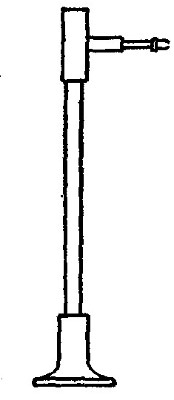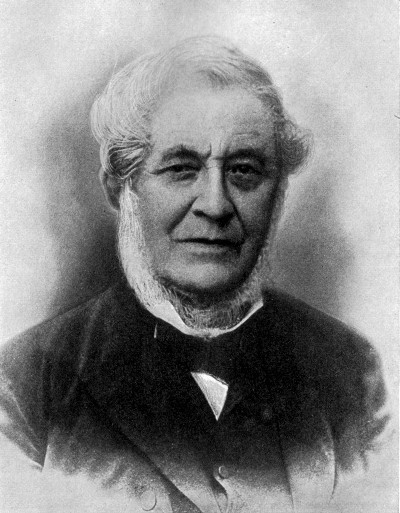The Project Gutenberg EBook of An Elementary Study of Chemistry, by
William McPherson and William Edwards Henderson
This eBook is for the use of anyone anywhere at no cost and with
almost no restrictions whatsoever. You may copy it, give it away or
re-use it under the terms of the Project Gutenberg License included
with this eBook or online at www.gutenberg.org
Title: An Elementary Study of Chemistry
Author: William McPherson
William Edwards Henderson
Release Date: March 18, 2007 [EBook #20848]
Language: English
Character set encoding: ISO-8859-1
*** START OF THIS PROJECT GUTENBERG EBOOK AN ELEMENTARY STUDY OF CHEMISTRY ***
Produced by Elaine Walker, Josephine Paolucci and the
Online Distributed Proofreading Team at http://www.pgdp.net
 ANTOINE LAURENT LAVOISIER
ANTOINE LAURENT LAVOISIER
Famous for his care in quantitative experiments, for demonstrating the true nature of
combustion, for introducing system into the naming and grouping of chemical substances. Executed (1794)
during the French Revolution because of his connection with the government
This picture is taken from a French engraving of 1799. The panel represents Lavoisier as he is being
arrested in his laboratory by the Revolutionary Committee
AN ELEMENTARY
STUDY OF CHEMISTRY
BY
WILLIAM McPHERSON, PH.D.
PROFESSOR OF CHEMISTRY, OHIO STATE UNIVERSITY
AND
WILLIAM EDWARDS HENDERSON, PH.D.
ASSOCIATE PROFESSOR OF CHEMISTRY, OHIO STATE UNIVERSITY
REVISED EDITION
GINN & COMPANY
BOSTON * NEW YORK * CHICAGO * LONDON
COPYRIGHT, 1905, 1906, BY
WILLIAM MCPHERSON AND WILLIAM E. HENDERSON
ALL RIGHTS RESERVED
The Athenæum Press
GINN & COMPANY * PROPRIETORS * BOSTON * U.S.A.
Transcriber's note: Minor typos have been corrected.
[Pg iii]
PREFACE
In offering this book to teachers of elementary chemistry the authors
lay no claim to any great originality. It has been their aim to prepare
a text-book constructed along lines which have become recognized as best
suited to an elementary treatment of the subject. At the same time they
have made a consistent effort to make the text clear in outline, simple
in style and language, conservatively modern in point of view, and
thoroughly teachable.
The question as to what shall be included in an elementary text on
chemistry is perhaps the most perplexing one which an author must
answer. While an enthusiastic chemist with a broad understanding of the
science is very apt to go beyond the capacity of the elementary student,
the authors of this text, after an experience of many years, cannot help
believing that the tendency has been rather in the other direction. In
many texts no mention at all is made of fundamental laws of chemical
action because their complete presentation is quite beyond the
comprehension of the student, whereas in many cases it is possible to
present the essential features of these laws in a way that will be of
real assistance in the understanding of the science. For example, it is
a difficult matter to deduce the law of mass action in any very simple
way; yet the elementary student can readily comprehend that reactions
are reversible, and that the point of equilibrium depends upon, rather
simple conditions. The authors believe that it is worth while to[Pg iv]
present such principles in even an elementary and partial manner because
they are of great assistance to the general student, and because they
make a foundation upon which the student who continues his studies to
more advanced courses can securely build.
The authors have no apologies to make for the extent to which they have
made use of the theory of electrolytic dissociation. It is inevitable
that in any rapidly developing science there will be differences of
opinion in regard to the value of certain theories. There can be no
question, however, that the outline of the theory of dissociation here
presented is in accord with the views of the very great majority of the
chemists of the present time. Moreover, its introduction to the extent
to which the authors have presented it simplifies rather than increases
the difficulties with which the development of the principles of the
science is attended.
The oxygen standard for atomic weights has been adopted throughout the
text. The International Committee, to which is assigned the duty of
yearly reporting a revised list of the atomic weights of the elements,
has adopted this standard for their report, and there is no longer any
authority for the older hydrogen standard. The authors do not believe
that the adoption of the oxygen standard introduces any real
difficulties in making perfectly clear the methods by which atomic
weights are calculated.
The problems appended to the various chapters have been chosen with a
view not only of fixing the principles developed in the text in the mind
of the student, but also of enabling him to answer such questions as
arise in his laboratory work. They are, therefore, more or less
practical in character. It is not necessary that all of them should[Pg v] be
solved, though with few exceptions the lists are not long. The answers
to the questions are not directly given in the text as a rule, but can
be inferred from the statements made. They therefore require independent
thought on the part of the student.
With very few exceptions only such experiments are included in the text
as cannot be easily carried out by the student. It is expected that
these will be performed by the teacher at the lecture table. Directions
for laboratory work by the student are published in a separate volume.
While the authors believe that the most important function of the
elementary text is to develop the principles of the science, they
recognize the importance of some discussion of the practical application
of these principles to our everyday life. Considerable space is
therefore devoted to this phase of chemistry. The teacher should
supplement this discussion whenever possible by having the class visit
different factories where chemical processes are employed.
Although this text is now for the first time offered to teachers of
elementary chemistry, it has nevertheless been used by a number of
teachers during the past three years. The present edition has been
largely rewritten in the light of the criticisms offered, and we desire
to express our thanks to the many teachers who have helped us in this
respect, especially to Dr. William Lloyd Evans of this laboratory, a
teacher of wide experience, for his continued interest and helpfulness.
We also very cordially solicit correspondence with teachers who may find
difficulties or inaccuracies in the text.
The authors wish to make acknowledgments for the photographs and
engravings of eminent chemists from which[Pg vi] the cuts included in the text
were taken; to Messrs. Elliott and Fry, London, England, for that of
Ramsay; to The Macmillan Company for those of Davy and Dalton, taken
from the Century Science Series; to the L. E. Knott Apparatus Company,
Boston, for that of Bunsen.
THE AUTHORS
OHIO STATE UNIVERSITY
COLUMBUS, OHIO
[Pg vii]
CONTENTS
| CHAPTER | PAGE |
| I. INTRODUCTION | 1 |
| II. OXYGEN | 13 |
| III. HYDROGEN | 28 |
| IV. WATER AND HYDROGEN DIOXIDE | 40 |
| V. THE ATOMIC THEORY | 59 |
| VI. CHEMICAL EQUATIONS AND CALCULATIONS | 68 |
| VII. NITROGEN AND THE RARE ELEMENTS IN THE ATMOSPHERE | 78 |
| VIII. THE ATMOSPHERE | 83 |
| IX. SOLUTIONS | 94 |
| X. ACIDS, BASES, AND SALTS; NEUTRALIZATION | 106 |
| XI. VALENCE | 116 |
| XII. COMPOUNDS OF NITROGEN | 122 |
| XIII. REVERSIBLE REACTIONS AND CHEMICAL EQUILIBRIUM | 137 |
| XIV. SULPHUR AND ITS COMPOUNDS | 143 |
| XV. PERIODIC LAW | 165 |
| XVI. THE CHLORINE FAMILY | 174 |
| XVII. CARBON AND SOME OF ITS SIMPLER COMPOUNDS | 196 |
| XVIII. FLAMES,—ILLUMINANTS | 213 |
| XIX. MOLECULAR WEIGHTS, ATOMIC WEIGHTS, FORMULAS | 223 |
| XX. THE PHOSPHORUS FAMILY | 238 |
| XXI. SILICON, TITANIUM, BORON | 257 |
| XXII. THE METALS | 267 |
| XXIII. THE ALKALI METALS | 274 |
| XXIV. THE ALKALINE-EARTH FAMILY | 300 |
| XXV. THE MAGNESIUM FAMILY | 316 |
| XXVI. THE ALUMINIUM FAMILY | 327 |
| XXVII. THE IRON FAMILY | 338 |
| XXVIII. COPPER, MERCURY, AND SILVER | 356 |
| XXIX. TIN AND LEAD | 370 |
| XXX. MANGANESE AND CHROMIUM | 379 |
| XXXI. GOLD AND THE PLATINUM FAMILY | 390 |
| XXXII. SOME SIMPLE ORGANIC COMPOUNDS | 397 |
| INDEX | 421 |
| APPENDIX A | Facing back cover |
| APPENDIX B | Inside back cover |
[Pg ix]
LIST OF FULL-PAGE ILLUSTRATIONS
| PAGE |
| ANTOINE LAURENT LAVOISIER | Frontispiece |
| JOSEPH PRIESTLEY | 14 |
| JOHN DALTON | 60 |
| WILLIAM RAMSAY | 82 |
| DMITRI IVANOVITCH MENDELÉEFF | 166 |
| HENRI MOISSAN | 176 |
| SIR HUMPHRY DAVY | 276 |
| ROBERT WILHELM BUNSEN | 298 |
[Pg 1]
AN ELEMENTARY STUDY OF CHEMISTRY
CHAPTER I
INTRODUCTION
The natural sciences. Before we advance very far in the study of nature,
it becomes evident that the one large study must be divided into a
number of more limited ones for the convenience of the investigator as
well as of the student. These more limited studies are called the
natural sciences.
Since the study of nature is divided in this way for mere convenience,
and not because there is any division in nature itself, it often happens
that the different sciences are very intimately related, and a thorough
knowledge of any one of them involves a considerable acquaintance with
several others. Thus the botanist must know something about animals as
well as about plants; the student of human physiology must know
something about physics as well as about the parts of the body.
Intimate relation of chemistry and physics. Physics and chemistry are
two sciences related in this close way, and it is not easy to make a
precise distinction between them. In a general way it may be said that
they are both concerned with inanimate matter rather than with living,
and more particularly with the changes which such matter[Pg 2] may be made to
undergo. These changes must be considered more closely before a
definition of the two sciences can be given.
Physical changes. One class of changes is not accompanied by an
alteration in the composition of matter. When a lump of coal is broken
the pieces do not differ from the original lump save in size. A rod of
iron may be broken into pieces; it may be magnetized; it may be heated
until it glows; it may be melted. In none of these changes has the
composition of the iron been affected. The pieces of iron, the
magnetized iron, the glowing iron, the melted iron, are just as truly
iron as was the original rod. Sugar may be dissolved in water, but
neither the sugar nor the water is changed in composition. The resulting
liquid has the sweet taste of sugar; moreover the water may be
evaporated by heating and the sugar recovered unchanged. Such changes
are called physical changes.
DEFINITION: Physical changes are those which do not involve a change in
the composition of the matter.
Chemical changes. Matter may undergo other changes in which its
composition is altered. When a lump of coal is burned ashes and
invisible gases are formed which are entirely different in composition
and properties from the original coal. A rod of iron when exposed to
moist air is gradually changed into rust, which is entirely different
from the original iron. When sugar is heated a black substance is formed
which is neither sweet nor soluble in water. Such changes are evidently
quite different from the physical changes just described, for in them
new substances are formed in place of the ones undergoing change.
Changes of this kind are called chemical changes.[Pg 3]
DEFINITION: Chemical changes are those which involve a change in the
composition of the matter.
How to distinguish between physical and chemical changes. It is not
always easy to tell to which class a given change belongs, and many
cases will require careful thought on the part of the student. The test
question in all cases is, Has the composition of the substance been
changed? Usually this can be answered by a study of the properties of
the substance before and after the change, since a change in composition
is attended by a change in properties. In some cases, however, only a
trained observer can decide the question.
Changes in physical state. One class of physical changes should be noted
with especial care, since it is likely to prove misleading. It is a
familiar fact that ice is changed into water, and water into steam, by
heating. Here we have three different substances,—the solid ice, the
liquid water, and the gaseous steam,—the properties of which differ
widely. The chemist can readily show, however, that these three bodies
have exactly the same composition, being composed of the same substances
in the same proportion. Hence the change from one of these substances
into another is a physical change. Many other substances may, under
suitable conditions, be changed from solids into liquids, or from
liquids into gases, without change in composition. Thus butter and wax
will melt when heated; alcohol and gasoline will evaporate when exposed
to the air. The three states—solid, liquid, and gas—are called the
three physical states of matter.
Physical and chemical properties. Many properties of a substance can be
noted without causing the substance to undergo chemical change, and are
therefore called its physical properties. Among these are its physical
state, color, odor, taste, size, shape, weight. Other properties are
only[Pg 4] discovered when the substance undergoes chemical change. These are
called its chemical properties. Thus we find that coal burns in air,
gunpowder explodes when ignited, milk sours when exposed to air.
Definition of physics and chemistry. It is now possible to make a
general distinction between physics and chemistry.
DEFINITION: Physics is the science which deals with those changes in
matter which do not involve a change in composition.
DEFINITION: Chemistry is the science which deals with those changes in
matter which do involve a change in composition.
Two factors in all changes. In all the changes which matter can undergo,
whether physical or chemical, two factors must be taken into account,
namely, energy and matter.
Energy. It is a familiar fact that certain bodies have the power to do
work. Thus water falling from a height upon a water wheel turns the
wheel and in this way does the work of the mills. Magnetized iron
attracts iron to itself and the motion of the iron as it moves towards
the magnet can be made to do work. When coal is burned it causes the
engine to move and transports the loaded cars from place to place. When
a body has this power to do work it is said to possess energy.
Law of conservation of energy. Careful experiments have shown that when
one body parts with its energy the energy is not destroyed but is
transferred to another body or system of bodies. Just as energy cannot
be destroyed, neither can it be created. If one body gains a certain
amount of energy, some other body has lost an equivalent amount.[Pg 5] These
facts are summed up in the law of conservation of energy which may be
stated thus: While energy can be changed from one form into another, it
cannot be created or destroyed.
Transformations of energy. Although energy can neither be created nor
destroyed, it is evident that it may assume many different forms. Thus
the falling water may turn the electric generator and produce a current
of electricity. The energy lost by the falling water is thus transformed
into the energy of the electric current. This in turn may be changed
into the energy of motion, as when the current is used for propelling
the cars, or into the energy of heat and light, as when it is used for
heating and lighting the cars. Again, the energy of coal may be
converted into energy of heat and subsequently of motion, as when it is
used as a fuel in steam engines.
Since the energy possessed by coal only becomes available when the coal
is made to undergo a chemical change, it is sometimes called chemical
energy. It is this form of energy in which we are especially interested
in the study of chemistry.
Matter. Matter may be defined as that which occupies space and possesses
weight. Like energy, matter may be changed oftentimes from one form into
another; and since in these transformations all the other physical
properties of a substance save weight are likely to change, the inquiry
arises, Does the weight also change? Much careful experimenting has
shown that it does not. The weight of the products formed in any change
in matter always equals the weight of the substances undergoing change.
Law of conservation of matter. The important truth just stated is
frequently referred to as the law of conservation[Pg 6] of matter, and this
law may be briefly stated thus: Matter can neither be created nor
destroyed, though it can be changed from one form into another.
Classification of matter. At first sight there appears to be no limit to
the varieties of matter of which the world is made. For convenience in
study we may classify all these varieties under three heads, namely,
mechanical mixtures, chemical compounds, and elements.
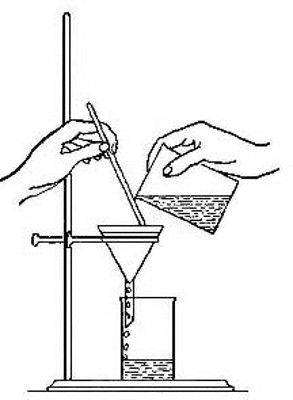 Fig. 1
Fig. 1
Mechanical mixtures. If equal bulks of common salt and iron filings are
thoroughly mixed together, a product is obtained which, judging by its
appearance, is a new substance. If it is examined more closely, however,
it will be seen to be merely a mixture of the salt and iron, each of
which substances retains its own peculiar properties. The mixture tastes
just like salt; the iron particles can be seen and their gritty
character detected. A magnet rubbed in the mixture draws out the iron
just as if the salt were not there. On the other hand, the salt can be
separated from the iron quite easily. Thus, if several grams of the
mixture are placed in a test tube, and the tube half filled with water
and thoroughly shaken, the salt dissolves in the water. The iron
particles can then be filtered from the liquid by pouring the entire
mixture upon a piece of filter paper folded so as to fit into the
interior of a funnel (Fig. 1). The paper retains the solid but allows
the clear liquid, known as the filtrate, to drain through. The iron
particles left upon the filter paper will be found to be identical with[Pg 7]
the original iron. The salt can be recovered from the filtrate by
evaporation of the water. To accomplish this the filtrate is poured into
a small evaporating dish and gently heated (Fig. 2) until the water has
disappeared, or evaporated. The solid left in the dish is identical in
every way with the original salt. Both the iron and the salt have thus
been recovered in their original condition. It is evident that no new
substance has been formed by rubbing the salt and iron together. The
product is called a mechanical mixture. Such mixtures are very common
in nature, almost all minerals, sands, and soils being examples of this
class of substances. It is at once apparent that there is no law
regulating the composition of a mechanical mixture, and no two mixtures
are likely to have exactly the same composition. The ingredients of a
mechanical mixture can usually be separated by mechanical means, such as
sifting, sorting, magnetic attraction, or by dissolving one constituent
and leaving the other unchanged.
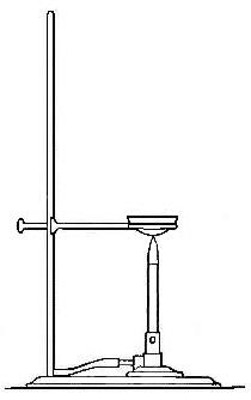 Fig. 2
Fig. 2
DEFINITION: A mechanical mixture is one in which the constituents
retain their original properties, no chemical action having taken place
when they were brought together.
Chemical compounds. If iron filings and powdered sulphur are thoroughly
ground together in a mortar, a yellowish-green substance results. It
might easily be taken to be a new body; but as in the case of the iron
and salt, the ingredients can readily be separated. A magnet draws out
the iron. Water does not dissolve the sulphur, but other liquids do, as,
for example, the liquid called carbon disulphide.[Pg 8] When the mixture is
treated with carbon disulphide the iron is left unchanged, and the
sulphur can be obtained again, after filtering off the iron, by
evaporating the liquid. The substance is, therefore, a mechanical
mixture.
If now a new portion of the mixture is placed in a dry test tube and
carefully heated in the flame of a Bunsen burner, as shown in Fig. 3, a
striking change takes place. The mixture begins to glow at some point,
the glow rapidly extending throughout the whole mass. If the test tube
is now broken and the product examined, it will be found to be a hard,
black, brittle substance, in no way recalling the iron or the sulphur.
The magnet no longer attracts it; carbon disulphide will not dissolve
sulphur from it. It is a new substance with new properties, resulting
from the chemical union of iron and sulphur, and is called iron
sulphide. Such substances are called chemical compounds, and differ
from mechanical mixtures in that the substances producing them lose
their own characteristic properties. We shall see later that the two
also differ in that the composition of a chemical compound never varies.
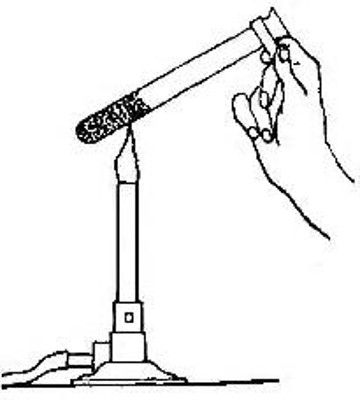 Fig. 3
Fig. 3
DEFINITION: A chemical compound is a substance the constituents of
which have lost their own characteristic properties, and which cannot be
separated save by a chemical change.
Elements. It has been seen that iron sulphide is composed of two
entirely different substances,—iron and sulphur. The question arises,
Do these substances in turn contain other substances, that is, are they
also chemical compounds?[Pg 9] Chemists have tried in a great many ways to
decompose them, but all their efforts have failed. Substances which have
resisted all efforts to decompose them into other substances are called
elements. It is not always easy to prove that a given substance is
really an element. Some way as yet untried may be successful in
decomposing it into other simpler forms of matter, and the supposed
element will then prove to be a compound. Water, lime, and many other
familiar compounds were at one time thought to be elements.
DEFINITION: An element is a substance which cannot be separated into
simpler substances by any known means.
Kinds of matter. While matter has been grouped in three classes for the
purpose of study, it will be apparent that there are really but two
distinct kinds of matter, namely, compounds and elements. A mechanical
mixture is not a third distinct kind of matter, but is made up of
varying quantities of either compounds or elements or both.
Alchemy. In olden times it was thought that some way could be found to
change one element into another, and a great many efforts were made to
accomplish this transformation. Most of these efforts were directed
toward changing the commoner metals into gold, and many fanciful ways
for doing this were described. The chemists of that time were called
alchemists, and the art which they practiced was called alchemy. The
alchemists gradually became convinced that the only way common metals
could be changed into gold was by the wonderful power of a magic
substance which they called the philosopher's stone, which would
accomplish this transformation by its mere touch and would in addition
give perpetual youth to its fortunate possessor. No one has ever found
such a stone, and no one has succeeded in changing one metal into
another.
Number of elements. The number of substances now considered to be
elements is not large—about eighty in all. Many of these are rare, and
very few of them make any[Pg 10] large fraction of the materials in the
earth's crust. Clarke gives the following estimate of the composition of
the earth's crust:
| Oxygen | 47.0% | Calcium | 3.5% |
| Silicon | 27.9 | Magnesium | 2.5 |
| Aluminium | 8.1 | Sodium | 2.7 |
| Iron | 4.7 | Potassium | 2.4 |
| Other elements | 1.2% |
A complete list of the elements is given in the Appendix. In this list
the more common of the elements are marked with an asterisk. It is not
necessary to study more than a third of the total number of elements to
gain a very good knowledge of chemistry.
Physical state of the elements. About ten of the elements are gases at
ordinary temperatures. Two—mercury and bromine—are liquids. The others
are all solids, though their melting points vary through wide limits,
from cæsium which melts at 26° to elements which do not melt save in the
intense heat of the electric furnace.
Occurrence of the elements. Comparatively few of the elements occur as
uncombined substances in nature, most of them being found in the form of
chemical compounds. When an element does occur by itself, as is the case
with gold, we say that it occurs in the free state or native; when
it is combined with other substances in the form of compounds, we say
that it occurs in the combined state, or in combination. In the
latter case there is usually little about the compound to suggest that
the element is present in it; for we have seen that elements lose their
own peculiar properties when they enter into combination with other
elements. It would never be suspected, for example, that the reddish,
earthy-looking iron ore contains iron.[Pg 11]
Names of elements. The names given to the elements have been selected in
a great many different ways. (1) Some names are very old and their
original meaning is obscure. Such names are iron, gold, and copper. (2)
Many names indicate some striking physical property of the element. The
name bromine, for example, is derived from a Greek word meaning a
stench, referring to the extremely unpleasant odor of the substance. The
name iodine comes from a word meaning violet, alluding to the beautiful
color of iodine vapor. (3) Some names indicate prominent chemical
properties of the elements. Thus, nitrogen means the producer of niter,
nitrogen being a constituent of niter or saltpeter. Hydrogen means water
former, signifying its presence in water. Argon means lazy or inert, the
element being so named because of its inactivity. (4) Other elements are
named from countries or localities, as germanium and scandium.
Symbols. In indicating the elements found in compounds it is
inconvenient to use such long names, and hence chemists have adopted a
system of abbreviations. These abbreviations are known as symbols,
each element having a distinctive symbol. (1) Sometimes the initial
letter of the name will suffice to indicate the element. Thus I stands
for iodine, C for carbon. (2) Usually it is necessary to add some other
characteristic letter to the symbol, since several names may begin with
the same letter. Thus C stands for carbon, Cl for chlorine, Cd for
cadmium, Ce for cerium, Cb for columbium. (3) Sometimes the symbol is an
abbreviation of the old Latin name. In this way Fe (ferrum) indicates
iron, Cu (cuprum), copper, Au (aurum), gold. The symbols are included in
the list of elements given in the Appendix. They will become familiar
through constant use.[Pg 12]
Chemical affinity the cause of chemical combination. The agency which
causes substances to combine and which holds them together when combined
is called chemical affinity. The experiments described in this
chapter, however, show that heat is often necessary to bring about
chemical action. The distinction between the cause producing chemical
action and the circumstances favoring it must be clearly made. Chemical
affinity is always the cause of chemical union. Many agencies may make
it possible for chemical affinity to act by overcoming circumstances
which stand in its way. Among these agencies are heat, light, and
electricity. As a rule, solution also promotes action between two
substances. Sometimes these agencies may overcome chemical attraction
and so occasion the decomposition of a compound.
EXERCISES
1. To what class of changes do the following belong? (a) The melting
of ice; (b) the souring of milk; (c) the burning of a candle; (d)
the explosion of gunpowder; (e) the corrosion of metals. What test
question must be applied in each of the above cases?
2. Give two additional examples (a) of chemical changes; (b) of
physical changes.
3. Is a chemical change always accompanied by a physical change? Is a
physical change always accompanied by a chemical change?
4. Give two or more characteristics of a chemical change.
5. (a) When a given weight of water freezes, does it absorb or evolve
heat? (b) When the resulting ice melts, is the total heat change the
same or different from that of freezing?
6. Give three examples of each of the following: (a) mechanical
mixtures; (b) chemical compounds; (c) elements.
7. Give the derivation of the names of the following elements: thorium,
gallium, selenium, uranium. (Consult dictionary.)
8. Give examples of chemical changes which are produced through the
agency of heat; of light; of electricity.
[Pg 13]
CHAPTER II
OXYGEN
History. The discovery of oxygen is generally attributed to the English
chemist Priestley, who in 1774 obtained the element by heating a
compound of mercury and oxygen, known as red oxide of mercury. It is
probable, however, that the Swedish chemist Scheele had previously
obtained it, although an account of his experiments was not published
until 1777. The name oxygen signifies acid former. It was given to the
element by the French chemist Lavoisier, since he believed that all
acids owe their characteristic properties to the presence of oxygen.
This view we now know to be incorrect.
Occurrence. Oxygen is by far the most abundant of all the elements. It
occurs both in the free and in the combined state. In the free state it
occurs in the air, 100 volumes of dry air containing about 21 volumes of
oxygen. In the combined state it forms eight ninths of water and nearly
one half of the rocks composing the earth's crust. It is also an
important constituent of the compounds which compose plant and animal
tissues; for example, about 66% by weight of the human body is oxygen.
Preparation. Although oxygen occurs in the free state in the atmosphere,
its separation from the nitrogen and other gases with which it is mixed
is such a difficult matter that in the laboratory it has been found more
convenient to prepare it from its compounds. The most important of the
laboratory methods are the following:[Pg 14]
1. Preparation from water. Water is a compound, consisting of 11.18%
hydrogen and 88.82% oxygen. It is easily separated into these
constituents by passing an electric current through it under suitable
conditions. The process will be described in the chapter on water. While
this method of preparation is a simple one, it is not economical.
2. Preparation from mercuric oxide. This method is of interest, since
it is the one which led to the discovery of oxygen. The oxide, which
consists of 7.4% oxygen and 92.6% mercury, is placed in a small, glass
test tube and heated. The compound is in this way decomposed into
mercury which collects on the sides of the glass tube, forming a silvery
mirror, and oxygen which, being a gas, escapes from the tube. The
presence of the oxygen is shown by lighting the end of a splint,
extinguishing the flame and bringing the glowing coal into the mouth of
the tube. The oxygen causes the glowing coal to burst into a flame.
In a similar way oxygen may be obtained from its compounds with
some of the other elements. Thus manganese dioxide, a black
compound of manganese and oxygen, when heated to about 700°,
loses one third of its oxygen, while barium dioxide, when
heated, loses one half of its oxygen.
3. Preparation from potassium chlorate (usual laboratory method).
Potassium chlorate is a white solid which consists of 31.9% potassium,
28.9% chlorine, and 39.2% oxygen. When heated it undergoes a series of
changes in which all the oxygen is finally set free, leaving a compound
of potassium and chlorine called potassium chloride. The change may be
represented as follows:
/ potassium \ (potassium /potassium \ (potassium
{ chlorine } chlorate) = { } chloride) + oxygen
\ oxygen / \ chlorine /
 JOSEPH PRIESTLEY (English) (1733-1804)
JOSEPH PRIESTLEY (English) (1733-1804)
School-teacher, theologian, philosopher, scientist; friend of Benjamin
Franklin; discoverer of oxygen; defender of the phlogiston theory; the
first to use mercury in a pneumatic trough, by which means he first
isolated in gaseous form hydrochloric acid, sulphur dioxide, and
ammonia
[Pg 15]
The evolution of the oxygen begins at about 400°. It has been found,
however, that if the potassium chlorate is mixed with about one fourth
its weight of manganese dioxide, the oxygen is given off at a much lower
temperature. Just how the manganese dioxide brings about this result is
not definitely known. The amount of oxygen obtained from a given weight
of potassium chlorate is exactly the same whether the manganese dioxide
is present or not. So far as can be detected the manganese dioxide
undergoes no change.
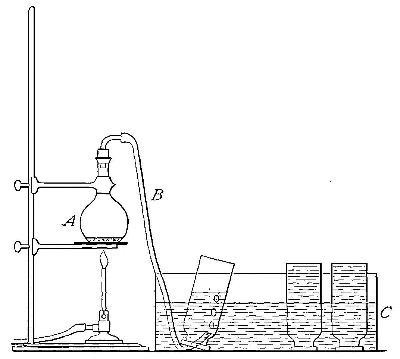 Fig. 4
Fig. 4
Directions for preparing oxygen. The manner of preparing oxygen from
potassium chlorate is illustrated in the accompanying diagram (Fig. 4).
A mixture consisting of one part of manganese dioxide and four parts of
potassium chlorate is placed in the flask A and gently heated. The
oxygen is evolved and escapes through the tube B. It is collected by
bringing over the end of the tube the mouth of a bottle completely
filled with water and inverted in a vessel of water, as shown in the
figure. The gas rises in the bottle and displaces the water. In the
preparation of large quantities of oxygen, a copper retort (Fig. 5) is
often substituted for the glass flask.
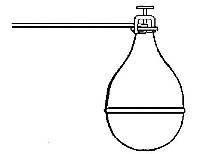 Fig. 5
Fig. 5
In the preparation of oxygen from potassium chlorate and manganese
dioxide, the materials used must be pure, otherwise a violent explosion
may occur. The purity of the materials is tested by heating a small
amount of the mixture in a test tube.
The collection of gases. The method used for collecting oxygen
illustrates the general method used for collecting such gases as are[Pg 16]
insoluble in water or nearly so. The vessel C (Fig. 4), containing the
water in which the bottles are inverted, is called a pneumatic trough.
Commercial methods of preparation. Oxygen can now be purchased stored
under great pressure in strong steel cylinders (Fig. 6). It is prepared
either by heating a mixture of potassium chlorate and manganese dioxide,
or by separating it from the nitrogen and other gases with which it is
mixed in the atmosphere. The methods employed for effecting this
separation will be described in subsequent chapters.
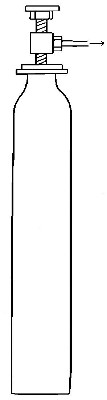 Fig. 6
Fig. 6
Physical properties. Oxygen is a colorless, odorless, tasteless gas,
slightly heavier than air. One liter of it, measured at a temperature of
0° and under a pressure of one atmosphere, weighs 1.4285 g., while under
similar conditions one liter of air weighs 1.2923 g. It is but slightly
soluble in water. Oxygen, like other gases, may be liquefied by applying
very great pressure to the highly cooled gas. When the pressure is
removed the liquid oxygen passes again into the gaseous state, since its
boiling point under ordinary atmospheric pressure is -182.5°.
Chemical properties. At ordinary temperatures oxygen is not very active
chemically. Most substances are either not at all affected by it, or the
action is so slow as to escape notice. At higher temperatures, however,
it is very active, and unites directly with most of the elements. This
activity may be shown by heating various substances until just ignited
and then bringing them into vessels of the gas, when they will burn with
great brilliancy. Thus a glowing splint introduced into a jar of oxygen
bursts into flame. Sulphur burns in the air with a very weak flame and
feeble light; in oxygen, however, the flame is increased in size and[Pg 17]
brightness. Substances which readily burn in air, such as phosphorus,
burn in oxygen with dazzling brilliancy. Even substances which burn in
air with great difficulty, such as iron, readily burn in oxygen.
The burning of a substance in oxygen is due to the rapid combination of
the substance or of the elements composing it with the oxygen. Thus,
when sulphur burns both the oxygen and sulphur disappear as such and
there is formed a compound of the two, which is an invisible gas, having
the characteristic odor of burning sulphur. Similarly, phosphorus on
burning forms a white solid compound of phosphorus and oxygen, while
iron forms a reddish-black compound of iron and oxygen.
Oxidation. The term oxidation is applied to the chemical change which
takes place when a substance, or one of its constituent parts, combines
with oxygen. This process may take place rapidly, as in the burning of
phosphorus, or slowly, as in the oxidation (or rusting) of iron when
exposed to the air. It is always accompanied by the liberation of heat.
The amount of heat liberated by the oxidation of a definite weight of
any given substance is always the same, being entirely independent of
the rapidity of the process. If the oxidation takes place slowly, the
heat is generated so slowly that it is difficult to detect it. If the
oxidation takes place rapidly, however, the heat is generated in such a
short interval of time that the substance may become white hot or burst
into a flame.
Combustion; kindling temperature. When oxidation takes place so rapidly
that the heat generated is sufficient to cause the substance to glow or
burst into a flame the process is called combustion. In order that any
substance may undergo combustion, it is necessary that it should be[Pg 18]
heated to a certain temperature, known as the kindling temperature.
This temperature varies widely for different bodies, but is always
definite for the same body. Thus the kindling temperature of phosphorus
is far lower than that of iron, but is definite for each. When any
portion of a substance is heated until it begins to burn the combustion
will continue without the further application of heat, provided the heat
generated by the process is sufficient to bring other parts of the
substance to the kindling temperature. On the other hand, if the heat
generated is not sufficient to maintain the kindling temperature,
combustion ceases.
Oxides. The compounds formed by the oxidation of any element are called
oxides. Thus in the combustion of sulphur, phosphorus, and iron, the
compounds formed are called respectively oxide of sulphur, oxide of
phosphorus, and oxide of iron. In general, then, an oxide is a compound
of oxygen with another element. A great many substances of this class
are known; in fact, the oxides of all the common elements have been
prepared, with the exception of those of fluorine and bromine. Some of
these are familiar compounds. Water, for example, is an oxide of
hydrogen, and lime an oxide of the metal calcium.
Products of combustion. The particular oxides formed by the combustion
of any substance are called products of combustion of that substance.
Thus oxide of sulphur is the product of the combustion of sulphur; oxide
of iron is the product of the combustion of iron. It is evident that the
products of the combustion of any substance must weigh more than the
original substance, the increase in weight corresponding to the amount
of oxygen taken up in the act of combustion. For example, when iron
burns the oxide of iron formed weighs more than the original iron.[Pg 19]
In some cases the products of combustion are invisible gases, so that
the substance undergoing combustion is apparently destroyed. Thus, when
a candle burns it is consumed, and so far as the eye can judge nothing
is formed during combustion. That invisible gases are formed, however,
and that the weight of these is greater than the weight of the candle
may be shown by the following experiment.
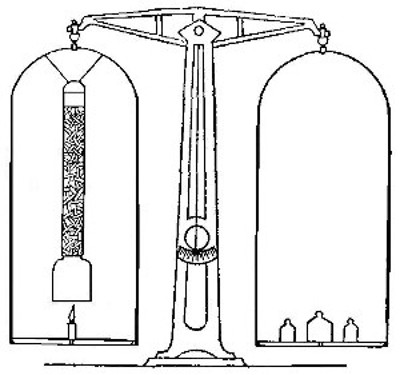 Fig. 7
Fig. 7
A lamp chimney is filled with sticks of the compound known as
sodium hydroxide (caustic soda), and suspended from the beam of
the balance, as shown in Fig. 7. A piece of candle is placed on
the balance pan so that the wick comes just below the chimney,
and the balance is brought to a level by adding weights to the
other pan. The candle is then lighted. The products formed pass
up through the chimney and are absorbed by the sodium
hydroxide. Although the candle burns away, the pan upon which
it rests slowly sinks, showing that the combustion is attended
by an increase in weight.
Combustion in air and in oxygen. Combustion in air and in
oxygen differs only in rapidity, the products formed being
exactly the same. That the process should take place less
rapidly in the former is readily understood, for the air is
only about one fifth oxygen, the remaining four fifths being
inert gases. Not only is less oxygen available, but much of the
heat is absorbed in raising the temperature of the inert gases
surrounding the substance undergoing combustion, and the
temperature reached in the combustion is therefore less.
Phlogiston theory of combustion. The French chemist Lavoisier
(1743-1794), who gave to oxygen its name was the first to show
that combustion is due to union with oxygen. Previous to his
time combustion was supposed to be due to the presence of a
substance or principle called phlogiston. One substance was
thought to be more combustible than another because it
contained more phlogiston. Coal, for example, was thought to be
very rich in phlogiston. The ashes[Pg 20] left after combustion would
not burn because all the phlogiston had escaped. If the
phlogiston could be restored in any way, the substance would
then become combustible again. Although this view seems absurd
to us in the light of our present knowledge, it formerly had
general acceptance. The discovery of oxygen led Lavoisier to
investigate the subject, and through his experiments he arrived
at the true explanation of combustion. The discovery of oxygen
together with the part it plays in combustion is generally
regarded as the most important discovery in the history of
chemistry. It marked the dawn of a new period in the growth of
the science.
Combustion in the broad sense. According to the definition given above,
the presence of oxygen is necessary for combustion. The term is
sometimes used, however, in a broader sense to designate any chemical
change attended by the evolution of heat and light. Thus iron and
sulphur, or hydrogen and chlorine under certain conditions, will combine
so rapidly that light is evolved, and the action is called a combustion.
Whenever combustion takes place in the air, however, the process is one
of oxidation.
Spontaneous combustion. The temperature reached in a given
chemical action, such as oxidation, depends upon the rate at
which the reaction takes place. This rate is usually increased
by raising the temperature of the substances taking part in the
action.
When a slow oxidation takes place under such conditions that
the heat generated is not lost by being conducted away, the
temperature of the substance undergoing oxidation is raised,
and this in turn hastens the rate of oxidation. The rise in
temperature may continue in this way until the kindling
temperature of the substance is reached, when combustion
begins. Combustion occurring in this way is called spontaneous
combustion.
Certain oils, such as the linseed oil used in paints, slowly
undergo oxidation at ordinary temperatures, and not
infrequently the origin of fires has been traced to the
spontaneous combustion of oily rags. The spontaneous combustion
of hay has been known to set barns on fire. Heaps of coal have
been found to be on fire when spontaneous combustion offered
the only possible explanation.
[Pg 21]
Importance of oxygen. 1. Oxygen is essential to life. Among living
organisms only certain minute forms of plant life can exist without it.
In the process of respiration the air is taken into the lungs where a
certain amount of oxygen is absorbed by the blood. It is then carried to
all parts of the body, oxidizing the worn-out tissues and changing them
into substances which may readily be eliminated from the body. The heat
generated by this oxidation is the source of the heat of the body. The
small amount of oxygen which water dissolves from the air supports all
the varied forms of aquatic animals.
2. Oxygen is also essential to decay. The process of decay is really a
kind of oxidation, but it will only take place in the presence of
certain minute forms of life known as bacteria. Just how these assist in
the oxidation is not known. By this process the dead products of animal
and vegetable life which collect on the surface of the earth are slowly
oxidized and so converted into harmless substances. In this way oxygen
acts as a great purifying agent.
3. Oxygen is also used in the treatment of certain diseases in which the
patient is unable to inhale sufficient air to supply the necessary
amount of oxygen.
OZONE
Preparation. When electric sparks are passed through oxygen or air a
small percentage of the oxygen is converted into a substance called
ozone, which differs greatly from oxygen in its properties. The same
change can also be brought about by certain chemical processes. Thus, if
some pieces of phosphorus are placed in a bottle and partially covered
with water, the presence of ozone may soon be detected in the air
contained in the bottle. The conversion of oxygen into ozone is attended
by a change in volume, 3 volumes of oxygen forming 2 volumes of ozone.
If the resulting ozone is heated to about 300°, the[Pg 22] reverse change
takes place, the 2 volumes of ozone being changed back into 3 volumes of
oxygen. It is possible that traces of ozone exist in the atmosphere,
although its presence there has not been definitely proved, the tests
formerly used for its detection having been shown to be unreliable.
Properties. As commonly prepared, ozone is mixed with a large excess of
oxygen. It is possible, however, to separate the ozone and thus obtain
it in pure form. The gas so obtained has the characteristic odor noticed
about electrical machines when in operation. By subjecting it to great
pressure and a low temperature, the gas condenses to a bluish liquid,
boiling at -119°. When unmixed with other gases ozone is very explosive,
changing back into oxygen with the liberation of heat. Its chemical
properties are similar to those of oxygen except that it is far more
active. Air or oxygen containing a small amount of ozone is now used in
place of oxygen in certain manufacturing processes.
The difference between oxygen and ozone. Experiments show that in
changing oxygen into ozone no other kind of matter is either added to
the oxygen or withdrawn from it. The question arises then, How can we
account for the difference in their properties? It must be remembered
that in all changes we have to take into account energy as well as
matter. By changing the amount of energy in a substance we change its
properties. That oxygen and ozone contain different amounts of energy
may be shown in a number of ways; for example, by the fact that the
conversion of ozone into oxygen is attended by the liberation of heat.
The passage of the electric sparks through oxygen has in some way
changed the energy content of the element and thus it has acquired new
properties. Oxygen and ozone must, therefore, be regarded as identical
so far as the kind of matter of which they are composed is concerned.
Their different properties are due to their different energy contents.
Allotropic states or forms of matter. Other elements besides oxygen may
exist in more than one form. These different forms of the same element
are called allotropic states or forms of the element. These forms
differ not only in physical properties but also in their energy
contents. Elements often exist in a variety of forms which look quite
different. These differences may be due to accidental causes, such as
the size or shape of the particles or the way in which the element was
prepared. Only such forms, however, as have different energy contents
are properly called allotropic forms.[Pg 23]
MEASUREMENT OF GAS VOLUMES
Standard conditions. It is a well-known fact that the volume occupied by
a definite weight of any gas can be altered by changing the temperature
of the gas or the pressure to which it is subjected. In measuring the
volume of gases it is therefore necessary, for the sake of accuracy, to
adopt some standard conditions of temperature and pressure. The
conditions agreed upon are (1) a temperature of 0°, and (2) a pressure
equal to the average pressure exerted by the atmosphere at the sea
level, that is, 1033.3 g. per square centimeter. These conditions of
temperature and pressure are known as the standard conditions, and
when the volume of a gas is given it is understood that the measurement
was made under these conditions, unless it is expressly stated
otherwise. For example, the weight of a liter of oxygen has been given
as 1.4285 g. This means that one liter of oxygen, measured at a
temperature of 0° and under a pressure of 1033.3 g. per square
centimeter, weighs 1.4285 g.
The conditions which prevail in the laboratory are never the standard
conditions. It becomes necessary, therefore, to find a way to calculate
the volume which a gas will occupy under standard conditions from the
volume which it occupies under any other conditions. This may be done in
accordance with the following laws.
Law of Charles. This law expresses the effect which a change in the
temperature of a gas has upon its volume. It may be stated as follows:
For every degree the temperature of a gas rises above zero the volume
of the gas is increased by 1/273 of the volume which it occupies at
zero; likewise for every degree the temperature of the gas falls below
zero the volume of the gas is decreased by 1/273 of the volume which it
occupies at zero, provided in both cases that the pressure to which the
gas is subjected remains constant.
If V represents the volume of gas at 0°, then the volume at 1° will be
V + 1/273 V; at 2° it will be V + 2/273 V; or, in general, the
volume v, at the temperature t, will be expressed by the formula
(1) v = V + t/273 V,
or (2) v = V(1 + (t/273)).
Since 1/273 = 0.00366, the formula may be written
Since the value of V (volume under standard conditions) is the one
usually sought, it is convenient to transpose the equation to the
following form:
(4) V = v/(1 + 0.00366t).
The following problem will serve as an illustration of the application
of this equation.
The volume of a gas at 20° is 750 cc.; find the volume it will occupy at
0°, the pressure remaining constant.
In this case, v = 750 cc. and t = 20. By substituting these values,
equation (4) becomes
V = 750/(1 + 0.00366 × 20) = 698.9 cc.
Law of Boyle. This law expresses the relation between the volume
occupied by a gas and the pressure to which it is subjected. It may be
stated as follows: The volume of a gas is inversely proportional to the
pressure under which it is measured, provided the temperature of the gas
remains constant.
If V represents the volume when subjected to a pressure P and v
represents its volume when the pressure is changed to p, then, in
accordance with the above law, V : v :: p : P, or VP = vp.
In other words, for a given weight of a gas the product of the numbers
representing its volume and the pressure to which it is subjected is a
constant.
Since the pressure of the atmosphere at any point is indicated by the
barometric reading, it is convenient in the solution of the problems to
substitute the latter for the pressure measured in grams per square
centimeter. The average reading of the barometer at the sea level is 760
mm., which corresponds to a pressure of 1033.3 g. per square centimeter.
The following problem will serve as an illustration of the application
of Boyle's law.
A gas occupies a volume of 500 cc. in a laboratory where the barometric
reading is 740 mm. What volume would it occupy if the atmospheric
pressure changed so that the reading became 750 mm.?
Substituting the values in the equation VP = vp, we have 500 × 740 =
v × 750, or v = 493.3 cc.
Variations in the volume of a gas due to changes both in temperature and
pressure. Inasmuch as corrections must be made as a rule[Pg 25] for both
temperature and pressure, it is convenient to combine the equations
given above for the corrections for each, so that the two corrections
may be made in one operation. The following equation is thus obtained:
(5) Vs = vp/(760(1 + 0.00366t)),
in which Vs represents the volume of a gas under standard
conditions and v, p, and t the volume, pressure, and temperature
respectively at which the gas was actually measured.
The following problem will serve to illustrate the application of this
equation.
A gas having a temperature of 20° occupies a volume of 500 cc. when
subjected to a pressure indicated by a barometric reading of 740 mm.
What volume would this gas occupy under standard conditions?
In this problem v = 500, p = 740, and t = 20. Substituting these
values in the above equation, we get
Vs = (500 × 740)/(760 (1 + 0.00366 × 20)) = 453.6 cc.
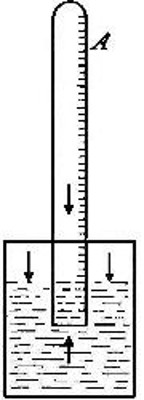 Fig. 8
Fig. 8
Variations in the volume of a gas due to the pressure of aqueous vapor.
In many cases gases are collected over water, as explained under the
preparation of oxygen. In such cases there is present in the gas a
certain amount of water vapor. This vapor exerts a definite pressure,
which acts in opposition to the atmospheric pressure and which therefore
must be subtracted from the latter in determining the effective pressure
upon the gas. Thus, suppose we wish to determine the pressure to which
the gas in tube A (Fig. 8) is subjected. The tube is raised or lowered
until the level of the water inside and outside the tube is the same.
The atmosphere presses down upon the surface of the water (as indicated
by the arrows), thus forcing the water upward within the tube with a
pressure equal to the atmospheric pressure. The full force of this
upward pressure, however, is not spent in compressing the gas within the
tube, for since it is collected over water it contains a certain amount
of water vapor. This water vapor exerts a pressure (as indicated by the
arrow within the tube) in opposition to[Pg 26] the upward pressure. It is
plain, therefore, that the effective pressure upon the gas is equal to
the atmospheric pressure less the pressure exerted by the aqueous vapor.
The pressure exerted by the aqueous vapor increases with the
temperature. The figures representing the extent of this pressure (often
called the tension of aqueous vapor) are given in the Appendix. They
express the pressure or tension in millimeters of mercury, just as the
atmospheric pressure is expressed in millimeters of mercury.
Representing the pressure of the aqueous vapor by a, formula (5)
becomes
(6) Vs = v(p - a)/(760(1 + 0.00366t)).
The following problem will serve to illustrate the method of applying
the correction for the pressure of the aqueous vapor.
The volume of a gas measured over water in a laboratory where the
temperature is 20° and the barometric reading is 740 mm. is 500 cc. What
volume would this occupy under standard conditions?
The pressure exerted by the aqueous vapor at 20° (see table in Appendix)
is equal to the pressure exerted by a column of mercury 17.4 mm. in
height. Substituting the values of v, t, p, and a in formula
(6), we have
(6) Vs = 500(740 - 17.4)/(760(1 + 0.00366 × 20)) = 442.9 cc.
Adjustment of tubes before reading gas volumes. In measuring the volumes
of gases collected in graduated tubes or other receivers, over a liquid
as illustrated in Fig. 8, the reading should be taken after raising or
lowering the tube containing the gas until the level of the liquid
inside and outside the tube is the same; for it is only under these
conditions that the upward pressure within the tube is the same as the
atmospheric pressure.
EXERCISES
1. What is the meaning of the following words? phlogiston, ozone,
phosphorus. (Consult dictionary.)
2. Can combustion take place without the emission of light?
3. Is the evolution of light always produced by combustion?
4. (a) What weight of oxygen can be obtained from 100 g. of water?
(b) What volume would this occupy under standard conditions?[Pg 27]
5. (a) What weight of oxygen can be obtained from 500g. of mercuric
oxide? (b) What volume would this occupy under standard conditions?
6. What weight of each of the following compounds is necessary to
prepare 50 l. of oxygen? (a) water; (b) mercuric oxide; (c)
potassium chlorate.
7. Reduce the following volumes to 0°, the pressure remaining constant:
(a) 150 cc. at 10°; (b) 840 cc. at 273°.
8. A certain volume of gas is measured when the temperature is 20°. At
what temperature will its volume be doubled?
9. Reduce the following volumes to standard conditions of pressure, the
temperature remaining constant: (a) 200 cc. at 740 mm.; (b) 500 l.
at 380 mm.
10. What is the weight of 1 l. of oxygen when the pressure is 750 mm.
and the temperature 0°?
11. Reduce the following volumes to standard conditions of temperature
and pressure: (a) 340 cc. at 12° and 753 mm; (b) 500 cc. at 15° and
740 mm.
12. What weight of potassium chlorate is necessary to prepare 250 l. of
oxygen at 20° and 750 mm.?
13. Assuming the cost of potassium chlorate and mercuric oxide to be
respectively $0.50 and $1.50 per kilogram, calculate the cost of
materials necessary for the preparation of 50 l. of oxygen from each of
the above compounds.
14. 100 g. of potassium chlorate and 25 g. of manganese dioxide were
heated in the preparation of oxygen. What products were left in the
flask, and how much of each was present?
[Pg 28]
CHAPTER III
HYDROGEN
Historical. The element hydrogen was first clearly recognized as a
distinct substance by the English investigator Cavendish, who in 1766
obtained it in a pure state, and showed it to be different from the
other inflammable airs or gases which had long been known. Lavoisier
gave it the name hydrogen, signifying water former, since it had been
found to be a constituent of water.
Occurrence. In the free state hydrogen is found in the atmosphere, but
only in traces. In the combined state it is widely distributed, being a
constituent of water as well as of all living organisms, and the
products derived from them, such as starch and sugar. About 10% of the
human body is hydrogen. Combined with carbon, it forms the substances
which constitute petroleum and natural gas.
It is an interesting fact that while hydrogen in the free state
occurs only in traces on the earth, it occurs in enormous
quantities in the gaseous matter surrounding the sun and
certain other stars.
Preparation from water. Hydrogen can be prepared from water by several
methods, the most important of which are the following.
1. By the electric current. As has been indicated in the preparation
of oxygen, water is easily separated into its constituents, hydrogen and
oxygen, by passing an electric current through it under certain
conditions.
2. By the action of certain metals. When brought into contact with
certain metals under appropriate conditions,[Pg 29] water gives up a portion
or the whole of its hydrogen, its place being taken by the metal. In the
case of a few of the metals this change occurs at ordinary temperatures.
Thus, if a bit of sodium is thrown on water, an action is seen to take
place at once, sufficient heat being generated to melt the sodium, which
runs about on the surface of the water. The change which takes place
consists in the displacement of one half of the hydrogen of the water by
the sodium, and may be represented as follows:
_ _ _ _
| hydrogen | | sodium |
sodium + | hydrogen |(water) = | hydrogen |(sodium hydroxide) + hydrogen
|_oxygen _| |_oxygen _|
The sodium hydroxide formed is a white solid which remains dissolved in
the undecomposed water, and may be obtained by evaporating the solution
to dryness. The hydrogen is evolved as a gas and may be collected by
suitable apparatus.
Other metals, such as magnesium and iron, decompose water rapidly, but
only at higher temperatures. When steam is passed over hot iron, for
example, the iron combines with the oxygen of the steam, thus displacing
the hydrogen. Experiments show that the change may be represented as
follows:
_ _
| hydrogen | _ _ _ _
iron + | hydrogen |(water) = | iron |(iron oxide) + | hydrogen |
|_oxygen _| |_oxygen _| |_hydrogen_|
The iron oxide formed is a reddish-black compound, identical with that
obtained by the combustion of iron in oxygen.
Directions for preparing hydrogen by the action of steam on
iron. The apparatus used in the preparation of hydrogen from
iron and[Pg 30] steam is shown in Fig. 9. A porcelain or iron tube
B, about 50 cm. in length and 2 cm. or 3 cm. in diameter, is
partially filled with fine iron wire or tacks and connected as
shown in the figure. The tube B is heated, slowly at first,
until the iron is red-hot. Steam is then conducted through the
tube by boiling the water in the flask A. The hot iron
combines with the oxygen in the steam, setting free the
hydrogen, which is collected over water. The gas which first
passes over is mixed with the air previously contained in the
flask and tube, and is allowed to escape, since a mixture of
hydrogen with oxygen or air explodes violently when brought in
contact with a flame. It is evident that the flask A must be
disconnected from the tube before the heat is withdrawn.
That the gas obtained is different from air and oxygen may be
shown by holding a bottle of it mouth downward and bringing a
lighted splint into it. The hydrogen is ignited and burns with
an almost colorless flame.
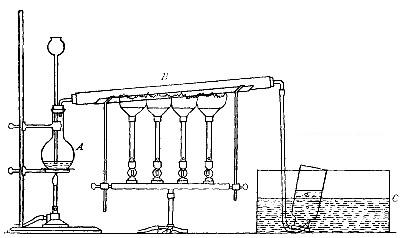 Fig. 9
Fig. 9
Preparation from acids (usual laboratory method). While hydrogen can
be prepared from water, either by the action of the electric current or
by the action of certain metals, these methods are not economical and
are therefore but little used. In the laboratory hydrogen is generally
prepared from compounds known as acids, all of which contain hydrogen.
When acids are brought in contact with certain metals, the metals
dissolve and set free the hydrogen[Pg 31] of the acid. Although this reaction
is a quite general one, it has been found most convenient in preparing
hydrogen by this method to use either zinc or iron as the metal and
either hydrochloric or sulphuric acid as the acid. Hydrochloric acid is
a compound consisting of 2.77% hydrogen and 97.23% chlorine, while
sulphuric acid consists of 2.05% hydrogen, 32.70% sulphur, and 65.25%
oxygen.
The changes which take place in the preparation of hydrogen from zinc
and sulphuric acid (diluted with water) may be represented as follows:
_ _ _ _
| hydrogen |(sulphuric | zinc |(zinc
zinc + | sulphur | acid) = | sulphur | sulphate) + hydrogen
|_oxygen _| |_oxygen _|
In other words, the zinc has taken the place of the hydrogen in
sulphuric acid. The resulting compound contains zinc, sulphur, and
oxygen, and is known as zinc sulphate. This remains dissolved in the
water present in the acid. It may be obtained in the form of a white
solid by evaporating the liquid left after the metal has passed into
solution.
When zinc and hydrochloric acid are used the following changes take
place:
_ _ _ _
| hydrogen |(hydrochloric | zinc |(zinc
zinc + |_chlorine_| acid) = |_chlorine_| chloride) + hydrogen
When iron is used the changes which take place are exactly similar to
those just given for zinc.
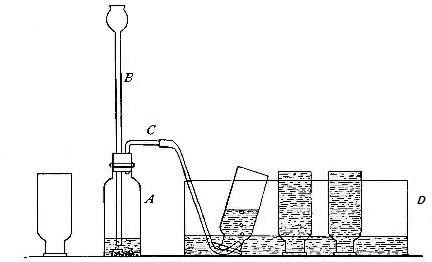 Fig. 10.
Fig. 10.
Directions for preparing hydrogen from acids. The preparation
of hydrogen from acids is carried out in the laboratory as
follows: The metal is placed in a flask or wide-mouthed bottle
A (Fig. 10) and the acid is added slowly through the funnel
tube B. The metal dissolves in the acid, while the hydrogen
which is liberated escapes through the exit tube C and is
collected over water. It is evident that the hydrogen[Pg 32] which
passes over first is mixed with the air from the bottle A.
Hence care must be taken not to bring a flame near the exit
tube, since, as has been stated previously, such a mixture
explodes with great violence when brought in contact with a
flame.
Precautions. Both sulphuric acid and zinc, if impure, are
likely to contain small amounts of arsenic. Such materials
should not be used in preparing hydrogen, since the arsenic
present combines with a portion of the hydrogen to form a very
poisonous gas known as arsine. On the other hand, chemically
pure sulphuric acid, i.e. sulphuric acid that is entirely free
from impurities, will not act upon chemically pure zinc. The
reaction may be started, however, by the addition of a few
drops of a solution of copper sulphate or platinum
tetrachloride.
Physical properties. Hydrogen is similar to oxygen in that it is a
colorless, tasteless, odorless gas. It is characterized by its extreme
lightness, being the lightest of all known substances. One liter of the
gas weighs only 0.08984 g. On comparing this weight with that of an
equal volume of oxygen, viz., 1.4285 g., the latter is found to be 15.88
times as heavy as hydrogen. Similarly, air is found to be 14.38 times as
heavy as hydrogen. Soap bubbles blown with hydrogen rapidly rise in the
air. On account of its lightness it is possible to pour it upward from
one bottle into another. Thus, if the bottle A (Fig. 11) is filled
with hydrogen, placed mouth downward by the side of bottle B,[Pg 33] filled
with air, and is then gradually inverted under B as indicated in the
figure, the hydrogen will flow upward into bottle B, displacing the
air. Its presence in bottle B may then be shown by bringing a lighted
splint to the mouth of the bottle, when the hydrogen will be ignited by
the flame. It is evident, from this experiment, that in order to retain
the gas in an open bottle the bottle must be placed mouth downward.
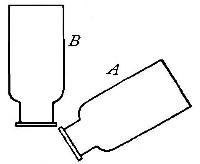 Fig. 11
Fig. 11
Hydrogen is far more difficult to liquefy than any other gas, with the
exception of helium, a rare element recently found to exist in the
atmosphere. The English scientist Dewar, however, in 1898 succeeded not
only in obtaining hydrogen in liquid state but also as a solid. Liquid
hydrogen is colorless and has a density of only 0.07. Its boiling point
under atmospheric pressure is -252°. Under diminished pressure the
temperature has been reduced to -262°. The solubility of hydrogen in
water is very slight, being still less than that of oxygen.
Pure hydrogen produces no injurious results when inhaled. Of course one
could not live in an atmosphere of the gas, since oxygen is essential to
respiration.
Chemical properties. At ordinary temperatures hydrogen is not an active
element. A mixture of hydrogen and chlorine, however, will combine with
explosive violence at ordinary temperature if exposed to the sunlight.
The union can be brought about also by heating. The product formed in
either case is hydrochloric acid. Under suitable conditions hydrogen
combines with nitrogen to form ammonia, and with sulphur to form the
foul-smelling gas, hydrogen sulphide. The affinity of hydrogen for
oxygen is so great that[Pg 34] a mixture of hydrogen and oxygen or hydrogen
and air explodes with great violence when heated to the kindling
temperature (about 612°). Nevertheless under proper conditions hydrogen
may be made to burn quietly in either oxygen or air. The resulting
hydrogen flame is almost colorless and is very hot. The combustion of
the hydrogen is, of course, due to its union with oxygen. The product of
the combustion is therefore a compound of hydrogen and oxygen. That this
compound is water may be shown easily by experiment.
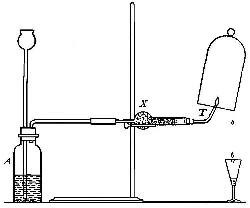 Fig. 12
Fig. 12
Directions for burning hydrogen in air. The combustion of
hydrogen in air may be carried out safely as follows: The
hydrogen is generated in the bottle A (Fig. 12), is dried by
conducting it through the tube X, filled with some substance
(generally calcium chloride) which has a great attraction for
moisture, and escapes through the tube T, the end of which is
drawn out to a jet. The hydrogen first liberated mixes with the
air contained in the generator. If a flame is brought near the
jet before this mixture has all escaped, a violent and very
dangerous explosion results, since the entire apparatus is
filled with the explosive mixture. On the other hand, if the
flame is not applied until all the air has been expelled, the
hydrogen is ignited and burns quietly, since only the small
amount of it which escapes from the jet can come in contact
with the oxygen of the air at any one time. By holding a cold,
dry bell jar or bottle over the flame, in the manner shown in
the figure, the steam formed by the combustion of the hydrogen
is condensed, the water collecting in drops on the sides of the
jar.
[Pg 35]
Precautions. In order to avoid danger it is absolutely necessary to
prove that the hydrogen is free from air before igniting it. This can be
done by testing small amounts of the escaping gas. A convenient and safe
method of doing this is to fill a test tube with the gas by inverting it
over the jet. The hydrogen, on account of its lightness, collects in the
tube, displacing the air. After holding it over the jet for a few
moments in order that it may be filled with the gas, the tube is gently
brought, mouth downward, to the flame of a burner placed not nearer than
an arm's length from the jet. If the hydrogen is mixed with air a slight
explosion occurs, but if pure it burns quietly in the tube. The
operation is repeated until the gas burns quietly, when the tube is
quickly brought back over the jet for an instant, whereby the escaping
hydrogen is ignited by the flame in the tube.
 . Fig. 13
. Fig. 13
A mixture of hydrogen and oxygen is explosive. That a mixture of
hydrogen and air is explosive may be shown safely as follows: A cork
through which passes a short glass tube about 1 cm. in diameter is
fitted air-tight into the tubule of a bell jar of 2 l. or 3 l. capacity.
(A thick glass bottle with bottom removed may be used.) The tube is
closed with a small rubber stopper and the bell jar filled with
hydrogen, the gas being collected over water. When entirely filled with
the gas the jar is removed from the water and supported by blocks of
wood in order to leave the bottom of the jar open, as shown in Fig. 13.
The stopper is now removed from the tube in the cork, and the hydrogen,
which on account of its lightness escapes from the tube, is at once
lighted. As the hydrogen escapes, the air flows in at the bottom of the
jar and mixes with the remaining portion of the hydrogen, so that a
mixture of the two soon forms, and a loud explosion results. The
explosion is not dangerous, since the bottom of the jar is open, thus
leaving room for the expansion of the hot gas.
Since air is only one fifth oxygen, the remainder being inert gases, it
may readily be inferred that a mixture of hydrogen with pure oxygen
would be far more explosive than a mixture of hydrogen with air. Such
mixtures should not be made except in small quantities and by
experienced workers.[Pg 36]
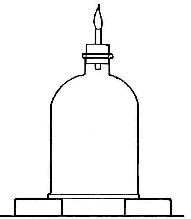
Hydrogen does not support combustion. While hydrogen is readily
combustible, it is not a supporter of combustion. In other words,
substances will not burn in it. This may be shown by bringing a lighted
candle supported by a stiff wire into a bottle or cylinder of the pure
gas, as shown in Fig. 14. The hydrogen is ignited by the flame of the
candle and burns at the mouth of the bottle, where it comes in contact
with the oxygen in the air. When the candle is thrust up into the gas,
its flame is extinguished on account of the absence of oxygen. If slowly
withdrawn, the candle is relighted as it passes through the layer of
burning hydrogen.
 Fig. 14
Fig. 14
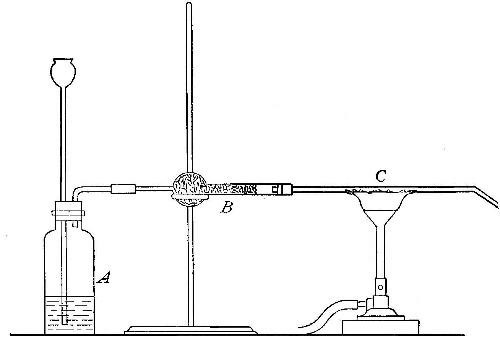 Fig. 15
Fig. 15
Reduction. On account of its great affinity for oxygen, hydrogen has the
power of abstracting it from many of its compounds. Thus, if a stream of
hydrogen, dried by passing through the tube B (Fig. 15), filled with[Pg 37]
calcium chloride, is conducted through the tube C containing some
copper oxide, heated to a moderate temperature, the hydrogen abstracts
the oxygen from the copper oxide. The change may be represented as
follows:
hydrogen + {copper} {hydrogen}
{oxygen}(copper oxide) = {oxygen }(water) + copper
The water formed collects in the cold portions of the tube C near its
end. In this experiment the copper oxide is said to undergo reduction.
Reduction may therefore be defined as the process of withdrawing oxygen
from a compound.
Relation of reduction to oxidation. At the same time that the copper
oxide is reduced it is clear that the hydrogen is oxidized, for it
combines with the oxygen given up by the copper oxide. The two processes
are therefore very closely related, and it usually happens that when one
substance is oxidized some other substance is reduced. That substance
which gives up its oxygen is called an oxidizing agent, while the
substance which unites with the oxygen is called a reducing agent.
The oxyhydrogen blowpipe. This is a form of apparatus used for burning
hydrogen in pure oxygen. As has been previously stated, the flame
produced by the combustion of hydrogen in the air is very hot. It is
evident that if pure oxygen is substituted for air, the temperature
reached will be much higher, since there are no inert gases to absorb
the heat. The oxyhydrogen blowpipe, used to effect this combination,
consists of a small tube placed within a larger one, as shown in Fig.
16.
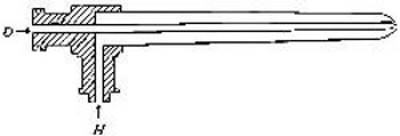 Fig. 16
Fig. 16
[Pg 38]
The hydrogen, stored under pressure, generally in steel cylinders, is
first passed through the outer tube and ignited at the open end of the
tube. The oxygen from a similar cylinder is then conducted through the
inner tube, and mixes with the hydrogen at the end of the tube. In order
to produce the maximum heat, the hydrogen and oxygen must be admitted to
the blowpipe in the exact proportion in which they combine, viz., 2
volumes of hydrogen to 1 of oxygen, or by weight, 1 part of hydrogen to
7.94 parts of oxygen. The intensity of the heat may be shown by bringing
into the flame pieces of metal such as iron wire or zinc. These burn
with great brilliancy. Even platinum, having a melting point of 1779°,
may be melted by the heat of the flame.
While the oxyhydrogen flame is intensely hot, it is almost non-luminous.
If directed against some infusible substance like ordinary lime (calcium
oxide), the heat is so intense that the lime becomes incandescent and
glows with a brilliant light. This is sometimes used as a source of
light, under the name of Drummond or lime light.
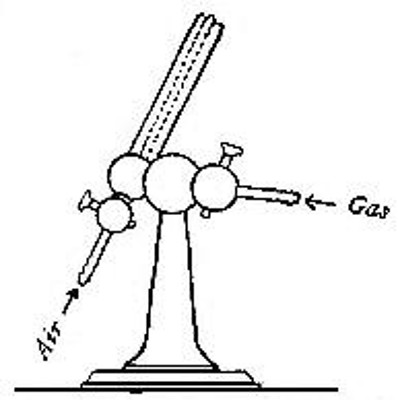 Fig. 17
Fig. 17
The blast lamp. A similar form of apparatus is commonly used in the
laboratory as a source of heat under the name blast lamp (Fig. 17).
This differs from the oxyhydrogen blowpipe only in the size of the
tubes. In place of the hydrogen and oxygen the more accessible coal gas
and air are respectively used. The former is composed largely of a
mixture of free hydrogen and gaseous compounds of carbon and hydrogen.
While the temperature of the flame is not so high as that of the
oxyhydrogen blowpipe, it nevertheless suffices for most chemical
operations carried out in the laboratory.
Uses of hydrogen. On account of its cost, hydrogen is but little used
for commercial purposes. It is sometimes used as a material for the
inflation of balloons, but usually the much cheaper coal gas is
substituted for it. Even hot air is often used when the duration of
ascension is very short. It has been used also as a source of heat and
light[Pg 39] in the oxyhydrogen blowpipe. Where the electric current is
available, however, this form of apparatus has been displaced almost
entirely by the electric light and electric furnace, which are much more
economical and more powerful sources of light and heat.
EXERCISES
1. Will a definite weight of iron decompose an unlimited weight of
steam?
2. Why is oxygen passed through the inner tube of the oxyhydrogen
blowpipe rather than the outer?
3. In Fig. 14, will the flame remain at the mouth of the tube?
4. From Fig. 15, suggest a way for determining experimentally the
quantity of water formed in the reaction.
5. Distinguish clearly between the following terms: oxidation,
reduction, combustion, and kindling temperature.
6. Is oxidation always accompanied by reduction?
7. What is the source of heat in the lime light? What is the exact use
of lime in this instrument?
8. In Fig. 12, why is it necessary to dry the hydrogen by means of the
calcium chloride in the tube X?
9. At what pressure would the weight of 1 l. of hydrogen be equal to
that of oxygen under standard conditions?
10. (a) What weight of hydrogen can be obtained from 150 g. of
sulphuric acid? (b) What volume would this occupy under standard
conditions? (c) The density of sulphuric acid is 1.84. What volume
would the 150 g. of the acid occupy?
11. How many liters of hydrogen can be obtained from 50 cc. of sulphuric
acid having a density of 1.84?
12. Suppose you wish to fill five liter bottles with hydrogen, the gas
to be collected over water in your laboratory, how many cubic
centimeters of sulphuric acid would be required?
[Pg 40]
CHAPTER IV
COMPOUNDS OF HYDROGEN AND OXYGEN; WATER AND HYDROGEN DIOXIDE
WATER
Historical. Water was long regarded as an element. In 1781 Cavendish
showed that it is formed by the union of hydrogen and oxygen. Being a
believer in the phlogiston theory, however, he failed to interpret his
results correctly. A few years later Lavoisier repeated Cavendish's
experiments and showed that water must be regarded as a compound of
hydrogen and oxygen.
General methods employed for the determination of the composition of a
compound. The composition of a compound may be determined by either of
two general processes these are known as analysis and synthesis.
1. Analysis is the process of decomposing a compound into its
constituents and determining what these constituents are. The analysis
is qualitative when it results in merely determining what elements
compose the compound; it is quantitative when the exact percentage of
each constituent is determined. Qualitative analysis must therefore
precede quantitative analysis, for it must be known what elements, are
in a compound before a method can be devised for determining exactly how
much of each is present.
2. Synthesis is the process of forming a compound from its constituent
parts. It is therefore the reverse of analysis. Like analysis, it may be
either qualitative or quantitative.[Pg 41]
Application of these methods to the determination of the composition of
water. The determination of the composition of water is a matter of
great interest not only because of the importance of the compound but
also because the methods employed illustrate the general methods of
analysis and synthesis.
Methods based on analysis. The methods based on analysis may be either
qualitative or quantitative in character.
 Fig. 18
Fig. 18
1. Qualitative analysis. As was stated in the study of oxygen, water
may be separated into its component parts by means of the electric
current. The form of apparatus ordinarily used for effecting this
analysis is shown in Fig. 18. A platinum wire, to the end of which is
attached a small piece of platinum foil (about 15 mm. by 25 mm.), is
fused through each of the tubes B and D, as shown in the figure. The
stopcocks at the ends of these tubes are opened and water, to which has
been added about one tenth of its volume of sulphuric acid, is poured
into the tube A until the side tubes B and D are completely
filled. The stopcocks are then closed. The platinum wires extending into
the tubes B and D are now connected with the wires leading from two
or three dichromate cells joined in series. The pieces of platinum foil
within the tubes thus become the electrodes, and the current flows from
one to the other through the acidulated water. As soon as the current
passes, bubbles of gas rise from each of the electrodes and collect in
the upper part of the tubes. The gas[Pg 42] rising from the negative electrode
is found to be hydrogen, while that from the positive electrode is
oxygen. It will be seen that the volume of the hydrogen is approximately
double that of the oxygen. Oxygen is more soluble in water than
hydrogen, and a very little of it is also lost by being converted into
ozone and other substances. It has been found that when the necessary
corrections are made for the error due to these facts, the volume of the
hydrogen is exactly double that of the oxygen.
Fig. 19 illustrates a simpler form of apparatus, which may be used in
place of that shown in Fig. 18. A glass or porcelain dish is partially
filled with water to which has been added the proper amount of acid. Two
tubes filled with the same liquid are inverted over the electrodes. The
gases resulting from the decomposition of the water collect in the
tubes.
 Fig. 19
Fig. 19
2. Quantitative analysis. The analysis just described is purely
qualitative and simply shows that water contains hydrogen and oxygen. It
does not prove the absence of other elements; indeed it does not prove
that the hydrogen and oxygen are present in the proportion in which they
are liberated by the electric current. The method may be made
quantitative, however, by weighing the water decomposed and also the
hydrogen and oxygen obtained in its decomposition. If the combined
weights of the hydrogen and oxygen exactly equal the weight of the water
decomposed, then it would[Pg 43] be proved that the water consists of hydrogen
and oxygen in the proportion in which they are liberated by the electric
current. This experiment is difficult to carry out, however, so that the
more accurate methods based on synthesis are used.
Methods based on synthesis. Two steps are necessary to ascertain the
exact composition of water by synthesis: (1) to show by qualitative
synthesis that water is formed by the union of oxygen with hydrogen; (2)
to determine by quantitative synthesis in what proportion the two
elements unite to form water. The fact that water is formed by the
combination of oxygen with hydrogen was proved in the preceding chapter.
The quantitative synthesis may be made as follows:
 Fig. 20
Fig. 20
The combination of the two gases is brought about in a tube called a
eudiometer. This is a graduated tube about 60 cm. long and 2 cm. wide,
closed at one end (Fig. 20). Near the closed end two platinum wires are
fused through the glass, the ends of the wires within the tube being
separated by a space of 2 mm or 3 mm. The tube is entirely filled with
mercury and inverted in a vessel of the same liquid. Pure hydrogen is
passed into the tube until it is about one fourth filled. The volume of
the gas is then read off on the scale and reduced to standard
conditions. Approximately an equal volume of pure oxygen is then
introduced and the volume again read off and reduced to standard
conditions. This gives the total volume of the two gases. From this the
volume of the oxygen introduced may be determined by[Pg 44] subtracting from
it the volume of the hydrogen. The combination of the two gases is now
brought about by connecting the two platinum wires with an induction
coil and passing a spark from one wire to the other. Immediately a
slight explosion occurs. The mercury in the tube is at first depressed
because of the expansion of the gases due to the heat generated, but at
once rebounds, taking the place of the gases which have combined to form
water. The volume of the water in the liquid state is so small that it
may be disregarded in the calculations. In order that the temperature of
the residual gas and the mercury may become uniform, the apparatus is
allowed to stand for a few minutes. The volume of the gas is then read
off and reduced to standard conditions, so that it may be compared with
the volumes of the hydrogen and oxygen originally taken. The residual
gas is then tested in order to ascertain whether it is hydrogen or
oxygen, experiments having proved that it is never a mixture of the two.
From the information thus obtained the composition of the water may be
calculated. Thus, suppose the readings were as follows:
| Volume of hydrogen taken | 20.3 cc. |
| Volume of hydrogen and oxygen | 38.7 |
| Volume of oxygen | 18.4 |
| Volume of gas left after combination has taken place (oxygen) | 8.3 |
The 20.3 cc. of hydrogen have combined with 18.4 cc. minus 8.3 cc. (or
10.1 cc.) of oxygen; or approximately 2 volumes of hydrogen have
combined with 1 of oxygen. Since oxygen is 15.88 times as heavy as
hydrogen, the proportion by weight in which the two gases combine is 1
part of hydrogen to 7.94 of oxygen.[Pg 45]
Precaution. If the two gases are introduced into the eudiometer in the
exact proportions in which they combine, after the combination has taken
place the liquid will rise and completely fill the tube. Under these
conditions, however, the tube is very likely to be broken by the sudden
upward rush of the liquid. Hence in performing the experiment care is
taken to introduce an excess of one of the gases.
A more convenient form of eudiometer. A form of eudiometer (Fig. 21)
different from that shown on page 43 is sometimes used to avoid the
calculations necessary in reducing the volumes of the gases to the same
conditions of temperature and pressure in order to make comparisons.
With this apparatus it is possible to take the readings of the volumes
under the same conditions of temperature and pressure, and thus compare
them directly. The apparatus (Fig. 21) is filled with mercury and the
gases introduced into the tube A. The experiment is carried out as in
the preceding one, except that before taking the reading of the gas
volumes, mercury is either added to the tube B or withdrawn from it by
means of the stopcock C, until it stands at exactly the same height in
both tubes. The gas inclosed in tube A is then under atmospheric
pressure; and since but a few minutes are required for performing the
experiment, the conditions of temperature and pressure may be regarded
as constant. Hence the volumes of the hydrogen and oxygen and of the
residual gas may be read off from the tube and directly compared.
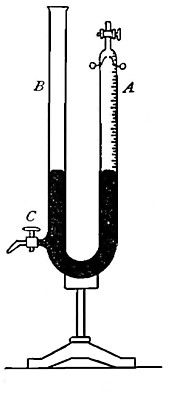 Fig. 21
Fig. 21
Method used by Berzelius and Dumas. The method used by these
investigators enables us to determine directly the proportion by weight
in which the hydrogen and oxygen combine. Fig. 22 illustrates the
apparatus used in making this determination. B is a glass tube
containing copper oxide. C and D are glass tubes filled with calcium
chloride, a substance which has great affinity for water.[Pg 46] The tubes B
and C, including their contents, are carefully weighed, and the
apparatus connected as shown in the figure. A slow current of pure
hydrogen is then passed through A, and that part of the tube B which
contains copper oxide is carefully heated. The hydrogen combines with
the oxygen present in the copper oxide to form water, which is absorbed
by the calcium chloride in tube C. The calcium chloride in tube D
prevents any moisture entering tube C from the air. The operation is
continued until an appreciable amount of water has been formed. The
tubes B and C are then weighed once more. The loss of weight in the
tube B will exactly equal the weight of oxygen taken up from the
copper oxide in the formation of the water. The gain in weight in the
tube C will exactly equal the weight of the water formed. The
difference in these weights will of course equal the weight of the
hydrogen present in the water formed.
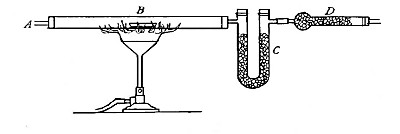 Fig. 22
Fig. 22
Dumas' results. The above method for the determination of the
composition of water was first used by Berzelius in 1820. The work was
repeated in 1843 by Dumas, the average of whose results is as follows:
| Weight of water formed | 236.36 g. |
| Oxygen given up by the copper oxide | 210.04 |
| ——— |
| Weight of hydrogen present in water | 26.32 |
[Pg 47]
According to this experiment the ratio of hydrogen to oxygen in water is
therefore 26.32 to 210.04, or as l to 7.98
Morley's results. The American chemist Morley has recently determined
the composition of water, extreme precautions being taken to use pure
materials and to eliminate all sources of error. The hydrogen and oxygen
which combined, as well as the water formed, were all accurately
weighed. According to Morley's results, 1 part of hydrogen by weight
combines with 7.94 parts of oxygen to form water.
Comparison of results obtained. From the above discussions it is easy to
see that it is by experiment alone that the composition of a compound
can be determined. Different methods may lead to slightly different
results. The more accurate the method chosen and the greater the skill
with which the experiment is carried out, the more accurate will be the
results. It is generally conceded by chemists that the results obtained
by Morley in reference to the composition of water are the most accurate
ones. In accordance with these results, then, water must be regarded as
a compound containing hydrogen and oxygen in the proportion of 1 part by
weight of hydrogen to 7.94 parts by weight of oxygen.
Relation between the volume of aqueous vapor and the volumes of the
hydrogen and oxygen which combine to form it. When the quantitative
synthesis of water is carried out in the eudiometer as described above,
the water vapor formed by the union of the hydrogen and oxygen at once
condenses. The volume of the resulting liquid is so small that it may be
disregarded in making the calculations. If, however, the experiment is
carried out at a temperature of 100° or above, the water-vapor formed is
not condensed and it thus becomes possible to compare the volume of the[Pg 48]
vapor with the volumes of hydrogen and oxygen which combined to form it.
This can be accomplished by surrounding the arm A of the eudiometer
(Fig. 23) with the tube B through which is passed the vapor obtained
by boiling some liquid which has a boiling point above 100°. In this way
it has been proved that 2 volumes of hydrogen and 1 volume of oxygen
combine to form exactly 2 volumes of water vapor, the volumes all being
measured under the same conditions of temperature and pressure. It will
be noted that the relation between these volumes may be expressed by
whole numbers. The significance of this very important fact will be
discussed in a subsequent chapter.
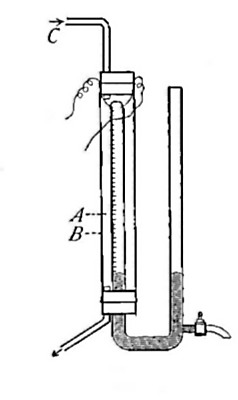 Fig. 23
Fig. 23
Occurrence of water. Water not only covers about three fourths of the
surface of the earth, and is present in the atmosphere in the form of
moisture, but it is also a common constituent of the soil and rocks and
of almost every form of animal and vegetable organism. The human body is
nearly 70% water. This is derived not only from the water which we drink
but also from the food which we eat, most of which contains a large
percentage of water. Thus potatoes contain about 78% of water, milk 85%,
beef over 50%, apples 84%, tomatoes 94%.
Impurities in water. Chemically pure water contains only hydrogen and
oxygen. Such a water never occurs in nature, however, for being a good
solvent, it takes up certain substances from the rocks and soil with
which it comes in contact. When such waters are evaporated these[Pg 49]
substances are deposited in the form of a residue. Even rain water,
which is the purest form occurring in nature, contains dust particles
and gases dissolved from the atmosphere. The foreign matter in water is
of two kinds, namely, mineral, such as common salt and limestone, and
organic, that is the products of animal and vegetable life.
Mineral matter in water. The amount and nature of the mineral
matter present in different waters vary greatly, depending on
the character of the rocks and soil with which the waters come
in contact. The more common of the substances present are
common salt and compounds of calcium, magnesium, and iron. One
liter of the average river water contains about 175 mg. of
mineral matter. Water from deep wells naturally contains more
mineral matter than river water, generally two or three times
as much, while sea water contains as much as 35,000 mg. to the
liter.
Effect of impurities on health. The mineral matter in water does not,
save in very exceptional cases, render the water injurious to the human
system. In fact the presence of a certain amount of such matter is
advantageous, supplying the mineral constituents necessary for the
formation of the solid tissues of the body. The presence of organic
matter, on the other hand, must always be regarded with suspicion. This
organic matter may consist not only of the products of animal and
vegetable life but also of certain microscopic forms of living organisms
which are likely to accompany such products. Contagious diseases are
known to be due to the presence in the body of minute living organisms
or germs. Each disease is caused by its own particular kind of germ.
Through sewage these germs may find their way from persons afflicted
with disease into the water supply, and it is principally through the
drinking water that certain of these diseases, especially typhoid fever,
are spread. It becomes of great importance, therefore, to be[Pg 50] able to
detect such matter when present in drinking water as well as to devise
methods whereby it can be removed or at least rendered harmless.
Analysis of water. The mineral analysis of a water is, as the
name suggests, simply the determination of the mineral matter
present. Sanitary analysis, on the other hand, is the
determination of the organic matter present. The physical
properties of a water give no conclusive evidence as to its
purity, since a water may be unfit for drinking purposes and
yet be perfectly clear and odorless. Neither can any reliance
be placed on the simple methods often given for testing the
purity of water. Only the trained chemist can carry out such
methods of analysis as can be relied upon.
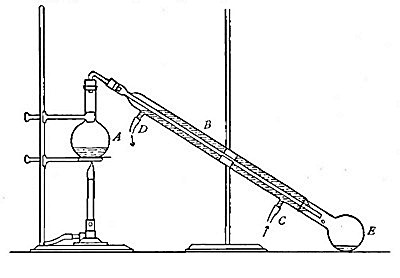 Fig. 24
Fig. 24
Purification of water. Three general methods are used for the
purification of water, namely, distillation, filtration, and
boiling.
1. Distillation. The most effective way of purifying natural waters is
by the process of distillation. This consists in boiling the water and
condensing the steam. Fig. 24 illustrates the process of distillation,
as commonly conducted[Pg 51] in the laboratory. Ordinary water is poured into
the flask A and boiled. The steam is conducted through the condenser
B, which consists essentially of a narrow glass tube sealed within a
larger one, the space between the two being filled with cold water,
which is admitted at C and escapes at D. The inner tube is thus kept
cool and the steam in passing through it is condensed. The water formed
by the condensation of the steam collects in the receiver E and is
known as distilled water. Such water is practically pure, since the
impurities are nonvolatile and remain in the flask A.
Commercial distillation. In preparing distilled water on a
large scale, the steam is generated in a boiler or other metal
container and condensed by passing it through a pipe made of
metal, generally tin. This pipe is wound into a spiral and is
surrounded by a current of cold water. Distilled water is used
by the chemist in almost all of his work. It is also used in
the manufacture of artificial ice and for drinking water.
Fractional distillation. In preparing distilled water, it is
evident that if the natural water contains some substance which
is volatile its vapor will pass over and be condensed with the
steam, so that the distillate will not be pure water. Even such
mixtures, however, may generally be separated by repeated
distillation. Thus, if a mixture of water (boiling point 100°)
and alcohol (boiling point 78°) is distilled, the alcohol,
having the lower boiling point, tends to distill first,
followed by the water. The separation of the two is not
perfect, however, but may be made nearly so by repeated
distillations. The process of separating a mixture of volatile
substances by distillation is known as fractional
distillation.
2. Filtration. The process of distillation practically removes all
nonvolatile foreign matter, mineral as well as organic. In purifying
water for drinking purposes, however, it is only necessary to eliminate
the latter or to render it harmless. This is ordinarily done either by
filtration or[Pg 52] boiling. In filtration the water is passed through some
medium which will retain the organic matter. Ordinary charcoal is a
porous substance and will condense within its pores the organic matter
in water if brought in contact with it. It is therefore well adapted to
the construction of filters. Such filters to be effective must be kept
clean, since it is evident that the charcoal is useless after its pores
are filled. A more effective type of filter is the Chamberlain-Pasteur
filter. In this the water is forced through a porous cylindrical cup,
the pores being so minute as to strain out the organic matter.
City filtration beds. For purifying the water supply of cities,
large filtration beds are prepared from sand and gravel, and
the water is allowed to filter through these. Some of the
impurities are strained out by the filter, while others are
decomposed by the action of certain kinds of bacteria present
in the sand. Fig. 25 shows a cross section of a portion of the
filter used in purifying the water supply of Philadelphia. The
water filters through the sand and gravel and passes into the
porous pipe A, from which it is pumped into the city mains.
The filters are covered to prevent the water from freezing in
cold weather.
 Fig. 25
Fig. 25
3. Boiling. A simpler and equally efficient method for purifying water
for drinking purposes consists in boiling the water. It is the germs in
water that render it dangerous to health. These germs are living forms
of matter. If the[Pg 53] water is boiled, the germs are killed and the water
rendered safe. While these germs are destroyed by heat, cold has little
effect upon them. Thus Dewar, in working with liquid hydrogen, exposed
some of these minute forms of life to the temperature of boiling
hydrogen (-252°) without killing them.
Self-purification of water. It has long been known that water
contaminated with organic matter tends to purify itself when exposed to
the air. This is due to the fact that the water takes up a small amount
of oxygen from the air, which gradually oxidizes the organic matter
present in the water. While water is undoubtedly purified in this way,
the method cannot be relied upon to purify a contaminated water so as to
render it safe for drinking purposes.
Physical properties. Pure water is an odorless and tasteless liquid,
colorless in thin layers, but having a bluish tinge when observed
through a considerable thickness. It solidifies at 0° and boils at 100°
under the normal pressure of one atmosphere. If the pressure is
increased, the boiling point is raised. When water is cooled it steadily
contracts until the temperature of 4° is reached: it then expands. Water
is remarkable for its ability to dissolve other substances, and is the
best solvent known. Solutions of solids in water are more frequently
employed in chemical work than are the solid substances, for chemical
action between substances goes on more readily when they are in solution
than it does when they are in the solid state.
Chemical properties. Water is a very stable substance, or, in other
words, it does not undergo decomposition readily. To decompose it into
its elements by heat alone requires a very high temperature; at 2500°,
for example, only about 5% of the entire amount is decomposed. Though
very[Pg 54] stable towards heat, water can be decomposed in other ways, as by
the action of the electrical current or by certain metals.
Heat of formation and heat of decomposition are equal. The fact
that a very high temperature is necessary to decompose water
into hydrogen and oxygen is in accord with the fact that a
great deal of heat is evolved by the union of hydrogen and
oxygen; for it has been proved that the heat necessary to
decompose a compound into its elements (heat of decomposition)
is equal to the heat evolved in the formation of a compound
from its elements (heat of formation).
Water of crystallization. When a solid is dissolved in water and the
resulting solution is allowed to evaporate, the solid separates out,
often in the form of crystals. It has been found that the crystals of
many compounds, although perfectly dry, give up a definite amount of
water when heated, the substance at the same time losing its crystalline
form. Such water is called water of crystallization. This varies in
amount with different compounds, but is perfectly definite for the same
compound. Thus, if a perfectly dry crystal of copper sulphate is
strongly heated in a tube, water is evolved and condenses on the sides
of the tube, the crystal crumbling to a light powder. The weight of the
water evolved is always equal to exactly 36.07% of the weight of copper
sulphate crystals heated. The water must therefore be in chemical
combination with the substance composing the crystal; for if simply
mixed with it or adhering to it, not only would the substance appear
moist but the amount present would undoubtedly vary. The combination,
however, must be a very weak one, since the water is often expelled by
even a gentle heat. Indeed, in some cases the water is given up on
simple exposure to air. Such compounds are said to be efflorescent.
Thus a crystal of sodium sulphate[Pg 55] (Glauber's salt) on exposure to air
crumbles to a fine powder, owing to the escape of its water of
crystallization. Other substances have just the opposite property: they
absorb moisture when exposed to the air. For example, if a bit of dry
calcium chloride is placed in moist air, in the course of a few hours it
will have absorbed sufficient moisture to dissolve it. Such substances
are said to be deliquescent. A deliquescent body serves as a good
drying or desiccating agent. We have already employed calcium chloride
as an agent for absorbing the moisture from hydrogen. Many substances,
as for example quartz, form crystals which contain no water of
crystallization.
Mechanically inclosed water. Water of crystallization must be
carefully distinguished from water which is mechanically
inclosed in a crystal and which can be removed by powdering the
crystal and drying. Thus, when crystals of common salt are
heated, the water inclosed in the crystal is changed into steam
and bursts the crystal with a crackling sound. Such crystals
are said to decrepitate. That this water is not combined is
proved by the fact that the amount present varies and that it
has all the properties of water.
Uses of water. The importance of water in its relation to life and
commerce is too well known to require comment. Its importance to the
chemist has also been pointed out. It remains to call attention to the
fact that it is used as a standard in many physical measurements. Thus
0° and 100° on the centigrade scale are respectively the freezing and
the boiling points of water under normal pressure. The weight of 1 cc.
of water at its point of greatest density is the unit of weight in the
metric system, namely, the gram. It is also taken as the unit for the
determination of the density of liquids and solids as well as for the
measurement of amounts of heat.[Pg 56]
HYDROGEN DIOXIDE
Composition. As has been shown, 1 part by weight of hydrogen combines
with 7.94 parts by weight of oxygen to form water. It is possible,
however, to obtain a second compound of hydrogen and oxygen differing
from water in composition in that 1 part by weight of hydrogen is
combined with 2 × 7.94, or 15.88 parts, of oxygen. This compound is
called hydrogen dioxide or hydrogen peroxide, the prefixes di- and
per- signifying that it contains more oxygen than hydrogen oxide,
which is the chemical name for water.
Preparation. Hydrogen dioxide cannot be prepared cheaply by the direct
union of hydrogen and oxygen, and indirect methods must therefore be
used. It is commonly prepared by the action of a solution of sulphuric
acid on barium dioxide. The change which takes place may be indicated as
follows:
| sulphuric acid | + | barium dioxide | = | barium sulphate | + | hydrogen dioxide |
| ——————— | | ——————— | | ——————— | | ——————— |
| hydrogen | | barium | | barium | | hydrogen |
| sulphur | | oxygen | | sulphur | | oxygen |
| oxygen | | oxygen |
In other words, the barium and hydrogen in the two compounds exchange
places. By this method a dilute solution of the dioxide in water is
obtained. It is possible to separate the dioxide from the water by
fractional distillation. This is attended with great difficulties,
however, since the pure dioxide is explosive. The distillation is
carried on under diminished pressure so as to lower the boiling points
as much as possible; otherwise the high temperature would decompose the
dioxide.[Pg 57]
Properties. Pure hydrogen dioxide is a colorless sirupy liquid having a
density of 1.49. Its most characteristic property is the ease with which
it decomposes into water and oxygen. One part by weight of hydrogen is
capable of holding firmly only 7.94 parts of oxygen. The additional 7.94
parts of oxygen present in hydrogen dioxide are therefore easily
evolved, the compound breaking down into water and oxygen. This
decomposition is attended by the generation of considerable heat. In
dilute solution hydrogen dioxide is fairly stable, although such a
solution should be kept in a dark, cool place, since both heat and light
aid in the decomposition of the dioxide.
Uses. Solutions of hydrogen dioxide are used largely as oxidizing
agents. The solution sold by druggists contains 3% of the dioxide and is
used in medicine as an antiseptic. Its use as an antiseptic depends upon
its oxidizing properties.
EXERCISES
1. Why does the chemist use distilled water in making solutions, rather
than filtered water?
2. How could you determine the total amount of solid matter dissolved in
a sample of water?
3. How could you determine whether a given sample of water is distilled
water?
4. How could the presence of air dissolved in water be detected?
5. How could the amount of water in a food such as bread or potato be
determined?
6. Would ice frozen from impure water necessarily be free from disease
germs?
7. Suppose that the maximum density of water were at 0° in place of 4°;
what effect would this have on the formation of ice on bodies of water?
8. Is it possible for a substance to contain both mechanically inclosed
water and water of crystallization?[Pg 58]
9. If steam is heated to 2000° and again cooled, has any chemical change
taken place in the steam?
10. Why is cold water passed into C instead of D (Fig. 24)?
11. Mention at least two advantages that a metal condenser has over a
glass condenser.
12. Draw a diagram of the apparatus used in your laboratory for
supplying distilled water.
13. 20 cc. of hydrogen and 7 cc. of oxygen are placed in a eudiometer
and the mixture exploded. (a) How many cubic centimeters of aqueous
vapor are formed? (b) What gas and how much of it remains in excess?
14. (a) What weight of water can be formed by the combustion of 100 L
of hydrogen, measured under standard conditions? (b)What volume of
oxygen would be required in (a)? (c)What weight of potassium
chlorate is necessary to prepare this amount of oxygen?
15. What weight of oxygen is present in 1 kg. of the ordinary hydrogen
dioxide solution? In the decomposition of this weight of the dioxide
into water and oxygen, what volume of oxygen (measured under standard
conditions) is evolved?
[Pg 59]
CHAPTER V
THE ATOMIC THEORY
Three fundamental laws of matter. Before we can gain any very definite
idea in regard to the structure of matter, and the way in which
different kinds of substances act chemically upon each other, it is
necessary to have clearly in view three fundamental laws of matter.
These laws have been established by experiment, and any conception which
may be formed concerning matter must therefore be in harmony with them.
The laws are as follows:
Law of conservation of matter. This law has already been touched upon in
the introductory chapter, and needs no further discussion. It will be
recalled that it may be stated thus: Matter can neither be created nor
destroyed, though it can be changed from one form into another.
Law of definite composition. In the earlier days of chemistry there was
much discussion as to whether the composition of a given compound is
always precisely the same or whether it is subject to some variation.
Two Frenchmen, Berthollet and Proust, were the leaders in this
discussion, and a great deal of most useful experimenting was done to
decide the question. Their experiments, as well as all succeeding ones,
have shown that the composition of a pure chemical compound is always
exactly the same. Water obtained by melting pure ice, condensing steam,
burning hydrogen in oxygen, has always 11.18% hydrogen and 88.82% oxygen
in it. Red oxide of mercury, from whatever source it is obtained,
contains 92.6%[Pg 60] mercury and 7.4% oxygen. This truth is known as the law
of definite composition, and may be stated thus: The composition of a
chemical compound never varies.
Law of multiple proportion. It has already been noted, however, that
hydrogen and oxygen combine in two different ratios to form water and
hydrogen dioxide respectively. It will be observed that this fact does
not contradict the law of definite composition, for entirely different
substances are formed. These compounds differ from each other in
composition, but the composition of each one is always constant. This
ability of two elements to unite in more than one ratio is very
frequently observed. Carbon and oxygen combine in two different ratios;
nitrogen and oxygen combine to form as many as five distinct compounds,
each with its own precise composition.
In the first decade of the last century John Dalton, an English
school-teacher and philosopher, endeavored to find some rule which holds
between the ratios in which two given substances combine. His studies
brought to light a very simple relation, which the following examples
will make clear. In water the hydrogen and oxygen are combined in the
ratio of 1 part by weight of hydrogen to 7.94 parts by weight of oxygen.
In hydrogen dioxide the 1 part by weight of hydrogen is combined with
15.88 parts by weight of oxygen. The ratio between the amounts of oxygen
which combine with the same amount of hydrogen to form water and
hydrogen dioxide respectively is therefore 7.94: 15.88, or 1: 2.
 JOHN DALTON (English) (1766-1844)
JOHN DALTON (English) (1766-1844)
Developed the atomic theory; made many studies on the properties and the
composition of gases. His book entitled "A New System of Chemical
Philosophy" had a large influence on the development of chemistry
[Pg 61]
Similarly, the element iron combines with oxygen to form two oxides, one
of which is black and the other red. By analysis it has been shown that
the former contains 1 part by weight of iron combined with 0.286 parts
by weight of oxygen, while the latter contains 1 part by weight of iron
combined with 0.429 parts by weight of oxygen. Here again we find that
the amounts of oxygen which combine with the same fixed amount of iron
to form the two compounds are in the ratio of small whole numbers, viz.,
2:3.
Many other examples of this simple relation might be given, since it has
been found to hold true in all cases where more than one compound is,
formed from the same elements. Dalton's law of multiple proportion
states these facts as follows: When any two elements, A and B,
combine to form more than one compound, the amounts of B which unite
with any fixed amount of A bear the ratio of small whole numbers to
each other.
Hypothesis necessary to explain the laws of matter. These three
generalizations are called laws, because they express in concise
language truths which are found by careful experiment to hold good in
all cases. They do not offer any explanation of the facts, but merely
state them. The human mind, however, does not rest content with the mere
bare facts, but seeks ever to learn the explanation of the facts. A
suggestion which is offered to explain such a set of facts is called an
hypothesis. The suggestion which Dalton offered to explain the three
laws of matter, called the atomic hypothesis, was prompted by his view
of the constitution of matter, and it involves three distinct
assumptions in regard to the nature of matter and chemical action.
Dalton could not prove these assumptions to be true, but he saw that if
they were true the laws of matter become very easy to understand.
Dalton's atomic hypothesis. The three assumptions which Dalton made in
regard to the nature of matter, and which together constitute the atomic
hypothesis, are these:[Pg 62]
1. All elements are made up of minute, independent particles which
Dalton designated as atoms.
2. All atoms of the same element have equal masses; those of different
elements have different masses; in any change to which an atom is
subjected its mass does not change.
3. When two or more elements unite to form a compound, the action
consists in the union of a definite small number of atoms of each
element to form a small particle of the compound. The smallest particles
of a given compound are therefore exactly alike in the number and kinds
of atoms which they contain, and larger masses of the substances are
simply aggregations of these least particles.
Molecules and atoms. Dalton applied the name atom not only to the minute
particles of the elements but also to the least particles of compounds.
Later Avogadro, an Italian scientist, pointed out the fact that the two
are different, since the smallest particle of an element is a unit,
while that of a compound must have at least two units in it. He
suggested the name molecule for the least particle of a compound which
can exist, retaining the name atom for the smallest particle of an
element. In accordance with this distinction, we may define the atom and
the molecule as follows: An atom is the smallest particle of an element
which can exist. A molecule is the smallest particle of a compound which
can exist. It will be shown in a subsequent chapter that sometimes two
or more atoms of the same element unite with each other to form
molecules of the element. While the term atom, therefore, is applicable
only to elements, the term molecule is applicable both to elements and
compounds.[Pg 63]
The atomic hypothesis and the laws of matter. Supposing the atomic
hypothesis to be true, let us now see if it is in harmony with the laws
of matter.
1. The atomic hypothesis and the law of conservation of matter. It is
evident that if the atoms never change their masses in any change which
they undergo, the total quantity of matter can never change and the law
of conservation of matter must follow.
2. The atomic hypothesis and the law of definite composition.
According to the third supposition, when iron combines with sulphur the
union is between definite numbers of the two kinds of atoms. In the
simplest case one atom of the one element combines with one atom of the
other. If the sulphur and the iron atoms never change their respective
masses when they unite to form a molecule of iron sulphide, all iron
sulphide molecules will have equal amounts of iron in them and also of
sulphur. Consequently any mass made up of iron sulphide molecules will
have the same fraction of iron by weight as do the individual iron
sulphide molecules. Iron sulphide, from whatever source, will have the
same composition, which is in accordance with the law of definite
composition.
3. The atomic hypothesis and the law of multiple proportion. But this
simplest case may not always be the only one. Under other conditions one
atom of iron might combine with two of sulphur to form a molecule of a
second compound. In such a case the one atom of iron would be in
combination with twice the mass of sulphur that is in the first
compound, since the sulphur atoms all have equal masses. What is true
for one molecule will be true for any number of them; consequently when
such quantities of these two compounds are selected as are found to
contain[Pg 64] the same amount of iron, the one will contain twice as much
sulphur as the other.
The combination between the atoms may of course take place in other
simple ratios. For example, two atoms of one element might combine with
three or with five of the other. In all such cases it is clear that the
law of multiple proportion must hold true. For on selecting such numbers
of the two kinds of molecules as have the same number of the one kind of
atoms, the numbers of the other kind of atoms will stand in some simple
ratio to each other, and their weights will therefore stand in the same
simple ratio.
Testing the hypothesis. Efforts have been made to find compounds which
do not conform to these laws, but all such attempts have resulted in
failure. If such compounds should be found, the laws would be no longer
true, and the hypothesis of Dalton would cease to possess value. When an
hypothesis has been tested in every way in which experiment can test it,
and is still found to be in harmony with the facts in the case, it is
termed a theory. We now speak of the atomic theory rather than of the
atomic hypothesis.
Value of a theory. The value of a theory is twofold. It aids in the
clear understanding of the laws of nature because it gives an
intelligent idea as to why these laws should be in operation.
A theory also leads to discoveries. It usually happens that in testing a
theory much valuable work is done, and many new facts are discovered.
Almost any theory in explaining given laws will involve a number of
consequences apart from the laws it seeks to explain. Experiment will
soon show whether these facts are as the theory predicts they will be.
Thus Dalton's atomic theory predicted many properties of gases which
experiment has since verified.[Pg 65]
Atomic weights. It would be of great advantage in the study of chemistry
if we could determine the weights of the different kinds of atoms. It is
evident that this cannot be done directly. They are so small that they
cannot be seen even with a most powerful microscope. It is calculated
that it would take 200,000,000 hydrogen atoms placed side by side to
make a row one centimeter long. No balance can weigh such minute
objects. It is possible, however, to determine their relative
weights,—that is, how much heavier one is than another. These relative
weights of the atoms are spoken of as the atomic weights of the
elements.
If elements were able to combine in only one way,—one atom of one with
one atom of another,—the problem of determining the atomic weights
would be very simple. We should merely have to take some one convenient
element as a standard, and find by experiment how much of each other
element would combine with a fixed weight of it. The ratios thus found
would be the same ratios as those between the atoms of the elements, and
thus we should have their relative atomic weights. The law of multiple
proportion calls attention to the fact that the atoms combine in other
ratios than 1: 1, and there is no direct way of telling which one, if
any, of the several compounds in a given case is the one consisting of a
single atom of each element.
If some way were to be found of telling how much heavier the entire
molecule of a compound is than the atom chosen as a standard,—that is,
of determining the molecular weights of compounds,—the problem could be
solved, though its solution would not be an entirely simple matter.
There are ways of determining the molecular weights of[Pg 66] compounds, and
there are other experiments which throw light directly upon the relative
weights of the atoms. These methods cannot be described until the facts
upon which they rest have been studied. It will be sufficient for the
present to assume that these methods are trustworthy.
Standard for atomic weights. Since the atomic weights are merely
relative to some one element chosen as a standard, it is evident that
any one of the elements may serve as this standard and that any
convenient value may be assigned to its atom. At one time oxygen was
taken as this standard, with the value 100, and the atomic weights of
the other elements were expressed in terms of this standard. It would
seem more rational to take the element of smallest atomic weight as the
standard and give it unit value; accordingly hydrogen was taken as the
standard with an atomic weight of 1. Very recently, however, this unit
has been replaced by oxygen, with an atomic weight of 16.
Why oxygen is chosen as the standard for atomic weights. In the
determination of the atomic weight of an element it is necessary to find
the weight of the element which combines with a definite weight of
another element, preferably the element chosen as the standard. Since
oxygen combines with the elements far more readily than does hydrogen to
form definite compounds, it is far better adapted for the standard
element, and has accordingly replaced hydrogen as the standard. Any
definite value might be given to the weight of the oxygen atom. In
assigning a value to it, however, it is convenient to choose a whole
number, and as small a number as possible without making the atomic
weight of any other element less than unity. For these reasons the
number 16 has been chosen as the atomic[Pg 67] weight of oxygen. This makes
the atomic weight of hydrogen equal to 1.008, so that there is but
little difference between taking oxygen as 16 and hydrogen as 1 for the
unit.
The atomic weights of the elements are given in the Appendix.
EXERCISES
1. Two compounds were found to have the following compositions: (a)
oxygen = 69.53%, nitrogen = 30.47%; (b) oxygen = 53.27%, nitrogen =
46.73%. Show that the law of multiple proportion holds in this case.
2. Two compounds were found to have the following compositions: (a)
oxygen = 43.64%, phosphorus = 56.36%; (b) oxygen = 56.35%, phosphorus
= 43.65%. Show that the law of multiple proportion holds in this case.
3. Why did Dalton assume that all the atoms of a given element have the
same weight?
[Pg 68]
CHAPTER VI
CHEMICAL EQUATIONS AND CALCULATIONS
Formulas. Since the molecule of any chemical compound consists of a
definite number of atoms, and this number never changes without
destroying the identity of the compound, it is very convenient to
represent the composition of a compound by indicating the composition of
its molecules. This can be done very easily by using the symbols of the
atoms to indicate the number and the kind of the atoms which constitute
the molecule. HgO will in this way represent mercuric oxide, a molecule
of which has been found to contain 1 atom each of mercury and oxygen.
H2O will represent water, the molecules of which consist of 1 atom of
oxygen and 2 of hydrogen, the subscript figure indicating the number of
the atoms of the element whose symbol precedes it. H2SO4 will
stand for sulphuric acid, the molecules of which contain 2 atoms of
hydrogen, 1 of sulphur, and 4 of oxygen. The combination of symbols
which represents the molecule of a substance is called its formula.
Equations. When a given substance undergoes a chemical change it is
possible to represent this change by the use of such symbols and
formulas. In a former chapter it was shown that mercuric oxide
decomposes when heated to form mercury and oxygen. This may be expressed
very briefly in the form of the equation
[Pg 69]
When water is electrolyzed two new substances, hydrogen and oxygen, are
formed from it. This statement in the form of an equation is
The coefficient before the symbol for hydrogen indicates that a single
molecule of water yields two atoms of hydrogen on decomposition.
In like manner the combination of sulphur with iron is expressed by the
equation
The decomposition of potassium chlorate by heat takes place as
represented by the equation
Reading of equations. Since equations are simply a kind of shorthand way
of indicating chemical changes which occur under certain conditions, in
reading an equation the full statement for which it stands should be
given. Equation (1) should be read, "Mercuric oxide when heated gives
mercury and oxygen"; equation (2) is equivalent to the statement, "When
electrolyzed, water produces hydrogen and oxygen"; equation (3), "When
heated together iron and sulphur unite to form iron sulphide"; equation
(4), "Potassium chlorate when heated yields potassium chloride and
oxygen."
Knowledge required for writing equations. In order to write such
equations correctly, a considerable amount of exact knowledge is
required. Thus, in equation (1) the fact that red oxide of mercury has
the composition represented by the formula HgO, that it is decomposed by
heat, that in this decomposition mercury and oxygen are formed and[Pg 70] no
other products,—all these facts must be ascertained by exact experiment
before the equation can be written. An equation expressing these facts
will then have much value.
Having obtained an equation describing the conduct of mercuric oxide on
being heated, it will not do to assume that other oxides will behave in
like manner. Iron oxide (FeO) resembles mercuric oxide in many respects,
but it undergoes no change at all when heated. Manganese dioxide, the
black substance used in the preparation of oxygen, has the formula
MnO2. When this substance is heated oxygen is set free, but the metal
manganese is not liberated; instead, a different oxide of manganese
containing less oxygen is produced. The equation representing the
reaction is
Classes of reactions. When a chemical change takes place in a substance
the substance is said to undergo a reaction. Although a great many
different reactions will be met in the study of chemistry, they may all
be grouped under the following heads.
1. Addition. This is the simplest kind of chemical action. It consists
in the union of two or more substances to produce a new substance. The
combination of iron with sulphur is an example:
2. Decomposition. This is the reverse of addition, the substance
undergoing reaction being parted into its constituents. The
decomposition of mercuric oxide is an example: HgO = Hg + O.
3. Substitution. It is sometimes possible for an element in the free
state to act upon a compound in such a way that[Pg 71] it takes the place of
one of the elements of the compound, liberating it in turn. In the study
of the element hydrogen it was pointed out that hydrogen is most
conveniently prepared by the action of sulphuric or hydrochloric acid
upon zinc. When sulphuric acid is used a substance called zinc sulphate,
having the composition represented by the formula ZnSO4, is formed
together with hydrogen. The equation is
When hydrochloric acid is used zinc chloride and hydrogen are the
products of reaction:
When iron is used in place of zinc the equation is
These reactions are quite similar, as is apparent from an examination of
the equations. In each case 1 atom of the metal replaces 2 atoms of
hydrogen in the acid, and the hydrogen escapes as a gas. When an element
in the free state, such as the zinc in the equations just given, takes
the place of some one element in a compound, setting it free from
chemical combination, the act is called substitution.
Other reactions illustrating substitution are the action of sodium on
water,
and the action of heated iron upon water,
4. Double decomposition. When barium dioxide (BaO2) is treated with
sulphuric acid two compounds are formed, namely, hydrogen dioxide
(H2O2) and barium sulphate (BaSO4). The equation is
BaO2 + H2SO4 = BaSO4 + H2O2.
[Pg 72]
In this reaction it will be seen that the two elements barium and
hydrogen simply exchange places. Such a reaction is called a double
decomposition. We shall meet with many examples of this kind of
chemical reactions.
Chemical equations are quantitative. The use of symbols and formulas in
expressing chemical changes has another great advantage. Thus, according
to the equation
1 molecule of water is decomposed into 2 atoms of hydrogen and 1 atom of
oxygen. But, as we have seen, the relative weights of the atoms are
known, that of hydrogen being 1.008, while that of oxygen is 16. The
molecule of water, being composed of 2 atoms of hydrogen and 1 atom of
oxygen, must therefore weigh relatively 2.016 + 16, or 18.016. The
amount of hydrogen in this molecule must be 2.016/18.016, or 11.18% of
the whole, while the amount of oxygen must be 16/18.018, or 88.82% of
the whole. Now, since any definite quantity of water is simply the sum
of a great many molecules of water, it is plain that the fractions
representing the relative amounts of hydrogen and oxygen present in a
molecule must likewise express the relative amounts of hydrogen and
oxygen present in any quantity of water. Thus, for example, in 20 g. of
water there are 2.016/18.016 × 20, or 2.238 g. of hydrogen, and
16/18.016 × 20, or 17.762 g. of oxygen. These results in reference to
the composition of water of course agree exactly with the facts obtained
by the experiments described in the chapter on water, for it is because
of those experiments that the values 1.008 and 16 are given to hydrogen
and oxygen respectively.
It is often easier to make calculations of this kind in the form of a
proportion rather than by fractions. Since the[Pg 73] molecule of water and
the two atoms of hydrogen which it contains have the ratio by weight of
18.016: 2.016, any mass of water has the same ratio between its total
weight and the weight of the hydrogen in it. Hence, to find the number
of grams (x) of hydrogen in 20 g. of water, we have the proportion
18.016 : 2.016 :: 20 g. : x (grams of hydrogen).
Solving for x, we get 2.238 for the number of grams of hydrogen.
Similarly, to find the amount (x) of oxygen present in the 20 g. of
water, we have the proportion
from which we find that x = 17.762 g.
Again, suppose we wish to find what weight of oxygen can be obtained
from 15 g. of mercuric oxide. The equation representing the
decomposition of mercuric oxide is
The relative weights of the mercury and oxygen atoms are respectively
200 and 16. The relative weight of the mercuric oxide molecule must
therefore be the sum of these, or 216. The molecule of mercuric oxide
and the atom of oxygen which it contains have the ratio 216: 16. This
same ratio must therefore hold between the weight of any given quantity
of mercuric oxide and that of the oxygen which it contains. Hence, to
find the weight of oxygen in 15 g. of mercuric oxide, we have the
proportion
216 : 16 :: 15 : x (grams of oxygen).
On the other hand, suppose we wish to prepare, say, 20 g. of oxygen. The
problem is to find out what weight of mercuric oxide will yield 20 g. of
oxygen. The following proportion evidently holds
216 : 16 :: x (grams of mercuric oxide) : 20;
from which we get x = 270.
In the preparation of hydrogen by the action of sulphuric acid upon
zinc, according to the equation,
Zn + H2SO4 = ZnSO4 + 2 H,
[Pg 74]
suppose that 50 g. of zinc are available; let it be required to
calculate the weight of hydrogen which can be obtained. It will be seen
that 1 atom of zinc will liberate 2 atoms of hydrogen. The ratio by
weight of a zinc to an hydrogen atom is 65.4: 1.008; of 1 zinc atom to 2
hydrogen atoms, 65.4: 2.016. Zinc and hydrogen will be related in this
reaction in this same ratio, however many atoms of zinc are concerned.
Consequently in the proportion
x will be the weight of hydrogen set free by 50 g. of zinc. The weight
of zinc sulphate produced at the same time can be found from the
proportion
where 161.46 is the molecular weight of the zinc sulphate, and x the
weight of zinc sulphate formed. In like manner, the weight of sulphuric
acid used up can be calculated from the proportion
These simple calculations are possible because the symbols and formulas
in the equations represent the relative weights of the substances
concerned in a chemical reaction. When once the relative weights of the
atoms have been determined, and it has been agreed to allow the symbols
to stand for these relative weights, an equation or formula making use
of the symbols becomes a statement of a definite numerical fact, and
calculations can be based on it.
Chemical equations not algebraic. Although chemical equations are
quantitative, it must be clearly understood that they are not algebraic.
A glance at the equations
7 + 4 = 11, 8 + 5 = 9 + 4
will show at once that they are true. The equations
HgO = Hg + O, FeO = Fe + O
are equally true in an algebraic sense, but experiment shows that only
the first is true chemically, for iron oxide (FeO)[Pg 75] cannot be directly
decomposed into iron and oxygen. Only such equations as have been found
by careful experiment to express a real chemical transformation, true
both for the kinds of substances as well as for the weights, have any
value.
Chemical formulas and equations, therefore, are a concise way of
representing qualitatively and quantitatively facts which have been
found by experiment to be true in reference to the composition of
substances and the changes which they undergo.
Formulas representing water of crystallization. An examination of
substances containing water of crystallization has shown that in every
case the water is present in such proportion by weight as can readily be
represented by a formula. For example, copper sulphate (CuSO4) and
water combine in the ratio of 1 molecule of the sulphate to 5 of water;
calcium sulphate (CaSO4) and water combine in the ratio 1: 2 to form
gypsum. These facts are expressed by writing the formulas for the two
substances with a period between them. Thus the formula for crystallized
copper sulphate is CuSO4·5H2O; that of gypsum is CaSO4·2H2O.
Heat of reaction. Attention has frequently been directed to the fact
that chemical changes are usually accompanied by heat changes. In
general it has been found that in every chemical action heat is either
absorbed or given off. By adopting a suitable unit for the measurement
of heat, the heat change during a chemical reaction can be expressed in
the equation for the reaction.
Heat cannot be measured by the use of a thermometer alone, since the
thermometer measures the intensity of heat, not its quantity. The
easiest way to measure a quantity of heat is to note how warm it will
make a definite amount of[Pg 76] a given substance chosen as a standard. Water
has been chosen as the standard, and the unit of heat is called a
calorie. A calorie is defined as the amount of heat required to raise
the temperature of one gram of water one degree.
By means of this unit it is easy to indicate the heat changes in a given
chemical reaction. The equation
2H + O = H2O + 68,300 cal.
means that when 2.016 g. of hydrogen combine with 16 g. of oxygen,
18.016 g. of water are formed and 68,300 cal. are set free.
C + 2S = CS2 - 19,000 cal.
means that an expenditure of 19,000 cal. is required to cause 12 g. of
carbon to unite with 64.12 g. of sulphur to form 76.12 g. of carbon
disulphide. In these equations it will be noted that the symbols stand
for as many grams of the substance as there are units in the weights of
the atoms represented by the symbols. This is always understood to be
the case in equations where the heat of reaction is given.
Conditions of a chemical action are not indicated by equations.
Equations do not tell the conditions under which a reaction will take
place. The equation
does not tell us that it is necessary to keep the mercuric oxide at a
high temperature in order that the decomposition may go on. The equation
in no way indicates the fact that the hydrochloric acid must be
dissolved in water before it will act upon the zinc. From the equation
[Pg 77]
it would not be suspected that the two gases hydrogen and chlorine will
unite instantly in the sunlight, but will stand mixed in the dark a long
time without change. It will therefore be necessary to pay much
attention to the details of the conditions under which a given reaction
occurs, as well as to the expression of the reaction in the form of an
equation.
EXERCISES
1. Calculate the percentage composition of the following substances:
(a) mercuric oxide; (b) potassium chlorate; (c) hydrochloric acid;
(d) sulphuric acid. Compare the results obtained with the compositions
as given in Chapters II and III.
2. Determine the percentage of copper, sulphur, oxygen, and water in
copper sulphate crystals. What weight of water can be obtained from 150
g. of this substance?
3. What weight of zinc can be dissolved in 10 g. of sulphuric acid? How
much zinc sulphate will be formed?
4. How many liters of hydrogen measured under standard conditions can be
obtained from the action of 8 g. of iron on 10 g. of sulphuric acid? How
much iron sulphate (FeSO4) will be formed?
5. 10 g. of zinc were used in the preparation of hydrogen; what weight
of iron will be required to prepare an equal volume?
6. How many grams of barium dioxide will be required to prepare 1 kg. of
common hydrogen dioxide solution? What weight of barium sulphate will be
formed at the same time?
7. What weight of the compound Mn3O4 will be formed by strongly
heating 25 g. of manganese dioxide? What volume of oxygen will be given
off at the same time, measured under standard conditions?
8. (a) What is the weight of 100 l. of hydrogen measured in a
laboratory in which the temperature is 20° and pressure 750 mm.? (b)
What weight of sulphuric acid is necessary to prepare this amount of
hydrogen? (c) The density of sulphuric acid is 1.84. Express the acid
required in (b) in cubic centimeters.
9. What weight of potassium chlorate is necessary to furnish sufficient
oxygen to fill four 200 cc. bottles in your laboratory (the gas to be
collected over water)?
[Pg 78]
CHAPTER VII
NITROGEN AND THE RARE ELEMENTS: ARGON, HELIUM, NEON, KRYPTON, XENON
Historical. Nitrogen was discovered by the English chemist Rutherford in
1772. A little later Scheele showed it to be a constituent of air, and
Lavoisier gave it the name azote, signifying that it would not support
life. The name nitrogen was afterwards given it because of its
presence in saltpeter or niter. The term azote and symbol Az are still
retained by the French chemists.
Occurrence. Air is composed principally of oxygen and nitrogen in the
free state, about 78 parts by volume out of every 100 parts being
nitrogen. Nitrogen also occurs in nature in the form of potassium
nitrate (KNO3)—commonly called saltpeter or niter—as well as in
sodium nitrate (NaNO3). Nitrogen is also an essential constituent of
all living organisms; for example, the human body contains about 2.4% of
nitrogen.
Preparation from air. Nitrogen can be prepared from air by the action of
some substance which will combine with the oxygen, leaving the nitrogen
free. Such a substance must be chosen, however, as will combine with the
oxygen to form a product which is not a gas, and which can be readily
separated from the nitrogen. The substances most commonly used for this
purpose are phosphorus and copper.
1. By the action of phosphorus. The method used for the preparation of
nitrogen by the action of phosphorus is as follows:[Pg 79]
The phosphorus is placed in a little porcelain dish, supported on a cork
and floated on water (Fig. 26). It is then ignited by contact with a hot
wire, and immediately a bell jar or bottle is brought over it so as to
confine a portion of the air. The phosphorus combines with the oxygen to
form an oxide of phosphorus, known as phosphorus pentoxide. This is a
white solid which floats about in the bell jar, but in a short time it
is all absorbed by the water, leaving the nitrogen. The withdrawal of
the oxygen is indicated by the rising of the water in the bell jar.
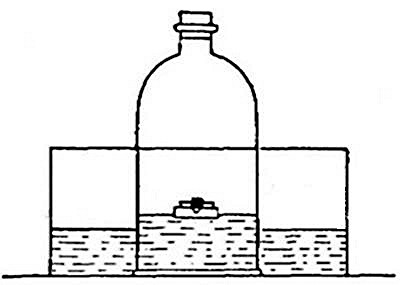 Fig. 26
Fig. 26
2. By the action of copper. The oxygen present in the air may also be
removed by passing air slowly through a heated tube containing copper.
The copper combines with the oxygen to form copper oxide, which is a
solid. The nitrogen passes on and may be collected over water.
Nitrogen obtained from air is not pure. Inasmuch as air, in
addition to oxygen and nitrogen, contains small amounts of
other gases, and since the phosphorus as well as the copper
removes only the oxygen, it is evident that the nitrogen
obtained by these methods is never quite pure. About 1% of the
product is composed of other gases, from which it is very
difficult to separate the nitrogen. The impure nitrogen so
obtained may, however, be used for a study of most of the
properties of nitrogen, since these are not materially affected
by the presence of the other gases.
Preparation from compounds of nitrogen. Pure nitrogen may be obtained
from certain compounds of the element. Thus, if heat is applied to the
compound ammonium nitrite (NH4NO2), the change represented in the
following equation takes place:
Physical properties. Nitrogen is similar to oxygen and hydrogen in that
it is a colorless, odorless, and tasteless gas. One liter of nitrogen
weighs 1.2501 g. It is almost insoluble in water. It can be obtained in
the form of a colorless liquid having a boiling point of -195° at
ordinary pressure. At -214° it solidifies.
Chemical properties. Nitrogen is characterized by its inertness. It is
neither combustible nor a supporter of combustion. At ordinary
temperatures it will not combine directly with any of the elements
except under rare conditions. At higher temperatures it combines with
magnesium, lithium, titanium, and a number of other elements. The
compounds formed are called nitrides, just as compounds of an element
with oxygen are called oxides. When it is mixed with oxygen and
subjected to the action of electric sparks, the two gases slowly combine
forming oxides of nitrogen. A mixture of nitrogen and hydrogen when
treated similarly forms ammonia, a gaseous compound of nitrogen and
hydrogen. Since we are constantly inhaling nitrogen, it is evident that
it is not poisonous. Nevertheless life would be impossible in an
atmosphere of pure nitrogen on account of the exclusion of the necessary
oxygen.
Argon, helium, neon, krypton, xenon. These are all rare
elements occurring in the air in very small quantities. Argon,
discovered in 1894, was the first one obtained. Lord Rayleigh,
an English scientist, while engaged in determining the exact
weights of various gases, observed that the nitrogen obtained
from the air is slightly heavier than pure nitrogen obtained
from its compounds. After repeating his experiments many times,
always with the same results, Rayleigh finally concluded that
the nitrogen which he had obtained from the air was not pure,
but was mixed with a small amount of some unknown gas, the
density of which is greater than that of nitrogen. Acting on
this assumption, Rayleigh, together with the[Pg 81] English chemist
Ramsay, attempted to separate the nitrogen from the unknown
gas. Knowing that nitrogen would combine with magnesium, they
passed the nitrogen obtained from the air and freed from all
known substances through tubes containing magnesium heated to
the necessary temperature. After repeating this operation, they
finally succeeded in obtaining from the atmospheric nitrogen a
small volume of gas which would not combine with magnesium and
hence could not be nitrogen. This proved to be a new element,
to which they gave the name argon. As predicted, this new
element was found to be heavier than nitrogen, its density as
compared with hydrogen as a standard being approximately 20,
that of nitrogen being only 14. About 1% of the atmospheric
nitrogen proved to be argon. The new element is characterized
by having no affinity for other elements. Even under the most
favorable conditions it has not been made to combine with any
other element. On this account it was given the name argon,
signifying lazy or idle. Like nitrogen, it is colorless,
odorless, and tasteless. It has been liquefied and solidified.
Its boiling point is -187°.
Helium was first found in the gases expelled from certain
minerals by heating. Through the agency of the spectroscope it
had been known to exist in the sun long before its presence on
the earth had been demonstrated,—a fact suggested by the name
helium, signifying the sun. Its existence in traces in the
atmosphere has also been proven. It was first liquefied by
Onnes in July, 1908. Its boiling point, namely -269°, is the
lowest temperature yet reached.
The remaining elements of this group—neon, krypton, and
xenon—have been obtained from liquid air. When liquid air is
allowed to boil, the constituents which are the most difficult
to liquefy, and which therefore have the lowest boiling points,
vaporize first, followed by the others in the order of their
boiling points. It is possible in this way to make at least a
partial separation of the air into its constituents, and Ramsay
thus succeeded in obtaining from liquid air not only the known
constituents, including argon and helium, but also the new
elements, neon, krypton, and xenon. These elements, as well as
helium, all proved to be similar to argon in that they are
without chemical activity, apparently forming no compounds
whatever. The percentages present in the air are very small.
The names, neon, krypton, xenon, signify respectively, new,
hidden, stranger.
[Pg 82]
EXERCISES
1. How could you distinguish between oxygen, hydrogen, and nitrogen?
2. Calculate the relative weights of nitrogen and oxygen; of nitrogen
and hydrogen.
3. In the preparation of nitrogen from the air, how would hydrogen do as
a substance for the removal of the oxygen?
4. What weight of nitrogen can be obtained from 10 l. of air measured
under the conditions of temperature and pressure which prevail in your
laboratory?
5. How many grams of ammonium nitrite are necessary in the preparation
of 20 l. of nitrogen measured over water under the conditions of
temperature and pressure which prevail in your laboratory?
6. If 10 l. of air, measured under standard conditions, is passed over
100 g. of hot copper, how much will the copper gain in weight?
 WILLIAM RAMSAY (Scotch) (1855-)
WILLIAM RAMSAY (Scotch) (1855-)
Has made many studies in the physical properties of substances;
discovered helium; together with Lord Rayleigh and others he discovered
argon, krypton, xenon, and neon; has contributed largely to the
knowledge of radio-active substances, showing that radium gradually
gives rise to helium; professor at University College, London
[Pg 83]
CHAPTER VIII
THE ATMOSPHERE
Atmosphere and air. The term atmosphere is applied to the gaseous
envelope surrounding the earth. The term air is generally applied to a
limited portion of this envelope, although the two words are often used
interchangeably. Many references have already been made to the
composition and properties of the atmosphere. These statements must now
be collected and discussed somewhat more in detail.
Air formerly regarded as an element. Like water, air was at first
regarded as elementary in character. Near the close of the eighteenth
century Scheele, Priestley, and Lavoisier showed by their experiments
that it is a mixture of at least two gases,—those which we now call
oxygen and nitrogen. By burning substances in an inclosed volume of air
and noting the contraction in volume due to the removal of the oxygen,
they were able to determine with some accuracy the relative volumes of
oxygen and nitrogen present in the air.
The constituents of the atmosphere. The constituents of the atmosphere
may be divided into two general groups: those which are essential to
life and those which are not essential.
1. Constituents essential to life. In addition to oxygen and nitrogen
at least two other substances, namely, carbon dioxide and water vapor,
must be present in the atmosphere in order that life may exist. The
former of these is a[Pg 84] gaseous compound of carbon and oxygen having the
formula CO2. Its properties will be discussed in detail in the
chapter on the compounds of carbon. Its presence in the air may be shown
by causing the air to bubble through a solution of calcium hydroxide
(Ca(OH)2), commonly called lime water. The carbon dioxide combines
with the calcium hydroxide in accordance with the following equation:
Ca(OH)2 + CO2 = CaCO3 + H2O.
The resulting calcium carbonate (CaCO3) is insoluble in water and
separates in the form of a white powder, which causes the solution to
appear milky.
The presence of water vapor is readily shown by its condensation on cold
objects as well as by the fact that a bit of calcium chloride when
exposed to the air becomes moist, and may even dissolve in the water
absorbed from the air.
2. Constituents not essential to life. In addition to the essential
constituents, the air contains small percentages of various other gases,
the presence of which so far as is known is not essential to life. This
list includes the rare elements, argon, helium, neon, krypton, and
xenon; also hydrogen, ammonia, hydrogen dioxide, and probably ozone.
Certain minute forms of life (germs) are also present, the decay of
organic matter being due to their presence.
Function of each of the essential constituents. (1) The oxygen
directly supports life through respiration. (2) The nitrogen,
on account of its inactivity, serves to dilute the oxygen, and
while contrary to the older views, it is possible that life
might continue to exist in the absence of the atmospheric
nitrogen, yet the conditions of life would be entirely changed.
Moreover, nitrogen is an essential constituent of all animal
and plant life. It was formerly supposed that neither animals
nor plants could assimilate the free nitrogen, but it has been
shown recently that the plants of at least one natural[Pg 85] order,
the Leguminosæ, to which belong the beans, peas, and clover,
have the power of directly assimilating the free nitrogen from
the atmosphere. This is accomplished through the agency of
groups of bacteria, which form colonies in little tubercles on
the roots of the plants. These bacteria probably assist in the
absorption of nitrogen by changing the free nitrogen into
compounds which can be assimilated by the plant. Fig. 27 shows
the tubercles on the roots of a variety of bean. (3) The
presence of water vapor in the air is necessary to prevent
excessive evaporation from both plants and animals. (4) Carbon
dioxide is an essential plant food.
 Fig. 27
Fig. 27
The quantitative analysis of air. A number of different methods have
been devised for the determination of the percentages of the
constituents present in the atmosphere. Among these are the following.
1. Determination of oxygen. (1) The oxygen is withdrawn from a
measured volume of air inclosed in a tube, by means of phosphorus.
To make the determination, a graduated tube is filled with
water and inverted in a vessel of water. Air is introduced into
the tube until it is partially filled with the gas. The volume
of the inclosed air is carefully noted and reduced to standard
conditions. A small piece of phosphorus is attached to a wire
and brought within the tube as shown in Fig. 28. After a few
hours the oxygen in the inclosed air will have combined with
the phosphorus, the water rising to take its place. The
phosphorus is removed and the volume is again noted and reduced
to standard conditions. The contraction in the volume of the
air is equal to the volume of oxygen absorbed.
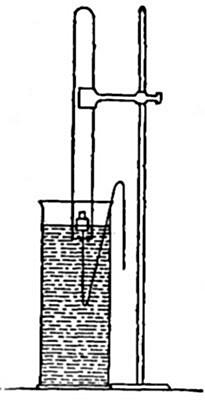 Fig. 28
Fig. 28
[Pg 86]
(2) The oxygen may also be estimated by passing a measured volume of air
through a tube containing copper heated to a high temperature. The
oxygen in the air combines with the copper to form copper oxide (CuO).
Hence the increase in the weight of the copper equals the weight of the
oxygen in the volume of air taken.
(3) A more accurate method is the following. A eudiometer tube is filled
with mercury and inverted in a vessel of the same liquid. A convenient
amount of air is then introduced into the tube and its volume accurately
noted. There is then introduced more than sufficient hydrogen to combine
with the oxygen present in the inclosed air, and the volume is again
accurately noted. The mixture is then exploded by an electric spark, and
the volume is once more taken. By subtracting this volume from the total
volume of the air and hydrogen there is obtained the contraction in
volume due to the union of the oxygen and hydrogen. The volume occupied
by the water formed by the union of the two gases is so small that it
may be disregarded in the calculation. Since oxygen and hydrogen combine
in the ratio 1: 2 by volume, it is evident that the contraction in
volume due to the combination is equal to the volume occupied by the
oxygen in the air contained in the tube, plus twice this volume of
hydrogen. In other words, one third of the total contraction is equal to
the volume occupied by the oxygen in the inclosed air. The following
example will make this clear:
| Volume of air in tube | 50.0 cc. |
| Volume after introducing hydrogen | 80.0 |
| Volume after combination of oxygen and hydrogen | 48.5 |
| Contraction in volume due to combination (80 cc.-48.5 cc.) | 31.5 |
| Volume of oxygen in 50 cc. of air (1/3 of 31.5) | 10.5 |
[Pg 87]
All these methods agree in showing that 100 volumes of dry air contain
approximately 21 volumes of oxygen.
2. Determination of nitrogen. If the gas left after the removal of
oxygen from a portion of air is passed over heated magnesium, the
nitrogen is withdrawn, argon and the other rare elements being left. It
may thus be shown that of the 79 volumes of gas left after the removal
of the oxygen from 100 volumes of air, approximately 78 are nitrogen and
0.93 argon. The other elements are present in such small quantities that
they may be neglected.
3. Determination of carbon dioxide. The percentage of carbon dioxide
in any given volume of air may be determined by passing the air over
calcium hydroxide or some other compound which will combine with the
carbon dioxide. The increase in the weight of the hydroxide equals the
weight of the carbon dioxide absorbed. The amount present in the open
normal air is from 3 to 4 parts by volume in 10,000 volumes of air, or
about 0.04%.
4. Determination of water vapor. The water vapor present in a given
volume of air may be determined by passing the air over calcium chloride
(or some other compound which has a strong affinity for water), and
noting the increase in the weight of the chloride. The amount present
varies not only with the locality, but there is a wide variation from
day to day in the same locality because of the winds and changes in
temperature.
Processes affecting the composition of the air. The most important of
these processes are the following.
1. Respiration. In the process of respiration some of the oxygen in
the inhaled air is absorbed by the blood and carried to all parts of the
body, where it combines with the carbon of the worn-out tissues. The
products of oxidation[Pg 88] are carried back to the lungs and exhaled in the
form of carbon dioxide. The amount exhaled by an adult averages about 20
l. per hour. Hence in a poorly ventilated room occupied by a number of
people the amount of carbon dioxide rapidly increases. While this gas is
not poisonous unless present in large amounts, nevertheless air
containing more than 15 parts in 10,000 is not fit for respiration.
2. Combustion. All of the ordinary forms of fuel contain large
percentages of carbon. On burning, this carbon combines with oxygen in
the air, forming carbon dioxide. Combustion and respiration, therefore,
tend to diminish the amount of oxygen in the air and to increase the
amount of carbon dioxide.
3. Action of plants. Plants have the power, when in the sunlight, of
absorbing carbon dioxide from the air, retaining the carbon and
returning at least a portion of the oxygen to the air. It will be
observed that these changes are just the opposite of those brought about
by the processes of respiration and combustion.
Poisonous effect of exhaled air. The differences in the
percentages of oxygen, carbon dioxide, and moisture present in
inhaled air and exhaled air are shown in the following
analyses.
| INHALED AIR | EXHALED AIR |
| Oxygen | 21.00% | 16.00% |
| Carbon dioxide | 0.04 | 4.38 |
| Moisture | variable | saturated |
The foul odor of respired air is due to the presence of a
certain amount of organic matter. It is possible that this
organic matter rather than the carbon dioxide is responsible
for the injurious effects which follow the respiration of
impure air. The extent of such organic impurities present may
be judged, however, by the amount of carbon dioxide present,
since the two are exhaled together.
The cycle of carbon in nature. Under the influence of sunlight,
the carbon dioxide absorbed from the air by plants reacts with
water[Pg 89] and small amounts of other substances absorbed from the
soil to form complex compounds of carbon which constitute the
essential part of the plant tissue. This reaction is attended
by the evolution of oxygen, which is restored to the air. The
compounds resulting from these changes are much richer in their
energy content than are the substances from which they are
formed; hence a certain amount of energy must have been
absorbed in their formation. The source of this energy is the
sun's rays.
If the plant is burned, the changes which took place in the
formation of the compounds present are largely reversed. The
carbon and hydrogen present combine with oxygen taken from the
air to form carbon dioxide and water, while the energy absorbed
from the sun's rays is liberated in the form of energy of heat.
If, on the other hand, the plant is used as food, the compounds
present are used in building up the tissues of the body. When
this tissue breaks down, the changes which it undergoes are
very similar to those which take place when the plant is
burned. The carbon and hydrogen combine with the inhaled oxygen
to form carbon dioxide and water, which are exhaled. The energy
possessed by the complex substances is liberated partly in the
form of energy of heat, which maintains the heat of the body,
and partly in the various forms of muscular energy. The carbon
originally absorbed from the air by the plant in the form of
carbon dioxide is thus restored to the air and is ready to
repeat the cycle of changes.
The composition of the air is constant. Notwithstanding the changes
constantly taking place which tend to alter the composition of the air,
the results of a great many analyses of air collected in the open fields
show that the percentages of oxygen and nitrogen as well as of carbon
dioxide are very nearly constant. Indeed, so constant are the
percentages of oxygen and nitrogen that the question has arisen, whether
these two elements are not combined in the air, forming a definite
chemical compound. That the two are not combined but are simply mixed
together can be shown in a number of ways, among which are the
following.[Pg 90]
1. When air dissolves in water it has been found that the ratio of
oxygen to nitrogen in the dissolved air is no longer 21: 78, but more
nearly 35: 65. If it were a chemical compound, the ratio of oxygen to
nitrogen would not be changed by solution in water.
2. A chemical compound in the form of a liquid has a definite boiling
point. Water, for example, boils at 100°. Moreover the steam which is
thus formed has the same composition as the water. The boiling point of
liquid air, on the other hand, gradually rises as the liquid boils, the
nitrogen escaping first followed by the oxygen. If the two were
combined, they would pass off together in the ratio in which they are
found in the air.
Why the air has a constant composition. If air is a mixture and changes
are constantly taking place which tend to modify its composition, how,
then, do we account for the constancy of composition which the analyses
reveal? This is explained by several facts. (1) The changes which are
caused by the processes of combustion and respiration, on the one hand,
and the action of plants, on the other, tend to equalize each other. (2)
The winds keep the air in constant motion and so prevent local changes.
(3) The volume of the air is so vast and the changes which occur are so
small compared with the total amount of air that they cannot be readily
detected. (4) Finally it must be noted that only air collected in the
open fields shows this constancy in composition. The air in a poorly
ventilated room occupied by a number of people rapidly changes in
composition.
The properties of the air. Inasmuch as air is composed principally of a
mixture of oxygen and nitrogen, which elements have already been
discussed, its properties may be inferred largely from those of the two
gases.[Pg 91] One liter weighs 1.2923 g. It is thus 14.38 times as heavy as
hydrogen. At the sea level it exerts an average pressure sufficient to
sustain a column of mercury 760 mm. in height. This is taken as the
standard pressure in determining the volumes of gases as well as the
boiling points of liquids. Water may be made to boil at any temperature
between 0° and considerably above 100° by simply varying the pressure.
It is only when the pressure upon it is equal to the normal pressure of
the atmosphere at the sea level, as indicated by a barometric reading of
760 mm., that it boils at 100°.
Preparation of liquid air. Attention has been called to the fact that
both oxygen and nitrogen can be obtained in the liquid state by strongly
cooling the gases and applying great pressure to them. Since air is
largely a mixture of these two gases, it can be liquefied by the same
methods.
The methods for liquefying air have been simplified greatly in
that the low temperature required is obtained by allowing a
portion of the compressed air to expand. The expansion of a gas
is always attended by the absorption of heat. In liquefying air
the apparatus is so constructed that the heat absorbed is
withdrawn from air already under great pressure. This process
is continued until the temperature is lowered to the point of
liquefaction.
 Fig. 29
Fig. 29
The Dewar bulb. It is not possible to preserve air in the liquid state
in a closed vessel, on account of the enormous pressure exerted by it in
its tendency to pass into the gaseous state. It may however be preserved
for some hours or even days before it will completely evaporate, by
simply placing it in an open vessel surrounded by a nonconducting
material. The most efficient vessel for this purpose is the Dewar bulb
shown in Fig. 29.[Pg 92] The air is withdrawn from the space between the two
walls, thus making it nonconducting.
Properties and uses of liquid air. When first prepared, liquid air is
cloudy because of the presence of particles of solid carbon dioxide.
These may be filtered off, leaving a liquid of slightly bluish color. It
begins to boil at about -190°, the nitrogen passing off first, gradually
followed by the oxygen, the last portions being nearly pure oxygen. To a
certain extent oxygen is now prepared in this way for commercial
purposes.
The extremely low temperature of liquid air may be inferred from the
fact that mercury when cooled by it is frozen to a mass so hard that it
may be used for driving nails.
Liquid air is used in the preparation of oxygen and as a cooling agent
in the study of the properties of matter at low temperatures. It has
thus been found that elements at extremely low temperatures largely lose
their chemical activity.
EXERCISES
1. When oxygen and nitrogen are mixed in the proportion in which they
exist in the atmosphere, heat is neither evolved nor absorbed by the
process. What important point does this suggest?
2. What essential constituent of the air is found in larger amount in
manufacturing districts than in the open country?
3. Can you suggest any reason why the growth of clover in a field
improves the soil?
4. Why are the inner walls of a Dewar bulb sometimes coated with a film
of silver?
5. To what is the blue color of liquid air due? Does this color increase
in intensity on standing?
6. When ice is placed in a vessel containing liquid air, the latter
boils violently. Explain.[Pg 93]
7. Taking the volumes of the oxygen and nitrogen in 100 volumes of air
as 21 and 78 respectively, calculate the percentages of these elements
present by weight.
8. Would combustion be more intense in liquid air than in the gaseous
substance?
9. A tube containing calcium chloride was found to weigh 30.1293 g. A
volume of air which weighed 15.2134 g. was passed through, after which
the weight of the tube was found to be 30.3405 g. What was the
percentage amount of moisture present in the air?
10. 10 l. of air measured at 20° and 740 mm. passed through lime water
caused the precipitation of 0.0102 g. of CaCO3. Find the number of
volumes of carbon dioxide in 10,000 volumes of the air.
[Pg 94]
CHAPTER IX
SOLUTIONS
Definitions. When a substance disappears in a liquid in such a way as to
thoroughly mix with it and to be lost to sight as an individual body,
the resulting liquid is called a solution. The liquid in which the
substance dissolves is called the solvent, while the dissolved
substance is called the solute.
Classes of solutions. Matter in any one of its physical states may
dissolve in a liquid, so that we may have solutions of gases, of
liquids, and of solids. Solutions of liquids in liquids are not often
mentioned in the following pages, but the other two classes will become
very familiar in the course of our study, and deserve special attention.
SOLUTION OF GASES IN LIQUIDS
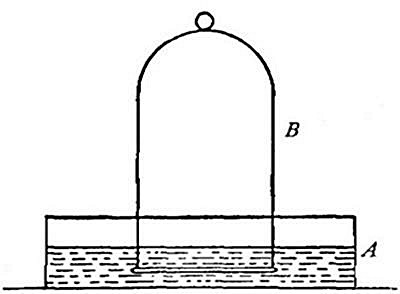 Fig. 30
Fig. 30
It has already been stated that oxygen, hydrogen, and nitrogen are
slightly soluble in water. Accurate study has led to the conclusion that
all gases are soluble to some extent not only in water but in many other
liquids. The amount of a gas which will dissolve in a liquid depends
upon a number of conditions, and these can best be understood by[Pg 95]
supposing a vessel B (Fig. 30), to be filled with the gas and inverted
over the liquid. Under these circumstances the gas cannot escape or
become mixed with another gas.
Circumstances affecting the solubility of gases. A number of
circumstances affect the solubility of a gas in a liquid.
1. Nature of the gas. Other conditions being equal, each gas has its
own peculiar solubility, just as it has its own special taste or odor.
The solubility of gases varies between wide limits, as will be seen from
the following table, but as a rule a given volume of a liquid will not
dissolve more than two or three times its own volume of a gas.
Solubility of Gases in Water
1 l. of water at 760 mm. pressure and at 0° will dissolve:
| Ammonia | 1148.00 l. |
| Hydrochloric acid | 503.00 |
| Sulphur dioxide | 79.79 |
| Carbon dioxide | 1.80 |
| Oxygen | 41.14 cc. |
| Hydrogen | 21.15 |
| Nitrogen | 20.03 |
In the case of very soluble gases, such as the first three in the table,
it is probable that chemical combination between the liquid and the gas
takes place.
2. Nature of the liquid. The character of the liquid has much
influence upon the solubility of a gas. Water, alcohol, and ether have
each its own peculiar solvent power. From the solubility of a gas in
water, no prediction can be made as to its solubility in other liquids.
3. Influence of pressure. It has been found that the weight of gas
which dissolves in a given case is proportional to the pressure exerted
upon the gas. If the[Pg 96] pressure is doubled, the weight of gas going into
solution is doubled; if the pressure is diminished to one half of its
original value, half of the dissolved gas will escape. Under high
pressure, large quantities of gas can be dissolved in a liquid, and when
the pressure is removed the gas escapes, causing the liquid to foam or
effervesce.
4. Influence of temperature. In general, the lower the temperature of
the liquid, the larger the quantity of gas which it can dissolve. 1000
volumes of water at 0° will dissolve 41.14 volumes of oxygen; at 50°,
18.37 volumes; at 100° none at all. While most gases can be expelled
from a liquid by boiling the solution, some cannot. For example, it is
not possible to expel hydrochloric acid gas completely from its solution
by boiling.
SOLUTION OF SOLIDS IN LIQUIDS
This is the most familiar class of solutions, since in the laboratory
substances are much more frequently used in the form of solutions than
in the solid state.
Circumstances affecting the solubility of a solid. The solubility of a
solid in a liquid depends upon several factors.
1. Nature of the solid. Other conditions being the same, solids vary
greatly in their solubility in liquids. This is illustrated in the
following table:
Table of Solubility of Solids at 18°
| 100 cc. of water will dissolve: |
| Calcium chloride | 71.0 g. |
| Sodium chloride | 35.9 |
| Potassium nitrate | 29.1 |
| Copper sulphate | 21.4 |
| Calcium sulphate | 0.207 |
[Pg 97]
No solids are absolutely insoluble, but the amount dissolved may be so
small as to be of no significance for most purposes. Thus barium
sulphate, one of the most insoluble of common substances, dissolves in
water to the extent of 1 part in 400,000.
2. Nature of the solvent. Liquids vary much in their power to dissolve
solids. Some are said to be good solvents, since they dissolve a great
variety of substances and considerable quantities of them. Others have
small solvent power, dissolving few substances, and those to a slight
extent only. Broadly speaking, water is the most general solvent, and
alcohol is perhaps second in solvent power.
3. Temperature. The weight of a solid which a given liquid can
dissolve varies with the temperature. Usually it increases rapidly as
the temperature rises, so that the boiling liquid dissolves several
times the weight which the cold liquid will dissolve. In some instances,
as in the case of common salt dissolved in water, the temperature has
little influence upon the solubility, and a few solids are more soluble
in cold water than in hot. The following examples will serve as
illustrations:
Table of Solubility at 0° and at 100°
100 cc. of water will dissolve:
| At 0° | At 100° |
| Calcium chloride | 49.6 g. | 155.0 g. |
| Sodium chloride | 35.7 | 39.8 |
| Potassium nitrate | 13.3 | 247.0 |
| Copper sulphate | 15.5 | 73.5 |
| Calcium sulphate | 0.205 | 0.217 |
| Calcium hydroxide | 0.173 | 0.079 |
Saturated solutions. A liquid will not dissolve an unlimited quantity of
a solid. On adding the solid to the liquid in small portions at[Pg 98] a time,
it will be found that a point is reached at which the liquid will not
dissolve more of the solid at that temperature. The solid and the
solution remain in contact with each other unchanged. This condition may
be described by saying that they are in equilibrium with each other. A
solution is said to be saturated when it remains unchanged in
concentration in contact with some of the solid. The weight of the solid
which will completely saturate a definite volume of a liquid at a given
temperature is called the solubility of the substance at that
temperature.
Supersaturated solutions. When a solution, saturated at a given
temperature, is allowed to cool it sometimes happens that no solid
crystallizes out. This is very likely to occur when the vessel used is
perfectly smooth and the solution is not disturbed in any way. Such a
solution is said to be supersaturated. That this condition is unstable
can be shown by adding a crystal of the solid to the solution. All of
the solid in excess of the quantity required to saturate the solution at
this temperature will at once crystallize out, leaving the solution
saturated. Supersaturation may also be overcome in many cases by
vigorously shaking or stirring the solution.
General physical properties of solutions. A few general statements may
be made in reference to the physical properties of solutions.
1. Distribution of the solid in the liquid. A solid, when dissolved,
tends to distribute itself uniformly through the liquid, so that every
part of the solution has the same concentration. The process goes on
very slowly unless hastened by stirring or shaking the solution. Thus,
if a few crystals of a highly colored substance such as copper sulphate
are placed in the bottom of a tall vessel full of water, it will take
weeks for the solution to become uniformly colored.
2. Boiling points of solutions. The boiling point of a liquid is
raised by the presence of a substance dissolved in it. In general the
extent to which the boiling point of a solvent is raised by a given
substance is proportional to the[Pg 99] concentration of the solution, that
is, to the weight of the substance dissolved in a definite weight of the
solvent.
3. Freezing points of solutions. A solution freezes at a lower
temperature than the pure solvent. The lowering of the freezing point
obeys the same law which holds for the raising of the boiling point: the
extent of lowering is proportional to the weight of dissolved substance,
that is, to the concentration of the solution.
Electrolysis of solutions. Pure water does not appreciably conduct the
electric current. If, however, certain substances such as common salt
are dissolved in the water, the resulting solutions are found to be
conductors of electricity. Such solutions are called electrolytes.
When the current passes through an electrolyte some chemical change
always takes place. This change is called electrolysis.
 Fig. 31
Fig. 31
The general method used in the electrolysis of a solution is illustrated
in Fig. 31. The vessel D contains the electrolyte. Two plates or rods,
A and B, made of suitable material, are connected with the wires
from a battery (or dynamo) and dipped into the electrolyte, as shown in
the figure. These plates or rods are called electrodes. The electrode
connected with the zinc plate of the battery is the negative electrode
or cathode, while that connected with the carbon plate is the positive
electrode or anode.
Theory of electrolytic dissociation. The facts which have just been
described in connection with solutions, together with many others, have
led chemists to adopt a theory of solutions called the theory of
electrolytic dissociation. The main assumptions in this theory are the
following.[Pg 100]
1. Formation of ions. Many compounds when dissolved in water undergo
an important change. A portion of their molecules fall apart, or
dissociate, into two or more parts, called ions. Thus sodium nitrate
(NaNO3) dissociates into the ions Na and NO3; sodium chloride,
into the ions Na and Cl. These ions are free to move about in the
solution independently of each other like independent molecules, and for
this reason were given the name ion, which signifies a wanderer.
2. The electrical charge of ions. Each ion carries a heavy electrical
charge, and in this respect differs from an atom or molecule. It is
evident that the sodium in the form of an ion must differ in some
important way from ordinary sodium, for sodium ions, formed from sodium
nitrate, give no visible evidence of their presence in water, whereas
metallic sodium at once decomposes the water. The electrical charge,
therefore, greatly modifies the usual chemical properties of the
element.
3. The positive charges equal the negative charges. The ions formed by
the dissociation of any molecule are of two kinds. One kind is charged
with positive electricity and the other with negative electricity;
moreover the sum of all the positive charges is always equal to the sum
of all the negative charges. The solution as a whole is therefore
electrically neutral. If we represent dissociation by the usual chemical
equations, with the electrical charges indicated by + and - signs
following the symbols, the dissociation of sodium chloride molecules is
represented thus:
The positive charge on each sodium ion exactly equals the negative
charge on each chlorine ion.[Pg 101] Sodium sulphate dissociates, as shown in
the equation
Here the positive charge on the two sodium ions equals the double
negative charge on the SO4 ion.
4. Not all compounds dissociate. Only those compounds dissociate whose
solutions form electrolytes. Thus salt dissociates when dissolved in
water, the resulting solution being an electrolyte. Sugar, on the other
hand, does not dissociate and its solution is not a conductor of the
electric current.
5. Extent of dissociation differs in different liquids. While
compounds most readily undergo dissociation in water, yet dissociation
often occurs to a limited extent when solution takes place in liquids
other than water. In the discussion of solutions it will be understood
that the solvent is water unless otherwise noted.
The theory of electrolytic dissociation and the properties of solutions.
In order to be of value, this theory must give a reasonable explanation
of the properties of solutions. Let us now see if the theory is in
harmony with certain of these properties.
The theory of electrolytic dissociation and the boiling and freezing
points of solutions. We have seen that the boiling point of a solution
of a substance is raised in proportion to the concentration of the
dissolved substance. This is but another way of saying that the change
in the boiling point of the solution is proportional to the number of
molecules of the dissolved substance present in the solution.
It has been found, however, that in the case of electrolytes the boiling
point is raised more than it should be to[Pg 102] conform to this law. If the
solute dissociates into ions, the reason for this becomes clear. Each
ion has the same effect on the boiling point as a molecule, and since
their number is greater than the number of molecules from which they
were formed, the effect on the boiling point is abnormally great.
In a similar way, the theory furnishes an explanation of the abnormal
lowering of the freezing point of electrolytes.
The theory of electrolytic dissociation and electrolysis. The changes
taking place during electrolysis harmonize very completely with the
theory of dissociation. This will become clear from a study of the
following examples.
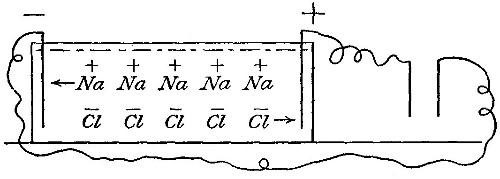 Fig. 32
Fig. 32
1. Electrolysis of sodium chloride. Fig. 32 represents a vessel in
which the electrolyte is a solution of sodium chloride (NaCl). According
to the dissociation theory the molecules of sodium chloride dissociate
into the ions Na+ and Cl-. The Na+ ions are attracted to the
cathode owing to its large negative charge. On coming into contact with
the cathode, the Na+ ions give up their positive charge and are then
ordinary sodium atoms. They immediately decompose the water according to
the equation
and hydrogen is evolved about the cathode.
The chlorine ions on being discharged at the anode in similar manner may
either be given off as chlorine gas, or may attack the water, as
represented in the equation
2. Electrolysis of water. The reason for the addition of sulphuric
acid to water in the preparation of oxygen and hydrogen by electrolysis
will now be clear. Water itself is not an electrolyte to an appreciable
extent; that is, it does not form enough ions to carry a current.
Sulphuric acid dissolved in water is an electrolyte, and dissociates
into the ions 2 H+ and SO4—. In the process of electrolysis of
the solution, the hydrogen ions travel to the cathode, and on being
discharged escape as hydrogen gas. The SO4 ions, when discharged at
the anode, act upon water, setting free oxygen and once more forming
sulphuric acid:
The sulphuric acid can again dissociate and the process repeat itself as
long as any water is left. Hence the hydrogen and oxygen set free in the
electrolysis of water really come directly from the acid but indirectly
from the water.
3. Electrolysis of sodium sulphate. In a similar way, sodium sulphate
(Na2SO4), when in solution, gives the ions 2 Na+ and
SO4—. On being discharged, the sodium atoms decompose water about
the cathode, as in the case of sodium chloride, while the SO4 ions
when discharged at the anode decompose the water, as represented in the
equation
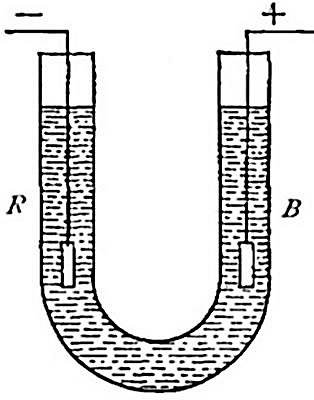 Fig. 33
Fig. 33
That new substances are formed at the cathode and anode may be shown in
the following way. A U-tube, such as is represented in Fig. 33, is
partially filled with a solution of sodium sulphate, and the liquid in
one arm is colored with red litmus, that in the other[Pg 104] with blue litmus.
An electrode placed in the red solution is made to serve as cathode,
while one in the blue solution is made the anode. On allowing the
current to pass, the blue solution turns red, while the red solution
turns blue. These are exactly the changes which would take place if
sodium hydroxide and sulphuric acid were to be set free at the
electrodes, as required by the theory.
The properties of electrolytes depend upon the ions present. When a
substance capable of dissociating into ions is dissolved in water, the
properties of the solution will depend upon two factors: (1) the ions
formed from the substance; (2) the undissociated molecules. Since the
ions are usually more active chemically than the molecules, most of the
chemical properties of an electrolyte are due to the ions rather than to
the molecules.
The solutions of any two substances which give the same ion will have
certain properties in common. Thus all solutions containing the copper
ion (Cu++) are blue, unless the color is modified by the presence of
ions or molecules having some other color.
EXERCISES
1. Distinguish clearly between the following terms: electrolysis,
electrolyte, electrolytic dissociation, ions, solute, solvent, solution,
saturated solution, and supersaturated solution.
2. Why does the water from some natural springs effervesce?
3. (a) Why does not the water of the ocean freeze? (b) Why will ice
and salt produce a lower temperature than ice alone?
4. Why does shaking or stirring make a solid dissolve more rapidly in a
liquid?
5. By experiment it was found that a certain volume of water was
saturated at 100° with 114 g. of potassium nitrate. On cooling to 0° a
portion of the substance crystallized. (a) How many grams of the
substance remained in solution? (b) What was the strength[Pg 105] of the
solution at 18°? (c) How much water had been used in the experiment?
6. (a) 10 g. of common salt were dissolved in water and the solution
evaporated to dryness; what weight of solid was left? (b) 10 g. of
zinc were dissolved in hydrochloric acid and the solution evaporated to
dryness; what weight of solid was left?
7. Account for the fact that sugar sometimes deposits from molasses,
even when no evaporation has taken place.
8. (a) From the standpoint of the theory of electrolytic dissociation,
write the simple equation for a dilute solution of copper sulphate
(CuSO4); this solution is blue. (b) In the same manner, write one
for sodium sulphate; this solution is colorless. (c) How would you
account for the color of the copper sulphate solution?
9. (a) As in the preceding exercise, write a simple equation for a
dilute solution of copper chloride (CuCl2); this solution is blue.
(b) In the same manner, write one for sodium chloride; this solution
is colorless. To what is the blue color due?
10. What component is present in concentrated sulphuric acid that is
almost wanting in very dilute sulphuric acid?
11. Why will vegetables cook faster when boiled in strong salt water
than when boiled in pure water?
12. How do you explain the foaming of soda water?
[Pg 106]
CHAPTER X
ACIDS, BASES, AND SALTS; NEUTRALIZATION
Acids, bases, and salts. The three classes of compounds known
respectively as acids, bases, and salts include the great majority of
the compounds with which we shall have to deal. It is important,
therefore, for us to consider each of these classes in a systematic way.
The individual members belonging to each class will be discussed in
detail in the appropriate places, but a few representatives of each
class will be described in this chapter with special reference to the
common properties in accordance with which they are classified.
The familiar acids. Hydrochloric acidis a gas composed of hydrogen and
chlorine, and has the formula HCl. The substance is very soluble in
water, and it is this solution which is usually called hydrochloric
acid. Nitric acid is a liquid composed of hydrogen, nitrogen, and
oxygen, having the formula HNO3. As sold commercially it is mixed
with about 32% of water. Sulphuric acid, whose composition is
represented by the formula H2SO4, is an oily liquid nearly twice
as heavy as water, and is commonly called oil of vitriol.
Characteristics of acids. (1) All acids contain hydrogen. (2) When
dissolved in water the molecules of the acid dissociate into two kinds
of ions. One of these is always hydrogen and is the cation (+), while
the other consists of the remainder of the molecule and is the anion
(-). (3) The solution tastes sour. (4) It has the power to change the[Pg 107]
color of certain substances called indicators. Thus blue litmus is
changed to red, and yellow methyl orange is changed to red. Since all
acids produce hydrogen cations, while the anions of each are different,
the properties which all acids have in common when in solution, such as
taste and action on indicators, must be attributed to the hydrogen ions.
DEFINITION: An acid is a substance which produces hydrogen ions when
dissolved in water or other dissociating liquids.
Undissociated acids. When acids are perfectly free from water, or are
dissolved in liquids like benzene which do not have the power of
dissociating them into ions, they should have no real acid properties.
This is found to be the case. Under these circumstances they do not
affect the color of indicators or have any of the properties
characteristic of acids.
The familiar bases. The bases most used in the laboratory are sodium
hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide
(Ca(OH)2). These are white solids, soluble in water, the latter
sparingly so. Some bases are very difficultly soluble in water. The very
soluble ones with most pronounced basic properties are sometimes called
the alkalis.
Characteristics of bases. (1) All bases contain hydrogen and oxygen. (2)
When dissolved in water the molecules of the base dissociate into two
kinds of ions. One of these is always composed of oxygen and hydrogen
and is the anion. It has the formula OH and is called the hydroxyl
ion. The remainder of the molecule, which usually consists of a single
atom, is the cation. (3) The solution of a base has[Pg 108] a soapy feel and a
brackish taste. (4) It reverses the color change produced in indicators
by acids, turning red litmus blue, and red methyl orange yellow. Since
all bases produce hydroxyl anions, while the cations of each are
different, the properties which all bases have in common when in
solution must be due to the hydroxyl ions.
DEFINITION: A base is a substance which produces hydroxyl ions when
dissolved in water or other dissociating liquids.
Undissociated bases. Bases, in the absence of water or when dissolved in
liquids which do not dissociate them, should have none of the properties
characteristic of this class of substances. This has been found to be
the case. For example, they have no effect upon indicators under these
circumstances.
Neutralization. When an acid and a base are brought together in solution
in proper proportion, the characteristic properties of each disappear.
The solution tastes neither sour nor brackish; it has no effect upon
indicators. There can therefore be neither hydrogen nor hydroxyl ions
present in the solution. A study of reactions of this kind has shown
that the hydrogen ions of the acid combine with the hydroxyl ions of the
base to form molecules of water, water being a substance which is not
appreciably dissociated into ions. This action of an acid on a base is
called neutralization. The following equations express the
neutralization of the three acids by three bases, water being formed in
each case.
Na+, OH- + H+, Cl- = Na+, Cl- + H2O.
K+, OH- + H+, NO3- = K+, NO3- + H2O.
Ca++, (OH)2— + H2++, SO4- = Ca++, SO4— + 2H2O.
[Pg 109]
DEFINITION: Neutralization consists in the union of the hydrogen ion of
an acid with the hydroxyl ion of a base to form water.
Salts. It will be noticed that in neutralization the anion of the acid
and the cation of the base are not changed. If, however, the water is
expelled by evaporation, these two ions slowly unite, and when the water
becomes saturated with the substance so produced, it separates in the
form of a solid called a salt.
DEFINITION: A salt is a substance formed by the union of the anion of
an acid with the cation of a base.
Characteristics of salts. (1) From the definition of a salt it will be
seen that there is no element or group of elements which characterize
salts. (2) Salts as a class have no peculiar taste. (3) In the absence
of all other substances they are without action on indicators. (4) When
dissolved in water they form two kinds of ions.
Heat of neutralization. If neutralization is due to the union
of hydrogen ions with hydroxyl ions, and nothing more, it
follows that when a given weight of water is formed in
neutralization, the heat set free should always be the same, no
matter from what acid and base the two kinds of ions have been
supplied. Careful experiments have shown that this is the case,
provided no other reactions take place at the same time. When
18g. of water are formed in neutralization, 13,700 cal. of heat
are set free. This is represented in the equations
Na+, OH- + H+, Cl- = Na+, Cl- + H2O + 13,700 cal.
K+, OH- + H+, NO3- = K+, NO3- + H2O + 13,700 cal.
Ca++, (OH)2- + H2++, SO4- = Ca++, SO4- + 2H2O + 2 × 13,700 cal.
Neutralization a quantitative act. Since neutralization is a definite
chemical act, each acid will require a perfectly definite weight of each
base for its neutralization. For[Pg 110] example, a given weight of sulphuric
acid will always require a definite weight of sodium hydroxide, in
accordance with the equation
H2, SO4 + 2Na, OH = Na2, SO4 + 2H2O.
Determination of the ratio in neutralization. The quantities of
acid and base required in neutralization may be determined in
the following way. Dilute solutions of the two substances are
prepared, the sulphuric acid being placed in one of the
burettes (Fig. 34) and the sodium hydroxide in the other. The
levels of the two liquids are then brought to the zero marks of
the burettes by means of the stopcocks. A measured volume of
the acid is drawn off into a beaker, a few drops of litmus
solution added, and the sodium hydroxide is run in drop by drop
until the red litmus just turns blue. The volume of the sodium
hydroxide consumed is then noted. If the concentrations of the
two solutions are known, it is easy to calculate what weight of
sodium hydroxide is required to neutralize a given weight of
sulphuric acid. By evaporating the neutralized solution to
dryness, the weight of the sodium sulphate formed can be
determined directly. Experiment shows that the weights are
always in accordance with the equation in the preceding
paragraph.
 Fig. 34
Fig. 34
Extent of dissociation. The question will naturally arise, When an acid,
base, or salt dissolves in water, do all the molecules dissociate into
ions, or only a part of them? The experiments by which this question can
be answered cannot be described here. It has been found, however, that
only a fraction of the molecules dissociate. The percentage which will
dissociate in a given case depends upon several conditions, the chief of
which are: (1) The concentration of the solution. In concentrated
solutions only a very small[Pg 111] percentage of dissociation occurs. As the
solution is diluted the percentage increases, and in very dilute
solutions it may be very large, though it is never complete in any
ordinary solution. (2) The nature of the dissolved compound. At equal
concentrations substances differ much among themselves in the percentage
of dissociation. The great majority of salts are about equally
dissociated. Acids and bases, on the contrary, show great differences.
Some are freely dissociated, while others are dissociated to but a
slight extent.
Strength of acids and bases. Since acid and basic properties are due to
hydrogen and hydroxyl ions respectively, the acid or base which will
produce the greatest percentage of these ions at a given concentration
must be regarded as the strongest representative of its class. The acids
and bases described in the foregoing paragraphs are all quite strong. In
10% solutions they are dissociated to about 50%, and this is also
approximately the extent to which most salts are dissociated at this
same concentration.
Partial neutralization. 1. Basic salts. The chemical action
between an acid and a base is not always as complete as has
been represented in the foregoing paragraphs. For example, if
the base magnesium hydroxide (Mg(OH)2) and hydrochloric acid
(HCl) are brought together in the ratio of an equal number of
molecules of each, there will be only half enough hydrogen ions
for the hydroxyl ions present.
Mg, (OH)2 + H, Cl = Mg, OH, Cl + H2O.
Magnesium, hydroxyl, and chlorine ions are left at the close of
the reaction, and under the proper conditions unite to form
molecules of the compound Mg(OH)Cl. This compound, when
dissolved, can form hydroxyl ions and therefore possesses basic
properties; it can also form the ions of a salt (Mg and Cl),
and has properties characteristic of salts. Substances of this
kind are called basic salts.
DEFINITION: A basic salt is a substance which can give the
ions both of a base and of a salt when dissolved in water.[Pg 112]
2. Acid salts. In a similar way, when sulphuric acid and
sodium hydroxide are brought together in the ratio of equal
numbers of the molecules of each, it is possible to have a
reaction expressed by the equation
Na, OH + H2, SO4 = Na, H, SO4 + H2O.
The ions remaining after all the hydroxyl ions have been used
up are those of an acid (H) and those of a salt (Na and
SO4). These unite to form the substance NaHSO4, and as
the solution becomes saturated with this substance through
evaporation, it separates in the form of crystals. In solution
this substance can give hydrogen ions, and therefore possesses
acid properties; it can also give the ions characteristic of a
salt. It is therefore called an acid salt.
DEFINITION: An acid salt is one which can give the ions of an
acid and of a salt when in solution.
3. Normal salts. Salts which are the products of complete
neutralization, such as Na2SO4, and which in solution can
give neither hydrogen nor hydroxyl ions, but only the ions of a
salt, are called normal salts to distinguish them from acid
and basic salts.
Methods of expressing reactions between compounds in solution. Chemical
equations representing reactions between substances in solution may
represent the details of the reaction, or they may simply indicate the
final products formed. In the latter case the formation of ions is not
indicated. Thus, if we wish to call attention to the details of the
reaction between sodium hydroxide and hydrochloric acid in solution, the
equation is written as follows:
Na+, OH- + H+, Cl- = Na+, Cl- + H2O.
On the other hand, if we wish simply to represent the final products
formed, the following is used.
Both of these methods will therefore be used:
Radicals. It has been emphasized that the hydroxyl group (OH) always
[Pg 113]forms the anion of a base, while the group NO3 forms the anion of
nitric acid and sodium nitrate; the group SO4, the anion of sulphuric
acid and calcium sulphate. A group of elements which in this way
constitutes a part of a molecule, acting as a unit in a chemical change,
or forming ions in solution, is called a radical. Some of these
radicals have been given special names, the names signifying the
elements present in the radical. Thus we have the hydroxyl radical (OH)
and the nitrate radical (NO3).
DEFINITION: A radical is a group of elements forming part of a
molecule, and acting as a unit in chemical reactions.
Names of acids, bases, and salts. Since acids, bases, and salts are so
intimately related to each other, it is very advantageous to give names
to the three classes in accordance with some fixed system. The system
universally adopted is as follows:
Naming of bases. All bases are called hydroxides. They are
distinguished from each other by prefixing the name of the element which
is in combination with the hydroxyl group. Examples: sodium hydroxide
(NaOH); calcium hydroxide (Ca(OH)2); copper hydroxide (Cu(OH)2).
Naming of acids. The method of naming acids depends upon whether the
acid consists of two elements or three.
1. Binary acids. Acids containing only one element in addition to
hydrogen are called binary acids. They are given names consisting of
the prefix hydro-, the name of the second element present, and the
termination -ic. Examples: hydrochloric acid (HCl); hydrosulphuric
acid (H2S).
2. Ternary acids. In addition to the two elements present in binary
acids, the great majority of acids also contain oxygen. They therefore
consist of three elements and[Pg 114] are called ternary acids. It usually
happens that the same three elements can unite in different proportions
to make several different acids. The most familiar one of these is given
a name ending in the suffix -ic, while the one with less oxygen is
given a similar name, but ending in the suffix -ous. Examples: nitric
acid (HNO3); nitrous acid (HNO2). In cases where more than two
acids are known, use is made of prefixes in addition to the two suffixes
-icand -ous. Thus the prefix per- signifies an acid still richer
in oxygen; the prefix hypo- signifies one with less oxygen.
Naming of salts. A salt derived from a binary acid is given a name
consisting of the names of the two elements composing it, with the
termination -ide. Example: sodium chloride (NaCl). All other binary
compounds are named in the same way.
A salt of a ternary acid is named in accordance with the acid from which
it is derived. A ternary acid with the termination -ic gives a salt
with the name ending in -ate, while an acid with termination -ous
gives a salt with the name ending in -ite. The following table will
make the application of these principles clear:
| ACIDS | SYMBOL | SALTS | SYMBOL |
| Hydrochloric | HCl | Sodium chloride | NaCl |
| Hypochlorous | HClO | Sodium hypochlorite | NaClO |
| Chlorous | HClO2 | Sodium chlorite | NaClO2 |
| Chloric | HClO3 | Sodium chlorate | NaClO3 |
| Perchloric | HClO4 | Sodium perchlorate | NaClO4 |
[Pg 115]
EXERCISES
1. 25 cc. of a solution containing 40 g. of sodium hydroxide per liter
was found to neutralize 25 cc. of a solution of hydrochloric acid. What
was the strength of the acid solution?
2. After neutralizing a solution of sodium hydroxide with nitric acid,
there remained after evaporation 100 g. of sodium nitrate. How much of
each substance had been used?
3. A solution contains 18 g. of hydrochloric acid per 100 cc. It
required 25 cc. of this solution to neutralize 30 cc. of a solution of
sodium hydroxide. What was the strength of the sodium hydroxide solution
in parts per hundred?
4. When perfectly dry sulphuric acid is treated with perfectly dry
sodium hydroxide, no chemical change takes place. Explain.
5. When cold, concentrated sulphuric acid is added to zinc, no change
takes place. Recall the action of dilute sulphuric acid on the same
metal. How do you account for the difference?
6. A solution of hydrochloric acid in benzene does not conduct the
electric current. When this solution is treated with zinc, will hydrogen
be evolved? Explain.
7. (a) Write equation for preparation of hydrogen from zinc and dilute
sulphuric acid. (b) Rewrite the same equation from the standpoint of
the theory of electrolytic dissociation, (c) Subtract the common
SO4 ion from both members of the equation, (d) From the resulting
equation, explain in what the preparation of hydrogen consists when
examined from the standpoint of this theory.
8. In the same manner as in the preceding exercise, explain in what the
action of sodium on water to give hydrogen consists.
[Pg 116]
CHAPTER XI
VALENCE
Definition of valence. A study of the formulas of various binary
compounds shows that the elements differ between themselves in the
number of atoms of other elements which they are able to hold in
combination. This is illustrated in the formulas
| HCl, | H2O, | H3N, | H4C. |
| (hydrochloric acid) | (water) | (ammonia) | (marsh gas) |
It will be noticed that while one atom of chlorine combines with one
atom of hydrogen, an atom of oxygen combines with two, an atom of
nitrogen with three, one of carbon with four. The number which expresses
this combining ratio between atoms is a definite property of each
element and is called its valence.
DEFINITION: The valence of an element is that property which determines
the number of the atoms of another element which its atom can hold in
combination.
Valence a numerical property. Valence is therefore merely a numerical
relation and does not convey any information in regard to the intensity
of the affinity between atoms. Judging by the heat liberated in their
union, oxygen has a far stronger affinity for hydrogen than does
nitrogen, but an atom of oxygen can combine with two atoms only of
hydrogen, while an atom of nitrogen can combine with three.[Pg 117]
Measure of valence. In expressing the valence of an element we must
select some standard for comparison, just as in the measurement of any
other numerical quantity. It has been found that an atom of hydrogen is
never able to hold in combination more than one atom of any other
element. Hydrogen is therefore taken as the standard, and other elements
are compared with it in determining their valence. A number of other
elements are like hydrogen in being able to combine with at most one
atom of other elements, and such elements are called univalent. Among
these are chlorine, iodine, and sodium. Elements such as oxygen,
calcium, and zinc, which can combine with two atoms of hydrogen or other
univalent elements, are said to be divalent. Similarly, we have
trivalent, tetravalent, pentavalent elements. None have a valence of
more than 8.
Indirect measure of valence. Many elements, especially among the metals,
do not readily form compounds with hydrogen, and their valence is not
easy to determine by direct comparison with the standard element. These
elements, however, combine with other univalent elements, such as
chlorine, and their valence can be determined from the compounds so
formed.
Variable valence. Many elements are able to exert different valences
under differing circumstances. Thus we have the compounds Cu2O and
CuO, CO and CO2, FeCl2 and FeCl3. It is not always possible to
assign a fixed valence to an element. Nevertheless each element tends to
exert some normal valence, and the compounds in which it has a valence
different from this are apt to be unstable and easily changed into
compounds in which the valence of the element is normal. The valences of
the various elements will become familiar as the elements are studied in
detail.[Pg 118]
Valence and combining ratios. When elements combine to form compounds,
the ratio in which they combine will be determined by their valences. In
those compounds which consist of two elements directly combined, the
union is between such numbers of the two atoms as have equal valences.
Elements of the same valence will therefore combine atom for atom.
Designating the valence of the atoms by Roman numerals placed above
their symbols, we have the formulas
| II II | II III | I II | IV IV |
| HCl, | ZnO, | BN, | CSi. |
A divalent element, on the other hand, will combine with two atoms of a
univalent element. Thus we have
(the numerals above each symbol representing the sum of the valences of
the atoms of the element present). A trivalent atom will combine with
three atoms of a univalent element, as in the compound
If a trivalent element combines with a divalent element, the union will
be between two atoms of the trivalent element and three of the divalent
element, since these numbers are the smallest which have equal valences.
Thus the oxide of the trivalent metal aluminium has the formula
Al2O3. Finally one atom of a tetravalent element such as carbon
will combine with four atoms of a univalent element, as in the compound
CH4, or with two atoms of a divalent element, as in the compound
CO2.
We have no knowledge as to why elements differ in their combining power,
and there is no way to determine their valences save by experiment.
Valence and the structure of compounds. Compounds will be met
from time to time which are apparent exceptions to the general
statements just made in regard to valence. Thus, from the
formula for hydrogen dioxide (H2O2), it might be
supposed that the oxygen is univalent; yet it is certainly[Pg 119]
divalent in water (H2O). That it may also be divalent in
H2O2 may be made clear as follows: The unit valence of
each element may be represented graphically by a line attached
to its symbol. Univalent hydrogen and divalent oxygen will then
have the symbols H- and -O-. When atoms combine, each unit
valence of one atom combines with a unit valence of another
atom. Thus the composition of water may be expressed by the
formula H-O-H, which is meant to show that each of the unit
valences of oxygen is satisfied with the unit valence of a
single hydrogen atom.
The chemical conduct of hydrogen dioxide leads to the
conclusion that the two oxygen atoms of its molecule are in
direct combination with each other, and in addition each is in
combination with a hydrogen atom. This may be expressed by the
formula H-O-O-H. The oxygen in the compound is therefore
divalent, just as it is in water. It will thus be seen that the
structure of a compound must be known before the valences of
the atoms making up the compound can be definitely decided
upon.
Such formulas as H-O-H and H-O-O-H are known as structural
formulas, because they are intended to show what is known in
regard to the arrangement of the atoms in the molecules.
Valence and the replacing power of atoms. Just as elements having the
same valence combine with each other atom for atom, so if they replace
each other in a chemical reaction they will do so in the same ratio.
This is seen in the following equations, in which a univalent hydrogen
atom is replaced by a univalent sodium atom:
NaOH + HCl = NaCl + H2O.
2NaOH + H2SO4 = Na2SO4 + 2H2O.
Na + H2O = NaOH + H.
Similarly, one atom of divalent calcium will replace two atoms of
univalent hydrogen or one of divalent zinc:
Ca(OH)2 + 2 HCl = CaCl2 + 2H2O.
CaCl2 + ZnSO4 = CaSO4 + ZnCl2.
[Pg 120]
In like manner, one atom of a trivalent element will replace three of a
univalent element, or two atoms will replace three atoms of a divalent
element.
Valence and its applications to formulas of salts. While the true nature
of valence is not understood and many questions connected with the
subject remain unanswered, yet many of the main facts are of much help
to the student. Thus the formula of a salt, differs from that of the
acid from which it is derived in that the hydrogen of the acid has been
replaced by a metal. If, then, it is known that a given metal forms a
normal salt with a certain acid, the formula of the salt can at once be
determined if the valence of the metal is known. Since sodium is
univalent, the sodium salts of the acids HCl and H2SO4 will be
respectively NaCl and Na2SO4. One atom of divalent zinc will
replace 2 hydrogen atoms, so that the corresponding zinc salts will be
ZnCl2 and ZnSO4.
The formula for aluminium sulphate is somewhat more difficult to
determine. Aluminium is trivalent, and the simplest ratio in which the
aluminium atom can replace the hydrogen in sulphuric acid is 2 atoms of
aluminium (6 valences) to 3 molecules of sulphuric acid (6 hydrogen
atoms). The formula of the sulphate will then be Al2(SO4)3.
Valence and its application to equation writing. It will be readily seen
that a knowledge of valence is also of very great assistance in writing
the equations for reactions of double decomposition. Thus, in the
general reaction between an acid and a base, the essential action is
between the univalent hydrogen ion and the univalent hydroxyl ion. The
base and the acid must always be taken in such proportions as to secure
an equal number of each of these ions. Thus, in the reaction between
ferric hydroxide (Fe(OH)3) and sulphuric acid (H2SO4), it will
be necessary to take 2 molecules of the former and 3 of the latter in
order to have an equal number of the two ions, namely, 6. The equation
will then be
2Fe(OH)3 + 3H2SO4 = Fe2(SO4)3 + 6H2O.
Under certain conditions the salts Al2(SO4)3 and CaCl2
undergo double decomposition, the two metals, aluminium and calcium,
exchanging places. The simplest ratio of exchange in this case is 2
atoms of aluminium (6 valences) and 3 atoms of calcium (6 valences).[Pg 121]
The reaction will therefore take place between 1 molecule of
Al2(SO4)3 and 3 of CaCl2, and the equation is as follows:
Al2(SO4)3 + 3 CaCl2 = 3CaSO4 + 2AlCl3.
EXERCISES
1. Sodium, calcium, and aluminium have valences of 1, 2, and 3
respectively; write the formulas of their chlorides, sulphates, and
phosphates (phosphoric acid = H3PO4), on the supposition that they
form salts having the normal composition.
2. Iron forms one series of salts in which it has a valence of 2, and
another series in which it has a valence of 3; write the formulas for
the two chlorides of iron, also for the two sulphates, on the
supposition that these have the normal composition.
3. Write the equation representing the neutralization of each of the
following bases by each of the acids whose formulas are given:
| NaOH | HCl |
| Ba(OH)2 | H2SO4 |
| Al(OH)3 | H3PO4 |
4. Silver acts as a univalent element and calcium as a divalent element
in the formation of their respective nitrates and chlorides. (a) Write
the formula for silver nitrate; for calcium chloride. (b) When
solutions of these two salts are mixed, the two metals, silver and
calcium, exchange places; write the equation for the reaction.
5. Antimony acts as a trivalent element in the formation of a
chloride. (a) What is the formula for antimony chloride? (b) When
hydrosulphuric acid (H2S) is passed into a solution of this chloride
the hydrogen and antimony exchange places; write the equation for the
reaction.
6. Lead has a valence of 2 and iron of 3 in the compounds known
respectively as lead nitrate and ferric sulphate. (a) Write the
formulas for these two compounds. (b) When their solutions are mixed
the two metals exchange places; write the equation for the reaction.
[Pg 122]
CHAPTER XII
COMPOUNDS OF NITROGEN
Occurrence. As has been stated in a former chapter, nitrogen constitutes
a large fraction of the atmosphere. The compounds of nitrogen, however,
cannot readily be obtained from this source, since at any ordinary
temperature nitrogen is able to combine directly with very few of the
elements.
In certain forms of combination nitrogen occurs in the soil from which
it is taken up by plants and built into complex substances composed
chiefly of carbon, hydrogen, oxygen, and nitrogen. Animals feeding on
these plants assimilate the nitrogenous matter, so that this element is
an essential constituent of both plants and animals.
Decomposition of organic matter by bacteria. When living matter dies and
undergoes decay complicated chemical reactions take place, one result of
which is that the nitrogen of the organic matter is set free either as
the element nitrogen, or in the form of simple compounds, such as
ammonia (NH3) or oxides of nitrogen. Experiment has shown that all
such processes of decay are due to the action of different kinds of
bacteria, each particular kind effecting a different change.
Decomposition of organic matter by heat. When organic matter is strongly
heated decomposition into simpler substances takes place in much the
same way as in the case of bacterial decomposition. Coal is a complex
substance of[Pg 123] vegetable origin, consisting largely of carbon, but also
containing hydrogen, oxygen, and nitrogen. When this is heated in a
closed vessel so that air is excluded, about one seventh of the nitrogen
is converted into ammonia, and this is the chief source from which
ammonia and its compounds are obtained.
COMPOUNDS OF NITROGEN WITH HYDROGEN
Ammonia (NH3). Several compounds consisting exclusively of nitrogen
and hydrogen are known, but only one, ammonia, need be considered here.
Preparation of ammonia. Ammonia is prepared in the laboratory by a
different method from the one which is used commercially.
1. Laboratory method. In the laboratory ammonia is prepared from
ammonium chloride, a compound having the formula NH4Cl, and obtained
in the manufacture of coal gas. As will be shown later in the chapter,
the group NH4 in this compound acts as a univalent radical and is
known as ammonium. When ammonium chloride is warmed with sodium
hydroxide, the ammonium and sodium change places, the reaction being
expressed in the following equation.
NH4Cl + NaOH = NaCl + NH4OH.
The ammonium hydroxide (NH4OH) so formed is unstable and breaks down
into water and ammonia.
Calcium hydroxide (Ca(OH)2) is frequently used in place of the more
expensive sodium hydroxide, the equations being
2NH4Cl + Ca(OH)2 = CaCl2 + 2NH4OH,
In the preparation, the ammonium chloride and calcium hydroxide
are mixed together and placed in a flask arranged as shown in
Fig. 35. The mixture is gently warmed, when ammonia is evolved
as a gas and is collected by displacement of air.
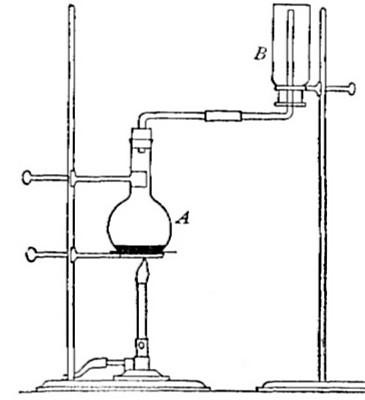 Fig. 35
Fig. 35
2. Commercial method. Nearly all the ammonia of commerce comes from
the gasworks. Ordinary illuminating gas is made by distilling coal, as
will be explained later, and among the products of this distillation a
solution of ammonia in water is obtained. This solution, known as gas
liquor, contains not only ammonia but other soluble substances. Most of
these combine chemically with lime, while ammonia does not; if then lime
is added to the gas liquor and the liquor is heated, the ammonia is
driven out from the mixture. It may be dissolved again in pure, cold
water, forming aqua ammonia, or the ammonia water of commerce.
Preparation from hydrogen and nitrogen. When electric sparks
are passed for some time through a mixture of hydrogen and
nitrogen, a small percentage of the two elements in the mixture
is changed into ammonia. The action soon ceases, however, for
the reason that ammonia is decomposed by the electric
discharge. The reaction expressed in the equation
N + 3H = NH3
can therefore go in either direction depending upon the
relative quantities of the substances present. This recalls the
similar change from oxygen into ozone, which soon ceases
because the ozone is in turn decomposed into oxygen.
[Pg 125]
Physical properties. Under ordinary conditions ammonia is a gas whose
density is 0.59. It is therefore little more than half as heavy as air.
It is easily condensed into a colorless liquid, and can now be purchased
in liquid form in steel cylinders. The gas is colorless and has a
strong, suffocating odor. It is extremely soluble in water, 1 l. of
water at 0° and 760 mm. pressure dissolving 1148 l. of the gas. In
dissolving this large volume of gas the water expands considerably, so
that the density of the solution is less than that of water, the
strongest solutions having a density of 0.88.
Chemical properties. Ammonia will not support combustion, nor will it
burn under ordinary conditions. In an atmosphere of oxygen it burns with
a feeble, yellowish flame. When quite dry it is not a very active
substance, but when moist it combines with a great many substances,
particularly with acids.
Uses. It has been stated that ammonia can be condensed to a liquid by
the application of pressure. If the pressure is removed from the liquid
so obtained, it rapidly passes again into the gaseous state and in so
doing absorbs a large amount of heat. Advantage is taken of this fact in
the preparation of artificial ice. Large quantities of ammonia are also
used in the preparation of ammonium compounds.
The manufacture of artificial ice. Fig. 36 illustrates the
method of preparing artificial ice. The ammonia gas is
liquefied in the pipes X by means of the pump Y. The heat
generated is absorbed by water flowing over the pipes. The
pipes lead into a large brine tank, a cross section of which is
shown in the figure. Into the brine (concentrated solution of
common salt) contained in this tank are dipped the vessels A,
B, C, filled with pure water. The pressure is removed from
the liquid ammonia as it passes into the pipes immersed in the[Pg 126]
brine, and the heat absorbed by the rapid evaporation of the
liquid lowers the temperature of the brine below zero. The
water in A, B, C is thereby frozen into cakes of ice. The
gaseous ammonia resulting from the evaporation of the liquid
ammonia is again condensed, so that the process is continuous.
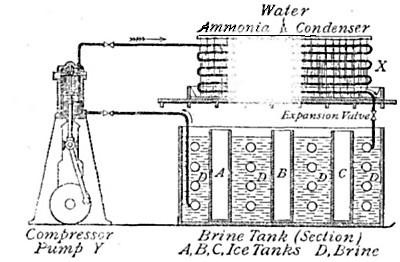 Fig. 36
Fig. 36
Ammonium hydroxide (NH4OH). The solution of ammonia in water is found
to have strong basic properties and therefore contains hydroxyl ions. It
turns red litmus blue; it has a soapy feel; it neutralizes acids,
forming salts with them. It seems probable, therefore, that when ammonia
dissolves in water it combines chemically with it according to the
equation
and that it is the substance NH4OH, called ammonium hydroxide, which
has the basic properties, dissociating into the ions NH4 and OH.
Ammonium hydroxide has never been obtained in a pure state. At every
attempt to isolate it the substance breaks up into water and ammonia,—
The ammonium radical. The radical NH4 plays the part of a metal in
many chemical reactions and is called ammonium. The ending -ium is
given to the name to indicate the metallic properties of the substance,
since the names[Pg 127] of the metals in general have that ending. The salts
formed by the action of the base ammonium hydroxide on acids are called
ammonium salts. Thus, with hydrochloric acid, ammonium chloride is
formed in accordance with the equation
NH4OH + HCl = NH4Cl + H2O.
Similarly, with nitric acid, ammonium nitrate (NH4NO3) is formed,
and with sulphuric acid, ammonium sulphate ((NH4)2S04).
It will be noticed that in the neutralization of ammonium hydroxide by
acids the group NH4 replaces one hydrogen atom of the acid, just as
sodium does. The group therefore acts as a univalent metal.
Combination of nitrogen with hydrogen by volume. Under suitable
conditions ammonia can be decomposed into nitrogen and hydrogen by
passing electric sparks through the gas. Accurate measurement has shown
that when ammonia is decomposed, two volumes of the gas yield one volume
of nitrogen and three volumes of hydrogen. Consequently, if the two
elements were to combine directly, one volume of nitrogen would combine
with three volumes of hydrogen to form two volumes of ammonia. Here, as
in the formation of steam from hydrogen and oxygen, small whole numbers
serve to indicate the relation between the volumes of combining gases
and that of the gaseous product.
COMPOUNDS OF NITROGEN WITH OXYGEN AND HYDROGEN
In addition to ammonium hydroxide, nitrogen forms several compounds with
hydrogen and oxygen, of which nitric acid (HNO3) and nitrous acid
[Pg 128](HNO2) are the most familiar.
Nitric acid (HNO3). Nitric acid is not found to any extent in nature,
but some of its salts, especially sodium nitrate (NaNO3) and
potassium nitrate (KNO3) are found in large quantities. From these
salts nitric acid can be obtained.
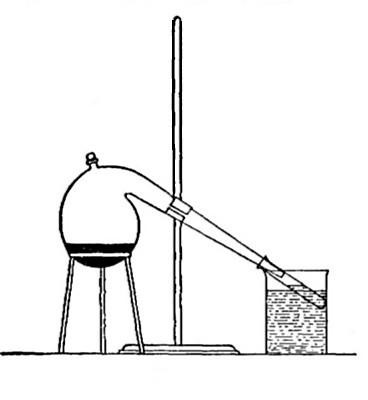 Fig. 37
Fig. 37
Preparation of nitric acid. When sodium nitrate is treated with
concentrated cold sulphuric acid, no chemical action seems to take
place. If, however, the mixture is heated in a retort, nitric acid is
given off as a vapor and may be easily condensed to a liquid by passing
the vapor into a tube surrounded by cold water, as shown in Fig. 37. An
examination of the liquid left in the retort shows that it contains
sodium acid sulphate (NaHSO4), so that the reaction may be
represented by the equation
NaNO3 + H2SO4 = NaHSO4 + HNO3.
If a smaller quantity of sulphuric acid is taken and the
mixture is heated to a high temperature, normal sodium sulphate
is formed:
2NaNO3 + H2SO4 = Na2SO4 + 2HNO3.
In this case, however, the higher temperature required
decomposes a part of the nitric acid.
The commercial preparation of nitric acid. Fig. 38 illustrates
a form of apparatus used in the preparation of nitric acid on a
large scale. Sodium nitrate and sulphuric acid are heated in
the iron retort A. The resulting acid vapors pass in the
direction indicated by the arrows, and are condensed in the
glass tubes B, which are covered with cloth kept cool by
streams of water. These tubes are inclined so that the liquid
resulting from the condensation of the vapors runs back into
C and is drawn off into large vessels (D).
[Pg 129]
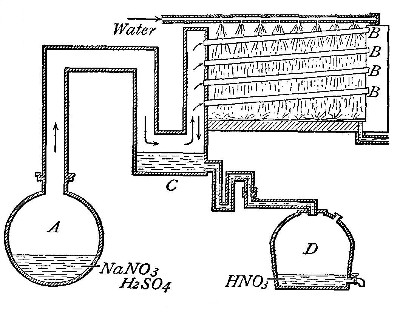 Fig. 38
Fig. 38
Physical properties of nitric acid. Pure nitric acid is a colorless
liquid, which boils at about 86° and has a density of 1.56. The
concentrated acid of commerce contains about 68% of the acid, the
remainder being water. Such a mixture has a density of 1.4. The
concentrated acid fumes somewhat in moist air, and has a sharp choking
odor.
Chemical properties. The most important chemical properties of nitric
acid are the following.
1. Acid properties. As the name indicates, this substance is an acid,
and has all the properties of that class of substances. It changes blue
litmus red and has a sour taste in dilute solutions. It forms hydrogen
ions in solution and neutralizes bases forming salts. It also acts upon
the oxides of most metals, forming a salt and water. It is one of the
strongest acids.
2. Decomposition on heating. When boiled, or exposed for some time to
sunlight, it suffers a partial decomposition according to the equation
The substance NO2, called nitrogen peroxide, is a brownish gas, which
is readily soluble in water and in nitric acid. It therefore dissolves
in the undecomposed acid, and imparts a yellowish or reddish color to
it. Concentrated[Pg 130] nitric acid highly charged with this substance is
called fuming nitric acid.
3. Oxidizing action. According to its formula, nitric acid contains a
large percentage of oxygen, and the reaction just mentioned shows that
the compound is not a very stable one, easily undergoing decomposition.
These properties should make it a good oxidizing agent, and we find that
this is the case. Under ordinary circumstances, when acting as an
oxidizing agent, it is decomposed according to the equation
The oxygen is taken up by the substance oxidized, and not set free, as
is indicated in the equation. Thus, if carbon is oxidized by nitric
acid, the oxygen combines with carbon, forming carbon dioxide (CO2):
4. Action on metals. We have seen that when an acid acts upon a metal
hydrogen is set free. Accordingly, when nitric acid acts upon a metal,
such as copper, we should expect the reaction to take place which is
expressed in the equation
Cu + 2HNO3 = Cu(NO3)2 + 2H.
This reaction does take place, but the hydrogen set free is immediately
oxidized to water by another portion of the nitric acid according to the
equation
As these two equations are written, two atoms of hydrogen are given off
in the first equation, while three are used up in the second. In order
that the hydrogen may be equal in[Pg 131] the two equations, we must multiply
the first by 3 and the second by 2. We shall then have
3Cu + 6HNO3 = 3Cu(NO3)2 + 6H,
2HNO3 + 6H = 4H2O + 2NO.
The two equations may now be combined into one by adding the quantities
on each side of the equality sign, canceling the hydrogen which is given
off in the one reaction and used up in the other. We shall then have the
equation
3Cu + 8HNO3 = 3Cu(NO3)2 + 2NO + 4H2O.
A number of other reactions may take place when nitric acid acts upon
metals, resulting in the formation of other oxides of nitrogen, free
nitrogen, or even ammonia. The reaction just given is, however, the
usual one.
Importance of steps in a reaction. This complete equation has
the advantage of making it possible to calculate very easily
the proportions in which the various substances enter into the
reaction or are formed in it. It is unsatisfactory in that it
does not give full information about the way in which the
reaction takes place. For example, it does not suggest that
hydrogen is at first formed, and subsequently transformed into
water. It is always much more important to remember the steps
in a chemical reaction than to remember the equation expressing
the complete action; for if these steps in the reaction are
understood, the complete equation is easily obtained in the
manner just described.
Salts of nitric acid,—nitrates. The salts of nitric acid are called
nitrates. Many of these salts will be described in the study of the
metals. They are all soluble in water, and when heated to a high
temperature undergo decomposition. In a few cases a nitrate on being
heated evolves oxygen, forming a nitrite:
[Pg 132]
In other cases the decomposition goes further, and the metal is left as
oxide:
Cu(NO3)2 = CuO + 2NO2 + O.
Nitrous acid (HNO2). It is an easy matter to obtain sodium nitrite
(NaNO2), as the reaction given on the previous page indicates.
Instead of merely heating the nitrate, it is better to heat it together
with a mild reducing agent, such as lead, when the reaction takes place
which is expressed by the equation
NaNO3 + Pb = PbO + NaNO2.
When sodium nitrite is treated with an acid, such as sulphuric acid, it
is decomposed and nitrous acid is set free:
NaNO2 + H2SO4 = NaHSO4 + HNO2.
The acid is very unstable, however, and decomposes readily into water
and nitrogen trioxide (N2O3):
Dilute solutions of the acid, however, can be obtained.
COMPOUNDS OF NITROGEN WITH OXYGEN
Nitrogen combines with oxygen to form five different oxides. The
formulas and names of these are as follows:
| N2O | nitrous oxide. |
| NO | nitric oxide. |
| NO2 | nitrogen peroxide. |
| N2O3 | nitrogen trioxide, or nitrous anhydride. |
| N2O5 | nitrogen pentoxide, or nitric anhydride. |
These will now be briefly discussed.
Nitrous oxide (laughing gas) (N2O). Ammonium nitrate, like all
nitrates, undergoes decomposition when heated; and owing to the fact
that it contains no metal, but does[Pg 133] contain both oxygen and hydrogen,
the reaction is a peculiar one. It is represented by the equation
The oxide of nitrogen so formed is called nitrous oxide or laughing gas.
It is a colorless gas having a slight odor. It is somewhat soluble in
water, and in solution has a slightly sweetish taste. It is easily
converted into a liquid and can be purchased in this form. When inhaled
it produces a kind of hysteria (hence the name "laughing gas"), and even
unconsciousness and insensibility to pain if taken in large amounts. It
has long been used as an anæsthetic for minor surgical operations, such
as those of dentistry, but owing to its unpleasant after effects it is
not so much in use now as formerly.
Chemically, nitrous oxide is remarkable for the fact that it is a very
energetic oxidizing agent. Substances such as carbon, sulphur, iron, and
phosphorus burn in it almost as brilliantly as in oxygen, forming oxides
and setting free nitrogen. Evidently the oxygen in nitrous oxide cannot
be held in very firm combination by the nitrogen.
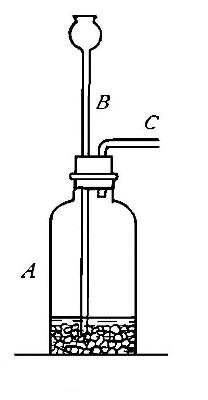 Fig. 39
Fig. 39
Nitric oxide (NO). We have seen that when nitric acid acts upon metals,
such as copper, the reaction represented by the following equation takes
place:
3Cu + 8HNO3 = 3Cu(NO3)3 + 2NO + 4H2O.
Nitric oxide is most conveniently prepared in this way. The metal is
placed in the flask A (Fig. 39) and the acid added slowly through the
funnel tube B. The gas escapes through C and is collected over
water.[Pg 134]
Pure nitric oxide is a colorless gas, slightly heavier than air, and is
practically insoluble in water. It is a difficult gas to liquefy. Unlike
nitrous oxide, nitric oxide does not part with its oxygen easily, and
burning substances introduced into this gas are usually extinguished. A
few substances like phosphorus, which have a very strong affinity for
oxygen and which are burning energetically in the air, will continue to
burn in an atmosphere of nitric oxide. In this case the nitric oxide
loses all of its oxygen and the nitrogen is set free as gas.
Action of nitric oxide with oxygen. When nitric oxide comes into contact
with oxygen or with the air, it at once combines with the oxygen even at
ordinary temperatures, forming a reddish-yellow gas of the formula
NO2, which is called nitrogen peroxide. This action is not energetic
enough to produce a flame, though considerable heat is set free.
Nitrogen peroxide (NO2). This gas, as we have just seen, is formed by
allowing nitric oxide to come into contact with oxygen. It can also be
made by heating certain nitrates, such as lead nitrate:
Pb(NO3)2 = PbO + 2NO2 + O.
It is a reddish-yellow gas of unpleasant odor, which is quite poisonous
when inhaled. It is heavier than air and is easily condensed to a
liquid. It dissolves in water, but this solution is not a mere physical
solution; the nitrogen peroxide is decomposed, forming a mixture of
nitric and nitrous acids:
2NO2 + H2O = HNO2 + HNO3.
Nitrogen peroxide will not combine with more oxygen; it will, however,
give up a part of its oxygen to burning substances, acting as an
oxidizing agent:
[Pg 135]
Acid anhydrides. The oxides N2O3 (nitrogen trioxide) and
N2O5 (nitrogen pentoxide) are rarely prepared and need not be
separately described. They bear a very interesting relation to the acids
of nitrogen. When dissolved in water they combine with the water,
forming acids:
N2O3 + H2O = 2HNO2,
N2O5 + H2O = 2HNO3.
On the other hand, nitrous acid very easily decomposes, yielding water
and nitrogen trioxide, and by suitable means nitric acid likewise may be
decomposed into water and nitrogen pentoxide:
2HNO2 = H2O + N2O3,
2HNO3 = H2O + N2O5.
In view of the close relation between these oxides and the corresponding
acids, they are called anhydrides of the acids, N2O3 being
nitrous anhydride and N2O5 nitric anhydride.
DEFINITION: Any oxide which will combine with water to form an acid, or
which together with water is formed by the decomposition of an acid, is
called an anhydride of that acid.
EXERCISES
1. Perfectly dry ammonia does not affect litmus paper. Explain.
2. Can ammonia be dried by passing the gas through concentrated
sulphuric acid? Explain.
3. Ammonium hydroxide is a weak base, i.e. it is not highly dissociated.
When it is neutralized by strong acids the heat of reaction is less than
when strong bases are so neutralized. Suggest some possible cause for
this.
4. Why is brine used in the manufacture of artificial ice?
5. Discuss the energy changes which take place in the manufacture of
artificial ice.[Pg 136]
6. What weight of ammonium chloride is necessary to furnish enough
ammonia to saturate 1 l. of water at 0° and 760 mm.?
7. What weight of sodium nitrate is necessary to prepare 100 cc. of
commercial nitric acid? What weight of potassium nitrate is necessary to
furnish the same weight of acid?
8. 100 l. of nitrogen peroxide were dissolved in water and neutralized
with sodium hydroxide. What substances were formed and how much of
each?(1 l. nitrogen peroxide weighs 2.05 grams.)
9. How many liters of nitrous oxide, measured under standard conditions,
can be prepared from 10 g. of ammonium nitrate?
10. What weight of copper is necessary to prepare 50 l. of nitric oxide
under standard conditions?
11. (a) Calculate the percentage composition of the oxides of
nitrogen. (b) What important law does this series of substances
illustrate?
12. Write the equations representing the reactions between ammonium
hydroxide, and sulphuric acid and nitric acid respectively, in
accordance with the theory of electrolytic dissociation.
13. In the same way, write the equations representing the reactions
between nitric acid and each of the following bases: NaOH, KOH,
NH4OH, Ca(OH)2.
[Pg 137]
CHAPTER XIII
REVERSIBLE REACTIONS AND CHEMICAL EQUILIBRIUM
Reversible reactions. The reactions so far considered have been
represented as continuing, when once started, until one or the other
substance taking part in the reaction has been used up. In some
reactions this is not the case. For example, we have seen that when
steam is passed over hot iron the reaction is represented by the
equation
On the other hand, when hydrogen is passed over hot iron oxide the
reverse reaction takes place:
The reaction can therefore go in either direction, depending upon the
conditions of the experiment. Such a reaction is called a reversible
reaction. It is represented by an equation with double arrows in place
of the equality sign, thus:
3Fe + 4H2O <--> Fe3O4 + 8H.
In a similar way, the equation
expresses the fact that under some conditions nitrogen may unite with
hydrogen to form ammonia, while under other conditions ammonia
decomposes into nitrogen and hydrogen.
The conversion of oxygen into ozone is also reversible and may be
represented thus:
[Pg 138]
Chemical equilibrium. Reversible reactions do not usually go on to
completion in one direction unless the conditions under which the
reaction takes place are very carefully chosen. Thus, if iron and steam
are confined in a heated tube, the steam acts upon the iron, producing
iron oxide and hydrogen. But these substances in turn act upon each
other to form iron and steam once more. When these two opposite
reactions go on at such rates that the weight of the iron changed into
iron oxide is just balanced by the weight of the iron oxide changed into
iron, there will be no further change in the relative weights of the
four substances present in the tube. The reaction is then said to have
reached an equilibrium.
Factors which determine the point of equilibrium. There are two factors
which have a great deal of influence in determining the point at which a
given reaction will reach equilibrium.
1. Influence of the chemical nature of the substances. If two
reversible reactions of the same general kind are selected, it has been
found that the point of equilibrium is different in the two cases. For
example, in the reactions represented by the equations
3Fe + 4H2O <--> Fe3O4 + 8H,
Zn + H2O <--> ZnO + 2H,
the equilibrium will be reached when very different quantities of the
iron and zinc have been changed into oxides. The individual chemical
properties of the iron and zinc have therefore marked influence upon the
point at which equilibrium will be reached.
2. Influence of relative mass. If the tube in which the reaction
3Fe + 4H2O <--> Fe3O4 + 8H
[Pg 139]
has come to an equilibrium is opened and more steam is admitted, an
additional quantity of the iron will be changed into iron oxide. If more
hydrogen is admitted, some of the oxide will be reduced to metal. The
point of equilibrium is therefore dependent upon the relative masses of
the substances taking part in the reaction. When one of the substances
is a solid, however, its mass has little influence, since it is only the
extent of its surface which can affect the reaction.
Conditions under which reversible reactions are complete. If, when the
equilibrium between iron and steam has been reached, the tube is opened
and a current of steam is passed in, the hydrogen is swept away as fast
as it is formed. The opposing reaction of hydrogen upon iron oxide must
therefore cease, and the action of steam on the iron will go on until
all of the iron has been transformed into iron oxide.
On the other hand, if a current of hydrogen is admitted into the tube,
the steam will be swept away by the hydrogen, and all of the iron oxide
will be reduced to iron. A reversible reaction can therefore be
completed in either direction when one of the products of the reaction
is removed as fast as it is formed.
Equilibrium in solution. When reactions take place in solution in water
the same general principles hold good. The matter is not so simple,
however, as in the case just described, owing to the fact that many of
the reactions in solution are due to the presence of ions. The
substances most commonly employed in solution are acids, bases, or
salts, and all of these undergo dissociation. Any equilibrium which may
be reached in solutions of these substances must take place between the
various ions formed, on the[Pg 140] one hand, and the undissociated molecules,
on the other. Thus, when nitric acid is dissolved in water, equilibrium
is reached in accordance with the equation
Conditions under which reversible reactions in solution are complete.
The equilibrium between substances in solution may be disturbed and the
reaction caused to go on in one direction to completion in either of
three ways.
1. A gas may be formed which escapes from the solution. When sodium
nitrate and sulphuric acid are brought together in solution all four
ions, Na+, NO3-, H+, SO4-, are formed. These ions are
free to rearrange themselves in various combinations. For example, the
H+ and the NO3- ions will reach the equilibrium
If the experiment is performed with very little water present, as is the
case in the preparation of nitric acid, the equilibrium will be reached
when most of the H+ and the NO3- ions have combined to form
undissociated HNO3.
Finally, if the mixture is now heated above the boiling point of nitric
acid, the acid distills away as fast as it is formed. More and more
H+ and NO3- ions will then combine, and the process will
continue until one or the other of them has all been removed from the
solution. The substance remaining is sodium acid sulphate (NaHSO4),
and the reaction can therefore be expressed by the equation
NaNO3 + H2SO4 = NaHSO4 + HNO3.
2. An insoluble solid may be formed. When hydrochloric acid (HCl) and
[Pg 141]silver nitrate (AgNO3) are brought together in solution the
following ions will be present: H+, Cl-, Ag+, NO3-. The
ions Ag+ and Cl- will then set up the equilibrium
But silver chloride (AgCl) is almost completely insoluble in water, and
as soon as a very little of it has formed the solution becomes
supersaturated, and the excess of the salt precipitates. More silver and
chlorine ions then unite, and this continues until practically all of
the silver or the chlorine ions have been removed from the solution. We
then say that the following reaction is complete:
AgNO3 + HCl = AgCl + HNO3.
3. Two different ions may form undissociated molecules. In the
neutralization of sodium hydroxide by hydrochloric acid the ions H+
and OH- come to the equilibrium
But since water is almost entirely undissociated, equilibrium can only
be reached when there are very few hydroxyl or hydrogen ions present.
Consequently the two ions keep uniting until one or the other of them is
practically removed from the solution. When this occurs the
neutralization expressed in the following equation is complete:
Preparation of acids. The principle of reversible reactions finds
practical application in the preparation of most of the common acids. An
acid is usually prepared by treating the most common of its salts with
some other acid of high boiling point. The mixture is then heated until
the lower boiling acid desired distills out. Owing to[Pg 142] its high boiling
point (338°), sulphuric acid is usually employed for this purpose, most
other acids boiling below that temperature.
EXERCISES
1. What would take place when solutions of silver nitrate and sodium
chloride are brought together? What other chlorides would act in the
same way?
2. Is the reaction expressed by the equation NH3 + H2O = NH4OH
reversible? If so, state the conditions under which it will go in each
direction.
3. Is the reaction expressed by the equation 2H + O = H2O reversible?
If so, state the conditions under which it will go in each direction.
4. Suggest a method for the preparation of hydrochloric acid.
[Pg 143]
CHAPTER XIV
SULPHUR AND ITS COMPOUNDS
Occurrence. The element sulphur has been known from the earliest times,
since it is widely distributed in nature and occurs in large quantities
in the uncombined form, especially in the neighborhood of volcanoes.
Sicily has long been famous for its sulphur mines, and smaller deposits
are found in Italy, Iceland, Mexico, and especially in Louisiana, where
it is mined extensively. In combination, sulphur occurs abundantly in
the form of sulphides and sulphates. In smaller amounts it is found in a
great variety of minerals, and it is a constituent of many animal and
vegetable substances.
Extraction of sulphur. Sulphur is prepared from the native substance,
the separation of crude sulphur from the rock and earthy materials with
which it is mixed being a very simple process. The ore from the mines is
merely heated until the sulphur melts and drains away from the earthy
impurities. The crude sulphur obtained in this way is distilled in a
retort-shaped vessel made of iron, the exit tube of which opens into a
cooling chamber of brickwork. When the sulphur vapor first enters the
cooling chamber it condenses as a fine crystalline powder called
flowers of sulphur. As the condensing chamber becomes warm, the
sulphur collects as a liquid in it, and is drawn off into cylindrical
molds, the product being called roll sulphur or brimstone.[Pg 144]
Physical properties. Roll sulphur is a pale yellow, crystalline solid,
without marked taste and with but a faint odor. It is insoluble in
water, but is freely soluble in a few liquids, notably in carbon
disulphide. Roll sulphur melts at 114.8°. Just above the melting point
it forms a rather thin, straw-colored liquid. As the temperature is
raised, this liquid turns darker in color and becomes thicker, until at
about 235° it is almost black and is so thick that the vessel containing
it can be inverted without danger of the liquid running out. At higher
temperatures it becomes thin once more, and boils at 448°, forming a
yellowish vapor. On cooling the same changes take place in reverse
order.
Varieties of sulphur. Sulphur is known in two general forms, crystalline
and amorphous. Each of these forms exists in definite modifications.
Crystalline sulphur. Sulphur occurs in two crystalline forms, namely,
rhombic sulphur and monoclinic sulphur.
1. Rhombic sulphur. When sulphur crystallizes from its solution in
carbon disulphide it separates in crystals which have the same color and
melting point as roll sulphur, and are rhombic in shape. Roll sulphur is
made up of minute rhombic crystals.
2. Monoclinic sulphur. When melted sulphur is allowed to cool until a
part of the liquid has solidified, and the remaining liquid is then
poured off, it is found that the solid sulphur remaining in the vessel
has assumed the form of fine needle-shaped crystals. These differ much
in appearance from the rhombic crystals obtained by crystallizing
sulphur from its solution in carbon disulphide. The needle-shaped form
is called monoclinic sulphur. The two varieties differ also in density
and in melting point, the monoclinic sulphur melting at 120°.[Pg 145]
Monoclinic and rhombic sulphur remain unchanged in contact with each
other at 96°. Above this temperature the rhombic changes into
monoclinic; at lower temperatures the monoclinic changes into rhombic.
The temperature 96° is therefore called the transition point of sulphur.
Heat is set free when monoclinic sulphur changes into rhombic.
Amorphous sulphur. Two varieties of amorphous sulphur can be readily
obtained. These are white sulphur and plastic sulphur.
1. White sulphur. Flowers of sulphur, the preparation of which has
been described, consists of a mixture of rhombic crystals and amorphous
particles. When treated with carbon disulphide, the crystals dissolve,
leaving the amorphous particles as a white residue.
2. Plastic sulphur. When boiling sulphur is poured into cold water it
assumes a gummy, doughlike form, which is quite elastic. This can be
seen in a very striking manner by distilling sulphur from a small,
short-necked retort, such as is represented in Fig. 40, and allowing the
liquid to run directly into water. In a few days it becomes quite
brittle and passes over into ordinary rhombic sulphur.
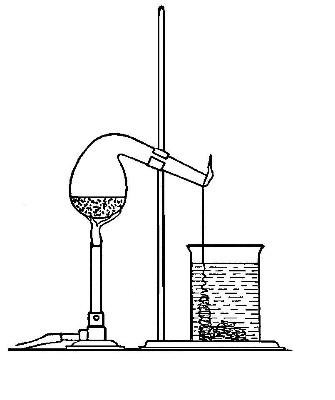 Fig. 40
Fig. 40
Chemical properties of sulphur. When sulphur is heated to its kindling
temperature in oxygen or in the air it burns with a pale blue flame,
forming sulphur dioxide (SO2). Small quantities of sulphur trioxide
[Pg 146](SO3) may also be formed in the combustion of sulphur. Most metals
when heated with sulphur combine directly with it, forming metallic
sulphides. In some cases the action is so energetic that the mass
becomes incandescent, as has been seen in the case of iron uniting with
sulphur. This property recalls the action of oxygen upon metals, and in
general the metals which combine readily with oxygen are apt to combine
quite readily with sulphur.
Uses of sulphur. Large quantities of sulphur are used as a germicide in
vineyards, also in the manufacture of gunpowder, matches, vulcanized
rubber, and sulphuric acid.
COMPOUNDS OF SULPHUR WITH HYDROGEN
Hydrosulphuric acid (H2S). This substance is a gas having the
composition expressed by the formula H2S and is commonly called
hydrogen sulphide. It is found in the vapors issuing from volcanoes, and
in solution in the so-called sulphur waters of many springs. It is
formed when organic matter containing sulphur undergoes decay, just as
ammonia is formed under similar circumstances from nitrogenous matter.
Preparation. Hydrosulphuric acid is prepared in the laboratory by
treating a sulphide with an acid. Iron sulphide (FeS) is usually
employed:
FeS + 2HCl = FeCl2 + H2S.
A convenient apparatus is shown in Fig. 41. A few lumps of iron sulphide
are placed in the bottle A, and dilute acid is added in small
quantities at a time through the funnel tube B, the gas escaping
through the tube C.
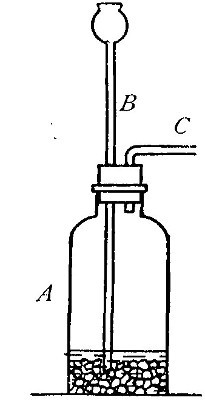 Fig. 41
Fig. 41
[Pg 147]
Explanation of the reaction. Iron sulphide is a salt of
hydrosulphuric acid, and this reaction is therefore similar to
the one which takes place when sulphuric acid acts upon a
nitrate. In both cases a salt and an acid are brought together,
and there is a tendency for the reaction to go on until a state
of equilibrium is reached. This equilibrium is constantly
disturbed by the escape of the gaseous acid set free, so that
the reaction goes on until all of the original salt has been
decomposed. The two reactions differ in that the first one is
complete at ordinary temperatures, while in the case of
sulphuric acid acting upon sodium nitrate, the reacting
substances must be heated so as to secure a temperature at
which nitric acid is a gas.
Physical properties. Hydrosulphuric acid is a colorless gas, having a
weak, disagreeable taste and an exceedingly offensive odor. It is rather
sparingly soluble in water at ordinary temperatures, about three volumes
dissolving in one of water. In boiling water it is not soluble at all.
In pure form it acts as a violent poison, and even when diluted largely
with air produces headache, dizziness, and nausea. It is a little
heavier than air, having a density of 1.18.
Chemical properties. The most important chemical properties of
hydrosulphuric acid are the following:
1. Acid properties. Hydrosulphuric acid is a weak acid. In solution in
water it turns blue litmus red and neutralizes bases, forming salts
called sulphides.
2. Action on oxygen. The elements composing hydrosulphuric acid have
each a strong affinity for oxygen, and are not held together very
firmly. Consequently the gas burns readily in oxygen or the air,
according to the equation
When there is not enough oxygen for both the sulphur and the hydrogen,
the latter element combines with the oxygen and the sulphur is set free:
[Pg 148]
3. Reducing action. Owing to the ease with which hydrosulphuric acid
decomposes and the strong affinity of both sulphur and hydrogen for
oxygen, the substance is a strong reducing agent, taking oxygen away
from many substances which contain it.
4. Action on metals. Hydrosulphuric acid acts towards metals in a way
very similar to water. Thus, when it is passed over heated iron in a
tube, the reaction is represented by the equation
Water in the form of steam, under similar circumstances, acts according
to the equation
Salts of hydrosulphuric acid,—sulphides. The salts of hydrosulphuric
acid, called sulphides, form an important class of salts. Many of them
are found abundantly in nature, and some of them are important ores.
They will be frequently mentioned in connection with the metals.
Most of the sulphides are insoluble in water, and some of them are
insoluble in acids. Consequently, when hydrosulphuric acid is passed
into a solution of a salt, it often happens that a sulphide is
precipitated. With copper chloride the equation is
CuCl2 + H2S = CuS + 2HCl.
Because of the fact that some metals are precipitated in this way as
sulphides while others are not, hydrosulphuric acid is extensively used
in the separation of the metals in the laboratory.
[Pg 149]
Explanation of the reaction. When hydrosulphuric acid and
copper chloride are brought together in solution, both copper
and sulphur ions are present, and these will come to an
equilibrium, as represented in the equation
Cu+ + S- <--> CuS.
Since copper sulphide is almost insoluble in water, as soon as
a very small quantity has formed the solution becomes
supersaturated, and the excess keeps precipitating until nearly
all the copper or sulphur ions have been removed from the
solution. With some other ions, such as iron, the sulphide
formed does not saturate the solution, and no precipitate
results.
OXIDES OF SULPHUR
Sulphur forms two well-known compounds with oxygen: sulphur dioxide
(SO2), sometimes called sulphurous anhydride; and sulphur trioxide
(SO3), frequently called sulphuric anhydride.
Sulphur dioxide (SO2). Sulphur dioxide occurs in nature in the gases
issuing from volcanoes, and in solution in the water of many springs. It
is likely to be found wherever sulphur compounds are undergoing
oxidation.
Preparation. Three general ways may be mentioned for the preparation of
sulphur dioxide:
1. By the combustion of sulphur. Sulphur dioxide is readily formed by
the combustion of sulphur in oxygen or the air:
It is also formed when substances containing sulphur are burned:
2. By the reduction of sulphuric acid. When concentrated sulphuric
acid is heated with certain metals, such as copper, part of the acid is
changed into copper sulphate, and part is reduced to sulphurous acid.
The latter then decomposes into sulphur dioxide and water, the complete
equation being
Cu + 2H2SO4 = CuSO4 + SO2 + 2H2O.
[Pg 150]
3. By the action of an acid on a sulphite. Sulphites are salts of
sulphurous acid (H2SO3). When a sulphite is treated with an acid,
sulphurous acid is set free, and being very unstable, decomposes into
water and sulphur dioxide. These reactions are expressed in the
equations
Na2SO3 + 2HCl = 2NaCl + H2SO3,
H2SO3 = H2O + SO2.
Explanation of the reaction. In this case we have two reversible
reactions depending on each other. In the first reaction,
(1) Na2SO3 + 2HCl <--> 2NaCl + H2SO3,
we should expect an equilibrium to result, for none of the four
substances in the equation are insoluble or volatile when water is
present to hold them in solution. But the quantity of the H2SO3 is
constantly diminishing, owing to the fact that it decomposes, as
represented in the equation
(2) H2SO3 <--> H2O + SO2,
and the sulphur dioxide, being a gas, escapes. No equilibrium can
therefore result, since the quantity of the sulphurous acid is
constantly being diminished because of the escape of sulphur dioxide.
Physical properties. Sulphur dioxide is a colorless gas, which at
ordinary temperatures is 2.2 times as heavy as air. It has a peculiar,
irritating odor. The gas is very soluble in water, one volume of water
dissolving eighty of the gas under standard conditions. It is easily
condensed to a colorless liquid, and can be purchased in this condition
stored in strong bottles, such as the one represented in Fig. 42.
 Fig. 42
Fig. 42
Chemical properties. Sulphur dioxide has a marked tendency to combine
with other substances, and is therefore an[Pg 151] active substance chemically.
It combines with oxygen gas, but not very easily. It can, however, take
oxygen away from some other substances, and is therefore a good reducing
agent. Its most marked chemical property is its ability to combine with
water to form sulphurous acid (H2SO3).
Sulphurous acid (H2SO3). When sulphur dioxide dissolves in water
it combines chemically with it to form sulphurous acid, an unstable
substance having the formula H3SO3. It is impossible to prepare
this acid in pure form, as it breaks down very easily into water and
sulphur dioxide. The reaction is therefore reversible, and is expressed
by the equation
Solutions of the acid in water have a number of interesting properties.
1. Acid properties. The solution has all the properties typical of an
acid. When neutralized by bases, sulphurous acid yields a series of
salts called sulphites.
2. Reducing properties. Solutions of sulphurous acid act as good
reducing agents. This is due to the fact that sulphurous acid has the
power of taking up oxygen from the air, or from substances rich in
oxygen, and is changed by this reaction into sulphuric acid:
H2SO3 + O = H2SO4,
H2SO3 + H2O2 = H2S04 + H2O.
3. Bleaching properties. Sulphurous acid has strong bleaching
properties, acting upon many colored substances in such a way as to
destroy their color. It is on this account used to bleach paper, straw
goods, and even such foods as canned corn.
4. Antiseptic properties. Sulphurous acid has marked antiseptic
properties, and on this account has the power[Pg 152] of arresting
fermentation. It is therefore used as a preservative.
Salts of sulphurous acid,—sulphites. The sulphites, like sulphurous
acid, have the power of taking up oxygen very readily, and are good
reducing agents. On account of this tendency, commercial sulphites are
often contaminated with sulphates. A great deal of sodium sulphite is
used in the bleaching industry, and as a reagent for softening paper
pulp.
Sulphur trioxide (SO3). When sulphur dioxide and oxygen are heated
together at a rather high temperature, a small amount of sulphur
trioxide (SO3) is formed, but the reaction is slow and incomplete.
If, however, the heating takes place in the presence of very fine
platinum dust, the reaction is rapid and nearly complete.
 Fig. 43
Fig. 43
Experimental preparation of sulphur trioxide. The experiment
can be performed by the use of the apparatus shown in Fig. 43,
the fine platinum being secured by moistening asbestos fiber
with a solution of platinum chloride and igniting it in a
flame. The fiber, covered with fine platinum, is placed in a
tube of hard glass, which is then heated with a burner to about
350°, while sulphur dioxide and air are passed into the tube.
Union takes place at once, and the strongly fuming sulphur
trioxide escapes from the jet at the end of the tube, and may
be condensed by surrounding the receiving tube with a freezing
mixture.
Properties of sulphur trioxide. Sulphur trioxide is a colorless liquid,
which solidifies at about 15° and boils at 46°.[Pg 153] A trace of moisture
causes it to solidify into a mass of silky white crystals, somewhat
resembling asbestos fiber in appearance. In contact with the air it
fumes strongly, and when thrown upon water it dissolves with a hissing
sound and the liberation of a great deal of heat. The product of this
reaction is sulphuric acid, so that sulphur trioxide is the anhydride of
that acid:
Catalysis. It has been found that many chemical reactions, such as the
union of sulphur dioxide with oxygen, are much influenced by the
presence of substances which do not themselves seem to take a part in
the reaction, and are left apparently unchanged after it has ceased.
These reactions go on very slowly under ordinary circumstances, but are
greatly hastened by the presence of the foreign substance. Substances
which hasten very slow reactions in this way are said to act as
catalytic agents or catalyzers, and the action is called catalysis.
Just how the action is brought about is not well understood.
DEFINITION: A catalyzer is a substance which changes the velocity of a
reaction, but does not change its products.
Examples of Catalysis. We have already had several instances of such
action. Oxygen and hydrogen combine with each other at ordinary
temperatures in the presence of platinum powder, while if no catalytic
agent is present they do not combine in appreciable quantities until a
rather high temperature is reached. Potassium chlorate, when heated with
manganese dioxide, gives up its oxygen at a much lower temperature than
when heated alone. Hydrogen dioxide decomposes very rapidly when
powdered manganese dioxide is sifted into its concentrated solution.[Pg 154]
On the other hand, the catalytic agent sometimes retards chemical
action. For example, a solution of hydrogen dioxide decomposes more
slowly when it contains a little phosphoric acid than when perfectly
pure. For this reason commercial hydrogen dioxide always contains
phosphoric acid.
Many reactions are brought about by the catalytic action of traces of
water. For example, phosphorus will not burn in oxygen in the absence of
all moisture. Hydrochloric acid will not unite with ammonia if the
reagents are perfectly dry. It is probable that many of the chemical
transformations in physiological processes, such as digestion, are
assisted by certain substances acting as catalytic agents. The principle
of catalysis is therefore very important.
Sulphuric acid (oil of vitriol) (H2SO4). Sulphuric acid is one
of the most important of all manufactured chemicals. Not only is it one
of the most common reagents in the laboratory, but enormous quantities
of it are used in many of the industries, especially in the refining of
petroleum, the manufacture of nitroglycerin, sodium carbonate, and
fertilizers.
Manufacture of sulphuric acid. 1. Contact process. The reactions
taking place in this process are represented by the following equations:
SO2 + O = SO3,
SO3 + H2O = H2SO4.
To bring about the first of these reactions rapidly, a catalyzer is
employed, and the process is carried out in the following way: Large
iron tubes are packed with some porous material, such as calcium and
magnesium sulphates, which contains a suitable catalytic substance
scattered through it. The catalyzers most used are platinum powder,[Pg 155]
vanadium oxide, and iron oxide. Purified sulphur dioxide and air are
passed through the tubes, which are kept at a temperature of about 350°.
Sulphur trioxide is formed, and as it issues from the tube it is
absorbed in water or dilute sulphuric acid. The process is continued
until all the water in the absorbing vessel has been changed into
sulphuric acid, so that a very concentrated acid is made in this way. An
excess of the trioxide may dissolve in the strong sulphuric acid,
forming what is known as fuming sulphuric acid.
2. Chamber process. The method of manufacture exclusively employed
until recent years, and still in very extensive use, is much more
complicated. The reactions are quite involved, but the conversion of
water, sulphur dioxide, and oxygen into sulphuric acid is accomplished
by the catalytic action of oxides of nitrogen. The reactions are brought
about in large lead-lined chambers, into which oxides of nitrogen,
sulphur dioxide, steam, and air are introduced in suitable proportions.
Reactions of the chamber process. In a very general way, the
various reactions which take place in the lead chambers may be
expressed in two equations. In the first reaction sulphur
dioxide, nitrogen peroxide, steam, and oxygen unite, as shown
in the equation
(1) 2SO2 + 2NO2 + H2O + O = 2SO2 (OH) (NO2).
The product formed in this reaction is called nitrosulphuric
acid or "chamber crystals." It actually separates on the walls
of the chambers when the process is not working properly. Under
normal conditions, it is decomposed as fast as it is formed by
the action of excess of steam, as shown in the equation
(2) 2SO2 (OH) (NO2) + H2O + O = 2H2SO4 + 2NO2.
The nitrogen dioxide formed in this reaction can now enter into
combination with a new quantity of sulphur dioxide, steam, and
oxygen, and the series of reactions go on indefinitely. Many
other reactions occur, but these two illustrate the principle
of the process.
[Pg 156]
The relation between sulphuric acid and nitrosulphuric acid can be seen
by comparing their structural formulas:
| O= -OH | O= -OH |
| S | S |
| O= -OH | O= -NO2 |
The latter may be regarded as derived from the former by the
substitution of the nitro group (NO2) for the hydroxyl group (OH).
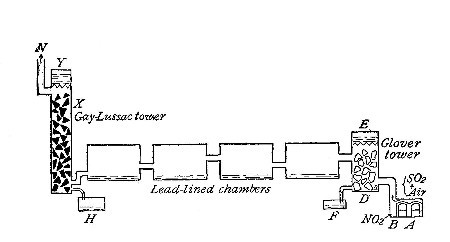 Fig. 44.
Fig. 44.
The sulphuric acid plant. Fig. 44 illustrates the simpler parts of a
plant used in the manufacture of sulphuric acid by the chamber process.
Sulphur or some sulphide, as FeS2, is burned in furnace A. The
resulting sulphur dioxide, together with air and some nitrogen peroxide,
are conducted into the large chambers, the capacity of each chamber
being about 75,000 cu. ft. Steam is also admitted into these chambers at
different points. These compounds react to form sulphuric acid,
according to the equations given above. The nitrogen left after the
withdrawal of the oxygen from the admitted air escapes through the
Gay-Lussac tower X. In order to prevent the escape of the oxides of
nitrogen regenerated in the reaction, the tower is filled with lumps of
coke, over which trickles concentrated sulphuric acid admitted from Y.
The nitrogen peroxide dissolves in the acid and the resulting solution
collects in H. This is pumped into E, where it is mixed with dilute
acid and allowed to trickle down through the chamber D (Glover tower),
which is filled with some acid-resisting rock. Here the nitrogen
peroxide is expelled from the solution by the action of the hot gases
entering from A, and together with them enters the first chamber
again. The acid from which the nitrogen peroxide is expelled collects in
F. Theoretically, a small amount of nitrogen peroxide would suffice to
prepare an unlimited amount of sulphuric acid; practically, some of it
escapes, and this is replaced by small amounts admitted at B.[Pg 157]
The sulphuric acid so formed, together with the excess of condensed
steam, collect upon the floor of the chambers in the form of a liquid
containing from 62% to 70% of sulphuric acid. The product is called
chamber acid and is quite impure; but for many purposes, such as the
manufacture of fertilizers, it needs no further treatment. It can be
concentrated by boiling it in vessels made of iron or platinum, which
resist the action of the acid, nearly all the water boiling off. Pure
concentrated acid can be made best by the contact process, while the
chamber process is cheaper for the dilute impure acid.
Physical properties. Sulphuric acid is a colorless, oily liquid, nearly
twice as heavy as water. The ordinary concentrated acid contains about
2% of water, has a density of 1.84, and boils at 338°. It is sometimes
called oil of vitriol, since it was formerly made by distilling a
substance called green vitriol.
Chemical properties. Sulphuric acid possesses chemical properties which
make it one of the most important of chemical substances.
1. Action as an acid. In dilute solution sulphuric acid acts as any
other acid, forming salts with oxides and hydroxides.
2. Action as an oxidizing agent. Sulphuric acid contains a large
percentage of oxygen and is, like nitric acid, a very good oxidizing
agent. When the concentrated acid is heated with sulphur, carbon, and
many other substances, oxidation takes place, the sulphuric acid
decomposing according to the equation
3. Action on metals. In dilute solution sulphuric acid acts upon many
metals, such as zinc, forming a sulphate and liberating hydrogen. When
the concentrated acid is employed the hydrogen set free is oxidized by a
new portion[Pg 158] of the acid, with the liberation of sulphur dioxide. With
copper the reactions are expressed by the equations
(1) Cu + H2SO4 = CuSO4 + 2H,
(2) H2SO4 + 2H = H2SO3 + H2O,
(3) H2SO3 = H2O + SO2.
By combining these equations the following one is obtained:
Cu + 2H2SO4 = CuSO4 + SO2 + 2H2O.
4. Action on salts. We have repeatedly seen that an acid of high
boiling point heated with the salt of some acid of lower boiling point
will drive out the low boiling acid. The boiling point of sulphuric acid
(338°) is higher than that of almost any common acid; hence it is used
largely in the preparation of other acids.
5. Action on water. Concentrated sulphuric acid has a very great
affinity for water, and is therefore an effective dehydrating agent.
Gases which have no chemical action upon sulphuric acid can be freed
from water vapor by bubbling them through the strong acid. When the acid
is diluted with water much heat is set free, and care must be taken to
keep the liquid thoroughly stirred during the mixing, and to pour the
acid into the water,—never the reverse.
Not only can sulphuric acid absorb water, but it will often withdraw the
elements hydrogen and oxygen from a compound containing them,
decomposing the compound, and combining with the water so formed. For
this reason most organic substances, such as sugar, wood, cotton, and
woolen fiber, and even flesh, all of which contain much oxygen and
hydrogen in addition to carbon, are charred or burned by the action of
the concentrated acid.[Pg 159]
Salts of sulphuric acid,—sulphates. The sulphates form a very important
class of salts, and many of them have commercial uses. Copperas (iron
sulphate), blue vitriol (copper sulphate), and Epsom salt (magnesium
sulphate) serve as examples. Many sulphates are important minerals,
prominent among these being gypsum (calcium sulphate) and barytes
(barium sulphate).
Thiosulphuric acid (H2S2O3); Thiosulphates. Many other
acids of sulphur containing oxygen are known, but none of them
are of great importance. Most of them cannot be prepared in a
pure state, and are known only through their salts. The most
important of these is thiosulphuric acid.
When sodium sulphite is boiled with sulphur the two substances
combine, forming a salt which has the composition represented
in the formula Na2S2O3:
Na2SO3 + S = Na2S2O3.
The substance is called sodium thiosulphate, and is a salt of
the easily decomposed acid H2S2O3, called
thiosulphuric acid. This reaction is quite similar to the
action of oxygen upon sulphites:
Na2SO3 + O = Na2SO4.
More commonly the salt is called sodium hyposulphite, or merely
"hypo." It is a white solid and is extensively used in
photography, in the bleaching industry, and as a disinfectant.
Monobasic and dibasic acids. Such acids as hydrochloric and nitric
acids, which have only one replaceable hydrogen atom in the molecule, or
in other words yield one hydrogen ion in solution, are called monobasic
acids. Acids yielding two hydrogen ions in solution are called dibasic
acids. Similarly, we may have tribasic and tetrabasic acids. The three
acids of sulphur are dibasic acids. It is therefore possible for each of
them to form both normal and acid salts. The acid salts can be made in
two ways: the acid may be treated with only half enough base to
neutralize it,—
NaOH + H2SO4 = NaHSO4 + H2O;
[Pg 160]
or a normal salt may be treated with the free acid,—
Na2SO4 + H2SO4 = 2NaHSO4.
Acid sulphites and sulphides may be made in the same ways.
Carbon disulphide (CS2). When sulphur vapor is passed over highly
heated carbon the two elements combine, forming carbon disulphide
(CS2), just as oxygen and carbon unite to form carbon dioxide
(CO2). The substance is a heavy, colorless liquid, possessing, when
pure, a pleasant ethereal odor. On standing for some time, especially
when exposed to sunlight, it undergoes a slight decomposition and
acquires a most disagreeable, rancid odor. It has the property of
dissolving many substances, such as gums, resins, and waxes, which are
insoluble in most liquids, and it is extensively used as a solvent for
such substances. It is also used as an insecticide. It boils at a low
temperature (46°), and its vapor is very inflammable, burning in the air
to form carbon dioxide and sulphur dioxide, according to the equation
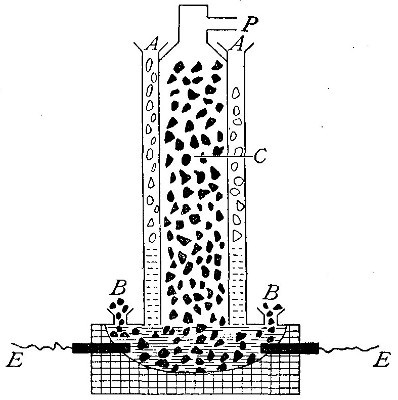 Fig. 45
Fig. 45
Commercial preparation of carbon disulphide. In the preparation
of carbon disulphide an electrical furnace is employed, such as
is represented in Fig. 45. The furnace is packed with carbon
C, and this is fed in through the hoppers B, as fast as
that which is present in the hearth of the furnace is used up.
Sulphur is introduced at A, and at the lower ends of the
tubes it is melted by the heat of the furnace and flows into
the hearth as a liquid. An electrical current is passed through
the carbon and melted sulphur from the electrodes E, heating
the charge. The vapors of carbon disulphide pass up through the
furnace and escape at D, from which they pass to a suitable
condensing apparatus.
[Pg 161]
Comparison of sulphur and oxygen. A comparison of the formulas and the
chemical properties of corresponding compounds of oxygen and sulphur
brings to light many striking similarities. The conduct of
hydrosulphuric acid and water toward many substances has been seen to be
very similar; the oxides and sulphides of the metals have analogous
formulas and undergo many parallel reactions. Carbon dioxide and
disulphide are prepared in similar ways and undergo many analogous
reactions. It is clear, therefore, that these two elements are far more
closely related to each other than to any of the other elements so far
studied.
Selenium and tellurium. These two very uncommon elements are still more
closely related to sulphur than is oxygen. They occur in comparatively
small quantities and are usually found associated with sulphur and
sulphides, either as the free elements or more commonly in combination
with metals. They form compounds with hydrogen of the formulas H2Se
and H2Te; these bodies are gases with properties very similar to
those of H2S. They also form oxides and oxygen acids which resemble
the corresponding sulphur compounds. The elements even have allotropic
forms corresponding very closely to those of sulphur. Tellurium is
sometimes found in combination with gold and copper, and occasions some
difficulties in the refining of these metals. The elements have very few
practical applications.
Crystallography. In order to understand the difference between the two
kinds of sulphur crystals, it is necessary to know something about
crystals in general and the forms which they may assume. An examination
of a large number of crystals has shown that although they may differ
much in geometric form, they can all be considered as modifications of a
few simple plans. The best way to understand the relation of one crystal
to another is to look upon every crystal as having its faces and angles
arranged in definite fashion about[Pg 162] certain imaginary lines drawn
through the crystal. These lines are called axes, and bear much the same
relation to a crystal as do the axis and parallels of latitude and
longitude to the earth and a geographical study of it. All crystals can
be referred to one of six simple plans or systems, which have their axes
as shown in the following drawings.
The names and characteristics of these systems are as follows:
1. Isometric or regular system (Fig. 46). Three equal axes, all at right
angles.
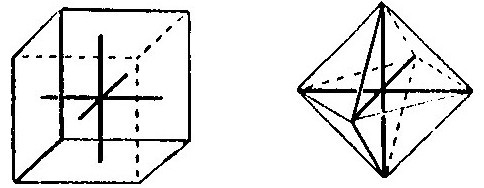 Fig. 46
Fig. 46
2. Tetragonal system (Fig. 47). Two equal axes and one of different
length, all at right angles to each other.
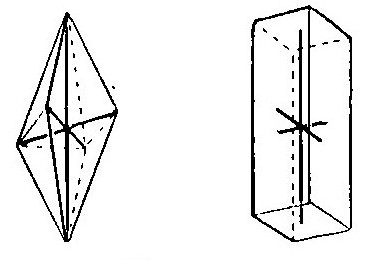 Fig. 47
Fig. 47
3. Orthorhombic system (Fig. 48). Three unequal axes, all at right
angles to each other.
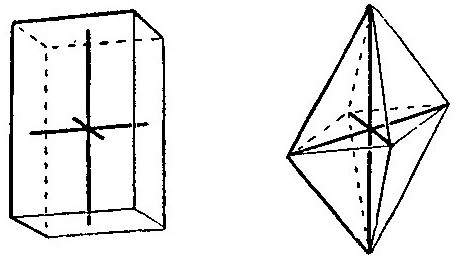 Fig. 48
Fig. 48
4. Monoclinic system (Fig. 49). Two axes at right angles, and a third at
right angles to one of these, but inclined to the other.
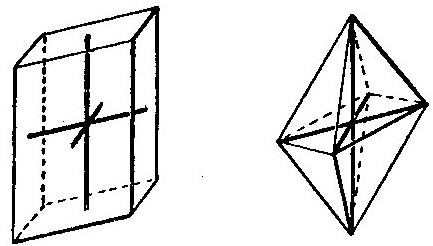 Fig. 49
Fig. 49
5. Triclinic system (Fig. 50). Three axes, all inclined to each other.
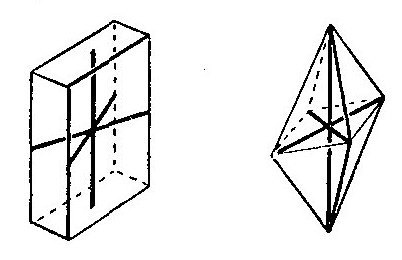 Fig. 50
Fig. 50
6. Hexagonal system (Fig. 51). Three equal axes in the same plane
intersecting at angles of 60°, and a fourth at right angles to all of
these.
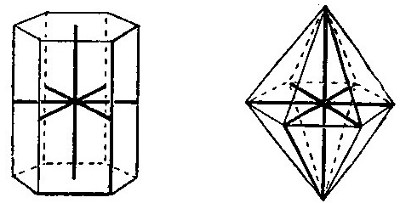 Fig. 51
Fig. 51
Every crystal can be imagined to have its faces and angles arranged in a
definite way around one of these systems of axes. A cube, for instance,
is referred to Plan 1, an axis ending in the center of each face; while
in a regular octohedron an axis ends in each solid angle. These forms
are shown in Fig. 46. It will be seen that both of these figures belong
to the same system, though they are very different in appearance. In the
same way, many geometric[Pg 163] forms may be derived from each of the systems,
and the light lines about the axes in the drawings show two of the
simplest forms of each of the systems.
In general a given substance always crystallizes in the same system, and
two corresponding faces of each crystal of it always make the same angle
with each other. A few substances, of which sulphur is an example,
crystallize in two different systems, and the crystals differ in such
physical properties as melting point and density. Such substances are
said to be dimorphous.
EXERCISES
1. (a) Would the same amount of heat be generated by the combustion of
1 g. of each of the allotropic modifications of sulphur? (b) Would the
same amount of sulphur dioxide be formed in each case?
2. Is the equation for the preparation of hydrosulphuric acid a
reversible one? As ordinarily carried out, does the reaction complete
itself?
3. Suppose that hydrosulphuric acid were a liquid, would it be necessary
to modify the method of preparation?
4. Can sulphuric acid be used to dry hydrosulphuric acid? Give reason
for answer.
5. Does dry hydrosulphuric acid react with litmus paper? State reason
for answer.
6. How many grams of iron sulphide are necessary to prepare 100 l. of
hydrosulphuric acid when the laboratory conditions are 17° and 740 mm.
pressure?
7. Suppose that the hydrogen in 1 l. of hydrosulphuric acid were
liberated; what volume would it occupy, the gases being measured under
the same conditions?
8. Write the equations representing the reaction between hydrosulphuric
acid and sodium hydroxide and ammonium hydroxide respectively.
9. Show that the preparation of sulphur dioxide from a sulphite is
similar in principle to the preparation of hydrogen sulphide.
10. (a) Does dry sulphur dioxide react with litmus paper? (b) How
can it be shown that a solution of sulphur dioxide in water acts like an
acid?[Pg 164]
11. (a) Calculate the percentage composition of sulphurous anhydride
and sulphuric anhydride. (b) Show how these two substances are in
harmony with the law of multiple proportion.
12. How many pounds of sulphur would be necessary in the preparation of
100 lb. of 98% sulphuric acid?
13. What weight of sulphur dioxide is necessary in the preparation of 1
kg. of sodium sulphite?
14. What weight of copper sulphate crystals can be obtained by
dissolving 1 kg. of copper in sulphuric acid and crystallizing the
product from water?
15. Write the names and formulas of the oxides and oxygen acids of
selenium and tellurium.
16. In the commercial preparation of carbon disulphide, what is the
function of the electric current?
17. If the Gay-Lussac tower were omitted from the sulphuric acid
factory, what effect would this have on the cost of production of
sulphuric acid?
[Pg 165]
CHAPTER XV
PERIODIC LAW
A number of the elements have now been studied somewhat closely. The
first three of these, oxygen, hydrogen, and nitrogen, while having some
physical properties in common with each other, have almost no point of
similarity as regards their chemical conduct. On the other hand, oxygen
and sulphur, while quite different physically, have much in common in
their chemical properties.
About eighty elements are now known. If all of these should have
properties as diverse as do oxygen, hydrogen, and nitrogen, the study of
chemistry would plainly be a very difficult and complicated one. If,
however, the elements can be classified in groups, the members of which
have very similar properties, the study will be very much simplified.
Earlier classification of the elements. Even at an early period efforts
were made to discover some natural principle in accordance with which
the elements could be classified. Two of these classifications may be
mentioned here.
1. Classification into metals and non-metals. The classification into
metals and non-metals most naturally suggested itself. This grouping was
based largely on physical properties, the metals being heavy, lustrous,
malleable, ductile, and good conductors of heat and electricity.
Elements possessing these properties are usually base-forming in
character, and the ability to form bases came to be regarded as a
characteristic property of the metals. The[Pg 166] non-metals possessed
physical properties which were the reverse of those of the metals, and
were acid-forming in character.
Not much was gained by this classification, and it was very imperfect.
Some metals, such as potassium, are very light; some non-metals, such as
iodine, have a high luster; some elements can form either an acid or a
base.
2. Classification into triad families. In 1825 Döbereiner observed
that an interesting relation exists between the atomic weights of
chemically similar elements. To illustrate, lithium, sodium, and
potassium resemble each other very closely, and the atomic weight of
sodium is almost exactly an arithmetical mean between those of the other
two: (7.03 + 39.15)/2 = 23.09. In many chemical and physical properties
sodium is midway between the other two.
A number of triad families were found, but among eighty elements, whose
atomic weights range all the way from 1 to 240, such agreements might be
mere chance. Moreover many elements did not appear to belong to such
families.
Periodic division. In 1869 the Russian chemist Mendeléeff devised an
arrangement of the elements based on their atomic weights, which has
proved to be of great service in the comparative study of the elements.
A few months later the German, Lothar Meyer, independently suggested the
same ideas. This arrangement brought to light a great generalization,
now known as the periodic law. An exact statement of the law will be
given after the method of arranging the elements has been described.
 DMITRI IVANOVITCH MENDELÉEFF (Russian) (1834-1907)
DMITRI IVANOVITCH MENDELÉEFF (Russian) (1834-1907)
Author of the periodic law; made many investigations on the physical
constants of elements and compounds; wrote an important book entitled
"Principles of Chemistry"; university professor and government
official
[Pg 167]
Arrangement of the periodic table. The arrangement suggested by
Mendeléeff, modified somewhat by more recent investigations, is as
follows: Beginning with lithium, which has an atomic weight of 7, the
elements are arranged in a horizontal row in the order of their atomic
weights, thus:
Li (7.03), Be (9.1), B (11), C (12), N (14.04), O (16), F (19).
These seven elements all differ markedly from each other. The eighth
element, sodium, is very similar to lithium. It is placed just under
lithium, and a new row follows:
Na(23.05), Mg (24.36), Al (27.1), Si (28.4), P (31), S (32.06),
Cl(35.45).
When the fifteenth element, potassium, is reached, it is placed under
sodium, to which it is very similar, and serves to begin a third row:
K (39.15), Ca (40.1), Sc (44.1,) Ti (48.1), V (51.2), Cr (52.1), Mn(55).
Not only is there a strong similarity between lithium, sodium, and
potassium, which have been placed in a vertical row because of this
resemblance, but the elements in the other vertical rows exhibit much of
the same kind of similarity among themselves, and evidently form little
natural groups.
The three elements following manganese, namely, iron, nickel, and
cobalt, have atomic weights near together, and are very similar
chemically. They do not strongly resemble any of the elements so far
considered, and are accordingly placed in a group by themselves,
following manganese. A new row is begun with copper, which somewhat
resembles the elements of the first vertical column. Following the fifth
and seventh rows are groups of three closely related elements, so that
the completed arrangement has the appearance represented in the table on
page 168.
[Pg 168]
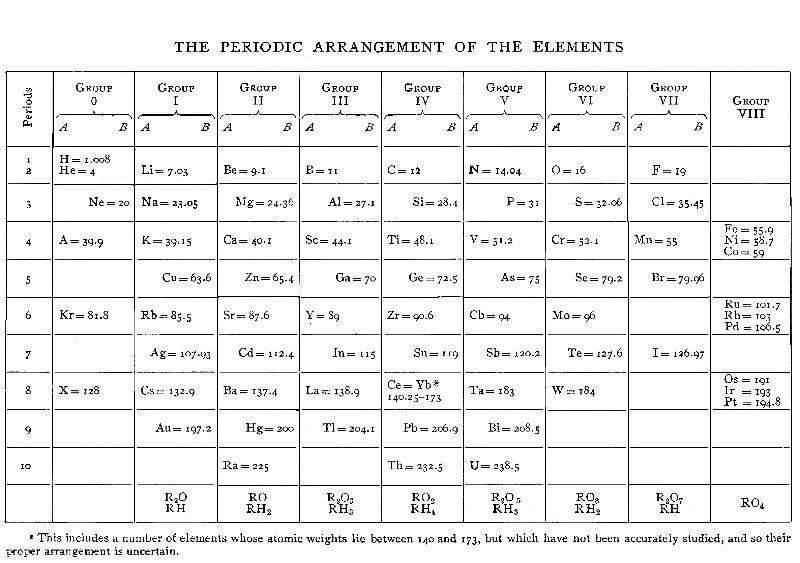 THE PERIODIC ARRANGEMENT OF THE ELEMENTS
THE PERIODIC ARRANGEMENT OF THE ELEMENTS
[Pg 169]
Place of the atmospheric elements. When argon was discovered it was seen
at once that there was no place in the table for an element of atomic
weight approximately 40. When the other inactive elements were found,
however, it became apparent that they form a group just preceding Group
1. They are accordingly arranged in this way in Group 0 (see table on
opposite page). A study of this table brings to light certain very
striking facts.
Properties of elements vary with atomic weights. There is evidently a
close relation between the properties of an element and its atomic
weight. Lithium, at the beginning of the first group, is a very strong
base-forming element, with pronounced metallic properties. Beryllium,
following lithium, is less strongly base-forming, while boron has some
base-forming and some acid-forming properties. In carbon all
base-forming properties have disappeared, and the acid-forming
properties are more marked than in boron. These become still more
emphasized as we pass through nitrogen and oxygen, until on reaching
fluorine we have one of the strongest acid-forming elements. The
properties of these seven elements therefore vary regularly with their
atomic weights, or, in mathematical language, are regular functions of
them.
Periodic law. The properties of the first seven elements vary
continuously—that is steadily—away from base-forming and toward
acid-forming properties. If lithium had the smallest atomic weight of
any of the elements, and fluorine the greatest, so that in passing from
one to the other we had included all the elements, we could say that the
properties of elements are continuous functions of their atomic weights.
But fluorine is an element of small atomic weight, and the one following
it, sodium, breaks the regular order, for in it reappear all the
characteristic properties of lithium. Magnesium, following sodium, bears
much the same relation to[Pg 170] beryllium that sodium does to lithium, and
the properties of the elements in the second row vary much as they do in
the first row until potassium is reached, when another repetition
begins. The properties of the elements do not vary continuously,
therefore, with atomic weights, but at regular intervals there is a
repetition, or period. This generalization is known as the periodic
law, and may be stated thus: The properties of elements are periodic
functions of their atomic weights.
The two families in a group. While all the elements in a given vertical
column bear a general resemblance to each other, it has been noticed
that those belonging to periods having even numbers are very strikingly
similar to each other. They are placed at the left side of the group
columns. In like manner, the elements belonging to the odd periods are
very similar and are arranged at the right side of the group columns.
Thus calcium, strontium, and barium are very much alike; so, too, are
magnesium, zinc, and cadmium. The resemblance between calcium and
magnesium, or strontium and zinc, is much less marked. This method of
arrangement therefore divides each group into two families, each
containing four or five members, between which there is a great
similarity.
Family resemblances. Let us now inquire more closely in what respects
the elements of a family resemble each other.
1. Valence. In general the valence of the elements in a family is the
same, and the formulas of their compounds are therefore similar. If we
know that the formula of sodium chloride is NaCl, it is pretty certain
that the formula of potassium chloride will be KCl—not KCl2 or
[Pg 171]KCl3. The general formulas R2O, RO, etc., placed below the
columns show the formulas of the oxides of the elements in the column
provided they form oxides. In like manner the formulas RH, RH2, etc.,
show the composition of the compounds formed with hydrogen or chlorine.
2. Chemical properties. The chemical properties of the members of a
family are quite similar. If one member is a metal, the others usually
are; if one is a non-metal, so, too, are the others. The families in the
first two columns consist of metals, while the elements found in the
last two columns form acids. There is in addition a certain regularity
in properties of the elements in each family. If the element at the head
of the family is a strong acid-forming element, this property is likely
to diminish gradually, as we pass to the members of the family with
higher atomic weights. Thus phosphorus is strongly acid-forming, arsenic
less so, antimony still less so, while bismuth has almost no
acid-forming properties. We shall meet with many illustrations of this
fact.
3. Physical properties. In the same way, the physical properties of
the members of a family are in general somewhat similar, and show a
regular gradation as we pass from element to element in the family. Thus
the densities of the members of the magnesium family are
Mg = 1.75, Zn = 7.00, Cd = 8.67, Hg = 13.6.
Their melting points are
Mg = 750°, Zn = 420°, Cd = 320°, Hg = -39.5°.
Value of the periodic law. The periodic law has proved of much value in
the development of the science of chemistry.
1. It simplifies study. It is at once evident that such regularities
very much simplify the study of chemistry.[Pg 172] A thorough study of one
element of a family makes the study of the other members a much easier
task, since so many of the properties and chemical reactions of the
elements are similar. Thus, having studied the element sulphur in some
detail, it is not necessary to study selenium and tellurium so closely,
for most of their properties can be predicted from the relation which
they sustain to sulphur.
2. It predicts new elements. When the periodic law was first
formulated there were a number of vacant places in the table which
evidently belonged to elements at that time unknown. From their position
in the table, Mendeléeff predicted with great precision the properties
of the elements which he felt sure would one day be discovered to fill
these places. Three of them, scandium, germanium, and gallium, were
found within fifteen years, and their properties agreed in a remarkable
way with the predictions of Mendeléeff. There are still some vacant
places in the table, especially among the heavier elements.
3. It corrects errors. The physical constants of many of the elements
did not at first agree with those demanded by the periodic law, and a
further study of many such cases showed that errors had been made. The
law has therefore done much service in indicating probable error.
Imperfections of the law. There still remain a good many features which
must be regarded as imperfections in the law. Most conspicuous is the
fact that the element hydrogen has no place in the table. In some of the
groups elements appear in one of the families, while all of their
properties show that they belong in the other. Thus sodium belongs with
lithium and not with copper; fluorine belongs with chlorine and not with
manganese. There are[Pg 173] two instances where the elements must be
transposed in order to make them fit into their proper group. According
to their atomic weights, tellurium should follow iodine, and argon
should follow potassium. Their properties show in each case that this
order must be reversed. The table separates some elements altogether
which, in many respects have closely agreeing properties. Iron,
chromium, and manganese are all in different groups, although they are
similar in many respects.
The system is therefore to be regarded as but a partial and imperfect
expression of some very important and fundamental relation between the
substances which we know as elements, the exact nature of this relation
being as yet not completely clear to us.
EXERCISES
1. Suppose that an element were discovered that filled the blank in
Group O, Period 5; what properties would it probably have?
2. Suppose that an element were discovered that filled the blank in
Group VI, Period 9, family B; what properties would it have?
3. Sulphur and oxygen both belong in Group VI, although in different
families; in what respects are the two similar?
[Pg 174]
CHAPTER XVI
THE CHLORINE FAMILY
| ATOMIC WEIGHT | MELTING POINT | BOILING POINT | COLOR AND STATE |
| Fluorine (F) | 19.00 | -223° | -187° | Pale yellowish gas. |
| Chlorine (Cl) | 35.45 | -102° | -33.6° | Greenish-yellow gas. |
| Bromine (Br) | 79.96 | -7° | 59° | Red liquid. |
| Iodine (I) | 126.97 | 107° | 175° | Purplish-black solid. |
The family. The four elements named in the above table form a strongly
marked family of elements and illustrate very clearly the way in which
the members of a family in a periodic group resemble each other, as well
as the character of the differences which we may expect to find between
the individual members.
1. Occurrence. These elements do not occur in nature in the free
state. The compounds of the last three elements of the family are found
extensively in sea water, and on this account the name halogens,
signifying "producers of sea salt," is sometimes applied to the family.
2. Properties. As will be seen by reference to the table, the melting
points and boiling points of the elements of the family increase with
their atomic weights. A somewhat similar gradation is noted in their
color and state. One atom of each of the elements combines with one atom
of hydrogen to form acids, which are gases very soluble in water. The
affinity of the elements for hydrogen is in[Pg 175] the inverse order of their
atomic weights, fluorine having the strongest affinity and iodine the
weakest. Only chlorine and iodine form oxides, and those of the former
element are very unstable. The elements of the group are univalent in
their compounds with hydrogen and the metals.
FLUORINE
Occurrence. The element fluorine occurs in nature most abundantly as the
mineral fluorspar (CaF2), as cryolite (Na3AlF6), and in the
complex mineral apatite (3 Ca3(PO4)2·CaF2).
Preparation. All attempts to isolate the element resulted in failure
until recent years. Methods similar to those which succeed in the
preparation of the other elements of the family cannot be used; for as
soon as the fluorine is liberated it combines with the materials of
which the apparatus is made or with the hydrogen of the water which is
always present. The preparation of fluorine was finally accomplished by
the French chemist Moissan by the electrolysis of hydrofluoric acid.
Perfectly dry hydrofluoric acid (HF) was condensed to a liquid and
placed in a U-shaped tube made of platinum (or copper), which was
furnished with electrodes and delivery tubes, as shown in Fig. 52. This
liquid is not an electrolyte, but becomes such when potassium fluoride
is dissolved in it. When this solution was electrolyzed hydrogen was set
free at the cathode and fluorine at the anode.
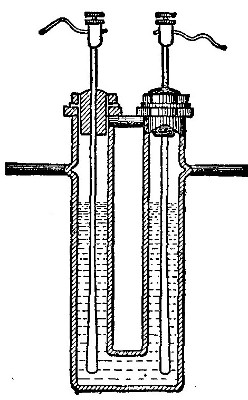 Fig. 52
Fig. 52
[Pg 176]
Properties. Fluorine is a gas of slightly yellowish color, and can be
condensed to a liquid boiling at -187° under atmospheric pressure. It
solidifies at -223°. It is extremely active chemically, being the most
active of all the elements at ordinary temperatures.
It combines with all the common elements save oxygen, very often with
incandescence and the liberation of much heat. It has a strong affinity
for hydrogen and is able to withdraw it from its compounds with other
elements. Because of its great activity it is extremely poisonous.
Fluorine does not form any oxides, neither does it form any oxygen
acids, in which respects it differs from the other members of the
family.
Hydrofluoric acid (HF). Hydrofluoric acid is readily obtained from
fluorspar by the action of concentrated sulphuric acid. The equation is
CaF2 + H2SO4 = CaSO4 + 2HF.
In its physical properties it resembles the binary acids of the other
elements of this family, being, however, more easily condensed to a
liquid. The anhydrous acid boils at 19° and can therefore be prepared at
ordinary pressures. It is soluble in all proportions in water, and a
concentrated solution—about 50%—is prepared for the market. Its fumes
are exceedingly irritating to the respiratory organs, and several
chemists have lost their lives by accidentally breathing them.
 HENRI MOISSAN (French) (1853-1907)
HENRI MOISSAN (French) (1853-1907)
Famous for his work with the electric furnace at high temperatures;
prepared artificial diamonds, together with many new binary compounds
such as carbides, silicides, borides, and nitrides; isolated fluorine
and studied its properties and its compounds very thoroughly
[Pg 177]
Chemical properties. Hydrofluoric acid, like other strong acids, readily
acts on bases and metallic oxides and forms the corresponding fluorides.
It also dissolves certain metals such as silver and copper. It acts very
vigorously upon organic matter, a single drop of the concentrated acid
making a sore on the skin which is very painful and slow in healing. Its
most characteristic property is its action upon silicon dioxide
(SiO2), with which it forms water and the gas silicon tetrafluoride
(SiF4), as shown in the equation
SiO2 + 4HF = SiF4 + 2H2O.
Glass consists of certain compounds of silicon, which are likewise acted
on by the acid so that it cannot be kept in glass bottles. It is
preserved in flasks made of wax or gutta-percha.
Etching. Advantage is taken of this reaction in etching designs
upon glass. The glass vessel is painted over with a protective
paint upon which the acid will not act, the parts which it is
desired to make opaque being left unprotected. A mixture of
fluorspar and sulphuric acid is then painted over the vessel
and after a few minutes the vessel is washed clean. Wherever
the hydrofluoric acid comes in contact with the glass it acts
upon it, destroying its luster and making it opaque, so that
the exposed design will be etched upon the clear glass. Frosted
glass globes are often made in this way.
The etching may also be effected by covering the glass with a
thin layer of paraffin, cutting the design through the wax and
then exposing the glass to the fumes of the acid.
Salts of hydrofluoric acid,—fluorides. A number of the fluorides are
known, but only one of them, calcium fluoride (CaF2), is of
importance. This is the well-known mineral fluorspar.
CHLORINE
Historical. While studying the action of hydrochloric acid upon the
mineral pyrolusite, in 1774, Scheele obtained a yellowish, gaseous
substance to which he gave a name in keeping with the phlogiston theory
then current. Later it was supposed to be a compound containing oxygen.
In[Pg 178] 1810, however, the English chemist Sir Humphry Davy proved it to be
an element and named it chlorine.
Occurrence. Chlorine does not occur free in nature, but its compounds
are widely distributed. For the most part it occurs in combination with
the metals in the form of chlorides, those of sodium, potassium, and
magnesium being most abundant. Nearly all salt water contains these
substances, particularly sodium chloride, and very large salt beds
consisting of chlorides are found in many parts of the world.
Preparation. Two general methods of preparing chlorine may be mentioned,
namely, the laboratory method and the electrolytic method.
1. Laboratory method. In the laboratory chlorine is made by warming
the mineral pyrolusite (manganese dioxide, MnO2) with concentrated
hydrochloric acid. The first reaction, which seems to be similar to the
action of acids upon oxides in general, is expressed in the equation
MnO2 + 4HCl = MnCl4 + 2H2O.
The manganese compound so formed is very unstable, however, and breaks
clown according to the equation
Instead of using hydrochloric acid in the preparation of chlorine it
will serve just as well to use a mixture of sodium chloride and
sulphuric acid, since these two react to form hydrochloric acid. The
following equations will then express the changes:
(1) 2NaCl + H2SO4 = Na2SO4 + 2HCl.
(2) MnO2 + 4 HCl = MnCl2 + 2Cl + 2H2O.
(3) MnCl2 + H2SO4 = MnSO4 + 2HCl.
[Pg 179]
Combining these equations, the following equation expressing the
complete reaction is obtained:
2NaCl + MnO2 + 2H2SO4 = MnSO4 + Na2SO4 + 2H2O + 2Cl.
Since the hydrochloric acid liberated in the third equation is free to
act upon manganese dioxide, it will be seen that all of the chlorine
originally present in the sodium chloride is set free.
The manganese dioxide and the hydrochloric acid are brought
together in a flask, as represented in Fig. 53, and a gentle
heat is applied. The rate of evolution of the gas is regulated
by the amount of heat applied, and the gas is collected by
displacement of air. As the equations show, only half of the
chlorine present in the hydrochloric acid is liberated.
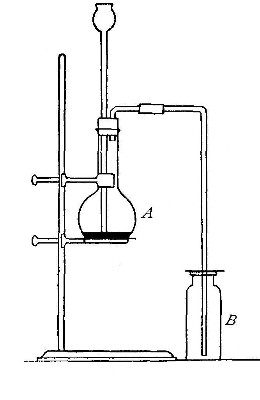 Fig. 53
Fig. 53
2. Electrolytic method. Under the discussion of electrolysis (p. 102)
it was shown that when a solution of sodium chloride is electrolyzed
chlorine is evolved at the anode, while the sodium set free at the
cathode reacts with the water to form hydrogen, which is evolved, and
sodium hydroxide, which remains in solution. A great deal of the
chlorine required in the chemical industries is now made in this way in
connection with the manufacture of sodium hydroxide.
Physical properties. Chlorine is a greenish-yellow gas, which has a
peculiar suffocating odor and produces a very violent effect upon the
throat and lungs. Even when inhaled in small quantities it often
produces all the symptoms of a[Pg 180] hard cold, and in larger quantities may
have serious and even fatal action. It is quite heavy (density = 2.45)
and can therefore be collected by displacement of air. One volume of
water under ordinary conditions dissolves about three volumes of
chlorine. The gas is readily liquefied, a pressure of six atmospheres
serving to liquefy it at 0°. It forms a yellowish liquid which
solidifies at -102°.
Chemical properties. At ordinary temperatures chlorine is far more
active chemically than any of the elements we have so far considered,
with the exception of fluorine; indeed, it is one of the most active of
all elements.
1. Action on metals. A great many metals combine directly with
chlorine, especially when hot. A strip of copper foil heated in a burner
flame and then dropped into chlorine burns with incandescence. Sodium
burns brilliantly when heated strongly in slightly moist chlorine. Gold
and silver are quickly tarnished by the gas.
2. Action on non-metals. Chlorine has likewise a strong affinity for
many of the non-metals. Thus phosphorus burns in a current of the gas,
while antimony and arsenic in the form of a fine powder at once burst
into flame when dropped into jars of the gas. The products formed in all
cases where chlorine combines with another element are called
chlorides.
3. Action on hydrogen. Chlorine has a strong affinity for hydrogen,
uniting with it to form hydrochloric acid. A jet of hydrogen burning in
the air continues to burn when introduced into a jar of chlorine, giving
a somewhat luminous flame. A mixture of the two gases explodes violently
when a spark is passed through it or when it is exposed to bright
sunlight. In the latter case it is the light and not the heat which
starts the action.[Pg 181]
4. Action on substances containing hydrogen. Not only will chlorine
combine directly with free hydrogen but it will often abstract the
element from its compounds. Thus, when chlorine is passed into a
solution containing hydrosulphuric acid, sulphur is precipitated and
Hydrochloric acid formed. The reaction is shown by the following
equation:
With ammonia the action is similar:
The same tendency is very strikingly seen in the action of chlorine upon
turpentine. The latter substance is largely made up of compounds having
the composition represented by the formula C10H16. When a strip of
paper moistened with warm turpentine is placed in a jar of chlorine
dense fumes of hydrochloric acid appear and a black deposit of carbon is
formed. Even water, which is a very stable compound, can be decomposed
by chlorine, the oxygen being liberated. This may be shown in the
following way:
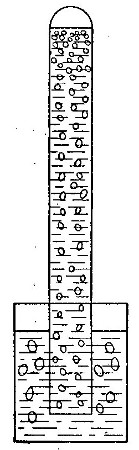 Fig. 54
Fig. 54
If a long tube of rather large diameter is filled with a strong
solution of chlorine in water and inverted in a vessel of the
same solution, as shown in Fig. 54, and the apparatus is placed
in bright sunlight, very soon bubbles of a gas will be observed
to rise through the solution and collect in the tube. An
examination of this gas will show that it is oxygen. It is
liberated from water in accordance with the following equation:
H2O + 2Cl = 2HCl + O.
5. Action on color substances,—bleaching action. If strips of
brightly colored cloth or some highly colored flowers are placed in
quite dry chlorine, no marked change[Pg 182] in color is noticed as a rule. If,
however, the cloth and flowers are first moistened, the color rapidly
disappears, that is, the objects are bleached. Evidently the moisture as
well as the chlorine is concerned in the action, and a study of the case
shows that the chlorine has combined with the hydrogen of the water. The
oxygen set free oxidizes the color substance, converting it into a
colorless compound. It is evident from this explanation that chlorine
will only bleach those substances which are changed into colorless
compounds by oxidation.
6. Action as a disinfectant. Chlorine has also marked germicidal
properties, and the free element, as well as compounds from which it is
easily liberated, are used as disinfectants.
Nascent state. It will be noticed that oxygen when set free from water
by chlorine is able to do what ordinary oxygen cannot do, for both the
cloth and the flowers are unchanged in the air which contains oxygen. It
is generally true that the activity of an element is greatest at the
instant of liberation from its compounds. To express this fact elements
at the instant of liberation are said to be in the nascent state. It
is nascent oxygen which does the bleaching.
Hydrochloric acid (muriatic acid) (HCl). The preparation of
hydrochloric acid may be discussed under two general heads:
1. Laboratory preparation. The product formed by the burning of
hydrogen in chlorine is the gas hydrochloric acid. This substance is
much more easily obtained, however, by treating common salt (sodium
chloride) with sulphuric acid. The following equation shows the
reaction:
2NaCl + H2SO4 = Na2SO4 + 2HCl.
[Pg 183]
The dry salt is placed in a flask furnished with a funnel tube and an
exit tube, the sulphuric acid is added, and the flask gently warmed. The
hydrochloric acid gas is rapidly given off and can be collected by
displacement of air. The same apparatus can be used as was employed in
the preparation of chlorine (Fig. 53).
When a solution of salt is treated with sulphuric acid there
is no very marked action. The hydrochloric acid formed is very
soluble in water, and so does not escape from the solution;
hence a state of equilibrium is soon reached between the four
substances represented in the equation. When concentrated
sulphuric acid, in which hydrochloric acid is not soluble, is
poured upon dry salt the reaction is complete.
2. Commercial preparation. Commercially, hydrochloric acid is prepared
in connection with the manufacture of sodium sulphate, the reaction
being the same as that just given. The reaction is carried out in a
furnace, and the hydrochloric acid as it escapes in the form of gas is
passed into water in which it dissolves, the solution forming the
hydrochloric acid of commerce. When the materials are pure a colorless
solution is obtained. The most concentrated solution has a density of
1.2 and contains 40% HCl. The commercial acid, often called muriatic
acid, is usually colored yellow by impurities.
Composition of hydrochloric acid. When a solution of hydrochloric acid
is electrolyzed in an apparatus similar to the one in which water was
electrolyzed (Fig. 18), chlorine collects at the anode and hydrogen at
the cathode. At first the chlorine dissolves in the water, but soon the
water in the one tube becomes saturated with it, and if the stopcocks
are left open until this is the case, and are then closed, it will be
seen that the two gases are set free in equal volumes.[Pg 184]
When measured volumes of the two gases are caused to unite it is found
that one volume of hydrogen combines with one of chlorine. Other
experiments show that the volume of hydrochloric acid formed is just
equal to the sum of the volumes of hydrogen and chlorine. Therefore one
volume of hydrogen combines with one volume of chlorine to form two
volumes of hydrochloric acid gas. Since chlorine is 35.18 times as heavy
as hydrogen, it follows that one part of hydrogen by weight combines
with 35.18 parts of chlorine to form 36.18 parts of hydrochloric acid.
Physical properties. Hydrochloric acid is a colorless gas which has an
irritating effect when inhaled, and possesses a sour, biting taste, but
no marked odor. It is heavier than air (density = 1.26) and is very
soluble in water. Under standard conditions 1 volume of water dissolves
about 500 volumes of the gas. On warming such a solution the gas
escapes, until at the boiling point the solution contains about 20% by
weight of HCl. Further boiling will not drive out any more acid, but the
solution will distill with unchanged concentration. A more dilute
solution than this will lose water on boiling until it has reached the
same concentration, 20%, and will then distill unchanged. Under high
pressure the gas can be liquefied, 28 atmospheres being required at 0°.
Under these conditions it forms a colorless liquid which is not very
active chemically. It boils at -80° and solidifies at -113°. The
solution of the gas in water is used almost entirely in the place of the
gas itself, since it is not only far more convenient but also more
active.
Chemical properties. The most important chemical properties of
hydrochloric acid are the following:
1. Action as an acid. In aqueous solution hydrochloric acid has very
strong acid properties; indeed, it is one of[Pg 185] the strongest acids. It
acts upon oxides and hydroxides, converting them into salts:
NaOH + HCl = NaCl + H2O,
CuO + 2HCl = CuCl2 + H2O.
It acts upon many metals, forming chlorides and liberating hydrogen:
Zn + 2HCl = ZnCl2 + 2H,
Al + 3HCl = AlCl3 + 3H.
Unlike nitric and sulphuric acids it has no oxidizing action, so that
when it acts on metals hydrogen is always given off.
2. Relation to combustion. Hydrochloric acid gas is not readily
decomposed, and is therefore neither combustible nor a supporter of
combustion.
3. Action on oxidizing agents. Although hydrochloric acid is
incombustible, it can be oxidized under some circumstances, in which
case the hydrogen combines with oxygen, while the chlorine is set free.
Thus, when a solution of hydrochloric acid acts upon manganese dioxide
part of the chlorine is set free:
MnO2 + 4HCl = MnCl2 + 2H2O + 2Cl.
Aqua regia. It has been seen that when nitric acid acts as an oxidizing
agent it usually decomposes, as represented in the equation
The oxygen so set free may act on hydrochloric acid:
The complete equation therefore is
2HNO3 + 6HCl = 4H2O + 2NO + 6Cl.
[Pg 186]
When concentrated nitric and hydrochloric acids are mixed this reaction
goes on slowly, chlorine and some other substances not represented in
the equation being formed. The mixture is known as aqua regia and is
commonly prepared by adding one volume of nitric acid to three volumes
of hydrochloric acid. It acts more powerfully upon metals and other
substances than either of the acids separately, and owes its strength
not to acid properties but to the action of the nascent chlorine which
it liberates. Consequently, when it acts upon metals such as gold it
converts them into chlorides, and the reaction can be represented by
such equations as
Salts of hydrochloric acid,—chlorides. The chlorides of all the metals
are known and many of them are very important compounds. Some of them
are found in nature, and all can be prepared by the general method of
preparing salts. Silver chloride, lead chloride, and mercurous chloride
are insoluble in water and acids, and can be prepared by adding
hydrochloric acid to solutions of compounds of the respective elements.
While the chlorides have formulas similar to the fluorides, their
properties are often quite different. This is seen in the solubility of
the salts. Those metals whose chlorides are insoluble form soluble
fluorides, while many of the metals which form soluble chlorides form
insoluble fluorides.
Compounds of chlorine with oxygen and hydrogen. Chlorine combines with
oxygen and hydrogen to form four different acids. They are all quite
unstable, and most of them cannot be prepared in pure form; their salts
can easily be made, however, and some of them will be met with in the[Pg 187]
study of the metals. The formulas and names of these acids are as
follows:
| HClO | hypochlorous acid. |
| HClO2 | chlorous acid. |
| HClO3 | chloric acid. |
| HClO4 | perchloric acid. |
Oxides of chlorine. Two oxides are known, having the formulas Cl2O
and ClO2. They decompose very easily and are good oxidizing agents.
BROMINE
Historical. Bromine was discovered in 1826 by the French chemist
Ballard, who isolated it from sea salt. He named it bromine (stench)
because of its unbearable fumes.
Occurrence. Bromine occurs almost entirely in the form of bromides,
especially as sodium bromide and magnesium bromide, which are found in
many salt springs and salt deposits. The Stassfurt deposits in Germany
and the salt waters of Ohio and Michigan are especially rich in
bromides.
Preparation of bromine. The laboratory method of preparing bromine is
essentially different from the commercial method.
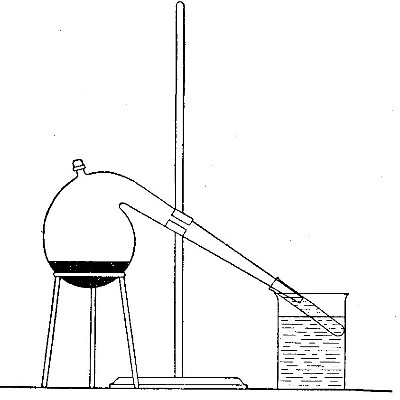 Fig. 55
Fig. 55
1. Laboratory method. As in the case of chlorine, bromine can be
prepared by the action of hydrobromic acid (HBr) on manganese dioxide.
Since hydrobromic acid is not an article of commerce, a mixture of
sulphuric acid[Pg 188] and a bromide is commonly substituted for it. The
materials are placed in a retort arranged as shown in Fig. 55. The end
of the retort just touches the surface of the water in the test tube. On
heating, the bromine distills over and is collected in the cold
receiver. The equation is
2NaBr + 2H2SO4 + MnO2 = Na2SO4 + MnSO4
+ 2H2O + 2Br.
2. Commercial method. Bromine is prepared commercially from the waters
of salt wells which are especially rich in bromides. On passing a
current of electricity through such waters the bromine is first
liberated. Any chlorine liberated, however, will assist in the reaction,
since free chlorine decomposes bromides, as shown in the equation
When the water containing the bromine is heated, the liberated bromine
distills over into the receiver.
Physical properties. Bromine is a dark red liquid about three times as
heavy as water. Its vapor has a very offensive odor and is most
irritating to the eyes and throat. The liquid boils at 59° and
solidifies at -7°; but even at ordinary temperatures it evaporates
rapidly, forming a reddish-brown gas very similar to nitrogen peroxide
in appearance. Bromine is somewhat soluble in water, 100 volumes of
water under ordinary conditions dissolving 1 volume of the liquid. It is
readily soluble in carbon disulphide, forming a yellow solution.
Chemical properties and uses. In chemical action bromine is very similar
to chlorine. It combines directly with many of the same elements with
which chlorine unites, but with less energy. It combines with hydrogen
and takes away[Pg 189] the latter element from some of its compounds, but not
so readily as does chlorine. Its bleaching properties are also less
marked.
Bromine finds many uses in the manufacture of organic drugs and
dyestuffs and in the preparation of bromides.
Hydrobromic acid (HBr). When sulphuric acid acts upon a bromide
hydrobromic acid is set free:
2NaBr + H2SO4 = Na2SO4 + 2HBr.
At the same time some bromine is set free, as may be seen from the red
fumes which appear, and from the odor. The explanation of this is found
in the fact that hydrobromic acid is much less stable than hydrochloric
acid, and is therefore more easily oxidized. Concentrated sulphuric acid
is a good oxidizing agent, and oxidizes a part of the hydrobromic acid,
liberating bromine:
H2SO4 + 2HBr = 2H2O + SO2 + 2Br.
Preparation of pure hydrobromic acid. A convenient way to make
pure hydrobromic acid is by the action of bromine upon moist
red phosphorus. This can be done with the apparatus shown in
Fig. 56. Bromine is put into the dropping funnel A, and red
phosphorus, together with enough water to cover it, is placed
in the flask B. By means of the stopcock the bromine is
allowed to flow drop by drop into the flask, the reaction
taking place without the application of heat. The equations are
(1) P + 3Br = PBr3,
(2) PBr3 + 3H2O = P(OH)3 + 3HBr.
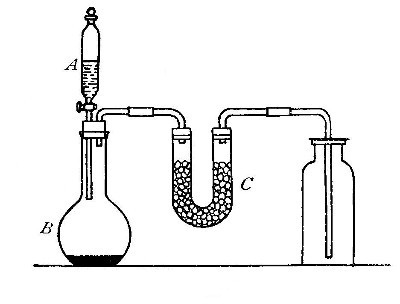 Fig. 56
Fig. 56
[Pg 190]
The U-tube C contains glass beads which have been moistened
with water and rubbed in red phosphorus. Any bromine escaping
action in the flask acts upon the phosphorus in the U-tube. The
hydrobromic acid is collected in the same way as hydrochloric
acid.
Properties. Hydrobromic acid very strikingly resembles hydrochloric acid
in physical and chemical properties. It is a colorless, strongly fuming
gas, heavier than hydrochloric acid and, like it, is very soluble in
water. Under standard conditions 1 volume of water dissolves 610 volumes
of the gas. Chemically, the chief point in which it differs from
hydrochloric acid is in the fact that it is much more easily oxidized,
so that bromine is more readily set free from it than chlorine is from
hydrochloric acid.
Salts of hydrobromic acid,—bromides. The bromides are very similar to
the chlorides in their properties. Chlorine acts upon both bromides and
free hydrobromic acid, liberating bromine from them:
KBr + Cl = KCl + Br,
HBr + Cl = HCl + Br.
Silver bromide is extensively used in photography, and the bromides of
sodium and potassium are used as drugs.
Oxygen compounds. No oxides of bromine are surely known, and
bromine does not form so many oxygen acids as chlorine does.
Salts of hypobromous acid (HBrO) and bromic acid (HBrO3) are
known.
IODINE
Historical. Iodine was discovered in 1812 by Courtois in the ashes of
certain sea plants. Its presence was revealed by its beautiful violet
vapor, and this suggested the name iodine (from the Greek for violet
appearance).
Occurrence. In the combined state iodine occurs in very small quantities
in sea water, from which it is absorbed by[Pg 191] certain sea plants, so that
it is found in their ashes. It occurs along with bromine in salt springs
and beds, and is also found in Chili saltpeter.
Preparation. Iodine may be prepared in a number of ways, the principal
methods being the following:
1. Laboratory method. Iodine can readily be prepared in the laboratory
from an iodide by the method used in preparing bromine, except that
sodium iodide is substituted for sodium bromide. It can also be made by
passing chlorine into a solution of an iodide.
 Fig. 57
Fig. 57
2. Commercial method. Commercially iodine was formerly prepared from
seaweed (kelp), but is now obtained almost entirely from the deposits of
Chili saltpeter. The crude saltpeter is dissolved in water and the
solution evaporated until the saltpeter crystallizes. The remaining
liquors, known as the "mother liquors," contain sodium iodate
(NaIO3), in which form the iodine is present in the saltpeter. The
chemical reaction by which the iodine is liberated from this compound is
a complicated one, depending on the fact that sulphurous acid acts upon
iodic acid, setting iodine free. This reaction is shown as follows:
2HIO3 + 5H2SO3 = 5H2SO4 + H2O + 2I.
Purification of iodine. Iodine can be purified very
conveniently in the following way. The crude iodine is placed
in an evaporating dish E (Fig. 57), and the dish is set upon
the sand bath S. The iodine is covered with the inverted
funnel F, and the sand bath is[Pg 192] gently heated with a Bunsen
burner. As the dish becomes warm the iodine rapidly evaporates
and condenses again on the cold surface of the funnel in
shining crystals.
This process, in which a solid is converted into a vapor and is
again condensed into a solid without passing through the liquid
state, is called sublimation.
Physical properties. Iodine is a purplish-black, shining, heavy solid
which crystallizes in brilliant plates. Even at ordinary temperatures it
gives off a beautiful violet vapor, which increases in amount as heat is
applied. It melts at 107° and boils at 175°. It is slightly soluble in
water, but readily dissolves in alcohol, forming a brown solution
(tincture of iodine), and in carbon disulphide, forming a violet
solution. The element has a strong, unpleasant odor, though by no means
as irritating as that of chlorine and bromine.
Chemical properties. Chemically iodine is quite similar to chlorine and
bromine, but is still less active than bromine. It combines directly
with many elements at ordinary temperatures. At elevated temperatures it
combines with hydrogen, but the reaction is reversible and the compound
formed is quite easily decomposed. Both chlorine and bromine displace it
from its salts:
KI + Br = KBr + I,
KI + Cl = KCl + I.
When even minute traces of iodine are added to thin starch paste a very
intense blue color develops, and this reaction forms a delicate test for
iodine. Iodine is extensively used in medicine, especially in the form
of a tincture. It is also largely used in the preparation of dyes and
organic drugs, iodoform, a substance used as an antiseptic, has the
[Pg 193]formula CHI3.
Hydriodic acid (HI). This acid cannot be prepared in pure condition by
the action of sulphuric acid upon an iodide, since the hydriodic acid
set free is oxidized by the sulphuric acid just as in the case of
hydrobromic acid, but to a much greater extent. It can be prepared in
exactly the same way as hydrobromic acid, iodine being substituted for
bromine. It can also be prepared by passing hydrosulphuric acid into
water in which iodine is suspended. The equation is
The hydriodic acid formed in this way dissolves in the water.
Properties and uses. Hydriodic acid resembles the corresponding acids of
chlorine and bromine in physical properties, being a strongly fuming,
colorless gas, readily soluble in water. Under standard conditions 1
volume of water dissolves about 460 volumes of the gas. It is, however,
more unstable than either hydrochloric or hydrobromic acids, and on
exposure to the air it gradually decomposes in accordance with the
equation
Owing to the slight affinity between iodine and hydrogen the acid easily
gives up its hydrogen and is therefore a strong reducing agent. This is
seen in its action on sulphuric acid.
The salts of hydriodic acid, the iodides, are, in general, similar to
the chlorides and bromides. Potassium iodide (KI) is the most familiar
of the iodides and is largely used in medicine.
Oxygen compounds. Iodine has a much greater affinity for oxygen
than has either chlorine or bromine. When heated with nitric
[Pg 194]acid it forms a stable oxide (I2O5). Salts of iodic acid
(HIO3) and periodic acid (HIO4) are easily prepared, and
the free acids are much more stable than the corresponding
acids of the other members of this family.
GAY-LUSSAC'S LAW OF VOLUMES
In the discussion of the composition of hydrochloric acid it was stated
that one volume of hydrogen combines with one volume of chlorine to form
two volumes of hydrochloric acid. With bromine and iodine similar
combining ratios hold good. These facts recall the simple volume
relations already noted in the study of the composition of steam and
ammonia. These relations may be represented graphically in the following
way:
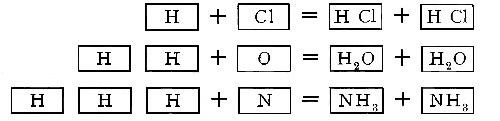 Graph
Graph
In the early part of the past century Gay-Lussac, a distinguished French
chemist, studied the volume relations of many combining gases, and
concluded that similar relations always hold. His observations are
summed up in the following law: When two gases combine chemically there
is always a simple ratio between their volumes, and between the volume
of either one of them and that of the product, provided it is a gas. By
a simple ratio is meant of course the ratio of small whole numbers, as
1 : 2, 2 : 3.
EXERCISES
1. How do we account for the fact that liquid hydrofluoric acid is not
an electrolyte?
2. Why does sulphuric acid liberate hydrofluoric acid from its salts?[Pg 195]
3. In the preparation of chlorine, what advantages are there in treating
manganese dioxide with a mixture of sodium chloride and sulphuric acid
rather than with hydrochloric acid?
4. Why must chlorine water be kept in the dark?
5. What is the derivation of the word nascent?
6. What substances studied are used as bleaching agents? To what is the
bleaching action due in each case?
7. What substances studied are used as disinfecting agents?
8. What is meant by the statement that hydrochloric acid is one of the
strongest acids?
9. What is the meaning of the phrase aqua regia?
10. Cl2O is the anhydride of what acid?
11. A solution of hydriodic acid on standing turns brown. How is this
accounted for?
12. How can bromine vapor and nitrogen peroxide be distinguished from
each other?
13. Write the equations for the reaction taking place when hydriodic
acid is prepared from iodine, phosphorus, and water.
14. From their behavior toward sulphuric acid, to what class of agents
do hydrobromic and hydriodic acids belong?
15. Give the derivation of the names of the elements of the chlorine
family.
16. Write the names and formulas for the binary acids of the group in
the order of the stability of the acids.
17. What is formed when a metal dissolves in each of the following?
nitric acid; dilute sulphuric acid; concentrated sulphuric acid;
hydrochloric acid; aqua regia.
18. How could you distinguish between a chloride, a bromide, and an
iodide?
19. What weight of sodium chloride is necessary to prepare sufficient
hydrochloric acid to saturate 1 l. of water under standard conditions?
20. On decomposition 100 l. of hydrochloric acid would yield how many
liters of hydrogen and chlorine respectively, the gases being measured
under the same conditions? Are your results in accord with the
experimental facts?
[Pg 196]
CHAPTER XVII
CARBON AND SOME OF ITS SIMPLER COMPOUNDS
The family. Carbon stands at the head of a family of elements in the
fourth group in the periodic table. The resemblances between the
elements of this family, while quite marked, are not so striking as in
the case of the elements of the chlorine family. With the exception of
carbon, these elements are comparatively rare, and need not be taken up
in detail in this chapter. Titanium will be referred to again in
connection with silicon which it very closely resembles.
Occurrence. Carbon is found in nature in the uncombined state in several
forms. The diamond is practically pure carbon, while graphite and coal
are largely carbon, but contain small amounts of other substances. Its
natural compounds are exceedingly numerous and occur as gases, liquids,
and solids. Carbon dioxide is its most familiar gaseous compound.
Natural gas and petroleum are largely compounds of carbon with hydrogen.
The carbonates, especially calcium carbonate, constitute great strata of
rocks, and are found in almost every locality. All living organisms,
both plant and animal, contain a large percentage of this element, and
the number of its compounds which go to make up all the vast variety of
animate nature is almost limitless. Over one hundred thousand definite
compounds containing carbon have been prepared. In the free state carbon
occurs in three allotropic forms, two of which are crystalline and one
amorphous.[Pg 197]
Crystalline carbon. Crystalline carbon occurs in two forms,—diamond and
graphite.
1. Diamond. Diamonds are found in considerable quantities in several
localities, especially in South Africa, the East Indies, and Brazil. The
crystals belong to the regular system, but the natural stones do not
show this very clearly. When found they are usually covered with a rough
coating which is removed in the process of cutting. Diamond cutting is
carried on most extensively in Holland.
The density of the diamond is 3.5, and, though brittle, it is one of the
hardest of substances. Black diamonds, as well as broken and imperfect
stones which are valueless as gems, are used for grinding hard
substances. Few chemical reagents have any action on the diamond, but
when heated in oxygen or the air it blackens and burns, forming carbon
dioxide.
Lavoisier first showed that carbon dioxide is formed by the combustion
of the diamond; and Sir Humphry Davy in 1814 showed that this is the
only product of combustion, and that the diamond is pure carbon.
The diamond as a gem. The pure diamond is perfectly transparent
and colorless, but many are tinted a variety of colors by
traces of foreign substances. Usually the colorless ones are
the most highly prized, although in some instances the color
adds to the value; thus the famous Hope diamond is a beautiful
blue. Light passing through a diamond is very much refracted,
and to this fact the stone owes its brilliancy and sparkle.
Artificial preparation of diamonds. Many attempts have been
made to produce diamonds artificially, but for a long time
these always ended in failure, graphite and not diamonds being
the product obtained. The French chemist Moissan, in his
extended study of chemistry at high temperatures, finally
succeeded (1893) in making some small ones. He accomplished
this by dissolving carbon in boiling iron and plunging the
crucible containing the mixture into water,[Pg 198] as shown in Fig.
58. Under these conditions the carbon crystallized in the iron
in the form of the diamond. The diamonds were then obtained by
dissolving away the iron in hydrochloric acid.
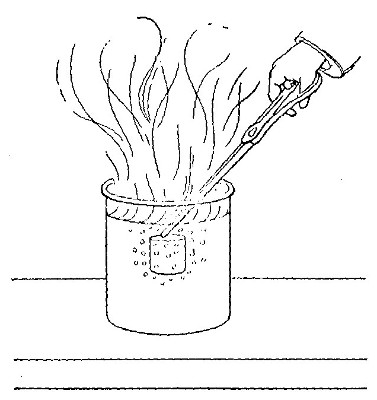 Fig. 58
Fig. 58
2. Graphite. This form of carbon is found in large quantities,
especially in Ceylon, Siberia, and in some localities of the United
States and Canada. It is a shining black substance, very soft and greasy
to the touch. Its density is about 2.15. It varies somewhat in
properties according to the locality in which it is found, and is more
easily attacked by reagents than is the diamond. It is also manufactured
by heating carbon with a small amount of iron (3%) in an electric
furnace. It is used in the manufacture of lead pencils and crucibles, as
a lubricant, and as a protective covering for iron in the form of a
polish or a paint.
Amorphous carbon. Although there are many varieties of amorphous carbon
known, they are not true allotropic modifications. They differ merely in
their degree of purity, their fineness of division, and in their mode of
preparation. These substances are of the greatest importance, owing to
their many uses in the arts and industries. As they occur in nature, or
are made artificially, they are nearly all impure carbon, the impurity
depending on the particular substance in question.
1. Pure carbon. Pure amorphous carbon is best prepared by charring
sugar. This is a substance consisting of carbon, hydrogen, and oxygen,
the latter two elements being present in the ratio of one oxygen atom to
two of hydrogen.[Pg 199] When sugar is strongly heated the oxygen and hydrogen
are driven off in the form of water and pure carbon is left behind.
Prepared in this way it is a soft, lustrous, very bulky, black powder.
2. Coal and coke. Coals of various kinds were probably formed from
vast accumulations of vegetable matter in former ages, which became
covered over with earthy material and were thus protected from rapid
decay. Under various natural agencies the organic matter was slowly
changed into coal. In anthracite these changes have gone the farthest,
and this variety of coal is nearly pure carbon. Soft or bituminous coals
contain considerable organic matter besides carbon and mineral
substances. When heated strongly out of contact with air the organic
matter is decomposed and the resulting volatile matter is driven off in
the form of gases and vapors, and only the mineral matter and carbon
remain behind. The gaseous product is chiefly illuminating gas and the
solid residue is coke. Some of the coke is found as a dense cake on
the sides and roof of the retort. This is called retort carbon and is
quite pure.
3. Charcoal. This is prepared from wood in the same way that coke is
made from coal. When the process is carried on in retorts the products
expelled by the heat are saved. Among these are many valuable substances
such as wood alcohol and acetic acid. Where timber is abundant the
process is carried out in a wasteful way, by merely covering piles of
wood with sod and setting the wood on fire. Some wood burns and the heat
from this decomposes the wood not burned, forming charcoal from it. The
charcoal, of course, contains the mineral part of the wood from which it
is formed.[Pg 200]
4. Bone black. This is sometimes called animal charcoal, and is made
by charring bones and animal refuse. The organic part of the materials
is thus decomposed and carbon is left in a very finely divided state,
scattered through the mineral part which consists largely of calcium
phosphate. For some uses this mineral part is removed by treatment with
hydrochloric acid and prolonged washing.
5. Lampblack. Lampblack and soot are products of imperfect combustion
of oil and coal, and are deposited from a smoky flame on a cold surface.
The carbon in this form is very finely divided and usually contains
various oily materials.
Properties. While the various forms of carbon differ in many properties,
especially in color and hardness, yet they are all odorless, tasteless
solids, insoluble in water and characterized by their stability towards
heat. Only in the intense heat of the electric arc does carbon
volatilize, passing directly from the solid state into a vapor. Owing to
this fact the inside surface of an incandescent light bulb after being
used for some time becomes coated with a dark film of carbon. It is not
acted on at ordinary temperatures by most reagents, but at a higher
temperature it combines directly with many of the elements, forming
compounds called carbides. When heated in the presence of sufficient
oxygen it burns, forming carbon dioxide.
Uses of carbon. The chief use of amorphous carbon is for fuel to furnish
heat and power for all the uses of civilization. An enormous quantity of
carbon in the form of the purer coals, coke, and charcoal is used as a
reducing agent in the manufacture of the various metals, especially in
the metallurgy of iron. Most of the metals are found in nature as
oxides, or in forms which can readily be[Pg 201] converted into oxides. When
these oxides are heated with carbon the oxygen is abstracted, leaving
the metal. Retort carbon and coke are used to make electric light
carbons and battery plates, while lampblack is used for indelible inks,
printer's ink, and black varnishes. Bone black and charcoal have the
property of absorbing large volumes of certain gases, as well as smaller
amounts of organic matter; hence they are used in filters to remove
noxious gases and objectionable colors and odors from water. Bone black
is used extensively in the sugar refineries to remove coloring matter
from the impure sugars.
Chemistry of carbon compounds. Carbon is remarkable for the very large
number of compounds which it forms with the other elements, especially
with oxygen and hydrogen. Compounds containing carbon are more numerous
than all others put together, and the chemistry of these substances
presents peculiarities not met with in the study of other substances.
For these reasons the systematic study of carbon compounds, or of
organic chemistryas it is usually called, must be deferred until the
student has gained some knowledge of the chemistry of other elements. An
acquaintance with a few of the most familiar carbon compounds is,
however, essential for the understanding of the general principles of
chemistry.
Compounds of carbon with hydrogen,—the hydrocarbons. Carbon unites with
hydrogen to form a very large number of compounds called hydrocarbons.
Petroleum and natural gas are essentially mixtures of a great variety of
these hydrocarbons. Many others are found in living plants, and still
others are produced by the decay of organic matter in the absence of
air. Only two of them, methane and acetylene, will be discussed here.[Pg 202]
Methane (marsh gas) (CH4). This is one of the most important of
these hydrocarbons, and constitutes about nine tenths of natural gas. As
its name suggests, it is formed in marshes by the decay of vegetable
matter under water, and bubbles of the gas are often seen to rise when
the dead leaves on the bottom of pools are stirred. It also collects in
mines, and, when mixed with air, is called fire damp by the miners
because of its great inflammability, damp being an old name for a gas.
It is formed when organic matter, such as coal or wood, is heated in
closed vessels, and is therefore a principal constituent of coal gas.
Preparation. Methane is prepared in the laboratory by heating sodium or
calcium acetate with soda-lime. Equal weights of fused sodium acetate
and soda-lime are thoroughly dried, then mixed and placed in a
good-sized, hard-glass test tube fitted with a one-holed stopper and
delivery tube. The mixture is gradually heated, and when the air has
been displaced from the tube the gas is collected in bottles by
displacement of water. Soda-lime is a mixture of sodium and calcium
hydroxides. Regarding it as sodium hydroxide alone, the equation is
NaC2H3O2 + NaOH = Na2CO3 + CH4.
Properties. Methane is a colorless, odorless gas whose density is 0.55.
It is difficult to liquefy, boiling at -155° under standard pressure,
and is almost insoluble in water. It burns with a pale blue flame,
liberating much heat, and when mixed with oxygen is very explosive.
Davy's safety lamp. In 1815 Sir Humphry Davy invented a lamp for the use
of miners, to prevent the dreadful mine explosions then common, due to
methane mixed with air. The invention consisted in surrounding the upper
part of the common miner's lamp with a mantle of wire gauze and the
lower part with glass (Fig. 59). It has been seen that two gases will
not combine until raised to their[Pg 203] kindling temperature, and if while
combining they are cooled below this point, the combination ceases. A
flame will not pass through a wire gauze because the metal, being a good
conductor of heat, takes away so much heat from the flame that the gases
are cooled below the kindling temperature. When a lamp so protected is
brought into an explosive mixture the gases inside the wire mantle burn
in a series of little explosions, giving warning to the miner that the
air is unsafe.
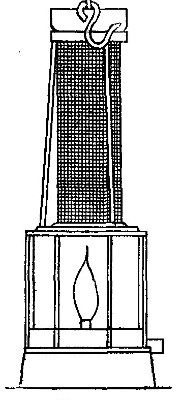 Fig. 59
Fig. 59
Acetylene (C2H2). This is a colorless gas usually having a
disagreeable odor due to impurities. It is now made in large quantities
from calcium carbide (CaC2). This substance is formed when coal and
lime are heated together in an electric furnace. When treated with water
the carbide is decomposed, yielding acetylene:
CaC2 + 2H2O = C2H2 + Ca(OH)2.
Under ordinary conditions the gas burns with a very smoky flame; in
burners constructed so as to secure a large amount of oxygen it burns
with a very brilliant white light, and hence is used as an illuminant.
Laboratory preparation. The gas can be prepared readily in a generator
such as is shown in Fig. 60. The inner tube contains fragments of
calcium carbide, while the outer one is filled with water. As long as
the stopcock is closed the water cannot rise in the inner tube. When the
stopcock is open the water rises, and, coming into contact with the
carbide in the inner tube, generates acetylene. This escapes through the
stopcock, and after the air has been expelled may be lighted as it
issues from the burner.
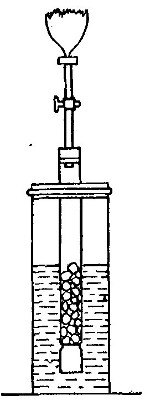 Fig. 60
Fig. 60
Carbon forms two oxides, namely, carbon dioxide (CO2) and carbon
monoxide (CO).[Pg 204]
Carbon dioxide (CO2). Carbon dioxide is present in the air to the
extent of about 3 parts in 10,000, and this apparently small amount is
of fundamental importance in nature. In some localities it escapes from
the earth in great quantities, and many spring waters carry large
amounts of it in solution. When these highly charged spring waters reach
the surface of the earth, and the pressure on them is removed, the
carbon dioxide escapes with effervescence. It is a product of the
oxidation of all organic matter, and is therefore formed in fires as
well as in the process of decay. It is thrown off from the lungs of all
animals in respiration, and is a product of many fermentation processes
such as vinegar making and brewing. Combined with metallic oxides it
forms vast deposits of carbonates in nature.
Preparation. In the laboratory carbon dioxide is always prepared by the
action of an acid upon a carbonate, usually calcium carbonate, the
apparatus shown in Fig. 39 serving the purpose very well. This reaction
might be expected to produce carbonic acid, thus:
CaCO3 + 2HCl = CaCl2 + H2CO3.
Carbonic acid is very unstable, however, and decomposes into its
anhydride, CO2, and water, thus:
The complete reaction is represented by the equation
CaCO3 + 2HCl = CaCl2 + CO2 + H2O.
Physical properties. Carbon dioxide is a colorless, practically odorless
gas whose density is 1.5. Its weight may be inferred from the fact that
it can be siphoned, or poured like water, from one vessel downward into
another. At 15°[Pg 205] and under ordinary pressure it dissolves in its own
volume of water and imparts a somewhat biting, pungent taste to it. It
is easily condensed, and is now prepared commercially in this form by
pumping the gas into steel cylinders (see Fig. 6) which are kept cold
during the process. When the liquid is permitted to escape into the air
part of it instantly evaporates, and in so doing absorbs so much heat
that another portion is solidified, the solid form strikingly resembling
snow in appearance. This snow is very cold and mercury can easily be
frozen with it.
Solid carbon dioxide. Cylinders of liquid carbon dioxide are
inexpensive, and should be available in every school. To demonstrate the
properties of solid carbon dioxide, the cylinder should be placed across
the table and supported in such a way that the stopcock end is several
inches lower than the other end. A loose bag is made by holding the
corners of a handkerchief around the neck of the stopcock, and the cock
is then turned on so that the gas rushes out in large quantities. Very
quickly a considerable quantity of the snow collects in the
handkerchief. To freeze mercury, press a piece of filter paper into a
small evaporating dish and pour the mercury upon it. Coil a flat spiral
upon the end of a wire, and dip the spiral into the mercury. Place a
quantity of solid carbon dioxide upon the mercury and pour 10 cc.-15 cc.
of ether over it. In a minute or two the mercury will solidify and may
be removed from the dish by the wire serving as a handle. The filter
paper is to prevent the mercury from sticking to the dish; the ether
dissolves the solid carbon dioxide and promotes its rapid conversion
into gas.
Chemical properties. Carbon dioxide is incombustible, since it is, like
water, a product of combustion. It does not support combustion, as does
nitrogen peroxide, because the oxygen in it is held in very firm
chemical union with the carbon. Very strong reducing agents, such as
highly heated carbon, can take away half of its oxygen:
[Pg 206]
Uses. The relation of carbon dioxide to plant life has been discussed in
a previous chapter. Water highly charged with carbon dioxide is used for
making soda water and similar beverages. Since it is a non-supporter of
combustion and can be generated readily, carbon dioxide is also used as
a fire extinguisher. Some of the portable fire extinguishers are simply
devices for generating large amounts of the gas. It is not necessary
that all the oxygen should be kept away from the fire in order to
smother it. A burning candle is extinguished in air which contains only
2.5% of carbon dioxide.
Carbonic acid (H2CO3). Like most of the oxides of the non-metallic
elements, carbon dioxide is an acid anhydride. It combines with water to
form an acid of the formula H2CO3, called carbonic acid:
The acid is, however, very unstable and cannot be isolated. Only a very
small amount of it is actually formed when carbon dioxide is passed into
water, as is evident from the small solubility of the gas. If, however,
a base is present in the water, salts of carbonic acid are formed, and
these are quite stable:
2NaOH + H2O + CO2 = Na2CO3 + 2H2O.
Action of carbon dioxide on bases. This conduct is explained by the
principles of reversible reactions. The equation
is a reversible equation, and the extent to which the reaction
progresses depends upon the relative concentrations of each of the three
factors in it. Equilibrium is ordinarily reached when very little
H2CO3 is formed. If a base is present in the water to combine with
the H2CO3 as fast as it is formed, all of the CO2 is converted
[Pg 207]into H2CO3, and thence into a carbonate.
Salts of carbonic acid,—carbonates. The carbonates form a very
important class of salts. They are found in large quantities in nature,
and are often used in chemical processes. Only the carbonates of sodium,
potassium, and ammonium are soluble, and these can be made by the action
of carbon dioxide on solutions of the bases, as has just been explained.
The insoluble carbonates are formed as precipitates when soluble salts
are treated with a solution of a soluble carbonate. Thus the insoluble
calcium carbonate can be made by bringing together solutions of calcium
chloride and sodium carbonate:
CaCl2 + Na2CO3 = CaCO3 + 2NaCl.
Most of the carbonates are decomposed by heat, yielding an oxide of the
metal and carbon dioxide. Thus lime (calcium oxide) is made by strongly
heating calcium carbonate:
Acid carbonates. Like all acids containing two acid hydrogen atoms,
carbonic acid can form both normal and acid salts. The acid carbonates
are made by treating a normal carbonate with an excess of carbonic acid.
With few exceptions they are very unstable, heat decomposing them even
when in solution.
Action of carbon dioxide on calcium hydroxide. If carbon dioxide is
passed into clear lime water, calcium carbonate is at first
precipitated:
H2O + CO2 = H2CO3,
Ca(OH)2 + H2CO3 = CaCO3 + 2H2O.
Advantage is taken of this reaction in testing for the presence of
carbon dioxide, as already explained in the chapter on the atmosphere.
If the current of carbon dioxide is continued, the precipitate[Pg 208] soon
dissolves, because the excess of carbonic acid forms calcium acid
carbonate which is soluble:
CaCO3 + H2CO3 = Ca(HCO3)2.
If now the solution is heated, the acid carbonate is decomposed and
calcium carbonate once more precipitated:
Ca(HCO3)2 = CaCO3 + H2CO3.
Carbon monoxide (CO). Carbon monoxide can be made in a number of ways,
the most important of which are the three following:
1. By the partial oxidation of carbon. If a slow current of air is
conducted over highly heated carbon, the monoxide is formed, thus:
It is therefore often formed in stoves when the air draught is
insufficient. Water gas, which contains large amounts of carbon
monoxide, is made by partially oxidizing carbon with steam:
2. By the partial reduction of carbon dioxide. When carbon dioxide is
conducted over highly heated carbon it is reduced to carbon monoxide by
the excess of carbon:
When coal is burning in a stove or grate carbon dioxide is at first
formed in the free supply of air, but as the hot gas rises through the
glowing coal it is reduced to carbon monoxide. When the carbon monoxide
reaches the free air above the coal it takes up oxygen to form carbon
dioxide, burning with the blue flame so familiar above a bed of coals,
especially in the case of hard coals.
3. By the decomposition of oxalic acid. In the laboratory carbon
monoxide is usually prepared by the action of[Pg 209] concentrated sulphuric
acid upon oxalic acid. The latter substance has the formula
C2H2O4. The sulphuric acid, owing to its affinity for water,
decomposes the oxalic acid, as represented in the equation
C2H2O4 + (H2SO4) = (H2SO4) + H2O + CO2 + CO.
Properties. Carbon monoxide is a light, colorless, almost odorless gas,
very difficult to liquefy. Chemically it is very active, combining
directly with a great many substances. It has a great affinity for
oxygen and is therefore combustible and a good reducing agent. Thus, if
carbon monoxide is passed over hot copper oxide, the copper is reduced
to the metallic state:
When inhaled it combines with the red coloring matter of the blood and
in this way prevents the absorption of oxygen, so that even a small
quantity of the gas may prove fatal.
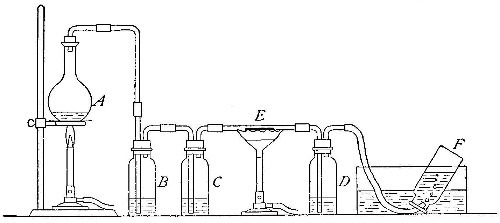 Fig. 61
Fig. 61
The reducing power of carbon monoxide. Fig. 61 illustrates a
method of showing the reducing power of carbon monoxide. The
gas is generated by gently heating 7 or 8 g. of oxalic acid
with 25 cc. of concentrated sulphuric acid in a 200 cc. flask
A. The bottle B contains a solution of sodium hydroxide,
which removes the carbon dioxide formed along with the
monoxide. C contains a solution of calcium hydroxide to show
that the carbon dioxide is completely removed. E is a
hard-glass tube containing 1 or 2 g. of copper oxide, which is
heated by a burner. The black copper oxide is reduced to
reddish metallic copper by the carbon monoxide, which is
thereby changed to carbon dioxide. The presence of the carbon
dioxide is shown by the precipitate in the calcium hydroxide
solution in D. Any unchanged carbon monoxide is collected
over water in F.
[Pg 210]
Carbon disulphide (CS2). Just as carbon combines with oxygen to form
carbon dioxide, so it combines with sulphur to form carbon disulphide
(CS2). This compound has been described in the chapter on sulphur.
Hydrocyanic acid (prussic acid)(HCN). Under the proper conditions
carbon unites with nitrogen and hydrogen to form the acid HCN, called
hydrocyanic acid. It is a weak, volatile acid, and is therefore easily
prepared by treating its salts with sulphuric acid:
KCN + H2SO4 = KHSO4 + HCN.
It is most familiar as a gas, though it condenses to a colorless liquid
boiling at 26°. It has a peculiar odor, suggesting bitter almonds, and
is extremely poisonous either when inhaled or when taken into the
stomach. A single drop may cause death. It dissolves readily in water,
its solution being commonly called prussic acid.
The salts of hydrocyanic acid are called cyanides, the cyanides of
sodium and potassium being the best known. These are white solids and
are extremely poisonous.
Solutions of potassium cyanide are alkaline. A solution of potassium
cyanide turns red litmus blue, and must therefore contain hydroxyl ions.
The presence of these ions is accounted for in the following way.
Although water is so little dissociated into its ions H+ and OH-
that for most purposes we may neglect the dissociation, it is
nevertheless measurably dissociated. Hydrocyanic acid is one of the
weakest of acids, and dissociates[Pg 211] to an extremely slight extent. When a
cyanide such as potassium cyanide dissolves it freely dissociates, and
the CN- ions must come to an equilibrium with the H+ ions derived
from the water:
The result of this equilibrium is that quite a number of H+ ions from
the water are converted into undissociated HCN molecules. But for every
H+ ion so removed an OH- ion remains free, and this will give the
solution alkaline properties.
EXERCISES
1. How can you prove that the composition of the different allotropic
forms of carbon is the same?
2. Are lampblack and bone black allotropic forms of carbon? Will equal
amounts of heat be liberated in the combustion of 1 g. of each?
3. How could you judge of the relative purity of different forms of
carbon?
4. Apart from its color, why should carbon be useful in the preparation
of inks and paints?
5. Could asbestos fibers be used to replace the wire in a safety lamp?
6. Why do most acids decompose carbonates?
7. What effect would doubling the pressure have upon the solubility of
carbon dioxide in water?
8. What compound would be formed by passing carbon dioxide into a
solution of ammonium hydroxide? Write the equation.
9. Write equations for the preparation of K2CO3; of BaCO3; of
MgCO3.
10. In what respects are carbonic and sulphurous acids similar?
11. Give three reasons why the reaction which takes place when a
solution of calcium acid carbonate is heated, completes itself.
12. How could you distinguish between carbonates and sulphites?
13. How could you distinguish between oxygen, hydrogen, nitrogen,
nitrous oxide, and carbon dioxide?[Pg 212]
14. Could a solution of sodium hydroxide be substituted for the solution
of calcium hydroxide in testing for carbon dioxide?
15. What weight of sodium hydroxide is necessary to neutralize the
carbonic acid formed by the action of hydrochloric acid on 100 g. of
calcium carbonate?
16. What weight of calcium carbonate would be necessary to prepare
sufficient carbon dioxide to saturate 10 l. of water at 15° and under
ordinary pressure?
17. On the supposition that calcium carbide costs 12 cents a kilogram,
what would be the cost of an amount sufficient to generate 100 l. of
acetylene measured at 20° and 740 mm.?
18. How would the volume of a definite amount of carbon monoxide compare
with the volume of carbon dioxide formed by its combustion, the
measurements being made under the same conditions?
[Pg 213]
CHAPTER XVIII
FLAMES,—ILLUMINANTS
Conditions necessary for flames. It has been seen that when two
substances unite chemically, with the production of light and heat, the
act of union is called combustion. When one of the substances undergoing
combustion remains solid at the temperature occasioned by the
combustion, light may be given off, but there is no flame. Thus iron
wire burning in oxygen throws off a shower of sparks and is brilliantly
incandescent, but no flame is seen. When, however, both of the
substances are gases or vapors at the temperature reached in the
combustion, the act of union is accompanied by a flame.
Flames from burning liquids or solids. Many substances which are liquids
or solids at ordinary temperatures burn with a flame because the heat of
combustion vaporizes them slowly, and the flame is due to the union of
this vapor with the gas supporting the combustion.
Supporter of combustion. That gas which surrounds the flame and
constitutes the atmosphere in which the combustion occurs is said to
support the combustion. The other gas which issues into this atmosphere
is said to be the combustible gas. Thus, in the ordinary combustion of
coal gas in the air the coal gas is said to be combustible, while the
air is regarded as the supporter of combustion. These terms are entirely
relative, however, for a jet of air issuing into an atmosphere of coal
gas will burn when ignited, the coal gas supporting the combustion.[Pg 214]
Ordinarily, when we say that a gas is combustible we mean that it is
combustible in an atmosphere of air.
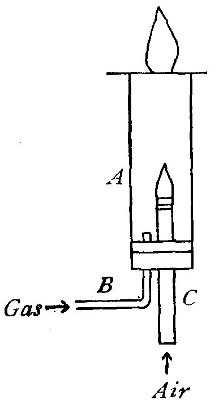 Fig. 62
Fig. 62
Either gas may be the supporter of combustion. That the terms
combustible and supporter of combustion are merely relative may be
shown in the following way: A lamp chimney A is fitted with a cork and
glass tubes, as shown in Fig. 62. The tube C should have a diameter of
from 12 to 15 mm. A thin sheet of asbestos in which is cut a circular
opening about 2 cm. in diameter is placed over the top of the chimney.
The opening in the asbestos is closed with the palm of the hand, and gas
is admitted to the chimney through the tube B. The air in the chimney
is soon expelled through the tube C, and the gas itself is then
lighted at the lower end of this tube. The hand is now removed from the
opening in the asbestos, when the flame at the end of the tube at once
rises and appears at the end within the chimney, as shown in the figure.
The excess of coal gas now escapes from the opening in the asbestos and
may be lighted. The flame at the top of the asbestos board is due to the
combustion of coal gas in air, while the flame within the chimney is due
to the combustion of air in coal gas, the air being drawn up through the
tube by the escaping gas.
Appearance of flames. The flame caused by the union of hydrogen and
oxygen is almost colorless and invisible. Chlorine and hydrogen combine
with a pale violet flame, carbon monoxide burns in oxygen with a blue
flame, while ammonia burns with a deep yellow flame. The color and
appearance of flames are therefore often quite characteristic of the
particular combustion which occasions them.
Structure of flames. When the gas undergoing combustion issues from a
round opening into an atmosphere of the gas supporting combustion, as is
the case with the burning Bunsen burner (Fig. 63), the flame is
generally[Pg 215] conical in outline. It consists of several distinct cones,
one within the other, the boundary between them being marked by
differences of color or luminosity. In the simplest flame, of which
hydrogen burning in oxygen is a good example, these cones are two in
number,—an inner one, formed by unburned gas, and an outer one, usually
more or less luminous, consisting of the combining gases. This outer one
is in turn surrounded by a third envelope of the products of combustion;
this envelope is sometimes invisible, as in the present case, but is
sometimes faintly luminous. The lower part of the inner cone of the
flame is quite cool and consists of unburned gas. Toward the top of the
inner cone the gas has become heated to a high temperature by the
burning envelope surrounding it. On reaching the supporter of combustion
on the outside it is far above its kindling temperature, and combustion
follows with the evolution of much heat. The region of combustion just
outside the inner cone is therefore the hottest part of the flame.
 Fig. 63
Fig. 63
Oxidizing and reducing flames. Since the tip of the outside cone
consists of very hot products of combustion mixed with oxygen from the
air, a substance capable of oxidation placed in this part of the flame
becomes very hot and is easily oxidized. The oxygen with which it
combines comes, of course, from the atmosphere, and not from the
products of combustion. This outer tip of the flame is called the
oxidizing flame.
At the tip of the inner cone the conditions are quite different. This
region consists of a highly heated combustible gas, which has not yet
reached a supply of oxygen.[Pg 216]
If a substance rich in oxygen, such as a metallic oxide, is placed in
this region of the flame, the heated gases combine with its oxygen and
the substance is reduced. This part of the flame is called the reducing
flame. These flames are used in testing certain substances, especially
minerals. For this purpose they are produced by blowing into a small
luminous Bunsen flame from one side through a blowpipe. This is a tube
of the shape shown in Fig. 64. The flame is directed in any desired way
and has the oxidizing and reducing regions very clearly marked (Fig.
65). It is non-luminous from the same causes which render the open
Bunsen burner flame non-luminous, the gases from the lungs serving to
furnish oxygen and to dilute the combustible gas.
 Fig. 64
Fig. 64
 Fig. 65
Fig. 65
Luminosity of flames. The luminosity of flames is due to a number of
distinct causes, and may therefore be increased or diminished in several
ways.
1. Presence of solid matter. The most obvious of these causes is the
presence in the flame of incandescent solid matter. Thus chalk dust
sifted into a non-luminous flame renders it luminous. When hydrocarbons
form a part of the combustible gas, as they do in nearly all
illuminating gases and oils, some carbon is usually set free in the
process of combustion. This is made very hot by the flame and becomes
incandescent, giving out light. In a well-regulated flame it is
afterward burned up, but when the supply of oxygen is insufficient it
escapes from the flame as lampblack or soot. That it is temporarily
present in a well-burning luminous flame may be demonstrated by holding
a cold object, such as a small evaporating dish, in the flame for a few
seconds. This cold object cools the carbon below its kindling
temperature, and it is deposited on the object as soot.[Pg 217]
2. Pressure. A second factor in the luminosity of flames is the
pressure under which the gases are burning. Under increased pressure
there is more matter in a given volume of a gas, and the chemical action
is more energetic than when the gases are rarefied. Consequently there
is more heat and light. A candle burning on a high mountain gives less
light than when it burns at the sea level.
If the gas is diluted with a non-combustible gas, the effect is the same
as if it is rarefied, for under these conditions there is less
combustible gas in a given volume.
3. Temperature. The luminosity also depends upon the temperature
attained in the combustion. In general the hotter the flame the greater
the luminosity; hence cooling the gases before combustion diminishes the
luminosity of the flame they will make, because it diminishes the
temperature attained in the combustion. Thus the luminosity of the
Bunsen flame is largely diminished by the air drawn up with the gas.
This is due in part to the fact that the burning gas is diluted and
cooled by the air drawn in. The oxygen thus introduced into the flame
also causes the combustion of the hot particles of carbon which would
otherwise tend to make the flame luminous.
Illuminating and fuel gases. A number of mixtures of combustible gases,
consisting largely of carbon compounds and hydrogen, find extensive use
for the production of light and heat. The three chief varieties are coal
gas, water gas, and natural gas. The use of acetylene gas has already
been referred to.
Coal gas. Coal gas is made by heating bituminous coal in large retorts
out of contact with the air. Soft or bituminous coal contains, in
addition to large amounts of carbon, considerable quantities of
compounds of hydrogen, oxygen, nitrogen, and sulphur. When distilled the
nitrogen is liberated partly in the form of ammonia and cyanides and
partly as free nitrogen gas; the sulphur is converted into hydrogen
sulphide, carbon disulphide, and oxides of sulphur; the oxygen into
water and oxides of carbon. The[Pg 218] remaining hydrogen is set free partly
as hydrogen and partly in combination with carbon in the form of
hydrocarbons. The most important of these is methane, with smaller
quantities of many others, some of which are liquids or solids at
ordinary temperatures. The great bulk of the carbon remains behind as
coke and retort carbon.
The manufacture of coal gas. In the manufacture of coal gas it is
necessary to separate from the volatile constituents formed by the
heating of the coal all those substances which are either solid or
liquid at ordinary temperature, since these would clog the gas pipes.
Certain gaseous constituents, such as hydrogen sulphide and ammonia,
must also be removed. The method used to accomplish this is shown in
Fig. 66. The coal is heated in air-tight retorts illustrated by A. The
volatile products escape through the pipe X and bubble into the tarry
liquid in the large pipe B, known as the hydraulic main, which runs
at right angles to the retorts. Here is deposited the greater portion of
the solid and liquid products, forming a tarry mass known as coal tar.
Much of the ammonia also remains dissolved in this liquid. The partially
purified gas then passes into the pipes C, which serve to cool it and
further remove the solid and liquid matter. The gas then passes into
D, which is filled with coke over which a jet of water is sprayed. The
water still further cools the gas and at the same time partially removes
such gaseous products as hydrogen sulphide and ammonia, which are
soluble in water. In E the gas passes over some material such as lime,
which removes the last portions of the sulphur compounds as well as much
of the carbon dioxide present. From E the gas passes into the large
gas holder F, from which it is distributed through pipes to the places
where it is burned.
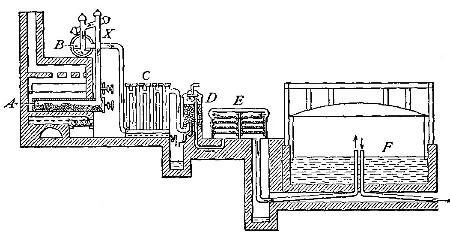 Fig. 66
Fig. 66
[Pg 219]
One ton of good gas coal yields approximately 10,000 cu. ft. of
gas, 1400 lb. of coke, 120 lb. of tar, and 20 gal. of
ammoniacal liquor.
Not only is the ammonia obtained in the manufacture of the gas
of great importance, but the coal tar also serves as the source
of many very useful substances, as will be explained in Chapter
XXXII.
Water gas. Water gas is essentially a mixture of carbon monoxide and
hydrogen. It is made by passing steam over very hot anthracite coal,
when the reaction shown in the following equation takes place:
When required merely to produce heat the gas is at once ready for use.
When made for illuminating purposes it must be enriched, that is,
illuminants must be added, since both carbon monoxide and hydrogen burn
with non-luminous flames. This is accomplished by passing it into
heaters containing highly heated petroleum oils. The gas takes up
hydrocarbon gases formed in the decomposition of the petroleum oils,
which make it burn with a luminous flame.
Water gas is very effective as a fuel, since both carbon monoxide and
hydrogen burn with very hot flames. It has little odor and is very
poisonous. Its use is therefore attended with some risk, since leaks in
pipes are very likely to escape notice.
Natural gas. This substance, so abundant in many localities, varies much
in composition, but is composed principally of methane. When used for
lighting purposes it is usually burned in a burner resembling an open
Bunsen, the illumination being furnished by an incandescent mantle. This
is the case in the familiar Welsbach burner. Contrary to statements
frequently made, natural gas contains no free hydrogen.[Pg 220]
TABLE SHOWING COMPOSITION OF GASES
| PENNSYLVANIA NATURAL GAS | COAL GAS | WATER GAS | ENRICHED WATER GAS |
| Hydrogen | | 41.3 | 52.88 | 30.00 |
| Methane | 90.64 | 43.6 | 2.16 | 24.00 |
| Illuminants | | 3.9 | | 12.05 |
| Carbon monoxide | | 6.4 | 36.80 | 29.00 |
| Carbon dioxide | 0.30 | 2.0 | 3.47 | 0.30 |
| Nitrogen | 9.06 | 1.2 | 4.69 | 2.50 |
| Oxygen | | 0.3 | | 1.50 |
| Hydrocarbon vapors | | 1.5 | | 1.50 |
These are analyses of actual samples, and may be taken as about
the average for the various kinds of gases. Any one of these
may vary considerably. The nitrogen and oxygen in most cases is
due to a slight admixture of air which is difficult to exclude
entirely in the manufacture and handling of gases.
Fuels. A variety of substances are used as fuels, the most important of
them being wood, coal, and the various gases mentioned above. Wood
consists mainly of compounds of carbon, hydrogen, and oxygen. The
composition of coal and the fuel gases has been given. Since these fuels
are composed principally of carbon and hydrogen or their compounds, the
chief products of combustion are carbon dioxide and water. The practice
of heating rooms with portable gas or oil stoves with no provision for
removing the products of combustion is to be condemned, since the carbon
dioxide is generated in sufficient quantities to render the air unfit
for breathing. Rooms so heated also become very damp from the large
amount of water vapor formed in the combustion, and which in[Pg 221] cold
weather condenses on the window glass, causing the glass to "sweat."
Both coal and wood contain a certain amount of mineral substances which
constitute the ashes.
The electric furnace. In recent years electric furnaces have come into
wide use in operations requiring a very high temperature. Temperatures
as high as 3500° can be easily reached, whereas the hottest oxyhydrogen
flame is not much above 2000°. These furnaces are constructed on one of
two general principles.
 Fig. 67
Fig. 67
1. Arc furnaces. In the one type the source of heat is an electric arc
formed between carbon electrodes separated a little from each other, as
shown in Fig. 67. The substance to be heated is placed in a vessel,
usually a graphite crucible, just below the arc. The electrodes and
crucible are surrounded by materials which fuse with great difficulty,
such as magnesium oxide, the walls of the furnace being so shaped as to
reflect the heat downwards upon the contents of the crucible.
 Fig. 68
Fig. 68
2. Resistance furnaces. In the other type of furnace the heat is
generated by the resistance offered to the current in its passage
through the furnace. In its simplest form it may be represented by Fig.
68. The furnace is merely a rectangular box built up of loose bricks.
The electrodes E, each consisting of a bundle of carbon rods, are
introduced through the sides of the furnace. The materials to be heated,
C, are filled into the furnace up to the electrodes, and a layer of
broken coke is arranged so as to extend from one electrode to the other.
More of the charge is then placed on top of the coke. In passing through
the broken coke the electrical current encounters great resistance. This
generates great heat, and the charge surrounding the coke is brought to
a very high temperature. The advantage of this type of furnace is that
the temperature can be regulated to any desired intensity.[Pg 222]
EXERCISES
1. Why does charcoal usually burn with no flame? How do you account for
the flame sometimes observed when it burns?
2. How do you account for the fact that a candle burns with a flame?
3. What two properties must the mantle used in the Welsbach lamp
possess?
4. (a) In what respects does the use of the Welsbach mantle resemble
that of lime in the calcium light? (b) If the mantle were made of
carbon, would it serve the same purpose?
5. Would anthracite coal be suitable for the manufacture of coal gas?
6. How could you prove the formation of carbon dioxide and water in the
combustion of illuminating gases?
7. Suggest a probable way in which natural gas has been formed.
8. Coal frequently contains a sulphide of iron. (a) What two sulphur
compounds are likely to be formed when gas is made from such coal? (b)
Suggest some suitable method for the removal of these compounds.
9. Why does the use of the bellows on the blacksmith's forge cause a
more intense heat?
10. What volume of oxygen is necessary to burn 100 l. of marsh gas and
what volume of carbon dioxide would be formed, all of the gases being
measured under standard conditions?
11. Suppose a cubic meter of Pennsylvania natural gas, measured under
standard conditions, were to be burned. How much water by weight would
result?
[Pg 223]
CHAPTER XIX
MOLECULAR WEIGHTS, ATOMIC WEIGHTS, FORMULAS
Introduction. In the chapter on The Atomic Theory, it was shown that if
it were true that two elements uniting to form a compound always
combined in the ratio of one atom of one element to one atom of the
other element, it would be a very easy matter to decide upon figures
which would represent the relative weights of the different atoms. It
would only be necessary to select some one element as a standard and
determine the weight of every element which combines with a definite
weight (say 1 g.) of the standard element. The figures so obtained would
evidently represent the relative weights of the atoms.
But the law of multiple proportion at once reminds us that two elements
may unite in several proportions; and there is no simple way to
determine the number of atoms present in the molecule of any compound.
Consequently the problem of deciding upon the relative atomic weights is
not an easy one. To the solution of this problem we must now turn.
Dalton's method of determining atomic weights. When Dalton first
advanced the atomic theory he attempted to solve this problem by very
simple methods. He thought that when only one compound of two elements
is known it is reasonable to suppose that it contains one atom of each
element. He therefore gave the formula HO to water, and HN to ammonia.
When more than two compounds were known he assumed that the most
familiar or the most stable one had the simple formula. He then
determined the atomic weight as[Pg 224] explained above. The results he
obtained were contradictory and very far from satisfactory, and it was
soon seen that some other method, resting on much more scientific
grounds, must be found to decide what compounds, if any, have a single
atom of each element present.
Determination of atomic weights. Three distinct steps are involved in
the determination of the atomic weight of an element: (1) determination
of the equivalent, (2) determination of molecular weights of its
compounds, and (3) deduction of the exact atomic weight from the
equivalent and molecular weights.
1. Determination of the equivalent. By the equivalent of an element is
meant the weight of the element which will combine with a fixed weight
of some other element chosen as a standard. It has already been
explained that oxygen has been selected as the standard element for
atomic weights, with a weight of 16. This same standard will serve very
well as a standard for equivalents. The equivalent of an element is the
weight of the element which will combine with 16 g. of oxygen. Thus 16
g. of oxygen combines with 16.03 g. of sulphur, 65.4 g. of zinc, 215.86
g. of silver, 70.9 g. of chlorine. These figures, therefore, represent
the equivalent weights of these elements.
Relation of atomic weights to equivalents. According to the atomic
theory combination always takes place between whole numbers of atoms.
Thus one atom unites with one other, or with two or three; or two atoms
may unite with three, or three with five, and so on.
When oxygen combines with zinc the combination must be between definite
numbers of the two kinds of atoms. Experiment shows that these two
elements combine in the ratio of 16 g. of oxygen to 65.4 g. of zinc. If
one atom of[Pg 225] oxygen combines with one atom of zinc, then this ratio must
be the ratio between the weights of the two atoms. If one atom of oxygen
combines with two atoms of zinc, then the ratio between the weights of
the two atoms will be 16: 32.7. If two atoms of oxygen combine with one
atom of zinc, the ratio by weight between the two atoms will be 8: 65.4.
It is evident, therefore, that the real atomic weight of an element must
be some multiple or submultiple of the equivalent; in other words, the
equivalent multiplied by 1/2, 1, 2, or 3 will give the atomic weight.
Combining weights. A very interesting relation holds good between the
equivalents of the various elements. We have just seen that the figures
16.03, 65.4, 215.86, and 70.9 are the equivalents respectively of
sulphur, zinc, silver, and chlorine. These same figures represent the
ratios by weight in which these elements combine among themselves. Thus
215.86 g. of silver combine with 70.9 g. of chlorine and with 2 × 16.03
g. of sulphur. 65.4 g. of zinc combine with 70.9 g. of chlorine and 2 ×
16.03 g. of sulphur.
By taking the equivalent or some multiple of it a value can be obtained
for each element which will represent its combining value, and for this
reason is called its combining weight. It is important to notice that
the fact that a combining weight can be obtained for each element is not
a part of a theory, but is the direct result of experiment.
Elements with more than one equivalent. It will be remembered that
oxygen combines with hydrogen in two ratios. In one case 16 g. of oxygen
combine with 2.016 g. of hydrogen to form water; in the other 16 g. of
oxygen combine with 1.008 g. of hydrogen to form hydrogen dioxide. The
equivalents of hydrogen are therefore 2.016 and 1.008. Barium combines
with oxygen in two proportions: in barium oxide the proportion is 16 g.
of oxygen to 137.4 g. of barium; in barium dioxide the proportion is 16
g. of oxygen to 68.7 g. of barium.[Pg 226]
In each case one equivalent is a simple multiple of the other, so the
fact that there may be two equivalents does not add to the uncertainty.
All we knew before was that the true atomic weight is some multiple of
the equivalent.
2. The determination of molecular weights. To decide the question as to
which multiple of the equivalent correctly represents the atomic weight
of an element, it has been found necessary to devise a method of
determining the molecular weights of compounds containing the element in
question. Since the molecular weight of a compound is merely the sum of
the weights of all the atoms present in it, it would seem to be
impossible to determine the molecular weight of a compound without first
knowing the atomic weights of the constituent atoms, and how many atoms
of each element are present in the molecule. But certain facts have been
discovered which suggest a way in which this can be done.
Avogadro's hypothesis. We have seen that the laws of Boyle, Charles, and
Gay-Lussac apply to all gases irrespective of their chemical character.
This would lead to the inference that the structure of gases must be
quite simple, and that it is much the same in all gases.
In 1811 Avogadro, an Italian physicist, suggested that if we assume all
gases under the same conditions of temperature and pressure to have the
same number of molecules in a given volume, we shall have a probable
explanation of the simplicity of the gas laws. It is difficult to prove
the truth of this hypothesis by a simple experiment, but there are so
many facts known which are in complete harmony with this suggestion that
there is little doubt that it expresses the truth. Avogadro's hypothesis
may be stated thus: Equal volumes of all gases under the same
conditions of temperature and pressure contain the same number of
molecules.
[Pg 227]
Avogadro's hypothesis and molecular weights. Assuming that Avogadro's
hypothesis is correct, we have a very simple means for deciding upon the
relative weights of molecules; for if equal volumes of two gases contain
the same number of molecules, the weights of the two volumes must be in
the same ratio as the weights of the individual molecules which they
contain. If we adopt some one gas as a standard, we can express the
weights of all other gases as compared with this one, and the same
figures will express the relative weights of the molecules of which the
gases are composed.
Oxygen as the standard. It is important that the same standard should be
adopted for the determination of molecular weights as has been decided
upon for atomic weights and equivalents, so that the three values may be
in harmony with each other. Accordingly it is best to adopt oxygen as
the standard element with which to compare the molecular weights of
other gases, being careful to keep the oxygen atom equal to 16.
The oxygen molecule contains two atoms. One point must not be
overlooked, however. We desire to have our unit, the oxygen atom,
equal to 16. The method of comparing the weights of gases just suggested
compares the molecules of the gases with the molecule of oxygen. Is
the molecule and the atom of oxygen the same thing? This question is
answered by the following considerations.
We have seen that when steam is formed by the union of oxygen and
hydrogen, two volumes of hydrogen combine with one volume of oxygen to
form two volumes of steam. Let us suppose that the one volume of oxygen
contains 100[Pg 228] molecules; then the two volumes of steam must, according
to Avogadro's hypothesis, contain 200 molecules. But each of these 200
molecules must contain at least one atom of oxygen, or 200 in all, and
these 200 atoms came from 100 molecules of oxygen. It follows that each
molecule of oxygen must contain at least two atoms of oxygen.
Evidently this reasoning merely shows that there are at least two
atoms in the oxygen molecule. There may be more than that, but as there
is no evidence to this effect, we assume that the molecule contains two
atoms only.
It is evident that if we wish to retain the value 16 for the atom of
oxygen we must take twice this value, or 32, for the value of the oxygen
molecule, when using it as a standard for molecular weights.
Determination of the molecular weights of gases from their weights
compared with oxygen. Assuming the molecular weight of oxygen to be 32,
Avogadro's hypothesis gives us a ready means for determining the
molecular weight of any other gas, for all that is required is to know
its weight compared with that of an equal volume of oxygen. For example,
1 l. of chlorine is found by experiment to weigh 2.216 times as much as
1 l. of oxygen. The molecular weight of chlorine must therefore be 2.216
×32, or 70.91.
If, instead of comparing the relative weights of 1 l. of the two gases,
we select such a volume of oxygen as will weigh 32 g., or the weight in
grams corresponding to the molecular weight of the gas, the calculation
is much simplified. It has been found that 32 g. of oxygen, under
standard conditions, measure 22.4 l. This same volume of hydrogen weighs
2.019 g.; of chlorine 70.9 g.; of hydrochloric acid 36.458 g. The
weights of these equal volumes must be proportional to their molecular
weights, and since[Pg 229] the weight of the oxygen is the same as the value of
its molecular weight, so too will the weights of the 22.4 l. of the
other gases be equal to the value of their molecular weights.
As a summary we can then make the following statement: The molecular
weight of any gas may be determined by calculating the weight of 22.4 l.
of the gas, measured under standard conditions.
Determination of molecular weights from density of gases. In an actual
experiment it is easier to determine the density of a gas than the
weight of a definite volume of it. The density of a gas is usually
defined as its weight compared with that of an equal volume of air.
Having determined the density of a gas, its weight compared with oxygen
may be determined by multiplying its density by the ratio between the
weights of air and oxygen. This ratio is 0.9046. To compare it with our
standard for atomic weights we must further multiply it by 32, since the
standard is 1/32 the weight of oxygen molecules. The steps then are
these:
1. Determine the density of the gas (its weight compared with air).
2. Multiply by 0.9046 to make the comparison with oxygen molecules.
3. Multiply by 32 to make the comparison with the unit for atomic
weights.
We have, then, the formula:
molecular weight = density × 0.9046 × 32;
or, still more briefly,
The value found by this method for the determination of molecular
weights will of course agree with those found[Pg 230] by calculating the weight
of 22.4 l. of the gas, since both methods depend on the same principles.
 Fig. 69
Fig. 69
Determination of densities of gases. The relative weights of
equal volumes of two gases can be easily determined. The
following is one of the methods used. A small flask, such as is
shown in Fig. 69, is filled with one of the gases, and after
the temperature and pressure have been noted the flask is
sealed up and weighed. The tip of the sealed end is then broken
off, the flask filled with the second gas, and its weight
determined. If the weight of the empty flask is subtracted from
these two weighings, the relative weights of the gases is
readily found.
3. Deduction of atomic weights from molecular weights and equivalents.
We have now seen how the equivalent of an element and the molecular
weight of compounds containing the element can be obtained. Let us see
how it is possible to decide which multiple of the equivalent really is
the true atomic weight. As an example, let us suppose that the
equivalent of nitrogen has been found to be 7.02 and that it is desired
to obtain its atomic weight. The next step is to obtain the molecular
weights of a large number of compounds containing nitrogen. The
following will serve:
| DENSITY BY EXPERIMENT | APPROXIMATE MOLECULAR WEIGHT (D. × 28.9) | PERCENTAGE OF NITROGEN BY EXPERIMENT | PART OF MOLECULAR WEIGHT DUE TO NITROGEN |
| Nitrogen gas | 0.9671 | 27.95 | 100.00 | 27.95 |
| Nitrous oxide | 1.527 | 44.13 | 63.70 | 27.11 |
| Nitric oxide | 1.0384 | 30.00 | 46.74 | 14.02 |
| Nitrogen peroxide | 1.580 | 45.66 | 30.49 | 13.90 |
| Ammonia | 0.591 | 17.05 | 82.28 | 14.03 |
| Nitric acid | 2.180 | 63.06 | 22.27 | 14.03 |
| Hydrocyanic acid | 0.930 | 26.87 | 51.90 | 13.94 |
[Pg 231]
Method of calculation. The densities of the various gases in the first
column of this table are determined by experiment, and are fairly
accurate but not entirely so. By multiplying these densities by 28.9 the
molecular weights of the compounds as given in the second column are
obtained. By chemical analysis it is possible to determine the
percentage composition of these substances, and the percentages of
nitrogen in them as determined by analysis are given in the third
column. If each of these molecular weights is multiplied in turn by the
percentage of nitrogen in the compound, the product will be the weight
of the nitrogen in the molecular weight of the compound. This will be
the sum of the weights of the nitrogen atoms in the molecule. These
values are given in the fourth column in the table.
If a large number of compounds containing nitrogen are studied in this
way, it is probable that there will be included in the list at least one
substance whose molecule contains a single nitrogen atom. In this case
the number in the fourth column will be the approximate atomic weight of
nitrogen. On comparing the values for nitrogen in the table it will be
seen that a number which is approximately 14 is the smallest, and that
the others are multiples of this. These compounds of higher value,
therefore, contain more than one nitrogen atom in the molecule.
Accurate determination of atomic weights. Molecular weights cannot be
determined very accurately, and consequently the part in them due to
nitrogen is a little uncertain, as will be seen in the table. All we can
tell by this method is that the true weight is very near 14. The
equivalent can however be determined very accurately, and we have seen
that it is some multiple or submultiple[Pg 232] of the true atomic weight.
Since molecular-weight determinations have shown that in the case of
nitrogen the atomic weight is near 14, and we have found the equivalent
to be 7.02, it is evident that the true atomic weight is twice the
equivalent, or 7.02 × 2 = 14.04.
Summary. These, then, are the steps necessary to establish the atomic
weight of an element.
1. Determine the equivalent accurately by analysis.
2. Determine the molecular weight of a large number of compounds of the
element, and by analysis the part of the molecular weight due to the
element. The smallest number so obtained will be approximately the
atomic weight.
3. Multiply the equivalent by the small whole number (usually 1, 2, or
3), which will make a number very close to the approximate atomic
weight. The figure so obtained will be the true atomic weight.
Molecular weights of the elements. It will be noticed that the molecular
weight of nitrogen obtained by multiplying its density by 28.9 is 28.08.
Yet the atomic weight of nitrogen as deduced from a study of its gaseous
compounds is 14.04. The simplest explanation that can be given for this
is that the gaseous nitrogen is made up of molecules, each of which
contains two atoms. In this respect it resembles oxygen; for we have
seen that an entirely different line of reasoning leads us to believe
that the molecule of oxygen contains two atoms. When we wish to indicate
molecules of these gases the symbols N2 and O2 should be used.
When we desire to merely show the weights taking part in a reaction this
is not necessary.
The vapor densities of many of the elements show that, like oxygen and
nitrogen, their molecules consist of two atoms. In other cases,
particularly among the metals,[Pg 233] the molecule and the atom are identical.
Still other elements have four atoms in their molecules.
While oxygen contains two atoms in its molecules, a study of ozone has
led to the conclusion that it has three. The formation of ozone from
oxygen can therefore be represented by the equation
Other methods of determining molecular weights. It will be noticed that
Avogadro's law gives us a method by which we can determine the relative
weights of the molecules of two gases because it enables us to tell when
we are dealing with an equal number of the two kinds of molecules. If by
any other means we can get this information, we can make use of the
knowledge so gained to determine the molecular weights of the two
substances.
Raoult's laws. Two laws have been discovered which give us just such
information. They are known as Raoult's laws, and can be stated as
follows:
1. When weights of substances which are proportional to their molecular
weights are dissolved in the same weight of solvent, the rise of the
boiling point is the same in each case.
2. When weights of substances which are proportional to their molecular
weights are dissolved in the same weight of solvent, the lowering of the
freezing point is the same in each case.
By taking advantage of these laws it is possible to determine when two
solutions contain the same number of molecules of two dissolved
substances, and consequently the relative molecular weights of the two
substances.
Law of Dulong and Petit. In 1819 Dulong and Petit discovered a very
interesting relation between the atomic[Pg 234] weight of an element and its
specific heat, which holds true for elements in the solid state. If
equal weights of two solids, say, lead and silver, are heated through
the same range of temperature, as from 10° to 20°, it is found that very
different amounts of heat are required. The amount of heat required to
change the temperature of a solid or a liquid by a definite amount
compared with the amount required to change the temperature of an equal
weight of water by the same amount is called its specific heat. Dulong
and Petit discovered the following law: The specific heat of an element
in the solid form multiplied by its atomic weight is approximately equal
to the constant 6.25. That is,
at. wt. × sp. ht. = 6.25.
Consequently,
| | 6.25 |
| at. wt. | = | ———— |
| | sp. ht. |
This law is not very accurate, but it is often possible by means of it
to decide upon what multiple of the equivalent is the real atomic
weight. Thus the specific heat of iron is found by experiment to be
0.112, and its equivalent is 27.95. 6.25 ÷ 0.112 = 55.8. We see,
therefore, that the atomic weight is twice the equivalent, or 55.9.
How formulas are determined. It will be well in connection with
molecular weights to consider how the formula of a compound is decided
upon, for the two subjects are very closely associated. Some examples
will make clear the method followed.
The molecular weight of a substance containing hydrogen and chlorine was
36.4. By analysis 36.4 parts of the substance was found to contain 1
part of hydrogen and 35.4 parts of chlorine. As these are the simple
atomic[Pg 235] weights of the two elements, the formula of the compound must be
HCl.
A substance consisting of oxygen and hydrogen was found to have a
molecular weight of 34. Analysis showed that in 34 parts of the
substance there were 2 parts of hydrogen and 32 parts of oxygen.
Dividing these figures by the atomic weights of the two elements, we get
2 ÷ 1 = 2 for H; 32 ÷ 16 = 2 for O. The formula is therefore H2O2.
A substance containing 2.04% H, 32.6% S, and 65.3% O was found to have a
molecular weight of 98. In these 98 parts of the substance there are 98
× 2.04% = 2 parts of H, 98 × 32.6% = 32 parts of S, and 98 × 65.3% = 64
parts of O. If the molecule weighs 98, the hydrogen atoms present must
together weigh 2, the sulphur atoms 32, and the oxygen atoms 64.
Dividing these figures by the respective atomic weights of the three
elements, we have, for H, 2 ÷ 1 = 2 atoms; for S, 32 ÷ 32 = 1 atom; for
O, 64 ÷ 16 = 4 atoms. Hence the formula is H2SO4.
We have, then, this general procedure: Find the percentage composition
of the substance and also its molecular weight. Multiply the molecular
weight successively by the percentage of each element present, to find
the amount of the element in the molecular weight of the compound. The
figures so obtained will be the respective parts of the molecular weight
due to the several atoms. Divide by the atomic weights of the respective
elements, and the quotient will be the number of atoms present.
Avogadro's hypothesis and chemical calculations. This law simplifies
many chemical calculations.
1. Application to volume relations in gaseous reactions. Since equal
volumes of gases contain an equal number of[Pg 236] molecules, it follows that
when an equal number of gaseous molecules of two or more gases take part
in a reaction, the reaction will involve equal volumes of the gases. In
the equation
since 1 molecule of each of the gases CO2 and CO is set free from
each molecule of oxalic acid, the two substances must always be set free
in equal volumes.
Acetylene burns in accordance with the equation
2C2H2 + 5O2 = 4CO2 + 2H2O.
Hence 2 volumes of acetylene will react with 5 volumes of oxygen to form
4 volumes of carbon dioxide and 2 volumes of steam. That the volume
relations may be correct a gaseous element must be given its molecular
formula. Thus oxygen must be written O2 and not 2O.
2. Application to weights of gases. It will be recalled that the
molecular weight of a gas is determined by ascertaining the weight of
22.4 l. of the gas. This weight in grams is called the gram-molecular
weight of a gas. If the molecular weight of any gas is known, the
weight of a liter of the gas under standard conditions may be determined
by dividing its gram-molecular weight by 22.4. Thus the gram-molecular
weight of a hydrochloric acid gas is 36.458. A liter of the gas will
therefore weigh 36.458 ÷ 22.4 = 1.627 g.
EXERCISES
1. From the following data calculate the atomic weight of sulphur. The
equivalent, as obtained by an analysis of sulphur dioxide, is 16.03. The
densities and compositions of a number of compounds containing sulphur
are as follows:[Pg 237]
| NAME | DENSITY | COMPOSITION BY PERCENTAGE |
| Hydrosulphuric acid | 1.1791 | S = 94.11 | H = 5.89 |
| Sulphur dioxide | 2.222 | S = 50.05 | O = 49.95 |
| Sulphur trioxide | 2.74 | S = 40.05 | O = 59.95 |
| Sulphur chloride | 4.70 | S = 47.48 | Cl = 52.52 |
| Sulphuryl chloride | 4.64 | S = 23.75 | Cl = 52.53 | O = 23.70 |
| Carbon disulphide | 2.68 | S = 84.24 | C = 15.76 |
2. Calculate the formulas for compounds of the following compositions:
| | | MOLECULAR WEIGHT |
| (1) S = 39.07% | O = 58.49% | H = 2.44% | 81.0 |
| (2) Ca = 29.40 | S = 23.56 | O = 47.04 | 136.2 |
| (3) K = 38.67 | N = 13.88 | O = 47.45 | 101.2 |
3. The molecular weight of ammonia is 17.06; of sulphur dioxide is
64.06; of chlorine is 70.9. From the molecular weight calculate the
weight of 1 l. of each of these gases. Compare your results with the
table on the back cover of the book.
4. From the molecular weight of the same gases calculate the density of
each, referred to air as a standard.
5. A mixture of 50 cc. of carbon monoxide and 50 cc. of oxygen was
exploded in a eudiometer, (a) What gases remained in the tube after
the explosion? (b) What was the volume of each?
6. In what proportion must acetylene and oxygen be mixed to produce the
greatest explosion?
7. Solve Problem 18, Chapter XVII, without using molecular weights.
Compare your results.
8. Solve Problem 10, Chapter XVIII, without using molecular weights.
Compare your results.
9. The specific heat of aluminium is 0.214; of lead is 0.031. From these
specific heats calculate the atomic weights of each of the elements.
[Pg 238]
CHAPTER XX
THE PHOSPHORUS FAMILY
| SYMBOL | ATOMIC WEIGHT | DENSITY | MELTING POINT |
| Phosphorus | P | 31.0 | 1.8 | 43.3° |
| Arsenic | As | 75.0 | 5.73 | — |
| Antimony | Sb | 120.2 | 6.7 | 432° |
| Bismuth | Bi | 208.5 | 9.8 | 270° |
The family. The elements constituting this family belong in the same
group with nitrogen and therefore resemble it in a general way. They
exhibit a regular gradation of physical properties, as is shown in the
above table. The same general gradation is also found in their chemical
properties, phosphorus being an acid-forming element, while bismuth is
essentially a metal. The other two elements are intermediate in
properties.
Compounds. In general the elements of the family form compounds having
similar composition, as is shown in the following table:
| PH3 | PCl3 | PCl5 | P2O3 | P2O5 |
| AsH3 | AsCl3 | AsCl5 | As2O3 | As2O5 |
| SbH3 | SbCl3 | SbCl5 | Sb2O3 | Sb2O5 |
| BiCl3 | BiCl5 | Bi2O3 | Bi2O5 |
In the case of phosphorus, arsenic, and antimony the oxides are acid
anhydrides. Salts of at least four acids of each of these three elements
are known, the free acid in[Pg 239] some instances being unstable. The relation
of these acids to the corresponding anhydrides may be illustrated as
follows, phosphorus being taken as an example:
P2O3 + 3H2O = 2H3PO3 (phosphorous acid).
P2O5 + 3H2O = 2H3PO4 (phosphoric acid).
P2O5 + 2H2O = H4P2O7 (pyrophosphoric acid).
P2O5 + H2O = 2HPO3 (metaphosphoric acid).
PHOSPHORUS
History. The element phosphorus was discovered by the alchemist Brand,
of Hamburg, in 1669, while searching for the philosopher's stone. Owing
to its peculiar properties and the secrecy which was maintained about
its preparation, it remained a very rare and costly substance until the
demand for it in the manufacture of matches brought about its production
on a large scale.
Occurrence. Owing to its great chemical activity phosphorus never occurs
free in nature. In the form of phosphates it is very abundant and widely
distributed. Phosphorite and sombrerite are mineral forms of calcium
phosphate, while apatite consists of calcium phosphate together with
calcium fluoride or chloride. These minerals form very large deposits
and are extensively mined for use as fertilizers. Calcium phosphate is a
constituent of all fertile soil, having been supplied to the soil by the
disintegration of rocks containing it. It is the chief mineral
constituent of bones of animals, and bone ash is therefore nearly pure
calcium phosphate.
Preparation. Phosphorus is now manufactured from bone ash or a pure
mineral phosphate by heating the phosphate with sand and carbon in an
electric furnace. The materials[Pg 240] are fed in at M (Fig. 70) by the feed
screw F. The phosphorus vapor escapes at P and is condensed under
water, while the calcium silicate is tapped off as a liquid at S. The
phosphorus obtained in this way is quite impure, and is purified by
distillation.
 Fig. 70
Fig. 70
Explanation of the reaction. To understand the reaction which
occurs, it must be remembered that a volatile acid anhydride is
expelled from its salts when heated with an anhydride which is
not volatile. Thus, when sodium carbonate and silicon dioxide
are heated together the following reaction takes place:
Na2CO3 + SiO2 = Na2SiO3 + CO2.
Silicon dioxide is a less volatile anhydride than phosphoric
anhydride (P2O5), and when strongly heated with a
phosphate the phosphoric anhydride is driven out, thus:
Ca3(PO4)2 + 3SiO2 = 3CaSiO3 + P2O5.
If carbon is added before the heat is applied, the P2O5
is reduced to phosphorus at the same time, according to the
equation
P2O5 + 5C = 2P + 5CO.
Physical properties. The purified phosphorus is a pale yellowish,
translucent, waxy solid which melts at 43.3° and boils at 269°. It can
therefore be cast into any convenient form under warm water, and is
usually sold in the market in the form of sticks. It is quite soft and
can be easily cut with a knife, but this must always be done while the
element is covered with water, since it is extremely inflammable, and
the friction of the knife blade is almost[Pg 241] sure to set it on fire if cut
in the air. It is not soluble in water, but is freely soluble in some
other liquids, notably in carbon disulphide. Its density is 1.8.
Chemical properties. Exposed to the air phosphorus slowly combines with
oxygen, and in so doing emits a pale light, or phosphorescence, which
can be seen only in a dark place. The heat of the room may easily raise
the temperature to the kindling point of phosphorus, when it burns with
a sputtering flame, giving off dense fumes of oxide of phosphorus. It
burns with dazzling brilliancy in oxygen, and combines directly with
many other elements, especially with sulphur and the halogens. On
account of its great affinity for oxygen it is always preserved under
water.
Phosphorus is very poisonous, from 0.2 to 0.3 gram being a fatal dose.
Ground up with flour and water or similar substances, it is often used
as a poison for rats and other vermin.
Precaution. The heat of the body is sufficient to raise
phosphorus above its kindling temperature, and for this reason
it should always be handled with forceps and never with the
bare fingers. Burns occasioned by it are very painful and slow
in healing.
Red phosphorus. On standing, yellow phosphorus gradually undergoes a
remarkable change, being converted into a dark red powder which has a
density of 2.1. It no longer takes fire easily, neither does it dissolve
in carbon disulphide. It is not poisonous and, in fact, seems to be an
entirely different substance. The velocity of this change increases with
rise in temperature, and the red phosphorus is therefore prepared by
heating the yellow just below the boiling point (250°-300°). When
distilled and quickly condensed the red form changes back to the yellow.
This is in accordance with the general rule that when a substance
capable[Pg 242] of existing in several allotropic forms is condensed from a gas
or crystallized from the liquid state, the more unstable variety forms
first, and this then passes into the more stable forms.
Matches. The chief use of phosphorus is in the manufacture of
matches. Common matches are made by first dipping the match
sticks into some inflammable substance, such as melted
paraffin, and afterward into a paste consisting of (1)
phosphorus, (2) some oxidizing substance, such as manganese
dioxide or potassium chlorate, and (3) a binding material,
usually some kind of glue. On friction the phosphorus is
ignited, the combustion being sustained by the oxidizing agent
and communicated to the wood by the burning paraffin. In
sulphur matches the paraffin is replaced by sulphur.
In safety matches red phosphorus, an oxidizing agent, and
some gritty material such as emery is placed on the side of the
box, while the match tip is provided as before with an
oxidizing agent and an easily oxidized substance, usually
antimony sulphide. The match cannot be ignited easily by
friction, save on the prepared surface.
Compounds of phosphorus with hydrogen. Phosphorus forms several
compounds with hydrogen, the best known of which is phosphine (PH3)
analogous to ammonia (NH3).
Preparation of phosphine. Phosphine is usually made by heating
phosphorus with a strong solution of potassium hydroxide, the reaction
being a complicated one.
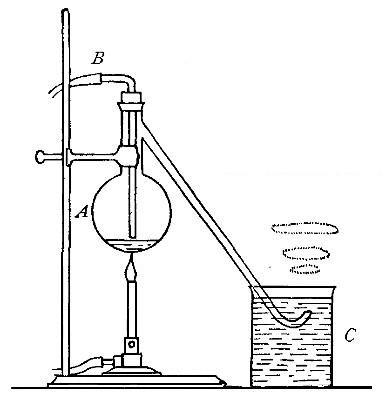 Fig. 71
Fig. 71
The experiment can be conveniently made in the apparatus shown
in Fig. 71. A strong solution of potassium hydroxide together
with several small bits of phosphorus are placed in the flask
A, and a current of coal gas is passed into the flask through
the tube B until[Pg 243] all the air has been displaced. The gas is
then turned off and the flask is heated. Phosphine is formed in
small quantities and escapes through the delivery tube, the
exit of which is just covered by the water in the vessel C.
Each bubble of the gas as it escapes into the air takes fire,
and the product of combustion (P2O5) forms beautiful
small rings, which float unbroken for a considerable time in
quiet air. The pure phosphine does not take fire spontaneously.
When prepared as directed above, impurities are present which
impart this property.
Properties. Phosphine is a gas of unpleasant odor and is exceedingly
poisonous. Like ammonia it forms salts with the halogen acids. Thus we
have phosphonium chloride (PH4Cl) analogous to ammonium chloride
(NH4Cl). The phosphonium salts are of but little importance.
Oxides of phosphorus. Phosphorus forms two well-known oxides,—the
trioxide (P2O3) and the pentoxide (P2O5), sometimes called
phosphoric anhydride. When phosphorus burns in an insufficient supply of
air the product is partially the trioxide; in oxygen or an excess of air
the pentoxide is formed. The pentoxide is much the better known of the
two. It is a snow-white, voluminous powder whose most marked property is
its great attraction for water. It has no chemical action upon most
gases, so that they can be very thoroughly dried by allowing them to
pass through properly arranged vessels containing phosphorus pentoxide.
Acids of phosphorus. The important acids of phosphorus are the
following:
| H3PO3 | phosphorous acid. |
| H3PO4 | phosphoric acid. |
| H4P2O7 | pyrophosphoric acid. |
| HPO3 | metaphosphoric acid. |
These may be regarded as combinations of the oxides of phosphorus with
water according to the equations given in the discussion of the
characteristics of the family.[Pg 244]
1. Phosphorous acid (H3PO3). Neither the acid nor its salts are
at all frequently met with in chemical operations. It can be easily
obtained, however, in the form of transparent crystals when phosphorus
trichloride is treated with water and the resulting solution is
evaporated:
PCl3 + 3H2O = H3PO3 + 3HCl.
Its most interesting property is its tendency to take up oxygen and pass
over into phosphoric acid.
2. Orthophosphoric acid (phosphoric acid) (H3PO4). This acid can
be obtained by dissolving phosphorus pentoxide in boiling water, as
represented in the equation
It is usually made by treating calcium phosphate with concentrated
sulphuric acid. The calcium sulphate produced in the reaction is nearly
insoluble, and can be filtered off, leaving the phosphoric acid in
solution. Very pure acid is made by oxidizing phosphorus with nitric
acid. It forms large colorless crystals which are exceedingly soluble in
water. Being a tribasic acid, it forms acid as well as normal salts.
Thus the following compounds of sodium are known:
| NaH2PO4 | monosodium hydrogen phosphate. |
| Na2HPO4 | disodium hydrogen phosphate. |
| Na3PO4 | normal sodium phosphate. |
These salts are sometimes called respectively primary, secondary, and
tertiary phosphates. They may be prepared by bringing together
phosphoric acid and appropriate quantities of sodium hydroxide.
Phosphoric acid also forms mixed salts, that is, salts containing two
different metals. The most familiar compound of this kind is microcosmic
[Pg 245]salt, which has the formula Na(NH4)HPO4.
Orthophosphates. The orthophosphates form an important class of salts.
The normal salts are nearly all insoluble and many of them occur in
nature. The secondary phosphates are as a rule insoluble, while most of
the primary salts are soluble.
3. Pyrophosphoric acid (H4P2O7). On heating orthophosphoric
acid to about 225° pyrophosphoric acid is formed in accordance with the
following equation:
It is a white crystalline solid. Its salts can be prepared by heating a
secondary phosphate:
2Na2HPO4 = Na4P2O7 + H2O.
4. Metaphosphoric acid (glacial phosphoric acid) (HPO3). This acid
is formed when orthophosphoric acid is heated above 400°:
It is also formed when phosphorus pentoxide is treated with cold water:
It is a white crystalline solid, and is so stable towards heat that it
can be fused and even volatilized without decomposition. On cooling from
the fused state it forms a glassy solid, and on this account is often
called glacial phosphoric acid. It possesses the property of dissolving
small quantities of metallic oxides, with the formation of compounds
which, in the case of certain metals, have characteristic colors. It is
therefore used in the detection of these metals.
While the secondary phosphates, on heating, give salts of pyrophosphoric
acid, the primary phosphates yield salts of metaphosphoric acid. The
equations representing these reactions are as follows:
2Na2HPO4 = Na4P3O7 + H2O,
NaH2PO4 = NaPO3 + H2O.
Fertilizers. When crops are produced year after year on the same field
certain constituents of the soil essential to plant growth are removed,
and the soil becomes impoverished and unproductive. To make the land
once more[Pg 246] fertile these constituents must be replaced. The calcium
phosphate of the mineral deposits or of bone ash serves well as a
material for restoring phosphorus to soils exhausted of that essential
element; but a more soluble substance, which the plants can more readily
assimilate, is desirable. It is better, therefore, to convert the
insoluble calcium phosphate into the soluble primary phosphate before it
is applied as fertilizer. It will be seen by reference to the formulas
for the orthophosphates (see page 244) that in a primary phosphate only
one hydrogen atom of phosphoric acid is replaced by a metal. Since the
calcium atom always replaces two hydrogen atoms, it might be thought
that there could be no primary calcium phosphate; but if the calcium
atom replaces one hydrogen atom from each of two molecules of phosphoric
acid, the salt Ca(H2PO4)2 will result, and this is a primary
phosphate. It can be made by treatment of the normal phosphate with the
necessary amount of sulphuric acid, calcium sulphate being formed at the
same time, thus:
Ca3(PO4)2 + 2H2SO4 = Ca(H2PO4)2 + 2CaSO4.
The resulting mixture is a powder, which is sold as a fertilizer under
the name of "superphosphate of lime."
ARSENIC
Occurrence. Arsenic occurs in considerable quantities in nature as the
native element, as the sulphides realgar (As2S2) and orpiment
(As2S3), as oxide (As2O3), and as a constituent of many
metallic sulphides, such as arsenopyrite (FeAsS).
Preparation. The element is prepared by purifying the native arsenic, or
by heating the arsenopyrite in iron tubes,[Pg 247] out of contact with air,
when the reaction expressed by the following equation occurs:
The arsenic, being volatile, condenses in chambers connected with the
heated tubes. It is also made from the oxide by reduction with carbon:
2As2O3 + 3C = 4As + 3CO2.
Properties. Arsenic is a steel-gray, metallic-looking substance of
density 5.73. Though resembling metals in appearance, it is quite
brittle, being easily powdered in a mortar. When strongly heated it
sublimes, that is, it passes into a vapor without melting, and condenses
again to a crystalline solid when the vapor is cooled. Like phosphorus
it can be obtained in several allotropic forms. It alloys readily with
some of the metals, and finds its chief use as an alloy with lead, which
is used for making shot, the alloy being harder than pure lead. When
heated on charcoal with the blowpipe it is converted into an oxide which
volatilizes, leaving the charcoal unstained by any oxide coating. It
burns readily in chlorine gas, forming arsenic trichloride,—
Unlike most of its compounds, the element itself is not poisonous.
Arsine (AsH3). When any compound containing arsenic is brought into
the presence of nascent hydrogen, arsine (AsH3), corresponding to
phosphine and ammonia, is formed. The reaction when oxide of arsenic is
so treated is
As2O3 + 12H = 2AsH3 + 3H2O.
[Pg 248]
Arsine is a gas with a peculiar garlic-like odor, and is intensely
poisonous. A single bubble of pure gas has been known to prove fatal. It
is an unstable compound, decomposing into its elements when heated to a
moderate temperature. It is combustible, burning with a pale
bluish-white flame to form arsenic trioxide and water when air is in
excess:
2AsH3 + 6O = As2O3 + 3H2O.
When the supply of air is deficient water and metallic arsenic are
formed:
These reactions make the detection of even minute quantities of arsenic
a very easy problem.
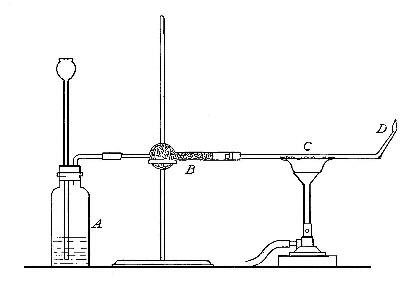 Fig. 72
Fig. 72
Marsh's test for arsenic. The method devised by Marsh for
detecting arsenic is most frequently used, the apparatus being
shown in Fig. 72. Hydrogen is generated in the flask A by the
action of dilute sulphuric acid on zinc, is dried by passing
over calcium chloride in the tube B, and after passing
through the hard-glass tube C is ignited at the jet D. If a
substance containing arsenic is now introduced into the
generator A, the arsenic is converted into arsine by the
action of the nascent hydrogen, and[Pg 249] passes to the jet along
with the hydrogen. If the tube C is strongly heated at some
point near the middle, the arsine is decomposed while passing
this point and the arsenic is deposited just beyond the heated
point in the form of a shining, brownish-black mirror. If the
tube is not heated, the arsine burns along with the hydrogen at
the jet. Under these conditions a small porcelain dish crowded
down into the flame is blackened by a spot of metallic arsenic,
for the arsine is decomposed by the heat of the flame, and the
arsenic, cooled below its kindling temperature by the cold
porcelain, deposits upon it as a black spot. Antimony conducts
itself in the same way as arsenic, but the antimony deposit is
more sooty in appearance. The two can also be distinguished by
the fact that sodium hypochlorite (NaClO) dissolves the arsenic
deposit, but not that formed by antimony.
Oxides of arsenic. Arsenic forms two oxides, As2O3 and
As2O5, corresponding to those of phosphorus. Of these arsenious
oxide, or arsenic trioxide (As2O3), is much better known, and is
the substance usually called white arsenic, or merely arsenic. It is
found as a mineral, but is usually obtained as a by-product in burning
pyrite in the sulphuric-acid industry. The pyrite has a small amount of
arsenopyrite in it, and when this is burned arsenious oxide is formed as
a vapor together with sulphur dioxide:
2FeAsS + 10O = Fe2O3 + As2O3 + 2SO2.
The arsenious oxide is condensed in appropriate chambers. It is a rather
heavy substance, obtained either as a crystalline powder or as large,
vitreous lumps, resembling lumps of porcelain in appearance. It is very
poisonous, from 0.2 to 0.3 g. being a fatal dose. It is frequently given
as a poison, since it is nearly tasteless and does not act very rapidly.
This slow action is due to the fact that it is not very soluble, and
hence is absorbed slowly by the system. Arsenious oxide is also used as
a chemical reagent in glass making and in the dye industry.[Pg 250]
Acids of arsenic. Like the corresponding oxides of phosphorus, the
oxides of arsenic are acid anhydrides. In solution they combine with
bases to form salts, corresponding to the salts of the acids of
phosphorus. Thus we have salts of the following acids:
| H3AsO3 | arsenious acid. |
| H3AsO4 | orthoarsenic acid. |
| H4As2O3 | pyroarsenic acid. |
| HAsO3 | metarsenic acid. |
Several other acids of arsenic are also known. Not all of these can be
obtained as free acids, since they tend to lose water and form the
oxides. Thus, instead of obtaining arsenious acid (H3AsO3), the
oxide As2O3 is obtained:
Salts of all the acids are known, however, and some of them have
commercial value. Most of them are insoluble, and some of the copper
salts, which are green, are used as pigments. Paris green, which has a
complicated formula, is a well-known insecticide.
Antidote for arsenical poisoning. The most efficient antidote for
arsenic poisoning is ferric hydroxide. It is prepared as needed,
according to the equation
Fe2(SO4)3 + 3Mg(OH)2 = 2Fe(OH)3 + 3MgSO4.
Sulphides of arsenic. When hydrogen sulphide is passed into an acidified
solution containing an arsenic compound the arsenic is precipitated as a
bright yellow sulphide, thus:
2H3AsO3 + 3H2S = As2S3 + 6H2O,
2H3AsO4 + 5H2S = As2S5 + 8H2O.
In this respect arsenic resembles the metallic elements, many of which
produce sulphides under similar conditions. The sulphides of arsenic,
both those produced artificially and those found in nature, are used as
yellow pigments.
ANTIMONY
Occurrence. Antimony occurs in nature chiefly as the sulphide
(Sb2S3), called stibnite, though it is also found as oxide and as
a constituent of many complex minerals.[Pg 251]
Preparation. Antimony is prepared from the sulphide in a very simple
manner. The sulphide is melted with scrap iron in a furnace, when the
iron combines with the sulphur to form a slag, or liquid layer of melted
iron sulphide, while the heavier liquid, antimony, settles to the bottom
and is drawn off from time to time. The reaction involved is represented
by the equation
Sb2S3 + 3Fe = 2Sb + 3FeS.
Physical properties. Antimony is a bluish-white, metallic-looking
substance whose density is 6.7. It is highly crystalline, hard, and very
brittle. It has a rather low melting point (432°) and expands very
noticeably on solidifying.
Chemical properties. In chemical properties antimony resembles arsenic
in many particulars. It forms the oxides Sb2O3 and Sb2O5,
and in addition Sb2O4. It combines with the halogen elements with
great energy, burning brilliantly in chlorine to form antimony
trichloride (SbCl3). When heated on charcoal with the blowpipe it is
oxidized and forms a coating of antimony oxide on the charcoal which has
a characteristic bluish-white color.
Stibine (SbH3). The gas stibine (SbH3) is formed under conditions
which are very similar to those which produce arsine, and it closely
resembles the latter compound, though it is still less stable. It is
very poisonous.
Acids of antimony. The oxides Sb_{2}O_{3} and Sb_{2}O_{5} are
weak acid anhydrides and are capable of forming two series of
acids corresponding in formulas to the acids of phosphorus and
arsenic. They are much weaker, however, and are of little
practical importance.
Sulphides of antimony. Antimony resembles arsenic in that
hydrogen sulphide precipitates it as a sulphide when conducted
into an acidified solution containing an antimony compound:
2SbCl3 + 3H2S = Sb2S3 + 6HCl,
The two sulphides of antimony are called the trisulphide and
the pentasulphide respectively. When prepared in this way they
are orange-colored substances, though the mineral stibnite is
black.
Metallic properties of antimony. The physical properties of the element
are those of a metal, and the fact that its sulphide is precipitated by
hydrogen sulphide shows that it acts like a metal in a chemical way.
Many other reactions show that antimony has more of the properties of a
metal than of a non-metal. The compound Sb(OH)3, corresponding to
arsenious acid, while able to act as a weak acid is also able to act as
a weak base with strong acids. For example, when treated with
concentrated hydrochloric acid antimony chloride is formed:
Sb(OH)3 + 3HCl = SbCl3 + 3H2O.
A number of elements act in this same way, their hydroxides under some
conditions being weak acids and under others weak bases.
ALLOYS
Some metals when melted together thoroughly intermix, and on cooling
form a homogeneous, metallic-appearing substance called an alloy. Not
all metals will mix in this way, and in some cases definite chemical
compounds are formed and separate out as the mixture solidifies, thus
destroying the uniform quality of the alloy. In general the melting
point of the alloy is below the average of the melting points of its
constituents, and it is often lower than any one of them.
Antimony forms alloys with many of the metals, and its chief commercial
use is for such purposes. It imparts to its alloys high density, rather
low melting point, and the[Pg 253] property of expanding on solidification.
Such an alloy is especially useful in type founding, where fine lines
are to be reproduced on a cast. Type metal consists of antimony, lead,
and tin. Babbitt metal, used for journal bearings in machinery, contains
the same metals in a different proportion together with a small
percentage of copper.
BISMUTH
Occurrence. Bismuth is usually found in the uncombined form in nature.
It also occurs as oxide and sulphide. Most of the bismuth of commerce
comes from Saxony, and from Mexico and Colorado, but it is not an
abundant element.
Preparation. It is prepared by merely heating the ore containing the
native bismuth and allowing the melted metal to run out into suitable
vessels. Other ores are converted into oxides and reduced by heating
with carbon.
Physical properties. Bismuth is a heavy, crystalline, brittle metal
nearly the color of silver, but with a slightly rosy tint which
distinguishes it from other metals. It melts at a low temperature (270°)
and has a density of 9.8. It is not acted upon by the air at ordinary
temperatures.
Chemical properties. When heated with the blowpipe on charcoal, bismuth
gives a coating of the oxide Bi2O3. This has a yellowish-brown
color which easily distinguishes it from the oxides formed by other
metals. It combines very readily with the halogen elements, powdered
bismuth burning readily in chlorine. It is not very easily acted upon by
hydrochloric acid, but nitric and sulphuric acids act upon it in the
same way that they do upon copper.
Uses. Bismuth finds its chief use as a constituent of alloys,
particularly in those of low melting point. Some[Pg 254] of these melt in hot
water. For example, Wood's metal, consisting of bismuth, lead, tin, and
cadmium, melts at 60.5°.
Compounds of bismuth. Unlike the other elements of this group, bismuth
has almost no acid properties. Its chief oxide, Bi2O3, is basic in
its properties. It dissolves in strong acids and forms salts of bismuth:
Bi2O3 + 6HCl = 2BiCl3 + 3H2O,
Bi2O3 + 6HNO3 = 2Bi(NO3)3 + 3H2O.
The nitrate and chloride of bismuth can be obtained as well-formed
colorless crystals. When treated with water the salts are decomposed in
the manner explained in the following paragraph.
HYDROLYSIS
Many salts such as those of antimony and bismuth form solutions which
are somewhat acid in reaction, and must therefore contain hydrogen ions.
This is accounted for by the same principle suggested to explain the
fact that solutions of potassium cyanide are alkaline in reaction (p.
210). Water forms an appreciable number of hydrogen and hydroxyl ions,
and very weak bases such as bismuth hydroxide are dissociated to but a
very slight extent. When Bi+++ ions from bismuth chloride, which
dissociates very readily, are brought in contact with the OH- ions
from water, the two come to the equilibrium expressed in the equation
Bi+++ + 3OH- <--> Bi(OH)3.
For every hydroxyl ion removed from the solution in this way a hydrogen
ion is left free, and the solution becomes acid in reaction.[Pg 255]
Reactions of this kind and that described under potassium cyanide are
called hydrolysis.
DEFINITION: Hydrolysis is the action of water upon a salt to form an
acid and a base, one of which is very slightly dissociated.
Conditions favoring hydrolysis. While hydrolysis is primarily due to the
slight extent to which either the acid or the base formed is
dissociated, several other factors have an influence upon the extent to
which it will take place.
1. Influence of mass. Since hydrolysis is a reversible reaction, the
relative masses of the reacting substances influence the point at which
equilibrium will be reached. In the equilibrium
BiCl3 + 3H2O <--> Bi(OH)3 + 3HCl
the addition of more water will result in the formation of more bismuth
hydroxide and hydrochloric acid. The addition of more hydrochloric acid
will convert some of the bismuth hydroxide into bismuth chloride.
2. Formation of insoluble substances. When one of the products of
hydrolysis is nearly insoluble in water the solution will become
saturated with it as soon as a very little has been formed. All in
excess of this will precipitate, and the reaction will go on until the
acid set free increases sufficiently to bring about an equilibrium. Thus
a considerable amount of bismuth and antimony hydroxides are
precipitated when water is added to the chlorides of these elements. The
greater the dilution the more hydroxide precipitates. The addition of
hydrochloric acid in considerable quantity will, however, redissolve the
precipitate.
Partial hydrolysis. In many cases the hydrolysis of a salt is only
partial, resulting in the formation of basic salts instead of the free
base. Most of these basic salts are insoluble in water, which accounts
for their ready formation. Thus bismuth chloride may hydrolyze by
successive steps, as shown in the equations
BiCl3 + H2O = Bi(OH)Cl2 + HCl,
BiCl3 + 2H2O = Bi(OH)2Cl + 2HCl,
BiCl3 + 3H2O = Bi(OH)3 + 3HCl.
The basic salt so formed may also lose water, as shown in the equation
[Pg 256]
The salt represented in the last equation is sometimes called bismuth
oxychloride, or bismuthyl chloride. The corresponding nitrate,
BiONO3, is largely used in medicine under the name of subnitrate of
bismuth. In these two compounds the group of atoms, BiO, acts as a
univalent metallic radical and is called bismuthyl. Similar basic
salts are formed by the hydrolysis of antimony salts.
EXERCISES
1. Name all the elements so far studied which possess allotropic forms.
2. What compounds would you expect phosphorus to form with bromine and
iodine? Write the equations showing the action of water on these
compounds.
3. In the preparation of phosphine, why is coal gas passed into the
flask? What other gases would serve the same purpose?
4. Give the formula for the salt which phosphine forms with hydriodic
acid. Give the name of the compound.
5. Could phosphoric acid be substituted for sulphuric acid in the
preparation of the common acids?
6. Write the equations for the preparation of the three sodium salts of
orthophosphoric acid.
7. Why does a solution of disodium hydrogen phosphate react alkaline?
8. On the supposition that bone ash is pure calcium phosphate, what
weight of it would be required in the preparation of 1 kg. of
phosphorus?
9. If arsenopyrite is heated in a current of air, what products are
formed?
10. (a) Write equations for the complete combustion of hydrosulphuric
acid, methane, and arsine. (b) In what respects are the reactions
similar?
11. Write the equations for all the reactions involved in Marsh's test
for arsenic.
12. Write the names and formulas for the acids of antimony.
13. Write the equations showing the hydrolysis of antimony trichloride;
of bismuth nitrate.
14. In what respects does nitrogen resemble the members of the
phosphorus family?
[Pg 257]
CHAPTER XXI
SILICON, TITANIUM, BORON
| SYMBOL | ATOMIC WEIGHT | DENSITY | CHLORIDES | OXIDES |
| Silicon | Si | 28.4 | 2.35 | SiCl4 | SiO |
| Titanium | Ti | 48.1 | 3.5 | TiCl4 | TiO |
| Boron | B | 11.0 | 2.45 | BCl3 | B2O3 |
General. Each of the three elements, silicon, titanium, and boron,
belongs to a separate periodic family, but they occur near together in
the periodic grouping and are very similar in both physical and chemical
properties. Since the other elements in their families are either so
rare that they cannot be studied in detail, or are best understood in
connection with other elements, it is convenient to consider these three
together at this point.
The three elements are very difficult to obtain in the free state, owing
to their strong attraction for other elements. They can be prepared by
the action of aluminium or magnesium on their oxides and in impure state
by reduction with carbon in an electric furnace. They are very hard and
melt only at the highest temperatures. At ordinary temperatures they are
not attacked by oxygen, but when strongly heated they burn with great
brilliancy. Silicon and boron are not attacked by acids under ordinary
conditions; titanium is easily dissolved by them.[Pg 258]
SILICON
Occurrence. Next to oxygen silicon is the most abundant element. It does
not occur free in nature, but its compounds are very abundant and of the
greatest importance. It occurs almost entirely in combination with
oxygen as silicon dioxide (SiO2), often called silica, or with oxygen
and various metals in the form of salts of silicic acids, or silicates.
These compounds form a large fraction of the earth's crust. Most plants
absorb small amounts of silica from the soil, and it is also found in
minute quantities in animal organisms.
Preparation. The element is most easily prepared by reducing pure
powdered quartz with magnesium powder:
Properties. As would be expected from its place in the periodic table,
silicon resembles carbon in many respects. It can be obtained in several
allotropic forms, corresponding to those of carbon. The crystallized
form is very hard, and is inactive toward reagents. The amorphous
variety has, in general, properties more similar to charcoal.
Compounds of silicon with hydrogen and the halogens. Silicon hydride
(SiH4) corresponds in formula to methane (CH4), but its properties
are more like those of phosphine (PH3). It is a very inflammable gas
of disagreeable odor, and, as ordinarily prepared, takes fire
spontaneously on account of the presence of impurities.
Silicon combines with the elements of the chlorine family to form such
compounds as SiCl4 and SiF4. Of these silicon fluoride is the most
familiar and interesting. As stated in the discussion of fluorine, it is
formed when[Pg 259] hydrofluoric acid acts upon silicon dioxide or a silicate.
With silica the reaction is thus expressed:
SiO2 + 4HF = SiF4 + 2H2O.
It is a very volatile, invisible, poisonous gas. In contact with water
it is partially decomposed, as shown in the equation
SiF4 + 4H2O = 4HF + Si(OH)4.
The hydrofluoric acid so formed combines with an additional amount of
silicon fluoride, forming the complex fluosilicic acid (H2SiF6),
thus:
Silicides. As the name indicates, silicides are binary compounds
consisting of silicon and some other element. They are very stable at
high temperatures, and are usually made by heating the appropriate
substances in an electric furnace. The most important one is
carborundum, which is a silicide of carbon of the formula CSi. It is
made by heating coke and sand, which is a form of silicon dioxide, in an
electric furnace, the process being extensively carried on at Niagara
Falls. The following equation represents the reaction
The substance so prepared consists of beautiful purplish-black crystals,
which are very hard. Carborundum is used as an abrasive, that is, as a
material for grinding and polishing very hard substances. Ferrosilicon
is a silicide of iron alloyed with an excess of iron, which finds
extensive use in the manufacture of certain kinds of steel.[Pg 260]
Manufacture of carborundum. The mixture of materials is heated in a
large resistance furnace for about thirty-six hours. After the reaction
is completed there is left a core of graphite G. Surrounding this core
is a layer of crystallized carborundum C, about 16 in. thick. Outside
this is a shell of amorphous carborundum A. The remaining materials
M are unchanged and are used for a new charge.
 Fig. 73
Fig. 73
Silicon dioxide (silica) (SiO2). This substance is found in a great
variety of forms in nature, both in the amorphous and in the crystalline
condition. In the form of quartz it is found in beautifully formed
six-sided prisms, sometimes of great size. When pure it is perfectly
transparent and colorless. Some colored varieties are given special
names, as amethyst (violet), rose quartz (pale pink), smoky or milky
quartz (colored and opaque). Other varieties of silicon dioxide, some of
which also contain water, are chalcedony, onyx, jasper, opal, agate, and
flint. Sand and sandstone are largely silicon dioxide.
Properties. As obtained by chemical processes silicon dioxide is an
amorphous white powder. In the crystallized state it is very hard and
has a density of 2.6. It is insoluble in water and in most chemical
reagents, and requires the hottest oxyhydrogen flame for fusion. Acids,
excepting hydrofluoric acid, have little action on it, and it requires
the most energetic reducing agents to deprive it of oxygen. It is the
anhydride of an acid, and consequently it dissolves in fused alkalis to
form silicates. Being nonvolatile, it will drive out most other
anhydrides when heated[Pg 261] to a high temperature with their salts,
especially when the silicates so formed are fusible. The following
equations illustrate this property:
Na2CO3 + SiO2 = Na2SiO3 + CO2,
Na2SO4 + SiO2 = Na2SiO3 + SO3.
Silicic acids. Silicon forms two simple acids, orthosilicic acid
(H4SiO4) and metasilicic acid (H2SiO3). Orthosilicic acid is
formed as a jelly-like mass when orthosilicates are treated with strong
acids such as hydrochloric. On attempting to dry this acid it loses
water, passing into metasilicic or common silicic acid:
Metasilicic acid when heated breaks up into silica and water, thus:
Salts of silicic acids,—silicates. A number of salts of the
orthosilicic and metasilicic acids occur in nature. Thus mica
(KAlSiO4) is a salt of orthosilicic acid.
Polysilicic acids. Silicon has the power to form a great many complex
acids which may be regarded as derived from the union of several
molecules of the orthosilicic acid, with the loss of water. Thus we have
3H4SiO4 = H4Si3O8 + 4H2O.
These acids cannot be prepared in the pure state, but their salts form
many of the crystalline rocks in nature. Feldspar, for example, has the
formula KAlSi3O8, and is a mixed salt of the acid
H4Si3O8, whose formation is represented in the equation above.
Kaolin has the formula Al2Si2O7·2H2O. Many other examples
will be met in the study of the metals.[Pg 262]
Glass. When sodium and calcium silicates, together with silicon dioxide,
are heated to a very high temperature, the mixture slowly fuses to a
transparent liquid, which on cooling passes into the solid called glass.
Instead of starting with sodium and calcium silicates it is more
convenient and economical to heat sodium carbonate (or sulphate) and
lime with an excess of clean sand, the silicates being formed during the
heating:
Na2CO3 + SiO2 = Na2SiO3 + CO2,
CaO + SiO2 = CaSiO3.
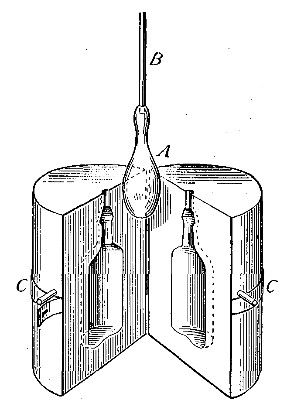 Fig. 74
Fig. 74
The mixture is heated below the fusing point for some time, so that the
escaping carbon dioxide may not spatter the hot liquid; the heat is then
increased and the mixture kept in a state of fusion until all gases
formed in the reaction have escaped.
Molding and blowing of glass. The way in which the melted mixture is
handled in the glass factory depends upon the character of the article
to be made. Many articles, such as bottles, are made by blowing the
plastic glass into hollow molds of the desired shape. The mold is first
opened, as shown in Fig. 74. A lump of plastic glass A on the hollow
rod B is lowered into the mold, which is then closed by the handles
C. By blowing into the tube the glass is blown into the shape of the
mold. The mold is then opened and the bottle lifted out. The neck of the
bottle must be cut off at the proper place and the sharp edges rounded
off in a flame.
Other objects, such as lamp chimneys, are made by getting a lump of
plastic glass on the end of a hollow iron rod and blowing it into the
desired shape without the help of a mold, great skill being required in
the manipulation of the glass. Window glass is made by blowing large
hollow cylinders about 6 ft. long and 1-1/2 ft. in diameter. These are
cut longitudinally, and are then placed in an oven and heated until they
soften, when they are flattened out into plates (Fig. 75). Plate glass
is cast into flat slabs, which are then ground and polished to perfectly
plane surfaces.[Pg 263]
Varieties of glass. The ingredients mentioned above make a soft,
easily fusible glass. If potassium carbonate is substituted for the
sodium carbonate, the glass is much harder and less easily fused;
increasing the amount of sand has somewhat the same effect. Potassium
glass is largely used in making chemical glassware, since it resists the
action of reagents better than the softer sodium glass. If lead oxide is
substituted for the whole or a part of the lime, the glass is very soft,
but has a high index of refraction and is valuable for making optical
instruments and artificial jewels.
 Fig. 75
Fig. 75
Coloring of glass. Various substances fused along with the glass
mixture give characteristic colors. The amber color of common bottles is
due to iron compounds in the glass; in other cases iron colors the glass
green. Cobalt compounds color it deep blue; those of manganese give it
an amethyst tint and uranium compounds impart a peculiar yellowish green
color. Since iron is nearly always present in the ingredients, glass is
usually slightly yellow. This color can be removed by adding the proper
amount of manganese dioxide, for the amethyst color of manganese and the
yellow of iron together produce white light.
Nature of glass. Glass is not a definite chemical compound and its
composition varies between wide limits. Fused glass is really a solution
of various silicates, such as those of calcium and lead, in fused sodium
or potassium silicate. A certain amount of silicon dioxide is also
present. This solution is then allowed to solidify under such conditions
of cooling that the dissolved substances do not separate from the
solvent. The compounds which are used to color the glass are sometimes
converted into silicates, which then dissolve in the glass, giving it a
uniform color. In other cases, as in the milky glasses which resemble
porcelain in appearance, the color or opaqueness is due to the finely
divided color material evenly distributed throughout the glass, but not
dissolved in it. Milky glass is made by mixing calcium fluoride, tin
oxide, or some other insoluble substance in the melted glass. Copper or
gold in metallic form scattered through glass gives it shades of red.[Pg 264]
TITANIUM
Titanium is a very widely distributed element in nature, being
found in almost all soils, in many rocks, and even in plant and
animal tissues. It is not very abundant in any one locality,
and it possesses little commercial value save in connection
with the iron industry. Its most common ore is rutile
(TiO2), which resembles silica in many respects.
In both physical and chemical properties titanium resembles
silicon, though it is somewhat more metallic in character. This
resemblance is most marked in the acids of titanium. It not
only forms metatitanic and orthotitanic acids but a great
variety of polytitanic acids as well.
BORON
Occurrence. Boron is never found free in nature. It occurs as boric acid
(H3BO3), and in salts of polyboric acids, which usually have very
complicated formulas.
Preparation and properties. Boron can be prepared from its oxide by
reduction with magnesium, exactly as in the case of silicon. It
resembles silicon very strikingly in its properties. It occurs in
several allotropic forms, is very hard when crystallized, and is rather
inactive toward reagents. It forms a hydride, BH3, and combines
directly with the elements of the chlorine family. Boron fluoride
(BF3) is very similar to silicon fluoride in its mode of formation
and chemical properties.
Boric oxide (B2O3). Boron forms one well-known oxide, B2O3,
called boric anhydride. It is formed as a glassy mass by heating boric
acid to a high temperature. It absorbs water very readily, uniting with
it to form boric acid again:
In this respect it differs from silicon dioxide, which will not combine
directly with water.[Pg 265]
Boric acid (H3BO3). This is found in nature in considerable
quantities and forms one of the chief sources of boron compounds. It is
found dissolved in the water of hot springs in some localities,
particularly in Italy. Being volatile with steam, the vapor which
escapes from these springs has some boric acid in it. It is easily
obtained from these sources by condensation and evaporation, the
necessary heat being supplied by other hot springs.
Boric acid crystallizes in pearly flakes, which are greasy to the touch.
In the laboratory it is easily prepared by treating a strong, hot
solution of borax with sulphuric acid. Boric acid being sparingly
soluble in water crystallizes out on cooling:
Na2B4O7 + 5H2O + H2SO4 = Na2SO4 + 4H3BO3.
The substance is a mild antiseptic, and on this account is often used in
medicine and as a preservative for canned foods and milk.
Metaboric and polyboric acids. When boric acid is gently heated it is
converted into metaboric acid (HBO2):
On heating metaboric acid to a somewhat higher temperature tetraboric
acid (H2B4O7) is formed:
Many other complex acids of boron are known.
Borax. Borax is the sodium salt of tetraboric acid, having the formula
Na2B4O7·10 H2O. It is found in some arid countries, as
southern California and Tibet, but is now made commercially from the
mineral colemanite, which is the calcium salt of a complex boric acid.
When this is treated with a solution of sodium carbonate, calcium[Pg 266]
carbonate is precipitated and borax crystallizes from the solution.
When heated borax at first swells up greatly, owing to the expulsion of
the water of crystallization, and then melts to a clear glass. This
glass has the property of easily dissolving many metallic oxides, and on
this account borax is used as a flux in soldering, for the purpose of
removing from the metallic surfaces to be soldered the film of oxide
with which they are likely to be covered. These oxides often give a
characteristic color to the clear borax glass, and borax beads are
therefore often used in testing for the presence of metals, instead of
the metaphosphoric acid bead already described.
The reason that metallic oxides dissolve in borax is that borax
contains an excess of acid anhydride, as can be more easily
seen if its formula is written 2NaBO2 + B2O3. The
metallic oxide combines with this excess of acid anhydride,
forming a mixed salt of metaboric acid.
Borax is extensively used as a constituent of enamels and glazes for
both metal ware and pottery. It is also used as a flux in soldering and
brazing, and in domestic ways it serves as a mild alkali, as a
preservative for meats, and in a great variety of less important
applications.
EXERCISES
1. Account for the fact that a solution of borax in water is alkaline.
2. What weight of water of crystallization does 1 kg. of borax contain?
3. When a concentrated solution of borax acts on silver nitrate a borate
of silver is formed. If the solution of borax is dilute, however, an
hydroxide of silver forms. Account for this difference in behavior.
[Pg 267]
CHAPTER XXII
THE METALS
The metals. The elements which remain to be considered are known
collectively as the metals. They are also called the base-forming
elements, since their hydroxides are bases. A metal may therefore be
defined as an element whose hydroxide is a base. When a base dissolves
in water the hydroxyl groups form the anions, while the metallic element
forms the cations. From this standpoint a metal can be defined as an
element capable of forming simple cations in solution.
The distinction between a metal and a non-metal is not a very sharp one,
since the hydroxides of a number of elements act as bases under some
conditions and as acids under others. We have seen that antimony is an
element of this kind.
Occurrence of metals in nature. A few of the metals are found in nature
in the free state. Among these are gold, platinum, and frequently
copper. They are usually found combined with other elements in the form
of oxides or salts of various acids. Silicates, carbonates, sulphides,
and sulphates are the most abundant salts. All inorganic substances
occurring in nature, whether they contain a metal or not, are called
minerals. Those minerals from which a useful substance can be
extracted are called ores of the substance. These two terms are most
frequently used in connection with the metals.[Pg 268]
Extraction of metals,—metallurgy. The process of extracting a metal
from its ores is called the metallurgy of the metal. The metallurgy of
each metal presents peculiarities of its own, but there are several
methods of general application which are very frequently employed.
1. Reduction of an oxide with carbon. Many of the metals occur in
nature in the form of oxides. When these oxides are heated to a high
temperature with carbon the oxygen combines with it and the metal is set
free. Iron, for example, occurs largely in the form of the oxide
Fe2O3. When this is heated with carbon the reaction expressed in
the following equation takes place:
Fe2O3 + 3 C = 2 Fe + 3 CO.
Many ores other than oxides may be changed into oxides which can then be
reduced by carbon. The conversion of such ores into oxides is generally
accomplished by heating, and this process is called roasting. Many
carbonates and hydroxides decompose directly into the oxide on heating.
Sulphides, on the other hand, must be heated in a current of air, the
oxygen of the air entering into the reaction. The following equations
will serve to illustrate these changes in the case of the ores of iron:
FeCO3 = FeO + CO2,
2Fe(OH)3 = Fe2O3 + 3H2O,
2FeS2 + 11O = Fe2O3 + 4SO2.
2. Reduction of an oxide with aluminium. Not all oxides, however, can
be reduced by carbon. In such cases aluminium may be used. Thus chromium
may be obtained in accordance with the following equation:
Cr2O3 + 2 Al = 2 Cr + Al2O3.
[Pg 269]
This method is a comparatively new one, having been brought into use by
the German chemist Goldschmidt; hence it is sometimes called the
Goldschmidt method.
3. Electrolysis. In recent years increasing use is being made of the
electric current in the preparation of metals. In some cases the
separation of the metal from its compounds is accomplished by passing
the current through a solution of a suitable salt of the metal, the
metal usually being deposited upon the cathode. In other cases the
current is passed through a fused salt of the metal, the chloride being
best adapted to this purpose.
Electro-chemical industries. Most of the electro-chemical industries of
the country are carried on where water power is abundant, since this
furnishes the cheapest means for the generation of electrical energy.
Niagara Falls is the most important locality in this country for such
industries, and many different electro-chemical products are
manufactured there. Some industries depend upon electrolytic processes,
while in others the electrical energy is used merely as a source of heat
in electric furnaces.
Preparation of compounds of the metals. Since the compounds of the
metals are so numerous and varied in character, there are many ways of
preparing them. In many cases the properties of the substance to be
prepared, or the material available for its preparation, suggest a
rather unusual way. There are, however, a number of general principles
which are constantly applied in the preparation of the compounds of the
metals, and a clear understanding of them will save much time and effort
in remembering the details in any given case. The most important of
these general methods for the preparation of compounds are the
following:[Pg 270]
1. By direct union of two elements. This is usually accomplished by
heating the two elements together. Thus the sulphides, chlorides, and
oxides of a metal can generally be obtained in this way. The following
equations serve as examples of this method:
Fe + S = FeS,
Mg + O = MgO,
Cu + 2Cl = CuCl2.
2. By the decomposition of a compound. This decomposition may be
brought about either by heat alone or by the combined action of heat and
a reducing agent. Thus when the nitrate of a metal is heated the oxide
of the metal is usually obtained. Copper nitrate, for example,
decomposes as follows:
Cu(NO3)2 = CuO + 2NO2 + O.
Similarly the carbonates of the metals yield oxides, thus:
Most of the hydroxides form an oxide and water when heated:
When heated with carbon, sulphates are reduced to sulphides, thus:
3. Methods based on equilibrium in solution. In the preparation of
compounds the first requisite is that the reactions chosen shall be of
such a kind as will go on to completion. In the chapter on chemical
equilibrium it was shown that reactions in solution may become complete
in either of three ways: (1) a gas may be formed which escapes from
solution; (2) an insoluble solid may be formed which precipitates; (3)
two different ions may combine to form[Pg 271] undissociated molecules. By the
judicious selection of materials these principles may be applied to the
preparation of a great variety of compounds, and illustrations of such
methods will very frequently be found in the subsequent pages.
4. By fusion methods. It sometimes happens that substances which are
insoluble in water and in acids, and which cannot therefore be brought
into double decomposition in the usual way, are soluble in other
liquids, and when dissolved in them can be decomposed and converted into
other desired compounds. Thus barium sulphate is not soluble in water,
and sulphuric acid, being less volatile than most other acids, cannot
easily be driven out from this salt When brought into contact with
melted sodium carbonate, however, it dissolves in it, and since barium
carbonate is insoluble in melted sodium carbonate, double decomposition
takes place:
Na2CO3 + BaSO4 = BaCO3 + Na2SO4.
On dissolving the cooled mixture in water the sodium sulphate formed in
the reaction, together with any excess of sodium carbonate which may be
present, dissolves. The barium carbonate can then be filtered off and
converted into any desired salt by the processes already described.
5. By the action of metals on salts of other metals. When a strip of
zinc is placed in a solution of a copper salt the copper is precipitated
and an equivalent quantity of zinc passes into solution:
In like manner copper will precipitate silver from its salts:
Cu + Ag2SO4 = 2Ag + CuSO4.
[Pg 272]
It is possible to tabulate the metals in such a way that any one of them
in the table will precipitate any one following it from its salts. The
following is a list of some of the commoner metals arranged in this way:
Zinc
Iron
Tin
Lead
Copper
Bismuth
Mercury
Silver
Gold
According to this table copper will precipitate bismuth, mercury,
silver, or gold from their salts, and will in turn be precipitated by
zinc, iron, tin, or lead. Advantage is taken of this principle in the
purification of some of the metals, and occasionally in the preparation
of metals and their compounds.
Important insoluble compounds. Since precipitates play so important a
part in the reactions which substances undergo, as well as in the
preparation of many chemical compounds, it is important to know what
substances are insoluble. Knowing this, we can in many cases predict
reactions under certain conditions, and are assisted in devising ways to
prepare desired compounds. While there is no general rule which will
enable one to foretell the solubility of any given compound,
nevertheless a few general statements can be made which will be of much
assistance.
1. Hydroxides. All hydroxides are insoluble save those of ammonium,
sodium, potassium, calcium, barium, and strontium.
2. Nitrates. All nitrates are soluble in water.
3. Chlorides. All chlorides are soluble save silver and mercurous
chlorides. (Lead chloride is but slightly soluble.)
4. Sulphates. All sulphates are soluble save those of barium,
strontium, and lead. (Sulphates of silver and calcium are only
moderately soluble.)[Pg 273]
5. Sulphides. All sulphides are insoluble save those of ammonium,
sodium, and potassium. The sulphides of calcium, barium, strontium, and
magnesium are insoluble in water, but are changed by hydrolysis into
acid sulphides which are soluble. On this account they cannot be
prepared by precipitation.
6. Carbonates, phosphates, and silicates. All normal carbonates,
phosphates, and silicates are insoluble save those of ammonium, sodium
and potassium.
EXERCISES
1. Write equations representing four different ways for preparing
Cu(NO3)2.
2. Write equations representing six different ways for preparing
ZnSO4.
3. Write equations for two reactions to illustrate each of the three
ways in which reactions in solutions may become complete.
4. Give one or more methods for preparing each of the following
compounds: CaCl2, PbCl2, BaSO4, CaCO3, (NH4)2S,
Ag2S, PbO, Cu(OH)2 (for solubilities, see last paragraph of
chapter). State in each case the general principle involved in the
method of preparation chosen.
[Pg 274]
CHAPTER XXIII
THE ALKALI METALS
| SYMBOL | ATOMIC WEIGHT | DENSITY | MELTING POINT | FIRST PREPARED |
| Lithium | Li | 7.03 | 0.59 | 186.° | Davy 1820 |
| Sodium | Na | 23.05 | 0.97 | 97.6° | " 1807 |
| Potassium | K | 39.15 | 0.87 | 62.5° | " 1807 |
| Rubidium | Rb | 85.5 | 1.52 | 38.5° | Bunsen 1861 |
| Cæsium | Cs | 132.9 | 1.88 | 26.5° | " 1860 |
The family. The metals listed in the above table constitute the even
family in Group I in the periodic arrangement of the elements, and
therefore form a natural family. The name alkali metals is commonly
applied to the family for the reason that the hydroxides of the most
familiar members of the family, namely sodium and potassium, have long
been called alkalis.
1. Occurrence. While none of these metals occur free in nature, their
compounds are very widely distributed, being especially abundant in sea
and mineral waters, in salt beds, and in many rocks. Only sodium and
potassium occur in abundance, the others being rarely found in any
considerable quantity.
2. Preparation. The metals are most conveniently prepared by the
electrolysis of their fused hydroxides or chlorides, though it is
possible to prepare them by reducing their oxides or carbonates with
carbon.[Pg 275]
3. Properties. They are soft, light metals, having low melting points
and small densities, as is indicated in the table. Their melting points
vary inversely with their atomic weights, while their densities (sodium
excepted) vary directly with these. The pure metals have a silvery
luster but tarnish at once when exposed to the air, owing to the
formation of a film of oxide upon the surface of the metal. They are
therefore preserved in some liquid, such as coal oil, which contains no
oxygen. Because of their strong affinity for oxygen they decompose water
with great ease, forming hydroxides and liberating hydrogen in
accordance with the equation
where M stands for any one of these metals. These hydroxides are white
solids; they are readily soluble in water and possess very strong basic
properties. These bases are nearly equal in strength, that is, they all
dissociate in water to about the same extent.
4. Compounds. The alkali metals almost always act as univalent
elements in the formation of compounds, the composition of which can be
represented by such formulas as MH, MCl, MNO3, M2SO4,
M3PO4. These compounds, when dissolved in water, dissociate in
such a way as to form simple, univalent metallic ions which are
colorless. With the exception of lithium these metals form very few
insoluble compounds, so that it is not often that precipitates
containing them are obtained. Only sodium and potassium will be studied
in detail, since the other metals of the family are of relatively small
importance.
The compounds of sodium and potassium are so similar in properties that
they can be used interchangeably for[Pg 276] most purposes. Other things being
equal, the sodium compounds are prepared in preference to those of
potassium, since they are cheaper. When a given sodium compound is
deliquescent, or is so soluble that it is difficult to purify, the
corresponding potassium compound is prepared in its stead, provided its
properties are more desirable in these respects.
SODIUM
Occurrence in nature. Large deposits of sodium chloride have been found
in various parts of the world, and the water of the ocean and of many
lakes and springs contains notable quantities of it. The element also
occurs as a constituent of many rocks and is therefore present in the
soil formed by their disintegration. The mineral cryolite
(Na3AlF6) is an important substance, and the nitrate, carbonate,
and borate also occur in nature.
Preparation. In 1807 Sir Humphry Davy succeeded in preparing very small
quantities of metallic sodium by the electrolysis of the fused
hydroxide. On account of the cost of electrical energy it was for many
years found more economical to prepare it by reducing the carbonate with
carbon in accordance with the following equation:
The cost of generating the electric current has been diminished to such
an extent, however, that it is now more economical to prepare sodium by
Davy's original method, namely, by the electrolysis of the fused
hydroxide or chloride. When the chloride is used the process is
difficult to manage, owing to the higher temperature required to keep
the electrolyte fused, and because of the corroding action of the fused
chloride upon the containing vessel.
 SIR HUMPHRY DAVY (English) (1778-1829)
SIR HUMPHRY DAVY (English) (1778-1829)
Isolated sodium, lithium, potassium, barium, strontium, and calcium by
means of electrolysis; demonstrated the elementary nature of chlorine;
invented the safety lamp; discovered the stupefying effects of nitrous
oxide
[Pg 277]
Technical preparation. The sodium hydroxide is melted in a
cylindrical iron vessel (Fig. 76) through the bottom of which
rises the cathode K. The anodes A, several in number, are
suspended around the cathode from above. A cylindrical vessel
C floats in the fused alkali directly over the cathode, and
under this cap the sodium and hydrogen liberated at the cathode
collect. The hydrogen escapes by lifting the cover, and the
sodium, protected from the air by the hydrogen, is skimmed or
drained off from time to time. Oxygen is set free upon the
anode and escapes into the air through the openings O without
coming into contact with the sodium or hydrogen. This process
is carried on extensively at Niagara Falls.
 Fig. 76
Fig. 76
Properties. Sodium is a silver-white metal about as heavy as water, and
so soft that it can be molded easily by the fingers or pressed into
wire. It is very active chemically, combining with most of the
non-metallic elements, such as oxygen and chlorine, with great energy.
It will often withdraw these elements from combination with other
elements, and is thus able to decompose water and the oxides and
chlorides of many metals.
Sodium peroxide (NaO). Since sodium is a univalent element we should
expect it to form an oxide of the formula Na2O. While such an oxide
can be prepared, the peroxide (NaO) is much better known. It is a
yellowish-white powder made by burning sodium in air. Its chief use is
as an oxidizing agent. When heated with oxidizable substances it gives
up a part of its oxygen, as shown in the equation
[Pg 278]
Water decomposes it in accordance with the equation
2NaO + 2H2O = 2NaOH + H2O2.
Acids act readily upon it, forming a sodium salt and hydrogen peroxide:
2NaO + 2HCl = 2NaCl + H2O2.
In these last two reactions the hydrogen dioxide formed may decompose
into water and oxygen if the temperature is allowed to rise:
Peroxides. It will be remembered that barium dioxide (BaO_{2})
yields hydrogen dioxide when treated with acids, and that
manganese dioxide gives up oxygen when heated with sulphuric
acid. Oxides which yield either hydrogen dioxide or oxygen when
treated with water or an acid are called peroxides.
Sodium hydroxide (caustic soda) (NaOH). 1. Preparation. Sodium
hydroxide is prepared commercially by several processes.
(a) In the older process, still in extensive use, sodium carbonate is
treated with calcium hydroxide suspended in water. Calcium carbonate is
precipitated according to the equation
Na2CO3 + Ca(OH)2 = CaCO3 + 2NaOH.
The dilute solution of sodium hydroxide, filtered from the calcium
carbonate, is evaporated to a paste and is then poured into molds to
solidify. It is sold in the form of slender sticks.
(b) The newer methods depend upon the electrolysis of sodium chloride.
In the Castner process a solution of salt is electrolyzed, the reaction
being expressed as follows:
NaCl + H2O = NaOH + H + Cl.
[Pg 279]
The chlorine escapes as a gas, and by an ingenious mechanical device the
sodium hydroxide is prevented from mixing with the salt in the solution.
In the Acker process the electrolyte is fused sodium chloride. The
chlorine is evolved as a gas at the anode, while the sodium alloys with
the melted lead which forms the cathode. When this alloy is treated with
water the following reaction takes place:
 Fig. 77
Fig. 77
Technical process. A sketch of an Acker furnace is represented in Fig.
77. The furnace is an irregularly shaped cast-iron box, divided into
three compartments, A, B, and C. Compartment A is lined with
magnesia brick. Compartments B and C are filled with melted lead,
which also covers the bottom of A to a depth of about an inch. Above
this layer in A is fused salt, into which dip carbon anodes D. The
metallic box and melted lead is the cathode.
When the furnace is in operation chlorine is evolved at the
anodes, and is drawn away through a pipe (not represented) to
the bleaching-powder chambers. Sodium is set free at the
surface of the melted lead in A, and at once alloys with it.
Through the pipe E a powerful jet of steam is driven through
the lead in B upwards[Pg 280] into the narrow tube F. This forces
the lead alloy up through the tube and over into the chamber
G.
In this process the steam is decomposed by the sodium in the
alloy, forming melted sodium hydroxide and hydrogen. The melted
lead and sodium hydroxide separate into two layers in G, and
the sodium hydroxide, being on top, overflows into tanks from
which it is drawn off and packed in metallic drums. The lead is
returned to the other compartments of the furnace by a pipe
leading from H to I. Compartment C serves merely as a
reservoir for excess of melted lead.
2. Properties. Sodium hydroxide is a white, crystalline, brittle
substance which rapidly absorbs water and carbon dioxide from the air.
As the name (caustic soda) indicates, it is a very corrosive substance,
having a disintegrating action on most animal and vegetable tissues. It
is a strong base. It is used in a great many chemical industries, and
under the name of lye is employed to a small extent as a cleansing agent
for household purposes.
Sodium chloride (common salt) (NaCl). 1. Preparation. Sodium
chloride, or common salt, is very widely distributed in nature. Thick
strata, evidently deposited at one time by the evaporation of salt
water, are found in many places. In the United States the most important
localities for salt are New York, Michigan, Ohio, and Kansas. Sometimes
the salt is mined, especially if it is in the pure form called rock
salt. More frequently a strong brine is pumped from deep wells sunk into
the salt deposit, and is then evaporated in large pans until the salt
crystallizes out. The crystals are in the form of small cubes and
contain no water of crystallization; some water is, however, held in
cavities in the crystals and causes the salt to decrepitate when heated.
2. Uses. Since salt is so abundant in nature it forms the starting
point in the preparation of all compounds[Pg 281] containing either sodium or
chlorine. This includes many substances of the highest importance to
civilization, such as soap, glass, hydrochloric acid, soda, and
bleaching powder. Enormous quantities of salt are therefore produced
each year. Small quantities are essential to the life of man and
animals. Pure salt does not absorb moisture; the fact that ordinary salt
becomes moist in air is not due to a property of the salt, but to
impurities commonly occurring in it, especially calcium and magnesium
chlorides.
Sodium sulphate (Glauber's salt) (Na2SO4·10H2O). This salt is
prepared by the action of sulphuric acid upon sodium chloride,
hydrochloric acid being formed at the same time:
2NaCl + H2SO4 = Na2SO4 + 2HCl.
Some sodium sulphate is prepared by the reaction represented in the
equation
MgSO4 + 2NaCl = Na2SO4 + MgCl2.
The magnesium sulphate required for this reaction is obtained in large
quantities in the manufacture of potassium chloride, and being of little
value for any other purpose is used in this way. The reaction depends
upon the fact that sodium sulphate is the least soluble of any of the
four factors in the equation, and therefore crystallizes out when hot,
saturated solutions of magnesium sulphate and sodium chloride are mixed
together and the resulting mixture cooled.
Sodium sulphate forms large efflorescent crystals. The salt is
extensively used in the manufacture of sodium carbonate and glass. Small
quantities are used in medicine.
Sodium sulphite (Na2SO3·7H2O). Sodium sulphite is prepared by
the action of sulphur dioxide upon solutions[Pg 282] of sodium hydroxide, the
reaction being analogous to the action of carbon dioxide upon sodium
hydroxide. Like the carbonate, the sulphite is readily decomposed by
acids:
Na2SO3 + 2HCl = 2NaCl + H2O + SO2.
Because of this reaction sodium sulphite is used as a convenient source
of sulphur dioxide. It is also used as a disinfectant and a
preservative.
Sodium thiosulphate (hyposulphite of soda or "hypo")
(Na2S2O3·5H2O). This salt, commonly called sodium
hyposulphite, or merely hypo, is made by boiling a solution of sodium
sulphite with sulphur:
It is used in photography and in the bleaching industry, to absorb the
excess of chlorine which is left upon the bleached fabrics.
Thio compounds. The prefix "thio" means sulphur. It is used to
designate substances which may be regarded as derived from
oxygen compounds by replacing the whole or a part of their
oxygen with sulphur. The thiosulphates may be regarded as
sulphates in which one atom of oxygen has been replaced by an
atom of sulphur. This may be seen by comparing the formula
Na2SO4 (sodium sulphate) with the formula
Na2S2O3 (sodium thiosulphate).
Sodium carbonate (sal soda)(Na2CO3·10H2O). There are two
different methods now employed in the manufacture of this important
substance.
1. Le Blanc process. This older process involves several distinct
reactions, as shown in the following equations.
(a) Sodium chloride is first converted into sodium sulphate:
2NaCl + H2SO4 = Na2SO4 + 2HCl.
[Pg 283]
(b) The sodium sulphate is next reduced to sulphide by heating it with
carbon:
Na2SO4 + 2C = Na2S + 2CO2.
(c) The sodium sulphide is then heated with calcium carbonate, when
double decomposition takes place:
Na2S + CaCO3 = CaS + Na2CO3.
Technical preparation of sodium carbonate. In a manufacturing
plant the last two reactions take place in one process. Sodium
sulphate, coal, and powdered limestone are heated together to a
rather high temperature. The coal reduces the sulphate to
sulphide, which in turn reacts upon the calcium carbonate. Some
limestone is decomposed by the heat, forming calcium oxide.
When treated with water the calcium oxide is changed into
hydroxide, and this prevents the water from decomposing the
insoluble calcium sulphide.
The crude product of the process is a hard black cake called
black ash. On digesting this mass with water the sodium
carbonate passes into solution. The pure carbonate is obtained
by evaporation of this solution, crystallizing from it in
crystals of the formula Na2CO3·10H2O. Since over 60%
of this salt is water, the crystals are sometimes heated until
it is driven off. The product is called calcined soda, and is,
of course, more valuable than the crystallized salt.
2. Solvay process. This more modern process depends upon the reactions
represented in the equations
NaCl + NH4HCO3 = NaHCO3 + NH4Cl,
2NaHCO3 = Na2CO3 + H2O + CO2.
The reason the first reaction takes place is that sodium hydrogen
carbonate is sparingly soluble in water, while the other compounds are
freely soluble. When strong solutions of sodium chloride and of ammonium
hydrogen carbonate are brought together the sparingly soluble sodium
hydrogen carbonate is precipitated. This is converted into the normal
carbonate by heating, the reaction being represented in the second
equation.[Pg 284]
Technical preparation. In the Solvay process a very
concentrated solution of salt is first saturated with ammonia
gas, and a current of carbon dioxide is then conducted into the
solution. In this way ammonium hydrogen carbonate is formed:
NH3 + H2O + CO2 = NH4HCO3.
This enters into double decomposition with the salt, as shown
in the first equation under the Solvay process. After the
sodium hydrogen carbonate has been precipitated the mother
liquors containing ammonium chloride are treated with lime:
2NH4Cl + CaO = CaCl2 + 2 NH3 + H2O.
The lime is obtained by burning limestone:
CaCO3 = CaO + CO2.
The ammonia and carbon dioxide evolved in the latter two
reactions are used in the preparation of an additional quantity
of ammonium hydrogen carbonate. It will thus be seen that there
is no loss of ammonia. The only materials permanently used up
are calcium carbonate and salt, while the only waste product is
calcium chloride.
Historical. In former times sodium carbonate was made by
burning seaweeds and extracting the carbonate from their ash.
On this account the salt was called soda ash, and the name is
still in common use. During the French Revolution this supply
was cut off, and in behalf of the French government Le Blanc
made a study of methods of preparing the carbonate directly
from salt. As a result he devised the method which bears his
name, and which was used exclusively for many years. It has
been replaced to a large extent by the Solvay process, which
has the advantage that the materials used are inexpensive, and
that the ammonium hydrogen carbonate used can be regenerated
from the products formed in the process. Much expense is also
saved in fuel, and the sodium hydrogen carbonate, which is the
first product of the process, has itself many commercial uses.
The Le Blanc process is still used, however, since the
hydrochloric acid generated is of value.
By-products. The substances obtained in a given process, aside
from the main product, are called the by-products. The success
of many processes depends upon the value of the by-products
formed.
Thus hydrochloric acid, a by-product in the Le Blanc process,
is valuable enough to make the process pay, even though sodium
carbonate can be made cheaper in other ways.
[Pg 285]
Properties of sodium carbonate. Sodium carbonate forms large crystals of
the formula Na2CO3 · 10 H2O. It has a mild alkaline reaction
and is used for laundry purposes under the name of washing soda. Mere
mention of the fact that it is used in the manufacture of glass, soap,
and many chemical reagents will indicate its importance in the
industries. It is one of the few soluble carbonates.
Sodium hydrogen carbonate (bicarbonate of soda) (NaHCO3). This
salt, commonly called bicarbonate of soda, or baking soda, is made by
the Solvay process, as explained above, or by passing carbon dioxide
into strong solutions of sodium carbonate:
Na2CO3 + H2O + CO2 = 2NaHCO3.
The bicarbonate, being sparingly soluble, crystallizes out. A mixture of
the bicarbonate with some substance (the compound known as cream of
tartar is generally used) which slowly reacts with it, liberating carbon
dioxide, is used largely in baking. The carbon dioxide generated forces
its way through the dough, thus making it porous and light.
Sodium nitrate (Chili saltpeter) (NaNO3). This substance is found
in nature in arid regions in a number of places, where it has been
formed apparently by the decay of organic substances in the presence of
air and sodium salts. The largest deposits are in Chili, and most of the
nitrate of commerce comes from that country. Smaller deposits occur in
California and Nevada. The commercial salt is prepared by dissolving the
crude nitrate in water,[Pg 286] allowing the insoluble earthy materials to
settle, and evaporating the clear solution so obtained to
crystallization. The soluble impurities remain for the most part in the
mother liquors.
Since this salt is the only nitrate found extensively in nature, it is
the material from which other nitrates as well as nitric acid are
prepared. It is used in enormous quantities in the manufacture of
sulphuric acid and potassium nitrate, and as a fertilizer.
Sodium phosphate (Na2HPO4·12H2O). Since phosphoric acid has
three replaceable hydrogen atoms, three sodium phosphates are
possible,—two acid salts and one normal. All three can be made without
difficulty, but disodium phosphate is the only one which is largely
used, and is the salt which is commonly called sodium phosphate. It is
made by the action of phosphoric acid on sodium carbonate:
Na2CO3 + H3PO4 = Na2HPO4 + CO2 + H2O.
It is interesting as being one of the few phosphates which are soluble
in water, and is the salt commonly used when a soluble phosphate is
needed.
Normal sodium phosphate (Na3PO4). Although this is a normal salt
its solution has a strongly alkaline reaction. This is due to the fact
that the salt hydrolyzes in solution into sodium hydroxide and disodium
phosphate, as represented in the equation
Na3PO4 + H2O = Na2HPO4 + NaOH.
Sodium hydroxide is strongly alkaline, while disodium phosphate is
nearly neutral in reaction. The solution as a whole is therefore
alkaline. The salt is prepared by adding a large excess of sodium
hydroxide to a solution of disodium[Pg 287] phosphate and evaporating to
crystallization. The excess of the sodium hydroxide reverses the
reaction of hydrolysis and the normal salt crystallizes out.
Sodium tetraborate (borax) (Na2B4O7·10H2O). The properties
of this important compound have been discussed under the head of boron.
POTASSIUM
Occurrence in nature. Potassium is a constituent of many common rocks
and minerals, and is therefore a rather abundant element, though not so
abundant as sodium. Feldspar, which occurs both by itself and as a
constituent of granite, contains considerable potassium. The element is
a constituent of all clay and of mica and also occurs in very large
deposits at Stassfurt, Germany, in the form of the chloride and
sulphate, associated with compounds of sodium and magnesium. In small
quantities it is found as nitrate and in many other forms.
The natural decomposition of rocks containing potassium gives rise to
various compounds of the element in all fertile soils. Its soluble
compounds are absorbed by growing plants and built up into complex
vegetable substances; when these are burned the potassium remains in the
ash in the form of the carbonate. Crude carbonate obtained from wood
ashes was formerly the chief source of potassium compounds; they are now
mostly prepared from the salts of the Stassfurt deposits.
Stassfurt salts. These salts form very extensive deposits in
middle and north Germany, the most noted locality for working
them being at Stassfurt. The deposits are very thick and rest
upon an enormous layer of common salt. They are in the form of
a series of strata, each consisting largely of a single mineral
salt. A cross section of[Pg 288] these deposits is shown in Fig. 78.
While these strata are salts from a chemical standpoint, they
are as solid and hard as many kinds of stone, and are mined as
stone or coal would be. Since the strata differ in general
appearance, each can be mined separately, and the various
minerals can be worked up by methods adapted to each particular
case. The chief minerals of commercial importance in these
deposits are the following:
| Sylvine | KCl. |
| Anhydrite | CaSO4. |
| Carnallite | KCl·MgCl2·6H2O. |
| Kainite | K2SO4·MgSO4·MgCl2·6H2O. |
| Polyhalite | K2SO4·MgSO4·2CaSO4·2H2O. |
| Kieserite | MgSO4·H2O. |
| Schönite | K2SO4·MgSO4·6H2O. |
Preparation and properties. The metal is prepared by the same method
used in the preparation of sodium. In most respects it is very similar
to sodium, the chief difference being that it is even more energetic in
its action upon other substances. The freshly cut, bright surface
instantly becomes dim through oxidation by the air. It decomposes water
very vigorously, the heat of reaction being sufficient to ignite the
hydrogen evolved. It is somewhat lighter than sodium and is preserved
under gasoline.
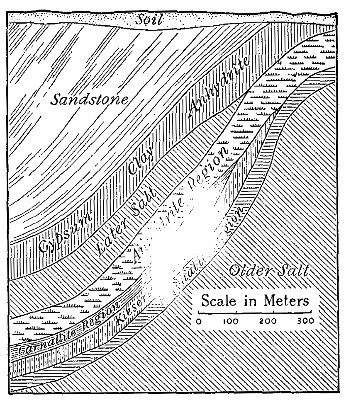 Fig. 78
Fig. 78
Potassium hydroxide (caustic potash) (KOH). Potassium hydroxide is
prepared by methods exactly similar to those[Pg 289] used in the preparation of
sodium hydroxide, which compound it closely resembles in both physical
and chemical properties. It is not used to any very great extent, being
replaced by the cheaper sodium hydroxide.
Action of the halogen elements on potassium hydroxide. When any one of
the three halogen elements—chlorine, bromine, and iodine—is added to a
solution of potassium hydroxide a reaction takes place, the nature of
which depends upon the conditions of the experiment. Thus, when chlorine
is passed into a cold dilute solution of potassium hydroxide the
reaction expressed by the following equation takes place:
(1) 2KOH + 2Cl = KCl + KClO + H2O.
If the solution of hydroxide is concentrated and hot, on the other hand,
the potassium hypochlorite formed according to equation (1) breaks down
as fast as formed:
(2) 3KClO = KClO3 + 2KCl.
Equation (1), after being multiplied by 3, may be combined with equation
(2), giving the following:
(3) 6KOH + 6Cl = 5KCl + KClO3 + 3H2O.
This represents in a single equation the action of chlorine on hot,
concentrated solutions of potassium hydroxide. By means of these
reactions one can prepare potassium chloride, potassium hypochlorite,
and potassium chlorate. By substituting bromine or iodine for chlorine
the corresponding compounds of these elements are obtained. Some of
these compounds can be obtained in cheaper ways.
If the halogen element is added to a solution of sodium hydroxide or
calcium hydroxide, the reaction which takes place is exactly similar to
that which takes place with[Pg 290] potassium hydroxide. It is possible,
therefore, to prepare in this way the sodium and calcium compounds
corresponding to the potassium compounds given above.
Potassium chloride (KCl). This salt occurs in nature in sea water, in
the mineral sylvine, and, combined with magnesium chloride, as
carnallite (KCl·MgCl2·6H2O). It is prepared from carnallite by
saturating boiling water with the mineral and allowing the solution to
cool. The mineral decomposes while in solution, and the potassium
chloride crystallizes out on cooling, while the very soluble magnesium
chloride remains in solution. The salt is very similar to sodium
chloride both in physical and chemical properties. It is used in the
preparation of nearly all other potassium salts, and, together with
potassium sulphate, is used as a fertilizer.
Potassium bromide (KBr). When bromine is added to a hot concentrated
solution of potassium hydroxide there is formed a mixture of potassium
bromide and potassium bromate in accordance with the reactions already
discussed. There is no special use for the bromate, so the solution is
evaporated to dryness, and the residue, consisting of a mixture of the
bromate and bromide, is strongly heated. This changes the bromate to
bromide, as follows:
The bromide is then crystallized from water, forming large colorless
crystals. It is used in medicine and in photography.
Potassium iodide (KI). Potassium iodide may be made by exactly the same
method as has just been described for the bromide, substituting iodine
for bromine. It is more frequently made as follows. Iron filings are
[Pg 291]treated with iodine, forming the compound Fe3I8; on boiling this
substance with potassium carbonate the reaction represented in the
following equation occurs:
Fe3I8 + 4K2CO3 = Fe3O4 + 8KI + 4CO2.
Potassium iodide finds its chief use in medicine.
Potassium chlorate (KClO3). This salt, as has just been explained,
can be made by the action of chlorine on strong potassium hydroxide
solutions. The chief use of potassium chlorate is as an oxidizing agent
in the manufacture of matches, fireworks, and explosives; it is also
used in the preparation of oxygen and in medicine.
Commercial preparation. By referring to the reaction between
chlorine and hot concentrated solutions of potassium hydroxide,
it will be seen that only one molecule of potassium chlorate is
formed from six molecules of potassium hydroxide. Partly
because of this poor yield and partly because the potassium
hydroxide is rather expensive, this process is not an
economical one for the preparation of potassium chlorate. The
commercial method is the following. Chlorine is passed into hot
solutions of calcium hydroxide, a compound which is very cheap.
The resulting calcium chloride and chlorate are both very
soluble. To the solution of these salts potassium chloride is
added, and as the solution cools the sparingly soluble
potassium chlorate crystallizes out:
Ca(ClO3)2 + 2KCl = 2KClO3 + CaCl2.
Electro-chemical processes are also used.
Potassium nitrate (saltpeter) (KNO3). This salt was formerly made
by allowing animal refuse to decompose in the open air in the presence
of wood ashes or earthy materials containing potassium. Under these
conditions the nitrogen in the organic matter is in part converted into
potassium nitrate, which was obtained by extracting the mass with water
and evaporating to crystallization. This crude and[Pg 292] slow process is now
almost entirely replaced by a manufacturing process in which the
potassium salt is made from Chili saltpeter:
NaNO3 + KCl = NaCl + KNO3.
This process has been made possible by the discovery of the Chili niter
beds and the potassium chloride of the Stassfurt deposits.
The reaction depends for its success upon the apparently
insignificant fact that sodium chloride is almost equally
soluble in cold and hot water. All four factors in the equation
are rather soluble in cold water, but in hot water sodium
chloride is far less soluble than the other three. When hot
saturated solutions of sodium nitrate and potassium chloride
are brought together, sodium chloride precipitates and can be
filtered off, leaving potassium nitrate in solution, together
with some sodium chloride. On cooling, potassium nitrate
crystallizes out, leaving small amounts of the other salts in
solution.
Potassium nitrate is a colorless salt which forms very large crystals.
It is stable in the air, and when heated is a good oxidizing agent,
giving up oxygen quite readily. Its chief use is in the manufacture of
gunpowder.
Gunpowder. The object sought for in the preparation of
gunpowder is to secure a solid substance which will remain
unchanged under ordinary conditions, but which will explode
readily when ignited, evolving a large volume of gas. When a
mixture of carbon and potassium nitrate is ignited a great deal
of gas is formed, as will be seen from the equation
2KNO3 + 3C = CO2 + CO + N2 + K2CO3.
By adding sulphur to the mixture the volume of gas formed in
the explosion is considerably increased:
2KNO3 + 3C + S = 3CO2 + N2 + K2S.
Gunpowder is simply a mechanical mixture of these three
substances in the proportion required for the above reaction.
While the equation represents the principal reaction, other
reactions also take place.[Pg 293] The gases formed in the explosion,
when measured under standard conditions, occupy about two
hundred and eighty times the volume of the original powder.
Potassium sulphide (K2S) is a solid substance, and it is
largely due to it that gunpowder gives off smoke and soot when
it explodes. Smokeless powder consists of organic substances
which, on explosion, give only colorless gases, and hence
produce no smoke. Sodium nitrate is cheaper than potassium
nitrate, but it is not adapted to the manufacture of the best
grades of powder, since it is somewhat deliquescent and does
not give up its oxygen so readily as does potassium nitrate. It
is used, however, in the cheaper grades of powder, such as are
employed for blasting.
Potassium cyanide (KCN). When animal matter containing nitrogen is
heated with iron and potassium carbonate, complicated changes occur
which result in the formation of a substance commonly called yellow
prussiate of potash, which has the formula K4FeC6N6. When this
substance is heated with potassium, potassium cyanide is formed:
K4FeC6N6 + 2 K = 6KCN + Fe.
Since sodium is much cheaper than potassium it is often used in place of
it:
K4FeC6N6 + 2Na = 4KCN + 2NaCN + Fe.
The mixture of cyanides so resulting serves most of the purposes of the
pure salt. It is used very extensively in several metallurgical
processes, particularly in the extraction of gold. Potassium cyanide is
a white solid characterized by its poisonous properties, and must be
used with extreme caution.
Potassium carbonate (potash) (K2CO3). This compound occurs in
wood ashes in small quantities. It cannot be prepared by the Solvay
process, since the acid carbonate is quite soluble in water, but is made
by the Le Blanc process. Its chief use is in the manufacture of other
potassium salts.[Pg 294]
Other salts of potassium. Among the other salts of potassium frequently
met with are the sulphate (K2SO4), the acid carbonate (KHCO3),
the acid sulphate (KHSO4), and the acid sulphite (KHSO3). These
are all white solids.
LITHIUM, RUBIDIUM, CÆSIUM
Of the three remaining elements of the family—lithium, rubidium, and
cæsium—lithium is by far the most common, the other two being very
rare. Lithium chloride and carbonate are not infrequently found in
natural mineral waters, and as these substances are supposed to increase
the medicinal value of the water, they are very often added to
artificial mineral waters in small quantities.
COMPOUNDS OF AMMONIUM
General. As explained in a previous chapter, when ammonia is passed into
water the two compounds combine to form the base NH4OH, known as
ammonium hydroxide. When this base is neutralized with acids there are
formed the corresponding salts, known as the ammonium salts. Since the
ammonium group is univalent, ammonium salts resemble those of the alkali
metals in formulas; they also resemble the latter salts very much in
their chemical properties, and may be conveniently described in
connection with them. Among the ammonium salts the chloride, sulphate,
carbonate, and sulphide are the most familiar.
Ammonium chloride (sal ammoniac) (NH4Cl). This substance is
obtained by neutralizing ammonium hydroxide with hydrochloric acid. It
is a colorless substance crystallizing in fine needles, and, like most
ammonium salts, is very soluble in water. When placed in a tube and
heated strongly it decomposes into hydrochloric acid and ammonia. When
these gases reach a cooler portion of the tube they[Pg 295] at once recombine,
and the resulting ammonium chloride is deposited on the sides of the
tube. In this way the salt can be separated from nonvolatile impurities.
Ammonium chloride is sometimes used in preparation of ammonia; it is
also used in making dry batteries and in the laboratory as a chemical
reagent.
Ammonium sulphate ((NH4)2SO4). This salt resembles the chloride
very closely, and, being cheaper, is used in place of it when possible.
It is used in large quantity as a fertilizer, the nitrogen which it
contains being a very valuable food for plants.
Ammonium carbonate ((NH4)2CO3). This salt, as well as the acid
carbonate (NH4HCO3), is used as a chemical reagent. They are
colorless solids, freely soluble in water. The normal carbonate is made
by heating ammonium chloride with powdered limestone (calcium
carbonate), the ammonium carbonate being obtained as a sublimate in
compact hard masses:
2NH4Cl + CaCO3 = (NH4)2CO3 + CaCl2.
The salt always smells of ammonia, since it slowly decomposes, as shown
in the equation
(NH4)2CO3 = NH4HCO3 + NH3.
The acid carbonate, or bicarbonate, is prepared by saturating a solution
of ammonium hydroxide with carbon dioxide:
It is a well-crystallized stable substance.
Ammonium sulphide ((NH4)2S). Ammonium sulphide is prepared by the
action of hydrosulphuric acid upon ammonium hydroxide:
2NH4OH + H2S = (NH4)2S + 2H2O.
[Pg 296]
If the action is allowed to continue until no more hydrosulphuric acid
is absorbed, the product is the acid sulphide, sometimes called the
hydrosulphide:
NH4OH + H2S = NH4HS + H2O.
If equal amounts of ammonium hydroxide and ammonium acid sulphide are
brought together, the normal sulphide is formed:
NH4OH + NH4HS = (NH4)2S + H2O
It has been obtained in the solid state, but only with great difficulty.
As used in the laboratory it is always in the form of a solution. It is
much used in the process of chemical analysis because it is a soluble
sulphide and easily prepared. On exposure to the air ammonium sulphide
slowly decomposes, being converted into ammonia, water, and sulphur:
(NH4)2S + O = 2NH3 + H2O + S.
As fast as the sulphur is liberated it combines with the unchanged
sulphide to form several different ammonium sulphides in which there are
from two to five sulphur atoms in the molecule, thus: (NH4)2S2,
(NH4)2S3, (NH4)2S5. These sulphides in turn decompose
by further action of oxygen, so that the final products of the reaction
are those given in the equation. A solution of these compounds is yellow
and is sometimes called yellow ammonium sulphide.
FLAME REACTION—SPECTROSCOPE
When compounds of either sodium or potassium are brought into
the non-luminous flame of a Bunsen burner the flame becomes
colored. Sodium compounds color it intensely yellow, while
those of potassium color it pale violet. When only one of these
elements is present it is[Pg 297] easy to identify it by this simple
test, but when both are present the intense color of the sodium
flame entirely conceals the pale tint characteristic of
potassium compounds.
It is possible to detect the potassium flame in such cases,
however, in the following way. When light is allowed to shine
through a very small hole or slit in some kind of a screen,
such as a piece of metal, upon a triangular prism of glass, the
light is bent or refracted out of its course instead of passing
straight through the glass. It thus comes out of the prism at
some angle to the line at which it entered. Yellow light is
bent more than red, and violet more than yellow. When light
made up of the yellow of sodium and the violet of potassium
shines through a slit upon such a prism, the yellow and the
violet lights come out at somewhat different angles, and so two
colored lines of light—a yellow line and a violet line—are
seen on looking into the prism in the proper direction. The
instrument used for separating the rays of light in this way is
called a spectroscope (Fig. 79). The material to be tested is
placed on a platinum wire and held in the colorless Bunsen
flame. The resulting light passes through the slit in the end
of tube B, and then through B to the prism. The resulting
lines of light are seen by looking into the tube A, which
contains a magnifying lens. Most elements give more than one
image of the slit, each having a different color, and the
series of colored lines due to an element is called its
spectrum.
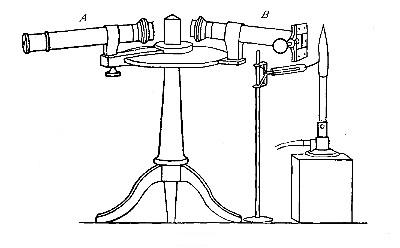 Fig. 79
Fig. 79
[Pg 298]
The spectra of the known elements have been carefully studied, and any
element which imparts a characteristic color to a flame, or has a
spectrum of its own, can be identified even when other elements are
present. Through the spectroscopic examination of certain minerals a
number of elements have been discovered by the observation of lines
which did not belong to any known element. A study of the substance then
brought to light the new element. Rubidium and cæsium were discovered in
this way, rubidium having bright red lines and cæsium a very intense
blue line. Lithium colors the flame deep red, and has a bright red line
in its spectrum.
EXERCISES
1. What is an alkali? Can a metal itself be an alkali?
2. Write equations showing how the following changes may be brought
about, giving the general principle involved in each change: NaCl -->
Na2SO3, Na2SO3 --> NaCl, NaCl --> NaBr, Na2SO4 -->
NaNO3, NaNO3 --> NaHCO3.
3. What carbonates are soluble?
4. State the conditions under which the reaction represented by the
following equation can be made to go in either direction:
Na2CO3 + H2O + CO2 <--> 2 NaHCO3.
5. Account for the fact that solutions of sodium carbonate and potassium
carbonate are alkaline.
6. What non-metallic element is obtained from the deposits of Chili
saltpeter?
7. Supposing concentrated hydrochloric acid (den. = 1.2) to be worth six
cents a pound, what is the value of the acid generated in the
preparation of 1 ton of sodium carbonate by the Le Blanc process?
8. What weight of sodium carbonate crystals will 1 kg. of the anhydrous
salt yield?
9. Write equations for the preparation of potassium hydroxide by three
different methods.
10. What would take place if a bit of potassium hydroxide were left
exposed to the air?
11. Write the equations for the reactions between sodium hydroxide and
bromine; between potassium hydroxide and iodine.
12. Write equations for the preparation of potassium sulphate; of
potassium acid carbonate.
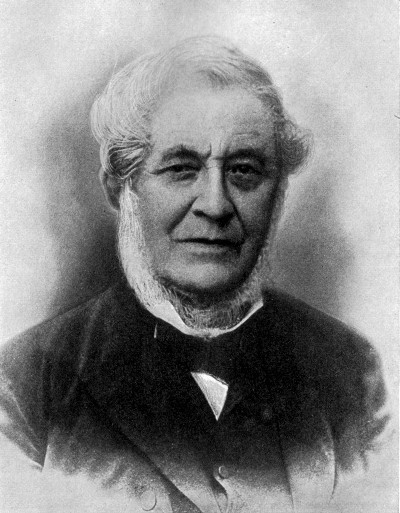 ROBERT WILHELM BUNSEN (German) (1811-1899)
ROBERT WILHELM BUNSEN (German) (1811-1899)
Invented many lecture-room and laboratory appliances (Bunsen burner);
invented the spectroscope and with it discovered rubidium and cæsium;
greatly perfected methods of electrolysis, inventing a new battery; made
many investigations among metallic and organic substances
[Pg 299]
13. What weight of carnallite would be necessary in the preparation of 1
ton of potassium carbonate?
14. Write the equations showing how ammonium chloride, ammonium
sulphate, ammonium carbonate, and ammonium nitrate may be prepared from
ammonium hydroxide.
15. Write an equation to represent the reaction involved in the
preparation of ammonia from ammonium chloride.
16. What substances already studied are prepared from the following
compounds? ammonium chloride; ammonium nitrate; ammonium nitrite; sodium
nitrate; sodium chloride.
17. How could you prove that the water in crystals of common salt is not
water of crystallization?
18. How could you distinguish between potassium chloride and potassium
iodide? between sodium chloride and ammonium chloride? between sodium
nitrate and potassium nitrate?
[Pg 300]
CHAPTER XXIV
THE ALKALINE-EARTH FAMILY
| SYMBOL | ATOMIC WEIGHT | DENSITY | MILLIGRAMS SOLUBLE IN 1 L OF WATER AT 18° | CARBONATE DECOMPOSES |
| | | | | SULPHATE | HYDROXIDE | |
| Calcium | Ca | 40.1 | 1.54 | 2070.00 | 1670. | At dull red heat |
| Strontium | Sr | 87.6 | 2.50 | 170.00 | 7460. | At white heat |
| Barium | Ba | 137.4 | 3.75 | 2.29 | 36300. | Scarcely at all |
The family. The alkaline-earth family consists of the very abundant
element calcium and the much rarer elements strontium and barium. They
are called the alkaline-earth metals because their properties are
between those of the alkali metals and the earth metals. The earth
metals will be discussed in a later chapter. The family is also
frequently called the calcium family.
1. Occurrence. These elements do not occur free in nature. Their most
abundant compounds are the carbonates and sulphates; calcium also occurs
in large quantities as the phosphate and silicate.
2. Preparation. The metals were first prepared by Davy in 1808 by
electrolysis. This method has again come into use in recent years.
Strontium and barium have as yet been obtained only in small quantities
and in the impure state, and many of their physical properties,[Pg 301] such as
their densities and melting points, are therefore imperfectly known.
3. Properties. The three metals resemble each other very closely. They
are silvery-white in color and are about as hard as lead. Their
densities increase with their atomic weights, as is shown in the table
on opposite page. Like the alkali metals they have a strong affinity for
oxygen, tarnishing in the air through oxidation. They decompose water at
ordinary temperatures, forming hydroxides and liberating hydrogen. When
ignited in the air they burn with brilliancy, forming oxides of the
general formula MO. These oxides readily combine with water, according
to the equation
Each of the elements has a characteristic spectrum, and the presence of
the metals can easily be detected by the spectroscope.
4. Compounds. The elements are divalent in almost all of their
compounds, and these compounds in solution give simple, divalent,
colorless ions. The corresponding salts of the three elements are very
similar to each other and show a regular variation in properties in
passing from calcium to strontium and from strontium to barium. This is
seen in the solubility of the sulphate and hydroxide, and in the ease of
decomposition of the carbonates, as given in the table. Unlike the
alkali metals, their normal carbonates and phosphates are insoluble in
water.
CALCIUM
Occurrence. The compounds of calcium are very abundant in nature, so
that the total amount of calcium in the earth's crust is very large. A
great many different compounds[Pg 302] containing the clement are known, the
most important of which are the following:
| Calcite (marble) | CaCO3. |
| Phosphorite | Ca3(PO4)2. |
| Fluorspar | CaF2. |
| Wollastonite | CaSiO3. |
| Gypsum | CaSO4·2H2O. |
| Anhydrite | CaSO4. |
Preparation. Calcium is now prepared by the electrolysis of the melted
chloride, the metal depositing in solid condition on the cathode. It is
a gray metal, considerably heavier and harder than sodium. It acts upon
water, forming calcium hydroxide and hydrogen, but the action does not
evolve sufficient heat to melt the metal. It promises to become a useful
substance, though no commercial applications for it have as yet been
found.
Calcium oxide (lime, quicklime) (CaO). Lime is prepared by strongly
heating calcium carbonate (limestone) in large furnaces called kilns:
When pure, lime is a white amorphous substance. Heated intensely, as in
the oxyhydrogen flame, it gives a brilliant light called the lime light.
Although it is a very difficultly fusible substance, yet in the electric
furnace it can be made to melt and even boil. Water acts upon lime with
the evolution of a great deal of heat,—hence the name quicklime, or
live lime,—the process being called slaking. The equation is
Lime readily absorbs moisture from the air, and is used to dry moist
gases, especially ammonia, which cannot be[Pg 303] dried by the usual
desiccating agents. It also absorbs carbon dioxide, forming the
carbonate
CaO + CO2 = CaCO3.
Lime exposed to air is therefore gradually converted into hydroxide and
carbonate, and will no longer slake with water. It is then said to be
air-slaked.
Limekilns. The older kiln, still in common use, consists of a
large cylindrical stack in which the limestone is loosely
packed. A fire is built at the base of the stack, and when the
burning is complete it is allowed to die out and the lime is
removed from the kiln. The newer kilns are constructed as shown
in Fig. 80. A number of fire boxes are built around the lower
part of the kiln, one of which is shown at B. The fire is
built on the grate F and the hot products of combustion are
drawn up through the stack, decomposing the limestone. The kiln
is charged at C, and sometimes fuel is added with the
limestone to cause combustion throughout the contents of the
kiln. The burned lime is raked out through openings in the
bottom of the stack, one of which is shown at D. The
advantage of this kind of a kiln over the older form is that
the process is continuous, limestone being charged in at the
top as fast as the lime is removed at the bottom.
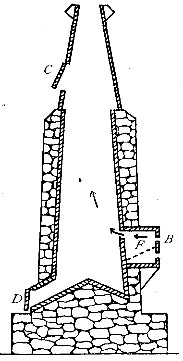 Fig. 80
Fig. 80
Calcium hydroxide (slaked lime) (Ca(OH)2). Pure calcium hydroxide
is a light white powder. It is sparingly soluble in water, forming a
solution called limewater, which is often used in medicine as a mild
alkali. Chemically, calcium hydroxide is a moderately strong base,
though not so strong as sodium hydroxide. Owing to its cheapness it is
much used in the[Pg 304] industries whenever an alkali is desired. A number of
its uses have already been mentioned. It is used in the preparation of
ammonia, bleaching powder, and potassium hydroxide. It is also used to
remove carbon dioxide and sulphur compounds from coal gas, to remove the
hair from hides in the tanneries (this recalls the caustic or corrosive
properties of sodium hydroxide), and for making mortar.
Mortar is a mixture of calcium hydroxide and sand. When it is exposed to
the air or spread upon porous materials moisture is removed from it
partly by absorption in the porous materials and partly by evaporation,
and the mortar becomes firm, or sets. At the same time carbon dioxide
is slowly absorbed from the air, forming hard calcium carbonate:
Ca(OH)2 + CO2 = CaCO3 + H2O.
By this combined action the mortar becomes very hard and adheres firmly
to the surface upon which it is spread. The sand serves to give body to
the mortar and makes it porous, so that the change into carbonate can
take place throughout the mass. It also prevents too much shrinkage.
Cement. When limestone to which clay and sand have been added in certain
proportions is burned until it is partly fused (some natural marl is
already of about the right composition), and the clinker so produced is
ground to powder, the product is called cement. When this material is
moistened it sets to a hard stone-like mass which retains its hardness
even when exposed to the continued action of water. It can be used for
under-water work, such as bridge piers, where mortar would quickly
soften. Several varieties of cement are made, the best known of which is
Portland cement.[Pg 305]
Growing importance of cement. Cement is rapidly coming into use for a
great variety of purposes. It is often used in place of mortar in the
construction of brick buildings. Mixed with crushed stone and sand it
forms concrete which is used in foundation work. It is also used in
making artificial stone, terra-cotta trimmings for buildings, artificial
stone walks and floors, and the like. It is being used more and more for
making many articles which were formerly made of wood or stone, and the
entire walls of buildings are sometimes made of cement blocks or of
concrete.
Calcium carbonate (CaCO3). This substance is found in a great many
natural forms to which various names have been given. They may be
classified under three heads:
1. Amorphous carbonate. This includes those forms which are not
markedly crystalline. Limestone is the most familiar of these and is a
grayish rock usually found in hard stratified masses. Whole mountain
ranges are sometimes made up of this material. It is always impure,
usually containing magnesium carbonate, clay, silica, iron and aluminium
compounds, and frequently fossil remains. Marl is a mixture of limestone
and clay. Pearls, chalk, coral, and shells are largely calcium
carbonate.
2. Hexagonal carbonate. Calcium carbonate crystallizes in the form of
rhomb-shaped crystals which belong to the hexagonal system. When very
pure and transparent the substance is called Iceland spar. Calcite is a
similar form, but somewhat opaque or clouded. Mexican onyx is a massive
variety, streaked or banded with colors due to impurities. Marble when
pure is made up of minute calcite crystals. Stalactites and stalagmites
are icicle-like forms sometimes found in caves.
3. Rhombic carbonate. Calcium carbonate sometimes crystallizes in
needle-shaped crystals belonging to the rhombic system. This is the
unstable form and tends to[Pg 306] go over into the other variety. Aragonite is
the most familiar example of this form.
Preparation and uses of calcium carbonate. In the laboratory pure
calcium carbonate can be prepared by treating a soluble calcium salt
with a soluble carbonate:
Na2CO3 + CaCl2 = CaCO3 + 2NaCl.
When prepared in this way it is a soft white powder often called
precipitated chalk, and is much used as a polishing powder. It is
insoluble in water, but dissolves in water saturated with carbon
dioxide, owing to the formation of the acid calcium carbonate which is
slightly soluble:
CaCO3 + H2CO3 = Ca(HCO3)2.
The natural varieties of calcium carbonate find many uses, such as in
the preparation of lime and carbon dioxide; in metallurgical operations,
especially in the blast furnaces; in the manufacture of soda, glass, and
crayon (which, in addition to chalk, usually contains clay and calcium
sulphate); for building stone and ballast for roads.
Calcium chloride (CaCl2). This salt occurs in considerable quantity
in sea water. It is obtained as a by-product in many technical
processes, as in the Solvay soda process. When crystallized from its
saturated solutions it forms colorless needles of the composition
CaCl2·6H2O. By evaporating a solution to dryness and heating to a
moderate temperature calcium chloride is obtained anhydrous as a white
porous mass. In this condition it absorbs water with great energy and is
a valuable drying agent.
Bleaching powder (CaOCl2). When chlorine acts upon a solution of
calcium hydroxide the reaction is similar to that which occurs between
chlorine and potassium hydroxide:
2Ca(OH)2 + 4Cl = CaCl2 + Ca(ClO)2 + 2H2O.
[Pg 307]
If, however, chlorine is conducted over calcium hydroxide in the form of
a dry powder, it is absorbed and a substance is formed which appears to
have the composition represented in the formula CaOCl2. This
substance is called bleaching powder, or hypochlorite of lime. It is
probably the calcium salt of both hydrochloric and hypochlorous acids,
so that its structure is represented by the formula
In solution this substance acts exactly like a mixture of calcium
chloride (CaCl2) and calcium hypochlorite (Ca(ClO)2), since it
dissociates to form the ions Ca++, Cl-, and ClO-.
Bleaching powder undergoes a number of reactions which make it an
important substance.
1. When treated with an acid it evolves chlorine:
/ClO
Ca + H2SO4 = CaSO4 + HCl + HClO,
\Cl
HCl + HClO = H2O + 2Cl.
This reaction can be employed in the preparation of chlorine, or the
nascent chlorine may be used as a bleaching agent.
2. It is slowly decomposed by the carbon dioxide of the air, yielding
calcium carbonate and chlorine:
CaOCl2 + CO2 = CaCO3 + 2Cl.
Owing to this slow action the substance is a good disinfectant.
3. When its solution is boiled the substance breaks down into calcium
chloride and chlorate:
6CaOCl2 = 5CaCl2 + Ca(ClO3)2.
This reaction is used in the preparation of potassium chlorate.[Pg 308]
Calcium fluoride (fluorspar) (CaF2). Fluorspar has already been
mentioned as the chief natural compound of fluorine. It is found in
large quantities in a number of localities, and is often crystallized in
perfect cubes of a light green or amethyst color. It can be melted
easily in a furnace, and is sometimes used in the fused condition in
metallurgical operations to protect a metal from the action of the air
during its reduction. It is used as the chief source of fluorine
compounds, especially hydrofluoric acid.
Calcium sulphate (gypsum) (CaSO4·2H2O). This abundant substance
occurs in very perfectly formed crystals or in massive deposits. It is
often found in solution in natural waters and in the sea water. Salts
deposited from sea water are therefore likely to contain this substance
(see Stassfurt salts).
It is very sparingly soluble in water, and is thrown down as a fine
white precipitate when any considerable amounts of a calcium salt and a
soluble sulphate (or sulphuric acid) are brought together in solution.
Its chief use is in the manufacture of plaster of Paris and of hollow
tiles for fireproof walls. Such material is called gypsite. It is also
used as a fertilizer.
Calcium sulphate, like the carbonate, occurs in many forms in nature.
Gypsum is a name given to all common varieties. Granular or massive
specimens are called alabaster, while all those which are well
crystallized are called selenite. Satin spar is still another variety
often seen in mineral collections.
Plaster of Paris. When gypsum is heated to about 115° it loses a portion
of its water of crystallization in accordance with the equation
2(CaSO4·2H2O) = 2CaSO4·H2O + 2H2O.
[Pg 309]
The product is a fine white powder called plaster of Paris. On being
moistened it again takes up this water, and in so doing first forms a
plastic mass, which soon becomes very firm and hard and regains its
crystalline structure. These properties make it very valuable as a
material for forming casts and stucco work, for cementing glass to
metals, and for other similar purposes. If overheated so that all water
is driven off, the process of taking up water is so slow that the
material is worthless. Such material is said to be dead burned. Plaster
of Paris is very extensively used as the finishing coat for plastered
walls.
Hard water. Waters containing compounds of calcium and magnesium in
solution are called hard waters because they feel harsh to the touch.
The hardness of water may be of two kinds,—(1) temporary hardness and
(2) permanent hardness.
1. Temporary hardness. We have seen that when water charged with
carbon dioxide comes in contact with limestone a certain amount of the
latter dissolves, owing to the formation of the soluble acid carbonate
of calcium. The hardness of such waters is said to be temporary, since
it may be removed by boiling. The heat changes the acid carbonate into
the insoluble normal carbonate which then precipitates, rendering the
water soft:
Ca(HCO3)2 = CaCO3 + H2O + CO2.
Such waters may also be softened by the addition of sufficient lime or
calcium hydroxide to convert the acid carbonate of calcium into the
normal carbonate. The equation representing the reaction is
Ca(HCO3)2 + Ca(OH)2 = 2CaCO3 + 2H2O.
[Pg 310]
2. Permanent hardness. The hardness of water may also be due to the
presence of calcium and magnesium sulphates or chlorides. Boiling the
water does not affect these salts; hence such waters are said to have
permanent hardness. They may be softened, however, by the addition of
sodium carbonate, which precipitates the calcium and magnesium as
insoluble carbonates:
CaSO4 + Na2CO3 = CaCO3 + Na2SO4.
This process is sometimes called "breaking" the water.
Commercial methods for softening water. The average water of a
city supply contains not only the acid carbonates of calcium
and magnesium but also the sulphates and chlorides of these
metals, together with other salts in smaller quantities. Such
waters are softened on a commercial scale by the addition of
the proper quantities of calcium hydroxide and sodium
carbonate. The calcium hydroxide is added first to precipitate
all the acid carbonates. After a short time the sodium
carbonate is added to precipitate the other soluble salts of
calcium and magnesium, together with any excess of calcium
hydroxide which may have been added. The quantity of calcium
hydroxide and sodium carbonate required is calculated from a
chemical analysis of the water. It will be noticed that the
water softened in this way will contain sodium sulphate and
chloride, but the presence of these salts is not objectionable.
Calcium carbide (CaC2). This substance is made by heating well-dried
coke and lime in an electrical furnace. The equation is
The pure carbide is a colorless, transparent, crystalline substance. In
contact with water it is decomposed with the evolution of pure acetylene
gas, having a pleasant ethereal odor. The commercial article is a dull
gray porous substance which contains many impurities. The acetylene
prepared from this substance has a very characteristic odor[Pg 311] due to
impurities, the chief of these being phosphine. It is used in
considerable quantities as a source of acetylene gas for illuminating
purposes.
Technical preparation. Fig. 81 represents a recent type of a
carbide furnace. The base of the furnace is provided with a
large block of carbon A, which serves as one of the
electrodes. The other electrodes B, several in number, are
arranged horizontally at some distance above this. A mixture of
coal and lime is fed into the furnace through the trap top C,
and in the lower part of the furnace this mixture becomes
intensely heated, forming liquid carbide. This is drawn off
through the taphole D.
The carbon monoxide formed in the reaction escapes through the
pipes E and is led back into the furnace. The pipes F
supply air, so that the monoxide burns as it reënters the
furnace and assists in heating the charge. The carbon dioxide
so formed, together with the nitrogen entering as air, escape
at G. An alternating current is used.
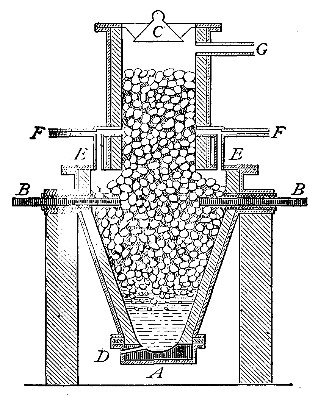 Fig. 81
Fig. 81
Calcium phosphate (Ca3(PO4)2). This important substance occurs
abundantly in nature as a constituent of apatite (3
Ca3(PO4)2·CaF2), in phosphate rock, and as the chief mineral
constituent of bones. Bone ash is therefore nearly pure calcium
phosphate. It is a white powder, insoluble in water, although it readily
dissolves in acids, being decomposed by them and converted into soluble
acid phosphates, as explained in connection with the acids of
phosphorus.[Pg 312]
STRONTIUM
Occurrence. Strontium occurs sparingly in nature, usually as
strontianite (SrCO3) and as celestite (SrSO4). Both minerals form
beautiful colorless crystals, though celestite is sometimes colored a
faint blue. Only a few of the compounds of strontium have any commercial
applications.
Strontium hydroxide (Sr(OH)2·8H2O). The method of preparation of
strontium hydroxide is analogous to that of calcium hydroxide. The
substance has the property of forming an insoluble compound with sugar,
which can easily be separated again into its constituents. It is
therefore sometimes used in the sugar refineries to extract sugar from
impure mother liquors from which the sugar will not crystallize.
Strontium nitrate (Sr(NO3)2·4H2O). This salt is prepared by
treating the native carbonate with nitric acid. When ignited with
combustible materials it imparts a brilliant crimson color to the flame,
and because of this property it is used in the manufacture of red
lights.
BARIUM
Barium is somewhat more abundant than strontium, occurring in nature
largely as barytes, or heavy spar (BaSO4), and witherite (BaCO3).
Like strontium, it closely resembles calcium both in the properties of
the metal and in the compounds which it forms.
Oxides of barium. Barium oxide (BaO) can be obtained by strongly heating
the nitrate:
Ba(NO3)2 = BaO + 2NO2 + O.
Heated to a low red heat in the air, the oxide combines with oxygen,
[Pg 313]forming the peroxide (BaO2). If the temperature is raised still
higher, or the pressure is reduced, oxygen is given off and the oxide is
once more formed. The reaction
is reversible and has been used as a means of separating oxygen from the
air. Treated with acids, barium peroxide yields hydrogen peroxide:
BaO2 + 2HCl = BaCl2 + H2O2.
Barium chloride (BaCl2·2H2O). Barium chloride is a white
well-crystallized substance which is easily prepared from the native
carbonate. It is largely used in the laboratory as a reagent to detect
the presence of sulphuric acid or soluble sulphates.
Barium sulphate (barytes) (BaSO4). Barium sulphate occurs in nature
in the form of heavy white crystals. It is precipitated as a crystalline
powder when a barium salt is added to a solution of a sulphate or
sulphuric acid:
BaCl2 + H2SO4 = BaSO4 + 2HCl.
This precipitate is used, as are also the finely ground native sulphate
and carbonate, as a pigment in paints. On account of its low cost it is
sometimes used as an adulterant of white lead, which is also a heavy
white substance.
Barium compounds color the flame green, and the nitrate (Ba(NO3)2)
is used in the manufacture of green lights. Soluble barium compounds are
poisonous.
RADIUM
Historical. In 1896 the French scientist Becquerel observed that the
mineral pitchblende possesses certain remarkable properties. It affects
photographic plates even in complete darkness, and discharges[Pg 314] a
gold-leaf electroscope when brought close to it. In 1898 Madam Curie
made a careful study of pitchblende to see if these properties belong to
it or to some unknown substance contained in it. She succeeded in
extracting from it a very small quantity of a substance containing a new
element which she named radium.
In 1910 Madam Curie succeeded in obtaining radium itself by the
electrolysis of radium chloride. It is a silver-white metal melting at
about 700°. It blackens in the air, forming a nitride, and decomposes
water. Its atomic weight is about 226.5.
Properties. Compounds of radium affect a photographic plate or
electroscope even through layers of paper or sheets of metal. They also
bring about chemical changes in substances placed near them.
Investigation of these strange properties has suggested that the radium
atoms are unstable and undergo a decomposition. As a result of this
decomposition very minute bodies, to which the name corpuscles has been
given, are projected from the radium atom with exceedingly great
velocity. It is to these corpuscles that the strange properties of
radium are due. It seems probable that the gas helium is in some way
formed during the decomposition of radium.
Two or three other elements, particularly uranium and thorium, have been
found to possess many of the properties of radium in smaller degree.
Radium and the atomic theory. If these views in regard to radium should
prove to be well founded, it will be necessary to modify in some
respects the conception of the atom as developed in a former chapter.
The atom would have to be regarded as a compound unit made up of several
parts. In a few cases, as in radium and uranium, it would appear that
this unit is unstable and undergoes transformation into more stable
combinations. This modification would not, in any essential way, be at
variance with the atomic theory as propounded by Dalton.
EXERCISES
1. What properties have the alkaline-earth metals in common with the
alkali metals? In what respects do they differ?
2. Write the equation for the reaction between calcium carbide and
water.
3. For what is calcium chlorate used?[Pg 315]
4. Could limestone be completely decomposed if heated in a closed
vessel?
5. Caves often occur in limestone. Account for their formation.
6. What is the significance of the term fluorspar? (Consult dictionary.)
7. Could calcium chloride be used in place of barium chloride in testing
for sulphates?
8. What weight of water is necessary to slake the lime obtained from 1
ton of pure calcium carbonate?
9. What weight of gypsum is necessary in the preparation of 1 ton of
plaster of Paris?
10. Write equations to represent the reactions involved in the
preparation of strontium hydroxide and strontium nitrate from
strontianite.
11. Write equations to represent the reactions involved in the
preparation of barium chloride from heavy spar.
12. Could barium hydroxide be used in place of calcium hydroxide in
testing for carbon dioxide?
[Pg 316]
CHAPTER XXV
THE MAGNESIUM FAMILY
| SYMBOL | ATOMIC WEIGHT | DENSITY | MELTING POINT | BOILING POINT | OXIDE |
| Magnesium | Mg | 24.36 | 1.75 | 750° | 920° | MgO |
| Zinc | Zn | 65.4 | 7.00 | 420° | 950° | ZnO |
| Cadmium | Cd | 112.4 | 8.67 | 320° | 778° | CdO |
The family. In the magnesium family are included the four elements:
magnesium, zinc, cadmium, and mercury. Between the first three of these
metals there is a close family resemblance, such as has been traced
between the members of the two preceding families. Mercury in some
respects is more similar to copper and will be studied in connection
with that metal.
1. Properties. When heated to a high temperature in the air each of
these metals combines with oxygen to form an oxide of the general
formula MO, in which M represents the metal. Magnesium decomposes
boiling water slowly, while zinc and cadmium have but little action on
it.
2. Compounds. The members of this group are divalent in nearly all
their compounds, so that the formulas of their salts resemble those of
the alkaline-earth metals. Like the alkaline-earth metals, their
carbonates and phosphates are insoluble in water. Their sulphates,
however, are readily soluble. Unlike both the alkali and alkaline-earth[Pg 317]
metals, their hydroxides are nearly insoluble in water. Most of their
compounds dissociate in such a way as to give a simple, colorless,
metallic ion.
MAGNESIUM
Occurrence. Magnesium is a very abundant element in nature, ranking a
little below calcium in this respect. Like calcium, it is a constituent
of many rocks and also occurs in the form of soluble salts.
Preparation. The metal magnesium, like most metals whose oxides are
difficult to reduce with carbon, was formerly prepared by heating the
anhydrous chloride with sodium:
MgCl2 + 2Na = 2NaCl + Mg.
It is now made by electrolysis, but instead of using as the electrolyte
the melted anhydrous chloride, which is difficult to obtain, the natural
mineral carnallite is used. This is melted in an iron pot which also
serves as the cathode in the electrolysis. A rod of carbon dipping into
the melted salt serves as the anode. The apparatus is very similar to
the one employed in the preparation of sodium.
Properties. Magnesium is a rather tough silvery-white metal of small
density. Air does not act rapidly upon it, but a thin film of oxide
forms upon its surface, dimming its bright luster. The common acids
dissolve it with the formation of the corresponding salts. It can be
ignited readily and in burning liberates much heat and gives a brilliant
white light. This light is very rich in the rays which affect
photographic plates, and the metal in the form of fine powder is
extensively used in the production of flash lights and for white lights
in pyrotechnic displays.[Pg 318]
Magnesium oxide (magnesia) (MgO). Magnesium oxide, sometimes called
magnesia or magnesia usta, resembles lime in many respects. It is much
more easily formed than lime and can be made in the same way,—by
igniting the carbonate. It is a white powder, very soft and light, and
is unchanged by heat even at very high temperatures. For this reason it
is used in the manufacture of crucibles, for lining furnaces, and for
other purposes where a refractory substance is needed. It combines with
water to form magnesium hydroxide, but much more slowly and with the
production of much less heat than in the case of calcium oxide.
Magnesium hydroxide (Mg(OH)2). The hydroxide formed in this way is
very slightly soluble in water, but enough dissolves to give the water
an alkaline reaction. Magnesium hydroxide is therefore a fairly strong
base. It is an amorphous white substance. Neither magnesia nor magnesium
salts have a very marked effect upon the system; and for this reason
magnesia is a very suitable antidote for poisoning by strong acids,
since any excess introduced into the system will have no injurious
effect.
Magnesium cement. A paste of magnesium hydroxide and water
slowly absorbs carbon dioxide from the air and becomes very
hard. The hardness of the product is increased by the presence
of a considerable amount of magnesium chloride in the paste.
The hydroxide, with or without the chloride, is used in the
preparation of cements for some purposes.
Magnesium carbonate (MgCO3). Magnesium carbonate is a very abundant
mineral. It occurs in a number of localities as magnesite, which is
usually amorphous, but sometimes forms pure crystals resembling calcite.
More commonly it is found associated with calcium carbonate.[Pg 319] The
mineral dolomite has the composition CaCO3·MgCO3. Limestone
containing smaller amounts of magnesium carbonate is known as dolomitic
limestone. Dolomite is one of the most common rocks, forming whole
mountain masses. It is harder and less readily attacked by acids than
limestone. It is valuable as a building stone and as ballast for
roadbeds and foundations. Like calcium carbonate, magnesium carbonate is
insoluble in water, though easily dissolved by acids.
Basic carbonate of magnesium. We should expect to find magnesium
carbonate precipitated when a soluble magnesium salt and a soluble
carbonate are brought together:
Na2CO3 + MgCl2 = MgCO3 + 2NaCl.
Instead of this, some carbon dioxide escapes and the product is found to
be a basic carbonate. The most common basic carbonate of magnesium has
the formula 4MgCO3·Mg(OH)2, and is sometimes called magnesia alba.
This compound is formed by the partial hydrolysis of the normal
carbonate at first precipitated:
5MgCO3 + 2H2O = 4MgCO3·Mg(OH)2 + H2CO3.
Magnesium chloride (MgCl2·6H2O). Magnesium chloride is found in
many natural waters and in many salt deposits (see Stassfurt salts). It
is obtained as a by-product in the manufacture of potassium chloride
from carnallite. As there is no very important use for it, large
quantities annually go to waste. When heated to drive off the water of
crystallization the chloride is decomposed as shown in the equation
MgCl2·6H2O = MgO + 2HCl + 5H2O.
[Pg 320]
Owing to the abundance of magnesium chloride, this reaction is being
used to some extent in the preparation of both magnesium oxide and
hydrochloric acid.
Boiler scale. When water which contains certain salts in
solution is evaporated in steam boilers, a hard insoluble
material called scale deposits in the boiler. The formation
of this scale may be due to several distinct causes.
1. To the deposit of calcium sulphate. This salt, while
sparingly soluble in cold water, is almost completely insoluble
in superheated water. Consequently it is precipitated when
water containing it is heated in a boiler.
2. To decomposition of acid carbonates. As we have seen,
calcium and magnesium acid carbonates are decomposed on
heating, forming insoluble normal carbonates:
Ca(HCO3)2 = CaCO3 + H2O + CO2.
3. To hydrolysis of magnesium salts. Magnesium chloride, and
to some extent magnesium sulphate, undergo hydrolysis when
superheated in solution, and the magnesium hydroxide, being
sparingly soluble, precipitates:
MgCl2 + 2H2O <--> Mg(OH)2 + 2HCl.
This scale adheres tightly to the boiler in compact layers and,
being a non-conductor of heat, causes much waste of fuel. It is
very difficult to remove, owing to its hardness and resistance
to reagents. Thick scale sometimes cracks, and the water coming
in contact with the overheated iron occasions an explosion.
Moreover, the acids set free in the hydrolysis of the magnesium
salts attack the iron tubes and rapidly corrode them. These
causes combine to make the formation of scale a matter which
occasions much trouble in cases where hard water is used in
steam boilers. Water containing such salts should be softened,
therefore, before being used in boilers.
Magnesium sulphate (Epsom salt) (MgSO4·7H2O). Like the chloride,
magnesium sulphate is found rather commonly in springs and in salt
deposits. A very large deposit of the almost pure salt has been found in
Wyoming. Its name[Pg 321] was given to it because of its abundant occurrence in
the waters of the Epsom springs in England.
Magnesium sulphate has many uses in the industries. It is used to a
small extent in the preparation of sodium and potassium sulphates, as a
coating for cotton cloth, in the dye industry, in tanning, and in the
manufacture of paints and laundry soaps. To some extent it is used in
medicine.
Magnesium silicates. Many silicates containing magnesium are known and
some of them are important substances. Serpentine, asbestos, talc, and
meerschaum are examples of such substances.
ZINC
Occurrence. Zinc never occurs free in nature. Its compounds have been
found in many different countries, but it is not a constituent of common
rocks and minerals, and its occurrence is rather local and confined to
definite deposits or pockets. It occurs chiefly in the following ores:
| Sphalerite (zinc blende) | ZnS. |
| Zincite | ZnO. |
| Smithsonite | ZnCO3. |
| Willemite | Zn2SiO4. |
| Franklinite | ZnO·Fe2O3. |
One fourth of the world's output of zinc comes from the United States,
Missouri being the largest producer.
Metallurgy. The ores employed in the preparation of zinc are chiefly the
sulphide, oxide, and carbonate. They are first roasted in the air, by
which process they are changed into oxide:
ZnCO3 = ZnO + CO2,
ZnS + 3O = ZnO + SO2.
[Pg 322]
The oxide is then mixed with coal dust, and the mixture is heated in
earthenware muffles or retorts, natural gas being used as fuel in many
cases. The oxide is reduced by this means to the metallic state, and the
zinc, being volatile at the high temperature reached, distills and is
collected in suitable receivers. At first the zinc collects in the form
of fine powder, called zinc dust or flowers of zinc, recalling the
formation under similar conditions of flowers of sulphur. Later, when
the whole apparatus has become warm, the zinc condenses to a liquid in
the receiver, from which it is drawn off into molds. Commercial zinc
often contains a number of impurities, especially carbon, arsenic, and
iron.
Physical properties. Pure zinc is a rather heavy bluish-white metal with
a high luster. It melts at about 420°, and if heated much above this
temperature in the air takes fire and burns with a very bright bluish
flame. It boils at about 950° and can therefore be purified by
distillation.
Many of the physical properties of zinc are much influenced by the
temperature and previous treatment of the metal. When cast into ingots
from the liquid state it becomes at ordinary temperatures quite hard,
brittle, and highly crystalline. At 150° it is malleable and can be
rolled into thin sheets; at higher temperatures it again becomes very
brittle. When once rolled into sheets it retains its softness and
malleability at ordinary temperatures. When melted and poured into water
it forms thin brittle flakes, and in this condition is called granulated
or mossy zinc.
Chemical properties. Zinc is tarnished superficially by moist air, but
beyond this is not affected by it. It does not decompose even boiling
water. When the metal is quite pure, sulphuric and hydrochloric acids
have scarcely any action upon it; when, however, it contains small[Pg 323]
amounts of other metals such as magnesium or arsenic, or when it is
merely in contact with metallic platinum, brisk action takes place and
hydrogen is evolved. For this reason, when pure zinc is used in the
preparation of hydrogen a few drops of platinum chloride are often added
to the solution to assist the chemical action. Nitric acid dissolves the
metal readily, with the formation of zinc nitrate and various reduction
products of nitric acid. The strong alkalis act upon zinc and liberate
hydrogen:
Zn + 2KOH = Zn(OK)2 + 2H.
The product of this reaction, potassium zincate, is a salt of zinc
hydroxide, which is thus seen to have acid properties, though it usually
acts as a base.
Uses of zinc. The metal has many familiar uses. Rolled into sheets, it
is used as a lining for vessels which are to contain water. As a thin
film upon the surface of iron (galvanized iron) it protects the iron
from rust. Iron is usually galvanized by dipping it into a bath of
melted zinc, but electrical methods are also employed. Zinc plates are
used in many forms of electrical batteries. In the laboratory zinc is
used in the preparation of hydrogen, and in the form of zinc dust as a
reducing agent.
One of the largest uses of zinc is in the manufacture of alloys. Brass,
an alloy of zinc and copper, is the most important of these; German
silver, consisting of copper, zinc, and nickel, has many uses; various
bronzes, coin metals, and bearing metals also contain zinc. Its ability
to alloy with silver finds application in the separation of silver from
lead (see silver).
Compounds of zinc. In general, the compounds of zinc are similar in
formula and appearance to those of magnesium,[Pg 324] but in other properties
they often differ markedly. A number of them have value in commercial
ways.
Zinc oxide (zinc white) (ZnO). Zinc oxide occurs in impure form in
nature, being colored red by manganese and iron compounds. It can be
prepared just like magnesium oxide, but is more often made by burning
the metal.
Zinc oxide is a pure white powder which becomes yellow on heating and
regains its white color when cold. It is much used as a white pigment in
paints, under the name of zinc white, and has the advantage over white
lead in that it is not changed in color by sulphur compounds, while lead
turns black. It is also used in the manufacture of rubber goods.
Commercial preparation of zinc oxide. Commercially it is often
made from franklinite in the following way. The franklinite is
mixed with coal and heated to a high temperature in a furnace,
by which process the zinc is set free and converted into vapor.
As the vapor leaves the furnace through a conduit it meets a
current of air and takes fire in it, forming zinc oxide. The
oxide passes on and is filtered from the air through canvas
bags, which allow the air to pass but retain the oxide. It is
thus made by burning the metal, though the metal is not
actually isolated in the process.
Soluble salts. The soluble salts of zinc can be made by dissolving the
metal or the oxide in the appropriate acid. They are all somewhat
poisonous. The sulphate and chloride are the most familiar.
Zinc sulphate (white vitriol) (ZnSO4·7H2O). This salt is readily
crystallized from strong solutions in transparent colorless crystals. It
is prepared commercially by careful roasting of the sulphide:
[Pg 325]
Zinc chloride (ZnCl2·H2O). When a solution of zinc chloride is
slowly evaporated a salt of the composition ZnCl2·H2O crystallizes
out. If the water is completely expelled by heat and the residue
distilled, the anhydrous chloride is obtained and may be cast into
sticks or broken into lumps. In this distillation, just as in heating
magnesium chloride, some of the chloride is decomposed:
The anhydrous chloride has a great affinity for water, and is used as a
dehydrating agent. It is also a germicide, and wood which is to be
exposed to conditions which favor decay, as, for example, railroad ties,
is often soaked in solutions of this salt.
Insoluble compounds. The insoluble compounds of zinc can be prepared by
precipitation. The most important are the sulphide, carbonate, and
hydroxide.
Zinc sulphide (ZnS). This substance occurs as the mineral sphalerite,
and is one of the most valued ores of zinc. Very large deposits occur in
southwestern Missouri. The natural mineral is found in large crystals or
masses, resembling resin in color and luster. When prepared by
precipitation the sulphide is white.
CADMIUM
The element. This element occurs in small quantities in some zinc ores.
In the course of the metallurgy of zinc the cadmium compounds undergo
chemical changes quite similar to those of the zinc compounds, and the
cadmium distills along with the zinc. Being more volatile, it comes over
with the first of the zinc and is prepared from the first portions of
the distillate by special methods of purification.[Pg 326] The element very
closely resembles zinc in most respects. Some of its alloys are
characterized by having low melting points.
Compounds of cadmium. Among the compounds of cadmium may be mentioned
the chloride (CdCl2·2H2O), the sulphate (3CdSO4·8H2O), and
the nitrate (Cd(NO3)2·4H2O). These are white solids soluble in
water. The sulphide (CdS) is a bright yellow substance which is
insoluble in water and in dilute acids. It is valuable as a pigment in
fine paints.
EXERCISES
1. What properties have the metals of the magnesium family in common
with the alkali metals; with the alkaline-earth metals?
2. Compare the action of the metals of the magnesium group on water with
that of the other metals studied.
3. What metals already studied are prepared by electrolysis?
4. Write the equations representing the reactions between magnesium and
hydrochloric acid; between magnesium and dilute sulphuric acid.
5. What property of magnesium was taken advantage of in the isolation of
argon?
6. With phosphoric acid magnesium forms salts similar to those of
calcium. Write the names and formulas of the corresponding magnesium
salts.
7. How could you distinguish between magnesium chloride and magnesium
sulphate? between Glauber's salts and Epsom salts?
8. What weight of carnallite is necessary in the preparation of 500 g.
of magnesium?
9. Account for the fact that paints made of zinc oxide are not colored
by hydrosulphuric acid.
10. What hydroxide studied, other than zinc hydroxide, has both acid and
basic properties?
11. Write equations showing how the following compounds of zinc may be
obtained from metallic zinc: the oxide, chloride, nitrate, carbonate,
sulphate, sulphide, hydroxide.
[Pg 327]
CHAPTER XXVI
THE ALUMINIUM FAMILY
The family. The element aluminium is the most abundant member of the
group of elements known as the aluminium family; indeed, the other
members of the family—gallium, indium, and thallium—are of such rare
occurrence that they need not be separately described. The elements of
the family are ordinarily trivalent, so that the formulas for their
compounds differ from those of the elements so far studied. Their
hydroxides are practically insoluble in water and are very weak bases;
indeed, the bases are so weak that their salts are often hydrolyzed into
free base and free acid in solution. The salts formed from these bases
usually contain water of crystallization, which cannot be driven off
without decomposing them more or less.
The trivalent metals, which in addition to aluminium include also iron
and chromium, are sometimes called the earth metals. The name refers
to the earthy appearance of the oxides of these metals, and to the fact
that many earths, soils, and rocks are composed in part of these
substances.
ALUMINIUM
Occurrence. Aluminium never occurs in the free state in nature, owing to
its great affinity for oxygen. In combined form, as oxides, silicates,
and a few other salts, it is both abundant and widely distributed, being
an essential[Pg 328] constituent of all soils and of most rocks excepting
limestone and sandstone. Cryolite (Na3AlF6), found in Greenland,
and bauxite, which is an aluminium hydroxide usually mixed with some
iron hydroxide, are important minerals. It is estimated that aluminium
composes about 8% of the earth's crust. In the industries the metal is
called aluminum, but its chemical name is aluminium.
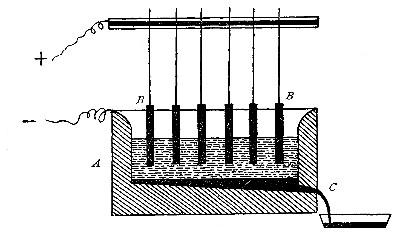 Fig. 82
Fig. 82
Preparation. Aluminium was first prepared by Wöhler, in 1827, by heating
anhydrous aluminium chloride with potassium:
This method was tried after it was found impossible to reduce the oxide
of aluminium with carbon. The metal possessed such interesting
properties and promised to be so useful that many efforts were made to
devise a cheap way of preparing it. The method which has proved most
successful consists in the electrolysis of the oxide dissolved in melted
cryolite.
[Pg 329]
Metallurgy. An iron box A (Fig. 82) about eight feet long and
six feet wide is connected with a powerful generator in such a
way as to serve as the cathode upon which the aluminium is
deposited. Three or four rows of carbon rods B dip into the
box and serve as the anodes. The box is partially filled with
cryolite and the current is turned on, generating enough heat
to melt the cryolite. Aluminium oxide is then added, and under
the influence of the electric current it decomposes into
aluminium and oxygen. The temperature is maintained above the
melting point of aluminium, and the liquid metal, being heavier
than cryolite, sinks to the bottom of the vessel, from which it
is tapped off from time to time through the tap hole C. The
oxygen in part escapes as gas, and in part combines with the
carbon of the anode, the combustion being very brilliant. The
process is carried on at Niagara Falls.
The largest expense in the process, apart from the cost of
electrical energy, is the preparation of aluminium oxide free
from other oxides, for most of the oxide found in nature is too
impure to serve without refining. Bauxite is the principal ore
used as a source of the aluminium because it is converted into
pure oxide without great difficulty. Since common clay is a
silicate of aluminium and is everywhere abundant, it might be
expected that this would be utilized in the preparation of
aluminium. It is, however, very difficult to extract the
aluminium from a silicate, and no practical method has been
found which will accomplish this.
Physical properties. Aluminium is a tin-white metal which melts at 640°
and is very light, having a density of 2.68. It is stiff and strong, and
with frequent annealing can be rolled into thin foil. It is a good
conductor of heat and electricity, though not so good as copper for a
given cross section of wire.
Chemical properties. Aluminium is not perceptibly acted on by boiling
water, and moist air merely dims its luster. Further action is prevented
in each case by the formation of an extremely thin film of oxide upon
the surface of the metal. It combines directly with chlorine, and when
heated in oxygen burns with great energy and the liberation of much
heat. It is therefore a good reducing agent. Hydrochloric acid acts upon
it, forming aluminium chloride:[Pg 330] nitric acid and dilute sulphuric acid
have almost no action on it, but hot, concentrated sulphuric acid acts
upon it in the same way as upon copper:
2Al + 6H2SO4 = Al2(SO4)3 + 6H2O + 3SO2.
Alkalis readily attack the metal, liberating hydrogen, as in the case of
zinc:
Al + 3KOH = Al(OK)3 + 3H.
Salt solutions, such as sea water, corrode the metal rapidly. It alloys
readily with other metals.
Uses of aluminium. These properties suggest many uses for the metal. Its
lightness, strength, and permanence make it well adapted for many
construction purposes. These same properties have led to its extensive
use in the manufacture of cooking utensils. The fact that it is easily
corroded by salt solutions is, however, a disadvantage. Owing to its
small resistance to electrical currents, it is replacing copper to some
extent in electrical construction, especially for trolley and power
wires. Some of its alloys have very valuable properties, and a
considerable part of the aluminium manufactured is used for this
purpose. Aluminium bronze, consisting of about 90% copper and 10%
aluminium, has a pure golden color, is strong and malleable, is easily
cast, and is permanent in the air. Considerable amounts of aluminium
steel are also made.
Goldschmidt reduction process. Aluminium is frequently employed as a
powerful reducing agent, many metallic oxides which resist reduction by
carbon being readily reduced by it. The aluminium in the form of a fine
powder is mixed with the metallic oxide, together with some substance
such as fluorspar to act as a flux. The mixture is ignited, and the
aluminium unites with the[Pg 331] oxygen of the metallic oxide, liberating the
metal. This collects in a fused condition under the flux.
An enormous quantity of heat is liberated in this reaction, and a
temperature as high as 3500° can be reached. The heat of the reaction is
turned to practical account in welding car rails, steel castings, and in
similar operations where an intense local heat is required. A mixture of
aluminium with various metallic oxides, ready prepared for such
purposes, is sold under the name of thermite.
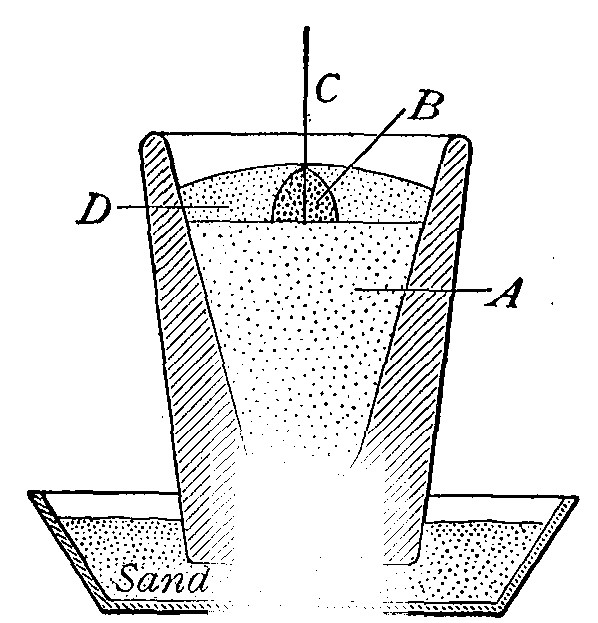 Fig. 83
Fig. 83
Preparation of chromium by the Goldschmidt method. A mixture of
chromium oxide and aluminium powder is placed in a Hessian
crucible (A, Fig. 83), and on top of it is placed a small
heap B of a mixture of sodium peroxide and aluminium, into
which is stuck a piece of magnesium ribbon C. Powdered
fluorspar D is placed around the sodium peroxide, after which
the crucible is set on a pan of sand and the magnesium ribbon
ignited. When the flame reaches the sodium peroxide mixture
combustion of the aluminium begins with almost explosive
violence, so that great care must be taken in the experiment.
The heat of this combustion starts the reaction in the chromium
oxide mixture, and the oxide is reduced to metallic chromium.
When the crucible has cooled a button of chromium will be found
in the bottom.
Aluminium oxide (Al2O3). This substance occurs in several forms in
nature. The relatively pure crystals are called corundum, while emery is
a variety colored dark gray or black, usually with iron compounds. In
transparent crystals, tinted different colors by traces of impurities,
it forms such precious stones as the sapphire, oriental ruby, topaz, and
amethyst. All these varieties are very[Pg 332] hard, falling little short of
the diamond in this respect. Chemically pure aluminium oxide can be made
by igniting the hydroxide, when it forms an amorphous white powder:
The natural varieties, corundum and emery, are used for cutting and
grinding purposes; the purest forms, together with the artificially
prepared oxide, are largely used in the preparation of aluminium.
Aluminium hydroxide (Al(OH)3). The hydroxide occurs in nature as the
mineral hydrargyllite, and in a partially dehydrated form called
bauxite. It can be prepared by adding ammonium hydroxide to any soluble
aluminium salt, forming a semi-transparent precipitate which is
insoluble in water but very hard to filter. It dissolves in most acids
to form soluble salts, and in the strong bases to form aluminates, as
indicated in the equations
Al(OH)3 + 3HCl = AlCl3 + 3H2O,
Al(OH)3 + 3NaOH = Al(ONa)3 + 3H2O.
It may act, therefore, either as a weak base or as a weak acid, its
action depending upon the character of the substances with which it is
in contact. When heated gently the hydroxide loses part of its hydrogen
and oxygen according to the equation
This substance, the formula of which is frequently written HAlO2, is
a more pronounced acid than is the hydroxide, and its salts are
frequently formed when aluminium compounds are fused with alkalis. The
magnesium salt Mg(AlO2)2 is called spinel, and many other of its
salts, called aluminates, are found in nature.[Pg 333]
When heated strongly the hydroxide is changed into oxide, which will not
again take up water on being moistened.
Mordants and dyeing. Aluminium hydroxide has the peculiar
property of combining with many soluble coloring materials and
forming insoluble products with them. On this account it is
often used as a filter to remove objectionable colors from
water. This property also leads to its wide use in the dye
industry. Many dyes will not adhere to natural fibers such as
cotton and wool, that is, will not "dye fast." If, however, the
cloth to be dyed is soaked in a solution of aluminium compounds
and then treated with ammonia, the aluminium salts which have
soaked into the fiber will be converted into the hydroxide,
which, being insoluble, remains in the body of it. If the fiber
is now dipped into a solution of the dye, the aluminium
hydroxide combines with the color material and fastens, or
"fixes," it upon the fiber. A substance which serves this
purpose is called a mordant, and aluminium salts,
particularly the acetate, are used in this way.
Aluminium chloride (AlCl3·6 H2O). This substance is prepared by
dissolving the hydroxide in hydrochloric acid and evaporating to
crystallization. When heated it is converted into the oxide, resembling
magnesium in this respect:
2(AlCl3·6 H2O) = Al2O3 + 6HCl + 9H2O.
The anhydrous chloride, which has some important uses, is made by
heating aluminium turnings in a current of chlorine.
Alums. Aluminium sulphate can be prepared by the action of sulphuric
acid upon aluminium hydroxide. It has the property of combining with the
sulphates of the alkali metals to form compounds called alums. Thus,
with potassium sulphate the reaction is expressed by the equation
K2SO4 + Al2(SO4)3 + 24H2O = 2(KAl(SO4)2·12H2O).
[Pg 334]
Under similar conditions ammonium sulphate yields ammonium alum:
(NH4)2SO4 + Al2(SO4)3 + 24H2O = 2(NH4Al(SO4)2·12H2O).
Other trivalent sulphates besides aluminium sulphate can form similar
compounds with the alkali sulphates, and these compounds are also called
alums, though they contain no aluminium. They all crystallize in
octahedra and contain twelve molecules of water of crystallization. The
alums most frequently prepared are the following:
| Potassium alum | KAl(SO4)2·12H2O. |
| Ammonium alum | NH4Al(SO4)2·12H2O. |
| Ammonium iron alum | NH4Fe(SO4)2·12H2O. |
| Potassium chrome alum | KCr(SO4)2·12H2O. |
An alum may therefore be regarded as a compound derived from two
molecules of sulphuric acid, in which one hydrogen atom has been
displaced by the univalent alkali atom, and the other three hydrogen
atoms by an atom of one of the trivalent metals, such as aluminium,
iron, or chromium.
Very large, well-formed crystals of an alum can be prepared by
suspending a small crystal by a thread in a saturated solution
of the alum, as shown in Fig. 84. The small crystal slowly
grows and assumes a very perfect form.
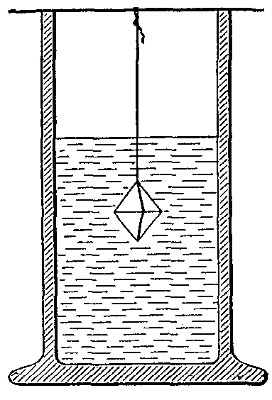 Fig. 84
Fig. 84
Other salts of aluminium. While aluminium hydroxide forms fairly stable
salts with strong acids, it is such a weak base that its salts with weak
acids are readily hydrolyzed. Thus, when an aluminium salt and a soluble
carbonate are[Pg 335] brought together in solution we should expect to have
aluminium carbonate precipitated according to the equation
3Na2CO3 + 2AlCl3 = Al2(CO3)3 + 6NaCl.
But if it is formed at all, it instantly begins to hydrolyze, the
products of the hydrolysis being aluminium hydroxide and carbonic acid,
Al2(CO3)3 + 6H2O = 2Al(OH)3 + 3H2CO3.
Similarly a soluble sulphide, instead of precipitating aluminium
sulphide (Al2S3), precipitates aluminium hydroxide; for hydrogen
sulphide is such a weak acid that the aluminium sulphide at first formed
hydrolyzes at once, forming aluminium hydroxide and hydrogen sulphide:
3Na2S + 2AlCl3 + 6H2O = 2Al(OH)3 + 6NaCl + 3H2S.
Alum baking powders. It is because of the hydrolysis of aluminium
carbonate that alum is used as a constituent of some baking powders. The
alum baking powders consist of a mixture of alum and sodium hydrogen
carbonate. When water is added the two compounds react together, forming
aluminium carbonate, which hydrolyzes into aluminium hydroxide and
carbonic acid. The carbon dioxide from the latter escapes through the
dough and in so doing raises it into a porous condition, which is the
end sought in the use of a baking powder.
Aluminium silicates. One of the most common constituents of rocks is
feldspar (KAlSi3O8), a mixed salt of potassium and aluminium with
the polysilicic acid (H4Si3O8). Under the influence of
moisture, carbon dioxide, and changes of temperature this substance is
constantly being broken down into soluble potassium compounds and
hydrated aluminium silicate. This compound has the formula
Al2Si2O7·2H2O. In relatively pure condition it is called
kaolin; in the impure state, mixed with sand and other[Pg 336] substances, it
forms common clay. Mica is another very abundant mineral, having varying
composition, but being essentially of the formula KAlSiO4.
Serpentine, talc, asbestos, and meerschaum are important complex
silicates of aluminium and magnesium, and granite is a mechanical
mixture of quartz, feldspar, and mica.
Ceramic industries. Many articles of greatest practical
importance, ranging from the roughest brick and tile to the
finest porcelain and chinaware, are made from some form of
kaolin, or clay. No very precise classification of such ware
can be made, as the products vary greatly in properties,
depending upon the materials used and the treatment during
manufacture.
Porcelain is made from the purest kaolin, to which must be
added some less pure, plastic kaolin, since the pure substance
is not sufficiently plastic. There is also added some more
fusible substance, such as feldspar, gypsum, or lime, together
with some pure quartz. The constituents must be ground very
fine, and when thoroughly mixed and moistened must make a
plastic mass which can be molded into any desired form. The
article molded from such materials is then burned. In this
process the article is slowly heated to a point at which it
begins to soften and almost fuse, and then it is allowed to
cool slowly. At this stage, a very thin vessel will be
translucent and have an almost glassy fracture; if, however, it
is somewhat thicker, or has not been heated quite so high, it
will still be porous, and partly on this account and partly to
improve its appearance it is usually glazed.
Glazing is accomplished by spreading upon the object a thin
layer of a more fusible mixture of the same materials as
compose the body of the object itself, and again heating until
the glaze melts to a transparent glassy coating upon the
surface of the vessel. In some cases fusible mixtures of quite
different composition from that used in fashioning the vessel
may be used as a glaze. Oxides of lead, zinc, and barium are
often used in this way.
When less carefully selected materials are used, or quite thick
vessels are made, various grades of stoneware are produced. The
inferior grades are glazed by throwing a quantity of common
salt into the kiln towards the end of the first firing. In the
form of vapor[Pg 337] the salt attacks the surface of the baked ware
and forms an easily fusible sodium silicate upon it, which
constitutes a glaze.
Vitrified bricks, made from clay or ground shale, are burned
until the materials begin to fuse superficially, forming their
own glaze. Other forms of brick and tile are not glazed at all,
but are left porous. The red color of ordinary brick and
earthenware is due to an oxide of iron formed in the burning
process.
The decorations upon china are sometimes painted upon the baked
ware and then glazed over, and sometimes painted upon the glaze
and burned in by a third firing. Care must be taken to use such
pigments as are not affected by a high heat and do not react
chemically with the constituents of the baked ware or the
glaze.
EXERCISES
1. What metals and compounds studied are prepared by electrolysis?
2. Write the equation for the reaction between aluminium and
hydrochloric acid; between aluminium and sulphuric acid (in two steps).
3. What hydroxides other than aluminium hydroxide have both acid and
basic properties?
4. Write equations showing the methods used for preparing aluminium
hydroxide and sulphate.
5. Write the general formula of an alum, representing an atom of an
alkali metal by X and an atom of a trivalent metal by Y.
6. What is meant by the term polysilicic acid, as used in the discussion
of aluminium silicates?
7. Compare the properties of the hydroxides of the different groups of
metals so far studied.
8. In what respects does aluminium oxide differ from calcium oxide in
properties?
9. Supposing bauxite to be 90% aluminium hydroxide, what weight of it is
necessary for the preparation of 100 kg. of aluminium?
[Pg 338]
CHAPTER XXVII
THE IRON FAMILY
| SYMBOL | ATOMIC WEIGHT | DENSITY | APPROXIMATE MELTING POINT | OXIDES |
| Iron | Fe | 55.9 | 7.93 | 1800° | FeO, Fe2O3 |
| Cobalt | Co | 59.0 | 8.55 | 1800° | CoO, Co2O3 |
| Nickel | Ni | 58.7 | 8.9 | 1600° | NiO, Ni2O3 |
The family. The elements iron, cobalt, and nickel form a group in the
eighth column of the periodic table. The atomic weights of the three are
very close together, and there is not the same gradual gradation in the
properties of the three elements that is noticed in the families in
which the atomic weights differ considerably in magnitude. The elements
are very similar in properties, the similarity being so great in the
case of nickel and cobalt that it is difficult to separate them by
chemical analysis.
The elements occur in nature chiefly as oxides and sulphides, though
they have been found in very small quantities in the native state,
usually in meteorites. Their sulphides, carbonates, and phosphates are
insoluble in water, the other common salts being soluble. Their salts
are usually highly colored, those of iron being yellow or light green as
a rule, those of nickel darker green, while cobalt salts are usually
rose colored. The metals are obtained by reducing the oxides with
carbon.[Pg 339]
IRON
Occurrence. The element iron has long been known, since its ores are
very abundant and it is not difficult to prepare the metal from them in
fairly pure condition. It occurs in nature in many forms of
combination,—in large deposits as oxides, sulphides, and carbonates,
and in smaller quantities in a great variety of minerals. Indeed, very
few rocks or soils are free from small amounts of iron, and it is
assimilated by plants and animals playing an important part in life
processes.
Metallurgy. It will be convenient to treat of the metallurgy of iron
under two heads,—Materials Used and Process.
Materials used. Four distinct materials are used in the metallurgy of
iron:
1. Iron ore. The ores most frequently used in the metallurgy
of iron are the following:
| Hematite | Fe2O3. |
| Magnetite | Fe3O4. |
| Siderite | FeCO3. |
| Limonite | 2Fe2O2·3H2O. |
These ores always contain impurities, such as silica,
sulphides, and earthy materials. All ores, with the exception
of the oxides, are first roasted to expel any water and carbon
dioxide present and to convert any sulphide into oxide.
2. Carbon. Carbon in some form is necessary both as a fuel
and as a reducing agent. In former times wood charcoal was used
to supply the carbon, but now anthracite coal or coke is almost
universally used.
3. Hot air. To maintain the high temperature required for the
reduction of iron a very active combustion of fuel[Pg 340] is
necessary. This is secured by forcing a strong blast of hot air
into the lower part of the furnace during the reduction
process.
4. Flux. (a) Purpose of the flux. All the materials which
enter the furnace must leave it again either in the form of
gases or as liquids. The iron is drawn off as the liquid metal
after its reduction. To secure the removal of the earthy matter
charged into the furnace along with the ore, materials are
added to the charge which will, at the high temperature of the
furnace, combine with the impurities in the ore, forming a
liquid. The material added for this purpose is called the
flux; the liquid produced from the flux and the ore is called
slag.
(b) Function of the slag. While the main purpose of adding
flux to the charge is to remove from the furnace in the form of
liquid slag the impurities originally present in the ore, the
slag thus produced serves several other functions. It keeps the
contents of the furnace in a state of fusion, thus preventing
clogging, and makes it possible for the small globules of iron
to run together with greater ease into one large liquid mass.
(c) Character of the slag. The slag is really a kind of
readily fusible glass, being essentially a calcium-aluminium
silicate. The ore usually contains silica and some aluminium
compounds, so that limestone (which also contains some silica
and aluminium) is added to furnish the calcium required for the
slag. If the ore and the limestone do not contain a sufficient
amount of silica and aluminium for the formation of the slag,
these ingredients are added in the form of sand and feldspar.
In the formation of slag from these materials the ore is freed
from the silica and aluminium which it contained.
[Pg 341]
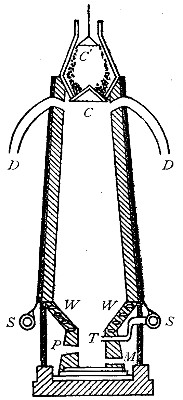 Fig. 85
Fig. 85
Process. The reduction of iron is carried out in large towers called
blast furnaces. The blast furnace (Fig. 85) is usually about 80 ft. high
and 20 ft. in internal diameter at its widest part, narrowing somewhat
both toward the top and toward the bottom. The walls are built of steel
and lined with fire-brick. The base is provided with a number of pipes
T, called tuyers, through which hot air can be forced into the
furnace. The tuyers are supplied from a large pipe S, which circles
the furnace as a girdle. The base has also an opening M, through which
the liquid metal can be drawn off from time to time, and a second
opening P, somewhat above the first, through which the excess of slag
overflows. The top is closed by a movable trap C and C', called the
cone, and through this the materials to be used are introduced. The
gases produced by the combustion of the fuel and the reduction of the
ore, together with the nitrogen of the air forced in through the tuyers,
escape through pipes D, called downcomer pipes, which leave the
furnace near the top. These gases are very hot and contain combustible
substances, principally carbon monoxide; they are therefore utilized as
fuel for the engines and also to heat the blast admitted through the
tuyers. The lower part of the furnace is often furnished with a water
jacket. This consists of a series of pipes W built into the walls,
through which water can be circulated to reduce their temperature.[Pg 342]
Charges consisting of coke (or anthracite coal), ore, and flux in proper
proportions are introduced into the furnace at intervals through the
trap top. The coke burns fiercely in the hot-air blast, giving an
intense heat and forming carbon monoxide. The ore, working down in the
furnace as the coke burns, becomes very hot, and by the combined
reducing action of the carbon and carbon monoxide is finally reduced to
metal and collects as a liquid in the bottom of the furnace, the slag
floating on the molten iron. After a considerable amount of the iron has
collected the slag is drawn off through the opening P. The molten iron
is then drawn off into large ladles and taken to the converters for the
manufacture of steel, or it is run out into sand molds, forming the bars
or ingots called "pigs." The process is a continuous one, and when once
started it is kept in operation for months or even years without
interruption.
It seems probable that the first product of combustion of the
carbon, at the point where the tuyers enter the furnace, is
carbon dioxide. This is at once reduced to carbon monoxide by
the intensely heated carbon present, so that no carbon dioxide
can be found at that point. For practical purposes, therefore,
we may consider that carbon monoxide is the first product of
combustion.
Varieties of iron. The iron of commerce is never pure, but contains
varying amounts of other elements, such as carbon, silicon, phosphorus,
sulphur, and manganese. These elements may either be alloyed with the
iron or may be combined with it in the form of definite chemical
compounds. In some instances, as in the case of graphite, the mixture
may be merely mechanical.
The properties of iron are very much modified by the presence of these
elements and by the form of the combination between them and the iron;
the way in which the[Pg 343] metal is treated during its preparation has also a
marked influence on its properties. Owing to these facts many kinds of
iron are recognized in commerce, the chief varieties being cast iron,
wrought iron, and steel.
Cast iron. The product of the blast furnace, prepared as just described,
is called cast iron. It varies considerably in composition, usually
containing from 90 to 95% iron, the remainder being largely carbon and
silicon with smaller amounts of phosphorus and sulphur. When the melted
metal from the blast furnace is allowed to cool rapidly most of the
carbon remains in chemical combination with the iron, and the product is
called white cast iron. If the cooling goes on slowly, the carbon
partially separates as flakes of graphite which remain scattered through
the metal. This product is softer and darker in color and is called gray
cast iron.
Properties of cast iron. Cast iron is hard, brittle, and rather easily
melted (melting point about 1100°). It cannot be welded or forged into
shape, but is easily cast in sand molds. It is strong and rigid but not
elastic. It is used for making castings and in the manufacture of other
kinds of iron. Cast iron, which contains the metal manganese up to the
extent of 20%, together with about 3% carbon, is called spiegel iron;
when more than this amount of manganese is present the product is called
ferromanganese. The ferromanganese may contain as much as 80% manganese.
These varieties of cast iron are much used in the manufacture of steel.
Wrought iron. Wrought iron is made by burning out from cast iron most of
the carbon, silicon, phosphorus, and sulphur which it contains. The
process is called puddling, and is carried out in a furnace
constructed as represented[Pg 344] in Fig. 86. The floor of the furnace F is
somewhat concave and is made of iron covered with a layer of iron oxide.
A long flame produced by burning fuel upon the grate G is directed
downward upon the materials placed upon the floor, and the draught is
maintained by the stack S. A is the ash box and T a trap to catch
the solid particles carried into the stack by the draught. Upon the
floor of the furnace is placed the charge of cast iron, together with a
small amount of material to make a slag. The iron is soon melted by the
flame directed upon it, and the sulphur, phosphorus, and silicon are
oxidized by the iron oxide, forming oxides which are anhydrides of
acids. These combine with the flux, which is basic in character, or with
the iron oxide, to form a slag. The carbon is also oxidized and escapes
as carbon dioxide. As the iron is freed from other elements it becomes
pasty, owing to the higher melting point of the purer iron, and in this
condition forms small lumps which are raked together into a larger one.
The large lump is then removed from the furnace and rolled or hammered
into bars, the slag; being squeezed out in this process. The product has
a stranded or fibrous structure. The product of a puddling furnace is
called wrought iron.
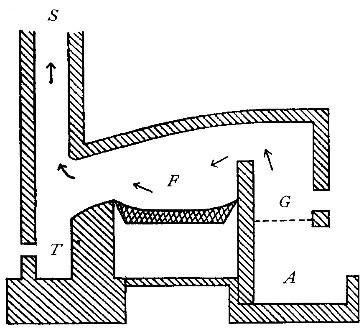 Fig. 86
Fig. 86
Properties of wrought iron. Wrought iron is nearly pure iron, usually
containing about 0.3% of other substances, chiefly carbon. It is tough,
malleable, and fibrous[Pg 345] in structure. It is easily bent and is not
elastic, so it will not sustain pressure as well as cast iron. It can be
drawn out into wire of great tensile strength, and can also be rolled
into thin sheets (sheet iron). It melts at a high temperature (about
1600°) and is therefore forged into shape rather than cast. If melted,
it would lose its fibrous structure and be changed into a low carbon
steel.
Steel. Steel, like wrought iron, is made by burning out from cast iron a
part of the carbon, silicon, phosphorus, and sulphur which it contains;
but the process is carried out in a very different way, and usually,
though not always, more carbon is found in steel than in wrought iron. A
number of processes are in use, but nearly all the steel of commerce is
made by one of the two following methods.
 Fig. 87
Fig. 87
1. Bessemer process. This process, invented about 1860, is by far the
most important. It is carried out in great egg-shaped crucibles called
converters (Fig. 87), each one of which will hold as much as 15 tons of
steel. The converter is built of steel and lined with silica. It is
mounted on trunnions T, so that it can be tipped over on its side for
filling and emptying. One of the trunnions is hollow and a pipe P
connects it with an air chamber A, which forms a false bottom to the
converter. The true bottom is perforated, so that air[Pg 346] can be forced in
by an air blast admitted through the trunnion and the air chamber.
White-hot, liquid cast iron from a blast furnace is run into the
converter through its open necklike top O, the converter being tipped
over to receive it; the air blast is then turned on and the converter
rotated to a nearly vertical position. The elements in the iron are
rapidly oxidized, the silicon first and then the carbon. The heat
liberated in the oxidation, largely due to the combustion of silicon,
keeps the iron in a molten condition. When the carbon is practically all
burned out cast iron or spiegel iron, containing a known percentage of
carbon, is added and allowed to mix thoroughly with the fluid. The steel
is then run into molds, and the ingots so formed are hammered or rolled
into rails or other forms. By this process any desired percentage of
carbon can be added to the steel. Low carbon steel, which does not
differ much from wrought iron in composition, is now made in this way
and is replacing the more expensive wrought iron for many purposes.
The basic lining process. When the cast iron contains
phosphorus and sulphur in appreciable quantities, the lining of
the converter is made of dolomite. The silicon and carbon burn,
followed by the phosphorus and sulphur, and the anhydrides of
acids so formed combine with the basic oxides of the lining,
forming a slag. This is known as the basic lining process.
2. Open-hearth process. In this process a furnace very similar to a
puddling furnace is used, but it is lined with silica or dolomite
instead of iron oxide. A charge consisting in part of old scrap iron of
any kind and in part of cast iron is melted in the furnace by a gas
flame. The silicon and carbon are slowly burned away, and when a test
shows that the desired percentage of carbon is present the steel[Pg 347] is run
out of the furnace. Steel may therefore be defined as the product of
the Bessemer or open-hearth processes.
Properties of steel. Bessemer and open-hearth steel usually contain only
a few tenths of a per cent of carbon, less than 0.1% silicon, and a very
much smaller quantity of phosphorus and sulphur. Any considerable amount
of the latter elements makes the steel brittle, the sulphur affecting it
when hot, and the phosphorus when cold. This kind of steel is used for
structural purposes, for rails, and for nearly all large steel articles.
It is hard, malleable, ductile, and melts at a lower temperature than
wrought iron. It can be forged into shape, rolled into sheets, or cast
in molds.
Relation of the three varieties of iron. It will be seen that wrought
iron is usually very nearly pure iron, while steel contains an
appreciable amount of alloy material, chiefly carbon, and cast iron
still more of the same substances. It is impossible, however, to assign
a given sample of iron to one of these three classes on the basis of its
chemical composition alone. A low carbon steel, for example, may contain
less carbon than a given sample of wrought iron. The real distinction
between the three is the process by which they are made. The product of
the blast furnace is cast iron; that of the puddling furnace is wrought
iron; that of the Bessemer and open-hearth methods is steel.
Tool steel. Steel designed for use in the manufacture of edged tools and
similar articles should be relatively free from silicon and phosphorus,
but should contain from 0.5 to 1.5% carbon. The percentage of carbon
should be regulated by the exact use to which the steel is to be put.
Steel of this character is usually made in small lots from either
Bessemer or open-hearth steel in the following way.[Pg 348]
A charge of melted steel is placed in a large crucible and the
calculated quantity of pure carbon is added. The carbon dissolves in the
steel, and when the solution is complete the metal is poured out of the
crucible. This is sometimes called crucible steel.
Tempering of steel. Steel containing from 0.5 to 1.5% carbon is
characterized by the property of "taking temper." When the hot steel is
suddenly cooled by plunging it into water or oil it becomes very hard
and brittle. On carefully reheating this hard form it gradually becomes
less brittle and softer, so that by regulating the temperature to which
steel is reheated in tempering almost any condition of temper demanded
for a given purpose, such as for making springs or cutting tools, can be
obtained.
Steel alloys. It has been found that small quantities of a number of
different elements when alloyed with steel very much improve its quality
for certain purposes, each element having a somewhat different effect.
Among the elements most used in this connection are manganese, silicon,
chromium, nickel, tungsten, and molybdenum.
The usual method for adding these elements to the steel is to first
prepare a very rich alloy of iron with the element to be added, and then
add enough of this alloy to a large quantity of the steel to bring it to
the desired composition. A rich alloy of iron with manganese or silicon
can be prepared directly in a blast furnace, and is called
ferromanganese or ferrosilicon. Similar alloys of iron with the other
elements mentioned are made in an electric furnace by reducing the mixed
oxides with carbon.
Pure iron. Perfectly pure iron is rarely prepared and is not adapted to
commercial uses. It can be made by reducing pure oxide of iron in a
current of hydrogen at a[Pg 349] high temperature. Prepared in this way it
forms a black powder; when melted it forms a tin-white metal which is
less fusible and more malleable than wrought iron. It is easily acted
upon by moist air.
Compounds of iron. Iron differs from the metals so far studied in that
it is able to form two series of compounds in which the iron has two
different valences. In the one series the iron is divalent and forms
compounds which in formulas and many chemical properties are similar to
the corresponding zinc compounds. It can also act as a trivalent metal,
and in this condition forms salts similar to those of aluminium. Those
compounds in which the iron is divalent are known as ferrous
compounds, while those in which it is trivalent are known as ferric.
Oxides of iron. Iron forms several oxides. Ferrous oxide (FeO) is not
found in nature, but can be prepared artificially in the form of a black
powder which easily takes up oxygen, forming ferric oxide:
Ferric oxide is the most abundant ore of iron and occurs in great
deposits, especially in the Lake Superior region. It is found in many
mineral varieties which vary in density and color, the most abundant
being hematite, which ranges in color from red to nearly black. When
prepared by chemical processes it forms a red powder which is used as a
paint pigment (Venetian red) and as a polishing powder (rouge).
Magnetite has the formula Fe3O4 and is a combination of FeO and
Fe2O3. It is a very valuable ore, but is less abundant than
hematite. It is sometimes called magnetic oxide of iron, or lodestone,
since it is a natural magnet.[Pg 350]
Ferrous salts. These salts are obtained by dissolving iron in the
appropriate acid, or, when insoluble, by precipitation. They are usually
light green in color and crystallize well. In chemical reactions they
are quite similar to the salts of magnesium and zinc, but differ from
them in one important respect, namely, that they are easily changed into
compounds in which the metal is trivalent. Thus ferrous chloride treated
with chlorine or aqua regia is changed into ferric chloride:
Ferrous hydroxide exposed to moist air is rapidly changed into ferric
hydroxide:
2Fe(OH)2 + H2O + O = 2Fe(OH)3.
Ferrous sulphate (copperas, green vitriol) (FeSO4·7H2O). Ferrous
sulphate is the most familiar ferrous compound. It is prepared
commercially as a by-product in the steel-plate mills. Steel plates are
cleaned by the action of dilute sulphuric acid upon them, and in the
process some of the iron dissolves. The liquors are concentrated and the
green vitriol separates from them.
Ferrous sulphide (FeS). Ferrous sulphide is sometimes found in nature as
a golden-yellow crystalline mineral. It is formed as a black precipitate
when a soluble sulphide and an iron salt are brought together in
solution:
FeSO4 + Na2S = FeS + Na2SO4.
It can also be made as a heavy dark-brown solid by fusing together the
requisite quantities of sulphur and iron. It is obtained as a by-product
in the metallurgy of lead:
[Pg 351]
It is used in the laboratory in the preparation of hydrosulphuric acid:
FeS + 2HCl = FeCl2 + H2S.
Iron disulphide (pyrites) (FeS2). This substance bears the same
relation to ferrous sulphide that hydrogen dioxide does to water. It
occurs abundantly in nature in the form of brass-yellow cubical crystals
and in compact masses. Sometimes the name "fool's gold" is applied to it
from its superficial resemblance to the precious metal. It is used in
very large quantities as a source of sulphur dioxide in the manufacture
of sulphuric acid, since it burns readily in the air, forming ferric
oxide and sulphur dioxide:
2FeS2 + 11O = Fe2O3 + 4SO2.
Ferrous carbonate (FeCO3). This compound occurs in nature as
siderite, and is a valuable ore. It will dissolve to some extent in
water containing carbon dioxide, just as will calcium carbonate, and
waters containing it are called chalybeate waters. These chalybeate
waters are supposed to possess certain medicinal virtues and form an
important class of mineral waters.
Ferric salts. Ferric salts are usually obtained by treating an acidified
solution of a ferrous salt with an oxidizing agent:
2FeCl2 + 2HCl + O = 2FeCl3 + H2O,
2FeSO4 + H2SO4 + O = Fe2(SO4)3 + H2O.
They are usually yellow or violet in color, are quite soluble, and as a
rule do not crystallize well. Heated with water in the absence of free
acid, they hydrolyze even more readily than the salts of aluminium. The
most familiar ferric salts are the chloride and the sulphate.[Pg 352]
Ferric chloride (FeCl3). This salt can be obtained most conveniently
by dissolving iron in hydrochloric acid and then passing chlorine into
the solution:
Fe + 2HCl = FeCl2 + 2H,
FeCl2 + Cl = FeCl3.
When the pure salt is heated with water it is partly hydrolyzed:
FeCl3 + 3 H2O <--> Fe(OH)3 + 3HCl.
This is a reversible reaction, however, and hydrolysis can therefore be
prevented by first adding a considerable amount of the soluble product
of the reaction, namely, hydrochloric acid.
Ferric sulphate (Fe2(SO4)3). This compound can be made by
treating an acid solution of green vitriol with an oxidizing agent. It
is difficult to crystallize and hard to obtain in pure condition. When
an alkali sulphate in proper quantity is added to ferric sulphate in
solution an iron alum is formed, and is easily obtained in large
crystals. The best known iron alums have the formulas
KFe(SO4)2·12H2O and NH4Fe(SO4)2·12H2O. They are
commonly used when a pure ferric salt is required.
Ferric hydroxide (Fe(OH)3). When solutions of ferric salts are
treated with ammonium hydroxide, ferric hydroxide is formed as a
rusty-red precipitate, insoluble in water.
Iron cyanides. A large number of complex cyanides containing iron are
known, the most important being potassium ferrocyanide, or yellow
prussiate of potash (K4FeC6N6), and potassium ferricyanide, or
red prussiate of potash (K3FeC6N6). These compounds are the
potassium salts of the complex acids of the formulas H4FeC6N6
[Pg 353]and H3FeC6N6.
Oxidation of ferrous salts. It has just been seen that when a ferrous
salt is treated with an oxidizing agent in the presence of a free acid a
ferric salt is formed:
2FeSO4 + H2SO4 + O = Fe2(SO4)3 + H2O.
In this reaction oxygen is used up, and the valence of the iron is
changed from 2 to 3. The same equation may be written
2Fe++, 2SO4- + 2H+, SO4- + O = 2Fe+++, 3SO4- + H2O.
Hydrogen ions have been oxidized to water, while the charge of each iron
ion has been increased from 2 to 3.
In a similar way the conversion of ferrous chloride into ferric chloride
may be written
Fe++, 2Cl- + Cl = Fe+++, + 3Cl-.
Here again the valence of the iron and the charge on the iron ion has
been increased from 2 to 3, though no oxygen has entered into the
reaction. As a rule, however, changes of this kind are brought about by
the use of an oxidizing agent, and are called oxidations.
The term "oxidation" is applied to all reactions in which the valence of
the metal of a compound is increased, or, in other words, to all
reactions in which the charge of a cation is increased.
Reduction of ferric salts. The changes which take place when a ferric
salt is converted into a ferrous salt are the reverse of the ones just
described. This is seen in the equation
In this reaction the valence of the iron has been changed from 3 to 2.
The same equation may be written
Fe+++, 3Cl- + H = Fe++, + H+ + 3Cl-
[Pg 354]
It will be seen that the charge of the iron ions has been diminished
from 3 to 2. Since these changes are the reverse of the oxidation
changes just considered, they are called reduction reactions. The term
"reduction" is applied to all processes in which the valence of the
metal of a compound is diminished, or, in other words, to all processes
in which the charge on the cations is diminished.
NICKEL AND COBALT
These elements occur sparingly in nature, usually combined with arsenic
or with arsenic and sulphur. Both elements have been found in the free
state in meteorites. Like iron they form two series of compounds, but
the salts corresponding to the ferrous salts are the most common, the
ones corresponding to the ferric salts being difficult to obtain. Thus
we have the chlorides NiCl2·6H2O and CoCl2·6H2O; the
sulphates NiSO4·7H2O and CoSO4·7H2O; the nitrates
Ni(NO3)2·6H2O and Co(NO3)2·6H2O.
Nickel is largely used as an alloy with other metals. Alloyed with
copper it forms coin metal from which five-cent pieces are made, with
copper and zinc it forms German silver, and when added to steel in small
quantities nickel steel is formed which is much superior to common steel
for certain purposes. When deposited by electrolysis upon the surface of
other metals such as iron, it forms a covering which will take a high
polish and protects the metal from rust, nickel not being acted upon by
moist air. Salts of nickel are usually green.
Compounds of cobalt fused with glass give it an intensely blue color. In
powdered form such glass is sometimes used[Pg 355] as a pigment called smalt.
Cobalt salts, which contain water of crystallization, are usually cherry
red in color; when dehydrated they become blue.
EXERCISES
1. In the manufacture of cast iron, why is the air heated before being
forced into the furnace?
2. Write the equations showing how each of the following compounds of
iron could be obtained from the metal itself: ferrous chloride, ferrous
hydroxide, ferrous sulphate, ferrous sulphide, ferrous carbonate, ferric
chloride, ferric sulphate, ferric hydroxide.
3. Account for the fact that a solution of sodium carbonate, when added
to a solution of a ferric salt, precipitates an hydroxide and not a
carbonate.
4. Calculate the percentage of iron in each of the common iron ores.
5. One ton of steel prepared by the Bessemer process is found by
analysis to contain 0.2% carbon. What is the minimum weight of carbon
which must be added in order that the steel may be made to take a
temper?
[Pg 356]
CHAPTER XXVIII
COPPER, MERCURY, AND SILVER
| | | | | | FORMULAS OF OXIDES |
| | SYMBOL | ATOMIC WEIGHT | DENSITY | MELTING POINT | "ous" | "ic" |
| Copper | Cu | 63.6 | 8.89 | 1084° | Cu2O | CuO |
| Mercury | Hg | 200.00 | 13.596 | -39.5° | Hg2O | HgO |
| Silver | Ag | 107.93 | 10.5 | 960° | Ag2O | AgO |
The family. By referring to the periodic arrangement of the elements
(page 168), it will be seen that mercury is not included in the same
family with copper and silver. Since the metallurgy of the three
elements is so similar, however, and since they resemble each other so
closely in chemical properties, it is convenient to class them together
for study.
1. Occurrence. The three elements occur in nature to some extent in
the free state, but are usually found as sulphides. Their ores are easy
to reduce.
2. Properties. They are heavy metals of high luster and are especially
good conductors of heat and electricity. They are not very active
chemically. Neither hydrochloric nor dilute sulphuric acid has any
appreciable action upon them. Concentrated sulphuric acid attacks all
three, forming metallic sulphates and evolving sulphur dioxide, while
nitric acid, both dilute and concentrated, converts them into nitrates
with the evolution of oxides of nitrogen.[Pg 357]
3. Two series of salts. Copper and mercury form oxides of the types
M2O and MO, as well as two series of salts. In one series the metals
are univalent and the salts have formulas like those of the sodium
salts. They are called cuprous and mercurous salts. In the other series
the metals are divalent and resemble magnesium salts in formulas. These
are called cupric and mercuric salts. Silver forms only one series of
salts, being always a univalent metal.
COPPER
Occurrence. The element copper has been used for various purposes since
the earliest days of history. It is often found in the metallic state in
nature, large masses of it occurring pure in the Lake Superior region
and in other places to a smaller extent. The most valuable ores are the
following:
| Cuprite | Cu2O. |
| Chalcocite | Cu2S. |
| Chalcopyrite | CuFeS2. |
| Bornite | Cu3FeS3. |
| Malachite | CuCO3·Cu(OH)2. |
| Azurite | 2CuCO3·Cu(OH)2. |
Metallurgy of copper. Ores containing little or no sulphur are easy to
reduce. They are first crushed and the earthy impurities washed away.
The concentrated ore is then mixed with carbon and heated in a furnace,
metallic copper resulting from the reduction of the copper oxide by the
hot carbon.
Metallurgy of sulphide ores. Much of the copper of commerce is
made from chalcopyrite and bornite, and these ores are more
difficult to work. They are first roasted in the air, by which
treatment much of the sulphur is burned to sulphur dioxide. The
roasted ore is then[Pg 358] melted in a small blast furnace or in an
open one like a puddling furnace. In melting, part of the iron
combines with silica to form a slag of iron silicate. The
product, called crude matte, contains about 50% copper together
with sulphur and iron. Further purification is commonly carried
on by a process very similar to the Bessemer process for steel.
The converter is lined with silica, and a charge of matte from
the melting furnace, together with sand, is introduced, and air
is blown into the mass. By this means the sulphur is
practically all burned out by the air, and the remaining iron
combines with silica and goes off as slag. The copper is poured
out of the converter and molded into anode plates for refining.
Refining of copper. Impure copper is purified by electrolysis. A large
plate of it, serving as an anode, is suspended in a tank facing a thin
plate of pure copper, which is the cathode. The tank is filled with a
solution of copper sulphate and sulphuric acid to serve as the
electrolyte. A current from a dynamo passes from the anode to the
cathode, and the copper, dissolving from the anode, is deposited upon
the cathode in pure form, while the impurities collect on the bottom of
the tank. Electrolytic copper is one of the purest of commercial metals
and is very nearly pure copper.
Recovery of gold and silver. Gold and silver are often present
in small quantities in copper ores, and in electrolytic
refining these metals collect in the muddy deposit on the
bottom of the tank. The mud is carefully worked over from time
to time and the precious metals extracted from it. A surprising
amount of gold and silver is obtained in this way.
Properties of copper. Copper is a rather heavy metal of density 8.9, and
has a characteristic reddish color. It is rather soft and is very
malleable, ductile, and flexible, yet tough and strong; it melts at
1084°. As a conductor of heat and electrical energy it is second only to
silver.[Pg 359]
Hydrochloric acid, dilute sulphuric acid, and fused alkalis are almost
without action upon it; nitric acid and hot, concentrated sulphuric
acid, however, readily dissolve it. In moist air it slowly becomes
covered with a thin layer of green basic carbonate; heated in the air it
is easily oxidized to black copper oxide (CuO).
Uses. Copper is extensively used for electrical purposes, for roofs and
cornices, for sheathing the bottom of ships, and for making alloys. In
the following table the composition of some of these alloys is
indicated:
COMPOSITION OF ALLOYS OF COPPER IN PERCENTAGES
| Aluminium bronze | copper (90 to 97%), aluminium (3 to 10%). |
| Brass | copper (63 to 73%), zinc (27 to 37%). |
| Bronze | copper (70 to 95%), zinc (1 to 25%), tin (1 to 18%). |
| German silver | copper (56 to 60%), zinc (20%), nickel (20 to 25%). |
| Gold coin | copper (10%), gold (90%). |
| Gun metal | copper (90%), tin (10%). |
| Nickel coin | copper (75%), nickel (25%) |
| Silver coin | copper (10%), silver (90%). |
Electrotyping. Matter is often printed from electrotype plates
which are prepared as follows. The matter is set up in type and
wax is firmly pressed down upon the face of it until a clear
impression is obtained. The impressed side of the wax is coated
with graphite and the impression is made the cathode in an
electrolytic cell containing a copper salt in solution. When
connected with a current the copper is deposited as a thin
sheet upon the letters in wax, and when detached is a perfect
copy of the type, the under part of the letters being hollow.
The sheet is strengthened by pouring on the under surface a
suitable amount of molten metal (commercial lead is used). The
sheet so strengthened is then used in printing.
Two series of copper compounds. Copper, like iron, forms two series of
compounds: in the cuprous compounds it is univalent; in the cupric it is
divalent. The cupric salts[Pg 360] are much the more common of the two, since
the cuprous salts pass readily into cupric by oxidation.
Cuprous compounds. The most important cuprous compound is the oxide
(Cu2O), which occurs in nature as ruby copper or cuprite. It is a
bright red substance and can easily be prepared by heating copper to a
high temperature in a limited supply of air. It is used for imparting a
ruby color to glass.
By treating cuprous oxide with different acids a number of cuprous salts
can be made. Many of these are insoluble in water, the chloride (CuCl)
being the best known. When suspended in dilute hydrochloric acid it is
changed into cupric chloride, the oxygen taking part in the reaction
being absorbed from the air:
2CuCl + 2HCl + O = 2CuCl2 + H2O.
Cupric compounds. Cupric salts are easily made by dissolving cupric
oxide in acids, or, when insoluble, by precipitation. Most of them are
blue or green in color, and the soluble ones crystallize well. Since
they are so much more familiar than the cuprous salts, they are
frequently called merely copper salts.
Cupric oxide (CuO). This is a black insoluble substance obtained by
heating copper in excess of air, or by igniting the hydroxide or
nitrate. It is used as an oxidizing agent.
Cupric hydroxide (Cu(OH)2). The hydroxide prepared by treating a
solution of a copper salt with sodium hydroxide is a light blue
insoluble substance which easily loses water and changes into the oxide.
Heat applied to the liquid containing the hydroxide suspended in it
serves to bring about the reaction represented by the equation
[Pg 361]
Cupric sulphate (blue vitriol) (CuSO4·5H2O). This substance,
called blue vitriol or bluestone, is obtained as a by-product in a
number of processes and is produced in very large quantities. It forms
large blue crystals, which lose water when heated and crumble to a white
powder. The salt finds many uses, especially in electrotyping and in
making electrical batteries.
Cupric sulphide (CuS). The insoluble black sulphide (CuS) is easily
prepared by the action of hydrosulphuric acid upon a solution of a
copper salt:
CuSO4 + H2S = CuS + H2SO4.
It is insoluble in water and dilute acids.
MERCURY
Occurrence. Mercury occurs in nature chiefly as the sulphide (HgS)
called cinnabar, and in globules of metal inclosed in the cinnabar. The
mercury mines of Spain have long been famous, California being the next
largest producer.
Metallurgy. Mercury is a volatile metal which has but little affinity
for oxygen. Sulphur, on the other hand, readily combines with oxygen.
These facts make the metallurgy of mercury very simple. The crushed ore,
mixed with a small amount of carbon to reduce any oxide or sulphate that
might be formed, is roasted in a current of air. The sulphur burns to
sulphur dioxide, while the mercury is converted into vapor and is
condensed in a series of condensing vessels. The metal is purified by
distillation.
Properties. Mercury is a heavy silvery liquid with a density of 13.596.
It boils at 357° and solidifies at -39.5°.[Pg 362] Small quantities of many
metals dissolve in it, forming liquid alloys, while with larger
quantities it forms solid alloys. The alloys of mercury are called
amalgams.
Toward acids mercury conducts itself very much like copper; it is easily
attacked by nitric and hot, concentrated sulphuric acids, while cold
sulphuric and hydrochloric acids have no effect on it.
Uses. Mercury is extensively used in the construction of scientific
instruments, such as the thermometer and barometer, and as a liquid over
which to collect gases which are soluble in water. The readiness with
which it alloys with silver and gold makes it very useful in the
extraction of these elements.
Compounds of mercury. Like copper, mercury forms two series of
compounds: the mercurous, of which mercurous chloride (HgCl) is an
example; and the mercuric, represented by mercuric chloride (HgCl2).
Mercuric oxide (HgO). Mercuric oxide can be obtained either as a
brick-red or as a yellow substance. When mercuric nitrate is heated
carefully the red modification is formed in accordance with the equation
Hg(NO3)2 = HgO + 2NO2 + O.
The yellow modification is prepared by adding a solution of a mercuric
salt to a solution of sodium or potassium hydroxide:
Hg(NO3)2 + 2NaOH = 2NaNO3 + Hg(OH)2,
Hg(OH)2 = HgO + H2O.
When heated the oxide darkens until it becomes almost black; at a higher
temperature it decomposes into mercury and oxygen. It was by this
reaction that oxygen was discovered.[Pg 363]
Mercurous chloride (calomel) (HgCl). Being insoluble, mercurous
chloride is precipitated as a white solid when a soluble chloride is
added to a solution of mercurous nitrate:
HgNO3 + NaCl = HgCl + NaNO3.
Commercially it is manufactured by heating a mixture of mercuric
chloride and mercury. When exposed to the light it slowly changes into
mercuric chloride and mercury:
It is therefore protected from the light by the use of colored bottles.
It is used in medicine.
Most mercurous salts are insoluble in water, the principal soluble one
being the nitrate, which is made by the action of cold, dilute nitric
acid on mercury.
Mercuric chloride (corrosive sublimate) (HgCl2). This substance can
be made by dissolving mercuric oxide in hydrochloric acid. On a
commercial scale it is made by subliming a mixture of common salt and
mercuric sulphate:
2NaCl + HgSO4 = HgCl2 + Na2SO4.
The mercuric chloride, being readily volatile, vaporizes and is
condensed again in cool vessels. Like mercurous chloride it is a white
solid, but differs from it in that it is soluble in water. It is
extremely poisonous and in dilute solutions is used as an antiseptic in
dressing wounds.
Mercuric sulphide (HgS). As cinnabar this substance forms the chief
native compound of mercury, occurring in red crystalline masses. By
passing hydrosulphuric acid into a solution of a mercuric salt it is
precipitated as a black powder, insoluble in water and acids. By other
means it can be prepared as a brilliant red powder known as vermilion,
which is used as a pigment in fine paints.[Pg 364]
The iodides of mercury. If a solution of potassium iodide is
added to solutions of a mercurous and a mercuric salt
respectively, the corresponding iodides are precipitated.
Mercuric iodide is the more important of the two, and as
prepared above is a red powder which changes to yellow on
heating to 150°. The yellow form on cooling changes back again
to the red form, or may be made to do so by rubbing it with a
knife blade or some other hard object.
SILVER
Occurrence. Silver is found in small quantities in the uncombined state;
usually, however, it occurs in combination with sulphur, either as the
sulphide (Ag2S) or as a small constituent of other sulphides,
especially those of lead and copper. It is also found alloyed with gold.
Metallurgy. Parkes's process. Silver is usually smelted in connection
with lead. The ores are worked over together, as described under lead,
and the lead and silver obtained as an alloy, the silver being present
in small quantity. The alloy is melted and metallic zinc is stirred in.
Zinc will alloy with silver but not with lead, and it is found that the
silver leaves the lead and, in the form of an alloy with zinc, forms as
a crust upon the lead and is skimmed off. This crust, which, of course,
contains lead adhering to it, is partially melted and the most of the
lead drained off. The zinc is removed by distillation, and the residue
is melted on an open hearth in a current of air; by this means the zinc
and lead remaining with the silver are changed into oxides and the
silver remains behind unaltered.
Amalgamation process. In some localities the old amalgamation
process is used. The silver ore is treated with common salt and
ferrous compounds, which process converts the silver first into
chloride and then into metallic silver. Mercury is then added
and thoroughly mixed with the mass, forming an amalgam with the
silver. After[Pg 365] some days the earthy materials are washed away
and the heavier amalgam is recovered. The mercury is distilled
off and the silver left in impure form.
Refining silver. The silver obtained by either of the above processes
may still contain copper, gold, and iron, and is refined by "parting"
with sulphuric acid. The metal is heated with strong sulphuric acid
which dissolves the silver, copper, and iron present, but not the gold.
In the solution of silver sulphate so obtained copper plates are
suspended, upon which the pure silver precipitates, the copper going
into solution as sulphate, as shown in the equation
Ag2SO4 + Cu = 2Ag + CuSO4.
The solution obtained as a by-product in this process furnishes most of
the blue vitriol of commerce. Silver is also refined by electrolytic
methods similar to those used in refining copper.
Properties of silver. Silver is a heavy, rather soft, white metal, very
ductile and malleable and capable of taking a high polish. It surpasses
all other metals as a conductor of heat and electricity, but is too
costly to find extensive use for such purposes. It melts at a little
lower temperature than copper (961°). It alloys readily with other heavy
metals, and when it is to be used for coinage a small amount of
copper—from 8 to 10%—is nearly always melted with it to give it
hardness.
It is not acted upon by water or air, but is quickly tarnished when in
contact with sulphur compounds, turning quite black in time.
Hydrochloric acid and fused alkalis do not act upon it, but nitric acid
and hot, concentrated sulphuric acid dissolve it with ease.[Pg 366]
 Fig. 88
Fig. 88
Electroplating. Since silver is not acted upon by water or air,
and has a pleasing appearance, it is used to coat various
articles made of cheaper metals. Such articles are said to be
silver plated. The process by which this is done is called
electroplating. It is carried on as follows: The object to be
plated (such as a spoon) is attached to a wire and dipped into
a solution of a silver salt. Electrical connection is made in
such a way that the article to be plated serves as the cathode,
while the anode is made up of one or more plates of silver
(Fig. 88, A). When a current is passed through the
electrolyte silver dissolves from the anode plate and deposits
on the cathode in the form of a closely adhering layer. By
making the proper change in the electrolyte and anode plate
objects may be plated with gold and other metals.
Compounds of silver. Silver forms two oxides but only one series of
salts, namely, the one which corresponds to the mercurous and cuprous
series.
Silver nitrate (lunar caustic) (AgNO3). This salt is easily
prepared by dissolving silver in nitric acid and evaporating the
resulting solution. It crystallizes in flat plates, and when heated
carefully can be melted without decomposition. When cast into sticks it
is called lunar caustic, for it has a very corrosive action on flesh,
and is sometimes used in surgery to burn away abnormal growths.
The alchemists designated the metals by the names of the
heavenly bodies. The moon (luna) was the symbol for silver;
hence the name "lunar caustic."
Silver sulphide (Ag2S). This occurs in nature and constitutes one of
the principal ores of silver. It can be[Pg 367] obtained in the form of a black
solid by passing hydrosulphuric acid through a solution of silver
nitrate.
Compounds of silver with the halogens. The chloride, bromide, and iodide
of silver are insoluble in water and acids, and are therefore
precipitated by bringing together a soluble halogen salt with silver
nitrate:
AgNO3 + KCl = AgCl + KNO3.
They are remarkable for the fact that they are very sensitive to the
action of light, undergoing a change of color and chemical composition
when exposed to sunlight, especially if in contact with organic matter
such as gelatin.
Photography. The art of photography is based on the fact that
the halogen compounds of silver are affected by the light,
particularly in the presence of organic matter. From a chemical
standpoint the processes involved may be described under two
heads: (1) the preparation of the negative; (2) the preparation
of the print.
1. Preparation of the negative. The plate used in the
preparation of the negative is made by spreading a thin layer
of gelatin, in which silver bromide is suspended (silver iodide
is sometimes added also), over a glass plate or celluloid film
and allowing it to dry. When the plate so prepared is placed in
a camera and the image of some object is focused upon it, the
silver salt undergoes a change which is proportional at each
point to the intensity of the light falling upon it. In this
way an image of the object photographed is produced upon the
plate, which is, however, invisible and is therefore called
"latent." It can be made visible by the process of developing.
To develop the image the exposed plate is immersed in a
solution of some reducing agent called the developer. The
developer reduces that portion of the silver salt which has
been affected by the light, depositing it in the form of black
metallic silver which closely adheres to the plate.
The unaffected silver salt, upon which the developer has no
action, must now be removed from the plate. This is done by
immersing the plate in a solution of sodium thiosulphate
(hypo). After the silver salt has been dissolved off, the plate
is washed with water and[Pg 368] dried. The plate so prepared is
called the negative because it is a picture of the object
photographed, with the lights exactly reversed. This is called
fixing the negative.
2. Preparation of the print. The print is made from paper
which is prepared in the same way as the negative plate. The
negative is placed upon this paper and exposed to the light in
such a way that the light must pass through the negative before
striking the paper. If the paper is coated with silver
chloride, a visible image is produced, in which case a
developer is not needed. The proofs are made in this way. In
order to make them permanent the unchanged silver chloride must
be dissolved off with sodium thiosulphate. The print is then
toned by dipping it into a solution of gold or platinum salts.
The silver on the print passes into solution, while the gold or
platinum takes its place. These metals give a characteristic
color or tone to the print, the gold making it reddish brown,
while the platinum gives it a steel-gray tone. If a silver
bromide paper is used in making the print, a latent image is
produced which must be developed as in the case of the negative
itself. The silver bromide is much more sensitive than the
chloride, so that the printing can be done in artificial light.
Since the darkest places on the negative cut off the most
light, it is evident that the lights of the print will be the
reverse of those of the negative, and will therefore correspond
to those of the object photographed. The print is therefore
called the positive.
EXERCISES
1. Account for the fact that copper has been used for so long a time.
2. Write equations for the action of concentrated sulphuric and nitric
acids upon the metals of this family.
3. How would you account for the fact that normal copper sulphate is
slightly acid to litmus?
4. Contrast the action of heat on cupric nitrate and mercuric nitrate.
5. State reasons why mercury is adapted for use in thermometers and
barometers.
6. How could you distinguish between mercurous chloride and mercuric
chloride?
7. Write equations for the preparation of mercuric and mercurous
iodides.[Pg 369]
8. How would you account for the fact that solutions of the different
salts of a metal usually have the same color?
9. Crude silver usually contains iron and lead. What would become of
these metals in refining by parting with sulphuric acid?
10. In the amalgamation process for extracting silver, how does ferrous
chloride convert silver chloride into silver? Write equation. Why is the
silver sulphide first changed into silver chloride?
11. What impurities would you expect to find in the copper sulphate
prepared from the refining of silver?
12. How could you prepare pure silver chloride from a silver coin?
13. Mercuric nitrate and silver nitrate are both white solids soluble in
water. How could you distinguish between them?
14. Account for the fact that sulphur waters turn a silver coin black;
also for the fact that a silver spoon is blackened by foods (eggs, for
example) containing sulphur.
15. When a solution of silver nitrate is added to a solution of
potassium chlorate no precipitate forms. How do you account for the fact
that a precipitate of silver chloride is not formed?
[Pg 370]
CHAPTER XXIX
TIN AND LEAD
| SYMBOL | ATOMIC WEIGHT | DENSITY | MELTING POINT | COMMON OXIDES |
| Tin | Sn | 119.0 | 7.35 | 235° | SnO SnO2 |
| Lead | Pb | 206.9 | 11.38 | 327° | PbO Pb3O4 PbO2 |
The family. Tin and lead, together with silicon and germanium, form a
family in Group IV of the periodic table. Silicon has been discussed
along with the non-metals, while germanium, on account of its rarity,
needs only to be mentioned.
The other family of Group IV includes carbon, already described, and a
number of rare elements.
TIN
Occurrence. Tin is found in nature chiefly as the oxide (SnO2),
called cassiterite or tinstone. The most famous mines are those of
Cornwall in England, and of the Malay Peninsula and East India Islands;
in small amounts tinstone is found in many other localities.
Metallurgy. The metallurgy of tin is very simple. The ore, separated as
far as possible from earthy materials, is mixed with carbon and heated
in a furnace, the reduction taking place readily. The equation is
[Pg 371]
The metal is often purified by carefully heating it until it is partly
melted; the pure tin melts first and can be drained away from the
impurities.
Properties. Pure tin, called block tin, is a soft white metal with a
silver-like appearance and luster; it melts readily (235°) and is
somewhat lighter than copper, having a density of 7.3. It is quite
malleable and can be rolled out into very thin sheets, forming tin foil;
most tin foil, however, contains a good deal of lead.
Under ordinary conditions it is quite unchanged by air or moisture, but
at a high temperature it burns in air, forming the oxide SnO2. Dilute
acids have no effect upon it, but concentrated acids attack it readily.
Concentrated hydrochloric acid changes it into the chloride
With sulphuric acid tin sulphate and sulphur dioxide are formed:
Sn + 2H2SO4 = SnSO4 + SO2 + 2H2O
Concentrated nitric acid oxidizes it, forming a white insoluble compound
of the formula H2SnO3, called metastannic acid:
3Sn + 4HNO3 + H2O = 3H2SnO3 + 4NO.
Uses of tin. A great deal of tin is made into tin plate by dipping thin
steel sheets into the melted metal. Owing to the way in which tin
resists the action of air and dilute acids, tin plate is used in many
ways, such as in roofing, and in the manufacture of tin cans, cooking
vessels, and similar articles.
Many useful alloys contain tin, some of which have been mentioned in
connection with copper. When tin is alloyed with other metals of low
melting point, soft, easily[Pg 372] melted alloys are formed which are used for
friction bearings in machinery; tin, antimony, lead, and bismuth are the
chief constituents of these alloys. Pewter and soft solder are alloys of
tin and lead.
Compounds of tin. Tin forms two series of compounds: the stannous, in
which the tin is divalent, illustrated in the compounds SnO, SnS,
SnCl2; the stannic, in which it is tetravalent as shown in the
compounds SnO2, SnS2. There is also an acid, H2SnO3, called
stannic acid, which forms a series of salts called stannates. While this
acid has the same composition as metastannic acid, the two are quite
different in their chemical properties. This difference is probably due
to the different arrangement of the atoms in the molecules of the two
substances. Only a few compounds of tin need be mentioned.
Stannic oxide (SnO2). Stannic oxide is of interest, since it is the
chief compound of tin found in nature. It is sometimes found in
good-sized crystals, but as prepared in the laboratory is a white
powder. When fused with potassium hydroxide it forms potassium stannate,
acting very much like silicon dioxide:
SnO2 + 2KOH = K2SnO3 + H2O.
Chlorides of tin. Stannous chloride is prepared by dissolving tin in
concentrated hydrochloric acid and evaporating the solution to
crystallization. The crystals which are obtained have the composition
SnCl2·2H2O, and are known as tin crystals. By treating a solution
of stannous chloride with aqua regia, stannic chloride is formed:
The salt which crystallizes from such a solution has the composition
[Pg 373]SnCl4·5H2O, and is known commercially as oxymuriate of tin. If
metallic tin is heated in a current of dry chlorine, the anhydrous
chloride (SnCl4) is obtained as a heavy colorless liquid which fumes
strongly on exposure to air.
The ease with which stannous chloride takes up chlorine to form stannic
chloride makes it a good reducing agent in many reactions, changing the
higher chlorides of metals to lower ones. Thus mercuric chloride is
changed into mercurous chloride:
SnCl2 + 2HgCl2 = SnCl4 + 2HgCl.
If the stannous chloride is in excess, the reaction may go further,
producing metallic mercury:
SnCl2 + 2HgCl = SnCl4 + 2Hg.
Ferric chloride is in like manner reduced to ferrous chloride:
SnCl3 + 2FeCl3 = SnCl4 + 2FeCl2.
The chlorides of tin, as well as the alkali stannates, are much used as
mordants in dyeing processes. The hydroxides of tin and free stannic
acid, which are easily liberated from these compounds, possess in very
marked degree the power of fixing dyes upon fibers, as explained under
aluminium.
LEAD
Occurrence. Lead is found in nature chiefly as the sulphide (PbS),
called galena; to a much smaller extent it occurs as carbonate,
sulphate, chromate, and in a few other forms. Practically all the lead
of commerce is made from galena, two general methods of metallurgy being
in use.
Metallurgy. 1. The sulphide is melted with scrap iron, when iron
sulphide and metallic lead are formed; the[Pg 374] liquid lead, being the
heavier, sinks to the bottom of the vessel and can be drawn off:
2. The sulphide is roasted in the air until a part of it has been
changed into oxide and sulphate. The air is then shut off and the
heating continued, the reactions indicated in the following equations
taking place:
2PbO + PbS = 3Pb + SO2,
PbSO4 + PbS = 2Pb + 2SO2.
The lead so prepared usually contains small amounts of silver, arsenic,
antimony, copper, and other metals. The silver is removed by Parkes's
method, as described under silver, and the other metals in various ways.
The lead of commerce is one of the purest commercial metals, containing
as a rule only a few tenths per cent of impurities.
Properties. Lead is a heavy metal (den. = 11.33) which has a brilliant
silvery luster on a freshly cut surface, but which soon tarnishes to a
dull blue-gray color. It is soft, easily fused (melting at 327°), and
quite malleable, but has little toughness or strength.
It is not acted upon to any great extent by the oxygen of the air under
ordinary conditions, but is changed into oxide at a high temperature.
With the exception of hydrochloric and sulphuric acids, most acids, even
very weak ones, act upon it, forming soluble lead salts. Hot,
concentrated hydrochloric and sulphuric acids also attack it to a slight
extent.
Uses. Lead is employed in the manufacture of lead pipes and in large
storage batteries. In the form of sheet lead it is used in lining the
chambers of sulphuric acid[Pg 375] works and in the preparation of paint
pigments. Some alloys of lead, such as solder and pewter (lead and tin),
shot (lead and arsenic), and soft bearing metals, are widely used. Type
metal consists of lead, antimony, and sometimes tin. Compounds of lead
form several important pigments.
Compounds of lead. In nearly all its compounds lead has a valence of 2,
but a few corresponding to stannic compounds have a valence of 4.
Lead oxides. Lead forms a number of oxides, the most important of which
are litharge, red lead or minium, and lead peroxide.
1. Litharge (PbO). This oxide forms when lead is oxidized at a rather
low temperature, and is obtained as a by-product in silver refining. It
is a pale yellow powder, and has a number of commercial uses. It is
easily soluble in nitric acid:
PbO + 2HNO3 = Pb(NO3)2 + H2O.
2. Red lead, or minium (Pb3O4). Minium is prepared by heating
lead (or litharge) to a high temperature in the air. It is a heavy
powder of a beautiful red color, and is much used as a pigment.
3. Lead peroxide (PbO2). This is left as a residue when minium is
heated with nitric acid:
Pb3O4 + 4HNO3 = 2Pb(NO3)2 + PbO2 + 2H2O.
It is a brown powder which easily gives up a part of its oxygen and,
like manganese dioxide and barium dioxide, is a good oxidizing agent.
Soluble salts of lead. The soluble salts of lead can be made by
dissolving litharge in acids. Lead acetate
(Pb(C2H3O2)2·3H2O), called sugar of lead, and lead
[Pg 376]nitrate (Pb(NO3)2) are the most familiar examples. They are while
crystalline solids and are poisonous in character.
Insoluble salts of lead; lead carbonate. While the normal carbonate of
lead (PbCO3) is found to some extent, in nature and can be prepared
in the laboratory, basic carbonates of varying composition are much more
easy to obtain. One of the simplest of these has the composition
2PbCO3·Pb(OH)2. A mixture of such carbonates is called white lead.
This is prepared on a large scale as a paint pigment and as a body for
paints which are to be colored with other substances.
White lead. White lead is an amorphous white substance which,
when mixed with oil, has great covering power, that is, it
spreads out in an even waxy film, free from streaks and lumps,
and covers the entire surface upon which it is spread. Its
disadvantage as a pigment lies in the fact that it gradually
blackens when exposed to sulphur compounds, which are often
present in the air, forming black lead sulphide (PbS).
Technical preparation of white lead. Different methods are used
in the preparation of white lead, but the old one known as the
Dutch process is still the principal one employed. In this
process, earthenware pots about ten inches high and of the
shape shown in Fig. 89 are used. In the bottom A is placed a
3% solution of acetic acid (vinegar answers the purpose very
well). The space above this is filled with thin, perforated,
circular pieces of lead, supported by the flange B of the
pot. These pots are placed close together on a bed of tan bark
on the floor of a room known as the corroding room. They are
covered over with boards, upon which tan bark is placed, and
another row of pots is placed on this. In this way the room is
filled. The white lead is formed by the fumes of the acetic
acid, together with the carbon dioxide set free in the
fermentation of the tan bark acting on the lead. About three
months are required to complete the process.
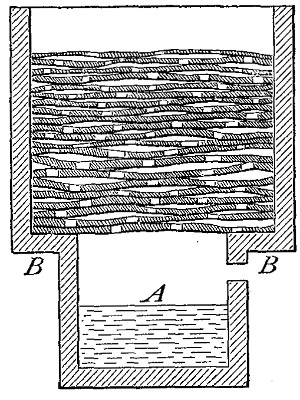 Fig. 89
Fig. 89
[Pg 377]
Lead sulphide (PbS). In nature this compound occurs in highly
crystalline condition, the crystals having much the same luster as pure
lead. It is readily prepared in the laboratory as a black precipitate,
by the action of hydrosulphuric acid upon soluble lead salts:
Pb(NO3)2 + H2S = PbS + 2HNO3.
It is insoluble both in water and in dilute acids.
Other insoluble salts. Lead chromate (PbCrO4) is a yellow substance
produced by the action of a soluble lead salt upon a soluble chromate,
thus:
K2CrO4 + Pb(NO3)2 = PbCrO4 + 2 KNO3.
It is used as a yellow pigment. Lead sulphate (PbSO4) is a white
substance sometimes found in nature and easily prepared by
precipitation. Lead chloride (PbCl2) is likewise a white substance
nearly insoluble in cold water, but readily soluble in boiling water.
Thorium and cerium. These elements are found in a few rare
minerals, especially in the monazite sand of the Carolinas and
Brazil. The oxides of these elements are used in the
preparation of the Welsbach mantles for gas lights, because of
the intense light given out when a mixture of the oxides is
heated. These mantles contain the oxides of cerium and thorium
in the ratio of about 1% of the former to 99% of the latter.
Compounds of thorium, like those of radium, are found to
possess radio-activity, but in a less degree.
EXERCISES
1. How could you detect lead if present in tin foil?
2. Stannous chloride reduces gold chloride (AuCl3) to gold. Give
equation.
3. What are the products of hydrolysis when stannic chloride is used as
a mordant?
4. How could you detect arsenic, antimony, or copper in lead?[Pg 378]
5. Why is lead so extensively used for making water pipes?
6. What sulphates other than lead are insoluble?
7. Could lead nitrate be used in place of barium chloride in testing for
sulphates?
8. How much lead peroxide could be obtained from 1 kg. of minium?
9. The purity of white lead is usually determined by observing the
volume of carbon dioxide given off when it is treated with an acid. What
acid should be used? On the supposition that it has the formula
2PbCO3·Pb(OH)2, how nearly pure was a sample if 1 g. gave 30 cc.
of carbon dioxide at 20° and 750 mm.?
10. Silicon belongs in the same family with tin and lead. In what
respects are these elements similar?
11. What weight of tin could be obtained by the reduction of 1 ton of
cassiterite?
12. What reaction would you expect to take place when lead peroxide is
treated with hydrochloric acid?
13. White lead is often adulterated with barytes. Suggest a method for
detecting it, if present, in a given example of white lead.
[Pg 379]
CHAPTER XXX
MANGANESE AND CHROMIUM
| SYMBOL | ATOMIC | WEIGHT DENSITY | MELTING POINT | FORMULAS OF ACIDS |
| Manganese | Mn | 55.0 | 8.01 | 1900° | H2MnO4 and HMnO4 |
| Chromium | Cr | 52.1 | 7.3 | 3000° | H2CrO4 and H2Cr2O7 |
General. Manganese and chromium, while belonging to different families,
have so many features in common in their chemical conduct that they may
be studied together with advantage. They differ from most of the
elements so far studied in that they can act either as acid-forming or
base-forming elements. As base-forming elements each of the metals forms
two series of salts. In the one series, designated by the suffix "ous,"
the metal is divalent; in the other series, designated by the suffix
"ic," the metal is trivalent. Only the manganous and the chromic salts,
however, are of importance. The acids in which these elements play the
part of a non-metal are unstable, but their salts are usually stable,
and some of them are important compounds.
MANGANESE
Occurrence. Manganese is found in nature chiefly as the dioxide MnO2,
called pyrolusite. In smaller amounts it occurs as the oxides
Mn2O3 and Mn3O4, and as the carbonate MnCO3. Some iron
ores also contain manganese.[Pg 380]
Preparation and properties. The element is difficult to prepare in pure
condition and has no commercial applications. It can be prepared,
however, by reducing the oxide with aluminium powder or by the use of
the electric furnace, with carbon as the reducing agent. The metal
somewhat resembles iron in appearance, but is harder, less fusible, and
more readily acted upon by air and moisture. Acids readily dissolve it,
forming manganous salts.
Oxides of manganese. The following oxides of manganese are known: MnO,
Mn2O3, Mn3O4, MnO2, and Mn2O7. Only one of these,
the dioxide, needs special mention.
Manganese dioxide (pyrolusite) (MnO2). This substance is the most
abundant manganese compound found in nature, and is the ore from which
all other compounds of manganese are made. It is a hard, brittle, black
substance which is valuable as an oxidizing agent. It will be recalled
that it is used in the preparation of chlorine and oxygen, in
decolorizing glass which contains iron, and in the manufacture of
ferromanganese.
Compounds containing manganese as a base-forming element. As has been
stated previously, manganese forms two series of salts. The most
important of these salts, all of which belong to the manganous series,
are the following:
| Manganous chloride | MnCl2·4H2O. |
| Manganous sulphide | MnS. |
| Manganous sulphate | MnSO4·4H2O. |
| Manganous carbonate | MnCO3. |
| Manganous hydroxide | Mn(OH)2. |
The chloride and sulphate may be prepared by heating the dioxide with
hydrochloric and sulphuric acids respectively:
MnO2 + 4HCl = MnCl2 + 2H2O + 2Cl,
MnO2 + H2SO4 = MnSO4 + H2O + O.
[Pg 381]
The sulphide, carbonate, and hydroxide, being insoluble, may be prepared
from a solution of the chloride or sulphate by precipitation with the
appropriate reagents. Most of the manganous salts are rose colored. They
not only have formulas similar to the ferrous salts, but resemble them
in many of their chemical properties.
Compounds containing manganese as an acid-forming element. Manganese
forms two unstable acids, namely, manganic acid and permanganic acid.
While these acids are of little interest, some of their salts,
especially the permanganates, are important compounds.
Manganic acid and manganates. When manganese dioxide is fused with an
alkali and an oxidizing agent a green compound is formed. The equation,
when caustic potash is used, is as follows:
MnO2 + 2KOH + O = K2MnO4 + H2O.
The green compound (K2MnO4) is called potassium manganate, and is
a salt of the unstable manganic acid (H2MnO4). The manganates are
all very unstable.
Permanganic acid and the permanganates. When carbon dioxide is passed
through a solution of a manganate a part of the manganese is changed
into manganese dioxide, while the remainder forms a salt of the unstable
acid HMnO4, called permanganic acid. The equation is
3K2MnO4 + 2CO2 = MnO2 + 2KMnO4 + 2K2CO3.
Potassium permanganate (KMnO4) crystallizes in purple-black needles
and is very soluble in water, forming an intensely purple solution. All
other permanganates, as well as permanganic acid itself, give solutions
of the same color.[Pg 382]
Oxidizing properties of the permanganates. The permanganates are
remarkable for their strong oxidizing properties. When used as an
oxidizing agent the permanganate is itself reduced, the exact character
of the products formed from it depending upon whether the oxidation
takes place (1) in an alkaline or neutral solution, or (2) in an acid
solution.
1. Oxidation in alkaline or neutral solution. When the solution is
either alkaline or neutral the potassium and the manganese of the
permanganate are both converted into hydroxides, as shown in the
equation
2KMnO4 + 5H2O = 2Mn(OH)4 + 2KOH + 3O.
2. Oxidation in acid solution. When free acid such as sulphuric is
present, the potassium and the manganese are both changed into salts of
the acid:
2KMnO4 + 3H2SO4 = K2SO4 + 2MnSO4 + 3H2O + 5O.
Under ordinary conditions, however, neither one of these reactions takes
place except in the presence of a third substance which is capable of
oxidation. The oxygen is not given off in the free state, as the
equations show, but is used up in effecting oxidation.
Potassium permanganate is particularly valuable as an oxidizing agent
not only because it acts readily either in acid or in alkaline solution,
but also because the reaction takes place so easily that often it is not
even necessary to heat the solution to secure action. The substance
finds many uses in the laboratory, especially in analytical work. It is
also used as an antiseptic as well as a disinfectant.[Pg 383]
CHROMIUM
Occurrence. The ore from which all chromium compounds are made is
chromite, or chrome iron ore (FeCr2O4). This is found most
abundantly in New Caledonia and Turkey. The element also occurs in small
quantities in many other minerals, especially in crocoisite (PbCrO4),
in which mineral it was first discovered.
Preparation. Chromium, like manganese, is very hard to reduce from its
ores, owing to its great affinity for oxygen. It can, however, be made
by the same methods which have proved successful with manganese.
Considerable quantities of an alloy of chromium with iron, called
ferrochromium, are now produced for the steel industry.
Properties. Chromium is a very hard metal of about the same density as
iron. It is one of the most infusible of the metals, requiring a
temperature little short of 3000° for fusion. At ordinary temperatures
air has little action on it; at higher temperatures, however, it burns
brilliantly. Nitric acid has no action on it, but hydrochloric and
dilute sulphuric acids dissolve it, liberating hydrogen.
Compounds containing chromium as a base-forming element. While chromium
forms two series of salts, chromous salts are difficult to prepare and
are of little importance. The most important of the chromic series are
the following:
| Chromic hydroxide | Cr(OH)3. |
| Chromic chloride | CrCl3·6H2O. |
| Chromic sulphate | Cr2(SO4)3. |
| Chrome alums |
Chromic hydroxide (Cr(OH)3). This substance, being insoluble, can be
obtained by precipitating a solution of the chloride or sulphate with a
soluble hydroxide. It is a[Pg 384] greenish substance which, like aluminium
hydroxide, dissolves in alkalis, forming soluble salts.
Dehydration of chromium hydroxide. When heated gently chromic
hydroxide loses a part of its oxygen and hydrogen, forming the
substance CrO·OH, which, like the corresponding aluminium
compound, has more pronounced acid properties than the
hydroxide. It forms a series of salts very similar to the
spinels; chromite is the ferrous salt of this acid, having the
formula Fe(CrO2)2. When heated to a higher temperature
chromic hydroxide is completely dehydrated, forming the
trioxide Cr2O3. This resembles the corresponding oxides
of aluminium and iron in many respects. It is a bright green
powder, and when ignited strongly becomes almost insoluble in
acids, as is also the case with aluminium oxide.
Chromic sulphate (Cr2(SO4)3). This compound is a violet-colored
solid which dissolves in water, forming a solution of the same color.
This solution, however, turns green on heating, owing to the formation
of basic salts. Chromic sulphate, like ferric and aluminium sulphates,
unites with the sulphates of the alkali metals to form alums, of which
the best known are potassium chrome alum (KCr(SO4)2·12H2O) and
ammonium chrome alum (NH4Cr(SO4)2·12H2O).
These form beautiful dark purple crystals and have some practical uses
in the tanning industry and in photography. A number of the salts of
chromium are also used in the dyeing industry, for they hydrolyze like
aluminium salts and the hydroxide forms a good mordant.
Hydrolysis of chromium salts. When ammonium sulphide is added
to a solution of a chromium salt, such as the sulphate,
chromium hydroxide precipitates instead of the sulphide. This
is due to the fact that chromic sulphide, like aluminium
sulphide, hydrolyzes in the presence of water, forming chromic
hydroxide and hydrosulphuric acid. Similarly, a soluble
carbonate precipitates a basic carbonate of chromium.
[Pg 385]
Compounds containing chromium as an acid-forming element. Like
manganese, chromium forms two unstable acids, namely, chromic acid and
dichromic acid. Their salts, the chromates and dichromates, are
important compounds.
Chromates. When a chromium compound is fused with an alkali and an
oxidizing agent a chromate is produced. When potassium hydroxide is used
as the alkali the equation is
2Cr(OH)3 + 4KOH + 3O = 2K2CrO4 + 5H2O.
This reaction recalls the formation of a manganate under similar
conditions.
Properties of chromates. The chromates are salts of the unstable chromic
acid (H2CrO4), and as a rule are yellow in color. Lead chromate
(PbCrO4) is the well-known pigment chrome yellow. Most of the
chromates are insoluble and can therefore be prepared by precipitation.
Thus, when a solution of potassium chromate is added to solutions of
lead nitrate and barium nitrate respectively, the reactions expressed by
the following equations occur:
Pb(NO3)2 + K2CrO4 = PbCrO4 + 2KNO3,
Ba(NO3)2 + K2CrO4 = BaCrO4 + 2KNO3.
The chromates of lead and barium separate as yellow precipitates. The
presence of either of these two metals can be detected by taking
advantage of these reactions.
Dichromates. When potassium chromate is treated with an acid the
potassium salt of the unstable dichromic acid (H2Cr2O7) is
formed:
2K2CrO4 + H2SO4 = K2Cr2O7 + K2SO4 + H2O.
[Pg 386]
The relation between the chromates and dichromates is the same as that
between the phosphates and the pyrophosphates. Potassium dichromate
might therefore be called potassium pyrochromate.
Potassium dichromate (K2Cr2O7). This is the best known
dichromate, and is the most familiar chromium compound. It forms large
crystals of a brilliant red color, and is rather sparingly soluble in
water. When treated with potassium hydroxide it is converted into the
chromate
K2Cr2O7 + 2KOH = 2K2CrO4 + H2O.
When added to a solution of lead or barium salt the corresponding
chromates (not dichromates) are precipitated. With barium nitrate the
equation is
2Ba(NO3)2 + K2Cr2O7 + H2O = 2BaCrO4 + 2KNO3 + 2HNO3.
Potassium dichromate finds use in many industries as an oxidizing agent,
especially in the preparation of organic substances, such as the dye
alizarin, and in the construction of several varieties of electric
batteries.
Sodium chromates. The reason why the potassium salt rather than
the sodium compound is used is that sodium chromate and
dichromate are so soluble that it is hard to prepare them pure.
This difficulty is being overcome now, and the sodium compounds
are replacing the corresponding potassium salts. This is of
advantage, since a sodium salt is cheaper than a potassium
salt, so far as raw materials go.
Oxidizing action of chromates and dichromates. When a dilute solution of
a chromate or dichromate is acidified with an acid, such as sulphuric
acid, no reaction apparently takes place. However, if there is present a
third substance capable of oxidation, the chromium compound gives up a[Pg 387]
portion of its oxygen to this substance. Since the chromate changes into
a dichromate in the presence of an acid, it will be sufficient to study
the action of the dichromates alone. The reaction takes place in two
steps. Thus, when a solution of ferrous sulphate is added to a solution
of potassium dichromate acidified with sulphuric acid, the reaction is
expressed by the following equations:
(1) K2Cr2O7 + 4H2SO4 = K2SO4 + Cr2(SO4)3 + 4H2O + 3O,
(2) 6FeSO4 + 3H2SO4 + 3O = 3Fe2(SO4)3 + 3H2O.
The dichromate decomposes in very much the same way as a permanganate
does, the potassium and chromium being both changed into salts in which
they play the part of metals, while part of the oxygen of the dichromate
is liberated.
By combining equations (1) and (2), the following is obtained:
K2Cr2O7 + 7H2SO4 + 6FeSO4 = K2SO4 + Cr2(SO4)3 + 3Fe2(SO4)3 + 7H20.
This reaction is often employed in the estimation of iron in iron ores.
Potassium chrome alum. It will be noticed that the oxidizing
action of potassium dichromate leaves potassium sulphate and
chromium sulphate as the products of the reaction. On
evaporating the solution these substances crystallize out as
potassium chrome alum, which substance is produced as a
by-product in the industries using potassium dichromate for
oxidizing purposes.
Chromic anhydride (CrO3). When concentrated sulphuric acid is added
to a strong solution of potassium dichromate, and the liquid allowed to
stand, deep red needle-shaped crystals appear which have the formula
[Pg 388]CrO3.This oxide of chromium is called chromic anhydride, since it
combines readily with water to form chromic acid:
It is therefore analogous to sulphur trioxide which forms sulphuric acid
in a similar way:
Chromic anhydride is a very strong oxidizing agent, giving up oxygen and
forming chromic oxide:
Rare elements of the family. Molybdenum, tungsten, and uranium
are three rather rare elements belonging in the same family
with chromium, and form many compounds which are similar in
formulas to the corresponding compounds of chromium. They can
play the part of metals and also form acids resembling chromic
acid in formula. Thus we have molybdic acid (H2MoO4), the
ammonium salt of which is (NH4)2MoO4. This salt has
the property of combining with phosphoric acid to form a very
complex substance which is insoluble in nitric acid. On this
account molybdic acid is often used in the estimation of the
phosphoric acid present in a substance. Like chromium, the
metals are difficult to prepare in pure condition. Alloys with
iron can be prepared by reducing the mixed oxides with carbon
in an electric furnace; these alloys are used to some extent in
preparing special kinds of steel.
EXERCISES
1. How does pyrolusite effect the decolorizing of glass containing iron?
2. Write the equations for the preparation of manganous chloride,
carbonate, and hydroxide.
3. Write the equations representing the reactions which take place when
ferrous sulphate is oxidized to ferric sulphate by potassium
permanganate in the presence of sulphuric acid.[Pg 389]
4. In the presence of sulphuric acid, oxalic acid is oxidized by
potassium permanganate according to the equation
Write the complete equation.
5. 10 g. of iron were dissolved in sulphuric acid and oxidized to ferric
sulphate by potassium permanganate. What weight of the permanganate was
required?
6. What weight of ferrochromium containing 40% chromium must be added to
a ton of steel to produce an alloy containing 1% of chromium?
7. Write the equation representing the action of ammonium sulphide upon
chromium sulphate.
8. Potassium chromate oxidizes hydrochloric acid, forming chlorine.
Write the complete equation.
9. Give the action of sulphuric acid on potassium dichromate (a) in
the presence of a large amount of water; (b) in the presence of a
small amount of water.
[Pg 390]
CHAPTER XXXI
GOLD AND THE PLATINUM FAMILY
| SYMBOL | ATOMIC WEIGHT | DENSITY | HIGHEST OXIDE | HIGHEST CHLORIDE | MELTING POINT |
| Ruthenium | Ru | 101.7 | 12.26 | RuO4 | RuCl4 | Electric arc |
| Rhodium | Rh | 103. | 12.1 | RhO2 | RhCl2 | Electric arc |
| Palladium | Pd | 106.5 | 11.8 | PdO2 | PdCl4 | 1500° |
| Iridium | Ir | 193. | 22.42 | IrO2 | IrCl4 | 1950° |
| Osmium | Os | 191. | 22.47 | OsO4 | OsCl4 | Electric arc |
| Platinum | Pt | 194.8 | 21.50 | PtO2 | PtCl4 | 1779° |
| Gold | Au | 197.2 | 19.30 | Au2O3 | AuCl3 | 1064° |
The family. Following iron, nickel, and cobalt in the eighth column of
the periodic table are two groups of three elements each. The metals of
the first of these groups—ruthenium, rhodium, and palladium—have
atomic weights near 100 and densities near 12. The metals of the other
group—iridium, osmium, and platinum—have atomic weights near 200 and
densities near 21. These six rare elements have very similar physical
properties and resemble each other chemically not only in the type of
compounds which they form but also in the great variety of them. They
occur closely associated in nature, usually as alloys of platinum in the
form of irregular metallic grains in sand and gravel. Platinum is by far
the most abundant of the six.
Although the periodic classification assigns gold to the silver-copper
group, its physical as well as many of its[Pg 391] chemical properties much
more closely resemble those of the platinum metals, and it can he
conveniently considered along with them. The four elements gold,
platinum, osmium, and iridium are the heaviest substances known, being
about twice as heavy as lead.
PLATINUM
Occurrence. About 90% of the platinum of commerce comes from Russia,
small amounts being produced in California, Brazil, and Australia.
Preparation. Native platinum is usually alloyed with gold and the
platinum metals. To separate the platinum the alloy is dissolved in aqua
regia, which converts the platinum into chloroplatinic acid
(H2PtCl6). Ammonium chloride is then added, which precipitates the
platinum as insoluble ammonium chloroplatinate:
H2PtCl6 + 2NH4Cl = (NH4)2PtCl6 + 2HCl.
Some iridium is also precipitated as a similar compound. On ignition the
double chloride is decomposed, leaving the platinum as a spongy metallic
mass, which is melted in an electric furnace and rolled or hammered into
the desired shape.
Physical properties. Platinum is a grayish-white metal of high luster,
and is very malleable and ductile. It melts in the oxyhydrogen blowpipe
and in the electric furnace; it is harder than gold and is a good
conductor of electricity. In finely divided form it has the ability to
absorb or occlude gases, especially oxygen and hydrogen. These gases,
when occluded, are in a very active condition resembling the nascent
state, and can combine with each other at ordinary[Pg 392] temperatures. A jet
of hydrogen or coal gas directed upon spongy platinum is at once
ignited.
Platinum as a catalytic agent. Platinum is remarkable for its
property of acting as a catalytic agent in a large number of
chemical reactions, and mention has been made of this use of
the metal in connection with the manufacture of sulphuric acid.
When desired for this purpose some porous or fibrous substance,
such as asbestos, is soaked in a solution of platinic chloride
and then ignited. The platinum compound is decomposed and the
platinum deposited in very finely divided form. Asbestos
prepared in this way is called platinized asbestos. The
catalytic action seems to be in part connected with the
property of absorbing gases and rendering them nascent. Some
other metals possess this same power, notably palladium, which
is remarkable for its ability to absorb hydrogen.
Chemical properties. Platinum is a very inactive element chemically, and
is not attacked by any of the common acids. Aqua regia slowly dissolves
it, forming platinic chloride (PtCl4), which in turn unites with the
hydrochloric acid present in the aqua regia, forming the compound
chloroplatinic acid (H2PtCl6). Platinum is attacked by fused
alkalis. It combines at higher temperatures with carbon and phosphorus
and alloys with many metals. It is readily attacked by chlorine but not
by oxidizing agents.
Applications. Platinum is very valuable as a material for the
manufacture of chemical utensils which are required to stand a high
temperature or the action of strong reagents. Platinum crucibles,
dishes, forceps, electrodes, and similar articles are indispensable in
the chemical laboratory. In the industries it is used for such purposes
as the manufacture of pans for evaporating sulphuric acid, wires for
sealing through incandescent light bulbs, and for making a great variety
of instruments. Unfortunately the supply[Pg 393] of the metal is very limited,
and the cost is steadily advancing, so that it is now more valuable than
gold.
Compounds. Platinum forms two series of salts of which platinous
chloride (PtCl2) and platinic chloride (PtCl4) are examples.
Platinates are also known. While a great variety of compounds of
platinum have been made, the substance is chiefly employed in the
metallic state.
Platinic chloride (PtCl4). Platinic chloride is an orange-colored,
soluble compound made by heating chloroplatinic acid in a current of
chlorine. If hydrochloric acid is added to a solution of the substance,
the two combine, forming chloroplatinic acid (H2PtCl6):
The potassium and ammonium salts of this acid are nearly insoluble in
water and alcohol. The acid is therefore used as a reagent to
precipitate potassium in analytical work. With potassium chloride the
equation is
2KCl + H2PtCl6 = K2PtCl6 + 2HCl.
Other metals of the family. The other members of the family
have few applications. Iridium is used in the form of a
platinum alloy, since the alloy is much harder than pure
platinum and is even less fusible. This alloy is sometimes used
to point gold pens. Osmium tetroxide (OsO4) is a very
volatile liquid and is used under the name of osmic acid as a
stain for sections in microscopy.
GOLD
Occurrence. Gold has been found in many localities, the most famous
being South Africa, Australia, Russia, and the United States. In this
country it is found in Alaska and in nearly half of the states of the
union, notably[Pg 394] in California, Colorado, and Nevada. It is usually found
in the native condition, frequently alloyed with silver; in combination
it is sometimes found as telluride (AuTe2), and in a few other
compounds.
Mining. Native gold occurs in the form of small grains or larger nuggets
in the sands of old rivers, or imbedded in quartz veins in rocks. In the
first case it is obtained in crude form by placer mining. The sand
containing the gold is shaken or stirred in troughs of running waters
called sluices. This sweeps away the sand but allows the heavier gold to
sink to the bottom of the sluice. Sometimes the sand containing the gold
is washed away from its natural location into the sluices by powerful
streams of water delivered under pressure from pipes. This is called
hydraulic mining. In vein mining the gold-bearing quartz is mined from
the veins, stamped into fine powder in stamping mills, and the gold
extracted by one of the processes to be described.
Extraction. 1. Amalgamation process. In the amalgamation process the
powder containing the gold is washed over a series of copper plates
whose surfaces have been amalgamated with mercury. The gold sticks to
the mercury or alloys with it, and after a time the gold and mercury are
scraped off and the mixture is distilled. The mercury distills off and
the gold is left in the retort ready for refining.
2. Chlorination process. When gold occurs along with metallic
sulphides it is often extracted by chlorination. The ore is first
roasted, and is then moistened and treated with chlorine. This dissolves
the gold but not the metallic oxides:
[Pg 395]
The gold chloride, being soluble, is extracted from the mixture with
water, and the gold is precipitated from the solution, usually by adding
ferrous sulphate:
AuCl3 + 3FeSO4 = Au + FeCl3 + Fe2(SO4)3.
3. Cyanide process. This process depends upon the fact that gold is
soluble in a solution of potassium cyanide in the presence of the oxygen
of the air. The powder from the stamping mills is treated with a very
dilute potassium cyanide solution which extracts the gold:
2Au + 4KCN + H2O + O = 2KOH + 2KAu(CN)2.
From this solution the gold can be obtained by electrolysis or by
precipitation with metallic zinc:
2KAu(CN)2 + Zn = K2Zn(CN)4 + 2Au.
Refining of gold. Gold is refined by three general methods:
1. Electrolysis. When gold is dissolved in a solution of potassium
cyanide, and the solution electrolyzed, the gold is deposited in very
pure condition on the cathode.
2. Cupellation. When the gold is alloyed with easily oxidizable
metals, such as copper or lead, it may be refined by cupellation. The
alloy is fused with an oxidizing flame on a shallow hearth made of bone
ash, which substance has the property of absorbing metallic oxides but
not the gold. Any silver which may be present remains alloyed with the
gold.
3. Parting with sulphuric acid. Gold may be separated from silver, as
well as from many other metals, by heating the alloy with concentrated
sulphuric acid. This dissolves the silver, while the gold is not
attacked.[Pg 396]
Physical properties. Gold is a very heavy bright yellow metal,
exceedingly malleable and ductile, and a good conductor of electricity.
It is quite soft and is usually alloyed with copper or silver to give it
the hardness required for most practical uses. The degree of fineness is
expressed in terms of carats, pure gold being twenty-four carats; the
gold used for jewelry is usually eighteen carats, eighteen parts being
gold and six parts copper or silver. Gold coinage is 90% gold and 10%
copper.
Chemical properties. Gold is not attacked by any one of the common
acids; aqua regia easily dissolves it, forming gold chloride (AuCl3),
which in turn combines with hydrochloric acid to form chlorauric acid
(HAuCl4). Fused alkalis also attack it. Most oxidizing agents are
without action upon it, and in general it is not an active element.
Compounds. The compounds of gold, though numerous and varied in
character, are of comparatively little importance and need not
be described in detail. The element forms two series of salts
in which it acts as a metal: in the aurous series the gold is
univalent, the chloride having the formula AuCl; in the auric
series it is trivalent, auric chloride having the formula
AuCl3. Gold also acts as an acid-forming element, forming
such compounds as potassium aurate (KAuO2). Its compounds
are very easily decomposed, however, metallic gold separating
from them.
EXERCISES
1. From the method of preparation of platinum, what metal is likely to
be alloyed with it?
2. The "platinum chloride" of the laboratory is made by dissolving
platinum in aqua regia. What is the compound?
3. How would you expect potassium aurate and platinate to be formed?
What precautions would this suggest in the use of platinum vessels?
4. Why must gold ores be roasted in the chlorination process?
[Pg 397]
CHAPTER XXXII
SOME SIMPLE ORGANIC COMPOUNDS
Division of chemistry into organic and inorganic. Chemistry is usually
divided into two great divisions,—organic and inorganic. The original
significance of these terms was entirely different from the meaning
which they have at the present time.
1. Original significance. The division into organic and inorganic was
originally made because it was believed that those substances which
constitute the essential parts of living organisms were built up under
the influence of the life force of the organism. Such substances,
therefore, should be regarded as different from those compounds prepared
in the laboratory or formed from the inorganic or mineral constituents
of the earth. In accordance with this view organic chemistry included
those substances formed by living organisms. Inorganic chemistry, on the
other hand, included all substances formed from the mineral portions of
the earth.
In 1828 the German chemist Wöhler prepared urea, a typical organic
compound, from inorganic materials. The synthesis of other so-called
organic compounds followed, and at present it is known that the same
chemical laws apply to all substances whether formed in the living
organism or prepared in the laboratory from inorganic constituents. The
terms "organic" and "inorganic" have therefore lost their original
significance.[Pg 398]
2. Present significance. The great majority of the compounds found in
living organisms contain carbon, and the term "organic chemistry," as
used at present, includes not only these compounds but all compounds of
carbon. Organic chemistry has become, therefore, the chemistry of the
compounds of carbon, all other substances being treated under the head
of inorganic chemistry. This separation of the compounds of carbon into
a group by themselves is made almost necessary by their great number,
over one hundred thousand having been recorded. For convenience some of
the simpler carbon compounds, such as the oxides and the carbonates, are
usually discussed in inorganic chemistry.
The grouping of compounds in classes. The study of organic chemistry is
much simplified by the fact that the large number of bodies included in
this field may be grouped in classes of similar compounds. It thus
becomes possible to study the properties of each class as a whole, in
much the same way as we study a group of elements. The most important of
these classes are the hydrocarbons, the alcohols, the aldehydes,
the acids, the ethereal salts, the ethers, the ketones, the
organic bases, and the carbohydrates. A few members of each of these
classes will now be discussed briefly.
THE HYDROCARBONS
Carbon and hydrogen combine to form a large number of compounds. These
compounds are known collectively as the hydrocarbons. They may be
divided into a number of groups or series, each being named from its
first member. Some of the groups are as follows:[Pg 399]
| METHANE SERIES |
| CH4 | methane |
| C2H6 | ethane |
| C3H8 | propane |
| C4H10 | butane |
| C5H12 | pentane |
| C6H14 | hexane |
| C7H16 | heptane |
| C8H18 | octane |
| ETHYLENE SERIES |
| C2H4 | ethylene |
| C3H6 | propylene |
| C4H8 | butylene |
| BENZENE SERIES |
| C6H6 | benzene |
| C7H8 | toluene |
| C8H10 | xylene |
| ACETYLENE SERIES |
| C2H2 | acetylene |
| C3H4 | allylene |
Only the lower members (that is, those which contain a small number of
carbon atoms) of the above groups are given. The methane series is the
most extensive, all of the compounds up to C24H50 being known.
It will be noticed that the successive members of each of the above
series differ by the group of atoms (CH2). Such a series is called an
homologous series. In general, it may be stated that the members of an
homologous series show a regular gradation in most physical properties
and are similar in chemical properties. Thus in the methane group the
first four members are gases at ordinary temperatures; those containing
from five to sixteen carbon atoms are liquids, the boiling points of
which increase with the number of carbon atoms present. Those containing
more than sixteen carbon atoms are solids.
Sources of the hydrocarbons. There are two chief sources of the
hydrocarbons, namely, (1) crude petroleum and (2) coal tar.
1. Crude petroleum. This is a liquid pumped from wells driven into the
earth in certain localities. Pennsylvania, Ohio, Kansas, California, and
Texas are the chief[Pg 400] oil-producing regions in the United States. The
crude petroleum consists largely of liquid hydrocarbons in which are
dissolved both gaseous and solid hydrocarbons. Before being used it must
be refined. In this process the petroleum is run into large iron stills
and subjected to fractional distillation. The various hydrocarbons
distill over in the general order of their boiling points. The
distillates which collect between certain limits of temperature are kept
separate and serve for different uses; they are further purified,
generally by washing with sulphuric acid, then with an alkali, and
finally with water. Among the products obtained from crude petroleum in
this way are the naphthas, including benzine and gasoline, kerosene or
coal oil, lubricating oils, vaseline, and paraffin. None of these
products are definite chemical compounds, but each consists of a mixture
of hydrocarbons, the boiling points of which lie within certain limits.
2. Coal tar. This product is obtained in the manufacture of coal gas,
as already explained. It is a complex mixture and is refined by the same
general method used in refining crude petroleum. The principal
hydrocarbons obtained from the coal tar are benzene, toluene,
naphthalene, and anthracene. In addition to the hydrocarbons, coal tar
contains many other compounds, such as carbolic acid and aniline.
Properties of the hydrocarbons. The lower members of the first two
series of hydrocarbons mentioned are all gases; the succeeding members
are liquids. In some series, as the methane series, the higher members
are solids. The preparation and properties of methane and acetylene have
been discussed in a previous chapter. Ethylene is present in small
quantities in coal gas and may be[Pg 401] obtained in the laboratory by
treating alcohol (C2H6O) with sulphuric acid:
Benzene, the first member of the benzene series, is a liquid boiling at
80°.
The hydrocarbons serve as the materials from which a large number of
compounds can be prepared; indeed, it has been proposed to call organic
chemistry the chemistry of the hydrocarbon derivatives.
Substitution products of the hydrocarbons. As a rule, at least a part of
the hydrogen in any hydrocarbon can be displaced by an equivalent amount
of certain elements or groups of elements. Thus the compounds CH3Cl,
CH2Cl2, CHCl3, CCl4 can be obtained from methane by
treatment with chlorine. Such compounds are called substitution
products.
Chloroform (CHCl3). This can be made by treating methane with
chlorine, as just indicated, although a much easier method consists in
treating alcohol or acetone (which see) with bleaching powder.
Chloroform is a heavy liquid having a pleasant odor and a sweetish
taste. It is largely used as a solvent and as an anæsthetic in surgery.
Iodoform (CHI3). This is a yellow crystalline solid obtained by
treating alcohol with iodine and an alkali. It has a characteristic odor
and is used as an antiseptic.
ALCOHOLS
When such a compound as CH3Cl is treated with silver hydroxide the
reaction expressed by the following equation takes place:
CH3Cl + AgOH = CH3OH + AgCl.
[Pg 402]
Similarly C2H5Cl will give C2H5OH and AgCl. The compounds
CH3OH and C2H5OH so obtained belong to the class of substances
known as alcohols. From their formulas it will be seen that they may
be regarded as derived from hydrocarbons by substituting the hydroxyl
group (OH) for hydrogen. Thus the alcohol CH3OH may be regarded as
derived from methane (CH4) by substituting the group OH for one atom
of hydrogen. A great many alcohols are known, and, like the
hydrocarbons, they may be grouped into series. The relation between the
first three members of the methane series and the corresponding alcohols
is shown in the following table:
| CH4 | (methane) | CH3OH | (methyl alcohol). |
| C2H6 | (ethane) | C2H5OH | (ethyl alcohol). |
| C3H8 | (propane) | C3H7OH | (propyl alcohol). |
Methyl alcohol (wood alcohol) (CH3OH). When wood is placed in an
air-tight retort and heated, a number of compounds are evolved, the most
important of which are the three liquids, methyl alcohol, acetic acid,
and acetone. Methyl alcohol is obtained entirely from this source, and
on this account is commonly called wood alcohol. It is a colorless
liquid which has a density of 0.79 and boils at 67°. It burns with an
almost colorless flame and is sometimes used for heating purposes, in
place of the more expensive ethyl alcohol. It is a good solvent for
organic substances and is used especially as a solvent in the
manufacture of varnishes. It is very poisonous.
Ethyl alcohol (common alcohol) (C2H5OH). 1. Preparation. This
compound may be prepared from glucose (C6H12O6), a sugar easily
obtained from starch. If some baker's yeast is added to a solution of
glucose and the temperature is maintained at about 30°, bubbles of gas
are[Pg 403] soon evolved, showing that a change is taking place. The yeast
contains a large number of minute organized bodies, which are really
forms of plant life. The plant grows in the glucose solution, and in so
doing secretes a substance known as zymase, which breaks down the
glucose in accordance with the following equation:
C6H12O6 = 2C2H5OH + 2CO2.
Laboratory preparation of alcohol. The formation of alcohol and
carbon dioxide from glucose may be shown as follows: About 100
g. of glucose are dissolved in a liter of water in flask A
(Fig. 90). This flask is connected with the bottle B, which
is partially filled with limewater. The tube C contains solid
sodium hydroxide. A little baker's yeast is now added to the
solution in flask A, and the apparatus is connected, as shown
in the figure. If the temperature is maintained at about 30°,
the reaction soon begins. The bubbles of gas escape through the
limewater in B. A precipitate of calcium carbonate soon forms
in the limewater, showing the presence of carbon dioxide. The
sodium hydroxide in tube C prevents the carbon dioxide in the
air from acting on the limewater. The alcohol remains in the
flask A and may be separated by fractional distillation.
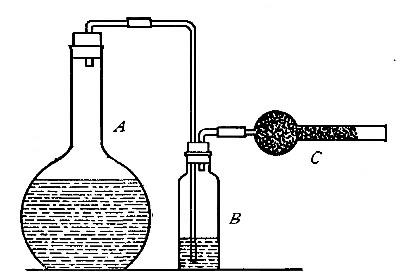 Fig. 90
Fig. 90
2. Properties. Ethyl alcohol is a colorless liquid with a pleasant
odor. It has a density of 0.78 and boils at 78°. It resembles methyl
alcohol in its general properties. It is sometimes used as a source of
heat, since its flame is very hot and does not deposit carbon, as the
flame from oil does. When taken into the system in small quantities[Pg 404] it
causes intoxication; in larger quantities it acts as a poison. The
intoxicating properties of such liquors as beer, wine, and whisky are
due to the alcohol present. Beer contains from 2 to 5% of alcohol, wine
from 5 to 20%, and whisky about 50%. The ordinary alcohol of the
druggist contains 94% of alcohol and 6% of water. When this is boiled
with lime and then distilled nearly all the water is removed, the
distillate being called absolute alcohol.
Commercial preparation of alcohol. Alcohol is prepared
commercially from starch obtained from corn or potatoes. The
starch is first converted into a sugar known as maltose, by the
action of malt, a substance prepared by moistening barley
with water, allowing it to germinate, and then drying it. There
is present in the malt a substance known as diastase, which has
the property of changing starch into maltose. This sugar, like
glucose, breaks down into alcohol and carbon dioxide in the
presence of yeast. The resulting alcohol is separated by
fractional distillation.
Denatured alcohol. The 94% alcohol is prepared at present at a
cost of about 35 cents per gallon, which is about half the cost
of the preparation of methyl alcohol. The government, however,
imposes a tax on all ethyl alcohol which amounts to $2.08 per
gallon on the 94% product. This increases its cost to such an
extent that it is not economical to use it for many purposes
for which it is adapted, such as a solvent in the preparation
of paints and varnishes and as a material for the preparation
of many important organic compounds. By an act of Congress in
1906, the tax was removed from denatured alcohol, that is
alcohol mixed with some substance which renders it unfit for
the purposes of a beverage but will not impair its use for
manufacturing purposes. Some of the European countries have
similar laws. The substances ordinarily used to denature
alcohol are wood alcohol and pyridine, the latter compound
having a very offensive odor.
Fermentation. The reaction which takes place in the preparation
of ethyl alcohol belongs to the class of changes known under
the general name of fermentation. Thus we say that the yeast
causes the glucose to ferment, and the process is known as
alcoholic fermentation. There are many kinds of fermentations,
and each is thought to be due to the presence of a definite
substance known[Pg 405] as an enzyme, which acts by catalysis. In
many cases, as in alcoholic fermentation, the change is brought
about by the action of minute forms of life. These probably
secrete the enzymes which cause the fermentation to take place.
Thus the yeast plant is supposed to bring about alcoholic
fermentation by secreting the enzyme known as zymase.
Glycerin (C3H5(OH)3). This compound may be regarded as derived
from propane (C3H8) by displacing three atoms of hydrogen by three
hydroxyl groups, and must therefore be regarded as an alcohol. It is
formed in the manufacture of soaps, as will be explained later. It is an
oily, colorless liquid having a sweetish taste. It is used in medicine
and in the manufacture of the explosives nitroglycerin and dynamite.
ALDEHYDES
When alcohols are treated with certain oxidizing agents two hydrogen
atoms are removed from each molecule of the alcohol. The resulting
compounds are known as aldehydes. The relation of the aldehydes derived
from methyl and ethyl alcohol to the alcohols themselves may be shown as
follows:
| Alcohols | {CH3OH | Corresponding aldehydes | {CH2O |
| {C2H5OH | {C2H4O |
The first of these (CH2O) is a gas known as formaldehyde. Its aqueous
solution is largely used as an antiseptic and disinfectant under the
name of formalin. Acetaldehyde (C2H4O) is a liquid boiling at
21°.
ACIDS
Like the other classes of organic compounds, the organic acids may be
arranged in homologous series. One of the most important of these series
is the fatty-acid series, the[Pg 406] name having been given to it because
the derivatives of certain of its members are constituents of the fats.
Some of the most important members of the series are given in the
following table. They are all monobasic, and this fact is expressed in
the formulas by separating the replaceable hydrogen atom from the rest
of the molecule:
| H·CHO2 | formic acid, a liquid boiling at 100°. |
| H·C2H3O | acetic acid, a liquid boiling at 118°. |
| H·C3H5O2 | propionic acid, a liquid boiling at 140°. |
| H·C4H7O2 | butyric acid, a liquid boiling at 163°. |
| H·C16H31O2 | palmitic acid, a solid melting at 62°. |
| H·C18H35O2 | stearic acid, a solid melting at 69°. |
Formic acid (H·CHO2). The name "formic" is derived from the Latin
formica, signifying ant. This name was given to the acid because it
was formerly obtained from a certain kind of ants. It is a colorless
liquid and occurs in many plants such as the stinging nettles. The
inflammation caused by the sting of the bee is due to formic acid.
Acetic acid (H·C2H3O2). Acetic acid is the acid present in
vinegar, the sour taste being due to it. It can be prepared by either of
the following methods.
1. Acetic fermentation. This consists in the change of alcohol into
acetic acid through the agency of a minute organism commonly called
mother of vinegar. The change is represented by the following equation:
C2H5OH + 2O = HC2H3O2 + H2O.
The various kinds of vinegars are all made by this process. In the
manufacture of cider vinegar the sugar present in the cider first
undergoes alcoholic fermentation; the resulting alcohol then undergoes
acetic fermentation. The amount of acetic acid present in vinegars
varies from 3 to 6%.[Pg 407]
2. From the distillation of wood. The liquid obtained by heating wood
in the absence of air contains a large amount of acetic acid, and this
can be separated readily in a pure state. This is the most economical
method for the preparation of the concentrated acid.
Acetic acid is a colorless liquid and has a strong pungent odor. Many of
its salts are well-known compounds. Lead acetate
(Pb(C2H3O2)2) is the ordinary sugar of lead. Sodium
acetate (NaC2H3O2) is a white solid largely used in making
chemical analyses. Copper acetate (Cu(C2H3O2)2) is a blue
solid. When copper is acted upon by acetic acid in the presence of air a
green basic acetate of copper is formed. This is commonly known as
verdigris. All acetates are soluble in water.
Butyric acid (H·C4H7O2). Derivatives of butyric acid are
present in butter and impart to it its characteristic flavor.
Palmitic and stearic acids. Ordinary fats consist principally of
derivatives of palmitic and stearic acids. When the fats are heated with
sodium hydroxide the sodium salts of these acids are formed. If
hydrochloric acid is added to a solution of the sodium salts, the free
palmitic and stearic acids are precipitated. They are white solids,
insoluble in water. Stearic acid is often used in making candles.
Acids belonging to other series. In addition to members of the
fatty-acid series, mention may be made of the following well-known
acids.
Oxalic acid (H2C2O4). This is a white solid which occurs in
nature in many plants, such as the sorrels. Its ammonium salt
((NH4)2C2O4) is used as a reagent for the detection of
calcium. When added to a solution of a calcium[Pg 408] compound the white,
insoluble calcium oxalate (CaC2O4) precipitates.
Tartaric acid (H2·C4H4O6). This compound occurs either in a
free state or in the form of its salts in many fruits. The potassium
acid salt (KHC4H4O6) occurs in the juice of grapes. When the
juice ferments in the manufacture of wine, this salt, being insoluble in
alcohol, separates out on the sides of the cask and in this form is
known as argol. This is more or less colored by the coloring matter of
the grape. When purified it forms a white solid and is sold under the
name of cream of tartar. The following are also well-known salts of
tartaric acid: potassium sodium tartrate (Rochelle salt)
(KNaC4H4O6), potassium antimonyl tartrate (tartar emetic)
(KSbOC4H4O6).
Cream of tartar baking powders. The so-called cream of tartar
baking powders consist of a mixture of cream of tartar,
bicarbonate of soda, and some starch or flour. When water is
added to this mixture the cream of tartar slowly acts upon the
soda present liberating carbon dioxide in accordance with the
following equation:
KHC4H4O6 + NaHCO3 = KNaC4H4O6 + H2O +
CO2.
The carbon dioxide evolved escapes through the dough, thus
making it light and porous.
Citric acid (H3·C6H5O7). This acid occurs in many fruits,
especially in lemons. It is a white solid, soluble in water, and is
often used as a substitute for lemons in making lemonade.
Lactic acid (H·C3H5O3). This is a liquid which is formed in the
souring of milk.
Oleic acid (H·C18H33O2). The derivatives of this acid
constitute the principal part of many oils and liquid fats. The acid
itself is an oily liquid.[Pg 409]
ETHEREAL SALTS
When acids are brought in contact with alcohols under certain conditions
a reaction takes place similar to that which takes place between acids
and bases. The following equations will serve as illustrations:
KOH + HNO3 = KNO3 + H2O,
CH3OH + HNO3 = CH3NO3 + H2O.
The resulting compounds of which methyl nitrate (CH3NO3) may be
taken as the type belong to the class known as ethereal salts, the
name having been given them because some of them possess pleasant
ethereal odors. It will be seen that the ethereal salts differ from
ordinary salts in that they contain a hydrocarbon radical, such as
CH3, C2H5, C3H5, in place of a metal.
The nitrates of glycerin (nitroglycerin). Nitric acid reacts with
glycerin in the same way that it reacts with a base containing three
hydroxyl groups such as Fe(OH)3:
Fe(OH)3 + 3HNO3 = Fe(NO3)3 + 3H2O,
C3H5(OH)3 + 3HNO3 = C3H5(NO3)3 + 3H2O.
The resulting nitrate (C3H5(NO3)3) is the main constituent
of nitroglycerin, a slightly yellowish oil characterized by its
explosive properties. Dynamite consists of porous earth which has
absorbed nitroglycerin, and its strength depends on the amount present.
It is used much more largely than nitroglycerin itself, since it does
not explode so readily by concussion and hence can be transported with
safety.
The fats. These are largely mixtures of the ethereal salts known
respectively as olein, palmitin, and stearin.[Pg 410] These salts may be
regarded as derived from oleic, palmitic, and stearic acids
respectively, by replacing the hydrogen of the acid with the glycerin
radical C3H5. Since this radical is trivalent and oleic, palmitic,
and stearic acids contain only one replaceable hydrogen atom to the
molecule, it is evident that three molecules of each acid must enter
into each molecule of the ethereal salt. The formulas for the acids and
the ethereal salts derived from each are as follows:
| HC18H33O2 | (oleic acid) |
| C8H6(C18H33O2)3, | (olein) |
| HC16H31O2 | (palmitic acid) |
| C3H5(C16H3102)3 | (palmitin) |
| HC18H35O2 | (stearic acid) |
| C3H5(C18H35O2)3 | (stearin) |
Olein is a liquid and is the main constituent of liquid fats. Palmitin
and stearin are solids.
Butter fat and oleomargarine. Butter fat consists principally of olein,
palmitin, and stearin. The flavor of the fat is due to the presence of a
small amount of butyrin, which is an ethereal salt of butyric acid.
Oleomargarine differs from butter mainly in the fact that a smaller
amount of butyrin is present. It is made from the fats obtained from
cattle and hogs. This fat is churned up with milk, or a small amount of
butter is added, in order to furnish sufficient butyrin to impart the
butter flavor.
Saponification. When an ethereal salt is heated with an alkali a
reaction expressed by the following equation takes place:
C2H5NO3 + KOH = C2H5OH + KNO3.
This process is known as saponification, since it is the one which
takes place in the manufacture of soaps. The ordinary soaps are made by
heating fats with a solution of[Pg 411] sodium hydroxide. The reactions
involved may be illustrated by the following equation representing the
reaction between palmitin and sodium hydroxide:
C3H5(C16H31O2)3 + 3 NaOH = 3 NaC16H31O2 + C3H5(OH)3.
In accordance with this equation the ethereal salts in the fats are
converted into glycerin and the sodium salts of the corresponding acids.
The sodium salts are separated and constitute the soaps. These salts are
soluble in water. When added to water containing calcium salts the
insoluble calcium palmitate and stearate are precipitated. Magnesium
salts act in a similar way. It is because of these facts that soap is
used up by hard waters.
ETHERS
When ethyl alcohol is heated to 140° with sulphuric acid the reaction
expressed by the following equation takes place:
2C2H5OH = (C2H5)2O + H2O.
The resulting compound, (C2H5)2O, is ordinary ether and is the
most important member of the class of compounds called ethers.
Ordinarily ether is a light, very inflammable liquid boiling at 35°. It
is used as a solvent for organic substances and as an anæsthetic in
surgical operations.
KETONES
The most common member of this group is acetone (C3H6O), a
colorless liquid obtained when wood is heated in the absence of air. It
is used in the preparation of other organic compounds, especially
chloroform.[Pg 412]
ORGANIC BASES
This group includes a number of compounds, all of which contain nitrogen
as well as carbon. They are characterized by combining directly with
acids to form salts, and in this respect they resemble ammonia. They
may, indeed, be regarded as derived from ammonia by displacing a part or
all of the hydrogen present in ammonia by hydrocarbon radicals. Among
the simplest of these compounds may be mentioned methylamine
(CH3NH2) and ethylamine (C2H5NH2). These two compounds
are gases and are formed in the distillation of wood and bones. Pyridine
(C5H6N) and quinoline (C9H7N) are liquids present in small
amounts in coal tar, and also in the liquid obtained by the distillation
of bones. Most of the compounds now classified under the general name of
alkaloids (which see) also belong to this group.
CARBOHYDRATES
The term "carbohydrate" is applied to a class of compounds which
includes the sugars, starch, and allied bodies These compounds contain
carbon, hydrogen, and oxygen the last two elements generally being
present in the proportion in which they combine to form water. The most
important members of this class are the following:
| Cane sugar | C12H22O11. |
| Milk sugar | C12H22O11. |
| Dextrose | C6H12O6. |
| Levulose | C6H12O6. |
| Cellulose | C6H10O5. |
| Starch | C6H1005. |
Cane sugar (C12H22O11). This is the well-known substance
commonly called sugar. It occurs in many plants[Pg 413] especially in the sugar
cane and sugar beet. It was formerly obtained almost entirely from the
sugar cane, but at present the greatest amount of it comes from the
sugar beet. The juice from the cane or beet contains the sugar in
solution along with many impurities. These impurities are removed, and
the resulting solution is then evaporated until the sugar crystallizes
out. The evaporation is conducted in closed vessels from which the air
is partially exhausted. In this way the boiling point of the solution is
lowered and the charring of the sugar is prevented. It is impossible to
remove all the sugar from the solution. In preparing sugar from sugar
cane the liquors left after separating as much of it as possible from
the juice of the cane constitute ordinary molasses. Maple sugar is made
by the evaporation of the sap obtained from a species of the maple tree.
Its sweetness is due to the presence of cane sugar, other products
present in the maple sap imparting the distinctive flavor.
When a solution of cane sugar is heated with hydrochloric or other
dilute mineral acid, two compounds, dextrose and levulose, are formed in
accordance with the following equation:
C12H22O11 + H2O = C6H12O6 + C6H12O6.
This same change is brought about by the action of an enzyme present in
the yeast plant. When yeast is added to a solution of cane sugar
fermentation is set up. The cane sugar, however, does not ferment
directly: the enzyme in the yeast first transforms the sugar into
dextrose and levulose, and these sugars then undergo alcoholic
fermentation.
When heated to 160° cane sugar melts; if the temperature is increased to
about 215°, a partial decomposition[Pg 414] takes place and a brown substance
known as caramel forms. This is used largely as a coloring matter.
Milk sugar (C12H22O11). This sugar is present in the milk of
all mammals. The average composition of cow's milk is as follows:
| Water | 87.17% |
| Casein (nitrogenous matter) | 3.56 |
| Butter fat | 3.64 |
| Milk sugar | 4.88 |
| Mineral matter | 0.75 |
When rennin, an enzyme obtained from the stomach of calves, is added
to milk, the casein separates and is used in the manufacture of cheese.
The remaining liquid contains the milk sugar which separates on
evaporation; it resembles cane sugar in appearance but is not so sweet
or soluble. The souring of milk is due to the fact that the milk sugar
present undergoes lactic fermentation in accordance with the equation
C12H22O11 + H2O = 4C3H6O3.
The lactic acid formed causes the separation of the casein, thus giving
the well-known appearance of sour milk.
Isomeric compounds. It will be observed that cane sugar and milk sugar
have the same formulas. Their difference in properties is due to the
different arrangement of the atoms in the molecule. Such compounds are
said to be isomeric. Dextrose and levulose are also isomeric.
Dextrose (grape sugar, glucose) (C6H12O6). This sugar is
present in many fruits and is commonly called grape sugar because of its
presence in grape juice. It can be obtained by heating cane sugar with
dilute acids, as[Pg 415] explained above; also by heating starch with dilute
acids, the change being as follows:
Pure dextrose is a white crystalline solid, readily soluble in water,
and is not so sweet as cane sugar. In the presence of yeast it undergoes
alcoholic fermentation. It is prepared from starch in large quantities,
and being less expensive than cane sugar, is used as a substitute for it
in the manufacture of jellies, jams, molasses, candy, and other sweets.
The product commonly sold under the name of glucose contains about 45%
of dextrose.
Levulose (fruit sugar)(C6H12O6). This sugar is a white solid
which occurs along with dextrose in fruits and honey. It undergoes
alcoholic fermentation in the presence of yeast.
Cellulose (C6H10O5). This forms the basis of all woody fibers.
Cotton and linen are nearly pure cellulose. It is insoluble in water,
alcohol, and dilute acids. Sulphuric acid slowly converts it into
dextrose. Nitric acid forms nitrates similar to nitroglycerin in
composition and explosive properties. These nitrates are variously known
as nitrocellulose, pyroxylin, and gun cotton. When exploded they yield
only colorless gases; hence they are used especially in the manufacture
of smokeless gunpowder. Collodion is a solution of nitrocellulose in a
mixture of alcohol and ether. Celluloid is a mixture of nitrocellulose
and camphor. Paper consists mainly of cellulose, the finer grades
being made from linen and cotton rags, and the cheaper grades from straw
and wood.
Starch (C6H10O5). This is by far the most abundant carbohydrate
found in nature, being present especially in[Pg 416] seeds and tubers. In the
United States it is obtained chiefly from corn, nearly 80% of which is
starch. In Europe it is obtained principally from the potato. It
consists of minute granules and is practically insoluble in cold water.
These granules differ somewhat in appearance, according to the source of
the starch, so that it is often possible to determine from what plant
the starch was obtained. When heated with water the granules burst and
the starch partially dissolves. Dilute acids, as well as certain
enzymes, convert it into dextrose or similar sugars. When seeds
germinate the starch present is converted into soluble sugars, which are
used as food for the growing plant.
Chemical changes in bread making. The average composition of wheat flour
is as follows:
| Water. | 13.8% |
| Protein (nitrogenous matter) | 7.9 |
| Fats | 1.4 |
| Starch | 76.4 |
| Mineral matter | 0.5 |
In making bread the flour is mixed with water and yeast, and the
resulting dough set aside in a warm place for a few hours. The yeast
first converts a portion of the starch into dextrose or a similar sugar,
which then undergoes alcoholic fermentation. The carbon dioxide formed
escapes through the dough, making it light and porous. The yeast plant
thrives best at about 30°; hence the necessity for having the dough in a
warm place. If the temperature rises above 50°, the vitality of the
yeast is destroyed and fermentation ceases. In baking the bread, the
heat expels the alcohol and also expands the bubbles of carbon dioxide
caught in the dough, thus increasing its lightness.[Pg 417]
SOME DERIVATIVES OF BENZENE
Attention has been called to the complex nature of coal tar. Among the
compounds present are the hydrocarbons, benzene, toluene, naphthalene,
and anthracene. These compounds are not only useful in themselves but
serve for the preparation of many other important compounds known under
the general name of coal-tar products.
Nitrobenzene (oil of myrbane) (C6H5NO2). When benzene is
treated with nitric acid a reaction takes place which is expressed by
the following equation:
C6H6 + HNO3 = C6H5NO2 + H2O.
The product C6H5NO2 is called nitrobenzene. It is a slightly
yellowish poisonous liquid, with a characteristic odor. Its main use is
in the manufacture of aniline.
Aniline (C6H5NH2). When nitrobenzene is heated with iron and
hydrochloric acid the hydrogen evolved by the action of the iron upon
the acid reduces the nitrobenzene in accordance with the following
equation:
C6H5NO2 + 6H = C6H5NH2 + 2H2O.
The resulting compound is known as aniline, a liquid boiling at 182°.
When first prepared it is colorless, but darkens on standing. Large
quantities of it are used in the manufacture of the aniline or coal-tar
dyes, which include many important compounds.
Carbolic acid (C6H5OH). This compound, sometimes known as
phenol, occurs in coal tar, and is also prepared from benzene. It
forms colorless crystals which are very soluble in water. It is strongly
corrosive and very poisonous.[Pg 418]
Naphthalene and anthracene. These are hydrocarbons occurring along with
benzene in coal tar. They are white solids, insoluble in water. The
well-known moth balls are made of naphthalene. Large quantities of
naphthalene are used in the preparation of indigo, a dye formerly
obtained from the indigo plant, but now largely prepared by laboratory
methods. Similarly anthracene is used in the preparation of the dye
alizarin, which was formerly obtained from the madder root.
THE ALKALOIDS
This term is applied to a group of compounds found in many plants and
trees. They all contain nitrogen, and most of them are characterized by
their power to combine with acids to form salts. This property is
indicated by the name alkaloids, which signifies alkali-like. The salts
are soluble in water, and on this account are more largely used than the
free alkaloids, which are insoluble in water. Many of the alkaloids are
used in medicine, some of the more important ones being given below.
Quinine. This alkaloid occurs along with a number of others in the bark
of certain trees which grow in districts in South America and also in
Java and other tropical islands. It is a white solid, and its sulphate
is used in medicine in the treatment of fevers.
Morphine. When incisions are made in the unripe capsules of one of the
varieties of the poppy plant, a milky juice exudes which soon thickens.
This is removed and partially dried. The resulting substance is the
ordinary opium which contains a number of alkaloids, the principal one
being morphine. This alkaloid is a white solid and is of great service
in medicine.[Pg 419]
Among the other alkaloids may be mentioned the following: Nicotine, a
very poisonous liquid, the salts of which occur in the leaves of the
tobacco plant; cocaine, a crystalline solid present in coca leaves and
used in medicine as a local anæsthetic; atropine, a solid present in
the berry of the deadly nightshade, and used in the treatment of
diseases of the eye; strychnine, a white, intensely poisonous solid
present in the seeds of the members of the Strychnos family.[Pg 421]
INDEX
Acetaldehyde 405
Acetic acid 406
Acetone 411
Acetylene 203
series 399
Acids 106
binary 113
characteristics 106
definition 107
dibasic 159
familiar 106
monobasic 159
nomenclature 113
organic 405
preparation 141
strength 111
ternary 113
undissociated 107
Acker furnace, 279
Agate 260
Air 83
a mechanical mixture 89
carbon dioxide in 87
changes in composition 87
liquid 91
nitrogen in 87
oxygen in 85
poisonous effects of exhaled 88
properties 90
quantitative analysis of 85
regarded as an element 83
standard for density 229
water vapor in 87
Alabaster 308
Alchemists 9
Alchemy 9
Alcohol, common 402
denatured 404
ethyl 402
methyl 402
wood 402
Alcohols 401
Aldehydes 405
Alizarin 418
Alkali 107, 274
family 274
Alkaline-earth family 300
Alkaloids 418
Allotropic forms 22
Alloys 252
Alum 333
ammonium 334
ammonium chrome 384
ammonium iron 352
baking powders 335
potassium 333
potassium chrome 384
potassium iron 352
Aluminates 332
Aluminium 327
bronze 330, 359
chloride 333
family 327
hydroxide 332
metallurgy 328
occurrence 327
oxide 331
preparation 328
properties 329
silicates 335
uses 330
Amalgam 362
Amethyst 260, 331
Ammonia 123
composition 127
preparation 123
properties 124
uses 125
Ammonium 126
acid carbonate 295
carbonate 295
chloride 294
[Pg 422]compounds 294
Ammonium hydrosulphide 296
hydroxide 126
molybdate 388
oxalate 407
sulphate 295
sulphide 295
sulphide, yellow 296
Analysis 40
Anhydride 135
carbonic 206
chromic 387
nitric 135
nitrous 135
phosphoric 243
sulphuric 153
Anhydrite 288
Aniline 417
Anion 106
Anode 99
Anthracene 418
Antimony 250
acids 251
alloys 253
chloride 252
metallic properties 252
occurrence 251
oxides 251
preparation 251
properties 251
sulphides 251
Apatite 175, 239, 311
Aqua ammonia 124
Aqua regia 185
Aqueous tension 25
Argon 80
Arsenic 246
acids 250
antidote 250
Marsh's test 248
occurrence 246
oxides 249
preparation 246
properties 247
sulphides 250
white 249
Arsenopyrites 246
Arsine 247
Asbestos 321, 336
Atmosphere 83
constituents 83
function of constituents 84
Atomic hypothesis 61
theory 59
and laws of matter 63
and radium 314
weights, 65
accurate determination 231
and general properties 167
and specific heats 233
calculation of 231
Dalton's method 223
direct determination 233
from molecular weights 230
relation to equivalent 224
standard for 66
steps in determining 224
Atoms 62
size 65
Atropine 419
Aurates 396
Avogadro's hypothesis 226
and chemical calculations 235
and molecular weights 227
Azote 78
Azurite 357
Babbitt metal 253
Bacteria 85
decomposition of organic matter by 122
nitrifying 85
Baking powders 285, 408
alum 335
soda 285
Barium 312
chloride 313
nitrate 313
oxides 312
sulphate 313
Barytes 312
Bases 107
characteristics 107
definition 108
familiar 107
nomenclature 113
organic 412
strength 113
[Pg 423]undissociated 108
Basic lining process 346
Bauxite 332
Beer 404
Benzene 417
derivatives 417
series 399
Benzine 400
Bessemer process 345
Bismuth 253
basic salts 255
chloride 253
nitrate 253
occurrence 253
oxides 254
preparation 253
salts, hydrolysis of 254
subnitrate 256
uses 253
Bismuthyl chloride 256
Blast furnace 341
lamp 38
Bleaching powder 306
Bleaching by chlorine 181
by sulphurous acid 152
Boiler scale 320
Bone ash 311
Bone black 200
Borax 265
bead 266
Bornite 357
Boron 257, 264
acids 265
fluoride 264
hydride 264
occurrence 264
oxides 264
preparation 264
properties 264
Brass 323
Bread making 416
Bromides 190
Bromine 187
occurrence 187
oxygen compounds 190
preparation 187
properties 188
Bronze 359
aluminium 330, 359
Butter fat 410
Butyric acid 407
By-product 284
Cadmium 325
compounds 326
Cæsium 294
Calamine 321
Calcite 305
Calcium 301
carbide 203, 310
carbonate 305
chloride 306
fluoride 308
hydroxide 303
occurrence 301
oxide 302
phosphate 246, 311
preparation 302
sulphate 308
Calomel 363
Calorie 76
Caramel 414
Carbohydrates 413
Carbolic acid 417
Carbon 196
allotropic forms 196
amorphous 198
compounds 196
crystalline forms 197
cycle in nature 88
dioxide 204
and bases 206
and plant life 88
in air 87
occurrence 204
preparation 204
properties 204
solid 204
disulphide 160, 210
family 196
hydrogen compounds 201
monoxide 208
occurrence 196
oxides 203
properties 200
pure 198
retort 199
uses 200
Carbonates 207
acid 207
Carbonic acid 206
Carborundum 259
Carnallite 288
[Pg 424]Casein 414
Cassiterite 370
Catalysis 153
Catalyzers 153
Cathode 99
Cation 106
Caustic potash 288
soda 278
Celestite 312
Celluloid 415
Cellulose 415
Cement 304
Ceramic industries 336
Cerium 377
Chalcedony 260
Chalcocite 357
Chalcopyrite 357
Chalk 305
Chamber acid 157
Changes, physical and chemical 2
Charcoal 199
Chemical affinity 12
changes 2
compounds 7
equilibrium 128
properties 3
Chemistry, definition 4
Chili saltpeter 191, 285
Chinaware 336
Chloric acid 187
Chlorides 186
Chlorine 177
bleaching action 181
chemical properties 180
family 174
historical 177
occurrence 178
oxides 187
oxygen acids 187
preparation 178
properties 179
Chloroform 401
Chloroplatinic acid 393
Chlorous acid 187
Chromates 385
Chrome alum 384
Chromic acid 388
anhydride 387
chloride 383
hydroxide 383
sulphate 384
sulphide 384
Chromite 383
Chromium 383
a base-forming element 383
an acid-forming element 385
occurrence 383
Cinnabar 363
Citric acid 408
Clay 336
Coal 199
gas 217
products 400
tar 218
Cobalt 354
compounds 354
Cocaine 419
Coke 199
Collodion 415
Colemanite 265
Combining weights 225
Combustion 17
broad sense 20
in air 19
phlogiston theory 19
products 18
spontaneous 20
supporters 213
Compounds, chemical 7
isomeric 414
of metals, preparation 265
structure of 118
Conservation of energy 4
of matter 5
Contact process 154
Converter, Bessemer 345
Copper 357
acetate 407
alloys of 359
family 356
hydroxide 360
metallurgy 357
occurrence 357
ores 357
oxide 360
properties 358
refining 358
sulphate 361
sulphide 361
uses 359
Copperas 350
Coral 305
[Pg 425]Corrosive sublimate 363
Corundum 331
Cream of tartar 408
Crocoisite 383
Cryolite 175, 328
Crystallization 98
water of 54, 75
Crystallography 161
Crystals 161
axes of 161
systems 162
Cupric compounds 360
Cuprite 360
Cuprous compounds 360
chloride 360
oxide 360
Cyanides 210
solutions are alkaline 210
Dalton's atomic hypothesis 61
Decay 21
Decomposition of organic matter 122
Decrepitation 55
Deliquescence 55
Density of gases 230
Desiccating agents 55
Developers 367
Dewar bulb 91
Dextrose 414
Diamond 197
Dichromates 385
Dichromic acid 385
Dimorphous substances 163
Dissociation 99
and boiling point 101
and freezing point 101
equations of 112
extent of 113
Distillation 50
Dogtooth spar 306
Dolomite 319
Double decomposition 71
Drummond light 38
Dyeing 333
Dynamite 409
Earth metals 327
Efflorescence 54
Electric furnace 221
Electro-chemical industries 269
Electrode 99
Electrolysis 99
of sodium chloride 102
of sodium sulphate 103
of water 41, 102
Electrolytes 99
Electrolytic dissociation 99
Electroplating 366
Electrotyping 359
Elements, definition 8
atomic weights 232
earlier classification 165
names 11
natural groups 165
number of 9
occurrence 10
periodic division 166
physical state 10
symbols of 11
Emery 331
Energy 4
and plant life 89
chemical 5
conservation of 4
transformation of 5
Enzyme 405
Epsom salts 320
Equations 68
are quantitative 72
knowledge requisite for 69
not algebraic 74
reading of 69
Equilibrium 138
chemical 138
in solution 139
point of 138
Equivalent 224
determination of 224
elements with more than one 225
relation to atomic weight 224
Etching 177
Ether 411
Ethereal salts 409
Ethers 411
Ethylamine 412
Ethylene series 399
Eudiometer 43
Evaporation 11
Families in periodic groups 170
[Pg 426]triads 165
Family resemblances 170
Fats 409
Fatty acid series 405
Feldspar 261, 335
Fermentation 404
acetic 406
alcoholic 404, 405
lactic 414
Ferric chloride 352
hydroxide 352
salts 351
reduction 353
sulphate 352
Ferrochromium, 383
Ferromanganese 343
Ferrosilicon 259
Ferrous carbonate 351
salts 350
oxidation of 353
sulphate 350
sulphide 350
Fertilizers 245
Filtration 6, 51
beds 52
Fire damp 202
Flames 213
appearance 214
blowpipe 216
Bunsen 214
conditions for 213
hydrogen 34
luminosity 216
oxidizing 214
oxyhydrogen 37
reactions 296
reducing 214
structure 214
Flash lights 317
Flint 260
Fluorides 177
Fluorine 175
Fluorspar 175, 308
Fluosilicic acid 259
Flux 340
Fool's gold 351
Formaldehyde 405
Formalin 405
Formic acid 406
Formulas 68
how determined 234
structural 119
Fractional distillation 51
Franklinite 321
Fuels 220
Furnace, arc 221
electric 221
resistance 221
Fusion methods 271
Galena 373
Gallium 327
Galvanized iron 323
Gas, collection of 15
coal 217
fuel 217
illuminating 217
measurement of 23
natural 219
purification of 218
water 219
Gases, table 220
Gasoline 400
German silver 323, 359
Germanium 370
Germs, effect of cold on 53
in air 84
in water 52
Glass 262
coloring of 263
etching of 177
molding of 263
nature of 263
varieties 263
Glauber's salt 281
Glazing 336
Glucose 414
Glycerin 405
nitrates of 409
Gold 393
alloys 396
chloride 396
coin 359
extraction of 394
in copper 358
mining 394
occurrence 393
properties 396
refining of 395
telluride 394
Goldschmidt method 269, 330
Gram-molecular weight 236
[Pg 427]Granite 336
Graphite 198
Gun cotton 415
metal 359
powder 292
Gypsite 308
Gypsum 308
Halogens 174
Hard water 309
Heat of reaction 75
Helium 80, 314
Hematite 339, 349
Homologous series 398
Hydriodic acid 193
Hydrobromic acid 189
Hydrocarbons 201, 398
properties 400
series 398
substitution products 401
Hydrochloric acid 182
composition 183
oxidation of 185
preparation 182
properties 184
salts 186
Hydrocyanic acid 210
Hydrofluoric acid 176
etching by 177
salts of 177
Hydrogen 28
dioxide 56
explosive with oxygen 35
occurrence 28
preparation from acids 30
preparation from water 28
properties 32
standard for atomic weights 66
standard for molecular weights 227
sulphide 146
uses 38
Hydrolysis 254
conditions affecting 255
partial 255
Hydrosulphuric acid 146
Hydroxyl radical 112
Hypochlorous acid 187
Hypothesis 61
Avogadro's 226
Dalton's 61
Ice manufacture 125
Iceland spar 305
Indigo 418
Indium 327
Insoluble compounds 272
Iodic acid 194
Iodides 193
Iodine 190
oxygen compounds 193
preparation 191
properties 192
tincture 192
Iodoform 192, 401
Ions 100
and electrolytes 104
Iridium 393
Iron 339
alum 352
cast 343
compounds 349
cyanides 352
disulphide 351
family 338
metallurgy 339
occurrence 339
ores 339
oxides 349
pure 348
varieties 342, 347
wrought 343
Jasper 260
Kainite 288
Kaolin 261, 335
Kerosene 400
Ketones 411
Kieserite 288
Kindling temperature 17
Krypton 80
Lactic acid 408
Lampblack 200
Laughing gas 132
Law, definition 61
of Boyle 24
of Charles 23
of combining volumes 194
of conservation of energy 4
of conservation of matter 5, 59
[Pg 428]of definite composition 59
of Dulong and Petit 233
of Gay-Lussac 194
of multiple proportion 60
of Raoult 233
periodic 169
Lead 373
acetate 375, 407
alloys 375
basic carbonate 376
carbonate 376
chloride 377
chromate 377
insoluble compounds 376
metallurgy 373
nitrate 375
occurrence 373
oxides 375
peroxide 375
properties 374
red 375
soluble salts 375
sugar of 375
sulphate 377
sulphide 377
white 376
Le Blanc soda process 282
Levulose 415
Lime 302
air-slaked 303
hypochlorite 307
kilns 303
slaked 303
Lime light 38
Limestone 305
Limewater 303
Limonite 339
Litharge 375
Lithium 294
Luminosity of flames 216
Lunar caustic 366
Magnesia 318
alba 319
usta 318
Magnesite 318
Magnesium 317
basic carbonate 319
carbonate 318
cement 318
chloride 319
family 316
hydroxide 318
oxide 318
silicates 321
sulphate 320
Magnetite 339, 349
Malachite 357
Manganates 381
Manganese 379
a base-forming element 380
an acid-forming element 381
in glass 263
occurrence 379
oxides 380
Manganic acid 381
Manganous salts 380
Marble 305
Marl 305
Marsh gas 202
Matches 242
Matte 358
Matter, classification 6
conservation 5
definition 5
kinds 9
Measurement of gases 23
Mechanical mixtures 6
Meerschaum 321, 336
Mercuric chloride 363
iodide 364
oxide 14, 362
sulphide 363
Mercurous chloride 363
Mercury 361
iodides 364
metallurgy 361
occurrence 361
oxides 362
uses 362
Metaboric acid 265
Metallurgy 268
Metals 165, 267
action on salts 271
definition 267
extraction 268
occurrence 267
preparation of compounds 269
reduction from ores 268
Metaphosphoric acid 245
Metarsenic acid 250
Metasilicic acid 261
[Pg 429]Metastannic acid 371
Methane 202, 399
Methylamine 412
Mexican onyx 305
Mica 261, 336
Microcosmic salt 244
Milk 414
Minerals 267
Minium 375
Mixed salts 244
Molasses 413
Molecular weights 226
boiling-point method 233
compared with oxygen 228
determination 226
freezing-point method 233
oxygen standard 227
of elements 232
vapor-density method 229
Molecule 62
Molybdenum 388
Molybdic acid 388
Monazite sand 377
Mordants 333
Morphine 418
Mortar 304
Moth balls 418
Muriatic acid 182
Naphthalene 418
Naphthas 400
Nascent state 182
Natural gas 219
sciences 1
Neon 80
Neutralization 108
a definite act 109
definition 109
heat of 109
partial 111
Niagara Falls 269, 329
Nickel 354
coin 359
compounds 354
plating 354
Nicotine 419
Nitrates 131
Nitric acid, 128
action on metals 130
decomposition 129
oxidizing action 130
preparation 128, 140
properties 129
salts 131
Nitric oxide 133
Nitrites 132
Nitrobenzene 417
Nitrocellulose 415
Nitrogen 78
compounds 122
in air 87
occurrence 78, 122
oxides 132
preparation 78
properties 80
Nitroglycerin 409
Nitrosulphuric acid 155
Nitrous acid 132
oxide 132
Non-metals 165
Oil of myrbane 417
of vitriol 154
Oleic acid 408
Olein 409
Oleomargarine 410
Onyx 260
Opal 260
Open-hearth process 346
Opium 418
Ores 267
Organic bases 412
chemistry 201, 397
matter, decomposition 122
Orpiment 246
Orthoarsenic acid 250
Orthophosphates 244
Orthophosphoric acid 244
Orthosilicic acid 261
Osmic acid 393
Osmium 393
tetroxide 393
Oxalic acid 407
Oxidation 17, 353
definition 18
Oxidizing agent 37
Oxygen 13
and ozone 22
commercial preparation 16
history 13
importance 21
in air estimation, 85
[Pg 430]in air function, 84
occurrence 13
preparation 13
properties 16
standard for atomic weights 66
two atoms in molecule 227
Oxyhydrogen blowpipe 37
Ozone 21, 137
Palladium 390
Palmitic acid 407
Palmitin 409
Paraffin 400
Paris green 250
Parkes's method for silver 364
Pearls 305
Perchloric acid 187
Periodic acid 194
Periodic division 166
groups 167
law 169
law, imperfections 172
law, value 171
table 168
table, arrangement 166
Permanent hardness 310
Permanganates 381
Permanganic acid 381
Peroxides 278
Petroleum 399
Pewter 372
Phenol 417
Philosopher's stone 9
Phlogiston 19
Phosphates 245
Phosphine 242
Phosphonium compounds 243
Phosphoric acid 244
Phosphorite 239
Phosphorous acid 244
Phosphorus 239
acids 243
family 238
hydrogen compounds 242
occurrence 239
oxides 243
preparation 239
properties 240
red 241
yellow 240
Photography 367
Physical changes 2
properties 3
properties and periodic groups 171
state 3
Physics 1, 4
Pitchblende 314
Plaster of Paris 308
Platinic chloride 393
Platinized asbestos 391
Platinous chloride 393
Platinum 391
a catalytic agent 152, 392
Pneumatic trough 16
Polyboric acid 265
Polyhalite 288
Polysilicic acids 261
Porcelain 336
Portland cement 304
Potash 293
Potassium 287
acid carbonate 294
acid sulphate 294
acid sulphite 294
alum, aluminium 334
alum, chrome 384
alum, iron 352
and plant life 287
aurate 396
bromide 290
carbonate 293
chlorate 291
chloride 290
chromate 385
cyanide 293
dichromate 386
ferricyanide 352
ferrocyanide 352
hydroxide 288
hydroxide, action of halogens 289
hypochlorite 289
iodide 290
manganate 381
nitrate 291
occurrence 287
permanganate 381
preparation 288
sulphate 294
Precipitated chalk 306
[Pg 431]Precipitation 140
Properties, chemical 3
physical 3
Prussic acid 210
Puddling 343
furnace 344
Pyridine 412
Pyrites 351
Pyrolusite 380
Pyrophosphoric acid 245
Quantitative equations 72
Quartz 260
Quicklime 302
Quinine 418
Quinoline 412
Radical 112
Radium 313
Reaction, classes 70
addition 70
completed 139
heat of 75
of decomposition 70
of double decomposition 71
of substitution 70
reversible 137
steps in 131
Realgar 246
Red lead 375
phosphorus 241
Reducing agent 37
Reduction 36, 354
Rennin 414
Resemblances, family 170
Respiration 87
Rhodium 390
Rochelle salts 408
Rouge 349
Rubidium 294
Ruby 331
Ruthenium 390
Rutile 264
Safety lamp 202
Sal ammoniac 294
soda 282
Salt 280
Saltpeter 291
Chili 285
Salts, 109
acid, 112
Salts basic 111
binary 114
characteristics 109
definition 109
insoluble 272
mixed 244
nomenclature 113
normal 112
preparation by precipitation 270
Sand 260
Sandstone 260
Saponification 410
Sapphire 331
Satinspar 308
Scale 320
Schönite 288
Selenite 308
Selenium 161
Serpentine 320, 336
Shot 247, 375
Siderite 339
Silica 260
Silicates 261
Silicic acids 261
Silicides 259
Silicon 258
acids 261
dioxide 260
fluoride 258
hydride 258
Silver 364
amalgamation process 364
bromide 367
chloride 367
coin 359
German 359
in copper ores 358
iodide 367
metallurgy 364
nitrate 366
oxide 366
parting of 365
refining 365
sulphide 366
Slag 340
Smalt 355
Smithsonite 321
Smokeless powder 293
Soaps 410
[Pg 432]Soda ash 284
Soda lime 202
Sodium 276
acetate 407
bicarbonate 285
carbonate 282
carbonate, historical 284
chloride 280
chromates 386
hydrogen carbonate 285
hydroxide 278
hyposulphite 282
iodate 191
nitrate 285
occurrence 276
peroxide 277
phosphates 286
preparation 276
properties 277
sulphate 281
sulphite 281
tetraborate 287
thiosulphate 282
Solder 372, 375
Solubility of gases 95
of solids 96
Solution 94
and chemical action 53
boiling point 98
classes 94
distribution of solids in 98
electrolysis of 99
freezing point 99
of gases in liquids 94
of solids in liquids 96
properties 98
saturated 97
supersaturated 98
Solvay soda process 283
Sombrerite 239
Spectroscope 296
Sphalerite 325
Spiegel iron 343
Spinel 332
Spontaneous combustion 20
Stalactites 305
Stalagmites 305
Standard conditions 23
Stannates 372
Stannic acid 372
chloride 372
oxide 372
Stannous chloride 372
Starch 415
Stassfurt salts 287
Stearic acid 407
Stearin 409
Steel 345
alloys 348
properties 347
tempering of 348
tool 347
Stibine 251
Stibnite 250
Stoneware 336
Strontianite 312
Strontium 312
hydroxide 312
nitrate 312
Structural formulas 119
Structure of compounds 119
Strychnine 419
Substitution 70
Sugars 412
cane 412
fruit 415
grape 414
milk 414
Sulphates 159
Sulphides 148
Sulphites 152
action of acids on 150
Sulphur 143
allotropic forms 144
chemical properties 145
comparison with oxygen 161
dioxide 149
preparation 149
properties 150
extraction 143
flowers of 143
occurrence 143
oxides 149
physical properties 144
trioxide 152
uses 146
varieties 144
Sulphuric acid 154
action as an acid 157
action on metals 157
action on organic matter 158
action on salts 158
[Pg 433]action on water 158
fuming 155
manufacture 154
oxidizing action 157
plant 156
properties 157
salts 159
Sulphuric anhydride 153
Sulphurous acid 151
Superphosphate of lime 246
Sylvine 288
Symbols 11
Synthesis 40
Table, alkali metals 274
alkaline-earth metals 300
alloys of copper 359
aqueous tension Appendix B
atomic weights Appendix A
chlorine family 174
composition of earth's crust 10
composition of fuel gases 220
constants of elements Appendix B
copper family 356
elements Appendix A
gold and platinum metals 390
hydrocarbons 399
magnesium family 316
manganese and chromium 379
periodic arrangement 168
phosphorus family 238
silicon family 257
solubility of gases in water 95
solubility of salts 96
solubility of salts at different temperatures 97
tin and lead 370
weights of gases Appendix B
Talc 321, 336
Tartar emetic 408
Tartaric acid 408
Tellurium 161
Temporary hardness 309
Ternary acids 113
salts 114
Tetraboric acid 265
Thallium 327
Theory, atomic 61
definition 64
value of 64
Thermite 331
Thio compounds 282
Thiosulphates 159
Thiosulphuric acid 159
Thorium 377
Tin 370
block 371
compounds 372
crystals 372
family 370
foil 371
metallurgy 370
plate 371
properties 371
uses 371
Titanium 257, 264
Topaz 331
Triad families 166
Tungsten 388
Type metal 253, 375
Uranium 388
Valence 116
a numerical property 116
and combining ratios 118
and equations 120
and formulas 120
and periodic groups 162
and structure 118
definition 116
indirectly determined 117
measure of 117
variable 117
Vaseline 400
Venetian red 349
Verdigris 407
Vermilion 363
Vinegar 406
Vitriol, blue 361
green 350
oil of 154
white 324
Volume and aqueous tension 25
and pressure 24
and temperature 23
of combining gases 194
Water 40
a compound 40
[Pg 434]and disease 49
catalytic action of 154
chalybeate 351
chemical properties 53
composition 47
composition by volume 44
composition by weight 47
dissociation of 210
distillation of 50
electrolysis of 41, 103
filtration of 51
gas 219
hard 309
historical 40
impurities in 48
in air 87
mineral 49
occurrence 48
of crystallization 54, 75
physical properties 53
purification of 50
qualitative analysis 41
quantitative analysis 42
river 49
sanitary analysis 50
self-purification 53
softening of 310
standard substance 55
synthesis 43
uses of 55
Weights, atomic 65
Welsbach mantles 219, 377
Whisky 404
Wine 404
Witherite 312
Wood alcohol 402
distillation 402
Wood's metal 254
Xenon 80
Yeast 403
Zinc 321
alloys of 323
blende 321
chloride 325
flowers of 322
metallurgy 321
occurrence 321
oxide 324
sulphate 324
sulphide 325
white 324
Zymase, 403
ANNOUNCEMENTS
AN ELEMENTARY STUDY OF CHEMISTRY
By WILLIAM McPHERSON, Professor of Chemistry in Ohio State University,
and WILLIAM E. HENDERSON, Associate Professor of Chemistry in Ohio State
University.
12mo. Cloth. 434 pages. Illustrated. List price, $1.25; mailing price,
$1.40
This book is the outgrowth of many years of experience in the teaching
of elementary chemistry. In its preparation the authors have steadfastly
kept in mind the limitations of the student to whom chemistry is a new
science. They have endeavored to present the subject in a clear,
well-graded way, passing in a natural and logical manner from principles
which are readily understood to those which are more difficult to grasp.
The language is simple and as free as possible from unusual and
technical phrases. Those which are unavoidable are carefully defined.
The outline is made very plain, and the paragraphing is designed to be
of real assistance to the student in his reading.
The book is in no way radical, either in the subject-matter selected or
in the method of treatment. At the same time it is in thorough harmony
with the most recent developments in chemistry, both in respect to
theory and discovery. Great care has been taken in the theoretical
portions to make the treatment simple and well within the reach of the
ability of an elementary student. The most recent discoveries have been
touched upon where they come within the scope of an elementary text.
Especial attention has been given to the practical applications of
chemistry, and to the description of the manufacturing processes in use
at the present time.
EXERCISES IN CHEMISTRY. By WILLIAM McPHERSON and WILLIAM E. HENDERSON.
(In press.)
GINN & COMPANY PUBLISHERS
A FIRST COURSE IN PHYSICS
By ROBERT A. MILLIKAN, Associate Professor of Physics, and HENRY G.
GALE, Assistant Professor of Physics in The University of Chicago
12mo, cloth, 488 pages, illustrated, $1.25
A LABORATORY COURSE IN PHYSICS
FOR SECONDARY SCHOOLS
By ROBERT A. MILLIKAN and HENRY G. GALE 12mo, flexible cloth, 134 pages,
illustrated, 40 cents
This one-year course in physics has grown out of the experience of the
authors in developing the work in physics at the School of Education of
The University of Chicago, and in dealing with the physics instruction
in affiliated high schools and academies.
The book is a simple, objective presentation of the subject as opposed
to a formal and mathematical one. It is intended for the third-year
high-school pupils and is therefore adapted in style and method of
treatment to the needs of students between the ages of fifteen and
eighteen. It especially emphasizes the historical and practical aspects
of the subject and connects the study very intimately with facts of
daily observation and experience.
The authors have made a careful distinction between the class of
experiments which are essentially laboratory problems and those which
belong more properly to the classroom and the lecture table. The former
are grouped into a Laboratory Manual which is designed for use in
connection with the text. The two books are not, however, organically
connected, each being complete in itself.
All the experiments included in the work have been carefully chosen with
reference to their usefulness as effective classroom demonstrations.
GINN AND COMPANY PUBLISHERS
APPENDIX A
LIST OF THE ELEMENTS, THEIR SYMBOLS, AND ATOMIC WEIGHTS
The more important elements are marked with an asterisk
O = 16
| *Antimony | Sb | 120.2 |
| *Argon | A | 39.9 |
| *Arsenic | As | 75.0 |
| *Barium | Ba | 137.4 |
| Beryllium | Be | 9.1 |
| *Bismuth | Bi | 208.5 |
| *Boron | B | 11.0 |
| *Bromine | Br | 79.96 |
| *Cadmium | Cd | 112.4 |
| Cæsium | Cs | 132.9 |
| *Calcium | Ca | 40.1 |
| *Carbon | C | 12.00 |
| Cerium | Ce | 140.25 |
| *Chlorine | Cl | 35.45 |
| *Chromium | Cr | 52.1 |
| *Cobalt | Co | 59.0 |
| Columbium | Cb | 94.0 |
| *Copper | Cu | 63.6 |
| Erbium | Er | 166.0 |
| *Fluorine | F | 19.0 |
| Gadolinium | Gd | 156.0 |
| Gallium | Ga | 70.0 |
| Germanium | Ge | 72.5 |
| *Gold | Au | 197.2 |
| Helium | He | 4.0 |
| *Hydrogen | H | 1.008 |
| Indium | In | 115.0 |
| *Iodine | I | 126.97 |
| Iridium | Ir | 193.0 |
| *Iron | Fe | 55.9 |
| Krypton | Kr | 81.8 |
| Lanthanum | La | 138.9 |
| *Lead | Pb | 206.9 |
| Lithium | Li | 7.03 |
| *Magnesium | Mg | 24.36 |
| *Manganese | Mn | 55.0 |
| *Mercury | Hg | 200.0 |
| Molybdenum | Mo | 96.0 |
| Neodymium | Nd | 143.6 |
| Neon | Ne | 20.0 |
| *Nickel | Ni | 58.7 |
| *Nitrogen | N | 14.04 |
| Osmium | Os | 191.0 |
| *Oxygen | O | 16.00 |
| Palladium | Pd | 106.5 |
| *Phosphorus | P | 31.0 |
| *Platinum | Pt | 194.8 |
| *Potassium | K | 39.15 |
| Praseodymium | Pr | 140.5 |
| Radium | Ra | 225.0 |
| Rhodium | Rh | 103.0 |
| Rubidium | Rb | 85.5 |
| Ruthenium | Ru | 101.7 |
| Samarium | Sm | 150.3 |
| Scandium | Sc | 44.1 |
| Selenium | Se | 79.2 |
| *Silicon | Si | 28.4 |
| *Silver | Ag | 107.93 |
| *Sodium | Na | 23.05 |
| *Strontium | Sr | 87.6 |
| *Sulphur | S | 32.06 |
| Tantalum | Ta | 183.0 |
| Tellurium | Te | 127.6 |
| Terbium | Tb | 160.0 |
| Thallium | Tl | 204.1 |
| Thorium | Th | 232.5 |
| Thulium | Tm | 171.0 |
| *Tin | Sn | 119.0 |
| Titanium | Ti | 48.1 |
| Tungsten | W | 184.0 |
| Uranium | U | 238.5 |
| Vanadium | V | 51.2 |
| Xenon | Xe | 128.0 |
| Ytterbium | Yb | 173.0 |
| Yttrium | Yt | 89.0 |
| *Zinc | Zn | 65.4 |
| Zirconium | Zr | 90.6 |
APPENDIX B
Tension of Aqueous Vapor expressed in Millimeters of Mercury
| TEMPERATURE | PRESSURE |
| 16 | 13.5 |
| 17 | 14.4 |
| 18 | 15.3 |
| 19 | 16.3 |
| 20 | 17.4 |
| 21 | 18.5 |
| 22 | 19.6 |
| 23 | 20.9 |
| 24 | 22.2 |
| 25 | 23.5 |
Weight of 1 Liter of Various Gases measured under Standard Conditions
| Acetylene | 1.1614 |
| Air | 1.2923 |
| Ammonia | 0.7617 |
| Carbon dioxide | 1.9641 |
| Carbon monoxide | 1.2499 |
| Chlorine | 3.1650 |
| Hydrocyanic acid | 1.2036 |
| Hydrochloric acid | 1.6275 |
| Hydrogen | 0.08984 |
| Hydrosulphuric acid | 1.5211 |
| Methane | 0.7157 |
| Nitric oxide | 1.3410 |
| Nitrogen | 1.2501 |
| Nitrous oxide | 1.9677 |
| Oxygen | 1.4285 |
| Sulphur dioxide | 2.8596 |
Densities and Melting Points of Some Common Elements
| DENSITY | MELTING POINT |
| Aluminium | 2.68 | 640 |
| Antimony | 6.70 | 432 |
| Arsenic | 5.73 | — |
| Barium | 3.75 | — |
| Bismuth | 9.80 | 270 |
| Boron | 2.45 | — |
| Cadmium | 8.67 | 320 |
| Cæsium | 1.88 | 26.5 |
| Calcium | 1.54 | — |
| Carbon, Diamond | 3.50 | — |
| " Graphite | 2.15 | — |
| " Charcoal | 1.80 | — |
| Chromium | 7.30 | 3000 |
| Cobalt | 8.55 | 1800 |
| Copper | 8.89 | 1084 |
| Gold | 19.30 | 1064 |
| Iridium | 22.42 | 1950 |
| Iron | 7.93 | 1800 |
| Lead | 11.38 | 327 |
| Lithium | 0.59 | 186 |
| Magnesium | 1.75 | 750 |
| Manganese | 8.01 | 1900 |
| Mercury | 13.596 | -39.5 |
| Nickel | 8.9 | 1600 |
| Osmium | 22.47 | — |
| Palladium | 11.80 | 1500 |
| Phosphorus | 1.80 | 45 |
| Platinum | 21.50 | 1779 |
| Potassium | 0.87 | 62.5 |
| Rhodium | 12.10 | — |
| Rubidium | 1.52 | 38.5 |
| Ruthenium | 12.26 | — |
| Silicon | 2.35 | — |
| Silver | 10.5 | 960 |
| Sodium | 0.97 | 97.6 |
| Strontium | 2.50 | — |
| Sulphur | 2.00 | 114.8 |
| Tin | 7.35 | 235 |
| Titanium | 3.50 | — |
| Zinc | 7.00 | 420 |
End of the Project Gutenberg EBook of An Elementary Study of Chemistry, by
William McPherson and William Edwards Henderson
*** END OF THIS PROJECT GUTENBERG EBOOK AN ELEMENTARY STUDY OF CHEMISTRY ***
***** This file should be named 20848-h.htm or 20848-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/2/0/8/4/20848/
Produced by Elaine Walker, Josephine Paolucci and the
Online Distributed Proofreading Team at http://www.pgdp.net
Updated editions will replace the previous one--the old editions
will be renamed.
Creating the works from public domain print editions means that no
one owns a United States copyright in these works, so the Foundation
(and you!) can copy and distribute it in the United States without
permission and without paying copyright royalties. Special rules,
set forth in the General Terms of Use part of this license, apply to
copying and distributing Project Gutenberg-tm electronic works to
protect the PROJECT GUTENBERG-tm concept and trademark. Project
Gutenberg is a registered trademark, and may not be used if you
charge for the eBooks, unless you receive specific permission. If you
do not charge anything for copies of this eBook, complying with the
rules is very easy. You may use this eBook for nearly any purpose
such as creation of derivative works, reports, performances and
research. They may be modified and printed and given away--you may do
practically ANYTHING with public domain eBooks. Redistribution is
subject to the trademark license, especially commercial
redistribution.
*** START: FULL LICENSE ***
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full Project
Gutenberg-tm License (available with this file or online at
http://gutenberg.org/license).
Section 1. General Terms of Use and Redistributing Project Gutenberg-tm
electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or destroy
all copies of Project Gutenberg-tm electronic works in your possession.
If you paid a fee for obtaining a copy of or access to a Project
Gutenberg-tm electronic work and you do not agree to be bound by the
terms of this agreement, you may obtain a refund from the person or
entity to whom you paid the fee as set forth in paragraph 1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this agreement
and help preserve free future access to Project Gutenberg-tm electronic
works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the Foundation"
or PGLAF), owns a compilation copyright in the collection of Project
Gutenberg-tm electronic works. Nearly all the individual works in the
collection are in the public domain in the United States. If an
individual work is in the public domain in the United States and you are
located in the United States, we do not claim a right to prevent you from
copying, distributing, performing, displaying or creating derivative
works based on the work as long as all references to Project Gutenberg
are removed. Of course, we hope that you will support the Project
Gutenberg-tm mission of promoting free access to electronic works by
freely sharing Project Gutenberg-tm works in compliance with the terms of
this agreement for keeping the Project Gutenberg-tm name associated with
the work. You can easily comply with the terms of this agreement by
keeping this work in the same format with its attached full Project
Gutenberg-tm License when you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are in
a constant state of change. If you are outside the United States, check
the laws of your country in addition to the terms of this agreement
before downloading, copying, displaying, performing, distributing or
creating derivative works based on this work or any other Project
Gutenberg-tm work. The Foundation makes no representations concerning
the copyright status of any work in any country outside the United
States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other immediate
access to, the full Project Gutenberg-tm License must appear prominently
whenever any copy of a Project Gutenberg-tm work (any work on which the
phrase "Project Gutenberg" appears, or with which the phrase "Project
Gutenberg" is associated) is accessed, displayed, performed, viewed,
copied or distributed:
This eBook is for the use of anyone anywhere at no cost and with
almost no restrictions whatsoever. You may copy it, give it away or
re-use it under the terms of the Project Gutenberg License included
with this eBook or online at www.gutenberg.org
1.E.2. If an individual Project Gutenberg-tm electronic work is derived
from the public domain (does not contain a notice indicating that it is
posted with permission of the copyright holder), the work can be copied
and distributed to anyone in the United States without paying any fees
or charges. If you are redistributing or providing access to a work
with the phrase "Project Gutenberg" associated with or appearing on the
work, you must comply either with the requirements of paragraphs 1.E.1
through 1.E.7 or obtain permission for the use of the work and the
Project Gutenberg-tm trademark as set forth in paragraphs 1.E.8 or
1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any additional
terms imposed by the copyright holder. Additional terms will be linked
to the Project Gutenberg-tm License for all works posted with the
permission of the copyright holder found at the beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including any
word processing or hypertext form. However, if you provide access to or
distribute copies of a Project Gutenberg-tm work in a format other than
"Plain Vanilla ASCII" or other format used in the official version
posted on the official Project Gutenberg-tm web site (www.gutenberg.org),
you must, at no additional cost, fee or expense to the user, provide a
copy, a means of exporting a copy, or a means of obtaining a copy upon
request, of the work in its original "Plain Vanilla ASCII" or other
form. Any alternate format must include the full Project Gutenberg-tm
License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works provided
that
- You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is
owed to the owner of the Project Gutenberg-tm trademark, but he
has agreed to donate royalties under this paragraph to the
Project Gutenberg Literary Archive Foundation. Royalty payments
must be paid within 60 days following each date on which you
prepare (or are legally required to prepare) your periodic tax
returns. Royalty payments should be clearly marked as such and
sent to the Project Gutenberg Literary Archive Foundation at the
address specified in Section 4, "Information about donations to
the Project Gutenberg Literary Archive Foundation."
- You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or
destroy all copies of the works possessed in a physical medium
and discontinue all use of and all access to other copies of
Project Gutenberg-tm works.
- You provide, in accordance with paragraph 1.F.3, a full refund of any
money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days
of receipt of the work.
- You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project Gutenberg-tm
electronic work or group of works on different terms than are set
forth in this agreement, you must obtain permission in writing from
both the Project Gutenberg Literary Archive Foundation and Michael
Hart, the owner of the Project Gutenberg-tm trademark. Contact the
Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
public domain works in creating the Project Gutenberg-tm
collection. Despite these efforts, Project Gutenberg-tm electronic
works, and the medium on which they may be stored, may contain
"Defects," such as, but not limited to, incomplete, inaccurate or
corrupt data, transcription errors, a copyright or other intellectual
property infringement, a defective or damaged disk or other medium, a
computer virus, or computer codes that damage or cannot be read by
your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH F3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium with
your written explanation. The person or entity that provided you with
the defective work may elect to provide a replacement copy in lieu of a
refund. If you received the work electronically, the person or entity
providing it to you may choose to give you a second opportunity to
receive the work electronically in lieu of a refund. If the second copy
is also defective, you may demand a refund in writing without further
opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS' WITH NO OTHER
WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO
WARRANTIES OF MERCHANTIBILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of damages.
If any disclaimer or limitation set forth in this agreement violates the
law of the state applicable to this agreement, the agreement shall be
interpreted to make the maximum disclaimer or limitation permitted by
the applicable state law. The invalidity or unenforceability of any
provision of this agreement shall not void the remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in accordance
with this agreement, and any volunteers associated with the production,
promotion and distribution of Project Gutenberg-tm electronic works,
harmless from all liability, costs and expenses, including legal fees,
that arise directly or indirectly from any of the following which you do
or cause to occur: (a) distribution of this or any Project Gutenberg-tm
work, (b) alteration, modification, or additions or deletions to any
Project Gutenberg-tm work, and (c) any Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of computers
including obsolete, old, middle-aged and new computers. It exists
because of the efforts of hundreds of volunteers and donations from
people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need, is critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future generations.
To learn more about the Project Gutenberg Literary Archive Foundation
and how your efforts and donations can help, see Sections 3 and 4
and the Foundation web page at http://www.pglaf.org.
Section 3. Information about the Project Gutenberg Literary Archive
Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Its 501(c)(3) letter is posted at
http://pglaf.org/fundraising. Contributions to the Project Gutenberg
Literary Archive Foundation are tax deductible to the full extent
permitted by U.S. federal laws and your state's laws.
The Foundation's principal office is located at 4557 Melan Dr. S.
Fairbanks, AK, 99712., but its volunteers and employees are scattered
throughout numerous locations. Its business office is located at
809 North 1500 West, Salt Lake City, UT 84116, (801) 596-1887, email
[email protected]. Email contact links and up to date contact
information can be found at the Foundation's web site and official
page at http://pglaf.org
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
[email protected]
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To
SEND DONATIONS or determine the status of compliance for any
particular state visit http://pglaf.org
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including checks, online payments and credit card donations.
To donate, please visit: http://pglaf.org/donate
Section 5. General Information About Project Gutenberg-tm electronic
works.
Professor Michael S. Hart is the originator of the Project Gutenberg-tm
concept of a library of electronic works that could be freely shared
with anyone. For thirty years, he produced and distributed Project
Gutenberg-tm eBooks with only a loose network of volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as Public Domain in the U.S.
unless a copyright notice is included. Thus, we do not necessarily
keep eBooks in compliance with any particular paper edition.
Most people start at our Web site which has the main PG search facility:
http://www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.
 ANTOINE LAURENT LAVOISIER
ANTOINE LAURENT LAVOISIER