The Project Gutenberg EBook of Photographic Reproduction Processes by P.C. Duchochois
This eBook is for the use of anyone anywhere at no cost and with almost no restrictions whatsoever. You may copy it, give it away or re-use it under the terms of the Project Gutenberg License included with this eBook or online at http://www.gutenberg.org/license
Title: Photographic Reproduction Processes Author: P.C. Duchochois Release Date: December 24, 2007 [Ebook #24016] Language: English Character set encoding: UTF-8 ***START OF THE PROJECT GUTENBERG EBOOK PHOTOGRAPHIC REPRODUCTION PROCESSES***
A Practical Treatise of the Photo-Impressions Without Silver Salts New York
The Scovill & Adams Company
423 Broome Street.
1891
Contents
- INTRODUCTION.
- THE DESIGNS.
- THE CYANOTYPE OR BLUE PROCESS.
- THE CYANOFER. (Pellet's Process.)
- THE BLACK OR INK PROCESS. (Ferro-tannate Process.)
- THE CUPROTYPE. (Burnett's Process.)
- THE ANILINE PROCESS.
- THE PRIMULINE OR DIAZOTYPE PROCESS.
- TRACING PROCESS ON METAL.
- GRAPHOTYPY.
- THE URANOTYPE.
- THE PLATINOTYPE.
- ARTIGUES' PROCESS
- THE CARBON PROCESS.
- APPENDIX.
Illustrations
Preparer's Note
Please remember that this book was published over a century ago, long before today's chemical safety standards. Please get expert advice before attempting to perform any of the procedures described in this book.
Authors Quoted
INTRODUCTION.
The photographic processes with the salts of iron are all derived from the researches of Sir John Herschel. The investigations of that great philosopher are so valuable, so full of instructions that we are led to reprint them, together with those of Mr. C. J. Burnett, on the salts of uranium, etc., as an Introduction. It will be seen that the process by which blue prints are to-day obtained is exactly that Sir John Herschel devised in 1840.
“It is no longer an insulated and anomalous affection of certain salts of silver or gold, but one which, doubtless, in a greater or less degree, pervades all nature, and connects itself intimately with the mechanism by which chemical combination and decomposition is operated. The general instability of organic combinations might lead us to expect the occurrence of numerous and remarkable cases of this affection among bodies of that class, but among metallic and other elements inorganically arranged, instances enough have already appeared, and more are daily presenting themselves, to justify its extension to all cases in which chemical elements may be supposed combined with a certain degree of laxity, and so to speak in a tottering equilibrium. There can be no doubt that the process, in a great majority, if not in all cases, which have been noticed among inorganic substances, is a deoxidizing one, so far as the more refrangible rays are concerned. It is obviously so in the cases of gold and silver. In the case of the bichromate of potash it is most probable that an atom of oxygen is parted with, and so of many others. A beautiful example of such deoxidizing action on a non-argentine compound has lately occurred to me in the examination of that interesting salt, the ferrosesquicyanuret [pg 8] of potassium described by Mr. Smee in the Philosophical Magazine, No. 109, September, 1840, and he has shown how to manufacture in abundance and purity, by voltaic action on the common or yellow ferrocyanuret. In this process nascent oxygen is absorbed, hydrogen given off, and the characters of the resulting compound in respect of the oxides of iron, forming as it does Prussian blue with proto salts, indicate an excess of electro-negative energy, a disposition to part with oxygen, or which is the same thing, to absorb hydrogen (in the presence of moisture), and thereby to return to its pristine state, under circumstances of moderate solicitation, such as the affinity of protoxide of iron (for instance) for an additional dose of oxygen, etc.”
“Paper simply washed with a solution of this salt is highly sensitive to the action of the light. Prussian blue is deposited (the base being necessarily supplied by the destruction of one portion of the acid, and the acid by the destruction of another). After half an hour or an hour's exposure to sunshine, a very beautiful negative photograph is the result, to fix which, all that is necessary is to soak it in water in which a little sulphate of soda is dissolved. While dry the impression is of a dove color or lavender blue, which has a curious and striking effect on the greenish yellow ground of the paper produced by the saline solution. After washing the ground color disappears and the photograph becomes bright blue on a white ground. If too long exposed, it gets ‘over-sunned,’ and the tint has a brownish or yellowish tendency, which, however, is removed in fixing; but no increase of intensity beyond a certain point is obtained by the continuance of exposure.”
“If paper be washed with a solution of ammonio-citrate of iron and dried and then a wash passed over it of the yellow ferro-cyanuret of potassium, there is no immediate formation of true Prussian blue, but the paper rapidly acquires a violet-purple color, which deepens after a few minutes, as it dries, to almost absolute blackness. In this state it is a positive photographic paper of high sensibility, and gives pictures of great depth and sharpness, but with this peculiarity, that they darken again spontaneously on exposure to the air in darkness, and are soon [pg 9] obliterated. The paper, however, remains susceptible to light, and capable of receiving other pictures, which in their turn fade, without any possibility (so far as I can see) of arresting them, which is to be regretted, as they are very beautiful, and the paper of such easy preparation. If washed with ammonia or its carbonate, they are for a few moments entirely obliterated, but presently reappear with reversed lights and shades. In this state they are fixed, and the ammonia, with all that it will dissolve, being removed by washing in water, their color becomes a pure Prussian blue, which deepens much by keeping. If the solution be mixed there results a very dark violet-colored ink, which may be kept uninjured in an opaque bottle, and will readily furnish by a single wash at a moment's notice the positive paper in question, which is most sensitive when wet.”
“It seems at first sight natural to refer these curious and complex changes to the instability of the cyanic compounds; and that this opinion is to a certain extent correct is proved by the photographic impressions obtained on papers to which no iron has been added beyond what exists in the ferrocyanic salts themselves. Nevertheless, the following experiments abundantly prove that in several of the changes above described, the immediate action of the solar rays is not exerted on these salts, but on the iron contained in the ferruginous solution added to them, which it deoxidizes or otherwise alters, thereby presenting it to the ferrocyanic salts in such a form as to precipitate the acids in combination with the peroxide, or protoxide of iron, as the case may be. To make this evident, all that is necessary is simply to leave out the ferrocyanate in the preparation of the paper, which thus becomes reduced to a simple washing over with the ammonio-citric solution. Paper so washed is of a bright yellow color, and is apparently little, but in reality highly sensitive to photographic action. Exposed to strong sunshine, for some time indeed, its bright yellow tint is dulled into an ochrey hue, or even to gray, but the change altogether amounts to a moderate percentage of the total light reflected, and in short exposures is such as would easily escape notice. Nevertheless, if a slip of this paper be held for only four or [pg 10] five seconds in the sun (the effect of which is quite imperceptible to the eye), and when withdrawn into the shade be washed over with the ferrosesquicyanate of potash, a considerable deposit of Prussian blue takes place on the sunned part, and none whatever on the rest; so that on washing the whole with water, a pretty strong blue impression is left, demonstrating the reduction of iron in that portion of the paper to the state of protoxide. The effect in question is not, it should be observed, peculiar to ammonio-nitrate of iron.”
“The ammonio and potasso-tartrate fully possess and the perchloride exactly neutralized partakes of the same property; but the experiment is far more neatly made and succeeds better with the other salts.”
“The varieties of cyanotype processes seem to be innumerable, but that which I shall now describe deserves particular notice not only for its pre-eminent beauty while in progress, but as illustrating the peculiar power of the ammoniacal and other parsalts of iron above-mentioned to receive a latent picture susceptible of development by a great variety of stimuli. This process consists in simply passing over the ammonio-citrated paper on which such a latent picture has been impressed, very sparingly and evenly, a wash of the solution of the common yellow ferrocyanate (prussiate) of potash. The latent picture, if not so faint as to be quite invisible (and for this purpose it should not be so), is negative. As soon as the liquid is applied, which cannot be in too thin a film, the negative picture vanishes, and by very slow degrees is replaced by a positive one of a violet blue color on a greenish yellow ground, which at a certain moment possesses a high degree of sharpness and singular beauty and delicacy of tint. If at this instant it be thrown into water, it passes immediately to a Prussian blue, losing, at the same time, however, much of its sharpness, and sometimes indeed becoming quite blotty and confused.”
“To prevent this confusion gum arabic may be added to the prussiated solution, by which it is hindered from spreading unmanageably within the pores of the paper, and the precipitated Prussian blue allowed time to agglomerate and fix itself on the fibers. By the use of this ingredient also, a much thinner [pg 9] and more equal film may be spread over the surface, and when perfectly dry, if not sufficiently developed, the application may be repeated. By operating thus I have occasionally (though rarely) succeeded in producing pictures of great beauty and richness of effect, which they retain (if not thrown in water) between the leaves of a portfolio, and have a certain degree of fixity—fading in strong light and recovering their tone in the dark. * * *”
“If paper be washed with a mixture of the solutions of ammonio-citrate of iron and ferrosesquicyanate (red prussiate) of potash, so as to contain the two salts in about equal proportions, and being then impressed with a picture, be thrown into water and dried, a negative blue picture will be produced. This picture I have found to be susceptible of a very curious transformation. To effect this it must be washed with a solution of protonitrate of mercury, which in a little time entirely discharges it. The nitrate being thoroughly washed out and the picture dried, a smooth iron is passed over it, somewhat hotter than is used for ironing linen, but not sufficiently so to scorch or injure the paper. The obliterated picture immediately reappears, not blue, but brown. If kept for some weeks in this state between the leaves of a portfolio, in complete darkness, it fades, and at length almost disappears. But what is very singular, a fresh application of heat revives and restores it to its full intensity.”
“This curious transformation is instructive in another way. It is not operated by light, at least not by light alone. A certain temperature must be attained, and that temperature suffices in complete darkness. Nevertheless, I find that on exposing to a very concentrated spectrum (collected by a lens of short focus) a slip of paper prepared as above (that is to say, by washing with the mixed solutions, exposure to sunshine, washing and discharging the uniform blue color so induced, as in the last article), its whiteness is changed to a brown over the whole region of the red and orange rays, but not beyond the luminous spectrum. Three conclusions seem unavoidable: first—that it is the heat of these rays, not their light, which operates the change; second—that this heat possesses a peculiar chemical quality which is not possessed by the purely calorific [pg 13] rays outside of the visible spectrum, though far more intense; and third—that the heat radiated from obscurely hot iron abounds especially in rays analogous to those of the region of the spectrum above indicated.”
Sir John Herschel then proceeds to show that whatever be the state of the iron in the double salts in question, its reduction by blue light to the state of protoxide is indicated by many other agents. “Thus, for example,” says Robert Hunt, “if a slip of paper prepared with the ammonio-citrate of iron be exposed partially to sunshine, and then washed with the bichromate of potash, the bichromate is deoxidized and precipitated upon the sunned portion, just as it would be if directly exposed to the sun's rays.”
“I have proved this fact with a great number of preparations of cobalt, nickel, bismuth, platinum and other salts which have been thought hitherto to be insensitive to the solar agency; but if they are partially sunned and then washed with nitrate of silver and put aside in the dark, the metallic silver is slowly reduced upon the sunned portion. In many instances days were required to produce the visible picture; and in one case paper being washed in the dark with neutral chloride of platinum was sunned and then washed in the dark with nitrate of silver; it was some weeks before the image made its appearance, but it was eventually perfectly developed, and, when quite so, remained permanently impressed upon the paper.”
The following process, discovered at the same time as the cyanotype, and termed chrysotype, is thus described by Sir John Herschel:
“In order to ascertain whether any portion of the iron in the double ammoniacal salt employed has really undergone deoxidation, I had recourse to a solution of gold, exactly neutralized by carbonate of soda. The proto-salts of iron, as is well known to chemists, precipitate gold in the metallic state. The effect proved exceedingly striking, and, as the experiment will probably be repeated by others, I shall here describe it ab initio. Paper is to be washed with a moderately concentrated solution [pg 13] of ammonio-citrate of iron and dried. The strength of solution should be such as to dry into a good yellow color, not at all brown. In this state it is ready to receive a photographic image, which may be impressed on it either from nature in the camera obscura, or from an engraving on a frame in sunshine. The image so impressed is, however, very faint, and sometimes hardly perceptible. The moment it is removed from the frame or camera, it must be washed over with a neutral solution of chloride of gold of such strength as to have about the color of a sherry wine. Instantly the picture appears, not, indeed, at once of its full intensity, but darkening with great rapidity up to a certain point, depending on the strength of the solutions used, etc. At this point nothing can surpass the sharpness and perfection of detail of the resulting photograph. To arrest this process and to fix the picture (so far at least as the further agency of light is concerned), it is to be thrown into water very slightly acidulated with sulphuric acid, and well soaked, dried, washed with hydrobromate of potash, rinsed and dried again. * * *”
“In point of direct sensibility, the chrysotype paper is certainly inferior to the calotype; but it is one of the most remarkable peculiarities of gold as a photographic ingredient, that extremely feeble impressions once made by light go on afterwards, darkening spontaneously and very slowly, apparently without limit so long as the least vestige of unreduced chloride of gold remains in the paper. To illustrate this curious and (so far as applications go) highly important property, I shall mention incidentally the results of some experiments made during the late fine weather on the habitudes of gold in presence of oxalic acid. It is well known to chemists that this acid, heated with solutions of gold, precipitates the metal in its metallic state; it is upon this property that Berzelius has founded his determination of the atomic weight of gold. Light, as well as heat, also operates this precipitation; but to render it effectual, several conditions are necessary:—First—the solution of gold should be neutral, or at most very slightly acid; secondly—the oxalic acid must be added in the form of a neutral oxalate; and thirdly—it must be present in a certain considerable quantity, which quantity [pg 14] must be greater the greater the amount of free acid present in the chloride. Under this condition, the gold is precipitated by light as a black powder if the liquid be in any bulk; and if merely washed over paper, a stain is produced, which, however feeble at first, under a certain dosage of the chloride, oxalate and free acid, goes on increasing from day to day and from week to week, when laid by in the dark and especially in a damp atmosphere, till it acquires almost the black of ink; the unsunned portion of the paper remaining unaffected, or so slightly as to render it almost certain that what little action of the kind exists is due to the effect of casual dispersed light incident in the preparation of the paper. I have before me a specimen of paper so treated in which the effect of thirty seconds' exposure to sunshine was quite invisible at first, and which is now of so intense a purple as may be well called black, while the unsunned portion has acquired comparatively but a slight brown. And (what is not a little remarkable, and indicates that in the time of exposure mentioned the maximum of effect was attained) other portions of the same paper exposed in graduated progression for longer times, viz., one minute, two minutes, and three minutes, are not in the least perceptible degree darker than the portion on which the light has acted during thirty seconds only.”
“If paper prepared as above recommended for the chrysotype, either with the ammonio-citrate or ammonio-tartrate of iron, and impressed, as in that process, with a latent picture, be washed with nitrate of silver instead of a solution of gold, a very sharp and beautiful picture is developed of great intensity. Its disclosure is not instantaneous; a few moments elapse without apparent effect; the dark shades are then first touched in, and by degrees the details appear, but much more slowly than in the case of gold. In two or three minutes, however, the maximum of distinctness will not fail to be obtained. The picture may be fixed by the hyposulphite of soda, which alone, I believe, can be fully depended on for fixing argentic photographs.”
“The best process for fixing the photographs prepared with gold is as follows: As soon as the picture is satisfactorily [pg 15] brought out by the auriferous liquid, it is to be rinsed in spring water, which must be three times renewed, letting it remain in the third water five or ten minutes. It is then to be blotted off and dried, after which it is to be washed on both sides with a somewhat weak solution of hydriodate of potash. If there be any free chloride of gold present in the pores of the paper it will be discolored, the lights passing to a ruddy brown; but they speedily whiten again spontaneously, or at all events on throwing it (after lying a minute or two) into fresh water, in which, being again rinsed and dried, it is now perfectly fixed.”
As the chrysotype will be no more referred to, we shall state, first, that the image can be developed with a plain solution of silver nitrate or one acidified with citric or any other organic acid, which generally gives a brown impression that can be toned with an acid or alkaline gold bath, the color varying with the solution employed; and secondly, that the process may be employed to obtain outlines of any picture on paper or canvas to be colored in oil-paints. The impression developed with gold terchloride is pale blue, quite permanent, and does not at all interfere with the work of the artist. The canvas should first be washed with a mixture of alcohol and aqueous ammonia, then dried and rubbed with pumice stone powder to give a tooth. The modus operandi suggests itself.
The researches of Mr. C. J. Burnett on the application of uranium salts and other compounds to photography are recorded in the Photographic Notes of Ths. Sutton for 1857. We give in the following lines the most interesting parts of the two papers of Mr. Burnett:
* * * “The next class of processes are dependent on the sensitiveness to light of the salts of uranic oxide or sesquioxide of uranium, U2O3.”
“In the first process, the paper being charged with the uranic salt and exposed to the solar influence under the negative to be copied, is washed with a solution of the ferridcyanide or [pg 16] red prussiate of potash. The ‘Harvest Scene’ in the exhibition, being from an albumen negative lent me by Mr. Ross, the well-known Edinburgh photographer, is an example, the salt of the sesquioxide of uranium being in this case the hydrofluate, and the time of exposure from the strength of the albumen negative fully an hour of good sunshine. I have used for the solution of the uranic oxide for this process a variety of acids with very similar results; the sensitiveness of the prepared paper to light varying much, however. For instance, a collodion negative with the hydrofluate paper producing a very good print in half an hour of unsteady sun, while with a paper prepared with the tartaric acid solution of the oxide, it gave an equally good impression in less than five minutes of the same intermitting sunshine, indicating thus a difference of sensitiveness of six to one in favor of the tartrate.”
“The rationale of this process is the reduction of the sesqui-oxide of uranium, U2O3, on those parts of the paper exposed to the solar influence, to a lower state of oxidation, the photo-oxide UO, the salts of which have the property of forming with soluble alkaline ferridcyanides a rich chocolate-brown precipitate, while the salts of the sesquioxide are destitute of this reaction. Hence the brown deposit on the parts of the picture on which the sun has been allowed to act when the developing solution is applied, and the absence of any such appearance on those parts which have been protected from its influence.”
“As to the manipulatory details of this process, the paper is floated on the solution in a dark room and hung up to dry, and then preserved from light in a portfolio. If carefully secluded from light it appears to keep well. After exposure for the proper time under this negative, there is in some cases scarcely any visible impression; while in other cases, particularly when using the tartaric solution, I have found the impression very distinguishable, of a brownish or blackish shade, although still quite faint. The development is best conducted by floating it, anything like rubbing the picture being very objectionable.”
“When the picture has fully come out, which is generally from three to ten minutes at the very most, it is removed from the [pg 17] developing bath, placed in cold water and washed very gently for a few minutes, the water being frequently changed till it ceases to acquire a yellow tinge from the dissolved red prussiate. The picture is then drained from the water, pressed between folds of blotting paper, dried (I dry in the dark), and the process is complete. * * * I may state, as one recommendation of this process to ladies and other lovers of clean hands, that any brown stains left by it on the fingers or elsewhere are at once removable by a little weak ammonia or soap and water. * * * I would particularly suggest, as deserving of notice, the development of the salts of sesquioxide of uranium, and still more iron, by the metals and metallic-cyanic alkaline salts, as also by the mellonides and nitro-prussides, and the latter also by itself and as developed by many metallic salts.”
“I have since had the opportunity of trying the nitro-prusside of sodium, which, by itself, gives a blue and white picture, in color like that obtained from the red prussiate of potash.”
“When mixed with a solution of ammonio-nitrate of copper, previous to its application to the paper, the color obtained is pale purplish pink or peach-blossom color. By mixing it in the same way with ammonio-oxalate of sesquioxide of iron, we get a dull green picture, changeable through intermediate stages into brown by alkaline carbonates, and that into a dirty black by gallic acid. It may be well to know that the blue of the picture given by the red prussiate in the process of Sir John Herschel may be considerably modified or entirely changed to another color, in many ways, without interfering with the purity of the white ground, by steeping the picture, after the undecomposed red prussiate has been washed out, in solution of salts of various metals, copper, uranium or cobalt, for instance, and that the colors so produced may be modified as desired, according to the stage at which the action is stopped.”
“There remains but one class of uranic photographs to be described, namely, that obtained when we develop with a salt of silver or gold (or platinum?). This class may be made to print much more rapidly than our ordinary silver printing process, approaching sometimes more nearly to the calotype development in this respect. We get the minutest details [pg 18] with great fidelity, and the picture is effectually fixed by a simple fresh hyposulphite solution, with a good color in many cases, or by ammonia, which will be considered an advantage by those who hold the hyposulphite an enemy to durability. Different shades of color are produced according to different solvent acids and different details. I have got a good black perfectly like that of an engraving, by the nitrate of uranic oxide, developed by ammonio-nitrate of silver (or plain nitrate) and fixed by plain hyposulphite without any coloring bath. * * * I have tried the hyposulphite of gold on some of the silver-developed prints prepared with the hydrofluate of the uranic oxide and fixed with ammonia, which had an exceedingly unpleasant raw-red color, a very agreeable gray was at once obtained. I have succeeded in getting very beautiful impressions by development of the uranic paper by chloride of gold alone.”
In another communication to the Photographic Notes, more interesting perhaps than the foregoing, Mr. Burnett says:
“The clearest and brightest of my results have been obtained by the action of gallic acid, tannin, or especially a mixture of tannin and carbonate of ammonia, potash or soda, on the blue pictures obtained by the solarization of paper prepared with ferridcyanide of potassium, ferrocyanide or ferridcyanide of ammonium. * * * I have also experimented with the bichromate and iron, with gallic, tannin and other developer; but I must confess to not having been, in this particular way, so successful as Mr. Sella appears to have been in the preservation of the whites, owing possibly to my not having taken the trouble to wash out sufficiently the iron before toning.”1
“I have experimented most extensively in many ways with the chromates and bichromates, and have succeeded in various ways in getting very good results. A very capital process for many purposes is to float or steep your paper in a mixed solution of bichromate of potash and sulphate of copper. As for E. Hunt's chromotype process,” 2 I have mixed gelatine, or occasionally grape sugar, or both, with the solution, but instead of developing it by a silver solution, as in the chromotype, wash out the salts unacted on by light, and develop by floating on a solution of ferrocyanide of potassium. The color of the red copper salt which now forms the picture may be modified or changed in many ways, viz., by soaking the picture, after the ferrocyanide of potassium has been washed out of the lights, in a solution of sulphate of iron (or the iron salt may, but not so advantageously, have been applied to the picture before the application of the ferrocyanide). Solutions of chloride of tin, gallic and tannic acids, alone or with alkalies or alkaline carbonates, may also be employed to modify or change the color. Instead of developing by ferrocyanide you may develop by the cobalt or chromo-cyanogen salts, or by an alkaline mellonide arsenite, etc. Sulphureted hydrogen, or a sulphide, will give a brown, or black tone, which may be protected against oxygen and dampness by a resinous varnish.
[pg 20]“Of all the simple pictures obtainable with bichromated papers, without complications or other tonings, those obtainable by the combination of a salt (say the sulphate) of manganese, with the bichromate in the paper preparation, are about the best; these pictures being, however, capable of being toned and modified in many different ways if desired. This may be accomplished by the use of toning baths of ferridcyanide or ferrocyanide, or other metal cyanogen salts, etc., or by either mixing the salts of other metals, as copper or iron, with the cyanic toning baths, or using them in the original solution, or by soaking the paper in them, as in Sella's process, previously to the application of the metal cyanic, mellonic or other toning baths. Alkalies and alkaline carbonates may also be used to remove the chromic acid, and leave a subsalt, or the very stable oxide or carbonate of manganese, which may be peroxidized by the use of chloride of lime, peroxide of hydrogen, or ozone.”
“In all the processes with metallic salts, alone with bichromates, the use of sized or unsized paper along with gelatine, etc., has some advantages. I have got good results by such processes on albumen paper, the albumen tending to prevent mealiness in the print; also on paper soaked in gelatine before the application of the bichromic solution. * * * There is great interest connected with the action of all such papers, along with the tannin and vegetable coloring matters. I have long been of opinion that by the steeping of papers or textile fabrics, containing the salts not only of iron, as recommended by Mr. Sella, but of tin, copper, bismuth, lead, etc., in solutions of cochineal, red cabbage, beetroot, grass or the most ordinary foliage, etc., that the most useful results might be obtained; though for certain permanence I am not sure but that some of the other processes which I have briefly run over with the cyanogen acid salts or metallic acid salts, as precipitators, may be more to be depended upon. The processes with precipitated oxides, such as the one with manganese and similar ones, with other metals which I have described, I also consider as deserving of more attention than almost any processes which have been stated, on the score of probable permanence; but perhaps the best process for black, or generally useful [pg 21] neutral tint, without silver, that has yet been offered to the public, I believe to be the process alluded to with the bichromate of potash and sulphate of copper, toned by an iron salt. * * * This process, the cuprotype (as also the uranotype and manganotype) is applicable perfectly to films of albumen or gelatine on glass or porcelain, textile fabrics, parchment, paper, tiles and many other substances besides paper.”
THE DESIGNS.
HOW TO MAKE A NEGATIVE DRAWING
The drawing paper for designs to be reproduced by the cyantotype and the other processes described in this book should be of a fine texture, free from opacities and very white; and, as the design must serve as a cliché it is a sine qua non that it be drawn with a very black ink and with well-fed lines, especially those which are very fine. To obtain a complete opacity, and, at the same time, to keep the ink quite fluid, which gives great facility to the designer, one adds some gamboge (or burnt sienna) to the India ink. The ink of Bourgeois, which is compounded with yellow and can be diluted as easily as India ink, is excellent, so is also the American ink of Higgins.3
As much as possible it is desirable to replace the colored lines indicating the constructions, the axis, projections, etc., by differently punctuated lines made with India ink. However, if the use of colors be obligatory on the original design, one should trace the red lines with very thick vermilion or sienna, the yellow lines with gamboge, and the blue and green lines with a thick mixture of Prussian blue and chrome yellow in different proportions.
One must abstain from applying washes of any tints on the original. If necessary they should be brushed over when the reproductions are made; moreover they can be often replaced by cross-lines more or less open, and the shadowing represented by thicker but not closer lines.
Tracing paper is recommended instead of linen, which latter, on account of its thickness and granulation, gives less satisfactory results in regard to the transparency of the ground and the continuity of the lines.
To reproduce a design on ordinary paper—not too thick—or [pg 26] an engraving, etc., the paper is rendered transparent by rubbing over on the back of the original a solution of 3 parts in volume of castor oil in 10 parts of alcohol, by means of a small sponge. When the paper is quite transparent, the oil in excess is removed by pressure between sheets of blotting paper, and the paper dried before the fire or spontaneously. The design so treated is not in the least injured, for it assumes its primitive condition by dissolving the oil from the paper by immersion into strong alcohol, which it is necessary to renew once or twice, then rinsing in alcoholized water if the drawing be in India ink, or simply in water in the case of an engraving, and finally drying between sheets of blotting paper.
Instead of an alcoholic solution of castor oil, vaseline can be employed. The paper is more transparent.
The method by which are made negative drawings, that is, those which can be used as negative clichés to reproduce the design in black lines on a white ground, is thus described by Mr. Cheysson, wlio originated it, in a manual published by the Department of Public Works of France, from which we have borrowed most of the above instructions for the drawing of designs suitable for the photo-reproduction processes:4
“One can avoid the necessity of making a negative from the original drawing by transforming the drawing itself into a negative.”
“To that effect it suffices to draw with lithographic ink, then to cover the paper with aniline brown, and, after drying, to wash it with turpentine oil which dissolves the lithographic ink without altering the aniline. The lines appear then white on a brown ground impervious to light (that is, non-actinic). The design is thus transformed into a negative, and can yield positive impressions with paper sensitized with silver salts, the ferriprussiate or the bichromate of potash. The lithographic ink should be very black and the lines well fed.”
“When the drawing is finished it is placed on a board lined with sheets of blotting paper, then one spreads all over it the aniline brown with a brush, and, lastly, after drying, the paper is carefully rubbed with a bung of cotton or a rag imbued with turpentine until the lines of the design are dissolved.”
[pg 27]In our practice we have often taken a negative cliché from drawings made in the ordinary manner, without the aid of the camera obscura (which would have been too expensive for drawings of a certain size), by simply printing a proof by contact on plain or albumenized silvered paper, and fixing, without toning, in a new solution of sodium thiosulphate, then washing as usual. The proofs thus obtained from designs drawn with an opaque ink, which allows a long insulation and, therefore, yields an intense reduction, are of a deep brick-red color, quite non-actinic, and give very good positives by the Artigues process.
N.B.—Paper in drying never assumes its original shape; it is, therefore, necessary to make the figures on the reproductions from plans when they are not on the originals.
CHOICE OF PAPER. SIZING.
In all the photographic processes by precipitation of metallic oxides the quality of the paper has a great influence on the results. When the paper is not well sized and not well calendered, the sensitizing solution is absorbed, instead of simply impregnating the surface of the paper, and not only the image is sunk in and its sharpness impaired, but good whites can never be obtained, especially if the image should be toned, owing to the impossibility of eliminating the metallic salts not acted on, that is, not reduced by the action of light which the fibers of the paper mechanically retain.
The “endless” rolls of paper, 54: inches wide—or “blue print paper,” as it is sometimes termed—of Blanchet fréres et Kléber, of Rives, better known as “Rives' paper”, that of Johannot, of Annonay (France), and the Steinbach (Saxe) paper are recommended.
For small prints from negatives in half tone the positive paper, 18×22 inches, of Rives or Saxe, should be preferred to the heavy kind. It is advisable to size it, so that the impressions be entirely formed on the surface of the paper. Moreover, an additional sizing is always advantageous, whatever be the photographic process employed, to prevent the imbibition of the sensitizing compound and to obtain more brilliant and [pg 28] vigorous images, for the iron, chromium, uranium and other metallic soluble salts require the presence of an organic matter (alcohol, ether, gum arabic, glucose, caseine, etc.) to be reduced by the agency of light; and as a consequence, the greater, within certain limits, of course, the amount of organic matters, and the more thoroughly they are mixed with the salts, the more sensitive the preparation and the better the results.
Arrowroot is the best sizing for our purposes. Gelatine may be employed, albumen also, but the coating should be insolubized when applied on the paper and dry.
Sizing with Arrowroot.—In a porcelain dish diffuse 4 parts of powdered arrowroot and one part of liquid glucose in 200 parts of distilled or rain water and dissolve by heat over an alcohol lamp, stirring all the while. Let the solution boil for an instant, and when the paste is homogeneous let it cool down and then remove the skin formed on its surface and strain it through a fine canvas. Now provide with three small sponges free from gritty matters and cleaned in water, and nail by the four corners, one over the other, felt size uppermost, as many sheets of paper as you wish to size on a board somewhat smaller than the paper. This done, with one of the sponges take a small quantity of the arrowroot and, brushing it length-way and cross-way, spread the paste into an even layer, then, by rubbing very lightly with the second sponge, efface the striae and smooth the coating as well as possible. The third sponge serves to remove the excess of paste when too much is at first spread on. From six to seven sheets of paper, 18×22, can be sized with the quantity of arrowroot paste above given.
Another, but not quite so effective a manner of sizing although sufficient for the cyanotype, is the following, employed by Mr. Pizzighelli for the paper used in the platinotypic process:
Ten parts of arrowroot are powdered in a mortar with a little water and then mixed by small quantities to 800 parts of boiling water. After a few minutes 200 parts of alcohol are added and the mixture filtered. The paper is immersed for two or three minutes in the warm solution and hung up to dry.
[pg 29]Sizing, with Gelatine.—Dissolve at a temperature of about 140 deg. Fahr. (60 deg. C.) 10 parts of good gelatine in 800 parts of water, then add 200 parts of alcohol and 3 parts of alum dissolved in a little water. Filter and prepare the paper by immersion as above directed. The gelatinized paper when dry should be prepared a second time and dried by hanging it up in the opposite direction in order to obtain an even coating.
THE CYANOTYPE OR BLUE PROCESS.
This process gives white impressions on a blue ground with diapositives or drawings on transparent or semi-transparent materials, and blue impressions on a white ground from negatives. It is commonly known under the names of “blue print process,” “negative ferrotype process” and “ferro-prussiate process.”
The process is indeed exceedingly simple. A sheet of paper, impregnated or sensitized, as it is termed, with a solution of ferric citrate and ferricyanate is impressed under a cliché,5 then immersed in pure water, whereby the image is developed and at the same time fixed. It is on account of the great advantages offered by its simplicity that this process is generally preferred by civil engineers and architects for the reproduction of their plans.
The sensitizing solution is prepared in mixing by equal volumes the two solutions following:
| A. | Iron, ammonio citrate | 20 parts |
| Water | 100 parts | |
| B. | Potassium ferricyanate (red prussiate) | 15 parts |
| Water | 100 parts |
Although the mixture keeps pretty well for a certain period in the dark, it is best to prepare only the quantity wanted for actual use.6
The paper is preferably sensitized in operating as follows:
Take hold of the paper by the two opposite corners and fold it into a loop, lay it on the iron solution, the center of the sheet first placed in contact with the liquid, and then gradually spread it by lowering the corners with a little pressure. No solution should run over on the back of the paper; it would be [pg 31] a cause of stain. This done, and without allowing the liquid to penetrate in the paper, immediately take hold of the two corners near the body and withdraw the paper by dragging it over on a glass rod for this purpose fixed on the edge of the tray. Now pin up the paper to dry, which should be done rapidly, and sensitize a second time in proceeding in the same manner. If this second sensitizing be found objectionable, let float the paper for no more than ten seconds; of course this method of sensitizing is not applicable to prepare larger sheets of paper. In this case the paper is pinned by the four corners on a drawing board or any other support, lined with blotting paper and quickly brushed over with a sponge sparingly imbued with the sensitizing mixture, so as to wet the paper with a very small excess of liquid.
The rationale of this manner of sensitizing is to impregnate only the very surface of the paper with the ferric salts, and thereby to obtain an intense blue with very good whites, which latter it would be impossible of obtaining should the sensitizing solution be allowed to reach in the fibers of the paper, for, in this condition, it is impossible, owing to the exigencies of the process, to wash out thoroughly the iron salts to prevent the chemical changes which cause the whites to be tinted blue. It is for this reason that better results are also obtained with well sized papers.
The sensitizing should be done by a very diffused daylight, and the drying, of course, in a dark room. When sensitized the paper is yellowish green. It should be well dried for keeping, and rolled or wrapped in orange or brown paper and preserved from the action of dampness and of the air. It does not keep well, however, no more than two or three months, perhaps, in good condition; but the sooner it is employed the finer the proofs, the better the whites and more rapidly is the paper impressed.
There is in the market a paper which keeps for a long time. It is prepared by adding a small quantity of gum arabic or of dextrine to the sensitizing solution. Good for the reproduction of line work, it does not give very satisfactory results for pictures in half tones.
[pg 32]The following compound gives a paper much more sensitive, but not keeping so long, than that prepared according to the formula previously given:
| Tartaric acid | 25 parts |
| Ferric chloride, solution at 45 deg. Baumé | 80 parts (in volume) |
| Water | 100 parts |
When the acid is dissolved, add gradually concentrated aqueous ammonia, just enough to neutralize the solution—170 volumes, about. The chemical change consists in the formation of ferric tartrate. Let cool the solution, then, after adding the following, keep it in the dark:
| Potassium ferricyanate | 21½ parts |
| Water | 100 parts |
Another and very sensitive preparation is the following:
| A. | Iron perchloride, cryst | 40 parts |
| Oxalic acid | 10 parts | |
| Water | 100 parts | |
| B. | Potassium ferricyanate | 20 parts |
| Water | 100 parts | |
| Mix |
Printing.—The process we describe yields negative impressions, that is a positive image from a negative cliché, and a negative image from a positive cliché, exactly as the silver printing-out process ordinarily employed in photography. Consequently, for the production of non-reversed proofs from plans, etc., the original drawing should be placed face downwards on the glass plate of the printing frame, and, upon the back, the sensitive paper is laid and pressed into perfect contact by means of a pad, felt or thick cloth.
The printing frame is that used by photographers. The lid is divided, according to the side, in two, three and even four sections, held by hinges and fastened for printing by as many cross-bars, in order that by opening one section, from time to time, the operator can follow the progressive changes resulting from the action of light on the iron salts. To print, the frame should be placed in the light in such a manner as the luminous rays fall perpendicularly upon the drawing or cliché. The reason of this is obvious, since the sensitive paper is not in direct [pg 33] contact with the design, but separated by the material upon which it is drawn.
During the insolation—whose time depends necessarily from the more or less transparency of the cliché, and, also, from the intensity of the light7—the paper assumes first a violet tint, which gradually intensifies to a dark shade; then this tint fades, becomes brownish, then pale lilac, while the parts under the lines—that is, the design—upon which the light has, therefore, no action, are visible by keeping the original yellow-green tint of the prepared paper. It is when the lilac color is produced that the exposure is sufficient.
To ascertain when the exposure is correct, a few black lines can be traced on one of the edges of the margin of the design, and strips of the sensitive paper placed upon them to serve as tests in operating, as it will be explained in the description of the Cyanofer process. When one of them is taken out and show, by being washed in water, a clear white line on a deep blue ground, the exposure is at an end. One understands that the blue color of the ground is more or less intense according to time of insolation, for the chemical actions between the reduced and the non-reduced iron salts is so much more complete as the salts acted on are more or less deoxidized, that is, reduced to ferrous salts; and that to obtain the maximum of effect, which, therefore, depends on the allowable time of exposure, the drawing ink should be opaque and non-actinic as far as possible, because when, on testing, the lines are tinted the exposure should be discontinued. However, a slight coloration of the lines is not very objectionable, for it disappears by a longer washing after the development.
The image is developed and fixed by washing in water two or three times renewed. The water must be free from calcareous salts; these salts converting the iron into carbonates which impart an ochrey tinge to the proof. Rain water—any water in which no precipitate is thrown down by the addition of a few drops of a weak solution of silver nitrate—may be used with safety.
[pg 34]During the development the ground takes a blue color which rapidly intensifies, while the iron compound, not acted on and imparting a yellow green tint to the design, is washed out from the white paper. If the print has not been sufficiently exposed the ground remains pale blue, more or less; the reason has been explained. In this case the development should be done quickly, as the blue is always discharged by washing. On the other hand, whenever the whites are tinted by excess of exposure, they can be cleared partly or entirely by a prolonged immersion in water, but the ground is also to some extent lightened.
When the proof is well developed and fixed, that is, when the soluble iron salts are eliminated, the blue color can be brightened by adding to the last but one washing water a small quantity of citric acid, or of potassium bisulphate, or a little of a solution of hypochlorite of lime (bleaching powder).
The action of light in this, as well as in the other photographic processes with metallic salts described in this work, is one of deoxidation, as shown by Herschel. The chemical changes which produce the blue precipitate is quite complicated. It is evident that both the ferric citrate and the ferric cyanate are partly reduced to ferrous salts under the luminous influence, and react in presence of water with the unreduced part of each of these compounds, the ferric citrate with the ferrous cyanate forming Prussian blue (ferric-ferrocyanate), and the ferric cyanate with the ferrous citrate giving rise to Turnbull's blue (ferrous ferricyanate). The blue of the print is consequently a mixture in a certain proportion of the two compounds; and as the color of Prussian blue is quite different from that of Turnbull's, it follows that by varying in a certain measure the percentage of the two ferric salts forming the sensitizing solution, the color of the blue may be varied thereby. Hence the difference in the formulas given by different authors.8
The blue color of the image can be changed into black or dark green. But to that purpose the paper should be, although [pg 35] not exactly necessary, well sized as before directed, and sensitized with extra care to prevent the imbibition of the iron solution into the paper. After exposure the proof should necessarily be thoroughly washed to eliminate the soluble iron salts, then immersed for a moment in water acidified with nitric acid, 1:100, and this done and without washing treated by a solution of aqueous ammonia at 2 per 100 of water. In this the blue color disappears, being changed into a red brownish tint, which indicates that the Turnbull's and Prussian blues are transformed, the former into ferroso-ferric hydrate, with formation of ferrocyanate, and the latter into ferric hydrate. It is by the action of tannin (gallotannic acid) on the ferric oxides thus formed that the black is produced, and by that of catechu-tannic acid contained in the extract of catechu that one obtains a dark green, almost black color.
To obtain the black tone it suffices to immerse the proof on its removal from the ammoniacal in a solution of tannin at 5 per 100 of water, and when toned, to wash it in a few changes of water.
The process to turn the blue color into a green was devised by Mr. Paul Roy. It is as follows: Dissolve 7 parts of borax in 100 parts of water, and acidify the solution with sulphuric acid added drop by drop until the litmus paper becomes red; then, in the same manner, neutralize with aqueous ammonia not in excess, but just enough to show an alkaline reaction; this done dissolve 1 part of powdered catechu and filter. In this the proof is immersed after development until the desired effect is attained. Wash, etc.
To clear the lines, or to make additions, or to write on the blue margin of the proof a solution of potassium oxalate is employed. It dissolves the blue without leaving scarcely any trace of it. The solution can be prepared by mixing the two solutions whose formula is given below:9
| A. | Oxalic acid | 10 parts |
| Water | 100 parts | |
| B. | Caustic potassa | 12½ parts |
| Water | 100 parts |
The blue prints are permanent. When drying they darken a little from oxidation; exposed to sunshine for some hours, they bleach considerably; but in the shade the faded pictures progressively absorb oxygen from the air and assume their original intensity and color in a period so much the longer as the insulation has been more prolonged; it may take weeks if the picture were much bleached.
THE CYANOFER. (Pellet's Process.)
This process gives blue impressions on a white ground from positive clichés, and white impressions on a blue ground from negative clichés. It is termed “positive ferrotype process.”
The cyanofer is an application of one of the numerous and useful inventions for which photography is indebted to A. Poitevin. In 1863 he discovered that certain organic substances were rendered insoluble by ferric chloride, and that they again became soluble; when under the influence of light the ferric chloride has been reduced to a ferrous salt. This curious phenomenon is the base of the process now to be described. As usual the process has been modified by compounding the sensitive solution in various ways and by minor details in the manner operating. But although these modifications have rendered the process easier to work with, there is not a great difference in the results obtained. We give two formulas. Aside from the addition of gum arabic, which was suggested by Mr. Pellet, and which constitutes the capital improvement of the process, the formula is substantially that devised by Mr. Poitevin.
Prepare three solutions as follows:
| A. | Gum arabic, best quality | 50 parts |
| Water | 170 parts | |
| B. | Tartaric acid | 12 parts |
| Water | 80 parts | |
| C. | Ferric chloride solution at 45 deg. Baumé | 35 parts in volume |
Mix gradually B to C, then C, by small quantities, in agitating briskly. It is important to prepare the solution as directed, for by adding the ferric chloride before tartaric acid, the gum arabic would be at once coagulated. When the ferric chloride is mixed, the solution at first thickens, but [pg 38] becomes sufficiently fluid for use in a certain period. It does not keep, and should be employed the day it is made if possible.
The paper, which should be well sized and calendered, and which, when not giving good results by too much absorbing the sensitive solution, must be starched as before directed, is coated either by brushing or by floating. By the first method a roll of paper five yards long can be prepared without great trouble, and give, perhaps, better results than if prepared by floating; but the latter method is by far the the most convenient: one does not generally prepare by brushing sheets of paper larger than about 30×40 inches.
For brushing, the paper is pinned on a board, then, with a large badger brush dipped in the sensitive solution, the latter is applied as evenly as possible; after which, by lightly passing the brush over, the striae are removed, the coating well equalized, and the paper hung up to dry. The coating should not be very thin, and, above all, not too thick, for then it would require an unusually long exposure to allow the light acting through the whole thickness of the film, which is a sine qua non to obtain a clear ground, i.e., not stained blue.
To prepare by floating, pour the solution in a shallow tray, which needs not to be more than 20×34 inches, 30 inches being the width of the drawing paper usually employed; then roll the paper and place it on the solution. Now, taking hold of it by two corners, draw it out slowly: the paper will unroll by itself. This operation can be done by diffused daylight, but, of course, the paper should be dried in a dark room. It dries rapidly. Endless rolls are prepared by machinery. To expose, the drawing is placed in the printing frame, face downwards, and the sensitive paper laid over it. The whole is then pressed into contact by interposing a cushion between the lid of the frame and the paper, and exposed so that the rays of light fall perpendicularly upon it.
The cyanofer preparation is quite sensitive. From half a minute to two minutes exposure, according to the intensity of the light and the thickness of the coating, is sufficient in sunshine to reproduce a drawing made on the ordinary tracing paper. In the shade, by a clear sky, the exposure is about [pg 39] five times longer, and varies from half an hour to an hour and more in cloudy weather, but then the design is seldom perfectly sharp.
The progresses of the impression is followed by opening one side of the printing frame and examining the proof. The exposure is sufficient when the paper is tinged brown on the parts corresponding to the ground of the design. The image appears then negative, that is, yellowish on a tinged ground.
Another and more safe method of ascertaining the correct time of exposure, which can be employed concurrently with the other, is to place a few strips of the same sheet of sensitive paper between the margin of the design, upon which a few lines have been traced, and the paper, and, without opening the frame, to draw one of them, from time to time, and dip it in the developing solution. If the whole strip be tinted blue, the proof is not sufficiently exposed; but if the lines soon appear with an intense coloration on the yellowish ground of the paper, and the latter do not turn blue in a minute, at the most, the exposure is right. By excess, the lines are with difficulty developed or broken.
For developing, we provide with three wooden trays lined with lead or gutta-percha, or, more economically, coated with yellow wax. The wax is melted, then applied very hot, and, when it is solidified and quite cold, the coating is equalized with a hot iron, whereby the cracks produced by the contraction of the wax when cooling are filled up.
One of these trays should contain a layer, about three-quarters of an inch thick, of an almost saturated solution of potassium ferrocyanate (the developer); the next be filled with water, and the third with water acidified by sulphuric acid in the proportion of three per cent. in volumes.
All this being ready, the margin of the proof is turned upwards—so as to form a disk of which the outside is the impressed surface—in order that the ferrocyanate solution does not find its way on the back of the proof, which would produce stains. Now the proof is laid, the lower edge first, on the developer, and gradually lowered upon it, when, taking immediately hold of it by the two corners nearest to the body, [pg 40] it is lifted out and held upright to allow one following the development of the image; and, presently, if any air-bubbles are seen on the proof, they should at once be touched up with a brush wetted with the ferrocyanate solution; the reason explains itself.
The image appears at once. As soon as the fine lines are well defined, the blue intense, and, especially, when the ground has a tendency to be tinged blue, the proof is placed in the tray filled with water and in this turned over two or three times, when it is immersed in the diluted sulphuric acid. In this bath the print acquires a deep blue coloration, consisting of Prussian blue, and the ground becomes tinted with a blue precipitate without adherence, which is easily washed off by throwing the liquid on the proof with a wooden spatula, or, better, by rubbing with a rag tied to a stick. When the ground is cleared, and after three or four minutes immersion to dissolve the iron salts acted on, the proof is rinsed in water several times renewed to free it from acid, and hung to dry.
There are two causes of failures in this process, viz., over and under-exposure. In the former case the fine lines are broken or washed out in clearing the proof (which may also arise from the drawing made with an ink not opaque enough); in the latter the ground is more or less stained.
The blue stains, the lines for corrections, etc., are erased with the the potassic oxalate (blue salving, as it is termed) whose formula has been given.
The additions, corrections and writing are made with a Prussian blue ink prepared by mixing the two following solutions:
| A. | Ferric chloride, dry | 4 parts |
| Water | 350 parts | |
| B. | Potassium ferrocyanate | 15 parts |
| Water | 250 parts |
The precipitate being collected on a filter and washed until the water commences to be tinged blue, is dissolved to the proper consistency in about 400 parts of water. This ink does not corrode steel pens.
It has been stated that the cyanofer process keeps for years [pg 41] if preserved from the combined action of dampness and the air. The writer found in his practice that the ferric salts in presence of the organic matters (the sizes) acts as does potassium bichromate and renders, in a certain period, the cyanofer film insoluble even after a prolonged insulation. Paper freshly prepared is always more sensitive and gives better whites and generally finer results.10
The prints can be toned black in operating as in the cyonotype, but the results are seldom good.
Captain Pizzighelli's formula is as follows: Prepare
| A. | Gum arabic | 15 parts |
| Water | 100 parts | |
| B. | Ammonia ferric citrate | 45 parts |
| Water | 100 parts | |
| C. | Ferric chloride | 45 parts |
| Water | 100 parts |
For sensitizing mix in order:
| Solution A | 100 parts |
| Solution B | 40 parts |
| Solution C | 20 parts |
The mixture very much thickens at first, but becomes sufficiently fluid for use in a few hours. It keeps well for two or three days. Leaving out B and replacing it by rain water, this makes also a good solution for the cyanotype.
THE BLACK OR INK PROCESS. (Ferro-tannate Process.)
This process gives black positive impressions on white ground from positive clichés, and negative impressions from negative clichés. It has been attributed to Mr. Colas, but in reality it was invented by Mr. Poitevin, who describes it as follows in his communication of May, 1860, to the Société Francaise de Photographie:
“I make a solution containing—”
| Iron perchloride, cryst | 10 parts |
| Tartaric acid | 3 parts |
| Water | 100 parts |
“I apply the paper on this mixture and let it dry spontaneously in the dark, and at the moment of using it I completely desiccate it at a gentle heat. Thus prepared the paper is of a deep yellow color. Light decolors it rapidly, and ten or twelve minutes' exposure through a positive cliché suffices to well impress it, that is, to reduce in the whites the iron perchloride to the state of protochloride.”
“To print, one is guided by the decoloration of the paper, and even for more facility I add to the solution of iron perchloride and tartaric acid a small quantity of a solution of potassium sulphocyanide for the purpose of obtaining a red tint, which is more visible and disappears also under the influence of light in proportion to the decomposition of the perchloride. One obtains then after exposure a red design on the white ground of the paper. This red color is not permanent. It even disappears by keeping the proof in the dark.”
“To develop and then to fix the design thus obtained I wash rapidly the paper in ordinary water, or better, in water holding chalk in suspension. The red coloration disappears, a part of the iron perchloride is washed out, and in the parts which have not been acted on by light the perchloride is transformed into sesquioxide. I replace then the water by solution of gallic acid or of tannin and the image progressively appears in ink-black. When I judge the image to be sufficiently intense I wash the proof in rain water, in preference to ordinary water, [pg 43] which might cause the gallic acid and tannin to turn brown. I sponge between sheets of blotting paper and let the proof dry spontaneously.”
“If in place of gallic acid I use a diluted solution of potassium ferricyanide (red prussiate of potash), Prussian blue is formed in the parts acted on by light. The preparation is even sensitive enough to permit one to obtain an impression in the camera obscura in developing by the ferricyanide.”
“As to the proofs in gallate (or tannate) of iron, they can be transformed into Prussian blue in a solution of potassium ferrocyanide (yellow prussiate of potash) slightly acidified by sulphuric acid.”
The paper most suitable for this process is that which has been previously well sized with starch, as explained in a special paragraph of this pamphlet. Paper prepared with a film of coagulated albumen gives also good results. It may be prepared by brushing as well as by floating, but in either case the paper should be wetted on the surface only and dried rapidly at a temperature of about 115 deg. Fahr. (46 deg. C.) and kept in a dry place. It does not keep for more than from ten to fifteen days, owing to the hygroscopicity of the iron compound. Mr. Colas, who prepares the paper for the Parisian market, I think, states that he avoids its deterioration by keeping it wrapped in blotting paper, between two sheets of India rubber, to exclude air and dampness. Silvered albumen and plain paper, well desiccated, could be kept in that way for a certain period, especially if the blotting paper is impregnated with sodium bicarbonate and well dried.
Mr. A. Fisch advises to discard the preliminary washing and to develop just on the removal of the proofs from the printing frame. In operating in this manner the development is best made by floating, taking care that the solution does not run off the back of the proof.
The developer may consist of a dilute solution of nutgalls or of
| Tannin or gallic acid | 4 parts |
| Oxalic acid | 0.15 parts |
| Water | 1,000 parts |
After developing the proof should be washed rapidly—under a jet of water, if possible—for were the iron salt and the [pg 44] reagent not soon removed, or any remain in the paper, the ground would be tinted violet. And whatever be the care taken, it very seldom occurs that the whites are pure when the proof is dry. This for half-tone pictures has not a great importance, but for the reproductions of plans it is sometimes objectionable. In fact it must be acknowledged that none of the processes now at our disposal—if we except the so-called Artigues process described further on—gives an entirely satisfactory result. A simple and expeditious process, yielding intense black impressions on a white ground, is yet to be found for the reproduction of plans, maps, etc., without resorting to a negative cliché or drawing.
THE CUPROTYPE. (Burnett's Process.)
This process gives positive impressions from negative clichés.
| Uranic nitrate | 10 parts |
| Cupric nitrate | 2 parts |
| Water | 100 parts |
Float for a minute strong, well-sized paper on this solution and let it dry spontaneously in the dark. Expose until the image is visible, then develop by floating on a solution of potassium ferricyanide at 5 per 100 of water—the image appears at once with a rich brown color. When developed, wash it in several changes of water until the unaltered salts are eliminated. The proof is then fixed, and, if too intense, can be reduced in water slightly acidified with hydrochloric acid. A fine black image is obtained by toning in a solution of platinic chloride at 1 per 100 of water.
The chemical actions giving rise to the formation of the metallic ferrocyanide, of which the image consists, are quite complicated. Under the luminous agency the uranic nitrate is first reduced, then the uranous oxide acts on the cupric nitrate, forming cupric oxide, which is finally reduced to the metallic state. This metal now converts the ferricyanate in the ferro compound, which, by another action, forms both cupric and uranic ferrocyanate.
The following uranium process gives black impressions:
In a saturated solution of tartaric acid dissolve freshly precipitated ferric oxide, and keep the solution—ferric tartrate—in the dark. To prepare the sensitizing solution, dissolve 20 parts of uranic nitrate and from 1 to 3 parts of tartaric acid in 100 parts of water, and add a small quantity of ferric tartrate, the proportion varying with the tint desired: an excess gives a blue black. With this solution brush the [pg 46] paper over, and, when dry, expose under the negative cliché, then develop with a solution of potassium ferricyanate at 4 per 100 of water. To fix, it suffices to wash in water, renewed three or four times.
As pointed out by Mr. B. J. Burnett (see Introduction), many photographic processes can be devised by basing them upon the various chemical changes, of which uranous oxide, reduced by light from the uranic nitrate or sulphate, is susceptible by means of metallic or organic reagents.
In the Appendix some of the most important processes, with or without silver salts as reagents, will be described.
THE ANILINE PROCESS.
The aniline process was published in 1865, by Mr. Willis, the inventor of the platinotype.11 It is based on the oxidation of aniline by chromic acid, thus: A sheet of paper brushed with a solution of potassium bichromate and sulphuric acid, dried, and after insolation under a cliché exposed to the fumes of aniline which, in reacting with the chromic compound not reduced by light, forms a blue-black image. The process gives, consequently, a positive impression from a positive cliché.
There are various methods of operating; we will briefly describe them.
| SENSITISING SOLUTION. | ||
|---|---|---|
| 1. | Potassium bichromate | 6 parts |
| Sulphuric acid | 6 parts | |
| Magnesium chloride | 10 parts | |
| Water | 150 parts | |
Willis recommended 10 parts of solid phosphoric acid instead of sulphuric acid; the latter forms a preparation about twice more rapidly reduced.
| 2. | Potassium bichromate | 10 parts |
| Manganous sulphate | 4 parts | |
| Potassium bisulphate | 20 parts | |
| Water | 300 parts | |
| 3. | Ammonium bichromate | 5 parts |
| Ammonium chloride | 5 parts | |
| Cupric sulphate | 1 part | |
| Sulphuric acid | 8 parts | |
| Water | 150 parts |
Good well-sized paper should be employed. Rives is too tender and absorbs too much. Steinbach is better. For small sizes, whatever be the paper selected, it is well to size it with starch and, if possible, to calender it on a hot steel plate, or, in lieu, to iron it. This is not, however, a sine qua non. [pg 48] The paper is sensitized by brushing or by floating. To sensitize by floating, it should be left but for a few seconds on the solution and removed by dragging it on a glass rod in order to remove the superfluous liquid. Only the surface of the paper should be impregnated, otherwise the whites would be more or less tinted and the image imbedded not as sharp.
Sensitized, the paper must be dried as rapidly as possible. It does not keep, and should be employed the day it is prepared or the day after, keeping it well wrapped in paper.
As said above, it is exposed under a positive cliché, plans, designs, etc., drawn on tracing paper or linen. The more transparent the material, the more rapid the chemical changes. During the insolation—and it is very short—the chromic compound is reduced, the parts corresponding to the ground, that is, the transparent parts of the cliché, are discolored, while those under the design remain unaltered; the image being, therefore, faintly visible, and being formed of the chromic mixture, it is developed by the fumes of aniline in a blue black tone. Therefore, if the paper be not sufficiently exposed, the ground is colored like the image, although not as deeply, since the dye formed is proportionate to the more or less quantity of unreduced compound, and if exposed too long the image is imperfectly developed or not at all by excess.
The discoloration of the ground, which turns to a greenish hue, easily indicates when the exposure is sufficient. But, to ascertain it, the beginner should use tests as in the cyanofer process. Mr. Endemann regulates the time of exposure by partly covering a strip of the sensitive paper with a piece of the tracing material upon which the design is made, and exposing the whole until the covered part of the paper assumes the same shade as the part directly exposed to light.
To develop the print is placed in the bottom of a tray, which is then covered with a lid upon which is pinned blotting paper well imbued with an aniline and benzine mixture, or the reverse; that is, exposing the print fastened to the lid and placing the aniline on the bottom of the tray. The tray should be hermetically closed; that is a condition to obtain a fine and equal coloration. For this purpose the lid should be [pg 49] well lined with sheets of blotting paper and a weight placed over it during the operation. Large prints are necessarily developed in a fumigating box made ad hoc. The aniline solution consists of
| Aniline (commercial for red) | 8 parts |
| Benzine, rectified | 100 parts |
In place of benzine, ether U.S.P., sp. grav. 0.837, may be used.
When the proof is not over-exposed the development commences in a few minutes. The image first takes a dirty black olive color which turns blue in water, then the tone darkens to a dark-brownish tint. The time of exposure to the aniline fumes depends on the time of insolation; if short, the ground is soon tinted, and consequently the development should then be stopped; if over-exposed, the development proceeds slowly. The darkest tone is obtained by a rather full exposure which admits a long fumigation. Sometimes the image takes a green color; it suffices then to wash the proof in water rendered alkaline by a few drops of aqueous ammonia to obtain the normal color.
To somewhat improve the tone of the image and, if objectionable, to remove the chromic oxide which tinges the ground greenish, the proof should be immersed in a dilute solution of sulphuric acid 1:100, then washed twice, and finally passed in ammoniacal water 1:100.
Mr. Hermann Endemann has published, in 1866, the following process in the Journal of the American Chemical Society, pp. 189 et seq.:
The paper, which must be well sized with glue, 1:50, is sensitized with the following solution and exposed when dry, but still slightly damp:
| A. | Potassium bicarbonate | 1 ounce or 480 parts |
| Salt | 1 ounce or 480 parts | |
| Sodium vanadate | 2/3 grain or 0.66 part | |
| Water | 20 ounces or 9,600 parts | |
| B. | Sulphuric acid | 2 ounces or 960 parts |
| Water | 10 ounces or 4,800 parts |
When cold mix to A.
[pg 50]“From the composition of the solution,” says Mr. Endemann, “it is evident that it must be strongly acid; but when this solution is exposed to light, in the presence of the organic substances of the paper, the acidity of the solution disappears, we obtain potassium and sodium sulphates, basic chromium sulphate, salt and vanadic acid. While, therefore, the unchanged parts of the paper remain acid, the changed parts acquire a neutral reaction, and while the first will readily assimilate bases, the second will not. Exposed in an atmosphere laden with water and aniline, the aniline will be absorbed in those parts where the solution remains acid and in proportion to the remaining acidity.”
To develop the image the paper is spread over the opening of a frame tightly placed on a pan, in the bottom of which is heated a solution of aniline in water, 1:50, until the image appears brown, and for further development in a box laden with steam water, which, according to Mr. Endemann, requires two hours to obtain a deep black coloration. To remove the chromium compound the picture is immersed in a solution of aqueous ammonia, 1:6, then washed and dried.
A few years ago the aniline process was improved by developing the image with the aniline-benzine mixture vaporized by steam in a box made specially for that purpose, whereby a reproduction can be obtained in less than ten minutes.
In the photographic department of Messrs Poulson & Eger's Hecia Architectural and Ornamental Iron Works, which is directed by Charles Bilordeaux, this process is worked in the following manner:
The developing is made of sheet iron with a door sliding up and down, it being balanced by a counterpoise, and provided with a chimney. In the box is a gutter, extending the whole length of the bottom, covered with muslin and connected to a steam pipe; there is also a coil similarly connected. After the insolation, which requires about one minute in sunshine, the print is suspended in the box, the muslin brushed over with the solution of aniline, and live steam allowed to pass through the gutter for only two minutes, whereby the aniline being vaporized acts on the chromic salt and develops the image; then the steam is allowed in the coil, and, in from three to four [pg 51] minutes, the paper is dry and the picture finished. The image stands on a slightly greenish ground, which is not objectionable for the purpose the reproductions are made.
The sensitizing solution is similar to that published by Mr. Endemann, viz.:
| Potassium bichromate | 460 grams |
| Sodium chloride | 460 grams |
| Ammonium vanadate | 0.75 gram |
| Sulphuric acid | 1 liter |
| Water | 13 liters |
THE PRIMULINE OR DIAZOTYPE PROCESS.
Primuline, discovered in 1887 by Mr. A. G. Green, an English chemist, is a dye of a primrose color, possessing a great affinity for cotton fibers, to which it is readily fixed by simply immersing the material for a few moments in a hot solution of the dye. If the material so dyed be placed in an acidified solution of nitrous oxide, the primuline is diazotized, forming a derivative compound of a deeper color, which fades in the light, and which in presence of amines and phenols gives rise to a variety of dyes whose color depends on the reagent employed, while, when acted on by light, the resulting compound is entirely deprived of this property. In other words, the diazotized primuline acts as a mordant only when not altered by the luminous action.
The chemical change light effects in the diazotized primuline is not well known. It is pretty certain, however, that nitrogen is set free, for if gelatine imbued with primuline be immersed in water after insulation, nitrogen is set free and can be collected as usual in a tub filled with water and inverted on the substance.
By itself diazotized primuline is slowly influenced by light, but quickly acted on in presence of organic substances. It is more sensitive when applied on cotton or paper than on wool, silk, linen, and such organic compounds as gelatine, albumen, caseine, starch, etc. Its sensitiveness is about one-tenth less with gelatine than with cotton.
The sensitiveness of diazotized primuline to light, when united to organic substances and the different colors which can be obtained with the unaltered compound, have given rise to an interesting printing method, the invention of Messrs. A. G. Green, C. F. Cross, and E. J. Bevan, which yields positive impressions from positive clichés. The manipulations of the process are simple:
In a certain quantity of rain water, kept at nearly the boiling [pg 53] temperature by an alcohol lamp placed under the vessel, dissolve per cent. 2 parts of commercial primuline, and in this immerse, by means of a glass rod, some pieces of calico—free from dressing—turning them over several times during the immersion. When the fibers are well imbued, which requires from four to five minutes, remove the calico with the glass rod and rinse it thoroughly in water. This done, wring out the superfluous liquid as much as possible, and, finally, immerse each piece separately in a solution of
| Sodium nitrite, commercial | 7 parts |
| Hydrochloric acid, commercial | 16 parts |
| Water | 100 parts |
After turning the pieces of calico two or three times over, they are rinsed to eliminate the acid, then drained and placed between sheets of blotting paper to dry. All this, except the impregnation with primuline, should be done in the dark room.
As said above, primuline is transformed by nitrous oxide into a diazotized compound, and consequently the material is now susceptible of being acted on by light. It does not keep, and should be exposed, etc., soon after its preparation.
Paper is impregnated with primuline either by floating or brushing. The best results are obtained with paper previously sized with arrowroot or gelatine in order to keep the image entirely on the surface of the paper.
Linen, silk and wool are treated as calico.
The clichés should be positive to obtain positive expressions and somewhat more opaque than those employed in the processes before described, else vigor and intensity could not be obtained. Here we must state that the primuline process seems to be better adapted for the reproductions of drawings, such as made for the black process, and of opaque photo-clichés in lines, or white and black, than for printing in half tone.
When the material to print upon is thick and wholly impregnated with diazotized primuline, it is advisable, since the insulation could not be prolonged to effect the change through, to expose the back of the material for a certain but [pg 54] short period in order to clear it. This is especially advantageous when the cliché is not of good intensity.
During the exposure, which varies from 30 seconds to 10 minutes and more by a dull light, the progresses of the luminous action is seen by the bleaching of the material which assumes a dingy coloration. But in order to ascertain when the decomposition is complete on the ground of the image, it is well to use tests as in the cyanofer process, dipping one of them in the developer from time to time.
The developers are compounded as follows:
| FOR RED. | |
|---|---|
| Beta-naphthol | 4 parts |
| Caustic potassa | 6 parts |
| Water | 500 parts |
Rub the alkali and the naphthol with a little water in a mortar and add the remainder of the water.
| FOR ORANGE. | |
|---|---|
| Resorcin | 3 parts |
| Water | 500 parts |
When dissolved add
| Caustic potassa | 5 parts |
| FOR YELLOW. | |
|---|---|
| Carbolic acid, cryst | 5 parts |
| Water | 500 parts |
| FOR PURPLE. | |
|---|---|
| Naphthylamine | 6 parts |
| Hydrochloric acid, in volume | 6 parts |
Mix in a mortar, then add
| Water | 500 parts |
| FOR BLACK. | |
|---|---|
| Eikonogen, white crystals | 6 parts |
| Water | 500 parts |
Pulverize the eikonogen, add the water and, at the same time, the material on its removal from the printing frame, and keep in motion until the development is effected.
| FOR BROWN. | |
|---|---|
| Pyrogallol | 5 parts |
| Water | 500 parts |
After the development, which requires but a few moments, it suffices to wash the material to fix the image by eliminating the soluble compounds. However, for purple the material should be passed in a dilute solution of tartaric acid and not washed afterwards; it should remain acid.
When it is desirable to obtain an impression in several colors, the various developers are thickened with starch, then locally applied with a brush on the image, which is always visible after exposure.
For printing on wood, glass and porcelain, see further on.
PRINTING ON WOOD, CANVAS, OPAL, AND TRANSPARENCIES
Printing on Wood.—To print on a wood block a design to be engraved on the same presents certain difficulties. In the first place, the sensitizing solution must not be absorbed by the wood, but remain wholly on its surface; then the photo film, although thick enough to produce an image sufficiently intense to be distinctly visible in all its details, should not scale or clip away under the graver, and not interfere in any way with the work of the artist; the least touch of the graver must reach the wood and make its impression. Lastly, the design should be permanent. These difficulties will be avoided by adhering to the instructions given in the lines following.
The solution to render impervious the surface of the wood consists of
| Common gelatine | 5 parts |
| Gum arabic | 3 parts |
| Castile soap | 3 parts |
| Water | 100 parts |
Dissolve by heat on a water bath.
To apply it, the wood is rubbed with fine sandpaper, then heated over a spirit lamp to about 86 deg. Fahr. (30 deg. C.) and upon it is poured in excess the liquefied and quite warm solution, which must be allowed to penetrate in the pores of the wood by letting it gelatinize, when it is wiped off clean. Nothing must remain on the surface of the wood. This done, and while still damp, the preparation is rendered insoluble by pouring over a solution of alum at 5 [pg 56] per 100 of water. The object of this preliminary operation is to render the wood impervious, and therefore to prevent the sensitizing solution to penetrate its texture. The wood is then heated again and its surface whitened with a little silver white or sulphate of barium, diffused in a small quantity of the following warm solution:
| Gelatine | 1 parts |
| Alum | 0.1 part |
| Water | 100 parts |
While wet, this is smoothed with a jeweler's brush, taking care to leave on the wood, a very thin layer of the mixture, only sufficient to obtain a white surface which, by contrasting with color of the wood assists the engraver in his work. The wood should now be allowed to dry thoroughly, when it is coated with a tepid solution of
| Isinglass | 3 parts |
| Water | 100 parts |
and dried.
Now the sensitizing process differs according as whether the cliché is positive or negative. In the former case the preparation is sensitized with the solution employed in the black process, proceeding afterwards as usual; in the latter, that is, when the cliché is negative, the best process is the cuprotype.12
For printing, special frames are employed to permit one to examine the progress of the impression from time to time without the possibility of either the wood block or the cliché moving. These frames open in two. The upper frame is provided with screws on the four sides to hold firmly the block when it is placed into contact with the cliché by means of the screws fixed on the cross bars. As to the cliché, if it is made on a glass plate, it is secured on the thick glass plate of the lower frame by two wooden bars against it pushed by screws.
[pg 57]When the block is ready for printing, the prepared side is usually concave. It is straightened by slightly wetting the back and resting it on one end, prepared side against the wall.
Printing on Canvas.—The canvas should be first brushed with a solution of aqueous ammonia in alcohol, 1:3, to remove greasiness until the thread just commences to show, then, when rinsed and dry, rubbed with fine sand to give a tooth, dusted, washed with a sponge and then coated with the following solution, proceeding afterwards as in the cuprotype process:
| Isinglass | 8 parts |
| Uranic nitrate | 5 parts |
| Copper nitrate | 2 parts |
| Water | 200 parts |
Printing on Opal, Celluloid, etc., is quite simple; it suffices to coat the material with the following gelatine solution, and, when the film is dry, to proceed in operating by any one of the processes before described.
The sensitizing compound may be incorporated to the gelatine solution, but we prefer not to do it and to sensitize the plates as they are wanted for use.
| A. | Gelatine | 4 parts |
| Water | 70 parts in volume |
Dissolve and mix little by little in order:
| B. | Chrome alum | 0.25 parts |
| Water, hot | 20 parts | |
| C. | Alcohol | 10 parts |
When coated place the plates on a level stand until the gelatine is set, and let them dry on a rack.
Transparencies.—Prepare the plate as directed above with
| A. | Gelatine | 6 parts |
| Water | 70 parts | |
| B. | Chrome alum | 0.3 part |
| Water, hot | 20 parts | |
| C. | Alcohol | 10 parts |
Sensitize with the uranic-copper solution employed in the cuprotype. By this process transparencies of a rich brown, [pg 58] not actinic, color are obtained. Consequently they can be used to reproduce negatives by the same process. For lantern slides they may be toned black by platinic chloride.
To strip off the picture, apply, first, on the glass plate a substratum of India rubber, 2 to 100 of benzole, coat with plain collodion, immerse the plate in water as soon as the film is set, and when greasiness has disappeared pour on the gelatine solution and proceed.
For tranferring on any material, a sheet of paper is immersed in a solution of India rubber cement in 20 parts of benzole, dried, coated with the gelatine solution, sensitized, etc., by operating in the ordinary manner. After development, the proof, being dry, is brushed over with alumed gelatine moderately warm, dried, immersed in tepid water until the gelatine is softened and tacky, when it is placed on the material and squeezed into contact. This done, the transfer should be allowed to dry thoroughly. Now, by imbuing the proof with benzole to dissolve the India rubber, the paper is easily stripped off, leaving behind the picture adhering to the material.
[pg 59]TRACING PROCESS ON METAL.
We call the attention of metal engravers to this process. It is well known that wood engravers have their original designs photographed on the block in order to save considerable time by not making the drawing themselves; moreover the cost is nominal, so to say, and the copy more true and perfect than it can be done by hand. Why should not the copper engraver and the aquafortist avail themselves of the same advantages? A few do it secretly, no doubt, but the generality not knowing the process, or, if so, not having tried it, think it is not possible or that it may spoil their plates. This is an error. It can be done and very easily by adhering to the following instructions:
Dissolve 2 parts of ammonium bichromate in 100 parts of water, and in this let soak for an hour or so 10 parts of Coignet's best gelatine, then dissolve on a water bath, filter through flannel, and the solution is ready for use.
Before being coated, the plate should necessarily be cleaned free from oxidation and greasy matters. This is done by immersing the plate for a few moments in a warm solution of common potash, then rinsing and rubbing it with chalk moistened with a little water, when after rinsing again and draining the plate should be immediately prepared.
To spread the gelatine solution in an even and thin layer, a tournette is employed. The most simple consists of a round wooden stick of which the upper part is carved in the form of a cup with an edge, or rim, about one quarter of an inch broad. On this rim is melted some gutta-percha, upon which the plate is pressed into contact and adhers quite firmly when the gutta-percha is solidified. The stick is perforated at the lower end and revolves on an iron pivot fixed at the bottom of the support, being held in the opening on the platform of the same, as shown in the diagram on the following page.
The plate being fastened to the tournette, the warm gelatine solution is flowed over it and spread to the edges by means of [pg 60] a glass rod or a piece of cardboard, avoiding air bubbles. This done the tournette is set into motion, and when the film is equalized, which is done in a moment, the plate is detached, placed on a leveled stand and slowly dried with the spirit lamp.
By a good light the exposure on the shade does not exceed twenty minutes with a pretty intense transparency, and should be regulated with a photometer. When the insulation is sufficient, the image is slightly visible, and should be so. The plate is then bordered with banking wax and bitten-in with a solution of ferric chloride at 45 deg. Baumé, or—
| Ferric chloride, crystal | 20 parts |
| Hydrochloric acid | 1 part |
| Water | 100 parts |
The parts of the gelatine film the most acted on are impermeable, so to say, and consequently do not allow the etching fluid to penetrate to the copper; while those the least impressed are permeated according as to their degree of insolation, Therefore, when the ferric chloride solution is poured upon the film and carefelly brushed over with a soft brush, in a few moments the image progressively appears, the deep blacks first, then the half tints, and lastly the most delicate details, the whole requiring but a few minutes. It is now that the etching [pg 61] action should be stopped by washing under the tap. However, should by excess of exposure, or any other cause, the details not appear within five or six minutes, the ferric chloride should nevertheless be washed off, for then it may find its way under the film and the plate would be spoiled. After washing the gelatine is dissolved in a solution of potash, etc., when the image would be found slightly engraved.
Should the image be in half-tints, it would be advisable to apply a grain of rosin on the gelatine film just before etching. To engrave on steel the operations are the same, but on its removal from the printing frame the plate should be soaked with water renewed several times until the bichromate is washed off. The film is then dried spontaneously and afterwards flowed for about two minutes with the Solution A, then, this being thrown away, with the Solution B, which is allowed to act for a similar period.
| A. | Nitric acid, pure | 120 parts |
| Silver nitrate | 6 parts | |
| Alcohol, 95 deg | 50 parts | |
| Water | 75 parts | |
| B. | Nitric acid, pure | 5 parts |
| Alcohol, 95 deg | 40 parts | |
| Water | 60 parts |
GRAPHOTYPY.
This process consists in converting a cliché in half tones into one in lines, which can be directly printed on paper, or impressed, by means of an ink transfer made as explained before, on a stone, or on a zinc or copper plate for etching in relief, or in intaglio, according as the cliché is negative or positive.
A cliché on gelatine, but preferably on a collodion film, is varnished with a solution of yellow wax and bitumen in benzole and turpentine-oil:
| Bitumen of Judaea | 8 parts |
| Yellow wax | 2 parts |
| Benzole | 40 parts |
| Turpentine oil | 60 parts (filter) |
then etched as done to engrave in the aquafortis manner, the corrections being made by applying with a brush some of the above varnish on the defective parts, which are worked over when the varnish is dry.
The tools are simply needles of various thickness ground in sharp square and round points of different sizes.
When the etching is finished, the parts which should form the ground, or white parts of the design, being covered with the bitumen varnish is non-actinic, or, in other words, does not admit the light acting on the sensitive plate preparation employed to reproduce the design, except by an exposure a good deal longer than that necessary to reduce the metallic salts.
The engraver will see at once that, although it greatly simplifies the copying work and, consequently, saves much time, this process does not, however, bind him to any rules and leaves him perfectly free to follow its inspirations and make such alterations as he thinks proper to produce artistic effects; in a word, the reproduction will no more be a picture taken [pg 63] by a mechanical process, so to say, but an original drawing reflecting his talent and characteristic manner.
A similar process much employed by photo engravers, and presenting the same advantages, is to convert an ordinary photograph on paper—or a blue print, as devised by the writer—into a design in lines by drawing with India ink, or the special ink of Higgins, and, this done, to wash off the photographic image, the design being afterwards reproduced by the ordinary processes as a negative or a positive cliché.
When the photograph is a silver print especially made for the purpose in question and, consequently not toned, but simply fixed in a new thiosulphate (hyposulphite) bath, and well washed—it is bleached by flowing over a solution of—
| Bichloride of mercury | 5 parts |
| Alcohol | 40 parts13 |
| Water | 100 parts |
If the photograph has been toned, i.e., colored by a deposit of gold, or if it was fixed in a thiosulphate bath in which toned prints have been fixed, then the image is dissolved by treatment in a solution of potassium cyanide in alcoholized water.
When a blue photograph is reduced, it is advisable before drawing upon it to first reduce its intensity by a prolonged immersion into water. Pale blue is a very actinic color which is not reproduced in photography, except by the ortho-chromatic process, or if it does, the impression being very weak, is not objectionable. When the image has not been sufficiently or not at all bleached, the blue is dissolved by an alcoholized solution of the blue solving.
[pg 70]THE URANOTYPE.
This process, devised by J. Wothly, in 1864, did not receive from the photographers the attention it merits, as it is always the case when a process is patented, and can be replaced by another equally practical which is not. It gives pictures of a very good tone, which are quite permanent; we have some made in 1866, which are suffered no change whatever, they seem to have been printed from yesterday.
The first process given by Wothly does not appear to be complete. It has been well described by H. Cooper and a gentleman who signs by the initial letter X.
The process published in 1865 by Wothly is as follows: A sheet of paper is sized by brushing with a paste made of 24 parts of arrowroot in 500 parts of water, to which are added a few drops of a solution of citric or tartaric acid, then coated with a collodion consisting of 100 cubic centimeters of plain collodion, a few drops of oil of turpentine and 30 cubic centimeters of the following sensitizing solution:
| Nitrate of uranium | 30 to 90 parts |
| Chloride of platinum | 2 parts |
| Alcohol | 180 parts |
The time of exposure is about that required for paper prepared with silver chloride. The image is bluish-black but weak. After washing the print is immersed in a solution containing 0.5 parts of chloride of gold for 2,000 parts of distilled water, and then fixed in a bath of sulphocyanate of potassium, which tones the image blue-black.
It may happen that the proof is slightly tinted red. This arises from a small quantity of lime in the paper which forms uranate of calcium.
To prevent the proofs turning yellow, it should be washed in an exceedingly weak solution of acetic acid.
If, after exposure, the print is immersed, without it being [pg 68] washed, in the gold bath, the image becomes rose-red, but the whites remain pure. The effect is peculiar.
H. COOPER'S PROCESS (1865).
| PREPARATION OF THE PAPER | |
|---|---|
| St. Vincent arrowroot | 200 grains |
| Boiling water | 10 ounces |
Crush the arrowroot to fine powder, then rub it to a paste with a little water, and let an assistant pour a few drams of boiling water while you keep stirring all the time; finally, let him add the rest of the boiling water, the operator still continuing the stirring. The paste is allowed to cool, and will be thicker when cold than when hot. Remove the upper portion entirely when quite cold, otherwise, if any left, it will give rise to streaks. The author insists upon the necessity of all these cares. Two sheets of paper are now placed side by side on a flat board, then the surface of the first is covered with the paste by means of a sponge, proceeding, before you leave it, all over the sheet in a horizontal direction; the second sheet is covered in a like manner. By the time the second sheet is pasted, the first one will be partially dry. The sponge is now drawn over each sheet, in succession, in a perpendicular direction in order to efface the streaks from the first sponging. If the paste drags in a slimy manner, it is too strong, and a fresh arrowroot must be prepared, because dilution only ends in failure. Why dry, the paper is rolled under moderate pressure, and when it lies smoothly the maximum pressure may be applied.
| PLAIN COLLODION. | |
|---|---|
| Alcohol | 12 ounces |
| Ether | 4 ounces |
| Pyroxyline | 80 grains |
| SENSITIVE COLLODION. | |
|---|---|
| Plain collodion | 1 ounce |
| Nitrate of uranium, pure | 30 grains |
| Nitrate or silver | 5 grains |
Add the uranium first, and as soon as it has dissolved all that [pg 69] it can, add a grain or two of soda, and when settled pour off the supernatant collodion and add the silver.14 To coat the paper with collodion, use a board with a handle beneath, such as is used by plasterers. On this place a sheet of paper, the edges being turned up about the sixteenth of an inch; this enables the whole of the sheet to be covered without spilling the collodion or allowing it to run on the back of the paper.
There is a marked difference in the appearance of the prints when they leave the pressure frame. Some samples of collodion cause the picture to print of a beautiful green, others of a rich brown, and some of a yellow or orange tint. The last take the longest of all to tone, and difficultly assume the tint of well toned silver prints,15 those printing to green or brown tone very rapidly.
After printing the pictures are placed in diluted sulphuric acid, 1 to 30 of water, until the high lights are perfectly clear and white; this takes from ten to fifteen minutes. After washing well under a stream of water, they are placed in the toning and fixing bath.
| TONING AND FIXING BATH. | |
|---|---|
| Sulphocyanide of ammonium | 1 ounce |
| Water | 12 ounces |
| Chloride of gold | 1 to 3 grains |
After removing from this bath, the prints are immersed for a few moments in water, and then rapidly washed.
[pg 70]| FORMULA FOR PREPARING THE PYROXYLINE | |
|---|---|
| Nitric acid, sp. gr. 1.30 | 12 fluid ounces |
| Sulphuric acid, sp. gr. 1.845 | 36 fluid ounces |
| Water | 8 fluid ounces |
| Temperature | 130 degrees Fahr. |
| Time of immersion | 15 minutes. |
X'S PROCESS (1865). (Secrets of the Uranotype)
Preparation of the Uranium Compound.—Precipitate the nitrate of uranium from its solution by concentrated liquid ammonia. Let settle the precipitate, decant, and wash in several changes of water. Dissolve it by heat in pure nitric acid, taking care not to add an excess of acid. The ammonio-nitrate of uranium salt is then crystallized and dried. Mix a solution of 6 drams of this salt, dissolved in 3 drams of water, to a solution of 15 grains of silver in 30 minims of water, and crystallize. This salt is called ammonio-nitrate of uranium and silver.
| SENSITIZING SOLUTION. | |
|---|---|
| Ammonio nitrate salt | 3 drams |
| Alcohol | 8 drams |
| Distilled water | 15 drops |
| Nitric acid, pure | 1 drop |
Plain Collodion.—Dissolve in a small quantity of ether 1 dram of Canada balsam and 1 dram of castor oil, filter and let evaporate the solution to the consistency of oil.
Of this, add 10 minims to a collodion made of
| Alcohol | 10 ounces |
| Ether | 20 ounces |
| Pyroxyline | 220 grains |
| SENSITIVE COLLODION | |
|---|---|
| Plain collodion | 12 drams |
| Sensitizing solution | 6 drams |
| Nitric acid | 2 or more drops |
Keep this collodion in the dark, as it is quite sensitive.
| PREPARATION OF THE PAPER | |
|---|---|
| Arrowroot, pulverized | 1 ounce |
| Water | 32 ounces |
| Solution of acetate of lead | 10 drops |
Heat to 100 deg. Fahr. and then add four ounces of albumen. The paper is floated on this solution for five minutes and hung up to dry. The sizing may also be applied with a sponge in the manner often described.
The proofs should be slightly over-printed and, before toning and fixing, placed for about ten minutes in the following solution:
| Distilled water | 40 ounces |
| Acetic acid | 1 ounce |
| Hydrochloric acid | 1 ounce |
After washing in several changes of water, the proofs may be toned in any toning bath, and then fixed with sulphocyanide of potassium, washing afterwards in the usual manner.
THE PLATINOTYPE.
This process, discovered by William Willis,16 yields very fine impressions which wholly consists of platinum and are, therefore, chemically permanent. It has been described theoretically and practically by Pizzighelli and Kübl in a paper for which the Vienna Photographic Society has awarded the Voightlander prize.17 The following is an abridgment of this important process, as described by the authors:
The paper, calendered or not,18 is sized with gelatine or arrowroot. The color of the proof with the latter size is brownish black, and bluish black with the former.
To prepare the gelatine solution 10 parts of gelatine are soaked in 800 parts of water and then dissolved at a temperature of 60 deg. C. (140 deg. Fahr.), when 200 parts of alcohol and 3 parts of alum are added and the solution filtered.
To prepare the arrowroot solution 10 parts of the substance are powdered in a mortar with a little water and mixed to 800 parts of boiling water, added gradually in stirring. After boiling for a few minutes 200 parts of alcohol are added and the mixture filtered.
These solutions are employed warm. The paper is immersed for two or three minutes and hung up to dry in a heated room, then immersed a second time and dried by hanging it up in the opposite direction, in order to obtain an even coating.
The potassic platinic chloride is an article of commerce. It should be soluble without residue in 6 parts of water and without acid reaction. In this proportion it constitutes the normal stock solution employed in the various formulas.
The standard ferric oxalate solution is also found in commerce. [pg 73] Treated by potassium ferricyanate it should not be colored blue, nor become turpid when diluted with one-tenth part of water and boiled. The former reaction indicates that it contains no ferrous salt, and the latter no basic oxalate.
The authors give the following instructions for preparing the ferric oxalate solution, to which they attach much importance:
Five hundred parts of ferric chloride are dissolved in 5,000 parts of water and heated to boiling, when a solution of soda is added until the liquid becomes alkaline.19 About 250 parts of caustic soda are generally employed for this purpose. The precipitate—ferric oxide—is now washed in warm water until the last washing water is quite neutral to test paper, then drained and mixed with 200 parts of pure crystallized oxalic acid. The mixture is then allowed to stand in the dark for several days at a temperature not exceeding 30 deg. C. (86 deg. Fahr.) At first the solution from green turns to a yellow green, and finally becomes almost brown. At this moment the excess of ferric oxide is filtered out and the liquor submitted to a quantitative analysis, the result of which leads to ascertain the quantity of ferric oxalate in 100 parts of the solution and the excess of oxalic acid. The solution should then be diluted with distilled water, such as it contains 20 parts of ferric oxalate per 100 parts of water, and oxalic acid must be added in the proportion of from 6 to 8 per 100 of the ferric oxalate, taking into account the quantity of acid the solution already contains. The solution should be kept in the dark. It is altered by light.20
| IRON CHLORATE SOLUTION | |
|---|---|
| Ferric oxalate solution | 100 parts |
| Potassium chlorate | 0.4 parts |
This solution is employed to obtain more contrasts.
[pg 74]| PREPARATION OF THE SENSITIZING SOLUTION | |
|---|---|
| Platinum solution | 12 parts |
| Ferric oxalate solution | 11 parts |
| Distilled water | 2 parts |
This solution gives very soft tones with intense black. To obtain more brilliancy we use the following proportions:
| Platinum solution | 12 parts |
| Ferric oxalate solution | 9 parts |
| Chlorate of iron solution | 3 parts |
| Distilled water | 2 parts |
To obtain results comparable to those which the silver printing out process gives, the following mixture is employed:
| Platinum solution | 12 parts |
| Ferric oxalate solution | 8 parts |
| Chlorate of iron solution | 4 parts |
| Distilled water | 8 parts |
For very weak negatives, reproductions of drawings, etc., we use—
| Platinum solution | 12 parts |
| Chlorate of iron | 11 parts |
| Distilled water | 2 parts21 |
To obtain proofs not completely black, as, for example, reproductions of lead drawings, the solution may be diluted with half or the whole volume of distilled water. But if the solution be applied on little absorbent surfaces or on paper strongly sized it is not advisable to dilute it.
Preparation, of the Paper.—The paper should be kept slightly moist in order that it does not too completely absorb the sensitizing solution. Therefore, when the atmosphere is very dry, it is well to keep the paper in a damp place, in the cellar for example. Before sensitizing, which should be done by a very diffused light, a quantity of the solution proportionate to the surface to be sensitized (about 15 c.c., for a whole sheet of Rives' or Saxe paper) must be measured, and spread with a large brush22 on the paper fixed with drawing [pg 75] pins on a board covered with a sheet of blotting paper. When well impregnated, the paper is hung up to dry in the dark room, and as soon as the apparent dampness of the surface has disappeared, it should be dried immediately at a temperature of 30—40 deg. C. (86—101 deg. Fahr). If the paper be dried too rapidly the sensitive compound remains on its surface, and in developing the image does not come out well. If, on the other hand, the drying is too slow, the solution penetrates too much in the paper and the image is wanting of vigor and does not appear very sharp. One cannot depart from this rule that the desiccation from the moment the solution has been applied until the paper is dry should last no more than from twelve to fifteen minutes.
The sensitized paper is hygroscopic and must be preserved in a calcium box. It is a conditio sine qua non that the paper must be quite dry before, during, and after printing, to obtain good results. Dampness is the greatest enemy in this process.
For printing a pad of India rubber should be placed over the platinum paper to prevent it from attracting the atmospheric moisture, and in damp weather it is even advisable to cover it with several sheets of blotting paper previously heated before the fire.
The platinum paper is at least three times more sensitive than the silver paper used in the printing-out process, under the reductive action of light the yellow color of the prepared paper turns brown and then becomes of a lighter color, nearly orange, so that the darker parts of the image often appears more luminous than the dark half tints. No rule can be given to regulate the insolation, but after a few trials it is easy to judge when it is right by observing the progress of the reduction and the color of the image. The orange color indicates the complete reduction of the ferric oxalate. When the details in the lights are faintly visible, the exposure is generally right.
The developer consists of an almost saturated solution of potassium oxalate acidified by oxalic acid, and for use heated to 80—85 deg. 0. (176—184 deg. Fahr.),23 in an agate glazed [pg 76] iron tray placed upon a water bath at the above temperature. By simply drawing the proof over it, the image is at once developed.24
When the proof is thought to be over-exposed, the oxalate solution can be employed at a lower temperature. If, on the contrary, it is under-exposed, the solution may be heated even to the boiling point.
The developer can be used over and over again. It should always have an acid reaction.
According to Mr. Borlinetto a sepia tone is obtained by using the following cold developer:
| Saturated solution of potassium oxalate | 120 parts |
| Saturated solution of copper chloride | 13 parts |
| Oxalic acid | 1.5 part |
After developing the proofs are immediately immersed for fixing in a solution of hydrochloric acid, 1 to 80 of water, renewed so long as the paper is tinged yellow (about three times), leaving the proofs ten minutes in each solution. Lastly, they are washed to remove the acid.
The platinotype has been still improved by Captain Pizzighelli, who devised the following methods of operating by which the impressions are obtained by the continuous action of light, that is, without development, thus rendering the platinotype just as simple as the ordinary printing-out silver process.
In these new processes to the sensitizing solution is added the alkaline oxalate, which effects the reduction of the platinous salt during the exposure to light. Consequently the prepared paper is insolated until the image appears as it should be, or—which is exceedingly useful in cloudy weather—until it is entirely visible but still deficient in delicate half tones, for in the dark the action proceeds and the image developing itself will be found finished in a period which may extend to a few hours. But it can be, however, developed in a few seconds by immersion in a cold or slightly warm solution of sodium carbonate, 1:25 of water. The image is fixed as directed in the foregoing process.
[pg 77]The paper, prepared exactly as in the former process and kept in the calcium box until wanted for use, should not be employed quite dry, but allowed to absorb a little moisture by hanging it in the dark room. Hence, the India rubber and other protecting pads can be dispensed with. They are even objectionable, for dampness is absolutely necessary to promote the chemical changes by which the image is developed.
| A. AMMONIO-FERRIC OXALATE SOLUTION | |
|---|---|
| Ferric oxalate solution | 100 parts |
| Neutral ammonium oxalate | 18 to 20 parts |
| B. SODIO-FERRIC OXALATE SOLUTION | |
|---|---|
| Ferric oxalate solution | 100 parts |
| Neutral sodium oxalate | 15 to 18 parts |
To prepare these two solutions the ammonium or sodium oxalate is dissolved by small quantities at a time, and when the emerald color due to the formation of the double oxalate commences to darken, the saturation being then complete, no more of either salt should be added. The solution is now well shaken with 3 parts of glycerine, allowed to settle and filtered.
Any one of the double oxalates can be used. The ammonium tends to produce softer pictures and bluish tones. To obtain more contrasts a little potassium chlorate may be added.
| C. IRON CHLORATE SOLUTION | |
|---|---|
| Solution B | 100 parts |
| Potassium chlorate | 0.4 part |
| D. MERCURIC SOLUTION. | |
|---|---|
| Mercuric chloride solution at 5:100 | 20 parts |
| Sodium oxalate solution at 3:100 | 40 parts |
| Glycerine | 2 parts |
| SENSITIZING SOLUTIONS. | |
|---|---|
| FOR BLACK TONES. | |
| Platinite solution, 1:6 | 5 parts |
| Solution B | 6 parts |
| Solution C | 2 parts |
| FOR SEPIA TONES. | |
|---|---|
| Platinite solution, 1:6 | 5 parts |
| Solution C | 4 parts |
| Solution D | 4 parts |
Intermediate tones are obtained by diminishing the dose of C and replacing it by an equal volume of B. For this process the paper should be sized with
| Arrowroot | 2 parts |
| Sodium oxalate at 3:100 | 100 parts |
To dispense with this preliminary sizing Captain Pizzighelli adds gum arabic to the platinite solution, whereby the sizing and sensitizing are done in one operation.
The gum arabic solutions are prepared as follows:
| E. | Gum arabic in powder | 40 parts |
| Sodium ferric oxalate solution, B | 40 parts | |
| Sodium oxalate solution at 3:100 | 100 parts | |
| Glycerine | 3 parts |
Place the glycerine and the gum arabic in a mortar, then, stirring with the pestle, dissolve by adding, little by little, the mixture, heated to 40—45 deg. C. (104—113 deg. Fahr.), of the solution of sodium ferric oxalate and sodium oxalate. Let stand for about two hours and grind again to dissolve entirely the gum arabic. Filter through muslin.
| F. Mercuric chloride solution, 5:100 | 20 parts |
| Sodium oxalate solution, 3:100 | 40 parts |
| Gum arabic in powder | 24 parts |
| Glycerine | 2 parts |
Dissolve as said above.
| SENSITIZING SOLUTIONS. | |
|---|---|
| FOR BLACK TONES. | |
| Platinite solution, 1:6 | 5 parts |
| Solution E | 6 parts |
| Solution C | 2 parts |
| FOR SEPIA TONES. | |
|---|---|
| Platinite solution, 1:6 | 5 parts |
| Solution C | 4 parts |
| Solution F | 4 parts |
Mix just before use. The solutions do not keep. The paper prepared by either one of these two processes can be exposed as in the old process, and the image developed bythe hot oxalate solution.
[pg 79]The preparation of wood, canvas, etc., for the platinotype printing need not to be described; it suggests itself.
CAUSES OF FAILURES.
The images are veiled.
This defect may result from various causes, viz.:
| 1st. | The stock ferric oxalate solution is impaired by a partial reduction of the ferric salt into ferrous oxalate. The solution should be preserved in an orange colored vial, and kept in the closet of the dark room. It should be tested from time to time for the ferrous salt with a solution of potassium ferricyanate. If it does not contain any ferrous oxalate it can be used by adding to it a little of the iron chlorate solution. |
|---|---|
| 2d. | The paper has been exposed to light during the sensitizing or the subsequent operations. One should bear in mind that the platinum paper is twice more sensitive than silvered paper. |
| 3d. | The sensitized paper has been dried at a temperature above 40 deg. C. (104. deg. Fahr.) |
| 4th. | Over-exposure. |
The proofs are not sharp.
| 1st. | The sensitive paper has absorbed moisture. |
|---|---|
| 2d. | It is too old. The paper cannot be kept good for over six weeks, unless special care be taken. |
According to Mr. Bory, the sensitive paper altered by keeping is restored to its original good quality by simply brushing it over with a solution of 0.05 parts of potassium chloride or the same quantity of potassium chlorate in 100 parts of distilled water, or a mixture of these two solutions, or one of iron chlorate.
By treating the insolated paper with these solutions, the image is destroyed, and the paper can be used again. One operates as for sensitizing, taking care to desiccate the paper, as it has been directed.
The proofs are brilliant during the development, but become dull in drying.
The paper not well sized. It has been dried too slowly.
[pg 80]Remember that it should be quite desiccated within fifteen minutes.
The paper is more or less yellow.
| 1st. | The paper tinted with ultramarine. |
|---|---|
| 2d. | The sensitizing solution or the developer are not sufficiently acid. |
| 3d. | The washing (fixing) in the solution of hydrochloric acid was not sufficient to eliminate the iron salts from the paper. |
The proofs harsh, devoid of half tones.
| 1st. | The sensitizing solution contains too much iron chlorate. |
|---|---|
| 2d. | Exposure too short. |
The paper is stained.
The brush not kept clean while sensitizing.
Black spots.
They are generally due to metallic dust in the paste of the paper, or from particles of undissolved salt in the platinite solution.
NB: No good results can be expected unless the paper be kept absolutely dry before, during and after exposure, when using the former (original) process.
Impaired sensitiveness of the paper, want of vigor, tinged whites, muddiness, indicate dampness.
[pg 81]ARTIGUES' PROCESS
The Artigues process, so called, is, without any doubt, the best to be employed for the reproduction of plans and drawings in lines. It is simple, expeditious, and yields black impressions on a very pure white ground which are absolutely permanent. And this is of the utmost importance when the copies are to be used for military purpose, or kept in archives, such as those of the Patent Office, for example. Should it not require the use of negative clichés, it would certainly supersede any of the processes previously described; moreover, as it will be seen, it can be employed for many other purposes than that of obtaining duplicates from original drawings. The objection is not even very great indeed, for the design can be, without great trouble, transformed into a negative by the aniline method described in the beginning of this work.
The Artigues process is an adaptation for the purposes in question of the carbon process invented by Poitevin. We shall describe it in extenso.
The paper can be prepared with any one of the following solutions:
| 1st. | Dissolve 2½ parts of ammonium bichromate and 5 parts of best gum arabic in 15 parts of water and neutralize with a few drops of concentrated aqueous ammonia; then add 100 parts in volume of whites of egg and a certain quantity of thick India ink, and, this done, beat the whole to a thick froth. In ten or twelve hours the albumen will be deposited and ready for use. The quantity of India ink added to the albumen should be such as the paper be black when coated, but, however, sufficiently transparent for one to see the shadow of objects placed on the back of it, and the coating should not be thick. This is important in order to allow the light acting through the whole thickness of the preparation when the paper is insolated under the cliché, for, if the film be too opaque or too thick (by addition [pg 82] of too much gum arabic), it would be only impressed on its surface, and the image dissolved during the development. The cause of this failure must be explained. Under the action of light the bichromate employed to sensitize the albumen is reduced into chromic oxide which render insoluble this organic substance—or any other, such as caseine, gelatine, gum arabic, etc.; therefore whenever the film is not acted on in its whole thickness, the subjacent part being still soluble, is necessary washed off and with it the superficial impressed part, that is, the image. |
|---|---|
| 2d. | Take 10 parts of lamp black and work it up in a mortar to the consistency of a thin paste by gradually pouring a little of a solution of from 6 to 8 parts of gum arabic and 1 part of liquid glucose in 100 parts of water, adding afterwards the remainder, into which 2½ parts of ammonium bichromate have been dissolved, and filter through flannel. With this, coat the paper by brushing so as to form a thin and uniform film, and pin it up to dry in the dark. |
These solutions keep well for a certain period. We have kept the albumen, which we prefer to use, for two months in good condition; but the sensitive paper does not for more than three or four days in taking the usual care. It is more practical—and this is recommended—to leave out the bichromate from the preparations, and to coat the paper, in quantity, beforehand, and for use to sensitize it with a solution of potassium bichromate at 3½ per cent. of water applied on the verso with a Buckle brush.25
The bichromate solution should be allowed to imbue the paper for about one minute, and having brushed it once more, the paper is pinned up to dry in the dark room. It can also be sensitized from the back by floating, if this manner is found more convenient.
When dry the paper is impressed under a negative cliché of good intensity until the design, well defined in all its details, is [pg 83] visible on the back of the paper, which requires an insolation of about two minutes in clear sunshine, and from eight to ten times longer in the shade. In cloudy weather the exposure to light is necessarily very long.
As explained before, the luminous action, by reducing the chromic salt in presence of certain organic substances, causes the latter to become insoluble; consequently if, on its removal from the printing frame, the proof be soaked in cold water, for, say, ten minutes, and, placing it on a glass plate or a smooth board, gently rubbed with a brush or a soft rag, the parts of the albumen or gum arabic preparation not acted on will dissolve, leaving behind the black image standing out on the white ground of the paper. This done, and when the unreduced bichromate is washed out in two changes of water, the operation is at an end.
As to the theory of this and similar processes, the insolubilization of the bichromate organic substance acted on by light was formerly attributed to the oxidation of the substance by the oxygen evolved during the reduction of the chromic salt into chromic oxide; but from the fact that oxidation generally tends to destroy organic matters, or to increase their solubility, it is more probable that it results from the formation of a peculiar compound of the substance with chromic oxide (J. W. Swan); moreover, gelatine imbued with an alkaline bichromate, then immersed first in a solution of ferrous sulphate and afterwards in hot water, is insolubilized with formation of chromium trioxide, Cr2O7K2+SO4Fe = SO4K2+C2O4Fe+C2O3 (Monckhoven). A similar but inverse action occurs, as shown by Poitevin, when gelatine rendered insoluble by ferric chloride becomes soluble by the transformation, under the influence of light, of the ferric salt into one at the minimum.
The writer has improved the above process by simplifying the modus operandi as follows:
Instead of compounding the preparation with gum arabic and the coloring matter, the albumen is simply clarified by beating the whites of eggs to a froth, etc., and the paper is coated by floating for one minute, then hung up to dry in a place free from dust. [pg 84]
If the reader has any objection for albumenizing his own paper, he can use the albumen paper found in the market for the printing-out silver process generally employed by photographers.
The paper is sensitized from the back with the potassium bichromate bath by floating or by brushing. When dry, it is exposed as usual, but for a shorter period than when the preparation contains the India ink or other coloring matters which impede the action of light.
The progress of the impression is followed by viewing, from time to time, the albumenized side of the paper. When the design is visible, well defined and brownish, the proof, being removed from the printing frame, is rubbed with very finely powdered, or, better, levigated graphite, and, this done, immersed in cold water for from fifteen to twenty minutes, when by gently rubbing it under a jet of water with a soft rag, or with a sponge imbued with water, the albumen is washed off from the parts not acted on, leaving the design on a perfectly white ground.
If instead of graphite, or any dry color insoluble in water, lithographic ink, much thinned with turpentine oil, be applied on the print in a light coating which permits one to see the design under it, and if, then, the print be soaked in water and afterwards developed as just directed, an image in greasy ink is obtained. And, furthermore, by replacing the printing by transfer ink, one readily obtains a transfer ready for the stone or a zinc plate to be etched in the ordinary manner.
As usual there are two causes of failures in these processes, viz., under and over-exposures. In the former case the image is partly washed off; in the latter the ground cannot be cleared. The reasons are obvious.
Mr. de Saint Florent gives the following processes:26 A sheet of albumenized or gelatinized paper is sensitized from the verso on a solution of potassium bichromate, dried in the dark and exposed under a positive cliché. After insolation, the proof is washed in water, to which are added few drops [pg 85] of ammonia, then inked all over with an ink consisting of 100 parts of liquid India ink, 7 parts of sulphuric acid and 3 parts of caustic potassa, and dried in a horizontal position. When quite dry, the proof is placed in water, and after an immersion of about ten minutes, rubbed with a soft brush: the image little by little appears, and if the time of exposure be right, it is soon entirely cleared, and, then, if not enough vigorous, it may be inked again. The gloss of the image is removed by means of a solution of caustic potassa at 10 per 100, and the proof finally washed with care.
If in lieu of albumen paper, one employs paper prepared with a thin coating of gelatine, and dissolves the not acted on gelatine in warm water, a very fine positive image is obtained by means of acidified inks which will fix themselves on the bare paper.
Positive impressions from positive clichés can also be obtained in operating in the following manner: On its removal from the printing frame the proof is washed, sponged between sheets of blotting paper, then covered with not acidified India ink mixed with potassium bichromate, and, when dry, exposed from the verso to the action of light. This done the image is cleared with a somewhat hard brush.
THE CARBON PROCESS.
The carbon tissue is seldom prepared by photographers. However, for the sake of completeness, we shall give the formula of the mixtures most generally employed, and describe the manner of coating the paper on a small scale.
Preparation of the Tissue.—The gelatine generally recommended to compound the mixture is the Nelson's autotype gelatine. Coignet's gold label gelatine, mixed with a more soluble product, such as Cox's gelatine, for example, gives also excellent results.
| Gelatine | 110 parts |
| Sugar | 25 parts |
| Soap, dry | 12 parts |
| Water | 350 parts |
The coloring substances consist of:
| FOR ENGRAVING BLACK. | |
|---|---|
| Lamp-black | 20 parts |
| Crimson lake | 2 parts |
| Indigo | 1 part |
| FOR WARM BLACK. | |
|---|---|
| Lamp-black | 3 parts |
| Crimson lake | 3 parts |
| Burnt amber | 2 parts |
| Indigo | 1 part |
| FOR SEPIA | |
|---|---|
| Lamp-black | 2 parts |
| Sepia of Cologne | 18 parts |
| FOR PHOTOGRAPHIC RED BROWN. | |
|---|---|
| India ink | 3 parts |
| Crimson lake | 4 parts |
| Van Dyck brown | 4 parts |
For blue, Turnbull's blue is employed; for yellow, light chrome yellow; for red, carmine dissolved in aqueous ammonia, evaporating, then adding water, etc. (See further on.)
[pg 87]To prepare the mixture, dissolve the sugar and soap in the cold water, add the gelatine, let it soak for an hour, then dissolve it in a water bath and mix by small quantity the colors finely ground together and wetted to the consistency of a paste. After filtering through flannel the mixture is ready for use.
For coating, the method devised by Mr. Alf. Harman has been found excellent in the hands of the writer, not only for the purpose in question, but also for coating paper with gelatinous or viscous (gum arabic) preparations.
“Take two tin dishes, such as used for the development of the carbon prints; arrange one on your bench tilted to an angle; the lower angle is intended to receive the warm water for keeping the gelatine mixture to a proper temperature. Into this angle of the tray arrange another tray somewhat smaller, and keep it from touching the bottom of the outer one by the insertion of any small article that will suggest itself. Into the inner tray the gelatine mixture is to be poured.”
“The actual making of the tissue can now be proceeded with, and is so simple and certain as not to be believed until put to the test. Purchase a roll of paper-hanger's lining paper of good quality, cut it into widths of about one and a half inch less than the width of your inner tray, and in length of, say, thirty inches. For the success of the operation it is necessary that the paper be rolled up the narrow way. Now having just sufficient water at a temperature of 100 deg. Fahr. (38 deg. C.) into the outer tray, pour the gelatine mixture into the inner one, and take one of the lengths of rolled paper, and, holding it by both ends, gently lower it on the surface of the gelatine; then at once slowly raise the end of the paper, which will unroll itself and become beautifully coated in far less time than it takes to describe. Twenty sheets may be coated in a quarter of an hour, and be equal in all respects to that made by the most expensive machine.”
In the description of this method of coating, Mr. Harman does not explain how the gelatine should be allowed to set before hanging up the paper to dry, which is, however, obviously important. It is as follows: Place on the tray a smooth board a little larger than the sheet of paper, leaving a small space at [pg 88] the end furthest from the body, and slowly, without a stop, draw off the paper, prepared side uppermost, on the board upon which it should remain until the gelatine is set. If the paper curls up, wet the back a little with a sponge before coating.
The following coating method, due to Mr. Chardon, is excellent for sheets of paper of the ordinary photographic size, 18×22 inches.
On a glass plate placed on a leveled stand, is laid a sheet of paper previously wetted, which is then flattened into contact with an India rubber squeegee, taking care to remove the air bubbles interposed. The quantity of gelatine necessary to coat the paper is regulated by means of a glass rod held by an iron lath, which serves to handle it; at each end of the rod is inserted a piece of an India rubber tube whose thickness regulates that of the gelatine layer. The mixture is poured from a small teapot, at the opening of which has been adapted a bent glass tube about three-sixteenths of an inch in diameter, between the rod and the lath, so that by a simultaneous motion, one can equalize the gelatine as it is poured on. When the gelatine is set the paper is hung up to dry. In drying, the gelatine contracts, and, necessarily, causes a deformation of the tissue, which curls up at the edges and loses its planimetry. To prevent this, while the gelatine is almost dry, the tissue is placed under pressure until quite desiccated. Dumoulin advises to apply on the film, while still soft and tacky, a wooden frame, which, by adhering to it. keeps the tissue perfectly plane as it dries.
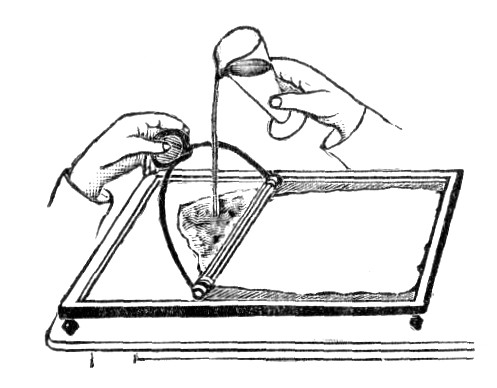
Sensitizing.—The tissue is sensitized in a bath of potassium bichromate. The degree of concentration of the bath, which varies from 2 to 5 per cent. of water, is important. The [pg 89] tissue sensitized in a weak bath is less rapidly acted on by light and yields more contrasts than when imbued in a concentrated one. The former should consequently be employed for printing weak negatives, and the latter for those which are intense. A bath compounded with 30 parts of potassium bichromate, 1,000 parts of water and 2 parts of aqueous ammonia, is used for printing negatives of the ordinary intensity, the tissue being, then practically of the same sensitiveness, a silvered paper insolated to obtain a print not over-exposed. For intense negatives the ammonia should be discarded and replaced by the same quantity of chromic acid.
The time of immersion has also a certain influence on the results. The less the tissue is allowed to absorb the solution the less sensitive it is, but also the more the tendency of the half tints to be washed off during the development. Generally the tissue should remain immersed until it lies flat and the edges just commence to curl up, unless white and black impressions are desired, but even then it is preferable to operate as said above, using a bath at 2 per cent.
For use the bichromate bath should be cooled down to 15 deg. C. (59 deg. Fahr.), and much lower in summer, say 10 deg. C. (50 deg. Fahr.), and kept at about this temperature by placing pieces of ice around the tray. At 20 deg. C. (68 deg. Fahr.) the prints are more or less granulated; above this the gelatine is softened and the reticulation greater; at 25 deg. C. (75 deg. Fahr.) it may dissolve.
The addition of alcohol to the bichromate bath—sometimes recommended to harden the film and allow it to stand a higher temperature, and to hasten the desiccation of the tissue—is objectionable, for the spirits tend to reduce the bichromate, which is transformed into the green salt, and, therefore, a partial or complete insolubilization of the gelatine is the result.
Aqueous ammonia added to the sensitizing solution has for its object to permit one to keep the sensitive tissue for a somewhat longer period, but it renders it less sensitive. If enough be added to turn the solution yellow weak prints are obtained.
The bichromate bath should be renewed often. It does [pg 90] not keep owing to the presence of gelatine and other organic matters which it dissolves and which cause the reduction of the chromic salt even in the dark. The tissue prepared in such a bath is not very sensitive and the image develops with difficulty, and even cannot be developed at all.
As said above, the tissue is well sensitized when its edges commence to curl up. It is then removed from the bath by drawing it on a glass rod fixed at the end of the tray, and placed, prepared side down, on a slightly waxed glass plate, rubbing it with an India rubber squeegee to remove the superflous liquid, when it is hung up to dry.
While wet the bichromated tissue is insensitive; the sensitizing can therefore be made by daylight, but the drying should of course be done in the dark room, that is in a room lighted by a candle or the sunlight filtered through a deep orange window glass.
Caution.—The soluble bichromates are very poisonous. By absorption they produce skin diseases not without danger and very difficult to cure. Hence when handling the wet tissue the fingers should be protected by India rubber tips, and any yellow, stains on the hands should be rubbed with a dilute solution of aqueous ammonia, and the hands well rinsed in water.
Drying.—When the tissue dries rapidly it adheres well on the support upon which it is applied for developing and yields brilliant images which are easily cleared. On the other hand, were it allowed to dry slowly the adherence would not be so complete, the image dull and developing with difficulty. They may even refuse to develop at all from the insolubilization of the gelatine.
In winter and in the cool days of spring and autumn, the gelatine dries quick enough in the air, but when the weather is warm and damp, the gelatine, drying very slowly, may be so softened as to run off, or to produce an entirely objectionable reticulation, or the defects above mentioned. This may be avoided by drying it pinned up in a box, or a closet, over quick-lime.
When dry, the tissue is generally wrinkled, brittle, breaks easily in handling and cannot be laid flat on the cliché; but by [pg 91] holding it over a basin of boiling water, the steam in a few moments rendering it sufficiently pliable to lay it flat between glass plates, where it should be kept under pressure until wanted for use.
The writer always dries the tissue in the following manner, which he devised about sixteen years ago.27 And not only the least trace of reticulation is avoided, but the tissue, drying quite flat, lies in perfect contact with the negative, which is quite important to obtain proofs exactly sharp all over.
A clean glass plate is rubbed with talc, or, which the writer prefers, flowed with a solution of28
| Yellow wax, pure | 1 part |
| Benzine, pure | 100 parts |
then strongly heated, allowed to cool and rubbed clean (apparently) with a piece of flannel. After once more repeating this operation the plate is coated with the following plain collodion:29
| Ether, conc. | 250 parts, in volume |
| Alcohol, 95 deg | 250 parts, in volume |
| Pyroxyline | 3 parts |
When the film is set, the plate is immersed in filtered water until greasiness has disappeared, when on its removal from the bichromate bath the tissue is laid, without draining, upon it and pressed into contact with the squeegee to remove the excess of liquid and, with it, the air bubbles interposited. The tissue is then allowed to dry in the air on the collodionized plate in the cold season, or, when the weather is warm and damp, in a box in the bottom of which is placed a quantity of quicklime in earthen dishes. When dry, the plates are placed one upon another, wrapped in paper and kept in a dry place. When wanted for use the tissue is stripped off and will be found quite flat with a beautiful surface to print upon.
[pg 92]One should avoid to keep the sensitized tissue in a moist and warm atmosphere, for in less than ten hours it becomes insoluble even in complete darkness. It should neither be kept in the air contaminated with gaseous reductive matters, such as the products of the combustion of coal gas and petroleum, sulphydric or sulphurous emanations from any source, the fumes of turpentine oil, etc., which, by reducing the chromic salt, cause the insolubilization of gelatine, prevent the print to adhere on the support or the clearing of the image, which may even refuse to develop.
The sensitive tissue keeps well for three or four weeks in cool and dry weather, and no more than eight or ten days in summer unless well desiccated and kept in a preservative box. If kept too long the image cannot be developed.
The Photometer.—The time of exposure is regulated by means of a photometer. Of all the photometers which have been devised for that purpose we do not know any one more practical than that suggested in 1876 by Mr. J. Loeffler, of Staten Island. It is made as follows: On a strip of a thin glass plate, 6×2 inches, make four or five negatives, 1½×1¼ inch, exposing each one exactly for the same period and developing in the usual manner, but without any intensification whatever. It is even advisable to reduce the intensity if they were opaque. Fix, etc., and apply a good hard varnish. Now cover the back of these negatives with strips of vegetable paper or transparent celluloid, or, better, of thin sheets of mica, in such a manner as there be one thickness on the second negative, two on the third, three on the fourth, etc., leaving the first one uncovered. Then place on the whole a glass plate of the same size as the first and border like a passe-partout.
The Negatives.—For the carbon process the negatives should be intenser than those intended for printing out on silver paper. However, good proofs may be obtained from any negatives, so to say, by varying the strength of the bichromate solution, as, also, by using the tissue freshly sensitized for weak negatives, in order to obtain vigor, and for strong negatives, the tissue two or three days after its preparation, [pg 93] when it yields better half tones. Printing dodges are also resorted to. That the most commonly employed consists to varnish the back of the negatives with a matt varnish, or to stretch on the same a sheet of mineral paper upon which the retouches are made by rubbing graphite, chrome yellow, pink or blue colors to strengthen the shadows or the whites, as the case requires. As a rule, it is advantageous to cover the printing frame with tissue paper, whatever be the quality of the negatives.
The negatives should be bordered with deep yellow or orange-red paper to form what is termed a “safe edge” upon which should rest the tissue in order to prevent the margin from being insolubilized by the reductive action of light. If this precaution were neglected it would be impossible to strip off the paper without tearing the proof when the tissue is applied on the support upon which the image is to be developed.
Before exposing it is advisable to ascertain what the printing qualities of the negative are by making on silvered paper a proof of it—not over-printed—and another of the photometer, both being exposed at the same time and for the same period. This done, compare the proof from the negative cliché with those of the photometer, and mark the negative with the number of that of the photometer to which it corresponds, stating the shade of the proof next to it; for example: No. 2; No. 3 faint, or commences to appear, etc. This No. 2 and the observation will indicate the intensity of the negative and serve as a guide for printing on the tissue, since, as before explained, the silver paper is practically of the same sensitiveness as the tissue prepared for negatives of the ordinary intensity.
Exposure.—To print, the tissue is laid over the negative, taking care that it covers the safe edge, and a strip of silvered paper placed in the photometer, then both the printing frame and the photometer are exposed to light side by side.
Unless the negative be weak, when more vigor is obtained by exposing in sunshine, the printing should be done in the shade. It is a well-known fact that the part of the bichromated [pg 94] film corresponding to the half tones in the lights are not sufficiently impressed in comparison to the blacks while impressed in direct sun's light in this as well as in the collotype, photogravure and other processes with the chromic salts, because the luminous action through the bare glass, or nearly so, which in the negative represent the shadows and half blacks, is more energetic in proportion than through the other parts, from which it results that these parts being most acted on are made deeply insoluble through the thickness of the film, and then require to be cleared by a treatment with water at a higher temperature than the parts representing the half tints in the lights of the picture, which are but superficially and slightly insoluble, can stand.
From time to time during the exposure the print in the photometer is examined, and when a certain picture is printed to a certain shade, or when the one next by commences to appear or is faintly printed, etc., the exposure of the tissue is sufficient. This, as the reader has already inferred, is a matter of experience, the guide being the knowledge of the intensity negative tested as above explained.
Development.—The carbon prints are developed either on a sheet of paper upon which it should remain (single or simple transfer), or on a provisory support to be afterwards transferred on paper or any other material (double transfer).
Simple Transfer.—This process is quite simple: The impressed tissue and a sheet of paper coated with alumed (insoluble) gelatine are immersed face to face in cold water, and when the tissue is softened both are removed, one superposed on the other, and the whole, being placed on a glass plate and covered with a thin oil cloth, is firmly pressed into contact with the squeegee. The rationale of applying under water the tissue on the gelatinized paper is to avoid the interposition of air bubbles.
To operate by simple transfer the tissue should be impressed under a reversed negative. The reason is obvious.
Double Transfer.—By this method the carbon prints are generally developed on porcelain or opal plates, which more easily than glass plates permit one to follow the progress of [pg 95] the development and to retouch the imperfections before transferring the picture on paper.
In order that the image does not adhere on the provisory support a little of the following mixture is spread over the plate, which is then pretty strongly heated, and, when it has cooled down, polished lightly with a piece of white flannel to obtain a very thin and even layer free from striæ. If the plate has not been used before for the purpose in question, it should be waxed a second time in the same manner:
| Yellow wax | 4 parts |
| Rosin | 1 part |
| Turpentine or benzine | 250 parts |
The plates can be developed on the plates so waxed, but for “full gloss,” that is, for enameled pictures, a film of collodion is applied on the plates, which then, instead of being waxed, should to be simply flowed with a solution of India rubber 1 to 100 of benzole:
| Ether | 250 parts |
| Alcohol | 250 parts |
| Castor oil | 1 part |
| Pyroxyline | 5 to 6 parts |
When the plate is coated and the collodion film set, it is immersed in water until greasiness has disappeared and wanted for use. Then the tissue, previously soaked in water, is applied upon it (taking care to avoid air bubbles) and squeezed, lightly at first, with some force afterwards, to insure a perfect contact.
Zinc plates are also employed as provisory supports instead of glass, opal or porcelain plates. The modus operandi is exactly the same.30 The plates should be well planed, free from scratches, etc., and well polished to obtain glossy pictures without one having recourse to a film of collodion. For matt pictures, i.e., without gloss whatever, the plate should be finely granulated, and when waxing a very light pressure should be exerted to remove the excess of wax, else it might be quite impossible to strip off the picture in transferring on paper.
[pg 96]For double transfer on biscuits, objects in alabaster, porcelain, wood, any even or curved rigid materials, flexible supports are employed to develop the pictures. These supports are prepared by fastening albumen paper on a board and evenly brushing over the following hot compound, filtered through flannel, which, when dry, is polished with a cloth:
| Stearine | 15 parts |
| Rosin | 3 parts |
| Alcohol | 100 parts |
The flexible supports should be waxed, then collodionized for full gloss, as the glass, porcelain and metallic plates.
Another method which the writer recommends is the following, due to Mr. Swan: Immerse a sheet of paper in a solution of India rubber, 4:100 of benzole, and let dry, which requires a few minutes. This is the flexible support. Then after exposure, brush over the India rubber solution on the carbon tissue, apply upon it the support when the benzole is evaporated, and pass the whole under a rolling press to secure adhesion, then develop. To transfer, soak the proof in tepid water, apply it on the material prepared, as it will be explained further on, and when dry, imbue the support from the back with benzole, to soften the India rubber, and strip.
To dispense with a rolling press, the proof may be developed on lacquered vegetable paper prepared by immersion in a solution of 10 parts of red shellac in 100 parts of alcohol. After developing the proof is coated with alumed gelatine, and when dry transferred as usual. To strip off it suffices to imbue the paper with alcohol in order to dissolve the shellac.
When the picture must be transferred on small spaces or on small objects the most simple method—the most effective, perhaps—is the following, devised some years ago by the writer and now employed for the ornaments of “articles de Paris:” Prepare the provisory support as usual, but with a thicker film of collodion; then, after developing and coloring, if necessary, the picture is coated with gelatine, to which may be added some zinc white or other colored substance to form a ground. This dry, strip off, immerse the pellicle in water to soften the gelatine and transfer on the material collodion side up.
[pg 97]The proofs should be developed within three or four hours after insolation, for the luminons action continues pretty actively in the dark, and this for a long time; thus: a proof rightly exposed in the morning behaves as one over-exposed if developed in the evening, and after a certain period either can not be developed or refuses to adhere on the support. However, the proofs can be kept for three weeks, may be more, before development, if the soluble bichromate be washed off, the tissue sponged and dried rapidly in the warm season. This capital improvement is due to Mr. Charles Brasseur.
It has been said that before being applied on the support the proof should be immersed in water to soften the tissue. The time which it should be allowed to absorb water has an importance which must not be neglected. If it do not remain long enough to be soaked through, small invisible air bubbles are formed on its surface, and interposing themselves between the image and the support, form minute, brilliant, silver-like spots on the finished picture; and, if the temperature of the water is above 20 deg. C. (68 deg. Fahr.), the image will be more or less reticulated. The temperature depends a good deal of the softness of the gelatine; 15 deg. C. (59 deg. Fahr.) is safe, except, however, when the thermometer is in the thirtieths (90th Fahr.), when the water should be cooled down a few degrees lower, but not at the melting ice temperature, for then the proof would not adhere well. As a rule, the tissue should remain in the cold water until it becomes flat and shows a tendency to curl up. It is at this very moment that it should be squeezed on the support.
The proofs should not be developed immediately after transferring. The adherence is greater and the pictures finer and devoid of defects when the development is made half an hour, and even an hour, after. If developed too soon the picture will be partly, and even entirely, washed off. Hence, a number of transfers can be prepared beforehand, placing them, face to face one upon another, in order that the tissue does not dry, which is quite essential.
To develop, the plate, with the tissue adhering to it, is placed in water heated to 30 deg. C. (80 deg. Fahr.), where it is [pg 98] left rocking the tray occasionally until the paper rises up by itself at the corners, when taking hold of it by one corner, it is stripped off, leaving behind the image buried in soluble gelatine. Should the paper offer any resistance whatever, the gelatine should be allowed to become more soluble by increasing the temperature of the water, or by a longer immersion. There is, in fact, no objection to this. The plate—and that is a good method—can be placed in an upright position in a tin box, made ad hoc, and left therein in warm water until the paper detaches itself and the image is partly developed and the bichromate washed off. This done, the plate is held in an inclined position on a tray filled with water at 35 deg. C. (95 deg. Fahr.), which is dashed with a wooden spoon on the image to clear it from the non-acted-on gelatine. Presently one can judge whether the exposure is right. If it is too short, the half tints in the shadows are washed off, unless the negative be too intense, when a similar effect also occurs in the whites. If it is too long, either the image is with difficulty cleared or remains undeveloped. In the latter case, it is recommended by some operators to increase the temperature of the developing water to near the boiling point, and, for local clearing, to pour it on. This we find objectionable, for the half tints are easily washed off. A better process, when the picture can not be cleared by water at 50 deg. (122 deg. Fahr.), or thereabout, is to use a solution of common salt at 5 or 6 per cent. of slightly warm water.31 It is even preferable to finish the development in a tepid solution of potassium sulpho-cyanide, 12:100. The dissolving action is long, but not only, as said above, the half tints are best preserved, but blistering and local washing-off are avoided.
After development the plate is rinsed under the tap, then flowed two or three times with a solution of chrome alum at 1 per cent. of water, then washed, and finally allowed to dry spontaneously.
[pg 99]It is objectionable to use a strong solution of alum, and in it to immerse the plate for any length of time; the gelatine is considerably hardened—which is not necessary—and more liable to crack by time in being thoroughly desiccated. We discard the common alum which we found liable to produce a slight reticulation.
Two defects are complained of by the beginners, viz., the want of adherence of the deep blacks, and, especially, the isolated and fine lines when the picture is a reproduction of an engraving, a drawing, etc., and the liability in half tone pictures of the delicate details being washed out. The first defects are avoided by pouring a solution of boric acid on the transitory support before applying the tissue and developing at a low temperature with salted water. The second from an imperfect knowledge of the properties of gelatine acted on by light in presence of a salt of chromic acid. One should bear in mind that the degree of solubility of gelatine so acted on, as also its degree of impermeability—which is important in certain processes of photogravure—is proportionate to the degree of insolation; thus, when not impressed, bichromated gelatine dissolves in water heated to about from 25 to 30 deg. C. (77 to 80 deg. Fahr.), and when acted on between 30 and 100 deg. C. (86 to 112 deg. Fahr.), according as to the degree of insolation, that is, of reduction of the chromic salt, the latter temperature being that of insolubility of the parts the most acted on. The very delicate half tints do not, generally, stand a temperature higher than 35 deg. C. (95 deg. Fahr.), and, therefore, as the degree of insolubility of the various parts cannot be ascertained, a priori, it is advisable during the development to increase gradually the temperature of the water from this degree, and not to exceed 45 deg. C. (113 deg. Fahr.), in order to obtain the most perfect result from a negative of good intensity. Indeed, by placing the supports on a rack and immersing the whole in water heated to 30 to 35 deg. C. (86 to 95 deg. Fahr.), the image will clear up by itself to perfection in a certain period. This method is excellent for proofs in lines. Those from the grained negatives employed in photogravure are still more perfectly developed [pg 100] in a tepid solution of potassium sulphocyanate, since the impressions wholly consist of insoluble parts (the lines) and gelatine not acted on.
Retouching.—The retouches are easily made. They should be done before transferring when working by the double transfer process.
The transparent spots, and any parts which should be altered, are retouched with the material of the tissue dissolved in warm water; the whites are cleared with a scraper; and any parts which are not intense enough, or which should be blended by the addition of half tints, are worked on the proof—to which a tooth has been given by rubbing with cuttle-fish powder—by means of a stump and an appropriate color, a mixture of lamp-black and carmine, for example, in very fine powder.
The proofs can also be colored by chemical means (see further on), or with water colors employed with a solution of chrome alum, 1 to 200 of water, or gilt, silvered or bronzed with metallic powders applied with the gilder's size thinned with turpentine on the proof previously coated with a thin layer of alumed gelatine.
Second Transfer.—To transfer, a sheet of enameled or simple transfer paper is immersed in tepid water until the gelatine is softened and feels slippery to the fingers. The support is then placed under water at ordinary temperatures—not under 16 deg. C. (60 deg. F.)—for two three minutes, then rubbed with a camel brush to remove the air bubbles, which might be formed on the surface of the image, when, without draining, the gelatinized paper is laid upon it, covered with the thin oil cloth, and pressed into contact with the squeegee, commencing in the center to the sweep off the water, then repeating the operation for the other half, as explained to apply the tissue on the provisory support. When the whole is quite dry, which requires three or four hours, the edges are cut with a penknife and the whole stripped off. It may happen that the proof is covered with minute, silver-like brilliant spots, which are nothing else than very small air bubbles interposited between the carbon proof and the transfer paper. [pg 101] They are caused by the gelatine paper not having been sufficiently softened or not laid on the proof with proper care. The defect may also arise from the transfer paper coated with not sufficiently thick gelatine.
To transfer on any rigid material, the proofs on flexible supports are coated by floating on the following gelatine solution, then allowed to dry, and, when wanted for use, immersed in tepid water to soften the gelatine and secure adherence:
| Gelatine | 50 parts |
| Water | 400 parts |
| Solution of chrome alum, 4:100 | 6 parts |
Development on Absorbing Materials.—The development of carbon prints on absorbent material—such as canvas and palettes to be painted in oil, etc.—cannot be made in the ordinary manner on account of the impossibility to eliminate entirely the chromic salt which tinges the material yellow. To turn the difficulty, it suffices to wash off in several changes of cold water all the unaltered bichromate from the prints on their removal from the printing frame, and to proceed as usual, or the prints can be allowed to dry and transferred at some future time.
Canvas should be prepared by brushing with a solution of aqueous ammonia in alcohol, 5:20, to remove greasiness until the thread is apparent, and, when dry, rubbed with sand to grain it—or to give a tooth, as it is termed—then rubbed dry with a solution of soluble glass, 1 to 10 of beer.32
Palettes should be rendered impervious, or nearly so, by flowing upon them a solution of alumed gelatine, which is allowed to penetrate into the pores of the wood and the excess scraped off when solidified, when the surface may be whitened, if necessary, as for printing on wood box, q.v.
Opals, porcelain, or ivory should be prepared with the following substratum:
| Gelatine | 50 parts |
| Water | 400 parts |
| Chrome alum, 4:100 | 6 parts |
Very fine carbon proofs having the appearance of pictures on opal plates are made by transferring in the following manner, devised by the author:
Develop on the ground surface of a glass or porcelain plate, well waxed, to obtain a matt picture, or in the ordinary manner for "full gloss," and when the image is retouched or colored, apply a thin coating of gelatine, let dry and coat with the following opaque collodion:
| A. | Ether, conc. | 100 parts |
| Alcohol, 95 deg | 90 parts | |
| Pyroxyline | 7 parts | |
| B. | White zinc in very fine powder | 9 parts |
| Castor oil | 3 parts | |
| Alcohol | 10 parts |
Grind in a mortar, adding ultramarine blue and carmine, or a little of any suitable coloring matters, and mix to A. When the collodion is dry, which requires a few hours, strip the whole or back with strong white or colored paper before stripping. A solution of gelatine with glycerine, white zinc, etc., may be substituted for collodion when the pictures are employed as ornaments on wood, etc. Carbon prints on celluloid are now made for similar purposes.
| OPAL GELATINE SOLUTION | |
|---|---|
| Gelatine | 150 parts |
| Glycerine | 15 parts |
| Zinc, white | 40 parts |
| Water | 600 parts |
To which some coloring matters may be added according to taste. Grind the white with the glycerine and a little water, mix to the gelatine dissolved in the remainder of water, and filter through canvas. Apply the mixture moderately hot, 30 deg. C. (86 deg. Fahr.)
Transparencies.—The transparencies are printed on a special tissue sold under the name of “diapositive.” It differs from the ordinary tissue in this, that the mixture contains a greater quantity of the color matter, India ink, which is ground exceedingly fine.
[pg 103]The proofs for transparencies should be printed deeper than those to be seen by reflection, and developed on thin glass plates, free from any defects, and coated with either one of the following substrata:
| Soluble glass | 5 parts |
| White of eggs | 15 parts |
| Water | 20 parts |
The whole is beaten up to a thick froth and allowed to subside, when the clear liquid is decanted, filtered through flannel and the glass plates coated. The substratum should be allowed to dry for a few hours, and rinsed under the tap before use.
The other substratum consists of
| Gelatine | 35 parts |
| Acetic acid, No. 8 | 250 parts |
| Alcohol, 95 deg | 50 parts |
| Water | 700 parts |
| Chrome alum, 4:100 | 60 parts |
Dissolve the gelatine in the acid at a moderate heat, add afterwards the alcohol and water, and lastly mix the chrome alum by small quantities at a time.
These substrata are employed to avoid the peeling off of the image. To prevent the entire desiccation of the gelatine, which is the cause of the defect above alluded to, it is advisable to add glycerine to the washing water after the image is cleared. Some operators recommend a coating of flexible collodion, that is, prepared with castor oil, for the purpose in question. We do not think that necessary when the transparencies are not exposed to sunshine. If anything should be applied we would prefer the encaustic.
Carbon transparencies are invaluable for reproducing negatives in the original size by the same (carbon) process, or for enlarging by the collodion or gelatine process. For these purposes they should be made on the special red tissue manufactured by the Autotype Company, of London, Eng. They can, however, be made on the ordinary tissues.
Whatever be the tissue employed, the transparencies for the reproduction of negatives are seldom opaque enough, and should be intensified. This is done by treating them with a [pg 104] very dilute solution of sodium permanganate, which colors them olive green.
Transparencies for lantern slides, etc., are best colored with the couleurs à l'albumine of L. Encausse, sold by J. Reygondaud, Paris (France). They are transparent.33
Toning and Intensifying.—The carbon proofs can be toned and at the same time intensified by reagents acting with chromic oxide.
The dyes or coloring matters precipitated are not opaque, and, as a consequence, not objectionable for transparencies. The following processes are the most employed:
Prepare three solutions as follows:
| A. | Ferric sulphate | 5 parts |
| Water | 100 parts | |
| B. | Sodium carbonate | 2 parts |
| Water | 100 parts | |
| C. | Gallic acid | 5 parts |
| Water | 100 parts |
Dissolve the gallic acid in warm water. Filter each solution. They keep well.
To tone, the plate is immersed for, say, ten minutes in A, then, after rinsing slightly, it is placed in B for the same period, rinsed again and flowed with C until the desired color is obtained. The tone is a splendid purple black color. If a solution of pyrogallol be substituted to that of gallic acid, the tone is green, and to a green bordering to black when a solution of catechu is used, the catechu exerting at the same time a tanning action on the gelatine. After toning, the plate should be thoroughly washed.
A similar process consists to wet the plate under the tap, then to flow over a mixture by about equal volumes of
| A. | Ferrous sulphate | 5 parts |
| Acetic acid, No. 8 | 5 parts | |
| Water | 100 parts, filter | |
| B. | Gallic acid | 5 parts |
| Water | 100 parts |
When toned, the plate is well washed, then flowed once with the alum solution and again washed. The tone by this process easily turns to an inky blue not very agreeable. The action should be stopped a little before the desired color is obtained.
It sometimes happens that the image in drying intensifies more then necessary. It can be cleared with a solution of oxalic or citric acid.
A brown sepia is obtained by toning first with potassium permanganate, 1 per cent. of water, then, after washing, with a solution of pyrogallol. If gallic acid be used instead of pyrogallol, the tone is black. By this process a great intensity is obtained. A dilute solution of ammonium sulphide can be employed as a clearing agent.
Pyrogallol and silver nitrate give a warm black tone.
Potassium bichromate followed by silver nitrate form a brick-red precipitate of some opacity.34
Chloride of nickel and potassium ferrocyanate produce a fine brown.
Lime water and alizarine dissolved in alcohol dye violet.
Alizarine and the caustic alkalies produce a variety of tints, from violet to purple, according to the concentration of the solutions.
Lead acetate and alizarine in ammoniacal solution dye purple.
Potassium ferrocyanide and uranium nitrate produce a warm sepia tone. With chloride of nickel the tone is brown.
Ammoniacal solution of coralline diluted with water gives carmine red.
Potassium bichromate and extract of indigo produce a fine greenish tone suitable for landscapes.
Extract of indigo colors blue35
Some of these reactions can be applied to the printing processes with the bichromates, etc. The paper should be coated with galatine. See the Appendix.
Other colorations can be obtained with dyes in utilizing (as shown by Persoz) chromous chromic oxide as a mordant: alizarine, Brazil and yellow wood (morus tinctoria), [pg 106] Fustet (rhus cotinus), etc. The extent of this work does not admit of describing the numerous processes which can be employed; they will suggest themselves to the chemist.
The alkalies employed with the dyes should be employed in diluted solutions, as being liable to produce reticulation. By applying the coloring matters and the mordants thickened with a little starch, the image can be colored with different colors. Lantern slides can be thus colored with great ease.
PREPARATION OF RED, YELLOW, OR BLUE TISSUES.
Red Tissue.—Dissolve 10 grams of carmine in 1 liter of aqueous ammonia and evaporate. When the smell of the alkali has almost disappeared, add 1 liter of rain water. Of this take 65 cubic centimeters, add 35 c.c.m. of rain water, and in the solution let soak for an hour 15 grams of very soluble gelatine, add 1 gram of sugar, and dissolve in a water bath. Filter, and take of the mixture a sufficient quantity (25 c.c.m. for a surface 18×24 centimeters) to cover a sheet of paper which has been previously applied upon a glass plate in the following manner: In a tray full of hot water, immerse the plate and the paper; remove the whole in such a manner as the paper remains in contact with the plate; rub out the excess of water with a squeegee, and flow the gelatine over the paper still damp. Let cool on a leveled stand, and when the gelatine is solidified to a consistent jelly, remove the paper from the plate and place it to dry in an oven heated at not over 24 or 25 deg. C.
It is desirable that in drying the paper does not curl up. To that end, apply over it, before it being removed from the plate a wooden frame to which the gelatine, still sticky, will sufficiently adhere to hold the tissue when it stretches in drying.
Yellow Tissue.—Pulverize to an impalpable powder 25 grams of light chrome yellow in tablets (water color), and gradually add in stirring 1 liter of rain water. Take 100 c.c.m. of this and into it let soak for an hour 15 grams of the same gelatine used for the red tissue, add 1 gram of sugar, then proceed as above.
[pg 107]Blue Tissue.—In a liquid consisting of 85 c.c.m. of rain water and from 12 to 15 c.c.m. of blue ink, such as sold by stationers, let soak for an hour 15 grams of the same gelatine and 1 gram of sugar, and proceed.
Preparation of Transfer Paper.—Two kinds of transfer paper are employed—the enamel and plain transfer paper.
To enamel the paper: Dissolve 100 parts of barium nitrate in 500 parts of water, and, on the other hand, 200 parts of sodium sulphate in the same quantity of water. Mix, wash well the precipitate—barium sulphate—by decantation, and when well drained, mix to the following solution:
| Gelatine, Coignet's | 300 parts |
| Glycerine | 80 parts |
| Ultramarine blue | 1 part |
| Crimson lake | 0.1 part |
| Water | 2,500 parts |
Let soak the gelatine for, say, one hour, dissolve by heat, then add by small quantities, stirring violently, 4 parts of chrome alum dissolved in 250 parts of hot water. Filter through flannel and coat the paper as directed to prepare the tissue. The mixture should be employed immediately after adding the chrome alum.
The plain transfer paper is prepared in the same manner, leaving out the barium sulphate and the coloring matters.
Preparation of the Silver Paper.—Immerse the paper for two minutes in a solution of—
| Sodium chloride (common salt, dry) | 2 parts |
| Lemon juice | 1 part |
| Water | 100 parts (filter) |
When dry and wanted for use, sensitize the salted paper by floating for one minute on—
| Silver nitrate | 8 parts |
| Nitric acid | 0.1 part |
| Water | 100 parts |
On its removal from the silver bath, sponge the paper between sheets of blotting paper and hang it up to dry.
[pg 108]| ENCAUSTIC FOR SINGLE TRANSFER PROOFS. | |
|---|---|
| White wax | 25 parts |
| Mastic | 3 parts |
| Turpentine | 100 parts |
Dissolve by heat, first the mastic, then the wax, and keep for use in a large mouthed vial.
| MATT VARNISH. | |
|---|---|
| Sandarac | 6 parts |
| Mastic | 6 parts |
| Lavender oil. | 0.5 parts |
| Ether | 100 parts |
When dissolved, add 30 parts of benzine. The opacity of the film varies with the quantity of benzine added; by excess the varnish dries transparent.
| WATER COLORS WHICH RESIST THE ACTION OF LIGHT. | ||||
|---|---|---|---|---|
| Red. | Indian red. | Light red. | ||
| Orange. | Mars yellow. | |||
| Blue. | Cobalt blue. | French blue. | Smalt. | New blue. |
| Brown. | Raw umber. | Burnt sienna. | ||
| Green | Terre verte. | |||
| Yellow. | Cadmium yellow. | Yellow ochre. | Roman ochre. | |
APPENDIX.
Although we intended to only describe the printing processes without the use of silver salts, we thought it would be well to complete this work by giving the most practical and interesting processes ever published to obtain permanent photographs; as they may give rise in the hand of experimenters to useful applications.
From time to time processes are published under “queer” names, which are based on the well known actions of reagents on the ferric salts reduced by light. They are derived from those described in the following pages.
We call specially the attention of the reader to the process of Poitevin, by which one can experiment with every ferric salts, citrate, lactate, oxalate, tartrate, benzoate, etc., by simply exciting with the corresponding acid. Observe that to obtain good results the paper should be strongly sized; it is a sine qua non, although not recommended by Poitevin.
C.J. BURNETT'S PROCESS(1857).
“A capital process for many purposes,” says Mr. Burnett, “is to float or steep the paper in a mixed solution of bichromate of potash and sulphate of copper, as for Hunt's chromotype process.36 I have mixed gelatine, or occasionally grape sugar, or both with the solution;37 but instead of developing it with nitrate of silver, as in chromotype, wash out the salt unaltered by light, and develop by floating on a solution of ferrocyanate of potassium. The purple red color of the copper salt which now forms the picture may be modified or changed [pg 110] in many ways,38 viz., by soaking the picture, after the ferrocyanate of potassium has been washed out of the lights, in a solution of sulphate of iron. Solutions of gallic acid, tannic acid with alkalies of carbonate, may also be employed to modify or change the color. This process has the advantage that one may regulate the exact tone (black or useful neutral tint) to the greatest nicety by the time we allow the print to remain in the iron toning bath.”
GODEFROY'S PROCESS (1858).
Float the paper upon the following solution for three minutes and hang it up to dry:
| Uranium nitrate | 30 to 60 parts |
| Silver nitrate | 8 parts |
| Water | 100 parts |
The sensitiveness increases in proportion to the quantity of uranium nitrate. With the above formula the paper can be exposed in the camera, or, for printing, under a negative cliché.
In printing an exposure of five seconds in diffused light gives an image perfectly visible, and a grayish black tone; ten seconds gives a vigorous image almost of a black color; in from fifteen to twenty seconds the image is very strong, with the color of an engraving. In sunshine the action is necessarily much more rapid.
The impression is developed by immersion in
| Ferrous sulphate | 8 parts |
| Tartaric acid | 4 parts |
| Sulphuric acid | 1 part |
| Water | 100 parts |
The image is rapidly developed. It is fixed by washing in water.
[pg 111]DE LA BLANCHERE'S PROCESS (1858).
| Uranium nitrate | 25 parts |
| Distilled water | 100 parts |
Filter the solution and keep it in the dark.
The paper should be sized with a gelatine solution at 5 per 100 of water, and, when dry, kept in the dark.40 It is sensitized by floating five minutes.
The exposure under a negative varies from fifteen to twenty minutes in the shade, and from one to three minutes, at the most, in sunshine. As a rule, it is advisable to somewhat underexpose in order that the development be regular, progressive, under control.
The image is developed by floating, or immersion in
| Silver nitrate | 2 parts |
| Distilled water | 100 parts |
| Nitrate acid, C.P. | a trace |
When the image is intense enough it is washed in several changes of water, then toned in a solution of gold at 1 per 1,000 of water acidified with traces of hydrochloride acid.41
The following bath develops slowly, and gives very rich purple tones without toning:
| Nitrate of silver | 3 parts |
| Nitrate of uranium | 1 part |
| Nitrate of cadmium | 1 part |
| Alcohol | 10 parts |
| Water | 100 parts |
| Nitric acid | traces |
The developing solutions should be as little acid as possible, but not neutral, for then the proofs would be veiled and grayish.
The image can also be developed in a solution of gold, or in a very weak solution of mercuric chloride at 1 per 10,000. The proof must be extremely well printed and left for from [pg 112] two to five minutes in the mercuric solution. If the time of exposure is right, the image will change but little in the solution, and will take, when treated with silver nitrate, the most splendid tones.
The proofs should be carefully washed when finished. If they were developed with silver, they must be immersed in diluted aqueous ammonia, which will perfectly clear the whites. If developed with chloride of gold, the water should be heated to 60 to 80 deg. C. (140 to 176 deg. Fahr.)
HOUDOY'S PROCESS (1858.)
The paper is floated upon a lukewarm solution of gelatine at 5:100, and when dry, on a bath of uranium at 10 or 15 per 100 of water. After exposure to the sun the image is developed with a solution of silver nitrate acidified with acetic acid. The exposure varies, according to the nature of the negative, from one to ten minutes; it must be long enough for the image being developed in from thirty to forty seconds. It is then removed from the silver bath and placed in the following:
| Ferrous sulphate | 3 to 8 parts |
| Acetic acid | 2 parts |
| Water | 100 parts |
In this bath the image takes a great vigor and appears entirely on the surface of the paper. When the proof has been too long exposed it should be washed slightly before placing it in the iron bath. Developed, the image is, generally, of a sepia tone, which can be turned to black by a solution of chloride of gold, 1:1,000, washing afterwards as usual.
NIÈPCE DE ST. VICTOR'S PROCESS (1859).
Red Prints.
Float the paper for fifteen or twenty seconds on a 20 per cent. solution of nitrate of uranium and dry before the fire in the dark room. This paper can be prepared many days before use. Expose in sunshine from eight to ten minutes, according [pg 113] to the intensity of the light and the quality of the negative, then wash in moderately warm water (50 to 60 deg. C.) for a few seconds. This done, immerse in a solution of red prussiate of potash at 2 per cent. of water; in a few moments the proof will become of a fine blood-red color, like “sanguine.” Wash, etc.
Green Prints.
Make a red print as above described, immerse it for a few minutes in a solution of nitrate of cobalt and dry it without washing. Fix then in a solution of sulphate of iron at 20 per cent. of water and 4 of sulphuric acid. Wash and dry before the fire.
Violet Prints.
Prepare the paper in the uranium bath, expose, wash and develop in a solution of chloride of gold, 1:200, until the proof has assumed a fine violet color. Wash in several changes of water.
Blue Prints.
Sensitize the paper with a red prussiate of potash solution at 20 per 100. Let dry, expose until the proof is slightly blue; immerse it for five or ten seconds in a saturated solution of bichloride of mercury, wash only once and immerse in a solution of oxalic acid—saturated when cold—heated to about 55 deg. C. Wash in three or four waters and let dry spontaneously.
Black Prints.
Float the paper on a mixture by equal volumes of a solution of iron perchloride and another of uranium nitrate, each at 10 per 100 of water. Expose and develop on a saturated solution of gallic acid.
DR. T.L. PHIPSON'S PROCESS (1861).
Take a solution of perchloride of iron and, having precipitated the peroxide with ammonia, collect the precipitate on [pg 114] a filter and wash it with boiling water. Add the precipitate in excess to a warm solution of oxalic acid. A beautiful emerald green solution is obtained, which must be a little concentrated by evaporation and then set aside in a dark room for use. The paper is floated for ten (?) minutes upon the green solution of ferric oxalate, to which has been added a little oxalate of ammonia and hung up to dry in the dark.
Expose under a negative for from ten to twenty minutes, according to the weather, and wash well the paper with rain water. Spring water will not do on account of the lime it may contain, which will form oxalate of lime in the paper (insoluble). When all the non-decomposed oxalate is washed from the proof, a feeble image of oxalate of protoxide of iron, scarcely visible, is left on the paper. To develop it and to obtain the vigor, the tone and color of silver prints proceed as follows:
Plunge the proof for a little while in a (weak) solution of permanganate of potassium to which a few drops of ammonia have been added; in the bath the image becomes brown and distinctly visible. It is then withdrawn and immersed in a solution of pyrogallic acid for half an hour, after which it is washed and dried.
The image thus obtained can hardly be distinguished from silver prints; the tone is soft, brilliant and permanent.
This process is quite original and interesting. The theory is as follows: Under the action of light the ferric oxalate is reduced in the ferrous salt, insoluble, which, after the print has been cleared from the ferric oxalate, is oxidized and reduced into ferric oxide by the alkalized permanganate, the latter then forming colored compounds with reagents.
It has been lately published in England under the name of “kallitype,” a new process—or old, ad libitum—which consists in developing the image in ferrous oxalate by a peculiar silver compound whose formula is given below. The paper is prepared by brushing with a strong solution of neutral ferric oxalate dried rapidly—which is a sine qua non when using deliquescent salts; and after exposure the image is developed, etc.
[pg 115]| Silver nitrate | 50 grains |
| Sodium citrate | 800 grains |
| Potassium bichromate | 1 to 2 grains |
| Water | 10 ounces |
“Dissolve the silver nitrate in 1 ounce of water, the citrate and bichromate in the remainder and mix. The precipitate—silver citrate and chromate—is then dissolved by adding 1 dram of ammonia .880, and after 35 drops of strong nitric acid has been added the solution is ready for use.”
This process reminds us that of Robert Hunt (1842), and that of more recent date (1863), of Borlinetto, who developed the image in black with a silver nitrate alcoholic solution, 1:500, and after washing the picture in a solution of citric acid, 1:10, fixed it by aqueous ammonia. But, although that is not absolutely necessary, we would advise one working this, or similar processes in which a silver salt is employed for developing, to fix the image, after treatment with citric acid to clear the proofs from iron salts, in a solution of ammonium sulphocyanate—which has not the injurious effect of sodium thiosulphate (hyposulphite)—in order to prevent the paper to be tinged by the reduction of the silver nitrate which is mechanically retained in its fiber.
The solution of ammonium sulphocyanate should be compounded with auric chloride to tone the picture at the same time it is fixed; thus:
| Ammonium sulphocyanate | 35 parts |
| Gold terchloride | 0.15 part |
| Water | 350 parts |
The solution can be used over again.
In the processes devised by Dr. Phipson, Monckhoven and other authorities, the double ammonio-ferric oxalate is rightly recommended instead of the simple oxalate. Not only is the preparation more sensitive to the luminous action, but better half tones are obtained. As usual, it is advantageous to size the paper with starch.
The ammonio-ferric oxalate is prepared by precipitating ferric chloride or sulphate by aqueous ammonia, then washing the precipitate collected on a filter until the washing water be [pg 116] neutral or does not evolve the smell of ammonia. The precipitate is then placed in an evaporating dish, and by small quantity is added a hot solution of ammonium oxalate until it is nearly (not entirely) dissolved, when the solution is set aside for a few hours, then filtered and evaporated to crystallization. For use, the crystals of ammonio-ferric oxalate are dissolved in the proportion of 1 for 5 of distilled water. The solution as well as the crystals should be kept in the dark.
If one object to the trouble of crystallizing, the solution can be prepared by dissolving the ferric oxide in a hot solution of 30 parts of ammonium oxalate and 25 parts of oxalic acid in 180 parts of water observing that the oxide must be in excess.42
The following sensitizing solution gives also excellent results:
| Ammonio-ferric oxalate | 10 parts |
| Ammonio-ferric lactate | 4 parts |
| Water | 100 parts |
After exposure, which varies from five to ten minutes, according to the intensity of the light and the printing quality of the negatives, the picture appears negative from formation of ferrous oxalate. It may be developed in a great many ways: by a solution of silver nitrate at 2 or 3 per cent. of water acidified slightly by an organic acid—citric acid, for example—or a diluted solution of ammonio-nitrate of silver, which most likely constitutes the best developer; the image is black and consists of metallic silver and ferric oxide, with formation of silver oxalate, which dissolve in the ammonia. If the print be treated by a weak solution of aqueous ammonia, the image turns green, then brown, and if, before the latter coloration is obtained, gallic acid or pyrogallol be added, the image becomes bluish-black or brown-black. In the same circumstances tannin (gallo-tannin) produces a blue-black image; catechu-tannin43 and quino-tannin give green, etc. Employed as [pg 117] a developer, potassium ferricyanate develops an image in prussian blue, and auric chloride one in the characteristic violet metallic gold. To fix the images obtained by the latter reactions, it suffices to wash them in a few changes of water, and, if developed with silver, they can be toned by any of the alkaline solutions of auric chloride used in the printing out silver process, etc.
The photographs obtained by all these processes are permanent.
DR. J.B. OBERNETTER'S PROCESS (1863).
| Copper chloride | 100 parts |
| Ferric chloride, sol. sp. gr. 1.5 | 13 parts |
| Hydrochloric acid, conc. C. P. | 12 parts |
| Water | 1,000 parts |
Float the paper on this solution for about two minutes and hang it up to dry. The keeping quality of the prepared paper is remarkable; it has been kept for two years without apparent change; its sensitiveness is at least one-third greater than that of silver albumen paper. Unless developed within an hour or two, the vigor of the proof is much impaired; after twenty-four hours a print can be taken over on the same.
When exposed, only a faint image is visible. It should be fixed in the following solution:
| Potassium sulphocyanate | 12 parts |
| Sulphuric acid, conc. | 1 part |
| Sensitizing solution | 10 to 12 parts |
| Water | 1,000 parts |
A print is floated on this solution, face downward, for three or four minutes, taking care to agitate the liquid as little as possible; the print is afterwards immersed and another one floated in its place, thus proceeding until all the prints are immersed or the solution can hold no more. A fresh solution is then added to strengthen it: the older the solution the more rapidly and better it works. In this developer copper cyanide is precipitated on the parts acted on by light, and this exactly in the proportion to the luminous action. The time of immersion depends on the method selected to finish the proofs; it its [pg 118] from five minutes to half an hour. If the proof is immersed for, say, twenty-four hours, the image comes out in a relief which may bring the shadows to two lines in depth. When well developed and thoroughly washed, the proof can be dried and the subsequent operations made at any convenient time.
Various processes may be employed to give to these proofs the tone required; thus: the prints well washed are placed in a solution of ferricyanate of potassium at 6 to 12 per 100 of water, where they take a red color increasing in intensity. If left over night the color becomes a splendid velvet deep red with perfect clear whites. To obtain the color of silver photographs one hour's immersion is sufficient. After this operation the proofs are washed until the water is no more tinged yellow.
By immersion in
| Ferrous sulphate | 100 parts |
| Iron sesquichloride | 40 parts |
| Hydrochloric acid | 80 parts |
| Water | 200 to 300 parts |
the proofs undergo the following gradation of colors: red, reddish violet, blue-violet, black and greenish black. As soon as the desired color is obtained, the proofs are washed in acidified water and dried.
The most beautiful purple violet is obtained by leaving the proofs in the iron solution until green-black, and then washing for a moment in a dilute solution of sub-acetate of lead.
A brown-black may be produced by treatment, after washing, with an ammoniacal solution of hypermanganate of potash.
A weak solution of nitrate of silver also yields very fine pictures, but the exposure should be very short, and the proofs must be fixed in water containing a small quantity of oxalate of ammonia.
In order to impart to the proofs the gloss of silver photographs, they should be albumenized in the ordinary manner, and the albumen insolubilized by well known means.
The chemical actions in this process I explain in the following manner: On the paper there are Fe2Cl3 and CuCl, the latter in excess. By the action of light, and according to the transparency [pg 119] of the negative, Fe2Cl3 is reduced to FeCl, while CuCl suffers no alteration.
If the paper be immediately placed in an absolutely dry room after exposure, the picture remains unchanged. In a moist atmosphere FeCl attracts moisture and, with a part of CuCl, is so decomposed that Fe2Cl3 is formed together with Cu2Cl.
After this action has commenced, if the proof be not immediately immersed in a solution of sulphocyanate of potassium, Cu2Cl passes over to a higher combination of chlorine, and the paper is again fit to be impressed anew by the action of light.
As long as FeCl or even Cu2Cl is present, if the print is immersed in the sulphocyanate solution, sulphocyanate of copper is immediately formed on the reduced parts, while on the others the sulphocyanide of copper, formed and dissolved by the sulphocyanide of potassium in excess, becomes decomposed with water in soluble sulphocyanide of copper and deposited as such on the parts already covered with the salt.
Frequently the prints appear yellow from formation of the double sulphocyanide of copper, but the color disappears by washing in water. Red coloration is due to decomposition into ferrocyanide of copper.
L. LIESEGANG'S PROCESS (1865).
Pour ammonia into a nitrate of uranium solution, wash the precipitate of uranate of ammonia in distilled water, then dissolve in citric acid.
Mix this solution of citrate of uranium and a little of a solution of chloride of gold with a paste prepared by dissolving tapioca in hot water. The quantity of chloride of gold must be small and the heat not too great, otherwise the gold would be reduced.
Spread the mixture with a sponge on the paper, which takes a brilliant yellow color, and expose when quite dry; the proofs have the delicacy and vigor of albumen prints.
The proofs come from the frame with a bluish-black color; they should not be toned, but merely fixed by washing until the yellow color of the paper has disappeared.
[pg 120]The color of the picture can be changed to a purple by a solution of chloride of tin.
GUARBASSI'S PROCESS (1867).
The paper is floated in the dark for four or five minutes on a saturated solution of bichromate of potash. When dry, it is printed a little longer than for silver prints and afterwards floated, face upwards, on a water bath until all the unaltered bichromate is dissolved. It is then immersed in the following solution, which improve by use and tones the pictures to a reddish color:
| Saturated solution nitrate of mercury, as free from acid as possible | 4 parts |
| Saturated solution bichromate of potash | 1 part |
| Distilled water | 28 parts |
This solution should be prepared, filtered and allowed to stand for some time before use. The print is left in the bath until it has assumed an intense red color, the whites remaining perfectly pure. It is then washed and put in another bath to obtain a brownish tint. This bath is thus composed:
| Conc. aqueous ammonia | 2 parts |
| Distilled water | 100 parts |
The print must be immersed at once, and when, in a short time, it has assumed the proper color, it should be washed immediately.
The picture is toned in a very diluted solution of chloride of gold, 1:7,000, in which the color passes from a light brown to a deep black or a violet black tone, when it is washed in two changes of water.
A. POITEVIN'S PROCESS (1870).
“I use a paper prepared with iron sesquioxide rendered sensitive to light by tartaric or, better, citric acid in concentrated solution. This paper, after desiccation and exposure to light, possesses the property of reducing the solution of silver nitrate and that of chloride of gold, and of turning blue with a solution of potassium ferncyanate in the parts where light [pg 121] has reduced the iron sesquichloride into the oxide at the minimum.”
“To coat the paper with an equal layer of iron sesqnioxide, I brush it with a tuft of fine linen dipped in a solution of iron perchloride at 10 or 12 per cent. of water, and dry the sheets in the dark. I immerse afterwards these sheets, one after the other, in a tray containing aqueous ammonia, in such a manner as to well wet each sheet successively. A sufficient number of sheets being immersed, I pour off the ammonia in a vial, and, in the tray, I wash them several times, and remove them one by one to hang them up to dry, even in full light, the iron sesquioxide not being sensitive to light.”
“The paper can be prepared in quantities beforehand. To use it I apply upon each sheet a solution of citric acid at 30 or 35 per cent. of water44—which may be done by daylight—and let them dry in the dark.”
“Exposed under a negative of the ordinary intensity, the paper is impressed in sunshine in a few minutes; in the shade it requires about the same time as chloride of silver paper.”
“After exposure the image is not visible, and without being obliged to shelter it from light, I immerse the print in a solution containing about 1 per cent. of silver nitrate. This solution can be used over and over again, by adding to it a little of the silver salt. It does not become turpid by use; it simply turns slightly green from formation of iron nitrate. The image appears soon and rapidly becomes vigorous; in half an hour it will be completely developed. When the exposure is sufficient the color is deep sepia, but not so intense if the quantity of citric acid is feeble. No fixing is necessary; it suffices to wash in several changes of waters.”
“The image can be toned with great facility by a weak solution of gold or of platinum chloride, or, better, by a mixture of these two salts. If the impressed paper be treated by a very diluted solution of potassium ferrocyanate, one obtains very pretty blue proofs.”
“A weak solution of gold chloride develops a violet image. A solution of platinum chloride has no effect.”
“All the various phases of this printing method can be followed in full (diffused) light; there is only the desiccation of the paper when sensitized with citric acid, which requires to be done in the dark.”