
The Project Gutenberg EBook of Scientific American Supplement, No. 623, December 10, 1887, by Various This eBook is for the use of anyone anywhere at no cost and with almost no restrictions whatsoever. You may copy it, give it away or re-use it under the terms of the Project Gutenberg License included with this eBook or online at www.gutenberg.org Title: Scientific American Supplement, No. 623, December 10, 1887 Author: Various Release Date: July 12, 2005 [EBook #16270] Language: English Character set encoding: ISO-8859-1 *** START OF THIS PROJECT GUTENBERG EBOOK SCIENTIFIC AMERICAN *** Produced by Juliet Sutherland and the Online Distributed Proofreading Team at www.pgdp.net.
The hot air engine, although theoretically recognized for some time past as the most economical means of converting heat into motive power, has up to the present met with little success. This is due to the fact that the arrangement of the motors of this class that have hitherto been constructed has been such as to render them but slightly practical. In the Benier hot air engine (illustrated herewith), however, obstacles that were once considered insurmountable have been overcome, and the motor presents many advantages over all the types that have preceded it. Among such advantages we shall cite the possibility of utilizing air at a high temperature (1,200 or 1,500 degrees), while the rubbing surfaces remain at a moderate temperature (60 or 80 degrees). The fire grate is placed in the interior of the cylinder, and is traversed by the cold air forced by a pump. The expanded hot gases fill the cylinder and act against the piston directly above the grate.
The type herewith illustrated is of 6 horse power. The motive cylinder, CC', is bolted to the extremity of the frame, A. Upon this latter is fixed a column, B, which carries a working beam, E. This latter transmits the motion of the piston, P, to the shaft, D. A pump, G, placed within the frame, forces a certain quantity of cold air at every revolution into the driving cylinder. The piston of this pump is actuated by the connecting rod, G', jointed to the lever, F', which receives its motion from the rod, F. A slide valve, b', actuated by a cam, regulates the entrance of the cold air into the pump during suction, as well as its introduction into the cylinder. There is a thrust upon the piston during its upward travel, and an escape of hot gas through the eduction valve, h, during the downward travel.
The cylinder is in two parts, C and C'. The piston, which is very long, rubs at its upper end against the sides of the cylinder, C. The lower end is of smaller diameter, and leaves an annular space between it and the cylinder. The grate is at the bottom of the cylinder, C'. The sides of the cylinder at the level of the fire box are protected with a lining of plumbago. When the piston is at the bottom of its travel, the eduction valve closes. The slide valve, b', establishes a communication between the pump chamber and the cylinder. The air contained in the pump is already compressed in the latter to a pressure of nearly a kilogramme at the moment of the communication. This air enters the cylinder, and the communication between the latter and the pump continues until all the air is forced into the driving cylinder, the piston of the pump being at the bottom of its travel, and that of the cylinder about midway.
The air forced by the pump piston enters the cylinder through two conduits, one of which leads a portion of it toward the top of the cylinder, and the other toward the bottom. The lower conduit debouches under the grate, and the air that passes through it traverses the fire box, and the hot gas fills the cylinder. The conduit that runs to the top debouches in the cylinder, C, at the lower limit of the surface rubbed by the piston. The air that traverses this conduit is distributed through the annular space between the piston and cylinder. The hot gas derived from combustion can therefore never introduce itself into this annular space, and consequently cannot come into contact with the rubbing surfaces of the cylinder and piston.
As the quantity of air introduced at every stroke is constant, the work developed at every stroke is varied by regulating the temperature of the gas that fills the cylinder. When the temperature falls, the pressure, and consequently the work developed, diminishes. This result is obtained by varying the respective quantities of air that pass through the fire box and around the piston. In measure as less air passes through the fire box, the quantity that passes around the piston augments by just so much, and the pressure diminishes. A valve, n', in the conduit that runs to the fire box is controlled by the regulator, L', in the interior of the column. When the work to be transmitted diminishes, the regulator closes the valve more or less, and the work developed diminishes.
The coke is put by shovelfuls into a hopper, I. Four buckets mounted upon the periphery of a wheel, I', traverse the coke, and, taking up a piece of it, let it fall upon the cover, J, of the slide valve, j, whence it falls into the cavity of the latter when it is uncovered, and from thence into the conduit, c', of the box, j', when the cavity of the valve is opposite the conduit. From the conduit, c', the coke falls upon the grate.
A small sight hole covered with glass, in the cover, J, permits the grate to be seen when the cavity of the valve is opposite c'.
As in gas engines, a current of water is made to flow around the cylinder, C', in order to keep the sides from getting too hot.
In order to set the engine in motion, we begin by opening the bottom, C, of the cylinder, C', to clean the grate. This done, we close C and introduce lighted charcoal through the conduit, c' (the valve being open). The valve is put in place, two or three revolutions are given to the fly wheel, and the motor starts. The feeding is afterward done with coke.
The parts that transmit motion operate under conditions analogous to those under which the same parts of a steam engine do. The air pump sucks and forces nothing but cold air, and nothing but cold air passes through the distributing slide valve. The pump and valve are therefore rendered very durable. The piston and cylinder, at the points where friction exists, are at a temperature of 60 or 80 degrees. These surfaces are protected against hot gas charged with dust.
The hot gas, which escapes from the cylinder through a valve, has previously been cooled by contact with the sides of the cylinder and by expansion. The eduction valve just mentioned works about like that of a steam engine, and it is only necessary to polish it now and then in order to keep it in good condition.—Annales Industrielles.
Mr. President and Ladies and Gentlemen: It has not been considered the duty of the speaker, in addressing the graduating class, to dwell on the triumphs of science or the advantage of a liberal education. These subjects have already been discussed, in connection with the regular courses of study, better, and more at length, than he could do. We propose rather to try and prepare the minds of the graduates for the practical problems before them.
All young men are impressed with the consciousness of higher powers as they increase their stores of knowledge, and this feeling perhaps reaches its maximum with those who have made a specialty of the investigation and application of physical laws. Young men who have learned how to harness the powers of nature and guide them to do their will are apt to belittle the difficulties they have yet to overcome, and have a false impression of the problems of life. This feeling is shown to a minimum extent by graduates of the Stevens Institute, on account of their careful practical training, in connection with the thorough study of principles; but it has been thought best for one from the outside world to supplement such teaching by calling to mind instances which may have a useful counteracting effect, and, like parables, serve the purpose of illustrative instruction.
Gentlemen of the Class of '87: It was the pleasure of the speaker to address the class of '79, under the title of "How to Succeed," some words of counsel and warning, which, if they left an impression of severity at the time, were apparently so well received afterward that he has been tempted to continue the general subject, with the title of "Your Future Problems." The notation of your future problems will not be found at once among the known quantities, but with x, y, and z, at the other end of the alphabet. Often word symbols will be applicable, expressing at times disappointment and pain, at other times renewed effort, and finally the active phases of individual thought and exertion.
The first serious problem with many of you will be to secure satisfactory engagements. This problem cannot be illustrated by parables. It needs, in general, patient, unremitting, and frequently long continued effort. It may be that the fame of some of you, that have already acquired the happy faculty of making yourselves immediately useful, has already gone abroad and the coveted positions been already assured. To be frank, we cannot promise you even a bed of roses. We have in mind an instance where a superior authority in a large business enterprise who had great respect, as he should have, for the attainments of young gentlemen who have had the opportunities of a technical education, deliberately ordered out a competent mechanical engineer, familiar with the designs required in a large repair shop, and sent in his place a young gentleman fresh from school and flushed with hope, but who from the very nature of the case could know little or nothing of his duties at that particular place. He was practically alone in the drawing room, and did not know where to find such drawings as were required, and candor requires it to be said that he desired to ask many questions about those he did find. The superintendent unfortunately had nothing to do with his appointment, and rather resented it. So he did not trust any of his work, and the new comer was obliged to learn his practical experience at that establishment, where he was known as the mechanical engineer, by having all his work done over by the pattern maker or others, under the eye of the superintendent or master mechanic, and be subjected all the time to the jealousies and annoyances incident to such a method of introduction.
His practical experience was certainly learned under difficulties which I trust none of you may experience. This statement is made that those of you who have not yet obtained positions may not envy those who have, and that each and all of you may be careful not to take a position so far above your experience, if not your capacity, as to become unpleasantly situated in the beginning. The educational facilities you have enjoyed are of such great value in some exceptional cases that the parties thus benefited may do you an injury by leading others to expect that you will be equally valuable in performing duties which require much more practical experience and knowledge of detail than it is possible that you could have obtained in the time you have been here.
The incident is ripe with suggestions. No matter how humble a position you may take in the beginning, you will be embarrassed in much the same way as the young gentleman in question, though it is hoped in a less degree. Your course of action should be first to learn to do as you are told, no matter what you think of it. And above everything keep your eyes and ears open to obtain practical knowledge of all that is going on about you. Let nothing escape you of an engineering nature, though it has connection with the business in hand. It may be your business the next day, and if you have taken advantage of the various opportunities to know all about that particular matter in every detail, you can intelligently act in relation to it, without embarrassment to yourself and with satisfaction to your superior.
Above all, avoid conflict with the practical force of the establishment into which you are introduced. It is better, as we have at another time advised, to establish friendly relations with the workmen and practical men with whom you have to do.
You are to be spared this evening any direct references to the "conceit of learning," but you are asked and advised to bear with the conceit of ignorance. You will find that practical men will be jealous of you on account of your opportunities, and at the same time jealous of their own practical information and experience, and that they may take some pains to hinder rather than aid you in your attempts to actively learn the practical details of the business. The most disagreeable man about the establishment to persons like you, who perhaps goes out of his way to insult you, and yet should be respected for his age, may be one who can be of greatest use to you. Cultivate his acquaintance. A kind word will generally be the best response to an offensive remark, though gentlemanly words of resentment may be necessary when others are present. Sometimes it will be sufficient to say, "I wish a little talk with you by yourself," which will put the bystanders at a distance and enable you to mature your plans. Ascertain as soon as possible that man's tastes; what he reads and what he delights in. Approach him as if you had no resentment and talk on his favorite topic. If rebuffed, tell a pleasant story, and persist from time to time in the attempt to please, until his hardened nature relaxes and he begins to feel and perhaps speaks to others favorably of you. St. Paul has said: "For though I be free from all men, yet have I made myself servant of all that I might gain the more." This is the keynote of policy, and though in humbling yourself you control and hide your true feelings, recollect that all your faculties are given you for proper use.
We have referred to some who have acquired the happy faculty of making themselves immediately useful. This is a much more difficult matter than the words imply. If one of you should be so fortunate as to be ordered to make certain tests almost like those you have already conducted here, or to tabulate the results of tests as you have done it here, or to make inspections akin to those which have been fully explained here, there is every probability the work would be done satisfactorily in the first instance. But let a much simpler case arise, for instance, if a superior hand one of you a letter with the simple instructions, "Get me the facts on that," you may be very much puzzled to know what is to be done and how to do it. It may be that the letter is a request for information in regard to certain work that was carried on in the past, in which case it will be necessary for you to hunt through old records, copy books, engineering notes, drawings, and the like, and get a list of all referring to the subject; to make an abstract of the letters and notes if they are at all complicated; and finally to lay the whole before the overworked superior in a business manner, that he largely from recollection, aided by the references and notes, can write an intelligent answer in a very brief period. The way not to do it would be to say, "Yes, sir," very promptly, go off and not more than half read the letter, do something and be back in five minutes with some question or ill-digested answer; then upon receiving a polite hint as to the method to be employed, go off and repeat the operation the next five minutes; then on receiving a short reply, in what appeared to be an unnecessary tone of voice, get a little flurried perhaps, do worse next time, and in the end feel very unpleasant without having accomplished much, and make the gentleman seeking assistance lament the difficulty in teaching young men practical work.
It is possible, on the contrary, for a young man to exceed his instructions and volunteer advice that has not been asked. If he has unfortunately gone too far for some time and been sharply spoken to, he may fail the next in not fully doing the work intended. Simply putting down a column of figures would not necessarily mean tabulating facts. The arrangement and rearrangement of the columns aid in classifying such facts, so that the results shown by them will be readily seen and a great deal of labor saved in examination. A good rule in a case of this kind is to try and find some work done by other parties of a similar nature, and thereby ascertain what is needed and expected. Reasonable questions to ascertain, where records are to be found and the kind of records accessible, are always proper if made at the proper time without interrupting an immediate train of thought; and with such information as a start, if a young man will endeavor to imagine himself in a place like that of the one who has finally to decide, and try to ascertain just what information will probably be required, then patiently go to work to find and present it in condensed shape, he from that moment really begins to be useful and his services will be rapidly appreciated. It is a good rule always to keep the memoranda obtained in accomplishing a result of this kind; so that if further information is required, the whole investigation need not be made over.
This remark suggests another line of thought. Some young men with quick perceptions get in the way at school of trusting their memories, and omit making complete notes of lectures or of the various tests illustrating their studies. This carelessness follows them into after life, and there are instances where young men, who can make certain kinds of investigations much better than their fellows, and promptly give a statement of the general nature of the results, have, when called on afterward for the details, forgotten then entirely, and their notes and memoranda, if preserved, being of little use, the labor is entirely lost. Such men necessarily have to learn more careful ways in after life. It is a good rule in this, as in the previous case, to make and copy complete records of everything in such shape that they may be convenient for reference and criticism afterward.
One of the important problems with which you will have to deal in the future is the labor question, and it is probable that your very first experience with it may be in direct antagonism with the opinions of many with whom you have heretofore been associated. It is an honor to the feelings of those who stand outside and witness this so-called struggle now in progress between capital and labor, that they believe the whole question can be settled by kindly treatment and reasonable argument. There are some cases that will yield to such treatment, and one's whole duty is not performed till all possible, reasonable, and humanitarian methods are adopted. There has been an excuse for the organization of labor, and it, to some small extent, still exists.
Time was that the surplus of unskilled labor was used on a mercantile basis to reduce wages to such an extent that it was almost impossible to rear a well nurtured, much less a well educated and well dressed family, and, moreover, the hours of labor in some branches of business were so long as to shorten the lives of operatives and make self-improvement impossible. The natural progress of civilizing influence did much to abate many of these evils, but the organization of labor removed sores that had not and perhaps could not have been reached in other ways. Having then an excuse for organization, and supported by the success made in directions where public sympathy was with them, is it to be wondered that they have gone too far in very many cases, and that the leadership of such organization has in many instances been captured by designing men, who control the masses to accomplish selfish ends? Whatever may have been the method of evolution, it is certain that the manufacturing operations of the present day have to meet with elements entirely antagonistic to their interests, and in very many ways antagonistic to the interests of the workingman. The members of many organizations, even of intelligent men, are blindly led by chiefs of various titles, of which perhaps the walking delegate is the most offensive one to reasonable people. This class of men claim the right to intrude themselves into the establishments owned by others, and on the most trivial grounds make demands more or less unreasonable, and order strikes and otherwise interfere with the work of manufacturers, much in the way that we have an idea that the agents of the barbarbous chieftains, feudal lords, and semi-civilized rulers collected taxes and laid burdens in earlier historical times. Necessarily these men must use their power so as to insure its permanency. If strikes are popular, strikes must be ordered. If funds run low, excuses for strikes, it is believed, in many cases are sought, so as to stir the pulses of those who sympathize with the labor cause.
Co-operation has been suggested as a cure for the evil, and there are cases where it has apparently succeeded, in connection with the earlier forms of labor organization. The ambition of later labor leaders almost prevents this remedy being of effect. It may be possible still with very intelligent workmen, isolated from the large mass of workmen in the country towns, to feel an interest in co-operation; but such inducements, or the higher ones of personal kindness to employes or their families, are not of much effect in large manufacturing centers. As soon as dissatisfaction exists in one mill or manufactory, all similar employes are ordered out. The final result will be that combinations of employers must follow the combination of employes, and those who have always been strong in the past will be stronger in the future, as has appeared to be the case in many contests that have already taken place. If there are any real abuses of power by the employers, such as requiring work for unusual hours or at less than living rates, the first thing to do is to correct these abuses, so that complaints will not be upon a sound foundation. Some men, when the labor epidemic strikes their places, have sufficient force of character and influence with their men to avert the blow for some time. Others find it is policy to compromise with the representatives until a plan of action, conciliatory, offensive, or defensive, can be determined upon. The whole matter must be considered one of policy rather than of principles. The class of men to be dealt with do not talk principles except as an excuse to secure their ends.
In spite of everything, there will be times when no compromise is possible and you will be called upon to take part in defending your employers' interests against what is called a "strike." You can do so with heart when you know the employes are all well paid, and particularly, as is frequently the case, when the labor organizers and walking delegates claim that some old, tried foreman shall be dismissed because they do like him, really because he has not been a tool in carrying out their plans, and they defiantly acknowledge that their war is against non-union labor, and that they have organized your men and forced a strike to require your establishment to become as it is called a "union shop." If your deluded employes were permitted simply to go away and let you alone, and you were permitted to employ others at the reasonable wages you were paying, the problem would be a simple one. The principal labor organizations claim that everything they do is by peaceable methods, but this, like many things said, is simply to deceive, for if you attempt to employ other assistants and carry on your business independently, you will surely find that well known roughs are assembled who never do anything without they are paid for it by somebody, that your men are assaulted by such persons, and while the labor organizers talk about peaceable methods and urge them aloud in public, in case one of the roughs is arrested, the loud talkers are the first to go bail for the defender, and you will feel morally sure that the sympathizing crowd with the roughs who make the assaults are all part of or tools of the organization.
At such times, you will find your old employes standing around the street corners, persuading other men not to go to work and thus interfere with what are called the true interests of labor. Any new employe who has to go in the street will be first met with inducements of other employment, with offers of money, afterward with threats, and, if opportunity occurs, with direct assault. All the features of persuasion, intimidation, and violence will be carried out as demanded, and strangers to everybody in the vicinity, but well known as experienced leaders in this kind of work in other places, be brought in to endeavor to make the strike a success. Then, young men, is the time to show your pluck, and our experience is that educated young men will do so every time. They can be depended upon to go straight ahead with duty through every danger, bearing patiently everything that may be said, defending themselves with nature's weapons as long as possible, and without fear using reserve weapons in case real danger of life is imminent.
In carrying through a very important strike against a mere desire to control and not to correct abuses, your speaker desires to pay the highest tribute to a number of educated young men, mostly from the technical schools, who fearlessly faced every danger, and by their example stimulated others to do their duty, and all participated in the results obtained by a great success.
We would not by such references fire your hearts to a desire to participate in such an unpleasant contest. It is the duty of all to study this problem intelligently and earnestly, with a view of overcoming the difficulties and permitting the prosperity of the country to go on. While conciliation may be best at some times, policy at another, and resistance at another, we must also be thinking of the best means to prevent further outbreaks. It would seem to be true policy not to interfere with organization, but to try and direct it into higher channels. Those of the humanitarians who claim that the disease will be rooted out eventually by a more general and better education are undoubtedly largely in the right, notwithstanding that some fairly educated men have acted against their best interests in affiliating with the labor organizations. It seems to the speaker that enough instances can be collected to show the utter folly of the present selfish system, based, as it is, entirely on getting all that is possible, independent of right in the matter, and by demanding equal wages for all men, tending to lower all to one common degradation, instead of rewarding industry and ability and advancing the cause of civilization.
Labor should not be organized for selfish ends, but for its own good, so as to secure steady and permanent employment, rather than prevent it by impracticable schemes and unwise methods, which will cripple manufacturers and all kinds of industry. The men should organize under the general laws of the State, so that their leaders will be responsible to the laws and can be indicted, tried, and punished in case they misappropriate funds or commit any breach of trust; and such laws should be amended if necessary, so that wise, responsible leaders of the organizations can contract to furnish labor for a certain time at a fixed price, when manufacturers can make calculations ahead as to the cost of labor the same as for the cost of material, and have such confidence that they will use all their energies to do a larger amount of business and benefit the workingman as well as themselves by furnishing steady employment. Such a plan as is here outlined can readily be carried into effect by selecting better men as leaders. It is well known how well the organization known as the locomotive brotherhood is conducted, and it should be an example to others. It has had its day of dissensions, when the best counsels did not prevail, which shows that any organization of the kind, no matter how well conducted, may be diverted by its leaders into improper channels.
When organized under the laws of the State and under by-laws designed to secure steady employment, rather than any artificial condition of things in regard to pay hours, and continuance of labor, the true interests of the workman will be advanced. It may be that some one of you will develop a talent in the direction of organization and be the means of aiding in the solution of this great problem. Please think of the matter seriously, watch the law of evolution while you are advancing your professional knowledge, and if the opportunity offers, do all you can to aid in a cause so important and beneficent.
One writer has criticised the technical schools because they do not teach mechanical intuition. The schools have enough to do in the time available if they teach principles and sufficient practice to enable the principles to be understood. The aptitude to design, which must be what is meant by mechanical intuition, requires very considerable practical experience, which you will readily learn if you do not keep yourself above it. If you have used your leisure hours to study why a certain piece of mechanism was made in a certain way rather than in another; if you have wondered why one part is thick in one place rather than in another, apparently in defiance of all rules of the strength of material; if you have endeavored to ascertain why a particular device is used rather than another more evident one; if you have thought and studied why a boss is thrown in here and there in designs to receive bolts or to lengthen a journal, and if you have in your mind, by repeated observation, a fair idea of how work is designed by other people, the so-called mechanical intuition will be learned and found to be the combination of common sense and good practice.
You will observe that some details have been copied for years and years, although thoughtful men would say they are not the best, simply because they are adapted to a large amount of work already done. This is particularly true of the rolling stock on railroads. The cost of a change in starting in a new country might be warranted, but it practically cannot be done when the parts must interchange with so much work done in other parts of the country. You will find in other cases that the direct strain to which a piece of mechanism is subjected is only one of the strains which occur in practice. A piece of metal may have been thickened where it customarily broke, and you may possibly surmise that certain jars took place that caused such breakages, or that particular point was where the abuse of the attendant was customarily applied.
Wherever you go you will find matters of this kind affecting designs staring you in the face, and you will soon see why a man who has learned his trade in the shop, and from there worked into the drawing room with much less technical information than you have, can get along as well as he does. Reserve your strength, however. Your time will come. Whenever there is a new departure to be taken, and matters to be worked out from the solid which require close computation of strains or the application of any principles, your education will put you far ahead, and if you have, during the period of what may be called your post-graduate course, which occurs during your early introduction into practical life, been careful to keep your eyes and ears open so as to learn all that a man in practical life has done, you will soon stand far ahead.
Reference was made to the use of leisure hours. Leisure hours can be spent in various ways. For instance, in studying the composition and resolution of forces and the laws of elasticity in a billiard room, the poetry of motion, etc., in a ball room, and the chemical properties of various malt and vinous extracts in another room; but the philosophical reason why certain engineering work is done in the way it is, and the proper way in which new work shall be done of a similar character and original work of any kind carried on, can only be learned by cultivating your powers of observation and ruminating on the facts collected in the privacy of one's own room, away from the allurements provided for those who have nothing to do. No one would recommend you to so separate yourself from the world as to sacrifice health and strength, or to become a recluse, even if you did learn all about a certain thing.
Remember, however, that the men who have accomplished most in this world worked the longest hours, and any one with a regular occupation must utilize his leisure hours to obtain prestige. The difference between one man and another of the same natural ability lies entirely in the amount of his information and the facility with which he can use it. Life is short, and you must realize that now is your opportunity. If any diversion in the way of pleasure or even certain kinds of congenial work is offered, consider it in connection with the question, "Will this be conducive to my higher aim?" This implies that you have a higher aim; and if you have it, and weigh everything in this way, you will find that every moment of exertion adds something to your storehouse of information and brings you nearer to the accomplishment of that higher aim.
In closing, we thank the ladies and gentlemen present for their close attention to details of special interest only to those engaged in technical study or practice.
We congratulate you, young gentlemen of the class of '87, for the success you have thus far obtained, and trust that you will persevere in well doing and win greater success in the future. We need hardly state that all that has been said was in a spirit of kindness, and we feel assured that much of it has been seconded by your parents, to whom no less than to all parents here present off or on the stage, the speaker not excepted, a serious, thoughtful problem has been, still is, and will continue to be to many, "What shall we do with our boys."—Stevens Indicator.
An address to the graduating class, Stevens Institute, Hoboken, N.J., 1887.
We were recently witness of an experiment made at Eragny Conflans on the steam yacht Flamboyante. It was a question of testing a new vaporizer or burner for liquid fuel. The experiment was a repetition of the one that the inventor, Mr. G. Dietrich, recently performed with success in the presence of Admirals Cloue and Miot.
The Flamboyante is 58 ft. in length, 9 ft. in width, draws 5 ft. of water, and has a displacement of 10 tons. She is provided with a double vertical engine supplied by a Belleville boiler that develops 28 horse power. The screw makes 200 revolutions per minute, and gives the yacht a speed of 6½ knots.
Mr. Dietrich's vaporizer appears to be very simple, and has given so good results that we have thought it of interest to give our readers a succinct description of it. In this apparatus, the inventor has endeavored to obtain an easy regulation of the two essential elements—naphtha and steam.
Fig. 1 represents the apparatus in section. The steam enters through the tubulure, A, and finds its way around the periphery of a tuyere, D. It escapes with great velocity, carries along the petroleum that runs from two lateral tubulures, B (Fig. 2), and throws it in a fine spray into the fireplace, through the nozzle, C (Fig. 1), which is flattened into the shape of a fan opened out horizontally. The mixture at once ignites in contact with the hot gases, and gives a beautiful, long, clear flame. The air necessary for the combustion is sucked through the interior of the nozzle, H, which is in front of the tuyere. It will be seen that the current of steam can be regulated by moving the tuyere, D, from or toward the eduction orifice. This is effected through a maneuver of the hand wheel, F. In the second place, the flow of the petroleum is made regular by revolving the hand wheel, G, which gives the piston, O, a to and fro motion in the tuyere, D.
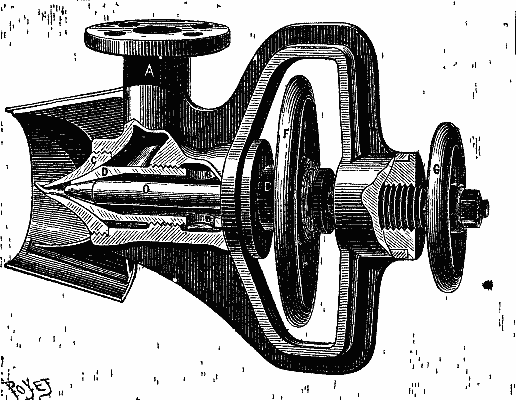
FIG. 1—THE DIETRICH PETROLEUM BURNER.
The regulation may be performed with the greatest ease. It is possible to instantly vary, together or separately, the steam and the petroleum. Under such circumstances, choking is not to be feared at the petroleum orifice, where, according to experiment, the thickness of the substance to be vaporized should not be less than 0.04 of an inch.
The petroleum might evidently be made to enter at A and the steam at B; but one of the conclusions of the experiments cited is that the performance is better when the jet of steam surrounds the petroleum. It will be understood, in fact, that by this means not a particle of the liquid can escape vaporization and, consequently, combustion. Moreover, as the jet of petroleum is completely surrounded by steam its flow can be increased within the widest limits, and this, in certain cases, may prevent an obstruction without much diminishing the useful effect of the burner.
The apparatus is easily and rapidly taken apart. It it is only necessary to remove the nozzle, C, in order to partially clean it. It would even seem that the cleaning might be done automatically by occasionally reversing the flow of the steam and petroleum. However efficacious such a method might prove, the apparatus as we have described it can be very easily applied to any generator. Fig. 2 represents it as applied to the front of a furnace provided with two doors. A metallic box, with two compartments, is placed on one side of the furnace, and is provided with two stuffing boxes that are capable of revolving around the steam and petroleum pipes. The latter thus form the pivots of the hinge that allows of the play of the vaporizers and piping.
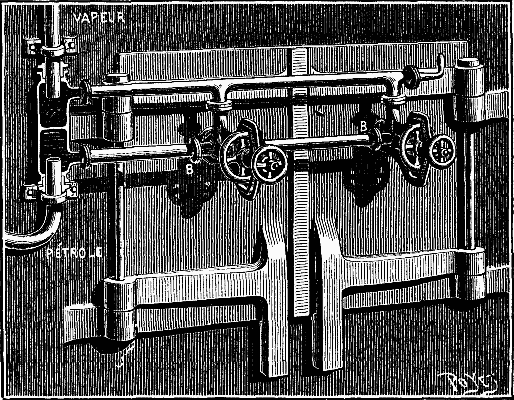
FIG. 2—THE BURNER APPLIED TO THE FURNACE OF A BOILER.
It was in this way that Mr. Dietrich arranged his apparatus in an experiment made upon a stationary boiler belonging to a Mr. Corpet. The experiment was satisfactory and led to the adoption of the arrangement shown in Fig. 3. The fire bridge is constructed of refractory bricks, and the majority of the grate bars are filled in with brick. The few free bars permit of the firing of the boiler and of access of air to the interior of the fire box. Under such circumstances, the combustion is very regular, the furnace does not roar, and the smoke-consuming qualities are perfect.
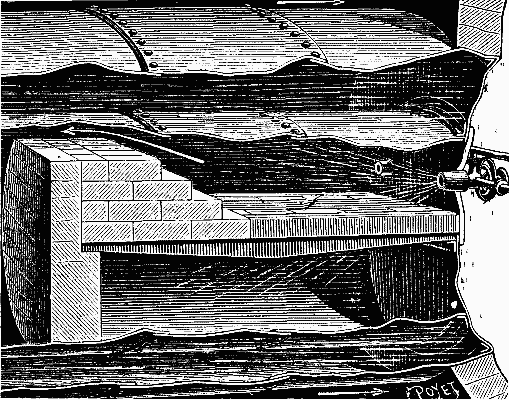
FIG. 3—APPLICATION OF THE BURNER TO A RETURN FLAME BOILER.
In the experiment on the Flamboyante, the boiler was provided with but one apparatus, and the grate remained covered with a layer of ignited coal that had been used for firing up in order to obtain the necessary pressure of steam to set the vaporizer in operation. This ignited coal appeared to very advantageously replace the refractory bricks, the role of which it exactly fulfilled. It has been found well, moreover, to break the flames by a few piles of bricks in the furnace, in order to obtain as intimate a mixture as possible of the inflammable gases.
It is to be remarked that firing up in order to obtain the necessary steam at first is a drawback that might be surmounted by using at the beginning of the operation a very small auxiliary boiler. The main furnace would then be fired by means of say a wad of cotton. But, in current practice, if a grate and fire be retained, the firing will perhaps be simpler.
With but one apparatus, the pressure in the Flamboyante's boiler rose in a few minutes from 6 to 25 pounds, and about a quarter of an hour after leaving the wharf the apparatus had been so regulated that there was no sign of smoke. This property of the Dietrich burner proceeds naturally from the use of a jet of steam to carry along the petroleum and air necessary for combustion. It is, in fact, an Orvis smoke consumer transformed, and applied in a special way.
It must be added that the regulating requires a certain amount of practice and even a certain amount of time at every change in the boat's running. So it is well to use two, and even three, apparatus, of a size adapted to that of the boiler. The regulation of the furnace temperature is then effected by extinguishing one or two, or even three, of the apparatus, according as it is desired to slow up more or less or to come to a standstill.
The oil used by Mr. De Dosme on his yacht comes from Comaille, near Antun. The price of it is quite low, and, seeing the feeble consumption (from 33 to 45 lb. for the yacht's boiler), it competes advantageously with the coal that Mr. De Dosme was formerly obliged to use.—La Nature.
[Continued from SUPPLEMENT, No. 622, page 9935.]
Many of the wheels that were still in use with the long hub were put into a lathe, and a groove was cut an inch and a half back from the face, leaving our cast collar, which was easily split off as before. (Fig. 24.)
With tender wheels, as with our car wheels, the case was different. Originally, the axle for the 5 ft. gauge was longer than for the 4 ft. 9 in.; but latterly the 5 ft. roads had used a great many master car builders' axles for the 4 ft. 9 in. gauge, namely, 6 ft. 11¼ in. over all, thus making the width of the truck the same as for 4 ft. 9 in. gauge. To do this a dished wheel, or rather a wheel with a greater dish by 1½ in. than previously used, was needed, so that the tread of the wheel could be at its proper place. (See Fig. 25.) There were, of course, many of the wheels with small dish and long axles still in use. Their treatment, however, when the day of change came, did not vary from that of the short axle.
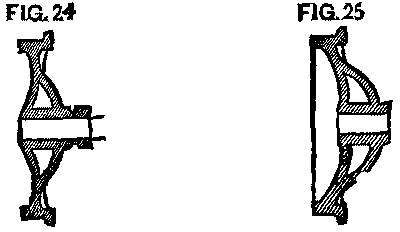
FIG. 24 and FIG. 25
It had been the rule for some years that all axles should be turned back 1½ in. further than needed; but unfortunately the rule had not been closely followed, and many were found not to be so turned. To make the matter worse, quite a number of the wheels were found to have been counterbored about ½ in. deep at the back end, and the axle turned up to fit this counterbore; a good idea to prevent the running in, in case the wheel worked loose, but bad from the standpoint of a change of gauge. In such cases the wheels had to be started off before the axle could be turned back, so that the wheels could be pushed on in their proper position. (Fig. 26.)
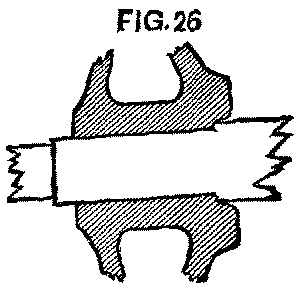
FIG. 26
If the work was done where they had a lathe large enough to swing a pair of wheels, they were pressed off but half an inch, the wheels swung in the lathe, the axles turned back 1½ in., and the wheels then pressed on 2 in. or 1½ in. inside of their first position.
Where no large lathe was in use, the wheels came entirely off before the axles could be turned back. The work in the former case was both the quicker and the cheaper. Where the large lathes were used they were either set down into the floor, so a pair of wheels would easily roll into place, or a raised platform was put before the lathe, with an incline up which the wheels were rolled and then taken to the lathe. These arrangements were found much quicker and cheaper than to hoist the wheels up, as is usually done.
In pressing the wheels on, where the axles had previously been turned back, much trouble was at first experienced because of the rust that had gathered upon the turned part behind the wheel, forming a ridge over or upon which the wheel must be pushed. Some of the roads, at the start, burst 10 or 15 per cent. of the wheels so pressed on. By saturating this surface with coal oil, however, it was found that the rust was easily removed and little trouble was had. It was found, sometimes, that upon axles newly turned back a careless workman would leave a ridge at the starting point of the turning. Frequently also the axles were a little sprung, so that the new turning would be a little scant upon one side when compared with the old surface, and upon the opposite side a little full. As an indication that these difficulties were overcome as they appeared, I will say that upon our line only 202 wheels burst out of nearly 27,000 pressed on—an exceedingly small percentage.
After the change upon the early roads they were troubled for weeks with hot boxes, caused, as we believed, by the changing of brasses. A brass once fitted to a journal will work upon it without trouble, but when placed upon some other journal will probably not fit. If the journal had been worn hollow (and it was surprising to see how many were so worn), the brass would be found worn down to fit it. (See Fig. 27. Exaggerated, of course.)
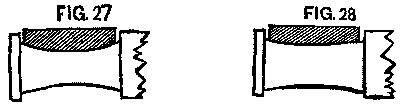
FIG. 27 and FIG. 28
The next wheel may have an axle worn little or none. (See Fig. 28)
Now, if these brasses are exchanged, we have the conditions as shown in Figs. 29 and 30, and we must expect they will heat. The remedy was simply to keep each brass upon its own journal. To do this the brasses were fastened to the axle by a piece of small wire, and went with it to the lathe and press. When its truck was reached, the brass was there with its journal. Worn-out brasses, of course, could not be put in, and new ones were substituted. The little trouble from that source that followed the change showed the efficacy of the remedy.
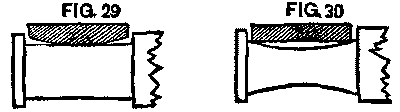
FIG. 29 and FIG. 30
The manner in which the tires of engines were to be changed, when the final day came, was a serious question. The old-fashioned fire upon the ground could not be thought of. The M. & O. had used a fire of pine under the wheel, which was covered by a box of sheet iron, so arranged that the flame and heat would be conveyed around the tire, and out at an aperture at the top. (Fig. 31.) Many thought this perfect, while others were not satisfied, and began experiments for something better. A device for using gas had been patented, but it was somewhat complicated, as well as expensive, and did not meet with general favor. A very simple device was soon hit upon. A two inch pipe was bent around in a circle a little larger than the outer rim of the wheel. Holes 1/10 in. in diameter and 3 or 4 in. apart were drilled through the pipe on the inside of the circle. To this pipe was fastened another with a branch or fork upon it. To one branch or fork was connected a gas pipe from the meter, while to the other was connected a pipe from an air pump. With the ordinary pressure of city gas upon this pipe it was found that the air pump must keep an air pressure of 40 pounds, that the air and gas might mix properly at the branch or fork, so we could get the best combustion and most heat from our "blowpipe," for such it was. (Fig. 32.)
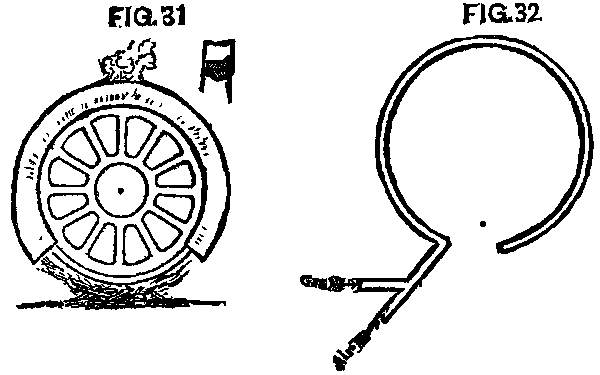
FIG. 31 and FIG. 32
We were able to heat a tire so it could be moved in ten to twenty minutes, and the machine may be said to have been satisfactory.
Gas, however, was not to be had at all places where it would be necessary to change tires, and the item of cost was considerable.
To reach a result as good, if possible, experiments were begun with coal oil (headlight oil). They were crude and unsatisfactory at first, but soon success was reached.
A pipe was bent to fit the lower half of a wheel pretty closely and then turned back under itself about the diameter of the pipe distant from it. This under part had holes 1/10 in. diameter and 3 or 4 in. apart drilled upon its upper side or under the upper pipe. Connected with the upper pipe at its center was a pipe which ran to one side and up to the can containing the kerosene. Between the can and the pipe under the wheel was a stop cock, by which the flow of oil could be controlled.
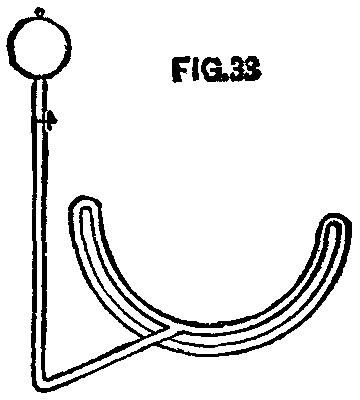
FIG. 33
To use the device, open the cock and let a small amount of oil flow; apply fire to the pipe under the wheel, and the oil in the upper pipe is converted into gas, which flows out of the small holes in the lower pipe, takes fire, and heats not only the tire, but the upper pipe, thus converting more oil into gas. We had here a lot of blue flame jets and the same result as with gas, but at less cost. We had also a machine that was inexpensive and easily handled anywhere. Boxes were placed over the upper parts of the wheels, that the heat might pass closely to the tire. This device was extensively used by our people, and with great satisfaction. In one way care had to be taken, viz.: That in starting the fire it did not smoke and cover the tire with carbon or "lampblack," which is a non-conductor of heat.
Experiments were made with air forced through gasoline, and with oil heated in a can to form gas. There was more danger in either of these than with our blowpipe device, and no better results were obtained, though the cost was greater.
With the change of the wheels, the brakes had to be changed the same amount, that is, each one set in 1½ in. This it was thought would either require new hangers or a change in the head or shoe in some way. We found that the hangers could easily be bent without removal. Fig. 34 shows three hangers after passing through the bending process. A short lever arranged to clasp the hanger just below the point, A, was the instrument; a forked "shore" is now placed, with the fork, against the point, A, and the other end against the car sill; press down on the lever and you bend the hanger at A; lower the lever to a point just below B, reverse the process, and you have the bend at B; the whole thing taking less than two minutes per hanger. A new bolt hole, of course, has been bored in the brake beam 1½ in. inside the old hole. It takes but a short time after this to change the position of the head and shoe.
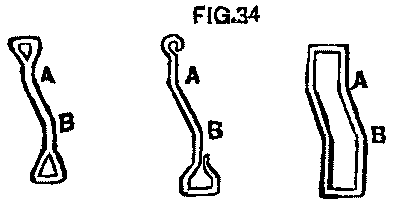
FIG. 34
Before the day of change, a portion of the spikes were drawn from the inside of the rail to be moved, and spike set 3 in. inside of the rail. As a rule two spikes were drawn and the third left. At least every third spike was set for the new gauge, and in some cases every other one.
There were several devices with which to set the spike. A small piece of iron 3 in. wide was common, and answered the purpose well. This had a handle, sometimes small, just large enough for the hand to clasp, while others had a handle long enough for a man to use it without stooping down. (See Figs. 35 and 36.) Another device is shown in Fig. 37, so arranged that the measurements were made from the head of the other rail. This was liked best, and, it is thought, gave the best results, as the moved rail was more likely to be in good line than when the measurements were taken from the flange.
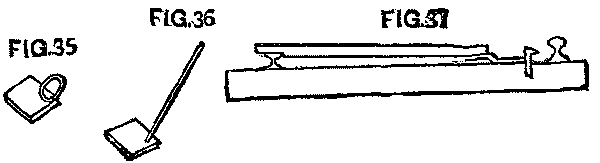
FIG. 35, FIG. 36 and FIG. 37
It was intended that great care should be taken in driving the spikes, that they were in the proper place, square with the rail, and left sticking up about an inch.
The ties, of course, were all adzed down before the day of change.
"Handspikes" were originally used to throw the rails, as were lining bars.
We found, however, that small "cant hooks" were more easily handled and did better work. The first were made like Fig. 38, with a spike in the end of a stick, while the hook was fastened with a bolt about 10 or 12 inches above the foot.
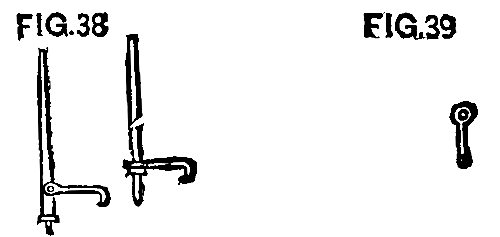
FIG. 38 and FIG. 39
We afterward made them of a 1¼ in. rod, 3½ ft. long, pointed at one end, with a ring shrunk on 1 ft. from the bottom. Then the hook was made with an eye, as shown in Fig. 39, which slipped down over the top of the main rod. This was simple and cheap, and the iron was to be used for repair purposes when this work was done.
Upon the system with which the writer was connected we had some branches where we could experiment upon the moving of the rail. Between Selma and Lauderdale the traffic was light, and at Lauderdale it connected with the Mobile & Ohio Railroad, which was narrow, and to which all freight had to be transferred, either by hoisting the cars or by handling through the house. By changing our gauge we would simply change the point of transfer to Selma. Here was a chance to experiment upon one hundred miles and cause little trouble to traffic. We could see the practical workings of our plans, and, at the same time, leave less to do on the final day. Upon the 20th of April we did this work. It had been our plan to do it somewhat earlier, but floods prevented.
Most of the rail was old chair iron, short, and consequently more time was used in making the change than would have been required had our work been on fishplate rail. Our sections here were about eight miles long, and we arranged our men on the basis blocked out by the committee, viz., 24 to 26 men to the section, consisting of 6 spike pullers, 4 throwing rails, 12 spikers, 2 to push the cars and carry water.
We soon found 5 ft. cars useless, and threw them into the ditch to be picked up at some future time.
The men were spread out so as not to be in each other's way, and when the organization was understood and conformed to, it worked well. One gang changed 5 miles in 5 hours and 10 minutes, including a number of switches. We found, however, and it was demonstrated still more strongly on later work, that after 5 or 6 miles the men began to lag.
We believed we had the best results when we had sections of about that length.
It was arranged that two sections, alternately, commenced work together at one point, working from each other and continuing until the force of another section was met, working from the opposite direction.
The foreman in charge was expected to examine the work and know that all was right. The push car which followed was a good test as to gauge.
A work train was started from each end with a small force (20 or 25 men) to run over the changed track. This train, of course, had been changed on a previous day to be ready for this work.
If a force was overtaken by this train with its work not done, the men on the train were at once spread out to aid in its completion. This done, the train ran on.
Not until this was done was a traffic train allowed to pass over the track. The same rule was followed upon all the work.
Upon the final day it was required that upon all high trestles and in tunnels the track should be full-spiked before being left or a train let over. This took extra time and labor, and possibly was not necessary; but it was a precaution on the side of safety.
Upon the day of the change of the Alabama Central Division (Selma to Lauderdale), superintendents of other divisions, with their road masters, supervisors, master mechanics and many section foremen, were sent over to see the organization and work and the preparations that had been made. Many of them lent a helping hand in the work. They saw here in practice what had only been theory before.
About a week before the general change that portion of the road between Rome, Ga., and Selma, Ala., about 200 miles, was changed, and again men from other divisions were sent to see and aid in the work. So when the final day came, the largest possible number of men were able to work understandingly.
On the last day of May the Memphis & Charleston, Knoxville & Ohio, and North Carolina branch were changed, and on June 1 the line from Bristol to Chattanooga and Brunswick.
Other roads changed their branch lines a day or two before the 1st of June; but the main lines, as a rule, were changed on that day.
It was a small matter to take care of the cars and arrange the train service so there should be no hitches. It was not expected that connections would move freight during the 48 hours prior to the change, and these days were spent in clearing the road of everything, and taking the cars to the points of rendezvous. All scheduled freight trains were abandoned on the day prior to the change, and only trains run to such points.
Upon the East Tennessee system these points were Knoxville, Rome, Atlanta, Macon, Huntsville, and Memphis, and to these points all cars must go, loaded or empty, and there they were parked upon the tracks prepared for the purpose. Passenger trains were run to points where it had been arranged to change them, generally to the general changing point.
Most of the Southern roads have double daily passenger service. Upon all roads one of these trains, upon the day of change, was abandoned, and upon some all. Some, even, did not run till next day.
We were able to start the day trains out by 10 or 11 o'clock A.M., and put them through in fair time. Of course, no freights were run that day, and the next day was used in getting the cars which had been changed out of the parks and into line. So our freight traffic over the entire South was suspended practically three days.
The work of changing was to commence at 3:30 A.M., but many of the men were in position at an earlier hour, and did commence work as soon as the last train was over, or an hour or so before the fixed time. Half-past three A.M., however, can be set down as the general hour of commencement.
For five or six hours in the cool morning the work went on briskly, the men working with much more than ordinary enthusiasm. But the day was warm, and after 9 or 10 A.M. it began to lag. All was done, however, before the day was over, and safe, so that trains could pass at full speed.
The men all received $1.50 for the work, whether it was finished early or late in the day, and were paid that afternoon as soon as the work was done. Tickets were given the men, which the nearest agent paid, remitting as cash to the treasurer.
On some lines it was deemed best to offer prizes to those who got through first.
Reports showed some very early finishes. But the facts seem to have been that under such encouragement the men were apt to pull too many spikes before the change and put too few in while changing. They were thus reported through early, but their work was not done, and they took great chances.
It was by most considered unwise to offer such prizes, preferring to have a little more time taken and be sure that all was safe. Such lines seemed to get their trains in motion with as much promptness as others. This, with freedom from accident, was the end sought.
It was found after the work had been done that there had been little inaccuracies in driving the gauge spike, to which the rail was thrown, probably from various causes. The rail to be moved may not always have been exactly in its proper place, and then the template in the hurry may not have been accurately placed, or the spike may have turned or twisted.
Whatever was the cause, it was found that frequently the line on the moved side was not perfect, and, of course, many spikes had to be drawn and the rail lined up and respiked. The more careful the work had been done, the less of this there was to do afterward. With rough track this was least seen. The nearer perfect, the more noticeable it was.
Of course, we all planned to get foreign cars home and have ours sent to us. But when the interchange stopped, we found we had many foreign cars, which, of course, had to be changed. This subject had come up in convention and it had been voted to charge three dollars per car when axles did not need turning, and five dollars where they did. By comparison with the cost of changing, as shown in this paper, it will be seen that to our company, at least, there was no loss at these figures.
The following tables will explain the work done upon the Louisville & Nashville and East Tennessee, Virginia & Georgia systems.
It is to be regretted that the writer has not at hand information regarding other roads, that fuller statements and comparisons might be made and the showings be of greater value.
The figures of the Mobile & Ohio are added, having been compiled from the annual report of that road.
MOBILE & OHIO RAILROAD.
(Compiled from Annual Report.)
| Number Changed. | Cost of Labor. | Cost of Material. | Total Cost. | Average Cost. | |
|---|---|---|---|---|---|
| Engines and tenders. | 47 | $ 8,031.42 | $ 7,276.86 | $15,308.28 | $325.70 |
| Pass., bag., ex. cars. | 55 | 439.37 | 104.25 | 542.62 | 9.87 |
| Freight cars, 1,361. Freight trucks, 107½. | 1,468½ | 5,719.03 | 739.57 | 6,458.60 | 4.40 |
| Lever and push cars. | 143 | 1,427.55 | 476.93 | 1,904.48 | 13.32 |
| Miles. | |||||
| Track (inc. sidings). | 583.5 | 17,109.53 | 7,275.14 | 24,384.87 | 41.79 |
| Bridges. | 583.5 | 1,896.60 | 190.00 | 2,086.60 | 3.58 |
| Track tools. | 583.5 | 170.72 | 1,405.74 | 1,576.46 | 2.70 |
| Shop tools. | 583.5 | 419.70 | 2,982.90 | 3,402.60 | 5.83 |
| Temp. side tracks. | 12.09 | 1,958.94 | 372.37 | 2,331.31 | 192.83 |
| Switching cars. | 1,398.18 | 16.50 | 1,414.68 | ||
| Car hoists. | 2,499.38 | 4,419.34 | 6,918.72 | ||
| Total cost. | $41,069.42 | $25,259.60 | $66,329.02 | ||
| Total average cost per mile. | $113.68 | ||||
LOUISVILLE & NASHVILLE RAILROAD.
(Compiled from Annual Report.)
| Miles of track | —Main line | 1,893.7 | ||
| —Side track | 196.3 | |||
| ———— | 2,090.0 | |||
| Track. | Total. | Cost per Mile. | ||
|---|---|---|---|---|
| Section labor | —Before day of change | $28,106.60 | ||
| —On day of change | 20,090.42 | |||
| —After day of change | 19,713.19 | |||
| ———— | $67,910.21 | $32.49 | ||
| Carpenter labor | 3,799.19 | 1.82 | ||
| Spikes | 20,873.70 | 9.99 | ||
| Switches | 6,331.85 | 3.03 | ||
| Tools | 2,749.50 | 1.31 | ||
| Hand cars and sundries | 5,691.39 | 2.72 | ||
| Total | $107,855.84 | $51.36 | ||
| Equipment. | ||||
| Number. | Total. | Average Cost. | ||
| Locomotives | 264 | $53,480.98 | $202.58 | |
| Cars (300 of these passenger—3.5%) | 8,537 | 49,577.20 | 5.81 | |
| Total cost | $210,414.02 | |||
| Total average cost per mile | $100.67 | |||
EAST TENNESSEE, VIRGINIA & GEORGIA SYSTEM.
| Number Changed. | Cost of Labor. | Cost of Material. | Total Cost. | Average Cost. | |
|---|---|---|---|---|---|
| Engines and tenders. | 180 | $ 8,227.47 | $ 2,904.30 | $ 11,131.77 | $ 61.82 |
| Pass., bag., and mail cars. | 168 | 734.93 | 59.67 | 794.60 | 4.73 |
| Freight cars and cabooses. | 5,175 | 17,425.57 | 1,224.08 | 18,649.65 | 3.60 |
| M. of W. cars. | 439 | 2,038.44 | 549.47 | 2,587.91 | 5.89 |
| Miles Track. | |||||
| Track (inc. sidings). | 1,532.7 | 27,718.17 | 40,912.09 | 68,630.26 | 44.78 |
| Bridges. | 1,532.7 | 1,808.57 | 200.00 | 2,008.57 | 1.31 |
| Track tools. | 1,532.7 | 194.48 | 2,573.83 | 2,768.31 | 1.80 |
| Storage tracks, inc. taking up. | 37.02 | 9,825.41 | 1,481.59 | 11,307.00 | 305.44 |
| Shop tools. | 472.20 | 2,728.30 | 3,200.50 | ||
| Total cost. | $68,445.24 | $52.633.33 | $121,078.57 | ||
| Total average cost per mile. | $ 79.06 | ||||
| Axles condemned | 577 |
| Wheels condemned | 754 |
| Wheels burst | 202 |
| New axles used | 1,102 |
| New wheels used | 2,783 |
| Axles turned back | 8,316 |
| Wheels pressed on without turning axle | 23,952 |
| New brasses used | 10,723 |
| Cars narrowed (not including lever or push cars) | 5,343 |
| Engines narrowed | 180 |
| Average cost of new centers and crank pins, etc | $264.46 |
| Average cost of cutting off hub and pressing wheels and new pins | 130.67 |
| Average cost of pressing old tires on old centers | 29.08 |
| Average cost of pressing old tires on broad centers | 31.83 |
| Average cost of labor putting on new tires | 22.94 |
COMPARATIVE STATEMENT OF AVERAGE COST OF VARIOUS ITEMS OF WORK.
| M. & O. R.R. | L. & N. R.R. | E.T.,V. & G. R.R. | Average. | |
|---|---|---|---|---|
| Engines and tenders—per engine | $325.70 | $202.58 | $61.82 | $196.70 |
| Pass., bag., and ex. cars—per car | 9.87 | 25.81 | 4.73 | 6.80 |
| Freight cars, per car | 4.40 | 35.81 | 3.60 | 4.60 |
| M. of W. cars, per car | 13.32 | 2.72 | 5.89 | 7.31 |
| Track (inc. sidings bridges, etc.), per mile | 45.37 | 47.83 | 46.09 | 46.26 |
| Track tools, per mile | 2.70 | 1.31 | 1.80 | 1.94 |
| Temporary side tracks, per mile | 192.83 | 305.44 | 249.13 | |
| Total per mile of track, inc. sidings | $113.68 | $100.67 | $ 79.06 | $ 97.80 |
NOTE—Since the preparation of this paper the general manager of the Norfolk & Western Railroad has kindly furnished the following items of expense for that line:
| No. | Cost. | Average Cost. | ||
|---|---|---|---|---|
| Engines and tenders | 95 | $37,730.00 | $397.16 | |
| Cars (all kinds) | 3,615 | 37,994.65 | 10.51 | |
| Track, miles (including sidings) | 597.5 | |||
| Labor | 25,296.96 | |||
| Tools and supplies | 3,531.12 | |||
| Changing M. of W. equipment | 813.13 | |||
| Switches | 571.67 | |||
| Spikes | 8,508.22 | |||
| Total track | $38,721,10 | 64.80 | ||
| Total | $114,445.75 | |||
| Total average cost per mile | $191.53 | |||
And the superintendent of the S.F. & W. R.R. has also furnished the expenses for that road:
| No. | Average Cost. | |
|---|---|---|
| Engines and tenders | 75 | $76.31 |
| Cars (passenger) | 95 | 4.67 |
| Cars (freight) | 1,133 | 3.88 |
| Track, including sidings | 601.76 | 44.49 |
Nothing was said about shop or other tools, storage tracks, or changing of maintenance of way equipment.
COMPARATIVE STATEMENT OF AVERAGE COST OF LABOR OF VARIOUS ITEMS OF WORK.
| M. & O. R.R. | L. & N. R.R. | E.T.,V. & G. R.R. | Average | |
| Engines and tenders. | $170.88 | Not divided | $45.71 | $108.29 |
| Pass., bag., and ex cars | 7.97 | 4.38 | 6.17 | |
| Freight cars | 3.89 | 3.36 | 3.62 | |
| M. of W. cars | 9.98 | 4.64 | 7.31 | |
| Miles track (including sidings, bridges, etc.) | 32.57 | $34.31 | 19.26 | 28.71 |
| Track tools, per mile | .30 | Not divided | .13 | .21 |
| Temporary tracks | 162.03 | 265.40 | 213.71 | |
| Total per mile of track | $70.38 | Not divided | $44.72 | $57.55 |
COMPARATIVE STATEMENT OF AVERAGE COST OF MATERIAL OF VARIOUS ITEMS OF WORK.
| M. & O. R.R. | L. & N. R.R. | E.T., V.& G. R.R. | Average | |
|---|---|---|---|---|
| Engines and tenders. | $154.82 | Not divided | $16.11 | $85.46 |
| Pass., bag., and ex cars | 1.90 | .35 | 1.12 | |
| Freight cars | .51 | .24 | .37 | |
| M. of W. cars | 3.34 | 1.25 | 2.30 | |
| Miles track (including sidings, bridges, etc.) | 12.80 | $13.02 | 26.88 | 17.55 |
| Track tools, per mile | 2.40 | Not divided | 1.67 | 2.03 |
| Temporary tracks | 162.03 | 40.04 | 101.03 | |
| Total per mile of track | $43.30 | Not divided | $34.34 | $38.82 |
SUMMARY OF STATEMENTS OF L.&N. AND E.T., V.&G. RAILWAYS.
| The mileage changed of the L&N. and E.T., V.& G. systems combined aggregates | 3,622 miles. |
| The total cost of these two roads. | $331,492.59 |
| Or an average per mile of | 91.52 |
| Total miles changed was about | 14,500 miles. |
| Which would give total cost, at same rate. | $1,327,040 |
We should really add to this a large sum for the great number of new locomotives which were purchased to replace old ones, that could not be changed, except at large cost, and which, when done, would have been light and undesirable.
Upon the basis of the work done upon the L. & N. and E.T., V. & G. systems, which, combined, cover about one-fourth the mileage changed, we have made the following estimates, which will, perhaps, convey a better idea of the extent of the work than can be obtained in any other way:
| Miles of track changed, about | 14,500 |
| Locomotives changed, about | 1,800 |
| Cars (pass, and freight) changed, about | 45,000 |
| New axles used, about | 9,000 |
| New wheels used, about | 20,000 |
| Axles turned back, about | 75,000 |
| Wheels pressed on without turning axles, about | 220,000 |
| New brasses used, about | 90,000 |
| Kegs of spikes used, about | 50,000 |
| Cost of material used, about | $600,000 |
| Cost of labor, about | 730,000 |
| Total cost of work, about | 1,330,000 |
| Amount expended on equipment, about | 650,000 |
| Amount expended on track, about | 680,000 |
| Amount expended on track on day of change in labor, about | 140,000 |
The work was done economically, and so quietly that the public hardly realized it was in progress. To the casual observer it was an every day transaction. It was, however, a work of great magnitude, requiring much thought and mechanical ability.
That it was ably handled is evidenced by the uniform success attained, the prompt changing at the agreed time, and the trifling inconvenience to the public.—Jour. Assn. Engineering Societies.
In our present issue, on page 9948, we give illustrations of two torpedo boats, the Azor and Halcon, which have lately been constructed by Messrs Yarrow & Co., of Poplar, for the Spanish government. They are 135 ft. in length by 14 ft. beam, being of the same dimensions as No. 80 torpedo boat, lately completed by the above firm for the Admiralty, which is the largest and fastest torpedo-boat in the British navy.
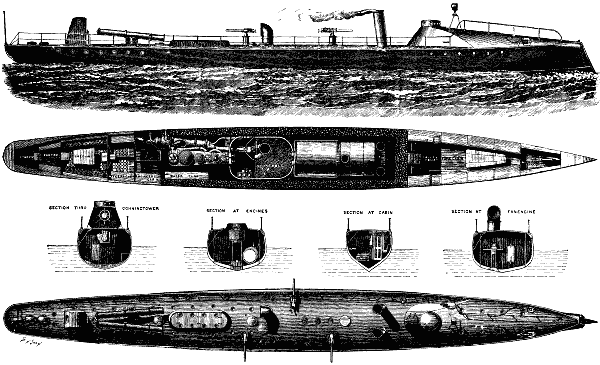
TORPEDO BOATS FOR THE SPANISH GOVERNMENT.
The general arrangement of these torpedo boats is sufficiently clear from the illustrations to need but little description. Suffice it to say that the engines are of the triple compound type, capable of indicating 1,550 horse power, steam being supplied by one large locomotive boiler, which our readers are already aware is in accordance with the usual practice of the makers, as, by using a single boiler, great simplification of the machinery takes place, and considerably less room is occupied than if two boilers were adopted. It is worthy of record that although in some torpedo boats, and indeed in a great number of them, trouble has been found with the locomotive type of boiler, still we have no hesitation in saying that this is due either to defective design or bad workmanship, and that, if properly designed and constructed, such difficulty does not occur. And it is a fact that Messrs. Yarrow & Co. have already constructed a great number of locomotive boilers of the exceptional size adopted in these two Spanish boats, and they have turned out in every respect, after actual service, perfectly satisfactory.
The forward part of the boat is provided with two torpedo-ejecting tubes, as usual, and near the stern, on deck, it is proposed to place turntables, with two torpedo guns for firing over the sides, as already adopted by several governments. The trials of the Azor took place about two months since, giving a speed during a run of two hours and three quarters, carrying a load of 17 tons, of 24 knots (over 27½ miles) per hour. Since her trial she has steamed out to Spain, having encountered, during a portion of the voyage very bad weather, when her sea going qualities were found to be admirable.
The Halcon, whose official trials took place lately, obtained a speed of 23.5 knots, carrying a load of 17 tons. It may be remarked that a speed of 24 knots, in a boat only 135 ft in length, under the Spanish conditions of trial, is by far the best result that has ever been obtained in a vessel of these dimensions There is, however, no doubt that had the length of the boat been greater, a still higher speed would have been obtained But it was desired by the authorities to keep within the smallest possible dimensions, so as to expose as little area as practicable to the fire of the enemy, it being clearly evident that this is a consideration of the first importance in an unprotected war vessel.
In conclusion, we would add that the hulls of these two Spanish boats are of much greater strength of construction than is usually adopted in torpedo boats, it having been found that for the sake of obtaining exceptional speeds, strength sufficient for actual service has often been injudiciously sacrificed And, judging from the numerous accidents which took place at the recent trials off Portland, we have no doubt that in the future naval authorities will be quite ready and willing to sacrifice a little speed so as to obtain vessels which are more trustworthy. The necessity for this, we feel convinced, will be conclusively shown if ever torpedo boats are engaged in actual warfare, and this not only as regards strength of hull, but also as regards the machinery, which at present is only capable of being handled successfully by men of exceptional training, who in times of war would not be readily procured—The Engineer.
In our SUPPLEMENT, No. 620 we gave an illustration of this ship, with some particulars. The interest expressed in naval circles for further information induces us to give still further engravings of this remarkable vessel, with additional information, for which we are indebted to the Engineer.
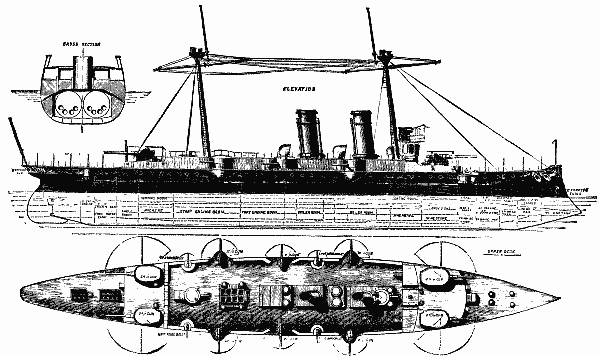
THE NEW SPANISH WAR SHIP REINA REGENTE.
We gave recently a short account of two of the trials of this vessel, and we are, by the courtesy of the builders—Messrs. Thomson, of Clydebank—enabled to lay further particulars before our readers this week. We give herewith engravings of the vessel, which will illustrate her salient points. The principal dimensions are as follows.
Length on water line, 317 ft., breadth, 50 ft. 7 in., depth moulded, 32 ft. 6 in., normal displacement, 4,800 tons, deep load displacement, 5,600* tons. We have before informed our readers that this vessel was designed by Messrs. Thomson, in competition with several other shipbuilding firms of this and other countries, in reply to an invitation of the Spanish government for a cruiser of the first class. The design submitted by the builders of the Reina Regente was accepted, and the vessel was contracted to be built in June of last year. The principal conditions of the contract were as follows.
The ship to steam at a speed of 20½ knots for four runs on the mile and for two hours continuously afterward. She was further to be capable of steaming for six hours continuously at a speed of 18½ knots, without any artificial means of producing draught. She was also to be capable of steaming a distance of at least 5,700 knots for 500 tons of coal, at some speed over 10 knots, to be chosen by the builders. Over the length of her machinery and magazine spaces she was to have a sloping deck extending to 6 ft. below the water line at the side, and formed of plates 4¾ in. thick. This deck was to extend to about 1 ft. above the water line, and the flat part to be 3-1/8 in. thick. Beyond the machinery and magazine spaces, the deck was to be gradually reduced to 3 in. thick at the ends. This deck is intended to protect the vitals of the ship, such as boilers, engines, powder magazines, steering gear, etc., from the effects of shot and shell, but the floating and stability maintaining power of the ship was to be dependent upon a similar structure raised above this protective deck to a height of about 5 ft. above the water.
This structure is covered by a water tight deck known as the main deck of the ship, on which the cabins and living spaces are arranged. The space between the main and protective deck is divided, as may be seen by reference to the protective deck plan, into many strong, water tight spaces, most of which are not more than about 500 cubic feet capacity. The spaces next to the ship's side are principally coal bunkers, and may, therefore, exclude largely any water that should enter. The first line of defense is formed inside these coal bunkers by a complete girdle of coffer dams, which can be worked from the main deck. These it is intended to fill with water and cellulose material, and as they are also minutely subdivided, the effects of damage by shot and consequent flooding may be localized to a considerable extent. The guns of the ship are to consist of four 20 centimeter Hontorio breech loading guns on Vavasseur carriages, six 12 centimeter guns, eight 6 pounder rapid firing, and eight or ten small guns for boats and mitrailleuse purposes, four of which are in the crow's nests at the top of the two masts of the ship. We may remark in passing that the builders saw their way at an early period of the construction to suggest an addition to the weight of the large sized guns, and there will actually be on the ship four 24 centimeter guns, instead of four 20 centimeter. The vessel was to carry five torpedo tubes, two forward in the bow, one in each broadside, and one aft. All these tubes to be fixed. To fulfill the speed condition, four boilers were necessary and two sets of triple expansion engines, capable of developing in all 12,000 horse power.
Now that the vessel has been completely tried, the promises by the builders may be compared with the results determined by the commission of Spanish officers appointed by the government of Spain to say whether the vessel fulfilled in all respects the conditions laid down in the contract. The mean speed attained for the two hours' run was 20.6 knots, as compared with 20.5 guaranteed, but this speed was obtained with 11,500 horse power instead of the 12,000 which the machinery is capable of developing. The officers of the Spanish commission were anxious not to have the vessel's machinery pressed beyond what was necessary to fulfill the speed conditions of the contract; but they saw enough to warrant them in expressing their belief that the vessel can easily do twenty-one knots when required, and she actually did this for some time during the trial.
During the natural draught trial the vessel obtained a mean speed of 18.68 knots, on an average of 94¾ revolutions—the forced draught having been done on an average of 105½ revolutions. The consumption trial, which lasted twelve hours, was made to determine the radius of action, when the ship showed that at a speed of 11.6 knots she could steam a distance of 5,900 knots. Further trials took place to test the evolutionary powers of the vessel, though these trials were not specified in the contract.
The vessel, as may be seen from the engravings, is fitted with a rudder of a new type, known as Thomson & Biles' rudder, with which it is claimed that all the advantage of a balanced rudder is obtained, while the ship loses the length due to the adoption of such a rudder. It is formed in the shape of the hull of the vessel, and as the partial balance of the lower foreside gradually reduces the strains, the rudder head may be made of very great service. As a matter of fact, this rudder is 230 ft. in area, and is probably the largest rudder fitted to a warship. The efficiency of it was shown in the turning trials, by its being able to bring the vessel round, when going at about nineteen knots, in half a circle in one minute twenty-three seconds, and a complete circle in two minutes fifty-eight seconds, the diameter of the circle being 350 yards. This result, we believe, is unrivaled, and makes this vessel equal in turning capabilities to many recent warships not much more than half her length.
Having had a certain measure of success with Eastman stripping films, I have been requested by your council to give a paper this evening dealing with the subject, and particularly with the method of working which my experience has found most successful. In according to their request, I feel I have imposed upon myself a somewhat difficult task.
There is, undoubtedly, a strong prejudice in the minds of most photographers, both amateur and professional, against a negative in which paper is used as a permanent support, on account of the inseparable "grain" and lack of brilliancy in the resulting prints; and the idea of the paper being used only as a temporary support does not seem to convey to their mind a correct impression of the true position of the matter.
It may be as well before entering into the technical details of the manipulation to consider briefly the advantages to be derived—which will be better appreciated after an actual trial.
My experience (which is at present limited) is that they are far superior to glass for all purposes except portraiture of the human form or instantaneous pictures where extreme rapidity is necessary, but for all ordinary cases of rapid exposure they are sufficiently quick. The first advantage, which I soon discovered, is their entire freedom from halation. This, with glass plates, is inseparable, and even when much labor has been bestowed on backing them, the halation is painfully apparent.
These films never frill, being made of emulsion which has been made insoluble. Compare the respective weights of the two substances—one plate weighing more than a dozen films of the same size.
Again, on comparing a stripping film negative with one on glass of the same exposure and subject, it will be found there is a greater sharpness or clearness in the detail, owing, I am of opinion, to the paper absorbing the light immediately it has penetrated the emulsion, the result being a brilliant negative. Landscapes on stripped films can be retouched or printed from on either side, and the advantage in this respect for carbon or mechanical printing is enormous. Now, imagine the tourist working with glass, and compare him to another working with films. The one works in harness, tugging, probably, a half hundredweight of glass with him from place to place, paying extra carriage, extra tips, and in a continual state of anxiety as to possible breakage, difficulty of packing, and having to be continually on the lookout for a dark place to change the plates, and, perhaps, on his return finds numbers of his plates damaged owing to friction on the surface; while the disciple of films, lightly burdened with only camera and slide, and his (say two hundred) films in his pockets, for they lie so compact together. Then the advantages to the tourists abroad, their name is "legion," not the least being the ease of guarding your exposed pictures from the custom house officials, who almost always seek to make matters disagreeable in this respect, and lastly, though not least, the ease with which the negatives can be stowed away in envelopes or albums, etc., when reference to them is easy in the extreme.
Now, having come (rightly, I think, you will admit) to the conclusion that films have these advantages, you naturally ask, What are their disadvantages? Remembering, then, that I am only advocating stripping films, I consider they have but two disadvantages: First, they entail some additional outlay in the way of apparatus, etc. Second, they are a little more trouble to finish than the glass negatives, which sink into insignificance when the manifold advantages are considered.
In order to deal effectively with the second objection I mentioned, viz., the extra trouble and perseverance, I propose, with your permission, to carry a negative through the different stages from exposure to completion, and in so doing I shall endeavor to make the process clear to you, and hope to enlist your attention.
The developer I use is slightly different to that of the Eastman company, and is as follows:
| A. | |
| Sulphite of soda. | 4 ounces. |
| To be dissolved in 8 ounces of hot distilled water, then rendered slightly acid with citric acid, then add— | |
| Pyrogallic acid. | 1 ounce. |
| Water to make up to | 10 ounces. |
| B. | |
| Pure carbonate of soda. | 1 ounce. |
| Water to make up in all to | 10 ounces. |
| C. | |
| Pure carbonate of potash. | 1 ounce. |
| Water to make up to | 10 ounces. |
| D. | |
| Bromide of potassium. | 1 ounce. |
| Water to make up to | 10 ounces. |
I have here two half-plate films exposed at 8:30 A.M. to-day, one with five and one with six seconds' exposure, subject chiefly middle distance. I take 90 minims A, 10 minims D, and 90 minims B, and make up to 2 ounces water. I do not soak the films in water. There is no need for it. In fact, it is prejudicial to do so. I place the films face uppermost in the dish, and pour on the developer on the center of the films. You will observe they lie perfectly flat, and are free from air bubbles. Rock the dish continually during development, and when the high lights are out add from 10 to 90 minims C, and finish development and fix. The negatives being complete, I ask you to observe that both are of equal quality, proving the latitude of exposure permissible.
I now coat a piece of glass half an inch larger all round than the negative with India rubber solution (see Eastman formula), and squeegee the negative face downward upon the rubber, interposing a sheet of blotting paper and oilskin between the negative and squeegee to prevent injury to the exposed rubber surface, and then place the negative under pressure with blotting paper interposed until moderately dry only.
I then pour hot water upon it, and, gently rocking the dish, you see the paper floats from the film without the necessity for pulling it with a pin, leaving the film negative on the glass. Now, the instructions say remove the remaining soluble gelatine with camel's hair brush, but, unless it requires intensifying, which no properly developed negative should require, you need not do so, but simply pour on the gelatine solution (see Eastman formula), well covering the edges of the film, and put on a level shelf to dry.
I will now take up a negative in this state on the glass, but dry, and carefully cut round the edges of the film, and you see I can readily pull off the film with its gelatine support. Having now passed through the whole of the process, it behooves us to consider for a few minutes the causes of failure in the hands of beginners and their remedies: 1. The rubber will not flow over glass? Solution too thick, glass greasy. 2. Rubber peels off on drying? Dirty glass. 3. Negative not dense enough? Use more bromide and longer development. 4. Gelatine cracks on being pulled off? Add more glycerine. 5. Gelatine not thick enough? Gelatine varnish too thin, not strong enough. 6. Does not dry sufficiently hard? Too much glycerine.—E.H. Jaques, Reported in Br. Jour. of Photography.
A communication to the Birmingham Photographic Society.
The following formulæ are for use with gelatino-chloride paper or plates. The quantities are in each case calculated for one ounce, three parts of each of the following solutions being employed and added to one part of solution of protosulphate of iron. Strength, 140 grains to the ounce.
| Slaty Blue. | |||
| 1.— | One part of the above solution to three parts of a solution of citrate of ammonia. | ||
| Greenish Brown. | |||
| 2.— | Citric acid. | 180 | grains |
| Carbonate of ammonia. | 50 | " | |
| 3.— | Citrate of ammonia. | 250 | grains. |
| Chloride of sodium. | 2 | " | |
| 4.— | Citrate of ammonia. | 250 | grains. |
| Chloride of sodium. | 4 | " | |
| Sepia Brown. | |||
| 5.— | Citrate of ammonia. | 250 | grains. |
| Chloride of sodium. | 8 | " | |
| Clear Red Brown. | |||
| 6.— | Citric acid. | 120 | grains. |
| Carbonate of magnesia. | 76 | " | |
| Warm Gray Brown. | |||
| 7.— | Citric acid. | 120 | grains. |
| Carbonate of soda. | 205 | " | |
| Deep Red Brown. | |||
| 8.— | Citric acid. | 120 | grains. |
| Carbonate of potash. | 117 | " | |
| Green Blue. | |||
| 9.— | Citric acid. | 90 | grains. |
| Carbonate of soda. | 154 | " | |
| Citrate of potash. | 24 | " | |
| Oxalate of potash. | 6 | " | |
| Sepia Red. | |||
| 10.— | Citric acid. | 80 | grains. |
| Carbonate of soda. | 135 | " | |
| Citrate of potash. | 12 | " | |
| Oxalate of potash. | 3 | " | |
| 11.— | Citric acid. | 108 | grains. |
| Carbonate of magnesia. | 68 | " | |
| Carbonate of potash. | 12 | " | |
| Oxalate of potash. | 3 | " | |
| Sepia Yellow. | |||
| 12.— | Citric acid. | 40 | grains. |
| Carbonate of magnesia. | 25 | " | |
| Citrate of ammonia. | 166 | " | |
| 13.— | Citric acid. | 120 | grains. |
| Carbonate of magnesia. | 72 | " | |
| Carbonate of ammonia. | 72 | " | |
| Chloride of sodium. | 8 | " | |
| Blue Black. | |||
| 14.— | Citric acid. | 120 | grains. |
| Carbonate of ammonia. | 70 | " | |
| Carbonate of magnesia. | 15 | " | |
| 15.— | Citric acid. | 120 | grains. |
| Carbonate of magnesia. | 38 | " | |
| Carbonate of ammonia. | 44 | " | |
| 16.— | Citric acid. | 90 | grains. |
| Carbonate of magnesia. | 57 | " | |
| Citrate of potash. | 54 | " | |
| Oxlate of potash. | 18 | " | |
| 17.— | Citric acid. | 72 | grains. |
| Carbonate of magnesia. | 45 | " | |
| Citrate of potash. | 54 | " | |
| Oxalate of potash. | 18 | " | |
| 18.— | Citric acid. | 60 | grains. |
| Carbonate of magnesia. | 38 | " | |
| Citrate of potash. | 68 | " | |
| Oxalate of potash. | 22 | " | |
| A more Intense Blue Black. | |||
| 19.— | Citric acid. | 30 | grains. |
| Carbonate of magnesia. | 18 | " | |
| Citrate of potash. | 100 | " | |
| Oxalate of potash. | 33 | " | |
| A Clearer Blue. | |||
| 20.— | Citrate of potash. | 136 | grains. |
| Oxalate of potash. | 44 | " | |
In the photographic exhibition at Florence, the firm of Corvan1 places on view a frame containing twenty proofs produced by the foregoing twenty formulæ, in such a way that the observer can compare the value of each tone and select that which pleases him best.—Le Moniteur de la Photographie, translated by British Jour. of Photo.
Does this mean Mr. A. Cowan?—Translator.
FIG. 1—ELEVATION.
At a recent meeting of the Industrial Society of Amiens, Mr. Schmidt, engineer of the Steam Users' Association, read a paper in which he described the process employed in the construction of a large chimney of peculiar character for the Rocourt distillery, at St. Quentin.
This chimney, which is cylindrical in form, is 140 feet in height, and has an internal diameter of 8½ feet from base to summit. The coal consumed for the nine generators varies between 860 and 1,200 pounds per hour and per 10 square feet of section.
The ground that was to support this chimney consisted of very aquiferous, cracked beds of marl, disintegrated by infiltrations of water from the distillery, and alternating with strata of clay. It became necessary, therefore, to build as light a chimney as possible. The problem was solved as follows, by Mr. Guendt, who was then superintendent of the Rocourt establishment.
Upon a wide concrete foundation a pedestal was built, in which were united the various smoke conduits, and upon this pedestal were erected four lattice girders, C, connected with each other by St. Andrew's crosses. The internal surface of these girders is vertical and the external is inclined. Within the framework there was built a five-inch thick masonry wall of bricks, made especially for the purpose. The masonry was then strengthened and its contact with the girders assured by numerous hoops, especially at the lower part; some of them internal, others external, to the surface of the girders, and others of angle irons, all in four parts.
FIG. 2—HORIZONTAL SECTION.
The anchors rest upon a cast iron foundation plate connected, through strong bolts embedded in the pedestal, with a second plate resting upon the concrete.
As the metallic framework was calculated for resisting the wind, the brick lining does not rest against it permanently above. The weight of the chimney is 1,112,200 pounds, and the foundation is about 515 square feet in area; and, consequently, the pressure upon the ground is about 900 pounds to the square inch. The cost was $3,840.
The chimney was built six years ago, and has withstood the most violent hurricanes.
The mounting of the iron framework was effected by means of a motor and two men, and took a month. The brick lining was built up in eight days by a mason and his assistant.
A chimney of the same size, all of brick, erected on the same foundation, would have weighed 2,459,600 pounds (say a load of 3,070 pounds to the square inch), and would have cost about $2,860.
FIG. 3—VERTICAL SECTION OF THE CHIMNEY.
The chimney of the Rocourt distillery is, therefore, lighter by half, and cost about a third more, than one of brick; but, at the present price of metal, the difference would be slight.—Annales Industrielles.
Considerable interest has been aroused lately in scientific and industrial circles by a report that separation of the oxygen and nitrogen of the air was being effected on a large scale in London by a process which promises to render the gases available for general application in the arts. The cheap manufacture of the compounds of nitrogen from the gas itself is still a dream of chemical enthusiasts; and though the pure gas is now available, the methods of making its compounds have yet to be devised. But the industrial processes which already depend directly or indirectly on the chemical union of bodies with atmospheric oxygen are innumerable.
In all these processes the action of the gas is impeded by the bulky presence of its fellow constituent of air, nitrogen. We may say, for instance, in homely phrase, that whenever a fire burns there are four volumes of nitrogen tending to extinguish it for every volume of oxygen supporting its combustion, and to the same degree the nitrogen interferes with all other processes of atmospheric oxidation, of which most metallurgical operations may be given as instances. If, then, it has become possible to remove this diluent gas simply and cheaply in order to give the oxygen free play in its various applications, we are doubtless on the eve of a revolution among some of the most extensive and familiar of the world's industries.
A series of chemical reactions has long been known by means of which oxygen could be separated out of air in the laboratory, and at various times processes based on these reactions have been patented for the production of oxygen on a large scale. Until recently, however, none of these methods gave sufficiently satisfactory results. The simplest and perhaps the best of them was based on the fact first noticed by Boussingault, that when baryta (BaO) is heated to low redness in a current of air, it takes up oxygen and becomes barium dioxide (BaO2), and that this dioxide at a higher temperature is reconverted into free oxygen and baryta, the latter being ready for use again. For many years it was assumed, however, by chemists that this ideally simple reaction was inapplicable on a commercial scale, owing to the gradual loss of power to absorb oxygen which was always found to take place in the baryta after a certain number of operations. About eight years ago Messrs. A. & L. Brin, who had studied chemistry under Boussingault, undertook experiments with the view of determining why the baryta lost its power of absorbing oxygen.
They found that it was owing to molecular and physical changes caused in it by impurities in the air used and by the high temperature employed for decomposing the dioxide. They discovered that by heating the dioxide in a partial vacuum the temperature necessary to drive off its oxygen was much reduced. They also found that by supplying the air to the baryta under a moderate pressure, its absorption of oxygen was greatly assisted. Under these conditions, and by carefully purifying the air before use, they found that it became possible to use the baryta an indefinite number of times. Thus the process became practically, as it was theoretically, continuous.
After securing patent protection for their process, Messrs. Brin erected a small producer in Paris, and successfully worked it for nearly three years without finding a renewal of the original charge of baryta once necessary. This producer was exhibited at the Inventions Exhibition in London, in 1885. Subsequently an English company was formed, and in the autumn of last year Brin's Oxygen Company began operations in Horseferry Road, Westminster, where a large and complete demonstration plant was erected, and the work commenced of developing the production and application of oxygen in the industrial world.
We give herewith details of the plant now working at Westminster. It is exceedingly simple. On the left of the side elevation and plan are shown the retorts, on the right is an arrangement of pumps for alternately supplying air under pressure and exhausting the oxygen from the retorts. As is shown in the plan, two sets of apparatus are worked side by side at Westminster, the seventy-two retorts shown in the drawings being divided into two systems of thirty-six. Each system is fed by the two pumps on the corresponding side of the boiler. Each set of retorts consists of six rows of six retorts each, one row above the other. They are heated by a small Wilson's producer, so that the attendant can easily regulate the supply of heat and obtain complete control over the temperature of the retorts. The retorts, A, are made of wrought iron and are about 10 ft long and 8 in. diameter. Experience, however, goes to prove that there is a limit to the diameter of the retorts beyond which the results become less satisfactory. This limit is probably somewhat under 8 in. Each retort is closely packed with baryta in lumps about the size of a walnut. The baryta is a heavy grayish porous substance prepared by carefully igniting the nitrate of barium; and of this each retort having the above dimensions holds about 125 lb. The retorts so charged are closed at each end by a gun metal lid riveted on so as to be air tight. From the center of each lid a bent gun metal pipe, B, connects each retort with the next of its series, so that air introduced into the end retort of any row may pass through the whole series of six retorts. Suppose now that the operations are to commence.
The retorts are first heated to a temperature of about 600° C. or faint redness, then the air pumps, C C, are started. Air is drawn by them through the purifier, D, where it is freed from carbon dioxide and moisture by the layers of quicklime and caustic soda with which the purifier is charged. The air is then forced along the pipe, E, into the small air vessel, F, which acts as a sort of cushion to prevent the baryta in the retorts being disturbed by the pulsation of the pumps. From this vessel the air passes by the pipe, G, and is distributed in the retorts as rapidly as possible at such a pressure that the nitrogen which passes out unabsorbed at the outlet registers about 15 lb. to the square inch. With the baryta so disposed in the retorts as to present as large a superficies as possible to the action of the air, it is found that in 1½ to 2 hours—during which time about 12,000 cub. ft of air have been passed through the retorts—the gas at the outlet fails to extinguish a glowing chip, indicating that oxygen is no longer being absorbed. The pumping now ceases, and the temperature of the retorts is raised to about 800° C. The workman is able to judge the temperature with sufficient accuracy by means of the small inspection holes, H, fitted with panes of mica, through which the color of the heat in the furnace can be distinguished. The pumps are now reversed and the process of exhaustion begins. At Westminster the pressure in the retorts is reduced to about 1½ in. of mercury. In this partial vacuum the oxygen is given off rapidly, and if forced by the pumps through another pipe and away into an ordinary gas holder, where it is stored for use. With powerful pumps such as are used in the plant under notice the whole of the oxygen can be drawn off in an hour, and from one charge a yield of about 2,000 cub. ft. is obtained. With a less perfect vacuum the time is longer—even as much as four hours. The whole operation of charging and exhausting the retorts can be completed in from three to four hours. As soon as the evolution of oxygen is finished, the doors, K, and ventilators, L, may be opened and the retorts cooled for recharging.
The cost of producing oxygen at Westminster, under specially expensive conditions, is high—about 12s. per 1,000 cub. ft. When we consider, however, that the cost should only embrace attendance, fuel, wear and tear, and a little lime and soda for the purifiers, that the consumption of fuel is small, the wear and tear light, and that the raw material—air—is obtained for nothing, it ought to be possible to produce the gas for a third or fourth of this amount in most of our great manufacturing centers, where the price of fuel is but a third of that demanded in London, and where provision could be made for economizing the waste heat, which is entirely lost in the Westminster installation. Moreover, in estimating this cost all the charges are thrown on the oxygen; were there any means of utilizing the 4,000 cub. ft. of nitrogen at present blown away as waste for every thousand cubic feet of oxygen produced, the nitrogen would of course bear its share of the cost.
The question of the application of the oxygen is one which must be determined in its manifold bearings mainly by the experiments of chemists and scientific men engaged in industrial work. Having ascertained the method by which and the limit of cost within which it is possible to use oxygen in their work, it can be seen whether by Brin's process the gas can be obtained within that limit.
Mr. S.R. Ogden, the manager of the corporation gasworks at Blackburn, has already made interesting experiments on the application of oxygen in the manufacture of illuminating gas. In order to purify coal gas from compounds of sulphur, it is passed through purifiers charged with layers of oxide of iron. When the oxide of iron has absorbed as much sulphur as it can combine with, it is described as "foul." It is then discharged and spread out in the open air, when, under the influence of the atmospheric oxygen, it is rapidly decomposed, the sulphur is separated out in the free state, and oxide of iron is reformed ready for use again in the purifiers. This process is called revivification, and it is repeated until the accumulation of sulphur in the oxide is so great (45 to 55 per cent.) that it can be profitably sold to the vitriol maker. Hawkins discovered that by introducing about 3 per cent. of air into the gas before passing it through the purifiers, the oxygen of the air introduced set free the sulphur from the iron as fast as it was absorbed. Thus the process of revivification could be carried on in the purifiers themselves simultaneously with the absorption of the sulphur impurities in the gas.
A great saving of labor was thus effected, and also an economy in the use of the iron oxide, which in this way could be left in the purifiers until charged with 75 per cent. of sulphur. Unfortunately it was found that this introduction of air for the sake of its oxygen meant also the introduction of much useless nitrogen, which materially reduced the illuminating power of the gas. To restore this illuminating power the gas had to be recarbureted, and this again meant cost in labor and material. Now, Mr. Ogden has found by a series of conclusive experiments made during a period of seventy-eight days upon a quantity of about 4,000,000 cub. ft. of gas, that by introducing 1 per cent. of oxygen into the gas instead of 3 per cent. of air, not only is the revivification in situ effected more satisfactorily than with air, but at the same time the illuminating power of the gas, so far from being decreased, is actually increased by one candle unit.
SIDE ELEVATION OF APPARATUS
GENERAL PLAN OF APPARATUS
THE PRODUCTION OF OXYGEN BY BRIN'S PROCESS.
So satisfied is he with his results that he has recommended the corporation to erect a plant for the production of oxygen at the Blackburn gas works, by which he estimates that the saving to the town on the year's make of gas will be something like £2,500. The practical observations of Mr. Ogden are being followed up by a series of exhaustive experiments by Mr. Valon, A.M. Inst. C.E., also a gas engineer. The make of an entire works at Westgate is being treated by him with oxygen. Mr. Valon has not yet published his report, as the experiments are not quite complete; but we understand that his results are even more satisfactory than those obtained at Blackburn.
In conclusion we may indicate a few other of the numerous possible applications of cheap oxygen which might be realized in the near future. The greatest illuminating effect from a given bulk of gas is obtained by mixing it with the requisite proportion of oxygen, and holding in the flame of the burning mixture a piece of some solid infusible and non-volatile substance, such as lime. This becomes heated to whiteness, and emits an intense light know as the Drummond light, used already for special purposes of illumination. By supplying oxygen in pipes laid by the side of the ordinary gas mains, it would be possible to fix small Drummond lights in place of the gas burners now used in houses; this would greatly reduce the consumption of gas and increase the light obtained, or even render possible the employment of cheap non-illuminating combustible gases other than coal gas for the purpose.
Two obstacles at present lie in the way of this consummation—the cost of the oxygen and the want of a convenient and completely refractory material to take the place of the lime. Messrs. Brin believe they have overcome the first obstacle, and are addressing themselves, we believe, to the removal of the second. Again, the intense heat which the combustion of carbon in cheap oxygen will place at the disposal of the metallurgist cannot fail to play an important part in his operations. There are many processes, too, of metal refining which ought to be facilitated by the use of the gas. Then the production of pure metallic oxides for the manufacture of paints, the bleaching of oils and fats, the reduction of refractory ores of the precious metals on a large scale, the conversion of iron into steel, and numberless other processes familiar to the specialists whose walk is in the byways of applied chemistry, should all profit by the employment of this energetic agent. Doubtless, too, the investigation into methods of producing the compounds of nitrogen so indispensable as plant foods, and for which we are now dependent on the supplies of the mineral world, may be stimulated by the fact that there is available by Brin's process a cheap and inexhaustible supply of pure nitrogen.—Industries.
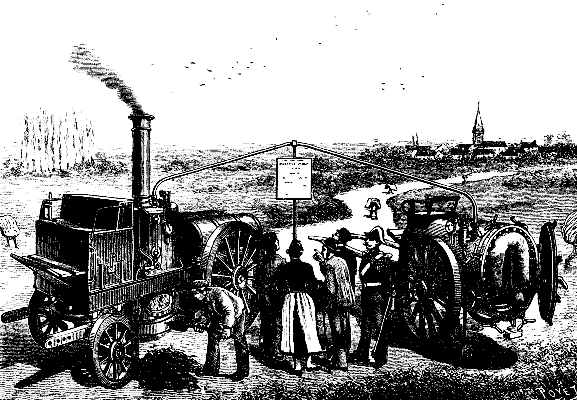
IMPROVED DISINFECTING APPARATUS.
We represent herewith a sanitary train that was very successfully used during the prevalence of an epidemic of sudor Anglicus in Poitou this year. It consisted of a movable stove and a boiler. In reality, to save time, such agricultural locomotives as could be found were utilized; but hereafter, apparatus like those shown in the engraving, and which are specially constructed to accompany the stoves, will be employed. We shall quote from a communication made by Prof. Brouardel to the Academy of Medicine on this subject, at its session of September 13:
In the country we can never think of disinfecting houses with sulphurous acid, as the peasants often have but a single room, in which the beds of the entire family are congregated. Every one knows that the agglomerations that compose the same department are often distant from each other and the chief town by from two to three miles or more. This is usually the case in the departments of Vienne, Haute Vienne, Indre, etc. To find a disinfecting place in the chief town of the department is still difficult, and to find one in each of the hamlets is absolutely impossible. Families in which there are invalids are obliged to carry clothing and bedding to the chief town to be disinfected, and to go after them after the expiration of twenty-four hours. This is not an easy thing to do.
It is easy to understand what difficulties must be met with in many cases, and so one has to be content to prescribe merely washing, and bleaching with lime—something that is simple and everywhere accepted, but insufficient. So, then, disinfection with sulphurous acid, which is easy in large cities, as was taught by the cholera epidemics of last year, is often difficult in the country. The objection has always be made to it, too, that it is of doubtful efficacy. It is not for us to examine this question here, but there is no doubt that damp steam alone, under pressure, effects a perfect disinfection, and that if this mode of disinfection could be applied in the rural districts (as it can be easily done in cities), the public health would be better protected in case of an epidemic.
In cities one or more stationary steam stoves can always be arranged; but in the country movable ones are necessary. From instructions given by Prof. Brouardel, Messrs. Geneste & Herscher have solved the problem of constructing such stoves in a few days, and four have been put at the disposal of the mission.
Dr. Thoinot, who directed this mission, in order to make an experiment with these apparatus, selected two points in which cases of sudor were still numerous, and in which the conditions were entirely different, and permitted of studying the working of the service and apparatus under various phases. One of these points was Dorat, chief town of Haute Vienne, a locality with a crowded population and presenting every desirable resource; and the other was the commune of Mauvieres, in Indre, where the population was scattered through several hamlets.
The first stove was operated at Dorat, on the 29th of June, and the second at Mauvieres, on the 1st of July. A gendarme accompanied the stove in all its movements and remained with it during the disinfecting experiments. The Dorat stove was operated on the 29th of June and the 1st, 2d, and 3d of July. On the 30th of June it proceeded to disinfect the commune of Darnac. The Mauvieres stove, in the first place, disinfected the chief town of this commune on the 1st of July, and on the next day it was taken to Poulets, a small hamlet, and a dependent of the commune of Mauvieres. All the linen and all the clothing of the sick of this locality, which had been the seat of sudor, especially infantile, was disinfected. On the 4th of July, the stove went to Concremiers, a commune about three miles distant, and there finished up the disinfection that until then had been performed in the ordinary way.
The epidemic was almost everywhere on the wane at this epoch; but we judge that the test of the stoves was sufficient.
We are able to advance the following statement boldly: For the application of disinfection in the rural districts, the movable stove is the most practical thing that we know of. It is easily used, can be taken to the smallest hamlets, and can be transported over the roughest roads. It inspires peasants with no distrust. The first repugnance is easily overcome, and every one, upon seeing that objects come from the stove unharmed, soon hastens to bring to it all the contaminated linen, etc., that he has in the house.
Further, we may add that the disinfection is accomplished in a quarter of an hour, and that it therefore keeps the peasant but a very short time from his work—an advantage that is greatly appreciated. Finally, a day well employed suffices to disinfect a small settlement completely. Upon the whole, disinfection by the stove under consideration is the only method that can always and everywhere be carried out.
We believe that it is called upon to render the greatest services in the future.
The movable stove, regarding which Prof. Brouardel expresses himself in the above terms, consists of a cylindrical chamber, 3½ feet in internal diameter and 5 feet in length, closed in front by a hermetically jointed door. This cylinder, which constitutes the disinfection chamber, is mounted upon wheels and is provided with shafts, so that it can easily be hauled by a horse or mule. The cylinder is of riveted iron plate, and is covered with a wooden jacket. The door is provided with a flange that enters a rubber lined groove in the cylinder, and to it are riveted wrought iron forks that receive the nuts of hinged bolts fixed upon the cylinder. The nuts are screwed up tight, and the flange of the door, compressing the rubber lining, renders the joint hermetical. The door, which is hinged, is provided with a handle, which, when the stove is closed, slides over an inclined plane fixed to the cylinder.
The steam enters a cast iron box in the stove through a rubber tube provided with a threaded coupling. The entrance of the steam is regulated by a cock. The box is provided with a safety and pressure gauge and a small pinge cock. In the interior of the stove the entrance of the steam is masked by a large tinned copper screen, which is situated at the upper part and preserves the objects under treatment from drops of water of condensation. These latter fall here and there from the screen, follow the sides of the cylinder, and collect at the bottom, from whence they are drawn off through a cock placed in the rear.
The sides are lined internally with wood, which prevents the objects to be infected from coming into contact with the metal. The objects to be treated are placed upon wire cloth shelves. The pinge cock likewise serves for drawing off the air or steam contained in the apparatus.
The stove is supported upon an axle through the intermedium of two angle irons riveted longitudinally upon the cylinder. The axle is cranked, and its wheels, which are of wood, are 4½ feet in diameter. The shafts are fixed to the angle irons. The apparatus is, in addition, provided with a seat, a brake, and prop rods before and behind to keep it horizontal when in operation.
The boiler that supplies this stove is vertical and is mounted upon four wheels. It is jacketed with wood, and is provided with a water level, two gauge cocks, a pressure gauge, two spring safety valves, a steam cock provided with a rubber tube that connects with that of the stove, an ash pan, and a smoke stack. In the rear there are two cylindrical water reservoirs that communicate with each other, and are designed to feed the boiler through an injector. Beneath these reservoirs there is a fuel box. In front there is a seat whose box serves to hold tools and various other objects.—La Nature.
We abstract the following from a paper on electric lighting by Prof. J.A. Fleeming, read before the Iron and Steel Institute, Manchester. The illustration is from Engineering.
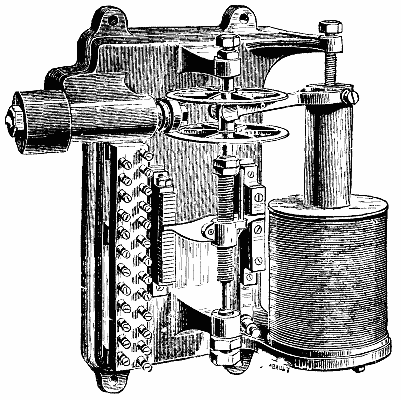
ELECTRICAL GOVERNOR.
One of the questions which most frequently occurs in reference to mill and factory lighting is whether the factory engines can be used to run the dynamo. As a broad, general rule, there can be no question that the best results are obtained by using a separate dynamo engine, controlled by a good governor, set apart for that purpose. With an ordinary shunt dynamo, the speed ought not to vary more than 2 or 3 per cent. of its normal value on either side of that value. Hence, if a dynamo has a normal speed of 1,000, it should certainly not vary over a greater range than from 970 to 980 to 1,020 to 1,030. In many cases there may be shafting from which the necessary power can be taken, and of which the speed is variable only within these limits. There are several devices by which it has been found possible to enable a dynamo to maintain a constant electromotive force, even if the speed of rotation varies over considerable limits. One of these is that (see illustration) due to Messrs. Trotter & Ravenshaw, and applicable to shunt or series machines.
In the circuit of the field magnet is placed a variable resistance. This resistance is thrown in or out by means of a motor device actuated by an electromotive force indicator. A plunger of soft iron is suspended from a spring, and hangs within a solenoid of wire, which solenoid is in connection with the terminals of the dynamo. Any increase or diminution of the electromotive force causes this iron to move in or out of the core, and its movement is made to connect or disconnect the gearing which throws in the field magnet resistance with a shaft driven by the engine itself. The principle of the apparatus is therefore that small variations of electromotive force are made to vary inversely the strength of the magnetic field through the intervention of a relay mechanism in which the power required to effect the movement is tapped from the engine.
With the aid of such a governor it is possible to drive a dynamo from a mill shaft providing the requisite power, but of which the speed of rotation is not sufficiently uniform to secure alone efficient regulation of electromotive force. Another device, patented by Mr. Crompton, is a modification of that method of field magnet winding commonly known as compound winding. The field magnets are wound over with two wires, one of which has a high resistance and is arranged as a shunt, and the other of which has a low resistance and is arranged in series. Instead, however, of the magnetizing powers of these coils being united in the same direction as an ordinary compound winding, they are opposed to one another. That is to say, the current in the shunt wire tends to magnetize the iron of the field magnets in an opposite direction to that of the series wire. It results from this that any slight increase of speed diminishes the strength of the magnetic field, and vice versa. Accordingly, within certain limits, the electromotive force of the dynamo is independent of the speed of rotation.
The object of this paper is to lay before you the results of some recent experiments in a comparatively new field of operation, but one that, judging from the results already attained, is destined to become of great importance and value in its practical application to various branches of industry.
I say "comparatively new" because the underlying principles involved in the experiments referred to have, to a certain extent, been employed (in, however, a somewhat restricted sense) for purposes analogous to those that form the basis of this communication.
As indicated by the title, the subject that will now occupy our attention is the use of the electric current as a means of increasing and varying the frictional adhesion of rolling contacts and other rubbing surfaces, and it is proposed to show how this effect may be produced, both by means of the direct action of the current itself and by its indirect action through the agency of electro-magnetism.
Probably the first instance in which the electric current was directly employed to vary the amount of friction between two rubbing surfaces was exemplified in Edison's electro-motograph, in which the variations in the strength of a telephonic current caused corresponding variations in friction between a revolving cylinder of moistened chalk and the free end of an adjustable contact arm whose opposite extremity was attached to the diaphragm of the receiving telephone. This device was extremely sensitive to the least changes in current strength, and if it were not for the complication introduced by the revolving cylinder, it is very likely that it would to-day be more generally used.
It has also been discovered more recently that in the operation of electric railways in which the track rails form part of the circuit, a considerable increase in the tractive adhesion of the driving wheels is manifested, due to the passage of the return current from the wheels into the track. In the Baltimore and Hampden electric railway, using the Daft "third rail" system, this increased tractive adhesion enables the motors to ascend without slipping a long grade of 350 feet to the mile, drawing two heavily loaded cars, which result, it is claimed, is not attainable by steam or other self-propelling motors of similar weight. In the two instances just cited the conditions are widely different, as regards the nature of the current employed, the mechanical properties of the surfaces in contact, and the electrical resistance and the working conditions of the respective circuits. In both, however, as clearly demonstrated by the experiments hereinafter referred to, the cause of the increased friction is substantially the same.
In order to ascertain the practical value of the electric current as a means of increasing mechanical friction, and, if possible, render it commercially and practically useful wherever such additional friction might be desirable, as for example in the transmission of power, etc., a series of experiments were entered into by the author, which, though not yet fully completed, are sufficiently advanced to show that an electric current, when properly applied, is capable of very materially increasing the mechanical friction of rotating bodies, in some cases as much as from 50 to 100 per cent., with a very economical expenditure of current; this increase depending upon the nature of the substances in contact and being capable of being raised by an increased flow of current.
Before entering into a description of the means by which this result is produced, and how it is proposed to apply this method practically to railway and other purposes, it may be well to give a general outline of what has so far been determined. These experiments have shown that the coefficient of friction between two conducting surfaces is very much increased by the passage therethrough of an electric current of low electromotive force and large volume, and this is especially noticeable between two rolling surfaces in peripheral contact with each other, or between a rolling and a stationary surface, as in the case of a driving wheel running upon a railway rail. This effect increases with the number of amperes of current flowing through the circuit, of which the two surfaces form part, and is not materially affected by the electromotive force, so long as the latter is sufficient to overcome the electrical resistance of the circuit. This increase in frictional adhesion is principally noticeable in iron, steel, and other metallic bodies, and is due to a molecular change in the conducting substances at their point of contact (which is also the point of greatest resistance in the circuit), caused by the heat developed at that point. This heat is ordinarily imperceptible, and becomes apparent only when the current strength is largely augmented. It is therefore probable that a portion of this increased tractive adhesion is due directly to the current itself aside from its heating effect, although I have not as yet been able to ascertain this definitely. The most economical and efficient results have been obtained by the employment of a transformed current of extremely low electromotive force (between ½ and 1 volt), but of very large volume or quantity, this latter being variable at will, so as to obtain different degrees of frictional resistance in the substances under observation.
These experiments were originally directed mainly toward an endeavor to increase the tractive adhesion of the driving wheels of locomotives and other vehicles, and to utilize the electric current for this purpose in such a manner as to render it entirely safe, practical, and economical. It will be apparent at once that a method of increasing the tractive power of the present steam locomotives by more than 50 per cent. without adding to their weight and without injury to the roadbed and wheel tires, such as is caused by the sand now commonly used, would prove of considerable value, and the same holds true with respect to electrically propelled street cars, especially as it has been found exceedingly difficult to secure sufficient tractive adhesion on street railways during the winter season, as well as at other times, on roads having grades of more than ordinary steepness. As this, therefore, is probably the most important use for this application of the electric current, it has been selected for illustrating this paper.
I have here a model car and track arranged to show the equipment and operation of the system as applied to railway motors. The current in the present instance is one of alternating polarity which is converted by this transformer into one having the required volume. The electromotive force of this secondary current is somewhat higher than is necessary. In practice it would be about half a volt. You will notice upon a closer inspection that one of the forward driving wheels is insulated from its axle, and the transformed current, after passing to a regulating switch under the control of the engineer or driver, goes to this insulated wheel, from which it enters the track rail, then through the rear pair of driving wheels and axles to the opposite rail, and then flows up through the forward uninsulated wheel, from the axle of which it returns by way of a contact brush to the opposite terminal of the secondary coil of the transformer. Thus the current is made to flow seriatim through all four of the driving wheels, completing its circuit through that portion of the rails lying between the two axles, and generating a sufficient amount of heat at each point of contact to produce the molecular change before referred to. By means of the regulating switch the engineer can control the amount of current flowing at any time, and can even increase its strength to such an extent, in wet or slippery weather, as to evaporate any moisture that may adhere to the surface of the rails at the point of contact with the wheels while the locomotive or motor car is under full speed.
It will be apparent that inasmuch as the "traction circuit" moves along with the locomotive, and is complete through its driving wheel base, the track rails in front and rear of the same are at all times entirely free from current, and no danger whatever can occur by coming in contact with the rails between successive motors. Moreover, the potential used in the present arrangement, while sufficient to overcome the extremely low resistance of the moving circuit, is too small to cause an appreciable loss of current from that portion of the rails in circuit, even under the most unfavorable conditions of the weather. In practice the primary current necessary is preferably generated by a small high speed alternating dynamo on the locomotive, the current being converted by means of an inductional transformer. To avoid the necessity for electrically bridging the rail joints, a modified arrangement may be employed, in which the electrical connection is made directly with a fixed collar on the forward and rear driving axles, the current dividing itself in parallel between the two rails in such a manner that, if a defective joint exists in the rail at one side, the circuit is still complete through the rail on the other; and as the rails usually break joints on opposite sides, this arrangement is found very effective. The insulation of the driving wheels is very easily effected in either case.
As the amount of additional tractive adhesion produced depends upon the quantity of current flowing rather than upon its pressure, the reason for transforming the current as described will be apparent, and its advantages over a direct current of higher tension and less quantity, both from an economical and practical standpoint, will for this reason be clear. The amount of heat produced at the point of contact between the wheels and rails is never large enough to injure or otherwise affect them, although it may be quite possible to increase the current sufficiently to produce a very considerable heating effect. The amount of current sent through the traction circuit will of course vary with the requirements, and as the extent to which the resistance to slipping may be increased is very great, this method is likely to prove of considerable value. While in some cases the use of such a method of increasing the tractive power of locomotives would be confined to ascending gradients and the movement of exceptionally heavy loads, in others it would prove useful as a constant factor in the work of transportation. In cases like that of the New York elevated railway system, where the traffic during certain hours is much beyond the capacity of the trains, and the structure unable to support the weight of heavier engines, a system like that just described would prove of very great benefit, as it would easily enable the present engines to draw two or three additional cars with far less slipping and lost motion than is the case with mechanical friction alone, at a cost for tractive current that is insignificant compared to the advantages gained. Other cases may be cited in which this method of increasing friction will probably be found useful, aside from its application to railway purposes, but these will naturally suggest themselves and need not be further dwelt upon.
In the course of the experiments above described, another and somewhat different method of increasing the traction of railway motors has been devised, which is more particularly adapted to electric motors for street railways, and is intended to be used in connection with a system of electric street railways now being developed by the author. In this system electro-magnetism provides the means whereby the increase in tractive adhesion is produced, and this result is attained in an entirely novel manner. Several attempts have heretofore been made to utilize magnetism for this purpose, but apparently without success, chiefly because of the crude and imperfect manner in which most of these attempts have been carried out.
The present system owes its efficiency to the formation of a complete and constantly closed magnetic circuit, moving with the vehicle and completed through the two driving axles, wheels, and that portion of the track rails lying between the two pairs of wheels, in a manner similar to that employed in the electrical method before shown. We have here a model of a second motor car equipped with the apparatus, mounted on a section of track and provided with means for measuring the amount of tractive force exerted both with and without the passage of the current.
You will notice that each axle of the motor car is wound with a helix of insulated wire, the helices in the present instance being divided to permit the attachment to the axles of the motor connections. The helices on both axles are so connected that, when energized, they induce magnetic lines of force that flow in the same direction through the magnetic circuit. There are, therefore, four points at which the circuit is maintained closed by the rolling wheels, and as the resistance to the flow of the lines of force is greatest at these points, the magnetic saturation there is more intense, and produces the most effective result just where it is most required. Now, when the battery circuit is closed through the helices, it will be observed that the torque, or pull, exerted by the motor car is fully twice that exerted by the motor with the traction circuit open, and, by increasing the battery current until the saturation point of the iron is reached, the tractive force is increased nearly 200 per cent., as shown by the dynamometer. A large portion of this resistance to the slipping or skidding of the driving wheels is undoubtedly due to direct magnetic attraction between the wheels and track, this attraction depending upon the degree of magnetic saturation and the relative mass of metal involved.
But by far the greatest proportion of the increased friction is purely the result of the change in position of the iron molecules due to the well known action of magnetism, which causes a direct and close interlocking action, so to speak, between the molecules of the two surfaces in contact. This may be illustrated by drawing a very thin knife blade over the poles of an ordinary electro-magnet, first with the current on and then off.
In the model before you, the helices are fixed firmly to, and revolve with, the axles, the connections being maintained by brushes bearing upon contact rings at each end of the helices. If desired, however, the axles may revolve loosely within the helices, and instead of the latter being connected for cumulative effects, they may be arranged in other ways so as to produce either subsequent or opposing magnetic forces, leaving certain portions of the circuit neutral and concentrating the lines of force wherever they maybe most desirable. Such a disposition will prove of advantage in some cases.
The amount of current required to obtain this increased adhesion in practice is extremely small, and may be entirely neglected when compared to the great benefits derived. The system is very simple and inexpensive, and the amount of traction secured is entirely within the control of the motor man, as in the electric system. It will be seen that the car here will not, with the traction circuit open, propel itself up hill when one end of the track is raised more than 5 inches above the table; but with the circuit energized it will readily ascend the track as you now see it, with one end about 13½, inches above the other in a length of three feet, or the equivalent of a 40 per cent. grade; and this could be increased still further if the motor had power enough to propel itself against the force of gravity on a steeper incline. As you will notice, the motor adheres very firmly to the track and requires a considerable push to force it down this 40 per cent. grade, whereas with the traction circuit open it slips down in very short order, notwithstanding the efforts of the driving mechanism to propel it up.
The resistance of the helices on this model is less than two ohms, and this will scarcely be exceeded when applied to a full sized car, the current from two or three cells of secondary batteries being probably sufficient to energize them.
The revolution of the driving axles and wheels is not interfered with in the slightest, because in the former the axle boxes are outside the path of the lines of force, and in the case of the latter because each wheel practically forms a single pole piece, and in revolving presents continuously a new point of contact, of the same polarity, to the rail; the flow of the lines of force being most intense through the lower half of the wheels, and on a perpendicular line connecting the center of the axle with the rail. In winter all that is necessary is to provide each motor car with a suitable brush for cleaning the track rails sufficiently to enable the wheels to make good contact therewith, and any tendency to slipping or skidding may be effectually checked. By this means it is easily possible to increase the tractive adhesion of an ordinary railway motor from 50 to 100 per cent., without any increase in the load or weight upon the track; for it must be remembered that even that portion of the increased friction due to direct attraction does not increase the weight upon the roadbed, as this attraction is mutual between the wheels and track rails; and if this car and track were placed upon a scale and the circuit closed, it would not weigh a single ounce more than with the circuit open.
It is obvious that this increase in friction between two moving surfaces can also be applied to check, as well as augment, the tractive power of a car or train of cars, and I have shown in connection with this model a system of braking that is intended to be used in conjunction with the electro-magnetic traction system just described. You will have noticed that in the experiments with the traction circuit the brake shoes here have remained idle; that is to say, they have not been attracted to the magnetized wheels. This is because a portion of the traction current has been circulating around this coil on the iron brake beam, inducing in the brake shoes magnetism of like polarity to that in the wheels to which they apply. They have therefore been repelled from the wheel tires instead of being attracted to them. Suppose now that it is desired to stop the motor car; instead of opening the traction circuit, the current flowing through the helices is simply reversed by means of this pole changing switch, whereupon the axles are magnetized in the opposite direction and the brake shoes are instantly drawn to the wheels with a very great pressure, as the current in the helices and brake coil now assist each other in setting up a very strong magnetic flow, sufficient to bring the motor car almost to an instant stop, if desired.
The same tractive force that has previously been applied to increase the tractive adhesion now exercises its influence upon the brake shoes and wheels, with the result of not only causing a very powerful pressure between the two surfaces due to the magnetic attraction, but offering an extremely large frictional resistance in virtue of the molecular interlocking action before referred to. As shown in the present instance, a portion of the current still flows through the traction circuit and prevents the skidding of the wheels.
The method thus described is equally applicable to increase the coefficient of friction in apparatus for the transmission of power, its chief advantage for this purpose being the ease and facility with which the amount of friction between the wheels can be varied to suit different requirements, or increased and diminished (either automatically or manually) according to the nature of the work being done. With soft iron contact surfaces the variation in friction is very rapid and sensitive to slight changes in current strength, and this fact may prove of value in connection with its application to regulating and measuring apparatus. In all cases the point to be observed is to maintain a closed magnetic circuit of low resistance through the two or more surfaces the friction of which it is desired to increase, and the same rule holds good with respect to the electric system, except that in the latter case the best effects are obtained when the area of surface in contact is smallest.
For large contact areas the magnetic system is found to be most economical, and this system might possibly be used to advantage to prevent slipping of short wire ropes and belts upon their driving pulleys, in cases where longer belts are inapplicable as in the driving of dynamos and other machinery. Experiments have also been, and are still being, made with the object of increasing friction by means of permanent magnetism, and also with a view to diminishing the friction of revolving and other moving surfaces, the results of which will probably form the subject matter of a subsequent paper.
Enough has been said to indicate that the development of these two methods of increasing mechanical friction opens up a new and extensive field of operation, and enables electricity to score another important point in the present age of progress. The great range and flexibility of this method peculiarly adapt it to the purposes we have considered and to numerous others that will doubtless suggest themselves to you. Its application to the increase of the tractive adhesion of railway motors is probably its most prominent and valuable feature at present, and is calculated to act as an important stimulus to the practical introduction of electric railways on our city streets, inasmuch as the claims heretofore made for cable traction in this respect are now no longer exclusively its own. On trunk line railways the use of sand and other objectionable traction-increasing appliances will be entirely dispensed with, and locomotives will be enabled to run at greater speed with less slipping of the wheels and less danger of derailment. Their tractive power can be nearly doubled without any increase in weight, enabling them to draw heavier trains and surmount steeper grades without imposing additional weight or strain upon bridges and other parts of the roadbed. Inertia of heavy trains can be more readily overcome, loss of time due to slippery tracks obviated, and the momentum of the train at full speed almost instantly checked by one and the same means.
Read before the American Association for the Advancement of Science. New York meeting, 1887.
Trials have been made at Havre with an electric launch built to the order of the French government by the Forges et Chantiers de la Mediterranée. The vessel, which has rather full lines, measures 28 ft. between perpendiculars and 9 ft. beam, and is 5 tons register.
The electromotor is the invention of Captain Krebs, who is already well known on account of his experiments in connection with navigable balloons, and of M. De Zédé, naval architect. The propeller shaft is not directly coupled with the spindle of the motor, but is geared to it by spur wheels in the ratio of 1 to 3, in order to allow of the employment of a light high-speed motor. The latter makes 850 revolutions per minute, and develops 12 horse power when driving the screw at 280 revolutions. Current is supplied by a new type of accumulators made by Messrs. Commelin & Desmazures. One hundred and thirty two of these accumulators are fitted in the bottom of the boat, the total weight being about 2 tons.
In ordering this boat the French government stipulated a speed of 6 knots to be maintained during three hours with an expenditure of 10 horse power. The result of the trials gave a speed of 6½ knots during five hours with 12 horse power, and sufficient charge was left in the accumulators to allow the boat to travel on the following day for four hours. This performance is exceedingly good, since it shows that one horse power hour has been obtained with less than 60 lb. of total weight of battery.
Leveling the ground, pulling down old buildings, and distributing light and air through her wide streets, Paris is slowly and continuously pursuing her transformation. At this moment it is an entire district, and not one of the least curious ones, that is disappearing, leaving no other trace of its existence than the circular walls that once inclosed the wheat market.
It is this building that, metamorphosed, is to become the Commercial Exchange that has been so earnestly demanded since 1880 by the commerce of Paris. The question, which was simple in the first place, and consisted in the conversion of the wheat market into a commercial exchange, became complicated by a project of enlarging the markets. It therefore became necessary to take possession, on the one hand, of sixty seven estates, of a total area of 116,715 square feet, to clear the exchange, and, on the other, of 49,965 square feet to clear the central markets. In other words, out of $5,000,000 voted by the common council for this work, $2,800,000 are devoted to the dispossessions necessitated by the new exchange, $1,800,000 to those necessitated by the markets, and $400,000 are appropriated to the wheat market.
The work of demolition began last spring, and the odd number side of Orleans street, Deux-Ecus street, from this latter to J.J. Rousseau street, Babille street, Mercier street, and Sortine street, now no longer exist. All this part is to-day but a desert, in whose center stands the iron trussing of the wheat market cupola. It is on these grounds that will be laid out the prolongation of Louvre street in a straight line to Coquilliere street.
Our engraving shows the present state of the work. What is seen of the wheat market will be preserved and utilized by Mr. Blondeau, the architect, who has obtained a grant from the commercial exchange to construct two edifices on two plots of an area of 32,220 square feet, fronting on Louvre street, and which will bring the city an annual rent of $60,000.
THE NEW COMMERCIAL EXCHANGE, PARIS.
Around the rotunda that still exists there was a circular wall 6½ feet in thickness. Mr. Blondeau has torn this down, and is now building another one appropriate to the new destination of the acquired estates. As for the trussing of the cupola, that is considered as a work of art, and care has been taken not to touch it. It was constructed at the beginning of this century, at an epoch when nothing but rudimentary tools were to be had for working iron, and it was, so to speak, forged. All the pieces were made with the hammer and were added one to the other in succession. This cupola will be glazed at the upper part, while the lower part will be covered with zinc. In the interior this part will be decorated with allegorical paintings representing the five divisions of the globe, with their commercial and industrial attributes. It was feared at one time that the hall, to which admission will be free, would not afford sufficient space, and the halls of the Bordeaux and Havre exchanges were cited. It is true that the hall of the wheat market has an area of but 11,825 square feet, but on utilizing the 5,000 feet of the circular gallery, which will not be occupied, it will reach 16,825 feet.
As for the tower which stands at one side of the edifice, that was built by Marie de Medici for the astrologer whom she brought with her to Paris from Florence. On account of its historic interest, this structure will be preserved. On either side of this tower, overlooking the roofs of the neighboring dwellings, are perceived the summit of a tower of St. Eustache church and a campanile of a pavilion of the markets.—L'Illustration.
Cocaine is manufactured from the dry leaves of the Erythroxylon coca, which grows in the valleys of the East Cordilleras of South America—i.e., in the interior of Peru and Bolivia. The fresh leaves contain 0.003 to 0.006 per cent of cocaine, which percentage decreases considerably if the leaves are stored any length of time before being worked up. On the other hand, the alkaloid can be transported and kept without decomposition. This circumstance caused the author to devise a simple process for the manufacture of crude cocaine on the spot, neither Peru nor Bolivia being suitable countries for complicated chemical operations. After many experiments, he hit upon the following plan: The disintegrated coca leaves are digested at 70° C. in closed vessels for two hours, with a very weak solution of sodium hydrate and petroleum (boiling between 200° and 250° C). The mass is filtered, pressed while still tepid, and the filtrate allowed to stand until the oil has completely separated from the aqueous solution. The oil is drawn off and carefully neutralized with very weak hydrochloric acid. A white bulky precipitate of cocaine hydrochloride is obtained, together with an aqueous solution of the same compound, while the petroleum is free from the alkaloid and may be used for the extraction of a fresh batch of leaves. The precipitate is dried, and by concentrating the aqueous solution a further quantity of the hydrochloride is obtained. Both can be shipped without risk of decomposition. The product is not quite pure, but contains some hygrine, traces of gum and other matters. Its percentage of alkaloid is 75 per cent., while chemically pure cocaine hydrochloride (C17H21NO4.2HCl) contains 80.6 per cent. of the alkaloid. The sodium hydrate solution cannot be replaced by milk of lime, nor can any other acid be used for neutralization. Alcohol or ether are not suitable for extraction. A repetition of the process with once-extracted coca leaves gave no further quantity of cocaine, proving that all the cocaine goes into solution by one treatment. The same process serves on the small scale for the valuation of coca leaves. 100 grms. of coca leaves are digested in a flask with 400 c.c. of water, 50 c.c. of 1/10 NaOH (10 grms. of NaOH in 100 c.c.) and 250 c.c. of petroleum. The flask is loosely covered and warmed on the water bath for two hours, shaking it from to time. The mass is then filtered, the residue pressed, and the filtrate allowed to separate in two layers. The oil layer is run into a bottle and titrated back with 1/100 HCl (1 grm. of HCl in 100 c.c.) until exactly neutral. The number of c.c. of hydrochloric acid required for titrating back multiplied by 0.42 gives the percentage of cocaine in the sample. The following are some of the results with different samples of coca leaves of various age:
| Contained per cent. of Cocaine. | ||||
| Coca leaves from | Mapiri, | 1 month old | 0.5% | =>Of the weight of the dry leaves. |
| " | Yungas | " | 0.5% | |
| " | Mapiri and Yungas | 6 months old | 0.4% | |
| " | Cuzco (Peru) | 6 months old | 0.3% | |
| " | Mapiri and Yungas | 1 year old | 0.3% | |
| " | Cuzco | " | 0.2% | |
| " | Mapiri and Yungas | 2 years old | 0.15% | |
Coca leaves from Yungas and Cuzco, three years old, contained no trace of the alkaloid, whereas fresh green leaves from Yungas contained 0.7 per cent. of the weight of the dry leaves. The same process is also applicable for the manufacture of quinine from poor quinine bark, with the single alteration that weak sulphuric acid must be used for the neutralization of the alkaline petroleum extract.—H.T. Pfeiffer, Chem. Zeit. 11.
[Continued from SUPPLEMENT, No. 622, page 9941.]
The succession of plants from the lower to the higher forms will be reviewed superficially, and chemical compounds noted where they appear.
When the germinating spores of the fungi, myxomycetes, rupture their walls and become masses of naked protoplasm, they are known as plasmodia. The plasmodium Æthalium septicum occurs in moist places, on heaps of tan or decaying barks. It is a soft, gelatinous mass of yellowish color, sometimes measuring several inches in length.
The plasmodium2 has been chemically analyzed, though not in a state of absolute purity. The table of Reinke and Rodewold gives an idea of its proximate constitution.
Many of the constituents given are always present in the living cells of higher plants. It cannot be too emphatically stated that where "biotic" force is manifested, these colloidal or albuminous compounds are found.
The simplest form of plant life is an undifferentiated individual, all of its functions being performed indifferently by all parts of its protoplasm.
The chemical basis of plasmodium is almost entirely composed of complex albuminous substances, and correlated with this structureless body are other compounds derived from them. Aside from the chemical substances which are always present in living matter, and are essential properties of protoplasm, we find no other compounds. In the higher organisms, where these functions are not performed indifferently, specialization of tissues is accompanied by many other kinds of bodies.
The algæ are a stage higher in the evolutionary scale than the undifferentiated noncellular plasmodium. The simple Alga protococcus3 may be regarded as a simple cell. All higher plants are masses of cells, varying in form, function, and chemical composition.
A typical living cell may be described as composed of a cell wall and contents. The cell wall is a firm, elastic membrane closed on all sides, and consists mainly of cellulose, water, and inorganic constituents. The contents consist of a semi-fluid colloidal substance, lying in contact with the inner surface of the membrane, and, like it, closed on all sides. This always is composed of albuminous substances. In the higher plants, at least, a nucleus occurs embedded in it; a watery liquid holding salts and saccharine substances in solution fills the space called the vacuole, inclosed by the protoplasm.
These simple plants may be seen as actively moving cells or as non-motile cells. The former consist of a minute mass of protoplasm, granular and mostly colored green, but clear and colorless at the more pointed end, and where it is prolonged into two delicate filaments called cilia. After moving actively for a time they come to rest, acquire a spherical form, and invest themselves with a firm membrane of cellulose. This firm, outer membrane of the Protococcus accompanies a higher differentiation of tissue and localization of function than is found in the plasmodium.
Hæatococcus and plasmodium come under the classes algæ and fungi of the Thallothyta group. The division4 of this group into two classes is based upon the presence of chlorophyl in algæ and its absence in fungi. Gelatinous starch is found in the algæ; the fungi contain a starchy substance called glycogen, which also occurs in the liver and muscles of animals. Structureless bodies, as æthalium, contain no true sugar. Stratified starch5 first appears in the phanerogams. Alkaloids have been found in fungi, and owe their presence doubtless to the richness of these plants in nitrogenous bodies.
In addition to the green coloring matter in algæ are found other coloring matters.6 The nature7 of these coloring matters is usually the same through whole families, which also resemble each other in their modes of reproduction.
In form, the algæ differ greatly from filaments or masses of cells; they live in the water and cover damp surfaces of rocks and wood. In these they are remarkable for their ramifications and colors and grow to a gigantic size.
The physiological functions of algæ and fungi depend upon their chemical differences.
These facts have been offered, simple as they are, as striking examples of chemical and structural opposition.
The fungi include very simple organisms, as well as others of tolerably high development, of most varied form, from the simple bacillus and yeast to the truffle, lichens, and mushrooms.
The cell membrane of this class contains no pure cellulose, but a modification called fungus cellulose. The membrane also contains an amyloid substance, amylomycin.8 Many of the chemical constituents found in the entire class are given in Die Pflanzenstoffe.9
Under the Schizomycetes to which the Micrococcus and Bacterium10 belong are found minute organisms differing much in form and in the coloring11 matters they produce, as that causing the red color of mouldy bread.
The class of lichens12 contains a number of different coloring substances, whose chemical composition has been examined. These substances are found separately in individuals differing in form. In the Polyporus13 an acid has been found peculiar to it, as in many plants special compounds are found. In the agariceæ the different kinds of vellum distinguish between species, and the color of the conidia is also of differential importance. In all cases of distinct characteristic habits of reproduction and form, one or more different chemical compounds is found.
In the next group of the musiceæ, or mosses, is an absence of some chemical compounds that were characteristic of the classes just described. Many of the albuminous substances are present. Starch14 is found often in large quantities, and also oily fats, which are contained in the oil bodies of the liverworts; wax,15 organic acids, including aconitic acid, and tannin, which is found for the first time at this evolutionary stage of the plant kingdom.
The vascular cryptogams are especially characterized by their mineral composition.16 The ash is extraordinarily rich in silicic acid and alumina.
| Equisetum 17 | silicic acid | 60 | per cent. |
| Aspidium | " | 13 | " |
| Asplenium | " | 35 | " |
| Osmunda | " | 53 | " |
| Lycopodium 18 | " | 14 | " |
| " | alumina | 26 to 27 | " |
| " | manganese | 2 to 2.5 | " |
These various plants contain acids and compounds peculiar to themselves.
As we ascend in the plant scale, we reach the phanerogams. These plants are characterized by the production of true seeds, and many chemical compounds not found in lower plants.
It will be convenient in speaking of these higher groups to follow M. Heckel's19 scheme of plant evolution. All these plants are grouped under three main divisions: apetalous, monocotyledonous, and dicotyledonous; and these main divisions are further subdivided.
It will be observed that these three main parallel columns are divided into three general horizontal planes.
On plane 1 are all plants of simplicity of floral elements, or parts; for example, the black walnut, with the simple flower contained in a catkin.
On plane 2 plants which have a multiplicity of floral elements, as the many petals and stamens of the rose; and finally, the higher plants, the orchids among the monocotyledons and the composite among the dicotyledonous plants, come under the third division of condensation of floral elements.
It will be impossible to take up in order for chemical consideration all these groups, and I shall restrict myself to pointing out the occurrence of certain constituents.
I desire now to call attention to chemical groups under the apetalous plants having simplicity of floral elements.
Cassuarina equisetifolia20 possibly contains tannin, since it is used for curing hides. The bark contains a dye. It is said to resemble Equisetum21 in appearance, and in this latter plant a yellow dye is found.
The Myrica22 contains ethereal oil, wax, resin, balsam, in all parts of the plant. The root contains in addition fats, tannin, and starch, also myricinic acid.
In the willow and poplar,23 a crystalline, bitter substance, salicin or populin, is found. This may be considered as the first appearance of a real glucoside, if tannin be excluded from the list.
The oak, walnut, beech, alder, and birch contain tannin in large quantities; in the case of the oak, ten to twelve per cent. Oak galls yield as much as seventy per cent.24 The numerous genera of pine and fir trees are remarkable for ethereal oil, resin, and camphor.
The plane25 trees contain caoutchouc and gum; peppers,26 ethereal oils, alkaloids, piperin, white resin, and malic acid. Datisca cannabina27 contains a coloring matter and another substance peculiar to itself, datiscin, a kind of starch, or allied to the glucosides.
Upon the same evolutionary plane among the monocotyledons, the dates and palms28 contain in large quantities special starches, and this is in harmony with the principles of the theory. Alkaloids and glucosides have not yet been discovered in them.
Other monocotyledonous groups with simplicity of floral elements, such as the typhaceæ, contain large quantities of starch; in the case of Typha latifolia29 12.5 per cent., and 1.5 per cent. gum. In the pollen of this same plant, 2.08 per cent. starch has been found.
Under the dicotyledonous groups, there are no plants with simplicity of floral elements.
Returning, now, to apetalous plants of multiplicity and simplification of floral elements, we find that the urticaceæ30 contain free formic acid; the hemp31 contains alkaloids; the hop,32 ethereal oil and resin; the rhubarb,33 crysophonic acid; and the begonias,34 chicarin and lapacho dyes. The highest apetalous plants contain camphors and oils; the highest of the monocotyledons contain a mucilage and oils; and the highest dicotyledons contain oils and special acids.
The trees yielding common camphor and borneol are from genera of the lauraceæ family; also sassafras camphor is from the same family. Small quantities of stereoptenes are widely distributed through the plant kingdom.
The gramineæ, or grasses, are especially characterized by the large quantities of sugar and silica they contain. The ash of the rice hull, for example, contains ninety eight per cent. silica.
The ranunculaceæ contain many plants which yield alkaloids, as Hydrastia canadensis, or Indian hemp, Helleborus, Delphinum, Aconitum, and the alkaloid berberine has been obtained from genera of this family.
The alkaloid35 furnishing families belong, with few exceptions, to the dicotyledons. The colchiceæ, from which is obtained veratrine, form an exception among the monocotyledons. The alkaloids of the fungus have already been noted.
36Among the greater number of plant families, no alkaloids have been found. In the labiatæ none has been discovered, nor in the compositæ among the highest plants.
One alkaloid is found in many genera of the loganiaceæ; berberine in genera of the berberidaceæ, ranunculaceæ, menispermaceæ, rutaceæ, papaveraceæ, anonaceæ.
Waxes are widely distributed in plants. They occur in quantities in some closely related families.
Ethereal oils occur in many families, in the bark, root, wood, leaf, flower, and fruit; particularly in myrtaceæ, laurineæ, cyperaceæ, crucifereæ, aurantiaceæ, labiatæ, and umbelliferæ.
Resins are found in most of the higher plants. Tropical plants are richer in resins than those of cold climates.
Chemical resemblance between groups, as indicating morphological relations, has been well shown. For example: the similarity37 of the viscid juices, and a like taste and smell, among cactaceæ and portulaceæ, indicate a closer relationship between these two orders than botanical classification would perhaps allow. This fact was corroborated by the discovery of irritable stamens in Portulaca and Opuntia, and other genera of cactaceæ.
Darwin38 states that in the compositæ the ray florets are more poisonous than the disk florets, in the ratio of about 3 to 2.
Comparing the cycadeæ and palmæ, the former are differently placed by different botanists, but the general resemblance is remarkable, and they both yield sago.
Chemical constituents of plants are found in varying quantities during stated periods of the year. Certain compounds present at one stage of growth are absent at another. Many facts could be brought forward to show the different chemical composition of plants in different stages of growth. The Thuja occidentalis39 in the juvenescent and adult form, offers an example where morphological and chemical differences go hand in hand. Analyses of this plant under both conditions show a striking difference.
Different parts of plants may contain distinct chemical compounds, and the comparative chemical study of plant orders comprises the analysis of all parts of plants of different species.
For example; four portions of the Yucca angustifolia40 were examined chemically; the bark and wood of the root and the base and blades of the leaves. Fixed oils were separated from each part. These were not identical; two were fluid at ordinary temperature, and two were solid. Their melting and solidifying points were not the same.
This difference in the physical character and chemical reaction of these fixed oils may be due to the presence of free fatty acid and glycerides in varying proportions in the four parts of the plants. It is of interest to note that, in the subterranean part of the Yucca, the oil extracted from the bark is solid at the ordinary temperature; from the wood it was of a less solid consistency; while the yellow base of the leaf contained an oil quite soft, and in the green leaf the oil is almost fluid.
Two new resins were extracted from the yellow and green parts of the leaf. It was proposed to name them yuccal and pyrophæal An examination of the contents of each extract showed a different quantitative and qualitative result.
Saponin was found in all parts of the plant.
Many of the above facts have been collected from the investigations of others. I have introduced these statements, selected from a mass of material, as evidences in favor of the view stated at the beginning of this paper.41 My own study has been directed toward the discovery of saponin in those plants where it was presumably to be found. The practical use of this theory in plant analysis will lead the chemists at once to a search for those compounds which morphology shows are probably present.
I have discovered saponin in all parts of the Yucca angustifolia, in the Y. filimentosa and Y. gloriosa, in several species of agavæ, and in plants belonging to the leguminosæ family.
The list42 of plants in which saponin has been discovered is given in the note. All these plants are contained in the middle plane of Heckel's scheme. No plants containing saponin have been found among apetalous groups. No plants have been found containing saponin among the lower monocotyledons.
The plane of saponin passes from the liliaceæ and allied groups to the rosales and higher dicotyledons.
Saponin belongs to a class of substances called glucosides. Under the action of dilute acids, it is split up into two substances, glucose and sopogenin. The chemical nature of this substance is not thoroughly understood. The commercial43 product is probably a mixture of several substances.
This complexity of chemical composition of saponin is admirably adapted for the nutrition of the plant, and it is associated with the corresponding complexity of the morphological elements of the plant's organs. According to M. Perrey,44 it seems that the power of a plant to direct the distribution of its carbon, hydrogen, and oxygen to form complex glucosides is indicative of its higher functions and developments.
The solvent action of saponin on resins has been already discussed. Saponin likewise acts as a solvent upon barium45 sulphate and calcium46 oxalate, and as a solvent of insoluble or slightly soluble salts would assist the plant in obtaining food, otherwise difficult of access.
Saponin is found in endogens and exogens. The line dividing these two groups is not always clearly defined. Statements pointing to this are found in the works of Haeckel, Bentham, and others.
Smilax belongs to a transition class, partaking somewhat of the nature of endogen and of exogen. It is worthy of note that this intermediate group of the sarsaparillas should contain saponin.
It is a significant fact that all the groups above named containing saponin belong to Heckel's middle division.
It may be suggested that saponin is thus a constructive element in developing the plant from the multiplicity of floral elements to the cephalization of those organs.
It has been observed that the composite occurs where the materials for growth are supplied in greatest abundance, and the more simple forms arise where sources of nutrition are remote. We may gather from this fact that the simpler organs of plants low in the evolutionary scale contain simpler non-nitrogenous chemical compounds for their nutrition.
The presence of saponin seems essential to the life of the plant where it is found, and it is an indispensable principle in the progression of certain lines of plants, passing from their lower to their higher stages.
Saponin is invariably absent where the floral elements are simple; it is invariably absent where the floral elements are condensed to their greatest extent. Its position is plainly that of a factor in the great middle realm of vegetable life, where the elements of the individual are striving to condense, and thus increase their physiological action and the economy of parts.
It may be suggested as a line of research to study what are the conditions which control the synthesis and gradual formation of saponin in plants. The simpler compounds of which this complex substance is built up, if located as compounds of lower plants, would indicate the lines of progression from the lower to the saponin groups.
In my paper47 read in Buffalo at the last meeting of the American Association for the Advancement of Science, various suggestions were offered why chemical compounds should be used as a means of botanical classification.
The botanical classifications based upon morphology are so frequently unsatisfactory, that efforts in some directions have been made to introduce other methods.48
There has been comparatively little study of the chemical principles of plants from a purely botanical view. It promises to become a new field of research.
The leguminosæ are conspicuous as furnishing us with important dyes, e.g., indigo, logwood, catechin. The former is obtained principally from different species of the genus Indigofera, and logwood from the Hæmatoxylon and Saraca indica.
The discovery49 of hæmatoxylin in the Saraca indica illustrates very well how this plant in its chemical, as well as botanical, character is related to the Hæmatoxylon campechianum; also, I found a substance like catechin in the Saraca. This compound is found in the acacias, to which class Saraca is related by its chemical position, as well as botanically. Saponin is found in both of these plants, as well as in many other plants of the leguminosæ. The leguminosæ come under the middle plane or multiplicity of floral elements, and the presence of saponin in these plants was to be expected.
From many of the facts above stated, it may be inferred that the chemical compounds of plants do not occur at random. Each stage of growth and development has its own particular chemistry.
It is said that many of the constituents found in plants are the result of destructive metabolism, and are of no further use in the plant's economy. This subject is by no means settled, and even should we be forced to accept that ground, it is a significant fact that certain cells, tissues, or organs peculiar to a plant secrete or excrete chemical compounds peculiar to them, which are to be found in one family, or in species closely allied to it.
It is a fact that the chemical compounds are there, no matter why or whence they came. They will serve our purposes of study and classification.
The result of experiment shows that the presence of certain compounds is essential to the vigor and development of all plants and particular compounds to the development of certain plants. Plant chemistry and morphology are related. Future investigations will demonstrate this relation.
In general terms, we may say that amides and carbohydrates are utilized in the manufacture of proteids. Organic acids cause a turgescence of cells. Glucosides may be a form of reserve food material.
Resins and waxes may serve only as protection to the surfaces of plants; coloring matters, as screens to shut off or admit certain of the sun's rays; but we are still far from penetrating the mystery of life.
A simple plant does what animals more highly endowed cannot do. From simplest substances they manufacture the most complex. We owe our existence to plants, as they do theirs to the air and soil.
The elements carbon, oxygen, hydrogen, and nitrogen pass through a cycle of changes from simple inorganic substances to the complex compounds of the living cell. Upon the decomposition of these bodies the elements return to their original state. During this transition those properties of protoplasm which were mentioned at the beginning, in turn, follow their path. From germination to death this course appears like a crescent, the other half of the circle closed from view. Where chemistry begins and ends it is difficult to say.—Jour. Fr. Inst.
A lecture delivered before the Franklin Institute, January 24, 1887.
Studien uber das Protoplasm, 1881.
Vines, p. 1. Rostafinski: Mem. de la Soc. des Sc. Nat. de Cherbourg, 1875. Strasburger: Zeitschr., xii, 1878.
Botany: Prantl and Vines. London, 1886, p. 110.
For the literature of starch, see p. 115, Die Pflanzenstoffe, von Hilger and Husemann.
Kutzing: Arch. Pharm., xli, 38. Kraus and Millardet: Bul. Soc. Sciences Nat., Strasbourg, 1868, 22. Sorby: Jour. Lin. Soc., xv, 34. J. Reinke: Jahrb. Wissenscht. Botan., x, B. 399. Phipson: Phar. Jour. Trans., clxii, 479.
Prantl and Vines, p. 111.
L. Crie: Compt. Rend., lxxxviii, 759 and 985. J. De Seynes, 820, 1043.
Page 279.
M. Nencki and F. Schaffer. N. Sieher: Jour. Pract. Chem., 23, 412.
E. Klein: Quar. Jour. Micros. Science, 1875, 381. O. Helm: Arch. Pharm., 1875, 19-24. G. Gugini: Gaz. Chem., 7, 4. W. Thorner: Bul. Ber, xi, 533.
Handbook of Dyeing. By W. Crookes, London, 1874. p. 367. Schunck: Ann. Chem. Pharm., 41, 157; 54, 261; 61, 72; 61, 64; 61, 78. Rochelder and Heldt, ibid., 48, 2; 48, 9. Stenhouse, ibid., 68, 57; 68, 72; 68, 97, 104; 125, 353. See also researches of Strecker, O. Hesse, Reymann, Liebermann, Lamparter, Knop, and Schnedermann.
Stahlschmidt.
E. Treffner: Inaugur. Diss. Dorpat, 1880.
W. Pfeffer: Flora, 1874.
Die Pflanzenstoffe, p. 323 W. Lange: Bul. Ber., xi, 822.
Ann. Chim. Phys., 41, 62, 208; Ann. Chim. Pharm., 77, 295.
Fluckiger: Pharmakognosie. Kamp: Ann. Chim. Pharm., 100, 300.
Revue Scientifiqe, 13 Mars, 1886.
Dictionary of Economic Plants. By J. Smith. London, 1882, p. 294.
Ibid., p. 160. Pharmakognosie des Pflanzenreichs, Wittstein, p. 736. Ann. Chem. Pharm., 77, 295.
Rabenhorst: Repert. Pharm., lx, 214. Moore: Chem. Centralbl., 1862, 779, Dana.
Johansen: Arch. Pharm., 3, ix, 210. Ibid., 3, ix 103. Bente: Berl. Ber., viii, 476. Braconnot: Ann. Chim. Phys., 2, 44, 296.
Wittstein; Pharm. des Pflanzenreichs, p. 249.
John; Ibid., p. 651.
Dulong. Oersted, Lucas, Pontet; Ibid., p. 640.
Braconnot: Ann. Chim. Phys., 2, 3. 277. Stenhouse: Ann. Chim. Phann., 198, 166
3 Pflanzenstoffe, p. 412.
Lecocq: Braconnot: Pharmacog. Pflan, p. 693.
Gorup-Besanez.
Siebold and Brodbury: Phar. Jour. Trans., 3, 590, 1881, 326.
Wagner: Jour. Prakt. Chem., 58, 352. B. Peters, v. Gohren: Jahresb. Agric., viii, 114; ix, 105; v. 58. Ann. Jour. Pharm., 4, 49.
Dragendorff: Pharm. Zeitschr. Russ., xvii, 65-97.
Bonssingault: Ann. Chim. Phys., 2, 27, 315. Erdmann: Jour. Pract. Chem., 71, 198.
Die Pflanzenstoffe, p. 21.
Ibid.
Meehan: Proc. Acad. Nat. Sciences.
Different forms of flowers on plants of the same species. Introduction.
Meehan: Proc. Acad. Nat. Sciences.
H.C. De S. Abbott: Trans. Amer. Philos. Soc., 1886.
For further facts confirming this theory, see "Comparative Chemistry of Higher and Lower Plants." By H.C. De S. Abbott. Amer. Naturalist, August, 1887.
Different genera and species of the following: Ranunculaceæ, Berberidaceæ, Carophyllaceæ, Polygalaceæ, Bromeliaceæ, Liliaceæ, Smilaceæ, Yuccas, Amaryllideæ, Leguminosæ, Primulaceæ, Rosaceæ, Sapindaceæ, Sapotaceæ
Kobert: Chem Ztg.
Compt. Rend., xciv, p. 1124.
Bul. de la Soc. Chim.
"Yucca angus." Trans. Am. Philos. Soc., Dec., 1885.
Botanical Gazette, October, 1886.
Borodin: Pharm. Jour. Trans., xvi, 369. Pax. Firemy: Ann. Sci. Nat., xiii.
H.C. De S. Abbott, Proc. Acad. Nat. Sciences, Nov. 30, 1886.
The author maintains that unsatisfactory results are obtained in determinations of starch when the method employed is based upon the inversion of sugar, formed as an intermediate product, since maltose, dextrose, and levulose are partly decomposed by boiling with dilute acids. He proposes to replace the methods hitherto employed by one which depends upon the formation of a barium salt of starch, to which he assigns the formula BaO.C24H40O20. This salt is sparingly soluble in water and insoluble in dilute alcohol.
In making a determination a weighed quantity of starch is saccharified with water, then mixed with an excess of normal baryta solution, dilute alcohol added to make up to a certain volume, and, after the precipitate has settled, the excess of baryta is titrated back with acid.
Titrating apparatus
The author also describes the apparatus he employs for storing and titrating with baryta solution. The latter is contained in the bottle, A, and the drying tube attached to the neck of the same is filled with quicklime. The burette, B, which is in direct connection with the bottle, may be filled with the solution by opening the stop cock, and the small drying tube, n, is filled with dry KOH, thus preventing the entrance of any CO2. Numbers are appended which seem to testify to the excellence of the method employed. The author finally gives a detailed account of the entire analysis of various cereals.—A.R. in Jour. Soc. Chem. Indus.
In the note on the constitution of alkaloids in a recent issue, we referred more especially to what we may term the less highly organized bases. Most of our knowledge, as we now have it, regarding such alkaloids as muscarine and choline has been acquired during the past dozen years. This is not exactly the case with the higher groups of alkaloids—the derivatives of pyridine and quinoline. It so happens that the oldest alkaloids are in these groups. They have, almost necessarily, been subjected to a longer period of attack, but the extreme complexity of their molecules, and the infinite number of differing parts or substances into which these molecules split up when attacked, are the main cause of the small progress which has been made in this department. All, however, yield one or more bodies or bases in common, while each has its distinctive and peculiar decomposition product. For example, cinchonine and quinine both afford the basic quinoline under certain conditions, but on oxidation of cinchonine, an acid—cinchoninic acid (C10H7NO2)—is the principal body formed, while in the case of quinine, quininic acid (C10H9NO3) is the principal product. The acquirement through experiment of such knowledge as that is, however, so much gained. We find, indeed, that obstacles are gradually being cleared away, and the actual synthetic formation of such alkaloids as piperidine and coniine is a proof that the chemist is on the right track in studying the decomposition products, and building up from them, theoretically, bodies of similar constitution. It is noteworthy that the synthesis of the alkaloids has led to some of the most brilliant discoveries of the present day, especially in the discovery of dye stuffs. Many of our quinine substitutes, such as thalline, for example, are the result of endeavors to make quinine artificially. If there is romance in chemistry at all, it is to be found certainly in this branch of it, which is generally considered the most uninteresting and unfathomable. We may take piperidine and coniine as examples of the methods followed in alkaloidal synthesis; these are pyridine bases. Pyridine has the formula C5H5N, that is, it is benzene with CH replaced by N. The relationship between these and piperidine is seen in the following formulæ:
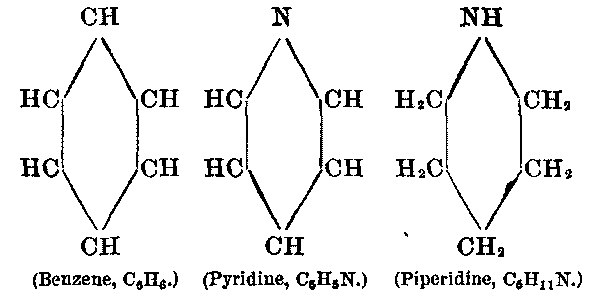
If we introduce six hydrogen atoms into pyridine, we convert it into piperidine. Ladenburg succeeded in so hydrogenizing pyridine by acting upon an alcoholic solution with sodium, and from the base which was formed he obtained a platinochloride which agreed with the similar double salt of piperidine. He has also prepared it from trimethyline cyanide by the action of sodium. Pentamethylinediamine is the principal intermediary product, and this gives piperidine when distilled with superheated steam. He has proved that the alkaloid so obtained is identical with that prepared from piperine. Another curious point which Ladenburg has lately proved is that cadaverine (one of the products of flesh decomposition) is identical with pentamethylinediamine, and that its imine is the same as piperidine. The synthesis of coniine by Ladenburg is one of the most notable achievements of modern chemistry. He at first supposed that this alkaloid was piperidine in which two hydrogen atoms were replaced by the isopropyl radical (C3H7), its formula being taken as C5H9(C3H7)NH. But he has since changed his view, as will be seen from what follows. In its synthesis 1,000 grammes of picoline were first converted into alphapicoline, 380 grammes being obtained. This was heated with paraldehyde, whereby it was converted into allylpyridine (48 grammes), and this by reduction with sodium yielded alpha-propylpyridine, a body in almost every respect identical with coniine. The more important difference was its optical inactivity, but he succeeded in splitting up a solution of the acid tartrate of the base by means of Penicillium glaucum. Crystals separated which had a dextro-rotatory power of [a]D = 31° 87' as compared with the [a]D = 13° 79' of natural coniine. This brief account conveys but a faint idea of the difficulties which were encountered in these researches. Optical methods of examination have proved of great value, and are destined to play an important part in such work.
Among the most complex alkaloids are those of the quinine group. As yet chemists have got no further with these than the oxidation products; but the study has afforded us several new antipyretics and many interesting facts. It has been found, for example, that artificial quinine-like bodies, which fluoresce and give the green color with chlorine water and ammonia, have antipyretic properties like quinine, but their secondary effects are so pernicious as to prevent their use. If, however, such bodies are hydrogenized or methylated they lose their fluorescing property, do not give the green color, and their secondary effects are removed. Knowledge of these facts led to the discovery of thalline. It is prepared from paraquinanisol, one of the objectionable bodies, by reduction with tin and hydrochloric acid. The following formulæ show the constitutional relationship of these compounds:
It is evident from the difficulties which have been encountered in this department of chemistry, and more especially from the costly nature of the work, that it will be many years before it will influence the manufacture of alkaloids from the drugs which yield them. Ladenburg has synthetized coniine, but he has not yet ventured to assert that his product will replace the natural alkaloid.—Chem. and Druggist.
The Southern California Advocate reports another magnificent donation of lands to the University of Southern California by Mr. D. Freeman, the owner of the Centinella ranch near Los Angeles—six hundred thousand dollars in all given to found a school of applied sciences, $100,000 for building and apparatus and $500,000 for endowment. The buildings will be in the vicinity of Inglewood, the new and beautiful town on the Ballona branch of the California Central.
The Hampshire Down breed of sheep originated about 80 years ago by a cross of South Downs on the horned, white-faced sheep which had for ages been native of the open, untilled, hilly stretch of land known as the Hampshire Downs, in the county of that name bordering on the English Channel, in the South of England. From time immemorial the South Downs had dark brown or black legs, matured early, produced the best of mutton and a fine quality of medium wool. The original Hampshire was larger, coarser, but hardier, slower to mature, with inferior flesh, and a longer but coarser wool. The South Down has always been remarkable for its power of transmitting its special characteristics to its progeny by other kinds of sheep, and hence it soon impressed its own characteristics on its progeny by the Hampshire. The horns of the original breed have disappeared; the face and legs have become dark, the frame has become more compact, the bones smaller, the back broader and straighter, the legs shorter, and the flesh and wool of better quality, while the superior hardiness and greater size, as well as the large head and Roman nose of the old breed, still remain. The Hampshires of to-day mature early and fatten readily. They clip from six to seven pounds of wool, suitable for combing, which is longer than South Down wool, but less fine. The mutton has a desirable proportion of fat and lean, and is juicy and fine flavored. The lambs are of large size and are usually dropped early and fed for market. Indeed, the Hampshire may be considered a larger and trifle coarser and hardier South Down. The breed is occasionally crossed with Cotswolds, when it produces a wool more valuable for worsted manufacturers than the pure Cotswold. Indeed, there is little doubt that in addition to South Down, the Hampshire has a dash of Cotswold blood in its composition. Considerable importations of the breed have been made into this country, but it has not become so popular as the South Down and some other English breeds. The excellent group shown is owned by Mr. James Wood, of Mount Kisco, New York.—Rural New-Yorker.
The Messrs. Repsold have established, and for the present seem likely to maintain, a practical monopoly in the construction of heliometers. That completed by them for the observatory of Yale College in 1882 leaves so little to be desired as to show excellence not to be the exclusive result of competition. In mere size it does not indeed take the highest rank. Its aperture is of only six inches, while that of the Oxford heliometer is of seven and a half; but the perfection of the arrangements adapting it to the twofold function of equatorial and micrometer stamps it as a model not easy to be surpassed. Steel has been almost exclusively used in the mounting. Recommended as the material for the objective cell by its quality of changing volume under variations of temperature nearly paripassu with glass, its employment was extended to the telescope tube and other portions of the mechanism. The optical part of the work was done by Merz, Alvan Clark having declined the responsibility of dividing the object lens. Its segments are separable to the extent of 2°, and through the contrivance of cylindrical slides (originally suggested by Bessel) perfect definition is preserved in all positions, giving a range of accurate measurement just six times that with a filar micrometer. (Gill, "Encyc. Brit.," vol. xvi., p. 253; Fischer, Sirius, vol. xvii., p. 145.)
This beautiful engine of research was in 1883 placed in the already practiced and skillful hands of Dr. Elkin. He lost no time in fixing upon a task suited both to test the powers of the new instrument and to employ them to the highest advantage.
The stars of the Pleiades have, from the earliest times, attracted the special notice of observers, whether savage or civilized. Hence, on the one hand, their prominence in stellar mythology all over the world; on the other, their unique interest for purposes of scientific study and comparison. They constitute an undoubted cluster; that is to say, they are really, and not simply in appearance, grouped together in space, so as to fall under the sway of prevailing mutual influences. And since there is, perhaps, no other stellar cluster so near the sun, the chance of perceptible displacements among them in a moderate lapse of time is greater than in any other similar case. Authentic data regarding them, besides, have now been so long garnered that their fruit may confidently be expected at least to begin to ripen.
Dr. Elkin determined, accordingly, to repeat the survey of the Pleiades executed by Bessel at Konigsberg during about twelve years previous to 1841. Wolf and Pritchard had, it is true, been beforehand with him; but the wide scattering of the grouped stars puts the filar micrometer at a disadvantage in measuring them, producing minute errors which the arduous conditions of the problem render of serious account. The heliometer, there can be no doubt, is the special instrument for the purpose, and it was, moreover, that employed by Bessel; so that the Konigsberg and Yale results are comparable in a stricter sense than any others so far obtained.
One of Bessel's fifty-three stars was omitted by Dr. Elkin as too faint for accurate determination. He added, however, seventeen stars from the Bonn Durchmusterung, so that his list comprised sixty-nine, down to 9.2 magnitude. Two independent triangulations were executed by him in 1884-85. For the first, four stars situated near the outskirts of the group, and marking the angles of quadrilateral by which it was inclosed, were chosen as reference points. The second rested upon measures of distance and position angle outward from Alcyone (η Tauri). Thus, two wholly unconnected sets of positions were secured, the close accordance of which testified strongly to the high quality of the entire work. They were combined, with nearly equal weights, in the final results. A fresh reduction of the Konigsberg observations, necessitated by recent improvements in the value of some of the corrections employed, was the preliminary to their comparison with those made, after an interval of forty-five years, at Yale College. The conclusions thus laboriously arrived at are not devoid of significance, and appear perfectly secure, so far as they go.
It has been known for some time that the stars of the Pleiades possess a small identical proper motion. Its direction, as ascertained by Newcomb in 1878, is about south-southeast; its amount is somewhat less than six seconds of arc in a century. The double star 61 Cygni, in fact, is displaced very nearly as much in one year as Alcyone with its train in one hundred. Nor is there much probability that this slow secular shifting is other than apparent; since it pretty accurately reverses the course of the sun's translation through space, it may be presumed that the backward current of movement in which the Pleiades seem to float is purely an effect of our own onward traveling.
Now the curious fact emerges from Dr. Elkin's inquiries that six of Bessel's stars are exempt from the general drift of the group. They are being progressively left behind. The inference is obvious that they do not in reality belong to, but are merely accidentally projected upon, it; or, rather, that it is projected upon them; for their apparent immobility (which, in two of the six, may be called absolute) shows them with tolerable certainty to be indefinitely more remote—so remote that the path, moderately estimated at 21,000,000,000 miles in length, traversed by the solar system during the forty-five years elapsed since the Konigsberg measures dwindles into visual insensibility when beheld from them. The brightest of these six far-off stars is just above the eighth (7.9) magnitude; the others range from 8.5 down to below the ninth.
A chart of the relative displacements indicated for Bessel's stars by the differences in their inter-mutual positions as determined at Konigsberg and Yale accompanies the paper before us. Divergences exceeding 0.40" (taken as the limit of probable error) are regarded as due to real motion; and this is the case with twenty-six stars besides the half dozen already mentioned as destined deserters from the group. With these last may be associated two stars surmised, for an opposite reason, to stand aloof from it. Instead of tarrying behind, they are hurrying on in front.
An excess of the proper movement of their companions belongs to them; and since that movement is presumably an effect of secular parallax, we are justified in inferring their possession of an extra share of it to signify their greater proximity to the sun. Hence, of all the stars in the Pleiades these are the most likely to have a measurable annual parallax. One is a star a little above the seventh magnitude, distinguished as s Pleiadum; the other, of about the eighth, is numbered 25 in Bessel's list. Dr. Elkin has not omitted to remark that the conjecture of their disconnection from the cluster is confirmed by the circumstance that its typical spectrum (as shown on Prof. Pickering's plates) is varied in s by the marked character of the K line. The spectrum of its fellow traveler (No. 25) is still undetermined.
It is improbable, however, that even these nearer stars are practicable subjects for the direct determination of annual parallax. By indirect means, however, we can obtain some idea of their distance. All that we want to know for the purpose is the rate of the sun's motion; its direction we may consider as given with approximate accuracy by Airy's investigation. Now, spectroscopic measurements of stellar movements of approach and recession will eventually afford ample materials from which to deduce the solar, velocity; though they are as yet not accurate or numerous enough to found any definitive conclusion upon. Nevertheless, M. Homann's preliminary result of fifteen miles a second as the speed with which our system travels in its vast orbit inspires confidence both from the trustworthiness of the determinations (Mr. Seabroke's) serving as its basis and from its intrinsic probability. Accepting it provisionally, we find the parallax of Alcyone = about 0.02', implying a distance of 954,000,000,000,000 miles and a light journey of 163 years. It is assumed that the whole of its proper motion of 2.61' in forty-five years is the visual projection of oar own movement toward a point in R.A. 261°, Decl. +25°.
Thus the parallax of the two stars which we suspect to lie between us and the stars forming the genuine group of the Pleiades, at perhaps two-thirds of their distance, can hardly exceed 0.03'. This is just half that found by Dr. Gill for ξ Toucani, which may be regarded as, up to this, the smallest annual displacement at all satisfactorily determined. And the error of the present estimate is more likely to be on the side of excess than of defect. That is, the stars in question can hardly be much nearer to us than is implied by an annual parallax of 0.03", and they may be considerably more remote.
Dr. Elkin concludes, from the minuteness of the detected changes of position among the Pleiades, that "the hopes of obtaining any clew to the internal mechanism of this cluster seem not likely to be realized in an immediate future;" remarking further: "The bright stars in especial seem to form an almost rigid system, as for only one is there really much evidence of motion, and in this case the total amount is barely 1 per century." This one mobile member of the naked eye group is Electra; and it is noticeable that the apparent direction of its displacement favors the hypothesis of leisurely orbital circulation round the leading star. The larger movements, however, ascribed to some of the fainter associated stars are far from harmonizing with this preconceived notion of what they ought to be.
On the contrary, so far as they are known at present, they force upon our minds the idea that the cluster may be undergoing some slow process of disintegration. M. Wolf's impression of incipient centrifugal tendencies among its components certainly derives some confirmation from Dr. Elkin's chart. Divergent movements are the most strongly marked; and the region round Alcyone suggests, at the first glance, rather a very confused area of radiation for a flight of meteors than the central seat of attraction of a revolving throng of suns.
There are many signs, however, that adjacent stars in the cluster do not pursue independent courses. "Community of drift" is visible in many distinct sets; while there is as yet no perceptible evidence, from orbital motion, of association into subordinate systems. The three eighth-magnitude stars, for instance, arranged in a small isosceles triangle near Alcyone, do not, as might have been expected a priori, constitute a real ternary group. They are all apparently traveling directly away from the large star close by them, in straight lines which may, of course, be the projections of closed curves; but their rates of travel are so different as to involve certain progressive separation. Obviously, the order and method of such movements as are just beginning to develop to our apprehension among the Pleiades will not prove easy to divine.—A.M. Clerke, in Nature.
"Determination of the Relative Positions of the Principal Stars in the Group of the Pleiades." By William L. Elkin. Transactions of the Astronomical Observatory of Yale University, Vol. I., Part I. (New Haven: 1887.)
I believe Prof. Ehrenberg was one of the first to examine, microscopically, deep sea dredgings, some of which were undertaken for the Atlantic cable expedition, 1857.
I propose to deal with the bottoms brought up from tropical waters of the Atlantic, a few years ago, during certain telegraph cable operations. These soundings were made for survey purposes, and not for any biological or chemical investigations. Still I think that this imperfect record may be a useful contribution to chemical science, bearing especially on marine operations.
Although there is little to be added to the chemistry of this subject, still I think there are few chemists who could successfully make an analysis of a deep sea "bottom" without some sacrifice of time and patience, to say nothing of the risk of wasting a valuable specimen.
The muds, clays, oozes, etc., from deep water are so very fine that they pass readily through the best kinds of filters, and it is necessary to wash out all traces of sea water as a preliminary. The specimen must be repeatedly washed by decantation, until the washings are perfectly free from chlorine, when the whole may be thrown onto a filter merely to drain. The turbid water which passes through is allowed to stand so that the suspended matter may settle, and after decanting the clear supernatant water, the residuum is again thrown on to the filter.
The washing and getting ready for the drying oven will, in some cases, require days to carry out, if we wish to avoid losing anything.
So far the proceeding is exactly the same, except draining on a filter, which would be adopted for preparing for the microscope. On no account should the opportunity be missed of mounting several slides permanently for microscopic examination. Drawings or photographic enlargements will render us independent of direct microscopic appeal, which is not at all times convenient.
The substance, if drained and allowed to dry on the filter, will adhere most tenaciously to it, so that it is better to complete the drying in a porcelain or platinum capsule, either by swilling the filter with a jet of water or by carefully removing with a spatula. The most strenuous care must be used not to contaminate the specimen with loose fibers from the filter.
The perfectly dried matter is best treated in exactly the same way as a residuum in water analysis. It is a common thing to ignite the residuum, and to put the loss down, if any, to water. This ought not to satisfy an accurate observer, since organic matter, carbonates—especially in presence of silica—will easily add to the loss. The best plan is to heat a small portion very cautiously, and note if any smell or alteration in color, due to carbon, etc., is perceptible, and to proceed accordingly.
I have seen some very satisfactory analyses made on board ship by a skillful use of the blowpipe, where liquid reagents would be very inconvenient to employ.
It will be necessary to say a few words as to the way in which soundings are made at sea. When the bottom consists of sand, mud, or other loose matter, it is easy enough to bring specimens to the surface, and, of course, we know in such a case that the bottom has been reached, but, in the event of the bottom being hard and rocky, it is not easy to say that our sounding has been successful: and here we meet with a difficulty which unfortunately is most unsatisfactorily provided for.
The lead is "cast," as the saying goes, "armed" for this emergency. An iron sinker is made with a hollow recess in the bottom; this is filled in with tallow, and on striking the bottom any loose matter may adhere by being pressed into the tallow. If the bottom is rocky or hard we get simply an imprint in the arming, and when such a result is obtained the usual construction is that "the bottom is rocky" or hard.
Now, this seems to me a point on which chemistry may give some very valuable help, for I am convinced that no sounding should be accepted unless evidence of the bottom itself is obtained. A few considerations will show that when we are working in very deep water, where there is a difficulty of knowing for certain that we have an "up and down" sounding, and the hardening of the "arming" by the cold and pressure, unless we bring up something we cannot be sure that we have touched the bottom; leaving the doubt on this point on one side, unless we use a very heavy sinker, so as to get an indication of the released strain when it touches the bottom, we encounter another complication.
Sir William Thomson's sounding wire has added the element of reliability to our soundings in this latter case. The note given out by the wire when the bottom is reached is perceptibly different when under strain, even if the dynamometer should give an unreliable indication.
It has been found that when a "bottom" has been recovered by the arming with tallow, the adherent grease seriously detracts from the value of the specimen for scientific purposes. Washing with perfectly pure bisulphide carbon will save the sounding, but of course any living organism is destroyed. As we have plenty of contrivances for bringing up loose "bottoms" without arming, we have nothing to fear on this score.
There is a great difficulty to explain the vast accumulations of clay deposits on the ocean bed, and it has been suggested that some minute organisms may produce these deposits, as others give us carbonate of lime. Is there not a very great probability of some of the apparently insoluble rocky formations being answerable for these accumulations?
We must not forget the peculiar changes which such an apparently stable substance as feldspar undergoes when disintegrated and exposed to the chemical action of sea water. As these deposits contain both sodium and potassium, our chemical operations must provide for the analytical results; in other respects the analysis can be proceeded with according to the operator's analytical knowledge.
Few operators are aware of the usefulness of an ordinary deep sea grapnel rope, as used for cable work, in recovering specimens of the fauna of any locality. The grapnel rope should be left down for a few months, so that the denizens of the deep may get used to it and make it their place of residence and attachment. The stench caused by their decomposition, unless the rope be kept in water, when hauled up will be in a few days intolerable, even to an individual with a sea-going stomach. I tried several chemical solutions for preserving specimens thus recovered, but nothing answered so well as the water itself drawn up from the same depth as the rope was recovered from.—Chem. News.
$2.50 a Year. Single Copies, 25 cts.
This is a Special Edition of the SCIENTIFIC AMERICAN, issued monthly—on the first day of the month. Each number contains about forty large quarto pages, equal to about two hundred ordinary book pages, forming, practically, a large and splendid Magazine of Architecture, richly adorned with elegant plates in colors and with fine engravings, illustrating the most interesting examples of modern Architectural Construction and allied subjects.
A special feature is the presentation in each number of a variety of the latest and best plans for private residences, city and country, including those of very moderate cost as well as the more expensive. Drawings in perspective and in color are given, together with full Plans, Specifications, Costs, Bills of Estimate, and Sheets of Details.
No other building paper contains so many plans, details, and specifications regularly presented as the SCIENTIFIC AMERICAN. Hundreds of dwellings have already been erected on the various plans we have issued during the past year, and many others are in process of construction.
Architects, Builders, and Owners will find this work valuable in furnishing fresh and useful suggestions. All who contemplate building or improving homes, or erecting structures of any kind, have before them in this work an almost endless series of the latest and best examples from which to make selections, thus saving time and money.
Many other subjects, including Sewerage, Piping, Lighting, Warming, Ventilating, Decorating, Laying out of Grounds, etc., are illustrated. An extensive Compendium of Manufacturers' Announcements is also given, in which the most reliable and approved Building Materials, Goods, Machines, Tools, and Appliances are described and illustrated, with addresses of the makers, etc.
The fullness, richness, cheapness, and convenience of this work have won for it the Largest Circulation of any Architectural publication in the world
MUNN& CO., Publishers,
361 Broadway, New York.
A Catalogue of valuable books on Architecture,
Building, Carpentry, Masonry, Heating, Warming,
Lighting, Ventilation, and all branches of industry
pertaining to the art of Building, is supplied free of
charge, sent to any address.
In connection with the publication of the BUILDING EDITION of the SCIENTIFIC AMERICAN, Messrs. Munn & Co. furnish plans and specifications for buildings of every kind, including Churches, Schools, Stores, Dwellings, Carriage Houses, Barns, etc.
In this work they are assisted by able and experienced architects. Full plans, details, and specifications for the various buildings illustrated in this paper can be supplied.
Those who contemplate building, or who wish to alter, improve, extend, or add to existing buildings, whether wings, porches, bay windows, or attic rooms, are invited to communicate with the undersigned. Our work extends to all parts of the country. Estimates, plans, and drawings promptly prepared. Terms moderate. Address
MUNN & CO., 361 BROADWAY, NEW YORK.
Published Weekly.
Terms of Subscription, $5 a year.
Sent by mail, postage prepaid, to subscribers in any part of the United States or Canada. Six dollars a year, sent, prepaid, to any foreign country.
All the back numbers of THE SUPPLEMENT, from the commencement, January 1, 1876, can be had. Price, 10 cents each.
All the back volumes of THE SUPPLEMENT can likewise be supplied. Two volumes are issued yearly. Price of each volume, $2.50 stitched in paper, or $3.50 bound in stiff covers.
COMBINED RATES.—One copy of SCIENTIFIC AMERICAN and one copy of SCIENTIFIC AMERICAN SUPPLEMENT, one year, postpaid, $7.00.
A liberal discount to booksellers, news agents, and canvassers.
MUNN & CO., Publishers,
361 Broadway, New York, N.Y.
In connection with the Scientific American, Messrs. MUNN & Co. are solicitors of American and Foreign Patents, have had 42 years' experience, and now have the largest establishment in the world. Patents are obtained on the best terms.
A special notice is made in the Scientific American of all inventions patented through this Agency, with the name and residence of the Patentee. By the immense circulation thus given, public attention is directed to the merits of the new patent, and sales or introduction often easily effected.
Any person who has made a new discovery or invention can ascertain, free of charge, whether a patent can probably be obtained, by writing to MUNN & CO.
We also send free our Hand Book about the Patent Laws, Patents, Caveats, Trade Marks, their costs, and how procured. Address
MUNN & CO.
361 Broadway, New York.
Branch Office, 622 and 624 F St., Washington, D.C.
End of the Project Gutenberg EBook of Scientific American Supplement, No.
623, December 10, 1887, by Various
*** END OF THIS PROJECT GUTENBERG EBOOK SCIENTIFIC AMERICAN ***
***** This file should be named 16270-h.htm or 16270-h.zip *****
This and all associated files of various formats will be found in:
https://www.gutenberg.org/1/6/2/7/16270/
Produced by Juliet Sutherland and the Online Distributed
Proofreading Team at www.pgdp.net.
Updated editions will replace the previous one--the old editions
will be renamed.
Creating the works from public domain print editions means that no
one owns a United States copyright in these works, so the Foundation
(and you!) can copy and distribute it in the United States without
permission and without paying copyright royalties. Special rules,
set forth in the General Terms of Use part of this license, apply to
copying and distributing Project Gutenberg-tm electronic works to
protect the PROJECT GUTENBERG-tm concept and trademark. Project
Gutenberg is a registered trademark, and may not be used if you
charge for the eBooks, unless you receive specific permission. If you
do not charge anything for copies of this eBook, complying with the
rules is very easy. You may use this eBook for nearly any purpose
such as creation of derivative works, reports, performances and
research. They may be modified and printed and given away--you may do
practically ANYTHING with public domain eBooks. Redistribution is
subject to the trademark license, especially commercial
redistribution.
*** START: FULL LICENSE ***
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full Project
Gutenberg-tm License (available with this file or online at
https://gutenberg.org/license).
Section 1. General Terms of Use and Redistributing Project Gutenberg-tm
electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or destroy
all copies of Project Gutenberg-tm electronic works in your possession.
If you paid a fee for obtaining a copy of or access to a Project
Gutenberg-tm electronic work and you do not agree to be bound by the
terms of this agreement, you may obtain a refund from the person or
entity to whom you paid the fee as set forth in paragraph 1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this agreement
and help preserve free future access to Project Gutenberg-tm electronic
works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the Foundation"
or PGLAF), owns a compilation copyright in the collection of Project
Gutenberg-tm electronic works. Nearly all the individual works in the
collection are in the public domain in the United States. If an
individual work is in the public domain in the United States and you are
located in the United States, we do not claim a right to prevent you from
copying, distributing, performing, displaying or creating derivative
works based on the work as long as all references to Project Gutenberg
are removed. Of course, we hope that you will support the Project
Gutenberg-tm mission of promoting free access to electronic works by
freely sharing Project Gutenberg-tm works in compliance with the terms of
this agreement for keeping the Project Gutenberg-tm name associated with
the work. You can easily comply with the terms of this agreement by
keeping this work in the same format with its attached full Project
Gutenberg-tm License when you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are in
a constant state of change. If you are outside the United States, check
the laws of your country in addition to the terms of this agreement
before downloading, copying, displaying, performing, distributing or
creating derivative works based on this work or any other Project
Gutenberg-tm work. The Foundation makes no representations concerning
the copyright status of any work in any country outside the United
States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other immediate
access to, the full Project Gutenberg-tm License must appear prominently
whenever any copy of a Project Gutenberg-tm work (any work on which the
phrase "Project Gutenberg" appears, or with which the phrase "Project
Gutenberg" is associated) is accessed, displayed, performed, viewed,
copied or distributed:
This eBook is for the use of anyone anywhere at no cost and with
almost no restrictions whatsoever. You may copy it, give it away or
re-use it under the terms of the Project Gutenberg License included
with this eBook or online at www.gutenberg.org
1.E.2. If an individual Project Gutenberg-tm electronic work is derived
from the public domain (does not contain a notice indicating that it is
posted with permission of the copyright holder), the work can be copied
and distributed to anyone in the United States without paying any fees
or charges. If you are redistributing or providing access to a work
with the phrase "Project Gutenberg" associated with or appearing on the
work, you must comply either with the requirements of paragraphs 1.E.1
through 1.E.7 or obtain permission for the use of the work and the
Project Gutenberg-tm trademark as set forth in paragraphs 1.E.8 or
1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any additional
terms imposed by the copyright holder. Additional terms will be linked
to the Project Gutenberg-tm License for all works posted with the
permission of the copyright holder found at the beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including any
word processing or hypertext form. However, if you provide access to or
distribute copies of a Project Gutenberg-tm work in a format other than
"Plain Vanilla ASCII" or other format used in the official version
posted on the official Project Gutenberg-tm web site (www.gutenberg.org),
you must, at no additional cost, fee or expense to the user, provide a
copy, a means of exporting a copy, or a means of obtaining a copy upon
request, of the work in its original "Plain Vanilla ASCII" or other
form. Any alternate format must include the full Project Gutenberg-tm
License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works provided
that
- You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is
owed to the owner of the Project Gutenberg-tm trademark, but he
has agreed to donate royalties under this paragraph to the
Project Gutenberg Literary Archive Foundation. Royalty payments
must be paid within 60 days following each date on which you
prepare (or are legally required to prepare) your periodic tax
returns. Royalty payments should be clearly marked as such and
sent to the Project Gutenberg Literary Archive Foundation at the
address specified in Section 4, "Information about donations to
the Project Gutenberg Literary Archive Foundation."
- You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or
destroy all copies of the works possessed in a physical medium
and discontinue all use of and all access to other copies of
Project Gutenberg-tm works.
- You provide, in accordance with paragraph 1.F.3, a full refund of any
money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days
of receipt of the work.
- You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project Gutenberg-tm
electronic work or group of works on different terms than are set
forth in this agreement, you must obtain permission in writing from
both the Project Gutenberg Literary Archive Foundation and Michael
Hart, the owner of the Project Gutenberg-tm trademark. Contact the
Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
public domain works in creating the Project Gutenberg-tm
collection. Despite these efforts, Project Gutenberg-tm electronic
works, and the medium on which they may be stored, may contain
"Defects," such as, but not limited to, incomplete, inaccurate or
corrupt data, transcription errors, a copyright or other intellectual
property infringement, a defective or damaged disk or other medium, a
computer virus, or computer codes that damage or cannot be read by
your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH F3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium with
your written explanation. The person or entity that provided you with
the defective work may elect to provide a replacement copy in lieu of a
refund. If you received the work electronically, the person or entity
providing it to you may choose to give you a second opportunity to
receive the work electronically in lieu of a refund. If the second copy
is also defective, you may demand a refund in writing without further
opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS' WITH NO OTHER
WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO
WARRANTIES OF MERCHANTIBILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of damages.
If any disclaimer or limitation set forth in this agreement violates the
law of the state applicable to this agreement, the agreement shall be
interpreted to make the maximum disclaimer or limitation permitted by
the applicable state law. The invalidity or unenforceability of any
provision of this agreement shall not void the remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in accordance
with this agreement, and any volunteers associated with the production,
promotion and distribution of Project Gutenberg-tm electronic works,
harmless from all liability, costs and expenses, including legal fees,
that arise directly or indirectly from any of the following which you do
or cause to occur: (a) distribution of this or any Project Gutenberg-tm
work, (b) alteration, modification, or additions or deletions to any
Project Gutenberg-tm work, and (c) any Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of computers
including obsolete, old, middle-aged and new computers. It exists
because of the efforts of hundreds of volunteers and donations from
people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need, is critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future generations.
To learn more about the Project Gutenberg Literary Archive Foundation
and how your efforts and donations can help, see Sections 3 and 4
and the Foundation web page at https://www.pglaf.org.
Section 3. Information about the Project Gutenberg Literary Archive
Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Its 501(c)(3) letter is posted at
https://pglaf.org/fundraising. Contributions to the Project Gutenberg
Literary Archive Foundation are tax deductible to the full extent
permitted by U.S. federal laws and your state's laws.
The Foundation's principal office is located at 4557 Melan Dr. S.
Fairbanks, AK, 99712., but its volunteers and employees are scattered
throughout numerous locations. Its business office is located at
809 North 1500 West, Salt Lake City, UT 84116, (801) 596-1887, email
[email protected]. Email contact links and up to date contact
information can be found at the Foundation's web site and official
page at https://pglaf.org
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
[email protected]
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To
SEND DONATIONS or determine the status of compliance for any
particular state visit https://pglaf.org
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including including checks, online payments and credit card
donations. To donate, please visit: https://pglaf.org/donate
Section 5. General Information About Project Gutenberg-tm electronic
works.
Professor Michael S. Hart was the originator of the Project Gutenberg-tm
concept of a library of electronic works that could be freely shared
with anyone. For thirty years, he produced and distributed Project
Gutenberg-tm eBooks with only a loose network of volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as Public Domain in the U.S.
unless a copyright notice is included. Thus, we do not necessarily
keep eBooks in compliance with any particular paper edition.
Most people start at our Web site which has the main PG search facility:
https://www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.