, 1960. | |
| 19 | Records of harvest mice, Reithrodontomys, from Central America, with description of a new subspecies from Nicaragua. By Sydney Anderson and J. Knox Jones, Jr. Pp. 519-529. January 14, 1960. [Pg iii] | |
| 20 | Small carnivores from San Josecito Cave (Pleistocene), Nuevo León, México. By E. Raymond Hall. Pp. 531-538, 1 figure in text. January 14, 1960. | |
| 21 | Pleistocene pocket gophers from San Josecito Cave, Nuevo León, México. By Robert J. Russell. Pp. 539-548, 1 figure in text. January 14, 1960., | |
| 22 | Review of the insectivores of Korea. By J. Knox Jones, Jr., and David H. Johnson. Pp. 549-578. February 23, 1960. | |
| More numbers will appear in volume 9. | ||
| Vol. 10 | 1 | Studies of birds killed in nocturnal migration. By Harrison B. Tordoff and Robert M. Mengel, Pp. 1-44, 6 figures in text, 2 tables. September 12, 1956. |
| 2 | Comparative breeding behavior of Ammospiza caudacuta and A. maritima. By Glen E. Woolfenden. Pp. 45-75, 6 plates, 1 figure. December 20, 1956. | |
| 3 | The forest habitat of the University of Kansas Natural History Reservation. By Henry S. Fitch and Ronald R. McGregor. Pp. 77-127, 2 plates, 7 figures in text, 4 tables. December 31, 1956. | |
| 4 | Aspects of reproduction and development in the prairie vole (Miorotus ochrogaster). By Henry S. Fitch. Pp. 129-161, 8 figures in text, 4 tables. December 19, 1957. | |
| 5 | Birds found on the Arctic slope of northern Alaska. By James W. Bee. Pp. 163-211, pls. 9-10, 1 figure in text. March 12, 1958. | |
| 6 | The wood rats of Colorado: distribution and ecology. By Robert B. Finley, Jr. Pp. 213-552, 34 plates, 8 figures in text, 35 tables. November 7, 1958. | |
| 7 | Home ranges and movements of the eastern cottontail in Kansas. By Donald W. Janes. Pp. 553-572, 4 plates, 3 figures in text. May 4, 1959. | |
| 8 | Natural history of the salamander, Aneides hardyi. By Richard F. Johnston and Schad Gerhard. Pp. 573-585. October 8, 1959. | |
| More numbers will appear in volume 10. | ||
| Vol. 11. | 1 | The systematic status of the colubrid snake, Leptodeira discolor Günther. By William E. Duellman. Pp. 1-9, 4 figs. July 14, 1958. |
| 2 | Natural history of the six-lined racerunner, Cnemidophorus sexlineatus. By Henry S. Fitch. Pp. 11-62, 9 figs., 9 tables. September 19, 1958. | |
| 3 | Home ranges, territories, and seasonal movements of vertebrates of the Natural History Reservation. By Henry S. Fitch. Pp. 63-326, 6 plates, 24 figures in text, 3 tables. December 12, 1958. | |
| 4 | A new snake of the genus Geophis from Chihuahua, Mexico. By John M. Legler. Pp. 327-334, 2 figures in text. January 28, 1959. | |
| 5 | A new tortoise, genus Gopherus, from north-central Mexico. By John M. Legler. Pp. 335-343. April 24, 1959. | |
| 6 | Fishes of Chautauqua, Cowley and Elk counties, Kansas. By Artie L. Metcalf. Pp. 345-400, 2 plates, 2 figures in text, 10 tables. May 6, 1959. | |
| 7 | Fishes of the Big Blue River Basin, Kansas. By W. L. Minakley. Pp. 401-442, 2 plates, 4 figures in text, 5 tables. May 8, 1959. | |
| 8 | Birds from Coahuila, Mexico. By Emil K. Urban. Pp. 443-516. August 1, 1959. | |
| 9 | Description of a new softshell turtle from the southeastern United States. By Robert G. Webb. Pp. 517-525, 2 pls., 1 figure in text. August 14, 1959. | |
| 10 | Natural history of the ornate box turtle, Terrapene ornata ornata Agassiz. By John M. Legler. Pp. 527-669, 16 pls., 29 figures in text. March 7, 1960. | |
| Index will follow. | ||
| Vol. 12. | 1 | Functional morphology of three bats: Eumops, Myotis, Macrotus. By Terry A. Vaughan. Pp. 1-153, 4 plates, 24 figures in text. July 8, 1959. |
| 2 | The ancestry of modern Amphibia: a review of the evidence. By Theodore H. Eaton, Jr. Pp. 155-180, 10 figures in text. July 10, 1959. | |
| 3 | The baculum in microtine rodents. By Sydney Anderson. Pp. 181-216, 49 figures in text. February 19, 1960. | |
| More numbers will appear in volume 12. | ||
The "UNIVERSITY OF KANSAS PUBLICATIONS - MUSEUM OF NATURAL HISTORY" listing was moved to the end of the document. In the original version, this listing was printed in the inside front and rear cover as well as the back. An enhanced version of the original cover (which just repeats the first page of the article) has been created using the illustrations contained within.
Except for the typographical corrections listed below, the following changes were made: The italics on the section label Color and Markings (Pg. 593) was removed as those were used to delimit the subsections. Based on the formatting used for other text in this publication, other minor typographica changes may also have been made where periods, commas, etc. were left out or inserted erroneously.
| Page | Correction |
| 542 | Plate 1 → Plate 15 |
| 568 | hiberation → hibernation |
| 580 | expresssed → expressed |
| 582 | rail → rain |
| 590 | spical → apical |
| 597 | Pl. 11 → Pl. 19 |
| 601 | mullberry → mulberry |
| 603 | an → and |
| 604 | monentarily → momentarily |
| 614 | detph → depth |
| 640 | presssure → pressure |
| 667 | retpiles → reptiles |